User login
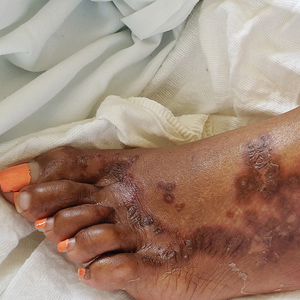
Erythematous Nodule With Central Erosions on the Calf
The Diagnosis: Osteoma Cutis
Osteoma cutis is the heterotopic development of cutaneous ossifications in the dermis or subcutaneous fat and presents as plaquelike, stony, hard nodules. It can manifest as either a primary or secondary condition based on the presence or absence of a prior skin insult at the lesion site. Primary osteoma cutis occurs in 15% of patients and arises either de novo or in association with any of several inflammatory, neoplastic, and metabolic diseases that provide a favorable environment for abnormal mesenchymal stem cell commitment to osteoid,1 including Albright hereditary osteodystrophy, myositis ossificans progressiva, and progressive osseous heteroplasia, which are all associated with mutations in the heterotrimeric G-protein alpha subunit encoding gene, GNAS. 1,2 It is suggested that an insufficiency of Gsα leads to uncontrolled negative regulation of nonosseous connective tissue differentiation, forming osteoid.3 Additionally, diseases involving gain-of-function mutations in the activin A receptor type 1 encoding gene, ACVR1, such as fibrodysplasia ossificans progressiva, have been associated with osteoma cutis.4 These mutations lead to decreased receptor affinity to molecular safeguards of bone morphogenetic protein signaling, ultimately contributing to progressive ectopic bone formation.5 Secondary osteoma cutis occurs in 85% of patients and develops at the site of prior skin damage due to inflammation, neoplasm, or trauma.6 It is believed that tissue damage and degeneration lead to mesenchymal stem cell proliferation and skeletogenicinducing factor recruitment forming cartilaginous tissue, later replaced by bone through endochondral ossification.7
Although osteoma cutis previously was believed to be rare, more recent radiologic studies suggest otherwise, detecting cutaneous osteomas in up to 42.1% of patients.8 Consequently, it is likely that osteoma cutis is underdiagnosed due to its subclinical nature. Our patient, however, presented with a solitary osteoma cutis with perforation of the epidermis, a rare phenomenon.9-12
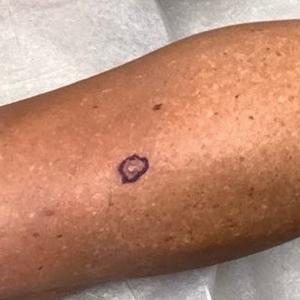
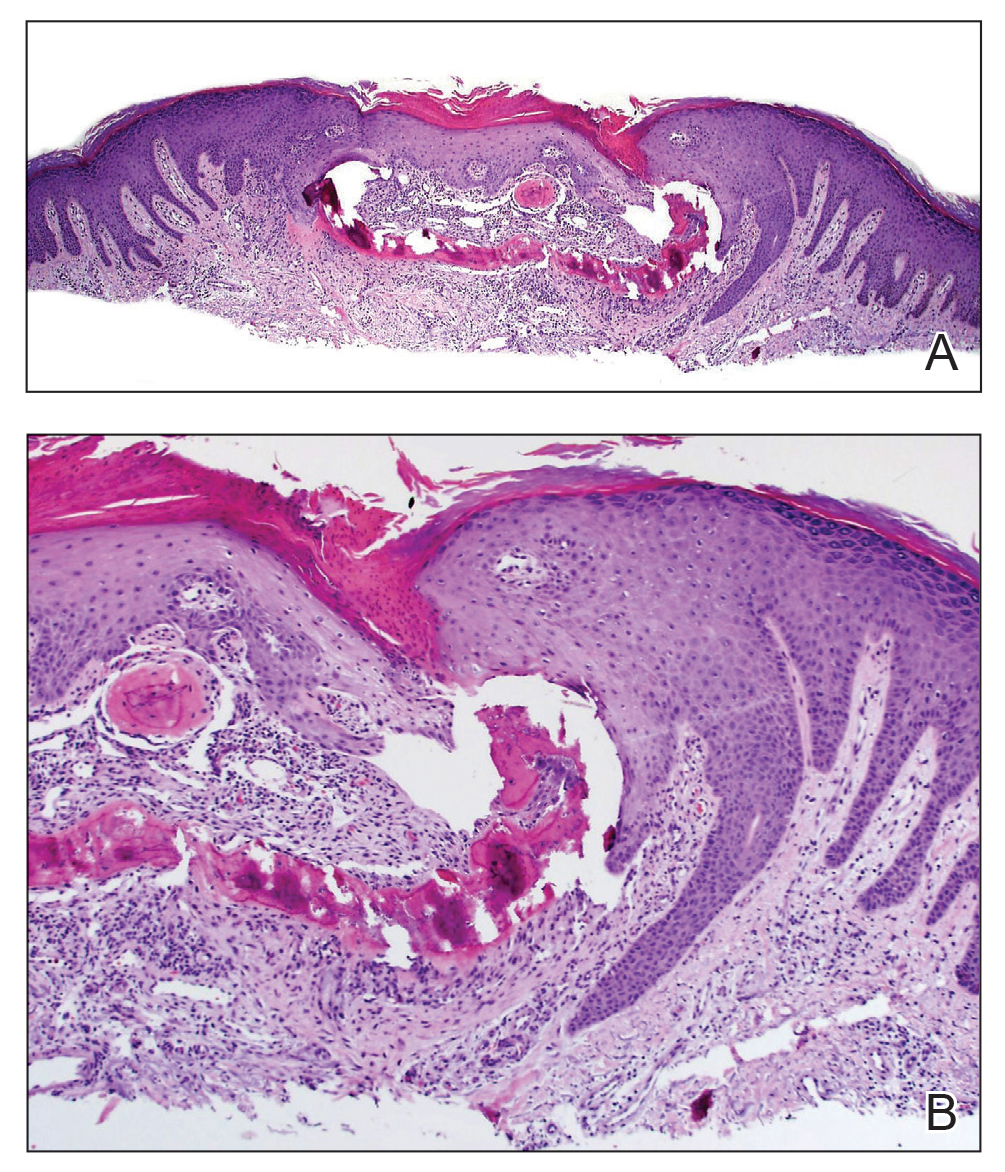
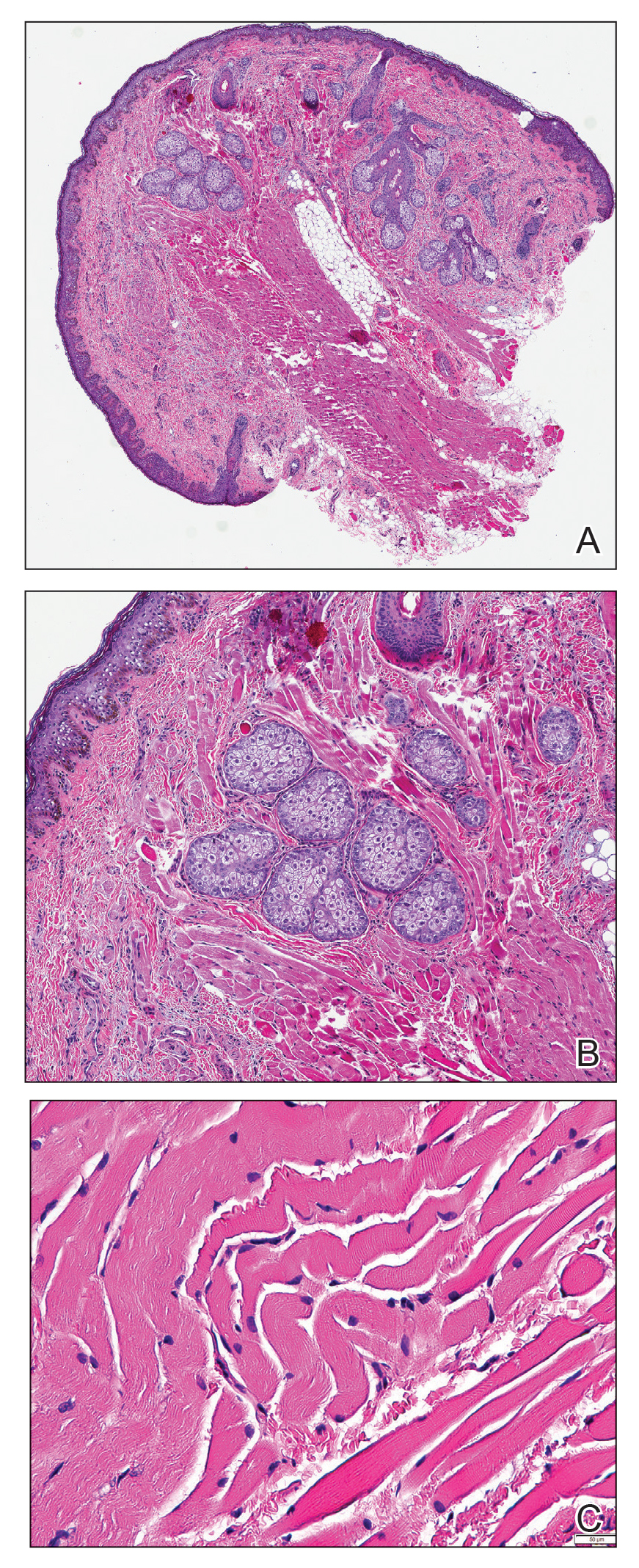
A shave biopsy in our patient revealed moderate to focally marked, irregular epidermal hyperplasia with a large focus of moderate, compact, parakeratotic crust overlying the epidermis in the center of the specimen. The papillary dermis in the center of the specimen revealed large foci of dark pink to purple bone fragments surrounded by moderate lymphocytic infiltrate with few foci perforating through the overlying epidermis (Figure, A). These findings were characteristic of osteoma cutis with perforation through the overlying epidermis.
The diagnosis of osteoma cutis at the age of 62 years suggested that the lesion was not primary in association with previously described diseases. Furthermore, the lack of phenotypic features of these diseases including obesity, developmental disability, and high parathyroid hormone levels essentially excluded this possibility. The presence of the lesion on the lower extremities initially may have suggested osteoma cutis secondary to chronic venous insufficiency13; however, the absence of visible varicose veins or obvious signs of stasis disease made this unlikely. No further cutaneous disorders at or around the lesion site clinically and histologically suggested that our patient’s lesion was primary and of idiopathic nature. Dermatofibroma can present similarly in appearance but would characteristically dimple centrally when pinched. Keratoacanthoma presents with central ulceration and keratin plugging. Pilomatricoma more commonly presents on the head and neck and less frequently as a firm nodule. Lastly, prurigo nodularis more commonly presents as a symmetrically diffuse rash compared to an isolated nodule.
Osteoma cutis is a cutaneous ossification that may be primary or secondary in nature and less rare than originally thought. Workup for potentially associated inflammatory, neoplastic, and metabolic diseases should be considered in patients with this condition. Perforating osteoma cutis is a rare variant that presents as solitary or multiple nodules with central erosion and crust. The mechanism of transepidermal elimination leading to skin perforation is hypothesized to involve epidermal hyperproliferation leading to upward movement.14 Shave biopsy establishes a definitive histopathologic diagnosis and often is curative. Given that lesions of osteoma cutis themselves are benign, removal may not be necessary.
- Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-yearold woman. osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO). JAMA Dermatol. 2013;149:975-976.
- Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483-484.
- Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99-106.
- Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191-205.
- Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542-22553.
- Roth SI, Stowell RE, Helwig EB, et al. Cutaneous ossification. report of 120 cases and review of the literature. Arch Pathol. 1963;76:44-54.
- Shimono K, Uchibe K, Kuboki T, et al. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Japanese Dental Science Review. 2014;50:1-8.
- Kim D, Franco GA, Shigehara H, et al. Benign miliary osteoma cutis of the face: a common incidental CT finding. AJNR Am J Neuroradiol. 2017;38:789-794.
- Basu P, Erickson CP, Calame A, et al. Osteoma cutis: an adverse event following tattoo placement. Cureus. 2019;11:E4323.
- Cohen PR. Perforating osteoma cutis: case report and literature review of patients with a solitary perforating osteoma cutis lesion. Dermatol Online J. 2018;24:13030/qt6kt5n92w.
- Hong SH, Kang HY. A case of perforating osteoma cutis. Ann Dermatol. 2003;15:153-155.
- Kim BK, Ahn SK. Acquired perforating osteoma cutis. Ann Dermatol. 2015;27:452-453.
- Lippmann HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279-288.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. J Cutan Pathol. 2009;36:591-593.
The Diagnosis: Osteoma Cutis
Osteoma cutis is the heterotopic development of cutaneous ossifications in the dermis or subcutaneous fat and presents as plaquelike, stony, hard nodules. It can manifest as either a primary or secondary condition based on the presence or absence of a prior skin insult at the lesion site. Primary osteoma cutis occurs in 15% of patients and arises either de novo or in association with any of several inflammatory, neoplastic, and metabolic diseases that provide a favorable environment for abnormal mesenchymal stem cell commitment to osteoid,1 including Albright hereditary osteodystrophy, myositis ossificans progressiva, and progressive osseous heteroplasia, which are all associated with mutations in the heterotrimeric G-protein alpha subunit encoding gene, GNAS. 1,2 It is suggested that an insufficiency of Gsα leads to uncontrolled negative regulation of nonosseous connective tissue differentiation, forming osteoid.3 Additionally, diseases involving gain-of-function mutations in the activin A receptor type 1 encoding gene, ACVR1, such as fibrodysplasia ossificans progressiva, have been associated with osteoma cutis.4 These mutations lead to decreased receptor affinity to molecular safeguards of bone morphogenetic protein signaling, ultimately contributing to progressive ectopic bone formation.5 Secondary osteoma cutis occurs in 85% of patients and develops at the site of prior skin damage due to inflammation, neoplasm, or trauma.6 It is believed that tissue damage and degeneration lead to mesenchymal stem cell proliferation and skeletogenicinducing factor recruitment forming cartilaginous tissue, later replaced by bone through endochondral ossification.7
Although osteoma cutis previously was believed to be rare, more recent radiologic studies suggest otherwise, detecting cutaneous osteomas in up to 42.1% of patients.8 Consequently, it is likely that osteoma cutis is underdiagnosed due to its subclinical nature. Our patient, however, presented with a solitary osteoma cutis with perforation of the epidermis, a rare phenomenon.9-12
A shave biopsy in our patient revealed moderate to focally marked, irregular epidermal hyperplasia with a large focus of moderate, compact, parakeratotic crust overlying the epidermis in the center of the specimen. The papillary dermis in the center of the specimen revealed large foci of dark pink to purple bone fragments surrounded by moderate lymphocytic infiltrate with few foci perforating through the overlying epidermis (Figure, A). These findings were characteristic of osteoma cutis with perforation through the overlying epidermis.

The diagnosis of osteoma cutis at the age of 62 years suggested that the lesion was not primary in association with previously described diseases. Furthermore, the lack of phenotypic features of these diseases including obesity, developmental disability, and high parathyroid hormone levels essentially excluded this possibility. The presence of the lesion on the lower extremities initially may have suggested osteoma cutis secondary to chronic venous insufficiency13; however, the absence of visible varicose veins or obvious signs of stasis disease made this unlikely. No further cutaneous disorders at or around the lesion site clinically and histologically suggested that our patient’s lesion was primary and of idiopathic nature. Dermatofibroma can present similarly in appearance but would characteristically dimple centrally when pinched. Keratoacanthoma presents with central ulceration and keratin plugging. Pilomatricoma more commonly presents on the head and neck and less frequently as a firm nodule. Lastly, prurigo nodularis more commonly presents as a symmetrically diffuse rash compared to an isolated nodule.
Osteoma cutis is a cutaneous ossification that may be primary or secondary in nature and less rare than originally thought. Workup for potentially associated inflammatory, neoplastic, and metabolic diseases should be considered in patients with this condition. Perforating osteoma cutis is a rare variant that presents as solitary or multiple nodules with central erosion and crust. The mechanism of transepidermal elimination leading to skin perforation is hypothesized to involve epidermal hyperproliferation leading to upward movement.14 Shave biopsy establishes a definitive histopathologic diagnosis and often is curative. Given that lesions of osteoma cutis themselves are benign, removal may not be necessary.
The Diagnosis: Osteoma Cutis
Osteoma cutis is the heterotopic development of cutaneous ossifications in the dermis or subcutaneous fat and presents as plaquelike, stony, hard nodules. It can manifest as either a primary or secondary condition based on the presence or absence of a prior skin insult at the lesion site. Primary osteoma cutis occurs in 15% of patients and arises either de novo or in association with any of several inflammatory, neoplastic, and metabolic diseases that provide a favorable environment for abnormal mesenchymal stem cell commitment to osteoid,1 including Albright hereditary osteodystrophy, myositis ossificans progressiva, and progressive osseous heteroplasia, which are all associated with mutations in the heterotrimeric G-protein alpha subunit encoding gene, GNAS. 1,2 It is suggested that an insufficiency of Gsα leads to uncontrolled negative regulation of nonosseous connective tissue differentiation, forming osteoid.3 Additionally, diseases involving gain-of-function mutations in the activin A receptor type 1 encoding gene, ACVR1, such as fibrodysplasia ossificans progressiva, have been associated with osteoma cutis.4 These mutations lead to decreased receptor affinity to molecular safeguards of bone morphogenetic protein signaling, ultimately contributing to progressive ectopic bone formation.5 Secondary osteoma cutis occurs in 85% of patients and develops at the site of prior skin damage due to inflammation, neoplasm, or trauma.6 It is believed that tissue damage and degeneration lead to mesenchymal stem cell proliferation and skeletogenicinducing factor recruitment forming cartilaginous tissue, later replaced by bone through endochondral ossification.7
Although osteoma cutis previously was believed to be rare, more recent radiologic studies suggest otherwise, detecting cutaneous osteomas in up to 42.1% of patients.8 Consequently, it is likely that osteoma cutis is underdiagnosed due to its subclinical nature. Our patient, however, presented with a solitary osteoma cutis with perforation of the epidermis, a rare phenomenon.9-12
A shave biopsy in our patient revealed moderate to focally marked, irregular epidermal hyperplasia with a large focus of moderate, compact, parakeratotic crust overlying the epidermis in the center of the specimen. The papillary dermis in the center of the specimen revealed large foci of dark pink to purple bone fragments surrounded by moderate lymphocytic infiltrate with few foci perforating through the overlying epidermis (Figure, A). These findings were characteristic of osteoma cutis with perforation through the overlying epidermis.

