User login
Sotagliflozin use in T2D patients linked with posthospitalization benefits in analysis
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
FROM ANNALS OF INTERNAL MEDICINE
Medicare rule changes allow for broader CGM use
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Bariatric surgery cuts insulin needs in type 1 diabetes with severe obesity
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
FROM ASMBS 2021
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
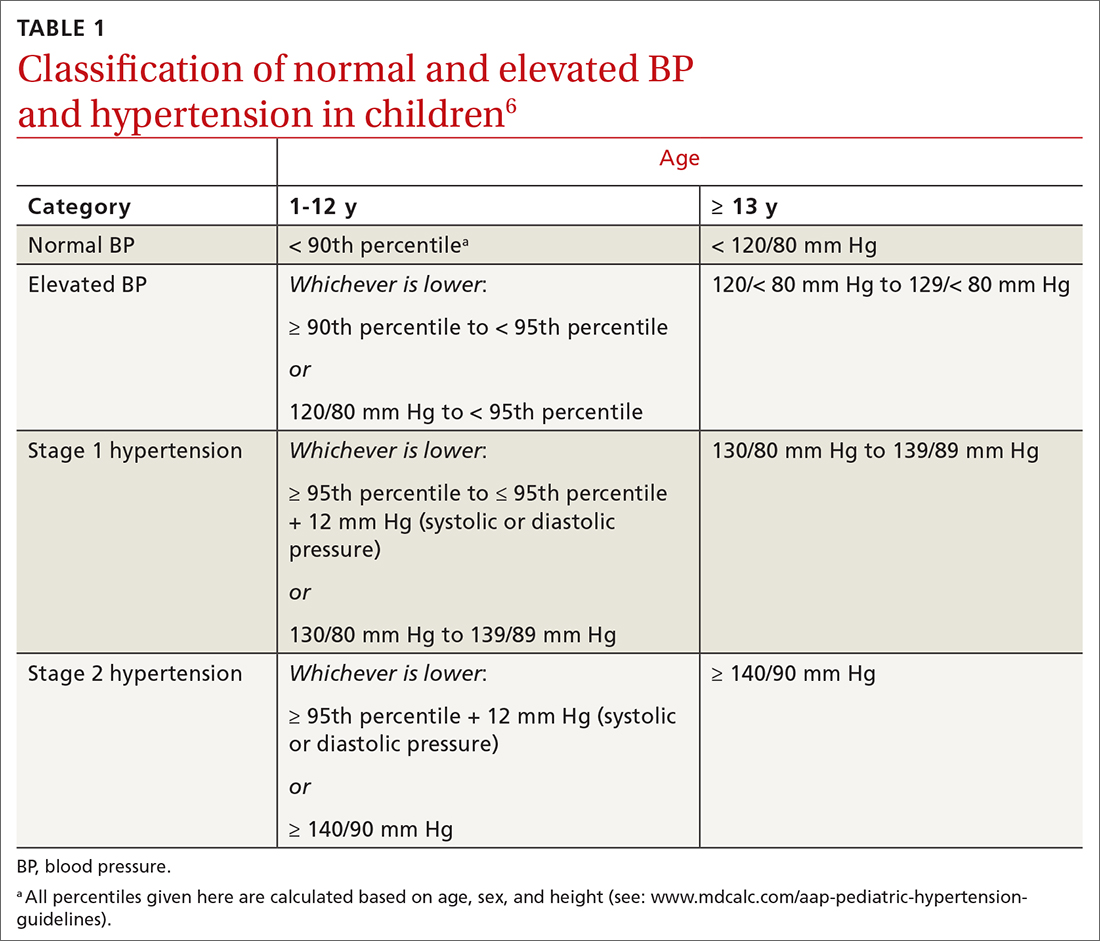
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
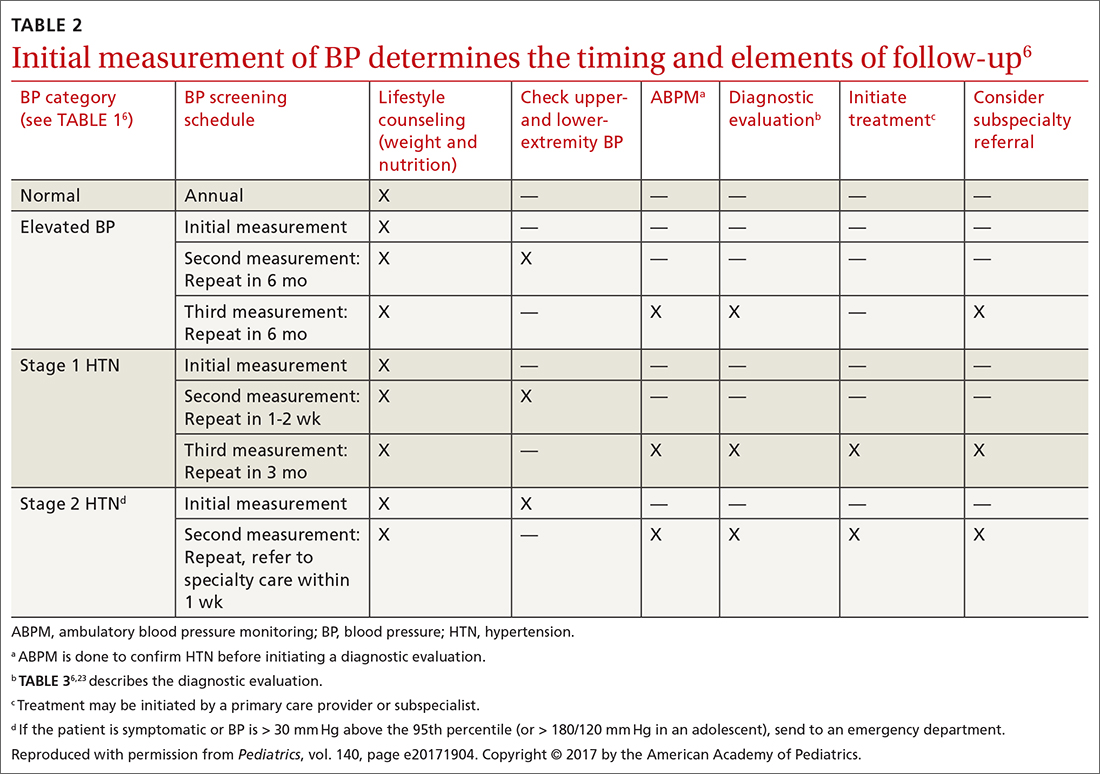
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
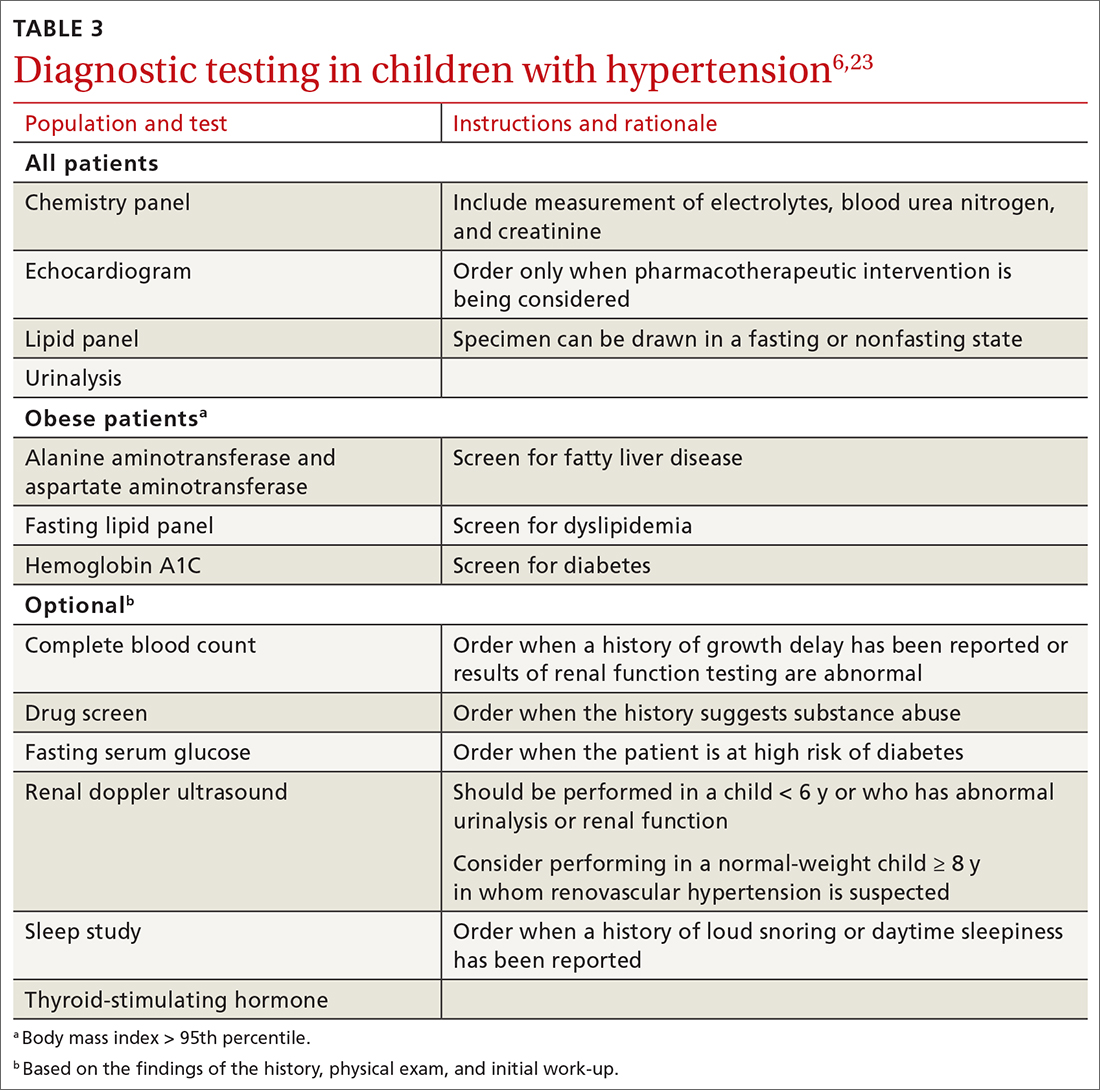
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
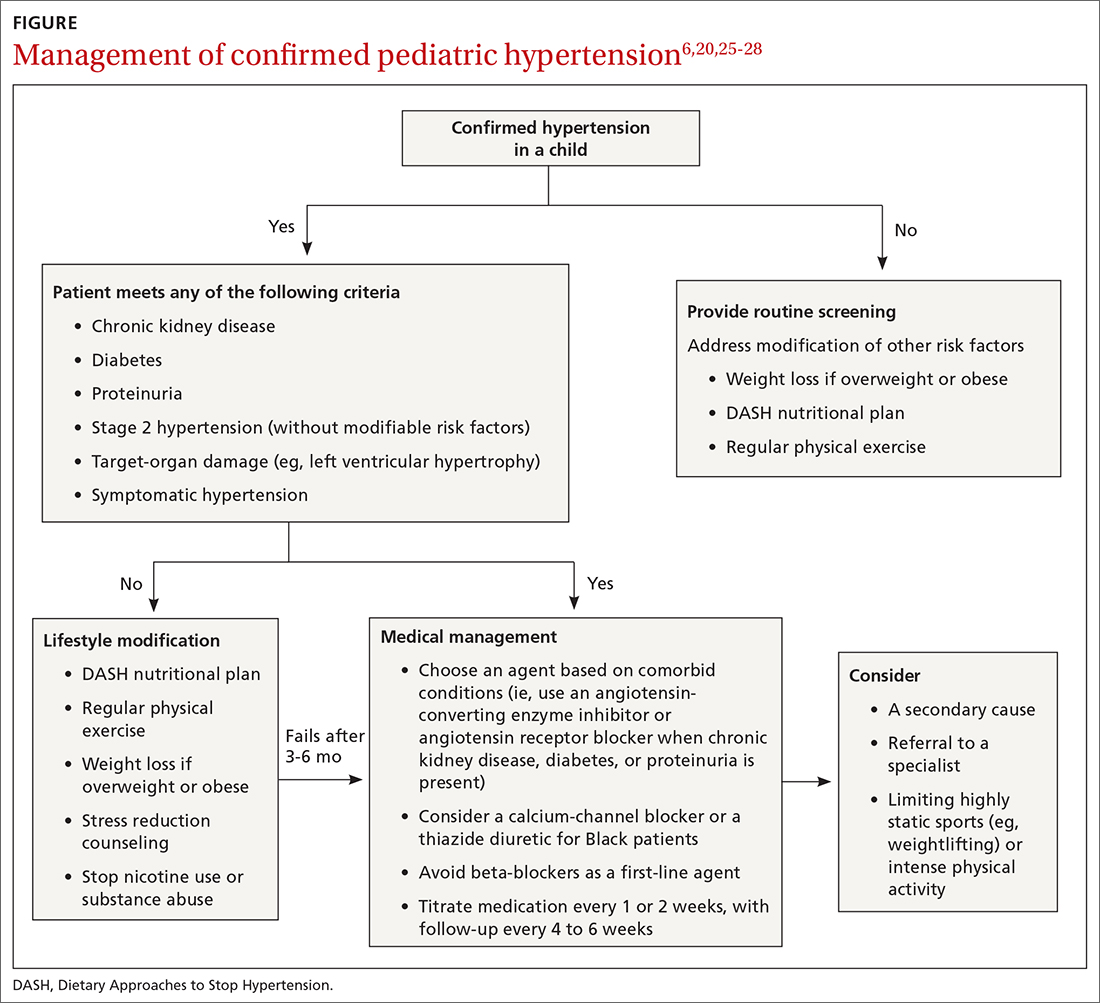
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Healthy with obesity? The latest study casts doubt
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
FROM DIABETOLOGIA
FDA: More metformin extended-release tablets recalled
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
FROM THE FOOD AND DRUG ADMINISTRATION
Eat two fruits a day, ward off diabetes?
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
‘Twincretin’ meets primary endpoints in five pivotal diabetes trials
The investigational, novel, injected once-weekly “twincretin” tirzepatide met its primary efficacy endpoint of significantly cutting hemoglobin A1c as well as its secondary weight-loss endpoint in patients with type 2 diabetes when compared with control patients in top-line results from each of five discrete pivotal trials.
The company developing tirzepatide, Lilly, announced these results in a series of four press releases issued during December 2020–May 2021. Scientific reports on the outcomes from four of these trials are scheduled during the American Diabetes Association’s Scientific Sessions being held virtually in late June 2021, with results from the fifth on track for a report during the annual meeting of the European Association for the Study of Diabetes in September 2021.
