User login
Not on My Watch!
The itchy patch of skin on this 60-year-old man’s forearm, beneath his watch, has troubled him intermittently for a year. It is unresponsive to topical medications, including tolnaftate cream and 1% hydrocortisone cream; the latter seemed to improve the condition initially, but with time, the patch grew larger and itchier.
The patient’s primary care provider believed the rash might be precancerous and prescribed 5-fluorouracil cream, which showed no benefit. The patient was then referred to a dermatologist, who performed a KOH examination. No fungal elements were found.
At that point, the patient decided to simply ignore the problem. That resolve ended when the itchiness increased—leading to his presentation today. He is in decent health but has had a kidney transplant and is taking the standard immunosuppressant (anti-rejection) medications.
EXAMINATION
A faintly pink, scaly rash is located on the patient’s forearm. It is unimpressive in appearance but very bothersome to the patient.

Despite the patient’s history, fungal origin remains a possibility, as does psoriasis. A punch biopsy is performed.
What is the diagnosis?
DISCUSSION
The biopsy results showed numerous fungal elements crowded around deeper aspects of hair follicles and a granulomatous reaction in the surrounding skin.
Normally, dermatophytes (the organisms that cause superficial fungal infections) are incapable of invading deeper tissues, making KOH exam a simple diagnostic method. But with immune suppression, the infection is able to invade deeper structures, making it more difficult to diagnose and treat. In this patient’s case, the use of steroid creams under occlusion (ie, the watch) had suppressed the body’s immune response to the infection.
The terminology used to describe this phenomenon varies according to the severity of infection. Tinea incognito might be used to describe this patient’s case, in which the steroid rendered the condition almost impossible to diagnose visually. With continued use of stronger topical steroids, the degree of inflammation might have been worse, involving deeper, larger nodules instead of faint pink scaling. In such cases, the term Majocchi fungal granuloma is used.
This patient moved his watch to the other arm temporarily and was successfully treated with twice-daily application of topical econazole cream and a two-week course of oral terbinafine (250 mg/d).
TAKE-HOME LEARNING POINTS
- Immunosuppression can make fungal infections more difficult to diagnose and treat.
- Dermatophytes don’t normally invade deeper structures and can usually be detected with a KOH exam of surface skin cells.
- If the patient is immunocompromised, a fungal diagnosis is best confirmed with a punch biopsy, and treatment achieved with a combination of oral and topical antifungal products.
- Mild cases of this kind are termed tinea incognito, while more severe cases are called Majocchi fungal granuloma.
The itchy patch of skin on this 60-year-old man’s forearm, beneath his watch, has troubled him intermittently for a year. It is unresponsive to topical medications, including tolnaftate cream and 1% hydrocortisone cream; the latter seemed to improve the condition initially, but with time, the patch grew larger and itchier.
The patient’s primary care provider believed the rash might be precancerous and prescribed 5-fluorouracil cream, which showed no benefit. The patient was then referred to a dermatologist, who performed a KOH examination. No fungal elements were found.
At that point, the patient decided to simply ignore the problem. That resolve ended when the itchiness increased—leading to his presentation today. He is in decent health but has had a kidney transplant and is taking the standard immunosuppressant (anti-rejection) medications.
EXAMINATION
A faintly pink, scaly rash is located on the patient’s forearm. It is unimpressive in appearance but very bothersome to the patient.

Despite the patient’s history, fungal origin remains a possibility, as does psoriasis. A punch biopsy is performed.
What is the diagnosis?
DISCUSSION
The biopsy results showed numerous fungal elements crowded around deeper aspects of hair follicles and a granulomatous reaction in the surrounding skin.
Normally, dermatophytes (the organisms that cause superficial fungal infections) are incapable of invading deeper tissues, making KOH exam a simple diagnostic method. But with immune suppression, the infection is able to invade deeper structures, making it more difficult to diagnose and treat. In this patient’s case, the use of steroid creams under occlusion (ie, the watch) had suppressed the body’s immune response to the infection.
The terminology used to describe this phenomenon varies according to the severity of infection. Tinea incognito might be used to describe this patient’s case, in which the steroid rendered the condition almost impossible to diagnose visually. With continued use of stronger topical steroids, the degree of inflammation might have been worse, involving deeper, larger nodules instead of faint pink scaling. In such cases, the term Majocchi fungal granuloma is used.
This patient moved his watch to the other arm temporarily and was successfully treated with twice-daily application of topical econazole cream and a two-week course of oral terbinafine (250 mg/d).
TAKE-HOME LEARNING POINTS
- Immunosuppression can make fungal infections more difficult to diagnose and treat.
- Dermatophytes don’t normally invade deeper structures and can usually be detected with a KOH exam of surface skin cells.
- If the patient is immunocompromised, a fungal diagnosis is best confirmed with a punch biopsy, and treatment achieved with a combination of oral and topical antifungal products.
- Mild cases of this kind are termed tinea incognito, while more severe cases are called Majocchi fungal granuloma.
The itchy patch of skin on this 60-year-old man’s forearm, beneath his watch, has troubled him intermittently for a year. It is unresponsive to topical medications, including tolnaftate cream and 1% hydrocortisone cream; the latter seemed to improve the condition initially, but with time, the patch grew larger and itchier.
The patient’s primary care provider believed the rash might be precancerous and prescribed 5-fluorouracil cream, which showed no benefit. The patient was then referred to a dermatologist, who performed a KOH examination. No fungal elements were found.
At that point, the patient decided to simply ignore the problem. That resolve ended when the itchiness increased—leading to his presentation today. He is in decent health but has had a kidney transplant and is taking the standard immunosuppressant (anti-rejection) medications.
EXAMINATION
A faintly pink, scaly rash is located on the patient’s forearm. It is unimpressive in appearance but very bothersome to the patient.

Despite the patient’s history, fungal origin remains a possibility, as does psoriasis. A punch biopsy is performed.
What is the diagnosis?
DISCUSSION
The biopsy results showed numerous fungal elements crowded around deeper aspects of hair follicles and a granulomatous reaction in the surrounding skin.
Normally, dermatophytes (the organisms that cause superficial fungal infections) are incapable of invading deeper tissues, making KOH exam a simple diagnostic method. But with immune suppression, the infection is able to invade deeper structures, making it more difficult to diagnose and treat. In this patient’s case, the use of steroid creams under occlusion (ie, the watch) had suppressed the body’s immune response to the infection.
The terminology used to describe this phenomenon varies according to the severity of infection. Tinea incognito might be used to describe this patient’s case, in which the steroid rendered the condition almost impossible to diagnose visually. With continued use of stronger topical steroids, the degree of inflammation might have been worse, involving deeper, larger nodules instead of faint pink scaling. In such cases, the term Majocchi fungal granuloma is used.
This patient moved his watch to the other arm temporarily and was successfully treated with twice-daily application of topical econazole cream and a two-week course of oral terbinafine (250 mg/d).
TAKE-HOME LEARNING POINTS
- Immunosuppression can make fungal infections more difficult to diagnose and treat.
- Dermatophytes don’t normally invade deeper structures and can usually be detected with a KOH exam of surface skin cells.
- If the patient is immunocompromised, a fungal diagnosis is best confirmed with a punch biopsy, and treatment achieved with a combination of oral and topical antifungal products.
- Mild cases of this kind are termed tinea incognito, while more severe cases are called Majocchi fungal granuloma.
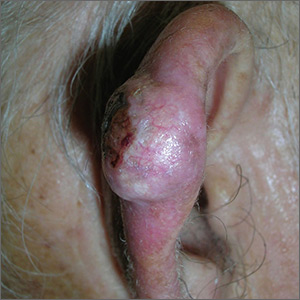
Growth on ear
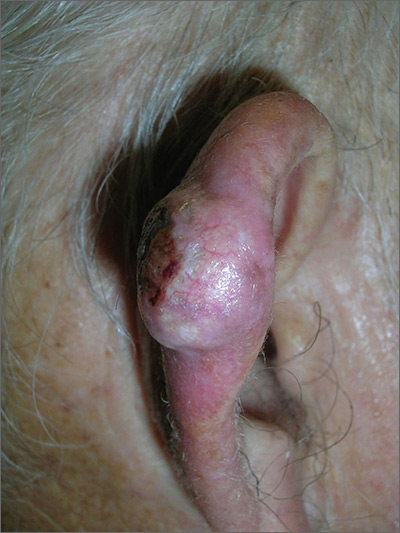
The FP suspected skin cancer, but was not sure if it was a basal cell carcinoma or a squamous cell carcinoma (SCC).
The growth was somewhat pearly with telangiectasias, but there was also a prominent area of keratin on the posterior aspect of the lesion. After injecting local anesthesia with 1% lidocaine and epinephrine, the physician performed a shave biopsy on one edge of the tumor. He was careful to avoid cutting the cartilage and decided that the diagnosis would be obtained without trying to remove the whole tumor. The bleeding did not stop with chemical hemostasis alone, so electrosurgery was used. The biopsy result showed an SCC of the keratoacanthoma type.
