User login
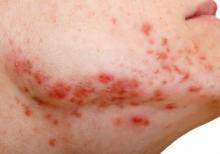
Abrocitinib highly effective as long-term monotherapy in AD
through 48 weeks of follow-up in the JADE EXTEND study, Kristian Reich, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The head-turning outcomes achieved at the higher studied dose of 200 mg once daily as monotherapy – namely, 87% of patients had an EASI-75 response, defined as at least a 75% reduction from baseline in Eczema Area and Severity Index score, and 62% had an EASI-90 response – herald a new era in the management of atopic dermatitis, predicted Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany).
“I think we will see an evolution in the treatment goals in atopic dermatitis. It’s really good to see nearly 90% of the patients achieved EASI-75 over time. I am completely convinced that if you ultimately want to have a happy patient, you will see treatment goals moving up. We have already seen this in psoriasis. I want to see drugs that give the majority of my patients an EASI-75. And ultimately I want to see EASI-90 for my patients,” he said.
Concurrent with his presentation at the EADV congress, Pfizer announced it has filed for marketing approval of abrocitinib at 100 mg and 200 mg once daily for the treatment of moderate to severe AD. The Food and Drug Administration has granted the application priority review status, with a decision due next April. The company has also filed for marketing approval with the European Medicines Agency.
The JADE EXTEND study is an ongoing extension of the previously reported phase 3, randomized, double-blind, placebo-controlled, 12-week JADE MONO-1 and JADE MONO-2 trials. The two trials included a total of 309 patients on abrocitinib at 200 mg/day and 314 on the selective Janus kinase (JAK) 1 inhibitor at 100 mg/day, 519 of whom subsequently entered the long-term extension study on their same dose. The 70% who required no supplemental topical therapy through 48 weeks were the focus of the analysis presented by Dr. Reich.
The proportion of strong responders increased up until the week 24 or 36 assessments, then remained steady until week 48. For example, the EASI-75 rate in patients on abrocitinib at 200 mg/day rose from 82.5% at week 16, to 86.2% at week 24, 90.1% at week 36, and reached 87.2% at week 48. The EASI-90 rates at the same time points were 56.7%, 64.5%, 65.5%, and 61.6%, respectively. And the EASI-100 rates were 24%, 31.6%, 29.6%, and 24%, respectively.
Not surprisingly, the EASI-75 rates in patients on abrocitinib at 100 mg/day were less robust: 64.4% at week 16, 75.5% at week 24, 74.5% at week 36, and 68% at week 48.
An Investigator’s Global Assessment score of 0 or 1 – that is, clear or almost clear – was achieved at week 16 in 55% of patients on 200 mg/day, 64.5% at week 24, 66% at week 36, and 60.5% at week 48. In patients on the 100-mg dose, the corresponding figures were 36.5%, 46.6%, 53.3%, and 45.2%.
A hallmark of all of the JAK inhibitors under study for AD is what Dr. Reich characterized as “an amazingly fast reduction of itch,” the dominant symptom of the disease. A clinically meaningful reduction of at least 4 points in the Peak Pruritus Numerical Rating Scale – a response of 4 or greater is considered clinically important – from the mean baseline score of 7.1 was present at week 12 in 56.3% of patients on abrocitinib at 200 mg, in 74.3% at week 16, and in 72.5% at week 48. The proportion of patients achieving this endpoint on 100 mg was 41.6% at week 12, 49.4% at week 16, and 52% at week 48.
Serious treatment-emergent adverse events occurred in 6.1% of JADE EXTEND participants on abrocitinib at 100 mg and 12.8% of those on 200 mg. These events included oral herpes and elevated creatine phosphokinase levels. The sole case of pulmonary embolism that occurred during the study was deemed unrelated to treatment.
“What this is telling me here is there are no signals that we haven’t seen earlier with this drug and with other JAK inhibitors before,” the dermatologist observed. “But I want to see more data. I want to see the overall safety, not just for a year, but for 2, 3, 4, and 5 years.”
Asked by an audience member if nonresponsiveness to one JAK inhibitor predicts nonresponse to others, Dr. Reich speculated that it’s likely to be so. He noted that all three of the JAK inhibitors furthest along in the developmental pipeline for atopic dermatitis – abrocitinib, baricitinib, and upadacitinib – are inhibitors of JAK 1, although baricitinib also targets JAK 2.
“I would think that if you really are a nonresponder to any of these that it will be hard to get a good response with the others. We’re not talking about antibodies here, where there may be different epitopes. The affinity is different, and we have seen that if you have no response to a weak TNF [tumor necrosis factor] inhibitor, you can still have a response to a strong TNF inhibitor. I don’t expect the same here,” according to Dr. Reich.
He reported serving as an adviser to and paid clinical research for Pfizer, which sponsored JADE EXTEND, as well as more than two dozen other pharmaceutical companies.
through 48 weeks of follow-up in the JADE EXTEND study, Kristian Reich, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The head-turning outcomes achieved at the higher studied dose of 200 mg once daily as monotherapy – namely, 87% of patients had an EASI-75 response, defined as at least a 75% reduction from baseline in Eczema Area and Severity Index score, and 62% had an EASI-90 response – herald a new era in the management of atopic dermatitis, predicted Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany).
“I think we will see an evolution in the treatment goals in atopic dermatitis. It’s really good to see nearly 90% of the patients achieved EASI-75 over time. I am completely convinced that if you ultimately want to have a happy patient, you will see treatment goals moving up. We have already seen this in psoriasis. I want to see drugs that give the majority of my patients an EASI-75. And ultimately I want to see EASI-90 for my patients,” he said.
Concurrent with his presentation at the EADV congress, Pfizer announced it has filed for marketing approval of abrocitinib at 100 mg and 200 mg once daily for the treatment of moderate to severe AD. The Food and Drug Administration has granted the application priority review status, with a decision due next April. The company has also filed for marketing approval with the European Medicines Agency.
The JADE EXTEND study is an ongoing extension of the previously reported phase 3, randomized, double-blind, placebo-controlled, 12-week JADE MONO-1 and JADE MONO-2 trials. The two trials included a total of 309 patients on abrocitinib at 200 mg/day and 314 on the selective Janus kinase (JAK) 1 inhibitor at 100 mg/day, 519 of whom subsequently entered the long-term extension study on their same dose. The 70% who required no supplemental topical therapy through 48 weeks were the focus of the analysis presented by Dr. Reich.
The proportion of strong responders increased up until the week 24 or 36 assessments, then remained steady until week 48. For example, the EASI-75 rate in patients on abrocitinib at 200 mg/day rose from 82.5% at week 16, to 86.2% at week 24, 90.1% at week 36, and reached 87.2% at week 48. The EASI-90 rates at the same time points were 56.7%, 64.5%, 65.5%, and 61.6%, respectively. And the EASI-100 rates were 24%, 31.6%, 29.6%, and 24%, respectively.
Not surprisingly, the EASI-75 rates in patients on abrocitinib at 100 mg/day were less robust: 64.4% at week 16, 75.5% at week 24, 74.5% at week 36, and 68% at week 48.
An Investigator’s Global Assessment score of 0 or 1 – that is, clear or almost clear – was achieved at week 16 in 55% of patients on 200 mg/day, 64.5% at week 24, 66% at week 36, and 60.5% at week 48. In patients on the 100-mg dose, the corresponding figures were 36.5%, 46.6%, 53.3%, and 45.2%.
