User login
Summertime and Mosquitoes Are Breeding
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia. Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases. Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia. Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases. Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia. Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases. Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
Using the Road Map
I had a premed college student with me, a young lady trying to figure out if medicine was for her, and what exactly a neurologist does.
The patient, a gentlemen in his mid-70s, had just left. He had some unusual symptoms. Not implausible, but the kind of case where the answers don’t come together easily. I’d ordered tests for the usual suspects and walked him up front.
When I got back she asked me “what do you think is wrong with him?”
Without thinking I said “I have no idea.” By this time I’d turned to some scheduling messages from my secretary, and didn’t register the student’s surprise for a moment.
I mean, I’m an attending physician. To her I’m the epitome of the career. I got accepted to (and survived) medical school. I made it through residency and fellowship and have almost 26 years of trench-earned experience behind me (hard to believe for me, too, sometimes). And yet I’d just said I didn’t know what was going on.
Reversing the roles and thinking back to the late 1980s, I probably would have felt the same way she did.
Of course “I have no idea” is a bit of unintentional hyperbole. I have some idea, just not a clear answer yet. I’d turned over the possible locations and causes, and so ordered tests to help narrow it down. As one of my attendings in residency used to say, “some days you need a rifle, some days a shotgun” to figure it out.
Being a doctor, even a good one (I hope I am, but not making any guarantees) doesn’t mean you know everything, or have the ability to figure it out immediately. Otherwise we wouldn’t need imaging, labs, and a host of other tests. Sherlock Holmes was a lot of things, but Watson was the doctor.
To those at the beginning of their careers, just like it was to us then, this is a revelation. Aren’t we supposed to know everything? We probably once believed we would, too, someday.
What combination of tests and decisions will hopefully lead us to the correct point.
Most of us realize that intuitively at this point, but it can be hard to explain to others. We have patients ask “what do you think is going on?” and we often have no answer other than “not sure yet, but I’ll try to find out.”
We don’t realize how far we’ve come until we see ourselves in someone who’s starting the same journey. And that’s something you can’t teach.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I had a premed college student with me, a young lady trying to figure out if medicine was for her, and what exactly a neurologist does.
The patient, a gentlemen in his mid-70s, had just left. He had some unusual symptoms. Not implausible, but the kind of case where the answers don’t come together easily. I’d ordered tests for the usual suspects and walked him up front.
When I got back she asked me “what do you think is wrong with him?”
Without thinking I said “I have no idea.” By this time I’d turned to some scheduling messages from my secretary, and didn’t register the student’s surprise for a moment.
I mean, I’m an attending physician. To her I’m the epitome of the career. I got accepted to (and survived) medical school. I made it through residency and fellowship and have almost 26 years of trench-earned experience behind me (hard to believe for me, too, sometimes). And yet I’d just said I didn’t know what was going on.
Reversing the roles and thinking back to the late 1980s, I probably would have felt the same way she did.
Of course “I have no idea” is a bit of unintentional hyperbole. I have some idea, just not a clear answer yet. I’d turned over the possible locations and causes, and so ordered tests to help narrow it down. As one of my attendings in residency used to say, “some days you need a rifle, some days a shotgun” to figure it out.
Being a doctor, even a good one (I hope I am, but not making any guarantees) doesn’t mean you know everything, or have the ability to figure it out immediately. Otherwise we wouldn’t need imaging, labs, and a host of other tests. Sherlock Holmes was a lot of things, but Watson was the doctor.
To those at the beginning of their careers, just like it was to us then, this is a revelation. Aren’t we supposed to know everything? We probably once believed we would, too, someday.
What combination of tests and decisions will hopefully lead us to the correct point.
Most of us realize that intuitively at this point, but it can be hard to explain to others. We have patients ask “what do you think is going on?” and we often have no answer other than “not sure yet, but I’ll try to find out.”
We don’t realize how far we’ve come until we see ourselves in someone who’s starting the same journey. And that’s something you can’t teach.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I had a premed college student with me, a young lady trying to figure out if medicine was for her, and what exactly a neurologist does.
The patient, a gentlemen in his mid-70s, had just left. He had some unusual symptoms. Not implausible, but the kind of case where the answers don’t come together easily. I’d ordered tests for the usual suspects and walked him up front.
When I got back she asked me “what do you think is wrong with him?”
Without thinking I said “I have no idea.” By this time I’d turned to some scheduling messages from my secretary, and didn’t register the student’s surprise for a moment.
I mean, I’m an attending physician. To her I’m the epitome of the career. I got accepted to (and survived) medical school. I made it through residency and fellowship and have almost 26 years of trench-earned experience behind me (hard to believe for me, too, sometimes). And yet I’d just said I didn’t know what was going on.
Reversing the roles and thinking back to the late 1980s, I probably would have felt the same way she did.
Of course “I have no idea” is a bit of unintentional hyperbole. I have some idea, just not a clear answer yet. I’d turned over the possible locations and causes, and so ordered tests to help narrow it down. As one of my attendings in residency used to say, “some days you need a rifle, some days a shotgun” to figure it out.
Being a doctor, even a good one (I hope I am, but not making any guarantees) doesn’t mean you know everything, or have the ability to figure it out immediately. Otherwise we wouldn’t need imaging, labs, and a host of other tests. Sherlock Holmes was a lot of things, but Watson was the doctor.
To those at the beginning of their careers, just like it was to us then, this is a revelation. Aren’t we supposed to know everything? We probably once believed we would, too, someday.
What combination of tests and decisions will hopefully lead us to the correct point.
Most of us realize that intuitively at this point, but it can be hard to explain to others. We have patients ask “what do you think is going on?” and we often have no answer other than “not sure yet, but I’ll try to find out.”
