User login
Topical ruxolitinib quickly relieves atopic dermatitis itch in Black patients
“Ruxolitinib cream monotherapy over 8 weeks was associated with rapid and considerable itch relief in Black or African American patients with AD and was well tolerated,” the study authors wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology.
AD can behave differently in different racial groups and can be especially bothersome in Black patients. AD has a prevalence of about 20% in Black children and 5%-10% in Black adults. Black children are roughly twice as likely to be diagnosed with AD, and to have severe AD, than White children, according to the authors.
Lead author Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and colleagues used pooled data from two identically designed phase 3 studies to describe the effects of the cream formulation of the Janus kinase (JAK) 1 and JAK 2 inhibitor ruxolitinib on itch in Black patients.
Topical ruxolitinib (Opzelura), 1.5%, was approved last September for treating AD in non-immunocompromised patients with mild to moderate AD, ages 12 years and older. In July 2022, it was approved for the treatment of nonsegmental vitiligo in the same age group.
FDA approval for AD was based on the results of the TRuE-AD1 and TRuE-AD2 double-blind randomized trials, which enrolled about 1,200 patients over age 12 with AD. These patients included 292 Black teenagers and adults between aged 12-71 years who had AD for 2 years or longer, with an Investigator’s Global Assessment (IGA) score of 2 or 3, with 3%-20% affected body surface area, excluding the scalp.
Of the 292 patients, those in the two treatment groups (n = 231) applied ruxolitinib cream twice a day for 8 weeks (0.75% in 118 patients and 1.5% in 113 patients) and 61 applied the vehicle. They used electronic diaries to record the worst level of itch they had experienced each day, from 0 (no itch) to 10 (worst imaginable itch). The main results were as follows:
- Mean itch numerical rating scale (NRS) scores at baseline were 5.3 and 5.4 for ruxolitinib cream 0.75% and 1.5%, respectively, and 5.7 for vehicle. Within about 12 hours of first application, mean itch NRS scores dropped –0.6 and –0.7 from baseline among those treated with ruxolitinib cream 0.75% and 1.5%, respectively, compared with –0.2 for those on the vehicle. At day 4, the decreases were –1.4 and –1.6 for ruxolitinib cream 0.75% and 1.5%, respectively, versus –0.6 for the vehicle (P = .026 and P = .005, respectively, vs. vehicle).
- At day 2, among the 187 patients with a baseline itch NRS score 4 or higher, more patients achieved 4-point or greater itch NRS improvement: 6.1% and 16.4% for ruxolitinib cream 0.75% and 1.5%, respectively versus 0% for vehicle. At day 7, the differences were 15.9% and 26.6% versus 3%, respectively. And by week 8, they increased to 30.1% and 43.2% versus 17.5% (P = .212 and P = .009), respectively.
- At week 2, 19% of patients in the 0.75% formulation group and 19.4% of patients in the 1.5% formulation group, compared with 5.3% in the vehicle group, reported no days of itch on question 1 of the Patient-Oriented Eczema Measure (POEM) questionnaire that evaluated various aspects of the disease over the previous week. By week 8, the differences grew to 34% and 30.8% versus 12.2%, respectively.
- Adverse events, reported by 14.4% and 22.1% of patients on 0.75% and 1.5% ruxolitinib, respectively, and by 32.8% of patients who received the vehicle, were headaches, upper respiratory tract infection, and application site pain.
Ruxolitinib may be an alternative to systemic immunosuppressives
Asked to comment on the results, Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, N.C., called itch “one of the major life disruptors in atopic dermatitis.”
Providers often assume that patients of different races respond similarly to treatment, but that is not always true, she noted in an email.
“This study proves ruxolitinib’s effectiveness in Black patients, who often have more severe atopic dermatitis signs and symptoms,” said Dr. McMichael, who was not involved in the study. “The fact that atopic dermatitis in patients of color has been singled out to examine efficacy is a great way to show that the findings are not just in those who have thinner plaques and potentially less longstanding thickening of the skin from scratching (lichenification),” she added.
Dr. McMichael welcomed the lack of systemic side effects and quick relief of itch with this treatment, noting that the effect on itch “is rare with other treatments and extremely rare with other topical medications.”
The effect of topical ruxolitinib on pruritus “was interesting and surprising because very few available topical medications can control itch,” she explained. “The strongest topical steroids can help with pruritus, but they have the risk for skin thinning (atrophy),” while topical ruxolitinib is not associated with skin atrophy.
“After topical steroids fail as first-line treatment, it is likely that more patients will be given this topical medication rather than be moved to immunosuppressive systemic medications,” she noted.
All study authors report relevant relationships with Incyte Corporation, which manufactures ruxolitinib and funded the study, and several authors report employment and shareholding interests in the company. Dr. McMichael reports no relevant relationship with the study.
A version of this article first appeared on Medscape.com.
“Ruxolitinib cream monotherapy over 8 weeks was associated with rapid and considerable itch relief in Black or African American patients with AD and was well tolerated,” the study authors wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology.
AD can behave differently in different racial groups and can be especially bothersome in Black patients. AD has a prevalence of about 20% in Black children and 5%-10% in Black adults. Black children are roughly twice as likely to be diagnosed with AD, and to have severe AD, than White children, according to the authors.
Lead author Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and colleagues used pooled data from two identically designed phase 3 studies to describe the effects of the cream formulation of the Janus kinase (JAK) 1 and JAK 2 inhibitor ruxolitinib on itch in Black patients.
Topical ruxolitinib (Opzelura), 1.5%, was approved last September for treating AD in non-immunocompromised patients with mild to moderate AD, ages 12 years and older. In July 2022, it was approved for the treatment of nonsegmental vitiligo in the same age group.
FDA approval for AD was based on the results of the TRuE-AD1 and TRuE-AD2 double-blind randomized trials, which enrolled about 1,200 patients over age 12 with AD. These patients included 292 Black teenagers and adults between aged 12-71 years who had AD for 2 years or longer, with an Investigator’s Global Assessment (IGA) score of 2 or 3, with 3%-20% affected body surface area, excluding the scalp.
Of the 292 patients, those in the two treatment groups (n = 231) applied ruxolitinib cream twice a day for 8 weeks (0.75% in 118 patients and 1.5% in 113 patients) and 61 applied the vehicle. They used electronic diaries to record the worst level of itch they had experienced each day, from 0 (no itch) to 10 (worst imaginable itch). The main results were as follows:
- Mean itch numerical rating scale (NRS) scores at baseline were 5.3 and 5.4 for ruxolitinib cream 0.75% and 1.5%, respectively, and 5.7 for vehicle. Within about 12 hours of first application, mean itch NRS scores dropped –0.6 and –0.7 from baseline among those treated with ruxolitinib cream 0.75% and 1.5%, respectively, compared with –0.2 for those on the vehicle. At day 4, the decreases were –1.4 and –1.6 for ruxolitinib cream 0.75% and 1.5%, respectively, versus –0.6 for the vehicle (P = .026 and P = .005, respectively, vs. vehicle).
- At day 2, among the 187 patients with a baseline itch NRS score 4 or higher, more patients achieved 4-point or greater itch NRS improvement: 6.1% and 16.4% for ruxolitinib cream 0.75% and 1.5%, respectively versus 0% for vehicle. At day 7, the differences were 15.9% and 26.6% versus 3%, respectively. And by week 8, they increased to 30.1% and 43.2% versus 17.5% (P = .212 and P = .009), respectively.
- At week 2, 19% of patients in the 0.75% formulation group and 19.4% of patients in the 1.5% formulation group, compared with 5.3% in the vehicle group, reported no days of itch on question 1 of the Patient-Oriented Eczema Measure (POEM) questionnaire that evaluated various aspects of the disease over the previous week. By week 8, the differences grew to 34% and 30.8% versus 12.2%, respectively.
- Adverse events, reported by 14.4% and 22.1% of patients on 0.75% and 1.5% ruxolitinib, respectively, and by 32.8% of patients who received the vehicle, were headaches, upper respiratory tract infection, and application site pain.
Ruxolitinib may be an alternative to systemic immunosuppressives
Asked to comment on the results, Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, N.C., called itch “one of the major life disruptors in atopic dermatitis.”
Providers often assume that patients of different races respond similarly to treatment, but that is not always true, she noted in an email.
“This study proves ruxolitinib’s effectiveness in Black patients, who often have more severe atopic dermatitis signs and symptoms,” said Dr. McMichael, who was not involved in the study. “The fact that atopic dermatitis in patients of color has been singled out to examine efficacy is a great way to show that the findings are not just in those who have thinner plaques and potentially less longstanding thickening of the skin from scratching (lichenification),” she added.
Dr. McMichael welcomed the lack of systemic side effects and quick relief of itch with this treatment, noting that the effect on itch “is rare with other treatments and extremely rare with other topical medications.”
The effect of topical ruxolitinib on pruritus “was interesting and surprising because very few available topical medications can control itch,” she explained. “The strongest topical steroids can help with pruritus, but they have the risk for skin thinning (atrophy),” while topical ruxolitinib is not associated with skin atrophy.
“After topical steroids fail as first-line treatment, it is likely that more patients will be given this topical medication rather than be moved to immunosuppressive systemic medications,” she noted.
All study authors report relevant relationships with Incyte Corporation, which manufactures ruxolitinib and funded the study, and several authors report employment and shareholding interests in the company. Dr. McMichael reports no relevant relationship with the study.
A version of this article first appeared on Medscape.com.
“Ruxolitinib cream monotherapy over 8 weeks was associated with rapid and considerable itch relief in Black or African American patients with AD and was well tolerated,” the study authors wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology.
AD can behave differently in different racial groups and can be especially bothersome in Black patients. AD has a prevalence of about 20% in Black children and 5%-10% in Black adults. Black children are roughly twice as likely to be diagnosed with AD, and to have severe AD, than White children, according to the authors.
Lead author Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and colleagues used pooled data from two identically designed phase 3 studies to describe the effects of the cream formulation of the Janus kinase (JAK) 1 and JAK 2 inhibitor ruxolitinib on itch in Black patients.
Topical ruxolitinib (Opzelura), 1.5%, was approved last September for treating AD in non-immunocompromised patients with mild to moderate AD, ages 12 years and older. In July 2022, it was approved for the treatment of nonsegmental vitiligo in the same age group.
FDA approval for AD was based on the results of the TRuE-AD1 and TRuE-AD2 double-blind randomized trials, which enrolled about 1,200 patients over age 12 with AD. These patients included 292 Black teenagers and adults between aged 12-71 years who had AD for 2 years or longer, with an Investigator’s Global Assessment (IGA) score of 2 or 3, with 3%-20% affected body surface area, excluding the scalp.
Of the 292 patients, those in the two treatment groups (n = 231) applied ruxolitinib cream twice a day for 8 weeks (0.75% in 118 patients and 1.5% in 113 patients) and 61 applied the vehicle. They used electronic diaries to record the worst level of itch they had experienced each day, from 0 (no itch) to 10 (worst imaginable itch). The main results were as follows:
- Mean itch numerical rating scale (NRS) scores at baseline were 5.3 and 5.4 for ruxolitinib cream 0.75% and 1.5%, respectively, and 5.7 for vehicle. Within about 12 hours of first application, mean itch NRS scores dropped –0.6 and –0.7 from baseline among those treated with ruxolitinib cream 0.75% and 1.5%, respectively, compared with –0.2 for those on the vehicle. At day 4, the decreases were –1.4 and –1.6 for ruxolitinib cream 0.75% and 1.5%, respectively, versus –0.6 for the vehicle (P = .026 and P = .005, respectively, vs. vehicle).
- At day 2, among the 187 patients with a baseline itch NRS score 4 or higher, more patients achieved 4-point or greater itch NRS improvement: 6.1% and 16.4% for ruxolitinib cream 0.75% and 1.5%, respectively versus 0% for vehicle. At day 7, the differences were 15.9% and 26.6% versus 3%, respectively. And by week 8, they increased to 30.1% and 43.2% versus 17.5% (P = .212 and P = .009), respectively.
- At week 2, 19% of patients in the 0.75% formulation group and 19.4% of patients in the 1.5% formulation group, compared with 5.3% in the vehicle group, reported no days of itch on question 1 of the Patient-Oriented Eczema Measure (POEM) questionnaire that evaluated various aspects of the disease over the previous week. By week 8, the differences grew to 34% and 30.8% versus 12.2%, respectively.
- Adverse events, reported by 14.4% and 22.1% of patients on 0.75% and 1.5% ruxolitinib, respectively, and by 32.8% of patients who received the vehicle, were headaches, upper respiratory tract infection, and application site pain.
Ruxolitinib may be an alternative to systemic immunosuppressives
Asked to comment on the results, Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, N.C., called itch “one of the major life disruptors in atopic dermatitis.”
Providers often assume that patients of different races respond similarly to treatment, but that is not always true, she noted in an email.
“This study proves ruxolitinib’s effectiveness in Black patients, who often have more severe atopic dermatitis signs and symptoms,” said Dr. McMichael, who was not involved in the study. “The fact that atopic dermatitis in patients of color has been singled out to examine efficacy is a great way to show that the findings are not just in those who have thinner plaques and potentially less longstanding thickening of the skin from scratching (lichenification),” she added.
Dr. McMichael welcomed the lack of systemic side effects and quick relief of itch with this treatment, noting that the effect on itch “is rare with other treatments and extremely rare with other topical medications.”
The effect of topical ruxolitinib on pruritus “was interesting and surprising because very few available topical medications can control itch,” she explained. “The strongest topical steroids can help with pruritus, but they have the risk for skin thinning (atrophy),” while topical ruxolitinib is not associated with skin atrophy.
“After topical steroids fail as first-line treatment, it is likely that more patients will be given this topical medication rather than be moved to immunosuppressive systemic medications,” she noted.
All study authors report relevant relationships with Incyte Corporation, which manufactures ruxolitinib and funded the study, and several authors report employment and shareholding interests in the company. Dr. McMichael reports no relevant relationship with the study.
A version of this article first appeared on Medscape.com.
FROM SID 2022
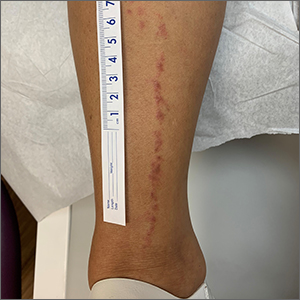
Linear leg rash

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846
Cultural humility required to optimize treatment of eczema patients with skin of color
INDIANAPOLIS – Treating atopic dermatitis (AD) in children and adolescents with skin of color requires an acumen that extends well beyond the skin, said Candrice R. Heath, MD, at the annual meeting of the Society for Pediatric Dermatology.
This involves the practice of cultural humility, which Dr. Heath defined as a commitment to learn about all aspects of patients to truly understand them, including their race, access to health care, and socioeconomic status.
“We can continue to prioritize learning about all different types of skin tones and hair types, but we really have to commit to advocating for what our patients deserve in every way,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said during her presentation at the meeting.
“That means advocating for kids to have access to better housing and for increasing health literacy programs in our hospitals, so that all our patients can understand what’s happening and how to navigate the health system,” she said. “It also means increasing diversity in our clinical trials by taking a few extra moments with the patient and family of color who might be eligible to participate in a clinical trial. We have work to do.”
To illustrate her points, she discussed the case of a 6-year-old Black patient, whose parents bring him into the clinic complaining about dark marks on the skin. The areas are itchy and the doctor figures, “this is a slam dunk; this is AD,” Dr. Heath said. “You talk about the diagnosis, and you give your treatment plan.
“But the issue is, in the parking lot when the patient’s family leaves, they feel like you didn’t help them at all,” she continued. “You didn’t understand what they came in for. They didn’t receive a treatment for what they came in for, because the initial complaint was dark marks on the skin, which is postinflammatory hyperpigmentation. We know that patients are distressed by this.”
As evidence, she cited a cross-sectional study that assessed the impact of hyperpigmentation and hyperchromia on quality of life in adults, published in the Journal of the American Academy of Dermatology. People who reported the highest levels of distress were women, those with postinflammatory hyperpigmentation, those with fewer formal years of education, and those who had higher out-of-pocket spending on skin-enhancing products.
“So, when you see hyperpigmentation in your AD patients of color, acknowledge it; say, ‘I see this pigmentation change,’ ” Dr. Heath advised. “Talk about how controlling the AD with a topical steroid or other treatment option can have a positive impact on that.”
However, she added that sometimes patients have steroid phobia, possibly because they believe the topical steroids are causing the pigmentation changes, “especially in cases of hypopigmentation, so I take the time to reassure patients so that they will not be fearful about using the medication.”
Parents of patients with skin of color who have AD may harbor other “invisible” concerns during office visits, she continued, including prior experiences with dermatologists that may not have been positive, difficulty accessing pediatric dermatologists, or a general mistrust of the health care system.
“All of that is going on in the room with your patients, particularly those with skin of color and those who feel marginalized,” said Dr. Heath, who is also a faculty scholar at Temple University medical school’s office of health equity, diversity and inclusion. “Of course, we can’t fix everything. But we can commit to approaching our visits with cultural humility.”
For patients with skin of color, she pointed out, other upstream effects impact AD care and outcomes, including well-documented socioeconomic factors.
“One of the equalizing factors is that we as pediatric dermatologists can think about increasing our education regarding skin of color,” Dr. Heath said.
For example, an analysis of data from the 2002 to 2012 National Inpatient Sample found that the main risk factors for inpatient hospitalization for AD were being non-White, having lowest-quartile household income, and having Medicaid or no insurance, researchers reported in 2018.
