User login
Hospitalized COVID-19 patients with GI symptoms have worse outcomes
Patients with COVID-19 who experience gastrointestinal symptoms have overall worse in-hospital complications but less cardiomyopathy and mortality, according to a new study.
About 20% of COVID-19 patients experience gastrointestinal symptoms, such as abdominal pain, diarrhea, nausea, and vomiting, which clinicians should consider when treating their hospitalized patients, wrote researchers led by Nikita Patil, MD, a hospitalist at Nash General Hospital–UNC Nash Healthcare in Rocky Mount, N.C., in Gastro Hep Advances.
“It’s important to know that certain complications are higher in people with GI symptoms,” she said in an interview. “Even without an increased risk of death, there are many problems that affect quality of life and lead to people not being able to do the things they were able to do before.”
Dr. Patil and colleagues analyzed the association of GI symptoms with adverse outcomes in 100,902 patients from the Cerner Real-World Data COVID-19 Database, which included hospital encounters and ED visits for COVID-19 between December 2019 to November 2020; the data were taken from EMRs at centers with which Cerner has a data use agreement. They also looked at factors associated with poor outcomes such as acute respiratory distress syndrome, sepsis, and ventilator requirement or oxygen dependence.
The average age of the patients was 52, and a higher proportion of patients with GI symptoms were 50 and older. Of those with GI symptoms, 54.5% were women. Overall, patients with GI symptoms were more likely to have higher Charlson Comorbidity Index scores and have comorbidities such as acute liver failure, gastroesophageal reflux disease, GI malignancy, and inflammatory bowel disease.
The research team found that COVID-19 patients with GI symptoms were more likely to have acute respiratory distress syndrome (odds ratio, 1.20; 95% confidence interval, 1.11-1.29), sepsis (OR, 1.19; 95% CI, 1.14-1.24), acute kidney injury (OR, 1.30; 95% CI, 1.24-1.36), venous thromboembolism (OR, 1.36; 95% CI, 1.22-1.52), and GI bleeding (OR 1.62; 95% CI, 1.47-1.79), as compared with COVID-19 patients without GI symptoms (P < .0001 for all comparisons). At the same time, those with GI symptoms were less likely to experience cardiomyopathy (OR, 0.87; 95% CI, 0.77-0.99; P = .027), respiratory failure (OR, 0.92; 95% CI, 0.88-0.95; P < .0001), or death (OR, 0.71; 95% CI, 0.67-0.75; P < .0001).
GI bleed was the most common GI complication, found among 2% of all patients, and was more likely in patients with GI symptoms than in those without (3.5% vs. 1.6%). Intestinal ischemia, pancreatitis, acute liver injury, and intestinal pseudo-obstruction weren’t associated with GI symptoms.
Among the 19,915 patients with GI symptoms, older age, higher Charlson Comorbidity Index scores, use of proton pump inhibitors, and use of H2 receptor antagonists were associated with higher mortality, acute respiratory distress syndrome, sepsis, and ventilator or oxygen requirement. Men with GI symptoms also had a higher risk of mortality, acute respiratory distress syndrome, and sepsis.
In particular, proton pump inhibitor use was associated with more than twice the risk of acute respiratory distress syndrome (OR, 2.19; 95% CI, 1.32-1.66; P < .0001). Similarly, H2 receptor antagonist use was associated with higher likelihood of death (OR, 1.78; 95% CI, 1.57-2.02), as well as more than three times the risk of acute respiratory distress syndrome (OR, 3.75; 95% CI, 3.29-4.28), more than twice the risk of sepsis (OR, 2.50; 95% CI, 2.28-2.73), and nearly twice the risk of ventilator or oxygen dependence (OR, 1.97; 95% CI, 1.68-2.30) (P < .0001 for all).
The findings could guide risk stratification, prognosis, and treatment decisions in COVID-19 patients with GI symptoms, as well as inform future research focused on risk mitigation and improvement of COVID-19 outcomes, Dr. Patil said.
“The protocols for COVID-19 treatment have changed over the past 2 years with blood thinners and steroids,” she said. “Although we likely can’t avoid anti-reflux medicines entirely, it’s something we need to be cognizant of and look out for in our hospitalized patients.”
One study limitation was its inclusion of only inpatient or ED encounters and, therefore, omission of those treated at home; this confers bias toward those with more aggressive disease, according to the authors.
The authors reported no grant support or funding sources for this study. One author declared grant support and consultant fees from several companies, including some medical and pharmaceutical companies, which were unrelated to this research. Dr. Patil reported no disclosures.
This article was updated Aug. 26, 2022.
Patients with COVID-19 who experience gastrointestinal symptoms have overall worse in-hospital complications but less cardiomyopathy and mortality, according to a new study.
About 20% of COVID-19 patients experience gastrointestinal symptoms, such as abdominal pain, diarrhea, nausea, and vomiting, which clinicians should consider when treating their hospitalized patients, wrote researchers led by Nikita Patil, MD, a hospitalist at Nash General Hospital–UNC Nash Healthcare in Rocky Mount, N.C., in Gastro Hep Advances.
“It’s important to know that certain complications are higher in people with GI symptoms,” she said in an interview. “Even without an increased risk of death, there are many problems that affect quality of life and lead to people not being able to do the things they were able to do before.”
Dr. Patil and colleagues analyzed the association of GI symptoms with adverse outcomes in 100,902 patients from the Cerner Real-World Data COVID-19 Database, which included hospital encounters and ED visits for COVID-19 between December 2019 to November 2020; the data were taken from EMRs at centers with which Cerner has a data use agreement. They also looked at factors associated with poor outcomes such as acute respiratory distress syndrome, sepsis, and ventilator requirement or oxygen dependence.
The average age of the patients was 52, and a higher proportion of patients with GI symptoms were 50 and older. Of those with GI symptoms, 54.5% were women. Overall, patients with GI symptoms were more likely to have higher Charlson Comorbidity Index scores and have comorbidities such as acute liver failure, gastroesophageal reflux disease, GI malignancy, and inflammatory bowel disease.
The research team found that COVID-19 patients with GI symptoms were more likely to have acute respiratory distress syndrome (odds ratio, 1.20; 95% confidence interval, 1.11-1.29), sepsis (OR, 1.19; 95% CI, 1.14-1.24), acute kidney injury (OR, 1.30; 95% CI, 1.24-1.36), venous thromboembolism (OR, 1.36; 95% CI, 1.22-1.52), and GI bleeding (OR 1.62; 95% CI, 1.47-1.79), as compared with COVID-19 patients without GI symptoms (P < .0001 for all comparisons). At the same time, those with GI symptoms were less likely to experience cardiomyopathy (OR, 0.87; 95% CI, 0.77-0.99; P = .027), respiratory failure (OR, 0.92; 95% CI, 0.88-0.95; P < .0001), or death (OR, 0.71; 95% CI, 0.67-0.75; P < .0001).
GI bleed was the most common GI complication, found among 2% of all patients, and was more likely in patients with GI symptoms than in those without (3.5% vs. 1.6%). Intestinal ischemia, pancreatitis, acute liver injury, and intestinal pseudo-obstruction weren’t associated with GI symptoms.
Among the 19,915 patients with GI symptoms, older age, higher Charlson Comorbidity Index scores, use of proton pump inhibitors, and use of H2 receptor antagonists were associated with higher mortality, acute respiratory distress syndrome, sepsis, and ventilator or oxygen requirement. Men with GI symptoms also had a higher risk of mortality, acute respiratory distress syndrome, and sepsis.
In particular, proton pump inhibitor use was associated with more than twice the risk of acute respiratory distress syndrome (OR, 2.19; 95% CI, 1.32-1.66; P < .0001). Similarly, H2 receptor antagonist use was associated with higher likelihood of death (OR, 1.78; 95% CI, 1.57-2.02), as well as more than three times the risk of acute respiratory distress syndrome (OR, 3.75; 95% CI, 3.29-4.28), more than twice the risk of sepsis (OR, 2.50; 95% CI, 2.28-2.73), and nearly twice the risk of ventilator or oxygen dependence (OR, 1.97; 95% CI, 1.68-2.30) (P < .0001 for all).
The findings could guide risk stratification, prognosis, and treatment decisions in COVID-19 patients with GI symptoms, as well as inform future research focused on risk mitigation and improvement of COVID-19 outcomes, Dr. Patil said.
“The protocols for COVID-19 treatment have changed over the past 2 years with blood thinners and steroids,” she said. “Although we likely can’t avoid anti-reflux medicines entirely, it’s something we need to be cognizant of and look out for in our hospitalized patients.”
One study limitation was its inclusion of only inpatient or ED encounters and, therefore, omission of those treated at home; this confers bias toward those with more aggressive disease, according to the authors.
The authors reported no grant support or funding sources for this study. One author declared grant support and consultant fees from several companies, including some medical and pharmaceutical companies, which were unrelated to this research. Dr. Patil reported no disclosures.
This article was updated Aug. 26, 2022.
Patients with COVID-19 who experience gastrointestinal symptoms have overall worse in-hospital complications but less cardiomyopathy and mortality, according to a new study.
About 20% of COVID-19 patients experience gastrointestinal symptoms, such as abdominal pain, diarrhea, nausea, and vomiting, which clinicians should consider when treating their hospitalized patients, wrote researchers led by Nikita Patil, MD, a hospitalist at Nash General Hospital–UNC Nash Healthcare in Rocky Mount, N.C., in Gastro Hep Advances.
“It’s important to know that certain complications are higher in people with GI symptoms,” she said in an interview. “Even without an increased risk of death, there are many problems that affect quality of life and lead to people not being able to do the things they were able to do before.”
Dr. Patil and colleagues analyzed the association of GI symptoms with adverse outcomes in 100,902 patients from the Cerner Real-World Data COVID-19 Database, which included hospital encounters and ED visits for COVID-19 between December 2019 to November 2020; the data were taken from EMRs at centers with which Cerner has a data use agreement. They also looked at factors associated with poor outcomes such as acute respiratory distress syndrome, sepsis, and ventilator requirement or oxygen dependence.
The average age of the patients was 52, and a higher proportion of patients with GI symptoms were 50 and older. Of those with GI symptoms, 54.5% were women. Overall, patients with GI symptoms were more likely to have higher Charlson Comorbidity Index scores and have comorbidities such as acute liver failure, gastroesophageal reflux disease, GI malignancy, and inflammatory bowel disease.
The research team found that COVID-19 patients with GI symptoms were more likely to have acute respiratory distress syndrome (odds ratio, 1.20; 95% confidence interval, 1.11-1.29), sepsis (OR, 1.19; 95% CI, 1.14-1.24), acute kidney injury (OR, 1.30; 95% CI, 1.24-1.36), venous thromboembolism (OR, 1.36; 95% CI, 1.22-1.52), and GI bleeding (OR 1.62; 95% CI, 1.47-1.79), as compared with COVID-19 patients without GI symptoms (P < .0001 for all comparisons). At the same time, those with GI symptoms were less likely to experience cardiomyopathy (OR, 0.87; 95% CI, 0.77-0.99; P = .027), respiratory failure (OR, 0.92; 95% CI, 0.88-0.95; P < .0001), or death (OR, 0.71; 95% CI, 0.67-0.75; P < .0001).
