User login
Is your patient using cocaine to self-medicate undiagnosed ADHD?
Attention-deficit/hyperactivity disorder (ADHD) often persists beyond childhood into adulthood. One of the therapeutic challenges of treating ADHD is identifying comorbidities, including underlying mood and anxiety disorders, and ongoing substance abuse. Effective treatment modalities tend to prioritize management of substance abuse, but the patient’s age may dictate the overall assessment plan.
So-called 'reward' center
Treating childhood ADHD with stimulants might reduce the risk for future drug abuse.1 It is estimated that approximately 10 million people with ADHD are undiagnosed in the United States2; characteristic ADHD symptoms—inattention, hyperactivity, impulsivity—can persist in adulthood, and affected persons might not meet societal expectations. Previously unidentified attention difficulties may emerge during early adulthood because of increasingly complex tasks at school and work.
Persons with undiagnosed ADHD might turn to potentially self-destructive means of placating inner tension. Cocaine has pharmacological properties in common with stimulants such as methylphenidate, which often is prescribed for ADHD. Cocaine and methylphenidate both work on altering brain chemistry with a similar mechanism of action, allowing for increased dopamine in the nucleus accumbens, also known as the “reward center” of the brain.
Adults with ADHD have a 300% higher risk of developing a substance use disorder than adults without ADHD.3 An estimated 15% to 25% of adults with substance abuse have comorbid ADHD. Although these patients abuse of a variety of substances including Cannabis and alcohol, cocaine is one of the most commonly abused substances among this population. These observations could point to a self-medication hypothesis.
Why self-medicate?
The self-medication hypothesis, formulated by Khantzian in 1985, was based on several clinical observations. Khantzian stated that an abuser’s drug of choice is not selected at random but, rather, by an inherent desire to suppress the attributes of the condition that seems to otherwise wreak havoc on his (her) life. Almost a century earlier, Freud mentioned that cocaine is an antidepressant. Among persons with ADHD who have not been given that diagnosis, or treated for the disorder, cocaine is a popular drug. Because of the antidepressant features of cocaine and its ability to produce a rapid increase of dopamine levels that exert a pro-euphoric effect, coupled with a seemingly paradoxical calming influence that leads to increased productivity, it is not surprising to find that cocaine is abused. Reportedly, persons who have not been treated because their ADHD is undiagnosed turn to cocaine because it improves attention, raises self-esteem, and allows users to harness a level of focus that they could not otherwise achieve.4
Mechanism of action
Methylphenidate reduces ADHD symptoms by increasing extracellular dopamine in the brain, acting by means of a mechanism that is similar to that of cocaine.5 By blocking reuptake of dopamine and allowing an extracellular surplus, users continue to experience the pleasurable effect the neuro-transmitter produces. Methylphenidate has been shown to be an even more potent inhibitor of the same autoreceptors. Injecting methylphenidate has been shown to produce a rapid release of dopamine similar to that of cocaine.5
However, methylphenidate causes a much slower increase in dopamine; its effect on the brain has been shown to be similar to that of cocaine without the increased abuse potential. Cocaine use remodels the brain by reconfiguring connections that are essential for craving and self-control.5 Therefore, substituting methylphenidate for cocaine could help ADHD patients by:
• improving overall executive functioning
• decreasing feelings of low self-worth
• increasing daily functioning
• minimizing craving and the risk of subsequent cocaine abuse.
Treatment recommendations
Carefully consider pharmacodynamics and pharmacokinetics when prescribing ADHD medication. In general, children and adolescents with ADHD respond more favorably to stimulants than adults do. In children, the mainstay of treatment is slow-dose stimulants such as methylphenidate; second-line treatments are immediate-release stimulants and atomoxetine, a selective norepinephrine reuptake inhibitor.6 Adults with ADHD might benefit from a nonstimulant, in part because of the presence of complex comorbidities.6 Modafinil often is prescribed for adults with ADHD.
Atomoxetine readily increases norepinephrine and dopamine in the prefrontal cortex as it bypasses the nucleus accumbens. Although atomoxetine is not a stimulant, the efficacy of the drug is based on its ability to increase norepinephrine through selective inhibition of the norepinephrine transporter. Norepinephrine modulates higher cortical functions—attention, executive function, arousal—that lead to a reduction in hyperactivity, inattention, and impulsivity.
Because dopamine is released in the prefrontal cortex—not in the nucleus accumbens—the addiction potential of atomoxetine is low.7 The drug might be an effective intervention for patients who are using cocaine to self-medicate. Stimulants such as methylphenidate have proven effective in safely mimicking the mechanism of action of cocaine. Nonstimulants, such as atomoxetine and modafinil, lack abuse potential and are excellent options for treating adults with ADHD.
Clinicians generally are advised to treat a patient’s underlying ADHD symptoms before addressing ongoing substance abuse. If a patient abruptly discontinues cocaine use before ADHD symptoms are properly controlled, her (his) condition might deteriorate further and the treatment plan might fail to progress. Some patients have experienced a reduction in craving for cocaine after they began stimulant therapy; these people no longer felt a need to self-medicate because their symptoms were being addressed.4
1. Jain S, Jain R, Islam J. Do stimulants for ADHD increase the risk of substance use disorders? Current Psychiatry. 2011;10(8):20-24.
2. Baskin S. Adult ADHD—A common disorder, often missed. http://www.stevebaskinmd.com/articles-about-adultadhd.html. Published 2009. Accessed November 5, 2014.
3. Tuzee M. Many adults who have ADHD go undiagnosed.
http://abclocal.go.com/kabc/story?section=news/health/your_health&id=7657326. Published September 8, 2010. Accessed October 9, 2014.
4. Plume D. The self medication hypothesis: ADHD & chronic cocaine abuse. A literature review. http://www.addcentre.co.uk/selfmedcocaine.htm. Published April 1995. Accessed October 9, 2014.
5. Searight HR, Burke JM. Adult attention deficit hyperactivity disorder. UpToDate. Updated Feb 2011. Accessed November 5, 2014.
6. Stahl SM. Attention deficit disorder and its treatment. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:884-897.
7. Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112-120.
Attention-deficit/hyperactivity disorder (ADHD) often persists beyond childhood into adulthood. One of the therapeutic challenges of treating ADHD is identifying comorbidities, including underlying mood and anxiety disorders, and ongoing substance abuse. Effective treatment modalities tend to prioritize management of substance abuse, but the patient’s age may dictate the overall assessment plan.
So-called 'reward' center
Treating childhood ADHD with stimulants might reduce the risk for future drug abuse.1 It is estimated that approximately 10 million people with ADHD are undiagnosed in the United States2; characteristic ADHD symptoms—inattention, hyperactivity, impulsivity—can persist in adulthood, and affected persons might not meet societal expectations. Previously unidentified attention difficulties may emerge during early adulthood because of increasingly complex tasks at school and work.
