User login
ASCO clinical practice guideline update incorporates Oncotype DX
In women with hormone receptor–positive, axillary node–negative breast cancer with Oncotype DX recurrence scores of less than 26, there is minimal to no benefit from chemotherapy, particularly for those greater than age 50 years, according to a clinical practice guideline update by the American Society of Clinical Oncology.
Furthermore, endocrine therapy alone may be offered for patients greater than age 50 years whose tumors have recurrence scores of less than 26, wrote Fabrice Andre, MD, PhD, of Paris Sud University and associates on the expert panel in the Journal of Clinical Oncology.
The panel members reviewed recently published findings from the Trial Assigning Individualized Options for Treatment (TAILORx), which evaluated the clinical utility of the Oncotype DX assay in women with early-stage invasive breast cancer.
“This focused update reviews and analyzes new data regarding these recommendations while applying the same criteria of clinical utility as described in the 2016 guideline,” they wrote.
The expert panel provided recommendations on how to integrate the results of the TAILORx study into clinical practice.
“For patients age 50 years or younger with Oncotype DX recurrence scores of 16-25, clinicians may offer chemoendocrine therapy” the panel wrote. “Patients with Oncotype DX recurrence scores of greater than 30 should be considered candidates for chemoendocrine therapy.”
In addition, on the basis of consensus they recommended that chemoendocrine therapy could be offered to patients with recurrence scores of 26-30.
The panel acknowledged that relevant literature on the use of Oncotype DX in this population will be reviewed over the upcoming months to address anticipated practice deviation related to biomarker testing.
More information on the guidelines is available on the ASCO website.
The study was funded by ASCO. The authors reported financial affiliations with AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Roche, and several others.
SOURCE: Andre F et al. J Clin Oncol. 2019 May 31. doi: 10.1200/JCO.19.00945.
In women with hormone receptor–positive, axillary node–negative breast cancer with Oncotype DX recurrence scores of less than 26, there is minimal to no benefit from chemotherapy, particularly for those greater than age 50 years, according to a clinical practice guideline update by the American Society of Clinical Oncology.
Furthermore, endocrine therapy alone may be offered for patients greater than age 50 years whose tumors have recurrence scores of less than 26, wrote Fabrice Andre, MD, PhD, of Paris Sud University and associates on the expert panel in the Journal of Clinical Oncology.
The panel members reviewed recently published findings from the Trial Assigning Individualized Options for Treatment (TAILORx), which evaluated the clinical utility of the Oncotype DX assay in women with early-stage invasive breast cancer.
“This focused update reviews and analyzes new data regarding these recommendations while applying the same criteria of clinical utility as described in the 2016 guideline,” they wrote.
The expert panel provided recommendations on how to integrate the results of the TAILORx study into clinical practice.
“For patients age 50 years or younger with Oncotype DX recurrence scores of 16-25, clinicians may offer chemoendocrine therapy” the panel wrote. “Patients with Oncotype DX recurrence scores of greater than 30 should be considered candidates for chemoendocrine therapy.”
In addition, on the basis of consensus they recommended that chemoendocrine therapy could be offered to patients with recurrence scores of 26-30.
The panel acknowledged that relevant literature on the use of Oncotype DX in this population will be reviewed over the upcoming months to address anticipated practice deviation related to biomarker testing.
More information on the guidelines is available on the ASCO website.
The study was funded by ASCO. The authors reported financial affiliations with AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Roche, and several others.
SOURCE: Andre F et al. J Clin Oncol. 2019 May 31. doi: 10.1200/JCO.19.00945.
In women with hormone receptor–positive, axillary node–negative breast cancer with Oncotype DX recurrence scores of less than 26, there is minimal to no benefit from chemotherapy, particularly for those greater than age 50 years, according to a clinical practice guideline update by the American Society of Clinical Oncology.
Furthermore, endocrine therapy alone may be offered for patients greater than age 50 years whose tumors have recurrence scores of less than 26, wrote Fabrice Andre, MD, PhD, of Paris Sud University and associates on the expert panel in the Journal of Clinical Oncology.
The panel members reviewed recently published findings from the Trial Assigning Individualized Options for Treatment (TAILORx), which evaluated the clinical utility of the Oncotype DX assay in women with early-stage invasive breast cancer.
“This focused update reviews and analyzes new data regarding these recommendations while applying the same criteria of clinical utility as described in the 2016 guideline,” they wrote.
The expert panel provided recommendations on how to integrate the results of the TAILORx study into clinical practice.
“For patients age 50 years or younger with Oncotype DX recurrence scores of 16-25, clinicians may offer chemoendocrine therapy” the panel wrote. “Patients with Oncotype DX recurrence scores of greater than 30 should be considered candidates for chemoendocrine therapy.”
In addition, on the basis of consensus they recommended that chemoendocrine therapy could be offered to patients with recurrence scores of 26-30.
The panel acknowledged that relevant literature on the use of Oncotype DX in this population will be reviewed over the upcoming months to address anticipated practice deviation related to biomarker testing.
More information on the guidelines is available on the ASCO website.
The study was funded by ASCO. The authors reported financial affiliations with AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Roche, and several others.
SOURCE: Andre F et al. J Clin Oncol. 2019 May 31. doi: 10.1200/JCO.19.00945.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Pediatric gastroesophageal reflux
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
NCCN publishes pediatric ALL guidelines
“The cure rate for pediatric ALL in the U.S. has risen from 0% in the 1960s to nearly 90% today. This is among the most profound medical success stories in history,” Patrick Brown, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, said in a statement announcing the guidelines. Dr. Brown chairs the NCCN Clinical Practice Guidelines for adult and pediatric ALL.
“Pediatric ALL survivors live a long time; we have to consider long-term effects as well,” Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital, Memphis, and vice chair of the guidelines committee, said in the statement.
The new recommendations highlight the importance of supportive care interventions in an effort to reduce the chances of patients experiencing severe adverse effects.
The pediatric ALL guidelines provide evidence-based recommendations about optimal treatment strategies for ALL to prolong survival in children affected, with a focus on treatment outside of clinical trials (Pediatric Acute Lymphoblastic Leukemia. NCCN.org, Version 1.2019, published May 30, 2019).
While treatment for ALL often includes long-term chemotherapy regimens that involve multiple stages, several novel treatment strategies are summarized in the guidelines, including various types of immunotherapy and targeted therapy.
The guidelines are intended to accompany the NCCN Guidelines for Adult ALL and integrate treatment recommendations for patients in overlapping age categories. The recommendations are organized based on risk level, which may also be associated with age.
“The highest risk [is] associated with those diagnosed within the first 12 months of life or between the ages 10 and 21 years old,” the guideline authors wrote.
Another unique aspect of the guidelines is the recognition of vulnerable populations, such as young infants or children with Down syndrome, who face distinct treatment challenges. The authors provide guidance on the best supportive care measures for these patients.
The NCCN is currently expanding the collection of clinical practice guidelines for additional pediatric malignancies. At present, they are planning to undertake a minimum of 90% of all incident pediatric cancers.
Upcoming guidelines include treatment recommendations for pediatric Burkitt lymphoma, and are scheduled for release later in 2019.
Future efforts include modifying the guidelines for use in low- and middle-income countries, with the goal of providing direction in resource-limited environments.
“We know that many, many children can be cured with inexpensive and widely-available therapies,” Dr. Brown said. “With the increasing global reach of the NCCN Guidelines, we can really pave the way for increasing the cure rates throughout the world.”
“The cure rate for pediatric ALL in the U.S. has risen from 0% in the 1960s to nearly 90% today. This is among the most profound medical success stories in history,” Patrick Brown, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, said in a statement announcing the guidelines. Dr. Brown chairs the NCCN Clinical Practice Guidelines for adult and pediatric ALL.
“Pediatric ALL survivors live a long time; we have to consider long-term effects as well,” Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital, Memphis, and vice chair of the guidelines committee, said in the statement.
The new recommendations highlight the importance of supportive care interventions in an effort to reduce the chances of patients experiencing severe adverse effects.
The pediatric ALL guidelines provide evidence-based recommendations about optimal treatment strategies for ALL to prolong survival in children affected, with a focus on treatment outside of clinical trials (Pediatric Acute Lymphoblastic Leukemia. NCCN.org, Version 1.2019, published May 30, 2019).
While treatment for ALL often includes long-term chemotherapy regimens that involve multiple stages, several novel treatment strategies are summarized in the guidelines, including various types of immunotherapy and targeted therapy.
The guidelines are intended to accompany the NCCN Guidelines for Adult ALL and integrate treatment recommendations for patients in overlapping age categories. The recommendations are organized based on risk level, which may also be associated with age.
“The highest risk [is] associated with those diagnosed within the first 12 months of life or between the ages 10 and 21 years old,” the guideline authors wrote.
Another unique aspect of the guidelines is the recognition of vulnerable populations, such as young infants or children with Down syndrome, who face distinct treatment challenges. The authors provide guidance on the best supportive care measures for these patients.
The NCCN is currently expanding the collection of clinical practice guidelines for additional pediatric malignancies. At present, they are planning to undertake a minimum of 90% of all incident pediatric cancers.
Upcoming guidelines include treatment recommendations for pediatric Burkitt lymphoma, and are scheduled for release later in 2019.
Future efforts include modifying the guidelines for use in low- and middle-income countries, with the goal of providing direction in resource-limited environments.
“We know that many, many children can be cured with inexpensive and widely-available therapies,” Dr. Brown said. “With the increasing global reach of the NCCN Guidelines, we can really pave the way for increasing the cure rates throughout the world.”
“The cure rate for pediatric ALL in the U.S. has risen from 0% in the 1960s to nearly 90% today. This is among the most profound medical success stories in history,” Patrick Brown, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, said in a statement announcing the guidelines. Dr. Brown chairs the NCCN Clinical Practice Guidelines for adult and pediatric ALL.