A separate multicenter study of 1,437 mother-child pairs with known AD found that non-Hispanic Black children and Hispanic children had greater odds of persistent AD than non-Hispanic White children, according to a 2019 study. Another large prospective cohort study published in 2019 found that AD prevalence and persistence is highest in U.S. urban children who are female or Black, and urban children with AD are more likely to have poor quality of life and asthma.
A few months after that study was published, researchers reported results from an analysis of data from the 2007-2008 National Survey of Children’s Health, which found that children who perceive the neighborhood they lived in as unsafe, unsupportive, or underdeveloped had a higher prevalence of AD and a higher severity of AD. The same year, a study of the social and economic risk factors for moderate to severe AD found that Black children were more likely to come from homes with a lower household income, lower parental education attainment, lack of home ownership, and live between two residences, and have exposure to smoke.
“Disease recognition is one thing, but we also want everyone to be aware of these other factors,” she said, “because some patients do need a little bit more care and help to be able to access the medications that they need and gain access to us.”
Follicular, nummular eczema
In her clinical experience, the most common clinical variants of AD in patients with skin of color is follicular eczema. “Examine the patient, apply your hand to the affected area, and you can feel the papules beneath your fingertips,” she advised.
“That’s what I teach my residents and medical students,” she said. “If you are looking for erythema to seal your diagnosis of AD, it may not happen. You may see more of a violaceous hue and sometimes you may not find it at all, depending on the patient’s skin tone. If I find an area of normal appearing skin and then look back at the area of active skin disease, I go back and forth until I’m able to train my eye to be able to see those violaceous and erythematous hues more easily.”
Nummular eczema can also be a challenge in AD patients with skin of color.
“I like to listen to buzz words,” Dr. Heath said. “If a parent says, ‘my child has been diagnosed with ringworm multiple times,’ I zoom in on that. We know that kids can get tinea corporis, but usually not multiple times. I ask about all the things that can be associated with AD, and often we do see these nummular plaques on the skin and do some education about that. I also talk to their pediatrician or send information to that person so that they can be aware that nummular eczema is a form of AD.”
She noted that AD of the scalp may be confused with tinea capitis, especially in young Black children with moderate to severe AD. In her experience, triamcinolone 0.1% ointment works well for AD of the scalp.
She concluded her presentation by noting that there is no easy solution to treating AD in young patients with skin of color. “It’s way more than just eczema. We can help people see AD in a different way. My goal is to see the value in challenging ourselves to understand the impact of what happens outside of the exam room on these patients.”
Dr. Heath disclosed that she has served as a consultant for several pharmaceutical companies, including Regeneron, Janssen, Arcutis, Johnson and Johnson, Cassiopea, and Lilly.
INDIANAPOLIS – Treating atopic dermatitis (AD) in children and adolescents with skin of color requires an acumen that extends well beyond the skin, said Candrice R. Heath, MD, at the annual meeting of the Society for Pediatric Dermatology.
This involves the practice of cultural humility, which Dr. Heath defined as a commitment to learn about all aspects of patients to truly understand them, including their race, access to health care, and socioeconomic status.
“We can continue to prioritize learning about all different types of skin tones and hair types, but we really have to commit to advocating for what our patients deserve in every way,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said during her presentation at the meeting.
“That means advocating for kids to have access to better housing and for increasing health literacy programs in our hospitals, so that all our patients can understand what’s happening and how to navigate the health system,” she said. “It also means increasing diversity in our clinical trials by taking a few extra moments with the patient and family of color who might be eligible to participate in a clinical trial. We have work to do.”
To illustrate her points, she discussed the case of a 6-year-old Black patient, whose parents bring him into the clinic complaining about dark marks on the skin. The areas are itchy and the doctor figures, “this is a slam dunk; this is AD,” Dr. Heath said. “You talk about the diagnosis, and you give your treatment plan.
“But the issue is, in the parking lot when the patient’s family leaves, they feel like you didn’t help them at all,” she continued. “You didn’t understand what they came in for. They didn’t receive a treatment for what they came in for, because the initial complaint was dark marks on the skin, which is postinflammatory hyperpigmentation. We know that patients are distressed by this.”
As evidence, she cited a cross-sectional study that assessed the impact of hyperpigmentation and hyperchromia on quality of life in adults, published in the Journal of the American Academy of Dermatology. People who reported the highest levels of distress were women, those with postinflammatory hyperpigmentation, those with fewer formal years of education, and those who had higher out-of-pocket spending on skin-enhancing products.
“So, when you see hyperpigmentation in your AD patients of color, acknowledge it; say, ‘I see this pigmentation change,’ ” Dr. Heath advised. “Talk about how controlling the AD with a topical steroid or other treatment option can have a positive impact on that.”
However, she added that sometimes patients have steroid phobia, possibly because they believe the topical steroids are causing the pigmentation changes, “especially in cases of hypopigmentation, so I take the time to reassure patients so that they will not be fearful about using the medication.”
Parents of patients with skin of color who have AD may harbor other “invisible” concerns during office visits, she continued, including prior experiences with dermatologists that may not have been positive, difficulty accessing pediatric dermatologists, or a general mistrust of the health care system.
“All of that is going on in the room with your patients, particularly those with skin of color and those who feel marginalized,” said Dr. Heath, who is also a faculty scholar at Temple University medical school’s office of health equity, diversity and inclusion. “Of course, we can’t fix everything. But we can commit to approaching our visits with cultural humility.”
For patients with skin of color, she pointed out, other upstream effects impact AD care and outcomes, including well-documented socioeconomic factors.
“One of the equalizing factors is that we as pediatric dermatologists can think about increasing our education regarding skin of color,” Dr. Heath said.
For example, an analysis of data from the 2002 to 2012 National Inpatient Sample found that the main risk factors for inpatient hospitalization for AD were being non-White, having lowest-quartile household income, and having Medicaid or no insurance, researchers reported in 2018.
A separate multicenter study of 1,437 mother-child pairs with known AD found that non-Hispanic Black children and Hispanic children had greater odds of persistent AD than non-Hispanic White children, according to a 2019 study. Another large prospective cohort study published in 2019 found that AD prevalence and persistence is highest in U.S. urban children who are female or Black, and urban children with AD are more likely to have poor quality of life and asthma.
A few months after that study was published, researchers reported results from an analysis of data from the 2007-2008 National Survey of Children’s Health, which found that children who perceive the neighborhood they lived in as unsafe, unsupportive, or underdeveloped had a higher prevalence of AD and a higher severity of AD. The same year, a study of the social and economic risk factors for moderate to severe AD found that Black children were more likely to come from homes with a lower household income, lower parental education attainment, lack of home ownership, and live between two residences, and have exposure to smoke.
“Disease recognition is one thing, but we also want everyone to be aware of these other factors,” she said, “because some patients do need a little bit more care and help to be able to access the medications that they need and gain access to us.”
Follicular, nummular eczema
In her clinical experience, the most common clinical variants of AD in patients with skin of color is follicular eczema. “Examine the patient, apply your hand to the affected area, and you can feel the papules beneath your fingertips,” she advised.
“That’s what I teach my residents and medical students,” she said. “If you are looking for erythema to seal your diagnosis of AD, it may not happen. You may see more of a violaceous hue and sometimes you may not find it at all, depending on the patient’s skin tone. If I find an area of normal appearing skin and then look back at the area of active skin disease, I go back and forth until I’m able to train my eye to be able to see those violaceous and erythematous hues more easily.”
Nummular eczema can also be a challenge in AD patients with skin of color.
“I like to listen to buzz words,” Dr. Heath said. “If a parent says, ‘my child has been diagnosed with ringworm multiple times,’ I zoom in on that. We know that kids can get tinea corporis, but usually not multiple times. I ask about all the things that can be associated with AD, and often we do see these nummular plaques on the skin and do some education about that. I also talk to their pediatrician or send information to that person so that they can be aware that nummular eczema is a form of AD.”
She noted that AD of the scalp may be confused with tinea capitis, especially in young Black children with moderate to severe AD. In her experience, triamcinolone 0.1% ointment works well for AD of the scalp.
She concluded her presentation by noting that there is no easy solution to treating AD in young patients with skin of color. “It’s way more than just eczema. We can help people see AD in a different way. My goal is to see the value in challenging ourselves to understand the impact of what happens outside of the exam room on these patients.”
Dr. Heath disclosed that she has served as a consultant for several pharmaceutical companies, including Regeneron, Janssen, Arcutis, Johnson and Johnson, Cassiopea, and Lilly.
INDIANAPOLIS – Treating atopic dermatitis (AD) in children and adolescents with skin of color requires an acumen that extends well beyond the skin, said Candrice R. Heath, MD, at the annual meeting of the Society for Pediatric Dermatology.
This involves the practice of cultural humility, which Dr. Heath defined as a commitment to learn about all aspects of patients to truly understand them, including their race, access to health care, and socioeconomic status.
“We can continue to prioritize learning about all different types of skin tones and hair types, but we really have to commit to advocating for what our patients deserve in every way,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said during her presentation at the meeting.
“That means advocating for kids to have access to better housing and for increasing health literacy programs in our hospitals, so that all our patients can understand what’s happening and how to navigate the health system,” she said. “It also means increasing diversity in our clinical trials by taking a few extra moments with the patient and family of color who might be eligible to participate in a clinical trial. We have work to do.”
To illustrate her points, she discussed the case of a 6-year-old Black patient, whose parents bring him into the clinic complaining about dark marks on the skin. The areas are itchy and the doctor figures, “this is a slam dunk; this is AD,” Dr. Heath said. “You talk about the diagnosis, and you give your treatment plan.
“But the issue is, in the parking lot when the patient’s family leaves, they feel like you didn’t help them at all,” she continued. “You didn’t understand what they came in for. They didn’t receive a treatment for what they came in for, because the initial complaint was dark marks on the skin, which is postinflammatory hyperpigmentation. We know that patients are distressed by this.”
As evidence, she cited a cross-sectional study that assessed the impact of hyperpigmentation and hyperchromia on quality of life in adults, published in the Journal of the American Academy of Dermatology. People who reported the highest levels of distress were women, those with postinflammatory hyperpigmentation, those with fewer formal years of education, and those who had higher out-of-pocket spending on skin-enhancing products.
“So, when you see hyperpigmentation in your AD patients of color, acknowledge it; say, ‘I see this pigmentation change,’ ” Dr. Heath advised. “Talk about how controlling the AD with a topical steroid or other treatment option can have a positive impact on that.”
However, she added that sometimes patients have steroid phobia, possibly because they believe the topical steroids are causing the pigmentation changes, “especially in cases of hypopigmentation, so I take the time to reassure patients so that they will not be fearful about using the medication.”
Parents of patients with skin of color who have AD may harbor other “invisible” concerns during office visits, she continued, including prior experiences with dermatologists that may not have been positive, difficulty accessing pediatric dermatologists, or a general mistrust of the health care system.
“All of that is going on in the room with your patients, particularly those with skin of color and those who feel marginalized,” said Dr. Heath, who is also a faculty scholar at Temple University medical school’s office of health equity, diversity and inclusion. “Of course, we can’t fix everything. But we can commit to approaching our visits with cultural humility.”
For patients with skin of color, she pointed out, other upstream effects impact AD care and outcomes, including well-documented socioeconomic factors.
“One of the equalizing factors is that we as pediatric dermatologists can think about increasing our education regarding skin of color,” Dr. Heath said.
For example, an analysis of data from the 2002 to 2012 National Inpatient Sample found that the main risk factors for inpatient hospitalization for AD were being non-White, having lowest-quartile household income, and having Medicaid or no insurance, researchers reported in 2018.
A separate multicenter study of 1,437 mother-child pairs with known AD found that non-Hispanic Black children and Hispanic children had greater odds of persistent AD than non-Hispanic White children, according to a 2019 study. Another large prospective cohort study published in 2019 found that AD prevalence and persistence is highest in U.S. urban children who are female or Black, and urban children with AD are more likely to have poor quality of life and asthma.
A few months after that study was published, researchers reported results from an analysis of data from the 2007-2008 National Survey of Children’s Health, which found that children who perceive the neighborhood they lived in as unsafe, unsupportive, or underdeveloped had a higher prevalence of AD and a higher severity of AD. The same year, a study of the social and economic risk factors for moderate to severe AD found that Black children were more likely to come from homes with a lower household income, lower parental education attainment, lack of home ownership, and live between two residences, and have exposure to smoke.
“Disease recognition is one thing, but we also want everyone to be aware of these other factors,” she said, “because some patients do need a little bit more care and help to be able to access the medications that they need and gain access to us.”
Follicular, nummular eczema
In her clinical experience, the most common clinical variants of AD in patients with skin of color is follicular eczema. “Examine the patient, apply your hand to the affected area, and you can feel the papules beneath your fingertips,” she advised.
“That’s what I teach my residents and medical students,” she said. “If you are looking for erythema to seal your diagnosis of AD, it may not happen. You may see more of a violaceous hue and sometimes you may not find it at all, depending on the patient’s skin tone. If I find an area of normal appearing skin and then look back at the area of active skin disease, I go back and forth until I’m able to train my eye to be able to see those violaceous and erythematous hues more easily.”
Nummular eczema can also be a challenge in AD patients with skin of color.
“I like to listen to buzz words,” Dr. Heath said. “If a parent says, ‘my child has been diagnosed with ringworm multiple times,’ I zoom in on that. We know that kids can get tinea corporis, but usually not multiple times. I ask about all the things that can be associated with AD, and often we do see these nummular plaques on the skin and do some education about that. I also talk to their pediatrician or send information to that person so that they can be aware that nummular eczema is a form of AD.”
She noted that AD of the scalp may be confused with tinea capitis, especially in young Black children with moderate to severe AD. In her experience, triamcinolone 0.1% ointment works well for AD of the scalp.
She concluded her presentation by noting that there is no easy solution to treating AD in young patients with skin of color. “It’s way more than just eczema. We can help people see AD in a different way. My goal is to see the value in challenging ourselves to understand the impact of what happens outside of the exam room on these patients.”
Dr. Heath disclosed that she has served as a consultant for several pharmaceutical companies, including Regeneron, Janssen, Arcutis, Johnson and Johnson, Cassiopea, and Lilly.
AT SPD 2022
COVID skin manifestations vary by type of variant, U.K. study finds
during the Omicron and Delta waves.
Among the key findings, the study shows that skin involvement during the Omicron wave was less frequent than during the Delta wave (11.4% vs. 17.6%), skin symptoms generally resolved more quickly, and that the risk for skin symptoms was similar whether patients had or had not been vaccinated, according to a team led by Alessia Visconti, PhD, a research fellow in the department of twin research and genetic epidemiology, King’s College, London.
These data are consistent with the experience of those dermatologists who have been following this area closely, according to Esther Freeman, MD, PhD, associate professor of dermatology at Harvard Medical School and director of MGH Global Health Dermatology at Massachusetts General Hospital, both in Boston.
“Anecdotally, we thought we were seeing fewer skin symptoms with Omicron versus Delta and the ancestral strains, and now this study shows it is true,” said Dr. Freeman, who is also principal investigator of the American Academy of Dermatology’s International Dermatology COVID-19 Registry.
The data also confirm that the skin is less likely to be involved than in past waves of COVID-19 infections.
“Up to this point, it was hard to know if we were seeing fewer referrals for COVID-related skin rashes or if clinicians had just become more comfortable with these rashes and were not referring them as often,” added Dr. Freeman, who was among the study coauthors.
Data captured from 348,691 patients
The data from the study was generated by 348,691 users in the United Kingdom of the ZOE COVID study app, a smartphone-based tool introduced relatively early in the pandemic. It asked users to provide demographic data, information on COVID-19 symptoms, including those involving the skin, and treatments. Of 33 COVID-related symptoms included in the app, five related to the skin (acral rash, burning rash, erythematopapular rash, urticarial rash, and unusual hair loss).
While the focus of this study was to compare skin manifestations during the Omicron wave with the Delta wave of COVID-19, the investigators also had data on the experience in 2020 with wild-type COVID-19 that preceded both variants. Overall, this showed a stepwise decline in skin symptoms overall, as well in as skin symptoms that occurred in the absence of systemic symptoms.
“The shift in the skin manifestations makes sense when you think about the change that is also being seen in the systemic symptoms,” said Dr. Freeman, referring to lower rates of cough and loss of smell but higher rates of sore throat and fatigue. “Omicron is achieving immune escape, which is why there is a shift in involved tissues,” she said in an interview.
Previous data collected during the wild-type COVID-19 stage of the pandemic by the same group of investigators showed that 17% of patients reported skin rash as the first symptom of COVID-19 infection, and 21% reported skin rash as the only clinical sign of infection.
In the Delta and Omicron waves, skin rash was an isolated initial symptom in only 0.8% and 0.5% of patients, respectively. (The authors noted that, in the United Kingdom, the first documented samples of the Delta variant were detected in October 2020, and the first documented samples of the Omicron variant were detected in November 2021.)
During the early stages of wild-type COVID, an acral rash was characteristic, occurring in 3.1% of patients, according to the U.K. data. In the Delta wave, acral rashes, at an incidence of 1.1% remained positively correlated with a diagnosis of COVID-19 infection. In the Omicron wave, acral rashes were observed in only 0.7% of patients and were no longer statistically correlated with a positive COVID diagnosis.
Characteristic cutaneous symptoms are evolving
Early in the course of the COVID-19 epidemic, more than 30 types of rashes were observed in patients with COVID-19 infection. Cutaneous symptoms continue to be diverse, but some, such as acral rash, are being seen less frequently. For example, the odds ratio of a positive COVID-19 diagnosis among those with an erythematopapular rash fell from 1.76 to 1.08 between the Delta and Omicron waves.