We don’t realize how far we’ve come until we see ourselves in someone who’s starting the same journey. And that’s something you can’t teach.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
ERISA Health Plan Lawsuits: Why Should We Care?
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.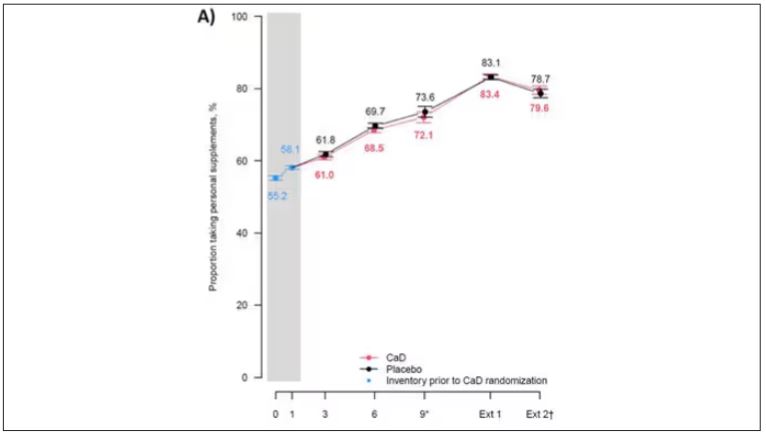
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.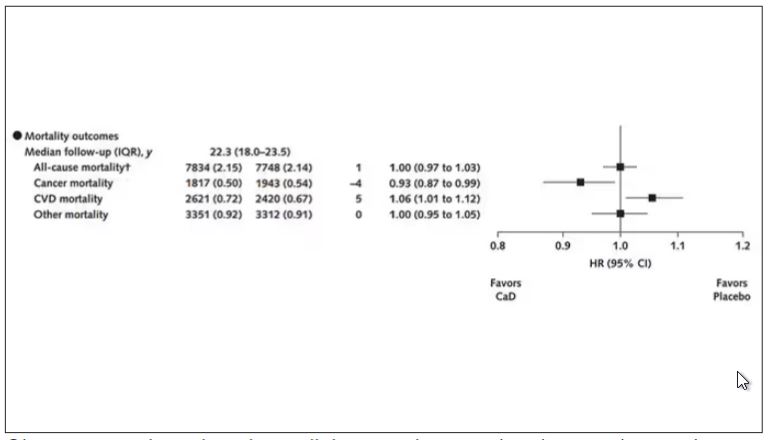
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.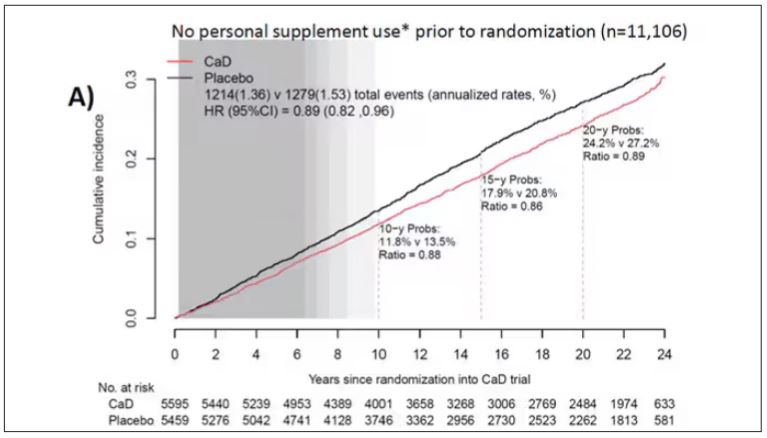
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.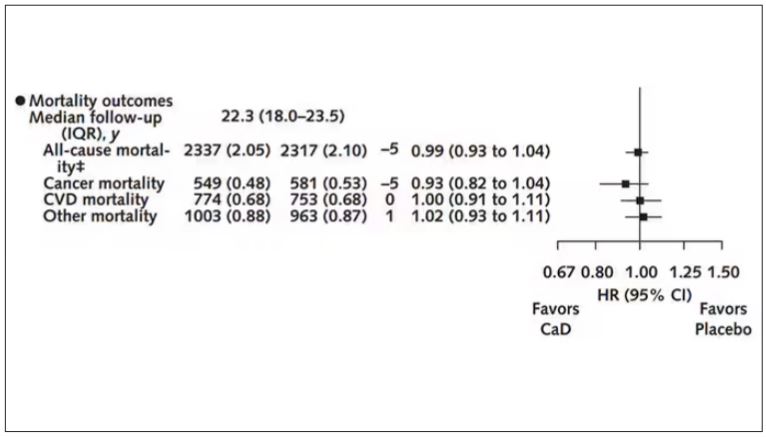
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
Survival Advantage of Adjuvant IO ‘Big News’ in Renal Cancer
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
The Urethra Is a Sex Organ; Why This Matters in Incontinence
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. We are coming to you live from the Mayo Clinic Urology Conference in Maui, Hawaii, with the world’s leading experts in men’s health, sexual health, and quality of life. I’m bringing in Dr. Allen Morey from the North Dallas area, one of the world’s leading experts in reconstructive urology. He deals with all things urinary incontinence, penile curvature, and sexual health.
Dr. Morey said something at this conference that really put my chin on the floor. He said, So, Dr. Morey, tell us more about why you made that comment and about the incontinence in men that you deal with all the time.
Allen F. Morey, MD: For many years, I’ve worked at cancer centers where, through the various treatments for prostate cancer, the men suffered from urinary incontinence and we put a lot of artificial urinary sphincters in those patients. I had one patient who asked, “Why do I keep having this erosion?” Of course, the erosion is where the cuff compresses the tissue surrounding the urethra, and the tissue gives way, leaving a hole in the urethra. I looked at him and noticed that he was pale. And I thought, Let me check his testosterone level. We started checking it on everybody who had this problem, and sure enough, the ones who had the cup erosion — who had the atrophic tissue around the urethra — most of them had low testosterone levels. Some of this was due to the cancer treatment, but in other men, it was just due to old age.
