User login
Hispanic diabetes patients receive less guideline-based care
based on data from more than 7,000 individuals.
Racial and ethnic disparities in diabetes care remain a pervasive health problem, and minorities including non-Hispanic Blacks and Hispanics experience higher rates of complications, including retinopathy and neuropathy, compared with other groups, Felippe Ottoni Marcondes, MD, of Massachusetts General Hospital, Boston, and colleagues noted in a poster presented at the annual meeting of the Society for General Internal Medicine.
Data from previous studies have shown that diabetes patients who receive guideline-directed preventive care soon after diagnosis can reduce their risk of complications, they said.
To identify disparities in the provision of guideline-directed preventive care, the researchers analyzed data from 7,341 individuals who participated in the National Health Interview Survey from 2011 to 2017. They reviewed associations between race/ethnicity and visits to an eye specialist, a foot specialist, and checks of blood pressure and cholesterol in the past year among individuals diagnosed with diabetes within the past 5 years.
Overall, Hispanics had significantly lower rates of insurance coverage (75.9%), compared with non-Hispanic Whites (93.2%) and non-Hispanic Blacks (88.1%; P < .001).
Hispanics also were significantly less likely than Whites to have had a prior year eye exam (odds ratio, 0.80) and blood pressure check (OR, 0.45), after controlling for variables including age, sex, socioeconomic status, health insurance, general health status, U.S. region, marital status, body mass index, and various comorbidities.
Although insurance coverage mediated 42.8% of the total effect of race/ethnicity on annual eye specialist visits for Hispanics as compared with Whites, there was no significant effect for Blacks, compared with Whites.
COVID concerns impact diabetes disparities
“As the diabetes epidemic continues in the U.S., it is important to bring to the front of the diabetes care conversation racial/ethnic disparities that persisted or have been only partially addressed,” Dr. Marcondes said in an interview. “It is also important to emphasize that patients with diabetes are at higher risk for COVID-19 hospitalizations, complications, and death, and COVID-19 has disproportionately affected racial/ethnic minorities, so racial/ethnic minorities with diabetes have compounded risk of complications not only from diabetes but also from COVID-19.
“Importantly, our study highlights disparities in health care that are likely the product of systemic inequalities in access to care and insurance coverage at a moment when conversations about the race/racism and their health impact are fresh in the minds of public and health policy officials and the general public,” he emphasized.
“Unfortunately, I cannot say that I am surprised by our findings,” Dr. Marcondes said. “We expected to see some differences in the receipt of care for racial/ethnic minorities compared to white individuals for those recently diagnosed with diabetes, and that is exactly what our findings show.”
However, “what was perhaps intriguing is that disparities in the receipt of guideline-directed care were greater for Hispanic compared to White individuals than for Black compared to White individuals,” said Dr. Marcondes. “The causes of these differences are many. Hispanic individuals are less likely than White and Black persons to have insurance coverage.” Other unmeasured factors include language barriers that Hispanic individuals may face, as well as the bias and discrimination experienced by Hispanic and Black individuals alike.
Focus on equitable early intervention
“There is plenty of evidence in the medical literature that Black and Hispanic individuals with diabetes, as well as other minorities, have higher risk of complications of diabetes such as retinopathy, nephropathy, as well as cardiovascular risk factors such as high blood pressure and cholesterol,” Dr. Marcondes said. “Yet, complications in the time that immediately follows the diagnosis of diabetes are likely to be low.”
To reduce the risk of complications in the future, “physicians and health providers need to focus on providing equitable, guideline-directed treatment for their minority patients recently diagnosed with diabetes,” Dr. Marcondes emphasized. “Intervening early in the disease course will hopefully lead to a decrease in the rate of complications for racial/ethnic minorities. Clinicians, especially primary care physicians and providers, need to be aware that they are often the first encounter of many patients with the health care system. Effective communication and unbiased language on the part of clinicians will lead to stronger patient-physician relationships that foster opportunity to discuss disease prevention.
“Additional research is needed to evaluate the attitudes and biases of primary care providers and access the impact of patient navigation resources when treating minority patients with diabetes,” he concluded.
Digging Deeper into Disparities
“In diabetes, there are known racial and ethnic disparities such that minorities receive suboptimal screening and treatment, and have worse outcomes,” said Scott J. Pilla, MD, of The Johns Hopkins University School of Medicine, Baltimore, in an interview.
“This study examines disparities in diabetes preventive measures in the U.S. using a national survey (NHIS) over the past decade. They took the important step of stratifying their analyses by health insurance and socioeconomic status which, in addition to race, may have a large impact,” said Dr. Pilla. However, “One critique of the poster is that it is unclear whether the researchers weighted their analyses to account for the nationally representative sampling of the NHIS survey,” he noted.
Dr. Pilla said the finding that Hispanic patients had fewer diabetes preventive measures lines up with previous research in this area.
“I was surprised that the disparities did not extend to black patients, who have been found to also receive suboptimal care compared to white patients in other studies,” he noted.
The message for clinical practice: “Minorities with diabetes are at a higher risk of adverse diabetes outcomes and may need extra support and resources to achieve their evidence-based diabetes prevention,” Dr. Pilla said.
