User login
Patient-Reported Outcomes of Azelaic Acid Foam 15% for Patients With Papulopustular Rosacea: Secondary Efficacy Results From a Randomized, Controlled, Double-blind, Phase 3 Trial
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
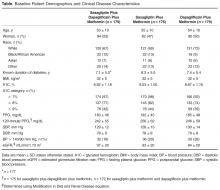
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.
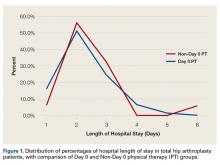
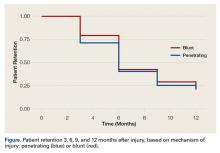
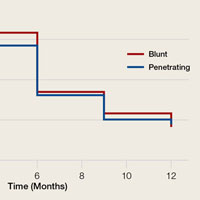
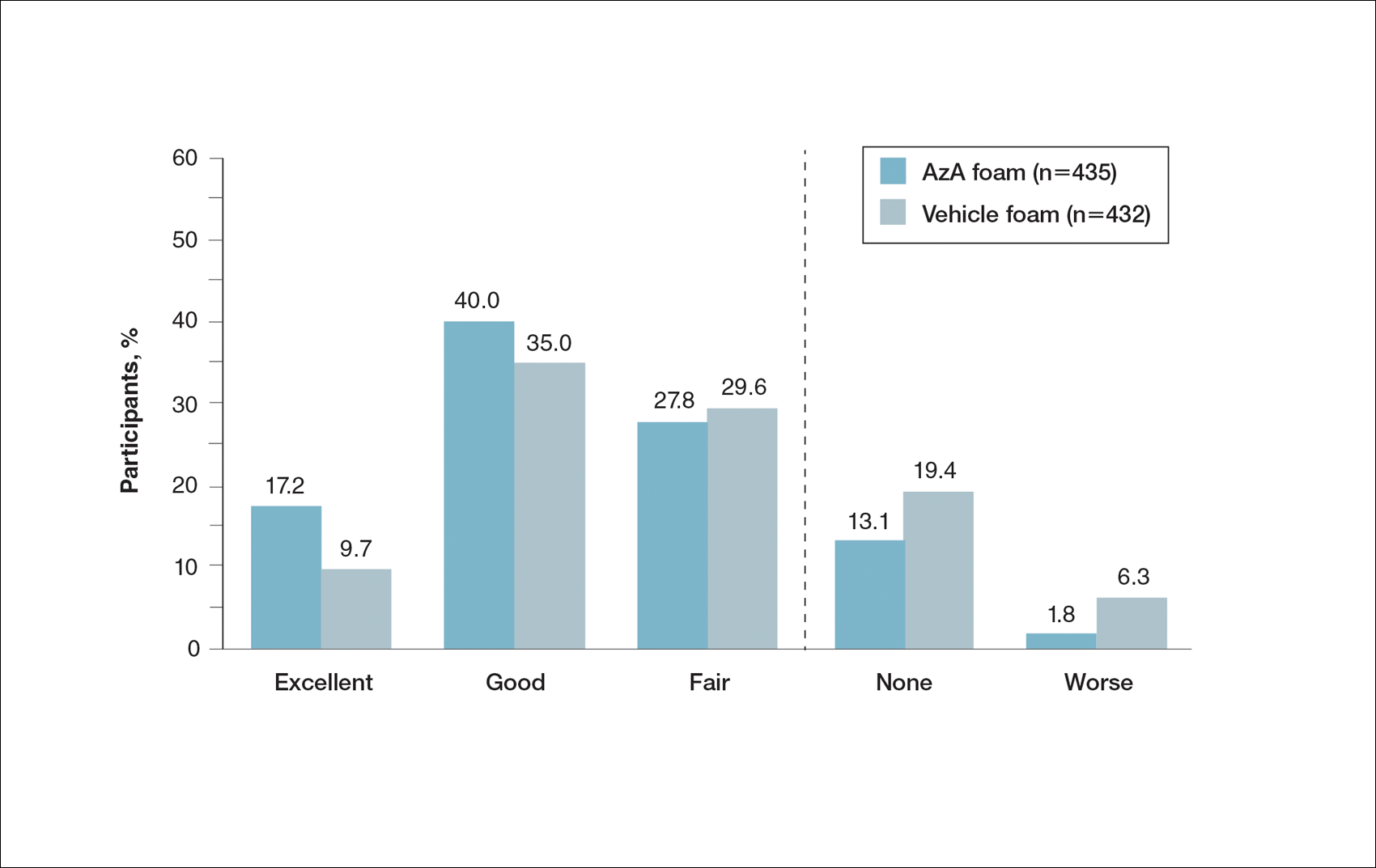
Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).
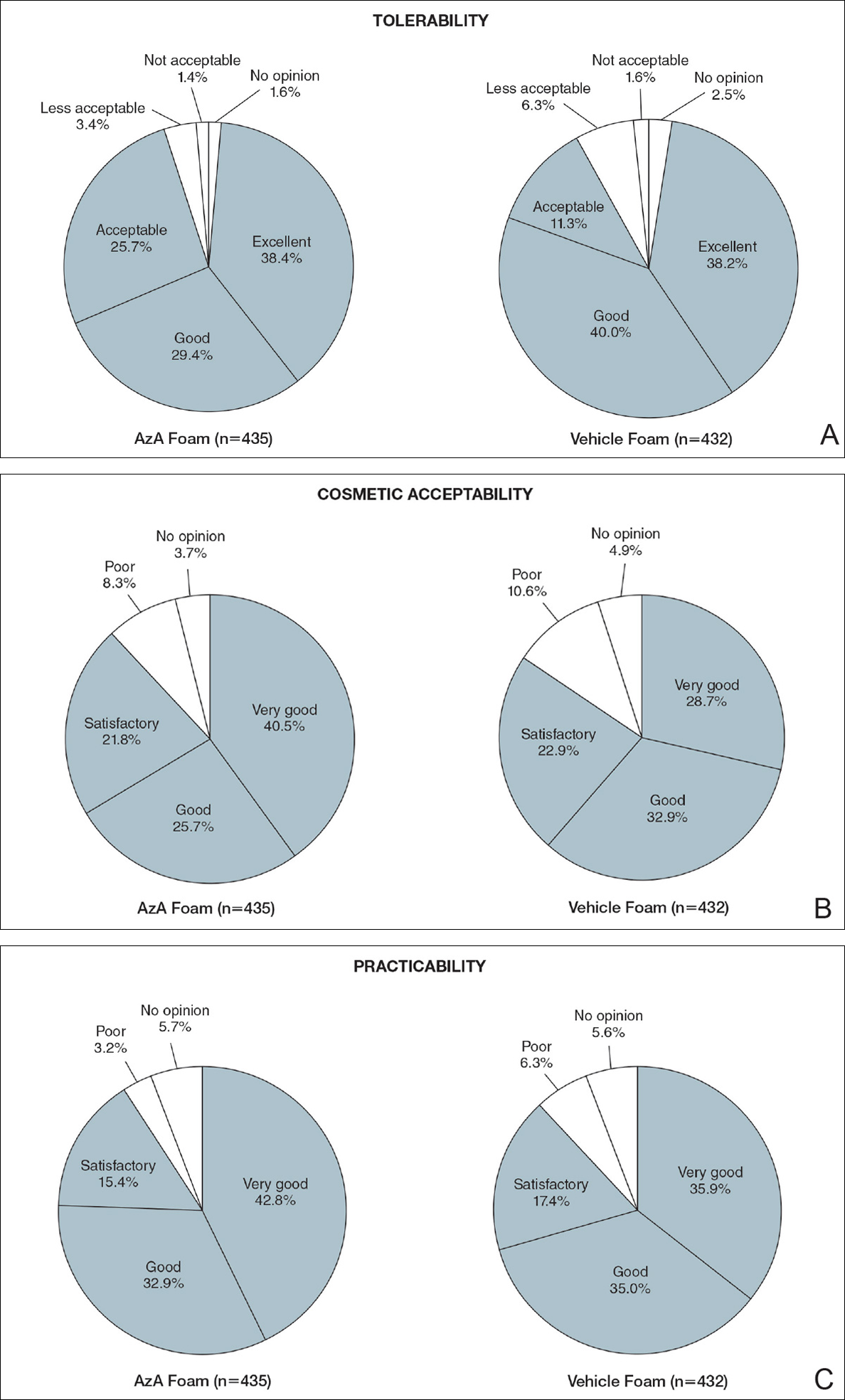
Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).
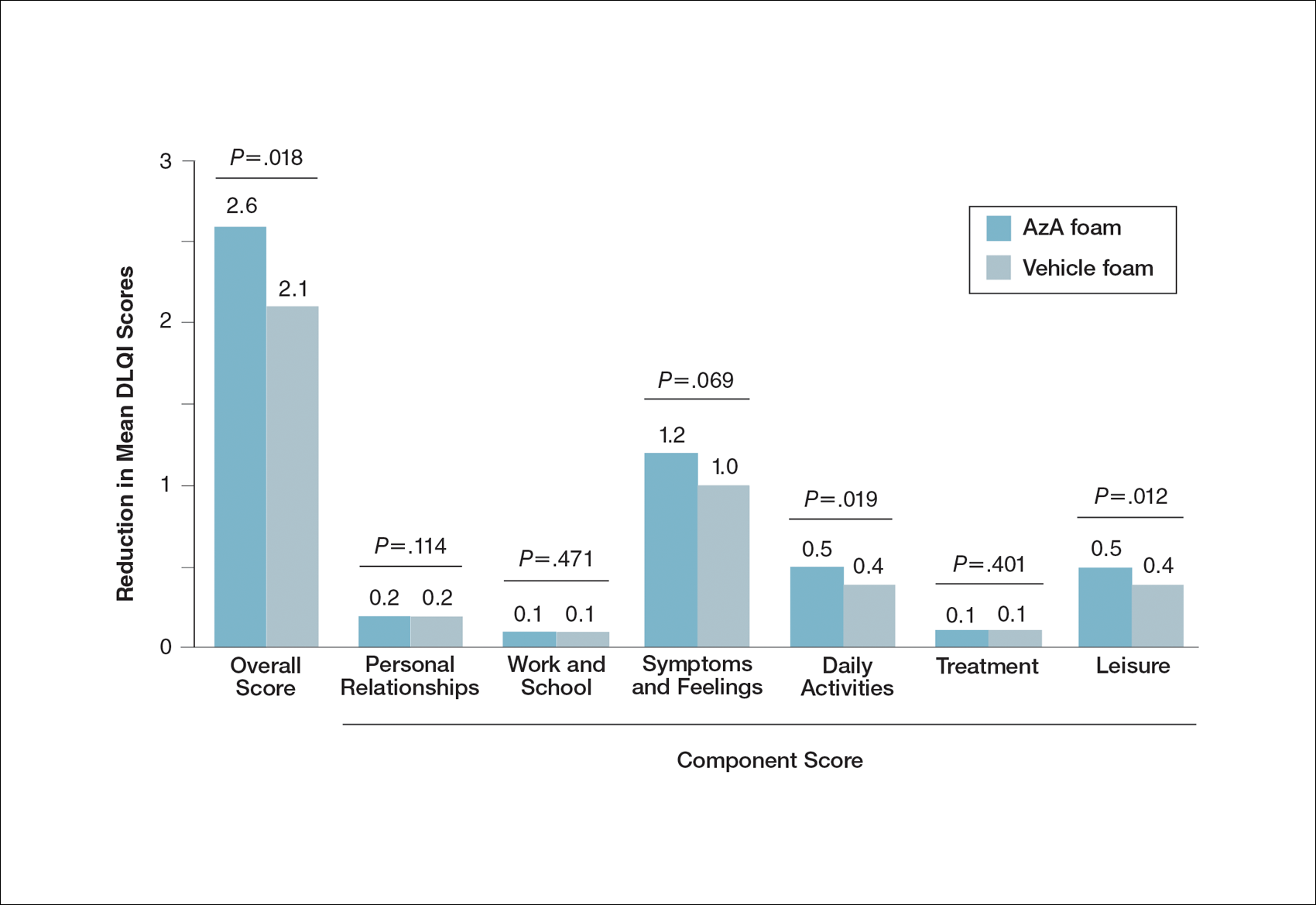
At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.
Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.
Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).

Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).

At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.

Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.
Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).

Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).

At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.

Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
Practice Points
- Patient perceptions of treatment are an important consideration in developing topical therapeutic strategies for papulopustular rosacea.
- A novel hydrophilic foam formulation of azelaic acid (AzA) provided substantial benefits in patient-reported measures of treatment response and quality of life.
- Patients reported high levels of satisfaction with the usability, tolerability, and practicability of AzA foam.
- The positive treatment experience described by patients parallels investigator-reported measures of clinical efficacy reported elsewhere.
Ceramic Femoral Heads for All Patients? An Argument for Cost Containment in Hip Surgery
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
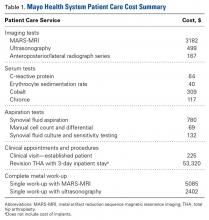
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
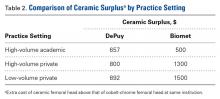
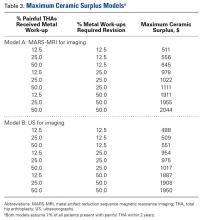
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Does Accelerated Physical Therapy After Elective Primary Hip and Knee Arthroplasty Facilitate Early Discharge?
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
Is It All in the Eye of the Beholder? Comparing Pulmonologists’ and Radiologists’ Performance
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
Fact or Fiction: Is Orthopedic Follow-Up Worse for Patients Who Sustain Penetrating Trauma?
There is a paucity of literature on how mechanism of injury may be associated with patient retention. Failure to attend outpatient clinics is a form of noncompliance and a major obstacle to safe, effective, and efficient healthcare delivery. Noncompliance may lead to increased patient morbidity and carries substantial financial implications for the healthcare system.1,2 In addition to these direct patient and healthcare issues, loss of patient follow-up or the belief of potential loss of follow-up of penetrating trauma patients may also significantly affect research studies. These patients often may be excluded from studies, even if they might otherwise meet inclusion criteria, because of concerns that they are unlikely to follow-up after leaving hospital. Is this myth or fact? To validate or to disprove this selection bias, we conducted a study in which we retrospectively evaluated long bone fractures caused by either penetrating or blunt trauma.
Methods
After obtaining Institutional Review Board approval for this study, we used the trauma database of an American College of Surgeons–verified level I trauma center in a major Midwest metropolitan area to compile a list of all cases of long bone fractures caused by penetrating trauma between 2006 and 2009 (N = 132). Gunshot wounds were the mechanism of injury for the penetrating trauma. We also compiled a list of control cases—long bone fractures caused by blunt trauma in patients demographically matched to the penetrating group patients on sex, race, and age (N = 104) (Table).
We retrospectively performed chart reviews to obtain patient follow-up data 3, 6, 9, and 12 months after injury from penetrating or blunt trauma. Patients scheduled to return on an as-needed basis were considered to have completed follow-up. The 2 groups were also statistically compared with respect to sex, race, age, surgical fixation, and history of tobacco, alcohol, or drug use.
SAS/STAT Version 8 (SAS Institute) was used to test the equality of survival functions (patient retention) for the penetrating and blunt trauma patient groups. A similar comparison was made for the categories of sex, race, and age. Pearson χ2 test was used to compare the 12-month survival rates of the 2 treatment groups across sex and race. Binary logistic regression was used to compare the 12-month survival rates of the 2 treatment groups removing the effect of age. A comparison of the frequency distributions of the 2 treatment groups with respect to alcohol use, tobacco use, drug use, and surgical intervention was also performed. Power analysis showed power of more than 90% in detecting at least a 20% difference in the follow-up rates between the penetrating and blunt trauma groups based on our sample size.
Results
There was no statistically significant difference (P = .736) between the penetrating and blunt trauma patients in terms of follow-up within 1 year after injury. At 1 year, 103 (78%) of the 132 penetrating trauma patients and 83 (80%) of the 104 blunt trauma patients were lost to follow-up (Figure).
Discussion
Trauma outcomes historically have been difficult to determine because of lack of patient follow-up. In a simulation series, Zelle and colleagues3 found that the turning point from significant to nonsignificant varied from 15% to 75% loss of follow-up, thus compromising the validity of a study. They and others have emphasized the importance of establishing research protocols to minimize follow-up loss and eliminate reporting bias, ensure randomization, and report accurate outcomes.3-7
Very few have tried to investigate factors associated with failure to follow up after trauma.1,2,4 Leukhardt and colleagues4 evaluated the medical services that trauma patients follow up with most often. Orthopedic surgery had the largest portion of follow-up visits (37%), followed by the trauma surgery clinic and the emergency department (19% each). The authors also found that penetrating trauma patients were more likely to follow up, though more than 90% of the authors’ patients had blunt trauma. Although our study did not support their finding, it does call into question the commonly held belief that penetrating trauma patients are less likely to follow up, as our study found no difference in follow-up between penetrating and blunt trauma patients.
One of the most interesting findings in this retrospective study is that almost 80% of patients were lost to follow-up regardless of mechanism of injury. Most prospective studies try to reduce loss to follow-up to below 10%. This difference may be attributable to having a dedicated research team and the resources required to ensure follow-up of research patients to improve follow-up beyond baseline values. At our institution, 13 prospective studies (most multicenter) are currently enrolling patients, and the worst loss to follow-up has been 30%. The majority of the studies have loss to follow-up of 15% or less. This low rate represents a significant difference from the 80% “baseline” clinical loss to follow-up for the blunt and penetrating trauma patients treated at our institution, based on the findings of this study. We have been improving follow-up by having dedicated research coordinators call patients to remind them of their appointments (all clinic patients who are not research patients receive a recorded reminder); by having the hospital agree that research patients can be seen without charge (by the facility or the physician), which helps defray costs to the patient; and by excluding patients the principal investigator thinks are unlikely to follow up. Patients unlikely to follow up are routinely excluded by all centers that enroll in prospective studies. Although it is difficult to quantitate, this factor may play a large role in reducing loss to follow-up. Penetrating trauma patients historically routinely biased investigators to exclude them from studies, regardless of whether being considered unlikely to follow-up was an exclusion criterion. Our study results suggest this bias may not be valid.
Our study evaluated the role of mechanism of injury, penetrating or blunt trauma, and the respective orthopedic follow-up. There was no statistically significant difference in the 1-year follow-up rate based on the mechanism of injury. Our study was conducted with a well-matched control group that eliminated potential confounding variables, such as sex, race, age, tobacco use, and alcohol use. Although the prevalence of drug use was higher in the penetrating trauma group, patient retention seemed not to be affected by it. Surprisingly, patient loss to follow-up was extremely high (almost 80%) for both the penetrating and blunt trauma patient groups at the 1-year mark. Our findings call into question the commonly accepted theory that patients with penetrating injuries are less likely to follow up, at least in an academic level I trauma center population. We suggest that the commonly held belief that penetrating trauma patients are less likely to follow up may not be valid and that, when prospective studies are designed, it may not be appropriate to exclude penetrating trauma patients on this basis alone.
The primary limitation of this study is that it was performed at a single institution. Eighty-five percent of blunt trauma patients and 93% of penetrating trauma patients live in the county that is predominantly served by our institution, and electronic medical records from all major hospitals in the metropolitan area are linked, suggesting that the large majority of patients lost to follow-up do not seek further medical care, at least not from local facilities in our metropolitan area. A prospective multicenter study is being designed to help us gain a better understanding of the variables that affect musculoskeletal trauma patient follow-up and learn interventional strategies that can be used to improve patient retention.