We started thinking that this is a causal relationship. And we tested it. I had a fellow who was a board-certified pathologist before becoming a urologist. He obtained some specimens from the urethra and did very sophisticated, elegant stains on that tissue. We found that it’s just erectile tissue surrounding the urethra. That’s why I call it a sex organ. I tell my patients, “When you are a teenager, this tissue is thin, like on your pinky finger. As you get older, it becomes thicker. And then as you get even older, and you may be having cancer treatment, that tissue is gone.” You can show them your little finger; it’s about that size. All the meat is off the bone. There’s nothing left protecting the urinary mucosa from the device.
That’s why it’s important to maintain the optimal health of those tissues and for them to remain dry. Because let’s face it: Urinary incontinence is a horrible quality of life. These are my happiest patients when we fix it. Before that, when they go on vacation, they have a separate suitcase filled with diapers. They can’t go anywhere. They can’t do anything. When they become incontinent after the prostatectomy, they gain weight. And when you put in the device to treat it, they lose weight and you can track it. The urinary incontinence patients really suffer. And we need to consider the medical optimization of those patients.
Dr. Rubin: It’s so important, and I love how analogous this is to our female patients. We know that incontinence is devastating to our female patients as well, and there’s a lot of hope. As we get older we start to pee every time we cough, laugh, or sneeze. Men are a little more bothered by it when it happens; they don’t expect it. Among women, it’s thought of as normal, but it’s not normal. There is so much we can do to help these patients, ranging from conservative treatment to surgical therapies.
The connection between hormones and urethral health is true on the female side as well. As you go through menopause, the urethral tissue thins out, gets dry, gets irritated, and can cause worsening incontinence, pain with sex, and genital urinary syndrome of menopause. It’s really important.
For our primary care doctors, how should we talk about stress incontinence in men? How do we diagnose it?
Dr. Morey: It’s easy to diagnose. Just do a quick history. Find out how many pads a day they are using. You have to ask the question, and then you have them stand up and look inside their underwear. You’ll see what kind of pad they are wearing. Is it just a shield or are they actually wearing full diapers? Then I have them do a standing cough test. I stand off to the side, holding a couple of towels, and have the patient cough four times. I can tell if it’s a full stream or just a couple of drops. Is it nothing but they are wearing a pad? You match up what you see with their experience, and in an instant it tells you how severe their problem is and it helps you direct them on to further treatment, because many patients have treatment fatigue. They’ve already been through the system. They have really suffered and they don’t know which way to go. They don’t know what’s available.
Dr. Rubin: On the female side, we have pelvic floor physical therapy. We have pads and devices that you can wear, and pessaries. We have surgical options, like bulking agents into the urethra as well as urethral slings, which can be quite helpful for women. So there’s a lot of hope out there for women, and from what I learned from you at this conference, there’s a lot of hope for men as well. So talk us through treatment, from conservative to surgical options.
Dr. Morey: There hasn’t been much innovation in male incontinence treatment over the past few decades, but we’re starting to see signs of new products appearing on the horizon, so I’m very optimistic that in the next 5 or 10 years, we’ll have more. But right now it comes down to slings and artificial sphincters, which are devices with little pumps and hydraulics, and they’re very good. But they’ve been around for 50 years, and they have this other potential risk factor of the erosion of the tissue.
We don’t have a pill that we can give the patient to tighten up those muscles. We can help them with overactive bladder. But maybe the hormonal influence is a way to optimize the health of the tissue so that these surgical treatments can really deliver the best outcomes. And as I always say, and having treated so many of these patients, it’s really a game of millimeters — how much coaptation you get. If you’re off by the slightest amount, that’s an unhappy patient. So it doesn’t take much to make it a lot better.
Dr. Rubin: There’s so much hope for our patients, and this can really have an effect on sexual health. You know the benefits you see in their quality of life and sexual health when you can stop leakage.
Dr. Morey: I always take care of the waterworks first. Many of the men have both urinary problems and erectile problems. Nobody feels sexy when they’re leaking urine all over their partner. So first we take care of that. And then, in the motivated younger patients, we bring them back and talk to them about potentially having a second operation.
Dr. Rubin: And so similarly, in women with urinary incontinence, it can have a major impact on sexual health — how they show up and how they talk to their partner. So, it is really important for our primary care docs to talk to patients about urinary incontinence and not just say, “Oh, well, you’re getting older. There’s nothing that you can do.” There’s actually no age at which there is nothing that we can do. And it’s really important to refer patients to those urologists who have extra training in incontinence and sexual health, because we do care about these quality-of-life measures and there is a lot we can do, ranging from conservative to more invasive treatments. But patients really should have options.
Dr. Morey: I heard during this meeting that urinary incontinence was the number-one source of treatment regret among patients who had their prostate treated for cancer. So, this is a really big deal for our patients. And it impacts wellness, quality of life, and overall well-being.
Dr. Rubin: When we are counseling patients for cancer surgeries or cancer treatments such as radiation therapy, it’s really hard for the patients who have never had urinary incontinence to imagine what that might be like. When you’re telling them they could have a stroke or a heart attack, or they could have erectile dysfunction or urinary incontinence, it all sounds similar to them. It could happen to someone else. It’s very hard to truly counsel patients on these quality-of-life issues that they’ve never encountered before.
Dr. Morey: We have found that it takes a long time for patients to get into our office for treatment, and it’s unbelievable — often 5 years in diapers before they find us.
Dr. Rubin: Hopefully videos like this will teach our docs and our patients that there is hope out there, that you don’t need to wait through years of suffering from incontinence. So, how does somebody find a reconstructive urologist or a sexual medicine urologist?
