User login
Family physician Joseph E. Scherger champions lifestyle change
Joseph E. Scherger, MD, MPH, is a family physician of 40 years and an avid runner who has carried over his passion for fitness and nutrition into treating patients.
He achieved this through moving to practicing functional medicine a decade ago.
According to Dr. Scherger, functional medicine “shifts the whole approach [to family medicine], recognizing that people’s chronic diseases, like hypertension and diabetes, are completely reversible, and the reason why is because they’re caused by what we eat and how we live.”
Practicing functional medicine continues to make working exciting for Dr. Scherger, he says.
“Now that I’ve shifted into nutrition and lifestyle, I feel like I’m a healer, you know? I’m not just refilling prescriptions anymore,” he said.
The burden of disease brought about by bad nutrition and our profit-hungry food industry is staggering, explained Dr. Scherger, As such, he encourages his patients to adopt lifestyle and nutritional changes that allow the body to become healthy again.
Dr. Scherger’s shift into lifestyle-oriented medicine reflects his own experiences with healthy living, and how it has impacted his life.
“I’m 70 years old, and I’m still running, and I feel the same as when I was 40 or 50.” He has completed 40 marathons, ten 50K and five 50-mile ultramarathon trail runs, and, although retired from long-distance running, he is currently training for an upcoming 5K Thanksgiving turkey trot with his 6-year-old grandson. “He loves it. He’s faster than I am, I have trouble keeping up with him,” he confessed.
Earlier days of career
“I’ve been very blessed to have a career that kept changing every 5-10 years,” he said. “I’ve been able to evolve in a way of shifting my interests from one area to another,” he said.
Dr. Scherger has held many positions in the medical field, from serving in the National Health Service Corps in Dixon, Calif., as a migrant health physician during 1978-1980, to being chair of graduate medical education at Eisenhower Medical Center in Rancho Mirage, Calif., from 2009 to 2015. In between, he taught at the University of California, Davis, and served as founding dean of the Florida State University College of Medicine.
Originally from Ohio, Dr. Scherger was born in 1950 in the small town of Delphos. He graduated from the University of Dayton in 1971 before attending medical school at University of California, Los Angeles, for 4 years. He then completed a family medicine residency and a masters in public health at the University of Washington, Seattle, in 1978.
A resident of the Golden State for 50 years now, Dr. Scherger describes himself as a “true Californian.” Currently, he is in practice at Eisenhower Health in La Quinta, Calif., where he is a core faculty member in the family medicine residency program. He is also a physician under the health center’s Primary Care 365 program, which offers patients regular communication with and increased access to their physicians, emphasizing on telemedicine. He also founded Restore Health – Disease Reversal, a wellness center in Indian Wells, Calif., that focuses on improving patients’ health through changes in nutrition and lifestyle.
Within his medical practice, Dr. Scherger is seen by colleagues as a doctor who not only advocates for his patients, but also goes above and beyond to solve their problems.
“He’s a leader, an advocate, and he inspires others to do what they do,” said Julia L. Martin, MD, a fellow family medicine practitioner who has been working with Dr. Scherger at the Eisenhower Medical Center for the past 5 years. “Being a physician is a very challenging role. You need to be patient and understanding in trying to investigate what the patient wants and work through that to try to find the solution. Dr. Scherger is really good at that.”
Inspiration for writing
Apart from his roles as a physician and faculty member, Dr. Scherger is also an author of two books: “40 Years in Family Medicine” (Scotts Valley, Calif.: CreateSpace, 2014) and “Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness” (Scotts Valley, Calif.: CreateSpace, 2015). He admits to not being a naturally gifted writer, and is more intrinsically skilled at speaking. When he was in medical school, however, a mentor told him that the written word is eternal, and this left a deep impression on him.
“When I think of something that’s worth writing about, that I think will be a contribution to my field, I don’t hesitate to begin to write and develop,” said Dr. Scherger. “ I’ve done some research that I’m proud of, but most of [my writings] are hopefully thoughtful essays to help move my field along, and it’s enormously satisfying to make these contributions.”
Awards and other contributions to family medicine
Dr. Scherger’s contributions to the field of family medicine have been recognized continuously over his career.
He has served on the board of directors of the American Academy of Family Physicians and the American Board of Family Medicine. He is also the recipient of numerous awards, such as being chosen as Family Physician of the Year by the American Academy of Family Physicians and the California Academy of Family Physicians in 1989. From 1988 to 1991, he was a fellow in the Kellogg National Fellowship Program.
While he has managed to reinvent his own practice and medical focus, Dr. Scherger is also concerned with the need to remodel the current state of primary care and family medicine. Regarding challenges facing the field, he mentions the burnout faced by many doctors.
Nowadays, the work of family medicine includes much more than those common acute illnesses – it includes preventive medicine, chronic illness management and mental health counseling. “Yet, somehow, the whole economic and schedule model is based on brief visits,” said Dr. Scherger. “I think the most common reason that a lot of family doctors are burned out is that they’re expected to see so many people a day, and they know they don’t have enough time to do a really good job.”
He elaborated: “The real challenge now for family practice is to be re-engineered to be for the modern age, and not be still stuck in a ‘make an appointment, come and get it’ model of care, which is outdated. So I’ve been working a long time in trying to reinvent primary care. And, you know, it’s hard to make those changes, and it’s still a work in progress.”
One of the ways Dr. Scherger has been working on the primary care model is to help redesign it for the computer age. He started doing telemedicine and online care in 1997, even though other doctors gave him pushback for doing so at the time. Today, in his practice, half of his patients are remote, and under Eisenhower’s Primary Care 365 service, he uses telemedicine to its fullest potential.
Dr. Martin calls Dr. Scherger an “innovator,” adding: “He really tries to find what works for a solution, in different ways – not just one cookie cutter way.”
Despite nearly 50 years of being a doctor, the profession has not gotten any less rewarding for Dr. Scherger, who says he does not intend to retire as long as he is any good at it.
“My mother always said, ‘Joe, your life should be dedicated to making the world a better place.’ I really took that to heart and realized that my greatest joy is to help other people.”
Joseph E. Scherger, MD, MPH, is a family physician of 40 years and an avid runner who has carried over his passion for fitness and nutrition into treating patients.
He achieved this through moving to practicing functional medicine a decade ago.
According to Dr. Scherger, functional medicine “shifts the whole approach [to family medicine], recognizing that people’s chronic diseases, like hypertension and diabetes, are completely reversible, and the reason why is because they’re caused by what we eat and how we live.”
Practicing functional medicine continues to make working exciting for Dr. Scherger, he says.
