User login
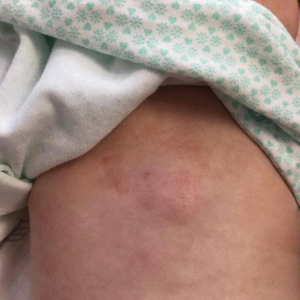
Diffuse Painful Plaques in the Setting of Chronic Lymphocytic Leukemia
The Diagnosis: Cutaneous Mycobacterium avium-intracellulare Complex Infection
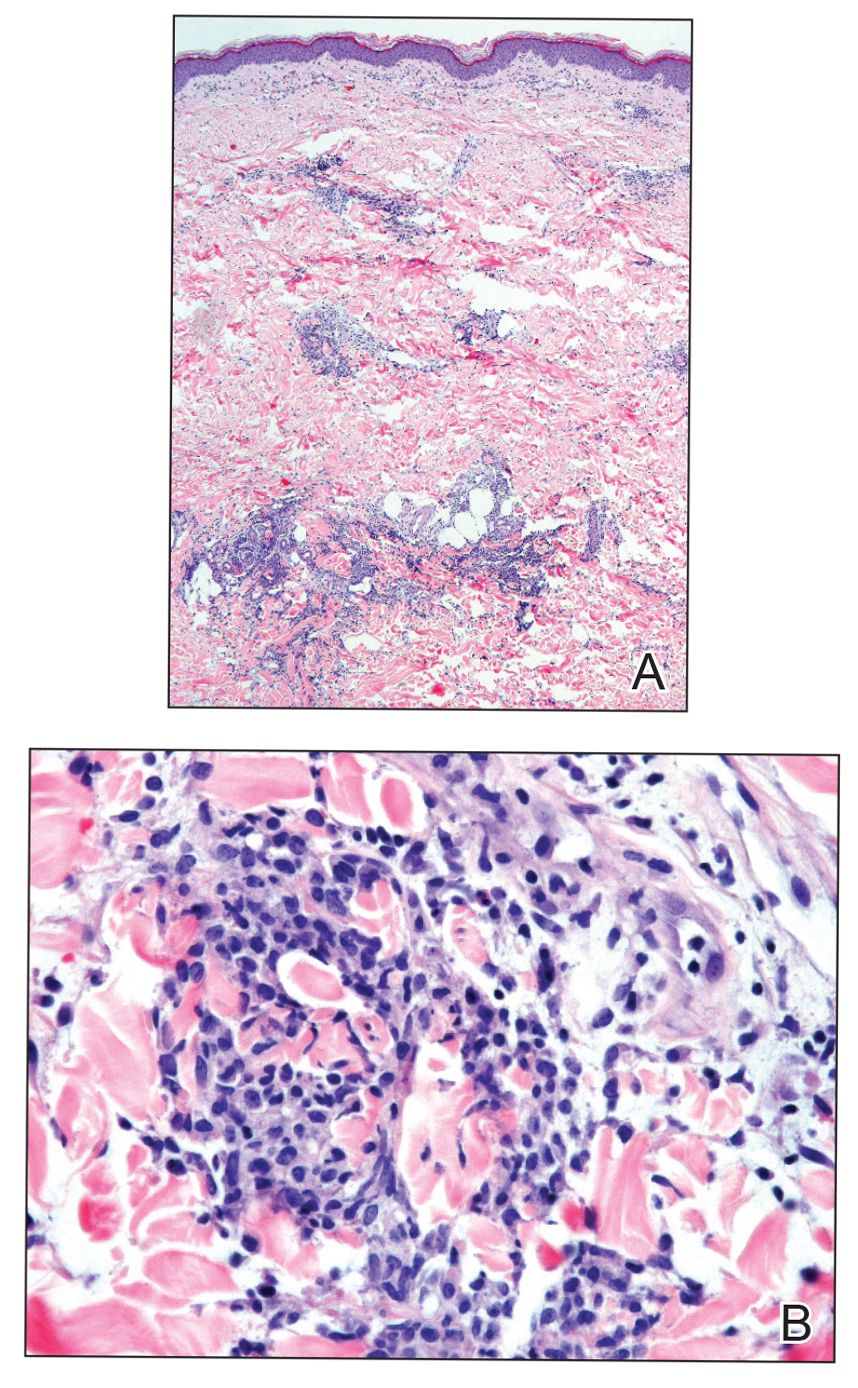
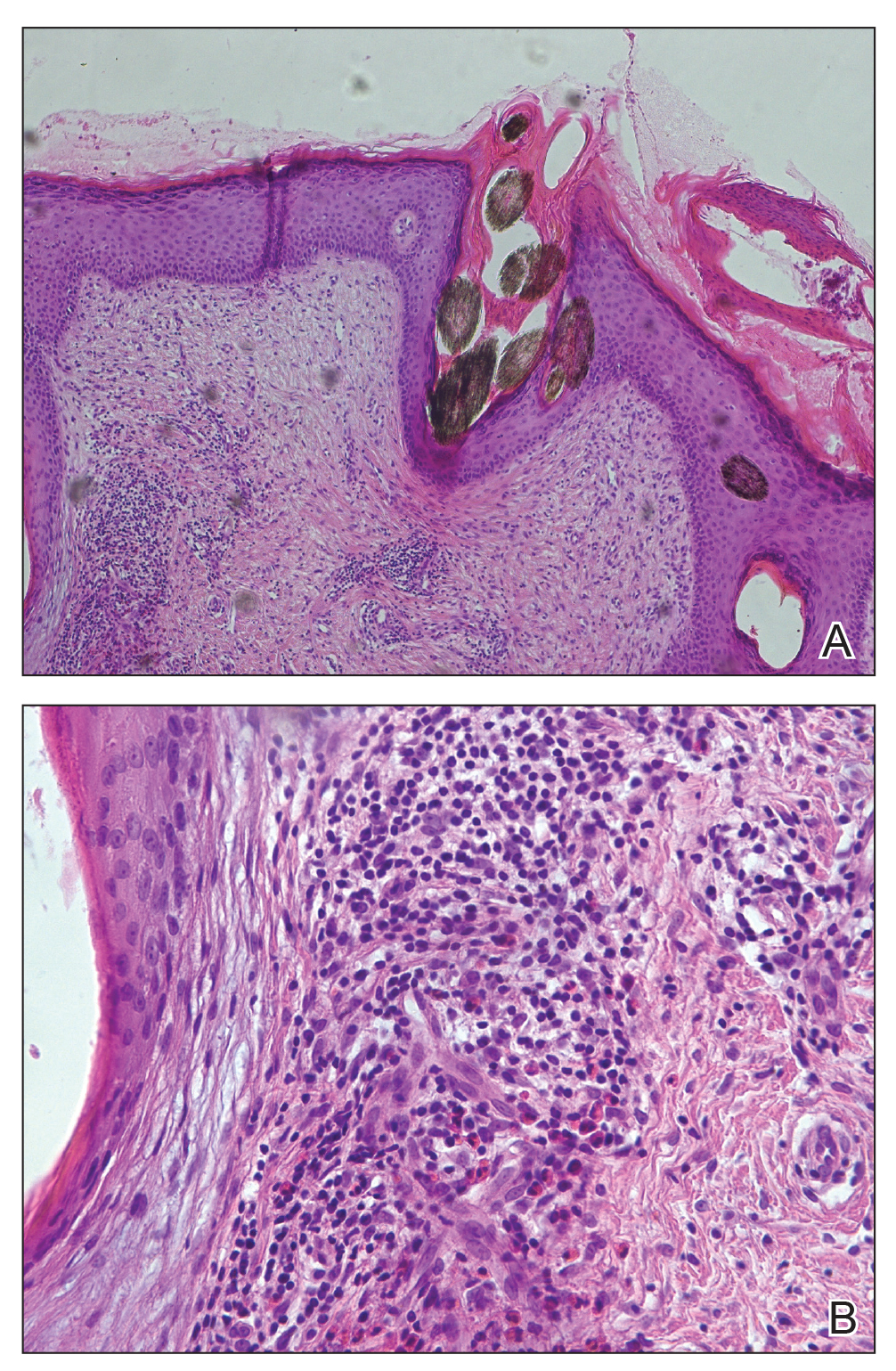
Histopathologic evaluation revealed superficial and deep perivascular and periadnexal inflammation. The epidermis exhibited some vacuolar interface change and effacement with relatively sparse dyskeratotic cells. A lymphohistiocytic inflammatory infiltrate surrounded the blood vessels, nerves, and adnexal structures and extended into the subcutaneous fat (Figure). Acid-fast, Grocott-Gomori methenamine-silver, Gram, Fite, Treponema pallidum, and Alcian blue stains were performed at our institution and were all negative. Biopsies sent to the National Hansen's Disease (Leprosy) Program demonstrated scattered extracellular acid-fast organisms on Fite staining in the specimen of the forearm. Polymerase chain reaction testing for Mycobacterium leprae DNA was negative. DNA sequencing of the 16S ribosomal RNA gene matched Mycobacterium avium-intracellulare complex (MAC). In the workup of the hepatic mass, the patient incidentally was found to have large-cell transformation of chronic lymphocytic leukemia (CLL) and therefore was treated with bendamustine and rituximab as an outpatient. The patient received 1 chemotherapy infusion every 4 weeks for a total of 10 rounds. At 10-week follow-up after 2 rounds of chemotherapy, all of the skin lesions had resolved despite no antibiotic therapy for atypical infections.
Disseminated infection with MAC is relatively rare in healthy as well as immunocompromised individuals. Clinical disease most commonly is seen as an opportunistic infection in patients with AIDS who have CD4 counts less than 50/mm3 (reference range, 500-1400/mm3) or in those with preexisting lung disease.1 Cutaneous involvement has been observed in only 14% of non-AIDS patients with disseminated MAC infection.2 In another study of 76 patients with MAC infection, only 2 involved the skin or soft tissue.3 Infection of the skin without concurrent pulmonary MAC infection is rare, though trauma may cause isolated skin infection. The cutaneous presentation of MAC infection is highly variable and may include erythematous papules, pustules, panniculitis, infiltrated plaques, verrucous lesions, and draining sinuses.3 The lesions have been reported to be painful.1
Cutaneous findings occur in up to 25% of patients with CLL, either due to the seeding of leukemic cells or other secondary lesions.4 Leukemia cutis, or skin involvement by B-cell CLL, most commonly presents in the head and neck region as chronic and relapsing erythematous papules and plaques.5 It histologically presents as monomorphic lymphocytic infiltrates accentuated around periadnexal and perivascular structures, with some extending into adipose tissue.2 In our case, histopathology demonstrated a lack of monomorphous infiltrate and thus was inconsistent with leukemia cutis. Similarly, lack of pale pink deposits and lack of neutrophilic infiltrates or degenerated collagen makes amyloidosis and palisaded neutrophilic granulomatous dermatitis incorrect diagnoses, respectively.
We hypothesize that the initially undetected worsening of CLL resulted in an immunocompromised state, which facilitated this unique presentation of cutaneous MAC infection in a human immunodeficiency virus-negative patient with no clinical symptoms of active pulmonary disease. The rash was the presenting sign of both the cutaneous MAC infection and worsening CLL. Additionally, our patient's cutaneous MAC facial involvement clinically resembled the leonine facies that is classic in lepromatous leprosy. Rare reports have been published addressing this similarity.6
Treatment of MAC pulmonary disease usually includes a combination of clarithromycin or azithromycin, rifampin, and ethambutol (for nodular/bronchiectatic disease), with or without amikacin or streptomycin.7 For limited pulmonary disease in patients with adequate pulmonary reserve, surgical resection may be considered in combination with the multidrug MAC pulmonary treatment regimen for 3 months to 1 year. Patients with localized MAC disease involving only the skin, soft tissue, tendons, and joints usually are treated with surgical excision in combination with clarithromycin, rifampin, and ethambutol for 6 to 12 months.7 In our patient, we believe that chemotherapy and the subsequent reconstituted immune system likely cleared the MAC infection without targeted antibiotic treatment.
Acknowledgments
The authors would like to thank David Scollard, MD, PhD, and Barbara Stryjewska, MD, from the National Hansen's Disease (Leprosy) Association (Baton Rouge, Louisiana).
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Plaza JA, Comfere NI, Gibson LE, et al. Unusual cutaneous manifestations of chronic lymphocytic leukemia. J Am Acad Dermatol. 2009;60:772-780.
- Sivanesan SP, Khera P, Buckthal-McCuin J, et al. Cutaneous Mycobacterium avium-intracellulare complex associated with immune reconstitution inflammatory syndrome. J Am Acad Dermatol. 2010;62:E25-E26.
- Horsburgh CR, Mason UG, Farhi DC, et al. Disseminated infection with Mycobacterium avium-intracellulare. a report of 13 cases and a review of the literature. Medicine (Baltimore). 1985;64:36-48.
- Bodle EE, Cunningham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:290-296.
- Boyd AS, Robbins J. Cutaneous Mycobacterium avium intracellulare infection in an HIV+ patient mimicking histoid leprosy. Am J Dermatopathol. 2005;27:39-41.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
The Diagnosis: Cutaneous Mycobacterium avium-intracellulare Complex Infection
Histopathologic evaluation revealed superficial and deep perivascular and periadnexal inflammation. The epidermis exhibited some vacuolar interface change and effacement with relatively sparse dyskeratotic cells. A lymphohistiocytic inflammatory infiltrate surrounded the blood vessels, nerves, and adnexal structures and extended into the subcutaneous fat (Figure). Acid-fast, Grocott-Gomori methenamine-silver, Gram, Fite, Treponema pallidum, and Alcian blue stains were performed at our institution and were all negative. Biopsies sent to the National Hansen's Disease (Leprosy) Program demonstrated scattered extracellular acid-fast organisms on Fite staining in the specimen of the forearm. Polymerase chain reaction testing for Mycobacterium leprae DNA was negative. DNA sequencing of the 16S ribosomal RNA gene matched Mycobacterium avium-intracellulare complex (MAC). In the workup of the hepatic mass, the patient incidentally was found to have large-cell transformation of chronic lymphocytic leukemia (CLL) and therefore was treated with bendamustine and rituximab as an outpatient. The patient received 1 chemotherapy infusion every 4 weeks for a total of 10 rounds. At 10-week follow-up after 2 rounds of chemotherapy, all of the skin lesions had resolved despite no antibiotic therapy for atypical infections.

Disseminated infection with MAC is relatively rare in healthy as well as immunocompromised individuals. Clinical disease most commonly is seen as an opportunistic infection in patients with AIDS who have CD4 counts less than 50/mm3 (reference range, 500-1400/mm3) or in those with preexisting lung disease.1 Cutaneous involvement has been observed in only 14% of non-AIDS patients with disseminated MAC infection.2 In another study of 76 patients with MAC infection, only 2 involved the skin or soft tissue.3 Infection of the skin without concurrent pulmonary MAC infection is rare, though trauma may cause isolated skin infection. The cutaneous presentation of MAC infection is highly variable and may include erythematous papules, pustules, panniculitis, infiltrated plaques, verrucous lesions, and draining sinuses.3 The lesions have been reported to be painful.1
Cutaneous findings occur in up to 25% of patients with CLL, either due to the seeding of leukemic cells or other secondary lesions.4 Leukemia cutis, or skin involvement by B-cell CLL, most commonly presents in the head and neck region as chronic and relapsing erythematous papules and plaques.5 It histologically presents as monomorphic lymphocytic infiltrates accentuated around periadnexal and perivascular structures, with some extending into adipose tissue.2 In our case, histopathology demonstrated a lack of monomorphous infiltrate and thus was inconsistent with leukemia cutis. Similarly, lack of pale pink deposits and lack of neutrophilic infiltrates or degenerated collagen makes amyloidosis and palisaded neutrophilic granulomatous dermatitis incorrect diagnoses, respectively.
We hypothesize that the initially undetected worsening of CLL resulted in an immunocompromised state, which facilitated this unique presentation of cutaneous MAC infection in a human immunodeficiency virus-negative patient with no clinical symptoms of active pulmonary disease. The rash was the presenting sign of both the cutaneous MAC infection and worsening CLL. Additionally, our patient's cutaneous MAC facial involvement clinically resembled the leonine facies that is classic in lepromatous leprosy. Rare reports have been published addressing this similarity.6
Treatment of MAC pulmonary disease usually includes a combination of clarithromycin or azithromycin, rifampin, and ethambutol (for nodular/bronchiectatic disease), with or without amikacin or streptomycin.7 For limited pulmonary disease in patients with adequate pulmonary reserve, surgical resection may be considered in combination with the multidrug MAC pulmonary treatment regimen for 3 months to 1 year. Patients with localized MAC disease involving only the skin, soft tissue, tendons, and joints usually are treated with surgical excision in combination with clarithromycin, rifampin, and ethambutol for 6 to 12 months.7 In our patient, we believe that chemotherapy and the subsequent reconstituted immune system likely cleared the MAC infection without targeted antibiotic treatment.
Acknowledgments
The authors would like to thank David Scollard, MD, PhD, and Barbara Stryjewska, MD, from the National Hansen's Disease (Leprosy) Association (Baton Rouge, Louisiana).
The Diagnosis: Cutaneous Mycobacterium avium-intracellulare Complex Infection
Histopathologic evaluation revealed superficial and deep perivascular and periadnexal inflammation. The epidermis exhibited some vacuolar interface change and effacement with relatively sparse dyskeratotic cells. A lymphohistiocytic inflammatory infiltrate surrounded the blood vessels, nerves, and adnexal structures and extended into the subcutaneous fat (Figure). Acid-fast, Grocott-Gomori methenamine-silver, Gram, Fite, Treponema pallidum, and Alcian blue stains were performed at our institution and were all negative. Biopsies sent to the National Hansen's Disease (Leprosy) Program demonstrated scattered extracellular acid-fast organisms on Fite staining in the specimen of the forearm. Polymerase chain reaction testing for Mycobacterium leprae DNA was negative. DNA sequencing of the 16S ribosomal RNA gene matched Mycobacterium avium-intracellulare complex (MAC). In the workup of the hepatic mass, the patient incidentally was found to have large-cell transformation of chronic lymphocytic leukemia (CLL) and therefore was treated with bendamustine and rituximab as an outpatient. The patient received 1 chemotherapy infusion every 4 weeks for a total of 10 rounds. At 10-week follow-up after 2 rounds of chemotherapy, all of the skin lesions had resolved despite no antibiotic therapy for atypical infections.