Dr. Morey: There are a couple of good websites out there, such as fixincontinence.com and edcure.org. The device manufacturers have pretty good information for patients.
Dr. Rubin: The Sexual Medicine Society of North America (SMSNA) has a great find-a-provider website, which provides a list of urologists who are trained in both sexual health and urinary incontinence, because they both matter. Our patients deeply care about these issues.
Rachel S. Rubin, MD, has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. We are coming to you live from the Mayo Clinic Urology Conference in Maui, Hawaii, with the world’s leading experts in men’s health, sexual health, and quality of life. I’m bringing in Dr. Allen Morey from the North Dallas area, one of the world’s leading experts in reconstructive urology. He deals with all things urinary incontinence, penile curvature, and sexual health.
Dr. Morey said something at this conference that really put my chin on the floor. He said, So, Dr. Morey, tell us more about why you made that comment and about the incontinence in men that you deal with all the time.
Allen F. Morey, MD: For many years, I’ve worked at cancer centers where, through the various treatments for prostate cancer, the men suffered from urinary incontinence and we put a lot of artificial urinary sphincters in those patients. I had one patient who asked, “Why do I keep having this erosion?” Of course, the erosion is where the cuff compresses the tissue surrounding the urethra, and the tissue gives way, leaving a hole in the urethra. I looked at him and noticed that he was pale. And I thought, Let me check his testosterone level. We started checking it on everybody who had this problem, and sure enough, the ones who had the cup erosion — who had the atrophic tissue around the urethra — most of them had low testosterone levels. Some of this was due to the cancer treatment, but in other men, it was just due to old age.
We started thinking that this is a causal relationship. And we tested it. I had a fellow who was a board-certified pathologist before becoming a urologist. He obtained some specimens from the urethra and did very sophisticated, elegant stains on that tissue. We found that it’s just erectile tissue surrounding the urethra. That’s why I call it a sex organ. I tell my patients, “When you are a teenager, this tissue is thin, like on your pinky finger. As you get older, it becomes thicker. And then as you get even older, and you may be having cancer treatment, that tissue is gone.” You can show them your little finger; it’s about that size. All the meat is off the bone. There’s nothing left protecting the urinary mucosa from the device.
That’s why it’s important to maintain the optimal health of those tissues and for them to remain dry. Because let’s face it: Urinary incontinence is a horrible quality of life. These are my happiest patients when we fix it. Before that, when they go on vacation, they have a separate suitcase filled with diapers. They can’t go anywhere. They can’t do anything. When they become incontinent after the prostatectomy, they gain weight. And when you put in the device to treat it, they lose weight and you can track it. The urinary incontinence patients really suffer. And we need to consider the medical optimization of those patients.
Dr. Rubin: It’s so important, and I love how analogous this is to our female patients. We know that incontinence is devastating to our female patients as well, and there’s a lot of hope. As we get older we start to pee every time we cough, laugh, or sneeze. Men are a little more bothered by it when it happens; they don’t expect it. Among women, it’s thought of as normal, but it’s not normal. There is so much we can do to help these patients, ranging from conservative treatment to surgical therapies.
The connection between hormones and urethral health is true on the female side as well. As you go through menopause, the urethral tissue thins out, gets dry, gets irritated, and can cause worsening incontinence, pain with sex, and genital urinary syndrome of menopause. It’s really important.
For our primary care doctors, how should we talk about stress incontinence in men? How do we diagnose it?
Dr. Morey: It’s easy to diagnose. Just do a quick history. Find out how many pads a day they are using. You have to ask the question, and then you have them stand up and look inside their underwear. You’ll see what kind of pad they are wearing. Is it just a shield or are they actually wearing full diapers? Then I have them do a standing cough test. I stand off to the side, holding a couple of towels, and have the patient cough four times. I can tell if it’s a full stream or just a couple of drops. Is it nothing but they are wearing a pad? You match up what you see with their experience, and in an instant it tells you how severe their problem is and it helps you direct them on to further treatment, because many patients have treatment fatigue. They’ve already been through the system. They have really suffered and they don’t know which way to go. They don’t know what’s available.
Dr. Rubin: On the female side, we have pelvic floor physical therapy. We have pads and devices that you can wear, and pessaries. We have surgical options, like bulking agents into the urethra as well as urethral slings, which can be quite helpful for women. So there’s a lot of hope out there for women, and from what I learned from you at this conference, there’s a lot of hope for men as well. So talk us through treatment, from conservative to surgical options.
Dr. Morey: There hasn’t been much innovation in male incontinence treatment over the past few decades, but we’re starting to see signs of new products appearing on the horizon, so I’m very optimistic that in the next 5 or 10 years, we’ll have more. But right now it comes down to slings and artificial sphincters, which are devices with little pumps and hydraulics, and they’re very good. But they’ve been around for 50 years, and they have this other potential risk factor of the erosion of the tissue.
We don’t have a pill that we can give the patient to tighten up those muscles. We can help them with overactive bladder. But maybe the hormonal influence is a way to optimize the health of the tissue so that these surgical treatments can really deliver the best outcomes. And as I always say, and having treated so many of these patients, it’s really a game of millimeters — how much coaptation you get. If you’re off by the slightest amount, that’s an unhappy patient. So it doesn’t take much to make it a lot better.
Dr. Rubin: There’s so much hope for our patients, and this can really have an effect on sexual health. You know the benefits you see in their quality of life and sexual health when you can stop leakage.
Dr. Morey: I always take care of the waterworks first. Many of the men have both urinary problems and erectile problems. Nobody feels sexy when they’re leaking urine all over their partner. So first we take care of that. And then, in the motivated younger patients, we bring them back and talk to them about potentially having a second operation.
