User login
COVID-19 has brought more complex, longer office visits
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
High-dose omega-3s tied to higher AFib risk
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Pacemakers after TAVR: No long-term survival differences
A comparison of long-term survival between patients who either did or did not undergo permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) revealed no differences, according to results of the SWEDEHEART observational study.
The nationwide population-based cohort study included all patients who underwent transfemoral TAVR in Sweden from 2008 to 2018.
Most frequent complications
While newer-generation aortic valve prostheses are less likely to necessitate PPI, the need for PPI is higher after TAVR than after surgical aortic valve replacement (SAVR), and the need for PPI remains the most frequent complication after TAVR, the study authors noted. Use of self-expandable valves, deep prosthetic valve implantation, preprocedural conduction disturbances, older age, and a high number of comorbidities are among the risk factors for PPI following TAVR.
With prior studies producing conflicting results, the authors stated, the impact of PPI after TAVR remains unknown. Expanding use of TAVR to include younger and low-risk patients with a long life expectancy underscores the importance of gaining greater understand of the impact of PPI after TAVR. Accordingly, the study was conducted to investigate long-term, clinically important outcomes in this post-TAVR population.
Out of 4,750 patients who underwent TAVR in the study period, 3,420 patients in SWEDEHEART met study criteria, with 481 (14.1%) undergoing PPI within 30 days after TAVR, and 2,939 not receiving a pacemaker. PPI exposure was defined as implantation of a permanent pacemaker or implantable cardioverter-defibrillator. The study primary outcome was all-cause mortality, with cardiovascular death, heart failure, and endocarditis as secondary outcomes. It was reported in JACC: Cardiovascular Interventions.
Similar survival
Mean patient age was 81.3 years (50.4% female). The rate for all-cause mortality in those with no pacemaker was 11.4 per 100 patient years and 13.1 for those with a pacemaker (hazard ratio, 1.04; 95% confidence interval, 0.89-1.23). The cardiovascular death rate in the no-pacemaker group was 6.0 per 100 patient years and 7.1 per 100 patient years in the pacemaker group (HR, 0.96; 95% CI, 0.75-1.23). For heart failure the rates were 4.5 per 100 patient years in the no pacemaker group and 6.3 in the pacemaker group (HR, 1.22; 95% CI, 0.93-1.672). Endocarditis rates were 1.2 and 1.1 per 100 patient years in the no pacemaker and pacemaker groups, respectively (HR, 0.93; 95% CI, 0.51-1.71).
The authors pointed out that their prior study had found PPI after SAVR in almost 25,000 patients to be associated with increased all-cause mortality and heart failure rates. Patients who undergo TAVR, however, are older and have more comorbidities than patients who undergo SAVR.
It is thus likely that patients who undergo TAVR die of other causes before the negative effects of their pacemaker become clinically evident.
Also, the incidence of conduction abnormalities increases with age, making it more likely that beneficial effects of pacemakers occur in older patients rather than younger ones, counterbalancing the detrimental effects to a larger extent.
Reduce PPI rates after TAVR
The study authors also observed that, although they did not find increased mortality or heart failure in patients who underwent PPI, PPI is associated with risks, including lead- and pocket-related complications, other traumatic complications, longer hospital stays and higher societal costs. These factors justify the search for strategies to reduce PPI rates after TAVR.
“Our study adds important information about the prognosis in patients who received a permanent pacemaker implantation following TAVR,” study author Natalie Glaser, MD, PhD, said in an interview. “Increased knowledge about prognosis after TAVR in different patient populations has important implications for preoperative risk stratification and can help to optimize postoperative follow-up and treatment for these patients.” Future studies, Dr. Glaser added, should include younger and low-risk patients with longer follow-up to confirm the present findings.
Balancing factors
In an accompanying editorial, Antonio J. Muñoz-García, MD, PhD, and Erika Muñoz-García, MD also noted factors potentially counterbalancing and masking adverse effects of PPI, echoing some mentioned by the study authors. Among those without PPI, 10%-50% develop new-onset left bundle branch block (LBBB) after TAVR. LBBB is a known marker of low long-term survival in TAVR populations, producing intraventricular dyssynchrony leading potentially to left ventricular dysfunction or development of complete atrioventricular block with higher mortality risk in those without pacemakers. PPI, as well, can be protective against unexpected death in those with advanced conduction disorders. Still, they point out, PPI can entail lead dysfunction, need for generator replacement, infection, and tricuspid valve regurgitation.
Commenting in an interview that an observed trend of a greater increase in events in the group of patients with pacemakers for the first 4 years is consistent with prior studies, Dr. Antonio Muñoz-García said: “This can be explained because long-term survival in the TAVI population is conditioned by comorbidities. It is true that the presence of a pacemaker can cause left ventricular ejection fraction to deteriorate and therefore condition heart failure and increased mortality. But the involvement of the pacemaker in left ventricular function in patients with TAVI is multifactorial and depends on the indication of the pacemaker, whether prophylactic or absolute, on the time-dependent pacing, whether or not the patients prior to TAVI present with alterations in atrioventricular conduction [and therefore could benefit from the implantation of pacemakers], as well as the forms of pacing optimization [resynchronization, hisian pacing, etc]. All of these are issues to be considered in clinical practice.”
The editorialists concluded: “To date, the impact of PPI on late clinical outcomes after TAVR remains controversial; however, this study to some extent helps clarify this controversy.” In accord with the study authors, they called for reductions in PPI rates and long-term clinical follow-up.
The study was funded by several Swedish research organizations. The study investigators and editorial authors declared having no disclosures.
A comparison of long-term survival between patients who either did or did not undergo permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) revealed no differences, according to results of the SWEDEHEART observational study.