While specific cutaneous symptoms are less predictive of a diagnosis of COVID-19, clinicians should not discount cutaneous symptoms as a sign of disease, according to Veronique Bataille, MD, PhD, a consultant dermatologist at King’s College.
“You need to keep an open mind” regarding cutaneous signs and a diagnosis of COVID-19, Dr. Bataille, one of the coauthors of the U.K. report, said in an interview. In general, she considers a low threshold of suspicion appropriate. “If the patient has no past history of skin disease and no other triggers for a rash, then, in a high prevalence area, COVID must be suspected.”
In most cases, the rash resolves on its own, but Dr. Bataille emphasized the need for individualized care. Even as the risk of life-threatening COVID-19 infections appears to be diminishing with current variants, cutaneous manifestations can be severe.
“There are cases of long COVID affecting the skin, such as urticaria or a lichenoid erythematopapular rash, both of which can be very pruritic and difficult to control,” she said.
Dr. Freeman echoed the importance of an individualized approach. She agreed that most cutaneous symptoms are self-limited, but there are exceptions and treatments vary for the different types of skin involvement. “I think another point to consider when examining skin lesions is monkey pox. The fact that these are overlapping outbreaks should not be ignored. You need to be alert for both.”
Dr. Visconti, Dr. Freeman, and Dr. Bataille reported no potential conflicts of interest.
during the Omicron and Delta waves.
Among the key findings, the study shows that skin involvement during the Omicron wave was less frequent than during the Delta wave (11.4% vs. 17.6%), skin symptoms generally resolved more quickly, and that the risk for skin symptoms was similar whether patients had or had not been vaccinated, according to a team led by Alessia Visconti, PhD, a research fellow in the department of twin research and genetic epidemiology, King’s College, London.
These data are consistent with the experience of those dermatologists who have been following this area closely, according to Esther Freeman, MD, PhD, associate professor of dermatology at Harvard Medical School and director of MGH Global Health Dermatology at Massachusetts General Hospital, both in Boston.
“Anecdotally, we thought we were seeing fewer skin symptoms with Omicron versus Delta and the ancestral strains, and now this study shows it is true,” said Dr. Freeman, who is also principal investigator of the American Academy of Dermatology’s International Dermatology COVID-19 Registry.
The data also confirm that the skin is less likely to be involved than in past waves of COVID-19 infections.
“Up to this point, it was hard to know if we were seeing fewer referrals for COVID-related skin rashes or if clinicians had just become more comfortable with these rashes and were not referring them as often,” added Dr. Freeman, who was among the study coauthors.
Data captured from 348,691 patients
The data from the study was generated by 348,691 users in the United Kingdom of the ZOE COVID study app, a smartphone-based tool introduced relatively early in the pandemic. It asked users to provide demographic data, information on COVID-19 symptoms, including those involving the skin, and treatments. Of 33 COVID-related symptoms included in the app, five related to the skin (acral rash, burning rash, erythematopapular rash, urticarial rash, and unusual hair loss).
While the focus of this study was to compare skin manifestations during the Omicron wave with the Delta wave of COVID-19, the investigators also had data on the experience in 2020 with wild-type COVID-19 that preceded both variants. Overall, this showed a stepwise decline in skin symptoms overall, as well in as skin symptoms that occurred in the absence of systemic symptoms.
“The shift in the skin manifestations makes sense when you think about the change that is also being seen in the systemic symptoms,” said Dr. Freeman, referring to lower rates of cough and loss of smell but higher rates of sore throat and fatigue. “Omicron is achieving immune escape, which is why there is a shift in involved tissues,” she said in an interview.
Previous data collected during the wild-type COVID-19 stage of the pandemic by the same group of investigators showed that 17% of patients reported skin rash as the first symptom of COVID-19 infection, and 21% reported skin rash as the only clinical sign of infection.
In the Delta and Omicron waves, skin rash was an isolated initial symptom in only 0.8% and 0.5% of patients, respectively. (The authors noted that, in the United Kingdom, the first documented samples of the Delta variant were detected in October 2020, and the first documented samples of the Omicron variant were detected in November 2021.)
During the early stages of wild-type COVID, an acral rash was characteristic, occurring in 3.1% of patients, according to the U.K. data. In the Delta wave, acral rashes, at an incidence of 1.1% remained positively correlated with a diagnosis of COVID-19 infection. In the Omicron wave, acral rashes were observed in only 0.7% of patients and were no longer statistically correlated with a positive COVID diagnosis.
Characteristic cutaneous symptoms are evolving
Early in the course of the COVID-19 epidemic, more than 30 types of rashes were observed in patients with COVID-19 infection. Cutaneous symptoms continue to be diverse, but some, such as acral rash, are being seen less frequently. For example, the odds ratio of a positive COVID-19 diagnosis among those with an erythematopapular rash fell from 1.76 to 1.08 between the Delta and Omicron waves.
While specific cutaneous symptoms are less predictive of a diagnosis of COVID-19, clinicians should not discount cutaneous symptoms as a sign of disease, according to Veronique Bataille, MD, PhD, a consultant dermatologist at King’s College.
“You need to keep an open mind” regarding cutaneous signs and a diagnosis of COVID-19, Dr. Bataille, one of the coauthors of the U.K. report, said in an interview. In general, she considers a low threshold of suspicion appropriate. “If the patient has no past history of skin disease and no other triggers for a rash, then, in a high prevalence area, COVID must be suspected.”
In most cases, the rash resolves on its own, but Dr. Bataille emphasized the need for individualized care. Even as the risk of life-threatening COVID-19 infections appears to be diminishing with current variants, cutaneous manifestations can be severe.
“There are cases of long COVID affecting the skin, such as urticaria or a lichenoid erythematopapular rash, both of which can be very pruritic and difficult to control,” she said.
Dr. Freeman echoed the importance of an individualized approach. She agreed that most cutaneous symptoms are self-limited, but there are exceptions and treatments vary for the different types of skin involvement. “I think another point to consider when examining skin lesions is monkey pox. The fact that these are overlapping outbreaks should not be ignored. You need to be alert for both.”
Dr. Visconti, Dr. Freeman, and Dr. Bataille reported no potential conflicts of interest.
during the Omicron and Delta waves.
Among the key findings, the study shows that skin involvement during the Omicron wave was less frequent than during the Delta wave (11.4% vs. 17.6%), skin symptoms generally resolved more quickly, and that the risk for skin symptoms was similar whether patients had or had not been vaccinated, according to a team led by Alessia Visconti, PhD, a research fellow in the department of twin research and genetic epidemiology, King’s College, London.
These data are consistent with the experience of those dermatologists who have been following this area closely, according to Esther Freeman, MD, PhD, associate professor of dermatology at Harvard Medical School and director of MGH Global Health Dermatology at Massachusetts General Hospital, both in Boston.
“Anecdotally, we thought we were seeing fewer skin symptoms with Omicron versus Delta and the ancestral strains, and now this study shows it is true,” said Dr. Freeman, who is also principal investigator of the American Academy of Dermatology’s International Dermatology COVID-19 Registry.
The data also confirm that the skin is less likely to be involved than in past waves of COVID-19 infections.
“Up to this point, it was hard to know if we were seeing fewer referrals for COVID-related skin rashes or if clinicians had just become more comfortable with these rashes and were not referring them as often,” added Dr. Freeman, who was among the study coauthors.
Data captured from 348,691 patients
The data from the study was generated by 348,691 users in the United Kingdom of the ZOE COVID study app, a smartphone-based tool introduced relatively early in the pandemic. It asked users to provide demographic data, information on COVID-19 symptoms, including those involving the skin, and treatments. Of 33 COVID-related symptoms included in the app, five related to the skin (acral rash, burning rash, erythematopapular rash, urticarial rash, and unusual hair loss).
While the focus of this study was to compare skin manifestations during the Omicron wave with the Delta wave of COVID-19, the investigators also had data on the experience in 2020 with wild-type COVID-19 that preceded both variants. Overall, this showed a stepwise decline in skin symptoms overall, as well in as skin symptoms that occurred in the absence of systemic symptoms.
“The shift in the skin manifestations makes sense when you think about the change that is also being seen in the systemic symptoms,” said Dr. Freeman, referring to lower rates of cough and loss of smell but higher rates of sore throat and fatigue. “Omicron is achieving immune escape, which is why there is a shift in involved tissues,” she said in an interview.
Previous data collected during the wild-type COVID-19 stage of the pandemic by the same group of investigators showed that 17% of patients reported skin rash as the first symptom of COVID-19 infection, and 21% reported skin rash as the only clinical sign of infection.
In the Delta and Omicron waves, skin rash was an isolated initial symptom in only 0.8% and 0.5% of patients, respectively. (The authors noted that, in the United Kingdom, the first documented samples of the Delta variant were detected in October 2020, and the first documented samples of the Omicron variant were detected in November 2021.)
During the early stages of wild-type COVID, an acral rash was characteristic, occurring in 3.1% of patients, according to the U.K. data. In the Delta wave, acral rashes, at an incidence of 1.1% remained positively correlated with a diagnosis of COVID-19 infection. In the Omicron wave, acral rashes were observed in only 0.7% of patients and were no longer statistically correlated with a positive COVID diagnosis.
Characteristic cutaneous symptoms are evolving
Early in the course of the COVID-19 epidemic, more than 30 types of rashes were observed in patients with COVID-19 infection. Cutaneous symptoms continue to be diverse, but some, such as acral rash, are being seen less frequently. For example, the odds ratio of a positive COVID-19 diagnosis among those with an erythematopapular rash fell from 1.76 to 1.08 between the Delta and Omicron waves.
While specific cutaneous symptoms are less predictive of a diagnosis of COVID-19, clinicians should not discount cutaneous symptoms as a sign of disease, according to Veronique Bataille, MD, PhD, a consultant dermatologist at King’s College.
“You need to keep an open mind” regarding cutaneous signs and a diagnosis of COVID-19, Dr. Bataille, one of the coauthors of the U.K. report, said in an interview. In general, she considers a low threshold of suspicion appropriate. “If the patient has no past history of skin disease and no other triggers for a rash, then, in a high prevalence area, COVID must be suspected.”
In most cases, the rash resolves on its own, but Dr. Bataille emphasized the need for individualized care. Even as the risk of life-threatening COVID-19 infections appears to be diminishing with current variants, cutaneous manifestations can be severe.
“There are cases of long COVID affecting the skin, such as urticaria or a lichenoid erythematopapular rash, both of which can be very pruritic and difficult to control,” she said.
Dr. Freeman echoed the importance of an individualized approach. She agreed that most cutaneous symptoms are self-limited, but there are exceptions and treatments vary for the different types of skin involvement. “I think another point to consider when examining skin lesions is monkey pox. The fact that these are overlapping outbreaks should not be ignored. You need to be alert for both.”
Dr. Visconti, Dr. Freeman, and Dr. Bataille reported no potential conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Devices to detect skin cancer: FDA advisers offer mixed views
.
So far, the U.S. Food and Drug Administration has cleared two devices. Both are computer-aided skin lesion classification devices meant to help clinicians assess cases of suspected melanoma.
Both were given a class III designation. That classification is intended for products that are considered to have a high risk of harm because of flawed design or implementation. Many such devices are under development, and there has been a proposal to include these devices in class II, which is less restrictive.
The FDA turned to one of its expert panels for advice. At a meeting held on Aug. 29, experts on the panel offered differing views and expressed concerns about the accuracy of these devices.
This was the second day of meetings of the general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee. On the previous day, the panel held a wide-ranging discussion about expanding use of skin lesion analyzer devices.
The FDA sought the expert panel’s advice concerning a field that appears to be heating up quickly after relatively quiet times.
Two devices have been approved by the FDA so far, but only one is still being promoted – SciBase AB’s Nevisense. The Swedish company announced in May 2020 that it had received FDA approval for Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection, an AI-based point-of-care system for the noninvasive evaluation of irregular moles.
The other device, known as MelaFind, was acquired by Strata Skin Sciences, but the company said in 2017 that it discontinued research and development, sales, and support activity related to the device, according to a filing with the Securities and Exchange Commission.
But there’s been a swell in recent years in the number of publications related to the use of AI and machine learning, which could give rise to new tools for aiding in the diagnosis of skin conditions, including cancer. Google is among the companies that are involved in these efforts.
So, the FDA asked the expert panel to discuss a series of questions related to how the agency should weigh the risks of computer-aided devices for melanoma diagnosis. The agency also asked the panel to provide feedback about how well risks associated with such devices and tools might be managed and to offer suggestions.
The discussion at the July 29 meeting spun beyond narrow questions about reclassification of the current class III devices to topics involving emerging technology, such as efforts to apply AI to dermatology.
“Innovation continues. Medical device developers are anxious to plan how they might be able to develop the level of evidence that would meet your expectations” for future products, Binita Ashar, MD, a senior official in FDA’s Center for Devices and Radiological Health, told the panel.
Company CEO backs tougher regulation
Simon Grant, the chief executive of SciBase, which markets Nevisense, the first and only skin cancer–detecting device currently on the U.S. market, sought to make a case for sticking with the tougher class III regulations.
Speaking during the public comment session, Mr. Grant said switching to class II designations would weaken the standards used in clearing products that analyze skin lesions so as to put patients at risk.
Under the FDA’s rules, the agency designates as class III devices that present potential unreasonable risk of illness or injury. Only about 10% of devices fall into this category. Such devices include implantable pacemakers and breast implants, as well as SciBase’s Nevisense.
About 43% of medical devices fall into the class II category, which includes powered wheelchairs and some pregnancy test kits, the FDA website says.
Class I medical devices pose minimal potential for harm and tend to be simpler in design. These include enema kits and elastic bandages, the FDA says.
Mr. Grant told the meeting that in his career he has worked on two class III products and about 20 class II products. (He had previously worked at medical startups Synectics Medical and Neoventa, as well as established multinationals such as Medtronic.)
“I can tell you that – practically – the FDA has many fewer sticks and much less control when it comes to class II devices,” he said. He offered an example of a manufacturer of a class II device having more latitude in making small changes to products without notifying the FDA.
In his hypothetical example, such a change could have unintended consequences, and “with AI systems, small changes can result in large and nonlinear or even random effects,” Mr. Grant said. “But it’s too late if the product is on the market and the harm has already occurred,” he said.
The American Society for Dermatologic Surgery Association also protested the reclassifying of approved computer-aided melanoma detection class III devices.
In a statement posted on the FDA website as part of the materials for the meeting, the ASDSA raised a series of concerns about the prospects of expanded U.S. use of tools for assisting in diagnosing melanoma, including ones that would be marketed to consumers.
“To the extent that algorithms and devices for patient self-diagnosis of skin lesions are already widely available, they should be required to include detailed disclaimers that include that they are for entertainment and educational purposes and not a diagnostic device, that they are not approved by dermatologists or a recognized medical regulatory authority for self-diagnosis,” the ASDSA said.
Devices and algorithms in screening tools “are not highly regulated and remain unproven. They may result in wrong diagnoses, missed diagnoses, or over- or underdiagnosis,” the ASDSA added. “Both patients at low risk and those at high risk are better served by scheduling an in-person examination with a board-certified dermatologist, who can also help them determine the appropriate future skin screening schedule that is most appropriate for them.”
‘Stepping stone’
However, there is strong consumer demand for better information about skin conditions, and many patients face hurdles in going to dermatologists.
Google research has shown that consumers are seeking “a stepping stone” between the information they can easily find online and what they could get from a medical professional, said Lily Peng, MD, PhD, a director of product management for the health AI team at Google. Dr. Peng was a scheduled presenter at the July 29 meeting.
Consumers often are looking for more information on common conditions such as acne and poison ivy, and they sometimes face challenges in getting access to clinicians, she said.
“There are many unmet needs for consumers experiencing skin issues, many of which are lower-acuity conditions. There’s a big opportunity to increase accessibility and relevance of health journeys for consumers,” Dr. Peng said. “We have heard from consumers that they would like to have a self-help tool for nonserious conditions so they can decide when to seek medical attention.”
Dr. Peng’s presentation was not directly related to the question of class II or class III designation for existing products. Instead, her talk served as a glimpse into the work already underway in creating apps and tools for consumers.
Google researchers have published a number of studies in recent years about the use of AI to improve dermatology diagnosis.
A 2020 article reported on Google’s test of a form of AI known as deep learning system (DLS) to provide a differential diagnosis of skin conditions. On 963 validation cases, where a rotating panel of three board-certified dermatologists defined the reference standard, the DLS was noninferior to six other dermatologists and was superior to six primary care physicians (PCPs) and six nurse practitioners (NPs), according to a summary of the article.
A 2021 report published in JAMA Network Open said that use of an AI tool was associated with a higher agreement rate with dermatologists’ reference diagnoses for both PCPs and NPs.
In a 2021 blog post, Google scientists wrote that their AI model that powers a tool for checking skin conditions had earned European clearance, known as a CE mark, as a class I medical device.
SkinVision has an app that the company says “is available worldwide (with the exception of the USA and Canada).” The firm’s website includes a link where people in the United States and Canada can sign up for notifications about when SkinVision will be available in these nations.
‘Not ready for prime time’
The FDA panel did not cast formal votes at the July 29 meeting. Rather, the members engaged in broad discussions about risks and potential benefits of new tools for aiding in the detection of skin cancer.
Among the key issues discussed was a question of whether the FDA could impose requirements and restrictions, known as special controls, to provide “reasonable assurance of safety and effectiveness” for computer-aided devices that provide adjunctive diagnostic information to dermatologists about lesions suspicious for melanoma.
Among the potential special controls would be clinical performance testing in regards to rates of the sensitivity (true-positive rate) and specificity (true-negative rate).