“Pediatric ALL survivors live a long time; we have to consider long-term effects as well,” Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital, Memphis, and vice chair of the guidelines committee, said in the statement.
The new recommendations highlight the importance of supportive care interventions in an effort to reduce the chances of patients experiencing severe adverse effects.
The pediatric ALL guidelines provide evidence-based recommendations about optimal treatment strategies for ALL to prolong survival in children affected, with a focus on treatment outside of clinical trials (Pediatric Acute Lymphoblastic Leukemia. NCCN.org, Version 1.2019, published May 30, 2019).
While treatment for ALL often includes long-term chemotherapy regimens that involve multiple stages, several novel treatment strategies are summarized in the guidelines, including various types of immunotherapy and targeted therapy.
The guidelines are intended to accompany the NCCN Guidelines for Adult ALL and integrate treatment recommendations for patients in overlapping age categories. The recommendations are organized based on risk level, which may also be associated with age.
“The highest risk [is] associated with those diagnosed within the first 12 months of life or between the ages 10 and 21 years old,” the guideline authors wrote.
Another unique aspect of the guidelines is the recognition of vulnerable populations, such as young infants or children with Down syndrome, who face distinct treatment challenges. The authors provide guidance on the best supportive care measures for these patients.
The NCCN is currently expanding the collection of clinical practice guidelines for additional pediatric malignancies. At present, they are planning to undertake a minimum of 90% of all incident pediatric cancers.
Upcoming guidelines include treatment recommendations for pediatric Burkitt lymphoma, and are scheduled for release later in 2019.
Future efforts include modifying the guidelines for use in low- and middle-income countries, with the goal of providing direction in resource-limited environments.
“We know that many, many children can be cured with inexpensive and widely-available therapies,” Dr. Brown said. “With the increasing global reach of the NCCN Guidelines, we can really pave the way for increasing the cure rates throughout the world.”
FROM THE NATIONAL COMPREHENSIVE CANCER NETWORK
Cholesterol guideline: Risk assessment gets personal
according to the 2018 update to U.S. guidelines for cholesterol management from the American Heart Association and the American College of Cardiology.
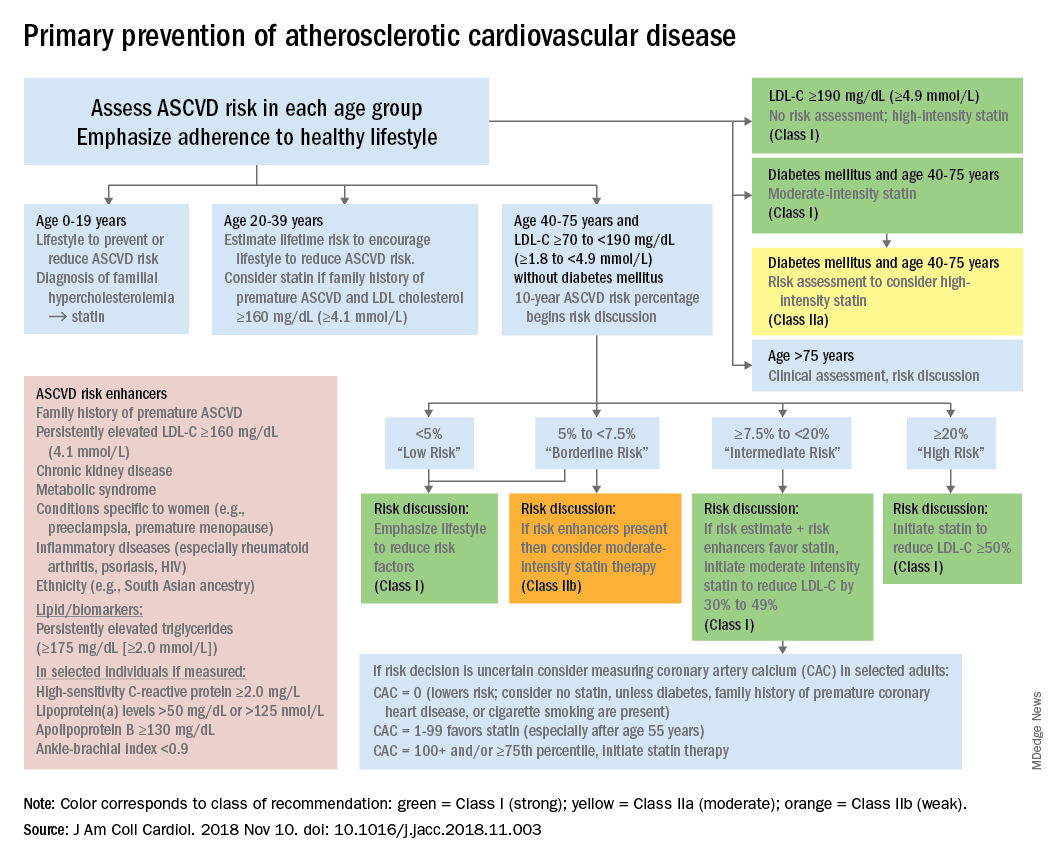
The guideline also emphasizes “careful adherence to lifestyle recommendations at an early age [to] reduce risk factor burden over the lifespan and decrease the need for preventive drug therapies later in life,” Scott M. Grundy, MD, PhD, and Neil J. Stone, MD, said in a synopsis of the document in the Annals of Internal Medicine.
For primary prevention in patients aged 40-75 years, estimation of 10-year ASCVD risk – introduced in the 2013 guidelines – and stratification into one of four categories should set the stage for clinician-patient discussion. A score of less than 5% indicates low risk and should prompt a risk discussion that emphasizes lifestyle recommendations. “Statins are clinically efficacious in the latter three categories, but the higher the risk, the stronger the statin indication,” said Dr. Grundy of the University of Texas, Dallas, and Dr. Stone of Northwestern University, Chicago.
When risk status is uncertain, measurement of coronary artery calcium should be considered in patients aged 40-75 years with LDL cholesterol levels of 70-189 mg/dL who do no not have diabetes, they noted.
The guideline emphasizes secondary prevention with “maximally tolerated doses of statins” and the use of nonstatin drugs such as ezetimibe and PCSK9 inhibitors for patients with very high ASCVD risk – defined as a history of multiple major events or one event and other high-risk conditions, Dr. Grundy and Dr. Stone wrote.
Financial support for the Guideline Writing Committee for the 2018 Cholesterol Guidelines came from the AHA and the ACC. Dr. Grundy and Dr. Stone said that they had no relevant conflicts of interest.
SOURCE: Grundy SM and Stone NJ. Ann Intern Med. 2019 May 28. doi: 10.7326/M19-0365.
according to the 2018 update to U.S. guidelines for cholesterol management from the American Heart Association and the American College of Cardiology.

The guideline also emphasizes “careful adherence to lifestyle recommendations at an early age [to] reduce risk factor burden over the lifespan and decrease the need for preventive drug therapies later in life,” Scott M. Grundy, MD, PhD, and Neil J. Stone, MD, said in a synopsis of the document in the Annals of Internal Medicine.
For primary prevention in patients aged 40-75 years, estimation of 10-year ASCVD risk – introduced in the 2013 guidelines – and stratification into one of four categories should set the stage for clinician-patient discussion. A score of less than 5% indicates low risk and should prompt a risk discussion that emphasizes lifestyle recommendations. “Statins are clinically efficacious in the latter three categories, but the higher the risk, the stronger the statin indication,” said Dr. Grundy of the University of Texas, Dallas, and Dr. Stone of Northwestern University, Chicago.
When risk status is uncertain, measurement of coronary artery calcium should be considered in patients aged 40-75 years with LDL cholesterol levels of 70-189 mg/dL who do no not have diabetes, they noted.
The guideline emphasizes secondary prevention with “maximally tolerated doses of statins” and the use of nonstatin drugs such as ezetimibe and PCSK9 inhibitors for patients with very high ASCVD risk – defined as a history of multiple major events or one event and other high-risk conditions, Dr. Grundy and Dr. Stone wrote.
Financial support for the Guideline Writing Committee for the 2018 Cholesterol Guidelines came from the AHA and the ACC. Dr. Grundy and Dr. Stone said that they had no relevant conflicts of interest.
SOURCE: Grundy SM and Stone NJ. Ann Intern Med. 2019 May 28. doi: 10.7326/M19-0365.
according to the 2018 update to U.S. guidelines for cholesterol management from the American Heart Association and the American College of Cardiology.

The guideline also emphasizes “careful adherence to lifestyle recommendations at an early age [to] reduce risk factor burden over the lifespan and decrease the need for preventive drug therapies later in life,” Scott M. Grundy, MD, PhD, and Neil J. Stone, MD, said in a synopsis of the document in the Annals of Internal Medicine.
For primary prevention in patients aged 40-75 years, estimation of 10-year ASCVD risk – introduced in the 2013 guidelines – and stratification into one of four categories should set the stage for clinician-patient discussion. A score of less than 5% indicates low risk and should prompt a risk discussion that emphasizes lifestyle recommendations. “Statins are clinically efficacious in the latter three categories, but the higher the risk, the stronger the statin indication,” said Dr. Grundy of the University of Texas, Dallas, and Dr. Stone of Northwestern University, Chicago.
When risk status is uncertain, measurement of coronary artery calcium should be considered in patients aged 40-75 years with LDL cholesterol levels of 70-189 mg/dL who do no not have diabetes, they noted.
The guideline emphasizes secondary prevention with “maximally tolerated doses of statins” and the use of nonstatin drugs such as ezetimibe and PCSK9 inhibitors for patients with very high ASCVD risk – defined as a history of multiple major events or one event and other high-risk conditions, Dr. Grundy and Dr. Stone wrote.
Financial support for the Guideline Writing Committee for the 2018 Cholesterol Guidelines came from the AHA and the ACC. Dr. Grundy and Dr. Stone said that they had no relevant conflicts of interest.
