User login
Minilaparoscopy is the next step in minimally invasive surgery
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
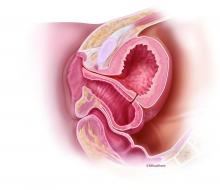
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
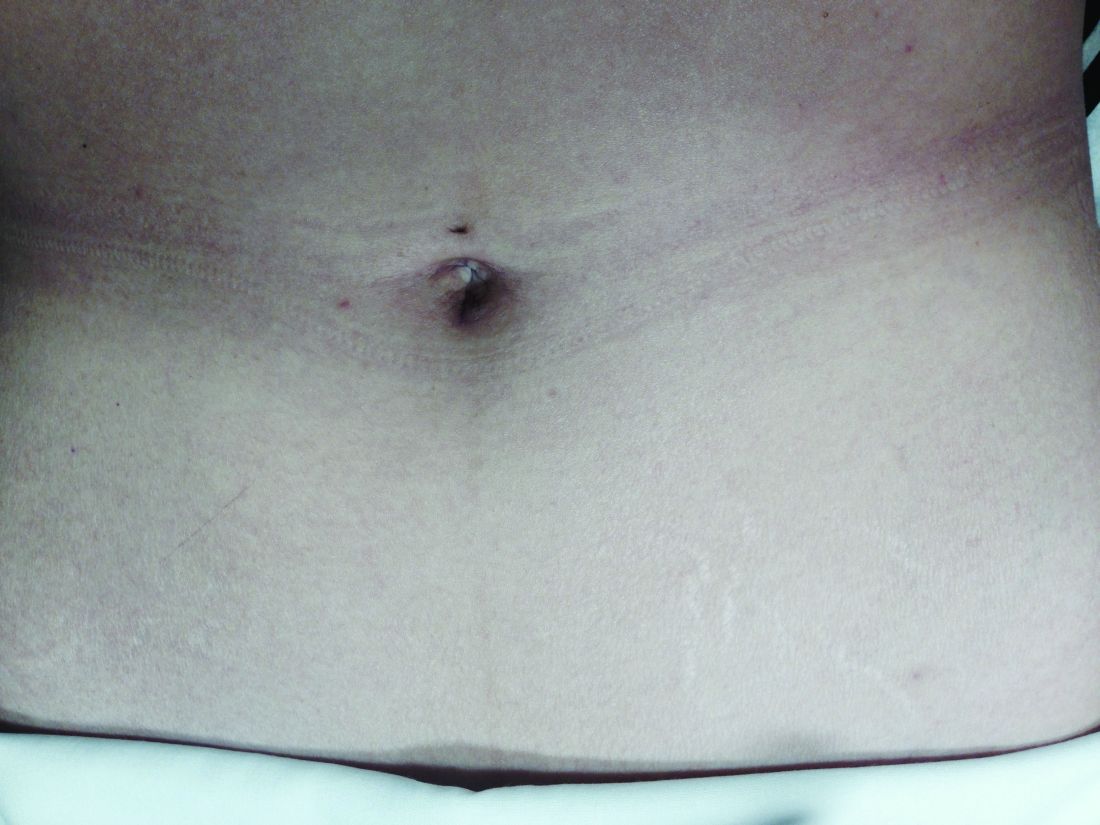
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
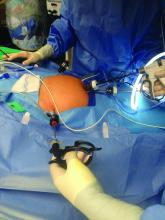
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
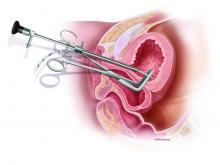
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
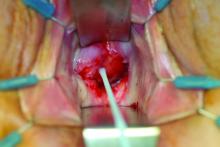
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”

Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”

Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minilaparoscopy is a relevant surgical technique
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
The value of low-dose aspirin for prevention of preeclampsia
Low-dose aspirin for the prevention of preeclampsia has been studied for more than 25 years, often with contradictory and confusing results. Studies have enrolled patients with varying levels of risk, assessed risk differently, and used different definitions of preeclampsia as well as a variety of aspirin dosages and treatment-initiation dates. Undoubtedly, this heterogeneity has made interpretation and comparisons difficult and frustrating.
Recently, systematic reviews and meta-analyses have improved our understanding of the role of low-dose aspirin, providing solid evidence that low-dose aspirin started after the first-trimester reduces the occurrence of preeclampsia in high-risk women. Data also suggest that low-dose aspirin reduces the incidence of fetal growth restriction and preterm birth in these women.
There is reasonable evidence, moreover, that low-dose aspirin provides similar benefit in women with modest levels of risk and that it’s best to begin aspirin use at 12-14 weeks’ gestation rather than later in the second trimester. Finally,
Despite this evidence and current recommendations for low-dose aspirin use by the U.S. Preventive Services Task Force and the American College of Obstetricians and Gynecologists, its use in practice is varied. Obstetricians and other obstetrics providers are not consistently making the recommendation, and pharmacists are not consistently supporting it.
Without more consistent initiation of low-dose aspirin prophylaxis and more consistent adherence, we are losing an opportunity to reduce serious maternal morbidity and mortality. We also are underutilizing an important tool for the reduction of racial and other health disparities relating to preterm birth, maternal death, and other complications of preeclampsia.
Dr. Lockwood: Epidemiology, etiology, and clinical value of aspirin
The use of low-dose aspirin can have a high impact, considering that preeclampsia complicates 3.4% of pregnancies nationally and accounts for at least 9% of maternal deaths (BMJ. 2013 Nov;347:f6564).
Preeclampsia also has been shown in multiple long-term epidemiologic studies to be a strong risk factor for future cardiovascular disease and metabolic disorders in women – especially when it occurs in multiple pregnancies or develops preterm. Moreover, it is associated with stillbirth, intrauterine growth restriction (IUGR), and oligohydramnios in the fetus (BMJ. 2013 Nov;347:f6564).
It is important to remember that criteria for a diagnosis of preeclampsia changed in 2013 such that the detection of proteinuria is no longer required. Preeclampsia is defined today as the new onset of hypertension and proteinuria, or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks in a previously normotensive woman, according to the ACOG Task Force on Hypertension in Pregnancy.
The leading risk factor appears to be previous preeclampsia. In a systematic review and meta-analysis of 92 cohort studies that looked at the pooled relative risk of developing preeclampsia in the presence or absence of 14 commonly reported and accepted risk factors, prior preeclampsia topped the list, putting patients at an eightfold increased risk (relative risk 8.4) (BMJ. 2016 Apr 19;353:i1753).
Nulliparity (relative risk, 2.1) and multiple gestation (RR, 2.9) presented lesser risks but still were significant, and preexisting medical conditions increased risk as well. Notably, both chronic hypertension and a body mass index (BMI) greater than 30 had a fivefold increased risk (RR, 5.1), and preexisting diabetes presented more than a threefold increased risk (RR, 3.7). The review covered more than 25 million pregnancies in 27 countries.
The etiology of preeclampsia still is not completely understood. There is evidence that underlying decidual inflammation, including increased activated macrophages and decreased uterine natural killer cells (uNK), promotes shallow placentation leading to incomplete uterine spiral artery remodeling, relative placental hypoxia, and progressive release of placental antiangiogenic substances such as soluble fms-like tyrosine kinase 1 (sFlt1) and endoglin (Am J Pathol. 2013 Sep;183[3]:841-56; Reprod Sci. 2015 Nov;22[11]:1461-7). The latter result in systemic endothelial cell damage, reduced endothelial prostacyclin (PGI2), and increased platelet thromboxane A2, triggering vasospasm and increased platelet turnover that ultimately lead to the typical signs and symptoms of preeclampsia.
The research focus traditionally has been on the placenta, but more recently the uterine decidual contribution has received more attention. A recent study published in the Proceedings of the National Academy of Sciences offers evidence that affected women have defective decidualization during and after severe preeclampsia, suggesting that the defect could be detected prior to conception.
Investigators isolated endometrial cells from women at the end of a pregnancy complicated by preeclampsia and found a transcriptional signature that persisted for years. They then linked the defect to impaired cytotrophoblast invasion (Proc Natl Acad Sci. 2017;114[40]:E8468-77). This elegant and provocative study suggests that it might be possible in the future to evaluate the endometrium and try to enhance stromal cell decidualization before pregnancy.
Currently, the rationale for using aspirin to prevent preeclampsia lies with its ability to inhibit platelet production of thromboxane and block NF-kB, a protein complex that plays a role in systemic and/or decidual inflammation. There likely are numerous mechanisms of action, however, including some that improve placentation.
Among the most recent studies on timing and dosage is a systematic review and meta-analysis of 45 randomized controlled trials with 20,909 women randomized to 50-150 mg aspirin daily or to placebo or no treatment. The investigators stratified the results by gestational age at the time of aspirin initiation and found that timing matters. Women who began aspirin at or before 16 weeks had the most significant reductions in preeclampsia (RR, 0.57) and severe preeclampsia (RR, 0.47), as well as fetal growth restriction (RR, 0.56), with a dose-response effect up to 150 mg.
When aspirin was initiated after 16 weeks, there was a much smaller reduction of preeclampsia (RR, 0.81) and no effects for severe preeclampsia or IUGR. Nor was there any dose-response effect (Am J Obstet Gynecol. 2017; 216[2]:110-20.e6).
In contrast, another recent meta-analysis of individual participant data on 32,217 women recruited in 31 randomized controlled trials found no significant difference among women who were randomized before 16 weeks versus those who were randomized at 16 weeks or later (Am J Obstet Gynecol. 2017 Feb;216[2]:121-8.e2). It’s important to note that this analysis covered other antiplatelet agents as well and that it stratified outcomes by gestational age with a slightly later cutoff point.
