User login
De-pathologizing gender identity: Psychiatry’s role
Treating patients who are transgender or gender diverse (TGGD) requires an understanding of the social and psychological factors that have a unique impact on this population. As clinicians, it is our responsibility to understand the social, cultural, and political issues our patients face, both historically and currently. In this article, we provide information about the nature of gender and gender identity as separate from biological sex and informed by a person’s perception of self as male, female, nonbinary, or other variation.
Psychiatrists must be aware of how individuals who are TGGD have been perceived, classified, and treated by the medical profession, as this history is often a source of mistrust and a barrier to treatment for patients who need psychiatric care. This includes awareness of the “gatekeeping” role that persists in medical institutions today: applying strict eligibility criteria to determine the “fitness” of individuals who are transgender to pursue medical transition, as compared to the informed-consent model that is widely applied to other medical interventions. Our review of minority stress theory, as applicable to this patient population, provides a context and framework for empathic approaches to care for patients who are TGGD. Recognizing barriers to care and ways in which we can create a supportive environment for treatment will allow for tailored approaches that better fit the unique needs of this patient population.
The gender binary
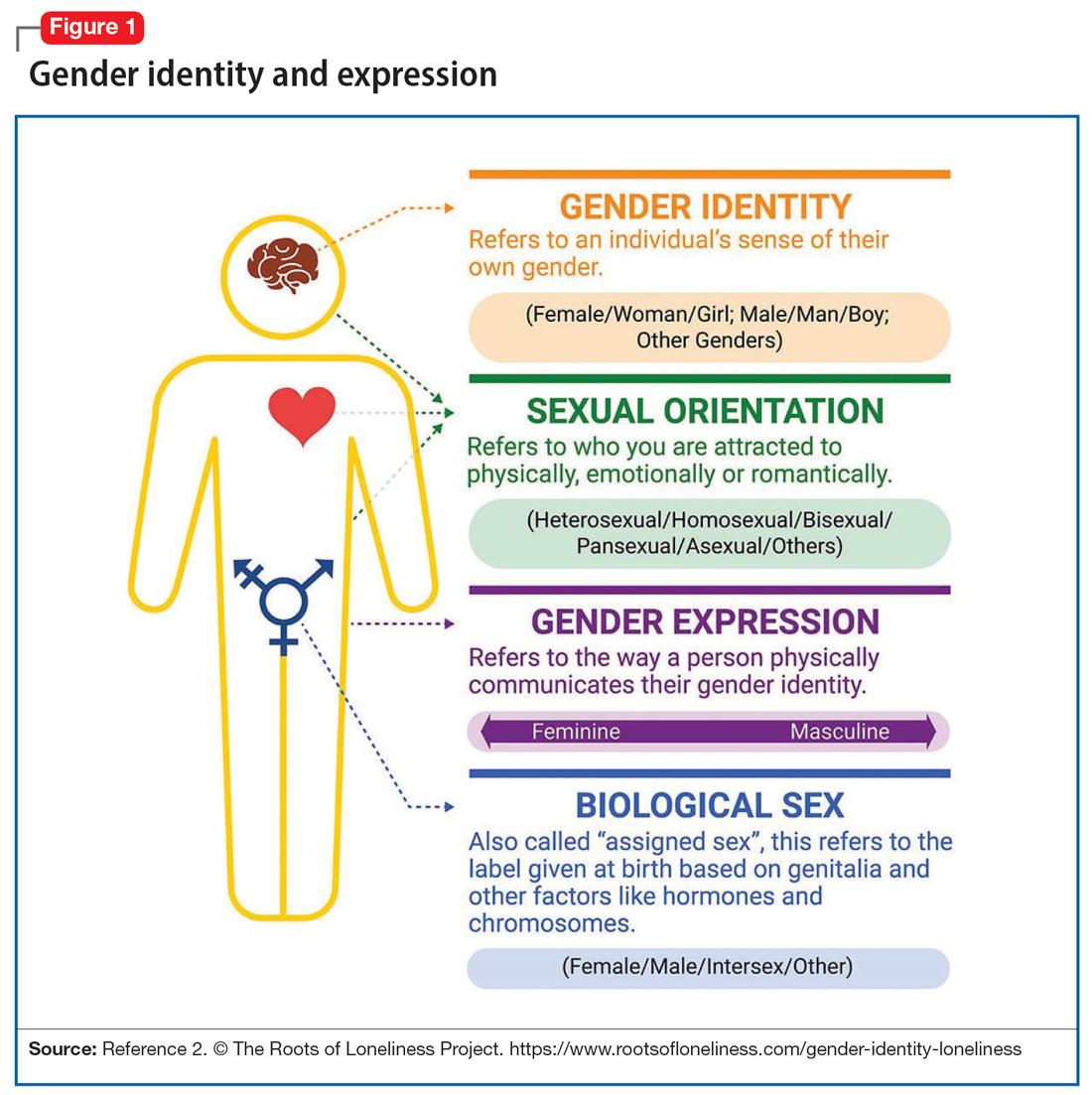
In Western societies, gender has often been viewed as “binary,” oppositional, and directly correlated with physical sex or presumed anatomy.1 The theory of gender essentialism insists that sex and gender are indistinguishable from one another and provide 2 “natural” and distinct categories: women and men. The “gender/sex” binary refers to the belief that individuals born with 2 X chromosomes will inherently develop into and fulfill the social roles of women, and those born with an X and a Y chromosome will develop into and fulfill the social roles of men.1 In this context, “sex” refers to biological characteristics of individuals, including combinations of sex chromosomes, anatomy, and the development of sex characteristics during puberty. The term “gender” refers to the social, cultural, and behavioral aspects of being a man, woman, both, or neither, and “gender identity” refers to one’s internal, individual sense of self and experience of gender (Figure 12). Many Western cultures are now facing destabilization of the gender/sex binary in social, political, and interpersonal contexts.1 This is perhaps most clearly seen in the battle for self-determination and protection by laws affecting individuals who are transgender as well as the determination of other groups to maintain traditional sex and gender roles, often through political action. Historically, individuals who are TGGD have been present in a variety of cultures. For example, most Native American cultures have revered other-gendered individuals, more recently referred to as “two-spirited.” Similarly, the Bugis people of South Sulawesi, Indonesia, recognize 5 genders that exist on a nonbinary spectrum.3
Despite its prevalence in Western society, scientific evidence for the gender/sex binary is lacking. The gender similarities hypothesis states that males and females are similar in most, but not all, psychological variables and is supported by multiple meta-analyses examining psychological gender differences.4 In a 2005 review of 46 meta-analyses of gender-differences, studied through behavior analysis, effect sizes for gender differences were trivial or small in almost 75% of examined variables.5 Analyzing for internal consistency among studies showing large gender/sex differences, Joel et al6 found that, on measures of personality traits, attitudes, interests, and behaviors were rarely homogenous in the brains of males or females. In fact, <1% of study participants showed only masculine or feminine traits, whereas 55% showed a combination, or mosaic, of these traits.6 These findings were supported by further research in behavioral neuroendocrinology that demonstrated a lack of hormonal evidence for 2 distinct sexes. Both estrogen (the “female” hormone) and testosterone (the “male” hormone) are produced by both biological males and females. Further, levels of estradiol do not significantly differ between males and females, and, in fact, in nonpregnant females, estradiol levels are more similar to those of males than to those of pregnant females.1 In the last decade, imaging studies of the human brain have shown that brain structure and connectivity in individuals who are transgender are more similar to those of their experienced gender than of their natal sex.7 In social analyses of intersex individuals (individuals born with ambiguous physical sex characteristics), surgical assignment into the binary gender system did not improve—and often worsened—feelings of isolation and shame.1
The National Institutes of Health defines gender as “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and time.”8 The World Health Organization (WHO) provides a similar definition, and the evidence to support this exists in social-role theory, social-identity theory, and the stereotype-content model. However, despite evidence disputing a gender/sex binary, this method of classifying individuals into a dyad persists in many areas of modern culture, from gender-specific physical spaces (bathrooms, classrooms, store brands), language (pronouns), and laws. This desire for categorization helps fulfill social and psychological needs of groups and individuals by providing group identities and giving structure to the complexity of modern-day life. Identity and group membership provide a sense of belonging, source of self-esteem, and avoidance of ambiguity. Binary gender stereotypes provide expectations that allow anticipation and prediction of our social environments.9 However, the harm of perpetuating the false gender/sex binary is well documented and includes social and economic penalties, extreme violence, and even death. The field of medicine has not been immune from practices that implicitly endorse the gender/sex connection, as seen in the erroneous use of gender in biomedical writings at the highest levels and evidenced in research examining “gender” differences in disease incidence.
Gender diversity as a pathology
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) has been a source of pathologizing gender diversity since the 1960s, with the introduction of “transsexualism” in DSM-II10 and “gender identity disorder of childhood” in DSM-III.11 These diagnoses were listed under the headings of “sexual deviations” and “psychosexual disorders” in the respective DSM editions. This illustrates how gender diversity was viewed as a mental illness/defect. As the DSM developed through various revisions, so have these diagnoses. DSM-IV used the diagnosis “gender identity disorder.”12 Psychiatry has evolved away from this line of thinking by focusing on the distress from biological sex characteristics that are “incongruent” with an individual’s gender identity, leading to the development of the gender dysphoria diagnosis.13 While this has been a positive step in psychiatry’s efforts to de-pathologize individuals who are gender-diverse, it raises the question: should such diagnoses be included in the DSM at all?
The gender dysphoria diagnosis continues to be needed by many individuals who are TGGD in order to access gender-affirming health care services. Mental health professionals are placed in a gatekeeping role by the expectation that they provide letters of “support” to indicate an individual is of sound mind and consistent gender identity to have services covered by insurance providers. In this way, the insurance industry and the field of medicine continue to believe that individuals who are TGGD need psychiatric permission and/or counsel regarding their gender identity. This can place psychiatry in a role of controlling access to necessary care while also creating a possible distrust in our ability to provide care to patients who are gender-diverse. This is particularly problematic given the high rates of depression, anxiety, trauma, and substance use within these communities.14 In the WHO’s ICD-11, gender dysphoria was changed to gender incongruence and is contained in the category of “Conditions related to sexual health.”15 This indicates continued evolution of how medicine views individuals who are TGGD, and offers hope that psychiatry and the DSM will follow suit.
Continue to: Minority stress theory
Minority stress theory
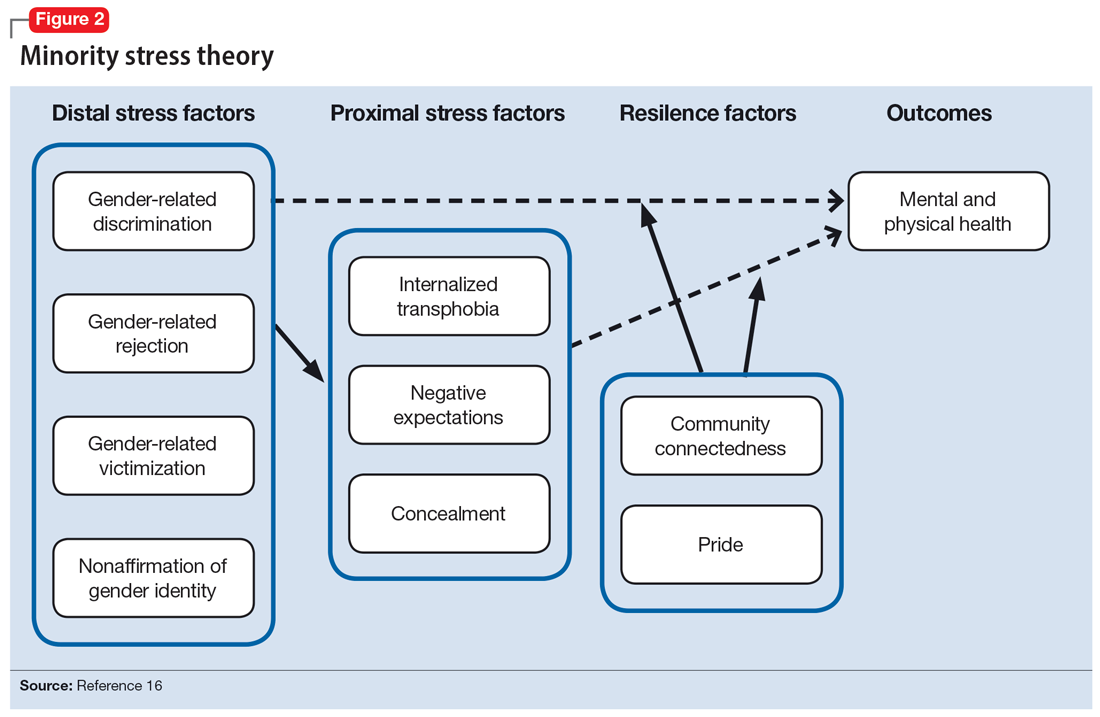
Ilan Meyer’s minority stress theory explores how cultural and social factors impact mental health functioning (Figure 216). Minority stress theory, which was originally developed for what at the time was described as the lesbian, gay, and bisexual communities, purports that the higher prevalence of mental health disorders among such individuals is likely due to social stigma, discrimination, and stressors associated with minority status. More recently, minority stress theory has been expanded to provide framework for individuals who are TGGD. Hendricks et al17 explain how distal, proximal, and resilience factors contribute to mental health outcomes among these individuals. Distal factors, such as gender-related discrimination, harassment, violence, and rejection, explain how systemic, cultural, and environmental events lead to overt stress. Proximal factors consist of an individual’s expectation and anticipation of negative and stressful events and the internalization of negative attitudes and prejudice (ie, internalized transphobia). Resilience factors consist of community connectedness and within-group identification and can help mediate the negative effects of distal and proximal factors.
