User login
A new and completely different pain medicine
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.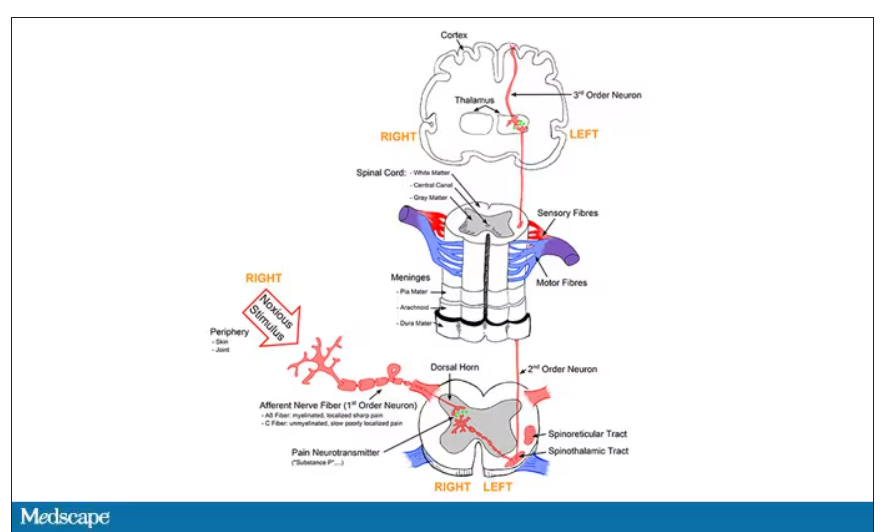
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.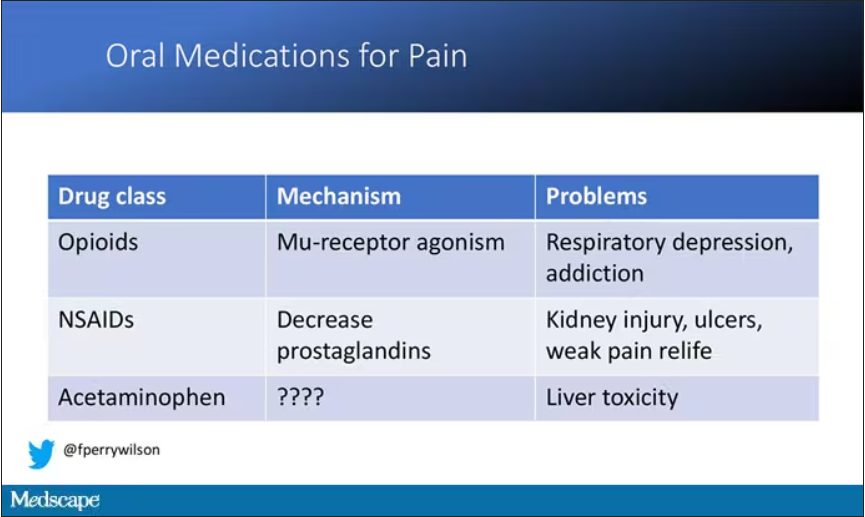
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.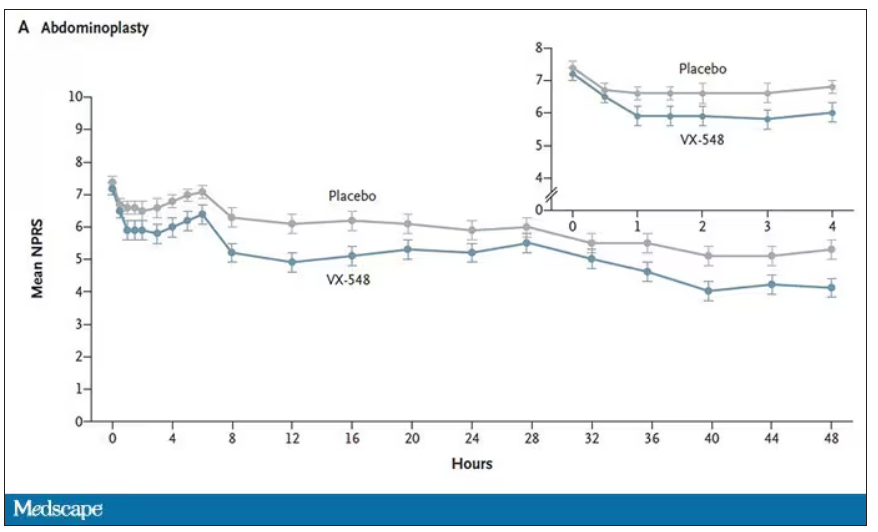
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.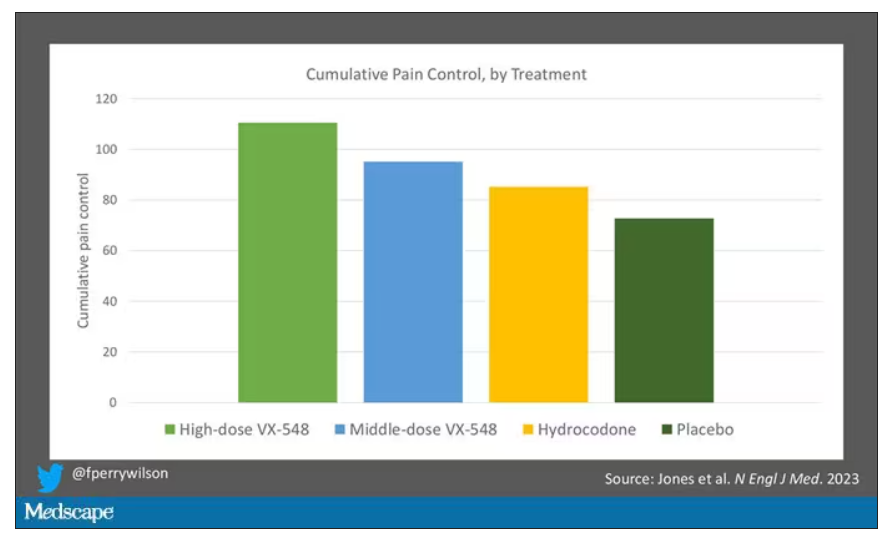
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.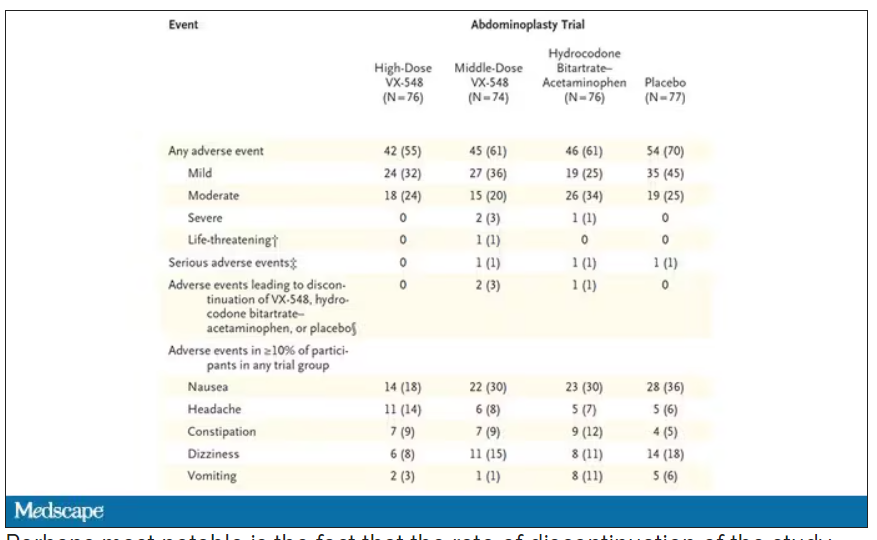
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prescribing lifestyle changes: When medicine isn’t enough
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
Will this trial help solve chronic back pain?
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Who owns your genes?
Who owns your genes? The assumption of any sane person would be that he or she owns his or her own genes. I mean, how dumb a question is that?
Yet, in 2007, Dov Michaeli, MD, PhD, described how an American company had claimed ownership of genetic materials and believed that it had the right to commercialize those naturally occurring bits of DNA. Myriad Genetics began by patenting mutations of BRCA. Dr. Michaeli issued a call for action to support early efforts to pass legislation to restore and preserve individual ownership of one’s own genes. This is a historically important quick read/watch/listen. Give it a click.
In related legislation, the Genetic Information Nondiscrimination Act (GINA), originally introduced by New York Rep. Louise Slaughter in 1995, was ultimately spearheaded by California Rep. Xavier Becerra (now Secretary of Health & Human Services) to passage by the House of Representatives on April 25, 2007, by a vote of 420-9-3. Led by Sen. Edward Kennedy of Massachusetts, it was passed by the Senate on April 24, 2008, by a vote of 95-0. President George W. Bush signed the bill into law on May 21, 2008.
GINA is a landmark piece of legislation that protects Americans. It prohibits employers and health insurers from discriminating against people on the basis of their genetic information, and it also prohibits the use of genetic information in life insurance and long-term care insurance.
Its impact has been immense. GINA has been indispensable in promoting progress in the field of human genetics. By safeguarding individuals against discrimination based on genetic information, it has encouraged broader participation in research, built public trust, and stimulated advancements in genetic testing and personalized medicine. GINA’s impact extends beyond borders and has influenced much of the rest of the world.
As important as GINA was to the field, more was needed. National legislation to protect ownership of genetic materials has, despite many attempts, still not become law in the United States. However, in our system of divided government and balance of power, we also have independent courts.
