User login
Which tube placement is best for a patient requiring enteral nutrition?
Comparative advantages of EN tubes
Case
A 68-year-old diabetic nonverbal female presents to the ED because of “seizure” 1 hour ago. On exam, her blood glucose is 200. She is unable to speak and has dysphagia because of a stroke she sustained last month. The patient’s husband adds that she hasn’t been eating and drinking sufficiently in the past couple of days. Imaging was negative for any acute intracranial bleeds or lesions. Labs showed a serum sodium level of 150 milliequivalents/L. D5W is started, and the following day, the patient has a sodium level of 154 milliequivalents/L.
Brief overview
Many hospitalized patients are unable to maintain hydration and/or nutritional status by mouth and will need enteral nutrition. Variables such as past medical history, swallowing ability, history of aspiration, prognosis, and functional capacity of each gastrointestinal segment will determine the best option for enteral nutrition for each patient. Each type of enteral tube feeding has advantages, disadvantages, and complications.
Overview of the data
Enteral nutrition should be started within 24-48 hours in a critically ill patient who is unable to maintain intake according to the American Society of Parenteral and Enteral Nutrition.1 This can be provided through a nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) tube, PEG tube with jejunal extension (PEG-J), or a percutaneous endoscopic jejunal (PEJ) tube.1
NG tubes are often the first method deployed because of their low cost and convenience. They are also suitable for the patient who requires this type of feeding for less than 4 weeks. However, NG tubes do require some patient cooperation (to place and maintain)and are contraindicated in some patients with orofacial trauma, upper GI tumors, inadequate lower esophageal sphincter tone, and gastroparesis.2
Another option is a PEG tube, which is a good alternative for patients who are sedated; ventilated; or have neurodegenerative processes, stroke with dysphagia, or head and neck cancers. These are typically recommended when enteral nutrition will be needed for more than 4 weeks. Disadvantages of PEG tubes include tube obstruction or displacement, gastroesophageal reflux, and leakage of gastric content around the percutaneous site or into the peritoneum.
PEG-J tubes, PEJ tubes, or jejunostomy tubes are best suited for patients with GI dysmotility, patients who have unsuccessfully undergone the aforementioned methods, patients with histories of partial gastrectomies, or patients with gastric or pancreatic cancers/multiple traumas. The PEG-J tube extends into the distal duodenum; because it is longer and more narrow, it is more likely to coil and occlude the flow of nutrients during feedings.2,3 Jejunal feeding methods incorporate a continuous pump controlled infusion; if set too rapidly, this could cause dumping syndrome. A benefit of jejunal nutrition is a lower risk of aspiration, compared with other enteral tubes.4
It is best to appraise the selected method for its efficacy and patient preference. The American College of Gastroenterology recommends starting with orogastric or nasogastric feeds, and switching to postpyloric or jejunal feeds for those intolerant to or at high risk for aspiration.5 The most important aspect is early enteral nutrition in hospitalized patients unable to maintain oral nutrition.
Application of the data to the original case
This is a severely hypernatremic diabetic patient unable to swallow. On day 2 of her hospitalization, the clinical team provided the patient with an NG tube for increased free-water intake to gradually decrease her serum sodium. By hospital day 4, the patient’s sodium had normalized. Considering the patient’s long-term prognosis and dysphagia, discussions were held with the patient and husband for PEG tube placement. The patient received a PEG tube and was subsequently discharged 2 days later.
Bottom line
Enteral nutrition is a common need among hospitalized patients. Modality of enteral nutrition will depend on the patient’s past medical history, anticipated duration, and preferences.
Dr. Basnet is the hospitalist program director for Apogee Physicians Group at Eastern New Mexico Medical Center in Roswell. Ms. Tayes is a third-year medical student at Burrell College of Osteopathic Medicine in Las Cruces, N.M., with interests in surgery, internal medicine, and emergency medicine. Ms. Gallivan is a third-year medical student at Burrell College of Osteopathic Medicine, with interests in cardiothoracic surgery, general surgery, and internal medicine.
References
1. Boullata JI et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J Parenter Enteral Nutr. 2016;1-89.
2. Kirby DF et al. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282.
3. Lazarus BA et al. Aspiration associated with long-term gastric versus jejunal feeding: A critical analysis of the literature. Arch Phys Med Rehabil. 1990;71:46.
4. Alkhawaja S et al. Postpyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015 Aug 4;(8):CD008875.
5. McCalve SA et al. ACG Clinical Guideline: Nutrition therapy in the hospitalized patient. Am J Gastroenterol. 2016;111:315-34. doi: 10.1038/ajg.2016.28.
Additional reading
Bellini LM. Nutrition Support in Advanced Lung Disease. UptoDate. https://www-uptodate-com.ezproxy.ad.bcomnm.org/contents/nutritional-support-in-advanced-lung-disease?. Published April 20, 2018.
Commercial Formulas for the Feeding Tube. The Oral Cancer Foundation. https://oralcancerfoundation.org/nutrition/commercial-formulas-feeding-tube/. Published June 5, 2018.
Marik Z. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11). doi: 10.1007/s00134-008-1213-6.
Wischmeyer PE. Enteral nutrition can be given to patients on vasopressors. Crit Care Med. 2020;48(1):122-5. doi: 10.1097/CCM.0000000000003965.
Comparative advantages of EN tubes
Comparative advantages of EN tubes
Case
A 68-year-old diabetic nonverbal female presents to the ED because of “seizure” 1 hour ago. On exam, her blood glucose is 200. She is unable to speak and has dysphagia because of a stroke she sustained last month. The patient’s husband adds that she hasn’t been eating and drinking sufficiently in the past couple of days. Imaging was negative for any acute intracranial bleeds or lesions. Labs showed a serum sodium level of 150 milliequivalents/L. D5W is started, and the following day, the patient has a sodium level of 154 milliequivalents/L.
Brief overview
Many hospitalized patients are unable to maintain hydration and/or nutritional status by mouth and will need enteral nutrition. Variables such as past medical history, swallowing ability, history of aspiration, prognosis, and functional capacity of each gastrointestinal segment will determine the best option for enteral nutrition for each patient. Each type of enteral tube feeding has advantages, disadvantages, and complications.
Overview of the data
Enteral nutrition should be started within 24-48 hours in a critically ill patient who is unable to maintain intake according to the American Society of Parenteral and Enteral Nutrition.1 This can be provided through a nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) tube, PEG tube with jejunal extension (PEG-J), or a percutaneous endoscopic jejunal (PEJ) tube.1
NG tubes are often the first method deployed because of their low cost and convenience. They are also suitable for the patient who requires this type of feeding for less than 4 weeks. However, NG tubes do require some patient cooperation (to place and maintain)and are contraindicated in some patients with orofacial trauma, upper GI tumors, inadequate lower esophageal sphincter tone, and gastroparesis.2
Another option is a PEG tube, which is a good alternative for patients who are sedated; ventilated; or have neurodegenerative processes, stroke with dysphagia, or head and neck cancers. These are typically recommended when enteral nutrition will be needed for more than 4 weeks. Disadvantages of PEG tubes include tube obstruction or displacement, gastroesophageal reflux, and leakage of gastric content around the percutaneous site or into the peritoneum.
PEG-J tubes, PEJ tubes, or jejunostomy tubes are best suited for patients with GI dysmotility, patients who have unsuccessfully undergone the aforementioned methods, patients with histories of partial gastrectomies, or patients with gastric or pancreatic cancers/multiple traumas. The PEG-J tube extends into the distal duodenum; because it is longer and more narrow, it is more likely to coil and occlude the flow of nutrients during feedings.2,3 Jejunal feeding methods incorporate a continuous pump controlled infusion; if set too rapidly, this could cause dumping syndrome. A benefit of jejunal nutrition is a lower risk of aspiration, compared with other enteral tubes.4
It is best to appraise the selected method for its efficacy and patient preference. The American College of Gastroenterology recommends starting with orogastric or nasogastric feeds, and switching to postpyloric or jejunal feeds for those intolerant to or at high risk for aspiration.5 The most important aspect is early enteral nutrition in hospitalized patients unable to maintain oral nutrition.
