User login
FDA okays Palforzia, first drug for peanut allergy in children
The Food and Drug Administration has approved the first drug to combat peanut allergy in children, (Palforzia, Aimmune Therapeutics), although those who take it must continue to avoid peanuts in their diets.
The peanut (Arachis hypogaea) allergen powder is also the first drug ever approved to treat a food allergy. It is not a cure, but it mitigates allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanuts, the FDA said in a news release.
Treatment with the oral powder, which is mixed into semisolid food – such as applesauce or yogurt – can be started in children aged 4 through 17 years who have a confirmed peanut allergy and then continued as a maintenance medication. Some 1 million American children have peanut allergy, and only a fifth will outgrow the allergy, the agency said.
“Because there is no cure, allergic individuals must strictly avoid exposure to prevent severe and potentially life-threatening reactions,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement.
An FDA advisory panel backed the medication in September 2019, but some committee members expressed concern about the large number of children in clinical trials who required epinephrine after receiving a dose of Palforzia.
The initial dose phase is given on a single day, while updosing consists of 11 increasing doses over several months. If the patient tolerates the first administration of an increased dose level, they may continue that dose daily at home. Daily maintenance begins after the completion of all updosing levels.
Palforzia will be available only through specially certified health care providers, health care settings, and pharmacies to patients enrolled in the REMS program, the agency said. Also, the initial dose escalation and first dose of each updosing level can be given only in a certified setting.
The agency said that patients or parents or caregivers must be counseled on the need for constant availability of injectable epinephrine, the need for continued dietary peanut avoidance, and on how to recognize the signs and symptoms of anaphylaxis.
‘Eagerly’ awaited
Palforzia’s effectiveness was based on a randomized, double-blind, placebo-controlled study involving about 500 peanut-allergic individuals that found that 67.2% of allergic patients tolerated an oral challenge with a single 600-mg dose of peanut protein with no more than mild allergic symptoms after 6 months of maintenance treatment, compared with 4% of placebo recipients, the FDA said.
In two double-blind, placebo-controlled studies looking at safety, the most commonly reported side effects among about 700 individuals involved in the research were abdominal pain, vomiting, nausea, tingling in the mouth, itching (including in the mouth and ears), cough, runny nose, throat irritation and tightness, hives, wheezing and shortness of breath, and anaphylaxis.
Palforzia should not be given to those with uncontrolled asthma and can’t be used for emergency treatment of allergic reactions, including anaphylaxis.
“The food allergy community has been eagerly awaiting an FDA-approved treatment that can help mitigate allergic reactions to peanut and, as allergists, we want nothing more than to have a treatment option to offer our patients that has demonstrated both the safety and efficacy to truly impact the lives of patients who live with peanut allergy,” said Christina Ciaccio, MD, chief of Allergy/Immunology and Pediatric Pulmonary Medicine at the University of Chicago Medical Center and Biological Sciences, in a company statement from Aimmune. “With today’s approval of Palforzia, we can – for the first time – offer children and teens with peanut allergy a proven medicine that employs an established therapeutic approach.”
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first drug to combat peanut allergy in children, (Palforzia, Aimmune Therapeutics), although those who take it must continue to avoid peanuts in their diets.
The peanut (Arachis hypogaea) allergen powder is also the first drug ever approved to treat a food allergy. It is not a cure, but it mitigates allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanuts, the FDA said in a news release.
Treatment with the oral powder, which is mixed into semisolid food – such as applesauce or yogurt – can be started in children aged 4 through 17 years who have a confirmed peanut allergy and then continued as a maintenance medication. Some 1 million American children have peanut allergy, and only a fifth will outgrow the allergy, the agency said.
“Because there is no cure, allergic individuals must strictly avoid exposure to prevent severe and potentially life-threatening reactions,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement.
An FDA advisory panel backed the medication in September 2019, but some committee members expressed concern about the large number of children in clinical trials who required epinephrine after receiving a dose of Palforzia.
