User login
Video-Based Coaching for Dermatology Resident Surgical Education
To the Editor:
Video-based coaching (VBC) involves a surgeon recording a surgery and then reviewing the video with a surgical coach; it is a form of education that is gaining popularity among surgical specialties.1 Video-based education is underutilized in dermatology residency training.2 We conducted a pilot study at our dermatology residency program to evaluate the efficacy and feasibility of VBC.
The University of Texas at Austin Dell Medical School institutional review board approved this study. All 4 first-year dermatology residents were recruited to participate in this study. Participants filled out a prestudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Participants used a head-mounted point-of-view camera to record themselves performing a wide local excision on the trunk or extremities of a live human patient. Participants then reviewed the recording on their own and scored themselves using the Objective Structured Assessment of Technical Skills (OSATS) scoring table (scored from 1 to 5, with 5 being the highest possible score for each element), which is a validated tool for assessing surgical skills (eTable 1).3 Given that there were no assistants participating in the surgery, this element of the OSATS scoring table was excluded, making a maximum possible score of 30 and a minimum possible score of 6. After scoring themselves, participants then had a 1-on-1 coaching session with a fellowship-trained dermatologic surgeon (M.F. or T.H.) via online teleconferencing.
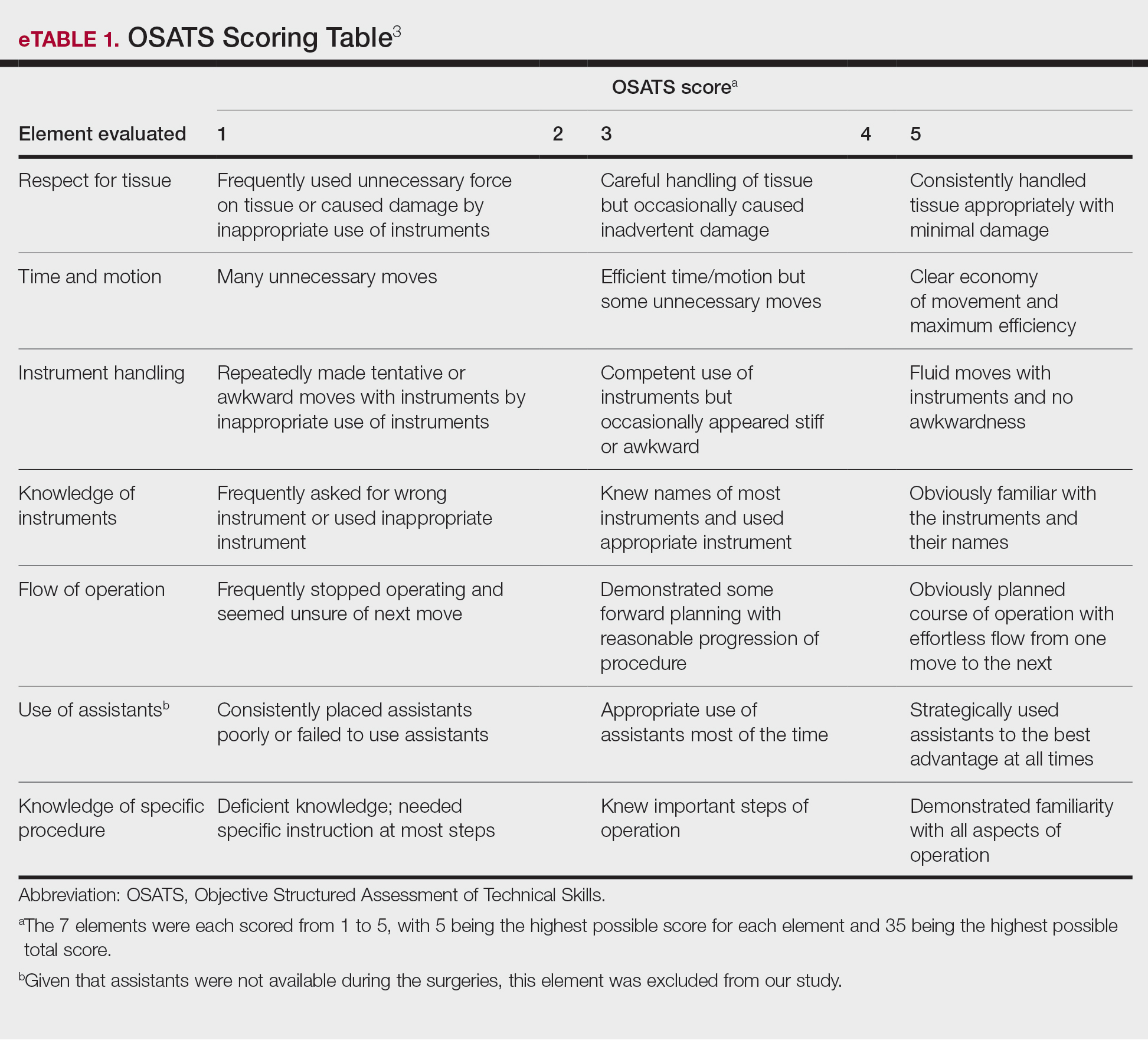
During the coaching session, participants and coaches reviewed the video. The surgical coaches also scored the residents using the OSATS, then residents and coaches discussed how the resident could improve using the OSATS scores as a guide. The residents then completed a poststudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Descriptive statistics were reported.
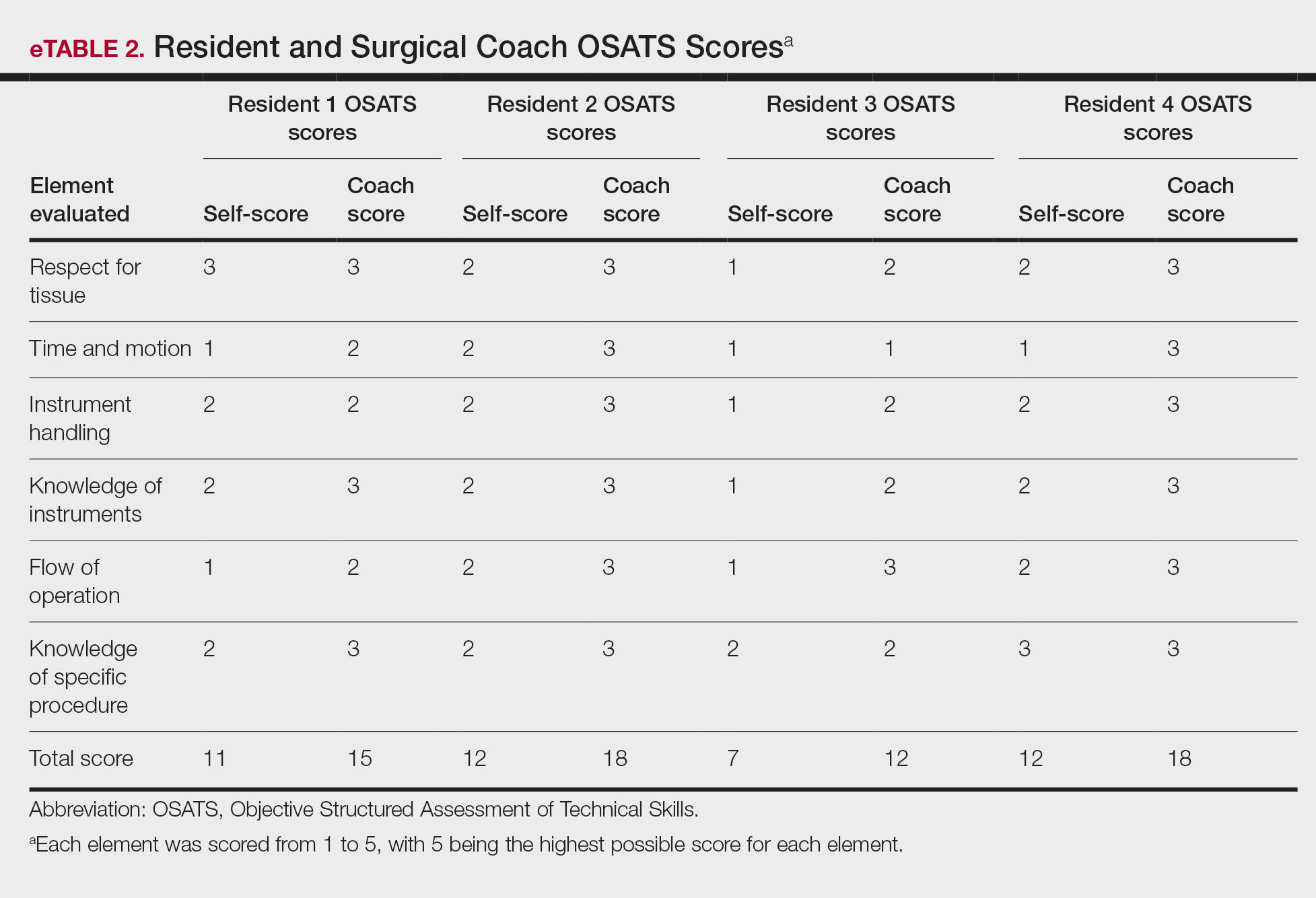
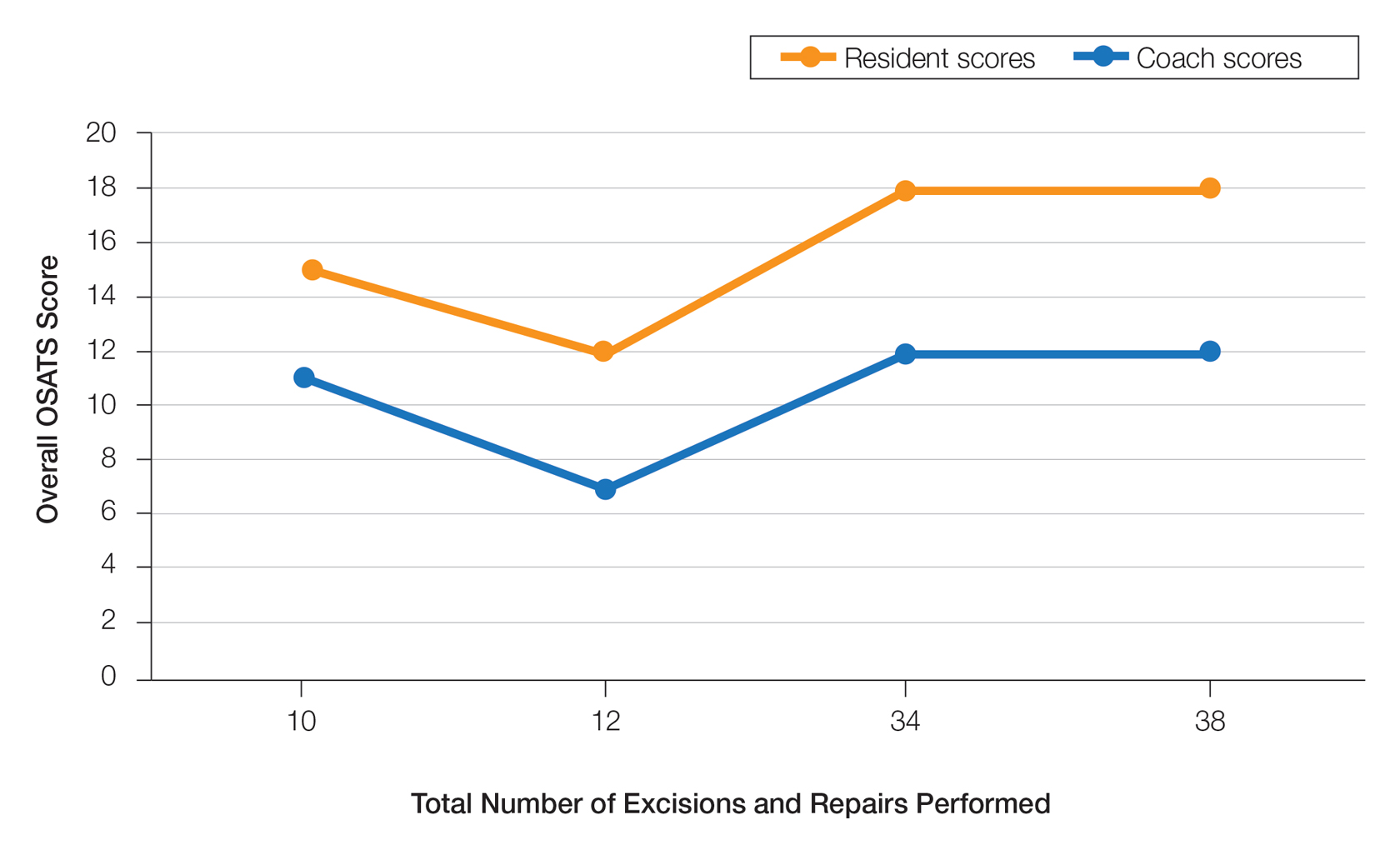
On average, residents spent 31.3 minutes reviewing their own surgeries and scoring themselves. The average time for a coaching session, which included time spent scoring, was 13.8 minutes. Residents scored themselves lower than the surgical coaches did by an average of 5.25 points (eTable 2). Residents gave themselves an average total score of 10.5, while their respective surgical coaches gave the residents an average score of 15.75. There was a trend of residents with greater surgical experience having higher OSATS scores (Figure). After the coaching session, 3 of 4 residents reported that they felt more confident in their surgical skills. All residents felt more confident in assessing their surgical skills and felt that VBC was an effective teaching measure. All residents agreed that VBC should be continued as part of their residency training.
Video-based coaching has the potential to provide several benefits for dermatology trainees. Because receiving feedback intraoperatively often can be distracting and incomplete, video review can instead allow the surgeon to focus on performing the surgery and then later focus on learning while reviewing the video.1,4 Feedback also can be more comprehensive and delivered without concern for time constraints or disturbing clinic flow as well as without the additional concern of the patient overhearing comments and feedback.3 Although independent video review in the absence of coaching can lead to improvement in surgical skills, the addition of VBC provides even greater potential educational benefit.4 During the COVID-19 pandemic, VBC allowed coaches to provide feedback without additional exposures. We utilized dermatologic surgery faculty as coaches, but this format of training also would apply to general dermatology faculty.
Another goal of VBC is to enhance a trainee’s ability to perform self-directed learning, which requires accurate self-assessment.4 Accurately assessing one’s own strengths empowers a trainee to act with appropriate confidence, while understanding one’s own weaknesses allows a trainee to effectively balance confidence and caution in daily practice.5 Interestingly, in our study all residents scored themselves lower than surgical coaches, but with 1 coaching session, the residents subsequently reported greater surgical confidence.
Time constraints can be a potential barrier to surgical coaching.4 Our study demonstrates that VBC requires minimal time investment. Increasing the speed of video playback allowed for efficient evaluation of resident surgeries without compromising the coach’s ability to provide comprehensive feedback. Our feedback sessions were performed virtually, which allowed for ease of scheduling between trainees and coaches.
Our pilot study demonstrated that VBC is relatively easy to implement in a dermatology residency training setting, leveraging relatively low-cost technologies and allowing for a means of learning that residents felt was effective. Video-based coaching requires minimal time investment from both trainees and coaches and has the potential to enhance surgical confidence. Our current study is limited by its small sample size. Future studies should include follow-up recordings and assess the efficacy of VBC in enhancing surgical skills.
- Greenberg CC, Dombrowski J, Dimick JB. Video-based surgical coaching: an emerging approach to performance improvement. JAMA Surg. 2016;151:282-283.
- Dai J, Bordeaux JS, Miller CJ, et al. Assessing surgical training and deliberate practice methods in dermatology residency: a survey of dermatology program directors. Dermatol Surg. 2016;42:977-984.
- Chitgopeker P, Sidey K, Aronson A, et al. Surgical skills video-based assessment tool for dermatology residents: a prospective pilot study. J Am Acad Dermatol. 2020;83:614-616.
- Bull NB, Silverman CD, Bonrath EM. Targeted surgical coaching can improve operative self-assessment ability: a single-blinded nonrandomized trial. Surgery. 2020;167:308-313.
- Eva KW, Regehr G. Self-assessment in the health professions: a reformulation and research agenda. Acad Med. 2005;80(10 suppl):S46-S54.
To the Editor:
Video-based coaching (VBC) involves a surgeon recording a surgery and then reviewing the video with a surgical coach; it is a form of education that is gaining popularity among surgical specialties.1 Video-based education is underutilized in dermatology residency training.2 We conducted a pilot study at our dermatology residency program to evaluate the efficacy and feasibility of VBC.
The University of Texas at Austin Dell Medical School institutional review board approved this study. All 4 first-year dermatology residents were recruited to participate in this study. Participants filled out a prestudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Participants used a head-mounted point-of-view camera to record themselves performing a wide local excision on the trunk or extremities of a live human patient. Participants then reviewed the recording on their own and scored themselves using the Objective Structured Assessment of Technical Skills (OSATS) scoring table (scored from 1 to 5, with 5 being the highest possible score for each element), which is a validated tool for assessing surgical skills (eTable 1).3 Given that there were no assistants participating in the surgery, this element of the OSATS scoring table was excluded, making a maximum possible score of 30 and a minimum possible score of 6. After scoring themselves, participants then had a 1-on-1 coaching session with a fellowship-trained dermatologic surgeon (M.F. or T.H.) via online teleconferencing.

