User login
Rheumatologist Volunteers Make a Difference to Those in Need at Home and Overseas
As a resident, rheumatologist Daniel Albert, MD, did his first volunteer mission to Afghanistan. The clinic had one portable chest x-ray machine, and physicians could order a complete blood count but no other laboratory studies.
“We could do sputum stains, but that was about it. You had to use your clinical acumen and make decisions based on examining the patient and taking a history,” said Dr. Albert, a professor of medicine and pediatrics at the Geisel School of Medicine at Dartmouth, Hanover, and The Dartmouth Institute in Lebanon, both in New Hampshire. Such tasks can be difficult in a non–English-speaking country.
“There’s a language barrier no matter where you are,” Dr. Albert said.
In Nashville, Tennessee, James Gore, MD, had an epiphany about opening a free rheumatology clinic during a church service. His priest was discussing St. Sampson the Hospitable’s story and closed with “you don’t have to change the world. All you have to do is your little part,” Dr. Gore said. He knew he didn’t need much: a computer, a stethoscope, and a printer for prescriptions.
When his church expanded its building space, Dr. Gore took the opportunity to achieve his goal.
“I didn’t feel responsible for the clinic to succeed, but I did feel responsible to try my best,” he said. That was 14 years ago. To date, the monthly clinic has served 1124 patients representing 55 counties in Tennessee and several other patients from Kentucky.
Volunteer work is a juggling act. Dr. Gore divides his time between the clinic and his work as associate professor of clinical medicine at Vanderbilt University Medical Center (VUMC), also in Nashville.
Dr. Albert often gave up his vacation time and had to balance commitments with his own medical practice and family to do his overseas missions. In his view, it’s worth the extra time and effort.
“It makes you a better physician because you make reasonable decisions and conclusions based on the resources available. Various places had various limitations, but none of them had the kind of resources that we routinely avail ourselves of in the US,” he said.
Tennessee Clients Get Access to Care, Medications
In some parts of the United States, good rheumatology care is hard to come by. One in four people in Tennessee have no health insurance. There’s a big need for rheumatology care in the state, Dr. Gore said.
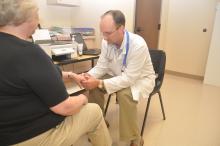
On the second Saturday of each month, he volunteers his services at the St. Sampson Medical Clinic at Holy Trinity Greek Orthodox Church, Nashville, Tennessee, from 9 AM to 4 PM, providing care for uninsured adult rheumatology patients.
Patients come by referral from a charity clinic or health department and appointment only. The clinic asks for a $10 payment for their visits. “If they can’t pay, we still see them. But we only take care of patients who don’t have insurance,” Dr. Gore said. Allowing patients to pay gives them an opportunity to show they are vested in their own care. Often, patients will donate extra in gratitude.
Dr. Gore, along with VUMC colleague and rheumatologist Narender Annapureddy, MD, and nurse practitioner Julie Barnes, treats a variety of rheumatic diseases. For Ms. Barnes, volunteering has many rewarding aspects, “as the patients would be unable to have the treatments they need without insurance,” she said.
“We have had patients waiting for many months or sometimes years and have not had a diagnosis, and in a short time, we have been able to diagnose and get them on specific treatment,” Dr. Annapureddy said.
Most people come in for rheumatoid arthritis (RA) and lupus and also positive antinuclear antibody tests. They also see patients with psoriatic arthritis, Sjögren’s disease, gout, scleroderma, Behçet disease, and leukocytoclastic vasculitis. On a typical clinic day, the team can treat up to 30-plus patients. The clinic recently expanded its services to include cardiology care, seeing about 10 patients each month.
Prior to St. Sampson, there were no volunteer clinics in Tennessee specifically dedicated to helping patients with rheumatologic disease. Untreated, these diseases may cause chronic, severe pain, lead to irreversible joint damage, and increase the risk for death.
Many patients have received medications such as adalimumab, etanercept, or tofacitinib for free. The drug companies will provide free medications, provided that they’re prescribed by a board-certified rheumatologist and the patient is uninsured and qualifies for the medication, Dr. Gore said.
Drugs like these can cost about $50,000 a year. “We have pharmacists that donate their time to help these patients get approved for those medicines,” Dr. Gore said. To date, more than 100 patients have received a biologic or targeted synthetic disease-modifying antirheumatic drug through the clinic.
The clinic has received more than $100,000 in donated professional fees, including $48,706 for consultations. Dr. Gore and colleagues relied on other volunteers to bring the clinic to life. He worked with his sister to develop an electronic medical record system that the clinic still uses today. “We did not buy expensive laptops or printers. I had a very generous volunteer, Damon Miltner, our IT guy, who set everything up to make our intranet secure,” he said.
The volunteer nurses, IT, and front desk all work together to make the clinic run efficiently, said Ms. Barnes, who also works as a nurse practitioner with Vanderbilt Rheumatology Cool Springs in Franklin, Tennessee. “We share a lunch together, all in a beautiful and holy church. I do not think of this as work, but as spending time with people who are appreciative and kind,” she said.
“It is amazing to see patients who are able to walk in by themselves after having used a cane for years,” Dr. Annapureddy said. “While doing this on weekends with young kids is challenging, having a supportive spouse who shares the same value makes it much easier to be able to do volunteer work.”
Working Outside Your Comfort Zone
Dr. Albert has traveled to all parts of the world to volunteer his services as a rheumatologist and general practitioner. This includes missions to Uganda, Rwanda, Ecuador, Peru, Nepal, and Borneo. He’s participated with several volunteer organizations, among them the International Student & Scholar Services program at the University of Pennsylvania, CARE, Global Volunteers, Project Amazonas, Asha Nepal, Health in Harmony, and several others.
Rheumatologists who volunteer in underdeveloped countries should be prepared to work outside of their specialty — and their comfort zone. In some instances, Dr. Albert took care of AIDS-related infectious diseases. “It’s not something I am particularly knowledgeable about, and I actually spent a fair amount of time reading about it before I went on the plane in order to get some comfort level.”
Dr. Albert often found himself doing more primary care and general pediatrics than rheumatology care. “I would see rheumatic conditions. But there’s not a lot of RA in developing countries, which is something that people have noted before. And the same goes for other autoimmune conditions. They’re just not that common.”
He did see a lot of septic arthritis and tuberculosis in Uganda. “We had a rheum clinic and saw a mixture of the consequences of septic arthritis and also a few RA and lupus patients.”
Limited resources are another thing to prepare for.
Whenever he traveled to a place that didn’t have a lot of resources, Dr. Albert would collect as many supplies as he could from the nearest hospital, pack them away, and try to get the supplies to the mission location.
Sometimes it worked out, and sometimes it didn’t, he said. “I probably had $10,000 worth of medical supplies when I went to Armenia, and American Airlines lost it. It ended up back in my apartment 3 months later. That was unfortunate because there was lot of good stuff there.”
He thought about FedEx-ing some supplies to a mission in Uganda, but it was astronomically expensive, so that didn’t work.
Luggage weight restrictions are another obstacle that sometimes requires a waiver. Dr. Albert once had to get the Red Cross to work with an airline to get a luggage waiver. “Other airlines were very good and didn’t have those kinds of restrictions. But most of the time I got some supplies to go with me, and sometimes that was a very helpful addition,” especially if the mission site was lacking in resources, he said.
When Charity Work Produces Success Stories
During one of his missions in Uganda with the University of Pennsylvania, Dr. Albert helped the Makerere University Medical School, Kampala, to establish a rheumatology clinic, which was affiliated with Mulago National Specialised Hospital. The clinic operated once a week for half a day, mostly treating patients with RA and lupus.
The mission also established an AIDS clinic. Many of the patients with musculoskeletal complaints also had HIV and were able to get antiretroviral drugs through the clinic, he said.
For Dr. Gore, seeing patients from more than half the counties in Tennessee was one of the clinic’s biggest accomplishments. “That was all through word of mouth,” he said.
In rheumatology, many patients may feel their condition is hopeless, Ms. Barnes noted. “There have been many patients that, through months of proper treatment, have normal lives. A high percentage would be disabled without the needed medical therapies.”
Dr. Gore has seen patients who literally couldn’t walk or had severe, painful psoriasis all over their body. The clinic would put them on medicine that would give them new life. The psoriasis would clear up, or their joints would heal, and they could walk again.
One of Dr. Gore’s patients, a woman in her mid-50s, got on an expensive medication that brought her arthritis into remission. She’s now able to care for her grandchildren.
The fact that the clinic, with the help of volunteer pharmacologists, can provide medications to enable patients to have a less destructive disease and improved quality of life “is a major reward,” Ms. Barnes said.
Balancing Your Priorities
Overseas missions can last for a few weeks to several months, depending on the mission, the organization, and the type of care involved.
Rheumatologists who want to volunteer need to do so in a way that doesn’t generate a lot of angst with supervisors or colleagues. Dr. Albert balanced this by keeping his missions reasonably short. “I would have someone cover my service. And since there’s reciprocity in the places I worked for, if they covered me for a month, I would cover them for a month, so it wasn’t a burden on anybody.”
“By and large, I used my vacation time to do it, and it does cost some money, but it’s a lot less than the cost of a typical vacation,” Dr. Albert said.
Volunteer work can also compete with family time. Dr. Albert ended up taking his family along on several of his missions to Ecuador and Uganda. He would tell the organization: “My family wants to come. Is there anything they can do while I’m working in the program? And they usually found an occupation.”
At St. Sampson, volunteering is also a family affair. “My wife acts as the administrator, so she’s the one that helps schedule patients and deals with a lot of the faxes.” It’s a big commitment for Dr. Gore’s family and for the church, which gives up a significant chunk of the building one Saturday a month.
“However, for us, I think that it’s a real manifestation of giving back and trying to help those in need and doing what we can do,” he said.
Volunteer Work Involves Prep Work
Establishing the St. Sampson clinic took some planning. Dr. Gore and colleagues had to fill out a 501(c)(3) application; establish a charter, bylaws, articles of incorporation, policies, and procedures; and obtain medical malpractice and general liability insurance.
The clinic was able to get financing from the Mid-South Chapter of the Lupus Foundation of America as well as in-kind donations from the church. “We’ve had a lot of different companies who were very generous in donating money and excited to help the clinic continue,” Dr. Gore said.
All volunteers sign a Health Insurance Portability and Accountability Act consent form.
Although the clinic operates for about 7 hours a month, it’s still important to have malpractice insurance, Dr. Gore said. He and his colleagues also have tail insurance that covers medical malpractice insurance for up to 7 years if the clinic closes.
“If somebody were to slip and fall and then try to sue the church, we have a separate policy for the clinic for that. We also have a director’s and officer’s insurance policy,” he said.
Anyone who volunteers abroad should get a travel medicine clinic consultation. “Most of the time, it’s of very little consequence. You might have to get [a] yellow fever vaccine” when traveling to certain parts of the world, Dr. Albert said.
“If you’re going into an area that is all volatile politically or in some way a threat to your personal security, I think you have to think very carefully about that,” he said, suggesting that doctors consult with the US Department of State about potential dangers.
Talk to other physicians who have gone on missions and your sponsoring institution. “By and large, you want to go with a large organization that’s been doing ongoing work,” Dr. Albert said.
Volunteer work teaches you about the breadth of humanist endeavors across the world, he noted. “The people that you deal with are very grateful for your help. Whether you’re successful or not, they’re still very appreciative of the efforts that you’re making to help.”
Dr. Albert and Dr. Gore had no disclosures. Dr. Annapureddy has done consulting for GlaxoSmithKline. Ms. Barnes had no disclosures.
A version of this article first appeared on Medscape.com.
As a resident, rheumatologist Daniel Albert, MD, did his first volunteer mission to Afghanistan. The clinic had one portable chest x-ray machine, and physicians could order a complete blood count but no other laboratory studies.
“We could do sputum stains, but that was about it. You had to use your clinical acumen and make decisions based on examining the patient and taking a history,” said Dr. Albert, a professor of medicine and pediatrics at the Geisel School of Medicine at Dartmouth, Hanover, and The Dartmouth Institute in Lebanon, both in New Hampshire. Such tasks can be difficult in a non–English-speaking country.
“There’s a language barrier no matter where you are,” Dr. Albert said.
In Nashville, Tennessee, James Gore, MD, had an epiphany about opening a free rheumatology clinic during a church service. His priest was discussing St. Sampson the Hospitable’s story and closed with “you don’t have to change the world. All you have to do is your little part,” Dr. Gore said. He knew he didn’t need much: a computer, a stethoscope, and a printer for prescriptions.
When his church expanded its building space, Dr. Gore took the opportunity to achieve his goal.
“I didn’t feel responsible for the clinic to succeed, but I did feel responsible to try my best,” he said. That was 14 years ago. To date, the monthly clinic has served 1124 patients representing 55 counties in Tennessee and several other patients from Kentucky.
Volunteer work is a juggling act. Dr. Gore divides his time between the clinic and his work as associate professor of clinical medicine at Vanderbilt University Medical Center (VUMC), also in Nashville.
Dr. Albert often gave up his vacation time and had to balance commitments with his own medical practice and family to do his overseas missions. In his view, it’s worth the extra time and effort.
“It makes you a better physician because you make reasonable decisions and conclusions based on the resources available. Various places had various limitations, but none of them had the kind of resources that we routinely avail ourselves of in the US,” he said.
Tennessee Clients Get Access to Care, Medications
In some parts of the United States, good rheumatology care is hard to come by. One in four people in Tennessee have no health insurance. There’s a big need for rheumatology care in the state, Dr. Gore said.
On the second Saturday of each month, he volunteers his services at the St. Sampson Medical Clinic at Holy Trinity Greek Orthodox Church, Nashville, Tennessee, from 9 AM to 4 PM, providing care for uninsured adult rheumatology patients.
Patients come by referral from a charity clinic or health department and appointment only. The clinic asks for a $10 payment for their visits. “If they can’t pay, we still see them. But we only take care of patients who don’t have insurance,” Dr. Gore said. Allowing patients to pay gives them an opportunity to show they are vested in their own care. Often, patients will donate extra in gratitude.
Dr. Gore, along with VUMC colleague and rheumatologist Narender Annapureddy, MD, and nurse practitioner Julie Barnes, treats a variety of rheumatic diseases. For Ms. Barnes, volunteering has many rewarding aspects, “as the patients would be unable to have the treatments they need without insurance,” she said.
“We have had patients waiting for many months or sometimes years and have not had a diagnosis, and in a short time, we have been able to diagnose and get them on specific treatment,” Dr. Annapureddy said.
Most people come in for rheumatoid arthritis (RA) and lupus and also positive antinuclear antibody tests. They also see patients with psoriatic arthritis, Sjögren’s disease, gout, scleroderma, Behçet disease, and leukocytoclastic vasculitis. On a typical clinic day, the team can treat up to 30-plus patients. The clinic recently expanded its services to include cardiology care, seeing about 10 patients each month.
Prior to St. Sampson, there were no volunteer clinics in Tennessee specifically dedicated to helping patients with rheumatologic disease. Untreated, these diseases may cause chronic, severe pain, lead to irreversible joint damage, and increase the risk for death.
Many patients have received medications such as adalimumab, etanercept, or tofacitinib for free. The drug companies will provide free medications, provided that they’re prescribed by a board-certified rheumatologist and the patient is uninsured and qualifies for the medication, Dr. Gore said.
Drugs like these can cost about $50,000 a year. “We have pharmacists that donate their time to help these patients get approved for those medicines,” Dr. Gore said. To date, more than 100 patients have received a biologic or targeted synthetic disease-modifying antirheumatic drug through the clinic.
The clinic has received more than $100,000 in donated professional fees, including $48,706 for consultations. Dr. Gore and colleagues relied on other volunteers to bring the clinic to life. He worked with his sister to develop an electronic medical record system that the clinic still uses today. “We did not buy expensive laptops or printers. I had a very generous volunteer, Damon Miltner, our IT guy, who set everything up to make our intranet secure,” he said.
The volunteer nurses, IT, and front desk all work together to make the clinic run efficiently, said Ms. Barnes, who also works as a nurse practitioner with Vanderbilt Rheumatology Cool Springs in Franklin, Tennessee. “We share a lunch together, all in a beautiful and holy church. I do not think of this as work, but as spending time with people who are appreciative and kind,” she said.
“It is amazing to see patients who are able to walk in by themselves after having used a cane for years,” Dr. Annapureddy said. “While doing this on weekends with young kids is challenging, having a supportive spouse who shares the same value makes it much easier to be able to do volunteer work.”
Working Outside Your Comfort Zone
Dr. Albert has traveled to all parts of the world to volunteer his services as a rheumatologist and general practitioner. This includes missions to Uganda, Rwanda, Ecuador, Peru, Nepal, and Borneo. He’s participated with several volunteer organizations, among them the International Student & Scholar Services program at the University of Pennsylvania, CARE, Global Volunteers, Project Amazonas, Asha Nepal, Health in Harmony, and several others.
Rheumatologists who volunteer in underdeveloped countries should be prepared to work outside of their specialty — and their comfort zone. In some instances, Dr. Albert took care of AIDS-related infectious diseases. “It’s not something I am particularly knowledgeable about, and I actually spent a fair amount of time reading about it before I went on the plane in order to get some comfort level.”
Dr. Albert often found himself doing more primary care and general pediatrics than rheumatology care. “I would see rheumatic conditions. But there’s not a lot of RA in developing countries, which is something that people have noted before. And the same goes for other autoimmune conditions. They’re just not that common.”
He did see a lot of septic arthritis and tuberculosis in Uganda. “We had a rheum clinic and saw a mixture of the consequences of septic arthritis and also a few RA and lupus patients.”
Limited resources are another thing to prepare for.
Whenever he traveled to a place that didn’t have a lot of resources, Dr. Albert would collect as many supplies as he could from the nearest hospital, pack them away, and try to get the supplies to the mission location.
Sometimes it worked out, and sometimes it didn’t, he said. “I probably had $10,000 worth of medical supplies when I went to Armenia, and American Airlines lost it. It ended up back in my apartment 3 months later. That was unfortunate because there was lot of good stuff there.”
He thought about FedEx-ing some supplies to a mission in Uganda, but it was astronomically expensive, so that didn’t work.
Luggage weight restrictions are another obstacle that sometimes requires a waiver. Dr. Albert once had to get the Red Cross to work with an airline to get a luggage waiver. “Other airlines were very good and didn’t have those kinds of restrictions. But most of the time I got some supplies to go with me, and sometimes that was a very helpful addition,” especially if the mission site was lacking in resources, he said.
When Charity Work Produces Success Stories
During one of his missions in Uganda with the University of Pennsylvania, Dr. Albert helped the Makerere University Medical School, Kampala, to establish a rheumatology clinic, which was affiliated with Mulago National Specialised Hospital. The clinic operated once a week for half a day, mostly treating patients with RA and lupus.
The mission also established an AIDS clinic. Many of the patients with musculoskeletal complaints also had HIV and were able to get antiretroviral drugs through the clinic, he said.
For Dr. Gore, seeing patients from more than half the counties in Tennessee was one of the clinic’s biggest accomplishments. “That was all through word of mouth,” he said.
In rheumatology, many patients may feel their condition is hopeless, Ms. Barnes noted. “There have been many patients that, through months of proper treatment, have normal lives. A high percentage would be disabled without the needed medical therapies.”
Dr. Gore has seen patients who literally couldn’t walk or had severe, painful psoriasis all over their body. The clinic would put them on medicine that would give them new life. The psoriasis would clear up, or their joints would heal, and they could walk again.
One of Dr. Gore’s patients, a woman in her mid-50s, got on an expensive medication that brought her arthritis into remission. She’s now able to care for her grandchildren.
The fact that the clinic, with the help of volunteer pharmacologists, can provide medications to enable patients to have a less destructive disease and improved quality of life “is a major reward,” Ms. Barnes said.
Balancing Your Priorities
Overseas missions can last for a few weeks to several months, depending on the mission, the organization, and the type of care involved.
Rheumatologists who want to volunteer need to do so in a way that doesn’t generate a lot of angst with supervisors or colleagues. Dr. Albert balanced this by keeping his missions reasonably short. “I would have someone cover my service. And since there’s reciprocity in the places I worked for, if they covered me for a month, I would cover them for a month, so it wasn’t a burden on anybody.”
“By and large, I used my vacation time to do it, and it does cost some money, but it’s a lot less than the cost of a typical vacation,” Dr. Albert said.
Volunteer work can also compete with family time. Dr. Albert ended up taking his family along on several of his missions to Ecuador and Uganda. He would tell the organization: “My family wants to come. Is there anything they can do while I’m working in the program? And they usually found an occupation.”
At St. Sampson, volunteering is also a family affair. “My wife acts as the administrator, so she’s the one that helps schedule patients and deals with a lot of the faxes.” It’s a big commitment for Dr. Gore’s family and for the church, which gives up a significant chunk of the building one Saturday a month.
“However, for us, I think that it’s a real manifestation of giving back and trying to help those in need and doing what we can do,” he said.
Volunteer Work Involves Prep Work
Establishing the St. Sampson clinic took some planning. Dr. Gore and colleagues had to fill out a 501(c)(3) application; establish a charter, bylaws, articles of incorporation, policies, and procedures; and obtain medical malpractice and general liability insurance.
The clinic was able to get financing from the Mid-South Chapter of the Lupus Foundation of America as well as in-kind donations from the church. “We’ve had a lot of different companies who were very generous in donating money and excited to help the clinic continue,” Dr. Gore said.
All volunteers sign a Health Insurance Portability and Accountability Act consent form.
Although the clinic operates for about 7 hours a month, it’s still important to have malpractice insurance, Dr. Gore said. He and his colleagues also have tail insurance that covers medical malpractice insurance for up to 7 years if the clinic closes.
“If somebody were to slip and fall and then try to sue the church, we have a separate policy for the clinic for that. We also have a director’s and officer’s insurance policy,” he said.
