User login
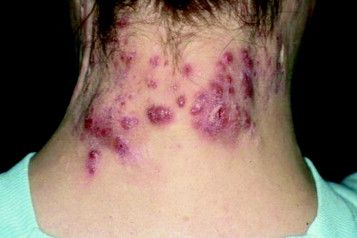
Keloids: Cutting is never enough
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Hyaluronic acid filler preferred for infraorbital hollowing
Most patients who responded to surveys reported being satisfied after off-label treatment with Juvéderm Voluma XC hyaluronic acid filler for infraorbital hollowing, a study finds.
Adverse effects were reported in 12% of patients.
The treatment’s “high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium,” reported Michael B. Hall, MD, and his associates at their private, ambulatory facial plastic and reconstructive surgery practice in Austin, Texas, in JAMA Facial Plastic Surgery.
According to the researchers, the Food and Drug Administration has not approved any soft-tissue fillers for the periorbital complex. At their practice, Dr. Hall and his associates treat infraorbital hollows with Juvéderm Voluma XC, which is approved by the FDA for certain types of cheek augmentation. Other studies have examined Belotero or Restylane as treatments for building volume in the periorbital area, the authors wrote, but research into cosmetic injections of Juvéderm Voluma XC is lacking.
For the new study, the researchers retrospectively analyzed the cases of 101 patients (aged 32-75 years, with average age of 54 years; 89% female; 54% Fitzpatrick skin type II; racial breakdown not reported) who were electively treated with the filler for infraorbital hollowing in 2016 and 2017. The patients received an average 1 mL of the treatment gel.
The patients were photographed and answered surveys, and they were evaluated using the Allergan Infraorbital Hollows Scale. Follow-up time averaged 12 months.
A total of 18 patients (18%) required touch-up within 3 months, and 2 required multiple touch-ups. A total of 12 subjects (12%) had adverse effects (including 3 who had more than one), which included bruising (10%), contour irregularities (2%), edema (3%) and Tyndall effect (1%). Hyaluronidase was required in 3 patients (3%), and 24 patients sought further treatment after 3 months.
The researchers sent two satisfaction surveys to the participants. A total of 41% responded to the FACE-Q Satisfaction With Eyes survey, and 42% responded to the FACE-Q Satisfaction With Decision survey.
Depending on the question, 70%-85% of the respondents to the Satisfaction With Eyes survey said they were “definitely” or “somewhat” satisfied with the treatment outcome.
The highest levels of dissatisfaction came in response to a questions about whether the subjects felt their eyes looked alert (not tired) or youthful. The highest levels of satisfaction were in response to questions about whether the subjects were happy with the shape, attractiveness, and openness of their eyes.
Depending on the question, 73%-85% of the subjects who took the Satisfaction With Decision survey reported that they “definitely” or “somewhat” agree with positive statements about the treatment. While differences were small, they agreed the most with a statement saying the procedure was “worth the time and effort.”
No external funding or remuneration was received. The study authors reported no relevant disclosures.
SOURCE: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
Most patients who responded to surveys reported being satisfied after off-label treatment with Juvéderm Voluma XC hyaluronic acid filler for infraorbital hollowing, a study finds.
Adverse effects were reported in 12% of patients.
The treatment’s “high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium,” reported Michael B. Hall, MD, and his associates at their private, ambulatory facial plastic and reconstructive surgery practice in Austin, Texas, in JAMA Facial Plastic Surgery.
According to the researchers, the Food and Drug Administration has not approved any soft-tissue fillers for the periorbital complex. At their practice, Dr. Hall and his associates treat infraorbital hollows with Juvéderm Voluma XC, which is approved by the FDA for certain types of cheek augmentation. Other studies have examined Belotero or Restylane as treatments for building volume in the periorbital area, the authors wrote, but research into cosmetic injections of Juvéderm Voluma XC is lacking.
For the new study, the researchers retrospectively analyzed the cases of 101 patients (aged 32-75 years, with average age of 54 years; 89% female; 54% Fitzpatrick skin type II; racial breakdown not reported) who were electively treated with the filler for infraorbital hollowing in 2016 and 2017. The patients received an average 1 mL of the treatment gel.
The patients were photographed and answered surveys, and they were evaluated using the Allergan Infraorbital Hollows Scale. Follow-up time averaged 12 months.
A total of 18 patients (18%) required touch-up within 3 months, and 2 required multiple touch-ups. A total of 12 subjects (12%) had adverse effects (including 3 who had more than one), which included bruising (10%), contour irregularities (2%), edema (3%) and Tyndall effect (1%). Hyaluronidase was required in 3 patients (3%), and 24 patients sought further treatment after 3 months.