June 13, 2023, was the 10th anniversary of another landmark event. The legal case is that of the Association for Molecular Pathology v. Myriad Genetics, a Salt Lake City–based biotech company that held patents on isolated DNA sequences associated with breast and ovarian cancer. The AMP, joined by several other organizations and researchers, challenged Myriad’s gene patents, arguing that human genes are naturally occurring and, therefore, should not be subject to patenting. In a unanimous decision, the Supreme Court held that naturally occurring DNA segments are products of nature and therefore are not eligible for patent protection.
This was a pivotal decision in the field of human genetics and had a broad impact on genetic research. The decision clarified that naturally occurring DNA sequences cannot be patented, which means that researchers are free to use these sequences in their research without fear of patent infringement. This has led to a vast increase in the amount of genetic research being conducted, and it has also led to the development of new genetic tests and treatments.
The numbers of genetic research papers published in scientific journals and of genetic tests available to consumers have increased significantly, while the cost of genetic testing has decreased significantly. The AMP v. Myriad decision is likely to continue to have an impact for many years to come.
Thank you, common sense, activist American molecular pathologists, Congress, the President, and the Supreme Court for siding with the people.Dr. Lundbert is editor in chief of Cancer Commons. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Who owns your genes? The assumption of any sane person would be that he or she owns his or her own genes. I mean, how dumb a question is that?
Yet, in 2007, Dov Michaeli, MD, PhD, described how an American company had claimed ownership of genetic materials and believed that it had the right to commercialize those naturally occurring bits of DNA. Myriad Genetics began by patenting mutations of BRCA. Dr. Michaeli issued a call for action to support early efforts to pass legislation to restore and preserve individual ownership of one’s own genes. This is a historically important quick read/watch/listen. Give it a click.
In related legislation, the Genetic Information Nondiscrimination Act (GINA), originally introduced by New York Rep. Louise Slaughter in 1995, was ultimately spearheaded by California Rep. Xavier Becerra (now Secretary of Health & Human Services) to passage by the House of Representatives on April 25, 2007, by a vote of 420-9-3. Led by Sen. Edward Kennedy of Massachusetts, it was passed by the Senate on April 24, 2008, by a vote of 95-0. President George W. Bush signed the bill into law on May 21, 2008.
GINA is a landmark piece of legislation that protects Americans. It prohibits employers and health insurers from discriminating against people on the basis of their genetic information, and it also prohibits the use of genetic information in life insurance and long-term care insurance.
Its impact has been immense. GINA has been indispensable in promoting progress in the field of human genetics. By safeguarding individuals against discrimination based on genetic information, it has encouraged broader participation in research, built public trust, and stimulated advancements in genetic testing and personalized medicine. GINA’s impact extends beyond borders and has influenced much of the rest of the world.
As important as GINA was to the field, more was needed. National legislation to protect ownership of genetic materials has, despite many attempts, still not become law in the United States. However, in our system of divided government and balance of power, we also have independent courts.
June 13, 2023, was the 10th anniversary of another landmark event. The legal case is that of the Association for Molecular Pathology v. Myriad Genetics, a Salt Lake City–based biotech company that held patents on isolated DNA sequences associated with breast and ovarian cancer. The AMP, joined by several other organizations and researchers, challenged Myriad’s gene patents, arguing that human genes are naturally occurring and, therefore, should not be subject to patenting. In a unanimous decision, the Supreme Court held that naturally occurring DNA segments are products of nature and therefore are not eligible for patent protection.
This was a pivotal decision in the field of human genetics and had a broad impact on genetic research. The decision clarified that naturally occurring DNA sequences cannot be patented, which means that researchers are free to use these sequences in their research without fear of patent infringement. This has led to a vast increase in the amount of genetic research being conducted, and it has also led to the development of new genetic tests and treatments.
The numbers of genetic research papers published in scientific journals and of genetic tests available to consumers have increased significantly, while the cost of genetic testing has decreased significantly. The AMP v. Myriad decision is likely to continue to have an impact for many years to come.
Thank you, common sense, activist American molecular pathologists, Congress, the President, and the Supreme Court for siding with the people.Dr. Lundbert is editor in chief of Cancer Commons. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Who owns your genes? The assumption of any sane person would be that he or she owns his or her own genes. I mean, how dumb a question is that?
Yet, in 2007, Dov Michaeli, MD, PhD, described how an American company had claimed ownership of genetic materials and believed that it had the right to commercialize those naturally occurring bits of DNA. Myriad Genetics began by patenting mutations of BRCA. Dr. Michaeli issued a call for action to support early efforts to pass legislation to restore and preserve individual ownership of one’s own genes. This is a historically important quick read/watch/listen. Give it a click.
In related legislation, the Genetic Information Nondiscrimination Act (GINA), originally introduced by New York Rep. Louise Slaughter in 1995, was ultimately spearheaded by California Rep. Xavier Becerra (now Secretary of Health & Human Services) to passage by the House of Representatives on April 25, 2007, by a vote of 420-9-3. Led by Sen. Edward Kennedy of Massachusetts, it was passed by the Senate on April 24, 2008, by a vote of 95-0. President George W. Bush signed the bill into law on May 21, 2008.
GINA is a landmark piece of legislation that protects Americans. It prohibits employers and health insurers from discriminating against people on the basis of their genetic information, and it also prohibits the use of genetic information in life insurance and long-term care insurance.
Its impact has been immense. GINA has been indispensable in promoting progress in the field of human genetics. By safeguarding individuals against discrimination based on genetic information, it has encouraged broader participation in research, built public trust, and stimulated advancements in genetic testing and personalized medicine. GINA’s impact extends beyond borders and has influenced much of the rest of the world.
As important as GINA was to the field, more was needed. National legislation to protect ownership of genetic materials has, despite many attempts, still not become law in the United States. However, in our system of divided government and balance of power, we also have independent courts.
June 13, 2023, was the 10th anniversary of another landmark event. The legal case is that of the Association for Molecular Pathology v. Myriad Genetics, a Salt Lake City–based biotech company that held patents on isolated DNA sequences associated with breast and ovarian cancer. The AMP, joined by several other organizations and researchers, challenged Myriad’s gene patents, arguing that human genes are naturally occurring and, therefore, should not be subject to patenting. In a unanimous decision, the Supreme Court held that naturally occurring DNA segments are products of nature and therefore are not eligible for patent protection.
This was a pivotal decision in the field of human genetics and had a broad impact on genetic research. The decision clarified that naturally occurring DNA sequences cannot be patented, which means that researchers are free to use these sequences in their research without fear of patent infringement. This has led to a vast increase in the amount of genetic research being conducted, and it has also led to the development of new genetic tests and treatments.
The numbers of genetic research papers published in scientific journals and of genetic tests available to consumers have increased significantly, while the cost of genetic testing has decreased significantly. The AMP v. Myriad decision is likely to continue to have an impact for many years to come.
Thank you, common sense, activist American molecular pathologists, Congress, the President, and the Supreme Court for siding with the people.Dr. Lundbert is editor in chief of Cancer Commons. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Babe Ruth’s unique cane, and why he used it
Babe Ruth was arguably the greatest athlete in American history.
Certainly, there have been, and always will be, many great figures in all sports. But none of them – Michael Jordan or LeBron James or Tom Brady – have ever, probably will never, dominate sports AND society in the way Babe Ruth did.
Ruth wasn’t an angel, nor did he claim to be. But he was a center of American life the way no athlete ever was or will be.
He was a remarkably good baseball player. In an era where home runs were rarities, he hit more than the entire rest of Major League Baseball combined. But he wasn’t just a slugger, he was an excellent play maker, fielder, and pitcher. (He was actually one of the best pitchers of his era, something else mostly forgotten today.)
Ruth retired in 1935. He never entirely left the limelight, with fans showing up even to watch him play golf in celebrity tournaments. In 1939 he spoke on July 4 at Lou Gehrig appreciation day as his former teammate was publicly dying of ALS.
In 1946 Ruth began having trouble swallowing and developed pain over his right eye. He was found to have nasopharyngeal carcinoma spreading down into his skull base and neck.
Even today surgery to remove cancer from that area is tricky. In 1946 it didn’t exist. An experimental treatment of combined radiation and chemotherapy – today standard – was tried, including a new folic acid derivative called teropterin. He improved somewhat – enough that he was an unnamed case study presented at a medical meeting – but had lost 80 pounds. After a brief respite he continued to go downhill. On June 13, 1948, he appeared at Yankee Stadium – the house that Ruth built – for the last time, where he was honored. He had difficulty walking and used a baseball bat as a cane. His pharynx was so damaged his voice could barely be heard. He died 2 months later on Aug. 16, 1948.
This isn’t a sports column, I’m not a sports writer, and this definitely ain’t Sport Illustrated. So why am I writing this?
Because Babe Ruth never knew he had cancer. Was never told he was dying. His family was afraid he’d harm himself if he knew, so his doctors were under strict instructions to keep the bad news from him.
Now, Ruth wasn’t stupid. Wild, unrepentant, hedonistic, and a lot of other things – but not stupid. He certainly must have figured it out with getting radiation, or chemotherapy, or his declining physical status. But none of his doctors or family ever told him he had cancer and was dying (what they did tell him I have no idea).
Let’s look at this as a case history: A 51-year-old male, possessed of all his mental faculties, presents with headaches, dysphonia, and dysphagia. Workup reveals advanced, inoperable, nasopharyngeal cancer. The family is willing to accept treatment, but understands the prognosis is poor. Family members request that, under no circumstances, he be told of the diagnosis or prognosis.
The fact that the patient is probably the biggest celebrity of his era shouldn’t make a difference, but it does.
