User login
When to suspect a severe skin reaction to an AED
NEW ORLEANS – Most skin eruptions in patients taking antiepileptic drugs (AEDs) are relatively benign. With close supervision, some patients with epilepsy may continue treatment despite having a benign drug rash, according to a lecture at the annual meeting of the American Epilepsy Society.
“When do you know that you’re not dealing with that kind of eruption?” said Jeanne M. Young, MD, assistant professor of dermatology at the University of Virginia in Charlottesville.
Signs and symptoms that raise concerns about severe cutaneous reactions include swelling of the face; lesions that are fluid-filled, dusky, or painful; mucus membrane involvement; and signs of systemic involvement.
Associations with anticonvulsants
Diffuse swelling of the face is a hallmark symptom of DRESS. Fluid-filled lesions such as pustules, vesicles, and bullae indicate a condition other than a benign drug eruption. Signs of systemic involvement may include fever, marked eosinophilia, transaminitis, and evidence of lymphocyte activation. “In general, I want to see systemic involvement that can’t be explained by the patient’s other systemic diseases,” Dr. Young said.
A 2018 study found that AEDs are associated with SJS and TEN, and the labels for lamotrigine and carbamazepine include black box warnings about the risk of severe cutaneous adverse events. Carbamazepine’s warning, which was added in 2007, notes that SJS and TEN are significantly more common in patients of Asian ancestry with the human leukocyte antigen allele HLA-B*1502 and that physicians should screen at-risk patients before starting treatment.
Benign drug rashes
Morbilliform drug eruptions, sometimes called benign exanthems, are “by far the most common drug rash that we see” and typically are “the rashes that people refer to as drug rashes,” Dr. Young said. The mechanisms appear to be primarily immune complex mediated and cell mediated. “When the drug is stopped, these rashes tend to go away quite predictably in 2-3 weeks.”
For any class of drug, about 1% of people taking that medication may have this type of reaction, Dr. Young said. “We expect to see erythematous papules and plaques that oftentimes become confluent on the skin.” These reactions generally occur 7-10 days after the first exposure to the medication, and most patients do not have other symptoms, although the rash may itch. In addition, patients may have erythroderma with desquamation. “I think it’s important to point out the difference between desquamation, which is loss of the stratum corneum, and epidermal sloughing, which is what you see in something like [SJS] or TEN, where you’re actually losing the entire epidermis,” Dr. Young said. Recovering from desquamation is “sort of like recovering from a sun burn, and it’s not particularly dangerous.” Management of morbilliform drug eruptions is largely symptomatic.
Treat through, taper, or rechallenge
In the case a benign drug rash, “if you feel like you … need to keep a patient on a drug, you do have that option with close supervision,” Dr. Young said. “Communicate that with the dermatologist. Say, ‘I have really struggled getting this patient stabilized. Can we keep them on this drug?’ ”
The dermatologist may not fully realize the implications of stopping an effective AED in a patient with seizures that have been difficult to control. If the drug rash is benign, treating through may be an option. Patients often resolve the rash while continuing the medication, which may be because of desensitization, Dr. Young said. If a patient’s symptoms are too great to continue the drug, neurologists have the option of slowly tapering the drug and reinitiating with a new drug, Dr. Young said. Neurologists also may choose to rechallenge.
If a patient is on several medications, making it difficult to elucidate a causative agent, after stopping those drugs and allowing the rash to resolve, “there is little danger in restarting a medication,” she said.
Benign rash or DRESS?
“When I see a morbilliform eruption, usually what’s on my mind is, ‘Is this just a drug rash or is this DRESS?’ ” Dr. Young said. DRESS often starts with a morbilliform eruption that is indistinguishable from a benign drug eruption.
“Timing is a major difference,” she said. “If a patient develops a morbilliform drug eruption much later than I would expect, then my suspicion [for DRESS] goes up.” Patients with DRESS often have fever and systemic symptoms. Proposed DRESS diagnostic criteria can be useful, but clinical judgment still plays a key role. If a patient does not satisfy diagnostic criteria but has some signs and is taking a drug that is associated with DRESS, “it is going to make me more suspicious and maybe make me recommend stopping that drug sooner,” she said. Anticonvulsants such as carbamazepine, lamotrigine, and phenobarbital are among the drugs most commonly associated with DRESS.
Toxic erythemas
Patients may present with toxic erythemas, such as fixed drug reactions, erythema multiforme, SJS, and TEN. These drug reactions appear similar on biopsy but have different courses.
A patient with a fixed drug reaction often has a single lesion, and the lesion will occur in the same location every time the patient is exposed to the drug. Patients may develop additional lesions with subsequent exposures. These lesions typically are large, erythematous, well-demarcated plaques with central duskiness. “They can be bullous in the center, and they typically will heal with pigmentation, which is unique to this particular drug reaction,” said Dr. Young. “When it gets more concerning and most important to differentiate is when you get generalized bullous fixed drug eruption.” Generalized bullous fixed drug eruptions mimic and are difficult to clinically distinguish from TEN, which has a much has a much poorer prognosis.
Patients with a fixed drug eruption are not as ill as patients with TEN and tend not to slough their skin to the extent seen with TEN. Interferon gamma, perforin, and Fas ligand have been implicated as mechanisms involved in fixed drug reactions. Unlike in TEN, regulatory T cells are abundant, which may explain why TEN and fixed drug reactions progress differently even though they appear to share pathologic mechanisms, Dr. Young said.
Erythema multiforme generally presents with classic target lesions and little mucosal involvement. Infections, most commonly herpes simplex virus (HSV) 1 and 2, may trigger erythema multiforme. Dr. Young recommends evaluating patients for HSV and checking serologies, even if patients have never had a herpes outbreak. “If you have no evidence for infection, you do have to consider discontinuing a medication,” she said.
Stevens–Johnson syndrome and TEN
SJS and TEN are “the rarest of the severe cutaneous adverse drug reactions” and have “the highest morbidity and mortality,” Dr. Young said. They appear to exist on a continuum where SJS may represent early TEN.
“This is a situation where you expect to see blistering of the skin [and] always mucosal involvement. You need to stop the drug immediately when you suspect this drug reaction,” Dr. Young said.
One reason to distinguish SJS or early TEN from later TEN is that high-dose steroids may play a role in the treatment of SJS or early TEN. “Once you get past about 10% total body surface area, there is good evidence that steroids actually increase morbidity and mortality,” she said.
If the eruption has occurred before, that factor suggests that a diagnosis of erythema multiforme or fixed drug reaction may be more likely than TEN.
An apparent lack of regulatory T cells in TEN could explain why patients with HIV infection are at much higher risk of developing SJS and TEN. Understanding the role that regulatory T cells play in severe drug eruptions may lead to new therapeutic options in the future, Dr. Young said.
Dr. Young had no disclosures.
NEW ORLEANS – Most skin eruptions in patients taking antiepileptic drugs (AEDs) are relatively benign. With close supervision, some patients with epilepsy may continue treatment despite having a benign drug rash, according to a lecture at the annual meeting of the American Epilepsy Society.
“When do you know that you’re not dealing with that kind of eruption?” said Jeanne M. Young, MD, assistant professor of dermatology at the University of Virginia in Charlottesville.
Signs and symptoms that raise concerns about severe cutaneous reactions include swelling of the face; lesions that are fluid-filled, dusky, or painful; mucus membrane involvement; and signs of systemic involvement.
Associations with anticonvulsants
Diffuse swelling of the face is a hallmark symptom of DRESS. Fluid-filled lesions such as pustules, vesicles, and bullae indicate a condition other than a benign drug eruption. Signs of systemic involvement may include fever, marked eosinophilia, transaminitis, and evidence of lymphocyte activation. “In general, I want to see systemic involvement that can’t be explained by the patient’s other systemic diseases,” Dr. Young said.
A 2018 study found that AEDs are associated with SJS and TEN, and the labels for lamotrigine and carbamazepine include black box warnings about the risk of severe cutaneous adverse events. Carbamazepine’s warning, which was added in 2007, notes that SJS and TEN are significantly more common in patients of Asian ancestry with the human leukocyte antigen allele HLA-B*1502 and that physicians should screen at-risk patients before starting treatment.
Benign drug rashes
Morbilliform drug eruptions, sometimes called benign exanthems, are “by far the most common drug rash that we see” and typically are “the rashes that people refer to as drug rashes,” Dr. Young said. The mechanisms appear to be primarily immune complex mediated and cell mediated. “When the drug is stopped, these rashes tend to go away quite predictably in 2-3 weeks.”
For any class of drug, about 1% of people taking that medication may have this type of reaction, Dr. Young said. “We expect to see erythematous papules and plaques that oftentimes become confluent on the skin.” These reactions generally occur 7-10 days after the first exposure to the medication, and most patients do not have other symptoms, although the rash may itch. In addition, patients may have erythroderma with desquamation. “I think it’s important to point out the difference between desquamation, which is loss of the stratum corneum, and epidermal sloughing, which is what you see in something like [SJS] or TEN, where you’re actually losing the entire epidermis,” Dr. Young said. Recovering from desquamation is “sort of like recovering from a sun burn, and it’s not particularly dangerous.” Management of morbilliform drug eruptions is largely symptomatic.
Treat through, taper, or rechallenge
In the case a benign drug rash, “if you feel like you … need to keep a patient on a drug, you do have that option with close supervision,” Dr. Young said. “Communicate that with the dermatologist. Say, ‘I have really struggled getting this patient stabilized. Can we keep them on this drug?’ ”
The dermatologist may not fully realize the implications of stopping an effective AED in a patient with seizures that have been difficult to control. If the drug rash is benign, treating through may be an option. Patients often resolve the rash while continuing the medication, which may be because of desensitization, Dr. Young said. If a patient’s symptoms are too great to continue the drug, neurologists have the option of slowly tapering the drug and reinitiating with a new drug, Dr. Young said. Neurologists also may choose to rechallenge.
If a patient is on several medications, making it difficult to elucidate a causative agent, after stopping those drugs and allowing the rash to resolve, “there is little danger in restarting a medication,” she said.
Benign rash or DRESS?
“When I see a morbilliform eruption, usually what’s on my mind is, ‘Is this just a drug rash or is this DRESS?’ ” Dr. Young said. DRESS often starts with a morbilliform eruption that is indistinguishable from a benign drug eruption.
“Timing is a major difference,” she said. “If a patient develops a morbilliform drug eruption much later than I would expect, then my suspicion [for DRESS] goes up.” Patients with DRESS often have fever and systemic symptoms. Proposed DRESS diagnostic criteria can be useful, but clinical judgment still plays a key role. If a patient does not satisfy diagnostic criteria but has some signs and is taking a drug that is associated with DRESS, “it is going to make me more suspicious and maybe make me recommend stopping that drug sooner,” she said. Anticonvulsants such as carbamazepine, lamotrigine, and phenobarbital are among the drugs most commonly associated with DRESS.
Toxic erythemas
Patients may present with toxic erythemas, such as fixed drug reactions, erythema multiforme, SJS, and TEN. These drug reactions appear similar on biopsy but have different courses.
A patient with a fixed drug reaction often has a single lesion, and the lesion will occur in the same location every time the patient is exposed to the drug. Patients may develop additional lesions with subsequent exposures. These lesions typically are large, erythematous, well-demarcated plaques with central duskiness. “They can be bullous in the center, and they typically will heal with pigmentation, which is unique to this particular drug reaction,” said Dr. Young. “When it gets more concerning and most important to differentiate is when you get generalized bullous fixed drug eruption.” Generalized bullous fixed drug eruptions mimic and are difficult to clinically distinguish from TEN, which has a much has a much poorer prognosis.
Patients with a fixed drug eruption are not as ill as patients with TEN and tend not to slough their skin to the extent seen with TEN. Interferon gamma, perforin, and Fas ligand have been implicated as mechanisms involved in fixed drug reactions. Unlike in TEN, regulatory T cells are abundant, which may explain why TEN and fixed drug reactions progress differently even though they appear to share pathologic mechanisms, Dr. Young said.
Erythema multiforme generally presents with classic target lesions and little mucosal involvement. Infections, most commonly herpes simplex virus (HSV) 1 and 2, may trigger erythema multiforme. Dr. Young recommends evaluating patients for HSV and checking serologies, even if patients have never had a herpes outbreak. “If you have no evidence for infection, you do have to consider discontinuing a medication,” she said.
Stevens–Johnson syndrome and TEN
SJS and TEN are “the rarest of the severe cutaneous adverse drug reactions” and have “the highest morbidity and mortality,” Dr. Young said. They appear to exist on a continuum where SJS may represent early TEN.
“This is a situation where you expect to see blistering of the skin [and] always mucosal involvement. You need to stop the drug immediately when you suspect this drug reaction,” Dr. Young said.
One reason to distinguish SJS or early TEN from later TEN is that high-dose steroids may play a role in the treatment of SJS or early TEN. “Once you get past about 10% total body surface area, there is good evidence that steroids actually increase morbidity and mortality,” she said.
If the eruption has occurred before, that factor suggests that a diagnosis of erythema multiforme or fixed drug reaction may be more likely than TEN.
An apparent lack of regulatory T cells in TEN could explain why patients with HIV infection are at much higher risk of developing SJS and TEN. Understanding the role that regulatory T cells play in severe drug eruptions may lead to new therapeutic options in the future, Dr. Young said.
Dr. Young had no disclosures.
NEW ORLEANS – Most skin eruptions in patients taking antiepileptic drugs (AEDs) are relatively benign. With close supervision, some patients with epilepsy may continue treatment despite having a benign drug rash, according to a lecture at the annual meeting of the American Epilepsy Society.
“When do you know that you’re not dealing with that kind of eruption?” said Jeanne M. Young, MD, assistant professor of dermatology at the University of Virginia in Charlottesville.
Signs and symptoms that raise concerns about severe cutaneous reactions include swelling of the face; lesions that are fluid-filled, dusky, or painful; mucus membrane involvement; and signs of systemic involvement.
Associations with anticonvulsants
Diffuse swelling of the face is a hallmark symptom of DRESS. Fluid-filled lesions such as pustules, vesicles, and bullae indicate a condition other than a benign drug eruption. Signs of systemic involvement may include fever, marked eosinophilia, transaminitis, and evidence of lymphocyte activation. “In general, I want to see systemic involvement that can’t be explained by the patient’s other systemic diseases,” Dr. Young said.
