User login
No evidence to guide selection of biologic for severe asthma
Although “biologics have been really revolutionary for the treatment of severe uncontrolled asthma, we still don’t have evidence to know the right drug for the right patient,” said Wendy Moore, MD, of Wake Forest University, Winston-Salem, N.C.
“You start with your best guess and then switch,” she said in an interview.
There are no real-world contemporary measurements of biologic therapy in the United States at this time, Dr. Moore explained during her presentation of findings from the CHRONICLE trial at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The agents have different targets: omalizumab targets immunoglobulin E, mepolizumab and reslizumab target interleukin (IL)-5, benralizumab targets the IL-5 receptor, and dupilumab targets the common receptor IL-4 receptor A for IL-4 and IL-13.
When the starting biologic doesn’t get the desired results, there is no evidence to show whether another will work better. What we say is, “This one is not working as well as I’d like, let’s try something new?” said Dr. Moore.
However, when looking at data on patients with severe asthma who change from one biologic to another, “I was actually pleased to see that only 10% are switching,” she said in an interview.
But, she added, “if you add that up with the 8% who are stopping, that means that almost 20% don’t get the clinical response they want.”
CHRONICLE trial
In the ongoing observational CHRONICLE trial, Dr. Moore and colleagues assessed biologic initiations, discontinuations, and switches to a different agent.
All 1,884 study participants had a diagnosis of severe asthma and were being treated by an allergist/immunologist or a pulmonologist. All were taking high-dose inhaled corticosteroids and additional controllers, or had received an Food and Drug Administration–approved monoclonal antibody, systemic corticosteroid, or another systemic immunosuppressant for at least half of the previous 12 months.
In the study cohort, 1,219 participants were receiving one biologic and 27 were receiving two.
Before November 2018, “it was almost universally all benralizumab being prescribed.” An earlier preference was omalizumab, which was prescribed to 99% of patients before November 2015 and to 45% from November 2017 to November 2018.
“As new drugs were introduced, patients were switched if the desired outcome was not achieved,” Dr. Moore explained.
Over the 2-year period from February 2018 to February 2020, 134 patients – about 10% of all participants taking a biologic – made 148 switches to another biologic.
“The most common reasons reported for switching were lack of efficacy, worsening of asthma control, or waning efficacy,” Dr. Moore reported.
Of the 101 patients (8%) who discontinued 106 biologics, reasons cited were a worsening of asthma symptoms, a desire to change to a cheaper medication, and a waning of effectiveness.
“It seems that the biologic used depended on when you started and whether you were prescribed by an immunologist or pulmonologist,” said Dr. Moore. “I don’t think we understand the perfect patient for any one of these drugs.”
Large-population studies need to be done on each of the drugs. “You have to look at who’s the super responder, the partial responder, compared with the nonresponders, for each medication, but those comparative studies are unlikely to happen,” she said.
In her own practice, her 175 patients are “pretty evenly split between dupilumab, benralizumab, and mepolizumab.”
I have opinions on what works, said Dr. Moore, but none of it is evidence-based. “Those with upper airway involvement with chronic sinusitis tend to do better with mepolizumab than benralizumab. My opinion,” she emphasized.
“People with nasal problems may do better with dupilumab and mepolizumab,” she added. “Also in my opinion.
“But more likely, the issue is you have a partial responder who’s on a T2 high drug but has a T2 low problem too.”
PATHWAY study
Findings from the phase 2B PATHWAY study showed that tezepelumab reduced exacerbations in patients with uncontrolled asthma better than inhaled corticosteroids, and improved forced expiratory volume in 1 second.
“Adherence was monitored very carefully,” said investigator Jonathan Corren, MD, of the University of California, Los Angeles, who presented the PATHWAY data. This could explain, in part, why some patients in the control group “showed improvement from baseline.”
Before switching to a biologic, “we should always consider some of these issues that might contribute to better asthma control, like patient adherence or the inability to use an inhaler properly,” Dr. Corren said.