I’m sure most of us would want to tell the patient. We live in an age of patient autonomy. . But what if the family has concerns that the patient would hurt himself, as Ruth’s family did?
This summer is 75 years since the Babe died. Medicine has changed a lot, but some questions never will.
What would you do?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Babe Ruth was arguably the greatest athlete in American history.
Certainly, there have been, and always will be, many great figures in all sports. But none of them – Michael Jordan or LeBron James or Tom Brady – have ever, probably will never, dominate sports AND society in the way Babe Ruth did.
Ruth wasn’t an angel, nor did he claim to be. But he was a center of American life the way no athlete ever was or will be.
He was a remarkably good baseball player. In an era where home runs were rarities, he hit more than the entire rest of Major League Baseball combined. But he wasn’t just a slugger, he was an excellent play maker, fielder, and pitcher. (He was actually one of the best pitchers of his era, something else mostly forgotten today.)
Ruth retired in 1935. He never entirely left the limelight, with fans showing up even to watch him play golf in celebrity tournaments. In 1939 he spoke on July 4 at Lou Gehrig appreciation day as his former teammate was publicly dying of ALS.
In 1946 Ruth began having trouble swallowing and developed pain over his right eye. He was found to have nasopharyngeal carcinoma spreading down into his skull base and neck.
Even today surgery to remove cancer from that area is tricky. In 1946 it didn’t exist. An experimental treatment of combined radiation and chemotherapy – today standard – was tried, including a new folic acid derivative called teropterin. He improved somewhat – enough that he was an unnamed case study presented at a medical meeting – but had lost 80 pounds. After a brief respite he continued to go downhill. On June 13, 1948, he appeared at Yankee Stadium – the house that Ruth built – for the last time, where he was honored. He had difficulty walking and used a baseball bat as a cane. His pharynx was so damaged his voice could barely be heard. He died 2 months later on Aug. 16, 1948.
This isn’t a sports column, I’m not a sports writer, and this definitely ain’t Sport Illustrated. So why am I writing this?
Because Babe Ruth never knew he had cancer. Was never told he was dying. His family was afraid he’d harm himself if he knew, so his doctors were under strict instructions to keep the bad news from him.
Now, Ruth wasn’t stupid. Wild, unrepentant, hedonistic, and a lot of other things – but not stupid. He certainly must have figured it out with getting radiation, or chemotherapy, or his declining physical status. But none of his doctors or family ever told him he had cancer and was dying (what they did tell him I have no idea).
Let’s look at this as a case history: A 51-year-old male, possessed of all his mental faculties, presents with headaches, dysphonia, and dysphagia. Workup reveals advanced, inoperable, nasopharyngeal cancer. The family is willing to accept treatment, but understands the prognosis is poor. Family members request that, under no circumstances, he be told of the diagnosis or prognosis.
The fact that the patient is probably the biggest celebrity of his era shouldn’t make a difference, but it does.
I’m sure most of us would want to tell the patient. We live in an age of patient autonomy. . But what if the family has concerns that the patient would hurt himself, as Ruth’s family did?
This summer is 75 years since the Babe died. Medicine has changed a lot, but some questions never will.
What would you do?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Babe Ruth was arguably the greatest athlete in American history.
Certainly, there have been, and always will be, many great figures in all sports. But none of them – Michael Jordan or LeBron James or Tom Brady – have ever, probably will never, dominate sports AND society in the way Babe Ruth did.
Ruth wasn’t an angel, nor did he claim to be. But he was a center of American life the way no athlete ever was or will be.
He was a remarkably good baseball player. In an era where home runs were rarities, he hit more than the entire rest of Major League Baseball combined. But he wasn’t just a slugger, he was an excellent play maker, fielder, and pitcher. (He was actually one of the best pitchers of his era, something else mostly forgotten today.)
Ruth retired in 1935. He never entirely left the limelight, with fans showing up even to watch him play golf in celebrity tournaments. In 1939 he spoke on July 4 at Lou Gehrig appreciation day as his former teammate was publicly dying of ALS.
In 1946 Ruth began having trouble swallowing and developed pain over his right eye. He was found to have nasopharyngeal carcinoma spreading down into his skull base and neck.
Even today surgery to remove cancer from that area is tricky. In 1946 it didn’t exist. An experimental treatment of combined radiation and chemotherapy – today standard – was tried, including a new folic acid derivative called teropterin. He improved somewhat – enough that he was an unnamed case study presented at a medical meeting – but had lost 80 pounds. After a brief respite he continued to go downhill. On June 13, 1948, he appeared at Yankee Stadium – the house that Ruth built – for the last time, where he was honored. He had difficulty walking and used a baseball bat as a cane. His pharynx was so damaged his voice could barely be heard. He died 2 months later on Aug. 16, 1948.
This isn’t a sports column, I’m not a sports writer, and this definitely ain’t Sport Illustrated. So why am I writing this?
Because Babe Ruth never knew he had cancer. Was never told he was dying. His family was afraid he’d harm himself if he knew, so his doctors were under strict instructions to keep the bad news from him.
Now, Ruth wasn’t stupid. Wild, unrepentant, hedonistic, and a lot of other things – but not stupid. He certainly must have figured it out with getting radiation, or chemotherapy, or his declining physical status. But none of his doctors or family ever told him he had cancer and was dying (what they did tell him I have no idea).
Let’s look at this as a case history: A 51-year-old male, possessed of all his mental faculties, presents with headaches, dysphonia, and dysphagia. Workup reveals advanced, inoperable, nasopharyngeal cancer. The family is willing to accept treatment, but understands the prognosis is poor. Family members request that, under no circumstances, he be told of the diagnosis or prognosis.
The fact that the patient is probably the biggest celebrity of his era shouldn’t make a difference, but it does.
I’m sure most of us would want to tell the patient. We live in an age of patient autonomy. . But what if the family has concerns that the patient would hurt himself, as Ruth’s family did?
This summer is 75 years since the Babe died. Medicine has changed a lot, but some questions never will.
What would you do?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The four questions you should ask about sexual health
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
New and emerging options for treating recurrent C. difficile
This transcript has been edited for clarity.
Clostridioides difficile is a toxin-based infection that takes up residence in the colon due to disturbed normal bowel flora, usually after antibiotics.
Recurrent C. difficile can happen in up to a quarter of patients who receive oral vancomycin as a treatment for their infection. It can also occur with treatment with the newer agent, fidaxomicin, although possibly in fewer patients. In general, relapses are indeed common.
When I trained at Johns Hopkins under John Bartlett, he took the approach that after the second – and always after the third – relapse, an extended course of oral therapy with vancomycin could help get patients out of trouble. He used the so-called extended pulse method, where patients would take the drug for approximately 4-6 weeks and gradually reduce the dose.
This approach can also be done with fidaxomicin. However, I’m not sure it works much better than vancomycin, and there are often hurdles to using fidaxomicin because of insurers not approving it because of the expense.
What other therapies are there?
There is bezlotoxumab, which is a human monoclonal antibody targeting C. difficile toxin B. I’ve used it a few times. It is given as a one-time infusion, and there are challenges regarding cost, the logistics of setting up the infusion, and insurance approval.
Fecal microbiota transplant
In recent years, fecal microbiota transplants (FMT) have received a lot of attention as a different avenue of treatment that could lower the potential for relapses, with success rates usually around 80%-90%. However, in the past few years, there have been some serious safety signals because of possible transmission of dangerous pathogens, often with drug resistance, with FMT.
I’m therefore pleased to say that newer fecal microbiota products are coming in fast and furious. I thought I’d spend a few minutes speaking about these.
OpenBiome, an organization dedicated to microbiome research, offers an investigational product from screened donors that has not received Food and Drug Administration approval. It’s been around for some time. It can be used in either upper or lower GI applications, and the organization cites about an 84% success rate using this product.
There are also two new FDA-approved products I think are worth knowing about. They’ve just been approved recently and we’re a little uncertain of where they’re going to end up in the treatment landscape.
The first is from Ferring, and it goes by fecal microbiota, live-jslm (Rebyota). This is a product from qualified and screened donors, the main component of which is Bacteroides, which is given as a single dose by enema.
The company did a phase 3 trial with a Bayesian primary analysis, which I think convinced the FDA to approve this product. The success rate in people with multiple relapses was 70.6%, compared with 57.5% with placebo. The estimated treatment effect was 13.1%. Of those who did respond, over 90% were kept free of relapse over a 6-month period.
The other product, also FDA approved, is from Seres. It was previously called SER-109, and is now called fecal microbiota spores, live-brpk (Vowst). Unlike the previous product, this is orally administered, with patients taking four capsules daily for 3 days. Again, these donor-derived firmicutes have been appropriately screened and are free of potential pathogens.
The phase 3 randomized clinical trial results were published in the New England Journal of Medicine. They showed that 12% of those taking this product had a relapse, compared with 40% of those taking placebo, which is about the range we tend to see in people who have had multiple relapses. The safety profile was similar to placebo.
So, how will people use these treatments?
I think the FDA imprimatur will be attractive to people, but the products, I believe, will be priced fairly expensively, in the under $10,000 range. The first (Rebyota) is a rectal infusion; it is a one-and-done treatment but creates logistical issues. Interestingly, it could be a billable procedure for infectious disease clinicians. The ease of oral administration for Vowst, no doubt, will be very appealing. Both of these are given after completing a course of treatment with vancomycin or fidaxomicin so as not to interfere with the microbiome product.
I’ll also briefly mention a paper published in JAMA on yet another microbiome product, called VE303. This product was based on eight commensal strains of Clostridia and was given orally in a phase 2 trial. Interestingly, this worked about the same as the oral product that is already FDA approved. The study showed a recurrence rate of 13.8% in the high-dose group, compared with 45.5% in the placebo group.
