User login
OTC topical ivermectin lotion earns FDA approval for head lice
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
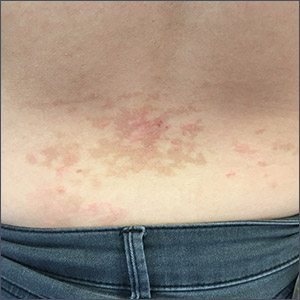
Pruritic rash on flank, back, and chest
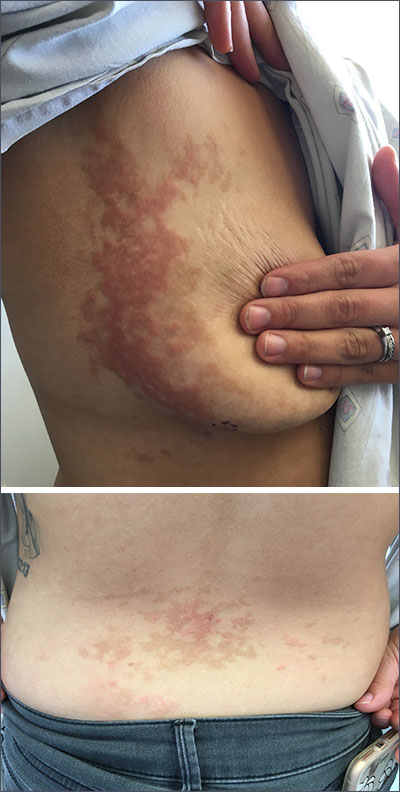
The patient was given a diagnosis of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30. It more commonly is seen in people of Asian descent.
While the pathophysiology remains unknown, the rash most commonly occurs in association with diabetes, ketosis, and more recently with ketogenic diets. Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal.
Other conditions can mimic prurigo pigmentosa such as urticaria, irritant contact dermatitis, confluent and reticulated papillomatosis, and pityriasis rosea.
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade. If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties. Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.
This patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
This case was adapted from: Croom D, Barlow T, Landers JT. Pruritic rash on chest and back. J Fam Pract. 2019;68:113-114,116

The patient was given a diagnosis of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30. It more commonly is seen in people of Asian descent.
While the pathophysiology remains unknown, the rash most commonly occurs in association with diabetes, ketosis, and more recently with ketogenic diets. Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal.
Other conditions can mimic prurigo pigmentosa such as urticaria, irritant contact dermatitis, confluent and reticulated papillomatosis, and pityriasis rosea.
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade. If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties. Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.
This patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
This case was adapted from: Croom D, Barlow T, Landers JT. Pruritic rash on chest and back. J Fam Pract. 2019;68:113-114,116

The patient was given a diagnosis of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30. It more commonly is seen in people of Asian descent.
While the pathophysiology remains unknown, the rash most commonly occurs in association with diabetes, ketosis, and more recently with ketogenic diets. Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal.
Other conditions can mimic prurigo pigmentosa such as urticaria, irritant contact dermatitis, confluent and reticulated papillomatosis, and pityriasis rosea.
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade. If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties. Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.
This patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
This case was adapted from: Croom D, Barlow T, Landers JT. Pruritic rash on chest and back. J Fam Pract. 2019;68:113-114,116
How to assess erythema in children with skin of color
When assessing inflammatory dermatoses in children with skin of color, it may be necessary to train the eye to recognize subtle changes and colors other than red, a doctor suggested at the virtual American Academy of Pediatrics annual meeting.
First, doctors should see whether they can detect any erythema, said Latanya T. Benjamin, MD, associate professor of pediatric dermatology at Florida Atlantic University, Boca Raton. “If the answer is no because of the background competing chromophore, then shift your focus off of the erythema and perhaps onto other colors that the skin can demonstrate,” such as red-brown, violaceous, or grayish hues.
Comparing involved areas with normal skin also may help. “Sometimes you can pick up subtleties in colors that way,” Dr. Benjamin said.
Finally, look for other changes that could relate to the patient’s condition. For example, when diagnosing acne, Dr. Benjamin looks for pigmentary sequelae like hyperpigmentation. “If a patient has atopic dermatitis, is there hypopigmentation on other areas of the face?”
Consider cutaneous T-cell lymphoma in the differential diagnosis of generalized hypopigmented patches and plaques in patients with darker skin types, Dr. Benjamin noted. Other diagnoses that may result in hypopigmentation include pityriasis alba, vitiligo, tinea versicolor, ash-leaf macules, Hansen’s disease, postinflammatory hypopigmentation secondary to atopic dermatitis, and tinea corporis.
Be sensitive to the fact that changes in skin color can be “very annoying or devastating to the family,” even with medically benign conditions such as pityriasis alba, Dr. Benjamin added.
Detecting redness in brown skin tones can take practice, Candrice R. Heath, MD, a member of the board of directors for the Skin of Color Society, commented in an interview.
Furthermore, presentations vary. For instance, depictions of atopic dermatitis in educational materials may focus on red patches and plaques but “miss that there are several presentations in those with darker skin tones, including follicular prominence, hyperpigmented plaques, and coin-shaped lesions,” said Dr. Heath, assistant professor of dermatology at Temple University, Philadelphia.
“The skin of color population is growing,” noted Dr. Heath. “By 2023, there will be more children with skin of color than without in the United States.”
While Dr. Heath has lectured about skin of color as it relates to pediatric patients for years, “now with the nation’s renewed interest in disparities in health care, it is the perfect time to highlight conditions that present more commonly in skin of color and present differently in those with skin of color.”
Dr. Benjamin had no conflicts of interest. Dr. Heath serves as associate editor of Cutis, which is owned by the same company as this publication.
When assessing inflammatory dermatoses in children with skin of color, it may be necessary to train the eye to recognize subtle changes and colors other than red, a doctor suggested at the virtual American Academy of Pediatrics annual meeting.
First, doctors should see whether they can detect any erythema, said Latanya T. Benjamin, MD, associate professor of pediatric dermatology at Florida Atlantic University, Boca Raton. “If the answer is no because of the background competing chromophore, then shift your focus off of the erythema and perhaps onto other colors that the skin can demonstrate,” such as red-brown, violaceous, or grayish hues.
Comparing involved areas with normal skin also may help. “Sometimes you can pick up subtleties in colors that way,” Dr. Benjamin said.
Finally, look for other changes that could relate to the patient’s condition. For example, when diagnosing acne, Dr. Benjamin looks for pigmentary sequelae like hyperpigmentation. “If a patient has atopic dermatitis, is there hypopigmentation on other areas of the face?”
Consider cutaneous T-cell lymphoma in the differential diagnosis of generalized hypopigmented patches and plaques in patients with darker skin types, Dr. Benjamin noted. Other diagnoses that may result in hypopigmentation include pityriasis alba, vitiligo, tinea versicolor, ash-leaf macules, Hansen’s disease, postinflammatory hypopigmentation secondary to atopic dermatitis, and tinea corporis.
Be sensitive to the fact that changes in skin color can be “very annoying or devastating to the family,” even with medically benign conditions such as pityriasis alba, Dr. Benjamin added.
Detecting redness in brown skin tones can take practice, Candrice R. Heath, MD, a member of the board of directors for the Skin of Color Society, commented in an interview.
Furthermore, presentations vary. For instance, depictions of atopic dermatitis in educational materials may focus on red patches and plaques but “miss that there are several presentations in those with darker skin tones, including follicular prominence, hyperpigmented plaques, and coin-shaped lesions,” said Dr. Heath, assistant professor of dermatology at Temple University, Philadelphia.
“The skin of color population is growing,” noted Dr. Heath. “By 2023, there will be more children with skin of color than without in the United States.”