A 2018 study found that AEDs are associated with SJS and TEN, and the labels for lamotrigine and carbamazepine include black box warnings about the risk of severe cutaneous adverse events. Carbamazepine’s warning, which was added in 2007, notes that SJS and TEN are significantly more common in patients of Asian ancestry with the human leukocyte antigen allele HLA-B*1502 and that physicians should screen at-risk patients before starting treatment.
Benign drug rashes
Morbilliform drug eruptions, sometimes called benign exanthems, are “by far the most common drug rash that we see” and typically are “the rashes that people refer to as drug rashes,” Dr. Young said. The mechanisms appear to be primarily immune complex mediated and cell mediated. “When the drug is stopped, these rashes tend to go away quite predictably in 2-3 weeks.”
For any class of drug, about 1% of people taking that medication may have this type of reaction, Dr. Young said. “We expect to see erythematous papules and plaques that oftentimes become confluent on the skin.” These reactions generally occur 7-10 days after the first exposure to the medication, and most patients do not have other symptoms, although the rash may itch. In addition, patients may have erythroderma with desquamation. “I think it’s important to point out the difference between desquamation, which is loss of the stratum corneum, and epidermal sloughing, which is what you see in something like [SJS] or TEN, where you’re actually losing the entire epidermis,” Dr. Young said. Recovering from desquamation is “sort of like recovering from a sun burn, and it’s not particularly dangerous.” Management of morbilliform drug eruptions is largely symptomatic.
Treat through, taper, or rechallenge
In the case a benign drug rash, “if you feel like you … need to keep a patient on a drug, you do have that option with close supervision,” Dr. Young said. “Communicate that with the dermatologist. Say, ‘I have really struggled getting this patient stabilized. Can we keep them on this drug?’ ”
The dermatologist may not fully realize the implications of stopping an effective AED in a patient with seizures that have been difficult to control. If the drug rash is benign, treating through may be an option. Patients often resolve the rash while continuing the medication, which may be because of desensitization, Dr. Young said. If a patient’s symptoms are too great to continue the drug, neurologists have the option of slowly tapering the drug and reinitiating with a new drug, Dr. Young said. Neurologists also may choose to rechallenge.
If a patient is on several medications, making it difficult to elucidate a causative agent, after stopping those drugs and allowing the rash to resolve, “there is little danger in restarting a medication,” she said.
Benign rash or DRESS?
“When I see a morbilliform eruption, usually what’s on my mind is, ‘Is this just a drug rash or is this DRESS?’ ” Dr. Young said. DRESS often starts with a morbilliform eruption that is indistinguishable from a benign drug eruption.
“Timing is a major difference,” she said. “If a patient develops a morbilliform drug eruption much later than I would expect, then my suspicion [for DRESS] goes up.” Patients with DRESS often have fever and systemic symptoms. Proposed DRESS diagnostic criteria can be useful, but clinical judgment still plays a key role. If a patient does not satisfy diagnostic criteria but has some signs and is taking a drug that is associated with DRESS, “it is going to make me more suspicious and maybe make me recommend stopping that drug sooner,” she said. Anticonvulsants such as carbamazepine, lamotrigine, and phenobarbital are among the drugs most commonly associated with DRESS.
Toxic erythemas
Patients may present with toxic erythemas, such as fixed drug reactions, erythema multiforme, SJS, and TEN. These drug reactions appear similar on biopsy but have different courses.
A patient with a fixed drug reaction often has a single lesion, and the lesion will occur in the same location every time the patient is exposed to the drug. Patients may develop additional lesions with subsequent exposures. These lesions typically are large, erythematous, well-demarcated plaques with central duskiness. “They can be bullous in the center, and they typically will heal with pigmentation, which is unique to this particular drug reaction,” said Dr. Young. “When it gets more concerning and most important to differentiate is when you get generalized bullous fixed drug eruption.” Generalized bullous fixed drug eruptions mimic and are difficult to clinically distinguish from TEN, which has a much has a much poorer prognosis.
Patients with a fixed drug eruption are not as ill as patients with TEN and tend not to slough their skin to the extent seen with TEN. Interferon gamma, perforin, and Fas ligand have been implicated as mechanisms involved in fixed drug reactions. Unlike in TEN, regulatory T cells are abundant, which may explain why TEN and fixed drug reactions progress differently even though they appear to share pathologic mechanisms, Dr. Young said.
Erythema multiforme generally presents with classic target lesions and little mucosal involvement. Infections, most commonly herpes simplex virus (HSV) 1 and 2, may trigger erythema multiforme. Dr. Young recommends evaluating patients for HSV and checking serologies, even if patients have never had a herpes outbreak. “If you have no evidence for infection, you do have to consider discontinuing a medication,” she said.
Stevens–Johnson syndrome and TEN
SJS and TEN are “the rarest of the severe cutaneous adverse drug reactions” and have “the highest morbidity and mortality,” Dr. Young said. They appear to exist on a continuum where SJS may represent early TEN.
“This is a situation where you expect to see blistering of the skin [and] always mucosal involvement. You need to stop the drug immediately when you suspect this drug reaction,” Dr. Young said.
One reason to distinguish SJS or early TEN from later TEN is that high-dose steroids may play a role in the treatment of SJS or early TEN. “Once you get past about 10% total body surface area, there is good evidence that steroids actually increase morbidity and mortality,” she said.
If the eruption has occurred before, that factor suggests that a diagnosis of erythema multiforme or fixed drug reaction may be more likely than TEN.
An apparent lack of regulatory T cells in TEN could explain why patients with HIV infection are at much higher risk of developing SJS and TEN. Understanding the role that regulatory T cells play in severe drug eruptions may lead to new therapeutic options in the future, Dr. Young said.
Dr. Young had no disclosures.
EXPERT ANALYSIS FROM AES 2018
A Faux Fungal Affliction
A 45-year-old woman is referred to dermatology for a “fungal infection” that has failed to respond to the following treatments: topical clotrimazole cream, topical miconazole cream, a 30-day course of oral terbinafine (250 mg/d), and a 2-month course of oral griseofulvin (unknown dose). The lesions are completely asymptomatic but quite worrisome to the patient since they manifested 6 months ago.
She has consulted at least 6 different providers—none of whom was a dermatologist but all of whom were certain of the diagnosis and thus felt no need to refer the patient. However, the passage of time and trail of ineffective treatments finally prompts the (albeit reluctant) decision to send the patient to dermatology.
On questioning, she denies any serious health problems, such as diabetes or immunosuppression. She has had no contact with any animals or children.
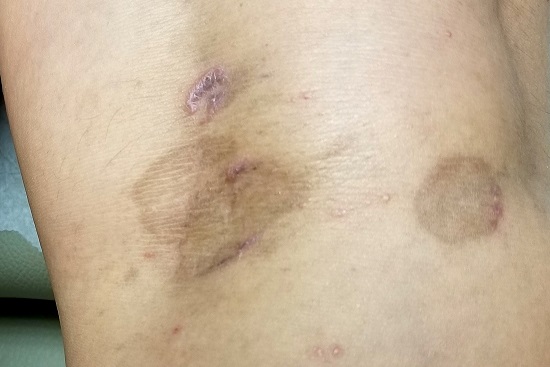
EXAMINATION
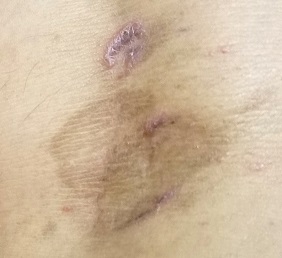
The lesions in question total 6; all are uniformly purplish brown, round, and macular, and they range from 5 mm to more than 3 cm. Most are located on the bilateral popliteal areas. The lesions have sharp, well-defined margins. Several have faintly raised papular margins that give the centers a slightly concave appearance.
Palpation reveals the complete absence of any surface disturbance, such as scaling or erosion. Thus, no KOH prep can be performed to check for fungal elements. Instead, a shave biopsy is performed, the results of which show a sawtooth-patterned lymphocytic infiltrate obliterating the normally smooth undulating dermoepidermal junction.
What’s the diagnosis?
DISCUSSION
This case effectively demonstrates the principle that, when confronted with round or annular lesions, some providers will rely on the diagnosis of “fungal” even when evidence (eg, failed treatment attempts) suggests otherwise. What that nonresponse should do is signal the need for an expanded differential—that is, a consideration of other diagnostic possibilities. This is a bedrock principle in every medical specialty, not just in dermatology.
In this case, the biopsy results clearly pointed to the correct diagnosis of lichen planus (LP), a common dermatosis well known to present in annular morphology. LP is a benign process, albeit one that is occasionally quite bothersome (eg, itching) and, rarely, widespread. LP’s more typical distribution is on volar wrists, in the sacral areas, and occasionally on genitals, so the inability to make a visual diagnosis in this case is forgivable.
Although LP’s etiology is unfortunately unknown, what is known is how to treat it: with topical steroids when necessary or “tincture of time,” as in this patient’s asymptomatic case. LP typically resolves on its own, and it has no worrisome import or connections to more serious disease.
But as always, the first step to correct diagnosis is to consider letting go of the old diagnosis—fungal infection—which was clearly incorrect given the lack of response to numerous antifungals. The second step is to consider other possibilities, which would include lichen planus, psoriasis, granuloma annulare, tinea versicolor, and necrobiosis. The third step is to perform a biopsy, which would establish the correct diagnosis with certainty and in turn, dictate correct treatment.
TAKE-HOME LEARNING POINTS
- There is an extensive differential for round or annular skin lesions that includes many nonfungal causes.
- When antifungals fail to help, consider other diagnostic possibilities.
- Perform a biopsy when a visual diagnosis is not possible.
- Lichen planus (LP) is a common benign inflammatory skin condition that can present with annular lesions.
A 45-year-old woman is referred to dermatology for a “fungal infection” that has failed to respond to the following treatments: topical clotrimazole cream, topical miconazole cream, a 30-day course of oral terbinafine (250 mg/d), and a 2-month course of oral griseofulvin (unknown dose). The lesions are completely asymptomatic but quite worrisome to the patient since they manifested 6 months ago.
She has consulted at least 6 different providers—none of whom was a dermatologist but all of whom were certain of the diagnosis and thus felt no need to refer the patient. However, the passage of time and trail of ineffective treatments finally prompts the (albeit reluctant) decision to send the patient to dermatology.
On questioning, she denies any serious health problems, such as diabetes or immunosuppression. She has had no contact with any animals or children.

EXAMINATION
The lesions in question total 6; all are uniformly purplish brown, round, and macular, and they range from 5 mm to more than 3 cm. Most are located on the bilateral popliteal areas. The lesions have sharp, well-defined margins. Several have faintly raised papular margins that give the centers a slightly concave appearance.
Palpation reveals the complete absence of any surface disturbance, such as scaling or erosion. Thus, no KOH prep can be performed to check for fungal elements. Instead, a shave biopsy is performed, the results of which show a sawtooth-patterned lymphocytic infiltrate obliterating the normally smooth undulating dermoepidermal junction.
What’s the diagnosis?
DISCUSSION
This case effectively demonstrates the principle that, when confronted with round or annular lesions, some providers will rely on the diagnosis of “fungal” even when evidence (eg, failed treatment attempts) suggests otherwise. What that nonresponse should do is signal the need for an expanded differential—that is, a consideration of other diagnostic possibilities. This is a bedrock principle in every medical specialty, not just in dermatology.
In this case, the biopsy results clearly pointed to the correct diagnosis of lichen planus (LP), a common dermatosis well known to present in annular morphology. LP is a benign process, albeit one that is occasionally quite bothersome (eg, itching) and, rarely, widespread. LP’s more typical distribution is on volar wrists, in the sacral areas, and occasionally on genitals, so the inability to make a visual diagnosis in this case is forgivable.
Although LP’s etiology is unfortunately unknown, what is known is how to treat it: with topical steroids when necessary or “tincture of time,” as in this patient’s asymptomatic case. LP typically resolves on its own, and it has no worrisome import or connections to more serious disease.
But as always, the first step to correct diagnosis is to consider letting go of the old diagnosis—fungal infection—which was clearly incorrect given the lack of response to numerous antifungals. The second step is to consider other possibilities, which would include lichen planus, psoriasis, granuloma annulare, tinea versicolor, and necrobiosis. The third step is to perform a biopsy, which would establish the correct diagnosis with certainty and in turn, dictate correct treatment.
TAKE-HOME LEARNING POINTS
- There is an extensive differential for round or annular skin lesions that includes many nonfungal causes.
- When antifungals fail to help, consider other diagnostic possibilities.
- Perform a biopsy when a visual diagnosis is not possible.
- Lichen planus (LP) is a common benign inflammatory skin condition that can present with annular lesions.
A 45-year-old woman is referred to dermatology for a “fungal infection” that has failed to respond to the following treatments: topical clotrimazole cream, topical miconazole cream, a 30-day course of oral terbinafine (250 mg/d), and a 2-month course of oral griseofulvin (unknown dose). The lesions are completely asymptomatic but quite worrisome to the patient since they manifested 6 months ago.
She has consulted at least 6 different providers—none of whom was a dermatologist but all of whom were certain of the diagnosis and thus felt no need to refer the patient. However, the passage of time and trail of ineffective treatments finally prompts the (albeit reluctant) decision to send the patient to dermatology.
On questioning, she denies any serious health problems, such as diabetes or immunosuppression. She has had no contact with any animals or children.

EXAMINATION
The lesions in question total 6; all are uniformly purplish brown, round, and macular, and they range from 5 mm to more than 3 cm. Most are located on the bilateral popliteal areas. The lesions have sharp, well-defined margins. Several have faintly raised papular margins that give the centers a slightly concave appearance.
Palpation reveals the complete absence of any surface disturbance, such as scaling or erosion. Thus, no KOH prep can be performed to check for fungal elements. Instead, a shave biopsy is performed, the results of which show a sawtooth-patterned lymphocytic infiltrate obliterating the normally smooth undulating dermoepidermal junction.
What’s the diagnosis?