“More research is needed to understand the root cause of racial and ethnic disparities in diabetes management to tease apart possible contributors including health insurance coverage, socioeconomic factors, cultural and community factors, and systemic racism. This will help inform targeted approaches to reducing disparities in diabetes care,” he emphasized.
The researchers had no relevant financial conflicts to disclose. Dr. Pilla had no financial conflicts to disclose.
based on data from more than 7,000 individuals.
Racial and ethnic disparities in diabetes care remain a pervasive health problem, and minorities including non-Hispanic Blacks and Hispanics experience higher rates of complications, including retinopathy and neuropathy, compared with other groups, Felippe Ottoni Marcondes, MD, of Massachusetts General Hospital, Boston, and colleagues noted in a poster presented at the annual meeting of the Society for General Internal Medicine.
Data from previous studies have shown that diabetes patients who receive guideline-directed preventive care soon after diagnosis can reduce their risk of complications, they said.
To identify disparities in the provision of guideline-directed preventive care, the researchers analyzed data from 7,341 individuals who participated in the National Health Interview Survey from 2011 to 2017. They reviewed associations between race/ethnicity and visits to an eye specialist, a foot specialist, and checks of blood pressure and cholesterol in the past year among individuals diagnosed with diabetes within the past 5 years.
Overall, Hispanics had significantly lower rates of insurance coverage (75.9%), compared with non-Hispanic Whites (93.2%) and non-Hispanic Blacks (88.1%; P < .001).
Hispanics also were significantly less likely than Whites to have had a prior year eye exam (odds ratio, 0.80) and blood pressure check (OR, 0.45), after controlling for variables including age, sex, socioeconomic status, health insurance, general health status, U.S. region, marital status, body mass index, and various comorbidities.
Although insurance coverage mediated 42.8% of the total effect of race/ethnicity on annual eye specialist visits for Hispanics as compared with Whites, there was no significant effect for Blacks, compared with Whites.
COVID concerns impact diabetes disparities
“As the diabetes epidemic continues in the U.S., it is important to bring to the front of the diabetes care conversation racial/ethnic disparities that persisted or have been only partially addressed,” Dr. Marcondes said in an interview. “It is also important to emphasize that patients with diabetes are at higher risk for COVID-19 hospitalizations, complications, and death, and COVID-19 has disproportionately affected racial/ethnic minorities, so racial/ethnic minorities with diabetes have compounded risk of complications not only from diabetes but also from COVID-19.
“Importantly, our study highlights disparities in health care that are likely the product of systemic inequalities in access to care and insurance coverage at a moment when conversations about the race/racism and their health impact are fresh in the minds of public and health policy officials and the general public,” he emphasized.
“Unfortunately, I cannot say that I am surprised by our findings,” Dr. Marcondes said. “We expected to see some differences in the receipt of care for racial/ethnic minorities compared to white individuals for those recently diagnosed with diabetes, and that is exactly what our findings show.”
However, “what was perhaps intriguing is that disparities in the receipt of guideline-directed care were greater for Hispanic compared to White individuals than for Black compared to White individuals,” said Dr. Marcondes. “The causes of these differences are many. Hispanic individuals are less likely than White and Black persons to have insurance coverage.” Other unmeasured factors include language barriers that Hispanic individuals may face, as well as the bias and discrimination experienced by Hispanic and Black individuals alike.
Focus on equitable early intervention
“There is plenty of evidence in the medical literature that Black and Hispanic individuals with diabetes, as well as other minorities, have higher risk of complications of diabetes such as retinopathy, nephropathy, as well as cardiovascular risk factors such as high blood pressure and cholesterol,” Dr. Marcondes said. “Yet, complications in the time that immediately follows the diagnosis of diabetes are likely to be low.”
To reduce the risk of complications in the future, “physicians and health providers need to focus on providing equitable, guideline-directed treatment for their minority patients recently diagnosed with diabetes,” Dr. Marcondes emphasized. “Intervening early in the disease course will hopefully lead to a decrease in the rate of complications for racial/ethnic minorities. Clinicians, especially primary care physicians and providers, need to be aware that they are often the first encounter of many patients with the health care system. Effective communication and unbiased language on the part of clinicians will lead to stronger patient-physician relationships that foster opportunity to discuss disease prevention.
“Additional research is needed to evaluate the attitudes and biases of primary care providers and access the impact of patient navigation resources when treating minority patients with diabetes,” he concluded.
Digging Deeper into Disparities
“In diabetes, there are known racial and ethnic disparities such that minorities receive suboptimal screening and treatment, and have worse outcomes,” said Scott J. Pilla, MD, of The Johns Hopkins University School of Medicine, Baltimore, in an interview.
“This study examines disparities in diabetes preventive measures in the U.S. using a national survey (NHIS) over the past decade. They took the important step of stratifying their analyses by health insurance and socioeconomic status which, in addition to race, may have a large impact,” said Dr. Pilla. However, “One critique of the poster is that it is unclear whether the researchers weighted their analyses to account for the nationally representative sampling of the NHIS survey,” he noted.
Dr. Pilla said the finding that Hispanic patients had fewer diabetes preventive measures lines up with previous research in this area.
“I was surprised that the disparities did not extend to black patients, who have been found to also receive suboptimal care compared to white patients in other studies,” he noted.
