User login
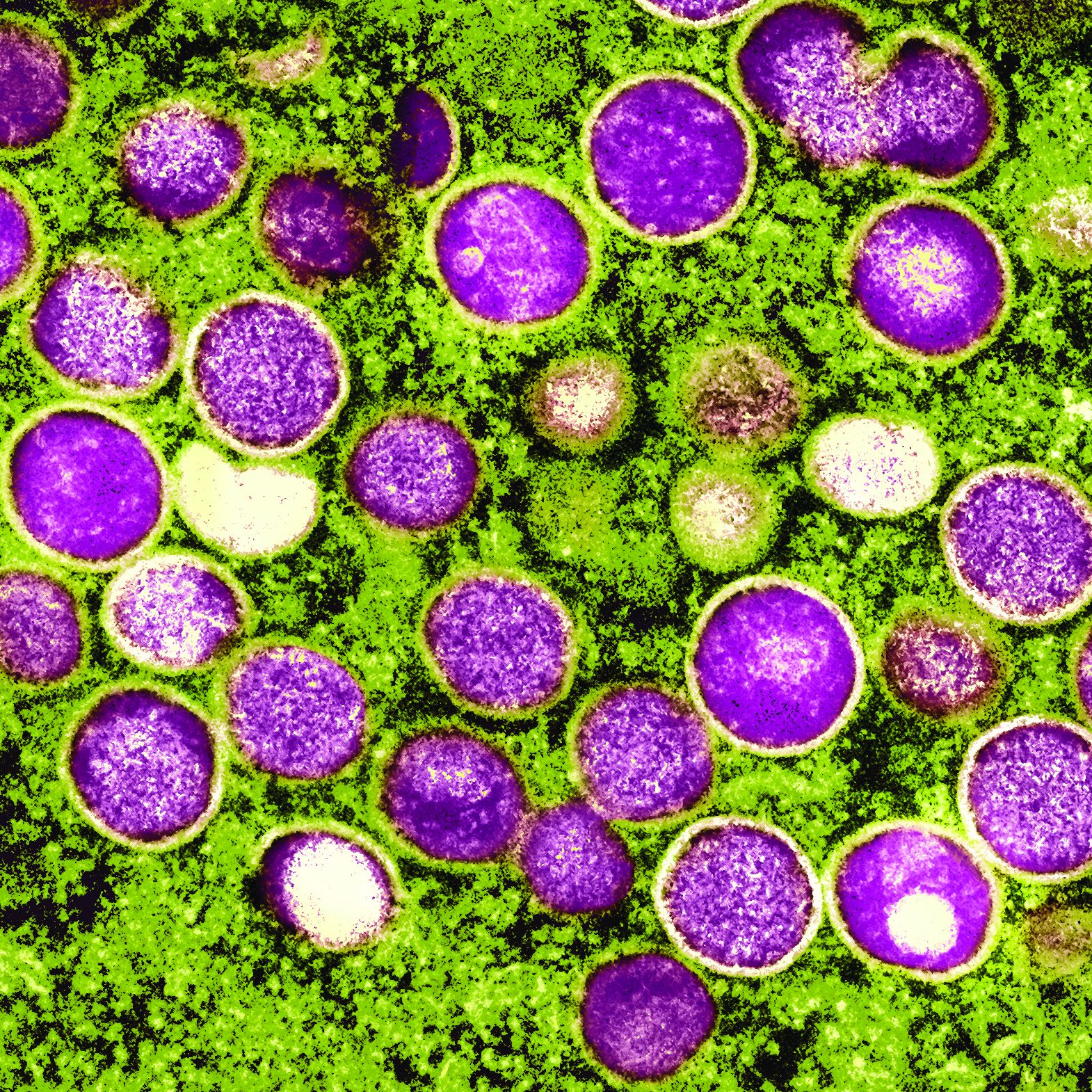
To gauge monkeypox spread, researchers eye cases in women
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
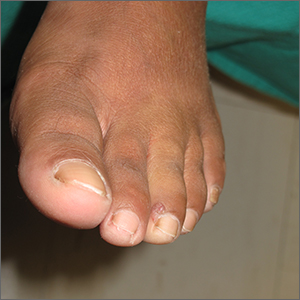
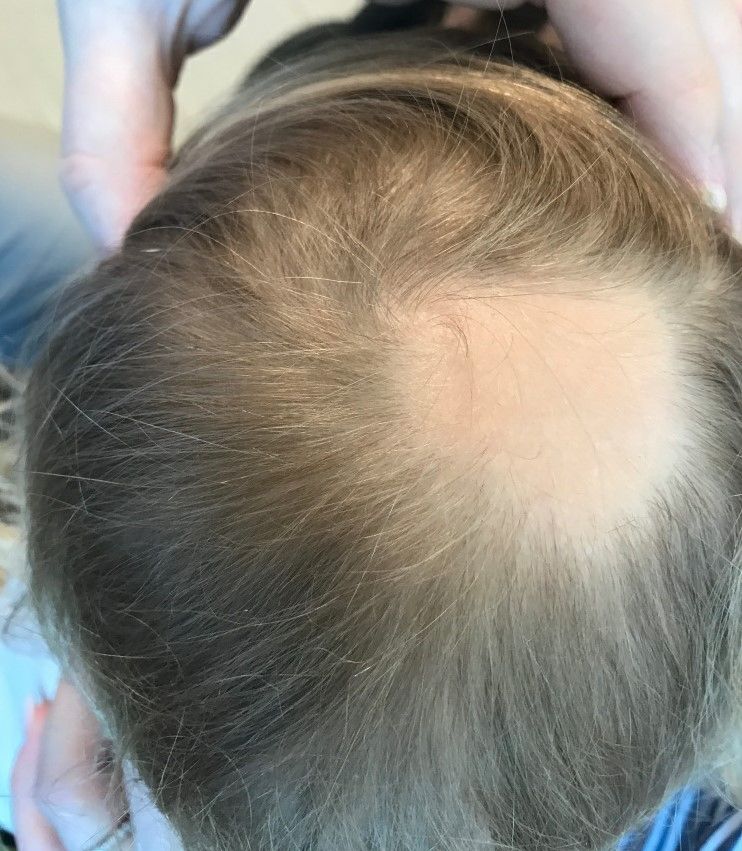
Toe growth
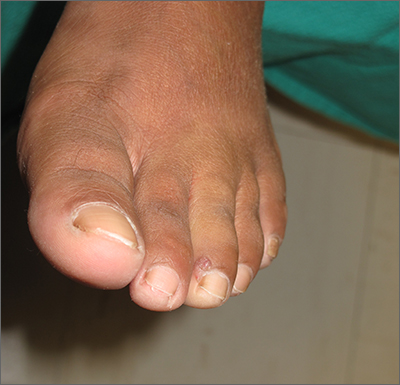
Shave biopsy was consistent with a solitary periungual angiofibroma, often termed a Koenen tumor. These can manifest as a soft pink papule (as with this patient), sometimes with a distal keratinaceous tip. At times, the nail bed and nail plate may be deformed because of the angiofibroma.