Application of the data to the original case
This is a severely hypernatremic diabetic patient unable to swallow. On day 2 of her hospitalization, the clinical team provided the patient with an NG tube for increased free-water intake to gradually decrease her serum sodium. By hospital day 4, the patient’s sodium had normalized. Considering the patient’s long-term prognosis and dysphagia, discussions were held with the patient and husband for PEG tube placement. The patient received a PEG tube and was subsequently discharged 2 days later.
Bottom line
Enteral nutrition is a common need among hospitalized patients. Modality of enteral nutrition will depend on the patient’s past medical history, anticipated duration, and preferences.
Dr. Basnet is the hospitalist program director for Apogee Physicians Group at Eastern New Mexico Medical Center in Roswell. Ms. Tayes is a third-year medical student at Burrell College of Osteopathic Medicine in Las Cruces, N.M., with interests in surgery, internal medicine, and emergency medicine. Ms. Gallivan is a third-year medical student at Burrell College of Osteopathic Medicine, with interests in cardiothoracic surgery, general surgery, and internal medicine.
References
1. Boullata JI et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J Parenter Enteral Nutr. 2016;1-89.
2. Kirby DF et al. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282.
3. Lazarus BA et al. Aspiration associated with long-term gastric versus jejunal feeding: A critical analysis of the literature. Arch Phys Med Rehabil. 1990;71:46.
4. Alkhawaja S et al. Postpyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015 Aug 4;(8):CD008875.
5. McCalve SA et al. ACG Clinical Guideline: Nutrition therapy in the hospitalized patient. Am J Gastroenterol. 2016;111:315-34. doi: 10.1038/ajg.2016.28.
Additional reading
Bellini LM. Nutrition Support in Advanced Lung Disease. UptoDate. https://www-uptodate-com.ezproxy.ad.bcomnm.org/contents/nutritional-support-in-advanced-lung-disease?. Published April 20, 2018.
Commercial Formulas for the Feeding Tube. The Oral Cancer Foundation. https://oralcancerfoundation.org/nutrition/commercial-formulas-feeding-tube/. Published June 5, 2018.
Marik Z. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11). doi: 10.1007/s00134-008-1213-6.
Wischmeyer PE. Enteral nutrition can be given to patients on vasopressors. Crit Care Med. 2020;48(1):122-5. doi: 10.1097/CCM.0000000000003965.
Case
A 68-year-old diabetic nonverbal female presents to the ED because of “seizure” 1 hour ago. On exam, her blood glucose is 200. She is unable to speak and has dysphagia because of a stroke she sustained last month. The patient’s husband adds that she hasn’t been eating and drinking sufficiently in the past couple of days. Imaging was negative for any acute intracranial bleeds or lesions. Labs showed a serum sodium level of 150 milliequivalents/L. D5W is started, and the following day, the patient has a sodium level of 154 milliequivalents/L.
Brief overview
Many hospitalized patients are unable to maintain hydration and/or nutritional status by mouth and will need enteral nutrition. Variables such as past medical history, swallowing ability, history of aspiration, prognosis, and functional capacity of each gastrointestinal segment will determine the best option for enteral nutrition for each patient. Each type of enteral tube feeding has advantages, disadvantages, and complications.
Overview of the data
Enteral nutrition should be started within 24-48 hours in a critically ill patient who is unable to maintain intake according to the American Society of Parenteral and Enteral Nutrition.1 This can be provided through a nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) tube, PEG tube with jejunal extension (PEG-J), or a percutaneous endoscopic jejunal (PEJ) tube.1
NG tubes are often the first method deployed because of their low cost and convenience. They are also suitable for the patient who requires this type of feeding for less than 4 weeks. However, NG tubes do require some patient cooperation (to place and maintain)and are contraindicated in some patients with orofacial trauma, upper GI tumors, inadequate lower esophageal sphincter tone, and gastroparesis.2
Another option is a PEG tube, which is a good alternative for patients who are sedated; ventilated; or have neurodegenerative processes, stroke with dysphagia, or head and neck cancers. These are typically recommended when enteral nutrition will be needed for more than 4 weeks. Disadvantages of PEG tubes include tube obstruction or displacement, gastroesophageal reflux, and leakage of gastric content around the percutaneous site or into the peritoneum.
PEG-J tubes, PEJ tubes, or jejunostomy tubes are best suited for patients with GI dysmotility, patients who have unsuccessfully undergone the aforementioned methods, patients with histories of partial gastrectomies, or patients with gastric or pancreatic cancers/multiple traumas. The PEG-J tube extends into the distal duodenum; because it is longer and more narrow, it is more likely to coil and occlude the flow of nutrients during feedings.2,3 Jejunal feeding methods incorporate a continuous pump controlled infusion; if set too rapidly, this could cause dumping syndrome. A benefit of jejunal nutrition is a lower risk of aspiration, compared with other enteral tubes.4
It is best to appraise the selected method for its efficacy and patient preference. The American College of Gastroenterology recommends starting with orogastric or nasogastric feeds, and switching to postpyloric or jejunal feeds for those intolerant to or at high risk for aspiration.5 The most important aspect is early enteral nutrition in hospitalized patients unable to maintain oral nutrition.
Application of the data to the original case
This is a severely hypernatremic diabetic patient unable to swallow. On day 2 of her hospitalization, the clinical team provided the patient with an NG tube for increased free-water intake to gradually decrease her serum sodium. By hospital day 4, the patient’s sodium had normalized. Considering the patient’s long-term prognosis and dysphagia, discussions were held with the patient and husband for PEG tube placement. The patient received a PEG tube and was subsequently discharged 2 days later.
Bottom line
Enteral nutrition is a common need among hospitalized patients. Modality of enteral nutrition will depend on the patient’s past medical history, anticipated duration, and preferences.
Dr. Basnet is the hospitalist program director for Apogee Physicians Group at Eastern New Mexico Medical Center in Roswell. Ms. Tayes is a third-year medical student at Burrell College of Osteopathic Medicine in Las Cruces, N.M., with interests in surgery, internal medicine, and emergency medicine. Ms. Gallivan is a third-year medical student at Burrell College of Osteopathic Medicine, with interests in cardiothoracic surgery, general surgery, and internal medicine.
References
1. Boullata JI et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J Parenter Enteral Nutr. 2016;1-89.
2. Kirby DF et al. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282.
3. Lazarus BA et al. Aspiration associated with long-term gastric versus jejunal feeding: A critical analysis of the literature. Arch Phys Med Rehabil. 1990;71:46.
4. Alkhawaja S et al. Postpyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015 Aug 4;(8):CD008875.
5. McCalve SA et al. ACG Clinical Guideline: Nutrition therapy in the hospitalized patient. Am J Gastroenterol. 2016;111:315-34. doi: 10.1038/ajg.2016.28.
Additional reading
Bellini LM. Nutrition Support in Advanced Lung Disease. UptoDate. https://www-uptodate-com.ezproxy.ad.bcomnm.org/contents/nutritional-support-in-advanced-lung-disease?. Published April 20, 2018.
Commercial Formulas for the Feeding Tube. The Oral Cancer Foundation. https://oralcancerfoundation.org/nutrition/commercial-formulas-feeding-tube/. Published June 5, 2018.
Marik Z. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11). doi: 10.1007/s00134-008-1213-6.
Wischmeyer PE. Enteral nutrition can be given to patients on vasopressors. Crit Care Med. 2020;48(1):122-5. doi: 10.1097/CCM.0000000000003965.
COVID-19: More hydroxychloroquine data from France, more questions
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Top 10 must-dos in ICU in COVID-19 include prone ventilation
As the first international guidelines on the management of critically ill patients with COVID-19 are understandably comprehensive, one expert involved in their development highlights the essential recommendations and explains the rationale behind prone ventilation.