Disseminated infection with MAC is relatively rare in healthy as well as immunocompromised individuals. Clinical disease most commonly is seen as an opportunistic infection in patients with AIDS who have CD4 counts less than 50/mm3 (reference range, 500-1400/mm3) or in those with preexisting lung disease.1 Cutaneous involvement has been observed in only 14% of non-AIDS patients with disseminated MAC infection.2 In another study of 76 patients with MAC infection, only 2 involved the skin or soft tissue.3 Infection of the skin without concurrent pulmonary MAC infection is rare, though trauma may cause isolated skin infection. The cutaneous presentation of MAC infection is highly variable and may include erythematous papules, pustules, panniculitis, infiltrated plaques, verrucous lesions, and draining sinuses.3 The lesions have been reported to be painful.1
Cutaneous findings occur in up to 25% of patients with CLL, either due to the seeding of leukemic cells or other secondary lesions.4 Leukemia cutis, or skin involvement by B-cell CLL, most commonly presents in the head and neck region as chronic and relapsing erythematous papules and plaques.5 It histologically presents as monomorphic lymphocytic infiltrates accentuated around periadnexal and perivascular structures, with some extending into adipose tissue.2 In our case, histopathology demonstrated a lack of monomorphous infiltrate and thus was inconsistent with leukemia cutis. Similarly, lack of pale pink deposits and lack of neutrophilic infiltrates or degenerated collagen makes amyloidosis and palisaded neutrophilic granulomatous dermatitis incorrect diagnoses, respectively.
We hypothesize that the initially undetected worsening of CLL resulted in an immunocompromised state, which facilitated this unique presentation of cutaneous MAC infection in a human immunodeficiency virus-negative patient with no clinical symptoms of active pulmonary disease. The rash was the presenting sign of both the cutaneous MAC infection and worsening CLL. Additionally, our patient's cutaneous MAC facial involvement clinically resembled the leonine facies that is classic in lepromatous leprosy. Rare reports have been published addressing this similarity.6
Treatment of MAC pulmonary disease usually includes a combination of clarithromycin or azithromycin, rifampin, and ethambutol (for nodular/bronchiectatic disease), with or without amikacin or streptomycin.7 For limited pulmonary disease in patients with adequate pulmonary reserve, surgical resection may be considered in combination with the multidrug MAC pulmonary treatment regimen for 3 months to 1 year. Patients with localized MAC disease involving only the skin, soft tissue, tendons, and joints usually are treated with surgical excision in combination with clarithromycin, rifampin, and ethambutol for 6 to 12 months.7 In our patient, we believe that chemotherapy and the subsequent reconstituted immune system likely cleared the MAC infection without targeted antibiotic treatment.
Acknowledgments
The authors would like to thank David Scollard, MD, PhD, and Barbara Stryjewska, MD, from the National Hansen's Disease (Leprosy) Association (Baton Rouge, Louisiana).
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Plaza JA, Comfere NI, Gibson LE, et al. Unusual cutaneous manifestations of chronic lymphocytic leukemia. J Am Acad Dermatol. 2009;60:772-780.
- Sivanesan SP, Khera P, Buckthal-McCuin J, et al. Cutaneous Mycobacterium avium-intracellulare complex associated with immune reconstitution inflammatory syndrome. J Am Acad Dermatol. 2010;62:E25-E26.
- Horsburgh CR, Mason UG, Farhi DC, et al. Disseminated infection with Mycobacterium avium-intracellulare. a report of 13 cases and a review of the literature. Medicine (Baltimore). 1985;64:36-48.
- Bodle EE, Cunningham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:290-296.
- Boyd AS, Robbins J. Cutaneous Mycobacterium avium intracellulare infection in an HIV+ patient mimicking histoid leprosy. Am J Dermatopathol. 2005;27:39-41.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Plaza JA, Comfere NI, Gibson LE, et al. Unusual cutaneous manifestations of chronic lymphocytic leukemia. J Am Acad Dermatol. 2009;60:772-780.
- Sivanesan SP, Khera P, Buckthal-McCuin J, et al. Cutaneous Mycobacterium avium-intracellulare complex associated with immune reconstitution inflammatory syndrome. J Am Acad Dermatol. 2010;62:E25-E26.
- Horsburgh CR, Mason UG, Farhi DC, et al. Disseminated infection with Mycobacterium avium-intracellulare. a report of 13 cases and a review of the literature. Medicine (Baltimore). 1985;64:36-48.
- Bodle EE, Cunningham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:290-296.
- Boyd AS, Robbins J. Cutaneous Mycobacterium avium intracellulare infection in an HIV+ patient mimicking histoid leprosy. Am J Dermatopathol. 2005;27:39-41.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.

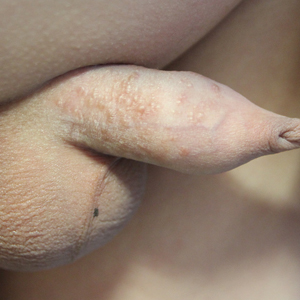
Nonhealing Ulcerative Hand Wound
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
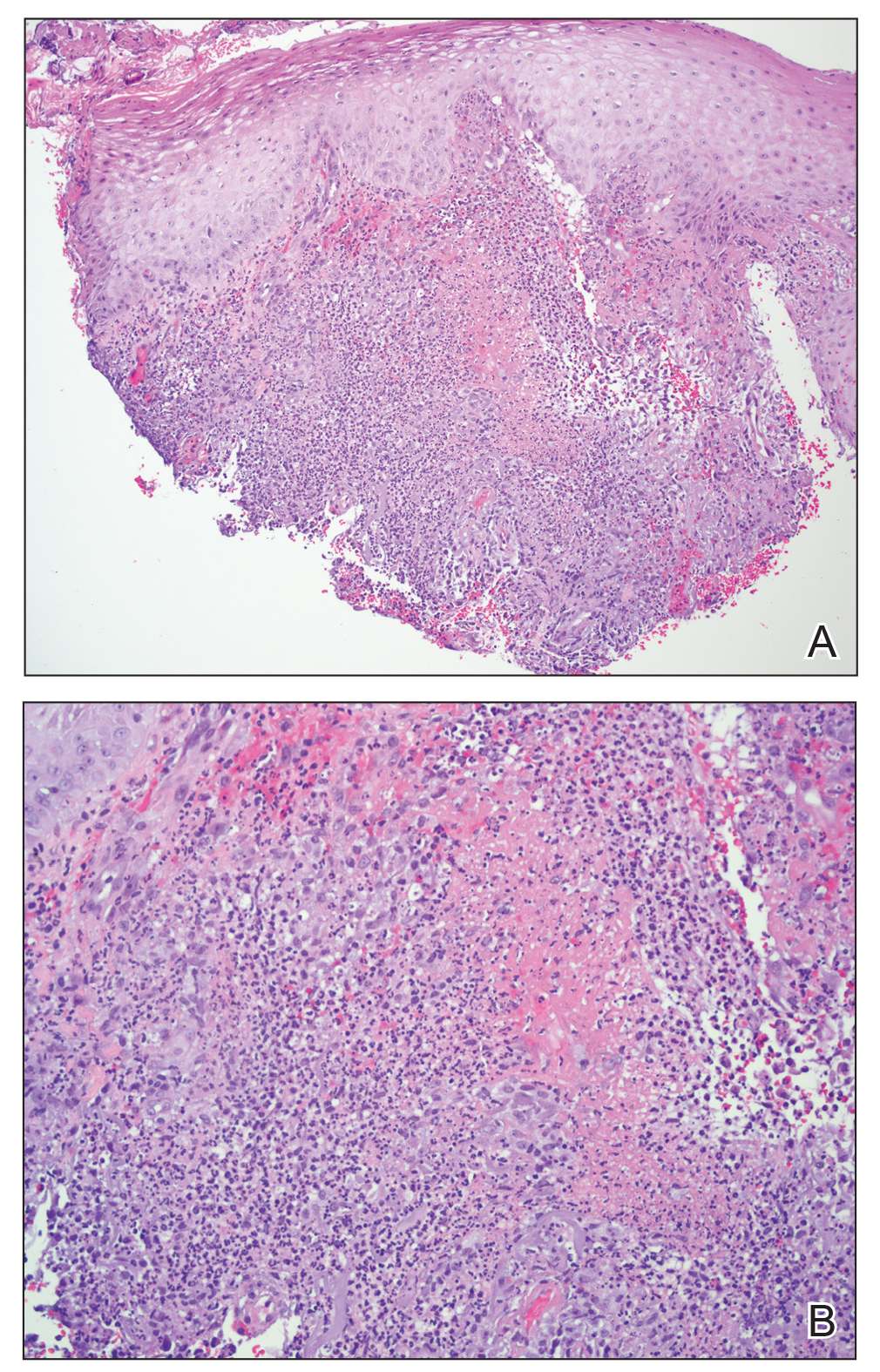
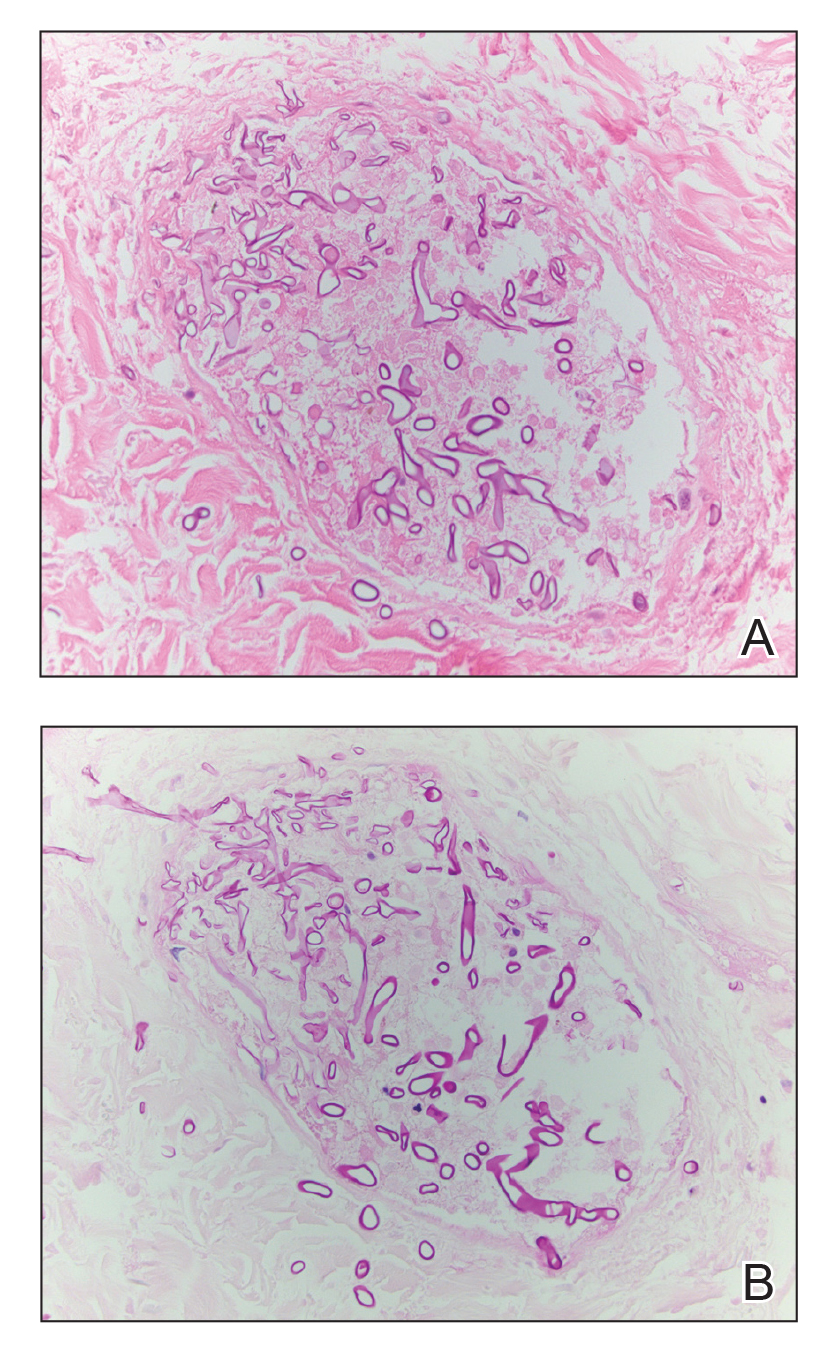
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.
Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.

Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.

Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
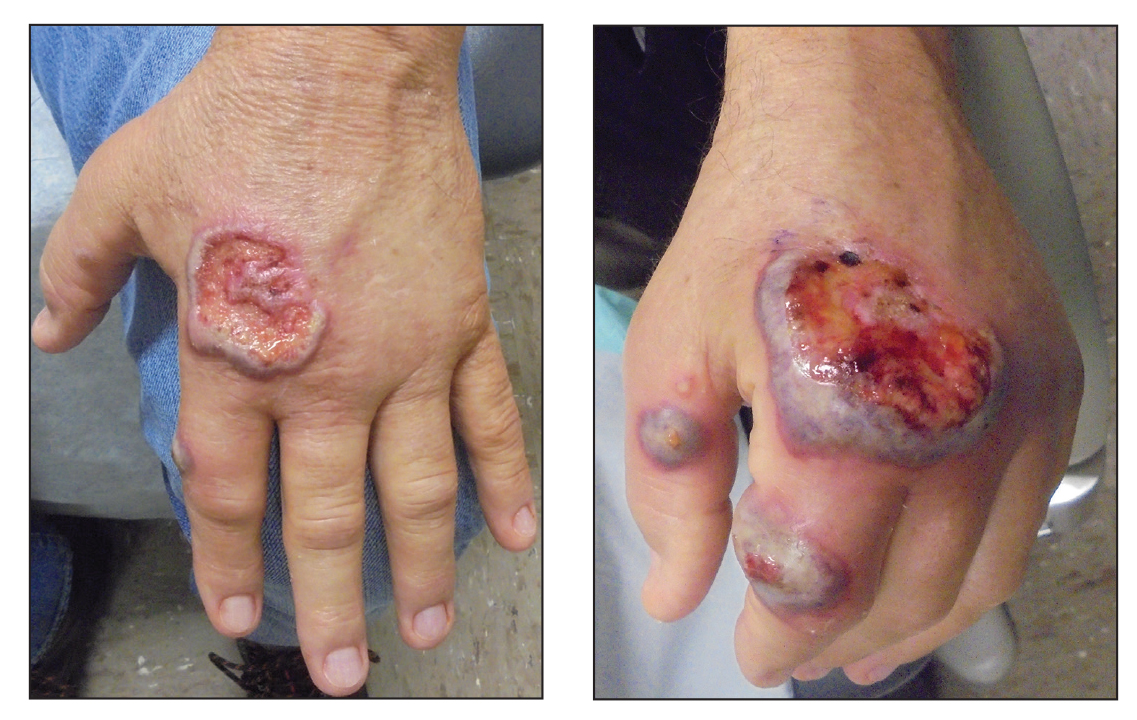
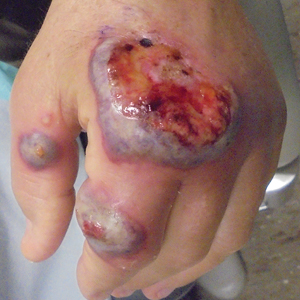
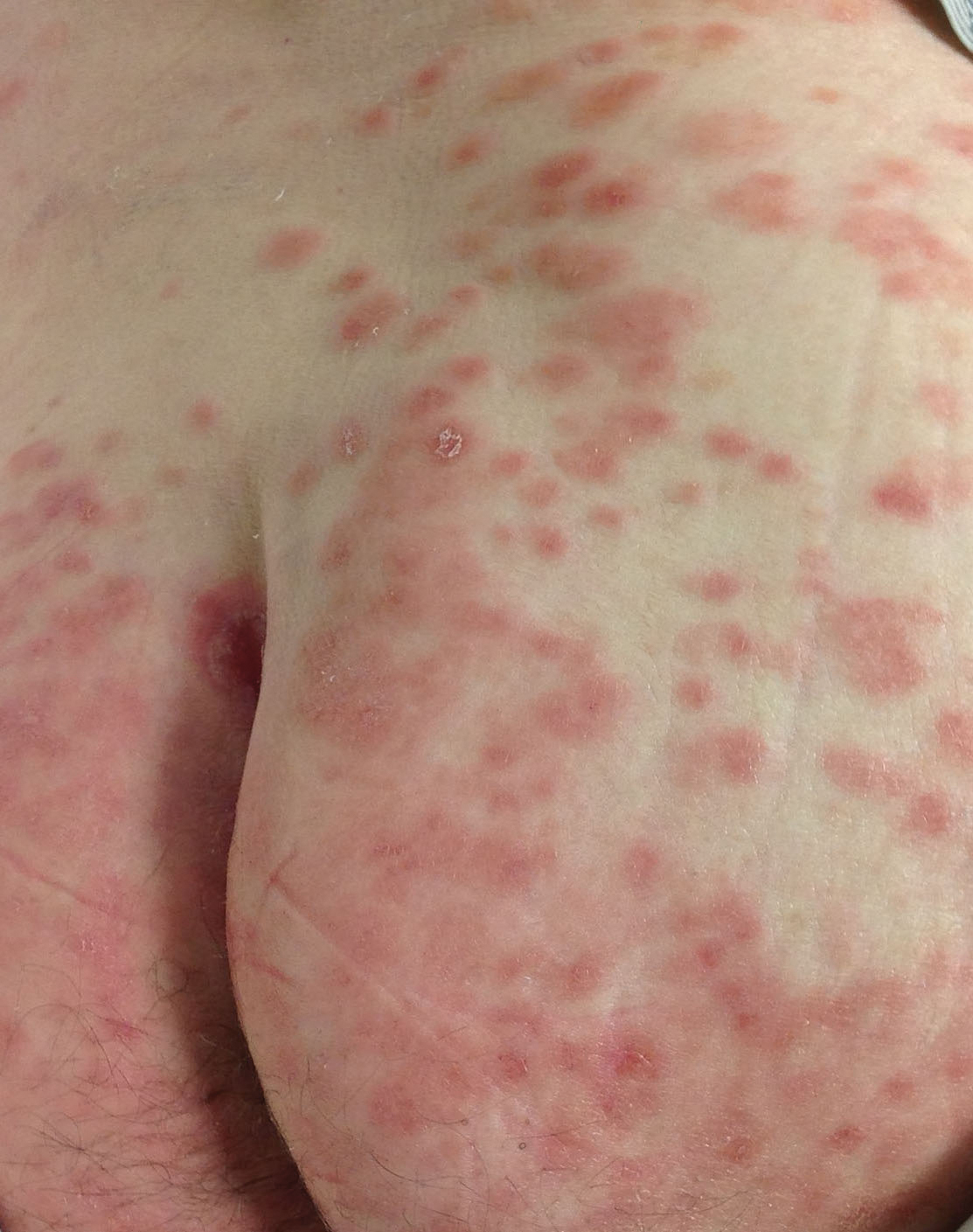
A 63-year-old man presented with an expanding wound on the dorsal aspect of the left hand after striking it on a wall. He sustained a small laceration that progressively became more edematous and developed a violaceous border. He presented to the emergency department the following day and was prescribed bacitracin with no improvement in the lesion. He returned to the emergency department after the symptoms worsened and was subsequently prescribed a 10-day course of oral trimethoprim-sulfamethoxazole (1600/320 mg) twice daily. Physical examination at a follow-up visit 11 days after the initial injury revealed an expanding, 4.3×5.0-cm, ulcerated wound with surrounding erythema and serosanguineous drainage (left). He was started on a 10-day course of amoxicillin–clavulanic acid (1750/250 mg) twice daily and underwent debridement the same day. On postoperative day 2 (13 days following the onset of symptoms), the wound had not improved, and 2 new 1-cm bullae on the left first and second fingers had progressed (right). Erythrocyte sedimentation rate (33 mm/h [reference range, 0–10 mm/h]) and C-reactive protein (3.701 mg/dL [reference range, 0–0.747 mg/dL]) were elevated; however, other laboratory studies, including a complete blood cell count, were within reference range. He remained afebrile, and a review of systems was normal. Punch biopsy specimens were obtained.
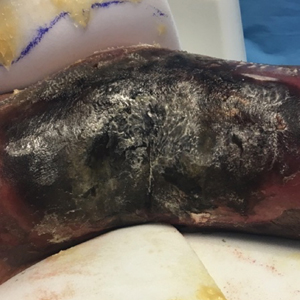
Extensive Purpura and Necrosis of the Leg
The Diagnosis: Disseminated Mucormycosis
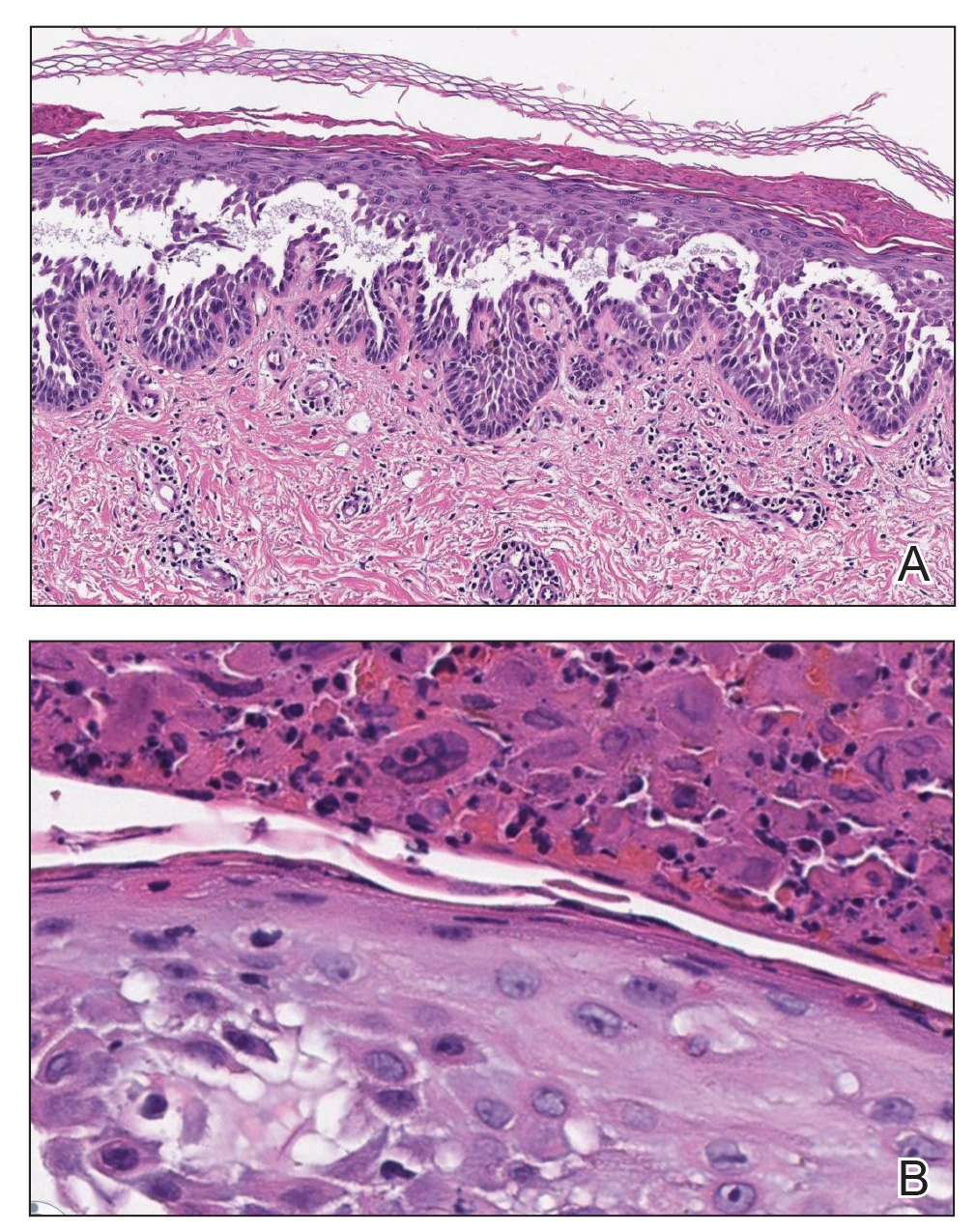
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.
Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.

Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.

Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
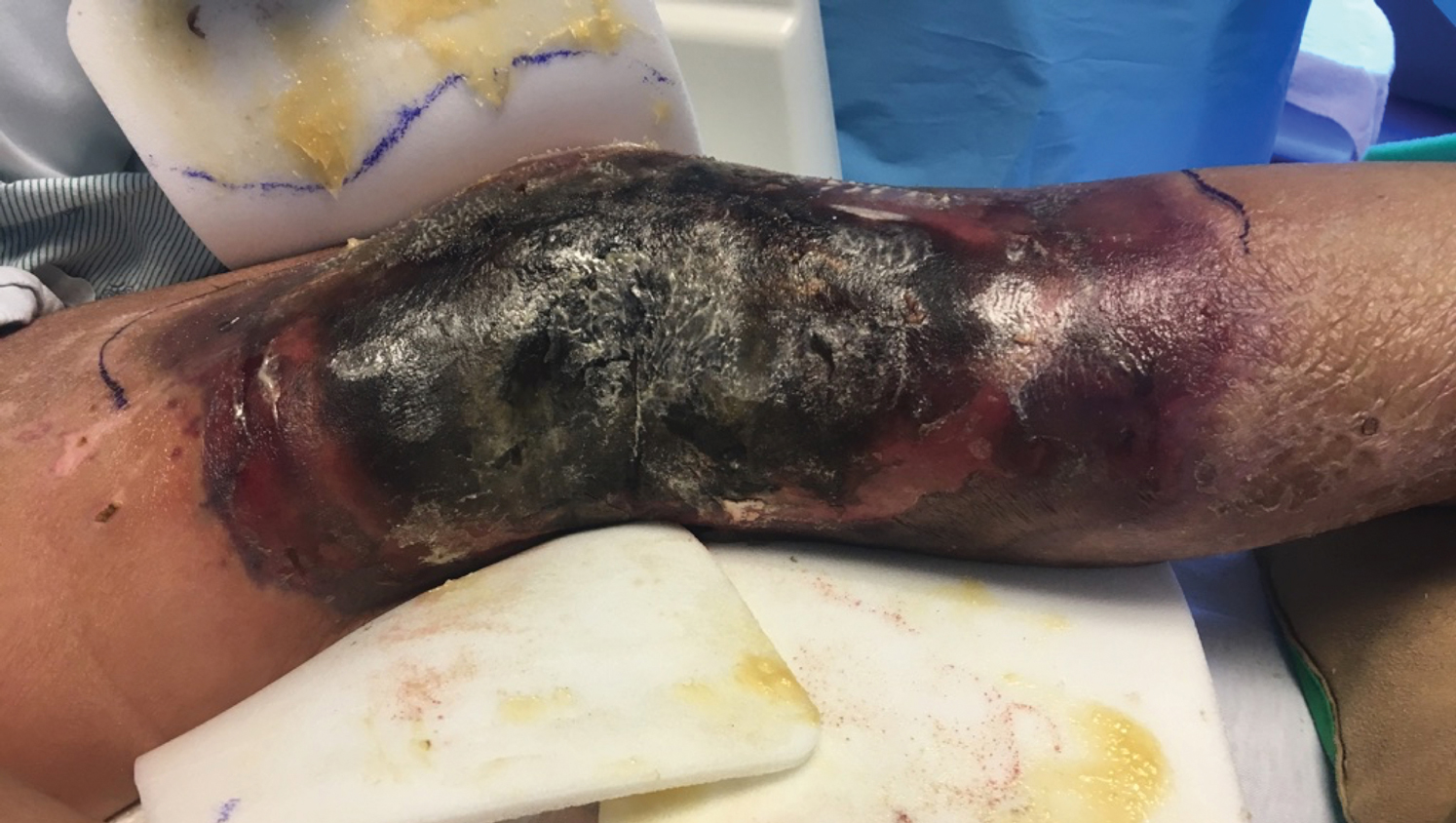
A 57-year-old woman presented with expanding purpura on the left leg of 2 weeks’ duration following a recent hematopoietic stem cell transplant for refractory diffuse large B-cell lymphoma. Prior to dermatologic consultation, the patient had been hospitalized for 2 months following the transplant due to Clostridium difficile colitis, Enterococcus faecium bacteremia, cardiac arrest, delayed engraftment with pancytopenia, and atypical hemolytic uremic syndrome with acute renal failure requiring hemodialysis and treatment with eculizumab. Her care team in the hospital initially noticed a small purpuric lesion on the posterior aspect of the left knee. The patient subsequently developed persistent fevers and expansion of the lesion, which prompted consultation of the dermatology service. Physical examination revealed a 22×10-cm, rectangular, indurated, purpuric plaque with central dusky, violaceous to black necrosis with superficial skin sloughing and peripheral dusky erythema extending from the inner thigh to the lower leg. The left distal leg felt cool, and both dorsalis pedis and posterior tibial pulses were absent. Laboratory test results revealed neutropenia and thrombocytopenia (white blood cell count, 0.2×103 /mm3 [reference range, 5–10×103 /mm3 ]; hematocrit, 23.2% [reference range, 41%–50%]; platelet count, 105×103 /µL [reference range, 150–350×103 /µL]). A punch biopsy was performed.
Painful Hemorrhagic Erosions
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.
Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).
Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.

Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).

Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.

Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).

Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
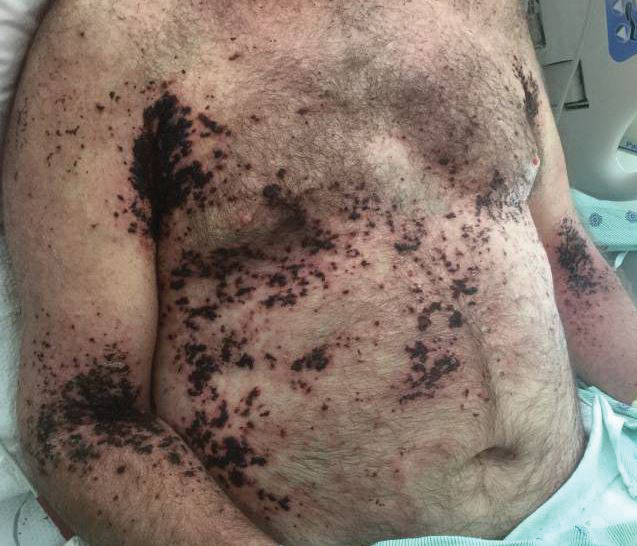
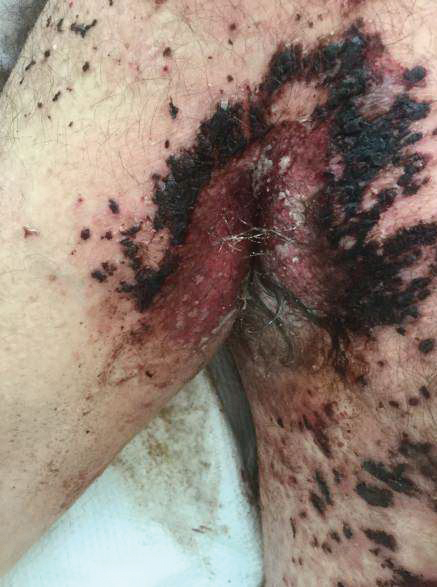
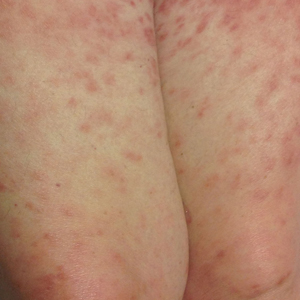
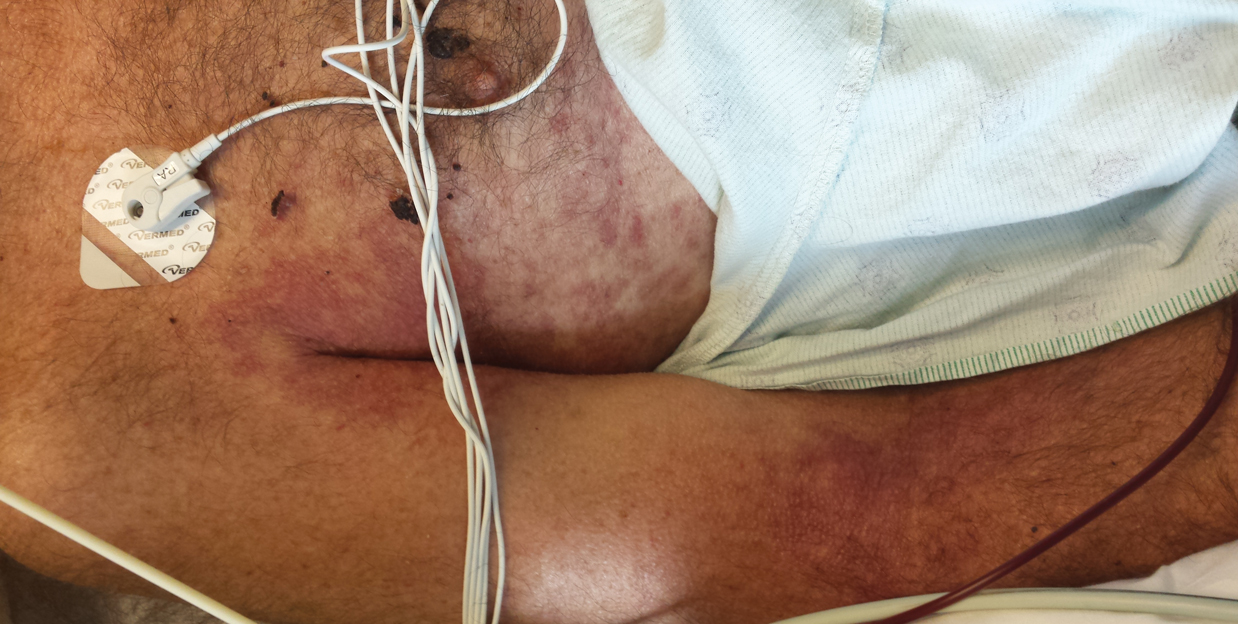
A 62-year-old man with a long-standing history (>40 years) of Hailey-Hailey disease was admitted from an outside hospital due to anemia (hemoglobin, 8.6 g/dL [reference range, 14.0–17.5 g/dL]), thrombocytopenia (platelets, 7×103 /µL [reference range, 150–350×103 /µL]), and worsening skin rash. The patient reported that his Hailey-Hailey disease worsened abruptly 1 month prior to admission and had progressed steadily since then. He described the rash as painful, especially with movement. Over the preceding month, he had been treated with topical triamcinolone, topical diphenhydramine, oral prednisone, fluconazole, and oral clindamycin, all without improvement. The skin lesions continued to worsen and persistently bled; he then presented to our institution for further care.
Physical examination demonstrated widespread shallow erosions with hemorrhagic drainage and crusting located on the lower back, chest, abdomen (top), axillae (bottom), groin, arms, and legs. No vesicles or pustules were noted. The patient had no cognitive dysfunction or focal neurologic deficits. A punch biopsy was performed.
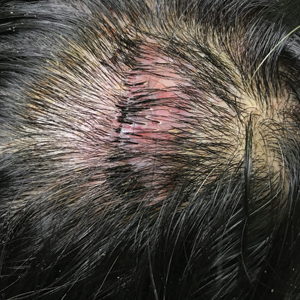
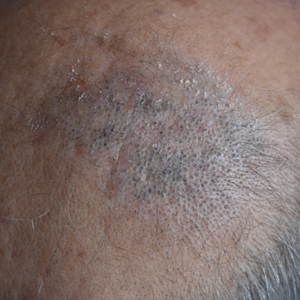
Erythematous Plaque on the Scalp With Alopecia
The Diagnosis: Tufted Hair Folliculitis
Dermoscopic examination revealed multiple hair tufts of 5 to 20 normal hairs emerging from single dilated follicular openings (Figure 1). The density of hair follicles was reduced with adherent yellow-white scales that encircled the dilated follicular orifices. Histopathology revealed hyperkeratosis and parakeratosis in the stratum corneum. Infiltration of lymphocytes, neutrophils, plasma cells, and eosinophils around the upper portions of the follicles also was found. Multiple hairs emerging from a single dilated follicular ostia with prominent fibrosis of the dermis were seen (Figure 2). Based on the clinical and histopathological findings, the patient was diagnosed with tufted hair folliculitis (THF). She was treated with minocycline 100 mg once daily and an intralesional betamethasone injection 5 mg once daily. After 2 weeks of treatment, the lesion improved and decreased in size to 1×1 cm in diameter; however, the hair tufts and scarring alopecia remained.
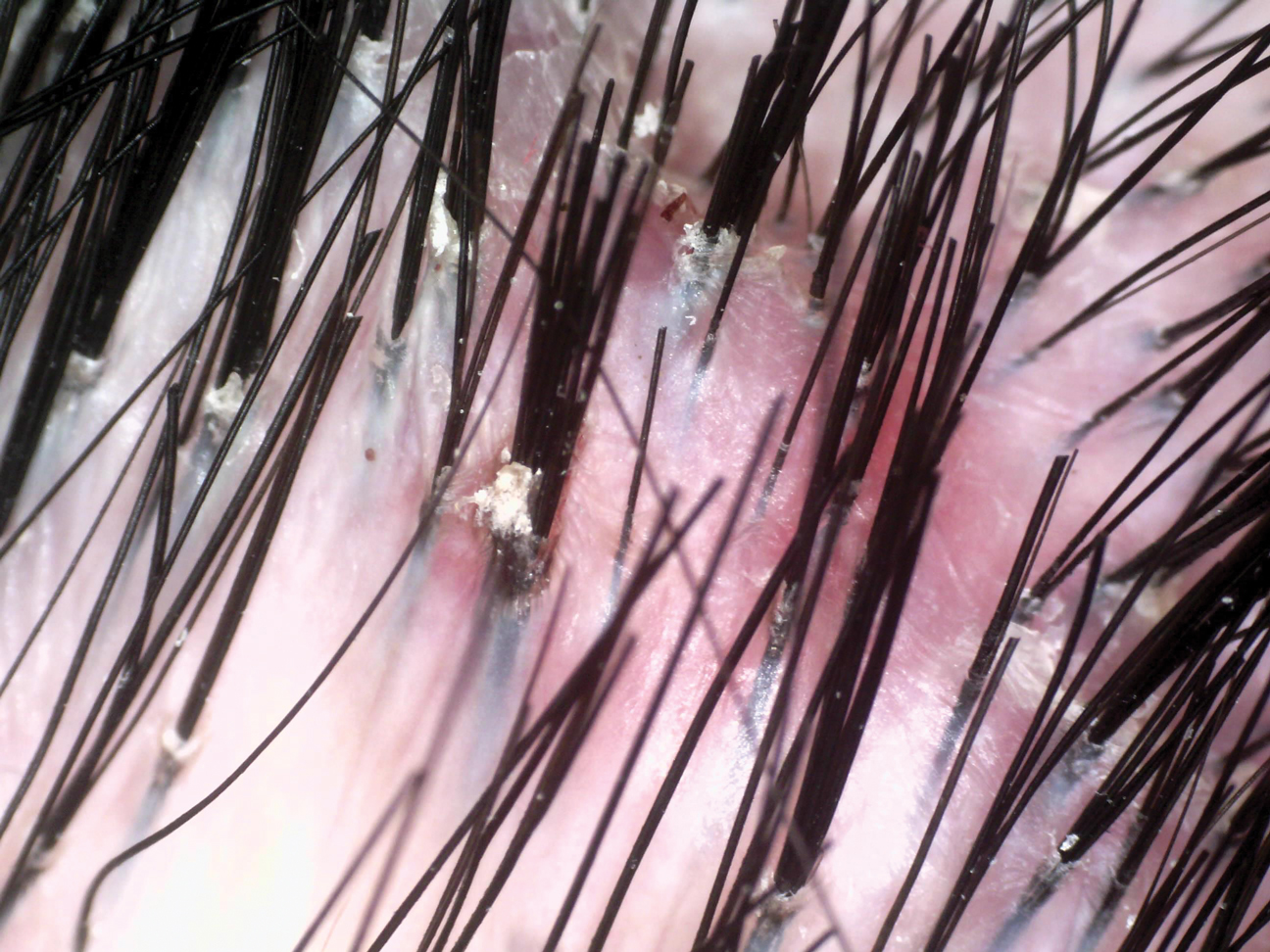
Tufted hair folliculitis is a rare inflammatory condition of the scalp characterized by a peculiar tufting of hair that was first described by Smith and Sanderson1 in 1978. Most patients present with a patch or plaque on the parietal or occipital region of the scalp. The condition may lead to the destruction of follicular units, resulting in permanent scarring alopecia.2 Histopathology in our patient revealed perifollicular inflammation, and several follicles could be seen converging toward a common follicular duct with a widely dilated opening, consistent with the diagnosis of THF.
The pathogenic mechanisms of THF are unclear. Primary hair tufting, local trauma, tinea capitis, nevoid malformation, and Staphylococcus aureus infection have been proposed as causative pathomechanisms.3 Typically there is no history of underlying disease or trauma on the scalp; however, secondary changes may have occurred following unrecognized trauma or repeated stimuli. Staphylococcal infections may play a notable role in inducing THF. Ekmekci and Koslu4 reported that a local inflammatory process led to the destruction of adjacent follicles, which subsequently amalgamated to form a common follicular duct due to local fibrosis and scarring. However, Powell et al5 found no evidence of local immune suppression or immune failure that could explain the abnormal host response to a certain presumptive superantigen. In our patient, the inflammatory injury was mild, and no purulent exudation was found from the dilated follicular openings. Because the patient had applied an antibiotic ointment prior to presentation, bacterial cultures from biopsy specimens were not appropriate.
The differential diagnosis of THF includes folliculitis decalvans, folliculitis keloidalis nuchae, dissecting cellulitis of the scalp, and follicular lichen planus.6 In our patient, folliculitis keloidalis nuchae and dissecting cellulitis of the scalp were excluded because no keloid or purulent inflammation was found. The diagnosis of follicular lichen planus was not taken into consideration because characteristic pathology such as liquefaction degeneration of basal cells was not observed. Folliculitis decalvans was considered to be a possible cause of the alopecia in our patient. It also was suggested that hair tufting could be a secondary phenomenon, occurring in several inflammatory disorders of the scalp. Powell et al5 concluded that THF should be considered as a distinctive clinicohistologic variant of folliculitis decalvans characterized by multiple hair tufts with patches of scarring alopecia. This hypothesis corresponded with our patient's clinical manifestation and histopathology.
Conventional treatment of THF includes topical antiseptics and oral antibiotics (eg, flucloxacillin, erythromycin, tetracycline, doxycycline), but reduction in hair bundling rarely has been observed after antibiotic treatment. Although good prognosis has been reported after surgical excision of the involved areas, it can only be performed in small lesions.6 Pranteda et al7 reported that combination therapy with oral rifampin and oral clindamycin can prevent relapse long-term. Combination therapy for 10 weeks also was effective in 10 of 18 patients with THF.5 Rifampin is an effective therapeutic modality to control the progression of THF as well as prevent relapse; however, long-term use should be avoided to prevent hepatic or renal side effects.7 Our patient was successfully treated with intralesional betamethasone and oral minocycline to reduce the inflammation and prevent the expansion of scarring alopecia.
Acknowledgment
The authors thank Xue Chen, MD (Beijing, China), for writing support.
- Smith NP, Sanderson KV. Tufted folliculitis of the scalp. J R Soc Med. 1978;71:606-608.
- Broshtilova V, Bardarov E, Kazandjieva J, et al. Tufted hair folliculitis: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:27-29.
- Gungor S, Yuksel T, Topal I. Tufted hair folliculitis associated with Melkersson-Rosenthal syndrome and hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2014;80:484-487.
- Ekmekci TR, Koslu A. Tufted hair folliculitis causing skullcap-pattern cicatricial alopecia. J Eur Acad Dermatol Venereol. 2006;20:227-229.
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186-188.
- Pranteda G, Grimaldi M, Palese E, et al. Tufted hair folliculitis: complete enduring response after treatment with rifampicin. J Dermatolog Treat. 2004;15:396-398.
The Diagnosis: Tufted Hair Folliculitis
Dermoscopic examination revealed multiple hair tufts of 5 to 20 normal hairs emerging from single dilated follicular openings (Figure 1). The density of hair follicles was reduced with adherent yellow-white scales that encircled the dilated follicular orifices. Histopathology revealed hyperkeratosis and parakeratosis in the stratum corneum. Infiltration of lymphocytes, neutrophils, plasma cells, and eosinophils around the upper portions of the follicles also was found. Multiple hairs emerging from a single dilated follicular ostia with prominent fibrosis of the dermis were seen (Figure 2). Based on the clinical and histopathological findings, the patient was diagnosed with tufted hair folliculitis (THF). She was treated with minocycline 100 mg once daily and an intralesional betamethasone injection 5 mg once daily. After 2 weeks of treatment, the lesion improved and decreased in size to 1×1 cm in diameter; however, the hair tufts and scarring alopecia remained.


Tufted hair folliculitis is a rare inflammatory condition of the scalp characterized by a peculiar tufting of hair that was first described by Smith and Sanderson1 in 1978. Most patients present with a patch or plaque on the parietal or occipital region of the scalp. The condition may lead to the destruction of follicular units, resulting in permanent scarring alopecia.2 Histopathology in our patient revealed perifollicular inflammation, and several follicles could be seen converging toward a common follicular duct with a widely dilated opening, consistent with the diagnosis of THF.
The pathogenic mechanisms of THF are unclear. Primary hair tufting, local trauma, tinea capitis, nevoid malformation, and Staphylococcus aureus infection have been proposed as causative pathomechanisms.3 Typically there is no history of underlying disease or trauma on the scalp; however, secondary changes may have occurred following unrecognized trauma or repeated stimuli. Staphylococcal infections may play a notable role in inducing THF. Ekmekci and Koslu4 reported that a local inflammatory process led to the destruction of adjacent follicles, which subsequently amalgamated to form a common follicular duct due to local fibrosis and scarring. However, Powell et al5 found no evidence of local immune suppression or immune failure that could explain the abnormal host response to a certain presumptive superantigen. In our patient, the inflammatory injury was mild, and no purulent exudation was found from the dilated follicular openings. Because the patient had applied an antibiotic ointment prior to presentation, bacterial cultures from biopsy specimens were not appropriate.
The differential diagnosis of THF includes folliculitis decalvans, folliculitis keloidalis nuchae, dissecting cellulitis of the scalp, and follicular lichen planus.6 In our patient, folliculitis keloidalis nuchae and dissecting cellulitis of the scalp were excluded because no keloid or purulent inflammation was found. The diagnosis of follicular lichen planus was not taken into consideration because characteristic pathology such as liquefaction degeneration of basal cells was not observed. Folliculitis decalvans was considered to be a possible cause of the alopecia in our patient. It also was suggested that hair tufting could be a secondary phenomenon, occurring in several inflammatory disorders of the scalp. Powell et al5 concluded that THF should be considered as a distinctive clinicohistologic variant of folliculitis decalvans characterized by multiple hair tufts with patches of scarring alopecia. This hypothesis corresponded with our patient's clinical manifestation and histopathology.
Conventional treatment of THF includes topical antiseptics and oral antibiotics (eg, flucloxacillin, erythromycin, tetracycline, doxycycline), but reduction in hair bundling rarely has been observed after antibiotic treatment. Although good prognosis has been reported after surgical excision of the involved areas, it can only be performed in small lesions.6 Pranteda et al7 reported that combination therapy with oral rifampin and oral clindamycin can prevent relapse long-term. Combination therapy for 10 weeks also was effective in 10 of 18 patients with THF.5 Rifampin is an effective therapeutic modality to control the progression of THF as well as prevent relapse; however, long-term use should be avoided to prevent hepatic or renal side effects.7 Our patient was successfully treated with intralesional betamethasone and oral minocycline to reduce the inflammation and prevent the expansion of scarring alopecia.
Acknowledgment
The authors thank Xue Chen, MD (Beijing, China), for writing support.
The Diagnosis: Tufted Hair Folliculitis
Dermoscopic examination revealed multiple hair tufts of 5 to 20 normal hairs emerging from single dilated follicular openings (Figure 1). The density of hair follicles was reduced with adherent yellow-white scales that encircled the dilated follicular orifices. Histopathology revealed hyperkeratosis and parakeratosis in the stratum corneum. Infiltration of lymphocytes, neutrophils, plasma cells, and eosinophils around the upper portions of the follicles also was found. Multiple hairs emerging from a single dilated follicular ostia with prominent fibrosis of the dermis were seen (Figure 2). Based on the clinical and histopathological findings, the patient was diagnosed with tufted hair folliculitis (THF). She was treated with minocycline 100 mg once daily and an intralesional betamethasone injection 5 mg once daily. After 2 weeks of treatment, the lesion improved and decreased in size to 1×1 cm in diameter; however, the hair tufts and scarring alopecia remained.


Tufted hair folliculitis is a rare inflammatory condition of the scalp characterized by a peculiar tufting of hair that was first described by Smith and Sanderson1 in 1978. Most patients present with a patch or plaque on the parietal or occipital region of the scalp. The condition may lead to the destruction of follicular units, resulting in permanent scarring alopecia.2 Histopathology in our patient revealed perifollicular inflammation, and several follicles could be seen converging toward a common follicular duct with a widely dilated opening, consistent with the diagnosis of THF.
The pathogenic mechanisms of THF are unclear. Primary hair tufting, local trauma, tinea capitis, nevoid malformation, and Staphylococcus aureus infection have been proposed as causative pathomechanisms.3 Typically there is no history of underlying disease or trauma on the scalp; however, secondary changes may have occurred following unrecognized trauma or repeated stimuli. Staphylococcal infections may play a notable role in inducing THF. Ekmekci and Koslu4 reported that a local inflammatory process led to the destruction of adjacent follicles, which subsequently amalgamated to form a common follicular duct due to local fibrosis and scarring. However, Powell et al5 found no evidence of local immune suppression or immune failure that could explain the abnormal host response to a certain presumptive superantigen. In our patient, the inflammatory injury was mild, and no purulent exudation was found from the dilated follicular openings. Because the patient had applied an antibiotic ointment prior to presentation, bacterial cultures from biopsy specimens were not appropriate.
The differential diagnosis of THF includes folliculitis decalvans, folliculitis keloidalis nuchae, dissecting cellulitis of the scalp, and follicular lichen planus.6 In our patient, folliculitis keloidalis nuchae and dissecting cellulitis of the scalp were excluded because no keloid or purulent inflammation was found. The diagnosis of follicular lichen planus was not taken into consideration because characteristic pathology such as liquefaction degeneration of basal cells was not observed. Folliculitis decalvans was considered to be a possible cause of the alopecia in our patient. It also was suggested that hair tufting could be a secondary phenomenon, occurring in several inflammatory disorders of the scalp. Powell et al5 concluded that THF should be considered as a distinctive clinicohistologic variant of folliculitis decalvans characterized by multiple hair tufts with patches of scarring alopecia. This hypothesis corresponded with our patient's clinical manifestation and histopathology.
Conventional treatment of THF includes topical antiseptics and oral antibiotics (eg, flucloxacillin, erythromycin, tetracycline, doxycycline), but reduction in hair bundling rarely has been observed after antibiotic treatment. Although good prognosis has been reported after surgical excision of the involved areas, it can only be performed in small lesions.6 Pranteda et al7 reported that combination therapy with oral rifampin and oral clindamycin can prevent relapse long-term. Combination therapy for 10 weeks also was effective in 10 of 18 patients with THF.5 Rifampin is an effective therapeutic modality to control the progression of THF as well as prevent relapse; however, long-term use should be avoided to prevent hepatic or renal side effects.7 Our patient was successfully treated with intralesional betamethasone and oral minocycline to reduce the inflammation and prevent the expansion of scarring alopecia.
Acknowledgment
The authors thank Xue Chen, MD (Beijing, China), for writing support.
- Smith NP, Sanderson KV. Tufted folliculitis of the scalp. J R Soc Med. 1978;71:606-608.
- Broshtilova V, Bardarov E, Kazandjieva J, et al. Tufted hair folliculitis: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:27-29.
- Gungor S, Yuksel T, Topal I. Tufted hair folliculitis associated with Melkersson-Rosenthal syndrome and hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2014;80:484-487.
- Ekmekci TR, Koslu A. Tufted hair folliculitis causing skullcap-pattern cicatricial alopecia. J Eur Acad Dermatol Venereol. 2006;20:227-229.
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186-188.
- Pranteda G, Grimaldi M, Palese E, et al. Tufted hair folliculitis: complete enduring response after treatment with rifampicin. J Dermatolog Treat. 2004;15:396-398.
- Smith NP, Sanderson KV. Tufted folliculitis of the scalp. J R Soc Med. 1978;71:606-608.
- Broshtilova V, Bardarov E, Kazandjieva J, et al. Tufted hair folliculitis: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:27-29.
- Gungor S, Yuksel T, Topal I. Tufted hair folliculitis associated with Melkersson-Rosenthal syndrome and hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2014;80:484-487.
- Ekmekci TR, Koslu A. Tufted hair folliculitis causing skullcap-pattern cicatricial alopecia. J Eur Acad Dermatol Venereol. 2006;20:227-229.
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186-188.
- Pranteda G, Grimaldi M, Palese E, et al. Tufted hair folliculitis: complete enduring response after treatment with rifampicin. J Dermatolog Treat. 2004;15:396-398.

