User login
Expert spotlights recent advances in the medical treatment of acne
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
FROM MOA 2020
Study highlights differences between White and Latino patients with psoriasis
in the same studies, according to new data presented at the virtual Skin of Color Update 2020.
“Our findings demonstrate that, though White psoriasis patients may have higher severity in certain body regions such as the trunk, axilla, and groin areas, Latino psoriasis patients have a greater distribution of involvement, particularly in their upper limbs,” reported Alyssa G. Ashbaugh, a third-year medical student at the University of California, Irvine.
The study also found that psoriasis had a greater adverse impact on well-being, as measured with the Dermatology Life Quality Index (DLQI). At entry into the trials from which these patients were drawn, the higher DLQI score, significantly lower quality of life, was nearly two times higher (13.78 vs. 7.31; P = .01) among the Latino patients, compared with White patients.
This is not the first study to show a greater negative impact from psoriasis on Latinos than Whites, according to Ms. Ashbaugh. For example, Latinos had the worse quality of life at baseline by DLQI score than White, Asians, or Black participants in a trial of etanercept that enrolled more than 2000 patients.
In this retrospective chart review, patient characteristics were evaluated in all 21 Latino patients enrolled in psoriasis clinical trials at the University of California, Irvine, in a recent period. They were matched by age and gender to an equal number of White patients participating in the same trials.
The mean age at diagnosis of psoriasis was older in the Latino group than in the White population (42.4 vs. 35.6 years; P = .20), but the difference did not reach statistical significance. The proportion of patients with severe disease on investigator global assessment was also greater but not significantly different in the Latino group, compared with the White group, respectively (42.9% vs. 28.6%; P = .10).
However, differences in the patterns of disease did reach significance. This included a lower mean Psoriasis Assessment Severity Index score of the trunk, axilla, and groin in Latinos (4.74 vs. 9.73; P = .02). But compared with White participants, Latinos had a higher mean percentage of body surface area involvement in the upper limbs (4.78 vs. 1.85; P = .004) and a higher percentage of total body surface area involvement (20.50 vs. 10.03; P = .02).
“While White patients were found to have lived many more years with psoriasis, it is important for future studies to examine whether this is due to earlier onset or delayed diagnosis, given the fact that minorities are less likely to have access to a dermatologist,” reported Ms. Ashbaugh, who performed this work under the guidance of the senior author, Natasha Mesinkovska, MD, PhD, with the department of dermatology, University of California, Irvine.
Overall, the study suggested that body surface coverage and severity is not similarly distributed in Latinos relative to Whites. Although Ms. Ashbaugh conceded that the small sample size and retrospective design of this study are important limitations, she believes that her study, along with previously published studies that suggest psoriasis characteristics may differ meaningfully by race or ethnicity, raises issues that should be explored in future studies designed to confirm differences and whether those differences should affect management.
Other studies have suggested “there are notable differences in the presentation of psoriasis between racial and ethnic groups with the Latino population often presenting to physicians with more severe psoriasis and increased body surface area involvement,” Ms. Ashbaugh noted. Although this appears to be one of the first studies to examine psoriasis characteristics in Latinos relative to Whites, she believes this is an area ripe for further analysis.
Psoriasis “is not a rare occurrence” in non-White populations even if U.S. data suggest that the prevalence in “people of color is lower than that of psoriasis in the U.S. white population,” Amy McMichael, MD, chair of the department of dermatology, Wake Forest Baptist Medical Center, Winston-Salem, N.C., commented in an interview after the meeting. She agreed that it cannot be assumed that psoriasis in skin of color has the same manifestations or responds to treatment in the same way as in White patients.
“Studies have suggested that lesion thickness and, often, extent of disease can be worse in patients of color. Few studies to date have examined the efficacy of treatments and impact of disease in these populations,” she said.
One exception was a study Dr. McMichael and colleagues published last year on the efficacy and safety of the interleukin-17 receptor A antagonist brodalumab for psoriasis in patients of color. The study showed that Black, Latino, and Asian patients participating in the AMAGINE-2 and AMAGINE-3 trials achieved similar outcomes as White participants.
“We published this study because this is one of the first, if not the first, to have enough patients of color to actually draw conclusions about the efficacy of the biologic as well as the patient-reported outcomes,” she explained.
Like the author of the evaluation of Latino patients undertaken at the University of California, Irvine, Dr. McMichael said studies of psoriasis specific to patients of color are needed.
“We cannot assume all patients of color will have the same outcomes as their Caucasian counterparts. It is imperative to include those of color in future psoriasis treatment trials in order to determine the efficacy of new medications,” she added, specifically calling for collection of data on patient-reported outcomes.
Ms. Ashbaugh has no relevant financial relationships to disclose. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Johnson & Johnson, and Aclaris.
in the same studies, according to new data presented at the virtual Skin of Color Update 2020.
“Our findings demonstrate that, though White psoriasis patients may have higher severity in certain body regions such as the trunk, axilla, and groin areas, Latino psoriasis patients have a greater distribution of involvement, particularly in their upper limbs,” reported Alyssa G. Ashbaugh, a third-year medical student at the University of California, Irvine.
The study also found that psoriasis had a greater adverse impact on well-being, as measured with the Dermatology Life Quality Index (DLQI). At entry into the trials from which these patients were drawn, the higher DLQI score, significantly lower quality of life, was nearly two times higher (13.78 vs. 7.31; P = .01) among the Latino patients, compared with White patients.
This is not the first study to show a greater negative impact from psoriasis on Latinos than Whites, according to Ms. Ashbaugh. For example, Latinos had the worse quality of life at baseline by DLQI score than White, Asians, or Black participants in a trial of etanercept that enrolled more than 2000 patients.
In this retrospective chart review, patient characteristics were evaluated in all 21 Latino patients enrolled in psoriasis clinical trials at the University of California, Irvine, in a recent period. They were matched by age and gender to an equal number of White patients participating in the same trials.
The mean age at diagnosis of psoriasis was older in the Latino group than in the White population (42.4 vs. 35.6 years; P = .20), but the difference did not reach statistical significance. The proportion of patients with severe disease on investigator global assessment was also greater but not significantly different in the Latino group, compared with the White group, respectively (42.9% vs. 28.6%; P = .10).
However, differences in the patterns of disease did reach significance. This included a lower mean Psoriasis Assessment Severity Index score of the trunk, axilla, and groin in Latinos (4.74 vs. 9.73; P = .02). But compared with White participants, Latinos had a higher mean percentage of body surface area involvement in the upper limbs (4.78 vs. 1.85; P = .004) and a higher percentage of total body surface area involvement (20.50 vs. 10.03; P = .02).