The nationwide population-based cohort study included all patients who underwent transfemoral TAVR in Sweden from 2008 to 2018.
Most frequent complications
While newer-generation aortic valve prostheses are less likely to necessitate PPI, the need for PPI is higher after TAVR than after surgical aortic valve replacement (SAVR), and the need for PPI remains the most frequent complication after TAVR, the study authors noted. Use of self-expandable valves, deep prosthetic valve implantation, preprocedural conduction disturbances, older age, and a high number of comorbidities are among the risk factors for PPI following TAVR.
With prior studies producing conflicting results, the authors stated, the impact of PPI after TAVR remains unknown. Expanding use of TAVR to include younger and low-risk patients with a long life expectancy underscores the importance of gaining greater understand of the impact of PPI after TAVR. Accordingly, the study was conducted to investigate long-term, clinically important outcomes in this post-TAVR population.
Out of 4,750 patients who underwent TAVR in the study period, 3,420 patients in SWEDEHEART met study criteria, with 481 (14.1%) undergoing PPI within 30 days after TAVR, and 2,939 not receiving a pacemaker. PPI exposure was defined as implantation of a permanent pacemaker or implantable cardioverter-defibrillator. The study primary outcome was all-cause mortality, with cardiovascular death, heart failure, and endocarditis as secondary outcomes. It was reported in JACC: Cardiovascular Interventions.
Similar survival
Mean patient age was 81.3 years (50.4% female). The rate for all-cause mortality in those with no pacemaker was 11.4 per 100 patient years and 13.1 for those with a pacemaker (hazard ratio, 1.04; 95% confidence interval, 0.89-1.23). The cardiovascular death rate in the no-pacemaker group was 6.0 per 100 patient years and 7.1 per 100 patient years in the pacemaker group (HR, 0.96; 95% CI, 0.75-1.23). For heart failure the rates were 4.5 per 100 patient years in the no pacemaker group and 6.3 in the pacemaker group (HR, 1.22; 95% CI, 0.93-1.672). Endocarditis rates were 1.2 and 1.1 per 100 patient years in the no pacemaker and pacemaker groups, respectively (HR, 0.93; 95% CI, 0.51-1.71).
The authors pointed out that their prior study had found PPI after SAVR in almost 25,000 patients to be associated with increased all-cause mortality and heart failure rates. Patients who undergo TAVR, however, are older and have more comorbidities than patients who undergo SAVR.
It is thus likely that patients who undergo TAVR die of other causes before the negative effects of their pacemaker become clinically evident.
Also, the incidence of conduction abnormalities increases with age, making it more likely that beneficial effects of pacemakers occur in older patients rather than younger ones, counterbalancing the detrimental effects to a larger extent.
Reduce PPI rates after TAVR
The study authors also observed that, although they did not find increased mortality or heart failure in patients who underwent PPI, PPI is associated with risks, including lead- and pocket-related complications, other traumatic complications, longer hospital stays and higher societal costs. These factors justify the search for strategies to reduce PPI rates after TAVR.
“Our study adds important information about the prognosis in patients who received a permanent pacemaker implantation following TAVR,” study author Natalie Glaser, MD, PhD, said in an interview. “Increased knowledge about prognosis after TAVR in different patient populations has important implications for preoperative risk stratification and can help to optimize postoperative follow-up and treatment for these patients.” Future studies, Dr. Glaser added, should include younger and low-risk patients with longer follow-up to confirm the present findings.
Balancing factors
In an accompanying editorial, Antonio J. Muñoz-García, MD, PhD, and Erika Muñoz-García, MD also noted factors potentially counterbalancing and masking adverse effects of PPI, echoing some mentioned by the study authors. Among those without PPI, 10%-50% develop new-onset left bundle branch block (LBBB) after TAVR. LBBB is a known marker of low long-term survival in TAVR populations, producing intraventricular dyssynchrony leading potentially to left ventricular dysfunction or development of complete atrioventricular block with higher mortality risk in those without pacemakers. PPI, as well, can be protective against unexpected death in those with advanced conduction disorders. Still, they point out, PPI can entail lead dysfunction, need for generator replacement, infection, and tricuspid valve regurgitation.
Commenting in an interview that an observed trend of a greater increase in events in the group of patients with pacemakers for the first 4 years is consistent with prior studies, Dr. Antonio Muñoz-García said: “This can be explained because long-term survival in the TAVI population is conditioned by comorbidities. It is true that the presence of a pacemaker can cause left ventricular ejection fraction to deteriorate and therefore condition heart failure and increased mortality. But the involvement of the pacemaker in left ventricular function in patients with TAVI is multifactorial and depends on the indication of the pacemaker, whether prophylactic or absolute, on the time-dependent pacing, whether or not the patients prior to TAVI present with alterations in atrioventricular conduction [and therefore could benefit from the implantation of pacemakers], as well as the forms of pacing optimization [resynchronization, hisian pacing, etc]. All of these are issues to be considered in clinical practice.”
The editorialists concluded: “To date, the impact of PPI on late clinical outcomes after TAVR remains controversial; however, this study to some extent helps clarify this controversy.” In accord with the study authors, they called for reductions in PPI rates and long-term clinical follow-up.
The study was funded by several Swedish research organizations. The study investigators and editorial authors declared having no disclosures.
A comparison of long-term survival between patients who either did or did not undergo permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) revealed no differences, according to results of the SWEDEHEART observational study.
The nationwide population-based cohort study included all patients who underwent transfemoral TAVR in Sweden from 2008 to 2018.
Most frequent complications
While newer-generation aortic valve prostheses are less likely to necessitate PPI, the need for PPI is higher after TAVR than after surgical aortic valve replacement (SAVR), and the need for PPI remains the most frequent complication after TAVR, the study authors noted. Use of self-expandable valves, deep prosthetic valve implantation, preprocedural conduction disturbances, older age, and a high number of comorbidities are among the risk factors for PPI following TAVR.
