User login
Type of insurance linked to length of survival after lung surgery
The study used public insurance status as a marker for low socioeconomic status (SES) and suggests that patients with combined insurance may constitute a separate population that deserves more attention.
Lower SES has been linked to later stage diagnoses and worse outcomes in NSCLC. Private insurance is a generally-accepted indicator of higher SES, while public insurance like Medicare or Medicaid, alone or in combination with private supplementary insurance, is an indicator of lower SES.
Although previous studies have found associations between patients having public health insurance and experiencing later-stage diagnoses and worse overall survival, there have been few studies of surgical outcomes, and almost no research has examined combination health insurance, according to Allison O. Dumitriu Carcoana, who presented the research during a poster session at the European Lung Cancer Congress 2023.
“This is an important insurance subgroup for us because the majority of our patients fall into this subgroup by being over 65 years old and thus qualifying for Medicare while also paying for a private supplement,” said Ms. Dumitriu Carcoana, who is a medical student at University of South Florida Health Morsani College of Medicine, Tampa.
A previous analysis by the group found an association between private insurance status and better discharge status, as well as higher 5-year overall survival. After accumulating an additional 278 patients, the researchers examined 10-year survival outcomes.
In the new analysis, 52% of 711 participants had combination insurance, while 28% had private insurance, and 20% had public insurance. The subgroups all had similar demographic and histological characteristics. The study was unique in that it found no between-group differences in higher stage at diagnosis, whereas previous studies have found a greater risk of higher stage diagnosis among individuals with public insurance. As expected, patients in the combined insurance group had a higher mean age (P less than .0001) and higher Charlson comorbidity index scores (P = .0014), which in turn was associated with lower 10-year survival. The group also had the highest percentage of former smokers, while the public insurance group had the highest percentage of current smokers (P = .0003).
At both 5 and 10 years, the private insurance group had better OS than the group with public (P less than .001) and the combination insurance group (P = .08). Public health insurance was associated with worse OS at 5 years (hazard ratio, 1.83; P less than .005) but not at 10 years (HR, 1.18; P = .51), while combination insurance was associated with worse OS at 10 years (HR, 1.72; P = .02).
“We think that patients with public health insurance having the worst 5-year overall survival, despite their lower ages and fewer comorbid conditions, compared with patients with combination insurance, highlights the impact of lower socioeconomic status on health outcomes. These patients had the same tumor characteristics, BMI, sex, and race as our patients in the other two insurance groups. The only other significant risk factor [the group had besides having a higher proportion of patients with lower socioeconomic status was that it had a higher proportion of current smokers]. But the multivariate analyses showed that insurance status was an independent predictor of survival, regardless of smoking status or other comorbidities,” said Ms. Dumitriu Carcoana.
“At 10 years post-operatively, the survival curves have shifted and the combination patients had the worst 10-year overall survival. We attribute this to their higher number of comorbid conditions and increased age. In practice, [this means that] the group of patients with public insurance type, but no supplement, should be identified clinically, and the clinical team can initiate a discussion,” Ms. Dumitriu Carcoana said.
“Do these patients feel that they can make follow-up appointments, keep up with medication costs, and make the right lifestyle decisions postoperatively on their current insurance plan? If not, can they afford a private supplement? In our cohort specifically, it may also be important to do more preoperative counseling on the importance of smoking cessation,” she added.
The study is interesting, but it has some important limitations, according to Raja Flores, MD, who was not involved with the study. The authors stated that there was no difference between the insurance groups with respect to mortality or cancer stage, which is the most important predictor of survival. However, the poster didn't include details of the authors' analysis, making it difficult to interpret, Dr. Flores said.
The fact that the study includes a single surgeon has some disadvantages in terms of broader applicability, but it also controls for surgical technique. “Different surgeons have different ways of doing things, so if you had the same surgeon doing it the same way every time, you can look at other variables like insurance (status) and stage,” said Dr. Flores.
The results may also provide an argument against using robotic surgery in patients who do not have insurance, especially since they have not been proven to be better than standard minimally invasive surgery with no robotic assistance. With uninsured patients, “you’re using taxpayer money for a more expensive procedure that isn’t proving to be any better,” Dr. Flores explained.
The study was performed at a single center and cannot prove causation due to its retrospective nature.
Ms. Dumitriu Carcoana and Dr. Flores have no relevant financial disclosures.
*This article was updated on 4/13/2023.
The study used public insurance status as a marker for low socioeconomic status (SES) and suggests that patients with combined insurance may constitute a separate population that deserves more attention.
Lower SES has been linked to later stage diagnoses and worse outcomes in NSCLC. Private insurance is a generally-accepted indicator of higher SES, while public insurance like Medicare or Medicaid, alone or in combination with private supplementary insurance, is an indicator of lower SES.
Although previous studies have found associations between patients having public health insurance and experiencing later-stage diagnoses and worse overall survival, there have been few studies of surgical outcomes, and almost no research has examined combination health insurance, according to Allison O. Dumitriu Carcoana, who presented the research during a poster session at the European Lung Cancer Congress 2023.
“This is an important insurance subgroup for us because the majority of our patients fall into this subgroup by being over 65 years old and thus qualifying for Medicare while also paying for a private supplement,” said Ms. Dumitriu Carcoana, who is a medical student at University of South Florida Health Morsani College of Medicine, Tampa.