“While White patients were found to have lived many more years with psoriasis, it is important for future studies to examine whether this is due to earlier onset or delayed diagnosis, given the fact that minorities are less likely to have access to a dermatologist,” reported Ms. Ashbaugh, who performed this work under the guidance of the senior author, Natasha Mesinkovska, MD, PhD, with the department of dermatology, University of California, Irvine.
Overall, the study suggested that body surface coverage and severity is not similarly distributed in Latinos relative to Whites. Although Ms. Ashbaugh conceded that the small sample size and retrospective design of this study are important limitations, she believes that her study, along with previously published studies that suggest psoriasis characteristics may differ meaningfully by race or ethnicity, raises issues that should be explored in future studies designed to confirm differences and whether those differences should affect management.
Other studies have suggested “there are notable differences in the presentation of psoriasis between racial and ethnic groups with the Latino population often presenting to physicians with more severe psoriasis and increased body surface area involvement,” Ms. Ashbaugh noted. Although this appears to be one of the first studies to examine psoriasis characteristics in Latinos relative to Whites, she believes this is an area ripe for further analysis.
Psoriasis “is not a rare occurrence” in non-White populations even if U.S. data suggest that the prevalence in “people of color is lower than that of psoriasis in the U.S. white population,” Amy McMichael, MD, chair of the department of dermatology, Wake Forest Baptist Medical Center, Winston-Salem, N.C., commented in an interview after the meeting. She agreed that it cannot be assumed that psoriasis in skin of color has the same manifestations or responds to treatment in the same way as in White patients.
“Studies have suggested that lesion thickness and, often, extent of disease can be worse in patients of color. Few studies to date have examined the efficacy of treatments and impact of disease in these populations,” she said.
One exception was a study Dr. McMichael and colleagues published last year on the efficacy and safety of the interleukin-17 receptor A antagonist brodalumab for psoriasis in patients of color. The study showed that Black, Latino, and Asian patients participating in the AMAGINE-2 and AMAGINE-3 trials achieved similar outcomes as White participants.
“We published this study because this is one of the first, if not the first, to have enough patients of color to actually draw conclusions about the efficacy of the biologic as well as the patient-reported outcomes,” she explained.
Like the author of the evaluation of Latino patients undertaken at the University of California, Irvine, Dr. McMichael said studies of psoriasis specific to patients of color are needed.
“We cannot assume all patients of color will have the same outcomes as their Caucasian counterparts. It is imperative to include those of color in future psoriasis treatment trials in order to determine the efficacy of new medications,” she added, specifically calling for collection of data on patient-reported outcomes.
Ms. Ashbaugh has no relevant financial relationships to disclose. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Johnson & Johnson, and Aclaris.
in the same studies, according to new data presented at the virtual Skin of Color Update 2020.
“Our findings demonstrate that, though White psoriasis patients may have higher severity in certain body regions such as the trunk, axilla, and groin areas, Latino psoriasis patients have a greater distribution of involvement, particularly in their upper limbs,” reported Alyssa G. Ashbaugh, a third-year medical student at the University of California, Irvine.
The study also found that psoriasis had a greater adverse impact on well-being, as measured with the Dermatology Life Quality Index (DLQI). At entry into the trials from which these patients were drawn, the higher DLQI score, significantly lower quality of life, was nearly two times higher (13.78 vs. 7.31; P = .01) among the Latino patients, compared with White patients.
This is not the first study to show a greater negative impact from psoriasis on Latinos than Whites, according to Ms. Ashbaugh. For example, Latinos had the worse quality of life at baseline by DLQI score than White, Asians, or Black participants in a trial of etanercept that enrolled more than 2000 patients.
In this retrospective chart review, patient characteristics were evaluated in all 21 Latino patients enrolled in psoriasis clinical trials at the University of California, Irvine, in a recent period. They were matched by age and gender to an equal number of White patients participating in the same trials.
The mean age at diagnosis of psoriasis was older in the Latino group than in the White population (42.4 vs. 35.6 years; P = .20), but the difference did not reach statistical significance. The proportion of patients with severe disease on investigator global assessment was also greater but not significantly different in the Latino group, compared with the White group, respectively (42.9% vs. 28.6%; P = .10).
However, differences in the patterns of disease did reach significance. This included a lower mean Psoriasis Assessment Severity Index score of the trunk, axilla, and groin in Latinos (4.74 vs. 9.73; P = .02). But compared with White participants, Latinos had a higher mean percentage of body surface area involvement in the upper limbs (4.78 vs. 1.85; P = .004) and a higher percentage of total body surface area involvement (20.50 vs. 10.03; P = .02).
“While White patients were found to have lived many more years with psoriasis, it is important for future studies to examine whether this is due to earlier onset or delayed diagnosis, given the fact that minorities are less likely to have access to a dermatologist,” reported Ms. Ashbaugh, who performed this work under the guidance of the senior author, Natasha Mesinkovska, MD, PhD, with the department of dermatology, University of California, Irvine.
Overall, the study suggested that body surface coverage and severity is not similarly distributed in Latinos relative to Whites. Although Ms. Ashbaugh conceded that the small sample size and retrospective design of this study are important limitations, she believes that her study, along with previously published studies that suggest psoriasis characteristics may differ meaningfully by race or ethnicity, raises issues that should be explored in future studies designed to confirm differences and whether those differences should affect management.
Other studies have suggested “there are notable differences in the presentation of psoriasis between racial and ethnic groups with the Latino population often presenting to physicians with more severe psoriasis and increased body surface area involvement,” Ms. Ashbaugh noted. Although this appears to be one of the first studies to examine psoriasis characteristics in Latinos relative to Whites, she believes this is an area ripe for further analysis.
Psoriasis “is not a rare occurrence” in non-White populations even if U.S. data suggest that the prevalence in “people of color is lower than that of psoriasis in the U.S. white population,” Amy McMichael, MD, chair of the department of dermatology, Wake Forest Baptist Medical Center, Winston-Salem, N.C., commented in an interview after the meeting. She agreed that it cannot be assumed that psoriasis in skin of color has the same manifestations or responds to treatment in the same way as in White patients.
“Studies have suggested that lesion thickness and, often, extent of disease can be worse in patients of color. Few studies to date have examined the efficacy of treatments and impact of disease in these populations,” she said.
One exception was a study Dr. McMichael and colleagues published last year on the efficacy and safety of the interleukin-17 receptor A antagonist brodalumab for psoriasis in patients of color. The study showed that Black, Latino, and Asian patients participating in the AMAGINE-2 and AMAGINE-3 trials achieved similar outcomes as White participants.
“We published this study because this is one of the first, if not the first, to have enough patients of color to actually draw conclusions about the efficacy of the biologic as well as the patient-reported outcomes,” she explained.
Like the author of the evaluation of Latino patients undertaken at the University of California, Irvine, Dr. McMichael said studies of psoriasis specific to patients of color are needed.