What do official guidelines say? The USPSTF’s recommendation, issued in 2014, calls for low-dose aspirin at 81 mg/day after 12 weeks’ gestation in women who have one or more high-risk factors, and consideration of such treatment in patients with “several” moderate-risk factors (Ann Intern Med. 2014 Dec 2;161[11]:819-26). In July 2018, ACOG reaffirmed its earlier support for low-dose aspirin in a committee opinion that recommends 81 mg/day beginning at 12-28 weeks’ gestation, optimally before 16 weeks’, for women who have one or more high-risk factors or more than one moderate-risk factor (Obstet Gynecol. 2018 Jul;132[1]:e44-e52).
My own take, based on published literature, including my own research, is that low-dose aspirin reduces the frequency of preeclampsia, particularly cases occurring preterm, as well as related IUGR, by approximately 10%-20% in moderate- and high-risk women. Regarding dose and gestational age for initiation, I have split the difference of what’s reflected in the literature and in guidelines. I advise 122 mg (a tablet-and-a-half) a day, starting at 12-14 weeks’, for patients at high and moderate levels of risk. For patients who are not seen until later, low-dose aspirin can be started up to 28 weeks’ gestation.
Dr. Abbott: Messaging and education to reduce disparities
Black women are not only more likely to develop preeclampsia, but they’re also more likely to have more severe complications and worse outcomes. In one analysis, black women with preeclampsia experienced an almost threefold higher risk of maternal mortality and intrauterine fetal death than did white women with the disorder (Hypertens Pregnancy. 2015 Nov;34[4]:506-15).
At Boston Medical Center, 30% of pregnant women have a diagnosis of preeclampsia or hypertension at term. In addition to 68% identifying as Hispanic/black or black, half of the families we care for have incomes less than $20,000, and 30% are non–English speaking. Low-dose prenatal aspirin is therefore an important tool for reducing racial health disparities as well as disparities created by health literacy, economic status, and language and cultural barriers. At BMC, New England’s largest safety-net hospital, we’ve found that the factors driving health disparities often overlap.
To increase the use of low-dose aspirin for women at moderate to high risk, we marry education about aspirin’s effectiveness and safety with education about the potential severity of hypertension and preeclampsia. We counsel patients who are hospitalized at delivery with gestational or chronic hypertension, or fetal growth restriction, about how preeclampsia can be very serious – contrary to what they’ve experienced or what friends or family may have shared. We also counsel them about signs and symptoms of severe preeclampsia that warrant consulting their provider. And overall, we deliberately use the term “prenatal aspirin” so that, over time and in the broader community, it will become associated with good prenatal care and risk reduction.
To counter perceived risks and dangers that we identified through focus groups and interviews, our patient education materials state that low-dose aspirin in pregnancy will not cause increased bleeding, does not reach the baby’s blood, does not increase the risk of miscarriage, and has not been shown to have negative effects on the baby’s initial development (www.prenatalaspirin.com/education-materials). We try to engage family members whenever possible, and we recognize that the black population has historical reasons to be concerned or suspicious that aspirin might not be safe for them.
Especially for underserved patients who receive prescriptions for low-dose aspirin, we must ensure that pharmacists will dispense the medication. A national survey of pharmacists (not yet published) found that over two-thirds were unaware of the USPSTF guidelines, and that only a minority would feel comfortable dispensing low-dose aspirin during pregnancy. In our community, some pharmacists have told patients to return to their physician and inquire more. Until recently, one of the major pharmacy chains placed a warning label on aspirin bottles being dispensed to women who also had an active prescription for prenatal vitamins.
We are working both with pharmacies and with pharmacy schools to impact the education of current and future pharmacists on guidelines and recommendations for low-dose aspirin prophylaxis. In addition, when I write a prescription for prenatal aspirin, starting at 12 weeks’ whenever possible, I include the message “for the purpose of trying to reduce pregnancy complications.”
Dr. Lockwood is senior vice president at University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa. He said he had no relevant financial disclosures or conflicts of interest. Dr. Abbot is a specialist in maternal-fetal medicine, the director of obstetrics and gynecology, and assistant dean for patient safety and quality improvement education at Boston Medical Center. She also is an associate professor of obstetrics and gynecology at Boston University. She disclosed a grant from the March of Dimes. Email them at [email protected].
Low-dose aspirin for the prevention of preeclampsia has been studied for more than 25 years, often with contradictory and confusing results. Studies have enrolled patients with varying levels of risk, assessed risk differently, and used different definitions of preeclampsia as well as a variety of aspirin dosages and treatment-initiation dates. Undoubtedly, this heterogeneity has made interpretation and comparisons difficult and frustrating.
Recently, systematic reviews and meta-analyses have improved our understanding of the role of low-dose aspirin, providing solid evidence that low-dose aspirin started after the first-trimester reduces the occurrence of preeclampsia in high-risk women. Data also suggest that low-dose aspirin reduces the incidence of fetal growth restriction and preterm birth in these women.
There is reasonable evidence, moreover, that low-dose aspirin provides similar benefit in women with modest levels of risk and that it’s best to begin aspirin use at 12-14 weeks’ gestation rather than later in the second trimester. Finally,
Despite this evidence and current recommendations for low-dose aspirin use by the U.S. Preventive Services Task Force and the American College of Obstetricians and Gynecologists, its use in practice is varied. Obstetricians and other obstetrics providers are not consistently making the recommendation, and pharmacists are not consistently supporting it.
Without more consistent initiation of low-dose aspirin prophylaxis and more consistent adherence, we are losing an opportunity to reduce serious maternal morbidity and mortality. We also are underutilizing an important tool for the reduction of racial and other health disparities relating to preterm birth, maternal death, and other complications of preeclampsia.
Dr. Lockwood: Epidemiology, etiology, and clinical value of aspirin
The use of low-dose aspirin can have a high impact, considering that preeclampsia complicates 3.4% of pregnancies nationally and accounts for at least 9% of maternal deaths (BMJ. 2013 Nov;347:f6564).
Preeclampsia also has been shown in multiple long-term epidemiologic studies to be a strong risk factor for future cardiovascular disease and metabolic disorders in women – especially when it occurs in multiple pregnancies or develops preterm. Moreover, it is associated with stillbirth, intrauterine growth restriction (IUGR), and oligohydramnios in the fetus (BMJ. 2013 Nov;347:f6564).
It is important to remember that criteria for a diagnosis of preeclampsia changed in 2013 such that the detection of proteinuria is no longer required. Preeclampsia is defined today as the new onset of hypertension and proteinuria, or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks in a previously normotensive woman, according to the ACOG Task Force on Hypertension in Pregnancy.
The leading risk factor appears to be previous preeclampsia. In a systematic review and meta-analysis of 92 cohort studies that looked at the pooled relative risk of developing preeclampsia in the presence or absence of 14 commonly reported and accepted risk factors, prior preeclampsia topped the list, putting patients at an eightfold increased risk (relative risk 8.4) (BMJ. 2016 Apr 19;353:i1753).
Nulliparity (relative risk, 2.1) and multiple gestation (RR, 2.9) presented lesser risks but still were significant, and preexisting medical conditions increased risk as well. Notably, both chronic hypertension and a body mass index (BMI) greater than 30 had a fivefold increased risk (RR, 5.1), and preexisting diabetes presented more than a threefold increased risk (RR, 3.7). The review covered more than 25 million pregnancies in 27 countries.
The etiology of preeclampsia still is not completely understood. There is evidence that underlying decidual inflammation, including increased activated macrophages and decreased uterine natural killer cells (uNK), promotes shallow placentation leading to incomplete uterine spiral artery remodeling, relative placental hypoxia, and progressive release of placental antiangiogenic substances such as soluble fms-like tyrosine kinase 1 (sFlt1) and endoglin (Am J Pathol. 2013 Sep;183[3]:841-56; Reprod Sci. 2015 Nov;22[11]:1461-7). The latter result in systemic endothelial cell damage, reduced endothelial prostacyclin (PGI2), and increased platelet thromboxane A2, triggering vasospasm and increased platelet turnover that ultimately lead to the typical signs and symptoms of preeclampsia.
The research focus traditionally has been on the placenta, but more recently the uterine decidual contribution has received more attention. A recent study published in the Proceedings of the National Academy of Sciences offers evidence that affected women have defective decidualization during and after severe preeclampsia, suggesting that the defect could be detected prior to conception.
Investigators isolated endometrial cells from women at the end of a pregnancy complicated by preeclampsia and found a transcriptional signature that persisted for years. They then linked the defect to impaired cytotrophoblast invasion (Proc Natl Acad Sci. 2017;114[40]:E8468-77). This elegant and provocative study suggests that it might be possible in the future to evaluate the endometrium and try to enhance stromal cell decidualization before pregnancy.
Currently, the rationale for using aspirin to prevent preeclampsia lies with its ability to inhibit platelet production of thromboxane and block NF-kB, a protein complex that plays a role in systemic and/or decidual inflammation. There likely are numerous mechanisms of action, however, including some that improve placentation.
Among the most recent studies on timing and dosage is a systematic review and meta-analysis of 45 randomized controlled trials with 20,909 women randomized to 50-150 mg aspirin daily or to placebo or no treatment. The investigators stratified the results by gestational age at the time of aspirin initiation and found that timing matters. Women who began aspirin at or before 16 weeks had the most significant reductions in preeclampsia (RR, 0.57) and severe preeclampsia (RR, 0.47), as well as fetal growth restriction (RR, 0.56), with a dose-response effect up to 150 mg.
When aspirin was initiated after 16 weeks, there was a much smaller reduction of preeclampsia (RR, 0.81) and no effects for severe preeclampsia or IUGR. Nor was there any dose-response effect (Am J Obstet Gynecol. 2017; 216[2]:110-20.e6).