The researchers sent two satisfaction surveys to the participants. A total of 41% responded to the FACE-Q Satisfaction With Eyes survey, and 42% responded to the FACE-Q Satisfaction With Decision survey.
Depending on the question, 70%-85% of the respondents to the Satisfaction With Eyes survey said they were “definitely” or “somewhat” satisfied with the treatment outcome.
The highest levels of dissatisfaction came in response to a questions about whether the subjects felt their eyes looked alert (not tired) or youthful. The highest levels of satisfaction were in response to questions about whether the subjects were happy with the shape, attractiveness, and openness of their eyes.
Depending on the question, 73%-85% of the subjects who took the Satisfaction With Decision survey reported that they “definitely” or “somewhat” agree with positive statements about the treatment. While differences were small, they agreed the most with a statement saying the procedure was “worth the time and effort.”
No external funding or remuneration was received. The study authors reported no relevant disclosures.
SOURCE: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
Most patients who responded to surveys reported being satisfied after off-label treatment with Juvéderm Voluma XC hyaluronic acid filler for infraorbital hollowing, a study finds.
Adverse effects were reported in 12% of patients.
The treatment’s “high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium,” reported Michael B. Hall, MD, and his associates at their private, ambulatory facial plastic and reconstructive surgery practice in Austin, Texas, in JAMA Facial Plastic Surgery.
According to the researchers, the Food and Drug Administration has not approved any soft-tissue fillers for the periorbital complex. At their practice, Dr. Hall and his associates treat infraorbital hollows with Juvéderm Voluma XC, which is approved by the FDA for certain types of cheek augmentation. Other studies have examined Belotero or Restylane as treatments for building volume in the periorbital area, the authors wrote, but research into cosmetic injections of Juvéderm Voluma XC is lacking.
For the new study, the researchers retrospectively analyzed the cases of 101 patients (aged 32-75 years, with average age of 54 years; 89% female; 54% Fitzpatrick skin type II; racial breakdown not reported) who were electively treated with the filler for infraorbital hollowing in 2016 and 2017. The patients received an average 1 mL of the treatment gel.
The patients were photographed and answered surveys, and they were evaluated using the Allergan Infraorbital Hollows Scale. Follow-up time averaged 12 months.
A total of 18 patients (18%) required touch-up within 3 months, and 2 required multiple touch-ups. A total of 12 subjects (12%) had adverse effects (including 3 who had more than one), which included bruising (10%), contour irregularities (2%), edema (3%) and Tyndall effect (1%). Hyaluronidase was required in 3 patients (3%), and 24 patients sought further treatment after 3 months.
The researchers sent two satisfaction surveys to the participants. A total of 41% responded to the FACE-Q Satisfaction With Eyes survey, and 42% responded to the FACE-Q Satisfaction With Decision survey.
Depending on the question, 70%-85% of the respondents to the Satisfaction With Eyes survey said they were “definitely” or “somewhat” satisfied with the treatment outcome.
The highest levels of dissatisfaction came in response to a questions about whether the subjects felt their eyes looked alert (not tired) or youthful. The highest levels of satisfaction were in response to questions about whether the subjects were happy with the shape, attractiveness, and openness of their eyes.
Depending on the question, 73%-85% of the subjects who took the Satisfaction With Decision survey reported that they “definitely” or “somewhat” agree with positive statements about the treatment. While differences were small, they agreed the most with a statement saying the procedure was “worth the time and effort.”
No external funding or remuneration was received. The study authors reported no relevant disclosures.
SOURCE: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
FROM JAMA FACIAL PLASTIC SURGERY
Key clinical point:
Major finding: Adverse effects occurred at a rate of 12%, and most who responded to surveys reported satisfaction postprocedure (70%-85%).
Study details: A retrospective observational study of 101 patients.
Disclosures: No external funding or remuneration was received. The study authors reported no relevant disclosures.
Source: Hall MB et al. JAMA Facial Plast Surg. 2018 Apr 5. doi:10.1001/jamafacial.2018.0230.
Surgical excision essential in severe hidradenitis suppurativa
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
What’s new in the latest melanoma guidelines
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dramatic improvements reported after surgery for hidradenitis suppurativa
A retrospective German study found that the majority of , with many saying they no longer suffered from everyday impairment from the disease.
“We were able to show that surgical therapy resulted in convincing improvement of life quality and long-term results for HS that are at least as effective as biologicals,” the researchers wrote. The study was published online in the Journal of the European Academy of Dermatology and Venereology.