“We cannot assume all patients of color will have the same outcomes as their Caucasian counterparts. It is imperative to include those of color in future psoriasis treatment trials in order to determine the efficacy of new medications,” she added, specifically calling for collection of data on patient-reported outcomes.
Ms. Ashbaugh has no relevant financial relationships to disclose. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Johnson & Johnson, and Aclaris.
FROM SOC 2020
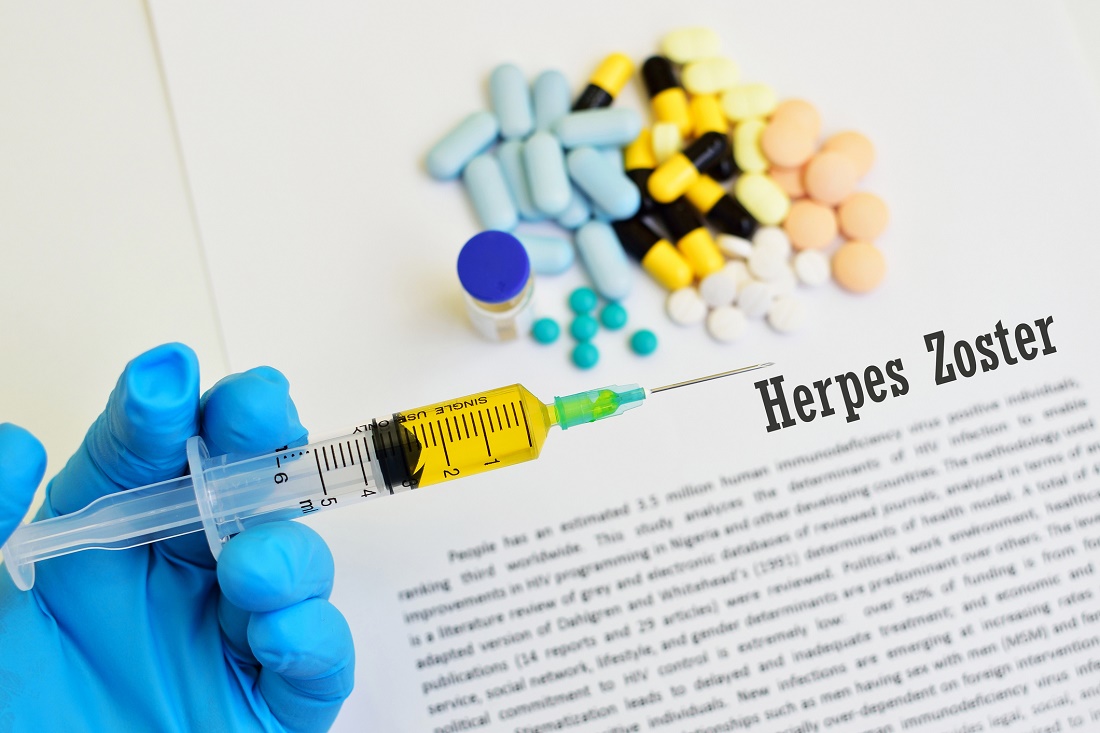
Shingrix effective in older adults with preexisting immune-mediated disorders
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
FROM RHEUMATOLOGY
Pregnancy studies on psoriasis, PsA medications pick up
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
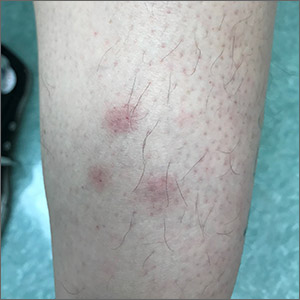
Recurrent leg lesions
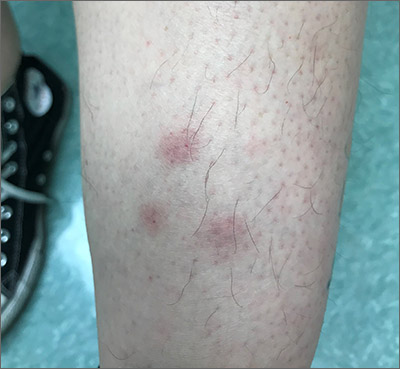
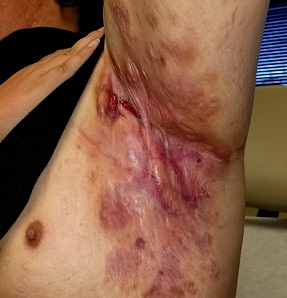
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Review finds evidence for beta-blockers for some rosacea symptoms
, while at the same time underscoring the paucity of evidence supporting their use, investigators reported.
“The evidence was highest for carvedilol and propranolol, two nonselective beta-blockers,” wrote the authors of the review, Jade G.M. Logger, MD, of the department of dermatology, Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. Their review is in the Journal of the American Academy of Dermatology.
The systematic review included a case control study of 53,927 patients and an equal number of controls that evaluated beta-blockers in general, but the remaining studies and case reports included only 106 patients in total. The largest was a prospective cohort study of propranolol in 63 patients. Other studies included a 15-patient randomized clinical trial of nadolol published 31 years ago and three single-patient case reports.
The studies included patients with a history of failed therapies; only a small number of beta-blockers were evaluated. Outcomes reported in the studies varied widely, which ruled out doing a meta-analysis. “Erythema and flushing were assessed by using a wide spectrum of mostly subjective clinical and patient-based scores, and method standardization was often missing,” the researchers stated.
“Most studies showed improved erythema and flushing after initiation of oral beta-blockers,” Dr. Logger and colleagues wrote. Treatment of facial erythema and flushing remains a clinical challenge despite approved therapies, for which poor response and reactivation are common. “Diminishing erythema and flushing in rosacea is challenging because it hardly responds to conventional anti-inflammatory treatment,” they noted.
“The study adds no new evidence to support the use of beta-blockers,” Diane M. Thiboutot, MD, professor of dermatology at Penn State University, Hershey, said in an interview. “As the authors point out, the nine studies reviewed were of low quality with a variety of outcome measures that precluded generation of a meta-analysis, which would have represented new information.”
Dr. Thiboutot is lead author of a 2019 update of management options for rosacea published by the National Rosacea Society Expert Committee last year.. Beta blockers are among the drugs that are sometimes prescribed off label to help rosacea-associated flushing, along with nonsteroidal anti-inflammatory drugs, antihistamines, and clonidine, according to the update.
Dr. Logger and coauthors noted that beta-blockers come with risks, and can aggravate asthma and psoriasis and are contraindicated in patients with heart failure, cardiogenic shock, and other cardiovascular diseases, along with hyperactive airway and Raynaud’s disease. “It is important to monitor patients for adverse effects, especially blood pressure and heart rate,” they stated. Carvedilol and propranolol may have more antioxidant and anti-inflammatory properties than other nonselective beta-blockers that may curtail rosacea manifestations, they wrote.
