User login
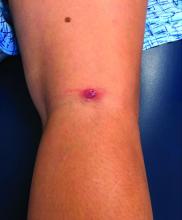
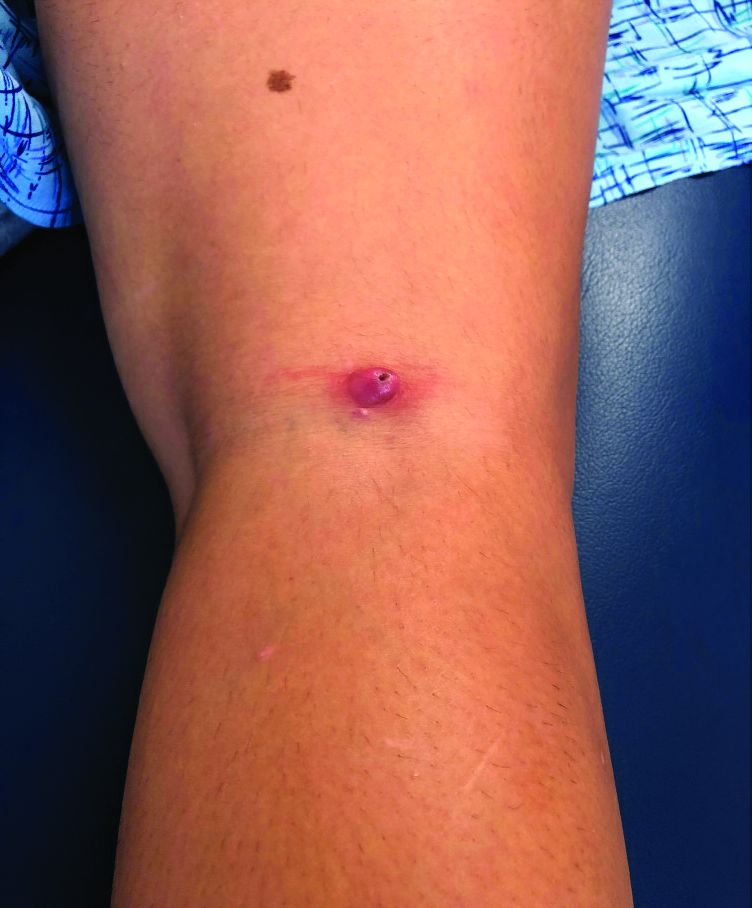
A teen girl presents with a pinkish-red bump on her right leg
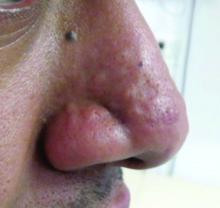
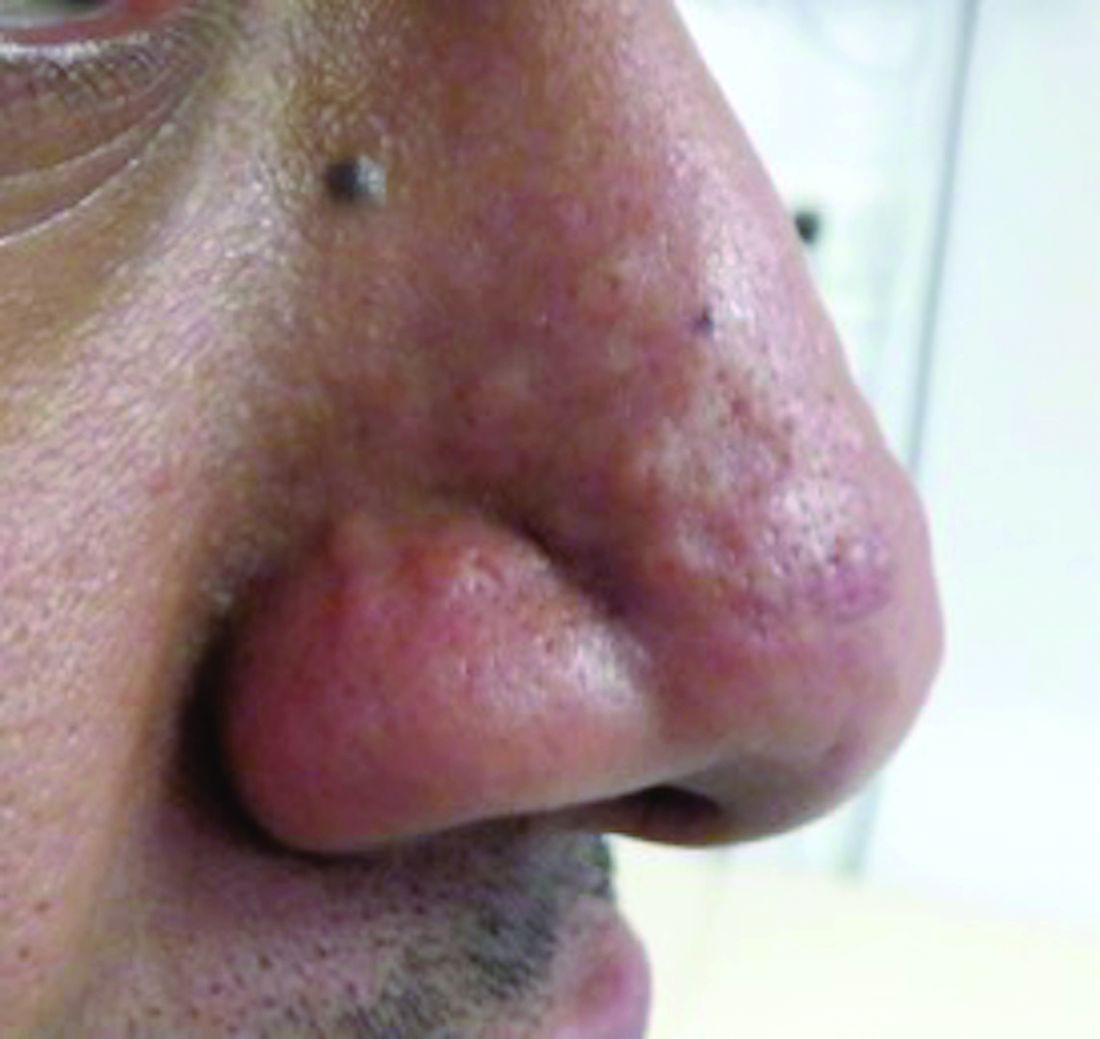
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
Biologics for psoriasis may also reduce coronary plaque
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
FROM CIRCULATION: CARDIOVASCULAR IMAGING
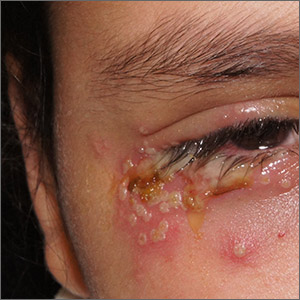
Painful periocular rash
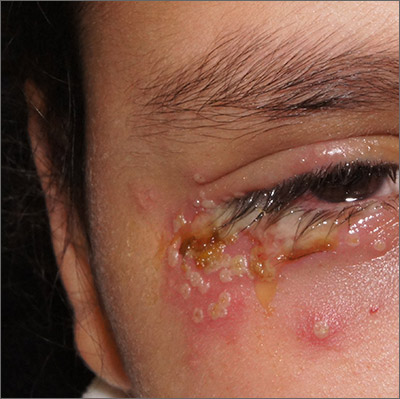
This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.