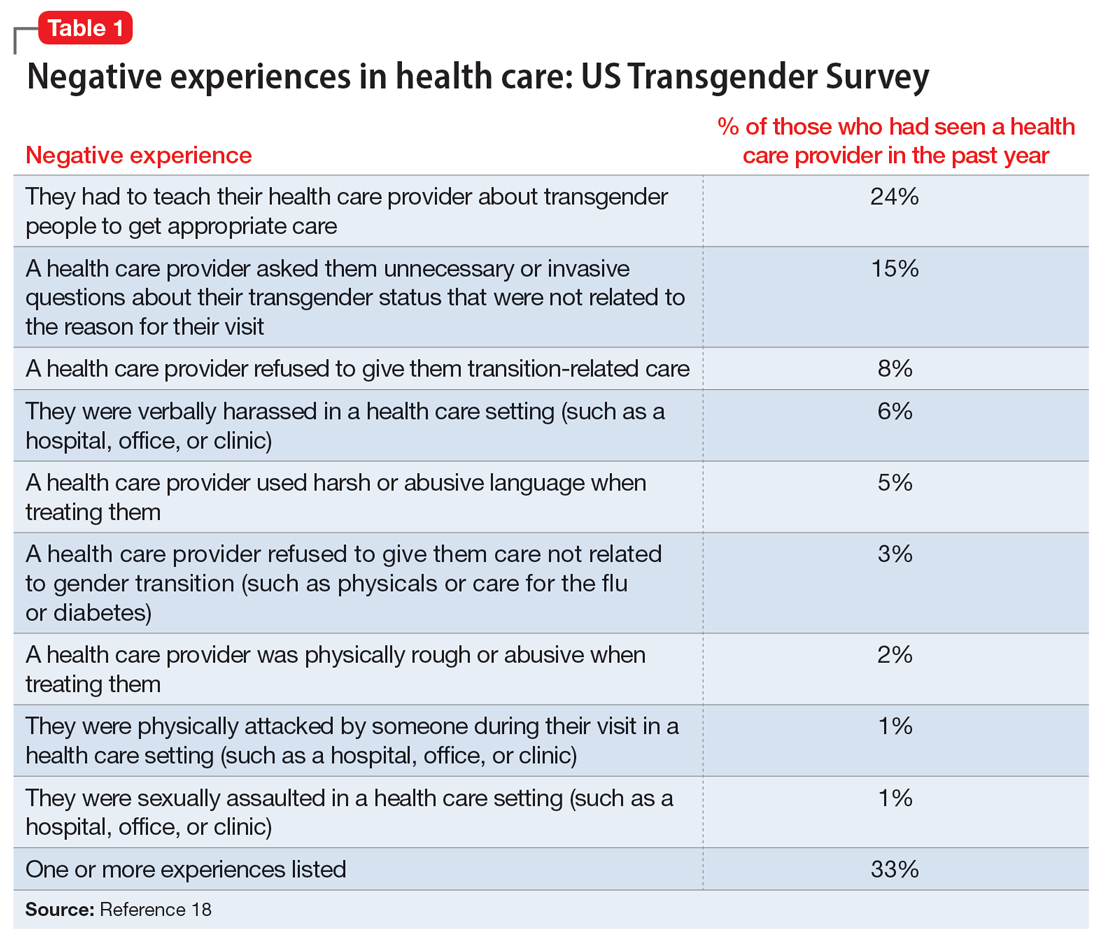
As clinicians, understanding our patients’ experiences and expectations can help us better engage with them and create an environment of safety and healing. Minority stress theory framework suggests that patients may start treatment with distrust or suspicion in light of previous negative experiences. They may also be likely to expect clinicians to be judgmental or to lack understanding of them. The 2015 US Transgender Survey found that 33% of individuals who are TGGD who sought medical treatment in the past year had at least 1 negative experience related to their gender identity (Table 118). Twenty-four percent reported having to educate their clinician about people who are TGGD, while 15% reported the health care professional asked invasive or unnecessary questions about their gender status that were unrelated to their visit. While psychiatry is often distinct from the larger medical field, it is important to understand the negative encounters individuals who are TGGD have likely experienced in medicine, and how those events may skew their feelings about psychiatric treatment. This is especially salient given the higher prevalence of various psychiatric disorders among individuals who are TGGD.18
According to the US Transgender Survey, 39% of participants were currently experiencing serious psychological distress, which is nearly 8 times the rate in the US population (5%).18 When extrapolated, this data indicates that we in psychiatry are likely to work with individuals who identify as TGGD, regardless of our expertise. Additionally, research indicates that having access to gender-affirming care—such as hormone replacement therapy, gender-affirming surgery, voice therapy, and other treatments—greatly improves mental health issues such as anxiety, depression, and suicidality among individuals who are TGGD.19,20 It is in this way we in psychiatry must do more than just care for our patients by becoming advocates for them to receive the care they need and deserve. While at times we may want to stay out of politics and other public discourse, it is becoming increasingly necessary as health care is entrenched in politics.
Clinical applicability
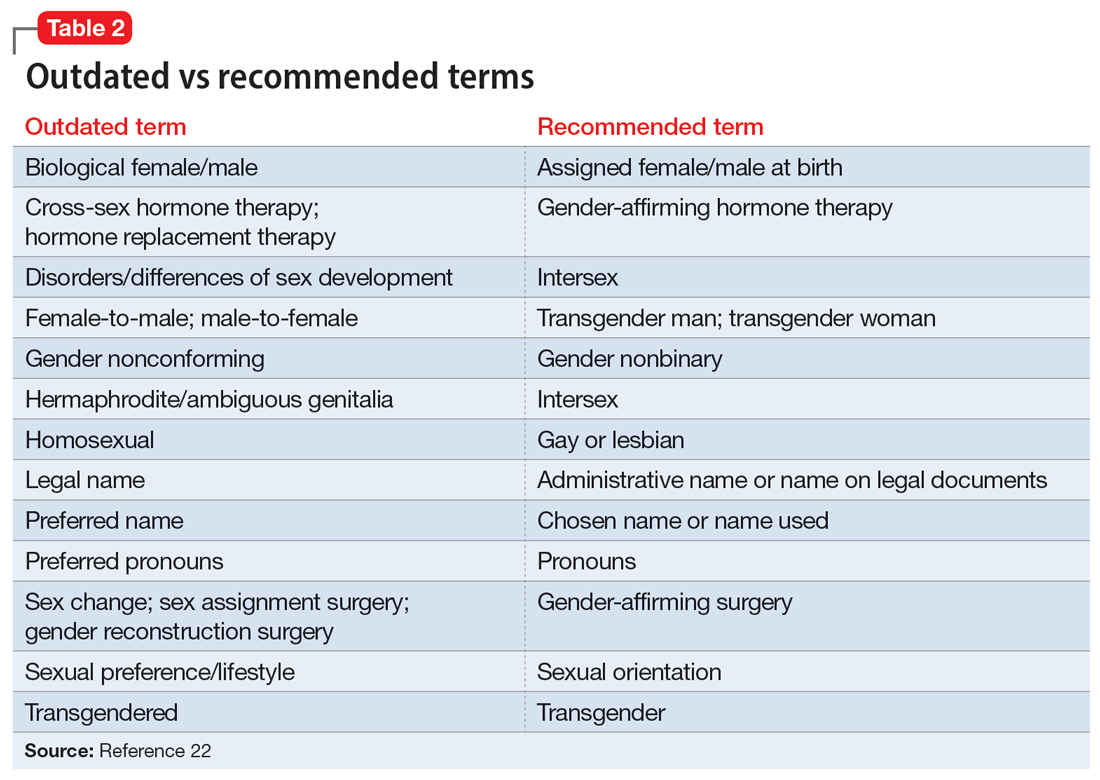
Because individuals who are TGGD experience higher rates of depression, anxiety, substance use, and other psychiatric disorders,14 it is increasingly likely that many clinicians will be presented with opportunities to treat such individuals. Despite high rates of psychiatric disorders, individuals who are TGGD often avoid treatment due to concerns about being pathologized, stereotyped, and/or encountering professionals who lack the knowledge to treat them as they are.21 Several studies recommend clinicians better equip themselves to appropriately provide services to individuals who are TGGD.21 Some advise seeking education to understand the unique needs of these patients and to help stay current with appropriate terminology and language (Table 222). This also implies not relying on patients to educate clinicians in understanding their specific needs and experiences.
Making assumptions about a patient’s identity is one of the most commonly reported issues by individuals who are TGGD. Therefore, it is critical to avoid making assumptions about patients based on binary stereotypes.23,24 We can circumvent these mistakes by asking every patient for their name and pronouns, and introducing ourselves with our pronouns. This illustrates an openness and understanding of the importance of identity and language, and makes it common practice from the outset. Integrating the use of gender-neutral language into paperwork, intake forms, charting, and conversation will also help avoid the pitfalls of misgendering and making false assumptions. This will also allow for support staff, medical assistants, and others to use correct language with patients. Having a patient’s used name and pronouns visible for everyone who works with the patient is necessary to effectively meet the patient’s needs. Additionally, understanding that the range of experiences and needs for individuals who are TGGD is heterogeneous can help reduce assumptions and ensure we are asking for needed information. It is also important to ask for only relevant information needed to provide treatment.
Continue to: Resources are widely available...
Resources are widely available to aid in the care of individuals who are TGGD. In 2022, the World Professional Association for Transgender Health released new guidelines—Standards of Care 8—for working with individuals who are TGGD.25 While these standards include a section dedicated to mental health, they also provide guidelines on education, assessments, specific demographic groups, hormone therapy, primary care, and sexual health. Additionally, while we may not want the role of gatekeeping for individuals to receive gender-affirming care, we work within a health care and insurance system that continues to require psychiatric assessment for such surgeries. In this role, we must do our part to educate ourselves in how to best provide these assessments and letters of support to help patients receive appropriate and life-saving care.
Finally, in order to provide a more comfortable and affirming space for individuals who are TGGD, develop ways to self-assess and monitor the policies, procedures, and language used within your practice, clinic, or institution. Monitoring the language used in charting to ensure consistency with the individual’s gender identity is important for our own understanding of the patient, and for patients to feel seen. This is especially true given patients’ access to medical records under the Cures Act. Moreover, it is essential to be cognizant of how you present clients to others in consultation or care coordination to ensure the patient is identified correctly and consistently by clinicians and staff.
Bottom Line
Understanding the social, cultural, and medical discrimination faced by patients who are transgender or gender diverse can make us better suited to engage and treat these individuals in an affirming and supportive way.
Related Resources
- World Professional Association of Transgender Health (WPATH) Standards of Care—8th edition. https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
- The Fenway Institute: National LGBTQIA+ Health Education Center. https://fenwayhealth.org/the-fenway-institute/education/the-national-lgbtia-health-education-center/
1. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113-1142. doi:10.1177/1745691620902442
2. The Roots of Loneliness Project. Accessed April 8, 2023. https://www.rootsofloneliness.com/gender-identity-loneliness
3. Davies SG. Challenging Gender Norms: Five Genders Among Bugis in Indonesia. Thomson Wadsworth; 2007.
4. Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60(6):581-592. doi:10.1037/0003-066X.60.6.581
5. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122:165-175. doi:10.1016/j.neubiorev.2020.22.018
6. Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468-15473. doi:10.1073/pnas.1509654112
7. Palmer BF, Clegg DJ. A universally accepted definition of gender will positively impact societal understanding, acceptance, and appropriateness of health care. Mayo Clin Proc. 2020;95(10):2235-2243. doi:10.1016/j.mayocp.2020.01.031
8. Office of Research on Women’s Health. Sex & Gender. National Institutes of Health. Accessed April 6, 2023. https://orwh.od.nih.gov/sex-gender
9. Morgenroth T, Sendén MG, Lindqvist A, et al. Defending the sex/gender binary: the role of gender identification and need for closure. Soc Psychol Pers Sci. 2021;12(5):731-740.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. American Psychiatric Association; 1968.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
14. Wanta JW, Niforatos JD, Durbak E, et al. Mental health diagnoses among transgender patients in the clinical setting: an all-payer electronic health record study. Transgend Health. 2019;4(1):313-315.