The initial dose phase is given on a single day, while updosing consists of 11 increasing doses over several months. If the patient tolerates the first administration of an increased dose level, they may continue that dose daily at home. Daily maintenance begins after the completion of all updosing levels.
Palforzia will be available only through specially certified health care providers, health care settings, and pharmacies to patients enrolled in the REMS program, the agency said. Also, the initial dose escalation and first dose of each updosing level can be given only in a certified setting.
The agency said that patients or parents or caregivers must be counseled on the need for constant availability of injectable epinephrine, the need for continued dietary peanut avoidance, and on how to recognize the signs and symptoms of anaphylaxis.
‘Eagerly’ awaited
Palforzia’s effectiveness was based on a randomized, double-blind, placebo-controlled study involving about 500 peanut-allergic individuals that found that 67.2% of allergic patients tolerated an oral challenge with a single 600-mg dose of peanut protein with no more than mild allergic symptoms after 6 months of maintenance treatment, compared with 4% of placebo recipients, the FDA said.
In two double-blind, placebo-controlled studies looking at safety, the most commonly reported side effects among about 700 individuals involved in the research were abdominal pain, vomiting, nausea, tingling in the mouth, itching (including in the mouth and ears), cough, runny nose, throat irritation and tightness, hives, wheezing and shortness of breath, and anaphylaxis.
Palforzia should not be given to those with uncontrolled asthma and can’t be used for emergency treatment of allergic reactions, including anaphylaxis.
“The food allergy community has been eagerly awaiting an FDA-approved treatment that can help mitigate allergic reactions to peanut and, as allergists, we want nothing more than to have a treatment option to offer our patients that has demonstrated both the safety and efficacy to truly impact the lives of patients who live with peanut allergy,” said Christina Ciaccio, MD, chief of Allergy/Immunology and Pediatric Pulmonary Medicine at the University of Chicago Medical Center and Biological Sciences, in a company statement from Aimmune. “With today’s approval of Palforzia, we can – for the first time – offer children and teens with peanut allergy a proven medicine that employs an established therapeutic approach.”
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first drug to combat peanut allergy in children, (Palforzia, Aimmune Therapeutics), although those who take it must continue to avoid peanuts in their diets.
The peanut (Arachis hypogaea) allergen powder is also the first drug ever approved to treat a food allergy. It is not a cure, but it mitigates allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanuts, the FDA said in a news release.
Treatment with the oral powder, which is mixed into semisolid food – such as applesauce or yogurt – can be started in children aged 4 through 17 years who have a confirmed peanut allergy and then continued as a maintenance medication. Some 1 million American children have peanut allergy, and only a fifth will outgrow the allergy, the agency said.
“Because there is no cure, allergic individuals must strictly avoid exposure to prevent severe and potentially life-threatening reactions,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement.
An FDA advisory panel backed the medication in September 2019, but some committee members expressed concern about the large number of children in clinical trials who required epinephrine after receiving a dose of Palforzia.
The initial dose phase is given on a single day, while updosing consists of 11 increasing doses over several months. If the patient tolerates the first administration of an increased dose level, they may continue that dose daily at home. Daily maintenance begins after the completion of all updosing levels.
Palforzia will be available only through specially certified health care providers, health care settings, and pharmacies to patients enrolled in the REMS program, the agency said. Also, the initial dose escalation and first dose of each updosing level can be given only in a certified setting.
The agency said that patients or parents or caregivers must be counseled on the need for constant availability of injectable epinephrine, the need for continued dietary peanut avoidance, and on how to recognize the signs and symptoms of anaphylaxis.
‘Eagerly’ awaited
Palforzia’s effectiveness was based on a randomized, double-blind, placebo-controlled study involving about 500 peanut-allergic individuals that found that 67.2% of allergic patients tolerated an oral challenge with a single 600-mg dose of peanut protein with no more than mild allergic symptoms after 6 months of maintenance treatment, compared with 4% of placebo recipients, the FDA said.