They called for large, prospective clinical trials to more accurately assess the efficacy of beta-blockers in rosacea patients. “Researchers should further focus on the determination of the optimal dosage, treatment duration, and long-term therapeutic effects for adequate treatment of erythema and flushing in rosacea,” they said.
Getting those trials is challenging, Dr. Thiboutot said. “Objective and even subjective measurement of transient and persistent facial erythema is extremely challenging, particularly in the setting of a prospective clinical trial.” The trials would have to control for a number of variables, including room conditions, patient diet, and timing of medication, and large trials require multiple sites,” which could add to the variability of the data,” she said in the interview. Funding such trials would be difficult because adding an indication for rosacea-related symptoms would have limited commercial potential, she added.
Nonetheless, the studies would be welcome, Dr. Thiboutot said. “If standardized outcome measures for facial erythema were to be developed, a study would be more feasible.”
Dr. Logger disclosed financial relationships with Galderma, AbbVie, Novartis, Janssen, and LEO Pharma; one author disclosed conducting clinical trials for AbbVie and Novartis; the third author disclosed relationships with Galderma, Cutanea Life Sciences, AbbVie, Novartis, and Janssen, with fees paid to his institution. Dr. Thiboutot disclosed a financial relationship with Galderma.
SOURCE: Logger JGM et al. J Am Acad Dermatol. 2020 Oct;83(4):1088-97.
, while at the same time underscoring the paucity of evidence supporting their use, investigators reported.
“The evidence was highest for carvedilol and propranolol, two nonselective beta-blockers,” wrote the authors of the review, Jade G.M. Logger, MD, of the department of dermatology, Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. Their review is in the Journal of the American Academy of Dermatology.
The systematic review included a case control study of 53,927 patients and an equal number of controls that evaluated beta-blockers in general, but the remaining studies and case reports included only 106 patients in total. The largest was a prospective cohort study of propranolol in 63 patients. Other studies included a 15-patient randomized clinical trial of nadolol published 31 years ago and three single-patient case reports.
The studies included patients with a history of failed therapies; only a small number of beta-blockers were evaluated. Outcomes reported in the studies varied widely, which ruled out doing a meta-analysis. “Erythema and flushing were assessed by using a wide spectrum of mostly subjective clinical and patient-based scores, and method standardization was often missing,” the researchers stated.
“Most studies showed improved erythema and flushing after initiation of oral beta-blockers,” Dr. Logger and colleagues wrote. Treatment of facial erythema and flushing remains a clinical challenge despite approved therapies, for which poor response and reactivation are common. “Diminishing erythema and flushing in rosacea is challenging because it hardly responds to conventional anti-inflammatory treatment,” they noted.
“The study adds no new evidence to support the use of beta-blockers,” Diane M. Thiboutot, MD, professor of dermatology at Penn State University, Hershey, said in an interview. “As the authors point out, the nine studies reviewed were of low quality with a variety of outcome measures that precluded generation of a meta-analysis, which would have represented new information.”
Dr. Thiboutot is lead author of a 2019 update of management options for rosacea published by the National Rosacea Society Expert Committee last year.. Beta blockers are among the drugs that are sometimes prescribed off label to help rosacea-associated flushing, along with nonsteroidal anti-inflammatory drugs, antihistamines, and clonidine, according to the update.
Dr. Logger and coauthors noted that beta-blockers come with risks, and can aggravate asthma and psoriasis and are contraindicated in patients with heart failure, cardiogenic shock, and other cardiovascular diseases, along with hyperactive airway and Raynaud’s disease. “It is important to monitor patients for adverse effects, especially blood pressure and heart rate,” they stated. Carvedilol and propranolol may have more antioxidant and anti-inflammatory properties than other nonselective beta-blockers that may curtail rosacea manifestations, they wrote.
They called for large, prospective clinical trials to more accurately assess the efficacy of beta-blockers in rosacea patients. “Researchers should further focus on the determination of the optimal dosage, treatment duration, and long-term therapeutic effects for adequate treatment of erythema and flushing in rosacea,” they said.
Getting those trials is challenging, Dr. Thiboutot said. “Objective and even subjective measurement of transient and persistent facial erythema is extremely challenging, particularly in the setting of a prospective clinical trial.” The trials would have to control for a number of variables, including room conditions, patient diet, and timing of medication, and large trials require multiple sites,” which could add to the variability of the data,” she said in the interview. Funding such trials would be difficult because adding an indication for rosacea-related symptoms would have limited commercial potential, she added.
Nonetheless, the studies would be welcome, Dr. Thiboutot said. “If standardized outcome measures for facial erythema were to be developed, a study would be more feasible.”
Dr. Logger disclosed financial relationships with Galderma, AbbVie, Novartis, Janssen, and LEO Pharma; one author disclosed conducting clinical trials for AbbVie and Novartis; the third author disclosed relationships with Galderma, Cutanea Life Sciences, AbbVie, Novartis, and Janssen, with fees paid to his institution. Dr. Thiboutot disclosed a financial relationship with Galderma.
SOURCE: Logger JGM et al. J Am Acad Dermatol. 2020 Oct;83(4):1088-97.
, while at the same time underscoring the paucity of evidence supporting their use, investigators reported.
“The evidence was highest for carvedilol and propranolol, two nonselective beta-blockers,” wrote the authors of the review, Jade G.M. Logger, MD, of the department of dermatology, Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. Their review is in the Journal of the American Academy of Dermatology.
The systematic review included a case control study of 53,927 patients and an equal number of controls that evaluated beta-blockers in general, but the remaining studies and case reports included only 106 patients in total. The largest was a prospective cohort study of propranolol in 63 patients. Other studies included a 15-patient randomized clinical trial of nadolol published 31 years ago and three single-patient case reports.
The studies included patients with a history of failed therapies; only a small number of beta-blockers were evaluated. Outcomes reported in the studies varied widely, which ruled out doing a meta-analysis. “Erythema and flushing were assessed by using a wide spectrum of mostly subjective clinical and patient-based scores, and method standardization was often missing,” the researchers stated.
“Most studies showed improved erythema and flushing after initiation of oral beta-blockers,” Dr. Logger and colleagues wrote. Treatment of facial erythema and flushing remains a clinical challenge despite approved therapies, for which poor response and reactivation are common. “Diminishing erythema and flushing in rosacea is challenging because it hardly responds to conventional anti-inflammatory treatment,” they noted.
“The study adds no new evidence to support the use of beta-blockers,” Diane M. Thiboutot, MD, professor of dermatology at Penn State University, Hershey, said in an interview. “As the authors point out, the nine studies reviewed were of low quality with a variety of outcome measures that precluded generation of a meta-analysis, which would have represented new information.”
