User login
Differences in COVID-19 Outcomes Among Patients With Type 1 Diabetes: First vs Later Surges
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.
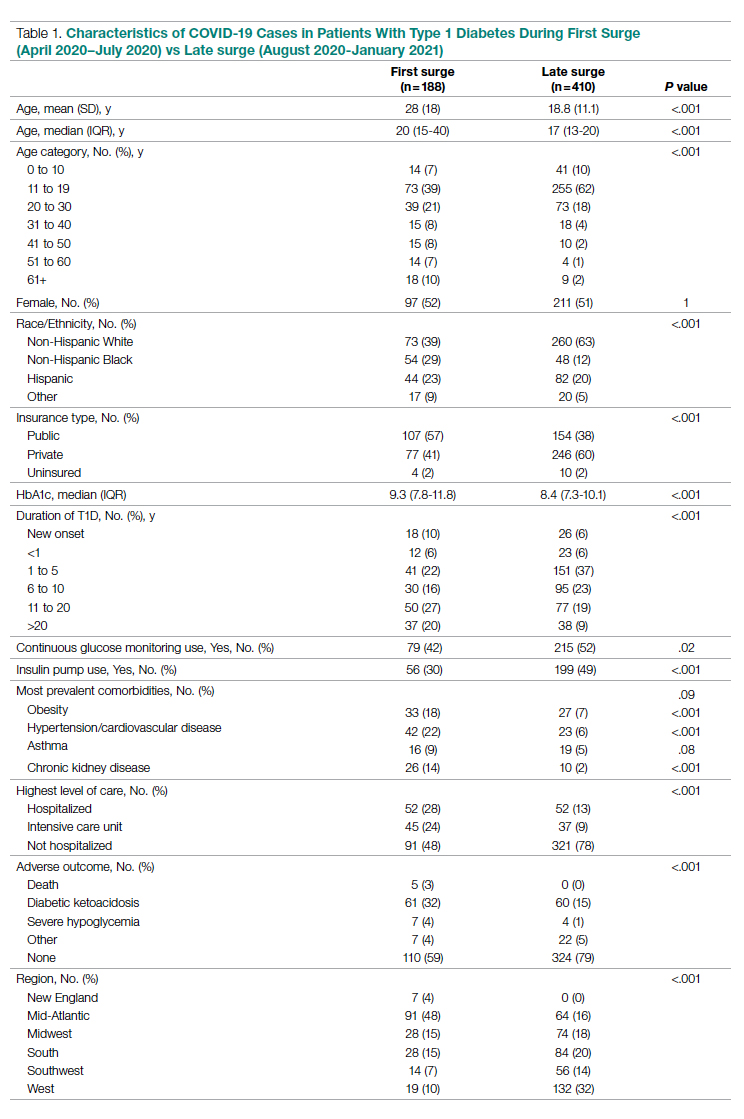
Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
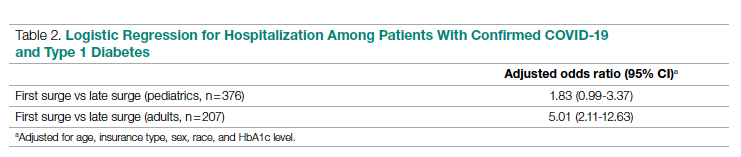
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.
Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
FDA okays first tubing-free ‘artificial pancreas’ Omnipod 5
The Food and Drug Administration has cleared the Omnipod 5 Automated Insulin Delivery System (Insulet), the third semiautomated closed-loop insulin delivery system in the United States and the first that is tubing free.
Omnipod 5 is cleared for people aged 6 years and older with type 1 diabetes. The system integrates the tubeless insulin delivery Pods with Dexcom G6 continuous glucose monitors (CGM) and a smartphone app or a separate controller device to automatically adjust insulin to minimize high and low blood glucose levels via SmartAdjust technology.
Within the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin up or down or pauses it based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
The Omnipod 5 becomes the third FDA-cleared semiautomated insulin delivery system in the United States, along with systems by Tandem and Medtronic. Others are available outside the United States. All of the currently marketed systems incorporate insulin pumps with tubing, whereas the tubeless Pods are worn directly on the body and changed every 3 days.
In a statement, JDRF, the type 1 diabetes advocacy organization, said: “Authorization of the Insulet Omnipod 5 is a huge win for the type 1 diabetes community. As the first tubeless hybrid closed-loop system to receive FDA clearance, this is a critical step forward in making day-to-day life better for people living with the disease.”
JDRF, which worked with the FDA to establish regulatory pathways for artificial pancreas technology, supported the development of the Omnipod 5 control algorithm through investigators in the JDRF Artificial Pancreas Consortium.
The Omnipod 5 will be available as a pharmacy product. It will be launched soon in limited market release and broadly thereafter.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Omnipod 5 Automated Insulin Delivery System (Insulet), the third semiautomated closed-loop insulin delivery system in the United States and the first that is tubing free.
Omnipod 5 is cleared for people aged 6 years and older with type 1 diabetes. The system integrates the tubeless insulin delivery Pods with Dexcom G6 continuous glucose monitors (CGM) and a smartphone app or a separate controller device to automatically adjust insulin to minimize high and low blood glucose levels via SmartAdjust technology.
Within the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin up or down or pauses it based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
The Omnipod 5 becomes the third FDA-cleared semiautomated insulin delivery system in the United States, along with systems by Tandem and Medtronic. Others are available outside the United States. All of the currently marketed systems incorporate insulin pumps with tubing, whereas the tubeless Pods are worn directly on the body and changed every 3 days.
In a statement, JDRF, the type 1 diabetes advocacy organization, said: “Authorization of the Insulet Omnipod 5 is a huge win for the type 1 diabetes community. As the first tubeless hybrid closed-loop system to receive FDA clearance, this is a critical step forward in making day-to-day life better for people living with the disease.”
JDRF, which worked with the FDA to establish regulatory pathways for artificial pancreas technology, supported the development of the Omnipod 5 control algorithm through investigators in the JDRF Artificial Pancreas Consortium.
The Omnipod 5 will be available as a pharmacy product. It will be launched soon in limited market release and broadly thereafter.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Omnipod 5 Automated Insulin Delivery System (Insulet), the third semiautomated closed-loop insulin delivery system in the United States and the first that is tubing free.
Omnipod 5 is cleared for people aged 6 years and older with type 1 diabetes. The system integrates the tubeless insulin delivery Pods with Dexcom G6 continuous glucose monitors (CGM) and a smartphone app or a separate controller device to automatically adjust insulin to minimize high and low blood glucose levels via SmartAdjust technology.
Within the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin up or down or pauses it based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
The Omnipod 5 becomes the third FDA-cleared semiautomated insulin delivery system in the United States, along with systems by Tandem and Medtronic. Others are available outside the United States. All of the currently marketed systems incorporate insulin pumps with tubing, whereas the tubeless Pods are worn directly on the body and changed every 3 days.
In a statement, JDRF, the type 1 diabetes advocacy organization, said: “Authorization of the Insulet Omnipod 5 is a huge win for the type 1 diabetes community. As the first tubeless hybrid closed-loop system to receive FDA clearance, this is a critical step forward in making day-to-day life better for people living with the disease.”
JDRF, which worked with the FDA to establish regulatory pathways for artificial pancreas technology, supported the development of the Omnipod 5 control algorithm through investigators in the JDRF Artificial Pancreas Consortium.
The Omnipod 5 will be available as a pharmacy product. It will be launched soon in limited market release and broadly thereafter.
A version of this article first appeared on Medscape.com.
More than 1 in 10 people in U.S. have diabetes, CDC says
More than 1 in 10 Americans have diabetes and over a third have prediabetes, according to updated statistics from the Centers for Disease Control and Prevention.
The National Diabetes Statistics Report includes data for 2017-2020 from several nationally representative sources on prevalence and incidence of diabetes and prediabetes, risk factors for complications, acute and long-term complications, and costs.
According to the new report, published on Jan. 25, a total of 37.3 million people in the United States have diabetes, or about 11.3% of the population. Of those, 28.7 million are diagnosed (including 28.5 million adults), while 8.5 million, or 23% of those with diabetes, are undiagnosed.