Tirzepatide is a “twincretin” because it combines in a single molecule two different gut-hormone activities. It works as both a glucagonlike peptide–1 receptor agonist (GLP-1 RA) and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
While diabetologists qualified their comments on these results because of the limited scope and format of the five reports to date, they also expressed enthusiasm over what the press releases said.
Results give hope
“It’s quite exciting, but of course we would like to go by the data that’s presented” at upcoming meetings, commented Robert A. Gabbay, MD, PhD, chief science and medical officer of the American Diabetes Association in Arlington, Va. “The idea of GLP-1 and GIP activities working together has been out there for a while, but without any therapeutic options that leverage this,” he said in an interview.
“The preliminary results give us hope that tirzepatide will be a very effective glucose-lowering agent, perhaps the most effective among all options currently available, including insulin,” commented Ildiko Lingvay, MD, a diabetologist and professor at the University of Texas Southwestern Medical Center, Dallas. “Tirzepatide might have the added benefit of clinically meaningful weight loss,” and “the adverse event profile seems to be in line with what we are accustomed to with the GLP-1 RA class. I look forward to seeing the full results. Tirzepatide promises to be a great addition for type 2 diabetes,” Dr. Lingvay said in an interview.
A rare head-to-head against semaglutide
The five phase 3, randomized controlled trials described by Lilly in its four press releases all belong to the SURPASS series of studies for this agent. Perhaps the most intriguing of the five were results from SURPASS-2, announced in a release on March 4. This trial randomized 1,879 patients from the United States or any of seven other countries