User login
Suicide rate higher than average for female clinicians
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.
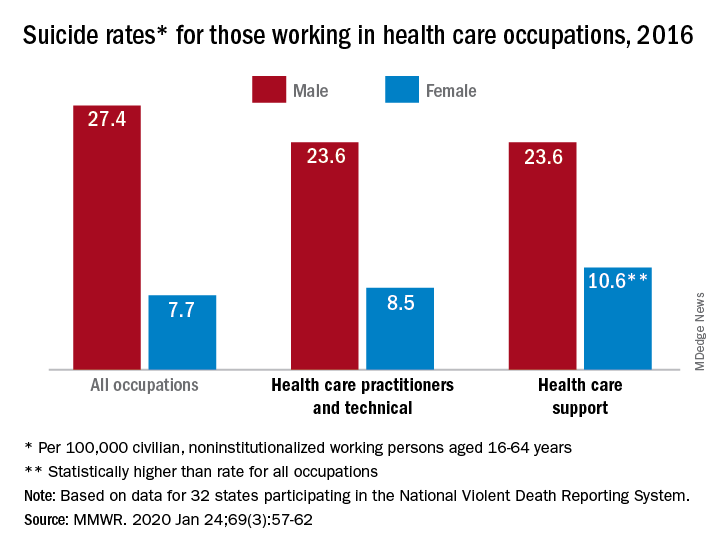
In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
FROM MMWR
Washington state patient is first U.S. case of novel coronavirus
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
REPORTING FROM CDC
FDA advisers set high bar for new opioids
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
Travelers to three U.S. airports to be screened for novel coronavirus
according to an announcement from the Centers for Disease Control and Prevention.
Starting today, Jan. 17, 2020, people traveling from Wuhan to New York (JFK), San Francisco (SFO), and Los Angeles (LAX) airports will be screened for symptoms associated with 2019-nCoV, which include fever, cough, and difficulty breathing.
“Based on the information that CDC has today, we believe the current risk for this virus to the general public is low,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a CDC telebriefing.
To date, 45 cases of 2019-nCoV have been reported in Wuhan, according to the CDC. The Wuhan Municipal Health Commission said 15 patients have been cured and discharged, 5 severe cases are still being treated, and 2 patients have died. Both deaths occurred in older patients, one of whom was aged 69 years and one aged 61 years. One of the patients was known to have underlying health conditions.
Three cases of 2019-nCoV have been confirmed outside of Wuhan, one in Japan and two in Thailand. All three were travelers from Wuhan.
The virus is believed to have originated at Wuhan South China Seafood City, a market that sold seafood, chickens, bats, cats, marmots, and other wild animals. (The market has since been closed and disinfected.) The origin suggests animal-to-human transmission of 2019-nCoV, but it appears that human-to-human transmission can occur as well.
“While most of these infections seem to be happening from animals to people, there is some indication that limited person-to-person spread is happening,” Dr. Messonnier said.
Because of this potential risk, the CDC is working with the Department of Homeland Security’s Customs and Border Protection to screen travelers from Wuhan to the United States. The CDC is deploying about 100 additional staff to JFK, SFO, and LAX, where direct flights (JFK and SFO) or connecting flights (LAX) from Wuhan land.
The CDC could not confirm if exit screening is planned for people traveling abroad from Wuhan.
At the U.S. airports, travelers from Wuhan will be given a questionnaire asking about symptoms of 2019-nCoV (fever, cough, and difficulty breathing). People who exhibit symptoms will be assessed and questioned further. If they are believed to have 2019-nCoV, they will be sent to designated hospitals, where they will be examined, and samples will be collected.
Samples from patients with suspected 2019-nCoV will be sent to the CDC for analysis. Chinese health authorities made the full genome of 2019-nCoV publicly available, which will allow the CDC to confirm any cases that may arise in the United States. The CDC is currently working on a test to detect 2019-nCoV, which can be distributed to state health departments.
Earlier this month, the CDC issued a Level 1 Travel Health Notice for travelers to Wuhan and a Health Alert on 2019-nCoV. The latest information on 2019-nCoV can be found on the CDC’s Novel Coronavirus 2019 webpage.
according to an announcement from the Centers for Disease Control and Prevention.
Starting today, Jan. 17, 2020, people traveling from Wuhan to New York (JFK), San Francisco (SFO), and Los Angeles (LAX) airports will be screened for symptoms associated with 2019-nCoV, which include fever, cough, and difficulty breathing.