“Now that I’ve shifted into nutrition and lifestyle, I feel like I’m a healer, you know? I’m not just refilling prescriptions anymore,” he said.
The burden of disease brought about by bad nutrition and our profit-hungry food industry is staggering, explained Dr. Scherger, As such, he encourages his patients to adopt lifestyle and nutritional changes that allow the body to become healthy again.
Dr. Scherger’s shift into lifestyle-oriented medicine reflects his own experiences with healthy living, and how it has impacted his life.
“I’m 70 years old, and I’m still running, and I feel the same as when I was 40 or 50.” He has completed 40 marathons, ten 50K and five 50-mile ultramarathon trail runs, and, although retired from long-distance running, he is currently training for an upcoming 5K Thanksgiving turkey trot with his 6-year-old grandson. “He loves it. He’s faster than I am, I have trouble keeping up with him,” he confessed.
Earlier days of career
“I’ve been very blessed to have a career that kept changing every 5-10 years,” he said. “I’ve been able to evolve in a way of shifting my interests from one area to another,” he said.
Dr. Scherger has held many positions in the medical field, from serving in the National Health Service Corps in Dixon, Calif., as a migrant health physician during 1978-1980, to being chair of graduate medical education at Eisenhower Medical Center in Rancho Mirage, Calif., from 2009 to 2015. In between, he taught at the University of California, Davis, and served as founding dean of the Florida State University College of Medicine.
Originally from Ohio, Dr. Scherger was born in 1950 in the small town of Delphos. He graduated from the University of Dayton in 1971 before attending medical school at University of California, Los Angeles, for 4 years. He then completed a family medicine residency and a masters in public health at the University of Washington, Seattle, in 1978.
A resident of the Golden State for 50 years now, Dr. Scherger describes himself as a “true Californian.” Currently, he is in practice at Eisenhower Health in La Quinta, Calif., where he is a core faculty member in the family medicine residency program. He is also a physician under the health center’s Primary Care 365 program, which offers patients regular communication with and increased access to their physicians, emphasizing on telemedicine. He also founded Restore Health – Disease Reversal, a wellness center in Indian Wells, Calif., that focuses on improving patients’ health through changes in nutrition and lifestyle.
Within his medical practice, Dr. Scherger is seen by colleagues as a doctor who not only advocates for his patients, but also goes above and beyond to solve their problems.
“He’s a leader, an advocate, and he inspires others to do what they do,” said Julia L. Martin, MD, a fellow family medicine practitioner who has been working with Dr. Scherger at the Eisenhower Medical Center for the past 5 years. “Being a physician is a very challenging role. You need to be patient and understanding in trying to investigate what the patient wants and work through that to try to find the solution. Dr. Scherger is really good at that.”
Inspiration for writing
Apart from his roles as a physician and faculty member, Dr. Scherger is also an author of two books: “40 Years in Family Medicine” (Scotts Valley, Calif.: CreateSpace, 2014) and “Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness” (Scotts Valley, Calif.: CreateSpace, 2015). He admits to not being a naturally gifted writer, and is more intrinsically skilled at speaking. When he was in medical school, however, a mentor told him that the written word is eternal, and this left a deep impression on him.
“When I think of something that’s worth writing about, that I think will be a contribution to my field, I don’t hesitate to begin to write and develop,” said Dr. Scherger. “ I’ve done some research that I’m proud of, but most of [my writings] are hopefully thoughtful essays to help move my field along, and it’s enormously satisfying to make these contributions.”
Awards and other contributions to family medicine
Dr. Scherger’s contributions to the field of family medicine have been recognized continuously over his career.
He has served on the board of directors of the American Academy of Family Physicians and the American Board of Family Medicine. He is also the recipient of numerous awards, such as being chosen as Family Physician of the Year by the American Academy of Family Physicians and the California Academy of Family Physicians in 1989. From 1988 to 1991, he was a fellow in the Kellogg National Fellowship Program.
While he has managed to reinvent his own practice and medical focus, Dr. Scherger is also concerned with the need to remodel the current state of primary care and family medicine. Regarding challenges facing the field, he mentions the burnout faced by many doctors.
Nowadays, the work of family medicine includes much more than those common acute illnesses – it includes preventive medicine, chronic illness management and mental health counseling. “Yet, somehow, the whole economic and schedule model is based on brief visits,” said Dr. Scherger. “I think the most common reason that a lot of family doctors are burned out is that they’re expected to see so many people a day, and they know they don’t have enough time to do a really good job.”
He elaborated: “The real challenge now for family practice is to be re-engineered to be for the modern age, and not be still stuck in a ‘make an appointment, come and get it’ model of care, which is outdated. So I’ve been working a long time in trying to reinvent primary care. And, you know, it’s hard to make those changes, and it’s still a work in progress.”
One of the ways Dr. Scherger has been working on the primary care model is to help redesign it for the computer age. He started doing telemedicine and online care in 1997, even though other doctors gave him pushback for doing so at the time. Today, in his practice, half of his patients are remote, and under Eisenhower’s Primary Care 365 service, he uses telemedicine to its fullest potential.
Dr. Martin calls Dr. Scherger an “innovator,” adding: “He really tries to find what works for a solution, in different ways – not just one cookie cutter way.”
Despite nearly 50 years of being a doctor, the profession has not gotten any less rewarding for Dr. Scherger, who says he does not intend to retire as long as he is any good at it.
“My mother always said, ‘Joe, your life should be dedicated to making the world a better place.’ I really took that to heart and realized that my greatest joy is to help other people.”
Joseph E. Scherger, MD, MPH, is a family physician of 40 years and an avid runner who has carried over his passion for fitness and nutrition into treating patients.
He achieved this through moving to practicing functional medicine a decade ago.
According to Dr. Scherger, functional medicine “shifts the whole approach [to family medicine], recognizing that people’s chronic diseases, like hypertension and diabetes, are completely reversible, and the reason why is because they’re caused by what we eat and how we live.”
Practicing functional medicine continues to make working exciting for Dr. Scherger, he says.
“Now that I’ve shifted into nutrition and lifestyle, I feel like I’m a healer, you know? I’m not just refilling prescriptions anymore,” he said.
The burden of disease brought about by bad nutrition and our profit-hungry food industry is staggering, explained Dr. Scherger, As such, he encourages his patients to adopt lifestyle and nutritional changes that allow the body to become healthy again.
Dr. Scherger’s shift into lifestyle-oriented medicine reflects his own experiences with healthy living, and how it has impacted his life.
“I’m 70 years old, and I’m still running, and I feel the same as when I was 40 or 50.” He has completed 40 marathons, ten 50K and five 50-mile ultramarathon trail runs, and, although retired from long-distance running, he is currently training for an upcoming 5K Thanksgiving turkey trot with his 6-year-old grandson. “He loves it. He’s faster than I am, I have trouble keeping up with him,” he confessed.