Periungual angiofibromas can occur sporadically in children and adults, it was a solitary finding in this case. Importantly, periungual angiofibromas may also occur as a visible sign of a multisystem genetic disorder known as tuberous sclerosis complex (TSC). TSC causes benign tumors to develop throughout the body (eg, skin, brain, heart, lungs). The condition can be mild or lead to serious disabilities, including seizures and developmental delays.
In isolation, periungual angiofibromas are benign but occasionally hurt or bleed from light trauma. In such cases, or for cosmetic reasons, patients may seek treatments. Complete excision of the lesion may include the affected portion of the nail bed or matrix. This is more easily repaired when the lesion is on the lateral nail fold, facilitating an en bloc fusiform excision and matrixectomy.1 Surgical excision of a lesion in the mid-proximal nail fold is much more likely to result in long-term nail deformity. Electrosurgery and various laser modalities have been successful as less invasive removal options.2 While expensive, topical sirolimus 1% has been used successfully in sporadic angiofibromas and those associated with TSC.
The patient in this case underwent lateral nail fold excision with complete removal of the tumor and repair with a side-to-side closure.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Tisa LM, Iurcotta A. Solitary periungual angiofibroma. An unusual case report. J Am Podiatr Med Assoc. 1993;83:679-80. doi: 10.7547/87507315-83-12-679
2. Boixeda P, Sánchez-Miralles E, Azaña JM, et al. CO2, argon, and pulsed dye laser treatment of angiofibromas. J Dermatol Surg Oncol. 1994;20:808-812. doi: 10.1111/j.1524- 4725.1994.tb03709.x

Shave biopsy was consistent with a solitary periungual angiofibroma, often termed a Koenen tumor. These can manifest as a soft pink papule (as with this patient), sometimes with a distal keratinaceous tip. At times, the nail bed and nail plate may be deformed because of the angiofibroma.
Periungual angiofibromas can occur sporadically in children and adults, it was a solitary finding in this case. Importantly, periungual angiofibromas may also occur as a visible sign of a multisystem genetic disorder known as tuberous sclerosis complex (TSC). TSC causes benign tumors to develop throughout the body (eg, skin, brain, heart, lungs). The condition can be mild or lead to serious disabilities, including seizures and developmental delays.
In isolation, periungual angiofibromas are benign but occasionally hurt or bleed from light trauma. In such cases, or for cosmetic reasons, patients may seek treatments. Complete excision of the lesion may include the affected portion of the nail bed or matrix. This is more easily repaired when the lesion is on the lateral nail fold, facilitating an en bloc fusiform excision and matrixectomy.1 Surgical excision of a lesion in the mid-proximal nail fold is much more likely to result in long-term nail deformity. Electrosurgery and various laser modalities have been successful as less invasive removal options.2 While expensive, topical sirolimus 1% has been used successfully in sporadic angiofibromas and those associated with TSC.
The patient in this case underwent lateral nail fold excision with complete removal of the tumor and repair with a side-to-side closure.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Shave biopsy was consistent with a solitary periungual angiofibroma, often termed a Koenen tumor. These can manifest as a soft pink papule (as with this patient), sometimes with a distal keratinaceous tip. At times, the nail bed and nail plate may be deformed because of the angiofibroma.
Periungual angiofibromas can occur sporadically in children and adults, it was a solitary finding in this case. Importantly, periungual angiofibromas may also occur as a visible sign of a multisystem genetic disorder known as tuberous sclerosis complex (TSC). TSC causes benign tumors to develop throughout the body (eg, skin, brain, heart, lungs). The condition can be mild or lead to serious disabilities, including seizures and developmental delays.
In isolation, periungual angiofibromas are benign but occasionally hurt or bleed from light trauma. In such cases, or for cosmetic reasons, patients may seek treatments. Complete excision of the lesion may include the affected portion of the nail bed or matrix. This is more easily repaired when the lesion is on the lateral nail fold, facilitating an en bloc fusiform excision and matrixectomy.1 Surgical excision of a lesion in the mid-proximal nail fold is much more likely to result in long-term nail deformity. Electrosurgery and various laser modalities have been successful as less invasive removal options.2 While expensive, topical sirolimus 1% has been used successfully in sporadic angiofibromas and those associated with TSC.