Lukas Kofler, MD, and associates from the department of dermatology at Eberhard Karls University’s University Medical Center, Tübingen, Germany, surveyed 910 of the facility’s patients who had undergone wide local excision for HS from 2006 to 2015. Surgery was “designed to reach into clinically disease-free subcutaneous fatty tissue,” followed by second intention healing, they wrote.
A total of 255 patients answered the survey, a response rate of 28%. There were 103 men and 152 women with a median age of 38 years (range, 14-66 years); 75% reported prior “nicotine abuse.” Almost half had been treated previously, most often with systemic antibiotics in 68%. The mean follow-up time was 57 months (range, 19-127 months);
All cases were Hurley grade III. Just over three-quarters of the patients described disease-related limitations in private life prior to surgery as “very strong” or “strong,” and 95% reported that their day-to-day life was impaired. Sixty percent said the disease impaired their work life (another 8% were not employed).
After surgery, 27% experienced postoperative complications, including minor bleeding, infection, and limited mobility; 65% experienced pain but 38% of the patients required analgesics postoperatively.
After surgery, 80% were satisfied or very satisfied with the results, and more than two-thirds were satisfied with the cosmetic results. Just over half said their private life was not impaired by the disease at all, compared with 3% who said so before surgery. After surgery, 20% reported being strongly or very strongly impaired, compared with 77% before surgery.
Nearly 70% reported that HS recurred after surgery, but 62% of those with recurrences said the disease was not as severe as before surgery.
“Surgery represents an important treatment option by itself but should also be part of combined therapeutic strategies, especially in severe disease stages. However, consistent approaches combining systemic and surgical treatments have not been established yet,” the authors noted.
The study had no funding source. The authors had no conflicts to disclose.
SOURCE: Kofler L et al. J Eur Acad Dermatol Venereol. 2018 Mar 23. doi: 10.1111/jdv.14892.
A retrospective German study found that the majority of , with many saying they no longer suffered from everyday impairment from the disease.
“We were able to show that surgical therapy resulted in convincing improvement of life quality and long-term results for HS that are at least as effective as biologicals,” the researchers wrote. The study was published online in the Journal of the European Academy of Dermatology and Venereology.
Lukas Kofler, MD, and associates from the department of dermatology at Eberhard Karls University’s University Medical Center, Tübingen, Germany, surveyed 910 of the facility’s patients who had undergone wide local excision for HS from 2006 to 2015. Surgery was “designed to reach into clinically disease-free subcutaneous fatty tissue,” followed by second intention healing, they wrote.
A total of 255 patients answered the survey, a response rate of 28%. There were 103 men and 152 women with a median age of 38 years (range, 14-66 years); 75% reported prior “nicotine abuse.” Almost half had been treated previously, most often with systemic antibiotics in 68%. The mean follow-up time was 57 months (range, 19-127 months);
All cases were Hurley grade III. Just over three-quarters of the patients described disease-related limitations in private life prior to surgery as “very strong” or “strong,” and 95% reported that their day-to-day life was impaired. Sixty percent said the disease impaired their work life (another 8% were not employed).
After surgery, 27% experienced postoperative complications, including minor bleeding, infection, and limited mobility; 65% experienced pain but 38% of the patients required analgesics postoperatively.
After surgery, 80% were satisfied or very satisfied with the results, and more than two-thirds were satisfied with the cosmetic results. Just over half said their private life was not impaired by the disease at all, compared with 3% who said so before surgery. After surgery, 20% reported being strongly or very strongly impaired, compared with 77% before surgery.
Nearly 70% reported that HS recurred after surgery, but 62% of those with recurrences said the disease was not as severe as before surgery.
“Surgery represents an important treatment option by itself but should also be part of combined therapeutic strategies, especially in severe disease stages. However, consistent approaches combining systemic and surgical treatments have not been established yet,” the authors noted.
The study had no funding source. The authors had no conflicts to disclose.
SOURCE: Kofler L et al. J Eur Acad Dermatol Venereol. 2018 Mar 23. doi: 10.1111/jdv.14892.
A retrospective German study found that the majority of , with many saying they no longer suffered from everyday impairment from the disease.
“We were able to show that surgical therapy resulted in convincing improvement of life quality and long-term results for HS that are at least as effective as biologicals,” the researchers wrote. The study was published online in the Journal of the European Academy of Dermatology and Venereology.
Lukas Kofler, MD, and associates from the department of dermatology at Eberhard Karls University’s University Medical Center, Tübingen, Germany, surveyed 910 of the facility’s patients who had undergone wide local excision for HS from 2006 to 2015. Surgery was “designed to reach into clinically disease-free subcutaneous fatty tissue,” followed by second intention healing, they wrote.