Dr. Rubin: And so similarly, in women with urinary incontinence, it can have a major impact on sexual health — how they show up and how they talk to their partner. So, it is really important for our primary care docs to talk to patients about urinary incontinence and not just say, “Oh, well, you’re getting older. There’s nothing that you can do.” There’s actually no age at which there is nothing that we can do. And it’s really important to refer patients to those urologists who have extra training in incontinence and sexual health, because we do care about these quality-of-life measures and there is a lot we can do, ranging from conservative to more invasive treatments. But patients really should have options.
Dr. Morey: I heard during this meeting that urinary incontinence was the number-one source of treatment regret among patients who had their prostate treated for cancer. So, this is a really big deal for our patients. And it impacts wellness, quality of life, and overall well-being.
Dr. Rubin: When we are counseling patients for cancer surgeries or cancer treatments such as radiation therapy, it’s really hard for the patients who have never had urinary incontinence to imagine what that might be like. When you’re telling them they could have a stroke or a heart attack, or they could have erectile dysfunction or urinary incontinence, it all sounds similar to them. It could happen to someone else. It’s very hard to truly counsel patients on these quality-of-life issues that they’ve never encountered before.
Dr. Morey: We have found that it takes a long time for patients to get into our office for treatment, and it’s unbelievable — often 5 years in diapers before they find us.
Dr. Rubin: Hopefully videos like this will teach our docs and our patients that there is hope out there, that you don’t need to wait through years of suffering from incontinence. So, how does somebody find a reconstructive urologist or a sexual medicine urologist?
Dr. Morey: There are a couple of good websites out there, such as fixincontinence.com and edcure.org. The device manufacturers have pretty good information for patients.
Dr. Rubin: The Sexual Medicine Society of North America (SMSNA) has a great find-a-provider website, which provides a list of urologists who are trained in both sexual health and urinary incontinence, because they both matter. Our patients deeply care about these issues.
Rachel S. Rubin, MD, has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. We are coming to you live from the Mayo Clinic Urology Conference in Maui, Hawaii, with the world’s leading experts in men’s health, sexual health, and quality of life. I’m bringing in Dr. Allen Morey from the North Dallas area, one of the world’s leading experts in reconstructive urology. He deals with all things urinary incontinence, penile curvature, and sexual health.
Dr. Morey said something at this conference that really put my chin on the floor. He said, So, Dr. Morey, tell us more about why you made that comment and about the incontinence in men that you deal with all the time.
Allen F. Morey, MD: For many years, I’ve worked at cancer centers where, through the various treatments for prostate cancer, the men suffered from urinary incontinence and we put a lot of artificial urinary sphincters in those patients. I had one patient who asked, “Why do I keep having this erosion?” Of course, the erosion is where the cuff compresses the tissue surrounding the urethra, and the tissue gives way, leaving a hole in the urethra. I looked at him and noticed that he was pale. And I thought, Let me check his testosterone level. We started checking it on everybody who had this problem, and sure enough, the ones who had the cup erosion — who had the atrophic tissue around the urethra — most of them had low testosterone levels. Some of this was due to the cancer treatment, but in other men, it was just due to old age.
We started thinking that this is a causal relationship. And we tested it. I had a fellow who was a board-certified pathologist before becoming a urologist. He obtained some specimens from the urethra and did very sophisticated, elegant stains on that tissue. We found that it’s just erectile tissue surrounding the urethra. That’s why I call it a sex organ. I tell my patients, “When you are a teenager, this tissue is thin, like on your pinky finger. As you get older, it becomes thicker. And then as you get even older, and you may be having cancer treatment, that tissue is gone.” You can show them your little finger; it’s about that size. All the meat is off the bone. There’s nothing left protecting the urinary mucosa from the device.
That’s why it’s important to maintain the optimal health of those tissues and for them to remain dry. Because let’s face it: Urinary incontinence is a horrible quality of life. These are my happiest patients when we fix it. Before that, when they go on vacation, they have a separate suitcase filled with diapers. They can’t go anywhere. They can’t do anything. When they become incontinent after the prostatectomy, they gain weight. And when you put in the device to treat it, they lose weight and you can track it. The urinary incontinence patients really suffer. And we need to consider the medical optimization of those patients.
Dr. Rubin: It’s so important, and I love how analogous this is to our female patients. We know that incontinence is devastating to our female patients as well, and there’s a lot of hope. As we get older we start to pee every time we cough, laugh, or sneeze. Men are a little more bothered by it when it happens; they don’t expect it. Among women, it’s thought of as normal, but it’s not normal. There is so much we can do to help these patients, ranging from conservative treatment to surgical therapies.
The connection between hormones and urethral health is true on the female side as well. As you go through menopause, the urethral tissue thins out, gets dry, gets irritated, and can cause worsening incontinence, pain with sex, and genital urinary syndrome of menopause. It’s really important.
For our primary care doctors, how should we talk about stress incontinence in men? How do we diagnose it?
Dr. Morey: It’s easy to diagnose. Just do a quick history. Find out how many pads a day they are using. You have to ask the question, and then you have them stand up and look inside their underwear. You’ll see what kind of pad they are wearing. Is it just a shield or are they actually wearing full diapers? Then I have them do a standing cough test. I stand off to the side, holding a couple of towels, and have the patient cough four times. I can tell if it’s a full stream or just a couple of drops. Is it nothing but they are wearing a pad? You match up what you see with their experience, and in an instant it tells you how severe their problem is and it helps you direct them on to further treatment, because many patients have treatment fatigue. They’ve already been through the system. They have really suffered and they don’t know which way to go. They don’t know what’s available.
Dr. Rubin: On the female side, we have pelvic floor physical therapy. We have pads and devices that you can wear, and pessaries. We have surgical options, like bulking agents into the urethra as well as urethral slings, which can be quite helpful for women. So there’s a lot of hope out there for women, and from what I learned from you at this conference, there’s a lot of hope for men as well. So talk us through treatment, from conservative to surgical options.