This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
COVID-19 outcomes no worse in patients on TNF inhibitors or methotrexate
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
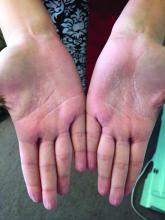
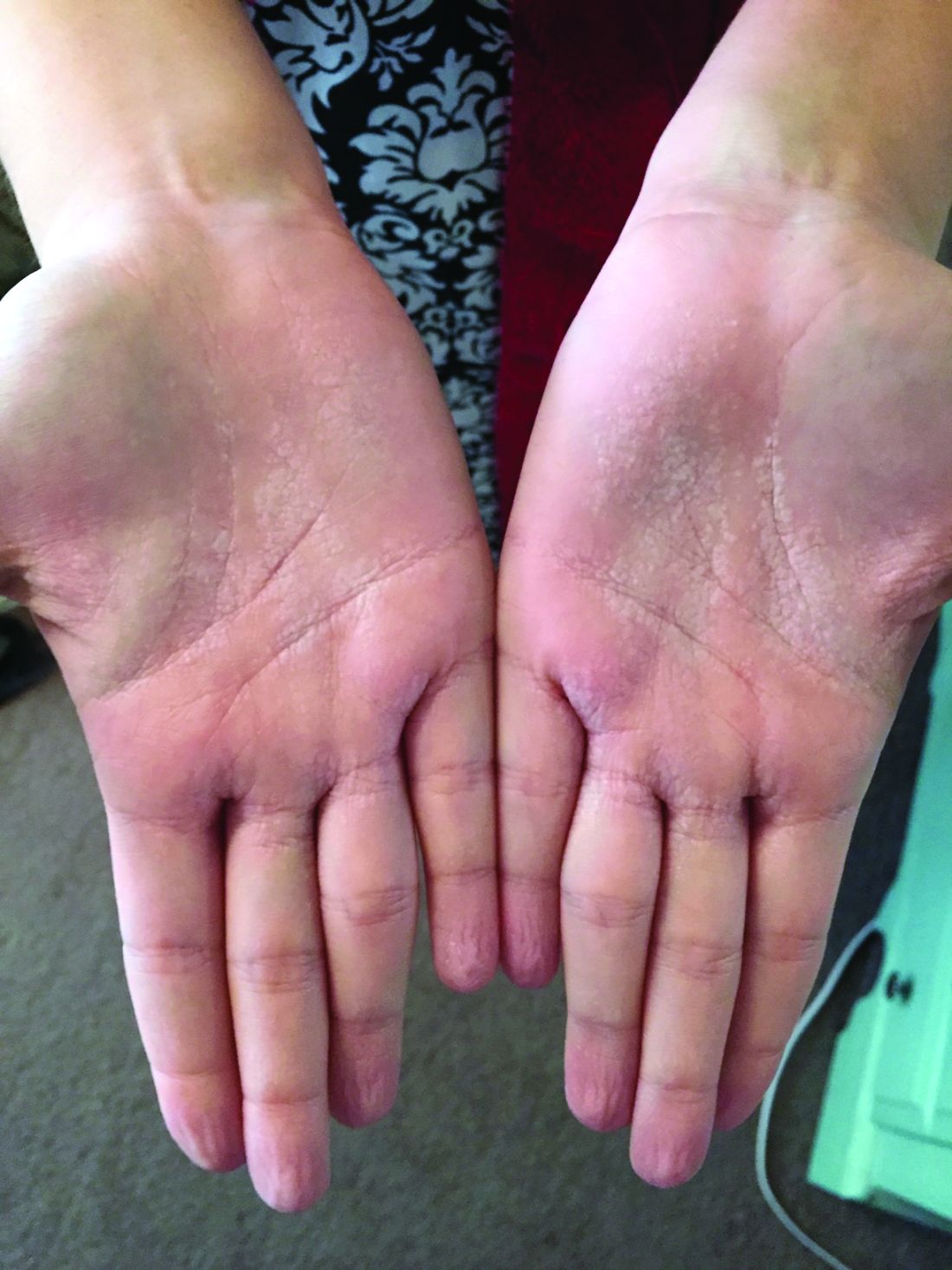
A woman with an asymptomatic eruption on her palms after exposure to water
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
Rash, muscle weakness, and confusion
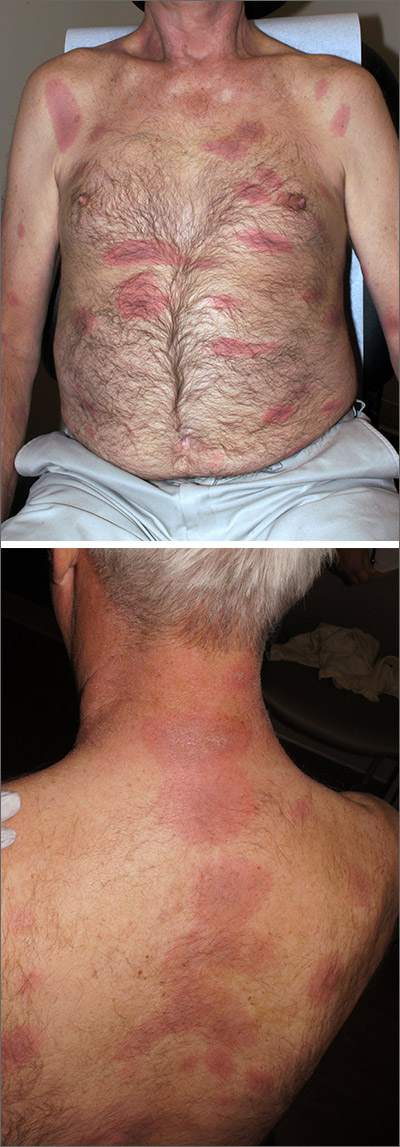
The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.