With prior studies producing conflicting results, the authors stated, the impact of PPI after TAVR remains unknown. Expanding use of TAVR to include younger and low-risk patients with a long life expectancy underscores the importance of gaining greater understand of the impact of PPI after TAVR. Accordingly, the study was conducted to investigate long-term, clinically important outcomes in this post-TAVR population.
Out of 4,750 patients who underwent TAVR in the study period, 3,420 patients in SWEDEHEART met study criteria, with 481 (14.1%) undergoing PPI within 30 days after TAVR, and 2,939 not receiving a pacemaker. PPI exposure was defined as implantation of a permanent pacemaker or implantable cardioverter-defibrillator. The study primary outcome was all-cause mortality, with cardiovascular death, heart failure, and endocarditis as secondary outcomes. It was reported in JACC: Cardiovascular Interventions.
Similar survival
Mean patient age was 81.3 years (50.4% female). The rate for all-cause mortality in those with no pacemaker was 11.4 per 100 patient years and 13.1 for those with a pacemaker (hazard ratio, 1.04; 95% confidence interval, 0.89-1.23). The cardiovascular death rate in the no-pacemaker group was 6.0 per 100 patient years and 7.1 per 100 patient years in the pacemaker group (HR, 0.96; 95% CI, 0.75-1.23). For heart failure the rates were 4.5 per 100 patient years in the no pacemaker group and 6.3 in the pacemaker group (HR, 1.22; 95% CI, 0.93-1.672). Endocarditis rates were 1.2 and 1.1 per 100 patient years in the no pacemaker and pacemaker groups, respectively (HR, 0.93; 95% CI, 0.51-1.71).
The authors pointed out that their prior study had found PPI after SAVR in almost 25,000 patients to be associated with increased all-cause mortality and heart failure rates. Patients who undergo TAVR, however, are older and have more comorbidities than patients who undergo SAVR.
It is thus likely that patients who undergo TAVR die of other causes before the negative effects of their pacemaker become clinically evident.
Also, the incidence of conduction abnormalities increases with age, making it more likely that beneficial effects of pacemakers occur in older patients rather than younger ones, counterbalancing the detrimental effects to a larger extent.
Reduce PPI rates after TAVR
The study authors also observed that, although they did not find increased mortality or heart failure in patients who underwent PPI, PPI is associated with risks, including lead- and pocket-related complications, other traumatic complications, longer hospital stays and higher societal costs. These factors justify the search for strategies to reduce PPI rates after TAVR.
“Our study adds important information about the prognosis in patients who received a permanent pacemaker implantation following TAVR,” study author Natalie Glaser, MD, PhD, said in an interview. “Increased knowledge about prognosis after TAVR in different patient populations has important implications for preoperative risk stratification and can help to optimize postoperative follow-up and treatment for these patients.” Future studies, Dr. Glaser added, should include younger and low-risk patients with longer follow-up to confirm the present findings.
Balancing factors
In an accompanying editorial, Antonio J. Muñoz-García, MD, PhD, and Erika Muñoz-García, MD also noted factors potentially counterbalancing and masking adverse effects of PPI, echoing some mentioned by the study authors. Among those without PPI, 10%-50% develop new-onset left bundle branch block (LBBB) after TAVR. LBBB is a known marker of low long-term survival in TAVR populations, producing intraventricular dyssynchrony leading potentially to left ventricular dysfunction or development of complete atrioventricular block with higher mortality risk in those without pacemakers. PPI, as well, can be protective against unexpected death in those with advanced conduction disorders. Still, they point out, PPI can entail lead dysfunction, need for generator replacement, infection, and tricuspid valve regurgitation.
Commenting in an interview that an observed trend of a greater increase in events in the group of patients with pacemakers for the first 4 years is consistent with prior studies, Dr. Antonio Muñoz-García said: “This can be explained because long-term survival in the TAVI population is conditioned by comorbidities. It is true that the presence of a pacemaker can cause left ventricular ejection fraction to deteriorate and therefore condition heart failure and increased mortality. But the involvement of the pacemaker in left ventricular function in patients with TAVI is multifactorial and depends on the indication of the pacemaker, whether prophylactic or absolute, on the time-dependent pacing, whether or not the patients prior to TAVI present with alterations in atrioventricular conduction [and therefore could benefit from the implantation of pacemakers], as well as the forms of pacing optimization [resynchronization, hisian pacing, etc]. All of these are issues to be considered in clinical practice.”
The editorialists concluded: “To date, the impact of PPI on late clinical outcomes after TAVR remains controversial; however, this study to some extent helps clarify this controversy.” In accord with the study authors, they called for reductions in PPI rates and long-term clinical follow-up.
The study was funded by several Swedish research organizations. The study investigators and editorial authors declared having no disclosures.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
‘Metabolically healthy obesity’ tied to substantial heart risk
In an analysis of almost 3 million people with no prior heart issues, there was a 34% increased risk for developing heart failure and a 33% increased risk for developing atrial fibrillation, it was reported at the annual meeting of the European Association for the Study of Diabetes.
There appeared to be no increase in the risk for heart attacks, ischemic stroke, or cardiovascular death, but the study’s 5-year follow-up period may have been too short to see such differences.
“Our findings highlight the importance of preventing poor metabolic health,” study investigator Laurent Fauchier, MD, PhD, of Centre Hospitalier Universitaire Trousseau (France), observed in a press release that highlighted his EASD presentation.
“Encouraging weight loss in people with obesity, regardless of whether or not they are ‘metabolically healthy,’ will help prevent atrial fibrillation and heart failure,” he suggested.
‘Metabolically healthy obesity’ – a misnomer?