The patient was referred for Mohs surgery because it was important to get clear margins, while attempting to preserve the ear anatomy. Also, SCC of the ear has a higher risk for metastasis, so a thorough exam of the head and neck for lymphadenopathy was performed. Fortunately, this SCC was well-differentiated and no metastases were clinically detected.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratocanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP suspected skin cancer, but was not sure if it was a basal cell carcinoma or a squamous cell carcinoma (SCC).
The growth was somewhat pearly with telangiectasias, but there was also a prominent area of keratin on the posterior aspect of the lesion. After injecting local anesthesia with 1% lidocaine and epinephrine, the physician performed a shave biopsy on one edge of the tumor. He was careful to avoid cutting the cartilage and decided that the diagnosis would be obtained without trying to remove the whole tumor. The bleeding did not stop with chemical hemostasis alone, so electrosurgery was used. The biopsy result showed an SCC of the keratoacanthoma type.
The patient was referred for Mohs surgery because it was important to get clear margins, while attempting to preserve the ear anatomy. Also, SCC of the ear has a higher risk for metastasis, so a thorough exam of the head and neck for lymphadenopathy was performed. Fortunately, this SCC was well-differentiated and no metastases were clinically detected.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratocanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP suspected skin cancer, but was not sure if it was a basal cell carcinoma or a squamous cell carcinoma (SCC).
The growth was somewhat pearly with telangiectasias, but there was also a prominent area of keratin on the posterior aspect of the lesion. After injecting local anesthesia with 1% lidocaine and epinephrine, the physician performed a shave biopsy on one edge of the tumor. He was careful to avoid cutting the cartilage and decided that the diagnosis would be obtained without trying to remove the whole tumor. The bleeding did not stop with chemical hemostasis alone, so electrosurgery was used. The biopsy result showed an SCC of the keratoacanthoma type.
The patient was referred for Mohs surgery because it was important to get clear margins, while attempting to preserve the ear anatomy. Also, SCC of the ear has a higher risk for metastasis, so a thorough exam of the head and neck for lymphadenopathy was performed. Fortunately, this SCC was well-differentiated and no metastases were clinically detected.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratocanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Three steps are involved in assessing a vesiculopustular lesion
LAKE TAHOE, CALIF. – Evaluation of an infant with a vesiculopustular lesion entails a pragmatic approach, with three assessment steps, according to pediatric dermatologist Lawrence A. Schachner, MD.
At the annual meeting of the Society for Pediatric Dermatology, Dr. Schachner noted that the differential diagnosis of noninfectious, usually benign neonatal vesiculopustular lesions includes acropustulosis of infancy; eosinophilic pustular folliculitis; erythema toxicum neonatorum; miliaria crystallina, rubra, or profunda; transient neonatal pustular melanosis; and neonatal sucking blisters. Noninfectious lesions that can be potentially serious include acrodermatitis enteropathica, bullous pemphigoid, epidermolysis bullosa, epidermolytic hyperkeratosis, incontinentia pigmenti, Langerhans cell histiocytosis, pemphigus vulgaris, pustular psoriasis, and urticarial pigmentosa.
Step 1 for evaluating vesiculopustular lesions involves drawing out the fluid for bacterial cultures and sensitivity, Gram stains, viral culture and/or serology, said Dr. Schachner, who directs the division of pediatric dermatology at the University of Miami.
Step 2 involves snipping off the lesion’s roof for a potassium hydroxide test to send for dermatopathology or frozen pathology.
Step 3 involves scraping the lesion’s base for viral cytology and cell identification. “If we can see a predominant cell type, that’s where the action is,” said Dr. Schachner, professor of pediatrics at the university. If cytology reveals polymorphic neutrophils, differential diagnoses include transient neonatal pustular melanosis, infantile acropustulosis, bullous impetigo, and pustular psoriasis. If cytology reveals eosinophils, differential diagnoses include eosinophilic pustular folliculitis of infancy, erythema toxicum neonatorum, incontinentia pigmenti, bullous pemphigoid, drug reactions, and arthropod bites. The presence of lymphocytes on cytology, meanwhile, may suggest a differential diagnosis miliaria or acrodermatitis enteropathica.
“When in doubt about the diagnosis, biopsy,” Dr. Schachner said. “This, to me, is a pragmatic approach.”
Dr. Schachner disclosed that he is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Stiefel Laboratories, Berg Pharma, Medimetrics, and Lilly. He is a consultant to Beiersdorf, Lexington, TopMD, Cutanea, Hoth Therapeutics, and Mustela.
[email protected]
LAKE TAHOE, CALIF. – Evaluation of an infant with a vesiculopustular lesion entails a pragmatic approach, with three assessment steps, according to pediatric dermatologist Lawrence A. Schachner, MD.
At the annual meeting of the Society for Pediatric Dermatology, Dr. Schachner noted that the differential diagnosis of noninfectious, usually benign neonatal vesiculopustular lesions includes acropustulosis of infancy; eosinophilic pustular folliculitis; erythema toxicum neonatorum; miliaria crystallina, rubra, or profunda; transient neonatal pustular melanosis; and neonatal sucking blisters. Noninfectious lesions that can be potentially serious include acrodermatitis enteropathica, bullous pemphigoid, epidermolysis bullosa, epidermolytic hyperkeratosis, incontinentia pigmenti, Langerhans cell histiocytosis, pemphigus vulgaris, pustular psoriasis, and urticarial pigmentosa.
Step 1 for evaluating vesiculopustular lesions involves drawing out the fluid for bacterial cultures and sensitivity, Gram stains, viral culture and/or serology, said Dr. Schachner, who directs the division of pediatric dermatology at the University of Miami.
Step 2 involves snipping off the lesion’s roof for a potassium hydroxide test to send for dermatopathology or frozen pathology.
Step 3 involves scraping the lesion’s base for viral cytology and cell identification. “If we can see a predominant cell type, that’s where the action is,” said Dr. Schachner, professor of pediatrics at the university. If cytology reveals polymorphic neutrophils, differential diagnoses include transient neonatal pustular melanosis, infantile acropustulosis, bullous impetigo, and pustular psoriasis. If cytology reveals eosinophils, differential diagnoses include eosinophilic pustular folliculitis of infancy, erythema toxicum neonatorum, incontinentia pigmenti, bullous pemphigoid, drug reactions, and arthropod bites. The presence of lymphocytes on cytology, meanwhile, may suggest a differential diagnosis miliaria or acrodermatitis enteropathica.
“When in doubt about the diagnosis, biopsy,” Dr. Schachner said. “This, to me, is a pragmatic approach.”
Dr. Schachner disclosed that he is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Stiefel Laboratories, Berg Pharma, Medimetrics, and Lilly. He is a consultant to Beiersdorf, Lexington, TopMD, Cutanea, Hoth Therapeutics, and Mustela.
[email protected]
LAKE TAHOE, CALIF. – Evaluation of an infant with a vesiculopustular lesion entails a pragmatic approach, with three assessment steps, according to pediatric dermatologist Lawrence A. Schachner, MD.
At the annual meeting of the Society for Pediatric Dermatology, Dr. Schachner noted that the differential diagnosis of noninfectious, usually benign neonatal vesiculopustular lesions includes acropustulosis of infancy; eosinophilic pustular folliculitis; erythema toxicum neonatorum; miliaria crystallina, rubra, or profunda; transient neonatal pustular melanosis; and neonatal sucking blisters. Noninfectious lesions that can be potentially serious include acrodermatitis enteropathica, bullous pemphigoid, epidermolysis bullosa, epidermolytic hyperkeratosis, incontinentia pigmenti, Langerhans cell histiocytosis, pemphigus vulgaris, pustular psoriasis, and urticarial pigmentosa.
Step 1 for evaluating vesiculopustular lesions involves drawing out the fluid for bacterial cultures and sensitivity, Gram stains, viral culture and/or serology, said Dr. Schachner, who directs the division of pediatric dermatology at the University of Miami.
Step 2 involves snipping off the lesion’s roof for a potassium hydroxide test to send for dermatopathology or frozen pathology.
Step 3 involves scraping the lesion’s base for viral cytology and cell identification. “If we can see a predominant cell type, that’s where the action is,” said Dr. Schachner, professor of pediatrics at the university. If cytology reveals polymorphic neutrophils, differential diagnoses include transient neonatal pustular melanosis, infantile acropustulosis, bullous impetigo, and pustular psoriasis. If cytology reveals eosinophils, differential diagnoses include eosinophilic pustular folliculitis of infancy, erythema toxicum neonatorum, incontinentia pigmenti, bullous pemphigoid, drug reactions, and arthropod bites. The presence of lymphocytes on cytology, meanwhile, may suggest a differential diagnosis miliaria or acrodermatitis enteropathica.
“When in doubt about the diagnosis, biopsy,” Dr. Schachner said. “This, to me, is a pragmatic approach.”
Dr. Schachner disclosed that he is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Stiefel Laboratories, Berg Pharma, Medimetrics, and Lilly. He is a consultant to Beiersdorf, Lexington, TopMD, Cutanea, Hoth Therapeutics, and Mustela.
[email protected]
REPORTING FROM SPD 2018
Bertilimumab granted orphan drug status for bullous pemphigoid
The , according to an announcement issued by the company that is developing the treatment.