A previous analysis by the group found an association between private insurance status and better discharge status, as well as higher 5-year overall survival. After accumulating an additional 278 patients, the researchers examined 10-year survival outcomes.
In the new analysis, 52% of 711 participants had combination insurance, while 28% had private insurance, and 20% had public insurance. The subgroups all had similar demographic and histological characteristics. The study was unique in that it found no between-group differences in higher stage at diagnosis, whereas previous studies have found a greater risk of higher stage diagnosis among individuals with public insurance. As expected, patients in the combined insurance group had a higher mean age (P less than .0001) and higher Charlson comorbidity index scores (P = .0014), which in turn was associated with lower 10-year survival. The group also had the highest percentage of former smokers, while the public insurance group had the highest percentage of current smokers (P = .0003).
At both 5 and 10 years, the private insurance group had better OS than the group with public (P less than .001) and the combination insurance group (P = .08). Public health insurance was associated with worse OS at 5 years (hazard ratio, 1.83; P less than .005) but not at 10 years (HR, 1.18; P = .51), while combination insurance was associated with worse OS at 10 years (HR, 1.72; P = .02).
“We think that patients with public health insurance having the worst 5-year overall survival, despite their lower ages and fewer comorbid conditions, compared with patients with combination insurance, highlights the impact of lower socioeconomic status on health outcomes. These patients had the same tumor characteristics, BMI, sex, and race as our patients in the other two insurance groups. The only other significant risk factor [the group had besides having a higher proportion of patients with lower socioeconomic status was that it had a higher proportion of current smokers]. But the multivariate analyses showed that insurance status was an independent predictor of survival, regardless of smoking status or other comorbidities,” said Ms. Dumitriu Carcoana.
“At 10 years post-operatively, the survival curves have shifted and the combination patients had the worst 10-year overall survival. We attribute this to their higher number of comorbid conditions and increased age. In practice, [this means that] the group of patients with public insurance type, but no supplement, should be identified clinically, and the clinical team can initiate a discussion,” Ms. Dumitriu Carcoana said.
“Do these patients feel that they can make follow-up appointments, keep up with medication costs, and make the right lifestyle decisions postoperatively on their current insurance plan? If not, can they afford a private supplement? In our cohort specifically, it may also be important to do more preoperative counseling on the importance of smoking cessation,” she added.
The study is interesting, but it has some important limitations, according to Raja Flores, MD, who was not involved with the study. The authors stated that there was no difference between the insurance groups with respect to mortality or cancer stage, which is the most important predictor of survival. However, the poster didn't include details of the authors' analysis, making it difficult to interpret, Dr. Flores said.
The fact that the study includes a single surgeon has some disadvantages in terms of broader applicability, but it also controls for surgical technique. “Different surgeons have different ways of doing things, so if you had the same surgeon doing it the same way every time, you can look at other variables like insurance (status) and stage,” said Dr. Flores.
The results may also provide an argument against using robotic surgery in patients who do not have insurance, especially since they have not been proven to be better than standard minimally invasive surgery with no robotic assistance. With uninsured patients, “you’re using taxpayer money for a more expensive procedure that isn’t proving to be any better,” Dr. Flores explained.
The study was performed at a single center and cannot prove causation due to its retrospective nature.
Ms. Dumitriu Carcoana and Dr. Flores have no relevant financial disclosures.
*This article was updated on 4/13/2023.
The study used public insurance status as a marker for low socioeconomic status (SES) and suggests that patients with combined insurance may constitute a separate population that deserves more attention.
Lower SES has been linked to later stage diagnoses and worse outcomes in NSCLC. Private insurance is a generally-accepted indicator of higher SES, while public insurance like Medicare or Medicaid, alone or in combination with private supplementary insurance, is an indicator of lower SES.
Although previous studies have found associations between patients having public health insurance and experiencing later-stage diagnoses and worse overall survival, there have been few studies of surgical outcomes, and almost no research has examined combination health insurance, according to Allison O. Dumitriu Carcoana, who presented the research during a poster session at the European Lung Cancer Congress 2023.
“This is an important insurance subgroup for us because the majority of our patients fall into this subgroup by being over 65 years old and thus qualifying for Medicare while also paying for a private supplement,” said Ms. Dumitriu Carcoana, who is a medical student at University of South Florida Health Morsani College of Medicine, Tampa.
A previous analysis by the group found an association between private insurance status and better discharge status, as well as higher 5-year overall survival. After accumulating an additional 278 patients, the researchers examined 10-year survival outcomes.
In the new analysis, 52% of 711 participants had combination insurance, while 28% had private insurance, and 20% had public insurance. The subgroups all had similar demographic and histological characteristics. The study was unique in that it found no between-group differences in higher stage at diagnosis, whereas previous studies have found a greater risk of higher stage diagnosis among individuals with public insurance. As expected, patients in the combined insurance group had a higher mean age (P less than .0001) and higher Charlson comorbidity index scores (P = .0014), which in turn was associated with lower 10-year survival. The group also had the highest percentage of former smokers, while the public insurance group had the highest percentage of current smokers (P = .0003).