I think this is exciting. And, of course, there is the expense.
But anything that can be done to help improve these patients is welcome, as once they get into the multiple-relapse phase, it is challenging to turn around. These commercialized products will hopefully become a bit more mainstream. Certainly, we’ll see how these will be utilized in the coming months and over the next few years.
Dr. Auwaerter is Clinical Director, Division of Infectious Diseases, Johns Hopkins University, Baltimore. He reported conflicts of interest with Gilead, Shionogi, and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Clostridioides difficile is a toxin-based infection that takes up residence in the colon due to disturbed normal bowel flora, usually after antibiotics.
Recurrent C. difficile can happen in up to a quarter of patients who receive oral vancomycin as a treatment for their infection. It can also occur with treatment with the newer agent, fidaxomicin, although possibly in fewer patients. In general, relapses are indeed common.
When I trained at Johns Hopkins under John Bartlett, he took the approach that after the second – and always after the third – relapse, an extended course of oral therapy with vancomycin could help get patients out of trouble. He used the so-called extended pulse method, where patients would take the drug for approximately 4-6 weeks and gradually reduce the dose.
This approach can also be done with fidaxomicin. However, I’m not sure it works much better than vancomycin, and there are often hurdles to using fidaxomicin because of insurers not approving it because of the expense.
What other therapies are there?
There is bezlotoxumab, which is a human monoclonal antibody targeting C. difficile toxin B. I’ve used it a few times. It is given as a one-time infusion, and there are challenges regarding cost, the logistics of setting up the infusion, and insurance approval.
Fecal microbiota transplant
In recent years, fecal microbiota transplants (FMT) have received a lot of attention as a different avenue of treatment that could lower the potential for relapses, with success rates usually around 80%-90%. However, in the past few years, there have been some serious safety signals because of possible transmission of dangerous pathogens, often with drug resistance, with FMT.
I’m therefore pleased to say that newer fecal microbiota products are coming in fast and furious. I thought I’d spend a few minutes speaking about these.
OpenBiome, an organization dedicated to microbiome research, offers an investigational product from screened donors that has not received Food and Drug Administration approval. It’s been around for some time. It can be used in either upper or lower GI applications, and the organization cites about an 84% success rate using this product.
There are also two new FDA-approved products I think are worth knowing about. They’ve just been approved recently and we’re a little uncertain of where they’re going to end up in the treatment landscape.
The first is from Ferring, and it goes by fecal microbiota, live-jslm (Rebyota). This is a product from qualified and screened donors, the main component of which is Bacteroides, which is given as a single dose by enema.
The company did a phase 3 trial with a Bayesian primary analysis, which I think convinced the FDA to approve this product. The success rate in people with multiple relapses was 70.6%, compared with 57.5% with placebo. The estimated treatment effect was 13.1%. Of those who did respond, over 90% were kept free of relapse over a 6-month period.
The other product, also FDA approved, is from Seres. It was previously called SER-109, and is now called fecal microbiota spores, live-brpk (Vowst). Unlike the previous product, this is orally administered, with patients taking four capsules daily for 3 days. Again, these donor-derived firmicutes have been appropriately screened and are free of potential pathogens.
The phase 3 randomized clinical trial results were published in the New England Journal of Medicine. They showed that 12% of those taking this product had a relapse, compared with 40% of those taking placebo, which is about the range we tend to see in people who have had multiple relapses. The safety profile was similar to placebo.
So, how will people use these treatments?
I think the FDA imprimatur will be attractive to people, but the products, I believe, will be priced fairly expensively, in the under $10,000 range. The first (Rebyota) is a rectal infusion; it is a one-and-done treatment but creates logistical issues. Interestingly, it could be a billable procedure for infectious disease clinicians. The ease of oral administration for Vowst, no doubt, will be very appealing. Both of these are given after completing a course of treatment with vancomycin or fidaxomicin so as not to interfere with the microbiome product.
I’ll also briefly mention a paper published in JAMA on yet another microbiome product, called VE303. This product was based on eight commensal strains of Clostridia and was given orally in a phase 2 trial. Interestingly, this worked about the same as the oral product that is already FDA approved. The study showed a recurrence rate of 13.8% in the high-dose group, compared with 45.5% in the placebo group.
I think this is exciting. And, of course, there is the expense.
But anything that can be done to help improve these patients is welcome, as once they get into the multiple-relapse phase, it is challenging to turn around. These commercialized products will hopefully become a bit more mainstream. Certainly, we’ll see how these will be utilized in the coming months and over the next few years.
Dr. Auwaerter is Clinical Director, Division of Infectious Diseases, Johns Hopkins University, Baltimore. He reported conflicts of interest with Gilead, Shionogi, and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Clostridioides difficile is a toxin-based infection that takes up residence in the colon due to disturbed normal bowel flora, usually after antibiotics.
Recurrent C. difficile can happen in up to a quarter of patients who receive oral vancomycin as a treatment for their infection. It can also occur with treatment with the newer agent, fidaxomicin, although possibly in fewer patients. In general, relapses are indeed common.
When I trained at Johns Hopkins under John Bartlett, he took the approach that after the second – and always after the third – relapse, an extended course of oral therapy with vancomycin could help get patients out of trouble. He used the so-called extended pulse method, where patients would take the drug for approximately 4-6 weeks and gradually reduce the dose.
This approach can also be done with fidaxomicin. However, I’m not sure it works much better than vancomycin, and there are often hurdles to using fidaxomicin because of insurers not approving it because of the expense.
What other therapies are there?
There is bezlotoxumab, which is a human monoclonal antibody targeting C. difficile toxin B. I’ve used it a few times. It is given as a one-time infusion, and there are challenges regarding cost, the logistics of setting up the infusion, and insurance approval.
Fecal microbiota transplant
In recent years, fecal microbiota transplants (FMT) have received a lot of attention as a different avenue of treatment that could lower the potential for relapses, with success rates usually around 80%-90%. However, in the past few years, there have been some serious safety signals because of possible transmission of dangerous pathogens, often with drug resistance, with FMT.
I’m therefore pleased to say that newer fecal microbiota products are coming in fast and furious. I thought I’d spend a few minutes speaking about these.
OpenBiome, an organization dedicated to microbiome research, offers an investigational product from screened donors that has not received Food and Drug Administration approval. It’s been around for some time. It can be used in either upper or lower GI applications, and the organization cites about an 84% success rate using this product.
There are also two new FDA-approved products I think are worth knowing about. They’ve just been approved recently and we’re a little uncertain of where they’re going to end up in the treatment landscape.
The first is from Ferring, and it goes by fecal microbiota, live-jslm (Rebyota). This is a product from qualified and screened donors, the main component of which is Bacteroides, which is given as a single dose by enema.
The company did a phase 3 trial with a Bayesian primary analysis, which I think convinced the FDA to approve this product. The success rate in people with multiple relapses was 70.6%, compared with 57.5% with placebo. The estimated treatment effect was 13.1%. Of those who did respond, over 90% were kept free of relapse over a 6-month period.
The other product, also FDA approved, is from Seres. It was previously called SER-109, and is now called fecal microbiota spores, live-brpk (Vowst). Unlike the previous product, this is orally administered, with patients taking four capsules daily for 3 days. Again, these donor-derived firmicutes have been appropriately screened and are free of potential pathogens.
The phase 3 randomized clinical trial results were published in the New England Journal of Medicine. They showed that 12% of those taking this product had a relapse, compared with 40% of those taking placebo, which is about the range we tend to see in people who have had multiple relapses. The safety profile was similar to placebo.
So, how will people use these treatments?
I think the FDA imprimatur will be attractive to people, but the products, I believe, will be priced fairly expensively, in the under $10,000 range. The first (Rebyota) is a rectal infusion; it is a one-and-done treatment but creates logistical issues. Interestingly, it could be a billable procedure for infectious disease clinicians. The ease of oral administration for Vowst, no doubt, will be very appealing. Both of these are given after completing a course of treatment with vancomycin or fidaxomicin so as not to interfere with the microbiome product.
I’ll also briefly mention a paper published in JAMA on yet another microbiome product, called VE303. This product was based on eight commensal strains of Clostridia and was given orally in a phase 2 trial. Interestingly, this worked about the same as the oral product that is already FDA approved. The study showed a recurrence rate of 13.8% in the high-dose group, compared with 45.5% in the placebo group.
I think this is exciting. And, of course, there is the expense.
But anything that can be done to help improve these patients is welcome, as once they get into the multiple-relapse phase, it is challenging to turn around. These commercialized products will hopefully become a bit more mainstream. Certainly, we’ll see how these will be utilized in the coming months and over the next few years.
Dr. Auwaerter is Clinical Director, Division of Infectious Diseases, Johns Hopkins University, Baltimore. He reported conflicts of interest with Gilead, Shionogi, and Medscape.
A version of this article first appeared on Medscape.com.
Sick humor
This past June, during the search for the Titan submersible, and since then, we’ve had a not-entirely-unexpected development: Sick humor.
There was a lot of it. The Subway owner who got reprimanded for putting “Our subs don’t implode” on his sign was minor league compared with other things circulating on the Internet. One example that was sent to me showed the late Stockton Rush, OceanGate’s co-owner, as the new spokesman for Cap’n Crunch.
Of course, this is nothing new. People have made jokes about awful situations since to the dawn of civilization.
Why do we do this?
Humor is a remarkably human trait. There’s evidence other mammals have it, but not to the extent we do. We’ve created a multitude of forms that vary between cultures. But there isn’t a civilization or culture on Earth that doesn’t have humor.