A panel of 39 experts from 12 countries from across the globe developed the 50 recommendations within four domains, under the auspices of the Surviving Sepsis Campaign. They are issued by the European Society of Intensive Care Medicine (ESICM), and will subsequently be published in the journal Intensive Care Medicine.
A central aspect of the guidance is what works, and what does not, in treating critically ill patients with COVID-19 in intensive care.
Ten of the recommendations cover potential pharmacotherapies, most of which have only weak or no evidence of benefit, as discussed in a recent perspective on Medscape. All 50 recommendations, along with the associated level of evidence, are detailed in table 2 in the paper.
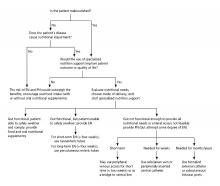
There is also an algorithm for the management of patients with acute hypoxemic respiratory failure secondary to COVID-19 (figure 2) and a summary of clinical practice recommendations (figure 3).
In an editorial in the Journal of the American Medical Association issued just days after these new guidelines, Francois Lamontagne, MD, MSc, and Derek C. Angus, MD, MPH, say they “represent an excellent first step toward optimal, evidence-informed care for patients with COVID-19.” Lamontagne is from Universitaire de Sherbrooke, Canada, and Angus is from University of Pittsburgh School of Medicine, Pennsylvania, and is an associate editor with JAMA.
Dealing With Tide of COVID-19 Patients, Protecting Healthcare Workers
Editor in chief of Intensive Care Medicine Giuseppe Citerio, MD, from University of Milano-Bicocca, Monza, Italy, said: “COVID-19 cases are rising rapidly worldwide, and so we are increasingly seeing that intensive care units [ICUs] have difficulty in dealing with the tide of patients.”
“We need more resource in ICUs, and quickly. This means more ventilators and more trained personnel. In the meantime, this guidance aims to rationalize our approach and to avoid unproven strategies,” he explains in a press release from ESICM.
“This is the first guidance to lay out what works and what doesn’t in treating coronavirus-infected patients in intensive care. It’s based on decades of research on acute respiratory infection being applied to COVID-19 patients,” added ESICM President-Elect Maurizio Cecconi, MD, from Humanitas University, Milan, Italy.
“At the same time as caring for patients, we need to make sure that health workers are following procedures which will allow themselves to be protected against infection,” he stressed.
“We must protect them, they are in the frontline. We cannot allow our healthcare workers to be at risk. On top of that, if they get infected they could also spread the disease further.”
Top-10 Recommendations
While all 50 recommendations are key to the successful management of COVID-19 patients, busy clinicians on the frontline need to zone in on those indispensable practical recommendations that they should implement immediately.
Medscape Medical News therefore asked lead author Waleed Alhazzani, MD, MSc, from the Division of Critical Care, McMaster University, Hamilton, Canada, to give his personal top 10, the first three of which are focused on limiting the spread of infection.
1. For healthcare workers performing aerosol-generating procedures1 on patients with COVID-19 in the ICU, we recommend using fitted respirator masks (N95 respirators, FFP2, or equivalent), as compared to surgical/medical masks, in addition to other personal protective equipment (eg, gloves, gown, and eye protection such as a face shield or safety goggles.
2. We recommend performing aerosol-generating procedures on ICU patients with COVID-19 in a negative-pressure room.
3. For healthcare workers providing usual care for nonventilated COVID-19 patients, we suggest using surgical/medical masks, as compared to respirator masks in addition to other personal protective equipment.
4. For healthcare workers performing endotracheal intubation on patients with COVID-19, we suggest using video guided laryngoscopy, over direct laryngoscopy, if available.
5. We recommend endotracheal intubation in patients with COVID-19, performed by healthcare workers experienced with airway management, to minimize the number of attempts and risk of transmission.
6. For intubated and mechanically ventilated adults with suspicion of COVID-19, we suggest obtaining endotracheal aspirates, over bronchial wash or bronchoalveolar lavage samples.
7. For adults with COVID-19 and acute hypoxemic respiratory failure, we suggest using high-flow nasal cannula [HFNC] over noninvasive positive pressure ventilation [NIPPV].
8. For adults with COVID-19 receiving NIPPV or HFNC, we recommend close monitoring for worsening of respiratory status and early intubation in a controlled setting if worsening occurs.
9. For mechanically ventilated adults with COVID-19 and moderate to severe acute respiratory distress syndrome [ARDS], we suggest prone ventilation for 12 to 16 hours over no prone ventilation.
10. For mechanically ventilated adults with COVID-19 and respiratory failure (without ARDS), we don’t recommend routine use of systemic corticosteroids.
1 This includes endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatment, manual ventilation before intubation, physical proning of the patient, disconnecting the patient from the ventilator, noninvasive positive pressure ventilation, tracheostomy, and cardiopulmonary resuscitation.
These choices are in broad agreement with those selected by Jason T. Poston, MD, University of Chicago, Illinois, and colleagues in their synopsis of these guidelines, published online March 26 in JAMA, although they also highlight another recommendation on infection control:
- For healthcare workers who are performing non-aerosol-generating procedures on mechanically ventilated (closed circuit) patients with COVID-19, we suggest using surgical/medical masks, as opposed to respirator masks, in addition to other personal protective equipment.
Importance of Prone Ventilation, Perhaps for Many Days
One recommendation singled out by both Alhazzani and coauthors, and Poston and colleagues, relates to prone ventilation for 12 to 16 hours in adults with moderate to severe ARDS receiving mechanical ventilation.
Michelle N. Gong, MD, MS, chief of critical care medicine at Montefiore Medical Center, New York City, also highlighted this practice in a live-stream interview with JAMA editor in chief Howard Bauchner, MD.
She explained that, in her institution, they have been “very aggressive about proning these patients as early as possible, but unlike some of the past ARDS patients…they tend to require many, many days of proning in order to get a response”.
Gong added that patients “may improve very rapidly when they are proned, but when we supinate them, they lose [the improvement] and then they get proned for upwards of 10 days or more, if need be.”
Alhazzani told Medscape Medical News that prone ventilation “is a simple intervention that requires training of healthcare providers but can be applied in most contexts.”
He explained that the recommendation “is driven by indirect evidence from ARDS,” not specifically those in COVID-19, with recent studies having shown that COVID-19 “can affect lung bases and may cause significant atelectasis and reduced lung compliance in the context of ARDS.”
“Prone ventilation has been shown to reduce mortality in patients with moderate to severe ARDS. Therefore, we issued a suggestion for clinicians to consider prone ventilation in this population.”
‘Impressively Thorough’ Recommendations, With Some Caveats
In their JAMA editorial, Lamontagne and Angus describe the recommendations as “impressively thorough and expansive.”
They note that they address resource scarcity, which “is likely to be a critical issue in low- and middle-income countries experiencing any reasonably large number of cases and in high-income countries experiencing a surge in the demand for critical care.”
The authors say, however, that a “weakness” of the guidelines is that they make recommendations for interventions that “lack supporting evidence.”
Consequently, “when prioritizing scarce resources, clinicians and healthcare systems will have to choose among options that have limited evidence to support them.”
“In future iterations of the guidelines, there should be more detailed recommendations for how clinicians should prioritize scarce resources, or include more recommendations against the use of unproven therapies.”
“The tasks ahead for the dissemination and uptake of optimal critical care are herculean,” Lamontagne and Angus say.
They include “a need to generate more robust evidence, consider carefully the application of that evidence across a wide variety of clinical circumstances, and generate supporting materials to ensure effective implementation of the guideline recommendations,” they conclude.
ESICM recommendations coauthor Yaseen Arabi is the principal investigator on a clinical trial for lopinavir/ritonavir and interferon in Middle East respiratory syndrome (MERS) and he was a nonpaid consultant on antiviral active for MERS- coronavirus (CoV) for Gilead Sciences and SAB Biotherapeutics. He is an investigator on REMAP-CAP trial and is a Board Members of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). Coauthor Eddy Fan declared receiving consultancy fees from ALung Technologies and MC3 Cardiopulmonary. Coauthor Maurizio Cecconi declared consultancy work with Edwards Lifesciences, Directed Systems, and Cheetah Medical.