‘Metabolically healthy obesity’, or MHO, has been suggested as a term to describe those who have a body mass index greater than 30 mg/m2 but no obvious metabolic abnormalities, such as hypertension, dyslipidemia, or diabetes. It’s a term that could cover around a third of people with obesity, but it’s one that not everyone agrees with.
“I don’t feel the label ‘MHO’ is useful,” Frederick Ho, PhD, who is part of team at the University of Glasgow (Scotland) that has done similar research in a U.K. population, said in an interview.
“Even if – and this is a big if– [people with obesity] are at no higher risk of heart attack or stroke, they are still at higher risk of many other diseases, including heart failure and respiratory diseases. The term ‘healthy’ is sometimes interpreted as no additional health risk at all, which is not true,” Dr. Ho, a research fellow in public health, qualified.
Hospital discharge records checked
For their analysis Dr. Fauchier and coinvestigators obtained the medical records of all patients who had been discharged from French hospitals in 2013 and who had at least 5 years’ worth of follow-up data. For inclusion, there had to be no prior history of major cardiovascular events (MACE), which included myocardial infarction (MI), heart failure, and ischemic stroke. Patients who were underweight or malnourished were excluded.
In all, around 2.8 million patients were included for the analysis, of whom 9.5% (n = 272,838) were classified as being obese and the remainder as ‘nonobese’ (n = 2,600,201). Patients were then subdivided according to whether they had diabetes, hypertension, and hyperlipidemia, with those who did not have any of these conditions being classified as ‘metabolically healthy’ and those who had all three as ‘metabolically unhealthy.’
The results, published in Diabetes, Obesity and Metabolism, showed that just under a third (32.8%) of the obese patients were ‘metabolically healthy,’ compared with 72.7% of those who were not obese.
The adjusted hazard ratio (aHR) for experiencing MACE with heart failure was 1.22 comparing those who were obese and ‘metabolically healthy’ with those who were not obese and had no metabolic abnormalities (95% confidence interval, 1.19-1.24). Corresponding aHRs for new-onset heart failure and new-onset atrial fibrillation were 1.34 (CI, 1.31-1.37) and 1.33 (CI, 1.30-1.37). For MI, ischemic stroke, and cardiovascular death aHRs were a respective 0.92 (CI, 0.87-0.98), 0.93 (CI, 0.88-0.98), and 0.99 (CI, 0.93-1.0).
Findings consistent with UK Biobank data
While these are observational associations that do not show cause and effect, they do agree with other recently published data from the UK Biobank as Dr. Ho pointed out. These data are “quite interesting and partly consistent with what we found previously, e.g., a higher heart failure risk,” he said.
“We’d expect people with ‘metabolically healthy’ obesity to develop heart attack and stroke a little later than those who were initially metabolically unhealthy,” Dr. Ho noted, observing that the study was very large, but it does has a relatively short period of follow up.
“This is partly because quite a few of those with ‘MHO’ would become metabolically unhealthy after a few years,” Dr. Ho added.
Importantly, he noted, “this study has omitted several important confounders, such as physical activity and diet, which are both strong predictors of MHO and cardiovascular outcomes.”
Naveed Sattar, FMedSci, FRCPath, FRCPGlas, FRSE, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, with whom Dr. Ho has collaborated, gave his thoughts on the topic in an interview.
“Carrying excess weight can give considerable risks for conditions such as heart failure or respiratory disease in ways (not yet fully understood) that are not captured by metabolic health factors,” he said.
“This means that even if someone were to be labeled as living with metabolically healthy obesity, losing weight may still benefit that individual in many ways and reduce their risk of several other important health outcomes. They may also feel better.”
Furthermore, he added: “Our Glasgow team has therefore strongly cautioned on the use of the term metabolically healthy obesity, and these new data do not change our view.”
Dr. Fauchier has acted as a speaker or consultant for AstraZeneca, Bayer, Bristol Myers Squibb Pfizer, Boehringer Ingelheim, Medtronic, Novartis, and XO. Dr. Ho had no relevant conflicts of interest. Dr. Sattar has received grants and personal fees from Boehringer Ingelheim, and personal fees from Amgen, AstraZeneca, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi.
In an analysis of almost 3 million people with no prior heart issues, there was a 34% increased risk for developing heart failure and a 33% increased risk for developing atrial fibrillation, it was reported at the annual meeting of the European Association for the Study of Diabetes.
There appeared to be no increase in the risk for heart attacks, ischemic stroke, or cardiovascular death, but the study’s 5-year follow-up period may have been too short to see such differences.
“Our findings highlight the importance of preventing poor metabolic health,” study investigator Laurent Fauchier, MD, PhD, of Centre Hospitalier Universitaire Trousseau (France), observed in a press release that highlighted his EASD presentation.
“Encouraging weight loss in people with obesity, regardless of whether or not they are ‘metabolically healthy,’ will help prevent atrial fibrillation and heart failure,” he suggested.
‘Metabolically healthy obesity’ – a misnomer?
‘Metabolically healthy obesity’, or MHO, has been suggested as a term to describe those who have a body mass index greater than 30 mg/m2 but no obvious metabolic abnormalities, such as hypertension, dyslipidemia, or diabetes. It’s a term that could cover around a third of people with obesity, but it’s one that not everyone agrees with.
“I don’t feel the label ‘MHO’ is useful,” Frederick Ho, PhD, who is part of team at the University of Glasgow (Scotland) that has done similar research in a U.K. population, said in an interview.
“Even if – and this is a big if– [people with obesity] are at no higher risk of heart attack or stroke, they are still at higher risk of many other diseases, including heart failure and respiratory diseases. The term ‘healthy’ is sometimes interpreted as no additional health risk at all, which is not true,” Dr. Ho, a research fellow in public health, qualified.