“Based on the information that CDC has today, we believe the current risk for this virus to the general public is low,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a CDC telebriefing.
To date, 45 cases of 2019-nCoV have been reported in Wuhan, according to the CDC. The Wuhan Municipal Health Commission said 15 patients have been cured and discharged, 5 severe cases are still being treated, and 2 patients have died. Both deaths occurred in older patients, one of whom was aged 69 years and one aged 61 years. One of the patients was known to have underlying health conditions.
Three cases of 2019-nCoV have been confirmed outside of Wuhan, one in Japan and two in Thailand. All three were travelers from Wuhan.
The virus is believed to have originated at Wuhan South China Seafood City, a market that sold seafood, chickens, bats, cats, marmots, and other wild animals. (The market has since been closed and disinfected.) The origin suggests animal-to-human transmission of 2019-nCoV, but it appears that human-to-human transmission can occur as well.
“While most of these infections seem to be happening from animals to people, there is some indication that limited person-to-person spread is happening,” Dr. Messonnier said.
Because of this potential risk, the CDC is working with the Department of Homeland Security’s Customs and Border Protection to screen travelers from Wuhan to the United States. The CDC is deploying about 100 additional staff to JFK, SFO, and LAX, where direct flights (JFK and SFO) or connecting flights (LAX) from Wuhan land.
The CDC could not confirm if exit screening is planned for people traveling abroad from Wuhan.
At the U.S. airports, travelers from Wuhan will be given a questionnaire asking about symptoms of 2019-nCoV (fever, cough, and difficulty breathing). People who exhibit symptoms will be assessed and questioned further. If they are believed to have 2019-nCoV, they will be sent to designated hospitals, where they will be examined, and samples will be collected.
Samples from patients with suspected 2019-nCoV will be sent to the CDC for analysis. Chinese health authorities made the full genome of 2019-nCoV publicly available, which will allow the CDC to confirm any cases that may arise in the United States. The CDC is currently working on a test to detect 2019-nCoV, which can be distributed to state health departments.
Earlier this month, the CDC issued a Level 1 Travel Health Notice for travelers to Wuhan and a Health Alert on 2019-nCoV. The latest information on 2019-nCoV can be found on the CDC’s Novel Coronavirus 2019 webpage.
according to an announcement from the Centers for Disease Control and Prevention.
Starting today, Jan. 17, 2020, people traveling from Wuhan to New York (JFK), San Francisco (SFO), and Los Angeles (LAX) airports will be screened for symptoms associated with 2019-nCoV, which include fever, cough, and difficulty breathing.
“Based on the information that CDC has today, we believe the current risk for this virus to the general public is low,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a CDC telebriefing.
To date, 45 cases of 2019-nCoV have been reported in Wuhan, according to the CDC. The Wuhan Municipal Health Commission said 15 patients have been cured and discharged, 5 severe cases are still being treated, and 2 patients have died. Both deaths occurred in older patients, one of whom was aged 69 years and one aged 61 years. One of the patients was known to have underlying health conditions.
Three cases of 2019-nCoV have been confirmed outside of Wuhan, one in Japan and two in Thailand. All three were travelers from Wuhan.
The virus is believed to have originated at Wuhan South China Seafood City, a market that sold seafood, chickens, bats, cats, marmots, and other wild animals. (The market has since been closed and disinfected.) The origin suggests animal-to-human transmission of 2019-nCoV, but it appears that human-to-human transmission can occur as well.
“While most of these infections seem to be happening from animals to people, there is some indication that limited person-to-person spread is happening,” Dr. Messonnier said.
Because of this potential risk, the CDC is working with the Department of Homeland Security’s Customs and Border Protection to screen travelers from Wuhan to the United States. The CDC is deploying about 100 additional staff to JFK, SFO, and LAX, where direct flights (JFK and SFO) or connecting flights (LAX) from Wuhan land.
The CDC could not confirm if exit screening is planned for people traveling abroad from Wuhan.
At the U.S. airports, travelers from Wuhan will be given a questionnaire asking about symptoms of 2019-nCoV (fever, cough, and difficulty breathing). People who exhibit symptoms will be assessed and questioned further. If they are believed to have 2019-nCoV, they will be sent to designated hospitals, where they will be examined, and samples will be collected.