The diagnosis of osteoma cutis at the age of 62 years suggested that the lesion was not primary in association with previously described diseases. Furthermore, the lack of phenotypic features of these diseases including obesity, developmental disability, and high parathyroid hormone levels essentially excluded this possibility. The presence of the lesion on the lower extremities initially may have suggested osteoma cutis secondary to chronic venous insufficiency13; however, the absence of visible varicose veins or obvious signs of stasis disease made this unlikely. No further cutaneous disorders at or around the lesion site clinically and histologically suggested that our patient’s lesion was primary and of idiopathic nature. Dermatofibroma can present similarly in appearance but would characteristically dimple centrally when pinched. Keratoacanthoma presents with central ulceration and keratin plugging. Pilomatricoma more commonly presents on the head and neck and less frequently as a firm nodule. Lastly, prurigo nodularis more commonly presents as a symmetrically diffuse rash compared to an isolated nodule.
Osteoma cutis is a cutaneous ossification that may be primary or secondary in nature and less rare than originally thought. Workup for potentially associated inflammatory, neoplastic, and metabolic diseases should be considered in patients with this condition. Perforating osteoma cutis is a rare variant that presents as solitary or multiple nodules with central erosion and crust. The mechanism of transepidermal elimination leading to skin perforation is hypothesized to involve epidermal hyperproliferation leading to upward movement.14 Shave biopsy establishes a definitive histopathologic diagnosis and often is curative. Given that lesions of osteoma cutis themselves are benign, removal may not be necessary.
- Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-yearold woman. osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO). JAMA Dermatol. 2013;149:975-976.
- Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483-484.
- Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99-106.
- Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191-205.
- Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542-22553.
- Roth SI, Stowell RE, Helwig EB, et al. Cutaneous ossification. report of 120 cases and review of the literature. Arch Pathol. 1963;76:44-54.
- Shimono K, Uchibe K, Kuboki T, et al. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Japanese Dental Science Review. 2014;50:1-8.
- Kim D, Franco GA, Shigehara H, et al. Benign miliary osteoma cutis of the face: a common incidental CT finding. AJNR Am J Neuroradiol. 2017;38:789-794.
- Basu P, Erickson CP, Calame A, et al. Osteoma cutis: an adverse event following tattoo placement. Cureus. 2019;11:E4323.
- Cohen PR. Perforating osteoma cutis: case report and literature review of patients with a solitary perforating osteoma cutis lesion. Dermatol Online J. 2018;24:13030/qt6kt5n92w.
- Hong SH, Kang HY. A case of perforating osteoma cutis. Ann Dermatol. 2003;15:153-155.
- Kim BK, Ahn SK. Acquired perforating osteoma cutis. Ann Dermatol. 2015;27:452-453.
- Lippmann HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279-288.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. J Cutan Pathol. 2009;36:591-593.
- Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-yearold woman. osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO). JAMA Dermatol. 2013;149:975-976.
- Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483-484.
- Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99-106.
- Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191-205.
- Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542-22553.
- Roth SI, Stowell RE, Helwig EB, et al. Cutaneous ossification. report of 120 cases and review of the literature. Arch Pathol. 1963;76:44-54.
- Shimono K, Uchibe K, Kuboki T, et al. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Japanese Dental Science Review. 2014;50:1-8.
- Kim D, Franco GA, Shigehara H, et al. Benign miliary osteoma cutis of the face: a common incidental CT finding. AJNR Am J Neuroradiol. 2017;38:789-794.
- Basu P, Erickson CP, Calame A, et al. Osteoma cutis: an adverse event following tattoo placement. Cureus. 2019;11:E4323.
- Cohen PR. Perforating osteoma cutis: case report and literature review of patients with a solitary perforating osteoma cutis lesion. Dermatol Online J. 2018;24:13030/qt6kt5n92w.
- Hong SH, Kang HY. A case of perforating osteoma cutis. Ann Dermatol. 2003;15:153-155.
- Kim BK, Ahn SK. Acquired perforating osteoma cutis. Ann Dermatol. 2015;27:452-453.
- Lippmann HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279-288.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. J Cutan Pathol. 2009;36:591-593.
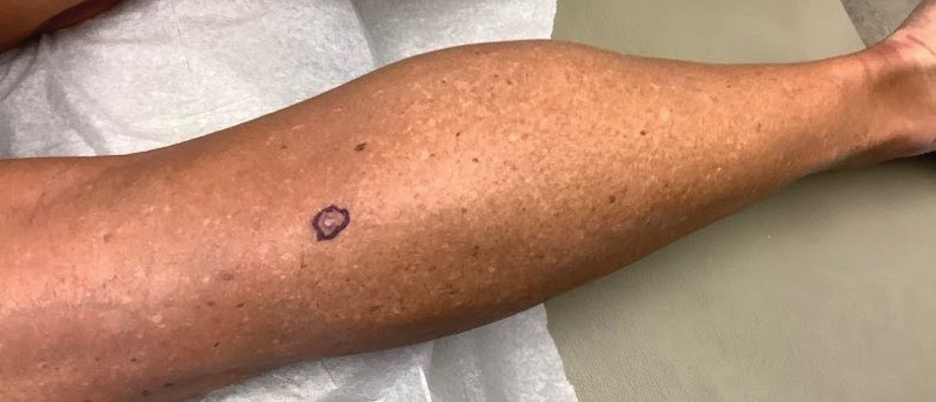
A 62-year-old woman presented with an irregular, erythematous, 4-mm nodule with central erosions on the left proximal calf of 2 months’ duration. The patient had a history of actinic keratoses and dysplastic nevi. She had no other notable medical history. She was not taking any medications and reported no history of trauma to the area. A shave biopsy of the lesion (encircled by black ink) was performed.
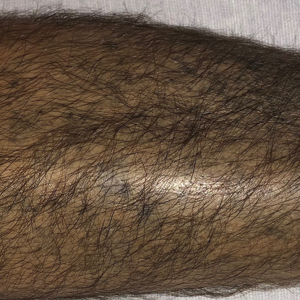
Linear Violaceous Papules in a Child
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
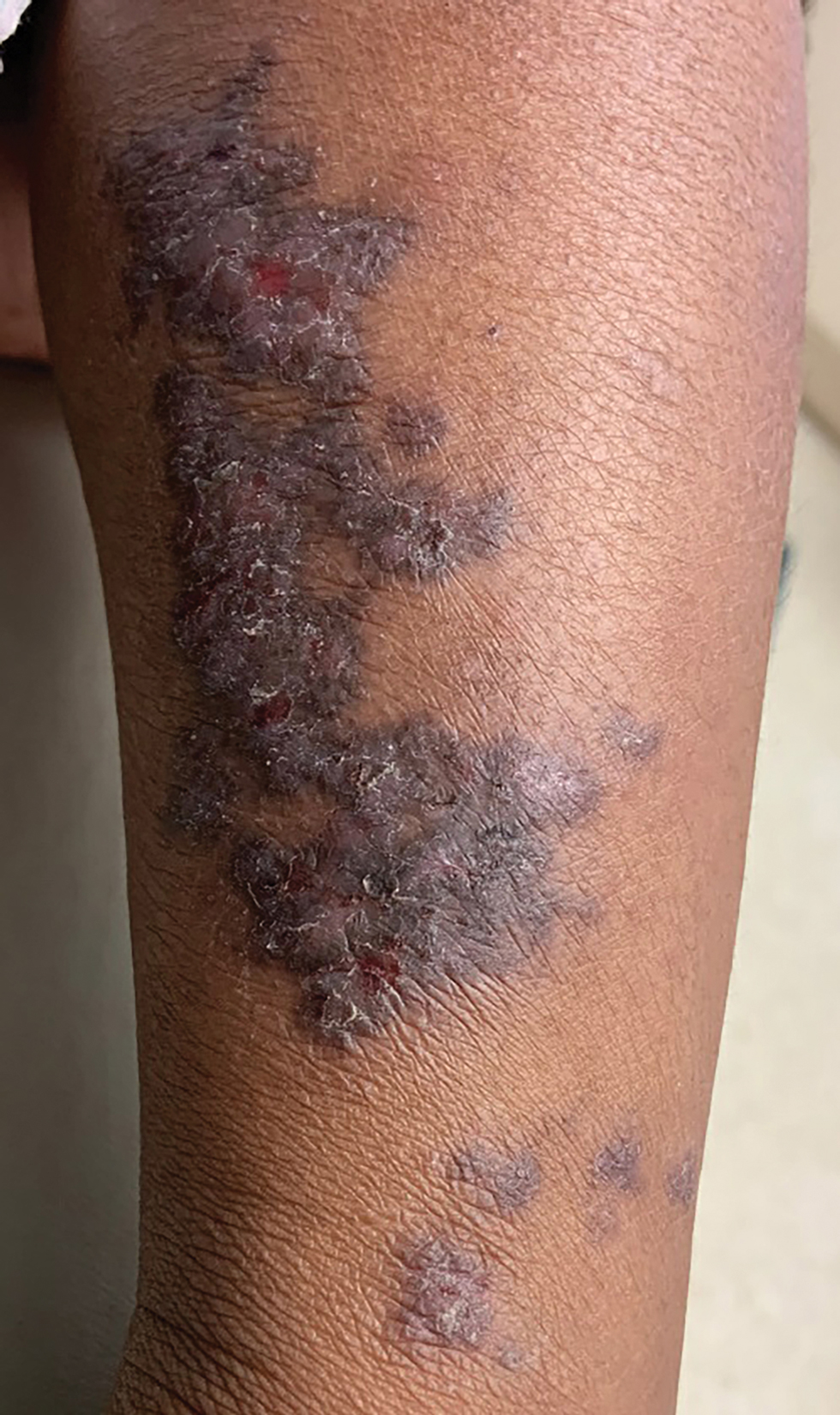
A 5-year-old Black girl presented to the dermatology clinic with a stable pruritic eruption on the right leg of 1 month’s duration. Over-the-counter hydrocortisone cream was applied for 3 days with no response. Physical examination revealed grouped, flat-topped, violaceous papules coalescing into plaques with overlying lacy white striae along the right lower leg, wrapping around to the right dorsal foot in a blaschkoid distribution. The patient was otherwise healthy and up-to-date on immunizations and had an unremarkable birth history.
Ulcer on the Leg
The Diagnosis: Calcinosis Cutis Due to Systemic Sclerosis Sine Scleroderma
Laboratory evaluation was notable for high titers of antinuclear antibodies (>1/320; reference range, 0–1/80) and positive anticentromere antibodies. There were no other relevant laboratory findings; phosphocalcic metabolism was within normal limits, and urinary sediment was normal. Biopsy of the edge of the ulcer revealed basophilic material compatible with calcium deposits. In a 3D volume rendering reconstruction from the lower limb scanner, grouped calcifications were observed in subcutaneous cellular tissue near the ulcer (Figure). The patient had a restrictive ventilatory pattern observed in a pulmonary function test. An esophageal motility study was normal.
The patient was diagnosed with systemic sclerosis sine scleroderma (ssSSc) type II because she met the 4 criteria established by Poormoghim et al1 : (1) Raynaud phenomenon or a peripheral vascular equivalent (ie, digital pitting scars, digital-tip ulcers, digital-tip gangrene, abnormal nail fold capillaries); (2) positive antinuclear antibodies; (3) distal esophageal hypomotility, small bowel hypomotility, pulmonary interstitial fibrosis, primary pulmonary arterial hypertension (without fibrosis), cardiac involvement typical of scleroderma, or renal failure; and (4) no other defined connective tissue or other disease as a cause of the prior conditions.
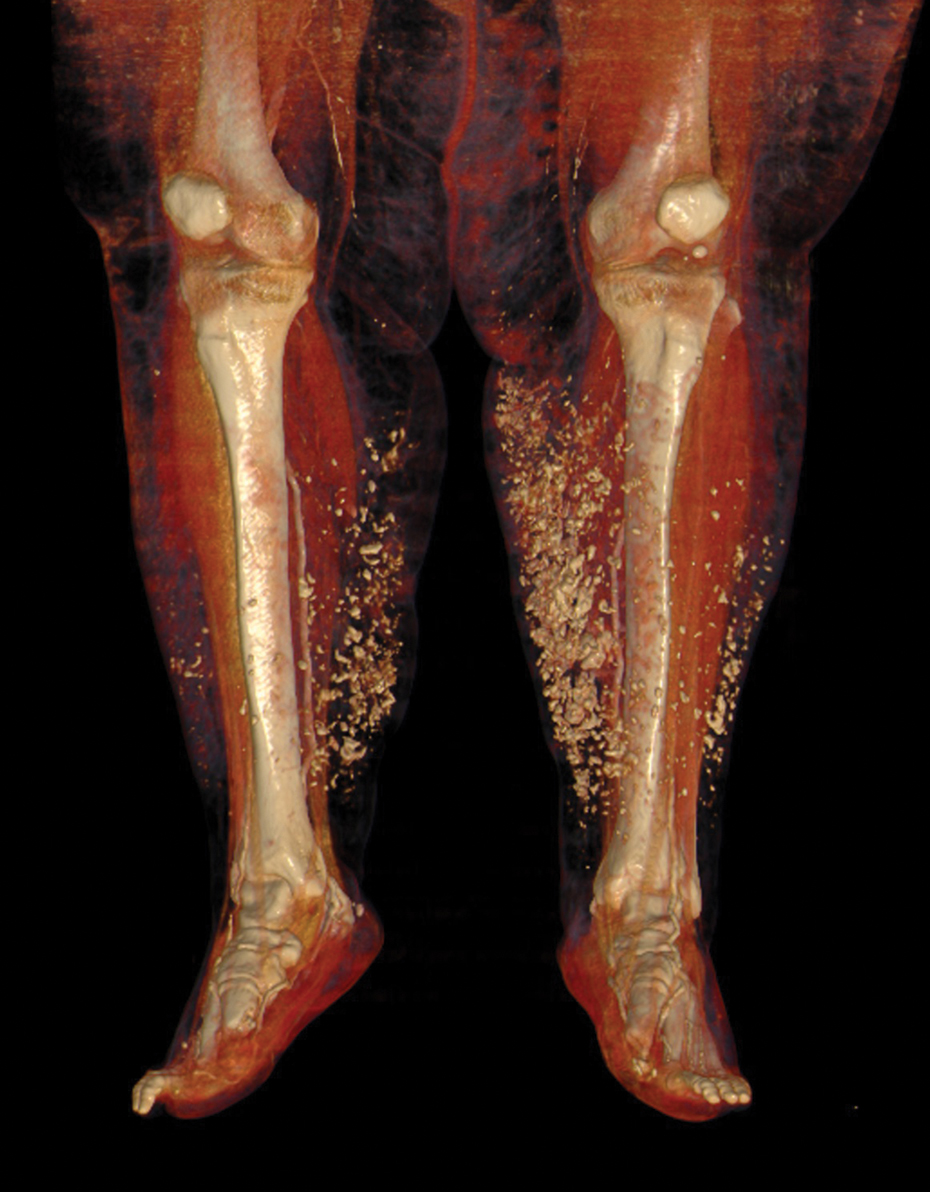
Systemic sclerosis is a chronic disease characterized by progressive fibrosis of the skin and other internal organs—especially the lungs, kidneys, digestive tract, and heart—as well as generalized vascular dysfunction. Cutaneous induration is its hallmark; however, up to 10% of affected patients have ssSSc.2 This entity is characterized by the total or partial absence of cutaneous manifestations of systemic sclerosis with the occurrence of internal organ involvement and serologic abnormalities. There are 3 types of ssSSc depending on the grade of skin involvement. Type I is characterized by the lack of any typical cutaneous stigmata of the disease. Type II is without sclerodactyly but can coexist with other cutaneous findings such as calcifications, telangiectases, or pitting scars. Type III is characterized clinically by internal organ involvement, typical of systemic sclerosis, that has appeared before skin changes.2
An abnormal deposit of calcium in the cutaneous and subcutaneous tissue is called calcinosis cutis. There are 5 subtypes of calcinosis cutis: dystrophic, metastatic, idiopathic, iatrogenic, and calciphylaxis. Dystrophic skin calcifications may appear in patients with connective tissue diseases such as dermatomyositis or systemic sclerosis.3 Up to 25% of patients with systemic sclerosis can develop calcinosis cutis due to local tissue damage, with normal phosphocalcic metabolism.3
Calcinosis cutis is more common in patients with systemic sclerosis and positive anticentromere antibodies.4 The calcifications usually are located in areas that are subject to repeated trauma, such as the fingers or arms, though other locations have been described such as cervical, paraspinal, or on the hips.5,6 Our patient developed calcifications on both legs, which represent atypical areas for this process.
Dermatomyositis also can present with calcinosis cutis. There are 4 patterns of calcification: superficial nodulelike calcified masses; deep calcified masses; deep sheetlike calcifications within the fascial planes; and a rare, diffuse, superficial lacy and reticular calcification that involves almost the entire body surface area.7 Patients with calcinosis cutis secondary to dermatomyositis usually develop proximal muscle weakness, high titers of creatine kinase, heliotrope rash, or interstitial lung disease with specific antibodies.
Calciphylaxis is a serious disorder involving the calcification of dermal and subcutaneous arterioles and capillaries. It presents with painful cutaneous areas of necrosis.
Venous ulcers also can present with secondary dystrophic calcification due to local tissue damage. These patients usually have cutaneous signs of chronic venous insufficiency. Our patient denied prior trauma to the area; therefore, a traumatic ulcer with secondary calcification was ruled out.
The most concerning complication of calcinosis cutis is the development of ulcers, which occurred in 154 of 316 calcinoses (48.7%) in patients with systemic sclerosis and secondary calcifications.8 These ulcers can cause disabling pain or become superinfected, as in our patient.
There currently is no drug capable of removing dystrophic calcifications, but diltiazem, minocycline, or colchicine can reduce their size and prevent their progression. In the event of neurologic compromise or intractable pain, the treatment of choice is surgical removal of the calcification.9 Curettage, intralesional sodium thiosulfate, and intravenous sodium thiosulfate also have been suggested as therapeutic options.10 Antibiotic treatment was carried out in our patient, which controlled the superinfection of the ulcers. Diltiazem also was started, with stabilization of the calcium deposits without a reduction in their size.
There are few studies evaluating the presence of nondigital ulcers in patients with systemic sclerosis. Shanmugam et al11 calculated a 4% (N=249) prevalence of ulcers in the lower limbs of systemic sclerosis patients. In a study by Bohelay et al12 of 45 patients, the estimated prevalence of lower limb ulcers was 12.8%, and the etiologies consisted of 22 cases of venous insufficiency (49%), 21 cases of ischemic causes (47%), and 2 cases of other causes (4%).
We present the case of a woman with ssSSc who developed dystrophic calcinosis cutis in atypical areas with secondary ulceration and superinfection. The skin usually plays a key role in the diagnosis of systemic sclerosis, as sclerodactyly and the characteristic generalized skin induration stand out in affected individuals. Although our patient was diagnosed with ssSSc, her skin manifestations also were crucial for the diagnosis, as she had ulcers on the lower limbs.
- Poormoghim H, Lucas M, Fertig N, et al. Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444-451.
- Kucharz EJ, Kopec´-Me˛ drek M. Systemic sclerosis sine scleroderma. Adv Clin Exp Med. 2017;26:875-880.
- Valenzuela A, Baron M, Herrick AL, et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: a scleroderma clinical trials consortium study. Semin Arthritis Rheum. 2016;46:344-349.
- D’Aoust J, Hudson M, Tatibouet S, et al. Clinical and serologic correlates of antiPM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheum. 2014;66:1608-1615.
- Contreras I, Sallés M, Mínguez S, et al. Hard paracervical tumor in a patient with limited systemic sclerosis. Rheumatol Clin. 2014; 10:336-337.
- Meriglier E, Lafourcade F, Gombert B, et al. Giant calcinosis revealing systemic sclerosis. Int J Rheum Dis. 2019;22:1787-1788.
- Chung CH. Calcinosis universalis in juvenile dermatomyositis [published online September 24, 2020]. Chonnam Med J. 2020;56:212-213.
- Bartoli F, Fiori G, Braschi F, et al. Calcinosis in systemic sclerosis: subsets, distribution and complications [published online May 30, 2016]. Rheumatology (Oxford). 2016;55:1610-1614.
- Jung H, Lee D, Cho J, et al. Surgical treatment of extensive tumoral calcinosis associated with systemic sclerosis. Korean J Thorac Cardiovasc Surg. 2015;48:151-154.
- Badawi AH, Patel V, Warner AE, et al. Dystrophic calcinosis cutis: treatment with intravenous sodium thiosulfate. Cutis. 2020;106:E15-E17.
- Shanmugam V, Price P, Attinger C, et al. Lower extremity ulcers in systemic sclerosis: features and response to therapy [published online August 18, 2010]. Int J Rheumatol. doi:10.1155/2010/747946
- Bohelay G, Blaise S, Levy P, et al. Lower-limb ulcers in systemic sclerosis: a multicentre retrospective case-control study. Acta Derm Venereol. 2018;98:677-682.
The Diagnosis: Calcinosis Cutis Due to Systemic Sclerosis Sine Scleroderma
Laboratory evaluation was notable for high titers of antinuclear antibodies (>1/320; reference range, 0–1/80) and positive anticentromere antibodies. There were no other relevant laboratory findings; phosphocalcic metabolism was within normal limits, and urinary sediment was normal. Biopsy of the edge of the ulcer revealed basophilic material compatible with calcium deposits. In a 3D volume rendering reconstruction from the lower limb scanner, grouped calcifications were observed in subcutaneous cellular tissue near the ulcer (Figure). The patient had a restrictive ventilatory pattern observed in a pulmonary function test. An esophageal motility study was normal.
The patient was diagnosed with systemic sclerosis sine scleroderma (ssSSc) type II because she met the 4 criteria established by Poormoghim et al1 : (1) Raynaud phenomenon or a peripheral vascular equivalent (ie, digital pitting scars, digital-tip ulcers, digital-tip gangrene, abnormal nail fold capillaries); (2) positive antinuclear antibodies; (3) distal esophageal hypomotility, small bowel hypomotility, pulmonary interstitial fibrosis, primary pulmonary arterial hypertension (without fibrosis), cardiac involvement typical of scleroderma, or renal failure; and (4) no other defined connective tissue or other disease as a cause of the prior conditions.

