User login
Analysis of Pharmacist Interventions Used to Resolve Safety Target of Polypharmacy (STOP) Drug Interactions
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
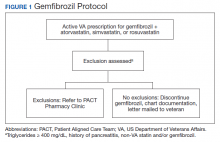
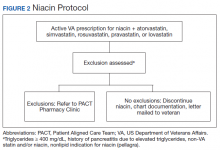
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
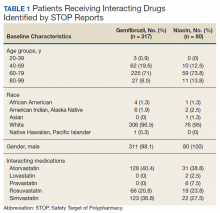
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
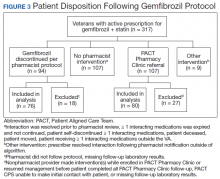
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
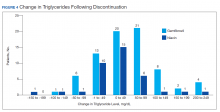
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.
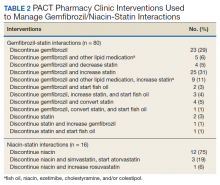
Of the 107 patients referred to the PACT Pharmacy Clinic, 80 (74.8%) had TG laboratory results available and were included in the analysis. These patients were followed by the PACT CPS until the drug interaction was resolved and confirmed to have TG levels at goal (< 500 mg/dL). Gemfibrozil doses ranged from 300 mg daily to 600 mg twice daily, with 70% (n = 56) of patients taking 600 mg twice daily. The PACT CPS made 148 interventions (Table 2). Twenty-three (29%) patients required only gemfibrozil discontinuation. The remaining 57 patients (71%) required at least 2 medication interventions. The PACT CPS generated 213 encounters for resolving drug interactions with a median of 2 encounters per patient.
Quality assurance review identified 5 patients (5.3%) who underwent gemfibrozil discontinuation by protocol, despite having criteria that would have recommended against discontinuation. In accordance with the protocol criteria, these patients were later referred to the PACT Pharmacy Clinic. None of these patients experienced a TG increase at or above the threshold of 500 mg/dL after gemfibrozil was initially discontinued but were excluded from the earlier analysis.
Niacin-Statin Interactions
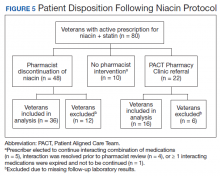
Pharmacists discontinued niacin by protocol for 48 patients (60.0%), and 22 patients (27.5%) were referred to the PACT Pharmacy Clinic (Figure 5). For the remaining 5 patients (6.3%), the interaction was either addressed outside the protocol prior to pharmacist review, or an interacting prescription was expired and not to be continued. Additionally, niacin was continued per prescriber preference in 5 patients (6.3%).
Thirty-six patients (75%) had TG laboratory results available following niacin discontinuation by protocol and were included in the analysis. Most patients’ (n = 33, 91.7%) TG levels decreased or increased by < 100 mg/dL. No patient had a TG level that increased higher than the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter, was 5.3 (2.5) months (range, 1.2-9.8). The pharmacists spent a mean of 15 minutes per patient resolving each interaction. The quality assurance review found no discrepancies in the pharmacists’ application of the protocol.
Of the 22 patients referred to the PACT Pharmacy Clinic, 16 (72.7%) patients had TG laboratory results available and were included in the analysis. As with the gemfibrozil interactions, these patients were followed by the PACT Pharmacy Clinic until the drug interaction was resolved and confirmed to have TGs at goal (< 500 mg/dL). Niacin doses ranged from 500 mg daily to 2,000 mg daily, with the majority of patients taking 1,000 mg daily. The PACT CPS made 23 interventions. The PACT CPS generated 46 encounters for resolving drug interactions with a median of 2 encounters per patient.
Discussion
Following gemfibrozil or niacin discontinuation by protocol, most patients with available laboratory results experienced either a decrease or modest TG elevation. The proportion of patients experiencing a decrease in TGs was unexpected but potentially multifactorial. Individual causes for the decrease in TGs were beyond the scope of this analysis. The retrospective design limited the ability to identify variables that could have impacted TG levels when gemfibrozil or niacin were started and discontinued. Although the treatment of TG levels is not indicated until it is ≥ 500 mg/dL, due to an increased risk of pancreatitis, both protocols excluded patients with a history of TGs ≥ 400 mg/dL.19 The lower threshold was set to compensate for anticipated increase in TG levels, following gemfibrozil or niacin discontinuation, and to minimize the number of patients with TG levels ≥ 500 mg/dL. The actual impact on patients’ TG levels supports the use of this lower threshold in the protocol.
When TG levels increased by 200 to 249 mg/dL after gemfibrozil or niacin discontinuation, patients were evaluated for possible underlying causes, which occurred for 4 gemfibrozil and 1 niacin patient. One patient started a β-blocker after gemfibrozil was initiated, and 3 patients were taking gemfibrozil prior to establishing care at the VA. The TG levels of the patient taking niacin correlated with an increased hemoglobin A1c. The TG level for only 1 patient taking gemfibrozil increased above the 500 mg/dL threshold. The patient had several comorbidities known to increase TG levels, but the comorbidities were previously well controlled. No additional medication changes were made at that time, and the TG levels on the next fasting lipid panel decreased to goal. The patient did not experience any negative clinical sequelae from the elevated TG levels.
Thirty-five patients (36%) who were referred to the PACT Pharmacy Clinic required only either gemfibrozil or niacin discontinuation. These patients were evaluated to identify whether adjustments to the protocols would have allowed for pharmacist discontinuation without referral to the PACT Pharmacy Clinic. Twenty-four of these patients (69%) had repeated TG levels ≥ 400 mg/dL prior to referral to the PACT Pharmacy Clinic. Additionally, there was no correlation between the gemfibrozil or niacin doses and the change in TG levels following discontinuation. These data indicate the protocols appropriately identified patients who did not have an indication for gemfibrozil or niacin.
In addition to drug interactions identified on the STOP report, the PACT CPS resolved 12 additional interactions involving simvastatin and gemfibrozil. Additionally, unnecessary lipid medications were deprescribed. The PACT CPS identified 13 patients who experienced myalgias, an ADR attributed to the gemfibrozil- statin interaction. Of those, 9 patients’ ADRs resolved after discontinuing gemfibrozil alone. For the remaining 4 patients, additional interventions to convert the patient to another statin were required to resolve the ADR.
Using pharmacists to address the drug interactions shifted workload from the prescribers and other primary care team members. The mean time spent to resolve both gemfibrozil and niacin interactions by protocol was 15.5 minutes. One hundred fortytwo patients (35.8%) had drug interactions resolved by protocol, saving the PACT CPS’ expertise for patients requiring individualized interventions. Drug interactions were resolved within 4 PACT CPS encounters for 93.8% of the patients taking gemfibrozil and within 3 PACT CPS encounters for 93.8% of the patients taking niacin.
The protocols allowed 12 additional pharmacists who did not have an ambulatory care scope of practice to assist the PACT CPS in mitigating the STOP drug interactions. These pharmacists otherwise would have been limited to making consultative recommendations. Simultaneously, the design allowed for the PACT pharmacists’ expertise to be allocated for patients most likely to require interventions beyond the protocols. This type of intraprofessional referral process is not well described in the medical literature. To the authors’ knowledge, the only studies described referrals from hospital pharmacists to community pharmacists during transitions of care on hospital discharge.20,21
Limitations
The results of this study are derived from a retrospective chart review at a single VA facility. The autonomous nature of PACT CPS interventions may be difficult to replicate in other settings that do not permit pharmacists the same prescriptive authority. This analysis was designed to demonstrate the impact of the pharmacist in resolving major drug interactions. Patients referred to the PACT Pharmacy Clinic who also had their lipid medications adjusted by a nonpharmacist provider were excluded. However, this may have minimized the impact of the PACT CPS on the patient care provided. As postintervention laboratory results were not available for all patients, some patients’ TG levels could have increased above the 500 mg/dL threshold but were not identified. The time investment was extensive and likely underestimates the true cost of implementing the interventions.
Because notification letters were used to instruct patients to stop gemfibrozil or niacin, several considerations need to be addressed when interpreting the follow-up laboratory results. First, we cannot confirm whether the patients received the letter or the exact date the letter was received. Additionally, we cannot confirm whether the patients followed the instructions to stop the interacting medications or the date the medications were stopped. It is possible some patients were still taking the interacting medication when the first laboratory was drawn. Should a patient have continued the interacting medication, most would have run out and been unable to obtain a refill within 90 days of receiving the letter, as this is the maximum amount dispensed at one time. The mean time to the first laboratory result for both gemfibrozil and niacin was 6.5 and 5.3 months, respectively. Approximately 85% of patients completed the first laboratory test at least 3 months after the letter was mailed.
The protocols were designed to assess whether gemfibrozil or niacin was indicated and did not assess whether the statin was indicated. Therefore, discontinuing the statin also could have resolved the interaction appropriately. However, due to characteristics of the patient population and recommendations in current lipid guidelines, it was more likely the statin would be indicated.22,23 The protocols also assumed that patients eligible for gemfibrozil or niacin discontinuation would not need additional changes to their lipid medications. The medication changes made by the PACT CPS may have gone beyond those minimally necessary to resolve the drug interaction and maintain TG goals. Patients who had gemfibrozil or niacin discontinued by protocol also may have benefited from additional optimization of their lipid medications.
Conclusions
This quality improvement analysis supports further evaluation of the complementary use of protocols and PACT CPS prescriptive authority to resolve statin drug interactions. The gemfibrozil and niacin protocols appropriately identified patients who were less likely to experience an adverse change in TG laboratory results. Patients more likely to require additional medication interventions were appropriately referred to the PACT Pharmacy Clinics for individualized care. These data support expanded roles for pharmacists, across various settings, to mitigate select drug interactions at the Truman VA.
Acknowledgments
This quality improvement project is the result of work supported with resources and use of the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Missouri.
1. The top 200 drugs of 2020 Provided by the ClinCalc DrugStats Database. http://clincalc.com/DrugStats /Top200Drugs.aspx. Updated February 11, 2017. Accessed May 12, 2020.