The patient in this case underwent lateral nail fold excision with complete removal of the tumor and repair with a side-to-side closure.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Tisa LM, Iurcotta A. Solitary periungual angiofibroma. An unusual case report. J Am Podiatr Med Assoc. 1993;83:679-80. doi: 10.7547/87507315-83-12-679
2. Boixeda P, Sánchez-Miralles E, Azaña JM, et al. CO2, argon, and pulsed dye laser treatment of angiofibromas. J Dermatol Surg Oncol. 1994;20:808-812. doi: 10.1111/j.1524- 4725.1994.tb03709.x
1. Tisa LM, Iurcotta A. Solitary periungual angiofibroma. An unusual case report. J Am Podiatr Med Assoc. 1993;83:679-80. doi: 10.7547/87507315-83-12-679
2. Boixeda P, Sánchez-Miralles E, Azaña JM, et al. CO2, argon, and pulsed dye laser treatment of angiofibromas. J Dermatol Surg Oncol. 1994;20:808-812. doi: 10.1111/j.1524- 4725.1994.tb03709.x
What are your treatment options when isotretinoin fails?
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
AT SPD 2022
Questionnaire for patients with psoriasis might identify risk of axial involvement
Preliminary findings are encouraging
NEW YORK – A questionnaire-based screening tool appears to accelerate the time to diagnosis of axial involvement in patients presenting with psoriasis but no clinical signs of joint pain, according to a study called ATTRACT that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
The risk of a delayed diagnosis of an axial component in patients with psoriasis, meaning a delay in the underlying diagnosis of psoriatic arthritis (PsA), is substantial, according to Devis Benfaremo, MD, of the department of clinical and molecular science at Marche Polytechnic University, Ancona, Italy.
There is “no consensus for the best strategy to achieve early detection of joint disease” in patients presenting with psoriasis, but Dr. Benfaremo pointed out that missing axial involvement is a particular problem because it is far more likely than swollen joints to be missed on clinical examination.
While about one in three patients with psoriasis have or will develop psoriatic arthritis, according to the National Psoriasis Foundation, delays in diagnosis are common, according to Dr. Benfaremo. In patients with undiagnosed PsA characterized by axial involvement alone, subtle symptoms can be overlooked or attributed to other causes.
There are several screening questionnaires to detect joint symptoms in patients presenting with psoriasis, such as the five-question Psoriasis Epidemiology Screening Tool, but the questionnaire tested in the ATTRACT trial is focused on detecting axial involvement specifically. It was characterized as the first to do so.
In the ongoing ATTRACT study, 253 patients with psoriasis but no history of PsA or axial disease have been enrolled so far. In the study, patients are screened for PsA based on a patient-completed yes-or-no questionnaire, which takes only a few minutes to complete.
“It is a validated questionnaire for axial [spondyloarthritis], but we have adopted it for detection of psoriasis patients with PsA,” Dr. Benfaremo explained.
The questionnaire for axial spondyloarthritis (axSpA) was initially evaluated and validated by Fabian Proft, MD, head of the clinical trials unit at Charité Hospital, Berlin. In addition to a patient self-completed questionnaire, Dr. Proft and coinvestigators have also created a related questionnaire to be administered by physicians.
In the ATTRACT study, patients completed the questionnaire on an electronic device in the waiting room. Positive answers to specific questions about symptoms, which addressed back pain and joint function as well as joint symptoms, divided patients into three groups:
- Group A patients did not respond positively to any of the symptom questions that would prompt suspicion of axial disease. These represented about one-third of those screened so far.
- Group B patients were those who answered positively to at least two questions that related to a high suspicion of axial involvement. These represented 45% of patients.
- The remaining patients were placed in Group C, a category of intermediate risk based on positive responses to some, but not all, questions relating to axial symptoms.
Those in group B are being referred to rheumatology. Patients in group C are given “conditional” eligibility based on the presence of additional risk factors.
AxSpA screening tool ‘makes sense’ for potential use in PsA
The primary outcome of the ATTRACT trial is early identification of axial PsA. Correctly identifying patients with or without peripheral joint involvement is one of several secondary outcomes. The identification of patients who fulfill Assessment Spondyloarthritis International Society (ASAS) criteria for axSpA is another secondary outcome.
Of the 114 patients placed in group B and analyzed so far, 87 have completed an assessment by a rheumatologist with laboratory analyses and imaging, as well as a clinical examination.
Of those 87 assessed by a rheumatologist, 17 did not have either axial or peripheral inflammation. Another 19 were diagnosed with axial disease, including 14 who met ASAS criteria. A total of 10 were classified as having PsA with peripheral inflammation, according to Classification for Psoriatic Arthritis criteria, and 41 are still being considered for a diagnosis of axial or peripheral PsA on the basis of further workup.
“Among the patients with axial PsA, only 10% had elevated C-reactive protein levels,” according to Dr. Benfaremo, echoing previous evidence that inflammatory biomarkers by themselves have limited value for identifying psoriasis patients at high risk of joint involvement.
The findings are preliminary, but Dr. Benfaremo reported that the questionnaire is showing promise for the routine stratification of patients who should be considered for a rheumatology consultation.
If further analyses validate the clinical utility of these stratifications, there is the potential for a substantial acceleration to the diagnosis of PsA.
When contacted to comment about this work, Dr. Proft said that there is an important need for new strategies reduce delay in the diagnosis of PsA among patients presenting with psoriasis. He thinks the screening tool he developed for axSpA “makes sense” as a potential tool in PsA.
“If validated, this could be a very useful for earlier identification of PsA,” Dr. Proft said. He reiterated the importance of focusing on axial involvement.
“Previous screening tools have focused on symptoms of PsA more generally, but inflammation in the peripheral joints is something that you can easily see in most patients,” he said.
In addition to the patient-completed questionnaire and the physician-administered questionnaire, Dr. Proft has also evaluated an online self-referral tool for patients.