Dr. Turner is an Orthopedic Surgeon, Rockwood Clinic, Spokane, Washington. Dr. Turner was a resident at the time the article was written. Dr. Hiatt is an Anesthesia Resident, University of Louisville Department of Anesthesiology and Perioperative Medicine, Louisville, Kentucky. Dr. Mullis is Chief of the Orthopaedic Trauma Service, Eskenazi Health, and Professor & Program Director, Indiana University School of Medicine Department of Orthopaedics, Indianapolis, Indiana.
Acknowledgments: This study was first reported in a poster presentation at the annual meeting of the Orthopaedic Trauma Association, October 2013, Phoenix, Arizona.
The authors gratefully acknowledge and thank Jyoti Sarkar, PhD, for his assistance with statistical analysis and manuscript preparation.
Am J Orthop. 2016;45(6):E331-E334. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sciberras N, Gregori A, Holt G. The ethical and practical challenges of patient noncompliance in orthopaedic surgery. J Bone Joint Surg Am. 2013;95(9):e61.
2. Sharma H, Crane E, Syme B, Foxworthy M. Non-compliance in orthopaedic surgery and its ethical challenges. Orthop Trauma. 2007;21(4):310-313.
3. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181.
4. Leukhardt WH, Golob JF, McCoy AM, Fadlalla AM, Malangoni MA, Claridge JA. Follow-up disparities after trauma: a real problem for outcomes research. Am J Surg. 2010;199(3):348-352.
5. Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000;21(5 suppl):226S-232S.
6. Smith JS, Watts HG. Methods for locating missing patients for the purpose of long-term clinical studies. J Bone Joint Surg Am. 1998;80(3):431-438.
7. Sprague S, Leece P, Bhandari M, Tornetta P 3rd, Schemitsch E, Swiontkowski MF; S.P.R.I.N.T. Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725.
There is a paucity of literature on how mechanism of injury may be associated with patient retention. Failure to attend outpatient clinics is a form of noncompliance and a major obstacle to safe, effective, and efficient healthcare delivery. Noncompliance may lead to increased patient morbidity and carries substantial financial implications for the healthcare system.1,2 In addition to these direct patient and healthcare issues, loss of patient follow-up or the belief of potential loss of follow-up of penetrating trauma patients may also significantly affect research studies. These patients often may be excluded from studies, even if they might otherwise meet inclusion criteria, because of concerns that they are unlikely to follow-up after leaving hospital. Is this myth or fact? To validate or to disprove this selection bias, we conducted a study in which we retrospectively evaluated long bone fractures caused by either penetrating or blunt trauma.
Methods
After obtaining Institutional Review Board approval for this study, we used the trauma database of an American College of Surgeons–verified level I trauma center in a major Midwest metropolitan area to compile a list of all cases of long bone fractures caused by penetrating trauma between 2006 and 2009 (N = 132). Gunshot wounds were the mechanism of injury for the penetrating trauma. We also compiled a list of control cases—long bone fractures caused by blunt trauma in patients demographically matched to the penetrating group patients on sex, race, and age (N = 104) (Table).
We retrospectively performed chart reviews to obtain patient follow-up data 3, 6, 9, and 12 months after injury from penetrating or blunt trauma. Patients scheduled to return on an as-needed basis were considered to have completed follow-up. The 2 groups were also statistically compared with respect to sex, race, age, surgical fixation, and history of tobacco, alcohol, or drug use.
SAS/STAT Version 8 (SAS Institute) was used to test the equality of survival functions (patient retention) for the penetrating and blunt trauma patient groups. A similar comparison was made for the categories of sex, race, and age. Pearson χ2 test was used to compare the 12-month survival rates of the 2 treatment groups across sex and race. Binary logistic regression was used to compare the 12-month survival rates of the 2 treatment groups removing the effect of age. A comparison of the frequency distributions of the 2 treatment groups with respect to alcohol use, tobacco use, drug use, and surgical intervention was also performed. Power analysis showed power of more than 90% in detecting at least a 20% difference in the follow-up rates between the penetrating and blunt trauma groups based on our sample size.
Results
There was no statistically significant difference (P = .736) between the penetrating and blunt trauma patients in terms of follow-up within 1 year after injury. At 1 year, 103 (78%) of the 132 penetrating trauma patients and 83 (80%) of the 104 blunt trauma patients were lost to follow-up (Figure).
Discussion
Trauma outcomes historically have been difficult to determine because of lack of patient follow-up. In a simulation series, Zelle and colleagues3 found that the turning point from significant to nonsignificant varied from 15% to 75% loss of follow-up, thus compromising the validity of a study. They and others have emphasized the importance of establishing research protocols to minimize follow-up loss and eliminate reporting bias, ensure randomization, and report accurate outcomes.3-7
Very few have tried to investigate factors associated with failure to follow up after trauma.1,2,4 Leukhardt and colleagues4 evaluated the medical services that trauma patients follow up with most often. Orthopedic surgery had the largest portion of follow-up visits (37%), followed by the trauma surgery clinic and the emergency department (19% each). The authors also found that penetrating trauma patients were more likely to follow up, though more than 90% of the authors’ patients had blunt trauma. Although our study did not support their finding, it does call into question the commonly held belief that penetrating trauma patients are less likely to follow up, as our study found no difference in follow-up between penetrating and blunt trauma patients.
One of the most interesting findings in this retrospective study is that almost 80% of patients were lost to follow-up regardless of mechanism of injury. Most prospective studies try to reduce loss to follow-up to below 10%. This difference may be attributable to having a dedicated research team and the resources required to ensure follow-up of research patients to improve follow-up beyond baseline values. At our institution, 13 prospective studies (most multicenter) are currently enrolling patients, and the worst loss to follow-up has been 30%. The majority of the studies have loss to follow-up of 15% or less. This low rate represents a significant difference from the 80% “baseline” clinical loss to follow-up for the blunt and penetrating trauma patients treated at our institution, based on the findings of this study. We have been improving follow-up by having dedicated research coordinators call patients to remind them of their appointments (all clinic patients who are not research patients receive a recorded reminder); by having the hospital agree that research patients can be seen without charge (by the facility or the physician), which helps defray costs to the patient; and by excluding patients the principal investigator thinks are unlikely to follow up. Patients unlikely to follow up are routinely excluded by all centers that enroll in prospective studies. Although it is difficult to quantitate, this factor may play a large role in reducing loss to follow-up. Penetrating trauma patients historically routinely biased investigators to exclude them from studies, regardless of whether being considered unlikely to follow-up was an exclusion criterion. Our study results suggest this bias may not be valid.
Our study evaluated the role of mechanism of injury, penetrating or blunt trauma, and the respective orthopedic follow-up. There was no statistically significant difference in the 1-year follow-up rate based on the mechanism of injury. Our study was conducted with a well-matched control group that eliminated potential confounding variables, such as sex, race, age, tobacco use, and alcohol use. Although the prevalence of drug use was higher in the penetrating trauma group, patient retention seemed not to be affected by it. Surprisingly, patient loss to follow-up was extremely high (almost 80%) for both the penetrating and blunt trauma patient groups at the 1-year mark. Our findings call into question the commonly accepted theory that patients with penetrating injuries are less likely to follow up, at least in an academic level I trauma center population. We suggest that the commonly held belief that penetrating trauma patients are less likely to follow up may not be valid and that, when prospective studies are designed, it may not be appropriate to exclude penetrating trauma patients on this basis alone.
The primary limitation of this study is that it was performed at a single institution. Eighty-five percent of blunt trauma patients and 93% of penetrating trauma patients live in the county that is predominantly served by our institution, and electronic medical records from all major hospitals in the metropolitan area are linked, suggesting that the large majority of patients lost to follow-up do not seek further medical care, at least not from local facilities in our metropolitan area. A prospective multicenter study is being designed to help us gain a better understanding of the variables that affect musculoskeletal trauma patient follow-up and learn interventional strategies that can be used to improve patient retention.
Dr. Turner is an Orthopedic Surgeon, Rockwood Clinic, Spokane, Washington. Dr. Turner was a resident at the time the article was written. Dr. Hiatt is an Anesthesia Resident, University of Louisville Department of Anesthesiology and Perioperative Medicine, Louisville, Kentucky. Dr. Mullis is Chief of the Orthopaedic Trauma Service, Eskenazi Health, and Professor & Program Director, Indiana University School of Medicine Department of Orthopaedics, Indianapolis, Indiana.
Acknowledgments: This study was first reported in a poster presentation at the annual meeting of the Orthopaedic Trauma Association, October 2013, Phoenix, Arizona.
The authors gratefully acknowledge and thank Jyoti Sarkar, PhD, for his assistance with statistical analysis and manuscript preparation.
Am J Orthop. 2016;45(6):E331-E334. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
There is a paucity of literature on how mechanism of injury may be associated with patient retention. Failure to attend outpatient clinics is a form of noncompliance and a major obstacle to safe, effective, and efficient healthcare delivery. Noncompliance may lead to increased patient morbidity and carries substantial financial implications for the healthcare system.1,2 In addition to these direct patient and healthcare issues, loss of patient follow-up or the belief of potential loss of follow-up of penetrating trauma patients may also significantly affect research studies. These patients often may be excluded from studies, even if they might otherwise meet inclusion criteria, because of concerns that they are unlikely to follow-up after leaving hospital. Is this myth or fact? To validate or to disprove this selection bias, we conducted a study in which we retrospectively evaluated long bone fractures caused by either penetrating or blunt trauma.
Methods
After obtaining Institutional Review Board approval for this study, we used the trauma database of an American College of Surgeons–verified level I trauma center in a major Midwest metropolitan area to compile a list of all cases of long bone fractures caused by penetrating trauma between 2006 and 2009 (N = 132). Gunshot wounds were the mechanism of injury for the penetrating trauma. We also compiled a list of control cases—long bone fractures caused by blunt trauma in patients demographically matched to the penetrating group patients on sex, race, and age (N = 104) (Table).
We retrospectively performed chart reviews to obtain patient follow-up data 3, 6, 9, and 12 months after injury from penetrating or blunt trauma. Patients scheduled to return on an as-needed basis were considered to have completed follow-up. The 2 groups were also statistically compared with respect to sex, race, age, surgical fixation, and history of tobacco, alcohol, or drug use.
SAS/STAT Version 8 (SAS Institute) was used to test the equality of survival functions (patient retention) for the penetrating and blunt trauma patient groups. A similar comparison was made for the categories of sex, race, and age. Pearson χ2 test was used to compare the 12-month survival rates of the 2 treatment groups across sex and race. Binary logistic regression was used to compare the 12-month survival rates of the 2 treatment groups removing the effect of age. A comparison of the frequency distributions of the 2 treatment groups with respect to alcohol use, tobacco use, drug use, and surgical intervention was also performed. Power analysis showed power of more than 90% in detecting at least a 20% difference in the follow-up rates between the penetrating and blunt trauma groups based on our sample size.
Results
There was no statistically significant difference (P = .736) between the penetrating and blunt trauma patients in terms of follow-up within 1 year after injury. At 1 year, 103 (78%) of the 132 penetrating trauma patients and 83 (80%) of the 104 blunt trauma patients were lost to follow-up (Figure).
Discussion
Trauma outcomes historically have been difficult to determine because of lack of patient follow-up. In a simulation series, Zelle and colleagues3 found that the turning point from significant to nonsignificant varied from 15% to 75% loss of follow-up, thus compromising the validity of a study. They and others have emphasized the importance of establishing research protocols to minimize follow-up loss and eliminate reporting bias, ensure randomization, and report accurate outcomes.3-7
Very few have tried to investigate factors associated with failure to follow up after trauma.1,2,4 Leukhardt and colleagues4 evaluated the medical services that trauma patients follow up with most often. Orthopedic surgery had the largest portion of follow-up visits (37%), followed by the trauma surgery clinic and the emergency department (19% each). The authors also found that penetrating trauma patients were more likely to follow up, though more than 90% of the authors’ patients had blunt trauma. Although our study did not support their finding, it does call into question the commonly held belief that penetrating trauma patients are less likely to follow up, as our study found no difference in follow-up between penetrating and blunt trauma patients.
One of the most interesting findings in this retrospective study is that almost 80% of patients were lost to follow-up regardless of mechanism of injury. Most prospective studies try to reduce loss to follow-up to below 10%. This difference may be attributable to having a dedicated research team and the resources required to ensure follow-up of research patients to improve follow-up beyond baseline values. At our institution, 13 prospective studies (most multicenter) are currently enrolling patients, and the worst loss to follow-up has been 30%. The majority of the studies have loss to follow-up of 15% or less. This low rate represents a significant difference from the 80% “baseline” clinical loss to follow-up for the blunt and penetrating trauma patients treated at our institution, based on the findings of this study. We have been improving follow-up by having dedicated research coordinators call patients to remind them of their appointments (all clinic patients who are not research patients receive a recorded reminder); by having the hospital agree that research patients can be seen without charge (by the facility or the physician), which helps defray costs to the patient; and by excluding patients the principal investigator thinks are unlikely to follow up. Patients unlikely to follow up are routinely excluded by all centers that enroll in prospective studies. Although it is difficult to quantitate, this factor may play a large role in reducing loss to follow-up. Penetrating trauma patients historically routinely biased investigators to exclude them from studies, regardless of whether being considered unlikely to follow-up was an exclusion criterion. Our study results suggest this bias may not be valid.
Our study evaluated the role of mechanism of injury, penetrating or blunt trauma, and the respective orthopedic follow-up. There was no statistically significant difference in the 1-year follow-up rate based on the mechanism of injury. Our study was conducted with a well-matched control group that eliminated potential confounding variables, such as sex, race, age, tobacco use, and alcohol use. Although the prevalence of drug use was higher in the penetrating trauma group, patient retention seemed not to be affected by it. Surprisingly, patient loss to follow-up was extremely high (almost 80%) for both the penetrating and blunt trauma patient groups at the 1-year mark. Our findings call into question the commonly accepted theory that patients with penetrating injuries are less likely to follow up, at least in an academic level I trauma center population. We suggest that the commonly held belief that penetrating trauma patients are less likely to follow up may not be valid and that, when prospective studies are designed, it may not be appropriate to exclude penetrating trauma patients on this basis alone.
The primary limitation of this study is that it was performed at a single institution. Eighty-five percent of blunt trauma patients and 93% of penetrating trauma patients live in the county that is predominantly served by our institution, and electronic medical records from all major hospitals in the metropolitan area are linked, suggesting that the large majority of patients lost to follow-up do not seek further medical care, at least not from local facilities in our metropolitan area. A prospective multicenter study is being designed to help us gain a better understanding of the variables that affect musculoskeletal trauma patient follow-up and learn interventional strategies that can be used to improve patient retention.
Dr. Turner is an Orthopedic Surgeon, Rockwood Clinic, Spokane, Washington. Dr. Turner was a resident at the time the article was written. Dr. Hiatt is an Anesthesia Resident, University of Louisville Department of Anesthesiology and Perioperative Medicine, Louisville, Kentucky. Dr. Mullis is Chief of the Orthopaedic Trauma Service, Eskenazi Health, and Professor & Program Director, Indiana University School of Medicine Department of Orthopaedics, Indianapolis, Indiana.
Acknowledgments: This study was first reported in a poster presentation at the annual meeting of the Orthopaedic Trauma Association, October 2013, Phoenix, Arizona.
The authors gratefully acknowledge and thank Jyoti Sarkar, PhD, for his assistance with statistical analysis and manuscript preparation.
Am J Orthop. 2016;45(6):E331-E334. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sciberras N, Gregori A, Holt G. The ethical and practical challenges of patient noncompliance in orthopaedic surgery. J Bone Joint Surg Am. 2013;95(9):e61.
2. Sharma H, Crane E, Syme B, Foxworthy M. Non-compliance in orthopaedic surgery and its ethical challenges. Orthop Trauma. 2007;21(4):310-313.
3. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181.
4. Leukhardt WH, Golob JF, McCoy AM, Fadlalla AM, Malangoni MA, Claridge JA. Follow-up disparities after trauma: a real problem for outcomes research. Am J Surg. 2010;199(3):348-352.
5. Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000;21(5 suppl):226S-232S.
6. Smith JS, Watts HG. Methods for locating missing patients for the purpose of long-term clinical studies. J Bone Joint Surg Am. 1998;80(3):431-438.
7. Sprague S, Leece P, Bhandari M, Tornetta P 3rd, Schemitsch E, Swiontkowski MF; S.P.R.I.N.T. Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725.
1. Sciberras N, Gregori A, Holt G. The ethical and practical challenges of patient noncompliance in orthopaedic surgery. J Bone Joint Surg Am. 2013;95(9):e61.
2. Sharma H, Crane E, Syme B, Foxworthy M. Non-compliance in orthopaedic surgery and its ethical challenges. Orthop Trauma. 2007;21(4):310-313.
3. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181.
4. Leukhardt WH, Golob JF, McCoy AM, Fadlalla AM, Malangoni MA, Claridge JA. Follow-up disparities after trauma: a real problem for outcomes research. Am J Surg. 2010;199(3):348-352.
5. Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000;21(5 suppl):226S-232S.
6. Smith JS, Watts HG. Methods for locating missing patients for the purpose of long-term clinical studies. J Bone Joint Surg Am. 1998;80(3):431-438.
7. Sprague S, Leece P, Bhandari M, Tornetta P 3rd, Schemitsch E, Swiontkowski MF; S.P.R.I.N.T. Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725.
Impact of a Musculoskeletal Clerkship on Orthopedic Surgery Applicant Diversity
As the United States becomes increasingly diverse, with predictions that by 2045 minorities will comprise 50% or more of the population,1 the demographics of the orthopedic surgery population will also likely diversify. It is important that orthopedic surgeons shift in their diversity as well. Lack of diversity in orthopedics (women and racial minorities are underrepresented) relative to the national population and other surgical specialties and their training programs is well documented.2-8
More concerning, the diversity of orthopedic residents does not compare favorably with that of medical school attendees.4,9 The difference suggests the greatest loss of potential diversity occurs during the transition from medical school to residency. A national study demonstrated that instruction in musculoskeletal medicine led to an increase in application rates nationally.10 However, the authors of that study stated they were unexpectedly limited by its large size—they could not validate the accuracy of curriculum data and could not differentiate between a 1-day required experience and a 4-week rotation.
In the present study, which accounted for curricular factors, we compared our medical students’ application rates to orthopedics residencies based on sex and race before and after introduction of a required third-year musculoskeletal clerkship. We hypothesized that making the curriculum a requirement would increase the number of applicants and increase the diversity of applicants in terms of both women and underrepresented minorities. This hypothesis was based on the rationale that these groups might not consider an orthopedics residency without first being directly exposed to orthopedics. We also wanted to determine what factors influenced applicants to choose orthopedic surgery.
Methods
Curriculum
Before 2006, third-year students spent 3 months completing a surgery clerkship. Some students interested in orthopedic surgery would have to wait until their fourth year to complete an elective in orthopedic surgery, and uninterested students would not be exposed at all. Starting in 2006, 1 month of the third-year surgery clerkship was required to be completed in musculoskeletal surgery: orthopedic surgery, plastic surgery, or neurosurgical spine. Plastic surgery was an option, as it exposed students to hand surgery and flap reconstruction.
During the 12-year study period, overall teaching hours in the preclinical curriculum did not change, and there were no other structural changes to the preclinical or clinical curriculum. The orthopedics department increased its faculty from 23 in 2000 to 34 in 2012. Number of female faculty increased from 1 to 3, representing a 4% to 9% increase in department faculty. Throughout the 12 years, there were no underrepresented minority faculty. Total number of residents increased from 26 in 2000 to 30 in 2012. Number of female residents varied year to year, from a low of 3 in the period 2003–2004 to a high of 11 in the period 2009–2010. Number of underrepresented minority residents varied yearly as well, from 1 to 2.