A 37-year-old woman presented with a 2×6-cm, firm, erythematous plaque on the parietal region of the scalp of 1 year’s duration. No history of injury to the scalp was noted. The patient noticed hair loss in the affected area in the month prior to presentation. She was afebrile and otherwise asymptomatic. She denied a family history of similar scalp disorders.
Erythematous Plaque on the Back of a Newborn
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).
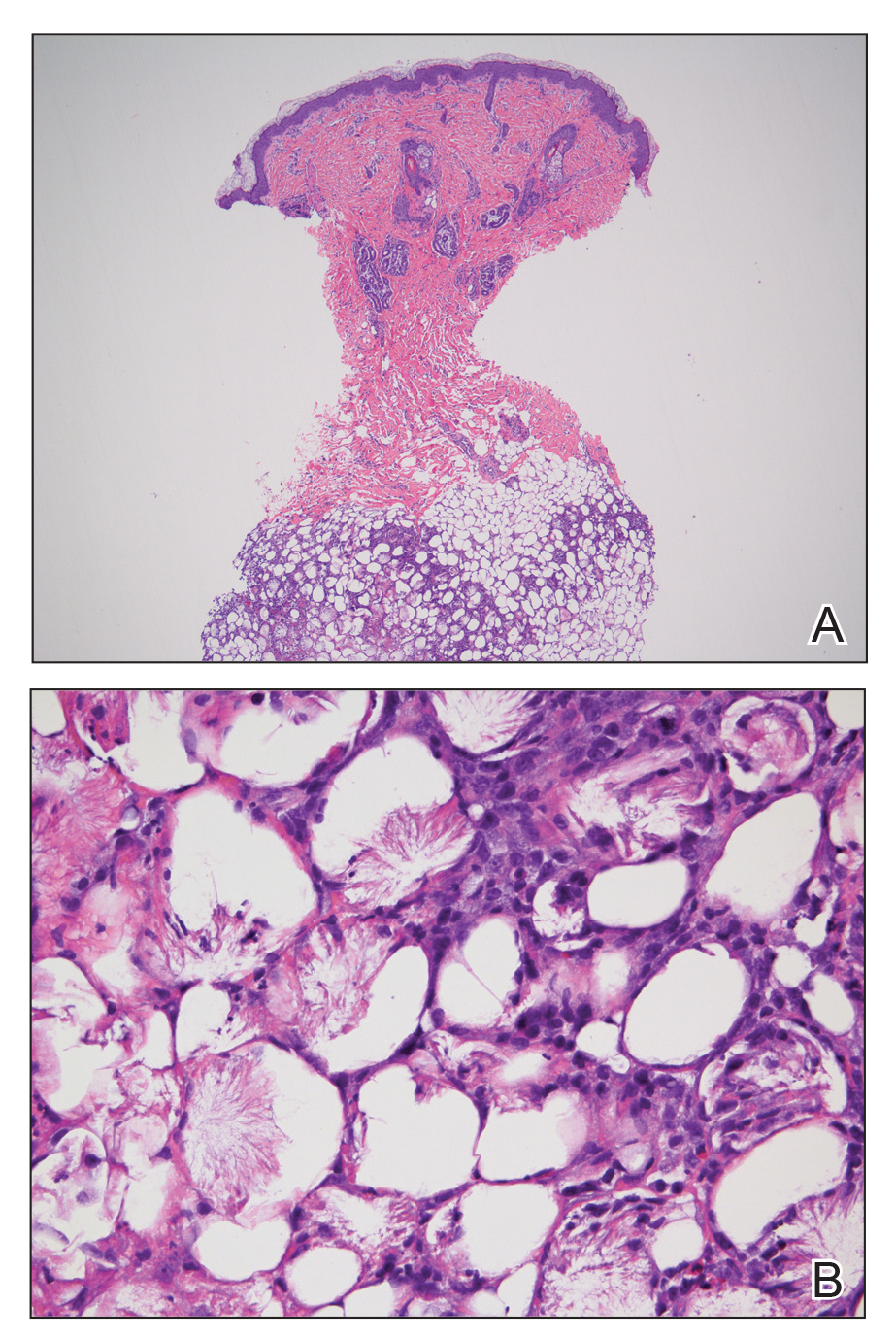
Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).

Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).

Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
An 8-day-old female infant presented with a mass on the lower back that had been present since birth. The patient was well appearing, alert, and active. Physical examination revealed a 6×5-cm, erythematous, ill-defined, indurated plaque on the lower thoracic back. There was no associated family history of similar findings. According to the mother, the patient was feeding well with no recent fever, irritability, or lethargy. The patient was born via elective induction of labor at term due to maternal intrauterine infection from chorioamnionitis. The birth was complicated by shoulder dystocia with an Erb palsy, and she was hospitalized for 5 days after delivery for management of hypotension and ABO isoimmunization and to rule out sepsis; blood cultures were negative for neonatal infection.
Asymptomatic Plaque on the Scalp
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.
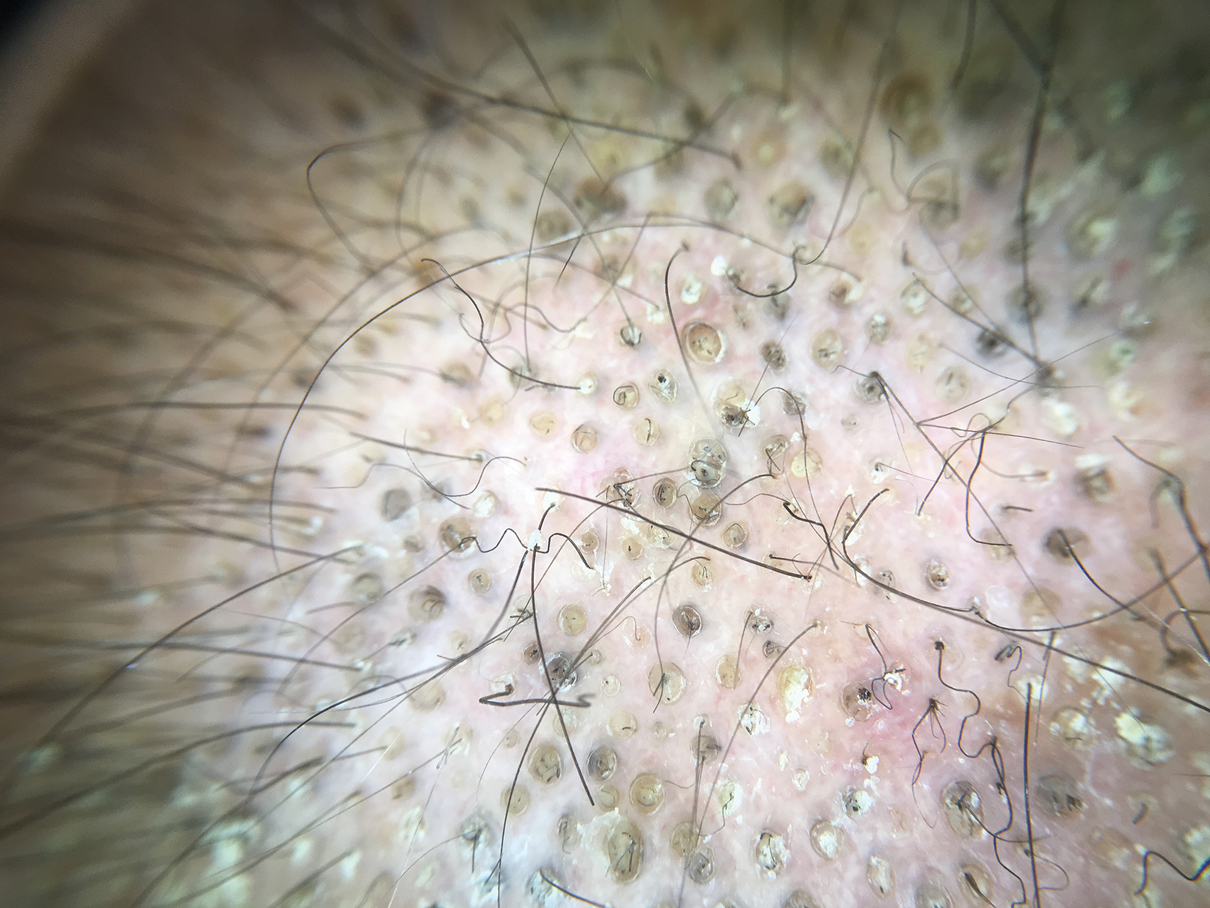
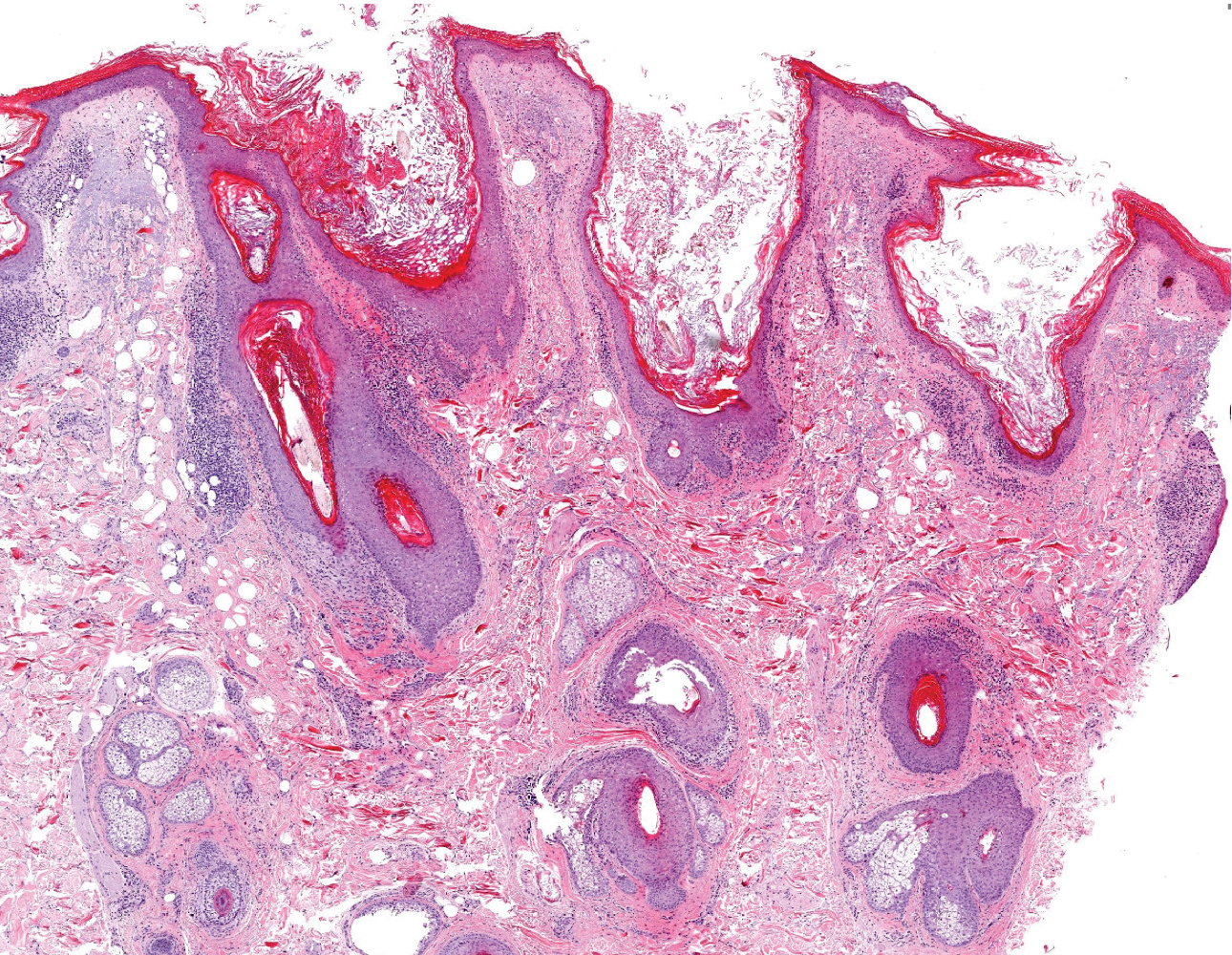
Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.


Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.


Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
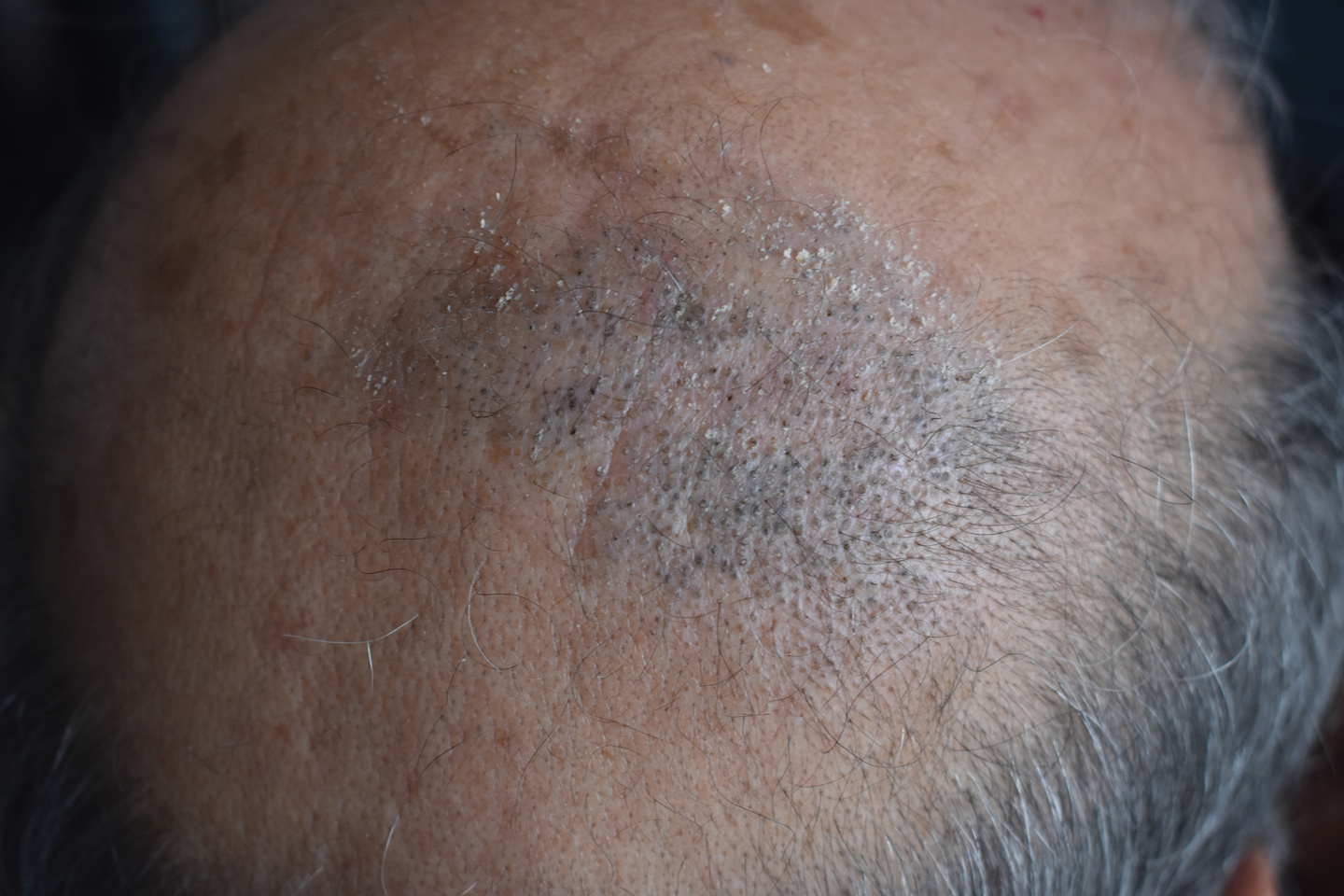
A 50-year-old man presented to the dermatology department with an asymptomatic plaque on the scalp that had been present since childhood. The size of the plaque gradually progressed initially but had notably increased in size in the last 6 months. There was no association with trauma or irritation. There was no family history of similar lesions. Physical examination revealed a 3.0×2.5-cm plaque on the vertex of the scalp consisting of aggregated pits plugged with keratinous material resembling comedones. There were no lesions elsewhere on the body. Dermoscopy and a 4-mm punch biopsy were performed.
Multiple Yellow-Brown Papules on the Penis
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.
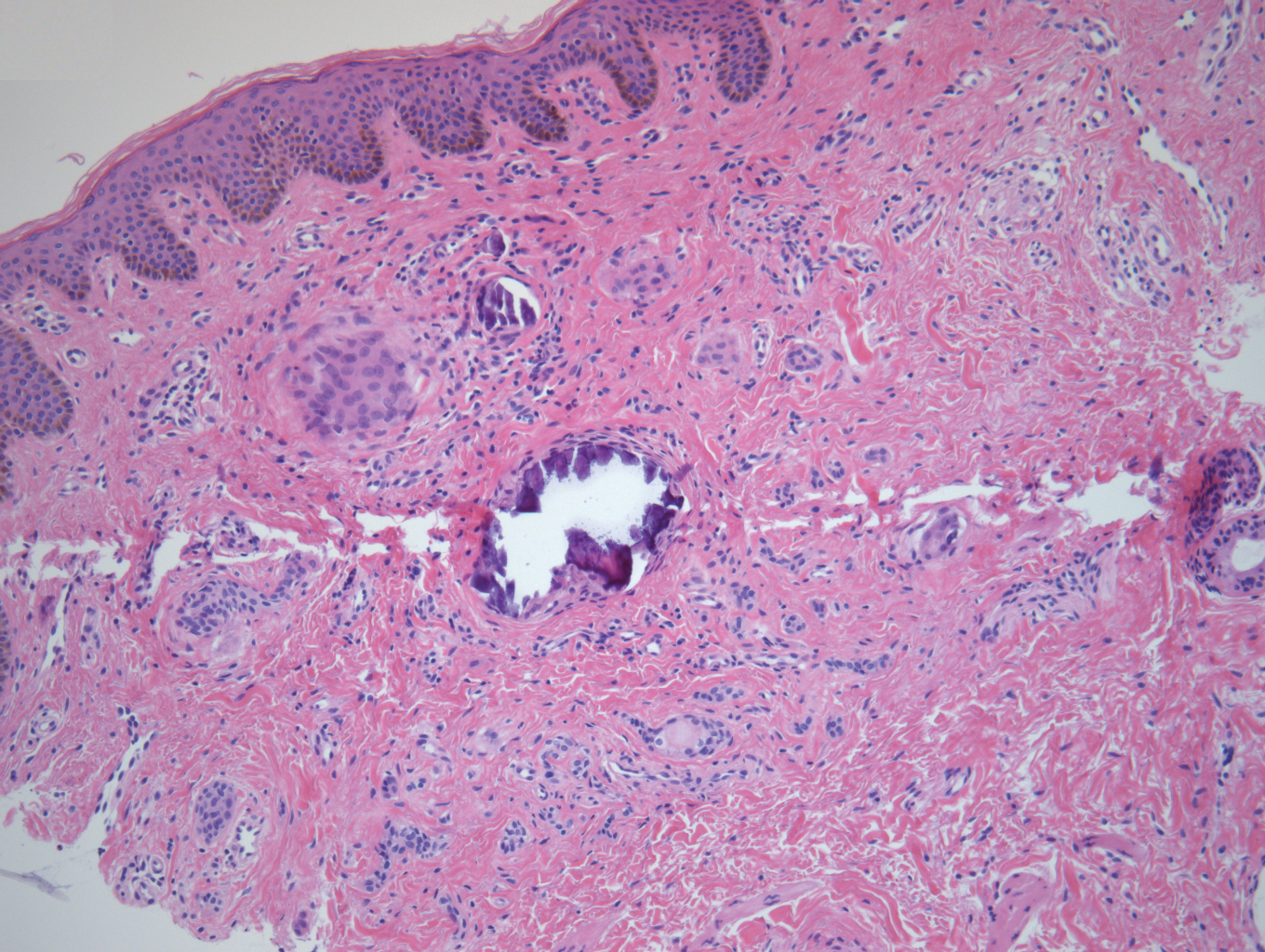
Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.

Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.

Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
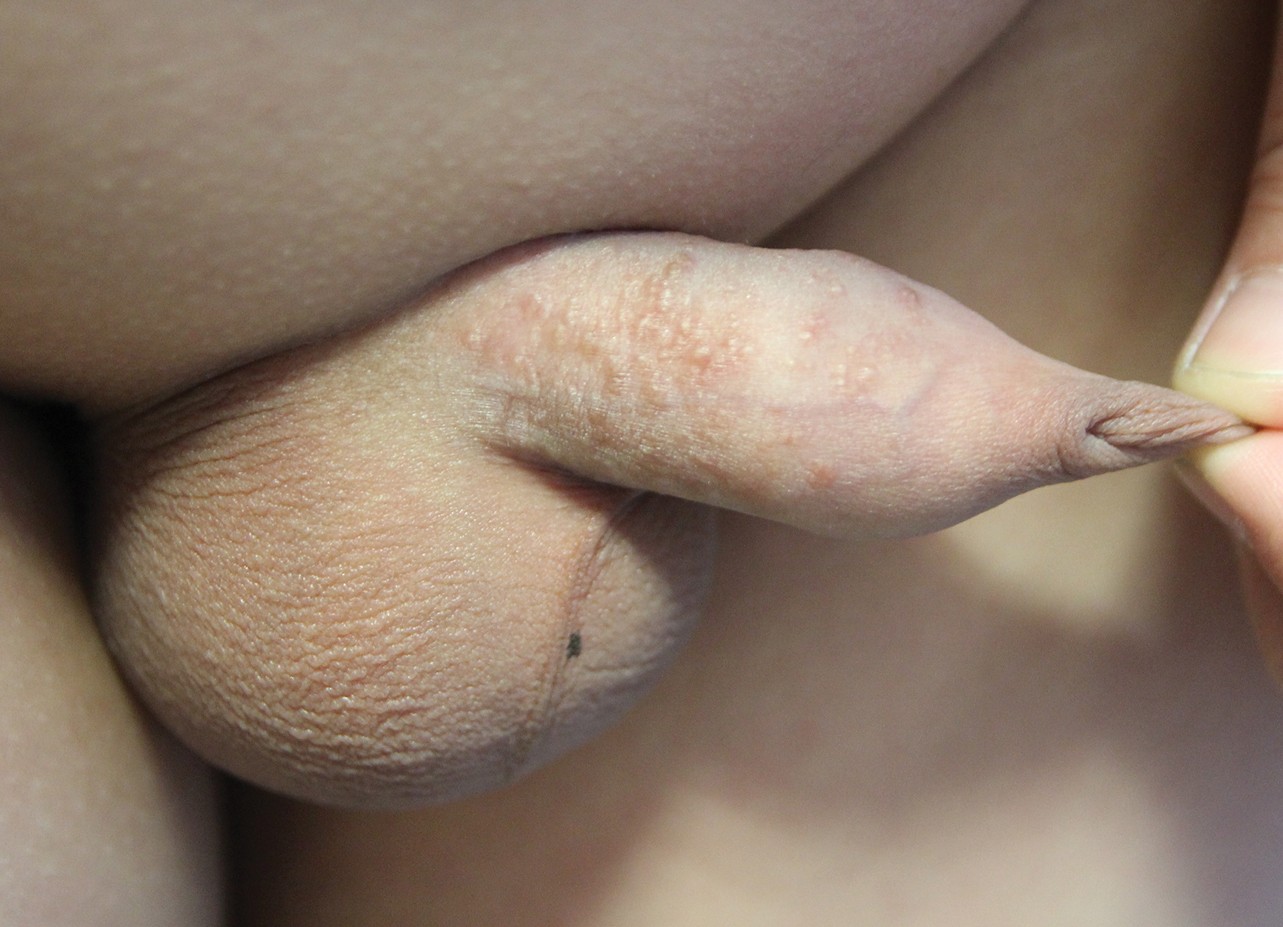
A 12-year-old boy presented with multiple asymptomatic, 0.1-cm, yellow-brown papules on the penile shaft of several years' duration. The lesions appeared suddenly. The patient had no history of trauma, injection, or an underlying disorder.
Erythematous Plaques on a Tattoo
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination demonstrated acanthosis and coarse hypergranulosis with enlarged keratinocytes exhibiting blue cytoplasmic discoloration (Figure), which was suggestive of acquired epidermodysplasia verruciformis (EV).

Acquired EV is a rare dermatologic condition associated with specific human papillomavirus (HPV) types that presents with recalcitrant lesions most commonly in the setting of immunosuppression.1 The most common HPV types associated with EV are HPV-5 and -8, but associations with HPV-3, -9, -10, -12, -14, -15, -17, -19 to -25, -36 to -38, -47, and -50 also have been reported.1,2 Acquired EV has been identified in individuals with human immunodeficiency virus, as well as in immunosuppressed patients with organ transplantation, Hodgkin lymphoma, systemic lupus erythematosus, and IgM deficiency, and in patients taking immunosuppressive medications such as tumor necrosis factor α inhibitors.1,3 The diagnosis is clinicopathological with potential polymerase chain reaction studies to identify underlying HPV types.
Acquired EV presents as hypopigmented to red, tinea versicolor-like macules or as verrucous, flat-topped papules on the trunk, arms, and/or legs.4 Histopathology reveals viral epidermal cytopathic changes, blue cytoplasm, and coarse hypogranulosis.4
There is no standardized treatment regimen for acquired EV, and no single approach has proven to yield an efficacious clinical outcome. Topical treatment options include steroids, retinoids, immunomodulators, cryotherapy, and electrosurgery, whereas retinoids or interferon alfa have been used as oral systemic therapy. Photodynamic therapy also has been shown to improve symptoms.3 Combination therapy such as interferon alfa with zidovudine or imiquimod with oral isotretinoin has shown better results than any single treatment.4 Due to the underlying HPV infection and its role in promotion of skin cancer development, lesions can characteristically undergo malignant transformations into Bowen disease but most commonly invasive squamous cell carcinoma (SCC), with initial lesions preferentially affecting sun-exposed areas due to the synergistic effect of UV light with EV-HPV lesions. The EV-HPV strains 5, 8, and 41 carry the highest oncogenic potential.5 Little is known of the true incidence of oncogenicity for acquired EV. Regardless, consistent sun protection and lifelong clinical examinations are critical for prognosis.5
The differential diagnosis of EV presenting in a tattoo includes allergic contact dermatitis, cutaneous sarcoidosis, pityriasis versicolor, and SCC. The pathology is critical to differentiate between these entities. The most frequently reported skin reactions to tattoo ink include inflammatory diseases (eg, allergic contact dermatitis, granulomatous reaction) or infectious diseases (eg, bacterial, viral, fungal).6 Allergic contact dermatitis, typically red pigment, is a common tattoo reaction. The most common histologic feature, however, is spongiosis, which results from intercellular edema. It often is limited to the lower epidermis but may affect the upper layers if the reaction is severe.7 Cutaneous sarcoidosis is a great masquerader that can present in various ways; however, its salient features on pathology are noncaseating granuloma involving the basal cell layer and epithelioid granuloma consisting of Langerhans giant cells.8 Although pityriasis versicolor can present in young immunocompromised adults, histologically salient features are the presence of both spores and hyphae in the stratum corneum.9 Although immunosuppression is a known risk factor for SCC, it is characterized histologically by hyperkeratosis, parakeratosis, and acanthosis with thickened and elongated rete ridges. Scattered atypical cells and frequent mitoses are present.10
- Schultz B, Nguyen CV, Jacobson-Dunlop E. Acquired epidermodysplasia verruciformis in setting of tumor necrosis factor-α inhibitor therapy. J Am Acad Dermatol Case Rep. 2018;4:805-807.
- DeVilliers EM, Fauquet C, Brocker TR, et al. Classification of papillomaviruses. Virology. 2004;324:17-27.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Berk DR, Bruckner AL, Lu D. Epidermodysplasia verruciform-like lesions in an HIV patient. Dermatol Online J. 2009;15:1.
- Napolitano M, Megna M, Cappello M, et al. Skin diseases and tattoos: a five-year experience. G Ital Dermatol Venereol. 2018;153:644-648.
- Nixon RL, Mowad CM, Marks JG Jr. Allergic contact dermatitis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:242-259.
- Ferringer T. Granulomatous and histiocytic diseases. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. China: Elsevier; 2019:175-176.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1329-1346.
- Soyer HP, Rigel DS, McMeniman E. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1887-1884.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination demonstrated acanthosis and coarse hypergranulosis with enlarged keratinocytes exhibiting blue cytoplasmic discoloration (Figure), which was suggestive of acquired epidermodysplasia verruciformis (EV).

Acquired EV is a rare dermatologic condition associated with specific human papillomavirus (HPV) types that presents with recalcitrant lesions most commonly in the setting of immunosuppression.1 The most common HPV types associated with EV are HPV-5 and -8, but associations with HPV-3, -9, -10, -12, -14, -15, -17, -19 to -25, -36 to -38, -47, and -50 also have been reported.1,2 Acquired EV has been identified in individuals with human immunodeficiency virus, as well as in immunosuppressed patients with organ transplantation, Hodgkin lymphoma, systemic lupus erythematosus, and IgM deficiency, and in patients taking immunosuppressive medications such as tumor necrosis factor α inhibitors.1,3 The diagnosis is clinicopathological with potential polymerase chain reaction studies to identify underlying HPV types.
Acquired EV presents as hypopigmented to red, tinea versicolor-like macules or as verrucous, flat-topped papules on the trunk, arms, and/or legs.4 Histopathology reveals viral epidermal cytopathic changes, blue cytoplasm, and coarse hypogranulosis.4
There is no standardized treatment regimen for acquired EV, and no single approach has proven to yield an efficacious clinical outcome. Topical treatment options include steroids, retinoids, immunomodulators, cryotherapy, and electrosurgery, whereas retinoids or interferon alfa have been used as oral systemic therapy. Photodynamic therapy also has been shown to improve symptoms.3 Combination therapy such as interferon alfa with zidovudine or imiquimod with oral isotretinoin has shown better results than any single treatment.4 Due to the underlying HPV infection and its role in promotion of skin cancer development, lesions can characteristically undergo malignant transformations into Bowen disease but most commonly invasive squamous cell carcinoma (SCC), with initial lesions preferentially affecting sun-exposed areas due to the synergistic effect of UV light with EV-HPV lesions. The EV-HPV strains 5, 8, and 41 carry the highest oncogenic potential.5 Little is known of the true incidence of oncogenicity for acquired EV. Regardless, consistent sun protection and lifelong clinical examinations are critical for prognosis.5
The differential diagnosis of EV presenting in a tattoo includes allergic contact dermatitis, cutaneous sarcoidosis, pityriasis versicolor, and SCC. The pathology is critical to differentiate between these entities. The most frequently reported skin reactions to tattoo ink include inflammatory diseases (eg, allergic contact dermatitis, granulomatous reaction) or infectious diseases (eg, bacterial, viral, fungal).6 Allergic contact dermatitis, typically red pigment, is a common tattoo reaction. The most common histologic feature, however, is spongiosis, which results from intercellular edema. It often is limited to the lower epidermis but may affect the upper layers if the reaction is severe.7 Cutaneous sarcoidosis is a great masquerader that can present in various ways; however, its salient features on pathology are noncaseating granuloma involving the basal cell layer and epithelioid granuloma consisting of Langerhans giant cells.8 Although pityriasis versicolor can present in young immunocompromised adults, histologically salient features are the presence of both spores and hyphae in the stratum corneum.9 Although immunosuppression is a known risk factor for SCC, it is characterized histologically by hyperkeratosis, parakeratosis, and acanthosis with thickened and elongated rete ridges. Scattered atypical cells and frequent mitoses are present.10
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination demonstrated acanthosis and coarse hypergranulosis with enlarged keratinocytes exhibiting blue cytoplasmic discoloration (Figure), which was suggestive of acquired epidermodysplasia verruciformis (EV).

