User login
Using Superficial Curettage to Diagnose Talon Noir
Using Superficial Curettage to Diagnose Talon Noir
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
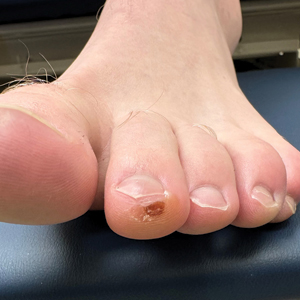
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
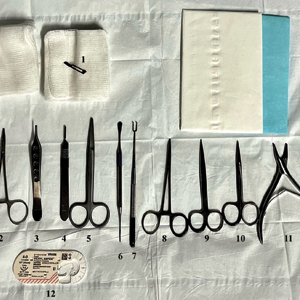
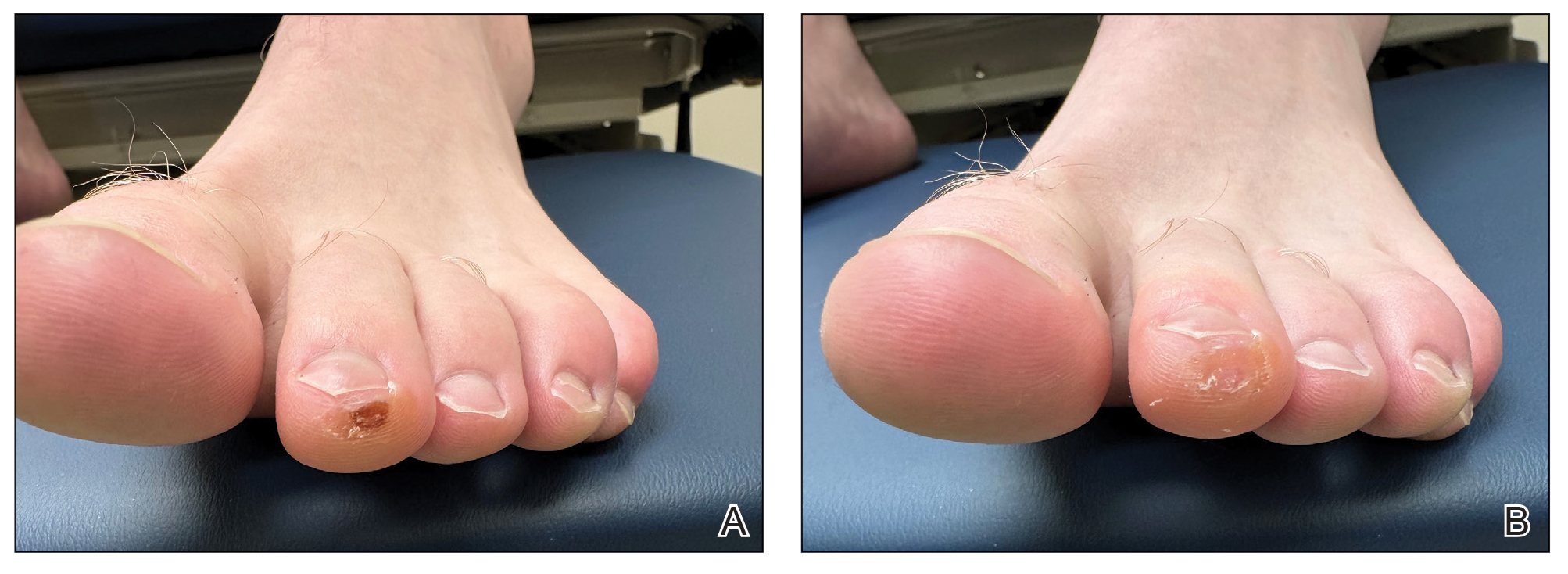
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.
Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.

Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.

Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
Using Superficial Curettage to Diagnose Talon Noir
Using Superficial Curettage to Diagnose Talon Noir
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Practice Gap
Dermatology encompasses a wide range of procedures performed in both clinical and surgical settings. One comprehensive review of ergonomics in dermatologic surgery found a high prevalence of musculoskeletal injuries (MSIs).1 A survey conducted in 2010 revealed that 90% of dermatologic surgeons experienced MSIs, which commonly resulted in neck, shoulder, and/or back pain.2
Prolonged abnormal static postures and repetitive motions, which are common in dermatologic practice, can lead to muscle imbalances and focal muscular ischemia, increasing physicians’ susceptibility to MSIs. When muscle fibers experience enough repeated focal ischemia, they may enter a constant state of contraction leading to myofascial pain syndrome (MPS); these painful areas are known as trigger points and often are refractory to traditional stretching.3
Musculoskeletal injuries can potentially impact dermatologists’ career longevity and satisfaction. To date, the literature on techniques and exercises that may prevent or alleviate MSIs is limited.1,4 We collaborated with a colleague in physical therapy (R.P.) to present stretching, mobility, and strengthening techniques and exercises dermatologists can perform both in and outside the procedure room to potentially reduce pain and prevent future MSIs.
The Techniques
Stretching and Mobility Exercises—When dermatologists adopt abnormal static postures, they are at risk for muscular imbalances caused by repetitive flexion and/or rotation in one direction. Over time, these repetitive movements can result in loss of flexibility in the direction opposite to that in which they are consistently positioned.3 Regular stretching offers physiologic benefits such as maintaining joint range of motion, increasing blood flow to muscles, and increasing synovial fluid production—all of which contribute to reduced risk for MSIs.3 Multiple studies and a systematic review have found that regular stretching throughout the day serves as an effective method for preventing and mitigating MSI pain in health care providers.1,3-5
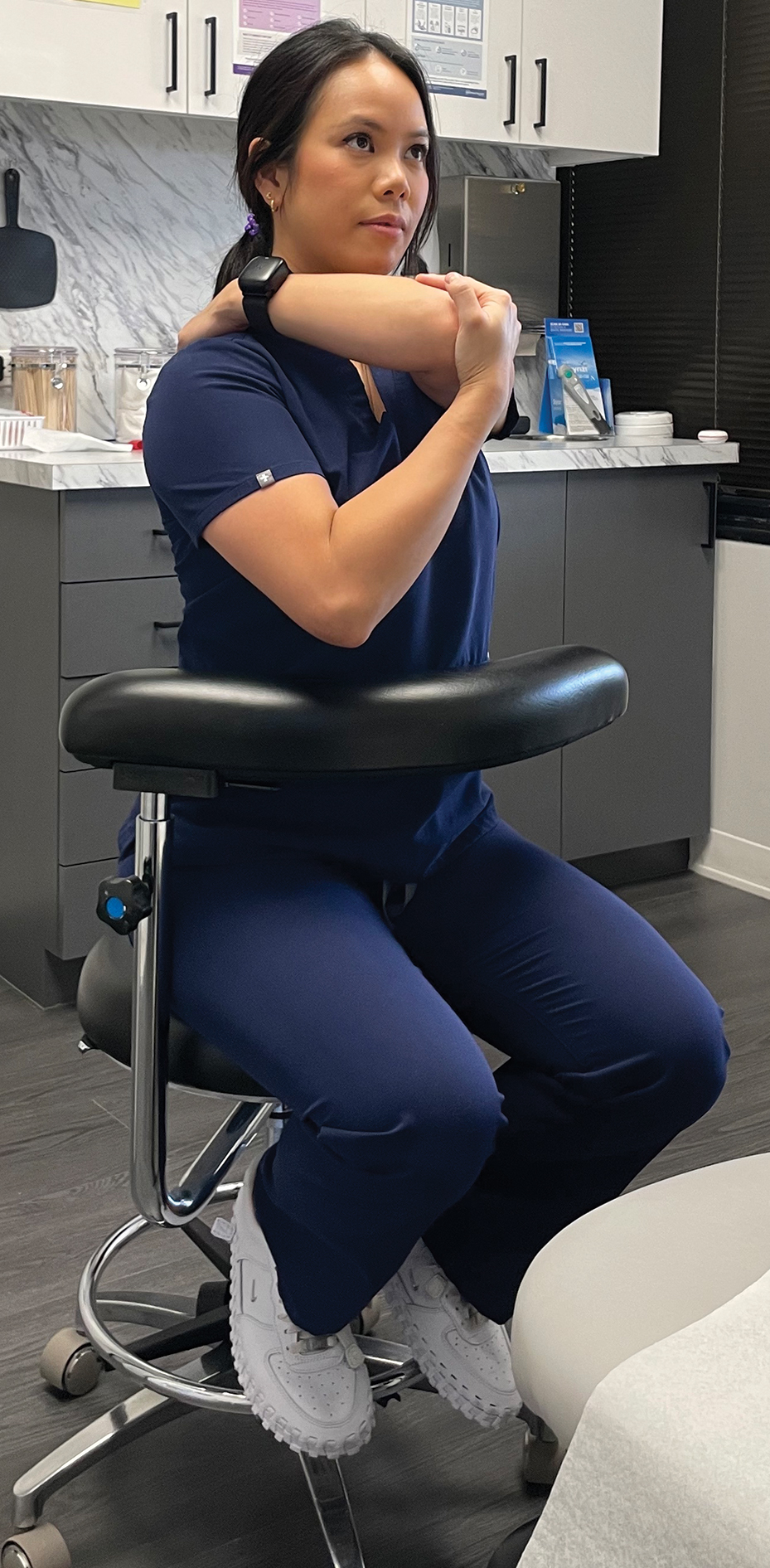
Considering the directional manner of MSIs induced by prolonged static positions, the most benefit will be derived from stretches or extension in the opposite direction of that in which the practitioner usually works. For most dermatologic surgeons, stretches should target the trapezius muscles, shoulders, and cervical musculature. Techniques such as the neck and shoulder combination stretch, the upper trapezius stretch, and the downward shoulder blade squeeze stretch can be performed regularly throughout the day.3,4 To perform the neck and shoulder combination stretch, place the arm in flexion to shoulder height and bend the elbow at a 90° angle. Gently pull the arm across the front of the body, point the head gazing in the direction of the shoulder being stretched, and hold for 10 to 20 seconds. Repeat with the other side (eFigure 1).
Some surgeons may experience pain that is refractory to stretching, potentially indicating the presence of MPS.3 Managing MPS via stretching alone may be a challenge. Physical therapists utilize various techniques to manually massage the tissue, but self-myofascial release—which involves the use of a tool such as a dense foam roller or massage ball, both of which can easily be purchased—may be convenient and effective for busy providers. To perform this technique, the operator lies with their back on a dense foam roller positioned perpendicular to the body and uses their legs to undulate or roll back and forth in a smooth motion (Figure 1). This may help to alleviate myofascial pain in the spinal intrinsic muscles, which often are prone to injury due to posture; it also warms the fascia and breaks up adhesions. Self-myofascial release may have similar acute analgesic effects to classic stretching while also helping to alleviate MPS.

