User login
Cardiovascular complications of systemic sclerosis: What to look for
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
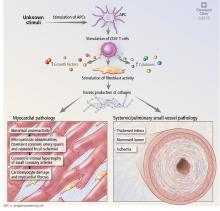
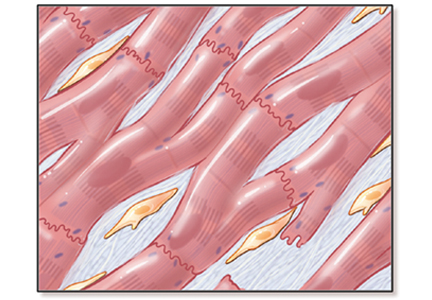
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
KEY POINTS
- Pulmonary hypertension is common in systemic sclerosis and carries a poor prognosis. Patients with systemic sclerosis should be screened regularly with echocardiography, followed, when necessary, by right heart catheterization to detect it early.
- Myocardial infarction and stroke are more common in patients with systemic sclerosis, and preventive measures are the same as for the general population.
- Right ventricular dysfunction secondary to pulmonary hypertension is common in systemic sclerosis; left ventricular dysfunction is less so. Routine echocardiography should include assessment of right and left ventricular function.
- Electrocardiography should be performed periodically, and urgently when indicated, to look for potentially dangerous arrhythmias.
Vulvar and gluteal manifestations of Crohn disease
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
Apple cider vinegar soaks fall short in atopic dermatitis
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
FROM PEDIATRIC DERMATOLOGY
Heart disease raises risk of severe cutaneous adverse reactions to allopurinol
Researchers have found that patients with heart disease have an increased risk of hospitalization for severe cutaneous adverse reactions to allopurinol, with factors like chronic kidney disease and high initial dosage adding to that risk.
“Physicians who prescribe allopurinol should look for these risk factors so that they may consider initiating lower-dosage allopurinol and other precautions, which may prevent this rare but serious adverse reaction,” Chio Yokose, MD, of Massachusetts General Hospital in Boston and coauthors wrote in the Canadian Medical Association Journal.
To further investigate known associations between heart disease and severe cutaneous adverse reactions to allopurinol – including Stevens-Johnson syndrome and toxic epidermal necrolysis – the researchers used an administrative database known as Population Data BC to conduct a cohort study of allopurinol initiators in British Columbia between 1997 and 2015. Individuals with a history of severe cutaneous adverse reactions before starting allopurinol were excluded.
Of the 130,325 allopurinol users identified, 109 were hospitalized for allopurinol-associated severe cutaneous adverse reactions within 3 months of starting the drug. One in 655 allopurinol users with heart disease were admitted to the hospital for allopurinol-associated severe cutaneous adverse reaction (risk ratio = 1.53 per 1,000; 95% confidence interval, 1.10-2.06), compared with 1 in 1,548 allopurinol users without heart disease (risk ratio = 0.65 per 1,000; 95% CI, 0.50-0.82).
After multivariable analysis, other significant associations with hospital admission included chronic kidney disease (relative risk, 1.88; 95% CI, 1.17-3.02) and an initial allopurinol dosage greater than 100 mg/day (RR, 2.78; 95% CI, 1.75-4.43). In addition, patients with heart disease, chronic kidney disease, and an initial dosage greater than 100 mg/day had an 11-fold higher risk of hospital admission (RR, 11.13; 95% CI, 4.66-26.58).
The authors acknowledged their study’s limitations, including potential misclassification of reactions and comorbidities that can stem from a reliance on ICD codes. However, they also noted that “any misclassification is expected to be nondifferential” and bias results toward the null accordingly.
The study was funded by the Canadian Institutes of Health Research. One author reported receiving a grant from the National Institutes of Health and research support from AstraZeneca, along with consulting fees from Takeda, Selecta Biosciences, and Horizon. No other conflicts of interest were reported.
SOURCE: Yokose C et al. CMAJ. 2019 Sep 30. doi: 10.1503/cmaj.190339.