Dr. Thiboutot is lead author of a 2019 update of management options for rosacea published by the National Rosacea Society Expert Committee last year.. Beta blockers are among the drugs that are sometimes prescribed off label to help rosacea-associated flushing, along with nonsteroidal anti-inflammatory drugs, antihistamines, and clonidine, according to the update.
Dr. Logger and coauthors noted that beta-blockers come with risks, and can aggravate asthma and psoriasis and are contraindicated in patients with heart failure, cardiogenic shock, and other cardiovascular diseases, along with hyperactive airway and Raynaud’s disease. “It is important to monitor patients for adverse effects, especially blood pressure and heart rate,” they stated. Carvedilol and propranolol may have more antioxidant and anti-inflammatory properties than other nonselective beta-blockers that may curtail rosacea manifestations, they wrote.
They called for large, prospective clinical trials to more accurately assess the efficacy of beta-blockers in rosacea patients. “Researchers should further focus on the determination of the optimal dosage, treatment duration, and long-term therapeutic effects for adequate treatment of erythema and flushing in rosacea,” they said.
Getting those trials is challenging, Dr. Thiboutot said. “Objective and even subjective measurement of transient and persistent facial erythema is extremely challenging, particularly in the setting of a prospective clinical trial.” The trials would have to control for a number of variables, including room conditions, patient diet, and timing of medication, and large trials require multiple sites,” which could add to the variability of the data,” she said in the interview. Funding such trials would be difficult because adding an indication for rosacea-related symptoms would have limited commercial potential, she added.
Nonetheless, the studies would be welcome, Dr. Thiboutot said. “If standardized outcome measures for facial erythema were to be developed, a study would be more feasible.”
Dr. Logger disclosed financial relationships with Galderma, AbbVie, Novartis, Janssen, and LEO Pharma; one author disclosed conducting clinical trials for AbbVie and Novartis; the third author disclosed relationships with Galderma, Cutanea Life Sciences, AbbVie, Novartis, and Janssen, with fees paid to his institution. Dr. Thiboutot disclosed a financial relationship with Galderma.
SOURCE: Logger JGM et al. J Am Acad Dermatol. 2020 Oct;83(4):1088-97.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Hair loss and scalp papules
The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.

The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
EMA panel backs baricitinib for moderate to severe atopic dermatitis
The
Baricitinib (Olumiant) is already approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis.
If approved in Europe, it will be the first Janus kinase (JAK) inhibitor and first oral medication indicated to treat patients with AD.
The CHMP’s positive opinion on baricitinib for AD was based on three phase 3, randomized, double-blind, placebo-controlled studies where the JAK inhibitor was used alone or in combination with topical treatments in adults with moderate to severe AD for whom topical treatments were insufficient or not tolerated. In all three studies, baricitinib was shown to be more effective than placebo in achieving skin that is “clear” or “almost clear” at 16 weeks.
“Patients living with AD face difficulties on a daily basis, and this CHMP opinion marks an important milestone in providing adult AD patients with a new potential treatment option,” Thomas Bieber, MD, PhD, professor of dermatology and allergy, University of Bonn (Germany), said in a company news release.
The most common side effects with baricitinib in clinical trials include increased LDL cholesterol, upper respiratory tract infections, and headache.
Patients receiving baricitinib, particularly in combination with immunosuppressants, are at risk of developing serious infections that may lead to hospitalization or death. If a serious infection develops, baricitinib should be stopped until the infection is controlled.
The CHMP’s positive opinion will be sent to the European Commission, which will adopt a final decision regarding an European Union–wide marketing authorization. Once granted, each member state will make decisions about price and reimbursement, taking into account the potential role/use of baricitinib in the context of that country’s national health system.
A version of this story originally appeared on Medscape.com.
The
Baricitinib (Olumiant) is already approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis.
If approved in Europe, it will be the first Janus kinase (JAK) inhibitor and first oral medication indicated to treat patients with AD.
The CHMP’s positive opinion on baricitinib for AD was based on three phase 3, randomized, double-blind, placebo-controlled studies where the JAK inhibitor was used alone or in combination with topical treatments in adults with moderate to severe AD for whom topical treatments were insufficient or not tolerated. In all three studies, baricitinib was shown to be more effective than placebo in achieving skin that is “clear” or “almost clear” at 16 weeks.
“Patients living with AD face difficulties on a daily basis, and this CHMP opinion marks an important milestone in providing adult AD patients with a new potential treatment option,” Thomas Bieber, MD, PhD, professor of dermatology and allergy, University of Bonn (Germany), said in a company news release.
The most common side effects with baricitinib in clinical trials include increased LDL cholesterol, upper respiratory tract infections, and headache.
Patients receiving baricitinib, particularly in combination with immunosuppressants, are at risk of developing serious infections that may lead to hospitalization or death. If a serious infection develops, baricitinib should be stopped until the infection is controlled.
The CHMP’s positive opinion will be sent to the European Commission, which will adopt a final decision regarding an European Union–wide marketing authorization. Once granted, each member state will make decisions about price and reimbursement, taking into account the potential role/use of baricitinib in the context of that country’s national health system.
A version of this story originally appeared on Medscape.com.
The
Baricitinib (Olumiant) is already approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis.
If approved in Europe, it will be the first Janus kinase (JAK) inhibitor and first oral medication indicated to treat patients with AD.
The CHMP’s positive opinion on baricitinib for AD was based on three phase 3, randomized, double-blind, placebo-controlled studies where the JAK inhibitor was used alone or in combination with topical treatments in adults with moderate to severe AD for whom topical treatments were insufficient or not tolerated. In all three studies, baricitinib was shown to be more effective than placebo in achieving skin that is “clear” or “almost clear” at 16 weeks.
“Patients living with AD face difficulties on a daily basis, and this CHMP opinion marks an important milestone in providing adult AD patients with a new potential treatment option,” Thomas Bieber, MD, PhD, professor of dermatology and allergy, University of Bonn (Germany), said in a company news release.
The most common side effects with baricitinib in clinical trials include increased LDL cholesterol, upper respiratory tract infections, and headache.
Patients receiving baricitinib, particularly in combination with immunosuppressants, are at risk of developing serious infections that may lead to hospitalization or death. If a serious infection develops, baricitinib should be stopped until the infection is controlled.
The CHMP’s positive opinion will be sent to the European Commission, which will adopt a final decision regarding an European Union–wide marketing authorization. Once granted, each member state will make decisions about price and reimbursement, taking into account the potential role/use of baricitinib in the context of that country’s national health system.
A version of this story originally appeared on Medscape.com.