Systemic sclerosis is a chronic disease characterized by progressive fibrosis of the skin and other internal organs—especially the lungs, kidneys, digestive tract, and heart—as well as generalized vascular dysfunction. Cutaneous induration is its hallmark; however, up to 10% of affected patients have ssSSc.2 This entity is characterized by the total or partial absence of cutaneous manifestations of systemic sclerosis with the occurrence of internal organ involvement and serologic abnormalities. There are 3 types of ssSSc depending on the grade of skin involvement. Type I is characterized by the lack of any typical cutaneous stigmata of the disease. Type II is without sclerodactyly but can coexist with other cutaneous findings such as calcifications, telangiectases, or pitting scars. Type III is characterized clinically by internal organ involvement, typical of systemic sclerosis, that has appeared before skin changes.2
An abnormal deposit of calcium in the cutaneous and subcutaneous tissue is called calcinosis cutis. There are 5 subtypes of calcinosis cutis: dystrophic, metastatic, idiopathic, iatrogenic, and calciphylaxis. Dystrophic skin calcifications may appear in patients with connective tissue diseases such as dermatomyositis or systemic sclerosis.3 Up to 25% of patients with systemic sclerosis can develop calcinosis cutis due to local tissue damage, with normal phosphocalcic metabolism.3
Calcinosis cutis is more common in patients with systemic sclerosis and positive anticentromere antibodies.4 The calcifications usually are located in areas that are subject to repeated trauma, such as the fingers or arms, though other locations have been described such as cervical, paraspinal, or on the hips.5,6 Our patient developed calcifications on both legs, which represent atypical areas for this process.
Dermatomyositis also can present with calcinosis cutis. There are 4 patterns of calcification: superficial nodulelike calcified masses; deep calcified masses; deep sheetlike calcifications within the fascial planes; and a rare, diffuse, superficial lacy and reticular calcification that involves almost the entire body surface area.7 Patients with calcinosis cutis secondary to dermatomyositis usually develop proximal muscle weakness, high titers of creatine kinase, heliotrope rash, or interstitial lung disease with specific antibodies.
Calciphylaxis is a serious disorder involving the calcification of dermal and subcutaneous arterioles and capillaries. It presents with painful cutaneous areas of necrosis.
Venous ulcers also can present with secondary dystrophic calcification due to local tissue damage. These patients usually have cutaneous signs of chronic venous insufficiency. Our patient denied prior trauma to the area; therefore, a traumatic ulcer with secondary calcification was ruled out.
The most concerning complication of calcinosis cutis is the development of ulcers, which occurred in 154 of 316 calcinoses (48.7%) in patients with systemic sclerosis and secondary calcifications.8 These ulcers can cause disabling pain or become superinfected, as in our patient.
There currently is no drug capable of removing dystrophic calcifications, but diltiazem, minocycline, or colchicine can reduce their size and prevent their progression. In the event of neurologic compromise or intractable pain, the treatment of choice is surgical removal of the calcification.9 Curettage, intralesional sodium thiosulfate, and intravenous sodium thiosulfate also have been suggested as therapeutic options.10 Antibiotic treatment was carried out in our patient, which controlled the superinfection of the ulcers. Diltiazem also was started, with stabilization of the calcium deposits without a reduction in their size.
There are few studies evaluating the presence of nondigital ulcers in patients with systemic sclerosis. Shanmugam et al11 calculated a 4% (N=249) prevalence of ulcers in the lower limbs of systemic sclerosis patients. In a study by Bohelay et al12 of 45 patients, the estimated prevalence of lower limb ulcers was 12.8%, and the etiologies consisted of 22 cases of venous insufficiency (49%), 21 cases of ischemic causes (47%), and 2 cases of other causes (4%).
We present the case of a woman with ssSSc who developed dystrophic calcinosis cutis in atypical areas with secondary ulceration and superinfection. The skin usually plays a key role in the diagnosis of systemic sclerosis, as sclerodactyly and the characteristic generalized skin induration stand out in affected individuals. Although our patient was diagnosed with ssSSc, her skin manifestations also were crucial for the diagnosis, as she had ulcers on the lower limbs.
The Diagnosis: Calcinosis Cutis Due to Systemic Sclerosis Sine Scleroderma
Laboratory evaluation was notable for high titers of antinuclear antibodies (>1/320; reference range, 0–1/80) and positive anticentromere antibodies. There were no other relevant laboratory findings; phosphocalcic metabolism was within normal limits, and urinary sediment was normal. Biopsy of the edge of the ulcer revealed basophilic material compatible with calcium deposits. In a 3D volume rendering reconstruction from the lower limb scanner, grouped calcifications were observed in subcutaneous cellular tissue near the ulcer (Figure). The patient had a restrictive ventilatory pattern observed in a pulmonary function test. An esophageal motility study was normal.
The patient was diagnosed with systemic sclerosis sine scleroderma (ssSSc) type II because she met the 4 criteria established by Poormoghim et al1 : (1) Raynaud phenomenon or a peripheral vascular equivalent (ie, digital pitting scars, digital-tip ulcers, digital-tip gangrene, abnormal nail fold capillaries); (2) positive antinuclear antibodies; (3) distal esophageal hypomotility, small bowel hypomotility, pulmonary interstitial fibrosis, primary pulmonary arterial hypertension (without fibrosis), cardiac involvement typical of scleroderma, or renal failure; and (4) no other defined connective tissue or other disease as a cause of the prior conditions.

Systemic sclerosis is a chronic disease characterized by progressive fibrosis of the skin and other internal organs—especially the lungs, kidneys, digestive tract, and heart—as well as generalized vascular dysfunction. Cutaneous induration is its hallmark; however, up to 10% of affected patients have ssSSc.2 This entity is characterized by the total or partial absence of cutaneous manifestations of systemic sclerosis with the occurrence of internal organ involvement and serologic abnormalities. There are 3 types of ssSSc depending on the grade of skin involvement. Type I is characterized by the lack of any typical cutaneous stigmata of the disease. Type II is without sclerodactyly but can coexist with other cutaneous findings such as calcifications, telangiectases, or pitting scars. Type III is characterized clinically by internal organ involvement, typical of systemic sclerosis, that has appeared before skin changes.2
An abnormal deposit of calcium in the cutaneous and subcutaneous tissue is called calcinosis cutis. There are 5 subtypes of calcinosis cutis: dystrophic, metastatic, idiopathic, iatrogenic, and calciphylaxis. Dystrophic skin calcifications may appear in patients with connective tissue diseases such as dermatomyositis or systemic sclerosis.3 Up to 25% of patients with systemic sclerosis can develop calcinosis cutis due to local tissue damage, with normal phosphocalcic metabolism.3
Calcinosis cutis is more common in patients with systemic sclerosis and positive anticentromere antibodies.4 The calcifications usually are located in areas that are subject to repeated trauma, such as the fingers or arms, though other locations have been described such as cervical, paraspinal, or on the hips.5,6 Our patient developed calcifications on both legs, which represent atypical areas for this process.
Dermatomyositis also can present with calcinosis cutis. There are 4 patterns of calcification: superficial nodulelike calcified masses; deep calcified masses; deep sheetlike calcifications within the fascial planes; and a rare, diffuse, superficial lacy and reticular calcification that involves almost the entire body surface area.7 Patients with calcinosis cutis secondary to dermatomyositis usually develop proximal muscle weakness, high titers of creatine kinase, heliotrope rash, or interstitial lung disease with specific antibodies.
Calciphylaxis is a serious disorder involving the calcification of dermal and subcutaneous arterioles and capillaries. It presents with painful cutaneous areas of necrosis.
Venous ulcers also can present with secondary dystrophic calcification due to local tissue damage. These patients usually have cutaneous signs of chronic venous insufficiency. Our patient denied prior trauma to the area; therefore, a traumatic ulcer with secondary calcification was ruled out.
The most concerning complication of calcinosis cutis is the development of ulcers, which occurred in 154 of 316 calcinoses (48.7%) in patients with systemic sclerosis and secondary calcifications.8 These ulcers can cause disabling pain or become superinfected, as in our patient.
There currently is no drug capable of removing dystrophic calcifications, but diltiazem, minocycline, or colchicine can reduce their size and prevent their progression. In the event of neurologic compromise or intractable pain, the treatment of choice is surgical removal of the calcification.9 Curettage, intralesional sodium thiosulfate, and intravenous sodium thiosulfate also have been suggested as therapeutic options.10 Antibiotic treatment was carried out in our patient, which controlled the superinfection of the ulcers. Diltiazem also was started, with stabilization of the calcium deposits without a reduction in their size.
There are few studies evaluating the presence of nondigital ulcers in patients with systemic sclerosis. Shanmugam et al11 calculated a 4% (N=249) prevalence of ulcers in the lower limbs of systemic sclerosis patients. In a study by Bohelay et al12 of 45 patients, the estimated prevalence of lower limb ulcers was 12.8%, and the etiologies consisted of 22 cases of venous insufficiency (49%), 21 cases of ischemic causes (47%), and 2 cases of other causes (4%).
We present the case of a woman with ssSSc who developed dystrophic calcinosis cutis in atypical areas with secondary ulceration and superinfection. The skin usually plays a key role in the diagnosis of systemic sclerosis, as sclerodactyly and the characteristic generalized skin induration stand out in affected individuals. Although our patient was diagnosed with ssSSc, her skin manifestations also were crucial for the diagnosis, as she had ulcers on the lower limbs.
- Poormoghim H, Lucas M, Fertig N, et al. Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444-451.
- Kucharz EJ, Kopec´-Me˛ drek M. Systemic sclerosis sine scleroderma. Adv Clin Exp Med. 2017;26:875-880.
- Valenzuela A, Baron M, Herrick AL, et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: a scleroderma clinical trials consortium study. Semin Arthritis Rheum. 2016;46:344-349.
- D’Aoust J, Hudson M, Tatibouet S, et al. Clinical and serologic correlates of antiPM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheum. 2014;66:1608-1615.
- Contreras I, Sallés M, Mínguez S, et al. Hard paracervical tumor in a patient with limited systemic sclerosis. Rheumatol Clin. 2014; 10:336-337.
- Meriglier E, Lafourcade F, Gombert B, et al. Giant calcinosis revealing systemic sclerosis. Int J Rheum Dis. 2019;22:1787-1788.
- Chung CH. Calcinosis universalis in juvenile dermatomyositis [published online September 24, 2020]. Chonnam Med J. 2020;56:212-213.
- Bartoli F, Fiori G, Braschi F, et al. Calcinosis in systemic sclerosis: subsets, distribution and complications [published online May 30, 2016]. Rheumatology (Oxford). 2016;55:1610-1614.
- Jung H, Lee D, Cho J, et al. Surgical treatment of extensive tumoral calcinosis associated with systemic sclerosis. Korean J Thorac Cardiovasc Surg. 2015;48:151-154.
- Badawi AH, Patel V, Warner AE, et al. Dystrophic calcinosis cutis: treatment with intravenous sodium thiosulfate. Cutis. 2020;106:E15-E17.
- Shanmugam V, Price P, Attinger C, et al. Lower extremity ulcers in systemic sclerosis: features and response to therapy [published online August 18, 2010]. Int J Rheumatol. doi:10.1155/2010/747946
- Bohelay G, Blaise S, Levy P, et al. Lower-limb ulcers in systemic sclerosis: a multicentre retrospective case-control study. Acta Derm Venereol. 2018;98:677-682.
- Poormoghim H, Lucas M, Fertig N, et al. Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444-451.
- Kucharz EJ, Kopec´-Me˛ drek M. Systemic sclerosis sine scleroderma. Adv Clin Exp Med. 2017;26:875-880.
- Valenzuela A, Baron M, Herrick AL, et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: a scleroderma clinical trials consortium study. Semin Arthritis Rheum. 2016;46:344-349.
- D’Aoust J, Hudson M, Tatibouet S, et al. Clinical and serologic correlates of antiPM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheum. 2014;66:1608-1615.
- Contreras I, Sallés M, Mínguez S, et al. Hard paracervical tumor in a patient with limited systemic sclerosis. Rheumatol Clin. 2014; 10:336-337.
- Meriglier E, Lafourcade F, Gombert B, et al. Giant calcinosis revealing systemic sclerosis. Int J Rheum Dis. 2019;22:1787-1788.
- Chung CH. Calcinosis universalis in juvenile dermatomyositis [published online September 24, 2020]. Chonnam Med J. 2020;56:212-213.
- Bartoli F, Fiori G, Braschi F, et al. Calcinosis in systemic sclerosis: subsets, distribution and complications [published online May 30, 2016]. Rheumatology (Oxford). 2016;55:1610-1614.
- Jung H, Lee D, Cho J, et al. Surgical treatment of extensive tumoral calcinosis associated with systemic sclerosis. Korean J Thorac Cardiovasc Surg. 2015;48:151-154.
- Badawi AH, Patel V, Warner AE, et al. Dystrophic calcinosis cutis: treatment with intravenous sodium thiosulfate. Cutis. 2020;106:E15-E17.
- Shanmugam V, Price P, Attinger C, et al. Lower extremity ulcers in systemic sclerosis: features and response to therapy [published online August 18, 2010]. Int J Rheumatol. doi:10.1155/2010/747946
- Bohelay G, Blaise S, Levy P, et al. Lower-limb ulcers in systemic sclerosis: a multicentre retrospective case-control study. Acta Derm Venereol. 2018;98:677-682.
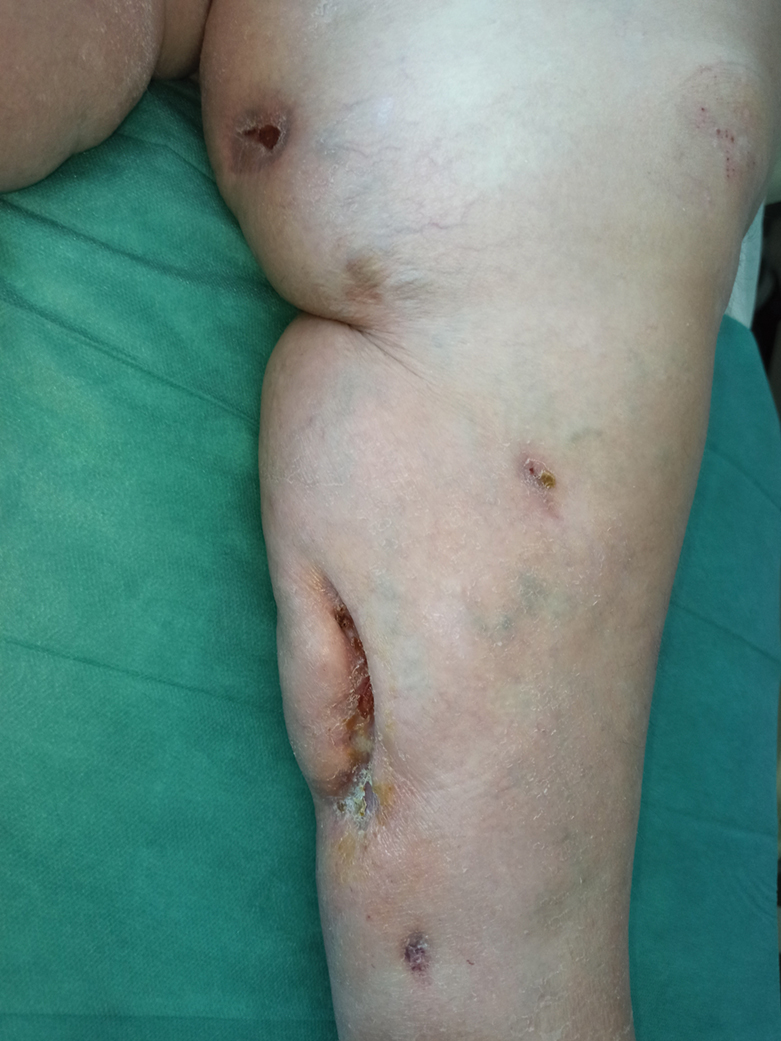
A 49-year-old woman with type 2 diabetes mellitus, morbid obesity, pulmonary fibrosis, and pulmonary arterial hypertension presented to our hospital with an ulcer on the left leg of unknown etiology that was superinfected by multidrug-resistant Klebsiella according to bacterial culture. She had an axillary temperature of 38.6 °C. She underwent amputation of the second and third toes on the left foot 5 years prior to presentation due to distal necrotic ulcers of ischemic origin. Physical examination revealed an 8×2-cm deep ulcer with abrupt edges on the left leg with fibrin and a purulent exudate. Deep palpation of the perilesional skin revealed indurated subcutaneous nodules. She also had scars on the fingertips of both hands with no induration on the rest of the skin surface. Capillaroscopy showed no pathologic findings. Blood cultures were performed, and she was admitted to the hospital for intravenous antibiotic therapy. During ulcer debridement, some solid whitish material was released.
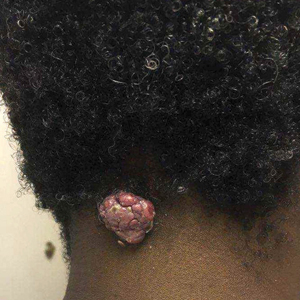
Flesh-Colored Papule in the Nose of a Child
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).
Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).

Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).

Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
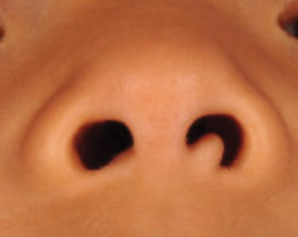
A 4-year-old girl presented to our clinic with an asymptomatic flesh-colored papule in the left nostril. The lesion had been present since birth and grew in relation to the patient with no rapid changes. There had been no pigmentation changes and no bleeding, pain, or itching. The patient’s birth and developmental history were normal. Physical examination revealed a singular, 10×5-mm, flesh-colored, pedunculated mass on the left nasal sill. There were no additional lesions present. An excisional biopsy was performed and submitted for pathologic diagnosis.
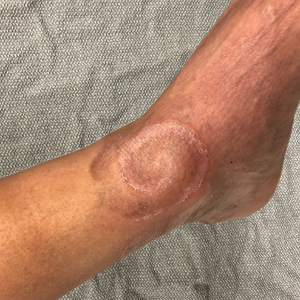
Tender Annular Plaque on the Thigh
The Diagnosis: Ecthyma Gangrenosum
Histopathology revealed basophilic bacterial rods around necrotic vessels with thrombosis and edema (Figure). Blood and tissue cultures grew Pseudomonas aeruginosa. Based on the histopathology and clinical presentation, a diagnosis of P aeruginosa–associated ecthyma gangrenosum (EG) was made. The patient’s symptoms resolved with intravenous cefepime, and he later was transitioned to oral levofloxacin for outpatient treatment.

Ecthyma gangrenosum is an uncommon cutaneous manifestation of bacteremia that most commonly occurs secondary to P aeruginosa in immunocompromised patients, particularly patients with severe neutropenia in the setting of recent chemotherapy.1,2 Ecthyma gangrenosum can occur anywhere on the body, predominantly in moist areas such as the axillae and groin; the arms and legs, such as in our patient, as well as the trunk and face also may be involved.3 Other causes of EG skin lesions include methicillin-resistant Staphylococcus aureus, Citrobacter freundii, Escherichia coli, fungi such as Candida, and viruses such as herpes simplex virus.2,4-6 Common predisposing conditions associated with EG include neutropenia, leukemia, HIV, diabetes mellitus, extensive burn wounds, and a history of immunosuppressive medications. It also has been known to occur in otherwise healthy, immunocompetent individuals with no difference in clinical manifestation.2
The diagnosis is clinicopathologic, with initial evaluation including blood and wound cultures as well as a complete blood cell count once EG is suspected. An excisional or punch biopsy is performed for confirmation, showing many gram-negative, rod-shaped bacteria in cases of pseudomonal EG.7 Histopathology is characterized by bacterial perivascular invasion that then leads to secondary arteriole thrombosis, tissue edema, and separation of the epidermis.7,8 Resultant ischemic necrosis results in the classic macroscopic appearance of an erythematous macule that rapidly progresses into a central necrotic lesion surrounded by an erythematous or violaceous halo after undergoing a hemorrhagic bullous stage.1,9 A Wood lamp can be used to expedite the diagnosis, as Pseudomonas bacteria excretes a pigment (pyoverdine) that fluoresces yellowish green.10
Ecthyma gangrenosum can be classified as a primary skin lesion that may or may not be followed by bacteremia or as a lesion secondary to pseudomonal bacteremia.11 Bacteremia has been reported in half of cases, with hematogenous metastasis of the infection, likely in manifestations with multiple bilateral lesions.2 Our patient’s presentation of a single lesion revealed a positive blood culture result. Lesions also can develop by direct inoculation of the epidermis causing local destruction of the surrounding tissue. The nonbacteremic form of EG has been associated with a lower mortality rate of around 15% compared to patients with bacteremia ranging from 38% to 96%.12 The presence of neutropenia is the most important prognostic factor for mortality at the time of diagnosis.13
Prompt empiric therapy should be initiated after obtaining wound and blood cultures in those with infection until the causative organism and its susceptibility are identified. Pseudomonal infections account for 4% of all cases of hospital-acquired bacteremia and are the third leading cause of gram-negative bloodstream infection.7 Initial broad-spectrum antibiotics include antipseudomonal β-lactams (piperacillin-tazobactam), cephalosporins (cefepime), fluoroquinolones (levofloxacin), and carbapenems (imipenem).1,7 Medical therapy alone may be sufficient without requiring extensive surgical debridement to remove necrotic tissue in some patients. Surgical debridement usually is warranted for lesions larger than 10 cm in diameter.3 Our patient was treated with intravenous cefepime with resolution and was followed with outpatient oral levofloxacin as appropriate. A high index of suspicion should be maintained for relapsing pseudomonal EG infection among patients with AIDS, as the reported recurrence rate is 57%.14
Clinically, the differential diagnosis of EG presenting in immunocompromised patients or individuals with underlying malignancy includes pyoderma gangrenosum, papulonecrotic tuberculid, and leukemia cutis. An erythematous rash with central necrosis presenting in a patient with systemic symptoms is pathognomonic for erythema migrans and should be considered as a diagnostic possibility in areas endemic for Lyme disease in the United States, including the northeastern, mid-Atlantic, and north-central regions.15 A thorough history, physical examination, basic laboratory studies, and histopathology are critical to differentiate between these entities with similar macroscopic features. Pyoderma gangrenosum histologically manifests as a noninfectious, deep, suppurative folliculitis with leukocytoclastic vasculitis in 40% of cases.16 Although papulonecrotic tuberculid can present with dermal necrosis resulting from a hypersensitivity reaction to antigenic components of mycobacteria, there typically are granulomatous infiltrates present and a lack of observed organisms on histopathology.17 Although leukemia cutis infrequently occurs in patients diagnosed with leukemia, its salient features on pathology are nodular or diffuse infiltrates of leukemic cells in the dermis and subcutis with a high nuclear-to-cytoplasmic ratio, often with prominent nucleoli.18 Lyme disease can present in various ways; however, cutaneous involvement in the primary lesion is histologically characterized by a perivascular lymphohistiocytic infiltrate containing plasma cells at the periphery of the expanding annular lesion and eosinophils present at the center.19
- Abdou A, Hassam B. Ecthyma gangrenosum [in French]. Pan Afr Med J. 2018;30:95. doi:10.11604/pamj.2018.30.95.6244
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis. 2015;34:633-639. doi:10.1007/s10096-014-2277-6
- Vaiman M, Lasarovitch T, Heller L, et al. Ecthyma gangrenosum versus ecthyma-like lesions: should we separate these conditions? Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:69-72. doi:10.15570 /actaapa.2015.18
- Reich HL, Williams Fadeyi D, Naik NS, et al. Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol. 2004;50(5 suppl): S114-S117. doi:10.1016/j.jaad.2003.09.019
- Hawkley T, Chang D, Pollard W, et al. Ecthyma gangrenosum caused by Citrobacter freundii [published online July 27, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220996
- Santhaseelan RG, Muralidhar V. Non-pseudomonal ecthyma gangrenosum caused by methicillin-resistant Staphylococcus aureus (MRSA) in a chronic alcoholic patient [published online August 3, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220983m
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections [published online May 29, 2018]. Drugs Context. 2018;7:212527. doi:10.7573/dic.212527
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662. doi:10.1097/DAD.0000000000000766
- Sarkar S, Patra AK, Mondal M. Ecthyma gangrenosum in the periorbital region in a previously healthy immunocompetent woman without bacteremia. Indian Dermatol Online J. 2016;7:36-39. doi:10.4103/2229-5178.174326
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Van den Broek PJ, Van der Meer JWM, Kunst MW. The pathogenesis of ecthyma gangrenosum. J Infect. 1979;1:263-267. doi:10.1016 /S0163-4453(79)91329-X
- Downey DM, O’Bryan MC, Burdette SD, et al. Ecthyma gangrenosum in a patient with toxic epidermal necrolysis. J Burn Care Res. 2007;28:198-202. doi:10.1097/BCR.0B013E31802CA481
- Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, et al. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92:698-700. doi:10.1590/abd1806-4841.20175580
- Khan MO, Montecalvo MA, Davis I, et al. Ecthyma gangrenosum in patients with acquired immunodeficiency syndrome. Cutis. 2000;66:121-123.
- Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998; 352:557-565.
- Su WP, Schroeter AL, Perry HO, et al. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13:323-330. doi:10.1111/j.1600-0560.1986.tb00466.x
- Tirumalae R, Yeliur IK, Antony M, et al. Papulonecrotic tuberculidclinicopathologic and molecular features of 12 Indian patients. Dermatol Pract Concept. 2014;4:17-22. doi:10.5826/dpc.0402a03
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis [published online August 15, 2015]. Dermatol Online J. 2015;21:13030/qt6m18g35f
- Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167-174. doi:10.4103/0019-5154.110822
The Diagnosis: Ecthyma Gangrenosum
Histopathology revealed basophilic bacterial rods around necrotic vessels with thrombosis and edema (Figure). Blood and tissue cultures grew Pseudomonas aeruginosa. Based on the histopathology and clinical presentation, a diagnosis of P aeruginosa–associated ecthyma gangrenosum (EG) was made. The patient’s symptoms resolved with intravenous cefepime, and he later was transitioned to oral levofloxacin for outpatient treatment.

Ecthyma gangrenosum is an uncommon cutaneous manifestation of bacteremia that most commonly occurs secondary to P aeruginosa in immunocompromised patients, particularly patients with severe neutropenia in the setting of recent chemotherapy.1,2 Ecthyma gangrenosum can occur anywhere on the body, predominantly in moist areas such as the axillae and groin; the arms and legs, such as in our patient, as well as the trunk and face also may be involved.3 Other causes of EG skin lesions include methicillin-resistant Staphylococcus aureus, Citrobacter freundii, Escherichia coli, fungi such as Candida, and viruses such as herpes simplex virus.2,4-6 Common predisposing conditions associated with EG include neutropenia, leukemia, HIV, diabetes mellitus, extensive burn wounds, and a history of immunosuppressive medications. It also has been known to occur in otherwise healthy, immunocompetent individuals with no difference in clinical manifestation.2
The diagnosis is clinicopathologic, with initial evaluation including blood and wound cultures as well as a complete blood cell count once EG is suspected. An excisional or punch biopsy is performed for confirmation, showing many gram-negative, rod-shaped bacteria in cases of pseudomonal EG.7 Histopathology is characterized by bacterial perivascular invasion that then leads to secondary arteriole thrombosis, tissue edema, and separation of the epidermis.7,8 Resultant ischemic necrosis results in the classic macroscopic appearance of an erythematous macule that rapidly progresses into a central necrotic lesion surrounded by an erythematous or violaceous halo after undergoing a hemorrhagic bullous stage.1,9 A Wood lamp can be used to expedite the diagnosis, as Pseudomonas bacteria excretes a pigment (pyoverdine) that fluoresces yellowish green.10
Ecthyma gangrenosum can be classified as a primary skin lesion that may or may not be followed by bacteremia or as a lesion secondary to pseudomonal bacteremia.11 Bacteremia has been reported in half of cases, with hematogenous metastasis of the infection, likely in manifestations with multiple bilateral lesions.2 Our patient’s presentation of a single lesion revealed a positive blood culture result. Lesions also can develop by direct inoculation of the epidermis causing local destruction of the surrounding tissue. The nonbacteremic form of EG has been associated with a lower mortality rate of around 15% compared to patients with bacteremia ranging from 38% to 96%.12 The presence of neutropenia is the most important prognostic factor for mortality at the time of diagnosis.13
Prompt empiric therapy should be initiated after obtaining wound and blood cultures in those with infection until the causative organism and its susceptibility are identified. Pseudomonal infections account for 4% of all cases of hospital-acquired bacteremia and are the third leading cause of gram-negative bloodstream infection.7 Initial broad-spectrum antibiotics include antipseudomonal β-lactams (piperacillin-tazobactam), cephalosporins (cefepime), fluoroquinolones (levofloxacin), and carbapenems (imipenem).1,7 Medical therapy alone may be sufficient without requiring extensive surgical debridement to remove necrotic tissue in some patients. Surgical debridement usually is warranted for lesions larger than 10 cm in diameter.3 Our patient was treated with intravenous cefepime with resolution and was followed with outpatient oral levofloxacin as appropriate. A high index of suspicion should be maintained for relapsing pseudomonal EG infection among patients with AIDS, as the reported recurrence rate is 57%.14
Clinically, the differential diagnosis of EG presenting in immunocompromised patients or individuals with underlying malignancy includes pyoderma gangrenosum, papulonecrotic tuberculid, and leukemia cutis. An erythematous rash with central necrosis presenting in a patient with systemic symptoms is pathognomonic for erythema migrans and should be considered as a diagnostic possibility in areas endemic for Lyme disease in the United States, including the northeastern, mid-Atlantic, and north-central regions.15 A thorough history, physical examination, basic laboratory studies, and histopathology are critical to differentiate between these entities with similar macroscopic features. Pyoderma gangrenosum histologically manifests as a noninfectious, deep, suppurative folliculitis with leukocytoclastic vasculitis in 40% of cases.16 Although papulonecrotic tuberculid can present with dermal necrosis resulting from a hypersensitivity reaction to antigenic components of mycobacteria, there typically are granulomatous infiltrates present and a lack of observed organisms on histopathology.17 Although leukemia cutis infrequently occurs in patients diagnosed with leukemia, its salient features on pathology are nodular or diffuse infiltrates of leukemic cells in the dermis and subcutis with a high nuclear-to-cytoplasmic ratio, often with prominent nucleoli.18 Lyme disease can present in various ways; however, cutaneous involvement in the primary lesion is histologically characterized by a perivascular lymphohistiocytic infiltrate containing plasma cells at the periphery of the expanding annular lesion and eosinophils present at the center.19
The Diagnosis: Ecthyma Gangrenosum
Histopathology revealed basophilic bacterial rods around necrotic vessels with thrombosis and edema (Figure). Blood and tissue cultures grew Pseudomonas aeruginosa. Based on the histopathology and clinical presentation, a diagnosis of P aeruginosa–associated ecthyma gangrenosum (EG) was made. The patient’s symptoms resolved with intravenous cefepime, and he later was transitioned to oral levofloxacin for outpatient treatment.