to 40 weeks of open-label treatment with one of three different dosages of tirzepatide administered by injection once weekly, or to the control group that received a weekly 1-mg injection of semaglutide (Ozempic), the highest dosage approved for controlling glycemia in patients with type 2 diabetes at the time the study launched.
In SURPASS-2 all three tested dosages of tirzepatide led to a significantly larger reduction, from baseline in A1c, compared with semaglutide, after 40 weeks, according to the Lilly release. Each of the three tirzepatide dosages also led to significantly greater weight loss from baseline, compared with semaglutide, and significantly greater percentages of patients who achieved an A1c of less than 7%, compared with semaglutide.
As an example, the highest tested tirzepatide dosage of 15 mg weekly led to an average A1c reduction from baseline of 2.46% and an average weight loss from baseline of 12.4 kg; 92% of patients achieved an A1c of less than 7%, and 51% had their A1c fall below 5.7% which indicates completely normalization of glycemic control. By comparison, the patients randomized to treatment with semaglutide had an average 1.86% reduction in their A1c level from baseline and a 6.2-kg average cut in body weight from baseline; 81% achieved an A1c of less than 7%, and 20% reached an A1c of less than 5.7%.
There are caveats
While these findings are notable as a rare example of an industry-sponsored head-to-head comparison of two new agents, the study comes with a few important asterisks.
First, it was open label, a curious limitation given that both agents are delivered by the same delivery method and schedule. “I cannot conclude based on this study that tirzepatide is superior because it was open label,” commented Anastassia Amaro, MD, medical director of Penn Metabolic Medicine at the University of Pennsylvania, Philadelphia.
“The gold standard is the double-blind study. An open-label design is a limitation,” agreed Dr. Gabbay.