The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
Chronic abdominal pain and diarrhea
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
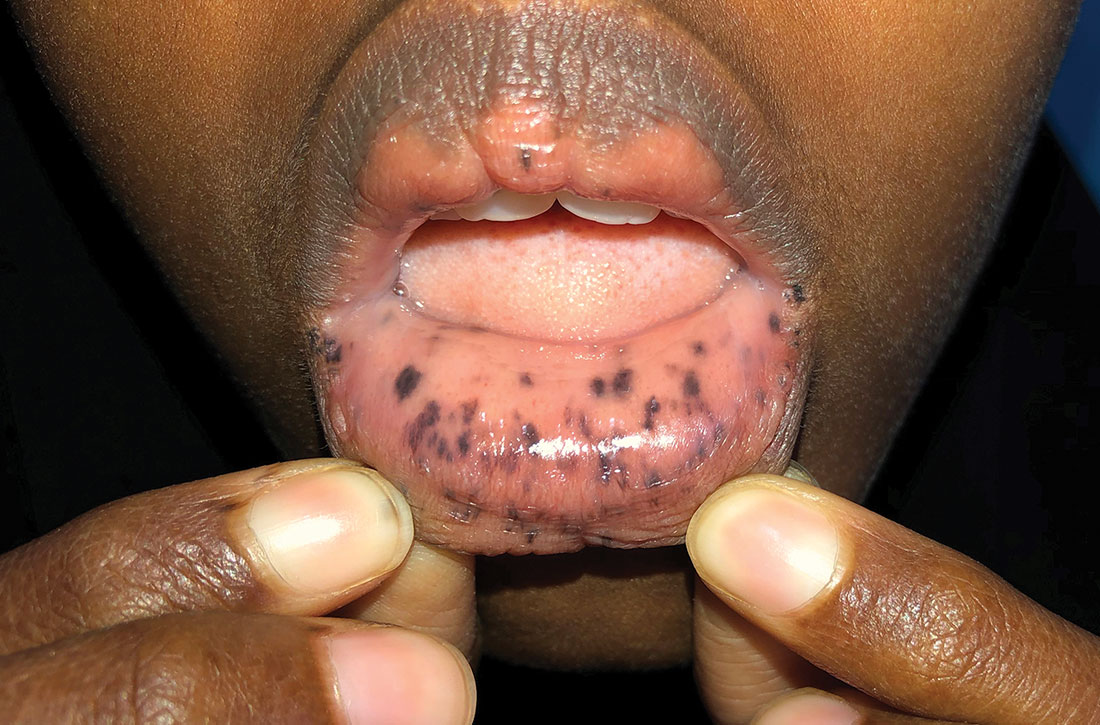
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
45-year-old man • fever • generalized rash • recent history of calcaneal osteomyelitis • Dx?
THE CASE
A 45-year-old man was admitted to the hospital with a fever and generalized rash. For the previous 2 weeks, he had been treated at a skilled nursing facility with IV vancomycin and cefepime for left calcaneal osteomyelitis. He reported that the rash was pruritic and started 2 days prior to hospital admission.
His past medical history was significant for type 2 diabetes mellitus and polysubstance drug abuse. Medical and travel history were otherwise unremarkable. The patient was taking the following medications at the time of presentation: hydrocodone-acetaminophen, cyclobenzaprine, melatonin, and metformin.
Initial vital signs included a temperature of 102.9°F; respiratory rate, 22 breaths/min; heart rate, 97 beats/min; and blood pressure, 89/50 mm Hg. Physical exam was notable for left anterior cervical and axillary lymphadenopathy. The patient had no facial edema, but he did have a diffuse, morbilliform rash on his bilateral upper and lower extremities, encompassing about 54% of his body surface area (FIGURE 1).
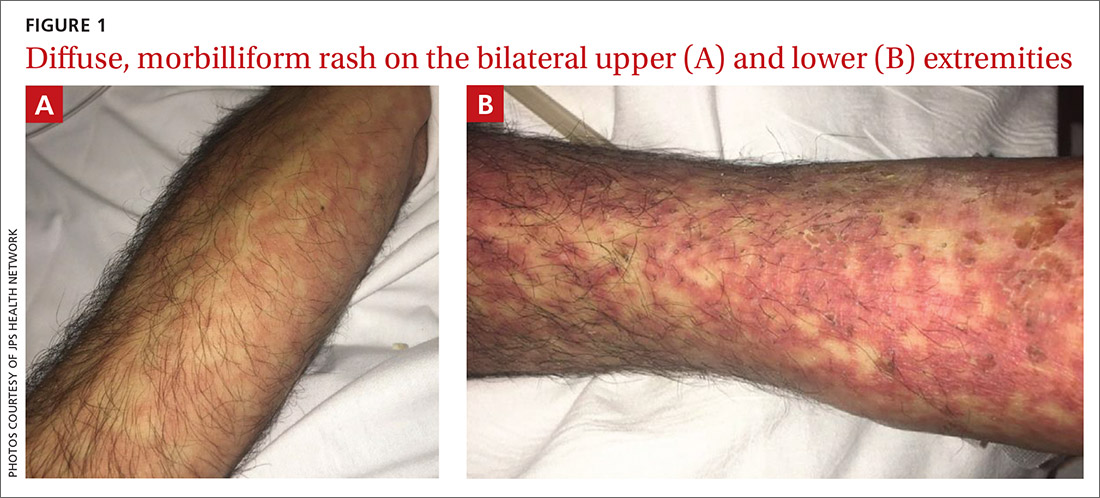
Laboratory studies revealed a white blood cell count of 4.7/mcL, with 3.4% eosinophils and 10.9% monocytes; an erythrocyte sedimentation rate of 60 mm/h; and a C-reactive protein level of 1 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated (AST: 95 U/L [normal range, 8 - 48 U/L]; ALT: 115 U/L [normal range: 7 - 55 U/L]). A chest x-ray was obtained and showed new lung infiltrates (FIGURE 2).