At both 5 and 10 years, the private insurance group had better OS than the group with public (P less than .001) and the combination insurance group (P = .08). Public health insurance was associated with worse OS at 5 years (hazard ratio, 1.83; P less than .005) but not at 10 years (HR, 1.18; P = .51), while combination insurance was associated with worse OS at 10 years (HR, 1.72; P = .02).
“We think that patients with public health insurance having the worst 5-year overall survival, despite their lower ages and fewer comorbid conditions, compared with patients with combination insurance, highlights the impact of lower socioeconomic status on health outcomes. These patients had the same tumor characteristics, BMI, sex, and race as our patients in the other two insurance groups. The only other significant risk factor [the group had besides having a higher proportion of patients with lower socioeconomic status was that it had a higher proportion of current smokers]. But the multivariate analyses showed that insurance status was an independent predictor of survival, regardless of smoking status or other comorbidities,” said Ms. Dumitriu Carcoana.
“At 10 years post-operatively, the survival curves have shifted and the combination patients had the worst 10-year overall survival. We attribute this to their higher number of comorbid conditions and increased age. In practice, [this means that] the group of patients with public insurance type, but no supplement, should be identified clinically, and the clinical team can initiate a discussion,” Ms. Dumitriu Carcoana said.
“Do these patients feel that they can make follow-up appointments, keep up with medication costs, and make the right lifestyle decisions postoperatively on their current insurance plan? If not, can they afford a private supplement? In our cohort specifically, it may also be important to do more preoperative counseling on the importance of smoking cessation,” she added.
The study is interesting, but it has some important limitations, according to Raja Flores, MD, who was not involved with the study. The authors stated that there was no difference between the insurance groups with respect to mortality or cancer stage, which is the most important predictor of survival. However, the poster didn't include details of the authors' analysis, making it difficult to interpret, Dr. Flores said.
The fact that the study includes a single surgeon has some disadvantages in terms of broader applicability, but it also controls for surgical technique. “Different surgeons have different ways of doing things, so if you had the same surgeon doing it the same way every time, you can look at other variables like insurance (status) and stage,” said Dr. Flores.