A second caveat is that the Food and Drug Administration recently approved a higher dosage of semaglutide (2.4 g once/week) for treating overweight or obesity in patients with type 2 diabetes and in those without diabetes but a different weight-related condition such as hypertension of hypercholesterolemia. This means that the tested comparator dosage of 1 mg/week is no longer the maximum that most patients treated with semaglutide for glycemic control can receive.
“The inevitable question” about this comparison study is “what about a higher semaglutide dose,” and how might tirzepatide perform relative to that, said Dr. Gabbay. The recently approved higher dosage of semaglutide “adds an interesting wrinkle.”
Lilly has launched a series of studies testing tirzepatide as a treatment for overweight or obesity in people without diabetes, but the results are not expected until sometime in 2022 or 2023.
And there’s a third caveat: Semaglutide has already shown its value for cardiovascular risk reduction in patients with type 2 diabetes in the SUSTAIN 6 trial with nearly 3,300 randomized patients followed for 2 years and reported in 2016. The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT with more than 12,000 patients with type 2 diabetes, is underway but its results are not expected until 2024.
Despite these important limitations, a blinded comparison of tirzepatide and higher-dose semaglutide is unlikely, Dr. Amaro predicted. “It’s not worth the expense,” she said in an interview. A more likely scenario will be that, if tirzepatide enters the U.S. market, decisions on whether to treat patients with it or semaglutide will pivot on factors like the cost for treatment to individual patients based on their insurance coverage and tolerability, suggested both Dr. Amaro and Dr. Gabbay. “Physicians will need to develop a sense for tirzepatide: Do patients tolerate it and are they happy using it?” Dr. Amaro said.
Tirzepatide versus insulin, or on top of insulin
The other four trials in patients with type 2 diabetes reported by Lilly in releases included SURPASS-1, which randomized 478 patients to treatment with tirzepatide or placebo as monotherapy; SURPASS-3, which randomized 1,444 patients to tirzepatide or insulin degludec (Tresiba) on top of background treatment with metformin; SURPASS-4, which randomized 2,002 patients with high cardiovascular disease risk to treatment with tirzepatide or insulin glargine (Lantus) on top of background treatment with one to three different oral drugs; and SURPASS-5, which randomized 475 patients to treatment with tirzepatide or placebo on top of background treatment with insulin glargine and optional addition of metformin. Altogether, the five trials randomized nearly 6,300 patients.
The studies that compared tirzepatide against two different types of insulin, and the third that tested tirzepatide on top of insulin glargine, are especially notable. “It’s good to see that the combination [of tirzepatide and insulin glargine] works without causing major adverse events,” said Dr. Amaro.
“These are fair and helpful comparisons. I applaud Lilly for doing the right kind of comparisons,” said Dr. Gabbay.
In total, the five studies “provide evidence that tirzepatide will be effective at all stages of type 2 diabetes and can safely be used in combination with other glucose-lowering agents, including insulin,” said Dr. Lingvay. The studies with active comparator agents “allow us to compare tirzepatide’s efficacy against established therapies.”
The SURPASS trials were sponsored by Lilly, which is developing tirzepatide. Dr. Gabbay had no relevant disclosures. Dr. Lingvay has received research funds, consulting and advisory fees, or other support from Lilly as well as from several other companies including Novo Nordisk, which markets semaglutide (Ozempic) and insulin degludec (Tresiba), and Sanofi, which markets insulin glargine (Lantus). Dr. Amaro has received research funding from Lilly and from Fractyl, and has been a consultant to and received research funding from Novo Nordisk.
The investigational, novel, injected once-weekly “twincretin” tirzepatide met its primary efficacy endpoint of significantly cutting hemoglobin A1c as well as its secondary weight-loss endpoint in patients with type 2 diabetes when compared with control patients in top-line results from each of five discrete pivotal trials.
The company developing tirzepatide, Lilly, announced these results in a series of four press releases issued during December 2020–May 2021. Scientific reports on the outcomes from four of these trials are scheduled during the American Diabetes Association’s Scientific Sessions being held virtually in late June 2021, with results from the fifth on track for a report during the annual meeting of the European Association for the Study of Diabetes in September 2021.
Tirzepatide is a “twincretin” because it combines in a single molecule two different gut-hormone activities. It works as both a glucagonlike peptide–1 receptor agonist (GLP-1 RA) and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
While diabetologists qualified their comments on these results because of the limited scope and format of the five reports to date, they also expressed enthusiasm over what the press releases said.
Results give hope
“It’s quite exciting, but of course we would like to go by the data that’s presented” at upcoming meetings, commented Robert A. Gabbay, MD, PhD, chief science and medical officer of the American Diabetes Association in Arlington, Va. “The idea of GLP-1 and GIP activities working together has been out there for a while, but without any therapeutic options that leverage this,” he said in an interview.
“The preliminary results give us hope that tirzepatide will be a very effective glucose-lowering agent, perhaps the most effective among all options currently available, including insulin,” commented Ildiko Lingvay, MD, a diabetologist and professor at the University of Texas Southwestern Medical Center, Dallas. “Tirzepatide might have the added benefit of clinically meaningful weight loss,” and “the adverse event profile seems to be in line with what we are accustomed to with the GLP-1 RA class. I look forward to seeing the full results. Tirzepatide promises to be a great addition for type 2 diabetes,” Dr. Lingvay said in an interview.
A rare head-to-head against semaglutide
The five phase 3, randomized controlled trials described by Lilly in its four press releases all belong to the SURPASS series of studies for this agent. Perhaps the most intriguing of the five were results from SURPASS-2, announced in a release on March 4. This trial randomized 1,879 patients from the United States or any of seven other countries to 40 weeks of open-label treatment with one of three different dosages of tirzepatide administered by injection once weekly, or to the control group that received a weekly 1-mg injection of semaglutide (Ozempic), the highest dosage approved for controlling glycemia in patients with type 2 diabetes at the time the study launched.
In SURPASS-2 all three tested dosages of tirzepatide led to a significantly larger reduction, from baseline in A1c, compared with semaglutide, after 40 weeks, according to the Lilly release. Each of the three tirzepatide dosages also led to significantly greater weight loss from baseline, compared with semaglutide, and significantly greater percentages of patients who achieved an A1c of less than 7%, compared with semaglutide.