Another 96 million adults have prediabetes, comprising 38.0% of the adult U.S. population, of whom only 19% are aware of their prediabetes status.
In a statement, the American Diabetes Association said the new CDC data “show an alarming increase of diabetes in our nation among adults,” while the high number with prediabetes who don’t know that they have it “is fueling the diabetes epidemic.”
Regarding the total estimated 1.84 million with type 1 diabetes, the advocacy organization JDRF said in a statement: “These data and additional statistical research reinforces the urgency to accelerate life-changing breakthroughs to cure, prevent, and treat [type 1 diabetes] and its complications.”
Overall, the ADA said, “the National Diabetes Statistics Report reaffirms why the ADA is dedicated to innovative research to find a cure for diabetes once and for all.”
Notable increases since 2019
These new data represent notable increases since the CDC’s 2019 Report Card, which gave the U.S. population with diabetes in 2018 as 34.2 million, or 10.5% of the population, including 7.3 million undiagnosed. The prediabetes prevalence that year was 88 million.
Among children and adolescents younger than 20 years, 283,000, or 35 per 10,000 U.S. youths, had diagnosed diabetes in 2019. Of those, 244,000 had type 1 diabetes. Another 1.6 million adults aged 20 and older also reported having type 1 diabetes, comprising 5.7% of U.S. adults with diagnosed diabetes.
A version of this article first appeared on Medscape.com.
More than 1 in 10 Americans have diabetes and over a third have prediabetes, according to updated statistics from the Centers for Disease Control and Prevention.
The National Diabetes Statistics Report includes data for 2017-2020 from several nationally representative sources on prevalence and incidence of diabetes and prediabetes, risk factors for complications, acute and long-term complications, and costs.
According to the new report, published on Jan. 25, a total of 37.3 million people in the United States have diabetes, or about 11.3% of the population. Of those, 28.7 million are diagnosed (including 28.5 million adults), while 8.5 million, or 23% of those with diabetes, are undiagnosed.
Another 96 million adults have prediabetes, comprising 38.0% of the adult U.S. population, of whom only 19% are aware of their prediabetes status.
In a statement, the American Diabetes Association said the new CDC data “show an alarming increase of diabetes in our nation among adults,” while the high number with prediabetes who don’t know that they have it “is fueling the diabetes epidemic.”
Regarding the total estimated 1.84 million with type 1 diabetes, the advocacy organization JDRF said in a statement: “These data and additional statistical research reinforces the urgency to accelerate life-changing breakthroughs to cure, prevent, and treat [type 1 diabetes] and its complications.”
Overall, the ADA said, “the National Diabetes Statistics Report reaffirms why the ADA is dedicated to innovative research to find a cure for diabetes once and for all.”
Notable increases since 2019
These new data represent notable increases since the CDC’s 2019 Report Card, which gave the U.S. population with diabetes in 2018 as 34.2 million, or 10.5% of the population, including 7.3 million undiagnosed. The prediabetes prevalence that year was 88 million.
Among children and adolescents younger than 20 years, 283,000, or 35 per 10,000 U.S. youths, had diagnosed diabetes in 2019. Of those, 244,000 had type 1 diabetes. Another 1.6 million adults aged 20 and older also reported having type 1 diabetes, comprising 5.7% of U.S. adults with diagnosed diabetes.
A version of this article first appeared on Medscape.com.
More than 1 in 10 Americans have diabetes and over a third have prediabetes, according to updated statistics from the Centers for Disease Control and Prevention.
The National Diabetes Statistics Report includes data for 2017-2020 from several nationally representative sources on prevalence and incidence of diabetes and prediabetes, risk factors for complications, acute and long-term complications, and costs.
According to the new report, published on Jan. 25, a total of 37.3 million people in the United States have diabetes, or about 11.3% of the population. Of those, 28.7 million are diagnosed (including 28.5 million adults), while 8.5 million, or 23% of those with diabetes, are undiagnosed.
Another 96 million adults have prediabetes, comprising 38.0% of the adult U.S. population, of whom only 19% are aware of their prediabetes status.
In a statement, the American Diabetes Association said the new CDC data “show an alarming increase of diabetes in our nation among adults,” while the high number with prediabetes who don’t know that they have it “is fueling the diabetes epidemic.”
Regarding the total estimated 1.84 million with type 1 diabetes, the advocacy organization JDRF said in a statement: “These data and additional statistical research reinforces the urgency to accelerate life-changing breakthroughs to cure, prevent, and treat [type 1 diabetes] and its complications.”
Overall, the ADA said, “the National Diabetes Statistics Report reaffirms why the ADA is dedicated to innovative research to find a cure for diabetes once and for all.”
Notable increases since 2019
These new data represent notable increases since the CDC’s 2019 Report Card, which gave the U.S. population with diabetes in 2018 as 34.2 million, or 10.5% of the population, including 7.3 million undiagnosed. The prediabetes prevalence that year was 88 million.
Among children and adolescents younger than 20 years, 283,000, or 35 per 10,000 U.S. youths, had diagnosed diabetes in 2019. Of those, 244,000 had type 1 diabetes. Another 1.6 million adults aged 20 and older also reported having type 1 diabetes, comprising 5.7% of U.S. adults with diagnosed diabetes.
A version of this article first appeared on Medscape.com.
Moderate-vigorous stepping seen to lower diabetes risk in older women
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
FROM DIABETES CARE
Does COVID-19 induce type 1 diabetes in kids? Jury still out
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
How to help adults meet dietary recommendations
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
Medtronic recalls HawkOne directional atherectomy system
Medtronic has recalled 95,110 HawkOne Directional Atherectomy Systems because of the risk of the guidewire within the catheter moving downward or prolapsing during use, which may damage the tip of the catheter.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The HawkOne Directional Atherectomy system is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
If the guideline moves downward or prolapses during use, the “catheter tip may break off or separate, and this could lead to serious adverse events, including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip,” the FDA says in a recall notice posted today on its website.
To date, there have been 55 injuries, no deaths, and 163 complaints reported for this device.
The recalled devices were distributed in the United States between Jan. 22, 2018 and Oct. 4, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Medtronic sent an urgent field safety notice to customers Dec. 6, 2021, requesting that they alert parties of the defect, review the instructions for use before using the device, and note the warnings and precautions listed in the letter.
Customers were also asked to complete the enclosed confirmation form and email to [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic has recalled 95,110 HawkOne Directional Atherectomy Systems because of the risk of the guidewire within the catheter moving downward or prolapsing during use, which may damage the tip of the catheter.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The HawkOne Directional Atherectomy system is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
If the guideline moves downward or prolapses during use, the “catheter tip may break off or separate, and this could lead to serious adverse events, including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip,” the FDA says in a recall notice posted today on its website.
To date, there have been 55 injuries, no deaths, and 163 complaints reported for this device.
The recalled devices were distributed in the United States between Jan. 22, 2018 and Oct. 4, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Medtronic sent an urgent field safety notice to customers Dec. 6, 2021, requesting that they alert parties of the defect, review the instructions for use before using the device, and note the warnings and precautions listed in the letter.
Customers were also asked to complete the enclosed confirmation form and email to [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic has recalled 95,110 HawkOne Directional Atherectomy Systems because of the risk of the guidewire within the catheter moving downward or prolapsing during use, which may damage the tip of the catheter.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The HawkOne Directional Atherectomy system is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
If the guideline moves downward or prolapses during use, the “catheter tip may break off or separate, and this could lead to serious adverse events, including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip,” the FDA says in a recall notice posted today on its website.
To date, there have been 55 injuries, no deaths, and 163 complaints reported for this device.
The recalled devices were distributed in the United States between Jan. 22, 2018 and Oct. 4, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Medtronic sent an urgent field safety notice to customers Dec. 6, 2021, requesting that they alert parties of the defect, review the instructions for use before using the device, and note the warnings and precautions listed in the letter.
Customers were also asked to complete the enclosed confirmation form and email to [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
‘We just have to keep them alive’: Transitioning youth with type 1 diabetes
“No one has asked young people what they want,” said Tabitha Randell, MBChB, an endocrinologist with Nottingham (England) University Hospitals NHS Trust, who specializes in treating teenagers with type 1 diabetes as they transition to adult care.