Earlier days of career
“I’ve been very blessed to have a career that kept changing every 5-10 years,” he said. “I’ve been able to evolve in a way of shifting my interests from one area to another,” he said.
Dr. Scherger has held many positions in the medical field, from serving in the National Health Service Corps in Dixon, Calif., as a migrant health physician during 1978-1980, to being chair of graduate medical education at Eisenhower Medical Center in Rancho Mirage, Calif., from 2009 to 2015. In between, he taught at the University of California, Davis, and served as founding dean of the Florida State University College of Medicine.
Originally from Ohio, Dr. Scherger was born in 1950 in the small town of Delphos. He graduated from the University of Dayton in 1971 before attending medical school at University of California, Los Angeles, for 4 years. He then completed a family medicine residency and a masters in public health at the University of Washington, Seattle, in 1978.
A resident of the Golden State for 50 years now, Dr. Scherger describes himself as a “true Californian.” Currently, he is in practice at Eisenhower Health in La Quinta, Calif., where he is a core faculty member in the family medicine residency program. He is also a physician under the health center’s Primary Care 365 program, which offers patients regular communication with and increased access to their physicians, emphasizing on telemedicine. He also founded Restore Health – Disease Reversal, a wellness center in Indian Wells, Calif., that focuses on improving patients’ health through changes in nutrition and lifestyle.
Within his medical practice, Dr. Scherger is seen by colleagues as a doctor who not only advocates for his patients, but also goes above and beyond to solve their problems.
“He’s a leader, an advocate, and he inspires others to do what they do,” said Julia L. Martin, MD, a fellow family medicine practitioner who has been working with Dr. Scherger at the Eisenhower Medical Center for the past 5 years. “Being a physician is a very challenging role. You need to be patient and understanding in trying to investigate what the patient wants and work through that to try to find the solution. Dr. Scherger is really good at that.”
Inspiration for writing
Apart from his roles as a physician and faculty member, Dr. Scherger is also an author of two books: “40 Years in Family Medicine” (Scotts Valley, Calif.: CreateSpace, 2014) and “Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness” (Scotts Valley, Calif.: CreateSpace, 2015). He admits to not being a naturally gifted writer, and is more intrinsically skilled at speaking. When he was in medical school, however, a mentor told him that the written word is eternal, and this left a deep impression on him.
“When I think of something that’s worth writing about, that I think will be a contribution to my field, I don’t hesitate to begin to write and develop,” said Dr. Scherger. “ I’ve done some research that I’m proud of, but most of [my writings] are hopefully thoughtful essays to help move my field along, and it’s enormously satisfying to make these contributions.”
Awards and other contributions to family medicine
Dr. Scherger’s contributions to the field of family medicine have been recognized continuously over his career.
He has served on the board of directors of the American Academy of Family Physicians and the American Board of Family Medicine. He is also the recipient of numerous awards, such as being chosen as Family Physician of the Year by the American Academy of Family Physicians and the California Academy of Family Physicians in 1989. From 1988 to 1991, he was a fellow in the Kellogg National Fellowship Program.
While he has managed to reinvent his own practice and medical focus, Dr. Scherger is also concerned with the need to remodel the current state of primary care and family medicine. Regarding challenges facing the field, he mentions the burnout faced by many doctors.
Nowadays, the work of family medicine includes much more than those common acute illnesses – it includes preventive medicine, chronic illness management and mental health counseling. “Yet, somehow, the whole economic and schedule model is based on brief visits,” said Dr. Scherger. “I think the most common reason that a lot of family doctors are burned out is that they’re expected to see so many people a day, and they know they don’t have enough time to do a really good job.”
He elaborated: “The real challenge now for family practice is to be re-engineered to be for the modern age, and not be still stuck in a ‘make an appointment, come and get it’ model of care, which is outdated. So I’ve been working a long time in trying to reinvent primary care. And, you know, it’s hard to make those changes, and it’s still a work in progress.”
One of the ways Dr. Scherger has been working on the primary care model is to help redesign it for the computer age. He started doing telemedicine and online care in 1997, even though other doctors gave him pushback for doing so at the time. Today, in his practice, half of his patients are remote, and under Eisenhower’s Primary Care 365 service, he uses telemedicine to its fullest potential.
Dr. Martin calls Dr. Scherger an “innovator,” adding: “He really tries to find what works for a solution, in different ways – not just one cookie cutter way.”
Despite nearly 50 years of being a doctor, the profession has not gotten any less rewarding for Dr. Scherger, who says he does not intend to retire as long as he is any good at it.
“My mother always said, ‘Joe, your life should be dedicated to making the world a better place.’ I really took that to heart and realized that my greatest joy is to help other people.”
SGLT2 inhibitor use rising in patients with DKD
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
FROM DIABETES CARE
Mediterranean diet slows progression of atherosclerosis in CHD
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Empagliflozin gets HFrEF approval from FDA
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.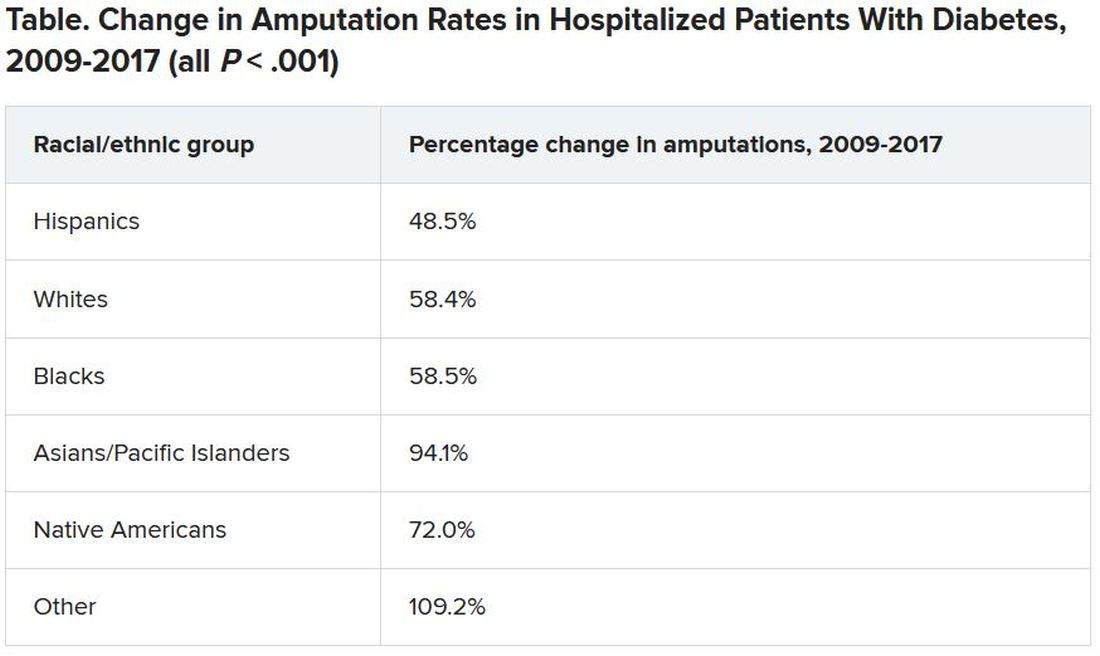
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin D pills do not alter kidney function in prediabetes
However, most of these adults with prediabetes plus obesity or overweight also had sufficient serum levels of 25-hydroxyvitamin D (25[OH]D) and a low risk for adverse kidney outcomes at study entry.