GI bleed was the most common GI complication, found among 2% of all patients, and was more likely in patients with GI symptoms than in those without (3.5% vs. 1.6%). Intestinal ischemia, pancreatitis, acute liver injury, and intestinal pseudo-obstruction weren’t associated with GI symptoms.
Among the 19,915 patients with GI symptoms, older age, higher Charlson Comorbidity Index scores, use of proton pump inhibitors, and use of H2 receptor antagonists were associated with higher mortality, acute respiratory distress syndrome, sepsis, and ventilator or oxygen requirement. Men with GI symptoms also had a higher risk of mortality, acute respiratory distress syndrome, and sepsis.
In particular, proton pump inhibitor use was associated with more than twice the risk of acute respiratory distress syndrome (OR, 2.19; 95% CI, 1.32-1.66; P < .0001). Similarly, H2 receptor antagonist use was associated with higher likelihood of death (OR, 1.78; 95% CI, 1.57-2.02), as well as more than three times the risk of acute respiratory distress syndrome (OR, 3.75; 95% CI, 3.29-4.28), more than twice the risk of sepsis (OR, 2.50; 95% CI, 2.28-2.73), and nearly twice the risk of ventilator or oxygen dependence (OR, 1.97; 95% CI, 1.68-2.30) (P < .0001 for all).
The findings could guide risk stratification, prognosis, and treatment decisions in COVID-19 patients with GI symptoms, as well as inform future research focused on risk mitigation and improvement of COVID-19 outcomes, Dr. Patil said.
“The protocols for COVID-19 treatment have changed over the past 2 years with blood thinners and steroids,” she said. “Although we likely can’t avoid anti-reflux medicines entirely, it’s something we need to be cognizant of and look out for in our hospitalized patients.”
One study limitation was its inclusion of only inpatient or ED encounters and, therefore, omission of those treated at home; this confers bias toward those with more aggressive disease, according to the authors.
The authors reported no grant support or funding sources for this study. One author declared grant support and consultant fees from several companies, including some medical and pharmaceutical companies, which were unrelated to this research. Dr. Patil reported no disclosures.
This article was updated Aug. 26, 2022.
FROM GASTRO HEP ADVANCES
Preparing for back to school amid monkeypox outbreak and ever-changing COVID landscape
Unlike last school year, there are now vaccines available for all over the age of 6 months, and home rapid antigen tests are more readily available. Additionally, many have now been exposed either by infection or vaccination to the virus.
The CDC has removed the recommendations for maintaining cohorts in the K-12 population. This changing landscape along with differing levels of personal risk make it challenging to counsel families about what to expect in terms of COVID this year.
The best defense that we currently have against COVID is the vaccine. Although it seems that many are susceptible to the virus despite the vaccine, those who have been vaccinated are less susceptible to serious disease, including young children.
As older children may be heading to college, it is important
to encourage them to isolate when they have symptoms, even when they test negative for COVID as we would all like to avoid being sick in general.
Additionally, they should pay attention to the COVID risk level in their area and wear masks, particularly when indoors, as the levels increase. College students should have a plan for where they can isolate when not feeling well. If anyone does test positive for COVID, they should follow the most recent quarantine guidelines, including wearing a well fitted mask when they do begin returning to activities.
Monkeypox
We now have a new health concern for this school year.
Monkeypox has come onto the scene with information changing as rapidly as information previously did for COVID. With this virus, we must particularly counsel those heading away to college to be careful to limit their exposure to this disease.
Dormitories and other congregate settings are high-risk locations for the spread of monkeypox. Particularly, students headed to stay in dormitories should be counseled about avoiding:
- sexual activity with those with lesions consistent with monkeypox;
- sharing eating and drinking utensils; and
- sleeping in the same bed as or sharing bedding or towels with anyone with a diagnosis of or lesions consistent with monkeypox.
Additionally, as with prevention of all infections, it is important to frequently wash hands or use alcohol-based sanitizer before eating, and avoid touching the face after using the restroom.
Guidance for those eligible for vaccines against monkeypox seems to be quickly changing as well.
At the time of this article, CDC guidance recommends the vaccine against monkeypox for:
- those considered to be at high risk for it, including those identified by public health officials as a contact of someone with monkeypox;
- those who are aware that a sexual partner had a diagnosis of monkeypox within the past 2 weeks;
- those with multiple sex partners in the past 2 weeks in an area with known monkeypox; and
- those whose jobs may expose them to monkeypox.
Currently, the CDC recommends the vaccine JYNNEOS, a two-dose vaccine that reaches maximum protection after fourteen days. Ultimately, guidance is likely to continue to quickly change for both COVID-19 and Monkeypox throughout the fall. It is possible that new vaccinations will become available, and families and physicians alike will have many questions.
Primary care offices should ensure that someone is keeping up to date with the latest guidance to share with the office so that physicians may share accurate information with their patients.
Families should be counseled that we anticipate information about monkeypox, particularly related to vaccinations, to continue to change, as it has during all stages of the COVID pandemic.
As always, patients should be reminded to continue regular routine vaccinations, including the annual influenza vaccine.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Unlike last school year, there are now vaccines available for all over the age of 6 months, and home rapid antigen tests are more readily available. Additionally, many have now been exposed either by infection or vaccination to the virus.
The CDC has removed the recommendations for maintaining cohorts in the K-12 population. This changing landscape along with differing levels of personal risk make it challenging to counsel families about what to expect in terms of COVID this year.
The best defense that we currently have against COVID is the vaccine. Although it seems that many are susceptible to the virus despite the vaccine, those who have been vaccinated are less susceptible to serious disease, including young children.
As older children may be heading to college, it is important
to encourage them to isolate when they have symptoms, even when they test negative for COVID as we would all like to avoid being sick in general.
Additionally, they should pay attention to the COVID risk level in their area and wear masks, particularly when indoors, as the levels increase. College students should have a plan for where they can isolate when not feeling well. If anyone does test positive for COVID, they should follow the most recent quarantine guidelines, including wearing a well fitted mask when they do begin returning to activities.
Monkeypox
We now have a new health concern for this school year.
Monkeypox has come onto the scene with information changing as rapidly as information previously did for COVID. With this virus, we must particularly counsel those heading away to college to be careful to limit their exposure to this disease.
Dormitories and other congregate settings are high-risk locations for the spread of monkeypox. Particularly, students headed to stay in dormitories should be counseled about avoiding:
- sexual activity with those with lesions consistent with monkeypox;
- sharing eating and drinking utensils; and
- sleeping in the same bed as or sharing bedding or towels with anyone with a diagnosis of or lesions consistent with monkeypox.
Additionally, as with prevention of all infections, it is important to frequently wash hands or use alcohol-based sanitizer before eating, and avoid touching the face after using the restroom.
Guidance for those eligible for vaccines against monkeypox seems to be quickly changing as well.
At the time of this article, CDC guidance recommends the vaccine against monkeypox for:
- those considered to be at high risk for it, including those identified by public health officials as a contact of someone with monkeypox;
- those who are aware that a sexual partner had a diagnosis of monkeypox within the past 2 weeks;
- those with multiple sex partners in the past 2 weeks in an area with known monkeypox; and
- those whose jobs may expose them to monkeypox.
Currently, the CDC recommends the vaccine JYNNEOS, a two-dose vaccine that reaches maximum protection after fourteen days. Ultimately, guidance is likely to continue to quickly change for both COVID-19 and Monkeypox throughout the fall. It is possible that new vaccinations will become available, and families and physicians alike will have many questions.
Primary care offices should ensure that someone is keeping up to date with the latest guidance to share with the office so that physicians may share accurate information with their patients.
Families should be counseled that we anticipate information about monkeypox, particularly related to vaccinations, to continue to change, as it has during all stages of the COVID pandemic.
As always, patients should be reminded to continue regular routine vaccinations, including the annual influenza vaccine.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Unlike last school year, there are now vaccines available for all over the age of 6 months, and home rapid antigen tests are more readily available. Additionally, many have now been exposed either by infection or vaccination to the virus.
The CDC has removed the recommendations for maintaining cohorts in the K-12 population. This changing landscape along with differing levels of personal risk make it challenging to counsel families about what to expect in terms of COVID this year.
The best defense that we currently have against COVID is the vaccine. Although it seems that many are susceptible to the virus despite the vaccine, those who have been vaccinated are less susceptible to serious disease, including young children.
As older children may be heading to college, it is important
to encourage them to isolate when they have symptoms, even when they test negative for COVID as we would all like to avoid being sick in general.
Additionally, they should pay attention to the COVID risk level in their area and wear masks, particularly when indoors, as the levels increase. College students should have a plan for where they can isolate when not feeling well. If anyone does test positive for COVID, they should follow the most recent quarantine guidelines, including wearing a well fitted mask when they do begin returning to activities.
Monkeypox
We now have a new health concern for this school year.
Monkeypox has come onto the scene with information changing as rapidly as information previously did for COVID. With this virus, we must particularly counsel those heading away to college to be careful to limit their exposure to this disease.
Dormitories and other congregate settings are high-risk locations for the spread of monkeypox. Particularly, students headed to stay in dormitories should be counseled about avoiding:
- sexual activity with those with lesions consistent with monkeypox;
- sharing eating and drinking utensils; and
- sleeping in the same bed as or sharing bedding or towels with anyone with a diagnosis of or lesions consistent with monkeypox.
Additionally, as with prevention of all infections, it is important to frequently wash hands or use alcohol-based sanitizer before eating, and avoid touching the face after using the restroom.
Guidance for those eligible for vaccines against monkeypox seems to be quickly changing as well.
At the time of this article, CDC guidance recommends the vaccine against monkeypox for:
- those considered to be at high risk for it, including those identified by public health officials as a contact of someone with monkeypox;
- those who are aware that a sexual partner had a diagnosis of monkeypox within the past 2 weeks;
- those with multiple sex partners in the past 2 weeks in an area with known monkeypox; and
- those whose jobs may expose them to monkeypox.
Currently, the CDC recommends the vaccine JYNNEOS, a two-dose vaccine that reaches maximum protection after fourteen days. Ultimately, guidance is likely to continue to quickly change for both COVID-19 and Monkeypox throughout the fall. It is possible that new vaccinations will become available, and families and physicians alike will have many questions.
Primary care offices should ensure that someone is keeping up to date with the latest guidance to share with the office so that physicians may share accurate information with their patients.
Families should be counseled that we anticipate information about monkeypox, particularly related to vaccinations, to continue to change, as it has during all stages of the COVID pandemic.
As always, patients should be reminded to continue regular routine vaccinations, including the annual influenza vaccine.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Implementation of a Virtual Huddle to Support Patient Care During the COVID-19 Pandemic
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
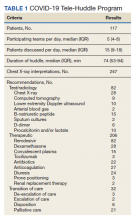
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
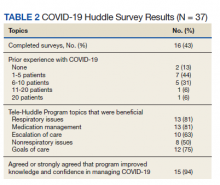
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
COVID to blame as U.S. life expectancy falls
All 50 states and the District of Columbia saw drops in life expectancy, according to the report from the Centers for Disease Control and Prevention’s National Center for Health Statistics.
The declines were mostly because of COVID-19 and “unintentional injuries,” such as drug overdoses.
The overall drop took national life expectancy from 78.8 years in 2019 to 77 years in 2020, the first year of the pandemic, ABC News reported.