Dr. Randell, who has set up a very successful specialist service in her hospital for such patients, said: “We consistently have the best, or the second best, outcomes in this country for our diabetes patients.” She believes this is one of the most important issues in modern endocrinology today.
Speaking at the Diabetes Professional Care conference in London at the end of 2021, and sharing her thoughts afterward with this news organization, she noted that in general there are “virtually no published outcomes” on how best to transition a patient with type 1 diabetes from pediatric to adult care.
“If you actually get them to transition – because some just drop out and disengage and there’s nothing you can do – none of them get lost. Some of them disengage in the adult clinic, but if you’re in the young diabetes service [in England] the rules are that if you miss a diabetes appointment you do not get discharged, as compared with the adult clinic, where if you miss an appointment, you are discharged.”
In the young diabetes clinic, doctors will “carry on trying to contact you, and get you back,” she explained. “And the patients do eventually come back in – it might be a year or 2, but they do come back. We’ve just got to keep them alive in the meantime!”
This issue needs tackling all over the world. Dr. Randell said she’s not aware of any one country – although there may be “pockets” of good care within a given country – that is doing this perfectly.
Across the pond, Grazia Aleppo, MD, division of endocrinology at Northwestern University, Chicago, agreed that transitioning pediatric patients with type 1 diabetes to adult care presents “unique challenges.”
Challenges when transitioning from pediatric to adult care
During childhood, type 1 diabetes management is largely supervised by patients’ parents and members of the pediatric diabetes care team, which may include diabetes educators, psychologists, or social workers, as well as pediatric endocrinologists.
When the patient with type 1 diabetes becomes a young adult and takes over management of their own health, Dr. Aleppo said, the care team may diminish along with the time spent in provider visits.
The adult endocrinology setting focuses more on self-management and autonomous functioning of the individual with diabetes.
Adult appointments are typically shorter, and the patient is usually expected to follow doctors’ suggestions independently, she noted. They are also expected to manage the practical aspects of their diabetes care, including prescriptions, diabetes supplies, laboratory tests, scheduling, and keeping appointments.
At the same time that the emerging adult needs to start asserting independence over their health care, they will also be going through a myriad of other important lifestyle changes, such as attending college, living on their own for the first time, and starting a career.
“With these fundamental differences and challenges, competing priorities, such as college, work and relationships, medical care may become of secondary importance and patients may become disengaged,” Dr. Aleppo explained.
As Dr. Randell has said, loss to follow-up is a big problem with this patient population, with disengagement from specialist services and worsening A1c across the transition, Dr. Aleppo noted. This makes addressing these patients’ specific needs extremely important.
Engage with kid, not disease; don’t palm them off on new recruits
“The really key thing these kids say is, ‘I do not want to be a disease,’” Dr. Randell said. “They want you to know that they are a person. Engage these kids!” she suggested. “Ask them: ‘How is your exam revision going?’ Find something positive to say, even if it’s just: ‘I’m glad you came today.’ ”
“If the first thing that you do is tell them off [for poor diabetes care], you are never going to see them again,” she cautioned.
Dr. Randell also said that role models with type 1 diabetes, such as Lila Moss – daughter of British supermodel Kate Moss – who was recently pictured wearing an insulin pump on her leg on the catwalk, are helping youngsters not feel so self-conscious about their diabetes.
“Let them know it’s not the end of the world, having [type 1] diabetes,” she emphasized.
And Partha Kar, MBBS, OBE, national specialty advisor, diabetes with NHS England, agreed wholeheartedly with Dr. Randall.
Reminiscing about his early days as a newly qualified endocrinologist, Dr. Kar, who works at Portsmouth (England) Hospital NHS Trust, noted that as a new member of staff he was given the youth with type 1 diabetes – those getting ready to transition to adult care – to look after.
But this is the exact opposite of what should be happening, he emphasized. “If you don’t think transition care is important, you shouldn’t be treating type 1 diabetes.”
He believes that every diabetes center “must have a young-adult team lead” and this job must not be given to the least experienced member of staff.
This lead “doesn’t need to be a doctor,” Dr. Kar stressed. “It can be a psychologist, or a diabetes nurse, or a pharmacist, or a dietician.”
In short, it must be someone experienced who loves working with this age group.
Dr. Randell agreed: “Make sure the team is interested in young people. It shouldn’t be the last person in who gets the job no one else wants.” Teens “are my favorite group to work with. They don’t take any nonsense.”
And she explained: “Young people like to get to know the person who’s going to take care of them. So, stay with them for their young adult years.” This can be “quite a fluid period,” with it normally extending to age 25, but in some cases, “it can be up to 32 years old.”
Preparing for the transition
To ease pediatric patients into the transition to adult care, Dr. Aleppo recommended that the pediatric diabetes team provide enough time so that any concerns the patient and their family may have can be addressed.
This should also include transferring management responsibilities to the young adult rather than their parent.
The pediatric provider should discuss with the patient available potential adult colleagues, personalizing these options to their needs, she said.
And the adult and pediatric clinicians should collaborate and provide important information beyond medical records or health summaries.
Adult providers should guide young adults on how to navigate the new practices, from scheduling follow-up appointments to policies regarding medication refills or supplies, to providing information about urgent numbers or email addresses for after-hours communications.
Dr. Kar reiterated that there are too few published outcomes in this patient group to guide the establishment of good transition services.
“Without data, we are dead on the ground. Without data, it’s all conjecture, anecdotes,” he said.
What he does know is that, in the latest national type 1 diabetes audit for England, “Diabetic ketoacidosis admissions ... are up in this age group,” which suggests these patients are not receiving adequate care.
Be a guide, not a gatekeeper
Dr. Kar stressed that, of the 8,760 hours in a year, the average patient with type 1 diabetes in the United Kingdom gets just “1-2 hours with you as a clinician, based on four appointments per year of 30 minutes each.”
“So you spend 0.02% of their time with individuals with type 1 diabetes. So, what’s the one thing you can do with that minimal contact? Be nice!”
Dr. Kar said he always has his email open to his adult patients and they are very respectful of his time. “They don’t email you at 1 a.m. That means every one of my patients has got support [from me]. Don’t be a barrier.”
“We have to fundamentally change the narrative. Doctors must have more empathy,” he said, stating that the one thing adolescents have constantly given feedback on has been, “Why don’t appointments start with: ‘How are you?’
“For a teenager, if you throw type 1 diabetes into the loop, it’s not easy,” he stressed. “Talk to them about something else. As a clinician, be a guide, not a gatekeeper. Give people the tools to self-manage better.”
Adult providers can meet these young adult patients “at their level,” Dr. Aleppo agreed.
“Pay attention to their immediate needs and focus on their present circumstances – whether how to get through their next semester in college, navigating job interviews, or handling having diabetes in the workplace.”
Paying attention to the mental health needs of these young patients is equally “paramount,” Dr. Aleppo said.
While access to mental health professionals may be challenging in the adult setting, providers should bring it up with their patients and offer counseling referrals.
“Diabetes impacts everything, and office appointments and conversations carry weight on these patients’ lives as a whole, not just on their diabetes,” she stressed. “A patient told me recently: ‘We’re learning to be adults,’ which can be hard enough, and with diabetes it can be even more challenging. Adult providers need to be aware of the patient’s ‘diabetes language’ in that often it is not what a patient is saying, rather how they are saying it that gives us information on what they truly need.
“As adult providers, we need to also train and teach our young patients to advocate for themselves on where to find resources that can help them navigate adulthood with diabetes,” she added.
One particularly helpful resource in the United States is the College Diabetes Network, a not-for-profit organization whose mission is to equip young adults with type 1 diabetes to successfully manage the challenging transition to independence at college and beyond.
“The sweetest thing that can happen to us as adult diabetes providers is when a patient – seen as an emerging adult during college – returns to your practice 10 years later after moving back and seeks you out for their diabetes care because of the relationship and trust you developed in those transitioning years,” Dr. Aleppo said.
Another resource is a freely available comic book series cocreated by Dr. Kar and colleague Mayank Patel, MBBS, an endocrinologist from University Hospital Southampton NHS Foundation Trust.