15. World Health Organization. International Statistical Classification of Diseases. 11th ed. World Health Organization; 2019.
16. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi:10.1037/0033-2909.129.5.674
17. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Profess Psychol: Res Pract. 2012;43(5):460-467. doi:10.1037/a0029597
18. James SE, Herman J, Keisling M, et al. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. Accessed April 6, 2023. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
19. Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618. doi:10.1001/jamasurg.2021.0952
20. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5(2):e220978. doi:10.1001/jamanetworkopen.2022.0978
21. Snow A, Cerel J, Loeffler DN, et al. Barriers to mental health care for transgender and gender-nonconforming adults: a systematic literature review. Health Soc Work. 2019;44(3):149-155. doi:10.1093/hsw/hlz016
22. National LGBTQIA+ Health Education Center. Accessed April 8, 2023. https://www.lgbtqiahealtheducation.org
23. Baldwin A, Dodge B, Schick VR, et al. Transgender and genderqueer individuals’ experiences with health care providers: what’s working, what’s not, and where do we go from here? J Health Care Poor Underserved. 2018;29(4):1300-1318. doi:10.1353/hpu.2018.0097
24. Kcomt L, Gorey KM, Barrett BJ, et al. Healthcare avoidance due to anticipated discrimination among transgender people: a call to create trans-affirmative environments. SSM-Popul Health. 2020;11:100608. doi:10.1016/j.ssmph.2020.100608
25. Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(Suppl 1):S1-S259.
Treating patients who are transgender or gender diverse (TGGD) requires an understanding of the social and psychological factors that have a unique impact on this population. As clinicians, it is our responsibility to understand the social, cultural, and political issues our patients face, both historically and currently. In this article, we provide information about the nature of gender and gender identity as separate from biological sex and informed by a person’s perception of self as male, female, nonbinary, or other variation.
Psychiatrists must be aware of how individuals who are TGGD have been perceived, classified, and treated by the medical profession, as this history is often a source of mistrust and a barrier to treatment for patients who need psychiatric care. This includes awareness of the “gatekeeping” role that persists in medical institutions today: applying strict eligibility criteria to determine the “fitness” of individuals who are transgender to pursue medical transition, as compared to the informed-consent model that is widely applied to other medical interventions. Our review of minority stress theory, as applicable to this patient population, provides a context and framework for empathic approaches to care for patients who are TGGD. Recognizing barriers to care and ways in which we can create a supportive environment for treatment will allow for tailored approaches that better fit the unique needs of this patient population.
The gender binary
In Western societies, gender has often been viewed as “binary,” oppositional, and directly correlated with physical sex or presumed anatomy.1 The theory of gender essentialism insists that sex and gender are indistinguishable from one another and provide 2 “natural” and distinct categories: women and men. The “gender/sex” binary refers to the belief that individuals born with 2 X chromosomes will inherently develop into and fulfill the social roles of women, and those born with an X and a Y chromosome will develop into and fulfill the social roles of men.1 In this context, “sex” refers to biological characteristics of individuals, including combinations of sex chromosomes, anatomy, and the development of sex characteristics during puberty. The term “gender” refers to the social, cultural, and behavioral aspects of being a man, woman, both, or neither, and “gender identity” refers to one’s internal, individual sense of self and experience of gender (Figure 12). Many Western cultures are now facing destabilization of the gender/sex binary in social, political, and interpersonal contexts.1 This is perhaps most clearly seen in the battle for self-determination and protection by laws affecting individuals who are transgender as well as the determination of other groups to maintain traditional sex and gender roles, often through political action. Historically, individuals who are TGGD have been present in a variety of cultures. For example, most Native American cultures have revered other-gendered individuals, more recently referred to as “two-spirited.” Similarly, the Bugis people of South Sulawesi, Indonesia, recognize 5 genders that exist on a nonbinary spectrum.3

Despite its prevalence in Western society, scientific evidence for the gender/sex binary is lacking. The gender similarities hypothesis states that males and females are similar in most, but not all, psychological variables and is supported by multiple meta-analyses examining psychological gender differences.4 In a 2005 review of 46 meta-analyses of gender-differences, studied through behavior analysis, effect sizes for gender differences were trivial or small in almost 75% of examined variables.5 Analyzing for internal consistency among studies showing large gender/sex differences, Joel et al6 found that, on measures of personality traits, attitudes, interests, and behaviors were rarely homogenous in the brains of males or females. In fact, <1% of study participants showed only masculine or feminine traits, whereas 55% showed a combination, or mosaic, of these traits.6 These findings were supported by further research in behavioral neuroendocrinology that demonstrated a lack of hormonal evidence for 2 distinct sexes. Both estrogen (the “female” hormone) and testosterone (the “male” hormone) are produced by both biological males and females. Further, levels of estradiol do not significantly differ between males and females, and, in fact, in nonpregnant females, estradiol levels are more similar to those of males than to those of pregnant females.1 In the last decade, imaging studies of the human brain have shown that brain structure and connectivity in individuals who are transgender are more similar to those of their experienced gender than of their natal sex.7 In social analyses of intersex individuals (individuals born with ambiguous physical sex characteristics), surgical assignment into the binary gender system did not improve—and often worsened—feelings of isolation and shame.1
The National Institutes of Health defines gender as “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and time.”8 The World Health Organization (WHO) provides a similar definition, and the evidence to support this exists in social-role theory, social-identity theory, and the stereotype-content model. However, despite evidence disputing a gender/sex binary, this method of classifying individuals into a dyad persists in many areas of modern culture, from gender-specific physical spaces (bathrooms, classrooms, store brands), language (pronouns), and laws. This desire for categorization helps fulfill social and psychological needs of groups and individuals by providing group identities and giving structure to the complexity of modern-day life. Identity and group membership provide a sense of belonging, source of self-esteem, and avoidance of ambiguity. Binary gender stereotypes provide expectations that allow anticipation and prediction of our social environments.9 However, the harm of perpetuating the false gender/sex binary is well documented and includes social and economic penalties, extreme violence, and even death. The field of medicine has not been immune from practices that implicitly endorse the gender/sex connection, as seen in the erroneous use of gender in biomedical writings at the highest levels and evidenced in research examining “gender” differences in disease incidence.
Gender diversity as a pathology
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) has been a source of pathologizing gender diversity since the 1960s, with the introduction of “transsexualism” in DSM-II10 and “gender identity disorder of childhood” in DSM-III.11 These diagnoses were listed under the headings of “sexual deviations” and “psychosexual disorders” in the respective DSM editions. This illustrates how gender diversity was viewed as a mental illness/defect. As the DSM developed through various revisions, so have these diagnoses. DSM-IV used the diagnosis “gender identity disorder.”12 Psychiatry has evolved away from this line of thinking by focusing on the distress from biological sex characteristics that are “incongruent” with an individual’s gender identity, leading to the development of the gender dysphoria diagnosis.13 While this has been a positive step in psychiatry’s efforts to de-pathologize individuals who are gender-diverse, it raises the question: should such diagnoses be included in the DSM at all?
The gender dysphoria diagnosis continues to be needed by many individuals who are TGGD in order to access gender-affirming health care services. Mental health professionals are placed in a gatekeeping role by the expectation that they provide letters of “support” to indicate an individual is of sound mind and consistent gender identity to have services covered by insurance providers. In this way, the insurance industry and the field of medicine continue to believe that individuals who are TGGD need psychiatric permission and/or counsel regarding their gender identity. This can place psychiatry in a role of controlling access to necessary care while also creating a possible distrust in our ability to provide care to patients who are gender-diverse. This is particularly problematic given the high rates of depression, anxiety, trauma, and substance use within these communities.14 In the WHO’s ICD-11, gender dysphoria was changed to gender incongruence and is contained in the category of “Conditions related to sexual health.”15 This indicates continued evolution of how medicine views individuals who are TGGD, and offers hope that psychiatry and the DSM will follow suit.
Continue to: Minority stress theory
Minority stress theory
Ilan Meyer’s minority stress theory explores how cultural and social factors impact mental health functioning (Figure 216). Minority stress theory, which was originally developed for what at the time was described as the lesbian, gay, and bisexual communities, purports that the higher prevalence of mental health disorders among such individuals is likely due to social stigma, discrimination, and stressors associated with minority status. More recently, minority stress theory has been expanded to provide framework for individuals who are TGGD. Hendricks et al17 explain how distal, proximal, and resilience factors contribute to mental health outcomes among these individuals. Distal factors, such as gender-related discrimination, harassment, violence, and rejection, explain how systemic, cultural, and environmental events lead to overt stress. Proximal factors consist of an individual’s expectation and anticipation of negative and stressful events and the internalization of negative attitudes and prejudice (ie, internalized transphobia). Resilience factors consist of community connectedness and within-group identification and can help mediate the negative effects of distal and proximal factors.

As clinicians, understanding our patients’ experiences and expectations can help us better engage with them and create an environment of safety and healing. Minority stress theory framework suggests that patients may start treatment with distrust or suspicion in light of previous negative experiences. They may also be likely to expect clinicians to be judgmental or to lack understanding of them. The 2015 US Transgender Survey found that 33% of individuals who are TGGD who sought medical treatment in the past year had at least 1 negative experience related to their gender identity (Table 118). Twenty-four percent reported having to educate their clinician about people who are TGGD, while 15% reported the health care professional asked invasive or unnecessary questions about their gender status that were unrelated to their visit. While psychiatry is often distinct from the larger medical field, it is important to understand the negative encounters individuals who are TGGD have likely experienced in medicine, and how those events may skew their feelings about psychiatric treatment. This is especially salient given the higher prevalence of various psychiatric disorders among individuals who are TGGD.18

According to the US Transgender Survey, 39% of participants were currently experiencing serious psychological distress, which is nearly 8 times the rate in the US population (5%).18 When extrapolated, this data indicates that we in psychiatry are likely to work with individuals who identify as TGGD, regardless of our expertise. Additionally, research indicates that having access to gender-affirming care—such as hormone replacement therapy, gender-affirming surgery, voice therapy, and other treatments—greatly improves mental health issues such as anxiety, depression, and suicidality among individuals who are TGGD.19,20 It is in this way we in psychiatry must do more than just care for our patients by becoming advocates for them to receive the care they need and deserve. While at times we may want to stay out of politics and other public discourse, it is becoming increasingly necessary as health care is entrenched in politics.
Clinical applicability
Because individuals who are TGGD experience higher rates of depression, anxiety, substance use, and other psychiatric disorders,14 it is increasingly likely that many clinicians will be presented with opportunities to treat such individuals. Despite high rates of psychiatric disorders, individuals who are TGGD often avoid treatment due to concerns about being pathologized, stereotyped, and/or encountering professionals who lack the knowledge to treat them as they are.21 Several studies recommend clinicians better equip themselves to appropriately provide services to individuals who are TGGD.21 Some advise seeking education to understand the unique needs of these patients and to help stay current with appropriate terminology and language (Table 222). This also implies not relying on patients to educate clinicians in understanding their specific needs and experiences.

Making assumptions about a patient’s identity is one of the most commonly reported issues by individuals who are TGGD. Therefore, it is critical to avoid making assumptions about patients based on binary stereotypes.23,24 We can circumvent these mistakes by asking every patient for their name and pronouns, and introducing ourselves with our pronouns. This illustrates an openness and understanding of the importance of identity and language, and makes it common practice from the outset. Integrating the use of gender-neutral language into paperwork, intake forms, charting, and conversation will also help avoid the pitfalls of misgendering and making false assumptions. This will also allow for support staff, medical assistants, and others to use correct language with patients. Having a patient’s used name and pronouns visible for everyone who works with the patient is necessary to effectively meet the patient’s needs. Additionally, understanding that the range of experiences and needs for individuals who are TGGD is heterogeneous can help reduce assumptions and ensure we are asking for needed information. It is also important to ask for only relevant information needed to provide treatment.
Continue to: Resources are widely available...
Resources are widely available to aid in the care of individuals who are TGGD. In 2022, the World Professional Association for Transgender Health released new guidelines—Standards of Care 8—for working with individuals who are TGGD.25 While these standards include a section dedicated to mental health, they also provide guidelines on education, assessments, specific demographic groups, hormone therapy, primary care, and sexual health. Additionally, while we may not want the role of gatekeeping for individuals to receive gender-affirming care, we work within a health care and insurance system that continues to require psychiatric assessment for such surgeries. In this role, we must do our part to educate ourselves in how to best provide these assessments and letters of support to help patients receive appropriate and life-saving care.
Finally, in order to provide a more comfortable and affirming space for individuals who are TGGD, develop ways to self-assess and monitor the policies, procedures, and language used within your practice, clinic, or institution. Monitoring the language used in charting to ensure consistency with the individual’s gender identity is important for our own understanding of the patient, and for patients to feel seen. This is especially true given patients’ access to medical records under the Cures Act. Moreover, it is essential to be cognizant of how you present clients to others in consultation or care coordination to ensure the patient is identified correctly and consistently by clinicians and staff.