States in the West and Northwest generally had higher life expectancy, with states in the South having the lowest.
Hawaii had the highest life expectancy at 80.7 years. It was followed by Washington, Minnesota, California, and Massachusetts. Mississippi had the lowest at 71.9 years, the figures show. The others in the bottom five were West Virginia, Louisiana, Alabama, and Kentucky.
In 2020, COVID-19 was the third-highest cause of death, leading to more than 350,000, the CDC reported earlier this year. At the same time, more people are dying annually from drug overdoses. A record 83,500 fatal overdoses were reported in 2020.
A version of this article first appeared on WebMD.com.
All 50 states and the District of Columbia saw drops in life expectancy, according to the report from the Centers for Disease Control and Prevention’s National Center for Health Statistics.
The declines were mostly because of COVID-19 and “unintentional injuries,” such as drug overdoses.
The overall drop took national life expectancy from 78.8 years in 2019 to 77 years in 2020, the first year of the pandemic, ABC News reported.
States in the West and Northwest generally had higher life expectancy, with states in the South having the lowest.
Hawaii had the highest life expectancy at 80.7 years. It was followed by Washington, Minnesota, California, and Massachusetts. Mississippi had the lowest at 71.9 years, the figures show. The others in the bottom five were West Virginia, Louisiana, Alabama, and Kentucky.
In 2020, COVID-19 was the third-highest cause of death, leading to more than 350,000, the CDC reported earlier this year. At the same time, more people are dying annually from drug overdoses. A record 83,500 fatal overdoses were reported in 2020.
A version of this article first appeared on WebMD.com.
All 50 states and the District of Columbia saw drops in life expectancy, according to the report from the Centers for Disease Control and Prevention’s National Center for Health Statistics.
The declines were mostly because of COVID-19 and “unintentional injuries,” such as drug overdoses.
The overall drop took national life expectancy from 78.8 years in 2019 to 77 years in 2020, the first year of the pandemic, ABC News reported.
States in the West and Northwest generally had higher life expectancy, with states in the South having the lowest.
Hawaii had the highest life expectancy at 80.7 years. It was followed by Washington, Minnesota, California, and Massachusetts. Mississippi had the lowest at 71.9 years, the figures show. The others in the bottom five were West Virginia, Louisiana, Alabama, and Kentucky.
In 2020, COVID-19 was the third-highest cause of death, leading to more than 350,000, the CDC reported earlier this year. At the same time, more people are dying annually from drug overdoses. A record 83,500 fatal overdoses were reported in 2020.
A version of this article first appeared on WebMD.com.
Metformin fails as early COVID-19 treatment but shows potential
Neither metformin, ivermectin, or fluvoxamine had any impact on reducing disease severity, hospitalization, or death from COVID-19, according to results from more than 1,000 overweight or obese adult patients in the COVID-OUT randomized trial.
However, metformin showed some potential in a secondary analysis.
Early treatment to prevent severe disease remains a goal in managing the ongoing COVID-19 pandemic, and biophysical modeling suggested that metformin, ivermectin, and fluvoxamine may serve as antivirals to help reduce severe disease in COVID-19 patients, Carolyn T. Bramante, MD, of the University of Minnesota, Minneapolis, and colleagues wrote.
“We started enrolling patients at the end of December 2020,” Dr. Bramante said in an interview. “At that time, even though vaccine data were coming out, we thought it was important to test early outpatient treatment with widely available safe medications with no interactions, because the virus would evolve and vaccine availability may be limited.”
In a study published in the New England Journal of Medicine, the researchers used a two-by-three factorial design to test the ability of metformin, ivermectin, and fluvoxamine to prevent severe COVID-19 infection in nonhospitalized adults aged 30-85 years. A total of 1,431 patients at six U.S. sites were enrolled within 3 days of a confirmed infection and less than 7 days after the start of symptoms, then randomized to one of six groups: metformin plus fluvoxamine; metformin plus ivermectin; metformin plus placebo; placebo plus fluvoxamine; placebo plus ivermectin; and placebo plus placebo.
A total of 1,323 patients were included in the primary analysis. The median age of the patients was 46 years, 56% were female (of whom 6% were pregnant), and all individuals met criteria for overweight or obesity. About half (52%) of the patients had been vaccinated against COVID-19.
The primary endpoint was a composite of hypoxemia, ED visit, hospitalization, or death. The analyses were adjusted for COVID-19 vaccination and other trial medications. Overall, the adjusted odds ratios of any primary event, compared with placebo, was 0.84 for metformin (P = .19), 1.05 for ivermectin (P = .78), and 0.94 for fluvoxamine (P = .75).
The researchers also conducted a prespecified secondary analysis of components of the primary endpoint. In this analysis, the aORs for an ED visit, hospitalization, or death was 0.58 for metformin, 1.39 for ivermectin, and 1.17 for fluvoxamine. The aORs for hospitalization or death were 0.47, 0.73, and 1.11 for metformin, ivermectin, and fluvoxamine, respectively. No medication-related serious adverse events were reported with any of the drugs during the study period.
The possible benefit for prevention of severe COVID-19 with metformin was a prespecified secondary endpoint, and therefore not definitive until more research has been completed, the researchers said. Metformin has demonstrated anti-inflammatory actions in previous studies, and has shown protective effects against COVID-19 lung injury in animal studies.
Previous observational studies also have shown an association between metformin use and less severe COVID-19 in patients already taking metformin. “The proposed mechanisms of action against COVID-19 for metformin include anti-inflammatory and antiviral activity and the prevention of hyperglycemia during acute illness,” they added.
The study findings were limited by several factors including the population age range and focus on overweight and obese patients, which may limit generalizability, the researchers noted. Other limitations include the disproportionately small percentage of Black and Latino patients and the potential lack of accuracy in identifying hypoxemia via home oxygen monitors.
However, the results demonstrate that none of the three repurposed drugs – metformin, ivermectin, and fluvoxamine – prevented primary events or reduced symptom severity in COVID-19, compared with placebos, the researchers concluded.
“Metformin had several streams of evidence supporting its use: in vitro, in silico [computer modeled], observational, and in tissue. We were not surprised to see that it reduced emergency department visits, hospitalization, and death,” Dr. Bramante said in an interview.
The take-home message for clinicians is to continue to look to guideline committees for direction on COVID-19 treatments, but to continue to consider metformin along with other treatments, she said.
“All research should be replicated, whether the primary outcome is positive or negative,” Dr. Bramante emphasized. “In this case, when our positive outcome was negative and secondary outcome was positive, a confirmatory trial for metformin is particularly important.”
Ineffective drugs are inefficient use of resources
“The results of the COVID-OUT trial provide persuasive additional data that increase the confidence and degree of certainty that fluvoxamine and ivermectin are not effective in preventing progression to severe disease,” wrote Salim S. Abdool Karim, MB, and Nikita Devnarain, PhD, of the Centre for the AIDS Programme of Research in South Africa, Durban, in an accompanying editorial.
At the start of the study, in 2020, data on the use of the three drugs to prevent severe COVID-19 were “either unavailable or equivocal,” they said. Since then, accumulating data support the current study findings of the nonefficacy of ivermectin and fluvoxamine, and the World Health Organization has advised against their use for COVID-19, although the WHO has not provided guidance for the use of metformin.
The authors called on clinicians to stop using ivermectin and fluvoxamine to treat COVID-19 patients.
“With respect to clinical decisions about COVID-19 treatment, some drug choices, especially those that have negative [World Health Organization] recommendations, are clearly wrong,” they wrote. “In keeping with evidence-based medical practice, patients with COVID-19 must be treated with efficacious medications; they deserve nothing less.”
The study was supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Lead author Dr. Bramante was supported the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Abdool Karim serves as a member of the World Health Organization Science Council. Dr. Devnarain had no financial conflicts to disclose.
Neither metformin, ivermectin, or fluvoxamine had any impact on reducing disease severity, hospitalization, or death from COVID-19, according to results from more than 1,000 overweight or obese adult patients in the COVID-OUT randomized trial.
However, metformin showed some potential in a secondary analysis.
Early treatment to prevent severe disease remains a goal in managing the ongoing COVID-19 pandemic, and biophysical modeling suggested that metformin, ivermectin, and fluvoxamine may serve as antivirals to help reduce severe disease in COVID-19 patients, Carolyn T. Bramante, MD, of the University of Minnesota, Minneapolis, and colleagues wrote.
“We started enrolling patients at the end of December 2020,” Dr. Bramante said in an interview. “At that time, even though vaccine data were coming out, we thought it was important to test early outpatient treatment with widely available safe medications with no interactions, because the virus would evolve and vaccine availability may be limited.”
In a study published in the New England Journal of Medicine, the researchers used a two-by-three factorial design to test the ability of metformin, ivermectin, and fluvoxamine to prevent severe COVID-19 infection in nonhospitalized adults aged 30-85 years. A total of 1,431 patients at six U.S. sites were enrolled within 3 days of a confirmed infection and less than 7 days after the start of symptoms, then randomized to one of six groups: metformin plus fluvoxamine; metformin plus ivermectin; metformin plus placebo; placebo plus fluvoxamine; placebo plus ivermectin; and placebo plus placebo.
A total of 1,323 patients were included in the primary analysis. The median age of the patients was 46 years, 56% were female (of whom 6% were pregnant), and all individuals met criteria for overweight or obesity. About half (52%) of the patients had been vaccinated against COVID-19.
The primary endpoint was a composite of hypoxemia, ED visit, hospitalization, or death. The analyses were adjusted for COVID-19 vaccination and other trial medications. Overall, the adjusted odds ratios of any primary event, compared with placebo, was 0.84 for metformin (P = .19), 1.05 for ivermectin (P = .78), and 0.94 for fluvoxamine (P = .75).
The researchers also conducted a prespecified secondary analysis of components of the primary endpoint. In this analysis, the aORs for an ED visit, hospitalization, or death was 0.58 for metformin, 1.39 for ivermectin, and 1.17 for fluvoxamine. The aORs for hospitalization or death were 0.47, 0.73, and 1.11 for metformin, ivermectin, and fluvoxamine, respectively. No medication-related serious adverse events were reported with any of the drugs during the study period.
The possible benefit for prevention of severe COVID-19 with metformin was a prespecified secondary endpoint, and therefore not definitive until more research has been completed, the researchers said. Metformin has demonstrated anti-inflammatory actions in previous studies, and has shown protective effects against COVID-19 lung injury in animal studies.
Previous observational studies also have shown an association between metformin use and less severe COVID-19 in patients already taking metformin. “The proposed mechanisms of action against COVID-19 for metformin include anti-inflammatory and antiviral activity and the prevention of hyperglycemia during acute illness,” they added.
The study findings were limited by several factors including the population age range and focus on overweight and obese patients, which may limit generalizability, the researchers noted. Other limitations include the disproportionately small percentage of Black and Latino patients and the potential lack of accuracy in identifying hypoxemia via home oxygen monitors.
However, the results demonstrate that none of the three repurposed drugs – metformin, ivermectin, and fluvoxamine – prevented primary events or reduced symptom severity in COVID-19, compared with placebos, the researchers concluded.
“Metformin had several streams of evidence supporting its use: in vitro, in silico [computer modeled], observational, and in tissue. We were not surprised to see that it reduced emergency department visits, hospitalization, and death,” Dr. Bramante said in an interview.