As detailed by this news organization in 2021, the series consists of three volumes: the first, Type 1: Origins, focuses on actual experiences of patients who have type 1 diabetes; the second, Type 1: Attack of the Ketones, is aimed at professionals who may provide care but have limited understanding of type 1 diabetes; and the third, Type 1 Mission 3: S.T.I.G.M.A., addresses the stigmas and misconceptions that patients with type 1 diabetes may face.
The idea for the first comic was inspired by a patient who compared having diabetes to being like the Marvel character The Hulk, said Dr. Kar, and has been expanded to include the additional volumes.
Dr. Kar and Dr. Patel have also just launched the fourth comic in the series, Type 1: Generations, to mark the 100-year anniversary since insulin was first given to a human.
“This is high priority”
Dr. Kar said the NHS in England has just appointed a national lead for type 1 diabetes in youth, Fulya Mehta, MD, of Alder Hey Children’s NHS Foundation Trust, Liverpool, England.
“If you have a plan, bring it to us,” he told the audience at the DPC conference, and “tell us, what is the one thing you would change? This is not a session we are doing just to tick a box. This is high priority.
“Encourage your colleagues to think about transition services. This is an absolute priority. We will be asking every center [in England] who is your transitioning lead?”
And he once again stressed that “a lead of transition service does not have to be a medic. This should be a multidisciplinary team. But they do need to be comfortable in that space. To that teenager, your job title means nothing. Give them time and space.”
Dr. Randell summed it up: “If we can work together, it’s only going to result in better outcomes. We need to blaze the trail for young people.”
Dr. Aleppo has reported serving as a consultant to Dexcom and Insulet and receiving support to Northwestern University from AstraZeneca, Dexcom, Eli Lilly, Fractyl Health, Insulet, and Novo Nordisk. Dr. Randell and Dr. Kar have no conflicts of interest.
A version of this article first appeared on Medscape.com.
“No one has asked young people what they want,” said Tabitha Randell, MBChB, an endocrinologist with Nottingham (England) University Hospitals NHS Trust, who specializes in treating teenagers with type 1 diabetes as they transition to adult care.
Dr. Randell, who has set up a very successful specialist service in her hospital for such patients, said: “We consistently have the best, or the second best, outcomes in this country for our diabetes patients.” She believes this is one of the most important issues in modern endocrinology today.
Speaking at the Diabetes Professional Care conference in London at the end of 2021, and sharing her thoughts afterward with this news organization, she noted that in general there are “virtually no published outcomes” on how best to transition a patient with type 1 diabetes from pediatric to adult care.
“If you actually get them to transition – because some just drop out and disengage and there’s nothing you can do – none of them get lost. Some of them disengage in the adult clinic, but if you’re in the young diabetes service [in England] the rules are that if you miss a diabetes appointment you do not get discharged, as compared with the adult clinic, where if you miss an appointment, you are discharged.”
In the young diabetes clinic, doctors will “carry on trying to contact you, and get you back,” she explained. “And the patients do eventually come back in – it might be a year or 2, but they do come back. We’ve just got to keep them alive in the meantime!”
This issue needs tackling all over the world. Dr. Randell said she’s not aware of any one country – although there may be “pockets” of good care within a given country – that is doing this perfectly.
Across the pond, Grazia Aleppo, MD, division of endocrinology at Northwestern University, Chicago, agreed that transitioning pediatric patients with type 1 diabetes to adult care presents “unique challenges.”
Challenges when transitioning from pediatric to adult care
During childhood, type 1 diabetes management is largely supervised by patients’ parents and members of the pediatric diabetes care team, which may include diabetes educators, psychologists, or social workers, as well as pediatric endocrinologists.
When the patient with type 1 diabetes becomes a young adult and takes over management of their own health, Dr. Aleppo said, the care team may diminish along with the time spent in provider visits.
The adult endocrinology setting focuses more on self-management and autonomous functioning of the individual with diabetes.
Adult appointments are typically shorter, and the patient is usually expected to follow doctors’ suggestions independently, she noted. They are also expected to manage the practical aspects of their diabetes care, including prescriptions, diabetes supplies, laboratory tests, scheduling, and keeping appointments.
At the same time that the emerging adult needs to start asserting independence over their health care, they will also be going through a myriad of other important lifestyle changes, such as attending college, living on their own for the first time, and starting a career.
“With these fundamental differences and challenges, competing priorities, such as college, work and relationships, medical care may become of secondary importance and patients may become disengaged,” Dr. Aleppo explained.
As Dr. Randell has said, loss to follow-up is a big problem with this patient population, with disengagement from specialist services and worsening A1c across the transition, Dr. Aleppo noted. This makes addressing these patients’ specific needs extremely important.
Engage with kid, not disease; don’t palm them off on new recruits
“The really key thing these kids say is, ‘I do not want to be a disease,’” Dr. Randell said. “They want you to know that they are a person. Engage these kids!” she suggested. “Ask them: ‘How is your exam revision going?’ Find something positive to say, even if it’s just: ‘I’m glad you came today.’ ”
“If the first thing that you do is tell them off [for poor diabetes care], you are never going to see them again,” she cautioned.
Dr. Randell also said that role models with type 1 diabetes, such as Lila Moss – daughter of British supermodel Kate Moss – who was recently pictured wearing an insulin pump on her leg on the catwalk, are helping youngsters not feel so self-conscious about their diabetes.
“Let them know it’s not the end of the world, having [type 1] diabetes,” she emphasized.
And Partha Kar, MBBS, OBE, national specialty advisor, diabetes with NHS England, agreed wholeheartedly with Dr. Randall.
Reminiscing about his early days as a newly qualified endocrinologist, Dr. Kar, who works at Portsmouth (England) Hospital NHS Trust, noted that as a new member of staff he was given the youth with type 1 diabetes – those getting ready to transition to adult care – to look after.
But this is the exact opposite of what should be happening, he emphasized. “If you don’t think transition care is important, you shouldn’t be treating type 1 diabetes.”
He believes that every diabetes center “must have a young-adult team lead” and this job must not be given to the least experienced member of staff.
This lead “doesn’t need to be a doctor,” Dr. Kar stressed. “It can be a psychologist, or a diabetes nurse, or a pharmacist, or a dietician.”
In short, it must be someone experienced who loves working with this age group.
Dr. Randell agreed: “Make sure the team is interested in young people. It shouldn’t be the last person in who gets the job no one else wants.” Teens “are my favorite group to work with. They don’t take any nonsense.”
And she explained: “Young people like to get to know the person who’s going to take care of them. So, stay with them for their young adult years.” This can be “quite a fluid period,” with it normally extending to age 25, but in some cases, “it can be up to 32 years old.”
Preparing for the transition
To ease pediatric patients into the transition to adult care, Dr. Aleppo recommended that the pediatric diabetes team provide enough time so that any concerns the patient and their family may have can be addressed.
This should also include transferring management responsibilities to the young adult rather than their parent.
The pediatric provider should discuss with the patient available potential adult colleagues, personalizing these options to their needs, she said.
And the adult and pediatric clinicians should collaborate and provide important information beyond medical records or health summaries.
Adult providers should guide young adults on how to navigate the new practices, from scheduling follow-up appointments to policies regarding medication refills or supplies, to providing information about urgent numbers or email addresses for after-hours communications.
Dr. Kar reiterated that there are too few published outcomes in this patient group to guide the establishment of good transition services.
“Without data, we are dead on the ground. Without data, it’s all conjecture, anecdotes,” he said.
What he does know is that, in the latest national type 1 diabetes audit for England, “Diabetic ketoacidosis admissions ... are up in this age group,” which suggests these patients are not receiving adequate care.
Be a guide, not a gatekeeper
Dr. Kar stressed that, of the 8,760 hours in a year, the average patient with type 1 diabetes in the United Kingdom gets just “1-2 hours with you as a clinician, based on four appointments per year of 30 minutes each.”
“So you spend 0.02% of their time with individuals with type 1 diabetes. So, what’s the one thing you can do with that minimal contact? Be nice!”
Dr. Kar said he always has his email open to his adult patients and they are very respectful of his time. “They don’t email you at 1 a.m. That means every one of my patients has got support [from me]. Don’t be a barrier.”
“We have to fundamentally change the narrative. Doctors must have more empathy,” he said, stating that the one thing adolescents have constantly given feedback on has been, “Why don’t appointments start with: ‘How are you?’