Some people have never been “shown how to use their inhalers properly,” said Moore. “Some of them come back fine when we show them.”
Dr. Moore has been on the advisory board for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Regeneron, and Sanofi. Dr. Corren reports receiving honoraria from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Although “biologics have been really revolutionary for the treatment of severe uncontrolled asthma, we still don’t have evidence to know the right drug for the right patient,” said Wendy Moore, MD, of Wake Forest University, Winston-Salem, N.C.
“You start with your best guess and then switch,” she said in an interview.
There are no real-world contemporary measurements of biologic therapy in the United States at this time, Dr. Moore explained during her presentation of findings from the CHRONICLE trial at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The agents have different targets: omalizumab targets immunoglobulin E, mepolizumab and reslizumab target interleukin (IL)-5, benralizumab targets the IL-5 receptor, and dupilumab targets the common receptor IL-4 receptor A for IL-4 and IL-13.
When the starting biologic doesn’t get the desired results, there is no evidence to show whether another will work better. What we say is, “This one is not working as well as I’d like, let’s try something new?” said Dr. Moore.
However, when looking at data on patients with severe asthma who change from one biologic to another, “I was actually pleased to see that only 10% are switching,” she said in an interview.
But, she added, “if you add that up with the 8% who are stopping, that means that almost 20% don’t get the clinical response they want.”
CHRONICLE trial
In the ongoing observational CHRONICLE trial, Dr. Moore and colleagues assessed biologic initiations, discontinuations, and switches to a different agent.
All 1,884 study participants had a diagnosis of severe asthma and were being treated by an allergist/immunologist or a pulmonologist. All were taking high-dose inhaled corticosteroids and additional controllers, or had received an Food and Drug Administration–approved monoclonal antibody, systemic corticosteroid, or another systemic immunosuppressant for at least half of the previous 12 months.
In the study cohort, 1,219 participants were receiving one biologic and 27 were receiving two.
Before November 2018, “it was almost universally all benralizumab being prescribed.” An earlier preference was omalizumab, which was prescribed to 99% of patients before November 2015 and to 45% from November 2017 to November 2018.
“As new drugs were introduced, patients were switched if the desired outcome was not achieved,” Dr. Moore explained.
Over the 2-year period from February 2018 to February 2020, 134 patients – about 10% of all participants taking a biologic – made 148 switches to another biologic.
“The most common reasons reported for switching were lack of efficacy, worsening of asthma control, or waning efficacy,” Dr. Moore reported.
Of the 101 patients (8%) who discontinued 106 biologics, reasons cited were a worsening of asthma symptoms, a desire to change to a cheaper medication, and a waning of effectiveness.
“It seems that the biologic used depended on when you started and whether you were prescribed by an immunologist or pulmonologist,” said Dr. Moore. “I don’t think we understand the perfect patient for any one of these drugs.”
Large-population studies need to be done on each of the drugs. “You have to look at who’s the super responder, the partial responder, compared with the nonresponders, for each medication, but those comparative studies are unlikely to happen,” she said.
In her own practice, her 175 patients are “pretty evenly split between dupilumab, benralizumab, and mepolizumab.”
I have opinions on what works, said Dr. Moore, but none of it is evidence-based. “Those with upper airway involvement with chronic sinusitis tend to do better with mepolizumab than benralizumab. My opinion,” she emphasized.
“People with nasal problems may do better with dupilumab and mepolizumab,” she added. “Also in my opinion.
“But more likely, the issue is you have a partial responder who’s on a T2 high drug but has a T2 low problem too.”
PATHWAY study
Findings from the phase 2B PATHWAY study showed that tezepelumab reduced exacerbations in patients with uncontrolled asthma better than inhaled corticosteroids, and improved forced expiratory volume in 1 second.
“Adherence was monitored very carefully,” said investigator Jonathan Corren, MD, of the University of California, Los Angeles, who presented the PATHWAY data. This could explain, in part, why some patients in the control group “showed improvement from baseline.”