The company, Immune Pharmaceuticals, plans to launch a pivotal phase 2/3 study in 2019, the company said in an Aug. 20 press release. Two phase 2 studies of bertilimumab are currently underway, one in patients with bullous pemphigoid, and another in patients with ulcerative colitis. Bertilimumab is “a first-in-class, fully human monoclonal antibody that targets and lowers levels of eotaxin-1, a chemokine that plays a role in immune responses and attracts eosinophils to the site of inflammation,” the statement said.“By neutralizing eotaxin-1, bertilimumab may prevent the migration of eosinophils and other cells, thus helping to relieve associated inflammatory conditions.”
The FDA’s Orphan Drug Designation program grants orphan status to drugs and biologics, which are “defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug,” according to the agency.
The , according to an announcement issued by the company that is developing the treatment.
The company, Immune Pharmaceuticals, plans to launch a pivotal phase 2/3 study in 2019, the company said in an Aug. 20 press release. Two phase 2 studies of bertilimumab are currently underway, one in patients with bullous pemphigoid, and another in patients with ulcerative colitis. Bertilimumab is “a first-in-class, fully human monoclonal antibody that targets and lowers levels of eotaxin-1, a chemokine that plays a role in immune responses and attracts eosinophils to the site of inflammation,” the statement said.“By neutralizing eotaxin-1, bertilimumab may prevent the migration of eosinophils and other cells, thus helping to relieve associated inflammatory conditions.”
The FDA’s Orphan Drug Designation program grants orphan status to drugs and biologics, which are “defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug,” according to the agency.
The , according to an announcement issued by the company that is developing the treatment.
The company, Immune Pharmaceuticals, plans to launch a pivotal phase 2/3 study in 2019, the company said in an Aug. 20 press release. Two phase 2 studies of bertilimumab are currently underway, one in patients with bullous pemphigoid, and another in patients with ulcerative colitis. Bertilimumab is “a first-in-class, fully human monoclonal antibody that targets and lowers levels of eotaxin-1, a chemokine that plays a role in immune responses and attracts eosinophils to the site of inflammation,” the statement said.“By neutralizing eotaxin-1, bertilimumab may prevent the migration of eosinophils and other cells, thus helping to relieve associated inflammatory conditions.”
The FDA’s Orphan Drug Designation program grants orphan status to drugs and biologics, which are “defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug,” according to the agency.
Alopecia areata linked to mental health disorders
Alopecia areata is associated with greater frequency of mental health disorders, according to a new analysis of U.S. hospitalizations.
Specifically, the analysis found, alopecia areata patients are at risk for any mental health disorder, anxiety disorders, attention-deficit/hyperactivity disorder, dementia, mood disorders, personality disorders, and suicide or intentionally self-inflicted injury. The report was published in the Journal of the American Academy of Dermatology.
The researchers worked with 87,053,155 adult and child records from the 2002-2012 National Inpatient Sample, which represents 20% of U.S. hospitalizations.
Overall, 5,605 patients had alopecia areata, which was the secondary diagnosis more than 99% of the time. Compared with inpatients without alopecia areata, those with the disorder were more likely to be younger (42.2 vs. 47.9 years; P less than .0001), female (61.7% vs. 58.6%; P = .0297), and uninsured (8.1% vs. 5.5%; P less than .0001). In addition, inpatients with alopecia areata had a greater frequency of mental health disorders (32.8% vs. 20.0%; P less than .0001) and were more likely to have a primary mental health diagnosis (5.5% vs. 2.2%; P less than .0001), reported Vivek Singam of Northwestern University, Chicago, and his associates.
Among 15 mental health or classes of disorders examined, alopecia areata patients were at greater risk in 13 of them. The only exceptions were delirium/dementia/amnestic/cognitive disorders and disorders diagnosed in infancy, childhood, or adolescence.
Alopecia areata patients with a mental health disorder had a mean hospital stay of 6.0 days (95% confidence interval, 5.4.-6.6) and hospitalization cost of $11,907 (95% CI, $10,312-$13,503).
Previous studies had shown similar relationships. However, previous studies showed lower risk of alopecia areata and schizophrenia and no increased risk of ADHD, compared with the current study’s findings. The authors could offer no explanation for those differences.
The strengths of the current analysis include its use of a large-scale, nationally representative cohort and its large sample size, as well its inclusion of a broad range of mental health disorders. Because of its cross-sectional design, the study could not establish the temporal relationship between alopecia areata and mental health disorders.
It is unclear whether psychosocial stress might cause or exacerbate alopecia areata, or whether alopecia areata can lead to or worsen mental health disorders.
The researchers called for additional studies to understand this relationship and potential mechanisms.
The Agency for Healthcare Research and Quality and the Dermatology Foundation funded the study. The researchers declared having no conflicts of interest.
SOURCE: Singam V et al. J Am Acad Dermatol. 2018 Aug 6. doi: 10.1016/j.jaad.2018.07.044.
Alopecia areata is associated with greater frequency of mental health disorders, according to a new analysis of U.S. hospitalizations.
Specifically, the analysis found, alopecia areata patients are at risk for any mental health disorder, anxiety disorders, attention-deficit/hyperactivity disorder, dementia, mood disorders, personality disorders, and suicide or intentionally self-inflicted injury. The report was published in the Journal of the American Academy of Dermatology.
The researchers worked with 87,053,155 adult and child records from the 2002-2012 National Inpatient Sample, which represents 20% of U.S. hospitalizations.
Overall, 5,605 patients had alopecia areata, which was the secondary diagnosis more than 99% of the time. Compared with inpatients without alopecia areata, those with the disorder were more likely to be younger (42.2 vs. 47.9 years; P less than .0001), female (61.7% vs. 58.6%; P = .0297), and uninsured (8.1% vs. 5.5%; P less than .0001). In addition, inpatients with alopecia areata had a greater frequency of mental health disorders (32.8% vs. 20.0%; P less than .0001) and were more likely to have a primary mental health diagnosis (5.5% vs. 2.2%; P less than .0001), reported Vivek Singam of Northwestern University, Chicago, and his associates.
Among 15 mental health or classes of disorders examined, alopecia areata patients were at greater risk in 13 of them. The only exceptions were delirium/dementia/amnestic/cognitive disorders and disorders diagnosed in infancy, childhood, or adolescence.
Alopecia areata patients with a mental health disorder had a mean hospital stay of 6.0 days (95% confidence interval, 5.4.-6.6) and hospitalization cost of $11,907 (95% CI, $10,312-$13,503).
Previous studies had shown similar relationships. However, previous studies showed lower risk of alopecia areata and schizophrenia and no increased risk of ADHD, compared with the current study’s findings. The authors could offer no explanation for those differences.
The strengths of the current analysis include its use of a large-scale, nationally representative cohort and its large sample size, as well its inclusion of a broad range of mental health disorders. Because of its cross-sectional design, the study could not establish the temporal relationship between alopecia areata and mental health disorders.
It is unclear whether psychosocial stress might cause or exacerbate alopecia areata, or whether alopecia areata can lead to or worsen mental health disorders.
The researchers called for additional studies to understand this relationship and potential mechanisms.
The Agency for Healthcare Research and Quality and the Dermatology Foundation funded the study. The researchers declared having no conflicts of interest.
SOURCE: Singam V et al. J Am Acad Dermatol. 2018 Aug 6. doi: 10.1016/j.jaad.2018.07.044.
Alopecia areata is associated with greater frequency of mental health disorders, according to a new analysis of U.S. hospitalizations.
Specifically, the analysis found, alopecia areata patients are at risk for any mental health disorder, anxiety disorders, attention-deficit/hyperactivity disorder, dementia, mood disorders, personality disorders, and suicide or intentionally self-inflicted injury. The report was published in the Journal of the American Academy of Dermatology.
The researchers worked with 87,053,155 adult and child records from the 2002-2012 National Inpatient Sample, which represents 20% of U.S. hospitalizations.
Overall, 5,605 patients had alopecia areata, which was the secondary diagnosis more than 99% of the time. Compared with inpatients without alopecia areata, those with the disorder were more likely to be younger (42.2 vs. 47.9 years; P less than .0001), female (61.7% vs. 58.6%; P = .0297), and uninsured (8.1% vs. 5.5%; P less than .0001). In addition, inpatients with alopecia areata had a greater frequency of mental health disorders (32.8% vs. 20.0%; P less than .0001) and were more likely to have a primary mental health diagnosis (5.5% vs. 2.2%; P less than .0001), reported Vivek Singam of Northwestern University, Chicago, and his associates.
Among 15 mental health or classes of disorders examined, alopecia areata patients were at greater risk in 13 of them. The only exceptions were delirium/dementia/amnestic/cognitive disorders and disorders diagnosed in infancy, childhood, or adolescence.
Alopecia areata patients with a mental health disorder had a mean hospital stay of 6.0 days (95% confidence interval, 5.4.-6.6) and hospitalization cost of $11,907 (95% CI, $10,312-$13,503).
Previous studies had shown similar relationships. However, previous studies showed lower risk of alopecia areata and schizophrenia and no increased risk of ADHD, compared with the current study’s findings. The authors could offer no explanation for those differences.