Samples from patients with suspected 2019-nCoV will be sent to the CDC for analysis. Chinese health authorities made the full genome of 2019-nCoV publicly available, which will allow the CDC to confirm any cases that may arise in the United States. The CDC is currently working on a test to detect 2019-nCoV, which can be distributed to state health departments.
Earlier this month, the CDC issued a Level 1 Travel Health Notice for travelers to Wuhan and a Health Alert on 2019-nCoV. The latest information on 2019-nCoV can be found on the CDC’s Novel Coronavirus 2019 webpage.
Flu activity declines for second straight week
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.
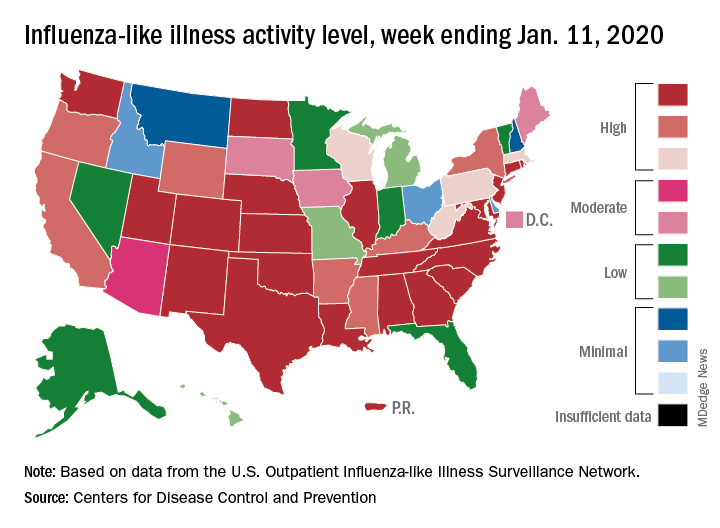
Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.

Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.

Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Two new cases of coronavirus pneumonia in Thailand, Japan
Health authorities in Wuhan, Hubei Province, China, identified the novel coronavirus, 2019-nCoV, responsible for the outbreak of a mysterious pneumonia that resulted in hospitalization of more than 40 patients and one death, according to the Centers for Disease Control and Prevention in a statement on the CDC website.
On Jan. 13, the Thailand’s Ministry of Public Health reported the first imported case of lab-confirmed 2019-nCoV from Wuhan. The Centers for Disease Control and Prevention stated: “The traveler with febrile illness was detected on the same day by thermal surveillance at Suvarnabhumi Airport, Thailand, and was hospitalized the same day. After temperature check and initial assessment, she was transferred to the hospital for further investigations and treatment.”
Samples from this patient tested positive for coronaviruses by reverse transcriptase-polymerase chain reaction. The genomic sequencing analysis was performed by Emerging Infectious Diseases Health Science Center, the Thai Red Cross Society, and the Thai National Institute of Health. The patient is reported to be in stable condition.
The New York Times has reported a case of 2019-nCoV in Japan in a traveler returning from Wuhan. That patient is reported to have recovered and been discharged.
Chinese health authorities transmitted the full genome of “2019 novel coronavirus,” or “2019-nCoV,” to GenBank, the genetic sequence database managed by the National Institutes of Health, and in the Global Initiative on Sharing All Influenza Data portal.
Coronaviruses are a large family of viruses. Most known human coronaviruses only cause mild respiratory disease, such as the common cold. But several coronaviruses have emerged to infect people and cause severe disease, such as has been seen with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The cases in the Wuhan pneumonia outbreak have tested negative for both SARS and MERS.
The outbreak in Wuhan appears to be contained. The World Health Organization reported that the Wuhan health authorities identified and followed 763 close contacts, including health care workers. No additional cases of infection with the novel coronavirus have been identified. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has issued a Level 1 travel alert and recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Health authorities in Wuhan, Hubei Province, China, identified the novel coronavirus, 2019-nCoV, responsible for the outbreak of a mysterious pneumonia that resulted in hospitalization of more than 40 patients and one death, according to the Centers for Disease Control and Prevention in a statement on the CDC website.
On Jan. 13, the Thailand’s Ministry of Public Health reported the first imported case of lab-confirmed 2019-nCoV from Wuhan. The Centers for Disease Control and Prevention stated: “The traveler with febrile illness was detected on the same day by thermal surveillance at Suvarnabhumi Airport, Thailand, and was hospitalized the same day. After temperature check and initial assessment, she was transferred to the hospital for further investigations and treatment.”