The message for clinical practice: “Minorities with diabetes are at a higher risk of adverse diabetes outcomes and may need extra support and resources to achieve their evidence-based diabetes prevention,” Dr. Pilla said.
“More research is needed to understand the root cause of racial and ethnic disparities in diabetes management to tease apart possible contributors including health insurance coverage, socioeconomic factors, cultural and community factors, and systemic racism. This will help inform targeted approaches to reducing disparities in diabetes care,” he emphasized.
The researchers had no relevant financial conflicts to disclose. Dr. Pilla had no financial conflicts to disclose.
based on data from more than 7,000 individuals.
Racial and ethnic disparities in diabetes care remain a pervasive health problem, and minorities including non-Hispanic Blacks and Hispanics experience higher rates of complications, including retinopathy and neuropathy, compared with other groups, Felippe Ottoni Marcondes, MD, of Massachusetts General Hospital, Boston, and colleagues noted in a poster presented at the annual meeting of the Society for General Internal Medicine.
Data from previous studies have shown that diabetes patients who receive guideline-directed preventive care soon after diagnosis can reduce their risk of complications, they said.
To identify disparities in the provision of guideline-directed preventive care, the researchers analyzed data from 7,341 individuals who participated in the National Health Interview Survey from 2011 to 2017. They reviewed associations between race/ethnicity and visits to an eye specialist, a foot specialist, and checks of blood pressure and cholesterol in the past year among individuals diagnosed with diabetes within the past 5 years.
Overall, Hispanics had significantly lower rates of insurance coverage (75.9%), compared with non-Hispanic Whites (93.2%) and non-Hispanic Blacks (88.1%; P < .001).
Hispanics also were significantly less likely than Whites to have had a prior year eye exam (odds ratio, 0.80) and blood pressure check (OR, 0.45), after controlling for variables including age, sex, socioeconomic status, health insurance, general health status, U.S. region, marital status, body mass index, and various comorbidities.
Although insurance coverage mediated 42.8% of the total effect of race/ethnicity on annual eye specialist visits for Hispanics as compared with Whites, there was no significant effect for Blacks, compared with Whites.
COVID concerns impact diabetes disparities
“As the diabetes epidemic continues in the U.S., it is important to bring to the front of the diabetes care conversation racial/ethnic disparities that persisted or have been only partially addressed,” Dr. Marcondes said in an interview. “It is also important to emphasize that patients with diabetes are at higher risk for COVID-19 hospitalizations, complications, and death, and COVID-19 has disproportionately affected racial/ethnic minorities, so racial/ethnic minorities with diabetes have compounded risk of complications not only from diabetes but also from COVID-19.
“Importantly, our study highlights disparities in health care that are likely the product of systemic inequalities in access to care and insurance coverage at a moment when conversations about the race/racism and their health impact are fresh in the minds of public and health policy officials and the general public,” he emphasized.
“Unfortunately, I cannot say that I am surprised by our findings,” Dr. Marcondes said. “We expected to see some differences in the receipt of care for racial/ethnic minorities compared to white individuals for those recently diagnosed with diabetes, and that is exactly what our findings show.”
However, “what was perhaps intriguing is that disparities in the receipt of guideline-directed care were greater for Hispanic compared to White individuals than for Black compared to White individuals,” said Dr. Marcondes. “The causes of these differences are many. Hispanic individuals are less likely than White and Black persons to have insurance coverage.” Other unmeasured factors include language barriers that Hispanic individuals may face, as well as the bias and discrimination experienced by Hispanic and Black individuals alike.
Focus on equitable early intervention
“There is plenty of evidence in the medical literature that Black and Hispanic individuals with diabetes, as well as other minorities, have higher risk of complications of diabetes such as retinopathy, nephropathy, as well as cardiovascular risk factors such as high blood pressure and cholesterol,” Dr. Marcondes said. “Yet, complications in the time that immediately follows the diagnosis of diabetes are likely to be low.”
To reduce the risk of complications in the future, “physicians and health providers need to focus on providing equitable, guideline-directed treatment for their minority patients recently diagnosed with diabetes,” Dr. Marcondes emphasized. “Intervening early in the disease course will hopefully lead to a decrease in the rate of complications for racial/ethnic minorities. Clinicians, especially primary care physicians and providers, need to be aware that they are often the first encounter of many patients with the health care system. Effective communication and unbiased language on the part of clinicians will lead to stronger patient-physician relationships that foster opportunity to discuss disease prevention.
“Additional research is needed to evaluate the attitudes and biases of primary care providers and access the impact of patient navigation resources when treating minority patients with diabetes,” he concluded.
Digging Deeper into Disparities
“In diabetes, there are known racial and ethnic disparities such that minorities receive suboptimal screening and treatment, and have worse outcomes,” said Scott J. Pilla, MD, of The Johns Hopkins University School of Medicine, Baltimore, in an interview.
“This study examines disparities in diabetes preventive measures in the U.S. using a national survey (NHIS) over the past decade. They took the important step of stratifying their analyses by health insurance and socioeconomic status which, in addition to race, may have a large impact,” said Dr. Pilla. However, “One critique of the poster is that it is unclear whether the researchers weighted their analyses to account for the nationally representative sampling of the NHIS survey,” he noted.
Dr. Pilla said the finding that Hispanic patients had fewer diabetes preventive measures lines up with previous research in this area.