JAMA Clinical Guidelines Synopsis coauthor Poston declares receiving honoraria for the CHEST Critical Care Board Review Course.
Editorialist Lamontagne reported receiving grants from the National Institute for Health Research (NIHR), Fonds de recherche du Québec-Santé, and the Lotte & John Hecht Foundation, unrelated to this work. Editorialist Angus participated in the development of Surviving Sepsis Campaign guidelines for sepsis, but had no role in the creation of the current COVID-19 guidelines, nor the decision to create these guidelines.
This article first appeared on Medscape.com.
As the first international guidelines on the management of critically ill patients with COVID-19 are understandably comprehensive, one expert involved in their development highlights the essential recommendations and explains the rationale behind prone ventilation.
A panel of 39 experts from 12 countries from across the globe developed the 50 recommendations within four domains, under the auspices of the Surviving Sepsis Campaign. They are issued by the European Society of Intensive Care Medicine (ESICM), and will subsequently be published in the journal Intensive Care Medicine.
A central aspect of the guidance is what works, and what does not, in treating critically ill patients with COVID-19 in intensive care.
Ten of the recommendations cover potential pharmacotherapies, most of which have only weak or no evidence of benefit, as discussed in a recent perspective on Medscape. All 50 recommendations, along with the associated level of evidence, are detailed in table 2 in the paper.
There is also an algorithm for the management of patients with acute hypoxemic respiratory failure secondary to COVID-19 (figure 2) and a summary of clinical practice recommendations (figure 3).
In an editorial in the Journal of the American Medical Association issued just days after these new guidelines, Francois Lamontagne, MD, MSc, and Derek C. Angus, MD, MPH, say they “represent an excellent first step toward optimal, evidence-informed care for patients with COVID-19.” Lamontagne is from Universitaire de Sherbrooke, Canada, and Angus is from University of Pittsburgh School of Medicine, Pennsylvania, and is an associate editor with JAMA.
Dealing With Tide of COVID-19 Patients, Protecting Healthcare Workers
Editor in chief of Intensive Care Medicine Giuseppe Citerio, MD, from University of Milano-Bicocca, Monza, Italy, said: “COVID-19 cases are rising rapidly worldwide, and so we are increasingly seeing that intensive care units [ICUs] have difficulty in dealing with the tide of patients.”
“We need more resource in ICUs, and quickly. This means more ventilators and more trained personnel. In the meantime, this guidance aims to rationalize our approach and to avoid unproven strategies,” he explains in a press release from ESICM.
“This is the first guidance to lay out what works and what doesn’t in treating coronavirus-infected patients in intensive care. It’s based on decades of research on acute respiratory infection being applied to COVID-19 patients,” added ESICM President-Elect Maurizio Cecconi, MD, from Humanitas University, Milan, Italy.
“At the same time as caring for patients, we need to make sure that health workers are following procedures which will allow themselves to be protected against infection,” he stressed.
“We must protect them, they are in the frontline. We cannot allow our healthcare workers to be at risk. On top of that, if they get infected they could also spread the disease further.”
Top-10 Recommendations
While all 50 recommendations are key to the successful management of COVID-19 patients, busy clinicians on the frontline need to zone in on those indispensable practical recommendations that they should implement immediately.
Medscape Medical News therefore asked lead author Waleed Alhazzani, MD, MSc, from the Division of Critical Care, McMaster University, Hamilton, Canada, to give his personal top 10, the first three of which are focused on limiting the spread of infection.
1. For healthcare workers performing aerosol-generating procedures1 on patients with COVID-19 in the ICU, we recommend using fitted respirator masks (N95 respirators, FFP2, or equivalent), as compared to surgical/medical masks, in addition to other personal protective equipment (eg, gloves, gown, and eye protection such as a face shield or safety goggles.
2. We recommend performing aerosol-generating procedures on ICU patients with COVID-19 in a negative-pressure room.
3. For healthcare workers providing usual care for nonventilated COVID-19 patients, we suggest using surgical/medical masks, as compared to respirator masks in addition to other personal protective equipment.
4. For healthcare workers performing endotracheal intubation on patients with COVID-19, we suggest using video guided laryngoscopy, over direct laryngoscopy, if available.
5. We recommend endotracheal intubation in patients with COVID-19, performed by healthcare workers experienced with airway management, to minimize the number of attempts and risk of transmission.
6. For intubated and mechanically ventilated adults with suspicion of COVID-19, we suggest obtaining endotracheal aspirates, over bronchial wash or bronchoalveolar lavage samples.
7. For adults with COVID-19 and acute hypoxemic respiratory failure, we suggest using high-flow nasal cannula [HFNC] over noninvasive positive pressure ventilation [NIPPV].
8. For adults with COVID-19 receiving NIPPV or HFNC, we recommend close monitoring for worsening of respiratory status and early intubation in a controlled setting if worsening occurs.
9. For mechanically ventilated adults with COVID-19 and moderate to severe acute respiratory distress syndrome [ARDS], we suggest prone ventilation for 12 to 16 hours over no prone ventilation.
10. For mechanically ventilated adults with COVID-19 and respiratory failure (without ARDS), we don’t recommend routine use of systemic corticosteroids.
1 This includes endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatment, manual ventilation before intubation, physical proning of the patient, disconnecting the patient from the ventilator, noninvasive positive pressure ventilation, tracheostomy, and cardiopulmonary resuscitation.
These choices are in broad agreement with those selected by Jason T. Poston, MD, University of Chicago, Illinois, and colleagues in their synopsis of these guidelines, published online March 26 in JAMA, although they also highlight another recommendation on infection control:
- For healthcare workers who are performing non-aerosol-generating procedures on mechanically ventilated (closed circuit) patients with COVID-19, we suggest using surgical/medical masks, as opposed to respirator masks, in addition to other personal protective equipment.
Importance of Prone Ventilation, Perhaps for Many Days
One recommendation singled out by both Alhazzani and coauthors, and Poston and colleagues, relates to prone ventilation for 12 to 16 hours in adults with moderate to severe ARDS receiving mechanical ventilation.
Michelle N. Gong, MD, MS, chief of critical care medicine at Montefiore Medical Center, New York City, also highlighted this practice in a live-stream interview with JAMA editor in chief Howard Bauchner, MD.
She explained that, in her institution, they have been “very aggressive about proning these patients as early as possible, but unlike some of the past ARDS patients…they tend to require many, many days of proning in order to get a response”.
Gong added that patients “may improve very rapidly when they are proned, but when we supinate them, they lose [the improvement] and then they get proned for upwards of 10 days or more, if need be.”
Alhazzani told Medscape Medical News that prone ventilation “is a simple intervention that requires training of healthcare providers but can be applied in most contexts.”
He explained that the recommendation “is driven by indirect evidence from ARDS,” not specifically those in COVID-19, with recent studies having shown that COVID-19 “can affect lung bases and may cause significant atelectasis and reduced lung compliance in the context of ARDS.”
“Prone ventilation has been shown to reduce mortality in patients with moderate to severe ARDS. Therefore, we issued a suggestion for clinicians to consider prone ventilation in this population.”
‘Impressively Thorough’ Recommendations, With Some Caveats
In their JAMA editorial, Lamontagne and Angus describe the recommendations as “impressively thorough and expansive.”
They note that they address resource scarcity, which “is likely to be a critical issue in low- and middle-income countries experiencing any reasonably large number of cases and in high-income countries experiencing a surge in the demand for critical care.”
The authors say, however, that a “weakness” of the guidelines is that they make recommendations for interventions that “lack supporting evidence.”
Consequently, “when prioritizing scarce resources, clinicians and healthcare systems will have to choose among options that have limited evidence to support them.”
“In future iterations of the guidelines, there should be more detailed recommendations for how clinicians should prioritize scarce resources, or include more recommendations against the use of unproven therapies.”