DISCUSSION
This case effectively demonstrates the principle that, when confronted with round or annular lesions, some providers will rely on the diagnosis of “fungal” even when evidence (eg, failed treatment attempts) suggests otherwise. What that nonresponse should do is signal the need for an expanded differential—that is, a consideration of other diagnostic possibilities. This is a bedrock principle in every medical specialty, not just in dermatology.
In this case, the biopsy results clearly pointed to the correct diagnosis of lichen planus (LP), a common dermatosis well known to present in annular morphology. LP is a benign process, albeit one that is occasionally quite bothersome (eg, itching) and, rarely, widespread. LP’s more typical distribution is on volar wrists, in the sacral areas, and occasionally on genitals, so the inability to make a visual diagnosis in this case is forgivable.
Although LP’s etiology is unfortunately unknown, what is known is how to treat it: with topical steroids when necessary or “tincture of time,” as in this patient’s asymptomatic case. LP typically resolves on its own, and it has no worrisome import or connections to more serious disease.
But as always, the first step to correct diagnosis is to consider letting go of the old diagnosis—fungal infection—which was clearly incorrect given the lack of response to numerous antifungals. The second step is to consider other possibilities, which would include lichen planus, psoriasis, granuloma annulare, tinea versicolor, and necrobiosis. The third step is to perform a biopsy, which would establish the correct diagnosis with certainty and in turn, dictate correct treatment.
TAKE-HOME LEARNING POINTS
- There is an extensive differential for round or annular skin lesions that includes many nonfungal causes.
- When antifungals fail to help, consider other diagnostic possibilities.
- Perform a biopsy when a visual diagnosis is not possible.
- Lichen planus (LP) is a common benign inflammatory skin condition that can present with annular lesions.
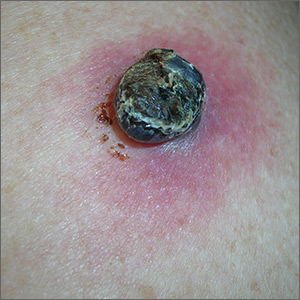
Irregular macule on back

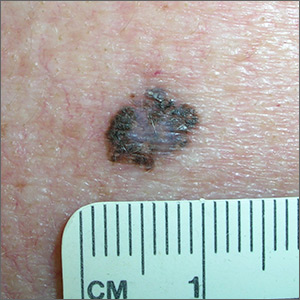
The FP suspected that this could be a melanoma and took out his dermatoscope. (See “Dermoscopy in family medicine: A primer.”) The FP was initially concerned about melanoma because the lesion appeared chaotic, with multiple colors and an irregular border. Going through the ABCDE criteria, he noted that the macule was Asymmetric, the Border was irregular, the Colors were varied, the Diameter was >6 mm, but the history was insufficient to say whether it was Enlarging. Of course, 4 out of 5 positive criteria requires a tissue diagnosis.
Dermoscopy added evidence for regression in the center and an atypical network in the brown and black areas. The FP performed a deep shave biopsy (saucerization) with 2 mm margins to provide full sampling for the pathologist. (See the Watch & Learn video on “Shave biopsy.”) The depth of the tissue biopsy was approximately 1 to 1.5 mm, which was adequate for a lesion of this type. The pathology report confirmed melanoma in situ.
On a return visit, the FP performed a local wide excision with 5 mm margins down to the deep fat. The surgical specimen revealed only the scar at the biopsy site with no remaining cancer. This reassured the patient because it should provide a cure very near 100%. The FP provided counseling about sun protection and the need for regular skin exams.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this could be a melanoma and took out his dermatoscope. (See “Dermoscopy in family medicine: A primer.”) The FP was initially concerned about melanoma because the lesion appeared chaotic, with multiple colors and an irregular border. Going through the ABCDE criteria, he noted that the macule was Asymmetric, the Border was irregular, the Colors were varied, the Diameter was >6 mm, but the history was insufficient to say whether it was Enlarging. Of course, 4 out of 5 positive criteria requires a tissue diagnosis.
Dermoscopy added evidence for regression in the center and an atypical network in the brown and black areas. The FP performed a deep shave biopsy (saucerization) with 2 mm margins to provide full sampling for the pathologist. (See the Watch & Learn video on “Shave biopsy.”) The depth of the tissue biopsy was approximately 1 to 1.5 mm, which was adequate for a lesion of this type. The pathology report confirmed melanoma in situ.
On a return visit, the FP performed a local wide excision with 5 mm margins down to the deep fat. The surgical specimen revealed only the scar at the biopsy site with no remaining cancer. This reassured the patient because it should provide a cure very near 100%. The FP provided counseling about sun protection and the need for regular skin exams.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this could be a melanoma and took out his dermatoscope. (See “Dermoscopy in family medicine: A primer.”) The FP was initially concerned about melanoma because the lesion appeared chaotic, with multiple colors and an irregular border. Going through the ABCDE criteria, he noted that the macule was Asymmetric, the Border was irregular, the Colors were varied, the Diameter was >6 mm, but the history was insufficient to say whether it was Enlarging. Of course, 4 out of 5 positive criteria requires a tissue diagnosis.
Dermoscopy added evidence for regression in the center and an atypical network in the brown and black areas. The FP performed a deep shave biopsy (saucerization) with 2 mm margins to provide full sampling for the pathologist. (See the Watch & Learn video on “Shave biopsy.”) The depth of the tissue biopsy was approximately 1 to 1.5 mm, which was adequate for a lesion of this type. The pathology report confirmed melanoma in situ.
On a return visit, the FP performed a local wide excision with 5 mm margins down to the deep fat. The surgical specimen revealed only the scar at the biopsy site with no remaining cancer. This reassured the patient because it should provide a cure very near 100%. The FP provided counseling about sun protection and the need for regular skin exams.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
New atopic dermatitis agents expand treatment options
GRAND CAYMAN, CAYMAN ISLANDS –
Moisturizers that confer skin barrier protection, lipid-replenishing topicals, and some biologics are available now or will soon be available for patients with AD, Joseph Fowler Jr., MD, said at the Caribbean Dermatology Symposium provided by Global Academy for Medical Education.
With the approval of dupilumab for moderate to severe disease in 2017, a biologic finally became available for treating AD, said Dr. Fowler of the University of Louisville (Ky.). While it’s not a cure and may take as long as 6 months to really kick in, “I think almost everyone gets some benefit from it. And although it’s not approved yet for anyone under 18, I’m sure it will be.”
He provided a brief rundown of dupilumab; crisaborole, another relatively new agent for AD; and some agents that are being investigated.
- Dupilumab. For AD, dupilumab, which inhibits interleukin-4 and interleukin-13 signaling, is usually started at 600 mg, then tapered to 300 mg subcutaneously every 2 weeks. Its pivotal data showed a mean 70% decrease in Eczema Area and Severity Index (EASI) scores over 16 weeks at that dose.*
“Again, I would say most patients do get benefit from this, but they might not see it for more than 3 months, and even up to 6 months. I’m not sure why, but some develop eye symptoms – I think these are more severe cases who also have respiratory atopy. I would also be interested to see if dupilumab might work on patients with chronic hand eczema,” he said.
- Crisaborole ointment 2%. A nonsteroidal topical phosphodiesterase 4 (PDE4) inhibitor approved in 2016 for mild to moderate AD in people aged 2 and older, crisaborole (Eucrisa) blocks the release of cyclic adenosine monophosphate (cAMP), which is elevated in AD. Lower cAMP levels lead to lower levels of inflammatory cytokines. In its pivotal phase 3 study, about 35% of patients achieved clinical success – an Investigator’s Static Global Assessment (ISGA) score of 0 or 1, or at least a two-grade improvement over baseline.
“In my opinion, it’s similar or slightly better than topical corticosteroids, and safer as well, especially in our younger patients, or when the face or intertriginous areas are involved,” Dr. Fowler said. “There is often some application site stinging and burning. If you put it in the fridge and get it good and cold when it goes on, that seems to moderate the sensation. It’s a good steroid-sparing option.”
Crisaborole is now being investigated for use in infants aged 3-24 months with mild to moderate AD.
- Tofacitinib ointment. This topical form of tofacitinib, an inhibitor of Janus kinase 1 and 3, is being evaluated in a placebo-controlled trial in adults with mild to moderate AD. There are also a few reports of oral tofacitinib improving AD, including a case report (Clin Exp Dermatol. 2017 Dec;42[8]:942-4). Dr. Fowler noted a small series of six adults with moderate to severe AD uncontrolled with methotrexate or azathioprine. The patients received oral tofacitinib 5 mg twice a day for 8-29 weeks; there was a mean 67% improvement in the Scoring Atopic Dermatitis (SCORAD) index.
- Ustekinumab. The interleukin-12 and -23 antagonist indicated for moderate to severe psoriasis has also made an appearance in the AD literature, including an Austrian report of three patients with severe AD who received 45 mg of ustekinumab (Stelara) subcutaneously at 0, 4 and 12 weeks. By week 16, all of them experienced a 50% reduction in their EASI score, with a marked reduction in interleukin-22 markers (J Am Acad Dermatol. 2017 Jan;76[1]:91-7.e3).
But no matter which therapy is chosen, regular moisturizing is critically important, Dr. Fowler remarked. Expensive prescription moisturizers are available, but he questioned whether they offer any cost-worthy extra benefit over a good nonprescription moisturizer.
“Do these super-moisturizers protect the skin barrier any more than petrolatum? I can’t answer that. They promise better results, but each patient and doc have to make the decision. If your patient can afford it, maybe some will be better for their skin, but really, it’s not as important as some of the other medications. So, I tell them, if cost is an issue, don’t worry about the fancy moisturizers.”
Dr. Fowler disclosed relationships with multiple pharmaceutical companies.
Global Academy and this news organization are owned by the same parent company.
Correction, 2/15/19: An earlier version of this article mischaracterized the chemical action of dupilumab.
GRAND CAYMAN, CAYMAN ISLANDS –
Moisturizers that confer skin barrier protection, lipid-replenishing topicals, and some biologics are available now or will soon be available for patients with AD, Joseph Fowler Jr., MD, said at the Caribbean Dermatology Symposium provided by Global Academy for Medical Education.
With the approval of dupilumab for moderate to severe disease in 2017, a biologic finally became available for treating AD, said Dr. Fowler of the University of Louisville (Ky.). While it’s not a cure and may take as long as 6 months to really kick in, “I think almost everyone gets some benefit from it. And although it’s not approved yet for anyone under 18, I’m sure it will be.”
He provided a brief rundown of dupilumab; crisaborole, another relatively new agent for AD; and some agents that are being investigated.
- Dupilumab. For AD, dupilumab, which inhibits interleukin-4 and interleukin-13 signaling, is usually started at 600 mg, then tapered to 300 mg subcutaneously every 2 weeks. Its pivotal data showed a mean 70% decrease in Eczema Area and Severity Index (EASI) scores over 16 weeks at that dose.*
“Again, I would say most patients do get benefit from this, but they might not see it for more than 3 months, and even up to 6 months. I’m not sure why, but some develop eye symptoms – I think these are more severe cases who also have respiratory atopy. I would also be interested to see if dupilumab might work on patients with chronic hand eczema,” he said.
- Crisaborole ointment 2%. A nonsteroidal topical phosphodiesterase 4 (PDE4) inhibitor approved in 2016 for mild to moderate AD in people aged 2 and older, crisaborole (Eucrisa) blocks the release of cyclic adenosine monophosphate (cAMP), which is elevated in AD. Lower cAMP levels lead to lower levels of inflammatory cytokines. In its pivotal phase 3 study, about 35% of patients achieved clinical success – an Investigator’s Static Global Assessment (ISGA) score of 0 or 1, or at least a two-grade improvement over baseline.
“In my opinion, it’s similar or slightly better than topical corticosteroids, and safer as well, especially in our younger patients, or when the face or intertriginous areas are involved,” Dr. Fowler said. “There is often some application site stinging and burning. If you put it in the fridge and get it good and cold when it goes on, that seems to moderate the sensation. It’s a good steroid-sparing option.”
Crisaborole is now being investigated for use in infants aged 3-24 months with mild to moderate AD.
- Tofacitinib ointment. This topical form of tofacitinib, an inhibitor of Janus kinase 1 and 3, is being evaluated in a placebo-controlled trial in adults with mild to moderate AD. There are also a few reports of oral tofacitinib improving AD, including a case report (Clin Exp Dermatol. 2017 Dec;42[8]:942-4). Dr. Fowler noted a small series of six adults with moderate to severe AD uncontrolled with methotrexate or azathioprine. The patients received oral tofacitinib 5 mg twice a day for 8-29 weeks; there was a mean 67% improvement in the Scoring Atopic Dermatitis (SCORAD) index.
- Ustekinumab. The interleukin-12 and -23 antagonist indicated for moderate to severe psoriasis has also made an appearance in the AD literature, including an Austrian report of three patients with severe AD who received 45 mg of ustekinumab (Stelara) subcutaneously at 0, 4 and 12 weeks. By week 16, all of them experienced a 50% reduction in their EASI score, with a marked reduction in interleukin-22 markers (J Am Acad Dermatol. 2017 Jan;76[1]:91-7.e3).
But no matter which therapy is chosen, regular moisturizing is critically important, Dr. Fowler remarked. Expensive prescription moisturizers are available, but he questioned whether they offer any cost-worthy extra benefit over a good nonprescription moisturizer.
“Do these super-moisturizers protect the skin barrier any more than petrolatum? I can’t answer that. They promise better results, but each patient and doc have to make the decision. If your patient can afford it, maybe some will be better for their skin, but really, it’s not as important as some of the other medications. So, I tell them, if cost is an issue, don’t worry about the fancy moisturizers.”
Dr. Fowler disclosed relationships with multiple pharmaceutical companies.
Global Academy and this news organization are owned by the same parent company.
Correction, 2/15/19: An earlier version of this article mischaracterized the chemical action of dupilumab.
GRAND CAYMAN, CAYMAN ISLANDS –
Moisturizers that confer skin barrier protection, lipid-replenishing topicals, and some biologics are available now or will soon be available for patients with AD, Joseph Fowler Jr., MD, said at the Caribbean Dermatology Symposium provided by Global Academy for Medical Education.