The strengths of the current analysis include its use of a large-scale, nationally representative cohort and its large sample size, as well its inclusion of a broad range of mental health disorders. Because of its cross-sectional design, the study could not establish the temporal relationship between alopecia areata and mental health disorders.
It is unclear whether psychosocial stress might cause or exacerbate alopecia areata, or whether alopecia areata can lead to or worsen mental health disorders.
The researchers called for additional studies to understand this relationship and potential mechanisms.
The Agency for Healthcare Research and Quality and the Dermatology Foundation funded the study. The researchers declared having no conflicts of interest.
SOURCE: Singam V et al. J Am Acad Dermatol. 2018 Aug 6. doi: 10.1016/j.jaad.2018.07.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Alopecia areata patients should be monitored closely for mental health disorders.
Major finding: Overall, 32.8% of hospitalized alopecia areata patients had a mental health disorder, compared with 20.0% of controls.
Study details: Retrospective analysis of 87,053,155 U.S. adults and children.
Disclosures: The Agency for Healthcare Research & Quality and the Dermatology Foundation funded the study. The researchers declared having no conflicts of interest.
Source: Singam V et al. J Am Acad Dermatol. 2018 Aug 6. doi: 10.1016/j.jaad.2018.07.044.
What is your treatment plan?
The treatment choice is oral terbinafine for tinea capitis with kerion, a scalp dermatophyte infection with a concurrent inflammatory process. Tinea capitis is a very common infection with the peak occurrence at age 3-7 years. Tinea capitis is caused by a variety of dermatophyte species, most commonly by Trichophyton tonsurans or Microsporum canis. T. tonsurans is an endothrix infection, invading the hair shaft and superficial hair while M. canis is an ectothrix infection. T. tonsurans has a person to person transmission; in contrast, M. canis is a zoonotic infection most commonly acquired from infected pets.1 The epidemiology of tinea capitis is affected by multiple factors, including immigration patterns. For example, in Montreal, a study showed a sixfold increase of African dermatophyte species, M. audouinii and T. soudanense.2 Similarly, global variation has increased prevalence of T. violaceum, more commonly found in Europe and Africa, which has been seen in immigrant populations in the United States.1 Kerion is thought to be a hypersensitivity reaction to dermatophytes. Often, misdiagnosis of kerion can result in unnecessary antibiotic prescription and delays in initiation of antifungal therapy.3,4
Tinea capitis can present with focal, “patchy,” well-demarcated hair loss and overlying scale, broken-off hairs at the scalp, and often with pustules. It may be associated with occipital or posterior cervical lymphadenopathy. which presents as a painful boggy scalp mass with or without purulent drainage. Kerions can have associated fever. It is important to differentiate tinea capitis with kerion from processes requiring a different treatment course.3,5 Bacterial folliculitis may have erythema and pustules but only rarely causes hair loss. Similarly, alopecia areata can present with focal alopecia but lacks the scalp inflammation and pustules. Scalp psoriasis is quite scaly, but is more thickened, and usually does not have pustules, nor would purulent drainage be evident. There is a broader differential for other inflammatory focal alopecias including discoid lupus, lichen planopilaris, and dissecting cellulitis of the scalp, which can have similar pus-filled lumps, and cause permanent hair loss, but may be differentiated by associated conditions such as acne conglobata, hidradenitis suppurativa, and pilonidal sinus.
While some clinicians advocate clinical diagnosis of tinea capitis, we advocate confirmation of infection by fungal culture, which can identify the causative organism and influence therapy selection.1 The presence of a fungal infection can be confirmed on potassium hydroxide wet mount prep if hyphae and small spores are seen.6,7 Wood lamp examination will fluoresce if there are ectothrix species, however a negative fluorescence does not differentiate between an endothrix species or lack of infection.
It is important that tinea capitis with kerion is treated with systemic antifungal treatments to allow resolution of the infection, recovery of hair growth, and to prevent or minimize scarring. Systemic antifungal options include terbinafine, griseofulvin, and azoles. Terbinafine is becoming the treatment of choice given shorter duration of treatment with similar efficacy.1T. tonsurans also is thought to respond better to terbinafine than to griseofulvin.8 Griseofulvin is the historical treatment of choice because of its history of clinical safety and no need for laboratory testing. It is important to note that higher doses of griseofulvin (20-25 mg/kg) are recommended, as older lower dose regimens have high rates of failure. Griseofulvin may be more effective for treatment of Microsporum spp than is terbinafine.8 Fluconazole is the only oral antifungal agent that is approved for patients younger than 2 years of age, however it has lower cure rates, compared with terbinafine and griseofulvin.1 Tinea capitis with kerion does not generally require antibiotics unless there is superimposed bacterial infection. Kerion also do not require incision or drainage, which may increase scarring and complicate the clinical course.
Management of tinea capitis should include evaluation of any other household members for coinfection. Depending on the dermatophyte involved there can be risk of person-to-person transmission.5 Families should be educated about fomite transmission via shared combs or hats. Additionally, if a zoophilic dermatophyte is suspected, pets also should be appropriately examined and treated. Topical antifungals are insufficient to eradicate tinea capitis but can be used as adjunctive therapy. Kerion can be treated with oral prednisone in addition to oral antifungals if the lesions are very painful, however there is limited data on this treatment option.9
Ms. Kaushik is a research fellow and Dr. Eichenfield is professor of dermatology and pediatrics in the division of pediatric and adolescent dermatology at Rady Children’s Hospital and the University of California, both in San Diego. They have no conflicts of interest or relevant financial disclosures.
References
1. “Red Book: Report of the Committee on Infectious Diseases,” 31st Edition, (Elk Grove Village, Ill.: American Academy of Pediatrics, 2018, p. 1264).
2. Pediatr Dermatol. 2018 May;35(3):323-8
3. Arch Dis Child. 2016 May;101(5):503.
4. IDCases. 2018 Jun 28;14:e00418.
5. Int J Dermatol. 2018 Jan;57(1):3-9.
6. Pediatr Rev. 2007 May;28(5):164-74.
7. Clin Cosmet Investig Dermatol. 2010;3:89-98.
8. Cochrane Database Syst Rev. 2016 May 12;(5):CD004685.
9. Med Mycol. 1999 Apr;37(2):97-9.
The treatment choice is oral terbinafine for tinea capitis with kerion, a scalp dermatophyte infection with a concurrent inflammatory process. Tinea capitis is a very common infection with the peak occurrence at age 3-7 years. Tinea capitis is caused by a variety of dermatophyte species, most commonly by Trichophyton tonsurans or Microsporum canis. T. tonsurans is an endothrix infection, invading the hair shaft and superficial hair while M. canis is an ectothrix infection. T. tonsurans has a person to person transmission; in contrast, M. canis is a zoonotic infection most commonly acquired from infected pets.1 The epidemiology of tinea capitis is affected by multiple factors, including immigration patterns. For example, in Montreal, a study showed a sixfold increase of African dermatophyte species, M. audouinii and T. soudanense.2 Similarly, global variation has increased prevalence of T. violaceum, more commonly found in Europe and Africa, which has been seen in immigrant populations in the United States.1 Kerion is thought to be a hypersensitivity reaction to dermatophytes. Often, misdiagnosis of kerion can result in unnecessary antibiotic prescription and delays in initiation of antifungal therapy.3,4
Tinea capitis can present with focal, “patchy,” well-demarcated hair loss and overlying scale, broken-off hairs at the scalp, and often with pustules. It may be associated with occipital or posterior cervical lymphadenopathy. which presents as a painful boggy scalp mass with or without purulent drainage. Kerions can have associated fever. It is important to differentiate tinea capitis with kerion from processes requiring a different treatment course.3,5 Bacterial folliculitis may have erythema and pustules but only rarely causes hair loss. Similarly, alopecia areata can present with focal alopecia but lacks the scalp inflammation and pustules. Scalp psoriasis is quite scaly, but is more thickened, and usually does not have pustules, nor would purulent drainage be evident. There is a broader differential for other inflammatory focal alopecias including discoid lupus, lichen planopilaris, and dissecting cellulitis of the scalp, which can have similar pus-filled lumps, and cause permanent hair loss, but may be differentiated by associated conditions such as acne conglobata, hidradenitis suppurativa, and pilonidal sinus.
While some clinicians advocate clinical diagnosis of tinea capitis, we advocate confirmation of infection by fungal culture, which can identify the causative organism and influence therapy selection.1 The presence of a fungal infection can be confirmed on potassium hydroxide wet mount prep if hyphae and small spores are seen.6,7 Wood lamp examination will fluoresce if there are ectothrix species, however a negative fluorescence does not differentiate between an endothrix species or lack of infection.
It is important that tinea capitis with kerion is treated with systemic antifungal treatments to allow resolution of the infection, recovery of hair growth, and to prevent or minimize scarring. Systemic antifungal options include terbinafine, griseofulvin, and azoles. Terbinafine is becoming the treatment of choice given shorter duration of treatment with similar efficacy.1T. tonsurans also is thought to respond better to terbinafine than to griseofulvin.8 Griseofulvin is the historical treatment of choice because of its history of clinical safety and no need for laboratory testing. It is important to note that higher doses of griseofulvin (20-25 mg/kg) are recommended, as older lower dose regimens have high rates of failure. Griseofulvin may be more effective for treatment of Microsporum spp than is terbinafine.8 Fluconazole is the only oral antifungal agent that is approved for patients younger than 2 years of age, however it has lower cure rates, compared with terbinafine and griseofulvin.1 Tinea capitis with kerion does not generally require antibiotics unless there is superimposed bacterial infection. Kerion also do not require incision or drainage, which may increase scarring and complicate the clinical course.