“The benefits of vitamin D might be greater in people with low blood vitamin D levels and/or reduced kidney function,” lead author Sun H. Kim, MD, Stanford (Calif.) University, speculated in a statement from the American Society of Nephrology.
The study was published online August 6 in the Clinical Journal of the American Society of Nephrology.
“The D2d study is unique because we recruited individuals with high-risk prediabetes, having two out of three abnormal glucose values, and we recruited more than 2,000 participants, representing the largest vitamin D diabetes prevention trial to date,” Dr. Kim pointed out.
Although the study did not show a benefit of vitamin D supplements on kidney function outcomes, 43% of participants were already taking up to 1,000 IU of vitamin D daily when they entered the study, she noted.
A subgroup analysis of individuals who were not taking vitamin D at study entry found that vitamin D supplements were associated with lowered proteinuria, “which means that it could have a beneficial effect on kidney health,” said Dr. Kim, cautioning that “additional studies are needed to look into this further.”
Effect of vitamin D on three kidney function outcomes
Although low levels of serum 25(OH)D are associated with kidney disease, few trials have evaluated how vitamin D supplements might affect kidney function, Dr. Kim and colleagues write.
The D2d trial, they note, found that vitamin D supplements did not lower the risk of incident diabetes in people with prediabetes recruited from medical centers across the United States, as previously reported in 2019.
However, since then, meta-analyses that included the D2d trial have reported a significant 11%-12% reduction in diabetes risk in people with prediabetes who took vitamin D supplements.
The current secondary analysis of D2d aimed to investigate whether vitamin D supplements affect kidney function in people with prediabetes.
A total of 2,166 participants in D2d with complete kidney function data were included in the analysis.
The three study outcomes were change in estimated glomerular filtration rate (eGFR) from baseline, change in urine albumin-to-creatinine ratio (UACR) from baseline, and worsening Kidney Disease: Improving Global Outcomes (KDIGO) risk score (which takes eGFR and UACR into account).
At baseline, patients were a mean age of 60, had a mean body mass index (BMI) of 32 kg/m2, and 44% were women.
Most (79%) had hypertension, 52% were receiving antihypertensives, and 33% were receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Participants had a mean serum 25(OH) level of 28 ng/mL.
They had a mean eGFR of 87 mL/min/1.73 m2 and a mean UACR of 11 mg/g. Only 10% had a moderate, high, or very high KDIGO risk score.
Participants were randomized to receive a daily gel pill containing 4,000 IU vitamin D3 (cholecalciferol) or placebo.
Medication adherence was high (83%) in both groups during a median follow-up of 2.9 years.
There was no significant between-group difference in the following kidney function outcomes:
- 28 patients in the vitamin D group and 30 patients in the placebo group had a worsening KDIGO risk score.
- The mean difference in eGFR from baseline was -1.0 mL/min/1.73 m2 in the vitamin D group and -0.1 mL/min/1.73 m2 in the placebo group.
- The mean difference in UACR from baseline was 2.7 mg/g in the vitamin D group and 2.0 mg/g in the placebo group.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, most of these adults with prediabetes plus obesity or overweight also had sufficient serum levels of 25-hydroxyvitamin D (25[OH]D) and a low risk for adverse kidney outcomes at study entry.
“The benefits of vitamin D might be greater in people with low blood vitamin D levels and/or reduced kidney function,” lead author Sun H. Kim, MD, Stanford (Calif.) University, speculated in a statement from the American Society of Nephrology.
The study was published online August 6 in the Clinical Journal of the American Society of Nephrology.
“The D2d study is unique because we recruited individuals with high-risk prediabetes, having two out of three abnormal glucose values, and we recruited more than 2,000 participants, representing the largest vitamin D diabetes prevention trial to date,” Dr. Kim pointed out.
Although the study did not show a benefit of vitamin D supplements on kidney function outcomes, 43% of participants were already taking up to 1,000 IU of vitamin D daily when they entered the study, she noted.
A subgroup analysis of individuals who were not taking vitamin D at study entry found that vitamin D supplements were associated with lowered proteinuria, “which means that it could have a beneficial effect on kidney health,” said Dr. Kim, cautioning that “additional studies are needed to look into this further.”
Effect of vitamin D on three kidney function outcomes
Although low levels of serum 25(OH)D are associated with kidney disease, few trials have evaluated how vitamin D supplements might affect kidney function, Dr. Kim and colleagues write.
The D2d trial, they note, found that vitamin D supplements did not lower the risk of incident diabetes in people with prediabetes recruited from medical centers across the United States, as previously reported in 2019.
However, since then, meta-analyses that included the D2d trial have reported a significant 11%-12% reduction in diabetes risk in people with prediabetes who took vitamin D supplements.
The current secondary analysis of D2d aimed to investigate whether vitamin D supplements affect kidney function in people with prediabetes.
A total of 2,166 participants in D2d with complete kidney function data were included in the analysis.
The three study outcomes were change in estimated glomerular filtration rate (eGFR) from baseline, change in urine albumin-to-creatinine ratio (UACR) from baseline, and worsening Kidney Disease: Improving Global Outcomes (KDIGO) risk score (which takes eGFR and UACR into account).
At baseline, patients were a mean age of 60, had a mean body mass index (BMI) of 32 kg/m2, and 44% were women.
Most (79%) had hypertension, 52% were receiving antihypertensives, and 33% were receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Participants had a mean serum 25(OH) level of 28 ng/mL.
They had a mean eGFR of 87 mL/min/1.73 m2 and a mean UACR of 11 mg/g. Only 10% had a moderate, high, or very high KDIGO risk score.