As an example, the highest tested tirzepatide dosage of 15 mg weekly led to an average A1c reduction from baseline of 2.46% and an average weight loss from baseline of 12.4 kg; 92% of patients achieved an A1c of less than 7%, and 51% had their A1c fall below 5.7% which indicates completely normalization of glycemic control. By comparison, the patients randomized to treatment with semaglutide had an average 1.86% reduction in their A1c level from baseline and a 6.2-kg average cut in body weight from baseline; 81% achieved an A1c of less than 7%, and 20% reached an A1c of less than 5.7%.
There are caveats
While these findings are notable as a rare example of an industry-sponsored head-to-head comparison of two new agents, the study comes with a few important asterisks.
First, it was open label, a curious limitation given that both agents are delivered by the same delivery method and schedule. “I cannot conclude based on this study that tirzepatide is superior because it was open label,” commented Anastassia Amaro, MD, medical director of Penn Metabolic Medicine at the University of Pennsylvania, Philadelphia.
“The gold standard is the double-blind study. An open-label design is a limitation,” agreed Dr. Gabbay.
A second caveat is that the Food and Drug Administration recently approved a higher dosage of semaglutide (2.4 g once/week) for treating overweight or obesity in patients with type 2 diabetes and in those without diabetes but a different weight-related condition such as hypertension of hypercholesterolemia. This means that the tested comparator dosage of 1 mg/week is no longer the maximum that most patients treated with semaglutide for glycemic control can receive.
“The inevitable question” about this comparison study is “what about a higher semaglutide dose,” and how might tirzepatide perform relative to that, said Dr. Gabbay. The recently approved higher dosage of semaglutide “adds an interesting wrinkle.”
Lilly has launched a series of studies testing tirzepatide as a treatment for overweight or obesity in people without diabetes, but the results are not expected until sometime in 2022 or 2023.
And there’s a third caveat: Semaglutide has already shown its value for cardiovascular risk reduction in patients with type 2 diabetes in the SUSTAIN 6 trial with nearly 3,300 randomized patients followed for 2 years and reported in 2016. The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT with more than 12,000 patients with type 2 diabetes, is underway but its results are not expected until 2024.
Despite these important limitations, a blinded comparison of tirzepatide and higher-dose semaglutide is unlikely, Dr. Amaro predicted. “It’s not worth the expense,” she said in an interview. A more likely scenario will be that, if tirzepatide enters the U.S. market, decisions on whether to treat patients with it or semaglutide will pivot on factors like the cost for treatment to individual patients based on their insurance coverage and tolerability, suggested both Dr. Amaro and Dr. Gabbay. “Physicians will need to develop a sense for tirzepatide: Do patients tolerate it and are they happy using it?” Dr. Amaro said.
Tirzepatide versus insulin, or on top of insulin
The other four trials in patients with type 2 diabetes reported by Lilly in releases included SURPASS-1, which randomized 478 patients to treatment with tirzepatide or placebo as monotherapy; SURPASS-3, which randomized 1,444 patients to tirzepatide or insulin degludec (Tresiba) on top of background treatment with metformin; SURPASS-4, which randomized 2,002 patients with high cardiovascular disease risk to treatment with tirzepatide or insulin glargine (Lantus) on top of background treatment with one to three different oral drugs; and SURPASS-5, which randomized 475 patients to treatment with tirzepatide or placebo on top of background treatment with insulin glargine and optional addition of metformin. Altogether, the five trials randomized nearly 6,300 patients.
The studies that compared tirzepatide against two different types of insulin, and the third that tested tirzepatide on top of insulin glargine, are especially notable. “It’s good to see that the combination [of tirzepatide and insulin glargine] works without causing major adverse events,” said Dr. Amaro.
“These are fair and helpful comparisons. I applaud Lilly for doing the right kind of comparisons,” said Dr. Gabbay.
In total, the five studies “provide evidence that tirzepatide will be effective at all stages of type 2 diabetes and can safely be used in combination with other glucose-lowering agents, including insulin,” said Dr. Lingvay. The studies with active comparator agents “allow us to compare tirzepatide’s efficacy against established therapies.”
The SURPASS trials were sponsored by Lilly, which is developing tirzepatide. Dr. Gabbay had no relevant disclosures. Dr. Lingvay has received research funds, consulting and advisory fees, or other support from Lilly as well as from several other companies including Novo Nordisk, which markets semaglutide (Ozempic) and insulin degludec (Tresiba), and Sanofi, which markets insulin glargine (Lantus). Dr. Amaro has received research funding from Lilly and from Fractyl, and has been a consultant to and received research funding from Novo Nordisk.
The investigational, novel, injected once-weekly “twincretin” tirzepatide met its primary efficacy endpoint of significantly cutting hemoglobin A1c as well as its secondary weight-loss endpoint in patients with type 2 diabetes when compared with control patients in top-line results from each of five discrete pivotal trials.
The company developing tirzepatide, Lilly, announced these results in a series of four press releases issued during December 2020–May 2021. Scientific reports on the outcomes from four of these trials are scheduled during the American Diabetes Association’s Scientific Sessions being held virtually in late June 2021, with results from the fifth on track for a report during the annual meeting of the European Association for the Study of Diabetes in September 2021.
Tirzepatide is a “twincretin” because it combines in a single molecule two different gut-hormone activities. It works as both a glucagonlike peptide–1 receptor agonist (GLP-1 RA) and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
While diabetologists qualified their comments on these results because of the limited scope and format of the five reports to date, they also expressed enthusiasm over what the press releases said.
Results give hope
“It’s quite exciting, but of course we would like to go by the data that’s presented” at upcoming meetings, commented Robert A. Gabbay, MD, PhD, chief science and medical officer of the American Diabetes Association in Arlington, Va. “The idea of GLP-1 and GIP activities working together has been out there for a while, but without any therapeutic options that leverage this,” he said in an interview.