SOURCE: Grundy SM and Stone NJ. Ann Intern Med. 2019 May 28. doi: 10.7326/M19-0365.
FROM THE ANNALS OF INTERNAL MEDICINE
Upcoming OA management guidelines reveal dearth of effective therapies
TORONTO – Draft guidelines for the management of OA developed by the Osteoarthritis Research Society International expose an inconvenient truth: Although a tremendous number of interventions are available for the treatment of OA, the cupboard is nearly bare when it comes to strongly recommended, evidence-based therapies.
Indeed, the sole strong, level Ia recommendation for pharmacotherapy of knee OA contained in the 2019 guidelines is for topical NSAIDs. The proposed guidelines contain no level Ia recommendations at all for nonpharmacologic treatment of knee OA. And for hip OA and polyarticular OA – the other two expressions of the disease addressed in the guidelines – there are no level Ia recommendations, pharmacologic or nonpharmacologic. The treatment recommendations for hip OA and polyarticular OA start at level Ib and drop-off in strength from there, Raveendhara R. Bannuru, MD, said at the OARSI 2019 World Congress. He and his fellow OARSI guideline panelists reviewed roughly 12,500 published abstracts before winnowing down the literature to 407 articles for data extraction. The voting panel was comprised of orthopedic surgeons, physical therapists, rheumatologists, primary care physicians, and sports medicine specialists from 10 countries. Panelists employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, resulting in guidelines which were categorized as either “strong,” that is, first-line, level Ia, a designation that required endorsement by at least 75% of panelists, or weaker “conditional” recommendations. Voting was conducted anonymously, Dr. Bannuru said in presenting highlights of the draft guidelines at the meeting sponsored by the Osteoarthritis Research Society International.
A challenge in coming up with evidence-based guidelines for OA management is that most of the existing research is focused on patients with knee OA and no comorbid conditions, he explained. The panelists wanted to create patient-centric guidelines, so they tackled hip OA and polyarticular OA as well, despite the paucity of good-quality data. And the guidelines separately address five common comorbidity scenarios for each of the three forms of OA: GI or cardiovascular comorbidity, frailty, comorbid widespread pain disorder/depression, and OA with no major comorbid conditions.
The draft guidelines feature one category of recommendations, known as the core recommendations, which are even stronger than the level Ia recommendations. The core recommendations are defined as key treatments deemed appropriate for nearly any OA patient at all points in treatment. The core recommendations are considered standard of care – the first interventions to utilize – to be supplemented by level Ia and Ib interventions added on as needed, with lower-level recommendations available when core plus levels Ia and Ib interventions don’t achieve the desired results.
The core recommendations include arthritis education, dietary weight management, and a structured, land-based exercise program involving strengthening and/or cardiovascular exercise and/or balance training. In a major departure from previous guidelines, mind-body exercise is categorized as a core recommendation, although just for patients with knee OA.
“Mind-body exercise is comprised of tai chi and yoga only. We do not recommend other things,” according to Dr. Bannuru, director of the Center for Treatment Comparison and Integrative Analysis at Tufts Medical Center, Boston.
For hip OA, mind-body exercise gets demoted to a level Ib nonpharmacologic recommendation across all five comorbidity categories, along with aquatic exercise, gait aids, and self-management programs.
Dr. Bannuru pointed out other highlights of the proposed guidelines: Opioids and acetaminophen are not recommended, duloxetine (Cymbalta) gets a conditional recommendation in OA patients with comorbid depression, and nonspecific NSAIDs are not recommended in OA patients with comorbid cardiovascular disease or frailty. When nonspecific NSAIDs are used, it should be at the lowest possible dose, for only 1-4 weeks, and in conjunction with a proton pump inhibitor, according to the draft guidelines.
Patient representatives asked the guideline panelists specifically about a number of interventions for OA popular in some circles but which the panel members are strongly opposed to because of unfavorable efficacy and safety profiles. The resulting “forget-about-it” list included colchicine, collagen, diacerein, doxycycline, dextrose prolotherapy, electrical stimulation, and electroacupuncture, with explanations provided as to why they deserve to be rejected.
The OA guidelines project was funded by the Arthritis Foundation, Versus Arthritis, and ReumaNederlands, with no industry funding.
TORONTO – Draft guidelines for the management of OA developed by the Osteoarthritis Research Society International expose an inconvenient truth: Although a tremendous number of interventions are available for the treatment of OA, the cupboard is nearly bare when it comes to strongly recommended, evidence-based therapies.
Indeed, the sole strong, level Ia recommendation for pharmacotherapy of knee OA contained in the 2019 guidelines is for topical NSAIDs. The proposed guidelines contain no level Ia recommendations at all for nonpharmacologic treatment of knee OA. And for hip OA and polyarticular OA – the other two expressions of the disease addressed in the guidelines – there are no level Ia recommendations, pharmacologic or nonpharmacologic. The treatment recommendations for hip OA and polyarticular OA start at level Ib and drop-off in strength from there, Raveendhara R. Bannuru, MD, said at the OARSI 2019 World Congress. He and his fellow OARSI guideline panelists reviewed roughly 12,500 published abstracts before winnowing down the literature to 407 articles for data extraction. The voting panel was comprised of orthopedic surgeons, physical therapists, rheumatologists, primary care physicians, and sports medicine specialists from 10 countries. Panelists employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, resulting in guidelines which were categorized as either “strong,” that is, first-line, level Ia, a designation that required endorsement by at least 75% of panelists, or weaker “conditional” recommendations. Voting was conducted anonymously, Dr. Bannuru said in presenting highlights of the draft guidelines at the meeting sponsored by the Osteoarthritis Research Society International.
A challenge in coming up with evidence-based guidelines for OA management is that most of the existing research is focused on patients with knee OA and no comorbid conditions, he explained. The panelists wanted to create patient-centric guidelines, so they tackled hip OA and polyarticular OA as well, despite the paucity of good-quality data. And the guidelines separately address five common comorbidity scenarios for each of the three forms of OA: GI or cardiovascular comorbidity, frailty, comorbid widespread pain disorder/depression, and OA with no major comorbid conditions.
The draft guidelines feature one category of recommendations, known as the core recommendations, which are even stronger than the level Ia recommendations. The core recommendations are defined as key treatments deemed appropriate for nearly any OA patient at all points in treatment. The core recommendations are considered standard of care – the first interventions to utilize – to be supplemented by level Ia and Ib interventions added on as needed, with lower-level recommendations available when core plus levels Ia and Ib interventions don’t achieve the desired results.
The core recommendations include arthritis education, dietary weight management, and a structured, land-based exercise program involving strengthening and/or cardiovascular exercise and/or balance training. In a major departure from previous guidelines, mind-body exercise is categorized as a core recommendation, although just for patients with knee OA.
“Mind-body exercise is comprised of tai chi and yoga only. We do not recommend other things,” according to Dr. Bannuru, director of the Center for Treatment Comparison and Integrative Analysis at Tufts Medical Center, Boston.
For hip OA, mind-body exercise gets demoted to a level Ib nonpharmacologic recommendation across all five comorbidity categories, along with aquatic exercise, gait aids, and self-management programs.
Dr. Bannuru pointed out other highlights of the proposed guidelines: Opioids and acetaminophen are not recommended, duloxetine (Cymbalta) gets a conditional recommendation in OA patients with comorbid depression, and nonspecific NSAIDs are not recommended in OA patients with comorbid cardiovascular disease or frailty. When nonspecific NSAIDs are used, it should be at the lowest possible dose, for only 1-4 weeks, and in conjunction with a proton pump inhibitor, according to the draft guidelines.
Patient representatives asked the guideline panelists specifically about a number of interventions for OA popular in some circles but which the panel members are strongly opposed to because of unfavorable efficacy and safety profiles. The resulting “forget-about-it” list included colchicine, collagen, diacerein, doxycycline, dextrose prolotherapy, electrical stimulation, and electroacupuncture, with explanations provided as to why they deserve to be rejected.
The OA guidelines project was funded by the Arthritis Foundation, Versus Arthritis, and ReumaNederlands, with no industry funding.
TORONTO – Draft guidelines for the management of OA developed by the Osteoarthritis Research Society International expose an inconvenient truth: Although a tremendous number of interventions are available for the treatment of OA, the cupboard is nearly bare when it comes to strongly recommended, evidence-based therapies.
Indeed, the sole strong, level Ia recommendation for pharmacotherapy of knee OA contained in the 2019 guidelines is for topical NSAIDs. The proposed guidelines contain no level Ia recommendations at all for nonpharmacologic treatment of knee OA. And for hip OA and polyarticular OA – the other two expressions of the disease addressed in the guidelines – there are no level Ia recommendations, pharmacologic or nonpharmacologic. The treatment recommendations for hip OA and polyarticular OA start at level Ib and drop-off in strength from there, Raveendhara R. Bannuru, MD, said at the OARSI 2019 World Congress. He and his fellow OARSI guideline panelists reviewed roughly 12,500 published abstracts before winnowing down the literature to 407 articles for data extraction. The voting panel was comprised of orthopedic surgeons, physical therapists, rheumatologists, primary care physicians, and sports medicine specialists from 10 countries. Panelists employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, resulting in guidelines which were categorized as either “strong,” that is, first-line, level Ia, a designation that required endorsement by at least 75% of panelists, or weaker “conditional” recommendations. Voting was conducted anonymously, Dr. Bannuru said in presenting highlights of the draft guidelines at the meeting sponsored by the Osteoarthritis Research Society International.
A challenge in coming up with evidence-based guidelines for OA management is that most of the existing research is focused on patients with knee OA and no comorbid conditions, he explained. The panelists wanted to create patient-centric guidelines, so they tackled hip OA and polyarticular OA as well, despite the paucity of good-quality data. And the guidelines separately address five common comorbidity scenarios for each of the three forms of OA: GI or cardiovascular comorbidity, frailty, comorbid widespread pain disorder/depression, and OA with no major comorbid conditions.