“The tasks ahead for the dissemination and uptake of optimal critical care are herculean,” Lamontagne and Angus say.
They include “a need to generate more robust evidence, consider carefully the application of that evidence across a wide variety of clinical circumstances, and generate supporting materials to ensure effective implementation of the guideline recommendations,” they conclude.
ESICM recommendations coauthor Yaseen Arabi is the principal investigator on a clinical trial for lopinavir/ritonavir and interferon in Middle East respiratory syndrome (MERS) and he was a nonpaid consultant on antiviral active for MERS- coronavirus (CoV) for Gilead Sciences and SAB Biotherapeutics. He is an investigator on REMAP-CAP trial and is a Board Members of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). Coauthor Eddy Fan declared receiving consultancy fees from ALung Technologies and MC3 Cardiopulmonary. Coauthor Maurizio Cecconi declared consultancy work with Edwards Lifesciences, Directed Systems, and Cheetah Medical.
JAMA Clinical Guidelines Synopsis coauthor Poston declares receiving honoraria for the CHEST Critical Care Board Review Course.
Editorialist Lamontagne reported receiving grants from the National Institute for Health Research (NIHR), Fonds de recherche du Québec-Santé, and the Lotte & John Hecht Foundation, unrelated to this work. Editorialist Angus participated in the development of Surviving Sepsis Campaign guidelines for sepsis, but had no role in the creation of the current COVID-19 guidelines, nor the decision to create these guidelines.
This article first appeared on Medscape.com.
As the first international guidelines on the management of critically ill patients with COVID-19 are understandably comprehensive, one expert involved in their development highlights the essential recommendations and explains the rationale behind prone ventilation.
A panel of 39 experts from 12 countries from across the globe developed the 50 recommendations within four domains, under the auspices of the Surviving Sepsis Campaign. They are issued by the European Society of Intensive Care Medicine (ESICM), and will subsequently be published in the journal Intensive Care Medicine.
A central aspect of the guidance is what works, and what does not, in treating critically ill patients with COVID-19 in intensive care.
Ten of the recommendations cover potential pharmacotherapies, most of which have only weak or no evidence of benefit, as discussed in a recent perspective on Medscape. All 50 recommendations, along with the associated level of evidence, are detailed in table 2 in the paper.
There is also an algorithm for the management of patients with acute hypoxemic respiratory failure secondary to COVID-19 (figure 2) and a summary of clinical practice recommendations (figure 3).
In an editorial in the Journal of the American Medical Association issued just days after these new guidelines, Francois Lamontagne, MD, MSc, and Derek C. Angus, MD, MPH, say they “represent an excellent first step toward optimal, evidence-informed care for patients with COVID-19.” Lamontagne is from Universitaire de Sherbrooke, Canada, and Angus is from University of Pittsburgh School of Medicine, Pennsylvania, and is an associate editor with JAMA.
Dealing With Tide of COVID-19 Patients, Protecting Healthcare Workers
Editor in chief of Intensive Care Medicine Giuseppe Citerio, MD, from University of Milano-Bicocca, Monza, Italy, said: “COVID-19 cases are rising rapidly worldwide, and so we are increasingly seeing that intensive care units [ICUs] have difficulty in dealing with the tide of patients.”
“We need more resource in ICUs, and quickly. This means more ventilators and more trained personnel. In the meantime, this guidance aims to rationalize our approach and to avoid unproven strategies,” he explains in a press release from ESICM.
“This is the first guidance to lay out what works and what doesn’t in treating coronavirus-infected patients in intensive care. It’s based on decades of research on acute respiratory infection being applied to COVID-19 patients,” added ESICM President-Elect Maurizio Cecconi, MD, from Humanitas University, Milan, Italy.
“At the same time as caring for patients, we need to make sure that health workers are following procedures which will allow themselves to be protected against infection,” he stressed.
“We must protect them, they are in the frontline. We cannot allow our healthcare workers to be at risk. On top of that, if they get infected they could also spread the disease further.”
Top-10 Recommendations
While all 50 recommendations are key to the successful management of COVID-19 patients, busy clinicians on the frontline need to zone in on those indispensable practical recommendations that they should implement immediately.
Medscape Medical News therefore asked lead author Waleed Alhazzani, MD, MSc, from the Division of Critical Care, McMaster University, Hamilton, Canada, to give his personal top 10, the first three of which are focused on limiting the spread of infection.
1. For healthcare workers performing aerosol-generating procedures1 on patients with COVID-19 in the ICU, we recommend using fitted respirator masks (N95 respirators, FFP2, or equivalent), as compared to surgical/medical masks, in addition to other personal protective equipment (eg, gloves, gown, and eye protection such as a face shield or safety goggles.
2. We recommend performing aerosol-generating procedures on ICU patients with COVID-19 in a negative-pressure room.
3. For healthcare workers providing usual care for nonventilated COVID-19 patients, we suggest using surgical/medical masks, as compared to respirator masks in addition to other personal protective equipment.
4. For healthcare workers performing endotracheal intubation on patients with COVID-19, we suggest using video guided laryngoscopy, over direct laryngoscopy, if available.
5. We recommend endotracheal intubation in patients with COVID-19, performed by healthcare workers experienced with airway management, to minimize the number of attempts and risk of transmission.
6. For intubated and mechanically ventilated adults with suspicion of COVID-19, we suggest obtaining endotracheal aspirates, over bronchial wash or bronchoalveolar lavage samples.
7. For adults with COVID-19 and acute hypoxemic respiratory failure, we suggest using high-flow nasal cannula [HFNC] over noninvasive positive pressure ventilation [NIPPV].
8. For adults with COVID-19 receiving NIPPV or HFNC, we recommend close monitoring for worsening of respiratory status and early intubation in a controlled setting if worsening occurs.
9. For mechanically ventilated adults with COVID-19 and moderate to severe acute respiratory distress syndrome [ARDS], we suggest prone ventilation for 12 to 16 hours over no prone ventilation.
10. For mechanically ventilated adults with COVID-19 and respiratory failure (without ARDS), we don’t recommend routine use of systemic corticosteroids.
1 This includes endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatment, manual ventilation before intubation, physical proning of the patient, disconnecting the patient from the ventilator, noninvasive positive pressure ventilation, tracheostomy, and cardiopulmonary resuscitation.
These choices are in broad agreement with those selected by Jason T. Poston, MD, University of Chicago, Illinois, and colleagues in their synopsis of these guidelines, published online March 26 in JAMA, although they also highlight another recommendation on infection control:
- For healthcare workers who are performing non-aerosol-generating procedures on mechanically ventilated (closed circuit) patients with COVID-19, we suggest using surgical/medical masks, as opposed to respirator masks, in addition to other personal protective equipment.
Importance of Prone Ventilation, Perhaps for Many Days
One recommendation singled out by both Alhazzani and coauthors, and Poston and colleagues, relates to prone ventilation for 12 to 16 hours in adults with moderate to severe ARDS receiving mechanical ventilation.
Michelle N. Gong, MD, MS, chief of critical care medicine at Montefiore Medical Center, New York City, also highlighted this practice in a live-stream interview with JAMA editor in chief Howard Bauchner, MD.
She explained that, in her institution, they have been “very aggressive about proning these patients as early as possible, but unlike some of the past ARDS patients…they tend to require many, many days of proning in order to get a response”.
Gong added that patients “may improve very rapidly when they are proned, but when we supinate them, they lose [the improvement] and then they get proned for upwards of 10 days or more, if need be.”
Alhazzani told Medscape Medical News that prone ventilation “is a simple intervention that requires training of healthcare providers but can be applied in most contexts.”
He explained that the recommendation “is driven by indirect evidence from ARDS,” not specifically those in COVID-19, with recent studies having shown that COVID-19 “can affect lung bases and may cause significant atelectasis and reduced lung compliance in the context of ARDS.”
“Prone ventilation has been shown to reduce mortality in patients with moderate to severe ARDS. Therefore, we issued a suggestion for clinicians to consider prone ventilation in this population.”