Management of tinea capitis should include evaluation of any other household members for coinfection. Depending on the dermatophyte involved there can be risk of person-to-person transmission.5 Families should be educated about fomite transmission via shared combs or hats. Additionally, if a zoophilic dermatophyte is suspected, pets also should be appropriately examined and treated. Topical antifungals are insufficient to eradicate tinea capitis but can be used as adjunctive therapy. Kerion can be treated with oral prednisone in addition to oral antifungals if the lesions are very painful, however there is limited data on this treatment option.9
Ms. Kaushik is a research fellow and Dr. Eichenfield is professor of dermatology and pediatrics in the division of pediatric and adolescent dermatology at Rady Children’s Hospital and the University of California, both in San Diego. They have no conflicts of interest or relevant financial disclosures.
References
1. “Red Book: Report of the Committee on Infectious Diseases,” 31st Edition, (Elk Grove Village, Ill.: American Academy of Pediatrics, 2018, p. 1264).
2. Pediatr Dermatol. 2018 May;35(3):323-8
3. Arch Dis Child. 2016 May;101(5):503.
4. IDCases. 2018 Jun 28;14:e00418.
5. Int J Dermatol. 2018 Jan;57(1):3-9.
6. Pediatr Rev. 2007 May;28(5):164-74.
7. Clin Cosmet Investig Dermatol. 2010;3:89-98.
8. Cochrane Database Syst Rev. 2016 May 12;(5):CD004685.
9. Med Mycol. 1999 Apr;37(2):97-9.
The treatment choice is oral terbinafine for tinea capitis with kerion, a scalp dermatophyte infection with a concurrent inflammatory process. Tinea capitis is a very common infection with the peak occurrence at age 3-7 years. Tinea capitis is caused by a variety of dermatophyte species, most commonly by Trichophyton tonsurans or Microsporum canis. T. tonsurans is an endothrix infection, invading the hair shaft and superficial hair while M. canis is an ectothrix infection. T. tonsurans has a person to person transmission; in contrast, M. canis is a zoonotic infection most commonly acquired from infected pets.1 The epidemiology of tinea capitis is affected by multiple factors, including immigration patterns. For example, in Montreal, a study showed a sixfold increase of African dermatophyte species, M. audouinii and T. soudanense.2 Similarly, global variation has increased prevalence of T. violaceum, more commonly found in Europe and Africa, which has been seen in immigrant populations in the United States.1 Kerion is thought to be a hypersensitivity reaction to dermatophytes. Often, misdiagnosis of kerion can result in unnecessary antibiotic prescription and delays in initiation of antifungal therapy.3,4
Tinea capitis can present with focal, “patchy,” well-demarcated hair loss and overlying scale, broken-off hairs at the scalp, and often with pustules. It may be associated with occipital or posterior cervical lymphadenopathy. which presents as a painful boggy scalp mass with or without purulent drainage. Kerions can have associated fever. It is important to differentiate tinea capitis with kerion from processes requiring a different treatment course.3,5 Bacterial folliculitis may have erythema and pustules but only rarely causes hair loss. Similarly, alopecia areata can present with focal alopecia but lacks the scalp inflammation and pustules. Scalp psoriasis is quite scaly, but is more thickened, and usually does not have pustules, nor would purulent drainage be evident. There is a broader differential for other inflammatory focal alopecias including discoid lupus, lichen planopilaris, and dissecting cellulitis of the scalp, which can have similar pus-filled lumps, and cause permanent hair loss, but may be differentiated by associated conditions such as acne conglobata, hidradenitis suppurativa, and pilonidal sinus.
While some clinicians advocate clinical diagnosis of tinea capitis, we advocate confirmation of infection by fungal culture, which can identify the causative organism and influence therapy selection.1 The presence of a fungal infection can be confirmed on potassium hydroxide wet mount prep if hyphae and small spores are seen.6,7 Wood lamp examination will fluoresce if there are ectothrix species, however a negative fluorescence does not differentiate between an endothrix species or lack of infection.
It is important that tinea capitis with kerion is treated with systemic antifungal treatments to allow resolution of the infection, recovery of hair growth, and to prevent or minimize scarring. Systemic antifungal options include terbinafine, griseofulvin, and azoles. Terbinafine is becoming the treatment of choice given shorter duration of treatment with similar efficacy.1T. tonsurans also is thought to respond better to terbinafine than to griseofulvin.8 Griseofulvin is the historical treatment of choice because of its history of clinical safety and no need for laboratory testing. It is important to note that higher doses of griseofulvin (20-25 mg/kg) are recommended, as older lower dose regimens have high rates of failure. Griseofulvin may be more effective for treatment of Microsporum spp than is terbinafine.8 Fluconazole is the only oral antifungal agent that is approved for patients younger than 2 years of age, however it has lower cure rates, compared with terbinafine and griseofulvin.1 Tinea capitis with kerion does not generally require antibiotics unless there is superimposed bacterial infection. Kerion also do not require incision or drainage, which may increase scarring and complicate the clinical course.
Management of tinea capitis should include evaluation of any other household members for coinfection. Depending on the dermatophyte involved there can be risk of person-to-person transmission.5 Families should be educated about fomite transmission via shared combs or hats. Additionally, if a zoophilic dermatophyte is suspected, pets also should be appropriately examined and treated. Topical antifungals are insufficient to eradicate tinea capitis but can be used as adjunctive therapy. Kerion can be treated with oral prednisone in addition to oral antifungals if the lesions are very painful, however there is limited data on this treatment option.9
Ms. Kaushik is a research fellow and Dr. Eichenfield is professor of dermatology and pediatrics in the division of pediatric and adolescent dermatology at Rady Children’s Hospital and the University of California, both in San Diego. They have no conflicts of interest or relevant financial disclosures.
References
1. “Red Book: Report of the Committee on Infectious Diseases,” 31st Edition, (Elk Grove Village, Ill.: American Academy of Pediatrics, 2018, p. 1264).
2. Pediatr Dermatol. 2018 May;35(3):323-8
3. Arch Dis Child. 2016 May;101(5):503.
4. IDCases. 2018 Jun 28;14:e00418.
5. Int J Dermatol. 2018 Jan;57(1):3-9.
6. Pediatr Rev. 2007 May;28(5):164-74.
7. Clin Cosmet Investig Dermatol. 2010;3:89-98.
8. Cochrane Database Syst Rev. 2016 May 12;(5):CD004685.
9. Med Mycol. 1999 Apr;37(2):97-9.
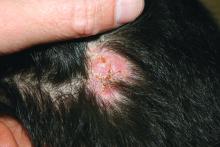
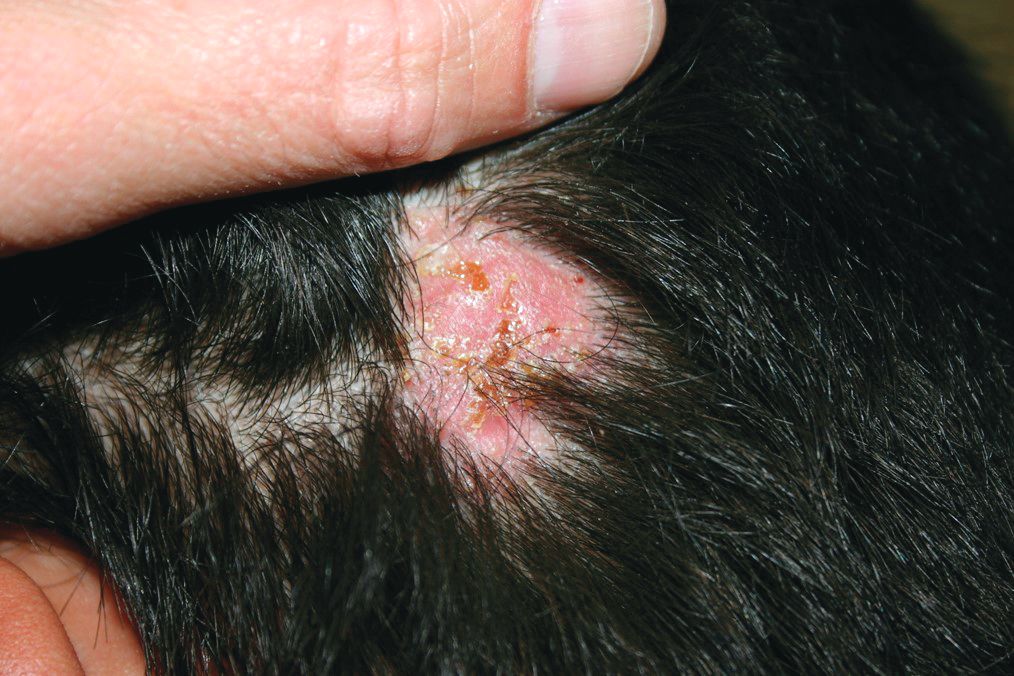
A 10-year-old otherwise-healthy male presents for a progressing lesion on his scalp. One month prior to coming in, he developed some peeling and itch followed by loss of hair. This had worsened, becoming a painful and boggy mass on the back of his head with focal alopecia. He went to the local ED, where he had plain films of his skull, which were normal and was prescribed cephalexin. He has not shown any improvement after starting the antibiotics. He has had no fevers in this time, but the pain persists.