Samples from this patient tested positive for coronaviruses by reverse transcriptase-polymerase chain reaction. The genomic sequencing analysis was performed by Emerging Infectious Diseases Health Science Center, the Thai Red Cross Society, and the Thai National Institute of Health. The patient is reported to be in stable condition.
The New York Times has reported a case of 2019-nCoV in Japan in a traveler returning from Wuhan. That patient is reported to have recovered and been discharged.
Chinese health authorities transmitted the full genome of “2019 novel coronavirus,” or “2019-nCoV,” to GenBank, the genetic sequence database managed by the National Institutes of Health, and in the Global Initiative on Sharing All Influenza Data portal.
Coronaviruses are a large family of viruses. Most known human coronaviruses only cause mild respiratory disease, such as the common cold. But several coronaviruses have emerged to infect people and cause severe disease, such as has been seen with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The cases in the Wuhan pneumonia outbreak have tested negative for both SARS and MERS.
The outbreak in Wuhan appears to be contained. The World Health Organization reported that the Wuhan health authorities identified and followed 763 close contacts, including health care workers. No additional cases of infection with the novel coronavirus have been identified. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has issued a Level 1 travel alert and recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Health authorities in Wuhan, Hubei Province, China, identified the novel coronavirus, 2019-nCoV, responsible for the outbreak of a mysterious pneumonia that resulted in hospitalization of more than 40 patients and one death, according to the Centers for Disease Control and Prevention in a statement on the CDC website.
On Jan. 13, the Thailand’s Ministry of Public Health reported the first imported case of lab-confirmed 2019-nCoV from Wuhan. The Centers for Disease Control and Prevention stated: “The traveler with febrile illness was detected on the same day by thermal surveillance at Suvarnabhumi Airport, Thailand, and was hospitalized the same day. After temperature check and initial assessment, she was transferred to the hospital for further investigations and treatment.”
Samples from this patient tested positive for coronaviruses by reverse transcriptase-polymerase chain reaction. The genomic sequencing analysis was performed by Emerging Infectious Diseases Health Science Center, the Thai Red Cross Society, and the Thai National Institute of Health. The patient is reported to be in stable condition.
The New York Times has reported a case of 2019-nCoV in Japan in a traveler returning from Wuhan. That patient is reported to have recovered and been discharged.
Chinese health authorities transmitted the full genome of “2019 novel coronavirus,” or “2019-nCoV,” to GenBank, the genetic sequence database managed by the National Institutes of Health, and in the Global Initiative on Sharing All Influenza Data portal.
Coronaviruses are a large family of viruses. Most known human coronaviruses only cause mild respiratory disease, such as the common cold. But several coronaviruses have emerged to infect people and cause severe disease, such as has been seen with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The cases in the Wuhan pneumonia outbreak have tested negative for both SARS and MERS.
The outbreak in Wuhan appears to be contained. The World Health Organization reported that the Wuhan health authorities identified and followed 763 close contacts, including health care workers. No additional cases of infection with the novel coronavirus have been identified. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has issued a Level 1 travel alert and recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
FDA committee rejects oxycodegol, new opioid designed for less abuse
A Food and Drug Administration advisory committee voted 27-0 on Jan. 14 against recommending agency approval of oxycodegol, a new type of opioid molecule designed to slow passage of the drug through the blood-brain barrier and hence cause less of a “high” and potentially blunt the drug’s abuse potential.
But the panel, which includes members from both the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee, unanimously shared the opinion that the data submitted by the drug developer, Nektar, failed to clearly demonstrate the drug’s safety and efficacy.
“There was a lot of ambiguity [in the data] about safety and efficacy, and this is too important an issue to have so much ambiguity,” summed up Ronald S. Litman, DO, professor of anesthesiology and critical care at the University of Pennsylvania in Philadelphia and chair of the combined committee.
“The subliminal message” that would surround oxycodegol if it received FDA approval would be that it is a “safer” opioid, “and that would make it a blockbuster drug, and for that to happen you need data that [prove] the benefits outweigh risks, but we didn’t see those data. It was all speculation, and we can’t approve a potential blockbuster opioid in the middle of a public health opioid crisis based on speculation,” commented Steve B. Meisel, PharmD, system director of medication safety for Fairview Health Services in Minneapolis and a committee member.