Why we developed it I’ll leave to others, though I assume a key part is that it strengthens bonds between people, helping them stick together in the groups that keep society moving forward.
Sick humor is part of this, though having grown up watching Monty Python and reading National Lampoon magazine I’m certainly guilty of enjoying it. To this day I think “Eating Raoul” is one of the greatest comedies ever.
It’s also pretty common in medicine. I’ve been involved in plenty of hospital situations that were quite unfunny, yet there are always jokes about it flying as we work.
I assume it’s a defense mechanism. Helping us cope with a bad situation as we do our best to deal with it. Using humor to put a block between the obvious realization that someday this could happen to us. To help psychologically shield us from something tragic.
Years ago I was trying to describe the plot of “Eating Raoul” and said “if you read about this sort of crime spree in a newspaper you’d be horrified. But the way it’s handled in the movie it’s hysterical.” Perhaps that’s as close to understanding sick humor as I’ll ever get. It makes the unfunny funny.
Perhaps the better phrase is the more generic “it’s human nature.”
Whether or not it’s funny depends on the person. There were plenty of people horrified by the Subway sign, enough that the owner had to change it. But there were also those who admitted they found it tasteless, but still got a laugh out of it. I’m sure the families of those lost on the Titan were justifiably upset, but the closer you get to a personal tragedy the more serious it is.
There’s a fine line, as National Lampoon put it, between funny and sick. But it’s also part of who we are.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past June, during the search for the Titan submersible, and since then, we’ve had a not-entirely-unexpected development: Sick humor.
There was a lot of it. The Subway owner who got reprimanded for putting “Our subs don’t implode” on his sign was minor league compared with other things circulating on the Internet. One example that was sent to me showed the late Stockton Rush, OceanGate’s co-owner, as the new spokesman for Cap’n Crunch.
Of course, this is nothing new. People have made jokes about awful situations since to the dawn of civilization.
Why do we do this?
Humor is a remarkably human trait. There’s evidence other mammals have it, but not to the extent we do. We’ve created a multitude of forms that vary between cultures. But there isn’t a civilization or culture on Earth that doesn’t have humor.
Why we developed it I’ll leave to others, though I assume a key part is that it strengthens bonds between people, helping them stick together in the groups that keep society moving forward.
Sick humor is part of this, though having grown up watching Monty Python and reading National Lampoon magazine I’m certainly guilty of enjoying it. To this day I think “Eating Raoul” is one of the greatest comedies ever.
It’s also pretty common in medicine. I’ve been involved in plenty of hospital situations that were quite unfunny, yet there are always jokes about it flying as we work.
I assume it’s a defense mechanism. Helping us cope with a bad situation as we do our best to deal with it. Using humor to put a block between the obvious realization that someday this could happen to us. To help psychologically shield us from something tragic.
Years ago I was trying to describe the plot of “Eating Raoul” and said “if you read about this sort of crime spree in a newspaper you’d be horrified. But the way it’s handled in the movie it’s hysterical.” Perhaps that’s as close to understanding sick humor as I’ll ever get. It makes the unfunny funny.
Perhaps the better phrase is the more generic “it’s human nature.”
Whether or not it’s funny depends on the person. There were plenty of people horrified by the Subway sign, enough that the owner had to change it. But there were also those who admitted they found it tasteless, but still got a laugh out of it. I’m sure the families of those lost on the Titan were justifiably upset, but the closer you get to a personal tragedy the more serious it is.
There’s a fine line, as National Lampoon put it, between funny and sick. But it’s also part of who we are.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past June, during the search for the Titan submersible, and since then, we’ve had a not-entirely-unexpected development: Sick humor.
There was a lot of it. The Subway owner who got reprimanded for putting “Our subs don’t implode” on his sign was minor league compared with other things circulating on the Internet. One example that was sent to me showed the late Stockton Rush, OceanGate’s co-owner, as the new spokesman for Cap’n Crunch.
Of course, this is nothing new. People have made jokes about awful situations since to the dawn of civilization.
Why do we do this?
Humor is a remarkably human trait. There’s evidence other mammals have it, but not to the extent we do. We’ve created a multitude of forms that vary between cultures. But there isn’t a civilization or culture on Earth that doesn’t have humor.
Why we developed it I’ll leave to others, though I assume a key part is that it strengthens bonds between people, helping them stick together in the groups that keep society moving forward.
Sick humor is part of this, though having grown up watching Monty Python and reading National Lampoon magazine I’m certainly guilty of enjoying it. To this day I think “Eating Raoul” is one of the greatest comedies ever.
It’s also pretty common in medicine. I’ve been involved in plenty of hospital situations that were quite unfunny, yet there are always jokes about it flying as we work.
I assume it’s a defense mechanism. Helping us cope with a bad situation as we do our best to deal with it. Using humor to put a block between the obvious realization that someday this could happen to us. To help psychologically shield us from something tragic.
Years ago I was trying to describe the plot of “Eating Raoul” and said “if you read about this sort of crime spree in a newspaper you’d be horrified. But the way it’s handled in the movie it’s hysterical.” Perhaps that’s as close to understanding sick humor as I’ll ever get. It makes the unfunny funny.
Perhaps the better phrase is the more generic “it’s human nature.”
Whether or not it’s funny depends on the person. There were plenty of people horrified by the Subway sign, enough that the owner had to change it. But there were also those who admitted they found it tasteless, but still got a laugh out of it. I’m sure the families of those lost on the Titan were justifiably upset, but the closer you get to a personal tragedy the more serious it is.
There’s a fine line, as National Lampoon put it, between funny and sick. But it’s also part of who we are.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pain mismanagement by the numbers
Despite my best efforts to cultivate acquaintances across a broader age group, my social circle still has the somewhat musty odor of septuagenarians. We try to talk about things beyond the weather and grandchildren but pain scenarios surface with unfortunate frequency. Arthritic joints ache, body parts wear out or become diseased and have to be removed or replaced. That stuff can hurt.
There are two pain-related themes that seem to crop up more frequently than you might expect. The first is the unfortunate side effects of opioid medication – most often gastric distress and vomiting, then of course there’s constipation. They seem so common that a good many of my acquaintances just plain refuse to take opioids when they have been prescribed postoperatively because of their vivid memories of the consequences or horror stories friends have told.
The second theme is the general annoyance with the damn “Please rate your pain from one to ten” request issued by every well-intentioned nurse. Do you mean the pain I am having right now, this second, or last night, or the average over the last day and a half? Or should I be comparing it with when I gave birth 70 years ago, or when I stubbed my toe getting out of the shower last week? And then what are you going to do with my guesstimated number?
It may surprise some of you that 40 years ago there wasn’t a pain scale fetish. But a few observant health care professionals realized that many of our patients were suffering because we weren’t adequately managing their pain. In postoperative situations this was slowing recovery and effecting outcomes. Like good pseudoscientists, they realized that we should first quantify the pain and the notion that no pain should go unrated came into being. Nor should pain go untreated, which is too frequently interpreted as meaning unmedicated.
For example a systematic review of 61 studies of juvenile idiopathic arthritis (JIA) published in the journal Pediatric Rheumatology found that there was positive relationship between pain and a child’s belief that pain causes harm, disability, and lack of control. Not surprisingly, stress was also associated with pain intensity.
It is a long paper and touches on numerous other associations of varying degrees of strength between parental, social, and other external factors. But, in general, they were not as consistent as those related to a child’s beliefs.
Before, or at least at the same time, we treat a patient’s pain, we should learn more about that patient – his or her concerns, beliefs, and stressors. You and I may have exactly the same hernia operation, but if you have a better understanding of why you are going to feel uncomfortable after the surgery, and understand that not every pain is the result of a complication, I suspect you are more likely to complain of less pain.
The recent JIA study doesn’t claim to suggest therapeutic methods. However, one wonders what the result would be if we could somehow alter a patient’s belief system so that he or she no longer sees pain as always harmful, nor does the patient see himself or herself as powerless to do anything about the pain. To do this experiment we must follow up our robotic request to “rate your pain” with a dialogue in which we learn more about the patient. Which means probing believes, fears, and stressors.
You can tell me this exercise would be unrealistic and time consuming. But I bet in the long run it will save time. Even if it doesn’t it is the better way to manage pain.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Despite my best efforts to cultivate acquaintances across a broader age group, my social circle still has the somewhat musty odor of septuagenarians. We try to talk about things beyond the weather and grandchildren but pain scenarios surface with unfortunate frequency. Arthritic joints ache, body parts wear out or become diseased and have to be removed or replaced. That stuff can hurt.
There are two pain-related themes that seem to crop up more frequently than you might expect. The first is the unfortunate side effects of opioid medication – most often gastric distress and vomiting, then of course there’s constipation. They seem so common that a good many of my acquaintances just plain refuse to take opioids when they have been prescribed postoperatively because of their vivid memories of the consequences or horror stories friends have told.
The second theme is the general annoyance with the damn “Please rate your pain from one to ten” request issued by every well-intentioned nurse. Do you mean the pain I am having right now, this second, or last night, or the average over the last day and a half? Or should I be comparing it with when I gave birth 70 years ago, or when I stubbed my toe getting out of the shower last week? And then what are you going to do with my guesstimated number?
It may surprise some of you that 40 years ago there wasn’t a pain scale fetish. But a few observant health care professionals realized that many of our patients were suffering because we weren’t adequately managing their pain. In postoperative situations this was slowing recovery and effecting outcomes. Like good pseudoscientists, they realized that we should first quantify the pain and the notion that no pain should go unrated came into being. Nor should pain go untreated, which is too frequently interpreted as meaning unmedicated.