Linezolid and meropenem were initiated for a presumed health care–associated pneumonia, and a sepsis work-up was initiated.
THE DIAGNOSIS
The patient’s rash and pruritus worsened after meropenem was introduced. A hepatitis panel was nonreactive except for prior hepatitis A exposure. Ultrasound of the liver and spleen was normal. Investigation of pneumonia pathogens including Legionella, Streptococcus, Mycoplasma, and Chlamydia psittaci did not reveal any causative agents. A skin biopsy revealed perivascular neutrophilic dermatitis with dyskeratosis.
The patient was diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome based on his fever, worsening morbilliform rash, lymphadenopathy, and elevated liver transaminase levels. Although he did not have marked eosinophilia, atypical lymphocytes were present. Serologies for human herpesvirus (HHV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were all unremarkable.
Continue to: During discussions...
During discussions with an infectious disease specialist, it was concluded that the patient’s DRESS syndrome was likely secondary to beta-lactam antibiotics. The patient had been receiving cefepime prior to hospitalization. Meropenem was discontinued and aztreonam was started, with continued linezolid. This patient did not have a reactivation of a herpesvirus (HHV-6, HHV-7, EBV, or CMV), which has been previously reported in cases of DRESS syndrome.
DISCUSSION
DRESS syndrome is a challenging diagnosis to make due to the multiplicity of presenting symptoms. Skin rash, lymphadenopathy, hepatic involvement, and hypereosinophilia are characteristic findings.1 Accurate diagnosis reduces fatal disease outcomes, which are estimated to occur in 5%-10% of cases.1,2
Causative agents. DRESS syndrome typically occurs 2 to 6 weeks after the introduction of the causative agent, commonly an aromatic anticonvulsant or antibiotic.3 The incidence of DRESS syndrome in patients using carbamazepine and phenytoin is estimated to be 1 to 5 per 10,000 patients. The incidence of DRESS syndrome in patients using antibiotics is unknown. Frequently, the inducing antibiotic is a beta-lactam, as in this case.4,5
The pathogenesis of DRESS syndrome is not well understood, although there appears to be an immune-mediated reaction that occurs in certain patients after viral reactivation, particularly with herpesviruses. In vitro studies have demonstrated that the culprit drug is able to induce viral reactivation leading to T-lymphocyte response and systemic inflammation, which occurs in multiple organs.6,7 Reported long-term sequelae of DRESS syndrome include immune-mediated diseases such as thyroiditis and type 1 diabetes. In addition, it is hypothesized that there is a genetic predisposition involving human leukocyte antigens that increases the likelihood that individuals will develop DRESS syndrome.5,8
Diagnosis. The
Continue to: Treatment
Treatment is aimed at stopping the causative agent and starting moderate- to high-dose systemic corticosteroids (from 0.5 to 2 mg/kg/d). If symptoms continue to progress, cyclosporine can be used. N-acetylcysteine may also be beneficial due to its ability to neutralize drug metabolites that can stimulate T-cell response.7 There has not been sufficient evidence to suggest that antiviral medication should be initiated.1,7
Our patient was treated with 2 mg/kg/d of prednisone, along with triamcinolone cream, diphenhydramine, and N-acetylcysteine. His rash improved dramatically during his hospital stay and at the subsequent 1-month follow-up was completely resolved.
THE TAKEAWAY
DRESS syndrome should be suspected in patients presenting with fever, rash, lymphadenopathy, pulmonary infiltrates, and liver involvement after initiation of drugs commonly associated with this syndrome. Our case reinforces previous clinical evidence that beta-lactam antibiotics are a common cause of DRESS syndrome; patients taking these medications should be closely monitored. Cross-reactions are frequent, and it is imperative that patients avoid related drugs to prevent recurrence. Although glucocorticoids are the mainstay of treatment, further studies are needed to assess the benefits of N-acetylcysteine.
CORRESPONDENCE
W. Jacob Cobb, MD, JPS Health Network, 1500 South Main Street, Fort Worth, TX, 76104; [email protected]
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Chen Y, Chiu H, Chu C. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373-1379.
3. Jeung Y-J, Lee J-Y, Oh M-J, et al. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and Stevens-Johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126.
4. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations [commentary]. Br J Dermatol. 2006;156:1083-1084.
5. Ben-Said B, Arnaud-Butel S, Rozières A, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta lactam antibiotics. J Dermatol Sci. 2015;80:71-74.
6. Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31.
7. Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir—a hypothesis. Med Sci Monit. 2012;18:CS57-CS62.
8. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898.
9. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
10. Bernard L, Eichenfield L. Drug-associated rashes. In: Zaoutis L, Chiang V, eds. Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Elsevier; 2010: 1005-1011.
11. Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol. 2011;77:3-6.
THE CASE
A 45-year-old man was admitted to the hospital with a fever and generalized rash. For the previous 2 weeks, he had been treated at a skilled nursing facility with IV vancomycin and cefepime for left calcaneal osteomyelitis. He reported that the rash was pruritic and started 2 days prior to hospital admission.
His past medical history was significant for type 2 diabetes mellitus and polysubstance drug abuse. Medical and travel history were otherwise unremarkable. The patient was taking the following medications at the time of presentation: hydrocodone-acetaminophen, cyclobenzaprine, melatonin, and metformin.
Initial vital signs included a temperature of 102.9°F; respiratory rate, 22 breaths/min; heart rate, 97 beats/min; and blood pressure, 89/50 mm Hg. Physical exam was notable for left anterior cervical and axillary lymphadenopathy. The patient had no facial edema, but he did have a diffuse, morbilliform rash on his bilateral upper and lower extremities, encompassing about 54% of his body surface area (FIGURE 1).

Laboratory studies revealed a white blood cell count of 4.7/mcL, with 3.4% eosinophils and 10.9% monocytes; an erythrocyte sedimentation rate of 60 mm/h; and a C-reactive protein level of 1 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated (AST: 95 U/L [normal range, 8 - 48 U/L]; ALT: 115 U/L [normal range: 7 - 55 U/L]). A chest x-ray was obtained and showed new lung infiltrates (FIGURE 2).

Linezolid and meropenem were initiated for a presumed health care–associated pneumonia, and a sepsis work-up was initiated.