Ecthyma gangrenosum is an uncommon cutaneous manifestation of bacteremia that most commonly occurs secondary to P aeruginosa in immunocompromised patients, particularly patients with severe neutropenia in the setting of recent chemotherapy.1,2 Ecthyma gangrenosum can occur anywhere on the body, predominantly in moist areas such as the axillae and groin; the arms and legs, such as in our patient, as well as the trunk and face also may be involved.3 Other causes of EG skin lesions include methicillin-resistant Staphylococcus aureus, Citrobacter freundii, Escherichia coli, fungi such as Candida, and viruses such as herpes simplex virus.2,4-6 Common predisposing conditions associated with EG include neutropenia, leukemia, HIV, diabetes mellitus, extensive burn wounds, and a history of immunosuppressive medications. It also has been known to occur in otherwise healthy, immunocompetent individuals with no difference in clinical manifestation.2
The diagnosis is clinicopathologic, with initial evaluation including blood and wound cultures as well as a complete blood cell count once EG is suspected. An excisional or punch biopsy is performed for confirmation, showing many gram-negative, rod-shaped bacteria in cases of pseudomonal EG.7 Histopathology is characterized by bacterial perivascular invasion that then leads to secondary arteriole thrombosis, tissue edema, and separation of the epidermis.7,8 Resultant ischemic necrosis results in the classic macroscopic appearance of an erythematous macule that rapidly progresses into a central necrotic lesion surrounded by an erythematous or violaceous halo after undergoing a hemorrhagic bullous stage.1,9 A Wood lamp can be used to expedite the diagnosis, as Pseudomonas bacteria excretes a pigment (pyoverdine) that fluoresces yellowish green.10
Ecthyma gangrenosum can be classified as a primary skin lesion that may or may not be followed by bacteremia or as a lesion secondary to pseudomonal bacteremia.11 Bacteremia has been reported in half of cases, with hematogenous metastasis of the infection, likely in manifestations with multiple bilateral lesions.2 Our patient’s presentation of a single lesion revealed a positive blood culture result. Lesions also can develop by direct inoculation of the epidermis causing local destruction of the surrounding tissue. The nonbacteremic form of EG has been associated with a lower mortality rate of around 15% compared to patients with bacteremia ranging from 38% to 96%.12 The presence of neutropenia is the most important prognostic factor for mortality at the time of diagnosis.13
Prompt empiric therapy should be initiated after obtaining wound and blood cultures in those with infection until the causative organism and its susceptibility are identified. Pseudomonal infections account for 4% of all cases of hospital-acquired bacteremia and are the third leading cause of gram-negative bloodstream infection.7 Initial broad-spectrum antibiotics include antipseudomonal β-lactams (piperacillin-tazobactam), cephalosporins (cefepime), fluoroquinolones (levofloxacin), and carbapenems (imipenem).1,7 Medical therapy alone may be sufficient without requiring extensive surgical debridement to remove necrotic tissue in some patients. Surgical debridement usually is warranted for lesions larger than 10 cm in diameter.3 Our patient was treated with intravenous cefepime with resolution and was followed with outpatient oral levofloxacin as appropriate. A high index of suspicion should be maintained for relapsing pseudomonal EG infection among patients with AIDS, as the reported recurrence rate is 57%.14
Clinically, the differential diagnosis of EG presenting in immunocompromised patients or individuals with underlying malignancy includes pyoderma gangrenosum, papulonecrotic tuberculid, and leukemia cutis. An erythematous rash with central necrosis presenting in a patient with systemic symptoms is pathognomonic for erythema migrans and should be considered as a diagnostic possibility in areas endemic for Lyme disease in the United States, including the northeastern, mid-Atlantic, and north-central regions.15 A thorough history, physical examination, basic laboratory studies, and histopathology are critical to differentiate between these entities with similar macroscopic features. Pyoderma gangrenosum histologically manifests as a noninfectious, deep, suppurative folliculitis with leukocytoclastic vasculitis in 40% of cases.16 Although papulonecrotic tuberculid can present with dermal necrosis resulting from a hypersensitivity reaction to antigenic components of mycobacteria, there typically are granulomatous infiltrates present and a lack of observed organisms on histopathology.17 Although leukemia cutis infrequently occurs in patients diagnosed with leukemia, its salient features on pathology are nodular or diffuse infiltrates of leukemic cells in the dermis and subcutis with a high nuclear-to-cytoplasmic ratio, often with prominent nucleoli.18 Lyme disease can present in various ways; however, cutaneous involvement in the primary lesion is histologically characterized by a perivascular lymphohistiocytic infiltrate containing plasma cells at the periphery of the expanding annular lesion and eosinophils present at the center.19
- Abdou A, Hassam B. Ecthyma gangrenosum [in French]. Pan Afr Med J. 2018;30:95. doi:10.11604/pamj.2018.30.95.6244
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis. 2015;34:633-639. doi:10.1007/s10096-014-2277-6
- Vaiman M, Lasarovitch T, Heller L, et al. Ecthyma gangrenosum versus ecthyma-like lesions: should we separate these conditions? Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:69-72. doi:10.15570 /actaapa.2015.18
- Reich HL, Williams Fadeyi D, Naik NS, et al. Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol. 2004;50(5 suppl): S114-S117. doi:10.1016/j.jaad.2003.09.019
- Hawkley T, Chang D, Pollard W, et al. Ecthyma gangrenosum caused by Citrobacter freundii [published online July 27, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220996
- Santhaseelan RG, Muralidhar V. Non-pseudomonal ecthyma gangrenosum caused by methicillin-resistant Staphylococcus aureus (MRSA) in a chronic alcoholic patient [published online August 3, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220983m
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections [published online May 29, 2018]. Drugs Context. 2018;7:212527. doi:10.7573/dic.212527
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662. doi:10.1097/DAD.0000000000000766
- Sarkar S, Patra AK, Mondal M. Ecthyma gangrenosum in the periorbital region in a previously healthy immunocompetent woman without bacteremia. Indian Dermatol Online J. 2016;7:36-39. doi:10.4103/2229-5178.174326
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Van den Broek PJ, Van der Meer JWM, Kunst MW. The pathogenesis of ecthyma gangrenosum. J Infect. 1979;1:263-267. doi:10.1016 /S0163-4453(79)91329-X
- Downey DM, O’Bryan MC, Burdette SD, et al. Ecthyma gangrenosum in a patient with toxic epidermal necrolysis. J Burn Care Res. 2007;28:198-202. doi:10.1097/BCR.0B013E31802CA481
- Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, et al. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92:698-700. doi:10.1590/abd1806-4841.20175580
- Khan MO, Montecalvo MA, Davis I, et al. Ecthyma gangrenosum in patients with acquired immunodeficiency syndrome. Cutis. 2000;66:121-123.
- Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998; 352:557-565.
- Su WP, Schroeter AL, Perry HO, et al. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13:323-330. doi:10.1111/j.1600-0560.1986.tb00466.x
- Tirumalae R, Yeliur IK, Antony M, et al. Papulonecrotic tuberculidclinicopathologic and molecular features of 12 Indian patients. Dermatol Pract Concept. 2014;4:17-22. doi:10.5826/dpc.0402a03
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis [published online August 15, 2015]. Dermatol Online J. 2015;21:13030/qt6m18g35f
- Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167-174. doi:10.4103/0019-5154.110822
- Abdou A, Hassam B. Ecthyma gangrenosum [in French]. Pan Afr Med J. 2018;30:95. doi:10.11604/pamj.2018.30.95.6244
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis. 2015;34:633-639. doi:10.1007/s10096-014-2277-6
- Vaiman M, Lasarovitch T, Heller L, et al. Ecthyma gangrenosum versus ecthyma-like lesions: should we separate these conditions? Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:69-72. doi:10.15570 /actaapa.2015.18
- Reich HL, Williams Fadeyi D, Naik NS, et al. Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol. 2004;50(5 suppl): S114-S117. doi:10.1016/j.jaad.2003.09.019
- Hawkley T, Chang D, Pollard W, et al. Ecthyma gangrenosum caused by Citrobacter freundii [published online July 27, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220996
- Santhaseelan RG, Muralidhar V. Non-pseudomonal ecthyma gangrenosum caused by methicillin-resistant Staphylococcus aureus (MRSA) in a chronic alcoholic patient [published online August 3, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220983m
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections [published online May 29, 2018]. Drugs Context. 2018;7:212527. doi:10.7573/dic.212527
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662. doi:10.1097/DAD.0000000000000766
- Sarkar S, Patra AK, Mondal M. Ecthyma gangrenosum in the periorbital region in a previously healthy immunocompetent woman without bacteremia. Indian Dermatol Online J. 2016;7:36-39. doi:10.4103/2229-5178.174326
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Van den Broek PJ, Van der Meer JWM, Kunst MW. The pathogenesis of ecthyma gangrenosum. J Infect. 1979;1:263-267. doi:10.1016 /S0163-4453(79)91329-X
- Downey DM, O’Bryan MC, Burdette SD, et al. Ecthyma gangrenosum in a patient with toxic epidermal necrolysis. J Burn Care Res. 2007;28:198-202. doi:10.1097/BCR.0B013E31802CA481
- Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, et al. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92:698-700. doi:10.1590/abd1806-4841.20175580
- Khan MO, Montecalvo MA, Davis I, et al. Ecthyma gangrenosum in patients with acquired immunodeficiency syndrome. Cutis. 2000;66:121-123.
- Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998; 352:557-565.
- Su WP, Schroeter AL, Perry HO, et al. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13:323-330. doi:10.1111/j.1600-0560.1986.tb00466.x
- Tirumalae R, Yeliur IK, Antony M, et al. Papulonecrotic tuberculidclinicopathologic and molecular features of 12 Indian patients. Dermatol Pract Concept. 2014;4:17-22. doi:10.5826/dpc.0402a03
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis [published online August 15, 2015]. Dermatol Online J. 2015;21:13030/qt6m18g35f
- Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167-174. doi:10.4103/0019-5154.110822
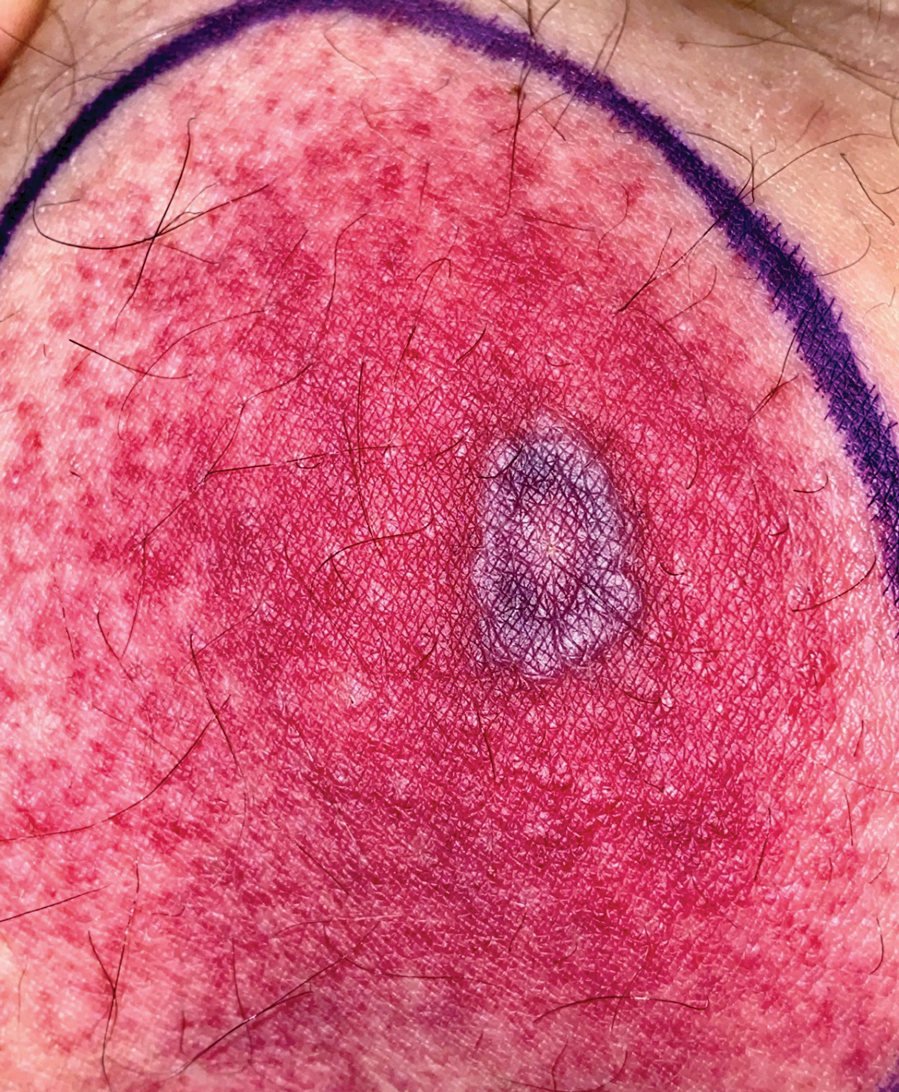
A 58-year-old man who was receiving gilteritinib therapy for relapsed acute myeloid leukemia presented to the emergency department with a painful, rapidly enlarging lesion on the right medial thigh of 2 days’ duration that was accompanied by fever (temperature, 39.2 °C) and body aches. Physical examination revealed a tender annular plaque with a dark violaceous halo overlying a larger area of erythema and induration. Laboratory evaluation revealed a white blood cell count of 600/μL (reference range, 4500–11,000/μL) and an absolute neutrophil count of 200/μL (reference range, 1800–7000/μL). A biopsy was performed.
Painful Psoriasiform Plaques
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).
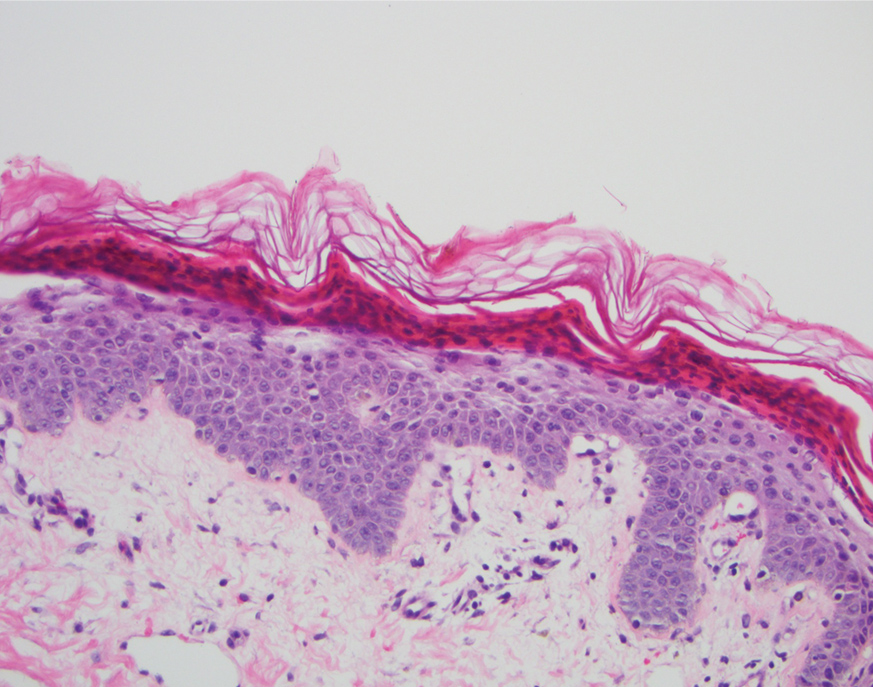
Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).

Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).

Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
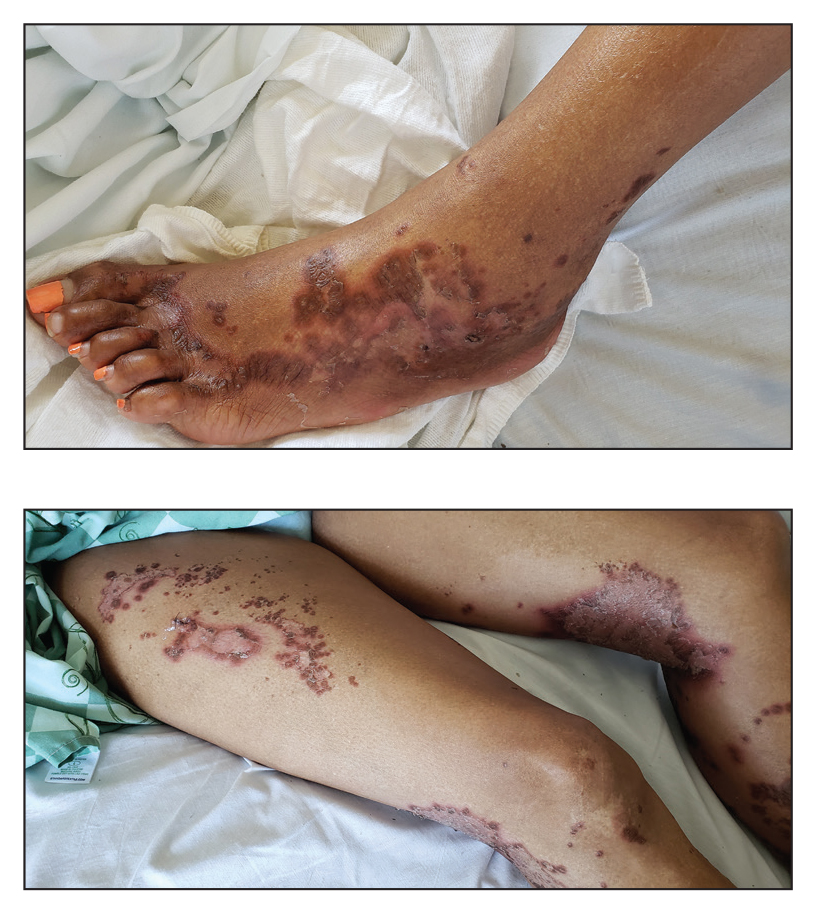
A 45-year-old woman presented to the emergency department with a painful skin eruption and malaise of 5 weeks’ duration. She had an orthotopic liver transplant 5 years prior for end-stage liver disease due to mixed nonalcoholic and alcoholic steatohepatitis and was on mycophenolate mofetil and tacrolimus for graft rejection prophylaxis. Her medical history also included Roux-en-Y gastric bypass 15 years prior, alcohol use disorder, hypothyroidism, and depression.
The exanthem began on the legs as pruritic, red, raised, exudative lesions that gradually crusted. Over the 2 weeks prior to the current presentation, the rash became tender as it spread to the feet, thighs, perianal skin, buttocks, and elbows. Triamcinolone ointment prescribed for a presumed nummular dermatitis effected marginal benefit. A review of systems was notable for a 15-pound weight loss over several weeks; lowgrade fever of 3 days’ duration; epigastric abdominal pain; and long-standing, frequent defecation of oily, foul-smelling feces.
Physical examination revealed a combination of flat-topped, violaceous papules and serpiginous, polycyclic, annular plaques coalescing to form larger psoriasiform plaques with hyperkeratotic rims and dusky borders on the dorsal aspect of the feet (top), lateral ankles, legs (bottom), lateral thighs, buttocks, perianal skin, and elbows. Bilateral angular cheilitis, a smooth and fissured tongue, and pitting of all fingernails were noted.
Chronic Hyperpigmented Patches on the Legs
The Diagnosis: Drug-Induced Hyperpigmentation
Additional history provided by the patient’s caretaker elucidated an extensive list of medications including chlorpromazine and minocycline, among several others. The caretaker revealed that the patient began treatment for acne vulgaris 2 years prior; despite the acne resolving, therapy was not discontinued. The blue-gray and brown pigmentation on our patient’s shins likely was attributed to a medication he was taking.
Both chlorpromazine and minocycline, among many other medications, are known to cause abnormal pigmentation of the skin.1 Minocycline is a tetracycline antibiotic prescribed for acne and other inflammatory cutaneous conditions. It is highly lipophilic, allowing it to reach high drug concentrations in the skin and nail unit.2 Patients taking minocycline long term and at high doses are at greatest risk for pigment deposition.3,4
Minocycline-induced hyperpigmentation is classified into 3 types. Type I describes blue-black deposition of pigment in acne scars and areas of inflammation, typically on facial skin.1,5 Histologically, type I stains positive for Perls Prussian blue, indicating an increased deposition of iron as hemosiderin,1 which likely occurs because minocycline is thought to play a role in defective clearance of hemosiderin from the dermis of injured tissue.5 Type II hyperpigmentation presents as bluegray pigment on the lower legs and occasionally the arms.6,7 Type II stains positive for both Perls Prussian blue and Fontana-Masson, demonstrating hemosiderin and melanin, respectively.6 The third form of hyperpigmentation results in diffuse, dark brown to gray pigmentation with a predilection for sun-exposed areas.8 Histology of type III shows increased pigment in the basal portion of the epidermis and brown-black pigment in macrophages of the dermis. Type III stains positive for Fontana-Masson and negative for Perls Prussian blue. The etiology of hyperpigmentation has been suspected to be caused by minocycline stimulating melanin production and/or deposition of minocycline-melanin complexes in dermal macrophages after a certain drug level; this largely is seen in patients receiving 100 to 200 mg daily as early as 1 year into treatment.8
Chlorpromazine is a typical antipsychotic that causes abnormal skin pigmentation in sun-exposed areas due to increased melanogenesis.9 Similar to type III minocyclineinduced hyperpigmentation, a histologic specimen may stain positive for Fontana-Masson yet negative for Perls Prussian blue. Lal et al10 demonstrated complete resolution of abnormal skin pigmentation within 5 years after stopping chlorpromazine. In contrast, minocyclineinduced hyperpigmentation may be permanent in some cases. There is substantial clinical and histologic overlap for drug-induced hyperpigmentation etiologies; it would behoove the clinician to focus on the most common locations affected and the generalized coloration.
Treatment of minocycline-induced hyperpigmentation includes the use of Q-switched lasers, specifically Q-switched ruby and Q-switched alexandrite.11 The use of the Q-switched Nd:YAG laser appears to be ineffective at clearing minocycline-induced pigmentation.7,11 In our patient, minocycline was discontinued immediately. Due to the patient’s critical condition, he deferred all other therapy. Erythema dyschromicum perstans, also referred to as ashy dermatosis, is an idiopathic form of hyperpigmentation.12 Lesions start as blue-gray to ashy gray macules, occasionally surrounded by a slightly erythematous, raised border.
Erythema dyschromicum perstans typically presents on the trunk, face, and arms of patients with Fitzpatrick skin types III and IV; it is considered a variant of lichen planus actinicus.12 Histologically, erythema dyschromicum perstans may mimic lichen planus pigmentosus (LPP); however, subtle differences exist to distinguish the 2 conditions. Erythema dyschromicum perstans demonstrates a mild lichenoid infiltrate, focal basal vacuolization at the dermoepidermal junction, and melanophage deposition.13 In contrast, LPP demonstrates pigmentary incontinence and a more severe inflammatory infiltrate. A perifollicular infiltrate and fibrosis also can be seen in LPP, which may explain the frontal fibrosing alopecia that often precedes LPP.13
Addison disease, also known as primary adrenal insufficiency, can cause diffuse hyperpigmentation in the skin, mucosae, and nail beds. The pigmentation is prominent in regions of naturally increased pigmentation, such as the flexural surfaces and intertriginous areas.14 Patients with adrenal insufficiency will have accompanying weight loss, hypotension, and fatigue, among other symptoms related to deficiency of cortisol and aldosterone. Skin biopsy shows acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, superficial perivascular lymphocytic infiltrate, basal melanin deposition, and superficial dermal macrophages.15
Confluent and reticulated papillomatosis is an uncommon dermatosis that presents with multiple hyperpigmented macules and papules that coalesce to form patches and plaques centrally with reticulation in the periphery.16 Confluent and reticulated papillomatosis commonly presents on the upper trunk, axillae, and neck, though involvement can include flexural surfaces as well as the lower trunk and legs.16,17 Biopsy demonstrates undulating hyperkeratosis, papillomatosis, acanthosis, and negative fungal staining.16
Pretibial myxedema most commonly is associated with Graves disease and presents as well-defined thickening and induration with overlying pink or purple-brown papules in the pretibial region.18 An acral surface and mucin deposition within the entire dermis may be appreciated on histology with staining for colloidal iron or Alcian blue.
- Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980;244:1103-1106. doi:10.1001/jama.1980.03310100021021
- Snodgrass A, Motaparthi K. Systemic antibacterial agents. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2020:69-98.
- Eisen D, Hakim MD. Minocycline-induced pigmentation. incidence, prevention and management. Drug Saf. 1998;18:431-440. doi:10.2165/00002018-199818060-00004
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
- Basler RS, Kohnen PW. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978;114:1695-1697.
- Ridgway HA, Sonnex TS, Kennedy CT, et al. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982;107:95-102. doi:10.1111/j.1365-2133.1982.tb00296.x
- Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
- Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3:244-247. doi:10.1016/s0190 -9622(80)80186-1
- Perry TL, Culling CF, Berry K, et al. 7-Hydroxychlorpromazine: potential toxic drug metabolite in psychiatric patients. Science. 1964;146:81-83. doi:10.1126/science.146.3640.81
- Lal S, Bloom D, Silver B, et al. Replacement of chlorpromazine with other neuroleptics: effect on abnormal skin pigmentation and ocular changes. J Psychiatry Neurosci. 1993;18:173-177.
- Tsao H, Busam K, Barnhill RL, et al. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250-1251.
- Knox JM, Dodge BG, Freeman RG. Erythema dyschromicum perstans. Arch Dermatol. 1968;97:262-272. doi:10.1001 /archderm.1968.01610090034006
- Rutnin S, Udompanich S, Pratumchart N, et al. Ashy dermatosis and lichen planus pigmentosus: the histopathological differences. Biomed Res Int. 2019;2019:5829185. doi:10.1155/2019/5829185
- Montgomery H, O’Leary PA. Pigmentation of the skin in Addison’s disease, acanthosis nigricans and hemochromatosis. Arch Derm Syphilol. 1930;21:970-984. doi:10.1001 /archderm.1930.01440120072005
- Fernandez-Flores A, Cassarino DS. Histopathologic findings of cutaneous hyperpigmentation in Addison disease and immunostain of the melanocytic population. Am J Dermatopathol. 2017;39:924-927. doi:10.1097/DAD.0000000000000937
- Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. a study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi:10.1111/j.1365-2133.2005.06955.x
- Jo S, Park HS, Cho S, et al. Updated diagnosis criteria for confluent and reticulated papillomatosis: a case report. Ann Dermatol. 2014; 26:409-410. doi:10.5021/ad.2014.26.3.409
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
The Diagnosis: Drug-Induced Hyperpigmentation
Additional history provided by the patient’s caretaker elucidated an extensive list of medications including chlorpromazine and minocycline, among several others. The caretaker revealed that the patient began treatment for acne vulgaris 2 years prior; despite the acne resolving, therapy was not discontinued. The blue-gray and brown pigmentation on our patient’s shins likely was attributed to a medication he was taking.
Both chlorpromazine and minocycline, among many other medications, are known to cause abnormal pigmentation of the skin.1 Minocycline is a tetracycline antibiotic prescribed for acne and other inflammatory cutaneous conditions. It is highly lipophilic, allowing it to reach high drug concentrations in the skin and nail unit.2 Patients taking minocycline long term and at high doses are at greatest risk for pigment deposition.3,4
Minocycline-induced hyperpigmentation is classified into 3 types. Type I describes blue-black deposition of pigment in acne scars and areas of inflammation, typically on facial skin.1,5 Histologically, type I stains positive for Perls Prussian blue, indicating an increased deposition of iron as hemosiderin,1 which likely occurs because minocycline is thought to play a role in defective clearance of hemosiderin from the dermis of injured tissue.5 Type II hyperpigmentation presents as bluegray pigment on the lower legs and occasionally the arms.6,7 Type II stains positive for both Perls Prussian blue and Fontana-Masson, demonstrating hemosiderin and melanin, respectively.6 The third form of hyperpigmentation results in diffuse, dark brown to gray pigmentation with a predilection for sun-exposed areas.8 Histology of type III shows increased pigment in the basal portion of the epidermis and brown-black pigment in macrophages of the dermis. Type III stains positive for Fontana-Masson and negative for Perls Prussian blue. The etiology of hyperpigmentation has been suspected to be caused by minocycline stimulating melanin production and/or deposition of minocycline-melanin complexes in dermal macrophages after a certain drug level; this largely is seen in patients receiving 100 to 200 mg daily as early as 1 year into treatment.8
Chlorpromazine is a typical antipsychotic that causes abnormal skin pigmentation in sun-exposed areas due to increased melanogenesis.9 Similar to type III minocyclineinduced hyperpigmentation, a histologic specimen may stain positive for Fontana-Masson yet negative for Perls Prussian blue. Lal et al10 demonstrated complete resolution of abnormal skin pigmentation within 5 years after stopping chlorpromazine. In contrast, minocyclineinduced hyperpigmentation may be permanent in some cases. There is substantial clinical and histologic overlap for drug-induced hyperpigmentation etiologies; it would behoove the clinician to focus on the most common locations affected and the generalized coloration.
Treatment of minocycline-induced hyperpigmentation includes the use of Q-switched lasers, specifically Q-switched ruby and Q-switched alexandrite.11 The use of the Q-switched Nd:YAG laser appears to be ineffective at clearing minocycline-induced pigmentation.7,11 In our patient, minocycline was discontinued immediately. Due to the patient’s critical condition, he deferred all other therapy. Erythema dyschromicum perstans, also referred to as ashy dermatosis, is an idiopathic form of hyperpigmentation.12 Lesions start as blue-gray to ashy gray macules, occasionally surrounded by a slightly erythematous, raised border.
Erythema dyschromicum perstans typically presents on the trunk, face, and arms of patients with Fitzpatrick skin types III and IV; it is considered a variant of lichen planus actinicus.12 Histologically, erythema dyschromicum perstans may mimic lichen planus pigmentosus (LPP); however, subtle differences exist to distinguish the 2 conditions. Erythema dyschromicum perstans demonstrates a mild lichenoid infiltrate, focal basal vacuolization at the dermoepidermal junction, and melanophage deposition.13 In contrast, LPP demonstrates pigmentary incontinence and a more severe inflammatory infiltrate. A perifollicular infiltrate and fibrosis also can be seen in LPP, which may explain the frontal fibrosing alopecia that often precedes LPP.13
Addison disease, also known as primary adrenal insufficiency, can cause diffuse hyperpigmentation in the skin, mucosae, and nail beds. The pigmentation is prominent in regions of naturally increased pigmentation, such as the flexural surfaces and intertriginous areas.14 Patients with adrenal insufficiency will have accompanying weight loss, hypotension, and fatigue, among other symptoms related to deficiency of cortisol and aldosterone. Skin biopsy shows acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, superficial perivascular lymphocytic infiltrate, basal melanin deposition, and superficial dermal macrophages.15
Confluent and reticulated papillomatosis is an uncommon dermatosis that presents with multiple hyperpigmented macules and papules that coalesce to form patches and plaques centrally with reticulation in the periphery.16 Confluent and reticulated papillomatosis commonly presents on the upper trunk, axillae, and neck, though involvement can include flexural surfaces as well as the lower trunk and legs.16,17 Biopsy demonstrates undulating hyperkeratosis, papillomatosis, acanthosis, and negative fungal staining.16
Pretibial myxedema most commonly is associated with Graves disease and presents as well-defined thickening and induration with overlying pink or purple-brown papules in the pretibial region.18 An acral surface and mucin deposition within the entire dermis may be appreciated on histology with staining for colloidal iron or Alcian blue.
The Diagnosis: Drug-Induced Hyperpigmentation
Additional history provided by the patient’s caretaker elucidated an extensive list of medications including chlorpromazine and minocycline, among several others. The caretaker revealed that the patient began treatment for acne vulgaris 2 years prior; despite the acne resolving, therapy was not discontinued. The blue-gray and brown pigmentation on our patient’s shins likely was attributed to a medication he was taking.
Both chlorpromazine and minocycline, among many other medications, are known to cause abnormal pigmentation of the skin.1 Minocycline is a tetracycline antibiotic prescribed for acne and other inflammatory cutaneous conditions. It is highly lipophilic, allowing it to reach high drug concentrations in the skin and nail unit.2 Patients taking minocycline long term and at high doses are at greatest risk for pigment deposition.3,4
Minocycline-induced hyperpigmentation is classified into 3 types. Type I describes blue-black deposition of pigment in acne scars and areas of inflammation, typically on facial skin.1,5 Histologically, type I stains positive for Perls Prussian blue, indicating an increased deposition of iron as hemosiderin,1 which likely occurs because minocycline is thought to play a role in defective clearance of hemosiderin from the dermis of injured tissue.5 Type II hyperpigmentation presents as bluegray pigment on the lower legs and occasionally the arms.6,7 Type II stains positive for both Perls Prussian blue and Fontana-Masson, demonstrating hemosiderin and melanin, respectively.6 The third form of hyperpigmentation results in diffuse, dark brown to gray pigmentation with a predilection for sun-exposed areas.8 Histology of type III shows increased pigment in the basal portion of the epidermis and brown-black pigment in macrophages of the dermis. Type III stains positive for Fontana-Masson and negative for Perls Prussian blue. The etiology of hyperpigmentation has been suspected to be caused by minocycline stimulating melanin production and/or deposition of minocycline-melanin complexes in dermal macrophages after a certain drug level; this largely is seen in patients receiving 100 to 200 mg daily as early as 1 year into treatment.8
Chlorpromazine is a typical antipsychotic that causes abnormal skin pigmentation in sun-exposed areas due to increased melanogenesis.9 Similar to type III minocyclineinduced hyperpigmentation, a histologic specimen may stain positive for Fontana-Masson yet negative for Perls Prussian blue. Lal et al10 demonstrated complete resolution of abnormal skin pigmentation within 5 years after stopping chlorpromazine. In contrast, minocyclineinduced hyperpigmentation may be permanent in some cases. There is substantial clinical and histologic overlap for drug-induced hyperpigmentation etiologies; it would behoove the clinician to focus on the most common locations affected and the generalized coloration.
Treatment of minocycline-induced hyperpigmentation includes the use of Q-switched lasers, specifically Q-switched ruby and Q-switched alexandrite.11 The use of the Q-switched Nd:YAG laser appears to be ineffective at clearing minocycline-induced pigmentation.7,11 In our patient, minocycline was discontinued immediately. Due to the patient’s critical condition, he deferred all other therapy. Erythema dyschromicum perstans, also referred to as ashy dermatosis, is an idiopathic form of hyperpigmentation.12 Lesions start as blue-gray to ashy gray macules, occasionally surrounded by a slightly erythematous, raised border.
Erythema dyschromicum perstans typically presents on the trunk, face, and arms of patients with Fitzpatrick skin types III and IV; it is considered a variant of lichen planus actinicus.12 Histologically, erythema dyschromicum perstans may mimic lichen planus pigmentosus (LPP); however, subtle differences exist to distinguish the 2 conditions. Erythema dyschromicum perstans demonstrates a mild lichenoid infiltrate, focal basal vacuolization at the dermoepidermal junction, and melanophage deposition.13 In contrast, LPP demonstrates pigmentary incontinence and a more severe inflammatory infiltrate. A perifollicular infiltrate and fibrosis also can be seen in LPP, which may explain the frontal fibrosing alopecia that often precedes LPP.13
Addison disease, also known as primary adrenal insufficiency, can cause diffuse hyperpigmentation in the skin, mucosae, and nail beds. The pigmentation is prominent in regions of naturally increased pigmentation, such as the flexural surfaces and intertriginous areas.14 Patients with adrenal insufficiency will have accompanying weight loss, hypotension, and fatigue, among other symptoms related to deficiency of cortisol and aldosterone. Skin biopsy shows acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, superficial perivascular lymphocytic infiltrate, basal melanin deposition, and superficial dermal macrophages.15
Confluent and reticulated papillomatosis is an uncommon dermatosis that presents with multiple hyperpigmented macules and papules that coalesce to form patches and plaques centrally with reticulation in the periphery.16 Confluent and reticulated papillomatosis commonly presents on the upper trunk, axillae, and neck, though involvement can include flexural surfaces as well as the lower trunk and legs.16,17 Biopsy demonstrates undulating hyperkeratosis, papillomatosis, acanthosis, and negative fungal staining.16
Pretibial myxedema most commonly is associated with Graves disease and presents as well-defined thickening and induration with overlying pink or purple-brown papules in the pretibial region.18 An acral surface and mucin deposition within the entire dermis may be appreciated on histology with staining for colloidal iron or Alcian blue.
- Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980;244:1103-1106. doi:10.1001/jama.1980.03310100021021
- Snodgrass A, Motaparthi K. Systemic antibacterial agents. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2020:69-98.
- Eisen D, Hakim MD. Minocycline-induced pigmentation. incidence, prevention and management. Drug Saf. 1998;18:431-440. doi:10.2165/00002018-199818060-00004
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
- Basler RS, Kohnen PW. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978;114:1695-1697.
- Ridgway HA, Sonnex TS, Kennedy CT, et al. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982;107:95-102. doi:10.1111/j.1365-2133.1982.tb00296.x
- Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
- Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3:244-247. doi:10.1016/s0190 -9622(80)80186-1
- Perry TL, Culling CF, Berry K, et al. 7-Hydroxychlorpromazine: potential toxic drug metabolite in psychiatric patients. Science. 1964;146:81-83. doi:10.1126/science.146.3640.81
- Lal S, Bloom D, Silver B, et al. Replacement of chlorpromazine with other neuroleptics: effect on abnormal skin pigmentation and ocular changes. J Psychiatry Neurosci. 1993;18:173-177.
- Tsao H, Busam K, Barnhill RL, et al. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250-1251.
- Knox JM, Dodge BG, Freeman RG. Erythema dyschromicum perstans. Arch Dermatol. 1968;97:262-272. doi:10.1001 /archderm.1968.01610090034006
- Rutnin S, Udompanich S, Pratumchart N, et al. Ashy dermatosis and lichen planus pigmentosus: the histopathological differences. Biomed Res Int. 2019;2019:5829185. doi:10.1155/2019/5829185
- Montgomery H, O’Leary PA. Pigmentation of the skin in Addison’s disease, acanthosis nigricans and hemochromatosis. Arch Derm Syphilol. 1930;21:970-984. doi:10.1001 /archderm.1930.01440120072005
- Fernandez-Flores A, Cassarino DS. Histopathologic findings of cutaneous hyperpigmentation in Addison disease and immunostain of the melanocytic population. Am J Dermatopathol. 2017;39:924-927. doi:10.1097/DAD.0000000000000937
- Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. a study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi:10.1111/j.1365-2133.2005.06955.x
- Jo S, Park HS, Cho S, et al. Updated diagnosis criteria for confluent and reticulated papillomatosis: a case report. Ann Dermatol. 2014; 26:409-410. doi:10.5021/ad.2014.26.3.409
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980;244:1103-1106. doi:10.1001/jama.1980.03310100021021
- Snodgrass A, Motaparthi K. Systemic antibacterial agents. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2020:69-98.
- Eisen D, Hakim MD. Minocycline-induced pigmentation. incidence, prevention and management. Drug Saf. 1998;18:431-440. doi:10.2165/00002018-199818060-00004
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
- Basler RS, Kohnen PW. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978;114:1695-1697.
- Ridgway HA, Sonnex TS, Kennedy CT, et al. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982;107:95-102. doi:10.1111/j.1365-2133.1982.tb00296.x
- Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
- Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3:244-247. doi:10.1016/s0190 -9622(80)80186-1
- Perry TL, Culling CF, Berry K, et al. 7-Hydroxychlorpromazine: potential toxic drug metabolite in psychiatric patients. Science. 1964;146:81-83. doi:10.1126/science.146.3640.81
- Lal S, Bloom D, Silver B, et al. Replacement of chlorpromazine with other neuroleptics: effect on abnormal skin pigmentation and ocular changes. J Psychiatry Neurosci. 1993;18:173-177.
- Tsao H, Busam K, Barnhill RL, et al. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250-1251.
- Knox JM, Dodge BG, Freeman RG. Erythema dyschromicum perstans. Arch Dermatol. 1968;97:262-272. doi:10.1001 /archderm.1968.01610090034006
- Rutnin S, Udompanich S, Pratumchart N, et al. Ashy dermatosis and lichen planus pigmentosus: the histopathological differences. Biomed Res Int. 2019;2019:5829185. doi:10.1155/2019/5829185
- Montgomery H, O’Leary PA. Pigmentation of the skin in Addison’s disease, acanthosis nigricans and hemochromatosis. Arch Derm Syphilol. 1930;21:970-984. doi:10.1001 /archderm.1930.01440120072005
- Fernandez-Flores A, Cassarino DS. Histopathologic findings of cutaneous hyperpigmentation in Addison disease and immunostain of the melanocytic population. Am J Dermatopathol. 2017;39:924-927. doi:10.1097/DAD.0000000000000937
- Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. a study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi:10.1111/j.1365-2133.2005.06955.x
- Jo S, Park HS, Cho S, et al. Updated diagnosis criteria for confluent and reticulated papillomatosis: a case report. Ann Dermatol. 2014; 26:409-410. doi:10.5021/ad.2014.26.3.409
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
A 37-year-old man with a history of cerebral palsy, bipolar disorder, and impulse control disorder presented to the emergency department with breathing difficulty and worsening malaise. The patient subsequently was intubated due to hypoxic respiratory failure and was found to be positive for SARS-CoV-2. He was admitted to the intensive care unit, and dermatology was consulted due to concern that the cutaneous findings were demonstrative of a vasculitic process. Physical examination revealed diffuse, symmetric, dark brown to blue-gray macules coalescing into patches on the anterior tibia (top) and covering the entire lower leg (bottom). The patches were mottled and did not blanch with pressure. According to the patient’s caretaker, the leg hyperpigmentation had been present for 2 years.
Pedunculated Tumor on the Posterior Neck
The Diagnosis: Nodular Hidradenoma
A biopsy of the nodule showed a large, fungating, well-circumscribed, multilobulated neoplasm composed of primarily monotonous eosinophilic cells in a background of keloidal stroma (Figure). There was a minority population of small, monotonous, clear cells within the lobules, and no glandular structures were noted. Neither cytological nor architectural atypia were evident. MART-1/Melan-A and S-100 stains were negative, consistent with a diagnosis of benign nodular hidradenoma.
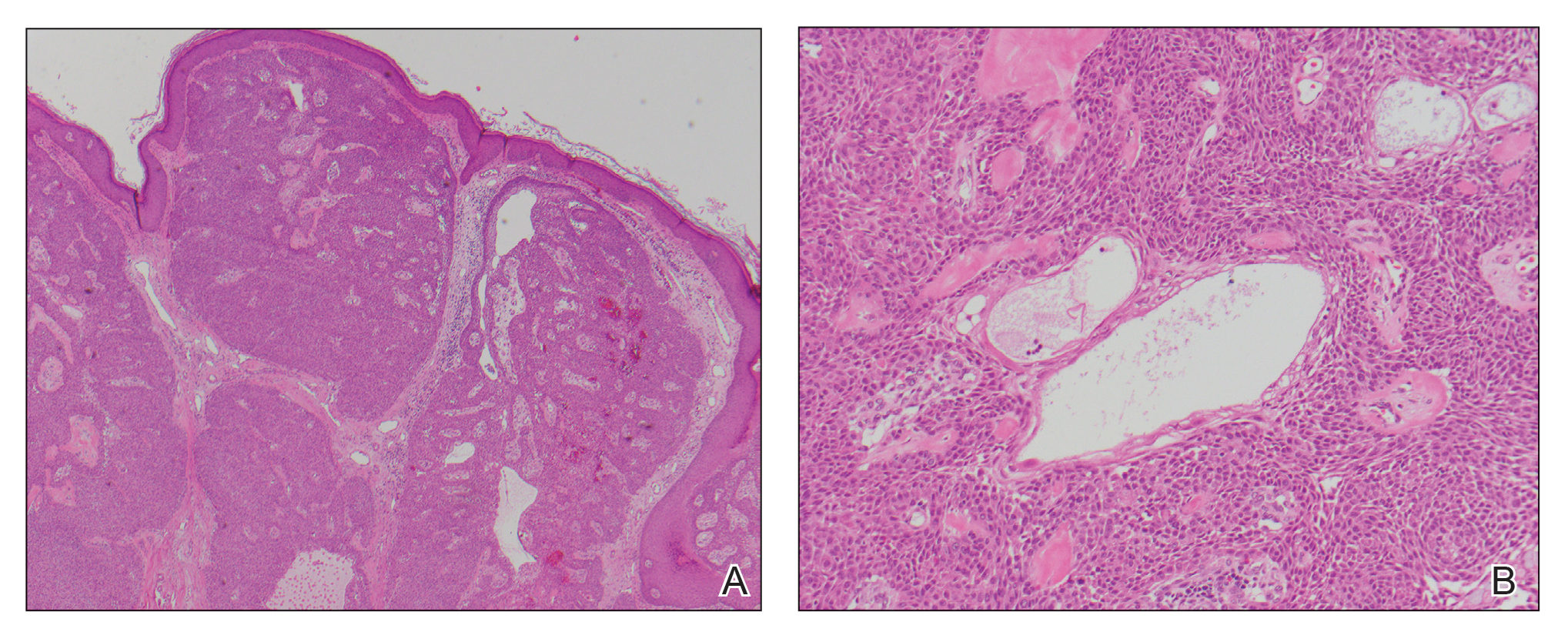
Nodular hidradenoma (also known as acrospiroma, solid-cystic hidradenoma, clear cell hidradenoma, and eccrine sweat gland adenoma) is a benign adnexal tumor of the apocrine or eccrine glands.1,2 Nodular hidradenoma can arise at any cutaneous site but most commonly arises on the head and anterior portion of the trunk and rarely on the extremities.2 It presents as a solitary nodular, cystic, or pedunculated mass that can reach up to several centimeters in diameter.2,3 Nodular hidradenoma more commonly affects women compared to men with a ratio of 1.7 to 1 and commonly presents between the third and fifth decades of life, with an average age at presentation of 37.2 years.2,4 There can be associated skin changes, including smoothening, thickening, ulceration, and bluish discoloration. Dermoscopy commonly shows a pinkish homogenous area that extends throughout the entire lesion. This homogenous area less commonly can be bluish, brownish, or pink-blue. Most nodular hidradenomas also can exhibit vascularization, with arborizing telangiectases, polymorphous atypical vessels, and linear irregular vessels being most common; however, this is not specific to nodular hidradenoma.3 Occasionally, tumors can drain serous or hemorrhagic fluid. Nodular hidradenoma commonly is a slow-growing tumor.5 Rapid increase in tumor size can be indicative of malignant transformation, hemorrhage into the tumor, or trauma to the area.2
Histologically, nodular hidradenoma consists of a circumscribed, nonencapsulated, multilobular tumor commonly found in the dermis and sometimes extending into the subcutaneous tissue. There usually is no epidermal attachment, and the overlying epidermis largely is normal. The tumor consists of large multilobulated areas of epithelial cells, tubular lamina, and large cystic areas filled with homogenous eosinophilic material.1 It notably is composed of 2 epithelial cell types: (1) fusiform cells with elongated vesicular nuclei and basophilic cytoplasm, and (2) large polygonal cells with round eccentric nuclei and eosinophilic, periodic acid–Schiff–positive cytoplasm that washes away during fixation, giving the appearance of clear cells.5 Both types of cells are small, monotonous, and void of mitosis or dyskeratosis. Although there can be ducts with apocrine secretion present within the lobulated tumor, they are not consistently found. Due to the varying features that are neither mandatory nor consistent to arrive at this diagnosis, some dermatopathologists view the term hidradenoma as a catch-all term that includes several different types of benign sweat gland tumors. Some authors divide the terminology into apocrine hidradenoma and eccrine hidradenoma based on whether the tumor is composed of solely clear mucinous cells, or poroid and cuticular cells, respectively.
Although nodular hidradenoma classically is a benign tumor, total surgical excision is recommended due to the rare risk for malignant transformation. Rarely, longstanding hidradenomas can metastasize to lymph nodes, bone, or viscera; in these instances, metastatic hidradenoma has a 5-year survival rate of 30%. Recurrence may occur in tumors that are inadequately excised, and the rate of recurrence is estimated to be approximately 10% of surgically excised tumors.5 However, utilization of Mohs micrographic surgery for excision of nodular hidradenoma is associated with a reduced recurrence rate.6
Keloids present as painful, sometimes pruritic, raised scars that extend beyond the boundary of the initial injury, commonly arising on the shoulder, upper arm, and chest. Histopathology reveals nodules of thick hyalinized collagen bundles, keloidal collagen with mucinous ground substance, and few fibroblasts.7
Metastatic renal cell carcinoma to the skin most commonly presents on the face and scalp as a nodular, rapidly growing, round to oval lesion that is flesh colored to reddish purple in a patient with history of renal cell carcinoma.8 Histopathology shows clusters of atypical, nucleated clear cells surrounded by chicken wire vasculature.8,9
Verruca vulgaris is caused by human papillomavirus and most commonly occurs on the hands and feet. It presents as a pink to white, sessile lesion with a verrucous surface and exophytic growths. Histopathology shows acanthosis; hypergranulosis; exophytic projections with a fibrovascular core; inward cupping of the rete ridges; and koilocytes, which are cells with an eccentric, raisinlike nucleus and vacuolated cytoplasm in the granular layer of the epidermis.10
Similar to nodular hidradenoma, nodular melanoma most commonly presents on the head and neck as a symmetric, elevated, amelanotic nodule, but in contrast to nodular hidradenoma, it typically is confined to a smaller diameter.11 Histologically, it is characterized by sheets of atypical, commonly epithelioid melanocytes with a lack of maturation and brisk mitotic activity extending through the epidermis and dermis with lateral extension limited to less than 3 rete ridges.12
- Patterson JW, Weedon D. Tumors of cutaneous appendages. In: Patterson JW, Weedon D. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020:951-1016.
- Ngo N, Susa M, Nakagawa T, et al. Malignant transformation of nodular hidradenoma in the lower leg. Case Rep Oncol. 2018;11:298-304. doi:10.1159/000489255
- Zaballos P, Gómez-Martín I, Martin JM, et al. Dermoscopy of adnexal tumors. Dermatol Clin. 2018;36:397-412. doi:10.1016/j .det.2018.05.007
- Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985; 12:15-20. doi:10.1016/s0190-9622(85)70002-3
- Stratigos AJ, Olbricht S, Kwan TH, et al. Nodular hidradenoma. Dermatol Surg. 1998;24:387-391. doi:10.1111/j.1524-4725.1998.tb04173.x
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach. Dermatol Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Lee JY-Y, Yang C-C, Chao S-C, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26:379-384. doi:10.1097/00000372-200410000-00006
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Jaitly V, Jahan-Tigh R, Belousova T, et al. Case report and literature review of nodular hidradenoma, a rare adnexal tumor that mimics breast carcinoma, in a 20-year-old woman. Lab Med. 2019;50:320-325. doi:10.1093/labmed/lmy084
- Betz SJ. HPV-related papillary lesions of the oral mucosa: a review. Head Neck Pathol. 2019;13:80-90. doi:10.1007/s12105-019-01003-7
- Kalkhoran S, Milne O, Zalaudek I, et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010;146:311-318. doi:10.1001/archdermatol.2009.369
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19(suppl 2):S34-S40. doi:10.1038 /modpathol.3800508
The Diagnosis: Nodular Hidradenoma
A biopsy of the nodule showed a large, fungating, well-circumscribed, multilobulated neoplasm composed of primarily monotonous eosinophilic cells in a background of keloidal stroma (Figure). There was a minority population of small, monotonous, clear cells within the lobules, and no glandular structures were noted. Neither cytological nor architectural atypia were evident. MART-1/Melan-A and S-100 stains were negative, consistent with a diagnosis of benign nodular hidradenoma.