With the approval of dupilumab for moderate to severe disease in 2017, a biologic finally became available for treating AD, said Dr. Fowler of the University of Louisville (Ky.). While it’s not a cure and may take as long as 6 months to really kick in, “I think almost everyone gets some benefit from it. And although it’s not approved yet for anyone under 18, I’m sure it will be.”
He provided a brief rundown of dupilumab; crisaborole, another relatively new agent for AD; and some agents that are being investigated.
- Dupilumab. For AD, dupilumab, which inhibits interleukin-4 and interleukin-13 signaling, is usually started at 600 mg, then tapered to 300 mg subcutaneously every 2 weeks. Its pivotal data showed a mean 70% decrease in Eczema Area and Severity Index (EASI) scores over 16 weeks at that dose.*
“Again, I would say most patients do get benefit from this, but they might not see it for more than 3 months, and even up to 6 months. I’m not sure why, but some develop eye symptoms – I think these are more severe cases who also have respiratory atopy. I would also be interested to see if dupilumab might work on patients with chronic hand eczema,” he said.
- Crisaborole ointment 2%. A nonsteroidal topical phosphodiesterase 4 (PDE4) inhibitor approved in 2016 for mild to moderate AD in people aged 2 and older, crisaborole (Eucrisa) blocks the release of cyclic adenosine monophosphate (cAMP), which is elevated in AD. Lower cAMP levels lead to lower levels of inflammatory cytokines. In its pivotal phase 3 study, about 35% of patients achieved clinical success – an Investigator’s Static Global Assessment (ISGA) score of 0 or 1, or at least a two-grade improvement over baseline.
“In my opinion, it’s similar or slightly better than topical corticosteroids, and safer as well, especially in our younger patients, or when the face or intertriginous areas are involved,” Dr. Fowler said. “There is often some application site stinging and burning. If you put it in the fridge and get it good and cold when it goes on, that seems to moderate the sensation. It’s a good steroid-sparing option.”
Crisaborole is now being investigated for use in infants aged 3-24 months with mild to moderate AD.
- Tofacitinib ointment. This topical form of tofacitinib, an inhibitor of Janus kinase 1 and 3, is being evaluated in a placebo-controlled trial in adults with mild to moderate AD. There are also a few reports of oral tofacitinib improving AD, including a case report (Clin Exp Dermatol. 2017 Dec;42[8]:942-4). Dr. Fowler noted a small series of six adults with moderate to severe AD uncontrolled with methotrexate or azathioprine. The patients received oral tofacitinib 5 mg twice a day for 8-29 weeks; there was a mean 67% improvement in the Scoring Atopic Dermatitis (SCORAD) index.
- Ustekinumab. The interleukin-12 and -23 antagonist indicated for moderate to severe psoriasis has also made an appearance in the AD literature, including an Austrian report of three patients with severe AD who received 45 mg of ustekinumab (Stelara) subcutaneously at 0, 4 and 12 weeks. By week 16, all of them experienced a 50% reduction in their EASI score, with a marked reduction in interleukin-22 markers (J Am Acad Dermatol. 2017 Jan;76[1]:91-7.e3).
But no matter which therapy is chosen, regular moisturizing is critically important, Dr. Fowler remarked. Expensive prescription moisturizers are available, but he questioned whether they offer any cost-worthy extra benefit over a good nonprescription moisturizer.
“Do these super-moisturizers protect the skin barrier any more than petrolatum? I can’t answer that. They promise better results, but each patient and doc have to make the decision. If your patient can afford it, maybe some will be better for their skin, but really, it’s not as important as some of the other medications. So, I tell them, if cost is an issue, don’t worry about the fancy moisturizers.”
Dr. Fowler disclosed relationships with multiple pharmaceutical companies.
Global Academy and this news organization are owned by the same parent company.
Correction, 2/15/19: An earlier version of this article mischaracterized the chemical action of dupilumab.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Hidradenitis suppurativa linked to increased lymphoma risk
Lymphomas appear to be up to four times more likely in patients with hidradenitis suppurativa than among the general population, Rachel Tannenbaum and her colleagues reported in a Research Letter in JAMA Dermatology.
The risks of Hodgkin (HL), non-Hodgkin (NHL), and cutaneous T-cell lymphoma (CTCL) all were significantly higher among patients with HS, wrote Ms. Tannenbaum, Andrew Strunk, and Amit Garg, MD. Males and older patients carried higher risks than females and younger patients, they found.
The team members, of Hofstra University, Hempstead, N.Y., conducted a health care database study comprising 55 million patients included in 27 integrated U.S. health care systems. All the subjects were at least 18 years old; records indicated active HS during the study period of 2013-2018. A regression analysis controlled for age and sex.
The database contained 62,690 patients with HS. The majority (74%) were female and were aged 44 years or younger (57%).
All three lymphomas were more common among HS patients than patients without HS, including non-Hodgkin lymphoma (0.40% vs. 0.35%,) Hodgkin lymphoma (0.17% vs. 0.09%), and cutaneous T-cell lymphoma (0.06% vs. 0.02%).
The multivariate analysis determined that HS patients were twice as likely to develop both non-Hodgkin and Hodgkin lymphoma (odds ratio, 2.0 and 2.21, respectively). They were four times more likely to develop cutaneous T-cell lymphoma (OR, 4.31).
All three lymphomas were more common among males than females: NHL, 0.62% vs. 0.32%; HL, 0.28% vs. 0.13%; and CTCL, 0.09% vs. 0.04%. This translated into significantly increased HS-associated risks, Ms. Tannenbaum and her coauthors noted. “For example, the [odds ratios] for the association between HS and HL were higher in males (OR, 2.97; 95% confidence interval, 2.22-3.99) than in females (OR, 1.86; 95% CI, 1.44-2.39) (P = .02),” they wrote.
Lymphomas were more common among HS patients in every age group. Patients with HS aged 45-64 years were 38% more likely to develop NHL, and those older than 65, about twice as likely (OR, 1.99).
“To our knowledge, this is the first investigation to systematically evaluate this association in a U.S. population of patients with HS,” the research team concluded.
The study was supported by a grant from AbbVie. Ms. Tannenbaum and Mr. Strunk reported no disclosures. Dr. Garg reported financial relationships with AbbVie and several other pharmaceutical companies.
SOURCE: Tannenbaum R et al. JAMA Dermatol. 2019 Jan 30. doi: 10.1001/jamadermatol.2018.5230.
Lymphomas appear to be up to four times more likely in patients with hidradenitis suppurativa than among the general population, Rachel Tannenbaum and her colleagues reported in a Research Letter in JAMA Dermatology.
The risks of Hodgkin (HL), non-Hodgkin (NHL), and cutaneous T-cell lymphoma (CTCL) all were significantly higher among patients with HS, wrote Ms. Tannenbaum, Andrew Strunk, and Amit Garg, MD. Males and older patients carried higher risks than females and younger patients, they found.
The team members, of Hofstra University, Hempstead, N.Y., conducted a health care database study comprising 55 million patients included in 27 integrated U.S. health care systems. All the subjects were at least 18 years old; records indicated active HS during the study period of 2013-2018. A regression analysis controlled for age and sex.
The database contained 62,690 patients with HS. The majority (74%) were female and were aged 44 years or younger (57%).
All three lymphomas were more common among HS patients than patients without HS, including non-Hodgkin lymphoma (0.40% vs. 0.35%,) Hodgkin lymphoma (0.17% vs. 0.09%), and cutaneous T-cell lymphoma (0.06% vs. 0.02%).
The multivariate analysis determined that HS patients were twice as likely to develop both non-Hodgkin and Hodgkin lymphoma (odds ratio, 2.0 and 2.21, respectively). They were four times more likely to develop cutaneous T-cell lymphoma (OR, 4.31).
All three lymphomas were more common among males than females: NHL, 0.62% vs. 0.32%; HL, 0.28% vs. 0.13%; and CTCL, 0.09% vs. 0.04%. This translated into significantly increased HS-associated risks, Ms. Tannenbaum and her coauthors noted. “For example, the [odds ratios] for the association between HS and HL were higher in males (OR, 2.97; 95% confidence interval, 2.22-3.99) than in females (OR, 1.86; 95% CI, 1.44-2.39) (P = .02),” they wrote.
Lymphomas were more common among HS patients in every age group. Patients with HS aged 45-64 years were 38% more likely to develop NHL, and those older than 65, about twice as likely (OR, 1.99).
“To our knowledge, this is the first investigation to systematically evaluate this association in a U.S. population of patients with HS,” the research team concluded.
The study was supported by a grant from AbbVie. Ms. Tannenbaum and Mr. Strunk reported no disclosures. Dr. Garg reported financial relationships with AbbVie and several other pharmaceutical companies.
SOURCE: Tannenbaum R et al. JAMA Dermatol. 2019 Jan 30. doi: 10.1001/jamadermatol.2018.5230.
Lymphomas appear to be up to four times more likely in patients with hidradenitis suppurativa than among the general population, Rachel Tannenbaum and her colleagues reported in a Research Letter in JAMA Dermatology.
The risks of Hodgkin (HL), non-Hodgkin (NHL), and cutaneous T-cell lymphoma (CTCL) all were significantly higher among patients with HS, wrote Ms. Tannenbaum, Andrew Strunk, and Amit Garg, MD. Males and older patients carried higher risks than females and younger patients, they found.
The team members, of Hofstra University, Hempstead, N.Y., conducted a health care database study comprising 55 million patients included in 27 integrated U.S. health care systems. All the subjects were at least 18 years old; records indicated active HS during the study period of 2013-2018. A regression analysis controlled for age and sex.
The database contained 62,690 patients with HS. The majority (74%) were female and were aged 44 years or younger (57%).
All three lymphomas were more common among HS patients than patients without HS, including non-Hodgkin lymphoma (0.40% vs. 0.35%,) Hodgkin lymphoma (0.17% vs. 0.09%), and cutaneous T-cell lymphoma (0.06% vs. 0.02%).
The multivariate analysis determined that HS patients were twice as likely to develop both non-Hodgkin and Hodgkin lymphoma (odds ratio, 2.0 and 2.21, respectively). They were four times more likely to develop cutaneous T-cell lymphoma (OR, 4.31).
All three lymphomas were more common among males than females: NHL, 0.62% vs. 0.32%; HL, 0.28% vs. 0.13%; and CTCL, 0.09% vs. 0.04%. This translated into significantly increased HS-associated risks, Ms. Tannenbaum and her coauthors noted. “For example, the [odds ratios] for the association between HS and HL were higher in males (OR, 2.97; 95% confidence interval, 2.22-3.99) than in females (OR, 1.86; 95% CI, 1.44-2.39) (P = .02),” they wrote.
Lymphomas were more common among HS patients in every age group. Patients with HS aged 45-64 years were 38% more likely to develop NHL, and those older than 65, about twice as likely (OR, 1.99).
“To our knowledge, this is the first investigation to systematically evaluate this association in a U.S. population of patients with HS,” the research team concluded.
The study was supported by a grant from AbbVie. Ms. Tannenbaum and Mr. Strunk reported no disclosures. Dr. Garg reported financial relationships with AbbVie and several other pharmaceutical companies.
SOURCE: Tannenbaum R et al. JAMA Dermatol. 2019 Jan 30. doi: 10.1001/jamadermatol.2018.5230.
FROM JAMA DERMATOLOGY
Key clinical point: Hidradenitis suppurativa appears to increase the risk of cutaneous T-cell lymphoma, Hodgkin, and non-Hodgkin lymphomas.
Major finding: Lymphomas are up to four times more common among patients with hidradenitis suppurativa than those without the chronic inflammatory disorder.
Study details: The database review comprised more than 55 million patients in 27 linked health care systems.
Disclosures: This study was supported by a grant from AbbVie. Ms. Tannenbaum and Mr. Strunk reported no disclosures. Dr. Garg reported financial relationships with AbbVie and several other pharmaceutical companies.
Source: Tannenbaum R et al. JAMA Dermatol. 2019 Jan 30. doi: 10.1001/jamadermatol.2018.5230.
What is your diagnosis?
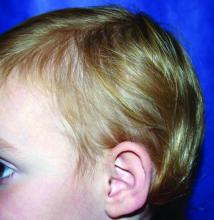
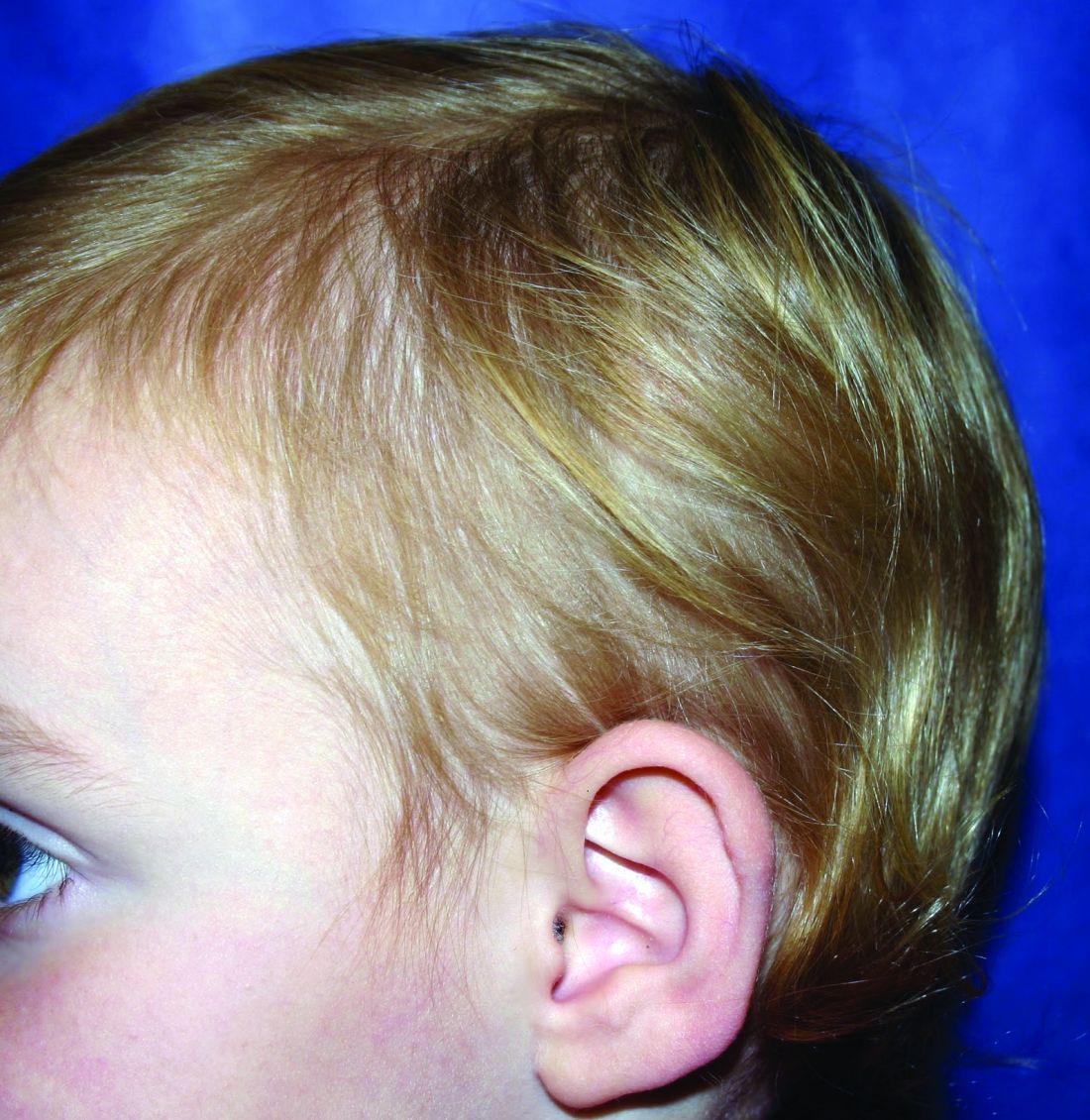
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at [email protected].