Strengthening Exercises—Musculoskeletal injuries often begin with fatigue in postural stabilizing muscles of the trunk and shoulders, leading the dermatologist to assume a slouched posture. Dermatologists should perform strengthening exercises targeting the trunk and shoulder girdle, which help to promote good working posture while optimizing the function of the arms and hands. Ideally, dermatologists should incorporate strengthening exercises 3 to 4 times per week in combination with daily stretching.
The 4-point kneeling alternate arm and leg extensions technique targets many muscle groups that commonly are affected in dermatologists and dermatologic surgeons. While on all fours, the operator positions the hands under the shoulders and the knees under the hips. The neck remains in line with the back with the eyes facing the floor. The abdominal muscles are then pulled up and in while simultaneously extending the left arm and right leg until both are parallel to the floor. This position should be held for 5 seconds and then repeated with the opposite contralateral extremities (Figure 2). Exercises specific to each muscle group also can be performed, such as planks to enhance truncal stability or scapular wall clocks to strengthen the shoulder girdle (eFigure 2). To perform scapular wall clocks, wrap a single resistance band around both wrists. Next, press the hands and elbows gently into a wall pointing superiorly and imagine there is a clock on the wall with 12 o’clock at the top and 6 o’clock at the bottom. Press the wrists outward on the band, keep the elbows straight, and reach out with the right hand while keeping the left hand stable. Move the right hand to the 1-, 3-, and 5-o’clock positions. Repeat with the left hand while holding the right hand stable. Move the left hand to the 11-, 9-, 7-, and 6-o’clock positions. Repeat these steps for 3 to 5 sets.


It is important to note that a decreased flow of oxygen and nutrients to muscles contributes to MSIs. Aerobic exercises increase blood flow and improve the ability of the muscles to utilize oxygen. Engaging in an enjoyable aerobic activity (eg, walking, running, swimming, cycling) 3 to 4 times per week can help prevent MSIs; however, as with any new exercise regimen (including the strengthening techniques described here), it is important to consult your primary care physician before getting started.
Practice Implications
As dermatologists progress in their careers, implementation of these techniques can mitigate MSIs and their sequelae. The long-term benefits of stretching, mobility, and strengthening exercises are dependent on having ergonomically suitable environmental factors. In addition to their own mechanics and posture, dermatologists must consider all elements that may affect the ergonomics of their daily practice, including operating room layout, instrumentation and workflow, and patient positioning. Through a consistent approach to prevention using the techniques described here, dermatologists can minimize the risk for MSIs and foster sustainability in their careers.
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097 /DSS.0000000000002295
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248. doi:10.1111/j.1524-4725.2011.02237.x
- Valachi B, Valachi K. Preventing musculoskeletal disorders in clinical dentistry: strategies to address the mechanisms leading to musculoskeletal disorders. J Am Dent Assoc. 2003;134:1604-1612. doi:10.14219/jada.archive.2003.0106
- Carley SK, Strauss JD, Vidal NY. Ergonomic solutions for dermatologists. Int J Womens Dermatol. 2021;7(5 part B):863-866. doi:10.1016/j.ijwd.2021.08.006
- da Costa BR, Vieira ER. Stretching to reduce work-related musculoskeletal disorders: a systematic review. J Rehabil Med. 2008;40:321-328. doi:10.2340/16501977-0204
Practice Gap
Dermatology encompasses a wide range of procedures performed in both clinical and surgical settings. One comprehensive review of ergonomics in dermatologic surgery found a high prevalence of musculoskeletal injuries (MSIs).1 A survey conducted in 2010 revealed that 90% of dermatologic surgeons experienced MSIs, which commonly resulted in neck, shoulder, and/or back pain.2
Prolonged abnormal static postures and repetitive motions, which are common in dermatologic practice, can lead to muscle imbalances and focal muscular ischemia, increasing physicians’ susceptibility to MSIs. When muscle fibers experience enough repeated focal ischemia, they may enter a constant state of contraction leading to myofascial pain syndrome (MPS); these painful areas are known as trigger points and often are refractory to traditional stretching.3
Musculoskeletal injuries can potentially impact dermatologists’ career longevity and satisfaction. To date, the literature on techniques and exercises that may prevent or alleviate MSIs is limited.1,4 We collaborated with a colleague in physical therapy (R.P.) to present stretching, mobility, and strengthening techniques and exercises dermatologists can perform both in and outside the procedure room to potentially reduce pain and prevent future MSIs.
The Techniques
Stretching and Mobility Exercises—When dermatologists adopt abnormal static postures, they are at risk for muscular imbalances caused by repetitive flexion and/or rotation in one direction. Over time, these repetitive movements can result in loss of flexibility in the direction opposite to that in which they are consistently positioned.3 Regular stretching offers physiologic benefits such as maintaining joint range of motion, increasing blood flow to muscles, and increasing synovial fluid production—all of which contribute to reduced risk for MSIs.3 Multiple studies and a systematic review have found that regular stretching throughout the day serves as an effective method for preventing and mitigating MSI pain in health care providers.1,3-5
Considering the directional manner of MSIs induced by prolonged static positions, the most benefit will be derived from stretches or extension in the opposite direction of that in which the practitioner usually works. For most dermatologic surgeons, stretches should target the trapezius muscles, shoulders, and cervical musculature. Techniques such as the neck and shoulder combination stretch, the upper trapezius stretch, and the downward shoulder blade squeeze stretch can be performed regularly throughout the day.3,4 To perform the neck and shoulder combination stretch, place the arm in flexion to shoulder height and bend the elbow at a 90° angle. Gently pull the arm across the front of the body, point the head gazing in the direction of the shoulder being stretched, and hold for 10 to 20 seconds. Repeat with the other side (eFigure 1).

Some surgeons may experience pain that is refractory to stretching, potentially indicating the presence of MPS.3 Managing MPS via stretching alone may be a challenge. Physical therapists utilize various techniques to manually massage the tissue, but self-myofascial release—which involves the use of a tool such as a dense foam roller or massage ball, both of which can easily be purchased—may be convenient and effective for busy providers. To perform this technique, the operator lies with their back on a dense foam roller positioned perpendicular to the body and uses their legs to undulate or roll back and forth in a smooth motion (Figure 1). This may help to alleviate myofascial pain in the spinal intrinsic muscles, which often are prone to injury due to posture; it also warms the fascia and breaks up adhesions. Self-myofascial release may have similar acute analgesic effects to classic stretching while also helping to alleviate MPS.

Strengthening Exercises—Musculoskeletal injuries often begin with fatigue in postural stabilizing muscles of the trunk and shoulders, leading the dermatologist to assume a slouched posture. Dermatologists should perform strengthening exercises targeting the trunk and shoulder girdle, which help to promote good working posture while optimizing the function of the arms and hands. Ideally, dermatologists should incorporate strengthening exercises 3 to 4 times per week in combination with daily stretching.
The 4-point kneeling alternate arm and leg extensions technique targets many muscle groups that commonly are affected in dermatologists and dermatologic surgeons. While on all fours, the operator positions the hands under the shoulders and the knees under the hips. The neck remains in line with the back with the eyes facing the floor. The abdominal muscles are then pulled up and in while simultaneously extending the left arm and right leg until both are parallel to the floor. This position should be held for 5 seconds and then repeated with the opposite contralateral extremities (Figure 2). Exercises specific to each muscle group also can be performed, such as planks to enhance truncal stability or scapular wall clocks to strengthen the shoulder girdle (eFigure 2). To perform scapular wall clocks, wrap a single resistance band around both wrists. Next, press the hands and elbows gently into a wall pointing superiorly and imagine there is a clock on the wall with 12 o’clock at the top and 6 o’clock at the bottom. Press the wrists outward on the band, keep the elbows straight, and reach out with the right hand while keeping the left hand stable. Move the right hand to the 1-, 3-, and 5-o’clock positions. Repeat with the left hand while holding the right hand stable. Move the left hand to the 11-, 9-, 7-, and 6-o’clock positions. Repeat these steps for 3 to 5 sets.