“I was surprised that the disparities did not extend to black patients, who have been found to also receive suboptimal care compared to white patients in other studies,” he noted.
The message for clinical practice: “Minorities with diabetes are at a higher risk of adverse diabetes outcomes and may need extra support and resources to achieve their evidence-based diabetes prevention,” Dr. Pilla said.
“More research is needed to understand the root cause of racial and ethnic disparities in diabetes management to tease apart possible contributors including health insurance coverage, socioeconomic factors, cultural and community factors, and systemic racism. This will help inform targeted approaches to reducing disparities in diabetes care,” he emphasized.
The researchers had no relevant financial conflicts to disclose. Dr. Pilla had no financial conflicts to disclose.
FROM SGIM 2021
Pros and cons of proposed recommendation for prediabetes and T2D screening
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
FDA panel supports islet cell treatment for type 1 diabetes
A Food and Drug Administration advisory panel has endorsed a pancreatic islet cell transplant therapy for the treatment of people with type 1 diabetes that can’t be managed with current therapies.
On April 15, the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee voted 12 to 4 in favor of approval of donislecel (Lantidra). There was one abstention. The panel regarded the drug as having “an overall favorable benefit-risk profile for some patients with type 1 diabetes.” The product consists of purified allogeneic pancreatic islets of Langerhans derived from cadaveric donors and is infused into the portal vein of the liver.
Benefits of the treatment include the potential for insulin independence and elimination of severe hypoglycemia. Risks are those associated with the surgical procedure and with long-term immunosuppression.
The therapy is manufactured by CellTrans. According to Jose Oberholzer, MD, the founder of CellTrans, the proposed indication is for adults with “brittle” type 1 diabetes who meet the American Diabetes Association’s (ADA) criteria for whole-organ pancreas-alone transplant (i.e., transplant of pancreas but not kidney).
The ADA criteria include the following: frequent, severe hypoglycemia, hyperglycemia, and/or ketoacidosis that requires medical attention; clinical or emotional problems regarding the use of exogenous insulin; and consistent failure of insulin-based management to prevent acute diabetes complications.
Success in two-thirds of patients in small studies
Dr. Oberholzer presented data from two single-arm open-label studies: a phase 1/2 trial initiated in 2004 with 10 patients, and a phase 3 study with 20 patients that began in 2007. The inclusion criteria differed somewhat between the two studies, but all 30 patients had hypoglycemic unawareness. Mean follow-up was 7.8 years for the phase 1/2 trial and 4.7 years for the phase 3 trial.
For all of the patients, C-peptide levels were positive after transplant. The composite endpoint for success – an A1c level of ≤ 6.5% and the absence of severe hypoglycemic episodes for 1 year – was met by 19 patients (63.3%). For five patients (16.7%), the target A1c level was not achieved, and seven patients (23.3%) experienced a severe episode of hypoglycemia.
Twenty of the 30 patients achieved insulin independence for at least 1 year.
Improvements were also seen at 1 year in mixed meal test outcomes, fasting blood glucose levels, and overall glycemic control. Graft survival 10 years post transplant was achieved by 60% of patients, Dr. Oberholzer said.
Adverse events not unexpected, but still of concern
Two patients died, one as a result of fulminant sepsis at 20 months post transplant, and the other as a result of severe dementia 9 years post transplant. Three patients experienced four serious procedure-related events, including one liver laceration and two hepatic hematomas. Elevations in portal pressure occurred in two patients.
Most adverse events were associated with immunosuppression. These included 178 infections in 26 of the 30 patients. The most common of these were herpes virus infections, Epstein-Barr virus infections, oral candidiasis, and cytomegalovirus infections. Twelve infections were severe. Renal function declined persistently in two patients (20%), and six (20%) experienced new-onset proteinuria at 1 year.
The adverse events related to the procedure and the problems associated with immunosuppression were not unexpected and were consistent with those described for patients receiving whole pancreas transplants, FDA reviewer Patricia Beaston, MD, said in her review of the CellTrans data.
Panel members support treatment for a small group of patients
During the discussion, several panel members pointed out that the target patient population for this treatment will likely be smaller today than it was when the two studies were initiated, given advances in diabetes care. Those advances include continuous glucose monitoring devices with alarms and closed-loop insulin delivery systems – the “artificial pancreas” that automatically suspends insulin delivery to prevent hypoglycemia.
Panel chair Lisa Butterfield, PhD, a surgeon and immunologist at the University of California, San Francisco, voted in favor of approval. But, she added, “I do support postapproval gathering of data to learn more about the product. ... I don’t know how many patients will really benefit, but I think it’s to be determined.”
Christopher K. Breuer, MD, a general and pediatric surgeon at the Center for Regenerative Medicine, Nationwide Children’s Hospital, Columbus, Ohio, said he supported approval for “two very small subpopulations where it would provide the only viable therapy”: those who are eligible for pancreas transplant but cannot tolerate a major operation, and those who already use the latest automated insulin delivery systems and still do not achieve acceptable glycemic control.
Temporary voting member David Harlan, MD, director of the University of Massachusetts Diabetes Center of Excellence, Worcester, Mass., voted no.
He noted that only about 100 whole pancreas-only transplants are performed annually in the United States and that such transplants are “very effective, so we’re talking about patients who aren’t pancreas transplant candidates who might get this.”