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at [email protected].
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at [email protected].
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
A 5-year-old female is brought to clinic for hair loss. The mother reports that when styling her daughter's hair, she has noticed areas of hair thinning, especially at the temples and at the occiput. The mother denies scale, pruritus, and erythema. The patient reports that her scalp does not hurt. She has never had a haircut because her hair hasn't grown long enough to cut. There is no history of specific bald spots. The patient has no personal history of psoriasis, seborrheic dermatitis, or autoimmune disease. No picking has been noted, and there is no history of compulsive behaviors or anxiety. The patient's mother has a history of Graves disease. The mother reports that the patient's older sister may have had hair thinning when she was younger as well, but no longer has thin hair.
The patient was previously seen by another provider who prescribed hydrocortisone 2.5% ointment, which the mother has been applying nightly without improvement.
The child is otherwise medically well and thriving, with no recent change in activity level and with growth parameters consistently around the 75th percentile for height and weight. On physical exam, the patient has blondish, fine hair with areas of poorly demarcated hair thinning at the left temple and at the occiput. The hair remaining at the occiput is normal in texture. There are no areas of complete hair loss. There is no scale, erythema, or abnormal pigmentation, and no cervical or occipital adenopathy. The patient has intact eyebrows and eyelashes.
Adolescence does not rule out bullous pemphigoid
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The course of adolescent bullous pemphigoid appears favorable, with long remission after the disease is controlled.
Major finding: The investigators found nine cases in children aged 10‐13 years, and five cases in children aged 14‐17 years.
Study details: A search in Medline detected 14 adolescents with a diagnosis of bullous pemphigoid.
Disclosures: No funding and no relevant financial disclosures were reported for the work.
Source: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Different disease features found with family history of psoriasis versus PsA
the results of a retrospective cohort study suggest.
A family history of psoriasis was associated with younger onset of psoriatic disease and the presence of enthesitis, while by contrast, a family history of psoriatic arthritis (PsA) was associated with lower risk of plaque psoriasis and higher risk of deformities, according to Dilek Solmaz, MD, of the University of Ottawa and her coauthors, who reported their findings in Arthritis Care & Research.
“The link between family history of psoriasis/psoriatic arthritis and pustular/plaque phenotypes may point to a different genetic background and pathogenic mechanisms in these subsets,” the investigators wrote.
Most, if not all, previous studies evaluating family history have grouped psoriasis and PsA together, according to Dr. Solmaz and her colleagues, rather than looking at the individual effects of psoriasis or PsA family history that may lead to unique disease phenotypes, as was done in the present study.
The investigators based their retrospective analysis on patients recruited in a longitudinal, multicenter database in Turkey and Canada. The mean age of patients in the study was 48 years; nearly 65% were female.
Out of 1,393 patients in the database, 444 had a family history of psoriasis or PsA. That included 335 patients with a psoriasis-only family history and 74 with a family history of PsA; another 35 patients weren’t sure about having a family history of PsA or psoriasis and were left out of the analysis.
Plaque psoriasis was more common in individuals with a family history of only psoriasis, while pustular psoriasis was more common in those with a PsA family history, the investigators reported.
In multivariate analyses, having a family member with psoriasis was a risk factor for younger age of psoriasis onset (odds ratio, 0.976; 95% confidence interval, 0.964-0.989; P less than .001) as well as a higher risk for enthesitis (OR, 1.931; 95% CI, 1.276-2.922; P = .002) when compared against patients without a family history of psoriasis.
Patients with a family history of PsA were more likely to have deformities (OR, 2.557; 95% CI, 1.250-5.234; P less than .010) and lower risk of plaque-type psoriasis (OR, 0.417; 95% CI, 0.213-0.816; P less than .011) than patients without a family history of PsA.
Disease onset was earlier among patients with a family history of psoriasis at a mean of 28.1 years versus 31.9 years for those with a family history of PsA (P less than .001).
Dr. Solmaz and her colleagues reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
SOURCE: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
the results of a retrospective cohort study suggest.
A family history of psoriasis was associated with younger onset of psoriatic disease and the presence of enthesitis, while by contrast, a family history of psoriatic arthritis (PsA) was associated with lower risk of plaque psoriasis and higher risk of deformities, according to Dilek Solmaz, MD, of the University of Ottawa and her coauthors, who reported their findings in Arthritis Care & Research.
“The link between family history of psoriasis/psoriatic arthritis and pustular/plaque phenotypes may point to a different genetic background and pathogenic mechanisms in these subsets,” the investigators wrote.
Most, if not all, previous studies evaluating family history have grouped psoriasis and PsA together, according to Dr. Solmaz and her colleagues, rather than looking at the individual effects of psoriasis or PsA family history that may lead to unique disease phenotypes, as was done in the present study.
The investigators based their retrospective analysis on patients recruited in a longitudinal, multicenter database in Turkey and Canada. The mean age of patients in the study was 48 years; nearly 65% were female.
Out of 1,393 patients in the database, 444 had a family history of psoriasis or PsA. That included 335 patients with a psoriasis-only family history and 74 with a family history of PsA; another 35 patients weren’t sure about having a family history of PsA or psoriasis and were left out of the analysis.
Plaque psoriasis was more common in individuals with a family history of only psoriasis, while pustular psoriasis was more common in those with a PsA family history, the investigators reported.
In multivariate analyses, having a family member with psoriasis was a risk factor for younger age of psoriasis onset (odds ratio, 0.976; 95% confidence interval, 0.964-0.989; P less than .001) as well as a higher risk for enthesitis (OR, 1.931; 95% CI, 1.276-2.922; P = .002) when compared against patients without a family history of psoriasis.
Patients with a family history of PsA were more likely to have deformities (OR, 2.557; 95% CI, 1.250-5.234; P less than .010) and lower risk of plaque-type psoriasis (OR, 0.417; 95% CI, 0.213-0.816; P less than .011) than patients without a family history of PsA.
Disease onset was earlier among patients with a family history of psoriasis at a mean of 28.1 years versus 31.9 years for those with a family history of PsA (P less than .001).
Dr. Solmaz and her colleagues reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
SOURCE: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
the results of a retrospective cohort study suggest.
A family history of psoriasis was associated with younger onset of psoriatic disease and the presence of enthesitis, while by contrast, a family history of psoriatic arthritis (PsA) was associated with lower risk of plaque psoriasis and higher risk of deformities, according to Dilek Solmaz, MD, of the University of Ottawa and her coauthors, who reported their findings in Arthritis Care & Research.
“The link between family history of psoriasis/psoriatic arthritis and pustular/plaque phenotypes may point to a different genetic background and pathogenic mechanisms in these subsets,” the investigators wrote.
Most, if not all, previous studies evaluating family history have grouped psoriasis and PsA together, according to Dr. Solmaz and her colleagues, rather than looking at the individual effects of psoriasis or PsA family history that may lead to unique disease phenotypes, as was done in the present study.
The investigators based their retrospective analysis on patients recruited in a longitudinal, multicenter database in Turkey and Canada. The mean age of patients in the study was 48 years; nearly 65% were female.
Out of 1,393 patients in the database, 444 had a family history of psoriasis or PsA. That included 335 patients with a psoriasis-only family history and 74 with a family history of PsA; another 35 patients weren’t sure about having a family history of PsA or psoriasis and were left out of the analysis.
Plaque psoriasis was more common in individuals with a family history of only psoriasis, while pustular psoriasis was more common in those with a PsA family history, the investigators reported.
In multivariate analyses, having a family member with psoriasis was a risk factor for younger age of psoriasis onset (odds ratio, 0.976; 95% confidence interval, 0.964-0.989; P less than .001) as well as a higher risk for enthesitis (OR, 1.931; 95% CI, 1.276-2.922; P = .002) when compared against patients without a family history of psoriasis.
Patients with a family history of PsA were more likely to have deformities (OR, 2.557; 95% CI, 1.250-5.234; P less than .010) and lower risk of plaque-type psoriasis (OR, 0.417; 95% CI, 0.213-0.816; P less than .011) than patients without a family history of PsA.
Disease onset was earlier among patients with a family history of psoriasis at a mean of 28.1 years versus 31.9 years for those with a family history of PsA (P less than .001).
Dr. Solmaz and her colleagues reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
SOURCE: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Family histories of psoriasis and psoriatic arthritis were linked to different skin phenotypes, disease severity, and musculoskeletal features.
Major finding: Compared with no family history, psoriasis family history was a risk factor for enthesitis (odds ratio, 1.931) and younger age of onset (OR, 0.976) while psoriatic arthritis family history was linked to higher risk of deformities (OR, 2.557) and lower risk of plaque-type psoriasis (OR, 0.417).
Study details: A retrospective analysis including 1,393 Turkish or Canadian patients enrolled in a psoriatic arthritis database.
Disclosures: The study authors reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
Source: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
Black lesion on arm
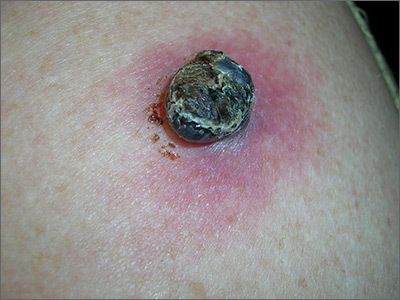
Due to the dark and rapidly growing nodule, the FP immediately worried about melanoma.
He thought that he should biopsy the entire lesion with an elliptical excision, so he scheduled the patient for a biopsy during some protected surgical time later that week. The patient did not show up for this appointment. Several calls were placed, and she returned for the biopsy the following week. The FP performed a narrow margin (2 mm) elliptical excision oriented to match the lymphatic drainage of the arm. He closed the excision with a 2-layer closure. (See the Watch & Learn video on elliptical excision.) The pathology report confirmed that it was a nodular melanoma that was 8 mm in depth. This was clearly an aggressive tumor, so the patient was referred to Surgical Oncology for sentinel lymph node biopsy. One node was positive for metastasis.
After a wide excision with 2 cm margins by Surgical Oncology, the patient underwent a course of chemotherapy and remained disease free 2 years later. She was carefully monitored for metastasis and new primary lesions by a multidisciplinary team that included family medicine, dermatology, and oncology.
While this FP handled the case in an excellent matter, he was fortunate to have the skills and time to be able to perform a full elliptical excision. It’s important to note that a 6 mm punch biopsy or a deep shave biopsy (saucerization) at the base of the thickest portion of this tumor would almost certainly have provided the same diagnosis of melanoma and at least showed that the tumor was thicker than 4 mm (an important cut-off for management). This could have been done on the day of original presentation and might have avoided the problem of the patient not showing up for the next appointment or a long delay to see a dermatologist.
FPs should be empowered to perform biopsies on the most worrisome of lesions as these biopsies can save lives. While incomplete sampling can result in false negative results and misdiagnosis, the protection against this is to not accept a benign pathology report in what appears to be an obvious malignancy. If this occurs, the next step is always complete excision. Having options and understanding potential sampling errors can help FPs diagnose patients more rapidly. This is essential when cancers are rapidly growing and delays of months for surgical appointments or referrals to specialists can worsen a prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Due to the dark and rapidly growing nodule, the FP immediately worried about melanoma.
He thought that he should biopsy the entire lesion with an elliptical excision, so he scheduled the patient for a biopsy during some protected surgical time later that week. The patient did not show up for this appointment. Several calls were placed, and she returned for the biopsy the following week. The FP performed a narrow margin (2 mm) elliptical excision oriented to match the lymphatic drainage of the arm. He closed the excision with a 2-layer closure. (See the Watch & Learn video on elliptical excision.) The pathology report confirmed that it was a nodular melanoma that was 8 mm in depth. This was clearly an aggressive tumor, so the patient was referred to Surgical Oncology for sentinel lymph node biopsy. One node was positive for metastasis.
After a wide excision with 2 cm margins by Surgical Oncology, the patient underwent a course of chemotherapy and remained disease free 2 years later. She was carefully monitored for metastasis and new primary lesions by a multidisciplinary team that included family medicine, dermatology, and oncology.
While this FP handled the case in an excellent matter, he was fortunate to have the skills and time to be able to perform a full elliptical excision. It’s important to note that a 6 mm punch biopsy or a deep shave biopsy (saucerization) at the base of the thickest portion of this tumor would almost certainly have provided the same diagnosis of melanoma and at least showed that the tumor was thicker than 4 mm (an important cut-off for management). This could have been done on the day of original presentation and might have avoided the problem of the patient not showing up for the next appointment or a long delay to see a dermatologist.