It is important to note that a decreased flow of oxygen and nutrients to muscles contributes to MSIs. Aerobic exercises increase blood flow and improve the ability of the muscles to utilize oxygen. Engaging in an enjoyable aerobic activity (eg, walking, running, swimming, cycling) 3 to 4 times per week can help prevent MSIs; however, as with any new exercise regimen (including the strengthening techniques described here), it is important to consult your primary care physician before getting started.
Practice Implications
As dermatologists progress in their careers, implementation of these techniques can mitigate MSIs and their sequelae. The long-term benefits of stretching, mobility, and strengthening exercises are dependent on having ergonomically suitable environmental factors. In addition to their own mechanics and posture, dermatologists must consider all elements that may affect the ergonomics of their daily practice, including operating room layout, instrumentation and workflow, and patient positioning. Through a consistent approach to prevention using the techniques described here, dermatologists can minimize the risk for MSIs and foster sustainability in their careers.
Practice Gap
Dermatology encompasses a wide range of procedures performed in both clinical and surgical settings. One comprehensive review of ergonomics in dermatologic surgery found a high prevalence of musculoskeletal injuries (MSIs).1 A survey conducted in 2010 revealed that 90% of dermatologic surgeons experienced MSIs, which commonly resulted in neck, shoulder, and/or back pain.2
Prolonged abnormal static postures and repetitive motions, which are common in dermatologic practice, can lead to muscle imbalances and focal muscular ischemia, increasing physicians’ susceptibility to MSIs. When muscle fibers experience enough repeated focal ischemia, they may enter a constant state of contraction leading to myofascial pain syndrome (MPS); these painful areas are known as trigger points and often are refractory to traditional stretching.3
Musculoskeletal injuries can potentially impact dermatologists’ career longevity and satisfaction. To date, the literature on techniques and exercises that may prevent or alleviate MSIs is limited.1,4 We collaborated with a colleague in physical therapy (R.P.) to present stretching, mobility, and strengthening techniques and exercises dermatologists can perform both in and outside the procedure room to potentially reduce pain and prevent future MSIs.
The Techniques
Stretching and Mobility Exercises—When dermatologists adopt abnormal static postures, they are at risk for muscular imbalances caused by repetitive flexion and/or rotation in one direction. Over time, these repetitive movements can result in loss of flexibility in the direction opposite to that in which they are consistently positioned.3 Regular stretching offers physiologic benefits such as maintaining joint range of motion, increasing blood flow to muscles, and increasing synovial fluid production—all of which contribute to reduced risk for MSIs.3 Multiple studies and a systematic review have found that regular stretching throughout the day serves as an effective method for preventing and mitigating MSI pain in health care providers.1,3-5
Considering the directional manner of MSIs induced by prolonged static positions, the most benefit will be derived from stretches or extension in the opposite direction of that in which the practitioner usually works. For most dermatologic surgeons, stretches should target the trapezius muscles, shoulders, and cervical musculature. Techniques such as the neck and shoulder combination stretch, the upper trapezius stretch, and the downward shoulder blade squeeze stretch can be performed regularly throughout the day.3,4 To perform the neck and shoulder combination stretch, place the arm in flexion to shoulder height and bend the elbow at a 90° angle. Gently pull the arm across the front of the body, point the head gazing in the direction of the shoulder being stretched, and hold for 10 to 20 seconds. Repeat with the other side (eFigure 1).

Some surgeons may experience pain that is refractory to stretching, potentially indicating the presence of MPS.3 Managing MPS via stretching alone may be a challenge. Physical therapists utilize various techniques to manually massage the tissue, but self-myofascial release—which involves the use of a tool such as a dense foam roller or massage ball, both of which can easily be purchased—may be convenient and effective for busy providers. To perform this technique, the operator lies with their back on a dense foam roller positioned perpendicular to the body and uses their legs to undulate or roll back and forth in a smooth motion (Figure 1). This may help to alleviate myofascial pain in the spinal intrinsic muscles, which often are prone to injury due to posture; it also warms the fascia and breaks up adhesions. Self-myofascial release may have similar acute analgesic effects to classic stretching while also helping to alleviate MPS.

Strengthening Exercises—Musculoskeletal injuries often begin with fatigue in postural stabilizing muscles of the trunk and shoulders, leading the dermatologist to assume a slouched posture. Dermatologists should perform strengthening exercises targeting the trunk and shoulder girdle, which help to promote good working posture while optimizing the function of the arms and hands. Ideally, dermatologists should incorporate strengthening exercises 3 to 4 times per week in combination with daily stretching.
The 4-point kneeling alternate arm and leg extensions technique targets many muscle groups that commonly are affected in dermatologists and dermatologic surgeons. While on all fours, the operator positions the hands under the shoulders and the knees under the hips. The neck remains in line with the back with the eyes facing the floor. The abdominal muscles are then pulled up and in while simultaneously extending the left arm and right leg until both are parallel to the floor. This position should be held for 5 seconds and then repeated with the opposite contralateral extremities (Figure 2). Exercises specific to each muscle group also can be performed, such as planks to enhance truncal stability or scapular wall clocks to strengthen the shoulder girdle (eFigure 2). To perform scapular wall clocks, wrap a single resistance band around both wrists. Next, press the hands and elbows gently into a wall pointing superiorly and imagine there is a clock on the wall with 12 o’clock at the top and 6 o’clock at the bottom. Press the wrists outward on the band, keep the elbows straight, and reach out with the right hand while keeping the left hand stable. Move the right hand to the 1-, 3-, and 5-o’clock positions. Repeat with the left hand while holding the right hand stable. Move the left hand to the 11-, 9-, 7-, and 6-o’clock positions. Repeat these steps for 3 to 5 sets.


It is important to note that a decreased flow of oxygen and nutrients to muscles contributes to MSIs. Aerobic exercises increase blood flow and improve the ability of the muscles to utilize oxygen. Engaging in an enjoyable aerobic activity (eg, walking, running, swimming, cycling) 3 to 4 times per week can help prevent MSIs; however, as with any new exercise regimen (including the strengthening techniques described here), it is important to consult your primary care physician before getting started.
Practice Implications
As dermatologists progress in their careers, implementation of these techniques can mitigate MSIs and their sequelae. The long-term benefits of stretching, mobility, and strengthening exercises are dependent on having ergonomically suitable environmental factors. In addition to their own mechanics and posture, dermatologists must consider all elements that may affect the ergonomics of their daily practice, including operating room layout, instrumentation and workflow, and patient positioning. Through a consistent approach to prevention using the techniques described here, dermatologists can minimize the risk for MSIs and foster sustainability in their careers.
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097 /DSS.0000000000002295
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248. doi:10.1111/j.1524-4725.2011.02237.x
- Valachi B, Valachi K. Preventing musculoskeletal disorders in clinical dentistry: strategies to address the mechanisms leading to musculoskeletal disorders. J Am Dent Assoc. 2003;134:1604-1612. doi:10.14219/jada.archive.2003.0106
- Carley SK, Strauss JD, Vidal NY. Ergonomic solutions for dermatologists. Int J Womens Dermatol. 2021;7(5 part B):863-866. doi:10.1016/j.ijwd.2021.08.006
- da Costa BR, Vieira ER. Stretching to reduce work-related musculoskeletal disorders: a systematic review. J Rehabil Med. 2008;40:321-328. doi:10.2340/16501977-0204
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097 /DSS.0000000000002295
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248. doi:10.1111/j.1524-4725.2011.02237.x
- Valachi B, Valachi K. Preventing musculoskeletal disorders in clinical dentistry: strategies to address the mechanisms leading to musculoskeletal disorders. J Am Dent Assoc. 2003;134:1604-1612. doi:10.14219/jada.archive.2003.0106
- Carley SK, Strauss JD, Vidal NY. Ergonomic solutions for dermatologists. Int J Womens Dermatol. 2021;7(5 part B):863-866. doi:10.1016/j.ijwd.2021.08.006
- da Costa BR, Vieira ER. Stretching to reduce work-related musculoskeletal disorders: a systematic review. J Rehabil Med. 2008;40:321-328. doi:10.2340/16501977-0204
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
PRACTICE GAP
Photography is an essential tool in modern dermatologic practice, aiding in the evaluation, documentation, and monitoring of nevi, skin cancers, and other cutaneous pathologies.1 With the rapid technologic advancement of smartphone cameras, high-quality clinical and dermoscopic images have become increasingly easy to attain; however, best practices for optimizing smartphone photography are limited in the medical literature. We have collated a series of recommendations to help fill this knowledge gap.
A search of PubMed articles indexed for MEDLINE was conducted using the terms clinical imaging AND smartphone, clinical photography AND smartphone, dermatology AND photography, dermatology AND imaging, dermoscopy AND photography, and dermoscopy AND imaging. We also consulted with Elizabeth Seiverling, MD (Annville, Pennsylvania) and Jennifer Stein, MD (New York, New York)—both renowned experts in the fields of dermatology, dermoscopy, and medical photography—via email and video meetings conducted during the period from June 1, 2022, through August 20, 2022. Our goal in creating this guide is to facilitate standardized yet simple ways to integrate smartphone photography into current dermatologic practice.
THE TECHNIQUE
Clinical Photography
Clinical images should be captured in a space with ample indirect natural light, such as a patient examination room with frosted or draped windows, ensuring patient privacy is maintained.1,2 The smartphone’s flash can be used if natural lighting is insufficient, but caution should be exercised when photographing patients with darker skin types, as the flash may create an undesired glare. To combat this, consider using a small clip-on light-emitting diode ring light positioned at a 45° angle for more uniform lighting and reduced glare (eFigures 1 and 2).2 This additional light source helps to distribute light evenly across the patient’s skin, enhancing detail visibility, minimizing harsh shadows, and ensuring a more accurate representation of skin pigmentation.2