Hospital discharge records checked
For their analysis Dr. Fauchier and coinvestigators obtained the medical records of all patients who had been discharged from French hospitals in 2013 and who had at least 5 years’ worth of follow-up data. For inclusion, there had to be no prior history of major cardiovascular events (MACE), which included myocardial infarction (MI), heart failure, and ischemic stroke. Patients who were underweight or malnourished were excluded.
In all, around 2.8 million patients were included for the analysis, of whom 9.5% (n = 272,838) were classified as being obese and the remainder as ‘nonobese’ (n = 2,600,201). Patients were then subdivided according to whether they had diabetes, hypertension, and hyperlipidemia, with those who did not have any of these conditions being classified as ‘metabolically healthy’ and those who had all three as ‘metabolically unhealthy.’
The results, published in Diabetes, Obesity and Metabolism, showed that just under a third (32.8%) of the obese patients were ‘metabolically healthy,’ compared with 72.7% of those who were not obese.
The adjusted hazard ratio (aHR) for experiencing MACE with heart failure was 1.22 comparing those who were obese and ‘metabolically healthy’ with those who were not obese and had no metabolic abnormalities (95% confidence interval, 1.19-1.24). Corresponding aHRs for new-onset heart failure and new-onset atrial fibrillation were 1.34 (CI, 1.31-1.37) and 1.33 (CI, 1.30-1.37). For MI, ischemic stroke, and cardiovascular death aHRs were a respective 0.92 (CI, 0.87-0.98), 0.93 (CI, 0.88-0.98), and 0.99 (CI, 0.93-1.0).
Findings consistent with UK Biobank data
While these are observational associations that do not show cause and effect, they do agree with other recently published data from the UK Biobank as Dr. Ho pointed out. These data are “quite interesting and partly consistent with what we found previously, e.g., a higher heart failure risk,” he said.
“We’d expect people with ‘metabolically healthy’ obesity to develop heart attack and stroke a little later than those who were initially metabolically unhealthy,” Dr. Ho noted, observing that the study was very large, but it does has a relatively short period of follow up.
“This is partly because quite a few of those with ‘MHO’ would become metabolically unhealthy after a few years,” Dr. Ho added.
Importantly, he noted, “this study has omitted several important confounders, such as physical activity and diet, which are both strong predictors of MHO and cardiovascular outcomes.”
Naveed Sattar, FMedSci, FRCPath, FRCPGlas, FRSE, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, with whom Dr. Ho has collaborated, gave his thoughts on the topic in an interview.
“Carrying excess weight can give considerable risks for conditions such as heart failure or respiratory disease in ways (not yet fully understood) that are not captured by metabolic health factors,” he said.
“This means that even if someone were to be labeled as living with metabolically healthy obesity, losing weight may still benefit that individual in many ways and reduce their risk of several other important health outcomes. They may also feel better.”
Furthermore, he added: “Our Glasgow team has therefore strongly cautioned on the use of the term metabolically healthy obesity, and these new data do not change our view.”
Dr. Fauchier has acted as a speaker or consultant for AstraZeneca, Bayer, Bristol Myers Squibb Pfizer, Boehringer Ingelheim, Medtronic, Novartis, and XO. Dr. Ho had no relevant conflicts of interest. Dr. Sattar has received grants and personal fees from Boehringer Ingelheim, and personal fees from Amgen, AstraZeneca, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi.
In an analysis of almost 3 million people with no prior heart issues, there was a 34% increased risk for developing heart failure and a 33% increased risk for developing atrial fibrillation, it was reported at the annual meeting of the European Association for the Study of Diabetes.
There appeared to be no increase in the risk for heart attacks, ischemic stroke, or cardiovascular death, but the study’s 5-year follow-up period may have been too short to see such differences.
“Our findings highlight the importance of preventing poor metabolic health,” study investigator Laurent Fauchier, MD, PhD, of Centre Hospitalier Universitaire Trousseau (France), observed in a press release that highlighted his EASD presentation.
“Encouraging weight loss in people with obesity, regardless of whether or not they are ‘metabolically healthy,’ will help prevent atrial fibrillation and heart failure,” he suggested.
‘Metabolically healthy obesity’ – a misnomer?
‘Metabolically healthy obesity’, or MHO, has been suggested as a term to describe those who have a body mass index greater than 30 mg/m2 but no obvious metabolic abnormalities, such as hypertension, dyslipidemia, or diabetes. It’s a term that could cover around a third of people with obesity, but it’s one that not everyone agrees with.
“I don’t feel the label ‘MHO’ is useful,” Frederick Ho, PhD, who is part of team at the University of Glasgow (Scotland) that has done similar research in a U.K. population, said in an interview.
“Even if – and this is a big if– [people with obesity] are at no higher risk of heart attack or stroke, they are still at higher risk of many other diseases, including heart failure and respiratory diseases. The term ‘healthy’ is sometimes interpreted as no additional health risk at all, which is not true,” Dr. Ho, a research fellow in public health, qualified.
Hospital discharge records checked
For their analysis Dr. Fauchier and coinvestigators obtained the medical records of all patients who had been discharged from French hospitals in 2013 and who had at least 5 years’ worth of follow-up data. For inclusion, there had to be no prior history of major cardiovascular events (MACE), which included myocardial infarction (MI), heart failure, and ischemic stroke. Patients who were underweight or malnourished were excluded.
In all, around 2.8 million patients were included for the analysis, of whom 9.5% (n = 272,838) were classified as being obese and the remainder as ‘nonobese’ (n = 2,600,201). Patients were then subdivided according to whether they had diabetes, hypertension, and hyperlipidemia, with those who did not have any of these conditions being classified as ‘metabolically healthy’ and those who had all three as ‘metabolically unhealthy.’