According to Nektar, oxycodegol is a new molecular entity that combines a morphinan pharmacophore, oxycodol, with a six-unit chain of polyethylene glycol, a design that slows movement of the drug into the brain by about an hour after an oral dose when compared with oxycodone. It works as a full mu opioid receptor agonist that inhibits the nociceptive pathways while it reduces reinforcing or euphoric effects and other negative CNS-mediated side effects because of its slowed entry into the central nervous system.
But, as became apparent during the course of the day’s presentations and questions, while the clinical evidence in general supported this mechanism, the data were flawed by many limitations and caveats. FDA staffers critiqued the company’s data set for a variety of issues, including testing dosages that were “arbitrary and flawed,” evidence for “high oral abuse potential” in one study, including “a high rate of euphoria” of 17% even at the recommended dosage level (and a higher euphoria incidence at a higher dosage), limited information on additional pain medications that enrolled patients had used both before enrollment and during the study, and possible enrollment bias. Many panel members also raised concerns that included the efficacy data coming from a single randomized study, incomplete data on possible hepatic toxicity, no data to address potential abuse via injection or inhalation, a very modest demonstrated increment in pain relief, and abuse potential studies that relied on a single dose of the drug.
On the other hand, many committee members, and others in the pain field, applauded the general concept behind oxycodegol and urged the developer to run additional studies and collect more data for a future FDA application.
There has been “a lot of controversy” about oxycodegol, often centered on the question “why do we need another opioid,” commented Steven P. Cohen, MD, professor of anesthesiology, neurology, and pain medicine and chief of pain medicine at Johns Hopkins Health in Baltimore. “Having another opioid on the market with a different pain indication and possibly lower abuse potential” would be welcome and “almost certainly would not result in more prescriptions or abuse,” said Dr. Cohen, who was not a committee member but addressed in an interview the need for new and effective pain medications that offer an alternative to conventional opioids. “Patients who might otherwise be prescribed a different opioid might receive oxycodegol instead,” he suggested. But first, the developing company will need to collect a lot more clinical data than it has reported so far.
A Food and Drug Administration advisory committee voted 27-0 on Jan. 14 against recommending agency approval of oxycodegol, a new type of opioid molecule designed to slow passage of the drug through the blood-brain barrier and hence cause less of a “high” and potentially blunt the drug’s abuse potential.
But the panel, which includes members from both the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee, unanimously shared the opinion that the data submitted by the drug developer, Nektar, failed to clearly demonstrate the drug’s safety and efficacy.
“There was a lot of ambiguity [in the data] about safety and efficacy, and this is too important an issue to have so much ambiguity,” summed up Ronald S. Litman, DO, professor of anesthesiology and critical care at the University of Pennsylvania in Philadelphia and chair of the combined committee.
“The subliminal message” that would surround oxycodegol if it received FDA approval would be that it is a “safer” opioid, “and that would make it a blockbuster drug, and for that to happen you need data that [prove] the benefits outweigh risks, but we didn’t see those data. It was all speculation, and we can’t approve a potential blockbuster opioid in the middle of a public health opioid crisis based on speculation,” commented Steve B. Meisel, PharmD, system director of medication safety for Fairview Health Services in Minneapolis and a committee member.
According to Nektar, oxycodegol is a new molecular entity that combines a morphinan pharmacophore, oxycodol, with a six-unit chain of polyethylene glycol, a design that slows movement of the drug into the brain by about an hour after an oral dose when compared with oxycodone. It works as a full mu opioid receptor agonist that inhibits the nociceptive pathways while it reduces reinforcing or euphoric effects and other negative CNS-mediated side effects because of its slowed entry into the central nervous system.
But, as became apparent during the course of the day’s presentations and questions, while the clinical evidence in general supported this mechanism, the data were flawed by many limitations and caveats. FDA staffers critiqued the company’s data set for a variety of issues, including testing dosages that were “arbitrary and flawed,” evidence for “high oral abuse potential” in one study, including “a high rate of euphoria” of 17% even at the recommended dosage level (and a higher euphoria incidence at a higher dosage), limited information on additional pain medications that enrolled patients had used both before enrollment and during the study, and possible enrollment bias. Many panel members also raised concerns that included the efficacy data coming from a single randomized study, incomplete data on possible hepatic toxicity, no data to address potential abuse via injection or inhalation, a very modest demonstrated increment in pain relief, and abuse potential studies that relied on a single dose of the drug.
On the other hand, many committee members, and others in the pain field, applauded the general concept behind oxycodegol and urged the developer to run additional studies and collect more data for a future FDA application.