The take-home message for clinicians is to continue to look to guideline committees for direction on COVID-19 treatments, but to continue to consider metformin along with other treatments, she said.
“All research should be replicated, whether the primary outcome is positive or negative,” Dr. Bramante emphasized. “In this case, when our positive outcome was negative and secondary outcome was positive, a confirmatory trial for metformin is particularly important.”
Ineffective drugs are inefficient use of resources
“The results of the COVID-OUT trial provide persuasive additional data that increase the confidence and degree of certainty that fluvoxamine and ivermectin are not effective in preventing progression to severe disease,” wrote Salim S. Abdool Karim, MB, and Nikita Devnarain, PhD, of the Centre for the AIDS Programme of Research in South Africa, Durban, in an accompanying editorial.
At the start of the study, in 2020, data on the use of the three drugs to prevent severe COVID-19 were “either unavailable or equivocal,” they said. Since then, accumulating data support the current study findings of the nonefficacy of ivermectin and fluvoxamine, and the World Health Organization has advised against their use for COVID-19, although the WHO has not provided guidance for the use of metformin.
The authors called on clinicians to stop using ivermectin and fluvoxamine to treat COVID-19 patients.
“With respect to clinical decisions about COVID-19 treatment, some drug choices, especially those that have negative [World Health Organization] recommendations, are clearly wrong,” they wrote. “In keeping with evidence-based medical practice, patients with COVID-19 must be treated with efficacious medications; they deserve nothing less.”
The study was supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Lead author Dr. Bramante was supported the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Abdool Karim serves as a member of the World Health Organization Science Council. Dr. Devnarain had no financial conflicts to disclose.
Neither metformin, ivermectin, or fluvoxamine had any impact on reducing disease severity, hospitalization, or death from COVID-19, according to results from more than 1,000 overweight or obese adult patients in the COVID-OUT randomized trial.
However, metformin showed some potential in a secondary analysis.
Early treatment to prevent severe disease remains a goal in managing the ongoing COVID-19 pandemic, and biophysical modeling suggested that metformin, ivermectin, and fluvoxamine may serve as antivirals to help reduce severe disease in COVID-19 patients, Carolyn T. Bramante, MD, of the University of Minnesota, Minneapolis, and colleagues wrote.
“We started enrolling patients at the end of December 2020,” Dr. Bramante said in an interview. “At that time, even though vaccine data were coming out, we thought it was important to test early outpatient treatment with widely available safe medications with no interactions, because the virus would evolve and vaccine availability may be limited.”
In a study published in the New England Journal of Medicine, the researchers used a two-by-three factorial design to test the ability of metformin, ivermectin, and fluvoxamine to prevent severe COVID-19 infection in nonhospitalized adults aged 30-85 years. A total of 1,431 patients at six U.S. sites were enrolled within 3 days of a confirmed infection and less than 7 days after the start of symptoms, then randomized to one of six groups: metformin plus fluvoxamine; metformin plus ivermectin; metformin plus placebo; placebo plus fluvoxamine; placebo plus ivermectin; and placebo plus placebo.
A total of 1,323 patients were included in the primary analysis. The median age of the patients was 46 years, 56% were female (of whom 6% were pregnant), and all individuals met criteria for overweight or obesity. About half (52%) of the patients had been vaccinated against COVID-19.
The primary endpoint was a composite of hypoxemia, ED visit, hospitalization, or death. The analyses were adjusted for COVID-19 vaccination and other trial medications. Overall, the adjusted odds ratios of any primary event, compared with placebo, was 0.84 for metformin (P = .19), 1.05 for ivermectin (P = .78), and 0.94 for fluvoxamine (P = .75).
The researchers also conducted a prespecified secondary analysis of components of the primary endpoint. In this analysis, the aORs for an ED visit, hospitalization, or death was 0.58 for metformin, 1.39 for ivermectin, and 1.17 for fluvoxamine. The aORs for hospitalization or death were 0.47, 0.73, and 1.11 for metformin, ivermectin, and fluvoxamine, respectively. No medication-related serious adverse events were reported with any of the drugs during the study period.
The possible benefit for prevention of severe COVID-19 with metformin was a prespecified secondary endpoint, and therefore not definitive until more research has been completed, the researchers said. Metformin has demonstrated anti-inflammatory actions in previous studies, and has shown protective effects against COVID-19 lung injury in animal studies.
Previous observational studies also have shown an association between metformin use and less severe COVID-19 in patients already taking metformin. “The proposed mechanisms of action against COVID-19 for metformin include anti-inflammatory and antiviral activity and the prevention of hyperglycemia during acute illness,” they added.
The study findings were limited by several factors including the population age range and focus on overweight and obese patients, which may limit generalizability, the researchers noted. Other limitations include the disproportionately small percentage of Black and Latino patients and the potential lack of accuracy in identifying hypoxemia via home oxygen monitors.
However, the results demonstrate that none of the three repurposed drugs – metformin, ivermectin, and fluvoxamine – prevented primary events or reduced symptom severity in COVID-19, compared with placebos, the researchers concluded.
“Metformin had several streams of evidence supporting its use: in vitro, in silico [computer modeled], observational, and in tissue. We were not surprised to see that it reduced emergency department visits, hospitalization, and death,” Dr. Bramante said in an interview.
The take-home message for clinicians is to continue to look to guideline committees for direction on COVID-19 treatments, but to continue to consider metformin along with other treatments, she said.
“All research should be replicated, whether the primary outcome is positive or negative,” Dr. Bramante emphasized. “In this case, when our positive outcome was negative and secondary outcome was positive, a confirmatory trial for metformin is particularly important.”
Ineffective drugs are inefficient use of resources
“The results of the COVID-OUT trial provide persuasive additional data that increase the confidence and degree of certainty that fluvoxamine and ivermectin are not effective in preventing progression to severe disease,” wrote Salim S. Abdool Karim, MB, and Nikita Devnarain, PhD, of the Centre for the AIDS Programme of Research in South Africa, Durban, in an accompanying editorial.
At the start of the study, in 2020, data on the use of the three drugs to prevent severe COVID-19 were “either unavailable or equivocal,” they said. Since then, accumulating data support the current study findings of the nonefficacy of ivermectin and fluvoxamine, and the World Health Organization has advised against their use for COVID-19, although the WHO has not provided guidance for the use of metformin.
The authors called on clinicians to stop using ivermectin and fluvoxamine to treat COVID-19 patients.
“With respect to clinical decisions about COVID-19 treatment, some drug choices, especially those that have negative [World Health Organization] recommendations, are clearly wrong,” they wrote. “In keeping with evidence-based medical practice, patients with COVID-19 must be treated with efficacious medications; they deserve nothing less.”
The study was supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Lead author Dr. Bramante was supported the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Abdool Karim serves as a member of the World Health Organization Science Council. Dr. Devnarain had no financial conflicts to disclose.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Pfizer seeks approval for updated COVID booster
Pfizer has sent an application to the Food and Drug Administration for emergency use authorization of its updated COVID-19 booster vaccine for the fall of 2022, the company announced on Aug. 22.
The vaccine, which is adapted for the BA.4 and BA.5 Omicron variants, would be meant for ages 12 and older. If authorized by the FDA, the doses could ship as soon as September.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent Omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the statement.
Earlier this year, the FDA ordered vaccine makers such as Pfizer and Moderna to update their shots to target BA.4 and BA.5, which are better at escaping immunity from earlier vaccines and previous infections.
The United States has a contract to buy 105 million of the Pfizer doses and 66 million of the Moderna doses, according to The Associated Press. Moderna is expected to file its FDA application soon as well.
The new shots target both the original spike protein on the coronavirus and the spike mutations carried by BA.4 and BA.5. For now, BA.5 is causing 89% of new infections in the United States, followed by BA.4.6 with 6.3% and BA.4 with 4.3%, according to the latest Centers for Disease Control and Prevention data.
There’s no way to tell if BA.5 will still be the dominant strain this winter or if new variant will replace it, the AP reported. But public health officials have supported the updated boosters as a way to target the most recent strains and increase immunity again.
On Aug. 15, Great Britain became the first country to authorize another one of Moderna’s updated vaccines, which adds protection against BA.1, or the original Omicron strain that became dominant in the winter of 2021-2022. European regulators are considering this shot, the AP reported, but the United States opted not to use this version since new Omicron variants have become dominant.
To approve the latest Pfizer shot, the FDA will rely on scientific testing of prior updates to the vaccine, rather than the newest boosters, to decide whether to fast-track the updated shots for fall, the AP reported. This method is like how flu vaccines are updated each year without large studies that take months.
Previously, Pfizer announced results from a study that found the earlier Omicron update significantly boosted antibodies capable of fighting the BA.1 variant and provided some protection against BA.4 and BA.5. The company’s latest FDA application contains that data and animal testing on the newest booster, the AP reported.
Pfizer will start a trial using the BA.4/BA.5 booster in coming weeks to get more data on how well the latest shot works. Moderna has begun a similar study.
The full results from these studies won’t be available before a fall booster campaign, which is why the FDA and public health officials have called for an updated shot to be ready for distribution in September.
“It’s clear that none of these vaccines are going to completely prevent infection,” Rachel Presti, MD, a researcher with the Moderna trial and an infectious diseases specialist at Washington University in St. Louis, told the AP.
But previous studies of variant booster candidates have shown that “you still get a broader immune response giving a variant booster than giving the same booster,” she said.
A version of this article first appeared on WebMD.com.
Pfizer has sent an application to the Food and Drug Administration for emergency use authorization of its updated COVID-19 booster vaccine for the fall of 2022, the company announced on Aug. 22.
The vaccine, which is adapted for the BA.4 and BA.5 Omicron variants, would be meant for ages 12 and older. If authorized by the FDA, the doses could ship as soon as September.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent Omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the statement.
Earlier this year, the FDA ordered vaccine makers such as Pfizer and Moderna to update their shots to target BA.4 and BA.5, which are better at escaping immunity from earlier vaccines and previous infections.
The United States has a contract to buy 105 million of the Pfizer doses and 66 million of the Moderna doses, according to The Associated Press. Moderna is expected to file its FDA application soon as well.
The new shots target both the original spike protein on the coronavirus and the spike mutations carried by BA.4 and BA.5. For now, BA.5 is causing 89% of new infections in the United States, followed by BA.4.6 with 6.3% and BA.4 with 4.3%, according to the latest Centers for Disease Control and Prevention data.
There’s no way to tell if BA.5 will still be the dominant strain this winter or if new variant will replace it, the AP reported. But public health officials have supported the updated boosters as a way to target the most recent strains and increase immunity again.
On Aug. 15, Great Britain became the first country to authorize another one of Moderna’s updated vaccines, which adds protection against BA.1, or the original Omicron strain that became dominant in the winter of 2021-2022. European regulators are considering this shot, the AP reported, but the United States opted not to use this version since new Omicron variants have become dominant.
To approve the latest Pfizer shot, the FDA will rely on scientific testing of prior updates to the vaccine, rather than the newest boosters, to decide whether to fast-track the updated shots for fall, the AP reported. This method is like how flu vaccines are updated each year without large studies that take months.