Anyone who volunteers abroad should get a travel medicine clinic consultation. “Most of the time, it’s of very little consequence. You might have to get [a] yellow fever vaccine” when traveling to certain parts of the world, Dr. Albert said.
“If you’re going into an area that is all volatile politically or in some way a threat to your personal security, I think you have to think very carefully about that,” he said, suggesting that doctors consult with the US Department of State about potential dangers.
Talk to other physicians who have gone on missions and your sponsoring institution. “By and large, you want to go with a large organization that’s been doing ongoing work,” Dr. Albert said.
Volunteer work teaches you about the breadth of humanist endeavors across the world, he noted. “The people that you deal with are very grateful for your help. Whether you’re successful or not, they’re still very appreciative of the efforts that you’re making to help.”
Dr. Albert and Dr. Gore had no disclosures. Dr. Annapureddy has done consulting for GlaxoSmithKline. Ms. Barnes had no disclosures.
A version of this article first appeared on Medscape.com.
As a resident, rheumatologist Daniel Albert, MD, did his first volunteer mission to Afghanistan. The clinic had one portable chest x-ray machine, and physicians could order a complete blood count but no other laboratory studies.
“We could do sputum stains, but that was about it. You had to use your clinical acumen and make decisions based on examining the patient and taking a history,” said Dr. Albert, a professor of medicine and pediatrics at the Geisel School of Medicine at Dartmouth, Hanover, and The Dartmouth Institute in Lebanon, both in New Hampshire. Such tasks can be difficult in a non–English-speaking country.
“There’s a language barrier no matter where you are,” Dr. Albert said.
In Nashville, Tennessee, James Gore, MD, had an epiphany about opening a free rheumatology clinic during a church service. His priest was discussing St. Sampson the Hospitable’s story and closed with “you don’t have to change the world. All you have to do is your little part,” Dr. Gore said. He knew he didn’t need much: a computer, a stethoscope, and a printer for prescriptions.
When his church expanded its building space, Dr. Gore took the opportunity to achieve his goal.
“I didn’t feel responsible for the clinic to succeed, but I did feel responsible to try my best,” he said. That was 14 years ago. To date, the monthly clinic has served 1124 patients representing 55 counties in Tennessee and several other patients from Kentucky.
Volunteer work is a juggling act. Dr. Gore divides his time between the clinic and his work as associate professor of clinical medicine at Vanderbilt University Medical Center (VUMC), also in Nashville.
Dr. Albert often gave up his vacation time and had to balance commitments with his own medical practice and family to do his overseas missions. In his view, it’s worth the extra time and effort.
“It makes you a better physician because you make reasonable decisions and conclusions based on the resources available. Various places had various limitations, but none of them had the kind of resources that we routinely avail ourselves of in the US,” he said.
Tennessee Clients Get Access to Care, Medications
In some parts of the United States, good rheumatology care is hard to come by. One in four people in Tennessee have no health insurance. There’s a big need for rheumatology care in the state, Dr. Gore said.
On the second Saturday of each month, he volunteers his services at the St. Sampson Medical Clinic at Holy Trinity Greek Orthodox Church, Nashville, Tennessee, from 9 AM to 4 PM, providing care for uninsured adult rheumatology patients.
Patients come by referral from a charity clinic or health department and appointment only. The clinic asks for a $10 payment for their visits. “If they can’t pay, we still see them. But we only take care of patients who don’t have insurance,” Dr. Gore said. Allowing patients to pay gives them an opportunity to show they are vested in their own care. Often, patients will donate extra in gratitude.
Dr. Gore, along with VUMC colleague and rheumatologist Narender Annapureddy, MD, and nurse practitioner Julie Barnes, treats a variety of rheumatic diseases. For Ms. Barnes, volunteering has many rewarding aspects, “as the patients would be unable to have the treatments they need without insurance,” she said.
“We have had patients waiting for many months or sometimes years and have not had a diagnosis, and in a short time, we have been able to diagnose and get them on specific treatment,” Dr. Annapureddy said.
Most people come in for rheumatoid arthritis (RA) and lupus and also positive antinuclear antibody tests. They also see patients with psoriatic arthritis, Sjögren’s disease, gout, scleroderma, Behçet disease, and leukocytoclastic vasculitis. On a typical clinic day, the team can treat up to 30-plus patients. The clinic recently expanded its services to include cardiology care, seeing about 10 patients each month.
Prior to St. Sampson, there were no volunteer clinics in Tennessee specifically dedicated to helping patients with rheumatologic disease. Untreated, these diseases may cause chronic, severe pain, lead to irreversible joint damage, and increase the risk for death.
Many patients have received medications such as adalimumab, etanercept, or tofacitinib for free. The drug companies will provide free medications, provided that they’re prescribed by a board-certified rheumatologist and the patient is uninsured and qualifies for the medication, Dr. Gore said.
Drugs like these can cost about $50,000 a year. “We have pharmacists that donate their time to help these patients get approved for those medicines,” Dr. Gore said. To date, more than 100 patients have received a biologic or targeted synthetic disease-modifying antirheumatic drug through the clinic.
The clinic has received more than $100,000 in donated professional fees, including $48,706 for consultations. Dr. Gore and colleagues relied on other volunteers to bring the clinic to life. He worked with his sister to develop an electronic medical record system that the clinic still uses today. “We did not buy expensive laptops or printers. I had a very generous volunteer, Damon Miltner, our IT guy, who set everything up to make our intranet secure,” he said.
The volunteer nurses, IT, and front desk all work together to make the clinic run efficiently, said Ms. Barnes, who also works as a nurse practitioner with Vanderbilt Rheumatology Cool Springs in Franklin, Tennessee. “We share a lunch together, all in a beautiful and holy church. I do not think of this as work, but as spending time with people who are appreciative and kind,” she said.
“It is amazing to see patients who are able to walk in by themselves after having used a cane for years,” Dr. Annapureddy said. “While doing this on weekends with young kids is challenging, having a supportive spouse who shares the same value makes it much easier to be able to do volunteer work.”
Working Outside Your Comfort Zone
Dr. Albert has traveled to all parts of the world to volunteer his services as a rheumatologist and general practitioner. This includes missions to Uganda, Rwanda, Ecuador, Peru, Nepal, and Borneo. He’s participated with several volunteer organizations, among them the International Student & Scholar Services program at the University of Pennsylvania, CARE, Global Volunteers, Project Amazonas, Asha Nepal, Health in Harmony, and several others.
Rheumatologists who volunteer in underdeveloped countries should be prepared to work outside of their specialty — and their comfort zone. In some instances, Dr. Albert took care of AIDS-related infectious diseases. “It’s not something I am particularly knowledgeable about, and I actually spent a fair amount of time reading about it before I went on the plane in order to get some comfort level.”
Dr. Albert often found himself doing more primary care and general pediatrics than rheumatology care. “I would see rheumatic conditions. But there’s not a lot of RA in developing countries, which is something that people have noted before. And the same goes for other autoimmune conditions. They’re just not that common.”
He did see a lot of septic arthritis and tuberculosis in Uganda. “We had a rheum clinic and saw a mixture of the consequences of septic arthritis and also a few RA and lupus patients.”
Limited resources are another thing to prepare for.
Whenever he traveled to a place that didn’t have a lot of resources, Dr. Albert would collect as many supplies as he could from the nearest hospital, pack them away, and try to get the supplies to the mission location.
Sometimes it worked out, and sometimes it didn’t, he said. “I probably had $10,000 worth of medical supplies when I went to Armenia, and American Airlines lost it. It ended up back in my apartment 3 months later. That was unfortunate because there was lot of good stuff there.”
He thought about FedEx-ing some supplies to a mission in Uganda, but it was astronomically expensive, so that didn’t work.
Luggage weight restrictions are another obstacle that sometimes requires a waiver. Dr. Albert once had to get the Red Cross to work with an airline to get a luggage waiver. “Other airlines were very good and didn’t have those kinds of restrictions. But most of the time I got some supplies to go with me, and sometimes that was a very helpful addition,” especially if the mission site was lacking in resources, he said.
When Charity Work Produces Success Stories
During one of his missions in Uganda with the University of Pennsylvania, Dr. Albert helped the Makerere University Medical School, Kampala, to establish a rheumatology clinic, which was affiliated with Mulago National Specialised Hospital. The clinic operated once a week for half a day, mostly treating patients with RA and lupus.
The mission also established an AIDS clinic. Many of the patients with musculoskeletal complaints also had HIV and were able to get antiretroviral drugs through the clinic, he said.
For Dr. Gore, seeing patients from more than half the counties in Tennessee was one of the clinic’s biggest accomplishments. “That was all through word of mouth,” he said.
In rheumatology, many patients may feel their condition is hopeless, Ms. Barnes noted. “There have been many patients that, through months of proper treatment, have normal lives. A high percentage would be disabled without the needed medical therapies.”
Dr. Gore has seen patients who literally couldn’t walk or had severe, painful psoriasis all over their body. The clinic would put them on medicine that would give them new life. The psoriasis would clear up, or their joints would heal, and they could walk again.
One of Dr. Gore’s patients, a woman in her mid-50s, got on an expensive medication that brought her arthritis into remission. She’s now able to care for her grandchildren.
The fact that the clinic, with the help of volunteer pharmacologists, can provide medications to enable patients to have a less destructive disease and improved quality of life “is a major reward,” Ms. Barnes said.
Balancing Your Priorities
Overseas missions can last for a few weeks to several months, depending on the mission, the organization, and the type of care involved.
Rheumatologists who want to volunteer need to do so in a way that doesn’t generate a lot of angst with supervisors or colleagues. Dr. Albert balanced this by keeping his missions reasonably short. “I would have someone cover my service. And since there’s reciprocity in the places I worked for, if they covered me for a month, I would cover them for a month, so it wasn’t a burden on anybody.”
“By and large, I used my vacation time to do it, and it does cost some money, but it’s a lot less than the cost of a typical vacation,” Dr. Albert said.
Volunteer work can also compete with family time. Dr. Albert ended up taking his family along on several of his missions to Ecuador and Uganda. He would tell the organization: “My family wants to come. Is there anything they can do while I’m working in the program? And they usually found an occupation.”
At St. Sampson, volunteering is also a family affair. “My wife acts as the administrator, so she’s the one that helps schedule patients and deals with a lot of the faxes.” It’s a big commitment for Dr. Gore’s family and for the church, which gives up a significant chunk of the building one Saturday a month.
“However, for us, I think that it’s a real manifestation of giving back and trying to help those in need and doing what we can do,” he said.
Volunteer Work Involves Prep Work
Establishing the St. Sampson clinic took some planning. Dr. Gore and colleagues had to fill out a 501(c)(3) application; establish a charter, bylaws, articles of incorporation, policies, and procedures; and obtain medical malpractice and general liability insurance.
The clinic was able to get financing from the Mid-South Chapter of the Lupus Foundation of America as well as in-kind donations from the church. “We’ve had a lot of different companies who were very generous in donating money and excited to help the clinic continue,” Dr. Gore said.
All volunteers sign a Health Insurance Portability and Accountability Act consent form.
Although the clinic operates for about 7 hours a month, it’s still important to have malpractice insurance, Dr. Gore said. He and his colleagues also have tail insurance that covers medical malpractice insurance for up to 7 years if the clinic closes.
“If somebody were to slip and fall and then try to sue the church, we have a separate policy for the clinic for that. We also have a director’s and officer’s insurance policy,” he said.
Anyone who volunteers abroad should get a travel medicine clinic consultation. “Most of the time, it’s of very little consequence. You might have to get [a] yellow fever vaccine” when traveling to certain parts of the world, Dr. Albert said.
“If you’re going into an area that is all volatile politically or in some way a threat to your personal security, I think you have to think very carefully about that,” he said, suggesting that doctors consult with the US Department of State about potential dangers.
Talk to other physicians who have gone on missions and your sponsoring institution. “By and large, you want to go with a large organization that’s been doing ongoing work,” Dr. Albert said.
Volunteer work teaches you about the breadth of humanist endeavors across the world, he noted. “The people that you deal with are very grateful for your help. Whether you’re successful or not, they’re still very appreciative of the efforts that you’re making to help.”
Dr. Albert and Dr. Gore had no disclosures. Dr. Annapureddy has done consulting for GlaxoSmithKline. Ms. Barnes had no disclosures.
A version of this article first appeared on Medscape.com.
Ustekinumab’s ‘Egregious’ Medicare Part B and D Pricing Differences Led to Federal Intervention
A US government report showed how a Medicare policy change made the drug ustekinumab (Stelara) for autoimmune diseases much more expensive, a finding that experts say illustrates the need for reforms created by the Inflation Reduction Act of 2022 (IRA).
The topline findings of an August report from the Department of Health and Human Services (HHS) about ustekinumab may seem somewhat surprising and a bit counterintuitive.
Ustekinumab costs spiked as Medicare pushed patients to get their supply through the Part D pharmacy program. The aim of Part D is to make medicines more affordable and accessible for patients. It runs on a model of insurers to negotiate deals for pharmaceuticals.
Earlier, many patients who needed ustekinumab had the drug covered by Medicare Part B. For many years, Medicare Part B has been largely a passive purchaser of medicines. Part B covers drugs administered by physicians. Its longtime model has been to add a premium of 6% to the reported average sales price to reimburse physicians who buy and administer the drug for patients.
But it was Part D, the Medicare program based on insurers’ negotiating clout, that saw a spike in ustekinumab costs after patients were shifted out of Part B coverage, where the cost of the medicine fell.
The average reported Part B cost for an ustekinumab injection slipped from $14,450 in 2016 to $12,912 by 2023, according to the report from HHS’ Office of Inspector General (OIG).
The Part D cost jumped in the same period. It rose by 84% from $17,717 in 2016 to $32,559 by 2023.
The IRA is intended to curb these kinds of increases in the future for drugs covered by Medicare, said Stacie B. Dusetzina, PhD, professor of health policy at Vanderbilt University School of Medicine, Nashville, Tennessee. The law demands companies pay rebates to Medicare if they increase drug prices faster than consumer inflation.
“That should at least help with some of this price growth that over time has seemed quite egregious,” Dr. Dusetzina told this news organization.
The IRA contains several provisions intended to curb rising drug costs for people enrolled in Medicare, including allowing the federal government to directly negotiate on some medicines.
Ustekinumab is one of the first 10 medicines that are subject to negotiations. Medicare will select as many as 15 additional drugs covered under Part D for negotiation in 2025, another 15 Part B and D drugs in 2026, and up to 20 drugs every year after that.
Earlier in August, the Centers for Medicare & Medicaid Services (CMS) announced the results of its first drug negotiations, with prices set to take effect in 2026. The Part D price for a 30-day supply of ustekinumab will be $4695 in 2026, a 66% reduction from the list price last year of $13,836.
Even at the negotiated price, ustekinumab’s cost will be high enough to trigger a new cap on out-of-pocket Part D spending, Dr. Dusetzina said.
Starting in 2025, Part D will have a cap of $2000 on individuals’ out-of-pocket costs, with annual adjustments in future years.
“It may not be better for someone who was filling this on Part B, who had a supplement [that covered their share of the ustekinumab cost], but it will be better for a lot of people that it’s covered under Part D,” Dr. Dusetzina said. “The good news is that at least from a beneficiary affordability standpoint, they’re going to have some price protection.”
OIG noted that the US Food and Drug Administration has approved three competing biosimilar versions of ustekinumab. These could also potentially work to lower costs.
‘A Complicated and Not Particularly Transparent Process’
OIG said it expects to release a report later this year with more detail about the decision that shifted ustekinumab coverage from Part B to Part D.
First cleared for US sales in 2009, ustekinumab is approved for psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It can be given subcutaneously or intravenously.
Part B does not generally cover self-administered drugs. The infused version of ustekinumab has been covered under Medicare Part B since it reached the market.
“However, Part B coverage of the subcutaneous versions has been less straightforward,” OIG said in the report.
In 2020, Medicare administrative contractors — the units or affiliates of insurers that for decades have processed Part B claims for the traditional Medicare programs — determined that subcutaneous ustekinumab did not meet the criteria for coverage under Part B. Implementation of this change was delayed due to the COVID public health emergency but has since taken effect.
The shift in ustekinumab coverage to Part D eroded financial protections of many people on Medicare when Part B covered the drug.
Almost 9 in 10 people enrolled in Medicare Part B have supplemental insurance such as Medigap, employer coverage, or Medicaid to fully or partially cover their cost-sharing requirements, the OIG report said. That means Part B coverage shielded many patients from high ustekinumab costs.
In contrast, patients who self-administered the drug at home under Part D coverage paid an average of almost $6000 out of pocket if they did not receive any type of financial assistance, OIG said.
“From a financial standpoint, as long as you have Part B coinsurance, it would be much cheaper to get the drug in your doctor’s office than getting it through a pharmacy, unless you qualify for the low-income subsidy,” OIG Regional Inspector General David Tawes, who supervised the team that produced the report, told this news organization.
OIG has previously reported that post–point-of-sale rebates paid by manufacturers sometimes lower the costs incurred by Part D plans by a significant margin. But this was not the case with ustekinumab. Instead, OIG said the gap between initial and actual costs of ustekinumab was reduced by less than one third even with rebates. Rebate information is considered confidential.
“The whole negotiation structure is a complicated and not particularly transparent process,” Mr. Tawes said.
Backchannel Discounts, Top-Line Prices
The IRA is bringing some more transparency to the process through negotiations, said Mariana P. Socal, MD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Patients who buy medicines that have been through the CMS negotiation process will be able to see if they are being charged correctly.
Dr. Socal noted that there’s something of a disconnect in discussions of Part D between how insurers and consumers view prices.
For Part D plans, the list prices represent the beginning of negotiations. They get rebates from drugmakers’ list prices for medicines, which insurers say work to lower premium costs.
“For plans, those prices are unrealistic. They are simply a sticker price. But for patients, for the Medicare beneficiaries, these prices are very real” because they are used to set copays, Dr. Socal said.
Dr. Dusetzina reported receiving funding from Arnold Ventures and the Commonwealth Fund for research related to drug pricing. Dr. Socal reported receiving funding from Arnold Ventures.
A version of this article first appeared on Medscape.com.
A US government report showed how a Medicare policy change made the drug ustekinumab (Stelara) for autoimmune diseases much more expensive, a finding that experts say illustrates the need for reforms created by the Inflation Reduction Act of 2022 (IRA).
The topline findings of an August report from the Department of Health and Human Services (HHS) about ustekinumab may seem somewhat surprising and a bit counterintuitive.
Ustekinumab costs spiked as Medicare pushed patients to get their supply through the Part D pharmacy program. The aim of Part D is to make medicines more affordable and accessible for patients. It runs on a model of insurers to negotiate deals for pharmaceuticals.
Earlier, many patients who needed ustekinumab had the drug covered by Medicare Part B. For many years, Medicare Part B has been largely a passive purchaser of medicines. Part B covers drugs administered by physicians. Its longtime model has been to add a premium of 6% to the reported average sales price to reimburse physicians who buy and administer the drug for patients.
But it was Part D, the Medicare program based on insurers’ negotiating clout, that saw a spike in ustekinumab costs after patients were shifted out of Part B coverage, where the cost of the medicine fell.
The average reported Part B cost for an ustekinumab injection slipped from $14,450 in 2016 to $12,912 by 2023, according to the report from HHS’ Office of Inspector General (OIG).
The Part D cost jumped in the same period. It rose by 84% from $17,717 in 2016 to $32,559 by 2023.
The IRA is intended to curb these kinds of increases in the future for drugs covered by Medicare, said Stacie B. Dusetzina, PhD, professor of health policy at Vanderbilt University School of Medicine, Nashville, Tennessee. The law demands companies pay rebates to Medicare if they increase drug prices faster than consumer inflation.
“That should at least help with some of this price growth that over time has seemed quite egregious,” Dr. Dusetzina told this news organization.
The IRA contains several provisions intended to curb rising drug costs for people enrolled in Medicare, including allowing the federal government to directly negotiate on some medicines.
Ustekinumab is one of the first 10 medicines that are subject to negotiations. Medicare will select as many as 15 additional drugs covered under Part D for negotiation in 2025, another 15 Part B and D drugs in 2026, and up to 20 drugs every year after that.
Earlier in August, the Centers for Medicare & Medicaid Services (CMS) announced the results of its first drug negotiations, with prices set to take effect in 2026. The Part D price for a 30-day supply of ustekinumab will be $4695 in 2026, a 66% reduction from the list price last year of $13,836.
Even at the negotiated price, ustekinumab’s cost will be high enough to trigger a new cap on out-of-pocket Part D spending, Dr. Dusetzina said.
Starting in 2025, Part D will have a cap of $2000 on individuals’ out-of-pocket costs, with annual adjustments in future years.
“It may not be better for someone who was filling this on Part B, who had a supplement [that covered their share of the ustekinumab cost], but it will be better for a lot of people that it’s covered under Part D,” Dr. Dusetzina said. “The good news is that at least from a beneficiary affordability standpoint, they’re going to have some price protection.”
OIG noted that the US Food and Drug Administration has approved three competing biosimilar versions of ustekinumab. These could also potentially work to lower costs.
‘A Complicated and Not Particularly Transparent Process’
OIG said it expects to release a report later this year with more detail about the decision that shifted ustekinumab coverage from Part B to Part D.
First cleared for US sales in 2009, ustekinumab is approved for psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It can be given subcutaneously or intravenously.
Part B does not generally cover self-administered drugs. The infused version of ustekinumab has been covered under Medicare Part B since it reached the market.
“However, Part B coverage of the subcutaneous versions has been less straightforward,” OIG said in the report.
In 2020, Medicare administrative contractors — the units or affiliates of insurers that for decades have processed Part B claims for the traditional Medicare programs — determined that subcutaneous ustekinumab did not meet the criteria for coverage under Part B. Implementation of this change was delayed due to the COVID public health emergency but has since taken effect.