“If we can diagnose PsA earlier in the course of disease, we can start treatment earlier, prevent or delay joint damage, and potentially improve outcomes for patients,” Dr. Proft said. He considers this an important direction of research.
Dr. Benfaremo and Dr. Proft reported no potential conflicts of interest.
Preliminary findings are encouraging
Preliminary findings are encouraging
NEW YORK – A questionnaire-based screening tool appears to accelerate the time to diagnosis of axial involvement in patients presenting with psoriasis but no clinical signs of joint pain, according to a study called ATTRACT that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
The risk of a delayed diagnosis of an axial component in patients with psoriasis, meaning a delay in the underlying diagnosis of psoriatic arthritis (PsA), is substantial, according to Devis Benfaremo, MD, of the department of clinical and molecular science at Marche Polytechnic University, Ancona, Italy.
There is “no consensus for the best strategy to achieve early detection of joint disease” in patients presenting with psoriasis, but Dr. Benfaremo pointed out that missing axial involvement is a particular problem because it is far more likely than swollen joints to be missed on clinical examination.
While about one in three patients with psoriasis have or will develop psoriatic arthritis, according to the National Psoriasis Foundation, delays in diagnosis are common, according to Dr. Benfaremo. In patients with undiagnosed PsA characterized by axial involvement alone, subtle symptoms can be overlooked or attributed to other causes.
There are several screening questionnaires to detect joint symptoms in patients presenting with psoriasis, such as the five-question Psoriasis Epidemiology Screening Tool, but the questionnaire tested in the ATTRACT trial is focused on detecting axial involvement specifically. It was characterized as the first to do so.
In the ongoing ATTRACT study, 253 patients with psoriasis but no history of PsA or axial disease have been enrolled so far. In the study, patients are screened for PsA based on a patient-completed yes-or-no questionnaire, which takes only a few minutes to complete.
“It is a validated questionnaire for axial [spondyloarthritis], but we have adopted it for detection of psoriasis patients with PsA,” Dr. Benfaremo explained.
The questionnaire for axial spondyloarthritis (axSpA) was initially evaluated and validated by Fabian Proft, MD, head of the clinical trials unit at Charité Hospital, Berlin. In addition to a patient self-completed questionnaire, Dr. Proft and coinvestigators have also created a related questionnaire to be administered by physicians.
In the ATTRACT study, patients completed the questionnaire on an electronic device in the waiting room. Positive answers to specific questions about symptoms, which addressed back pain and joint function as well as joint symptoms, divided patients into three groups:
- Group A patients did not respond positively to any of the symptom questions that would prompt suspicion of axial disease. These represented about one-third of those screened so far.
- Group B patients were those who answered positively to at least two questions that related to a high suspicion of axial involvement. These represented 45% of patients.
- The remaining patients were placed in Group C, a category of intermediate risk based on positive responses to some, but not all, questions relating to axial symptoms.
Those in group B are being referred to rheumatology. Patients in group C are given “conditional” eligibility based on the presence of additional risk factors.
AxSpA screening tool ‘makes sense’ for potential use in PsA
The primary outcome of the ATTRACT trial is early identification of axial PsA. Correctly identifying patients with or without peripheral joint involvement is one of several secondary outcomes. The identification of patients who fulfill Assessment Spondyloarthritis International Society (ASAS) criteria for axSpA is another secondary outcome.
Of the 114 patients placed in group B and analyzed so far, 87 have completed an assessment by a rheumatologist with laboratory analyses and imaging, as well as a clinical examination.
Of those 87 assessed by a rheumatologist, 17 did not have either axial or peripheral inflammation. Another 19 were diagnosed with axial disease, including 14 who met ASAS criteria. A total of 10 were classified as having PsA with peripheral inflammation, according to Classification for Psoriatic Arthritis criteria, and 41 are still being considered for a diagnosis of axial or peripheral PsA on the basis of further workup.
“Among the patients with axial PsA, only 10% had elevated C-reactive protein levels,” according to Dr. Benfaremo, echoing previous evidence that inflammatory biomarkers by themselves have limited value for identifying psoriasis patients at high risk of joint involvement.
The findings are preliminary, but Dr. Benfaremo reported that the questionnaire is showing promise for the routine stratification of patients who should be considered for a rheumatology consultation.
If further analyses validate the clinical utility of these stratifications, there is the potential for a substantial acceleration to the diagnosis of PsA.
When contacted to comment about this work, Dr. Proft said that there is an important need for new strategies reduce delay in the diagnosis of PsA among patients presenting with psoriasis. He thinks the screening tool he developed for axSpA “makes sense” as a potential tool in PsA.
“If validated, this could be a very useful for earlier identification of PsA,” Dr. Proft said. He reiterated the importance of focusing on axial involvement.
“Previous screening tools have focused on symptoms of PsA more generally, but inflammation in the peripheral joints is something that you can easily see in most patients,” he said.
In addition to the patient-completed questionnaire and the physician-administered questionnaire, Dr. Proft has also evaluated an online self-referral tool for patients.
“If we can diagnose PsA earlier in the course of disease, we can start treatment earlier, prevent or delay joint damage, and potentially improve outcomes for patients,” Dr. Proft said. He considers this an important direction of research.
Dr. Benfaremo and Dr. Proft reported no potential conflicts of interest.
NEW YORK – A questionnaire-based screening tool appears to accelerate the time to diagnosis of axial involvement in patients presenting with psoriasis but no clinical signs of joint pain, according to a study called ATTRACT that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
The risk of a delayed diagnosis of an axial component in patients with psoriasis, meaning a delay in the underlying diagnosis of psoriatic arthritis (PsA), is substantial, according to Devis Benfaremo, MD, of the department of clinical and molecular science at Marche Polytechnic University, Ancona, Italy.