A hallmark of all of the JAK inhibitors under study for AD is what Dr. Reich characterized as “an amazingly fast reduction of itch,” the dominant symptom of the disease. A clinically meaningful reduction of at least 4 points in the Peak Pruritus Numerical Rating Scale – a response of 4 or greater is considered clinically important – from the mean baseline score of 7.1 was present at week 12 in 56.3% of patients on abrocitinib at 200 mg, in 74.3% at week 16, and in 72.5% at week 48. The proportion of patients achieving this endpoint on 100 mg was 41.6% at week 12, 49.4% at week 16, and 52% at week 48.
Serious treatment-emergent adverse events occurred in 6.1% of JADE EXTEND participants on abrocitinib at 100 mg and 12.8% of those on 200 mg. These events included oral herpes and elevated creatine phosphokinase levels. The sole case of pulmonary embolism that occurred during the study was deemed unrelated to treatment.
“What this is telling me here is there are no signals that we haven’t seen earlier with this drug and with other JAK inhibitors before,” the dermatologist observed. “But I want to see more data. I want to see the overall safety, not just for a year, but for 2, 3, 4, and 5 years.”
Asked by an audience member if nonresponsiveness to one JAK inhibitor predicts nonresponse to others, Dr. Reich speculated that it’s likely to be so. He noted that all three of the JAK inhibitors furthest along in the developmental pipeline for atopic dermatitis – abrocitinib, baricitinib, and upadacitinib – are inhibitors of JAK 1, although baricitinib also targets JAK 2.
“I would think that if you really are a nonresponder to any of these that it will be hard to get a good response with the others. We’re not talking about antibodies here, where there may be different epitopes. The affinity is different, and we have seen that if you have no response to a weak TNF [tumor necrosis factor] inhibitor, you can still have a response to a strong TNF inhibitor. I don’t expect the same here,” according to Dr. Reich.
He reported serving as an adviser to and paid clinical research for Pfizer, which sponsored JADE EXTEND, as well as more than two dozen other pharmaceutical companies.
through 48 weeks of follow-up in the JADE EXTEND study, Kristian Reich, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The head-turning outcomes achieved at the higher studied dose of 200 mg once daily as monotherapy – namely, 87% of patients had an EASI-75 response, defined as at least a 75% reduction from baseline in Eczema Area and Severity Index score, and 62% had an EASI-90 response – herald a new era in the management of atopic dermatitis, predicted Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany).
“I think we will see an evolution in the treatment goals in atopic dermatitis. It’s really good to see nearly 90% of the patients achieved EASI-75 over time. I am completely convinced that if you ultimately want to have a happy patient, you will see treatment goals moving up. We have already seen this in psoriasis. I want to see drugs that give the majority of my patients an EASI-75. And ultimately I want to see EASI-90 for my patients,” he said.
Concurrent with his presentation at the EADV congress, Pfizer announced it has filed for marketing approval of abrocitinib at 100 mg and 200 mg once daily for the treatment of moderate to severe AD. The Food and Drug Administration has granted the application priority review status, with a decision due next April. The company has also filed for marketing approval with the European Medicines Agency.
The JADE EXTEND study is an ongoing extension of the previously reported phase 3, randomized, double-blind, placebo-controlled, 12-week JADE MONO-1 and JADE MONO-2 trials. The two trials included a total of 309 patients on abrocitinib at 200 mg/day and 314 on the selective Janus kinase (JAK) 1 inhibitor at 100 mg/day, 519 of whom subsequently entered the long-term extension study on their same dose. The 70% who required no supplemental topical therapy through 48 weeks were the focus of the analysis presented by Dr. Reich.
The proportion of strong responders increased up until the week 24 or 36 assessments, then remained steady until week 48. For example, the EASI-75 rate in patients on abrocitinib at 200 mg/day rose from 82.5% at week 16, to 86.2% at week 24, 90.1% at week 36, and reached 87.2% at week 48. The EASI-90 rates at the same time points were 56.7%, 64.5%, 65.5%, and 61.6%, respectively. And the EASI-100 rates were 24%, 31.6%, 29.6%, and 24%, respectively.
Not surprisingly, the EASI-75 rates in patients on abrocitinib at 100 mg/day were less robust: 64.4% at week 16, 75.5% at week 24, 74.5% at week 36, and 68% at week 48.
An Investigator’s Global Assessment score of 0 or 1 – that is, clear or almost clear – was achieved at week 16 in 55% of patients on 200 mg/day, 64.5% at week 24, 66% at week 36, and 60.5% at week 48. In patients on the 100-mg dose, the corresponding figures were 36.5%, 46.6%, 53.3%, and 45.2%.
A hallmark of all of the JAK inhibitors under study for AD is what Dr. Reich characterized as “an amazingly fast reduction of itch,” the dominant symptom of the disease. A clinically meaningful reduction of at least 4 points in the Peak Pruritus Numerical Rating Scale – a response of 4 or greater is considered clinically important – from the mean baseline score of 7.1 was present at week 12 in 56.3% of patients on abrocitinib at 200 mg, in 74.3% at week 16, and in 72.5% at week 48. The proportion of patients achieving this endpoint on 100 mg was 41.6% at week 12, 49.4% at week 16, and 52% at week 48.
Serious treatment-emergent adverse events occurred in 6.1% of JADE EXTEND participants on abrocitinib at 100 mg and 12.8% of those on 200 mg. These events included oral herpes and elevated creatine phosphokinase levels. The sole case of pulmonary embolism that occurred during the study was deemed unrelated to treatment.
“What this is telling me here is there are no signals that we haven’t seen earlier with this drug and with other JAK inhibitors before,” the dermatologist observed. “But I want to see more data. I want to see the overall safety, not just for a year, but for 2, 3, 4, and 5 years.”
Asked by an audience member if nonresponsiveness to one JAK inhibitor predicts nonresponse to others, Dr. Reich speculated that it’s likely to be so. He noted that all three of the JAK inhibitors furthest along in the developmental pipeline for atopic dermatitis – abrocitinib, baricitinib, and upadacitinib – are inhibitors of JAK 1, although baricitinib also targets JAK 2.
“I would think that if you really are a nonresponder to any of these that it will be hard to get a good response with the others. We’re not talking about antibodies here, where there may be different epitopes. The affinity is different, and we have seen that if you have no response to a weak TNF [tumor necrosis factor] inhibitor, you can still have a response to a strong TNF inhibitor. I don’t expect the same here,” according to Dr. Reich.
He reported serving as an adviser to and paid clinical research for Pfizer, which sponsored JADE EXTEND, as well as more than two dozen other pharmaceutical companies.
FROM THE EADV CONGRESS
Topical tapinarof effective in pivotal psoriasis trials
in two identical pivotal phase 3, randomized trials, Mark G. Lebwohl, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Tapinarof cream has the potential to be a first-in-class topical therapeutic aryl hydrocarbon receptor modulating agent and will provide physicians and patients with a novel nonsteroidal topical treatment option that’s effective and well tolerated,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dermavant Sciences, the company developing topical tapinarof for treatment of atopic dermatitis as well as psoriasis, announced that upon completion of an ongoing long-term extension study the company plans to file for approval of the drug for psoriasis in 2021.
The two pivotal phase 3 trials, PSOARING 1 and PSOARING 2, randomized a total of 1,025 patients with plaque psoriasis to once-daily tapinarof cream 1% or its vehicle. “This was a fairly difficult group of patients,” Dr. Lebwohl said. Roughly 80% had moderate psoriasis as defined by a baseline Physician Global Assessment (PGA) score of 3, with the remainder split evenly between mild and severe disease. Participants averaged 8% body surface area involvement. Body mass index was on average greater than 31 kg/m2.
The primary efficacy endpoint was a PGA score of 0 or 1 – that is, clear or almost clear – plus at least a 2-grade improvement in PGA from baseline at week 12. This was achieved in 35.4% of patients on tapinarof cream once daily in PSOARING 1 and 40.2% in PSOARING 2, compared with 6.0% and 6.3% of vehicle-treated controls, a highly significant difference (both P < .0001).