There has been “a lot of controversy” about oxycodegol, often centered on the question “why do we need another opioid,” commented Steven P. Cohen, MD, professor of anesthesiology, neurology, and pain medicine and chief of pain medicine at Johns Hopkins Health in Baltimore. “Having another opioid on the market with a different pain indication and possibly lower abuse potential” would be welcome and “almost certainly would not result in more prescriptions or abuse,” said Dr. Cohen, who was not a committee member but addressed in an interview the need for new and effective pain medications that offer an alternative to conventional opioids. “Patients who might otherwise be prescribed a different opioid might receive oxycodegol instead,” he suggested. But first, the developing company will need to collect a lot more clinical data than it has reported so far.
A Food and Drug Administration advisory committee voted 27-0 on Jan. 14 against recommending agency approval of oxycodegol, a new type of opioid molecule designed to slow passage of the drug through the blood-brain barrier and hence cause less of a “high” and potentially blunt the drug’s abuse potential.
But the panel, which includes members from both the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee, unanimously shared the opinion that the data submitted by the drug developer, Nektar, failed to clearly demonstrate the drug’s safety and efficacy.
“There was a lot of ambiguity [in the data] about safety and efficacy, and this is too important an issue to have so much ambiguity,” summed up Ronald S. Litman, DO, professor of anesthesiology and critical care at the University of Pennsylvania in Philadelphia and chair of the combined committee.
“The subliminal message” that would surround oxycodegol if it received FDA approval would be that it is a “safer” opioid, “and that would make it a blockbuster drug, and for that to happen you need data that [prove] the benefits outweigh risks, but we didn’t see those data. It was all speculation, and we can’t approve a potential blockbuster opioid in the middle of a public health opioid crisis based on speculation,” commented Steve B. Meisel, PharmD, system director of medication safety for Fairview Health Services in Minneapolis and a committee member.
According to Nektar, oxycodegol is a new molecular entity that combines a morphinan pharmacophore, oxycodol, with a six-unit chain of polyethylene glycol, a design that slows movement of the drug into the brain by about an hour after an oral dose when compared with oxycodone. It works as a full mu opioid receptor agonist that inhibits the nociceptive pathways while it reduces reinforcing or euphoric effects and other negative CNS-mediated side effects because of its slowed entry into the central nervous system.
But, as became apparent during the course of the day’s presentations and questions, while the clinical evidence in general supported this mechanism, the data were flawed by many limitations and caveats. FDA staffers critiqued the company’s data set for a variety of issues, including testing dosages that were “arbitrary and flawed,” evidence for “high oral abuse potential” in one study, including “a high rate of euphoria” of 17% even at the recommended dosage level (and a higher euphoria incidence at a higher dosage), limited information on additional pain medications that enrolled patients had used both before enrollment and during the study, and possible enrollment bias. Many panel members also raised concerns that included the efficacy data coming from a single randomized study, incomplete data on possible hepatic toxicity, no data to address potential abuse via injection or inhalation, a very modest demonstrated increment in pain relief, and abuse potential studies that relied on a single dose of the drug.
On the other hand, many committee members, and others in the pain field, applauded the general concept behind oxycodegol and urged the developer to run additional studies and collect more data for a future FDA application.
There has been “a lot of controversy” about oxycodegol, often centered on the question “why do we need another opioid,” commented Steven P. Cohen, MD, professor of anesthesiology, neurology, and pain medicine and chief of pain medicine at Johns Hopkins Health in Baltimore. “Having another opioid on the market with a different pain indication and possibly lower abuse potential” would be welcome and “almost certainly would not result in more prescriptions or abuse,” said Dr. Cohen, who was not a committee member but addressed in an interview the need for new and effective pain medications that offer an alternative to conventional opioids. “Patients who might otherwise be prescribed a different opioid might receive oxycodegol instead,” he suggested. But first, the developing company will need to collect a lot more clinical data than it has reported so far.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Drop in flu activity may not signal seasonal peak
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.