Bottom Line
Understanding the social, cultural, and medical discrimination faced by patients who are transgender or gender diverse can make us better suited to engage and treat these individuals in an affirming and supportive way.
Related Resources
- World Professional Association of Transgender Health (WPATH) Standards of Care—8th edition. https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
- The Fenway Institute: National LGBTQIA+ Health Education Center. https://fenwayhealth.org/the-fenway-institute/education/the-national-lgbtia-health-education-center/
Treating patients who are transgender or gender diverse (TGGD) requires an understanding of the social and psychological factors that have a unique impact on this population. As clinicians, it is our responsibility to understand the social, cultural, and political issues our patients face, both historically and currently. In this article, we provide information about the nature of gender and gender identity as separate from biological sex and informed by a person’s perception of self as male, female, nonbinary, or other variation.
Psychiatrists must be aware of how individuals who are TGGD have been perceived, classified, and treated by the medical profession, as this history is often a source of mistrust and a barrier to treatment for patients who need psychiatric care. This includes awareness of the “gatekeeping” role that persists in medical institutions today: applying strict eligibility criteria to determine the “fitness” of individuals who are transgender to pursue medical transition, as compared to the informed-consent model that is widely applied to other medical interventions. Our review of minority stress theory, as applicable to this patient population, provides a context and framework for empathic approaches to care for patients who are TGGD. Recognizing barriers to care and ways in which we can create a supportive environment for treatment will allow for tailored approaches that better fit the unique needs of this patient population.
The gender binary
In Western societies, gender has often been viewed as “binary,” oppositional, and directly correlated with physical sex or presumed anatomy.1 The theory of gender essentialism insists that sex and gender are indistinguishable from one another and provide 2 “natural” and distinct categories: women and men. The “gender/sex” binary refers to the belief that individuals born with 2 X chromosomes will inherently develop into and fulfill the social roles of women, and those born with an X and a Y chromosome will develop into and fulfill the social roles of men.1 In this context, “sex” refers to biological characteristics of individuals, including combinations of sex chromosomes, anatomy, and the development of sex characteristics during puberty. The term “gender” refers to the social, cultural, and behavioral aspects of being a man, woman, both, or neither, and “gender identity” refers to one’s internal, individual sense of self and experience of gender (Figure 12). Many Western cultures are now facing destabilization of the gender/sex binary in social, political, and interpersonal contexts.1 This is perhaps most clearly seen in the battle for self-determination and protection by laws affecting individuals who are transgender as well as the determination of other groups to maintain traditional sex and gender roles, often through political action. Historically, individuals who are TGGD have been present in a variety of cultures. For example, most Native American cultures have revered other-gendered individuals, more recently referred to as “two-spirited.” Similarly, the Bugis people of South Sulawesi, Indonesia, recognize 5 genders that exist on a nonbinary spectrum.3

Despite its prevalence in Western society, scientific evidence for the gender/sex binary is lacking. The gender similarities hypothesis states that males and females are similar in most, but not all, psychological variables and is supported by multiple meta-analyses examining psychological gender differences.4 In a 2005 review of 46 meta-analyses of gender-differences, studied through behavior analysis, effect sizes for gender differences were trivial or small in almost 75% of examined variables.5 Analyzing for internal consistency among studies showing large gender/sex differences, Joel et al6 found that, on measures of personality traits, attitudes, interests, and behaviors were rarely homogenous in the brains of males or females. In fact, <1% of study participants showed only masculine or feminine traits, whereas 55% showed a combination, or mosaic, of these traits.6 These findings were supported by further research in behavioral neuroendocrinology that demonstrated a lack of hormonal evidence for 2 distinct sexes. Both estrogen (the “female” hormone) and testosterone (the “male” hormone) are produced by both biological males and females. Further, levels of estradiol do not significantly differ between males and females, and, in fact, in nonpregnant females, estradiol levels are more similar to those of males than to those of pregnant females.1 In the last decade, imaging studies of the human brain have shown that brain structure and connectivity in individuals who are transgender are more similar to those of their experienced gender than of their natal sex.7 In social analyses of intersex individuals (individuals born with ambiguous physical sex characteristics), surgical assignment into the binary gender system did not improve—and often worsened—feelings of isolation and shame.1
The National Institutes of Health defines gender as “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and time.”8 The World Health Organization (WHO) provides a similar definition, and the evidence to support this exists in social-role theory, social-identity theory, and the stereotype-content model. However, despite evidence disputing a gender/sex binary, this method of classifying individuals into a dyad persists in many areas of modern culture, from gender-specific physical spaces (bathrooms, classrooms, store brands), language (pronouns), and laws. This desire for categorization helps fulfill social and psychological needs of groups and individuals by providing group identities and giving structure to the complexity of modern-day life. Identity and group membership provide a sense of belonging, source of self-esteem, and avoidance of ambiguity. Binary gender stereotypes provide expectations that allow anticipation and prediction of our social environments.9 However, the harm of perpetuating the false gender/sex binary is well documented and includes social and economic penalties, extreme violence, and even death. The field of medicine has not been immune from practices that implicitly endorse the gender/sex connection, as seen in the erroneous use of gender in biomedical writings at the highest levels and evidenced in research examining “gender” differences in disease incidence.
Gender diversity as a pathology
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) has been a source of pathologizing gender diversity since the 1960s, with the introduction of “transsexualism” in DSM-II10 and “gender identity disorder of childhood” in DSM-III.11 These diagnoses were listed under the headings of “sexual deviations” and “psychosexual disorders” in the respective DSM editions. This illustrates how gender diversity was viewed as a mental illness/defect. As the DSM developed through various revisions, so have these diagnoses. DSM-IV used the diagnosis “gender identity disorder.”12 Psychiatry has evolved away from this line of thinking by focusing on the distress from biological sex characteristics that are “incongruent” with an individual’s gender identity, leading to the development of the gender dysphoria diagnosis.13 While this has been a positive step in psychiatry’s efforts to de-pathologize individuals who are gender-diverse, it raises the question: should such diagnoses be included in the DSM at all?
The gender dysphoria diagnosis continues to be needed by many individuals who are TGGD in order to access gender-affirming health care services. Mental health professionals are placed in a gatekeeping role by the expectation that they provide letters of “support” to indicate an individual is of sound mind and consistent gender identity to have services covered by insurance providers. In this way, the insurance industry and the field of medicine continue to believe that individuals who are TGGD need psychiatric permission and/or counsel regarding their gender identity. This can place psychiatry in a role of controlling access to necessary care while also creating a possible distrust in our ability to provide care to patients who are gender-diverse. This is particularly problematic given the high rates of depression, anxiety, trauma, and substance use within these communities.14 In the WHO’s ICD-11, gender dysphoria was changed to gender incongruence and is contained in the category of “Conditions related to sexual health.”15 This indicates continued evolution of how medicine views individuals who are TGGD, and offers hope that psychiatry and the DSM will follow suit.
Continue to: Minority stress theory
Minority stress theory
Ilan Meyer’s minority stress theory explores how cultural and social factors impact mental health functioning (Figure 216). Minority stress theory, which was originally developed for what at the time was described as the lesbian, gay, and bisexual communities, purports that the higher prevalence of mental health disorders among such individuals is likely due to social stigma, discrimination, and stressors associated with minority status. More recently, minority stress theory has been expanded to provide framework for individuals who are TGGD. Hendricks et al17 explain how distal, proximal, and resilience factors contribute to mental health outcomes among these individuals. Distal factors, such as gender-related discrimination, harassment, violence, and rejection, explain how systemic, cultural, and environmental events lead to overt stress. Proximal factors consist of an individual’s expectation and anticipation of negative and stressful events and the internalization of negative attitudes and prejudice (ie, internalized transphobia). Resilience factors consist of community connectedness and within-group identification and can help mediate the negative effects of distal and proximal factors.

As clinicians, understanding our patients’ experiences and expectations can help us better engage with them and create an environment of safety and healing. Minority stress theory framework suggests that patients may start treatment with distrust or suspicion in light of previous negative experiences. They may also be likely to expect clinicians to be judgmental or to lack understanding of them. The 2015 US Transgender Survey found that 33% of individuals who are TGGD who sought medical treatment in the past year had at least 1 negative experience related to their gender identity (Table 118). Twenty-four percent reported having to educate their clinician about people who are TGGD, while 15% reported the health care professional asked invasive or unnecessary questions about their gender status that were unrelated to their visit. While psychiatry is often distinct from the larger medical field, it is important to understand the negative encounters individuals who are TGGD have likely experienced in medicine, and how those events may skew their feelings about psychiatric treatment. This is especially salient given the higher prevalence of various psychiatric disorders among individuals who are TGGD.18

According to the US Transgender Survey, 39% of participants were currently experiencing serious psychological distress, which is nearly 8 times the rate in the US population (5%).18 When extrapolated, this data indicates that we in psychiatry are likely to work with individuals who identify as TGGD, regardless of our expertise. Additionally, research indicates that having access to gender-affirming care—such as hormone replacement therapy, gender-affirming surgery, voice therapy, and other treatments—greatly improves mental health issues such as anxiety, depression, and suicidality among individuals who are TGGD.19,20 It is in this way we in psychiatry must do more than just care for our patients by becoming advocates for them to receive the care they need and deserve. While at times we may want to stay out of politics and other public discourse, it is becoming increasingly necessary as health care is entrenched in politics.
Clinical applicability
Because individuals who are TGGD experience higher rates of depression, anxiety, substance use, and other psychiatric disorders,14 it is increasingly likely that many clinicians will be presented with opportunities to treat such individuals. Despite high rates of psychiatric disorders, individuals who are TGGD often avoid treatment due to concerns about being pathologized, stereotyped, and/or encountering professionals who lack the knowledge to treat them as they are.21 Several studies recommend clinicians better equip themselves to appropriately provide services to individuals who are TGGD.21 Some advise seeking education to understand the unique needs of these patients and to help stay current with appropriate terminology and language (Table 222). This also implies not relying on patients to educate clinicians in understanding their specific needs and experiences.

Making assumptions about a patient’s identity is one of the most commonly reported issues by individuals who are TGGD. Therefore, it is critical to avoid making assumptions about patients based on binary stereotypes.23,24 We can circumvent these mistakes by asking every patient for their name and pronouns, and introducing ourselves with our pronouns. This illustrates an openness and understanding of the importance of identity and language, and makes it common practice from the outset. Integrating the use of gender-neutral language into paperwork, intake forms, charting, and conversation will also help avoid the pitfalls of misgendering and making false assumptions. This will also allow for support staff, medical assistants, and others to use correct language with patients. Having a patient’s used name and pronouns visible for everyone who works with the patient is necessary to effectively meet the patient’s needs. Additionally, understanding that the range of experiences and needs for individuals who are TGGD is heterogeneous can help reduce assumptions and ensure we are asking for needed information. It is also important to ask for only relevant information needed to provide treatment.
Continue to: Resources are widely available...
Resources are widely available to aid in the care of individuals who are TGGD. In 2022, the World Professional Association for Transgender Health released new guidelines—Standards of Care 8—for working with individuals who are TGGD.25 While these standards include a section dedicated to mental health, they also provide guidelines on education, assessments, specific demographic groups, hormone therapy, primary care, and sexual health. Additionally, while we may not want the role of gatekeeping for individuals to receive gender-affirming care, we work within a health care and insurance system that continues to require psychiatric assessment for such surgeries. In this role, we must do our part to educate ourselves in how to best provide these assessments and letters of support to help patients receive appropriate and life-saving care.
Finally, in order to provide a more comfortable and affirming space for individuals who are TGGD, develop ways to self-assess and monitor the policies, procedures, and language used within your practice, clinic, or institution. Monitoring the language used in charting to ensure consistency with the individual’s gender identity is important for our own understanding of the patient, and for patients to feel seen. This is especially true given patients’ access to medical records under the Cures Act. Moreover, it is essential to be cognizant of how you present clients to others in consultation or care coordination to ensure the patient is identified correctly and consistently by clinicians and staff.
Bottom Line
Understanding the social, cultural, and medical discrimination faced by patients who are transgender or gender diverse can make us better suited to engage and treat these individuals in an affirming and supportive way.