Persons with undiagnosed ADHD might turn to potentially self-destructive means of placating inner tension. Cocaine has pharmacological properties in common with stimulants such as methylphenidate, which often is prescribed for ADHD. Cocaine and methylphenidate both work on altering brain chemistry with a similar mechanism of action, allowing for increased dopamine in the nucleus accumbens, also known as the “reward center” of the brain.
Adults with ADHD have a 300% higher risk of developing a substance use disorder than adults without ADHD.3 An estimated 15% to 25% of adults with substance abuse have comorbid ADHD. Although these patients abuse of a variety of substances including Cannabis and alcohol, cocaine is one of the most commonly abused substances among this population. These observations could point to a self-medication hypothesis.
Why self-medicate?
The self-medication hypothesis, formulated by Khantzian in 1985, was based on several clinical observations. Khantzian stated that an abuser’s drug of choice is not selected at random but, rather, by an inherent desire to suppress the attributes of the condition that seems to otherwise wreak havoc on his (her) life. Almost a century earlier, Freud mentioned that cocaine is an antidepressant. Among persons with ADHD who have not been given that diagnosis, or treated for the disorder, cocaine is a popular drug. Because of the antidepressant features of cocaine and its ability to produce a rapid increase of dopamine levels that exert a pro-euphoric effect, coupled with a seemingly paradoxical calming influence that leads to increased productivity, it is not surprising to find that cocaine is abused. Reportedly, persons who have not been treated because their ADHD is undiagnosed turn to cocaine because it improves attention, raises self-esteem, and allows users to harness a level of focus that they could not otherwise achieve.4
Mechanism of action
Methylphenidate reduces ADHD symptoms by increasing extracellular dopamine in the brain, acting by means of a mechanism that is similar to that of cocaine.5 By blocking reuptake of dopamine and allowing an extracellular surplus, users continue to experience the pleasurable effect the neuro-transmitter produces. Methylphenidate has been shown to be an even more potent inhibitor of the same autoreceptors. Injecting methylphenidate has been shown to produce a rapid release of dopamine similar to that of cocaine.5
However, methylphenidate causes a much slower increase in dopamine; its effect on the brain has been shown to be similar to that of cocaine without the increased abuse potential. Cocaine use remodels the brain by reconfiguring connections that are essential for craving and self-control.5 Therefore, substituting methylphenidate for cocaine could help ADHD patients by:
• improving overall executive functioning
• decreasing feelings of low self-worth
• increasing daily functioning
• minimizing craving and the risk of subsequent cocaine abuse.
Treatment recommendations
Carefully consider pharmacodynamics and pharmacokinetics when prescribing ADHD medication. In general, children and adolescents with ADHD respond more favorably to stimulants than adults do. In children, the mainstay of treatment is slow-dose stimulants such as methylphenidate; second-line treatments are immediate-release stimulants and atomoxetine, a selective norepinephrine reuptake inhibitor.6 Adults with ADHD might benefit from a nonstimulant, in part because of the presence of complex comorbidities.6 Modafinil often is prescribed for adults with ADHD.
Atomoxetine readily increases norepinephrine and dopamine in the prefrontal cortex as it bypasses the nucleus accumbens. Although atomoxetine is not a stimulant, the efficacy of the drug is based on its ability to increase norepinephrine through selective inhibition of the norepinephrine transporter. Norepinephrine modulates higher cortical functions—attention, executive function, arousal—that lead to a reduction in hyperactivity, inattention, and impulsivity.
Because dopamine is released in the prefrontal cortex—not in the nucleus accumbens—the addiction potential of atomoxetine is low.7 The drug might be an effective intervention for patients who are using cocaine to self-medicate. Stimulants such as methylphenidate have proven effective in safely mimicking the mechanism of action of cocaine. Nonstimulants, such as atomoxetine and modafinil, lack abuse potential and are excellent options for treating adults with ADHD.
Clinicians generally are advised to treat a patient’s underlying ADHD symptoms before addressing ongoing substance abuse. If a patient abruptly discontinues cocaine use before ADHD symptoms are properly controlled, her (his) condition might deteriorate further and the treatment plan might fail to progress. Some patients have experienced a reduction in craving for cocaine after they began stimulant therapy; these people no longer felt a need to self-medicate because their symptoms were being addressed.4
Attention-deficit/hyperactivity disorder (ADHD) often persists beyond childhood into adulthood. One of the therapeutic challenges of treating ADHD is identifying comorbidities, including underlying mood and anxiety disorders, and ongoing substance abuse. Effective treatment modalities tend to prioritize management of substance abuse, but the patient’s age may dictate the overall assessment plan.
So-called 'reward' center
Treating childhood ADHD with stimulants might reduce the risk for future drug abuse.1 It is estimated that approximately 10 million people with ADHD are undiagnosed in the United States2; characteristic ADHD symptoms—inattention, hyperactivity, impulsivity—can persist in adulthood, and affected persons might not meet societal expectations. Previously unidentified attention difficulties may emerge during early adulthood because of increasingly complex tasks at school and work.
Persons with undiagnosed ADHD might turn to potentially self-destructive means of placating inner tension. Cocaine has pharmacological properties in common with stimulants such as methylphenidate, which often is prescribed for ADHD. Cocaine and methylphenidate both work on altering brain chemistry with a similar mechanism of action, allowing for increased dopamine in the nucleus accumbens, also known as the “reward center” of the brain.
Adults with ADHD have a 300% higher risk of developing a substance use disorder than adults without ADHD.3 An estimated 15% to 25% of adults with substance abuse have comorbid ADHD. Although these patients abuse of a variety of substances including Cannabis and alcohol, cocaine is one of the most commonly abused substances among this population. These observations could point to a self-medication hypothesis.
Why self-medicate?