“The preliminary results give us hope that tirzepatide will be a very effective glucose-lowering agent, perhaps the most effective among all options currently available, including insulin,” commented Ildiko Lingvay, MD, a diabetologist and professor at the University of Texas Southwestern Medical Center, Dallas. “Tirzepatide might have the added benefit of clinically meaningful weight loss,” and “the adverse event profile seems to be in line with what we are accustomed to with the GLP-1 RA class. I look forward to seeing the full results. Tirzepatide promises to be a great addition for type 2 diabetes,” Dr. Lingvay said in an interview.
A rare head-to-head against semaglutide
The five phase 3, randomized controlled trials described by Lilly in its four press releases all belong to the SURPASS series of studies for this agent. Perhaps the most intriguing of the five were results from SURPASS-2, announced in a release on March 4. This trial randomized 1,879 patients from the United States or any of seven other countries to 40 weeks of open-label treatment with one of three different dosages of tirzepatide administered by injection once weekly, or to the control group that received a weekly 1-mg injection of semaglutide (Ozempic), the highest dosage approved for controlling glycemia in patients with type 2 diabetes at the time the study launched.
In SURPASS-2 all three tested dosages of tirzepatide led to a significantly larger reduction, from baseline in A1c, compared with semaglutide, after 40 weeks, according to the Lilly release. Each of the three tirzepatide dosages also led to significantly greater weight loss from baseline, compared with semaglutide, and significantly greater percentages of patients who achieved an A1c of less than 7%, compared with semaglutide.
As an example, the highest tested tirzepatide dosage of 15 mg weekly led to an average A1c reduction from baseline of 2.46% and an average weight loss from baseline of 12.4 kg; 92% of patients achieved an A1c of less than 7%, and 51% had their A1c fall below 5.7% which indicates completely normalization of glycemic control. By comparison, the patients randomized to treatment with semaglutide had an average 1.86% reduction in their A1c level from baseline and a 6.2-kg average cut in body weight from baseline; 81% achieved an A1c of less than 7%, and 20% reached an A1c of less than 5.7%.
There are caveats
While these findings are notable as a rare example of an industry-sponsored head-to-head comparison of two new agents, the study comes with a few important asterisks.
First, it was open label, a curious limitation given that both agents are delivered by the same delivery method and schedule. “I cannot conclude based on this study that tirzepatide is superior because it was open label,” commented Anastassia Amaro, MD, medical director of Penn Metabolic Medicine at the University of Pennsylvania, Philadelphia.
“The gold standard is the double-blind study. An open-label design is a limitation,” agreed Dr. Gabbay.
A second caveat is that the Food and Drug Administration recently approved a higher dosage of semaglutide (2.4 g once/week) for treating overweight or obesity in patients with type 2 diabetes and in those without diabetes but a different weight-related condition such as hypertension of hypercholesterolemia. This means that the tested comparator dosage of 1 mg/week is no longer the maximum that most patients treated with semaglutide for glycemic control can receive.
“The inevitable question” about this comparison study is “what about a higher semaglutide dose,” and how might tirzepatide perform relative to that, said Dr. Gabbay. The recently approved higher dosage of semaglutide “adds an interesting wrinkle.”
Lilly has launched a series of studies testing tirzepatide as a treatment for overweight or obesity in people without diabetes, but the results are not expected until sometime in 2022 or 2023.
And there’s a third caveat: Semaglutide has already shown its value for cardiovascular risk reduction in patients with type 2 diabetes in the SUSTAIN 6 trial with nearly 3,300 randomized patients followed for 2 years and reported in 2016. The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT with more than 12,000 patients with type 2 diabetes, is underway but its results are not expected until 2024.
Despite these important limitations, a blinded comparison of tirzepatide and higher-dose semaglutide is unlikely, Dr. Amaro predicted. “It’s not worth the expense,” she said in an interview. A more likely scenario will be that, if tirzepatide enters the U.S. market, decisions on whether to treat patients with it or semaglutide will pivot on factors like the cost for treatment to individual patients based on their insurance coverage and tolerability, suggested both Dr. Amaro and Dr. Gabbay. “Physicians will need to develop a sense for tirzepatide: Do patients tolerate it and are they happy using it?” Dr. Amaro said.
Tirzepatide versus insulin, or on top of insulin
The other four trials in patients with type 2 diabetes reported by Lilly in releases included SURPASS-1, which randomized 478 patients to treatment with tirzepatide or placebo as monotherapy; SURPASS-3, which randomized 1,444 patients to tirzepatide or insulin degludec (Tresiba) on top of background treatment with metformin; SURPASS-4, which randomized 2,002 patients with high cardiovascular disease risk to treatment with tirzepatide or insulin glargine (Lantus) on top of background treatment with one to three different oral drugs; and SURPASS-5, which randomized 475 patients to treatment with tirzepatide or placebo on top of background treatment with insulin glargine and optional addition of metformin. Altogether, the five trials randomized nearly 6,300 patients.
The studies that compared tirzepatide against two different types of insulin, and the third that tested tirzepatide on top of insulin glargine, are especially notable. “It’s good to see that the combination [of tirzepatide and insulin glargine] works without causing major adverse events,” said Dr. Amaro.
“These are fair and helpful comparisons. I applaud Lilly for doing the right kind of comparisons,” said Dr. Gabbay.
In total, the five studies “provide evidence that tirzepatide will be effective at all stages of type 2 diabetes and can safely be used in combination with other glucose-lowering agents, including insulin,” said Dr. Lingvay. The studies with active comparator agents “allow us to compare tirzepatide’s efficacy against established therapies.”
The SURPASS trials were sponsored by Lilly, which is developing tirzepatide. Dr. Gabbay had no relevant disclosures. Dr. Lingvay has received research funds, consulting and advisory fees, or other support from Lilly as well as from several other companies including Novo Nordisk, which markets semaglutide (Ozempic) and insulin degludec (Tresiba), and Sanofi, which markets insulin glargine (Lantus). Dr. Amaro has received research funding from Lilly and from Fractyl, and has been a consultant to and received research funding from Novo Nordisk.