Before switching to a biologic, “we should always consider some of these issues that might contribute to better asthma control, like patient adherence or the inability to use an inhaler properly,” Dr. Corren said.
Some people have never been “shown how to use their inhalers properly,” said Moore. “Some of them come back fine when we show them.”
Dr. Moore has been on the advisory board for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Regeneron, and Sanofi. Dr. Corren reports receiving honoraria from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Although “biologics have been really revolutionary for the treatment of severe uncontrolled asthma, we still don’t have evidence to know the right drug for the right patient,” said Wendy Moore, MD, of Wake Forest University, Winston-Salem, N.C.
“You start with your best guess and then switch,” she said in an interview.
There are no real-world contemporary measurements of biologic therapy in the United States at this time, Dr. Moore explained during her presentation of findings from the CHRONICLE trial at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The agents have different targets: omalizumab targets immunoglobulin E, mepolizumab and reslizumab target interleukin (IL)-5, benralizumab targets the IL-5 receptor, and dupilumab targets the common receptor IL-4 receptor A for IL-4 and IL-13.
When the starting biologic doesn’t get the desired results, there is no evidence to show whether another will work better. What we say is, “This one is not working as well as I’d like, let’s try something new?” said Dr. Moore.
However, when looking at data on patients with severe asthma who change from one biologic to another, “I was actually pleased to see that only 10% are switching,” she said in an interview.
But, she added, “if you add that up with the 8% who are stopping, that means that almost 20% don’t get the clinical response they want.”
CHRONICLE trial
In the ongoing observational CHRONICLE trial, Dr. Moore and colleagues assessed biologic initiations, discontinuations, and switches to a different agent.
All 1,884 study participants had a diagnosis of severe asthma and were being treated by an allergist/immunologist or a pulmonologist. All were taking high-dose inhaled corticosteroids and additional controllers, or had received an Food and Drug Administration–approved monoclonal antibody, systemic corticosteroid, or another systemic immunosuppressant for at least half of the previous 12 months.
In the study cohort, 1,219 participants were receiving one biologic and 27 were receiving two.
Before November 2018, “it was almost universally all benralizumab being prescribed.” An earlier preference was omalizumab, which was prescribed to 99% of patients before November 2015 and to 45% from November 2017 to November 2018.
“As new drugs were introduced, patients were switched if the desired outcome was not achieved,” Dr. Moore explained.
Over the 2-year period from February 2018 to February 2020, 134 patients – about 10% of all participants taking a biologic – made 148 switches to another biologic.
“The most common reasons reported for switching were lack of efficacy, worsening of asthma control, or waning efficacy,” Dr. Moore reported.
Of the 101 patients (8%) who discontinued 106 biologics, reasons cited were a worsening of asthma symptoms, a desire to change to a cheaper medication, and a waning of effectiveness.
“It seems that the biologic used depended on when you started and whether you were prescribed by an immunologist or pulmonologist,” said Dr. Moore. “I don’t think we understand the perfect patient for any one of these drugs.”
Large-population studies need to be done on each of the drugs. “You have to look at who’s the super responder, the partial responder, compared with the nonresponders, for each medication, but those comparative studies are unlikely to happen,” she said.
In her own practice, her 175 patients are “pretty evenly split between dupilumab, benralizumab, and mepolizumab.”
I have opinions on what works, said Dr. Moore, but none of it is evidence-based. “Those with upper airway involvement with chronic sinusitis tend to do better with mepolizumab than benralizumab. My opinion,” she emphasized.
“People with nasal problems may do better with dupilumab and mepolizumab,” she added. “Also in my opinion.
“But more likely, the issue is you have a partial responder who’s on a T2 high drug but has a T2 low problem too.”
PATHWAY study
Findings from the phase 2B PATHWAY study showed that tezepelumab reduced exacerbations in patients with uncontrolled asthma better than inhaled corticosteroids, and improved forced expiratory volume in 1 second.
“Adherence was monitored very carefully,” said investigator Jonathan Corren, MD, of the University of California, Los Angeles, who presented the PATHWAY data. This could explain, in part, why some patients in the control group “showed improvement from baseline.”