The FDA could also look at requirements on software validation and verification and cybersecurity testing, as well as directions on labeling so as to mitigate risk.
Dermatologists serving on the panel called for caution in proceeding with steps that would make it easier for companies to market tools for aiding in melanoma diagnosis than it would be within the class III framework used for MelaFind and Nevisense.
Many expressed concerns about the need to design studies that would answer questions about how well new tools could accurately identify concerning lesions.
The phrase “not ready for prime time” was used at least three times during the discussion.
FDA panelist Maral Skelsey, MD, a skin cancer specialist from Chevy Chase, Maryland, said that over the years, she had used both Nevisense and MelaFind.
She said she had found MelaFind “unusable,” owing in large part to the high number of false positives it generated. The device also was limited as to where on patients’ bodies it could be used.
However, she spoke with enthusiasm about the prospects for better devices to aid in diagnosis of skin lesions. “It’s an area where we’re on the verge, and we really need these devices. There’s a need for patients to be able to examine themselves, for nondermatologists to be able to assess lesions,” Dr. Skelsey said.
But this field is “just not ready for prime time” yet, even with special controls, Dr. Skelsey said. To loosen approval standards too quickly could be a “detriment to what’s coming down the pipeline,” she said.
“It’s harmful to things that are likely to be around the corner,” she said.
FDA panelist Renata Block, PA-C, who works in a Chicago dermatology practice, pressed for maintaining a class III designation. “We are not ready for prime time yet, though the data that is coming down the pipeline on what we have is quite exciting,” Ms. Block said.
FDA panelist Karla V. Ballman, PhD, a statistician from Weill Cornell Medicine, New York, said there would need to be a clear standard for clinical performance before proceeding toward reclassification of devices for aid in detecting melanoma. “I just don’t think it’s ready for prime time at this point and should remain in class III,” she said.
But there was support from some panelists for the idea of a lower bar for clearance, combined with special controls to ensure patient safety.
In expressing her view, FDA panelist Katalin Roth, MD, JD, professor of medicine, George Washington University, Washington, said she was an outlier in her support for the agency’s view that these risks could be managed and that future tools could allow more patients to take a step on the pathway toward critical diagnoses.
“I deal with a lot of people with cancer as a palliative care physician,” Dr. Roth said. “I think what we’re missing here is the issue of time. Melanoma is a terrible disease, and missing the diagnosis is a terrible thing, but I think special controls would be sufficient to counter the concerns of my colleagues on the committee.”
The FDA’s Dr. Ashar ended the meeting with questions posed to one panelist, Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center in New York.
Dr. Rotemberg has for years been working in the field of research on developing AI and other computer-based tools for detecting and diagnosing melanoma, the deadliest form of skin cancer.
She has been publicly skeptical of the performance of commercial apps that scan moles and other lesions and that claim to identify which are cancerous. A May blog post on the Memorial Sloan Kettering website highlighted a recent British Journal of Dermatology article in which Dr. Rotemberg and coauthors reported on their evaluations of commercial apps. They judged them to be on average only 59% accurate, the blog post said.
However, during an earlier discussion at the meeting, she had spoken more positively about the prospects for using special controls in the near term to mitigate risk, although she said she would have a “very long list” of these requirements.
In the closing exchange with Dr. Ashar, Dr. Rotemberg outlined steps that could potentially ensure the safe use of tools to aid in melanoma screening. These included a need for postmarketing surveillance, which would require evaluation over time of algorithms used in tools meant to detect skin cancer.
“We need to have a mechanism for sampling,” Dr. Rotemberg said. “Most of our data is electronic now anyway, so comparing an algorithm and performance with biopsy results should not be that challenging.”
A version of this article first appeared on Medscape.com.
.
So far, the U.S. Food and Drug Administration has cleared two devices. Both are computer-aided skin lesion classification devices meant to help clinicians assess cases of suspected melanoma.
Both were given a class III designation. That classification is intended for products that are considered to have a high risk of harm because of flawed design or implementation. Many such devices are under development, and there has been a proposal to include these devices in class II, which is less restrictive.
The FDA turned to one of its expert panels for advice. At a meeting held on Aug. 29, experts on the panel offered differing views and expressed concerns about the accuracy of these devices.
This was the second day of meetings of the general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee. On the previous day, the panel held a wide-ranging discussion about expanding use of skin lesion analyzer devices.
The FDA sought the expert panel’s advice concerning a field that appears to be heating up quickly after relatively quiet times.
Two devices have been approved by the FDA so far, but only one is still being promoted – SciBase AB’s Nevisense. The Swedish company announced in May 2020 that it had received FDA approval for Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection, an AI-based point-of-care system for the noninvasive evaluation of irregular moles.
The other device, known as MelaFind, was acquired by Strata Skin Sciences, but the company said in 2017 that it discontinued research and development, sales, and support activity related to the device, according to a filing with the Securities and Exchange Commission.
But there’s been a swell in recent years in the number of publications related to the use of AI and machine learning, which could give rise to new tools for aiding in the diagnosis of skin conditions, including cancer. Google is among the companies that are involved in these efforts.
So, the FDA asked the expert panel to discuss a series of questions related to how the agency should weigh the risks of computer-aided devices for melanoma diagnosis. The agency also asked the panel to provide feedback about how well risks associated with such devices and tools might be managed and to offer suggestions.
The discussion at the July 29 meeting spun beyond narrow questions about reclassification of the current class III devices to topics involving emerging technology, such as efforts to apply AI to dermatology.
“Innovation continues. Medical device developers are anxious to plan how they might be able to develop the level of evidence that would meet your expectations” for future products, Binita Ashar, MD, a senior official in FDA’s Center for Devices and Radiological Health, told the panel.
Company CEO backs tougher regulation
Simon Grant, the chief executive of SciBase, which markets Nevisense, the first and only skin cancer–detecting device currently on the U.S. market, sought to make a case for sticking with the tougher class III regulations.
Speaking during the public comment session, Mr. Grant said switching to class II designations would weaken the standards used in clearing products that analyze skin lesions so as to put patients at risk.
Under the FDA’s rules, the agency designates as class III devices that present potential unreasonable risk of illness or injury. Only about 10% of devices fall into this category. Such devices include implantable pacemakers and breast implants, as well as SciBase’s Nevisense.
About 43% of medical devices fall into the class II category, which includes powered wheelchairs and some pregnancy test kits, the FDA website says.
Class I medical devices pose minimal potential for harm and tend to be simpler in design. These include enema kits and elastic bandages, the FDA says.
Mr. Grant told the meeting that in his career he has worked on two class III products and about 20 class II products. (He had previously worked at medical startups Synectics Medical and Neoventa, as well as established multinationals such as Medtronic.)
“I can tell you that – practically – the FDA has many fewer sticks and much less control when it comes to class II devices,” he said. He offered an example of a manufacturer of a class II device having more latitude in making small changes to products without notifying the FDA.
In his hypothetical example, such a change could have unintended consequences, and “with AI systems, small changes can result in large and nonlinear or even random effects,” Mr. Grant said. “But it’s too late if the product is on the market and the harm has already occurred,” he said.
The American Society for Dermatologic Surgery Association also protested the reclassifying of approved computer-aided melanoma detection class III devices.
In a statement posted on the FDA website as part of the materials for the meeting, the ASDSA raised a series of concerns about the prospects of expanded U.S. use of tools for assisting in diagnosing melanoma, including ones that would be marketed to consumers.
“To the extent that algorithms and devices for patient self-diagnosis of skin lesions are already widely available, they should be required to include detailed disclaimers that include that they are for entertainment and educational purposes and not a diagnostic device, that they are not approved by dermatologists or a recognized medical regulatory authority for self-diagnosis,” the ASDSA said.
Devices and algorithms in screening tools “are not highly regulated and remain unproven. They may result in wrong diagnoses, missed diagnoses, or over- or underdiagnosis,” the ASDSA added. “Both patients at low risk and those at high risk are better served by scheduling an in-person examination with a board-certified dermatologist, who can also help them determine the appropriate future skin screening schedule that is most appropriate for them.”
‘Stepping stone’
However, there is strong consumer demand for better information about skin conditions, and many patients face hurdles in going to dermatologists.
Google research has shown that consumers are seeking “a stepping stone” between the information they can easily find online and what they could get from a medical professional, said Lily Peng, MD, PhD, a director of product management for the health AI team at Google. Dr. Peng was a scheduled presenter at the July 29 meeting.
Consumers often are looking for more information on common conditions such as acne and poison ivy, and they sometimes face challenges in getting access to clinicians, she said.
“There are many unmet needs for consumers experiencing skin issues, many of which are lower-acuity conditions. There’s a big opportunity to increase accessibility and relevance of health journeys for consumers,” Dr. Peng said. “We have heard from consumers that they would like to have a self-help tool for nonserious conditions so they can decide when to seek medical attention.”
Dr. Peng’s presentation was not directly related to the question of class II or class III designation for existing products. Instead, her talk served as a glimpse into the work already underway in creating apps and tools for consumers.
Google researchers have published a number of studies in recent years about the use of AI to improve dermatology diagnosis.
A 2020 article reported on Google’s test of a form of AI known as deep learning system (DLS) to provide a differential diagnosis of skin conditions. On 963 validation cases, where a rotating panel of three board-certified dermatologists defined the reference standard, the DLS was noninferior to six other dermatologists and was superior to six primary care physicians (PCPs) and six nurse practitioners (NPs), according to a summary of the article.
A 2021 report published in JAMA Network Open said that use of an AI tool was associated with a higher agreement rate with dermatologists’ reference diagnoses for both PCPs and NPs.
In a 2021 blog post, Google scientists wrote that their AI model that powers a tool for checking skin conditions had earned European clearance, known as a CE mark, as a class I medical device.
SkinVision has an app that the company says “is available worldwide (with the exception of the USA and Canada).” The firm’s website includes a link where people in the United States and Canada can sign up for notifications about when SkinVision will be available in these nations.
‘Not ready for prime time’
The FDA panel did not cast formal votes at the July 29 meeting. Rather, the members engaged in broad discussions about risks and potential benefits of new tools for aiding in the detection of skin cancer.
Among the key issues discussed was a question of whether the FDA could impose requirements and restrictions, known as special controls, to provide “reasonable assurance of safety and effectiveness” for computer-aided devices that provide adjunctive diagnostic information to dermatologists about lesions suspicious for melanoma.
Among the potential special controls would be clinical performance testing in regards to rates of the sensitivity (true-positive rate) and specificity (true-negative rate).
The FDA could also look at requirements on software validation and verification and cybersecurity testing, as well as directions on labeling so as to mitigate risk.
Dermatologists serving on the panel called for caution in proceeding with steps that would make it easier for companies to market tools for aiding in melanoma diagnosis than it would be within the class III framework used for MelaFind and Nevisense.
Many expressed concerns about the need to design studies that would answer questions about how well new tools could accurately identify concerning lesions.
The phrase “not ready for prime time” was used at least three times during the discussion.
FDA panelist Maral Skelsey, MD, a skin cancer specialist from Chevy Chase, Maryland, said that over the years, she had used both Nevisense and MelaFind.
She said she had found MelaFind “unusable,” owing in large part to the high number of false positives it generated. The device also was limited as to where on patients’ bodies it could be used.
However, she spoke with enthusiasm about the prospects for better devices to aid in diagnosis of skin lesions. “It’s an area where we’re on the verge, and we really need these devices. There’s a need for patients to be able to examine themselves, for nondermatologists to be able to assess lesions,” Dr. Skelsey said.
But this field is “just not ready for prime time” yet, even with special controls, Dr. Skelsey said. To loosen approval standards too quickly could be a “detriment to what’s coming down the pipeline,” she said.
“It’s harmful to things that are likely to be around the corner,” she said.
FDA panelist Renata Block, PA-C, who works in a Chicago dermatology practice, pressed for maintaining a class III designation. “We are not ready for prime time yet, though the data that is coming down the pipeline on what we have is quite exciting,” Ms. Block said.
FDA panelist Karla V. Ballman, PhD, a statistician from Weill Cornell Medicine, New York, said there would need to be a clear standard for clinical performance before proceeding toward reclassification of devices for aid in detecting melanoma. “I just don’t think it’s ready for prime time at this point and should remain in class III,” she said.
But there was support from some panelists for the idea of a lower bar for clearance, combined with special controls to ensure patient safety.
In expressing her view, FDA panelist Katalin Roth, MD, JD, professor of medicine, George Washington University, Washington, said she was an outlier in her support for the agency’s view that these risks could be managed and that future tools could allow more patients to take a step on the pathway toward critical diagnoses.
“I deal with a lot of people with cancer as a palliative care physician,” Dr. Roth said. “I think what we’re missing here is the issue of time. Melanoma is a terrible disease, and missing the diagnosis is a terrible thing, but I think special controls would be sufficient to counter the concerns of my colleagues on the committee.”
The FDA’s Dr. Ashar ended the meeting with questions posed to one panelist, Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center in New York.
Dr. Rotemberg has for years been working in the field of research on developing AI and other computer-based tools for detecting and diagnosing melanoma, the deadliest form of skin cancer.
She has been publicly skeptical of the performance of commercial apps that scan moles and other lesions and that claim to identify which are cancerous. A May blog post on the Memorial Sloan Kettering website highlighted a recent British Journal of Dermatology article in which Dr. Rotemberg and coauthors reported on their evaluations of commercial apps. They judged them to be on average only 59% accurate, the blog post said.
However, during an earlier discussion at the meeting, she had spoken more positively about the prospects for using special controls in the near term to mitigate risk, although she said she would have a “very long list” of these requirements.
In the closing exchange with Dr. Ashar, Dr. Rotemberg outlined steps that could potentially ensure the safe use of tools to aid in melanoma screening. These included a need for postmarketing surveillance, which would require evaluation over time of algorithms used in tools meant to detect skin cancer.
“We need to have a mechanism for sampling,” Dr. Rotemberg said. “Most of our data is electronic now anyway, so comparing an algorithm and performance with biopsy results should not be that challenging.”
A version of this article first appeared on Medscape.com.
.
So far, the U.S. Food and Drug Administration has cleared two devices. Both are computer-aided skin lesion classification devices meant to help clinicians assess cases of suspected melanoma.
Both were given a class III designation. That classification is intended for products that are considered to have a high risk of harm because of flawed design or implementation. Many such devices are under development, and there has been a proposal to include these devices in class II, which is less restrictive.
The FDA turned to one of its expert panels for advice. At a meeting held on Aug. 29, experts on the panel offered differing views and expressed concerns about the accuracy of these devices.
This was the second day of meetings of the general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee. On the previous day, the panel held a wide-ranging discussion about expanding use of skin lesion analyzer devices.
The FDA sought the expert panel’s advice concerning a field that appears to be heating up quickly after relatively quiet times.
Two devices have been approved by the FDA so far, but only one is still being promoted – SciBase AB’s Nevisense. The Swedish company announced in May 2020 that it had received FDA approval for Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection, an AI-based point-of-care system for the noninvasive evaluation of irregular moles.
The other device, known as MelaFind, was acquired by Strata Skin Sciences, but the company said in 2017 that it discontinued research and development, sales, and support activity related to the device, according to a filing with the Securities and Exchange Commission.
But there’s been a swell in recent years in the number of publications related to the use of AI and machine learning, which could give rise to new tools for aiding in the diagnosis of skin conditions, including cancer. Google is among the companies that are involved in these efforts.
So, the FDA asked the expert panel to discuss a series of questions related to how the agency should weigh the risks of computer-aided devices for melanoma diagnosis. The agency also asked the panel to provide feedback about how well risks associated with such devices and tools might be managed and to offer suggestions.
The discussion at the July 29 meeting spun beyond narrow questions about reclassification of the current class III devices to topics involving emerging technology, such as efforts to apply AI to dermatology.
“Innovation continues. Medical device developers are anxious to plan how they might be able to develop the level of evidence that would meet your expectations” for future products, Binita Ashar, MD, a senior official in FDA’s Center for Devices and Radiological Health, told the panel.
Company CEO backs tougher regulation
Simon Grant, the chief executive of SciBase, which markets Nevisense, the first and only skin cancer–detecting device currently on the U.S. market, sought to make a case for sticking with the tougher class III regulations.
Speaking during the public comment session, Mr. Grant said switching to class II designations would weaken the standards used in clearing products that analyze skin lesions so as to put patients at risk.
Under the FDA’s rules, the agency designates as class III devices that present potential unreasonable risk of illness or injury. Only about 10% of devices fall into this category. Such devices include implantable pacemakers and breast implants, as well as SciBase’s Nevisense.
About 43% of medical devices fall into the class II category, which includes powered wheelchairs and some pregnancy test kits, the FDA website says.
Class I medical devices pose minimal potential for harm and tend to be simpler in design. These include enema kits and elastic bandages, the FDA says.
Mr. Grant told the meeting that in his career he has worked on two class III products and about 20 class II products. (He had previously worked at medical startups Synectics Medical and Neoventa, as well as established multinationals such as Medtronic.)
“I can tell you that – practically – the FDA has many fewer sticks and much less control when it comes to class II devices,” he said. He offered an example of a manufacturer of a class II device having more latitude in making small changes to products without notifying the FDA.
In his hypothetical example, such a change could have unintended consequences, and “with AI systems, small changes can result in large and nonlinear or even random effects,” Mr. Grant said. “But it’s too late if the product is on the market and the harm has already occurred,” he said.
The American Society for Dermatologic Surgery Association also protested the reclassifying of approved computer-aided melanoma detection class III devices.
In a statement posted on the FDA website as part of the materials for the meeting, the ASDSA raised a series of concerns about the prospects of expanded U.S. use of tools for assisting in diagnosing melanoma, including ones that would be marketed to consumers.