Participants were randomized to receive a daily gel pill containing 4,000 IU vitamin D3 (cholecalciferol) or placebo.
Medication adherence was high (83%) in both groups during a median follow-up of 2.9 years.
There was no significant between-group difference in the following kidney function outcomes:
- 28 patients in the vitamin D group and 30 patients in the placebo group had a worsening KDIGO risk score.
- The mean difference in eGFR from baseline was -1.0 mL/min/1.73 m2 in the vitamin D group and -0.1 mL/min/1.73 m2 in the placebo group.
- The mean difference in UACR from baseline was 2.7 mg/g in the vitamin D group and 2.0 mg/g in the placebo group.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, most of these adults with prediabetes plus obesity or overweight also had sufficient serum levels of 25-hydroxyvitamin D (25[OH]D) and a low risk for adverse kidney outcomes at study entry.
“The benefits of vitamin D might be greater in people with low blood vitamin D levels and/or reduced kidney function,” lead author Sun H. Kim, MD, Stanford (Calif.) University, speculated in a statement from the American Society of Nephrology.
The study was published online August 6 in the Clinical Journal of the American Society of Nephrology.
“The D2d study is unique because we recruited individuals with high-risk prediabetes, having two out of three abnormal glucose values, and we recruited more than 2,000 participants, representing the largest vitamin D diabetes prevention trial to date,” Dr. Kim pointed out.
Although the study did not show a benefit of vitamin D supplements on kidney function outcomes, 43% of participants were already taking up to 1,000 IU of vitamin D daily when they entered the study, she noted.
A subgroup analysis of individuals who were not taking vitamin D at study entry found that vitamin D supplements were associated with lowered proteinuria, “which means that it could have a beneficial effect on kidney health,” said Dr. Kim, cautioning that “additional studies are needed to look into this further.”
Effect of vitamin D on three kidney function outcomes
Although low levels of serum 25(OH)D are associated with kidney disease, few trials have evaluated how vitamin D supplements might affect kidney function, Dr. Kim and colleagues write.
The D2d trial, they note, found that vitamin D supplements did not lower the risk of incident diabetes in people with prediabetes recruited from medical centers across the United States, as previously reported in 2019.
However, since then, meta-analyses that included the D2d trial have reported a significant 11%-12% reduction in diabetes risk in people with prediabetes who took vitamin D supplements.
The current secondary analysis of D2d aimed to investigate whether vitamin D supplements affect kidney function in people with prediabetes.
A total of 2,166 participants in D2d with complete kidney function data were included in the analysis.
The three study outcomes were change in estimated glomerular filtration rate (eGFR) from baseline, change in urine albumin-to-creatinine ratio (UACR) from baseline, and worsening Kidney Disease: Improving Global Outcomes (KDIGO) risk score (which takes eGFR and UACR into account).
At baseline, patients were a mean age of 60, had a mean body mass index (BMI) of 32 kg/m2, and 44% were women.
Most (79%) had hypertension, 52% were receiving antihypertensives, and 33% were receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Participants had a mean serum 25(OH) level of 28 ng/mL.
They had a mean eGFR of 87 mL/min/1.73 m2 and a mean UACR of 11 mg/g. Only 10% had a moderate, high, or very high KDIGO risk score.
Participants were randomized to receive a daily gel pill containing 4,000 IU vitamin D3 (cholecalciferol) or placebo.
Medication adherence was high (83%) in both groups during a median follow-up of 2.9 years.
There was no significant between-group difference in the following kidney function outcomes:
- 28 patients in the vitamin D group and 30 patients in the placebo group had a worsening KDIGO risk score.
- The mean difference in eGFR from baseline was -1.0 mL/min/1.73 m2 in the vitamin D group and -0.1 mL/min/1.73 m2 in the placebo group.
- The mean difference in UACR from baseline was 2.7 mg/g in the vitamin D group and 2.0 mg/g in the placebo group.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves rapid-acting insulin, Lyumjev, for pump use
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
Brain memory signals appear to regulate metabolism
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low glycemic diet improves A1c, other risk factors in diabetes
A diet rich in vegetables and low in carbs – a so-called low glycemic index (GI) diet – is associated with clinically significant benefits beyond those provided by existing medications for people with type 1 and type 2 diabetes, compared with a higher glycemic diet, findings from a new meta-analysis show.
“Although the effects were small, which is not surprising in clinical trials in nutrition, they were clinically meaningful improvements for which our certainty in the effects were moderate to high,” first author Laura Chiavaroli, PhD, of the department of nutritional sciences, Temerty Faculty of Medicine, University of Toronto, said in an interview.
The GI rates foods on the basis of how quickly they affect blood glucose levels.
Fruits, vegetables, and whole grains have a low GI. They also help to regulate blood sugar levels. Such foods are linked to a reduced risk for heart disease among people with diabetes.
But guidelines on this – such as those from the European Association for the Study of Diabetes – reflect research published more than 15 years ago, before several key trials were published.
Dr. Chiavaroli and colleagues identified 27 randomized controlled trials – the most recent of which was published in May 2021 – that involved a total of 1,617 adults with type 1 or 2 diabetes. For the patients in these trials, diabetes was moderately controlled with glucose-lowering drugs or insulin. All of the included trials examined the effects of a low GI diet or a low glycemic load (GL) diet for people with diabetes over a period 3 or more weeks. The majority of patients in the studies were overweight or had obesity, and they were largely middle-aged.
The meta-analysis, which included new data, was published Aug. 5 in The BMJ. The study “expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE [grading of recommendations assessment, development, and evaluation],” Dr. Chiavaroli and colleagues noted.
“The available evidence provides a good indication of the likely benefit in this population and supports existing recommendations for the use of low GI dietary patterns in the management of diabetes,” they emphasized.
Improvements in A1c, fasting glucose, cholesterol, and triglycerides
Overall, compared with people who consumed diets with higher GI/GL ratings, for those who consumed lower glycemic diets, glycemic control was significantly improved, as reflected in A1c level, which was the primary outcome of the study (mean difference, –0.31%; P < .001).
This “would meet the threshold of ≥ 0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications,” the authors noted.
Those who consumed low glycemic diets also showed improvements in secondary outcomes, including fasting glucose level, which was reduced by 0.36 mmol/L (–6.5 mg/dL), a 6% reduction in low-density cholesterol (LDL-C) (–0.17 mmol/L), and a fall in triglyceride levels (–0.09 mmol/L).
They also lost marginally more body weight, at –0.66 kg (–1.5 pounds). Body mass index was lower by –0.38, and inflammation was reduced (C-reactive protein, –.41 mg/L; all P < .05).