Data Collection
After this study was granted exempt status by our Institutional Review Board, we obtained student data from our registrar. Data included graduation year, self-identified sex and race, exposure to orthopedic surgery during clerkships, and matching residency specialty. National data were obtained from the Electronic Residency Application Service for the periods 2002–2007 and 2009–2012. These data included all US allopathic medical students’ self-identified sex and race, and applied-to primary residency specialty. National data from 2008 and national data on sex differences in orthopedic applications from 2009 were not available.
Graduates who matched into orthopedic surgery were asked to complete an anonymous survey on what influenced their decision to apply to orthopedic surgery and when this decision was made. Our goal with the survey was to substantiate or refute the conclusion that application rates depended on third-year exposure to musculoskeletal medicine.
Statistical Methods
Students were divided into 2 groups: precurriculum (graduated within 7-year period, 2000–2006) and postcurriculum (graduated within 6-year period, 2007–2012). A 2-sample test for proportions was used to compare percentage of total students who applied to orthopedics in each group. In the group of students who applied to orthopedics, we compared precurriculum and postcurriculum proportions of women and underrepresented minorities (non-white, non-Asian). We also compared these proportions with national data (using 2-sample tests for proportions) to determine if any change in diversity of our institution’s applicants was mirroring a national trend. Our definition of underrepresented minority was based on work that showed that the proportion of Asian matriculants in medical school and the proportion of applicants to orthopedics are higher than their respective national proportions.5 Survey data are reported descriptively. Statistical significance was defined with a 2-tailed α of 0.05 for all tests.
Results
Over the 2000–2012 period, 1507 students from our institution successfully applied to residency programs: 792 in the precurriculum group and 715 in the postcurriculum group. Of these students, 91 successfully applied to orthopedic surgery: 48 in the precurriculum group (applied before introduction of the required clerkship) and 43 in the postcurriculum group (applied afterward).
Over the 2002–2012 period, 10,100 US allopathic medical students applied to orthopedic residency programs: 4769 students between 2002 and 2006 and 5331 students between 2007 and 2012.
Before the musculoskeletal clerkship was required, 317 (40%) of the 792 precurriculum students were exposed to orthopedics during their third year. During this period, 42 of the 48 orthopedic surgery applicants completed an orthopedic surgery rotation during their third year of medical school. After the clerkship was required, 465 (65%) of the 715 postcurriculum students were exposed to orthopedics during their third year, including all 43 orthopedic surgery applicants (100% of students were exposed to musculoskeletal surgery, including plastic surgery and neurologic spine). The 25% increase in exposure to orthopedic surgery during the third year was statistically significant (P < .0001), but there was no resultant increase in overall percentage of students applying to orthopedic residencies (6% in each case; P = .98).
Over the 12-year study period, the proportion of female medical students at our institution declined from 50% (395/792) to 46% (328/715) (P = .13). However, there was an 81% relative increase, from 17% (8/48) before introduction of the clerkship to 30% (13/43) afterward, in the proportion of female applicants to orthopedic surgery. This contrasted with national data showing the percentage of female applicants to orthopedic surgery remained stable from 2002–2006 (14%, 675/4758) to 2007–2012 (15%, 643/4277). Before the clerkship was required, the proportion of female applicants from our institution was similar to national rates (P = .50). Afterward, our institution produced a significantly higher proportion of female applicants compared with the national proportion (P = .026).
Over the 12-year period, our self-identified underrepresented minority medical student population increased significantly (P = .02), from 13% (103/792) to 17% (124/715). The relative proportion of underrepresented minority orthopedic surgery applicants increased by 101%, from 10% (5/48) before the clerkship was required to 21% (9/43) afterward. Nationally, over the same period, underrepresented minorities’ orthopedic surgery application rates increased significantly (P < .001), from 16% (763/4769) to 19% (1002/5331). The proportion of underrepresented racial minorities that applied did not differ significantly between our institution and nationally for the years either before (P = .97) or after (P = .68) introduction of the curriculum.
Surveys were completed by 58 (64%) of 91 graduates (21 women, 70 men). Respondents’ characteristics are listed in Table 4.
Discussion
Orthopedic surgery needs a more diverse workforce11-17 in order to better mirror the population served, bring care to underserved areas,18-26 and provide better training environments.27 Several hypotheses about the lack of diversity have been posited: stereotypes about the specialty,28-31 lack of interest among minority medical students, and lack of exposure to the specialty.5,6,32,33
Lack of exposure deserves scrutiny, as a large proportion of medical students who choose to apply to orthopedic surgery make their decision before entering medical school, which is not typical.33 Such a finding suggests that exposure to orthopedic surgery is lacking, especially given that an orthopedic surgery rotation is usually not required during the clinical years. The idea that increased exposure to orthopedics affects application patterns is logical, as clinical exposure has been shown to play a role in medical students’ choice of specialty.34
Exposure helps in several key areas. Firsthand experience can help dispel stereotypes, such as the idea that success in orthopedic surgery depends on physical strength and that only former athletes pursue orthopedics.28-31 Authors have also reported on a perceived negative bias against women: Orthopedics is an “old boys’ network”; women will not fit in and need not apply; the orthopedic lifestyle is difficult and not conducive to a satisfying personal life.9 Requiring exposure ensures that all students, but especially women, can gain firsthand experience that can show these stereotypes to be false. Beyond dispelling these stereotypes, exposure to orthopedic surgery is essential for women, as studies have shown that clinical rotations play a larger role in determining specialty choice for women compared to men,33 and this would be particularly critical for specialties they may not be initially considering.
A national study found that requiring an orthopedic/musculoskeletal clerkship led to a 12% relative increase in the application rate, from 5.1% to 5.7%, and to an increase in applicant diversity (race, sex).10 However, the investigators could not determine individual reasons for specialty choice or the exact nature of each institution’s musculoskeletal curriculum. Confirming these factors, we found an 81% increase in number of female applicants and a 101% increase in number of underrepresented minority applicants after introduction of the required third-year musculoskeletal surgery clerkship at our institution.
We were unable to replicate the 12% relative increase in the overall application rate; our orthopedic surgery match rate remained 6%. Our findings cannot directly explain this, but we have several hypotheses. First, whereas other studies measured the application rate, we measured the successful match rate, given our data structure. This difference in data definition could account for some of the discrepancy. Second, we did not account for individuals’ academic success, and career counseling is paramount in decisions regarding residency specialties. It is possible we are substituting qualified female and underrepresented minority candidates for less-than-qualified male applicants. Third, the 25% increase in medical student exposure to orthopedic surgery led to a corresponding increase in number of orthopedic faculty providing undergraduate medical education. Some of these faculty could have been inexperienced in undergraduate medical education, and thus the teaching environment may not have been optimal.
Our study had several limitations. First, our institution has limited racial diversity. Over the past 12 years, only 15% of our students have been underrepresented minorities. (Nationally, the proportion is closer to 18%.) This may have limited the ability of our orthopedic rotation to affect the proportion of underrepresented minority applicants. Second, this study involved medical students at only one institution, which limits generalizability of findings. Third, we were unable to obtain records specifying which faculty and residents interacted with which medical students, and the increased number of female faculty and residents coinciding with the curriculum change may also be a factor. However, we expect that, without the curriculum change, these students would have had smaller odds of interacting with these potential female role models in orthopedics, negating any affect they may have had. Last, although we contacted former students to ask about their reasons for choosing the orthopedics residency, those findings are limited by a potential respondent selection bias.
The qualities and characteristics of successful orthopedic surgeons, as presented in both medical and lay cultures, are subject to numerous stereotypes. By increasing medical student exposure to orthopedics during the third year of medical school, we are giving a larger proportion of our students direct clinical experience in a field they may not have been considering. This exposure allows students to interact with mentors who can be positive role models—orthopedic surgeons who are dispelling stereotypes. By increasing medical student exposure and reaching students who may not have been considering orthopedics, we have increased diversity among our applicants. Third-year medical students’ exposure to orthopedic surgery is essential in promoting a more diverse workforce.
Am J Orthop. 2016;45(6):E347-E351. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. US Census Bureau. 2012 National Population Projections: Summary Tables. http://www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed April 15, 2013.
2. Blakemore LC, Hall JM, Biermann JS. Women in surgical residency training programs. J Bone Joint Surg Am. 2003;85(12):2477-2480.
3. Day CS, Lage DE, Ahn CS. Diversity based on race, ethnicity, and sex between academic orthopaedic surgery and other specialties: a comparative study. J Bone Joint Surg Am. 2010;92(13):2328-2335.
4. Lewis VO, Scherl SA, O’Connor MI. Women in orthopaedics—way behind the number curve. J Bone Joint Surg Am. 2012;94(5):e30.
5. Okike K, Utuk ME, White AA. Racial and ethnic diversity in orthopaedic surgery residency programs. J Bone Joint Surg Am. 2011;93(18):e107.
6. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
7. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the US 2008. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009.
8. White AA 3rd. Alfred R. Shands, Jr., lecture: our humanitarian orthopaedic opportunity. J Bone Joint Surg Am. 2002;84(3):478-484.
9. Templeton K, Wood VJ, Haynes R. Women and minorities in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S37-S41.
10. Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86(10):2335-2338.
11. Dykes DC, White AA. Getting to equal: strategies to understand and eliminate general and orthopaedic healthcare disparities. Clin Orthop Relat Res. 2009;467(10):2598-2605.
12. Gebhardt MC. Improving diversity in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S49-S50.
13. Hammond RA. The moral imperatives for diversity. Clin Orthop Relat Res. 1999;(362):102-106.
14. Lindsey RW. The role of the department chair in promoting diversity. J Am Acad Orthop Surg. 2007;15(suppl 1):S65-S69.
15. Satcher RL. African Americans and orthopaedic surgery. A resident’s perspective. Clin Orthop Relat Res. 1999;(362):114-116.
16. White AA. Justifications and needs for diversity in orthopaedics. Clin Orthop Relat Res. 1999;(362):22-33.
17. White AA. Resident selection: are we putting the cart before the horse? Clin Orthop Relat Res. 2002;(399):255-259.
18. Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14(4):558-566.
19. Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931-938.
20. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
21. Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305-1310.
22. Moy E, Bartman BA. Physician race and care of minority and medically indigent patients. JAMA. 1995;273(19):1515-1520.
23. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13-S17.
24. Rowley DL, Jenkins BC, Frazier E. Utilization of joint arthroplasty: racial and ethnic disparities in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S43-S48.
25. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349(14):1350-1359.
26. Steel N, Clark A, Lang LA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998-2004. J Gerontol A Biol Sci Med Sci. 2008;63(6):629-634.
27. Whitla DK, Orfield G, Silen W, Teperow C, Howard C, Reede J. Educational benefits of diversity in medical school: a survey of students. Acad Med. 2003;78(5):460-466.
28. Barrett DS. Are orthopaedic surgeons gorillas? Br Med J. 1988;297(6664):1638-1639.
29. Brenkel IJ, Pearse M, Gregg PJ. A “cracking” complication of hemiarthroplasty of the hip. Br Med J. 1986;293(6562):1648.
30. Fox JS, Bell GR, Sweeney PJ. Are orthopaedic surgeons really gorillas? Br Med J. 1990;301(6766):1425-1426.
31. Subramanian P, Kantharuban S, Subramanian V, Willis-Owen SA, Willis-Owen CA. Orthopaedic surgeons: as strong as an ox and almost twice as clever? Multicentre prospective comparative study. Br Med J. 2011;343:d7506.
32. Baldwin K, Namdari S, Bowers A, Keenan MA, Levin LS, Ahn J. Factors affecting interest in orthopedics among female medical students: a prospective analysis. Orthopedics. 2011;34(12):e919-e932.
33. Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94(11):e78.
34. Wilson FC. Teaching by residents. Clin Orthop Relat Res. 2007;(454):247-250.
As the United States becomes increasingly diverse, with predictions that by 2045 minorities will comprise 50% or more of the population,1 the demographics of the orthopedic surgery population will also likely diversify. It is important that orthopedic surgeons shift in their diversity as well. Lack of diversity in orthopedics (women and racial minorities are underrepresented) relative to the national population and other surgical specialties and their training programs is well documented.2-8
More concerning, the diversity of orthopedic residents does not compare favorably with that of medical school attendees.4,9 The difference suggests the greatest loss of potential diversity occurs during the transition from medical school to residency. A national study demonstrated that instruction in musculoskeletal medicine led to an increase in application rates nationally.10 However, the authors of that study stated they were unexpectedly limited by its large size—they could not validate the accuracy of curriculum data and could not differentiate between a 1-day required experience and a 4-week rotation.
In the present study, which accounted for curricular factors, we compared our medical students’ application rates to orthopedics residencies based on sex and race before and after introduction of a required third-year musculoskeletal clerkship. We hypothesized that making the curriculum a requirement would increase the number of applicants and increase the diversity of applicants in terms of both women and underrepresented minorities. This hypothesis was based on the rationale that these groups might not consider an orthopedics residency without first being directly exposed to orthopedics. We also wanted to determine what factors influenced applicants to choose orthopedic surgery.
Methods
Curriculum
Before 2006, third-year students spent 3 months completing a surgery clerkship. Some students interested in orthopedic surgery would have to wait until their fourth year to complete an elective in orthopedic surgery, and uninterested students would not be exposed at all. Starting in 2006, 1 month of the third-year surgery clerkship was required to be completed in musculoskeletal surgery: orthopedic surgery, plastic surgery, or neurosurgical spine. Plastic surgery was an option, as it exposed students to hand surgery and flap reconstruction.
During the 12-year study period, overall teaching hours in the preclinical curriculum did not change, and there were no other structural changes to the preclinical or clinical curriculum. The orthopedics department increased its faculty from 23 in 2000 to 34 in 2012. Number of female faculty increased from 1 to 3, representing a 4% to 9% increase in department faculty. Throughout the 12 years, there were no underrepresented minority faculty. Total number of residents increased from 26 in 2000 to 30 in 2012. Number of female residents varied year to year, from a low of 3 in the period 2003–2004 to a high of 11 in the period 2009–2010. Number of underrepresented minority residents varied yearly as well, from 1 to 2.
Data Collection
After this study was granted exempt status by our Institutional Review Board, we obtained student data from our registrar. Data included graduation year, self-identified sex and race, exposure to orthopedic surgery during clerkships, and matching residency specialty. National data were obtained from the Electronic Residency Application Service for the periods 2002–2007 and 2009–2012. These data included all US allopathic medical students’ self-identified sex and race, and applied-to primary residency specialty. National data from 2008 and national data on sex differences in orthopedic applications from 2009 were not available.
Graduates who matched into orthopedic surgery were asked to complete an anonymous survey on what influenced their decision to apply to orthopedic surgery and when this decision was made. Our goal with the survey was to substantiate or refute the conclusion that application rates depended on third-year exposure to musculoskeletal medicine.
Statistical Methods
Students were divided into 2 groups: precurriculum (graduated within 7-year period, 2000–2006) and postcurriculum (graduated within 6-year period, 2007–2012). A 2-sample test for proportions was used to compare percentage of total students who applied to orthopedics in each group. In the group of students who applied to orthopedics, we compared precurriculum and postcurriculum proportions of women and underrepresented minorities (non-white, non-Asian). We also compared these proportions with national data (using 2-sample tests for proportions) to determine if any change in diversity of our institution’s applicants was mirroring a national trend. Our definition of underrepresented minority was based on work that showed that the proportion of Asian matriculants in medical school and the proportion of applicants to orthopedics are higher than their respective national proportions.5 Survey data are reported descriptively. Statistical significance was defined with a 2-tailed α of 0.05 for all tests.
Results
Over the 2000–2012 period, 1507 students from our institution successfully applied to residency programs: 792 in the precurriculum group and 715 in the postcurriculum group. Of these students, 91 successfully applied to orthopedic surgery: 48 in the precurriculum group (applied before introduction of the required clerkship) and 43 in the postcurriculum group (applied afterward).
Over the 2002–2012 period, 10,100 US allopathic medical students applied to orthopedic residency programs: 4769 students between 2002 and 2006 and 5331 students between 2007 and 2012.
Before the musculoskeletal clerkship was required, 317 (40%) of the 792 precurriculum students were exposed to orthopedics during their third year. During this period, 42 of the 48 orthopedic surgery applicants completed an orthopedic surgery rotation during their third year of medical school. After the clerkship was required, 465 (65%) of the 715 postcurriculum students were exposed to orthopedics during their third year, including all 43 orthopedic surgery applicants (100% of students were exposed to musculoskeletal surgery, including plastic surgery and neurologic spine). The 25% increase in exposure to orthopedic surgery during the third year was statistically significant (P < .0001), but there was no resultant increase in overall percentage of students applying to orthopedic residencies (6% in each case; P = .98).
Over the 12-year study period, the proportion of female medical students at our institution declined from 50% (395/792) to 46% (328/715) (P = .13). However, there was an 81% relative increase, from 17% (8/48) before introduction of the clerkship to 30% (13/43) afterward, in the proportion of female applicants to orthopedic surgery. This contrasted with national data showing the percentage of female applicants to orthopedic surgery remained stable from 2002–2006 (14%, 675/4758) to 2007–2012 (15%, 643/4277). Before the clerkship was required, the proportion of female applicants from our institution was similar to national rates (P = .50). Afterward, our institution produced a significantly higher proportion of female applicants compared with the national proportion (P = .026).
Over the 12-year period, our self-identified underrepresented minority medical student population increased significantly (P = .02), from 13% (103/792) to 17% (124/715). The relative proportion of underrepresented minority orthopedic surgery applicants increased by 101%, from 10% (5/48) before the clerkship was required to 21% (9/43) afterward. Nationally, over the same period, underrepresented minorities’ orthopedic surgery application rates increased significantly (P < .001), from 16% (763/4769) to 19% (1002/5331). The proportion of underrepresented racial minorities that applied did not differ significantly between our institution and nationally for the years either before (P = .97) or after (P = .68) introduction of the curriculum.
Surveys were completed by 58 (64%) of 91 graduates (21 women, 70 men). Respondents’ characteristics are listed in Table 4.
Discussion
Orthopedic surgery needs a more diverse workforce11-17 in order to better mirror the population served, bring care to underserved areas,18-26 and provide better training environments.27 Several hypotheses about the lack of diversity have been posited: stereotypes about the specialty,28-31 lack of interest among minority medical students, and lack of exposure to the specialty.5,6,32,33
Lack of exposure deserves scrutiny, as a large proportion of medical students who choose to apply to orthopedic surgery make their decision before entering medical school, which is not typical.33 Such a finding suggests that exposure to orthopedic surgery is lacking, especially given that an orthopedic surgery rotation is usually not required during the clinical years. The idea that increased exposure to orthopedics affects application patterns is logical, as clinical exposure has been shown to play a role in medical students’ choice of specialty.34
Exposure helps in several key areas. Firsthand experience can help dispel stereotypes, such as the idea that success in orthopedic surgery depends on physical strength and that only former athletes pursue orthopedics.28-31 Authors have also reported on a perceived negative bias against women: Orthopedics is an “old boys’ network”; women will not fit in and need not apply; the orthopedic lifestyle is difficult and not conducive to a satisfying personal life.9 Requiring exposure ensures that all students, but especially women, can gain firsthand experience that can show these stereotypes to be false. Beyond dispelling these stereotypes, exposure to orthopedic surgery is essential for women, as studies have shown that clinical rotations play a larger role in determining specialty choice for women compared to men,33 and this would be particularly critical for specialties they may not be initially considering.