In two double-blind, placebo-controlled studies looking at safety, the most commonly reported side effects among about 700 individuals involved in the research were abdominal pain, vomiting, nausea, tingling in the mouth, itching (including in the mouth and ears), cough, runny nose, throat irritation and tightness, hives, wheezing and shortness of breath, and anaphylaxis.
Palforzia should not be given to those with uncontrolled asthma and can’t be used for emergency treatment of allergic reactions, including anaphylaxis.
“The food allergy community has been eagerly awaiting an FDA-approved treatment that can help mitigate allergic reactions to peanut and, as allergists, we want nothing more than to have a treatment option to offer our patients that has demonstrated both the safety and efficacy to truly impact the lives of patients who live with peanut allergy,” said Christina Ciaccio, MD, chief of Allergy/Immunology and Pediatric Pulmonary Medicine at the University of Chicago Medical Center and Biological Sciences, in a company statement from Aimmune. “With today’s approval of Palforzia, we can – for the first time – offer children and teens with peanut allergy a proven medicine that employs an established therapeutic approach.”
This article first appeared on Medscape.com.
CDC: Opioid prescribing and use rates down since 2010
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
FROM MMWR SURVEILLANCE SUMMARIES
CDC: First person-to-person spread of novel coronavirus in U.S.
A Chicago woman in her 60s who tested positive for the 2019 Novel Coronavirus (2019-nCoV) after returning from Wuhan, China, earlier this month has infected her husband, becoming the first known instance of person-to-person transmission of the 2019-nCoV in the United States.
“Limited person-to-person spread of this new virus outside of China has already been seen in nine close contacts, where travelers were infected and transmitted the virus to someone else,” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during a press briefing on Jan. 30, 2020. “However, the full picture of how easy and how sustainable this virus can spread is unclear. Today’s news underscores the important risk-dependent exposure. The vast majority of Americans have not had recent travel to China, where sustained human-to-human transmission is occurring. Individuals who are close personal contacts of cases, though, could have a risk.”
The affected man, also in his 60s, is the spouse of the first confirmed travel-associated case of 2019-nCoV to be reported in the state of Illinois, according to Ngozi O. Ezike, MD, director of the Illinois Department of Public Health. The man had no history of recent travel to China. “This person-to-person spread was between two very close contacts: a wife and husband,” said Dr. Ezike, who added that 21 individuals in the state are under investigation for 2019-nCoV. “The virus is not spreading widely across the community. At this time, we are not recommending that people in the general public take additional precautions such as canceling activities or avoiding going out. While there is concern with this second case, public health officials are actively monitoring close contacts, including health care workers, and we believe that people in Illinois are at low risk.”
Jennifer Layden, MD, state epidemiologist at the Illinois Department of Public Health, said that the infected Chicago woman returned from Wuhan, China on Jan. 13, 2020. She is hospitalized in stable condition “and continues to do well,” Dr. Layden said. “Public health officials have been actively and closely monitoring individuals who had contacts with her, including her husband, who had close contact for symptoms. He recently began reporting symptoms and was immediately admitted to the hospital and placed in an isolation room, where he is in stable condition. We are actively monitoring individuals such as health care workers, household contacts, and others who were in contact with either of the confirmed cases in the goal to contain and reduce the risk of additional transmission.”
Nancy Messonnier, MD, director, National Center for Immunization and Respiratory Diseases, expects that more cases of 2019-nCoV will transpire in the United States.
“More cases means the potential for more person-to-person spread,” Dr. Messonnier said. “We’re trying to strike a balance in our response right now. We want to be aggressive, but we want our actions to be evidence-based and appropriate for the current circumstance. For example, CDC does not currently recommend use of face masks for the general public. The virus is not spreading in the general community.”
A Chicago woman in her 60s who tested positive for the 2019 Novel Coronavirus (2019-nCoV) after returning from Wuhan, China, earlier this month has infected her husband, becoming the first known instance of person-to-person transmission of the 2019-nCoV in the United States.