FPs should be empowered to perform biopsies on the most worrisome of lesions as these biopsies can save lives. While incomplete sampling can result in false negative results and misdiagnosis, the protection against this is to not accept a benign pathology report in what appears to be an obvious malignancy. If this occurs, the next step is always complete excision. Having options and understanding potential sampling errors can help FPs diagnose patients more rapidly. This is essential when cancers are rapidly growing and delays of months for surgical appointments or referrals to specialists can worsen a prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Due to the dark and rapidly growing nodule, the FP immediately worried about melanoma.
He thought that he should biopsy the entire lesion with an elliptical excision, so he scheduled the patient for a biopsy during some protected surgical time later that week. The patient did not show up for this appointment. Several calls were placed, and she returned for the biopsy the following week. The FP performed a narrow margin (2 mm) elliptical excision oriented to match the lymphatic drainage of the arm. He closed the excision with a 2-layer closure. (See the Watch & Learn video on elliptical excision.) The pathology report confirmed that it was a nodular melanoma that was 8 mm in depth. This was clearly an aggressive tumor, so the patient was referred to Surgical Oncology for sentinel lymph node biopsy. One node was positive for metastasis.
After a wide excision with 2 cm margins by Surgical Oncology, the patient underwent a course of chemotherapy and remained disease free 2 years later. She was carefully monitored for metastasis and new primary lesions by a multidisciplinary team that included family medicine, dermatology, and oncology.
While this FP handled the case in an excellent matter, he was fortunate to have the skills and time to be able to perform a full elliptical excision. It’s important to note that a 6 mm punch biopsy or a deep shave biopsy (saucerization) at the base of the thickest portion of this tumor would almost certainly have provided the same diagnosis of melanoma and at least showed that the tumor was thicker than 4 mm (an important cut-off for management). This could have been done on the day of original presentation and might have avoided the problem of the patient not showing up for the next appointment or a long delay to see a dermatologist.
FPs should be empowered to perform biopsies on the most worrisome of lesions as these biopsies can save lives. While incomplete sampling can result in false negative results and misdiagnosis, the protection against this is to not accept a benign pathology report in what appears to be an obvious malignancy. If this occurs, the next step is always complete excision. Having options and understanding potential sampling errors can help FPs diagnose patients more rapidly. This is essential when cancers are rapidly growing and delays of months for surgical appointments or referrals to specialists can worsen a prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Psoriatic arthritis eludes early diagnosis
Most patients with psoriatic arthritis first present with psoriasis only. Their skin disorder precedes any joint involvement, often by several years. That suggests targeting interventions to patients with psoriasis to prevent or slow their progression to psoriatic arthritis, as well as following psoriatic patients closely to diagnose psoriatic arthritis quickly when it first appears. It’s a simple and attractive management premise that’s been challenging to apply in practice.
It’s not that clinicians aren’t motivated to diagnose psoriatic arthritis (PsA) in patients early, hopefully as soon as it appears. The susceptibility of patients with psoriasis to develop PsA is well described, with an annual progression rate of about 3%, and adverse consequences result from even a 6-month delay in diagnosis.
“Some physicians still don’t ask psoriasis patients about joint pain, or their symptoms are misinterpreted as something else,” said Lihi Eder, MD, a rheumatologist at Women’s College Research Institute, Toronto, and the University of Toronto. “Although there is increased awareness about PsA, there are still delays in diagnosis,” she said in an interview.
“Often there is a massive delay in diagnosis, and we know from a number of studies that longer duration of symptoms before diagnosis is associated with poorer outcomes,” said Laura C. Coates, MBChB, PhD, a rheumatologist at the University of Oxford (England). The delay to PsA diagnosis is generally “longer than for equivalent rheumatoid arthritis patients. PsA patients take longer to ask a primary care physician for help, longer to get a referral to a rheumatologist, and longer to get a diagnosis” from a rheumatologist. “We need to educate patients with psoriasis about their risk so that they seek help, educate GPs about whom to refer, and educate rheumatologists about diagnosis,” Dr. Coates said.
“It is very important to diagnose PsA as early as possible. We know that a delay in diagnosis and treatment can lead to worse outcomes and joint damage,” said Soumya M. Reddy, MD, codirector of the Psoriasis and Psoriatic Arthritis Center at New York University Langone Health in New York. “The heterogeneity of clinical manifestations of PsA can make it difficult to diagnose, and in some cases this leads to delayed diagnosis.”
“We are increasingly interested in the concept of preventing PsA. Psoriasis is a unique disease state in which we have an at-risk population where 30% will develop an inflammatory and potentially damaging arthritis. This may become important as our skin treatments may also treat musculoskeletal components of the disease,” said Joseph F. Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital in Boston and double board certified in dermatology and rheumatology.
Focusing on patients with psoriasis
Treatment of psoriasis makes sense to address several quality-of-life issues, but controlling the severity of psoriatic skin manifestations gives patients no guarantees about their possible progression to PsA.
“So many people have mild psoriasis that, although they are less likely, proportionately, to get PsA, we still see many in the clinic,” said Dr. Coates. “Patients with severe skin involvement have a good reason to get treatment, which could help test whether a drug slows progression to arthritis. But it’s unethical to not treat severe psoriasis just to have a comparison group.” Aside from skin psoriasis, “we don’t have any other good markers,” Dr. Coates noted.
PsA can develop in patients who had psoriasis in the past but without currently active disease. “At the level of individual patients you can’t say someone is protected from developing PsA because their psoriasis is inactive,” Dr. Eder said. “No study has looked at whether treatment of psoriasis reduces the risk for progression to PsA. We don’t know whether any treatments that reduce inflammation in psoriasis also reduce progression to PsA.” Regardless, treating psoriasis is important because it improves quality of life and may have a beneficial, long-term effects on possible cardiovascular disease effects from psoriasis, Dr. Eder said.
Her research group is now studying the associations among different biological drugs taken by patients with psoriasis and their subsequent incidence of PsA with use of medical records from about 4 million Israeli residents. Other studies are also looking at this, but they are all observational and therefore are subject to unidentified confounders, she noted. A more definitive demonstration of PsA protection would need a randomized trial.
“The idea of preventing the transition from psoriasis to PsA is an exciting concept. But currently, there are no established treatments for preventing transition of psoriasis to PsA. It’s an area of active research, and the holy grail of psoriatic disease research. We do not yet know whether successful treatment of skin psoriasis delays the onset of arthritis,” Dr. Reddy said in an interview.
“The risk of developing PsA increases as the severity of skin psoriasis increases, measured by percentage of body surface area affected. However, there is only a weak correlation between the severity of skin disease and the severity of PsA,” said Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania in Philadelphia.
Widening the PsA diagnostic net
What’s elusive is some other parameter to identify the 3% of psoriasis patients who will develop PsA during the next year – or at least a better way to find patients as soon as they have what is identifiably PsA.
The diagnostic definition for PsA that many experts now accept is actually a set of classification criteria, CASPAR (Classification Criteria for Psoriatic Arthritis) (Arthritis Rheum. 2006 Aug;54[8]:2665-73). But in actual practice, diagnosing PsA relies heavily on clinical skill, case recognition, and ruling out mimickers.
“The CASPAR criteria were developed as classification criteria to use in studies, but they are also quite helpful in diagnosing PsA. It is the agreed on definition of PsA at the moment,” said Alexis Ogdie, MD, director of the psoriatic arthritis clinic at the University of Pennsylvania.
“CASPAR fills the role at present for clinical trials, but the diagnosis remains clinical, based on history, physical findings, and supported by lab and radiology findings,” Dr. Merola said. “A clinical prediction tool and a proper biomarker would be of great value.”
“We certainly use CASPAR criteria in the clinic, but they do not include all PsA patients; sometimes we diagnose PsA in patients who don’t meet the CASPAR criteria,” Dr. Coates said.
“In practice, rheumatologists mostly use their clinical judgment: a patient with history and findings typical of PsA after ruling out other causes. But distinguishing inflammatory from noninflammatory arthritis – like osteoarthritis or fibromyalgia – can be quite difficult; that’s the main challenge,” Dr. Eder said. “Rheumatologists rely on their clinical skill and experience. PsA can be difficult to diagnose. There is no biomarker for it. PsA is complex [to diagnose], and that’s why it’s diagnosed later. A primary care physician might get a negative result on a rheumatoid factor test and think that rules out PsA, but it doesn’t.”
Dr. Eder and others suggest that ultrasound may be a useful tool for earlier diagnosis of PsA. Ultrasound is more sensitive than a physical examination and can detect inflammation in joints and entheses, she noted. Another effective method may be more systematic use of screening questionnaires, Dr. Coates said, or simply more systematic questioning of patients.
“Questionnaires are not a high priority for a primary care physician who may have only a few patients with psoriasis. Even some dermatologists may not use the questionnaires because they take time to administer and assess. But just asking a psoriasis patient whether they have joint symptoms is enough,” Dr. Eder said. All clinicians who encounter patients with psoriasis should ask about musculoskeletal symptoms and refer when appropriate to a rheumatologist, Dr. Reddy said.
The earliest indicators of PsA
Evidence supporting ultrasound’s value for early PsA diagnosis came in a report at the most recent annual meeting of the American College of Rheumatology in Chicago in October 2018. Researchers from the University of Rochester (New York) used ultrasound to examine 78 patients with psoriasis but without PsA or musculoskeletal symptoms and 25 healthy controls. They found ultrasound abnormalities in almost half of the patients with psoriasis, significantly more than in the controls (Thiele R et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2904).
Another report at the ACR annual meeting last October looked at the incidence of physician visits for nonspecific musculoskeletal symptoms during each of the 5 years preceding diagnosis of PsA. A prior report from Dr. Eder and her associates had documented in an observational cohort of 410 patients with psoriasis that, prior to development of PsA, patients often had nonspecific musculoskeletal symptoms of joint pain, fatigue, and stiffness that constituted a “preclinical” phase (Arthritis Rheumatol. 2017 March;69[3]:622-9).
In October, Dr. Eder reported how the appearance of musculoskeletal symptoms played out in terms of physician visits. She and her associates analyzed data from an Ontario health insurance database that included about 430,000 Ontario patients seen by 466 primary care physicians, which included 462 patients with a new diagnosis of PsA and 2,310 matched controls. The results showed that, in every year during the 5 years preceding diagnosis of PsA, the patients who would wind up getting diagnosed had roughly twice the number of visits to a primary care physician each year for nonspecific musculoskeletal issues. A similar pattern of doubled visits occurred for people prior to their PsA diagnosis going to physicians who specialize in musculoskeletal conditions, and when the analysis focused on visits to rheumatologists, patients who went on to get diagnosed with PsA had a nearly sevenfold increased rate of these visits, compared with controls, for each of the 5 years preceding their PsA diagnosis (Eder L et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 967).
These results “highlight that in many patients PsA is not an acute disease that starts suddenly. In many patients, there is a period when the patient experiences musculoskeletal symptoms and sees a primary care physician or rheumatologist and may be diagnosed with something that is not PsA. That means that the delay in diagnosis [of PsA] may have happened because the patients were misdiagnosed. It reinforces the need for better diagnostic tools,” Dr. Eder said. “We have focused on getting these patients to see a rheumatologist earlier, but that may not be enough. These patients may not receive routine follow-up; we need to do more follow-up on patients like these.” Diagnosing PsA early means earlier treatment, a better chance of reaching remission, less chance of permanent joint damage, and better quality of life.
The challenges of making an early diagnosis were also documented in a study reported by Dr. Ogdie during the June 2018 annual congress of the European League Against Rheumatism. Dr. Ogdie reported on the survey responses of 203 patients who said they had been diagnosed with PsA whose index diagnosis was a median of 6 years before they completed the survey. A total of 195 of these patients, or 96%, said that they had received at least one misdiagnosis prior to their PsA diagnosis (Odgie A et al. Ann Rheum Dis. 2018;77[Suppl 2]:163. Abstract THU0292). The most common misdiagnoses were psychosomatic disease, reported by 27% of the patients; osteoarthritis in 22%; anxiety or depression in 18%; and an orthopedic problem in 18%. (Patients could report more than one type of misdiagnosis.)
The results “showed that patients often had substantial delays and misdiagnoses before they received a PsA diagnosis,” Dr. Ogdie and her associates concluded. Although the CASPAR classification criteria may be the agreed on PsA definition, recent findings suggest a pre-PsA stage exists with musculoskeletal and other abnormalities. “How may we diagnose ‘pre-PsA’? How might we capture this transition phase from psoriasis to PsA before the CASPAR criteria are fulfilled,” she wondered in an interview. “If we could stop PsA before it is clinically relevant, that could dramatically change the course of the disease. This is a big need in the field right now.”
Weight loss and other interventions
Aside from treating psoriasis and perhaps putting a patient with psoriasis in a PsA-prevention trial, one of the best ways to prevent PsA may be weight loss.
Results from “some studies suggest that being overweight increases the risk for developing PsA. Obesity also exacerbates skin psoriasis, makes treatment less effective, and further increases the risk of cardiometabolic diseases associated with psoriasis,” Dr. Gelfand said. “All patients with psoriatic disease should be educated about the importance of maintaining a healthy body weight.”
“Several studies suggest that obesity is a risk factor for developing PsA. Obesity likely plays a role in driving or contributing to inflammation in psoriatic disease,” said Dr. Reddy, who noted that other PsA risk factors include nail psoriasis and first-degree relatives with PsA. Dr. Ogdie also cited uveitis and prior joint trauma as other risk factors.
“Strong observational data link obesity and PsA incidence. I talk to psoriasis patients about weight control, and selected patients could even consider bariatric surgery,” Dr. Eder said. Losing at least 5% of body mass index can make a difference, she added.
At the 2018 annual meeting of the American College of Rheumatology, researchers from the University of Bath (England) reported results from a retrospective, observational study of more than 90,000 people with recent-onset psoriasis; they found that people with an obese BMI had twice the rate of progression to PsA when compared with people with a normal BMI. Overweight people had a nearly 80% higher rate of incident PsA (Green A et al. Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2134).
