User login
Clinician violence: Virtual reality to the rescue?
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
Even mild COVID is hard on the brain
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
Increased anxiety and depression after menstruation
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
Two cups of coffee increase heart dangers with hypertension
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF AMERICAN HEART ASSOCIATION
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
‘Sighing’ tops mindfulness for reduced stress, improved mood
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
Mental health system failing kids leaving ED
Only 56% of children enrolled in Medicaid received any outpatient follow-up within 30 days after a mental health emergency department discharge, according to results of a large study released in Pediatrics.
Fewer than one-third (31.2%) had an outpatient visit within a week after a mental health ED discharge.
Researchers conducted a retrospective study of 28,551 children ages 6-17 years old who had mental health discharges from EDs from January 2018 to June 2019.
The researchers, led by Jennifer A. Hoffmann, MD, MS, with the division of emergency medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, Chicago, also analyzed the effect that having a timely follow-up had on whether the child was likely to return to the ED.
Follow-up within 30 days cuts risk of quick return to ED
They found that follow-up within 30 days was linked with a 26% decreased risk of return within 5 days of the initial ED discharge (hazard ratio, 0.74; 95% confidence interval, 0.63-0.91).
The researchers also found racial disparities in the data. The odds for getting follow-up outpatient care were lower for non-Hispanic Black children, for children with fee-for-service insurance, and for children with no previous mental health outpatient visits.
The numbers were particularly striking for Black children, who were 10% less likely to get outpatient follow-up than their White counterparts.
In addition, 27% of all children in this sample returned to the ED for mental health-related symptoms within 6 months, 20% spent more than 48 hours in the ED for their initial mental health visit, and children with 14 or more mental health outpatient visits had five times higher adjusted odds of follow-up within 7 days and 9.5 times higher adjusted odds of follow-up within 30 days, compared with children with no outpatient mental health visits in the previous year.
A ‘mental health system of care in crisis’
In an accompanying editorial, Hannah E. Karpman, MSW, PhD, with the department of pediatrics, University of Massachusetts, Worcester, and colleagues said those statistics help expose other signs of “a pediatric mental health system of care in crisis.”
If one in five children are spending more than 2 days in the ED for their initial mental health visit, they wrote, that signals the follow-up care they need is not readily available.
The 27% returning to the ED shows that, even if the children are getting outpatient services, that environment is failing them, they noted.
Additionally, 28% of children presented with more than four mental health diagnoses, “suggesting poor diagnostic specificity or perhaps inadequate diagnostic categories to characterize their needs.”
The authors called for interventions that link patients to outpatient care within 5 days of a mental health ED discharge.
The editorialists wrote: “We believe it is time for a “child mental health moonshot,” and call on the field and its funders to come together to launch the next wave of bold mental health research for the benefit of these children and their families who so desperately need our support.”
Things may even be worse in light of COVID
David Rettew, MD, a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland, said in an interview the numbers won’t surprise clinicians who support these children or the patients’ families.
He added that he wouldn’t be surprised if things are even worse now after this study’s data collection, “as COVID and other factors have driven more mental health professionals away from many of the people who need them the most.”
The study does present new evidence that quick access to care is particularly tough for young people who aren’t already established in care, he noted.
“As wait lists grow at outpatient clinics, we are seeing ever stronger need for centers willing and able to provide actual mental health assessment and treatment for people right ‘off the street,’” he said.
Dr. Rettew emphasized that, because mental health conditions rarely improve quickly, having a timely follow-up appointment is important, but won’t likely bring quick improvement.
He agreed with the editorialists’ argument and emphasized, “not only do we need to focus on more rapid care, but also more comprehensive and effective care.
“For an adolescent in crisis, achieving stability often involves more than a medication tweak and a supportive conversation,” Dr. Rettew said. “Rather, it can require an intensive multimodal approach that addresses things like family financial stressors, parental mental health and substance use concerns, school supports, and health promotion or lifestyle changes. What we desperately need are more teams that can quickly intervene on all these levels.”
Addressing problems before crisis is essential
Ideally, teams would address these issues before a crisis. That helps support the “moonshot” charge the editorialists suggest, which “would significantly disrupt the current way we value different components of our health care system,” Dr. Rettew said.
He highlighted a statistic that may get lost in the data: Nearly 40% of youth in enough danger to need an ED visit had no more than one health-related appointment of any kind in the previous year.
“To me, this speaks volumes about the need for earlier involvement before things escalate to the level of an emergency,” Dr. Rettew said.
The authors and editorialists declared no relevant financial relationships. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Only 56% of children enrolled in Medicaid received any outpatient follow-up within 30 days after a mental health emergency department discharge, according to results of a large study released in Pediatrics.