THE DIAGNOSIS
The patient’s rash and pruritus worsened after meropenem was introduced. A hepatitis panel was nonreactive except for prior hepatitis A exposure. Ultrasound of the liver and spleen was normal. Investigation of pneumonia pathogens including Legionella, Streptococcus, Mycoplasma, and Chlamydia psittaci did not reveal any causative agents. A skin biopsy revealed perivascular neutrophilic dermatitis with dyskeratosis.
The patient was diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome based on his fever, worsening morbilliform rash, lymphadenopathy, and elevated liver transaminase levels. Although he did not have marked eosinophilia, atypical lymphocytes were present. Serologies for human herpesvirus (HHV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were all unremarkable.
Continue to: During discussions...
During discussions with an infectious disease specialist, it was concluded that the patient’s DRESS syndrome was likely secondary to beta-lactam antibiotics. The patient had been receiving cefepime prior to hospitalization. Meropenem was discontinued and aztreonam was started, with continued linezolid. This patient did not have a reactivation of a herpesvirus (HHV-6, HHV-7, EBV, or CMV), which has been previously reported in cases of DRESS syndrome.
DISCUSSION
DRESS syndrome is a challenging diagnosis to make due to the multiplicity of presenting symptoms. Skin rash, lymphadenopathy, hepatic involvement, and hypereosinophilia are characteristic findings.1 Accurate diagnosis reduces fatal disease outcomes, which are estimated to occur in 5%-10% of cases.1,2
Causative agents. DRESS syndrome typically occurs 2 to 6 weeks after the introduction of the causative agent, commonly an aromatic anticonvulsant or antibiotic.3 The incidence of DRESS syndrome in patients using carbamazepine and phenytoin is estimated to be 1 to 5 per 10,000 patients. The incidence of DRESS syndrome in patients using antibiotics is unknown. Frequently, the inducing antibiotic is a beta-lactam, as in this case.4,5
The pathogenesis of DRESS syndrome is not well understood, although there appears to be an immune-mediated reaction that occurs in certain patients after viral reactivation, particularly with herpesviruses. In vitro studies have demonstrated that the culprit drug is able to induce viral reactivation leading to T-lymphocyte response and systemic inflammation, which occurs in multiple organs.6,7 Reported long-term sequelae of DRESS syndrome include immune-mediated diseases such as thyroiditis and type 1 diabetes. In addition, it is hypothesized that there is a genetic predisposition involving human leukocyte antigens that increases the likelihood that individuals will develop DRESS syndrome.5,8
Diagnosis. The

Continue to: Treatment
Treatment is aimed at stopping the causative agent and starting moderate- to high-dose systemic corticosteroids (from 0.5 to 2 mg/kg/d). If symptoms continue to progress, cyclosporine can be used. N-acetylcysteine may also be beneficial due to its ability to neutralize drug metabolites that can stimulate T-cell response.7 There has not been sufficient evidence to suggest that antiviral medication should be initiated.1,7
Our patient was treated with 2 mg/kg/d of prednisone, along with triamcinolone cream, diphenhydramine, and N-acetylcysteine. His rash improved dramatically during his hospital stay and at the subsequent 1-month follow-up was completely resolved.
THE TAKEAWAY
DRESS syndrome should be suspected in patients presenting with fever, rash, lymphadenopathy, pulmonary infiltrates, and liver involvement after initiation of drugs commonly associated with this syndrome. Our case reinforces previous clinical evidence that beta-lactam antibiotics are a common cause of DRESS syndrome; patients taking these medications should be closely monitored. Cross-reactions are frequent, and it is imperative that patients avoid related drugs to prevent recurrence. Although glucocorticoids are the mainstay of treatment, further studies are needed to assess the benefits of N-acetylcysteine.
CORRESPONDENCE
W. Jacob Cobb, MD, JPS Health Network, 1500 South Main Street, Fort Worth, TX, 76104; [email protected]
THE CASE
A 45-year-old man was admitted to the hospital with a fever and generalized rash. For the previous 2 weeks, he had been treated at a skilled nursing facility with IV vancomycin and cefepime for left calcaneal osteomyelitis. He reported that the rash was pruritic and started 2 days prior to hospital admission.
His past medical history was significant for type 2 diabetes mellitus and polysubstance drug abuse. Medical and travel history were otherwise unremarkable. The patient was taking the following medications at the time of presentation: hydrocodone-acetaminophen, cyclobenzaprine, melatonin, and metformin.
Initial vital signs included a temperature of 102.9°F; respiratory rate, 22 breaths/min; heart rate, 97 beats/min; and blood pressure, 89/50 mm Hg. Physical exam was notable for left anterior cervical and axillary lymphadenopathy. The patient had no facial edema, but he did have a diffuse, morbilliform rash on his bilateral upper and lower extremities, encompassing about 54% of his body surface area (FIGURE 1).

Laboratory studies revealed a white blood cell count of 4.7/mcL, with 3.4% eosinophils and 10.9% monocytes; an erythrocyte sedimentation rate of 60 mm/h; and a C-reactive protein level of 1 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated (AST: 95 U/L [normal range, 8 - 48 U/L]; ALT: 115 U/L [normal range: 7 - 55 U/L]). A chest x-ray was obtained and showed new lung infiltrates (FIGURE 2).

Linezolid and meropenem were initiated for a presumed health care–associated pneumonia, and a sepsis work-up was initiated.
THE DIAGNOSIS
The patient’s rash and pruritus worsened after meropenem was introduced. A hepatitis panel was nonreactive except for prior hepatitis A exposure. Ultrasound of the liver and spleen was normal. Investigation of pneumonia pathogens including Legionella, Streptococcus, Mycoplasma, and Chlamydia psittaci did not reveal any causative agents. A skin biopsy revealed perivascular neutrophilic dermatitis with dyskeratosis.
The patient was diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome based on his fever, worsening morbilliform rash, lymphadenopathy, and elevated liver transaminase levels. Although he did not have marked eosinophilia, atypical lymphocytes were present. Serologies for human herpesvirus (HHV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were all unremarkable.
Continue to: During discussions...
During discussions with an infectious disease specialist, it was concluded that the patient’s DRESS syndrome was likely secondary to beta-lactam antibiotics. The patient had been receiving cefepime prior to hospitalization. Meropenem was discontinued and aztreonam was started, with continued linezolid. This patient did not have a reactivation of a herpesvirus (HHV-6, HHV-7, EBV, or CMV), which has been previously reported in cases of DRESS syndrome.