There is “no consensus for the best strategy to achieve early detection of joint disease” in patients presenting with psoriasis, but Dr. Benfaremo pointed out that missing axial involvement is a particular problem because it is far more likely than swollen joints to be missed on clinical examination.
While about one in three patients with psoriasis have or will develop psoriatic arthritis, according to the National Psoriasis Foundation, delays in diagnosis are common, according to Dr. Benfaremo. In patients with undiagnosed PsA characterized by axial involvement alone, subtle symptoms can be overlooked or attributed to other causes.
There are several screening questionnaires to detect joint symptoms in patients presenting with psoriasis, such as the five-question Psoriasis Epidemiology Screening Tool, but the questionnaire tested in the ATTRACT trial is focused on detecting axial involvement specifically. It was characterized as the first to do so.
In the ongoing ATTRACT study, 253 patients with psoriasis but no history of PsA or axial disease have been enrolled so far. In the study, patients are screened for PsA based on a patient-completed yes-or-no questionnaire, which takes only a few minutes to complete.
“It is a validated questionnaire for axial [spondyloarthritis], but we have adopted it for detection of psoriasis patients with PsA,” Dr. Benfaremo explained.
The questionnaire for axial spondyloarthritis (axSpA) was initially evaluated and validated by Fabian Proft, MD, head of the clinical trials unit at Charité Hospital, Berlin. In addition to a patient self-completed questionnaire, Dr. Proft and coinvestigators have also created a related questionnaire to be administered by physicians.
In the ATTRACT study, patients completed the questionnaire on an electronic device in the waiting room. Positive answers to specific questions about symptoms, which addressed back pain and joint function as well as joint symptoms, divided patients into three groups:
- Group A patients did not respond positively to any of the symptom questions that would prompt suspicion of axial disease. These represented about one-third of those screened so far.
- Group B patients were those who answered positively to at least two questions that related to a high suspicion of axial involvement. These represented 45% of patients.
- The remaining patients were placed in Group C, a category of intermediate risk based on positive responses to some, but not all, questions relating to axial symptoms.
Those in group B are being referred to rheumatology. Patients in group C are given “conditional” eligibility based on the presence of additional risk factors.
AxSpA screening tool ‘makes sense’ for potential use in PsA
The primary outcome of the ATTRACT trial is early identification of axial PsA. Correctly identifying patients with or without peripheral joint involvement is one of several secondary outcomes. The identification of patients who fulfill Assessment Spondyloarthritis International Society (ASAS) criteria for axSpA is another secondary outcome.
Of the 114 patients placed in group B and analyzed so far, 87 have completed an assessment by a rheumatologist with laboratory analyses and imaging, as well as a clinical examination.
Of those 87 assessed by a rheumatologist, 17 did not have either axial or peripheral inflammation. Another 19 were diagnosed with axial disease, including 14 who met ASAS criteria. A total of 10 were classified as having PsA with peripheral inflammation, according to Classification for Psoriatic Arthritis criteria, and 41 are still being considered for a diagnosis of axial or peripheral PsA on the basis of further workup.
“Among the patients with axial PsA, only 10% had elevated C-reactive protein levels,” according to Dr. Benfaremo, echoing previous evidence that inflammatory biomarkers by themselves have limited value for identifying psoriasis patients at high risk of joint involvement.
The findings are preliminary, but Dr. Benfaremo reported that the questionnaire is showing promise for the routine stratification of patients who should be considered for a rheumatology consultation.
If further analyses validate the clinical utility of these stratifications, there is the potential for a substantial acceleration to the diagnosis of PsA.
When contacted to comment about this work, Dr. Proft said that there is an important need for new strategies reduce delay in the diagnosis of PsA among patients presenting with psoriasis. He thinks the screening tool he developed for axSpA “makes sense” as a potential tool in PsA.
“If validated, this could be a very useful for earlier identification of PsA,” Dr. Proft said. He reiterated the importance of focusing on axial involvement.
“Previous screening tools have focused on symptoms of PsA more generally, but inflammation in the peripheral joints is something that you can easily see in most patients,” he said.
In addition to the patient-completed questionnaire and the physician-administered questionnaire, Dr. Proft has also evaluated an online self-referral tool for patients.
“If we can diagnose PsA earlier in the course of disease, we can start treatment earlier, prevent or delay joint damage, and potentially improve outcomes for patients,” Dr. Proft said. He considers this an important direction of research.
Dr. Benfaremo and Dr. Proft reported no potential conflicts of interest.
AT GRAPPA 2022
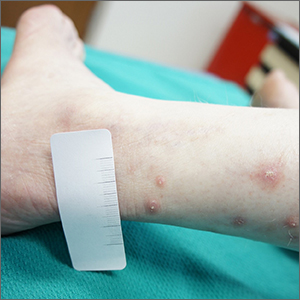
Topical gene therapy for dystrophic epidermolysis bullosa shows promise
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
AT SPD 2022
Clinical characteristics of recurrent RIME elucidated in chart review
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
A toddler presents with patchy hair loss
Given the history of sudden hair loss, with the exam revealing a well-circumscribed patch of focal alopecia without cutaneous inflammation, hairs with a narrow base and broad distal shaft, the diagnosis is alopecia areata (AA).