When a magnified image is required (eg, to capture suspicious lesions with unique and detailed findings such as irregular borders or atypical pigmentation), use the smartphone’s digital zoom function rather than physically moving the camera lens closer to the subject. Moving the camera too close can cause proximity distortion, artificially enlarging objects close to the lens and degrading the quality of the image.1,2 Unnecessary camera features such as portrait mode, live focus, and filters should be turned off to maintain image accuracy. It also is important to avoid excessive manual adjustments to exposure and brightness settings.1,2 The tap-to-focus feature that is integrated into many smartphone cameras can be utilized to ensure the capture of sharp, focused images. After verifying the image preview on the smartphone display, take the photograph. Immediately review the captured image to ensure it is clear and well lit and accurately depicts the area of interest, including its color, texture, and any relevant details, without glare or distortion. If the image does not meet these criteria, promptly reattempt to achieve the desired quality.
Dermoscopic Photography
Dermoscopy, which enables magnified examination of skin lesions, is increasingly being utilized in dermatology. While traditional dermoscopic photography requires specialized equipment, such as large single-lens reflex cameras with dedicated dermoscopic lens attachments, smartphone cameras now can be used to obtain dermoscopic images of reasonable quality.3,4 Adhering to specific practices can help to optimize the quality of dermoscopic images obtained via this technique.
Before capturing an image, it is essential to prepare both the lesion and the surrounding skin. Ensure the area is cleaned thoroughly and trim any hairs that may obscure the image. Apply an interface fluid such as rubbing alcohol or ultrasonography gel to improve image clarity by reducing surface tension and reflections, minimizing glare, and ensuring even light transmission throughout the lesion.5 As recommended for clinical photography, images should be captured in a space with ample indirect light. For best results, we recommend utilizing the primary photo capture option instead of portrait or panoramic mode or additional settings. It is crucial to disable features such as live focus, filters, night mode, and flash, as they may alter image accuracy; however, use of the tap-to-focus feature or manual settings adjustment is encouraged to ensure a high-resolution photograph.
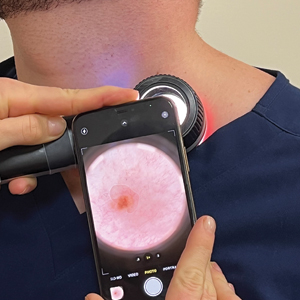
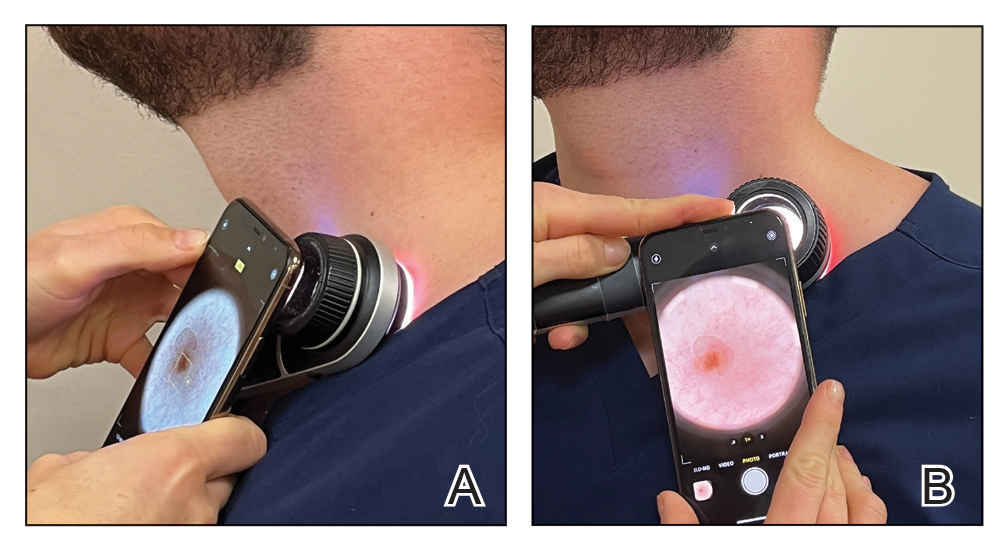
Once these smartphone settings have been verified, position the dermatoscope directly over the lesion of interest. Next, place the smartphone camera lens directly against the eyepiece of the dermatoscope (Figure). Center the lesion in the field of view on the screen. Most smartphones enable adjustment to the image magnification on the photo capture screen. A single tap on the screen should populate the zoom options (eg, ×0.5, ×1, ×3) and allow for adjustment. For the majority of dermoscopic photographs, we recommend standard ×1 magnification, as it typically provides a clear and accurate representation of the lesion without introducing the possibility of image distortion. To obtain a close-up image, use the smartphone’s digital zoom function prior to taking the photograph rather than zooming in on the image after it has been captured; however, to minimize proximity distortion and maintain optimal image quality, avoid exceeding the halfway point on the camera’s zoom dial. After verifying the image preview on the smartphone display, capture the photograph. Immediate review is recommended to allow for prompt reattempt at capturing the image if needed.
PRACTICE IMPLICATIONS
The inherent convenience and accessibility offered by smartphone photography further solidifies its status as a valuable tool in modern dermatologic practice. By adhering to the best practices outlined in this guide, dermatologists can utilize smartphones to capture high-quality clinical and dermoscopic images that support accurate diagnosis and enhance patient care. This approach helps streamline workflows, enhance consistency in image quality, and standardize image capture across different settings and providers.
Additionally, smartphone photography can enhance both education and telemedicine by enabling physicians to easily share high-quality images with colleagues for virtual consultations, second opinions, and collaborative diagnoses. This sharing of images fosters learning opportunities, supports knowledge exchange, and allows for real-time feedback—all of which can improve clinical decision-making. Moreover, it broadens access to dermatologic expertise, strengthens communication between health care providers, and facilitates timely decision-making. As a result, patients benefit from more efficient, accurate, and collaborative care.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123. doi:10.1001 /jamadermatol.2019.3849
- Alvarado SM, Flessland P, Grant-Kels JM, et al. Practical strategies for improving clinical photography of dark skin. J Am Acad Dermatol. 2022;86:E21-E23. doi:10.1016/j.jaad.2021.09.001
- Pagliarello C, Feliciani C, Fantini C, et al. Use of the dermoscope as a smartphone close-up lens and LED annular macro ring flash. J Am Acad Dermatol. 2016;75:E27–E28. doi:10.1016/j.jaad .2015.12.04
- Zuo KJ, Guo D, Rao J. Mobile teledermatology: a promising future in clinical practice. J Cutan Med Surg. 2013;17:387-391. doi:10.2310/7750.2013.13030
- Gewirtzman AJ, Saurat J-H, Braun RP. An evaluation of dermscopy fluids and application techniques. Br J Dermatol. 2003;149:59-63. doi:10.1046/j.1365-2133.2003.05366.x
PRACTICE GAP
Photography is an essential tool in modern dermatologic practice, aiding in the evaluation, documentation, and monitoring of nevi, skin cancers, and other cutaneous pathologies.1 With the rapid technologic advancement of smartphone cameras, high-quality clinical and dermoscopic images have become increasingly easy to attain; however, best practices for optimizing smartphone photography are limited in the medical literature. We have collated a series of recommendations to help fill this knowledge gap.
A search of PubMed articles indexed for MEDLINE was conducted using the terms clinical imaging AND smartphone, clinical photography AND smartphone, dermatology AND photography, dermatology AND imaging, dermoscopy AND photography, and dermoscopy AND imaging. We also consulted with Elizabeth Seiverling, MD (Annville, Pennsylvania) and Jennifer Stein, MD (New York, New York)—both renowned experts in the fields of dermatology, dermoscopy, and medical photography—via email and video meetings conducted during the period from June 1, 2022, through August 20, 2022. Our goal in creating this guide is to facilitate standardized yet simple ways to integrate smartphone photography into current dermatologic practice.
THE TECHNIQUE
Clinical Photography
Clinical images should be captured in a space with ample indirect natural light, such as a patient examination room with frosted or draped windows, ensuring patient privacy is maintained.1,2 The smartphone’s flash can be used if natural lighting is insufficient, but caution should be exercised when photographing patients with darker skin types, as the flash may create an undesired glare. To combat this, consider using a small clip-on light-emitting diode ring light positioned at a 45° angle for more uniform lighting and reduced glare (eFigures 1 and 2).2 This additional light source helps to distribute light evenly across the patient’s skin, enhancing detail visibility, minimizing harsh shadows, and ensuring a more accurate representation of skin pigmentation.2