The self-medication hypothesis, formulated by Khantzian in 1985, was based on several clinical observations. Khantzian stated that an abuser’s drug of choice is not selected at random but, rather, by an inherent desire to suppress the attributes of the condition that seems to otherwise wreak havoc on his (her) life. Almost a century earlier, Freud mentioned that cocaine is an antidepressant. Among persons with ADHD who have not been given that diagnosis, or treated for the disorder, cocaine is a popular drug. Because of the antidepressant features of cocaine and its ability to produce a rapid increase of dopamine levels that exert a pro-euphoric effect, coupled with a seemingly paradoxical calming influence that leads to increased productivity, it is not surprising to find that cocaine is abused. Reportedly, persons who have not been treated because their ADHD is undiagnosed turn to cocaine because it improves attention, raises self-esteem, and allows users to harness a level of focus that they could not otherwise achieve.4
Mechanism of action
Methylphenidate reduces ADHD symptoms by increasing extracellular dopamine in the brain, acting by means of a mechanism that is similar to that of cocaine.5 By blocking reuptake of dopamine and allowing an extracellular surplus, users continue to experience the pleasurable effect the neuro-transmitter produces. Methylphenidate has been shown to be an even more potent inhibitor of the same autoreceptors. Injecting methylphenidate has been shown to produce a rapid release of dopamine similar to that of cocaine.5
However, methylphenidate causes a much slower increase in dopamine; its effect on the brain has been shown to be similar to that of cocaine without the increased abuse potential. Cocaine use remodels the brain by reconfiguring connections that are essential for craving and self-control.5 Therefore, substituting methylphenidate for cocaine could help ADHD patients by:
• improving overall executive functioning
• decreasing feelings of low self-worth
• increasing daily functioning
• minimizing craving and the risk of subsequent cocaine abuse.
Treatment recommendations
Carefully consider pharmacodynamics and pharmacokinetics when prescribing ADHD medication. In general, children and adolescents with ADHD respond more favorably to stimulants than adults do. In children, the mainstay of treatment is slow-dose stimulants such as methylphenidate; second-line treatments are immediate-release stimulants and atomoxetine, a selective norepinephrine reuptake inhibitor.6 Adults with ADHD might benefit from a nonstimulant, in part because of the presence of complex comorbidities.6 Modafinil often is prescribed for adults with ADHD.
Atomoxetine readily increases norepinephrine and dopamine in the prefrontal cortex as it bypasses the nucleus accumbens. Although atomoxetine is not a stimulant, the efficacy of the drug is based on its ability to increase norepinephrine through selective inhibition of the norepinephrine transporter. Norepinephrine modulates higher cortical functions—attention, executive function, arousal—that lead to a reduction in hyperactivity, inattention, and impulsivity.
Because dopamine is released in the prefrontal cortex—not in the nucleus accumbens—the addiction potential of atomoxetine is low.7 The drug might be an effective intervention for patients who are using cocaine to self-medicate. Stimulants such as methylphenidate have proven effective in safely mimicking the mechanism of action of cocaine. Nonstimulants, such as atomoxetine and modafinil, lack abuse potential and are excellent options for treating adults with ADHD.
Clinicians generally are advised to treat a patient’s underlying ADHD symptoms before addressing ongoing substance abuse. If a patient abruptly discontinues cocaine use before ADHD symptoms are properly controlled, her (his) condition might deteriorate further and the treatment plan might fail to progress. Some patients have experienced a reduction in craving for cocaine after they began stimulant therapy; these people no longer felt a need to self-medicate because their symptoms were being addressed.4
1. Jain S, Jain R, Islam J. Do stimulants for ADHD increase the risk of substance use disorders? Current Psychiatry. 2011;10(8):20-24.
2. Baskin S. Adult ADHD—A common disorder, often missed. http://www.stevebaskinmd.com/articles-about-adultadhd.html. Published 2009. Accessed November 5, 2014.
3. Tuzee M. Many adults who have ADHD go undiagnosed.
http://abclocal.go.com/kabc/story?section=news/health/your_health&id=7657326. Published September 8, 2010. Accessed October 9, 2014.
4. Plume D. The self medication hypothesis: ADHD & chronic cocaine abuse. A literature review. http://www.addcentre.co.uk/selfmedcocaine.htm. Published April 1995. Accessed October 9, 2014.
5. Searight HR, Burke JM. Adult attention deficit hyperactivity disorder. UpToDate. Updated Feb 2011. Accessed November 5, 2014.
6. Stahl SM. Attention deficit disorder and its treatment. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:884-897.
7. Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112-120.
1. Jain S, Jain R, Islam J. Do stimulants for ADHD increase the risk of substance use disorders? Current Psychiatry. 2011;10(8):20-24.
2. Baskin S. Adult ADHD—A common disorder, often missed. http://www.stevebaskinmd.com/articles-about-adultadhd.html. Published 2009. Accessed November 5, 2014.
3. Tuzee M. Many adults who have ADHD go undiagnosed.
http://abclocal.go.com/kabc/story?section=news/health/your_health&id=7657326. Published September 8, 2010. Accessed October 9, 2014.
4. Plume D. The self medication hypothesis: ADHD & chronic cocaine abuse. A literature review. http://www.addcentre.co.uk/selfmedcocaine.htm. Published April 1995. Accessed October 9, 2014.
5. Searight HR, Burke JM. Adult attention deficit hyperactivity disorder. UpToDate. Updated Feb 2011. Accessed November 5, 2014.
6. Stahl SM. Attention deficit disorder and its treatment. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:884-897.
7. Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112-120.
Self-management of mental illness? It’s possible
Patients with chronic illness, such as diabetes, are expected, and taught how, to participate in managing their disease. On the other hand, patients with serious mental illness historically have been thought of as passive recipients of care. Attitudes of care providers, patients, and family members are changing, however, and, in the last decade, the Illness Management and Recovery (IMR) program has been developed for treating patients with serious mental illness, such as schizophrenia, using principles of chronic disease management.1 Consider using this evidence-based psychosocial treatment modality for your patients with schizophrenia.
Basic philosophy
A core assumption of IMR is realistic optimism that recovery is possible. Recovery, in this context, means that a patient can have a meaningful life despite having a serious illness. In IMR, patients engage in developing and tracking their progress toward personally meaningful goals; that progress is broken down into small steps and worked on over the course of the program.
Critical components
IMR addresses practical matters of living with schizophrenia, including coping with symptoms and collaborating with providers. The program combines elements from 6 areas:
• psychoeducation
• cognitive-behavioral approaches to medication management and other treatment targets
• motivational interviewing
• relapse prevention planning
• social skills training
• coping skills to manage persistent symptoms.
The original curriculum, which is available free of charge, comprises 10 topics (Table).2 Of note, the newest version, IMR-3, includes an additional module focused on healthy lifestyles.3
Implementation
Although typically delivered as a structured weekly group intervention for 9 to 12 months, IMR can be taught individually. Involving a supportive person such as a family member, case manager, or residential staff member can be useful. Physicians can select modules tailored to the patient’s needs. Any physician who wants to bring IMR to her (his) patient can download the SAMHSA Illness Management and Recovery Evidence-Based Practices KIT (Knowledge Informing Transformation).2 This free download provides the full curriculum with handouts, and tips and training tools related to implementation and evaluation of IMR in a typical mental health care setting.