On physical exam, he is noted to have a hairless patch on a boggy left occipital mass, which is tender to palpation. There is a small amount of overlying honey-colored crusting. He has associated posterior occipital nontender lymphadenopathy.
The patient's older sister has a small area of scalp hair loss.
This Baby's Got Flare
At birth, this child had a lesion on his shoulder that now—a year later—has doubled in size. His parents report no systemic symptoms or medication use for their son. They say that the child exhibits no distress; he does not attempt to scratch at the affected patch of skin. However, they observe that if the lesion is touched, it swells and then (within minutes) returns to normal.
There is no family history of similar problems. However, both the patient and his mother are highly atopic.
EXAMINATION
The lesion—a low, orange, oval plaque—measures about 3.5 x 2 cm. Barely palpable, it urticates when stroked with a fingernail edge but does not appear to cause any discomfort.

No other lesions of note are found. The child appears quite healthy and is in no distress.
What is the diagnosis?
DISCUSSION
Mastocytosis is caused by a localized accumulation of mast cells (a type of white blood cell) and CD34-positive mast cell precursors, which are normally present but widely scattered and sparse. This child has the most common form of cutaneous mastocytosis, which can manifest with solitary lesions or with dozens or hundreds of scattered lesions (the latter known as urticaria pigmentosa). Both types are typically benign and self-limited.
When stroked, mast cell lesions degranulate portions of the cell, releasing histamine precursors and leukotrienes (eg, IL 1 and IL 31). In most cases, stroking merely leads to short-lived urtication. But if the problem is more widespread (eg, urticaria pigmentosa) and the lesions are sufficiently traumatized, the release of these substances can lead to problems such as hypotension, malaise, fever, and abdominal pain.
Fortunately, this is rare, as is systemic mastocytosis—a condition in which mast cells infiltrate internal organs and bone marrow, interrupting normal function and, in the extreme, leading to mast cell leukemia. Our patient is not at risk for these complications; his lesion should resolve completely by age 3.
The differential for this patient’s lesion includes congenital nevus, lichen aureus, and café au lait spot.
TAKE-HOME LEARNING POINTS
- Cutaneous mastocytosis manifests as a reddish orange maculopapular patch, which urticates upon forceful stroking.
- Stroking the lesion degranulates the mast cells comprising it, leading to the release of histamine precursors.
- Mast cells can infiltrate internal organs and bone marrow, leading, in the extreme, to mast cell leukemia.
- Urticaria pigmentosa is a variation of mastocytosis in which hundreds of such lesions develop all over the body.
At birth, this child had a lesion on his shoulder that now—a year later—has doubled in size. His parents report no systemic symptoms or medication use for their son. They say that the child exhibits no distress; he does not attempt to scratch at the affected patch of skin. However, they observe that if the lesion is touched, it swells and then (within minutes) returns to normal.
There is no family history of similar problems. However, both the patient and his mother are highly atopic.
EXAMINATION
The lesion—a low, orange, oval plaque—measures about 3.5 x 2 cm. Barely palpable, it urticates when stroked with a fingernail edge but does not appear to cause any discomfort.

No other lesions of note are found. The child appears quite healthy and is in no distress.
What is the diagnosis?
DISCUSSION
Mastocytosis is caused by a localized accumulation of mast cells (a type of white blood cell) and CD34-positive mast cell precursors, which are normally present but widely scattered and sparse. This child has the most common form of cutaneous mastocytosis, which can manifest with solitary lesions or with dozens or hundreds of scattered lesions (the latter known as urticaria pigmentosa). Both types are typically benign and self-limited.
When stroked, mast cell lesions degranulate portions of the cell, releasing histamine precursors and leukotrienes (eg, IL 1 and IL 31). In most cases, stroking merely leads to short-lived urtication. But if the problem is more widespread (eg, urticaria pigmentosa) and the lesions are sufficiently traumatized, the release of these substances can lead to problems such as hypotension, malaise, fever, and abdominal pain.
Fortunately, this is rare, as is systemic mastocytosis—a condition in which mast cells infiltrate internal organs and bone marrow, interrupting normal function and, in the extreme, leading to mast cell leukemia. Our patient is not at risk for these complications; his lesion should resolve completely by age 3.
The differential for this patient’s lesion includes congenital nevus, lichen aureus, and café au lait spot.
TAKE-HOME LEARNING POINTS
- Cutaneous mastocytosis manifests as a reddish orange maculopapular patch, which urticates upon forceful stroking.
- Stroking the lesion degranulates the mast cells comprising it, leading to the release of histamine precursors.
- Mast cells can infiltrate internal organs and bone marrow, leading, in the extreme, to mast cell leukemia.
- Urticaria pigmentosa is a variation of mastocytosis in which hundreds of such lesions develop all over the body.
At birth, this child had a lesion on his shoulder that now—a year later—has doubled in size. His parents report no systemic symptoms or medication use for their son. They say that the child exhibits no distress; he does not attempt to scratch at the affected patch of skin. However, they observe that if the lesion is touched, it swells and then (within minutes) returns to normal.
There is no family history of similar problems. However, both the patient and his mother are highly atopic.
EXAMINATION
The lesion—a low, orange, oval plaque—measures about 3.5 x 2 cm. Barely palpable, it urticates when stroked with a fingernail edge but does not appear to cause any discomfort.

No other lesions of note are found. The child appears quite healthy and is in no distress.
What is the diagnosis?
DISCUSSION
Mastocytosis is caused by a localized accumulation of mast cells (a type of white blood cell) and CD34-positive mast cell precursors, which are normally present but widely scattered and sparse. This child has the most common form of cutaneous mastocytosis, which can manifest with solitary lesions or with dozens or hundreds of scattered lesions (the latter known as urticaria pigmentosa). Both types are typically benign and self-limited.
When stroked, mast cell lesions degranulate portions of the cell, releasing histamine precursors and leukotrienes (eg, IL 1 and IL 31). In most cases, stroking merely leads to short-lived urtication. But if the problem is more widespread (eg, urticaria pigmentosa) and the lesions are sufficiently traumatized, the release of these substances can lead to problems such as hypotension, malaise, fever, and abdominal pain.
Fortunately, this is rare, as is systemic mastocytosis—a condition in which mast cells infiltrate internal organs and bone marrow, interrupting normal function and, in the extreme, leading to mast cell leukemia. Our patient is not at risk for these complications; his lesion should resolve completely by age 3.
The differential for this patient’s lesion includes congenital nevus, lichen aureus, and café au lait spot.
TAKE-HOME LEARNING POINTS
- Cutaneous mastocytosis manifests as a reddish orange maculopapular patch, which urticates upon forceful stroking.
- Stroking the lesion degranulates the mast cells comprising it, leading to the release of histamine precursors.
- Mast cells can infiltrate internal organs and bone marrow, leading, in the extreme, to mast cell leukemia.
- Urticaria pigmentosa is a variation of mastocytosis in which hundreds of such lesions develop all over the body.
Psoriasis registry study provides more data on infliximab’s infection risk
that led to hospitalization, the use of intravenous antimicrobial therapy, or death, according to a prospective cohort study of cases in the United Kingdom and the Republic of Ireland.
The new data suggest a risk associated with infliximab treatment that previous clinical trials and observational studies were insufficiently powered to detect, according to the investigators, led by Zenas Yiu, of the University of Manchester (England). They found no associations between infection risk and treatment with etanercept, adalimumab, or ustekinumab, and they noted that there are no such data yet on more recently approved biologic therapies for psoriasis, such as secukinumab or ixekizumab.
The British Association of Dermatologists (BAD) recommends infliximab, a tumor necrosis factor (TNF)–blocker, only for severe cases of psoriasis (Psoriasis Area and Severity Index greater than or equal to 20 and a Dermatology Life Quality Index greater than 18), or when other biologics fail or cannot be used.
To address the insufficient power of earlier studies, the researchers used data from the BAD Biologic Interventions Register (BADBIR), a large, prospective psoriasis registry in the United Kingdom and Ireland established in 2007. The analysis included 3,421 subjects in the nonbiologic systemic therapy cohort, and 422 subjects in the all-lines infliximab cohort. The median follow-up period was 1.49 person-years (interquartile range, 2.50 person-years) for the all-lines (not just first-line) infliximab group, and 1.51 person-years (1.84 person-years) for the nonbiologics group.*
Treatment with infliximab was associated with a statistically significant increased risk of serious infection (defined as an infection associated with prolonged hospitalization or use of IV antimicrobial therapy; or an infection that resulted in death), with an adjusted hazard ratio of 1.95 (95% confidence interval, 1.01-3.75), compared with nonbiologic systemic treatments. The risk was higher in the first 6 months (adjusted HR, 3.49; 95% CI, 1.14-10.70), and from 6 months to 1 year (aHR, 2.99; 95% CI, 1.10-8.14,) but did not reach statistical significance at 1 year to 2 years (aHR, 2.03; 95% CI, 0.61-6.79).