The draft guidelines feature one category of recommendations, known as the core recommendations, which are even stronger than the level Ia recommendations. The core recommendations are defined as key treatments deemed appropriate for nearly any OA patient at all points in treatment. The core recommendations are considered standard of care – the first interventions to utilize – to be supplemented by level Ia and Ib interventions added on as needed, with lower-level recommendations available when core plus levels Ia and Ib interventions don’t achieve the desired results.
The core recommendations include arthritis education, dietary weight management, and a structured, land-based exercise program involving strengthening and/or cardiovascular exercise and/or balance training. In a major departure from previous guidelines, mind-body exercise is categorized as a core recommendation, although just for patients with knee OA.
“Mind-body exercise is comprised of tai chi and yoga only. We do not recommend other things,” according to Dr. Bannuru, director of the Center for Treatment Comparison and Integrative Analysis at Tufts Medical Center, Boston.
For hip OA, mind-body exercise gets demoted to a level Ib nonpharmacologic recommendation across all five comorbidity categories, along with aquatic exercise, gait aids, and self-management programs.
Dr. Bannuru pointed out other highlights of the proposed guidelines: Opioids and acetaminophen are not recommended, duloxetine (Cymbalta) gets a conditional recommendation in OA patients with comorbid depression, and nonspecific NSAIDs are not recommended in OA patients with comorbid cardiovascular disease or frailty. When nonspecific NSAIDs are used, it should be at the lowest possible dose, for only 1-4 weeks, and in conjunction with a proton pump inhibitor, according to the draft guidelines.
Patient representatives asked the guideline panelists specifically about a number of interventions for OA popular in some circles but which the panel members are strongly opposed to because of unfavorable efficacy and safety profiles. The resulting “forget-about-it” list included colchicine, collagen, diacerein, doxycycline, dextrose prolotherapy, electrical stimulation, and electroacupuncture, with explanations provided as to why they deserve to be rejected.
The OA guidelines project was funded by the Arthritis Foundation, Versus Arthritis, and ReumaNederlands, with no industry funding.
REPORTING FROM OARSI 2019
Stroke policy recommendations incorporate advances in endovascular therapy
Stroke centers need to collaborate within their regions to assure best practices and optimal access to comprehensive stroke centers as well as newly-designated thrombectomy-capable stroke centers, according to an updated policy statement from the American Stroke Association published in Stroke.
Opeolu Adeoye, MD, associate professor of emergency medicine and neurosurgery at the University of Cincinnati – and chair of the policy statement writing group – and coauthors updated the ASA’s 2005 recommendations for policy makers and public health care agencies to reflect current evidence, the increased availability of endovascular therapy, and new stroke center certifications.
“We have seen monumental advancements in acute stroke care over the past 14 years, and our concept of a comprehensive stroke system of care has evolved as a result,” Dr. Adeoye said in a news release.
While a recommendation to support the initiation of stroke prevention regimens remains unchanged from the 2005 recommendations, the 2019 update emphasizes a need to support long-term adherence to such regimens. To that end, researchers should examine the potential benefits of stroke prevention efforts that incorporate social media, gamification, and other technologies and principles to promote healthy behavior, the authors suggested. Furthermore, technology may allow for the passive surveillance of baseline behaviors and enable researchers to track changes in behavior over time.
Thrombectomy-capable centers
Thrombectomy-capable stroke centers, which have capabilities between those of primary stroke centers and comprehensive stroke centers, provide a relatively new level of acute stroke care. In communities that do not otherwise have access to thrombectomy, these centers play a clear role. In communities with comprehensive stroke centers, their role “is more controversial, and routing plans for patients with a suspected LVO [large vessel occlusion] should always seek the center of highest capability when travel time differences are short,” the statement says.
Timely parenchymal and arterial imaging via CT or MRI are needed to identify the subset of patients who may benefit from thrombectomy. All centers managing stroke patients should develop a plan for the definitive identification and treatment of these patients. Imaging techniques that assess penumbral patterns to identify candidates for endovascular therapy between 6 and 24 hours after patients were last known to be normal “merit broader adoption,” the statement says.
Hospitals without thrombectomy capability should have transfer protocols to allow the rapid treatment of these patients to hospitals with the appropriate level of care. In rural facilities that lack 24/7 imaging and radiology capabilities, this may mean rapid transfer of patients with clinically suspected LVO to hospitals where their work-up may be expedited.
To improve process, centers providing thrombectomy should rigorously track patient flow at all time points from presentation to imaging to intervention. Reperfusion rates, procedural complications, and patient clinical outcomes must be tracked and reported.
Travel times
Triage paradigms and protocols should be developed to ensure that emergency medical service (EMS) providers are able to rapidly identify all patients with a known or suspected stroke and to assess them with a validated and standardized instrument for stroke screening such as FAST (Face, Arm, Speech, Time), Los Angeles Prehospital Stroke Screen, or Cincinnati Prehospital Stroke Scale.
In prehospital patients who screen positive for suspected stroke, a standard prehospital stroke severity assessment tool such as the Cincinnati Stroke Triage Assessment Tool, Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, or Field Assessment Stroke Triage for Emergency Destination should be used. “Further research is needed to establish the most effective prehospital stroke severity triage scale,” the authors noted. In all cases, EMS should notify hospitals that a stroke patient is en route.
“When there are several intravenous alteplase–capable hospitals in a well-defined geographic region, extra transportation times to reach a facility capable of endovascular thrombectomy should be limited to no more than 15 minutes in patients with a prehospital stroke severity score suggestive of LVO,” according to the recommendations. “When several hospital options exist within similar travel times, EMS should seek care at the facility capable of offering the highest level of stroke care. Further research is needed to establish travel time parameters for hospital bypass in cases of prehospital suspicion of LVO.”
Outcomes and discharge
Centers should track various treatment and patient outcomes, and all patients discharged to their homes should have appropriate follow-up with specialized stroke services and primary care and be screened for postacute complications.
Government institutions should standardize the organization of stroke care, ensure that stroke patients receive timely care at appropriate hospitals, and facilitate access to secondary prevention and rehabilitation resources after stroke, the authors wrote.
“Programs geared at further improving the knowledge of the public, encouraging primordial and primary prevention, advancing and facilitating acute therapy, improving secondary prevention and recovery from stroke, and reducing disparities in stroke care should be actively developed in a coordinated and collaborative fashion by providers and policymakers at the local, state, and national levels,” the authors concluded. “Such efforts will continue to mitigate the effects of stroke on society.”
Dr. Adeoye had no disclosures. Some coauthors reported research grants and consultant or advisory board positions.
SOURCE: Adeoye O et al. Stroke. 2019 May 20. doi: 10.1161/STR.0000000000000173.
When determining where to transport a patient with stroke, uncertainty about the patient’s diagnosis and eligibility for thrombectomy is a necessary consideration, said Robert A. Harrington, MD, of Stanford University (Calif.), in an accompanying editorial.
In lieu of better data, stroke systems should follow the recommendation of the Mission: Lifeline Severity-based Stroke Triage Algorithm for emergency medical services to avoid more than 15 minutes of additional travel time to transport a patient to a center that can perform endovascular therapy when the patient may be eligible for intravenous tissue plasminogen activator (tPA), said Dr. Harrington.
Delays in initiating tPA could lead to some patients not receiving treatment. “Some patients with suspected LVO [large vessel occlusion] either will not have thrombectomy or will not be eligible for it, and they also run the risk of not receiving any acute reperfusion therapy. Consequently, transport algorithms and models must take into account the uncertainty in prehospital diagnosis when considering the most appropriate facility,” he said.
Forthcoming acute stroke guidelines “will recommend intravenous tPA for all eligible subjects” because administration of tPA before endovascular thrombectomy does not appear to be harmful, Dr. Harrington noted.
Ultimately, approaches to routing patients may vary by region. “It is up to local and regional communities ... to define how best to implement these elements into a stroke system of care that meets their needs and resources and to define the types of hospitals that should qualify as points of entry for patients with suspected LVO strokes,” Dr. Harrington said.
A group convened by the American Heart Association and American Stroke Association is drafting further guiding principles for stroke systems of care in various regional settings.
Dr. Harrington is president-elect of the American Heart Association. He reported receiving research grants from AstraZeneca and Bristol-Myers Squibb.
When determining where to transport a patient with stroke, uncertainty about the patient’s diagnosis and eligibility for thrombectomy is a necessary consideration, said Robert A. Harrington, MD, of Stanford University (Calif.), in an accompanying editorial.
In lieu of better data, stroke systems should follow the recommendation of the Mission: Lifeline Severity-based Stroke Triage Algorithm for emergency medical services to avoid more than 15 minutes of additional travel time to transport a patient to a center that can perform endovascular therapy when the patient may be eligible for intravenous tissue plasminogen activator (tPA), said Dr. Harrington.
Delays in initiating tPA could lead to some patients not receiving treatment. “Some patients with suspected LVO [large vessel occlusion] either will not have thrombectomy or will not be eligible for it, and they also run the risk of not receiving any acute reperfusion therapy. Consequently, transport algorithms and models must take into account the uncertainty in prehospital diagnosis when considering the most appropriate facility,” he said.
Forthcoming acute stroke guidelines “will recommend intravenous tPA for all eligible subjects” because administration of tPA before endovascular thrombectomy does not appear to be harmful, Dr. Harrington noted.
Ultimately, approaches to routing patients may vary by region. “It is up to local and regional communities ... to define how best to implement these elements into a stroke system of care that meets their needs and resources and to define the types of hospitals that should qualify as points of entry for patients with suspected LVO strokes,” Dr. Harrington said.
A group convened by the American Heart Association and American Stroke Association is drafting further guiding principles for stroke systems of care in various regional settings.