During the coaching session, participants and coaches reviewed the video. The surgical coaches also scored the residents using the OSATS, then residents and coaches discussed how the resident could improve using the OSATS scores as a guide. The residents then completed a poststudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Descriptive statistics were reported.

On average, residents spent 31.3 minutes reviewing their own surgeries and scoring themselves. The average time for a coaching session, which included time spent scoring, was 13.8 minutes. Residents scored themselves lower than the surgical coaches did by an average of 5.25 points (eTable 2). Residents gave themselves an average total score of 10.5, while their respective surgical coaches gave the residents an average score of 15.75. There was a trend of residents with greater surgical experience having higher OSATS scores (Figure). After the coaching session, 3 of 4 residents reported that they felt more confident in their surgical skills. All residents felt more confident in assessing their surgical skills and felt that VBC was an effective teaching measure. All residents agreed that VBC should be continued as part of their residency training.

Video-based coaching has the potential to provide several benefits for dermatology trainees. Because receiving feedback intraoperatively often can be distracting and incomplete, video review can instead allow the surgeon to focus on performing the surgery and then later focus on learning while reviewing the video.1,4 Feedback also can be more comprehensive and delivered without concern for time constraints or disturbing clinic flow as well as without the additional concern of the patient overhearing comments and feedback.3 Although independent video review in the absence of coaching can lead to improvement in surgical skills, the addition of VBC provides even greater potential educational benefit.4 During the COVID-19 pandemic, VBC allowed coaches to provide feedback without additional exposures. We utilized dermatologic surgery faculty as coaches, but this format of training also would apply to general dermatology faculty.
Another goal of VBC is to enhance a trainee’s ability to perform self-directed learning, which requires accurate self-assessment.4 Accurately assessing one’s own strengths empowers a trainee to act with appropriate confidence, while understanding one’s own weaknesses allows a trainee to effectively balance confidence and caution in daily practice.5 Interestingly, in our study all residents scored themselves lower than surgical coaches, but with 1 coaching session, the residents subsequently reported greater surgical confidence.
Time constraints can be a potential barrier to surgical coaching.4 Our study demonstrates that VBC requires minimal time investment. Increasing the speed of video playback allowed for efficient evaluation of resident surgeries without compromising the coach’s ability to provide comprehensive feedback. Our feedback sessions were performed virtually, which allowed for ease of scheduling between trainees and coaches.
Our pilot study demonstrated that VBC is relatively easy to implement in a dermatology residency training setting, leveraging relatively low-cost technologies and allowing for a means of learning that residents felt was effective. Video-based coaching requires minimal time investment from both trainees and coaches and has the potential to enhance surgical confidence. Our current study is limited by its small sample size. Future studies should include follow-up recordings and assess the efficacy of VBC in enhancing surgical skills.
To the Editor:
Video-based coaching (VBC) involves a surgeon recording a surgery and then reviewing the video with a surgical coach; it is a form of education that is gaining popularity among surgical specialties.1 Video-based education is underutilized in dermatology residency training.2 We conducted a pilot study at our dermatology residency program to evaluate the efficacy and feasibility of VBC.
The University of Texas at Austin Dell Medical School institutional review board approved this study. All 4 first-year dermatology residents were recruited to participate in this study. Participants filled out a prestudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Participants used a head-mounted point-of-view camera to record themselves performing a wide local excision on the trunk or extremities of a live human patient. Participants then reviewed the recording on their own and scored themselves using the Objective Structured Assessment of Technical Skills (OSATS) scoring table (scored from 1 to 5, with 5 being the highest possible score for each element), which is a validated tool for assessing surgical skills (eTable 1).3 Given that there were no assistants participating in the surgery, this element of the OSATS scoring table was excluded, making a maximum possible score of 30 and a minimum possible score of 6. After scoring themselves, participants then had a 1-on-1 coaching session with a fellowship-trained dermatologic surgeon (M.F. or T.H.) via online teleconferencing.

During the coaching session, participants and coaches reviewed the video. The surgical coaches also scored the residents using the OSATS, then residents and coaches discussed how the resident could improve using the OSATS scores as a guide. The residents then completed a poststudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Descriptive statistics were reported.

On average, residents spent 31.3 minutes reviewing their own surgeries and scoring themselves. The average time for a coaching session, which included time spent scoring, was 13.8 minutes. Residents scored themselves lower than the surgical coaches did by an average of 5.25 points (eTable 2). Residents gave themselves an average total score of 10.5, while their respective surgical coaches gave the residents an average score of 15.75. There was a trend of residents with greater surgical experience having higher OSATS scores (Figure). After the coaching session, 3 of 4 residents reported that they felt more confident in their surgical skills. All residents felt more confident in assessing their surgical skills and felt that VBC was an effective teaching measure. All residents agreed that VBC should be continued as part of their residency training.

Video-based coaching has the potential to provide several benefits for dermatology trainees. Because receiving feedback intraoperatively often can be distracting and incomplete, video review can instead allow the surgeon to focus on performing the surgery and then later focus on learning while reviewing the video.1,4 Feedback also can be more comprehensive and delivered without concern for time constraints or disturbing clinic flow as well as without the additional concern of the patient overhearing comments and feedback.3 Although independent video review in the absence of coaching can lead to improvement in surgical skills, the addition of VBC provides even greater potential educational benefit.4 During the COVID-19 pandemic, VBC allowed coaches to provide feedback without additional exposures. We utilized dermatologic surgery faculty as coaches, but this format of training also would apply to general dermatology faculty.
Another goal of VBC is to enhance a trainee’s ability to perform self-directed learning, which requires accurate self-assessment.4 Accurately assessing one’s own strengths empowers a trainee to act with appropriate confidence, while understanding one’s own weaknesses allows a trainee to effectively balance confidence and caution in daily practice.5 Interestingly, in our study all residents scored themselves lower than surgical coaches, but with 1 coaching session, the residents subsequently reported greater surgical confidence.
Time constraints can be a potential barrier to surgical coaching.4 Our study demonstrates that VBC requires minimal time investment. Increasing the speed of video playback allowed for efficient evaluation of resident surgeries without compromising the coach’s ability to provide comprehensive feedback. Our feedback sessions were performed virtually, which allowed for ease of scheduling between trainees and coaches.
Our pilot study demonstrated that VBC is relatively easy to implement in a dermatology residency training setting, leveraging relatively low-cost technologies and allowing for a means of learning that residents felt was effective. Video-based coaching requires minimal time investment from both trainees and coaches and has the potential to enhance surgical confidence. Our current study is limited by its small sample size. Future studies should include follow-up recordings and assess the efficacy of VBC in enhancing surgical skills.
- Greenberg CC, Dombrowski J, Dimick JB. Video-based surgical coaching: an emerging approach to performance improvement. JAMA Surg. 2016;151:282-283.
- Dai J, Bordeaux JS, Miller CJ, et al. Assessing surgical training and deliberate practice methods in dermatology residency: a survey of dermatology program directors. Dermatol Surg. 2016;42:977-984.
- Chitgopeker P, Sidey K, Aronson A, et al. Surgical skills video-based assessment tool for dermatology residents: a prospective pilot study. J Am Acad Dermatol. 2020;83:614-616.
- Bull NB, Silverman CD, Bonrath EM. Targeted surgical coaching can improve operative self-assessment ability: a single-blinded nonrandomized trial. Surgery. 2020;167:308-313.
- Eva KW, Regehr G. Self-assessment in the health professions: a reformulation and research agenda. Acad Med. 2005;80(10 suppl):S46-S54.
- Greenberg CC, Dombrowski J, Dimick JB. Video-based surgical coaching: an emerging approach to performance improvement. JAMA Surg. 2016;151:282-283.
- Dai J, Bordeaux JS, Miller CJ, et al. Assessing surgical training and deliberate practice methods in dermatology residency: a survey of dermatology program directors. Dermatol Surg. 2016;42:977-984.
- Chitgopeker P, Sidey K, Aronson A, et al. Surgical skills video-based assessment tool for dermatology residents: a prospective pilot study. J Am Acad Dermatol. 2020;83:614-616.
- Bull NB, Silverman CD, Bonrath EM. Targeted surgical coaching can improve operative self-assessment ability: a single-blinded nonrandomized trial. Surgery. 2020;167:308-313.
- Eva KW, Regehr G. Self-assessment in the health professions: a reformulation and research agenda. Acad Med. 2005;80(10 suppl):S46-S54.
PRACTICE POINTS
- Video-based coaching (VBC) for surgical procedures is an up-and-coming form of medical education that allows a “coach” to provide thoughtful and in-depth feedback while reviewing a recording with the surgeon in a private setting. This format has potential utility in teaching dermatology resident surgeons being coached by a dermatology faculty member.
- We performed a pilot study demonstrating that VBC can be performed easily with a minimal time investment for both the surgeon and the coach. Dermatology residents not only felt that VBC was an effective teaching method but also should become a formal part of their education.
Perceived Benefits of a Research Fellowship for Dermatology Residency Applicants: Outcomes of a Faculty-Reported Survey
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.
None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).
Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
PRACTICE POINTS
- Many medical students seeking to match into a dermatology residency program complete a research fellowship (RF).
- Completion of an RF can give a competitive advantage to applicants even though most advisors acknowledge that these applicants are not likely to be involved in research throughout their career, perform better on standardized examinations, or provide better patient care.
- The decision to recommend an RF represents an extremely complex topic and should be tailored to each individual applicant.
Assessment of the Efficacy of Tranexamic Acid Solution 5% in the Treatment of Melasma in Patients of South Asian Descent
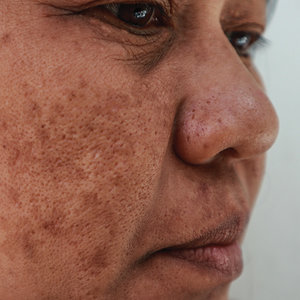
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