Alopecia areata (AA) is a nonscarring alopecia, within a set of diseases characterized by the preservation of hair follicles and therefore the potential for future hair regrowth.1 AA is believed to be caused by a breakdown of the immune-privileged nature of hair follicles, resulting in T-lymphocytes targeting the hair follicle directly, shifting follicles to early catagen or telogen phase, but sparing follicular stem cells, thereby allowing the follicle to regenerate in the future.1-3 Risk factors include family history of AA, thyroid disorders, as well as iron and vitamin D deficiency.4,5 It characteristically presents with focal, well-demarcated patches of hair loss in the scalp, typically with background skin normal to slightly pink.3,6 Exam can show “exclamation point” hairs consisting of hairs that are narrow at their base and wide at the distal end.3,7 Patients may also exhibit eyebrow and eyelash loss as well as nail changes including nail pitting and splitting.8 Diagnosis is typically made clinically but is supported by a positive hair pull test, where hairs are pulled from the periphery of an alopecic lesion; the presence of greater than 10% of hairs plucked from the scalp indicates a positive result.9,10
What’s the differential diagnosis?
The differential diagnosis of AA includes other nonscarring alopecias such as trichotillomania and telogen effluvium. Other possible diagnoses include lichen planopilaris and tinea capitis.
Trichotillomania results in irregularly bordered hair loss and broken hairs of different lengths because of an internal urge to remove one’s hair, resulting in nonscarring alopecia. It can be associated with obsessive-compulsive disorder, anxiety, or other body-altering behaviors like skin picking and nail biting (characterized as body-focused repetitive behavior disorders). Treatments include reassurance and education, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs. Toddlers can engage in hair pulling behavior and trichotillomania can be difficult to differentiate from AA. However, the absence of broken hairs of varying lengths makes trichotillomania less likely in this patient.
Telogen effluvium is another form of nonscarring alopecia that presents as diffuse hair thinning across the entire scalp in response to acute psychological or physiological stress, hormonal changes, certain medications, systemic illness, or nutritional deficiency. The timing between the triggering event and hair loss can vary from weeks to months. Diagnosis requires detailed history-taking and may include evaluation for endocrinologic hair thinning (e.g. thyroid function tests) to identify reversible causes. Treatment involves directing therapy to the underlying etiology and most cases of telogen effluvium are self-limited. The presence of a well-circumscribed patch of hair loss in this patient makes AA more likely.
Lichen planopilaris (LPP) is a scarring, irreversible alopecia caused by T-lymphocytes attacking follicular hair stem cells. It is characterized by hair loss, pruritus, burning pain, scalp scaling, and multifocal scarring. Exam shows patches of alopecia with loss of follicular ostia centrally and perifollicular scale and erythema at the borders. Diagnosis is aided by biopsy of the affected scalp. Treatment of LPP requires the use of potent and superpotent topical corticosteroids and intralesional corticosteroids to decrease scalp inflammation and prevent further progression. The presence of follicular ostia and absence of perifollicular scale in this patient makes LPP highly unlikely.
Tinea capitis is a fungal infection of the scalp caused by dermatophytes including Trychophyton tonsurans and Microsporum canis. It presents with patches of alopecia with overlying scale and broken hairs and can have associated cervical and occipital lymphadenopathy. Diagnosis can involve skin scraping and KOH prep to visualize branching hyphae as well as fungal culture to identify the causative organism. Because dermatophytes in tinea capitis invade hair follicles, topical antifungals are ineffective because of their lack of penetration. Therefore, systemic antifungals including oral terbinafine and griseofulvin are considered first-line agents for treatment.
What’s the management plan?
The diagnosis of AA is usually a clinical one, though assessment of alternative diagnoses is appropriate dependent on signs and symptoms. Workup of AA can include thyroid studies because of the association with autoimmune thyroid disease, though studies suggest limited screening benefits in children.11 Given its variable and unpredictable course, management can include “watchful waiting” because of its potential for spontaneous remission.6 For limited patchy loss, active treatment with mid to superpotent topical steroids or intralesional triamcinolone acetonide in older children and adolescents is reasonable.12 Other treatment options include topical or low-dose oral minoxidil and immunotherapy with diphenylcyclopropenone or squaric acid (inducing an allergic contact dermatitis).12 Management of therapies for more extensive AA is evolving, with ongoing studies of oral JAK-inhibitors and biologic agents.12,13
Our patient was started on topical fluocinonide 0.05% solution and achieved good disease control and hair regrowth over the course of 3 months.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology at the university and Rady Children’s Hospital. They had no disclosures.
References
1. Bernardez C et al. Actas Dermosifiliogr. 2015;106(3):158-67.
2. Rajabi F et al. Br J Dermatol. 2018;179(5):1033-48.
3. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):1-12.
4. Lee S et al. J Am Acad Dermatol. 2019;80(2):466-77 e16.
5. MacLean KJ and Tidman MJ. Practitioner. 2013;257(1764):29-32, 3.
6. Pratt CH et al. Nat Rev Dis Primers. 2017;3:17011.
7. Gilhar A et al. N Engl J Med. 2012;366(16):1515-25.
8. Wyrwich KW et al. Am J Clin Dermatol. 2020;21(5):725-32.
9. Spano F and Donovan JC. Can Fam Physician. 2015;61(9):751-5.
10. Mounsey AL and Reed SW. Am Fam Physician. 2009;80(4):356-62.
11. Hordinsky MK. J Investig Dermatol Symp Proc. 2015;17(2):44-6.
12. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):15-24.
13. Zhou C et al. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Given the history of sudden hair loss, with the exam revealing a well-circumscribed patch of focal alopecia without cutaneous inflammation, hairs with a narrow base and broad distal shaft, the diagnosis is alopecia areata (AA).