Dr. Harrington is president-elect of the American Heart Association. He reported receiving research grants from AstraZeneca and Bristol-Myers Squibb.
When determining where to transport a patient with stroke, uncertainty about the patient’s diagnosis and eligibility for thrombectomy is a necessary consideration, said Robert A. Harrington, MD, of Stanford University (Calif.), in an accompanying editorial.
In lieu of better data, stroke systems should follow the recommendation of the Mission: Lifeline Severity-based Stroke Triage Algorithm for emergency medical services to avoid more than 15 minutes of additional travel time to transport a patient to a center that can perform endovascular therapy when the patient may be eligible for intravenous tissue plasminogen activator (tPA), said Dr. Harrington.
Delays in initiating tPA could lead to some patients not receiving treatment. “Some patients with suspected LVO [large vessel occlusion] either will not have thrombectomy or will not be eligible for it, and they also run the risk of not receiving any acute reperfusion therapy. Consequently, transport algorithms and models must take into account the uncertainty in prehospital diagnosis when considering the most appropriate facility,” he said.
Forthcoming acute stroke guidelines “will recommend intravenous tPA for all eligible subjects” because administration of tPA before endovascular thrombectomy does not appear to be harmful, Dr. Harrington noted.
Ultimately, approaches to routing patients may vary by region. “It is up to local and regional communities ... to define how best to implement these elements into a stroke system of care that meets their needs and resources and to define the types of hospitals that should qualify as points of entry for patients with suspected LVO strokes,” Dr. Harrington said.
A group convened by the American Heart Association and American Stroke Association is drafting further guiding principles for stroke systems of care in various regional settings.
Dr. Harrington is president-elect of the American Heart Association. He reported receiving research grants from AstraZeneca and Bristol-Myers Squibb.
Stroke centers need to collaborate within their regions to assure best practices and optimal access to comprehensive stroke centers as well as newly-designated thrombectomy-capable stroke centers, according to an updated policy statement from the American Stroke Association published in Stroke.
Opeolu Adeoye, MD, associate professor of emergency medicine and neurosurgery at the University of Cincinnati – and chair of the policy statement writing group – and coauthors updated the ASA’s 2005 recommendations for policy makers and public health care agencies to reflect current evidence, the increased availability of endovascular therapy, and new stroke center certifications.
“We have seen monumental advancements in acute stroke care over the past 14 years, and our concept of a comprehensive stroke system of care has evolved as a result,” Dr. Adeoye said in a news release.
While a recommendation to support the initiation of stroke prevention regimens remains unchanged from the 2005 recommendations, the 2019 update emphasizes a need to support long-term adherence to such regimens. To that end, researchers should examine the potential benefits of stroke prevention efforts that incorporate social media, gamification, and other technologies and principles to promote healthy behavior, the authors suggested. Furthermore, technology may allow for the passive surveillance of baseline behaviors and enable researchers to track changes in behavior over time.
Thrombectomy-capable centers
Thrombectomy-capable stroke centers, which have capabilities between those of primary stroke centers and comprehensive stroke centers, provide a relatively new level of acute stroke care. In communities that do not otherwise have access to thrombectomy, these centers play a clear role. In communities with comprehensive stroke centers, their role “is more controversial, and routing plans for patients with a suspected LVO [large vessel occlusion] should always seek the center of highest capability when travel time differences are short,” the statement says.
Timely parenchymal and arterial imaging via CT or MRI are needed to identify the subset of patients who may benefit from thrombectomy. All centers managing stroke patients should develop a plan for the definitive identification and treatment of these patients. Imaging techniques that assess penumbral patterns to identify candidates for endovascular therapy between 6 and 24 hours after patients were last known to be normal “merit broader adoption,” the statement says.
Hospitals without thrombectomy capability should have transfer protocols to allow the rapid treatment of these patients to hospitals with the appropriate level of care. In rural facilities that lack 24/7 imaging and radiology capabilities, this may mean rapid transfer of patients with clinically suspected LVO to hospitals where their work-up may be expedited.
To improve process, centers providing thrombectomy should rigorously track patient flow at all time points from presentation to imaging to intervention. Reperfusion rates, procedural complications, and patient clinical outcomes must be tracked and reported.
Travel times
Triage paradigms and protocols should be developed to ensure that emergency medical service (EMS) providers are able to rapidly identify all patients with a known or suspected stroke and to assess them with a validated and standardized instrument for stroke screening such as FAST (Face, Arm, Speech, Time), Los Angeles Prehospital Stroke Screen, or Cincinnati Prehospital Stroke Scale.
In prehospital patients who screen positive for suspected stroke, a standard prehospital stroke severity assessment tool such as the Cincinnati Stroke Triage Assessment Tool, Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, or Field Assessment Stroke Triage for Emergency Destination should be used. “Further research is needed to establish the most effective prehospital stroke severity triage scale,” the authors noted. In all cases, EMS should notify hospitals that a stroke patient is en route.
“When there are several intravenous alteplase–capable hospitals in a well-defined geographic region, extra transportation times to reach a facility capable of endovascular thrombectomy should be limited to no more than 15 minutes in patients with a prehospital stroke severity score suggestive of LVO,” according to the recommendations. “When several hospital options exist within similar travel times, EMS should seek care at the facility capable of offering the highest level of stroke care. Further research is needed to establish travel time parameters for hospital bypass in cases of prehospital suspicion of LVO.”
Outcomes and discharge
Centers should track various treatment and patient outcomes, and all patients discharged to their homes should have appropriate follow-up with specialized stroke services and primary care and be screened for postacute complications.
Government institutions should standardize the organization of stroke care, ensure that stroke patients receive timely care at appropriate hospitals, and facilitate access to secondary prevention and rehabilitation resources after stroke, the authors wrote.
“Programs geared at further improving the knowledge of the public, encouraging primordial and primary prevention, advancing and facilitating acute therapy, improving secondary prevention and recovery from stroke, and reducing disparities in stroke care should be actively developed in a coordinated and collaborative fashion by providers and policymakers at the local, state, and national levels,” the authors concluded. “Such efforts will continue to mitigate the effects of stroke on society.”
Dr. Adeoye had no disclosures. Some coauthors reported research grants and consultant or advisory board positions.
SOURCE: Adeoye O et al. Stroke. 2019 May 20. doi: 10.1161/STR.0000000000000173.
Stroke centers need to collaborate within their regions to assure best practices and optimal access to comprehensive stroke centers as well as newly-designated thrombectomy-capable stroke centers, according to an updated policy statement from the American Stroke Association published in Stroke.
Opeolu Adeoye, MD, associate professor of emergency medicine and neurosurgery at the University of Cincinnati – and chair of the policy statement writing group – and coauthors updated the ASA’s 2005 recommendations for policy makers and public health care agencies to reflect current evidence, the increased availability of endovascular therapy, and new stroke center certifications.
“We have seen monumental advancements in acute stroke care over the past 14 years, and our concept of a comprehensive stroke system of care has evolved as a result,” Dr. Adeoye said in a news release.
While a recommendation to support the initiation of stroke prevention regimens remains unchanged from the 2005 recommendations, the 2019 update emphasizes a need to support long-term adherence to such regimens. To that end, researchers should examine the potential benefits of stroke prevention efforts that incorporate social media, gamification, and other technologies and principles to promote healthy behavior, the authors suggested. Furthermore, technology may allow for the passive surveillance of baseline behaviors and enable researchers to track changes in behavior over time.
Thrombectomy-capable centers
Thrombectomy-capable stroke centers, which have capabilities between those of primary stroke centers and comprehensive stroke centers, provide a relatively new level of acute stroke care. In communities that do not otherwise have access to thrombectomy, these centers play a clear role. In communities with comprehensive stroke centers, their role “is more controversial, and routing plans for patients with a suspected LVO [large vessel occlusion] should always seek the center of highest capability when travel time differences are short,” the statement says.
Timely parenchymal and arterial imaging via CT or MRI are needed to identify the subset of patients who may benefit from thrombectomy. All centers managing stroke patients should develop a plan for the definitive identification and treatment of these patients. Imaging techniques that assess penumbral patterns to identify candidates for endovascular therapy between 6 and 24 hours after patients were last known to be normal “merit broader adoption,” the statement says.
Hospitals without thrombectomy capability should have transfer protocols to allow the rapid treatment of these patients to hospitals with the appropriate level of care. In rural facilities that lack 24/7 imaging and radiology capabilities, this may mean rapid transfer of patients with clinically suspected LVO to hospitals where their work-up may be expedited.
To improve process, centers providing thrombectomy should rigorously track patient flow at all time points from presentation to imaging to intervention. Reperfusion rates, procedural complications, and patient clinical outcomes must be tracked and reported.
Travel times
Triage paradigms and protocols should be developed to ensure that emergency medical service (EMS) providers are able to rapidly identify all patients with a known or suspected stroke and to assess them with a validated and standardized instrument for stroke screening such as FAST (Face, Arm, Speech, Time), Los Angeles Prehospital Stroke Screen, or Cincinnati Prehospital Stroke Scale.
In prehospital patients who screen positive for suspected stroke, a standard prehospital stroke severity assessment tool such as the Cincinnati Stroke Triage Assessment Tool, Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, or Field Assessment Stroke Triage for Emergency Destination should be used. “Further research is needed to establish the most effective prehospital stroke severity triage scale,” the authors noted. In all cases, EMS should notify hospitals that a stroke patient is en route.
“When there are several intravenous alteplase–capable hospitals in a well-defined geographic region, extra transportation times to reach a facility capable of endovascular thrombectomy should be limited to no more than 15 minutes in patients with a prehospital stroke severity score suggestive of LVO,” according to the recommendations. “When several hospital options exist within similar travel times, EMS should seek care at the facility capable of offering the highest level of stroke care. Further research is needed to establish travel time parameters for hospital bypass in cases of prehospital suspicion of LVO.”