In contrast, another recent meta-analysis of individual participant data on 32,217 women recruited in 31 randomized controlled trials found no significant difference among women who were randomized before 16 weeks versus those who were randomized at 16 weeks or later (Am J Obstet Gynecol. 2017 Feb;216[2]:121-8.e2). It’s important to note that this analysis covered other antiplatelet agents as well and that it stratified outcomes by gestational age with a slightly later cutoff point.
What do official guidelines say? The USPSTF’s recommendation, issued in 2014, calls for low-dose aspirin at 81 mg/day after 12 weeks’ gestation in women who have one or more high-risk factors, and consideration of such treatment in patients with “several” moderate-risk factors (Ann Intern Med. 2014 Dec 2;161[11]:819-26). In July 2018, ACOG reaffirmed its earlier support for low-dose aspirin in a committee opinion that recommends 81 mg/day beginning at 12-28 weeks’ gestation, optimally before 16 weeks’, for women who have one or more high-risk factors or more than one moderate-risk factor (Obstet Gynecol. 2018 Jul;132[1]:e44-e52).
My own take, based on published literature, including my own research, is that low-dose aspirin reduces the frequency of preeclampsia, particularly cases occurring preterm, as well as related IUGR, by approximately 10%-20% in moderate- and high-risk women. Regarding dose and gestational age for initiation, I have split the difference of what’s reflected in the literature and in guidelines. I advise 122 mg (a tablet-and-a-half) a day, starting at 12-14 weeks’, for patients at high and moderate levels of risk. For patients who are not seen until later, low-dose aspirin can be started up to 28 weeks’ gestation.
Dr. Abbott: Messaging and education to reduce disparities
Black women are not only more likely to develop preeclampsia, but they’re also more likely to have more severe complications and worse outcomes. In one analysis, black women with preeclampsia experienced an almost threefold higher risk of maternal mortality and intrauterine fetal death than did white women with the disorder (Hypertens Pregnancy. 2015 Nov;34[4]:506-15).
At Boston Medical Center, 30% of pregnant women have a diagnosis of preeclampsia or hypertension at term. In addition to 68% identifying as Hispanic/black or black, half of the families we care for have incomes less than $20,000, and 30% are non–English speaking. Low-dose prenatal aspirin is therefore an important tool for reducing racial health disparities as well as disparities created by health literacy, economic status, and language and cultural barriers. At BMC, New England’s largest safety-net hospital, we’ve found that the factors driving health disparities often overlap.
To increase the use of low-dose aspirin for women at moderate to high risk, we marry education about aspirin’s effectiveness and safety with education about the potential severity of hypertension and preeclampsia. We counsel patients who are hospitalized at delivery with gestational or chronic hypertension, or fetal growth restriction, about how preeclampsia can be very serious – contrary to what they’ve experienced or what friends or family may have shared. We also counsel them about signs and symptoms of severe preeclampsia that warrant consulting their provider. And overall, we deliberately use the term “prenatal aspirin” so that, over time and in the broader community, it will become associated with good prenatal care and risk reduction.
To counter perceived risks and dangers that we identified through focus groups and interviews, our patient education materials state that low-dose aspirin in pregnancy will not cause increased bleeding, does not reach the baby’s blood, does not increase the risk of miscarriage, and has not been shown to have negative effects on the baby’s initial development (www.prenatalaspirin.com/education-materials). We try to engage family members whenever possible, and we recognize that the black population has historical reasons to be concerned or suspicious that aspirin might not be safe for them.
Especially for underserved patients who receive prescriptions for low-dose aspirin, we must ensure that pharmacists will dispense the medication. A national survey of pharmacists (not yet published) found that over two-thirds were unaware of the USPSTF guidelines, and that only a minority would feel comfortable dispensing low-dose aspirin during pregnancy. In our community, some pharmacists have told patients to return to their physician and inquire more. Until recently, one of the major pharmacy chains placed a warning label on aspirin bottles being dispensed to women who also had an active prescription for prenatal vitamins.
We are working both with pharmacies and with pharmacy schools to impact the education of current and future pharmacists on guidelines and recommendations for low-dose aspirin prophylaxis. In addition, when I write a prescription for prenatal aspirin, starting at 12 weeks’ whenever possible, I include the message “for the purpose of trying to reduce pregnancy complications.”
Dr. Lockwood is senior vice president at University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa. He said he had no relevant financial disclosures or conflicts of interest. Dr. Abbot is a specialist in maternal-fetal medicine, the director of obstetrics and gynecology, and assistant dean for patient safety and quality improvement education at Boston Medical Center. She also is an associate professor of obstetrics and gynecology at Boston University. She disclosed a grant from the March of Dimes. Email them at [email protected].
Low-dose aspirin for the prevention of preeclampsia has been studied for more than 25 years, often with contradictory and confusing results. Studies have enrolled patients with varying levels of risk, assessed risk differently, and used different definitions of preeclampsia as well as a variety of aspirin dosages and treatment-initiation dates. Undoubtedly, this heterogeneity has made interpretation and comparisons difficult and frustrating.
Recently, systematic reviews and meta-analyses have improved our understanding of the role of low-dose aspirin, providing solid evidence that low-dose aspirin started after the first-trimester reduces the occurrence of preeclampsia in high-risk women. Data also suggest that low-dose aspirin reduces the incidence of fetal growth restriction and preterm birth in these women.
There is reasonable evidence, moreover, that low-dose aspirin provides similar benefit in women with modest levels of risk and that it’s best to begin aspirin use at 12-14 weeks’ gestation rather than later in the second trimester. Finally,
Despite this evidence and current recommendations for low-dose aspirin use by the U.S. Preventive Services Task Force and the American College of Obstetricians and Gynecologists, its use in practice is varied. Obstetricians and other obstetrics providers are not consistently making the recommendation, and pharmacists are not consistently supporting it.
Without more consistent initiation of low-dose aspirin prophylaxis and more consistent adherence, we are losing an opportunity to reduce serious maternal morbidity and mortality. We also are underutilizing an important tool for the reduction of racial and other health disparities relating to preterm birth, maternal death, and other complications of preeclampsia.
Dr. Lockwood: Epidemiology, etiology, and clinical value of aspirin
The use of low-dose aspirin can have a high impact, considering that preeclampsia complicates 3.4% of pregnancies nationally and accounts for at least 9% of maternal deaths (BMJ. 2013 Nov;347:f6564).
Preeclampsia also has been shown in multiple long-term epidemiologic studies to be a strong risk factor for future cardiovascular disease and metabolic disorders in women – especially when it occurs in multiple pregnancies or develops preterm. Moreover, it is associated with stillbirth, intrauterine growth restriction (IUGR), and oligohydramnios in the fetus (BMJ. 2013 Nov;347:f6564).
It is important to remember that criteria for a diagnosis of preeclampsia changed in 2013 such that the detection of proteinuria is no longer required. Preeclampsia is defined today as the new onset of hypertension and proteinuria, or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks in a previously normotensive woman, according to the ACOG Task Force on Hypertension in Pregnancy.
The leading risk factor appears to be previous preeclampsia. In a systematic review and meta-analysis of 92 cohort studies that looked at the pooled relative risk of developing preeclampsia in the presence or absence of 14 commonly reported and accepted risk factors, prior preeclampsia topped the list, putting patients at an eightfold increased risk (relative risk 8.4) (BMJ. 2016 Apr 19;353:i1753).
Nulliparity (relative risk, 2.1) and multiple gestation (RR, 2.9) presented lesser risks but still were significant, and preexisting medical conditions increased risk as well. Notably, both chronic hypertension and a body mass index (BMI) greater than 30 had a fivefold increased risk (RR, 5.1), and preexisting diabetes presented more than a threefold increased risk (RR, 3.7). The review covered more than 25 million pregnancies in 27 countries.
The etiology of preeclampsia still is not completely understood. There is evidence that underlying decidual inflammation, including increased activated macrophages and decreased uterine natural killer cells (uNK), promotes shallow placentation leading to incomplete uterine spiral artery remodeling, relative placental hypoxia, and progressive release of placental antiangiogenic substances such as soluble fms-like tyrosine kinase 1 (sFlt1) and endoglin (Am J Pathol. 2013 Sep;183[3]:841-56; Reprod Sci. 2015 Nov;22[11]:1461-7). The latter result in systemic endothelial cell damage, reduced endothelial prostacyclin (PGI2), and increased platelet thromboxane A2, triggering vasospasm and increased platelet turnover that ultimately lead to the typical signs and symptoms of preeclampsia.
The research focus traditionally has been on the placenta, but more recently the uterine decidual contribution has received more attention. A recent study published in the Proceedings of the National Academy of Sciences offers evidence that affected women have defective decidualization during and after severe preeclampsia, suggesting that the defect could be detected prior to conception.
Investigators isolated endometrial cells from women at the end of a pregnancy complicated by preeclampsia and found a transcriptional signature that persisted for years. They then linked the defect to impaired cytotrophoblast invasion (Proc Natl Acad Sci. 2017;114[40]:E8468-77). This elegant and provocative study suggests that it might be possible in the future to evaluate the endometrium and try to enhance stromal cell decidualization before pregnancy.
Currently, the rationale for using aspirin to prevent preeclampsia lies with its ability to inhibit platelet production of thromboxane and block NF-kB, a protein complex that plays a role in systemic and/or decidual inflammation. There likely are numerous mechanisms of action, however, including some that improve placentation.