“Limited person-to-person spread of this new virus outside of China has already been seen in nine close contacts, where travelers were infected and transmitted the virus to someone else,” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during a press briefing on Jan. 30, 2020. “However, the full picture of how easy and how sustainable this virus can spread is unclear. Today’s news underscores the important risk-dependent exposure. The vast majority of Americans have not had recent travel to China, where sustained human-to-human transmission is occurring. Individuals who are close personal contacts of cases, though, could have a risk.”
The affected man, also in his 60s, is the spouse of the first confirmed travel-associated case of 2019-nCoV to be reported in the state of Illinois, according to Ngozi O. Ezike, MD, director of the Illinois Department of Public Health. The man had no history of recent travel to China. “This person-to-person spread was between two very close contacts: a wife and husband,” said Dr. Ezike, who added that 21 individuals in the state are under investigation for 2019-nCoV. “The virus is not spreading widely across the community. At this time, we are not recommending that people in the general public take additional precautions such as canceling activities or avoiding going out. While there is concern with this second case, public health officials are actively monitoring close contacts, including health care workers, and we believe that people in Illinois are at low risk.”
Jennifer Layden, MD, state epidemiologist at the Illinois Department of Public Health, said that the infected Chicago woman returned from Wuhan, China on Jan. 13, 2020. She is hospitalized in stable condition “and continues to do well,” Dr. Layden said. “Public health officials have been actively and closely monitoring individuals who had contacts with her, including her husband, who had close contact for symptoms. He recently began reporting symptoms and was immediately admitted to the hospital and placed in an isolation room, where he is in stable condition. We are actively monitoring individuals such as health care workers, household contacts, and others who were in contact with either of the confirmed cases in the goal to contain and reduce the risk of additional transmission.”
Nancy Messonnier, MD, director, National Center for Immunization and Respiratory Diseases, expects that more cases of 2019-nCoV will transpire in the United States.
“More cases means the potential for more person-to-person spread,” Dr. Messonnier said. “We’re trying to strike a balance in our response right now. We want to be aggressive, but we want our actions to be evidence-based and appropriate for the current circumstance. For example, CDC does not currently recommend use of face masks for the general public. The virus is not spreading in the general community.”
A Chicago woman in her 60s who tested positive for the 2019 Novel Coronavirus (2019-nCoV) after returning from Wuhan, China, earlier this month has infected her husband, becoming the first known instance of person-to-person transmission of the 2019-nCoV in the United States.
“Limited person-to-person spread of this new virus outside of China has already been seen in nine close contacts, where travelers were infected and transmitted the virus to someone else,” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during a press briefing on Jan. 30, 2020. “However, the full picture of how easy and how sustainable this virus can spread is unclear. Today’s news underscores the important risk-dependent exposure. The vast majority of Americans have not had recent travel to China, where sustained human-to-human transmission is occurring. Individuals who are close personal contacts of cases, though, could have a risk.”
The affected man, also in his 60s, is the spouse of the first confirmed travel-associated case of 2019-nCoV to be reported in the state of Illinois, according to Ngozi O. Ezike, MD, director of the Illinois Department of Public Health. The man had no history of recent travel to China. “This person-to-person spread was between two very close contacts: a wife and husband,” said Dr. Ezike, who added that 21 individuals in the state are under investigation for 2019-nCoV. “The virus is not spreading widely across the community. At this time, we are not recommending that people in the general public take additional precautions such as canceling activities or avoiding going out. While there is concern with this second case, public health officials are actively monitoring close contacts, including health care workers, and we believe that people in Illinois are at low risk.”
Jennifer Layden, MD, state epidemiologist at the Illinois Department of Public Health, said that the infected Chicago woman returned from Wuhan, China on Jan. 13, 2020. She is hospitalized in stable condition “and continues to do well,” Dr. Layden said. “Public health officials have been actively and closely monitoring individuals who had contacts with her, including her husband, who had close contact for symptoms. He recently began reporting symptoms and was immediately admitted to the hospital and placed in an isolation room, where he is in stable condition. We are actively monitoring individuals such as health care workers, household contacts, and others who were in contact with either of the confirmed cases in the goal to contain and reduce the risk of additional transmission.”