Outcomes and discharge
Centers should track various treatment and patient outcomes, and all patients discharged to their homes should have appropriate follow-up with specialized stroke services and primary care and be screened for postacute complications.
Government institutions should standardize the organization of stroke care, ensure that stroke patients receive timely care at appropriate hospitals, and facilitate access to secondary prevention and rehabilitation resources after stroke, the authors wrote.
“Programs geared at further improving the knowledge of the public, encouraging primordial and primary prevention, advancing and facilitating acute therapy, improving secondary prevention and recovery from stroke, and reducing disparities in stroke care should be actively developed in a coordinated and collaborative fashion by providers and policymakers at the local, state, and national levels,” the authors concluded. “Such efforts will continue to mitigate the effects of stroke on society.”
Dr. Adeoye had no disclosures. Some coauthors reported research grants and consultant or advisory board positions.
SOURCE: Adeoye O et al. Stroke. 2019 May 20. doi: 10.1161/STR.0000000000000173.
FROM STROKE
New JIA, JIA-associated uveitis guidelines address knowledge gaps
while attempting to address gaps in the clinical screening, monitoring, and treatment of these diseases.
The first guideline – which was published simultaneously in Arthritis Care & Research and Arthritis & Rheumatology – offered 39 recommendations for treating children and adolescents with JIA and nonsystemic polyarthritis, sacroiliitis, and enthesitis. Due to the low quality of the supporting evidence, 31 of the recommendations were labeled as conditional.
“While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences,” wrote first author Sarah Ringold, MD, of Seattle Children’s Hospital and her coauthors.
These recommendations serve as updates to an initial set on JIA treatment in 2011 and a 2013 addition on the treatment of systemic arthritis. In addition, the ACR has since adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to generate its recommendations, which was described in an interview with Dr. Ringold as providing “greater transparency around the decisions made during the recommendation development process.”
“An important difference from the 2011 guidelines is that is that initial NSAID monotherapy is no longer recommended for children with polyarthritis, given the established benefits of early initiation of DMARD [disease-modifying antirheumatic drug] therapy for these patients,” she added. “In addition, these guidelines also support inactive disease as a treatment goal for children with polyarthritis, with treatment escalation recommended for patients with low disease activity.”
As general recommendations for patients with JIA and polyarthritis, the coauthors strongly recommend using triamcinolone hexacetonide over triamcinolone acetonide for intraarticular glucocorticoid injections, along with using infliximab in combination with a DMARD. Despite the quality of the supporting evidence being very low, they also strongly recommend against adding chronic low-dose glucocorticoids to treatment because of well-known adverse effects like growth suppression, weight gain, osteopenia, and cataract.
Other strong recommendations include choosing treatment with an NSAID in children and adolescents with JIA and active sacroiliitis; adding a tumor necrosis factor inhibitor (TNFi) rather than continued NSAID monotherapy and avoiding methotrexate monotherapy in children and adolescents with active sacroiliitis despite NSAIDs; and choosing NSAID treatment in children and adolescents with JIA and active enthesitis.
Recommendations on JIA with associated uveitis
The second guideline – also published in Arthritis Care & Research and Arthritis & Rheumatology – focused on the screening, monitoring, and treatment of JIA with associated uveitis. Their 19 recommendations serve as updates to 2006 recommendations from the American Academy of Pediatrics on routine ophthalmic screening in children with arthritis and 2012 recommendations from the German Uveitis in Childhood Study Group on proposed ophthalmic screening schedules, neither of which addressed the monitoring of children with an established diagnosis of uveitis or the treatment of uveitis.
“Although the quality of evidence was very low, and most recommendations were therefore conditional, this guideline fills an important clinical gap in the care of children with JIA-associated uveitis and may be updated as better evidence becomes available,” wrote first author Sheila T. Angeles-Han, MD, of the Cincinnati Children’s Hospital Medical Center and her coauthors.
Their strong recommendations include ophthalmic monitoring at least every 3 months in children and adolescents with JIA and controlled uveitis on stable therapy; monitoring within 1 month after each change of topical glucocorticoids in patients who are tapering or discontinuing that treatment; and monitoring within 2 months of changing systemic therapy for patients who are tapering or discontinuing that treatment.
They also strongly recommend education on the warning signs of acute anterior uveitis for children and adolescents with spondyloarthritis, along with tapering topical glucocorticoids before systemic therapy in children and adolescents with JIA and chronic anterior uveitis who are still on 1-2 drops a day of glucocorticoids.
“Our biggest message is that it is critical that uveitis is controlled, since persistent active uveitis can lead to sight-threatening complications and permanent vision loss,” Dr. Angeles-Han said in an interview. “It is important that ocular inflammation is controlled early, exposure to long-term topical glucocorticoids is limited, and that systemic treatment is started promptly.
“We also emphasize that close communication and collaboration between pediatric rheumatologists and ophthalmologists is important to ensure optimal vision outcomes,” she added.
These guidelines also factored in feedback from a patient and parent panel, particularly in regard to recommendations with a low level of supporting evidence.
“This partnership highlighted the importance of incorporating parent and patient preferences into treatment decisions and the need for shared decision-making approaches,” Dr. Ringold said.
Both guidelines were supported by the American College of Rheumatology and the Arthritis Foundation. Several authors reported support from the National Institutes of Health, the Rheumatology Research Foundation, and the Fundación Bechara. Several authors also reported receiving consulting and speaking fees, along with research grants, from numerous pharmaceutical companies.
SOURCES: Ringold S et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870 ; Angeles-Han ST et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23871 .
while attempting to address gaps in the clinical screening, monitoring, and treatment of these diseases.
The first guideline – which was published simultaneously in Arthritis Care & Research and Arthritis & Rheumatology – offered 39 recommendations for treating children and adolescents with JIA and nonsystemic polyarthritis, sacroiliitis, and enthesitis. Due to the low quality of the supporting evidence, 31 of the recommendations were labeled as conditional.
“While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences,” wrote first author Sarah Ringold, MD, of Seattle Children’s Hospital and her coauthors.
These recommendations serve as updates to an initial set on JIA treatment in 2011 and a 2013 addition on the treatment of systemic arthritis. In addition, the ACR has since adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to generate its recommendations, which was described in an interview with Dr. Ringold as providing “greater transparency around the decisions made during the recommendation development process.”
“An important difference from the 2011 guidelines is that is that initial NSAID monotherapy is no longer recommended for children with polyarthritis, given the established benefits of early initiation of DMARD [disease-modifying antirheumatic drug] therapy for these patients,” she added. “In addition, these guidelines also support inactive disease as a treatment goal for children with polyarthritis, with treatment escalation recommended for patients with low disease activity.”
As general recommendations for patients with JIA and polyarthritis, the coauthors strongly recommend using triamcinolone hexacetonide over triamcinolone acetonide for intraarticular glucocorticoid injections, along with using infliximab in combination with a DMARD. Despite the quality of the supporting evidence being very low, they also strongly recommend against adding chronic low-dose glucocorticoids to treatment because of well-known adverse effects like growth suppression, weight gain, osteopenia, and cataract.
Other strong recommendations include choosing treatment with an NSAID in children and adolescents with JIA and active sacroiliitis; adding a tumor necrosis factor inhibitor (TNFi) rather than continued NSAID monotherapy and avoiding methotrexate monotherapy in children and adolescents with active sacroiliitis despite NSAIDs; and choosing NSAID treatment in children and adolescents with JIA and active enthesitis.
Recommendations on JIA with associated uveitis
The second guideline – also published in Arthritis Care & Research and Arthritis & Rheumatology – focused on the screening, monitoring, and treatment of JIA with associated uveitis. Their 19 recommendations serve as updates to 2006 recommendations from the American Academy of Pediatrics on routine ophthalmic screening in children with arthritis and 2012 recommendations from the German Uveitis in Childhood Study Group on proposed ophthalmic screening schedules, neither of which addressed the monitoring of children with an established diagnosis of uveitis or the treatment of uveitis.
“Although the quality of evidence was very low, and most recommendations were therefore conditional, this guideline fills an important clinical gap in the care of children with JIA-associated uveitis and may be updated as better evidence becomes available,” wrote first author Sheila T. Angeles-Han, MD, of the Cincinnati Children’s Hospital Medical Center and her coauthors.
Their strong recommendations include ophthalmic monitoring at least every 3 months in children and adolescents with JIA and controlled uveitis on stable therapy; monitoring within 1 month after each change of topical glucocorticoids in patients who are tapering or discontinuing that treatment; and monitoring within 2 months of changing systemic therapy for patients who are tapering or discontinuing that treatment.
They also strongly recommend education on the warning signs of acute anterior uveitis for children and adolescents with spondyloarthritis, along with tapering topical glucocorticoids before systemic therapy in children and adolescents with JIA and chronic anterior uveitis who are still on 1-2 drops a day of glucocorticoids.
“Our biggest message is that it is critical that uveitis is controlled, since persistent active uveitis can lead to sight-threatening complications and permanent vision loss,” Dr. Angeles-Han said in an interview. “It is important that ocular inflammation is controlled early, exposure to long-term topical glucocorticoids is limited, and that systemic treatment is started promptly.
“We also emphasize that close communication and collaboration between pediatric rheumatologists and ophthalmologists is important to ensure optimal vision outcomes,” she added.
These guidelines also factored in feedback from a patient and parent panel, particularly in regard to recommendations with a low level of supporting evidence.
“This partnership highlighted the importance of incorporating parent and patient preferences into treatment decisions and the need for shared decision-making approaches,” Dr. Ringold said.
Both guidelines were supported by the American College of Rheumatology and the Arthritis Foundation. Several authors reported support from the National Institutes of Health, the Rheumatology Research Foundation, and the Fundación Bechara. Several authors also reported receiving consulting and speaking fees, along with research grants, from numerous pharmaceutical companies.
SOURCES: Ringold S et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870 ; Angeles-Han ST et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23871 .
while attempting to address gaps in the clinical screening, monitoring, and treatment of these diseases.