Related Resources
- World Professional Association of Transgender Health (WPATH) Standards of Care—8th edition. https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
- The Fenway Institute: National LGBTQIA+ Health Education Center. https://fenwayhealth.org/the-fenway-institute/education/the-national-lgbtia-health-education-center/
1. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113-1142. doi:10.1177/1745691620902442
2. The Roots of Loneliness Project. Accessed April 8, 2023. https://www.rootsofloneliness.com/gender-identity-loneliness
3. Davies SG. Challenging Gender Norms: Five Genders Among Bugis in Indonesia. Thomson Wadsworth; 2007.
4. Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60(6):581-592. doi:10.1037/0003-066X.60.6.581
5. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122:165-175. doi:10.1016/j.neubiorev.2020.22.018
6. Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468-15473. doi:10.1073/pnas.1509654112
7. Palmer BF, Clegg DJ. A universally accepted definition of gender will positively impact societal understanding, acceptance, and appropriateness of health care. Mayo Clin Proc. 2020;95(10):2235-2243. doi:10.1016/j.mayocp.2020.01.031
8. Office of Research on Women’s Health. Sex & Gender. National Institutes of Health. Accessed April 6, 2023. https://orwh.od.nih.gov/sex-gender
9. Morgenroth T, Sendén MG, Lindqvist A, et al. Defending the sex/gender binary: the role of gender identification and need for closure. Soc Psychol Pers Sci. 2021;12(5):731-740.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. American Psychiatric Association; 1968.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
14. Wanta JW, Niforatos JD, Durbak E, et al. Mental health diagnoses among transgender patients in the clinical setting: an all-payer electronic health record study. Transgend Health. 2019;4(1):313-315.
15. World Health Organization. International Statistical Classification of Diseases. 11th ed. World Health Organization; 2019.
16. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi:10.1037/0033-2909.129.5.674
17. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Profess Psychol: Res Pract. 2012;43(5):460-467. doi:10.1037/a0029597
18. James SE, Herman J, Keisling M, et al. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. Accessed April 6, 2023. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
19. Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618. doi:10.1001/jamasurg.2021.0952
20. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5(2):e220978. doi:10.1001/jamanetworkopen.2022.0978
21. Snow A, Cerel J, Loeffler DN, et al. Barriers to mental health care for transgender and gender-nonconforming adults: a systematic literature review. Health Soc Work. 2019;44(3):149-155. doi:10.1093/hsw/hlz016
22. National LGBTQIA+ Health Education Center. Accessed April 8, 2023. https://www.lgbtqiahealtheducation.org
23. Baldwin A, Dodge B, Schick VR, et al. Transgender and genderqueer individuals’ experiences with health care providers: what’s working, what’s not, and where do we go from here? J Health Care Poor Underserved. 2018;29(4):1300-1318. doi:10.1353/hpu.2018.0097
24. Kcomt L, Gorey KM, Barrett BJ, et al. Healthcare avoidance due to anticipated discrimination among transgender people: a call to create trans-affirmative environments. SSM-Popul Health. 2020;11:100608. doi:10.1016/j.ssmph.2020.100608
25. Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(Suppl 1):S1-S259.
1. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113-1142. doi:10.1177/1745691620902442
2. The Roots of Loneliness Project. Accessed April 8, 2023. https://www.rootsofloneliness.com/gender-identity-loneliness
3. Davies SG. Challenging Gender Norms: Five Genders Among Bugis in Indonesia. Thomson Wadsworth; 2007.
4. Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60(6):581-592. doi:10.1037/0003-066X.60.6.581
5. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122:165-175. doi:10.1016/j.neubiorev.2020.22.018
6. Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468-15473. doi:10.1073/pnas.1509654112
7. Palmer BF, Clegg DJ. A universally accepted definition of gender will positively impact societal understanding, acceptance, and appropriateness of health care. Mayo Clin Proc. 2020;95(10):2235-2243. doi:10.1016/j.mayocp.2020.01.031
8. Office of Research on Women’s Health. Sex & Gender. National Institutes of Health. Accessed April 6, 2023. https://orwh.od.nih.gov/sex-gender
9. Morgenroth T, Sendén MG, Lindqvist A, et al. Defending the sex/gender binary: the role of gender identification and need for closure. Soc Psychol Pers Sci. 2021;12(5):731-740.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. American Psychiatric Association; 1968.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
14. Wanta JW, Niforatos JD, Durbak E, et al. Mental health diagnoses among transgender patients in the clinical setting: an all-payer electronic health record study. Transgend Health. 2019;4(1):313-315.
15. World Health Organization. International Statistical Classification of Diseases. 11th ed. World Health Organization; 2019.
16. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi:10.1037/0033-2909.129.5.674
17. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Profess Psychol: Res Pract. 2012;43(5):460-467. doi:10.1037/a0029597
18. James SE, Herman J, Keisling M, et al. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. Accessed April 6, 2023. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
19. Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618. doi:10.1001/jamasurg.2021.0952
20. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5(2):e220978. doi:10.1001/jamanetworkopen.2022.0978
21. Snow A, Cerel J, Loeffler DN, et al. Barriers to mental health care for transgender and gender-nonconforming adults: a systematic literature review. Health Soc Work. 2019;44(3):149-155. doi:10.1093/hsw/hlz016
22. National LGBTQIA+ Health Education Center. Accessed April 8, 2023. https://www.lgbtqiahealtheducation.org
23. Baldwin A, Dodge B, Schick VR, et al. Transgender and genderqueer individuals’ experiences with health care providers: what’s working, what’s not, and where do we go from here? J Health Care Poor Underserved. 2018;29(4):1300-1318. doi:10.1353/hpu.2018.0097
24. Kcomt L, Gorey KM, Barrett BJ, et al. Healthcare avoidance due to anticipated discrimination among transgender people: a call to create trans-affirmative environments. SSM-Popul Health. 2020;11:100608. doi:10.1016/j.ssmph.2020.100608
25. Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(Suppl 1):S1-S259.
Guidelines for assessing cancer risk may need updating
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
FROM AACR 2023
Poor representation of patients with darker skin phototypes in laser and light device studies
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Racial disparities in cardiotoxicity after chemotherapy
a research review indicates.
“It’s important that both patients and clinicians be aware of these disparities so that more meaningful conversations around long-term cardiac health and cancer treatment can take place,” lead investigator Wondewossen Gebeyehu, with the University of Toronto, said in an interview.
However, patients “should not avoid chemotherapy, as the most important thing is making sure they get the best cancer treatment possible, and studies already show Black patients may get less optimal cancer treatments,” Mr. Gebeyehu added in a statement.
Ana Barac, MD, PHD, chair of cardio-oncology at Inova Schar Cancer Institute and Inova Heart and Vascular Institute, Fairfax, Va., who wasn’t involved in the study, agreed.
“The most important message is to look at preexisting cardiovascular disease, oncology diagnosis, and be aware of existing disparities in a specific cancer and CVD,” Barac said in an interview.
“What should NOT happen is to overinterpret this report of cardiotoxicity as an indication to modify/avoid planned cancer treatment to decrease cardiotoxicity. This approach could worsen oncology outcomes and lead to undertreatment of cancer, therefore posing real danger,” said Dr. Barac.
The study was presented at the American College of Cardiology Advancing the Cardiovascular Care of the Oncology Patient 2023 conference.
Causes unclear
Chemotherapy is known to increase the risk of cardiovascular heart failure and other forms of CVD, but less is known about racial disparities in the incidence of chemotherapy-induced cardiotoxicity.
Mr. Gebeyehu and colleagues conducted a systematic review and meta-analysis of the available literature to assess racial disparities in CV adverse effects among cancer patients who were treated with chemotherapeutic agents. They screened 7,057 studies, fully reviewed 57, and included 24 studies, representing 683,749 participants, in their analysis.
Breast cancer was the most commonly reported malignancy. Other common malignancies were prostate, kidney, and hematologic malignancies such as leukemia and lymphoma.
Chemotherapeutic agents included anthracyclines (doxorubicin, daunorubicin), trastuzumab, and hormonal therapies.
Black race or African ancestry was associated with increased odds of chemotherapy-associated cardiotoxicity (odds ratio, 1.71; 95% confidence interval, 1.40-2.10), as well as congestive heart failure (OR, 1.92; 95% CI, 1.68-2.19).
Mr. Gebeyehu said in an interview that it’s hard to speculate on causation with an analysis of preexisting data such as this. “Our initial analysis that we’ve reported on so far are unadjusted values, meaning they don’t adjust for those potential underlying factors,” he noted.
“However, some of the studies individually controlled for socioeconomic factors and still found increased vulnerability to chemotherapy-associated cardiotoxicity in patients of Black race or African ancestry,” Mr. Gebeyehu said.
“It’s certainly possible that a mix of both biological and socioeconomic factors are interacting to lead to these disparities. One example could be the underrepresentation of Black patients in clinical trials to develop drugs. These could lead to chemotherapeutic agents being poorly optimized in this population relative to other racial/ethnic groups,” he added.
Dr. Barac said this study adds to the growing body of evidence about the importance of racial disparities in CVD and cancer outcomes.
“It is important to note that only the unadjusted odds ratio was reported and that much more detail is needed to understand what may be underlying the disparities. It is critically important to await the adjusted analysis, as well as details of the type of cancers and treatment used, before clinical implications can be discussed,” said Dr. Barac, who served as codirector of the conference.
“The risk of cardiotoxicity needs to be presented in the context of the oncology and CV disease burden, as both can influence the risk, and there could be a synergistic effect of disparities,” Dr. Barac added.
The study had no specific funding. Mr. Gebeyehu and Dr. Barac disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
a research review indicates.
“It’s important that both patients and clinicians be aware of these disparities so that more meaningful conversations around long-term cardiac health and cancer treatment can take place,” lead investigator Wondewossen Gebeyehu, with the University of Toronto, said in an interview.
However, patients “should not avoid chemotherapy, as the most important thing is making sure they get the best cancer treatment possible, and studies already show Black patients may get less optimal cancer treatments,” Mr. Gebeyehu added in a statement.
Ana Barac, MD, PHD, chair of cardio-oncology at Inova Schar Cancer Institute and Inova Heart and Vascular Institute, Fairfax, Va., who wasn’t involved in the study, agreed.
“The most important message is to look at preexisting cardiovascular disease, oncology diagnosis, and be aware of existing disparities in a specific cancer and CVD,” Barac said in an interview.
“What should NOT happen is to overinterpret this report of cardiotoxicity as an indication to modify/avoid planned cancer treatment to decrease cardiotoxicity. This approach could worsen oncology outcomes and lead to undertreatment of cancer, therefore posing real danger,” said Dr. Barac.
The study was presented at the American College of Cardiology Advancing the Cardiovascular Care of the Oncology Patient 2023 conference.
Causes unclear
Chemotherapy is known to increase the risk of cardiovascular heart failure and other forms of CVD, but less is known about racial disparities in the incidence of chemotherapy-induced cardiotoxicity.
Mr. Gebeyehu and colleagues conducted a systematic review and meta-analysis of the available literature to assess racial disparities in CV adverse effects among cancer patients who were treated with chemotherapeutic agents. They screened 7,057 studies, fully reviewed 57, and included 24 studies, representing 683,749 participants, in their analysis.
Breast cancer was the most commonly reported malignancy. Other common malignancies were prostate, kidney, and hematologic malignancies such as leukemia and lymphoma.
Chemotherapeutic agents included anthracyclines (doxorubicin, daunorubicin), trastuzumab, and hormonal therapies.
Black race or African ancestry was associated with increased odds of chemotherapy-associated cardiotoxicity (odds ratio, 1.71; 95% confidence interval, 1.40-2.10), as well as congestive heart failure (OR, 1.92; 95% CI, 1.68-2.19).
Mr. Gebeyehu said in an interview that it’s hard to speculate on causation with an analysis of preexisting data such as this. “Our initial analysis that we’ve reported on so far are unadjusted values, meaning they don’t adjust for those potential underlying factors,” he noted.
“However, some of the studies individually controlled for socioeconomic factors and still found increased vulnerability to chemotherapy-associated cardiotoxicity in patients of Black race or African ancestry,” Mr. Gebeyehu said.
“It’s certainly possible that a mix of both biological and socioeconomic factors are interacting to lead to these disparities. One example could be the underrepresentation of Black patients in clinical trials to develop drugs. These could lead to chemotherapeutic agents being poorly optimized in this population relative to other racial/ethnic groups,” he added.
Dr. Barac said this study adds to the growing body of evidence about the importance of racial disparities in CVD and cancer outcomes.
“It is important to note that only the unadjusted odds ratio was reported and that much more detail is needed to understand what may be underlying the disparities. It is critically important to await the adjusted analysis, as well as details of the type of cancers and treatment used, before clinical implications can be discussed,” said Dr. Barac, who served as codirector of the conference.
“The risk of cardiotoxicity needs to be presented in the context of the oncology and CV disease burden, as both can influence the risk, and there could be a synergistic effect of disparities,” Dr. Barac added.
The study had no specific funding. Mr. Gebeyehu and Dr. Barac disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
a research review indicates.
“It’s important that both patients and clinicians be aware of these disparities so that more meaningful conversations around long-term cardiac health and cancer treatment can take place,” lead investigator Wondewossen Gebeyehu, with the University of Toronto, said in an interview.
However, patients “should not avoid chemotherapy, as the most important thing is making sure they get the best cancer treatment possible, and studies already show Black patients may get less optimal cancer treatments,” Mr. Gebeyehu added in a statement.
Ana Barac, MD, PHD, chair of cardio-oncology at Inova Schar Cancer Institute and Inova Heart and Vascular Institute, Fairfax, Va., who wasn’t involved in the study, agreed.