2. Wiggins BS, Saseen JJ, Page RL 2nd, et al; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468‐e495. doi:10.1161/CIR.0000000000000456
3. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf. 2011;10(6):871‐882. doi:10.1517/14740338.2011.583916
4. US Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. https://www.fda.gov/Drugs/DrugSafety /ucm256581.htm. Updated December 15, 2017. Accessed May 12, 2020.
5. US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov /Drugs/DrugSafety/ucm293101.htm. Updated January 19, 2016. Accessed May 12, 2020.
6. US Food and Drug Administration Federal Register. AbbVie Inc. et al; withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://www.federalregister .gov/documents/2016/04/18/2016-08887/abbvie-inc -et-al-withdrawal-of-approval-of-indications-related -to-the-coadministration-with-statins. Published April 18, 2016. Accessed May 12, 2020.
7. Lamprecht DG Jr, Todd BA, Denham AM, Ruppe LK, Stadler SL. Clinical pharmacist patient-safety initiative to reduce against-label prescribing of statins with cyclosporine. Ann Pharmacother. 2017;51(2):140‐145. doi:10.1177/1060028016675352
8. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drugdrug interactions in patients with heart failure: A randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647‐652. doi:10.1016/j.ijcard.2015.10.206
9. Tuchscherer RM, Nair K, Ghushchyan V, Saseen JJ. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs. 2015;15(1):27‐34. doi:10.1007/s40256-014-0096-x
10. Alford JC, Saseen JJ, Allen RR, Nair KV. Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States Food and Drug Administration dose restrictions. Pharmacotherapy. 2012;32(7):623‐630. doi:10.1002/j.1875-9114.2011.01090.x
11. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [published correction appears in JAMA 2000 Mar 8;283(10):1293]. JAMA. 1999;282(3):267‐270. doi:10.1001/jama.282.3.267
12. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014‐2018. doi:10.1001/archinte.163.17.2014
13. Humphries TL, Carroll N, Chester EA, Magid D, Rocho B. Evaluation of an electronic critical drug interaction program coupled with active pharmacist intervention. Ann Pharmacother. 2007;41(12):1979‐1985. doi:10.1345/aph.1K349
14. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
15. Lipitor [package insert]. New York, NY: Pfizer; 2017.
16. Crestor [package insert]. Wilmington, DE: AstraZeneca; 2018.
17. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
18. Wolters Kluwer Health, Lexi-Drugs, Lexicomp. Pravastatin. www.online.lexi.com. [Source not verified.]
19. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726
20. Ferguson J, Seston L, Ashcroft DM. Refer-to-pharmacy: a qualitative study exploring the implementation of an electronic transfer of care initiative to improve medicines optimisation following hospital discharge. BMC Health Serv Res. 2018;18(1):424. doi:10.1186/s12913-018-3262-z
21. Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm. 2019;15(3):267‐278. doi:10.1016/j.sapharm.2018.05.001
22. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction guideline summary. https://www.healthquality.va.gov /guidelines/CD/lipids/LipidSumOptSinglePg31Aug15.pdf. Published 2014. Accessed May 14, 2020.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014 Jun 24;129(25) (suppl 2):S46-48] [published correction appears in Circulation. 2015 Dec 22;132(25):e396]. Circulation. 2014;129(25)(suppl 2): S1‐S45. doi:10.1161/01.cir.0000437738.63853.7a
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.
Of the 107 patients referred to the PACT Pharmacy Clinic, 80 (74.8%) had TG laboratory results available and were included in the analysis. These patients were followed by the PACT CPS until the drug interaction was resolved and confirmed to have TG levels at goal (< 500 mg/dL). Gemfibrozil doses ranged from 300 mg daily to 600 mg twice daily, with 70% (n = 56) of patients taking 600 mg twice daily. The PACT CPS made 148 interventions (Table 2). Twenty-three (29%) patients required only gemfibrozil discontinuation. The remaining 57 patients (71%) required at least 2 medication interventions. The PACT CPS generated 213 encounters for resolving drug interactions with a median of 2 encounters per patient.
Quality assurance review identified 5 patients (5.3%) who underwent gemfibrozil discontinuation by protocol, despite having criteria that would have recommended against discontinuation. In accordance with the protocol criteria, these patients were later referred to the PACT Pharmacy Clinic. None of these patients experienced a TG increase at or above the threshold of 500 mg/dL after gemfibrozil was initially discontinued but were excluded from the earlier analysis.
Niacin-Statin Interactions
Pharmacists discontinued niacin by protocol for 48 patients (60.0%), and 22 patients (27.5%) were referred to the PACT Pharmacy Clinic (Figure 5). For the remaining 5 patients (6.3%), the interaction was either addressed outside the protocol prior to pharmacist review, or an interacting prescription was expired and not to be continued. Additionally, niacin was continued per prescriber preference in 5 patients (6.3%).
Thirty-six patients (75%) had TG laboratory results available following niacin discontinuation by protocol and were included in the analysis. Most patients’ (n = 33, 91.7%) TG levels decreased or increased by < 100 mg/dL. No patient had a TG level that increased higher than the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter, was 5.3 (2.5) months (range, 1.2-9.8). The pharmacists spent a mean of 15 minutes per patient resolving each interaction. The quality assurance review found no discrepancies in the pharmacists’ application of the protocol.
Of the 22 patients referred to the PACT Pharmacy Clinic, 16 (72.7%) patients had TG laboratory results available and were included in the analysis. As with the gemfibrozil interactions, these patients were followed by the PACT Pharmacy Clinic until the drug interaction was resolved and confirmed to have TGs at goal (< 500 mg/dL). Niacin doses ranged from 500 mg daily to 2,000 mg daily, with the majority of patients taking 1,000 mg daily. The PACT CPS made 23 interventions. The PACT CPS generated 46 encounters for resolving drug interactions with a median of 2 encounters per patient.
Discussion
Following gemfibrozil or niacin discontinuation by protocol, most patients with available laboratory results experienced either a decrease or modest TG elevation. The proportion of patients experiencing a decrease in TGs was unexpected but potentially multifactorial. Individual causes for the decrease in TGs were beyond the scope of this analysis. The retrospective design limited the ability to identify variables that could have impacted TG levels when gemfibrozil or niacin were started and discontinued. Although the treatment of TG levels is not indicated until it is ≥ 500 mg/dL, due to an increased risk of pancreatitis, both protocols excluded patients with a history of TGs ≥ 400 mg/dL.19 The lower threshold was set to compensate for anticipated increase in TG levels, following gemfibrozil or niacin discontinuation, and to minimize the number of patients with TG levels ≥ 500 mg/dL. The actual impact on patients’ TG levels supports the use of this lower threshold in the protocol.
When TG levels increased by 200 to 249 mg/dL after gemfibrozil or niacin discontinuation, patients were evaluated for possible underlying causes, which occurred for 4 gemfibrozil and 1 niacin patient. One patient started a β-blocker after gemfibrozil was initiated, and 3 patients were taking gemfibrozil prior to establishing care at the VA. The TG levels of the patient taking niacin correlated with an increased hemoglobin A1c. The TG level for only 1 patient taking gemfibrozil increased above the 500 mg/dL threshold. The patient had several comorbidities known to increase TG levels, but the comorbidities were previously well controlled. No additional medication changes were made at that time, and the TG levels on the next fasting lipid panel decreased to goal. The patient did not experience any negative clinical sequelae from the elevated TG levels.
Thirty-five patients (36%) who were referred to the PACT Pharmacy Clinic required only either gemfibrozil or niacin discontinuation. These patients were evaluated to identify whether adjustments to the protocols would have allowed for pharmacist discontinuation without referral to the PACT Pharmacy Clinic. Twenty-four of these patients (69%) had repeated TG levels ≥ 400 mg/dL prior to referral to the PACT Pharmacy Clinic. Additionally, there was no correlation between the gemfibrozil or niacin doses and the change in TG levels following discontinuation. These data indicate the protocols appropriately identified patients who did not have an indication for gemfibrozil or niacin.
In addition to drug interactions identified on the STOP report, the PACT CPS resolved 12 additional interactions involving simvastatin and gemfibrozil. Additionally, unnecessary lipid medications were deprescribed. The PACT CPS identified 13 patients who experienced myalgias, an ADR attributed to the gemfibrozil- statin interaction. Of those, 9 patients’ ADRs resolved after discontinuing gemfibrozil alone. For the remaining 4 patients, additional interventions to convert the patient to another statin were required to resolve the ADR.
Using pharmacists to address the drug interactions shifted workload from the prescribers and other primary care team members. The mean time spent to resolve both gemfibrozil and niacin interactions by protocol was 15.5 minutes. One hundred fortytwo patients (35.8%) had drug interactions resolved by protocol, saving the PACT CPS’ expertise for patients requiring individualized interventions. Drug interactions were resolved within 4 PACT CPS encounters for 93.8% of the patients taking gemfibrozil and within 3 PACT CPS encounters for 93.8% of the patients taking niacin.
The protocols allowed 12 additional pharmacists who did not have an ambulatory care scope of practice to assist the PACT CPS in mitigating the STOP drug interactions. These pharmacists otherwise would have been limited to making consultative recommendations. Simultaneously, the design allowed for the PACT pharmacists’ expertise to be allocated for patients most likely to require interventions beyond the protocols. This type of intraprofessional referral process is not well described in the medical literature. To the authors’ knowledge, the only studies described referrals from hospital pharmacists to community pharmacists during transitions of care on hospital discharge.20,21
Limitations
The results of this study are derived from a retrospective chart review at a single VA facility. The autonomous nature of PACT CPS interventions may be difficult to replicate in other settings that do not permit pharmacists the same prescriptive authority. This analysis was designed to demonstrate the impact of the pharmacist in resolving major drug interactions. Patients referred to the PACT Pharmacy Clinic who also had their lipid medications adjusted by a nonpharmacist provider were excluded. However, this may have minimized the impact of the PACT CPS on the patient care provided. As postintervention laboratory results were not available for all patients, some patients’ TG levels could have increased above the 500 mg/dL threshold but were not identified. The time investment was extensive and likely underestimates the true cost of implementing the interventions.