Dr. Morey: There hasn’t been much innovation in male incontinence treatment over the past few decades, but we’re starting to see signs of new products appearing on the horizon, so I’m very optimistic that in the next 5 or 10 years, we’ll have more. But right now it comes down to slings and artificial sphincters, which are devices with little pumps and hydraulics, and they’re very good. But they’ve been around for 50 years, and they have this other potential risk factor of the erosion of the tissue.
We don’t have a pill that we can give the patient to tighten up those muscles. We can help them with overactive bladder. But maybe the hormonal influence is a way to optimize the health of the tissue so that these surgical treatments can really deliver the best outcomes. And as I always say, and having treated so many of these patients, it’s really a game of millimeters — how much coaptation you get. If you’re off by the slightest amount, that’s an unhappy patient. So it doesn’t take much to make it a lot better.
Dr. Rubin: There’s so much hope for our patients, and this can really have an effect on sexual health. You know the benefits you see in their quality of life and sexual health when you can stop leakage.
Dr. Morey: I always take care of the waterworks first. Many of the men have both urinary problems and erectile problems. Nobody feels sexy when they’re leaking urine all over their partner. So first we take care of that. And then, in the motivated younger patients, we bring them back and talk to them about potentially having a second operation.
Dr. Rubin: And so similarly, in women with urinary incontinence, it can have a major impact on sexual health — how they show up and how they talk to their partner. So, it is really important for our primary care docs to talk to patients about urinary incontinence and not just say, “Oh, well, you’re getting older. There’s nothing that you can do.” There’s actually no age at which there is nothing that we can do. And it’s really important to refer patients to those urologists who have extra training in incontinence and sexual health, because we do care about these quality-of-life measures and there is a lot we can do, ranging from conservative to more invasive treatments. But patients really should have options.
Dr. Morey: I heard during this meeting that urinary incontinence was the number-one source of treatment regret among patients who had their prostate treated for cancer. So, this is a really big deal for our patients. And it impacts wellness, quality of life, and overall well-being.
Dr. Rubin: When we are counseling patients for cancer surgeries or cancer treatments such as radiation therapy, it’s really hard for the patients who have never had urinary incontinence to imagine what that might be like. When you’re telling them they could have a stroke or a heart attack, or they could have erectile dysfunction or urinary incontinence, it all sounds similar to them. It could happen to someone else. It’s very hard to truly counsel patients on these quality-of-life issues that they’ve never encountered before.
Dr. Morey: We have found that it takes a long time for patients to get into our office for treatment, and it’s unbelievable — often 5 years in diapers before they find us.