“The most important message is to look at preexisting cardiovascular disease, oncology diagnosis, and be aware of existing disparities in a specific cancer and CVD,” Barac said in an interview.
“What should NOT happen is to overinterpret this report of cardiotoxicity as an indication to modify/avoid planned cancer treatment to decrease cardiotoxicity. This approach could worsen oncology outcomes and lead to undertreatment of cancer, therefore posing real danger,” said Dr. Barac.
The study was presented at the American College of Cardiology Advancing the Cardiovascular Care of the Oncology Patient 2023 conference.
Causes unclear
Chemotherapy is known to increase the risk of cardiovascular heart failure and other forms of CVD, but less is known about racial disparities in the incidence of chemotherapy-induced cardiotoxicity.
Mr. Gebeyehu and colleagues conducted a systematic review and meta-analysis of the available literature to assess racial disparities in CV adverse effects among cancer patients who were treated with chemotherapeutic agents. They screened 7,057 studies, fully reviewed 57, and included 24 studies, representing 683,749 participants, in their analysis.
Breast cancer was the most commonly reported malignancy. Other common malignancies were prostate, kidney, and hematologic malignancies such as leukemia and lymphoma.
Chemotherapeutic agents included anthracyclines (doxorubicin, daunorubicin), trastuzumab, and hormonal therapies.
Black race or African ancestry was associated with increased odds of chemotherapy-associated cardiotoxicity (odds ratio, 1.71; 95% confidence interval, 1.40-2.10), as well as congestive heart failure (OR, 1.92; 95% CI, 1.68-2.19).
Mr. Gebeyehu said in an interview that it’s hard to speculate on causation with an analysis of preexisting data such as this. “Our initial analysis that we’ve reported on so far are unadjusted values, meaning they don’t adjust for those potential underlying factors,” he noted.
“However, some of the studies individually controlled for socioeconomic factors and still found increased vulnerability to chemotherapy-associated cardiotoxicity in patients of Black race or African ancestry,” Mr. Gebeyehu said.
“It’s certainly possible that a mix of both biological and socioeconomic factors are interacting to lead to these disparities. One example could be the underrepresentation of Black patients in clinical trials to develop drugs. These could lead to chemotherapeutic agents being poorly optimized in this population relative to other racial/ethnic groups,” he added.
Dr. Barac said this study adds to the growing body of evidence about the importance of racial disparities in CVD and cancer outcomes.
“It is important to note that only the unadjusted odds ratio was reported and that much more detail is needed to understand what may be underlying the disparities. It is critically important to await the adjusted analysis, as well as details of the type of cancers and treatment used, before clinical implications can be discussed,” said Dr. Barac, who served as codirector of the conference.
“The risk of cardiotoxicity needs to be presented in the context of the oncology and CV disease burden, as both can influence the risk, and there could be a synergistic effect of disparities,” Dr. Barac added.
The study had no specific funding. Mr. Gebeyehu and Dr. Barac disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Breast cancer screening advice ‘dangerous’ for black women
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
African ancestry genetically linked to worse CRC outcomes
, a disparity attributed to many factors, including socioeconomic, environmental, and genetic influences, as well as less access to care.
Results from a new genomic study provide greater clarity regarding the genetic piece of the puzzle: Persons of African background tend to have fewer targetable alterations, compared with patients of other races.
The findings were presented in a briefing and scientific poster session at the annual meeting of the American Association for Cancer Research.
Overall, the numbers to date show a clear trend: The incidence of and mortality from CRC are higher among Black patients than other populations. However, the extent to which genetic difference plays a role in these disparities remains unclear.
In the current study, researchers from Memorial Sloan Kettering (MSK) Cancer Center in New York explored how germline and somatic genomic alterations differ among patients of African ancestry, in comparison with those of European and other heritage, and how those differences might influence CRC outcomes.
Lead author Henry Walch, MS, a computational biologist at MSK, and colleagues compared genomic profiles among nearly 3,800 patients with CRC who were treated at MSK from 2014 to 2022. Patients in the study were classified by genetic ancestry as European (3,201 patients), African (236 patients), East Asian (253 patients), and South Asian (89 patients).
Tumor and normal tissues from the patients underwent next-generation DNA sequencing with a panel that covers 505 cancer-associated genes.
An analysis of overall survival by genetic ancestry confirmed findings from other studies: Overall survival was significantly worse among patients of African ancestry than among those of other groups (median 45.7 vs. 67.1 months).
The investigators used a precision oncology knowledge base (OncoKB) to assign levels of therapeutic actionability for each genomic alteration that was identified. The highest assigned value was for drugs that have been approved by the U.S. Food and Drug Administration and that target FDA-recognized biomarkers. The lowest value was assigned to biomarkers for which there was “compelling biological evidence” that the particular biomarker predicted response to a drug.
The team found that the percentage of patients who qualified for immunotherapy on the basis of microsatellite instability or high tumor mutational burden was significantly lower among patients of African heritage, compared with those of European heritage (13.5% vs. 20.4%; P = .008).
Compared with those of European ancestry, patients of African ancestry had significantly fewer actionable alterations (5.6% vs. 11.2%; P = .01). This difference was largely driven by the lack of targetable BRAF mutations (1.8% vs. 5.0%).
Mutations in APC, the most frequently altered gene in CRC, are typically associated with cancer outcomes, but the authors found that overall survival was similar for patients of African heritage regardless of whether they had altered or wild-type APC (median overall survival, 45.0 months for altered APC vs. 45.9 months for wild-type APC; P = .91). However, a significant association between APC status and overall survival was observed for patients of European ancestry (median, 64.6 months for altered APC vs. 45.6 months for wild-type APC; P < .0001).
Analyses that accounted for sex, age, primary tumor location, and stage at diagnosis also showed an association between APC status and overall survival for patients of European heritage (hazard ratio, 0.64), but not for patients of African heritage (HR, 0.74, P = .492).
Mr. Walch noted that a limitation of the study is that information regarding comprehensive treatment, environmental exposures, lifestyle, and socioeconomic factors was not available for the analysis but that these elements likely play an important role in patient outcomes.
“This is a complex problem involving many unseen factors, and the genomic landscape is a piece of a much larger puzzle,” said Mr. Walch. He noted that future studies will incorporate these factors into the models “with the ultimate goal of identifying opportunities to intervene and improve outcomes.”
Briefing moderator Lisa Newman, MD, MPH, of Weill Cornell Medicine and New York–Presbyterian, in New York, commented that Mr. Walch presented “some very compelling data that demonstrate the importance of including individuals from diverse backgrounds into [cancer] research.”
The study was funded in part by a Chris4Life Early Career Investigator Award Grant from the Colorectal Cancer Alliance for Francisco Sanchez-Vega, PhD, senior author of the study. Dr. Sanchez-Vega was also supported by an AACR-Minority and Minority-serving Institution Faculty Scholar in Cancer Research Award. Mr. Walch and Dr. Newman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a disparity attributed to many factors, including socioeconomic, environmental, and genetic influences, as well as less access to care.
Results from a new genomic study provide greater clarity regarding the genetic piece of the puzzle: Persons of African background tend to have fewer targetable alterations, compared with patients of other races.
The findings were presented in a briefing and scientific poster session at the annual meeting of the American Association for Cancer Research.
Overall, the numbers to date show a clear trend: The incidence of and mortality from CRC are higher among Black patients than other populations. However, the extent to which genetic difference plays a role in these disparities remains unclear.
In the current study, researchers from Memorial Sloan Kettering (MSK) Cancer Center in New York explored how germline and somatic genomic alterations differ among patients of African ancestry, in comparison with those of European and other heritage, and how those differences might influence CRC outcomes.
Lead author Henry Walch, MS, a computational biologist at MSK, and colleagues compared genomic profiles among nearly 3,800 patients with CRC who were treated at MSK from 2014 to 2022. Patients in the study were classified by genetic ancestry as European (3,201 patients), African (236 patients), East Asian (253 patients), and South Asian (89 patients).
Tumor and normal tissues from the patients underwent next-generation DNA sequencing with a panel that covers 505 cancer-associated genes.
An analysis of overall survival by genetic ancestry confirmed findings from other studies: Overall survival was significantly worse among patients of African ancestry than among those of other groups (median 45.7 vs. 67.1 months).
The investigators used a precision oncology knowledge base (OncoKB) to assign levels of therapeutic actionability for each genomic alteration that was identified. The highest assigned value was for drugs that have been approved by the U.S. Food and Drug Administration and that target FDA-recognized biomarkers. The lowest value was assigned to biomarkers for which there was “compelling biological evidence” that the particular biomarker predicted response to a drug.
The team found that the percentage of patients who qualified for immunotherapy on the basis of microsatellite instability or high tumor mutational burden was significantly lower among patients of African heritage, compared with those of European heritage (13.5% vs. 20.4%; P = .008).
Compared with those of European ancestry, patients of African ancestry had significantly fewer actionable alterations (5.6% vs. 11.2%; P = .01). This difference was largely driven by the lack of targetable BRAF mutations (1.8% vs. 5.0%).
Mutations in APC, the most frequently altered gene in CRC, are typically associated with cancer outcomes, but the authors found that overall survival was similar for patients of African heritage regardless of whether they had altered or wild-type APC (median overall survival, 45.0 months for altered APC vs. 45.9 months for wild-type APC; P = .91). However, a significant association between APC status and overall survival was observed for patients of European ancestry (median, 64.6 months for altered APC vs. 45.6 months for wild-type APC; P < .0001).
Analyses that accounted for sex, age, primary tumor location, and stage at diagnosis also showed an association between APC status and overall survival for patients of European heritage (hazard ratio, 0.64), but not for patients of African heritage (HR, 0.74, P = .492).
Mr. Walch noted that a limitation of the study is that information regarding comprehensive treatment, environmental exposures, lifestyle, and socioeconomic factors was not available for the analysis but that these elements likely play an important role in patient outcomes.
“This is a complex problem involving many unseen factors, and the genomic landscape is a piece of a much larger puzzle,” said Mr. Walch. He noted that future studies will incorporate these factors into the models “with the ultimate goal of identifying opportunities to intervene and improve outcomes.”
Briefing moderator Lisa Newman, MD, MPH, of Weill Cornell Medicine and New York–Presbyterian, in New York, commented that Mr. Walch presented “some very compelling data that demonstrate the importance of including individuals from diverse backgrounds into [cancer] research.”
The study was funded in part by a Chris4Life Early Career Investigator Award Grant from the Colorectal Cancer Alliance for Francisco Sanchez-Vega, PhD, senior author of the study. Dr. Sanchez-Vega was also supported by an AACR-Minority and Minority-serving Institution Faculty Scholar in Cancer Research Award. Mr. Walch and Dr. Newman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a disparity attributed to many factors, including socioeconomic, environmental, and genetic influences, as well as less access to care.
Results from a new genomic study provide greater clarity regarding the genetic piece of the puzzle: Persons of African background tend to have fewer targetable alterations, compared with patients of other races.
The findings were presented in a briefing and scientific poster session at the annual meeting of the American Association for Cancer Research.
Overall, the numbers to date show a clear trend: The incidence of and mortality from CRC are higher among Black patients than other populations. However, the extent to which genetic difference plays a role in these disparities remains unclear.
In the current study, researchers from Memorial Sloan Kettering (MSK) Cancer Center in New York explored how germline and somatic genomic alterations differ among patients of African ancestry, in comparison with those of European and other heritage, and how those differences might influence CRC outcomes.
Lead author Henry Walch, MS, a computational biologist at MSK, and colleagues compared genomic profiles among nearly 3,800 patients with CRC who were treated at MSK from 2014 to 2022. Patients in the study were classified by genetic ancestry as European (3,201 patients), African (236 patients), East Asian (253 patients), and South Asian (89 patients).
Tumor and normal tissues from the patients underwent next-generation DNA sequencing with a panel that covers 505 cancer-associated genes.
An analysis of overall survival by genetic ancestry confirmed findings from other studies: Overall survival was significantly worse among patients of African ancestry than among those of other groups (median 45.7 vs. 67.1 months).
The investigators used a precision oncology knowledge base (OncoKB) to assign levels of therapeutic actionability for each genomic alteration that was identified. The highest assigned value was for drugs that have been approved by the U.S. Food and Drug Administration and that target FDA-recognized biomarkers. The lowest value was assigned to biomarkers for which there was “compelling biological evidence” that the particular biomarker predicted response to a drug.
The team found that the percentage of patients who qualified for immunotherapy on the basis of microsatellite instability or high tumor mutational burden was significantly lower among patients of African heritage, compared with those of European heritage (13.5% vs. 20.4%; P = .008).
Compared with those of European ancestry, patients of African ancestry had significantly fewer actionable alterations (5.6% vs. 11.2%; P = .01). This difference was largely driven by the lack of targetable BRAF mutations (1.8% vs. 5.0%).