DISCUSSION
DRESS syndrome is a challenging diagnosis to make due to the multiplicity of presenting symptoms. Skin rash, lymphadenopathy, hepatic involvement, and hypereosinophilia are characteristic findings.1 Accurate diagnosis reduces fatal disease outcomes, which are estimated to occur in 5%-10% of cases.1,2
Causative agents. DRESS syndrome typically occurs 2 to 6 weeks after the introduction of the causative agent, commonly an aromatic anticonvulsant or antibiotic.3 The incidence of DRESS syndrome in patients using carbamazepine and phenytoin is estimated to be 1 to 5 per 10,000 patients. The incidence of DRESS syndrome in patients using antibiotics is unknown. Frequently, the inducing antibiotic is a beta-lactam, as in this case.4,5
The pathogenesis of DRESS syndrome is not well understood, although there appears to be an immune-mediated reaction that occurs in certain patients after viral reactivation, particularly with herpesviruses. In vitro studies have demonstrated that the culprit drug is able to induce viral reactivation leading to T-lymphocyte response and systemic inflammation, which occurs in multiple organs.6,7 Reported long-term sequelae of DRESS syndrome include immune-mediated diseases such as thyroiditis and type 1 diabetes. In addition, it is hypothesized that there is a genetic predisposition involving human leukocyte antigens that increases the likelihood that individuals will develop DRESS syndrome.5,8
Diagnosis. The

Continue to: Treatment
Treatment is aimed at stopping the causative agent and starting moderate- to high-dose systemic corticosteroids (from 0.5 to 2 mg/kg/d). If symptoms continue to progress, cyclosporine can be used. N-acetylcysteine may also be beneficial due to its ability to neutralize drug metabolites that can stimulate T-cell response.7 There has not been sufficient evidence to suggest that antiviral medication should be initiated.1,7
Our patient was treated with 2 mg/kg/d of prednisone, along with triamcinolone cream, diphenhydramine, and N-acetylcysteine. His rash improved dramatically during his hospital stay and at the subsequent 1-month follow-up was completely resolved.
THE TAKEAWAY
DRESS syndrome should be suspected in patients presenting with fever, rash, lymphadenopathy, pulmonary infiltrates, and liver involvement after initiation of drugs commonly associated with this syndrome. Our case reinforces previous clinical evidence that beta-lactam antibiotics are a common cause of DRESS syndrome; patients taking these medications should be closely monitored. Cross-reactions are frequent, and it is imperative that patients avoid related drugs to prevent recurrence. Although glucocorticoids are the mainstay of treatment, further studies are needed to assess the benefits of N-acetylcysteine.
CORRESPONDENCE
W. Jacob Cobb, MD, JPS Health Network, 1500 South Main Street, Fort Worth, TX, 76104; [email protected]
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Chen Y, Chiu H, Chu C. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373-1379.
3. Jeung Y-J, Lee J-Y, Oh M-J, et al. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and Stevens-Johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126.
4. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations [commentary]. Br J Dermatol. 2006;156:1083-1084.
5. Ben-Said B, Arnaud-Butel S, Rozières A, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta lactam antibiotics. J Dermatol Sci. 2015;80:71-74.
6. Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31.
7. Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir—a hypothesis. Med Sci Monit. 2012;18:CS57-CS62.
8. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898.
9. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
10. Bernard L, Eichenfield L. Drug-associated rashes. In: Zaoutis L, Chiang V, eds. Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Elsevier; 2010: 1005-1011.
11. Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol. 2011;77:3-6.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Chen Y, Chiu H, Chu C. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373-1379.
3. Jeung Y-J, Lee J-Y, Oh M-J, et al. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and Stevens-Johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126.
4. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations [commentary]. Br J Dermatol. 2006;156:1083-1084.
5. Ben-Said B, Arnaud-Butel S, Rozières A, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta lactam antibiotics. J Dermatol Sci. 2015;80:71-74.
6. Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31.
7. Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir—a hypothesis. Med Sci Monit. 2012;18:CS57-CS62.
8. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898.
9. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
10. Bernard L, Eichenfield L. Drug-associated rashes. In: Zaoutis L, Chiang V, eds. Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Elsevier; 2010: 1005-1011.
11. Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol. 2011;77:3-6.
Post-acne nasal papules described in a series of patients
, but researchers believe the condition could be an underrecognized problem, affecting patients with skin of color in particular, according to the authors of a case series published in Pediatric Dermatology.
Jorge Roman, MD, and coauthors in the department of dermatology at New York (N.Y.) University identified 20 patients with a history of acne who had nasal papules, in a retrospective review of electronic medical records at NYU over 1 year (April 2018 to April 2019). The presentation ranged from “a few, small skin-colored papules to large, dome-shaped papulonodules, to more extensive rhinophymatous-like” changes with some patients having papular lesions on the chin in addition to the nose, they wrote in the report.
These papules greatly resembled angiofibromas, but appear to be a sequela of acne, according to the authors. In five patients who had biopsies, the results showed “a dome-shaped proliferation of spindle and stellate-shaped cells with thickened collagen bundles and dilated thin-walled blood vessels,” the authors wrote. “The histopathological findings of these nasal papules were indistinguishable from those of a conventional angiofibroma.”
In addition, the patients did not have evidence of underlying genetic conditions that could explain the angiofibroma-like lesions. “Although acne has not previously been implicated in the development of angiofibromas, based on the data available for our patients, it seems extremely unlikely that the lesions would be related to anything else,” Dr. Roman, a dermatology resident at New York University, said in an interview.
He said he first recognized the nasal papules in clinic as a first-year resident, but was surprised to find a lack of information on the condition. “Dermatology has a name for just about every skin disease imaginable, so I found it very odd when I couldn’t find much describing this condition,” he said. “There was a large disparity between what we were seeing in clinic and what was reported in the literature.”
Nearly all the patients were Hispanic (17 of 20) and adolescent males (17 patients), with a median age of 16 years at the time of presentation. There were two Black patients and one Asian patient. Race and ethnicity were not mentioned in two previous reports describing papular acne scarring, but Dr. Roman and colleagues noted that in their clinic, the condition appeared to affect adolescent patients with skin of color predominantly.
Reasons why nasal papules may be underreported are unclear, Dr. Roman noted. One possible explanation is lower use of dermatologic care among patients with skin of color. “Interestingly, previous research has shown that racial minorities are lower utilizers of dermatologic care. It is possible that the patient demographic most afflicted by this condition face significant barriers when seeking care,” he said.