Researchers have found that patients with heart disease have an increased risk of hospitalization for severe cutaneous adverse reactions to allopurinol, with factors like chronic kidney disease and high initial dosage adding to that risk.
“Physicians who prescribe allopurinol should look for these risk factors so that they may consider initiating lower-dosage allopurinol and other precautions, which may prevent this rare but serious adverse reaction,” Chio Yokose, MD, of Massachusetts General Hospital in Boston and coauthors wrote in the Canadian Medical Association Journal.
To further investigate known associations between heart disease and severe cutaneous adverse reactions to allopurinol – including Stevens-Johnson syndrome and toxic epidermal necrolysis – the researchers used an administrative database known as Population Data BC to conduct a cohort study of allopurinol initiators in British Columbia between 1997 and 2015. Individuals with a history of severe cutaneous adverse reactions before starting allopurinol were excluded.
Of the 130,325 allopurinol users identified, 109 were hospitalized for allopurinol-associated severe cutaneous adverse reactions within 3 months of starting the drug. One in 655 allopurinol users with heart disease were admitted to the hospital for allopurinol-associated severe cutaneous adverse reaction (risk ratio = 1.53 per 1,000; 95% confidence interval, 1.10-2.06), compared with 1 in 1,548 allopurinol users without heart disease (risk ratio = 0.65 per 1,000; 95% CI, 0.50-0.82).
After multivariable analysis, other significant associations with hospital admission included chronic kidney disease (relative risk, 1.88; 95% CI, 1.17-3.02) and an initial allopurinol dosage greater than 100 mg/day (RR, 2.78; 95% CI, 1.75-4.43). In addition, patients with heart disease, chronic kidney disease, and an initial dosage greater than 100 mg/day had an 11-fold higher risk of hospital admission (RR, 11.13; 95% CI, 4.66-26.58).
The authors acknowledged their study’s limitations, including potential misclassification of reactions and comorbidities that can stem from a reliance on ICD codes. However, they also noted that “any misclassification is expected to be nondifferential” and bias results toward the null accordingly.
The study was funded by the Canadian Institutes of Health Research. One author reported receiving a grant from the National Institutes of Health and research support from AstraZeneca, along with consulting fees from Takeda, Selecta Biosciences, and Horizon. No other conflicts of interest were reported.
SOURCE: Yokose C et al. CMAJ. 2019 Sep 30. doi: 10.1503/cmaj.190339.
Researchers have found that patients with heart disease have an increased risk of hospitalization for severe cutaneous adverse reactions to allopurinol, with factors like chronic kidney disease and high initial dosage adding to that risk.
“Physicians who prescribe allopurinol should look for these risk factors so that they may consider initiating lower-dosage allopurinol and other precautions, which may prevent this rare but serious adverse reaction,” Chio Yokose, MD, of Massachusetts General Hospital in Boston and coauthors wrote in the Canadian Medical Association Journal.
To further investigate known associations between heart disease and severe cutaneous adverse reactions to allopurinol – including Stevens-Johnson syndrome and toxic epidermal necrolysis – the researchers used an administrative database known as Population Data BC to conduct a cohort study of allopurinol initiators in British Columbia between 1997 and 2015. Individuals with a history of severe cutaneous adverse reactions before starting allopurinol were excluded.
Of the 130,325 allopurinol users identified, 109 were hospitalized for allopurinol-associated severe cutaneous adverse reactions within 3 months of starting the drug. One in 655 allopurinol users with heart disease were admitted to the hospital for allopurinol-associated severe cutaneous adverse reaction (risk ratio = 1.53 per 1,000; 95% confidence interval, 1.10-2.06), compared with 1 in 1,548 allopurinol users without heart disease (risk ratio = 0.65 per 1,000; 95% CI, 0.50-0.82).