The prespecified secondary endpoint was a 75% improvement in Psoriasis Area and Severity Index (PASI) score from baseline to week 12. The PASI 75 rates were 36.1% and 47.6% with tapinarof, significantly better than the 10.2% and 6.9% rates in controls.
The most common adverse event associated with tapinarof was folliculitis, which occurred in 20.6% of treated patients in PSOARING 1 and in 15.7% in PSOARING 2. More than 98% of cases were mild or moderate. The folliculitis led to study discontinuation in only 1.8% and 0.9% of subjects in the two trials.
The other noteworthy adverse event was contact dermatitis. It occurred in 3.8% and 4.7% of tapinarof-treated patients, again with low study discontinuation rates of 1.5% and 2.2%.
During the audience discussion, Linda Stein Gold, MD, lead investigator for PSOARING 2, was asked about this folliculitis. She said the mechanism is unclear, as is the best management. She encountered it in patients, didn’t treat it, and it went away on its own. It’s not a bacterial folliculitis; when cultured it invariably proved culture negative, she noted.
The comparative efficacy of tapinarof cream versus the potent and superpotent topical corticosteroids commonly used in the treatment of psoriasis hasn’t been evaluated in head-to-head studies. Her experience and that of the other investigators has been that tapinarof’s efficacy is comparably strong, “but we don’t have the steroid side effects,” said Dr. Stein Gold, director of dermatology clinical research at Henry Ford Health System in Detroit.
In an interview, Dr. Lebwohl said tapinarof, if approved, could help meet a major unmet need for new and better topical therapies for psoriasis.
“You keep hearing about all these biologic agents and small-molecule pills coming out, but the majority of patients still only need topical therapy,” he observed.
Moreover, even when patients with more severe disease achieve a PASI 75 or PASI 90 response with systemic therapy, they usually still need supplemental topical therapy to get them closer to the goal of clear skin.
The superpotent steroids that are the current mainstay of topical therapy come with predictable side effects that dictate a 2- to 4-week limit on their approved use. Also, they’re not supposed to be applied to the face or to intertriginous sites, including the groin, axillae, and under the breasts. In contrast, tapinarof has proved safe and effective in these sensitive areas.
Asked to predict how tapinarof is likely to be used in clinical practice, Dr. Lebwohl replied: “The efficacy was equivalent to strong topical steroids, so I think it could be used first line in place of topical steroids. And in particular, in patients with psoriasis at facial and intertriginous sites, I think an argument can be made for insisting that it be first line.”
He also expects that physicians will end up utilizing tapinarof for a varied group of steroid-responsive dermatoses beyond psoriasis and atopic dermatitis.
“It clearly reduces inflammation, which is why I would expect it would work well for those,” the dermatologist said.
The mechanism of action of tapinarof has been worked out. The drug enters the cell and binds to the aryl hydrocarbon receptor, forming a complex that enters the nucleus. There it joins with the aryl hydrocarbon receptor nuclear translocator, which regulates gene expression so as to reduce production of inflammatory cytokines while promoting an increase in skin barrier proteins, which is why tapinarof is also being developed as an atopic dermatitis therapy.
Dr. Lebwohl and Dr. Stein Gold reported receiving research funds from and serving as consultants to Dermavant Sciences as well as numerous other pharmaceutical companies.
in two identical pivotal phase 3, randomized trials, Mark G. Lebwohl, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Tapinarof cream has the potential to be a first-in-class topical therapeutic aryl hydrocarbon receptor modulating agent and will provide physicians and patients with a novel nonsteroidal topical treatment option that’s effective and well tolerated,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dermavant Sciences, the company developing topical tapinarof for treatment of atopic dermatitis as well as psoriasis, announced that upon completion of an ongoing long-term extension study the company plans to file for approval of the drug for psoriasis in 2021.
The two pivotal phase 3 trials, PSOARING 1 and PSOARING 2, randomized a total of 1,025 patients with plaque psoriasis to once-daily tapinarof cream 1% or its vehicle. “This was a fairly difficult group of patients,” Dr. Lebwohl said. Roughly 80% had moderate psoriasis as defined by a baseline Physician Global Assessment (PGA) score of 3, with the remainder split evenly between mild and severe disease. Participants averaged 8% body surface area involvement. Body mass index was on average greater than 31 kg/m2.
The primary efficacy endpoint was a PGA score of 0 or 1 – that is, clear or almost clear – plus at least a 2-grade improvement in PGA from baseline at week 12. This was achieved in 35.4% of patients on tapinarof cream once daily in PSOARING 1 and 40.2% in PSOARING 2, compared with 6.0% and 6.3% of vehicle-treated controls, a highly significant difference (both P < .0001).
The prespecified secondary endpoint was a 75% improvement in Psoriasis Area and Severity Index (PASI) score from baseline to week 12. The PASI 75 rates were 36.1% and 47.6% with tapinarof, significantly better than the 10.2% and 6.9% rates in controls.
The most common adverse event associated with tapinarof was folliculitis, which occurred in 20.6% of treated patients in PSOARING 1 and in 15.7% in PSOARING 2. More than 98% of cases were mild or moderate. The folliculitis led to study discontinuation in only 1.8% and 0.9% of subjects in the two trials.
The other noteworthy adverse event was contact dermatitis. It occurred in 3.8% and 4.7% of tapinarof-treated patients, again with low study discontinuation rates of 1.5% and 2.2%.
During the audience discussion, Linda Stein Gold, MD, lead investigator for PSOARING 2, was asked about this folliculitis. She said the mechanism is unclear, as is the best management. She encountered it in patients, didn’t treat it, and it went away on its own. It’s not a bacterial folliculitis; when cultured it invariably proved culture negative, she noted.
The comparative efficacy of tapinarof cream versus the potent and superpotent topical corticosteroids commonly used in the treatment of psoriasis hasn’t been evaluated in head-to-head studies. Her experience and that of the other investigators has been that tapinarof’s efficacy is comparably strong, “but we don’t have the steroid side effects,” said Dr. Stein Gold, director of dermatology clinical research at Henry Ford Health System in Detroit.
In an interview, Dr. Lebwohl said tapinarof, if approved, could help meet a major unmet need for new and better topical therapies for psoriasis.
“You keep hearing about all these biologic agents and small-molecule pills coming out, but the majority of patients still only need topical therapy,” he observed.
Moreover, even when patients with more severe disease achieve a PASI 75 or PASI 90 response with systemic therapy, they usually still need supplemental topical therapy to get them closer to the goal of clear skin.
The superpotent steroids that are the current mainstay of topical therapy come with predictable side effects that dictate a 2- to 4-week limit on their approved use. Also, they’re not supposed to be applied to the face or to intertriginous sites, including the groin, axillae, and under the breasts. In contrast, tapinarof has proved safe and effective in these sensitive areas.
Asked to predict how tapinarof is likely to be used in clinical practice, Dr. Lebwohl replied: “The efficacy was equivalent to strong topical steroids, so I think it could be used first line in place of topical steroids. And in particular, in patients with psoriasis at facial and intertriginous sites, I think an argument can be made for insisting that it be first line.”
He also expects that physicians will end up utilizing tapinarof for a varied group of steroid-responsive dermatoses beyond psoriasis and atopic dermatitis.
“It clearly reduces inflammation, which is why I would expect it would work well for those,” the dermatologist said.