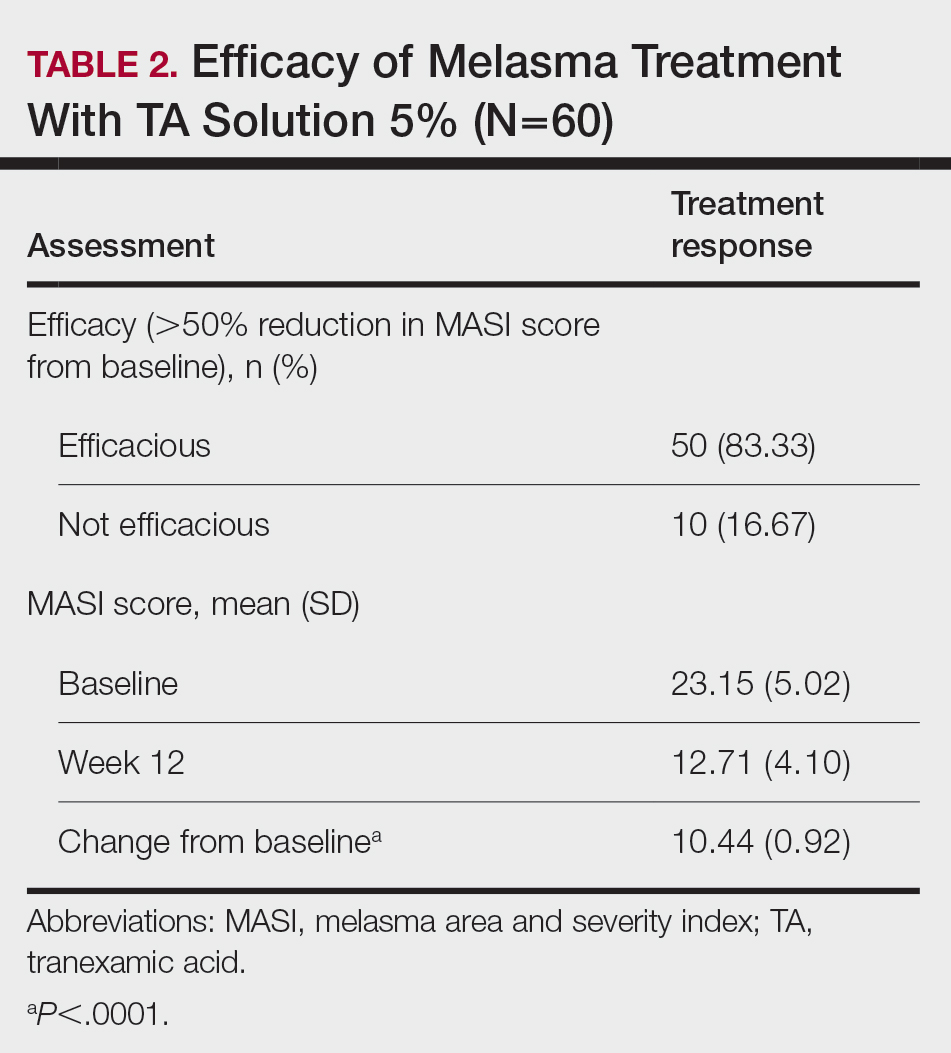
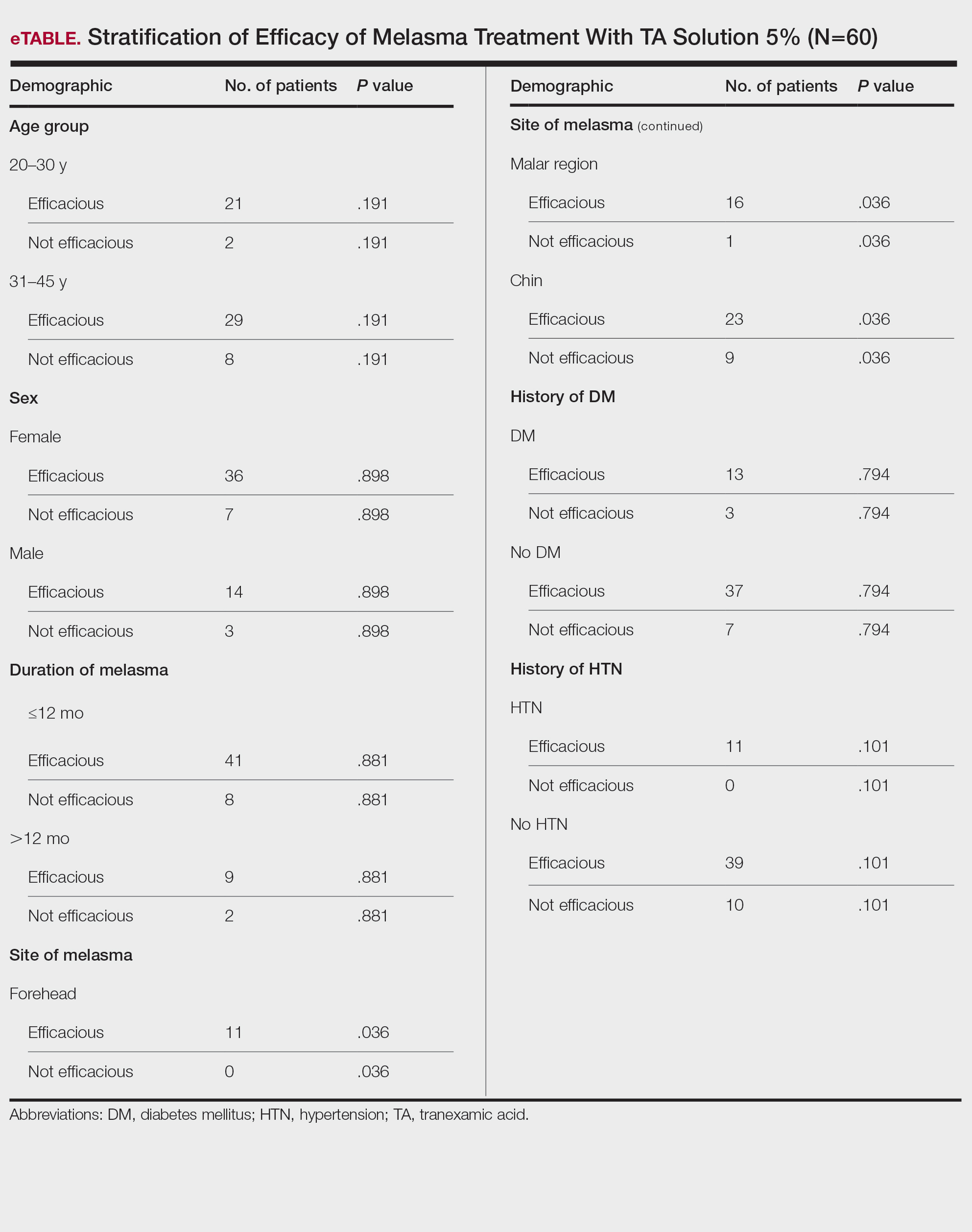
Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).
Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19
Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
PRATICE POINTS
- Tranexamic acid (TA) solution 5% is an efficacious treatment for skin of color patients with melasma.
- Topical TA is a treatment alternative for patients who may not be able to tolerate oral TA.
- Our study revealed the greatest efficacy for TA solution 5% was seen on the forehead and malar region, with less efficacy on the chin.
Endocrine Mucin-Producing Sweat Gland Carcinoma and Primary Cutaneous Mucinous Carcinoma: A Case Series
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and
Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.
Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.
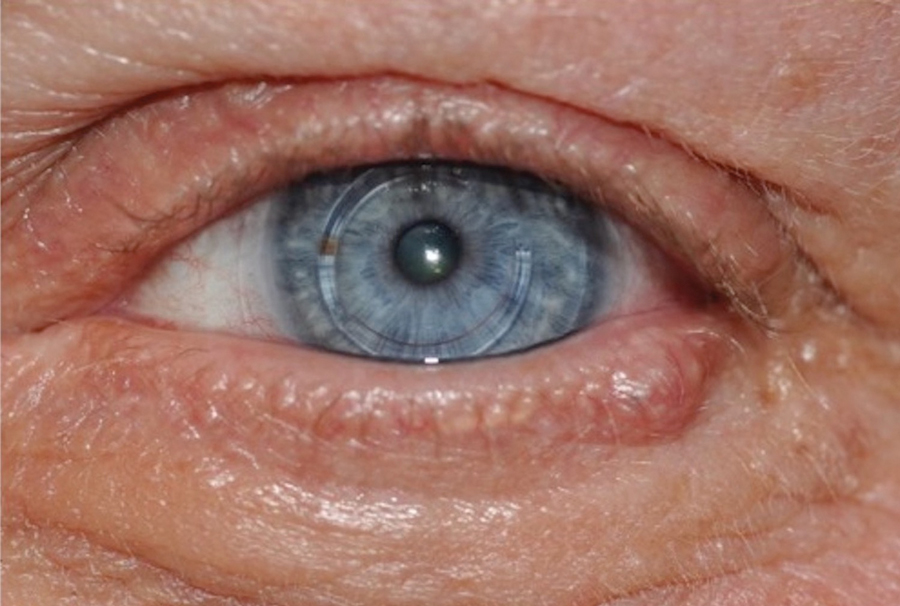
Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.
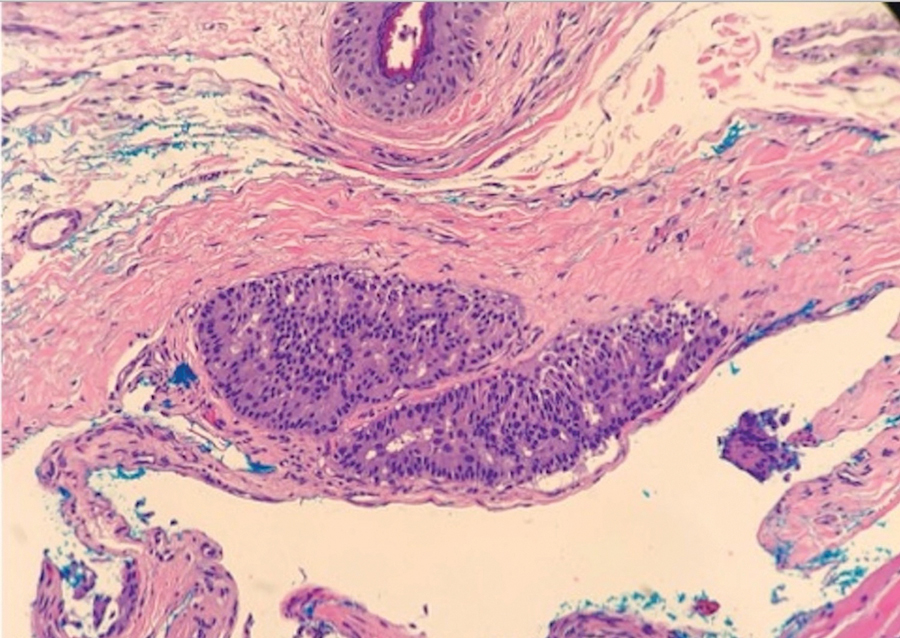
Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.
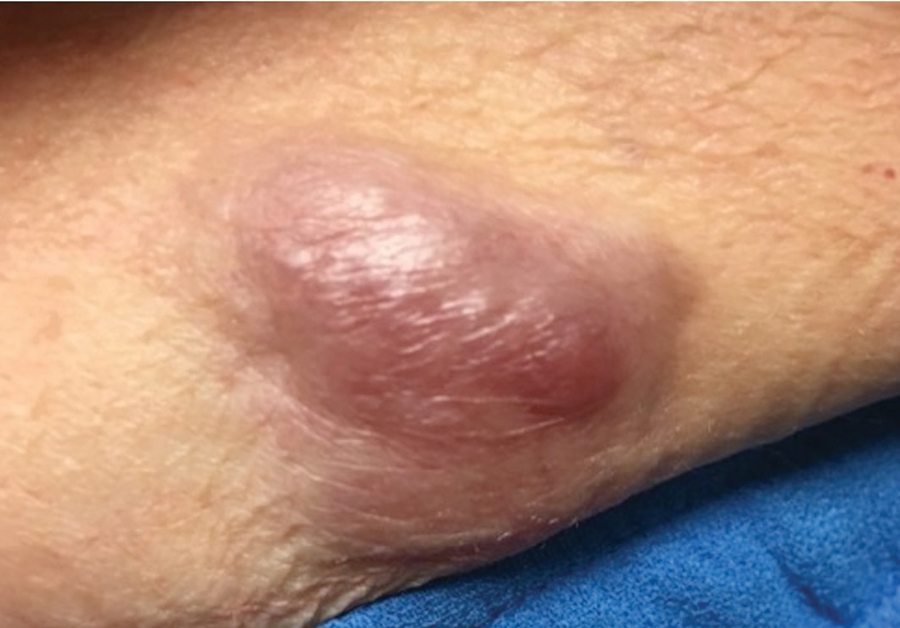
Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.
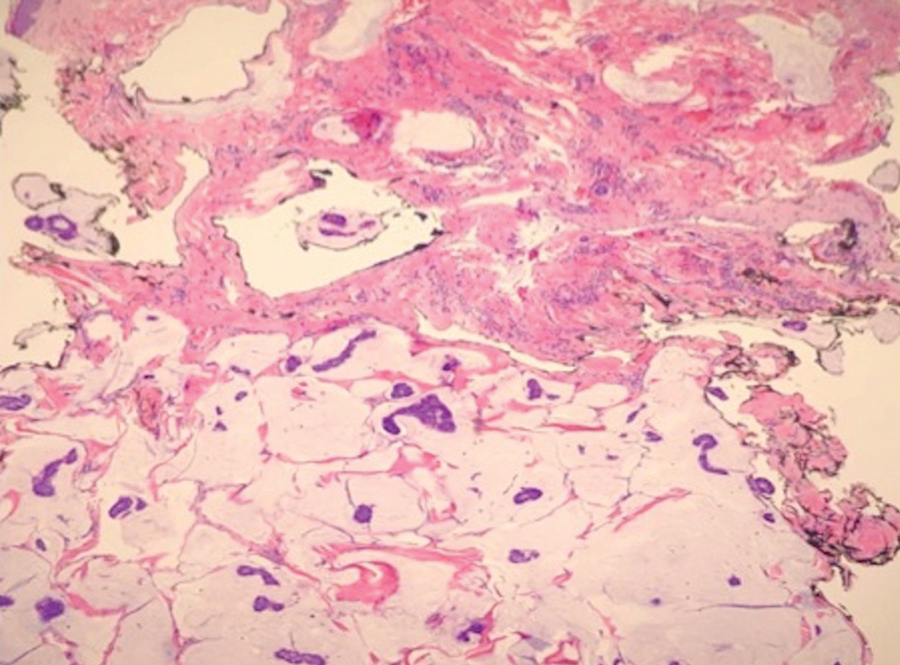
Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.
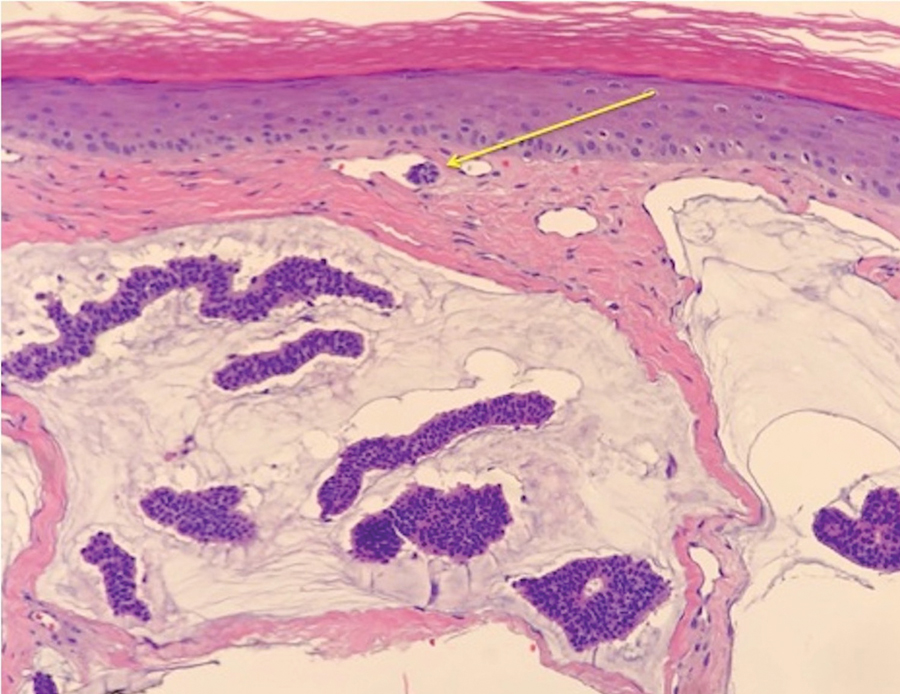
Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Practice Points
- Endocrine mucin-producing sweat gland carcinoma and primary cutaneous mucinous carcinoma are rare low-grade neoplasms thought to arise from apocrine glands that are morphologically and immunohistochemically analogous to ductal carcinoma in situ and mucinous carcinoma of the breast, respectively.
- Management involves a metastatic workup and either wide local excision with margins greater than 5 mm or Mohs micrographic surgery in anatomically sensitive areas.
Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
A worthwhile tool in evaluating worrisome lesions
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.
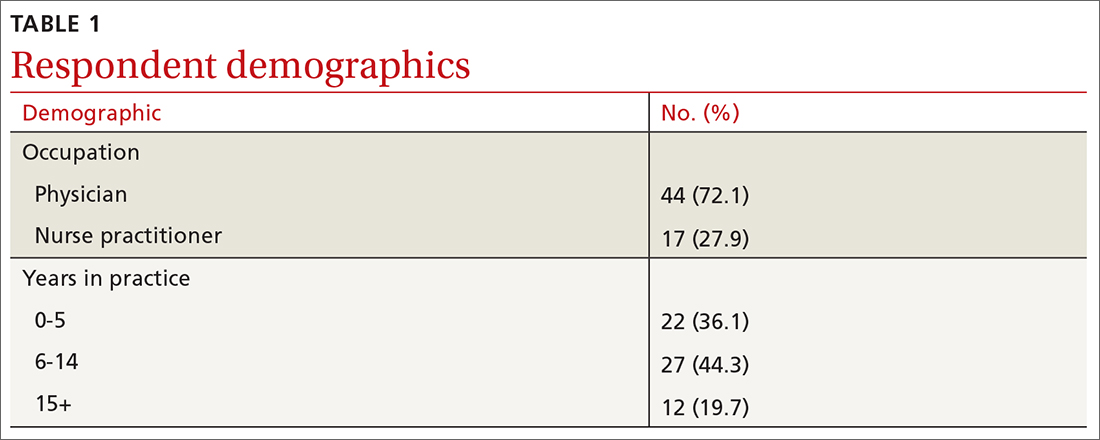
When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.
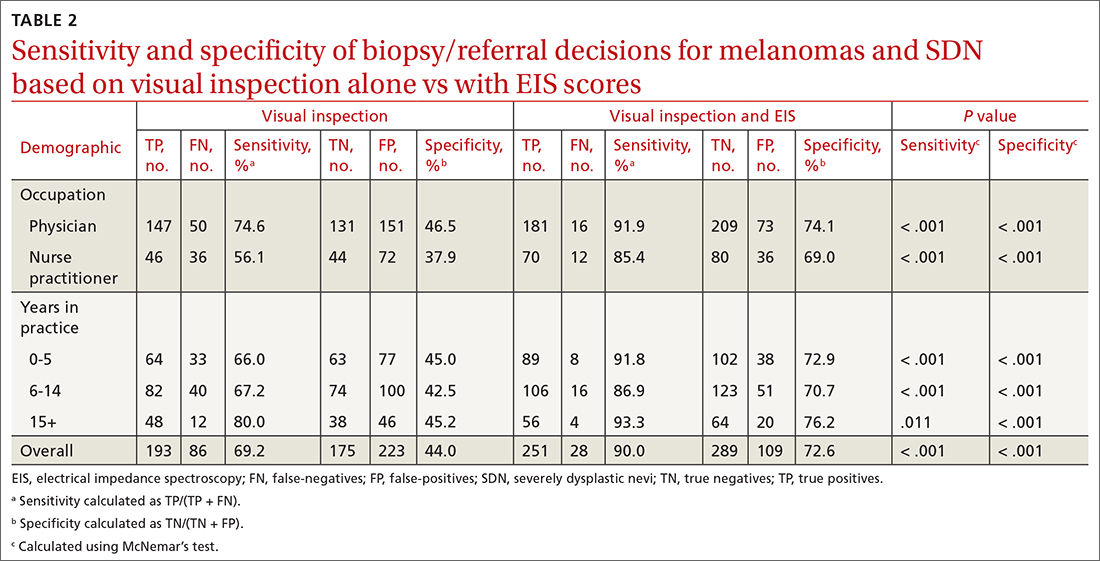
All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).
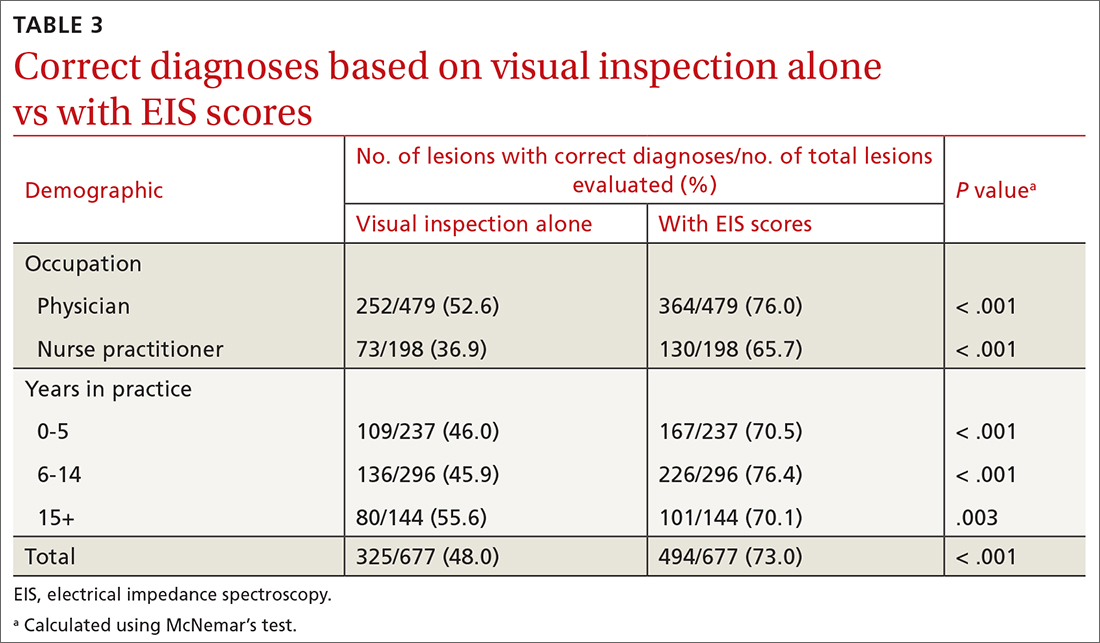
Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).
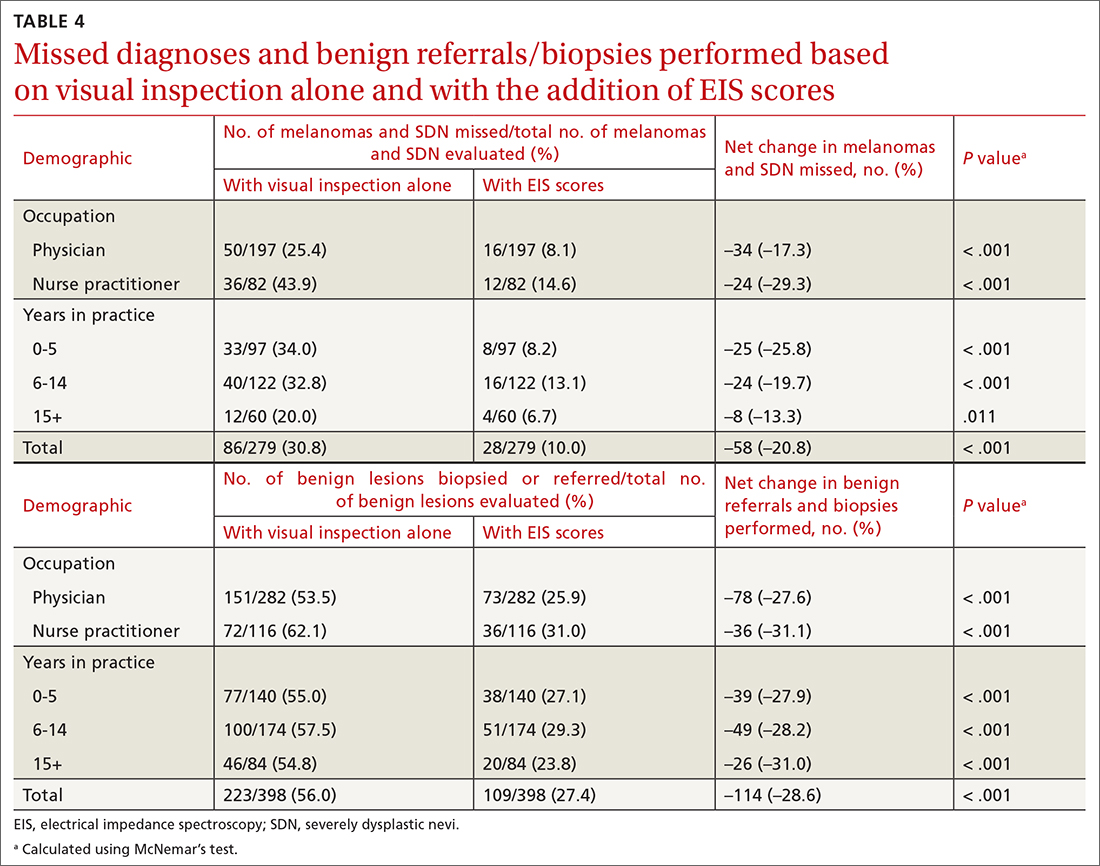
Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.