Among the most recent studies on timing and dosage is a systematic review and meta-analysis of 45 randomized controlled trials with 20,909 women randomized to 50-150 mg aspirin daily or to placebo or no treatment. The investigators stratified the results by gestational age at the time of aspirin initiation and found that timing matters. Women who began aspirin at or before 16 weeks had the most significant reductions in preeclampsia (RR, 0.57) and severe preeclampsia (RR, 0.47), as well as fetal growth restriction (RR, 0.56), with a dose-response effect up to 150 mg.
When aspirin was initiated after 16 weeks, there was a much smaller reduction of preeclampsia (RR, 0.81) and no effects for severe preeclampsia or IUGR. Nor was there any dose-response effect (Am J Obstet Gynecol. 2017; 216[2]:110-20.e6).
In contrast, another recent meta-analysis of individual participant data on 32,217 women recruited in 31 randomized controlled trials found no significant difference among women who were randomized before 16 weeks versus those who were randomized at 16 weeks or later (Am J Obstet Gynecol. 2017 Feb;216[2]:121-8.e2). It’s important to note that this analysis covered other antiplatelet agents as well and that it stratified outcomes by gestational age with a slightly later cutoff point.
What do official guidelines say? The USPSTF’s recommendation, issued in 2014, calls for low-dose aspirin at 81 mg/day after 12 weeks’ gestation in women who have one or more high-risk factors, and consideration of such treatment in patients with “several” moderate-risk factors (Ann Intern Med. 2014 Dec 2;161[11]:819-26). In July 2018, ACOG reaffirmed its earlier support for low-dose aspirin in a committee opinion that recommends 81 mg/day beginning at 12-28 weeks’ gestation, optimally before 16 weeks’, for women who have one or more high-risk factors or more than one moderate-risk factor (Obstet Gynecol. 2018 Jul;132[1]:e44-e52).
My own take, based on published literature, including my own research, is that low-dose aspirin reduces the frequency of preeclampsia, particularly cases occurring preterm, as well as related IUGR, by approximately 10%-20% in moderate- and high-risk women. Regarding dose and gestational age for initiation, I have split the difference of what’s reflected in the literature and in guidelines. I advise 122 mg (a tablet-and-a-half) a day, starting at 12-14 weeks’, for patients at high and moderate levels of risk. For patients who are not seen until later, low-dose aspirin can be started up to 28 weeks’ gestation.
Dr. Abbott: Messaging and education to reduce disparities
Black women are not only more likely to develop preeclampsia, but they’re also more likely to have more severe complications and worse outcomes. In one analysis, black women with preeclampsia experienced an almost threefold higher risk of maternal mortality and intrauterine fetal death than did white women with the disorder (Hypertens Pregnancy. 2015 Nov;34[4]:506-15).
At Boston Medical Center, 30% of pregnant women have a diagnosis of preeclampsia or hypertension at term. In addition to 68% identifying as Hispanic/black or black, half of the families we care for have incomes less than $20,000, and 30% are non–English speaking. Low-dose prenatal aspirin is therefore an important tool for reducing racial health disparities as well as disparities created by health literacy, economic status, and language and cultural barriers. At BMC, New England’s largest safety-net hospital, we’ve found that the factors driving health disparities often overlap.
To increase the use of low-dose aspirin for women at moderate to high risk, we marry education about aspirin’s effectiveness and safety with education about the potential severity of hypertension and preeclampsia. We counsel patients who are hospitalized at delivery with gestational or chronic hypertension, or fetal growth restriction, about how preeclampsia can be very serious – contrary to what they’ve experienced or what friends or family may have shared. We also counsel them about signs and symptoms of severe preeclampsia that warrant consulting their provider. And overall, we deliberately use the term “prenatal aspirin” so that, over time and in the broader community, it will become associated with good prenatal care and risk reduction.
To counter perceived risks and dangers that we identified through focus groups and interviews, our patient education materials state that low-dose aspirin in pregnancy will not cause increased bleeding, does not reach the baby’s blood, does not increase the risk of miscarriage, and has not been shown to have negative effects on the baby’s initial development (www.prenatalaspirin.com/education-materials). We try to engage family members whenever possible, and we recognize that the black population has historical reasons to be concerned or suspicious that aspirin might not be safe for them.
Especially for underserved patients who receive prescriptions for low-dose aspirin, we must ensure that pharmacists will dispense the medication. A national survey of pharmacists (not yet published) found that over two-thirds were unaware of the USPSTF guidelines, and that only a minority would feel comfortable dispensing low-dose aspirin during pregnancy. In our community, some pharmacists have told patients to return to their physician and inquire more. Until recently, one of the major pharmacy chains placed a warning label on aspirin bottles being dispensed to women who also had an active prescription for prenatal vitamins.
We are working both with pharmacies and with pharmacy schools to impact the education of current and future pharmacists on guidelines and recommendations for low-dose aspirin prophylaxis. In addition, when I write a prescription for prenatal aspirin, starting at 12 weeks’ whenever possible, I include the message “for the purpose of trying to reduce pregnancy complications.”
Dr. Lockwood is senior vice president at University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa. He said he had no relevant financial disclosures or conflicts of interest. Dr. Abbot is a specialist in maternal-fetal medicine, the director of obstetrics and gynecology, and assistant dean for patient safety and quality improvement education at Boston Medical Center. She also is an associate professor of obstetrics and gynecology at Boston University. She disclosed a grant from the March of Dimes. Email them at [email protected].
Aspirin has myriad benefits
Some of our readers might remember the old saying, “Take two aspirin and call me in the morning,” as advice physicians gave to patients experiencing a minor malady. Aspirin often has been called a “wonder drug” as its uses continue to expand. From its first recorded use in the Ebers papyrus as an anti-inflammatory agent, to its first use in a clinical trial showing that it induces remission of fever and joint inflammation, to the discovery that it could prevent death from heart attack, to its anticancer properties, aspirin remains one of the most researched drugs in use today. According to ClinicalTrials.gov, there are over 465 active and nearly 1,000 completed aspirin-related clinical trials around the world.
Despite its myriad benefits, aspirin has been linked to bleeding, nausea, and gastrointestinal ulcers. Additionally, more research is needed to determine the risks/benefits of daily aspirin in younger adults (under age 50 years) or older adults (over age 70 years), although the ASPREE (Aspirin in Reducing Events in the Elderly) trial, expected to be completed in 2019, is working to determine the effects of daily low-dose aspirin (100 mg) on the health of people over age 65.
It is tempting to consider aspirin one of modern medicine’s so-called silver bullets, and, for women with a history of gestational hypertension and preeclampsia, it just might be. Aspirin use, especially daily aspirin, is typically not recommended during pregnancy, and most ob.gyns. will include aspirin on the “do not take” list they give to their patients during prenatal examinations. Women at risk for developing preeclampsia are the exceptions to this general rule, and a number of clinical studies have indicated that use of low-dose aspirin can help prevent disease as well as secondary outcomes for mother (i.e., placental abruption, antepartum hemorrhage) and baby (i.e., intrauterine growth restriction, stillbirth). In addition, aspirin is an easily obtainable, low-cost preventive measure for any patient at high risk.
To discuss the value of low-dose aspirin to prevent preeclampsia and how ob.gyns. can educate their patients and other health care professionals about its benefits, we have invited Charles J. Lockwood, MD, MHCM, senior vice president of University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa, and Jodi F. Abbott, MD, MSc, MHCM, director of obstetrics and gynecology at Boston Medical Center, and associate professor of obstetrics and gynecology at Boston University, to coauthor this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Some of our readers might remember the old saying, “Take two aspirin and call me in the morning,” as advice physicians gave to patients experiencing a minor malady. Aspirin often has been called a “wonder drug” as its uses continue to expand. From its first recorded use in the Ebers papyrus as an anti-inflammatory agent, to its first use in a clinical trial showing that it induces remission of fever and joint inflammation, to the discovery that it could prevent death from heart attack, to its anticancer properties, aspirin remains one of the most researched drugs in use today. According to ClinicalTrials.gov, there are over 465 active and nearly 1,000 completed aspirin-related clinical trials around the world.
Despite its myriad benefits, aspirin has been linked to bleeding, nausea, and gastrointestinal ulcers. Additionally, more research is needed to determine the risks/benefits of daily aspirin in younger adults (under age 50 years) or older adults (over age 70 years), although the ASPREE (Aspirin in Reducing Events in the Elderly) trial, expected to be completed in 2019, is working to determine the effects of daily low-dose aspirin (100 mg) on the health of people over age 65.
It is tempting to consider aspirin one of modern medicine’s so-called silver bullets, and, for women with a history of gestational hypertension and preeclampsia, it just might be. Aspirin use, especially daily aspirin, is typically not recommended during pregnancy, and most ob.gyns. will include aspirin on the “do not take” list they give to their patients during prenatal examinations. Women at risk for developing preeclampsia are the exceptions to this general rule, and a number of clinical studies have indicated that use of low-dose aspirin can help prevent disease as well as secondary outcomes for mother (i.e., placental abruption, antepartum hemorrhage) and baby (i.e., intrauterine growth restriction, stillbirth). In addition, aspirin is an easily obtainable, low-cost preventive measure for any patient at high risk.
To discuss the value of low-dose aspirin to prevent preeclampsia and how ob.gyns. can educate their patients and other health care professionals about its benefits, we have invited Charles J. Lockwood, MD, MHCM, senior vice president of University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa, and Jodi F. Abbott, MD, MSc, MHCM, director of obstetrics and gynecology at Boston Medical Center, and associate professor of obstetrics and gynecology at Boston University, to coauthor this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Some of our readers might remember the old saying, “Take two aspirin and call me in the morning,” as advice physicians gave to patients experiencing a minor malady. Aspirin often has been called a “wonder drug” as its uses continue to expand. From its first recorded use in the Ebers papyrus as an anti-inflammatory agent, to its first use in a clinical trial showing that it induces remission of fever and joint inflammation, to the discovery that it could prevent death from heart attack, to its anticancer properties, aspirin remains one of the most researched drugs in use today. According to ClinicalTrials.gov, there are over 465 active and nearly 1,000 completed aspirin-related clinical trials around the world.