IMR is well-accepted by most participants; studies report a median 63% of patients complete IMR.4 Attendance for the intervention appears to be better in a group, rather than an individual, format. A number of patient characteristics, including older age, lower hostility, fewer psychotic symptoms, and more education, have been identified as predictors of better attendance to manualized psychosocial treatments, such as IMR.5
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mueser KT, Meyer PS, Penn DL, et al. The Illness Management and Recovery program: rationale, development, and preliminary findings. Schizophr Bull. 2006;32(suppl 1):S32-S43.
2. SAMSHA. Illness Management and Recovery Evidence-Based Practices (EBP) KIT. http://store.samhsa.gov/product/Illness-Management-and-Recovery-Evidence-Based-Practices-EBP-KIT/SMA09-4463. Published March 2010. Accessed October 16, 2014.
3. Mueser KT, Gingerich S. Illness Management and Recovery. 3rd ed. Center City, MN: Hazelden; 2011.
4. McGuire AB, Kukla M, Green A, et al. Illness management and recovery: a review of the literature. Psychiatr Serv. 2014;65(2):171-179.
5. McGuire AB, Bonfils KA, Kukla M, et al. Measuring participation in an evidence-based practice: illness management and recovery group attendance. Psychiatry Res. 2013;210(3):684-689.
Patients with chronic illness, such as diabetes, are expected, and taught how, to participate in managing their disease. On the other hand, patients with serious mental illness historically have been thought of as passive recipients of care. Attitudes of care providers, patients, and family members are changing, however, and, in the last decade, the Illness Management and Recovery (IMR) program has been developed for treating patients with serious mental illness, such as schizophrenia, using principles of chronic disease management.1 Consider using this evidence-based psychosocial treatment modality for your patients with schizophrenia.
Basic philosophy
A core assumption of IMR is realistic optimism that recovery is possible. Recovery, in this context, means that a patient can have a meaningful life despite having a serious illness. In IMR, patients engage in developing and tracking their progress toward personally meaningful goals; that progress is broken down into small steps and worked on over the course of the program.
Critical components
IMR addresses practical matters of living with schizophrenia, including coping with symptoms and collaborating with providers. The program combines elements from 6 areas:
• psychoeducation
• cognitive-behavioral approaches to medication management and other treatment targets
• motivational interviewing
• relapse prevention planning
• social skills training
• coping skills to manage persistent symptoms.
The original curriculum, which is available free of charge, comprises 10 topics (Table).2 Of note, the newest version, IMR-3, includes an additional module focused on healthy lifestyles.3
Implementation
Although typically delivered as a structured weekly group intervention for 9 to 12 months, IMR can be taught individually. Involving a supportive person such as a family member, case manager, or residential staff member can be useful. Physicians can select modules tailored to the patient’s needs. Any physician who wants to bring IMR to her (his) patient can download the SAMHSA Illness Management and Recovery Evidence-Based Practices KIT (Knowledge Informing Transformation).2 This free download provides the full curriculum with handouts, and tips and training tools related to implementation and evaluation of IMR in a typical mental health care setting.
IMR is well-accepted by most participants; studies report a median 63% of patients complete IMR.4 Attendance for the intervention appears to be better in a group, rather than an individual, format. A number of patient characteristics, including older age, lower hostility, fewer psychotic symptoms, and more education, have been identified as predictors of better attendance to manualized psychosocial treatments, such as IMR.5
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Patients with chronic illness, such as diabetes, are expected, and taught how, to participate in managing their disease. On the other hand, patients with serious mental illness historically have been thought of as passive recipients of care. Attitudes of care providers, patients, and family members are changing, however, and, in the last decade, the Illness Management and Recovery (IMR) program has been developed for treating patients with serious mental illness, such as schizophrenia, using principles of chronic disease management.1 Consider using this evidence-based psychosocial treatment modality for your patients with schizophrenia.
Basic philosophy
A core assumption of IMR is realistic optimism that recovery is possible. Recovery, in this context, means that a patient can have a meaningful life despite having a serious illness. In IMR, patients engage in developing and tracking their progress toward personally meaningful goals; that progress is broken down into small steps and worked on over the course of the program.
Critical components
IMR addresses practical matters of living with schizophrenia, including coping with symptoms and collaborating with providers. The program combines elements from 6 areas:
• psychoeducation
• cognitive-behavioral approaches to medication management and other treatment targets
• motivational interviewing
• relapse prevention planning
• social skills training
• coping skills to manage persistent symptoms.
The original curriculum, which is available free of charge, comprises 10 topics (Table).2 Of note, the newest version, IMR-3, includes an additional module focused on healthy lifestyles.3
Implementation
Although typically delivered as a structured weekly group intervention for 9 to 12 months, IMR can be taught individually. Involving a supportive person such as a family member, case manager, or residential staff member can be useful. Physicians can select modules tailored to the patient’s needs. Any physician who wants to bring IMR to her (his) patient can download the SAMHSA Illness Management and Recovery Evidence-Based Practices KIT (Knowledge Informing Transformation).2 This free download provides the full curriculum with handouts, and tips and training tools related to implementation and evaluation of IMR in a typical mental health care setting.
IMR is well-accepted by most participants; studies report a median 63% of patients complete IMR.4 Attendance for the intervention appears to be better in a group, rather than an individual, format. A number of patient characteristics, including older age, lower hostility, fewer psychotic symptoms, and more education, have been identified as predictors of better attendance to manualized psychosocial treatments, such as IMR.5
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mueser KT, Meyer PS, Penn DL, et al. The Illness Management and Recovery program: rationale, development, and preliminary findings. Schizophr Bull. 2006;32(suppl 1):S32-S43.
2. SAMSHA. Illness Management and Recovery Evidence-Based Practices (EBP) KIT. http://store.samhsa.gov/product/Illness-Management-and-Recovery-Evidence-Based-Practices-EBP-KIT/SMA09-4463. Published March 2010. Accessed October 16, 2014.
3. Mueser KT, Gingerich S. Illness Management and Recovery. 3rd ed. Center City, MN: Hazelden; 2011.
4. McGuire AB, Kukla M, Green A, et al. Illness management and recovery: a review of the literature. Psychiatr Serv. 2014;65(2):171-179.
5. McGuire AB, Bonfils KA, Kukla M, et al. Measuring participation in an evidence-based practice: illness management and recovery group attendance. Psychiatry Res. 2013;210(3):684-689.
1. Mueser KT, Meyer PS, Penn DL, et al. The Illness Management and Recovery program: rationale, development, and preliminary findings. Schizophr Bull. 2006;32(suppl 1):S32-S43.
2. SAMSHA. Illness Management and Recovery Evidence-Based Practices (EBP) KIT. http://store.samhsa.gov/product/Illness-Management-and-Recovery-Evidence-Based-Practices-EBP-KIT/SMA09-4463. Published March 2010. Accessed October 16, 2014.