‘Impressively Thorough’ Recommendations, With Some Caveats
In their JAMA editorial, Lamontagne and Angus describe the recommendations as “impressively thorough and expansive.”
They note that they address resource scarcity, which “is likely to be a critical issue in low- and middle-income countries experiencing any reasonably large number of cases and in high-income countries experiencing a surge in the demand for critical care.”
The authors say, however, that a “weakness” of the guidelines is that they make recommendations for interventions that “lack supporting evidence.”
Consequently, “when prioritizing scarce resources, clinicians and healthcare systems will have to choose among options that have limited evidence to support them.”
“In future iterations of the guidelines, there should be more detailed recommendations for how clinicians should prioritize scarce resources, or include more recommendations against the use of unproven therapies.”
“The tasks ahead for the dissemination and uptake of optimal critical care are herculean,” Lamontagne and Angus say.
They include “a need to generate more robust evidence, consider carefully the application of that evidence across a wide variety of clinical circumstances, and generate supporting materials to ensure effective implementation of the guideline recommendations,” they conclude.
ESICM recommendations coauthor Yaseen Arabi is the principal investigator on a clinical trial for lopinavir/ritonavir and interferon in Middle East respiratory syndrome (MERS) and he was a nonpaid consultant on antiviral active for MERS- coronavirus (CoV) for Gilead Sciences and SAB Biotherapeutics. He is an investigator on REMAP-CAP trial and is a Board Members of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). Coauthor Eddy Fan declared receiving consultancy fees from ALung Technologies and MC3 Cardiopulmonary. Coauthor Maurizio Cecconi declared consultancy work with Edwards Lifesciences, Directed Systems, and Cheetah Medical.
JAMA Clinical Guidelines Synopsis coauthor Poston declares receiving honoraria for the CHEST Critical Care Board Review Course.
Editorialist Lamontagne reported receiving grants from the National Institute for Health Research (NIHR), Fonds de recherche du Québec-Santé, and the Lotte & John Hecht Foundation, unrelated to this work. Editorialist Angus participated in the development of Surviving Sepsis Campaign guidelines for sepsis, but had no role in the creation of the current COVID-19 guidelines, nor the decision to create these guidelines.
This article first appeared on Medscape.com.
Critical care and COVID-19: Dr. Matt Aldrich
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
At U.S. Ground Zero for coronavirus, a hospital is transformed
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.
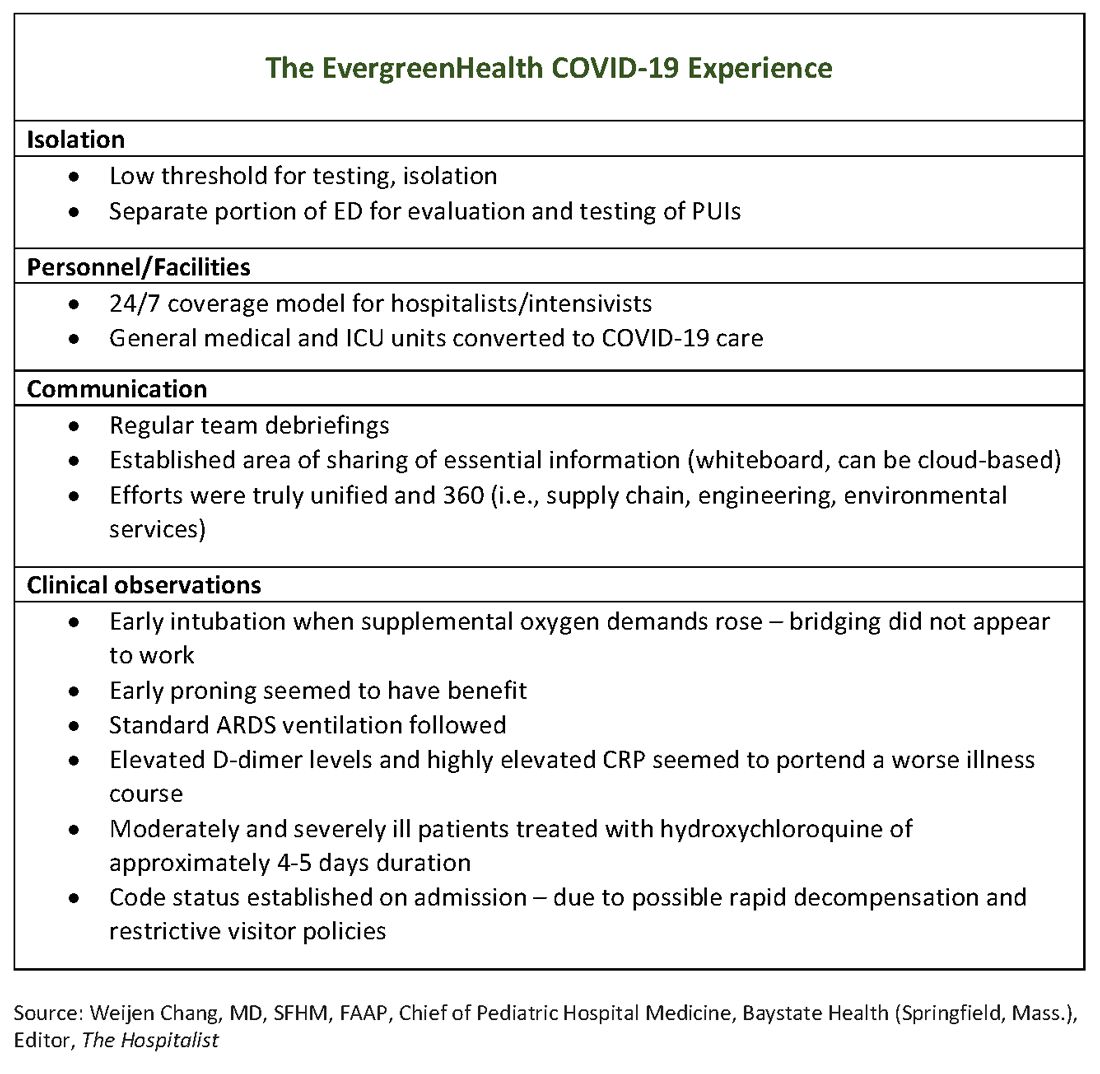
The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
Lessons from Seattle: Prepping a critical care system for COVID-19
What can the nation’s critical care systems do to prepare for the worst of the COVID-19 pandemic?
Mark Tonelli, MD, is professor of medicine and section head of the University of Washington Medical Center’s division of pulmonary, critical care, and sleep medicine. In an audio interview, Dr. Tonelli outlines exactly how the University of Washington and the region’s other health systems are readying their critical care departments for the demands of the COVID-19 pandemic. And he offers advice from the front lines for health systems nationwide as they prep their own critical care systems.
To listen to the interview, click the play button below.
What can the nation’s critical care systems do to prepare for the worst of the COVID-19 pandemic?
Mark Tonelli, MD, is professor of medicine and section head of the University of Washington Medical Center’s division of pulmonary, critical care, and sleep medicine. In an audio interview, Dr. Tonelli outlines exactly how the University of Washington and the region’s other health systems are readying their critical care departments for the demands of the COVID-19 pandemic. And he offers advice from the front lines for health systems nationwide as they prep their own critical care systems.
To listen to the interview, click the play button below.
What can the nation’s critical care systems do to prepare for the worst of the COVID-19 pandemic?
Mark Tonelli, MD, is professor of medicine and section head of the University of Washington Medical Center’s division of pulmonary, critical care, and sleep medicine. In an audio interview, Dr. Tonelli outlines exactly how the University of Washington and the region’s other health systems are readying their critical care departments for the demands of the COVID-19 pandemic. And he offers advice from the front lines for health systems nationwide as they prep their own critical care systems.
To listen to the interview, click the play button below.