While Dr. Heath has lectured about skin of color as it relates to pediatric patients for years, “now with the nation’s renewed interest in disparities in health care, it is the perfect time to highlight conditions that present more commonly in skin of color and present differently in those with skin of color.”
Dr. Benjamin had no conflicts of interest. Dr. Heath serves as associate editor of Cutis, which is owned by the same company as this publication.
When assessing inflammatory dermatoses in children with skin of color, it may be necessary to train the eye to recognize subtle changes and colors other than red, a doctor suggested at the virtual American Academy of Pediatrics annual meeting.
First, doctors should see whether they can detect any erythema, said Latanya T. Benjamin, MD, associate professor of pediatric dermatology at Florida Atlantic University, Boca Raton. “If the answer is no because of the background competing chromophore, then shift your focus off of the erythema and perhaps onto other colors that the skin can demonstrate,” such as red-brown, violaceous, or grayish hues.
Comparing involved areas with normal skin also may help. “Sometimes you can pick up subtleties in colors that way,” Dr. Benjamin said.
Finally, look for other changes that could relate to the patient’s condition. For example, when diagnosing acne, Dr. Benjamin looks for pigmentary sequelae like hyperpigmentation. “If a patient has atopic dermatitis, is there hypopigmentation on other areas of the face?”
Consider cutaneous T-cell lymphoma in the differential diagnosis of generalized hypopigmented patches and plaques in patients with darker skin types, Dr. Benjamin noted. Other diagnoses that may result in hypopigmentation include pityriasis alba, vitiligo, tinea versicolor, ash-leaf macules, Hansen’s disease, postinflammatory hypopigmentation secondary to atopic dermatitis, and tinea corporis.
Be sensitive to the fact that changes in skin color can be “very annoying or devastating to the family,” even with medically benign conditions such as pityriasis alba, Dr. Benjamin added.
Detecting redness in brown skin tones can take practice, Candrice R. Heath, MD, a member of the board of directors for the Skin of Color Society, commented in an interview.
Furthermore, presentations vary. For instance, depictions of atopic dermatitis in educational materials may focus on red patches and plaques but “miss that there are several presentations in those with darker skin tones, including follicular prominence, hyperpigmented plaques, and coin-shaped lesions,” said Dr. Heath, assistant professor of dermatology at Temple University, Philadelphia.
“The skin of color population is growing,” noted Dr. Heath. “By 2023, there will be more children with skin of color than without in the United States.”
While Dr. Heath has lectured about skin of color as it relates to pediatric patients for years, “now with the nation’s renewed interest in disparities in health care, it is the perfect time to highlight conditions that present more commonly in skin of color and present differently in those with skin of color.”
Dr. Benjamin had no conflicts of interest. Dr. Heath serves as associate editor of Cutis, which is owned by the same company as this publication.
FROM AAP 2020
Red hair in women linked to elevated CRP levels in Nurses’ Health Study
Red-haired women were significantly more likely than were women with nonred hair to have elevated levels of C-reactive protein that may increase risk for cardiovascular conditions, according to data from nearly 9,000 women participating in the Nurses’ Health Study.
“Positive associations between red hair and cardiovascular disease and cancer in women, but not men, have been reported,” wrote Rebecca I. Hartman, MD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
In a study published in the Journal of Investigative Dermatology, they reviewed data from the Nurses’ Health Study, a 1976 cohort study of 121,700 women registered nurses in the United States. They analyzed blood specimens from 8,994 women that were collected between 1989 and 1990. Participants’ natural hair color was determined by asking them their natural hair color at age 21 years, with choices of red, blonde, light brown, dark brown, or black. Overall, dark brown/black hair was the most common color (45%) and 390 of the women (4.3%) had red hair.
The average CRP levels were significantly higher for women with red hair (3.7 mg/L), compared with those with blonde (3.3 mg/L), light brown (3.0 mg/mL), or dark brown/black (3.2 mg/L).
Using the CRP levels for red-haired women as a reference, women with blond, light brown, and dark brown/black hair averaged significantly lower CRP levels than those of red-haired women in an age-adjusted model (–15.2%, –18/1%, and –14.2%, respectively) and in a multivariate analysis (–12.7%, –14.1%, and –10.9%, respectively).
Non-red-haired women had significantly lower odds of high CRP levels compared with red-haired women, with odds ratios of 0.62, 0.60, and 0.67 for women with blonde, light brown, and dark brown/black hair, respectively, in multivariate analysis, the researchers found.
The study was limited by several factors including the use of self-reports for hair color and the relative homogeneity of the Nurses’ Health Study, which has a population of mostly white, female health professionals, the researchers noted.
However, the findings of significantly increased CRP levels “could potentially explain a prior report of increased risks of cardiovascular disease and cancer in red-haired women,” they said. “Although, we observed similar associations in the NHS between red hair and cardiovascular disease and cancer, they were not statistically significant,” they added.
Additional studies are needed to validate and examine the clinical significance of the results, they concluded.
“Elevated CRP levels, a marker of inflammation, have been associated with increased risk for several diseases, including colon cancer and heart disease,” lead author Dr. Hartman said in an interview. “Another study suggested red-haired women have elevated risks of cardiovascular disease and cancer. We wanted to see if different levels of inflammation in red-haired women could possibly explain these findings.”
She said she was not surprised by the findings, “as they were in line with our hypothesis.” In addition, “animal studies suggest that the gene most responsible for red hair, MC1R, may be linked to inflammation,” she said.
While red-haired women were found to have higher CRP levels in the study, “the underlying mechanism and clinical significance remain unknown,” and more research is needed, Dr. Hartman emphasized. “First, our findings need to be validated in women and also examined in men. If our findings are validated, future studies should examine the mechanism of CRP elevation in red-haired women, and whether these women have elevated risks of colon cancer and heart disease,” she said.
“If red-haired women do have increased levels of inflammation, and as a result have elevated risks of colon cancer and heart disease, then future interventions can focus on enhanced screening and possibly chemoprevention in this population,” she added.
The study was supported by the National Institutes of Health. Lead author Dr. Hartman was supported by an American Skin Association Research Grant.
SOURCE: Hartman RI et al. J Invest Dermatol. 2020 Oct 12. doi: 10.1016/j.jid.2020.09.015.
Red-haired women were significantly more likely than were women with nonred hair to have elevated levels of C-reactive protein that may increase risk for cardiovascular conditions, according to data from nearly 9,000 women participating in the Nurses’ Health Study.
“Positive associations between red hair and cardiovascular disease and cancer in women, but not men, have been reported,” wrote Rebecca I. Hartman, MD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
In a study published in the Journal of Investigative Dermatology, they reviewed data from the Nurses’ Health Study, a 1976 cohort study of 121,700 women registered nurses in the United States. They analyzed blood specimens from 8,994 women that were collected between 1989 and 1990. Participants’ natural hair color was determined by asking them their natural hair color at age 21 years, with choices of red, blonde, light brown, dark brown, or black. Overall, dark brown/black hair was the most common color (45%) and 390 of the women (4.3%) had red hair.
The average CRP levels were significantly higher for women with red hair (3.7 mg/L), compared with those with blonde (3.3 mg/L), light brown (3.0 mg/mL), or dark brown/black (3.2 mg/L).
Using the CRP levels for red-haired women as a reference, women with blond, light brown, and dark brown/black hair averaged significantly lower CRP levels than those of red-haired women in an age-adjusted model (–15.2%, –18/1%, and –14.2%, respectively) and in a multivariate analysis (–12.7%, –14.1%, and –10.9%, respectively).
Non-red-haired women had significantly lower odds of high CRP levels compared with red-haired women, with odds ratios of 0.62, 0.60, and 0.67 for women with blonde, light brown, and dark brown/black hair, respectively, in multivariate analysis, the researchers found.