Due to a low level of awareness of acne-related nasal papules, “clinicians may not recognize it as an acne-related scarring process. This is significant, as early recognition and treatment can prevent the development or progression of these potentially disfiguring sequelae,” Dr. Roman said.
Although the results are from a small case series at a single center, Dr. Roman said this condition may be more prevalent than realized. “Having been raised in a predominately Latino community in Texas, I can easily recall seeing people with these papules growing up. I don’t think it would be surprising for dermatologists reading our paper to say, ‘I’ve seen this in clinic before,’ ” he said.
Regarding treatment, there is an ongoing investigation into what treatments are effective for the acne-related nasal papules. “Physical treatment modalities such as ablative laser or surgical removal seem to be the most efficacious,” Dr. Roman said. “In the future, a prospective clinical study will help to better define the prevalence and risk factors for the condition,” he said.
He and coauthors reported no conflicts of interest. No funding source was listed.
SOURCE: Roman J et al. Pediatr Dermatol. 2020 Aug 7. doi: 10.1111/pde.14319.
, but researchers believe the condition could be an underrecognized problem, affecting patients with skin of color in particular, according to the authors of a case series published in Pediatric Dermatology.
Jorge Roman, MD, and coauthors in the department of dermatology at New York (N.Y.) University identified 20 patients with a history of acne who had nasal papules, in a retrospective review of electronic medical records at NYU over 1 year (April 2018 to April 2019). The presentation ranged from “a few, small skin-colored papules to large, dome-shaped papulonodules, to more extensive rhinophymatous-like” changes with some patients having papular lesions on the chin in addition to the nose, they wrote in the report.
These papules greatly resembled angiofibromas, but appear to be a sequela of acne, according to the authors. In five patients who had biopsies, the results showed “a dome-shaped proliferation of spindle and stellate-shaped cells with thickened collagen bundles and dilated thin-walled blood vessels,” the authors wrote. “The histopathological findings of these nasal papules were indistinguishable from those of a conventional angiofibroma.”
In addition, the patients did not have evidence of underlying genetic conditions that could explain the angiofibroma-like lesions. “Although acne has not previously been implicated in the development of angiofibromas, based on the data available for our patients, it seems extremely unlikely that the lesions would be related to anything else,” Dr. Roman, a dermatology resident at New York University, said in an interview.
He said he first recognized the nasal papules in clinic as a first-year resident, but was surprised to find a lack of information on the condition. “Dermatology has a name for just about every skin disease imaginable, so I found it very odd when I couldn’t find much describing this condition,” he said. “There was a large disparity between what we were seeing in clinic and what was reported in the literature.”
Nearly all the patients were Hispanic (17 of 20) and adolescent males (17 patients), with a median age of 16 years at the time of presentation. There were two Black patients and one Asian patient. Race and ethnicity were not mentioned in two previous reports describing papular acne scarring, but Dr. Roman and colleagues noted that in their clinic, the condition appeared to affect adolescent patients with skin of color predominantly.
Reasons why nasal papules may be underreported are unclear, Dr. Roman noted. One possible explanation is lower use of dermatologic care among patients with skin of color. “Interestingly, previous research has shown that racial minorities are lower utilizers of dermatologic care. It is possible that the patient demographic most afflicted by this condition face significant barriers when seeking care,” he said.
Due to a low level of awareness of acne-related nasal papules, “clinicians may not recognize it as an acne-related scarring process. This is significant, as early recognition and treatment can prevent the development or progression of these potentially disfiguring sequelae,” Dr. Roman said.
Although the results are from a small case series at a single center, Dr. Roman said this condition may be more prevalent than realized. “Having been raised in a predominately Latino community in Texas, I can easily recall seeing people with these papules growing up. I don’t think it would be surprising for dermatologists reading our paper to say, ‘I’ve seen this in clinic before,’ ” he said.
Regarding treatment, there is an ongoing investigation into what treatments are effective for the acne-related nasal papules. “Physical treatment modalities such as ablative laser or surgical removal seem to be the most efficacious,” Dr. Roman said. “In the future, a prospective clinical study will help to better define the prevalence and risk factors for the condition,” he said.
He and coauthors reported no conflicts of interest. No funding source was listed.
SOURCE: Roman J et al. Pediatr Dermatol. 2020 Aug 7. doi: 10.1111/pde.14319.
, but researchers believe the condition could be an underrecognized problem, affecting patients with skin of color in particular, according to the authors of a case series published in Pediatric Dermatology.
Jorge Roman, MD, and coauthors in the department of dermatology at New York (N.Y.) University identified 20 patients with a history of acne who had nasal papules, in a retrospective review of electronic medical records at NYU over 1 year (April 2018 to April 2019). The presentation ranged from “a few, small skin-colored papules to large, dome-shaped papulonodules, to more extensive rhinophymatous-like” changes with some patients having papular lesions on the chin in addition to the nose, they wrote in the report.
These papules greatly resembled angiofibromas, but appear to be a sequela of acne, according to the authors. In five patients who had biopsies, the results showed “a dome-shaped proliferation of spindle and stellate-shaped cells with thickened collagen bundles and dilated thin-walled blood vessels,” the authors wrote. “The histopathological findings of these nasal papules were indistinguishable from those of a conventional angiofibroma.”
In addition, the patients did not have evidence of underlying genetic conditions that could explain the angiofibroma-like lesions. “Although acne has not previously been implicated in the development of angiofibromas, based on the data available for our patients, it seems extremely unlikely that the lesions would be related to anything else,” Dr. Roman, a dermatology resident at New York University, said in an interview.
He said he first recognized the nasal papules in clinic as a first-year resident, but was surprised to find a lack of information on the condition. “Dermatology has a name for just about every skin disease imaginable, so I found it very odd when I couldn’t find much describing this condition,” he said. “There was a large disparity between what we were seeing in clinic and what was reported in the literature.”
Nearly all the patients were Hispanic (17 of 20) and adolescent males (17 patients), with a median age of 16 years at the time of presentation. There were two Black patients and one Asian patient. Race and ethnicity were not mentioned in two previous reports describing papular acne scarring, but Dr. Roman and colleagues noted that in their clinic, the condition appeared to affect adolescent patients with skin of color predominantly.
Reasons why nasal papules may be underreported are unclear, Dr. Roman noted. One possible explanation is lower use of dermatologic care among patients with skin of color. “Interestingly, previous research has shown that racial minorities are lower utilizers of dermatologic care. It is possible that the patient demographic most afflicted by this condition face significant barriers when seeking care,” he said.