Nodular hidradenoma (also known as acrospiroma, solid-cystic hidradenoma, clear cell hidradenoma, and eccrine sweat gland adenoma) is a benign adnexal tumor of the apocrine or eccrine glands.1,2 Nodular hidradenoma can arise at any cutaneous site but most commonly arises on the head and anterior portion of the trunk and rarely on the extremities.2 It presents as a solitary nodular, cystic, or pedunculated mass that can reach up to several centimeters in diameter.2,3 Nodular hidradenoma more commonly affects women compared to men with a ratio of 1.7 to 1 and commonly presents between the third and fifth decades of life, with an average age at presentation of 37.2 years.2,4 There can be associated skin changes, including smoothening, thickening, ulceration, and bluish discoloration. Dermoscopy commonly shows a pinkish homogenous area that extends throughout the entire lesion. This homogenous area less commonly can be bluish, brownish, or pink-blue. Most nodular hidradenomas also can exhibit vascularization, with arborizing telangiectases, polymorphous atypical vessels, and linear irregular vessels being most common; however, this is not specific to nodular hidradenoma.3 Occasionally, tumors can drain serous or hemorrhagic fluid. Nodular hidradenoma commonly is a slow-growing tumor.5 Rapid increase in tumor size can be indicative of malignant transformation, hemorrhage into the tumor, or trauma to the area.2
Histologically, nodular hidradenoma consists of a circumscribed, nonencapsulated, multilobular tumor commonly found in the dermis and sometimes extending into the subcutaneous tissue. There usually is no epidermal attachment, and the overlying epidermis largely is normal. The tumor consists of large multilobulated areas of epithelial cells, tubular lamina, and large cystic areas filled with homogenous eosinophilic material.1 It notably is composed of 2 epithelial cell types: (1) fusiform cells with elongated vesicular nuclei and basophilic cytoplasm, and (2) large polygonal cells with round eccentric nuclei and eosinophilic, periodic acid–Schiff–positive cytoplasm that washes away during fixation, giving the appearance of clear cells.5 Both types of cells are small, monotonous, and void of mitosis or dyskeratosis. Although there can be ducts with apocrine secretion present within the lobulated tumor, they are not consistently found. Due to the varying features that are neither mandatory nor consistent to arrive at this diagnosis, some dermatopathologists view the term hidradenoma as a catch-all term that includes several different types of benign sweat gland tumors. Some authors divide the terminology into apocrine hidradenoma and eccrine hidradenoma based on whether the tumor is composed of solely clear mucinous cells, or poroid and cuticular cells, respectively.
Although nodular hidradenoma classically is a benign tumor, total surgical excision is recommended due to the rare risk for malignant transformation. Rarely, longstanding hidradenomas can metastasize to lymph nodes, bone, or viscera; in these instances, metastatic hidradenoma has a 5-year survival rate of 30%. Recurrence may occur in tumors that are inadequately excised, and the rate of recurrence is estimated to be approximately 10% of surgically excised tumors.5 However, utilization of Mohs micrographic surgery for excision of nodular hidradenoma is associated with a reduced recurrence rate.6
Keloids present as painful, sometimes pruritic, raised scars that extend beyond the boundary of the initial injury, commonly arising on the shoulder, upper arm, and chest. Histopathology reveals nodules of thick hyalinized collagen bundles, keloidal collagen with mucinous ground substance, and few fibroblasts.7
Metastatic renal cell carcinoma to the skin most commonly presents on the face and scalp as a nodular, rapidly growing, round to oval lesion that is flesh colored to reddish purple in a patient with history of renal cell carcinoma.8 Histopathology shows clusters of atypical, nucleated clear cells surrounded by chicken wire vasculature.8,9
Verruca vulgaris is caused by human papillomavirus and most commonly occurs on the hands and feet. It presents as a pink to white, sessile lesion with a verrucous surface and exophytic growths. Histopathology shows acanthosis; hypergranulosis; exophytic projections with a fibrovascular core; inward cupping of the rete ridges; and koilocytes, which are cells with an eccentric, raisinlike nucleus and vacuolated cytoplasm in the granular layer of the epidermis.10
Similar to nodular hidradenoma, nodular melanoma most commonly presents on the head and neck as a symmetric, elevated, amelanotic nodule, but in contrast to nodular hidradenoma, it typically is confined to a smaller diameter.11 Histologically, it is characterized by sheets of atypical, commonly epithelioid melanocytes with a lack of maturation and brisk mitotic activity extending through the epidermis and dermis with lateral extension limited to less than 3 rete ridges.12
The Diagnosis: Nodular Hidradenoma
A biopsy of the nodule showed a large, fungating, well-circumscribed, multilobulated neoplasm composed of primarily monotonous eosinophilic cells in a background of keloidal stroma (Figure). There was a minority population of small, monotonous, clear cells within the lobules, and no glandular structures were noted. Neither cytological nor architectural atypia were evident. MART-1/Melan-A and S-100 stains were negative, consistent with a diagnosis of benign nodular hidradenoma.

Nodular hidradenoma (also known as acrospiroma, solid-cystic hidradenoma, clear cell hidradenoma, and eccrine sweat gland adenoma) is a benign adnexal tumor of the apocrine or eccrine glands.1,2 Nodular hidradenoma can arise at any cutaneous site but most commonly arises on the head and anterior portion of the trunk and rarely on the extremities.2 It presents as a solitary nodular, cystic, or pedunculated mass that can reach up to several centimeters in diameter.2,3 Nodular hidradenoma more commonly affects women compared to men with a ratio of 1.7 to 1 and commonly presents between the third and fifth decades of life, with an average age at presentation of 37.2 years.2,4 There can be associated skin changes, including smoothening, thickening, ulceration, and bluish discoloration. Dermoscopy commonly shows a pinkish homogenous area that extends throughout the entire lesion. This homogenous area less commonly can be bluish, brownish, or pink-blue. Most nodular hidradenomas also can exhibit vascularization, with arborizing telangiectases, polymorphous atypical vessels, and linear irregular vessels being most common; however, this is not specific to nodular hidradenoma.3 Occasionally, tumors can drain serous or hemorrhagic fluid. Nodular hidradenoma commonly is a slow-growing tumor.5 Rapid increase in tumor size can be indicative of malignant transformation, hemorrhage into the tumor, or trauma to the area.2
Histologically, nodular hidradenoma consists of a circumscribed, nonencapsulated, multilobular tumor commonly found in the dermis and sometimes extending into the subcutaneous tissue. There usually is no epidermal attachment, and the overlying epidermis largely is normal. The tumor consists of large multilobulated areas of epithelial cells, tubular lamina, and large cystic areas filled with homogenous eosinophilic material.1 It notably is composed of 2 epithelial cell types: (1) fusiform cells with elongated vesicular nuclei and basophilic cytoplasm, and (2) large polygonal cells with round eccentric nuclei and eosinophilic, periodic acid–Schiff–positive cytoplasm that washes away during fixation, giving the appearance of clear cells.5 Both types of cells are small, monotonous, and void of mitosis or dyskeratosis. Although there can be ducts with apocrine secretion present within the lobulated tumor, they are not consistently found. Due to the varying features that are neither mandatory nor consistent to arrive at this diagnosis, some dermatopathologists view the term hidradenoma as a catch-all term that includes several different types of benign sweat gland tumors. Some authors divide the terminology into apocrine hidradenoma and eccrine hidradenoma based on whether the tumor is composed of solely clear mucinous cells, or poroid and cuticular cells, respectively.
Although nodular hidradenoma classically is a benign tumor, total surgical excision is recommended due to the rare risk for malignant transformation. Rarely, longstanding hidradenomas can metastasize to lymph nodes, bone, or viscera; in these instances, metastatic hidradenoma has a 5-year survival rate of 30%. Recurrence may occur in tumors that are inadequately excised, and the rate of recurrence is estimated to be approximately 10% of surgically excised tumors.5 However, utilization of Mohs micrographic surgery for excision of nodular hidradenoma is associated with a reduced recurrence rate.6
Keloids present as painful, sometimes pruritic, raised scars that extend beyond the boundary of the initial injury, commonly arising on the shoulder, upper arm, and chest. Histopathology reveals nodules of thick hyalinized collagen bundles, keloidal collagen with mucinous ground substance, and few fibroblasts.7
Metastatic renal cell carcinoma to the skin most commonly presents on the face and scalp as a nodular, rapidly growing, round to oval lesion that is flesh colored to reddish purple in a patient with history of renal cell carcinoma.8 Histopathology shows clusters of atypical, nucleated clear cells surrounded by chicken wire vasculature.8,9
Verruca vulgaris is caused by human papillomavirus and most commonly occurs on the hands and feet. It presents as a pink to white, sessile lesion with a verrucous surface and exophytic growths. Histopathology shows acanthosis; hypergranulosis; exophytic projections with a fibrovascular core; inward cupping of the rete ridges; and koilocytes, which are cells with an eccentric, raisinlike nucleus and vacuolated cytoplasm in the granular layer of the epidermis.10
Similar to nodular hidradenoma, nodular melanoma most commonly presents on the head and neck as a symmetric, elevated, amelanotic nodule, but in contrast to nodular hidradenoma, it typically is confined to a smaller diameter.11 Histologically, it is characterized by sheets of atypical, commonly epithelioid melanocytes with a lack of maturation and brisk mitotic activity extending through the epidermis and dermis with lateral extension limited to less than 3 rete ridges.12
- Patterson JW, Weedon D. Tumors of cutaneous appendages. In: Patterson JW, Weedon D. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020:951-1016.
- Ngo N, Susa M, Nakagawa T, et al. Malignant transformation of nodular hidradenoma in the lower leg. Case Rep Oncol. 2018;11:298-304. doi:10.1159/000489255
- Zaballos P, Gómez-Martín I, Martin JM, et al. Dermoscopy of adnexal tumors. Dermatol Clin. 2018;36:397-412. doi:10.1016/j .det.2018.05.007
- Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985; 12:15-20. doi:10.1016/s0190-9622(85)70002-3
- Stratigos AJ, Olbricht S, Kwan TH, et al. Nodular hidradenoma. Dermatol Surg. 1998;24:387-391. doi:10.1111/j.1524-4725.1998.tb04173.x
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach. Dermatol Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Lee JY-Y, Yang C-C, Chao S-C, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26:379-384. doi:10.1097/00000372-200410000-00006
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Jaitly V, Jahan-Tigh R, Belousova T, et al. Case report and literature review of nodular hidradenoma, a rare adnexal tumor that mimics breast carcinoma, in a 20-year-old woman. Lab Med. 2019;50:320-325. doi:10.1093/labmed/lmy084
- Betz SJ. HPV-related papillary lesions of the oral mucosa: a review. Head Neck Pathol. 2019;13:80-90. doi:10.1007/s12105-019-01003-7
- Kalkhoran S, Milne O, Zalaudek I, et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010;146:311-318. doi:10.1001/archdermatol.2009.369
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19(suppl 2):S34-S40. doi:10.1038 /modpathol.3800508
- Patterson JW, Weedon D. Tumors of cutaneous appendages. In: Patterson JW, Weedon D. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020:951-1016.
- Ngo N, Susa M, Nakagawa T, et al. Malignant transformation of nodular hidradenoma in the lower leg. Case Rep Oncol. 2018;11:298-304. doi:10.1159/000489255
- Zaballos P, Gómez-Martín I, Martin JM, et al. Dermoscopy of adnexal tumors. Dermatol Clin. 2018;36:397-412. doi:10.1016/j .det.2018.05.007
- Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985; 12:15-20. doi:10.1016/s0190-9622(85)70002-3
- Stratigos AJ, Olbricht S, Kwan TH, et al. Nodular hidradenoma. Dermatol Surg. 1998;24:387-391. doi:10.1111/j.1524-4725.1998.tb04173.x
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach. Dermatol Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Lee JY-Y, Yang C-C, Chao S-C, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26:379-384. doi:10.1097/00000372-200410000-00006
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Jaitly V, Jahan-Tigh R, Belousova T, et al. Case report and literature review of nodular hidradenoma, a rare adnexal tumor that mimics breast carcinoma, in a 20-year-old woman. Lab Med. 2019;50:320-325. doi:10.1093/labmed/lmy084
- Betz SJ. HPV-related papillary lesions of the oral mucosa: a review. Head Neck Pathol. 2019;13:80-90. doi:10.1007/s12105-019-01003-7
- Kalkhoran S, Milne O, Zalaudek I, et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010;146:311-318. doi:10.1001/archdermatol.2009.369
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19(suppl 2):S34-S40. doi:10.1038 /modpathol.3800508