A national study found that requiring an orthopedic/musculoskeletal clerkship led to a 12% relative increase in the application rate, from 5.1% to 5.7%, and to an increase in applicant diversity (race, sex).10 However, the investigators could not determine individual reasons for specialty choice or the exact nature of each institution’s musculoskeletal curriculum. Confirming these factors, we found an 81% increase in number of female applicants and a 101% increase in number of underrepresented minority applicants after introduction of the required third-year musculoskeletal surgery clerkship at our institution.
We were unable to replicate the 12% relative increase in the overall application rate; our orthopedic surgery match rate remained 6%. Our findings cannot directly explain this, but we have several hypotheses. First, whereas other studies measured the application rate, we measured the successful match rate, given our data structure. This difference in data definition could account for some of the discrepancy. Second, we did not account for individuals’ academic success, and career counseling is paramount in decisions regarding residency specialties. It is possible we are substituting qualified female and underrepresented minority candidates for less-than-qualified male applicants. Third, the 25% increase in medical student exposure to orthopedic surgery led to a corresponding increase in number of orthopedic faculty providing undergraduate medical education. Some of these faculty could have been inexperienced in undergraduate medical education, and thus the teaching environment may not have been optimal.
Our study had several limitations. First, our institution has limited racial diversity. Over the past 12 years, only 15% of our students have been underrepresented minorities. (Nationally, the proportion is closer to 18%.) This may have limited the ability of our orthopedic rotation to affect the proportion of underrepresented minority applicants. Second, this study involved medical students at only one institution, which limits generalizability of findings. Third, we were unable to obtain records specifying which faculty and residents interacted with which medical students, and the increased number of female faculty and residents coinciding with the curriculum change may also be a factor. However, we expect that, without the curriculum change, these students would have had smaller odds of interacting with these potential female role models in orthopedics, negating any affect they may have had. Last, although we contacted former students to ask about their reasons for choosing the orthopedics residency, those findings are limited by a potential respondent selection bias.
The qualities and characteristics of successful orthopedic surgeons, as presented in both medical and lay cultures, are subject to numerous stereotypes. By increasing medical student exposure to orthopedics during the third year of medical school, we are giving a larger proportion of our students direct clinical experience in a field they may not have been considering. This exposure allows students to interact with mentors who can be positive role models—orthopedic surgeons who are dispelling stereotypes. By increasing medical student exposure and reaching students who may not have been considering orthopedics, we have increased diversity among our applicants. Third-year medical students’ exposure to orthopedic surgery is essential in promoting a more diverse workforce.
Am J Orthop. 2016;45(6):E347-E351. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
As the United States becomes increasingly diverse, with predictions that by 2045 minorities will comprise 50% or more of the population,1 the demographics of the orthopedic surgery population will also likely diversify. It is important that orthopedic surgeons shift in their diversity as well. Lack of diversity in orthopedics (women and racial minorities are underrepresented) relative to the national population and other surgical specialties and their training programs is well documented.2-8
More concerning, the diversity of orthopedic residents does not compare favorably with that of medical school attendees.4,9 The difference suggests the greatest loss of potential diversity occurs during the transition from medical school to residency. A national study demonstrated that instruction in musculoskeletal medicine led to an increase in application rates nationally.10 However, the authors of that study stated they were unexpectedly limited by its large size—they could not validate the accuracy of curriculum data and could not differentiate between a 1-day required experience and a 4-week rotation.
In the present study, which accounted for curricular factors, we compared our medical students’ application rates to orthopedics residencies based on sex and race before and after introduction of a required third-year musculoskeletal clerkship. We hypothesized that making the curriculum a requirement would increase the number of applicants and increase the diversity of applicants in terms of both women and underrepresented minorities. This hypothesis was based on the rationale that these groups might not consider an orthopedics residency without first being directly exposed to orthopedics. We also wanted to determine what factors influenced applicants to choose orthopedic surgery.
Methods
Curriculum
Before 2006, third-year students spent 3 months completing a surgery clerkship. Some students interested in orthopedic surgery would have to wait until their fourth year to complete an elective in orthopedic surgery, and uninterested students would not be exposed at all. Starting in 2006, 1 month of the third-year surgery clerkship was required to be completed in musculoskeletal surgery: orthopedic surgery, plastic surgery, or neurosurgical spine. Plastic surgery was an option, as it exposed students to hand surgery and flap reconstruction.
During the 12-year study period, overall teaching hours in the preclinical curriculum did not change, and there were no other structural changes to the preclinical or clinical curriculum. The orthopedics department increased its faculty from 23 in 2000 to 34 in 2012. Number of female faculty increased from 1 to 3, representing a 4% to 9% increase in department faculty. Throughout the 12 years, there were no underrepresented minority faculty. Total number of residents increased from 26 in 2000 to 30 in 2012. Number of female residents varied year to year, from a low of 3 in the period 2003–2004 to a high of 11 in the period 2009–2010. Number of underrepresented minority residents varied yearly as well, from 1 to 2.
Data Collection
After this study was granted exempt status by our Institutional Review Board, we obtained student data from our registrar. Data included graduation year, self-identified sex and race, exposure to orthopedic surgery during clerkships, and matching residency specialty. National data were obtained from the Electronic Residency Application Service for the periods 2002–2007 and 2009–2012. These data included all US allopathic medical students’ self-identified sex and race, and applied-to primary residency specialty. National data from 2008 and national data on sex differences in orthopedic applications from 2009 were not available.
Graduates who matched into orthopedic surgery were asked to complete an anonymous survey on what influenced their decision to apply to orthopedic surgery and when this decision was made. Our goal with the survey was to substantiate or refute the conclusion that application rates depended on third-year exposure to musculoskeletal medicine.
Statistical Methods
Students were divided into 2 groups: precurriculum (graduated within 7-year period, 2000–2006) and postcurriculum (graduated within 6-year period, 2007–2012). A 2-sample test for proportions was used to compare percentage of total students who applied to orthopedics in each group. In the group of students who applied to orthopedics, we compared precurriculum and postcurriculum proportions of women and underrepresented minorities (non-white, non-Asian). We also compared these proportions with national data (using 2-sample tests for proportions) to determine if any change in diversity of our institution’s applicants was mirroring a national trend. Our definition of underrepresented minority was based on work that showed that the proportion of Asian matriculants in medical school and the proportion of applicants to orthopedics are higher than their respective national proportions.5 Survey data are reported descriptively. Statistical significance was defined with a 2-tailed α of 0.05 for all tests.
Results
Over the 2000–2012 period, 1507 students from our institution successfully applied to residency programs: 792 in the precurriculum group and 715 in the postcurriculum group. Of these students, 91 successfully applied to orthopedic surgery: 48 in the precurriculum group (applied before introduction of the required clerkship) and 43 in the postcurriculum group (applied afterward).
Over the 2002–2012 period, 10,100 US allopathic medical students applied to orthopedic residency programs: 4769 students between 2002 and 2006 and 5331 students between 2007 and 2012.
Before the musculoskeletal clerkship was required, 317 (40%) of the 792 precurriculum students were exposed to orthopedics during their third year. During this period, 42 of the 48 orthopedic surgery applicants completed an orthopedic surgery rotation during their third year of medical school. After the clerkship was required, 465 (65%) of the 715 postcurriculum students were exposed to orthopedics during their third year, including all 43 orthopedic surgery applicants (100% of students were exposed to musculoskeletal surgery, including plastic surgery and neurologic spine). The 25% increase in exposure to orthopedic surgery during the third year was statistically significant (P < .0001), but there was no resultant increase in overall percentage of students applying to orthopedic residencies (6% in each case; P = .98).
Over the 12-year study period, the proportion of female medical students at our institution declined from 50% (395/792) to 46% (328/715) (P = .13). However, there was an 81% relative increase, from 17% (8/48) before introduction of the clerkship to 30% (13/43) afterward, in the proportion of female applicants to orthopedic surgery. This contrasted with national data showing the percentage of female applicants to orthopedic surgery remained stable from 2002–2006 (14%, 675/4758) to 2007–2012 (15%, 643/4277). Before the clerkship was required, the proportion of female applicants from our institution was similar to national rates (P = .50). Afterward, our institution produced a significantly higher proportion of female applicants compared with the national proportion (P = .026).
Over the 12-year period, our self-identified underrepresented minority medical student population increased significantly (P = .02), from 13% (103/792) to 17% (124/715). The relative proportion of underrepresented minority orthopedic surgery applicants increased by 101%, from 10% (5/48) before the clerkship was required to 21% (9/43) afterward. Nationally, over the same period, underrepresented minorities’ orthopedic surgery application rates increased significantly (P < .001), from 16% (763/4769) to 19% (1002/5331). The proportion of underrepresented racial minorities that applied did not differ significantly between our institution and nationally for the years either before (P = .97) or after (P = .68) introduction of the curriculum.
Surveys were completed by 58 (64%) of 91 graduates (21 women, 70 men). Respondents’ characteristics are listed in Table 4.
Discussion
Orthopedic surgery needs a more diverse workforce11-17 in order to better mirror the population served, bring care to underserved areas,18-26 and provide better training environments.27 Several hypotheses about the lack of diversity have been posited: stereotypes about the specialty,28-31 lack of interest among minority medical students, and lack of exposure to the specialty.5,6,32,33
Lack of exposure deserves scrutiny, as a large proportion of medical students who choose to apply to orthopedic surgery make their decision before entering medical school, which is not typical.33 Such a finding suggests that exposure to orthopedic surgery is lacking, especially given that an orthopedic surgery rotation is usually not required during the clinical years. The idea that increased exposure to orthopedics affects application patterns is logical, as clinical exposure has been shown to play a role in medical students’ choice of specialty.34
Exposure helps in several key areas. Firsthand experience can help dispel stereotypes, such as the idea that success in orthopedic surgery depends on physical strength and that only former athletes pursue orthopedics.28-31 Authors have also reported on a perceived negative bias against women: Orthopedics is an “old boys’ network”; women will not fit in and need not apply; the orthopedic lifestyle is difficult and not conducive to a satisfying personal life.9 Requiring exposure ensures that all students, but especially women, can gain firsthand experience that can show these stereotypes to be false. Beyond dispelling these stereotypes, exposure to orthopedic surgery is essential for women, as studies have shown that clinical rotations play a larger role in determining specialty choice for women compared to men,33 and this would be particularly critical for specialties they may not be initially considering.
A national study found that requiring an orthopedic/musculoskeletal clerkship led to a 12% relative increase in the application rate, from 5.1% to 5.7%, and to an increase in applicant diversity (race, sex).10 However, the investigators could not determine individual reasons for specialty choice or the exact nature of each institution’s musculoskeletal curriculum. Confirming these factors, we found an 81% increase in number of female applicants and a 101% increase in number of underrepresented minority applicants after introduction of the required third-year musculoskeletal surgery clerkship at our institution.
We were unable to replicate the 12% relative increase in the overall application rate; our orthopedic surgery match rate remained 6%. Our findings cannot directly explain this, but we have several hypotheses. First, whereas other studies measured the application rate, we measured the successful match rate, given our data structure. This difference in data definition could account for some of the discrepancy. Second, we did not account for individuals’ academic success, and career counseling is paramount in decisions regarding residency specialties. It is possible we are substituting qualified female and underrepresented minority candidates for less-than-qualified male applicants. Third, the 25% increase in medical student exposure to orthopedic surgery led to a corresponding increase in number of orthopedic faculty providing undergraduate medical education. Some of these faculty could have been inexperienced in undergraduate medical education, and thus the teaching environment may not have been optimal.
Our study had several limitations. First, our institution has limited racial diversity. Over the past 12 years, only 15% of our students have been underrepresented minorities. (Nationally, the proportion is closer to 18%.) This may have limited the ability of our orthopedic rotation to affect the proportion of underrepresented minority applicants. Second, this study involved medical students at only one institution, which limits generalizability of findings. Third, we were unable to obtain records specifying which faculty and residents interacted with which medical students, and the increased number of female faculty and residents coinciding with the curriculum change may also be a factor. However, we expect that, without the curriculum change, these students would have had smaller odds of interacting with these potential female role models in orthopedics, negating any affect they may have had. Last, although we contacted former students to ask about their reasons for choosing the orthopedics residency, those findings are limited by a potential respondent selection bias.
The qualities and characteristics of successful orthopedic surgeons, as presented in both medical and lay cultures, are subject to numerous stereotypes. By increasing medical student exposure to orthopedics during the third year of medical school, we are giving a larger proportion of our students direct clinical experience in a field they may not have been considering. This exposure allows students to interact with mentors who can be positive role models—orthopedic surgeons who are dispelling stereotypes. By increasing medical student exposure and reaching students who may not have been considering orthopedics, we have increased diversity among our applicants. Third-year medical students’ exposure to orthopedic surgery is essential in promoting a more diverse workforce.
Am J Orthop. 2016;45(6):E347-E351. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. US Census Bureau. 2012 National Population Projections: Summary Tables. http://www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed April 15, 2013.
2. Blakemore LC, Hall JM, Biermann JS. Women in surgical residency training programs. J Bone Joint Surg Am. 2003;85(12):2477-2480.
3. Day CS, Lage DE, Ahn CS. Diversity based on race, ethnicity, and sex between academic orthopaedic surgery and other specialties: a comparative study. J Bone Joint Surg Am. 2010;92(13):2328-2335.
4. Lewis VO, Scherl SA, O’Connor MI. Women in orthopaedics—way behind the number curve. J Bone Joint Surg Am. 2012;94(5):e30.
5. Okike K, Utuk ME, White AA. Racial and ethnic diversity in orthopaedic surgery residency programs. J Bone Joint Surg Am. 2011;93(18):e107.
6. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
7. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the US 2008. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009.
8. White AA 3rd. Alfred R. Shands, Jr., lecture: our humanitarian orthopaedic opportunity. J Bone Joint Surg Am. 2002;84(3):478-484.
9. Templeton K, Wood VJ, Haynes R. Women and minorities in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S37-S41.
10. Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86(10):2335-2338.
11. Dykes DC, White AA. Getting to equal: strategies to understand and eliminate general and orthopaedic healthcare disparities. Clin Orthop Relat Res. 2009;467(10):2598-2605.
12. Gebhardt MC. Improving diversity in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S49-S50.
13. Hammond RA. The moral imperatives for diversity. Clin Orthop Relat Res. 1999;(362):102-106.
14. Lindsey RW. The role of the department chair in promoting diversity. J Am Acad Orthop Surg. 2007;15(suppl 1):S65-S69.
15. Satcher RL. African Americans and orthopaedic surgery. A resident’s perspective. Clin Orthop Relat Res. 1999;(362):114-116.
16. White AA. Justifications and needs for diversity in orthopaedics. Clin Orthop Relat Res. 1999;(362):22-33.
17. White AA. Resident selection: are we putting the cart before the horse? Clin Orthop Relat Res. 2002;(399):255-259.
18. Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14(4):558-566.
19. Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931-938.
20. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
21. Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305-1310.
22. Moy E, Bartman BA. Physician race and care of minority and medically indigent patients. JAMA. 1995;273(19):1515-1520.
23. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13-S17.
24. Rowley DL, Jenkins BC, Frazier E. Utilization of joint arthroplasty: racial and ethnic disparities in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S43-S48.
25. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349(14):1350-1359.
26. Steel N, Clark A, Lang LA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998-2004. J Gerontol A Biol Sci Med Sci. 2008;63(6):629-634.
27. Whitla DK, Orfield G, Silen W, Teperow C, Howard C, Reede J. Educational benefits of diversity in medical school: a survey of students. Acad Med. 2003;78(5):460-466.
28. Barrett DS. Are orthopaedic surgeons gorillas? Br Med J. 1988;297(6664):1638-1639.
29. Brenkel IJ, Pearse M, Gregg PJ. A “cracking” complication of hemiarthroplasty of the hip. Br Med J. 1986;293(6562):1648.
30. Fox JS, Bell GR, Sweeney PJ. Are orthopaedic surgeons really gorillas? Br Med J. 1990;301(6766):1425-1426.
31. Subramanian P, Kantharuban S, Subramanian V, Willis-Owen SA, Willis-Owen CA. Orthopaedic surgeons: as strong as an ox and almost twice as clever? Multicentre prospective comparative study. Br Med J. 2011;343:d7506.
32. Baldwin K, Namdari S, Bowers A, Keenan MA, Levin LS, Ahn J. Factors affecting interest in orthopedics among female medical students: a prospective analysis. Orthopedics. 2011;34(12):e919-e932.
33. Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94(11):e78.
34. Wilson FC. Teaching by residents. Clin Orthop Relat Res. 2007;(454):247-250.
1. US Census Bureau. 2012 National Population Projections: Summary Tables. http://www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed April 15, 2013.
2. Blakemore LC, Hall JM, Biermann JS. Women in surgical residency training programs. J Bone Joint Surg Am. 2003;85(12):2477-2480.
3. Day CS, Lage DE, Ahn CS. Diversity based on race, ethnicity, and sex between academic orthopaedic surgery and other specialties: a comparative study. J Bone Joint Surg Am. 2010;92(13):2328-2335.
4. Lewis VO, Scherl SA, O’Connor MI. Women in orthopaedics—way behind the number curve. J Bone Joint Surg Am. 2012;94(5):e30.
5. Okike K, Utuk ME, White AA. Racial and ethnic diversity in orthopaedic surgery residency programs. J Bone Joint Surg Am. 2011;93(18):e107.
6. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
7. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the US 2008. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009.
8. White AA 3rd. Alfred R. Shands, Jr., lecture: our humanitarian orthopaedic opportunity. J Bone Joint Surg Am. 2002;84(3):478-484.
9. Templeton K, Wood VJ, Haynes R. Women and minorities in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S37-S41.
10. Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86(10):2335-2338.
11. Dykes DC, White AA. Getting to equal: strategies to understand and eliminate general and orthopaedic healthcare disparities. Clin Orthop Relat Res. 2009;467(10):2598-2605.
12. Gebhardt MC. Improving diversity in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S49-S50.
13. Hammond RA. The moral imperatives for diversity. Clin Orthop Relat Res. 1999;(362):102-106.
14. Lindsey RW. The role of the department chair in promoting diversity. J Am Acad Orthop Surg. 2007;15(suppl 1):S65-S69.
15. Satcher RL. African Americans and orthopaedic surgery. A resident’s perspective. Clin Orthop Relat Res. 1999;(362):114-116.
16. White AA. Justifications and needs for diversity in orthopaedics. Clin Orthop Relat Res. 1999;(362):22-33.
17. White AA. Resident selection: are we putting the cart before the horse? Clin Orthop Relat Res. 2002;(399):255-259.
18. Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14(4):558-566.
19. Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931-938.
20. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
21. Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305-1310.
22. Moy E, Bartman BA. Physician race and care of minority and medically indigent patients. JAMA. 1995;273(19):1515-1520.
23. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13-S17.
24. Rowley DL, Jenkins BC, Frazier E. Utilization of joint arthroplasty: racial and ethnic disparities in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S43-S48.
25. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349(14):1350-1359.
26. Steel N, Clark A, Lang LA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998-2004. J Gerontol A Biol Sci Med Sci. 2008;63(6):629-634.
27. Whitla DK, Orfield G, Silen W, Teperow C, Howard C, Reede J. Educational benefits of diversity in medical school: a survey of students. Acad Med. 2003;78(5):460-466.