Before switching to a biologic, “we should always consider some of these issues that might contribute to better asthma control, like patient adherence or the inability to use an inhaler properly,” Dr. Corren said.
Some people have never been “shown how to use their inhalers properly,” said Moore. “Some of them come back fine when we show them.”
Dr. Moore has been on the advisory board for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Regeneron, and Sanofi. Dr. Corren reports receiving honoraria from AstraZeneca.
A version of this article originally appeared on Medscape.com.
An assessment of asthma drugs in pregnancy
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Severe Asthma: Changing the Game
In this supplement to CHEST Physician, Dr. Sandra Adams investigates the following topics:
- Difficult-to-control vs severe asthma
- T2-high inflammatory endotype
- T2-low endotype
- Biologic therapies in severe asthma
- Treatment follow-up and assessment
Click here to read.
Author
Sandra G. Adams, MD, MS, FCCP
Division of Pulmonary
Diseases and Critical Care Medicine
UT Health San Antonio
Staff Physician,
Care System
San Antonio, TX
In this supplement to CHEST Physician, Dr. Sandra Adams investigates the following topics:
- Difficult-to-control vs severe asthma
- T2-high inflammatory endotype
- T2-low endotype
- Biologic therapies in severe asthma
- Treatment follow-up and assessment
Click here to read.
Author
Sandra G. Adams, MD, MS, FCCP
Division of Pulmonary
Diseases and Critical Care Medicine
UT Health San Antonio
Staff Physician,
Care System
San Antonio, TX
In this supplement to CHEST Physician, Dr. Sandra Adams investigates the following topics:
- Difficult-to-control vs severe asthma
- T2-high inflammatory endotype
- T2-low endotype
- Biologic therapies in severe asthma
- Treatment follow-up and assessment
Click here to read.
Author
Sandra G. Adams, MD, MS, FCCP
Division of Pulmonary
Diseases and Critical Care Medicine
UT Health San Antonio
Staff Physician,
Care System
San Antonio, TX
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
More data on impact of corticosteroids on COVID-19 mortality in patients with COPD
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
FROM LANCET RESPIRATORY MEDICINE
Daily Recap: Lifestyle vs. genes in breast cancer showdown; Big pharma sues over insulin affordability law
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Black women at highest risk for asthma
Women are much more likely than men to have asthma, and , according to the Centers for Disease Control and Prevention.
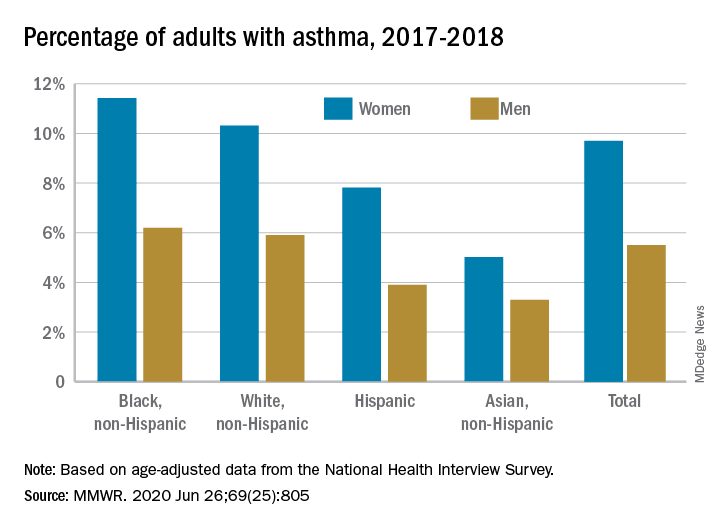
Among all women aged 18 years and older, 9.7% reported that they currently had asthma in 2017-2018, compared with 5.5% of men, based on age-adjusted data from the National Health Interview Survey.