When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.

All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).

Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).

Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.

When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.

All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).

Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).

Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
Is the Altman Rule a proxy for glycemic load?
ABSTRACT
Background: The Altman Rule, a simple tool for consumers seeking to make healthier packaged food choices at the point of sale, applies to packaged carbohydrates. According to the Altman Rule, a food is a healthier option if it has at least 3 g of fiber per serving and the grams of fiber plus the grams of protein exceed the grams of sugar per serving. This study sought to evaluate whether the Altman Rule is a valid proxy for glycemic load (GL).
Methods: We compared the binary outcome of whether a food item meets the Altman Rule with the GL of all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research Database (University of Minnesota, Version 2010). We examined the percentage of foods in low-, medium-, and high-GL categories that met the Altman Rule.
Results: There were 1235 foods (342 cereals, 305 chips, 379 crackers, and 209 granola bars) in this analysis. There was a significant relationship between the GL of foods and the Altman Rule (P < .001) in that most low-GL (68%), almost half of medium-GL (48%), and very few high-GL (7%) foods met the criteria of the rule.
Conclusions: The Altman Rule is a reasonable proxy for GL and can be a useful and accessible tool for consumers interested in buying healthier packaged carbohydrate foods.
Nutrition can be complicated for consumers interested in making healthier choices at the grocery store. Consumers may have difficulty identifying more nutritious options, especially when food labels are adorned with claims such as “Good Source of Fiber” or “Heart Healthy.”1 In addition, when reading food labels, consumers may find it difficult to decipher which data to prioritize when carbohydrates, total sugars, added sugars, total dietary fiber, soluble fiber, and insoluble fiber are all listed.
The concept of glycemic load (GL) is an important consideration, especially for people with diabetes. GL approximates the blood sugar response to different foods. A food with a high GL is digested quickly, and its carbohydrates are taken into the bloodstream rapidly. This leads to a spike and subsequent drop in blood sugars, which can cause symptoms of hyperglycemia and hypoglycemia in a person with diabetes.2,3 Despite its usefulness, GL may be too complicated for a consumer to understand, and it does not appear anywhere on the food label. Since GL is calculated using pooled blood sugar response from individuals after the ingestion of the particular food, estimation of the GL is not intuitable.4
Point-of-sale tools. People seeking to lose weight, control diabetes, improve dyslipidemia and/or blood pressure, and/or decrease their risk for heart disease may benefit from point-of-sale tools such as the Altman Rule, which simplifies and encourages the selection of more nutritious foods.1 Other tools—such as Guiding Stars (https://guidingstars.com), NuVal (www.nuval.com), and different variations of traffic lights—have been created to help consumers make more informed and healthier food choices.5-8 However, Guiding Stars and NuVal are based on complicated algorithms that are not entirely transparent and not accessible to the average consumer.6,7 Evaluations of these nutrition tools indicate that consumers tend to underrate the healthiness of some foods, such as raw almonds and salmon, and overrate the healthiness of others, such as fruit punch and diet soda, when using traffic light systems.6 Furthermore, these nutrition tools are not available in many supermarkets. Previous research suggests that the use of point-of-sale nutrition apps decreases with the time and effort involved in using an app.9
Continue to: The Altman Rule
The Altman Rule was developed by a family physician (author WA) to provide a more accessible tool for people interested in choosing healthier prepackaged carbohydrate foods while shopping. Since the user does not need to have a smartphone, and they are not required to download or understand an app for each purchase, the Altman Rule may be more usable compared with more complicated alternatives.