After multivariable analysis, other significant associations with hospital admission included chronic kidney disease (relative risk, 1.88; 95% CI, 1.17-3.02) and an initial allopurinol dosage greater than 100 mg/day (RR, 2.78; 95% CI, 1.75-4.43). In addition, patients with heart disease, chronic kidney disease, and an initial dosage greater than 100 mg/day had an 11-fold higher risk of hospital admission (RR, 11.13; 95% CI, 4.66-26.58).
The authors acknowledged their study’s limitations, including potential misclassification of reactions and comorbidities that can stem from a reliance on ICD codes. However, they also noted that “any misclassification is expected to be nondifferential” and bias results toward the null accordingly.
The study was funded by the Canadian Institutes of Health Research. One author reported receiving a grant from the National Institutes of Health and research support from AstraZeneca, along with consulting fees from Takeda, Selecta Biosciences, and Horizon. No other conflicts of interest were reported.
SOURCE: Yokose C et al. CMAJ. 2019 Sep 30. doi: 10.1503/cmaj.190339.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Review looks at natural course of alopecia areata in young children
, according to a retrospective chart review of 125 children.
Almost 90% of the children presented between aged 2 and 4 years, compared with 11.9% between ages 1 and 2 years, and 1.6% aged under 1 year, “in keeping with the existing literature,” the study authors reported in Pediatric Dermatology. “A high percentage of patients continued to have mild, patchy alopecia at their follow‐up visits,” they added.
Epidemiologic studies of children with alopecia areata are few and have not focused on the youngest patients, said Sneha Rangu, of the section of dermatology at Children’s Hospital of Philadelphia, and coauthors. They performed a retrospective chart review of 125 patients, who initially presented at the hospital with alopecia areata between Jan. 1, 2016, and June 1, 2018, when they were younger than 4 years. Patients who received systemic therapy or topical JAK inhibitors for alopecia were excluded. Severity was measured with the Severity of Alopecia Tool (SALT) score, to monitor progression of hair loss, analyzing scores at the initial presentation, at 3-6 months, at 1 year, and at 2 or more years.
Almost 70% were female, which the authors said was similar to other studies that have found alopecia areata is more prevalent in females; and 86.6% were between ages 2 and 4 years when they first presented. The initial diagnosis was alopecia areata in 72.0%, alopecia totalis in 8.8%, and alopecia universalis in 19.2%. Of the 41 boys, 39% had alopecia totalis or alopecia universalis, as did 22% of the girls, which suggested that boys presenting under aged 4 years were more likely to have more severe disease, or that “guardians of boys are more likely to present for therapy when disease is more severe,” the authors wrote.
About 40% of the children presented with a history of atopic dermatitis, and 4% had an autoimmune disease (vitiligo, celiac disease, or type 1 diabetes). Twenty-eight percent of patients had a family history of alopecia areata, 27.2% had a family history of other autoimmune diseases, and 32% had a family history of hypothyroidism.
At the first visit, 57.6% had patch‐stage alopecia and SALT scores in the mild range (0%‐24% hair loss), which was present in a high proportion of these patients at follow-up: 49.4% at 3-6 months, 39.5% at 1 year, and 42.9% at two or more years.
At the first visit, 28% had high SALT scores (50%-100% hair loss), increasing to 36% at 3-6 months, 41.8% at 1 year, and 46.4% at 2 or more years. They calculated that for those with more than 50% hair loss at the initial presentation, the likelihood of being in a high category of hair loss, as measured by increasing SALT scores, was significantly higher at 1 year (odds ratio, 1.85, P =.033) and at 2 or more years (OR, 2.29, P = .038).
“While there is a likelihood of increasing disease severity, those with higher severity at initial presentation are likely to stay severe after one or 2 years,” the authors noted.
They concluded that their results add to the understanding of the epidemiology of alopecia areata in children “and perhaps can provide clinicians and families with a better sense of prognosis for progression in the youngest patients presenting with alopecia areata.”
They said the retrospective design and small sample size were among the study’s limitations. They had no conflicts of interest to disclose.
SOURCE: Rangu S et al. Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990.