Moreover, Dr. Harlan said, “I’ve seen the awful things that can happen in posttransplant recipients. It’s really hard to get that informed consent from someone when you’re asking them to consider a future that they don’t know. When it works, it’s great. When it doesn’t work, it can be catastrophic. I just worry about opening Pandora’s box.”
The only other diabetes specialist on the panel, temporary voting member Ellen Leschek, MD, said she “reluctantly voted yes because a few people could benefit, but I think it’s a much smaller number than the company may believe.”
Dr. Leschek, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., said she’s concerned that “if it’s approved, too many people will get treated this way, when in fact, for a lot of those people, the risks will outweigh the benefits.”
Sandy Feng, MD, PhD, of the department of surgery at the University of California, San Francisco, pointed out that with regard to immunosuppressive therapy, “We’re concerned about the toxicity of what we currently use, but there are additional therapies being developed that might mitigate those toxicities that would be beneficial to this population.”
Dr. Feng, who voted yes, also said, “I do pancreas transplants. I can tell you that there is nothing that [patients with type 1 diabetes] like more than the freedom from dealing with the entire insulin issue. That has made a large impression on me over the last 20-plus years of clinical practice, so I do think this can help some people and will be incredibly meaningful to those people.”
FDA advisory panel members are vetted for conflicts of interest, and special waivers are granted if necessary. No such waivers were granted for this meeting.
A version of this article first appeared on Medscape.com.
A Food and Drug Administration advisory panel has endorsed a pancreatic islet cell transplant therapy for the treatment of people with type 1 diabetes that can’t be managed with current therapies.
On April 15, the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee voted 12 to 4 in favor of approval of donislecel (Lantidra). There was one abstention. The panel regarded the drug as having “an overall favorable benefit-risk profile for some patients with type 1 diabetes.” The product consists of purified allogeneic pancreatic islets of Langerhans derived from cadaveric donors and is infused into the portal vein of the liver.
Benefits of the treatment include the potential for insulin independence and elimination of severe hypoglycemia. Risks are those associated with the surgical procedure and with long-term immunosuppression.
The therapy is manufactured by CellTrans. According to Jose Oberholzer, MD, the founder of CellTrans, the proposed indication is for adults with “brittle” type 1 diabetes who meet the American Diabetes Association’s (ADA) criteria for whole-organ pancreas-alone transplant (i.e., transplant of pancreas but not kidney).
The ADA criteria include the following: frequent, severe hypoglycemia, hyperglycemia, and/or ketoacidosis that requires medical attention; clinical or emotional problems regarding the use of exogenous insulin; and consistent failure of insulin-based management to prevent acute diabetes complications.
Success in two-thirds of patients in small studies
Dr. Oberholzer presented data from two single-arm open-label studies: a phase 1/2 trial initiated in 2004 with 10 patients, and a phase 3 study with 20 patients that began in 2007. The inclusion criteria differed somewhat between the two studies, but all 30 patients had hypoglycemic unawareness. Mean follow-up was 7.8 years for the phase 1/2 trial and 4.7 years for the phase 3 trial.
For all of the patients, C-peptide levels were positive after transplant. The composite endpoint for success – an A1c level of ≤ 6.5% and the absence of severe hypoglycemic episodes for 1 year – was met by 19 patients (63.3%). For five patients (16.7%), the target A1c level was not achieved, and seven patients (23.3%) experienced a severe episode of hypoglycemia.
Twenty of the 30 patients achieved insulin independence for at least 1 year.
Improvements were also seen at 1 year in mixed meal test outcomes, fasting blood glucose levels, and overall glycemic control. Graft survival 10 years post transplant was achieved by 60% of patients, Dr. Oberholzer said.
Adverse events not unexpected, but still of concern
Two patients died, one as a result of fulminant sepsis at 20 months post transplant, and the other as a result of severe dementia 9 years post transplant. Three patients experienced four serious procedure-related events, including one liver laceration and two hepatic hematomas. Elevations in portal pressure occurred in two patients.
Most adverse events were associated with immunosuppression. These included 178 infections in 26 of the 30 patients. The most common of these were herpes virus infections, Epstein-Barr virus infections, oral candidiasis, and cytomegalovirus infections. Twelve infections were severe. Renal function declined persistently in two patients (20%), and six (20%) experienced new-onset proteinuria at 1 year.
The adverse events related to the procedure and the problems associated with immunosuppression were not unexpected and were consistent with those described for patients receiving whole pancreas transplants, FDA reviewer Patricia Beaston, MD, said in her review of the CellTrans data.
Panel members support treatment for a small group of patients
During the discussion, several panel members pointed out that the target patient population for this treatment will likely be smaller today than it was when the two studies were initiated, given advances in diabetes care. Those advances include continuous glucose monitoring devices with alarms and closed-loop insulin delivery systems – the “artificial pancreas” that automatically suspends insulin delivery to prevent hypoglycemia.
Panel chair Lisa Butterfield, PhD, a surgeon and immunologist at the University of California, San Francisco, voted in favor of approval. But, she added, “I do support postapproval gathering of data to learn more about the product. ... I don’t know how many patients will really benefit, but I think it’s to be determined.”