Alopecia areata (AA) is a nonscarring alopecia, within a set of diseases characterized by the preservation of hair follicles and therefore the potential for future hair regrowth.1 AA is believed to be caused by a breakdown of the immune-privileged nature of hair follicles, resulting in T-lymphocytes targeting the hair follicle directly, shifting follicles to early catagen or telogen phase, but sparing follicular stem cells, thereby allowing the follicle to regenerate in the future.1-3 Risk factors include family history of AA, thyroid disorders, as well as iron and vitamin D deficiency.4,5 It characteristically presents with focal, well-demarcated patches of hair loss in the scalp, typically with background skin normal to slightly pink.3,6 Exam can show “exclamation point” hairs consisting of hairs that are narrow at their base and wide at the distal end.3,7 Patients may also exhibit eyebrow and eyelash loss as well as nail changes including nail pitting and splitting.8 Diagnosis is typically made clinically but is supported by a positive hair pull test, where hairs are pulled from the periphery of an alopecic lesion; the presence of greater than 10% of hairs plucked from the scalp indicates a positive result.9,10
What’s the differential diagnosis?
The differential diagnosis of AA includes other nonscarring alopecias such as trichotillomania and telogen effluvium. Other possible diagnoses include lichen planopilaris and tinea capitis.
Trichotillomania results in irregularly bordered hair loss and broken hairs of different lengths because of an internal urge to remove one’s hair, resulting in nonscarring alopecia. It can be associated with obsessive-compulsive disorder, anxiety, or other body-altering behaviors like skin picking and nail biting (characterized as body-focused repetitive behavior disorders). Treatments include reassurance and education, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs. Toddlers can engage in hair pulling behavior and trichotillomania can be difficult to differentiate from AA. However, the absence of broken hairs of varying lengths makes trichotillomania less likely in this patient.
Telogen effluvium is another form of nonscarring alopecia that presents as diffuse hair thinning across the entire scalp in response to acute psychological or physiological stress, hormonal changes, certain medications, systemic illness, or nutritional deficiency. The timing between the triggering event and hair loss can vary from weeks to months. Diagnosis requires detailed history-taking and may include evaluation for endocrinologic hair thinning (e.g. thyroid function tests) to identify reversible causes. Treatment involves directing therapy to the underlying etiology and most cases of telogen effluvium are self-limited. The presence of a well-circumscribed patch of hair loss in this patient makes AA more likely.
Lichen planopilaris (LPP) is a scarring, irreversible alopecia caused by T-lymphocytes attacking follicular hair stem cells. It is characterized by hair loss, pruritus, burning pain, scalp scaling, and multifocal scarring. Exam shows patches of alopecia with loss of follicular ostia centrally and perifollicular scale and erythema at the borders. Diagnosis is aided by biopsy of the affected scalp. Treatment of LPP requires the use of potent and superpotent topical corticosteroids and intralesional corticosteroids to decrease scalp inflammation and prevent further progression. The presence of follicular ostia and absence of perifollicular scale in this patient makes LPP highly unlikely.
Tinea capitis is a fungal infection of the scalp caused by dermatophytes including Trychophyton tonsurans and Microsporum canis. It presents with patches of alopecia with overlying scale and broken hairs and can have associated cervical and occipital lymphadenopathy. Diagnosis can involve skin scraping and KOH prep to visualize branching hyphae as well as fungal culture to identify the causative organism. Because dermatophytes in tinea capitis invade hair follicles, topical antifungals are ineffective because of their lack of penetration. Therefore, systemic antifungals including oral terbinafine and griseofulvin are considered first-line agents for treatment.
What’s the management plan?
The diagnosis of AA is usually a clinical one, though assessment of alternative diagnoses is appropriate dependent on signs and symptoms. Workup of AA can include thyroid studies because of the association with autoimmune thyroid disease, though studies suggest limited screening benefits in children.11 Given its variable and unpredictable course, management can include “watchful waiting” because of its potential for spontaneous remission.6 For limited patchy loss, active treatment with mid to superpotent topical steroids or intralesional triamcinolone acetonide in older children and adolescents is reasonable.12 Other treatment options include topical or low-dose oral minoxidil and immunotherapy with diphenylcyclopropenone or squaric acid (inducing an allergic contact dermatitis).12 Management of therapies for more extensive AA is evolving, with ongoing studies of oral JAK-inhibitors and biologic agents.12,13
Our patient was started on topical fluocinonide 0.05% solution and achieved good disease control and hair regrowth over the course of 3 months.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology at the university and Rady Children’s Hospital. They had no disclosures.
References
1. Bernardez C et al. Actas Dermosifiliogr. 2015;106(3):158-67.
2. Rajabi F et al. Br J Dermatol. 2018;179(5):1033-48.
3. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):1-12.
4. Lee S et al. J Am Acad Dermatol. 2019;80(2):466-77 e16.
5. MacLean KJ and Tidman MJ. Practitioner. 2013;257(1764):29-32, 3.
6. Pratt CH et al. Nat Rev Dis Primers. 2017;3:17011.
7. Gilhar A et al. N Engl J Med. 2012;366(16):1515-25.
8. Wyrwich KW et al. Am J Clin Dermatol. 2020;21(5):725-32.
9. Spano F and Donovan JC. Can Fam Physician. 2015;61(9):751-5.
10. Mounsey AL and Reed SW. Am Fam Physician. 2009;80(4):356-62.
11. Hordinsky MK. J Investig Dermatol Symp Proc. 2015;17(2):44-6.
12. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):15-24.
13. Zhou C et al. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Given the history of sudden hair loss, with the exam revealing a well-circumscribed patch of focal alopecia without cutaneous inflammation, hairs with a narrow base and broad distal shaft, the diagnosis is alopecia areata (AA).