Despite its myriad benefits, aspirin has been linked to bleeding, nausea, and gastrointestinal ulcers. Additionally, more research is needed to determine the risks/benefits of daily aspirin in younger adults (under age 50 years) or older adults (over age 70 years), although the ASPREE (Aspirin in Reducing Events in the Elderly) trial, expected to be completed in 2019, is working to determine the effects of daily low-dose aspirin (100 mg) on the health of people over age 65.
It is tempting to consider aspirin one of modern medicine’s so-called silver bullets, and, for women with a history of gestational hypertension and preeclampsia, it just might be. Aspirin use, especially daily aspirin, is typically not recommended during pregnancy, and most ob.gyns. will include aspirin on the “do not take” list they give to their patients during prenatal examinations. Women at risk for developing preeclampsia are the exceptions to this general rule, and a number of clinical studies have indicated that use of low-dose aspirin can help prevent disease as well as secondary outcomes for mother (i.e., placental abruption, antepartum hemorrhage) and baby (i.e., intrauterine growth restriction, stillbirth). In addition, aspirin is an easily obtainable, low-cost preventive measure for any patient at high risk.
To discuss the value of low-dose aspirin to prevent preeclampsia and how ob.gyns. can educate their patients and other health care professionals about its benefits, we have invited Charles J. Lockwood, MD, MHCM, senior vice president of University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa, and Jodi F. Abbott, MD, MSc, MHCM, director of obstetrics and gynecology at Boston Medical Center, and associate professor of obstetrics and gynecology at Boston University, to coauthor this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
The diagnosis and surgical repair of vesicovaginal fistula
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
A rare but debilitating diagnosis in developed countries
Vesicovaginal fistula continues to be the most common form of genitourinary fistula, with resultant diminishment in quality of life secondary to physical and psychosocial distress. While it has been reported that 1 million women in Sub-Saharan Africa have untreated vesicovaginal fistula secondary to obstetric trauma, vesicovaginal fistulas are relatively rare in the United States. Per the United States National Hospital Discharge Survey, in 2007, fewer than 5,000 vesicovaginal fistula repairs were performed out of over 2.3 million procedures involving the female urinary and genital system.
The rarity of the diagnosis is also reflected in data collected from the English National Health Service, where vesicovaginal fistula occurred in 1 in 788 hysterectomies (although more common in radical hysterectomy, at 1 in 87).
In a recent systematic review and meta-analysis on the management of vesicovaginal fistulas in women following benign gynecologic surgery, Bodner-Adler et al. evaluated 282 full-text articles to identify 124 studies for inclusion (PLoS One. 2017 Feb 22;12[2]:e0171554). Only ten studies involved solely conservative management with prolonged bladder drainage. Dismal success was noted: 8%. Surgery was performed in 96.4% of cases (1379/1430); transvaginal in 39%, transabdominal/transvesical in 36%, laparoscopic/robotic approach in 15%, and transabdominal/transvaginal in 3%. Overall success rate in these surgical cases was 97.98% (95% confidence interval, 96.13%-99.29%); with similar procedural success: transvaginal, 89.96%-97.49%; transabdominal/transvesical, 94.55%-99.18%; and laparoscopic/robotic, 96.85%-99.99%. Studies are very limited comparing the various surgical techniques, with only one study comparing transvaginal, transabdominal, and laparoscopic approaches. Interestingly, in this study, the laparoscopic approach was noted to have the least morbidity (Ou CS et al. J Lapraoendosc Adv Surg Tech A. 2004 Feb;14(1):17-21).
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Alan D. Garely, MD, FACOG, FACS, of the Icahn School of Medicine at Mount Sinai, New York. Dr. Garely has served on the board of directors for the American Urogynecologic Society, serves as chair of the gynecology and obstetrics advisory board for the American College of Surgeons, and has published numerous papers and book chapters.
It is a pleasure to welcome Dr. Garely to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He has no disclosures related to this column.
Vesicovaginal fistula continues to be the most common form of genitourinary fistula, with resultant diminishment in quality of life secondary to physical and psychosocial distress. While it has been reported that 1 million women in Sub-Saharan Africa have untreated vesicovaginal fistula secondary to obstetric trauma, vesicovaginal fistulas are relatively rare in the United States. Per the United States National Hospital Discharge Survey, in 2007, fewer than 5,000 vesicovaginal fistula repairs were performed out of over 2.3 million procedures involving the female urinary and genital system.
The rarity of the diagnosis is also reflected in data collected from the English National Health Service, where vesicovaginal fistula occurred in 1 in 788 hysterectomies (although more common in radical hysterectomy, at 1 in 87).
In a recent systematic review and meta-analysis on the management of vesicovaginal fistulas in women following benign gynecologic surgery, Bodner-Adler et al. evaluated 282 full-text articles to identify 124 studies for inclusion (PLoS One. 2017 Feb 22;12[2]:e0171554). Only ten studies involved solely conservative management with prolonged bladder drainage. Dismal success was noted: 8%. Surgery was performed in 96.4% of cases (1379/1430); transvaginal in 39%, transabdominal/transvesical in 36%, laparoscopic/robotic approach in 15%, and transabdominal/transvaginal in 3%. Overall success rate in these surgical cases was 97.98% (95% confidence interval, 96.13%-99.29%); with similar procedural success: transvaginal, 89.96%-97.49%; transabdominal/transvesical, 94.55%-99.18%; and laparoscopic/robotic, 96.85%-99.99%. Studies are very limited comparing the various surgical techniques, with only one study comparing transvaginal, transabdominal, and laparoscopic approaches. Interestingly, in this study, the laparoscopic approach was noted to have the least morbidity (Ou CS et al. J Lapraoendosc Adv Surg Tech A. 2004 Feb;14(1):17-21).
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Alan D. Garely, MD, FACOG, FACS, of the Icahn School of Medicine at Mount Sinai, New York. Dr. Garely has served on the board of directors for the American Urogynecologic Society, serves as chair of the gynecology and obstetrics advisory board for the American College of Surgeons, and has published numerous papers and book chapters.
It is a pleasure to welcome Dr. Garely to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He has no disclosures related to this column.
Vesicovaginal fistula continues to be the most common form of genitourinary fistula, with resultant diminishment in quality of life secondary to physical and psychosocial distress. While it has been reported that 1 million women in Sub-Saharan Africa have untreated vesicovaginal fistula secondary to obstetric trauma, vesicovaginal fistulas are relatively rare in the United States. Per the United States National Hospital Discharge Survey, in 2007, fewer than 5,000 vesicovaginal fistula repairs were performed out of over 2.3 million procedures involving the female urinary and genital system.
The rarity of the diagnosis is also reflected in data collected from the English National Health Service, where vesicovaginal fistula occurred in 1 in 788 hysterectomies (although more common in radical hysterectomy, at 1 in 87).
In a recent systematic review and meta-analysis on the management of vesicovaginal fistulas in women following benign gynecologic surgery, Bodner-Adler et al. evaluated 282 full-text articles to identify 124 studies for inclusion (PLoS One. 2017 Feb 22;12[2]:e0171554). Only ten studies involved solely conservative management with prolonged bladder drainage. Dismal success was noted: 8%. Surgery was performed in 96.4% of cases (1379/1430); transvaginal in 39%, transabdominal/transvesical in 36%, laparoscopic/robotic approach in 15%, and transabdominal/transvaginal in 3%. Overall success rate in these surgical cases was 97.98% (95% confidence interval, 96.13%-99.29%); with similar procedural success: transvaginal, 89.96%-97.49%; transabdominal/transvesical, 94.55%-99.18%; and laparoscopic/robotic, 96.85%-99.99%. Studies are very limited comparing the various surgical techniques, with only one study comparing transvaginal, transabdominal, and laparoscopic approaches. Interestingly, in this study, the laparoscopic approach was noted to have the least morbidity (Ou CS et al. J Lapraoendosc Adv Surg Tech A. 2004 Feb;14(1):17-21).
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Alan D. Garely, MD, FACOG, FACS, of the Icahn School of Medicine at Mount Sinai, New York. Dr. Garely has served on the board of directors for the American Urogynecologic Society, serves as chair of the gynecology and obstetrics advisory board for the American College of Surgeons, and has published numerous papers and book chapters.
It is a pleasure to welcome Dr. Garely to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He has no disclosures related to this column.
How congenital heart disease affects brain development
Congenital heart disease (CHD) is the most common congenital anomaly, with an estimated incidence of 6-12 per 1,000 live births. It is also the congenital anomaly that most often leads to death or significant morbidity. Advances in surgical procedures and operating room care as well as specialized care in the ICU have led to significant improvements in survival over the past 10-20 years – even for the most complex cases of CHD. We now expect the majority of newborns with CHD not only to survive, but to grow up into adulthood.
The focus of clinical research has thus transitioned from survival to issues of long-term morbidity and outcomes, and the more recent literature has clearly shown us that children with CHD are at high risk of learning disabilities and other neurodevelopmental abnormalities. The prevalence of impairment rises with the complexity of CHD, from a prevalence of approximately 20% in mild CHD to as much as 75% in severe CHD. Almost all neonates and infants who undergo palliative surgical procedures have neurodevelopmental impairments.
The neurobehavioral “signature” of CHD includes cognitive defects (usually mild), short attention span, fine and gross motor delays, speech and language delays, visual motor integration, and executive function deficits. Executive function deficits and attention deficits are among the problems that often do not present in children until they reach middle school and beyond, when they are expected to learn more complicated material and handle more complex tasks. Long-term surveillance and care have thus become a major focus at our institution and others throughout the country.