The mechanism of action of tapinarof has been worked out. The drug enters the cell and binds to the aryl hydrocarbon receptor, forming a complex that enters the nucleus. There it joins with the aryl hydrocarbon receptor nuclear translocator, which regulates gene expression so as to reduce production of inflammatory cytokines while promoting an increase in skin barrier proteins, which is why tapinarof is also being developed as an atopic dermatitis therapy.
Dr. Lebwohl and Dr. Stein Gold reported receiving research funds from and serving as consultants to Dermavant Sciences as well as numerous other pharmaceutical companies.
in two identical pivotal phase 3, randomized trials, Mark G. Lebwohl, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Tapinarof cream has the potential to be a first-in-class topical therapeutic aryl hydrocarbon receptor modulating agent and will provide physicians and patients with a novel nonsteroidal topical treatment option that’s effective and well tolerated,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dermavant Sciences, the company developing topical tapinarof for treatment of atopic dermatitis as well as psoriasis, announced that upon completion of an ongoing long-term extension study the company plans to file for approval of the drug for psoriasis in 2021.
The two pivotal phase 3 trials, PSOARING 1 and PSOARING 2, randomized a total of 1,025 patients with plaque psoriasis to once-daily tapinarof cream 1% or its vehicle. “This was a fairly difficult group of patients,” Dr. Lebwohl said. Roughly 80% had moderate psoriasis as defined by a baseline Physician Global Assessment (PGA) score of 3, with the remainder split evenly between mild and severe disease. Participants averaged 8% body surface area involvement. Body mass index was on average greater than 31 kg/m2.
The primary efficacy endpoint was a PGA score of 0 or 1 – that is, clear or almost clear – plus at least a 2-grade improvement in PGA from baseline at week 12. This was achieved in 35.4% of patients on tapinarof cream once daily in PSOARING 1 and 40.2% in PSOARING 2, compared with 6.0% and 6.3% of vehicle-treated controls, a highly significant difference (both P < .0001).
The prespecified secondary endpoint was a 75% improvement in Psoriasis Area and Severity Index (PASI) score from baseline to week 12. The PASI 75 rates were 36.1% and 47.6% with tapinarof, significantly better than the 10.2% and 6.9% rates in controls.
The most common adverse event associated with tapinarof was folliculitis, which occurred in 20.6% of treated patients in PSOARING 1 and in 15.7% in PSOARING 2. More than 98% of cases were mild or moderate. The folliculitis led to study discontinuation in only 1.8% and 0.9% of subjects in the two trials.
The other noteworthy adverse event was contact dermatitis. It occurred in 3.8% and 4.7% of tapinarof-treated patients, again with low study discontinuation rates of 1.5% and 2.2%.
During the audience discussion, Linda Stein Gold, MD, lead investigator for PSOARING 2, was asked about this folliculitis. She said the mechanism is unclear, as is the best management. She encountered it in patients, didn’t treat it, and it went away on its own. It’s not a bacterial folliculitis; when cultured it invariably proved culture negative, she noted.
The comparative efficacy of tapinarof cream versus the potent and superpotent topical corticosteroids commonly used in the treatment of psoriasis hasn’t been evaluated in head-to-head studies. Her experience and that of the other investigators has been that tapinarof’s efficacy is comparably strong, “but we don’t have the steroid side effects,” said Dr. Stein Gold, director of dermatology clinical research at Henry Ford Health System in Detroit.
In an interview, Dr. Lebwohl said tapinarof, if approved, could help meet a major unmet need for new and better topical therapies for psoriasis.
“You keep hearing about all these biologic agents and small-molecule pills coming out, but the majority of patients still only need topical therapy,” he observed.
Moreover, even when patients with more severe disease achieve a PASI 75 or PASI 90 response with systemic therapy, they usually still need supplemental topical therapy to get them closer to the goal of clear skin.
The superpotent steroids that are the current mainstay of topical therapy come with predictable side effects that dictate a 2- to 4-week limit on their approved use. Also, they’re not supposed to be applied to the face or to intertriginous sites, including the groin, axillae, and under the breasts. In contrast, tapinarof has proved safe and effective in these sensitive areas.
Asked to predict how tapinarof is likely to be used in clinical practice, Dr. Lebwohl replied: “The efficacy was equivalent to strong topical steroids, so I think it could be used first line in place of topical steroids. And in particular, in patients with psoriasis at facial and intertriginous sites, I think an argument can be made for insisting that it be first line.”
He also expects that physicians will end up utilizing tapinarof for a varied group of steroid-responsive dermatoses beyond psoriasis and atopic dermatitis.
“It clearly reduces inflammation, which is why I would expect it would work well for those,” the dermatologist said.
The mechanism of action of tapinarof has been worked out. The drug enters the cell and binds to the aryl hydrocarbon receptor, forming a complex that enters the nucleus. There it joins with the aryl hydrocarbon receptor nuclear translocator, which regulates gene expression so as to reduce production of inflammatory cytokines while promoting an increase in skin barrier proteins, which is why tapinarof is also being developed as an atopic dermatitis therapy.
Dr. Lebwohl and Dr. Stein Gold reported receiving research funds from and serving as consultants to Dermavant Sciences as well as numerous other pharmaceutical companies.
FROM THE EADV CONGRESS
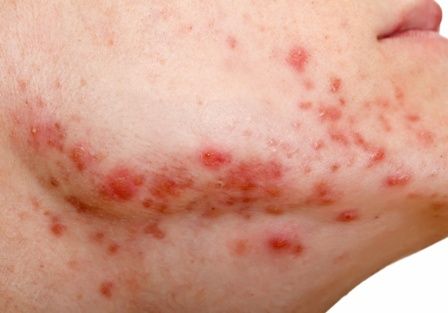
Who’s at risk for depression on isotretinoin?
A Sanaa Butt, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
This was, however, the sole identifiable risk factor for treatment-limiting depressive symptoms in acne patients on isotretinoin in the study of 3,151 consecutive acne patients taking isotretinoin. There was no significant difference between those who did or did not develop depression on the oral retinoid in terms of age, gender, or daily dose of the drug at the time it was discontinued.
“Depressive symptoms occurred at any time from the date of initiation of isotretinoin up to 6 months into therapy, with no identifiable peak time period,” said Dr. Butt, a dermatologist with the U.K. National Health Service Tayside district at Ninewells Hospital, Dundee, Scotland. “Lower doses appear not to be protective,” she added.
The Tayside district has a catchment of roughly 450,000 people. The local population tends to stay put because Tayside is an economically disadvantaged and remote part of Scotland. There are very few private practice dermatologists in the area, so Dr. Butt and coinvestigators are confident their observational study of NHS patients captured the great majority of isotretinoin users in northern Scotland.
The investigators utilized software to analyze the contents of more than 8,000 digitized letters exchanged between NHS Tayside dermatologists and general practitioners during 2005-2018, zeroing in on 3,151 consecutive patients on isotretinoin for acne and 158 on the drug for other conditions, most often rosacea or folliculitis. They then drilled down further through the letters, electronically searching for key words such as suicide, depression, and anxiety. In this way, they ultimately identified 30 patients who discontinued the drug because they developed depressive symptoms. All 30 were on the drug for acne.
The annual incidence of treatment-limiting depressive mood changes was 0.96%, a figure that remained steady over the 13-year study period, even though prescribing of isotretinoin increased over time. This flat incidence rate effectively rules out the potential for confounding because of assessor bias, especially since many different NHS dermatologists were prescribing the drug, Dr. Butt said.
Half of acne patients prescribed isotretinoin were female and 50% were male. And 15 cases of treatment discontinuation caused by development of depressive symptoms occurred in females, 15 in males. A history of past depressive illness was present in 9.3% of females who started on isotretinoin and in 4.5% of the males. The relative risk of treatment-limiting depressive mood changes was increased 790% among females with a prior history of depressive illness and 440% in males with such a history.