For example a systematic review of 61 studies of juvenile idiopathic arthritis (JIA) published in the journal Pediatric Rheumatology found that there was positive relationship between pain and a child’s belief that pain causes harm, disability, and lack of control. Not surprisingly, stress was also associated with pain intensity.
It is a long paper and touches on numerous other associations of varying degrees of strength between parental, social, and other external factors. But, in general, they were not as consistent as those related to a child’s beliefs.
Before, or at least at the same time, we treat a patient’s pain, we should learn more about that patient – his or her concerns, beliefs, and stressors. You and I may have exactly the same hernia operation, but if you have a better understanding of why you are going to feel uncomfortable after the surgery, and understand that not every pain is the result of a complication, I suspect you are more likely to complain of less pain.
The recent JIA study doesn’t claim to suggest therapeutic methods. However, one wonders what the result would be if we could somehow alter a patient’s belief system so that he or she no longer sees pain as always harmful, nor does the patient see himself or herself as powerless to do anything about the pain. To do this experiment we must follow up our robotic request to “rate your pain” with a dialogue in which we learn more about the patient. Which means probing believes, fears, and stressors.
You can tell me this exercise would be unrealistic and time consuming. But I bet in the long run it will save time. Even if it doesn’t it is the better way to manage pain.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Despite my best efforts to cultivate acquaintances across a broader age group, my social circle still has the somewhat musty odor of septuagenarians. We try to talk about things beyond the weather and grandchildren but pain scenarios surface with unfortunate frequency. Arthritic joints ache, body parts wear out or become diseased and have to be removed or replaced. That stuff can hurt.
There are two pain-related themes that seem to crop up more frequently than you might expect. The first is the unfortunate side effects of opioid medication – most often gastric distress and vomiting, then of course there’s constipation. They seem so common that a good many of my acquaintances just plain refuse to take opioids when they have been prescribed postoperatively because of their vivid memories of the consequences or horror stories friends have told.
The second theme is the general annoyance with the damn “Please rate your pain from one to ten” request issued by every well-intentioned nurse. Do you mean the pain I am having right now, this second, or last night, or the average over the last day and a half? Or should I be comparing it with when I gave birth 70 years ago, or when I stubbed my toe getting out of the shower last week? And then what are you going to do with my guesstimated number?
It may surprise some of you that 40 years ago there wasn’t a pain scale fetish. But a few observant health care professionals realized that many of our patients were suffering because we weren’t adequately managing their pain. In postoperative situations this was slowing recovery and effecting outcomes. Like good pseudoscientists, they realized that we should first quantify the pain and the notion that no pain should go unrated came into being. Nor should pain go untreated, which is too frequently interpreted as meaning unmedicated.
For example a systematic review of 61 studies of juvenile idiopathic arthritis (JIA) published in the journal Pediatric Rheumatology found that there was positive relationship between pain and a child’s belief that pain causes harm, disability, and lack of control. Not surprisingly, stress was also associated with pain intensity.
It is a long paper and touches on numerous other associations of varying degrees of strength between parental, social, and other external factors. But, in general, they were not as consistent as those related to a child’s beliefs.
Before, or at least at the same time, we treat a patient’s pain, we should learn more about that patient – his or her concerns, beliefs, and stressors. You and I may have exactly the same hernia operation, but if you have a better understanding of why you are going to feel uncomfortable after the surgery, and understand that not every pain is the result of a complication, I suspect you are more likely to complain of less pain.
The recent JIA study doesn’t claim to suggest therapeutic methods. However, one wonders what the result would be if we could somehow alter a patient’s belief system so that he or she no longer sees pain as always harmful, nor does the patient see himself or herself as powerless to do anything about the pain. To do this experiment we must follow up our robotic request to “rate your pain” with a dialogue in which we learn more about the patient. Which means probing believes, fears, and stressors.
You can tell me this exercise would be unrealistic and time consuming. But I bet in the long run it will save time. Even if it doesn’t it is the better way to manage pain.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A primer on gender-affirming care for transgender youth
Over the past few years, there has been rampant misinformation regarding gender-affirming care for transgender youth. In particular, there has been confusion regarding how care is administered, and what types of care are considered at various stages of development. This primer will help you understand the developmental approach to supporting transgender youth.
While people generally think of medical and surgical aspects of gender-affirming care, other domains can be just as important. For example, a 2020 publication in The Lancet Public Health found that access to gender-congruent government identification documents was associated with lower odds of severe psychological distress and suicidality.1
Considerations for prepubertal children
The youngest developmental stage at which a young person may seek care regarding gender diversity is the prepubertal childhood stage. Guidelines set forth by The Endocrine Society and The World Professional Association for Transgender Health make it clear that no medical or surgical interventions are considered at this developmental stage.2,3 However, some young people may choose to pursue a “social transition.” Though this may sound like one thing, social transition can mean very different things for different people. It may include any combination of adopting a new name, pronouns, hairstyle, clothing, etc. Young people may also choose to pursue these various aspects of social transition in all settings, or sometimes only in some settings (for example, only at home if they don’t yet feel comfortable doing so at school). Research so far shows that prepubertal children who are allowed to socially transition have levels of anxiety and depression nearly indistinguishable from their cisgender peers.4 While some in the past have raised the question of whether a social transition increases a child’s degree of gender incongruence and thus their likelihood to “persist” in a transgender identity, research has suggested this is not the case, and that gender identity does not meaningfully differ before and after a social transition.5 It’s worth noting, that “desistance” of a young person’s transgender identity is generally not considered an ethical goal and that gender identity conversion efforts (that is, attempts to force transgender people to be cisgender) have been labeled unethical by the American Academy of Child & Adolescent Psychiatry.
Sadly, transgender children are victims of bullying at high rates in their schools and communities. Creating safe and affirming school and community environments can be some of the highest yield ways in which providers can support the mental health of gender-diverse youth at this stage. Gender Spectrum is an excellent nonprofit that provides resources to help families and communities with some of these nonmedical supports.
Early adolescence and pubertal suppression
The earliest gender-affirming medical intervention that may be considered is pubertal suppression. Pubertal suppression is achieved with gonadotropin-releasing hormone agonists. This class of medications is Food and Drug Administration approved in pediatrics for precocious puberty – a condition in which young people enter puberty much earlier than expected (sometimes as early as age 3). For that condition, the rationale is to delay puberty until the patient reaches a more developmentally normative age for puberty to begin. The rationale for pubertal suppression for adolescent gender dysphoria is somewhat similar – these medications allow for the temporary pausing of puberty, which can be particularly helpful for adolescents who are having severe negative psychological reactions to the ways in which their bodies are developing. The major advantage here is that pubertal suppression can be reversed (if the medication is stopped, endogenous puberty will proceed), whereas puberty itself cannot be easily reversed (resulting in adult transgender people needing surgery and other interventions later in life, if these changes can be fully undone at all). As with all medications, puberty blockers do carry known side effects, including falling behind on bone density (sex hormones are needed to mineralize bones). Because of this, it is generally recommended that adolescents have their bone density monitored during treatment, pursue avenues to improve bone health (for example, exercise), and either stop the puberty blocker to undergo endogenous puberty or start gender-affirming hormones (estrogen or testosterone) by around age 16.
It is also important to note that, under current guidelines, an adolescent must first undergo a comprehensive biopsychosocial mental health evaluation prior to starting pubertal suppression to ensure the clinical team has a comprehensive understanding of the adolescent’s mental health, that all potential gender supports that are needed are put into place, and that the adolescent and their guardians have a strong understanding of the medical intervention, its risks, side effects, and potential benefits. In addition, consent must be provided by parents or legal guardians, whereas adolescents themselves provide assent. Several studies have linked access to pubertal suppression, when indicated for gender dysphoria, to improved mental health outcomes (for example, van der Miesen and colleagues, Turban and colleagues, de Vries and colleagues, and Costa and colleagues).6-9
Later adolescence and gender-affirming hormones
Later in adolescence, transgender youth may be candidates for gender-affirming hormone treatment (for example, estrogen or testosterone) to induce pubertal changes that align with their gender identities. Once again, under current guidelines, a comprehensive mental health biopsychosocial evaluation must be conducted prior to initiation of these treatments. Part of this evaluation includes fertility counseling and consideration of fertility preservation (for example, oocyte or semen cryopreservation), given the potential for these medications to impact fertility. It also involves discussion of several of the physiologic changes from these medications that can be irreversible (for example, voice changes from testosterone are particularly difficult to reverse in the future). Tables of the physical changes from these medications, when they begin after starting, and when they generally reach their maximum are available in the Endocrine Society guidelines.2 The past endocrine society guidelines recommended not initiating gender-affirming hormones until age 16. The most recent guidelines note that there may be instances in which providers may consider starting them as early as age 13 (for example, to reduce risk of falling behind on bone density, or if a patient is having psychological distress related to their peers going through puberty while they are still in a prepubertal state). The latest World Professional Association for Transgender Health Standards of Care removed specific age cutoffs, highlighting the importance of a multidisciplinary team of mental health and hormone prescribing providers working together to understand the best course of action for a particular patient. As with pubertal suppression, several studies have linked access to gender-affirming hormones to improve mental health for adolescents with gender dysphoria (for example, Turban and colleagues, Chen and colleagues, de Vries and colleagues, Allen and colleagues, and Tordoff and colleagues).10-14
Gender-affirming surgeries
The vast majority of gender-affirming surgeries are not considered until adulthood. The most notable exception to this is masculinizing top surgery for trans masculine and nonbinary adolescents. As with all surgeries, this is a major decision, and requires agreement from a mental health provider, a medical provider, and the surgeon. Early research suggests such surgeries result in improved chest dysphoria and that regret rates appear to be low.15,16 While the latest World Professional Association for Transgender Health similarly removed strict age cutoffs for gender-affirming surgery, again noting the importance of individualized care, I suspect most will read this change in the context of the Endocrine Society guidelines and past WPATH guidelines that noted gender-affirming genital surgeries are not offered until adulthood (a rare exception perhaps being someone pursuing a gender-affirming vaginoplasty at say age 17 in the summer prior to college to avoid needing to take off from school for surgical recovery). Gender-affirming genital surgeries are generally much more involved surgeries with prolonged recovery times.