Waist circumference a marker for NAFL in type 1 diabetes
It follows that, as the prevalence of obesity among people with type 1 diabetes mellitus (T1DM) increases, so would the incidence of nonalcoholic fatty liver (NAFL), as it does in type 2 diabetes.
However, researchers in Finland report that the incidence of NAFL in T1DM is much lower, and that the use of the waist-to-height ratio to calculate midsection girth could be a low-cost alternative to MRI and computed tomography to more precisely diagnose NAFL in T1DM.
In a cross-sectional analysis of 121 adults with T1DM in the Finnish Diabetic Nephropathy study, known as FinnDiane, researchers from the University of Helsinki report in Diabetes Care that a waist-to-height ratio of 0.5 showed a relatively high rate of accuracy for identifying NAFL that was statistically significant (P = .04).
Lead author Erika B. Parente, MD, PhD, a researcher at the Folkhälsän Research Center in Helsinki, noted that the findings do not identify any causality between what the researchers called visceral adiposity and NAFL. “As long as they have accumulation of fat in the center of body and they can develop this low-grade inflammation that also goes to insulin-load sensitivity, people with T1DM can accumulate fat in the liver as do people with T2DM and the general population,” she said in an interview.
These findings build on her group’s previous work published in Scientific Reports showing a strong relationship between waist-to-height ratio and visceral fat percentage in adults with T1DM. The most recent FinnDiane analysis found no similar relationship between NAFL and fat tissue in the hips, arms and legs, and total adipose tissue.
Better than BMI as a measure
“We also found that waist-to-height ratio is better than body mass index to identify those individuals at higher risk of having NAFL,” Dr. Parente said. However, it’s not possible to predict which patients referred to imaging evaluation after being screened by waist-to-height ratio of 0.5 will surely have NAFL, she added.
That answer, she said, would require a longitudinal and cost-effectiveness study with larger population.
The waist-to-height ratio cutoff of 0.5 showed an 86% sensitivity and 55% specificity for NAFL, whereas BMI of 26.6 kg/m2 showed an 79% sensitivity and 57% specificity.
“The most important message from our research is that health care professionals should be aware that individuals with T1DM can have NAFL, and waist-to-height ratio may help to identify those at higher risk,” she said.
The prevalence of NAFL among the adults with T1DM in the study was 11.6%, which is lower than the prevalence other studies reported in T2DM – 76% in a U.S. study – and in the general population – ranging from 19% to 46%. This underscores, Dr. Parente noted, the importance of using waist-to-height ratio in T1DM patients to determine the status of NAFL.
She said that few studies have investigated the consequences of NAFL in T1DM, pointing to two that linked NAFL with chronic kidney disease and cardiovascular disease in T1DM (Diabetes Care. 2014;37:1729-36; J Hepatol. 2010;53:713-8). “Most of the studies about the consequences of NAFL included people with T2DM,” she said. “From our research, we cannot conclude about the impact of NAFL in cardiovascular or kidney complications in our population because this is a cross-sectional study.”
That question may be answered by a future follow-up study of the ongoing FinnDiane study, she said.
The study is a “good reminder” that people with central adiposity and metabolic syndrome can develop NAFL disease, said Jeanne Marie Clark, MD, MPH, of Johns Hopkins University, Baltimore. “Even patients we may not think of having insulin resistance, such as those with T1DM.”
However, Dr. Clark added, “I do not think we can really determine which measure of central adiposity is best.” She noted that the study was “pretty small” with only 14 patients who had NAFL disease. “Waist-to-height ratio is certainly a reasonable option,” she added. “Waist circumference alone is known to be a strong predictor. I would say some measure is better than none, and it should be more routine in clinical practice.”
Dr. Parente disclosed financial relationships with Eli Lilly, Abbott, AstraZeneca, Sanofi, and Boehringer Ingelheim. Two of eight coauthors disclosed financial relationships with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Fresenius, GE Healthcare, Medscape, Merck Sharpe and Dohme, Mundipharma, Novo Nordisk, Peer-Voice, Sanofi, and Sciarc. The remaining coauthors had no disclosures.
Dr. Clark had no disclosures.
It follows that, as the prevalence of obesity among people with type 1 diabetes mellitus (T1DM) increases, so would the incidence of nonalcoholic fatty liver (NAFL), as it does in type 2 diabetes.
However, researchers in Finland report that the incidence of NAFL in T1DM is much lower, and that the use of the waist-to-height ratio to calculate midsection girth could be a low-cost alternative to MRI and computed tomography to more precisely diagnose NAFL in T1DM.
In a cross-sectional analysis of 121 adults with T1DM in the Finnish Diabetic Nephropathy study, known as FinnDiane, researchers from the University of Helsinki report in Diabetes Care that a waist-to-height ratio of 0.5 showed a relatively high rate of accuracy for identifying NAFL that was statistically significant (P = .04).
Lead author Erika B. Parente, MD, PhD, a researcher at the Folkhälsän Research Center in Helsinki, noted that the findings do not identify any causality between what the researchers called visceral adiposity and NAFL. “As long as they have accumulation of fat in the center of body and they can develop this low-grade inflammation that also goes to insulin-load sensitivity, people with T1DM can accumulate fat in the liver as do people with T2DM and the general population,” she said in an interview.
These findings build on her group’s previous work published in Scientific Reports showing a strong relationship between waist-to-height ratio and visceral fat percentage in adults with T1DM. The most recent FinnDiane analysis found no similar relationship between NAFL and fat tissue in the hips, arms and legs, and total adipose tissue.
Better than BMI as a measure
“We also found that waist-to-height ratio is better than body mass index to identify those individuals at higher risk of having NAFL,” Dr. Parente said. However, it’s not possible to predict which patients referred to imaging evaluation after being screened by waist-to-height ratio of 0.5 will surely have NAFL, she added.
That answer, she said, would require a longitudinal and cost-effectiveness study with larger population.
The waist-to-height ratio cutoff of 0.5 showed an 86% sensitivity and 55% specificity for NAFL, whereas BMI of 26.6 kg/m2 showed an 79% sensitivity and 57% specificity.
“The most important message from our research is that health care professionals should be aware that individuals with T1DM can have NAFL, and waist-to-height ratio may help to identify those at higher risk,” she said.