The first guideline – which was published simultaneously in Arthritis Care & Research and Arthritis & Rheumatology – offered 39 recommendations for treating children and adolescents with JIA and nonsystemic polyarthritis, sacroiliitis, and enthesitis. Due to the low quality of the supporting evidence, 31 of the recommendations were labeled as conditional.
“While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences,” wrote first author Sarah Ringold, MD, of Seattle Children’s Hospital and her coauthors.
These recommendations serve as updates to an initial set on JIA treatment in 2011 and a 2013 addition on the treatment of systemic arthritis. In addition, the ACR has since adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to generate its recommendations, which was described in an interview with Dr. Ringold as providing “greater transparency around the decisions made during the recommendation development process.”
“An important difference from the 2011 guidelines is that is that initial NSAID monotherapy is no longer recommended for children with polyarthritis, given the established benefits of early initiation of DMARD [disease-modifying antirheumatic drug] therapy for these patients,” she added. “In addition, these guidelines also support inactive disease as a treatment goal for children with polyarthritis, with treatment escalation recommended for patients with low disease activity.”
As general recommendations for patients with JIA and polyarthritis, the coauthors strongly recommend using triamcinolone hexacetonide over triamcinolone acetonide for intraarticular glucocorticoid injections, along with using infliximab in combination with a DMARD. Despite the quality of the supporting evidence being very low, they also strongly recommend against adding chronic low-dose glucocorticoids to treatment because of well-known adverse effects like growth suppression, weight gain, osteopenia, and cataract.
Other strong recommendations include choosing treatment with an NSAID in children and adolescents with JIA and active sacroiliitis; adding a tumor necrosis factor inhibitor (TNFi) rather than continued NSAID monotherapy and avoiding methotrexate monotherapy in children and adolescents with active sacroiliitis despite NSAIDs; and choosing NSAID treatment in children and adolescents with JIA and active enthesitis.
Recommendations on JIA with associated uveitis
The second guideline – also published in Arthritis Care & Research and Arthritis & Rheumatology – focused on the screening, monitoring, and treatment of JIA with associated uveitis. Their 19 recommendations serve as updates to 2006 recommendations from the American Academy of Pediatrics on routine ophthalmic screening in children with arthritis and 2012 recommendations from the German Uveitis in Childhood Study Group on proposed ophthalmic screening schedules, neither of which addressed the monitoring of children with an established diagnosis of uveitis or the treatment of uveitis.
“Although the quality of evidence was very low, and most recommendations were therefore conditional, this guideline fills an important clinical gap in the care of children with JIA-associated uveitis and may be updated as better evidence becomes available,” wrote first author Sheila T. Angeles-Han, MD, of the Cincinnati Children’s Hospital Medical Center and her coauthors.
Their strong recommendations include ophthalmic monitoring at least every 3 months in children and adolescents with JIA and controlled uveitis on stable therapy; monitoring within 1 month after each change of topical glucocorticoids in patients who are tapering or discontinuing that treatment; and monitoring within 2 months of changing systemic therapy for patients who are tapering or discontinuing that treatment.
They also strongly recommend education on the warning signs of acute anterior uveitis for children and adolescents with spondyloarthritis, along with tapering topical glucocorticoids before systemic therapy in children and adolescents with JIA and chronic anterior uveitis who are still on 1-2 drops a day of glucocorticoids.
“Our biggest message is that it is critical that uveitis is controlled, since persistent active uveitis can lead to sight-threatening complications and permanent vision loss,” Dr. Angeles-Han said in an interview. “It is important that ocular inflammation is controlled early, exposure to long-term topical glucocorticoids is limited, and that systemic treatment is started promptly.
“We also emphasize that close communication and collaboration between pediatric rheumatologists and ophthalmologists is important to ensure optimal vision outcomes,” she added.
These guidelines also factored in feedback from a patient and parent panel, particularly in regard to recommendations with a low level of supporting evidence.
“This partnership highlighted the importance of incorporating parent and patient preferences into treatment decisions and the need for shared decision-making approaches,” Dr. Ringold said.
Both guidelines were supported by the American College of Rheumatology and the Arthritis Foundation. Several authors reported support from the National Institutes of Health, the Rheumatology Research Foundation, and the Fundación Bechara. Several authors also reported receiving consulting and speaking fees, along with research grants, from numerous pharmaceutical companies.
SOURCES: Ringold S et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870 ; Angeles-Han ST et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23871 .
FROM ARTHRITIS CARE & RESEARCH
ACOG guidance addresses cardiac contributors to maternal mortality
NASHVILLE, TENN. – according to new comprehensive guidance from the American College of Obstetricians and Gynecologists.
The toolkit algorithm is called the California Improving Health Care Response to Cardiovascular Disease in Pregnancy and Postpartum Toolkit. It was developed by the Cardiovascular Disease in Pregnancy Postpartum Task Force to serve as a resource for obstetrics, primary care and emergency medicine providers who provide prenatal care or interact with women during the postpartum period. It incldues an overview of clinical assessment and comprehensive management strategies for cardiovascular disease based on risk factors and presenting symptoms.
The guidance also calls for all pregnant and postpartum women with known or suspected CVD to undergo further evaluation by a “Pregnancy Heart Team that includes a cardiologist and maternal–fetal medicine subspecialist, or both, and other subspecialists as necessary.” The guidance was issued in Practice Bulletin 212, Pregnancy and Heart Disease, which is published in the May edition of Obstetrics & Gynecology (Obstet Gynecol. 2019 May;133[5]:e320-e356).
In all, 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period are included in the guidance.
ACOG president Lisa Hollier, MD, convened the task force that developed this guidance to address cardiac contributors to maternal mortality, she said during a press briefing at the ACOG annual clinical and scientific meeting.
“When I began my presidency a year ago, my goal was to bring together a multidisciplinary group of clinicians ... to create clinical guidance that would make a difference in the lives of women," said Dr. Hollier, who is also a professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
Part of her presidential initiative was centered on eliminating preventable maternal death, and this guidance has the potential to make strides toward that goal, she said. When it comes to CVD in pregnancy, “there is so much we can do to prevent negative outcomes and ensure that moms go home with their babies and are around to see them grow up,” she noted.
CVD is the leading cause of death in pregnant women and women in the postpartum period, accounting for 26.5% of U.S. pregnancy-related deaths.
“It’s critical that we as physicians and health care professionals develop expertise in recognizing the signs and symptoms so that we can save women’s lives,” she said in the press breifing. Dr. Hollier also implored her colleagues to “start using this guidance immediately and prevent more women from dying from cardiovascular complications of pregnancy.”
In this video interview, Dr. Hollier further explains the need for the guidance and its potential for improving maternal mortality rates.
Dr. Hollier reported having no relevant disclosures.
SOURCE: Hollier L et al., Obstet Gynecol. 2019 May;133[5]:e320-56.
NASHVILLE, TENN. – according to new comprehensive guidance from the American College of Obstetricians and Gynecologists.
The toolkit algorithm is called the California Improving Health Care Response to Cardiovascular Disease in Pregnancy and Postpartum Toolkit. It was developed by the Cardiovascular Disease in Pregnancy Postpartum Task Force to serve as a resource for obstetrics, primary care and emergency medicine providers who provide prenatal care or interact with women during the postpartum period. It incldues an overview of clinical assessment and comprehensive management strategies for cardiovascular disease based on risk factors and presenting symptoms.
The guidance also calls for all pregnant and postpartum women with known or suspected CVD to undergo further evaluation by a “Pregnancy Heart Team that includes a cardiologist and maternal–fetal medicine subspecialist, or both, and other subspecialists as necessary.” The guidance was issued in Practice Bulletin 212, Pregnancy and Heart Disease, which is published in the May edition of Obstetrics & Gynecology (Obstet Gynecol. 2019 May;133[5]:e320-e356).
In all, 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period are included in the guidance.
ACOG president Lisa Hollier, MD, convened the task force that developed this guidance to address cardiac contributors to maternal mortality, she said during a press briefing at the ACOG annual clinical and scientific meeting.
“When I began my presidency a year ago, my goal was to bring together a multidisciplinary group of clinicians ... to create clinical guidance that would make a difference in the lives of women," said Dr. Hollier, who is also a professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
Part of her presidential initiative was centered on eliminating preventable maternal death, and this guidance has the potential to make strides toward that goal, she said. When it comes to CVD in pregnancy, “there is so much we can do to prevent negative outcomes and ensure that moms go home with their babies and are around to see them grow up,” she noted.
CVD is the leading cause of death in pregnant women and women in the postpartum period, accounting for 26.5% of U.S. pregnancy-related deaths.
“It’s critical that we as physicians and health care professionals develop expertise in recognizing the signs and symptoms so that we can save women’s lives,” she said in the press breifing. Dr. Hollier also implored her colleagues to “start using this guidance immediately and prevent more women from dying from cardiovascular complications of pregnancy.”
In this video interview, Dr. Hollier further explains the need for the guidance and its potential for improving maternal mortality rates.
Dr. Hollier reported having no relevant disclosures.
SOURCE: Hollier L et al., Obstet Gynecol. 2019 May;133[5]:e320-56.
NASHVILLE, TENN. – according to new comprehensive guidance from the American College of Obstetricians and Gynecologists.
The toolkit algorithm is called the California Improving Health Care Response to Cardiovascular Disease in Pregnancy and Postpartum Toolkit. It was developed by the Cardiovascular Disease in Pregnancy Postpartum Task Force to serve as a resource for obstetrics, primary care and emergency medicine providers who provide prenatal care or interact with women during the postpartum period. It incldues an overview of clinical assessment and comprehensive management strategies for cardiovascular disease based on risk factors and presenting symptoms.