The results may also provide an argument against using robotic surgery in patients who do not have insurance, especially since they have not been proven to be better than standard minimally invasive surgery with no robotic assistance. With uninsured patients, “you’re using taxpayer money for a more expensive procedure that isn’t proving to be any better,” Dr. Flores explained.
The study was performed at a single center and cannot prove causation due to its retrospective nature.
Ms. Dumitriu Carcoana and Dr. Flores have no relevant financial disclosures.
*This article was updated on 4/13/2023.
FROM ELCC 2023
Melasma
THE COMPARISON
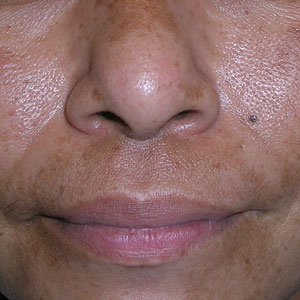
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.
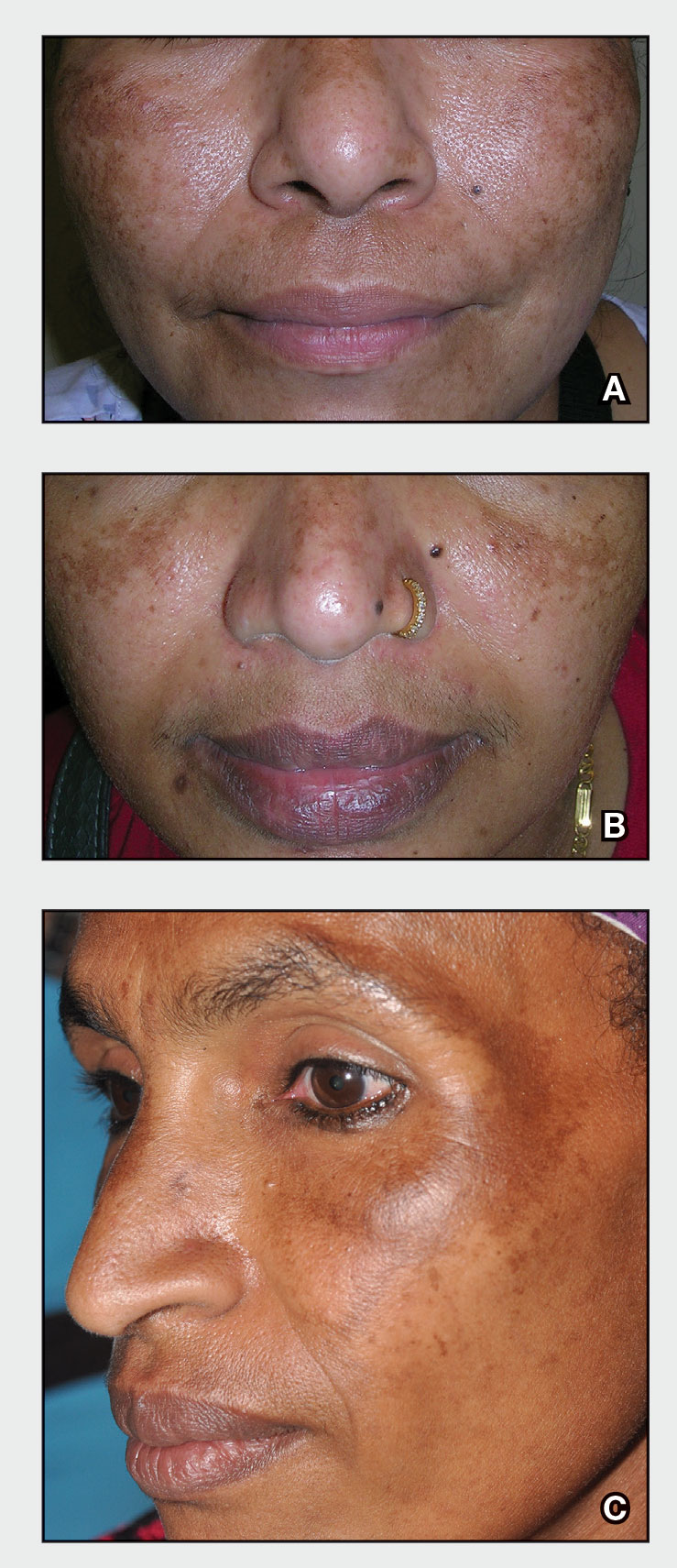
Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
Treatment of Frontal Fibrosing Alopecia in Black Patients: A Systematic Review
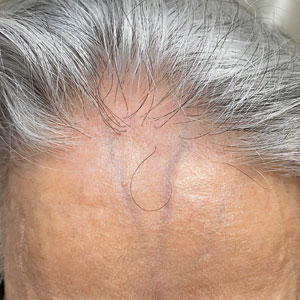
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
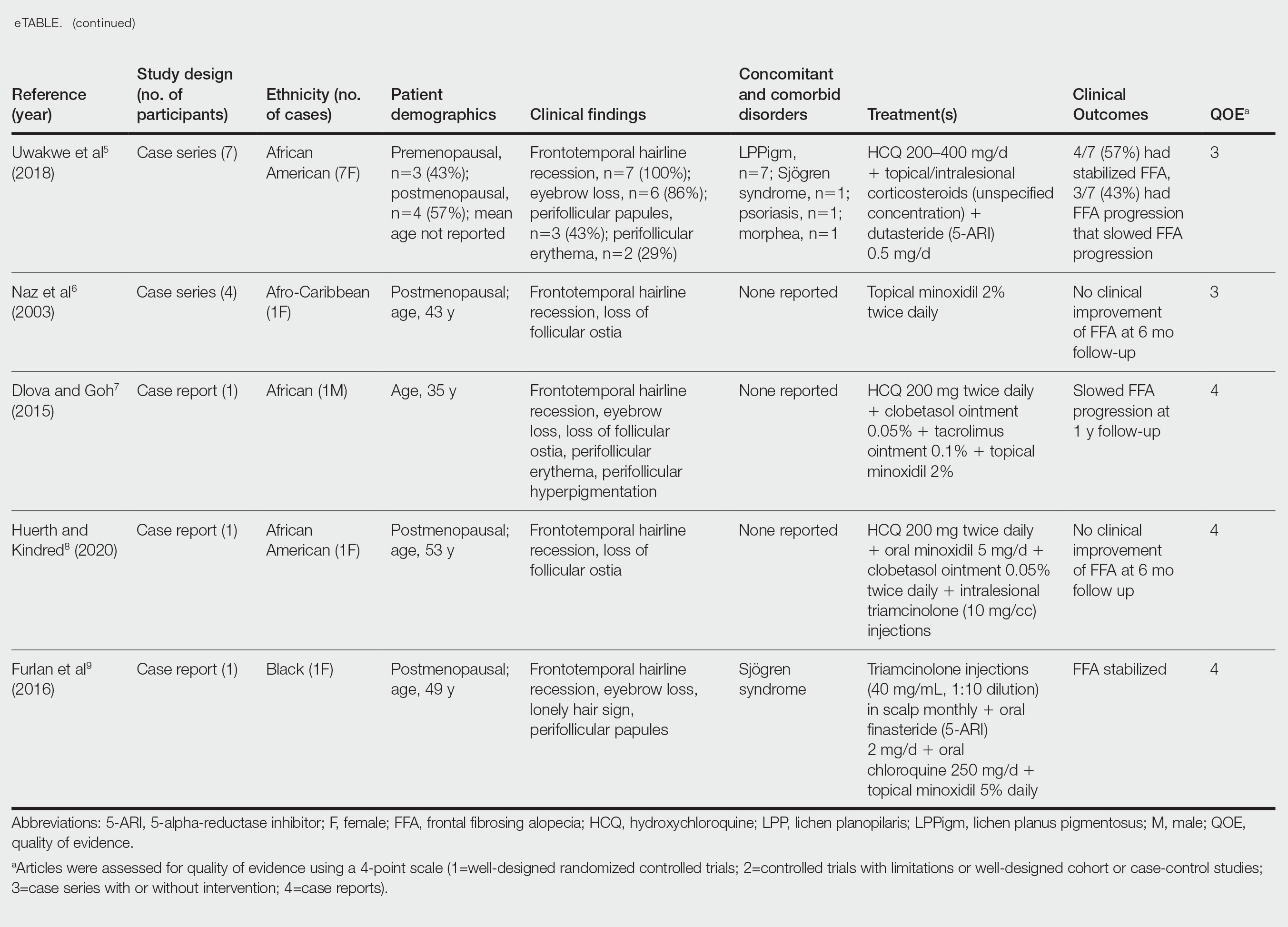
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
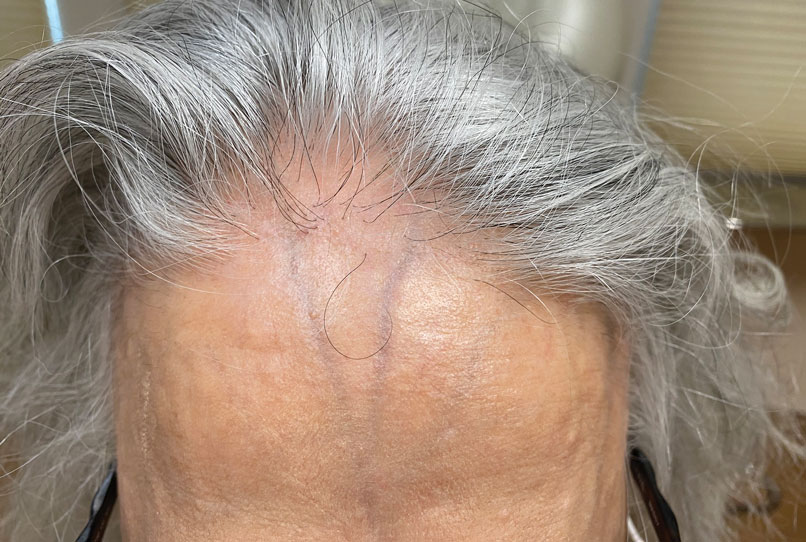
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.
The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.
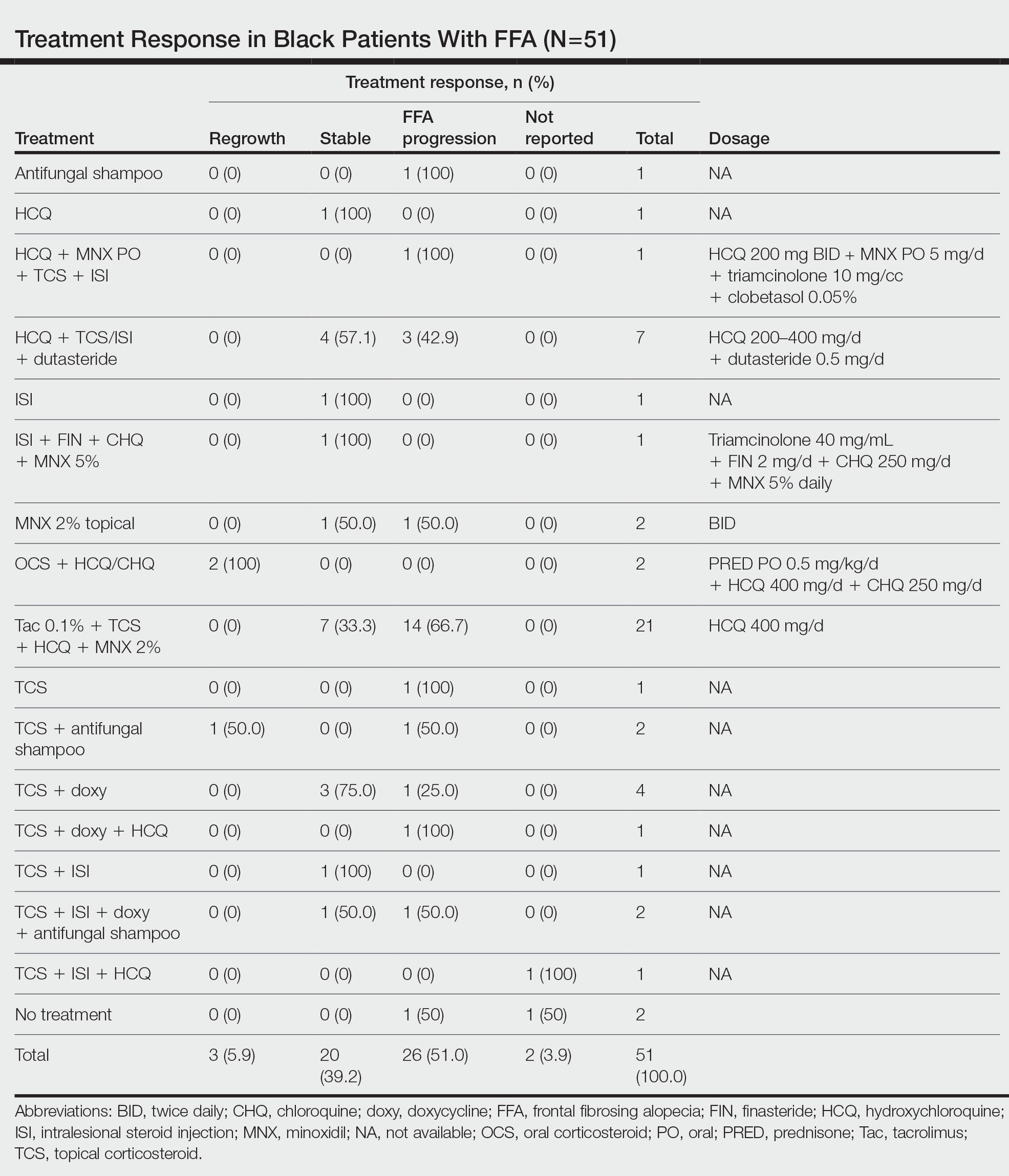
Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.

- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Practice Points
- Treatment of frontal fibrosing alopecia (FFA) is challenging, and there are no evidence-based treatment guidelines available. Patients with skin of color (SOC) may have varying responses to treatment modalities.
- Special consideration should be taken when treating FFA in patients with SOC.
- Histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
The importance of diversity in psychiatry
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
Expect increased demand for experienced dermatologic care of Asian skin
NEW ORLEANS – With the Asian population estimated to increase to 41 million by 2050 in the United States, expect the demand for experienced dermatologic care of patients with Asian skin to increase in the coming years, Hye Jin (Leah) Chung, MD, said at the annual meeting of the American Academy of Dermatology.
“Asians account for about 60% of the global population,” said Dr. Chung, assistant professor of dermatology at Harvard Medical School, and director of the Asian Skin Clinic at Beth Israel Deaconess Medical Center, Boston. Along with the estimate that Asians are expected to make up 25% of Canada’s population by 2036, “we will most likely encounter more Asian skin type patients in North America,” Dr. Chung said, noting that the Asian population “is very diverse, ranging from skin type 3 in Far East Asia to skin type 5 in India.”
During her presentation, she provided tips for treating hypertrophic scars and keloids in this patient population when intralesional corticosteroids fail. Typically, her first option is to combine an intralesional corticosteroid with 5-fluorouracil (5-FU), a pyrimidine analogue with antimetabolite activity. 5-FU “can cause cell apoptosis of endothelial cells and fibroblasts (which steroids cannot), cell cycle arrest, and TGF-beta [transforming growth factor beta]-induced COL1A2 transcription,” Dr. Chung said. The recommended ratio between 5-FU and steroids in the literature is variable, from a 9:1 ratio to a 1:1 ratio. “In my practice I do not inject more than 100 mg at a time,” she said. Several studies of this approach led by Asian investigators used weekly injections, “but that’s not practical in the U.S. I usually do monthly injections.”
A large systematic review and meta-analysis confirmed that the combination of intralesional triamcinolone acetonide and 5-FU achieved a better efficacy and fewer complications than triamcinolone alone for treating hypertrophic scars and keloids. Potential side effects from 5-FU injections include pain/pruritus, transient hyperpigmentation (especially in skin types 4-6), ulceration, teratogenicity, and transient alopecia.
A more recent meta-analysis comparing the efficacy of multiple drug injections for hypertrophic scars and keloids confirmed that the combination of triamcinolone and 5-FU was superior to bleomycin, verapamil, 5-FU alone, and triamcinolone alone. “And, there was no difference between 5-FU/steroid combination and botulinum toxin A,” Dr. Chung added. “Some parts of the world are using botulinum toxin with mixed results. Based on the amount of toxin required for keloids, this would be cost prohibitive in the U.S.”
Another approach to treating hypertrophic scars and keloids in Asian skin is laser-assisted drug delivery. “First, you can use a fractional ablative laser to create a hole in the epidermis and dermis,” Dr. Chung said. “Then you can apply the suspension topically to the holes. You can also use a steroid ointment or cream after laser treatment for drug delivery.”
Combining pulsed dye laser with steroid injections is another option. Pulsed dye lasers coagulate microvasculature within keloid tissue, “which can cause tissue hypoxia and can decrease growth factors or cytokines for fibrosis within the tissue,” Dr. Chung said. At the cellular level, pulsed dye laser alone can decrease connective tissue growth factor (CTGF), TGF-beta 1, proliferating cell nuclear antigen, and collagen III, and increases matrix metalloproteinase–13 (MMP-13), P53, ERK and p38 MAPK, apoptosis, blockade of AP-1 transcription, and cell cycle changes.
In 2004, plastic surgeons in Korea described a new approach for removing earlobe keloids, which they termed a “keloid fillet flap”. For the procedure, about 50% of the keloid margin is incised with a #15 scalpel blade. “Then you dissect the keloid from the surrounding tissue with a blade or curved scissors,” Dr. Chung said. “Next, you excise the keloid, so you have some dead space. After hemostasis you place the fillet flap to cover the wound. After you trim the redundant tissue, you can close it with epidermal sutures.”
In her clinical experience, she finds the fillet flap “very helpful for fast recovery” and it is associated with less pain. “Several studies have confirmed an excellent improvement of keloids, low recurrence rate, and rare side effects from a fillet flap and adjuvant intralesional corticosteroids. Occasionally, you may see flap necrosis but usually patients do well with topical antibiotics or petrolatum jelly.”