The study was limited by several factors including the use of self-reports for hair color and the relative homogeneity of the Nurses’ Health Study, which has a population of mostly white, female health professionals, the researchers noted.
However, the findings of significantly increased CRP levels “could potentially explain a prior report of increased risks of cardiovascular disease and cancer in red-haired women,” they said. “Although, we observed similar associations in the NHS between red hair and cardiovascular disease and cancer, they were not statistically significant,” they added.
Additional studies are needed to validate and examine the clinical significance of the results, they concluded.
“Elevated CRP levels, a marker of inflammation, have been associated with increased risk for several diseases, including colon cancer and heart disease,” lead author Dr. Hartman said in an interview. “Another study suggested red-haired women have elevated risks of cardiovascular disease and cancer. We wanted to see if different levels of inflammation in red-haired women could possibly explain these findings.”
She said she was not surprised by the findings, “as they were in line with our hypothesis.” In addition, “animal studies suggest that the gene most responsible for red hair, MC1R, may be linked to inflammation,” she said.
While red-haired women were found to have higher CRP levels in the study, “the underlying mechanism and clinical significance remain unknown,” and more research is needed, Dr. Hartman emphasized. “First, our findings need to be validated in women and also examined in men. If our findings are validated, future studies should examine the mechanism of CRP elevation in red-haired women, and whether these women have elevated risks of colon cancer and heart disease,” she said.
“If red-haired women do have increased levels of inflammation, and as a result have elevated risks of colon cancer and heart disease, then future interventions can focus on enhanced screening and possibly chemoprevention in this population,” she added.
The study was supported by the National Institutes of Health. Lead author Dr. Hartman was supported by an American Skin Association Research Grant.
SOURCE: Hartman RI et al. J Invest Dermatol. 2020 Oct 12. doi: 10.1016/j.jid.2020.09.015.
Red-haired women were significantly more likely than were women with nonred hair to have elevated levels of C-reactive protein that may increase risk for cardiovascular conditions, according to data from nearly 9,000 women participating in the Nurses’ Health Study.
“Positive associations between red hair and cardiovascular disease and cancer in women, but not men, have been reported,” wrote Rebecca I. Hartman, MD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
In a study published in the Journal of Investigative Dermatology, they reviewed data from the Nurses’ Health Study, a 1976 cohort study of 121,700 women registered nurses in the United States. They analyzed blood specimens from 8,994 women that were collected between 1989 and 1990. Participants’ natural hair color was determined by asking them their natural hair color at age 21 years, with choices of red, blonde, light brown, dark brown, or black. Overall, dark brown/black hair was the most common color (45%) and 390 of the women (4.3%) had red hair.
The average CRP levels were significantly higher for women with red hair (3.7 mg/L), compared with those with blonde (3.3 mg/L), light brown (3.0 mg/mL), or dark brown/black (3.2 mg/L).
Using the CRP levels for red-haired women as a reference, women with blond, light brown, and dark brown/black hair averaged significantly lower CRP levels than those of red-haired women in an age-adjusted model (–15.2%, –18/1%, and –14.2%, respectively) and in a multivariate analysis (–12.7%, –14.1%, and –10.9%, respectively).
Non-red-haired women had significantly lower odds of high CRP levels compared with red-haired women, with odds ratios of 0.62, 0.60, and 0.67 for women with blonde, light brown, and dark brown/black hair, respectively, in multivariate analysis, the researchers found.
The study was limited by several factors including the use of self-reports for hair color and the relative homogeneity of the Nurses’ Health Study, which has a population of mostly white, female health professionals, the researchers noted.
However, the findings of significantly increased CRP levels “could potentially explain a prior report of increased risks of cardiovascular disease and cancer in red-haired women,” they said. “Although, we observed similar associations in the NHS between red hair and cardiovascular disease and cancer, they were not statistically significant,” they added.
Additional studies are needed to validate and examine the clinical significance of the results, they concluded.
“Elevated CRP levels, a marker of inflammation, have been associated with increased risk for several diseases, including colon cancer and heart disease,” lead author Dr. Hartman said in an interview. “Another study suggested red-haired women have elevated risks of cardiovascular disease and cancer. We wanted to see if different levels of inflammation in red-haired women could possibly explain these findings.”
She said she was not surprised by the findings, “as they were in line with our hypothesis.” In addition, “animal studies suggest that the gene most responsible for red hair, MC1R, may be linked to inflammation,” she said.
While red-haired women were found to have higher CRP levels in the study, “the underlying mechanism and clinical significance remain unknown,” and more research is needed, Dr. Hartman emphasized. “First, our findings need to be validated in women and also examined in men. If our findings are validated, future studies should examine the mechanism of CRP elevation in red-haired women, and whether these women have elevated risks of colon cancer and heart disease,” she said.
“If red-haired women do have increased levels of inflammation, and as a result have elevated risks of colon cancer and heart disease, then future interventions can focus on enhanced screening and possibly chemoprevention in this population,” she added.
The study was supported by the National Institutes of Health. Lead author Dr. Hartman was supported by an American Skin Association Research Grant.
SOURCE: Hartman RI et al. J Invest Dermatol. 2020 Oct 12. doi: 10.1016/j.jid.2020.09.015.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
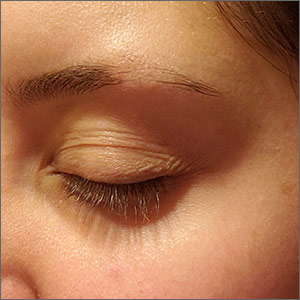
Eyebrow hair loss
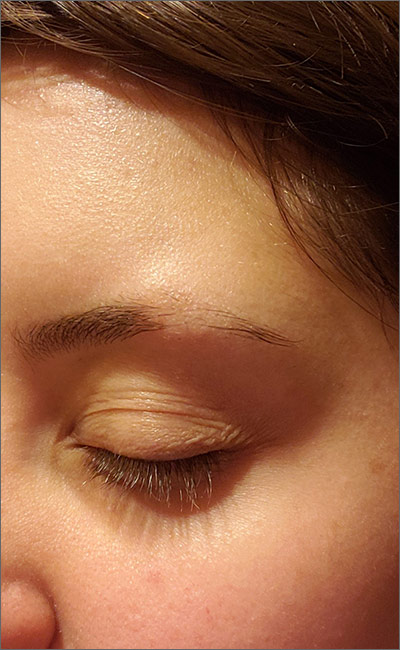
Although it appeared that the hair loss was preceded by some dry skin, the patch of hair loss was smooth and nonscarring, consistent with a diagnosis of alopecia areata (AA).
AA typically is found on the scalp in solitary areas but can affect the whole body, including the eyelashes and eyebrows. Affected hair typically is narrower at the proximal end, resembling an exclamation point, as the hair fails to grow and falls out. Sometimes, patches of alopecia may coalesce into a larger area. Nail changes may be noted, as well. Nails may become brittle, with pitting and/or longitudinal ridges. Patients are usually asymptomatic but may complain of pruritus.
AA is believed to be an autoimmune disorder. It affects males and females of all ages but is more common in children and young adults. AA is believed to result from a T-cell–mediated immune response that transitions the hair follicles from the growth phase to the resting phase. This leads to sudden hair loss and inhibition of regrowth of the hair. However, the hair follicle is not permanently destroyed as in other processes of alopecia. There is also an association between AA and other autoimmune disorders such as vitiligo, thyroid disease, and lupus.
The diagnosis usually is made clinically, as in this patient, but a definitive diagnosis can be made by biopsy and pathology. Other differential diagnoses to consider are trichotillomania, tinea, traumatic alopecia, and lupus.