“To the extent that algorithms and devices for patient self-diagnosis of skin lesions are already widely available, they should be required to include detailed disclaimers that include that they are for entertainment and educational purposes and not a diagnostic device, that they are not approved by dermatologists or a recognized medical regulatory authority for self-diagnosis,” the ASDSA said.
Devices and algorithms in screening tools “are not highly regulated and remain unproven. They may result in wrong diagnoses, missed diagnoses, or over- or underdiagnosis,” the ASDSA added. “Both patients at low risk and those at high risk are better served by scheduling an in-person examination with a board-certified dermatologist, who can also help them determine the appropriate future skin screening schedule that is most appropriate for them.”
‘Stepping stone’
However, there is strong consumer demand for better information about skin conditions, and many patients face hurdles in going to dermatologists.
Google research has shown that consumers are seeking “a stepping stone” between the information they can easily find online and what they could get from a medical professional, said Lily Peng, MD, PhD, a director of product management for the health AI team at Google. Dr. Peng was a scheduled presenter at the July 29 meeting.
Consumers often are looking for more information on common conditions such as acne and poison ivy, and they sometimes face challenges in getting access to clinicians, she said.
“There are many unmet needs for consumers experiencing skin issues, many of which are lower-acuity conditions. There’s a big opportunity to increase accessibility and relevance of health journeys for consumers,” Dr. Peng said. “We have heard from consumers that they would like to have a self-help tool for nonserious conditions so they can decide when to seek medical attention.”
Dr. Peng’s presentation was not directly related to the question of class II or class III designation for existing products. Instead, her talk served as a glimpse into the work already underway in creating apps and tools for consumers.
Google researchers have published a number of studies in recent years about the use of AI to improve dermatology diagnosis.
A 2020 article reported on Google’s test of a form of AI known as deep learning system (DLS) to provide a differential diagnosis of skin conditions. On 963 validation cases, where a rotating panel of three board-certified dermatologists defined the reference standard, the DLS was noninferior to six other dermatologists and was superior to six primary care physicians (PCPs) and six nurse practitioners (NPs), according to a summary of the article.
A 2021 report published in JAMA Network Open said that use of an AI tool was associated with a higher agreement rate with dermatologists’ reference diagnoses for both PCPs and NPs.
In a 2021 blog post, Google scientists wrote that their AI model that powers a tool for checking skin conditions had earned European clearance, known as a CE mark, as a class I medical device.
SkinVision has an app that the company says “is available worldwide (with the exception of the USA and Canada).” The firm’s website includes a link where people in the United States and Canada can sign up for notifications about when SkinVision will be available in these nations.
‘Not ready for prime time’
The FDA panel did not cast formal votes at the July 29 meeting. Rather, the members engaged in broad discussions about risks and potential benefits of new tools for aiding in the detection of skin cancer.
Among the key issues discussed was a question of whether the FDA could impose requirements and restrictions, known as special controls, to provide “reasonable assurance of safety and effectiveness” for computer-aided devices that provide adjunctive diagnostic information to dermatologists about lesions suspicious for melanoma.
Among the potential special controls would be clinical performance testing in regards to rates of the sensitivity (true-positive rate) and specificity (true-negative rate).
The FDA could also look at requirements on software validation and verification and cybersecurity testing, as well as directions on labeling so as to mitigate risk.
Dermatologists serving on the panel called for caution in proceeding with steps that would make it easier for companies to market tools for aiding in melanoma diagnosis than it would be within the class III framework used for MelaFind and Nevisense.
Many expressed concerns about the need to design studies that would answer questions about how well new tools could accurately identify concerning lesions.
The phrase “not ready for prime time” was used at least three times during the discussion.
FDA panelist Maral Skelsey, MD, a skin cancer specialist from Chevy Chase, Maryland, said that over the years, she had used both Nevisense and MelaFind.
She said she had found MelaFind “unusable,” owing in large part to the high number of false positives it generated. The device also was limited as to where on patients’ bodies it could be used.
However, she spoke with enthusiasm about the prospects for better devices to aid in diagnosis of skin lesions. “It’s an area where we’re on the verge, and we really need these devices. There’s a need for patients to be able to examine themselves, for nondermatologists to be able to assess lesions,” Dr. Skelsey said.
But this field is “just not ready for prime time” yet, even with special controls, Dr. Skelsey said. To loosen approval standards too quickly could be a “detriment to what’s coming down the pipeline,” she said.
“It’s harmful to things that are likely to be around the corner,” she said.
FDA panelist Renata Block, PA-C, who works in a Chicago dermatology practice, pressed for maintaining a class III designation. “We are not ready for prime time yet, though the data that is coming down the pipeline on what we have is quite exciting,” Ms. Block said.
FDA panelist Karla V. Ballman, PhD, a statistician from Weill Cornell Medicine, New York, said there would need to be a clear standard for clinical performance before proceeding toward reclassification of devices for aid in detecting melanoma. “I just don’t think it’s ready for prime time at this point and should remain in class III,” she said.
But there was support from some panelists for the idea of a lower bar for clearance, combined with special controls to ensure patient safety.
In expressing her view, FDA panelist Katalin Roth, MD, JD, professor of medicine, George Washington University, Washington, said she was an outlier in her support for the agency’s view that these risks could be managed and that future tools could allow more patients to take a step on the pathway toward critical diagnoses.
“I deal with a lot of people with cancer as a palliative care physician,” Dr. Roth said. “I think what we’re missing here is the issue of time. Melanoma is a terrible disease, and missing the diagnosis is a terrible thing, but I think special controls would be sufficient to counter the concerns of my colleagues on the committee.”
The FDA’s Dr. Ashar ended the meeting with questions posed to one panelist, Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center in New York.
Dr. Rotemberg has for years been working in the field of research on developing AI and other computer-based tools for detecting and diagnosing melanoma, the deadliest form of skin cancer.
She has been publicly skeptical of the performance of commercial apps that scan moles and other lesions and that claim to identify which are cancerous. A May blog post on the Memorial Sloan Kettering website highlighted a recent British Journal of Dermatology article in which Dr. Rotemberg and coauthors reported on their evaluations of commercial apps. They judged them to be on average only 59% accurate, the blog post said.
However, during an earlier discussion at the meeting, she had spoken more positively about the prospects for using special controls in the near term to mitigate risk, although she said she would have a “very long list” of these requirements.
In the closing exchange with Dr. Ashar, Dr. Rotemberg outlined steps that could potentially ensure the safe use of tools to aid in melanoma screening. These included a need for postmarketing surveillance, which would require evaluation over time of algorithms used in tools meant to detect skin cancer.
“We need to have a mechanism for sampling,” Dr. Rotemberg said. “Most of our data is electronic now anyway, so comparing an algorithm and performance with biopsy results should not be that challenging.”
A version of this article first appeared on Medscape.com.
Skin-picking, hair-pulling disorders: Diagnostic criteria, prevalence, and treatment
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
AT SPD 2022
Banana Boat recalls scalp sunscreen spray
.
The company announced a voluntary recall for three batches of the Banana Boat Hair & Scalp Spray SPF 30, which came in 6-ounce bottles and was sold across the U.S. through various retailers and online, according to a recall alert by the Food and Drug Administration.
The three batches have a UPC label of 0-79656-04041-8 and fall under the lot codes 20016AF, 20084BF, and 21139AF, with the expiration dates of December 2022, February 2023, and April 2024, respectively.
“An internal review found that some samples of the product contained trace levels of benzene. While benzene is not an ingredient in any Banana Boat products, the review showed the unexpected levels of benzene came from the propellant that sprays the product out of the can,” according to the recall notice.
“Importantly, no other batches of Hair & Scalp (either before or after these batch codes) and no other Banana Boat products are in the scope of this recall and may continue to be used by consumers safely and as intended,” the company wrote.
Benzene is classified as a human carcinogen, the FDA wrote. Exposure to benzene can occur through the nose, mouth, and skin, and it can result in serious conditions such as leukemia, bone marrow cancer, and blood disorders.
“Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources,” the company said. “Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines.”
Edgewell said it hasn’t received any reports of bad events related to the recall. The company has told retailers to remove the affected batches from shelves.
Banana Boat will reimburse consumers who purchased a product with one of the affected lot codes, which are on the bottom of the can. In the meantime, consumers should stop using the affected product right away and discard it.
The recall comes a little over a year after Johnson & Johnson recalled five sunscreens due to low levels of benzene, according to The Associated Press. That recall included Aveeno and Neutrogena products in spray cans.
Consumers with questions about the recall can contact Edgewell Personal Care at 888-686-3988 Monday through Friday, 9 a.m. to 6 p.m. ET. People can also read more at the Banana Boat FAQ page or file for a refund directly on the Banana Boat Recall page.
A version of this article first appeared on WebMD.com.
.
The company announced a voluntary recall for three batches of the Banana Boat Hair & Scalp Spray SPF 30, which came in 6-ounce bottles and was sold across the U.S. through various retailers and online, according to a recall alert by the Food and Drug Administration.
The three batches have a UPC label of 0-79656-04041-8 and fall under the lot codes 20016AF, 20084BF, and 21139AF, with the expiration dates of December 2022, February 2023, and April 2024, respectively.
“An internal review found that some samples of the product contained trace levels of benzene. While benzene is not an ingredient in any Banana Boat products, the review showed the unexpected levels of benzene came from the propellant that sprays the product out of the can,” according to the recall notice.
“Importantly, no other batches of Hair & Scalp (either before or after these batch codes) and no other Banana Boat products are in the scope of this recall and may continue to be used by consumers safely and as intended,” the company wrote.
Benzene is classified as a human carcinogen, the FDA wrote. Exposure to benzene can occur through the nose, mouth, and skin, and it can result in serious conditions such as leukemia, bone marrow cancer, and blood disorders.
“Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources,” the company said. “Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines.”
Edgewell said it hasn’t received any reports of bad events related to the recall. The company has told retailers to remove the affected batches from shelves.
Banana Boat will reimburse consumers who purchased a product with one of the affected lot codes, which are on the bottom of the can. In the meantime, consumers should stop using the affected product right away and discard it.
The recall comes a little over a year after Johnson & Johnson recalled five sunscreens due to low levels of benzene, according to The Associated Press. That recall included Aveeno and Neutrogena products in spray cans.
Consumers with questions about the recall can contact Edgewell Personal Care at 888-686-3988 Monday through Friday, 9 a.m. to 6 p.m. ET. People can also read more at the Banana Boat FAQ page or file for a refund directly on the Banana Boat Recall page.
A version of this article first appeared on WebMD.com.
.
The company announced a voluntary recall for three batches of the Banana Boat Hair & Scalp Spray SPF 30, which came in 6-ounce bottles and was sold across the U.S. through various retailers and online, according to a recall alert by the Food and Drug Administration.
The three batches have a UPC label of 0-79656-04041-8 and fall under the lot codes 20016AF, 20084BF, and 21139AF, with the expiration dates of December 2022, February 2023, and April 2024, respectively.
“An internal review found that some samples of the product contained trace levels of benzene. While benzene is not an ingredient in any Banana Boat products, the review showed the unexpected levels of benzene came from the propellant that sprays the product out of the can,” according to the recall notice.
“Importantly, no other batches of Hair & Scalp (either before or after these batch codes) and no other Banana Boat products are in the scope of this recall and may continue to be used by consumers safely and as intended,” the company wrote.
Benzene is classified as a human carcinogen, the FDA wrote. Exposure to benzene can occur through the nose, mouth, and skin, and it can result in serious conditions such as leukemia, bone marrow cancer, and blood disorders.
“Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources,” the company said. “Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines.”
Edgewell said it hasn’t received any reports of bad events related to the recall. The company has told retailers to remove the affected batches from shelves.
Banana Boat will reimburse consumers who purchased a product with one of the affected lot codes, which are on the bottom of the can. In the meantime, consumers should stop using the affected product right away and discard it.
The recall comes a little over a year after Johnson & Johnson recalled five sunscreens due to low levels of benzene, according to The Associated Press. That recall included Aveeno and Neutrogena products in spray cans.
Consumers with questions about the recall can contact Edgewell Personal Care at 888-686-3988 Monday through Friday, 9 a.m. to 6 p.m. ET. People can also read more at the Banana Boat FAQ page or file for a refund directly on the Banana Boat Recall page.
A version of this article first appeared on WebMD.com.
Topical roflumilast approved for psoriasis in adults and adolescents
.
Roflumilast is a selective inhibitor of phosphodiesterase 4 (PDE4), the first approved for treating psoriasis, according to manufacturer Arcutis Biotherapeutics. The company announced the approval on July 29. Oral roflumilast (Daliresp ) was approved in 2011 for treating chronic obstructive pulmonary disease (COPD).“It’s a breakthrough topical therapy,” says Mark G. Lebwohl, MD, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, and principal investigator in trials of topical roflumilast. In an interview, Dr. Lebwohl noted that the treatment significantly reduced psoriasis symptoms in both short- and long-term trials.
In addition, two features of this treatment set it apart from other topical psoriasis treatments, he said. Roflumilast is not a steroid, so does not have the risk for topical steroid-related side effects associated with chronic use, and, in clinical trials, topical roflumilast was effective in treating psoriasis in intertriginous areas, including the buttocks, underarms, and beneath the breasts, which are difficult to treat.
FDA approval is based on data from two phase 3 randomized, double-blind, vehicle-controlled trials, according to Arcutis. The primary endpoint was Investigator Global Assessment (IGA) success, defined as clear or almost clear with at least a two-grade improvement from baseline, and at least a two-grade IGA score improvement from baseline at 8 weeks.
At 8 weeks, 42.4% and 37.5% of the patients treated with topical roflumilast achieved an IGA success rate compared with 6.1% and 6.9% in the control groups, respectively (P < .0001 for both studies).
Treated patients also experienced significant improvements compared with those in the vehicle groups in secondary endpoints in the trials: Those included Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index–75 (PASI-75), reductions in itch based on the Worst Itch–Numerical Rating Scale (WI-NRS), and self-reported psoriasis symptoms diary (PSD).
In the studies, 72% and 68% of patients treated with roflumilast met the I-IGA endpoint at 8 weeks versus 14% and 17%, respectively, of those on vehicle (P < .0001 for both studies).
In addition, by week 2, some participants treated with roflumilast had experienced reduced itchiness in both studies. At 8 weeks, among those with a WI-NRS score of 4 or more at baseline, 67% and 69% of the treated patients had at least a four-point reduction in the WI-NRS versus 26% and 33%, respectively, among those on vehicle (P < .0001 for both studies), according to the company.
In general, the cream was well tolerated. There were reports of diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), application-site pain (1%), upper respiratory tract infections (1%), and urinary tract infections (1%). However, Dr. Lebwohl noted that these events were also observed in the control group.
“The study was unequivocal about the improvement in the intertriginous sites,” Dr. Lebwohl said. He contrasts that to the data from other nonsteroidal topicals, which he said can be associated with a rash or irritation in sensitive areas.
Dr. Lebwohl noted that PDE4 is an enzyme that increases inflammation and decreases anti-inflammatory mediators and that inhibiting PDE4 may interrupt some of the inflammation response responsible for psoriasis symptoms, as it has for other conditions such as atopic dermatitis. Data from the 8-week phase 3 trials and yearlong phase 2b, open-label studies support that hypothesis.
“I’m always excited for new psoriasis treatments to broaden our treatment armamentarium,” said Lauren E. Ploch, MD, a dermatologist who practices in Augusta, Ga., and Aiken, S.C., who was asked to comment on the approval.
Even a symptom that seems benign, like itching, Dr. Ploch added, can lead to reduced sleep and increased irritability. Referring to the data on the treatment in the sensitive, intertriginous areas, she noted that the skin in these areas is often thinner, so treatment with steroids can cause further thinning and damage to the skin. If roflumilast doesn’t cause burning, itching, or thinning, it will be a great option to treat these areas, she said in an interview. She was not involved in the trials.
Roflumilast cream will be marketed under the trade name Zoryve, and is expected to be available by mid-August, according to Arcutis.
Roflumilast cream is also under review in Canada for treatment of plaque psoriasis in adults and adolescents.
The studies were funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving grant support and consulting fees from Arcutis. Dr. Ploch reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Roflumilast is a selective inhibitor of phosphodiesterase 4 (PDE4), the first approved for treating psoriasis, according to manufacturer Arcutis Biotherapeutics. The company announced the approval on July 29. Oral roflumilast (Daliresp ) was approved in 2011 for treating chronic obstructive pulmonary disease (COPD).“It’s a breakthrough topical therapy,” says Mark G. Lebwohl, MD, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, and principal investigator in trials of topical roflumilast. In an interview, Dr. Lebwohl noted that the treatment significantly reduced psoriasis symptoms in both short- and long-term trials.
In addition, two features of this treatment set it apart from other topical psoriasis treatments, he said. Roflumilast is not a steroid, so does not have the risk for topical steroid-related side effects associated with chronic use, and, in clinical trials, topical roflumilast was effective in treating psoriasis in intertriginous areas, including the buttocks, underarms, and beneath the breasts, which are difficult to treat.
FDA approval is based on data from two phase 3 randomized, double-blind, vehicle-controlled trials, according to Arcutis. The primary endpoint was Investigator Global Assessment (IGA) success, defined as clear or almost clear with at least a two-grade improvement from baseline, and at least a two-grade IGA score improvement from baseline at 8 weeks.
At 8 weeks, 42.4% and 37.5% of the patients treated with topical roflumilast achieved an IGA success rate compared with 6.1% and 6.9% in the control groups, respectively (P < .0001 for both studies).