The Altman Rule can be used with nutrition labels that feature serving information and calories in enlarged and bold type, in compliance with the most recent US Food and Drug Administration (FDA) guideline from 2016. Many foods with high fiber also have high amounts of sugar, so the criteria of the Altman Rule includes a 2-step process requiring (1) a minimum of 3 g of total dietary fiber per serving and (2) the sum of the grams of fiber plus the grams of protein per serving to be greater than the total grams of sugar (not grams of added sugar or grams of carbohydrate) per serving (FIGURE 1A). Unlike the relatively complicated formula related to GL, this 2-part rule can be applied in seconds while shopping (FIGURE 1B).
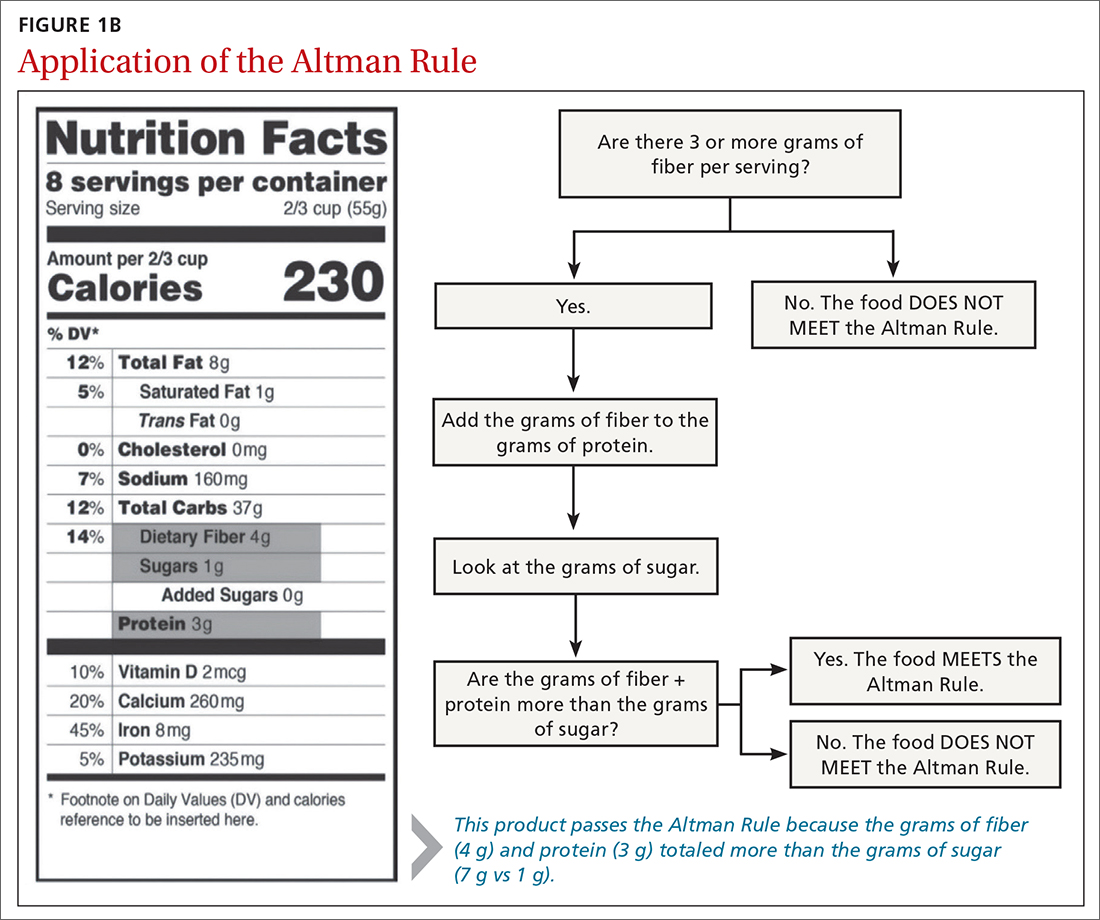
The rule is intended only to be used for packaged carbohydrate products, such as bread, muffins, bagels, pasta, rice, oatmeal, cereals, snack bars, chips, and crackers. It does not apply to whole foods, such as meat, dairy, fruits, or vegetables. These foods are excluded to prevent any consumer confusion related to the nutritional content of whole foods (eg, an apple may have more sugar than fiber and protein combined, but it is still a nutritious option).
This study aimed to determine if the Altman Rule is a reasonable proxy for the more complicated concept of GL. We calculated the relationship between the GL of commercially available packaged carbohydrate foods and whether those foods met the Altman Rule.
METHODS
The Altman Rule was tested by comparing the binary outcome of the rule (meets/does not meet) with data on all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research (NDSR) Database (University of Minnesota, Version 2010).
Continue to: To account for differences...
To account for differences in serving size, we used the standard of 50 g for each product as 1 serving. We used 50 g (about 1.7 oz) to help compare the different foods and between foods within the same group. Additionally, 50 g is close to 1 serving for most foods in these groups; it is about the size of a typical granola bar, three-quarters to 2 cups of cereal, 10 to 12 crackers, and 15 to 25 chips. We determined the GL for each product by multiplying the number of available carbohydrates (total carbohydrate – dietary fiber) by the product’s glycemic index/100. In general, GL is categorized as low (≤ 10), medium (11-19), or high (≥ 20).
We applied the Altman Rule to categorize each product as meeting or not meeting the rule. We compared the proportion of foods meeting the Altman Rule, stratified by GL and by specific foods, and used chi-square to determine if differences were statistically significant. These data were collected and analyzed in the summer of 2019.
RESULTS
There were 1235 foods (342 breakfast cereals, 305 chips, 379 crackers, and 209 granola bars) used for this analysis. There is a significant relationship between the GL of foods and the Altman Rule in that most low-GL (68%), almost half of medium-GL (48%), and only a few high-GL foods (7%) met the rule (P < .001) (TABLE 1). There was also a significant relationship between “meeting the Altman Rule” and GL within each food type (P < .001) (TABLE 2).
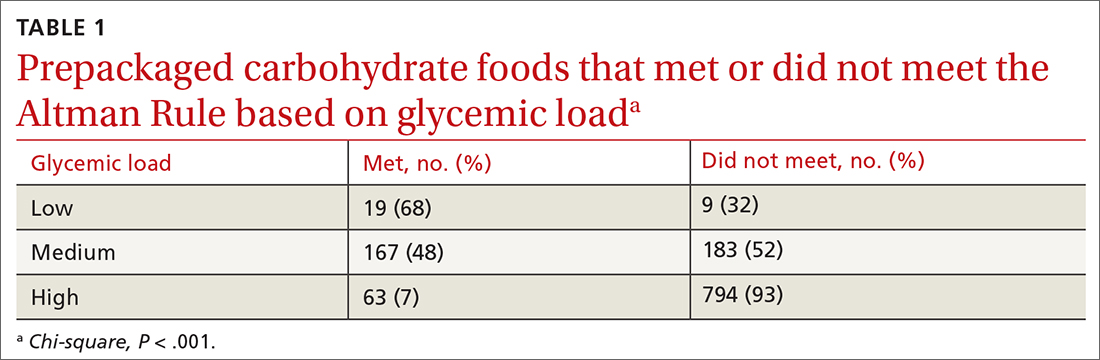
The medium-GL foods were the second largest category of foods we calculated; thus we further broke them into binary categories of
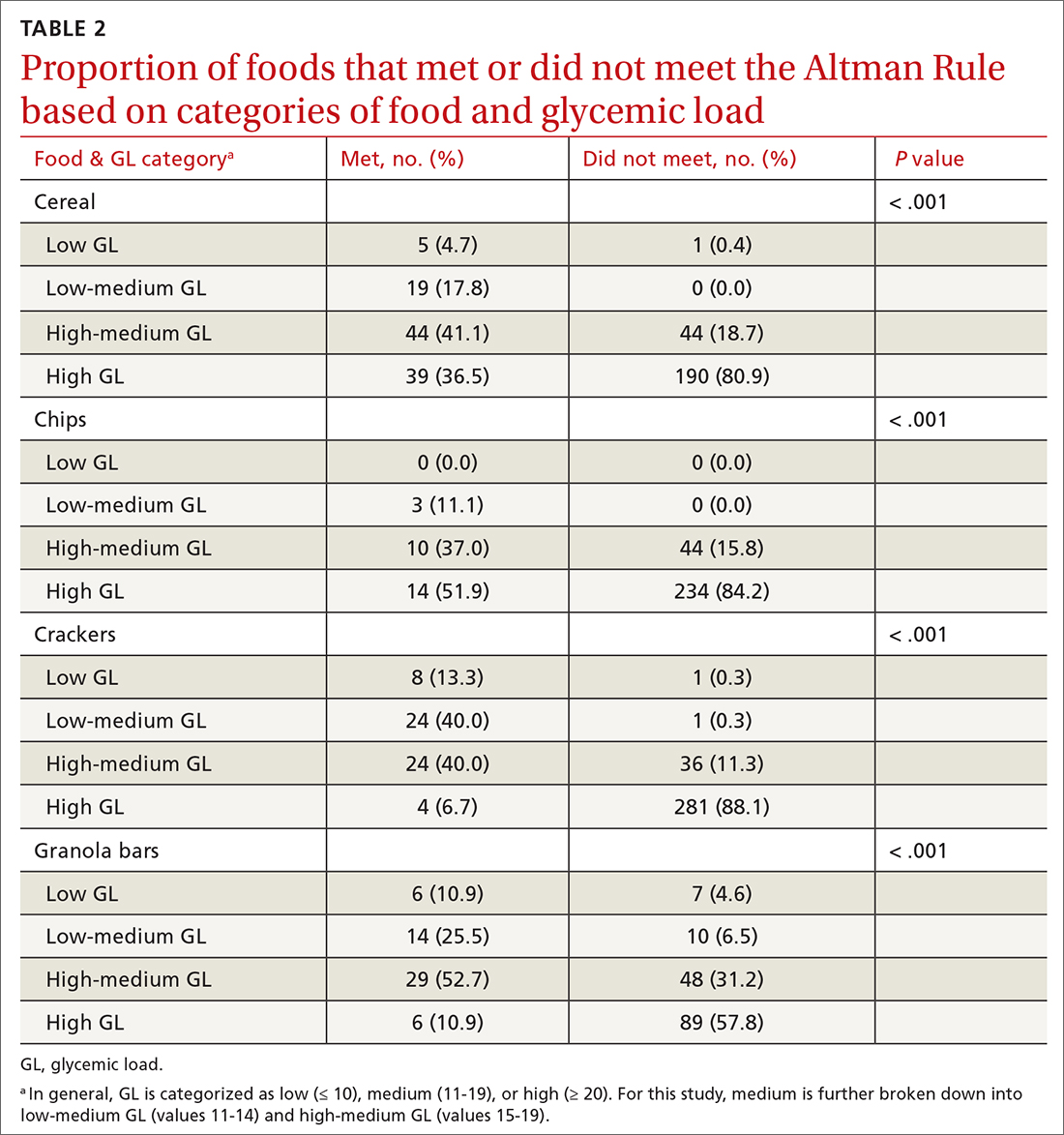
Foods that met the rule were more likely to be low GL and foods that did not pass the rule were more likely high GL. Within the medium-GL category, foods that met the rule were more likely to be low-medium GL.
Continue to: The findings within food categories...
The findings within food categories showed that very few cereals, chips, crackers, and granola bars were low GL. For every food category, except granola bars, far more low-GL foods met the Altman Rule than those that did not. At the same time, very few high-GL foods met the Altman Rule. The category with the most individual high-GL food items meeting the Altman Rule was cereal. This was also the subcategory with the largest percentage of high-GL food items meeting the Altman Rule. Thirty-nine cereals that were high GL met the rule, but more than 4 times as many high-GL cereals did not (n = 190).
DISCUSSION
Marketing and nutrition messaging create consumer confusion that makes it challenging to identify packaged food items that are more nutrient dense. The Altman Rule simplifies food choices that have become unnecessarily complex. Our findings suggest this 2-step rule is a reasonable proxy for the more complicated and less accessible GL for packaged carbohydrates, such as cereals, chips, crackers, and snack bars. Foods that meet the rule are likely low or low-medium GL and thus are foods that are likely to be healthier choices.
Of note, only 9% of chips (n = 27) passed the Altman Rule, likely due to their low dietary fiber content, which was typical of chips. If a food item does not have at least 3 grams of total dietary fiber per serving, it does not pass the Altman Rule, regardless of how much protein or sugar is in the product. This may be considered a strength or a weakness of the Altman Rule. Few nutrition-dense foods are low in fiber, but some foods could be nutritious but do not meet the Altman Rule due to having < 3 g of fiber.
With the high prevalence of chronic diseases such as hypertension, diabetes, hyperlipidemia, and cardiovascular disease, it is essential to help consumers prevent chronic disease altogether or manage their chronic disease by providing tools to identify healthier food choices. The tool also has a place in clinical medicine for use by physicians and other health care professionals. Research shows that physicians find both time and lack of knowledge/resources to be a barrier to providing nutritional counseling to patients.10 Since the Altman Rule can be shared and explained with very little time and without extensive nutritional knowledge, it meets these needs.
Limitations
Glycemic load. We acknowledge that the Altman Rule is not foolproof and that assessing this rule based on GL has some limitations. GL is not a perfect or comprehensive way to measure the nutritional value of a food. For example, fruits such as watermelon and grapes are nutritionally dense. However, they contain high amounts of natural sugars—and as such, their GL is relatively high, which could lead a consumer to perceive them as unhealthy. Nevertheless, GL is both a useful and accepted tool and a reasonable way to assess the validity of the rule, specifically when assessing packaged carbohydrates. The simplicity of the Altman Rule and its relationship with GL makes it such that consumers are more likely to make a healthier food choice using it.9
Continue to: Specificity and sensitivity
Specificity and sensitivity. There are other limitations to the Altman Rule, given that a small number of high-GL foods meet the rule. For example, some granola bars had high dietary protein, which offset a high sugar content just enough to pass the rule despite a higher GL. As such, concluding that a snack bar is a healthier choice because it meets the Altman Rule when it has high amounts of sugar may not be appropriate. This limitation could be considered a lack of specificity (the rule includes food it ought not to include). Another limitation to consider would be a lack of sensitivity, given that only 68% of low-GL foods passed the Altman Rule. Since GL is associated with carbohydrate content, foods with a low carbohydrate count often have little to no fiber and thus would fall into the category of foods that did not meet the Altman Rule but had low GL. In this case, however, the low amount of fiber may render the Altman Rule a better indicator of a healthier food choice than the GL.
Hidden sugars. Foods with sugar alcohols and artificial sweeteners may be as deleterious as caloric alternatives while not being accounted for when reporting the grams of sugar per serving on the nutrition label.7 This may represent an exception to the Altman Rule, as foods that are not healthier choices may pass the rule because the sugar content on the nutrition label is, in a sense, artificially lowered. Future research may investigate the hypothesis that these foods are nutritionally inferior despite meeting the Altman Rule.
The sample. Our study also was limited to working only with foods that were included in the NDSR database up to 2010. This limitation is mitigated by the fact that the sample size was large (> 1000 packaged food items were included in our analyses). The study also could be limited by the food categories that were analyzed; food categories such as bread, rice, pasta, and bagels were not included.
The objective of this research was to investigate the relationship between GL and the Altman Rule, rather than to conduct an exhaustive analysis of the Altman Rule for every possible food category. Studying the relationship between the Altman Rule and GL in other categories of food is an objective for future research. The data so far support a relationship between these entities. The likelihood of the nutrition facts of foods changing without the GL changing (or vice versa) is very low. As such, the Altman Rule still seems to be a reasonable proxy of GL.
CONCLUSIONS
Research indicates that point-of-sale tools, such as Guiding Stars, NuVal, and other stoplight tools, can successfully alter consumers’ behaviors.9 These tools can be helpful but are not available in many supermarkets. Despite the limitations, the Altman Rule is a useful decision aid that is accessible to all consumers no matter where they live or shop and is easy to use and remember.
The Altman rule can be used in clinical practice by health care professionals, such as physicians, nurse practitioners, physician assistants, dietitians, and health coaches. It also has the potential to be used in commercial settings, such as grocery stores, to help consumers easily identify healthier convenience foods. This has public health implications, as the rule can both empower consumers and potentially incentivize food manufacturers to upgrade their products nutritionally.
Additional research would be useful to evaluate consumers’ preferences and perceptions about how user-friendly the Altman Rule is at the point of sale with packaged carbohydrate foods. This would help to further understand how the use of information on food packaging can motivate healthier decisions—thereby helping to alleviate the burden of chronic disease.
CORRESPONDENCE
Kimberly R. Dong, DrPH, MS, RDN, Tufts University School of Medicine, Department of Public Health and Community Medicine, 136 Harrison Avenue, MV Building, Boston, MA 02111; [email protected]
1. Hersey JC, Wohlgenant KC, Arsenault JE, et al. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. 2013;71:1-14. doi: 10.1111/nure.12000
2. Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. doi: 10.1056/NEJMoa2007123
3. Brand-Miller J, Hayne S, Petocz P, et al. Low–glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261-2267. doi: 10.2337/diacare.26.8.2261
4. Matthan NR, Ausman LM, Meng H, et al. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004-1013. doi: 10.3945/ajcn.116.137208
5. Sonnenberg L, Gelsomin E, Levy DE, et al. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev Med. 2013;57:253-257. doi: 10.1016/j.ypmed.2013.07.001
6. Savoie N, Barlow K, Harvey KL, et al. Consumer perceptions of front-of-package labelling systems and healthiness of foods. Can J Public Health. 2013;104:e359-e363. doi: 10.17269/cjph.104.4027
7. Fischer LM, Sutherland LA, Kaley LA, et al. Development and implementation of the Guiding Stars nutrition guidance program. Am J Health Promot. 2011;26:e55-e63. doi: 10.4278/ajhp.100709-QUAL-238
8. Maubach N, Hoek J, Mather D. Interpretive front-of-pack nutrition labels. Comparing competing recommendations. Appetite. 2014;82:67-77. doi: 10.1016/j.appet.2014.07.006
9. Chan J, McMahon E, Brimblecombe J. Point‐of‐sale nutrition information interventions in food retail stores to promote healthier food purchase and intake: a systematic review. Obes Rev. 2021;22. doi: 10.1111/obr.13311
10. Mathioudakis N, Bashura H, Boyér L, et al. Development, implementation, and evaluation of a physician-targeted inpatient glycemic management curriculum. J Med Educ Curric Dev. 2019;6:238212051986134. doi: 10.1177/2382120519861342
ABSTRACT
Background: The Altman Rule, a simple tool for consumers seeking to make healthier packaged food choices at the point of sale, applies to packaged carbohydrates. According to the Altman Rule, a food is a healthier option if it has at least 3 g of fiber per serving and the grams of fiber plus the grams of protein exceed the grams of sugar per serving. This study sought to evaluate whether the Altman Rule is a valid proxy for glycemic load (GL).
Methods: We compared the binary outcome of whether a food item meets the Altman Rule with the GL of all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research Database (University of Minnesota, Version 2010). We examined the percentage of foods in low-, medium-, and high-GL categories that met the Altman Rule.
Results: There were 1235 foods (342 cereals, 305 chips, 379 crackers, and 209 granola bars) in this analysis. There was a significant relationship between the GL of foods and the Altman Rule (P < .001) in that most low-GL (68%), almost half of medium-GL (48%), and very few high-GL (7%) foods met the criteria of the rule.
Conclusions: The Altman Rule is a reasonable proxy for GL and can be a useful and accessible tool for consumers interested in buying healthier packaged carbohydrate foods.
Nutrition can be complicated for consumers interested in making healthier choices at the grocery store. Consumers may have difficulty identifying more nutritious options, especially when food labels are adorned with claims such as “Good Source of Fiber” or “Heart Healthy.”1 In addition, when reading food labels, consumers may find it difficult to decipher which data to prioritize when carbohydrates, total sugars, added sugars, total dietary fiber, soluble fiber, and insoluble fiber are all listed.
The concept of glycemic load (GL) is an important consideration, especially for people with diabetes. GL approximates the blood sugar response to different foods. A food with a high GL is digested quickly, and its carbohydrates are taken into the bloodstream rapidly. This leads to a spike and subsequent drop in blood sugars, which can cause symptoms of hyperglycemia and hypoglycemia in a person with diabetes.2,3 Despite its usefulness, GL may be too complicated for a consumer to understand, and it does not appear anywhere on the food label. Since GL is calculated using pooled blood sugar response from individuals after the ingestion of the particular food, estimation of the GL is not intuitable.4
Point-of-sale tools. People seeking to lose weight, control diabetes, improve dyslipidemia and/or blood pressure, and/or decrease their risk for heart disease may benefit from point-of-sale tools such as the Altman Rule, which simplifies and encourages the selection of more nutritious foods.1 Other tools—such as Guiding Stars (https://guidingstars.com), NuVal (www.nuval.com), and different variations of traffic lights—have been created to help consumers make more informed and healthier food choices.5-8 However, Guiding Stars and NuVal are based on complicated algorithms that are not entirely transparent and not accessible to the average consumer.6,7 Evaluations of these nutrition tools indicate that consumers tend to underrate the healthiness of some foods, such as raw almonds and salmon, and overrate the healthiness of others, such as fruit punch and diet soda, when using traffic light systems.6 Furthermore, these nutrition tools are not available in many supermarkets. Previous research suggests that the use of point-of-sale nutrition apps decreases with the time and effort involved in using an app.9
Continue to: The Altman Rule
The Altman Rule was developed by a family physician (author WA) to provide a more accessible tool for people interested in choosing healthier prepackaged carbohydrate foods while shopping. Since the user does not need to have a smartphone, and they are not required to download or understand an app for each purchase, the Altman Rule may be more usable compared with more complicated alternatives.