Because notification letters were used to instruct patients to stop gemfibrozil or niacin, several considerations need to be addressed when interpreting the follow-up laboratory results. First, we cannot confirm whether the patients received the letter or the exact date the letter was received. Additionally, we cannot confirm whether the patients followed the instructions to stop the interacting medications or the date the medications were stopped. It is possible some patients were still taking the interacting medication when the first laboratory was drawn. Should a patient have continued the interacting medication, most would have run out and been unable to obtain a refill within 90 days of receiving the letter, as this is the maximum amount dispensed at one time. The mean time to the first laboratory result for both gemfibrozil and niacin was 6.5 and 5.3 months, respectively. Approximately 85% of patients completed the first laboratory test at least 3 months after the letter was mailed.
The protocols were designed to assess whether gemfibrozil or niacin was indicated and did not assess whether the statin was indicated. Therefore, discontinuing the statin also could have resolved the interaction appropriately. However, due to characteristics of the patient population and recommendations in current lipid guidelines, it was more likely the statin would be indicated.22,23 The protocols also assumed that patients eligible for gemfibrozil or niacin discontinuation would not need additional changes to their lipid medications. The medication changes made by the PACT CPS may have gone beyond those minimally necessary to resolve the drug interaction and maintain TG goals. Patients who had gemfibrozil or niacin discontinued by protocol also may have benefited from additional optimization of their lipid medications.
Conclusions
This quality improvement analysis supports further evaluation of the complementary use of protocols and PACT CPS prescriptive authority to resolve statin drug interactions. The gemfibrozil and niacin protocols appropriately identified patients who were less likely to experience an adverse change in TG laboratory results. Patients more likely to require additional medication interventions were appropriately referred to the PACT Pharmacy Clinics for individualized care. These data support expanded roles for pharmacists, across various settings, to mitigate select drug interactions at the Truman VA.
Acknowledgments
This quality improvement project is the result of work supported with resources and use of the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Missouri.
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.
Of the 107 patients referred to the PACT Pharmacy Clinic, 80 (74.8%) had TG laboratory results available and were included in the analysis. These patients were followed by the PACT CPS until the drug interaction was resolved and confirmed to have TG levels at goal (< 500 mg/dL). Gemfibrozil doses ranged from 300 mg daily to 600 mg twice daily, with 70% (n = 56) of patients taking 600 mg twice daily. The PACT CPS made 148 interventions (Table 2). Twenty-three (29%) patients required only gemfibrozil discontinuation. The remaining 57 patients (71%) required at least 2 medication interventions. The PACT CPS generated 213 encounters for resolving drug interactions with a median of 2 encounters per patient.
Quality assurance review identified 5 patients (5.3%) who underwent gemfibrozil discontinuation by protocol, despite having criteria that would have recommended against discontinuation. In accordance with the protocol criteria, these patients were later referred to the PACT Pharmacy Clinic. None of these patients experienced a TG increase at or above the threshold of 500 mg/dL after gemfibrozil was initially discontinued but were excluded from the earlier analysis.
Niacin-Statin Interactions
Pharmacists discontinued niacin by protocol for 48 patients (60.0%), and 22 patients (27.5%) were referred to the PACT Pharmacy Clinic (Figure 5). For the remaining 5 patients (6.3%), the interaction was either addressed outside the protocol prior to pharmacist review, or an interacting prescription was expired and not to be continued. Additionally, niacin was continued per prescriber preference in 5 patients (6.3%).
Thirty-six patients (75%) had TG laboratory results available following niacin discontinuation by protocol and were included in the analysis. Most patients’ (n = 33, 91.7%) TG levels decreased or increased by < 100 mg/dL. No patient had a TG level that increased higher than the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter, was 5.3 (2.5) months (range, 1.2-9.8). The pharmacists spent a mean of 15 minutes per patient resolving each interaction. The quality assurance review found no discrepancies in the pharmacists’ application of the protocol.
Of the 22 patients referred to the PACT Pharmacy Clinic, 16 (72.7%) patients had TG laboratory results available and were included in the analysis. As with the gemfibrozil interactions, these patients were followed by the PACT Pharmacy Clinic until the drug interaction was resolved and confirmed to have TGs at goal (< 500 mg/dL). Niacin doses ranged from 500 mg daily to 2,000 mg daily, with the majority of patients taking 1,000 mg daily. The PACT CPS made 23 interventions. The PACT CPS generated 46 encounters for resolving drug interactions with a median of 2 encounters per patient.
Discussion
Following gemfibrozil or niacin discontinuation by protocol, most patients with available laboratory results experienced either a decrease or modest TG elevation. The proportion of patients experiencing a decrease in TGs was unexpected but potentially multifactorial. Individual causes for the decrease in TGs were beyond the scope of this analysis. The retrospective design limited the ability to identify variables that could have impacted TG levels when gemfibrozil or niacin were started and discontinued. Although the treatment of TG levels is not indicated until it is ≥ 500 mg/dL, due to an increased risk of pancreatitis, both protocols excluded patients with a history of TGs ≥ 400 mg/dL.19 The lower threshold was set to compensate for anticipated increase in TG levels, following gemfibrozil or niacin discontinuation, and to minimize the number of patients with TG levels ≥ 500 mg/dL. The actual impact on patients’ TG levels supports the use of this lower threshold in the protocol.
When TG levels increased by 200 to 249 mg/dL after gemfibrozil or niacin discontinuation, patients were evaluated for possible underlying causes, which occurred for 4 gemfibrozil and 1 niacin patient. One patient started a β-blocker after gemfibrozil was initiated, and 3 patients were taking gemfibrozil prior to establishing care at the VA. The TG levels of the patient taking niacin correlated with an increased hemoglobin A1c. The TG level for only 1 patient taking gemfibrozil increased above the 500 mg/dL threshold. The patient had several comorbidities known to increase TG levels, but the comorbidities were previously well controlled. No additional medication changes were made at that time, and the TG levels on the next fasting lipid panel decreased to goal. The patient did not experience any negative clinical sequelae from the elevated TG levels.
Thirty-five patients (36%) who were referred to the PACT Pharmacy Clinic required only either gemfibrozil or niacin discontinuation. These patients were evaluated to identify whether adjustments to the protocols would have allowed for pharmacist discontinuation without referral to the PACT Pharmacy Clinic. Twenty-four of these patients (69%) had repeated TG levels ≥ 400 mg/dL prior to referral to the PACT Pharmacy Clinic. Additionally, there was no correlation between the gemfibrozil or niacin doses and the change in TG levels following discontinuation. These data indicate the protocols appropriately identified patients who did not have an indication for gemfibrozil or niacin.
In addition to drug interactions identified on the STOP report, the PACT CPS resolved 12 additional interactions involving simvastatin and gemfibrozil. Additionally, unnecessary lipid medications were deprescribed. The PACT CPS identified 13 patients who experienced myalgias, an ADR attributed to the gemfibrozil- statin interaction. Of those, 9 patients’ ADRs resolved after discontinuing gemfibrozil alone. For the remaining 4 patients, additional interventions to convert the patient to another statin were required to resolve the ADR.
Using pharmacists to address the drug interactions shifted workload from the prescribers and other primary care team members. The mean time spent to resolve both gemfibrozil and niacin interactions by protocol was 15.5 minutes. One hundred fortytwo patients (35.8%) had drug interactions resolved by protocol, saving the PACT CPS’ expertise for patients requiring individualized interventions. Drug interactions were resolved within 4 PACT CPS encounters for 93.8% of the patients taking gemfibrozil and within 3 PACT CPS encounters for 93.8% of the patients taking niacin.
The protocols allowed 12 additional pharmacists who did not have an ambulatory care scope of practice to assist the PACT CPS in mitigating the STOP drug interactions. These pharmacists otherwise would have been limited to making consultative recommendations. Simultaneously, the design allowed for the PACT pharmacists’ expertise to be allocated for patients most likely to require interventions beyond the protocols. This type of intraprofessional referral process is not well described in the medical literature. To the authors’ knowledge, the only studies described referrals from hospital pharmacists to community pharmacists during transitions of care on hospital discharge.20,21
Limitations
The results of this study are derived from a retrospective chart review at a single VA facility. The autonomous nature of PACT CPS interventions may be difficult to replicate in other settings that do not permit pharmacists the same prescriptive authority. This analysis was designed to demonstrate the impact of the pharmacist in resolving major drug interactions. Patients referred to the PACT Pharmacy Clinic who also had their lipid medications adjusted by a nonpharmacist provider were excluded. However, this may have minimized the impact of the PACT CPS on the patient care provided. As postintervention laboratory results were not available for all patients, some patients’ TG levels could have increased above the 500 mg/dL threshold but were not identified. The time investment was extensive and likely underestimates the true cost of implementing the interventions.
Because notification letters were used to instruct patients to stop gemfibrozil or niacin, several considerations need to be addressed when interpreting the follow-up laboratory results. First, we cannot confirm whether the patients received the letter or the exact date the letter was received. Additionally, we cannot confirm whether the patients followed the instructions to stop the interacting medications or the date the medications were stopped. It is possible some patients were still taking the interacting medication when the first laboratory was drawn. Should a patient have continued the interacting medication, most would have run out and been unable to obtain a refill within 90 days of receiving the letter, as this is the maximum amount dispensed at one time. The mean time to the first laboratory result for both gemfibrozil and niacin was 6.5 and 5.3 months, respectively. Approximately 85% of patients completed the first laboratory test at least 3 months after the letter was mailed.
The protocols were designed to assess whether gemfibrozil or niacin was indicated and did not assess whether the statin was indicated. Therefore, discontinuing the statin also could have resolved the interaction appropriately. However, due to characteristics of the patient population and recommendations in current lipid guidelines, it was more likely the statin would be indicated.22,23 The protocols also assumed that patients eligible for gemfibrozil or niacin discontinuation would not need additional changes to their lipid medications. The medication changes made by the PACT CPS may have gone beyond those minimally necessary to resolve the drug interaction and maintain TG goals. Patients who had gemfibrozil or niacin discontinued by protocol also may have benefited from additional optimization of their lipid medications.