The prevalence of NAFL among the adults with T1DM in the study was 11.6%, which is lower than the prevalence other studies reported in T2DM – 76% in a U.S. study – and in the general population – ranging from 19% to 46%. This underscores, Dr. Parente noted, the importance of using waist-to-height ratio in T1DM patients to determine the status of NAFL.
She said that few studies have investigated the consequences of NAFL in T1DM, pointing to two that linked NAFL with chronic kidney disease and cardiovascular disease in T1DM (Diabetes Care. 2014;37:1729-36; J Hepatol. 2010;53:713-8). “Most of the studies about the consequences of NAFL included people with T2DM,” she said. “From our research, we cannot conclude about the impact of NAFL in cardiovascular or kidney complications in our population because this is a cross-sectional study.”
That question may be answered by a future follow-up study of the ongoing FinnDiane study, she said.
The study is a “good reminder” that people with central adiposity and metabolic syndrome can develop NAFL disease, said Jeanne Marie Clark, MD, MPH, of Johns Hopkins University, Baltimore. “Even patients we may not think of having insulin resistance, such as those with T1DM.”
However, Dr. Clark added, “I do not think we can really determine which measure of central adiposity is best.” She noted that the study was “pretty small” with only 14 patients who had NAFL disease. “Waist-to-height ratio is certainly a reasonable option,” she added. “Waist circumference alone is known to be a strong predictor. I would say some measure is better than none, and it should be more routine in clinical practice.”
Dr. Parente disclosed financial relationships with Eli Lilly, Abbott, AstraZeneca, Sanofi, and Boehringer Ingelheim. Two of eight coauthors disclosed financial relationships with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Fresenius, GE Healthcare, Medscape, Merck Sharpe and Dohme, Mundipharma, Novo Nordisk, Peer-Voice, Sanofi, and Sciarc. The remaining coauthors had no disclosures.
Dr. Clark had no disclosures.
It follows that, as the prevalence of obesity among people with type 1 diabetes mellitus (T1DM) increases, so would the incidence of nonalcoholic fatty liver (NAFL), as it does in type 2 diabetes.
However, researchers in Finland report that the incidence of NAFL in T1DM is much lower, and that the use of the waist-to-height ratio to calculate midsection girth could be a low-cost alternative to MRI and computed tomography to more precisely diagnose NAFL in T1DM.
In a cross-sectional analysis of 121 adults with T1DM in the Finnish Diabetic Nephropathy study, known as FinnDiane, researchers from the University of Helsinki report in Diabetes Care that a waist-to-height ratio of 0.5 showed a relatively high rate of accuracy for identifying NAFL that was statistically significant (P = .04).
Lead author Erika B. Parente, MD, PhD, a researcher at the Folkhälsän Research Center in Helsinki, noted that the findings do not identify any causality between what the researchers called visceral adiposity and NAFL. “As long as they have accumulation of fat in the center of body and they can develop this low-grade inflammation that also goes to insulin-load sensitivity, people with T1DM can accumulate fat in the liver as do people with T2DM and the general population,” she said in an interview.
These findings build on her group’s previous work published in Scientific Reports showing a strong relationship between waist-to-height ratio and visceral fat percentage in adults with T1DM. The most recent FinnDiane analysis found no similar relationship between NAFL and fat tissue in the hips, arms and legs, and total adipose tissue.
Better than BMI as a measure
“We also found that waist-to-height ratio is better than body mass index to identify those individuals at higher risk of having NAFL,” Dr. Parente said. However, it’s not possible to predict which patients referred to imaging evaluation after being screened by waist-to-height ratio of 0.5 will surely have NAFL, she added.
That answer, she said, would require a longitudinal and cost-effectiveness study with larger population.
The waist-to-height ratio cutoff of 0.5 showed an 86% sensitivity and 55% specificity for NAFL, whereas BMI of 26.6 kg/m2 showed an 79% sensitivity and 57% specificity.
“The most important message from our research is that health care professionals should be aware that individuals with T1DM can have NAFL, and waist-to-height ratio may help to identify those at higher risk,” she said.
The prevalence of NAFL among the adults with T1DM in the study was 11.6%, which is lower than the prevalence other studies reported in T2DM – 76% in a U.S. study – and in the general population – ranging from 19% to 46%. This underscores, Dr. Parente noted, the importance of using waist-to-height ratio in T1DM patients to determine the status of NAFL.
She said that few studies have investigated the consequences of NAFL in T1DM, pointing to two that linked NAFL with chronic kidney disease and cardiovascular disease in T1DM (Diabetes Care. 2014;37:1729-36; J Hepatol. 2010;53:713-8). “Most of the studies about the consequences of NAFL included people with T2DM,” she said. “From our research, we cannot conclude about the impact of NAFL in cardiovascular or kidney complications in our population because this is a cross-sectional study.”
That question may be answered by a future follow-up study of the ongoing FinnDiane study, she said.
The study is a “good reminder” that people with central adiposity and metabolic syndrome can develop NAFL disease, said Jeanne Marie Clark, MD, MPH, of Johns Hopkins University, Baltimore. “Even patients we may not think of having insulin resistance, such as those with T1DM.”
However, Dr. Clark added, “I do not think we can really determine which measure of central adiposity is best.” She noted that the study was “pretty small” with only 14 patients who had NAFL disease. “Waist-to-height ratio is certainly a reasonable option,” she added. “Waist circumference alone is known to be a strong predictor. I would say some measure is better than none, and it should be more routine in clinical practice.”
Dr. Parente disclosed financial relationships with Eli Lilly, Abbott, AstraZeneca, Sanofi, and Boehringer Ingelheim. Two of eight coauthors disclosed financial relationships with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Fresenius, GE Healthcare, Medscape, Merck Sharpe and Dohme, Mundipharma, Novo Nordisk, Peer-Voice, Sanofi, and Sciarc. The remaining coauthors had no disclosures.
Dr. Clark had no disclosures.
FROM DIABETES CARE
‘Remarkable’ response to diabetes drug in resistant bipolar depression
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.