When a magnified image is required (eg, to capture suspicious lesions with unique and detailed findings such as irregular borders or atypical pigmentation), use the smartphone’s digital zoom function rather than physically moving the camera lens closer to the subject. Moving the camera too close can cause proximity distortion, artificially enlarging objects close to the lens and degrading the quality of the image.1,2 Unnecessary camera features such as portrait mode, live focus, and filters should be turned off to maintain image accuracy. It also is important to avoid excessive manual adjustments to exposure and brightness settings.1,2 The tap-to-focus feature that is integrated into many smartphone cameras can be utilized to ensure the capture of sharp, focused images. After verifying the image preview on the smartphone display, take the photograph. Immediately review the captured image to ensure it is clear and well lit and accurately depicts the area of interest, including its color, texture, and any relevant details, without glare or distortion. If the image does not meet these criteria, promptly reattempt to achieve the desired quality.
Dermoscopic Photography
Dermoscopy, which enables magnified examination of skin lesions, is increasingly being utilized in dermatology. While traditional dermoscopic photography requires specialized equipment, such as large single-lens reflex cameras with dedicated dermoscopic lens attachments, smartphone cameras now can be used to obtain dermoscopic images of reasonable quality.3,4 Adhering to specific practices can help to optimize the quality of dermoscopic images obtained via this technique.
Before capturing an image, it is essential to prepare both the lesion and the surrounding skin. Ensure the area is cleaned thoroughly and trim any hairs that may obscure the image. Apply an interface fluid such as rubbing alcohol or ultrasonography gel to improve image clarity by reducing surface tension and reflections, minimizing glare, and ensuring even light transmission throughout the lesion.5 As recommended for clinical photography, images should be captured in a space with ample indirect light. For best results, we recommend utilizing the primary photo capture option instead of portrait or panoramic mode or additional settings. It is crucial to disable features such as live focus, filters, night mode, and flash, as they may alter image accuracy; however, use of the tap-to-focus feature or manual settings adjustment is encouraged to ensure a high-resolution photograph.
Once these smartphone settings have been verified, position the dermatoscope directly over the lesion of interest. Next, place the smartphone camera lens directly against the eyepiece of the dermatoscope (Figure). Center the lesion in the field of view on the screen. Most smartphones enable adjustment to the image magnification on the photo capture screen. A single tap on the screen should populate the zoom options (eg, ×0.5, ×1, ×3) and allow for adjustment. For the majority of dermoscopic photographs, we recommend standard ×1 magnification, as it typically provides a clear and accurate representation of the lesion without introducing the possibility of image distortion. To obtain a close-up image, use the smartphone’s digital zoom function prior to taking the photograph rather than zooming in on the image after it has been captured; however, to minimize proximity distortion and maintain optimal image quality, avoid exceeding the halfway point on the camera’s zoom dial. After verifying the image preview on the smartphone display, capture the photograph. Immediate review is recommended to allow for prompt reattempt at capturing the image if needed.

PRACTICE IMPLICATIONS
The inherent convenience and accessibility offered by smartphone photography further solidifies its status as a valuable tool in modern dermatologic practice. By adhering to the best practices outlined in this guide, dermatologists can utilize smartphones to capture high-quality clinical and dermoscopic images that support accurate diagnosis and enhance patient care. This approach helps streamline workflows, enhance consistency in image quality, and standardize image capture across different settings and providers.
Additionally, smartphone photography can enhance both education and telemedicine by enabling physicians to easily share high-quality images with colleagues for virtual consultations, second opinions, and collaborative diagnoses. This sharing of images fosters learning opportunities, supports knowledge exchange, and allows for real-time feedback—all of which can improve clinical decision-making. Moreover, it broadens access to dermatologic expertise, strengthens communication between health care providers, and facilitates timely decision-making. As a result, patients benefit from more efficient, accurate, and collaborative care.
PRACTICE GAP
Photography is an essential tool in modern dermatologic practice, aiding in the evaluation, documentation, and monitoring of nevi, skin cancers, and other cutaneous pathologies.1 With the rapid technologic advancement of smartphone cameras, high-quality clinical and dermoscopic images have become increasingly easy to attain; however, best practices for optimizing smartphone photography are limited in the medical literature. We have collated a series of recommendations to help fill this knowledge gap.
A search of PubMed articles indexed for MEDLINE was conducted using the terms clinical imaging AND smartphone, clinical photography AND smartphone, dermatology AND photography, dermatology AND imaging, dermoscopy AND photography, and dermoscopy AND imaging. We also consulted with Elizabeth Seiverling, MD (Annville, Pennsylvania) and Jennifer Stein, MD (New York, New York)—both renowned experts in the fields of dermatology, dermoscopy, and medical photography—via email and video meetings conducted during the period from June 1, 2022, through August 20, 2022. Our goal in creating this guide is to facilitate standardized yet simple ways to integrate smartphone photography into current dermatologic practice.
THE TECHNIQUE
Clinical Photography
Clinical images should be captured in a space with ample indirect natural light, such as a patient examination room with frosted or draped windows, ensuring patient privacy is maintained.1,2 The smartphone’s flash can be used if natural lighting is insufficient, but caution should be exercised when photographing patients with darker skin types, as the flash may create an undesired glare. To combat this, consider using a small clip-on light-emitting diode ring light positioned at a 45° angle for more uniform lighting and reduced glare (eFigures 1 and 2).2 This additional light source helps to distribute light evenly across the patient’s skin, enhancing detail visibility, minimizing harsh shadows, and ensuring a more accurate representation of skin pigmentation.2


When a magnified image is required (eg, to capture suspicious lesions with unique and detailed findings such as irregular borders or atypical pigmentation), use the smartphone’s digital zoom function rather than physically moving the camera lens closer to the subject. Moving the camera too close can cause proximity distortion, artificially enlarging objects close to the lens and degrading the quality of the image.1,2 Unnecessary camera features such as portrait mode, live focus, and filters should be turned off to maintain image accuracy. It also is important to avoid excessive manual adjustments to exposure and brightness settings.1,2 The tap-to-focus feature that is integrated into many smartphone cameras can be utilized to ensure the capture of sharp, focused images. After verifying the image preview on the smartphone display, take the photograph. Immediately review the captured image to ensure it is clear and well lit and accurately depicts the area of interest, including its color, texture, and any relevant details, without glare or distortion. If the image does not meet these criteria, promptly reattempt to achieve the desired quality.
Dermoscopic Photography
Dermoscopy, which enables magnified examination of skin lesions, is increasingly being utilized in dermatology. While traditional dermoscopic photography requires specialized equipment, such as large single-lens reflex cameras with dedicated dermoscopic lens attachments, smartphone cameras now can be used to obtain dermoscopic images of reasonable quality.3,4 Adhering to specific practices can help to optimize the quality of dermoscopic images obtained via this technique.
Before capturing an image, it is essential to prepare both the lesion and the surrounding skin. Ensure the area is cleaned thoroughly and trim any hairs that may obscure the image. Apply an interface fluid such as rubbing alcohol or ultrasonography gel to improve image clarity by reducing surface tension and reflections, minimizing glare, and ensuring even light transmission throughout the lesion.5 As recommended for clinical photography, images should be captured in a space with ample indirect light. For best results, we recommend utilizing the primary photo capture option instead of portrait or panoramic mode or additional settings. It is crucial to disable features such as live focus, filters, night mode, and flash, as they may alter image accuracy; however, use of the tap-to-focus feature or manual settings adjustment is encouraged to ensure a high-resolution photograph.
Once these smartphone settings have been verified, position the dermatoscope directly over the lesion of interest. Next, place the smartphone camera lens directly against the eyepiece of the dermatoscope (Figure). Center the lesion in the field of view on the screen. Most smartphones enable adjustment to the image magnification on the photo capture screen. A single tap on the screen should populate the zoom options (eg, ×0.5, ×1, ×3) and allow for adjustment. For the majority of dermoscopic photographs, we recommend standard ×1 magnification, as it typically provides a clear and accurate representation of the lesion without introducing the possibility of image distortion. To obtain a close-up image, use the smartphone’s digital zoom function prior to taking the photograph rather than zooming in on the image after it has been captured; however, to minimize proximity distortion and maintain optimal image quality, avoid exceeding the halfway point on the camera’s zoom dial. After verifying the image preview on the smartphone display, capture the photograph. Immediate review is recommended to allow for prompt reattempt at capturing the image if needed.