The shift in ustekinumab coverage to Part D eroded financial protections of many people on Medicare when Part B covered the drug.
Almost 9 in 10 people enrolled in Medicare Part B have supplemental insurance such as Medigap, employer coverage, or Medicaid to fully or partially cover their cost-sharing requirements, the OIG report said. That means Part B coverage shielded many patients from high ustekinumab costs.
In contrast, patients who self-administered the drug at home under Part D coverage paid an average of almost $6000 out of pocket if they did not receive any type of financial assistance, OIG said.
“From a financial standpoint, as long as you have Part B coinsurance, it would be much cheaper to get the drug in your doctor’s office than getting it through a pharmacy, unless you qualify for the low-income subsidy,” OIG Regional Inspector General David Tawes, who supervised the team that produced the report, told this news organization.
OIG has previously reported that post–point-of-sale rebates paid by manufacturers sometimes lower the costs incurred by Part D plans by a significant margin. But this was not the case with ustekinumab. Instead, OIG said the gap between initial and actual costs of ustekinumab was reduced by less than one third even with rebates. Rebate information is considered confidential.
“The whole negotiation structure is a complicated and not particularly transparent process,” Mr. Tawes said.
Backchannel Discounts, Top-Line Prices
The IRA is bringing some more transparency to the process through negotiations, said Mariana P. Socal, MD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Patients who buy medicines that have been through the CMS negotiation process will be able to see if they are being charged correctly.
Dr. Socal noted that there’s something of a disconnect in discussions of Part D between how insurers and consumers view prices.
For Part D plans, the list prices represent the beginning of negotiations. They get rebates from drugmakers’ list prices for medicines, which insurers say work to lower premium costs.
“For plans, those prices are unrealistic. They are simply a sticker price. But for patients, for the Medicare beneficiaries, these prices are very real” because they are used to set copays, Dr. Socal said.
Dr. Dusetzina reported receiving funding from Arnold Ventures and the Commonwealth Fund for research related to drug pricing. Dr. Socal reported receiving funding from Arnold Ventures.
A version of this article first appeared on Medscape.com.
A US government report showed how a Medicare policy change made the drug ustekinumab (Stelara) for autoimmune diseases much more expensive, a finding that experts say illustrates the need for reforms created by the Inflation Reduction Act of 2022 (IRA).
The topline findings of an August report from the Department of Health and Human Services (HHS) about ustekinumab may seem somewhat surprising and a bit counterintuitive.
Ustekinumab costs spiked as Medicare pushed patients to get their supply through the Part D pharmacy program. The aim of Part D is to make medicines more affordable and accessible for patients. It runs on a model of insurers to negotiate deals for pharmaceuticals.
Earlier, many patients who needed ustekinumab had the drug covered by Medicare Part B. For many years, Medicare Part B has been largely a passive purchaser of medicines. Part B covers drugs administered by physicians. Its longtime model has been to add a premium of 6% to the reported average sales price to reimburse physicians who buy and administer the drug for patients.
But it was Part D, the Medicare program based on insurers’ negotiating clout, that saw a spike in ustekinumab costs after patients were shifted out of Part B coverage, where the cost of the medicine fell.
The average reported Part B cost for an ustekinumab injection slipped from $14,450 in 2016 to $12,912 by 2023, according to the report from HHS’ Office of Inspector General (OIG).
The Part D cost jumped in the same period. It rose by 84% from $17,717 in 2016 to $32,559 by 2023.
The IRA is intended to curb these kinds of increases in the future for drugs covered by Medicare, said Stacie B. Dusetzina, PhD, professor of health policy at Vanderbilt University School of Medicine, Nashville, Tennessee. The law demands companies pay rebates to Medicare if they increase drug prices faster than consumer inflation.
“That should at least help with some of this price growth that over time has seemed quite egregious,” Dr. Dusetzina told this news organization.
The IRA contains several provisions intended to curb rising drug costs for people enrolled in Medicare, including allowing the federal government to directly negotiate on some medicines.
Ustekinumab is one of the first 10 medicines that are subject to negotiations. Medicare will select as many as 15 additional drugs covered under Part D for negotiation in 2025, another 15 Part B and D drugs in 2026, and up to 20 drugs every year after that.
Earlier in August, the Centers for Medicare & Medicaid Services (CMS) announced the results of its first drug negotiations, with prices set to take effect in 2026. The Part D price for a 30-day supply of ustekinumab will be $4695 in 2026, a 66% reduction from the list price last year of $13,836.
Even at the negotiated price, ustekinumab’s cost will be high enough to trigger a new cap on out-of-pocket Part D spending, Dr. Dusetzina said.
Starting in 2025, Part D will have a cap of $2000 on individuals’ out-of-pocket costs, with annual adjustments in future years.
“It may not be better for someone who was filling this on Part B, who had a supplement [that covered their share of the ustekinumab cost], but it will be better for a lot of people that it’s covered under Part D,” Dr. Dusetzina said. “The good news is that at least from a beneficiary affordability standpoint, they’re going to have some price protection.”
OIG noted that the US Food and Drug Administration has approved three competing biosimilar versions of ustekinumab. These could also potentially work to lower costs.
‘A Complicated and Not Particularly Transparent Process’
OIG said it expects to release a report later this year with more detail about the decision that shifted ustekinumab coverage from Part B to Part D.
First cleared for US sales in 2009, ustekinumab is approved for psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It can be given subcutaneously or intravenously.
Part B does not generally cover self-administered drugs. The infused version of ustekinumab has been covered under Medicare Part B since it reached the market.
“However, Part B coverage of the subcutaneous versions has been less straightforward,” OIG said in the report.
In 2020, Medicare administrative contractors — the units or affiliates of insurers that for decades have processed Part B claims for the traditional Medicare programs — determined that subcutaneous ustekinumab did not meet the criteria for coverage under Part B. Implementation of this change was delayed due to the COVID public health emergency but has since taken effect.
The shift in ustekinumab coverage to Part D eroded financial protections of many people on Medicare when Part B covered the drug.
Almost 9 in 10 people enrolled in Medicare Part B have supplemental insurance such as Medigap, employer coverage, or Medicaid to fully or partially cover their cost-sharing requirements, the OIG report said. That means Part B coverage shielded many patients from high ustekinumab costs.
In contrast, patients who self-administered the drug at home under Part D coverage paid an average of almost $6000 out of pocket if they did not receive any type of financial assistance, OIG said.
“From a financial standpoint, as long as you have Part B coinsurance, it would be much cheaper to get the drug in your doctor’s office than getting it through a pharmacy, unless you qualify for the low-income subsidy,” OIG Regional Inspector General David Tawes, who supervised the team that produced the report, told this news organization.
OIG has previously reported that post–point-of-sale rebates paid by manufacturers sometimes lower the costs incurred by Part D plans by a significant margin. But this was not the case with ustekinumab. Instead, OIG said the gap between initial and actual costs of ustekinumab was reduced by less than one third even with rebates. Rebate information is considered confidential.
“The whole negotiation structure is a complicated and not particularly transparent process,” Mr. Tawes said.
Backchannel Discounts, Top-Line Prices
The IRA is bringing some more transparency to the process through negotiations, said Mariana P. Socal, MD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Patients who buy medicines that have been through the CMS negotiation process will be able to see if they are being charged correctly.
Dr. Socal noted that there’s something of a disconnect in discussions of Part D between how insurers and consumers view prices.
For Part D plans, the list prices represent the beginning of negotiations. They get rebates from drugmakers’ list prices for medicines, which insurers say work to lower premium costs.
“For plans, those prices are unrealistic. They are simply a sticker price. But for patients, for the Medicare beneficiaries, these prices are very real” because they are used to set copays, Dr. Socal said.
Dr. Dusetzina reported receiving funding from Arnold Ventures and the Commonwealth Fund for research related to drug pricing. Dr. Socal reported receiving funding from Arnold Ventures.
A version of this article first appeared on Medscape.com.
From Scrubs to Social Media: How Some Med Students Become Influencers
A medical student’s life is an endless cycle of classes, exams, clinical rotations, and residency preparation. On TikTok and Instagram, among other sites, they share medical school experiences and lessons learned in the classroom and advocate for causes such as increased diversity and gender rights in the medical field.
This news organization caught up with a few social media influencers with a large online following to learn how medical students can effectively use social media to build a professional brand and network. Most of the students interviewed said that their social media platforms offered an opportunity to educate others about significant medical developments, feel part of a community with a like-minded audience, and network with doctors who may lead them to a future residency or career path.
Many med students said that they built their large audiences by creating a platform for people of their ethnic background, nationality, race, gender, or simply what others weren’t already talking about. They said they saw a niche in social media that was missing or others hadn’t tackled in the same way.
When Joel Bervell began med school in 2020, he questioned some of the lessons he learned about how race is used in medical practice, which didn’t make sense to him. So, he began his own research. He had about 2000 followers on Instagram at the time.
Mr. Bervell read a new study about pulse oximeters and how they often produce misleading readings on patients with dark skin.
He wondered why he hadn’t learned this in medical school, so he posted it on TikTok. Within 24 hours, about 500,000 people viewed it. Most of the comments were from doctors, nurses, and physician assistants who said they weren’t aware of the disparity.
While his initial posts detailed his journey to medical school and a day-in-the-life of a medical student, he transitioned to posts primarily about race, health equity, and what he perceives as racial bias in medicine.
Now, the fourth-year Ghanaian-American student at the Elson S. Floyd College of Medicine at Washington State University Spokane has close to 1.2 million followers on Instagram and TikTok combined. He frequently visits the White House to advise on social media’s influence on healthcare and has appeared on the Kelly Clarkson Show, Good Morning America, CNN, and ABC, among others.
He said he also uses social media to translate complex medical information for a general audience, many of whom access health information online so they can manage their own healthcare. He sees his social media work as an extension of his medical education, allowing him to delve deeper into subjects and report on them as if he were publishing research in a medical journal.
“When I came to medical school, yes, I wanted to be a doctor. But I also wanted to impact people.” Social media allows him to educate many more people than individual patients, the 29-year-old told this news organization.
Inspiring Minorities
Tabhata Paulet, 27, started her TikTok presence as a premed student in 2021. She aimed to provide free resources to help low-income, first-generation Latinx students like herself study for standardized exams.
“I always looked online for guidance and resources, and the medical influencers did not share a similar background. So, I shared my story and what I had to do as a first-generation and first person in my family to become a physician. I did not have access to the same resources as my peers,” said Ms. Paulet, who was born in Peru and came to New Jersey as a child.
Students who are Hispanic, Latinx, or of Spanish origin made up 6.8% of total medical school enrollment in 2023-2024, up slightly from 6.7% in 2022-2023, according to the Association of American Medical Colleges (AAMC).
Ms. Paulet’s online presence grew when she began documenting her experiences as a first-year medical student, bridging the language barrier for Spanish-speaking patients so they could understand their diagnosis and treatment. She often posts about health disparity and barriers to care for underserved communities.
Most of her nearly 22,000 followers are Hispanic, said the now fourth-year student at Rutgers New Jersey Medical School in Newark, New Jersey. “I talk a lot about my interesting Spanish-speaking patients ... and how sometimes speaking their native language truly makes a difference in their care.”
She believes that she serves an important role in social media. “It can be very inspirational for those who come after you [in med school] to see someone from a similar culture and upbringing.”
Creating a Community
It was during a therapy session 4 years ago that Jeremy “JP” Scott decided to share Instagram posts about his experiences as a nontraditional medical student. The 37-year-old was studying at Ross University School of Medicine in Barbados and was feeling lonely as an international medical student training to be a doctor as a second career.
Before starting med school, Mr. Scott was an adjunct professor and lab supervisor at the University of Hartford Biology Department, West Hartford, Connecticut, and then a research assistant and lab manager at the Wistar Institute in Philadelphia.
Although he wanted to follow his mother’s path to becoming a doctor, it was more difficult than he envisioned, said the fourth-year student who completed clinical rotations in the United States and is now applying for residencies.
“I talked about how medical school is not what it appears to be ... There are a lot of challenges we are going through,” especially as people of color, he said.
Mr. Scott believes social media helps people feel included and less alone. He said many of his followers are med students and physicians.
His posts often focus on LGBTQIA+ pride and being a minority as a Black man in medicine.
“The pandemic spurred a lot of us. We had a racial reckoning in our country at the time. It inspired us to talk as Black creators and Black medical students.”
Black or African American medical students made up 8.5% of total med school enrollment in 2023-2024, a slight increase from 2022 to 2023, according to AAMC figures. Black men represented 7% of total enrollment in 2023-2024, while Black women represented 9.8%.
After only a handful of online posts in which Mr. Scott candidly discussed his mental health struggles and relationships, he attracted the attention of several medical apparel companies, including the popular FIGS scrubs. He’s now an ambassador for the company, which supports him and his content.
“My association with FIGS has helped attract a wider online audience, increasing my presence.” Today, he has 14,000 Instagram followers. “It opened up so many opportunities,” Mr. Scott said. One example is working with the national LGBTQIA+ community.
“The goal was never to be a social media influencer, to gain sponsorships or photo opportunities,” he said.
“My job, first, is as a medical student. Everything else is second. I am not trying to be a professional social media personality. I’m trying to be an actual physician.” He also tries to separate JP “social media” from Jeremy, the medical student.
“On Instagram, anyone can pull it up and see what you’re doing. The last thing I want is for them to think that I’m not serious about what I’m doing, that I’m not here to learn and become a doctor.”
Benefits and Drawbacks
Ms. Paulet said her social media following helped her connect with leaders in the Latinx medical community, including an obstetrics anesthesiologist, her intended specialty. “I don’t think I’d be able to do that without a social media platform.”
Her online activity also propelled her from regional to national leadership in the Latino Medical Student Association (LMSA). She now also runs their Instagram page, which has 14,000 followers.
Mr. Bervell believes social media is a great way to network. He’s connected with people he wouldn’t have met otherwise, including physicians. “I think it will help me get into a residency,” he said. “It allows people to know who you are ... They will be able to tell in a few videos the type of doctor I want to be.”
On the other hand, Mr. Bervell is aware of the negative impacts of social media on mental health. “You can get lost in social media.” For that reason, he often tries to disconnect. “I can go days without my phone.”
Posting on social media can be time-consuming, Mr. Bervell admitted. He said he spent about 2 hours a day researching, editing, and posting on TikTok when he first started building his following. Now, he spends about 2-3 hours a week creating videos. “I don’t post every day anymore. I don’t have the time.”
When she started building her TikTok presence, Ms. Paulet said she devoted 15 hours a week to the endeavor, but now she spends 10-12 hours a week posting online, including on LMSA’s Instagram page. “Whenever you are done with an exam or have a study break, this is something fun to do.” She also says you never know who you’re going to inspire when you put yourself out there.
“Talk about your journey, rotations, or your experience in your first or second year of medical school. Talk about milestones like board exams.”
Word to the Wise
Some students may be concerned that their posts might affect a potential residency program. But the medical students interviewed say they want to find programs that align with their values and accept them for who they are.
Mr. Scott said he’s not worried about someone not liking him because of who he is. “I am Black and openly gay. If it’s a problem, I don’t need to work with you or your institution.”
Mr. Bervell stressed that medical students should stay professional online. “I reach 5-10 million people a month, and I have to think: Would I want them to see this? You have to know at all times that someone is watching. I’m very careful about how I post. I script out every video.”
Mr. Scott agreed. He advises those interested in becoming medical influencers to know what they can’t post online. For example, to ensure safety and privacy, Mr. Scott doesn’t take photos in the hospital, show his medical badge, or post patient information. “You want to be respectful of your future medical profession,” he said.
“If it’s something my mother would be ashamed of, I don’t need to post about it.”
A version of this article first appeared on Medscape.com.
A medical student’s life is an endless cycle of classes, exams, clinical rotations, and residency preparation. On TikTok and Instagram, among other sites, they share medical school experiences and lessons learned in the classroom and advocate for causes such as increased diversity and gender rights in the medical field.
This news organization caught up with a few social media influencers with a large online following to learn how medical students can effectively use social media to build a professional brand and network. Most of the students interviewed said that their social media platforms offered an opportunity to educate others about significant medical developments, feel part of a community with a like-minded audience, and network with doctors who may lead them to a future residency or career path.
Many med students said that they built their large audiences by creating a platform for people of their ethnic background, nationality, race, gender, or simply what others weren’t already talking about. They said they saw a niche in social media that was missing or others hadn’t tackled in the same way.
When Joel Bervell began med school in 2020, he questioned some of the lessons he learned about how race is used in medical practice, which didn’t make sense to him. So, he began his own research. He had about 2000 followers on Instagram at the time.
Mr. Bervell read a new study about pulse oximeters and how they often produce misleading readings on patients with dark skin.
He wondered why he hadn’t learned this in medical school, so he posted it on TikTok. Within 24 hours, about 500,000 people viewed it. Most of the comments were from doctors, nurses, and physician assistants who said they weren’t aware of the disparity.
While his initial posts detailed his journey to medical school and a day-in-the-life of a medical student, he transitioned to posts primarily about race, health equity, and what he perceives as racial bias in medicine.
Now, the fourth-year Ghanaian-American student at the Elson S. Floyd College of Medicine at Washington State University Spokane has close to 1.2 million followers on Instagram and TikTok combined. He frequently visits the White House to advise on social media’s influence on healthcare and has appeared on the Kelly Clarkson Show, Good Morning America, CNN, and ABC, among others.
He said he also uses social media to translate complex medical information for a general audience, many of whom access health information online so they can manage their own healthcare. He sees his social media work as an extension of his medical education, allowing him to delve deeper into subjects and report on them as if he were publishing research in a medical journal.
“When I came to medical school, yes, I wanted to be a doctor. But I also wanted to impact people.” Social media allows him to educate many more people than individual patients, the 29-year-old told this news organization.
Inspiring Minorities
Tabhata Paulet, 27, started her TikTok presence as a premed student in 2021. She aimed to provide free resources to help low-income, first-generation Latinx students like herself study for standardized exams.
“I always looked online for guidance and resources, and the medical influencers did not share a similar background. So, I shared my story and what I had to do as a first-generation and first person in my family to become a physician. I did not have access to the same resources as my peers,” said Ms. Paulet, who was born in Peru and came to New Jersey as a child.
Students who are Hispanic, Latinx, or of Spanish origin made up 6.8% of total medical school enrollment in 2023-2024, up slightly from 6.7% in 2022-2023, according to the Association of American Medical Colleges (AAMC).
Ms. Paulet’s online presence grew when she began documenting her experiences as a first-year medical student, bridging the language barrier for Spanish-speaking patients so they could understand their diagnosis and treatment. She often posts about health disparity and barriers to care for underserved communities.
Most of her nearly 22,000 followers are Hispanic, said the now fourth-year student at Rutgers New Jersey Medical School in Newark, New Jersey. “I talk a lot about my interesting Spanish-speaking patients ... and how sometimes speaking their native language truly makes a difference in their care.”
She believes that she serves an important role in social media. “It can be very inspirational for those who come after you [in med school] to see someone from a similar culture and upbringing.”
Creating a Community
It was during a therapy session 4 years ago that Jeremy “JP” Scott decided to share Instagram posts about his experiences as a nontraditional medical student. The 37-year-old was studying at Ross University School of Medicine in Barbados and was feeling lonely as an international medical student training to be a doctor as a second career.
Before starting med school, Mr. Scott was an adjunct professor and lab supervisor at the University of Hartford Biology Department, West Hartford, Connecticut, and then a research assistant and lab manager at the Wistar Institute in Philadelphia.
Although he wanted to follow his mother’s path to becoming a doctor, it was more difficult than he envisioned, said the fourth-year student who completed clinical rotations in the United States and is now applying for residencies.
“I talked about how medical school is not what it appears to be ... There are a lot of challenges we are going through,” especially as people of color, he said.
Mr. Scott believes social media helps people feel included and less alone. He said many of his followers are med students and physicians.
His posts often focus on LGBTQIA+ pride and being a minority as a Black man in medicine.
“The pandemic spurred a lot of us. We had a racial reckoning in our country at the time. It inspired us to talk as Black creators and Black medical students.”
Black or African American medical students made up 8.5% of total med school enrollment in 2023-2024, a slight increase from 2022 to 2023, according to AAMC figures. Black men represented 7% of total enrollment in 2023-2024, while Black women represented 9.8%.
After only a handful of online posts in which Mr. Scott candidly discussed his mental health struggles and relationships, he attracted the attention of several medical apparel companies, including the popular FIGS scrubs. He’s now an ambassador for the company, which supports him and his content.
“My association with FIGS has helped attract a wider online audience, increasing my presence.” Today, he has 14,000 Instagram followers. “It opened up so many opportunities,” Mr. Scott said. One example is working with the national LGBTQIA+ community.
“The goal was never to be a social media influencer, to gain sponsorships or photo opportunities,” he said.
“My job, first, is as a medical student. Everything else is second. I am not trying to be a professional social media personality. I’m trying to be an actual physician.” He also tries to separate JP “social media” from Jeremy, the medical student.
“On Instagram, anyone can pull it up and see what you’re doing. The last thing I want is for them to think that I’m not serious about what I’m doing, that I’m not here to learn and become a doctor.”
Benefits and Drawbacks
Ms. Paulet said her social media following helped her connect with leaders in the Latinx medical community, including an obstetrics anesthesiologist, her intended specialty. “I don’t think I’d be able to do that without a social media platform.”
Her online activity also propelled her from regional to national leadership in the Latino Medical Student Association (LMSA). She now also runs their Instagram page, which has 14,000 followers.
Mr. Bervell believes social media is a great way to network. He’s connected with people he wouldn’t have met otherwise, including physicians. “I think it will help me get into a residency,” he said. “It allows people to know who you are ... They will be able to tell in a few videos the type of doctor I want to be.”
On the other hand, Mr. Bervell is aware of the negative impacts of social media on mental health. “You can get lost in social media.” For that reason, he often tries to disconnect. “I can go days without my phone.”