Previously, Pfizer announced results from a study that found the earlier Omicron update significantly boosted antibodies capable of fighting the BA.1 variant and provided some protection against BA.4 and BA.5. The company’s latest FDA application contains that data and animal testing on the newest booster, the AP reported.
Pfizer will start a trial using the BA.4/BA.5 booster in coming weeks to get more data on how well the latest shot works. Moderna has begun a similar study.
The full results from these studies won’t be available before a fall booster campaign, which is why the FDA and public health officials have called for an updated shot to be ready for distribution in September.
“It’s clear that none of these vaccines are going to completely prevent infection,” Rachel Presti, MD, a researcher with the Moderna trial and an infectious diseases specialist at Washington University in St. Louis, told the AP.
But previous studies of variant booster candidates have shown that “you still get a broader immune response giving a variant booster than giving the same booster,” she said.
A version of this article first appeared on WebMD.com.
Pfizer has sent an application to the Food and Drug Administration for emergency use authorization of its updated COVID-19 booster vaccine for the fall of 2022, the company announced on Aug. 22.
The vaccine, which is adapted for the BA.4 and BA.5 Omicron variants, would be meant for ages 12 and older. If authorized by the FDA, the doses could ship as soon as September.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent Omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the statement.
Earlier this year, the FDA ordered vaccine makers such as Pfizer and Moderna to update their shots to target BA.4 and BA.5, which are better at escaping immunity from earlier vaccines and previous infections.
The United States has a contract to buy 105 million of the Pfizer doses and 66 million of the Moderna doses, according to The Associated Press. Moderna is expected to file its FDA application soon as well.
The new shots target both the original spike protein on the coronavirus and the spike mutations carried by BA.4 and BA.5. For now, BA.5 is causing 89% of new infections in the United States, followed by BA.4.6 with 6.3% and BA.4 with 4.3%, according to the latest Centers for Disease Control and Prevention data.
There’s no way to tell if BA.5 will still be the dominant strain this winter or if new variant will replace it, the AP reported. But public health officials have supported the updated boosters as a way to target the most recent strains and increase immunity again.
On Aug. 15, Great Britain became the first country to authorize another one of Moderna’s updated vaccines, which adds protection against BA.1, or the original Omicron strain that became dominant in the winter of 2021-2022. European regulators are considering this shot, the AP reported, but the United States opted not to use this version since new Omicron variants have become dominant.
To approve the latest Pfizer shot, the FDA will rely on scientific testing of prior updates to the vaccine, rather than the newest boosters, to decide whether to fast-track the updated shots for fall, the AP reported. This method is like how flu vaccines are updated each year without large studies that take months.
Previously, Pfizer announced results from a study that found the earlier Omicron update significantly boosted antibodies capable of fighting the BA.1 variant and provided some protection against BA.4 and BA.5. The company’s latest FDA application contains that data and animal testing on the newest booster, the AP reported.
Pfizer will start a trial using the BA.4/BA.5 booster in coming weeks to get more data on how well the latest shot works. Moderna has begun a similar study.
The full results from these studies won’t be available before a fall booster campaign, which is why the FDA and public health officials have called for an updated shot to be ready for distribution in September.
“It’s clear that none of these vaccines are going to completely prevent infection,” Rachel Presti, MD, a researcher with the Moderna trial and an infectious diseases specialist at Washington University in St. Louis, told the AP.
But previous studies of variant booster candidates have shown that “you still get a broader immune response giving a variant booster than giving the same booster,” she said.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases fall again, ED rates rebound for some
The 7-day average percentage of ED visits with diagnosed COVID, which had reached a post-Omicron high of 3.5% in late July for those aged 12-15, began to fall and was down to 3.0% on Aug. 12. That trend reversed, however, and the rate was up to 3.6% on Aug. 19, the last date for which data are available from the Centers for Disease Control and Prevention.
That change of COVID fortunes cannot yet be seen for all children. The 7-day average ED visit rate for those aged 0-11 years peaked at 6.8% during the last week of July and has continued to fall, dropping from 5.7% on Aug. 12 to 5.1% on Aug. 19. Children aged 16-17 years seem to be taking a middle path: Their ED-visit rate declined from late July into mid-August but held steady over the last week, according to the CDC’s COVID Data Tracker.
There is a hint of the same trend regarding new admissions among children aged 0-17 years. The national rate, which had declined in recent weeks, ticked up from 0.42 to 0.43 new admissions per 100,000 population over the last week of available data, the CDC said.
Weekly cases fall below 80,000
New cases in general were down by 8.5% from the previous week, dropping from 87,902 for the week of Aug. 5-11 to 79,525 for Aug. 12-18. That marked the second straight week with fewer cases after a 4-week period that saw weekly totals increase from almost 68,000 to nearly 97,000, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP and CHA put the cumulative number of child COVID-19 cases at just under 14.4 million since the pandemic began, which represents 18.4% of cases among all ages. The CDC estimates that there have been almost 14.7 million cases in children aged 0-17 years, as well as 1,750 deaths, of which 14 were reported in the last week (Aug. 16-22).
The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases (2.9 million) than children aged 5-11 years (5.6 million cases) and 12-15 (3.0 million cases) but more deaths: 548 so far, versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds, the COVID Data Tracker shows. Those aged 0-4 make up 6% of the total U.S. population, compared with 8.7% and 5.1%, respectively, for the older children.
Most younger children still not vaccinated
Although it may not qualify as a big push to vaccinate children before the start of the new school year, first-time vaccinations did rise somewhat in late July and August for children aged 5-17 years. Among children younger than 5 years, though, initial doses of the vaccine fell during the second full week of August, especially in 2- to 4-year-olds, based on the CDC data.
Through almost 2 months of vaccine eligibility, 4.8% of children under age 5 have received at least one dose and 0.9% are fully vaccinated as of Aug. 17. The current rates are 37.8% (one dose) and 30.4% (completed) for those aged 5-11 and 70.5% and 60.3% for 12- to 17-year-olds.
The 7-day average percentage of ED visits with diagnosed COVID, which had reached a post-Omicron high of 3.5% in late July for those aged 12-15, began to fall and was down to 3.0% on Aug. 12. That trend reversed, however, and the rate was up to 3.6% on Aug. 19, the last date for which data are available from the Centers for Disease Control and Prevention.
That change of COVID fortunes cannot yet be seen for all children. The 7-day average ED visit rate for those aged 0-11 years peaked at 6.8% during the last week of July and has continued to fall, dropping from 5.7% on Aug. 12 to 5.1% on Aug. 19. Children aged 16-17 years seem to be taking a middle path: Their ED-visit rate declined from late July into mid-August but held steady over the last week, according to the CDC’s COVID Data Tracker.
There is a hint of the same trend regarding new admissions among children aged 0-17 years. The national rate, which had declined in recent weeks, ticked up from 0.42 to 0.43 new admissions per 100,000 population over the last week of available data, the CDC said.
Weekly cases fall below 80,000
New cases in general were down by 8.5% from the previous week, dropping from 87,902 for the week of Aug. 5-11 to 79,525 for Aug. 12-18. That marked the second straight week with fewer cases after a 4-week period that saw weekly totals increase from almost 68,000 to nearly 97,000, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP and CHA put the cumulative number of child COVID-19 cases at just under 14.4 million since the pandemic began, which represents 18.4% of cases among all ages. The CDC estimates that there have been almost 14.7 million cases in children aged 0-17 years, as well as 1,750 deaths, of which 14 were reported in the last week (Aug. 16-22).
The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases (2.9 million) than children aged 5-11 years (5.6 million cases) and 12-15 (3.0 million cases) but more deaths: 548 so far, versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds, the COVID Data Tracker shows. Those aged 0-4 make up 6% of the total U.S. population, compared with 8.7% and 5.1%, respectively, for the older children.
Most younger children still not vaccinated
Although it may not qualify as a big push to vaccinate children before the start of the new school year, first-time vaccinations did rise somewhat in late July and August for children aged 5-17 years. Among children younger than 5 years, though, initial doses of the vaccine fell during the second full week of August, especially in 2- to 4-year-olds, based on the CDC data.

Through almost 2 months of vaccine eligibility, 4.8% of children under age 5 have received at least one dose and 0.9% are fully vaccinated as of Aug. 17. The current rates are 37.8% (one dose) and 30.4% (completed) for those aged 5-11 and 70.5% and 60.3% for 12- to 17-year-olds.
The 7-day average percentage of ED visits with diagnosed COVID, which had reached a post-Omicron high of 3.5% in late July for those aged 12-15, began to fall and was down to 3.0% on Aug. 12. That trend reversed, however, and the rate was up to 3.6% on Aug. 19, the last date for which data are available from the Centers for Disease Control and Prevention.
That change of COVID fortunes cannot yet be seen for all children. The 7-day average ED visit rate for those aged 0-11 years peaked at 6.8% during the last week of July and has continued to fall, dropping from 5.7% on Aug. 12 to 5.1% on Aug. 19. Children aged 16-17 years seem to be taking a middle path: Their ED-visit rate declined from late July into mid-August but held steady over the last week, according to the CDC’s COVID Data Tracker.
There is a hint of the same trend regarding new admissions among children aged 0-17 years. The national rate, which had declined in recent weeks, ticked up from 0.42 to 0.43 new admissions per 100,000 population over the last week of available data, the CDC said.
Weekly cases fall below 80,000
New cases in general were down by 8.5% from the previous week, dropping from 87,902 for the week of Aug. 5-11 to 79,525 for Aug. 12-18. That marked the second straight week with fewer cases after a 4-week period that saw weekly totals increase from almost 68,000 to nearly 97,000, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP and CHA put the cumulative number of child COVID-19 cases at just under 14.4 million since the pandemic began, which represents 18.4% of cases among all ages. The CDC estimates that there have been almost 14.7 million cases in children aged 0-17 years, as well as 1,750 deaths, of which 14 were reported in the last week (Aug. 16-22).
The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases (2.9 million) than children aged 5-11 years (5.6 million cases) and 12-15 (3.0 million cases) but more deaths: 548 so far, versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds, the COVID Data Tracker shows. Those aged 0-4 make up 6% of the total U.S. population, compared with 8.7% and 5.1%, respectively, for the older children.
Most younger children still not vaccinated
Although it may not qualify as a big push to vaccinate children before the start of the new school year, first-time vaccinations did rise somewhat in late July and August for children aged 5-17 years. Among children younger than 5 years, though, initial doses of the vaccine fell during the second full week of August, especially in 2- to 4-year-olds, based on the CDC data.

Through almost 2 months of vaccine eligibility, 4.8% of children under age 5 have received at least one dose and 0.9% are fully vaccinated as of Aug. 17. The current rates are 37.8% (one dose) and 30.4% (completed) for those aged 5-11 and 70.5% and 60.3% for 12- to 17-year-olds.
Regular physical activity may fight infection, illness from COVID: Study
New research suggests that regular physical activity can help lower the risk of COVID-19 infection and its severity, with a weekly tally of 150 minutes of moderate, or 75 minutes of vigorous, physical activity affording the best protection.
“, with potential benefits to reduce the risk of severe COVID-19,” say Antonio García-Hermoso, PhD, Public University of Navarra, Pamplona, Spain, and colleagues.