Dr. Chung also discussed her approach to treating papular scars in Asian patients. She described papular scars as underrecognized, anetoderma-like scars on the central face and trunk. “They comprise about 11% of all acne scars but up to 19% of patients with such scars may not recall a history of acne,” she said. Biopsies of papular scars reveal marked reduction or thinning of elastic fibers around hair follicles.
“Papular scars are difficult to treat,” she said. “If you have a conventional Er:YAG or CO2 laser, you can create tiny holes within the scars,” she said, referring to studies on these approaches. Another treatment option is needle-guided radiofrequency, she noted.
Dr. Chung reported having no relevant financial disclosures.
NEW ORLEANS – With the Asian population estimated to increase to 41 million by 2050 in the United States, expect the demand for experienced dermatologic care of patients with Asian skin to increase in the coming years, Hye Jin (Leah) Chung, MD, said at the annual meeting of the American Academy of Dermatology.
“Asians account for about 60% of the global population,” said Dr. Chung, assistant professor of dermatology at Harvard Medical School, and director of the Asian Skin Clinic at Beth Israel Deaconess Medical Center, Boston. Along with the estimate that Asians are expected to make up 25% of Canada’s population by 2036, “we will most likely encounter more Asian skin type patients in North America,” Dr. Chung said, noting that the Asian population “is very diverse, ranging from skin type 3 in Far East Asia to skin type 5 in India.”
During her presentation, she provided tips for treating hypertrophic scars and keloids in this patient population when intralesional corticosteroids fail. Typically, her first option is to combine an intralesional corticosteroid with 5-fluorouracil (5-FU), a pyrimidine analogue with antimetabolite activity. 5-FU “can cause cell apoptosis of endothelial cells and fibroblasts (which steroids cannot), cell cycle arrest, and TGF-beta [transforming growth factor beta]-induced COL1A2 transcription,” Dr. Chung said. The recommended ratio between 5-FU and steroids in the literature is variable, from a 9:1 ratio to a 1:1 ratio. “In my practice I do not inject more than 100 mg at a time,” she said. Several studies of this approach led by Asian investigators used weekly injections, “but that’s not practical in the U.S. I usually do monthly injections.”
A large systematic review and meta-analysis confirmed that the combination of intralesional triamcinolone acetonide and 5-FU achieved a better efficacy and fewer complications than triamcinolone alone for treating hypertrophic scars and keloids. Potential side effects from 5-FU injections include pain/pruritus, transient hyperpigmentation (especially in skin types 4-6), ulceration, teratogenicity, and transient alopecia.
A more recent meta-analysis comparing the efficacy of multiple drug injections for hypertrophic scars and keloids confirmed that the combination of triamcinolone and 5-FU was superior to bleomycin, verapamil, 5-FU alone, and triamcinolone alone. “And, there was no difference between 5-FU/steroid combination and botulinum toxin A,” Dr. Chung added. “Some parts of the world are using botulinum toxin with mixed results. Based on the amount of toxin required for keloids, this would be cost prohibitive in the U.S.”
Another approach to treating hypertrophic scars and keloids in Asian skin is laser-assisted drug delivery. “First, you can use a fractional ablative laser to create a hole in the epidermis and dermis,” Dr. Chung said. “Then you can apply the suspension topically to the holes. You can also use a steroid ointment or cream after laser treatment for drug delivery.”
Combining pulsed dye laser with steroid injections is another option. Pulsed dye lasers coagulate microvasculature within keloid tissue, “which can cause tissue hypoxia and can decrease growth factors or cytokines for fibrosis within the tissue,” Dr. Chung said. At the cellular level, pulsed dye laser alone can decrease connective tissue growth factor (CTGF), TGF-beta 1, proliferating cell nuclear antigen, and collagen III, and increases matrix metalloproteinase–13 (MMP-13), P53, ERK and p38 MAPK, apoptosis, blockade of AP-1 transcription, and cell cycle changes.
In 2004, plastic surgeons in Korea described a new approach for removing earlobe keloids, which they termed a “keloid fillet flap”. For the procedure, about 50% of the keloid margin is incised with a #15 scalpel blade. “Then you dissect the keloid from the surrounding tissue with a blade or curved scissors,” Dr. Chung said. “Next, you excise the keloid, so you have some dead space. After hemostasis you place the fillet flap to cover the wound. After you trim the redundant tissue, you can close it with epidermal sutures.”
In her clinical experience, she finds the fillet flap “very helpful for fast recovery” and it is associated with less pain. “Several studies have confirmed an excellent improvement of keloids, low recurrence rate, and rare side effects from a fillet flap and adjuvant intralesional corticosteroids. Occasionally, you may see flap necrosis but usually patients do well with topical antibiotics or petrolatum jelly.”
Dr. Chung also discussed her approach to treating papular scars in Asian patients. She described papular scars as underrecognized, anetoderma-like scars on the central face and trunk. “They comprise about 11% of all acne scars but up to 19% of patients with such scars may not recall a history of acne,” she said. Biopsies of papular scars reveal marked reduction or thinning of elastic fibers around hair follicles.
“Papular scars are difficult to treat,” she said. “If you have a conventional Er:YAG or CO2 laser, you can create tiny holes within the scars,” she said, referring to studies on these approaches. Another treatment option is needle-guided radiofrequency, she noted.
Dr. Chung reported having no relevant financial disclosures.