Dr. Butt reported having no financial conflicts regarding her NHS-funded study.
A Sanaa Butt, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
This was, however, the sole identifiable risk factor for treatment-limiting depressive symptoms in acne patients on isotretinoin in the study of 3,151 consecutive acne patients taking isotretinoin. There was no significant difference between those who did or did not develop depression on the oral retinoid in terms of age, gender, or daily dose of the drug at the time it was discontinued.
“Depressive symptoms occurred at any time from the date of initiation of isotretinoin up to 6 months into therapy, with no identifiable peak time period,” said Dr. Butt, a dermatologist with the U.K. National Health Service Tayside district at Ninewells Hospital, Dundee, Scotland. “Lower doses appear not to be protective,” she added.
The Tayside district has a catchment of roughly 450,000 people. The local population tends to stay put because Tayside is an economically disadvantaged and remote part of Scotland. There are very few private practice dermatologists in the area, so Dr. Butt and coinvestigators are confident their observational study of NHS patients captured the great majority of isotretinoin users in northern Scotland.
The investigators utilized software to analyze the contents of more than 8,000 digitized letters exchanged between NHS Tayside dermatologists and general practitioners during 2005-2018, zeroing in on 3,151 consecutive patients on isotretinoin for acne and 158 on the drug for other conditions, most often rosacea or folliculitis. They then drilled down further through the letters, electronically searching for key words such as suicide, depression, and anxiety. In this way, they ultimately identified 30 patients who discontinued the drug because they developed depressive symptoms. All 30 were on the drug for acne.
The annual incidence of treatment-limiting depressive mood changes was 0.96%, a figure that remained steady over the 13-year study period, even though prescribing of isotretinoin increased over time. This flat incidence rate effectively rules out the potential for confounding because of assessor bias, especially since many different NHS dermatologists were prescribing the drug, Dr. Butt said.
Half of acne patients prescribed isotretinoin were female and 50% were male. And 15 cases of treatment discontinuation caused by development of depressive symptoms occurred in females, 15 in males. A history of past depressive illness was present in 9.3% of females who started on isotretinoin and in 4.5% of the males. The relative risk of treatment-limiting depressive mood changes was increased 790% among females with a prior history of depressive illness and 440% in males with such a history.
Dr. Butt reported having no financial conflicts regarding her NHS-funded study.
A Sanaa Butt, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
This was, however, the sole identifiable risk factor for treatment-limiting depressive symptoms in acne patients on isotretinoin in the study of 3,151 consecutive acne patients taking isotretinoin. There was no significant difference between those who did or did not develop depression on the oral retinoid in terms of age, gender, or daily dose of the drug at the time it was discontinued.
“Depressive symptoms occurred at any time from the date of initiation of isotretinoin up to 6 months into therapy, with no identifiable peak time period,” said Dr. Butt, a dermatologist with the U.K. National Health Service Tayside district at Ninewells Hospital, Dundee, Scotland. “Lower doses appear not to be protective,” she added.
The Tayside district has a catchment of roughly 450,000 people. The local population tends to stay put because Tayside is an economically disadvantaged and remote part of Scotland. There are very few private practice dermatologists in the area, so Dr. Butt and coinvestigators are confident their observational study of NHS patients captured the great majority of isotretinoin users in northern Scotland.
The investigators utilized software to analyze the contents of more than 8,000 digitized letters exchanged between NHS Tayside dermatologists and general practitioners during 2005-2018, zeroing in on 3,151 consecutive patients on isotretinoin for acne and 158 on the drug for other conditions, most often rosacea or folliculitis. They then drilled down further through the letters, electronically searching for key words such as suicide, depression, and anxiety. In this way, they ultimately identified 30 patients who discontinued the drug because they developed depressive symptoms. All 30 were on the drug for acne.
The annual incidence of treatment-limiting depressive mood changes was 0.96%, a figure that remained steady over the 13-year study period, even though prescribing of isotretinoin increased over time. This flat incidence rate effectively rules out the potential for confounding because of assessor bias, especially since many different NHS dermatologists were prescribing the drug, Dr. Butt said.
Half of acne patients prescribed isotretinoin were female and 50% were male. And 15 cases of treatment discontinuation caused by development of depressive symptoms occurred in females, 15 in males. A history of past depressive illness was present in 9.3% of females who started on isotretinoin and in 4.5% of the males. The relative risk of treatment-limiting depressive mood changes was increased 790% among females with a prior history of depressive illness and 440% in males with such a history.
Dr. Butt reported having no financial conflicts regarding her NHS-funded study.
FROM THE EADV CONGRESS
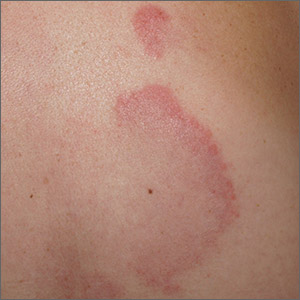
Arcuate eruption on the back
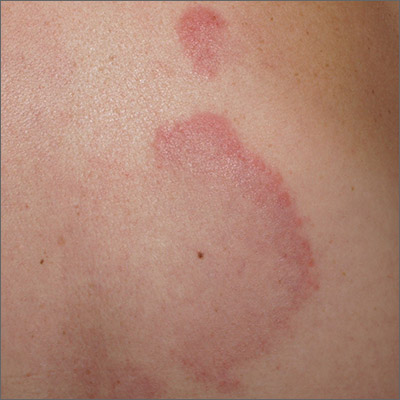
A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.

A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
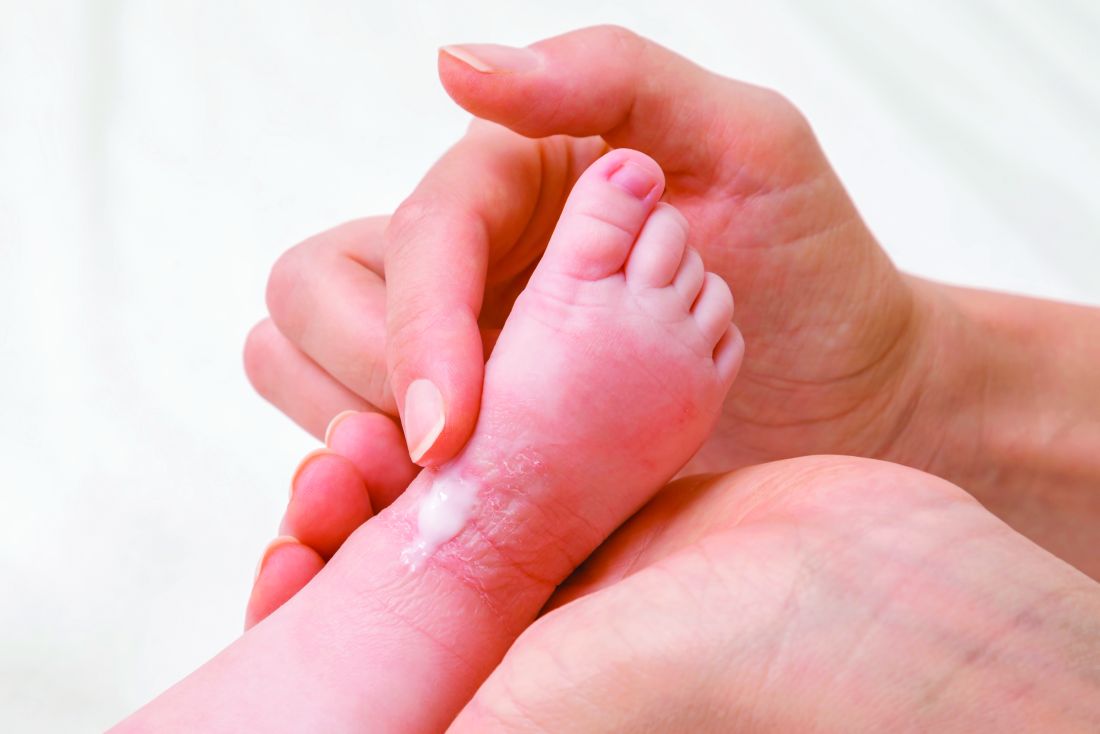
‘Soak-and-smear’ AD protocol backed by evidence
The most effective initial step for clearing atopic dermatitis in infants and young children involves daily bathing, followed by immediate application of a moisturizer, topical steroid, or both, according to an expert speaking at the virtual annual Coastal Dermatology Symposium.