3. Mueser KT, Gingerich S. Illness Management and Recovery. 3rd ed. Center City, MN: Hazelden; 2011.
4. McGuire AB, Kukla M, Green A, et al. Illness management and recovery: a review of the literature. Psychiatr Serv. 2014;65(2):171-179.
5. McGuire AB, Bonfils KA, Kukla M, et al. Measuring participation in an evidence-based practice: illness management and recovery group attendance. Psychiatry Res. 2013;210(3):684-689.
Potentially dangerous mix: Antiretrovirals and drugs of abuse
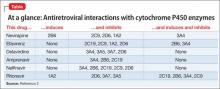
Patients with HIV often receive antiretroviral therapy, which includes non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Opiate, amphetamine, cocaine, and Cannabis abuse are common among this population.1 Many of these substances and antiretroviral medications undergo hepatic metabolism by cytochrome P450 (CYP) isoenzymes, which could lead to adverse events (Table2).
MDMA
The synthetic derivative of the amphetamine 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy” or “Molly”]) is metabolized by CYP2D6; thus, coadministration of MDMA with ritonavir can result in MDMA toxicity. This can induce a dangerous, potentially fatal serotonin syndrome characterized by tachycardia, arrhythmia, hyperthermia, seizures, myocardial infarction, rhabdomyolysis, renal or liver failure, and death.3
Opiates
Opiates are metabolized by CYP2D6 and, sometimes by CYP3A4. Metabolism of opiates, such as oxycodone, is decreased when these drugs are coadministered with a CYP2D6 inhibitor—potentially leading to toxicity.
Analgesic effect may be augmented when a CYP2D6 inhibitor is started, and decreased when the agent is stopped. Inducers of CYP2D6 or CYP3A4 can lead to decreased analgesia and oxycodone withdrawal.
Efavirenz can cause methadone withdrawal. Methadone inhibition of CYP2D6 and CYP3A4 can increase the serum level of antiretroviral medications, with adverse effects and resulting poor compliance.4
Cannabis
Tetrahydrocannabinol is metabolized by CYP3A4 and CYP2D6. Cannabis and CYP3A4 inhibitor co-utilization can cause toxicity, evidenced by paranoia, hallucinations, delusions, depersonalization, tachycardia, and orthostatic hypotension. Co-exposure of antiretroviral agents in occasional Cannabis users yields only a small change metabolically; however, nonadherence has been reported more frequently in heavy users.5
Education can help
The variability of CYP genotypes makes it important to understand drug metabolism as an aid to improving outcomes among patients with HIV who abuse drugs. Because of the risk for adverse effects, discuss the dangers of substance abuse with patients for whom antiretroviral therapy has been prescribed.
Clinical education should improve compliance and prognosis in patients with HIV. Advise drug users about pharmaceutical effects and risks of co-utilization. This might help some patients limit the use of illicit substances—and will help physicians manage pharmacotherapy with greater safety.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arvguidelines/22/hiv-and-illicit-drug-users. Updated March 27, 2012. Accessed July 26, 2013.
2. Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583-598.
3. Papaseit E, Vázquez A, Pérez-Mañá C, et al. Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine,ecstasy) toxicity caused by ritonavir (RTV).
Intensive Care Med. 2012;38(7):1239-1240.
4. Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598-1613.
5. Bonn-Miller MO, Oser ML, Bucossi MM, et al. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1-10.
3,4-methylenedioxymethamphetamine, opiates
Patients with HIV often receive antiretroviral therapy, which includes non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Opiate, amphetamine, cocaine, and Cannabis abuse are common among this population.1 Many of these substances and antiretroviral medications undergo hepatic metabolism by cytochrome P450 (CYP) isoenzymes, which could lead to adverse events (Table2).
MDMA
The synthetic derivative of the amphetamine 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy” or “Molly”]) is metabolized by CYP2D6; thus, coadministration of MDMA with ritonavir can result in MDMA toxicity. This can induce a dangerous, potentially fatal serotonin syndrome characterized by tachycardia, arrhythmia, hyperthermia, seizures, myocardial infarction, rhabdomyolysis, renal or liver failure, and death.3
Opiates
Opiates are metabolized by CYP2D6 and, sometimes by CYP3A4. Metabolism of opiates, such as oxycodone, is decreased when these drugs are coadministered with a CYP2D6 inhibitor—potentially leading to toxicity.
Analgesic effect may be augmented when a CYP2D6 inhibitor is started, and decreased when the agent is stopped. Inducers of CYP2D6 or CYP3A4 can lead to decreased analgesia and oxycodone withdrawal.
Efavirenz can cause methadone withdrawal. Methadone inhibition of CYP2D6 and CYP3A4 can increase the serum level of antiretroviral medications, with adverse effects and resulting poor compliance.4
Cannabis
Tetrahydrocannabinol is metabolized by CYP3A4 and CYP2D6. Cannabis and CYP3A4 inhibitor co-utilization can cause toxicity, evidenced by paranoia, hallucinations, delusions, depersonalization, tachycardia, and orthostatic hypotension. Co-exposure of antiretroviral agents in occasional Cannabis users yields only a small change metabolically; however, nonadherence has been reported more frequently in heavy users.5
Education can help
The variability of CYP genotypes makes it important to understand drug metabolism as an aid to improving outcomes among patients with HIV who abuse drugs. Because of the risk for adverse effects, discuss the dangers of substance abuse with patients for whom antiretroviral therapy has been prescribed.
Clinical education should improve compliance and prognosis in patients with HIV. Advise drug users about pharmaceutical effects and risks of co-utilization. This might help some patients limit the use of illicit substances—and will help physicians manage pharmacotherapy with greater safety.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Patients with HIV often receive antiretroviral therapy, which includes non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Opiate, amphetamine, cocaine, and Cannabis abuse are common among this population.1 Many of these substances and antiretroviral medications undergo hepatic metabolism by cytochrome P450 (CYP) isoenzymes, which could lead to adverse events (Table2).
MDMA
The synthetic derivative of the amphetamine 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy” or “Molly”]) is metabolized by CYP2D6; thus, coadministration of MDMA with ritonavir can result in MDMA toxicity. This can induce a dangerous, potentially fatal serotonin syndrome characterized by tachycardia, arrhythmia, hyperthermia, seizures, myocardial infarction, rhabdomyolysis, renal or liver failure, and death.3
Opiates
Opiates are metabolized by CYP2D6 and, sometimes by CYP3A4. Metabolism of opiates, such as oxycodone, is decreased when these drugs are coadministered with a CYP2D6 inhibitor—potentially leading to toxicity.
Analgesic effect may be augmented when a CYP2D6 inhibitor is started, and decreased when the agent is stopped. Inducers of CYP2D6 or CYP3A4 can lead to decreased analgesia and oxycodone withdrawal.