, according to a retrospective chart review of 125 children.
Almost 90% of the children presented between aged 2 and 4 years, compared with 11.9% between ages 1 and 2 years, and 1.6% aged under 1 year, “in keeping with the existing literature,” the study authors reported in Pediatric Dermatology. “A high percentage of patients continued to have mild, patchy alopecia at their follow‐up visits,” they added.
Epidemiologic studies of children with alopecia areata are few and have not focused on the youngest patients, said Sneha Rangu, of the section of dermatology at Children’s Hospital of Philadelphia, and coauthors. They performed a retrospective chart review of 125 patients, who initially presented at the hospital with alopecia areata between Jan. 1, 2016, and June 1, 2018, when they were younger than 4 years. Patients who received systemic therapy or topical JAK inhibitors for alopecia were excluded. Severity was measured with the Severity of Alopecia Tool (SALT) score, to monitor progression of hair loss, analyzing scores at the initial presentation, at 3-6 months, at 1 year, and at 2 or more years.
Almost 70% were female, which the authors said was similar to other studies that have found alopecia areata is more prevalent in females; and 86.6% were between ages 2 and 4 years when they first presented. The initial diagnosis was alopecia areata in 72.0%, alopecia totalis in 8.8%, and alopecia universalis in 19.2%. Of the 41 boys, 39% had alopecia totalis or alopecia universalis, as did 22% of the girls, which suggested that boys presenting under aged 4 years were more likely to have more severe disease, or that “guardians of boys are more likely to present for therapy when disease is more severe,” the authors wrote.
About 40% of the children presented with a history of atopic dermatitis, and 4% had an autoimmune disease (vitiligo, celiac disease, or type 1 diabetes). Twenty-eight percent of patients had a family history of alopecia areata, 27.2% had a family history of other autoimmune diseases, and 32% had a family history of hypothyroidism.
At the first visit, 57.6% had patch‐stage alopecia and SALT scores in the mild range (0%‐24% hair loss), which was present in a high proportion of these patients at follow-up: 49.4% at 3-6 months, 39.5% at 1 year, and 42.9% at two or more years.
At the first visit, 28% had high SALT scores (50%-100% hair loss), increasing to 36% at 3-6 months, 41.8% at 1 year, and 46.4% at 2 or more years. They calculated that for those with more than 50% hair loss at the initial presentation, the likelihood of being in a high category of hair loss, as measured by increasing SALT scores, was significantly higher at 1 year (odds ratio, 1.85, P =.033) and at 2 or more years (OR, 2.29, P = .038).
“While there is a likelihood of increasing disease severity, those with higher severity at initial presentation are likely to stay severe after one or 2 years,” the authors noted.
They concluded that their results add to the understanding of the epidemiology of alopecia areata in children “and perhaps can provide clinicians and families with a better sense of prognosis for progression in the youngest patients presenting with alopecia areata.”
They said the retrospective design and small sample size were among the study’s limitations. They had no conflicts of interest to disclose.
SOURCE: Rangu S et al. Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990.
, according to a retrospective chart review of 125 children.
Almost 90% of the children presented between aged 2 and 4 years, compared with 11.9% between ages 1 and 2 years, and 1.6% aged under 1 year, “in keeping with the existing literature,” the study authors reported in Pediatric Dermatology. “A high percentage of patients continued to have mild, patchy alopecia at their follow‐up visits,” they added.
Epidemiologic studies of children with alopecia areata are few and have not focused on the youngest patients, said Sneha Rangu, of the section of dermatology at Children’s Hospital of Philadelphia, and coauthors. They performed a retrospective chart review of 125 patients, who initially presented at the hospital with alopecia areata between Jan. 1, 2016, and June 1, 2018, when they were younger than 4 years. Patients who received systemic therapy or topical JAK inhibitors for alopecia were excluded. Severity was measured with the Severity of Alopecia Tool (SALT) score, to monitor progression of hair loss, analyzing scores at the initial presentation, at 3-6 months, at 1 year, and at 2 or more years.