A total of 255 patients answered the survey, a response rate of 28%. There were 103 men and 152 women with a median age of 38 years (range, 14-66 years); 75% reported prior “nicotine abuse.” Almost half had been treated previously, most often with systemic antibiotics in 68%. The mean follow-up time was 57 months (range, 19-127 months);
All cases were Hurley grade III. Just over three-quarters of the patients described disease-related limitations in private life prior to surgery as “very strong” or “strong,” and 95% reported that their day-to-day life was impaired. Sixty percent said the disease impaired their work life (another 8% were not employed).
After surgery, 27% experienced postoperative complications, including minor bleeding, infection, and limited mobility; 65% experienced pain but 38% of the patients required analgesics postoperatively.
After surgery, 80% were satisfied or very satisfied with the results, and more than two-thirds were satisfied with the cosmetic results. Just over half said their private life was not impaired by the disease at all, compared with 3% who said so before surgery. After surgery, 20% reported being strongly or very strongly impaired, compared with 77% before surgery.
Nearly 70% reported that HS recurred after surgery, but 62% of those with recurrences said the disease was not as severe as before surgery.
“Surgery represents an important treatment option by itself but should also be part of combined therapeutic strategies, especially in severe disease stages. However, consistent approaches combining systemic and surgical treatments have not been established yet,” the authors noted.
The study had no funding source. The authors had no conflicts to disclose.
SOURCE: Kofler L et al. J Eur Acad Dermatol Venereol. 2018 Mar 23. doi: 10.1111/jdv.14892.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY.
Key clinical point: Patients with hidradenitis suppurativa (HS) report dramatic improvement after radical surgery.
Major finding: The percentage reporting strong or very strong impairment of private life fell from 77% before surgery to 20% afterward.
Study details: A retrospective survey of 255 patients who had undergone surgery for HS (Hurley stage III).
Disclosures: The study had no funding source. The authors had no conflicts to disclose.
Source: Kofler L et al. J Eur Acad Dermatol Venereol. 2018 Mar 23. doi: 10.1111/jdv.14892.
VIDEO: Bioimpedance provides accurate assessment of Mohs surgical margins
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
REPORTING FROM AAD 18
Key clinical point: Bioimpedance spectroscopy showed excellent diagnostic accuracy for cancer cells on Mohs surgical margins.
Major finding: Bioimpedance spectroscopy had a sensitivity of 95.6% and specificity of 99.1% compared with Mohs histology.
Study details: A single-center pilot study with 151 Mohs surgical specimens taken from 50 patients.
Disclosures: The study was funded by NovaScan, the company developing the device tested in the study. Dr. Rigel has been a consultant to NovaScan and to Castle Biosciences, DermTech, Ferndale, Myriad, and Neutrogena, and has received research support from Castle and Neutrogena.
Source: Svoboda R et al. Poster 7304.
Topical imiquimod helps clear blurred lines in lentigo maligna excision
according to a study from the University of Utah.
Lentigo maligna is a subtype of melanoma in situ, usually occurring in the head and neck regions, the researchers said.
“Neoadjuvant topical imiquimod 5% cream applied 5 times weekly for 8 weeks was associated with decreased MDCs in LM treatment sites compared with the MDCs of negative control sites,” wrote Shadai Flores of the University of Utah, Salt Lake City, and her colleagues.
Previously, the ability to distinguish between the surgical border and surrounding background melanocytic hyperplasia was uncertain. Because of this uncertainty, LM removal required an average margin of 7.2 mm. Another study showed that topical imiquimod 5% cream enabled the removal of most LM tumors with 2-mm margins. This study “sought to evaluate MDCs in imiquimod-treated LM and negative control biopsy specimens to determine if there was a measurable difference in melanocyte density,” the researchers wrote in a research letter published in JAMA Dermatology.
The study prospectively followed 52 cases of LM treated with imiquimod 5% topical cream 5 days per week for 8 weeks followed by conservative staged excisions with 2-mm margins. Treatment with imiquimod 5% of LM was followed by a 2- to 4-month recuperation period before surgery could be performed. All patients in the study were treated by one Mohs surgeon at the Huntsman Cancer Institute at the university.
To establish an MDC baseline, a 10-mm long fusiform biopsy was taken as a negative control. The negative control sample site and the LM site were separated by approximately 6 cm, found on the same side of the body, and showed similar color changes. After a negative control was taken, an LM lesion was resected and subsequently quadrisected. The MDCs then were concurrently counted by the researchers and compared with the negative controls.