28. Barrett DS. Are orthopaedic surgeons gorillas? Br Med J. 1988;297(6664):1638-1639.
29. Brenkel IJ, Pearse M, Gregg PJ. A “cracking” complication of hemiarthroplasty of the hip. Br Med J. 1986;293(6562):1648.
30. Fox JS, Bell GR, Sweeney PJ. Are orthopaedic surgeons really gorillas? Br Med J. 1990;301(6766):1425-1426.
31. Subramanian P, Kantharuban S, Subramanian V, Willis-Owen SA, Willis-Owen CA. Orthopaedic surgeons: as strong as an ox and almost twice as clever? Multicentre prospective comparative study. Br Med J. 2011;343:d7506.
32. Baldwin K, Namdari S, Bowers A, Keenan MA, Levin LS, Ahn J. Factors affecting interest in orthopedics among female medical students: a prospective analysis. Orthopedics. 2011;34(12):e919-e932.
33. Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94(11):e78.
34. Wilson FC. Teaching by residents. Clin Orthop Relat Res. 2007;(454):247-250.
Emerging Cataract Surgery Practice Patterns in the Veterans Health Administration
The rates of cataract surgery, the most commonly performed ophthalmic procedure in the U.S., have increased in the past few decades with an estimated rate of 1,100 surgeries per 100,000 people in 2011.1,2 Several emerging practices have the potential to radically impact the efficacy, safety, and cost of cataract surgery.3-5 These practices include femtosecond laser-assisted cataract surgery, intracameral antibiotics, and bilateral same-day cataract surgery.
The femtosecond laser is capable of producing precise incisions in the cornea for access by surgical instruments and reduction of astigmatism. Laser pulses also can create a perfectly round incision of the anterior lens capsule, which surrounds and supports the crystalline lens, and make incisions into the cataractous lens to facilitate disassembly for easy removal of fragments.
Placement of antibiotics internally into the anterior chamber, the space between the crystalline lens and the posterior cornea (intracameral space), is a more direct method to prevent bacterial infection within the eye (endophthalmitis), compared with current external methods, including injections under the conjunctiva (subconjunctival) and/or use of antibiotic drops directly onto the eye surface (topical).6
Routine cataract surgery is typically staged, with a period of time between sequential surgeries of 1 week or more to allow for observation of infection (delayed sequential surgery). In view of the very low rate of infection and the impact of staged surgery on patients, including additional visits and copays, some surgeons have begun to perform bilateral surgery (immediate sequential bilateral surgery, using separate patient safety checklists, surgical preps, instruments, and medications) on the same day for patients with significant cataracts in both eyes to promote rapid restoration of binocular vision as well reduce the number of patient visits.
The extent of adaptation of femtosecond laser surgery, intracameral antibiotics, and immediate sequential bilateral surgery in the U.S. is currently unknown.7,8 To provide an updated snapshot of these cataract surgery practices, the authors report on the results of a brief survey administered to ophthalmology section chiefs in the VHA, the largest integrated health care system and the largest provider of health care training in the U.S.
Methods
Following institutional review board approval from the Providence VA Medical Center, the office of the National Program Director of VA Ophthalmology provided a list of all VHA ophthalmology section chiefs and their contact information. The study targeted section chiefs because they are responsible for all eye surgery performed at their respective VAMCs. The survey queried the section chiefs on femtosecond laser-assisted cataract surgery, intracameral antibiotics, immediate sequential bilateral cataract surgery, and resident training at their institutions (Table).
The survey was administered using the web-based Research Electronic Data Capture (REDCap) software.9 The initial survey was e-mailed in April 2015, followed by 2 reminder e-mails 1 week apart and then 2 phone calls 1 week apart to nonresponders.
The survey responses were stored anonymously in the REDCap database and analyzed using descriptive statistics.
Results
The original list from the office of the National Program Director included 114 ophthalmology section chiefs (excluding one of the authors). After follow-up phone calls, 9 individuals were identified who were not ophthalmologists (eg, optometrists or nonophthalmic surgeons) or who were incorrectly listed as section chiefs, and 9 were duplicates from institutions that were represented twice on the contact list. These 18 individuals, none of whom had responded to the survey, were removed from the eligible sample. Hence, the analysis included 86% (95/111) of the VAMCs where cataract surgery is performed.10 Sixty-five responses were received for an overall response rate of 68% (65/96), including 1 ophthalmologist who responded to the survey twice.
Most section chiefs (86%, 56/65) trained ophthalmology residents at their respective medical centers (Table). Eleven VAMCs (17%) offered femtosecond laser-assisted cataract surgery; 8 of those 11 (73%) also offered resident training in this surgery. At 12 VAMCs (18%), cataract surgeons used intracameral antibiotics, which included vancomycin (4), cefuroxime (4), moxifloxacin (3), and unspecified (1); at 10 of these VAMCs (83%), surgeons used intracameral and postoperative topical antibiotics concomitantly; 8 VAMCs (67%) compounded the intracameral antibiotics—either in the hospital pharmacy (5) or within the operating room (3). The 2 most common reasons cited for not using intracameral antibiotics were risk of dilution error (28%; 15/53) and a lack of evidence for use (25%; 13/53). Only 2 medical centers (3.1%) offered immediate sequential bilateral cataract surgery.
Discussion
This survey provides updated information on the role of emerging cataract surgery practices in the VHA. These trends may impact future U.S. cataract surgery practice patterns given the large number of ophthalmology residents who receive training in the VHA.
Only 17% of VAMCs offered femtosecond laser-assisted cataract surgery. Reasons for this low rate may include (a) the high cost of the femtosecond laser units (the lowest average cost of a laser is $400,000, while the average costs of services can be $40,000 or more per year); and (b) the lack of evidence that a femtosecond laser improves cataract surgery outcomes relative to standard phacoemulsification.4,11-15 Another potential barrier to procurement of femtosecond lasers is the emphasis within VHA to increase access to care for the many newly enrolled veterans, which this technology does not address. However, most of the VAMCs with a femtosecond laser unit offered resident training in this technique, confirming early reports on the potential for incorporating femtosecond laser-assisted cataract surgery into ophthalmic graduate medical education.16
In 2007, the multicenter, prospective, randomized European Society of Cataract and Refractive Surgery Endophthalmitis Study demonstrated that intracameral cefuroxime was associated with a 5-fold decrease in the risk of postoperative endophthalmitis.17 In 2011, a statement from the American Society of Cataract and Refractive Surgery (ASCRS) Cataract Clinical Committee noted that the method of antibiotic prophylaxis with the strongest evidence base is “a direct intracameral bolus at the conclusion of surgery.”18 However, surgeons used intracameral antibiotics in only 19% of VAMCs. Although this is a higher rate than those reported in older surveys of VHA ophthalmologists (14%)7 and ASCRS members (15%), it is still significantly lower than the 74% reported in a recent survey of the European Society of Cataract and Refractive Surgeons.3,8
The most common reasons given for not using intracameral antibiotics included risk of a dilution error when preparing the antibiotics and lack of evidence supporting their effectiveness. Less common reasons included risk of contamination, lack of pharmacy approval, and increasing bacterial resistance to commonly used antibiotics. Most of these concerns have been previously cited as barriers to the adoption of intracameral antibiotics.19 The availability of a prepackaged intracameral antibiotic (eg, cefuroxime in Europe) would help address the risks of compounding dilution errors and contamination in the U.S.6 The publication of 3 large observational studies in 2016 has also significantly strengthened the evidence base supporting the use of intracameral antibiotics.20-22
Only 2 VAMCs (3%) offered immediate sequential bilateral cataract surgery. The advocates of this practice have touted its potential cost savings, patient convenience, and the opportunity for more rapid visual rehabilitation.23 Recently, several multicenter, randomized clinical trials have reported similar refractive outcomes, complication rates, and patient satisfaction for immediate and delayed bilateral cataract surgery.24,25 Hence, it is possible that rates of immediate sequential bilateral cataract surgery may increase in the VHA over the next few years.
Strengths/Limitations
A strength of this survey is its high response rate (67.7%), which exceeds the 53% and 33% rates reported in previous surveys of cataract surgery practice patterns among VHA ophthalmologistsand ASCRS members, respectively.7,8 Another strength is lack of financial incentive for adaptation of any new practices by VHA surgeons, suggesting that these decisions have been made to improve patient safety, quality of care, and/or resident education. A limitation of this study is that its findings may not be generalizable to ophthalmologists practicing in the private sector or in teaching hospitals outside the VHA.
Conclusion
This study suggests that femtosecond laser-assisted cataract surgery, intracameral antibiotics, and immediate sequential bilateral cataract surgery have limited roles in VHA cataract surgery. More research and clinical experience are needed to understand the barriers to more widespread acceptance and to assess the impact of these emerging practices on cataract surgery in the U.S.
1. Lindstrom R. Thoughts on cataract surgery: 2015. http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422/. Published March 9, 2015. Accessed June 23, 2016.
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383-1389.
3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142.
4. Quiñones A, Gleitsmann K, Freeman M, et al. Benefits and Harms of Femtosecond Laser Assisted Cataract Surgery: A Systematic Review. VA-ESP Project #05-225; 2013. Washington, DC: Department of Veterans Affairs; 2013.
5. Naseri A, McLeod S. Benefits of and barriers to immediate sequential cataract surgery. JAMA Ophthalmol. 2014;132(11):1362-1363.
6. Brage-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-2142.
7. Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg. 2012;38(4):705-709.
8. Chang DF, Braga-Mele R, Mamalis N, et al; ASCRS Clinical Cataract Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33(10):1801-1805.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. French DD, Margo CE, Campbell RR. Do ophthalmology training programs affect corrective procedure rates after cataract surgery? Am J Med Qual. 2013;28(3):250-255.
11. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753-1763.
12. Abouzeid H, Ferrini W. Femtosecond-laser assisted cataract surgery: a review. Acta Ophthalmol. 2014;92(7):597-603.
13. Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol. 2014;25(1):49-53.
14. Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Experiment Ophthalmol. 2016;44(6):472-480.
15. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123(1):178-182.
16. Cohen MN, Intili A, Ni N, Blecher MH. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol. 2015;26(1):56-60.
17. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
18. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
19. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132(11):1269-1270.
20. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294.
21. Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308.
22. Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123(2):295-301.
23. Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132(11):1359-1362.
24. Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37(6):992-1002.
25. Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-1742.
The rates of cataract surgery, the most commonly performed ophthalmic procedure in the U.S., have increased in the past few decades with an estimated rate of 1,100 surgeries per 100,000 people in 2011.1,2 Several emerging practices have the potential to radically impact the efficacy, safety, and cost of cataract surgery.3-5 These practices include femtosecond laser-assisted cataract surgery, intracameral antibiotics, and bilateral same-day cataract surgery.
The femtosecond laser is capable of producing precise incisions in the cornea for access by surgical instruments and reduction of astigmatism. Laser pulses also can create a perfectly round incision of the anterior lens capsule, which surrounds and supports the crystalline lens, and make incisions into the cataractous lens to facilitate disassembly for easy removal of fragments.
Placement of antibiotics internally into the anterior chamber, the space between the crystalline lens and the posterior cornea (intracameral space), is a more direct method to prevent bacterial infection within the eye (endophthalmitis), compared with current external methods, including injections under the conjunctiva (subconjunctival) and/or use of antibiotic drops directly onto the eye surface (topical).6
Routine cataract surgery is typically staged, with a period of time between sequential surgeries of 1 week or more to allow for observation of infection (delayed sequential surgery). In view of the very low rate of infection and the impact of staged surgery on patients, including additional visits and copays, some surgeons have begun to perform bilateral surgery (immediate sequential bilateral surgery, using separate patient safety checklists, surgical preps, instruments, and medications) on the same day for patients with significant cataracts in both eyes to promote rapid restoration of binocular vision as well reduce the number of patient visits.
The extent of adaptation of femtosecond laser surgery, intracameral antibiotics, and immediate sequential bilateral surgery in the U.S. is currently unknown.7,8 To provide an updated snapshot of these cataract surgery practices, the authors report on the results of a brief survey administered to ophthalmology section chiefs in the VHA, the largest integrated health care system and the largest provider of health care training in the U.S.
Methods
Following institutional review board approval from the Providence VA Medical Center, the office of the National Program Director of VA Ophthalmology provided a list of all VHA ophthalmology section chiefs and their contact information. The study targeted section chiefs because they are responsible for all eye surgery performed at their respective VAMCs. The survey queried the section chiefs on femtosecond laser-assisted cataract surgery, intracameral antibiotics, immediate sequential bilateral cataract surgery, and resident training at their institutions (Table).
The survey was administered using the web-based Research Electronic Data Capture (REDCap) software.9 The initial survey was e-mailed in April 2015, followed by 2 reminder e-mails 1 week apart and then 2 phone calls 1 week apart to nonresponders.
The survey responses were stored anonymously in the REDCap database and analyzed using descriptive statistics.
Results
The original list from the office of the National Program Director included 114 ophthalmology section chiefs (excluding one of the authors). After follow-up phone calls, 9 individuals were identified who were not ophthalmologists (eg, optometrists or nonophthalmic surgeons) or who were incorrectly listed as section chiefs, and 9 were duplicates from institutions that were represented twice on the contact list. These 18 individuals, none of whom had responded to the survey, were removed from the eligible sample. Hence, the analysis included 86% (95/111) of the VAMCs where cataract surgery is performed.10 Sixty-five responses were received for an overall response rate of 68% (65/96), including 1 ophthalmologist who responded to the survey twice.
Most section chiefs (86%, 56/65) trained ophthalmology residents at their respective medical centers (Table). Eleven VAMCs (17%) offered femtosecond laser-assisted cataract surgery; 8 of those 11 (73%) also offered resident training in this surgery. At 12 VAMCs (18%), cataract surgeons used intracameral antibiotics, which included vancomycin (4), cefuroxime (4), moxifloxacin (3), and unspecified (1); at 10 of these VAMCs (83%), surgeons used intracameral and postoperative topical antibiotics concomitantly; 8 VAMCs (67%) compounded the intracameral antibiotics—either in the hospital pharmacy (5) or within the operating room (3). The 2 most common reasons cited for not using intracameral antibiotics were risk of dilution error (28%; 15/53) and a lack of evidence for use (25%; 13/53). Only 2 medical centers (3.1%) offered immediate sequential bilateral cataract surgery.
Discussion
This survey provides updated information on the role of emerging cataract surgery practices in the VHA. These trends may impact future U.S. cataract surgery practice patterns given the large number of ophthalmology residents who receive training in the VHA.
Only 17% of VAMCs offered femtosecond laser-assisted cataract surgery. Reasons for this low rate may include (a) the high cost of the femtosecond laser units (the lowest average cost of a laser is $400,000, while the average costs of services can be $40,000 or more per year); and (b) the lack of evidence that a femtosecond laser improves cataract surgery outcomes relative to standard phacoemulsification.4,11-15 Another potential barrier to procurement of femtosecond lasers is the emphasis within VHA to increase access to care for the many newly enrolled veterans, which this technology does not address. However, most of the VAMCs with a femtosecond laser unit offered resident training in this technique, confirming early reports on the potential for incorporating femtosecond laser-assisted cataract surgery into ophthalmic graduate medical education.16
In 2007, the multicenter, prospective, randomized European Society of Cataract and Refractive Surgery Endophthalmitis Study demonstrated that intracameral cefuroxime was associated with a 5-fold decrease in the risk of postoperative endophthalmitis.17 In 2011, a statement from the American Society of Cataract and Refractive Surgery (ASCRS) Cataract Clinical Committee noted that the method of antibiotic prophylaxis with the strongest evidence base is “a direct intracameral bolus at the conclusion of surgery.”18 However, surgeons used intracameral antibiotics in only 19% of VAMCs. Although this is a higher rate than those reported in older surveys of VHA ophthalmologists (14%)7 and ASCRS members (15%), it is still significantly lower than the 74% reported in a recent survey of the European Society of Cataract and Refractive Surgeons.3,8
The most common reasons given for not using intracameral antibiotics included risk of a dilution error when preparing the antibiotics and lack of evidence supporting their effectiveness. Less common reasons included risk of contamination, lack of pharmacy approval, and increasing bacterial resistance to commonly used antibiotics. Most of these concerns have been previously cited as barriers to the adoption of intracameral antibiotics.19 The availability of a prepackaged intracameral antibiotic (eg, cefuroxime in Europe) would help address the risks of compounding dilution errors and contamination in the U.S.6 The publication of 3 large observational studies in 2016 has also significantly strengthened the evidence base supporting the use of intracameral antibiotics.20-22
Only 2 VAMCs (3%) offered immediate sequential bilateral cataract surgery. The advocates of this practice have touted its potential cost savings, patient convenience, and the opportunity for more rapid visual rehabilitation.23 Recently, several multicenter, randomized clinical trials have reported similar refractive outcomes, complication rates, and patient satisfaction for immediate and delayed bilateral cataract surgery.24,25 Hence, it is possible that rates of immediate sequential bilateral cataract surgery may increase in the VHA over the next few years.
Strengths/Limitations
A strength of this survey is its high response rate (67.7%), which exceeds the 53% and 33% rates reported in previous surveys of cataract surgery practice patterns among VHA ophthalmologistsand ASCRS members, respectively.7,8 Another strength is lack of financial incentive for adaptation of any new practices by VHA surgeons, suggesting that these decisions have been made to improve patient safety, quality of care, and/or resident education. A limitation of this study is that its findings may not be generalizable to ophthalmologists practicing in the private sector or in teaching hospitals outside the VHA.
Conclusion
This study suggests that femtosecond laser-assisted cataract surgery, intracameral antibiotics, and immediate sequential bilateral cataract surgery have limited roles in VHA cataract surgery. More research and clinical experience are needed to understand the barriers to more widespread acceptance and to assess the impact of these emerging practices on cataract surgery in the U.S.
The rates of cataract surgery, the most commonly performed ophthalmic procedure in the U.S., have increased in the past few decades with an estimated rate of 1,100 surgeries per 100,000 people in 2011.1,2 Several emerging practices have the potential to radically impact the efficacy, safety, and cost of cataract surgery.3-5 These practices include femtosecond laser-assisted cataract surgery, intracameral antibiotics, and bilateral same-day cataract surgery.
The femtosecond laser is capable of producing precise incisions in the cornea for access by surgical instruments and reduction of astigmatism. Laser pulses also can create a perfectly round incision of the anterior lens capsule, which surrounds and supports the crystalline lens, and make incisions into the cataractous lens to facilitate disassembly for easy removal of fragments.
Placement of antibiotics internally into the anterior chamber, the space between the crystalline lens and the posterior cornea (intracameral space), is a more direct method to prevent bacterial infection within the eye (endophthalmitis), compared with current external methods, including injections under the conjunctiva (subconjunctival) and/or use of antibiotic drops directly onto the eye surface (topical).6
Routine cataract surgery is typically staged, with a period of time between sequential surgeries of 1 week or more to allow for observation of infection (delayed sequential surgery). In view of the very low rate of infection and the impact of staged surgery on patients, including additional visits and copays, some surgeons have begun to perform bilateral surgery (immediate sequential bilateral surgery, using separate patient safety checklists, surgical preps, instruments, and medications) on the same day for patients with significant cataracts in both eyes to promote rapid restoration of binocular vision as well reduce the number of patient visits.
The extent of adaptation of femtosecond laser surgery, intracameral antibiotics, and immediate sequential bilateral surgery in the U.S. is currently unknown.7,8 To provide an updated snapshot of these cataract surgery practices, the authors report on the results of a brief survey administered to ophthalmology section chiefs in the VHA, the largest integrated health care system and the largest provider of health care training in the U.S.