Three-step approach may help relieve one of the itchiest vulvar conditions
A three-step approach may help relieve itch in patients with lichen simplex chronicus, “one of the itchiest conditions that we ever see on the vulva,” an expert advised at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
For some patients, such as those with excessive sweating or underlying psoriasis, seeing a dermatologist may be beneficial, physicians at the meeting suggested.
Treatment should aim to optimize epithelial barrier function, reduce inflammation, and stop scratching, Lynette Margesson, MD, said in a lecture at the biennial meeting, which is held by the International Society for the Study of Vulvovaginal Disease (ISSVD). “With this condition, please look always for more than one problem.”
said Dr. Margesson, an obstetrician and gynecologist at Geisel School of Medicine at Dartmouth in Hanover, N.H. “It is because of chronic rubbing and scratching on top of something else.”
It may develop on top of atopic dermatitis, psoriasis, or contact dermatitis, as well as infection, lichen sclerosus, lichen planus, or neoplasia.
Lichen simplex chronicus is characterized by years of relentless itching, and patients may wake up at night scratching. The skin looks and feels leathery, and the condition can be localized or around the entire vulva. Heat, humidity, stress, and irritants may exacerbate the condition.
Patients often try to wash the rash away with scrubbers and cleansers, which only makes it worse, Dr. Margesson said.
To get patients better, improve barrier function, such as by controlling infections, reducing sweating, avoiding irritants, and stopping excessive hygiene. Immediate therapy may include soaks, cool compresses, and ointments.
A superpotent steroid taper (e.g., clobetasol 0.05% ointment), a prednisone taper, or intramuscular triamcinolone may reduce inflammation. Dr. Margesson usually uses clobetasol, although this treatment or halobetasol can burn if patients have open skin. In such cases, she uses prednisone or intramuscular triamcinolone.
Sedating medications may help patients stop scratching, especially at night. Hydroxyzine, doxepin, or amitriptyline 2-3 hours before bedtime can help. Scratching can be a form of obsessive-compulsive disorder, and a small dose of citalopram may help during the day. Patients with significant psychological factors can be difficult to manage and tend to relapse easily, Dr. Margesson said.
If lichen simplex chronicus recurs, test for infections and allergies. “Maybe they need a mild corticosteroid all the time, like 2.5% hydrocortisone to alternate with your superpotent steroid so you can use it longer without thinning the skin,” she suggested.
Although Dr. Margesson does not often treat hyperhidrosis, addressing excessive sweating can make a big difference for patients, she said.
If a gynecologist identifies a patient who may benefit from treatment of hyperhidrosis but has limited experience with medications for this condition, it might make sense to work with a dermatologist, Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., suggested during a panel discussion. Most dermatologists treat hyperhidrosis regularly, she said.
Dermatologists also may help treat patients with psoriasis who need systemic medication, Dr. Margesson said.
“In terms of ... doing the lab monitoring and knowing what side effects to look out for, your colleagues who use these medicines more are going to be more comfortable with that,” Dr. Venkatesan said. They also may have more experience navigating insurance denials to obtain a therapy. “Don’t think you are passing the buck to someone else. Sometimes that is the right thing to do, to get that help from someone else.”
Dr. Margesson is an author for UpToDate. Dr. Venkatesan had no conflicts of interest.
A three-step approach may help relieve itch in patients with lichen simplex chronicus, “one of the itchiest conditions that we ever see on the vulva,” an expert advised at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
For some patients, such as those with excessive sweating or underlying psoriasis, seeing a dermatologist may be beneficial, physicians at the meeting suggested.
Treatment should aim to optimize epithelial barrier function, reduce inflammation, and stop scratching, Lynette Margesson, MD, said in a lecture at the biennial meeting, which is held by the International Society for the Study of Vulvovaginal Disease (ISSVD). “With this condition, please look always for more than one problem.”
said Dr. Margesson, an obstetrician and gynecologist at Geisel School of Medicine at Dartmouth in Hanover, N.H. “It is because of chronic rubbing and scratching on top of something else.”
It may develop on top of atopic dermatitis, psoriasis, or contact dermatitis, as well as infection, lichen sclerosus, lichen planus, or neoplasia.
Lichen simplex chronicus is characterized by years of relentless itching, and patients may wake up at night scratching. The skin looks and feels leathery, and the condition can be localized or around the entire vulva. Heat, humidity, stress, and irritants may exacerbate the condition.
Patients often try to wash the rash away with scrubbers and cleansers, which only makes it worse, Dr. Margesson said.
To get patients better, improve barrier function, such as by controlling infections, reducing sweating, avoiding irritants, and stopping excessive hygiene. Immediate therapy may include soaks, cool compresses, and ointments.
A superpotent steroid taper (e.g., clobetasol 0.05% ointment), a prednisone taper, or intramuscular triamcinolone may reduce inflammation. Dr. Margesson usually uses clobetasol, although this treatment or halobetasol can burn if patients have open skin. In such cases, she uses prednisone or intramuscular triamcinolone.
Sedating medications may help patients stop scratching, especially at night. Hydroxyzine, doxepin, or amitriptyline 2-3 hours before bedtime can help. Scratching can be a form of obsessive-compulsive disorder, and a small dose of citalopram may help during the day. Patients with significant psychological factors can be difficult to manage and tend to relapse easily, Dr. Margesson said.
If lichen simplex chronicus recurs, test for infections and allergies. “Maybe they need a mild corticosteroid all the time, like 2.5% hydrocortisone to alternate with your superpotent steroid so you can use it longer without thinning the skin,” she suggested.
Although Dr. Margesson does not often treat hyperhidrosis, addressing excessive sweating can make a big difference for patients, she said.
If a gynecologist identifies a patient who may benefit from treatment of hyperhidrosis but has limited experience with medications for this condition, it might make sense to work with a dermatologist, Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., suggested during a panel discussion. Most dermatologists treat hyperhidrosis regularly, she said.
Dermatologists also may help treat patients with psoriasis who need systemic medication, Dr. Margesson said.
“In terms of ... doing the lab monitoring and knowing what side effects to look out for, your colleagues who use these medicines more are going to be more comfortable with that,” Dr. Venkatesan said. They also may have more experience navigating insurance denials to obtain a therapy. “Don’t think you are passing the buck to someone else. Sometimes that is the right thing to do, to get that help from someone else.”
Dr. Margesson is an author for UpToDate. Dr. Venkatesan had no conflicts of interest.
A three-step approach may help relieve itch in patients with lichen simplex chronicus, “one of the itchiest conditions that we ever see on the vulva,” an expert advised at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
For some patients, such as those with excessive sweating or underlying psoriasis, seeing a dermatologist may be beneficial, physicians at the meeting suggested.