Fewer than one-third (31.2%) had an outpatient visit within a week after a mental health ED discharge.
Researchers conducted a retrospective study of 28,551 children ages 6-17 years old who had mental health discharges from EDs from January 2018 to June 2019.
The researchers, led by Jennifer A. Hoffmann, MD, MS, with the division of emergency medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, Chicago, also analyzed the effect that having a timely follow-up had on whether the child was likely to return to the ED.
Follow-up within 30 days cuts risk of quick return to ED
They found that follow-up within 30 days was linked with a 26% decreased risk of return within 5 days of the initial ED discharge (hazard ratio, 0.74; 95% confidence interval, 0.63-0.91).
The researchers also found racial disparities in the data. The odds for getting follow-up outpatient care were lower for non-Hispanic Black children, for children with fee-for-service insurance, and for children with no previous mental health outpatient visits.
The numbers were particularly striking for Black children, who were 10% less likely to get outpatient follow-up than their White counterparts.
In addition, 27% of all children in this sample returned to the ED for mental health-related symptoms within 6 months, 20% spent more than 48 hours in the ED for their initial mental health visit, and children with 14 or more mental health outpatient visits had five times higher adjusted odds of follow-up within 7 days and 9.5 times higher adjusted odds of follow-up within 30 days, compared with children with no outpatient mental health visits in the previous year.
A ‘mental health system of care in crisis’
In an accompanying editorial, Hannah E. Karpman, MSW, PhD, with the department of pediatrics, University of Massachusetts, Worcester, and colleagues said those statistics help expose other signs of “a pediatric mental health system of care in crisis.”
If one in five children are spending more than 2 days in the ED for their initial mental health visit, they wrote, that signals the follow-up care they need is not readily available.
The 27% returning to the ED shows that, even if the children are getting outpatient services, that environment is failing them, they noted.
Additionally, 28% of children presented with more than four mental health diagnoses, “suggesting poor diagnostic specificity or perhaps inadequate diagnostic categories to characterize their needs.”
The authors called for interventions that link patients to outpatient care within 5 days of a mental health ED discharge.
The editorialists wrote: “We believe it is time for a “child mental health moonshot,” and call on the field and its funders to come together to launch the next wave of bold mental health research for the benefit of these children and their families who so desperately need our support.”
Things may even be worse in light of COVID
David Rettew, MD, a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland, said in an interview the numbers won’t surprise clinicians who support these children or the patients’ families.
He added that he wouldn’t be surprised if things are even worse now after this study’s data collection, “as COVID and other factors have driven more mental health professionals away from many of the people who need them the most.”
The study does present new evidence that quick access to care is particularly tough for young people who aren’t already established in care, he noted.
“As wait lists grow at outpatient clinics, we are seeing ever stronger need for centers willing and able to provide actual mental health assessment and treatment for people right ‘off the street,’” he said.
Dr. Rettew emphasized that, because mental health conditions rarely improve quickly, having a timely follow-up appointment is important, but won’t likely bring quick improvement.
He agreed with the editorialists’ argument and emphasized, “not only do we need to focus on more rapid care, but also more comprehensive and effective care.
“For an adolescent in crisis, achieving stability often involves more than a medication tweak and a supportive conversation,” Dr. Rettew said. “Rather, it can require an intensive multimodal approach that addresses things like family financial stressors, parental mental health and substance use concerns, school supports, and health promotion or lifestyle changes. What we desperately need are more teams that can quickly intervene on all these levels.”
Addressing problems before crisis is essential
Ideally, teams would address these issues before a crisis. That helps support the “moonshot” charge the editorialists suggest, which “would significantly disrupt the current way we value different components of our health care system,” Dr. Rettew said.
He highlighted a statistic that may get lost in the data: Nearly 40% of youth in enough danger to need an ED visit had no more than one health-related appointment of any kind in the previous year.
“To me, this speaks volumes about the need for earlier involvement before things escalate to the level of an emergency,” Dr. Rettew said.
The authors and editorialists declared no relevant financial relationships. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Only 56% of children enrolled in Medicaid received any outpatient follow-up within 30 days after a mental health emergency department discharge, according to results of a large study released in Pediatrics.
Fewer than one-third (31.2%) had an outpatient visit within a week after a mental health ED discharge.
Researchers conducted a retrospective study of 28,551 children ages 6-17 years old who had mental health discharges from EDs from January 2018 to June 2019.
The researchers, led by Jennifer A. Hoffmann, MD, MS, with the division of emergency medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, Chicago, also analyzed the effect that having a timely follow-up had on whether the child was likely to return to the ED.