Methods
Following institutional review board approval from the Providence VA Medical Center, the office of the National Program Director of VA Ophthalmology provided a list of all VHA ophthalmology section chiefs and their contact information. The study targeted section chiefs because they are responsible for all eye surgery performed at their respective VAMCs. The survey queried the section chiefs on femtosecond laser-assisted cataract surgery, intracameral antibiotics, immediate sequential bilateral cataract surgery, and resident training at their institutions (Table).
The survey was administered using the web-based Research Electronic Data Capture (REDCap) software.9 The initial survey was e-mailed in April 2015, followed by 2 reminder e-mails 1 week apart and then 2 phone calls 1 week apart to nonresponders.
The survey responses were stored anonymously in the REDCap database and analyzed using descriptive statistics.
Results
The original list from the office of the National Program Director included 114 ophthalmology section chiefs (excluding one of the authors). After follow-up phone calls, 9 individuals were identified who were not ophthalmologists (eg, optometrists or nonophthalmic surgeons) or who were incorrectly listed as section chiefs, and 9 were duplicates from institutions that were represented twice on the contact list. These 18 individuals, none of whom had responded to the survey, were removed from the eligible sample. Hence, the analysis included 86% (95/111) of the VAMCs where cataract surgery is performed.10 Sixty-five responses were received for an overall response rate of 68% (65/96), including 1 ophthalmologist who responded to the survey twice.
Most section chiefs (86%, 56/65) trained ophthalmology residents at their respective medical centers (Table). Eleven VAMCs (17%) offered femtosecond laser-assisted cataract surgery; 8 of those 11 (73%) also offered resident training in this surgery. At 12 VAMCs (18%), cataract surgeons used intracameral antibiotics, which included vancomycin (4), cefuroxime (4), moxifloxacin (3), and unspecified (1); at 10 of these VAMCs (83%), surgeons used intracameral and postoperative topical antibiotics concomitantly; 8 VAMCs (67%) compounded the intracameral antibiotics—either in the hospital pharmacy (5) or within the operating room (3). The 2 most common reasons cited for not using intracameral antibiotics were risk of dilution error (28%; 15/53) and a lack of evidence for use (25%; 13/53). Only 2 medical centers (3.1%) offered immediate sequential bilateral cataract surgery.
Discussion
This survey provides updated information on the role of emerging cataract surgery practices in the VHA. These trends may impact future U.S. cataract surgery practice patterns given the large number of ophthalmology residents who receive training in the VHA.
Only 17% of VAMCs offered femtosecond laser-assisted cataract surgery. Reasons for this low rate may include (a) the high cost of the femtosecond laser units (the lowest average cost of a laser is $400,000, while the average costs of services can be $40,000 or more per year); and (b) the lack of evidence that a femtosecond laser improves cataract surgery outcomes relative to standard phacoemulsification.4,11-15 Another potential barrier to procurement of femtosecond lasers is the emphasis within VHA to increase access to care for the many newly enrolled veterans, which this technology does not address. However, most of the VAMCs with a femtosecond laser unit offered resident training in this technique, confirming early reports on the potential for incorporating femtosecond laser-assisted cataract surgery into ophthalmic graduate medical education.16
In 2007, the multicenter, prospective, randomized European Society of Cataract and Refractive Surgery Endophthalmitis Study demonstrated that intracameral cefuroxime was associated with a 5-fold decrease in the risk of postoperative endophthalmitis.17 In 2011, a statement from the American Society of Cataract and Refractive Surgery (ASCRS) Cataract Clinical Committee noted that the method of antibiotic prophylaxis with the strongest evidence base is “a direct intracameral bolus at the conclusion of surgery.”18 However, surgeons used intracameral antibiotics in only 19% of VAMCs. Although this is a higher rate than those reported in older surveys of VHA ophthalmologists (14%)7 and ASCRS members (15%), it is still significantly lower than the 74% reported in a recent survey of the European Society of Cataract and Refractive Surgeons.3,8
The most common reasons given for not using intracameral antibiotics included risk of a dilution error when preparing the antibiotics and lack of evidence supporting their effectiveness. Less common reasons included risk of contamination, lack of pharmacy approval, and increasing bacterial resistance to commonly used antibiotics. Most of these concerns have been previously cited as barriers to the adoption of intracameral antibiotics.19 The availability of a prepackaged intracameral antibiotic (eg, cefuroxime in Europe) would help address the risks of compounding dilution errors and contamination in the U.S.6 The publication of 3 large observational studies in 2016 has also significantly strengthened the evidence base supporting the use of intracameral antibiotics.20-22
Only 2 VAMCs (3%) offered immediate sequential bilateral cataract surgery. The advocates of this practice have touted its potential cost savings, patient convenience, and the opportunity for more rapid visual rehabilitation.23 Recently, several multicenter, randomized clinical trials have reported similar refractive outcomes, complication rates, and patient satisfaction for immediate and delayed bilateral cataract surgery.24,25 Hence, it is possible that rates of immediate sequential bilateral cataract surgery may increase in the VHA over the next few years.
Strengths/Limitations
A strength of this survey is its high response rate (67.7%), which exceeds the 53% and 33% rates reported in previous surveys of cataract surgery practice patterns among VHA ophthalmologistsand ASCRS members, respectively.7,8 Another strength is lack of financial incentive for adaptation of any new practices by VHA surgeons, suggesting that these decisions have been made to improve patient safety, quality of care, and/or resident education. A limitation of this study is that its findings may not be generalizable to ophthalmologists practicing in the private sector or in teaching hospitals outside the VHA.
Conclusion
This study suggests that femtosecond laser-assisted cataract surgery, intracameral antibiotics, and immediate sequential bilateral cataract surgery have limited roles in VHA cataract surgery. More research and clinical experience are needed to understand the barriers to more widespread acceptance and to assess the impact of these emerging practices on cataract surgery in the U.S.
1. Lindstrom R. Thoughts on cataract surgery: 2015. http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422/. Published March 9, 2015. Accessed June 23, 2016.
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383-1389.
3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142.
4. Quiñones A, Gleitsmann K, Freeman M, et al. Benefits and Harms of Femtosecond Laser Assisted Cataract Surgery: A Systematic Review. VA-ESP Project #05-225; 2013. Washington, DC: Department of Veterans Affairs; 2013.
5. Naseri A, McLeod S. Benefits of and barriers to immediate sequential cataract surgery. JAMA Ophthalmol. 2014;132(11):1362-1363.
6. Brage-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-2142.
7. Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg. 2012;38(4):705-709.
8. Chang DF, Braga-Mele R, Mamalis N, et al; ASCRS Clinical Cataract Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33(10):1801-1805.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. French DD, Margo CE, Campbell RR. Do ophthalmology training programs affect corrective procedure rates after cataract surgery? Am J Med Qual. 2013;28(3):250-255.
11. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753-1763.
12. Abouzeid H, Ferrini W. Femtosecond-laser assisted cataract surgery: a review. Acta Ophthalmol. 2014;92(7):597-603.
13. Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol. 2014;25(1):49-53.
14. Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Experiment Ophthalmol. 2016;44(6):472-480.
15. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123(1):178-182.
16. Cohen MN, Intili A, Ni N, Blecher MH. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol. 2015;26(1):56-60.
17. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
18. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
19. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132(11):1269-1270.
20. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294.
21. Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308.
22. Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123(2):295-301.
23. Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132(11):1359-1362.
24. Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37(6):992-1002.
25. Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-1742.
1. Lindstrom R. Thoughts on cataract surgery: 2015. http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422/. Published March 9, 2015. Accessed June 23, 2016.
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383-1389.
3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142.
4. Quiñones A, Gleitsmann K, Freeman M, et al. Benefits and Harms of Femtosecond Laser Assisted Cataract Surgery: A Systematic Review. VA-ESP Project #05-225; 2013. Washington, DC: Department of Veterans Affairs; 2013.
5. Naseri A, McLeod S. Benefits of and barriers to immediate sequential cataract surgery. JAMA Ophthalmol. 2014;132(11):1362-1363.
6. Brage-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-2142.
7. Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg. 2012;38(4):705-709.
8. Chang DF, Braga-Mele R, Mamalis N, et al; ASCRS Clinical Cataract Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33(10):1801-1805.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. French DD, Margo CE, Campbell RR. Do ophthalmology training programs affect corrective procedure rates after cataract surgery? Am J Med Qual. 2013;28(3):250-255.
11. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753-1763.
12. Abouzeid H, Ferrini W. Femtosecond-laser assisted cataract surgery: a review. Acta Ophthalmol. 2014;92(7):597-603.
13. Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol. 2014;25(1):49-53.
14. Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Experiment Ophthalmol. 2016;44(6):472-480.
15. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123(1):178-182.
16. Cohen MN, Intili A, Ni N, Blecher MH. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol. 2015;26(1):56-60.
17. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
18. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
19. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132(11):1269-1270.
20. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294.
21. Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308.
22. Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123(2):295-301.
23. Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132(11):1359-1362.
24. Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37(6):992-1002.
25. Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-1742.
High-Grade Articular, Bursal, and Intratendinous Partial-Thickness Rotator Cuff Tears: A Retrospective Study Comparing Functional Outcomes After Completion and Repair
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
Historical Patterns and Variation in Treatment of Injuries in NFL (National Football League) Players and NCAA (National Collegiate Athletic Association) Division I Football Players
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
Quality Measure Attainment After Add-on Therapy of Both Saxagliptin and Dapagliflozin to Metformin Versus Single Add-On of Saxagliptin or Dapagliflozin
From the Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA (Dr. Blonde), and AstraZeneca, Gaithersburg, MD (Drs. Sheehan, Barrett, and Garcia-Sanchez).
Abstract
- Objective: To evaluate diabetes care quality measure attainment, specifically, blood glucose and blood pressure (BP) control, with saxagliptin, a dipeptidyl peptidase-4 inhibitor, and dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, added singly or as dual add-on therapy in patients with type 2 diabetes inadequately controlled with metformin alone.
- Methods: Analysis of a phase 3, randomized, double-blind, active-controlled, parallel-group trial was conducted. Patients were randomized 1:1:1 to receive saxagliptin 5 mg/d plus dapagliflozin 10 mg/d, saxagliptin 5 mg/d, or dapagliflozin 10 mg/d as add-on to metformin 1500 to 2000 mg/d. Assessments included attainment of individual and composite glycated hemoglobin (A1C) and BP measures at 24 weeks of treatment.
- Results: Compared with single add-on saxagliptin or dapagliflozin, dual add-on saxagliptin plus dapagliflozin to metformin was associated with significantly more patients attaining the individual quality measures of A1C < 7% and A1C < 8%. Similarly, dual add-on saxagliptin plus dapagliflozin was associated with significantly more patients attaining the composite quality measures A1C < 7% and BP < 140/90 mm Hg and A1C < 8% and BP < 140/90 mmHg (vs saxagliptin plus metformin).
- Conclusion: Dual add-on saxagliptin plus dapagliflozin to metformin was associated with a higher proportion of patients achieving glycemic and BP quality measures compared with single add-on saxagliptin or dapagliflozin.
Assessment of performance is a focus of many health care organizations as a means to evaluate and improve the quality of health care. Standardized performance measures have been developed to improve quality of care as well as to allow for comparative assessment of health plans and to support pay for performance models [1]. A widely used set of performance measures is the Healthcare Effectiveness Data and Information Set or HEDIS [2,3], measures that are maintained by the National Committee for Quality Assurance [4,5] and used by most US health plans [6].
Type 2 diabetes (T2D) is a focus of quality measure assessment and performance improvement because of its high prevalence, substantial personal and economic impact on society, high morbidity and mortality, and because it is a condition that requires coordinated care. Important outcome measures for diabetes include blood glucose control and blood pressure (BP) control. HEDIS measures for T2D include a glycated hemoglobin (A1C) > 9%, indicating poor glucose control, < 8%, indicating good control, and < 7%, a more stringent measure of good glycemic control. The HEDIS measure for BP in T2D is < 140/90 mm Hg, which is considered good BP control. All of these HEDIS measures are currently or were previously (A1C < 7%) endorsed by the National Quality Forum [1,7–10]. Endorsement of a quality measure by the NQF indicates that the measure has been thoroughly evaluated, meets specific criteria, and is based on recognized standards of care grounded in evidence-based medicine [1].
A number of oral agents are utilized in the treatment of diabetes. Saxagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, and dapagliflozin, an oral sodium-glucose cotransporter-2 (SGLT-2) inhibitor, are indicated as adjuncts to diet and exercise in adults with T2D [11,12]. Saxagliptin inhibits DPP-4, and thereby reduces fasting and postprandial glucose concentrations by preventing degradation of the incretin hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide [13]. Dapagliflozin reduces blood glucose concentrations by inhibiting glucose reabsorption in the proximal tubule of the kidney, which results in enhanced urinary glucose excretion [14]. Because their mechanisms of action are glucose-dependent, both saxagliptin and dapagliflozin have a low intrinsic potential to cause hypoglycemia [13,14]. In a randomized, double-blind study of patients with T2D inadequately controlled with metformin, Rosenstock et al assessed the efficacy and safety of dual add-on of saxagliptin plus dapagliflozin versus saxagliptin and dapagliflozin added on alone (ClinicalTrials.gov identifier, NCT01606007) [15]. The dual add-on therapy resulted in a greater adjusted mean reduction from baseline in A1C at week 24 (–1.47%) compared with either saxagliptin (–0.88%) or dapagliflozin (–1.20%) alone added to metformin; the difference for dual add-on saxagliptin plus dapagliflozin to metformin vs. single add-on saxagliptin and single add-on dapagliflozin was –0.59% (P < 0.001) and –0.27% (P = 0.0166), respectively. The incidence of adverse events was similar across the 3 treatment groups, and hypo-glycemia was infrequent (1%), with no reports of severe hypoglycemia events (symptomatic events with glucose ≤ 54 mg/dL requiring assistance).
In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
Study Design and Patients
Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.
Results
Patients
Individual Quality Measures
The dual addition of saxagliptin plus dapagliflozin to metformin resulted in a significantly greater proportion of patients achieving A1C < 8.0% compared with saxagliptin plus metformin (71.2% vs 49.1%; P < 0.001; NNT 5 [95% CI 3–8]) or dapagliflozin plus metformin (60.1%; P = 0.033; NNT 9 [95% CI 5–85]; Figure 2). Similar results (proportions of patients: 66.4% vs 40.0% vs 51.9%; P ≤ 0.02; NNTs 4 [95% CI 3–7]) and 7 [95% CI 4–34]) were attained when the analysis excluded patients with baseline A1C < 8.0%.
Significantly fewer patients had A1C > 9% (a measure of poor glycemic control) with saxagliptin plus dapagliflozin plus metformin (12.4%) compared with saxagliptin plus metformin (22.3%; P = 0.017; NNT –10 [95% CI –50 to –6]; Figure 2). The proportion of patients with A1C > 9% was similar for both regimens that included dapagliflozin (12.4% vs 10.4%; P = 0.616).
No significant difference was observed among treatment groups in the proportion of patients with BP < 140/90 mm Hg (Figure 2). However, most patients had BP < 140/90 mm Hg (72%–82%) at baseline, which was generally maintained at week 24.
Composite Quality Measures
Discussion
This post hoc analysis evaluated attainment of glycemic and BP quality measures for diabetes. A significantly greater proportion of patients achieved the individual quality measures of A1C < 7% and A1C < 8% with dual add-on saxagliptin plus dapagliflozin to metformin compared with single add-on saxagliptin or dapagliflozin to metformin after 24 weeks. Similar results were seen when the analysis excluded patients with A1C < 7% and < 8% at baseline. All measures of good glycemic control had clinically relevant NNTs ≤ 10 after 24 weeks with saxagliptin plus dapagliflozin plus metformin compared with saxagliptin or dapagliflozin plus metformin, regardless of baseline status. Very few patients experienced lackof improvement in glycemic control, evidenced by small proportions of patients with A1C > 9%.
There was little difference in BP between dual add-on saxagliptin plus dapagliflozin or single add-on saxagliptin or dapagliflozin to metformin. The proportion of patients who attained the BP quality measure of BP < 140/90 mm Hg was similar across the 3 treatments, as might be expected because most patients already met this target at baseline. However, as might be expected based on the mild diuretic effect and weight loss associated with SGLT-2 inhibitors [16,17], trends in BP favored groups treated with dapagliflozin.
Attainment of multiple treatment targets is desirable in reducing complications of diabetes. A significantly greater proportion of patients achieved both A1C < 7% and BP < 140/90 mm Hg when both saxagliptin and dapagliflozin were added to metformin compared with single-agent addition of either saxagliptin or dapagliflozin plus metformin. Similarly, a significantly greater proportion of patients achieved both A1C < 8% and BP < 140/90 mm Hg with dual addition of saxagliptin and dapagliflozin plus metformin compared with saxagliptin plus metformin. There was also a numerically greater number of patients who achieved both of these goals with triple therapy compared with dapagliflozin plus metformin, but this finding did not reach statistical significance. Clinically relevant NNT values ≤ 10 were observed for both composite outcomes for saxagliptin plus dapagliflozin plus metformin compared with saxagliptin plus metformin or dapagliflozin plus metformin after 24 weeks.
Despite advances in the medical management of T2D, a report published in 2013 showed that between 2007 and 2010, only 53% of patients achieved an A1C < 7.0% and only 19% simultaneously achieved all 3 American Diabetes Association (ADA) goals recommended for most patients at that time: A1C < 7.0%, BP < 130/80 mm Hg, and low-density lipoprotein cholesterol LDL-C < 100 mg/dL [18]. These data highlight a need for new approaches to help patients attain glycemic, BP, and cholesterol goals. Our results demonstrated that a higher proportion of patients attained glycemic and BP quality measures with dual add-on saxagliptin plus dapagliflozin compared with single add-on saxagliptin or dapagliflozin to metformin. As a result of recent updates for cholesterol management from the American College of Cardiology and the American Heart Association [19], attainment of a cholesterol level was retired as a diabetes quality measure and replaced with a recommendation for statin therapy use [20,21]. Although the current analysis did not include assessment of LDL, DPP-4 inhibitors have demonstrated neutral effects on lipids [22,23], and SGLT-2 inhibitors have demonstrated generally modest increases in LDL-C (placebo-adjusted change from baseline: 4.5%–8.0% for canagliflozin 100 and 300 mg/d, 3.9% for dapagliflozin 10 mg, and 2.3%–4.2% for empagliflozin 10 and 25 mg/d) [12,24,25], as well as increases in high-density lipoprotein cholesterol and reductions in triglycerides [26].
Current ADA guidelines recommend an individualized, stepwise approach to treatment with sequential addition of single oral antihyperglycemic agents for patients who do not achieve their glycemic goal in 3 months [27]. Although T2D may progress at different rates in different patients, T2D does generally progress over time [28], and the ADA and American Association of Clinical Endocrinologists treatment guidelines recommend initial dual add-on therapy for individuals with higher A1C, which is suggestive of more advanced disease [27,29]. For individuals requiring initial combination therapy, guidelines note that antihyperglycemic agents that have a low risk of hypoglycemia and low potential for weight gain should be preferentially selected [29]. Attainment of A1C ≤ 7%, the guideline recommendation considered appropriate for many patients, is associated with reductions in microvascular disease and, if attained soon after diagnosis of diabetes, studies have shown reductions in macrovascular disease with long-term follow-up [27,30,31]. However, it may be challenging to achieve A1C < 7% with the addition of single oral antihyperglycemic agents, especially in patients with higher A1C [32]. Less stringent A1C goals (eg, A1C < 8%) may be appropriate in individuals with a long duration of diabetes that is difficult to control, history of severe hypoglycemia, limited life expectancy, numerous comorbidities, and extensive complications or comorbidities, especially cardiovascular disease [27]. Given the shift toward individualized treatment plans with patient-specific treatment goals, it is valuable to understand how different treatment strategies effect attainment of guideline-recommended less stringent and more stringent glycemic targets that may be appropriate for certain patients.