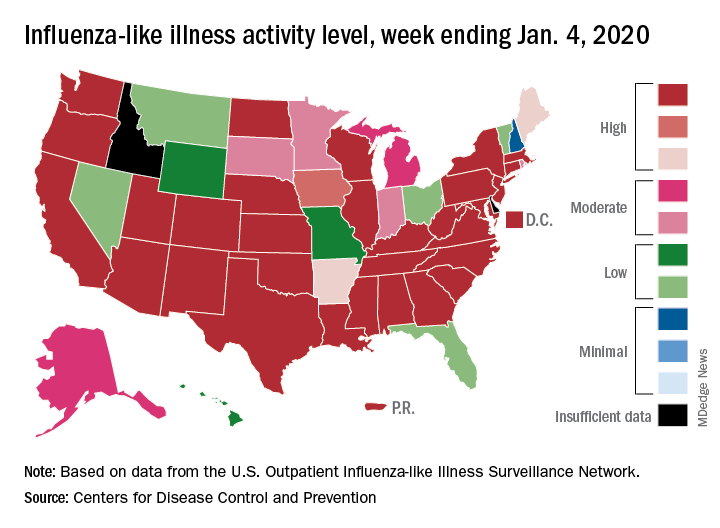
Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.

Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.

Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
FDA approves diazepam nasal spray for seizure clusters
The drug may be administered by a care partner outside of a medical setting for the treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern. The formulation is the first nasal spray approved by the FDA as a rescue treatment for people with epilepsy aged 6 years and older, according to Neurelis, the developer of the drug. Midazolam nasal spray, approved in May 2019, is indicated for patients with epilepsy aged 12 years and older.
Investigators evaluated the safety of diazepam nasal spray in a long-term, open-label, repeat-dose, clinical trial. The study enrolled 130 patients aged 6 years and older; more than 2,000 seizures were treated. The drug generally was safe and well tolerated, and the most common adverse reactions were somnolence, headache, and nasal discomfort.
The FDA has granted Valtoco 7 years of orphan drug exclusivity. In the United States, about 170,000 patients with epilepsy are at risk of cluster or acute repetitive seizures, the company said. Until recently, approved rescue medications had been rectally administered.
Patients may receive a second dose of diazepam nasal spray at least 4 hours after an initial dose if needed, but caregivers should not use more than two doses to treat a single episode, according to the prescribing information. In addition, the prescribing information recommends that diazepam nasal spray be used for no more than one episode every 5 days and no more than five episodes per month.
The drug may be administered by a care partner outside of a medical setting for the treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern. The formulation is the first nasal spray approved by the FDA as a rescue treatment for people with epilepsy aged 6 years and older, according to Neurelis, the developer of the drug. Midazolam nasal spray, approved in May 2019, is indicated for patients with epilepsy aged 12 years and older.
Investigators evaluated the safety of diazepam nasal spray in a long-term, open-label, repeat-dose, clinical trial. The study enrolled 130 patients aged 6 years and older; more than 2,000 seizures were treated. The drug generally was safe and well tolerated, and the most common adverse reactions were somnolence, headache, and nasal discomfort.
The FDA has granted Valtoco 7 years of orphan drug exclusivity. In the United States, about 170,000 patients with epilepsy are at risk of cluster or acute repetitive seizures, the company said. Until recently, approved rescue medications had been rectally administered.
Patients may receive a second dose of diazepam nasal spray at least 4 hours after an initial dose if needed, but caregivers should not use more than two doses to treat a single episode, according to the prescribing information. In addition, the prescribing information recommends that diazepam nasal spray be used for no more than one episode every 5 days and no more than five episodes per month.
The drug may be administered by a care partner outside of a medical setting for the treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern. The formulation is the first nasal spray approved by the FDA as a rescue treatment for people with epilepsy aged 6 years and older, according to Neurelis, the developer of the drug. Midazolam nasal spray, approved in May 2019, is indicated for patients with epilepsy aged 12 years and older.
Investigators evaluated the safety of diazepam nasal spray in a long-term, open-label, repeat-dose, clinical trial. The study enrolled 130 patients aged 6 years and older; more than 2,000 seizures were treated. The drug generally was safe and well tolerated, and the most common adverse reactions were somnolence, headache, and nasal discomfort.
The FDA has granted Valtoco 7 years of orphan drug exclusivity. In the United States, about 170,000 patients with epilepsy are at risk of cluster or acute repetitive seizures, the company said. Until recently, approved rescue medications had been rectally administered.
Patients may receive a second dose of diazepam nasal spray at least 4 hours after an initial dose if needed, but caregivers should not use more than two doses to treat a single episode, according to the prescribing information. In addition, the prescribing information recommends that diazepam nasal spray be used for no more than one episode every 5 days and no more than five episodes per month.
FDA approves avapritinib for adults with GIST with PDGFRA mutation
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.