Follow-up within 30 days cuts risk of quick return to ED
They found that follow-up within 30 days was linked with a 26% decreased risk of return within 5 days of the initial ED discharge (hazard ratio, 0.74; 95% confidence interval, 0.63-0.91).
The researchers also found racial disparities in the data. The odds for getting follow-up outpatient care were lower for non-Hispanic Black children, for children with fee-for-service insurance, and for children with no previous mental health outpatient visits.
The numbers were particularly striking for Black children, who were 10% less likely to get outpatient follow-up than their White counterparts.
In addition, 27% of all children in this sample returned to the ED for mental health-related symptoms within 6 months, 20% spent more than 48 hours in the ED for their initial mental health visit, and children with 14 or more mental health outpatient visits had five times higher adjusted odds of follow-up within 7 days and 9.5 times higher adjusted odds of follow-up within 30 days, compared with children with no outpatient mental health visits in the previous year.
A ‘mental health system of care in crisis’
In an accompanying editorial, Hannah E. Karpman, MSW, PhD, with the department of pediatrics, University of Massachusetts, Worcester, and colleagues said those statistics help expose other signs of “a pediatric mental health system of care in crisis.”
If one in five children are spending more than 2 days in the ED for their initial mental health visit, they wrote, that signals the follow-up care they need is not readily available.
The 27% returning to the ED shows that, even if the children are getting outpatient services, that environment is failing them, they noted.
Additionally, 28% of children presented with more than four mental health diagnoses, “suggesting poor diagnostic specificity or perhaps inadequate diagnostic categories to characterize their needs.”
The authors called for interventions that link patients to outpatient care within 5 days of a mental health ED discharge.
The editorialists wrote: “We believe it is time for a “child mental health moonshot,” and call on the field and its funders to come together to launch the next wave of bold mental health research for the benefit of these children and their families who so desperately need our support.”
Things may even be worse in light of COVID
David Rettew, MD, a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland, said in an interview the numbers won’t surprise clinicians who support these children or the patients’ families.
He added that he wouldn’t be surprised if things are even worse now after this study’s data collection, “as COVID and other factors have driven more mental health professionals away from many of the people who need them the most.”
The study does present new evidence that quick access to care is particularly tough for young people who aren’t already established in care, he noted.
“As wait lists grow at outpatient clinics, we are seeing ever stronger need for centers willing and able to provide actual mental health assessment and treatment for people right ‘off the street,’” he said.
Dr. Rettew emphasized that, because mental health conditions rarely improve quickly, having a timely follow-up appointment is important, but won’t likely bring quick improvement.
He agreed with the editorialists’ argument and emphasized, “not only do we need to focus on more rapid care, but also more comprehensive and effective care.
“For an adolescent in crisis, achieving stability often involves more than a medication tweak and a supportive conversation,” Dr. Rettew said. “Rather, it can require an intensive multimodal approach that addresses things like family financial stressors, parental mental health and substance use concerns, school supports, and health promotion or lifestyle changes. What we desperately need are more teams that can quickly intervene on all these levels.”
Addressing problems before crisis is essential
Ideally, teams would address these issues before a crisis. That helps support the “moonshot” charge the editorialists suggest, which “would significantly disrupt the current way we value different components of our health care system,” Dr. Rettew said.
He highlighted a statistic that may get lost in the data: Nearly 40% of youth in enough danger to need an ED visit had no more than one health-related appointment of any kind in the previous year.
“To me, this speaks volumes about the need for earlier involvement before things escalate to the level of an emergency,” Dr. Rettew said.
The authors and editorialists declared no relevant financial relationships. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
FROM PEDIATRICS
TMS tied to ‘marked’ antidepressant, anxiolytic effects in anxious depression
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Primary care providers are increasingly addressing mental health concerns
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
FROM HEALTH AFFAIRS
Psychiatric illnesses share common brain network
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.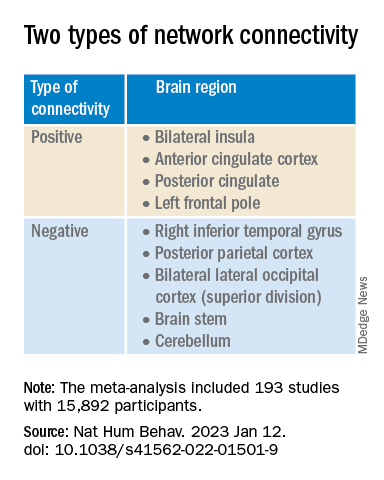
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE HUMAN BEHAVIOR