The Altman Rule can be used with nutrition labels that feature serving information and calories in enlarged and bold type, in compliance with the most recent US Food and Drug Administration (FDA) guideline from 2016. Many foods with high fiber also have high amounts of sugar, so the criteria of the Altman Rule includes a 2-step process requiring (1) a minimum of 3 g of total dietary fiber per serving and (2) the sum of the grams of fiber plus the grams of protein per serving to be greater than the total grams of sugar (not grams of added sugar or grams of carbohydrate) per serving (FIGURE 1A). Unlike the relatively complicated formula related to GL, this 2-part rule can be applied in seconds while shopping (FIGURE 1B).

The rule is intended only to be used for packaged carbohydrate products, such as bread, muffins, bagels, pasta, rice, oatmeal, cereals, snack bars, chips, and crackers. It does not apply to whole foods, such as meat, dairy, fruits, or vegetables. These foods are excluded to prevent any consumer confusion related to the nutritional content of whole foods (eg, an apple may have more sugar than fiber and protein combined, but it is still a nutritious option).
This study aimed to determine if the Altman Rule is a reasonable proxy for the more complicated concept of GL. We calculated the relationship between the GL of commercially available packaged carbohydrate foods and whether those foods met the Altman Rule.
METHODS
The Altman Rule was tested by comparing the binary outcome of the rule (meets/does not meet) with data on all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research (NDSR) Database (University of Minnesota, Version 2010).
Continue to: To account for differences...
To account for differences in serving size, we used the standard of 50 g for each product as 1 serving. We used 50 g (about 1.7 oz) to help compare the different foods and between foods within the same group. Additionally, 50 g is close to 1 serving for most foods in these groups; it is about the size of a typical granola bar, three-quarters to 2 cups of cereal, 10 to 12 crackers, and 15 to 25 chips. We determined the GL for each product by multiplying the number of available carbohydrates (total carbohydrate – dietary fiber) by the product’s glycemic index/100. In general, GL is categorized as low (≤ 10), medium (11-19), or high (≥ 20).
We applied the Altman Rule to categorize each product as meeting or not meeting the rule. We compared the proportion of foods meeting the Altman Rule, stratified by GL and by specific foods, and used chi-square to determine if differences were statistically significant. These data were collected and analyzed in the summer of 2019.
RESULTS
There were 1235 foods (342 breakfast cereals, 305 chips, 379 crackers, and 209 granola bars) used for this analysis. There is a significant relationship between the GL of foods and the Altman Rule in that most low-GL (68%), almost half of medium-GL (48%), and only a few high-GL foods (7%) met the rule (P < .001) (TABLE 1). There was also a significant relationship between “meeting the Altman Rule” and GL within each food type (P < .001) (TABLE 2).

The medium-GL foods were the second largest category of foods we calculated; thus we further broke them into binary categories of

Foods that met the rule were more likely to be low GL and foods that did not pass the rule were more likely high GL. Within the medium-GL category, foods that met the rule were more likely to be low-medium GL.
Continue to: The findings within food categories...
The findings within food categories showed that very few cereals, chips, crackers, and granola bars were low GL. For every food category, except granola bars, far more low-GL foods met the Altman Rule than those that did not. At the same time, very few high-GL foods met the Altman Rule. The category with the most individual high-GL food items meeting the Altman Rule was cereal. This was also the subcategory with the largest percentage of high-GL food items meeting the Altman Rule. Thirty-nine cereals that were high GL met the rule, but more than 4 times as many high-GL cereals did not (n = 190).
DISCUSSION
Marketing and nutrition messaging create consumer confusion that makes it challenging to identify packaged food items that are more nutrient dense. The Altman Rule simplifies food choices that have become unnecessarily complex. Our findings suggest this 2-step rule is a reasonable proxy for the more complicated and less accessible GL for packaged carbohydrates, such as cereals, chips, crackers, and snack bars. Foods that meet the rule are likely low or low-medium GL and thus are foods that are likely to be healthier choices.
Of note, only 9% of chips (n = 27) passed the Altman Rule, likely due to their low dietary fiber content, which was typical of chips. If a food item does not have at least 3 grams of total dietary fiber per serving, it does not pass the Altman Rule, regardless of how much protein or sugar is in the product. This may be considered a strength or a weakness of the Altman Rule. Few nutrition-dense foods are low in fiber, but some foods could be nutritious but do not meet the Altman Rule due to having < 3 g of fiber.
With the high prevalence of chronic diseases such as hypertension, diabetes, hyperlipidemia, and cardiovascular disease, it is essential to help consumers prevent chronic disease altogether or manage their chronic disease by providing tools to identify healthier food choices. The tool also has a place in clinical medicine for use by physicians and other health care professionals. Research shows that physicians find both time and lack of knowledge/resources to be a barrier to providing nutritional counseling to patients.10 Since the Altman Rule can be shared and explained with very little time and without extensive nutritional knowledge, it meets these needs.
Limitations
Glycemic load. We acknowledge that the Altman Rule is not foolproof and that assessing this rule based on GL has some limitations. GL is not a perfect or comprehensive way to measure the nutritional value of a food. For example, fruits such as watermelon and grapes are nutritionally dense. However, they contain high amounts of natural sugars—and as such, their GL is relatively high, which could lead a consumer to perceive them as unhealthy. Nevertheless, GL is both a useful and accepted tool and a reasonable way to assess the validity of the rule, specifically when assessing packaged carbohydrates. The simplicity of the Altman Rule and its relationship with GL makes it such that consumers are more likely to make a healthier food choice using it.9
Continue to: Specificity and sensitivity
Specificity and sensitivity. There are other limitations to the Altman Rule, given that a small number of high-GL foods meet the rule. For example, some granola bars had high dietary protein, which offset a high sugar content just enough to pass the rule despite a higher GL. As such, concluding that a snack bar is a healthier choice because it meets the Altman Rule when it has high amounts of sugar may not be appropriate. This limitation could be considered a lack of specificity (the rule includes food it ought not to include). Another limitation to consider would be a lack of sensitivity, given that only 68% of low-GL foods passed the Altman Rule. Since GL is associated with carbohydrate content, foods with a low carbohydrate count often have little to no fiber and thus would fall into the category of foods that did not meet the Altman Rule but had low GL. In this case, however, the low amount of fiber may render the Altman Rule a better indicator of a healthier food choice than the GL.
Hidden sugars. Foods with sugar alcohols and artificial sweeteners may be as deleterious as caloric alternatives while not being accounted for when reporting the grams of sugar per serving on the nutrition label.7 This may represent an exception to the Altman Rule, as foods that are not healthier choices may pass the rule because the sugar content on the nutrition label is, in a sense, artificially lowered. Future research may investigate the hypothesis that these foods are nutritionally inferior despite meeting the Altman Rule.
The sample. Our study also was limited to working only with foods that were included in the NDSR database up to 2010. This limitation is mitigated by the fact that the sample size was large (> 1000 packaged food items were included in our analyses). The study also could be limited by the food categories that were analyzed; food categories such as bread, rice, pasta, and bagels were not included.
The objective of this research was to investigate the relationship between GL and the Altman Rule, rather than to conduct an exhaustive analysis of the Altman Rule for every possible food category. Studying the relationship between the Altman Rule and GL in other categories of food is an objective for future research. The data so far support a relationship between these entities. The likelihood of the nutrition facts of foods changing without the GL changing (or vice versa) is very low. As such, the Altman Rule still seems to be a reasonable proxy of GL.
CONCLUSIONS
Research indicates that point-of-sale tools, such as Guiding Stars, NuVal, and other stoplight tools, can successfully alter consumers’ behaviors.9 These tools can be helpful but are not available in many supermarkets. Despite the limitations, the Altman Rule is a useful decision aid that is accessible to all consumers no matter where they live or shop and is easy to use and remember.
The Altman rule can be used in clinical practice by health care professionals, such as physicians, nurse practitioners, physician assistants, dietitians, and health coaches. It also has the potential to be used in commercial settings, such as grocery stores, to help consumers easily identify healthier convenience foods. This has public health implications, as the rule can both empower consumers and potentially incentivize food manufacturers to upgrade their products nutritionally.
Additional research would be useful to evaluate consumers’ preferences and perceptions about how user-friendly the Altman Rule is at the point of sale with packaged carbohydrate foods. This would help to further understand how the use of information on food packaging can motivate healthier decisions—thereby helping to alleviate the burden of chronic disease.
CORRESPONDENCE
Kimberly R. Dong, DrPH, MS, RDN, Tufts University School of Medicine, Department of Public Health and Community Medicine, 136 Harrison Avenue, MV Building, Boston, MA 02111; [email protected]
ABSTRACT
Background: The Altman Rule, a simple tool for consumers seeking to make healthier packaged food choices at the point of sale, applies to packaged carbohydrates. According to the Altman Rule, a food is a healthier option if it has at least 3 g of fiber per serving and the grams of fiber plus the grams of protein exceed the grams of sugar per serving. This study sought to evaluate whether the Altman Rule is a valid proxy for glycemic load (GL).
Methods: We compared the binary outcome of whether a food item meets the Altman Rule with the GL of all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research Database (University of Minnesota, Version 2010). We examined the percentage of foods in low-, medium-, and high-GL categories that met the Altman Rule.
Results: There were 1235 foods (342 cereals, 305 chips, 379 crackers, and 209 granola bars) in this analysis. There was a significant relationship between the GL of foods and the Altman Rule (P < .001) in that most low-GL (68%), almost half of medium-GL (48%), and very few high-GL (7%) foods met the criteria of the rule.
Conclusions: The Altman Rule is a reasonable proxy for GL and can be a useful and accessible tool for consumers interested in buying healthier packaged carbohydrate foods.
Nutrition can be complicated for consumers interested in making healthier choices at the grocery store. Consumers may have difficulty identifying more nutritious options, especially when food labels are adorned with claims such as “Good Source of Fiber” or “Heart Healthy.”1 In addition, when reading food labels, consumers may find it difficult to decipher which data to prioritize when carbohydrates, total sugars, added sugars, total dietary fiber, soluble fiber, and insoluble fiber are all listed.
The concept of glycemic load (GL) is an important consideration, especially for people with diabetes. GL approximates the blood sugar response to different foods. A food with a high GL is digested quickly, and its carbohydrates are taken into the bloodstream rapidly. This leads to a spike and subsequent drop in blood sugars, which can cause symptoms of hyperglycemia and hypoglycemia in a person with diabetes.2,3 Despite its usefulness, GL may be too complicated for a consumer to understand, and it does not appear anywhere on the food label. Since GL is calculated using pooled blood sugar response from individuals after the ingestion of the particular food, estimation of the GL is not intuitable.4
Point-of-sale tools. People seeking to lose weight, control diabetes, improve dyslipidemia and/or blood pressure, and/or decrease their risk for heart disease may benefit from point-of-sale tools such as the Altman Rule, which simplifies and encourages the selection of more nutritious foods.1 Other tools—such as Guiding Stars (https://guidingstars.com), NuVal (www.nuval.com), and different variations of traffic lights—have been created to help consumers make more informed and healthier food choices.5-8 However, Guiding Stars and NuVal are based on complicated algorithms that are not entirely transparent and not accessible to the average consumer.6,7 Evaluations of these nutrition tools indicate that consumers tend to underrate the healthiness of some foods, such as raw almonds and salmon, and overrate the healthiness of others, such as fruit punch and diet soda, when using traffic light systems.6 Furthermore, these nutrition tools are not available in many supermarkets. Previous research suggests that the use of point-of-sale nutrition apps decreases with the time and effort involved in using an app.9
Continue to: The Altman Rule
The Altman Rule was developed by a family physician (author WA) to provide a more accessible tool for people interested in choosing healthier prepackaged carbohydrate foods while shopping. Since the user does not need to have a smartphone, and they are not required to download or understand an app for each purchase, the Altman Rule may be more usable compared with more complicated alternatives.