Treated patients also experienced significant improvements compared with those in the vehicle groups in secondary endpoints in the trials: Those included Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index–75 (PASI-75), reductions in itch based on the Worst Itch–Numerical Rating Scale (WI-NRS), and self-reported psoriasis symptoms diary (PSD).
In the studies, 72% and 68% of patients treated with roflumilast met the I-IGA endpoint at 8 weeks versus 14% and 17%, respectively, of those on vehicle (P < .0001 for both studies).
In addition, by week 2, some participants treated with roflumilast had experienced reduced itchiness in both studies. At 8 weeks, among those with a WI-NRS score of 4 or more at baseline, 67% and 69% of the treated patients had at least a four-point reduction in the WI-NRS versus 26% and 33%, respectively, among those on vehicle (P < .0001 for both studies), according to the company.
In general, the cream was well tolerated. There were reports of diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), application-site pain (1%), upper respiratory tract infections (1%), and urinary tract infections (1%). However, Dr. Lebwohl noted that these events were also observed in the control group.
“The study was unequivocal about the improvement in the intertriginous sites,” Dr. Lebwohl said. He contrasts that to the data from other nonsteroidal topicals, which he said can be associated with a rash or irritation in sensitive areas.
Dr. Lebwohl noted that PDE4 is an enzyme that increases inflammation and decreases anti-inflammatory mediators and that inhibiting PDE4 may interrupt some of the inflammation response responsible for psoriasis symptoms, as it has for other conditions such as atopic dermatitis. Data from the 8-week phase 3 trials and yearlong phase 2b, open-label studies support that hypothesis.
“I’m always excited for new psoriasis treatments to broaden our treatment armamentarium,” said Lauren E. Ploch, MD, a dermatologist who practices in Augusta, Ga., and Aiken, S.C., who was asked to comment on the approval.
Even a symptom that seems benign, like itching, Dr. Ploch added, can lead to reduced sleep and increased irritability. Referring to the data on the treatment in the sensitive, intertriginous areas, she noted that the skin in these areas is often thinner, so treatment with steroids can cause further thinning and damage to the skin. If roflumilast doesn’t cause burning, itching, or thinning, it will be a great option to treat these areas, she said in an interview. She was not involved in the trials.
Roflumilast cream will be marketed under the trade name Zoryve, and is expected to be available by mid-August, according to Arcutis.
Roflumilast cream is also under review in Canada for treatment of plaque psoriasis in adults and adolescents.
The studies were funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving grant support and consulting fees from Arcutis. Dr. Ploch reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Roflumilast is a selective inhibitor of phosphodiesterase 4 (PDE4), the first approved for treating psoriasis, according to manufacturer Arcutis Biotherapeutics. The company announced the approval on July 29. Oral roflumilast (Daliresp ) was approved in 2011 for treating chronic obstructive pulmonary disease (COPD).“It’s a breakthrough topical therapy,” says Mark G. Lebwohl, MD, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, and principal investigator in trials of topical roflumilast. In an interview, Dr. Lebwohl noted that the treatment significantly reduced psoriasis symptoms in both short- and long-term trials.
In addition, two features of this treatment set it apart from other topical psoriasis treatments, he said. Roflumilast is not a steroid, so does not have the risk for topical steroid-related side effects associated with chronic use, and, in clinical trials, topical roflumilast was effective in treating psoriasis in intertriginous areas, including the buttocks, underarms, and beneath the breasts, which are difficult to treat.
FDA approval is based on data from two phase 3 randomized, double-blind, vehicle-controlled trials, according to Arcutis. The primary endpoint was Investigator Global Assessment (IGA) success, defined as clear or almost clear with at least a two-grade improvement from baseline, and at least a two-grade IGA score improvement from baseline at 8 weeks.
At 8 weeks, 42.4% and 37.5% of the patients treated with topical roflumilast achieved an IGA success rate compared with 6.1% and 6.9% in the control groups, respectively (P < .0001 for both studies).
Treated patients also experienced significant improvements compared with those in the vehicle groups in secondary endpoints in the trials: Those included Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index–75 (PASI-75), reductions in itch based on the Worst Itch–Numerical Rating Scale (WI-NRS), and self-reported psoriasis symptoms diary (PSD).
In the studies, 72% and 68% of patients treated with roflumilast met the I-IGA endpoint at 8 weeks versus 14% and 17%, respectively, of those on vehicle (P < .0001 for both studies).
In addition, by week 2, some participants treated with roflumilast had experienced reduced itchiness in both studies. At 8 weeks, among those with a WI-NRS score of 4 or more at baseline, 67% and 69% of the treated patients had at least a four-point reduction in the WI-NRS versus 26% and 33%, respectively, among those on vehicle (P < .0001 for both studies), according to the company.
In general, the cream was well tolerated. There were reports of diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), application-site pain (1%), upper respiratory tract infections (1%), and urinary tract infections (1%). However, Dr. Lebwohl noted that these events were also observed in the control group.
“The study was unequivocal about the improvement in the intertriginous sites,” Dr. Lebwohl said. He contrasts that to the data from other nonsteroidal topicals, which he said can be associated with a rash or irritation in sensitive areas.
Dr. Lebwohl noted that PDE4 is an enzyme that increases inflammation and decreases anti-inflammatory mediators and that inhibiting PDE4 may interrupt some of the inflammation response responsible for psoriasis symptoms, as it has for other conditions such as atopic dermatitis. Data from the 8-week phase 3 trials and yearlong phase 2b, open-label studies support that hypothesis.
“I’m always excited for new psoriasis treatments to broaden our treatment armamentarium,” said Lauren E. Ploch, MD, a dermatologist who practices in Augusta, Ga., and Aiken, S.C., who was asked to comment on the approval.
Even a symptom that seems benign, like itching, Dr. Ploch added, can lead to reduced sleep and increased irritability. Referring to the data on the treatment in the sensitive, intertriginous areas, she noted that the skin in these areas is often thinner, so treatment with steroids can cause further thinning and damage to the skin. If roflumilast doesn’t cause burning, itching, or thinning, it will be a great option to treat these areas, she said in an interview. She was not involved in the trials.
Roflumilast cream will be marketed under the trade name Zoryve, and is expected to be available by mid-August, according to Arcutis.
Roflumilast cream is also under review in Canada for treatment of plaque psoriasis in adults and adolescents.
The studies were funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving grant support and consulting fees from Arcutis. Dr. Ploch reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA panel urges caution with skin cancer–detecting tools
A and how to address longstanding issues of racial equity in this field of medicine.
The Food and Drug Administration has scheduled two meetings to gather expert feedback about managing an expected expansion in the use of skin lesion apps and devices. Outside of the United States, there are apps promoted as being able to help spot skin lesions that should trigger a medical visit.
The general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee began work on this topic on July 28, with a wide-ranging discussion about potential expanded use of computer-aided, skin lesion analyzer (SLA) devices. On Friday, the panel is considering an FDA proposal to shift the designation for an approved device for aiding dermatologists in skin cancer diagnoses from the most stringent regulatory category, class III, to the less restrictive class II.
The FDA called the meeting amid growing interest in using technology to aid in finding cancers, with some of these products already marketed to consumers outside of the United States. There are presently no legally marketed, FDA-cleared or FDA-approved SLA devices indicated for use by clinicians other than dermatologists or the lay public, the agency said in a briefing memo for the meeting. There are two devices with FDA approval, though, for aiding dermatologists. The FDA approved SciBase’s Nevisense in 2017 and Mela Sciences’ MelaFind, which has fallen out of use, in 2012. Both are class III devices.
But some companies intend to offer products for consumers in the United States. The company SkinVision, for example, has developed an app of the same name, which is intended to detect suspicious-looking skin spots via smartphone photos. SkinVision’s website says the product has been offered to consumers in Australia for remote skin checks since 2015. People in the Netherlands and United Kingdom also can use SkinVision, according to the company’s website. SkinVision says the company is working on providing the app for U.S. customers, “but we are not quite there yet.”
During the meeting, FDA panelists repeatedly emphasized the potential risks of these devices in terms of sensitivity (how often a test correctly generates a positive result) and of specificity (how often a test correctly generates a negative result).
New tools intended to aid in detection of skin cancer might produce too many false positives and thus trigger floods of worried patients seeking care and often facing unnecessary biopsies, the FDA panelists said. But more worrisome would be FDA clearance of tools that delivered too many false negative results, leaving people unaware of their cancers.
The standards would have to be set very high for new products, especially those intended for consumers, said FDA panelist Murad Alam, MD, a dermatologist and vice chair of the department of dermatology at Northwestern University, Chicago. Current technologies for analyzing skin lesions are not yet up to that task. Dr. Alam likened the situation to the hopes for self-driving cars.
“It sounds great in principle. If you read the predictions from 20 years ago, it should already have happened,” Dr. Alam said. “But we’re still struggling with that because there are serious points of failure.”
FDA panelist Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York, also argued for well-designed studies to understand how consumers and clinicians would react to new tools.
“We have to define what prospective information, in the intended use setting, we need to feel comfortable saying that these tools could be in a layperson’s hand or a primary care person’s hand,” Dr. Rotemberg said.
The studies would not need to be large, especially in the case of nonmelanoma skin cancer, which is common, she added.
“There’s too much nuance here for us to be able to say: ‘This is what would happen,’ without testing it,” Dr. Rotemberg said. “I do not think these prospective studies would be very burdensome, but they would help us understand what the burden would be and what the costs would be and what the potential harms would be.”
Because of rules against disclosing corporate information, the FDA cannot tell the public about the kinds of inquiries it already may have fielded from companies interested in selling skin cancer detection tools.
But in response to a question during the FDA meeting, Binita Ashar, MD, a top official in the FDA’s Center for Devices and Radiological Health, said there is interest in having these kinds of products sold in the United States as well.
“I can tell you that this a very timely discussion and questions that we’re posing to you are the questions that we’re encountering or that we have been grappling with,” Dr. Ashar said.
FDA panelists noted that many patients cannot get access easily to dermatology visits.
Companies seeking to develop SLA devices likely will market their tools as attempts to fill a gap that now exists in medical care.
But there will be challenges ahead in explaining to patients how to interpret readings from these tools, the FDA panelists said. Consumers should know these tools are meant to assist in diagnosis, and not to make it.
“I’m not sure the layperson will hear that,” said FDA panelist Paula E. Bourelly, MD, a dermatologist from Olney, Md.
As a result, use of SLA tools could create tension between physicians and patients, with consumers demanding biopsies after seeing readings they don’t understand.
“I do have great concerns about the layperson feeling overly confident and reducing the provider to a technician,” she said.
The FDA panelists were not asked to cast formal votes on any issues discussed during the meeting They instead engaged in broad discussions around questions posed by the FDA in three key areas:
- What standards should be used to confirm lesion diagnosis in clinical testing of the accuracy of SLA devices?
- What would be acceptable true false-positive and false-negative results (sensitivity and specificity) for different diagnoses and users?
- How can the FDA address health equity considerations based on variable incidence of skin lesions?
Developing standards
The FDA asked the panel to consider several scenarios for SLA devices and to discuss how standards might vary depending on the user of the device, whether it would be dermatologists, other clinicians, or consumers.
The agency sought comments in particular about using histological diagnosis (core specimen processing with a consensus diagnosis from an expert dermatopathologist panel). In the briefing document for the meeting, the FDA argued that this approach provides the greatest certainty in the diagnosis.
“Device developers, however, cite concerns, both practical and ethical, in requiring biopsy of all lesions, particularly those that appear benign,” the FDA said. “They have proposed alternate means of defining ground truth, including consensus opinion of experts (of visual or dermoscopic examination of the lesion[s]), opinion of one expert (visual or dermoscopic examination), or other methods.”
In summarizing the discussion on this question, the FDA panel chairman, Hobart W. Harris, MD, MPH, a surgeon from the University of California, San Francisco, noted that there was broad support for histological data in clinical trials of SLA devices, with some allowance for cases where more hybrid approaches would be used.
There were also suggestions offered about designing trials and the need for biopsies of lesions that are clearly benign, as this would help gather data to help in developing algorithms.
Dr. Alam said care should be taken in explaining to study participants that they might have to undergo biopsies that they didn’t need, as part of the larger effort to gather data. This should be detailed in the consent form, he said.
“But I also think this is a relatively minor risk,” Dr. Alam said, comparing these biopsies to the blood samples that patients in many clinical studies routinely give.
“Are all of those blood draws necessary to track the change in whatever parameters that are being tracked? Probably not,” Dr. Alam said. “I think it would be possible to explain to a reasonable patient what this entails.”
Dr. Alam noted that companies might face extra hurdles in enrolling study participants and keeping them in the trials if the FDA seeks this kind of biopsy data. “But I don’t think inconvenience to the study sponsor is a good argument” for not seeking this kind of data, he added.
Leaving a loophole where certain kinds of clearly benign lesions don’t require a biopsy would eventually erode the quality of the research done on these devices. “That bar will be moved to accommodate the convenience of the sponsor, to make the study feasible,” Dr. Alam said. “And pretty soon, you’ll be missing a lot of patients that really should have biopsies.”
Acceptable rates of false positives, false negatives
The FDA panel chair noted that his colleagues had strongly urged review standards that would require that the devices improve on the rates of successful catches of suspicious lesions and lower false positives. But they did not endorse specific targets regarding the sensitivity and specificity rates.
“No one seems to be comfortable with providing or preordaining” these targets, Dr. Harris said.
Panelist Deneen Hesser, MSHSA, RN, urged a deep recognition of the power of a FDA clearance in the view of consumers.
“We need to be cognizant of what the term ‘FDA approved’ means to the lay individual,” said Ms. Hesser, who served as the patient representative on the panel. “A patient who sees that those tools are FDA approved will assume that each of those is the gold standard” in terms of expectations for delivering accurate results.
Like many of the panelists, Dr. Rotemberg urged the FDA to gather data about how patients would react to different messages encoded in consumer-oriented products.
“If the device says: ‘You should see a dermatologist for this’ and no other information, that’s very different from [saying]: ‘That lesion is suspicious for melanoma,’ ” Dr. Rotemberg said.
Despite the likely difficulties in conducting trials, the FDA needs to have the data to answer key questions about patient and physician reactions to readings from new tools, Dr. Rotemberg said.
“We don’t know how many additional biopsies we would cause with a specificity of 80%” for a new SLA tool, Dr. Rotemberg said, giving an example. “We don’t know how confident a dermatologist might be to say: ‘Actually, I’m not suspicious about that lesion and we can just fudge it or not biopsy it.’ We don’t know any of that until we study it in real life.”
The panelists also urged the FDA to seek to ensure that new tools used in analyzing skin lesions improve the quality of diagnosis.
Addressing equity
The FDA also asked the panel to weigh in on whether the agency should clear SLA tools in cases where the existing study data is drawn heavily from people considered to be at higher risk for skin cancer.
“To ensure generalizability across the entire U.S. population, should FDA require SLAs indicated for use beyond cancerous lesions be tested in a representative U.S. population?” the FDA asked.
The three most common skin cancers – melanoma, basal cell carcinoma, and squamous cell carcinoma – are more prevalent in people with Fitzpatrick I and II skin types, who tend to get sunburns, not tans. But people of color are more likely to develop melanoma in areas that are not sun exposed, such as the sole of the foot or under fingernails or toenails.
“Due in part to lower expected risk and screening, these melanomas are often detected late,” the FDA said in the briefing document.
There was broad consensus among panelists that the FDA should encourage companies to enroll people with all skin types and tones.
But they also looked for ways that the FDA could clear devices based on initial studies conducted largely with people considered to be at higher risk, with the agency then requiring follow-up trials to see how these products would work for the general U.S. population.
A version of this article first appeared on Medscape.com.
A and how to address longstanding issues of racial equity in this field of medicine.
The Food and Drug Administration has scheduled two meetings to gather expert feedback about managing an expected expansion in the use of skin lesion apps and devices. Outside of the United States, there are apps promoted as being able to help spot skin lesions that should trigger a medical visit.
The general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee began work on this topic on July 28, with a wide-ranging discussion about potential expanded use of computer-aided, skin lesion analyzer (SLA) devices. On Friday, the panel is considering an FDA proposal to shift the designation for an approved device for aiding dermatologists in skin cancer diagnoses from the most stringent regulatory category, class III, to the less restrictive class II.
The FDA called the meeting amid growing interest in using technology to aid in finding cancers, with some of these products already marketed to consumers outside of the United States. There are presently no legally marketed, FDA-cleared or FDA-approved SLA devices indicated for use by clinicians other than dermatologists or the lay public, the agency said in a briefing memo for the meeting. There are two devices with FDA approval, though, for aiding dermatologists. The FDA approved SciBase’s Nevisense in 2017 and Mela Sciences’ MelaFind, which has fallen out of use, in 2012. Both are class III devices.
But some companies intend to offer products for consumers in the United States. The company SkinVision, for example, has developed an app of the same name, which is intended to detect suspicious-looking skin spots via smartphone photos. SkinVision’s website says the product has been offered to consumers in Australia for remote skin checks since 2015. People in the Netherlands and United Kingdom also can use SkinVision, according to the company’s website. SkinVision says the company is working on providing the app for U.S. customers, “but we are not quite there yet.”
During the meeting, FDA panelists repeatedly emphasized the potential risks of these devices in terms of sensitivity (how often a test correctly generates a positive result) and of specificity (how often a test correctly generates a negative result).
New tools intended to aid in detection of skin cancer might produce too many false positives and thus trigger floods of worried patients seeking care and often facing unnecessary biopsies, the FDA panelists said. But more worrisome would be FDA clearance of tools that delivered too many false negative results, leaving people unaware of their cancers.