Hints have also emerged that new approaches to treating psoriasis also could help to keep PsA precursors at bay. One recent example from researchers at the University of Leeds (England) was a phase 2 study of 73 patients with moderate to severe psoriasis but no PsA who underwent ultrasound screening of their entheses for signs of inflammatory changes. The 23 patients underwent 52 weeks of treatment with the drug ustekinumab (Stelara), an antagonist of interleukin-12 and -23 that is approved for U.S. marketing to treat both psoriasis and PsA. After 24 weeks on treatment, their mean inflammation scores had dropped by more than 40%, and the effect persisted through 52 weeks of treatment (Arthritis Rheum. 2018 Nov 22. doi: 10.1002/art.40778).
Despite this promise, the researchers “haven’t looked long enough or in enough people to see whether this actually stops patients from developing PsA,” Dr. Coates commented. It also remains unclear whether this or another ultrasound abnormality detectable in joints or entheses is a reliable predictor of PsA, she noted.
“We still have a lot to learn about how to classify patients as high risk” for PsA, Dr. Ogdie concluded.
Dr. Eder has received research and educational grants from AbbVie, Amgen, Celgene, Lilly, Novartis, and UCB. Dr. Coates has received honoraria, research funding, or both from more than a dozen companies. Dr. Reddy has been a consultant to AbbVie, Novartis, Pfizer, and UCB. Dr. Merola has been a consultant to AbbVie, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Samumed, Sanofi, and UCB and has received research grants from Aclaris, Biogen, Incyte, Novartis, Pfizer, and Sanofi. Dr. Gelfand has been a consultant, adviser, or both to more than a dozen companies. Dr. Ogdie has been a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Lilly, Novartis, Pfizer, and Takeda.
Most patients with psoriatic arthritis first present with psoriasis only. Their skin disorder precedes any joint involvement, often by several years. That suggests targeting interventions to patients with psoriasis to prevent or slow their progression to psoriatic arthritis, as well as following psoriatic patients closely to diagnose psoriatic arthritis quickly when it first appears. It’s a simple and attractive management premise that’s been challenging to apply in practice.
It’s not that clinicians aren’t motivated to diagnose psoriatic arthritis (PsA) in patients early, hopefully as soon as it appears. The susceptibility of patients with psoriasis to develop PsA is well described, with an annual progression rate of about 3%, and adverse consequences result from even a 6-month delay in diagnosis.
“Some physicians still don’t ask psoriasis patients about joint pain, or their symptoms are misinterpreted as something else,” said Lihi Eder, MD, a rheumatologist at Women’s College Research Institute, Toronto, and the University of Toronto. “Although there is increased awareness about PsA, there are still delays in diagnosis,” she said in an interview.
“Often there is a massive delay in diagnosis, and we know from a number of studies that longer duration of symptoms before diagnosis is associated with poorer outcomes,” said Laura C. Coates, MBChB, PhD, a rheumatologist at the University of Oxford (England). The delay to PsA diagnosis is generally “longer than for equivalent rheumatoid arthritis patients. PsA patients take longer to ask a primary care physician for help, longer to get a referral to a rheumatologist, and longer to get a diagnosis” from a rheumatologist. “We need to educate patients with psoriasis about their risk so that they seek help, educate GPs about whom to refer, and educate rheumatologists about diagnosis,” Dr. Coates said.
“It is very important to diagnose PsA as early as possible. We know that a delay in diagnosis and treatment can lead to worse outcomes and joint damage,” said Soumya M. Reddy, MD, codirector of the Psoriasis and Psoriatic Arthritis Center at New York University Langone Health in New York. “The heterogeneity of clinical manifestations of PsA can make it difficult to diagnose, and in some cases this leads to delayed diagnosis.”
“We are increasingly interested in the concept of preventing PsA. Psoriasis is a unique disease state in which we have an at-risk population where 30% will develop an inflammatory and potentially damaging arthritis. This may become important as our skin treatments may also treat musculoskeletal components of the disease,” said Joseph F. Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital in Boston and double board certified in dermatology and rheumatology.
Focusing on patients with psoriasis
Treatment of psoriasis makes sense to address several quality-of-life issues, but controlling the severity of psoriatic skin manifestations gives patients no guarantees about their possible progression to PsA.
“So many people have mild psoriasis that, although they are less likely, proportionately, to get PsA, we still see many in the clinic,” said Dr. Coates. “Patients with severe skin involvement have a good reason to get treatment, which could help test whether a drug slows progression to arthritis. But it’s unethical to not treat severe psoriasis just to have a comparison group.” Aside from skin psoriasis, “we don’t have any other good markers,” Dr. Coates noted.
PsA can develop in patients who had psoriasis in the past but without currently active disease. “At the level of individual patients you can’t say someone is protected from developing PsA because their psoriasis is inactive,” Dr. Eder said. “No study has looked at whether treatment of psoriasis reduces the risk for progression to PsA. We don’t know whether any treatments that reduce inflammation in psoriasis also reduce progression to PsA.” Regardless, treating psoriasis is important because it improves quality of life and may have a beneficial, long-term effects on possible cardiovascular disease effects from psoriasis, Dr. Eder said.
Her research group is now studying the associations among different biological drugs taken by patients with psoriasis and their subsequent incidence of PsA with use of medical records from about 4 million Israeli residents. Other studies are also looking at this, but they are all observational and therefore are subject to unidentified confounders, she noted. A more definitive demonstration of PsA protection would need a randomized trial.
“The idea of preventing the transition from psoriasis to PsA is an exciting concept. But currently, there are no established treatments for preventing transition of psoriasis to PsA. It’s an area of active research, and the holy grail of psoriatic disease research. We do not yet know whether successful treatment of skin psoriasis delays the onset of arthritis,” Dr. Reddy said in an interview.
“The risk of developing PsA increases as the severity of skin psoriasis increases, measured by percentage of body surface area affected. However, there is only a weak correlation between the severity of skin disease and the severity of PsA,” said Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania in Philadelphia.
Widening the PsA diagnostic net
What’s elusive is some other parameter to identify the 3% of psoriasis patients who will develop PsA during the next year – or at least a better way to find patients as soon as they have what is identifiably PsA.
The diagnostic definition for PsA that many experts now accept is actually a set of classification criteria, CASPAR (Classification Criteria for Psoriatic Arthritis) (Arthritis Rheum. 2006 Aug;54[8]:2665-73). But in actual practice, diagnosing PsA relies heavily on clinical skill, case recognition, and ruling out mimickers.
“The CASPAR criteria were developed as classification criteria to use in studies, but they are also quite helpful in diagnosing PsA. It is the agreed on definition of PsA at the moment,” said Alexis Ogdie, MD, director of the psoriatic arthritis clinic at the University of Pennsylvania.
“CASPAR fills the role at present for clinical trials, but the diagnosis remains clinical, based on history, physical findings, and supported by lab and radiology findings,” Dr. Merola said. “A clinical prediction tool and a proper biomarker would be of great value.”
“We certainly use CASPAR criteria in the clinic, but they do not include all PsA patients; sometimes we diagnose PsA in patients who don’t meet the CASPAR criteria,” Dr. Coates said.
“In practice, rheumatologists mostly use their clinical judgment: a patient with history and findings typical of PsA after ruling out other causes. But distinguishing inflammatory from noninflammatory arthritis – like osteoarthritis or fibromyalgia – can be quite difficult; that’s the main challenge,” Dr. Eder said. “Rheumatologists rely on their clinical skill and experience. PsA can be difficult to diagnose. There is no biomarker for it. PsA is complex [to diagnose], and that’s why it’s diagnosed later. A primary care physician might get a negative result on a rheumatoid factor test and think that rules out PsA, but it doesn’t.”
Dr. Eder and others suggest that ultrasound may be a useful tool for earlier diagnosis of PsA. Ultrasound is more sensitive than a physical examination and can detect inflammation in joints and entheses, she noted. Another effective method may be more systematic use of screening questionnaires, Dr. Coates said, or simply more systematic questioning of patients.
“Questionnaires are not a high priority for a primary care physician who may have only a few patients with psoriasis. Even some dermatologists may not use the questionnaires because they take time to administer and assess. But just asking a psoriasis patient whether they have joint symptoms is enough,” Dr. Eder said. All clinicians who encounter patients with psoriasis should ask about musculoskeletal symptoms and refer when appropriate to a rheumatologist, Dr. Reddy said.
The earliest indicators of PsA
Evidence supporting ultrasound’s value for early PsA diagnosis came in a report at the most recent annual meeting of the American College of Rheumatology in Chicago in October 2018. Researchers from the University of Rochester (New York) used ultrasound to examine 78 patients with psoriasis but without PsA or musculoskeletal symptoms and 25 healthy controls. They found ultrasound abnormalities in almost half of the patients with psoriasis, significantly more than in the controls (Thiele R et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2904).
Another report at the ACR annual meeting last October looked at the incidence of physician visits for nonspecific musculoskeletal symptoms during each of the 5 years preceding diagnosis of PsA. A prior report from Dr. Eder and her associates had documented in an observational cohort of 410 patients with psoriasis that, prior to development of PsA, patients often had nonspecific musculoskeletal symptoms of joint pain, fatigue, and stiffness that constituted a “preclinical” phase (Arthritis Rheumatol. 2017 March;69[3]:622-9).
In October, Dr. Eder reported how the appearance of musculoskeletal symptoms played out in terms of physician visits. She and her associates analyzed data from an Ontario health insurance database that included about 430,000 Ontario patients seen by 466 primary care physicians, which included 462 patients with a new diagnosis of PsA and 2,310 matched controls. The results showed that, in every year during the 5 years preceding diagnosis of PsA, the patients who would wind up getting diagnosed had roughly twice the number of visits to a primary care physician each year for nonspecific musculoskeletal issues. A similar pattern of doubled visits occurred for people prior to their PsA diagnosis going to physicians who specialize in musculoskeletal conditions, and when the analysis focused on visits to rheumatologists, patients who went on to get diagnosed with PsA had a nearly sevenfold increased rate of these visits, compared with controls, for each of the 5 years preceding their PsA diagnosis (Eder L et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 967).
These results “highlight that in many patients PsA is not an acute disease that starts suddenly. In many patients, there is a period when the patient experiences musculoskeletal symptoms and sees a primary care physician or rheumatologist and may be diagnosed with something that is not PsA. That means that the delay in diagnosis [of PsA] may have happened because the patients were misdiagnosed. It reinforces the need for better diagnostic tools,” Dr. Eder said. “We have focused on getting these patients to see a rheumatologist earlier, but that may not be enough. These patients may not receive routine follow-up; we need to do more follow-up on patients like these.” Diagnosing PsA early means earlier treatment, a better chance of reaching remission, less chance of permanent joint damage, and better quality of life.
The challenges of making an early diagnosis were also documented in a study reported by Dr. Ogdie during the June 2018 annual congress of the European League Against Rheumatism. Dr. Ogdie reported on the survey responses of 203 patients who said they had been diagnosed with PsA whose index diagnosis was a median of 6 years before they completed the survey. A total of 195 of these patients, or 96%, said that they had received at least one misdiagnosis prior to their PsA diagnosis (Odgie A et al. Ann Rheum Dis. 2018;77[Suppl 2]:163. Abstract THU0292). The most common misdiagnoses were psychosomatic disease, reported by 27% of the patients; osteoarthritis in 22%; anxiety or depression in 18%; and an orthopedic problem in 18%. (Patients could report more than one type of misdiagnosis.)
The results “showed that patients often had substantial delays and misdiagnoses before they received a PsA diagnosis,” Dr. Ogdie and her associates concluded. Although the CASPAR classification criteria may be the agreed on PsA definition, recent findings suggest a pre-PsA stage exists with musculoskeletal and other abnormalities. “How may we diagnose ‘pre-PsA’? How might we capture this transition phase from psoriasis to PsA before the CASPAR criteria are fulfilled,” she wondered in an interview. “If we could stop PsA before it is clinically relevant, that could dramatically change the course of the disease. This is a big need in the field right now.”
Weight loss and other interventions
Aside from treating psoriasis and perhaps putting a patient with psoriasis in a PsA-prevention trial, one of the best ways to prevent PsA may be weight loss.
Results from “some studies suggest that being overweight increases the risk for developing PsA. Obesity also exacerbates skin psoriasis, makes treatment less effective, and further increases the risk of cardiometabolic diseases associated with psoriasis,” Dr. Gelfand said. “All patients with psoriatic disease should be educated about the importance of maintaining a healthy body weight.”
“Several studies suggest that obesity is a risk factor for developing PsA. Obesity likely plays a role in driving or contributing to inflammation in psoriatic disease,” said Dr. Reddy, who noted that other PsA risk factors include nail psoriasis and first-degree relatives with PsA. Dr. Ogdie also cited uveitis and prior joint trauma as other risk factors.
“Strong observational data link obesity and PsA incidence. I talk to psoriasis patients about weight control, and selected patients could even consider bariatric surgery,” Dr. Eder said. Losing at least 5% of body mass index can make a difference, she added.
At the 2018 annual meeting of the American College of Rheumatology, researchers from the University of Bath (England) reported results from a retrospective, observational study of more than 90,000 people with recent-onset psoriasis; they found that people with an obese BMI had twice the rate of progression to PsA when compared with people with a normal BMI. Overweight people had a nearly 80% higher rate of incident PsA (Green A et al. Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2134).
Hints have also emerged that new approaches to treating psoriasis also could help to keep PsA precursors at bay. One recent example from researchers at the University of Leeds (England) was a phase 2 study of 73 patients with moderate to severe psoriasis but no PsA who underwent ultrasound screening of their entheses for signs of inflammatory changes. The 23 patients underwent 52 weeks of treatment with the drug ustekinumab (Stelara), an antagonist of interleukin-12 and -23 that is approved for U.S. marketing to treat both psoriasis and PsA. After 24 weeks on treatment, their mean inflammation scores had dropped by more than 40%, and the effect persisted through 52 weeks of treatment (Arthritis Rheum. 2018 Nov 22. doi: 10.1002/art.40778).
Despite this promise, the researchers “haven’t looked long enough or in enough people to see whether this actually stops patients from developing PsA,” Dr. Coates commented. It also remains unclear whether this or another ultrasound abnormality detectable in joints or entheses is a reliable predictor of PsA, she noted.