Efavirenz can cause methadone withdrawal. Methadone inhibition of CYP2D6 and CYP3A4 can increase the serum level of antiretroviral medications, with adverse effects and resulting poor compliance.4
Cannabis
Tetrahydrocannabinol is metabolized by CYP3A4 and CYP2D6. Cannabis and CYP3A4 inhibitor co-utilization can cause toxicity, evidenced by paranoia, hallucinations, delusions, depersonalization, tachycardia, and orthostatic hypotension. Co-exposure of antiretroviral agents in occasional Cannabis users yields only a small change metabolically; however, nonadherence has been reported more frequently in heavy users.5
Education can help
The variability of CYP genotypes makes it important to understand drug metabolism as an aid to improving outcomes among patients with HIV who abuse drugs. Because of the risk for adverse effects, discuss the dangers of substance abuse with patients for whom antiretroviral therapy has been prescribed.
Clinical education should improve compliance and prognosis in patients with HIV. Advise drug users about pharmaceutical effects and risks of co-utilization. This might help some patients limit the use of illicit substances—and will help physicians manage pharmacotherapy with greater safety.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arvguidelines/22/hiv-and-illicit-drug-users. Updated March 27, 2012. Accessed July 26, 2013.
2. Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583-598.
3. Papaseit E, Vázquez A, Pérez-Mañá C, et al. Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine,ecstasy) toxicity caused by ritonavir (RTV).
Intensive Care Med. 2012;38(7):1239-1240.
4. Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598-1613.
5. Bonn-Miller MO, Oser ML, Bucossi MM, et al. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1-10.
1. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arvguidelines/22/hiv-and-illicit-drug-users. Updated March 27, 2012. Accessed July 26, 2013.
2. Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583-598.
3. Papaseit E, Vázquez A, Pérez-Mañá C, et al. Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine,ecstasy) toxicity caused by ritonavir (RTV).
Intensive Care Med. 2012;38(7):1239-1240.
4. Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598-1613.
5. Bonn-Miller MO, Oser ML, Bucossi MM, et al. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1-10.
3,4-methylenedioxymethamphetamine, opiates
3,4-methylenedioxymethamphetamine, opiates
How to assess the merits of psychological and neuropsychological test evaluations
Psychological and neuropsychological test evaluations, like all consultative diagnostic services, can vary in quality and clinical utility. Many of these examinations provide valuable insights and helpful recommendations; regrettably, some assessments are only marginally beneficial and can contribute to diagnostic confusion and uncertainty.
When weighing the pros and cons of evaluations, consider these best practices.
Gold-standard tests ought to be in-cluded in the assessment. These include (but are not limited to) the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV); Wechsler Memory Scale-Fourth Edition (WMS-IV); Delis-Kaplan Executive Function System (D-KEFS); Wechsler Individual Achievement Test-Third Edition (WIAT-III); and the Minnesota Multiphasic Personality Inventory-2 (MMPI-2). These tests have a strong evidence base that:
• demonstrates good reliability (ie, produce consistent and accurate scores across examiners and time intervals and are relatively free of measurement error)
• demonstrates good validity (ie, have been shown to measure aspects of psychological and neuropsychological functioning that they claim to measure).
Many gold-standard tests are normed on national samples and are stratified by age, sex, ethnicity or race, educational level, and geographic region. They also include normative data based on the performance of patients who have neuropsychiatric syndromes often seen by psychiatrists in practice.1
The test battery ought to comprise cognitive and neuropsychological measures as well as affective and behavioral measures. When feasible, these tests should be supplemented by informant-based measures of neuropsychiatric functioning to obtain a comprehensive assessment of the patient’s capacities and skills.
An estimated premorbid baseline should be established. This is done by taking a relevant history and administering tests, such as the National Adult Reading Test (NART), that can be used to compare against current test performance. This testing-in-context approach helps differentiate long-term limitations in information processing, which might be attributed to a DSM-5 intellectual disability, specific learning disorder, or other neurodevelopmental disorder, from a known or suspected recent neurobehavioral change.
Tests in the assessment should tap a broad set of neurobehavioral functions. Doing so ensures that, when a patient is referred with a change in cognition or other aspects of mental status, it will be easier to determine whether clinically significant score discrepancies exist across different ability and skill domains. Such dissociations in performance can have important implications for the differential diagnosis and everyday functioning.
Tests that are sensitive to a patient’s over-reporting of symptoms should be used as part of the evaluation in cases of suspected malingering—especially subtle simulation that might elude identification with brief screening-level measures.2 These tests can include the Test of Memory Malingering (TOMM) and the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2).
Test recommendations ought to be grounded in findings; practical; and relatively easy to implement. They also should be consistent with the treatment setting and the patient’s lifestyle, values, and treatment preferences.3
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Geisinger KF, Bracken BA, Carlson JF, et al, eds. APA handbook of testing and assessment in psychology. Washington, DC: American Psychological Association Press; 2013.
2. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency department. Current Psychiatry. 2013;12(10):33-38,40.
3. McHugh RK, Whitton SW, Peckham AD, et al. Patient p for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602.
Psychological and neuropsychological test evaluations, like all consultative diagnostic services, can vary in quality and clinical utility. Many of these examinations provide valuable insights and helpful recommendations; regrettably, some assessments are only marginally beneficial and can contribute to diagnostic confusion and uncertainty.
When weighing the pros and cons of evaluations, consider these best practices.
Gold-standard tests ought to be in-cluded in the assessment. These include (but are not limited to) the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV); Wechsler Memory Scale-Fourth Edition (WMS-IV); Delis-Kaplan Executive Function System (D-KEFS); Wechsler Individual Achievement Test-Third Edition (WIAT-III); and the Minnesota Multiphasic Personality Inventory-2 (MMPI-2). These tests have a strong evidence base that:
• demonstrates good reliability (ie, produce consistent and accurate scores across examiners and time intervals and are relatively free of measurement error)
• demonstrates good validity (ie, have been shown to measure aspects of psychological and neuropsychological functioning that they claim to measure).
Many gold-standard tests are normed on national samples and are stratified by age, sex, ethnicity or race, educational level, and geographic region. They also include normative data based on the performance of patients who have neuropsychiatric syndromes often seen by psychiatrists in practice.1
The test battery ought to comprise cognitive and neuropsychological measures as well as affective and behavioral measures. When feasible, these tests should be supplemented by informant-based measures of neuropsychiatric functioning to obtain a comprehensive assessment of the patient’s capacities and skills.