Of the 52 LM specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls. The median MDC from post–imiquimod-treated sites was 14.4, with a range of 0.5-26.6. This showed marked improvement over the negative controls, which had a median MDC of 20.0 (range of 9.0-36.7). A 2-tailed paired t test revealed that the results displayed statistical significance (P less than .001). Residual LM was seen in the central areas of 9 (17%) specimens, but 43 (83%) had no indication of residual LM.
“The decreased melanocytic hyperplasia in imiquimod-treated sites reduced ambiguity in making a distinction between the border of the excised LM and background melanocytic hyperplasia,” noted Ms. Flores and her colleagues.
The authors had no conflicts of interest.
SOURCE: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
according to a study from the University of Utah.
Lentigo maligna is a subtype of melanoma in situ, usually occurring in the head and neck regions, the researchers said.
“Neoadjuvant topical imiquimod 5% cream applied 5 times weekly for 8 weeks was associated with decreased MDCs in LM treatment sites compared with the MDCs of negative control sites,” wrote Shadai Flores of the University of Utah, Salt Lake City, and her colleagues.
Previously, the ability to distinguish between the surgical border and surrounding background melanocytic hyperplasia was uncertain. Because of this uncertainty, LM removal required an average margin of 7.2 mm. Another study showed that topical imiquimod 5% cream enabled the removal of most LM tumors with 2-mm margins. This study “sought to evaluate MDCs in imiquimod-treated LM and negative control biopsy specimens to determine if there was a measurable difference in melanocyte density,” the researchers wrote in a research letter published in JAMA Dermatology.
The study prospectively followed 52 cases of LM treated with imiquimod 5% topical cream 5 days per week for 8 weeks followed by conservative staged excisions with 2-mm margins. Treatment with imiquimod 5% of LM was followed by a 2- to 4-month recuperation period before surgery could be performed. All patients in the study were treated by one Mohs surgeon at the Huntsman Cancer Institute at the university.
To establish an MDC baseline, a 10-mm long fusiform biopsy was taken as a negative control. The negative control sample site and the LM site were separated by approximately 6 cm, found on the same side of the body, and showed similar color changes. After a negative control was taken, an LM lesion was resected and subsequently quadrisected. The MDCs then were concurrently counted by the researchers and compared with the negative controls.
Of the 52 LM specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls. The median MDC from post–imiquimod-treated sites was 14.4, with a range of 0.5-26.6. This showed marked improvement over the negative controls, which had a median MDC of 20.0 (range of 9.0-36.7). A 2-tailed paired t test revealed that the results displayed statistical significance (P less than .001). Residual LM was seen in the central areas of 9 (17%) specimens, but 43 (83%) had no indication of residual LM.
“The decreased melanocytic hyperplasia in imiquimod-treated sites reduced ambiguity in making a distinction between the border of the excised LM and background melanocytic hyperplasia,” noted Ms. Flores and her colleagues.
The authors had no conflicts of interest.
SOURCE: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
according to a study from the University of Utah.
Lentigo maligna is a subtype of melanoma in situ, usually occurring in the head and neck regions, the researchers said.
“Neoadjuvant topical imiquimod 5% cream applied 5 times weekly for 8 weeks was associated with decreased MDCs in LM treatment sites compared with the MDCs of negative control sites,” wrote Shadai Flores of the University of Utah, Salt Lake City, and her colleagues.
Previously, the ability to distinguish between the surgical border and surrounding background melanocytic hyperplasia was uncertain. Because of this uncertainty, LM removal required an average margin of 7.2 mm. Another study showed that topical imiquimod 5% cream enabled the removal of most LM tumors with 2-mm margins. This study “sought to evaluate MDCs in imiquimod-treated LM and negative control biopsy specimens to determine if there was a measurable difference in melanocyte density,” the researchers wrote in a research letter published in JAMA Dermatology.
The study prospectively followed 52 cases of LM treated with imiquimod 5% topical cream 5 days per week for 8 weeks followed by conservative staged excisions with 2-mm margins. Treatment with imiquimod 5% of LM was followed by a 2- to 4-month recuperation period before surgery could be performed. All patients in the study were treated by one Mohs surgeon at the Huntsman Cancer Institute at the university.
To establish an MDC baseline, a 10-mm long fusiform biopsy was taken as a negative control. The negative control sample site and the LM site were separated by approximately 6 cm, found on the same side of the body, and showed similar color changes. After a negative control was taken, an LM lesion was resected and subsequently quadrisected. The MDCs then were concurrently counted by the researchers and compared with the negative controls.