“If they are really severe, you can do it twice-daily, but there are several studies that show there is not a huge benefit of twice-daily over once-daily,” said Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland.
He called this technique “soak-and-smear.” The “smear” is performed immediately after the bath when the skin is still damp, he said. When clearing is the goal, and the child has moderate to severe atopic dermatitis (AD), 0.1% triamcinolone or a similar medium potency topical steroid can be applied, and after clearing, the steroid can be switched for a moisturizer, according to Dr. Simpson.
Rather than restricting application to areas of greatest skin involvement, “put it all over,” he advised.
The clearing regimen should be continued “for a couple of more days” after the lesions have resolved, with a return visit in about a week to confirm clearing and reinforce the next steps for keeping patients clear, he added.
The next steps depend on severity. According to Dr. Simpson, severity is defined less by the extent of skin involvement at the baseline examination than the speed at which symptoms return.
For those with only mild symptoms after an extended period of clearing, moisturizer might be sufficient to prevent a significant relapse. For children with a more rapid relapse, it will be necessary to reintroduce topical steroid either every day, every other day, or twice per week.
Whether with moisturizer or with topical steroids, the soak-and-smear technique has now been validated in a recently published crossover randomized trial.
In the trial, children aged 6 months to 11 years, with moderate to severe AD, were randomized to a twice-daily bath, called the “wet method,” versus a twice-weekly bath, called the “dry method.” Both groups received a cleanser and moisturizer along with a low-potency topical steroid as needed.
After 2 weeks, the 40 evaluable patients were crossed over to the opposite bathing technique. The wet, or soak-and-smear approach, was associated with a highly significant reduction in the primary endpoint of SCORing Atopic Dermatitis (SCORAD) index, compared with the dry method (95% confidence interval, 14.9-27.6; P less than .0001). In a secondary analysis, this translated into a 30% relative reduction in favor of the wet method.
In addition, there was improvement in a caregiver assessment of the Atopic Dermatitis Quickscore (ADQ). These data show that “twice-daily baths with topical steroids and moisturizer can help in more moderate to severe population,” said Dr. Simpson, who noted that he has participated in open-label studies with the same soak-and-smear technique that have produced similar results.
Once children are clear, Dr. Simpson recommends a maintenance strategy individualized for severity. In many cases, this will involve moisturizers applied after the bath, supplemented intermittently, such as once or twice per week, with topical steroids. However, if parents find themselves resorting to daily steroids to maintain control, “that’s when you incorporate the TCIs [topical calcineurin inhibitors].”
TCIs “can help you stay at twice-per-week topical steroids,” Dr. Simpson said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
TCIs also help patients avoid steroid withdrawal, a particularly common phenomenon when topical steroids are applied repeatedly to the face. He recommended a proactive approach. By applying TCIs to areas where skin lesions frequently recur, such as the eyelids, flares can often be prevented.
Repeated applications of TCIs “is perfectly safe and effective, and there are many studies that show proactive treatment is very effective and can prevent you from having to use too much topical steroids” or move to a systemic steroid, Dr. Simpson said.
These steps have been highly effective for sustained control even in challenging cases of AD, but he emphasized the importance of explaining the rationale to parents and eliciting their adherence to these treatment steps. Writing out the instructions will reduce confusion and help parents keep their children clear, he added.
Lawrence F. Eichenfield, MD, professor of pediatrics and dermatology at the University of California, San Diego, agreed that this recently published crossover trial has been helpful in counseling parents about how to manage AD in their children.
“Many times, pediatricians tell parents to avoid bathing because they feel that bathing will dry out the skin,” he said. The crossover study, by showing better control of AD with frequent bathing, dispels that notion, although he is not convinced that bathing at this frequency is necessary.
“I have not advised anyone to do twice-daily bathing, with rare exceptions, on the basis on this study, but, basically, I think that whether people do daily bathing or every other day bathing, it is pretty reasonable that bathing might help as long as they are applying moisturizer immediately afterward,” he said.
Dr. Simpson reports financial relationships with AbbVie, Celgene Dermira, Genentech, GlaxoSmithKline, Incyte, Lilly, Medimmune, Pfizer, Regeneron/Sanofi, and Tioga.
This publication and Global Academy for Medical Education are owned by the same parent company.
The most effective initial step for clearing atopic dermatitis in infants and young children involves daily bathing, followed by immediate application of a moisturizer, topical steroid, or both, according to an expert speaking at the virtual annual Coastal Dermatology Symposium.
“If they are really severe, you can do it twice-daily, but there are several studies that show there is not a huge benefit of twice-daily over once-daily,” said Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland.
He called this technique “soak-and-smear.” The “smear” is performed immediately after the bath when the skin is still damp, he said. When clearing is the goal, and the child has moderate to severe atopic dermatitis (AD), 0.1% triamcinolone or a similar medium potency topical steroid can be applied, and after clearing, the steroid can be switched for a moisturizer, according to Dr. Simpson.
Rather than restricting application to areas of greatest skin involvement, “put it all over,” he advised.
The clearing regimen should be continued “for a couple of more days” after the lesions have resolved, with a return visit in about a week to confirm clearing and reinforce the next steps for keeping patients clear, he added.
The next steps depend on severity. According to Dr. Simpson, severity is defined less by the extent of skin involvement at the baseline examination than the speed at which symptoms return.
For those with only mild symptoms after an extended period of clearing, moisturizer might be sufficient to prevent a significant relapse. For children with a more rapid relapse, it will be necessary to reintroduce topical steroid either every day, every other day, or twice per week.
Whether with moisturizer or with topical steroids, the soak-and-smear technique has now been validated in a recently published crossover randomized trial.
In the trial, children aged 6 months to 11 years, with moderate to severe AD, were randomized to a twice-daily bath, called the “wet method,” versus a twice-weekly bath, called the “dry method.” Both groups received a cleanser and moisturizer along with a low-potency topical steroid as needed.
After 2 weeks, the 40 evaluable patients were crossed over to the opposite bathing technique. The wet, or soak-and-smear approach, was associated with a highly significant reduction in the primary endpoint of SCORing Atopic Dermatitis (SCORAD) index, compared with the dry method (95% confidence interval, 14.9-27.6; P less than .0001). In a secondary analysis, this translated into a 30% relative reduction in favor of the wet method.
In addition, there was improvement in a caregiver assessment of the Atopic Dermatitis Quickscore (ADQ). These data show that “twice-daily baths with topical steroids and moisturizer can help in more moderate to severe population,” said Dr. Simpson, who noted that he has participated in open-label studies with the same soak-and-smear technique that have produced similar results.
Once children are clear, Dr. Simpson recommends a maintenance strategy individualized for severity. In many cases, this will involve moisturizers applied after the bath, supplemented intermittently, such as once or twice per week, with topical steroids. However, if parents find themselves resorting to daily steroids to maintain control, “that’s when you incorporate the TCIs [topical calcineurin inhibitors].”