Given the substantial proportion of young people who openly identify as transgender,17 and the proliferation of misinformation, political rhetoric, and legislation that can impact gender-affirming care for adolescents with gender dysphoria,18 it is essential that providers have accurate, up-to-date information on what this care entails and how it is provided.
Dr. Turban is director of the gender psychiatry program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on Twitter @jack_turban.
References
1. Malta M et al. Lancet Public Health. 2020 Apr;5(4):e178-9.
2. Hembree WC et al. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
3. Coleman E et al. Int J Transgend Health. 2022 Sep 6;23(Suppl 1):S1-259.
4. Durwood L et al. J Am Acad Child Adolesc Psychiatry. 2017 Feb;56(2):116-23.e2.
5. Rae JR et al. Psychol Sci. 2019 May;30(5):669-81.
6. van der Miesen AIR et al. J Adolesc Health. 2020 Jun;66(6):699-704.
7. Turban JL et al. Pediatrics. 2020 Feb;145(2):e20191725.
8. de Vries ALC et al. J Sex Med. 2011 Aug;8(8):2276-83.
9. Costa R et al. J Sex Med. 2015 Nov;12(11):2206-14.
10. Turban JL et al. PLoS One. 2022 Jan 12;17(1):e0261039.
11. Chen D et al. N Engl J Med. 2023;388:240-50.
12. de Vries ALC et al. Pediatrics. 2014 Oct;134(4):696-70.
13. Allen LR et al. Clin Pract Pediatr Psychol. 2019. doi: 10.1037/cpp0000288.
14. Tordoff DM et al. JAMA Netw Open. 2022 Feb 1;5(2):e220978.
15. Olson-Kennedy J et al. JAMA Pediatr. 2018;172(5):431-6.
16. Tang A et al. Ann Plast Surg. 2022 May;88(4 Suppl):S325-31
17. Johns MM et al. Morb Mortal Wkly Rep. 2019 Jan 25;68(3):67-71.
18. Turban JL et al. JAMA. 2021;325(22):2251-2.
Over the past few years, there has been rampant misinformation regarding gender-affirming care for transgender youth. In particular, there has been confusion regarding how care is administered, and what types of care are considered at various stages of development. This primer will help you understand the developmental approach to supporting transgender youth.
While people generally think of medical and surgical aspects of gender-affirming care, other domains can be just as important. For example, a 2020 publication in The Lancet Public Health found that access to gender-congruent government identification documents was associated with lower odds of severe psychological distress and suicidality.1
Considerations for prepubertal children
The youngest developmental stage at which a young person may seek care regarding gender diversity is the prepubertal childhood stage. Guidelines set forth by The Endocrine Society and The World Professional Association for Transgender Health make it clear that no medical or surgical interventions are considered at this developmental stage.2,3 However, some young people may choose to pursue a “social transition.” Though this may sound like one thing, social transition can mean very different things for different people. It may include any combination of adopting a new name, pronouns, hairstyle, clothing, etc. Young people may also choose to pursue these various aspects of social transition in all settings, or sometimes only in some settings (for example, only at home if they don’t yet feel comfortable doing so at school). Research so far shows that prepubertal children who are allowed to socially transition have levels of anxiety and depression nearly indistinguishable from their cisgender peers.4 While some in the past have raised the question of whether a social transition increases a child’s degree of gender incongruence and thus their likelihood to “persist” in a transgender identity, research has suggested this is not the case, and that gender identity does not meaningfully differ before and after a social transition.5 It’s worth noting, that “desistance” of a young person’s transgender identity is generally not considered an ethical goal and that gender identity conversion efforts (that is, attempts to force transgender people to be cisgender) have been labeled unethical by the American Academy of Child & Adolescent Psychiatry.
Sadly, transgender children are victims of bullying at high rates in their schools and communities. Creating safe and affirming school and community environments can be some of the highest yield ways in which providers can support the mental health of gender-diverse youth at this stage. Gender Spectrum is an excellent nonprofit that provides resources to help families and communities with some of these nonmedical supports.
Early adolescence and pubertal suppression
The earliest gender-affirming medical intervention that may be considered is pubertal suppression. Pubertal suppression is achieved with gonadotropin-releasing hormone agonists. This class of medications is Food and Drug Administration approved in pediatrics for precocious puberty – a condition in which young people enter puberty much earlier than expected (sometimes as early as age 3). For that condition, the rationale is to delay puberty until the patient reaches a more developmentally normative age for puberty to begin. The rationale for pubertal suppression for adolescent gender dysphoria is somewhat similar – these medications allow for the temporary pausing of puberty, which can be particularly helpful for adolescents who are having severe negative psychological reactions to the ways in which their bodies are developing. The major advantage here is that pubertal suppression can be reversed (if the medication is stopped, endogenous puberty will proceed), whereas puberty itself cannot be easily reversed (resulting in adult transgender people needing surgery and other interventions later in life, if these changes can be fully undone at all). As with all medications, puberty blockers do carry known side effects, including falling behind on bone density (sex hormones are needed to mineralize bones). Because of this, it is generally recommended that adolescents have their bone density monitored during treatment, pursue avenues to improve bone health (for example, exercise), and either stop the puberty blocker to undergo endogenous puberty or start gender-affirming hormones (estrogen or testosterone) by around age 16.
It is also important to note that, under current guidelines, an adolescent must first undergo a comprehensive biopsychosocial mental health evaluation prior to starting pubertal suppression to ensure the clinical team has a comprehensive understanding of the adolescent’s mental health, that all potential gender supports that are needed are put into place, and that the adolescent and their guardians have a strong understanding of the medical intervention, its risks, side effects, and potential benefits. In addition, consent must be provided by parents or legal guardians, whereas adolescents themselves provide assent. Several studies have linked access to pubertal suppression, when indicated for gender dysphoria, to improved mental health outcomes (for example, van der Miesen and colleagues, Turban and colleagues, de Vries and colleagues, and Costa and colleagues).6-9
Later adolescence and gender-affirming hormones
Later in adolescence, transgender youth may be candidates for gender-affirming hormone treatment (for example, estrogen or testosterone) to induce pubertal changes that align with their gender identities. Once again, under current guidelines, a comprehensive mental health biopsychosocial evaluation must be conducted prior to initiation of these treatments. Part of this evaluation includes fertility counseling and consideration of fertility preservation (for example, oocyte or semen cryopreservation), given the potential for these medications to impact fertility. It also involves discussion of several of the physiologic changes from these medications that can be irreversible (for example, voice changes from testosterone are particularly difficult to reverse in the future). Tables of the physical changes from these medications, when they begin after starting, and when they generally reach their maximum are available in the Endocrine Society guidelines.2 The past endocrine society guidelines recommended not initiating gender-affirming hormones until age 16. The most recent guidelines note that there may be instances in which providers may consider starting them as early as age 13 (for example, to reduce risk of falling behind on bone density, or if a patient is having psychological distress related to their peers going through puberty while they are still in a prepubertal state). The latest World Professional Association for Transgender Health Standards of Care removed specific age cutoffs, highlighting the importance of a multidisciplinary team of mental health and hormone prescribing providers working together to understand the best course of action for a particular patient. As with pubertal suppression, several studies have linked access to gender-affirming hormones to improve mental health for adolescents with gender dysphoria (for example, Turban and colleagues, Chen and colleagues, de Vries and colleagues, Allen and colleagues, and Tordoff and colleagues).10-14
Gender-affirming surgeries
The vast majority of gender-affirming surgeries are not considered until adulthood. The most notable exception to this is masculinizing top surgery for trans masculine and nonbinary adolescents. As with all surgeries, this is a major decision, and requires agreement from a mental health provider, a medical provider, and the surgeon. Early research suggests such surgeries result in improved chest dysphoria and that regret rates appear to be low.15,16 While the latest World Professional Association for Transgender Health similarly removed strict age cutoffs for gender-affirming surgery, again noting the importance of individualized care, I suspect most will read this change in the context of the Endocrine Society guidelines and past WPATH guidelines that noted gender-affirming genital surgeries are not offered until adulthood (a rare exception perhaps being someone pursuing a gender-affirming vaginoplasty at say age 17 in the summer prior to college to avoid needing to take off from school for surgical recovery). Gender-affirming genital surgeries are generally much more involved surgeries with prolonged recovery times.
Given the substantial proportion of young people who openly identify as transgender,17 and the proliferation of misinformation, political rhetoric, and legislation that can impact gender-affirming care for adolescents with gender dysphoria,18 it is essential that providers have accurate, up-to-date information on what this care entails and how it is provided.
Dr. Turban is director of the gender psychiatry program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on Twitter @jack_turban.
References
1. Malta M et al. Lancet Public Health. 2020 Apr;5(4):e178-9.
2. Hembree WC et al. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
3. Coleman E et al. Int J Transgend Health. 2022 Sep 6;23(Suppl 1):S1-259.
4. Durwood L et al. J Am Acad Child Adolesc Psychiatry. 2017 Feb;56(2):116-23.e2.
5. Rae JR et al. Psychol Sci. 2019 May;30(5):669-81.
6. van der Miesen AIR et al. J Adolesc Health. 2020 Jun;66(6):699-704.