Mutations in APC, the most frequently altered gene in CRC, are typically associated with cancer outcomes, but the authors found that overall survival was similar for patients of African heritage regardless of whether they had altered or wild-type APC (median overall survival, 45.0 months for altered APC vs. 45.9 months for wild-type APC; P = .91). However, a significant association between APC status and overall survival was observed for patients of European ancestry (median, 64.6 months for altered APC vs. 45.6 months for wild-type APC; P < .0001).
Analyses that accounted for sex, age, primary tumor location, and stage at diagnosis also showed an association between APC status and overall survival for patients of European heritage (hazard ratio, 0.64), but not for patients of African heritage (HR, 0.74, P = .492).
Mr. Walch noted that a limitation of the study is that information regarding comprehensive treatment, environmental exposures, lifestyle, and socioeconomic factors was not available for the analysis but that these elements likely play an important role in patient outcomes.
“This is a complex problem involving many unseen factors, and the genomic landscape is a piece of a much larger puzzle,” said Mr. Walch. He noted that future studies will incorporate these factors into the models “with the ultimate goal of identifying opportunities to intervene and improve outcomes.”
Briefing moderator Lisa Newman, MD, MPH, of Weill Cornell Medicine and New York–Presbyterian, in New York, commented that Mr. Walch presented “some very compelling data that demonstrate the importance of including individuals from diverse backgrounds into [cancer] research.”
The study was funded in part by a Chris4Life Early Career Investigator Award Grant from the Colorectal Cancer Alliance for Francisco Sanchez-Vega, PhD, senior author of the study. Dr. Sanchez-Vega was also supported by an AACR-Minority and Minority-serving Institution Faculty Scholar in Cancer Research Award. Mr. Walch and Dr. Newman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AACR 2023
Circulating DNA has promise for cancer detection, but faces challenges
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
FROM AACR 2023
Use age, not weight, to screen for diabetes; assess over 35s
Universal screening of all U.S. adults aged 35-70 years for prediabetes and type 2 diabetes, regardless of body mass index, would provide the fairest means of detection, according to a new analysis.
This would better detect prediabetes and diabetes in ethnic groups that have a higher risk of diabetes at lower cutoffs. Compared with White individuals, Black or Hispanic adults have a higher risk of developing type 2 diabetes at a younger age, and Asian, Hispanic, and Black Americans all have a higher risk of developing it at a lower BMI.
In the new study, researchers examined six different screening scenarios in a nationally representative sample without diabetes.
They compared screening for prediabetes and type 2 diabetes using criteria from the 2021 U.S. Preventive Services Task Force (USPSTF) recommendations with the 2015 USPSTF recommendations, as well as four other screening thresholds with lower age or weight.
Universal screening for prediabetes and diabetes at age 35-70, regardless of BMI – which appears to be the sweet spot for most equitable detection in different races – may be easier to put into practice because it will mean clinicians don’t have to remember alternate cutoffs for different patient groups, the researchers suggested.
“All major racial and ethnic minority groups develop diabetes at lower weights than White adults, and it’s most pronounced for Asian Americans,” lead author Matthew J. O’Brien, MD, explained in a press release.
“If we make decisions about diabetes testing based on weight we will miss some people from racial and ethnic minority groups who are developing prediabetes and diabetes at lower weights,” said Dr. O’Brien, of Northwestern University, Chicago.
Going forward, to achieve equity in diagnosing prediabetes and diabetes “also requires addressing structural barriers [facing racial and ethnic minorities], which include not having a usual source of primary care, lacking health insurance, or having copays for screening tests based on insurance coverage,” the authors noted in their paper, published online in the American Journal of Preventive Medicine.
There is also a need for further study to examine the cost-effectiveness of any approach, and to study the impact of screening criteria on diagnosis, treatment, and outcomes in diverse populations.
Nationally representative sample, six screening scenarios
In the overall U.S. population, 81% of adults with prediabetes are unaware they have it, said Dr. O’Brien and colleagues, and 23% of diabetes cases are undiagnosed.
And Black, Hispanic, or Asian individuals have a nearly twofold higher prevalence of diabetes compared with White individuals.
The 2021 USPSTF recommendations state that clinicians should screen asymptomatic adults aged 35-70 years with overweight/obesity (BMI ≥ 25 kg/m2) and “should consider screening at an earlier age in persons from groups with disproportionately high incidence and prevalence (American Indian/Alaska Native, Asian American, Black, Hispanic/Latino, or Native Hawaiian/Pacific Islander persons) or in persons who have a family history of diabetes, a history of gestational diabetes, or a history of polycystic ovarian syndrome, and at a lower BMI in Asian American persons. Data suggest that a BMI of 23 or greater may be an appropriate cut point in Asian American persons.”
Dr. O’Brien and colleagues identified 3,243 nonpregnant adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) in 2017-2020 and had an A1c blood test. (Half also had a fasting plasma glucose test.)
First, they compared screening using the more recent and earlier USPSTF criteria: BMI of at least 25 kg/m2 and age 35-70 (2021 criteria) and BMI of at least 25 kg/m2 and age 40-70 (2015 criteria).
They estimated that 13.9 million more adults would be eligible for screening using the 2021 versus the 2015 screening criteria.
The increases in screening eligibility were highest in Hispanic individuals (30.6%), followed by Asian individuals (17.9%), White individuals (14.0%), and Black individuals (13.9%).
Using the USPSTF 2021 versus 2015 screening criteria resulted in marginally higher sensitivity (58.6% vs. 52.9%) but lower specificity (69.3% vs. 76.4%) overall, as well as within each racial group.
Next, the researchers examined screening at two lower age cutoffs and two lower BMI cutoffs: BMI of at least 25 kg/m2 and age 30-70, BMI of at least 25 kg/m2 and age 18-70, age 35-70 and BMI of at least 23 kg/m2, and age 35-70 and any BMI.
Screening at these lower age and weight thresholds resulted in even greater sensitivity and lower specificity than using the 2021 USPSTF criteria, especially among Hispanic, non-Hispanic Black, and Asian adults.
However, screening all adults aged 35-70 years regardless of BMI yielded the most equitable detection of prediabetes and diabetes – with a sensitivity of 67.8% and a specificity of 52.1% in the overall population, and a sensitivity of 70.1%, 70.4%, 68.4%, and 67.6%, and a specificity of 53.8%, 59.9%, 56.2%, and 48.9%, in the Asian, Black, Hispanic, and White subgroups, respectively.
The American Diabetes Association currently recommends screening all adults aged ≥ 35 years, or at any age if they have overweight/obesity and an additional diabetes risk factor, the researchers noted.
The study was partly funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Universal screening of all U.S. adults aged 35-70 years for prediabetes and type 2 diabetes, regardless of body mass index, would provide the fairest means of detection, according to a new analysis.
This would better detect prediabetes and diabetes in ethnic groups that have a higher risk of diabetes at lower cutoffs. Compared with White individuals, Black or Hispanic adults have a higher risk of developing type 2 diabetes at a younger age, and Asian, Hispanic, and Black Americans all have a higher risk of developing it at a lower BMI.
In the new study, researchers examined six different screening scenarios in a nationally representative sample without diabetes.
They compared screening for prediabetes and type 2 diabetes using criteria from the 2021 U.S. Preventive Services Task Force (USPSTF) recommendations with the 2015 USPSTF recommendations, as well as four other screening thresholds with lower age or weight.
Universal screening for prediabetes and diabetes at age 35-70, regardless of BMI – which appears to be the sweet spot for most equitable detection in different races – may be easier to put into practice because it will mean clinicians don’t have to remember alternate cutoffs for different patient groups, the researchers suggested.
“All major racial and ethnic minority groups develop diabetes at lower weights than White adults, and it’s most pronounced for Asian Americans,” lead author Matthew J. O’Brien, MD, explained in a press release.
“If we make decisions about diabetes testing based on weight we will miss some people from racial and ethnic minority groups who are developing prediabetes and diabetes at lower weights,” said Dr. O’Brien, of Northwestern University, Chicago.
Going forward, to achieve equity in diagnosing prediabetes and diabetes “also requires addressing structural barriers [facing racial and ethnic minorities], which include not having a usual source of primary care, lacking health insurance, or having copays for screening tests based on insurance coverage,” the authors noted in their paper, published online in the American Journal of Preventive Medicine.
There is also a need for further study to examine the cost-effectiveness of any approach, and to study the impact of screening criteria on diagnosis, treatment, and outcomes in diverse populations.
Nationally representative sample, six screening scenarios
In the overall U.S. population, 81% of adults with prediabetes are unaware they have it, said Dr. O’Brien and colleagues, and 23% of diabetes cases are undiagnosed.
And Black, Hispanic, or Asian individuals have a nearly twofold higher prevalence of diabetes compared with White individuals.
The 2021 USPSTF recommendations state that clinicians should screen asymptomatic adults aged 35-70 years with overweight/obesity (BMI ≥ 25 kg/m2) and “should consider screening at an earlier age in persons from groups with disproportionately high incidence and prevalence (American Indian/Alaska Native, Asian American, Black, Hispanic/Latino, or Native Hawaiian/Pacific Islander persons) or in persons who have a family history of diabetes, a history of gestational diabetes, or a history of polycystic ovarian syndrome, and at a lower BMI in Asian American persons. Data suggest that a BMI of 23 or greater may be an appropriate cut point in Asian American persons.”
Dr. O’Brien and colleagues identified 3,243 nonpregnant adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) in 2017-2020 and had an A1c blood test. (Half also had a fasting plasma glucose test.)
First, they compared screening using the more recent and earlier USPSTF criteria: BMI of at least 25 kg/m2 and age 35-70 (2021 criteria) and BMI of at least 25 kg/m2 and age 40-70 (2015 criteria).
They estimated that 13.9 million more adults would be eligible for screening using the 2021 versus the 2015 screening criteria.
The increases in screening eligibility were highest in Hispanic individuals (30.6%), followed by Asian individuals (17.9%), White individuals (14.0%), and Black individuals (13.9%).
Using the USPSTF 2021 versus 2015 screening criteria resulted in marginally higher sensitivity (58.6% vs. 52.9%) but lower specificity (69.3% vs. 76.4%) overall, as well as within each racial group.
Next, the researchers examined screening at two lower age cutoffs and two lower BMI cutoffs: BMI of at least 25 kg/m2 and age 30-70, BMI of at least 25 kg/m2 and age 18-70, age 35-70 and BMI of at least 23 kg/m2, and age 35-70 and any BMI.
Screening at these lower age and weight thresholds resulted in even greater sensitivity and lower specificity than using the 2021 USPSTF criteria, especially among Hispanic, non-Hispanic Black, and Asian adults.
However, screening all adults aged 35-70 years regardless of BMI yielded the most equitable detection of prediabetes and diabetes – with a sensitivity of 67.8% and a specificity of 52.1% in the overall population, and a sensitivity of 70.1%, 70.4%, 68.4%, and 67.6%, and a specificity of 53.8%, 59.9%, 56.2%, and 48.9%, in the Asian, Black, Hispanic, and White subgroups, respectively.
The American Diabetes Association currently recommends screening all adults aged ≥ 35 years, or at any age if they have overweight/obesity and an additional diabetes risk factor, the researchers noted.
The study was partly funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Universal screening of all U.S. adults aged 35-70 years for prediabetes and type 2 diabetes, regardless of body mass index, would provide the fairest means of detection, according to a new analysis.
This would better detect prediabetes and diabetes in ethnic groups that have a higher risk of diabetes at lower cutoffs. Compared with White individuals, Black or Hispanic adults have a higher risk of developing type 2 diabetes at a younger age, and Asian, Hispanic, and Black Americans all have a higher risk of developing it at a lower BMI.
In the new study, researchers examined six different screening scenarios in a nationally representative sample without diabetes.
They compared screening for prediabetes and type 2 diabetes using criteria from the 2021 U.S. Preventive Services Task Force (USPSTF) recommendations with the 2015 USPSTF recommendations, as well as four other screening thresholds with lower age or weight.
Universal screening for prediabetes and diabetes at age 35-70, regardless of BMI – which appears to be the sweet spot for most equitable detection in different races – may be easier to put into practice because it will mean clinicians don’t have to remember alternate cutoffs for different patient groups, the researchers suggested.
“All major racial and ethnic minority groups develop diabetes at lower weights than White adults, and it’s most pronounced for Asian Americans,” lead author Matthew J. O’Brien, MD, explained in a press release.
“If we make decisions about diabetes testing based on weight we will miss some people from racial and ethnic minority groups who are developing prediabetes and diabetes at lower weights,” said Dr. O’Brien, of Northwestern University, Chicago.
Going forward, to achieve equity in diagnosing prediabetes and diabetes “also requires addressing structural barriers [facing racial and ethnic minorities], which include not having a usual source of primary care, lacking health insurance, or having copays for screening tests based on insurance coverage,” the authors noted in their paper, published online in the American Journal of Preventive Medicine.
There is also a need for further study to examine the cost-effectiveness of any approach, and to study the impact of screening criteria on diagnosis, treatment, and outcomes in diverse populations.