There was also an increased risk of serious infection with infliximab compared with methotrexate (aHR, 2.96; 95% CI, 1.58-5.57).
“Given our findings of a higher risk of serious infection associated with infliximab, we provide real-world evidence to reinforce the position of infliximab in the psoriasis treatment hierarchy,” the authors wrote, adding that “patients with severe psoriasis who fulfill the criteria for the prescription of infliximab should be counseled” about the risk of serious infection.
Dr. Yiu disclosed having received nonfinancial support form Novartis, two authors had no disclosures, and the remainder had various disclosures related to pharmaceutical companies. BADBIR is funded by BAD, which receives funding from Pfizer, Janssen Cilag, AbbVie, Novartis, Samsung Bioepis and Eli Lilly for providing pharmacovigilance services.
SOURCE: Yiu ZZN et al. Br J Dermatol. 2018 Aug 2. doi: 10.1111/bjd.17036.
*This article was updated to correctly indicate that the median follow-up period was 1.49 person-years (interquartile range, 2.50 person-years) for the all-lines (not just first-line) infliximab group, and 1.51 person-years (1.84 person-years) for the nonbiologics group.
that led to hospitalization, the use of intravenous antimicrobial therapy, or death, according to a prospective cohort study of cases in the United Kingdom and the Republic of Ireland.
The new data suggest a risk associated with infliximab treatment that previous clinical trials and observational studies were insufficiently powered to detect, according to the investigators, led by Zenas Yiu, of the University of Manchester (England). They found no associations between infection risk and treatment with etanercept, adalimumab, or ustekinumab, and they noted that there are no such data yet on more recently approved biologic therapies for psoriasis, such as secukinumab or ixekizumab.
The British Association of Dermatologists (BAD) recommends infliximab, a tumor necrosis factor (TNF)–blocker, only for severe cases of psoriasis (Psoriasis Area and Severity Index greater than or equal to 20 and a Dermatology Life Quality Index greater than 18), or when other biologics fail or cannot be used.
To address the insufficient power of earlier studies, the researchers used data from the BAD Biologic Interventions Register (BADBIR), a large, prospective psoriasis registry in the United Kingdom and Ireland established in 2007. The analysis included 3,421 subjects in the nonbiologic systemic therapy cohort, and 422 subjects in the all-lines infliximab cohort. The median follow-up period was 1.49 person-years (interquartile range, 2.50 person-years) for the all-lines (not just first-line) infliximab group, and 1.51 person-years (1.84 person-years) for the nonbiologics group.*
Treatment with infliximab was associated with a statistically significant increased risk of serious infection (defined as an infection associated with prolonged hospitalization or use of IV antimicrobial therapy; or an infection that resulted in death), with an adjusted hazard ratio of 1.95 (95% confidence interval, 1.01-3.75), compared with nonbiologic systemic treatments. The risk was higher in the first 6 months (adjusted HR, 3.49; 95% CI, 1.14-10.70), and from 6 months to 1 year (aHR, 2.99; 95% CI, 1.10-8.14,) but did not reach statistical significance at 1 year to 2 years (aHR, 2.03; 95% CI, 0.61-6.79).
There was also an increased risk of serious infection with infliximab compared with methotrexate (aHR, 2.96; 95% CI, 1.58-5.57).
“Given our findings of a higher risk of serious infection associated with infliximab, we provide real-world evidence to reinforce the position of infliximab in the psoriasis treatment hierarchy,” the authors wrote, adding that “patients with severe psoriasis who fulfill the criteria for the prescription of infliximab should be counseled” about the risk of serious infection.
Dr. Yiu disclosed having received nonfinancial support form Novartis, two authors had no disclosures, and the remainder had various disclosures related to pharmaceutical companies. BADBIR is funded by BAD, which receives funding from Pfizer, Janssen Cilag, AbbVie, Novartis, Samsung Bioepis and Eli Lilly for providing pharmacovigilance services.
SOURCE: Yiu ZZN et al. Br J Dermatol. 2018 Aug 2. doi: 10.1111/bjd.17036.
*This article was updated to correctly indicate that the median follow-up period was 1.49 person-years (interquartile range, 2.50 person-years) for the all-lines (not just first-line) infliximab group, and 1.51 person-years (1.84 person-years) for the nonbiologics group.
that led to hospitalization, the use of intravenous antimicrobial therapy, or death, according to a prospective cohort study of cases in the United Kingdom and the Republic of Ireland.
The new data suggest a risk associated with infliximab treatment that previous clinical trials and observational studies were insufficiently powered to detect, according to the investigators, led by Zenas Yiu, of the University of Manchester (England). They found no associations between infection risk and treatment with etanercept, adalimumab, or ustekinumab, and they noted that there are no such data yet on more recently approved biologic therapies for psoriasis, such as secukinumab or ixekizumab.
The British Association of Dermatologists (BAD) recommends infliximab, a tumor necrosis factor (TNF)–blocker, only for severe cases of psoriasis (Psoriasis Area and Severity Index greater than or equal to 20 and a Dermatology Life Quality Index greater than 18), or when other biologics fail or cannot be used.
To address the insufficient power of earlier studies, the researchers used data from the BAD Biologic Interventions Register (BADBIR), a large, prospective psoriasis registry in the United Kingdom and Ireland established in 2007. The analysis included 3,421 subjects in the nonbiologic systemic therapy cohort, and 422 subjects in the all-lines infliximab cohort. The median follow-up period was 1.49 person-years (interquartile range, 2.50 person-years) for the all-lines (not just first-line) infliximab group, and 1.51 person-years (1.84 person-years) for the nonbiologics group.*
Treatment with infliximab was associated with a statistically significant increased risk of serious infection (defined as an infection associated with prolonged hospitalization or use of IV antimicrobial therapy; or an infection that resulted in death), with an adjusted hazard ratio of 1.95 (95% confidence interval, 1.01-3.75), compared with nonbiologic systemic treatments. The risk was higher in the first 6 months (adjusted HR, 3.49; 95% CI, 1.14-10.70), and from 6 months to 1 year (aHR, 2.99; 95% CI, 1.10-8.14,) but did not reach statistical significance at 1 year to 2 years (aHR, 2.03; 95% CI, 0.61-6.79).
There was also an increased risk of serious infection with infliximab compared with methotrexate (aHR, 2.96; 95% CI, 1.58-5.57).
“Given our findings of a higher risk of serious infection associated with infliximab, we provide real-world evidence to reinforce the position of infliximab in the psoriasis treatment hierarchy,” the authors wrote, adding that “patients with severe psoriasis who fulfill the criteria for the prescription of infliximab should be counseled” about the risk of serious infection.
Dr. Yiu disclosed having received nonfinancial support form Novartis, two authors had no disclosures, and the remainder had various disclosures related to pharmaceutical companies. BADBIR is funded by BAD, which receives funding from Pfizer, Janssen Cilag, AbbVie, Novartis, Samsung Bioepis and Eli Lilly for providing pharmacovigilance services.
SOURCE: Yiu ZZN et al. Br J Dermatol. 2018 Aug 2. doi: 10.1111/bjd.17036.
*This article was updated to correctly indicate that the median follow-up period was 1.49 person-years (interquartile range, 2.50 person-years) for the all-lines (not just first-line) infliximab group, and 1.51 person-years (1.84 person-years) for the nonbiologics group.
FROM BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The study reinforces British guidelines that infliximab should be restricted to most severe cases.
Major finding: Infliximab was associated with a hazard ratio of 1.95 for severe infections, compared with non-biologic systemic therapies.
Study details: Prospective cohort analysis of a psoriasis treatment database of 3,843 individuals.
Disclosures: Dr. Yiu disclosed having received non-financial support form Novartis, two authors had no disclosures, and the remainder had various disclosures related to pharmaceutical companies. BADBIR is funded by BAD, which receives funding from Pfizer, Janssen Cilag, AbbVie, Novartis, Samsung Bioepis and Eli Lilly for providing pharmacovigilance services.
Source: Yiu ZZN et al. Br J Dermatol. 2018 Aug 2. doi: 10.1111/bjd.17036.
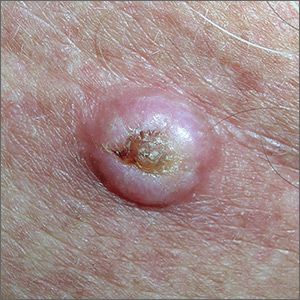
Growth on chest
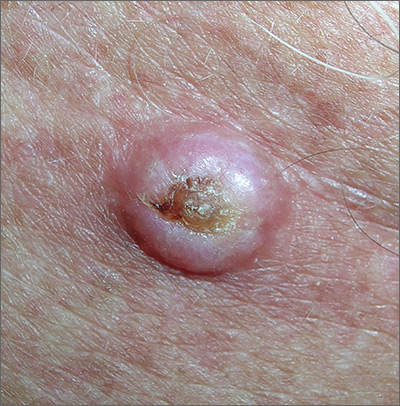
The FP noted that the lesion had a central keratin core (resembling a volcano) surrounded by a somewhat pearly raised border. He suspected that this was a squamous cell carcinoma (SCC) of the keratoacanthoma type and recommended a biopsy.