Due to a low level of awareness of acne-related nasal papules, “clinicians may not recognize it as an acne-related scarring process. This is significant, as early recognition and treatment can prevent the development or progression of these potentially disfiguring sequelae,” Dr. Roman said.
Although the results are from a small case series at a single center, Dr. Roman said this condition may be more prevalent than realized. “Having been raised in a predominately Latino community in Texas, I can easily recall seeing people with these papules growing up. I don’t think it would be surprising for dermatologists reading our paper to say, ‘I’ve seen this in clinic before,’ ” he said.
Regarding treatment, there is an ongoing investigation into what treatments are effective for the acne-related nasal papules. “Physical treatment modalities such as ablative laser or surgical removal seem to be the most efficacious,” Dr. Roman said. “In the future, a prospective clinical study will help to better define the prevalence and risk factors for the condition,” he said.
He and coauthors reported no conflicts of interest. No funding source was listed.
SOURCE: Roman J et al. Pediatr Dermatol. 2020 Aug 7. doi: 10.1111/pde.14319.
FROM PEDIATRIC DERMATOLOGY
Papules on a child’s chest
Potassium hydroxide preparation of expressed contents revealed no molluscum bodies, but rather, small-caliber, trapped, and coiled hairs consistent with eruptive vellus hair cysts (EVHCs).
Vellus hairs are fine, light-colored (nonterminal) hairs that normally are found on the face, trunk, and limbs. EVHCs are typically asymptomatic papules containing an entrapped vellus hair. They form from entrapment of the hair follicle just proximal to the infundibulum, leading to hair bulb atrophy and dilation of the follicle. EVHCs typically are dome-shaped, flesh-colored soft papules that are 2 to 3 mm in size.
Diagnostic confusion can arise with molluscum contagiosum because those lesions also tend to be umbilicated and flesh colored. EVHCs can appear at any age but are more common in the first 3 decades of life. Familial types exist, presenting earlier in life than sporadic types. The differential diagnosis includes molluscum contagiosum, folliculitis, and steatocystoma multiplex. Rare genodermatoses have been associated with EVHCs, including autosomal dominant familial keratin gene mutation, anhidrotic and hidrotic ectodermal dysplasia, and pachyonychia congenita.
Up to 25% of cases spontaneously resolve, so patients can be counseled to observe the lesions. Alternative options including topical emollients or various topical keratolytics, including ammonium lactate 20% once to twice daily, topical urea, or topical salicylic acid until the lesions are flat and smooth. Topical and systemic retinoids have been reported to have limited success. Individual lesions of concern may be removed surgically by excision, curettage, cryotherapy, or laser but will likely leave a minor scar.
The patient and his family were reassured of the benign nature of the lesions and instructed to treat the papules with topical ammonium lactate 20% twice daily until the next appointment in 12 weeks. At follow-up, many, but not all, of the lesions had resolved.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.

Potassium hydroxide preparation of expressed contents revealed no molluscum bodies, but rather, small-caliber, trapped, and coiled hairs consistent with eruptive vellus hair cysts (EVHCs).
Vellus hairs are fine, light-colored (nonterminal) hairs that normally are found on the face, trunk, and limbs. EVHCs are typically asymptomatic papules containing an entrapped vellus hair. They form from entrapment of the hair follicle just proximal to the infundibulum, leading to hair bulb atrophy and dilation of the follicle. EVHCs typically are dome-shaped, flesh-colored soft papules that are 2 to 3 mm in size.
Diagnostic confusion can arise with molluscum contagiosum because those lesions also tend to be umbilicated and flesh colored. EVHCs can appear at any age but are more common in the first 3 decades of life. Familial types exist, presenting earlier in life than sporadic types. The differential diagnosis includes molluscum contagiosum, folliculitis, and steatocystoma multiplex. Rare genodermatoses have been associated with EVHCs, including autosomal dominant familial keratin gene mutation, anhidrotic and hidrotic ectodermal dysplasia, and pachyonychia congenita.
Up to 25% of cases spontaneously resolve, so patients can be counseled to observe the lesions. Alternative options including topical emollients or various topical keratolytics, including ammonium lactate 20% once to twice daily, topical urea, or topical salicylic acid until the lesions are flat and smooth. Topical and systemic retinoids have been reported to have limited success. Individual lesions of concern may be removed surgically by excision, curettage, cryotherapy, or laser but will likely leave a minor scar.
The patient and his family were reassured of the benign nature of the lesions and instructed to treat the papules with topical ammonium lactate 20% twice daily until the next appointment in 12 weeks. At follow-up, many, but not all, of the lesions had resolved.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Potassium hydroxide preparation of expressed contents revealed no molluscum bodies, but rather, small-caliber, trapped, and coiled hairs consistent with eruptive vellus hair cysts (EVHCs).
Vellus hairs are fine, light-colored (nonterminal) hairs that normally are found on the face, trunk, and limbs. EVHCs are typically asymptomatic papules containing an entrapped vellus hair. They form from entrapment of the hair follicle just proximal to the infundibulum, leading to hair bulb atrophy and dilation of the follicle. EVHCs typically are dome-shaped, flesh-colored soft papules that are 2 to 3 mm in size.
Diagnostic confusion can arise with molluscum contagiosum because those lesions also tend to be umbilicated and flesh colored. EVHCs can appear at any age but are more common in the first 3 decades of life. Familial types exist, presenting earlier in life than sporadic types. The differential diagnosis includes molluscum contagiosum, folliculitis, and steatocystoma multiplex. Rare genodermatoses have been associated with EVHCs, including autosomal dominant familial keratin gene mutation, anhidrotic and hidrotic ectodermal dysplasia, and pachyonychia congenita.
Up to 25% of cases spontaneously resolve, so patients can be counseled to observe the lesions. Alternative options including topical emollients or various topical keratolytics, including ammonium lactate 20% once to twice daily, topical urea, or topical salicylic acid until the lesions are flat and smooth. Topical and systemic retinoids have been reported to have limited success. Individual lesions of concern may be removed surgically by excision, curettage, cryotherapy, or laser but will likely leave a minor scar.
The patient and his family were reassured of the benign nature of the lesions and instructed to treat the papules with topical ammonium lactate 20% twice daily until the next appointment in 12 weeks. At follow-up, many, but not all, of the lesions had resolved.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.