The Altman Rule can be used with nutrition labels that feature serving information and calories in enlarged and bold type, in compliance with the most recent US Food and Drug Administration (FDA) guideline from 2016. Many foods with high fiber also have high amounts of sugar, so the criteria of the Altman Rule includes a 2-step process requiring (1) a minimum of 3 g of total dietary fiber per serving and (2) the sum of the grams of fiber plus the grams of protein per serving to be greater than the total grams of sugar (not grams of added sugar or grams of carbohydrate) per serving (FIGURE 1A). Unlike the relatively complicated formula related to GL, this 2-part rule can be applied in seconds while shopping (FIGURE 1B).

The rule is intended only to be used for packaged carbohydrate products, such as bread, muffins, bagels, pasta, rice, oatmeal, cereals, snack bars, chips, and crackers. It does not apply to whole foods, such as meat, dairy, fruits, or vegetables. These foods are excluded to prevent any consumer confusion related to the nutritional content of whole foods (eg, an apple may have more sugar than fiber and protein combined, but it is still a nutritious option).
This study aimed to determine if the Altman Rule is a reasonable proxy for the more complicated concept of GL. We calculated the relationship between the GL of commercially available packaged carbohydrate foods and whether those foods met the Altman Rule.
METHODS
The Altman Rule was tested by comparing the binary outcome of the rule (meets/does not meet) with data on all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research (NDSR) Database (University of Minnesota, Version 2010).
Continue to: To account for differences...
To account for differences in serving size, we used the standard of 50 g for each product as 1 serving. We used 50 g (about 1.7 oz) to help compare the different foods and between foods within the same group. Additionally, 50 g is close to 1 serving for most foods in these groups; it is about the size of a typical granola bar, three-quarters to 2 cups of cereal, 10 to 12 crackers, and 15 to 25 chips. We determined the GL for each product by multiplying the number of available carbohydrates (total carbohydrate – dietary fiber) by the product’s glycemic index/100. In general, GL is categorized as low (≤ 10), medium (11-19), or high (≥ 20).
We applied the Altman Rule to categorize each product as meeting or not meeting the rule. We compared the proportion of foods meeting the Altman Rule, stratified by GL and by specific foods, and used chi-square to determine if differences were statistically significant. These data were collected and analyzed in the summer of 2019.
RESULTS
There were 1235 foods (342 breakfast cereals, 305 chips, 379 crackers, and 209 granola bars) used for this analysis. There is a significant relationship between the GL of foods and the Altman Rule in that most low-GL (68%), almost half of medium-GL (48%), and only a few high-GL foods (7%) met the rule (P < .001) (TABLE 1). There was also a significant relationship between “meeting the Altman Rule” and GL within each food type (P < .001) (TABLE 2).

The medium-GL foods were the second largest category of foods we calculated; thus we further broke them into binary categories of