Of the 52 LM specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls. The median MDC from post–imiquimod-treated sites was 14.4, with a range of 0.5-26.6. This showed marked improvement over the negative controls, which had a median MDC of 20.0 (range of 9.0-36.7). A 2-tailed paired t test revealed that the results displayed statistical significance (P less than .001). Residual LM was seen in the central areas of 9 (17%) specimens, but 43 (83%) had no indication of residual LM.
“The decreased melanocytic hyperplasia in imiquimod-treated sites reduced ambiguity in making a distinction between the border of the excised LM and background melanocytic hyperplasia,” noted Ms. Flores and her colleagues.
The authors had no conflicts of interest.
SOURCE: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
FROM JAMA DERMATOLOGY
Key clinical point: Neoadjuvant, topical imiquimod 5% cream is associated with a decrease in melanocyte density counts (MDC).
Major finding: Of 52 patient specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls.
Study details: A prospective study of 52 cases of lentigo maligna treated with imiquimod 5% topical cream 5 days per week for 8 weeks, followed by conservative staged excisions with 2-mm margins.
Disclosures: The authors had no conflicts of interest.
Source: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
Minority of dermatologists prescribe majority of opioids
Opioid prescribing among dermatologists is concentrated among a relative few, with surgeons and those in Southern states overrepresented, according to a study of Medicare Part D data for 2014.
“A small number of dermatologists account for a large percentage of the prescriptions. And it’s concentrated among dermatology surgeons, who are likely giving a standard prescription for a 4-day course of opioids after surgery,” senior author Arash Mostaghimi, MD, the director of inpatient services in the dermatology department at Brigham and Women’s Hospital, Boston, said in a press release issued by the hospital.
A closer look at those 115 top prescribers shows that 108, or 94%, worked in a surgical practice, 72% were located in Southern states, and 86% were male. Among all the dermatologists in the study, those in the South also had an opioid prescribing rate (2.77 claims/1,000 Medicare beneficiaries) that was more than triple the rate in the Northeast (0.83 claims/1,000 beneficiaries), the investigators said.
they noted, and may have created, based on consumption habits after Mohs microsurgery, a reservoir of more than 490,000 unused pills “that poses a substantial risk for future misuse.”
The study was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences. The investigators did not report any conflicts of interest.
SOURCE: Cao S et al. JAMA Dermatol. 2018 Feb 7. doi: 10.1001/jamadermatol.2017.5835.
Opioid prescribing among dermatologists is concentrated among a relative few, with surgeons and those in Southern states overrepresented, according to a study of Medicare Part D data for 2014.
“A small number of dermatologists account for a large percentage of the prescriptions. And it’s concentrated among dermatology surgeons, who are likely giving a standard prescription for a 4-day course of opioids after surgery,” senior author Arash Mostaghimi, MD, the director of inpatient services in the dermatology department at Brigham and Women’s Hospital, Boston, said in a press release issued by the hospital.
A closer look at those 115 top prescribers shows that 108, or 94%, worked in a surgical practice, 72% were located in Southern states, and 86% were male. Among all the dermatologists in the study, those in the South also had an opioid prescribing rate (2.77 claims/1,000 Medicare beneficiaries) that was more than triple the rate in the Northeast (0.83 claims/1,000 beneficiaries), the investigators said.
they noted, and may have created, based on consumption habits after Mohs microsurgery, a reservoir of more than 490,000 unused pills “that poses a substantial risk for future misuse.”
The study was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences. The investigators did not report any conflicts of interest.
SOURCE: Cao S et al. JAMA Dermatol. 2018 Feb 7. doi: 10.1001/jamadermatol.2017.5835.
Opioid prescribing among dermatologists is concentrated among a relative few, with surgeons and those in Southern states overrepresented, according to a study of Medicare Part D data for 2014.
“A small number of dermatologists account for a large percentage of the prescriptions. And it’s concentrated among dermatology surgeons, who are likely giving a standard prescription for a 4-day course of opioids after surgery,” senior author Arash Mostaghimi, MD, the director of inpatient services in the dermatology department at Brigham and Women’s Hospital, Boston, said in a press release issued by the hospital.
A closer look at those 115 top prescribers shows that 108, or 94%, worked in a surgical practice, 72% were located in Southern states, and 86% were male. Among all the dermatologists in the study, those in the South also had an opioid prescribing rate (2.77 claims/1,000 Medicare beneficiaries) that was more than triple the rate in the Northeast (0.83 claims/1,000 beneficiaries), the investigators said.
they noted, and may have created, based on consumption habits after Mohs microsurgery, a reservoir of more than 490,000 unused pills “that poses a substantial risk for future misuse.”