At the same time, evidence has increased in the past 5-10 years that adverse neurodevelopmental outcomes in children with complex CHD may stem from genetic factors as well as compromise to the brain in utero because of altered blood flow, compromise at the time of delivery, and insults during and after corrective or palliative surgery. Surgical strategies and operating room teams have become significantly better at protecting the brain, and new research now is directed toward understanding the neurologic abnormalities that are present in newborns prior to surgical intervention.
Increasingly, researchers are now focused on looking at the in utero origins of brain impairments in children with CHD and trying to understand specific prenatal causes, mechanisms, and potentially modifiable factors. We’re asking what we can do during pregnancy to improve neurodevelopmental outcomes.
Impaired brain growth
The question of how CHD affects blood flow to the fetal brain is an important one. We found some time ago in a study using Doppler ultrasound that 44% of fetuses with CHD had blood flow abnormalities in the middle cerebral artery at some point in the late second or third trimester, suggesting that the blood vessels had dilated to allow more cerebral perfusion. This phenomenon, termed “brain sparing,” is believed to be an autoregulatory mechanism that occurs as a result of diminished oxygen delivery or inadequate blood flow to the brain (Pediatr Cardiol. 2003 Jan;24[5]:436-43).
Subsequent studies have similarly documented abnormal cerebral blood flow in fetuses with various types of congenital heart lesions. What is left to be determined is whether this autoregulatory mechanism is adequate to maintain perfusion in the presence of specific, high-risk CHD.
Abnormalities were more often seen in CHD with obstructed aortic flow, such as hypoplastic left heart syndrome (HLHS) in which the aorta is perfused retrograde through the fetal ductus arteriosus (Circulation. 2010 Jan 4;121:26-33).
Other fetal imaging studies have similarly demonstrated a progressive third-trimester decrease in both cortical gray and white matter and in gyrification (cortical folding) (Cereb Cortex. 2013;23:2932-43), as well as decreased cerebral oxygen delivery and consumption (Circulation. 2015;131:1313-23) in fetuses with severe CHD. It appears that the brain may start out normal in size, but in the third trimester, the accelerated metabolic demands that come with rapid growth and development are not sufficiently met by the fetal cardiovascular circulation in CHD.
In the newborn with CHD, preoperative brain imaging studies have demonstrated structural abnormalities suggesting delayed development (for example, microcephaly and a widened operculum), microstructural abnormalities suggesting abnormal myelination and neuroaxonal development, and lower brain maturity scores (a composite score that combines multiple factors, such as myelination and cortical in-folding, to represent “brain age”).
Moreover, some of the newborn brain imaging studies have correlated brain MRI findings with neonatal neurodevelopmental assessments. For instance, investigators found that full-term newborns with CHD had decreased gray matter brain volume and increased cerebrospinal fluid volume and that these impairments were associated with poor behavioral state regulation and poor visual orienting (J Pediatr. 2014;164:1121-7).
Interestingly, it has been found that the full-term baby with specific complex CHD, including newborns with single ventricle CHD or transposition of the great arteries, is more likely to have a brain maturity score that is equivalent to that of a baby born at 35 weeks’ gestation. This means that, in some infants with CHD, the brain has lagged in growth by about a month, resulting in a pattern of disturbed development and subsequent injury that is similar to that of premature infants.
It also means that infants with CHD and an immature brain are especially vulnerable to brain injury when open-heart surgery is needed. In short, we now appreciate that the brain in patients with CHD is likely more fragile than we previously thought – and that this fragility is prenatal in its origins.
Delivery room planning
Ideally, our goal is to find ways of changing the circulation in utero to improve cerebral oxygenation and blood flow, and, consequently, improve brain development and long-term neurocognitive function. Despite significant efforts in this area, we’re not there yet.
Examples of strategies that are being tested include catheter intervention to open the aortic valve in utero for fetuses with critical aortic stenosis. This procedure currently is being performed to try to prevent progression of the valve abnormality to HLHS, but it has not been determined whether the intervention affects cerebral blood flow. Maternal oxygen therapy has been shown to change cerebral blood flow in the short term for fetuses with HLHS, but its long-term use has not been studied. At the time of birth, to prevent injury in the potentially more fragile brain of the newborn with CHD, what we can do is to identify those fetuses who are more likely to be at risk for hypoxia low cardiac output and hemodynamic compromise in the delivery room, and plan for specialized delivery room and perinatal management beyond standard neonatal care.
Most newborns with CHD are assigned to Level 1; they have no predicted risk of compromise in the delivery room – or even in the first couple weeks of life – and can deliver at a local hospital with neonatal evaluation and then consult with the pediatric cardiologist. Defects include shunt lesions such as septal defects or mild valve abnormalities.
Patients assigned to Level 2 have minimal risk of compromise in the delivery room but are expected to require postnatal surgery, cardiac catheterization, or another procedure before going home. They can be stabilized by the neonatologist, usually with initiation of a prostaglandin infusion, before transfer to the cardiac center for the planned intervention. Defects include single ventricle CHD and severe Tetralogy of Fallot.
Fetuses assigned to Level 3 and Level 4 are expected to have hemodynamic instability at cord clamping, requiring immediate specialty care in the delivery room that is likely to include urgent cardiac catheterization or surgical intervention. These defects are rare and include diagnoses such as transposition of the great arteries, HLHS with a restrictive or closed foramen ovale, and CHD with associated heart failure and hydrops.
We have found that fetal echocardiography accurately predicts postnatal risk and the need for specialized delivery room care in newborns diagnosed in utero with CHD and that level-of-care protocols ensure safe delivery and optimize fetal outcomes (J Am Soc Echocardiogr. 2015;28:1339-49; Am J Cardiol. 2013;111:737-47).
Such delivery planning, which is coordinated between obstetric, neonatal, cardiology, and surgical services with specialty teams as needed (for example, cardiac intensive care, interventional cardiology, and cardiac surgery), is recommended in a 2014 AHA statement on the diagnosis and treatment of fetal cardiac disease. In recent years it has become the standard of care in many health systems (Circulation. 2014;129[21]:2183-242).
The effect of maternal stress on the in utero environment is also getting increased attention in pediatric cardiology. Alterations in neurocognitive development and fetal and child cardiovascular health are likely to be associated with maternal stress during pregnancy, and studies have shown that maternal stress is high with prenatal diagnoses of CHD. We have to ask: Is stress a modifiable risk factor? There must be ways in which we can do better with prenatal counseling and support after a fetal diagnosis of CHD.
Screening for CHD
Initiating strategies to improve neurodevelopmental outcomes in infants with CHD rests partly on identifying babies with CHD before birth through improved fetal cardiac screening. Research cited in the 2014 AHA statement indicates that nearly all women giving birth to babies with CHD in the United States have obstetric ultrasound examinations in the second or third trimesters, but that only about 30% of the fetuses are diagnosed prenatally.
Current indications for referral for a fetal echocardiogram – in addition to suspicion of a structural heart abnormality on obstetric ultrasound – include maternal factors, such as diabetes mellitus, that raise the risk of CHD above the baseline population risk for low-risk pregnancies.
Women with pregestational diabetes mellitus have a nearly fivefold increase in CHD, compared with the general population (3%-5%), and should be referred for fetal echocardiography. Women with gestational diabetes mellitus have no or minimally increased risk for fetal CHD, but it has been shown that there is an increased risk for cardiac hypertrophy – particularly late in gestation – if glycemic levels are poorly controlled. The 2014 AHA guidelines recommend that fetal echocardiographic evaluation be considered in those who have HbA1c levels greater than 6% in the second half of pregnancy.
Dr. Mary T. Donofrio is a pediatric cardiologist and director of the fetal heart program and critical care delivery program at Children’s National Medical Center, Washington. She reported that she has no disclosures relevant to this article.
Congenital heart disease (CHD) is the most common congenital anomaly, with an estimated incidence of 6-12 per 1,000 live births. It is also the congenital anomaly that most often leads to death or significant morbidity. Advances in surgical procedures and operating room care as well as specialized care in the ICU have led to significant improvements in survival over the past 10-20 years – even for the most complex cases of CHD. We now expect the majority of newborns with CHD not only to survive, but to grow up into adulthood.
The focus of clinical research has thus transitioned from survival to issues of long-term morbidity and outcomes, and the more recent literature has clearly shown us that children with CHD are at high risk of learning disabilities and other neurodevelopmental abnormalities. The prevalence of impairment rises with the complexity of CHD, from a prevalence of approximately 20% in mild CHD to as much as 75% in severe CHD. Almost all neonates and infants who undergo palliative surgical procedures have neurodevelopmental impairments.
The neurobehavioral “signature” of CHD includes cognitive defects (usually mild), short attention span, fine and gross motor delays, speech and language delays, visual motor integration, and executive function deficits. Executive function deficits and attention deficits are among the problems that often do not present in children until they reach middle school and beyond, when they are expected to learn more complicated material and handle more complex tasks. Long-term surveillance and care have thus become a major focus at our institution and others throughout the country.
At the same time, evidence has increased in the past 5-10 years that adverse neurodevelopmental outcomes in children with complex CHD may stem from genetic factors as well as compromise to the brain in utero because of altered blood flow, compromise at the time of delivery, and insults during and after corrective or palliative surgery. Surgical strategies and operating room teams have become significantly better at protecting the brain, and new research now is directed toward understanding the neurologic abnormalities that are present in newborns prior to surgical intervention.
Increasingly, researchers are now focused on looking at the in utero origins of brain impairments in children with CHD and trying to understand specific prenatal causes, mechanisms, and potentially modifiable factors. We’re asking what we can do during pregnancy to improve neurodevelopmental outcomes.