Almost 70% were female, which the authors said was similar to other studies that have found alopecia areata is more prevalent in females; and 86.6% were between ages 2 and 4 years when they first presented. The initial diagnosis was alopecia areata in 72.0%, alopecia totalis in 8.8%, and alopecia universalis in 19.2%. Of the 41 boys, 39% had alopecia totalis or alopecia universalis, as did 22% of the girls, which suggested that boys presenting under aged 4 years were more likely to have more severe disease, or that “guardians of boys are more likely to present for therapy when disease is more severe,” the authors wrote.
About 40% of the children presented with a history of atopic dermatitis, and 4% had an autoimmune disease (vitiligo, celiac disease, or type 1 diabetes). Twenty-eight percent of patients had a family history of alopecia areata, 27.2% had a family history of other autoimmune diseases, and 32% had a family history of hypothyroidism.
At the first visit, 57.6% had patch‐stage alopecia and SALT scores in the mild range (0%‐24% hair loss), which was present in a high proportion of these patients at follow-up: 49.4% at 3-6 months, 39.5% at 1 year, and 42.9% at two or more years.
At the first visit, 28% had high SALT scores (50%-100% hair loss), increasing to 36% at 3-6 months, 41.8% at 1 year, and 46.4% at 2 or more years. They calculated that for those with more than 50% hair loss at the initial presentation, the likelihood of being in a high category of hair loss, as measured by increasing SALT scores, was significantly higher at 1 year (odds ratio, 1.85, P =.033) and at 2 or more years (OR, 2.29, P = .038).
“While there is a likelihood of increasing disease severity, those with higher severity at initial presentation are likely to stay severe after one or 2 years,” the authors noted.
They concluded that their results add to the understanding of the epidemiology of alopecia areata in children “and perhaps can provide clinicians and families with a better sense of prognosis for progression in the youngest patients presenting with alopecia areata.”
They said the retrospective design and small sample size were among the study’s limitations. They had no conflicts of interest to disclose.
SOURCE: Rangu S et al. Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990.
FROM PEDIATRIC DERMATOLOGY
Click for Credit: Psoriasis relief; Stress & CV problems; more
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Marks on lower leg
The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Parent survey sheds light on suboptimal compliance with eczema medications
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
FROM PEDIATRIC DERMATOLOGY
Does this patient have bacterial conjunctivitis?
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
Rash on lower legs and abdomen
The FP suspected leukocytoclastic vasculitis (LCV) and, with the patient’s consent, performed a 4-mm punch biopsy on a well-developed lesion on the abdomen. Biopsies on the abdomen heal faster than the legs and may provide a better specimen to the pathologist. (See the Watch & Learn video on “Punch biopsy.”)
The biopsy confirmed the diagnosis of LCV. This is the most commonly seen form of small vessel vasculitis. LCV causes acute inflammation and necrosis of venules in the dermis. The term leukocytoclastic vasculitis describes the histologic pattern produced when leukocytes break apart into fragments. The purpura begins as asymptomatic localized areas of cutaneous hemorrhage that become palpable.
Discrete lesions are most commonly seen on the lower extremities, but they may occur on any dependent area. Small lesions may itch and be painful, but nodules, ulcers, and bullae may be more painful. Lesions appear in crops, last for 1 to 4 weeks, and may heal with residual scarring and hyperpigmentation. Patients may experience a single episode caused by a drug reaction or viral infection or have multiple episodes associated with rheumatologic diseases. LCV usually is self-limited and confined to the skin.
To make the diagnosis, look for the presence of 3 or more of the following:
- age > 16 years;
- use of a possible offending drug in temporal relation to the symptoms;
- palpable purpura;
- maculopapular rash; and
- neutrophils around an arteriole or venule in a biopsy of a skin lesion.