The proportion of black, non-Hispanic women with asthma, however, was even higher, at 11.4%. White non-Hispanic women were next at 10.3%, followed by Hispanic (7.8%) and Asian (5.0%) women, the CDC reported June 26 in the Morbidity and Mortality Weekly Report.
The same pattern held for men: 6.2% of black men had asthma in 2017-2018, compared with 5.9% of whites, 3.9% of Hispanics, and 3.3% of Asian men, the CDC said.
SOURCE: MMWR. 2020 Jun 26;69(25):805.
Women are much more likely than men to have asthma, and , according to the Centers for Disease Control and Prevention.

Among all women aged 18 years and older, 9.7% reported that they currently had asthma in 2017-2018, compared with 5.5% of men, based on age-adjusted data from the National Health Interview Survey.
The proportion of black, non-Hispanic women with asthma, however, was even higher, at 11.4%. White non-Hispanic women were next at 10.3%, followed by Hispanic (7.8%) and Asian (5.0%) women, the CDC reported June 26 in the Morbidity and Mortality Weekly Report.
The same pattern held for men: 6.2% of black men had asthma in 2017-2018, compared with 5.9% of whites, 3.9% of Hispanics, and 3.3% of Asian men, the CDC said.
SOURCE: MMWR. 2020 Jun 26;69(25):805.
Women are much more likely than men to have asthma, and , according to the Centers for Disease Control and Prevention.

Among all women aged 18 years and older, 9.7% reported that they currently had asthma in 2017-2018, compared with 5.5% of men, based on age-adjusted data from the National Health Interview Survey.
The proportion of black, non-Hispanic women with asthma, however, was even higher, at 11.4%. White non-Hispanic women were next at 10.3%, followed by Hispanic (7.8%) and Asian (5.0%) women, the CDC reported June 26 in the Morbidity and Mortality Weekly Report.
The same pattern held for men: 6.2% of black men had asthma in 2017-2018, compared with 5.9% of whites, 3.9% of Hispanics, and 3.3% of Asian men, the CDC said.
SOURCE: MMWR. 2020 Jun 26;69(25):805.
FROM MMWR
Asthma leads spending on avoidable pediatric inpatient stays
according to the Agency for Healthcare Research and Quality.

The cost of potentially avoidable visits for asthma that year was $278 million, versus $284 million combined for the other three conditions “that evidence suggests may be avoidable, in part, through timely and quality primary and preventive care,” Kimberly W. McDermott, PhD, and H. Joanna Jiang, PhD, said in an AHRQ statistical brief.
Those three other conditions are diabetes short-term complications, gastroenteritis, and urinary tract infections (UTIs). Neonatal stays were excluded from the analysis, Dr. McDermott of IBM Watson Health and Dr. Jiang of the AHRQ noted.
The state inpatient databases of the AHRQ’s Healthcare Cost and Utilization Project included 1.4 million inpatient stays among children aged 3 months to 17 years in 2017, of which 8% (108,300) were deemed potentially preventable. Hospital charges for the preventable stays came to $561.6 million, or 3% of the $20 billion in total costs for all nonneonatal stays, they said.
Rates of potentially avoidable stays for asthma (159 per 100,000 population), gastroenteritis (90 per 100,000), and UTIs (41 per 100,000) were highest for children aged 0-4 years and generally decreased with age, but diabetes stays increased with age, rising from 12 per 100,000 in children aged 5-9 years to 38 per 100,000 for those 15-17 years old, the researchers said.
Black children had a much higher rate of potentially avoidable stays for asthma (218 per 100,000) than did Hispanic children (74), Asian/Pacific Islander children (46), or white children (43), but children classified as other race/ethnicity were higher still: 380 per 100,000. Rates for children classified as other race/ethnicity were highest for the other three conditions as well, they reported.
Comparisons by sex for the four conditions ended up in a 2-2 tie: Girls had higher rates for diabetes (28 vs. 23) and UTIs (35 vs. 8), and boys had higher rates for asthma (96 vs. 67) and gastroenteritis (38 vs. 35), Dr. McDermott and Dr. Jiang reported.