TCIs “can help you stay at twice-per-week topical steroids,” Dr. Simpson said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
TCIs also help patients avoid steroid withdrawal, a particularly common phenomenon when topical steroids are applied repeatedly to the face. He recommended a proactive approach. By applying TCIs to areas where skin lesions frequently recur, such as the eyelids, flares can often be prevented.
Repeated applications of TCIs “is perfectly safe and effective, and there are many studies that show proactive treatment is very effective and can prevent you from having to use too much topical steroids” or move to a systemic steroid, Dr. Simpson said.
These steps have been highly effective for sustained control even in challenging cases of AD, but he emphasized the importance of explaining the rationale to parents and eliciting their adherence to these treatment steps. Writing out the instructions will reduce confusion and help parents keep their children clear, he added.
Lawrence F. Eichenfield, MD, professor of pediatrics and dermatology at the University of California, San Diego, agreed that this recently published crossover trial has been helpful in counseling parents about how to manage AD in their children.
“Many times, pediatricians tell parents to avoid bathing because they feel that bathing will dry out the skin,” he said. The crossover study, by showing better control of AD with frequent bathing, dispels that notion, although he is not convinced that bathing at this frequency is necessary.
“I have not advised anyone to do twice-daily bathing, with rare exceptions, on the basis on this study, but, basically, I think that whether people do daily bathing or every other day bathing, it is pretty reasonable that bathing might help as long as they are applying moisturizer immediately afterward,” he said.
Dr. Simpson reports financial relationships with AbbVie, Celgene Dermira, Genentech, GlaxoSmithKline, Incyte, Lilly, Medimmune, Pfizer, Regeneron/Sanofi, and Tioga.
This publication and Global Academy for Medical Education are owned by the same parent company.
The most effective initial step for clearing atopic dermatitis in infants and young children involves daily bathing, followed by immediate application of a moisturizer, topical steroid, or both, according to an expert speaking at the virtual annual Coastal Dermatology Symposium.
“If they are really severe, you can do it twice-daily, but there are several studies that show there is not a huge benefit of twice-daily over once-daily,” said Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland.
He called this technique “soak-and-smear.” The “smear” is performed immediately after the bath when the skin is still damp, he said. When clearing is the goal, and the child has moderate to severe atopic dermatitis (AD), 0.1% triamcinolone or a similar medium potency topical steroid can be applied, and after clearing, the steroid can be switched for a moisturizer, according to Dr. Simpson.
Rather than restricting application to areas of greatest skin involvement, “put it all over,” he advised.
The clearing regimen should be continued “for a couple of more days” after the lesions have resolved, with a return visit in about a week to confirm clearing and reinforce the next steps for keeping patients clear, he added.
The next steps depend on severity. According to Dr. Simpson, severity is defined less by the extent of skin involvement at the baseline examination than the speed at which symptoms return.
For those with only mild symptoms after an extended period of clearing, moisturizer might be sufficient to prevent a significant relapse. For children with a more rapid relapse, it will be necessary to reintroduce topical steroid either every day, every other day, or twice per week.
Whether with moisturizer or with topical steroids, the soak-and-smear technique has now been validated in a recently published crossover randomized trial.
In the trial, children aged 6 months to 11 years, with moderate to severe AD, were randomized to a twice-daily bath, called the “wet method,” versus a twice-weekly bath, called the “dry method.” Both groups received a cleanser and moisturizer along with a low-potency topical steroid as needed.
After 2 weeks, the 40 evaluable patients were crossed over to the opposite bathing technique. The wet, or soak-and-smear approach, was associated with a highly significant reduction in the primary endpoint of SCORing Atopic Dermatitis (SCORAD) index, compared with the dry method (95% confidence interval, 14.9-27.6; P less than .0001). In a secondary analysis, this translated into a 30% relative reduction in favor of the wet method.
In addition, there was improvement in a caregiver assessment of the Atopic Dermatitis Quickscore (ADQ). These data show that “twice-daily baths with topical steroids and moisturizer can help in more moderate to severe population,” said Dr. Simpson, who noted that he has participated in open-label studies with the same soak-and-smear technique that have produced similar results.
Once children are clear, Dr. Simpson recommends a maintenance strategy individualized for severity. In many cases, this will involve moisturizers applied after the bath, supplemented intermittently, such as once or twice per week, with topical steroids. However, if parents find themselves resorting to daily steroids to maintain control, “that’s when you incorporate the TCIs [topical calcineurin inhibitors].”
TCIs “can help you stay at twice-per-week topical steroids,” Dr. Simpson said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
TCIs also help patients avoid steroid withdrawal, a particularly common phenomenon when topical steroids are applied repeatedly to the face. He recommended a proactive approach. By applying TCIs to areas where skin lesions frequently recur, such as the eyelids, flares can often be prevented.
Repeated applications of TCIs “is perfectly safe and effective, and there are many studies that show proactive treatment is very effective and can prevent you from having to use too much topical steroids” or move to a systemic steroid, Dr. Simpson said.
These steps have been highly effective for sustained control even in challenging cases of AD, but he emphasized the importance of explaining the rationale to parents and eliciting their adherence to these treatment steps. Writing out the instructions will reduce confusion and help parents keep their children clear, he added.
Lawrence F. Eichenfield, MD, professor of pediatrics and dermatology at the University of California, San Diego, agreed that this recently published crossover trial has been helpful in counseling parents about how to manage AD in their children.
“Many times, pediatricians tell parents to avoid bathing because they feel that bathing will dry out the skin,” he said. The crossover study, by showing better control of AD with frequent bathing, dispels that notion, although he is not convinced that bathing at this frequency is necessary.
“I have not advised anyone to do twice-daily bathing, with rare exceptions, on the basis on this study, but, basically, I think that whether people do daily bathing or every other day bathing, it is pretty reasonable that bathing might help as long as they are applying moisturizer immediately afterward,” he said.
Dr. Simpson reports financial relationships with AbbVie, Celgene Dermira, Genentech, GlaxoSmithKline, Incyte, Lilly, Medimmune, Pfizer, Regeneron/Sanofi, and Tioga.
This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Twelve medical groups pen letter opposing UHC copay accumulator program
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
ACR leads outcry against the insurer’s proposed move
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Birch bark derivative gel found effective for EB, in phase 3 study
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Hand eczema: Pan-JAK inhibitor delgocitinib shows dose-dependent response in phase 2b trial
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE EADV CONGRESS
No lab monitoring needed in adolescents on dupilumab
, Michael J. Cork, MBBS, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These reassuring results from the ongoing LIBERTY AD PED-OLE study confirm that, as previously established in adults, no blood monitoring is required in adolescents on the monoclonal antibody, which inhibits signaling of interleukins-4 and -13, said Dr. Cork, professor of dermatology and head of Sheffield Dermatology Research at the University of Sheffield (England).
“The practical importance of this finding is that there are no other systemic drugs available that don’t require blood samples. Cyclosporine, methotrexate, and the others used for atopic dermatitis require a lot of blood monitoring, and they’re off-license anyway for use in children and adolescents,” he said in an interview.
Many pediatric patients are afraid of needles and have an intense dislike of blood draws. And in a pandemic, no one wants to come into the office for blood draws if they don’t need to.
“Blood draws are very different from the injection for dupilumab. Taking a blood sample is much more painful for children. The needle in the autoinjector is really, really tiny; you can hardly feel it, and with the autoinjector you can’t even see it,” noted Dr. Cork, who is both a pediatric and adult dermatologist.