Posting on social media can be time-consuming, Mr. Bervell admitted. He said he spent about 2 hours a day researching, editing, and posting on TikTok when he first started building his following. Now, he spends about 2-3 hours a week creating videos. “I don’t post every day anymore. I don’t have the time.”
When she started building her TikTok presence, Ms. Paulet said she devoted 15 hours a week to the endeavor, but now she spends 10-12 hours a week posting online, including on LMSA’s Instagram page. “Whenever you are done with an exam or have a study break, this is something fun to do.” She also says you never know who you’re going to inspire when you put yourself out there.
“Talk about your journey, rotations, or your experience in your first or second year of medical school. Talk about milestones like board exams.”
Word to the Wise
Some students may be concerned that their posts might affect a potential residency program. But the medical students interviewed say they want to find programs that align with their values and accept them for who they are.
Mr. Scott said he’s not worried about someone not liking him because of who he is. “I am Black and openly gay. If it’s a problem, I don’t need to work with you or your institution.”
Mr. Bervell stressed that medical students should stay professional online. “I reach 5-10 million people a month, and I have to think: Would I want them to see this? You have to know at all times that someone is watching. I’m very careful about how I post. I script out every video.”
Mr. Scott agreed. He advises those interested in becoming medical influencers to know what they can’t post online. For example, to ensure safety and privacy, Mr. Scott doesn’t take photos in the hospital, show his medical badge, or post patient information. “You want to be respectful of your future medical profession,” he said.
“If it’s something my mother would be ashamed of, I don’t need to post about it.”
A version of this article first appeared on Medscape.com.
A medical student’s life is an endless cycle of classes, exams, clinical rotations, and residency preparation. On TikTok and Instagram, among other sites, they share medical school experiences and lessons learned in the classroom and advocate for causes such as increased diversity and gender rights in the medical field.
This news organization caught up with a few social media influencers with a large online following to learn how medical students can effectively use social media to build a professional brand and network. Most of the students interviewed said that their social media platforms offered an opportunity to educate others about significant medical developments, feel part of a community with a like-minded audience, and network with doctors who may lead them to a future residency or career path.
Many med students said that they built their large audiences by creating a platform for people of their ethnic background, nationality, race, gender, or simply what others weren’t already talking about. They said they saw a niche in social media that was missing or others hadn’t tackled in the same way.
When Joel Bervell began med school in 2020, he questioned some of the lessons he learned about how race is used in medical practice, which didn’t make sense to him. So, he began his own research. He had about 2000 followers on Instagram at the time.
Mr. Bervell read a new study about pulse oximeters and how they often produce misleading readings on patients with dark skin.
He wondered why he hadn’t learned this in medical school, so he posted it on TikTok. Within 24 hours, about 500,000 people viewed it. Most of the comments were from doctors, nurses, and physician assistants who said they weren’t aware of the disparity.
While his initial posts detailed his journey to medical school and a day-in-the-life of a medical student, he transitioned to posts primarily about race, health equity, and what he perceives as racial bias in medicine.
Now, the fourth-year Ghanaian-American student at the Elson S. Floyd College of Medicine at Washington State University Spokane has close to 1.2 million followers on Instagram and TikTok combined. He frequently visits the White House to advise on social media’s influence on healthcare and has appeared on the Kelly Clarkson Show, Good Morning America, CNN, and ABC, among others.
He said he also uses social media to translate complex medical information for a general audience, many of whom access health information online so they can manage their own healthcare. He sees his social media work as an extension of his medical education, allowing him to delve deeper into subjects and report on them as if he were publishing research in a medical journal.
“When I came to medical school, yes, I wanted to be a doctor. But I also wanted to impact people.” Social media allows him to educate many more people than individual patients, the 29-year-old told this news organization.
Inspiring Minorities
Tabhata Paulet, 27, started her TikTok presence as a premed student in 2021. She aimed to provide free resources to help low-income, first-generation Latinx students like herself study for standardized exams.
“I always looked online for guidance and resources, and the medical influencers did not share a similar background. So, I shared my story and what I had to do as a first-generation and first person in my family to become a physician. I did not have access to the same resources as my peers,” said Ms. Paulet, who was born in Peru and came to New Jersey as a child.
Students who are Hispanic, Latinx, or of Spanish origin made up 6.8% of total medical school enrollment in 2023-2024, up slightly from 6.7% in 2022-2023, according to the Association of American Medical Colleges (AAMC).
Ms. Paulet’s online presence grew when she began documenting her experiences as a first-year medical student, bridging the language barrier for Spanish-speaking patients so they could understand their diagnosis and treatment. She often posts about health disparity and barriers to care for underserved communities.
Most of her nearly 22,000 followers are Hispanic, said the now fourth-year student at Rutgers New Jersey Medical School in Newark, New Jersey. “I talk a lot about my interesting Spanish-speaking patients ... and how sometimes speaking their native language truly makes a difference in their care.”
She believes that she serves an important role in social media. “It can be very inspirational for those who come after you [in med school] to see someone from a similar culture and upbringing.”
Creating a Community
It was during a therapy session 4 years ago that Jeremy “JP” Scott decided to share Instagram posts about his experiences as a nontraditional medical student. The 37-year-old was studying at Ross University School of Medicine in Barbados and was feeling lonely as an international medical student training to be a doctor as a second career.
Before starting med school, Mr. Scott was an adjunct professor and lab supervisor at the University of Hartford Biology Department, West Hartford, Connecticut, and then a research assistant and lab manager at the Wistar Institute in Philadelphia.
Although he wanted to follow his mother’s path to becoming a doctor, it was more difficult than he envisioned, said the fourth-year student who completed clinical rotations in the United States and is now applying for residencies.
“I talked about how medical school is not what it appears to be ... There are a lot of challenges we are going through,” especially as people of color, he said.
Mr. Scott believes social media helps people feel included and less alone. He said many of his followers are med students and physicians.
His posts often focus on LGBTQIA+ pride and being a minority as a Black man in medicine.
“The pandemic spurred a lot of us. We had a racial reckoning in our country at the time. It inspired us to talk as Black creators and Black medical students.”
Black or African American medical students made up 8.5% of total med school enrollment in 2023-2024, a slight increase from 2022 to 2023, according to AAMC figures. Black men represented 7% of total enrollment in 2023-2024, while Black women represented 9.8%.
After only a handful of online posts in which Mr. Scott candidly discussed his mental health struggles and relationships, he attracted the attention of several medical apparel companies, including the popular FIGS scrubs. He’s now an ambassador for the company, which supports him and his content.
“My association with FIGS has helped attract a wider online audience, increasing my presence.” Today, he has 14,000 Instagram followers. “It opened up so many opportunities,” Mr. Scott said. One example is working with the national LGBTQIA+ community.
“The goal was never to be a social media influencer, to gain sponsorships or photo opportunities,” he said.
“My job, first, is as a medical student. Everything else is second. I am not trying to be a professional social media personality. I’m trying to be an actual physician.” He also tries to separate JP “social media” from Jeremy, the medical student.
“On Instagram, anyone can pull it up and see what you’re doing. The last thing I want is for them to think that I’m not serious about what I’m doing, that I’m not here to learn and become a doctor.”
Benefits and Drawbacks
Ms. Paulet said her social media following helped her connect with leaders in the Latinx medical community, including an obstetrics anesthesiologist, her intended specialty. “I don’t think I’d be able to do that without a social media platform.”
Her online activity also propelled her from regional to national leadership in the Latino Medical Student Association (LMSA). She now also runs their Instagram page, which has 14,000 followers.
Mr. Bervell believes social media is a great way to network. He’s connected with people he wouldn’t have met otherwise, including physicians. “I think it will help me get into a residency,” he said. “It allows people to know who you are ... They will be able to tell in a few videos the type of doctor I want to be.”
On the other hand, Mr. Bervell is aware of the negative impacts of social media on mental health. “You can get lost in social media.” For that reason, he often tries to disconnect. “I can go days without my phone.”
Posting on social media can be time-consuming, Mr. Bervell admitted. He said he spent about 2 hours a day researching, editing, and posting on TikTok when he first started building his following. Now, he spends about 2-3 hours a week creating videos. “I don’t post every day anymore. I don’t have the time.”
When she started building her TikTok presence, Ms. Paulet said she devoted 15 hours a week to the endeavor, but now she spends 10-12 hours a week posting online, including on LMSA’s Instagram page. “Whenever you are done with an exam or have a study break, this is something fun to do.” She also says you never know who you’re going to inspire when you put yourself out there.
“Talk about your journey, rotations, or your experience in your first or second year of medical school. Talk about milestones like board exams.”
Word to the Wise
Some students may be concerned that their posts might affect a potential residency program. But the medical students interviewed say they want to find programs that align with their values and accept them for who they are.
Mr. Scott said he’s not worried about someone not liking him because of who he is. “I am Black and openly gay. If it’s a problem, I don’t need to work with you or your institution.”
Mr. Bervell stressed that medical students should stay professional online. “I reach 5-10 million people a month, and I have to think: Would I want them to see this? You have to know at all times that someone is watching. I’m very careful about how I post. I script out every video.”
Mr. Scott agreed. He advises those interested in becoming medical influencers to know what they can’t post online. For example, to ensure safety and privacy, Mr. Scott doesn’t take photos in the hospital, show his medical badge, or post patient information. “You want to be respectful of your future medical profession,” he said.
“If it’s something my mother would be ashamed of, I don’t need to post about it.”
A version of this article first appeared on Medscape.com.
Beyond One-Size-Fits-All: Precision Psychiatry Is Here
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
No Surprises Act: Private Equity Scores Big in Arbitrations
Four organizations owned by private equity firms — including two provider groups — dominated the No Surprises Act’s disputed bill arbitration process in its first year, filing about 70% of 657,040 cases against insurers in 2023, a new report finds.
The findings, recently published in Health Affairs, suggest that private equity–owned organizations are forcefully challenging insurers about payments for certain kinds of out-of-network care.
Their fighting stance has paid off: The percentage of resolved arbitration cases won by providers jumped from 72% in the first quarter of 2023 to 85% in the last quarter, and they were awarded a median of more than 300% the contracted in-network rates for the services in question.
With many more out-of-network bills disputed by providers than expected, “the system is not working exactly the way it was anticipated when this law was written,” lead author Jack Hoadley, PhD, a research professor emeritus at Georgetown University’s McCourt School of Public Policy, Washington, DC, told this news organization.
And, he said, the public and the federal government may end up paying a price.
Congress passed the No Surprises Act in 2020 and then-President Donald Trump signed it. The landmark bill, which went into effect in 2022, was designed to protect patients from unexpected and often exorbitant “surprise” bills after they received some kinds of out-of-network care.
Now, many types of providers are forbidden from billing patients beyond normal in-network costs. In these cases, health plans and out-of-network providers — who don’t have mutual agreements — must wrangle over payment amounts, which are intended to not exceed inflation-adjusted 2019 median levels.
A binding arbitration process kicks in when a provider and a health plan fail to agree about how much the plan will pay for a service. Then, a third-party arbitrator is called in to make a ruling that’s binding. The process is controversial, and a flurry of lawsuits from providers have challenged it.
The new report, which updates an earlier analysis, examines data about disputed cases from all of 2023.
Of the 657,040 new cases filed in 2023, about 70% came from four private equity-funded organizations: Team Health, SCP Health, Radiology Partners, and Envision, which each provide physician services.
About half of the 2023 cases were from just four states: Texas, Florida, Tennessee, and Georgia. The report says the four organizations are especially active in those states. In contrast, Connecticut, Maryland, Massachusetts, and Washington state each had just 1500 or fewer cases filed last year.
Health plans challenged a third of cases as ineligible, and 22% of all resolved cases were deemed ineligible.
Providers won 80% of resolved challenges in 2023, although it’s not clear how much money they reaped. Still, it’s clear that “in the vast majority of the cases, insurers have to pay larger amounts to the provider,” Dr. Hoadley said.
Radiologists made a median of at least 500% of the in-network rate in their cases. Surgeons and neurologists made even more money — a median of at least 800% of the in-network rate. Overall, providers made 322%-350% of in-network rates, depending on the quarter.
Dr. Hoadley cautioned that only a small percentage of medical payments are disputed. In those cases, “the amount that the insurer offers is accepted, and that’s the end of the story.”
Why are the providers often reaping much more than typical payments for in-network services? It’s “really hard to know,” Dr. Hoadley said. But one factor, he said, may be the fact that providers are able to offer evidence challenging that amounts that insurers say they paid previously: “Hey, when we were in network, we were paid this much.”
It’s not clear whether the dispute-and-arbitration system will cost insurers — and patients — more in the long run. The Congressional Budget Office actually thought the No Surprises Act might lower the growth of premiums slightly and save the federal government money, Dr. Hoadley said, but that could potentially not happen. The flood of litigation also contributes to uncertainty, he said.
Alan Sager, PhD, professor of Health Law, Policy, and Management at Boston University School of Public Health, told this news organization that premiums are bound to rise as insurers react to higher costs. He also expects that providers will question the value of being in-network. “If you’re out-of-network and can obtain much higher payments, why would any doctor or hospital remain in-network, especially since they don’t lose out on patient volume?”
Why are provider groups owned by private equity firms so aggressive at challenging health plans? Loren Adler, a fellow and associate director of the Brookings Institution’s Center on Health Policy, told this news organization that these companies play large roles in fields affected by the No Surprises Act. These include emergency medicine, radiology, and anesthesiology, said Mr. Adler, who’s also studied the No Surprises Act’s dispute/arbitration system.
Mr. Adler added that larger companies “are better suited to deal with technical complexities of this process and spend the sort of upfront money to go through it.”
In the big picture, Mr. Adler said, the new study “raises question of whether Congress at some point wants to try to basically bring prices from the arbitration process back in line with average in-network prices.”
The study was funded by the Commonwealth Fund and Arnold Ventures. Dr. Hoadley, Dr. Sager, and Mr. Adler had no disclosures.
A version of this article first appeared on Medscape.com.
Four organizations owned by private equity firms — including two provider groups — dominated the No Surprises Act’s disputed bill arbitration process in its first year, filing about 70% of 657,040 cases against insurers in 2023, a new report finds.
The findings, recently published in Health Affairs, suggest that private equity–owned organizations are forcefully challenging insurers about payments for certain kinds of out-of-network care.
Their fighting stance has paid off: The percentage of resolved arbitration cases won by providers jumped from 72% in the first quarter of 2023 to 85% in the last quarter, and they were awarded a median of more than 300% the contracted in-network rates for the services in question.
With many more out-of-network bills disputed by providers than expected, “the system is not working exactly the way it was anticipated when this law was written,” lead author Jack Hoadley, PhD, a research professor emeritus at Georgetown University’s McCourt School of Public Policy, Washington, DC, told this news organization.
And, he said, the public and the federal government may end up paying a price.
Congress passed the No Surprises Act in 2020 and then-President Donald Trump signed it. The landmark bill, which went into effect in 2022, was designed to protect patients from unexpected and often exorbitant “surprise” bills after they received some kinds of out-of-network care.
Now, many types of providers are forbidden from billing patients beyond normal in-network costs. In these cases, health plans and out-of-network providers — who don’t have mutual agreements — must wrangle over payment amounts, which are intended to not exceed inflation-adjusted 2019 median levels.
A binding arbitration process kicks in when a provider and a health plan fail to agree about how much the plan will pay for a service. Then, a third-party arbitrator is called in to make a ruling that’s binding. The process is controversial, and a flurry of lawsuits from providers have challenged it.
The new report, which updates an earlier analysis, examines data about disputed cases from all of 2023.
Of the 657,040 new cases filed in 2023, about 70% came from four private equity-funded organizations: Team Health, SCP Health, Radiology Partners, and Envision, which each provide physician services.
About half of the 2023 cases were from just four states: Texas, Florida, Tennessee, and Georgia. The report says the four organizations are especially active in those states. In contrast, Connecticut, Maryland, Massachusetts, and Washington state each had just 1500 or fewer cases filed last year.
Health plans challenged a third of cases as ineligible, and 22% of all resolved cases were deemed ineligible.
Providers won 80% of resolved challenges in 2023, although it’s not clear how much money they reaped. Still, it’s clear that “in the vast majority of the cases, insurers have to pay larger amounts to the provider,” Dr. Hoadley said.
Radiologists made a median of at least 500% of the in-network rate in their cases. Surgeons and neurologists made even more money — a median of at least 800% of the in-network rate. Overall, providers made 322%-350% of in-network rates, depending on the quarter.
Dr. Hoadley cautioned that only a small percentage of medical payments are disputed. In those cases, “the amount that the insurer offers is accepted, and that’s the end of the story.”
Why are the providers often reaping much more than typical payments for in-network services? It’s “really hard to know,” Dr. Hoadley said. But one factor, he said, may be the fact that providers are able to offer evidence challenging that amounts that insurers say they paid previously: “Hey, when we were in network, we were paid this much.”
It’s not clear whether the dispute-and-arbitration system will cost insurers — and patients — more in the long run. The Congressional Budget Office actually thought the No Surprises Act might lower the growth of premiums slightly and save the federal government money, Dr. Hoadley said, but that could potentially not happen. The flood of litigation also contributes to uncertainty, he said.
Alan Sager, PhD, professor of Health Law, Policy, and Management at Boston University School of Public Health, told this news organization that premiums are bound to rise as insurers react to higher costs. He also expects that providers will question the value of being in-network. “If you’re out-of-network and can obtain much higher payments, why would any doctor or hospital remain in-network, especially since they don’t lose out on patient volume?”
Why are provider groups owned by private equity firms so aggressive at challenging health plans? Loren Adler, a fellow and associate director of the Brookings Institution’s Center on Health Policy, told this news organization that these companies play large roles in fields affected by the No Surprises Act. These include emergency medicine, radiology, and anesthesiology, said Mr. Adler, who’s also studied the No Surprises Act’s dispute/arbitration system.
Mr. Adler added that larger companies “are better suited to deal with technical complexities of this process and spend the sort of upfront money to go through it.”
In the big picture, Mr. Adler said, the new study “raises question of whether Congress at some point wants to try to basically bring prices from the arbitration process back in line with average in-network prices.”
The study was funded by the Commonwealth Fund and Arnold Ventures. Dr. Hoadley, Dr. Sager, and Mr. Adler had no disclosures.
A version of this article first appeared on Medscape.com.
Four organizations owned by private equity firms — including two provider groups — dominated the No Surprises Act’s disputed bill arbitration process in its first year, filing about 70% of 657,040 cases against insurers in 2023, a new report finds.
The findings, recently published in Health Affairs, suggest that private equity–owned organizations are forcefully challenging insurers about payments for certain kinds of out-of-network care.
Their fighting stance has paid off: The percentage of resolved arbitration cases won by providers jumped from 72% in the first quarter of 2023 to 85% in the last quarter, and they were awarded a median of more than 300% the contracted in-network rates for the services in question.
With many more out-of-network bills disputed by providers than expected, “the system is not working exactly the way it was anticipated when this law was written,” lead author Jack Hoadley, PhD, a research professor emeritus at Georgetown University’s McCourt School of Public Policy, Washington, DC, told this news organization.
And, he said, the public and the federal government may end up paying a price.
Congress passed the No Surprises Act in 2020 and then-President Donald Trump signed it. The landmark bill, which went into effect in 2022, was designed to protect patients from unexpected and often exorbitant “surprise” bills after they received some kinds of out-of-network care.
Now, many types of providers are forbidden from billing patients beyond normal in-network costs. In these cases, health plans and out-of-network providers — who don’t have mutual agreements — must wrangle over payment amounts, which are intended to not exceed inflation-adjusted 2019 median levels.
A binding arbitration process kicks in when a provider and a health plan fail to agree about how much the plan will pay for a service. Then, a third-party arbitrator is called in to make a ruling that’s binding. The process is controversial, and a flurry of lawsuits from providers have challenged it.
The new report, which updates an earlier analysis, examines data about disputed cases from all of 2023.
Of the 657,040 new cases filed in 2023, about 70% came from four private equity-funded organizations: Team Health, SCP Health, Radiology Partners, and Envision, which each provide physician services.
About half of the 2023 cases were from just four states: Texas, Florida, Tennessee, and Georgia. The report says the four organizations are especially active in those states. In contrast, Connecticut, Maryland, Massachusetts, and Washington state each had just 1500 or fewer cases filed last year.
Health plans challenged a third of cases as ineligible, and 22% of all resolved cases were deemed ineligible.
Providers won 80% of resolved challenges in 2023, although it’s not clear how much money they reaped. Still, it’s clear that “in the vast majority of the cases, insurers have to pay larger amounts to the provider,” Dr. Hoadley said.
Radiologists made a median of at least 500% of the in-network rate in their cases. Surgeons and neurologists made even more money — a median of at least 800% of the in-network rate. Overall, providers made 322%-350% of in-network rates, depending on the quarter.
Dr. Hoadley cautioned that only a small percentage of medical payments are disputed. In those cases, “the amount that the insurer offers is accepted, and that’s the end of the story.”
Why are the providers often reaping much more than typical payments for in-network services? It’s “really hard to know,” Dr. Hoadley said. But one factor, he said, may be the fact that providers are able to offer evidence challenging that amounts that insurers say they paid previously: “Hey, when we were in network, we were paid this much.”
It’s not clear whether the dispute-and-arbitration system will cost insurers — and patients — more in the long run. The Congressional Budget Office actually thought the No Surprises Act might lower the growth of premiums slightly and save the federal government money, Dr. Hoadley said, but that could potentially not happen. The flood of litigation also contributes to uncertainty, he said.
Alan Sager, PhD, professor of Health Law, Policy, and Management at Boston University School of Public Health, told this news organization that premiums are bound to rise as insurers react to higher costs. He also expects that providers will question the value of being in-network. “If you’re out-of-network and can obtain much higher payments, why would any doctor or hospital remain in-network, especially since they don’t lose out on patient volume?”