SOURCE: McDermott KW, Jiang HJ. HCUP Statistical Brief #259. June 2020.
according to the Agency for Healthcare Research and Quality.

The cost of potentially avoidable visits for asthma that year was $278 million, versus $284 million combined for the other three conditions “that evidence suggests may be avoidable, in part, through timely and quality primary and preventive care,” Kimberly W. McDermott, PhD, and H. Joanna Jiang, PhD, said in an AHRQ statistical brief.
Those three other conditions are diabetes short-term complications, gastroenteritis, and urinary tract infections (UTIs). Neonatal stays were excluded from the analysis, Dr. McDermott of IBM Watson Health and Dr. Jiang of the AHRQ noted.
The state inpatient databases of the AHRQ’s Healthcare Cost and Utilization Project included 1.4 million inpatient stays among children aged 3 months to 17 years in 2017, of which 8% (108,300) were deemed potentially preventable. Hospital charges for the preventable stays came to $561.6 million, or 3% of the $20 billion in total costs for all nonneonatal stays, they said.
Rates of potentially avoidable stays for asthma (159 per 100,000 population), gastroenteritis (90 per 100,000), and UTIs (41 per 100,000) were highest for children aged 0-4 years and generally decreased with age, but diabetes stays increased with age, rising from 12 per 100,000 in children aged 5-9 years to 38 per 100,000 for those 15-17 years old, the researchers said.
Black children had a much higher rate of potentially avoidable stays for asthma (218 per 100,000) than did Hispanic children (74), Asian/Pacific Islander children (46), or white children (43), but children classified as other race/ethnicity were higher still: 380 per 100,000. Rates for children classified as other race/ethnicity were highest for the other three conditions as well, they reported.
Comparisons by sex for the four conditions ended up in a 2-2 tie: Girls had higher rates for diabetes (28 vs. 23) and UTIs (35 vs. 8), and boys had higher rates for asthma (96 vs. 67) and gastroenteritis (38 vs. 35), Dr. McDermott and Dr. Jiang reported.
SOURCE: McDermott KW, Jiang HJ. HCUP Statistical Brief #259. June 2020.
according to the Agency for Healthcare Research and Quality.

The cost of potentially avoidable visits for asthma that year was $278 million, versus $284 million combined for the other three conditions “that evidence suggests may be avoidable, in part, through timely and quality primary and preventive care,” Kimberly W. McDermott, PhD, and H. Joanna Jiang, PhD, said in an AHRQ statistical brief.
Those three other conditions are diabetes short-term complications, gastroenteritis, and urinary tract infections (UTIs). Neonatal stays were excluded from the analysis, Dr. McDermott of IBM Watson Health and Dr. Jiang of the AHRQ noted.
The state inpatient databases of the AHRQ’s Healthcare Cost and Utilization Project included 1.4 million inpatient stays among children aged 3 months to 17 years in 2017, of which 8% (108,300) were deemed potentially preventable. Hospital charges for the preventable stays came to $561.6 million, or 3% of the $20 billion in total costs for all nonneonatal stays, they said.
Rates of potentially avoidable stays for asthma (159 per 100,000 population), gastroenteritis (90 per 100,000), and UTIs (41 per 100,000) were highest for children aged 0-4 years and generally decreased with age, but diabetes stays increased with age, rising from 12 per 100,000 in children aged 5-9 years to 38 per 100,000 for those 15-17 years old, the researchers said.
Black children had a much higher rate of potentially avoidable stays for asthma (218 per 100,000) than did Hispanic children (74), Asian/Pacific Islander children (46), or white children (43), but children classified as other race/ethnicity were higher still: 380 per 100,000. Rates for children classified as other race/ethnicity were highest for the other three conditions as well, they reported.
Comparisons by sex for the four conditions ended up in a 2-2 tie: Girls had higher rates for diabetes (28 vs. 23) and UTIs (35 vs. 8), and boys had higher rates for asthma (96 vs. 67) and gastroenteritis (38 vs. 35), Dr. McDermott and Dr. Jiang reported.