Foods that met the rule were more likely to be low GL and foods that did not pass the rule were more likely high GL. Within the medium-GL category, foods that met the rule were more likely to be low-medium GL.
Continue to: The findings within food categories...
The findings within food categories showed that very few cereals, chips, crackers, and granola bars were low GL. For every food category, except granola bars, far more low-GL foods met the Altman Rule than those that did not. At the same time, very few high-GL foods met the Altman Rule. The category with the most individual high-GL food items meeting the Altman Rule was cereal. This was also the subcategory with the largest percentage of high-GL food items meeting the Altman Rule. Thirty-nine cereals that were high GL met the rule, but more than 4 times as many high-GL cereals did not (n = 190).
DISCUSSION
Marketing and nutrition messaging create consumer confusion that makes it challenging to identify packaged food items that are more nutrient dense. The Altman Rule simplifies food choices that have become unnecessarily complex. Our findings suggest this 2-step rule is a reasonable proxy for the more complicated and less accessible GL for packaged carbohydrates, such as cereals, chips, crackers, and snack bars. Foods that meet the rule are likely low or low-medium GL and thus are foods that are likely to be healthier choices.
Of note, only 9% of chips (n = 27) passed the Altman Rule, likely due to their low dietary fiber content, which was typical of chips. If a food item does not have at least 3 grams of total dietary fiber per serving, it does not pass the Altman Rule, regardless of how much protein or sugar is in the product. This may be considered a strength or a weakness of the Altman Rule. Few nutrition-dense foods are low in fiber, but some foods could be nutritious but do not meet the Altman Rule due to having < 3 g of fiber.
With the high prevalence of chronic diseases such as hypertension, diabetes, hyperlipidemia, and cardiovascular disease, it is essential to help consumers prevent chronic disease altogether or manage their chronic disease by providing tools to identify healthier food choices. The tool also has a place in clinical medicine for use by physicians and other health care professionals. Research shows that physicians find both time and lack of knowledge/resources to be a barrier to providing nutritional counseling to patients.10 Since the Altman Rule can be shared and explained with very little time and without extensive nutritional knowledge, it meets these needs.
Limitations
Glycemic load. We acknowledge that the Altman Rule is not foolproof and that assessing this rule based on GL has some limitations. GL is not a perfect or comprehensive way to measure the nutritional value of a food. For example, fruits such as watermelon and grapes are nutritionally dense. However, they contain high amounts of natural sugars—and as such, their GL is relatively high, which could lead a consumer to perceive them as unhealthy. Nevertheless, GL is both a useful and accepted tool and a reasonable way to assess the validity of the rule, specifically when assessing packaged carbohydrates. The simplicity of the Altman Rule and its relationship with GL makes it such that consumers are more likely to make a healthier food choice using it.9
Continue to: Specificity and sensitivity
Specificity and sensitivity. There are other limitations to the Altman Rule, given that a small number of high-GL foods meet the rule. For example, some granola bars had high dietary protein, which offset a high sugar content just enough to pass the rule despite a higher GL. As such, concluding that a snack bar is a healthier choice because it meets the Altman Rule when it has high amounts of sugar may not be appropriate. This limitation could be considered a lack of specificity (the rule includes food it ought not to include). Another limitation to consider would be a lack of sensitivity, given that only 68% of low-GL foods passed the Altman Rule. Since GL is associated with carbohydrate content, foods with a low carbohydrate count often have little to no fiber and thus would fall into the category of foods that did not meet the Altman Rule but had low GL. In this case, however, the low amount of fiber may render the Altman Rule a better indicator of a healthier food choice than the GL.
Hidden sugars. Foods with sugar alcohols and artificial sweeteners may be as deleterious as caloric alternatives while not being accounted for when reporting the grams of sugar per serving on the nutrition label.7 This may represent an exception to the Altman Rule, as foods that are not healthier choices may pass the rule because the sugar content on the nutrition label is, in a sense, artificially lowered. Future research may investigate the hypothesis that these foods are nutritionally inferior despite meeting the Altman Rule.
The sample. Our study also was limited to working only with foods that were included in the NDSR database up to 2010. This limitation is mitigated by the fact that the sample size was large (> 1000 packaged food items were included in our analyses). The study also could be limited by the food categories that were analyzed; food categories such as bread, rice, pasta, and bagels were not included.
The objective of this research was to investigate the relationship between GL and the Altman Rule, rather than to conduct an exhaustive analysis of the Altman Rule for every possible food category. Studying the relationship between the Altman Rule and GL in other categories of food is an objective for future research. The data so far support a relationship between these entities. The likelihood of the nutrition facts of foods changing without the GL changing (or vice versa) is very low. As such, the Altman Rule still seems to be a reasonable proxy of GL.
CONCLUSIONS
Research indicates that point-of-sale tools, such as Guiding Stars, NuVal, and other stoplight tools, can successfully alter consumers’ behaviors.9 These tools can be helpful but are not available in many supermarkets. Despite the limitations, the Altman Rule is a useful decision aid that is accessible to all consumers no matter where they live or shop and is easy to use and remember.
The Altman rule can be used in clinical practice by health care professionals, such as physicians, nurse practitioners, physician assistants, dietitians, and health coaches. It also has the potential to be used in commercial settings, such as grocery stores, to help consumers easily identify healthier convenience foods. This has public health implications, as the rule can both empower consumers and potentially incentivize food manufacturers to upgrade their products nutritionally.
Additional research would be useful to evaluate consumers’ preferences and perceptions about how user-friendly the Altman Rule is at the point of sale with packaged carbohydrate foods. This would help to further understand how the use of information on food packaging can motivate healthier decisions—thereby helping to alleviate the burden of chronic disease.
CORRESPONDENCE
Kimberly R. Dong, DrPH, MS, RDN, Tufts University School of Medicine, Department of Public Health and Community Medicine, 136 Harrison Avenue, MV Building, Boston, MA 02111; [email protected]
1. Hersey JC, Wohlgenant KC, Arsenault JE, et al. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. 2013;71:1-14. doi: 10.1111/nure.12000
2. Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. doi: 10.1056/NEJMoa2007123
3. Brand-Miller J, Hayne S, Petocz P, et al. Low–glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261-2267. doi: 10.2337/diacare.26.8.2261
4. Matthan NR, Ausman LM, Meng H, et al. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004-1013. doi: 10.3945/ajcn.116.137208
5. Sonnenberg L, Gelsomin E, Levy DE, et al. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev Med. 2013;57:253-257. doi: 10.1016/j.ypmed.2013.07.001
6. Savoie N, Barlow K, Harvey KL, et al. Consumer perceptions of front-of-package labelling systems and healthiness of foods. Can J Public Health. 2013;104:e359-e363. doi: 10.17269/cjph.104.4027
7. Fischer LM, Sutherland LA, Kaley LA, et al. Development and implementation of the Guiding Stars nutrition guidance program. Am J Health Promot. 2011;26:e55-e63. doi: 10.4278/ajhp.100709-QUAL-238
8. Maubach N, Hoek J, Mather D. Interpretive front-of-pack nutrition labels. Comparing competing recommendations. Appetite. 2014;82:67-77. doi: 10.1016/j.appet.2014.07.006
9. Chan J, McMahon E, Brimblecombe J. Point‐of‐sale nutrition information interventions in food retail stores to promote healthier food purchase and intake: a systematic review. Obes Rev. 2021;22. doi: 10.1111/obr.13311
10. Mathioudakis N, Bashura H, Boyér L, et al. Development, implementation, and evaluation of a physician-targeted inpatient glycemic management curriculum. J Med Educ Curric Dev. 2019;6:238212051986134. doi: 10.1177/2382120519861342
1. Hersey JC, Wohlgenant KC, Arsenault JE, et al. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. 2013;71:1-14. doi: 10.1111/nure.12000
2. Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. doi: 10.1056/NEJMoa2007123
3. Brand-Miller J, Hayne S, Petocz P, et al. Low–glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261-2267. doi: 10.2337/diacare.26.8.2261
4. Matthan NR, Ausman LM, Meng H, et al. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004-1013. doi: 10.3945/ajcn.116.137208
5. Sonnenberg L, Gelsomin E, Levy DE, et al. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev Med. 2013;57:253-257. doi: 10.1016/j.ypmed.2013.07.001
6. Savoie N, Barlow K, Harvey KL, et al. Consumer perceptions of front-of-package labelling systems and healthiness of foods. Can J Public Health. 2013;104:e359-e363. doi: 10.17269/cjph.104.4027
7. Fischer LM, Sutherland LA, Kaley LA, et al. Development and implementation of the Guiding Stars nutrition guidance program. Am J Health Promot. 2011;26:e55-e63. doi: 10.4278/ajhp.100709-QUAL-238
8. Maubach N, Hoek J, Mather D. Interpretive front-of-pack nutrition labels. Comparing competing recommendations. Appetite. 2014;82:67-77. doi: 10.1016/j.appet.2014.07.006
9. Chan J, McMahon E, Brimblecombe J. Point‐of‐sale nutrition information interventions in food retail stores to promote healthier food purchase and intake: a systematic review. Obes Rev. 2021;22. doi: 10.1111/obr.13311
10. Mathioudakis N, Bashura H, Boyér L, et al. Development, implementation, and evaluation of a physician-targeted inpatient glycemic management curriculum. J Med Educ Curric Dev. 2019;6:238212051986134. doi: 10.1177/2382120519861342
Demographic Characteristics of Veterans Diagnosed With Breast and Gynecologic Cancers: A Comparative Analysis With the General Population
PURPOSE
This project aims to describe the demographics of Veterans diagnosed with breast and gynecologic cancers and assess differences compared to the general population.
BACKGROUND
With an increasing number of women Veterans enrolling in the VA, it is crucial for oncologists to be prepared to provide care for VeterS32 • SEPTEMBER 2023 www.mdedge.com/fedprac/avaho NOTES ans diagnosed with breast and gynecologic cancers. Despite the rising incidence of these cancers among Veterans, there is limited characterization of the demographic profile of this population. Understanding the unique characteristics of Veterans with these malignancies, distinct from the general population, is essential for the Veterans Administration (VA) to develop programs and enhance care for these patients.
METHODS/DATA ANALYSIS
Consult records from the VA Corporate Data Warehouse between January 1, 2021, and December 31, 2022, were analyzed to identify Veterans with newly diagnosed breast, uterine, ovarian, cervical, and vulvovaginal cancer. Demographic were evaluated. Data on the general population were obtained data from SEER (Surveillance, Epidemiology, and End Results) 19 database for 2020.
RESULTS
A total of 3,304 Veterans diagnosed with breast cancer and 918 Veterans with gynecologic cancers were identified (uterine, n = 365; cervical, n = 344, ovarian, n = 177; vulvovaginal, n = 32). Veterans were found to be younger than the general population, with a mean age at diagnosis of 59 for Veterans with breast cancer to 63 for non-veterans. Among those with gynecologic cancers, the mean age at diagnosis for Veterans was 55 compared to 61 for non-veterans. Male breast cancer cases were more prevalent among Veterans, accounting for 11% in the VA compared to 1% in SEER. The Veteran cohort also displayed a higher proportion of Black patients, with 30% of breast cancer cases in the VA being Black compared to 12% in SEER.
CONCLUSIONS/IMPLICATIONS
Veterans diagnosed with breast and gynecologic cancers exhibit unique demographic characteristics compared to the general population. They tend to be younger and have a higher representation of Black patients. The incidence of male breast cancer is notably higher among Veterans. As the prevalence of these cancer types continue to rise among Veterans, it is vital for oncologists to be aware of and adequately address the unique health needs of this population. These findings emphasize the importance of tailored strategies and programs to provide optimal care for Veterans with breast and gynecologic cancers.
PURPOSE
This project aims to describe the demographics of Veterans diagnosed with breast and gynecologic cancers and assess differences compared to the general population.
BACKGROUND
With an increasing number of women Veterans enrolling in the VA, it is crucial for oncologists to be prepared to provide care for VeterS32 • SEPTEMBER 2023 www.mdedge.com/fedprac/avaho NOTES ans diagnosed with breast and gynecologic cancers. Despite the rising incidence of these cancers among Veterans, there is limited characterization of the demographic profile of this population. Understanding the unique characteristics of Veterans with these malignancies, distinct from the general population, is essential for the Veterans Administration (VA) to develop programs and enhance care for these patients.
METHODS/DATA ANALYSIS
Consult records from the VA Corporate Data Warehouse between January 1, 2021, and December 31, 2022, were analyzed to identify Veterans with newly diagnosed breast, uterine, ovarian, cervical, and vulvovaginal cancer. Demographic were evaluated. Data on the general population were obtained data from SEER (Surveillance, Epidemiology, and End Results) 19 database for 2020.
RESULTS
A total of 3,304 Veterans diagnosed with breast cancer and 918 Veterans with gynecologic cancers were identified (uterine, n = 365; cervical, n = 344, ovarian, n = 177; vulvovaginal, n = 32). Veterans were found to be younger than the general population, with a mean age at diagnosis of 59 for Veterans with breast cancer to 63 for non-veterans. Among those with gynecologic cancers, the mean age at diagnosis for Veterans was 55 compared to 61 for non-veterans. Male breast cancer cases were more prevalent among Veterans, accounting for 11% in the VA compared to 1% in SEER. The Veteran cohort also displayed a higher proportion of Black patients, with 30% of breast cancer cases in the VA being Black compared to 12% in SEER.
CONCLUSIONS/IMPLICATIONS
Veterans diagnosed with breast and gynecologic cancers exhibit unique demographic characteristics compared to the general population. They tend to be younger and have a higher representation of Black patients. The incidence of male breast cancer is notably higher among Veterans. As the prevalence of these cancer types continue to rise among Veterans, it is vital for oncologists to be aware of and adequately address the unique health needs of this population. These findings emphasize the importance of tailored strategies and programs to provide optimal care for Veterans with breast and gynecologic cancers.
PURPOSE
This project aims to describe the demographics of Veterans diagnosed with breast and gynecologic cancers and assess differences compared to the general population.
BACKGROUND
With an increasing number of women Veterans enrolling in the VA, it is crucial for oncologists to be prepared to provide care for VeterS32 • SEPTEMBER 2023 www.mdedge.com/fedprac/avaho NOTES ans diagnosed with breast and gynecologic cancers. Despite the rising incidence of these cancers among Veterans, there is limited characterization of the demographic profile of this population. Understanding the unique characteristics of Veterans with these malignancies, distinct from the general population, is essential for the Veterans Administration (VA) to develop programs and enhance care for these patients.
METHODS/DATA ANALYSIS
Consult records from the VA Corporate Data Warehouse between January 1, 2021, and December 31, 2022, were analyzed to identify Veterans with newly diagnosed breast, uterine, ovarian, cervical, and vulvovaginal cancer. Demographic were evaluated. Data on the general population were obtained data from SEER (Surveillance, Epidemiology, and End Results) 19 database for 2020.
RESULTS
A total of 3,304 Veterans diagnosed with breast cancer and 918 Veterans with gynecologic cancers were identified (uterine, n = 365; cervical, n = 344, ovarian, n = 177; vulvovaginal, n = 32). Veterans were found to be younger than the general population, with a mean age at diagnosis of 59 for Veterans with breast cancer to 63 for non-veterans. Among those with gynecologic cancers, the mean age at diagnosis for Veterans was 55 compared to 61 for non-veterans. Male breast cancer cases were more prevalent among Veterans, accounting for 11% in the VA compared to 1% in SEER. The Veteran cohort also displayed a higher proportion of Black patients, with 30% of breast cancer cases in the VA being Black compared to 12% in SEER.
CONCLUSIONS/IMPLICATIONS
Veterans diagnosed with breast and gynecologic cancers exhibit unique demographic characteristics compared to the general population. They tend to be younger and have a higher representation of Black patients. The incidence of male breast cancer is notably higher among Veterans. As the prevalence of these cancer types continue to rise among Veterans, it is vital for oncologists to be aware of and adequately address the unique health needs of this population. These findings emphasize the importance of tailored strategies and programs to provide optimal care for Veterans with breast and gynecologic cancers.
Impact of Socioeconomic Disparities and Facility Type on Overall Survival in Stage I vs Stage IV Amelanotic Melanoma: An Analysis of the National Cancer Database
PURPOSE
This study addresses a gap in knowledge regarding socioeconomic factors, facility type, and overall survival in stage I vs stage IV Amelanotic Melanoma.
BACKGROUND
Amelanotic Melanoma (AM) is a rare form of melanoma that lacks pigment and accounts for approximately 5% of melanomas. Light skin color and increasing age are important risk factors. Although curable when diagnosed early, it is often missed or mistaken for other benign conditions. A study investigating the impact of facility type on overall survival between stage I vs stage IV AM has yet to be done.
METHODS
This is a retrospective study of patients diagnosed with Amelanotic Melanoma (ICD-8730) between 2004 and 2020 in the National Cancer Database (NCDB) to compare demographic features and overall survival (n = 2147). Exclusion criteria included missing data.
DATA ANALYSIS
Descriptive statistics for all AM patients were collected. Median household income and facility type were compared between patients diagnosed with stage I and stage IV AM using Pearson Chi- Square test. Breslow thickness and overall survival between stage I and stage IV were evaluated using independent t-test and Kaplan-Meier test, respectively. All variables were evaluated for a significance of P < .05.
RESULTS
Most cases analyzed were White (98.1%), male (58.6%), and had Medicare as the primary payor at diagnosis (51.1%). Of 2147 cases, 497 were stage I (23.1%) and 164 were stage IV AM (7.6%) with a mean age at diagnosis of 66.05 and 63.72 years, respectively. There was a significant difference in overall survival between stage I (mean = 118.7 months) and stage 4 (mean = 42.4 months, P < 0.001). The average Breslow thickness was 1.17mm in stage I and 2.59mm in stage IV (P<0.05). More patients diagnosed at stage I used academic facilities than those diagnosed at stage IV (43.9% vs 33.8%, P<0.05). Most patients diagnosed at stage I were high income compared to patients diagnosed at stage IV (55% vs 43.2%, P<0.05).
CONCLUSIONS
With the overall survival of stage IV AM being significantly worse, we hope this study can provide a starting point in the study and prevention of disparities in the early diagnosis of AM.
PURPOSE
This study addresses a gap in knowledge regarding socioeconomic factors, facility type, and overall survival in stage I vs stage IV Amelanotic Melanoma.
BACKGROUND
Amelanotic Melanoma (AM) is a rare form of melanoma that lacks pigment and accounts for approximately 5% of melanomas. Light skin color and increasing age are important risk factors. Although curable when diagnosed early, it is often missed or mistaken for other benign conditions. A study investigating the impact of facility type on overall survival between stage I vs stage IV AM has yet to be done.
METHODS
This is a retrospective study of patients diagnosed with Amelanotic Melanoma (ICD-8730) between 2004 and 2020 in the National Cancer Database (NCDB) to compare demographic features and overall survival (n = 2147). Exclusion criteria included missing data.
DATA ANALYSIS
Descriptive statistics for all AM patients were collected. Median household income and facility type were compared between patients diagnosed with stage I and stage IV AM using Pearson Chi- Square test. Breslow thickness and overall survival between stage I and stage IV were evaluated using independent t-test and Kaplan-Meier test, respectively. All variables were evaluated for a significance of P < .05.
RESULTS
Most cases analyzed were White (98.1%), male (58.6%), and had Medicare as the primary payor at diagnosis (51.1%). Of 2147 cases, 497 were stage I (23.1%) and 164 were stage IV AM (7.6%) with a mean age at diagnosis of 66.05 and 63.72 years, respectively. There was a significant difference in overall survival between stage I (mean = 118.7 months) and stage 4 (mean = 42.4 months, P < 0.001). The average Breslow thickness was 1.17mm in stage I and 2.59mm in stage IV (P<0.05). More patients diagnosed at stage I used academic facilities than those diagnosed at stage IV (43.9% vs 33.8%, P<0.05). Most patients diagnosed at stage I were high income compared to patients diagnosed at stage IV (55% vs 43.2%, P<0.05).
CONCLUSIONS
With the overall survival of stage IV AM being significantly worse, we hope this study can provide a starting point in the study and prevention of disparities in the early diagnosis of AM.
PURPOSE
This study addresses a gap in knowledge regarding socioeconomic factors, facility type, and overall survival in stage I vs stage IV Amelanotic Melanoma.
BACKGROUND
Amelanotic Melanoma (AM) is a rare form of melanoma that lacks pigment and accounts for approximately 5% of melanomas. Light skin color and increasing age are important risk factors. Although curable when diagnosed early, it is often missed or mistaken for other benign conditions. A study investigating the impact of facility type on overall survival between stage I vs stage IV AM has yet to be done.
METHODS
This is a retrospective study of patients diagnosed with Amelanotic Melanoma (ICD-8730) between 2004 and 2020 in the National Cancer Database (NCDB) to compare demographic features and overall survival (n = 2147). Exclusion criteria included missing data.
DATA ANALYSIS
Descriptive statistics for all AM patients were collected. Median household income and facility type were compared between patients diagnosed with stage I and stage IV AM using Pearson Chi- Square test. Breslow thickness and overall survival between stage I and stage IV were evaluated using independent t-test and Kaplan-Meier test, respectively. All variables were evaluated for a significance of P < .05.
RESULTS
Most cases analyzed were White (98.1%), male (58.6%), and had Medicare as the primary payor at diagnosis (51.1%). Of 2147 cases, 497 were stage I (23.1%) and 164 were stage IV AM (7.6%) with a mean age at diagnosis of 66.05 and 63.72 years, respectively. There was a significant difference in overall survival between stage I (mean = 118.7 months) and stage 4 (mean = 42.4 months, P < 0.001). The average Breslow thickness was 1.17mm in stage I and 2.59mm in stage IV (P<0.05). More patients diagnosed at stage I used academic facilities than those diagnosed at stage IV (43.9% vs 33.8%, P<0.05). Most patients diagnosed at stage I were high income compared to patients diagnosed at stage IV (55% vs 43.2%, P<0.05).
CONCLUSIONS
With the overall survival of stage IV AM being significantly worse, we hope this study can provide a starting point in the study and prevention of disparities in the early diagnosis of AM.