Conclusions
This quality improvement analysis supports further evaluation of the complementary use of protocols and PACT CPS prescriptive authority to resolve statin drug interactions. The gemfibrozil and niacin protocols appropriately identified patients who were less likely to experience an adverse change in TG laboratory results. Patients more likely to require additional medication interventions were appropriately referred to the PACT Pharmacy Clinics for individualized care. These data support expanded roles for pharmacists, across various settings, to mitigate select drug interactions at the Truman VA.
Acknowledgments
This quality improvement project is the result of work supported with resources and use of the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Missouri.
1. The top 200 drugs of 2020 Provided by the ClinCalc DrugStats Database. http://clincalc.com/DrugStats /Top200Drugs.aspx. Updated February 11, 2017. Accessed May 12, 2020.
2. Wiggins BS, Saseen JJ, Page RL 2nd, et al; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468‐e495. doi:10.1161/CIR.0000000000000456
3. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf. 2011;10(6):871‐882. doi:10.1517/14740338.2011.583916
4. US Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. https://www.fda.gov/Drugs/DrugSafety /ucm256581.htm. Updated December 15, 2017. Accessed May 12, 2020.
5. US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov /Drugs/DrugSafety/ucm293101.htm. Updated January 19, 2016. Accessed May 12, 2020.
6. US Food and Drug Administration Federal Register. AbbVie Inc. et al; withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://www.federalregister .gov/documents/2016/04/18/2016-08887/abbvie-inc -et-al-withdrawal-of-approval-of-indications-related -to-the-coadministration-with-statins. Published April 18, 2016. Accessed May 12, 2020.
7. Lamprecht DG Jr, Todd BA, Denham AM, Ruppe LK, Stadler SL. Clinical pharmacist patient-safety initiative to reduce against-label prescribing of statins with cyclosporine. Ann Pharmacother. 2017;51(2):140‐145. doi:10.1177/1060028016675352
8. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drugdrug interactions in patients with heart failure: A randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647‐652. doi:10.1016/j.ijcard.2015.10.206
9. Tuchscherer RM, Nair K, Ghushchyan V, Saseen JJ. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs. 2015;15(1):27‐34. doi:10.1007/s40256-014-0096-x
10. Alford JC, Saseen JJ, Allen RR, Nair KV. Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States Food and Drug Administration dose restrictions. Pharmacotherapy. 2012;32(7):623‐630. doi:10.1002/j.1875-9114.2011.01090.x
11. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [published correction appears in JAMA 2000 Mar 8;283(10):1293]. JAMA. 1999;282(3):267‐270. doi:10.1001/jama.282.3.267
12. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014‐2018. doi:10.1001/archinte.163.17.2014
13. Humphries TL, Carroll N, Chester EA, Magid D, Rocho B. Evaluation of an electronic critical drug interaction program coupled with active pharmacist intervention. Ann Pharmacother. 2007;41(12):1979‐1985. doi:10.1345/aph.1K349
14. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
15. Lipitor [package insert]. New York, NY: Pfizer; 2017.
16. Crestor [package insert]. Wilmington, DE: AstraZeneca; 2018.
17. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
18. Wolters Kluwer Health, Lexi-Drugs, Lexicomp. Pravastatin. www.online.lexi.com. [Source not verified.]
19. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726
20. Ferguson J, Seston L, Ashcroft DM. Refer-to-pharmacy: a qualitative study exploring the implementation of an electronic transfer of care initiative to improve medicines optimisation following hospital discharge. BMC Health Serv Res. 2018;18(1):424. doi:10.1186/s12913-018-3262-z
21. Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm. 2019;15(3):267‐278. doi:10.1016/j.sapharm.2018.05.001
22. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction guideline summary. https://www.healthquality.va.gov /guidelines/CD/lipids/LipidSumOptSinglePg31Aug15.pdf. Published 2014. Accessed May 14, 2020.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014 Jun 24;129(25) (suppl 2):S46-48] [published correction appears in Circulation. 2015 Dec 22;132(25):e396]. Circulation. 2014;129(25)(suppl 2): S1‐S45. doi:10.1161/01.cir.0000437738.63853.7a
1. The top 200 drugs of 2020 Provided by the ClinCalc DrugStats Database. http://clincalc.com/DrugStats /Top200Drugs.aspx. Updated February 11, 2017. Accessed May 12, 2020.
2. Wiggins BS, Saseen JJ, Page RL 2nd, et al; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468‐e495. doi:10.1161/CIR.0000000000000456
3. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf. 2011;10(6):871‐882. doi:10.1517/14740338.2011.583916
4. US Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. https://www.fda.gov/Drugs/DrugSafety /ucm256581.htm. Updated December 15, 2017. Accessed May 12, 2020.
5. US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov /Drugs/DrugSafety/ucm293101.htm. Updated January 19, 2016. Accessed May 12, 2020.
6. US Food and Drug Administration Federal Register. AbbVie Inc. et al; withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://www.federalregister .gov/documents/2016/04/18/2016-08887/abbvie-inc -et-al-withdrawal-of-approval-of-indications-related -to-the-coadministration-with-statins. Published April 18, 2016. Accessed May 12, 2020.
7. Lamprecht DG Jr, Todd BA, Denham AM, Ruppe LK, Stadler SL. Clinical pharmacist patient-safety initiative to reduce against-label prescribing of statins with cyclosporine. Ann Pharmacother. 2017;51(2):140‐145. doi:10.1177/1060028016675352
8. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drugdrug interactions in patients with heart failure: A randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647‐652. doi:10.1016/j.ijcard.2015.10.206
9. Tuchscherer RM, Nair K, Ghushchyan V, Saseen JJ. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs. 2015;15(1):27‐34. doi:10.1007/s40256-014-0096-x
10. Alford JC, Saseen JJ, Allen RR, Nair KV. Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States Food and Drug Administration dose restrictions. Pharmacotherapy. 2012;32(7):623‐630. doi:10.1002/j.1875-9114.2011.01090.x
11. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [published correction appears in JAMA 2000 Mar 8;283(10):1293]. JAMA. 1999;282(3):267‐270. doi:10.1001/jama.282.3.267
12. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014‐2018. doi:10.1001/archinte.163.17.2014
13. Humphries TL, Carroll N, Chester EA, Magid D, Rocho B. Evaluation of an electronic critical drug interaction program coupled with active pharmacist intervention. Ann Pharmacother. 2007;41(12):1979‐1985. doi:10.1345/aph.1K349
14. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
15. Lipitor [package insert]. New York, NY: Pfizer; 2017.
16. Crestor [package insert]. Wilmington, DE: AstraZeneca; 2018.
17. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
18. Wolters Kluwer Health, Lexi-Drugs, Lexicomp. Pravastatin. www.online.lexi.com. [Source not verified.]
19. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726
20. Ferguson J, Seston L, Ashcroft DM. Refer-to-pharmacy: a qualitative study exploring the implementation of an electronic transfer of care initiative to improve medicines optimisation following hospital discharge. BMC Health Serv Res. 2018;18(1):424. doi:10.1186/s12913-018-3262-z
21. Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm. 2019;15(3):267‐278. doi:10.1016/j.sapharm.2018.05.001
22. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction guideline summary. https://www.healthquality.va.gov /guidelines/CD/lipids/LipidSumOptSinglePg31Aug15.pdf. Published 2014. Accessed May 14, 2020.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014 Jun 24;129(25) (suppl 2):S46-48] [published correction appears in Circulation. 2015 Dec 22;132(25):e396]. Circulation. 2014;129(25)(suppl 2): S1‐S45. doi:10.1161/01.cir.0000437738.63853.7a
Biologics may carry melanoma risk for patients with immune-mediated inflammatory diseases
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
FROM JAMA DERMATOLOGY
Steroid-Induced Sleep Disturbance and Delirium: A Focused Review for Critically Ill Patients
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
TNF inhibitor plus methotrexate surpassed methotrexate monotherapy in PsA
Adding a tumor necrosis factor inhibitor to the treatment regimen of patients with psoriatic arthritis who failed to reach minimal disease activity on methotrexate monotherapy after 4 or more weeks had more than triple the rate of minimal disease activity after 16 weeks, compared with patients who had their methotrexate dosage escalated but received no second drug, in a multicenter, randomized study with 245 patients.
After 16 weeks, 42% of 123 patients with psoriatic arthritis (PsA) treated with methotrexate and the tumor necrosis factor (TNF) inhibitor adalimumab achieved minimal disease activity, compared with 13% of 122 patients randomized to receive escalated methotrexate monotherapy to their maximally tolerated dosage or to a maximum of 25 mg/week, Laura C. Coates, MBChB, PhD, reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The findings are “supportive of the EULAR recommendations” for managing patients with PsA, said Dr. Coates, a rheumatologist at the University of Oxford (England). The EULAR recommendations call for starting a biologic disease-modifying antirheumatic drug (bDMARD) in patients with PsA and peripheral arthritis and “inadequate response to at least one [conventional synthetic] DMARD,” such as methotrexate (Ann Rheum Dis. 2019 Jun;79[6]:700-12). “A proportion of patients treated with methotrexate do well, but for those struggling on methotrexate, these results support use of a TNF inhibitor. It’s a balance of cost and benefit. If TNF inhibitors were as cheap as methotrexate, I suspect that would be first line more frequently,” Dr. Coates said in an interview. In contrast, the PsA management recommendations from the American College of Rheumatology make treatment with a TNF inhibitor first line, before starting with what these guidelines call an oral small molecule, the same as a conventional synthetic DMARD such as methotrexate (Arthritis Rheumatol. 2019 Jan;71[1]:5-32).
“It’s a well-known fact that adalimumab is more effective than methotrexate in [PsA] patients who do not respond sufficiently well to methotrexate. Patients failing on methotrexate have been escalated to a TNF inhibitor for years,” commented Robert B.M. Landewé, MD, a rheumatologist and professor of medicine at the University of Amsterdam, and a coauthor of the EULAR PsA treatment recommendations. “In the Netherlands and in my practice, every [PsA] patient starts on methotrexate until a dosage of at least 15 mg/week, but if they don’t have sufficient response we escalate to adding a TNF inhibitor,” he said in an interview. “A significant proportion of patients with PsA respond well to moderate to higher dosages of methotrexate,” and this monotherapy with escalation of methotrexate can be safely continued for more than 3 months in many patients without the risk of “losing too much time by waiting” to start a bDMARD.