PRACTICE IMPLICATIONS
The inherent convenience and accessibility offered by smartphone photography further solidifies its status as a valuable tool in modern dermatologic practice. By adhering to the best practices outlined in this guide, dermatologists can utilize smartphones to capture high-quality clinical and dermoscopic images that support accurate diagnosis and enhance patient care. This approach helps streamline workflows, enhance consistency in image quality, and standardize image capture across different settings and providers.
Additionally, smartphone photography can enhance both education and telemedicine by enabling physicians to easily share high-quality images with colleagues for virtual consultations, second opinions, and collaborative diagnoses. This sharing of images fosters learning opportunities, supports knowledge exchange, and allows for real-time feedback—all of which can improve clinical decision-making. Moreover, it broadens access to dermatologic expertise, strengthens communication between health care providers, and facilitates timely decision-making. As a result, patients benefit from more efficient, accurate, and collaborative care.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123. doi:10.1001 /jamadermatol.2019.3849
- Alvarado SM, Flessland P, Grant-Kels JM, et al. Practical strategies for improving clinical photography of dark skin. J Am Acad Dermatol. 2022;86:E21-E23. doi:10.1016/j.jaad.2021.09.001
- Pagliarello C, Feliciani C, Fantini C, et al. Use of the dermoscope as a smartphone close-up lens and LED annular macro ring flash. J Am Acad Dermatol. 2016;75:E27–E28. doi:10.1016/j.jaad .2015.12.04
- Zuo KJ, Guo D, Rao J. Mobile teledermatology: a promising future in clinical practice. J Cutan Med Surg. 2013;17:387-391. doi:10.2310/7750.2013.13030
- Gewirtzman AJ, Saurat J-H, Braun RP. An evaluation of dermscopy fluids and application techniques. Br J Dermatol. 2003;149:59-63. doi:10.1046/j.1365-2133.2003.05366.x
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123. doi:10.1001 /jamadermatol.2019.3849
- Alvarado SM, Flessland P, Grant-Kels JM, et al. Practical strategies for improving clinical photography of dark skin. J Am Acad Dermatol. 2022;86:E21-E23. doi:10.1016/j.jaad.2021.09.001
- Pagliarello C, Feliciani C, Fantini C, et al. Use of the dermoscope as a smartphone close-up lens and LED annular macro ring flash. J Am Acad Dermatol. 2016;75:E27–E28. doi:10.1016/j.jaad .2015.12.04
- Zuo KJ, Guo D, Rao J. Mobile teledermatology: a promising future in clinical practice. J Cutan Med Surg. 2013;17:387-391. doi:10.2310/7750.2013.13030
- Gewirtzman AJ, Saurat J-H, Braun RP. An evaluation of dermscopy fluids and application techniques. Br J Dermatol. 2003;149:59-63. doi:10.1046/j.1365-2133.2003.05366.x
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
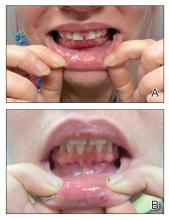
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
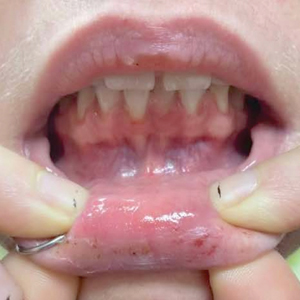
Pinto Bean Pressure Wraps: A Novel Approach to Treating Digital Warts
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.
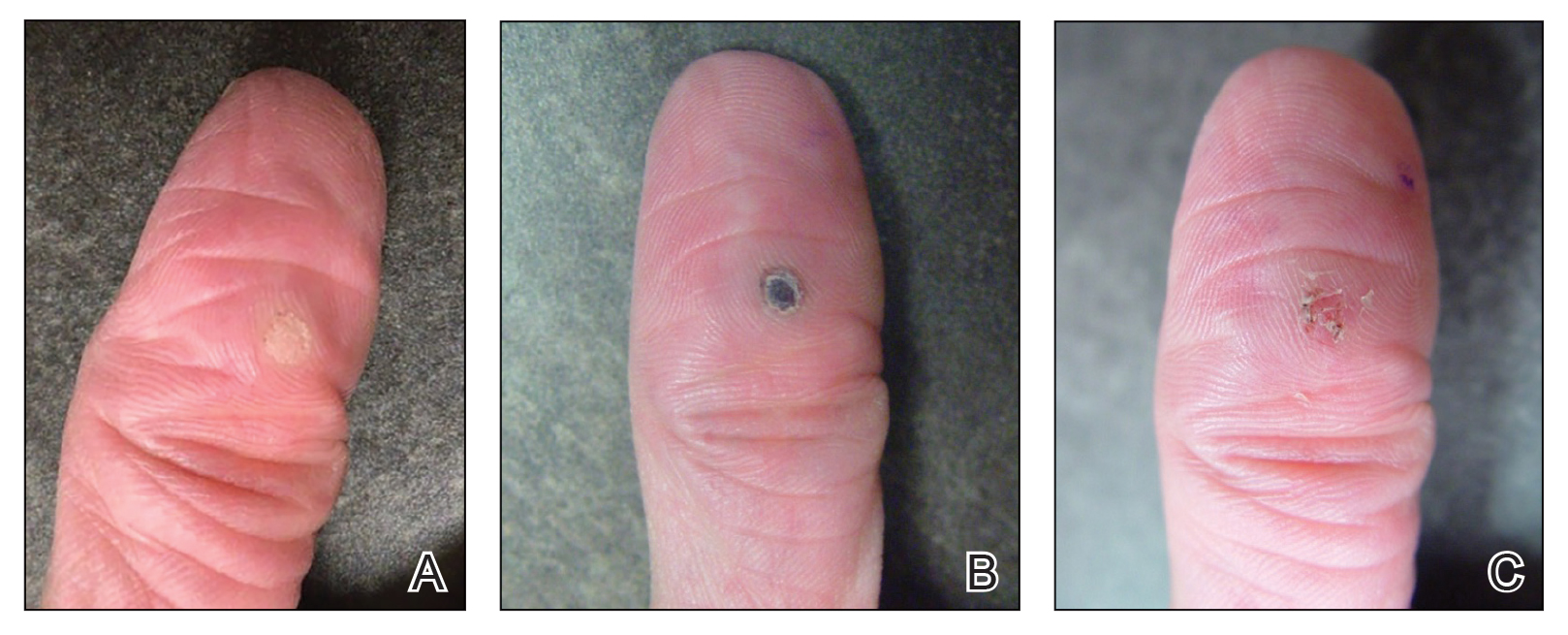
What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.


What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.


What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
Nailing the Nail Biopsy: Surgical Instruments and Their Function in Nail Biopsy Procedures
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.
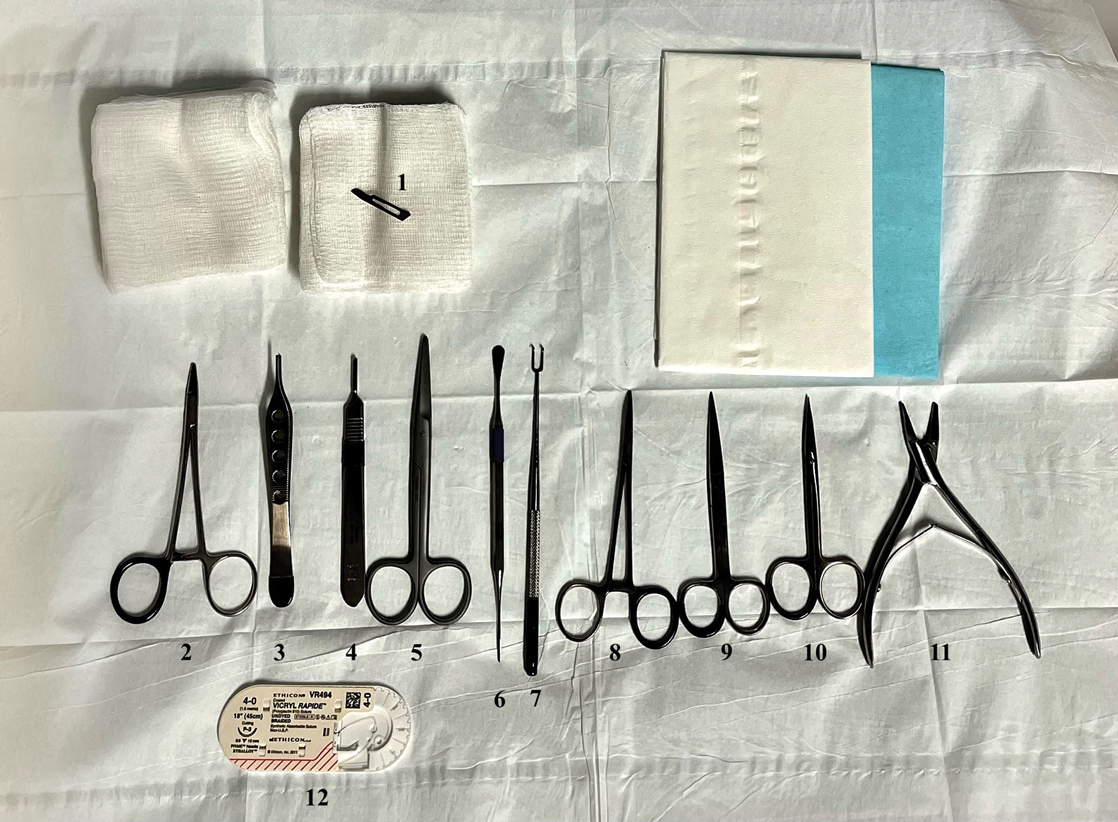
After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.