“For a teenager, if you throw type 1 diabetes into the loop, it’s not easy,” he stressed. “Talk to them about something else. As a clinician, be a guide, not a gatekeeper. Give people the tools to self-manage better.”
Adult providers can meet these young adult patients “at their level,” Dr. Aleppo agreed.
“Pay attention to their immediate needs and focus on their present circumstances – whether how to get through their next semester in college, navigating job interviews, or handling having diabetes in the workplace.”
Paying attention to the mental health needs of these young patients is equally “paramount,” Dr. Aleppo said.
While access to mental health professionals may be challenging in the adult setting, providers should bring it up with their patients and offer counseling referrals.
“Diabetes impacts everything, and office appointments and conversations carry weight on these patients’ lives as a whole, not just on their diabetes,” she stressed. “A patient told me recently: ‘We’re learning to be adults,’ which can be hard enough, and with diabetes it can be even more challenging. Adult providers need to be aware of the patient’s ‘diabetes language’ in that often it is not what a patient is saying, rather how they are saying it that gives us information on what they truly need.
“As adult providers, we need to also train and teach our young patients to advocate for themselves on where to find resources that can help them navigate adulthood with diabetes,” she added.
One particularly helpful resource in the United States is the College Diabetes Network, a not-for-profit organization whose mission is to equip young adults with type 1 diabetes to successfully manage the challenging transition to independence at college and beyond.
“The sweetest thing that can happen to us as adult diabetes providers is when a patient – seen as an emerging adult during college – returns to your practice 10 years later after moving back and seeks you out for their diabetes care because of the relationship and trust you developed in those transitioning years,” Dr. Aleppo said.
Another resource is a freely available comic book series cocreated by Dr. Kar and colleague Mayank Patel, MBBS, an endocrinologist from University Hospital Southampton NHS Foundation Trust.
As detailed by this news organization in 2021, the series consists of three volumes: the first, Type 1: Origins, focuses on actual experiences of patients who have type 1 diabetes; the second, Type 1: Attack of the Ketones, is aimed at professionals who may provide care but have limited understanding of type 1 diabetes; and the third, Type 1 Mission 3: S.T.I.G.M.A., addresses the stigmas and misconceptions that patients with type 1 diabetes may face.
The idea for the first comic was inspired by a patient who compared having diabetes to being like the Marvel character The Hulk, said Dr. Kar, and has been expanded to include the additional volumes.
Dr. Kar and Dr. Patel have also just launched the fourth comic in the series, Type 1: Generations, to mark the 100-year anniversary since insulin was first given to a human.
“This is high priority”
Dr. Kar said the NHS in England has just appointed a national lead for type 1 diabetes in youth, Fulya Mehta, MD, of Alder Hey Children’s NHS Foundation Trust, Liverpool, England.
“If you have a plan, bring it to us,” he told the audience at the DPC conference, and “tell us, what is the one thing you would change? This is not a session we are doing just to tick a box. This is high priority.
“Encourage your colleagues to think about transition services. This is an absolute priority. We will be asking every center [in England] who is your transitioning lead?”
And he once again stressed that “a lead of transition service does not have to be a medic. This should be a multidisciplinary team. But they do need to be comfortable in that space. To that teenager, your job title means nothing. Give them time and space.”
Dr. Randell summed it up: “If we can work together, it’s only going to result in better outcomes. We need to blaze the trail for young people.”
Dr. Aleppo has reported serving as a consultant to Dexcom and Insulet and receiving support to Northwestern University from AstraZeneca, Dexcom, Eli Lilly, Fractyl Health, Insulet, and Novo Nordisk. Dr. Randell and Dr. Kar have no conflicts of interest.
A version of this article first appeared on Medscape.com.
“No one has asked young people what they want,” said Tabitha Randell, MBChB, an endocrinologist with Nottingham (England) University Hospitals NHS Trust, who specializes in treating teenagers with type 1 diabetes as they transition to adult care.
Dr. Randell, who has set up a very successful specialist service in her hospital for such patients, said: “We consistently have the best, or the second best, outcomes in this country for our diabetes patients.” She believes this is one of the most important issues in modern endocrinology today.
Speaking at the Diabetes Professional Care conference in London at the end of 2021, and sharing her thoughts afterward with this news organization, she noted that in general there are “virtually no published outcomes” on how best to transition a patient with type 1 diabetes from pediatric to adult care.
“If you actually get them to transition – because some just drop out and disengage and there’s nothing you can do – none of them get lost. Some of them disengage in the adult clinic, but if you’re in the young diabetes service [in England] the rules are that if you miss a diabetes appointment you do not get discharged, as compared with the adult clinic, where if you miss an appointment, you are discharged.”
In the young diabetes clinic, doctors will “carry on trying to contact you, and get you back,” she explained. “And the patients do eventually come back in – it might be a year or 2, but they do come back. We’ve just got to keep them alive in the meantime!”
This issue needs tackling all over the world. Dr. Randell said she’s not aware of any one country – although there may be “pockets” of good care within a given country – that is doing this perfectly.
Across the pond, Grazia Aleppo, MD, division of endocrinology at Northwestern University, Chicago, agreed that transitioning pediatric patients with type 1 diabetes to adult care presents “unique challenges.”
Challenges when transitioning from pediatric to adult care
During childhood, type 1 diabetes management is largely supervised by patients’ parents and members of the pediatric diabetes care team, which may include diabetes educators, psychologists, or social workers, as well as pediatric endocrinologists.
When the patient with type 1 diabetes becomes a young adult and takes over management of their own health, Dr. Aleppo said, the care team may diminish along with the time spent in provider visits.
The adult endocrinology setting focuses more on self-management and autonomous functioning of the individual with diabetes.
Adult appointments are typically shorter, and the patient is usually expected to follow doctors’ suggestions independently, she noted. They are also expected to manage the practical aspects of their diabetes care, including prescriptions, diabetes supplies, laboratory tests, scheduling, and keeping appointments.
At the same time that the emerging adult needs to start asserting independence over their health care, they will also be going through a myriad of other important lifestyle changes, such as attending college, living on their own for the first time, and starting a career.
“With these fundamental differences and challenges, competing priorities, such as college, work and relationships, medical care may become of secondary importance and patients may become disengaged,” Dr. Aleppo explained.
As Dr. Randell has said, loss to follow-up is a big problem with this patient population, with disengagement from specialist services and worsening A1c across the transition, Dr. Aleppo noted. This makes addressing these patients’ specific needs extremely important.
Engage with kid, not disease; don’t palm them off on new recruits
“The really key thing these kids say is, ‘I do not want to be a disease,’” Dr. Randell said. “They want you to know that they are a person. Engage these kids!” she suggested. “Ask them: ‘How is your exam revision going?’ Find something positive to say, even if it’s just: ‘I’m glad you came today.’ ”
“If the first thing that you do is tell them off [for poor diabetes care], you are never going to see them again,” she cautioned.
Dr. Randell also said that role models with type 1 diabetes, such as Lila Moss – daughter of British supermodel Kate Moss – who was recently pictured wearing an insulin pump on her leg on the catwalk, are helping youngsters not feel so self-conscious about their diabetes.
“Let them know it’s not the end of the world, having [type 1] diabetes,” she emphasized.
And Partha Kar, MBBS, OBE, national specialty advisor, diabetes with NHS England, agreed wholeheartedly with Dr. Randall.
Reminiscing about his early days as a newly qualified endocrinologist, Dr. Kar, who works at Portsmouth (England) Hospital NHS Trust, noted that as a new member of staff he was given the youth with type 1 diabetes – those getting ready to transition to adult care – to look after.
But this is the exact opposite of what should be happening, he emphasized. “If you don’t think transition care is important, you shouldn’t be treating type 1 diabetes.”
He believes that every diabetes center “must have a young-adult team lead” and this job must not be given to the least experienced member of staff.
This lead “doesn’t need to be a doctor,” Dr. Kar stressed. “It can be a psychologist, or a diabetes nurse, or a pharmacist, or a dietician.”
In short, it must be someone experienced who loves working with this age group.
Dr. Randell agreed: “Make sure the team is interested in young people. It shouldn’t be the last person in who gets the job no one else wants.” Teens “are my favorite group to work with. They don’t take any nonsense.”