An estimated premorbid baseline should be established. This is done by taking a relevant history and administering tests, such as the National Adult Reading Test (NART), that can be used to compare against current test performance. This testing-in-context approach helps differentiate long-term limitations in information processing, which might be attributed to a DSM-5 intellectual disability, specific learning disorder, or other neurodevelopmental disorder, from a known or suspected recent neurobehavioral change.
Tests in the assessment should tap a broad set of neurobehavioral functions. Doing so ensures that, when a patient is referred with a change in cognition or other aspects of mental status, it will be easier to determine whether clinically significant score discrepancies exist across different ability and skill domains. Such dissociations in performance can have important implications for the differential diagnosis and everyday functioning.
Tests that are sensitive to a patient’s over-reporting of symptoms should be used as part of the evaluation in cases of suspected malingering—especially subtle simulation that might elude identification with brief screening-level measures.2 These tests can include the Test of Memory Malingering (TOMM) and the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2).
Test recommendations ought to be grounded in findings; practical; and relatively easy to implement. They also should be consistent with the treatment setting and the patient’s lifestyle, values, and treatment preferences.3
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychological and neuropsychological test evaluations, like all consultative diagnostic services, can vary in quality and clinical utility. Many of these examinations provide valuable insights and helpful recommendations; regrettably, some assessments are only marginally beneficial and can contribute to diagnostic confusion and uncertainty.
When weighing the pros and cons of evaluations, consider these best practices.
Gold-standard tests ought to be in-cluded in the assessment. These include (but are not limited to) the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV); Wechsler Memory Scale-Fourth Edition (WMS-IV); Delis-Kaplan Executive Function System (D-KEFS); Wechsler Individual Achievement Test-Third Edition (WIAT-III); and the Minnesota Multiphasic Personality Inventory-2 (MMPI-2). These tests have a strong evidence base that:
• demonstrates good reliability (ie, produce consistent and accurate scores across examiners and time intervals and are relatively free of measurement error)
• demonstrates good validity (ie, have been shown to measure aspects of psychological and neuropsychological functioning that they claim to measure).
Many gold-standard tests are normed on national samples and are stratified by age, sex, ethnicity or race, educational level, and geographic region. They also include normative data based on the performance of patients who have neuropsychiatric syndromes often seen by psychiatrists in practice.1
The test battery ought to comprise cognitive and neuropsychological measures as well as affective and behavioral measures. When feasible, these tests should be supplemented by informant-based measures of neuropsychiatric functioning to obtain a comprehensive assessment of the patient’s capacities and skills.
An estimated premorbid baseline should be established. This is done by taking a relevant history and administering tests, such as the National Adult Reading Test (NART), that can be used to compare against current test performance. This testing-in-context approach helps differentiate long-term limitations in information processing, which might be attributed to a DSM-5 intellectual disability, specific learning disorder, or other neurodevelopmental disorder, from a known or suspected recent neurobehavioral change.
Tests in the assessment should tap a broad set of neurobehavioral functions. Doing so ensures that, when a patient is referred with a change in cognition or other aspects of mental status, it will be easier to determine whether clinically significant score discrepancies exist across different ability and skill domains. Such dissociations in performance can have important implications for the differential diagnosis and everyday functioning.
Tests that are sensitive to a patient’s over-reporting of symptoms should be used as part of the evaluation in cases of suspected malingering—especially subtle simulation that might elude identification with brief screening-level measures.2 These tests can include the Test of Memory Malingering (TOMM) and the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2).
Test recommendations ought to be grounded in findings; practical; and relatively easy to implement. They also should be consistent with the treatment setting and the patient’s lifestyle, values, and treatment preferences.3
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Geisinger KF, Bracken BA, Carlson JF, et al, eds. APA handbook of testing and assessment in psychology. Washington, DC: American Psychological Association Press; 2013.
2. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency department. Current Psychiatry. 2013;12(10):33-38,40.
3. McHugh RK, Whitton SW, Peckham AD, et al. Patient p for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602.
1. Geisinger KF, Bracken BA, Carlson JF, et al, eds. APA handbook of testing and assessment in psychology. Washington, DC: American Psychological Association Press; 2013.
2. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency department. Current Psychiatry. 2013;12(10):33-38,40.
3. McHugh RK, Whitton SW, Peckham AD, et al. Patient p for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602.
Be prepared to adjust dosing of psychotropics after bariatric surgery
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
Will finding the depression−inflammation link lead to tailored treatments for MDD?
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
How to document SUICIDE risk
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
Help your patient with hoarding disorder move the clutter to the curb
Hoarding disorder (HD), categorized in DSM-5 under obsessive-compulsive and related disorders, is defined as the “persistent difficulty discarding or parting with possessions, regardless of their actual value.”1 Hoarders feel that they need to save items, and experience distress when discarding them. Prevalence of HD among the general population is 2% to 5%.
Compulsive hoarders usually keep old items in their home that they do not intend to use. In severe cases, the clutter is so great that areas of the home cannot be used or entered. Hoarders tend to isolate themselves and usually do not invite people home, perhaps because they are embarrassed about the clutter or anxious that someone might try to clean the house. Hoarders may travel long distances to collect items others have discarded.
Hoarding can lead to psychiatric disorders and social problems. Hoarders tend to not develop attachment with people because they are more attached to their possessions. They may avoid social interactions; in turn, others avoid them. This isolation can lead to depression, anxiety, and substance abuse. Hoarders may be evicted from their home if the clutter makes the house dangerous or unfit to live in it. Compulsive hoarding is detrimental to the hoarder and the health and well-being of family members. Hoarding can coexist or can be result of other psychiatric disorders (Table).
Neural mechanism in hoarding
Hoarders may start to accumulate and store large quantities of items because of a cognitive deficit, such as trouble making decisions or poor recognition or acknowledgement of the situation, or maladaptive thoughts. Tolin et al1 found the anterior cingulate cortex and insula was stimulus-dependent in patients with HD. Functional MRI showed when patients with HD were shown an item that was their possession, they exhibited an abnormal brain activity, compared with low activity when the items shown were not theirs.
Interventions
Choice of treatment depends on the age of the patient and severity of illness: behavioral, medical, or a combination of both. For an uncomplicated case, management can begin with behavioral modification.
Behavioral modifications. HD can stem from any of several variables, including greater response latency for decision-making about possessions and maladaptive beliefs about, and emotional attachment to, possessions, which can lead to intense emotional experiences about the prospect of losing those possessions.2 Cognitive-behavioral therapy has shown promising results for treating HD by addressing the aforementioned factors. A step-by-step approach usually is feasible and convenient for the therapist and patient. It involves gradual mental detachment from items to accommodate the patient’s pace.2
Pharmacotherapy. There is no clear evidence for treating HD with any particular drug. Hoarders are less likely to use psychotropics, possibly because of poor insight (eg, they do not realize the potentially dangerous living conditions hoarding creates).3 Because HD is related to obsessive-compulsive disorder, it is intuitive to consider a selective serotonin reuptake inhibitor.