No significant differences were observed between the groups in blood insulin level, high-density lipoprotein cholesterol level, waist circumference, or blood pressure.
Three of the studies showed that participants developed a preference for the low GI diet. “In recent years, there has been a growing interest in whole-food plant-based diets, and there are more options, for example, for pulse-based products,” Dr. Chiavaroli said.
This meta-analysis should support the recommendation of the low-glycemic diet, particularly among people with diabetes, she reiterated.
Will larger randomized trial show effect on outcomes?
The authors noted, however, that to determine whether these small improvements in intermediate cardiometabolic risk factors observed with low GI diets translate to reductions in cardiovascular disease, nephropathy, and retinopathy among people with diabetes, larger randomized trials are needed.
One such trial, the Low Glycemic Index Diet for Type 2 Diabetics, includes 169 high-risk patients with type 2 diabetes and subclinical atherosclerosis. The investigators are evaluating the effect of a low GI diet on the progression of atherosclerosis, as assessed by vascular MRI over 3 years.
“We await the results,” they said.
The study received funding from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD) as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. The study was also supported by the Canadian Institutes of Health Research through the Canada-wide Human Nutrition Trialists’ Network. The Diet, Digestive Tract, and Disease (3D) Center, which is funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the study.
A version of this article first appeared on Medscape.com.
A diet rich in vegetables and low in carbs – a so-called low glycemic index (GI) diet – is associated with clinically significant benefits beyond those provided by existing medications for people with type 1 and type 2 diabetes, compared with a higher glycemic diet, findings from a new meta-analysis show.
“Although the effects were small, which is not surprising in clinical trials in nutrition, they were clinically meaningful improvements for which our certainty in the effects were moderate to high,” first author Laura Chiavaroli, PhD, of the department of nutritional sciences, Temerty Faculty of Medicine, University of Toronto, said in an interview.
The GI rates foods on the basis of how quickly they affect blood glucose levels.
Fruits, vegetables, and whole grains have a low GI. They also help to regulate blood sugar levels. Such foods are linked to a reduced risk for heart disease among people with diabetes.
But guidelines on this – such as those from the European Association for the Study of Diabetes – reflect research published more than 15 years ago, before several key trials were published.
Dr. Chiavaroli and colleagues identified 27 randomized controlled trials – the most recent of which was published in May 2021 – that involved a total of 1,617 adults with type 1 or 2 diabetes. For the patients in these trials, diabetes was moderately controlled with glucose-lowering drugs or insulin. All of the included trials examined the effects of a low GI diet or a low glycemic load (GL) diet for people with diabetes over a period 3 or more weeks. The majority of patients in the studies were overweight or had obesity, and they were largely middle-aged.
The meta-analysis, which included new data, was published Aug. 5 in The BMJ. The study “expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE [grading of recommendations assessment, development, and evaluation],” Dr. Chiavaroli and colleagues noted.
“The available evidence provides a good indication of the likely benefit in this population and supports existing recommendations for the use of low GI dietary patterns in the management of diabetes,” they emphasized.
Improvements in A1c, fasting glucose, cholesterol, and triglycerides
Overall, compared with people who consumed diets with higher GI/GL ratings, for those who consumed lower glycemic diets, glycemic control was significantly improved, as reflected in A1c level, which was the primary outcome of the study (mean difference, –0.31%; P < .001).
This “would meet the threshold of ≥ 0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications,” the authors noted.
Those who consumed low glycemic diets also showed improvements in secondary outcomes, including fasting glucose level, which was reduced by 0.36 mmol/L (–6.5 mg/dL), a 6% reduction in low-density cholesterol (LDL-C) (–0.17 mmol/L), and a fall in triglyceride levels (–0.09 mmol/L).
They also lost marginally more body weight, at –0.66 kg (–1.5 pounds). Body mass index was lower by –0.38, and inflammation was reduced (C-reactive protein, –.41 mg/L; all P < .05).
No significant differences were observed between the groups in blood insulin level, high-density lipoprotein cholesterol level, waist circumference, or blood pressure.
Three of the studies showed that participants developed a preference for the low GI diet. “In recent years, there has been a growing interest in whole-food plant-based diets, and there are more options, for example, for pulse-based products,” Dr. Chiavaroli said.
This meta-analysis should support the recommendation of the low-glycemic diet, particularly among people with diabetes, she reiterated.
Will larger randomized trial show effect on outcomes?
The authors noted, however, that to determine whether these small improvements in intermediate cardiometabolic risk factors observed with low GI diets translate to reductions in cardiovascular disease, nephropathy, and retinopathy among people with diabetes, larger randomized trials are needed.
One such trial, the Low Glycemic Index Diet for Type 2 Diabetics, includes 169 high-risk patients with type 2 diabetes and subclinical atherosclerosis. The investigators are evaluating the effect of a low GI diet on the progression of atherosclerosis, as assessed by vascular MRI over 3 years.
“We await the results,” they said.
The study received funding from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD) as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. The study was also supported by the Canadian Institutes of Health Research through the Canada-wide Human Nutrition Trialists’ Network. The Diet, Digestive Tract, and Disease (3D) Center, which is funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the study.
A version of this article first appeared on Medscape.com.
A diet rich in vegetables and low in carbs – a so-called low glycemic index (GI) diet – is associated with clinically significant benefits beyond those provided by existing medications for people with type 1 and type 2 diabetes, compared with a higher glycemic diet, findings from a new meta-analysis show.
“Although the effects were small, which is not surprising in clinical trials in nutrition, they were clinically meaningful improvements for which our certainty in the effects were moderate to high,” first author Laura Chiavaroli, PhD, of the department of nutritional sciences, Temerty Faculty of Medicine, University of Toronto, said in an interview.
The GI rates foods on the basis of how quickly they affect blood glucose levels.
Fruits, vegetables, and whole grains have a low GI. They also help to regulate blood sugar levels. Such foods are linked to a reduced risk for heart disease among people with diabetes.
But guidelines on this – such as those from the European Association for the Study of Diabetes – reflect research published more than 15 years ago, before several key trials were published.
Dr. Chiavaroli and colleagues identified 27 randomized controlled trials – the most recent of which was published in May 2021 – that involved a total of 1,617 adults with type 1 or 2 diabetes. For the patients in these trials, diabetes was moderately controlled with glucose-lowering drugs or insulin. All of the included trials examined the effects of a low GI diet or a low glycemic load (GL) diet for people with diabetes over a period 3 or more weeks. The majority of patients in the studies were overweight or had obesity, and they were largely middle-aged.
The meta-analysis, which included new data, was published Aug. 5 in The BMJ. The study “expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE [grading of recommendations assessment, development, and evaluation],” Dr. Chiavaroli and colleagues noted.