A 56-year-old man presented with a progressively enlarging lesion on the posterior neck of 8 months’ duration. He reported localized pruritus of the lesion that improved with triamcinolone cream 0.05% and oral hydroxyzine as well as occasional irritation of the mass with oozing of clear fluid and blood. He denied associated pain and constitutional symptoms. Physical examination revealed a 2.5-cm, nodular, pedunculated, rubbery mass with foci of crusting on the central posterior neck. The mass was flesh colored to pink, and no lymphadenopathy was noted on physical examination.
Velvety Plaques on the Abdomen and Extremities
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.
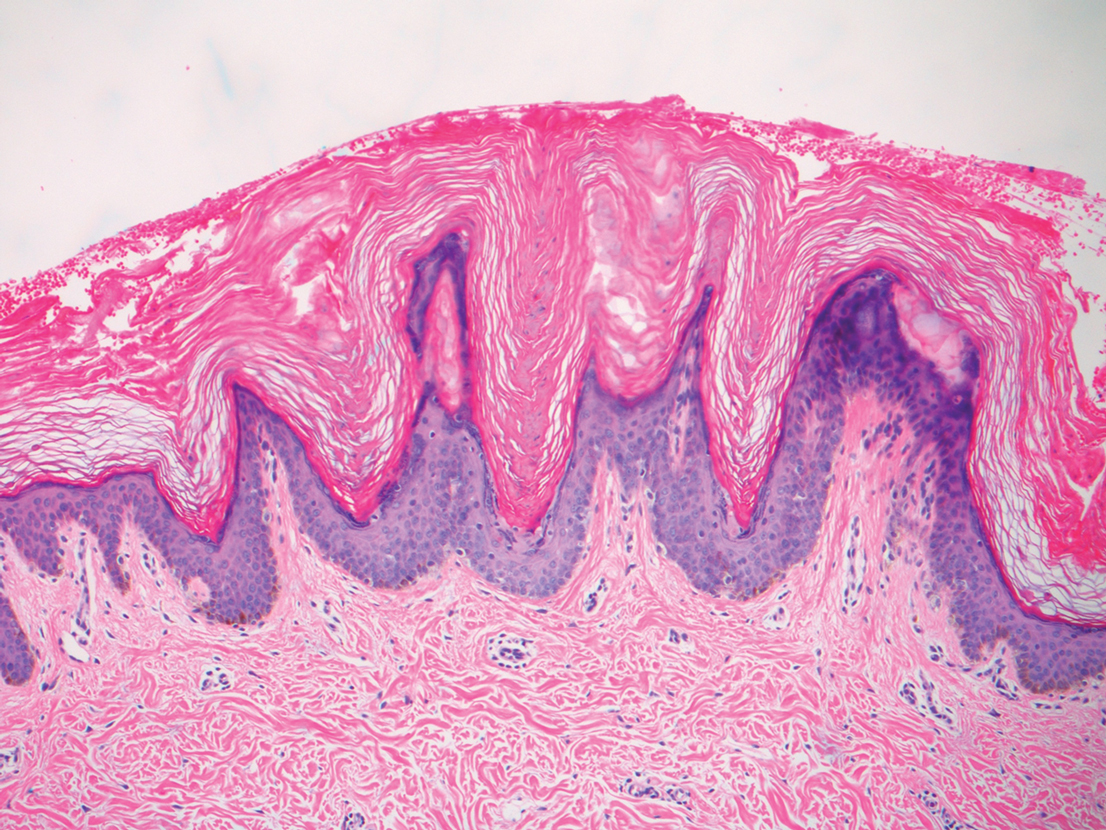
Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.

Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.

Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
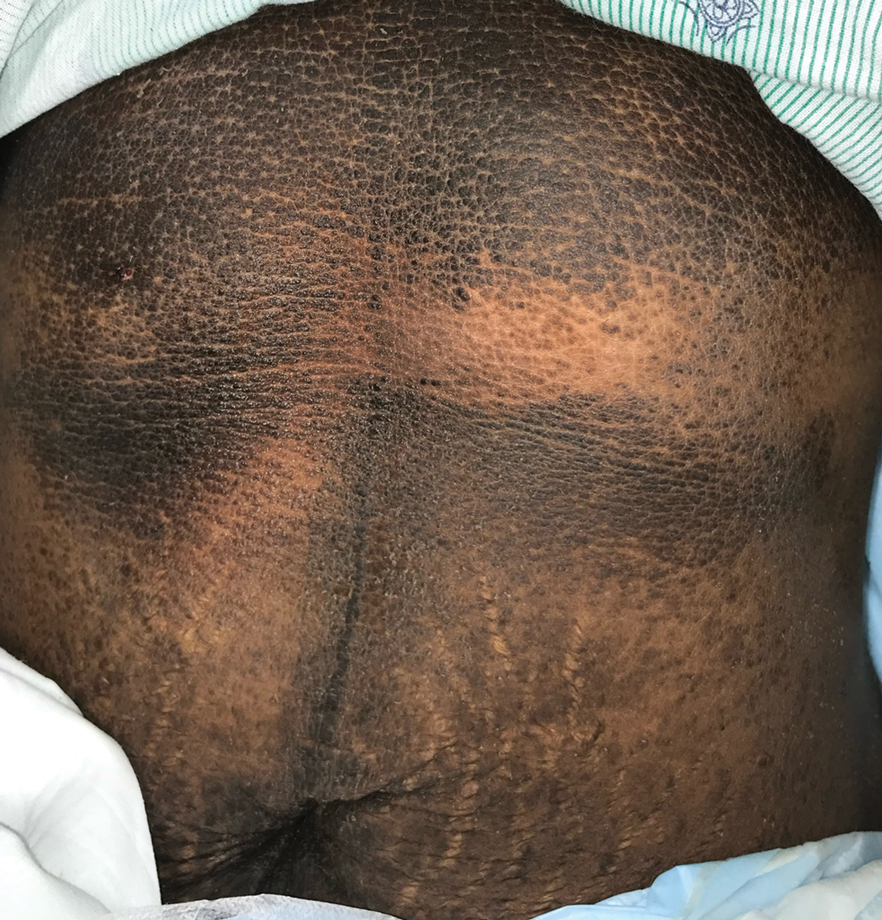
A 28-year-old woman was admitted to the medicine service with bilateral pedal numbness and ataxia, as well as an asymptomatic rash on the neck, chest, abdomen, and extremities of a few months’ duration. The patient was seen by an outside dermatologist for the same rash 1 month prior, at which time a punch biopsy of the right forearm was suggestive of psoriasiform dermatitis; however, the rash failed to improve with topical ammonium lactate and corticosteroids. During the current admission, the patient was found to have low methylmalonic acid and vitamin B1 levels; however, vitamin B12, thyroid studies, rapid plasma reagin test, and inflammatory markers, as well as central and peripheral imaging and nerve conduction studies were normal.
Dermatology was consulted. Physical examination revealed retention hyperkeratosis on the neck that was wipeable with 70% isopropyl alcohol, as well as nonwipeable, thin, reticulated plaques on the mid chest and thick velvety plaques on the abdomen and bilateral extremities. There was notable sparing of areas with natural occlusion such as the back and body folds. A punch biopsy of the abdomen was performed.
Spiral Plaque on the Left Ankle
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
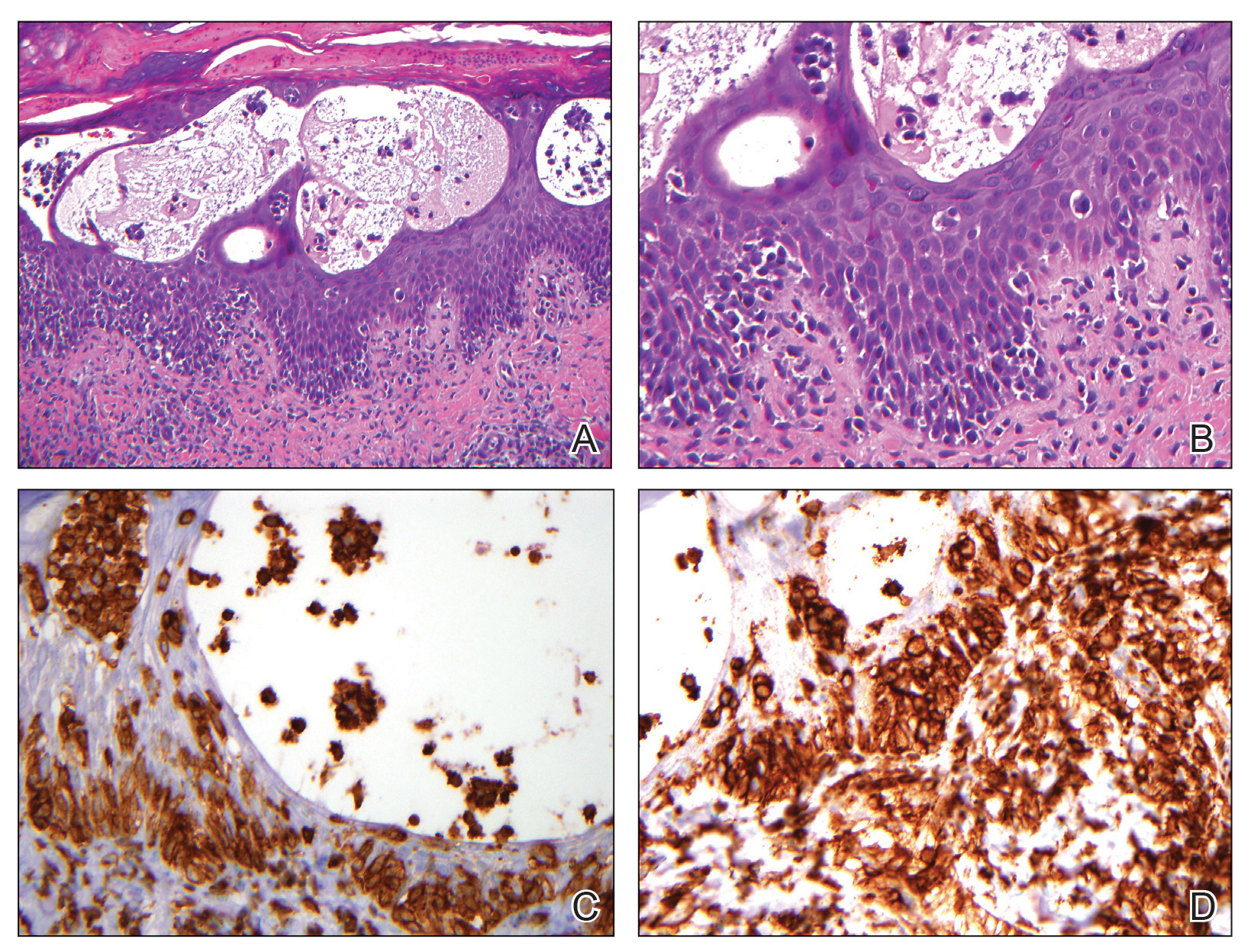
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.

Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.

Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
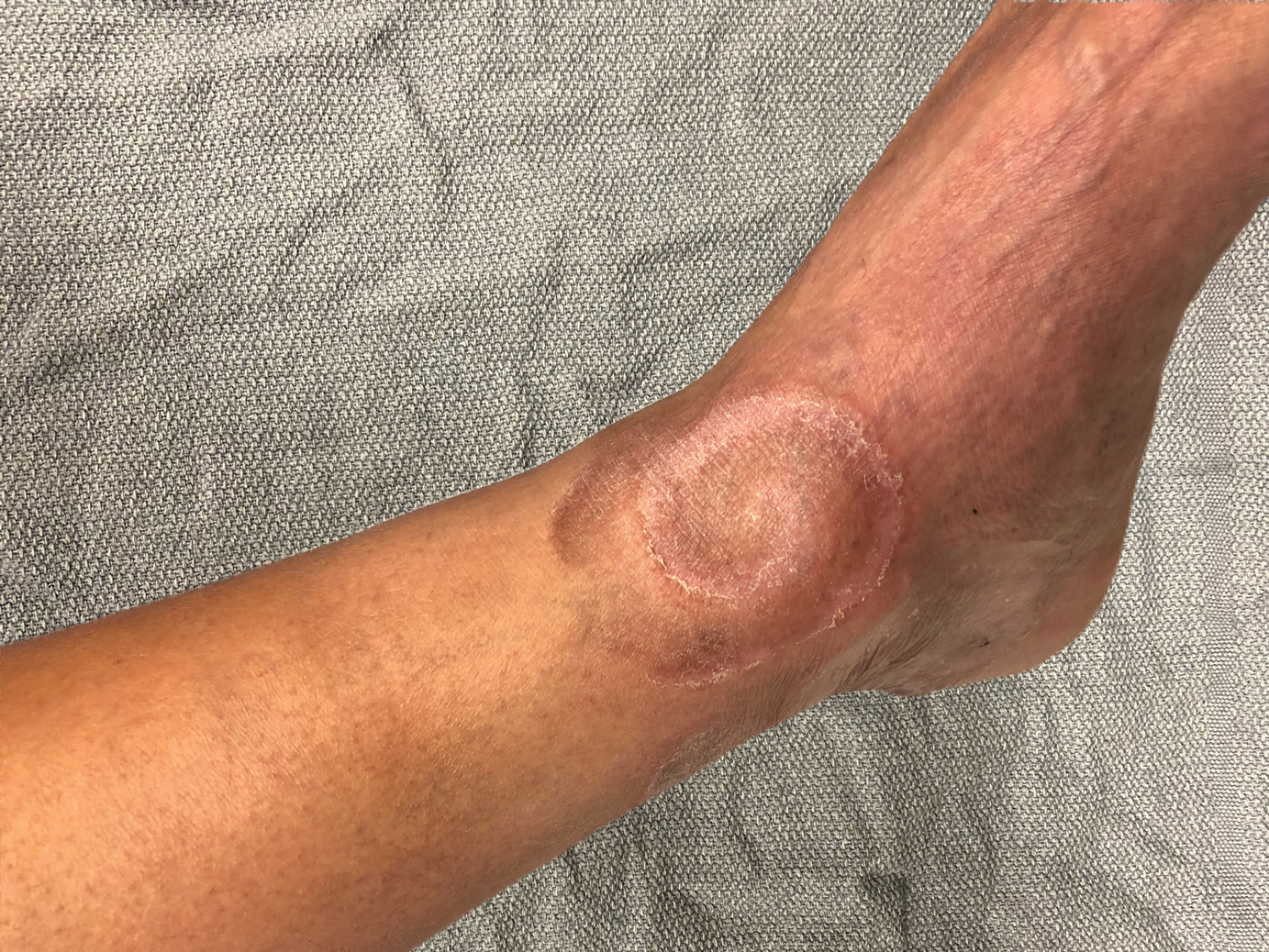
A 60-year-old man presented with a whorl-like plaque on the left ankle that he had noticed while undergoing treatment with narrowband UVB every other week and nitrogen mustard gel daily for stage IB cutaneous T-cell lymphoma, mycosis fungoides type. He denied pain, pruritus, and any other associated symptoms at the site. He denied recent illness, new medications, or changes in diet. His medical history included multiple sclerosis, vascular disease, and stroke. Physical examination revealed an 8×6-cm, welldemarcated, slightly scaly, erythematous plaque with a spiral appearance and peripheral hyperpigmentation involving the left ankle. The remainder of the examination was notable for well-controlled mycosis fungoides with several hyperpigmented patches at sites of prior involvement on the trunk and upper and lower extremities. No cervical, axillary, or inguinal lymphadenopathy was noted. A 4-mm punch biopsy was performed and sent for histopathologic examination.