Nationally representative sample, six screening scenarios
In the overall U.S. population, 81% of adults with prediabetes are unaware they have it, said Dr. O’Brien and colleagues, and 23% of diabetes cases are undiagnosed.
And Black, Hispanic, or Asian individuals have a nearly twofold higher prevalence of diabetes compared with White individuals.
The 2021 USPSTF recommendations state that clinicians should screen asymptomatic adults aged 35-70 years with overweight/obesity (BMI ≥ 25 kg/m2) and “should consider screening at an earlier age in persons from groups with disproportionately high incidence and prevalence (American Indian/Alaska Native, Asian American, Black, Hispanic/Latino, or Native Hawaiian/Pacific Islander persons) or in persons who have a family history of diabetes, a history of gestational diabetes, or a history of polycystic ovarian syndrome, and at a lower BMI in Asian American persons. Data suggest that a BMI of 23 or greater may be an appropriate cut point in Asian American persons.”
Dr. O’Brien and colleagues identified 3,243 nonpregnant adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) in 2017-2020 and had an A1c blood test. (Half also had a fasting plasma glucose test.)
First, they compared screening using the more recent and earlier USPSTF criteria: BMI of at least 25 kg/m2 and age 35-70 (2021 criteria) and BMI of at least 25 kg/m2 and age 40-70 (2015 criteria).
They estimated that 13.9 million more adults would be eligible for screening using the 2021 versus the 2015 screening criteria.
The increases in screening eligibility were highest in Hispanic individuals (30.6%), followed by Asian individuals (17.9%), White individuals (14.0%), and Black individuals (13.9%).
Using the USPSTF 2021 versus 2015 screening criteria resulted in marginally higher sensitivity (58.6% vs. 52.9%) but lower specificity (69.3% vs. 76.4%) overall, as well as within each racial group.
Next, the researchers examined screening at two lower age cutoffs and two lower BMI cutoffs: BMI of at least 25 kg/m2 and age 30-70, BMI of at least 25 kg/m2 and age 18-70, age 35-70 and BMI of at least 23 kg/m2, and age 35-70 and any BMI.
Screening at these lower age and weight thresholds resulted in even greater sensitivity and lower specificity than using the 2021 USPSTF criteria, especially among Hispanic, non-Hispanic Black, and Asian adults.
However, screening all adults aged 35-70 years regardless of BMI yielded the most equitable detection of prediabetes and diabetes – with a sensitivity of 67.8% and a specificity of 52.1% in the overall population, and a sensitivity of 70.1%, 70.4%, 68.4%, and 67.6%, and a specificity of 53.8%, 59.9%, 56.2%, and 48.9%, in the Asian, Black, Hispanic, and White subgroups, respectively.
The American Diabetes Association currently recommends screening all adults aged ≥ 35 years, or at any age if they have overweight/obesity and an additional diabetes risk factor, the researchers noted.
The study was partly funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
AHA statement targets nuance in CVD risk assessment of women
In a new scientific statement, the American Heart Association highlighted the importance of incorporating nonbiological risk factors and social determinants of health in cardiovascular disease (CVD) risk assessment for women, particularly women from different racial and ethnic backgrounds.
CVD risk assessment in women is multifaceted and goes well beyond traditional risk factors to include sex-specific biological risk factors, as well as social, behavioral, and environmental factors, the writing group noted.
They said a greater focus on addressing all CVD risk factors among women from underrepresented races and ethnicities is warranted to avert future CVD.
The scientific statement was published online in Circulation.
Look beyond traditional risk factors
“Risk assessment is the first step in preventing heart disease, yet there are many limitations to traditional risk factors and their ability to comprehensively estimate a woman’s risk for cardiovascular disease,” Jennifer H. Mieres, MD, vice chair of the writing group and professor of cardiology at Hofstra University, Hempstead, N.Y., said in a news release.
“The delivery of equitable cardiovascular health care for women depends on improving the knowledge and awareness of all members of the healthcare team about the full spectrum of cardiovascular risk factors for women, including female-specific and female-predominant risk factors,” Dr. Mieres added.
Female-specific factors that should be included in CVD risk assessment include pregnancy-related conditions such as preeclampsia, preterm delivery, and gestational diabetes, the writing group said.
Other factors include menstrual cycle history; types of birth control and/or hormone replacement therapy used; polycystic ovarian syndrome (PCOS), which affects 10% of women of reproductive age and is associated with increased CVD risk; and autoimmune disorders, depression, and PTSD, all of which are more common in women and are also associated with higher risk for CVD.
The statement also highlights the key role that social determinants of health (SDOH) play in the development of CVD in women, particularly women from diverse racial and ethnic backgrounds. SDOH include education level, economic stability, neighborhood safety, working conditions, environmental hazards, and access to quality health care.
“It is critical that risk assessment be expanded to include [SDOH] as risk factors if we are to improve health outcomes in all women,” Laxmi Mehta, MD, chair of the writing group and director of preventative cardiology and women’s cardiovascular health at Ohio State University Wexner Medical Center, Columbus, said in the news release.
“It is also important for the health care team to consider [SDOH] when working with women on shared decisions about cardiovascular disease prevention and treatment,” Dr. Mehta noted.
No one-size-fits-all approach
The statement highlighted significant differences in CVD risk among women of different racial and ethnic backgrounds and provides detailed CV risk factor profiles for non-Hispanic Black, Hispanic/Latinx, Asian and American Indian/Alaska Native women.
It noted that language barriers, discrimination, acculturation, and health care access disproportionately affect women of underrepresented racial and ethnic groups. These factors result in a higher prevalence of CVD and significant challenges in CVD diagnosis and treatment.
“When customizing CVD prevention and treatment strategies to improve cardiovascular health for women, a one-size-fits-all approach is unlikely to be successful,” Dr. Mieres said.
“We must be cognizant of the complex interplay of sex, race and ethnicity, as well as social determinants of health, and how they impact the risk of cardiovascular disease and adverse outcomes in order to avert future CVD morbidity and mortality,” Dr. Mieres added.
Looking ahead, the writing group said future CVD prevention guidelines could be strengthened by including culturally-specific lifestyle recommendations.
They also said community-based approaches, faith-based community partnerships, and peer support to encourage a healthy lifestyle could play a key role in preventing CVD among all women.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology, the Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article first appeared on Medscape.com.
In a new scientific statement, the American Heart Association highlighted the importance of incorporating nonbiological risk factors and social determinants of health in cardiovascular disease (CVD) risk assessment for women, particularly women from different racial and ethnic backgrounds.
CVD risk assessment in women is multifaceted and goes well beyond traditional risk factors to include sex-specific biological risk factors, as well as social, behavioral, and environmental factors, the writing group noted.
They said a greater focus on addressing all CVD risk factors among women from underrepresented races and ethnicities is warranted to avert future CVD.
The scientific statement was published online in Circulation.
Look beyond traditional risk factors
“Risk assessment is the first step in preventing heart disease, yet there are many limitations to traditional risk factors and their ability to comprehensively estimate a woman’s risk for cardiovascular disease,” Jennifer H. Mieres, MD, vice chair of the writing group and professor of cardiology at Hofstra University, Hempstead, N.Y., said in a news release.
“The delivery of equitable cardiovascular health care for women depends on improving the knowledge and awareness of all members of the healthcare team about the full spectrum of cardiovascular risk factors for women, including female-specific and female-predominant risk factors,” Dr. Mieres added.
Female-specific factors that should be included in CVD risk assessment include pregnancy-related conditions such as preeclampsia, preterm delivery, and gestational diabetes, the writing group said.
Other factors include menstrual cycle history; types of birth control and/or hormone replacement therapy used; polycystic ovarian syndrome (PCOS), which affects 10% of women of reproductive age and is associated with increased CVD risk; and autoimmune disorders, depression, and PTSD, all of which are more common in women and are also associated with higher risk for CVD.
The statement also highlights the key role that social determinants of health (SDOH) play in the development of CVD in women, particularly women from diverse racial and ethnic backgrounds. SDOH include education level, economic stability, neighborhood safety, working conditions, environmental hazards, and access to quality health care.
“It is critical that risk assessment be expanded to include [SDOH] as risk factors if we are to improve health outcomes in all women,” Laxmi Mehta, MD, chair of the writing group and director of preventative cardiology and women’s cardiovascular health at Ohio State University Wexner Medical Center, Columbus, said in the news release.
“It is also important for the health care team to consider [SDOH] when working with women on shared decisions about cardiovascular disease prevention and treatment,” Dr. Mehta noted.
No one-size-fits-all approach
The statement highlighted significant differences in CVD risk among women of different racial and ethnic backgrounds and provides detailed CV risk factor profiles for non-Hispanic Black, Hispanic/Latinx, Asian and American Indian/Alaska Native women.
It noted that language barriers, discrimination, acculturation, and health care access disproportionately affect women of underrepresented racial and ethnic groups. These factors result in a higher prevalence of CVD and significant challenges in CVD diagnosis and treatment.
“When customizing CVD prevention and treatment strategies to improve cardiovascular health for women, a one-size-fits-all approach is unlikely to be successful,” Dr. Mieres said.
“We must be cognizant of the complex interplay of sex, race and ethnicity, as well as social determinants of health, and how they impact the risk of cardiovascular disease and adverse outcomes in order to avert future CVD morbidity and mortality,” Dr. Mieres added.
Looking ahead, the writing group said future CVD prevention guidelines could be strengthened by including culturally-specific lifestyle recommendations.
They also said community-based approaches, faith-based community partnerships, and peer support to encourage a healthy lifestyle could play a key role in preventing CVD among all women.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology, the Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article first appeared on Medscape.com.
In a new scientific statement, the American Heart Association highlighted the importance of incorporating nonbiological risk factors and social determinants of health in cardiovascular disease (CVD) risk assessment for women, particularly women from different racial and ethnic backgrounds.
CVD risk assessment in women is multifaceted and goes well beyond traditional risk factors to include sex-specific biological risk factors, as well as social, behavioral, and environmental factors, the writing group noted.
They said a greater focus on addressing all CVD risk factors among women from underrepresented races and ethnicities is warranted to avert future CVD.
The scientific statement was published online in Circulation.
Look beyond traditional risk factors
“Risk assessment is the first step in preventing heart disease, yet there are many limitations to traditional risk factors and their ability to comprehensively estimate a woman’s risk for cardiovascular disease,” Jennifer H. Mieres, MD, vice chair of the writing group and professor of cardiology at Hofstra University, Hempstead, N.Y., said in a news release.
“The delivery of equitable cardiovascular health care for women depends on improving the knowledge and awareness of all members of the healthcare team about the full spectrum of cardiovascular risk factors for women, including female-specific and female-predominant risk factors,” Dr. Mieres added.
Female-specific factors that should be included in CVD risk assessment include pregnancy-related conditions such as preeclampsia, preterm delivery, and gestational diabetes, the writing group said.
Other factors include menstrual cycle history; types of birth control and/or hormone replacement therapy used; polycystic ovarian syndrome (PCOS), which affects 10% of women of reproductive age and is associated with increased CVD risk; and autoimmune disorders, depression, and PTSD, all of which are more common in women and are also associated with higher risk for CVD.
The statement also highlights the key role that social determinants of health (SDOH) play in the development of CVD in women, particularly women from diverse racial and ethnic backgrounds. SDOH include education level, economic stability, neighborhood safety, working conditions, environmental hazards, and access to quality health care.
“It is critical that risk assessment be expanded to include [SDOH] as risk factors if we are to improve health outcomes in all women,” Laxmi Mehta, MD, chair of the writing group and director of preventative cardiology and women’s cardiovascular health at Ohio State University Wexner Medical Center, Columbus, said in the news release.
“It is also important for the health care team to consider [SDOH] when working with women on shared decisions about cardiovascular disease prevention and treatment,” Dr. Mehta noted.
No one-size-fits-all approach
The statement highlighted significant differences in CVD risk among women of different racial and ethnic backgrounds and provides detailed CV risk factor profiles for non-Hispanic Black, Hispanic/Latinx, Asian and American Indian/Alaska Native women.
It noted that language barriers, discrimination, acculturation, and health care access disproportionately affect women of underrepresented racial and ethnic groups. These factors result in a higher prevalence of CVD and significant challenges in CVD diagnosis and treatment.
“When customizing CVD prevention and treatment strategies to improve cardiovascular health for women, a one-size-fits-all approach is unlikely to be successful,” Dr. Mieres said.
“We must be cognizant of the complex interplay of sex, race and ethnicity, as well as social determinants of health, and how they impact the risk of cardiovascular disease and adverse outcomes in order to avert future CVD morbidity and mortality,” Dr. Mieres added.
Looking ahead, the writing group said future CVD prevention guidelines could be strengthened by including culturally-specific lifestyle recommendations.
They also said community-based approaches, faith-based community partnerships, and peer support to encourage a healthy lifestyle could play a key role in preventing CVD among all women.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology, the Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Melasma
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.
Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.