COVID-19 critical care guideline offers support for frontline clinicians
The 49 recommendations and statements it included are geared to “support hospital clinicians managing critically ill adults with COVID-19 in the ICU. The target users of this guideline are frontline clinicians, allied health professionals, and policy makers involved in the care of patients with COVID-19 in the ICU,” said the document, written by a panel of 36 experts organized by the Surviving Sepsis Campaign, a joint program of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine.
The document divides the recommendations into four categories: infection control, which includes 3 “best-practice” statements and 5 “weak” recommendations; hemodynamics with 2 “strong” recommendations and 13 weak ones; ventilation, with 1 best-practice statement, 6 strong recommendations, and 12 weak recommendations; and therapy with 7 weak recommendations. The guidelines also included five management questions considered by the writing panel without arriving at a recommendation because of insufficient evidence.
Useful guide nonspecialists
Some critical care medicine physicians saw the new guidelines as offering no surprises, but providing a very useful resource to guide management, especially for clinicians who may become involved in caring for COVID-19 patients despite having little experience caring for patients with acute respiratory distress syndrome (ARDS).
“For those of us who manage ARDS patients all the time, this is not a lot of new information, but many critically ill COVID-19 patients are now being cared for by physicians who have not cared for these patients before,” commented Mangala Narasimhan, DO, FCCP, a critical care medicine physician at Long Island Jewish Medical Center in New Hyde Park, N.Y. In fact, Dr, Narasimhan and associates took the new guidelines soon after their release and used them to create a one-page summary sheet to give to all their colleagues who are now seeing COVID-19 patients, she said in an interview. “The guidelines are very important for clinicians who are suddenly taking care of a roomful of patients with ARDS.”
“A lot of people want to know this information,” agreed David M. Ferraro, MD, FCCP, a pulmonologist and critical care medicine physician at National Jewish Health in Denver.
Perhaps the only potentially controversial aspect of the guidelines are a couple of weak recommendations that suggest using a high-flow nasal cannula (HFNC) rather than noninvasive positive pressure ventilation (NIPPV) in patients with acute hypoxemic respiratory failure who have not fully responded to conventional oxygen therapy. “This is controversial, and some of my colleagues are debating this,” said Dr. Narasimhan, but she noted that her clinic has decided to follow the recommended preference for HFNC, which seemed to have modest advantages over NIPPV in a recent meta-analysis (Intensive Care Med. 2019 May;45[5]:563-72).
Another issue with NIPPV is the higher risk for viral dispersion it seems to have, compared with a HFNC, said Dr. Ferraro. If a patient’s mask comes off during NIPPV, it creates a substantial risk for aerosolization of virus. That risk is likely lower with HFNC, especially a HFNC system that uses a small cannula without heating or humidification of the gas flow. “I’d recommend against NIPPV,” Dr. Ferraro said.
He also highlighted the value of quickly forgoing continued use of either of these ventilatory approaches in a declining patient and having a low threshold to switch to intubation. “Many clinicians now favor erring on the side of early intubation,” he noted, an approach that the new guidelines endorsed in a best-practice statement: “In adults with COVID-19 receiving NIPPV or HFNC we recommend close monitoring for worsening respiratory status and early intubation in a controlled setting if worsening occurs.”
One aspect of the COVID-19 pandemic that the new guidelines don’t address are some of the challenges being faced from skyrocketing numbers of patients and inadequate supplies and manpower to meet their acute clinical needs. “We need recommendations on how systems should manage when they are overwhelmed,” commented Dr. Ferraro, an omission that he also saw in the COVID-19 management guidance released on March 13, 2020, by the World Health Organization.
“Neither document gets into this in depth, but that wasn’t in their scope,” Dr. Ferraro acknowledged. He said that recommendations on how to deal with scarce resources, inadequate staffing, and the health of clinicians are probably best handled on a state or local level rather than trying to create recommendations that are applicable to the entire U.S. health system.
Dr. Narasimhan and Dr. Ferraro reported that they had no disclosures.
The 49 recommendations and statements it included are geared to “support hospital clinicians managing critically ill adults with COVID-19 in the ICU. The target users of this guideline are frontline clinicians, allied health professionals, and policy makers involved in the care of patients with COVID-19 in the ICU,” said the document, written by a panel of 36 experts organized by the Surviving Sepsis Campaign, a joint program of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine.
The document divides the recommendations into four categories: infection control, which includes 3 “best-practice” statements and 5 “weak” recommendations; hemodynamics with 2 “strong” recommendations and 13 weak ones; ventilation, with 1 best-practice statement, 6 strong recommendations, and 12 weak recommendations; and therapy with 7 weak recommendations. The guidelines also included five management questions considered by the writing panel without arriving at a recommendation because of insufficient evidence.
Useful guide nonspecialists
Some critical care medicine physicians saw the new guidelines as offering no surprises, but providing a very useful resource to guide management, especially for clinicians who may become involved in caring for COVID-19 patients despite having little experience caring for patients with acute respiratory distress syndrome (ARDS).
“For those of us who manage ARDS patients all the time, this is not a lot of new information, but many critically ill COVID-19 patients are now being cared for by physicians who have not cared for these patients before,” commented Mangala Narasimhan, DO, FCCP, a critical care medicine physician at Long Island Jewish Medical Center in New Hyde Park, N.Y. In fact, Dr, Narasimhan and associates took the new guidelines soon after their release and used them to create a one-page summary sheet to give to all their colleagues who are now seeing COVID-19 patients, she said in an interview. “The guidelines are very important for clinicians who are suddenly taking care of a roomful of patients with ARDS.”
“A lot of people want to know this information,” agreed David M. Ferraro, MD, FCCP, a pulmonologist and critical care medicine physician at National Jewish Health in Denver.
Perhaps the only potentially controversial aspect of the guidelines are a couple of weak recommendations that suggest using a high-flow nasal cannula (HFNC) rather than noninvasive positive pressure ventilation (NIPPV) in patients with acute hypoxemic respiratory failure who have not fully responded to conventional oxygen therapy. “This is controversial, and some of my colleagues are debating this,” said Dr. Narasimhan, but she noted that her clinic has decided to follow the recommended preference for HFNC, which seemed to have modest advantages over NIPPV in a recent meta-analysis (Intensive Care Med. 2019 May;45[5]:563-72).
Another issue with NIPPV is the higher risk for viral dispersion it seems to have, compared with a HFNC, said Dr. Ferraro. If a patient’s mask comes off during NIPPV, it creates a substantial risk for aerosolization of virus. That risk is likely lower with HFNC, especially a HFNC system that uses a small cannula without heating or humidification of the gas flow. “I’d recommend against NIPPV,” Dr. Ferraro said.
He also highlighted the value of quickly forgoing continued use of either of these ventilatory approaches in a declining patient and having a low threshold to switch to intubation. “Many clinicians now favor erring on the side of early intubation,” he noted, an approach that the new guidelines endorsed in a best-practice statement: “In adults with COVID-19 receiving NIPPV or HFNC we recommend close monitoring for worsening respiratory status and early intubation in a controlled setting if worsening occurs.”
One aspect of the COVID-19 pandemic that the new guidelines don’t address are some of the challenges being faced from skyrocketing numbers of patients and inadequate supplies and manpower to meet their acute clinical needs. “We need recommendations on how systems should manage when they are overwhelmed,” commented Dr. Ferraro, an omission that he also saw in the COVID-19 management guidance released on March 13, 2020, by the World Health Organization.
“Neither document gets into this in depth, but that wasn’t in their scope,” Dr. Ferraro acknowledged. He said that recommendations on how to deal with scarce resources, inadequate staffing, and the health of clinicians are probably best handled on a state or local level rather than trying to create recommendations that are applicable to the entire U.S. health system.
Dr. Narasimhan and Dr. Ferraro reported that they had no disclosures.
The 49 recommendations and statements it included are geared to “support hospital clinicians managing critically ill adults with COVID-19 in the ICU. The target users of this guideline are frontline clinicians, allied health professionals, and policy makers involved in the care of patients with COVID-19 in the ICU,” said the document, written by a panel of 36 experts organized by the Surviving Sepsis Campaign, a joint program of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine.