“Regular physical activity seemed to be related to a lower risk of COVID-19 infection, Dr. García-Hermoso said in an interview. “There is evidence that regular physical activity might contribute to a more effective immune response, providing enhanced protective immunity to infections, which could explain the relationship between exercise consistency with COVID-19 infection.”
Regular exercise may also help to boost the body’s anti-inflammatory responses, as well as cardiorespiratory and muscular fitness, all of which may explain its beneficial effects on COVID-19 severity, the researchers say.
The study was published online in the British Journal of Sports Medicine.
Strong protection from COVID?
A growing body of evidence suggests that increased physical activity may modulate the course of COVID-19 infection and reduce the risk of poor outcomes. The new analysis is the first to systematically evaluate and pool data on the effect of regular physical activity on COVID-19 outcomes.
The findings are based on data from 16 studies with over 1.8 million adults (53% women, mean age 53 years).
Individuals who included regular physical activity in their weekly routine had an 11% lower risk for infection with SARS-CoV-2 (hazard ratio, 0.89; 95% confidence interval, 0.84-0.95), compared with inactive peers.
The physically active adults also had a 36% (HR, 0.64; 95% CI, 0.54-0.76) lower risk of being hospitalized, a 44% (HR, 0.66; 95% CI, 0.58-0.77) lower risk for severe COVID-19 illness, and a 43% (HR, 0.57; 95% CI, 0.46-0.71) lower risk of dying from COVID-19 than their inactive peers.
The greatest protective effect occurs with achieving at least 500 metabolic equivalent of task (MET) minutes per week of physical activity – equivalent to 150 minutes of moderate-intensity or 75 min of vigorous-intensity physical activity per week – with no added benefit beyond this level.
The researchers caution that the analysis included observational studies, differing study designs, subjective assessments of physical activity levels, and concerned only the Beta and Delta variants of SARS-CoV-2, not Omicron.
Despite these limitations, the researchers say their findings “may help guide physicians and health care policymakers in making recommendations and developing guidelines with respect to the degree of physical activity that can help reduce the risk of infectivity, hospitalization, severity, and mortality of COVID-19 at both the individual and the population level, especially in high-risk patients.”
Helpful, but not a panacea
Reached for comment, Sean Heffron, MD, a preventive cardiologist and assistant professor of medicine at NYU Langone Health, New York, said the study “supports the well-established nonlinear association of increasing physical activity with adverse outcomes from a diverse array of diseases, including infectious diseases, such as COVID-19.”
The observation is not particularly surprising, he said.
“It is as I would suspect. They compiled data from a large number of studies published over the past several years that all had consistent findings,” Dr. Heffron said.
“The take-away from a public health standpoint is that being physically active improves health in myriad ways. That being said, it is not a panacea, so additional measures (masking, vaccinations, etc.) are important for everyone,” he said.
Also weighing in, Joseph Herrera, DO, chair of the department of rehabilitation for Mount Sinai Health System, New York, said, “If you are physically fit, your body is more resilient and better prepared to handle the stressors of COVID or any other disease process.”
For now, however, the question of whether physical fitness is actually protective against COVID remains unclear. “I’m just not sure right now,” Dr. Herrera said in an interview.
He said he has treated athletes in professional sports – including the National Football League and Major League Baseball – and some of them have had long COVID and have not returned to play. “These are athletes at the peak of fitness and their career.”
Nonetheless, Dr. Herrera said a good public health message in general is to stay fit or get fit.
“That’s something I preach all the time,” he told this news organization.
Dr. García-Hermoso agreed. “In contrast to the vast majority of drugs, exercise is free of adverse effects. It’s time to consider exercise as medicine. It’s never too late to start being physically active.”
The study had no specific funding. Dr. García-Hermoso, Dr. Heffron, and Dr. Herrera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests that regular physical activity can help lower the risk of COVID-19 infection and its severity, with a weekly tally of 150 minutes of moderate, or 75 minutes of vigorous, physical activity affording the best protection.
“, with potential benefits to reduce the risk of severe COVID-19,” say Antonio García-Hermoso, PhD, Public University of Navarra, Pamplona, Spain, and colleagues.
“Regular physical activity seemed to be related to a lower risk of COVID-19 infection, Dr. García-Hermoso said in an interview. “There is evidence that regular physical activity might contribute to a more effective immune response, providing enhanced protective immunity to infections, which could explain the relationship between exercise consistency with COVID-19 infection.”
Regular exercise may also help to boost the body’s anti-inflammatory responses, as well as cardiorespiratory and muscular fitness, all of which may explain its beneficial effects on COVID-19 severity, the researchers say.
The study was published online in the British Journal of Sports Medicine.
Strong protection from COVID?
A growing body of evidence suggests that increased physical activity may modulate the course of COVID-19 infection and reduce the risk of poor outcomes. The new analysis is the first to systematically evaluate and pool data on the effect of regular physical activity on COVID-19 outcomes.
The findings are based on data from 16 studies with over 1.8 million adults (53% women, mean age 53 years).
Individuals who included regular physical activity in their weekly routine had an 11% lower risk for infection with SARS-CoV-2 (hazard ratio, 0.89; 95% confidence interval, 0.84-0.95), compared with inactive peers.
The physically active adults also had a 36% (HR, 0.64; 95% CI, 0.54-0.76) lower risk of being hospitalized, a 44% (HR, 0.66; 95% CI, 0.58-0.77) lower risk for severe COVID-19 illness, and a 43% (HR, 0.57; 95% CI, 0.46-0.71) lower risk of dying from COVID-19 than their inactive peers.
The greatest protective effect occurs with achieving at least 500 metabolic equivalent of task (MET) minutes per week of physical activity – equivalent to 150 minutes of moderate-intensity or 75 min of vigorous-intensity physical activity per week – with no added benefit beyond this level.
The researchers caution that the analysis included observational studies, differing study designs, subjective assessments of physical activity levels, and concerned only the Beta and Delta variants of SARS-CoV-2, not Omicron.
Despite these limitations, the researchers say their findings “may help guide physicians and health care policymakers in making recommendations and developing guidelines with respect to the degree of physical activity that can help reduce the risk of infectivity, hospitalization, severity, and mortality of COVID-19 at both the individual and the population level, especially in high-risk patients.”
Helpful, but not a panacea
Reached for comment, Sean Heffron, MD, a preventive cardiologist and assistant professor of medicine at NYU Langone Health, New York, said the study “supports the well-established nonlinear association of increasing physical activity with adverse outcomes from a diverse array of diseases, including infectious diseases, such as COVID-19.”
The observation is not particularly surprising, he said.
“It is as I would suspect. They compiled data from a large number of studies published over the past several years that all had consistent findings,” Dr. Heffron said.
“The take-away from a public health standpoint is that being physically active improves health in myriad ways. That being said, it is not a panacea, so additional measures (masking, vaccinations, etc.) are important for everyone,” he said.
Also weighing in, Joseph Herrera, DO, chair of the department of rehabilitation for Mount Sinai Health System, New York, said, “If you are physically fit, your body is more resilient and better prepared to handle the stressors of COVID or any other disease process.”
For now, however, the question of whether physical fitness is actually protective against COVID remains unclear. “I’m just not sure right now,” Dr. Herrera said in an interview.
He said he has treated athletes in professional sports – including the National Football League and Major League Baseball – and some of them have had long COVID and have not returned to play. “These are athletes at the peak of fitness and their career.”
Nonetheless, Dr. Herrera said a good public health message in general is to stay fit or get fit.
“That’s something I preach all the time,” he told this news organization.
Dr. García-Hermoso agreed. “In contrast to the vast majority of drugs, exercise is free of adverse effects. It’s time to consider exercise as medicine. It’s never too late to start being physically active.”
The study had no specific funding. Dr. García-Hermoso, Dr. Heffron, and Dr. Herrera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests that regular physical activity can help lower the risk of COVID-19 infection and its severity, with a weekly tally of 150 minutes of moderate, or 75 minutes of vigorous, physical activity affording the best protection.
“, with potential benefits to reduce the risk of severe COVID-19,” say Antonio García-Hermoso, PhD, Public University of Navarra, Pamplona, Spain, and colleagues.
“Regular physical activity seemed to be related to a lower risk of COVID-19 infection, Dr. García-Hermoso said in an interview. “There is evidence that regular physical activity might contribute to a more effective immune response, providing enhanced protective immunity to infections, which could explain the relationship between exercise consistency with COVID-19 infection.”
Regular exercise may also help to boost the body’s anti-inflammatory responses, as well as cardiorespiratory and muscular fitness, all of which may explain its beneficial effects on COVID-19 severity, the researchers say.
The study was published online in the British Journal of Sports Medicine.
Strong protection from COVID?
A growing body of evidence suggests that increased physical activity may modulate the course of COVID-19 infection and reduce the risk of poor outcomes. The new analysis is the first to systematically evaluate and pool data on the effect of regular physical activity on COVID-19 outcomes.
The findings are based on data from 16 studies with over 1.8 million adults (53% women, mean age 53 years).
Individuals who included regular physical activity in their weekly routine had an 11% lower risk for infection with SARS-CoV-2 (hazard ratio, 0.89; 95% confidence interval, 0.84-0.95), compared with inactive peers.
The physically active adults also had a 36% (HR, 0.64; 95% CI, 0.54-0.76) lower risk of being hospitalized, a 44% (HR, 0.66; 95% CI, 0.58-0.77) lower risk for severe COVID-19 illness, and a 43% (HR, 0.57; 95% CI, 0.46-0.71) lower risk of dying from COVID-19 than their inactive peers.
The greatest protective effect occurs with achieving at least 500 metabolic equivalent of task (MET) minutes per week of physical activity – equivalent to 150 minutes of moderate-intensity or 75 min of vigorous-intensity physical activity per week – with no added benefit beyond this level.
The researchers caution that the analysis included observational studies, differing study designs, subjective assessments of physical activity levels, and concerned only the Beta and Delta variants of SARS-CoV-2, not Omicron.
Despite these limitations, the researchers say their findings “may help guide physicians and health care policymakers in making recommendations and developing guidelines with respect to the degree of physical activity that can help reduce the risk of infectivity, hospitalization, severity, and mortality of COVID-19 at both the individual and the population level, especially in high-risk patients.”
Helpful, but not a panacea
Reached for comment, Sean Heffron, MD, a preventive cardiologist and assistant professor of medicine at NYU Langone Health, New York, said the study “supports the well-established nonlinear association of increasing physical activity with adverse outcomes from a diverse array of diseases, including infectious diseases, such as COVID-19.”
The observation is not particularly surprising, he said.
“It is as I would suspect. They compiled data from a large number of studies published over the past several years that all had consistent findings,” Dr. Heffron said.
“The take-away from a public health standpoint is that being physically active improves health in myriad ways. That being said, it is not a panacea, so additional measures (masking, vaccinations, etc.) are important for everyone,” he said.
Also weighing in, Joseph Herrera, DO, chair of the department of rehabilitation for Mount Sinai Health System, New York, said, “If you are physically fit, your body is more resilient and better prepared to handle the stressors of COVID or any other disease process.”
For now, however, the question of whether physical fitness is actually protective against COVID remains unclear. “I’m just not sure right now,” Dr. Herrera said in an interview.
He said he has treated athletes in professional sports – including the National Football League and Major League Baseball – and some of them have had long COVID and have not returned to play. “These are athletes at the peak of fitness and their career.”