Nancy Messonnier, MD, director, National Center for Immunization and Respiratory Diseases, expects that more cases of 2019-nCoV will transpire in the United States.
“More cases means the potential for more person-to-person spread,” Dr. Messonnier said. “We’re trying to strike a balance in our response right now. We want to be aggressive, but we want our actions to be evidence-based and appropriate for the current circumstance. For example, CDC does not currently recommend use of face masks for the general public. The virus is not spreading in the general community.”
CDC: Risk in U.S. from 2019-nCoV remains low
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
HHS: Coronavirus risk low in U.S., vaccine development underway
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
CDC: Five confirmed 2019-nCoV cases in the U.S.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Zika virus: Birth defects rose fourfold in U.S. hardest-hit areas
according to the Centers for Disease Control and Prevention.
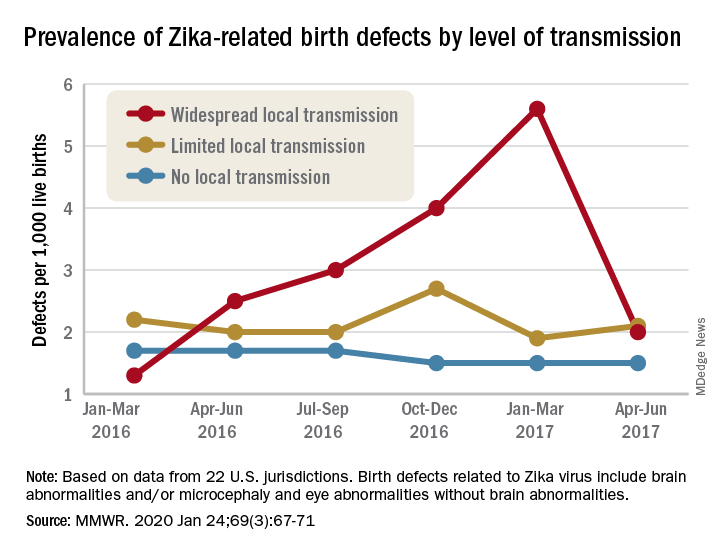
That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
FROM MMWR
EVALI update warns of chemicals in vaping products
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
FROM MMWR
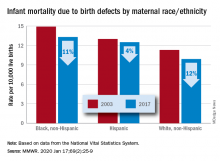
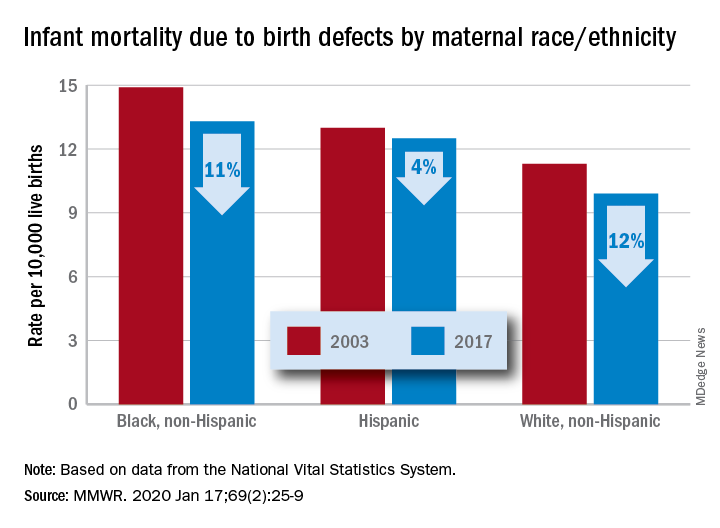
Infant deaths from birth defects decline, but some disparities widen
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
FROM MMWR
FDA approves first treatment for advanced epithelioid sarcoma
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.