The document divides the recommendations into four categories: infection control, which includes 3 “best-practice” statements and 5 “weak” recommendations; hemodynamics with 2 “strong” recommendations and 13 weak ones; ventilation, with 1 best-practice statement, 6 strong recommendations, and 12 weak recommendations; and therapy with 7 weak recommendations. The guidelines also included five management questions considered by the writing panel without arriving at a recommendation because of insufficient evidence.
Useful guide nonspecialists
Some critical care medicine physicians saw the new guidelines as offering no surprises, but providing a very useful resource to guide management, especially for clinicians who may become involved in caring for COVID-19 patients despite having little experience caring for patients with acute respiratory distress syndrome (ARDS).
“For those of us who manage ARDS patients all the time, this is not a lot of new information, but many critically ill COVID-19 patients are now being cared for by physicians who have not cared for these patients before,” commented Mangala Narasimhan, DO, FCCP, a critical care medicine physician at Long Island Jewish Medical Center in New Hyde Park, N.Y. In fact, Dr, Narasimhan and associates took the new guidelines soon after their release and used them to create a one-page summary sheet to give to all their colleagues who are now seeing COVID-19 patients, she said in an interview. “The guidelines are very important for clinicians who are suddenly taking care of a roomful of patients with ARDS.”
“A lot of people want to know this information,” agreed David M. Ferraro, MD, FCCP, a pulmonologist and critical care medicine physician at National Jewish Health in Denver.
Perhaps the only potentially controversial aspect of the guidelines are a couple of weak recommendations that suggest using a high-flow nasal cannula (HFNC) rather than noninvasive positive pressure ventilation (NIPPV) in patients with acute hypoxemic respiratory failure who have not fully responded to conventional oxygen therapy. “This is controversial, and some of my colleagues are debating this,” said Dr. Narasimhan, but she noted that her clinic has decided to follow the recommended preference for HFNC, which seemed to have modest advantages over NIPPV in a recent meta-analysis (Intensive Care Med. 2019 May;45[5]:563-72).
Another issue with NIPPV is the higher risk for viral dispersion it seems to have, compared with a HFNC, said Dr. Ferraro. If a patient’s mask comes off during NIPPV, it creates a substantial risk for aerosolization of virus. That risk is likely lower with HFNC, especially a HFNC system that uses a small cannula without heating or humidification of the gas flow. “I’d recommend against NIPPV,” Dr. Ferraro said.
He also highlighted the value of quickly forgoing continued use of either of these ventilatory approaches in a declining patient and having a low threshold to switch to intubation. “Many clinicians now favor erring on the side of early intubation,” he noted, an approach that the new guidelines endorsed in a best-practice statement: “In adults with COVID-19 receiving NIPPV or HFNC we recommend close monitoring for worsening respiratory status and early intubation in a controlled setting if worsening occurs.”
One aspect of the COVID-19 pandemic that the new guidelines don’t address are some of the challenges being faced from skyrocketing numbers of patients and inadequate supplies and manpower to meet their acute clinical needs. “We need recommendations on how systems should manage when they are overwhelmed,” commented Dr. Ferraro, an omission that he also saw in the COVID-19 management guidance released on March 13, 2020, by the World Health Organization.
“Neither document gets into this in depth, but that wasn’t in their scope,” Dr. Ferraro acknowledged. He said that recommendations on how to deal with scarce resources, inadequate staffing, and the health of clinicians are probably best handled on a state or local level rather than trying to create recommendations that are applicable to the entire U.S. health system.
Dr. Narasimhan and Dr. Ferraro reported that they had no disclosures.
Hyperoxia in the ICU: Is less more?
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
Here’s what ICUs are putting up against COVID-19
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.
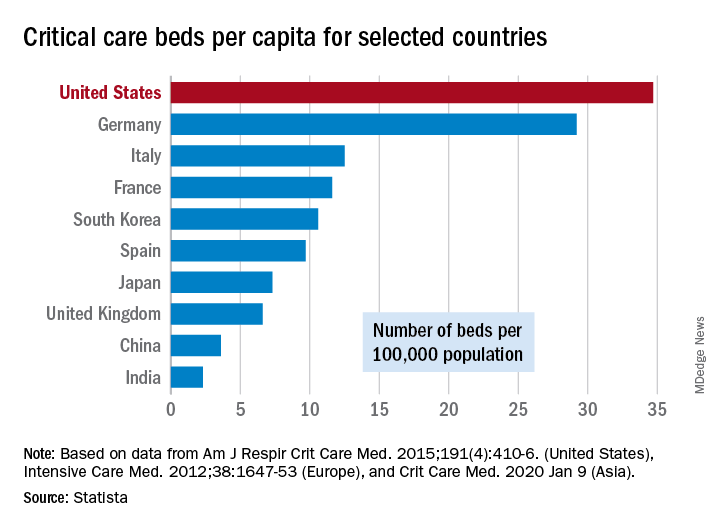
That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.
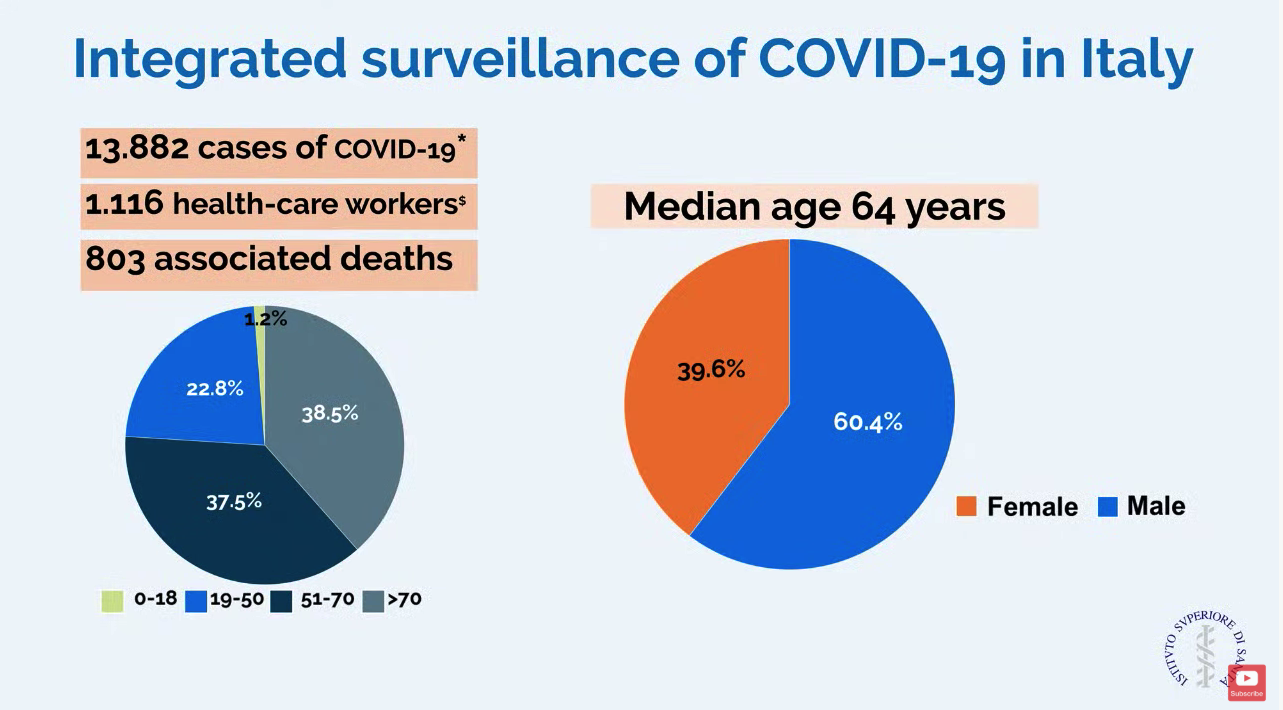
Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
REPORTING FROM JAMA LIVE STREAM