And she explained: “Young people like to get to know the person who’s going to take care of them. So, stay with them for their young adult years.” This can be “quite a fluid period,” with it normally extending to age 25, but in some cases, “it can be up to 32 years old.”
Preparing for the transition
To ease pediatric patients into the transition to adult care, Dr. Aleppo recommended that the pediatric diabetes team provide enough time so that any concerns the patient and their family may have can be addressed.
This should also include transferring management responsibilities to the young adult rather than their parent.
The pediatric provider should discuss with the patient available potential adult colleagues, personalizing these options to their needs, she said.
And the adult and pediatric clinicians should collaborate and provide important information beyond medical records or health summaries.
Adult providers should guide young adults on how to navigate the new practices, from scheduling follow-up appointments to policies regarding medication refills or supplies, to providing information about urgent numbers or email addresses for after-hours communications.
Dr. Kar reiterated that there are too few published outcomes in this patient group to guide the establishment of good transition services.
“Without data, we are dead on the ground. Without data, it’s all conjecture, anecdotes,” he said.
What he does know is that, in the latest national type 1 diabetes audit for England, “Diabetic ketoacidosis admissions ... are up in this age group,” which suggests these patients are not receiving adequate care.
Be a guide, not a gatekeeper
Dr. Kar stressed that, of the 8,760 hours in a year, the average patient with type 1 diabetes in the United Kingdom gets just “1-2 hours with you as a clinician, based on four appointments per year of 30 minutes each.”
“So you spend 0.02% of their time with individuals with type 1 diabetes. So, what’s the one thing you can do with that minimal contact? Be nice!”
Dr. Kar said he always has his email open to his adult patients and they are very respectful of his time. “They don’t email you at 1 a.m. That means every one of my patients has got support [from me]. Don’t be a barrier.”
“We have to fundamentally change the narrative. Doctors must have more empathy,” he said, stating that the one thing adolescents have constantly given feedback on has been, “Why don’t appointments start with: ‘How are you?’
“For a teenager, if you throw type 1 diabetes into the loop, it’s not easy,” he stressed. “Talk to them about something else. As a clinician, be a guide, not a gatekeeper. Give people the tools to self-manage better.”
Adult providers can meet these young adult patients “at their level,” Dr. Aleppo agreed.
“Pay attention to their immediate needs and focus on their present circumstances – whether how to get through their next semester in college, navigating job interviews, or handling having diabetes in the workplace.”
Paying attention to the mental health needs of these young patients is equally “paramount,” Dr. Aleppo said.
While access to mental health professionals may be challenging in the adult setting, providers should bring it up with their patients and offer counseling referrals.
“Diabetes impacts everything, and office appointments and conversations carry weight on these patients’ lives as a whole, not just on their diabetes,” she stressed. “A patient told me recently: ‘We’re learning to be adults,’ which can be hard enough, and with diabetes it can be even more challenging. Adult providers need to be aware of the patient’s ‘diabetes language’ in that often it is not what a patient is saying, rather how they are saying it that gives us information on what they truly need.
“As adult providers, we need to also train and teach our young patients to advocate for themselves on where to find resources that can help them navigate adulthood with diabetes,” she added.
One particularly helpful resource in the United States is the College Diabetes Network, a not-for-profit organization whose mission is to equip young adults with type 1 diabetes to successfully manage the challenging transition to independence at college and beyond.
“The sweetest thing that can happen to us as adult diabetes providers is when a patient – seen as an emerging adult during college – returns to your practice 10 years later after moving back and seeks you out for their diabetes care because of the relationship and trust you developed in those transitioning years,” Dr. Aleppo said.
Another resource is a freely available comic book series cocreated by Dr. Kar and colleague Mayank Patel, MBBS, an endocrinologist from University Hospital Southampton NHS Foundation Trust.
As detailed by this news organization in 2021, the series consists of three volumes: the first, Type 1: Origins, focuses on actual experiences of patients who have type 1 diabetes; the second, Type 1: Attack of the Ketones, is aimed at professionals who may provide care but have limited understanding of type 1 diabetes; and the third, Type 1 Mission 3: S.T.I.G.M.A., addresses the stigmas and misconceptions that patients with type 1 diabetes may face.
The idea for the first comic was inspired by a patient who compared having diabetes to being like the Marvel character The Hulk, said Dr. Kar, and has been expanded to include the additional volumes.
Dr. Kar and Dr. Patel have also just launched the fourth comic in the series, Type 1: Generations, to mark the 100-year anniversary since insulin was first given to a human.
“This is high priority”
Dr. Kar said the NHS in England has just appointed a national lead for type 1 diabetes in youth, Fulya Mehta, MD, of Alder Hey Children’s NHS Foundation Trust, Liverpool, England.
“If you have a plan, bring it to us,” he told the audience at the DPC conference, and “tell us, what is the one thing you would change? This is not a session we are doing just to tick a box. This is high priority.
“Encourage your colleagues to think about transition services. This is an absolute priority. We will be asking every center [in England] who is your transitioning lead?”
And he once again stressed that “a lead of transition service does not have to be a medic. This should be a multidisciplinary team. But they do need to be comfortable in that space. To that teenager, your job title means nothing. Give them time and space.”
Dr. Randell summed it up: “If we can work together, it’s only going to result in better outcomes. We need to blaze the trail for young people.”
Dr. Aleppo has reported serving as a consultant to Dexcom and Insulet and receiving support to Northwestern University from AstraZeneca, Dexcom, Eli Lilly, Fractyl Health, Insulet, and Novo Nordisk. Dr. Randell and Dr. Kar have no conflicts of interest.
A version of this article first appeared on Medscape.com.
‘Artificial pancreas’ life-changing in kids with type 1 diabetes
A semiautomated insulin delivery system improved glycemic control in young children with type 1 diabetes aged 1-7 years without increasing hypoglycemia.
“Hybrid closed-loop” systems – comprising an insulin pump, a continuous glucose monitor (CGM), and software enabling communication that semiautomates insulin delivery based on glucose levels – have been shown to improve glucose control in older children and adults.
The technology, also known as an artificial pancreas, has been less studied in very young children even though it may uniquely benefit them, said the authors of the new study, led by Julia Ware, MD, of the Wellcome Trust–Medical Research Council Institute of Metabolic Science and the University of Cambridge (England). The findings were published online Jan. 19, 2022, in the New England Journal of Medicine.
“Very young children are extremely vulnerable to changes in their blood sugar levels. High levels in particular can have potentially lasting consequences to their brain development. On top of that, diabetes is very challenging to manage in this age group, creating a huge burden for families,” she said in a University of Cambridge statement.
There is “high variability of insulin requirements, marked insulin sensitivity, and unpredictable eating and activity patterns,” Dr. Ware and colleagues noted.
“Caregiver fear of hypoglycemia, particularly overnight, is common and, coupled with young children’s unawareness that hypoglycemia is occurring, contributes to children not meeting the recommended glycemic targets or having difficulty maintaining recommended glycemic control unless caregivers can provide constant monitoring. These issues often lead to ... reduced quality of life for the whole family,” they added.
Except for mealtimes, device is fully automated
The new multicenter, randomized, crossover trial was conducted at seven centers across Austria, Germany, Luxembourg, and the United Kingdom in 2019-2020.
The trial compared the safety and efficacy of hybrid closed-loop therapy with sensor-augmented pump therapy (that is, without the device communication, as a control). All 74 children used the CamAPS FX hybrid closed-loop system for 16 weeks, and then used the control treatment for 16 weeks. The children were a mean age of 5.6 years and had a baseline hemoglobin A1c of 7.3% (56.6 mmol/mol).
The hybrid closed-loop system consisted of components that are commercially available in Europe: the Sooil insulin pump (Dana Diabecare RS) and the Dexcom G6 CGM, along with an unlocked Samsung Galaxy 8 smartphone housing an app (CamAPS FX, CamDiab) that runs the Cambridge proprietary model predictive control algorithm.
The smartphone communicates wirelessly with both the pump and the CGM transmitter and automatically adjusts the pump’s insulin delivery based on real-time sensor glucose readings. It also issues alarms if glucose levels fall below or rise above user-specified thresholds. This functionality was disabled during the study control periods.