SOURCE: McDermott KW, Jiang HJ. HCUP Statistical Brief #259. June 2020.
Kids with food allergies the newest victims of COVID-19?
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
By the numbers: Asthma-COPD overlap deaths
Death rates for combined asthma and chronic obstructive pulmonary disease declined during 1999-2016, but the risk remains higher among women, compared with men, and in certain occupations, according to a recent report from the Centers for Disease Control and Prevention.

There is also an association between mortality and nonworking status among adults aged 25-64 years, which “suggests that asthma-COPD overlap might be associated with substantial morbidity,” Katelynn E. Dodd, MPH, and associates at the CDC’s National Institute for Occupational Safety and Health said in the Morbidity and Mortality Weekly Report. “These patients have been reported to have worse health outcomes than do those with asthma or COPD alone.”
For females with asthma-COPD overlap, the age-adjusted death rate among adults aged 25 years and older dropped from 7.71 per million in 1999 to 4.01 in 2016, with corresponding rates of 6.70 and 3.01 per million for males, they reported.
In 1999-2016, a total of 18,766 U.S. decedents aged ≥25 years had both asthma and COPD assigned as the underlying or contributing cause of death (12,028 women and 6,738 men), for an overall death rate of 5.03 per million persons (women, 5.59; men, 4.30), data from the National Vital Statistics System show.
Additional analysis, based on the calculation of proportionate mortality ratios (PMRs), also showed that mortality varied by occupational status and age for both males and females, the investigators said, noting that workplace exposures, such as dusts and secondhand smoke, are known to cause both asthma and COPD.
The PMR represents the observed number of deaths from asthma-COPD overlap in a specified industry or occupation, divided by the expected number of deaths, so a value over 1.0 indicates that there were more deaths associated with the condition than expected, Ms. Dodd and her associates explained.
Among female decedents, the occupation with the highest PMR that was statistically significant was bartending at 3.28. For men, the highest significant PMR, 5.64, occurred in logging workers. Those rates, however, only applied to one of the two age groups: 25-64 years in women and ≥65 in men, based on data from the National Occupational Mortality Surveillance, which included information from 26 states for the years 1999, 2003, 2004, and 2007-2014.
Occupationally speaking, the one area of common ground between males and females was lack of occupation. PMRs for those aged 25-64 years “were significantly elevated among men (1.98) and women (1.79) who were unemployed, never worked, or were disabled workers,” they said. PMRs were elevated for nonworking older males and females but were not significant.
The elevated PMRs suggest “that asthma-COPD overlap might be associated with substantial morbidity resulting in loss of employment [because] retired and unemployed persons might have left the workforce because of severe asthma or COPD,” the investigators wrote.
SOURCE: Dodd KE et al. MMWR. 2020 Jun 5. 69(22):670-9.
Death rates for combined asthma and chronic obstructive pulmonary disease declined during 1999-2016, but the risk remains higher among women, compared with men, and in certain occupations, according to a recent report from the Centers for Disease Control and Prevention.

There is also an association between mortality and nonworking status among adults aged 25-64 years, which “suggests that asthma-COPD overlap might be associated with substantial morbidity,” Katelynn E. Dodd, MPH, and associates at the CDC’s National Institute for Occupational Safety and Health said in the Morbidity and Mortality Weekly Report. “These patients have been reported to have worse health outcomes than do those with asthma or COPD alone.”
For females with asthma-COPD overlap, the age-adjusted death rate among adults aged 25 years and older dropped from 7.71 per million in 1999 to 4.01 in 2016, with corresponding rates of 6.70 and 3.01 per million for males, they reported.
In 1999-2016, a total of 18,766 U.S. decedents aged ≥25 years had both asthma and COPD assigned as the underlying or contributing cause of death (12,028 women and 6,738 men), for an overall death rate of 5.03 per million persons (women, 5.59; men, 4.30), data from the National Vital Statistics System show.