The results, published in Diabetes, Obesity and Metabolism, showed that just under a third (32.8%) of the obese patients were ‘metabolically healthy,’ compared with 72.7% of those who were not obese.
The adjusted hazard ratio (aHR) for experiencing MACE with heart failure was 1.22 comparing those who were obese and ‘metabolically healthy’ with those who were not obese and had no metabolic abnormalities (95% confidence interval, 1.19-1.24). Corresponding aHRs for new-onset heart failure and new-onset atrial fibrillation were 1.34 (CI, 1.31-1.37) and 1.33 (CI, 1.30-1.37). For MI, ischemic stroke, and cardiovascular death aHRs were a respective 0.92 (CI, 0.87-0.98), 0.93 (CI, 0.88-0.98), and 0.99 (CI, 0.93-1.0).
Findings consistent with UK Biobank data
While these are observational associations that do not show cause and effect, they do agree with other recently published data from the UK Biobank as Dr. Ho pointed out. These data are “quite interesting and partly consistent with what we found previously, e.g., a higher heart failure risk,” he said.
“We’d expect people with ‘metabolically healthy’ obesity to develop heart attack and stroke a little later than those who were initially metabolically unhealthy,” Dr. Ho noted, observing that the study was very large, but it does has a relatively short period of follow up.
“This is partly because quite a few of those with ‘MHO’ would become metabolically unhealthy after a few years,” Dr. Ho added.
Importantly, he noted, “this study has omitted several important confounders, such as physical activity and diet, which are both strong predictors of MHO and cardiovascular outcomes.”
Naveed Sattar, FMedSci, FRCPath, FRCPGlas, FRSE, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, with whom Dr. Ho has collaborated, gave his thoughts on the topic in an interview.
“Carrying excess weight can give considerable risks for conditions such as heart failure or respiratory disease in ways (not yet fully understood) that are not captured by metabolic health factors,” he said.
“This means that even if someone were to be labeled as living with metabolically healthy obesity, losing weight may still benefit that individual in many ways and reduce their risk of several other important health outcomes. They may also feel better.”
Furthermore, he added: “Our Glasgow team has therefore strongly cautioned on the use of the term metabolically healthy obesity, and these new data do not change our view.”
Dr. Fauchier has acted as a speaker or consultant for AstraZeneca, Bayer, Bristol Myers Squibb Pfizer, Boehringer Ingelheim, Medtronic, Novartis, and XO. Dr. Ho had no relevant conflicts of interest. Dr. Sattar has received grants and personal fees from Boehringer Ingelheim, and personal fees from Amgen, AstraZeneca, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi.
FROM EASD 2021
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
Alcohol ups risk for atrial fibrillation episode hours later
Consuming alcohol increases the risk for an atrial fibrillation (AF) episode hours later, according to a study published online Aug. 30 in Annals of Internal Medicine.
Past research has associated long-term alcohol consumption with the development of AF, and abstinence from alcohol has been associated with a lower overall AF burden. However, lead study author Greg Marcus, MD, a cardioelectrophysiolgist at the University of California, San Francisco, noted that many patients say that alcohol is a trigger for discrete AF episodes.
To test whether that was possible, the researchers enrolled 100 patients who had a history of AF events and who consumed at least one drink per month. Participants wore a transdermal alcohol sensor and an ambulatory, single-lead electrocardiogram device for 4 weeks. They were instructed to press a button on the electrocardiogram device each time they consumed a standard alcoholic beverage. In addition, blood samples were tested for phosphatidylethanol (PEth) at the participants’ 2-week and 4-week visits. PEth is a phospholipid formed in the blood after alcohol intake. It remains in the blood for up to 4 weeks after alcohol consumption.
The study findings confirmed what the patients had reported. The odds of an AF episode were 38% greater with every 0.1% increase in peak blood alcohol concentration over the previous 12 hours (odds ratio [OR], 1.38; 95% confidence interval, 1.04-1.83; P = .024). Moreover, an episode of AF was associated with twofold greater odds (OR, 2.02; 95% CI, 1.38-3.17) of having consumed one alcoholic drink in the past 4 hours. It was associated with more than threefold greater odds of having consumed two or more drinks (OR, 3.58; 95% CI, 1.63-7.89).
“The major takeaway is, among atrial fibrillation patients, consuming alcohol substantially heightened their risk for any given atrial fibrillation event in the subsequent few hours,” Dr. Marcus said. “The more alcohol consumed, the higher that risk.”
The acute effect of alcohol on these arrhythmias also means that modifying alcohol consumption could immediately benefit some patients. “These data combined with other evidence suggest that recommending minimizing or completely eliminating alcohol will likely be helpful to them,” Dr. Marcus said.
The study’s reliance on wearables and sensors was impressive, said Mariann R. Piano, PhD, director of the Center for Research Development and Scholarship, Vanderbilt University, Nashville, Tenn. Often, these types of studies are “self-reported and confounded by recall bias,” she said. But this study passively documented arrhythmia events and blood alcohol level without any patient input. The additional measures of alcohol consumption were used to validate the blood alcohol sensor.
The study’s focus on patients with a history of AF highlighted a high-risk patient group, according to Dr. Piano, who coauthored an editorial about the study. However, the findings may not be applicable to the general population.
Dr. Marcus said alcohol’s role in causing these types of arrhythmias is probably a matter of degree. AF patients are more prone to events than is the general population and are therefore more sensitive to alcohol, he said. But excessive alcohol consumption could increase the chance of AF in the general population.
The study is not without its limitations, however. For instance, “it would have been really ideal if we knew what that blood alcohol was” before an episode, Dr. Piano said. The number of drinks is a good start, but two drinks can affect persons differently, depending on their weight and height. Also, baseline PEth values suggest that patients had been drinking before the study, she said. Ideally, patients could have been asked to abstain from alcohol for a period before the study to determine a negative baseline PEth value and minimize the effects of previous drinking on AF episodes.