The standards would have to be set very high for new products, especially those intended for consumers, said FDA panelist Murad Alam, MD, a dermatologist and vice chair of the department of dermatology at Northwestern University, Chicago. Current technologies for analyzing skin lesions are not yet up to that task. Dr. Alam likened the situation to the hopes for self-driving cars.
“It sounds great in principle. If you read the predictions from 20 years ago, it should already have happened,” Dr. Alam said. “But we’re still struggling with that because there are serious points of failure.”
FDA panelist Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York, also argued for well-designed studies to understand how consumers and clinicians would react to new tools.
“We have to define what prospective information, in the intended use setting, we need to feel comfortable saying that these tools could be in a layperson’s hand or a primary care person’s hand,” Dr. Rotemberg said.
The studies would not need to be large, especially in the case of nonmelanoma skin cancer, which is common, she added.
“There’s too much nuance here for us to be able to say: ‘This is what would happen,’ without testing it,” Dr. Rotemberg said. “I do not think these prospective studies would be very burdensome, but they would help us understand what the burden would be and what the costs would be and what the potential harms would be.”
Because of rules against disclosing corporate information, the FDA cannot tell the public about the kinds of inquiries it already may have fielded from companies interested in selling skin cancer detection tools.
But in response to a question during the FDA meeting, Binita Ashar, MD, a top official in the FDA’s Center for Devices and Radiological Health, said there is interest in having these kinds of products sold in the United States as well.
“I can tell you that this a very timely discussion and questions that we’re posing to you are the questions that we’re encountering or that we have been grappling with,” Dr. Ashar said.
FDA panelists noted that many patients cannot get access easily to dermatology visits.
Companies seeking to develop SLA devices likely will market their tools as attempts to fill a gap that now exists in medical care.
But there will be challenges ahead in explaining to patients how to interpret readings from these tools, the FDA panelists said. Consumers should know these tools are meant to assist in diagnosis, and not to make it.
“I’m not sure the layperson will hear that,” said FDA panelist Paula E. Bourelly, MD, a dermatologist from Olney, Md.
As a result, use of SLA tools could create tension between physicians and patients, with consumers demanding biopsies after seeing readings they don’t understand.
“I do have great concerns about the layperson feeling overly confident and reducing the provider to a technician,” she said.
The FDA panelists were not asked to cast formal votes on any issues discussed during the meeting They instead engaged in broad discussions around questions posed by the FDA in three key areas:
- What standards should be used to confirm lesion diagnosis in clinical testing of the accuracy of SLA devices?
- What would be acceptable true false-positive and false-negative results (sensitivity and specificity) for different diagnoses and users?
- How can the FDA address health equity considerations based on variable incidence of skin lesions?
Developing standards
The FDA asked the panel to consider several scenarios for SLA devices and to discuss how standards might vary depending on the user of the device, whether it would be dermatologists, other clinicians, or consumers.
The agency sought comments in particular about using histological diagnosis (core specimen processing with a consensus diagnosis from an expert dermatopathologist panel). In the briefing document for the meeting, the FDA argued that this approach provides the greatest certainty in the diagnosis.
“Device developers, however, cite concerns, both practical and ethical, in requiring biopsy of all lesions, particularly those that appear benign,” the FDA said. “They have proposed alternate means of defining ground truth, including consensus opinion of experts (of visual or dermoscopic examination of the lesion[s]), opinion of one expert (visual or dermoscopic examination), or other methods.”
In summarizing the discussion on this question, the FDA panel chairman, Hobart W. Harris, MD, MPH, a surgeon from the University of California, San Francisco, noted that there was broad support for histological data in clinical trials of SLA devices, with some allowance for cases where more hybrid approaches would be used.
There were also suggestions offered about designing trials and the need for biopsies of lesions that are clearly benign, as this would help gather data to help in developing algorithms.
Dr. Alam said care should be taken in explaining to study participants that they might have to undergo biopsies that they didn’t need, as part of the larger effort to gather data. This should be detailed in the consent form, he said.
“But I also think this is a relatively minor risk,” Dr. Alam said, comparing these biopsies to the blood samples that patients in many clinical studies routinely give.
“Are all of those blood draws necessary to track the change in whatever parameters that are being tracked? Probably not,” Dr. Alam said. “I think it would be possible to explain to a reasonable patient what this entails.”
Dr. Alam noted that companies might face extra hurdles in enrolling study participants and keeping them in the trials if the FDA seeks this kind of biopsy data. “But I don’t think inconvenience to the study sponsor is a good argument” for not seeking this kind of data, he added.
Leaving a loophole where certain kinds of clearly benign lesions don’t require a biopsy would eventually erode the quality of the research done on these devices. “That bar will be moved to accommodate the convenience of the sponsor, to make the study feasible,” Dr. Alam said. “And pretty soon, you’ll be missing a lot of patients that really should have biopsies.”
Acceptable rates of false positives, false negatives
The FDA panel chair noted that his colleagues had strongly urged review standards that would require that the devices improve on the rates of successful catches of suspicious lesions and lower false positives. But they did not endorse specific targets regarding the sensitivity and specificity rates.
“No one seems to be comfortable with providing or preordaining” these targets, Dr. Harris said.
Panelist Deneen Hesser, MSHSA, RN, urged a deep recognition of the power of a FDA clearance in the view of consumers.
“We need to be cognizant of what the term ‘FDA approved’ means to the lay individual,” said Ms. Hesser, who served as the patient representative on the panel. “A patient who sees that those tools are FDA approved will assume that each of those is the gold standard” in terms of expectations for delivering accurate results.
Like many of the panelists, Dr. Rotemberg urged the FDA to gather data about how patients would react to different messages encoded in consumer-oriented products.
“If the device says: ‘You should see a dermatologist for this’ and no other information, that’s very different from [saying]: ‘That lesion is suspicious for melanoma,’ ” Dr. Rotemberg said.
Despite the likely difficulties in conducting trials, the FDA needs to have the data to answer key questions about patient and physician reactions to readings from new tools, Dr. Rotemberg said.
“We don’t know how many additional biopsies we would cause with a specificity of 80%” for a new SLA tool, Dr. Rotemberg said, giving an example. “We don’t know how confident a dermatologist might be to say: ‘Actually, I’m not suspicious about that lesion and we can just fudge it or not biopsy it.’ We don’t know any of that until we study it in real life.”
The panelists also urged the FDA to seek to ensure that new tools used in analyzing skin lesions improve the quality of diagnosis.
Addressing equity
The FDA also asked the panel to weigh in on whether the agency should clear SLA tools in cases where the existing study data is drawn heavily from people considered to be at higher risk for skin cancer.
“To ensure generalizability across the entire U.S. population, should FDA require SLAs indicated for use beyond cancerous lesions be tested in a representative U.S. population?” the FDA asked.
The three most common skin cancers – melanoma, basal cell carcinoma, and squamous cell carcinoma – are more prevalent in people with Fitzpatrick I and II skin types, who tend to get sunburns, not tans. But people of color are more likely to develop melanoma in areas that are not sun exposed, such as the sole of the foot or under fingernails or toenails.
“Due in part to lower expected risk and screening, these melanomas are often detected late,” the FDA said in the briefing document.
There was broad consensus among panelists that the FDA should encourage companies to enroll people with all skin types and tones.
But they also looked for ways that the FDA could clear devices based on initial studies conducted largely with people considered to be at higher risk, with the agency then requiring follow-up trials to see how these products would work for the general U.S. population.
A version of this article first appeared on Medscape.com.
A and how to address longstanding issues of racial equity in this field of medicine.
The Food and Drug Administration has scheduled two meetings to gather expert feedback about managing an expected expansion in the use of skin lesion apps and devices. Outside of the United States, there are apps promoted as being able to help spot skin lesions that should trigger a medical visit.
The general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee began work on this topic on July 28, with a wide-ranging discussion about potential expanded use of computer-aided, skin lesion analyzer (SLA) devices. On Friday, the panel is considering an FDA proposal to shift the designation for an approved device for aiding dermatologists in skin cancer diagnoses from the most stringent regulatory category, class III, to the less restrictive class II.
The FDA called the meeting amid growing interest in using technology to aid in finding cancers, with some of these products already marketed to consumers outside of the United States. There are presently no legally marketed, FDA-cleared or FDA-approved SLA devices indicated for use by clinicians other than dermatologists or the lay public, the agency said in a briefing memo for the meeting. There are two devices with FDA approval, though, for aiding dermatologists. The FDA approved SciBase’s Nevisense in 2017 and Mela Sciences’ MelaFind, which has fallen out of use, in 2012. Both are class III devices.
But some companies intend to offer products for consumers in the United States. The company SkinVision, for example, has developed an app of the same name, which is intended to detect suspicious-looking skin spots via smartphone photos. SkinVision’s website says the product has been offered to consumers in Australia for remote skin checks since 2015. People in the Netherlands and United Kingdom also can use SkinVision, according to the company’s website. SkinVision says the company is working on providing the app for U.S. customers, “but we are not quite there yet.”
During the meeting, FDA panelists repeatedly emphasized the potential risks of these devices in terms of sensitivity (how often a test correctly generates a positive result) and of specificity (how often a test correctly generates a negative result).
New tools intended to aid in detection of skin cancer might produce too many false positives and thus trigger floods of worried patients seeking care and often facing unnecessary biopsies, the FDA panelists said. But more worrisome would be FDA clearance of tools that delivered too many false negative results, leaving people unaware of their cancers.
The standards would have to be set very high for new products, especially those intended for consumers, said FDA panelist Murad Alam, MD, a dermatologist and vice chair of the department of dermatology at Northwestern University, Chicago. Current technologies for analyzing skin lesions are not yet up to that task. Dr. Alam likened the situation to the hopes for self-driving cars.
“It sounds great in principle. If you read the predictions from 20 years ago, it should already have happened,” Dr. Alam said. “But we’re still struggling with that because there are serious points of failure.”
FDA panelist Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York, also argued for well-designed studies to understand how consumers and clinicians would react to new tools.
“We have to define what prospective information, in the intended use setting, we need to feel comfortable saying that these tools could be in a layperson’s hand or a primary care person’s hand,” Dr. Rotemberg said.
The studies would not need to be large, especially in the case of nonmelanoma skin cancer, which is common, she added.
“There’s too much nuance here for us to be able to say: ‘This is what would happen,’ without testing it,” Dr. Rotemberg said. “I do not think these prospective studies would be very burdensome, but they would help us understand what the burden would be and what the costs would be and what the potential harms would be.”
Because of rules against disclosing corporate information, the FDA cannot tell the public about the kinds of inquiries it already may have fielded from companies interested in selling skin cancer detection tools.
But in response to a question during the FDA meeting, Binita Ashar, MD, a top official in the FDA’s Center for Devices and Radiological Health, said there is interest in having these kinds of products sold in the United States as well.
“I can tell you that this a very timely discussion and questions that we’re posing to you are the questions that we’re encountering or that we have been grappling with,” Dr. Ashar said.
FDA panelists noted that many patients cannot get access easily to dermatology visits.
Companies seeking to develop SLA devices likely will market their tools as attempts to fill a gap that now exists in medical care.
But there will be challenges ahead in explaining to patients how to interpret readings from these tools, the FDA panelists said. Consumers should know these tools are meant to assist in diagnosis, and not to make it.
“I’m not sure the layperson will hear that,” said FDA panelist Paula E. Bourelly, MD, a dermatologist from Olney, Md.
As a result, use of SLA tools could create tension between physicians and patients, with consumers demanding biopsies after seeing readings they don’t understand.
“I do have great concerns about the layperson feeling overly confident and reducing the provider to a technician,” she said.
The FDA panelists were not asked to cast formal votes on any issues discussed during the meeting They instead engaged in broad discussions around questions posed by the FDA in three key areas:
- What standards should be used to confirm lesion diagnosis in clinical testing of the accuracy of SLA devices?
- What would be acceptable true false-positive and false-negative results (sensitivity and specificity) for different diagnoses and users?
- How can the FDA address health equity considerations based on variable incidence of skin lesions?
Developing standards
The FDA asked the panel to consider several scenarios for SLA devices and to discuss how standards might vary depending on the user of the device, whether it would be dermatologists, other clinicians, or consumers.
The agency sought comments in particular about using histological diagnosis (core specimen processing with a consensus diagnosis from an expert dermatopathologist panel). In the briefing document for the meeting, the FDA argued that this approach provides the greatest certainty in the diagnosis.
“Device developers, however, cite concerns, both practical and ethical, in requiring biopsy of all lesions, particularly those that appear benign,” the FDA said. “They have proposed alternate means of defining ground truth, including consensus opinion of experts (of visual or dermoscopic examination of the lesion[s]), opinion of one expert (visual or dermoscopic examination), or other methods.”
In summarizing the discussion on this question, the FDA panel chairman, Hobart W. Harris, MD, MPH, a surgeon from the University of California, San Francisco, noted that there was broad support for histological data in clinical trials of SLA devices, with some allowance for cases where more hybrid approaches would be used.
There were also suggestions offered about designing trials and the need for biopsies of lesions that are clearly benign, as this would help gather data to help in developing algorithms.
Dr. Alam said care should be taken in explaining to study participants that they might have to undergo biopsies that they didn’t need, as part of the larger effort to gather data. This should be detailed in the consent form, he said.
“But I also think this is a relatively minor risk,” Dr. Alam said, comparing these biopsies to the blood samples that patients in many clinical studies routinely give.
“Are all of those blood draws necessary to track the change in whatever parameters that are being tracked? Probably not,” Dr. Alam said. “I think it would be possible to explain to a reasonable patient what this entails.”
Dr. Alam noted that companies might face extra hurdles in enrolling study participants and keeping them in the trials if the FDA seeks this kind of biopsy data. “But I don’t think inconvenience to the study sponsor is a good argument” for not seeking this kind of data, he added.
Leaving a loophole where certain kinds of clearly benign lesions don’t require a biopsy would eventually erode the quality of the research done on these devices. “That bar will be moved to accommodate the convenience of the sponsor, to make the study feasible,” Dr. Alam said. “And pretty soon, you’ll be missing a lot of patients that really should have biopsies.”
Acceptable rates of false positives, false negatives
The FDA panel chair noted that his colleagues had strongly urged review standards that would require that the devices improve on the rates of successful catches of suspicious lesions and lower false positives. But they did not endorse specific targets regarding the sensitivity and specificity rates.
“No one seems to be comfortable with providing or preordaining” these targets, Dr. Harris said.
Panelist Deneen Hesser, MSHSA, RN, urged a deep recognition of the power of a FDA clearance in the view of consumers.
“We need to be cognizant of what the term ‘FDA approved’ means to the lay individual,” said Ms. Hesser, who served as the patient representative on the panel. “A patient who sees that those tools are FDA approved will assume that each of those is the gold standard” in terms of expectations for delivering accurate results.
Like many of the panelists, Dr. Rotemberg urged the FDA to gather data about how patients would react to different messages encoded in consumer-oriented products.
“If the device says: ‘You should see a dermatologist for this’ and no other information, that’s very different from [saying]: ‘That lesion is suspicious for melanoma,’ ” Dr. Rotemberg said.
Despite the likely difficulties in conducting trials, the FDA needs to have the data to answer key questions about patient and physician reactions to readings from new tools, Dr. Rotemberg said.
“We don’t know how many additional biopsies we would cause with a specificity of 80%” for a new SLA tool, Dr. Rotemberg said, giving an example. “We don’t know how confident a dermatologist might be to say: ‘Actually, I’m not suspicious about that lesion and we can just fudge it or not biopsy it.’ We don’t know any of that until we study it in real life.”
The panelists also urged the FDA to seek to ensure that new tools used in analyzing skin lesions improve the quality of diagnosis.
Addressing equity
The FDA also asked the panel to weigh in on whether the agency should clear SLA tools in cases where the existing study data is drawn heavily from people considered to be at higher risk for skin cancer.
“To ensure generalizability across the entire U.S. population, should FDA require SLAs indicated for use beyond cancerous lesions be tested in a representative U.S. population?” the FDA asked.
The three most common skin cancers – melanoma, basal cell carcinoma, and squamous cell carcinoma – are more prevalent in people with Fitzpatrick I and II skin types, who tend to get sunburns, not tans. But people of color are more likely to develop melanoma in areas that are not sun exposed, such as the sole of the foot or under fingernails or toenails.
“Due in part to lower expected risk and screening, these melanomas are often detected late,” the FDA said in the briefing document.
There was broad consensus among panelists that the FDA should encourage companies to enroll people with all skin types and tones.
But they also looked for ways that the FDA could clear devices based on initial studies conducted largely with people considered to be at higher risk, with the agency then requiring follow-up trials to see how these products would work for the general U.S. population.
A version of this article first appeared on Medscape.com.
Avoiding harm in the diagnosis and treatment of food allergies
INDIANAPOLIS – If there’s one truth that David R. Stukus, MD, has come to realize from his 2 years as director of a food allergy treatment center, it’s that
“When they’re given a diagnosis of food allergy, many families do not receive proper education to help them understand the risk as well as self-management and prognosis,” he said at the annual meeting of the Society for Pediatric Dermatology. “They are left to fend for themselves, which leads to increased anxiety. If they don’t understand what it means to manage their child’s food allergy, they’re going to think that they’re a ticking time bomb,” said Dr. Stukus, director of the Food Allergy Treatment Center and professor of pediatrics in the division of allergy and immunology at Nationwide Children’s Hospital in Columbus, Ohio.
During his presentation, he toured clinicians through best practices to diagnose and treat food allergies and shared cautionary tales of unsupported claims, unnecessary testing, and potential harm to misdiagnosed patients.