Christopher K. Breuer, MD, a general and pediatric surgeon at the Center for Regenerative Medicine, Nationwide Children’s Hospital, Columbus, Ohio, said he supported approval for “two very small subpopulations where it would provide the only viable therapy”: those who are eligible for pancreas transplant but cannot tolerate a major operation, and those who already use the latest automated insulin delivery systems and still do not achieve acceptable glycemic control.
Temporary voting member David Harlan, MD, director of the University of Massachusetts Diabetes Center of Excellence, Worcester, Mass., voted no.
He noted that only about 100 whole pancreas-only transplants are performed annually in the United States and that such transplants are “very effective, so we’re talking about patients who aren’t pancreas transplant candidates who might get this.”
Moreover, Dr. Harlan said, “I’ve seen the awful things that can happen in posttransplant recipients. It’s really hard to get that informed consent from someone when you’re asking them to consider a future that they don’t know. When it works, it’s great. When it doesn’t work, it can be catastrophic. I just worry about opening Pandora’s box.”
The only other diabetes specialist on the panel, temporary voting member Ellen Leschek, MD, said she “reluctantly voted yes because a few people could benefit, but I think it’s a much smaller number than the company may believe.”
Dr. Leschek, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., said she’s concerned that “if it’s approved, too many people will get treated this way, when in fact, for a lot of those people, the risks will outweigh the benefits.”
Sandy Feng, MD, PhD, of the department of surgery at the University of California, San Francisco, pointed out that with regard to immunosuppressive therapy, “We’re concerned about the toxicity of what we currently use, but there are additional therapies being developed that might mitigate those toxicities that would be beneficial to this population.”
Dr. Feng, who voted yes, also said, “I do pancreas transplants. I can tell you that there is nothing that [patients with type 1 diabetes] like more than the freedom from dealing with the entire insulin issue. That has made a large impression on me over the last 20-plus years of clinical practice, so I do think this can help some people and will be incredibly meaningful to those people.”
FDA advisory panel members are vetted for conflicts of interest, and special waivers are granted if necessary. No such waivers were granted for this meeting.
A version of this article first appeared on Medscape.com.
A Food and Drug Administration advisory panel has endorsed a pancreatic islet cell transplant therapy for the treatment of people with type 1 diabetes that can’t be managed with current therapies.
On April 15, the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee voted 12 to 4 in favor of approval of donislecel (Lantidra). There was one abstention. The panel regarded the drug as having “an overall favorable benefit-risk profile for some patients with type 1 diabetes.” The product consists of purified allogeneic pancreatic islets of Langerhans derived from cadaveric donors and is infused into the portal vein of the liver.
Benefits of the treatment include the potential for insulin independence and elimination of severe hypoglycemia. Risks are those associated with the surgical procedure and with long-term immunosuppression.
The therapy is manufactured by CellTrans. According to Jose Oberholzer, MD, the founder of CellTrans, the proposed indication is for adults with “brittle” type 1 diabetes who meet the American Diabetes Association’s (ADA) criteria for whole-organ pancreas-alone transplant (i.e., transplant of pancreas but not kidney).
The ADA criteria include the following: frequent, severe hypoglycemia, hyperglycemia, and/or ketoacidosis that requires medical attention; clinical or emotional problems regarding the use of exogenous insulin; and consistent failure of insulin-based management to prevent acute diabetes complications.
Success in two-thirds of patients in small studies
Dr. Oberholzer presented data from two single-arm open-label studies: a phase 1/2 trial initiated in 2004 with 10 patients, and a phase 3 study with 20 patients that began in 2007. The inclusion criteria differed somewhat between the two studies, but all 30 patients had hypoglycemic unawareness. Mean follow-up was 7.8 years for the phase 1/2 trial and 4.7 years for the phase 3 trial.
For all of the patients, C-peptide levels were positive after transplant. The composite endpoint for success – an A1c level of ≤ 6.5% and the absence of severe hypoglycemic episodes for 1 year – was met by 19 patients (63.3%). For five patients (16.7%), the target A1c level was not achieved, and seven patients (23.3%) experienced a severe episode of hypoglycemia.
Twenty of the 30 patients achieved insulin independence for at least 1 year.
Improvements were also seen at 1 year in mixed meal test outcomes, fasting blood glucose levels, and overall glycemic control. Graft survival 10 years post transplant was achieved by 60% of patients, Dr. Oberholzer said.
Adverse events not unexpected, but still of concern
Two patients died, one as a result of fulminant sepsis at 20 months post transplant, and the other as a result of severe dementia 9 years post transplant. Three patients experienced four serious procedure-related events, including one liver laceration and two hepatic hematomas. Elevations in portal pressure occurred in two patients.
Most adverse events were associated with immunosuppression. These included 178 infections in 26 of the 30 patients. The most common of these were herpes virus infections, Epstein-Barr virus infections, oral candidiasis, and cytomegalovirus infections. Twelve infections were severe. Renal function declined persistently in two patients (20%), and six (20%) experienced new-onset proteinuria at 1 year.
The adverse events related to the procedure and the problems associated with immunosuppression were not unexpected and were consistent with those described for patients receiving whole pancreas transplants, FDA reviewer Patricia Beaston, MD, said in her review of the CellTrans data.