Dr. Coates said that her practice was to look for some level of response to methotrexate by 12 weeks on treatment and for achievement of minimal disease activity within 24 weeks of treatment. If these targets are not reached, she then adds a TNF inhibitor.
The CONTROL study ran at 60 sites in the United States and in 12 other countries and enrolled patients with active PsA despite treatment with methotrexate for at least 4 weeks and no history of treatment with a bDMARD. Patients received either 40 mg adalimumab every other week plus 15 mg of methotrexate weekly, or maximum-tolerated methotrexate up to 25 mg/week. The results also showed that the primary endpoint of the rate of achieved minimal disease activity seen overall in each of the two study arms was consistent in both the roughly half of patients who had been on methotrexate monotherapy for 3 months or less before entering the study as well as those who had been on initial methotrexate monotherapy for a longer period. Other secondary endpoints examined also showed significantly better responses to adding adalimumab, including a tripling of the rate at which patients achieved complete resolution of their Psoriasis Area and Severity Index score, which occurred in 30% of patients on the TNF inhibitor plus methotrexate and in 9% of those on methotrexate monotherapy.
The results seen in the CONTROL study with adalimumab would likely be similar using a different TNF inhibitor or an agent that’s an adalimumab biosimilar, Dr. Coates said. The only patients with PsA and not achieving minimal disease activity on methotrexate monotherapy who should not then receive add-on treatment with a TNF inhibitor are those known to have a safety exclusion for this drug class or patients for whom the incremental cost poses a barrier, she added. In addition, patients with more substantial skin involvement may get greater benefit from a different class of bDMARD, such as a drug that inhibits interleukin-17 or IL-12 and -23 as recommended by the EULAR panel.
“We still get very good results with a TNF inhibitor for psoriasis, but in patients with severe psoriasis there is an argument to use a different drug,” Dr. Coates acknowledged. Skin responses with an IL-17 inhibitor or an IL-12/23 inhibitor “are far better” than with a TNF inhibitor, said Dr. Landewé. He also added the caution that longer-term use of adalimumab “may induce aggravation of PsA in a significant number of patients.”
CONTROL was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Coates has been a consultant to AbbVie, as well as to Amgen, Biogen, Boehringer Ingelheim, Celgene, Jansen, Novartis, Pfizer, and UCB. Dr. Landewé has been a consultant to AbbVie, as well as to Eli Lilly, Novartis, Pfizer, and UCB.
SOURCE: Coates LC et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:33, Abstract OP0050.
Adding a tumor necrosis factor inhibitor to the treatment regimen of patients with psoriatic arthritis who failed to reach minimal disease activity on methotrexate monotherapy after 4 or more weeks had more than triple the rate of minimal disease activity after 16 weeks, compared with patients who had their methotrexate dosage escalated but received no second drug, in a multicenter, randomized study with 245 patients.
After 16 weeks, 42% of 123 patients with psoriatic arthritis (PsA) treated with methotrexate and the tumor necrosis factor (TNF) inhibitor adalimumab achieved minimal disease activity, compared with 13% of 122 patients randomized to receive escalated methotrexate monotherapy to their maximally tolerated dosage or to a maximum of 25 mg/week, Laura C. Coates, MBChB, PhD, reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The findings are “supportive of the EULAR recommendations” for managing patients with PsA, said Dr. Coates, a rheumatologist at the University of Oxford (England). The EULAR recommendations call for starting a biologic disease-modifying antirheumatic drug (bDMARD) in patients with PsA and peripheral arthritis and “inadequate response to at least one [conventional synthetic] DMARD,” such as methotrexate (Ann Rheum Dis. 2019 Jun;79[6]:700-12). “A proportion of patients treated with methotrexate do well, but for those struggling on methotrexate, these results support use of a TNF inhibitor. It’s a balance of cost and benefit. If TNF inhibitors were as cheap as methotrexate, I suspect that would be first line more frequently,” Dr. Coates said in an interview. In contrast, the PsA management recommendations from the American College of Rheumatology make treatment with a TNF inhibitor first line, before starting with what these guidelines call an oral small molecule, the same as a conventional synthetic DMARD such as methotrexate (Arthritis Rheumatol. 2019 Jan;71[1]:5-32).
“It’s a well-known fact that adalimumab is more effective than methotrexate in [PsA] patients who do not respond sufficiently well to methotrexate. Patients failing on methotrexate have been escalated to a TNF inhibitor for years,” commented Robert B.M. Landewé, MD, a rheumatologist and professor of medicine at the University of Amsterdam, and a coauthor of the EULAR PsA treatment recommendations. “In the Netherlands and in my practice, every [PsA] patient starts on methotrexate until a dosage of at least 15 mg/week, but if they don’t have sufficient response we escalate to adding a TNF inhibitor,” he said in an interview. “A significant proportion of patients with PsA respond well to moderate to higher dosages of methotrexate,” and this monotherapy with escalation of methotrexate can be safely continued for more than 3 months in many patients without the risk of “losing too much time by waiting” to start a bDMARD.
Dr. Coates said that her practice was to look for some level of response to methotrexate by 12 weeks on treatment and for achievement of minimal disease activity within 24 weeks of treatment. If these targets are not reached, she then adds a TNF inhibitor.
The CONTROL study ran at 60 sites in the United States and in 12 other countries and enrolled patients with active PsA despite treatment with methotrexate for at least 4 weeks and no history of treatment with a bDMARD. Patients received either 40 mg adalimumab every other week plus 15 mg of methotrexate weekly, or maximum-tolerated methotrexate up to 25 mg/week. The results also showed that the primary endpoint of the rate of achieved minimal disease activity seen overall in each of the two study arms was consistent in both the roughly half of patients who had been on methotrexate monotherapy for 3 months or less before entering the study as well as those who had been on initial methotrexate monotherapy for a longer period. Other secondary endpoints examined also showed significantly better responses to adding adalimumab, including a tripling of the rate at which patients achieved complete resolution of their Psoriasis Area and Severity Index score, which occurred in 30% of patients on the TNF inhibitor plus methotrexate and in 9% of those on methotrexate monotherapy.
The results seen in the CONTROL study with adalimumab would likely be similar using a different TNF inhibitor or an agent that’s an adalimumab biosimilar, Dr. Coates said. The only patients with PsA and not achieving minimal disease activity on methotrexate monotherapy who should not then receive add-on treatment with a TNF inhibitor are those known to have a safety exclusion for this drug class or patients for whom the incremental cost poses a barrier, she added. In addition, patients with more substantial skin involvement may get greater benefit from a different class of bDMARD, such as a drug that inhibits interleukin-17 or IL-12 and -23 as recommended by the EULAR panel.
“We still get very good results with a TNF inhibitor for psoriasis, but in patients with severe psoriasis there is an argument to use a different drug,” Dr. Coates acknowledged. Skin responses with an IL-17 inhibitor or an IL-12/23 inhibitor “are far better” than with a TNF inhibitor, said Dr. Landewé. He also added the caution that longer-term use of adalimumab “may induce aggravation of PsA in a significant number of patients.”
CONTROL was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Coates has been a consultant to AbbVie, as well as to Amgen, Biogen, Boehringer Ingelheim, Celgene, Jansen, Novartis, Pfizer, and UCB. Dr. Landewé has been a consultant to AbbVie, as well as to Eli Lilly, Novartis, Pfizer, and UCB.
SOURCE: Coates LC et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:33, Abstract OP0050.
Adding a tumor necrosis factor inhibitor to the treatment regimen of patients with psoriatic arthritis who failed to reach minimal disease activity on methotrexate monotherapy after 4 or more weeks had more than triple the rate of minimal disease activity after 16 weeks, compared with patients who had their methotrexate dosage escalated but received no second drug, in a multicenter, randomized study with 245 patients.
After 16 weeks, 42% of 123 patients with psoriatic arthritis (PsA) treated with methotrexate and the tumor necrosis factor (TNF) inhibitor adalimumab achieved minimal disease activity, compared with 13% of 122 patients randomized to receive escalated methotrexate monotherapy to their maximally tolerated dosage or to a maximum of 25 mg/week, Laura C. Coates, MBChB, PhD, reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The findings are “supportive of the EULAR recommendations” for managing patients with PsA, said Dr. Coates, a rheumatologist at the University of Oxford (England). The EULAR recommendations call for starting a biologic disease-modifying antirheumatic drug (bDMARD) in patients with PsA and peripheral arthritis and “inadequate response to at least one [conventional synthetic] DMARD,” such as methotrexate (Ann Rheum Dis. 2019 Jun;79[6]:700-12). “A proportion of patients treated with methotrexate do well, but for those struggling on methotrexate, these results support use of a TNF inhibitor. It’s a balance of cost and benefit. If TNF inhibitors were as cheap as methotrexate, I suspect that would be first line more frequently,” Dr. Coates said in an interview. In contrast, the PsA management recommendations from the American College of Rheumatology make treatment with a TNF inhibitor first line, before starting with what these guidelines call an oral small molecule, the same as a conventional synthetic DMARD such as methotrexate (Arthritis Rheumatol. 2019 Jan;71[1]:5-32).
“It’s a well-known fact that adalimumab is more effective than methotrexate in [PsA] patients who do not respond sufficiently well to methotrexate. Patients failing on methotrexate have been escalated to a TNF inhibitor for years,” commented Robert B.M. Landewé, MD, a rheumatologist and professor of medicine at the University of Amsterdam, and a coauthor of the EULAR PsA treatment recommendations. “In the Netherlands and in my practice, every [PsA] patient starts on methotrexate until a dosage of at least 15 mg/week, but if they don’t have sufficient response we escalate to adding a TNF inhibitor,” he said in an interview. “A significant proportion of patients with PsA respond well to moderate to higher dosages of methotrexate,” and this monotherapy with escalation of methotrexate can be safely continued for more than 3 months in many patients without the risk of “losing too much time by waiting” to start a bDMARD.