In this case, the use of ibuprofen was the most likely precipitating event. Blood and urine tests did not show any renal or other organ system involvement. The patient was warned to not use ibuprofen in the future and that acetaminophen is a safer option for him. He was given topical triamcinolone cream 0.1% to apply twice daily for symptomatic relief. In this case, oral prednisone was not prescribed because the numerous potential adverse effects of prednisone outweighed the benefits. The vasculitis resolved in 4 weeks without any sequelae.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected leukocytoclastic vasculitis (LCV) and, with the patient’s consent, performed a 4-mm punch biopsy on a well-developed lesion on the abdomen. Biopsies on the abdomen heal faster than the legs and may provide a better specimen to the pathologist. (See the Watch & Learn video on “Punch biopsy.”)
The biopsy confirmed the diagnosis of LCV. This is the most commonly seen form of small vessel vasculitis. LCV causes acute inflammation and necrosis of venules in the dermis. The term leukocytoclastic vasculitis describes the histologic pattern produced when leukocytes break apart into fragments. The purpura begins as asymptomatic localized areas of cutaneous hemorrhage that become palpable.
Discrete lesions are most commonly seen on the lower extremities, but they may occur on any dependent area. Small lesions may itch and be painful, but nodules, ulcers, and bullae may be more painful. Lesions appear in crops, last for 1 to 4 weeks, and may heal with residual scarring and hyperpigmentation. Patients may experience a single episode caused by a drug reaction or viral infection or have multiple episodes associated with rheumatologic diseases. LCV usually is self-limited and confined to the skin.
To make the diagnosis, look for the presence of 3 or more of the following:
- age > 16 years;
- use of a possible offending drug in temporal relation to the symptoms;
- palpable purpura;
- maculopapular rash; and
- neutrophils around an arteriole or venule in a biopsy of a skin lesion.
In this case, the use of ibuprofen was the most likely precipitating event. Blood and urine tests did not show any renal or other organ system involvement. The patient was warned to not use ibuprofen in the future and that acetaminophen is a safer option for him. He was given topical triamcinolone cream 0.1% to apply twice daily for symptomatic relief. In this case, oral prednisone was not prescribed because the numerous potential adverse effects of prednisone outweighed the benefits. The vasculitis resolved in 4 weeks without any sequelae.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected leukocytoclastic vasculitis (LCV) and, with the patient’s consent, performed a 4-mm punch biopsy on a well-developed lesion on the abdomen. Biopsies on the abdomen heal faster than the legs and may provide a better specimen to the pathologist. (See the Watch & Learn video on “Punch biopsy.”)
The biopsy confirmed the diagnosis of LCV. This is the most commonly seen form of small vessel vasculitis. LCV causes acute inflammation and necrosis of venules in the dermis. The term leukocytoclastic vasculitis describes the histologic pattern produced when leukocytes break apart into fragments. The purpura begins as asymptomatic localized areas of cutaneous hemorrhage that become palpable.
Discrete lesions are most commonly seen on the lower extremities, but they may occur on any dependent area. Small lesions may itch and be painful, but nodules, ulcers, and bullae may be more painful. Lesions appear in crops, last for 1 to 4 weeks, and may heal with residual scarring and hyperpigmentation. Patients may experience a single episode caused by a drug reaction or viral infection or have multiple episodes associated with rheumatologic diseases. LCV usually is self-limited and confined to the skin.
To make the diagnosis, look for the presence of 3 or more of the following:
- age > 16 years;
- use of a possible offending drug in temporal relation to the symptoms;
- palpable purpura;
- maculopapular rash; and
- neutrophils around an arteriole or venule in a biopsy of a skin lesion.
In this case, the use of ibuprofen was the most likely precipitating event. Blood and urine tests did not show any renal or other organ system involvement. The patient was warned to not use ibuprofen in the future and that acetaminophen is a safer option for him. He was given topical triamcinolone cream 0.1% to apply twice daily for symptomatic relief. In this case, oral prednisone was not prescribed because the numerous potential adverse effects of prednisone outweighed the benefits. The vasculitis resolved in 4 weeks without any sequelae.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/