The study was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences. The investigators did not report any conflicts of interest.
SOURCE: Cao S et al. JAMA Dermatol. 2018 Feb 7. doi: 10.1001/jamadermatol.2017.5835.
FROM JAMA DERMATOLOGY
Multisite, same-day cryolipolysis treatments don’t skew lipids, liver enzymes
Multiple, same-day cryolipolysis treatments don’t adversely affect serum lipids or liver enzymes, either acutely or after 12 weeks.
Among all the lipids measured, only triglycerides showed a significant increase, jumping from a mean 77 mg/dL to 83.4 mg/dL. The just over 6 mg/dL increase was “clinically trivial” and driven by a single patient in the 35-subject study, Kenneth B. Klein, MD, and his colleagues reported in Lasers in Surgery and Medicine.
Results of the small, prospective study are reassuring, if not surprising, the authors wrote. Even four flank treatments, as performed in this cohort, release only about 160 g of fat, at a rate of less than 2 g/day. “To put this figure in perspective, the typical American diet contains at least 75 g of fat per day. Moreover, it has been shown that consuming as much as 261 grams of fat per day causes no ill effects or laboratory abnormalities.”
The study included 35 men and women with a mean age of 45 years but a range of 20-67 years. The mean body mass index was 24.7 kg/m2, although the range was quite wide, at 18-29.7 kg/m2. All subjects underwent cryolipolysis of the lower abdomen and both flanks. Blood was drawn for analysis at baseline and at weeks 1, 4, and 14 after treatment. One patient didn’t complete the treatment because it was impossible to draw enough flank tissue into the applicator.
Patients experienced the expected procedural side effects of erythema, numbness, and edema, with a few reports of tingling and bruising. All of these resolved spontaneously. Immediately after the procedure, the mean pain score was 4 on a 1-10 scale; by week 12, there were no reports of pain.
Other than the triglyceride change in the single patient, there were no statistically or clinically significant changes from baseline to week 12 in total cholesterol (mean 186.5 mg/dL vs. 189.2 mg/dL), HDL cholesterol (71 mg/dL vs. 73.8 mg/dL), LDL cholesterol (100 mg/dL vs. 102.5 mg/dL), or very low-density cholesterol (15.7 mg/dL vs. 16.8 mg/dL). Although the mean triglyceride change was statistically significant, the final measurement was still well below the upper limit of reference (150 mg/dL).
The only liver enzyme change of note occurred in one patient, who had one sharp increase in aminotransferase. That was related to alcohol consumption the night before the week 12 blood draw.
The study was sponsored by Zeltiq Aesthetics, which manufactures the device used in the trial. Dr. Klein is a consultant to Zeltiq. All three coauthors were paid investigators; in addition, one of them, Eric P. Bachelor, MD, is on the Zeltiq speakers’ bureau.
SOURCE: Klein KB et al. Lasers Surg Med. 2017 Sep;49(7):640-4.
Multiple, same-day cryolipolysis treatments don’t adversely affect serum lipids or liver enzymes, either acutely or after 12 weeks.
Among all the lipids measured, only triglycerides showed a significant increase, jumping from a mean 77 mg/dL to 83.4 mg/dL. The just over 6 mg/dL increase was “clinically trivial” and driven by a single patient in the 35-subject study, Kenneth B. Klein, MD, and his colleagues reported in Lasers in Surgery and Medicine.
Results of the small, prospective study are reassuring, if not surprising, the authors wrote. Even four flank treatments, as performed in this cohort, release only about 160 g of fat, at a rate of less than 2 g/day. “To put this figure in perspective, the typical American diet contains at least 75 g of fat per day. Moreover, it has been shown that consuming as much as 261 grams of fat per day causes no ill effects or laboratory abnormalities.”
The study included 35 men and women with a mean age of 45 years but a range of 20-67 years. The mean body mass index was 24.7 kg/m2, although the range was quite wide, at 18-29.7 kg/m2. All subjects underwent cryolipolysis of the lower abdomen and both flanks. Blood was drawn for analysis at baseline and at weeks 1, 4, and 14 after treatment. One patient didn’t complete the treatment because it was impossible to draw enough flank tissue into the applicator.
Patients experienced the expected procedural side effects of erythema, numbness, and edema, with a few reports of tingling and bruising. All of these resolved spontaneously. Immediately after the procedure, the mean pain score was 4 on a 1-10 scale; by week 12, there were no reports of pain.