Dr. Coates said that her practice was to look for some level of response to methotrexate by 12 weeks on treatment and for achievement of minimal disease activity within 24 weeks of treatment. If these targets are not reached, she then adds a TNF inhibitor.
The CONTROL study ran at 60 sites in the United States and in 12 other countries and enrolled patients with active PsA despite treatment with methotrexate for at least 4 weeks and no history of treatment with a bDMARD. Patients received either 40 mg adalimumab every other week plus 15 mg of methotrexate weekly, or maximum-tolerated methotrexate up to 25 mg/week. The results also showed that the primary endpoint of the rate of achieved minimal disease activity seen overall in each of the two study arms was consistent in both the roughly half of patients who had been on methotrexate monotherapy for 3 months or less before entering the study as well as those who had been on initial methotrexate monotherapy for a longer period. Other secondary endpoints examined also showed significantly better responses to adding adalimumab, including a tripling of the rate at which patients achieved complete resolution of their Psoriasis Area and Severity Index score, which occurred in 30% of patients on the TNF inhibitor plus methotrexate and in 9% of those on methotrexate monotherapy.
The results seen in the CONTROL study with adalimumab would likely be similar using a different TNF inhibitor or an agent that’s an adalimumab biosimilar, Dr. Coates said. The only patients with PsA and not achieving minimal disease activity on methotrexate monotherapy who should not then receive add-on treatment with a TNF inhibitor are those known to have a safety exclusion for this drug class or patients for whom the incremental cost poses a barrier, she added. In addition, patients with more substantial skin involvement may get greater benefit from a different class of bDMARD, such as a drug that inhibits interleukin-17 or IL-12 and -23 as recommended by the EULAR panel.
“We still get very good results with a TNF inhibitor for psoriasis, but in patients with severe psoriasis there is an argument to use a different drug,” Dr. Coates acknowledged. Skin responses with an IL-17 inhibitor or an IL-12/23 inhibitor “are far better” than with a TNF inhibitor, said Dr. Landewé. He also added the caution that longer-term use of adalimumab “may induce aggravation of PsA in a significant number of patients.”
CONTROL was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Coates has been a consultant to AbbVie, as well as to Amgen, Biogen, Boehringer Ingelheim, Celgene, Jansen, Novartis, Pfizer, and UCB. Dr. Landewé has been a consultant to AbbVie, as well as to Eli Lilly, Novartis, Pfizer, and UCB.
SOURCE: Coates LC et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:33, Abstract OP0050.
FROM EULAR 2020 E-CONGRESS
FDA approves new antibiotic for HABP/VABP treatment
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
Lancet, NEJM retract studies on hydroxychloroquine for COVID-19
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
‘Promising’ durvalumab results spark phase 3 trial in mesothelioma
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
FROM ASCO 2020
Acute lymphoblastic leukemia can be successfully treated in the frail elderly
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
FDA okays emergency use for Impella RP in COVID-19 right heart failure
The Food and Drug Administration issued an emergency use authorization for use of the Impella RP heart pump system in COVID-19 patients with right heart failure or decompensation, Abiomed announced June 1.
“Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE [pulmonary embolism],” the letter noted.
It cited, for example, use of the temporary heart pump in a 59-year-old woman suffering from COVID-19 who went into right ventricular failure and became hypotensive after an acute PE was removed. After placement of the device, the patient experienced a “dramatic and immediate” improvement in arterial pressure and the device was removed on the fifth day, according to Amir Kaki, MD, and Ted Schreiber, MD, of Ascension St. John Hospital, Detroit, whose review of the case has been posted online.
“Acute pulmonary embolism is clearly being recognized as a life-threatening manifestation of COVID-19. Impella RP is an important tool to help cardiologists save lives during this pandemic,” Dr. Kaki said in the letter. “As we have demonstrated in our series of patients, early recognition of right ventricular dysfunction and early placement of the Impella RP for patients who are hypotensive can be lifesaving.”
Other data cited in support of the Impella RP emergency use authorization (EUA) include a 2019 series of hemodynamically unstable patients with PE in Japan and a 2017 case report of a 47-year-old man with right ventricular failure, profound shock, and a massive PE.
The FDA granted premarket approval of the Impella RP system in 2017 to provide temporary right ventricular support for up to 14 days in patients with a body surface area of at least 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, MI, heart transplant, or open-heart surgery.
The EUA indication for the Impella RP system is to provide temporary right ventricular support for up to 14 days in critical care patients with a body surface area of at least 1.5 m2 for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19, including PE.
The Impella RP is authorized only for emergency use under the EUA and only for the duration of the circumstances justifying use of EUAs, the letter noted.
Last year, concerns were raised about off-indication use after interim results from a postapproval study suggested a higher risk for death than seen in premarket studies treated with the temporary heart pump.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration issued an emergency use authorization for use of the Impella RP heart pump system in COVID-19 patients with right heart failure or decompensation, Abiomed announced June 1.
“Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE [pulmonary embolism],” the letter noted.
It cited, for example, use of the temporary heart pump in a 59-year-old woman suffering from COVID-19 who went into right ventricular failure and became hypotensive after an acute PE was removed. After placement of the device, the patient experienced a “dramatic and immediate” improvement in arterial pressure and the device was removed on the fifth day, according to Amir Kaki, MD, and Ted Schreiber, MD, of Ascension St. John Hospital, Detroit, whose review of the case has been posted online.
“Acute pulmonary embolism is clearly being recognized as a life-threatening manifestation of COVID-19. Impella RP is an important tool to help cardiologists save lives during this pandemic,” Dr. Kaki said in the letter. “As we have demonstrated in our series of patients, early recognition of right ventricular dysfunction and early placement of the Impella RP for patients who are hypotensive can be lifesaving.”
Other data cited in support of the Impella RP emergency use authorization (EUA) include a 2019 series of hemodynamically unstable patients with PE in Japan and a 2017 case report of a 47-year-old man with right ventricular failure, profound shock, and a massive PE.
The FDA granted premarket approval of the Impella RP system in 2017 to provide temporary right ventricular support for up to 14 days in patients with a body surface area of at least 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, MI, heart transplant, or open-heart surgery.
The EUA indication for the Impella RP system is to provide temporary right ventricular support for up to 14 days in critical care patients with a body surface area of at least 1.5 m2 for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19, including PE.
The Impella RP is authorized only for emergency use under the EUA and only for the duration of the circumstances justifying use of EUAs, the letter noted.
Last year, concerns were raised about off-indication use after interim results from a postapproval study suggested a higher risk for death than seen in premarket studies treated with the temporary heart pump.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration issued an emergency use authorization for use of the Impella RP heart pump system in COVID-19 patients with right heart failure or decompensation, Abiomed announced June 1.
“Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE [pulmonary embolism],” the letter noted.
It cited, for example, use of the temporary heart pump in a 59-year-old woman suffering from COVID-19 who went into right ventricular failure and became hypotensive after an acute PE was removed. After placement of the device, the patient experienced a “dramatic and immediate” improvement in arterial pressure and the device was removed on the fifth day, according to Amir Kaki, MD, and Ted Schreiber, MD, of Ascension St. John Hospital, Detroit, whose review of the case has been posted online.
“Acute pulmonary embolism is clearly being recognized as a life-threatening manifestation of COVID-19. Impella RP is an important tool to help cardiologists save lives during this pandemic,” Dr. Kaki said in the letter. “As we have demonstrated in our series of patients, early recognition of right ventricular dysfunction and early placement of the Impella RP for patients who are hypotensive can be lifesaving.”
Other data cited in support of the Impella RP emergency use authorization (EUA) include a 2019 series of hemodynamically unstable patients with PE in Japan and a 2017 case report of a 47-year-old man with right ventricular failure, profound shock, and a massive PE.
The FDA granted premarket approval of the Impella RP system in 2017 to provide temporary right ventricular support for up to 14 days in patients with a body surface area of at least 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, MI, heart transplant, or open-heart surgery.
The EUA indication for the Impella RP system is to provide temporary right ventricular support for up to 14 days in critical care patients with a body surface area of at least 1.5 m2 for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19, including PE.
The Impella RP is authorized only for emergency use under the EUA and only for the duration of the circumstances justifying use of EUAs, the letter noted.
Last year, concerns were raised about off-indication use after interim results from a postapproval study suggested a higher risk for death than seen in premarket studies treated with the temporary heart pump.
A version of this article originally appeared on Medscape.com.
More evidence hydroxychloroquine is ineffective, harmful in COVID-19
Hydroxychloroquine and chloroquine, with or without azithromycin or clarithromycin, offer no benefit in treating patients with COVID-19 and, instead, are associated with ventricular arrhythmias and higher rates of mortality, according to a major new international study.
In the largest observational study of its kind, including close to 100,000 people in 671 hospitals on six continents, investigators compared outcomes in 15,000 patients with COVID-19 treated with hydroxychloroquine and chloroquine alone or in combination with a macrolide with 80,000 control patients with COVID-19 not receiving these agents.
Treatment with any of these medications, either alone or in combination, was associated with increased death during hospitalization; compared with about 10% in control group patients, mortality rates ranged from more than 16% to almost 24% in the treated groups.
Patients treated with hydroxychloroquine plus a macrolide showed the highest rates of serious cardiac arrhythmias, and, even after accounting for demographic factors and comorbidities, this combination was found to be associated with a more than 5-fold increase in the risk of developing a serious arrhythmia while in the hospital.
“In this real-world study, the biggest yet, we looked at 100,000 patients [with COVID-19] across six continents and found not the slightest hint of benefits and only risks, and the data is pretty straightforward,” study coauthor Frank Ruschitzka, MD, director of the Heart Center at University Hospital, Zürich, said in an interview. The study was published online May 22 in The Lancet.
‘Inconclusive’ evidence
The absence of an effective treatment for COVID-19 has led to the “repurposing” of the antimalarial drug chloroquine and its analogue hydroxychloroquine, which is used for treating autoimmune disease, but this approach is based on anecdotal evidence or open-label randomized trials that have been “largely inconclusive,” the authors wrote.