Why are provider groups owned by private equity firms so aggressive at challenging health plans? Loren Adler, a fellow and associate director of the Brookings Institution’s Center on Health Policy, told this news organization that these companies play large roles in fields affected by the No Surprises Act. These include emergency medicine, radiology, and anesthesiology, said Mr. Adler, who’s also studied the No Surprises Act’s dispute/arbitration system.
Mr. Adler added that larger companies “are better suited to deal with technical complexities of this process and spend the sort of upfront money to go through it.”
In the big picture, Mr. Adler said, the new study “raises question of whether Congress at some point wants to try to basically bring prices from the arbitration process back in line with average in-network prices.”
The study was funded by the Commonwealth Fund and Arnold Ventures. Dr. Hoadley, Dr. Sager, and Mr. Adler had no disclosures.
A version of this article first appeared on Medscape.com.
The New Formula for Stronger, Longer-Lasting Vaccines
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Alcohol-Associated Liver Disease’s Changing Demographics
, accounting for approximately 5% of all disease and injury. In the United States, the prevalence of ALD has increased since 2014, and the trajectory accelerated during the COVID-19 pandemic.
ALD encompasses a spectrum of diseases that includes steatosis, fibrosis, cirrhosis, and hepatocellular carcinoma, as well as related complications. Although earlier stages of ALD may be asymptomatic, hepatologists and gastroenterologists rarely see patients at this point.
“Unfortunately, patients with ALD more often present in late stages of disease (decompensated cirrhosis) as compared with other chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease or hepatitis C,” Doug A. Simonetto, MD, associate professor of medicine and director of the Gastroenterology and Hepatology Fellowship Program at the Mayo Clinic, Rochester, Minnesota, told this news organization.
Recent data have identified three demographic groups experiencing higher rates of ALD relative to previous periods and who may therefore require special attention. Understanding what makes these groups increasingly susceptible to ALD may allow for improved screening, earlier diagnosis, and potentially the prevention of its most dire consequences.
As Women Consume More Alcohol, ALD Follows
Historically, men have had higher rates of alcohol use, heavy drinking, and alcohol disorders than women. But this gender gap has begun to narrow.
Men born in the early 1900s were 2.2 times more likely to drink alcohol and 3.6 times more likely to experience alcohol-related harms than women, according to a 2016 meta-analysis. By the end of the 1990s, however, women’s drinking had begun to catch up. Men still led in these categories, but only by 1.1 and 1.3 times, respectively.
Rates of binge drinking (defined as at least five drinks in men or at least four drinks in women in an approximately 2-hour period) are also converging between the sexes. The authors of a longitudinal analysis hypothesized that an uptick in young women reporting drinking for social reasons — from 53% in 1987 to 87% in 2020 — was a possible cause.
Greater alcohol consumption among women has translated into higher rates of ALD. Analyzing data from the Global Burden of Disease Study 2019, which looked at hundreds of diseases across 204 countries and territories, researchers reported that the worldwide prevalence of ALD among young women (15-49 years) rose within the past decade. Those in the 20- to 24-year-old age group had the most significant increases in ALD prevalence rates.
Recent US statistics highlight the relative imbalance in ALD’s impact on women, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“The age-adjusted death rate from alcohol-associated liver cirrhosis increased by 47% between 2000 and 2019, with larger increases for females than for males (83.5% compared to 33%),” Dr. Koob told this news organization. “Larger increases for women are consistent with a general increase in alcohol use among adult women and larger increases in alcohol-related emergency department visits, hospitalizations, and deaths.”
Physiologically, women have a higher risk than men of developing ALD and more severe disease, even at lower levels of alcohol exposure. According to a 2021 review, several proposed mechanisms might play a role, including differences in alcohol metabolism and first-pass metabolism, hormones, and endotoxin and Kupffer cell activation.
Crucially, women are less likely than men to receive in-person therapy or approved medications for alcohol use disorder, according to a 2019 analysis of over 66,000 privately insured adult patients.
Certain Ethnic, Racial Minorities Have Higher Rates of ALD
In the United States, rates of ALD and associated complications are higher among certain minority groups, most prominently Hispanic and Native American individuals.
A 2021 analysis of three large US databases found that Hispanic ethnicity was associated with a 17% increased risk for acute-on-chronic liver failure in patients with ALD-related admissions.
Data also show that Hispanic and White patients have a higher proportion of alcoholic hepatitis than African American patients. And for Hispanic patients admitted for alcoholic hepatitis, they incur significantly more total hospital costs despite having similar mortality rates as White patients.
ALD-related mortality appears higher within certain subgroups of Hispanic patient populations. NIAAA surveillance reports track deaths resulting from cirrhosis in the White, Black, and Hispanic populations. From 2000 to 2019, these statistics show that although death rates from cirrhosis decreased for Hispanic White men, they increased for Hispanic White women, Dr. Koob said.
The latest data show that Native American populations are experiencing ALD at relatively higher rates than other racial/ethnic groups as well. An analysis of nearly 200,000 cirrhosis-related hospitalizations found that ALD, including alcoholic hepatitis, was the most common etiology in American Indian/Alaska Native patients. A separate analysis of the National Inpatient Sample database revealed that discharges resulting from ALD were disproportionately higher among Native American women.
As with Hispanic populations, ALD-associated mortality rates are also higher in Native American populations. The death rate from ALD increased for all racial and ethnic groups by 23.4% from 2019 to 2020, but the biggest increase occurred in the American Indian or Alaska Native populations (34.3% increase, from 20.1 to 27 per 100,000 people). Additionally, over the first two decades of the 21st century, mortality rates resulting from cirrhosis were highest among the American Indian and Alaska Native populations, according to a recently published systematic analysis of US health disparities across five racial/ethnic groups.
Discrepancies in these and other minority groups may be due partly to genetic mechanisms, such as the relatively higher frequency of the PNPLA3 G/G polymorphism, a known risk factor for the development of advanced ALD, among those with Native American ancestry. A host of complex socioeconomic factors, such as income discrepancies and access to care, likely contribute too.
Evidence suggests that alcohol screening interventions are not applied equally across various racial and ethnic groups, Dr. Koob noted.
“For instance, Subbaraman and colleagues reported that, compared to non-Hispanic White patients, those who identify as Hispanic, Black, or other race or ethnicity were less likely to be screened for alcohol use during visits to healthcare providers. This was particularly true for those with a high school education or less,” he told this news organization. “However, other studies have not found such disparities.”
ALD Rates High in Young Adults, but the Tide May Be Changing
Globally, the prevalence of ALD has increased among both adolescents and young adults since the beginning of the 21st century. The global incidence of alcohol-associated hepatitis in recent years has been greatest among those aged 15-44 years.
In the United States, the increasing rate of ALD-related hospitalizations is primarily driven by the rise in cases of alcoholic hepatitis and acute-on-chronic liver failure among those aged 35 years and younger.
ALD is now the most common indication for liver transplant in those younger than 40 years of age, having increased fourfold between 2003 and 2018.
From 2009 to 2016, people aged 25-34 years experienced the highest average annual increase in cirrhosis-related mortality (10.5%), a trend the authors noted was “driven entirely by alcohol-related liver disease.”
Younger adults may be more susceptible to ALD due to the way they drink.
In a 2021 analysis of the National Health and Nutrition Examination Survey database, the weighted prevalence of harmful alcohol use was 29.3% in those younger than 35 years, compared with 16.9% in those aged 35-64 years. Higher blood alcohol levels resulting from binge drinking may make patients more susceptible to bacterial translocation and liver fibrosis and can increase the likelihood of cirrhosis in those with an underlying metabolic syndrome.
Yet, Dr. Koob said, thinking of “young adults” as one cohort may be misguided because he’s found very different attitudes toward alcohol within that population. Cross-sectional survey data obtained from more than 180,000 young adults indicated that alcohol abstinence increased between 2002 and 2018. Young adults report various reasons for not drinking, ranging from lack of interest to financial and situational barriers (eg, not wanting to interfere with school or work).
“The tide is coming in and out at the same time,” he said. “Younger people under the age of 25 are drinking less each year, are increasingly interested in things like Dry January, and more than half view moderate levels of consumption as unhealthy. People who are 26 years and older are drinking more, are not as interested in cutting back or taking breaks, and are less likely to consider 1 or 2 drinks per day as potentially unhealthy.”
Dr. Koob would like to believe the positive trends around alcohol in the under-25 set prove not only resilient, but someday, dominant.
“We have seen historic increases in alcohol consumption in the last few years — the largest increases in more than 50 years. But we are hopeful that, as the younger cohorts age, we will see lower levels of drinking by adults in mid-life and beyond.”
A version of this article first appeared on Medscape.com.
, accounting for approximately 5% of all disease and injury. In the United States, the prevalence of ALD has increased since 2014, and the trajectory accelerated during the COVID-19 pandemic.
ALD encompasses a spectrum of diseases that includes steatosis, fibrosis, cirrhosis, and hepatocellular carcinoma, as well as related complications. Although earlier stages of ALD may be asymptomatic, hepatologists and gastroenterologists rarely see patients at this point.
“Unfortunately, patients with ALD more often present in late stages of disease (decompensated cirrhosis) as compared with other chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease or hepatitis C,” Doug A. Simonetto, MD, associate professor of medicine and director of the Gastroenterology and Hepatology Fellowship Program at the Mayo Clinic, Rochester, Minnesota, told this news organization.
Recent data have identified three demographic groups experiencing higher rates of ALD relative to previous periods and who may therefore require special attention. Understanding what makes these groups increasingly susceptible to ALD may allow for improved screening, earlier diagnosis, and potentially the prevention of its most dire consequences.
As Women Consume More Alcohol, ALD Follows
Historically, men have had higher rates of alcohol use, heavy drinking, and alcohol disorders than women. But this gender gap has begun to narrow.
Men born in the early 1900s were 2.2 times more likely to drink alcohol and 3.6 times more likely to experience alcohol-related harms than women, according to a 2016 meta-analysis. By the end of the 1990s, however, women’s drinking had begun to catch up. Men still led in these categories, but only by 1.1 and 1.3 times, respectively.
Rates of binge drinking (defined as at least five drinks in men or at least four drinks in women in an approximately 2-hour period) are also converging between the sexes. The authors of a longitudinal analysis hypothesized that an uptick in young women reporting drinking for social reasons — from 53% in 1987 to 87% in 2020 — was a possible cause.
Greater alcohol consumption among women has translated into higher rates of ALD. Analyzing data from the Global Burden of Disease Study 2019, which looked at hundreds of diseases across 204 countries and territories, researchers reported that the worldwide prevalence of ALD among young women (15-49 years) rose within the past decade. Those in the 20- to 24-year-old age group had the most significant increases in ALD prevalence rates.
Recent US statistics highlight the relative imbalance in ALD’s impact on women, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“The age-adjusted death rate from alcohol-associated liver cirrhosis increased by 47% between 2000 and 2019, with larger increases for females than for males (83.5% compared to 33%),” Dr. Koob told this news organization. “Larger increases for women are consistent with a general increase in alcohol use among adult women and larger increases in alcohol-related emergency department visits, hospitalizations, and deaths.”
Physiologically, women have a higher risk than men of developing ALD and more severe disease, even at lower levels of alcohol exposure. According to a 2021 review, several proposed mechanisms might play a role, including differences in alcohol metabolism and first-pass metabolism, hormones, and endotoxin and Kupffer cell activation.
Crucially, women are less likely than men to receive in-person therapy or approved medications for alcohol use disorder, according to a 2019 analysis of over 66,000 privately insured adult patients.
Certain Ethnic, Racial Minorities Have Higher Rates of ALD
In the United States, rates of ALD and associated complications are higher among certain minority groups, most prominently Hispanic and Native American individuals.
A 2021 analysis of three large US databases found that Hispanic ethnicity was associated with a 17% increased risk for acute-on-chronic liver failure in patients with ALD-related admissions.
Data also show that Hispanic and White patients have a higher proportion of alcoholic hepatitis than African American patients. And for Hispanic patients admitted for alcoholic hepatitis, they incur significantly more total hospital costs despite having similar mortality rates as White patients.
ALD-related mortality appears higher within certain subgroups of Hispanic patient populations. NIAAA surveillance reports track deaths resulting from cirrhosis in the White, Black, and Hispanic populations. From 2000 to 2019, these statistics show that although death rates from cirrhosis decreased for Hispanic White men, they increased for Hispanic White women, Dr. Koob said.
The latest data show that Native American populations are experiencing ALD at relatively higher rates than other racial/ethnic groups as well. An analysis of nearly 200,000 cirrhosis-related hospitalizations found that ALD, including alcoholic hepatitis, was the most common etiology in American Indian/Alaska Native patients. A separate analysis of the National Inpatient Sample database revealed that discharges resulting from ALD were disproportionately higher among Native American women.
As with Hispanic populations, ALD-associated mortality rates are also higher in Native American populations. The death rate from ALD increased for all racial and ethnic groups by 23.4% from 2019 to 2020, but the biggest increase occurred in the American Indian or Alaska Native populations (34.3% increase, from 20.1 to 27 per 100,000 people). Additionally, over the first two decades of the 21st century, mortality rates resulting from cirrhosis were highest among the American Indian and Alaska Native populations, according to a recently published systematic analysis of US health disparities across five racial/ethnic groups.
Discrepancies in these and other minority groups may be due partly to genetic mechanisms, such as the relatively higher frequency of the PNPLA3 G/G polymorphism, a known risk factor for the development of advanced ALD, among those with Native American ancestry. A host of complex socioeconomic factors, such as income discrepancies and access to care, likely contribute too.
Evidence suggests that alcohol screening interventions are not applied equally across various racial and ethnic groups, Dr. Koob noted.
“For instance, Subbaraman and colleagues reported that, compared to non-Hispanic White patients, those who identify as Hispanic, Black, or other race or ethnicity were less likely to be screened for alcohol use during visits to healthcare providers. This was particularly true for those with a high school education or less,” he told this news organization. “However, other studies have not found such disparities.”
ALD Rates High in Young Adults, but the Tide May Be Changing
Globally, the prevalence of ALD has increased among both adolescents and young adults since the beginning of the 21st century. The global incidence of alcohol-associated hepatitis in recent years has been greatest among those aged 15-44 years.
In the United States, the increasing rate of ALD-related hospitalizations is primarily driven by the rise in cases of alcoholic hepatitis and acute-on-chronic liver failure among those aged 35 years and younger.
ALD is now the most common indication for liver transplant in those younger than 40 years of age, having increased fourfold between 2003 and 2018.
From 2009 to 2016, people aged 25-34 years experienced the highest average annual increase in cirrhosis-related mortality (10.5%), a trend the authors noted was “driven entirely by alcohol-related liver disease.”
Younger adults may be more susceptible to ALD due to the way they drink.
In a 2021 analysis of the National Health and Nutrition Examination Survey database, the weighted prevalence of harmful alcohol use was 29.3% in those younger than 35 years, compared with 16.9% in those aged 35-64 years. Higher blood alcohol levels resulting from binge drinking may make patients more susceptible to bacterial translocation and liver fibrosis and can increase the likelihood of cirrhosis in those with an underlying metabolic syndrome.
Yet, Dr. Koob said, thinking of “young adults” as one cohort may be misguided because he’s found very different attitudes toward alcohol within that population. Cross-sectional survey data obtained from more than 180,000 young adults indicated that alcohol abstinence increased between 2002 and 2018. Young adults report various reasons for not drinking, ranging from lack of interest to financial and situational barriers (eg, not wanting to interfere with school or work).
“The tide is coming in and out at the same time,” he said. “Younger people under the age of 25 are drinking less each year, are increasingly interested in things like Dry January, and more than half view moderate levels of consumption as unhealthy. People who are 26 years and older are drinking more, are not as interested in cutting back or taking breaks, and are less likely to consider 1 or 2 drinks per day as potentially unhealthy.”
Dr. Koob would like to believe the positive trends around alcohol in the under-25 set prove not only resilient, but someday, dominant.
“We have seen historic increases in alcohol consumption in the last few years — the largest increases in more than 50 years. But we are hopeful that, as the younger cohorts age, we will see lower levels of drinking by adults in mid-life and beyond.”
A version of this article first appeared on Medscape.com.
, accounting for approximately 5% of all disease and injury. In the United States, the prevalence of ALD has increased since 2014, and the trajectory accelerated during the COVID-19 pandemic.
ALD encompasses a spectrum of diseases that includes steatosis, fibrosis, cirrhosis, and hepatocellular carcinoma, as well as related complications. Although earlier stages of ALD may be asymptomatic, hepatologists and gastroenterologists rarely see patients at this point.
“Unfortunately, patients with ALD more often present in late stages of disease (decompensated cirrhosis) as compared with other chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease or hepatitis C,” Doug A. Simonetto, MD, associate professor of medicine and director of the Gastroenterology and Hepatology Fellowship Program at the Mayo Clinic, Rochester, Minnesota, told this news organization.
Recent data have identified three demographic groups experiencing higher rates of ALD relative to previous periods and who may therefore require special attention. Understanding what makes these groups increasingly susceptible to ALD may allow for improved screening, earlier diagnosis, and potentially the prevention of its most dire consequences.
As Women Consume More Alcohol, ALD Follows
Historically, men have had higher rates of alcohol use, heavy drinking, and alcohol disorders than women. But this gender gap has begun to narrow.
Men born in the early 1900s were 2.2 times more likely to drink alcohol and 3.6 times more likely to experience alcohol-related harms than women, according to a 2016 meta-analysis. By the end of the 1990s, however, women’s drinking had begun to catch up. Men still led in these categories, but only by 1.1 and 1.3 times, respectively.
Rates of binge drinking (defined as at least five drinks in men or at least four drinks in women in an approximately 2-hour period) are also converging between the sexes. The authors of a longitudinal analysis hypothesized that an uptick in young women reporting drinking for social reasons — from 53% in 1987 to 87% in 2020 — was a possible cause.
Greater alcohol consumption among women has translated into higher rates of ALD. Analyzing data from the Global Burden of Disease Study 2019, which looked at hundreds of diseases across 204 countries and territories, researchers reported that the worldwide prevalence of ALD among young women (15-49 years) rose within the past decade. Those in the 20- to 24-year-old age group had the most significant increases in ALD prevalence rates.
Recent US statistics highlight the relative imbalance in ALD’s impact on women, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“The age-adjusted death rate from alcohol-associated liver cirrhosis increased by 47% between 2000 and 2019, with larger increases for females than for males (83.5% compared to 33%),” Dr. Koob told this news organization. “Larger increases for women are consistent with a general increase in alcohol use among adult women and larger increases in alcohol-related emergency department visits, hospitalizations, and deaths.”
Physiologically, women have a higher risk than men of developing ALD and more severe disease, even at lower levels of alcohol exposure. According to a 2021 review, several proposed mechanisms might play a role, including differences in alcohol metabolism and first-pass metabolism, hormones, and endotoxin and Kupffer cell activation.
Crucially, women are less likely than men to receive in-person therapy or approved medications for alcohol use disorder, according to a 2019 analysis of over 66,000 privately insured adult patients.
Certain Ethnic, Racial Minorities Have Higher Rates of ALD
In the United States, rates of ALD and associated complications are higher among certain minority groups, most prominently Hispanic and Native American individuals.
A 2021 analysis of three large US databases found that Hispanic ethnicity was associated with a 17% increased risk for acute-on-chronic liver failure in patients with ALD-related admissions.
Data also show that Hispanic and White patients have a higher proportion of alcoholic hepatitis than African American patients. And for Hispanic patients admitted for alcoholic hepatitis, they incur significantly more total hospital costs despite having similar mortality rates as White patients.
ALD-related mortality appears higher within certain subgroups of Hispanic patient populations. NIAAA surveillance reports track deaths resulting from cirrhosis in the White, Black, and Hispanic populations. From 2000 to 2019, these statistics show that although death rates from cirrhosis decreased for Hispanic White men, they increased for Hispanic White women, Dr. Koob said.
The latest data show that Native American populations are experiencing ALD at relatively higher rates than other racial/ethnic groups as well. An analysis of nearly 200,000 cirrhosis-related hospitalizations found that ALD, including alcoholic hepatitis, was the most common etiology in American Indian/Alaska Native patients. A separate analysis of the National Inpatient Sample database revealed that discharges resulting from ALD were disproportionately higher among Native American women.
As with Hispanic populations, ALD-associated mortality rates are also higher in Native American populations. The death rate from ALD increased for all racial and ethnic groups by 23.4% from 2019 to 2020, but the biggest increase occurred in the American Indian or Alaska Native populations (34.3% increase, from 20.1 to 27 per 100,000 people). Additionally, over the first two decades of the 21st century, mortality rates resulting from cirrhosis were highest among the American Indian and Alaska Native populations, according to a recently published systematic analysis of US health disparities across five racial/ethnic groups.
Discrepancies in these and other minority groups may be due partly to genetic mechanisms, such as the relatively higher frequency of the PNPLA3 G/G polymorphism, a known risk factor for the development of advanced ALD, among those with Native American ancestry. A host of complex socioeconomic factors, such as income discrepancies and access to care, likely contribute too.
Evidence suggests that alcohol screening interventions are not applied equally across various racial and ethnic groups, Dr. Koob noted.
“For instance, Subbaraman and colleagues reported that, compared to non-Hispanic White patients, those who identify as Hispanic, Black, or other race or ethnicity were less likely to be screened for alcohol use during visits to healthcare providers. This was particularly true for those with a high school education or less,” he told this news organization. “However, other studies have not found such disparities.”
ALD Rates High in Young Adults, but the Tide May Be Changing
Globally, the prevalence of ALD has increased among both adolescents and young adults since the beginning of the 21st century. The global incidence of alcohol-associated hepatitis in recent years has been greatest among those aged 15-44 years.
In the United States, the increasing rate of ALD-related hospitalizations is primarily driven by the rise in cases of alcoholic hepatitis and acute-on-chronic liver failure among those aged 35 years and younger.