Moving forward, this research should inform how clinicians care for their AF patients, both experts agree. “We need to talk to patients about how much they drink,” Dr. Piano said. In addition, patients should be advised to closely monitor what they’re drinking.
“This definitely sharpens the focus of the importance of a thorough alcohol history when we see an atrial fibrillation patient and to counsel them to reduce or eliminate alcohol, even among those that don’t have alcohol use disorders,” Dr. Marcus said.
Preliminary results of the study were presented as a late-breaking clinical trials presentation at the American College of Cardiology meeting in May.
Dr. Marcus has received grants from Baylis, Jawbone, and Eight Sleep and has received personal fees from InCarda and Johnson & Johnson. Coauthors have received personal fees from VivaLNK, Huba Pharmaceuticals, Johnson & Johnson, and Merck and grants from Samsung and Amgen Inc. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Consuming alcohol increases the risk for an atrial fibrillation (AF) episode hours later, according to a study published online Aug. 30 in Annals of Internal Medicine.
Past research has associated long-term alcohol consumption with the development of AF, and abstinence from alcohol has been associated with a lower overall AF burden. However, lead study author Greg Marcus, MD, a cardioelectrophysiolgist at the University of California, San Francisco, noted that many patients say that alcohol is a trigger for discrete AF episodes.
To test whether that was possible, the researchers enrolled 100 patients who had a history of AF events and who consumed at least one drink per month. Participants wore a transdermal alcohol sensor and an ambulatory, single-lead electrocardiogram device for 4 weeks. They were instructed to press a button on the electrocardiogram device each time they consumed a standard alcoholic beverage. In addition, blood samples were tested for phosphatidylethanol (PEth) at the participants’ 2-week and 4-week visits. PEth is a phospholipid formed in the blood after alcohol intake. It remains in the blood for up to 4 weeks after alcohol consumption.
The study findings confirmed what the patients had reported. The odds of an AF episode were 38% greater with every 0.1% increase in peak blood alcohol concentration over the previous 12 hours (odds ratio [OR], 1.38; 95% confidence interval, 1.04-1.83; P = .024). Moreover, an episode of AF was associated with twofold greater odds (OR, 2.02; 95% CI, 1.38-3.17) of having consumed one alcoholic drink in the past 4 hours. It was associated with more than threefold greater odds of having consumed two or more drinks (OR, 3.58; 95% CI, 1.63-7.89).
“The major takeaway is, among atrial fibrillation patients, consuming alcohol substantially heightened their risk for any given atrial fibrillation event in the subsequent few hours,” Dr. Marcus said. “The more alcohol consumed, the higher that risk.”
The acute effect of alcohol on these arrhythmias also means that modifying alcohol consumption could immediately benefit some patients. “These data combined with other evidence suggest that recommending minimizing or completely eliminating alcohol will likely be helpful to them,” Dr. Marcus said.
The study’s reliance on wearables and sensors was impressive, said Mariann R. Piano, PhD, director of the Center for Research Development and Scholarship, Vanderbilt University, Nashville, Tenn. Often, these types of studies are “self-reported and confounded by recall bias,” she said. But this study passively documented arrhythmia events and blood alcohol level without any patient input. The additional measures of alcohol consumption were used to validate the blood alcohol sensor.
The study’s focus on patients with a history of AF highlighted a high-risk patient group, according to Dr. Piano, who coauthored an editorial about the study. However, the findings may not be applicable to the general population.
Dr. Marcus said alcohol’s role in causing these types of arrhythmias is probably a matter of degree. AF patients are more prone to events than is the general population and are therefore more sensitive to alcohol, he said. But excessive alcohol consumption could increase the chance of AF in the general population.
The study is not without its limitations, however. For instance, “it would have been really ideal if we knew what that blood alcohol was” before an episode, Dr. Piano said. The number of drinks is a good start, but two drinks can affect persons differently, depending on their weight and height. Also, baseline PEth values suggest that patients had been drinking before the study, she said. Ideally, patients could have been asked to abstain from alcohol for a period before the study to determine a negative baseline PEth value and minimize the effects of previous drinking on AF episodes.
Moving forward, this research should inform how clinicians care for their AF patients, both experts agree. “We need to talk to patients about how much they drink,” Dr. Piano said. In addition, patients should be advised to closely monitor what they’re drinking.
“This definitely sharpens the focus of the importance of a thorough alcohol history when we see an atrial fibrillation patient and to counsel them to reduce or eliminate alcohol, even among those that don’t have alcohol use disorders,” Dr. Marcus said.
Preliminary results of the study were presented as a late-breaking clinical trials presentation at the American College of Cardiology meeting in May.
Dr. Marcus has received grants from Baylis, Jawbone, and Eight Sleep and has received personal fees from InCarda and Johnson & Johnson. Coauthors have received personal fees from VivaLNK, Huba Pharmaceuticals, Johnson & Johnson, and Merck and grants from Samsung and Amgen Inc. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Consuming alcohol increases the risk for an atrial fibrillation (AF) episode hours later, according to a study published online Aug. 30 in Annals of Internal Medicine.
Past research has associated long-term alcohol consumption with the development of AF, and abstinence from alcohol has been associated with a lower overall AF burden. However, lead study author Greg Marcus, MD, a cardioelectrophysiolgist at the University of California, San Francisco, noted that many patients say that alcohol is a trigger for discrete AF episodes.