While food allergies can be serious and life-threatening, they are also manageable, he continued. It doesn’t mean that children with food allergies can’t go to school, attend baseball games, or participate in activities that any other child would. “Telling someone to adopt a restricted diet is not a benign recommendation,” he said. “That can cause real harm.”
Dr. Stukus defined food allergy as an immunologic response to an allergen that results in reproducible symptoms with every exposure. “Most commonly we’re going to see IgE-mediated food allergies, which often occur within minutes of eating certain foods,” he said.
Food intolerance, on the other hand, is a nonimmunologic response to a food that causes gastrointestinal symptoms with exposure. “This can come and go over time,” he said. “The most common example is lactose intolerance.”
Then there’s food sensitivity, which Dr. Stukus said is not a medical term but a marketing term often applied to a variety of symptoms without evidence to support its use.
“On the Internet you will find many companies marketing food sensitivity tests,” he said. “Gluten-free foods are now a billion-dollar industry. There are no validated tests to diagnose food sensitivity. All the blood tests measure IgG, which is memory antibody. If you eat a food, it is a normal response to produce IgG to it, but these companies will test all these things and when it comes back elevated, they say ‘Aha! This is your food sensitivity and this is why you’re not sleeping well at night.’ ” To illustrate the harm that can come from food allergy tests he discussed a 6-year-old girl who presented to his clinic several years ago with typical symptoms of allergic rhinitis. The parent reported a history of sneezing around dogs, itchy, watery eyes in the spring, recurrent cough, and frequent upper respiratory infections.
The referring physician had ordered an allergy panel, which flagged a long list of foods that the girl was supposedly allergic to, including banana, egg white, cod, and peanut. “This family was told to take all of these foods out of her diet,” Dr. Stukus said. “Interestingly, she had been seen by this physician for evaluation of environmental allergies, but the only ones included in the test were cat, cockroach, dog, and dust mite. They didn’t even include the spring pollen allergies. You want to avoid tests like this.”
Food sensitization is not the same as food allergy, he continued, noting that about 30% of all children will have detectable IgE toward peanuts, milk, egg, and shrimp, but that only about 5% are truly allergic to those foods.
“If we go by IgE testing alone, we’re going to overdiagnose the vast majority of people with food allergies that they don’t actually have,” he said. “Food allergy is diagnosed by the history and then confirmed by testing. With IgE-mediated food allergies we know that milk, egg, wheat, soy, finned fish, shellfish, and peanuts account for more than 90% of all food allergy reactions. Can any food potentially cause a food allergy? Yes, potentially, but we know that most fruits and vegetables and grains are very unlikely to cause an allergy.”
IgE-mediated food allergies are objective, immediate onset, and reproducible with every exposure to the offending food, no matter what form. Typical symptoms include hives, swelling, vomiting, runny nose/congestion, wheezing, hypotension, and anaphylaxis.
“We can also accurately identify infants that are more at risk to develop food allergies,” Dr. Stukus said. Infants with refractory atopic dermatitis often progress from eczema to food allergies to allergic rhinitis and asthma, the so-called “allergic march.” “Family history does have a role as well, but it’s not as significant,” he said. As for diagnostic tools, skin prick testing detects the presence of specific IgE bound to cutaneous mast cells and has a high negative predictive value and a low positive predictive value (around 50%).
With serum-specific IgE testing, levels of IgE for food and/or inhalant allergen can be obtained conveniently through routine venipuncture. Results are reported in ranges from 0.1 kU/L to 100 kU/L, and some are reported as arbitrary classes in levels of severity from 1 to 5.
“I highly discourage anybody from paying attention to arbitrary classes [on these reports],” Dr. Stukus said. “Those are meaningless. The absolute value is all that matters.”
He added that both skin and blood testing have high rates of false positive results. “We really need to use the history to help guide what tests we do; they were never designed to be used as screening tests, yet they’re used as screening tests on a regular basis,” he said. “There is also no indication to do shotgun testing. The reason why is because we see lots of cross reactivity on testing. If we have someone with peanut allergy and we start doing specific IgE testing for all legumes, more often than not we’re going to find detectable IgE, but it’s much less likely that they actually have clinical reactivity to foods like soy and beans.”
Dr. Stukus advises clinicians to consider certain questions before they order an allergen panel, the first being: Do I have the knowledge and experience to properly interpret the results?
“If you don’t know how to interpret the test, you probably shouldn’t order it in the first place,” he said. “If you do have the knowledge to interpret the results, will the results help to determine the diagnosis or change management? If not, why are you testing just to test? There is zero clinical indication to order a food allergy panel.” Dr. Stukus recommended a review of unproven tests for adverse reactions to foods published in 2018 in The Journal of Allergy and Clinical Immunology.
According to Dr. Stukus, potential harms from unproven food allergy tests include cost, unnecessary dietary avoidance, and a delay in diagnosis for the underlying condition. During the COVID-19 pandemic, he observed an increase in the number of patients with orthorexia, which he described as an eating disorder characterized by having an unsafe obsession with healthy food that becomes deeply rooted in the individual’s way of thinking to the point that it interferes with daily life.
“If you take someone who has anxiety at baseline, and then you give them a list of foods that they allegedly can’t eat, that’s going to cause worse anxiety,” he added. “We’re seeing that from the results of these tests.”
Dr. Stukus disclosed that he is a consultant for Before Brands, Kaleo, and Novartis. He is also associate editor of the Annals of Allergy, Asthma and Immunology.
INDIANAPOLIS – If there’s one truth that David R. Stukus, MD, has come to realize from his 2 years as director of a food allergy treatment center, it’s that
“When they’re given a diagnosis of food allergy, many families do not receive proper education to help them understand the risk as well as self-management and prognosis,” he said at the annual meeting of the Society for Pediatric Dermatology. “They are left to fend for themselves, which leads to increased anxiety. If they don’t understand what it means to manage their child’s food allergy, they’re going to think that they’re a ticking time bomb,” said Dr. Stukus, director of the Food Allergy Treatment Center and professor of pediatrics in the division of allergy and immunology at Nationwide Children’s Hospital in Columbus, Ohio.
During his presentation, he toured clinicians through best practices to diagnose and treat food allergies and shared cautionary tales of unsupported claims, unnecessary testing, and potential harm to misdiagnosed patients.
While food allergies can be serious and life-threatening, they are also manageable, he continued. It doesn’t mean that children with food allergies can’t go to school, attend baseball games, or participate in activities that any other child would. “Telling someone to adopt a restricted diet is not a benign recommendation,” he said. “That can cause real harm.”
Dr. Stukus defined food allergy as an immunologic response to an allergen that results in reproducible symptoms with every exposure. “Most commonly we’re going to see IgE-mediated food allergies, which often occur within minutes of eating certain foods,” he said.
Food intolerance, on the other hand, is a nonimmunologic response to a food that causes gastrointestinal symptoms with exposure. “This can come and go over time,” he said. “The most common example is lactose intolerance.”
Then there’s food sensitivity, which Dr. Stukus said is not a medical term but a marketing term often applied to a variety of symptoms without evidence to support its use.
“On the Internet you will find many companies marketing food sensitivity tests,” he said. “Gluten-free foods are now a billion-dollar industry. There are no validated tests to diagnose food sensitivity. All the blood tests measure IgG, which is memory antibody. If you eat a food, it is a normal response to produce IgG to it, but these companies will test all these things and when it comes back elevated, they say ‘Aha! This is your food sensitivity and this is why you’re not sleeping well at night.’ ” To illustrate the harm that can come from food allergy tests he discussed a 6-year-old girl who presented to his clinic several years ago with typical symptoms of allergic rhinitis. The parent reported a history of sneezing around dogs, itchy, watery eyes in the spring, recurrent cough, and frequent upper respiratory infections.
The referring physician had ordered an allergy panel, which flagged a long list of foods that the girl was supposedly allergic to, including banana, egg white, cod, and peanut. “This family was told to take all of these foods out of her diet,” Dr. Stukus said. “Interestingly, she had been seen by this physician for evaluation of environmental allergies, but the only ones included in the test were cat, cockroach, dog, and dust mite. They didn’t even include the spring pollen allergies. You want to avoid tests like this.”
Food sensitization is not the same as food allergy, he continued, noting that about 30% of all children will have detectable IgE toward peanuts, milk, egg, and shrimp, but that only about 5% are truly allergic to those foods.
“If we go by IgE testing alone, we’re going to overdiagnose the vast majority of people with food allergies that they don’t actually have,” he said. “Food allergy is diagnosed by the history and then confirmed by testing. With IgE-mediated food allergies we know that milk, egg, wheat, soy, finned fish, shellfish, and peanuts account for more than 90% of all food allergy reactions. Can any food potentially cause a food allergy? Yes, potentially, but we know that most fruits and vegetables and grains are very unlikely to cause an allergy.”
IgE-mediated food allergies are objective, immediate onset, and reproducible with every exposure to the offending food, no matter what form. Typical symptoms include hives, swelling, vomiting, runny nose/congestion, wheezing, hypotension, and anaphylaxis.
“We can also accurately identify infants that are more at risk to develop food allergies,” Dr. Stukus said. Infants with refractory atopic dermatitis often progress from eczema to food allergies to allergic rhinitis and asthma, the so-called “allergic march.” “Family history does have a role as well, but it’s not as significant,” he said. As for diagnostic tools, skin prick testing detects the presence of specific IgE bound to cutaneous mast cells and has a high negative predictive value and a low positive predictive value (around 50%).
With serum-specific IgE testing, levels of IgE for food and/or inhalant allergen can be obtained conveniently through routine venipuncture. Results are reported in ranges from 0.1 kU/L to 100 kU/L, and some are reported as arbitrary classes in levels of severity from 1 to 5.
“I highly discourage anybody from paying attention to arbitrary classes [on these reports],” Dr. Stukus said. “Those are meaningless. The absolute value is all that matters.”
He added that both skin and blood testing have high rates of false positive results. “We really need to use the history to help guide what tests we do; they were never designed to be used as screening tests, yet they’re used as screening tests on a regular basis,” he said. “There is also no indication to do shotgun testing. The reason why is because we see lots of cross reactivity on testing. If we have someone with peanut allergy and we start doing specific IgE testing for all legumes, more often than not we’re going to find detectable IgE, but it’s much less likely that they actually have clinical reactivity to foods like soy and beans.”
Dr. Stukus advises clinicians to consider certain questions before they order an allergen panel, the first being: Do I have the knowledge and experience to properly interpret the results?
“If you don’t know how to interpret the test, you probably shouldn’t order it in the first place,” he said. “If you do have the knowledge to interpret the results, will the results help to determine the diagnosis or change management? If not, why are you testing just to test? There is zero clinical indication to order a food allergy panel.” Dr. Stukus recommended a review of unproven tests for adverse reactions to foods published in 2018 in The Journal of Allergy and Clinical Immunology.
According to Dr. Stukus, potential harms from unproven food allergy tests include cost, unnecessary dietary avoidance, and a delay in diagnosis for the underlying condition. During the COVID-19 pandemic, he observed an increase in the number of patients with orthorexia, which he described as an eating disorder characterized by having an unsafe obsession with healthy food that becomes deeply rooted in the individual’s way of thinking to the point that it interferes with daily life.
“If you take someone who has anxiety at baseline, and then you give them a list of foods that they allegedly can’t eat, that’s going to cause worse anxiety,” he added. “We’re seeing that from the results of these tests.”
Dr. Stukus disclosed that he is a consultant for Before Brands, Kaleo, and Novartis. He is also associate editor of the Annals of Allergy, Asthma and Immunology.
INDIANAPOLIS – If there’s one truth that David R. Stukus, MD, has come to realize from his 2 years as director of a food allergy treatment center, it’s that
“When they’re given a diagnosis of food allergy, many families do not receive proper education to help them understand the risk as well as self-management and prognosis,” he said at the annual meeting of the Society for Pediatric Dermatology. “They are left to fend for themselves, which leads to increased anxiety. If they don’t understand what it means to manage their child’s food allergy, they’re going to think that they’re a ticking time bomb,” said Dr. Stukus, director of the Food Allergy Treatment Center and professor of pediatrics in the division of allergy and immunology at Nationwide Children’s Hospital in Columbus, Ohio.
During his presentation, he toured clinicians through best practices to diagnose and treat food allergies and shared cautionary tales of unsupported claims, unnecessary testing, and potential harm to misdiagnosed patients.
While food allergies can be serious and life-threatening, they are also manageable, he continued. It doesn’t mean that children with food allergies can’t go to school, attend baseball games, or participate in activities that any other child would. “Telling someone to adopt a restricted diet is not a benign recommendation,” he said. “That can cause real harm.”
Dr. Stukus defined food allergy as an immunologic response to an allergen that results in reproducible symptoms with every exposure. “Most commonly we’re going to see IgE-mediated food allergies, which often occur within minutes of eating certain foods,” he said.
Food intolerance, on the other hand, is a nonimmunologic response to a food that causes gastrointestinal symptoms with exposure. “This can come and go over time,” he said. “The most common example is lactose intolerance.”
Then there’s food sensitivity, which Dr. Stukus said is not a medical term but a marketing term often applied to a variety of symptoms without evidence to support its use.
“On the Internet you will find many companies marketing food sensitivity tests,” he said. “Gluten-free foods are now a billion-dollar industry. There are no validated tests to diagnose food sensitivity. All the blood tests measure IgG, which is memory antibody. If you eat a food, it is a normal response to produce IgG to it, but these companies will test all these things and when it comes back elevated, they say ‘Aha! This is your food sensitivity and this is why you’re not sleeping well at night.’ ” To illustrate the harm that can come from food allergy tests he discussed a 6-year-old girl who presented to his clinic several years ago with typical symptoms of allergic rhinitis. The parent reported a history of sneezing around dogs, itchy, watery eyes in the spring, recurrent cough, and frequent upper respiratory infections.
The referring physician had ordered an allergy panel, which flagged a long list of foods that the girl was supposedly allergic to, including banana, egg white, cod, and peanut. “This family was told to take all of these foods out of her diet,” Dr. Stukus said. “Interestingly, she had been seen by this physician for evaluation of environmental allergies, but the only ones included in the test were cat, cockroach, dog, and dust mite. They didn’t even include the spring pollen allergies. You want to avoid tests like this.”
Food sensitization is not the same as food allergy, he continued, noting that about 30% of all children will have detectable IgE toward peanuts, milk, egg, and shrimp, but that only about 5% are truly allergic to those foods.
“If we go by IgE testing alone, we’re going to overdiagnose the vast majority of people with food allergies that they don’t actually have,” he said. “Food allergy is diagnosed by the history and then confirmed by testing. With IgE-mediated food allergies we know that milk, egg, wheat, soy, finned fish, shellfish, and peanuts account for more than 90% of all food allergy reactions. Can any food potentially cause a food allergy? Yes, potentially, but we know that most fruits and vegetables and grains are very unlikely to cause an allergy.”
IgE-mediated food allergies are objective, immediate onset, and reproducible with every exposure to the offending food, no matter what form. Typical symptoms include hives, swelling, vomiting, runny nose/congestion, wheezing, hypotension, and anaphylaxis.
“We can also accurately identify infants that are more at risk to develop food allergies,” Dr. Stukus said. Infants with refractory atopic dermatitis often progress from eczema to food allergies to allergic rhinitis and asthma, the so-called “allergic march.” “Family history does have a role as well, but it’s not as significant,” he said. As for diagnostic tools, skin prick testing detects the presence of specific IgE bound to cutaneous mast cells and has a high negative predictive value and a low positive predictive value (around 50%).
With serum-specific IgE testing, levels of IgE for food and/or inhalant allergen can be obtained conveniently through routine venipuncture. Results are reported in ranges from 0.1 kU/L to 100 kU/L, and some are reported as arbitrary classes in levels of severity from 1 to 5.
“I highly discourage anybody from paying attention to arbitrary classes [on these reports],” Dr. Stukus said. “Those are meaningless. The absolute value is all that matters.”
He added that both skin and blood testing have high rates of false positive results. “We really need to use the history to help guide what tests we do; they were never designed to be used as screening tests, yet they’re used as screening tests on a regular basis,” he said. “There is also no indication to do shotgun testing. The reason why is because we see lots of cross reactivity on testing. If we have someone with peanut allergy and we start doing specific IgE testing for all legumes, more often than not we’re going to find detectable IgE, but it’s much less likely that they actually have clinical reactivity to foods like soy and beans.”
Dr. Stukus advises clinicians to consider certain questions before they order an allergen panel, the first being: Do I have the knowledge and experience to properly interpret the results?
“If you don’t know how to interpret the test, you probably shouldn’t order it in the first place,” he said. “If you do have the knowledge to interpret the results, will the results help to determine the diagnosis or change management? If not, why are you testing just to test? There is zero clinical indication to order a food allergy panel.” Dr. Stukus recommended a review of unproven tests for adverse reactions to foods published in 2018 in The Journal of Allergy and Clinical Immunology.
According to Dr. Stukus, potential harms from unproven food allergy tests include cost, unnecessary dietary avoidance, and a delay in diagnosis for the underlying condition. During the COVID-19 pandemic, he observed an increase in the number of patients with orthorexia, which he described as an eating disorder characterized by having an unsafe obsession with healthy food that becomes deeply rooted in the individual’s way of thinking to the point that it interferes with daily life.
“If you take someone who has anxiety at baseline, and then you give them a list of foods that they allegedly can’t eat, that’s going to cause worse anxiety,” he added. “We’re seeing that from the results of these tests.”
Dr. Stukus disclosed that he is a consultant for Before Brands, Kaleo, and Novartis. He is also associate editor of the Annals of Allergy, Asthma and Immunology.
AT SPD 2022