Nonetheless, Dr. Herrera said a good public health message in general is to stay fit or get fit.
“That’s something I preach all the time,” he told this news organization.
Dr. García-Hermoso agreed. “In contrast to the vast majority of drugs, exercise is free of adverse effects. It’s time to consider exercise as medicine. It’s never too late to start being physically active.”
The study had no specific funding. Dr. García-Hermoso, Dr. Heffron, and Dr. Herrera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BRITISH JOURNAL OF SPORTS MEDICINE
Guidelines on GLP1RAs and continuous glucose monitors are among biggest news in diabetes
glucagonlike peptide-1 receptor agonists (GLP1RAs) and continuous glucose monitoring (CGM) technology. I am hoping my discussion about these major advances in this edition of Highlights will be helpful to those caring for patients with diabetes.
Tirzepatide
The first GLP1RA, exenatide, was released in April 2005. Since then, numerous daily and weekly drugs of this class have been developed. We’ve learned they are effective glucose lowering drugs, and the weekly agents dulaglutide and semaglutide have shown impressive weight reduction properties as well as cardiovascular benefits.
Secondary outcomes have also shown renal benefits to these agents, and studies for primary renal efficacy are pending. Due to all of these properties, the GLP1RAs are recommended as the first injectable for the treatment of type 2 diabetes, prior to insulin initiation.1
The next generation of these agents are a combination of a GLP1RA and a glucose-dependent insulinotropic polypeptide (GIP). Glucagonlike peptide-1 (GLP-1) stimulates insulin secretion, inhibits glucagon secretion, delays gastric emptying, and has central effects inducing satiety.
We now understand that GIP is the main incretin hormone in those without diabetes, causative of most of the incretin effects. But the insulin response after GIP secretion in type 2 diabetes is strongly reduced. It is now appreciated that this poor effect of GIP can be reduced when used in combination with a GLP1RA. This combination incretin, called by some a “twincretin,” is the basis for the drug tirzepatide which was approved by the Food and Drug Administration in May of 2022.
The data supporting this agent for both diabetes and obesity are impressive. For example, in a 40-week study with a baseline HbA1c of 8.0%, those randomized to tirzepatide at 5 mg, 10 mg, and 15 mg had HbA1c reductions of 1.87%, 1.89%, and 2.07% respectively.2 Over 81% at all doses had HbA1c levels less than 6.5% at 40 weeks.
For the 5-mg, 10-mg, and 15-mg doses, weight change from baseline was 7.9%, 9.3%, and 11.0% respectively. Like older GLP1RAs, gastrointestinal side effects were the main problem. For the three doses, 3%, 5%, and 7%, respectively, had to stop the drug, compared with the 3% who stopped taking the placebo. In another study, tirzepatide was noninferior or superior at all three doses compared with semaglutide 1 mg weekly.3
In a population without diabetes, with 40% of patients having prediabetes, weight loss percentages for the three doses were 15.0%, 19.5%, and 20.9% respectively.4 Discontinuation percentages due to side effects were 4%-7%. The exciting part is we now have a drug that approaches weight loss from bariatric surgery. The cardiovascular and renal outcome trials are now underway, but the enthusiasm for this drug is clear from the data.
Like other GLP1RAs, the key is to start low and go slowly. It is recommended to start tirzepatide at 2.5 mg four times a week, then increase to 5 mg. Due to gastrointestinal side effects, some patients will do better at the lower dose before increasing. For those switching from another GLP1RA, there are no data to guide us but, in my practice, I start those patients at 5 mg weekly.
Continuous glucose monitoring
Data continue to accumulate that this form of glycemic self-monitoring is effective to reduce HbA1c levels and minimize hypoglycemia in both type 1 and type 2 diabetes. The most important change to the 2022 American Diabetes Association (ADA) standards of care is recognizing CGM as level A evidence for those receiving basal insulin without mealtime insulin.5 There are four CGMs on the market, but most of the market uses the Dexcom G6 or the Libre 2. Both of these devices will be updated within the next few months to newer generation sensors.
While there are similarities and differences between the two devices, by late 2022 and early 2023 changes to both will reduce the dissimilarities.
The next generation Libre (Libre 3) will be continuous, and “scanning” will no longer be required. For those unable to get insurance to cover CGM, the Libre will continue to be more affordable than the Dexcom. Alerts will be present on both, but the Dexcom G7 will be approved for both the arm and the abdomen. The Dexcom also can communicate with several automated insulin delivery systems and data can be shared real-time with family members.
For clinicians just starting patients on this technology, my suggestion is to focus on one system so both the provider and staff can become familiar with it. It is key to review downloaded glucose metrics, in addition to the “ambulatory glucose profile,” a graphic overview of daily glycemia where patterns can be identified. It is also helpful to ask for assistance from endocrinologists who have experience with CGMs, in addition to the representatives of the companies.
COVID-19 and new-onset diabetes
From the beginning of the COVID 19 pandemic in 2020, it was clear that stress hyperglycemia and glucose dysregulation was an important observation for those infected. What was not known at the time is that for some, the hyperglycemia continued, and permanent diabetes ensued.
In one study of over 2.7 million U.S. veterans, men infected with COVID-19, but not women, were at a higher risk of new incident diabetes at 120 days after infection compared to no infection (odds ratio for men = 2.56).6
Another literature review using meta-analyses and cross-sectional studies concluded new-onset diabetes following COVID-19 infection can have a varied phenotype, with no risk factors, presenting from diabetic ketoacidosis to milder forms of diabetes.7
The current thought is that COVID-19 binds to the ACE2 and TMPRSS2 receptors which appear to be located on the beta-cells in the islet, resulting in insulin deficiency, in addition to the insulin resistance that seems to persist after the acute infection. Much more needs to be learned about this, but clinicians need to appreciate this appears to be a new form of diabetes and optimal treatments are not yet clear.
Dr. Hirsch is an endocrinologist, professor of medicine, and diabetes treatment and teaching chair at the University of Washington, Seattle. He has received research grant support from Dexcom and Insulet and has provided consulting to Abbott, Roche, Lifescan, and GWave. You can contact him at [email protected].
References
1. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125-S143.
2. Rosenstock J et al. Efficacy and safety of a novel GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet. 2021;398:143-55.
3. Frias JP et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-15.
4. Jastreboff AM et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205-16.
5. American Diabetes Association Professional Practice Committee. Diabetes technology: Standards of Medical Care in Diabetes–2022. Diabetes Care. 2022;45(Suppl 1):S97-S112.
6. Wander PL et al. The incidence of diabetes in 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care 2022;45:782-8.
7. Joshi SC and Pozzilli P. COVID-19 induced diabetes: A novel presentation. Diabetes Res Clin Pract. 2022 Aug 6;191:110034.
glucagonlike peptide-1 receptor agonists (GLP1RAs) and continuous glucose monitoring (CGM) technology. I am hoping my discussion about these major advances in this edition of Highlights will be helpful to those caring for patients with diabetes.
Tirzepatide
The first GLP1RA, exenatide, was released in April 2005. Since then, numerous daily and weekly drugs of this class have been developed. We’ve learned they are effective glucose lowering drugs, and the weekly agents dulaglutide and semaglutide have shown impressive weight reduction properties as well as cardiovascular benefits.
Secondary outcomes have also shown renal benefits to these agents, and studies for primary renal efficacy are pending. Due to all of these properties, the GLP1RAs are recommended as the first injectable for the treatment of type 2 diabetes, prior to insulin initiation.1
The next generation of these agents are a combination of a GLP1RA and a glucose-dependent insulinotropic polypeptide (GIP). Glucagonlike peptide-1 (GLP-1) stimulates insulin secretion, inhibits glucagon secretion, delays gastric emptying, and has central effects inducing satiety.
We now understand that GIP is the main incretin hormone in those without diabetes, causative of most of the incretin effects. But the insulin response after GIP secretion in type 2 diabetes is strongly reduced. It is now appreciated that this poor effect of GIP can be reduced when used in combination with a GLP1RA. This combination incretin, called by some a “twincretin,” is the basis for the drug tirzepatide which was approved by the Food and Drug Administration in May of 2022.
The data supporting this agent for both diabetes and obesity are impressive. For example, in a 40-week study with a baseline HbA1c of 8.0%, those randomized to tirzepatide at 5 mg, 10 mg, and 15 mg had HbA1c reductions of 1.87%, 1.89%, and 2.07% respectively.2 Over 81% at all doses had HbA1c levels less than 6.5% at 40 weeks.
For the 5-mg, 10-mg, and 15-mg doses, weight change from baseline was 7.9%, 9.3%, and 11.0% respectively. Like older GLP1RAs, gastrointestinal side effects were the main problem. For the three doses, 3%, 5%, and 7%, respectively, had to stop the drug, compared with the 3% who stopped taking the placebo. In another study, tirzepatide was noninferior or superior at all three doses compared with semaglutide 1 mg weekly.3
In a population without diabetes, with 40% of patients having prediabetes, weight loss percentages for the three doses were 15.0%, 19.5%, and 20.9% respectively.4 Discontinuation percentages due to side effects were 4%-7%. The exciting part is we now have a drug that approaches weight loss from bariatric surgery. The cardiovascular and renal outcome trials are now underway, but the enthusiasm for this drug is clear from the data.
Like other GLP1RAs, the key is to start low and go slowly. It is recommended to start tirzepatide at 2.5 mg four times a week, then increase to 5 mg. Due to gastrointestinal side effects, some patients will do better at the lower dose before increasing. For those switching from another GLP1RA, there are no data to guide us but, in my practice, I start those patients at 5 mg weekly.
Continuous glucose monitoring
Data continue to accumulate that this form of glycemic self-monitoring is effective to reduce HbA1c levels and minimize hypoglycemia in both type 1 and type 2 diabetes. The most important change to the 2022 American Diabetes Association (ADA) standards of care is recognizing CGM as level A evidence for those receiving basal insulin without mealtime insulin.5 There are four CGMs on the market, but most of the market uses the Dexcom G6 or the Libre 2. Both of these devices will be updated within the next few months to newer generation sensors.
While there are similarities and differences between the two devices, by late 2022 and early 2023 changes to both will reduce the dissimilarities.
The next generation Libre (Libre 3) will be continuous, and “scanning” will no longer be required. For those unable to get insurance to cover CGM, the Libre will continue to be more affordable than the Dexcom. Alerts will be present on both, but the Dexcom G7 will be approved for both the arm and the abdomen. The Dexcom also can communicate with several automated insulin delivery systems and data can be shared real-time with family members.
For clinicians just starting patients on this technology, my suggestion is to focus on one system so both the provider and staff can become familiar with it. It is key to review downloaded glucose metrics, in addition to the “ambulatory glucose profile,” a graphic overview of daily glycemia where patterns can be identified. It is also helpful to ask for assistance from endocrinologists who have experience with CGMs, in addition to the representatives of the companies.
COVID-19 and new-onset diabetes
From the beginning of the COVID 19 pandemic in 2020, it was clear that stress hyperglycemia and glucose dysregulation was an important observation for those infected. What was not known at the time is that for some, the hyperglycemia continued, and permanent diabetes ensued.