ALD is now the most common indication for liver transplant in those younger than 40 years of age, having increased fourfold between 2003 and 2018.
From 2009 to 2016, people aged 25-34 years experienced the highest average annual increase in cirrhosis-related mortality (10.5%), a trend the authors noted was “driven entirely by alcohol-related liver disease.”
Younger adults may be more susceptible to ALD due to the way they drink.
In a 2021 analysis of the National Health and Nutrition Examination Survey database, the weighted prevalence of harmful alcohol use was 29.3% in those younger than 35 years, compared with 16.9% in those aged 35-64 years. Higher blood alcohol levels resulting from binge drinking may make patients more susceptible to bacterial translocation and liver fibrosis and can increase the likelihood of cirrhosis in those with an underlying metabolic syndrome.
Yet, Dr. Koob said, thinking of “young adults” as one cohort may be misguided because he’s found very different attitudes toward alcohol within that population. Cross-sectional survey data obtained from more than 180,000 young adults indicated that alcohol abstinence increased between 2002 and 2018. Young adults report various reasons for not drinking, ranging from lack of interest to financial and situational barriers (eg, not wanting to interfere with school or work).
“The tide is coming in and out at the same time,” he said. “Younger people under the age of 25 are drinking less each year, are increasingly interested in things like Dry January, and more than half view moderate levels of consumption as unhealthy. People who are 26 years and older are drinking more, are not as interested in cutting back or taking breaks, and are less likely to consider 1 or 2 drinks per day as potentially unhealthy.”
Dr. Koob would like to believe the positive trends around alcohol in the under-25 set prove not only resilient, but someday, dominant.
“We have seen historic increases in alcohol consumption in the last few years — the largest increases in more than 50 years. But we are hopeful that, as the younger cohorts age, we will see lower levels of drinking by adults in mid-life and beyond.”
A version of this article first appeared on Medscape.com.
From Baghdad to Boston: The Making of a Blood Cancer Specialist
Today, she practices hematology at Massachusetts General Hospital, Boston, and is a leading advocate for palliative care in oncology.
In an interview, Dr. El-Jawahri spoke about her journey from Baghdad to Boston and the future of palliative medicine in hematology.
Question: Where did you grow up?
Dr. El-Jawahri: My family is from Baghdad, Iraq, and I was born there. We moved to the States when I was 14. I came to Michigan not speaking a word of English. My parents — my father is a mechanical engineer, and my mom is a computer engineer — chose to live in a very white neighborhood in Farmington Hills, in the suburbs of Detroit. The neighborhood did not have any immigrants or Arab Americans. There are a lot of Arab Americans in Michigan, but they chose for me not to hang out with them early on so that I could learn the language. It was a really good choice.
Question: What happened to your college friend?
Dr. El-Jawahri: She had a brain tumor and ended up receiving intensive care at the end of life. We had a lot of conversations about her wishes and desires, but none of those were honored. Her ending was not something that she wanted, nor did it honor her memory.
Question: What do you think went wrong?
Dr. El-Jawahri: She was getting treatment for her family’s sake. The idea of losing her was too hard for them. I remember vividly the conversations where she would say, “I just hope I don’t end up in the hospital at the end of life.” We had that conversation explicitly. But because we were young, her family was very involved in her care. A lot of the decision-making was very complicated.
Question: How did this experience change your career path?
Dr. El-Jawahri: I went into medicine specifically to become an oncologist and cure cancer. The naive 20-year-old in me said, “Nobody should die this miserable death. I’m going to go in, and I’m going to cure it.”
Question: How did palliative medicine become your major focus?
Dr. El-Jawahri: During my first year at Harvard Medical School, I took a course that’s called “Living With Life-Threatening Illness.” It allows medical students to spend their entire first year getting to know a patient living with a serious illness. We’d spend weekly coffee or lunch breaks with them, where we’d hear about their experiences. After every weekly meeting with a patient, we also had a group meeting with several students and group facilitators to talk about — and process — the interactions we had with patients. I was assigned a woman who was living with metastatic breast cancer. I was also introduced to the field of palliative care and how it helps patients manage complex symptoms and process and cope with a difficult diagnosis. It also cultivates the understanding to make informed decisions about their care. That’s when I knew what I wanted to do for the rest of my life — figure out ways to integrate these palliative and supportive care concepts and improve the lived experience of patients and families within the oncology setting.
Question: What happened next?
Dr. El-Jawahri: When I was a first-year intern, I went to residency at Massachusetts General Hospital. I was on an oncology service and admitted a young college student who was diagnosed with acute myeloid leukemia. She was an athlete, and every time she went up the stairs to her dorm, she was getting very short of breath. She went to a walk-in clinic because when you’re 20 and you’re healthy, you don’t think you need anything. They did some blood work, and 2 hours later, they called her and said, “You probably have leukemia. You need to go to the emergency department immediately.” There she saw an emergency doctor who said, “You will be admitted to the hospital. You have leukemia. I’m calling an oncologist, and you’ll probably have to start chemotherapy within the next day or two.”
Question: What was that experience like for the patient?
Dr. El-Jawahri: I’ve never seen someone so scared. The first question she asked me was about her family, who were from North Carolina. She said, “It feels like everybody thinks that I’m dying. Do you think my family will have time to get here?” They were in a car driving over. This is not a unique story in this population. Unfortunately, these patients experience the most traumatic way of being diagnosed and probably the most traumatic experience in oncology. They’re being abducted into a hospital environment, losing all control and starting immediate therapy. Then, for the first 4-6 weeks, they experience immense toxicity, side effects like nausea, vomiting, diarrhea, and mucositis, where they have painful mouth and throat sores that require intravenous pain medications. This causes real posttraumatic stress. After seeing that woman, I made the decision to work in leukemia and transplants to try to make things a little bit better for these patients.
Question: How did the patient fare?
Dr. El-Jawahri: She actually did great and was cured of her disease. Many of our patients with leukemia, especially younger ones, do well in terms of survival. But they struggle with the trauma of their diagnosis and the distress of the acute treatment period. Even in the curative setting, helping patients to cope with a traumatic diagnosis can have a big impact on their quality of life, how they feel, and their long-term outcomes in terms of psychological stress, depression, anxiety, and posttraumatic stress. But so often, our patients with leukemia are not offered palliative care and supportive care because they’re going to be cured.
Question: What is an important lesson from your research into palliative care in hematology?
Dr. El-Jawahri: We can make things better for patients and families by integrating palliative care clinicians into the care of patients. Patients receiving palliative care are more likely to document their end-of-life preferences and discuss them with their clinicians, and they’re less likely to be hospitalized at the end of life. When you ask patients with cancer where do they want to die, many of patients say, “I want to die at home. I don’t want to be in a hospital.” A lot of the work I’m doing now is focused on creating digital apps with components of palliative care and supportive care interventions. Patients can administer these interventions to themselves and learn how to effectively cope and deal with their illness. Some patients may do well with a digital app, but others may actually need the in-person touch. Some may need a hybrid approach. One of the other future directions for us is thinking about how we optimize supportive care interventions. Which ones do we give to which patient?
Question: Considering all that you’ve learned since college, how do you think your sick friend should have been treated?
Dr. El-Jawahri: She was neither introduced to the term palliative care nor to palliative care specialists. Now the standard of care — especially in patients with advanced cancer — is to integrate palliative care clinicians early in the course of illness. We would have loved for her to have a palliative care clinician who didn’t replace the oncologist but rather helped the patient, family, and oncologist communicate more effectively with one another. We hear all the time from patients who say different things to their oncologist than to their palliative care clinician. It’s not like my friend wasn’t able to communicate with her oncologist. But maybe part of it was that she wanted to not disappoint her oncologist [by ending treatment].
Question: Could you tell me about the research you presented at ASCO 2024 regarding 115 adult patients with acute myeloid leukemia and high-risk myelodysplastic syndrome who were receiving non-intensive chemotherapy?
Dr. El-Jawahri: These patients receive therapy that requires frequent clinic visits and often substantially impairs their quality of life. We know this population often does not engage in any timely discussion with their clinicians about their end-of-life care preferences. This multisite randomized clinical trial assigned patients to receive usual oncology care [with palliative care consultations only upon request] vs to see palliative care clinicians monthly in the outpatient setting and twice weekly every time they were hospitalized. The intervention focused on how to help patients manage their symptoms and end-of-life communication in particular. The primary outcome of the study was time from the documentation of end-of-life care preferences to death.
Question: What did you learn?
Dr. El-Jawahri: This is one of the first studies to highlight the impact of palliative care integration on end-of-life care preferences and discussions and documentation in this population. Patients receiving the palliative care intervention were much more likely to discuss their end-of-life care preferences (96.5% vs 68.4%; P < .001). More importantly, those receiving the intervention had a much longer time from documentation of end-of-life care preferences to death. On average, patients in the palliative care intervention group vs the usual care group had a mean of 41 vs 1.5 days from documentation of their preferences to death (P < .001). In the intervention group, these conversations were happening early enough for patients to plan, talk to their families, and discuss their wishes. In the usual care group, they were happening acutely while these patients were dying. We also learned that patients receiving palliative care intervention were less likely to be hospitalized at the end of life (70.6% vs 91.9%; P = .031) and had better quality of life (138.6 vs 125.5; P = .010).
Question: What’s next for your research in this area?
Dr. El-Jawahri: We are doing a large-scale randomized, comparative effectiveness trial of specialty palliative care vs primary palliative care in 11,150 patients with acute myeloid leukemia across 20 institutions in the United States. We expect results in 2028.
Question: What are you hoping to understand?
Dr. El-Jawahri: We will never have enough specialty palliative care clinicians to take care of all patients with serious illness. As a result, we have to learn how palliative care works: How does it improve outcomes? How do we potentially take what palliative care clinicians do and try to integrate it into regular oncology practice? A lot of the work that I’m excited about now regards what we call primary palliative care. How do we train oncology clinicians to incorporate palliative care skills in their practices so we’re able to better meet the needs of our patients and their families? What we’d love to understand from future research is which patient populations need specialty palliative care and which patients can do just fine with an oncology clinician who has a lot of good palliative care skills integrated into their practice.
Dr. El-Jawahri disclosed consulting for Incyte and Novartis.
A version of this article first appeared on Medscape.com.
Today, she practices hematology at Massachusetts General Hospital, Boston, and is a leading advocate for palliative care in oncology.
In an interview, Dr. El-Jawahri spoke about her journey from Baghdad to Boston and the future of palliative medicine in hematology.
Question: Where did you grow up?
Dr. El-Jawahri: My family is from Baghdad, Iraq, and I was born there. We moved to the States when I was 14. I came to Michigan not speaking a word of English. My parents — my father is a mechanical engineer, and my mom is a computer engineer — chose to live in a very white neighborhood in Farmington Hills, in the suburbs of Detroit. The neighborhood did not have any immigrants or Arab Americans. There are a lot of Arab Americans in Michigan, but they chose for me not to hang out with them early on so that I could learn the language. It was a really good choice.
Question: What happened to your college friend?
Dr. El-Jawahri: She had a brain tumor and ended up receiving intensive care at the end of life. We had a lot of conversations about her wishes and desires, but none of those were honored. Her ending was not something that she wanted, nor did it honor her memory.
Question: What do you think went wrong?
Dr. El-Jawahri: She was getting treatment for her family’s sake. The idea of losing her was too hard for them. I remember vividly the conversations where she would say, “I just hope I don’t end up in the hospital at the end of life.” We had that conversation explicitly. But because we were young, her family was very involved in her care. A lot of the decision-making was very complicated.
Question: How did this experience change your career path?
Dr. El-Jawahri: I went into medicine specifically to become an oncologist and cure cancer. The naive 20-year-old in me said, “Nobody should die this miserable death. I’m going to go in, and I’m going to cure it.”
Question: How did palliative medicine become your major focus?
Dr. El-Jawahri: During my first year at Harvard Medical School, I took a course that’s called “Living With Life-Threatening Illness.” It allows medical students to spend their entire first year getting to know a patient living with a serious illness. We’d spend weekly coffee or lunch breaks with them, where we’d hear about their experiences. After every weekly meeting with a patient, we also had a group meeting with several students and group facilitators to talk about — and process — the interactions we had with patients. I was assigned a woman who was living with metastatic breast cancer. I was also introduced to the field of palliative care and how it helps patients manage complex symptoms and process and cope with a difficult diagnosis. It also cultivates the understanding to make informed decisions about their care. That’s when I knew what I wanted to do for the rest of my life — figure out ways to integrate these palliative and supportive care concepts and improve the lived experience of patients and families within the oncology setting.
Question: What happened next?
Dr. El-Jawahri: When I was a first-year intern, I went to residency at Massachusetts General Hospital. I was on an oncology service and admitted a young college student who was diagnosed with acute myeloid leukemia. She was an athlete, and every time she went up the stairs to her dorm, she was getting very short of breath. She went to a walk-in clinic because when you’re 20 and you’re healthy, you don’t think you need anything. They did some blood work, and 2 hours later, they called her and said, “You probably have leukemia. You need to go to the emergency department immediately.” There she saw an emergency doctor who said, “You will be admitted to the hospital. You have leukemia. I’m calling an oncologist, and you’ll probably have to start chemotherapy within the next day or two.”
Question: What was that experience like for the patient?
Dr. El-Jawahri: I’ve never seen someone so scared. The first question she asked me was about her family, who were from North Carolina. She said, “It feels like everybody thinks that I’m dying. Do you think my family will have time to get here?” They were in a car driving over. This is not a unique story in this population. Unfortunately, these patients experience the most traumatic way of being diagnosed and probably the most traumatic experience in oncology. They’re being abducted into a hospital environment, losing all control and starting immediate therapy. Then, for the first 4-6 weeks, they experience immense toxicity, side effects like nausea, vomiting, diarrhea, and mucositis, where they have painful mouth and throat sores that require intravenous pain medications. This causes real posttraumatic stress. After seeing that woman, I made the decision to work in leukemia and transplants to try to make things a little bit better for these patients.
Question: How did the patient fare?
Dr. El-Jawahri: She actually did great and was cured of her disease. Many of our patients with leukemia, especially younger ones, do well in terms of survival. But they struggle with the trauma of their diagnosis and the distress of the acute treatment period. Even in the curative setting, helping patients to cope with a traumatic diagnosis can have a big impact on their quality of life, how they feel, and their long-term outcomes in terms of psychological stress, depression, anxiety, and posttraumatic stress. But so often, our patients with leukemia are not offered palliative care and supportive care because they’re going to be cured.
Question: What is an important lesson from your research into palliative care in hematology?
Dr. El-Jawahri: We can make things better for patients and families by integrating palliative care clinicians into the care of patients. Patients receiving palliative care are more likely to document their end-of-life preferences and discuss them with their clinicians, and they’re less likely to be hospitalized at the end of life. When you ask patients with cancer where do they want to die, many of patients say, “I want to die at home. I don’t want to be in a hospital.” A lot of the work I’m doing now is focused on creating digital apps with components of palliative care and supportive care interventions. Patients can administer these interventions to themselves and learn how to effectively cope and deal with their illness. Some patients may do well with a digital app, but others may actually need the in-person touch. Some may need a hybrid approach. One of the other future directions for us is thinking about how we optimize supportive care interventions. Which ones do we give to which patient?
Question: Considering all that you’ve learned since college, how do you think your sick friend should have been treated?
Dr. El-Jawahri: She was neither introduced to the term palliative care nor to palliative care specialists. Now the standard of care — especially in patients with advanced cancer — is to integrate palliative care clinicians early in the course of illness. We would have loved for her to have a palliative care clinician who didn’t replace the oncologist but rather helped the patient, family, and oncologist communicate more effectively with one another. We hear all the time from patients who say different things to their oncologist than to their palliative care clinician. It’s not like my friend wasn’t able to communicate with her oncologist. But maybe part of it was that she wanted to not disappoint her oncologist [by ending treatment].
Question: Could you tell me about the research you presented at ASCO 2024 regarding 115 adult patients with acute myeloid leukemia and high-risk myelodysplastic syndrome who were receiving non-intensive chemotherapy?
Dr. El-Jawahri: These patients receive therapy that requires frequent clinic visits and often substantially impairs their quality of life. We know this population often does not engage in any timely discussion with their clinicians about their end-of-life care preferences. This multisite randomized clinical trial assigned patients to receive usual oncology care [with palliative care consultations only upon request] vs to see palliative care clinicians monthly in the outpatient setting and twice weekly every time they were hospitalized. The intervention focused on how to help patients manage their symptoms and end-of-life communication in particular. The primary outcome of the study was time from the documentation of end-of-life care preferences to death.
Question: What did you learn?
Dr. El-Jawahri: This is one of the first studies to highlight the impact of palliative care integration on end-of-life care preferences and discussions and documentation in this population. Patients receiving the palliative care intervention were much more likely to discuss their end-of-life care preferences (96.5% vs 68.4%; P < .001). More importantly, those receiving the intervention had a much longer time from documentation of end-of-life care preferences to death. On average, patients in the palliative care intervention group vs the usual care group had a mean of 41 vs 1.5 days from documentation of their preferences to death (P < .001). In the intervention group, these conversations were happening early enough for patients to plan, talk to their families, and discuss their wishes. In the usual care group, they were happening acutely while these patients were dying. We also learned that patients receiving palliative care intervention were less likely to be hospitalized at the end of life (70.6% vs 91.9%; P = .031) and had better quality of life (138.6 vs 125.5; P = .010).
Question: What’s next for your research in this area?
Dr. El-Jawahri: We are doing a large-scale randomized, comparative effectiveness trial of specialty palliative care vs primary palliative care in 11,150 patients with acute myeloid leukemia across 20 institutions in the United States. We expect results in 2028.
Question: What are you hoping to understand?
Dr. El-Jawahri: We will never have enough specialty palliative care clinicians to take care of all patients with serious illness. As a result, we have to learn how palliative care works: How does it improve outcomes? How do we potentially take what palliative care clinicians do and try to integrate it into regular oncology practice? A lot of the work that I’m excited about now regards what we call primary palliative care. How do we train oncology clinicians to incorporate palliative care skills in their practices so we’re able to better meet the needs of our patients and their families? What we’d love to understand from future research is which patient populations need specialty palliative care and which patients can do just fine with an oncology clinician who has a lot of good palliative care skills integrated into their practice.
Dr. El-Jawahri disclosed consulting for Incyte and Novartis.
A version of this article first appeared on Medscape.com.
Today, she practices hematology at Massachusetts General Hospital, Boston, and is a leading advocate for palliative care in oncology.
In an interview, Dr. El-Jawahri spoke about her journey from Baghdad to Boston and the future of palliative medicine in hematology.
Question: Where did you grow up?
Dr. El-Jawahri: My family is from Baghdad, Iraq, and I was born there. We moved to the States when I was 14. I came to Michigan not speaking a word of English. My parents — my father is a mechanical engineer, and my mom is a computer engineer — chose to live in a very white neighborhood in Farmington Hills, in the suburbs of Detroit. The neighborhood did not have any immigrants or Arab Americans. There are a lot of Arab Americans in Michigan, but they chose for me not to hang out with them early on so that I could learn the language. It was a really good choice.
Question: What happened to your college friend?
Dr. El-Jawahri: She had a brain tumor and ended up receiving intensive care at the end of life. We had a lot of conversations about her wishes and desires, but none of those were honored. Her ending was not something that she wanted, nor did it honor her memory.
Question: What do you think went wrong?
Dr. El-Jawahri: She was getting treatment for her family’s sake. The idea of losing her was too hard for them. I remember vividly the conversations where she would say, “I just hope I don’t end up in the hospital at the end of life.” We had that conversation explicitly. But because we were young, her family was very involved in her care. A lot of the decision-making was very complicated.
Question: How did this experience change your career path?
Dr. El-Jawahri: I went into medicine specifically to become an oncologist and cure cancer. The naive 20-year-old in me said, “Nobody should die this miserable death. I’m going to go in, and I’m going to cure it.”
Question: How did palliative medicine become your major focus?
Dr. El-Jawahri: During my first year at Harvard Medical School, I took a course that’s called “Living With Life-Threatening Illness.” It allows medical students to spend their entire first year getting to know a patient living with a serious illness. We’d spend weekly coffee or lunch breaks with them, where we’d hear about their experiences. After every weekly meeting with a patient, we also had a group meeting with several students and group facilitators to talk about — and process — the interactions we had with patients. I was assigned a woman who was living with metastatic breast cancer. I was also introduced to the field of palliative care and how it helps patients manage complex symptoms and process and cope with a difficult diagnosis. It also cultivates the understanding to make informed decisions about their care. That’s when I knew what I wanted to do for the rest of my life — figure out ways to integrate these palliative and supportive care concepts and improve the lived experience of patients and families within the oncology setting.
Question: What happened next?
Dr. El-Jawahri: When I was a first-year intern, I went to residency at Massachusetts General Hospital. I was on an oncology service and admitted a young college student who was diagnosed with acute myeloid leukemia. She was an athlete, and every time she went up the stairs to her dorm, she was getting very short of breath. She went to a walk-in clinic because when you’re 20 and you’re healthy, you don’t think you need anything. They did some blood work, and 2 hours later, they called her and said, “You probably have leukemia. You need to go to the emergency department immediately.” There she saw an emergency doctor who said, “You will be admitted to the hospital. You have leukemia. I’m calling an oncologist, and you’ll probably have to start chemotherapy within the next day or two.”
Question: What was that experience like for the patient?
Dr. El-Jawahri: I’ve never seen someone so scared. The first question she asked me was about her family, who were from North Carolina. She said, “It feels like everybody thinks that I’m dying. Do you think my family will have time to get here?” They were in a car driving over. This is not a unique story in this population. Unfortunately, these patients experience the most traumatic way of being diagnosed and probably the most traumatic experience in oncology. They’re being abducted into a hospital environment, losing all control and starting immediate therapy. Then, for the first 4-6 weeks, they experience immense toxicity, side effects like nausea, vomiting, diarrhea, and mucositis, where they have painful mouth and throat sores that require intravenous pain medications. This causes real posttraumatic stress. After seeing that woman, I made the decision to work in leukemia and transplants to try to make things a little bit better for these patients.