In addition to quality measures that assess glucose lowering with pharmacotherapy, it is important to consider measures that assess other aspects of diabetes care. For example, quality measures related to hypoglycemia and hyperglycemia may help avoid potentially adverse glucose levels, and quality measures related to weight may provide insight on treatment and lifestyle efforts directed at weight loss and management. NQF-endorsed measures of hypoglycemia and hyperglycemia are currently moving through annual review and are paired measures, intended to be interpreted with respect to one another to ensure balanced outcomes [33,34]. This underscores the value of efficacious antihyperglycemic agents with low intrinsic potential for hypoglycemia. Although this analysis did not include quality measures related to hypoglycemia or weight, future studies evaluating these aspects of diabetes care will likely further contribute to a more comprehensive and holistic treatment approach.
In addition to assessing a broad range of quality measures, an important aspect of care to consider is patient affordability. Affordability for an individual patient will depend on access in the patient’s individual plan, the financial resources of the patient, and the potential for medical cost offsets from improved control of the patient’s disease. For example, fixed-dose combination products are associated with increased patient adherence and may increase pharmacy costs but decrease medical costs [35].
Limitations of this study include the post hoc design and that quality measure attainment was assessed over a shorter duration of time (24 weeks) than is commonly assessed in the real-world/community setting (~12 months).
Dual add-on therapy with oral antihyperglycemic agents that have complementary mechanisms of action should lead to enhanced reductions in A1C. The results reported here and from the primary study, in which saxagliptin and dapagliflozin added to metformin significantly reduced mean A1C from baseline to week 24 compared with single add-on saxagliptin or dapagliflozin [15], showed that greater reductions in A1C were attained with the coadministration of saxagliptin and dapagliflozin. The glucuretic effect of SGLT-2 inhibitors has been associated with increased plasma glucagon concentrations and increased endogenous glucose production, which may impair the full glucose-lowering potential of SGLT-2 inhibitors [36,37]. Administering saxagliptin with dapagliflozin as dual therapy was shown to blunt the rise in plasma glucagon caused by dapagliflozin [38], and this may have contributed to the greater glucose control achieved with dual add-on of these 2 antihyperglycemic drugs [15].
By targeting multiple aspects of the underlying pathophysiology in T2D, greater improvements in A1C can be achieved. Dual add-on saxagliptin plus dapagliflozin to metformin resulted in a greater proportion of patients achieving NQF-endorsed HEDIS quality measures, as well as A1C < 7% (no longer an NQF-endorsed measure). As health care shifts to a more value-based payment structure, measuring quality outcomes will assume a greater role in guiding decision making and influence the care that patients receive. Understanding how antihyperglycemic medication regimens affect quality measures can help clinicians make informed decisions.
Corresponding author: Lawrence Blonde, MD, Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA.
Funding/support: This study was supported by AstraZeneca. Medical writing support for the preparation of this manuscript was provided by Lauren D’Angelo, PhD, and Janet Matsuura, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA), with funding from AstraZeneca.
Financial disclosures: Dr. Blonde has received grant and research support from AstraZeneca, Jansen Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis and has received honoraria for participating as a speaker from AstraZeneca, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis as well as honoraria for consultant work from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis. R. Garcia-Sanchez is an employee of AstraZeneca. J. Sheehan and Y. C. Barrett were employees of AstraZeneca at the time of this research.
1. National Quality Forum. ABCs of measurement. Accessed 11 Mar 2016 at www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx.
2. National Committee for Quality Assurance. HEDIS measure development process. Accessed 14 Mar 2016 at www.ncqa.org/tabid/414/Default.aspx.
3. National Committee for Quality Assurance. HEDIS measures. Accessed 11 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
4. National Committee for Quality Assurance. About NCQA: overview. Accessed 14 Mar 2016 at www.ncqa.org/AboutNCQA.aspx.
5. National Committee for Quality Assurance. Health care program evaluations. Accessed 11 Mar 2016 at www.ncqa.org/Programs.aspx.
6. National Committee for Quality Assurance. HEDIS and Performance Measurement. Accessed 14 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement.aspx.
7. National Committee for Quality Assurance. HEDIS 2015 technical specifications for ACO measurement. Washington, DC: National Committee for Quality Assurance; 2014.
8. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (> 9.0%). NQF identifier: 0059. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
9. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) control (< 8.0%). NQF identifier: 0575. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
10. National Quality Forum. Comprehensive diabetes care: blood pressure control (< 140/90 mm Hg). NQF identifier: 0061. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
11. Onglyza(saxagliptin). Full prescribing information. AstraZeneca, Wilmington, DE; 2014.
12. Farxiga (dapagliflozin). Full prescribing information. AstraZeneca, Wilmington, DE; March 2015.
13. Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009;26:488–99.
14. Kasichayanula S, Liu X, Lacreta F, et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014;53:17–27.
15. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2014;38:376–83.
16. Bailey CJ. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. British Journal of Diabetes & Vascular Disease 2010;10:193-9.
17. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53.
18. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9.
19. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
20. National Quality Forum. Comprehensive diabetes care (composite). NQF identifier: #0731. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
21. National Quality Forum. Optimal diabetes care (composite measure). NQF identifier: 0729. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206.
23. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25.
24. Invokana(canagliflzoin). Full prescribing information. Janssen Pharmaceuticals, Titusville, NJ; 2013.
25. Jardiance(empagliflozin). Full prescribing information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly, Ingelheim, Germany and Indianapolis, IN; 2014.
26. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100.
27. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016;39:S1–S119.
28. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32:S151–S6.
29. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 executive summary. Endocr Pract 2016;22:84–113.
30. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
31. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
32. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007;30:890–5.
33. National Quality Forum. Glycemic control: hypoglycemia. NQF identifier: 2363. Accessed 29 Oct 2015 at www.qualityforum.org/QPS/QPSTool.aspx.
34. National Quality Forum. Glycemic control: hyperglycemia. NQF identifier: 2362. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
35. Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009;11:527–33.
36. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508.
37. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.
38. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 2014;20:1187–97.
From the Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA (Dr. Blonde), and AstraZeneca, Gaithersburg, MD (Drs. Sheehan, Barrett, and Garcia-Sanchez).
Abstract
- Objective: To evaluate diabetes care quality measure attainment, specifically, blood glucose and blood pressure (BP) control, with saxagliptin, a dipeptidyl peptidase-4 inhibitor, and dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, added singly or as dual add-on therapy in patients with type 2 diabetes inadequately controlled with metformin alone.
- Methods: Analysis of a phase 3, randomized, double-blind, active-controlled, parallel-group trial was conducted. Patients were randomized 1:1:1 to receive saxagliptin 5 mg/d plus dapagliflozin 10 mg/d, saxagliptin 5 mg/d, or dapagliflozin 10 mg/d as add-on to metformin 1500 to 2000 mg/d. Assessments included attainment of individual and composite glycated hemoglobin (A1C) and BP measures at 24 weeks of treatment.
- Results: Compared with single add-on saxagliptin or dapagliflozin, dual add-on saxagliptin plus dapagliflozin to metformin was associated with significantly more patients attaining the individual quality measures of A1C < 7% and A1C < 8%. Similarly, dual add-on saxagliptin plus dapagliflozin was associated with significantly more patients attaining the composite quality measures A1C < 7% and BP < 140/90 mm Hg and A1C < 8% and BP < 140/90 mmHg (vs saxagliptin plus metformin).
- Conclusion: Dual add-on saxagliptin plus dapagliflozin to metformin was associated with a higher proportion of patients achieving glycemic and BP quality measures compared with single add-on saxagliptin or dapagliflozin.
Assessment of performance is a focus of many health care organizations as a means to evaluate and improve the quality of health care. Standardized performance measures have been developed to improve quality of care as well as to allow for comparative assessment of health plans and to support pay for performance models [1]. A widely used set of performance measures is the Healthcare Effectiveness Data and Information Set or HEDIS [2,3], measures that are maintained by the National Committee for Quality Assurance [4,5] and used by most US health plans [6].
Type 2 diabetes (T2D) is a focus of quality measure assessment and performance improvement because of its high prevalence, substantial personal and economic impact on society, high morbidity and mortality, and because it is a condition that requires coordinated care. Important outcome measures for diabetes include blood glucose control and blood pressure (BP) control. HEDIS measures for T2D include a glycated hemoglobin (A1C) > 9%, indicating poor glucose control, < 8%, indicating good control, and < 7%, a more stringent measure of good glycemic control. The HEDIS measure for BP in T2D is < 140/90 mm Hg, which is considered good BP control. All of these HEDIS measures are currently or were previously (A1C < 7%) endorsed by the National Quality Forum [1,7–10]. Endorsement of a quality measure by the NQF indicates that the measure has been thoroughly evaluated, meets specific criteria, and is based on recognized standards of care grounded in evidence-based medicine [1].
A number of oral agents are utilized in the treatment of diabetes. Saxagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, and dapagliflozin, an oral sodium-glucose cotransporter-2 (SGLT-2) inhibitor, are indicated as adjuncts to diet and exercise in adults with T2D [11,12]. Saxagliptin inhibits DPP-4, and thereby reduces fasting and postprandial glucose concentrations by preventing degradation of the incretin hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide [13]. Dapagliflozin reduces blood glucose concentrations by inhibiting glucose reabsorption in the proximal tubule of the kidney, which results in enhanced urinary glucose excretion [14]. Because their mechanisms of action are glucose-dependent, both saxagliptin and dapagliflozin have a low intrinsic potential to cause hypoglycemia [13,14]. In a randomized, double-blind study of patients with T2D inadequately controlled with metformin, Rosenstock et al assessed the efficacy and safety of dual add-on of saxagliptin plus dapagliflozin versus saxagliptin and dapagliflozin added on alone (ClinicalTrials.gov identifier, NCT01606007) [15]. The dual add-on therapy resulted in a greater adjusted mean reduction from baseline in A1C at week 24 (–1.47%) compared with either saxagliptin (–0.88%) or dapagliflozin (–1.20%) alone added to metformin; the difference for dual add-on saxagliptin plus dapagliflozin to metformin vs. single add-on saxagliptin and single add-on dapagliflozin was –0.59% (P < 0.001) and –0.27% (P = 0.0166), respectively. The incidence of adverse events was similar across the 3 treatment groups, and hypo-glycemia was infrequent (1%), with no reports of severe hypoglycemia events (symptomatic events with glucose ≤ 54 mg/dL requiring assistance).
In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
Study Design and Patients
Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.
Results
Patients
Individual Quality Measures
The dual addition of saxagliptin plus dapagliflozin to metformin resulted in a significantly greater proportion of patients achieving A1C < 8.0% compared with saxagliptin plus metformin (71.2% vs 49.1%; P < 0.001; NNT 5 [95% CI 3–8]) or dapagliflozin plus metformin (60.1%; P = 0.033; NNT 9 [95% CI 5–85]; Figure 2). Similar results (proportions of patients: 66.4% vs 40.0% vs 51.9%; P ≤ 0.02; NNTs 4 [95% CI 3–7]) and 7 [95% CI 4–34]) were attained when the analysis excluded patients with baseline A1C < 8.0%.
Significantly fewer patients had A1C > 9% (a measure of poor glycemic control) with saxagliptin plus dapagliflozin plus metformin (12.4%) compared with saxagliptin plus metformin (22.3%; P = 0.017; NNT –10 [95% CI –50 to –6]; Figure 2). The proportion of patients with A1C > 9% was similar for both regimens that included dapagliflozin (12.4% vs 10.4%; P = 0.616).
No significant difference was observed among treatment groups in the proportion of patients with BP < 140/90 mm Hg (Figure 2). However, most patients had BP < 140/90 mm Hg (72%–82%) at baseline, which was generally maintained at week 24.
Composite Quality Measures
Discussion
This post hoc analysis evaluated attainment of glycemic and BP quality measures for diabetes. A significantly greater proportion of patients achieved the individual quality measures of A1C < 7% and A1C < 8% with dual add-on saxagliptin plus dapagliflozin to metformin compared with single add-on saxagliptin or dapagliflozin to metformin after 24 weeks. Similar results were seen when the analysis excluded patients with A1C < 7% and < 8% at baseline. All measures of good glycemic control had clinically relevant NNTs ≤ 10 after 24 weeks with saxagliptin plus dapagliflozin plus metformin compared with saxagliptin or dapagliflozin plus metformin, regardless of baseline status. Very few patients experienced lackof improvement in glycemic control, evidenced by small proportions of patients with A1C > 9%.
There was little difference in BP between dual add-on saxagliptin plus dapagliflozin or single add-on saxagliptin or dapagliflozin to metformin. The proportion of patients who attained the BP quality measure of BP < 140/90 mm Hg was similar across the 3 treatments, as might be expected because most patients already met this target at baseline. However, as might be expected based on the mild diuretic effect and weight loss associated with SGLT-2 inhibitors [16,17], trends in BP favored groups treated with dapagliflozin.
Attainment of multiple treatment targets is desirable in reducing complications of diabetes. A significantly greater proportion of patients achieved both A1C < 7% and BP < 140/90 mm Hg when both saxagliptin and dapagliflozin were added to metformin compared with single-agent addition of either saxagliptin or dapagliflozin plus metformin. Similarly, a significantly greater proportion of patients achieved both A1C < 8% and BP < 140/90 mm Hg with dual addition of saxagliptin and dapagliflozin plus metformin compared with saxagliptin plus metformin. There was also a numerically greater number of patients who achieved both of these goals with triple therapy compared with dapagliflozin plus metformin, but this finding did not reach statistical significance. Clinically relevant NNT values ≤ 10 were observed for both composite outcomes for saxagliptin plus dapagliflozin plus metformin compared with saxagliptin plus metformin or dapagliflozin plus metformin after 24 weeks.
Despite advances in the medical management of T2D, a report published in 2013 showed that between 2007 and 2010, only 53% of patients achieved an A1C < 7.0% and only 19% simultaneously achieved all 3 American Diabetes Association (ADA) goals recommended for most patients at that time: A1C < 7.0%, BP < 130/80 mm Hg, and low-density lipoprotein cholesterol LDL-C < 100 mg/dL [18]. These data highlight a need for new approaches to help patients attain glycemic, BP, and cholesterol goals. Our results demonstrated that a higher proportion of patients attained glycemic and BP quality measures with dual add-on saxagliptin plus dapagliflozin compared with single add-on saxagliptin or dapagliflozin to metformin. As a result of recent updates for cholesterol management from the American College of Cardiology and the American Heart Association [19], attainment of a cholesterol level was retired as a diabetes quality measure and replaced with a recommendation for statin therapy use [20,21]. Although the current analysis did not include assessment of LDL, DPP-4 inhibitors have demonstrated neutral effects on lipids [22,23], and SGLT-2 inhibitors have demonstrated generally modest increases in LDL-C (placebo-adjusted change from baseline: 4.5%–8.0% for canagliflozin 100 and 300 mg/d, 3.9% for dapagliflozin 10 mg, and 2.3%–4.2% for empagliflozin 10 and 25 mg/d) [12,24,25], as well as increases in high-density lipoprotein cholesterol and reductions in triglycerides [26].
Current ADA guidelines recommend an individualized, stepwise approach to treatment with sequential addition of single oral antihyperglycemic agents for patients who do not achieve their glycemic goal in 3 months [27]. Although T2D may progress at different rates in different patients, T2D does generally progress over time [28], and the ADA and American Association of Clinical Endocrinologists treatment guidelines recommend initial dual add-on therapy for individuals with higher A1C, which is suggestive of more advanced disease [27,29]. For individuals requiring initial combination therapy, guidelines note that antihyperglycemic agents that have a low risk of hypoglycemia and low potential for weight gain should be preferentially selected [29]. Attainment of A1C ≤ 7%, the guideline recommendation considered appropriate for many patients, is associated with reductions in microvascular disease and, if attained soon after diagnosis of diabetes, studies have shown reductions in macrovascular disease with long-term follow-up [27,30,31]. However, it may be challenging to achieve A1C < 7% with the addition of single oral antihyperglycemic agents, especially in patients with higher A1C [32]. Less stringent A1C goals (eg, A1C < 8%) may be appropriate in individuals with a long duration of diabetes that is difficult to control, history of severe hypoglycemia, limited life expectancy, numerous comorbidities, and extensive complications or comorbidities, especially cardiovascular disease [27]. Given the shift toward individualized treatment plans with patient-specific treatment goals, it is valuable to understand how different treatment strategies effect attainment of guideline-recommended less stringent and more stringent glycemic targets that may be appropriate for certain patients.
In addition to quality measures that assess glucose lowering with pharmacotherapy, it is important to consider measures that assess other aspects of diabetes care. For example, quality measures related to hypoglycemia and hyperglycemia may help avoid potentially adverse glucose levels, and quality measures related to weight may provide insight on treatment and lifestyle efforts directed at weight loss and management. NQF-endorsed measures of hypoglycemia and hyperglycemia are currently moving through annual review and are paired measures, intended to be interpreted with respect to one another to ensure balanced outcomes [33,34]. This underscores the value of efficacious antihyperglycemic agents with low intrinsic potential for hypoglycemia. Although this analysis did not include quality measures related to hypoglycemia or weight, future studies evaluating these aspects of diabetes care will likely further contribute to a more comprehensive and holistic treatment approach.
In addition to assessing a broad range of quality measures, an important aspect of care to consider is patient affordability. Affordability for an individual patient will depend on access in the patient’s individual plan, the financial resources of the patient, and the potential for medical cost offsets from improved control of the patient’s disease. For example, fixed-dose combination products are associated with increased patient adherence and may increase pharmacy costs but decrease medical costs [35].
Limitations of this study include the post hoc design and that quality measure attainment was assessed over a shorter duration of time (24 weeks) than is commonly assessed in the real-world/community setting (~12 months).
Dual add-on therapy with oral antihyperglycemic agents that have complementary mechanisms of action should lead to enhanced reductions in A1C. The results reported here and from the primary study, in which saxagliptin and dapagliflozin added to metformin significantly reduced mean A1C from baseline to week 24 compared with single add-on saxagliptin or dapagliflozin [15], showed that greater reductions in A1C were attained with the coadministration of saxagliptin and dapagliflozin. The glucuretic effect of SGLT-2 inhibitors has been associated with increased plasma glucagon concentrations and increased endogenous glucose production, which may impair the full glucose-lowering potential of SGLT-2 inhibitors [36,37]. Administering saxagliptin with dapagliflozin as dual therapy was shown to blunt the rise in plasma glucagon caused by dapagliflozin [38], and this may have contributed to the greater glucose control achieved with dual add-on of these 2 antihyperglycemic drugs [15].
By targeting multiple aspects of the underlying pathophysiology in T2D, greater improvements in A1C can be achieved. Dual add-on saxagliptin plus dapagliflozin to metformin resulted in a greater proportion of patients achieving NQF-endorsed HEDIS quality measures, as well as A1C < 7% (no longer an NQF-endorsed measure). As health care shifts to a more value-based payment structure, measuring quality outcomes will assume a greater role in guiding decision making and influence the care that patients receive. Understanding how antihyperglycemic medication regimens affect quality measures can help clinicians make informed decisions.
Corresponding author: Lawrence Blonde, MD, Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA.