Panel members support treatment for a small group of patients
During the discussion, several panel members pointed out that the target patient population for this treatment will likely be smaller today than it was when the two studies were initiated, given advances in diabetes care. Those advances include continuous glucose monitoring devices with alarms and closed-loop insulin delivery systems – the “artificial pancreas” that automatically suspends insulin delivery to prevent hypoglycemia.
Panel chair Lisa Butterfield, PhD, a surgeon and immunologist at the University of California, San Francisco, voted in favor of approval. But, she added, “I do support postapproval gathering of data to learn more about the product. ... I don’t know how many patients will really benefit, but I think it’s to be determined.”
Christopher K. Breuer, MD, a general and pediatric surgeon at the Center for Regenerative Medicine, Nationwide Children’s Hospital, Columbus, Ohio, said he supported approval for “two very small subpopulations where it would provide the only viable therapy”: those who are eligible for pancreas transplant but cannot tolerate a major operation, and those who already use the latest automated insulin delivery systems and still do not achieve acceptable glycemic control.
Temporary voting member David Harlan, MD, director of the University of Massachusetts Diabetes Center of Excellence, Worcester, Mass., voted no.
He noted that only about 100 whole pancreas-only transplants are performed annually in the United States and that such transplants are “very effective, so we’re talking about patients who aren’t pancreas transplant candidates who might get this.”
Moreover, Dr. Harlan said, “I’ve seen the awful things that can happen in posttransplant recipients. It’s really hard to get that informed consent from someone when you’re asking them to consider a future that they don’t know. When it works, it’s great. When it doesn’t work, it can be catastrophic. I just worry about opening Pandora’s box.”
The only other diabetes specialist on the panel, temporary voting member Ellen Leschek, MD, said she “reluctantly voted yes because a few people could benefit, but I think it’s a much smaller number than the company may believe.”
Dr. Leschek, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., said she’s concerned that “if it’s approved, too many people will get treated this way, when in fact, for a lot of those people, the risks will outweigh the benefits.”