Additional agents used to treat COVID-19 are second-generation macrolides (azithromycin or clarithromycin), in combination with chloroquine or hydroxychloroquine, “despite limited evidence” and the risk for ventricular arrhythmias, the authors noted.
“Our primary question was whether there was any associated benefits of the use of hydroxychloroquine, chloroquine, or a combined regimen with macrolides in treating COVID-19, and — if there was no benefit — would there be harm?” lead author Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, said in an interview.
The investigators used data from a multinational registry comprising 671 hospitals that included patients (n = 96,032; mean age 53.8 years; 46.3% female) who had been hospitalized between Dec. 20, 2019, and April 14, 2020, with confirmed COVID-19 infection.
They also collected data about demographics, underlying comorbidities, and medical history, and medications that patients were taking at baseline.
Patients receiving treatment (n = 14,888) were divided into four groups: those receiving chloroquine alone (n = 1,868), those receiving chloroquine with a macrolide (n = 3,783), those receiving hydroxychloroquine alone (n = 3,016) and those receiving hydroxychloroquine with a macrolide (n = 6,221).
The remaining patients not treated with these regimens (n = 81,144) were regarded as the control group.
Most patients (65.9%) came from North America, followed by Europe (17.39%), Asia (7.9%), Africa (4.6%), South America (3.7%), and Australia (0.6%). Most (66.9%) were white, followed by patients of Asian origin (14.1%), black patients (9.4%), and Hispanic patients (6.2%).
Comorbidities and underlying conditions included obesity, hyperlipidemia, and hypertension in about 30%.
Comorbidities and underlying conditions
The investigators conducted multiple analyses to control for confounding variables, including Cox proportional hazards regression and propensity score matching analyses.
“In an observational study, there is always a chance of residual confounding, which is why we did propensity score based matched analyses,” Dr. Ruschitzka explained.
No significant differences were found in distribution of demographics and comorbidities between the groups.
As good as it gets
“We found no benefit in any of the four treatment regimens for hospitalized patients with COVID-19, but we did notice higher rates of death and serious ventricular arrhythmias in these patients, compared to the controls,” Dr. Mehra reported.
Of the patients in the control group, roughly 9.3% died during their hospitalization, compared with 16.4% of patients treated with chloroquine alone, 18.0% of those treated with hydroxychloroquine alone, 22.2% of those treated with chloroquine and a macrolide, and 23.8% of those treated with hydroxychloroquine and a macrolide.
After accounting for confounding variables, the researchers estimated that the excess mortality risk attributable to use of the drug regimen ranged from 34% to 45%.
Patients treated with any of the four regimens sustained more serious arrhythmias, compared with those in the control group (0.35), with the biggest increase seen in the group treated with the combination of hydroxychloroquine plus a macrolide (8.1%), followed by chloroquine with a macrolide (6.5%), hydroxychloroquine alone (6.1%), and chloroquine alone (4.3%).
“We were fairly reassured that, although the study was observational, the signals were robust and consistent across all regions of the world in diverse populations, and we did not see any muting of that signal, depending on region,” Dr. Mehra said.
“Two months ago, we were all scratching our heads about how to treat patients with COVID-19, and then came a drug [hydroxychloroquine] with some anecdotal evidence, but now we have 2 months more experience, and we looked to science to provide some answer,” Dr. Ruschitzka said.
“Although this was not a randomized, controlled trial, so we do not have a definite answer, the data provided in this [large, multinational] real-world study is as good as it gets and the best data we have,” he concluded.
“Let the science speak for itself”
Commenting on the study in an interview, Christian Funck-Brentano, MD, from the Hospital Pitié-Salpêtrière and Sorbonne University, both in Paris, said that, although the study is observational and therefore not as reliable as a randomized controlled trial, it is “nevertheless well-documented, studied a huge amount of people, and utilized several sensitivity methods, all of which showed the same results.”
Dr. Funck-Brentano, who is the coauthor of an accompanying editorial in The Lancet and was not involved with the study, said that “we now have no evidence that hydroxychloroquine and chloroquine alone or in combination with a macrolide do any good and we have potential evidence that they do harm and kill people.”
Also commenting on the study in an interview, David Holtgrave, PhD, dean of the School of Public Health at the State University of New York at Albany, said that, “while no one observational study alone would lead to a firm clinical recommendation, I think it is helpful for physicians and public health officials to be aware of the findings of the peer-reviewed observational studies to date and the National Institutes of Health COVID-19 treatment guidelines and the Food and Drug Administration’s statement of drug safety concern about hydroxychloroquine to inform their decision-making as we await the results of randomized clinical trials of these drugs for the treatment of COVID-19,” said Dr. Holtgrave, who was not involved with the study.
He added that, to his knowledge, there are “still no published studies of prophylactic use of these drugs to prevent COVID-19.”
Dr. Mehra emphasized that a cardinal principle of practicing medicine is “first do no harm” and “even in situations where you believe a desperate disease calls for desperate measures, responsible physicians should take a step back and ask if we are doing harm, and until we can say we aren’t, I don’t think it’s wise to push something like this in the absence of good efficacy data.”
Dr. Ruschitzka added that those who are encouraging the use of these agents “should review their decision based on today’s data and let the science speak for itself.”
The study was supported by the William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston. Dr. Mehra reported personal fees from Abbott, Medtronic, Janssen, Mesoblast, Portola, Bayer, Baim Institute for Clinical Research, NuPulseCV, FineHeart, Leviticus, Roivant, and Triple Gene. Dr. Ruschitzka was paid for time spent as a committee member for clinical trials, advisory boards, other forms of consulting, and lectures or presentations; these payments were made directly to the University of Zürich and no personal payments were received in relation to these trials or other activities. Dr. Funck-Brentano, his coauthor, and Dr. Holtgrave declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hydroxychloroquine and chloroquine, with or without azithromycin or clarithromycin, offer no benefit in treating patients with COVID-19 and, instead, are associated with ventricular arrhythmias and higher rates of mortality, according to a major new international study.
In the largest observational study of its kind, including close to 100,000 people in 671 hospitals on six continents, investigators compared outcomes in 15,000 patients with COVID-19 treated with hydroxychloroquine and chloroquine alone or in combination with a macrolide with 80,000 control patients with COVID-19 not receiving these agents.
Treatment with any of these medications, either alone or in combination, was associated with increased death during hospitalization; compared with about 10% in control group patients, mortality rates ranged from more than 16% to almost 24% in the treated groups.
Patients treated with hydroxychloroquine plus a macrolide showed the highest rates of serious cardiac arrhythmias, and, even after accounting for demographic factors and comorbidities, this combination was found to be associated with a more than 5-fold increase in the risk of developing a serious arrhythmia while in the hospital.
“In this real-world study, the biggest yet, we looked at 100,000 patients [with COVID-19] across six continents and found not the slightest hint of benefits and only risks, and the data is pretty straightforward,” study coauthor Frank Ruschitzka, MD, director of the Heart Center at University Hospital, Zürich, said in an interview. The study was published online May 22 in The Lancet.
‘Inconclusive’ evidence
The absence of an effective treatment for COVID-19 has led to the “repurposing” of the antimalarial drug chloroquine and its analogue hydroxychloroquine, which is used for treating autoimmune disease, but this approach is based on anecdotal evidence or open-label randomized trials that have been “largely inconclusive,” the authors wrote.
Additional agents used to treat COVID-19 are second-generation macrolides (azithromycin or clarithromycin), in combination with chloroquine or hydroxychloroquine, “despite limited evidence” and the risk for ventricular arrhythmias, the authors noted.
“Our primary question was whether there was any associated benefits of the use of hydroxychloroquine, chloroquine, or a combined regimen with macrolides in treating COVID-19, and — if there was no benefit — would there be harm?” lead author Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, said in an interview.
The investigators used data from a multinational registry comprising 671 hospitals that included patients (n = 96,032; mean age 53.8 years; 46.3% female) who had been hospitalized between Dec. 20, 2019, and April 14, 2020, with confirmed COVID-19 infection.
They also collected data about demographics, underlying comorbidities, and medical history, and medications that patients were taking at baseline.
Patients receiving treatment (n = 14,888) were divided into four groups: those receiving chloroquine alone (n = 1,868), those receiving chloroquine with a macrolide (n = 3,783), those receiving hydroxychloroquine alone (n = 3,016) and those receiving hydroxychloroquine with a macrolide (n = 6,221).
The remaining patients not treated with these regimens (n = 81,144) were regarded as the control group.
Most patients (65.9%) came from North America, followed by Europe (17.39%), Asia (7.9%), Africa (4.6%), South America (3.7%), and Australia (0.6%). Most (66.9%) were white, followed by patients of Asian origin (14.1%), black patients (9.4%), and Hispanic patients (6.2%).
Comorbidities and underlying conditions included obesity, hyperlipidemia, and hypertension in about 30%.
Comorbidities and underlying conditions
The investigators conducted multiple analyses to control for confounding variables, including Cox proportional hazards regression and propensity score matching analyses.
“In an observational study, there is always a chance of residual confounding, which is why we did propensity score based matched analyses,” Dr. Ruschitzka explained.
No significant differences were found in distribution of demographics and comorbidities between the groups.
As good as it gets
“We found no benefit in any of the four treatment regimens for hospitalized patients with COVID-19, but we did notice higher rates of death and serious ventricular arrhythmias in these patients, compared to the controls,” Dr. Mehra reported.
Of the patients in the control group, roughly 9.3% died during their hospitalization, compared with 16.4% of patients treated with chloroquine alone, 18.0% of those treated with hydroxychloroquine alone, 22.2% of those treated with chloroquine and a macrolide, and 23.8% of those treated with hydroxychloroquine and a macrolide.
After accounting for confounding variables, the researchers estimated that the excess mortality risk attributable to use of the drug regimen ranged from 34% to 45%.
Patients treated with any of the four regimens sustained more serious arrhythmias, compared with those in the control group (0.35), with the biggest increase seen in the group treated with the combination of hydroxychloroquine plus a macrolide (8.1%), followed by chloroquine with a macrolide (6.5%), hydroxychloroquine alone (6.1%), and chloroquine alone (4.3%).