7. Turban JL et al. Pediatrics. 2020 Feb;145(2):e20191725.
8. de Vries ALC et al. J Sex Med. 2011 Aug;8(8):2276-83.
9. Costa R et al. J Sex Med. 2015 Nov;12(11):2206-14.
10. Turban JL et al. PLoS One. 2022 Jan 12;17(1):e0261039.
11. Chen D et al. N Engl J Med. 2023;388:240-50.
12. de Vries ALC et al. Pediatrics. 2014 Oct;134(4):696-70.
13. Allen LR et al. Clin Pract Pediatr Psychol. 2019. doi: 10.1037/cpp0000288.
14. Tordoff DM et al. JAMA Netw Open. 2022 Feb 1;5(2):e220978.
15. Olson-Kennedy J et al. JAMA Pediatr. 2018;172(5):431-6.
16. Tang A et al. Ann Plast Surg. 2022 May;88(4 Suppl):S325-31
17. Johns MM et al. Morb Mortal Wkly Rep. 2019 Jan 25;68(3):67-71.
18. Turban JL et al. JAMA. 2021;325(22):2251-2.
Over the past few years, there has been rampant misinformation regarding gender-affirming care for transgender youth. In particular, there has been confusion regarding how care is administered, and what types of care are considered at various stages of development. This primer will help you understand the developmental approach to supporting transgender youth.
While people generally think of medical and surgical aspects of gender-affirming care, other domains can be just as important. For example, a 2020 publication in The Lancet Public Health found that access to gender-congruent government identification documents was associated with lower odds of severe psychological distress and suicidality.1
Considerations for prepubertal children
The youngest developmental stage at which a young person may seek care regarding gender diversity is the prepubertal childhood stage. Guidelines set forth by The Endocrine Society and The World Professional Association for Transgender Health make it clear that no medical or surgical interventions are considered at this developmental stage.2,3 However, some young people may choose to pursue a “social transition.” Though this may sound like one thing, social transition can mean very different things for different people. It may include any combination of adopting a new name, pronouns, hairstyle, clothing, etc. Young people may also choose to pursue these various aspects of social transition in all settings, or sometimes only in some settings (for example, only at home if they don’t yet feel comfortable doing so at school). Research so far shows that prepubertal children who are allowed to socially transition have levels of anxiety and depression nearly indistinguishable from their cisgender peers.4 While some in the past have raised the question of whether a social transition increases a child’s degree of gender incongruence and thus their likelihood to “persist” in a transgender identity, research has suggested this is not the case, and that gender identity does not meaningfully differ before and after a social transition.5 It’s worth noting, that “desistance” of a young person’s transgender identity is generally not considered an ethical goal and that gender identity conversion efforts (that is, attempts to force transgender people to be cisgender) have been labeled unethical by the American Academy of Child & Adolescent Psychiatry.
Sadly, transgender children are victims of bullying at high rates in their schools and communities. Creating safe and affirming school and community environments can be some of the highest yield ways in which providers can support the mental health of gender-diverse youth at this stage. Gender Spectrum is an excellent nonprofit that provides resources to help families and communities with some of these nonmedical supports.
Early adolescence and pubertal suppression
The earliest gender-affirming medical intervention that may be considered is pubertal suppression. Pubertal suppression is achieved with gonadotropin-releasing hormone agonists. This class of medications is Food and Drug Administration approved in pediatrics for precocious puberty – a condition in which young people enter puberty much earlier than expected (sometimes as early as age 3). For that condition, the rationale is to delay puberty until the patient reaches a more developmentally normative age for puberty to begin. The rationale for pubertal suppression for adolescent gender dysphoria is somewhat similar – these medications allow for the temporary pausing of puberty, which can be particularly helpful for adolescents who are having severe negative psychological reactions to the ways in which their bodies are developing. The major advantage here is that pubertal suppression can be reversed (if the medication is stopped, endogenous puberty will proceed), whereas puberty itself cannot be easily reversed (resulting in adult transgender people needing surgery and other interventions later in life, if these changes can be fully undone at all). As with all medications, puberty blockers do carry known side effects, including falling behind on bone density (sex hormones are needed to mineralize bones). Because of this, it is generally recommended that adolescents have their bone density monitored during treatment, pursue avenues to improve bone health (for example, exercise), and either stop the puberty blocker to undergo endogenous puberty or start gender-affirming hormones (estrogen or testosterone) by around age 16.
It is also important to note that, under current guidelines, an adolescent must first undergo a comprehensive biopsychosocial mental health evaluation prior to starting pubertal suppression to ensure the clinical team has a comprehensive understanding of the adolescent’s mental health, that all potential gender supports that are needed are put into place, and that the adolescent and their guardians have a strong understanding of the medical intervention, its risks, side effects, and potential benefits. In addition, consent must be provided by parents or legal guardians, whereas adolescents themselves provide assent. Several studies have linked access to pubertal suppression, when indicated for gender dysphoria, to improved mental health outcomes (for example, van der Miesen and colleagues, Turban and colleagues, de Vries and colleagues, and Costa and colleagues).6-9
Later adolescence and gender-affirming hormones
Later in adolescence, transgender youth may be candidates for gender-affirming hormone treatment (for example, estrogen or testosterone) to induce pubertal changes that align with their gender identities. Once again, under current guidelines, a comprehensive mental health biopsychosocial evaluation must be conducted prior to initiation of these treatments. Part of this evaluation includes fertility counseling and consideration of fertility preservation (for example, oocyte or semen cryopreservation), given the potential for these medications to impact fertility. It also involves discussion of several of the physiologic changes from these medications that can be irreversible (for example, voice changes from testosterone are particularly difficult to reverse in the future). Tables of the physical changes from these medications, when they begin after starting, and when they generally reach their maximum are available in the Endocrine Society guidelines.2 The past endocrine society guidelines recommended not initiating gender-affirming hormones until age 16. The most recent guidelines note that there may be instances in which providers may consider starting them as early as age 13 (for example, to reduce risk of falling behind on bone density, or if a patient is having psychological distress related to their peers going through puberty while they are still in a prepubertal state). The latest World Professional Association for Transgender Health Standards of Care removed specific age cutoffs, highlighting the importance of a multidisciplinary team of mental health and hormone prescribing providers working together to understand the best course of action for a particular patient. As with pubertal suppression, several studies have linked access to gender-affirming hormones to improve mental health for adolescents with gender dysphoria (for example, Turban and colleagues, Chen and colleagues, de Vries and colleagues, Allen and colleagues, and Tordoff and colleagues).10-14
Gender-affirming surgeries
The vast majority of gender-affirming surgeries are not considered until adulthood. The most notable exception to this is masculinizing top surgery for trans masculine and nonbinary adolescents. As with all surgeries, this is a major decision, and requires agreement from a mental health provider, a medical provider, and the surgeon. Early research suggests such surgeries result in improved chest dysphoria and that regret rates appear to be low.15,16 While the latest World Professional Association for Transgender Health similarly removed strict age cutoffs for gender-affirming surgery, again noting the importance of individualized care, I suspect most will read this change in the context of the Endocrine Society guidelines and past WPATH guidelines that noted gender-affirming genital surgeries are not offered until adulthood (a rare exception perhaps being someone pursuing a gender-affirming vaginoplasty at say age 17 in the summer prior to college to avoid needing to take off from school for surgical recovery). Gender-affirming genital surgeries are generally much more involved surgeries with prolonged recovery times.
Given the substantial proportion of young people who openly identify as transgender,17 and the proliferation of misinformation, political rhetoric, and legislation that can impact gender-affirming care for adolescents with gender dysphoria,18 it is essential that providers have accurate, up-to-date information on what this care entails and how it is provided.
Dr. Turban is director of the gender psychiatry program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on Twitter @jack_turban.
References
1. Malta M et al. Lancet Public Health. 2020 Apr;5(4):e178-9.
2. Hembree WC et al. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
3. Coleman E et al. Int J Transgend Health. 2022 Sep 6;23(Suppl 1):S1-259.
4. Durwood L et al. J Am Acad Child Adolesc Psychiatry. 2017 Feb;56(2):116-23.e2.
5. Rae JR et al. Psychol Sci. 2019 May;30(5):669-81.
6. van der Miesen AIR et al. J Adolesc Health. 2020 Jun;66(6):699-704.
7. Turban JL et al. Pediatrics. 2020 Feb;145(2):e20191725.
8. de Vries ALC et al. J Sex Med. 2011 Aug;8(8):2276-83.
9. Costa R et al. J Sex Med. 2015 Nov;12(11):2206-14.
10. Turban JL et al. PLoS One. 2022 Jan 12;17(1):e0261039.
11. Chen D et al. N Engl J Med. 2023;388:240-50.
12. de Vries ALC et al. Pediatrics. 2014 Oct;134(4):696-70.
13. Allen LR et al. Clin Pract Pediatr Psychol. 2019. doi: 10.1037/cpp0000288.
14. Tordoff DM et al. JAMA Netw Open. 2022 Feb 1;5(2):e220978.
15. Olson-Kennedy J et al. JAMA Pediatr. 2018;172(5):431-6.
16. Tang A et al. Ann Plast Surg. 2022 May;88(4 Suppl):S325-31
17. Johns MM et al. Morb Mortal Wkly Rep. 2019 Jan 25;68(3):67-71.
18. Turban JL et al. JAMA. 2021;325(22):2251-2.