Other than the triglyceride change in the single patient, there were no statistically or clinically significant changes from baseline to week 12 in total cholesterol (mean 186.5 mg/dL vs. 189.2 mg/dL), HDL cholesterol (71 mg/dL vs. 73.8 mg/dL), LDL cholesterol (100 mg/dL vs. 102.5 mg/dL), or very low-density cholesterol (15.7 mg/dL vs. 16.8 mg/dL). Although the mean triglyceride change was statistically significant, the final measurement was still well below the upper limit of reference (150 mg/dL).
The only liver enzyme change of note occurred in one patient, who had one sharp increase in aminotransferase. That was related to alcohol consumption the night before the week 12 blood draw.
The study was sponsored by Zeltiq Aesthetics, which manufactures the device used in the trial. Dr. Klein is a consultant to Zeltiq. All three coauthors were paid investigators; in addition, one of them, Eric P. Bachelor, MD, is on the Zeltiq speakers’ bureau.
SOURCE: Klein KB et al. Lasers Surg Med. 2017 Sep;49(7):640-4.
Multiple, same-day cryolipolysis treatments don’t adversely affect serum lipids or liver enzymes, either acutely or after 12 weeks.
Among all the lipids measured, only triglycerides showed a significant increase, jumping from a mean 77 mg/dL to 83.4 mg/dL. The just over 6 mg/dL increase was “clinically trivial” and driven by a single patient in the 35-subject study, Kenneth B. Klein, MD, and his colleagues reported in Lasers in Surgery and Medicine.
Results of the small, prospective study are reassuring, if not surprising, the authors wrote. Even four flank treatments, as performed in this cohort, release only about 160 g of fat, at a rate of less than 2 g/day. “To put this figure in perspective, the typical American diet contains at least 75 g of fat per day. Moreover, it has been shown that consuming as much as 261 grams of fat per day causes no ill effects or laboratory abnormalities.”
The study included 35 men and women with a mean age of 45 years but a range of 20-67 years. The mean body mass index was 24.7 kg/m2, although the range was quite wide, at 18-29.7 kg/m2. All subjects underwent cryolipolysis of the lower abdomen and both flanks. Blood was drawn for analysis at baseline and at weeks 1, 4, and 14 after treatment. One patient didn’t complete the treatment because it was impossible to draw enough flank tissue into the applicator.
Patients experienced the expected procedural side effects of erythema, numbness, and edema, with a few reports of tingling and bruising. All of these resolved spontaneously. Immediately after the procedure, the mean pain score was 4 on a 1-10 scale; by week 12, there were no reports of pain.
Other than the triglyceride change in the single patient, there were no statistically or clinically significant changes from baseline to week 12 in total cholesterol (mean 186.5 mg/dL vs. 189.2 mg/dL), HDL cholesterol (71 mg/dL vs. 73.8 mg/dL), LDL cholesterol (100 mg/dL vs. 102.5 mg/dL), or very low-density cholesterol (15.7 mg/dL vs. 16.8 mg/dL). Although the mean triglyceride change was statistically significant, the final measurement was still well below the upper limit of reference (150 mg/dL).
The only liver enzyme change of note occurred in one patient, who had one sharp increase in aminotransferase. That was related to alcohol consumption the night before the week 12 blood draw.
The study was sponsored by Zeltiq Aesthetics, which manufactures the device used in the trial. Dr. Klein is a consultant to Zeltiq. All three coauthors were paid investigators; in addition, one of them, Eric P. Bachelor, MD, is on the Zeltiq speakers’ bureau.
SOURCE: Klein KB et al. Lasers Surg Med. 2017 Sep;49(7):640-4.
FROM LASERS IN SURGERY AND MEDICINE
Key clinical point:
Major finding: There were no statistically or clinically significant changes from baseline to week 12 in total cholesterol (mean 186.5 mg/dL vs. 189.2 mg/dL), HDL cholesterol (71 mg/dL vs. 73.8 mg/dL), LDL cholesterol (100 mg/dL vs. 102.5 mg/dL), or VLDL cholesterol (15.7 mg/dL vs. 16.8 mg/dL).
Data source: A small prospective study of 35 men and women who underwent cryolipolysis of the lower abdomen and both flanks.
Disclosures: The study was sponsored by Zeltiq Aesthetics, which manufactures the device used in the trial. Dr. Klein is a consultant for Zeltiq. All three coauthors were paid investigators; in addition, one of them, Eric Bachelor, MD, is on the Zeltiq speakers’ bureau.
Source: Klein KB et al. Lasers Surg Med. 2017 Sep;49(7):640-4.
Best practices address latest trends in PDT, skin cancer treatment
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
REPORTING FROM ODAC 2018