Impaired brain growth
The question of how CHD affects blood flow to the fetal brain is an important one. We found some time ago in a study using Doppler ultrasound that 44% of fetuses with CHD had blood flow abnormalities in the middle cerebral artery at some point in the late second or third trimester, suggesting that the blood vessels had dilated to allow more cerebral perfusion. This phenomenon, termed “brain sparing,” is believed to be an autoregulatory mechanism that occurs as a result of diminished oxygen delivery or inadequate blood flow to the brain (Pediatr Cardiol. 2003 Jan;24[5]:436-43).
Subsequent studies have similarly documented abnormal cerebral blood flow in fetuses with various types of congenital heart lesions. What is left to be determined is whether this autoregulatory mechanism is adequate to maintain perfusion in the presence of specific, high-risk CHD.
Abnormalities were more often seen in CHD with obstructed aortic flow, such as hypoplastic left heart syndrome (HLHS) in which the aorta is perfused retrograde through the fetal ductus arteriosus (Circulation. 2010 Jan 4;121:26-33).
Other fetal imaging studies have similarly demonstrated a progressive third-trimester decrease in both cortical gray and white matter and in gyrification (cortical folding) (Cereb Cortex. 2013;23:2932-43), as well as decreased cerebral oxygen delivery and consumption (Circulation. 2015;131:1313-23) in fetuses with severe CHD. It appears that the brain may start out normal in size, but in the third trimester, the accelerated metabolic demands that come with rapid growth and development are not sufficiently met by the fetal cardiovascular circulation in CHD.
In the newborn with CHD, preoperative brain imaging studies have demonstrated structural abnormalities suggesting delayed development (for example, microcephaly and a widened operculum), microstructural abnormalities suggesting abnormal myelination and neuroaxonal development, and lower brain maturity scores (a composite score that combines multiple factors, such as myelination and cortical in-folding, to represent “brain age”).
Moreover, some of the newborn brain imaging studies have correlated brain MRI findings with neonatal neurodevelopmental assessments. For instance, investigators found that full-term newborns with CHD had decreased gray matter brain volume and increased cerebrospinal fluid volume and that these impairments were associated with poor behavioral state regulation and poor visual orienting (J Pediatr. 2014;164:1121-7).
Interestingly, it has been found that the full-term baby with specific complex CHD, including newborns with single ventricle CHD or transposition of the great arteries, is more likely to have a brain maturity score that is equivalent to that of a baby born at 35 weeks’ gestation. This means that, in some infants with CHD, the brain has lagged in growth by about a month, resulting in a pattern of disturbed development and subsequent injury that is similar to that of premature infants.
It also means that infants with CHD and an immature brain are especially vulnerable to brain injury when open-heart surgery is needed. In short, we now appreciate that the brain in patients with CHD is likely more fragile than we previously thought – and that this fragility is prenatal in its origins.
Delivery room planning
Ideally, our goal is to find ways of changing the circulation in utero to improve cerebral oxygenation and blood flow, and, consequently, improve brain development and long-term neurocognitive function. Despite significant efforts in this area, we’re not there yet.
Examples of strategies that are being tested include catheter intervention to open the aortic valve in utero for fetuses with critical aortic stenosis. This procedure currently is being performed to try to prevent progression of the valve abnormality to HLHS, but it has not been determined whether the intervention affects cerebral blood flow. Maternal oxygen therapy has been shown to change cerebral blood flow in the short term for fetuses with HLHS, but its long-term use has not been studied. At the time of birth, to prevent injury in the potentially more fragile brain of the newborn with CHD, what we can do is to identify those fetuses who are more likely to be at risk for hypoxia low cardiac output and hemodynamic compromise in the delivery room, and plan for specialized delivery room and perinatal management beyond standard neonatal care.
Most newborns with CHD are assigned to Level 1; they have no predicted risk of compromise in the delivery room – or even in the first couple weeks of life – and can deliver at a local hospital with neonatal evaluation and then consult with the pediatric cardiologist. Defects include shunt lesions such as septal defects or mild valve abnormalities.
Patients assigned to Level 2 have minimal risk of compromise in the delivery room but are expected to require postnatal surgery, cardiac catheterization, or another procedure before going home. They can be stabilized by the neonatologist, usually with initiation of a prostaglandin infusion, before transfer to the cardiac center for the planned intervention. Defects include single ventricle CHD and severe Tetralogy of Fallot.
Fetuses assigned to Level 3 and Level 4 are expected to have hemodynamic instability at cord clamping, requiring immediate specialty care in the delivery room that is likely to include urgent cardiac catheterization or surgical intervention. These defects are rare and include diagnoses such as transposition of the great arteries, HLHS with a restrictive or closed foramen ovale, and CHD with associated heart failure and hydrops.
We have found that fetal echocardiography accurately predicts postnatal risk and the need for specialized delivery room care in newborns diagnosed in utero with CHD and that level-of-care protocols ensure safe delivery and optimize fetal outcomes (J Am Soc Echocardiogr. 2015;28:1339-49; Am J Cardiol. 2013;111:737-47).
Such delivery planning, which is coordinated between obstetric, neonatal, cardiology, and surgical services with specialty teams as needed (for example, cardiac intensive care, interventional cardiology, and cardiac surgery), is recommended in a 2014 AHA statement on the diagnosis and treatment of fetal cardiac disease. In recent years it has become the standard of care in many health systems (Circulation. 2014;129[21]:2183-242).
The effect of maternal stress on the in utero environment is also getting increased attention in pediatric cardiology. Alterations in neurocognitive development and fetal and child cardiovascular health are likely to be associated with maternal stress during pregnancy, and studies have shown that maternal stress is high with prenatal diagnoses of CHD. We have to ask: Is stress a modifiable risk factor? There must be ways in which we can do better with prenatal counseling and support after a fetal diagnosis of CHD.
Screening for CHD
Initiating strategies to improve neurodevelopmental outcomes in infants with CHD rests partly on identifying babies with CHD before birth through improved fetal cardiac screening. Research cited in the 2014 AHA statement indicates that nearly all women giving birth to babies with CHD in the United States have obstetric ultrasound examinations in the second or third trimesters, but that only about 30% of the fetuses are diagnosed prenatally.
Current indications for referral for a fetal echocardiogram – in addition to suspicion of a structural heart abnormality on obstetric ultrasound – include maternal factors, such as diabetes mellitus, that raise the risk of CHD above the baseline population risk for low-risk pregnancies.
Women with pregestational diabetes mellitus have a nearly fivefold increase in CHD, compared with the general population (3%-5%), and should be referred for fetal echocardiography. Women with gestational diabetes mellitus have no or minimally increased risk for fetal CHD, but it has been shown that there is an increased risk for cardiac hypertrophy – particularly late in gestation – if glycemic levels are poorly controlled. The 2014 AHA guidelines recommend that fetal echocardiographic evaluation be considered in those who have HbA1c levels greater than 6% in the second half of pregnancy.
Dr. Mary T. Donofrio is a pediatric cardiologist and director of the fetal heart program and critical care delivery program at Children’s National Medical Center, Washington. She reported that she has no disclosures relevant to this article.
Congenital heart disease (CHD) is the most common congenital anomaly, with an estimated incidence of 6-12 per 1,000 live births. It is also the congenital anomaly that most often leads to death or significant morbidity. Advances in surgical procedures and operating room care as well as specialized care in the ICU have led to significant improvements in survival over the past 10-20 years – even for the most complex cases of CHD. We now expect the majority of newborns with CHD not only to survive, but to grow up into adulthood.
The focus of clinical research has thus transitioned from survival to issues of long-term morbidity and outcomes, and the more recent literature has clearly shown us that children with CHD are at high risk of learning disabilities and other neurodevelopmental abnormalities. The prevalence of impairment rises with the complexity of CHD, from a prevalence of approximately 20% in mild CHD to as much as 75% in severe CHD. Almost all neonates and infants who undergo palliative surgical procedures have neurodevelopmental impairments.
The neurobehavioral “signature” of CHD includes cognitive defects (usually mild), short attention span, fine and gross motor delays, speech and language delays, visual motor integration, and executive function deficits. Executive function deficits and attention deficits are among the problems that often do not present in children until they reach middle school and beyond, when they are expected to learn more complicated material and handle more complex tasks. Long-term surveillance and care have thus become a major focus at our institution and others throughout the country.
At the same time, evidence has increased in the past 5-10 years that adverse neurodevelopmental outcomes in children with complex CHD may stem from genetic factors as well as compromise to the brain in utero because of altered blood flow, compromise at the time of delivery, and insults during and after corrective or palliative surgery. Surgical strategies and operating room teams have become significantly better at protecting the brain, and new research now is directed toward understanding the neurologic abnormalities that are present in newborns prior to surgical intervention.
Increasingly, researchers are now focused on looking at the in utero origins of brain impairments in children with CHD and trying to understand specific prenatal causes, mechanisms, and potentially modifiable factors. We’re asking what we can do during pregnancy to improve neurodevelopmental outcomes.
Impaired brain growth
The question of how CHD affects blood flow to the fetal brain is an important one. We found some time ago in a study using Doppler ultrasound that 44% of fetuses with CHD had blood flow abnormalities in the middle cerebral artery at some point in the late second or third trimester, suggesting that the blood vessels had dilated to allow more cerebral perfusion. This phenomenon, termed “brain sparing,” is believed to be an autoregulatory mechanism that occurs as a result of diminished oxygen delivery or inadequate blood flow to the brain (Pediatr Cardiol. 2003 Jan;24[5]:436-43).