In one study of over 2.7 million U.S. veterans, men infected with COVID-19, but not women, were at a higher risk of new incident diabetes at 120 days after infection compared to no infection (odds ratio for men = 2.56).6
Another literature review using meta-analyses and cross-sectional studies concluded new-onset diabetes following COVID-19 infection can have a varied phenotype, with no risk factors, presenting from diabetic ketoacidosis to milder forms of diabetes.7
The current thought is that COVID-19 binds to the ACE2 and TMPRSS2 receptors which appear to be located on the beta-cells in the islet, resulting in insulin deficiency, in addition to the insulin resistance that seems to persist after the acute infection. Much more needs to be learned about this, but clinicians need to appreciate this appears to be a new form of diabetes and optimal treatments are not yet clear.
Dr. Hirsch is an endocrinologist, professor of medicine, and diabetes treatment and teaching chair at the University of Washington, Seattle. He has received research grant support from Dexcom and Insulet and has provided consulting to Abbott, Roche, Lifescan, and GWave. You can contact him at [email protected].
References
1. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125-S143.
2. Rosenstock J et al. Efficacy and safety of a novel GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet. 2021;398:143-55.
3. Frias JP et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-15.
4. Jastreboff AM et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205-16.
5. American Diabetes Association Professional Practice Committee. Diabetes technology: Standards of Medical Care in Diabetes–2022. Diabetes Care. 2022;45(Suppl 1):S97-S112.
6. Wander PL et al. The incidence of diabetes in 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care 2022;45:782-8.
7. Joshi SC and Pozzilli P. COVID-19 induced diabetes: A novel presentation. Diabetes Res Clin Pract. 2022 Aug 6;191:110034.
glucagonlike peptide-1 receptor agonists (GLP1RAs) and continuous glucose monitoring (CGM) technology. I am hoping my discussion about these major advances in this edition of Highlights will be helpful to those caring for patients with diabetes.
Tirzepatide
The first GLP1RA, exenatide, was released in April 2005. Since then, numerous daily and weekly drugs of this class have been developed. We’ve learned they are effective glucose lowering drugs, and the weekly agents dulaglutide and semaglutide have shown impressive weight reduction properties as well as cardiovascular benefits.
Secondary outcomes have also shown renal benefits to these agents, and studies for primary renal efficacy are pending. Due to all of these properties, the GLP1RAs are recommended as the first injectable for the treatment of type 2 diabetes, prior to insulin initiation.1
The next generation of these agents are a combination of a GLP1RA and a glucose-dependent insulinotropic polypeptide (GIP). Glucagonlike peptide-1 (GLP-1) stimulates insulin secretion, inhibits glucagon secretion, delays gastric emptying, and has central effects inducing satiety.
We now understand that GIP is the main incretin hormone in those without diabetes, causative of most of the incretin effects. But the insulin response after GIP secretion in type 2 diabetes is strongly reduced. It is now appreciated that this poor effect of GIP can be reduced when used in combination with a GLP1RA. This combination incretin, called by some a “twincretin,” is the basis for the drug tirzepatide which was approved by the Food and Drug Administration in May of 2022.
The data supporting this agent for both diabetes and obesity are impressive. For example, in a 40-week study with a baseline HbA1c of 8.0%, those randomized to tirzepatide at 5 mg, 10 mg, and 15 mg had HbA1c reductions of 1.87%, 1.89%, and 2.07% respectively.2 Over 81% at all doses had HbA1c levels less than 6.5% at 40 weeks.
For the 5-mg, 10-mg, and 15-mg doses, weight change from baseline was 7.9%, 9.3%, and 11.0% respectively. Like older GLP1RAs, gastrointestinal side effects were the main problem. For the three doses, 3%, 5%, and 7%, respectively, had to stop the drug, compared with the 3% who stopped taking the placebo. In another study, tirzepatide was noninferior or superior at all three doses compared with semaglutide 1 mg weekly.3
In a population without diabetes, with 40% of patients having prediabetes, weight loss percentages for the three doses were 15.0%, 19.5%, and 20.9% respectively.4 Discontinuation percentages due to side effects were 4%-7%. The exciting part is we now have a drug that approaches weight loss from bariatric surgery. The cardiovascular and renal outcome trials are now underway, but the enthusiasm for this drug is clear from the data.
Like other GLP1RAs, the key is to start low and go slowly. It is recommended to start tirzepatide at 2.5 mg four times a week, then increase to 5 mg. Due to gastrointestinal side effects, some patients will do better at the lower dose before increasing. For those switching from another GLP1RA, there are no data to guide us but, in my practice, I start those patients at 5 mg weekly.
Continuous glucose monitoring
Data continue to accumulate that this form of glycemic self-monitoring is effective to reduce HbA1c levels and minimize hypoglycemia in both type 1 and type 2 diabetes. The most important change to the 2022 American Diabetes Association (ADA) standards of care is recognizing CGM as level A evidence for those receiving basal insulin without mealtime insulin.5 There are four CGMs on the market, but most of the market uses the Dexcom G6 or the Libre 2. Both of these devices will be updated within the next few months to newer generation sensors.
While there are similarities and differences between the two devices, by late 2022 and early 2023 changes to both will reduce the dissimilarities.
The next generation Libre (Libre 3) will be continuous, and “scanning” will no longer be required. For those unable to get insurance to cover CGM, the Libre will continue to be more affordable than the Dexcom. Alerts will be present on both, but the Dexcom G7 will be approved for both the arm and the abdomen. The Dexcom also can communicate with several automated insulin delivery systems and data can be shared real-time with family members.
For clinicians just starting patients on this technology, my suggestion is to focus on one system so both the provider and staff can become familiar with it. It is key to review downloaded glucose metrics, in addition to the “ambulatory glucose profile,” a graphic overview of daily glycemia where patterns can be identified. It is also helpful to ask for assistance from endocrinologists who have experience with CGMs, in addition to the representatives of the companies.
COVID-19 and new-onset diabetes
From the beginning of the COVID 19 pandemic in 2020, it was clear that stress hyperglycemia and glucose dysregulation was an important observation for those infected. What was not known at the time is that for some, the hyperglycemia continued, and permanent diabetes ensued.
In one study of over 2.7 million U.S. veterans, men infected with COVID-19, but not women, were at a higher risk of new incident diabetes at 120 days after infection compared to no infection (odds ratio for men = 2.56).6
Another literature review using meta-analyses and cross-sectional studies concluded new-onset diabetes following COVID-19 infection can have a varied phenotype, with no risk factors, presenting from diabetic ketoacidosis to milder forms of diabetes.7
The current thought is that COVID-19 binds to the ACE2 and TMPRSS2 receptors which appear to be located on the beta-cells in the islet, resulting in insulin deficiency, in addition to the insulin resistance that seems to persist after the acute infection. Much more needs to be learned about this, but clinicians need to appreciate this appears to be a new form of diabetes and optimal treatments are not yet clear.
Dr. Hirsch is an endocrinologist, professor of medicine, and diabetes treatment and teaching chair at the University of Washington, Seattle. He has received research grant support from Dexcom and Insulet and has provided consulting to Abbott, Roche, Lifescan, and GWave. You can contact him at [email protected].
References
1. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125-S143.
2. Rosenstock J et al. Efficacy and safety of a novel GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet. 2021;398:143-55.
3. Frias JP et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-15.
4. Jastreboff AM et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205-16.
5. American Diabetes Association Professional Practice Committee. Diabetes technology: Standards of Medical Care in Diabetes–2022. Diabetes Care. 2022;45(Suppl 1):S97-S112.
6. Wander PL et al. The incidence of diabetes in 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care 2022;45:782-8.
7. Joshi SC and Pozzilli P. COVID-19 induced diabetes: A novel presentation. Diabetes Res Clin Pract. 2022 Aug 6;191:110034.
Most people with Omicron don’t know they’re infected
Most people with Omicron likely don’t know it.
That’s according to a study in JAMA Network Open, which says 56% of people who have the Omicron variant of the coronavirus are unaware of their infection.
And it has an upside and a downside, depending on how you look at it, according to Time magazine.
“It’s good news, in some ways, since ) in vaccinated people,” Time says. “The downside is that many people are likely spreading the virus unintentionally.”
The study looked at 210 hospital patients and employees in the Los Angeles area. More than half who tested positive didn’t know it – because they had no symptoms, or they assumed they merely had a cold or allergies.
“The findings support early data from around the world suggesting that throughout the pandemic, anywhere from 25% to 40% of SARS-CoV-2 infections have been asymptomatic, which presents challenges for public health officials trying to control the spread of the virus,” Time reports.
The study found that awareness of infection rose after at-home tests became available this year. About three-quarters of people in January and February didn’t know their status, for example.
“Findings of this study suggest that low rates of Omicron variant infection awareness may be a key contributor to rapid transmission of the virus within communities,” the authors wrote. “Given that unawareness of active infection precludes self-initiated interventions, such as testing and self-isolation, even modest levels of undiagnosed infection can contribute to substantial population-level transmission.”
A version of this article first appeared on WebMD.com.
Most people with Omicron likely don’t know it.
That’s according to a study in JAMA Network Open, which says 56% of people who have the Omicron variant of the coronavirus are unaware of their infection.
And it has an upside and a downside, depending on how you look at it, according to Time magazine.
“It’s good news, in some ways, since ) in vaccinated people,” Time says. “The downside is that many people are likely spreading the virus unintentionally.”
The study looked at 210 hospital patients and employees in the Los Angeles area. More than half who tested positive didn’t know it – because they had no symptoms, or they assumed they merely had a cold or allergies.
“The findings support early data from around the world suggesting that throughout the pandemic, anywhere from 25% to 40% of SARS-CoV-2 infections have been asymptomatic, which presents challenges for public health officials trying to control the spread of the virus,” Time reports.
The study found that awareness of infection rose after at-home tests became available this year. About three-quarters of people in January and February didn’t know their status, for example.
“Findings of this study suggest that low rates of Omicron variant infection awareness may be a key contributor to rapid transmission of the virus within communities,” the authors wrote. “Given that unawareness of active infection precludes self-initiated interventions, such as testing and self-isolation, even modest levels of undiagnosed infection can contribute to substantial population-level transmission.”
A version of this article first appeared on WebMD.com.
Most people with Omicron likely don’t know it.
That’s according to a study in JAMA Network Open, which says 56% of people who have the Omicron variant of the coronavirus are unaware of their infection.
And it has an upside and a downside, depending on how you look at it, according to Time magazine.
“It’s good news, in some ways, since ) in vaccinated people,” Time says. “The downside is that many people are likely spreading the virus unintentionally.”
The study looked at 210 hospital patients and employees in the Los Angeles area. More than half who tested positive didn’t know it – because they had no symptoms, or they assumed they merely had a cold or allergies.
“The findings support early data from around the world suggesting that throughout the pandemic, anywhere from 25% to 40% of SARS-CoV-2 infections have been asymptomatic, which presents challenges for public health officials trying to control the spread of the virus,” Time reports.
The study found that awareness of infection rose after at-home tests became available this year. About three-quarters of people in January and February didn’t know their status, for example.
“Findings of this study suggest that low rates of Omicron variant infection awareness may be a key contributor to rapid transmission of the virus within communities,” the authors wrote. “Given that unawareness of active infection precludes self-initiated interventions, such as testing and self-isolation, even modest levels of undiagnosed infection can contribute to substantial population-level transmission.”
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN