User login
For SCC, legs are a high-risk anatomic site in women
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Keeping the Differential at Hand
ANSWER
The correct answer is granuloma annulare (GA; choice “a”).
DISCUSSION
One of the most difficult concepts to grasp in dermatologic diagnosis is that almost all lesions and conditions—even the most common—have a broad range of morphologic presentations. These will often differ from the textbook photos. In this digital age, a simple Internet search will provide many results showing a diverse morphologic spectrum for many diseases, conditions, and lesions.
GA is a good example of how the presentation can vary. It has raised rolled margins and delled (gently concave) centers. The brownish red color is typical, but this patient showed a deeper red than most cases of GA. Occasionally, the red color is even deeper, with large patches of darkened skin and no palpable component.
Unfortunately, the misdiagnosis of fungal infection in this patient is typical. But dermatophytosis (otherwise known as “ringworm”)—the most common fungal skin infection—involves the epidermis. This means the patient would have scaly skin, probably with a well-defined margin—factors missing in this case. In addition, this patient reported no contact with sources of infection: animals, children, or immunosuppressive agents.
Ultimately, the most basic information that this patient's past providers neglected was a differential diagnosis. By establishing a differential, there was a clear decision to biopsy, which not only revealed the correct diagnosis but effectively ruled out the other options.
In defense of the patient’s other providers, I must admit that as a young primary care provider, I made this same mistake for exactly the same reasons.
TREATMENT
Most cases of GA are mild and self-limited, requiring no treatment. This is fortunate because no effective treatment exists. Topical steroids and cryotherapy will lighten the lesion, but GA nearly always resolves on its own.
ANSWER
The correct answer is granuloma annulare (GA; choice “a”).
DISCUSSION
One of the most difficult concepts to grasp in dermatologic diagnosis is that almost all lesions and conditions—even the most common—have a broad range of morphologic presentations. These will often differ from the textbook photos. In this digital age, a simple Internet search will provide many results showing a diverse morphologic spectrum for many diseases, conditions, and lesions.
GA is a good example of how the presentation can vary. It has raised rolled margins and delled (gently concave) centers. The brownish red color is typical, but this patient showed a deeper red than most cases of GA. Occasionally, the red color is even deeper, with large patches of darkened skin and no palpable component.
Unfortunately, the misdiagnosis of fungal infection in this patient is typical. But dermatophytosis (otherwise known as “ringworm”)—the most common fungal skin infection—involves the epidermis. This means the patient would have scaly skin, probably with a well-defined margin—factors missing in this case. In addition, this patient reported no contact with sources of infection: animals, children, or immunosuppressive agents.
Ultimately, the most basic information that this patient's past providers neglected was a differential diagnosis. By establishing a differential, there was a clear decision to biopsy, which not only revealed the correct diagnosis but effectively ruled out the other options.
In defense of the patient’s other providers, I must admit that as a young primary care provider, I made this same mistake for exactly the same reasons.
TREATMENT
Most cases of GA are mild and self-limited, requiring no treatment. This is fortunate because no effective treatment exists. Topical steroids and cryotherapy will lighten the lesion, but GA nearly always resolves on its own.
ANSWER
The correct answer is granuloma annulare (GA; choice “a”).
DISCUSSION
One of the most difficult concepts to grasp in dermatologic diagnosis is that almost all lesions and conditions—even the most common—have a broad range of morphologic presentations. These will often differ from the textbook photos. In this digital age, a simple Internet search will provide many results showing a diverse morphologic spectrum for many diseases, conditions, and lesions.
GA is a good example of how the presentation can vary. It has raised rolled margins and delled (gently concave) centers. The brownish red color is typical, but this patient showed a deeper red than most cases of GA. Occasionally, the red color is even deeper, with large patches of darkened skin and no palpable component.
Unfortunately, the misdiagnosis of fungal infection in this patient is typical. But dermatophytosis (otherwise known as “ringworm”)—the most common fungal skin infection—involves the epidermis. This means the patient would have scaly skin, probably with a well-defined margin—factors missing in this case. In addition, this patient reported no contact with sources of infection: animals, children, or immunosuppressive agents.
Ultimately, the most basic information that this patient's past providers neglected was a differential diagnosis. By establishing a differential, there was a clear decision to biopsy, which not only revealed the correct diagnosis but effectively ruled out the other options.
In defense of the patient’s other providers, I must admit that as a young primary care provider, I made this same mistake for exactly the same reasons.
TREATMENT
Most cases of GA are mild and self-limited, requiring no treatment. This is fortunate because no effective treatment exists. Topical steroids and cryotherapy will lighten the lesion, but GA nearly always resolves on its own.
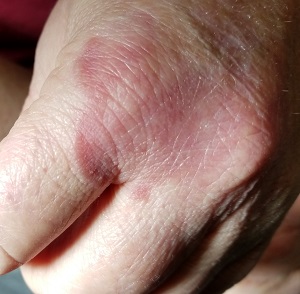
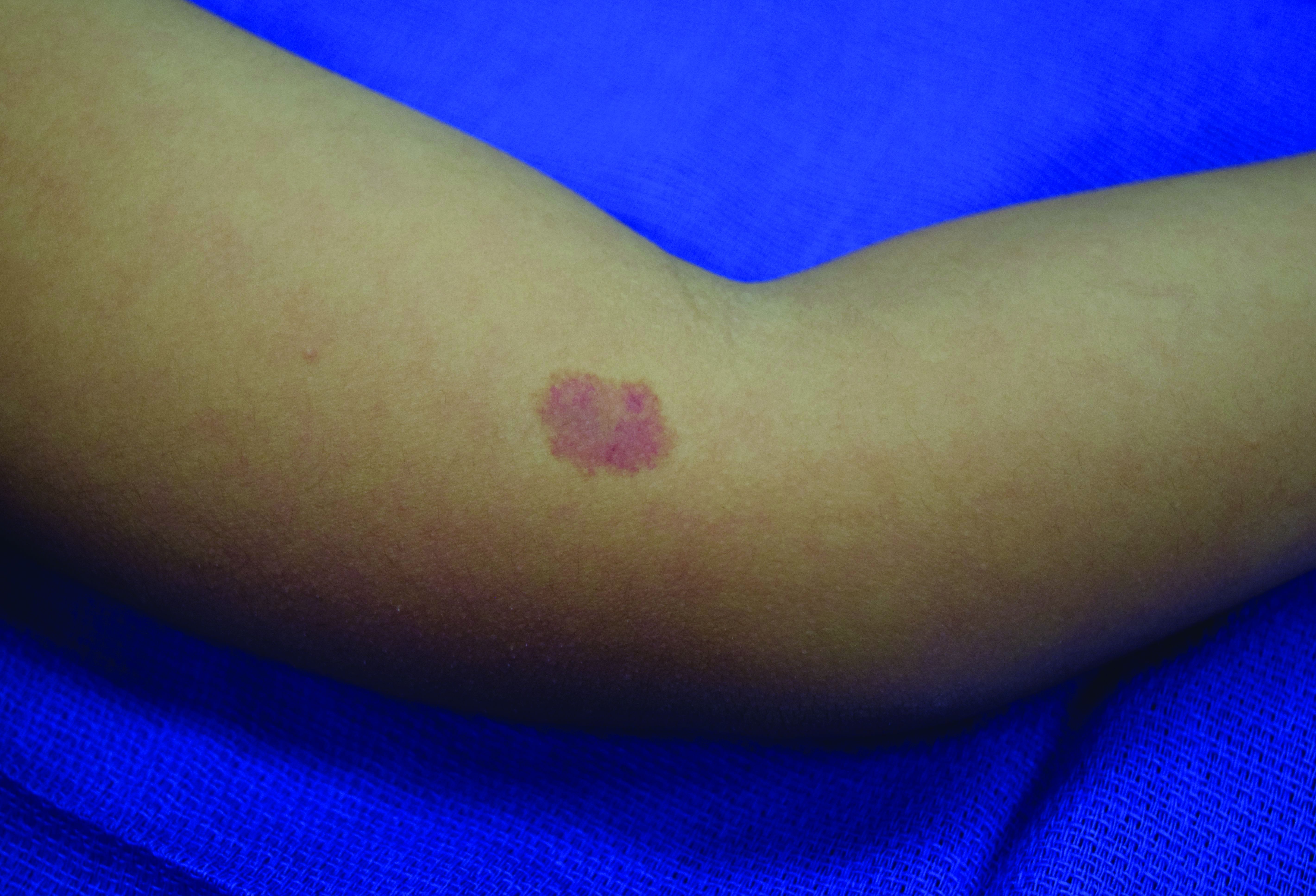
Several months ago, an asymptomatic rash slowly manifested on a 60-year-old woman’s hand. The rash—diagnosed previously as a fungal infection—continues to grow despite application of multiple antifungal creams, including tolnaftate, clotrimazole, and terbinafine. In addition, she was treated with a 1-month course of oral terbinafine 250 mg/d. Unfortunately, no treatment has provided her relief.
The patient is in otherwise good health. She denies any injury to the area. She also reports no exposure to children or animals.
The rash is a reddish brown, annular patch of skin that covers most of the dorsum of her left hand. The borders are slightly raised and thickened. It is nontender and readily blanchable. It is intradermal, with no surface disturbance such as scaling.
Punch biopsy shows palisaded granulomatous features, with no epidermal changes. Stains for fungi and bacteria fail to demonstrate any organisms.
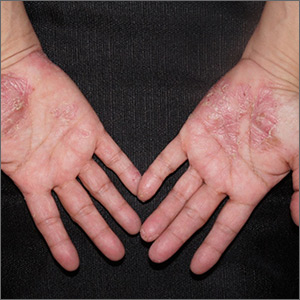
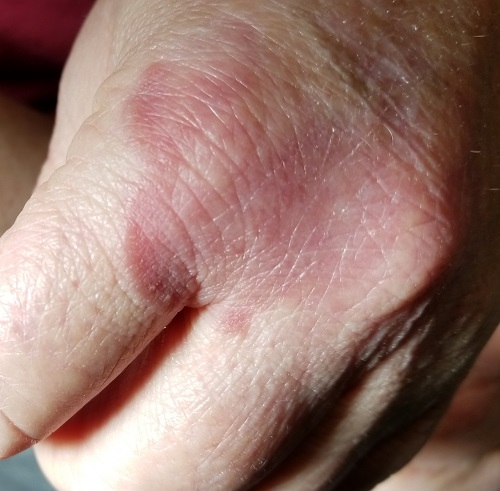
Painful pustules on hands
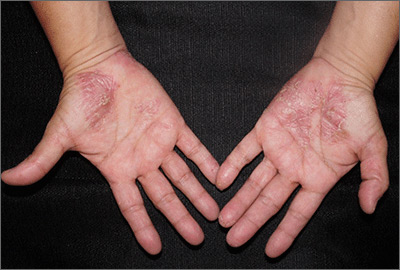
Close inspection of the plaques revealed multiple small pustules and mahogany colored macules on a broad, well-demarcated scaly red plaque. This pattern was consistent with palmoplantar pustular psoriasis.
Palmoplantar psoriasis is a chronic disease that may manifest at any age and in both sexes. The diagnosis may be clinical, as it was in this case. Bacterial culture of a pustule may demonstrate staphylococcus superinfection—a problem more likely to occur in smokers. More widespread disease, spiking fevers, or tachycardia could indicate a need for hospitalization for further work-up and management.
With disease localized to this patient’s hands and feet, the physician considered dyshidrotic eczema as part of the differential diagnosis. However dyshidrotic eczema, which presents with clear tapioca-like vesicles, is profoundly itchy; pustular psoriasis is often painful. Other differences: The vesicles in dyshidrotic eczema do not uniformly form pustules nor do they form brown macules upon healing, as occurs in palmoplantar pustular psoriasis.
Treatment for very limited disease includes topical ultrapotent steroids, topical retinoids, or phototherapy—either alone or in combination. However, more extensive disease, even if limited to hands and feet, merits systemic therapy with acitretin, methotrexate, or biologic agents. (Acitretin and methotrexate are reliable teratogens and should not be used in women who are, or may become, pregnant.) Individuals with known superinfection often benefit from antibiotic therapy, as well.
In this case, the patient had already undergone a tubal ligation for contraception. Therefore, she was started on acitretin 25 mg PO daily. Patients on systemic retinoids undergo laboratory surveillance for transaminitis and hypertriglyceridemia regularly. Adverse effects are generally well tolerated, but include photosensitivity and dry skin, lips, and eyes. The patient improved significantly on 25 mg/d and the dosage was reduced to 10 mg/d after 3 months. She currently remains clear on this regimen.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355-370.

Close inspection of the plaques revealed multiple small pustules and mahogany colored macules on a broad, well-demarcated scaly red plaque. This pattern was consistent with palmoplantar pustular psoriasis.
Palmoplantar psoriasis is a chronic disease that may manifest at any age and in both sexes. The diagnosis may be clinical, as it was in this case. Bacterial culture of a pustule may demonstrate staphylococcus superinfection—a problem more likely to occur in smokers. More widespread disease, spiking fevers, or tachycardia could indicate a need for hospitalization for further work-up and management.
With disease localized to this patient’s hands and feet, the physician considered dyshidrotic eczema as part of the differential diagnosis. However dyshidrotic eczema, which presents with clear tapioca-like vesicles, is profoundly itchy; pustular psoriasis is often painful. Other differences: The vesicles in dyshidrotic eczema do not uniformly form pustules nor do they form brown macules upon healing, as occurs in palmoplantar pustular psoriasis.
Treatment for very limited disease includes topical ultrapotent steroids, topical retinoids, or phototherapy—either alone or in combination. However, more extensive disease, even if limited to hands and feet, merits systemic therapy with acitretin, methotrexate, or biologic agents. (Acitretin and methotrexate are reliable teratogens and should not be used in women who are, or may become, pregnant.) Individuals with known superinfection often benefit from antibiotic therapy, as well.
In this case, the patient had already undergone a tubal ligation for contraception. Therefore, she was started on acitretin 25 mg PO daily. Patients on systemic retinoids undergo laboratory surveillance for transaminitis and hypertriglyceridemia regularly. Adverse effects are generally well tolerated, but include photosensitivity and dry skin, lips, and eyes. The patient improved significantly on 25 mg/d and the dosage was reduced to 10 mg/d after 3 months. She currently remains clear on this regimen.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

Close inspection of the plaques revealed multiple small pustules and mahogany colored macules on a broad, well-demarcated scaly red plaque. This pattern was consistent with palmoplantar pustular psoriasis.
Palmoplantar psoriasis is a chronic disease that may manifest at any age and in both sexes. The diagnosis may be clinical, as it was in this case. Bacterial culture of a pustule may demonstrate staphylococcus superinfection—a problem more likely to occur in smokers. More widespread disease, spiking fevers, or tachycardia could indicate a need for hospitalization for further work-up and management.
With disease localized to this patient’s hands and feet, the physician considered dyshidrotic eczema as part of the differential diagnosis. However dyshidrotic eczema, which presents with clear tapioca-like vesicles, is profoundly itchy; pustular psoriasis is often painful. Other differences: The vesicles in dyshidrotic eczema do not uniformly form pustules nor do they form brown macules upon healing, as occurs in palmoplantar pustular psoriasis.
Treatment for very limited disease includes topical ultrapotent steroids, topical retinoids, or phototherapy—either alone or in combination. However, more extensive disease, even if limited to hands and feet, merits systemic therapy with acitretin, methotrexate, or biologic agents. (Acitretin and methotrexate are reliable teratogens and should not be used in women who are, or may become, pregnant.) Individuals with known superinfection often benefit from antibiotic therapy, as well.
In this case, the patient had already undergone a tubal ligation for contraception. Therefore, she was started on acitretin 25 mg PO daily. Patients on systemic retinoids undergo laboratory surveillance for transaminitis and hypertriglyceridemia regularly. Adverse effects are generally well tolerated, but include photosensitivity and dry skin, lips, and eyes. The patient improved significantly on 25 mg/d and the dosage was reduced to 10 mg/d after 3 months. She currently remains clear on this regimen.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355-370.
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355-370.
Poor image quality may limit televulvology care
Seeing patients with vulvar problems via telemedicine can lead to efficient and successful care, but there are challenges and limitations with this approach, doctors are finding.
Image quality is one key factor that determines whether a clinician can assess and manage a condition remotely, said Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif. Other issues may be especially relevant to televulvology, including privacy concerns.
“Who is helping with the positioning? Who is the photographer? Is the patient comfortable with having photos taken of this part of their body and submitted, even if they know it is submitted securely? Because they might not be,” Dr. Venkatesan said in a lecture at a virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
When quality photographs from referring providers are available, Dr. Venkatesan has conducted virtual new consultations. “But sometimes I will do a virtual telemedicine visit as the first visit and then figure out, okay, this isn’t really sufficient. I need to see them in person.”
Melissa Mauskar, MD, assistant professor of dermatology and obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas, described a case early on during the COVID-19 pandemic that illustrates a limitation of virtual visits.
A patient sent in a photograph that appeared to show lichen sclerosus. “There looked like some classic lichen sclerosus changes,” Dr. Mauskar said during a discussion at the meeting. “But she was having a lot of pain, and after a week, her pain still was not better.”
Dr. Mauskar brought the patient into the office and ultimately diagnosed a squamous cell carcinoma. “What I thought was a normal erosion was actually an ulcerated plaque,” she said.
Like Dr. Venkatesan, Dr. Mauskar has found that image quality can be uneven. Photographs may be out of focus. Video visits have been a mixed bag. Some are successful. Other times, Dr. Mauskar has to tell the patient she needs to see her in the office.
Certain clinical scenarios require a vaginal exam, Dr. Venkatesan noted. Although some type of assessment may be possible if a patient is with a primary care provider during the telemedicine visit, the examination may not be equivalent. Doctors also should anticipate where a patient might go to have a biopsy if one is necessary.
Another telemedicine caveat pertains to patient counseling. When using store-and-forward telemedicine systems, advising patients in a written report can be challenging. “Is there an easy way ... to counsel patients how to apply their topical medications?” Dr. Venkatesan said.
Excellent care is possible
Vulvology is a small part of Dr. Venkatesan’s general dermatology practice, which has used telemedicine extensively since the pandemic.
In recent years, Dr. Venkatesan’s clinic began encouraging providers in their health system to submit photographs with referrals. “That has really paid off now because we have been able to help provide a lot of excellent quality care for patients without them having to come in,” she said. “We may be able to say: ‘These are excellent photos. We know what this patient has. We can manage it. They don’t need to come see us in person.’ ” That could be the case for certain types of acne, eczema, and psoriasis.
In other cases, they may be able to provide initial advice remotely but still want to see the patient. For a patient with severe acne, “I may be able to tell the referring doctor: ‘Please start the patient on these three medicines. It will take 2 months for those medicines to start working and then we will plan to have an in-person dermatology visit.’ ” In this case, telemedicine essentially replaces one in-person visit.
If photographs are poor, the differential diagnosis is broad, a procedure is required, the doctor needs to touch the lesion, or more involved history taking or counseling are required, the patient may need to go into the office.
Beyond its public health advantages during a pandemic, telemedicine can improve access for patients who live far away, lack transportation, or are unable to take time off from work. It also can decrease patient wait times. “Once we started doing some telemedicine work … we went from having a 5-month wait time for patients to see us in person to a 72-hour wait time for providing some care for patients if they had good photos as part of their referral,” Dr. Venkatesan said.
Telemedicine has been used in inpatient and outpatient dermatology settings. Primary care providers who consult with dermatologists using a store-and-forward telemedicine system may improve their dermatology knowledge and feel more confident in their ability to diagnose and manage dermatologic conditions, research indicates.
In obstetrics and gynecology, telemedicine may play a role in preconception, contraception, and medical abortion care, prenatal visits, well-woman exams, mental health, and pre- and postoperative counseling, a recent review suggests.
Image quality is key
“Quality of the image is so critical for being able to provide good care, especially in such a visual exam field as dermatology,” Dr. Venkatesan said.
To that end, doctors have offered recommendations on how to photograph skin conditions. A guide shared by the mobile telehealth system company ClickMedix suggests focusing on the area of importance, capturing the extent of involvement, and including involved and uninvolved areas.
Good lighting and checking the image resolution can help, Dr. Venkatesan offered. Nevertheless, patients may have difficulty photographing themselves. If a patient is with their primary care doctor, “we are much more likely to be able to get good quality photos,” she said.
Dr. Venkatesan is a paid consultant for DirectDerm, a store-and-forward teledermatology company. Dr. Mauskar had no relevant disclosures.
Seeing patients with vulvar problems via telemedicine can lead to efficient and successful care, but there are challenges and limitations with this approach, doctors are finding.
Image quality is one key factor that determines whether a clinician can assess and manage a condition remotely, said Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif. Other issues may be especially relevant to televulvology, including privacy concerns.
“Who is helping with the positioning? Who is the photographer? Is the patient comfortable with having photos taken of this part of their body and submitted, even if they know it is submitted securely? Because they might not be,” Dr. Venkatesan said in a lecture at a virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
When quality photographs from referring providers are available, Dr. Venkatesan has conducted virtual new consultations. “But sometimes I will do a virtual telemedicine visit as the first visit and then figure out, okay, this isn’t really sufficient. I need to see them in person.”
Melissa Mauskar, MD, assistant professor of dermatology and obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas, described a case early on during the COVID-19 pandemic that illustrates a limitation of virtual visits.
A patient sent in a photograph that appeared to show lichen sclerosus. “There looked like some classic lichen sclerosus changes,” Dr. Mauskar said during a discussion at the meeting. “But she was having a lot of pain, and after a week, her pain still was not better.”
Dr. Mauskar brought the patient into the office and ultimately diagnosed a squamous cell carcinoma. “What I thought was a normal erosion was actually an ulcerated plaque,” she said.
Like Dr. Venkatesan, Dr. Mauskar has found that image quality can be uneven. Photographs may be out of focus. Video visits have been a mixed bag. Some are successful. Other times, Dr. Mauskar has to tell the patient she needs to see her in the office.
Certain clinical scenarios require a vaginal exam, Dr. Venkatesan noted. Although some type of assessment may be possible if a patient is with a primary care provider during the telemedicine visit, the examination may not be equivalent. Doctors also should anticipate where a patient might go to have a biopsy if one is necessary.
Another telemedicine caveat pertains to patient counseling. When using store-and-forward telemedicine systems, advising patients in a written report can be challenging. “Is there an easy way ... to counsel patients how to apply their topical medications?” Dr. Venkatesan said.
Excellent care is possible
Vulvology is a small part of Dr. Venkatesan’s general dermatology practice, which has used telemedicine extensively since the pandemic.
In recent years, Dr. Venkatesan’s clinic began encouraging providers in their health system to submit photographs with referrals. “That has really paid off now because we have been able to help provide a lot of excellent quality care for patients without them having to come in,” she said. “We may be able to say: ‘These are excellent photos. We know what this patient has. We can manage it. They don’t need to come see us in person.’ ” That could be the case for certain types of acne, eczema, and psoriasis.
In other cases, they may be able to provide initial advice remotely but still want to see the patient. For a patient with severe acne, “I may be able to tell the referring doctor: ‘Please start the patient on these three medicines. It will take 2 months for those medicines to start working and then we will plan to have an in-person dermatology visit.’ ” In this case, telemedicine essentially replaces one in-person visit.
If photographs are poor, the differential diagnosis is broad, a procedure is required, the doctor needs to touch the lesion, or more involved history taking or counseling are required, the patient may need to go into the office.
Beyond its public health advantages during a pandemic, telemedicine can improve access for patients who live far away, lack transportation, or are unable to take time off from work. It also can decrease patient wait times. “Once we started doing some telemedicine work … we went from having a 5-month wait time for patients to see us in person to a 72-hour wait time for providing some care for patients if they had good photos as part of their referral,” Dr. Venkatesan said.
Telemedicine has been used in inpatient and outpatient dermatology settings. Primary care providers who consult with dermatologists using a store-and-forward telemedicine system may improve their dermatology knowledge and feel more confident in their ability to diagnose and manage dermatologic conditions, research indicates.
In obstetrics and gynecology, telemedicine may play a role in preconception, contraception, and medical abortion care, prenatal visits, well-woman exams, mental health, and pre- and postoperative counseling, a recent review suggests.
Image quality is key
“Quality of the image is so critical for being able to provide good care, especially in such a visual exam field as dermatology,” Dr. Venkatesan said.
To that end, doctors have offered recommendations on how to photograph skin conditions. A guide shared by the mobile telehealth system company ClickMedix suggests focusing on the area of importance, capturing the extent of involvement, and including involved and uninvolved areas.
Good lighting and checking the image resolution can help, Dr. Venkatesan offered. Nevertheless, patients may have difficulty photographing themselves. If a patient is with their primary care doctor, “we are much more likely to be able to get good quality photos,” she said.
Dr. Venkatesan is a paid consultant for DirectDerm, a store-and-forward teledermatology company. Dr. Mauskar had no relevant disclosures.
Seeing patients with vulvar problems via telemedicine can lead to efficient and successful care, but there are challenges and limitations with this approach, doctors are finding.
Image quality is one key factor that determines whether a clinician can assess and manage a condition remotely, said Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif. Other issues may be especially relevant to televulvology, including privacy concerns.
“Who is helping with the positioning? Who is the photographer? Is the patient comfortable with having photos taken of this part of their body and submitted, even if they know it is submitted securely? Because they might not be,” Dr. Venkatesan said in a lecture at a virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
When quality photographs from referring providers are available, Dr. Venkatesan has conducted virtual new consultations. “But sometimes I will do a virtual telemedicine visit as the first visit and then figure out, okay, this isn’t really sufficient. I need to see them in person.”
Melissa Mauskar, MD, assistant professor of dermatology and obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas, described a case early on during the COVID-19 pandemic that illustrates a limitation of virtual visits.
A patient sent in a photograph that appeared to show lichen sclerosus. “There looked like some classic lichen sclerosus changes,” Dr. Mauskar said during a discussion at the meeting. “But she was having a lot of pain, and after a week, her pain still was not better.”
Dr. Mauskar brought the patient into the office and ultimately diagnosed a squamous cell carcinoma. “What I thought was a normal erosion was actually an ulcerated plaque,” she said.
Like Dr. Venkatesan, Dr. Mauskar has found that image quality can be uneven. Photographs may be out of focus. Video visits have been a mixed bag. Some are successful. Other times, Dr. Mauskar has to tell the patient she needs to see her in the office.
Certain clinical scenarios require a vaginal exam, Dr. Venkatesan noted. Although some type of assessment may be possible if a patient is with a primary care provider during the telemedicine visit, the examination may not be equivalent. Doctors also should anticipate where a patient might go to have a biopsy if one is necessary.
Another telemedicine caveat pertains to patient counseling. When using store-and-forward telemedicine systems, advising patients in a written report can be challenging. “Is there an easy way ... to counsel patients how to apply their topical medications?” Dr. Venkatesan said.
Excellent care is possible
Vulvology is a small part of Dr. Venkatesan’s general dermatology practice, which has used telemedicine extensively since the pandemic.
In recent years, Dr. Venkatesan’s clinic began encouraging providers in their health system to submit photographs with referrals. “That has really paid off now because we have been able to help provide a lot of excellent quality care for patients without them having to come in,” she said. “We may be able to say: ‘These are excellent photos. We know what this patient has. We can manage it. They don’t need to come see us in person.’ ” That could be the case for certain types of acne, eczema, and psoriasis.
In other cases, they may be able to provide initial advice remotely but still want to see the patient. For a patient with severe acne, “I may be able to tell the referring doctor: ‘Please start the patient on these three medicines. It will take 2 months for those medicines to start working and then we will plan to have an in-person dermatology visit.’ ” In this case, telemedicine essentially replaces one in-person visit.
If photographs are poor, the differential diagnosis is broad, a procedure is required, the doctor needs to touch the lesion, or more involved history taking or counseling are required, the patient may need to go into the office.
Beyond its public health advantages during a pandemic, telemedicine can improve access for patients who live far away, lack transportation, or are unable to take time off from work. It also can decrease patient wait times. “Once we started doing some telemedicine work … we went from having a 5-month wait time for patients to see us in person to a 72-hour wait time for providing some care for patients if they had good photos as part of their referral,” Dr. Venkatesan said.
Telemedicine has been used in inpatient and outpatient dermatology settings. Primary care providers who consult with dermatologists using a store-and-forward telemedicine system may improve their dermatology knowledge and feel more confident in their ability to diagnose and manage dermatologic conditions, research indicates.
In obstetrics and gynecology, telemedicine may play a role in preconception, contraception, and medical abortion care, prenatal visits, well-woman exams, mental health, and pre- and postoperative counseling, a recent review suggests.
Image quality is key
“Quality of the image is so critical for being able to provide good care, especially in such a visual exam field as dermatology,” Dr. Venkatesan said.
To that end, doctors have offered recommendations on how to photograph skin conditions. A guide shared by the mobile telehealth system company ClickMedix suggests focusing on the area of importance, capturing the extent of involvement, and including involved and uninvolved areas.
Good lighting and checking the image resolution can help, Dr. Venkatesan offered. Nevertheless, patients may have difficulty photographing themselves. If a patient is with their primary care doctor, “we are much more likely to be able to get good quality photos,” she said.
Dr. Venkatesan is a paid consultant for DirectDerm, a store-and-forward teledermatology company. Dr. Mauskar had no relevant disclosures.
FROM THE ISSVD BIENNIAL CONFERENCE
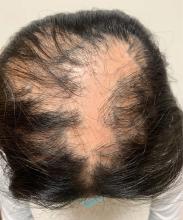
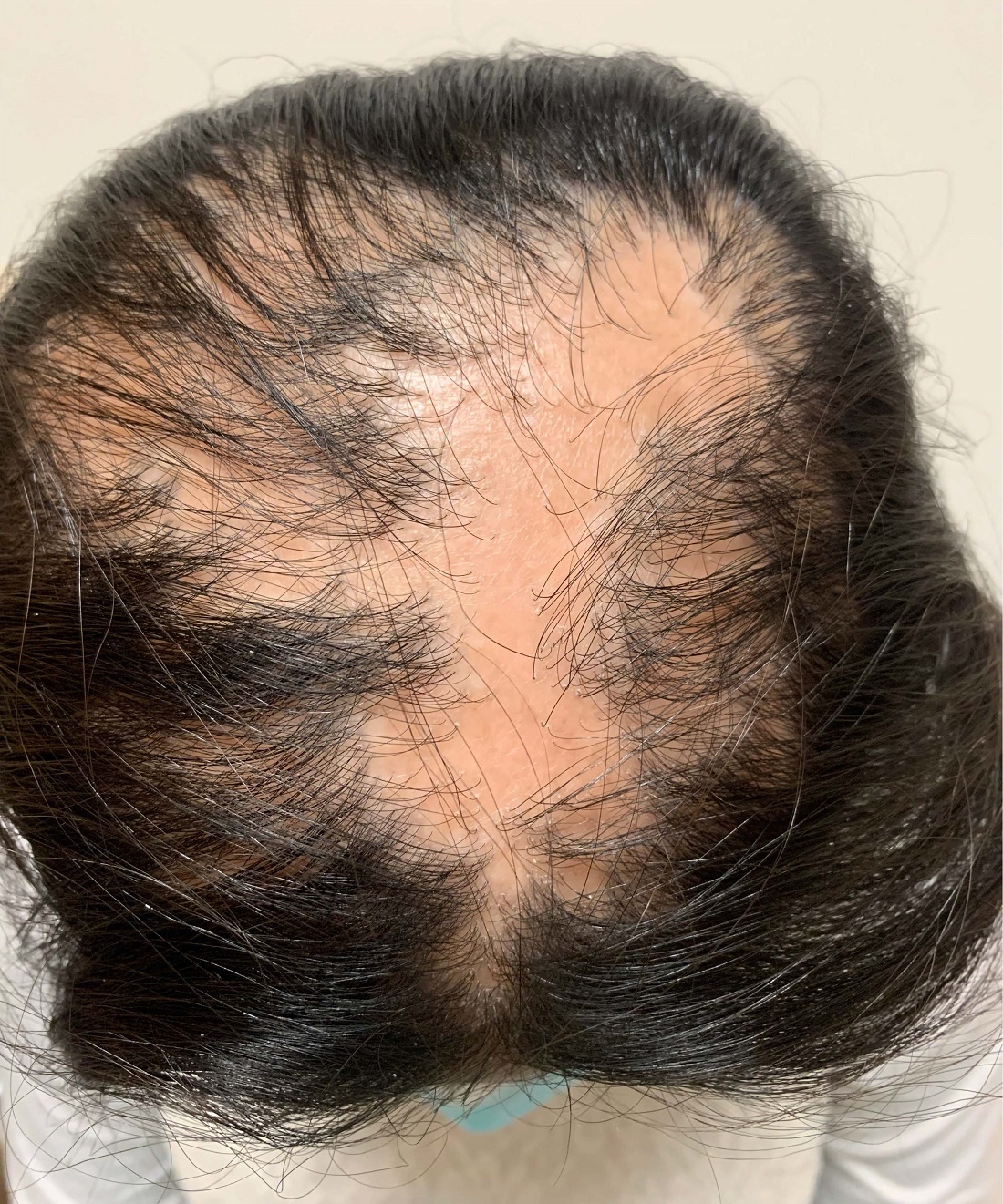
An 11-year-old female with a 3-year history of alopecia
Given the longstanding scarring alopecia, with negative fungal cultures and with perifollicular erythema and scaling, this diagnosis is most consistent with lichen planopilaris.
Lichen planopilaris (LPP) is considered one of the primary scarring alopecias, a group of diseases characterized by inflammation and subsequent irreversible hair loss.1 LPP specifically is believed to be caused by dysfunction of cell-mediated immunity, resulting in T lymphocytes attacking follicular hair stem cells.2 It typically presents with hair loss, pruritus, scaling, burning pain, and tenderness of the scalp when active,1,3 with exam showing perifollicular scale and erythema on the borders of the patches of alopecia.4,5 Over time, scarring of the scalp develops with loss of follicular ostia.1 Definitive diagnosis typically requires punch biopsy of the affected scalp, as such can determine the presence or absence of inflammation in affected areas of the scalp.1
What’s the treatment plan?
Given that LPP is an autoimmune inflammatory disease process, the goal of treatment is to calm down the inflammation of the scalp to prevent further progression of a patient’s hair loss. This is typically achieved with superpotent topical corticosteroids, such as clobetasol applied directly to the scalp, and/or intralesional corticosteroids, such as triamcinolone acetonide suspension injected directly to the affected scalp.3,6,7 Other treatment options include systemic agents, such as hydroxychloroquine, methotrexate, mycophenolate mofetil, pioglitazone, and doxycycline.3,6 Hair loss is not reversible as loss of follicular ostia and hair stem cells results in permanent scarring.1 Management often requires a referral to dermatology for aggressive treatment to prevent further hair loss.
What’s the differential diagnosis?
The differential diagnosis of lichen planopilaris includes other scarring alopecias, including central centrifugal cicatricial alopecia, discoid lupus erythematosus, folliculitis decalvans. While nonscarring, alopecia areata, trichotillomania, and telogen effluvium are discussed below as well.
Central centrifugal cicatricial alopecia is very rare in pediatrics, and is a type of asymptomatic scarring alopecia that begins at the vertex of the scalp, spreading centrifugally and resulting in shiny plaque development. Treatment involves reduction of hair grooming as well as topical and intralesional steroids.
Discoid lupus erythematosus presents as scaling erythematous plaques on the face and scalp that result in skin pigment changes and atrophy over time. Scalp involvement results in scarring alopecia. Treatment includes the use of high-potency topical corticosteroids, topical calcineurin inhibitors, and hydroxychloroquine.
Folliculitis decalvans is another form of scarring alopecia believed to be caused by an inflammatory response to Staphylococcus aureus in the scalp, resulting in the formation of scarring of the scalp and perifollicular pustules. Treatment is topical antibiotics and intralesional steroids.
Alopecia areata is a form of nonscarring alopecia resulting in small round patches of partially reversible hair loss characterized by the pathognomonic finding of so-called exclamation point hairs that are broader distally and taper toward the scalp on physical exam. Considered an autoimmune disorder, it varies greatly in extent and course. While focal hair loss is the hallmark of this disease, usually hair follicles are present.
Trichotillosis, also known as trichotillomania (hair pulling), results in alopecia with irregular borders and broken hairs of different lengths secondary to the urge to remove or pull one’s own hair, resulting in nonscarring alopecia. It may be associated with stress or anxiety, obsessive-compulsive disorders, or other repetitive body-altering behaviors. Treatments include reassurance and education as it can be self-limited in some, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs.
Our patient underwent scalp punch biopsy to confirm the diagnosis and was started on potent topical corticosteroids with good disease control.
Dr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university, and he is chief of pediatric and adolescent dermatology at the hospital. Neither of the doctors had any relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2005 Jul. doi: 10.1016/j.jaad.2004.06.015.
2. J Pathol. 2013 Oct. doi: 10.1002/path.4233.
3. Pediatr Dermatol. 2015 Sep-Oct. doi: 10.1111/pde.12624.
4. J Am Acad Dermatol. 2004 Jan. doi: 10.1016/j.jaad.2003.04.001.
5. J Am Acad Dermatol. 1992 Dec. doi: 10.1016/0190-9622(92)70290-v.
6. Clin Cosmet Investig Dermatol. 2018 Feb 27. doi: 10.2147/CCID.S137870.
7. Semin Cutan Med Surg. 2009 Mar. doi: 10.1016/j.sder.2008.12.006.
Given the longstanding scarring alopecia, with negative fungal cultures and with perifollicular erythema and scaling, this diagnosis is most consistent with lichen planopilaris.
Lichen planopilaris (LPP) is considered one of the primary scarring alopecias, a group of diseases characterized by inflammation and subsequent irreversible hair loss.1 LPP specifically is believed to be caused by dysfunction of cell-mediated immunity, resulting in T lymphocytes attacking follicular hair stem cells.2 It typically presents with hair loss, pruritus, scaling, burning pain, and tenderness of the scalp when active,1,3 with exam showing perifollicular scale and erythema on the borders of the patches of alopecia.4,5 Over time, scarring of the scalp develops with loss of follicular ostia.1 Definitive diagnosis typically requires punch biopsy of the affected scalp, as such can determine the presence or absence of inflammation in affected areas of the scalp.1
What’s the treatment plan?
Given that LPP is an autoimmune inflammatory disease process, the goal of treatment is to calm down the inflammation of the scalp to prevent further progression of a patient’s hair loss. This is typically achieved with superpotent topical corticosteroids, such as clobetasol applied directly to the scalp, and/or intralesional corticosteroids, such as triamcinolone acetonide suspension injected directly to the affected scalp.3,6,7 Other treatment options include systemic agents, such as hydroxychloroquine, methotrexate, mycophenolate mofetil, pioglitazone, and doxycycline.3,6 Hair loss is not reversible as loss of follicular ostia and hair stem cells results in permanent scarring.1 Management often requires a referral to dermatology for aggressive treatment to prevent further hair loss.
What’s the differential diagnosis?
The differential diagnosis of lichen planopilaris includes other scarring alopecias, including central centrifugal cicatricial alopecia, discoid lupus erythematosus, folliculitis decalvans. While nonscarring, alopecia areata, trichotillomania, and telogen effluvium are discussed below as well.
Central centrifugal cicatricial alopecia is very rare in pediatrics, and is a type of asymptomatic scarring alopecia that begins at the vertex of the scalp, spreading centrifugally and resulting in shiny plaque development. Treatment involves reduction of hair grooming as well as topical and intralesional steroids.
Discoid lupus erythematosus presents as scaling erythematous plaques on the face and scalp that result in skin pigment changes and atrophy over time. Scalp involvement results in scarring alopecia. Treatment includes the use of high-potency topical corticosteroids, topical calcineurin inhibitors, and hydroxychloroquine.
Folliculitis decalvans is another form of scarring alopecia believed to be caused by an inflammatory response to Staphylococcus aureus in the scalp, resulting in the formation of scarring of the scalp and perifollicular pustules. Treatment is topical antibiotics and intralesional steroids.
Alopecia areata is a form of nonscarring alopecia resulting in small round patches of partially reversible hair loss characterized by the pathognomonic finding of so-called exclamation point hairs that are broader distally and taper toward the scalp on physical exam. Considered an autoimmune disorder, it varies greatly in extent and course. While focal hair loss is the hallmark of this disease, usually hair follicles are present.
Trichotillosis, also known as trichotillomania (hair pulling), results in alopecia with irregular borders and broken hairs of different lengths secondary to the urge to remove or pull one’s own hair, resulting in nonscarring alopecia. It may be associated with stress or anxiety, obsessive-compulsive disorders, or other repetitive body-altering behaviors. Treatments include reassurance and education as it can be self-limited in some, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs.
Our patient underwent scalp punch biopsy to confirm the diagnosis and was started on potent topical corticosteroids with good disease control.
Dr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university, and he is chief of pediatric and adolescent dermatology at the hospital. Neither of the doctors had any relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2005 Jul. doi: 10.1016/j.jaad.2004.06.015.
2. J Pathol. 2013 Oct. doi: 10.1002/path.4233.
3. Pediatr Dermatol. 2015 Sep-Oct. doi: 10.1111/pde.12624.
4. J Am Acad Dermatol. 2004 Jan. doi: 10.1016/j.jaad.2003.04.001.
5. J Am Acad Dermatol. 1992 Dec. doi: 10.1016/0190-9622(92)70290-v.
6. Clin Cosmet Investig Dermatol. 2018 Feb 27. doi: 10.2147/CCID.S137870.
7. Semin Cutan Med Surg. 2009 Mar. doi: 10.1016/j.sder.2008.12.006.
Given the longstanding scarring alopecia, with negative fungal cultures and with perifollicular erythema and scaling, this diagnosis is most consistent with lichen planopilaris.
Lichen planopilaris (LPP) is considered one of the primary scarring alopecias, a group of diseases characterized by inflammation and subsequent irreversible hair loss.1 LPP specifically is believed to be caused by dysfunction of cell-mediated immunity, resulting in T lymphocytes attacking follicular hair stem cells.2 It typically presents with hair loss, pruritus, scaling, burning pain, and tenderness of the scalp when active,1,3 with exam showing perifollicular scale and erythema on the borders of the patches of alopecia.4,5 Over time, scarring of the scalp develops with loss of follicular ostia.1 Definitive diagnosis typically requires punch biopsy of the affected scalp, as such can determine the presence or absence of inflammation in affected areas of the scalp.1
What’s the treatment plan?
Given that LPP is an autoimmune inflammatory disease process, the goal of treatment is to calm down the inflammation of the scalp to prevent further progression of a patient’s hair loss. This is typically achieved with superpotent topical corticosteroids, such as clobetasol applied directly to the scalp, and/or intralesional corticosteroids, such as triamcinolone acetonide suspension injected directly to the affected scalp.3,6,7 Other treatment options include systemic agents, such as hydroxychloroquine, methotrexate, mycophenolate mofetil, pioglitazone, and doxycycline.3,6 Hair loss is not reversible as loss of follicular ostia and hair stem cells results in permanent scarring.1 Management often requires a referral to dermatology for aggressive treatment to prevent further hair loss.
What’s the differential diagnosis?
The differential diagnosis of lichen planopilaris includes other scarring alopecias, including central centrifugal cicatricial alopecia, discoid lupus erythematosus, folliculitis decalvans. While nonscarring, alopecia areata, trichotillomania, and telogen effluvium are discussed below as well.
Central centrifugal cicatricial alopecia is very rare in pediatrics, and is a type of asymptomatic scarring alopecia that begins at the vertex of the scalp, spreading centrifugally and resulting in shiny plaque development. Treatment involves reduction of hair grooming as well as topical and intralesional steroids.
Discoid lupus erythematosus presents as scaling erythematous plaques on the face and scalp that result in skin pigment changes and atrophy over time. Scalp involvement results in scarring alopecia. Treatment includes the use of high-potency topical corticosteroids, topical calcineurin inhibitors, and hydroxychloroquine.
Folliculitis decalvans is another form of scarring alopecia believed to be caused by an inflammatory response to Staphylococcus aureus in the scalp, resulting in the formation of scarring of the scalp and perifollicular pustules. Treatment is topical antibiotics and intralesional steroids.
Alopecia areata is a form of nonscarring alopecia resulting in small round patches of partially reversible hair loss characterized by the pathognomonic finding of so-called exclamation point hairs that are broader distally and taper toward the scalp on physical exam. Considered an autoimmune disorder, it varies greatly in extent and course. While focal hair loss is the hallmark of this disease, usually hair follicles are present.
Trichotillosis, also known as trichotillomania (hair pulling), results in alopecia with irregular borders and broken hairs of different lengths secondary to the urge to remove or pull one’s own hair, resulting in nonscarring alopecia. It may be associated with stress or anxiety, obsessive-compulsive disorders, or other repetitive body-altering behaviors. Treatments include reassurance and education as it can be self-limited in some, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs.
Our patient underwent scalp punch biopsy to confirm the diagnosis and was started on potent topical corticosteroids with good disease control.
Dr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university, and he is chief of pediatric and adolescent dermatology at the hospital. Neither of the doctors had any relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2005 Jul. doi: 10.1016/j.jaad.2004.06.015.
2. J Pathol. 2013 Oct. doi: 10.1002/path.4233.
3. Pediatr Dermatol. 2015 Sep-Oct. doi: 10.1111/pde.12624.
4. J Am Acad Dermatol. 2004 Jan. doi: 10.1016/j.jaad.2003.04.001.
5. J Am Acad Dermatol. 1992 Dec. doi: 10.1016/0190-9622(92)70290-v.
6. Clin Cosmet Investig Dermatol. 2018 Feb 27. doi: 10.2147/CCID.S137870.
7. Semin Cutan Med Surg. 2009 Mar. doi: 10.1016/j.sder.2008.12.006.
An 11-year-old female is seen in clinic with a 3-year history of alopecia. The patient recently immigrated to the United States from Afghanistan. Prior to immigrating, she was evaluated for "scarring alopecia" and had been treated with oral and topical steroids as well as oral and topical antifungals. When active, she had itching and tenderness. She is not actively losing any hair at this time, but she has not regrown any of her hair. The patient has no family members with alopecia. She reports some burning pain and itching of her scalp, and denies any muscle pain or weakness or sun sensitivity.
On physical exam, you see 50% loss of hair on the superior scalp with preservation of the anterior hair line. Patches of hair can be seen throughout, with segments of smooth-skinned alopecia, without pustules. There is a loss of the follicle pattern in scarred areas, and magnification or "dermoscopy" shows perifollicular erythema and scaling at the border of the affected scalp. Labs are all within normal limits. Bacterial and fungal cultures of the scalp do not grow organisms.
Purple toe lesion
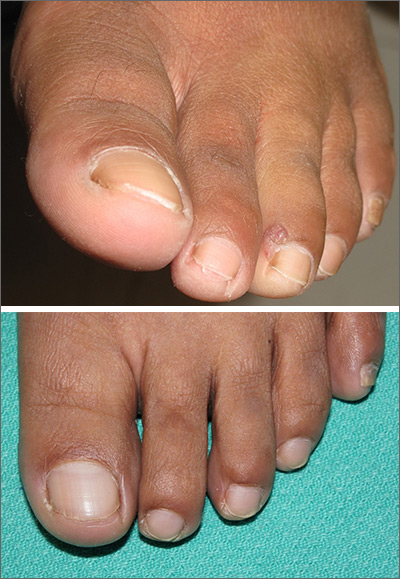
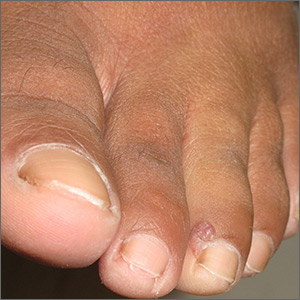
An excisional biopsy revealed that this was an eccrine poroma, a benign neoplasm of sweat gland tissue in the epidermis.
This lesion was clearly not a wart, as it lacked the common verrucous and keratotic features one would expect, and it did not respond to wart treatments. Other diagnoses that might be considered with a lesion like this include pyogenic granuloma, periungual fibroma, and squamous cell carcinoma.
Poromas are well demarcated papules that grow slowly or are stable in size. They are most commonly flesh colored and smooth, but poromas may also appear verrucous, pigmented, or ulcerated. They are usually found on acral skin—particularly the palms or soles. Due to their location, poromas bleed easily, which is what usually prompts patients to seek care. Friable lesions can mimic acral melanoma.
Poromas occur most often in patients over 40 years of age and are evenly distributed among sexes and skin types. Cases have been associated with trauma, radiation, and chemotherapy. Although exceedingly rare, malignant transformation can occur in the form of eccrine porocarcinoma—a larger tumor that can grow rapidly and that has metastatic potential.
Treatment is optional—but desirable—when lesions are painful or bleed easily. Surgical excision is curative. Shave biopsy or curettage coupled with electrocautery of the base is also curative. Recurrence rates are very low with either method of treatment.
In this case, an excisional biopsy of the lateral nail fold was both diagnostic and curative (second image). The patient remained clear 9 months after treatment.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.

An excisional biopsy revealed that this was an eccrine poroma, a benign neoplasm of sweat gland tissue in the epidermis.
This lesion was clearly not a wart, as it lacked the common verrucous and keratotic features one would expect, and it did not respond to wart treatments. Other diagnoses that might be considered with a lesion like this include pyogenic granuloma, periungual fibroma, and squamous cell carcinoma.
Poromas are well demarcated papules that grow slowly or are stable in size. They are most commonly flesh colored and smooth, but poromas may also appear verrucous, pigmented, or ulcerated. They are usually found on acral skin—particularly the palms or soles. Due to their location, poromas bleed easily, which is what usually prompts patients to seek care. Friable lesions can mimic acral melanoma.
Poromas occur most often in patients over 40 years of age and are evenly distributed among sexes and skin types. Cases have been associated with trauma, radiation, and chemotherapy. Although exceedingly rare, malignant transformation can occur in the form of eccrine porocarcinoma—a larger tumor that can grow rapidly and that has metastatic potential.
Treatment is optional—but desirable—when lesions are painful or bleed easily. Surgical excision is curative. Shave biopsy or curettage coupled with electrocautery of the base is also curative. Recurrence rates are very low with either method of treatment.
In this case, an excisional biopsy of the lateral nail fold was both diagnostic and curative (second image). The patient remained clear 9 months after treatment.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

An excisional biopsy revealed that this was an eccrine poroma, a benign neoplasm of sweat gland tissue in the epidermis.
This lesion was clearly not a wart, as it lacked the common verrucous and keratotic features one would expect, and it did not respond to wart treatments. Other diagnoses that might be considered with a lesion like this include pyogenic granuloma, periungual fibroma, and squamous cell carcinoma.
Poromas are well demarcated papules that grow slowly or are stable in size. They are most commonly flesh colored and smooth, but poromas may also appear verrucous, pigmented, or ulcerated. They are usually found on acral skin—particularly the palms or soles. Due to their location, poromas bleed easily, which is what usually prompts patients to seek care. Friable lesions can mimic acral melanoma.
Poromas occur most often in patients over 40 years of age and are evenly distributed among sexes and skin types. Cases have been associated with trauma, radiation, and chemotherapy. Although exceedingly rare, malignant transformation can occur in the form of eccrine porocarcinoma—a larger tumor that can grow rapidly and that has metastatic potential.
Treatment is optional—but desirable—when lesions are painful or bleed easily. Surgical excision is curative. Shave biopsy or curettage coupled with electrocautery of the base is also curative. Recurrence rates are very low with either method of treatment.
In this case, an excisional biopsy of the lateral nail fold was both diagnostic and curative (second image). The patient remained clear 9 months after treatment.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
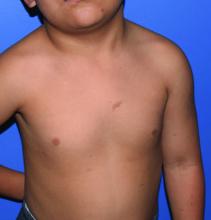
Tiny papules on trunk and genitals
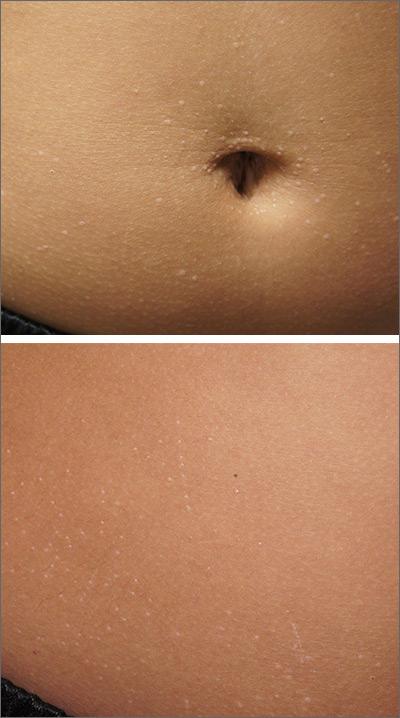
The miniscule papules arising suddenly on the trunk and genitals with linear arrays and clusters are clinically consistent with lichen nitidus, an uncommon eruption without a clear etiology.
Presentations may be focal or widespread and range from mildly itchy to asymptomatic. Children and young adults are most often affected. Linear arrays may appear in response to the trauma of scratching, which is termed the Koebner phenomenon. The differential diagnosis includes molluscum contagiosum, lichen planus, and lichen spinulosis. Usually these conditions can be distinguished clinically, but a biopsy would differentiate them, if needed. It’s worth noting, too, that lichen nitidus papules are monomorphic and lack the umbilication that is seen with molluscum contagiosum.
Cases of lichen nitidus clear up spontaneously, although usually months to years after diagnosis. Lichen nitidus is not contagious. Reassurance is, however, important as many patients may have experienced misdiagnosis and have concerns about sexual transmission because of the location of the papules on their genitals.
Treatment is often unnecessary. However, if itching is problematic, topical steroids and other topical antipruritics may be used. Topical hydrocortisone 2.5% cream or ointment for skin folds and genitals may be safely used, as well as topical triamcinolone 0.1% for the trunk and extremities. Pramoxine lotion (Sarna) is an over-the-counter nonsteroidal antipruritic. Oral nonsedating antihistamines can also be used as an adjunct.
This patient was reassured that the lesions were not contagious. Due to the itching, he was started on the pramoxine lotion twice daily, as needed, and the lesions cleared in about 6 months.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160.

The miniscule papules arising suddenly on the trunk and genitals with linear arrays and clusters are clinically consistent with lichen nitidus, an uncommon eruption without a clear etiology.
Presentations may be focal or widespread and range from mildly itchy to asymptomatic. Children and young adults are most often affected. Linear arrays may appear in response to the trauma of scratching, which is termed the Koebner phenomenon. The differential diagnosis includes molluscum contagiosum, lichen planus, and lichen spinulosis. Usually these conditions can be distinguished clinically, but a biopsy would differentiate them, if needed. It’s worth noting, too, that lichen nitidus papules are monomorphic and lack the umbilication that is seen with molluscum contagiosum.
Cases of lichen nitidus clear up spontaneously, although usually months to years after diagnosis. Lichen nitidus is not contagious. Reassurance is, however, important as many patients may have experienced misdiagnosis and have concerns about sexual transmission because of the location of the papules on their genitals.
Treatment is often unnecessary. However, if itching is problematic, topical steroids and other topical antipruritics may be used. Topical hydrocortisone 2.5% cream or ointment for skin folds and genitals may be safely used, as well as topical triamcinolone 0.1% for the trunk and extremities. Pramoxine lotion (Sarna) is an over-the-counter nonsteroidal antipruritic. Oral nonsedating antihistamines can also be used as an adjunct.
This patient was reassured that the lesions were not contagious. Due to the itching, he was started on the pramoxine lotion twice daily, as needed, and the lesions cleared in about 6 months.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

The miniscule papules arising suddenly on the trunk and genitals with linear arrays and clusters are clinically consistent with lichen nitidus, an uncommon eruption without a clear etiology.
Presentations may be focal or widespread and range from mildly itchy to asymptomatic. Children and young adults are most often affected. Linear arrays may appear in response to the trauma of scratching, which is termed the Koebner phenomenon. The differential diagnosis includes molluscum contagiosum, lichen planus, and lichen spinulosis. Usually these conditions can be distinguished clinically, but a biopsy would differentiate them, if needed. It’s worth noting, too, that lichen nitidus papules are monomorphic and lack the umbilication that is seen with molluscum contagiosum.
Cases of lichen nitidus clear up spontaneously, although usually months to years after diagnosis. Lichen nitidus is not contagious. Reassurance is, however, important as many patients may have experienced misdiagnosis and have concerns about sexual transmission because of the location of the papules on their genitals.
Treatment is often unnecessary. However, if itching is problematic, topical steroids and other topical antipruritics may be used. Topical hydrocortisone 2.5% cream or ointment for skin folds and genitals may be safely used, as well as topical triamcinolone 0.1% for the trunk and extremities. Pramoxine lotion (Sarna) is an over-the-counter nonsteroidal antipruritic. Oral nonsedating antihistamines can also be used as an adjunct.
This patient was reassured that the lesions were not contagious. Due to the itching, he was started on the pramoxine lotion twice daily, as needed, and the lesions cleared in about 6 months.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160.
Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160.
What happened to melanoma care during COVID-19 sequestration
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
REPORTING FROM THE CUTANEOUS MALIGNANCIES FORUM
How to assess and relieve that perplexing rashless itch
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.
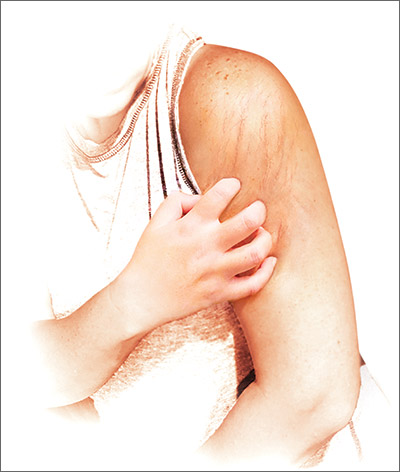
An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2
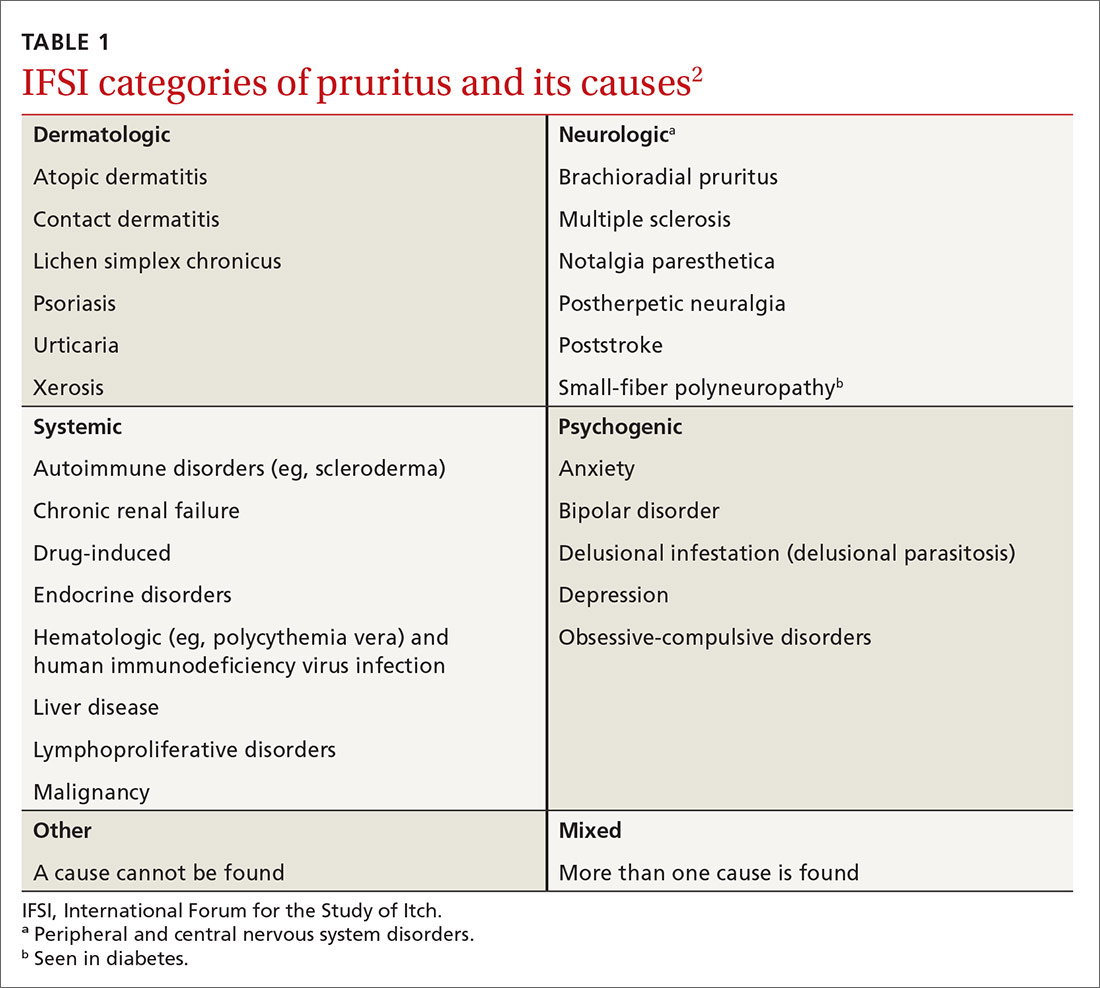
A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2
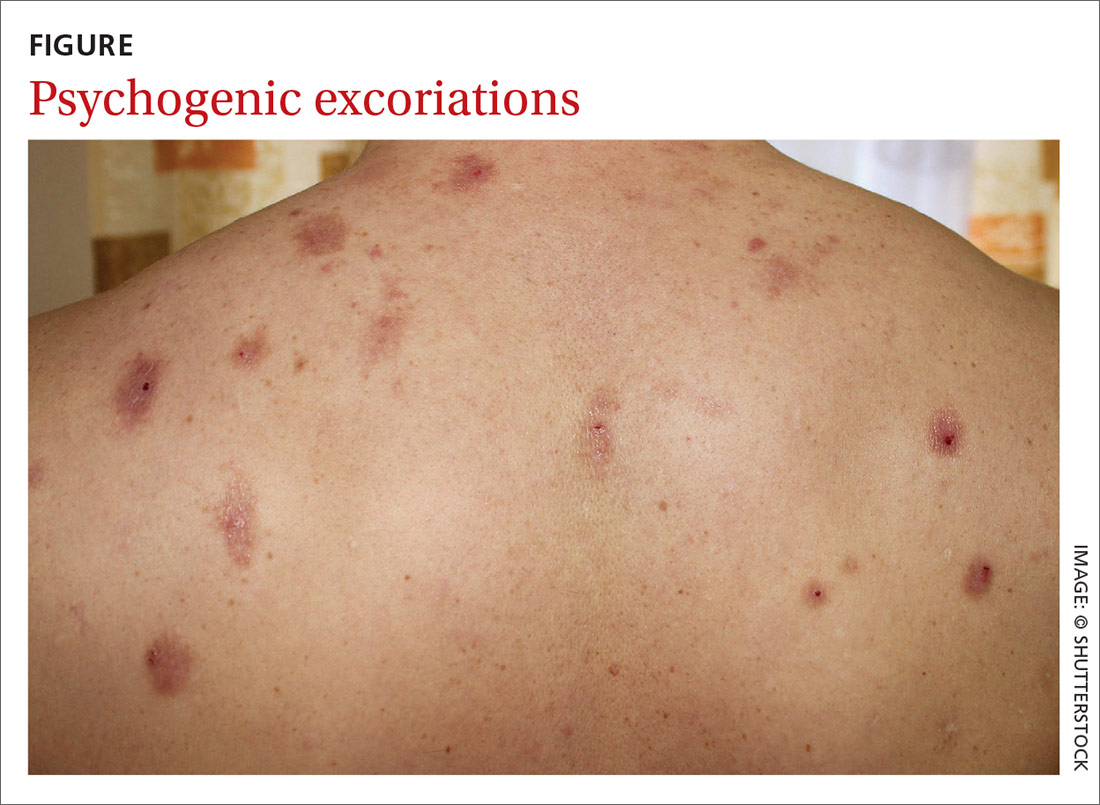
Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17
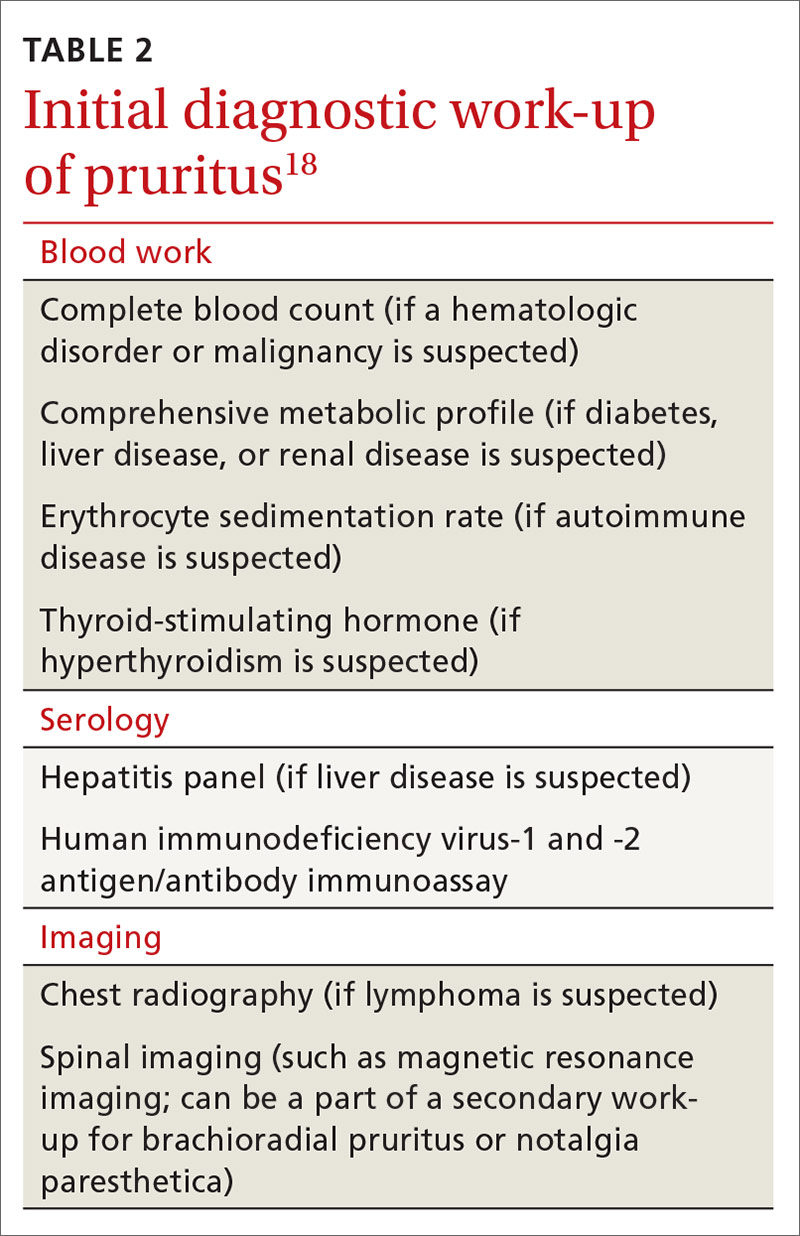
Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7
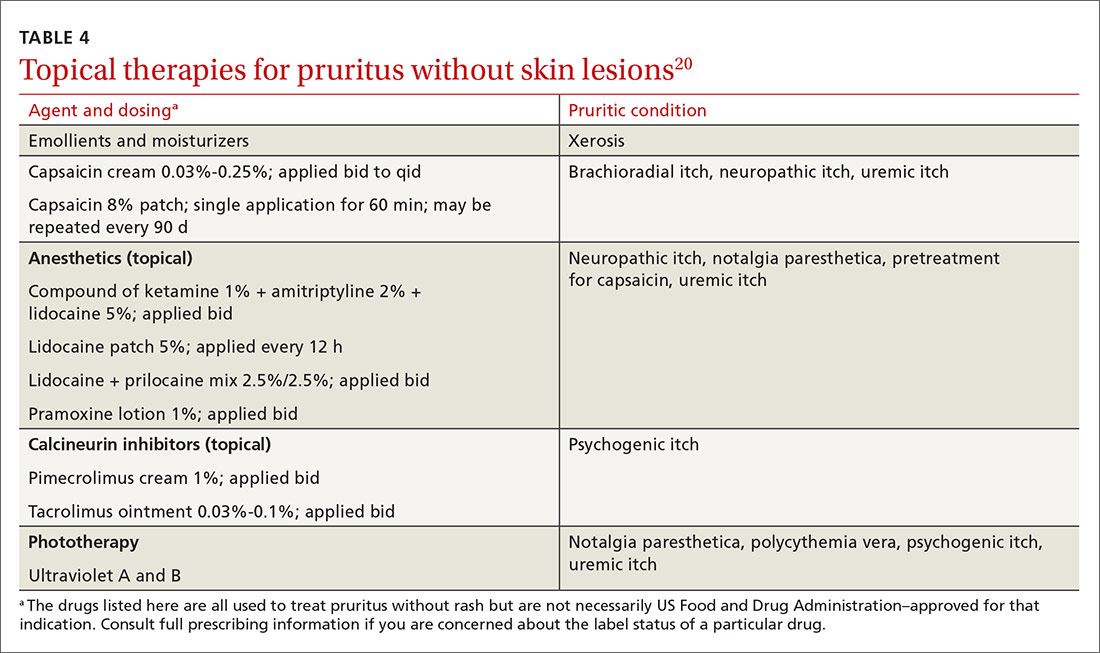
Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
PRACTICE RECOMMENDATIONS
› Undertake a diagnostic work-up for systemic causes of pruritus in patients who have a chronic, generalized itch and abnormal findings on physical examination. C
› Prescribe gabapentin for its effectiveness in treating pruritus caused by uremic and neurologic itch. B
› Consider prescribing one of the bile-acid sequestrants in patients with cholestatic pruritus because these agents can provide moderate relief of the symptom. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 4-year-old presented to our pediatric dermatology clinic for evaluation of asymptomatic "brown spots."
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.