“We still have a lot to learn about how to classify patients as high risk” for PsA, Dr. Ogdie concluded.
Dr. Eder has received research and educational grants from AbbVie, Amgen, Celgene, Lilly, Novartis, and UCB. Dr. Coates has received honoraria, research funding, or both from more than a dozen companies. Dr. Reddy has been a consultant to AbbVie, Novartis, Pfizer, and UCB. Dr. Merola has been a consultant to AbbVie, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Samumed, Sanofi, and UCB and has received research grants from Aclaris, Biogen, Incyte, Novartis, Pfizer, and Sanofi. Dr. Gelfand has been a consultant, adviser, or both to more than a dozen companies. Dr. Ogdie has been a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Lilly, Novartis, Pfizer, and Takeda.
Most patients with psoriatic arthritis first present with psoriasis only. Their skin disorder precedes any joint involvement, often by several years. That suggests targeting interventions to patients with psoriasis to prevent or slow their progression to psoriatic arthritis, as well as following psoriatic patients closely to diagnose psoriatic arthritis quickly when it first appears. It’s a simple and attractive management premise that’s been challenging to apply in practice.
It’s not that clinicians aren’t motivated to diagnose psoriatic arthritis (PsA) in patients early, hopefully as soon as it appears. The susceptibility of patients with psoriasis to develop PsA is well described, with an annual progression rate of about 3%, and adverse consequences result from even a 6-month delay in diagnosis.
“Some physicians still don’t ask psoriasis patients about joint pain, or their symptoms are misinterpreted as something else,” said Lihi Eder, MD, a rheumatologist at Women’s College Research Institute, Toronto, and the University of Toronto. “Although there is increased awareness about PsA, there are still delays in diagnosis,” she said in an interview.
“Often there is a massive delay in diagnosis, and we know from a number of studies that longer duration of symptoms before diagnosis is associated with poorer outcomes,” said Laura C. Coates, MBChB, PhD, a rheumatologist at the University of Oxford (England). The delay to PsA diagnosis is generally “longer than for equivalent rheumatoid arthritis patients. PsA patients take longer to ask a primary care physician for help, longer to get a referral to a rheumatologist, and longer to get a diagnosis” from a rheumatologist. “We need to educate patients with psoriasis about their risk so that they seek help, educate GPs about whom to refer, and educate rheumatologists about diagnosis,” Dr. Coates said.
“It is very important to diagnose PsA as early as possible. We know that a delay in diagnosis and treatment can lead to worse outcomes and joint damage,” said Soumya M. Reddy, MD, codirector of the Psoriasis and Psoriatic Arthritis Center at New York University Langone Health in New York. “The heterogeneity of clinical manifestations of PsA can make it difficult to diagnose, and in some cases this leads to delayed diagnosis.”
“We are increasingly interested in the concept of preventing PsA. Psoriasis is a unique disease state in which we have an at-risk population where 30% will develop an inflammatory and potentially damaging arthritis. This may become important as our skin treatments may also treat musculoskeletal components of the disease,” said Joseph F. Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital in Boston and double board certified in dermatology and rheumatology.
Focusing on patients with psoriasis
Treatment of psoriasis makes sense to address several quality-of-life issues, but controlling the severity of psoriatic skin manifestations gives patients no guarantees about their possible progression to PsA.
“So many people have mild psoriasis that, although they are less likely, proportionately, to get PsA, we still see many in the clinic,” said Dr. Coates. “Patients with severe skin involvement have a good reason to get treatment, which could help test whether a drug slows progression to arthritis. But it’s unethical to not treat severe psoriasis just to have a comparison group.” Aside from skin psoriasis, “we don’t have any other good markers,” Dr. Coates noted.
PsA can develop in patients who had psoriasis in the past but without currently active disease. “At the level of individual patients you can’t say someone is protected from developing PsA because their psoriasis is inactive,” Dr. Eder said. “No study has looked at whether treatment of psoriasis reduces the risk for progression to PsA. We don’t know whether any treatments that reduce inflammation in psoriasis also reduce progression to PsA.” Regardless, treating psoriasis is important because it improves quality of life and may have a beneficial, long-term effects on possible cardiovascular disease effects from psoriasis, Dr. Eder said.
Her research group is now studying the associations among different biological drugs taken by patients with psoriasis and their subsequent incidence of PsA with use of medical records from about 4 million Israeli residents. Other studies are also looking at this, but they are all observational and therefore are subject to unidentified confounders, she noted. A more definitive demonstration of PsA protection would need a randomized trial.
“The idea of preventing the transition from psoriasis to PsA is an exciting concept. But currently, there are no established treatments for preventing transition of psoriasis to PsA. It’s an area of active research, and the holy grail of psoriatic disease research. We do not yet know whether successful treatment of skin psoriasis delays the onset of arthritis,” Dr. Reddy said in an interview.
“The risk of developing PsA increases as the severity of skin psoriasis increases, measured by percentage of body surface area affected. However, there is only a weak correlation between the severity of skin disease and the severity of PsA,” said Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania in Philadelphia.
Widening the PsA diagnostic net
What’s elusive is some other parameter to identify the 3% of psoriasis patients who will develop PsA during the next year – or at least a better way to find patients as soon as they have what is identifiably PsA.
The diagnostic definition for PsA that many experts now accept is actually a set of classification criteria, CASPAR (Classification Criteria for Psoriatic Arthritis) (Arthritis Rheum. 2006 Aug;54[8]:2665-73). But in actual practice, diagnosing PsA relies heavily on clinical skill, case recognition, and ruling out mimickers.
“The CASPAR criteria were developed as classification criteria to use in studies, but they are also quite helpful in diagnosing PsA. It is the agreed on definition of PsA at the moment,” said Alexis Ogdie, MD, director of the psoriatic arthritis clinic at the University of Pennsylvania.
“CASPAR fills the role at present for clinical trials, but the diagnosis remains clinical, based on history, physical findings, and supported by lab and radiology findings,” Dr. Merola said. “A clinical prediction tool and a proper biomarker would be of great value.”
“We certainly use CASPAR criteria in the clinic, but they do not include all PsA patients; sometimes we diagnose PsA in patients who don’t meet the CASPAR criteria,” Dr. Coates said.
“In practice, rheumatologists mostly use their clinical judgment: a patient with history and findings typical of PsA after ruling out other causes. But distinguishing inflammatory from noninflammatory arthritis – like osteoarthritis or fibromyalgia – can be quite difficult; that’s the main challenge,” Dr. Eder said. “Rheumatologists rely on their clinical skill and experience. PsA can be difficult to diagnose. There is no biomarker for it. PsA is complex [to diagnose], and that’s why it’s diagnosed later. A primary care physician might get a negative result on a rheumatoid factor test and think that rules out PsA, but it doesn’t.”
Dr. Eder and others suggest that ultrasound may be a useful tool for earlier diagnosis of PsA. Ultrasound is more sensitive than a physical examination and can detect inflammation in joints and entheses, she noted. Another effective method may be more systematic use of screening questionnaires, Dr. Coates said, or simply more systematic questioning of patients.
“Questionnaires are not a high priority for a primary care physician who may have only a few patients with psoriasis. Even some dermatologists may not use the questionnaires because they take time to administer and assess. But just asking a psoriasis patient whether they have joint symptoms is enough,” Dr. Eder said. All clinicians who encounter patients with psoriasis should ask about musculoskeletal symptoms and refer when appropriate to a rheumatologist, Dr. Reddy said.
The earliest indicators of PsA
Evidence supporting ultrasound’s value for early PsA diagnosis came in a report at the most recent annual meeting of the American College of Rheumatology in Chicago in October 2018. Researchers from the University of Rochester (New York) used ultrasound to examine 78 patients with psoriasis but without PsA or musculoskeletal symptoms and 25 healthy controls. They found ultrasound abnormalities in almost half of the patients with psoriasis, significantly more than in the controls (Thiele R et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2904).
Another report at the ACR annual meeting last October looked at the incidence of physician visits for nonspecific musculoskeletal symptoms during each of the 5 years preceding diagnosis of PsA. A prior report from Dr. Eder and her associates had documented in an observational cohort of 410 patients with psoriasis that, prior to development of PsA, patients often had nonspecific musculoskeletal symptoms of joint pain, fatigue, and stiffness that constituted a “preclinical” phase (Arthritis Rheumatol. 2017 March;69[3]:622-9).
In October, Dr. Eder reported how the appearance of musculoskeletal symptoms played out in terms of physician visits. She and her associates analyzed data from an Ontario health insurance database that included about 430,000 Ontario patients seen by 466 primary care physicians, which included 462 patients with a new diagnosis of PsA and 2,310 matched controls. The results showed that, in every year during the 5 years preceding diagnosis of PsA, the patients who would wind up getting diagnosed had roughly twice the number of visits to a primary care physician each year for nonspecific musculoskeletal issues. A similar pattern of doubled visits occurred for people prior to their PsA diagnosis going to physicians who specialize in musculoskeletal conditions, and when the analysis focused on visits to rheumatologists, patients who went on to get diagnosed with PsA had a nearly sevenfold increased rate of these visits, compared with controls, for each of the 5 years preceding their PsA diagnosis (Eder L et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 967).
These results “highlight that in many patients PsA is not an acute disease that starts suddenly. In many patients, there is a period when the patient experiences musculoskeletal symptoms and sees a primary care physician or rheumatologist and may be diagnosed with something that is not PsA. That means that the delay in diagnosis [of PsA] may have happened because the patients were misdiagnosed. It reinforces the need for better diagnostic tools,” Dr. Eder said. “We have focused on getting these patients to see a rheumatologist earlier, but that may not be enough. These patients may not receive routine follow-up; we need to do more follow-up on patients like these.” Diagnosing PsA early means earlier treatment, a better chance of reaching remission, less chance of permanent joint damage, and better quality of life.
The challenges of making an early diagnosis were also documented in a study reported by Dr. Ogdie during the June 2018 annual congress of the European League Against Rheumatism. Dr. Ogdie reported on the survey responses of 203 patients who said they had been diagnosed with PsA whose index diagnosis was a median of 6 years before they completed the survey. A total of 195 of these patients, or 96%, said that they had received at least one misdiagnosis prior to their PsA diagnosis (Odgie A et al. Ann Rheum Dis. 2018;77[Suppl 2]:163. Abstract THU0292). The most common misdiagnoses were psychosomatic disease, reported by 27% of the patients; osteoarthritis in 22%; anxiety or depression in 18%; and an orthopedic problem in 18%. (Patients could report more than one type of misdiagnosis.)
The results “showed that patients often had substantial delays and misdiagnoses before they received a PsA diagnosis,” Dr. Ogdie and her associates concluded. Although the CASPAR classification criteria may be the agreed on PsA definition, recent findings suggest a pre-PsA stage exists with musculoskeletal and other abnormalities. “How may we diagnose ‘pre-PsA’? How might we capture this transition phase from psoriasis to PsA before the CASPAR criteria are fulfilled,” she wondered in an interview. “If we could stop PsA before it is clinically relevant, that could dramatically change the course of the disease. This is a big need in the field right now.”
Weight loss and other interventions
Aside from treating psoriasis and perhaps putting a patient with psoriasis in a PsA-prevention trial, one of the best ways to prevent PsA may be weight loss.
Results from “some studies suggest that being overweight increases the risk for developing PsA. Obesity also exacerbates skin psoriasis, makes treatment less effective, and further increases the risk of cardiometabolic diseases associated with psoriasis,” Dr. Gelfand said. “All patients with psoriatic disease should be educated about the importance of maintaining a healthy body weight.”
“Several studies suggest that obesity is a risk factor for developing PsA. Obesity likely plays a role in driving or contributing to inflammation in psoriatic disease,” said Dr. Reddy, who noted that other PsA risk factors include nail psoriasis and first-degree relatives with PsA. Dr. Ogdie also cited uveitis and prior joint trauma as other risk factors.
“Strong observational data link obesity and PsA incidence. I talk to psoriasis patients about weight control, and selected patients could even consider bariatric surgery,” Dr. Eder said. Losing at least 5% of body mass index can make a difference, she added.
At the 2018 annual meeting of the American College of Rheumatology, researchers from the University of Bath (England) reported results from a retrospective, observational study of more than 90,000 people with recent-onset psoriasis; they found that people with an obese BMI had twice the rate of progression to PsA when compared with people with a normal BMI. Overweight people had a nearly 80% higher rate of incident PsA (Green A et al. Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2134).
Hints have also emerged that new approaches to treating psoriasis also could help to keep PsA precursors at bay. One recent example from researchers at the University of Leeds (England) was a phase 2 study of 73 patients with moderate to severe psoriasis but no PsA who underwent ultrasound screening of their entheses for signs of inflammatory changes. The 23 patients underwent 52 weeks of treatment with the drug ustekinumab (Stelara), an antagonist of interleukin-12 and -23 that is approved for U.S. marketing to treat both psoriasis and PsA. After 24 weeks on treatment, their mean inflammation scores had dropped by more than 40%, and the effect persisted through 52 weeks of treatment (Arthritis Rheum. 2018 Nov 22. doi: 10.1002/art.40778).
Despite this promise, the researchers “haven’t looked long enough or in enough people to see whether this actually stops patients from developing PsA,” Dr. Coates commented. It also remains unclear whether this or another ultrasound abnormality detectable in joints or entheses is a reliable predictor of PsA, she noted.
“We still have a lot to learn about how to classify patients as high risk” for PsA, Dr. Ogdie concluded.
Dr. Eder has received research and educational grants from AbbVie, Amgen, Celgene, Lilly, Novartis, and UCB. Dr. Coates has received honoraria, research funding, or both from more than a dozen companies. Dr. Reddy has been a consultant to AbbVie, Novartis, Pfizer, and UCB. Dr. Merola has been a consultant to AbbVie, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Samumed, Sanofi, and UCB and has received research grants from Aclaris, Biogen, Incyte, Novartis, Pfizer, and Sanofi. Dr. Gelfand has been a consultant, adviser, or both to more than a dozen companies. Dr. Ogdie has been a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Lilly, Novartis, Pfizer, and Takeda.