Dr. Rubin: Hopefully videos like this will teach our docs and our patients that there is hope out there, that you don’t need to wait through years of suffering from incontinence. So, how does somebody find a reconstructive urologist or a sexual medicine urologist?
Dr. Morey: There are a couple of good websites out there, such as fixincontinence.com and edcure.org. The device manufacturers have pretty good information for patients.
Dr. Rubin: The Sexual Medicine Society of North America (SMSNA) has a great find-a-provider website, which provides a list of urologists who are trained in both sexual health and urinary incontinence, because they both matter. Our patients deeply care about these issues.
Rachel S. Rubin, MD, has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, Endo.
A version of this article appeared on Medscape.com.
Attrition in Youth Sports
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.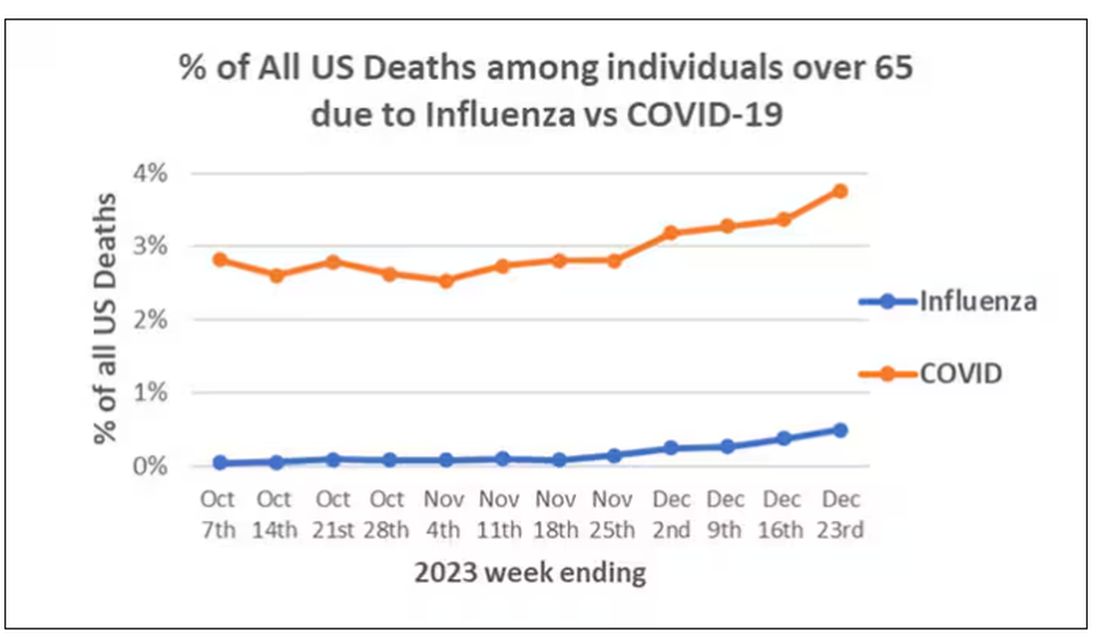
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).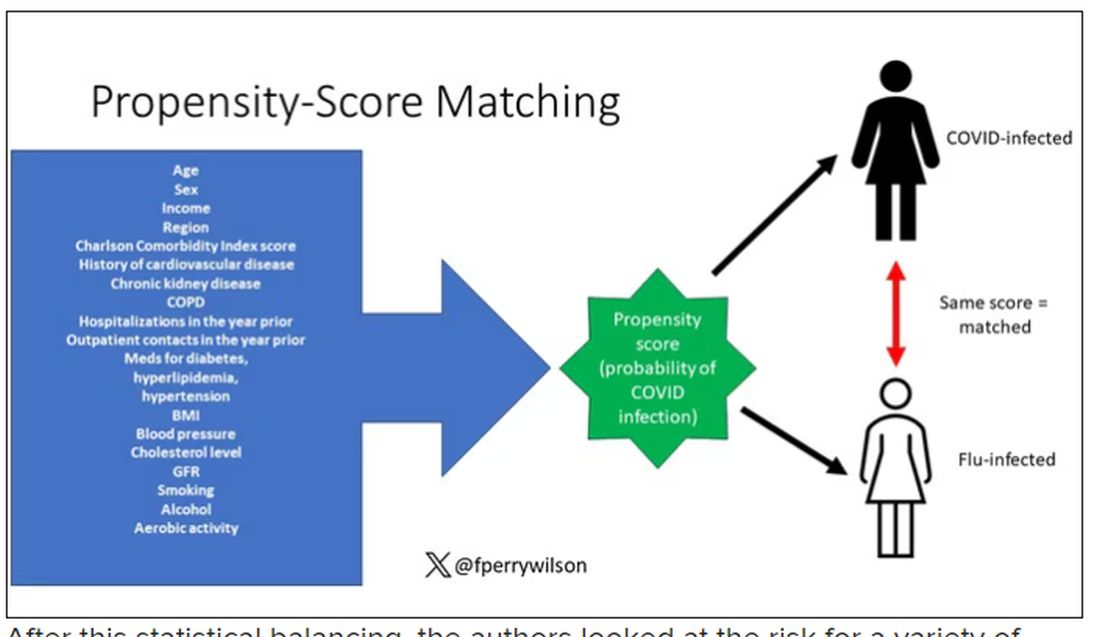
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.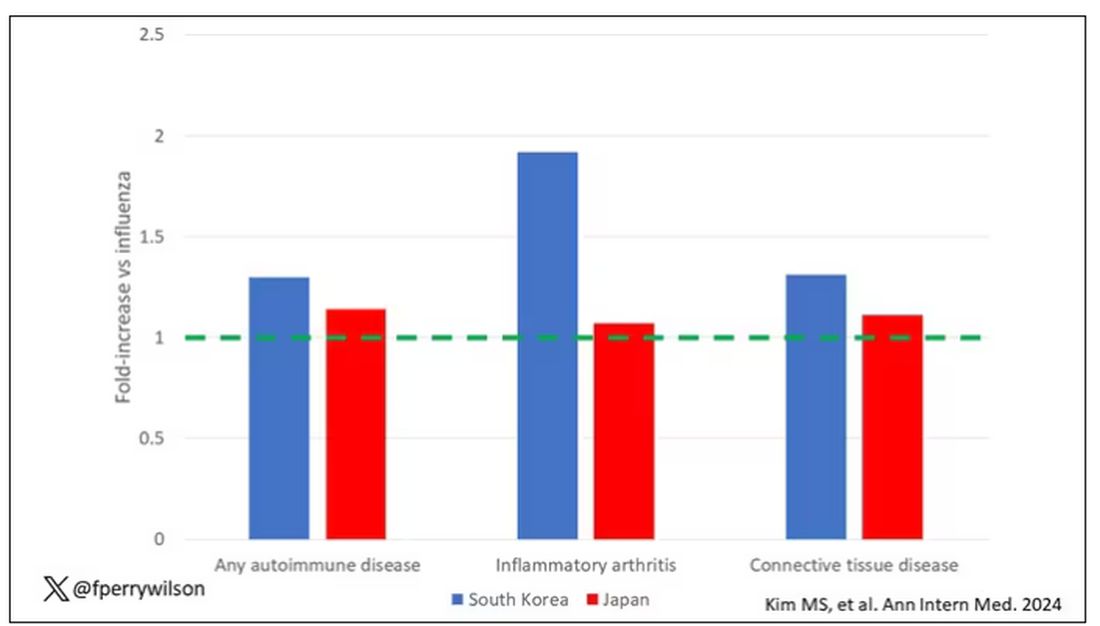
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.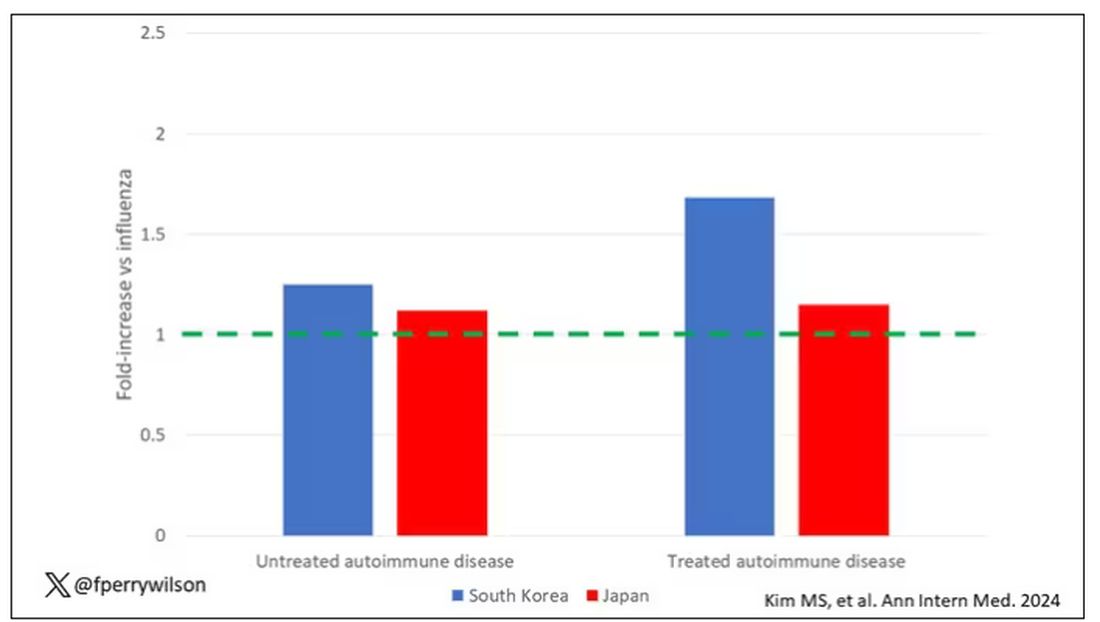
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.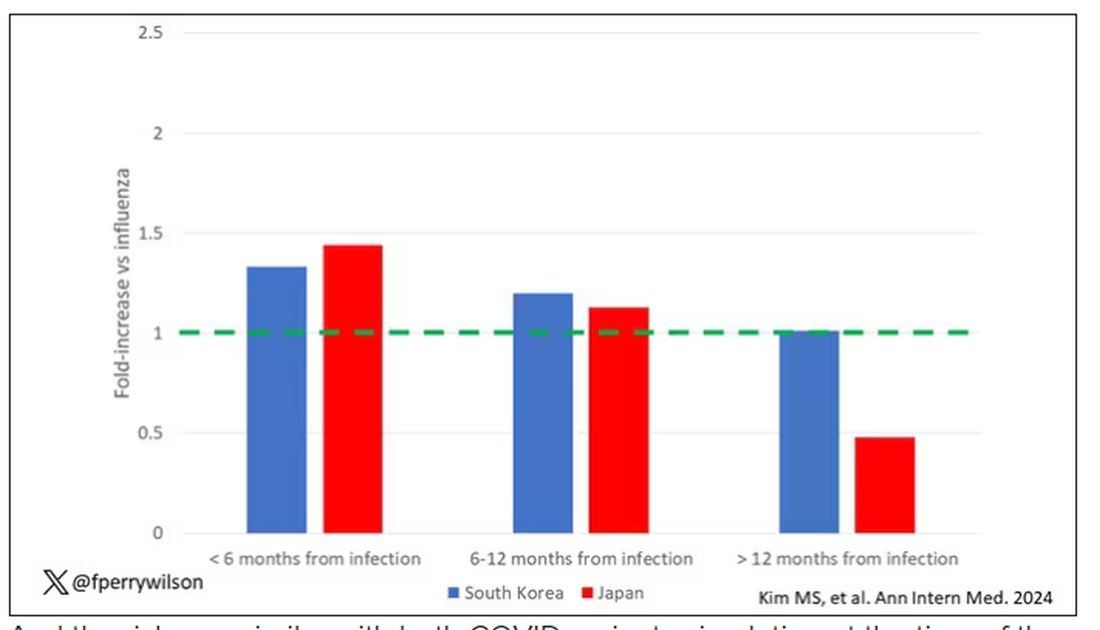
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?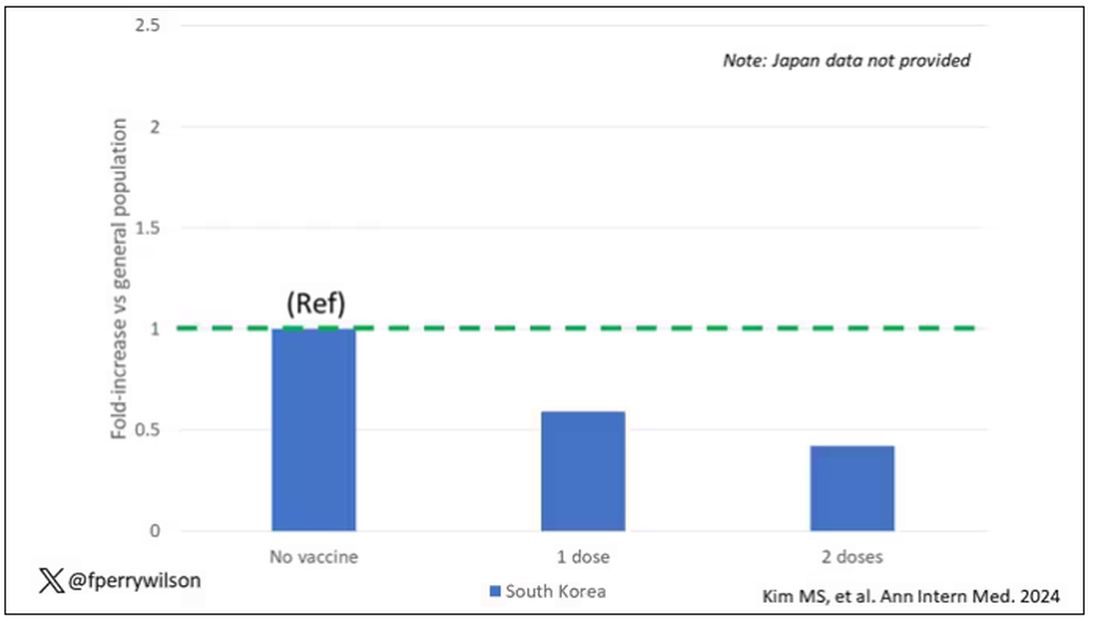
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
What’s Changed in Asthma Treatment? Quite a Bit
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
The Small Office Vibe
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].