Additional analysis, based on the calculation of proportionate mortality ratios (PMRs), also showed that mortality varied by occupational status and age for both males and females, the investigators said, noting that workplace exposures, such as dusts and secondhand smoke, are known to cause both asthma and COPD.
The PMR represents the observed number of deaths from asthma-COPD overlap in a specified industry or occupation, divided by the expected number of deaths, so a value over 1.0 indicates that there were more deaths associated with the condition than expected, Ms. Dodd and her associates explained.
Among female decedents, the occupation with the highest PMR that was statistically significant was bartending at 3.28. For men, the highest significant PMR, 5.64, occurred in logging workers. Those rates, however, only applied to one of the two age groups: 25-64 years in women and ≥65 in men, based on data from the National Occupational Mortality Surveillance, which included information from 26 states for the years 1999, 2003, 2004, and 2007-2014.
Occupationally speaking, the one area of common ground between males and females was lack of occupation. PMRs for those aged 25-64 years “were significantly elevated among men (1.98) and women (1.79) who were unemployed, never worked, or were disabled workers,” they said. PMRs were elevated for nonworking older males and females but were not significant.
The elevated PMRs suggest “that asthma-COPD overlap might be associated with substantial morbidity resulting in loss of employment [because] retired and unemployed persons might have left the workforce because of severe asthma or COPD,” the investigators wrote.
SOURCE: Dodd KE et al. MMWR. 2020 Jun 5. 69(22):670-9.
Death rates for combined asthma and chronic obstructive pulmonary disease declined during 1999-2016, but the risk remains higher among women, compared with men, and in certain occupations, according to a recent report from the Centers for Disease Control and Prevention.

There is also an association between mortality and nonworking status among adults aged 25-64 years, which “suggests that asthma-COPD overlap might be associated with substantial morbidity,” Katelynn E. Dodd, MPH, and associates at the CDC’s National Institute for Occupational Safety and Health said in the Morbidity and Mortality Weekly Report. “These patients have been reported to have worse health outcomes than do those with asthma or COPD alone.”
For females with asthma-COPD overlap, the age-adjusted death rate among adults aged 25 years and older dropped from 7.71 per million in 1999 to 4.01 in 2016, with corresponding rates of 6.70 and 3.01 per million for males, they reported.
In 1999-2016, a total of 18,766 U.S. decedents aged ≥25 years had both asthma and COPD assigned as the underlying or contributing cause of death (12,028 women and 6,738 men), for an overall death rate of 5.03 per million persons (women, 5.59; men, 4.30), data from the National Vital Statistics System show.
Additional analysis, based on the calculation of proportionate mortality ratios (PMRs), also showed that mortality varied by occupational status and age for both males and females, the investigators said, noting that workplace exposures, such as dusts and secondhand smoke, are known to cause both asthma and COPD.
The PMR represents the observed number of deaths from asthma-COPD overlap in a specified industry or occupation, divided by the expected number of deaths, so a value over 1.0 indicates that there were more deaths associated with the condition than expected, Ms. Dodd and her associates explained.
Among female decedents, the occupation with the highest PMR that was statistically significant was bartending at 3.28. For men, the highest significant PMR, 5.64, occurred in logging workers. Those rates, however, only applied to one of the two age groups: 25-64 years in women and ≥65 in men, based on data from the National Occupational Mortality Surveillance, which included information from 26 states for the years 1999, 2003, 2004, and 2007-2014.
Occupationally speaking, the one area of common ground between males and females was lack of occupation. PMRs for those aged 25-64 years “were significantly elevated among men (1.98) and women (1.79) who were unemployed, never worked, or were disabled workers,” they said. PMRs were elevated for nonworking older males and females but were not significant.
The elevated PMRs suggest “that asthma-COPD overlap might be associated with substantial morbidity resulting in loss of employment [because] retired and unemployed persons might have left the workforce because of severe asthma or COPD,” the investigators wrote.
SOURCE: Dodd KE et al. MMWR. 2020 Jun 5. 69(22):670-9.
FROM MMWR