This report from the ongoing LIBERTY AD PED-OLE study included 105 patients aged 12-17 years who completed 52 weeks on dupilumab (Dupixent) with assessments of hematologic and serum chemistry parameters at baseline and weeks 16 and 52.
“The results were anticipated, but we want to know the drug is safe in every age group. The immune system is different in different age groups, so we have to be really careful,” Dr. Cork said.
The clinical side-effect profile was the same as in adults, consisting mainly of mild conjunctivitis and injection-site reactions. It’s a much less problematic side effect picture than with the older drugs.
“We’re finding the conjunctivitis to be slightly less severe than in adults, maybe because we’ve learned from the first trials in adults and from clinical experience to use prophylactic therapy. There would be no child going on dupilumab now – and no adult – that I wouldn’t put on prophylactic eye drops with replacement tears. I start them 2 weeks before I start dupilumab,” the dermatologist explained.
He and others with extensive experience using the biologic agent also work closely with an ophthalmologist.
“If we see an eye problem before going on dupilumab we get an assessment and then ophthalmologic monitoring during treatment,” Dr. Cork said.
As a dermatologist specializing in atopic dermatitis, he confessed to feeling deprived over the years as he watched the multitude of targeted biologic agents being developed for psoriasis. When he became involved in the first pediatric clinical trials of dupilumab, he had a realization: “It’s a miraculous treatment.”
“The first child I put on dupilumab spent 70 days in the hospital for IV antibiotics in the prior year. Seventy days! He almost died from MRSA septicemia. His serum IgE was 155,000 kU/L. And his IgE just went down and down and down as the dupilumab took effect. It was just incredible,” he recalled.
Dr. Cork reported receiving research funding from and serving as a consultant to Sanofi and Regeneron, which fund the LIBERTY AD PED-OLE study, as well as numerous other pharmaceutical companies.
SOURCE: Cork MJ. EADV 2020, Abstract 1772.
, Michael J. Cork, MBBS, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These reassuring results from the ongoing LIBERTY AD PED-OLE study confirm that, as previously established in adults, no blood monitoring is required in adolescents on the monoclonal antibody, which inhibits signaling of interleukins-4 and -13, said Dr. Cork, professor of dermatology and head of Sheffield Dermatology Research at the University of Sheffield (England).
“The practical importance of this finding is that there are no other systemic drugs available that don’t require blood samples. Cyclosporine, methotrexate, and the others used for atopic dermatitis require a lot of blood monitoring, and they’re off-license anyway for use in children and adolescents,” he said in an interview.
Many pediatric patients are afraid of needles and have an intense dislike of blood draws. And in a pandemic, no one wants to come into the office for blood draws if they don’t need to.
“Blood draws are very different from the injection for dupilumab. Taking a blood sample is much more painful for children. The needle in the autoinjector is really, really tiny; you can hardly feel it, and with the autoinjector you can’t even see it,” noted Dr. Cork, who is both a pediatric and adult dermatologist.
This report from the ongoing LIBERTY AD PED-OLE study included 105 patients aged 12-17 years who completed 52 weeks on dupilumab (Dupixent) with assessments of hematologic and serum chemistry parameters at baseline and weeks 16 and 52.
“The results were anticipated, but we want to know the drug is safe in every age group. The immune system is different in different age groups, so we have to be really careful,” Dr. Cork said.
The clinical side-effect profile was the same as in adults, consisting mainly of mild conjunctivitis and injection-site reactions. It’s a much less problematic side effect picture than with the older drugs.
“We’re finding the conjunctivitis to be slightly less severe than in adults, maybe because we’ve learned from the first trials in adults and from clinical experience to use prophylactic therapy. There would be no child going on dupilumab now – and no adult – that I wouldn’t put on prophylactic eye drops with replacement tears. I start them 2 weeks before I start dupilumab,” the dermatologist explained.
He and others with extensive experience using the biologic agent also work closely with an ophthalmologist.
“If we see an eye problem before going on dupilumab we get an assessment and then ophthalmologic monitoring during treatment,” Dr. Cork said.
As a dermatologist specializing in atopic dermatitis, he confessed to feeling deprived over the years as he watched the multitude of targeted biologic agents being developed for psoriasis. When he became involved in the first pediatric clinical trials of dupilumab, he had a realization: “It’s a miraculous treatment.”
“The first child I put on dupilumab spent 70 days in the hospital for IV antibiotics in the prior year. Seventy days! He almost died from MRSA septicemia. His serum IgE was 155,000 kU/L. And his IgE just went down and down and down as the dupilumab took effect. It was just incredible,” he recalled.
Dr. Cork reported receiving research funding from and serving as a consultant to Sanofi and Regeneron, which fund the LIBERTY AD PED-OLE study, as well as numerous other pharmaceutical companies.
SOURCE: Cork MJ. EADV 2020, Abstract 1772.
, Michael J. Cork, MBBS, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These reassuring results from the ongoing LIBERTY AD PED-OLE study confirm that, as previously established in adults, no blood monitoring is required in adolescents on the monoclonal antibody, which inhibits signaling of interleukins-4 and -13, said Dr. Cork, professor of dermatology and head of Sheffield Dermatology Research at the University of Sheffield (England).
“The practical importance of this finding is that there are no other systemic drugs available that don’t require blood samples. Cyclosporine, methotrexate, and the others used for atopic dermatitis require a lot of blood monitoring, and they’re off-license anyway for use in children and adolescents,” he said in an interview.
Many pediatric patients are afraid of needles and have an intense dislike of blood draws. And in a pandemic, no one wants to come into the office for blood draws if they don’t need to.
“Blood draws are very different from the injection for dupilumab. Taking a blood sample is much more painful for children. The needle in the autoinjector is really, really tiny; you can hardly feel it, and with the autoinjector you can’t even see it,” noted Dr. Cork, who is both a pediatric and adult dermatologist.
This report from the ongoing LIBERTY AD PED-OLE study included 105 patients aged 12-17 years who completed 52 weeks on dupilumab (Dupixent) with assessments of hematologic and serum chemistry parameters at baseline and weeks 16 and 52.
“The results were anticipated, but we want to know the drug is safe in every age group. The immune system is different in different age groups, so we have to be really careful,” Dr. Cork said.
The clinical side-effect profile was the same as in adults, consisting mainly of mild conjunctivitis and injection-site reactions. It’s a much less problematic side effect picture than with the older drugs.
“We’re finding the conjunctivitis to be slightly less severe than in adults, maybe because we’ve learned from the first trials in adults and from clinical experience to use prophylactic therapy. There would be no child going on dupilumab now – and no adult – that I wouldn’t put on prophylactic eye drops with replacement tears. I start them 2 weeks before I start dupilumab,” the dermatologist explained.
He and others with extensive experience using the biologic agent also work closely with an ophthalmologist.
“If we see an eye problem before going on dupilumab we get an assessment and then ophthalmologic monitoring during treatment,” Dr. Cork said.
As a dermatologist specializing in atopic dermatitis, he confessed to feeling deprived over the years as he watched the multitude of targeted biologic agents being developed for psoriasis. When he became involved in the first pediatric clinical trials of dupilumab, he had a realization: “It’s a miraculous treatment.”
“The first child I put on dupilumab spent 70 days in the hospital for IV antibiotics in the prior year. Seventy days! He almost died from MRSA septicemia. His serum IgE was 155,000 kU/L. And his IgE just went down and down and down as the dupilumab took effect. It was just incredible,” he recalled.
Dr. Cork reported receiving research funding from and serving as a consultant to Sanofi and Regeneron, which fund the LIBERTY AD PED-OLE study, as well as numerous other pharmaceutical companies.
SOURCE: Cork MJ. EADV 2020, Abstract 1772.
FROM THE EADV CONGRESS