Question: How did the patient fare?
Dr. El-Jawahri: She actually did great and was cured of her disease. Many of our patients with leukemia, especially younger ones, do well in terms of survival. But they struggle with the trauma of their diagnosis and the distress of the acute treatment period. Even in the curative setting, helping patients to cope with a traumatic diagnosis can have a big impact on their quality of life, how they feel, and their long-term outcomes in terms of psychological stress, depression, anxiety, and posttraumatic stress. But so often, our patients with leukemia are not offered palliative care and supportive care because they’re going to be cured.
Question: What is an important lesson from your research into palliative care in hematology?
Dr. El-Jawahri: We can make things better for patients and families by integrating palliative care clinicians into the care of patients. Patients receiving palliative care are more likely to document their end-of-life preferences and discuss them with their clinicians, and they’re less likely to be hospitalized at the end of life. When you ask patients with cancer where do they want to die, many of patients say, “I want to die at home. I don’t want to be in a hospital.” A lot of the work I’m doing now is focused on creating digital apps with components of palliative care and supportive care interventions. Patients can administer these interventions to themselves and learn how to effectively cope and deal with their illness. Some patients may do well with a digital app, but others may actually need the in-person touch. Some may need a hybrid approach. One of the other future directions for us is thinking about how we optimize supportive care interventions. Which ones do we give to which patient?
Question: Considering all that you’ve learned since college, how do you think your sick friend should have been treated?
Dr. El-Jawahri: She was neither introduced to the term palliative care nor to palliative care specialists. Now the standard of care — especially in patients with advanced cancer — is to integrate palliative care clinicians early in the course of illness. We would have loved for her to have a palliative care clinician who didn’t replace the oncologist but rather helped the patient, family, and oncologist communicate more effectively with one another. We hear all the time from patients who say different things to their oncologist than to their palliative care clinician. It’s not like my friend wasn’t able to communicate with her oncologist. But maybe part of it was that she wanted to not disappoint her oncologist [by ending treatment].
Question: Could you tell me about the research you presented at ASCO 2024 regarding 115 adult patients with acute myeloid leukemia and high-risk myelodysplastic syndrome who were receiving non-intensive chemotherapy?
Dr. El-Jawahri: These patients receive therapy that requires frequent clinic visits and often substantially impairs their quality of life. We know this population often does not engage in any timely discussion with their clinicians about their end-of-life care preferences. This multisite randomized clinical trial assigned patients to receive usual oncology care [with palliative care consultations only upon request] vs to see palliative care clinicians monthly in the outpatient setting and twice weekly every time they were hospitalized. The intervention focused on how to help patients manage their symptoms and end-of-life communication in particular. The primary outcome of the study was time from the documentation of end-of-life care preferences to death.
Question: What did you learn?
Dr. El-Jawahri: This is one of the first studies to highlight the impact of palliative care integration on end-of-life care preferences and discussions and documentation in this population. Patients receiving the palliative care intervention were much more likely to discuss their end-of-life care preferences (96.5% vs 68.4%; P < .001). More importantly, those receiving the intervention had a much longer time from documentation of end-of-life care preferences to death. On average, patients in the palliative care intervention group vs the usual care group had a mean of 41 vs 1.5 days from documentation of their preferences to death (P < .001). In the intervention group, these conversations were happening early enough for patients to plan, talk to their families, and discuss their wishes. In the usual care group, they were happening acutely while these patients were dying. We also learned that patients receiving palliative care intervention were less likely to be hospitalized at the end of life (70.6% vs 91.9%; P = .031) and had better quality of life (138.6 vs 125.5; P = .010).
Question: What’s next for your research in this area?
Dr. El-Jawahri: We are doing a large-scale randomized, comparative effectiveness trial of specialty palliative care vs primary palliative care in 11,150 patients with acute myeloid leukemia across 20 institutions in the United States. We expect results in 2028.
Question: What are you hoping to understand?
Dr. El-Jawahri: We will never have enough specialty palliative care clinicians to take care of all patients with serious illness. As a result, we have to learn how palliative care works: How does it improve outcomes? How do we potentially take what palliative care clinicians do and try to integrate it into regular oncology practice? A lot of the work that I’m excited about now regards what we call primary palliative care. How do we train oncology clinicians to incorporate palliative care skills in their practices so we’re able to better meet the needs of our patients and their families? What we’d love to understand from future research is which patient populations need specialty palliative care and which patients can do just fine with an oncology clinician who has a lot of good palliative care skills integrated into their practice.
Dr. El-Jawahri disclosed consulting for Incyte and Novartis.
A version of this article first appeared on Medscape.com.
We Asked 7 Doctors: How Do You Get Patients to Exercise?
We know exercise can be a powerful medical intervention. Now scientists are finally starting to understand why.
A recent study in rats found that exercise positively changes virtually every tissue in the body. The research was part of a large National Institutes of Health initiative called MoTrPAC (Molecular Transducers of Physical Activity Consortium) to understand how physical activity improves health and prevents disease. As part of the project, a large human study is also underway.
“What was mind-blowing to me was just how much every organ changed,” said cardiologist Euan A. Ashley, MD, professor of medicine at Stanford University, Stanford, California, and the study’s lead author. “You really are a different person on exercise.”
The study examined hundreds of previously sedentary rats that exercised on a treadmill for 8 weeks. Their tissues were compared with a control group of rats that stayed sedentary.
Your patients, unlike lab animals, can’t be randomly assigned to run on a treadmill until you switch the machine off.
So how do you persuade your patients to become more active?
We asked seven doctors what works for them. They shared 10 of their most effective persuasion tactics.
1. Focus on the First Step
“It’s easy to say you want to change behavior,” said Jordan Metzl, MD, a sports medicine specialist at the Hospital for Special Surgery in New York City who instructs medical students on how to prescribe exercise. “It’s much more difficult to do it.”
He compares it with moving a tractor tire from point A to point B. The hardest part is lifting the tire off the ground and starting to move it. “Once it’s rolling, it takes much less effort to keep it going in the same direction,” he said.
How much exercise a patient does is irrelevant until they’ve given that tire its first push.
“Any amount of exercise is better than nothing,” Dr. Ashley said. “Let’s just start with that. Making the move from sitting a lot to standing more has genuine health benefits.”
2. Mind Your Language
Many patients have a deep-rooted aversion to words and phrases associated with physical activity.
“Exercise” is one. “Working out” is another.
“I often tell them they just have to start moving,” said Chris Raynor, MD, an orthopedic surgeon based in Ottawa, Ontario. “Don’t think about it as working out. Think about it as just moving. Start with something they already like doing and work from there.”
3. Make It Manageable
This also applies to patients who’re injured and either waiting for or recovering from surgery.
“Joints like motion,” said Rachel M. Frank, MD, an orthopedic surgeon at the University of Colorado Sports Medicine, Denver, Colorado. “The more mobile you can be, the easier your recovery’s going to be.”
That can be a challenge for a patient who wasn’t active before the injury, especially if he or she is fixed on the idea that exercise doesn’t matter unless they do it for 30-45 minutes at a time.
“I try to break it down into manageable bits they can do at home,” Dr. Frank said. “I say, ‘Look, you brush your teeth twice a day, right? Can you do these exercises for 5 or 10 minutes before or after you brush your teeth?’ ”
4. Connect Their Interests to Their Activity Level
Chad Waterbury, DPT, thought he knew how to motivate a postsurgical patient to become more active and improve her odds for a full recovery. He told her she’d feel better and have more energy — all the usual selling points.
None of it impressed her.
But one day she mentioned that she’d recently become a grandmother for the first time. Dr. Waterbury, a physical therapist based in Los Angeles, noticed how she lit up when she talked about her new granddaughter.
“So I started giving her scenarios, like taking her daughter to Disneyland when she’s 9 or 10. You have to be somewhat fit to do something like that.”
It worked, and Dr. Waterbury learned a fundamental lesson in motivation. “You have to connect the exercise to something that’s important in their life,” he said.
5. Don’t Let a Crisis Go to Waste
“There are very few things more motivating than having a heart attack,” Dr. Ashley said. “For the vast majority of people, that’s a very sobering moment where they reassess everything in their lives.”
There’ll never be a better time to persuade a patient to become more active. In his cardiology practice, Dr. Ashley has seen a lot of patients make that switch.
“They really do start to prioritize their health in a way they never did before,” he said.
6. Emphasize the Practical Over the Ideal
Not all patients attach negative feelings to working out. For some, it’s the goal.
Todd Ivan, MD, calls it the “ ’I need to get to the gym’ lament”: Something they’ve aspired to but rarely if ever done.
“I tell them I’d welcome a half-hour walk every day to get started,” said Dr. Ivan, a consultation-liaison psychiatrist at Summa Health in Akron, Ohio. “It’s a way to introduce the idea that fitness begins with small adjustments.”
7. Go Beneath the Surface
“Exercise doesn’t generally result in great weight loss,” said endocrinologist Karl Nadolsky, DO, an obesity specialist and co-host of the Docs Who Lift podcast.
But a lot of his patients struggle to break that connection. It’s understandable, given how many times they’ve been told they’d weigh less if they moved more.
Dr. Nadolsky tells them it’s what’s on the inside that counts. “I explain it as very literal, meaning their physical health, metabolic health, and mental health.”
By reframing physical activity with an internal rather than external focus — the plumbing and wiring vs the shutters and shingles — he gives them permission to approach exercise as a health upgrade rather than yet another part of their lifelong struggle to lose weight.
“A significant number of our patients respond well to that,” he said.
8. Appeal to Their Intellect
Some patients think like doctors: No matter how reluctant they may be to change their mind about something, they’ll respond to evidence.
Dr. Frank has learned to identify these scientifically inclined patients. “I’ll flood them with data,” she said. “I’ll say, ‘These studies show that if you do x, y, z, your outcome will be better.’ ”
Dr. Ashley takes a similar approach when his patients give him the most common reason for not exercising: “I don’t have time.”
He tells them that exercise doesn’t take time. It gives you time.
That’s according to a 2012 study of more than 650,000 adults that associated physical activity with an increased lifespan.
As one of the authors said in an interview, a middle-aged person who gets 150 minutes a week of moderate exercise will, on average, gain 7 more minutes of life for each minute of exercise, compared with someone who doesn’t get any exercise.
The strategy works because it brings patients out of their day-to-day lives and into the future, Dr. Ashley said.
“What about your entire life?” he asks them. “You’re actually in this world for 80-plus years, you hope. How are you going to spend that? You have to think about that when you’re in your 40s and 50s.”
9. Show Them the Money
Illness and injury, on top of everything else, can be really expensive.
Even with good insurance, a health problem that requires surgery and/or hospitalization might cost thousands of dollars out of pocket. With mediocre insurance, it might be tens of thousands.
Sometimes, Dr. Frank said, it helps to remind patients of the price they paid for their treatment. “I’ll say, ‘Let’s get moving so you don’t have to pay for this again.’ ”
Protecting their investment can be a powerful motivation.
10. Make It a Team Effort
While the doctors we interviewed have a wide range of specialties — cardiology, sports medicine, psychiatry, endocrinology, orthopedics, and physical therapy — their patients have one thing in common.
They don’t want to be in a doctor’s office. It means they have something, need something, or broke something.
It might be a treatable condition that’s merely inconvenient or a life-threatening event that’s flat-out terrifying.
Whatever it is, it pulls them out of their normal world. It can be a lonely, disorienting experience.
Sometimes the best thing a doctor can do is stay connected with the patient. “This is like a team sport,” Dr. Frank tells her patients. “I’m going to be your coach, but you’re the captain of the team.”
In some cases, she’ll ask the patient to message her on the portal after completing the daily or weekly exercises. That alone might motivate the patient — especially when she responds to their messages.
After all, nobody wants to let the coach down.
A version of this article first appeared on Medscape.com.
We know exercise can be a powerful medical intervention. Now scientists are finally starting to understand why.
A recent study in rats found that exercise positively changes virtually every tissue in the body. The research was part of a large National Institutes of Health initiative called MoTrPAC (Molecular Transducers of Physical Activity Consortium) to understand how physical activity improves health and prevents disease. As part of the project, a large human study is also underway.
“What was mind-blowing to me was just how much every organ changed,” said cardiologist Euan A. Ashley, MD, professor of medicine at Stanford University, Stanford, California, and the study’s lead author. “You really are a different person on exercise.”
The study examined hundreds of previously sedentary rats that exercised on a treadmill for 8 weeks. Their tissues were compared with a control group of rats that stayed sedentary.
Your patients, unlike lab animals, can’t be randomly assigned to run on a treadmill until you switch the machine off.
So how do you persuade your patients to become more active?
We asked seven doctors what works for them. They shared 10 of their most effective persuasion tactics.
1. Focus on the First Step
“It’s easy to say you want to change behavior,” said Jordan Metzl, MD, a sports medicine specialist at the Hospital for Special Surgery in New York City who instructs medical students on how to prescribe exercise. “It’s much more difficult to do it.”
He compares it with moving a tractor tire from point A to point B. The hardest part is lifting the tire off the ground and starting to move it. “Once it’s rolling, it takes much less effort to keep it going in the same direction,” he said.
How much exercise a patient does is irrelevant until they’ve given that tire its first push.
“Any amount of exercise is better than nothing,” Dr. Ashley said. “Let’s just start with that. Making the move from sitting a lot to standing more has genuine health benefits.”
2. Mind Your Language
Many patients have a deep-rooted aversion to words and phrases associated with physical activity.
“Exercise” is one. “Working out” is another.
“I often tell them they just have to start moving,” said Chris Raynor, MD, an orthopedic surgeon based in Ottawa, Ontario. “Don’t think about it as working out. Think about it as just moving. Start with something they already like doing and work from there.”
3. Make It Manageable
This also applies to patients who’re injured and either waiting for or recovering from surgery.
“Joints like motion,” said Rachel M. Frank, MD, an orthopedic surgeon at the University of Colorado Sports Medicine, Denver, Colorado. “The more mobile you can be, the easier your recovery’s going to be.”
That can be a challenge for a patient who wasn’t active before the injury, especially if he or she is fixed on the idea that exercise doesn’t matter unless they do it for 30-45 minutes at a time.
“I try to break it down into manageable bits they can do at home,” Dr. Frank said. “I say, ‘Look, you brush your teeth twice a day, right? Can you do these exercises for 5 or 10 minutes before or after you brush your teeth?’ ”
4. Connect Their Interests to Their Activity Level
Chad Waterbury, DPT, thought he knew how to motivate a postsurgical patient to become more active and improve her odds for a full recovery. He told her she’d feel better and have more energy — all the usual selling points.
None of it impressed her.
But one day she mentioned that she’d recently become a grandmother for the first time. Dr. Waterbury, a physical therapist based in Los Angeles, noticed how she lit up when she talked about her new granddaughter.
“So I started giving her scenarios, like taking her daughter to Disneyland when she’s 9 or 10. You have to be somewhat fit to do something like that.”
It worked, and Dr. Waterbury learned a fundamental lesson in motivation. “You have to connect the exercise to something that’s important in their life,” he said.
5. Don’t Let a Crisis Go to Waste
“There are very few things more motivating than having a heart attack,” Dr. Ashley said. “For the vast majority of people, that’s a very sobering moment where they reassess everything in their lives.”
There’ll never be a better time to persuade a patient to become more active. In his cardiology practice, Dr. Ashley has seen a lot of patients make that switch.
“They really do start to prioritize their health in a way they never did before,” he said.
6. Emphasize the Practical Over the Ideal
Not all patients attach negative feelings to working out. For some, it’s the goal.
Todd Ivan, MD, calls it the “ ’I need to get to the gym’ lament”: Something they’ve aspired to but rarely if ever done.
“I tell them I’d welcome a half-hour walk every day to get started,” said Dr. Ivan, a consultation-liaison psychiatrist at Summa Health in Akron, Ohio. “It’s a way to introduce the idea that fitness begins with small adjustments.”
7. Go Beneath the Surface
“Exercise doesn’t generally result in great weight loss,” said endocrinologist Karl Nadolsky, DO, an obesity specialist and co-host of the Docs Who Lift podcast.
But a lot of his patients struggle to break that connection. It’s understandable, given how many times they’ve been told they’d weigh less if they moved more.
Dr. Nadolsky tells them it’s what’s on the inside that counts. “I explain it as very literal, meaning their physical health, metabolic health, and mental health.”
By reframing physical activity with an internal rather than external focus — the plumbing and wiring vs the shutters and shingles — he gives them permission to approach exercise as a health upgrade rather than yet another part of their lifelong struggle to lose weight.
“A significant number of our patients respond well to that,” he said.
8. Appeal to Their Intellect
Some patients think like doctors: No matter how reluctant they may be to change their mind about something, they’ll respond to evidence.
Dr. Frank has learned to identify these scientifically inclined patients. “I’ll flood them with data,” she said. “I’ll say, ‘These studies show that if you do x, y, z, your outcome will be better.’ ”
Dr. Ashley takes a similar approach when his patients give him the most common reason for not exercising: “I don’t have time.”
He tells them that exercise doesn’t take time. It gives you time.
That’s according to a 2012 study of more than 650,000 adults that associated physical activity with an increased lifespan.
As one of the authors said in an interview, a middle-aged person who gets 150 minutes a week of moderate exercise will, on average, gain 7 more minutes of life for each minute of exercise, compared with someone who doesn’t get any exercise.
The strategy works because it brings patients out of their day-to-day lives and into the future, Dr. Ashley said.
“What about your entire life?” he asks them. “You’re actually in this world for 80-plus years, you hope. How are you going to spend that? You have to think about that when you’re in your 40s and 50s.”
9. Show Them the Money
Illness and injury, on top of everything else, can be really expensive.
Even with good insurance, a health problem that requires surgery and/or hospitalization might cost thousands of dollars out of pocket. With mediocre insurance, it might be tens of thousands.
Sometimes, Dr. Frank said, it helps to remind patients of the price they paid for their treatment. “I’ll say, ‘Let’s get moving so you don’t have to pay for this again.’ ”
Protecting their investment can be a powerful motivation.
10. Make It a Team Effort
While the doctors we interviewed have a wide range of specialties — cardiology, sports medicine, psychiatry, endocrinology, orthopedics, and physical therapy — their patients have one thing in common.
They don’t want to be in a doctor’s office. It means they have something, need something, or broke something.
It might be a treatable condition that’s merely inconvenient or a life-threatening event that’s flat-out terrifying.
Whatever it is, it pulls them out of their normal world. It can be a lonely, disorienting experience.
Sometimes the best thing a doctor can do is stay connected with the patient. “This is like a team sport,” Dr. Frank tells her patients. “I’m going to be your coach, but you’re the captain of the team.”
In some cases, she’ll ask the patient to message her on the portal after completing the daily or weekly exercises. That alone might motivate the patient — especially when she responds to their messages.
After all, nobody wants to let the coach down.
A version of this article first appeared on Medscape.com.
We know exercise can be a powerful medical intervention. Now scientists are finally starting to understand why.
A recent study in rats found that exercise positively changes virtually every tissue in the body. The research was part of a large National Institutes of Health initiative called MoTrPAC (Molecular Transducers of Physical Activity Consortium) to understand how physical activity improves health and prevents disease. As part of the project, a large human study is also underway.
“What was mind-blowing to me was just how much every organ changed,” said cardiologist Euan A. Ashley, MD, professor of medicine at Stanford University, Stanford, California, and the study’s lead author. “You really are a different person on exercise.”
The study examined hundreds of previously sedentary rats that exercised on a treadmill for 8 weeks. Their tissues were compared with a control group of rats that stayed sedentary.
Your patients, unlike lab animals, can’t be randomly assigned to run on a treadmill until you switch the machine off.
So how do you persuade your patients to become more active?
We asked seven doctors what works for them. They shared 10 of their most effective persuasion tactics.
1. Focus on the First Step
“It’s easy to say you want to change behavior,” said Jordan Metzl, MD, a sports medicine specialist at the Hospital for Special Surgery in New York City who instructs medical students on how to prescribe exercise. “It’s much more difficult to do it.”
He compares it with moving a tractor tire from point A to point B. The hardest part is lifting the tire off the ground and starting to move it. “Once it’s rolling, it takes much less effort to keep it going in the same direction,” he said.
How much exercise a patient does is irrelevant until they’ve given that tire its first push.
“Any amount of exercise is better than nothing,” Dr. Ashley said. “Let’s just start with that. Making the move from sitting a lot to standing more has genuine health benefits.”
2. Mind Your Language
Many patients have a deep-rooted aversion to words and phrases associated with physical activity.
“Exercise” is one. “Working out” is another.
“I often tell them they just have to start moving,” said Chris Raynor, MD, an orthopedic surgeon based in Ottawa, Ontario. “Don’t think about it as working out. Think about it as just moving. Start with something they already like doing and work from there.”
3. Make It Manageable
This also applies to patients who’re injured and either waiting for or recovering from surgery.
“Joints like motion,” said Rachel M. Frank, MD, an orthopedic surgeon at the University of Colorado Sports Medicine, Denver, Colorado. “The more mobile you can be, the easier your recovery’s going to be.”
That can be a challenge for a patient who wasn’t active before the injury, especially if he or she is fixed on the idea that exercise doesn’t matter unless they do it for 30-45 minutes at a time.
“I try to break it down into manageable bits they can do at home,” Dr. Frank said. “I say, ‘Look, you brush your teeth twice a day, right? Can you do these exercises for 5 or 10 minutes before or after you brush your teeth?’ ”
4. Connect Their Interests to Their Activity Level
Chad Waterbury, DPT, thought he knew how to motivate a postsurgical patient to become more active and improve her odds for a full recovery. He told her she’d feel better and have more energy — all the usual selling points.
None of it impressed her.