“The available evidence provides a good indication of the likely benefit in this population and supports existing recommendations for the use of low GI dietary patterns in the management of diabetes,” they emphasized.
Improvements in A1c, fasting glucose, cholesterol, and triglycerides
Overall, compared with people who consumed diets with higher GI/GL ratings, for those who consumed lower glycemic diets, glycemic control was significantly improved, as reflected in A1c level, which was the primary outcome of the study (mean difference, –0.31%; P < .001).
This “would meet the threshold of ≥ 0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications,” the authors noted.
Those who consumed low glycemic diets also showed improvements in secondary outcomes, including fasting glucose level, which was reduced by 0.36 mmol/L (–6.5 mg/dL), a 6% reduction in low-density cholesterol (LDL-C) (–0.17 mmol/L), and a fall in triglyceride levels (–0.09 mmol/L).
They also lost marginally more body weight, at –0.66 kg (–1.5 pounds). Body mass index was lower by –0.38, and inflammation was reduced (C-reactive protein, –.41 mg/L; all P < .05).
No significant differences were observed between the groups in blood insulin level, high-density lipoprotein cholesterol level, waist circumference, or blood pressure.
Three of the studies showed that participants developed a preference for the low GI diet. “In recent years, there has been a growing interest in whole-food plant-based diets, and there are more options, for example, for pulse-based products,” Dr. Chiavaroli said.
This meta-analysis should support the recommendation of the low-glycemic diet, particularly among people with diabetes, she reiterated.
Will larger randomized trial show effect on outcomes?
The authors noted, however, that to determine whether these small improvements in intermediate cardiometabolic risk factors observed with low GI diets translate to reductions in cardiovascular disease, nephropathy, and retinopathy among people with diabetes, larger randomized trials are needed.
One such trial, the Low Glycemic Index Diet for Type 2 Diabetics, includes 169 high-risk patients with type 2 diabetes and subclinical atherosclerosis. The investigators are evaluating the effect of a low GI diet on the progression of atherosclerosis, as assessed by vascular MRI over 3 years.
“We await the results,” they said.
The study received funding from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD) as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. The study was also supported by the Canadian Institutes of Health Research through the Canada-wide Human Nutrition Trialists’ Network. The Diet, Digestive Tract, and Disease (3D) Center, which is funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the study.
A version of this article first appeared on Medscape.com.
Obesity leads to depression via social and metabolic factors
New research provides further evidence that a high body mass index (BMI) leads to depressed mood and poor well-being via social and physical factors.
Obesity and depression are “major global health challenges; our findings suggest that reducing obesity will lower depression and improve well-being,” co–lead author Jessica O’Loughlin, PhD student, University of Exeter Medical School, United Kingdom, told this news organization.
“Doctors should consider both the biological consequences of having a higher BMI as well as the social implications when treating patients with obesity in order to help reduce the odds of them developing depression,” Ms. O’Loughlin added.
The study was published online July 16 in Human Molecular Genetics.
Large body of evidence
A large body of evidence indicates that higher BMI leads to depression.
Ms. O’Loughlin and colleagues leveraged genetic data from more than 145,000 individuals in the UK Biobank and Mendelian randomization to determine whether the causal link between high BMI and depression is the result of psychosocial pathways, physical pathways, or both.
The analysis showed that a genetically determined 1 standard deviation higher BMI (4.6 kg/m2) was associated with higher likelihood of depression (odds ratio, 1.50; 95% confidence interval, 1.15-1.95) and lower well-being (beta, -0.15; 95% CI, -0.26 to -0.04).
Using genetics to distinguish metabolic and psychosocial effects, the results also indicate that, even in the absence of adverse metabolic effects, “higher adiposity remains causal to depression and lowers wellbeing,” the researchers report.
“ and when using genetic variants that make you fatter but metabolically healthier (favorable adiposity genetic variants),” said Ms. O’Loughlin.
“Although we can’t tell which factor plays a bigger role in the adiposity-depression relationship, our analysis suggests that both physical and social factors (e.g., social stigma) play a role in the relationship between higher BMI and higher odds of depression,” she added.
In contrast, there was little evidence that higher BMI in the presence or absence of adverse metabolic consequences causes generalized anxiety disorder.
“Finding ways to support people to lose weight could benefit their mental health as well as their physical health,” co–lead author Francesco Casanova, PhD, with the University of Exeter, said in a statement.
Unexpected finding
Reached for comment, Samoon Ahmad, MD, professor, department of psychiatry, New York University, said that “multiple studies have shown a correlation between stress, obesity, inflammation, overall well-being, and psychiatric disorders, particularly depressive and anxiety disorders.”
He said this new study is important for three reasons.
“The first is the cohort size. There were over 145,000 participants involved in the study, which is significant and serves to make its conclusions stronger,” Dr. Ahmad noted.
“The second point is that the authors found that the correlation between higher adiposity and depression and lower well-being scores occurred even in patients without adverse metabolic effects,” he said in an interview.
“Of note, obesity significantly increases the risk of developing type 2 diabetes, hypertension, and a host of other illnesses as well as inflammatory conditions, which can all have a negative impact on quality of life. Consequently, these can contribute to depression as well as anxiety,” Dr. Ahmad added.
“Interestingly, what this study suggests is that even people without these additional stressors are reporting higher rates of depression and lower scores of well-being, while higher adiposity is the common denominator,” he noted.
“Third, the paper found little to no correlation between higher adiposity and generalized anxiety disorder. This comes as a complete surprise because anxiety and depression are very common comorbidities,” Dr. Ahmad said.
“Moreover, numerous studies as well as clinical data suggest that obesity leads to chronic inflammation, which in turn is associated with less favorable metabolic profiles, and that anxiety and depressive disorders may in some way be psychiatric manifestations of inflammation. To see one but not the other was quite an unexpected finding,” Dr. Ahmad said.
The study was funded by the Academy of Medical Sciences. Ms. O’Loughlin, Dr. Casanova, and Dr. Ahmad have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research provides further evidence that a high body mass index (BMI) leads to depressed mood and poor well-being via social and physical factors.
Obesity and depression are “major global health challenges; our findings suggest that reducing obesity will lower depression and improve well-being,” co–lead author Jessica O’Loughlin, PhD student, University of Exeter Medical School, United Kingdom, told this news organization.
“Doctors should consider both the biological consequences of having a higher BMI as well as the social implications when treating patients with obesity in order to help reduce the odds of them developing depression,” Ms. O’Loughlin added.
The study was published online July 16 in Human Molecular Genetics.