The guidance also calls for all pregnant and postpartum women with known or suspected CVD to undergo further evaluation by a “Pregnancy Heart Team that includes a cardiologist and maternal–fetal medicine subspecialist, or both, and other subspecialists as necessary.” The guidance was issued in Practice Bulletin 212, Pregnancy and Heart Disease, which is published in the May edition of Obstetrics & Gynecology (Obstet Gynecol. 2019 May;133[5]:e320-e356).
In all, 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period are included in the guidance.
ACOG president Lisa Hollier, MD, convened the task force that developed this guidance to address cardiac contributors to maternal mortality, she said during a press briefing at the ACOG annual clinical and scientific meeting.
“When I began my presidency a year ago, my goal was to bring together a multidisciplinary group of clinicians ... to create clinical guidance that would make a difference in the lives of women," said Dr. Hollier, who is also a professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
Part of her presidential initiative was centered on eliminating preventable maternal death, and this guidance has the potential to make strides toward that goal, she said. When it comes to CVD in pregnancy, “there is so much we can do to prevent negative outcomes and ensure that moms go home with their babies and are around to see them grow up,” she noted.
CVD is the leading cause of death in pregnant women and women in the postpartum period, accounting for 26.5% of U.S. pregnancy-related deaths.
“It’s critical that we as physicians and health care professionals develop expertise in recognizing the signs and symptoms so that we can save women’s lives,” she said in the press breifing. Dr. Hollier also implored her colleagues to “start using this guidance immediately and prevent more women from dying from cardiovascular complications of pregnancy.”
In this video interview, Dr. Hollier further explains the need for the guidance and its potential for improving maternal mortality rates.
Dr. Hollier reported having no relevant disclosures.
SOURCE: Hollier L et al., Obstet Gynecol. 2019 May;133[5]:e320-56.
REPORTING FROM ACOG 2019
Experts propose new definition and recommendations for Alzheimer’s-like disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
FROM BRAIN
Are guidelines relevant?
The recent publication of the changes and current levels of evidence for the guidelines from the American College of Cardiology, American Heart Association, and the European Society of Cardiology once again indicates that the level of high-quality scientific data supporting the decision making process is limited.
Of the thousands of recommendations among the guidelines provided, level A data – which is supported by at least two randomized, controlled trials (RCT), arguably the gold standard for the proof of benefit of a treatment program – was available in only 8.5% of ACC/AHA recommendations and in 14.2% of ESC recommendations. The recent review covering guidelines published from 2008 to 2018 has changed very little since the previous review carried out on data collected between 1999 and 2014 (JAMA. 2019 Mar 19;321[11]:1069-80). Admittedly, by the nature of their design, RCTs represent a very special subset of any disease process but they are the best we can do. The remainder of the guidelines is supported by nonrandomized data and consensus opinion of “experts.” The absence of randomized data in the rest of the guidelines creates an area of uncertainty between clinical practice and outcomes.
Guidelines have been with us for almost 30 years and have provided a near “cookbook recipe” for the treatment of almost the totality of cardiovascular care, both in the United States and Europe. There is little evidence that guideline dissemination results in a beneficial effect on cardiovascular care, instead reflecting the contemporary published research and informed opinion of our generation.
Despite the limitation of hard data based on RCTs, cardiovascular mortality has leveled off in the last few years. Although there are a number of areas that could be investigated with more focused and practical RCTs, there has been little enthusiasm by either the National Heart, Lung, and Blood Institute or the pharmaceutical or device industries to investigate existing cardiovascular drugs and/or devices to better elucidate nuanced therapy for subsets of heart disease that remain in the uncertain data areas. In fact, the current report shows that there has been little change in guidelines in the last 10 years. Most effort has been focused on new drug development and application.
Much of the advance in cardiovascular care and decrease in mortality is a result of the development of new drugs for the treatment of hypertension, management of hypercholesterolemia, and MI, together with coronary angiography and its interventional permutations of the previous 50 years. Those advances are now part of our knowledge base, our therapeutic DNA, and in the absence of some new scientific breakthrough, there is little to expect from future guideline development. Medical students and house officers are being taught as fact what we found as exciting new therapy just a few short years ago. Guidelines are now part of textbook knowledge and until new clinical outcome are reported, guidelines will probably serve a useful reference material but will not lead to much change in clinical care.
Even so, treating the patient in the clinic and in the hospital still requires the doctor to practice the “art” of medicine and to synthesize therapy based on the paucity of scientific data and quasi knowledge acquired over a generation.
The appreciation that much is unknown gives the doctor the humility required to be sensitive to patient needs and their symptoms and the aspiration for new, science-based data. This is still part of the excitement of being a doctor.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The recent publication of the changes and current levels of evidence for the guidelines from the American College of Cardiology, American Heart Association, and the European Society of Cardiology once again indicates that the level of high-quality scientific data supporting the decision making process is limited.
Of the thousands of recommendations among the guidelines provided, level A data – which is supported by at least two randomized, controlled trials (RCT), arguably the gold standard for the proof of benefit of a treatment program – was available in only 8.5% of ACC/AHA recommendations and in 14.2% of ESC recommendations. The recent review covering guidelines published from 2008 to 2018 has changed very little since the previous review carried out on data collected between 1999 and 2014 (JAMA. 2019 Mar 19;321[11]:1069-80). Admittedly, by the nature of their design, RCTs represent a very special subset of any disease process but they are the best we can do. The remainder of the guidelines is supported by nonrandomized data and consensus opinion of “experts.” The absence of randomized data in the rest of the guidelines creates an area of uncertainty between clinical practice and outcomes.
Guidelines have been with us for almost 30 years and have provided a near “cookbook recipe” for the treatment of almost the totality of cardiovascular care, both in the United States and Europe. There is little evidence that guideline dissemination results in a beneficial effect on cardiovascular care, instead reflecting the contemporary published research and informed opinion of our generation.
Despite the limitation of hard data based on RCTs, cardiovascular mortality has leveled off in the last few years. Although there are a number of areas that could be investigated with more focused and practical RCTs, there has been little enthusiasm by either the National Heart, Lung, and Blood Institute or the pharmaceutical or device industries to investigate existing cardiovascular drugs and/or devices to better elucidate nuanced therapy for subsets of heart disease that remain in the uncertain data areas. In fact, the current report shows that there has been little change in guidelines in the last 10 years. Most effort has been focused on new drug development and application.
Much of the advance in cardiovascular care and decrease in mortality is a result of the development of new drugs for the treatment of hypertension, management of hypercholesterolemia, and MI, together with coronary angiography and its interventional permutations of the previous 50 years. Those advances are now part of our knowledge base, our therapeutic DNA, and in the absence of some new scientific breakthrough, there is little to expect from future guideline development. Medical students and house officers are being taught as fact what we found as exciting new therapy just a few short years ago. Guidelines are now part of textbook knowledge and until new clinical outcome are reported, guidelines will probably serve a useful reference material but will not lead to much change in clinical care.
Even so, treating the patient in the clinic and in the hospital still requires the doctor to practice the “art” of medicine and to synthesize therapy based on the paucity of scientific data and quasi knowledge acquired over a generation.
The appreciation that much is unknown gives the doctor the humility required to be sensitive to patient needs and their symptoms and the aspiration for new, science-based data. This is still part of the excitement of being a doctor.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The recent publication of the changes and current levels of evidence for the guidelines from the American College of Cardiology, American Heart Association, and the European Society of Cardiology once again indicates that the level of high-quality scientific data supporting the decision making process is limited.
Of the thousands of recommendations among the guidelines provided, level A data – which is supported by at least two randomized, controlled trials (RCT), arguably the gold standard for the proof of benefit of a treatment program – was available in only 8.5% of ACC/AHA recommendations and in 14.2% of ESC recommendations. The recent review covering guidelines published from 2008 to 2018 has changed very little since the previous review carried out on data collected between 1999 and 2014 (JAMA. 2019 Mar 19;321[11]:1069-80). Admittedly, by the nature of their design, RCTs represent a very special subset of any disease process but they are the best we can do. The remainder of the guidelines is supported by nonrandomized data and consensus opinion of “experts.” The absence of randomized data in the rest of the guidelines creates an area of uncertainty between clinical practice and outcomes.
Guidelines have been with us for almost 30 years and have provided a near “cookbook recipe” for the treatment of almost the totality of cardiovascular care, both in the United States and Europe. There is little evidence that guideline dissemination results in a beneficial effect on cardiovascular care, instead reflecting the contemporary published research and informed opinion of our generation.
Despite the limitation of hard data based on RCTs, cardiovascular mortality has leveled off in the last few years. Although there are a number of areas that could be investigated with more focused and practical RCTs, there has been little enthusiasm by either the National Heart, Lung, and Blood Institute or the pharmaceutical or device industries to investigate existing cardiovascular drugs and/or devices to better elucidate nuanced therapy for subsets of heart disease that remain in the uncertain data areas. In fact, the current report shows that there has been little change in guidelines in the last 10 years. Most effort has been focused on new drug development and application.
Much of the advance in cardiovascular care and decrease in mortality is a result of the development of new drugs for the treatment of hypertension, management of hypercholesterolemia, and MI, together with coronary angiography and its interventional permutations of the previous 50 years. Those advances are now part of our knowledge base, our therapeutic DNA, and in the absence of some new scientific breakthrough, there is little to expect from future guideline development. Medical students and house officers are being taught as fact what we found as exciting new therapy just a few short years ago. Guidelines are now part of textbook knowledge and until new clinical outcome are reported, guidelines will probably serve a useful reference material but will not lead to much change in clinical care.
Even so, treating the patient in the clinic and in the hospital still requires the doctor to practice the “art” of medicine and to synthesize therapy based on the paucity of scientific data and quasi knowledge acquired over a generation.
The appreciation that much is unknown gives the doctor the humility required to be sensitive to patient needs and their symptoms and the aspiration for new, science-based data. This is still part of the excitement of being a doctor.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.