Treatment should aim to optimize epithelial barrier function, reduce inflammation, and stop scratching, Lynette Margesson, MD, said in a lecture at the biennial meeting, which is held by the International Society for the Study of Vulvovaginal Disease (ISSVD). “With this condition, please look always for more than one problem.”
said Dr. Margesson, an obstetrician and gynecologist at Geisel School of Medicine at Dartmouth in Hanover, N.H. “It is because of chronic rubbing and scratching on top of something else.”
It may develop on top of atopic dermatitis, psoriasis, or contact dermatitis, as well as infection, lichen sclerosus, lichen planus, or neoplasia.
Lichen simplex chronicus is characterized by years of relentless itching, and patients may wake up at night scratching. The skin looks and feels leathery, and the condition can be localized or around the entire vulva. Heat, humidity, stress, and irritants may exacerbate the condition.
Patients often try to wash the rash away with scrubbers and cleansers, which only makes it worse, Dr. Margesson said.
To get patients better, improve barrier function, such as by controlling infections, reducing sweating, avoiding irritants, and stopping excessive hygiene. Immediate therapy may include soaks, cool compresses, and ointments.
A superpotent steroid taper (e.g., clobetasol 0.05% ointment), a prednisone taper, or intramuscular triamcinolone may reduce inflammation. Dr. Margesson usually uses clobetasol, although this treatment or halobetasol can burn if patients have open skin. In such cases, she uses prednisone or intramuscular triamcinolone.
Sedating medications may help patients stop scratching, especially at night. Hydroxyzine, doxepin, or amitriptyline 2-3 hours before bedtime can help. Scratching can be a form of obsessive-compulsive disorder, and a small dose of citalopram may help during the day. Patients with significant psychological factors can be difficult to manage and tend to relapse easily, Dr. Margesson said.
If lichen simplex chronicus recurs, test for infections and allergies. “Maybe they need a mild corticosteroid all the time, like 2.5% hydrocortisone to alternate with your superpotent steroid so you can use it longer without thinning the skin,” she suggested.
Although Dr. Margesson does not often treat hyperhidrosis, addressing excessive sweating can make a big difference for patients, she said.
If a gynecologist identifies a patient who may benefit from treatment of hyperhidrosis but has limited experience with medications for this condition, it might make sense to work with a dermatologist, Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., suggested during a panel discussion. Most dermatologists treat hyperhidrosis regularly, she said.
Dermatologists also may help treat patients with psoriasis who need systemic medication, Dr. Margesson said.
“In terms of ... doing the lab monitoring and knowing what side effects to look out for, your colleagues who use these medicines more are going to be more comfortable with that,” Dr. Venkatesan said. They also may have more experience navigating insurance denials to obtain a therapy. “Don’t think you are passing the buck to someone else. Sometimes that is the right thing to do, to get that help from someone else.”
Dr. Margesson is an author for UpToDate. Dr. Venkatesan had no conflicts of interest.
FROM ISSVD BIENNIAL CONFERENCE
What a Slow Boil Got Him
ANSWER
The correct answer is hidradenitis suppurativa (HS; choice “c”).
DISCUSSION
HS (also known as acne inversa) is a relatively common condition first described in 1839 by French physician Frederick Velpeau, who thought it was probably a form of acne. Because HS only affects intertriginous areas (groin, axillae, inframammary, intergluteal, and abdominal folds rich in apocrine glands), during the next 130 years, it was presumed that this disease was solely caused by malfunctioning apocrine glands.
Though experts disagree about the true etiology of HS, most agree that it is caused by several environmental and genetic factors leading to plugged or malfunctioning hair follicles and apocrine glands that become inflamed, swollen, painful, and purulent. In severe cases, tracts develop between lesions, resulting in the formation of ropy, linear hypertrophic scarring. Early on and in milder cases, comedones can manifest—often in multiples—along with widely scattered cysts. It appears that a case can be made for a genetic predisposition to develop HS because at least one-third of affected patients report a positive family history.
Females (usually postpubescent) are affected at 3 times the rate of males. Hormones, especially androgens, appear to play a role. This assertion is bolstered not only by the age of onset, but also because HS has been observed to improve after pregnancy and after menopause.
Though not causative, smoking and obesity have a strong statistical correlation with HS as exacerbating factors. Many patients with HS find movement from exercise too painful, which may contribute to obesity.
Because HS has a wide range of clinical presentations, the Hurley Staging scale can help clinicians distinguish the condition’s severity by stages I, II, or III. Stage I manifests with scant signs of disease, including comedones and sparsely separated small cysts. Stage II includes numerous painful cysts with tract formation between lesions. Stage III involves symptoms described in the other stages as well as multiple large, painful, draining cysts; widespread erythema; and extensive hypertrophic scarring.
Regarding the differential, the culture would have identified community-acquired methicillin-resistant Staphylococcus aureus (choice “a”) or flesh-eating bacterial infection (choice “d”), but neither would be as chronic as the patient’s disease. Acne conglobata (choice “b”) manifests with chronic dense acne largely confined to the trunk. Interestingly, acne conglobata, HS, dissecting cellulitis of the scalp, and pilonidal cyst are collectively known as the follicular occlusion tetrad.
TREATMENT
Treatment for HS is limited. Adalimumab is the most recent and promising biologic, though its use comes at a high cost ($60,000/y) and with an adverse effect profile that should give any prescriber pause. Also, the best one could hope for in using adalimumab is about a 30% improvement in the patient.
Before using a biologic, other options should at least be considered. Though not entirely satisfactory, there are oral antibiotics (minocycline, trimethoprim/sulfa, or a combination of rifampin and clindamycin) or intralesional steroids. Incision and drainage of individual lesions and isotretinoin therapy can temporarily relieve pain, but they are not effective as long-term therapies. Large-scale surgical extirpation of the affected tissue can be considered in extreme cases, but there can be recurrences and associated morbidity (eg, chronic lymphedema in the affected arm, extreme scarring, and postoperative pain).
ANSWER
The correct answer is hidradenitis suppurativa (HS; choice “c”).
DISCUSSION
HS (also known as acne inversa) is a relatively common condition first described in 1839 by French physician Frederick Velpeau, who thought it was probably a form of acne. Because HS only affects intertriginous areas (groin, axillae, inframammary, intergluteal, and abdominal folds rich in apocrine glands), during the next 130 years, it was presumed that this disease was solely caused by malfunctioning apocrine glands.
Though experts disagree about the true etiology of HS, most agree that it is caused by several environmental and genetic factors leading to plugged or malfunctioning hair follicles and apocrine glands that become inflamed, swollen, painful, and purulent. In severe cases, tracts develop between lesions, resulting in the formation of ropy, linear hypertrophic scarring. Early on and in milder cases, comedones can manifest—often in multiples—along with widely scattered cysts. It appears that a case can be made for a genetic predisposition to develop HS because at least one-third of affected patients report a positive family history.