A shave biopsy was performed, and the result confirmed the diagnosis of SCC of the keratoacanthoma type. (See the Watch & Learn video on “Shave biopsy.”) The pathologist added that the lesion was well-differentiated (which was to be expected with a keratoacanthoma). While some keratoacanthomas actually resolve spontaneously, the standard of care is to treat them with either excision, electrodesiccation and curettage, or cryotherapy. On the follow-up visit, these options were presented to the patient and he decided to have cryotherapy. He accepted the risk of hypopigmentation in the treated area, but said that he preferred to avoid surgery.
The area was anesthetized with an injection of 1% lidocaine and epinephrine, and cryotherapy was performed with a 5 mm margin. The area was frozen with liquid nitrogen spray for 30 seconds and allowed to thaw. A second freeze for 30 seconds was then applied. Sun protection and sun avoidance were encouraged. At a 1-year follow-up, there was no recurrence and there was hypopigmentation at the treatment site.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratoacanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the lesion had a central keratin core (resembling a volcano) surrounded by a somewhat pearly raised border. He suspected that this was a squamous cell carcinoma (SCC) of the keratoacanthoma type and recommended a biopsy.
A shave biopsy was performed, and the result confirmed the diagnosis of SCC of the keratoacanthoma type. (See the Watch & Learn video on “Shave biopsy.”) The pathologist added that the lesion was well-differentiated (which was to be expected with a keratoacanthoma). While some keratoacanthomas actually resolve spontaneously, the standard of care is to treat them with either excision, electrodesiccation and curettage, or cryotherapy. On the follow-up visit, these options were presented to the patient and he decided to have cryotherapy. He accepted the risk of hypopigmentation in the treated area, but said that he preferred to avoid surgery.
The area was anesthetized with an injection of 1% lidocaine and epinephrine, and cryotherapy was performed with a 5 mm margin. The area was frozen with liquid nitrogen spray for 30 seconds and allowed to thaw. A second freeze for 30 seconds was then applied. Sun protection and sun avoidance were encouraged. At a 1-year follow-up, there was no recurrence and there was hypopigmentation at the treatment site.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratoacanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the lesion had a central keratin core (resembling a volcano) surrounded by a somewhat pearly raised border. He suspected that this was a squamous cell carcinoma (SCC) of the keratoacanthoma type and recommended a biopsy.
A shave biopsy was performed, and the result confirmed the diagnosis of SCC of the keratoacanthoma type. (See the Watch & Learn video on “Shave biopsy.”) The pathologist added that the lesion was well-differentiated (which was to be expected with a keratoacanthoma). While some keratoacanthomas actually resolve spontaneously, the standard of care is to treat them with either excision, electrodesiccation and curettage, or cryotherapy. On the follow-up visit, these options were presented to the patient and he decided to have cryotherapy. He accepted the risk of hypopigmentation in the treated area, but said that he preferred to avoid surgery.
The area was anesthetized with an injection of 1% lidocaine and epinephrine, and cryotherapy was performed with a 5 mm margin. The area was frozen with liquid nitrogen spray for 30 seconds and allowed to thaw. A second freeze for 30 seconds was then applied. Sun protection and sun avoidance were encouraged. At a 1-year follow-up, there was no recurrence and there was hypopigmentation at the treatment site.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratoacanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Climbing the therapeutic ladder in eczema-related itch
WASHINGTON – Currently available including antihistamines and an oral antiemetic approved for preventing chemotherapy-related nausea and vomiting, Peter Lio, MD, said at a symposium presented by the Coalition United for Better Eczema Care (CUBE-C).
There are four basic areas of treatment, which Dr. Lio, a dermatologist at Northwestern University, Chicago, referred to as the “itch therapeutic ladder.” In a video interview at the meeting, he reviewed the treatments, starting with topical therapies, which include camphor and menthol, strontium-containing topicals, as well as “dilute bleach-type products” that seem to have some anti-inflammatory and anti-itch effects.
The next levels: oral medications – antihistamines, followed by “more intense” options that may carry more risks, such as the antidepressant mirtazapine, and aprepitant, a neurokinin-1 receptor antagonist approved for the prevention of chemotherapy-induced and postoperative nausea and vomiting. Gabapentin and naltrexone can also be helpful for certain populations; all are used off-label, he pointed out.
Dr. Lio, formally trained in acupuncture, often uses alternative therapies as the fourth rung of the ladder. These include using a specific acupressure point, which he said “seems to give a little bit of relief.”
In the interview, he also discussed considerations in children with atopic dermatitis and exciting treatments in development, such as biologics that target “one of the master itch cytokines,” interleukin-31.
“Itch is such an important part of this disease because we know not only is it one of the key pieces that pushes the disease forward and keeps these cycles going, but also contributes a huge amount to the morbidity,” he said.
CUBE-C, established by the National Eczema Association (NEA), is a “network of cross-specialty leaders, patients and caregivers, constructing an educational curriculum based on standards of effective treatment and disease management,” according to the NEA.
The symposium was supported by an educational grant from Sanofi Genzyme, Regeneron Pharmaceuticals, and Pfizer. Dr. Lio reported serving as a speaker, consultant, and/or advisor for companies developing and marketing atopic dermatitis therapies and products.
WASHINGTON – Currently available including antihistamines and an oral antiemetic approved for preventing chemotherapy-related nausea and vomiting, Peter Lio, MD, said at a symposium presented by the Coalition United for Better Eczema Care (CUBE-C).
There are four basic areas of treatment, which Dr. Lio, a dermatologist at Northwestern University, Chicago, referred to as the “itch therapeutic ladder.” In a video interview at the meeting, he reviewed the treatments, starting with topical therapies, which include camphor and menthol, strontium-containing topicals, as well as “dilute bleach-type products” that seem to have some anti-inflammatory and anti-itch effects.
The next levels: oral medications – antihistamines, followed by “more intense” options that may carry more risks, such as the antidepressant mirtazapine, and aprepitant, a neurokinin-1 receptor antagonist approved for the prevention of chemotherapy-induced and postoperative nausea and vomiting. Gabapentin and naltrexone can also be helpful for certain populations; all are used off-label, he pointed out.
Dr. Lio, formally trained in acupuncture, often uses alternative therapies as the fourth rung of the ladder. These include using a specific acupressure point, which he said “seems to give a little bit of relief.”
In the interview, he also discussed considerations in children with atopic dermatitis and exciting treatments in development, such as biologics that target “one of the master itch cytokines,” interleukin-31.
“Itch is such an important part of this disease because we know not only is it one of the key pieces that pushes the disease forward and keeps these cycles going, but also contributes a huge amount to the morbidity,” he said.
CUBE-C, established by the National Eczema Association (NEA), is a “network of cross-specialty leaders, patients and caregivers, constructing an educational curriculum based on standards of effective treatment and disease management,” according to the NEA.
The symposium was supported by an educational grant from Sanofi Genzyme, Regeneron Pharmaceuticals, and Pfizer. Dr. Lio reported serving as a speaker, consultant, and/or advisor for companies developing and marketing atopic dermatitis therapies and products.
WASHINGTON – Currently available including antihistamines and an oral antiemetic approved for preventing chemotherapy-related nausea and vomiting, Peter Lio, MD, said at a symposium presented by the Coalition United for Better Eczema Care (CUBE-C).
There are four basic areas of treatment, which Dr. Lio, a dermatologist at Northwestern University, Chicago, referred to as the “itch therapeutic ladder.” In a video interview at the meeting, he reviewed the treatments, starting with topical therapies, which include camphor and menthol, strontium-containing topicals, as well as “dilute bleach-type products” that seem to have some anti-inflammatory and anti-itch effects.
The next levels: oral medications – antihistamines, followed by “more intense” options that may carry more risks, such as the antidepressant mirtazapine, and aprepitant, a neurokinin-1 receptor antagonist approved for the prevention of chemotherapy-induced and postoperative nausea and vomiting. Gabapentin and naltrexone can also be helpful for certain populations; all are used off-label, he pointed out.
Dr. Lio, formally trained in acupuncture, often uses alternative therapies as the fourth rung of the ladder. These include using a specific acupressure point, which he said “seems to give a little bit of relief.”
In the interview, he also discussed considerations in children with atopic dermatitis and exciting treatments in development, such as biologics that target “one of the master itch cytokines,” interleukin-31.
“Itch is such an important part of this disease because we know not only is it one of the key pieces that pushes the disease forward and keeps these cycles going, but also contributes a huge amount to the morbidity,” he said.
CUBE-C, established by the National Eczema Association (NEA), is a “network of cross-specialty leaders, patients and caregivers, constructing an educational curriculum based on standards of effective treatment and disease management,” according to the NEA.
The symposium was supported by an educational grant from Sanofi Genzyme, Regeneron Pharmaceuticals, and Pfizer. Dr. Lio reported serving as a speaker, consultant, and/or advisor for companies developing and marketing atopic dermatitis therapies and products.