After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.

After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
Enhanced Care for Pediatric Patients With Generalized Lichen Planus: Diagnosis and Treatment Tips
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.
The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Customized Dermal Curette: An Alternative and Effective Shaving Tool in Nail Surgery
Practice Gap
Longitudinal melanonychia (LM) is characterized by the presence of a dark brown, longitudinal, pigmented band on the nail unit, often caused by melanocytic activation or melanocytic hyperplasia in the nail matrix. Distinguishing between benign and early malignant LM is crucial due to their similar clinical presentations.1 Hence, surgical excision of the pigmented nail matrix followed by histopathologic examination is a common procedure aimed at managing LM and reducing the risk for delayed diagnosis of subungual melanoma.
Tangential matrix excision combined with the nail window technique has emerged as a common and favored surgical strategy for managing LM.2 This method is highly valued for its ability to minimize the risk for severe permanent nail dystrophy and effectively reduce postsurgical pigmentation recurrence.
The procedure begins with the creation of a matrix window along the lateral edge of the pigmented band followed by 1 lateral incision carefully made on each side of the nail fold. This meticulous approach allows for the complete exposure of the pigmented lesion. Subsequently, the nail fold is separated from the dorsal surface of the nail plate to facilitate access to the pigmented nail matrix. Finally, the target pigmented area is excised using a scalpel.
Despite the recognized efficacy of this procedure, challenges do arise, particularly when the width of the pigmented matrix lesion is narrow. Holding the scalpel horizontally to ensure precise excision can prove to be demanding, leading to difficulty achieving complete lesion removal and obtaining the desired cosmetic outcomes. As such, there is a clear need to explore alternative tools that can effectively address these challenges while ensuring optimal surgical outcomes for patients with LM. We propose the use of the customized dermal curette.
The Technique
An improved curette tool is a practical solution for complete removal of the pigmented nail matrix. This enhanced instrument is crafted from a sterile disposable dermal curette with its top flattened using a needle holder(Figure 1). Termed the customized dermal curette, this device is a simple yet accurate tool for the precise excision of pigmented lesions within the nail matrix. Importantly, it offers versatility by accommodating different widths of pigmented lesions through the availability of various sizes of dermal curettes (Figure 2).

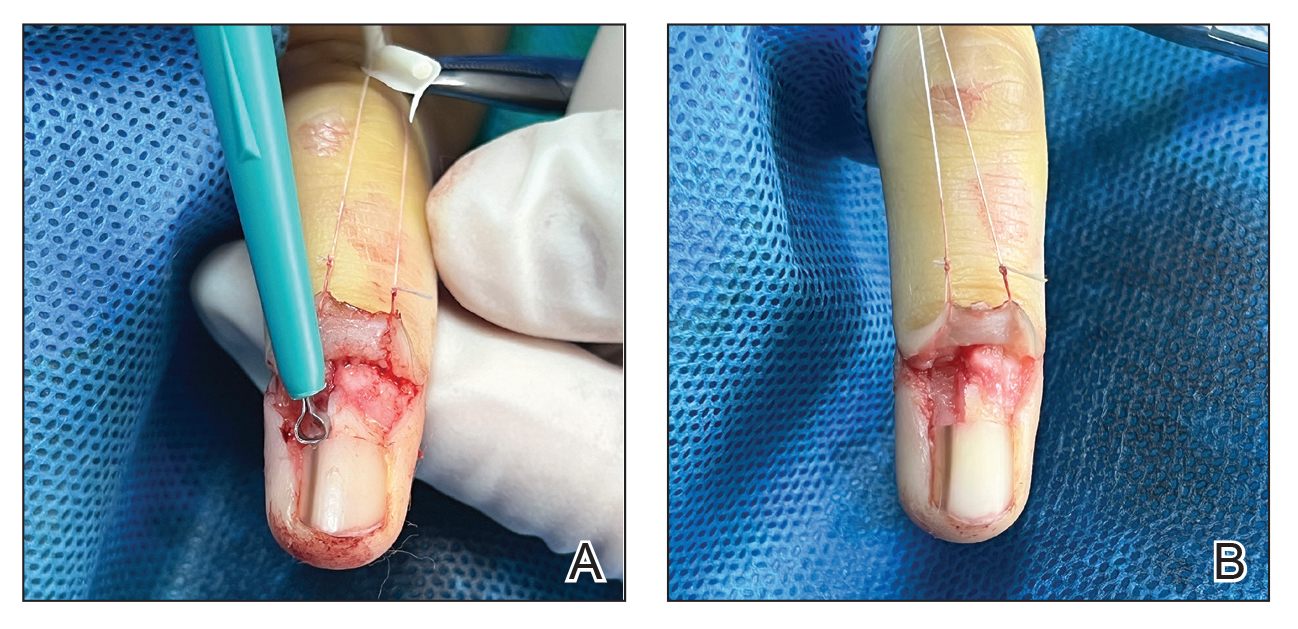
Histopathologically, we have found that the scalpel technique may lead to variable tissue removal, resulting in differences in tissue thickness, fragility, and completeness (Figure 3A). Conversely, the customized dermal curette consistently provides more accurate tissue excision, resulting in uniform tissue thickness and integrity (Figure 3B).
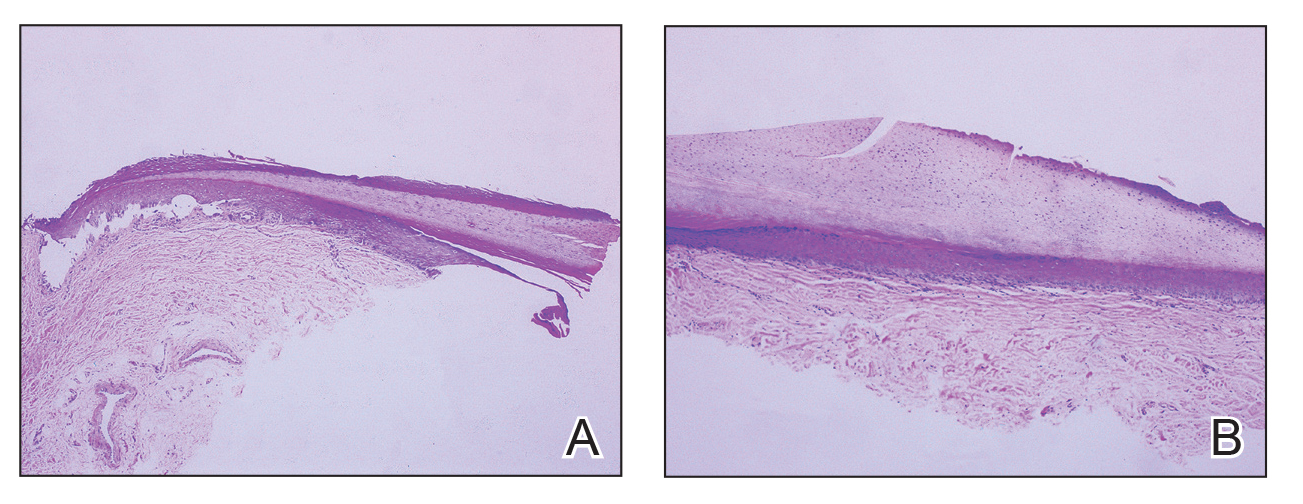
Practice Implications
Compared to the traditional scalpel, this modified tool offers distinct advantages. Specifically, the customized dermal curette provides enhanced maneuverability and control during the procedure, thereby improving the overall efficacy of the excision process. It also offers a more accurate approach to completely remove pigmented bands, which reduces the risk for postoperative recurrence. The simplicity, affordability, and ease of operation associated with customized dermal curettes holds promise as an effective alternative for tissue shaving, especially in cases involving narrow pigmented matrix lesions, thereby addressing a notable practice gap and enhancing patient care.
- Tan WC, Wang DY, Seghers AC, et al. Should we biopsy melanonychia striata in Asian children? a retrospective observational study. Pediatr Dermatol. 2019;36:864-868. doi:10.1111/pde.13934
- Zhou Y, Chen W, Liu ZR, et al. Modified shave surgery combined with nail window technique for the treatment of longitudinal melanonychia: evaluation of the method on a series of 67 cases. J Am Acad Dermatol. 2019;81:717-722. doi:10.1016/j.jaad.2019.03.065
Practice Gap
Longitudinal melanonychia (LM) is characterized by the presence of a dark brown, longitudinal, pigmented band on the nail unit, often caused by melanocytic activation or melanocytic hyperplasia in the nail matrix. Distinguishing between benign and early malignant LM is crucial due to their similar clinical presentations.1 Hence, surgical excision of the pigmented nail matrix followed by histopathologic examination is a common procedure aimed at managing LM and reducing the risk for delayed diagnosis of subungual melanoma.
Tangential matrix excision combined with the nail window technique has emerged as a common and favored surgical strategy for managing LM.2 This method is highly valued for its ability to minimize the risk for severe permanent nail dystrophy and effectively reduce postsurgical pigmentation recurrence.
The procedure begins with the creation of a matrix window along the lateral edge of the pigmented band followed by 1 lateral incision carefully made on each side of the nail fold. This meticulous approach allows for the complete exposure of the pigmented lesion. Subsequently, the nail fold is separated from the dorsal surface of the nail plate to facilitate access to the pigmented nail matrix. Finally, the target pigmented area is excised using a scalpel.
Despite the recognized efficacy of this procedure, challenges do arise, particularly when the width of the pigmented matrix lesion is narrow. Holding the scalpel horizontally to ensure precise excision can prove to be demanding, leading to difficulty achieving complete lesion removal and obtaining the desired cosmetic outcomes. As such, there is a clear need to explore alternative tools that can effectively address these challenges while ensuring optimal surgical outcomes for patients with LM. We propose the use of the customized dermal curette.
The Technique
An improved curette tool is a practical solution for complete removal of the pigmented nail matrix. This enhanced instrument is crafted from a sterile disposable dermal curette with its top flattened using a needle holder(Figure 1). Termed the customized dermal curette, this device is a simple yet accurate tool for the precise excision of pigmented lesions within the nail matrix. Importantly, it offers versatility by accommodating different widths of pigmented lesions through the availability of various sizes of dermal curettes (Figure 2).