But one day she mentioned that she’d recently become a grandmother for the first time. Dr. Waterbury, a physical therapist based in Los Angeles, noticed how she lit up when she talked about her new granddaughter.
“So I started giving her scenarios, like taking her daughter to Disneyland when she’s 9 or 10. You have to be somewhat fit to do something like that.”
It worked, and Dr. Waterbury learned a fundamental lesson in motivation. “You have to connect the exercise to something that’s important in their life,” he said.
5. Don’t Let a Crisis Go to Waste
“There are very few things more motivating than having a heart attack,” Dr. Ashley said. “For the vast majority of people, that’s a very sobering moment where they reassess everything in their lives.”
There’ll never be a better time to persuade a patient to become more active. In his cardiology practice, Dr. Ashley has seen a lot of patients make that switch.
“They really do start to prioritize their health in a way they never did before,” he said.
6. Emphasize the Practical Over the Ideal
Not all patients attach negative feelings to working out. For some, it’s the goal.
Todd Ivan, MD, calls it the “ ’I need to get to the gym’ lament”: Something they’ve aspired to but rarely if ever done.
“I tell them I’d welcome a half-hour walk every day to get started,” said Dr. Ivan, a consultation-liaison psychiatrist at Summa Health in Akron, Ohio. “It’s a way to introduce the idea that fitness begins with small adjustments.”
7. Go Beneath the Surface
“Exercise doesn’t generally result in great weight loss,” said endocrinologist Karl Nadolsky, DO, an obesity specialist and co-host of the Docs Who Lift podcast.
But a lot of his patients struggle to break that connection. It’s understandable, given how many times they’ve been told they’d weigh less if they moved more.
Dr. Nadolsky tells them it’s what’s on the inside that counts. “I explain it as very literal, meaning their physical health, metabolic health, and mental health.”
By reframing physical activity with an internal rather than external focus — the plumbing and wiring vs the shutters and shingles — he gives them permission to approach exercise as a health upgrade rather than yet another part of their lifelong struggle to lose weight.
“A significant number of our patients respond well to that,” he said.
8. Appeal to Their Intellect
Some patients think like doctors: No matter how reluctant they may be to change their mind about something, they’ll respond to evidence.
Dr. Frank has learned to identify these scientifically inclined patients. “I’ll flood them with data,” she said. “I’ll say, ‘These studies show that if you do x, y, z, your outcome will be better.’ ”
Dr. Ashley takes a similar approach when his patients give him the most common reason for not exercising: “I don’t have time.”
He tells them that exercise doesn’t take time. It gives you time.
That’s according to a 2012 study of more than 650,000 adults that associated physical activity with an increased lifespan.
As one of the authors said in an interview, a middle-aged person who gets 150 minutes a week of moderate exercise will, on average, gain 7 more minutes of life for each minute of exercise, compared with someone who doesn’t get any exercise.
The strategy works because it brings patients out of their day-to-day lives and into the future, Dr. Ashley said.
“What about your entire life?” he asks them. “You’re actually in this world for 80-plus years, you hope. How are you going to spend that? You have to think about that when you’re in your 40s and 50s.”
9. Show Them the Money
Illness and injury, on top of everything else, can be really expensive.
Even with good insurance, a health problem that requires surgery and/or hospitalization might cost thousands of dollars out of pocket. With mediocre insurance, it might be tens of thousands.
Sometimes, Dr. Frank said, it helps to remind patients of the price they paid for their treatment. “I’ll say, ‘Let’s get moving so you don’t have to pay for this again.’ ”
Protecting their investment can be a powerful motivation.
10. Make It a Team Effort
While the doctors we interviewed have a wide range of specialties — cardiology, sports medicine, psychiatry, endocrinology, orthopedics, and physical therapy — their patients have one thing in common.
They don’t want to be in a doctor’s office. It means they have something, need something, or broke something.
It might be a treatable condition that’s merely inconvenient or a life-threatening event that’s flat-out terrifying.
Whatever it is, it pulls them out of their normal world. It can be a lonely, disorienting experience.
Sometimes the best thing a doctor can do is stay connected with the patient. “This is like a team sport,” Dr. Frank tells her patients. “I’m going to be your coach, but you’re the captain of the team.”
In some cases, she’ll ask the patient to message her on the portal after completing the daily or weekly exercises. That alone might motivate the patient — especially when she responds to their messages.
After all, nobody wants to let the coach down.
A version of this article first appeared on Medscape.com.
Could Baseline MRIs Reshape Prostate Cancer Risk Assessment?
The multicenter, real-world trial showed that men with low-risk or favorable intermediate-risk disease who had higher Prostate Imaging Reporting and Data System (PI-RADS) scores at baseline were more likely to be reclassified with more aggressive disease on a future biopsy, wrote lead author Kiran R. Nandalur, MD and colleagues. The study was published in The Journal of Urology.
This means that without MRI, some cases of prostate cancer are being labeled as lower-risk than they actually are.
The investigators noted that MRI is increasingly being used to choose patients who are appropriate for active surveillance instead of treatment, but related clinical data are scarce.
Although PI-RADS is the preferred metric for characterizing prostate tumors via MRI, “most previous studies on the prognostic implications of baseline PI-RADS score included smaller populations from academic centers, limited inclusion of clinical and pathologic data into models, and/or [are] ambiguous on the implications of PI-RADS score,” they wrote.
These knowledge gaps prompted the present study.
How Were Baseline MRI Findings Related to Prostate Cancer Disease Risk?
The dataset included 1491 men with prostate cancer that was diagnosed at 46 hospital-based, academic, or private practice urology groups. All had low-risk or favorable intermediate-risk disease and had undergone MRI within 6 months before or after initial biopsy, along with enrollment in active surveillance.
“A novel aspect of this study was that the MRIs were not read by dedicated prostate MRI experts at academic institutions, but rather a mix of community and academic radiologists,” Dr. Nandalur, medical director of Corewell Health East Radiology, Royal Oak, Michigan, said in an interview.
After traditional risk factors were accounted for, baseline PI-RADS (four or more lesions) was significantly associated with increased likelihood of biopsy reclassification to high-grade prostate cancer on surveillance biopsy (hazard ratio, 2.3; 95% CI 1.6-3.2; P < .001).
“These patients with suspicious lesions on their initial MRI were more than twice as likely to have higher-grade disease within 5 years,” Nandalur noted. “This result was not only seen in the low-risk group but also in the favorable intermediate-risk group, which hasn’t been shown before.”
Grade group 2 vs 1 and increasing age were also associated with significantly increased risk for reclassification to a more aggressive cancer type.
How Might These Findings Improve Outcomes in Patients With Prostate Cancer?
Currently, 60%-70% of patients with low-risk disease choose active surveillance over immediate treatment, whereas 20% with favorable intermediate-risk disease choose active surveillance, according to Dr. Nandalur.
For low-risk patients, PI-RADS score is unlikely to change this decision, although surveillance intervals could be adjusted in accordance with risk. More notably, those with favorable intermediate-risk disease may benefit from considering PI-RADS score when choosing between active surveillance and immediate treatment.
“Most of the management strategies for prostate cancer are based on just your lab values and your pathology,” Dr. Nandalur said, “but this study shows that maybe we should start taking MRI into account — into the general paradigm of management of prostate cancer.”
Ideally, he added, prospective studies will confirm these findings, although such studies can be challenging to perform and similar data have historically been sufficient to reshape clinical practice.
“We are hoping that [baseline PI-RADS score] will be adopted into the NCCN [National Comprehensive Cancer Network] guidelines,” Dr. Nandalur said.
How Likely Are These Findings to Reshape Clinical Practice?
“The study’s large, multicenter cohort and its focus on the prognostic value of baseline MRI in active surveillance make it a crucial contribution to the field, providing evidence that can potentially refine patient management strategies in clinical practice,” Ismail Baris Turkbey, MD, FSAR, head of MRI Section, Molecular Imaging Branch, National Cancer Institute, Rockville, Maryland, said in a written comment.
“The findings from this study are likely to have a significant impact on clinical practice and potentially influence future guidelines in the management of localized prostate cancer, particularly in the context of active surveillance,” Dr. Turkbey said. “MRI, already a commonly used imaging modality in prostate cancer management, may become an even more integral part of the initial assessment and ongoing monitoring of patients with low or favorable-intermediate risk prostate cancer.”
Dr. Turkbey noted several strengths of the study.
First, the size and the diversity of the cohort, along with the variety of treatment centers, support generalizability of findings. Second, the study pinpoints a “critical aspect” of active surveillance by uncovering the link between baseline MRI findings and later risk reclassification. Finally, the study also showed that increasing age was associated with higher likelihood of risk reclassification, “further emphasizing the need for personalized risk assessment” among these patients.
What Were Some Limitations of This Study?
“One important limitation is the lack of inter-reader agreement for PI-RADS evaluations for baseline MRIs,” Dr. Turkbey said. “Variation of PI-RADS is quite known, and centralized evaluations could have made this study stronger. Same applies for centralized quality evaluation of MRIs using The Prostate Imaging Quality (PI-QUAL) score. These items are difficult to do in a multicenter prospective data registry, and maybe authors will consider including these additional analyses in their future work.”
How Does This New Approach to Prostate Cancer Risk Assessment Compare With Recent Advances in AI-Based Risk Assessment?
Over the past few years, artificial intelligence (AI)–assisted risk assessment in prostate cancer has been gaining increasing attention. Recently, for example, Artera, a self-styled “precision medicine company,” released the first AI tool to help patients choose between active surveillance and active treatment on the basis of analysis of digital pathology images.
When asked to compare this approach with the methods used in the present study, Dr. Nandalur called the AI model “a step forward” but noted that it still relies on conventional risk criteria.
“Our data show imaging with MRI has independent prognostic information for prostate cancer patients considering active surveillance, over and above these traditional factors,” he said. “Moreover, this predictive ability of MRI was seen in low and favorable intermediate risk groups, so the additive value is broad.”
Still, he predicted that the future will not involve a binary choice, but a combination approach.
“The exciting aspect is that MRI results can eventually be added to this novel AI model and further improve prediction models for patients,” Dr. Nandalur said. “The combination of recent AI models and MRI will likely represent the future paradigm for prostate cancer patients considering active surveillance versus immediate treatment.”
The study was supported by Blue Cross and Blue Shield of Michigan. The investigators and Dr. Turkbey reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
The multicenter, real-world trial showed that men with low-risk or favorable intermediate-risk disease who had higher Prostate Imaging Reporting and Data System (PI-RADS) scores at baseline were more likely to be reclassified with more aggressive disease on a future biopsy, wrote lead author Kiran R. Nandalur, MD and colleagues. The study was published in The Journal of Urology.
This means that without MRI, some cases of prostate cancer are being labeled as lower-risk than they actually are.
The investigators noted that MRI is increasingly being used to choose patients who are appropriate for active surveillance instead of treatment, but related clinical data are scarce.
Although PI-RADS is the preferred metric for characterizing prostate tumors via MRI, “most previous studies on the prognostic implications of baseline PI-RADS score included smaller populations from academic centers, limited inclusion of clinical and pathologic data into models, and/or [are] ambiguous on the implications of PI-RADS score,” they wrote.
These knowledge gaps prompted the present study.
How Were Baseline MRI Findings Related to Prostate Cancer Disease Risk?
The dataset included 1491 men with prostate cancer that was diagnosed at 46 hospital-based, academic, or private practice urology groups. All had low-risk or favorable intermediate-risk disease and had undergone MRI within 6 months before or after initial biopsy, along with enrollment in active surveillance.
“A novel aspect of this study was that the MRIs were not read by dedicated prostate MRI experts at academic institutions, but rather a mix of community and academic radiologists,” Dr. Nandalur, medical director of Corewell Health East Radiology, Royal Oak, Michigan, said in an interview.
After traditional risk factors were accounted for, baseline PI-RADS (four or more lesions) was significantly associated with increased likelihood of biopsy reclassification to high-grade prostate cancer on surveillance biopsy (hazard ratio, 2.3; 95% CI 1.6-3.2; P < .001).
“These patients with suspicious lesions on their initial MRI were more than twice as likely to have higher-grade disease within 5 years,” Nandalur noted. “This result was not only seen in the low-risk group but also in the favorable intermediate-risk group, which hasn’t been shown before.”
Grade group 2 vs 1 and increasing age were also associated with significantly increased risk for reclassification to a more aggressive cancer type.
How Might These Findings Improve Outcomes in Patients With Prostate Cancer?
Currently, 60%-70% of patients with low-risk disease choose active surveillance over immediate treatment, whereas 20% with favorable intermediate-risk disease choose active surveillance, according to Dr. Nandalur.
For low-risk patients, PI-RADS score is unlikely to change this decision, although surveillance intervals could be adjusted in accordance with risk. More notably, those with favorable intermediate-risk disease may benefit from considering PI-RADS score when choosing between active surveillance and immediate treatment.
“Most of the management strategies for prostate cancer are based on just your lab values and your pathology,” Dr. Nandalur said, “but this study shows that maybe we should start taking MRI into account — into the general paradigm of management of prostate cancer.”
Ideally, he added, prospective studies will confirm these findings, although such studies can be challenging to perform and similar data have historically been sufficient to reshape clinical practice.
“We are hoping that [baseline PI-RADS score] will be adopted into the NCCN [National Comprehensive Cancer Network] guidelines,” Dr. Nandalur said.
How Likely Are These Findings to Reshape Clinical Practice?
“The study’s large, multicenter cohort and its focus on the prognostic value of baseline MRI in active surveillance make it a crucial contribution to the field, providing evidence that can potentially refine patient management strategies in clinical practice,” Ismail Baris Turkbey, MD, FSAR, head of MRI Section, Molecular Imaging Branch, National Cancer Institute, Rockville, Maryland, said in a written comment.
“The findings from this study are likely to have a significant impact on clinical practice and potentially influence future guidelines in the management of localized prostate cancer, particularly in the context of active surveillance,” Dr. Turkbey said. “MRI, already a commonly used imaging modality in prostate cancer management, may become an even more integral part of the initial assessment and ongoing monitoring of patients with low or favorable-intermediate risk prostate cancer.”
Dr. Turkbey noted several strengths of the study.
First, the size and the diversity of the cohort, along with the variety of treatment centers, support generalizability of findings. Second, the study pinpoints a “critical aspect” of active surveillance by uncovering the link between baseline MRI findings and later risk reclassification. Finally, the study also showed that increasing age was associated with higher likelihood of risk reclassification, “further emphasizing the need for personalized risk assessment” among these patients.
What Were Some Limitations of This Study?
“One important limitation is the lack of inter-reader agreement for PI-RADS evaluations for baseline MRIs,” Dr. Turkbey said. “Variation of PI-RADS is quite known, and centralized evaluations could have made this study stronger. Same applies for centralized quality evaluation of MRIs using The Prostate Imaging Quality (PI-QUAL) score. These items are difficult to do in a multicenter prospective data registry, and maybe authors will consider including these additional analyses in their future work.”
How Does This New Approach to Prostate Cancer Risk Assessment Compare With Recent Advances in AI-Based Risk Assessment?
Over the past few years, artificial intelligence (AI)–assisted risk assessment in prostate cancer has been gaining increasing attention. Recently, for example, Artera, a self-styled “precision medicine company,” released the first AI tool to help patients choose between active surveillance and active treatment on the basis of analysis of digital pathology images.
When asked to compare this approach with the methods used in the present study, Dr. Nandalur called the AI model “a step forward” but noted that it still relies on conventional risk criteria.
“Our data show imaging with MRI has independent prognostic information for prostate cancer patients considering active surveillance, over and above these traditional factors,” he said. “Moreover, this predictive ability of MRI was seen in low and favorable intermediate risk groups, so the additive value is broad.”
Still, he predicted that the future will not involve a binary choice, but a combination approach.
“The exciting aspect is that MRI results can eventually be added to this novel AI model and further improve prediction models for patients,” Dr. Nandalur said. “The combination of recent AI models and MRI will likely represent the future paradigm for prostate cancer patients considering active surveillance versus immediate treatment.”
The study was supported by Blue Cross and Blue Shield of Michigan. The investigators and Dr. Turkbey reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
The multicenter, real-world trial showed that men with low-risk or favorable intermediate-risk disease who had higher Prostate Imaging Reporting and Data System (PI-RADS) scores at baseline were more likely to be reclassified with more aggressive disease on a future biopsy, wrote lead author Kiran R. Nandalur, MD and colleagues. The study was published in The Journal of Urology.
This means that without MRI, some cases of prostate cancer are being labeled as lower-risk than they actually are.
The investigators noted that MRI is increasingly being used to choose patients who are appropriate for active surveillance instead of treatment, but related clinical data are scarce.
Although PI-RADS is the preferred metric for characterizing prostate tumors via MRI, “most previous studies on the prognostic implications of baseline PI-RADS score included smaller populations from academic centers, limited inclusion of clinical and pathologic data into models, and/or [are] ambiguous on the implications of PI-RADS score,” they wrote.
These knowledge gaps prompted the present study.
How Were Baseline MRI Findings Related to Prostate Cancer Disease Risk?
The dataset included 1491 men with prostate cancer that was diagnosed at 46 hospital-based, academic, or private practice urology groups. All had low-risk or favorable intermediate-risk disease and had undergone MRI within 6 months before or after initial biopsy, along with enrollment in active surveillance.
“A novel aspect of this study was that the MRIs were not read by dedicated prostate MRI experts at academic institutions, but rather a mix of community and academic radiologists,” Dr. Nandalur, medical director of Corewell Health East Radiology, Royal Oak, Michigan, said in an interview.
After traditional risk factors were accounted for, baseline PI-RADS (four or more lesions) was significantly associated with increased likelihood of biopsy reclassification to high-grade prostate cancer on surveillance biopsy (hazard ratio, 2.3; 95% CI 1.6-3.2; P < .001).
“These patients with suspicious lesions on their initial MRI were more than twice as likely to have higher-grade disease within 5 years,” Nandalur noted. “This result was not only seen in the low-risk group but also in the favorable intermediate-risk group, which hasn’t been shown before.”
Grade group 2 vs 1 and increasing age were also associated with significantly increased risk for reclassification to a more aggressive cancer type.
How Might These Findings Improve Outcomes in Patients With Prostate Cancer?
Currently, 60%-70% of patients with low-risk disease choose active surveillance over immediate treatment, whereas 20% with favorable intermediate-risk disease choose active surveillance, according to Dr. Nandalur.
For low-risk patients, PI-RADS score is unlikely to change this decision, although surveillance intervals could be adjusted in accordance with risk. More notably, those with favorable intermediate-risk disease may benefit from considering PI-RADS score when choosing between active surveillance and immediate treatment.
“Most of the management strategies for prostate cancer are based on just your lab values and your pathology,” Dr. Nandalur said, “but this study shows that maybe we should start taking MRI into account — into the general paradigm of management of prostate cancer.”
Ideally, he added, prospective studies will confirm these findings, although such studies can be challenging to perform and similar data have historically been sufficient to reshape clinical practice.
“We are hoping that [baseline PI-RADS score] will be adopted into the NCCN [National Comprehensive Cancer Network] guidelines,” Dr. Nandalur said.
How Likely Are These Findings to Reshape Clinical Practice?
“The study’s large, multicenter cohort and its focus on the prognostic value of baseline MRI in active surveillance make it a crucial contribution to the field, providing evidence that can potentially refine patient management strategies in clinical practice,” Ismail Baris Turkbey, MD, FSAR, head of MRI Section, Molecular Imaging Branch, National Cancer Institute, Rockville, Maryland, said in a written comment.
“The findings from this study are likely to have a significant impact on clinical practice and potentially influence future guidelines in the management of localized prostate cancer, particularly in the context of active surveillance,” Dr. Turkbey said. “MRI, already a commonly used imaging modality in prostate cancer management, may become an even more integral part of the initial assessment and ongoing monitoring of patients with low or favorable-intermediate risk prostate cancer.”
Dr. Turkbey noted several strengths of the study.
First, the size and the diversity of the cohort, along with the variety of treatment centers, support generalizability of findings. Second, the study pinpoints a “critical aspect” of active surveillance by uncovering the link between baseline MRI findings and later risk reclassification. Finally, the study also showed that increasing age was associated with higher likelihood of risk reclassification, “further emphasizing the need for personalized risk assessment” among these patients.
What Were Some Limitations of This Study?
“One important limitation is the lack of inter-reader agreement for PI-RADS evaluations for baseline MRIs,” Dr. Turkbey said. “Variation of PI-RADS is quite known, and centralized evaluations could have made this study stronger. Same applies for centralized quality evaluation of MRIs using The Prostate Imaging Quality (PI-QUAL) score. These items are difficult to do in a multicenter prospective data registry, and maybe authors will consider including these additional analyses in their future work.”
How Does This New Approach to Prostate Cancer Risk Assessment Compare With Recent Advances in AI-Based Risk Assessment?
Over the past few years, artificial intelligence (AI)–assisted risk assessment in prostate cancer has been gaining increasing attention. Recently, for example, Artera, a self-styled “precision medicine company,” released the first AI tool to help patients choose between active surveillance and active treatment on the basis of analysis of digital pathology images.
When asked to compare this approach with the methods used in the present study, Dr. Nandalur called the AI model “a step forward” but noted that it still relies on conventional risk criteria.
“Our data show imaging with MRI has independent prognostic information for prostate cancer patients considering active surveillance, over and above these traditional factors,” he said. “Moreover, this predictive ability of MRI was seen in low and favorable intermediate risk groups, so the additive value is broad.”
Still, he predicted that the future will not involve a binary choice, but a combination approach.
“The exciting aspect is that MRI results can eventually be added to this novel AI model and further improve prediction models for patients,” Dr. Nandalur said. “The combination of recent AI models and MRI will likely represent the future paradigm for prostate cancer patients considering active surveillance versus immediate treatment.”
The study was supported by Blue Cross and Blue Shield of Michigan. The investigators and Dr. Turkbey reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF UROLOGY