Sandy Feng, MD, PhD, of the department of surgery at the University of California, San Francisco, pointed out that with regard to immunosuppressive therapy, “We’re concerned about the toxicity of what we currently use, but there are additional therapies being developed that might mitigate those toxicities that would be beneficial to this population.”
Dr. Feng, who voted yes, also said, “I do pancreas transplants. I can tell you that there is nothing that [patients with type 1 diabetes] like more than the freedom from dealing with the entire insulin issue. That has made a large impression on me over the last 20-plus years of clinical practice, so I do think this can help some people and will be incredibly meaningful to those people.”
FDA advisory panel members are vetted for conflicts of interest, and special waivers are granted if necessary. No such waivers were granted for this meeting.
A version of this article first appeared on Medscape.com.
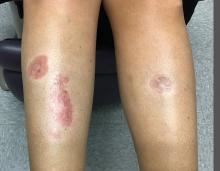
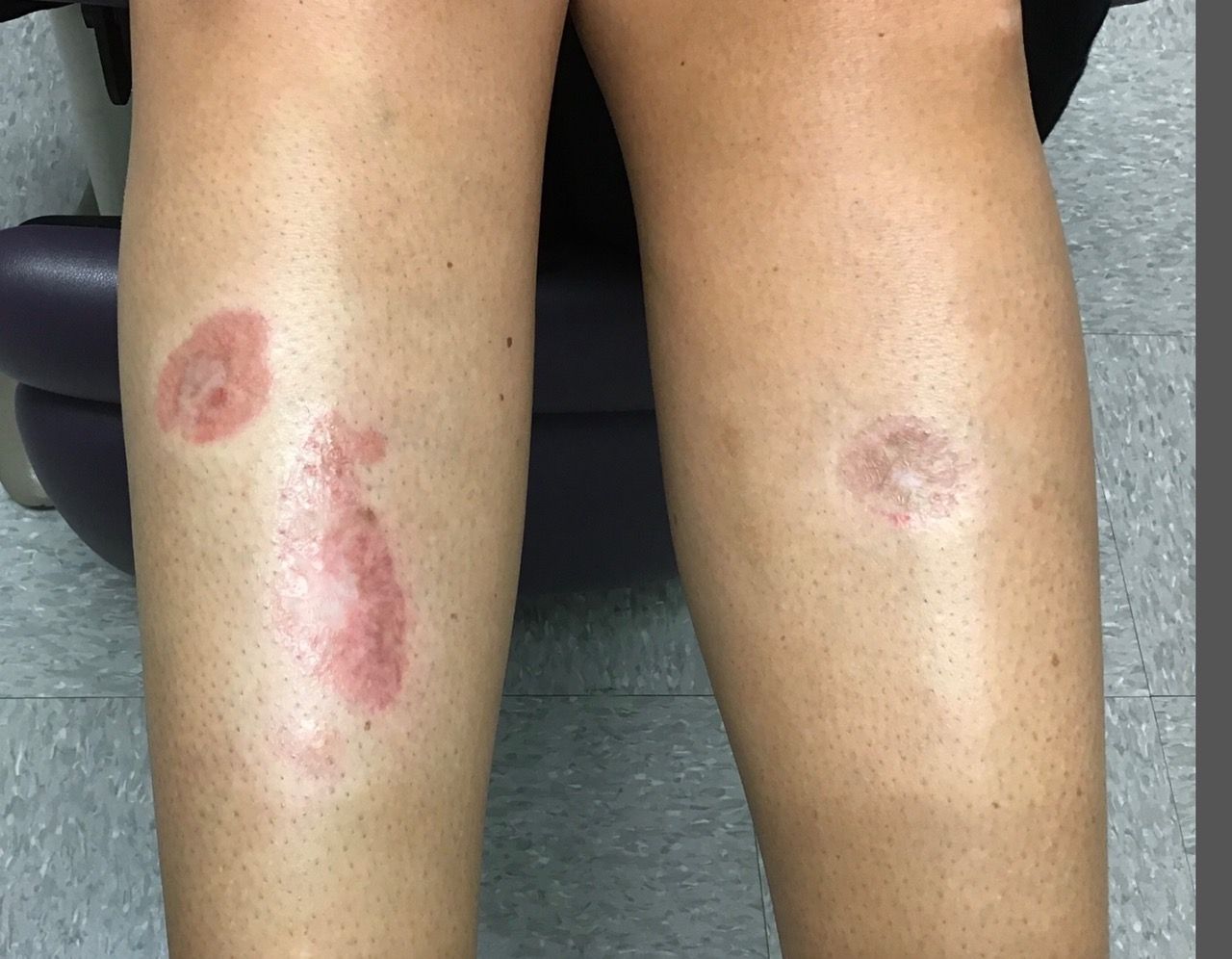
A woman with a history of diabetes, and plaques on both shins
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
Enhancing Diabetes Self-Management Education and Psychological Services for Veterans With Comorbid Chronic Health and Mental Health Conditions
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
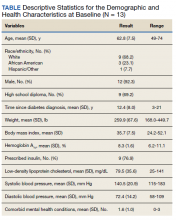
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
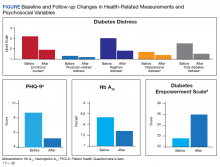
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
Integrating primary care into a community mental health center
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
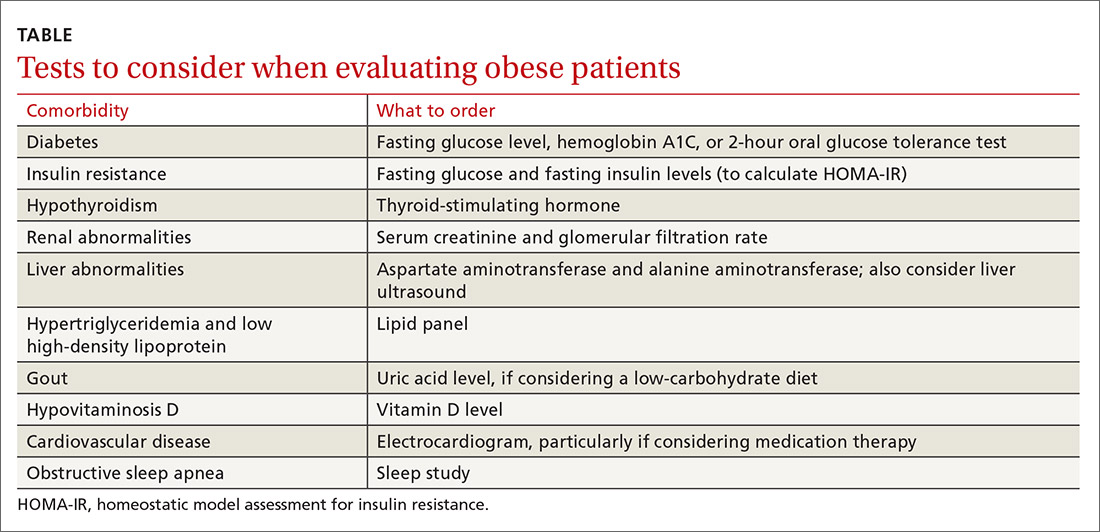
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table

Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table

Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cardiovascular disease remains leading cause of type 2 diabetes mortality
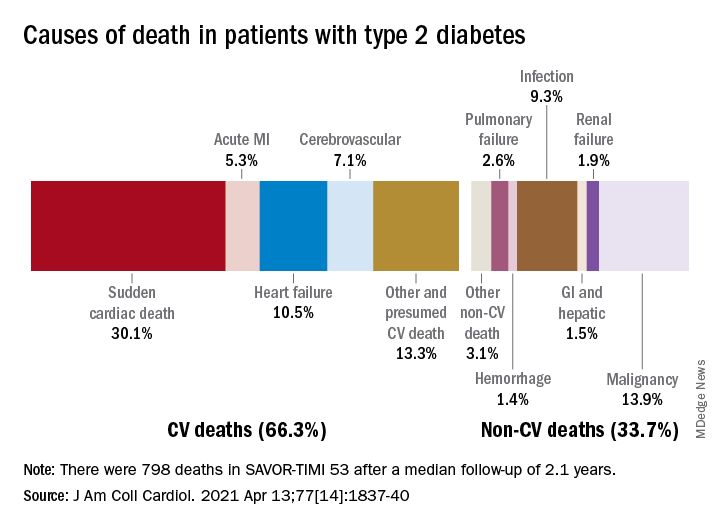
Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Six pregnancy complications flag later heart disease risk
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
FDA approves new ready-to-inject glucagon product
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.