Histopathologically, we have found that the scalpel technique may lead to variable tissue removal, resulting in differences in tissue thickness, fragility, and completeness (Figure 3A). Conversely, the customized dermal curette consistently provides more accurate tissue excision, resulting in uniform tissue thickness and integrity (Figure 3B).

Practice Implications
Compared to the traditional scalpel, this modified tool offers distinct advantages. Specifically, the customized dermal curette provides enhanced maneuverability and control during the procedure, thereby improving the overall efficacy of the excision process. It also offers a more accurate approach to completely remove pigmented bands, which reduces the risk for postoperative recurrence. The simplicity, affordability, and ease of operation associated with customized dermal curettes holds promise as an effective alternative for tissue shaving, especially in cases involving narrow pigmented matrix lesions, thereby addressing a notable practice gap and enhancing patient care.
Practice Gap
Longitudinal melanonychia (LM) is characterized by the presence of a dark brown, longitudinal, pigmented band on the nail unit, often caused by melanocytic activation or melanocytic hyperplasia in the nail matrix. Distinguishing between benign and early malignant LM is crucial due to their similar clinical presentations.1 Hence, surgical excision of the pigmented nail matrix followed by histopathologic examination is a common procedure aimed at managing LM and reducing the risk for delayed diagnosis of subungual melanoma.
Tangential matrix excision combined with the nail window technique has emerged as a common and favored surgical strategy for managing LM.2 This method is highly valued for its ability to minimize the risk for severe permanent nail dystrophy and effectively reduce postsurgical pigmentation recurrence.
The procedure begins with the creation of a matrix window along the lateral edge of the pigmented band followed by 1 lateral incision carefully made on each side of the nail fold. This meticulous approach allows for the complete exposure of the pigmented lesion. Subsequently, the nail fold is separated from the dorsal surface of the nail plate to facilitate access to the pigmented nail matrix. Finally, the target pigmented area is excised using a scalpel.
Despite the recognized efficacy of this procedure, challenges do arise, particularly when the width of the pigmented matrix lesion is narrow. Holding the scalpel horizontally to ensure precise excision can prove to be demanding, leading to difficulty achieving complete lesion removal and obtaining the desired cosmetic outcomes. As such, there is a clear need to explore alternative tools that can effectively address these challenges while ensuring optimal surgical outcomes for patients with LM. We propose the use of the customized dermal curette.
The Technique
An improved curette tool is a practical solution for complete removal of the pigmented nail matrix. This enhanced instrument is crafted from a sterile disposable dermal curette with its top flattened using a needle holder(Figure 1). Termed the customized dermal curette, this device is a simple yet accurate tool for the precise excision of pigmented lesions within the nail matrix. Importantly, it offers versatility by accommodating different widths of pigmented lesions through the availability of various sizes of dermal curettes (Figure 2).


Histopathologically, we have found that the scalpel technique may lead to variable tissue removal, resulting in differences in tissue thickness, fragility, and completeness (Figure 3A). Conversely, the customized dermal curette consistently provides more accurate tissue excision, resulting in uniform tissue thickness and integrity (Figure 3B).

Practice Implications
Compared to the traditional scalpel, this modified tool offers distinct advantages. Specifically, the customized dermal curette provides enhanced maneuverability and control during the procedure, thereby improving the overall efficacy of the excision process. It also offers a more accurate approach to completely remove pigmented bands, which reduces the risk for postoperative recurrence. The simplicity, affordability, and ease of operation associated with customized dermal curettes holds promise as an effective alternative for tissue shaving, especially in cases involving narrow pigmented matrix lesions, thereby addressing a notable practice gap and enhancing patient care.
- Tan WC, Wang DY, Seghers AC, et al. Should we biopsy melanonychia striata in Asian children? a retrospective observational study. Pediatr Dermatol. 2019;36:864-868. doi:10.1111/pde.13934
- Zhou Y, Chen W, Liu ZR, et al. Modified shave surgery combined with nail window technique for the treatment of longitudinal melanonychia: evaluation of the method on a series of 67 cases. J Am Acad Dermatol. 2019;81:717-722. doi:10.1016/j.jaad.2019.03.065
- Tan WC, Wang DY, Seghers AC, et al. Should we biopsy melanonychia striata in Asian children? a retrospective observational study. Pediatr Dermatol. 2019;36:864-868. doi:10.1111/pde.13934
- Zhou Y, Chen W, Liu ZR, et al. Modified shave surgery combined with nail window technique for the treatment of longitudinal melanonychia: evaluation of the method on a series of 67 cases. J Am Acad Dermatol. 2019;81:717-722. doi:10.1016/j.jaad.2019.03.065
Two Techniques to Avoid Cyst Spray During Excision
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.
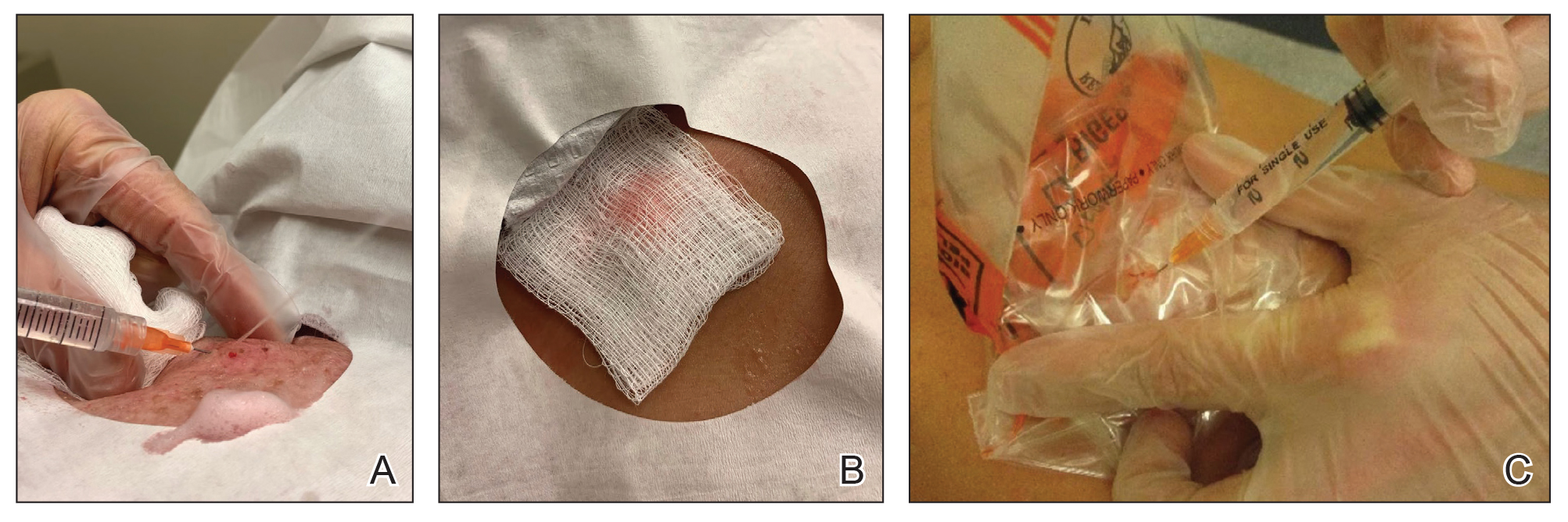
Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.

Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.

Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
Need a Wood Lamp Alternative? Grab Your Smartphone
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).
Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9