Acquired EV is a rare dermatologic condition associated with specific human papillomavirus (HPV) types that presents with recalcitrant lesions most commonly in the setting of immunosuppression.1 The most common HPV types associated with EV are HPV-5 and -8, but associations with HPV-3, -9, -10, -12, -14, -15, -17, -19 to -25, -36 to -38, -47, and -50 also have been reported.1,2 Acquired EV has been identified in individuals with human immunodeficiency virus, as well as in immunosuppressed patients with organ transplantation, Hodgkin lymphoma, systemic lupus erythematosus, and IgM deficiency, and in patients taking immunosuppressive medications such as tumor necrosis factor α inhibitors.1,3 The diagnosis is clinicopathological with potential polymerase chain reaction studies to identify underlying HPV types.
Acquired EV presents as hypopigmented to red, tinea versicolor-like macules or as verrucous, flat-topped papules on the trunk, arms, and/or legs.4 Histopathology reveals viral epidermal cytopathic changes, blue cytoplasm, and coarse hypogranulosis.4
There is no standardized treatment regimen for acquired EV, and no single approach has proven to yield an efficacious clinical outcome. Topical treatment options include steroids, retinoids, immunomodulators, cryotherapy, and electrosurgery, whereas retinoids or interferon alfa have been used as oral systemic therapy. Photodynamic therapy also has been shown to improve symptoms.3 Combination therapy such as interferon alfa with zidovudine or imiquimod with oral isotretinoin has shown better results than any single treatment.4 Due to the underlying HPV infection and its role in promotion of skin cancer development, lesions can characteristically undergo malignant transformations into Bowen disease but most commonly invasive squamous cell carcinoma (SCC), with initial lesions preferentially affecting sun-exposed areas due to the synergistic effect of UV light with EV-HPV lesions. The EV-HPV strains 5, 8, and 41 carry the highest oncogenic potential.5 Little is known of the true incidence of oncogenicity for acquired EV. Regardless, consistent sun protection and lifelong clinical examinations are critical for prognosis.5
The differential diagnosis of EV presenting in a tattoo includes allergic contact dermatitis, cutaneous sarcoidosis, pityriasis versicolor, and SCC. The pathology is critical to differentiate between these entities. The most frequently reported skin reactions to tattoo ink include inflammatory diseases (eg, allergic contact dermatitis, granulomatous reaction) or infectious diseases (eg, bacterial, viral, fungal).6 Allergic contact dermatitis, typically red pigment, is a common tattoo reaction. The most common histologic feature, however, is spongiosis, which results from intercellular edema. It often is limited to the lower epidermis but may affect the upper layers if the reaction is severe.7 Cutaneous sarcoidosis is a great masquerader that can present in various ways; however, its salient features on pathology are noncaseating granuloma involving the basal cell layer and epithelioid granuloma consisting of Langerhans giant cells.8 Although pityriasis versicolor can present in young immunocompromised adults, histologically salient features are the presence of both spores and hyphae in the stratum corneum.9 Although immunosuppression is a known risk factor for SCC, it is characterized histologically by hyperkeratosis, parakeratosis, and acanthosis with thickened and elongated rete ridges. Scattered atypical cells and frequent mitoses are present.10
- Schultz B, Nguyen CV, Jacobson-Dunlop E. Acquired epidermodysplasia verruciformis in setting of tumor necrosis factor-α inhibitor therapy. J Am Acad Dermatol Case Rep. 2018;4:805-807.
- DeVilliers EM, Fauquet C, Brocker TR, et al. Classification of papillomaviruses. Virology. 2004;324:17-27.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Berk DR, Bruckner AL, Lu D. Epidermodysplasia verruciform-like lesions in an HIV patient. Dermatol Online J. 2009;15:1.
- Napolitano M, Megna M, Cappello M, et al. Skin diseases and tattoos: a five-year experience. G Ital Dermatol Venereol. 2018;153:644-648.
- Nixon RL, Mowad CM, Marks JG Jr. Allergic contact dermatitis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:242-259.
- Ferringer T. Granulomatous and histiocytic diseases. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. China: Elsevier; 2019:175-176.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1329-1346.
- Soyer HP, Rigel DS, McMeniman E. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1887-1884.
- Schultz B, Nguyen CV, Jacobson-Dunlop E. Acquired epidermodysplasia verruciformis in setting of tumor necrosis factor-α inhibitor therapy. J Am Acad Dermatol Case Rep. 2018;4:805-807.
- DeVilliers EM, Fauquet C, Brocker TR, et al. Classification of papillomaviruses. Virology. 2004;324:17-27.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Berk DR, Bruckner AL, Lu D. Epidermodysplasia verruciform-like lesions in an HIV patient. Dermatol Online J. 2009;15:1.
- Napolitano M, Megna M, Cappello M, et al. Skin diseases and tattoos: a five-year experience. G Ital Dermatol Venereol. 2018;153:644-648.
- Nixon RL, Mowad CM, Marks JG Jr. Allergic contact dermatitis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:242-259.
- Ferringer T. Granulomatous and histiocytic diseases. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. China: Elsevier; 2019:175-176.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1329-1346.
- Soyer HP, Rigel DS, McMeniman E. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1887-1884.
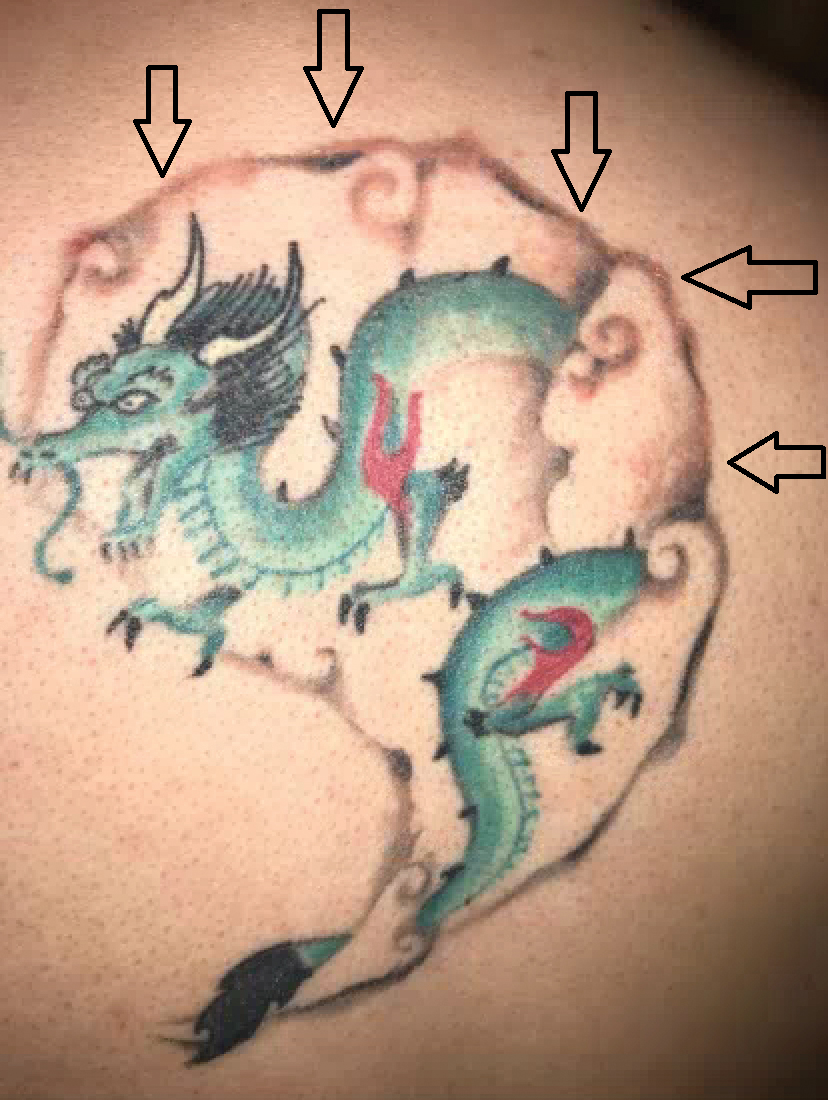
A 29-year-old man presented with increased redness, dryness, and pruritus at the periphery of a tattoo (arrows) on the upper back of 4 months' duration. He was diagnosed with human immunodeficiency virus 8 months prior to presentation and had a history of cystic fibrosis, eczema, and genital molluscum contagiosum. Laboratory analysis 1 month prior revealed a CD4 count of 42 cells/mm3 (reference range, 500-1200 cells/mm3), and the viral load was 2388 copies/mL (reference range, 20-10,000,000 copies/mL). Physical examination revealed multiple erythematous, eczematous, linear plaques along the dark gray lines of the tattoo. A 1.1.2 ×0.7.2 ×0.1-cm shave biopsy specimen was obtained. After the biopsy, tretinoin cream 0.1% and betamethasone dipropionate ointment 0.05% were prescribed to be alternately applied on the tattoo lesions until resolution.
Severe Gingival Swelling and Erythema
The Diagnosis: Plasma Cell Gingivitis
Microscopic analysis demonstrated an acanthotic stratified squamous epithelium with an edematous fibrous stroma containing dense perivascular infiltrates of plasma cells and lymphocytes (Figure 1). Immunohistochemical analysis with kappa, lambda, and CD79a immunostains indicated a polyclonal proliferation of plasma cells that excluded monoclonal plasma cell neoplasia (Figure 2). Direct immunofluorescence (DIF) was negative. Serum enzyme-linked immunosorbent assay for bullous pemphigoid 180 and 230 antibodies as well as desmoglein 1 and 3 antibodies was normal. The cumulative findings were consistent with plasma cell gingivitis (PCG). It was recommended that the patient avoid possible foods (eg, citrus) and oral hygiene products (eg, mint-flavored toothpaste) that could trigger PCG. With patient compliance to an elimination diet for 3 months, the condition resolved (Figure 3).
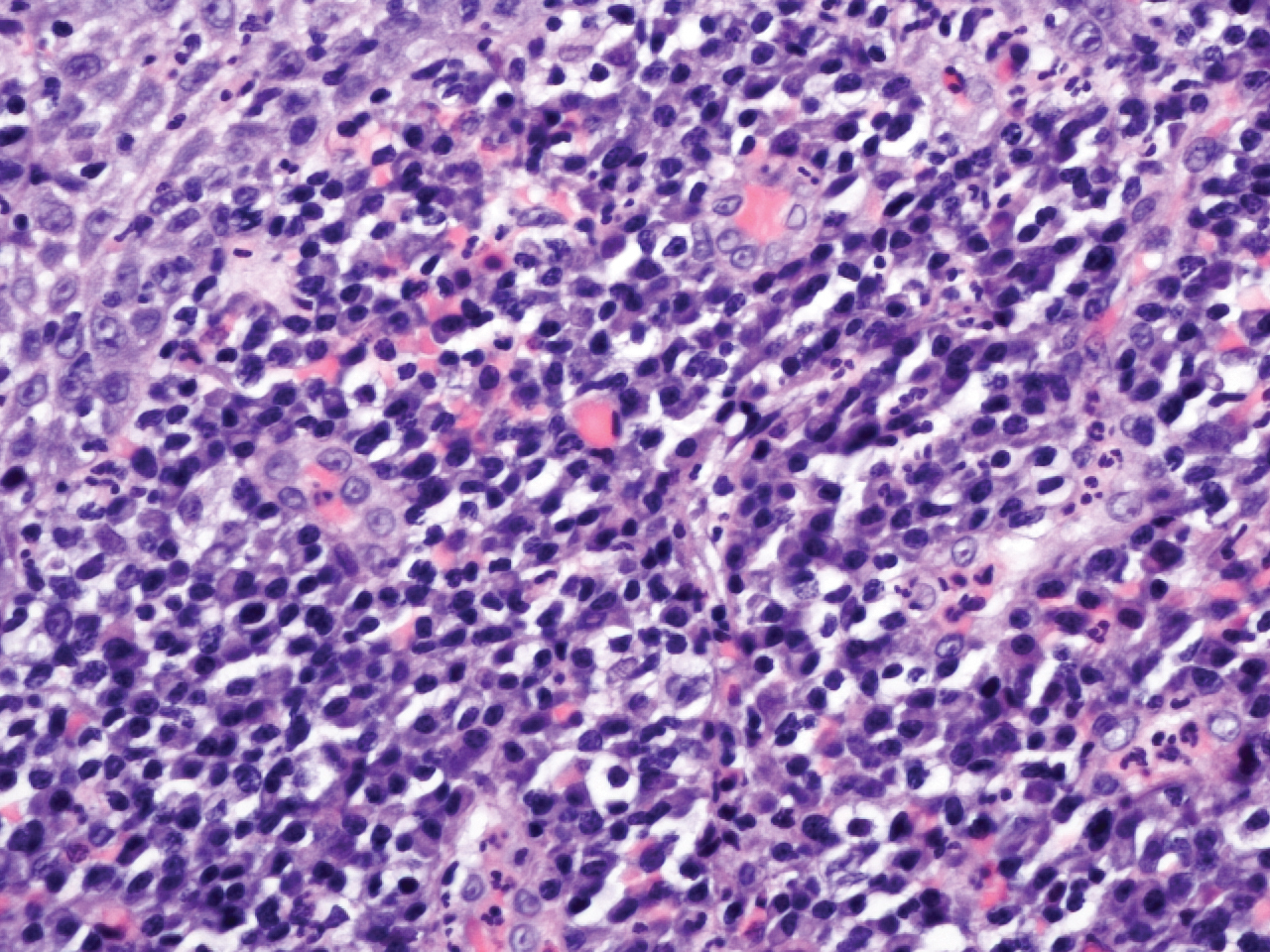
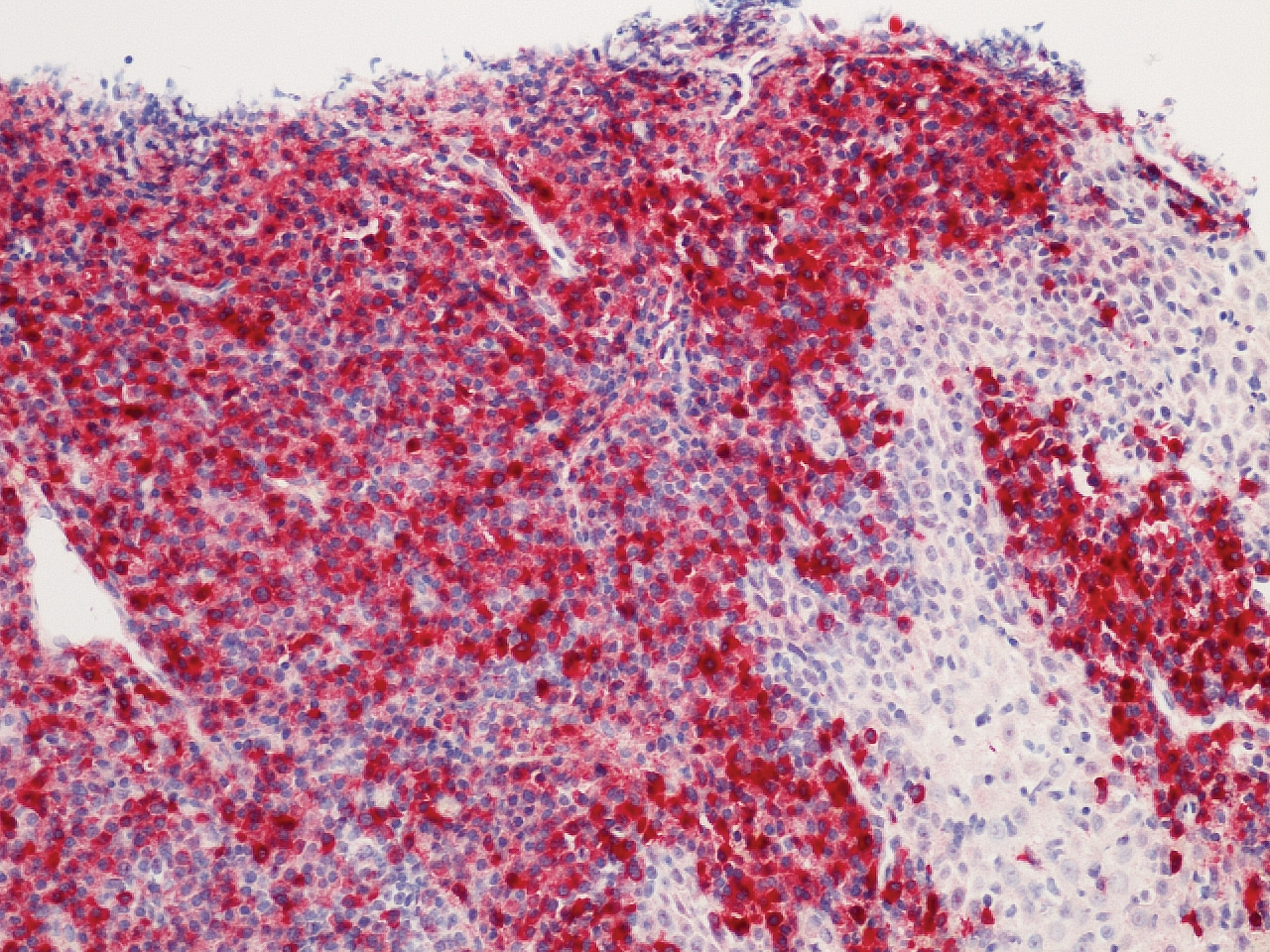
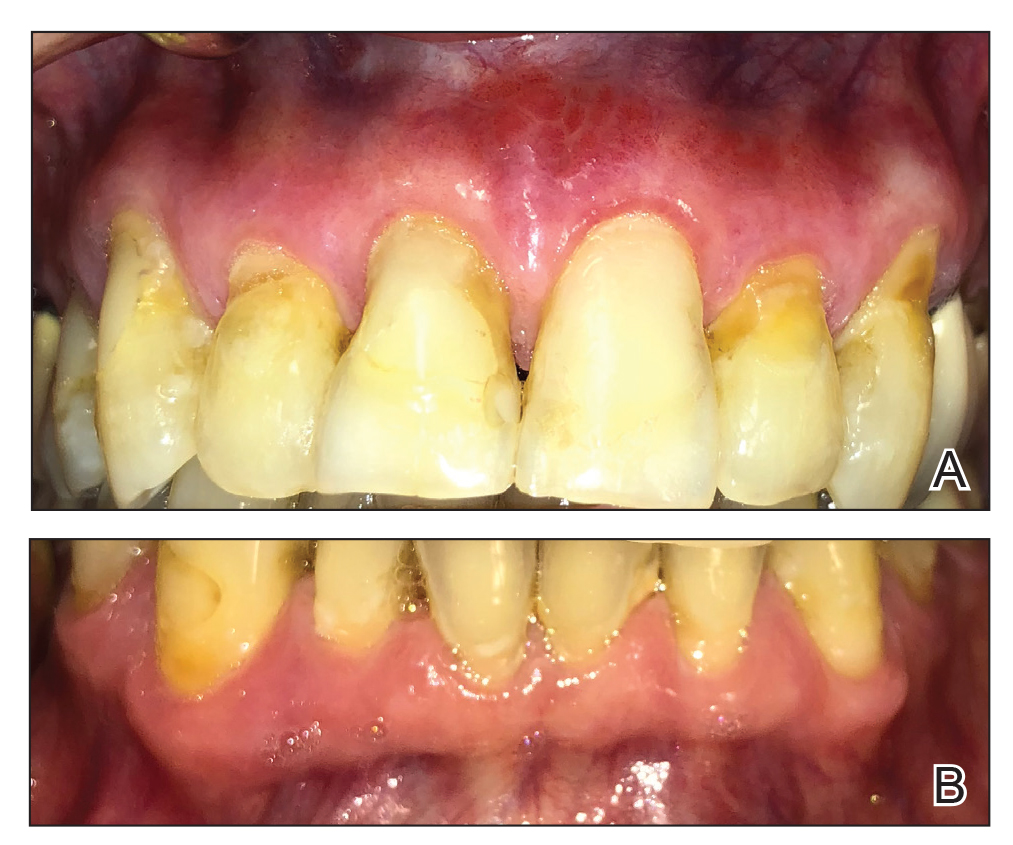
Plasma cell gingivitis is a rare condition characterized by generalized edema and erythema of the attached gingiva. It was described in the 1960s and classified into 3 types based on etiology: (1) hypersensitivity (most common), (2) neoplastic, and (3) PCG of unknown origin.1,2 Spices, herbs, and flavoring agents are implicated as potential triggers of hypersensitivity PCG, while neoplastic PCG is associated with monoclonal plasma cell neoplasms, such as multiple myeloma and extramedullary plasmacytoma.2,3 Histologically, a diffuse subepithelial infiltrate of a polyclonal mixture of plasma cells typically is observed in hypersensitivity PCG.3 The plasma cell infiltration in hypersensitivity PCG is a benign reactive process without known risk for development of plasma cell malignancy, but the presence of a notable number of plasma cells may require special tissue staining to rule out the possibility of associated neoplasia.2,3 There are no standardized protocols for management of PCG.4 Elimination of potential allergens, including flavored oral hygiene products, may result in resolution of hypersensitivity PCG lesions, as exemplified in our patient.1 Neoplastic PCG responds to treatment of the underlying malignancy.5 Topical, intralesional, and/or systemic steroids may be considered in symptomatic cases of PCG.4
Clinical presentation of PCG can mimic immune-mediated mucocutaneous diseases such as mucous membrane pemphigoid (MMP), pemphigus vulgaris (PV), and oral lichen planus; microscopic analysis is needed to establish the diagnosis.6 Mucous membrane pemphigoid is a chronic autoimmune blistering disease involving the mucous membranes with possible cutaneous involvement. It is characterized by a complement-mediated autoantibody process against one or several antigens in the epithelial basement membrane. The oral mucosa is involved in 85% of MMP patients, and 65% of patients experience complications involving the ocular conjunctiva. Intraorally, MMP typically manifests as painful erosions, ulcerations, desquamative gingivitis, and/or occasionally intact blisters. Ocular complications include conjunctivitis and corneal erosions that often scar, resulting in blindness in approximately 15% of patients with ocular involvement. Microscopic features of MMP classically exhibit subepithelial separation with a mixed inflammatory cell infiltrate on routine analysis and linear deposition of IgG, IgA, or C3 within the basement membrane zone on DIF. Treatment of MMP involves topical or systemic immunosuppressants to control symptoms, minimize complications, and alter disease progression.6
Pemphigus vulgaris is an autoimmune vesiculobullous disease that affects the oral mucosa with or without cutaneous involvement.7 Desmogleins 1 and 3, transmembrane glycoproteins of desmosomes that convene cell-to-cell adhesion, are identified as antigens in PV. Antibodies against these desmoglein proteins result in intraepithelial separation, which leads to blister formation.7 Oral manifestations of PV include mucosal erosions and ulcerations as well as desquamative gingivitis. Bullae rarely are seen in the oral cavity, as they tend to rupture, leaving nonhealing ulcerations.8 Histologically, PV is characterized by acantholysis of the suprabasal cell layers with an intact basement membrane zone on routine examination. The distinctive microscopic feature of PV is the detection of cell surface-bound IgG within the epidermis on DIF.7 Treatment of PV may include topical and/or systemic corticosteroids and other immunosuppressants. Rituximab, a monoclonal antibody, has been successful in the management of PV.8
Oral lichen planus is a T-cell mediated autoimmune condition that leads to subepithelial lymphocytic infiltration and excessive keratinocyte apoptosis.9 Women typically are affected more often than men, and 75% of patients also have cutaneous manifestations of the condition. Desquamation and/or erythema of the gingiva may be the initial manifestation of oral lichen planus.9 Other commonly involved sites include the buccal mucosa, tongue, and palate. Biopsy of affected tissues typically demonstrates degeneration of the basal cell layer with subjacent bandlike lymphocytic infiltration on routine staining. Linear fibrinogen at the basement membrane zone usually is observed on DIF. Topical corticosteroids are considered first-line therapy, but systemic therapy including corticosteroids, steroid-sparing agents, or immunomodulators may be used in severe cases.9
There are 3 variants of plasma cell neoplasms including multiple myeloma, medullary plasmacytoma (also known as solitary bone plasmacytoma), and extramedullary plasmacytoma (EMP).10 Extramedullary plasmacytoma, sometimes referred to as extraosseous plasmacytoma, is described as a solitary or multiple plasma cell neoplasm contained in the soft tissue. Its occurrence is rare, accounting for only 3% of plasma cell neoplasms. Approximately 90% of EMPs affect the head and neck region, and males are affected 4 times more often than females. The oral cavity is one of the sites of clinical presentation; the gingival tissue infrequently is affected. When EMP affects the gingiva, it can mimic any form of gingivitis as well as other benign inflammatory conditions, such as pyogenic granuloma. Biopsy is the gold standard diagnostic method for differentiating EMP from other conditions, and specific immunohistochemical stains are essential for the diagnosis. Extramedullary plasmacytoma has the best prognosis among plasma cell neoplasms, despite the risk for progression to multiple myeloma. Extramedullary plasmacytoma lesions are very sensitive to radiotherapy, and the 10-year survival rate is approximately 70%.10
- Sollecito TP, Greenberg MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690-693.
- Gargiulo AV, Ladone JA, Ladone PA, et al. Case report: plasma cell gingivitis A. CDS Rev. 1995;88:22-23.
- Abhishek K, Rashmi J. Plasma cell gingivitis associated with inflammatory cheilitis: a report on a rare case. Ethiop J Health Sci. 2013;23:183-187.
- Arduino PG, D'Aiuto F, Cavallito C, et al. Professional oral hygiene as a therapeutic option for pediatric patients with plasma cell gingivitis: preliminary results of a prospective case series. J Periodontol. 2011;82:1670-1675.
- Nayak A, Nayak MT. Multiple myeloma with an unusual oral presentation. J Exp Ther Oncol. 2016;11:199-206.
- Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:75-97.
- Cizenski JD, Michel P, Watson IT, et al. Spectrum of orocutaneous disease associations: immune-mediated conditions. J Am Acad Dermatol. 2017;77:795-806.
- Stoopler ET, Sollecito TP. Recurrent gingival and oral mucosal lesions. JAMA. 2014;312:1794-1795.
- Nair SK, Faizuddin M, Jayanthi D, et al. Extramedullary plasmacytoma of gingiva and soft tissue in neck. J Clin Diagn Res. 2014;8:ZD16-ZD18.
The Diagnosis: Plasma Cell Gingivitis
Microscopic analysis demonstrated an acanthotic stratified squamous epithelium with an edematous fibrous stroma containing dense perivascular infiltrates of plasma cells and lymphocytes (Figure 1). Immunohistochemical analysis with kappa, lambda, and CD79a immunostains indicated a polyclonal proliferation of plasma cells that excluded monoclonal plasma cell neoplasia (Figure 2). Direct immunofluorescence (DIF) was negative. Serum enzyme-linked immunosorbent assay for bullous pemphigoid 180 and 230 antibodies as well as desmoglein 1 and 3 antibodies was normal. The cumulative findings were consistent with plasma cell gingivitis (PCG). It was recommended that the patient avoid possible foods (eg, citrus) and oral hygiene products (eg, mint-flavored toothpaste) that could trigger PCG. With patient compliance to an elimination diet for 3 months, the condition resolved (Figure 3).