Large body of evidence
A large body of evidence indicates that higher BMI leads to depression.
Ms. O’Loughlin and colleagues leveraged genetic data from more than 145,000 individuals in the UK Biobank and Mendelian randomization to determine whether the causal link between high BMI and depression is the result of psychosocial pathways, physical pathways, or both.
The analysis showed that a genetically determined 1 standard deviation higher BMI (4.6 kg/m2) was associated with higher likelihood of depression (odds ratio, 1.50; 95% confidence interval, 1.15-1.95) and lower well-being (beta, -0.15; 95% CI, -0.26 to -0.04).
Using genetics to distinguish metabolic and psychosocial effects, the results also indicate that, even in the absence of adverse metabolic effects, “higher adiposity remains causal to depression and lowers wellbeing,” the researchers report.
“ and when using genetic variants that make you fatter but metabolically healthier (favorable adiposity genetic variants),” said Ms. O’Loughlin.
“Although we can’t tell which factor plays a bigger role in the adiposity-depression relationship, our analysis suggests that both physical and social factors (e.g., social stigma) play a role in the relationship between higher BMI and higher odds of depression,” she added.
In contrast, there was little evidence that higher BMI in the presence or absence of adverse metabolic consequences causes generalized anxiety disorder.
“Finding ways to support people to lose weight could benefit their mental health as well as their physical health,” co–lead author Francesco Casanova, PhD, with the University of Exeter, said in a statement.
Unexpected finding
Reached for comment, Samoon Ahmad, MD, professor, department of psychiatry, New York University, said that “multiple studies have shown a correlation between stress, obesity, inflammation, overall well-being, and psychiatric disorders, particularly depressive and anxiety disorders.”
He said this new study is important for three reasons.
“The first is the cohort size. There were over 145,000 participants involved in the study, which is significant and serves to make its conclusions stronger,” Dr. Ahmad noted.
“The second point is that the authors found that the correlation between higher adiposity and depression and lower well-being scores occurred even in patients without adverse metabolic effects,” he said in an interview.
“Of note, obesity significantly increases the risk of developing type 2 diabetes, hypertension, and a host of other illnesses as well as inflammatory conditions, which can all have a negative impact on quality of life. Consequently, these can contribute to depression as well as anxiety,” Dr. Ahmad added.
“Interestingly, what this study suggests is that even people without these additional stressors are reporting higher rates of depression and lower scores of well-being, while higher adiposity is the common denominator,” he noted.
“Third, the paper found little to no correlation between higher adiposity and generalized anxiety disorder. This comes as a complete surprise because anxiety and depression are very common comorbidities,” Dr. Ahmad said.
“Moreover, numerous studies as well as clinical data suggest that obesity leads to chronic inflammation, which in turn is associated with less favorable metabolic profiles, and that anxiety and depressive disorders may in some way be psychiatric manifestations of inflammation. To see one but not the other was quite an unexpected finding,” Dr. Ahmad said.
The study was funded by the Academy of Medical Sciences. Ms. O’Loughlin, Dr. Casanova, and Dr. Ahmad have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research provides further evidence that a high body mass index (BMI) leads to depressed mood and poor well-being via social and physical factors.
Obesity and depression are “major global health challenges; our findings suggest that reducing obesity will lower depression and improve well-being,” co–lead author Jessica O’Loughlin, PhD student, University of Exeter Medical School, United Kingdom, told this news organization.
“Doctors should consider both the biological consequences of having a higher BMI as well as the social implications when treating patients with obesity in order to help reduce the odds of them developing depression,” Ms. O’Loughlin added.
The study was published online July 16 in Human Molecular Genetics.
Large body of evidence
A large body of evidence indicates that higher BMI leads to depression.
Ms. O’Loughlin and colleagues leveraged genetic data from more than 145,000 individuals in the UK Biobank and Mendelian randomization to determine whether the causal link between high BMI and depression is the result of psychosocial pathways, physical pathways, or both.
The analysis showed that a genetically determined 1 standard deviation higher BMI (4.6 kg/m2) was associated with higher likelihood of depression (odds ratio, 1.50; 95% confidence interval, 1.15-1.95) and lower well-being (beta, -0.15; 95% CI, -0.26 to -0.04).
Using genetics to distinguish metabolic and psychosocial effects, the results also indicate that, even in the absence of adverse metabolic effects, “higher adiposity remains causal to depression and lowers wellbeing,” the researchers report.
“ and when using genetic variants that make you fatter but metabolically healthier (favorable adiposity genetic variants),” said Ms. O’Loughlin.
“Although we can’t tell which factor plays a bigger role in the adiposity-depression relationship, our analysis suggests that both physical and social factors (e.g., social stigma) play a role in the relationship between higher BMI and higher odds of depression,” she added.
In contrast, there was little evidence that higher BMI in the presence or absence of adverse metabolic consequences causes generalized anxiety disorder.
“Finding ways to support people to lose weight could benefit their mental health as well as their physical health,” co–lead author Francesco Casanova, PhD, with the University of Exeter, said in a statement.
Unexpected finding
Reached for comment, Samoon Ahmad, MD, professor, department of psychiatry, New York University, said that “multiple studies have shown a correlation between stress, obesity, inflammation, overall well-being, and psychiatric disorders, particularly depressive and anxiety disorders.”
He said this new study is important for three reasons.
“The first is the cohort size. There were over 145,000 participants involved in the study, which is significant and serves to make its conclusions stronger,” Dr. Ahmad noted.
“The second point is that the authors found that the correlation between higher adiposity and depression and lower well-being scores occurred even in patients without adverse metabolic effects,” he said in an interview.
“Of note, obesity significantly increases the risk of developing type 2 diabetes, hypertension, and a host of other illnesses as well as inflammatory conditions, which can all have a negative impact on quality of life. Consequently, these can contribute to depression as well as anxiety,” Dr. Ahmad added.
“Interestingly, what this study suggests is that even people without these additional stressors are reporting higher rates of depression and lower scores of well-being, while higher adiposity is the common denominator,” he noted.
“Third, the paper found little to no correlation between higher adiposity and generalized anxiety disorder. This comes as a complete surprise because anxiety and depression are very common comorbidities,” Dr. Ahmad said.
“Moreover, numerous studies as well as clinical data suggest that obesity leads to chronic inflammation, which in turn is associated with less favorable metabolic profiles, and that anxiety and depressive disorders may in some way be psychiatric manifestations of inflammation. To see one but not the other was quite an unexpected finding,” Dr. Ahmad said.
The study was funded by the Academy of Medical Sciences. Ms. O’Loughlin, Dr. Casanova, and Dr. Ahmad have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
