In almost half of cases, AA is self-resolving; therefore, in first episodes of localized disease, watchful waiting is appropriate. Treatment is tailored to the individual patient and may be difficult. Intralesional corticosteroids often are used for mild cases. Typically, 10 mg/mL of a glucocorticoid is injected into the mid-dermis every 4 to 6 weeks; however, this treatment carries a risk of transient or even permanent atrophy of the injection site. Potent topical corticosteroids often are used, especially in children who do not tolerate intralesional injections, and success can be variable. Other topical treatments to consider are photochemotherapy (psoralen plus UVA), or an irritant agent such as anthralin and a vasodilator such as minoxidil. Regrowth can be expected in a few months to a year, but recurrence is common.
Image courtesy of Stacy Nguy, MD, and text courtesy of Stacy Nguy, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Papadopoulos AJ, Schwartz RA, Janniger C. Alopecia areata: pathogenesis, diagnosis, and therapy. Am J Clin Dermatol. 2000;1:101-105

Although it appeared that the hair loss was preceded by some dry skin, the patch of hair loss was smooth and nonscarring, consistent with a diagnosis of alopecia areata (AA).
AA typically is found on the scalp in solitary areas but can affect the whole body, including the eyelashes and eyebrows. Affected hair typically is narrower at the proximal end, resembling an exclamation point, as the hair fails to grow and falls out. Sometimes, patches of alopecia may coalesce into a larger area. Nail changes may be noted, as well. Nails may become brittle, with pitting and/or longitudinal ridges. Patients are usually asymptomatic but may complain of pruritus.
AA is believed to be an autoimmune disorder. It affects males and females of all ages but is more common in children and young adults. AA is believed to result from a T-cell–mediated immune response that transitions the hair follicles from the growth phase to the resting phase. This leads to sudden hair loss and inhibition of regrowth of the hair. However, the hair follicle is not permanently destroyed as in other processes of alopecia. There is also an association between AA and other autoimmune disorders such as vitiligo, thyroid disease, and lupus.
The diagnosis usually is made clinically, as in this patient, but a definitive diagnosis can be made by biopsy and pathology. Other differential diagnoses to consider are trichotillomania, tinea, traumatic alopecia, and lupus.
In almost half of cases, AA is self-resolving; therefore, in first episodes of localized disease, watchful waiting is appropriate. Treatment is tailored to the individual patient and may be difficult. Intralesional corticosteroids often are used for mild cases. Typically, 10 mg/mL of a glucocorticoid is injected into the mid-dermis every 4 to 6 weeks; however, this treatment carries a risk of transient or even permanent atrophy of the injection site. Potent topical corticosteroids often are used, especially in children who do not tolerate intralesional injections, and success can be variable. Other topical treatments to consider are photochemotherapy (psoralen plus UVA), or an irritant agent such as anthralin and a vasodilator such as minoxidil. Regrowth can be expected in a few months to a year, but recurrence is common.
Image courtesy of Stacy Nguy, MD, and text courtesy of Stacy Nguy, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Although it appeared that the hair loss was preceded by some dry skin, the patch of hair loss was smooth and nonscarring, consistent with a diagnosis of alopecia areata (AA).
AA typically is found on the scalp in solitary areas but can affect the whole body, including the eyelashes and eyebrows. Affected hair typically is narrower at the proximal end, resembling an exclamation point, as the hair fails to grow and falls out. Sometimes, patches of alopecia may coalesce into a larger area. Nail changes may be noted, as well. Nails may become brittle, with pitting and/or longitudinal ridges. Patients are usually asymptomatic but may complain of pruritus.
AA is believed to be an autoimmune disorder. It affects males and females of all ages but is more common in children and young adults. AA is believed to result from a T-cell–mediated immune response that transitions the hair follicles from the growth phase to the resting phase. This leads to sudden hair loss and inhibition of regrowth of the hair. However, the hair follicle is not permanently destroyed as in other processes of alopecia. There is also an association between AA and other autoimmune disorders such as vitiligo, thyroid disease, and lupus.
The diagnosis usually is made clinically, as in this patient, but a definitive diagnosis can be made by biopsy and pathology. Other differential diagnoses to consider are trichotillomania, tinea, traumatic alopecia, and lupus.
In almost half of cases, AA is self-resolving; therefore, in first episodes of localized disease, watchful waiting is appropriate. Treatment is tailored to the individual patient and may be difficult. Intralesional corticosteroids often are used for mild cases. Typically, 10 mg/mL of a glucocorticoid is injected into the mid-dermis every 4 to 6 weeks; however, this treatment carries a risk of transient or even permanent atrophy of the injection site. Potent topical corticosteroids often are used, especially in children who do not tolerate intralesional injections, and success can be variable. Other topical treatments to consider are photochemotherapy (psoralen plus UVA), or an irritant agent such as anthralin and a vasodilator such as minoxidil. Regrowth can be expected in a few months to a year, but recurrence is common.
Image courtesy of Stacy Nguy, MD, and text courtesy of Stacy Nguy, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Papadopoulos AJ, Schwartz RA, Janniger C. Alopecia areata: pathogenesis, diagnosis, and therapy. Am J Clin Dermatol. 2000;1:101-105
Papadopoulos AJ, Schwartz RA, Janniger C. Alopecia areata: pathogenesis, diagnosis, and therapy. Am J Clin Dermatol. 2000;1:101-105
Systemic sclerosis patients share their perspectives and needs in treatment trials
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
FROM AN FDA PATIENT-FOCUSED DRUG DEVELOPMENT MEETING
Baricitinib reduces adult atopic dermatitis severity in phase 3 study
in the phase 3, double-blind, placebo-controlled, BREEZE-AD7 study.
The study enrolled patients with inadequate responses to topical corticosteroids, according to Kristian Reich, MD, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his coauthors.
First test of baricitinib plus topical steroids
Baricitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, inhibits several cytokines in AD pathogenesis, and in two monotherapy studies (BREEZE-AD1 and BREEZE-AD2), it was superior to placebo for reducing several AD clinical signs and symptoms. The current BREEZE-AD7 study is the first to test baricitinib plus background topical corticosteroid therapy, more closely mirroring clinical practice, the authors noted.
BREEZE-AD7 was conducted at 68 centers in 10 countries in Asia, Australia, Europe, and South America. It included 329 adults with moderate to severe AD (mean age around 34 years, and around 34% were female) with inadequate responses to topical corticosteroids documented within the last 6 months. They were randomized 1:1:1 to daily baricitinib 4 mg, daily baricitinib 2 mg, or placebo for 16 weeks. All patients received moderate- and/or low-potency topical corticosteroids (such as 0.1%triamcinolone cream and 2.5% hydrocortisone ointment, respectively) for active lesions.
Significant benefit at 4 mg
At week 16, 31% of AD patients receiving baricitinib 4 mg achieved Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 (clear) or 1 (almost clear) versus 15% in the placebo group (odds ratio, 2.8; 95% confidence interval, 1.4-5.6; P = .004). Among patients receiving baricitinib 2 mg, 24% achieved vI-GA-AD scores of 0 or 1 (OR, 1.9; 95% CI, 0.9-3.9; P = .08).
The same pattern of improving scores from placebo to baricitinib 2 mg to baricitinib 4 mg persisted, as reflected with secondary endpoints at week 16. Among patients receiving baricitinib 4 mg, 48% achieved Eczema Area Severity Index (EASI) 75 responses, versus 43% and 23% in 2 mg and placebo groups, respectively. Percent changes from baseline in total EASI score were –67%, –58%, –45% for baricitinib 4 mg, baricitinib 2 mg, and placebo, respectively; the proportion of patients achieving 4-point or greater improvements in Itch Numeric Rating Scale (NRS) was 44%, 38%, and 20% for baricitinib 4 mg, baricitinib 2 mg and placebo, respectively.
Similarly, mean change from baseline on the Skin Pain numeric rating scale was –3.7, –3.2, and –2.1 for baricitinib 4 mg, baricitinib 2 mg and placebo. Nighttime itch awakenings were also reduced in a similar progression from placebo to the higher baricitinib dose.
Adverse events dose related
Treatment-related adverse events were reported more frequently in the baricitinib groups (58% baricitinib 4 mg, 56% baricitinib 2 mg) versus placebo 38%. Nasopharyngitis was most common, followed by oral herpes, upper respiratory tract infection, acne, diarrhea, and back pain. Serious adverse event rates were similar across treatment groups. Permanent discontinuation rates were low at 5% for baricitinib 4 mg, 0% for baricitinib 2 mg, and 1% for placebo. The side-effect profile for baricitinib was consistent with prior studies, Dr. Reich and his coauthors reported.
The authors noted further, “data in this study suggest that patients with AD treated with baricitinib may be able to reduce the frequency and total quantity of concomitant TCSs [topical corticosteroids] used, thus mitigating concerns associated with continual or sustained application of topical treatments.”
“Overall, this study provides further evidence to support the efficacy and safety profile of baricitinib for the treatment of moderate-severe AD,” commented one of the authors, Jonathan I. Silverberg, MD, PhD, MPH, of the department of dermatology at George Washington University in Washington.
“In particular, this study shows that adding topical corticosteroids to baricitinib increases the rate of treatment success compared with the efficacy seen in baricitinib monotherapy studies. These data will be important to guide the use of baricitinib with topical corticosteroids in clinical practice. I think these data are also important because they show that baricitinib 4 mg may be more effective than 2 mg in some patients,” he said in an interview.
In late September, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended approval of oral baricitinib for adults with moderate to severe AD who are candidates for systemic therapy. Baricitinib is approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis. If approved in Europe, it will be the first JAK inhibitor and first oral medication indicated to treat patients with AD.
The study was funded by Eli Lilly and Company under license from Incyte Corporation. Dr. Reich reported receiving fees to the institution for participation in clinical trials from Eli Lilly and Company during the conduct of the study and personal fees for lectures. Dr. Silverberg reported receiving fees from Eli Lilly and Company during the conduct of the study, and fees from companies outside of this work. Other authors also reported disclosures related to Eli Lilly and other pharmaceutical companies, and several authors were Eli Lilly employees.
SOURCE: Reich K et al. JAMA Dermatol. 2020 Sep 30. doi: 10.1001/jamadermatol.2020.3260.
in the phase 3, double-blind, placebo-controlled, BREEZE-AD7 study.
The study enrolled patients with inadequate responses to topical corticosteroids, according to Kristian Reich, MD, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his coauthors.
First test of baricitinib plus topical steroids
Baricitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, inhibits several cytokines in AD pathogenesis, and in two monotherapy studies (BREEZE-AD1 and BREEZE-AD2), it was superior to placebo for reducing several AD clinical signs and symptoms. The current BREEZE-AD7 study is the first to test baricitinib plus background topical corticosteroid therapy, more closely mirroring clinical practice, the authors noted.
BREEZE-AD7 was conducted at 68 centers in 10 countries in Asia, Australia, Europe, and South America. It included 329 adults with moderate to severe AD (mean age around 34 years, and around 34% were female) with inadequate responses to topical corticosteroids documented within the last 6 months. They were randomized 1:1:1 to daily baricitinib 4 mg, daily baricitinib 2 mg, or placebo for 16 weeks. All patients received moderate- and/or low-potency topical corticosteroids (such as 0.1%triamcinolone cream and 2.5% hydrocortisone ointment, respectively) for active lesions.
Significant benefit at 4 mg
At week 16, 31% of AD patients receiving baricitinib 4 mg achieved Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 (clear) or 1 (almost clear) versus 15% in the placebo group (odds ratio, 2.8; 95% confidence interval, 1.4-5.6; P = .004). Among patients receiving baricitinib 2 mg, 24% achieved vI-GA-AD scores of 0 or 1 (OR, 1.9; 95% CI, 0.9-3.9; P = .08).
The same pattern of improving scores from placebo to baricitinib 2 mg to baricitinib 4 mg persisted, as reflected with secondary endpoints at week 16. Among patients receiving baricitinib 4 mg, 48% achieved Eczema Area Severity Index (EASI) 75 responses, versus 43% and 23% in 2 mg and placebo groups, respectively. Percent changes from baseline in total EASI score were –67%, –58%, –45% for baricitinib 4 mg, baricitinib 2 mg, and placebo, respectively; the proportion of patients achieving 4-point or greater improvements in Itch Numeric Rating Scale (NRS) was 44%, 38%, and 20% for baricitinib 4 mg, baricitinib 2 mg and placebo, respectively.
Similarly, mean change from baseline on the Skin Pain numeric rating scale was –3.7, –3.2, and –2.1 for baricitinib 4 mg, baricitinib 2 mg and placebo. Nighttime itch awakenings were also reduced in a similar progression from placebo to the higher baricitinib dose.
Adverse events dose related
Treatment-related adverse events were reported more frequently in the baricitinib groups (58% baricitinib 4 mg, 56% baricitinib 2 mg) versus placebo 38%. Nasopharyngitis was most common, followed by oral herpes, upper respiratory tract infection, acne, diarrhea, and back pain. Serious adverse event rates were similar across treatment groups. Permanent discontinuation rates were low at 5% for baricitinib 4 mg, 0% for baricitinib 2 mg, and 1% for placebo. The side-effect profile for baricitinib was consistent with prior studies, Dr. Reich and his coauthors reported.
The authors noted further, “data in this study suggest that patients with AD treated with baricitinib may be able to reduce the frequency and total quantity of concomitant TCSs [topical corticosteroids] used, thus mitigating concerns associated with continual or sustained application of topical treatments.”
“Overall, this study provides further evidence to support the efficacy and safety profile of baricitinib for the treatment of moderate-severe AD,” commented one of the authors, Jonathan I. Silverberg, MD, PhD, MPH, of the department of dermatology at George Washington University in Washington.
“In particular, this study shows that adding topical corticosteroids to baricitinib increases the rate of treatment success compared with the efficacy seen in baricitinib monotherapy studies. These data will be important to guide the use of baricitinib with topical corticosteroids in clinical practice. I think these data are also important because they show that baricitinib 4 mg may be more effective than 2 mg in some patients,” he said in an interview.
In late September, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended approval of oral baricitinib for adults with moderate to severe AD who are candidates for systemic therapy. Baricitinib is approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis. If approved in Europe, it will be the first JAK inhibitor and first oral medication indicated to treat patients with AD.
The study was funded by Eli Lilly and Company under license from Incyte Corporation. Dr. Reich reported receiving fees to the institution for participation in clinical trials from Eli Lilly and Company during the conduct of the study and personal fees for lectures. Dr. Silverberg reported receiving fees from Eli Lilly and Company during the conduct of the study, and fees from companies outside of this work. Other authors also reported disclosures related to Eli Lilly and other pharmaceutical companies, and several authors were Eli Lilly employees.
SOURCE: Reich K et al. JAMA Dermatol. 2020 Sep 30. doi: 10.1001/jamadermatol.2020.3260.
in the phase 3, double-blind, placebo-controlled, BREEZE-AD7 study.
The study enrolled patients with inadequate responses to topical corticosteroids, according to Kristian Reich, MD, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his coauthors.
First test of baricitinib plus topical steroids
Baricitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, inhibits several cytokines in AD pathogenesis, and in two monotherapy studies (BREEZE-AD1 and BREEZE-AD2), it was superior to placebo for reducing several AD clinical signs and symptoms. The current BREEZE-AD7 study is the first to test baricitinib plus background topical corticosteroid therapy, more closely mirroring clinical practice, the authors noted.
BREEZE-AD7 was conducted at 68 centers in 10 countries in Asia, Australia, Europe, and South America. It included 329 adults with moderate to severe AD (mean age around 34 years, and around 34% were female) with inadequate responses to topical corticosteroids documented within the last 6 months. They were randomized 1:1:1 to daily baricitinib 4 mg, daily baricitinib 2 mg, or placebo for 16 weeks. All patients received moderate- and/or low-potency topical corticosteroids (such as 0.1%triamcinolone cream and 2.5% hydrocortisone ointment, respectively) for active lesions.
Significant benefit at 4 mg
At week 16, 31% of AD patients receiving baricitinib 4 mg achieved Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 (clear) or 1 (almost clear) versus 15% in the placebo group (odds ratio, 2.8; 95% confidence interval, 1.4-5.6; P = .004). Among patients receiving baricitinib 2 mg, 24% achieved vI-GA-AD scores of 0 or 1 (OR, 1.9; 95% CI, 0.9-3.9; P = .08).
The same pattern of improving scores from placebo to baricitinib 2 mg to baricitinib 4 mg persisted, as reflected with secondary endpoints at week 16. Among patients receiving baricitinib 4 mg, 48% achieved Eczema Area Severity Index (EASI) 75 responses, versus 43% and 23% in 2 mg and placebo groups, respectively. Percent changes from baseline in total EASI score were –67%, –58%, –45% for baricitinib 4 mg, baricitinib 2 mg, and placebo, respectively; the proportion of patients achieving 4-point or greater improvements in Itch Numeric Rating Scale (NRS) was 44%, 38%, and 20% for baricitinib 4 mg, baricitinib 2 mg and placebo, respectively.
Similarly, mean change from baseline on the Skin Pain numeric rating scale was –3.7, –3.2, and –2.1 for baricitinib 4 mg, baricitinib 2 mg and placebo. Nighttime itch awakenings were also reduced in a similar progression from placebo to the higher baricitinib dose.
Adverse events dose related
Treatment-related adverse events were reported more frequently in the baricitinib groups (58% baricitinib 4 mg, 56% baricitinib 2 mg) versus placebo 38%. Nasopharyngitis was most common, followed by oral herpes, upper respiratory tract infection, acne, diarrhea, and back pain. Serious adverse event rates were similar across treatment groups. Permanent discontinuation rates were low at 5% for baricitinib 4 mg, 0% for baricitinib 2 mg, and 1% for placebo. The side-effect profile for baricitinib was consistent with prior studies, Dr. Reich and his coauthors reported.
The authors noted further, “data in this study suggest that patients with AD treated with baricitinib may be able to reduce the frequency and total quantity of concomitant TCSs [topical corticosteroids] used, thus mitigating concerns associated with continual or sustained application of topical treatments.”
“Overall, this study provides further evidence to support the efficacy and safety profile of baricitinib for the treatment of moderate-severe AD,” commented one of the authors, Jonathan I. Silverberg, MD, PhD, MPH, of the department of dermatology at George Washington University in Washington.
“In particular, this study shows that adding topical corticosteroids to baricitinib increases the rate of treatment success compared with the efficacy seen in baricitinib monotherapy studies. These data will be important to guide the use of baricitinib with topical corticosteroids in clinical practice. I think these data are also important because they show that baricitinib 4 mg may be more effective than 2 mg in some patients,” he said in an interview.
In late September, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended approval of oral baricitinib for adults with moderate to severe AD who are candidates for systemic therapy. Baricitinib is approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis. If approved in Europe, it will be the first JAK inhibitor and first oral medication indicated to treat patients with AD.
The study was funded by Eli Lilly and Company under license from Incyte Corporation. Dr. Reich reported receiving fees to the institution for participation in clinical trials from Eli Lilly and Company during the conduct of the study and personal fees for lectures. Dr. Silverberg reported receiving fees from Eli Lilly and Company during the conduct of the study, and fees from companies outside of this work. Other authors also reported disclosures related to Eli Lilly and other pharmaceutical companies, and several authors were Eli Lilly employees.
SOURCE: Reich K et al. JAMA Dermatol. 2020 Sep 30. doi: 10.1001/jamadermatol.2020.3260.
FROM JAMA DERMATOLOGY
Was This Tattoo a Rash Choice?
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.

Weeks ago, a 43-year-old man received a birthday tattoo of his choice: a geometric pattern etched in blue ink on his wrist. Unfortunately, a rash began to develop within the lines of the tattoo. The rash itches, but its appearance is of greater concern to the patient. He’s gotten some relief from topical creams, although the rash quickly returns with cessation of treatment.
Past medical history is notable for arthritis affecting his left elbow and right heel. He also has intermittent rashes that manifest on his elbows and knees, but these are partially relieved by a steroid cream (triamcinolone 0.1%).
His tattoo is located on the extensor right wrist. The affected areas show a brisk, red, inflammatory response, which—in several locations—is also scaly. There is no tenderness or induration on palpation of the rash and no palpable adenopathy in local nodal locations (epitrochlear and axillary). Elsewhere, 5 of his 10 fingernails demonstrate pitting; 2 show onycholysis and oil spotting. His scalp, knees, and elbows are free of any notable changes.
A teen presents with a severe, tender rash on the extremities
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
She started taking lithium for depression and anxiety 3 weeks prior to her developing the rash. She denies taking any other medications, supplements, or recreational drugs.
She denied any prior history of photosensitivity, no history of mouth ulcers, joint pain, muscle weakness, hair loss, or any other symptoms.
Besides her brother, there are no other affected family members, and no history of immune bullous disorders or other skin conditions.
On physical exam, the girl appears in a lot of pain and is uncomfortable. The skin is red and hot, and there are tense bullae on the neck, arms, and legs. There are no ocular or mucosal lesions.
Rapidly developing vesicular eruption
A 23-month-old girl with a history of well-controlled atopic dermatitis was admitted to the hospital with fever and a widespread vesicular eruption of 2 days’ duration. Two days prior to admission, the patient had 3 episodes of nonbloody diarrhea and redness in the diaper area. The child’s parents reported that the red areas spread to her arms and legs later that day, and that she subsequently developed a fever, cough, and rhinorrhea. She was taken to an urgent care facility where she was diagnosed with vulvovaginitis and an upper respiratory infection; amoxicillin was prescribed. Shortly thereafter, the patient developed more lesions in and around the mouth, as well as on the trunk, prompting the parents to bring her to the emergency department.
The history revealed that the patient had spent time with her aunt and cousins who had “red spots” on their palms and soles. The patient’s sister had a flare of “cold sores,” about 2 weeks prior to the current presentation. The patient had received a varicella zoster virus (VZV) vaccine several months earlier.
Physical examination was notable for an uncomfortable infant with erythematous macules on the bilateral palms and soles and an erythematous hard palate. The child also had scattered vesicles on an erythematous base with confluent crusted plaques on her lips, perioral skin (FIGURE 1A), abdomen, back, buttocks, arms, legs (FIGURE 1B), and dorsal aspects of her hands and feet.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema coxsackium
Given the history of atopic dermatitis; prodromal diarrhea/rhinorrhea; papulovesicular eruption involving areas of prior dermatitis as well as the palms, soles, and mouth; recent contacts with suspected hand-foot-mouth disease (HFMD); and history of VZV vaccination, the favored diagnosis was eczema coxsackium.
Eczema coxsackium is an atypical form of HFMD that occurs in patients with a history of eczema. Classic HFMD usually is caused by coxsackievirus A16 or enterovirus 71, while atypical HFMD often is caused by coxsackievirus A6.1,2,3 Patients with HFMD present with painful oral vesicles and ulcers and a papulovesicular eruption on the palms, soles, and sometimes the buttocks and genitalia. Patients may have prodromal fever, fussiness, and diarrhea. Painful oral lesions may result in poor oral intake.1,2
Differential includes viral eruptions
Other conditions may manifest similarly to eczema coxsackium and must be ruled out before initiating proper treatment.
Eczema herpeticum (EH). In atypical HFMD, the virus can show tropism for active or previously inflamed areas of eczematous skin, leading to a widespread vesicular eruption, which can be difficult to distinguish from EH.1 Similar to EH, eczema coxsackium does not exclusively affect children with atopic dermatitis. It also has been described in adults and patients with Darier disease, incontinentia pigmenti, and epidermolytic ichthyosis.4-6
In cases of vesicular eruptions in eczema patients, it is imperative to rule out EH. One prospective study of atypical HFMD compared similarities of the conditions. Both have a predilection for mucosa during primary infection and develop vesicular eruptions on cutaneous eczematous skin.1 One key difference between eczema coxsackium and EH is that EH tends to produce intraoral vesicles beyond simple erythema; it also tends to predominate in the area of the head and neck.7
Continue to: Eczema varicellicum
Eczema varicellicum has been reported, and it has been suggested that some cases of EH may actually be caused by VZV as the 2 are clinically indistinguishable and less than half of EH cases are diagnosed with laboratory confirmation.8
Confirm Dx before you treat
To guide management, cases of suspected eczema coxsackium should be confirmed, and HSV/VZV should be ruled out.9 Testing modalities include swabbing vesicular fluid for enterovirus polymerase chain reaction (PCR) analysis (preferred modality), oropharyngeal swab up to 2 weeks after infection, or viral isolate from stool samples up to 3 months after infection.2,3
Treatment for eczema coxsackium involves supportive care such as intravenous (IV) hydration and antipyretics. Some studies show potential benefit with IV immunoglobulin in treating severe HFMD, while other studies show the exacerbation of widespread HFMD with this treatment.7,10
Prompt diagnosis and treatment for eczema coxsackium is critical to prevent unnecessary antiviral therapy and to help guide monitoring for associated morbidities including Gianotti-Crosti syndrome–like eruptions, purpuric eruptions, and onychomadesis.
Our patient. Because EH was in the differential, our patient was started on empiric IV acyclovir 10 mg/kg every 8 hours while test results were pending. In addition, she received acetaminophen, IV fluids, gentle sponge baths, and diligent emollient application. Scraping from a vesicle revealed negative herpes simplex virus 1/2 PCR, negative VZV direct fluorescent antibody, and a positive enterovirus PCR—confirming the diagnosis of eczema coxsackium. Interestingly, a viral culture was negative in our patient, consistent with prior reports of enterovirus being difficult to culture.11
With confirmation of the diagnosis of eczema coxsackium, the IV acyclovir was discontinued, and symptoms resolved after 7 days.
CORRESPONDENCE
Shane M. Swink, DO, MS, Division of Dermatology, 1200 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected]
1. Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429-437.
2. Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491.
3. Horsten H, Fisker N, Bygu, A. Eczema coxsackium caused by coxsackievirus A6. Pediatr Dermatol. 2016;33:230-231.
4. Jefferson J, Grossberg A. Incontinentia pigmenti coxsackium. Pediatr Dermatol. 2016;33:E280-E281.
5. Ganguly S, Kuruvila S. Eczema coxsackium. Indian J Dermatol. 2016;61:682-683.
6. Harris P, Wang AD, Yin M, et al. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043.
7. Wollenberg A, Zoch C, Wetzel S, et al. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198-205.
8. Austin TA, Steele RW. Eczema varicella/zoster (varicellicum). Clin Pediatr. 2017;56:579-581.
9. Leung DYM. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
10. Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8:E64024.
11. Mathes EF, Oza V, Frieden IJ, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:149-157.
A 23-month-old girl with a history of well-controlled atopic dermatitis was admitted to the hospital with fever and a widespread vesicular eruption of 2 days’ duration. Two days prior to admission, the patient had 3 episodes of nonbloody diarrhea and redness in the diaper area. The child’s parents reported that the red areas spread to her arms and legs later that day, and that she subsequently developed a fever, cough, and rhinorrhea. She was taken to an urgent care facility where she was diagnosed with vulvovaginitis and an upper respiratory infection; amoxicillin was prescribed. Shortly thereafter, the patient developed more lesions in and around the mouth, as well as on the trunk, prompting the parents to bring her to the emergency department.
The history revealed that the patient had spent time with her aunt and cousins who had “red spots” on their palms and soles. The patient’s sister had a flare of “cold sores,” about 2 weeks prior to the current presentation. The patient had received a varicella zoster virus (VZV) vaccine several months earlier.
Physical examination was notable for an uncomfortable infant with erythematous macules on the bilateral palms and soles and an erythematous hard palate. The child also had scattered vesicles on an erythematous base with confluent crusted plaques on her lips, perioral skin (FIGURE 1A), abdomen, back, buttocks, arms, legs (FIGURE 1B), and dorsal aspects of her hands and feet.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema coxsackium
Given the history of atopic dermatitis; prodromal diarrhea/rhinorrhea; papulovesicular eruption involving areas of prior dermatitis as well as the palms, soles, and mouth; recent contacts with suspected hand-foot-mouth disease (HFMD); and history of VZV vaccination, the favored diagnosis was eczema coxsackium.
Eczema coxsackium is an atypical form of HFMD that occurs in patients with a history of eczema. Classic HFMD usually is caused by coxsackievirus A16 or enterovirus 71, while atypical HFMD often is caused by coxsackievirus A6.1,2,3 Patients with HFMD present with painful oral vesicles and ulcers and a papulovesicular eruption on the palms, soles, and sometimes the buttocks and genitalia. Patients may have prodromal fever, fussiness, and diarrhea. Painful oral lesions may result in poor oral intake.1,2
Differential includes viral eruptions
Other conditions may manifest similarly to eczema coxsackium and must be ruled out before initiating proper treatment.
Eczema herpeticum (EH). In atypical HFMD, the virus can show tropism for active or previously inflamed areas of eczematous skin, leading to a widespread vesicular eruption, which can be difficult to distinguish from EH.1 Similar to EH, eczema coxsackium does not exclusively affect children with atopic dermatitis. It also has been described in adults and patients with Darier disease, incontinentia pigmenti, and epidermolytic ichthyosis.4-6
In cases of vesicular eruptions in eczema patients, it is imperative to rule out EH. One prospective study of atypical HFMD compared similarities of the conditions. Both have a predilection for mucosa during primary infection and develop vesicular eruptions on cutaneous eczematous skin.1 One key difference between eczema coxsackium and EH is that EH tends to produce intraoral vesicles beyond simple erythema; it also tends to predominate in the area of the head and neck.7
Continue to: Eczema varicellicum
Eczema varicellicum has been reported, and it has been suggested that some cases of EH may actually be caused by VZV as the 2 are clinically indistinguishable and less than half of EH cases are diagnosed with laboratory confirmation.8
Confirm Dx before you treat
To guide management, cases of suspected eczema coxsackium should be confirmed, and HSV/VZV should be ruled out.9 Testing modalities include swabbing vesicular fluid for enterovirus polymerase chain reaction (PCR) analysis (preferred modality), oropharyngeal swab up to 2 weeks after infection, or viral isolate from stool samples up to 3 months after infection.2,3
Treatment for eczema coxsackium involves supportive care such as intravenous (IV) hydration and antipyretics. Some studies show potential benefit with IV immunoglobulin in treating severe HFMD, while other studies show the exacerbation of widespread HFMD with this treatment.7,10
Prompt diagnosis and treatment for eczema coxsackium is critical to prevent unnecessary antiviral therapy and to help guide monitoring for associated morbidities including Gianotti-Crosti syndrome–like eruptions, purpuric eruptions, and onychomadesis.
Our patient. Because EH was in the differential, our patient was started on empiric IV acyclovir 10 mg/kg every 8 hours while test results were pending. In addition, she received acetaminophen, IV fluids, gentle sponge baths, and diligent emollient application. Scraping from a vesicle revealed negative herpes simplex virus 1/2 PCR, negative VZV direct fluorescent antibody, and a positive enterovirus PCR—confirming the diagnosis of eczema coxsackium. Interestingly, a viral culture was negative in our patient, consistent with prior reports of enterovirus being difficult to culture.11
With confirmation of the diagnosis of eczema coxsackium, the IV acyclovir was discontinued, and symptoms resolved after 7 days.
CORRESPONDENCE
Shane M. Swink, DO, MS, Division of Dermatology, 1200 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected]
A 23-month-old girl with a history of well-controlled atopic dermatitis was admitted to the hospital with fever and a widespread vesicular eruption of 2 days’ duration. Two days prior to admission, the patient had 3 episodes of nonbloody diarrhea and redness in the diaper area. The child’s parents reported that the red areas spread to her arms and legs later that day, and that she subsequently developed a fever, cough, and rhinorrhea. She was taken to an urgent care facility where she was diagnosed with vulvovaginitis and an upper respiratory infection; amoxicillin was prescribed. Shortly thereafter, the patient developed more lesions in and around the mouth, as well as on the trunk, prompting the parents to bring her to the emergency department.
The history revealed that the patient had spent time with her aunt and cousins who had “red spots” on their palms and soles. The patient’s sister had a flare of “cold sores,” about 2 weeks prior to the current presentation. The patient had received a varicella zoster virus (VZV) vaccine several months earlier.
Physical examination was notable for an uncomfortable infant with erythematous macules on the bilateral palms and soles and an erythematous hard palate. The child also had scattered vesicles on an erythematous base with confluent crusted plaques on her lips, perioral skin (FIGURE 1A), abdomen, back, buttocks, arms, legs (FIGURE 1B), and dorsal aspects of her hands and feet.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema coxsackium
Given the history of atopic dermatitis; prodromal diarrhea/rhinorrhea; papulovesicular eruption involving areas of prior dermatitis as well as the palms, soles, and mouth; recent contacts with suspected hand-foot-mouth disease (HFMD); and history of VZV vaccination, the favored diagnosis was eczema coxsackium.
Eczema coxsackium is an atypical form of HFMD that occurs in patients with a history of eczema. Classic HFMD usually is caused by coxsackievirus A16 or enterovirus 71, while atypical HFMD often is caused by coxsackievirus A6.1,2,3 Patients with HFMD present with painful oral vesicles and ulcers and a papulovesicular eruption on the palms, soles, and sometimes the buttocks and genitalia. Patients may have prodromal fever, fussiness, and diarrhea. Painful oral lesions may result in poor oral intake.1,2
Differential includes viral eruptions
Other conditions may manifest similarly to eczema coxsackium and must be ruled out before initiating proper treatment.
Eczema herpeticum (EH). In atypical HFMD, the virus can show tropism for active or previously inflamed areas of eczematous skin, leading to a widespread vesicular eruption, which can be difficult to distinguish from EH.1 Similar to EH, eczema coxsackium does not exclusively affect children with atopic dermatitis. It also has been described in adults and patients with Darier disease, incontinentia pigmenti, and epidermolytic ichthyosis.4-6
In cases of vesicular eruptions in eczema patients, it is imperative to rule out EH. One prospective study of atypical HFMD compared similarities of the conditions. Both have a predilection for mucosa during primary infection and develop vesicular eruptions on cutaneous eczematous skin.1 One key difference between eczema coxsackium and EH is that EH tends to produce intraoral vesicles beyond simple erythema; it also tends to predominate in the area of the head and neck.7
Continue to: Eczema varicellicum
Eczema varicellicum has been reported, and it has been suggested that some cases of EH may actually be caused by VZV as the 2 are clinically indistinguishable and less than half of EH cases are diagnosed with laboratory confirmation.8
Confirm Dx before you treat
To guide management, cases of suspected eczema coxsackium should be confirmed, and HSV/VZV should be ruled out.9 Testing modalities include swabbing vesicular fluid for enterovirus polymerase chain reaction (PCR) analysis (preferred modality), oropharyngeal swab up to 2 weeks after infection, or viral isolate from stool samples up to 3 months after infection.2,3
Treatment for eczema coxsackium involves supportive care such as intravenous (IV) hydration and antipyretics. Some studies show potential benefit with IV immunoglobulin in treating severe HFMD, while other studies show the exacerbation of widespread HFMD with this treatment.7,10
Prompt diagnosis and treatment for eczema coxsackium is critical to prevent unnecessary antiviral therapy and to help guide monitoring for associated morbidities including Gianotti-Crosti syndrome–like eruptions, purpuric eruptions, and onychomadesis.
Our patient. Because EH was in the differential, our patient was started on empiric IV acyclovir 10 mg/kg every 8 hours while test results were pending. In addition, she received acetaminophen, IV fluids, gentle sponge baths, and diligent emollient application. Scraping from a vesicle revealed negative herpes simplex virus 1/2 PCR, negative VZV direct fluorescent antibody, and a positive enterovirus PCR—confirming the diagnosis of eczema coxsackium. Interestingly, a viral culture was negative in our patient, consistent with prior reports of enterovirus being difficult to culture.11
With confirmation of the diagnosis of eczema coxsackium, the IV acyclovir was discontinued, and symptoms resolved after 7 days.
CORRESPONDENCE
Shane M. Swink, DO, MS, Division of Dermatology, 1200 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected]
1. Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429-437.
2. Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491.
3. Horsten H, Fisker N, Bygu, A. Eczema coxsackium caused by coxsackievirus A6. Pediatr Dermatol. 2016;33:230-231.
4. Jefferson J, Grossberg A. Incontinentia pigmenti coxsackium. Pediatr Dermatol. 2016;33:E280-E281.
5. Ganguly S, Kuruvila S. Eczema coxsackium. Indian J Dermatol. 2016;61:682-683.
6. Harris P, Wang AD, Yin M, et al. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043.
7. Wollenberg A, Zoch C, Wetzel S, et al. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198-205.
8. Austin TA, Steele RW. Eczema varicella/zoster (varicellicum). Clin Pediatr. 2017;56:579-581.
9. Leung DYM. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
10. Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8:E64024.
11. Mathes EF, Oza V, Frieden IJ, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:149-157.
1. Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429-437.
2. Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491.
3. Horsten H, Fisker N, Bygu, A. Eczema coxsackium caused by coxsackievirus A6. Pediatr Dermatol. 2016;33:230-231.
4. Jefferson J, Grossberg A. Incontinentia pigmenti coxsackium. Pediatr Dermatol. 2016;33:E280-E281.
5. Ganguly S, Kuruvila S. Eczema coxsackium. Indian J Dermatol. 2016;61:682-683.
6. Harris P, Wang AD, Yin M, et al. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043.
7. Wollenberg A, Zoch C, Wetzel S, et al. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198-205.
8. Austin TA, Steele RW. Eczema varicella/zoster (varicellicum). Clin Pediatr. 2017;56:579-581.
9. Leung DYM. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
10. Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8:E64024.
11. Mathes EF, Oza V, Frieden IJ, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:149-157.