Alopecia areata (AA) is a nonscarring alopecia, within a set of diseases characterized by the preservation of hair follicles and therefore the potential for future hair regrowth.1 AA is believed to be caused by a breakdown of the immune-privileged nature of hair follicles, resulting in T-lymphocytes targeting the hair follicle directly, shifting follicles to early catagen or telogen phase, but sparing follicular stem cells, thereby allowing the follicle to regenerate in the future.1-3 Risk factors include family history of AA, thyroid disorders, as well as iron and vitamin D deficiency.4,5 It characteristically presents with focal, well-demarcated patches of hair loss in the scalp, typically with background skin normal to slightly pink.3,6 Exam can show “exclamation point” hairs consisting of hairs that are narrow at their base and wide at the distal end.3,7 Patients may also exhibit eyebrow and eyelash loss as well as nail changes including nail pitting and splitting.8 Diagnosis is typically made clinically but is supported by a positive hair pull test, where hairs are pulled from the periphery of an alopecic lesion; the presence of greater than 10% of hairs plucked from the scalp indicates a positive result.9,10
What’s the differential diagnosis?
The differential diagnosis of AA includes other nonscarring alopecias such as trichotillomania and telogen effluvium. Other possible diagnoses include lichen planopilaris and tinea capitis.
Trichotillomania results in irregularly bordered hair loss and broken hairs of different lengths because of an internal urge to remove one’s hair, resulting in nonscarring alopecia. It can be associated with obsessive-compulsive disorder, anxiety, or other body-altering behaviors like skin picking and nail biting (characterized as body-focused repetitive behavior disorders). Treatments include reassurance and education, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs. Toddlers can engage in hair pulling behavior and trichotillomania can be difficult to differentiate from AA. However, the absence of broken hairs of varying lengths makes trichotillomania less likely in this patient.
Telogen effluvium is another form of nonscarring alopecia that presents as diffuse hair thinning across the entire scalp in response to acute psychological or physiological stress, hormonal changes, certain medications, systemic illness, or nutritional deficiency. The timing between the triggering event and hair loss can vary from weeks to months. Diagnosis requires detailed history-taking and may include evaluation for endocrinologic hair thinning (e.g. thyroid function tests) to identify reversible causes. Treatment involves directing therapy to the underlying etiology and most cases of telogen effluvium are self-limited. The presence of a well-circumscribed patch of hair loss in this patient makes AA more likely.
Lichen planopilaris (LPP) is a scarring, irreversible alopecia caused by T-lymphocytes attacking follicular hair stem cells. It is characterized by hair loss, pruritus, burning pain, scalp scaling, and multifocal scarring. Exam shows patches of alopecia with loss of follicular ostia centrally and perifollicular scale and erythema at the borders. Diagnosis is aided by biopsy of the affected scalp. Treatment of LPP requires the use of potent and superpotent topical corticosteroids and intralesional corticosteroids to decrease scalp inflammation and prevent further progression. The presence of follicular ostia and absence of perifollicular scale in this patient makes LPP highly unlikely.
Tinea capitis is a fungal infection of the scalp caused by dermatophytes including Trychophyton tonsurans and Microsporum canis. It presents with patches of alopecia with overlying scale and broken hairs and can have associated cervical and occipital lymphadenopathy. Diagnosis can involve skin scraping and KOH prep to visualize branching hyphae as well as fungal culture to identify the causative organism. Because dermatophytes in tinea capitis invade hair follicles, topical antifungals are ineffective because of their lack of penetration. Therefore, systemic antifungals including oral terbinafine and griseofulvin are considered first-line agents for treatment.
What’s the management plan?
The diagnosis of AA is usually a clinical one, though assessment of alternative diagnoses is appropriate dependent on signs and symptoms. Workup of AA can include thyroid studies because of the association with autoimmune thyroid disease, though studies suggest limited screening benefits in children.11 Given its variable and unpredictable course, management can include “watchful waiting” because of its potential for spontaneous remission.6 For limited patchy loss, active treatment with mid to superpotent topical steroids or intralesional triamcinolone acetonide in older children and adolescents is reasonable.12 Other treatment options include topical or low-dose oral minoxidil and immunotherapy with diphenylcyclopropenone or squaric acid (inducing an allergic contact dermatitis).12 Management of therapies for more extensive AA is evolving, with ongoing studies of oral JAK-inhibitors and biologic agents.12,13
Our patient was started on topical fluocinonide 0.05% solution and achieved good disease control and hair regrowth over the course of 3 months.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology at the university and Rady Children’s Hospital. They had no disclosures.
References
1. Bernardez C et al. Actas Dermosifiliogr. 2015;106(3):158-67.
2. Rajabi F et al. Br J Dermatol. 2018;179(5):1033-48.
3. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):1-12.
4. Lee S et al. J Am Acad Dermatol. 2019;80(2):466-77 e16.
5. MacLean KJ and Tidman MJ. Practitioner. 2013;257(1764):29-32, 3.
6. Pratt CH et al. Nat Rev Dis Primers. 2017;3:17011.
7. Gilhar A et al. N Engl J Med. 2012;366(16):1515-25.
8. Wyrwich KW et al. Am J Clin Dermatol. 2020;21(5):725-32.
9. Spano F and Donovan JC. Can Fam Physician. 2015;61(9):751-5.
10. Mounsey AL and Reed SW. Am Fam Physician. 2009;80(4):356-62.
11. Hordinsky MK. J Investig Dermatol Symp Proc. 2015;17(2):44-6.
12. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):15-24.
13. Zhou C et al. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Examination findings of the scalp demonstrate a well-circumscribed alopecic patch on the vertex scalp without erythema or scale. Closer inspection of the patch with magnification or 'dermoscopy' reveals hair follicle ostia and hairs that are broader distally and narrower at their base. Nails and rest of the skin exam are unremarkable.
Leg lesions

A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561

A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
Milium cysts on hands; hypertrichosis on face
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda

The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda

The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda

The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
NAFLD strongly correlated with psoriasis, PsA; risk linked to severity
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
AT GRAPPA 2022