Funding/support: This study was supported by AstraZeneca. Medical writing support for the preparation of this manuscript was provided by Lauren D’Angelo, PhD, and Janet Matsuura, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA), with funding from AstraZeneca.
Financial disclosures: Dr. Blonde has received grant and research support from AstraZeneca, Jansen Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis and has received honoraria for participating as a speaker from AstraZeneca, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis as well as honoraria for consultant work from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis. R. Garcia-Sanchez is an employee of AstraZeneca. J. Sheehan and Y. C. Barrett were employees of AstraZeneca at the time of this research.
From the Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA (Dr. Blonde), and AstraZeneca, Gaithersburg, MD (Drs. Sheehan, Barrett, and Garcia-Sanchez).
Abstract
- Objective: To evaluate diabetes care quality measure attainment, specifically, blood glucose and blood pressure (BP) control, with saxagliptin, a dipeptidyl peptidase-4 inhibitor, and dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, added singly or as dual add-on therapy in patients with type 2 diabetes inadequately controlled with metformin alone.
- Methods: Analysis of a phase 3, randomized, double-blind, active-controlled, parallel-group trial was conducted. Patients were randomized 1:1:1 to receive saxagliptin 5 mg/d plus dapagliflozin 10 mg/d, saxagliptin 5 mg/d, or dapagliflozin 10 mg/d as add-on to metformin 1500 to 2000 mg/d. Assessments included attainment of individual and composite glycated hemoglobin (A1C) and BP measures at 24 weeks of treatment.
- Results: Compared with single add-on saxagliptin or dapagliflozin, dual add-on saxagliptin plus dapagliflozin to metformin was associated with significantly more patients attaining the individual quality measures of A1C < 7% and A1C < 8%. Similarly, dual add-on saxagliptin plus dapagliflozin was associated with significantly more patients attaining the composite quality measures A1C < 7% and BP < 140/90 mm Hg and A1C < 8% and BP < 140/90 mmHg (vs saxagliptin plus metformin).
- Conclusion: Dual add-on saxagliptin plus dapagliflozin to metformin was associated with a higher proportion of patients achieving glycemic and BP quality measures compared with single add-on saxagliptin or dapagliflozin.
Assessment of performance is a focus of many health care organizations as a means to evaluate and improve the quality of health care. Standardized performance measures have been developed to improve quality of care as well as to allow for comparative assessment of health plans and to support pay for performance models [1]. A widely used set of performance measures is the Healthcare Effectiveness Data and Information Set or HEDIS [2,3], measures that are maintained by the National Committee for Quality Assurance [4,5] and used by most US health plans [6].
Type 2 diabetes (T2D) is a focus of quality measure assessment and performance improvement because of its high prevalence, substantial personal and economic impact on society, high morbidity and mortality, and because it is a condition that requires coordinated care. Important outcome measures for diabetes include blood glucose control and blood pressure (BP) control. HEDIS measures for T2D include a glycated hemoglobin (A1C) > 9%, indicating poor glucose control, < 8%, indicating good control, and < 7%, a more stringent measure of good glycemic control. The HEDIS measure for BP in T2D is < 140/90 mm Hg, which is considered good BP control. All of these HEDIS measures are currently or were previously (A1C < 7%) endorsed by the National Quality Forum [1,7–10]. Endorsement of a quality measure by the NQF indicates that the measure has been thoroughly evaluated, meets specific criteria, and is based on recognized standards of care grounded in evidence-based medicine [1].
A number of oral agents are utilized in the treatment of diabetes. Saxagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, and dapagliflozin, an oral sodium-glucose cotransporter-2 (SGLT-2) inhibitor, are indicated as adjuncts to diet and exercise in adults with T2D [11,12]. Saxagliptin inhibits DPP-4, and thereby reduces fasting and postprandial glucose concentrations by preventing degradation of the incretin hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide [13]. Dapagliflozin reduces blood glucose concentrations by inhibiting glucose reabsorption in the proximal tubule of the kidney, which results in enhanced urinary glucose excretion [14]. Because their mechanisms of action are glucose-dependent, both saxagliptin and dapagliflozin have a low intrinsic potential to cause hypoglycemia [13,14]. In a randomized, double-blind study of patients with T2D inadequately controlled with metformin, Rosenstock et al assessed the efficacy and safety of dual add-on of saxagliptin plus dapagliflozin versus saxagliptin and dapagliflozin added on alone (ClinicalTrials.gov identifier, NCT01606007) [15]. The dual add-on therapy resulted in a greater adjusted mean reduction from baseline in A1C at week 24 (–1.47%) compared with either saxagliptin (–0.88%) or dapagliflozin (–1.20%) alone added to metformin; the difference for dual add-on saxagliptin plus dapagliflozin to metformin vs. single add-on saxagliptin and single add-on dapagliflozin was –0.59% (P < 0.001) and –0.27% (P = 0.0166), respectively. The incidence of adverse events was similar across the 3 treatment groups, and hypo-glycemia was infrequent (1%), with no reports of severe hypoglycemia events (symptomatic events with glucose ≤ 54 mg/dL requiring assistance).
In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
Study Design and Patients
Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.
Results
Patients
Individual Quality Measures
The dual addition of saxagliptin plus dapagliflozin to metformin resulted in a significantly greater proportion of patients achieving A1C < 8.0% compared with saxagliptin plus metformin (71.2% vs 49.1%; P < 0.001; NNT 5 [95% CI 3–8]) or dapagliflozin plus metformin (60.1%; P = 0.033; NNT 9 [95% CI 5–85]; Figure 2). Similar results (proportions of patients: 66.4% vs 40.0% vs 51.9%; P ≤ 0.02; NNTs 4 [95% CI 3–7]) and 7 [95% CI 4–34]) were attained when the analysis excluded patients with baseline A1C < 8.0%.
Significantly fewer patients had A1C > 9% (a measure of poor glycemic control) with saxagliptin plus dapagliflozin plus metformin (12.4%) compared with saxagliptin plus metformin (22.3%; P = 0.017; NNT –10 [95% CI –50 to –6]; Figure 2). The proportion of patients with A1C > 9% was similar for both regimens that included dapagliflozin (12.4% vs 10.4%; P = 0.616).
No significant difference was observed among treatment groups in the proportion of patients with BP < 140/90 mm Hg (Figure 2). However, most patients had BP < 140/90 mm Hg (72%–82%) at baseline, which was generally maintained at week 24.
Composite Quality Measures
Discussion
This post hoc analysis evaluated attainment of glycemic and BP quality measures for diabetes. A significantly greater proportion of patients achieved the individual quality measures of A1C < 7% and A1C < 8% with dual add-on saxagliptin plus dapagliflozin to metformin compared with single add-on saxagliptin or dapagliflozin to metformin after 24 weeks. Similar results were seen when the analysis excluded patients with A1C < 7% and < 8% at baseline. All measures of good glycemic control had clinically relevant NNTs ≤ 10 after 24 weeks with saxagliptin plus dapagliflozin plus metformin compared with saxagliptin or dapagliflozin plus metformin, regardless of baseline status. Very few patients experienced lackof improvement in glycemic control, evidenced by small proportions of patients with A1C > 9%.
There was little difference in BP between dual add-on saxagliptin plus dapagliflozin or single add-on saxagliptin or dapagliflozin to metformin. The proportion of patients who attained the BP quality measure of BP < 140/90 mm Hg was similar across the 3 treatments, as might be expected because most patients already met this target at baseline. However, as might be expected based on the mild diuretic effect and weight loss associated with SGLT-2 inhibitors [16,17], trends in BP favored groups treated with dapagliflozin.
Attainment of multiple treatment targets is desirable in reducing complications of diabetes. A significantly greater proportion of patients achieved both A1C < 7% and BP < 140/90 mm Hg when both saxagliptin and dapagliflozin were added to metformin compared with single-agent addition of either saxagliptin or dapagliflozin plus metformin. Similarly, a significantly greater proportion of patients achieved both A1C < 8% and BP < 140/90 mm Hg with dual addition of saxagliptin and dapagliflozin plus metformin compared with saxagliptin plus metformin. There was also a numerically greater number of patients who achieved both of these goals with triple therapy compared with dapagliflozin plus metformin, but this finding did not reach statistical significance. Clinically relevant NNT values ≤ 10 were observed for both composite outcomes for saxagliptin plus dapagliflozin plus metformin compared with saxagliptin plus metformin or dapagliflozin plus metformin after 24 weeks.
Despite advances in the medical management of T2D, a report published in 2013 showed that between 2007 and 2010, only 53% of patients achieved an A1C < 7.0% and only 19% simultaneously achieved all 3 American Diabetes Association (ADA) goals recommended for most patients at that time: A1C < 7.0%, BP < 130/80 mm Hg, and low-density lipoprotein cholesterol LDL-C < 100 mg/dL [18]. These data highlight a need for new approaches to help patients attain glycemic, BP, and cholesterol goals. Our results demonstrated that a higher proportion of patients attained glycemic and BP quality measures with dual add-on saxagliptin plus dapagliflozin compared with single add-on saxagliptin or dapagliflozin to metformin. As a result of recent updates for cholesterol management from the American College of Cardiology and the American Heart Association [19], attainment of a cholesterol level was retired as a diabetes quality measure and replaced with a recommendation for statin therapy use [20,21]. Although the current analysis did not include assessment of LDL, DPP-4 inhibitors have demonstrated neutral effects on lipids [22,23], and SGLT-2 inhibitors have demonstrated generally modest increases in LDL-C (placebo-adjusted change from baseline: 4.5%–8.0% for canagliflozin 100 and 300 mg/d, 3.9% for dapagliflozin 10 mg, and 2.3%–4.2% for empagliflozin 10 and 25 mg/d) [12,24,25], as well as increases in high-density lipoprotein cholesterol and reductions in triglycerides [26].
Current ADA guidelines recommend an individualized, stepwise approach to treatment with sequential addition of single oral antihyperglycemic agents for patients who do not achieve their glycemic goal in 3 months [27]. Although T2D may progress at different rates in different patients, T2D does generally progress over time [28], and the ADA and American Association of Clinical Endocrinologists treatment guidelines recommend initial dual add-on therapy for individuals with higher A1C, which is suggestive of more advanced disease [27,29]. For individuals requiring initial combination therapy, guidelines note that antihyperglycemic agents that have a low risk of hypoglycemia and low potential for weight gain should be preferentially selected [29]. Attainment of A1C ≤ 7%, the guideline recommendation considered appropriate for many patients, is associated with reductions in microvascular disease and, if attained soon after diagnosis of diabetes, studies have shown reductions in macrovascular disease with long-term follow-up [27,30,31]. However, it may be challenging to achieve A1C < 7% with the addition of single oral antihyperglycemic agents, especially in patients with higher A1C [32]. Less stringent A1C goals (eg, A1C < 8%) may be appropriate in individuals with a long duration of diabetes that is difficult to control, history of severe hypoglycemia, limited life expectancy, numerous comorbidities, and extensive complications or comorbidities, especially cardiovascular disease [27]. Given the shift toward individualized treatment plans with patient-specific treatment goals, it is valuable to understand how different treatment strategies effect attainment of guideline-recommended less stringent and more stringent glycemic targets that may be appropriate for certain patients.
In addition to quality measures that assess glucose lowering with pharmacotherapy, it is important to consider measures that assess other aspects of diabetes care. For example, quality measures related to hypoglycemia and hyperglycemia may help avoid potentially adverse glucose levels, and quality measures related to weight may provide insight on treatment and lifestyle efforts directed at weight loss and management. NQF-endorsed measures of hypoglycemia and hyperglycemia are currently moving through annual review and are paired measures, intended to be interpreted with respect to one another to ensure balanced outcomes [33,34]. This underscores the value of efficacious antihyperglycemic agents with low intrinsic potential for hypoglycemia. Although this analysis did not include quality measures related to hypoglycemia or weight, future studies evaluating these aspects of diabetes care will likely further contribute to a more comprehensive and holistic treatment approach.
In addition to assessing a broad range of quality measures, an important aspect of care to consider is patient affordability. Affordability for an individual patient will depend on access in the patient’s individual plan, the financial resources of the patient, and the potential for medical cost offsets from improved control of the patient’s disease. For example, fixed-dose combination products are associated with increased patient adherence and may increase pharmacy costs but decrease medical costs [35].
Limitations of this study include the post hoc design and that quality measure attainment was assessed over a shorter duration of time (24 weeks) than is commonly assessed in the real-world/community setting (~12 months).
Dual add-on therapy with oral antihyperglycemic agents that have complementary mechanisms of action should lead to enhanced reductions in A1C. The results reported here and from the primary study, in which saxagliptin and dapagliflozin added to metformin significantly reduced mean A1C from baseline to week 24 compared with single add-on saxagliptin or dapagliflozin [15], showed that greater reductions in A1C were attained with the coadministration of saxagliptin and dapagliflozin. The glucuretic effect of SGLT-2 inhibitors has been associated with increased plasma glucagon concentrations and increased endogenous glucose production, which may impair the full glucose-lowering potential of SGLT-2 inhibitors [36,37]. Administering saxagliptin with dapagliflozin as dual therapy was shown to blunt the rise in plasma glucagon caused by dapagliflozin [38], and this may have contributed to the greater glucose control achieved with dual add-on of these 2 antihyperglycemic drugs [15].
By targeting multiple aspects of the underlying pathophysiology in T2D, greater improvements in A1C can be achieved. Dual add-on saxagliptin plus dapagliflozin to metformin resulted in a greater proportion of patients achieving NQF-endorsed HEDIS quality measures, as well as A1C < 7% (no longer an NQF-endorsed measure). As health care shifts to a more value-based payment structure, measuring quality outcomes will assume a greater role in guiding decision making and influence the care that patients receive. Understanding how antihyperglycemic medication regimens affect quality measures can help clinicians make informed decisions.
Corresponding author: Lawrence Blonde, MD, Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA.
Funding/support: This study was supported by AstraZeneca. Medical writing support for the preparation of this manuscript was provided by Lauren D’Angelo, PhD, and Janet Matsuura, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA), with funding from AstraZeneca.
Financial disclosures: Dr. Blonde has received grant and research support from AstraZeneca, Jansen Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis and has received honoraria for participating as a speaker from AstraZeneca, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis as well as honoraria for consultant work from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis. R. Garcia-Sanchez is an employee of AstraZeneca. J. Sheehan and Y. C. Barrett were employees of AstraZeneca at the time of this research.
1. National Quality Forum. ABCs of measurement. Accessed 11 Mar 2016 at www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx.
2. National Committee for Quality Assurance. HEDIS measure development process. Accessed 14 Mar 2016 at www.ncqa.org/tabid/414/Default.aspx.
3. National Committee for Quality Assurance. HEDIS measures. Accessed 11 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
4. National Committee for Quality Assurance. About NCQA: overview. Accessed 14 Mar 2016 at www.ncqa.org/AboutNCQA.aspx.
5. National Committee for Quality Assurance. Health care program evaluations. Accessed 11 Mar 2016 at www.ncqa.org/Programs.aspx.
6. National Committee for Quality Assurance. HEDIS and Performance Measurement. Accessed 14 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement.aspx.
7. National Committee for Quality Assurance. HEDIS 2015 technical specifications for ACO measurement. Washington, DC: National Committee for Quality Assurance; 2014.
8. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (> 9.0%). NQF identifier: 0059. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
9. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) control (< 8.0%). NQF identifier: 0575. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
10. National Quality Forum. Comprehensive diabetes care: blood pressure control (< 140/90 mm Hg). NQF identifier: 0061. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
11. Onglyza(saxagliptin). Full prescribing information. AstraZeneca, Wilmington, DE; 2014.
12. Farxiga (dapagliflozin). Full prescribing information. AstraZeneca, Wilmington, DE; March 2015.
13. Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009;26:488–99.
14. Kasichayanula S, Liu X, Lacreta F, et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014;53:17–27.
15. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2014;38:376–83.
16. Bailey CJ. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. British Journal of Diabetes & Vascular Disease 2010;10:193-9.
17. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53.
18. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9.
19. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
20. National Quality Forum. Comprehensive diabetes care (composite). NQF identifier: #0731. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
21. National Quality Forum. Optimal diabetes care (composite measure). NQF identifier: 0729. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206.
23. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25.
24. Invokana(canagliflzoin). Full prescribing information. Janssen Pharmaceuticals, Titusville, NJ; 2013.
25. Jardiance(empagliflozin). Full prescribing information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly, Ingelheim, Germany and Indianapolis, IN; 2014.
26. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100.
27. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016;39:S1–S119.
28. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32:S151–S6.
29. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 executive summary. Endocr Pract 2016;22:84–113.
30. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
31. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
32. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007;30:890–5.
33. National Quality Forum. Glycemic control: hypoglycemia. NQF identifier: 2363. Accessed 29 Oct 2015 at www.qualityforum.org/QPS/QPSTool.aspx.
34. National Quality Forum. Glycemic control: hyperglycemia. NQF identifier: 2362. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
35. Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009;11:527–33.
36. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508.
37. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.
38. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 2014;20:1187–97.
1. National Quality Forum. ABCs of measurement. Accessed 11 Mar 2016 at www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx.
2. National Committee for Quality Assurance. HEDIS measure development process. Accessed 14 Mar 2016 at www.ncqa.org/tabid/414/Default.aspx.
3. National Committee for Quality Assurance. HEDIS measures. Accessed 11 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
4. National Committee for Quality Assurance. About NCQA: overview. Accessed 14 Mar 2016 at www.ncqa.org/AboutNCQA.aspx.
5. National Committee for Quality Assurance. Health care program evaluations. Accessed 11 Mar 2016 at www.ncqa.org/Programs.aspx.
6. National Committee for Quality Assurance. HEDIS and Performance Measurement. Accessed 14 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement.aspx.
7. National Committee for Quality Assurance. HEDIS 2015 technical specifications for ACO measurement. Washington, DC: National Committee for Quality Assurance; 2014.
8. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (> 9.0%). NQF identifier: 0059. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
9. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) control (< 8.0%). NQF identifier: 0575. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
10. National Quality Forum. Comprehensive diabetes care: blood pressure control (< 140/90 mm Hg). NQF identifier: 0061. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
11. Onglyza(saxagliptin). Full prescribing information. AstraZeneca, Wilmington, DE; 2014.
12. Farxiga (dapagliflozin). Full prescribing information. AstraZeneca, Wilmington, DE; March 2015.
13. Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009;26:488–99.
14. Kasichayanula S, Liu X, Lacreta F, et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014;53:17–27.
15. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2014;38:376–83.
16. Bailey CJ. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. British Journal of Diabetes & Vascular Disease 2010;10:193-9.
17. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53.
18. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9.
19. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
20. National Quality Forum. Comprehensive diabetes care (composite). NQF identifier: #0731. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
21. National Quality Forum. Optimal diabetes care (composite measure). NQF identifier: 0729. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206.
23. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25.
24. Invokana(canagliflzoin). Full prescribing information. Janssen Pharmaceuticals, Titusville, NJ; 2013.
25. Jardiance(empagliflozin). Full prescribing information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly, Ingelheim, Germany and Indianapolis, IN; 2014.
26. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100.
27. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016;39:S1–S119.
28. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32:S151–S6.
29. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 executive summary. Endocr Pract 2016;22:84–113.
30. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
31. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
32. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007;30:890–5.
33. National Quality Forum. Glycemic control: hypoglycemia. NQF identifier: 2363. Accessed 29 Oct 2015 at www.qualityforum.org/QPS/QPSTool.aspx.
34. National Quality Forum. Glycemic control: hyperglycemia. NQF identifier: 2362. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
35. Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009;11:527–33.
36. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508.
37. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.
38. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 2014;20:1187–97.