Senior investigator Roman Hovorka, PhD, who developed the CamAPS FX app, explained in the University of Cambridge statement that the app “makes predictions about what it thinks is likely to happen next based on past experience. It learns how much insulin the child needs per day and how this changes at different times of the day.
“It then uses this [information] to adjust insulin levels to help achieve ideal blood sugar levels. Other than at mealtimes, it is fully automated, so parents do not need to continually monitor their child’s blood sugar levels.”
Indeed, the time spent in target glucose range (70-180 mg/dL) during the 16-week closed-loop period was 8.7 percentage points higher than during the control period (P < .001).
That difference translates to “a clinically meaningful 125 minutes per day,” and represented around three-quarters of their day (71.6%) in the target range, the investigators wrote.
The mean adjusted difference in time spent above 180 mg/dL was 8.5 percentage points lower with the closed-loop, also a significant difference (P < .001). Time spent below 70 mg/dL did not differ significantly between the two interventions (P = .74).
At the end of the study periods, the mean adjusted between-treatment difference in A1c was –0.4 percentage points, significantly lower following the closed-loop, compared with the control period (P < .001).
That percentage point difference (equivalent to 3.9 mmol/mol) “is important in a population of patients who had tight glycemic control at baseline. This result was observed without an increase in the time spent in a hypoglycemic state,” Dr. Ware and colleagues noted.
Median glucose sensor use was 99% during the closed-loop period and 96% during the control periods. During the closed-loop periods, the system was in closed-loop mode 95% of the time.
This finding supports longer-term usability in this age group and compares well with use in older children, they said.
One serious hypoglycemic episode, attributed to parental error rather than system malfunction, occurred during the closed-loop period. There were no episodes of diabetic ketoacidosis. Rates of other adverse events didn’t differ between the two periods.
“CamAPS FX led to improvements in several measures, including hyperglycemia and average blood sugar levels, without increasing the risk of hypos. This is likely to have important benefits for those children who use it,” Dr. Ware summarized.
Sleep quality could improve for children and caregivers
Reductions in time spent in hyperglycemia without increasing hypoglycemia could minimize the risk for neurocognitive deficits that have been reported among young children with type 1 diabetes, the authors speculated.
In addition, they noted that because 80% of overnight sensor readings were within target range and less than 3% were below 70 mg/dL, sleep quality could improve for both the children and their parents. This, in turn, “would confer associated quality of life benefits.”
“Parents have described our artificial pancreas as ‘life changing’ as it meant they were able to relax and spend less time worrying about their child’s blood sugar levels, particularly at nighttime. They tell us it gives them more time to do what any ‘normal’ family can do, to play and do fun things with their children,” observed Dr. Ware.
The CamAPS FX has been commercialized by CamDiab, a spin-out company set up by Dr. Hovorka. It is currently available through several NHS trusts across the United Kingdom, including Cambridge University Hospitals NHS Foundation Trust, and is expected to be more widely available soon.
The study was supported by the European Commission within the Horizon 2020 Framework Program, the NIHR Cambridge Biomedical Research Centre, and JDRF. Dr. Ware had no further disclosures. Dr. Hovorka has reported acting as consultant for Abbott Diabetes Care, BD, Dexcom, being a speaker for Novo Nordisk and Eli Lilly, and receiving royalty payments from B. Braun for software. He is director of CamDiab.
A version of this article first appeared on Medscape.com.
A semiautomated insulin delivery system improved glycemic control in young children with type 1 diabetes aged 1-7 years without increasing hypoglycemia.
“Hybrid closed-loop” systems – comprising an insulin pump, a continuous glucose monitor (CGM), and software enabling communication that semiautomates insulin delivery based on glucose levels – have been shown to improve glucose control in older children and adults.
The technology, also known as an artificial pancreas, has been less studied in very young children even though it may uniquely benefit them, said the authors of the new study, led by Julia Ware, MD, of the Wellcome Trust–Medical Research Council Institute of Metabolic Science and the University of Cambridge (England). The findings were published online Jan. 19, 2022, in the New England Journal of Medicine.
“Very young children are extremely vulnerable to changes in their blood sugar levels. High levels in particular can have potentially lasting consequences to their brain development. On top of that, diabetes is very challenging to manage in this age group, creating a huge burden for families,” she said in a University of Cambridge statement.
There is “high variability of insulin requirements, marked insulin sensitivity, and unpredictable eating and activity patterns,” Dr. Ware and colleagues noted.
“Caregiver fear of hypoglycemia, particularly overnight, is common and, coupled with young children’s unawareness that hypoglycemia is occurring, contributes to children not meeting the recommended glycemic targets or having difficulty maintaining recommended glycemic control unless caregivers can provide constant monitoring. These issues often lead to ... reduced quality of life for the whole family,” they added.
Except for mealtimes, device is fully automated
The new multicenter, randomized, crossover trial was conducted at seven centers across Austria, Germany, Luxembourg, and the United Kingdom in 2019-2020.
The trial compared the safety and efficacy of hybrid closed-loop therapy with sensor-augmented pump therapy (that is, without the device communication, as a control). All 74 children used the CamAPS FX hybrid closed-loop system for 16 weeks, and then used the control treatment for 16 weeks. The children were a mean age of 5.6 years and had a baseline hemoglobin A1c of 7.3% (56.6 mmol/mol).
The hybrid closed-loop system consisted of components that are commercially available in Europe: the Sooil insulin pump (Dana Diabecare RS) and the Dexcom G6 CGM, along with an unlocked Samsung Galaxy 8 smartphone housing an app (CamAPS FX, CamDiab) that runs the Cambridge proprietary model predictive control algorithm.
The smartphone communicates wirelessly with both the pump and the CGM transmitter and automatically adjusts the pump’s insulin delivery based on real-time sensor glucose readings. It also issues alarms if glucose levels fall below or rise above user-specified thresholds. This functionality was disabled during the study control periods.
Senior investigator Roman Hovorka, PhD, who developed the CamAPS FX app, explained in the University of Cambridge statement that the app “makes predictions about what it thinks is likely to happen next based on past experience. It learns how much insulin the child needs per day and how this changes at different times of the day.
“It then uses this [information] to adjust insulin levels to help achieve ideal blood sugar levels. Other than at mealtimes, it is fully automated, so parents do not need to continually monitor their child’s blood sugar levels.”
Indeed, the time spent in target glucose range (70-180 mg/dL) during the 16-week closed-loop period was 8.7 percentage points higher than during the control period (P < .001).
That difference translates to “a clinically meaningful 125 minutes per day,” and represented around three-quarters of their day (71.6%) in the target range, the investigators wrote.
The mean adjusted difference in time spent above 180 mg/dL was 8.5 percentage points lower with the closed-loop, also a significant difference (P < .001). Time spent below 70 mg/dL did not differ significantly between the two interventions (P = .74).
At the end of the study periods, the mean adjusted between-treatment difference in A1c was –0.4 percentage points, significantly lower following the closed-loop, compared with the control period (P < .001).
That percentage point difference (equivalent to 3.9 mmol/mol) “is important in a population of patients who had tight glycemic control at baseline. This result was observed without an increase in the time spent in a hypoglycemic state,” Dr. Ware and colleagues noted.
Median glucose sensor use was 99% during the closed-loop period and 96% during the control periods. During the closed-loop periods, the system was in closed-loop mode 95% of the time.
This finding supports longer-term usability in this age group and compares well with use in older children, they said.
One serious hypoglycemic episode, attributed to parental error rather than system malfunction, occurred during the closed-loop period. There were no episodes of diabetic ketoacidosis. Rates of other adverse events didn’t differ between the two periods.
“CamAPS FX led to improvements in several measures, including hyperglycemia and average blood sugar levels, without increasing the risk of hypos. This is likely to have important benefits for those children who use it,” Dr. Ware summarized.
Sleep quality could improve for children and caregivers
Reductions in time spent in hyperglycemia without increasing hypoglycemia could minimize the risk for neurocognitive deficits that have been reported among young children with type 1 diabetes, the authors speculated.
In addition, they noted that because 80% of overnight sensor readings were within target range and less than 3% were below 70 mg/dL, sleep quality could improve for both the children and their parents. This, in turn, “would confer associated quality of life benefits.”
“Parents have described our artificial pancreas as ‘life changing’ as it meant they were able to relax and spend less time worrying about their child’s blood sugar levels, particularly at nighttime. They tell us it gives them more time to do what any ‘normal’ family can do, to play and do fun things with their children,” observed Dr. Ware.
The CamAPS FX has been commercialized by CamDiab, a spin-out company set up by Dr. Hovorka. It is currently available through several NHS trusts across the United Kingdom, including Cambridge University Hospitals NHS Foundation Trust, and is expected to be more widely available soon.
The study was supported by the European Commission within the Horizon 2020 Framework Program, the NIHR Cambridge Biomedical Research Centre, and JDRF. Dr. Ware had no further disclosures. Dr. Hovorka has reported acting as consultant for Abbott Diabetes Care, BD, Dexcom, being a speaker for Novo Nordisk and Eli Lilly, and receiving royalty payments from B. Braun for software. He is director of CamDiab.
A version of this article first appeared on Medscape.com.
A semiautomated insulin delivery system improved glycemic control in young children with type 1 diabetes aged 1-7 years without increasing hypoglycemia.
“Hybrid closed-loop” systems – comprising an insulin pump, a continuous glucose monitor (CGM), and software enabling communication that semiautomates insulin delivery based on glucose levels – have been shown to improve glucose control in older children and adults.
The technology, also known as an artificial pancreas, has been less studied in very young children even though it may uniquely benefit them, said the authors of the new study, led by Julia Ware, MD, of the Wellcome Trust–Medical Research Council Institute of Metabolic Science and the University of Cambridge (England). The findings were published online Jan. 19, 2022, in the New England Journal of Medicine.
“Very young children are extremely vulnerable to changes in their blood sugar levels. High levels in particular can have potentially lasting consequences to their brain development. On top of that, diabetes is very challenging to manage in this age group, creating a huge burden for families,” she said in a University of Cambridge statement.
There is “high variability of insulin requirements, marked insulin sensitivity, and unpredictable eating and activity patterns,” Dr. Ware and colleagues noted.
“Caregiver fear of hypoglycemia, particularly overnight, is common and, coupled with young children’s unawareness that hypoglycemia is occurring, contributes to children not meeting the recommended glycemic targets or having difficulty maintaining recommended glycemic control unless caregivers can provide constant monitoring. These issues often lead to ... reduced quality of life for the whole family,” they added.
Except for mealtimes, device is fully automated
The new multicenter, randomized, crossover trial was conducted at seven centers across Austria, Germany, Luxembourg, and the United Kingdom in 2019-2020.
The trial compared the safety and efficacy of hybrid closed-loop therapy with sensor-augmented pump therapy (that is, without the device communication, as a control). All 74 children used the CamAPS FX hybrid closed-loop system for 16 weeks, and then used the control treatment for 16 weeks. The children were a mean age of 5.6 years and had a baseline hemoglobin A1c of 7.3% (56.6 mmol/mol).
The hybrid closed-loop system consisted of components that are commercially available in Europe: the Sooil insulin pump (Dana Diabecare RS) and the Dexcom G6 CGM, along with an unlocked Samsung Galaxy 8 smartphone housing an app (CamAPS FX, CamDiab) that runs the Cambridge proprietary model predictive control algorithm.
The smartphone communicates wirelessly with both the pump and the CGM transmitter and automatically adjusts the pump’s insulin delivery based on real-time sensor glucose readings. It also issues alarms if glucose levels fall below or rise above user-specified thresholds. This functionality was disabled during the study control periods.
Senior investigator Roman Hovorka, PhD, who developed the CamAPS FX app, explained in the University of Cambridge statement that the app “makes predictions about what it thinks is likely to happen next based on past experience. It learns how much insulin the child needs per day and how this changes at different times of the day.
“It then uses this [information] to adjust insulin levels to help achieve ideal blood sugar levels. Other than at mealtimes, it is fully automated, so parents do not need to continually monitor their child’s blood sugar levels.”
Indeed, the time spent in target glucose range (70-180 mg/dL) during the 16-week closed-loop period was 8.7 percentage points higher than during the control period (P < .001).
That difference translates to “a clinically meaningful 125 minutes per day,” and represented around three-quarters of their day (71.6%) in the target range, the investigators wrote.
The mean adjusted difference in time spent above 180 mg/dL was 8.5 percentage points lower with the closed-loop, also a significant difference (P < .001). Time spent below 70 mg/dL did not differ significantly between the two interventions (P = .74).
At the end of the study periods, the mean adjusted between-treatment difference in A1c was –0.4 percentage points, significantly lower following the closed-loop, compared with the control period (P < .001).
That percentage point difference (equivalent to 3.9 mmol/mol) “is important in a population of patients who had tight glycemic control at baseline. This result was observed without an increase in the time spent in a hypoglycemic state,” Dr. Ware and colleagues noted.
Median glucose sensor use was 99% during the closed-loop period and 96% during the control periods. During the closed-loop periods, the system was in closed-loop mode 95% of the time.
This finding supports longer-term usability in this age group and compares well with use in older children, they said.
One serious hypoglycemic episode, attributed to parental error rather than system malfunction, occurred during the closed-loop period. There were no episodes of diabetic ketoacidosis. Rates of other adverse events didn’t differ between the two periods.
“CamAPS FX led to improvements in several measures, including hyperglycemia and average blood sugar levels, without increasing the risk of hypos. This is likely to have important benefits for those children who use it,” Dr. Ware summarized.
Sleep quality could improve for children and caregivers
Reductions in time spent in hyperglycemia without increasing hypoglycemia could minimize the risk for neurocognitive deficits that have been reported among young children with type 1 diabetes, the authors speculated.
In addition, they noted that because 80% of overnight sensor readings were within target range and less than 3% were below 70 mg/dL, sleep quality could improve for both the children and their parents. This, in turn, “would confer associated quality of life benefits.”
“Parents have described our artificial pancreas as ‘life changing’ as it meant they were able to relax and spend less time worrying about their child’s blood sugar levels, particularly at nighttime. They tell us it gives them more time to do what any ‘normal’ family can do, to play and do fun things with their children,” observed Dr. Ware.
The CamAPS FX has been commercialized by CamDiab, a spin-out company set up by Dr. Hovorka. It is currently available through several NHS trusts across the United Kingdom, including Cambridge University Hospitals NHS Foundation Trust, and is expected to be more widely available soon.
The study was supported by the European Commission within the Horizon 2020 Framework Program, the NIHR Cambridge Biomedical Research Centre, and JDRF. Dr. Ware had no further disclosures. Dr. Hovorka has reported acting as consultant for Abbott Diabetes Care, BD, Dexcom, being a speaker for Novo Nordisk and Eli Lilly, and receiving royalty payments from B. Braun for software. He is director of CamDiab.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Advances in Diabetes and Cardiovascular Care
Real-World Experience With Automated Insulin Pumps
Continuous Blood Glucose Monitoring for T2DM
Statin-Induced Adverse Effects
Long QT and Cardiac Arrest After Pulmonary Edema
And more online
• Clinical Impact of U-500 Insulin Initiation
• Diabetes Self-Management Education
• SGLT2 Inhibitors, T2DM, and Heart Failure
• Alirocumab Use in Statin-Intolerant Veterans
• K Pneumoniae-Induced Aortitis
Real-World Experience With Automated Insulin Pumps
Continuous Blood Glucose Monitoring for T2DM
Statin-Induced Adverse Effects
Long QT and Cardiac Arrest After Pulmonary Edema
And more online
• Clinical Impact of U-500 Insulin Initiation
• Diabetes Self-Management Education
• SGLT2 Inhibitors, T2DM, and Heart Failure
• Alirocumab Use in Statin-Intolerant Veterans
• K Pneumoniae-Induced Aortitis
Real-World Experience With Automated Insulin Pumps
Continuous Blood Glucose Monitoring for T2DM
Statin-Induced Adverse Effects
Long QT and Cardiac Arrest After Pulmonary Edema
And more online
• Clinical Impact of U-500 Insulin Initiation
• Diabetes Self-Management Education
• SGLT2 Inhibitors, T2DM, and Heart Failure
• Alirocumab Use in Statin-Intolerant Veterans
• K Pneumoniae-Induced Aortitis