To test whether that was possible, the researchers enrolled 100 patients who had a history of AF events and who consumed at least one drink per month. Participants wore a transdermal alcohol sensor and an ambulatory, single-lead electrocardiogram device for 4 weeks. They were instructed to press a button on the electrocardiogram device each time they consumed a standard alcoholic beverage. In addition, blood samples were tested for phosphatidylethanol (PEth) at the participants’ 2-week and 4-week visits. PEth is a phospholipid formed in the blood after alcohol intake. It remains in the blood for up to 4 weeks after alcohol consumption.
The study findings confirmed what the patients had reported. The odds of an AF episode were 38% greater with every 0.1% increase in peak blood alcohol concentration over the previous 12 hours (odds ratio [OR], 1.38; 95% confidence interval, 1.04-1.83; P = .024). Moreover, an episode of AF was associated with twofold greater odds (OR, 2.02; 95% CI, 1.38-3.17) of having consumed one alcoholic drink in the past 4 hours. It was associated with more than threefold greater odds of having consumed two or more drinks (OR, 3.58; 95% CI, 1.63-7.89).
“The major takeaway is, among atrial fibrillation patients, consuming alcohol substantially heightened their risk for any given atrial fibrillation event in the subsequent few hours,” Dr. Marcus said. “The more alcohol consumed, the higher that risk.”
The acute effect of alcohol on these arrhythmias also means that modifying alcohol consumption could immediately benefit some patients. “These data combined with other evidence suggest that recommending minimizing or completely eliminating alcohol will likely be helpful to them,” Dr. Marcus said.
The study’s reliance on wearables and sensors was impressive, said Mariann R. Piano, PhD, director of the Center for Research Development and Scholarship, Vanderbilt University, Nashville, Tenn. Often, these types of studies are “self-reported and confounded by recall bias,” she said. But this study passively documented arrhythmia events and blood alcohol level without any patient input. The additional measures of alcohol consumption were used to validate the blood alcohol sensor.
The study’s focus on patients with a history of AF highlighted a high-risk patient group, according to Dr. Piano, who coauthored an editorial about the study. However, the findings may not be applicable to the general population.
Dr. Marcus said alcohol’s role in causing these types of arrhythmias is probably a matter of degree. AF patients are more prone to events than is the general population and are therefore more sensitive to alcohol, he said. But excessive alcohol consumption could increase the chance of AF in the general population.
The study is not without its limitations, however. For instance, “it would have been really ideal if we knew what that blood alcohol was” before an episode, Dr. Piano said. The number of drinks is a good start, but two drinks can affect persons differently, depending on their weight and height. Also, baseline PEth values suggest that patients had been drinking before the study, she said. Ideally, patients could have been asked to abstain from alcohol for a period before the study to determine a negative baseline PEth value and minimize the effects of previous drinking on AF episodes.
Moving forward, this research should inform how clinicians care for their AF patients, both experts agree. “We need to talk to patients about how much they drink,” Dr. Piano said. In addition, patients should be advised to closely monitor what they’re drinking.
“This definitely sharpens the focus of the importance of a thorough alcohol history when we see an atrial fibrillation patient and to counsel them to reduce or eliminate alcohol, even among those that don’t have alcohol use disorders,” Dr. Marcus said.
Preliminary results of the study were presented as a late-breaking clinical trials presentation at the American College of Cardiology meeting in May.
Dr. Marcus has received grants from Baylis, Jawbone, and Eight Sleep and has received personal fees from InCarda and Johnson & Johnson. Coauthors have received personal fees from VivaLNK, Huba Pharmaceuticals, Johnson & Johnson, and Merck and grants from Samsung and Amgen Inc. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
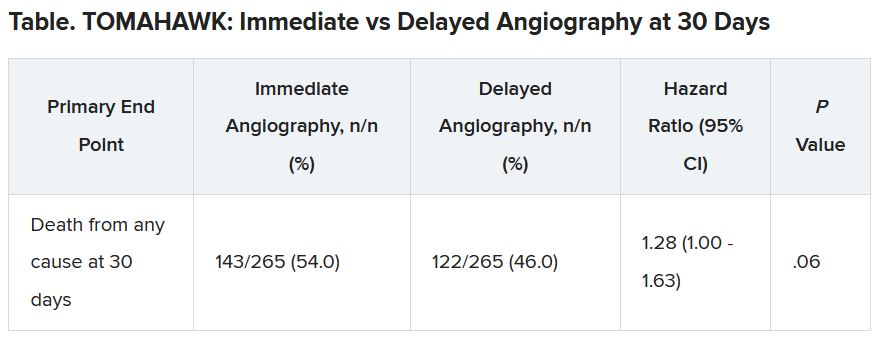
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
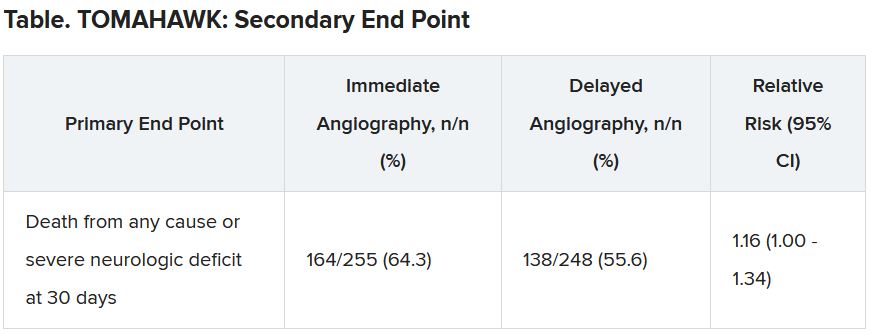
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
LOOP trial undercuts value of long-term continuous ECG screening for AFib
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Coffee drinking in midlife tied to heart benefits
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
APAF-CRT: ‘Ablate and pace’ cuts mortality in narrow-QRS HF, permanent AFib
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.