Plasma cell gingivitis is a rare condition characterized by generalized edema and erythema of the attached gingiva. It was described in the 1960s and classified into 3 types based on etiology: (1) hypersensitivity (most common), (2) neoplastic, and (3) PCG of unknown origin.1,2 Spices, herbs, and flavoring agents are implicated as potential triggers of hypersensitivity PCG, while neoplastic PCG is associated with monoclonal plasma cell neoplasms, such as multiple myeloma and extramedullary plasmacytoma.2,3 Histologically, a diffuse subepithelial infiltrate of a polyclonal mixture of plasma cells typically is observed in hypersensitivity PCG.3 The plasma cell infiltration in hypersensitivity PCG is a benign reactive process without known risk for development of plasma cell malignancy, but the presence of a notable number of plasma cells may require special tissue staining to rule out the possibility of associated neoplasia.2,3 There are no standardized protocols for management of PCG.4 Elimination of potential allergens, including flavored oral hygiene products, may result in resolution of hypersensitivity PCG lesions, as exemplified in our patient.1 Neoplastic PCG responds to treatment of the underlying malignancy.5 Topical, intralesional, and/or systemic steroids may be considered in symptomatic cases of PCG.4
Clinical presentation of PCG can mimic immune-mediated mucocutaneous diseases such as mucous membrane pemphigoid (MMP), pemphigus vulgaris (PV), and oral lichen planus; microscopic analysis is needed to establish the diagnosis.6 Mucous membrane pemphigoid is a chronic autoimmune blistering disease involving the mucous membranes with possible cutaneous involvement. It is characterized by a complement-mediated autoantibody process against one or several antigens in the epithelial basement membrane. The oral mucosa is involved in 85% of MMP patients, and 65% of patients experience complications involving the ocular conjunctiva. Intraorally, MMP typically manifests as painful erosions, ulcerations, desquamative gingivitis, and/or occasionally intact blisters. Ocular complications include conjunctivitis and corneal erosions that often scar, resulting in blindness in approximately 15% of patients with ocular involvement. Microscopic features of MMP classically exhibit subepithelial separation with a mixed inflammatory cell infiltrate on routine analysis and linear deposition of IgG, IgA, or C3 within the basement membrane zone on DIF. Treatment of MMP involves topical or systemic immunosuppressants to control symptoms, minimize complications, and alter disease progression.6
Pemphigus vulgaris is an autoimmune vesiculobullous disease that affects the oral mucosa with or without cutaneous involvement.7 Desmogleins 1 and 3, transmembrane glycoproteins of desmosomes that convene cell-to-cell adhesion, are identified as antigens in PV. Antibodies against these desmoglein proteins result in intraepithelial separation, which leads to blister formation.7 Oral manifestations of PV include mucosal erosions and ulcerations as well as desquamative gingivitis. Bullae rarely are seen in the oral cavity, as they tend to rupture, leaving nonhealing ulcerations.8 Histologically, PV is characterized by acantholysis of the suprabasal cell layers with an intact basement membrane zone on routine examination. The distinctive microscopic feature of PV is the detection of cell surface-bound IgG within the epidermis on DIF.7 Treatment of PV may include topical and/or systemic corticosteroids and other immunosuppressants. Rituximab, a monoclonal antibody, has been successful in the management of PV.8
Oral lichen planus is a T-cell mediated autoimmune condition that leads to subepithelial lymphocytic infiltration and excessive keratinocyte apoptosis.9 Women typically are affected more often than men, and 75% of patients also have cutaneous manifestations of the condition. Desquamation and/or erythema of the gingiva may be the initial manifestation of oral lichen planus.9 Other commonly involved sites include the buccal mucosa, tongue, and palate. Biopsy of affected tissues typically demonstrates degeneration of the basal cell layer with subjacent bandlike lymphocytic infiltration on routine staining. Linear fibrinogen at the basement membrane zone usually is observed on DIF. Topical corticosteroids are considered first-line therapy, but systemic therapy including corticosteroids, steroid-sparing agents, or immunomodulators may be used in severe cases.9
There are 3 variants of plasma cell neoplasms including multiple myeloma, medullary plasmacytoma (also known as solitary bone plasmacytoma), and extramedullary plasmacytoma (EMP).10 Extramedullary plasmacytoma, sometimes referred to as extraosseous plasmacytoma, is described as a solitary or multiple plasma cell neoplasm contained in the soft tissue. Its occurrence is rare, accounting for only 3% of plasma cell neoplasms. Approximately 90% of EMPs affect the head and neck region, and males are affected 4 times more often than females. The oral cavity is one of the sites of clinical presentation; the gingival tissue infrequently is affected. When EMP affects the gingiva, it can mimic any form of gingivitis as well as other benign inflammatory conditions, such as pyogenic granuloma. Biopsy is the gold standard diagnostic method for differentiating EMP from other conditions, and specific immunohistochemical stains are essential for the diagnosis. Extramedullary plasmacytoma has the best prognosis among plasma cell neoplasms, despite the risk for progression to multiple myeloma. Extramedullary plasmacytoma lesions are very sensitive to radiotherapy, and the 10-year survival rate is approximately 70%.10
The Diagnosis: Plasma Cell Gingivitis
Microscopic analysis demonstrated an acanthotic stratified squamous epithelium with an edematous fibrous stroma containing dense perivascular infiltrates of plasma cells and lymphocytes (Figure 1). Immunohistochemical analysis with kappa, lambda, and CD79a immunostains indicated a polyclonal proliferation of plasma cells that excluded monoclonal plasma cell neoplasia (Figure 2). Direct immunofluorescence (DIF) was negative. Serum enzyme-linked immunosorbent assay for bullous pemphigoid 180 and 230 antibodies as well as desmoglein 1 and 3 antibodies was normal. The cumulative findings were consistent with plasma cell gingivitis (PCG). It was recommended that the patient avoid possible foods (eg, citrus) and oral hygiene products (eg, mint-flavored toothpaste) that could trigger PCG. With patient compliance to an elimination diet for 3 months, the condition resolved (Figure 3).



Plasma cell gingivitis is a rare condition characterized by generalized edema and erythema of the attached gingiva. It was described in the 1960s and classified into 3 types based on etiology: (1) hypersensitivity (most common), (2) neoplastic, and (3) PCG of unknown origin.1,2 Spices, herbs, and flavoring agents are implicated as potential triggers of hypersensitivity PCG, while neoplastic PCG is associated with monoclonal plasma cell neoplasms, such as multiple myeloma and extramedullary plasmacytoma.2,3 Histologically, a diffuse subepithelial infiltrate of a polyclonal mixture of plasma cells typically is observed in hypersensitivity PCG.3 The plasma cell infiltration in hypersensitivity PCG is a benign reactive process without known risk for development of plasma cell malignancy, but the presence of a notable number of plasma cells may require special tissue staining to rule out the possibility of associated neoplasia.2,3 There are no standardized protocols for management of PCG.4 Elimination of potential allergens, including flavored oral hygiene products, may result in resolution of hypersensitivity PCG lesions, as exemplified in our patient.1 Neoplastic PCG responds to treatment of the underlying malignancy.5 Topical, intralesional, and/or systemic steroids may be considered in symptomatic cases of PCG.4
Clinical presentation of PCG can mimic immune-mediated mucocutaneous diseases such as mucous membrane pemphigoid (MMP), pemphigus vulgaris (PV), and oral lichen planus; microscopic analysis is needed to establish the diagnosis.6 Mucous membrane pemphigoid is a chronic autoimmune blistering disease involving the mucous membranes with possible cutaneous involvement. It is characterized by a complement-mediated autoantibody process against one or several antigens in the epithelial basement membrane. The oral mucosa is involved in 85% of MMP patients, and 65% of patients experience complications involving the ocular conjunctiva. Intraorally, MMP typically manifests as painful erosions, ulcerations, desquamative gingivitis, and/or occasionally intact blisters. Ocular complications include conjunctivitis and corneal erosions that often scar, resulting in blindness in approximately 15% of patients with ocular involvement. Microscopic features of MMP classically exhibit subepithelial separation with a mixed inflammatory cell infiltrate on routine analysis and linear deposition of IgG, IgA, or C3 within the basement membrane zone on DIF. Treatment of MMP involves topical or systemic immunosuppressants to control symptoms, minimize complications, and alter disease progression.6
Pemphigus vulgaris is an autoimmune vesiculobullous disease that affects the oral mucosa with or without cutaneous involvement.7 Desmogleins 1 and 3, transmembrane glycoproteins of desmosomes that convene cell-to-cell adhesion, are identified as antigens in PV. Antibodies against these desmoglein proteins result in intraepithelial separation, which leads to blister formation.7 Oral manifestations of PV include mucosal erosions and ulcerations as well as desquamative gingivitis. Bullae rarely are seen in the oral cavity, as they tend to rupture, leaving nonhealing ulcerations.8 Histologically, PV is characterized by acantholysis of the suprabasal cell layers with an intact basement membrane zone on routine examination. The distinctive microscopic feature of PV is the detection of cell surface-bound IgG within the epidermis on DIF.7 Treatment of PV may include topical and/or systemic corticosteroids and other immunosuppressants. Rituximab, a monoclonal antibody, has been successful in the management of PV.8
Oral lichen planus is a T-cell mediated autoimmune condition that leads to subepithelial lymphocytic infiltration and excessive keratinocyte apoptosis.9 Women typically are affected more often than men, and 75% of patients also have cutaneous manifestations of the condition. Desquamation and/or erythema of the gingiva may be the initial manifestation of oral lichen planus.9 Other commonly involved sites include the buccal mucosa, tongue, and palate. Biopsy of affected tissues typically demonstrates degeneration of the basal cell layer with subjacent bandlike lymphocytic infiltration on routine staining. Linear fibrinogen at the basement membrane zone usually is observed on DIF. Topical corticosteroids are considered first-line therapy, but systemic therapy including corticosteroids, steroid-sparing agents, or immunomodulators may be used in severe cases.9
There are 3 variants of plasma cell neoplasms including multiple myeloma, medullary plasmacytoma (also known as solitary bone plasmacytoma), and extramedullary plasmacytoma (EMP).10 Extramedullary plasmacytoma, sometimes referred to as extraosseous plasmacytoma, is described as a solitary or multiple plasma cell neoplasm contained in the soft tissue. Its occurrence is rare, accounting for only 3% of plasma cell neoplasms. Approximately 90% of EMPs affect the head and neck region, and males are affected 4 times more often than females. The oral cavity is one of the sites of clinical presentation; the gingival tissue infrequently is affected. When EMP affects the gingiva, it can mimic any form of gingivitis as well as other benign inflammatory conditions, such as pyogenic granuloma. Biopsy is the gold standard diagnostic method for differentiating EMP from other conditions, and specific immunohistochemical stains are essential for the diagnosis. Extramedullary plasmacytoma has the best prognosis among plasma cell neoplasms, despite the risk for progression to multiple myeloma. Extramedullary plasmacytoma lesions are very sensitive to radiotherapy, and the 10-year survival rate is approximately 70%.10
- Sollecito TP, Greenberg MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690-693.
- Gargiulo AV, Ladone JA, Ladone PA, et al. Case report: plasma cell gingivitis A. CDS Rev. 1995;88:22-23.
- Abhishek K, Rashmi J. Plasma cell gingivitis associated with inflammatory cheilitis: a report on a rare case. Ethiop J Health Sci. 2013;23:183-187.
- Arduino PG, D'Aiuto F, Cavallito C, et al. Professional oral hygiene as a therapeutic option for pediatric patients with plasma cell gingivitis: preliminary results of a prospective case series. J Periodontol. 2011;82:1670-1675.
- Nayak A, Nayak MT. Multiple myeloma with an unusual oral presentation. J Exp Ther Oncol. 2016;11:199-206.
- Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:75-97.
- Cizenski JD, Michel P, Watson IT, et al. Spectrum of orocutaneous disease associations: immune-mediated conditions. J Am Acad Dermatol. 2017;77:795-806.
- Stoopler ET, Sollecito TP. Recurrent gingival and oral mucosal lesions. JAMA. 2014;312:1794-1795.
- Nair SK, Faizuddin M, Jayanthi D, et al. Extramedullary plasmacytoma of gingiva and soft tissue in neck. J Clin Diagn Res. 2014;8:ZD16-ZD18.
- Sollecito TP, Greenberg MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690-693.
- Gargiulo AV, Ladone JA, Ladone PA, et al. Case report: plasma cell gingivitis A. CDS Rev. 1995;88:22-23.
- Abhishek K, Rashmi J. Plasma cell gingivitis associated with inflammatory cheilitis: a report on a rare case. Ethiop J Health Sci. 2013;23:183-187.
- Arduino PG, D'Aiuto F, Cavallito C, et al. Professional oral hygiene as a therapeutic option for pediatric patients with plasma cell gingivitis: preliminary results of a prospective case series. J Periodontol. 2011;82:1670-1675.
- Nayak A, Nayak MT. Multiple myeloma with an unusual oral presentation. J Exp Ther Oncol. 2016;11:199-206.
- Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:75-97.
- Cizenski JD, Michel P, Watson IT, et al. Spectrum of orocutaneous disease associations: immune-mediated conditions. J Am Acad Dermatol. 2017;77:795-806.
- Stoopler ET, Sollecito TP. Recurrent gingival and oral mucosal lesions. JAMA. 2014;312:1794-1795.
- Nair SK, Faizuddin M, Jayanthi D, et al. Extramedullary plasmacytoma of gingiva and soft tissue in neck. J Clin Diagn Res. 2014;8:ZD16-ZD18.
A 62-year-old man presented to an oral medicine specialist with gingival inflammation of at least 1 year's duration. He reported mild discomfort when consuming spicy foods and denied associated extraoral lesions. His medical history revealed hypertension, hypothyroidism, and psoriasis. Medications included lisinopril 10 mg and levothyroxine 100 µg daily. No known drug allergies were reported. His family and social history were noncontributory, and a detailed review of systems was unremarkable. Extraoral examination revealed no lymphadenopathy, salivary gland enlargement, or thyromegaly. Intraoral examination revealed diffuse enlargement of the maxillary and mandibular gingiva accompanied by severe erythema and bleeding on provocation. A 3-mm punch biopsy of the gingiva was performed for routine analysis and direct immunofluorescence.