There is still a need for more research on management of HD.
Disclosure
Dr. Silman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tolin DF, Stevens MC, Villavicencio AL, et al. Neural mechanism of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832-841.
2. Tolin DF, Frost RO, Steketee G. An open trial of cognitivebehavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461-1470.
3. Brakoulias V, Starcevic V, Berle D, et al. The use of psychotropic agents for the symptoms of obsessivecompulsive disorder. Australas Psychiatry. 2013;21(2): 117-121.
Hoarding disorder (HD), categorized in DSM-5 under obsessive-compulsive and related disorders, is defined as the “persistent difficulty discarding or parting with possessions, regardless of their actual value.”1 Hoarders feel that they need to save items, and experience distress when discarding them. Prevalence of HD among the general population is 2% to 5%.
Compulsive hoarders usually keep old items in their home that they do not intend to use. In severe cases, the clutter is so great that areas of the home cannot be used or entered. Hoarders tend to isolate themselves and usually do not invite people home, perhaps because they are embarrassed about the clutter or anxious that someone might try to clean the house. Hoarders may travel long distances to collect items others have discarded.
Hoarding can lead to psychiatric disorders and social problems. Hoarders tend to not develop attachment with people because they are more attached to their possessions. They may avoid social interactions; in turn, others avoid them. This isolation can lead to depression, anxiety, and substance abuse. Hoarders may be evicted from their home if the clutter makes the house dangerous or unfit to live in it. Compulsive hoarding is detrimental to the hoarder and the health and well-being of family members. Hoarding can coexist or can be result of other psychiatric disorders (Table).
Neural mechanism in hoarding
Hoarders may start to accumulate and store large quantities of items because of a cognitive deficit, such as trouble making decisions or poor recognition or acknowledgement of the situation, or maladaptive thoughts. Tolin et al1 found the anterior cingulate cortex and insula was stimulus-dependent in patients with HD. Functional MRI showed when patients with HD were shown an item that was their possession, they exhibited an abnormal brain activity, compared with low activity when the items shown were not theirs.
Interventions
Choice of treatment depends on the age of the patient and severity of illness: behavioral, medical, or a combination of both. For an uncomplicated case, management can begin with behavioral modification.
Behavioral modifications. HD can stem from any of several variables, including greater response latency for decision-making about possessions and maladaptive beliefs about, and emotional attachment to, possessions, which can lead to intense emotional experiences about the prospect of losing those possessions.2 Cognitive-behavioral therapy has shown promising results for treating HD by addressing the aforementioned factors. A step-by-step approach usually is feasible and convenient for the therapist and patient. It involves gradual mental detachment from items to accommodate the patient’s pace.2
Pharmacotherapy. There is no clear evidence for treating HD with any particular drug. Hoarders are less likely to use psychotropics, possibly because of poor insight (eg, they do not realize the potentially dangerous living conditions hoarding creates).3 Because HD is related to obsessive-compulsive disorder, it is intuitive to consider a selective serotonin reuptake inhibitor.
There is still a need for more research on management of HD.
Disclosure
Dr. Silman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Hoarding disorder (HD), categorized in DSM-5 under obsessive-compulsive and related disorders, is defined as the “persistent difficulty discarding or parting with possessions, regardless of their actual value.”1 Hoarders feel that they need to save items, and experience distress when discarding them. Prevalence of HD among the general population is 2% to 5%.
Compulsive hoarders usually keep old items in their home that they do not intend to use. In severe cases, the clutter is so great that areas of the home cannot be used or entered. Hoarders tend to isolate themselves and usually do not invite people home, perhaps because they are embarrassed about the clutter or anxious that someone might try to clean the house. Hoarders may travel long distances to collect items others have discarded.
Hoarding can lead to psychiatric disorders and social problems. Hoarders tend to not develop attachment with people because they are more attached to their possessions. They may avoid social interactions; in turn, others avoid them. This isolation can lead to depression, anxiety, and substance abuse. Hoarders may be evicted from their home if the clutter makes the house dangerous or unfit to live in it. Compulsive hoarding is detrimental to the hoarder and the health and well-being of family members. Hoarding can coexist or can be result of other psychiatric disorders (Table).
Neural mechanism in hoarding
Hoarders may start to accumulate and store large quantities of items because of a cognitive deficit, such as trouble making decisions or poor recognition or acknowledgement of the situation, or maladaptive thoughts. Tolin et al1 found the anterior cingulate cortex and insula was stimulus-dependent in patients with HD. Functional MRI showed when patients with HD were shown an item that was their possession, they exhibited an abnormal brain activity, compared with low activity when the items shown were not theirs.
Interventions
Choice of treatment depends on the age of the patient and severity of illness: behavioral, medical, or a combination of both. For an uncomplicated case, management can begin with behavioral modification.
Behavioral modifications. HD can stem from any of several variables, including greater response latency for decision-making about possessions and maladaptive beliefs about, and emotional attachment to, possessions, which can lead to intense emotional experiences about the prospect of losing those possessions.2 Cognitive-behavioral therapy has shown promising results for treating HD by addressing the aforementioned factors. A step-by-step approach usually is feasible and convenient for the therapist and patient. It involves gradual mental detachment from items to accommodate the patient’s pace.2
Pharmacotherapy. There is no clear evidence for treating HD with any particular drug. Hoarders are less likely to use psychotropics, possibly because of poor insight (eg, they do not realize the potentially dangerous living conditions hoarding creates).3 Because HD is related to obsessive-compulsive disorder, it is intuitive to consider a selective serotonin reuptake inhibitor.
There is still a need for more research on management of HD.
Disclosure
Dr. Silman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tolin DF, Stevens MC, Villavicencio AL, et al. Neural mechanism of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832-841.
2. Tolin DF, Frost RO, Steketee G. An open trial of cognitivebehavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461-1470.
3. Brakoulias V, Starcevic V, Berle D, et al. The use of psychotropic agents for the symptoms of obsessivecompulsive disorder. Australas Psychiatry. 2013;21(2): 117-121.
1. Tolin DF, Stevens MC, Villavicencio AL, et al. Neural mechanism of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832-841.
2. Tolin DF, Frost RO, Steketee G. An open trial of cognitivebehavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461-1470.
3. Brakoulias V, Starcevic V, Berle D, et al. The use of psychotropic agents for the symptoms of obsessivecompulsive disorder. Australas Psychiatry. 2013;21(2): 117-121.
8 tests rolled into a mnemonic to detect weakness in suspected conversion disorder
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
Should lithium and ECT be used concurrently in geriatric patients?
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.