“We were fairly reassured that, although the study was observational, the signals were robust and consistent across all regions of the world in diverse populations, and we did not see any muting of that signal, depending on region,” Dr. Mehra said.
“Two months ago, we were all scratching our heads about how to treat patients with COVID-19, and then came a drug [hydroxychloroquine] with some anecdotal evidence, but now we have 2 months more experience, and we looked to science to provide some answer,” Dr. Ruschitzka said.
“Although this was not a randomized, controlled trial, so we do not have a definite answer, the data provided in this [large, multinational] real-world study is as good as it gets and the best data we have,” he concluded.
“Let the science speak for itself”
Commenting on the study in an interview, Christian Funck-Brentano, MD, from the Hospital Pitié-Salpêtrière and Sorbonne University, both in Paris, said that, although the study is observational and therefore not as reliable as a randomized controlled trial, it is “nevertheless well-documented, studied a huge amount of people, and utilized several sensitivity methods, all of which showed the same results.”
Dr. Funck-Brentano, who is the coauthor of an accompanying editorial in The Lancet and was not involved with the study, said that “we now have no evidence that hydroxychloroquine and chloroquine alone or in combination with a macrolide do any good and we have potential evidence that they do harm and kill people.”
Also commenting on the study in an interview, David Holtgrave, PhD, dean of the School of Public Health at the State University of New York at Albany, said that, “while no one observational study alone would lead to a firm clinical recommendation, I think it is helpful for physicians and public health officials to be aware of the findings of the peer-reviewed observational studies to date and the National Institutes of Health COVID-19 treatment guidelines and the Food and Drug Administration’s statement of drug safety concern about hydroxychloroquine to inform their decision-making as we await the results of randomized clinical trials of these drugs for the treatment of COVID-19,” said Dr. Holtgrave, who was not involved with the study.
He added that, to his knowledge, there are “still no published studies of prophylactic use of these drugs to prevent COVID-19.”
Dr. Mehra emphasized that a cardinal principle of practicing medicine is “first do no harm” and “even in situations where you believe a desperate disease calls for desperate measures, responsible physicians should take a step back and ask if we are doing harm, and until we can say we aren’t, I don’t think it’s wise to push something like this in the absence of good efficacy data.”
Dr. Ruschitzka added that those who are encouraging the use of these agents “should review their decision based on today’s data and let the science speak for itself.”
The study was supported by the William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston. Dr. Mehra reported personal fees from Abbott, Medtronic, Janssen, Mesoblast, Portola, Bayer, Baim Institute for Clinical Research, NuPulseCV, FineHeart, Leviticus, Roivant, and Triple Gene. Dr. Ruschitzka was paid for time spent as a committee member for clinical trials, advisory boards, other forms of consulting, and lectures or presentations; these payments were made directly to the University of Zürich and no personal payments were received in relation to these trials or other activities. Dr. Funck-Brentano, his coauthor, and Dr. Holtgrave declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hydroxychloroquine and chloroquine, with or without azithromycin or clarithromycin, offer no benefit in treating patients with COVID-19 and, instead, are associated with ventricular arrhythmias and higher rates of mortality, according to a major new international study.
In the largest observational study of its kind, including close to 100,000 people in 671 hospitals on six continents, investigators compared outcomes in 15,000 patients with COVID-19 treated with hydroxychloroquine and chloroquine alone or in combination with a macrolide with 80,000 control patients with COVID-19 not receiving these agents.
Treatment with any of these medications, either alone or in combination, was associated with increased death during hospitalization; compared with about 10% in control group patients, mortality rates ranged from more than 16% to almost 24% in the treated groups.
Patients treated with hydroxychloroquine plus a macrolide showed the highest rates of serious cardiac arrhythmias, and, even after accounting for demographic factors and comorbidities, this combination was found to be associated with a more than 5-fold increase in the risk of developing a serious arrhythmia while in the hospital.
“In this real-world study, the biggest yet, we looked at 100,000 patients [with COVID-19] across six continents and found not the slightest hint of benefits and only risks, and the data is pretty straightforward,” study coauthor Frank Ruschitzka, MD, director of the Heart Center at University Hospital, Zürich, said in an interview. The study was published online May 22 in The Lancet.
‘Inconclusive’ evidence
The absence of an effective treatment for COVID-19 has led to the “repurposing” of the antimalarial drug chloroquine and its analogue hydroxychloroquine, which is used for treating autoimmune disease, but this approach is based on anecdotal evidence or open-label randomized trials that have been “largely inconclusive,” the authors wrote.
Additional agents used to treat COVID-19 are second-generation macrolides (azithromycin or clarithromycin), in combination with chloroquine or hydroxychloroquine, “despite limited evidence” and the risk for ventricular arrhythmias, the authors noted.
“Our primary question was whether there was any associated benefits of the use of hydroxychloroquine, chloroquine, or a combined regimen with macrolides in treating COVID-19, and — if there was no benefit — would there be harm?” lead author Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, said in an interview.
The investigators used data from a multinational registry comprising 671 hospitals that included patients (n = 96,032; mean age 53.8 years; 46.3% female) who had been hospitalized between Dec. 20, 2019, and April 14, 2020, with confirmed COVID-19 infection.
They also collected data about demographics, underlying comorbidities, and medical history, and medications that patients were taking at baseline.
Patients receiving treatment (n = 14,888) were divided into four groups: those receiving chloroquine alone (n = 1,868), those receiving chloroquine with a macrolide (n = 3,783), those receiving hydroxychloroquine alone (n = 3,016) and those receiving hydroxychloroquine with a macrolide (n = 6,221).
The remaining patients not treated with these regimens (n = 81,144) were regarded as the control group.
Most patients (65.9%) came from North America, followed by Europe (17.39%), Asia (7.9%), Africa (4.6%), South America (3.7%), and Australia (0.6%). Most (66.9%) were white, followed by patients of Asian origin (14.1%), black patients (9.4%), and Hispanic patients (6.2%).
Comorbidities and underlying conditions included obesity, hyperlipidemia, and hypertension in about 30%.
Comorbidities and underlying conditions
The investigators conducted multiple analyses to control for confounding variables, including Cox proportional hazards regression and propensity score matching analyses.
“In an observational study, there is always a chance of residual confounding, which is why we did propensity score based matched analyses,” Dr. Ruschitzka explained.
No significant differences were found in distribution of demographics and comorbidities between the groups.
As good as it gets
“We found no benefit in any of the four treatment regimens for hospitalized patients with COVID-19, but we did notice higher rates of death and serious ventricular arrhythmias in these patients, compared to the controls,” Dr. Mehra reported.
Of the patients in the control group, roughly 9.3% died during their hospitalization, compared with 16.4% of patients treated with chloroquine alone, 18.0% of those treated with hydroxychloroquine alone, 22.2% of those treated with chloroquine and a macrolide, and 23.8% of those treated with hydroxychloroquine and a macrolide.
After accounting for confounding variables, the researchers estimated that the excess mortality risk attributable to use of the drug regimen ranged from 34% to 45%.
Patients treated with any of the four regimens sustained more serious arrhythmias, compared with those in the control group (0.35), with the biggest increase seen in the group treated with the combination of hydroxychloroquine plus a macrolide (8.1%), followed by chloroquine with a macrolide (6.5%), hydroxychloroquine alone (6.1%), and chloroquine alone (4.3%).
“We were fairly reassured that, although the study was observational, the signals were robust and consistent across all regions of the world in diverse populations, and we did not see any muting of that signal, depending on region,” Dr. Mehra said.
“Two months ago, we were all scratching our heads about how to treat patients with COVID-19, and then came a drug [hydroxychloroquine] with some anecdotal evidence, but now we have 2 months more experience, and we looked to science to provide some answer,” Dr. Ruschitzka said.
“Although this was not a randomized, controlled trial, so we do not have a definite answer, the data provided in this [large, multinational] real-world study is as good as it gets and the best data we have,” he concluded.
“Let the science speak for itself”
Commenting on the study in an interview, Christian Funck-Brentano, MD, from the Hospital Pitié-Salpêtrière and Sorbonne University, both in Paris, said that, although the study is observational and therefore not as reliable as a randomized controlled trial, it is “nevertheless well-documented, studied a huge amount of people, and utilized several sensitivity methods, all of which showed the same results.”
Dr. Funck-Brentano, who is the coauthor of an accompanying editorial in The Lancet and was not involved with the study, said that “we now have no evidence that hydroxychloroquine and chloroquine alone or in combination with a macrolide do any good and we have potential evidence that they do harm and kill people.”
Also commenting on the study in an interview, David Holtgrave, PhD, dean of the School of Public Health at the State University of New York at Albany, said that, “while no one observational study alone would lead to a firm clinical recommendation, I think it is helpful for physicians and public health officials to be aware of the findings of the peer-reviewed observational studies to date and the National Institutes of Health COVID-19 treatment guidelines and the Food and Drug Administration’s statement of drug safety concern about hydroxychloroquine to inform their decision-making as we await the results of randomized clinical trials of these drugs for the treatment of COVID-19,” said Dr. Holtgrave, who was not involved with the study.
He added that, to his knowledge, there are “still no published studies of prophylactic use of these drugs to prevent COVID-19.”
Dr. Mehra emphasized that a cardinal principle of practicing medicine is “first do no harm” and “even in situations where you believe a desperate disease calls for desperate measures, responsible physicians should take a step back and ask if we are doing harm, and until we can say we aren’t, I don’t think it’s wise to push something like this in the absence of good efficacy data.”
Dr. Ruschitzka added that those who are encouraging the use of these agents “should review their decision based on today’s data and let the science speak for itself.”
The study was supported by the William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston. Dr. Mehra reported personal fees from Abbott, Medtronic, Janssen, Mesoblast, Portola, Bayer, Baim Institute for Clinical Research, NuPulseCV, FineHeart, Leviticus, Roivant, and Triple Gene. Dr. Ruschitzka was paid for time spent as a committee member for clinical trials, advisory boards, other forms of consulting, and lectures or presentations; these payments were made directly to the University of Zürich and no personal payments were received in relation to these trials or other activities. Dr. Funck-Brentano, his coauthor, and Dr. Holtgrave declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.