Females (usually postpubescent) are affected at 3 times the rate of males. Hormones, especially androgens, appear to play a role. This assertion is bolstered not only by the age of onset, but also because HS has been observed to improve after pregnancy and after menopause.
Though not causative, smoking and obesity have a strong statistical correlation with HS as exacerbating factors. Many patients with HS find movement from exercise too painful, which may contribute to obesity.
Because HS has a wide range of clinical presentations, the Hurley Staging scale can help clinicians distinguish the condition’s severity by stages I, II, or III. Stage I manifests with scant signs of disease, including comedones and sparsely separated small cysts. Stage II includes numerous painful cysts with tract formation between lesions. Stage III involves symptoms described in the other stages as well as multiple large, painful, draining cysts; widespread erythema; and extensive hypertrophic scarring.
Regarding the differential, the culture would have identified community-acquired methicillin-resistant Staphylococcus aureus (choice “a”) or flesh-eating bacterial infection (choice “d”), but neither would be as chronic as the patient’s disease. Acne conglobata (choice “b”) manifests with chronic dense acne largely confined to the trunk. Interestingly, acne conglobata, HS, dissecting cellulitis of the scalp, and pilonidal cyst are collectively known as the follicular occlusion tetrad.
TREATMENT
Treatment for HS is limited. Adalimumab is the most recent and promising biologic, though its use comes at a high cost ($60,000/y) and with an adverse effect profile that should give any prescriber pause. Also, the best one could hope for in using adalimumab is about a 30% improvement in the patient.
Before using a biologic, other options should at least be considered. Though not entirely satisfactory, there are oral antibiotics (minocycline, trimethoprim/sulfa, or a combination of rifampin and clindamycin) or intralesional steroids. Incision and drainage of individual lesions and isotretinoin therapy can temporarily relieve pain, but they are not effective as long-term therapies. Large-scale surgical extirpation of the affected tissue can be considered in extreme cases, but there can be recurrences and associated morbidity (eg, chronic lymphedema in the affected arm, extreme scarring, and postoperative pain).
ANSWER
The correct answer is hidradenitis suppurativa (HS; choice “c”).
DISCUSSION
HS (also known as acne inversa) is a relatively common condition first described in 1839 by French physician Frederick Velpeau, who thought it was probably a form of acne. Because HS only affects intertriginous areas (groin, axillae, inframammary, intergluteal, and abdominal folds rich in apocrine glands), during the next 130 years, it was presumed that this disease was solely caused by malfunctioning apocrine glands.
Though experts disagree about the true etiology of HS, most agree that it is caused by several environmental and genetic factors leading to plugged or malfunctioning hair follicles and apocrine glands that become inflamed, swollen, painful, and purulent. In severe cases, tracts develop between lesions, resulting in the formation of ropy, linear hypertrophic scarring. Early on and in milder cases, comedones can manifest—often in multiples—along with widely scattered cysts. It appears that a case can be made for a genetic predisposition to develop HS because at least one-third of affected patients report a positive family history.
Females (usually postpubescent) are affected at 3 times the rate of males. Hormones, especially androgens, appear to play a role. This assertion is bolstered not only by the age of onset, but also because HS has been observed to improve after pregnancy and after menopause.
Though not causative, smoking and obesity have a strong statistical correlation with HS as exacerbating factors. Many patients with HS find movement from exercise too painful, which may contribute to obesity.
Because HS has a wide range of clinical presentations, the Hurley Staging scale can help clinicians distinguish the condition’s severity by stages I, II, or III. Stage I manifests with scant signs of disease, including comedones and sparsely separated small cysts. Stage II includes numerous painful cysts with tract formation between lesions. Stage III involves symptoms described in the other stages as well as multiple large, painful, draining cysts; widespread erythema; and extensive hypertrophic scarring.
Regarding the differential, the culture would have identified community-acquired methicillin-resistant Staphylococcus aureus (choice “a”) or flesh-eating bacterial infection (choice “d”), but neither would be as chronic as the patient’s disease. Acne conglobata (choice “b”) manifests with chronic dense acne largely confined to the trunk. Interestingly, acne conglobata, HS, dissecting cellulitis of the scalp, and pilonidal cyst are collectively known as the follicular occlusion tetrad.
TREATMENT
Treatment for HS is limited. Adalimumab is the most recent and promising biologic, though its use comes at a high cost ($60,000/y) and with an adverse effect profile that should give any prescriber pause. Also, the best one could hope for in using adalimumab is about a 30% improvement in the patient.
Before using a biologic, other options should at least be considered. Though not entirely satisfactory, there are oral antibiotics (minocycline, trimethoprim/sulfa, or a combination of rifampin and clindamycin) or intralesional steroids. Incision and drainage of individual lesions and isotretinoin therapy can temporarily relieve pain, but they are not effective as long-term therapies. Large-scale surgical extirpation of the affected tissue can be considered in extreme cases, but there can be recurrences and associated morbidity (eg, chronic lymphedema in the affected arm, extreme scarring, and postoperative pain).
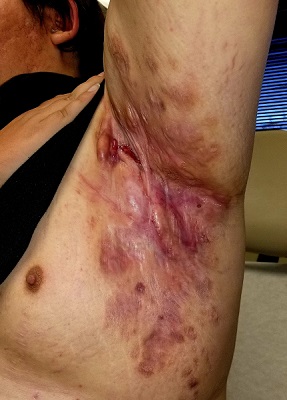
Boils first manifested on this 25-year-old man’s axillae when he was 13 (at the onset of puberty). As he grew older, they appeared in other intertriginous areas, including the groin and between the buttocks. As he became increasingly overweight, the folds under his breasts and abdomen became affected.
Over the years, several primary care providers consistently diagnosed him with either boils or staph infection. They also would reliably admonish him regarding his weight and lack of cleanliness. Treatment included various oral antibiotics that would only calm the condition for a short time. At no point was he referred to dermatology. Family history revealed similar skin problems in his mother and 1 sister.
Recently, his lesions have grown more numerous and painful. They drain pustular fluid, which makes it difficult for him to function in classrooms or at work. Even walking is excruciatingly painful. As a result, he is more sedentary. He also has started smoking.
On examination, the patient is 5 ft 8 in and grossly obese (weight, 300 lb). His affect is decidedly morose. His gait is ponderous and halting. His type 4 skin is swarthy, sweaty, and oily.
All intertriginous areas are similarly affected with large, ropy keloidal and hypertrophic scars, as well as diffuse bright red erythema and edema. There are dozens of single and multiple comedones, along with multiple draining sinuses.
A bacterial culture is collected from the fluid draining from the lesions. The results show a mixed, normal, cutaneous flora—including staph epidermidis—with no predominant organism.