Subsequent studies have similarly documented abnormal cerebral blood flow in fetuses with various types of congenital heart lesions. What is left to be determined is whether this autoregulatory mechanism is adequate to maintain perfusion in the presence of specific, high-risk CHD.
Abnormalities were more often seen in CHD with obstructed aortic flow, such as hypoplastic left heart syndrome (HLHS) in which the aorta is perfused retrograde through the fetal ductus arteriosus (Circulation. 2010 Jan 4;121:26-33).
Other fetal imaging studies have similarly demonstrated a progressive third-trimester decrease in both cortical gray and white matter and in gyrification (cortical folding) (Cereb Cortex. 2013;23:2932-43), as well as decreased cerebral oxygen delivery and consumption (Circulation. 2015;131:1313-23) in fetuses with severe CHD. It appears that the brain may start out normal in size, but in the third trimester, the accelerated metabolic demands that come with rapid growth and development are not sufficiently met by the fetal cardiovascular circulation in CHD.
In the newborn with CHD, preoperative brain imaging studies have demonstrated structural abnormalities suggesting delayed development (for example, microcephaly and a widened operculum), microstructural abnormalities suggesting abnormal myelination and neuroaxonal development, and lower brain maturity scores (a composite score that combines multiple factors, such as myelination and cortical in-folding, to represent “brain age”).
Moreover, some of the newborn brain imaging studies have correlated brain MRI findings with neonatal neurodevelopmental assessments. For instance, investigators found that full-term newborns with CHD had decreased gray matter brain volume and increased cerebrospinal fluid volume and that these impairments were associated with poor behavioral state regulation and poor visual orienting (J Pediatr. 2014;164:1121-7).
Interestingly, it has been found that the full-term baby with specific complex CHD, including newborns with single ventricle CHD or transposition of the great arteries, is more likely to have a brain maturity score that is equivalent to that of a baby born at 35 weeks’ gestation. This means that, in some infants with CHD, the brain has lagged in growth by about a month, resulting in a pattern of disturbed development and subsequent injury that is similar to that of premature infants.
It also means that infants with CHD and an immature brain are especially vulnerable to brain injury when open-heart surgery is needed. In short, we now appreciate that the brain in patients with CHD is likely more fragile than we previously thought – and that this fragility is prenatal in its origins.
Delivery room planning
Ideally, our goal is to find ways of changing the circulation in utero to improve cerebral oxygenation and blood flow, and, consequently, improve brain development and long-term neurocognitive function. Despite significant efforts in this area, we’re not there yet.
Examples of strategies that are being tested include catheter intervention to open the aortic valve in utero for fetuses with critical aortic stenosis. This procedure currently is being performed to try to prevent progression of the valve abnormality to HLHS, but it has not been determined whether the intervention affects cerebral blood flow. Maternal oxygen therapy has been shown to change cerebral blood flow in the short term for fetuses with HLHS, but its long-term use has not been studied. At the time of birth, to prevent injury in the potentially more fragile brain of the newborn with CHD, what we can do is to identify those fetuses who are more likely to be at risk for hypoxia low cardiac output and hemodynamic compromise in the delivery room, and plan for specialized delivery room and perinatal management beyond standard neonatal care.
Most newborns with CHD are assigned to Level 1; they have no predicted risk of compromise in the delivery room – or even in the first couple weeks of life – and can deliver at a local hospital with neonatal evaluation and then consult with the pediatric cardiologist. Defects include shunt lesions such as septal defects or mild valve abnormalities.
Patients assigned to Level 2 have minimal risk of compromise in the delivery room but are expected to require postnatal surgery, cardiac catheterization, or another procedure before going home. They can be stabilized by the neonatologist, usually with initiation of a prostaglandin infusion, before transfer to the cardiac center for the planned intervention. Defects include single ventricle CHD and severe Tetralogy of Fallot.
Fetuses assigned to Level 3 and Level 4 are expected to have hemodynamic instability at cord clamping, requiring immediate specialty care in the delivery room that is likely to include urgent cardiac catheterization or surgical intervention. These defects are rare and include diagnoses such as transposition of the great arteries, HLHS with a restrictive or closed foramen ovale, and CHD with associated heart failure and hydrops.
We have found that fetal echocardiography accurately predicts postnatal risk and the need for specialized delivery room care in newborns diagnosed in utero with CHD and that level-of-care protocols ensure safe delivery and optimize fetal outcomes (J Am Soc Echocardiogr. 2015;28:1339-49; Am J Cardiol. 2013;111:737-47).
Such delivery planning, which is coordinated between obstetric, neonatal, cardiology, and surgical services with specialty teams as needed (for example, cardiac intensive care, interventional cardiology, and cardiac surgery), is recommended in a 2014 AHA statement on the diagnosis and treatment of fetal cardiac disease. In recent years it has become the standard of care in many health systems (Circulation. 2014;129[21]:2183-242).
The effect of maternal stress on the in utero environment is also getting increased attention in pediatric cardiology. Alterations in neurocognitive development and fetal and child cardiovascular health are likely to be associated with maternal stress during pregnancy, and studies have shown that maternal stress is high with prenatal diagnoses of CHD. We have to ask: Is stress a modifiable risk factor? There must be ways in which we can do better with prenatal counseling and support after a fetal diagnosis of CHD.
Screening for CHD
Initiating strategies to improve neurodevelopmental outcomes in infants with CHD rests partly on identifying babies with CHD before birth through improved fetal cardiac screening. Research cited in the 2014 AHA statement indicates that nearly all women giving birth to babies with CHD in the United States have obstetric ultrasound examinations in the second or third trimesters, but that only about 30% of the fetuses are diagnosed prenatally.
Current indications for referral for a fetal echocardiogram – in addition to suspicion of a structural heart abnormality on obstetric ultrasound – include maternal factors, such as diabetes mellitus, that raise the risk of CHD above the baseline population risk for low-risk pregnancies.
Women with pregestational diabetes mellitus have a nearly fivefold increase in CHD, compared with the general population (3%-5%), and should be referred for fetal echocardiography. Women with gestational diabetes mellitus have no or minimally increased risk for fetal CHD, but it has been shown that there is an increased risk for cardiac hypertrophy – particularly late in gestation – if glycemic levels are poorly controlled. The 2014 AHA guidelines recommend that fetal echocardiographic evaluation be considered in those who have HbA1c levels greater than 6% in the second half of pregnancy.
Dr. Mary T. Donofrio is a pediatric cardiologist and director of the fetal heart program and critical care delivery program at Children’s National Medical Center, Washington. She reported that she has no disclosures relevant to this article.
How better imaging technology for prenatal diagnoses can improve outcomes
We live during an unprecedented time in the history of ob.gyn. practice. Only a relatively short time ago, the only way ob.gyns. could assess the health of the fetus was through the invasive and risky procedures of the amniocentesis and, later, chorionic villus sampling. A woman who might eventually have had a baby with a congenital abnormality would not have known of her fetus’s defect until after birth, when successful intervention might have been extremely difficult to achieve or even too late. At the time, in utero evaluation could be done only by static, low-resolution sonographic images of the fetus. By today’s standards of imaging technology, these once-revolutionary pictures are almost tantamount to cave paintings.
Therefore, while it is imperative that we employ all available technologies and techniques possible to detect and diagnose potential fetal developmental defects, we must also bear in mind that no test is ever infallible. It is our obligation to provide the very best information based on expert and thorough review.
This month we have invited Mary Donofrio, MD, director of the fetal heart program at Children’s National Medical Center, Washington, to discuss how the latest advances in imaging technology have enabled us to screen for and diagnose congenital heart diseases, and improve outcomes for mother and baby.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
We live during an unprecedented time in the history of ob.gyn. practice. Only a relatively short time ago, the only way ob.gyns. could assess the health of the fetus was through the invasive and risky procedures of the amniocentesis and, later, chorionic villus sampling. A woman who might eventually have had a baby with a congenital abnormality would not have known of her fetus’s defect until after birth, when successful intervention might have been extremely difficult to achieve or even too late. At the time, in utero evaluation could be done only by static, low-resolution sonographic images of the fetus. By today’s standards of imaging technology, these once-revolutionary pictures are almost tantamount to cave paintings.
Therefore, while it is imperative that we employ all available technologies and techniques possible to detect and diagnose potential fetal developmental defects, we must also bear in mind that no test is ever infallible. It is our obligation to provide the very best information based on expert and thorough review.
This month we have invited Mary Donofrio, MD, director of the fetal heart program at Children’s National Medical Center, Washington, to discuss how the latest advances in imaging technology have enabled us to screen for and diagnose congenital heart diseases, and improve outcomes for mother and baby.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
We live during an unprecedented time in the history of ob.gyn. practice. Only a relatively short time ago, the only way ob.gyns. could assess the health of the fetus was through the invasive and risky procedures of the amniocentesis and, later, chorionic villus sampling. A woman who might eventually have had a baby with a congenital abnormality would not have known of her fetus’s defect until after birth, when successful intervention might have been extremely difficult to achieve or even too late. At the time, in utero evaluation could be done only by static, low-resolution sonographic images of the fetus. By today’s standards of imaging technology, these once-revolutionary pictures are almost tantamount to cave paintings.
Therefore, while it is imperative that we employ all available technologies and techniques possible to detect and diagnose potential fetal developmental defects, we must also bear in mind that no test is ever infallible. It is our obligation to provide the very best information based on expert and thorough review.
This month we have invited Mary Donofrio, MD, director of the fetal heart program at Children’s National Medical Center, Washington, to discuss how the latest advances in imaging technology have enabled us to screen for and diagnose congenital heart diseases, and improve outcomes for mother and baby.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Ureteral reimplantation for injuries not easily managed with stenting
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The diagnosis and treatment of ureteral injury
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.