NEW ORLEANS – With the Asian population estimated to increase to 41 million by 2050 in the United States, expect the demand for experienced dermatologic care of patients with Asian skin to increase in the coming years, Hye Jin (Leah) Chung, MD, said at the annual meeting of the American Academy of Dermatology.
“Asians account for about 60% of the global population,” said Dr. Chung, assistant professor of dermatology at Harvard Medical School, and director of the Asian Skin Clinic at Beth Israel Deaconess Medical Center, Boston. Along with the estimate that Asians are expected to make up 25% of Canada’s population by 2036, “we will most likely encounter more Asian skin type patients in North America,” Dr. Chung said, noting that the Asian population “is very diverse, ranging from skin type 3 in Far East Asia to skin type 5 in India.”
During her presentation, she provided tips for treating hypertrophic scars and keloids in this patient population when intralesional corticosteroids fail. Typically, her first option is to combine an intralesional corticosteroid with 5-fluorouracil (5-FU), a pyrimidine analogue with antimetabolite activity. 5-FU “can cause cell apoptosis of endothelial cells and fibroblasts (which steroids cannot), cell cycle arrest, and TGF-beta [transforming growth factor beta]-induced COL1A2 transcription,” Dr. Chung said. The recommended ratio between 5-FU and steroids in the literature is variable, from a 9:1 ratio to a 1:1 ratio. “In my practice I do not inject more than 100 mg at a time,” she said. Several studies of this approach led by Asian investigators used weekly injections, “but that’s not practical in the U.S. I usually do monthly injections.”
A large systematic review and meta-analysis confirmed that the combination of intralesional triamcinolone acetonide and 5-FU achieved a better efficacy and fewer complications than triamcinolone alone for treating hypertrophic scars and keloids. Potential side effects from 5-FU injections include pain/pruritus, transient hyperpigmentation (especially in skin types 4-6), ulceration, teratogenicity, and transient alopecia.
A more recent meta-analysis comparing the efficacy of multiple drug injections for hypertrophic scars and keloids confirmed that the combination of triamcinolone and 5-FU was superior to bleomycin, verapamil, 5-FU alone, and triamcinolone alone. “And, there was no difference between 5-FU/steroid combination and botulinum toxin A,” Dr. Chung added. “Some parts of the world are using botulinum toxin with mixed results. Based on the amount of toxin required for keloids, this would be cost prohibitive in the U.S.”
Another approach to treating hypertrophic scars and keloids in Asian skin is laser-assisted drug delivery. “First, you can use a fractional ablative laser to create a hole in the epidermis and dermis,” Dr. Chung said. “Then you can apply the suspension topically to the holes. You can also use a steroid ointment or cream after laser treatment for drug delivery.”
Combining pulsed dye laser with steroid injections is another option. Pulsed dye lasers coagulate microvasculature within keloid tissue, “which can cause tissue hypoxia and can decrease growth factors or cytokines for fibrosis within the tissue,” Dr. Chung said. At the cellular level, pulsed dye laser alone can decrease connective tissue growth factor (CTGF), TGF-beta 1, proliferating cell nuclear antigen, and collagen III, and increases matrix metalloproteinase–13 (MMP-13), P53, ERK and p38 MAPK, apoptosis, blockade of AP-1 transcription, and cell cycle changes.
In 2004, plastic surgeons in Korea described a new approach for removing earlobe keloids, which they termed a “keloid fillet flap”. For the procedure, about 50% of the keloid margin is incised with a #15 scalpel blade. “Then you dissect the keloid from the surrounding tissue with a blade or curved scissors,” Dr. Chung said. “Next, you excise the keloid, so you have some dead space. After hemostasis you place the fillet flap to cover the wound. After you trim the redundant tissue, you can close it with epidermal sutures.”
In her clinical experience, she finds the fillet flap “very helpful for fast recovery” and it is associated with less pain. “Several studies have confirmed an excellent improvement of keloids, low recurrence rate, and rare side effects from a fillet flap and adjuvant intralesional corticosteroids. Occasionally, you may see flap necrosis but usually patients do well with topical antibiotics or petrolatum jelly.”
Dr. Chung also discussed her approach to treating papular scars in Asian patients. She described papular scars as underrecognized, anetoderma-like scars on the central face and trunk. “They comprise about 11% of all acne scars but up to 19% of patients with such scars may not recall a history of acne,” she said. Biopsies of papular scars reveal marked reduction or thinning of elastic fibers around hair follicles.
“Papular scars are difficult to treat,” she said. “If you have a conventional Er:YAG or CO2 laser, you can create tiny holes within the scars,” she said, referring to studies on these approaches. Another treatment option is needle-guided radiofrequency, she noted.
Dr. Chung reported having no relevant financial disclosures.
AT AAD 2023
Sleep duration of Black infants increased by intervention
An intervention tailored for Black first-time mothers helped increase their infants’ sleep time, researchers have found, a notable result as many studies have shown Black infants get less sleep on average than White infants.
Less sleep has historically put Black children at higher risk for negative outcomes including obesity and poorer social-emotional functioning and cognitive development. These disparities persist into adulthood, the researchers note, as previous studies have shown.
Justin A. Lavner, PhD, with the department of psychology at the University of Georgia in Athens, led this post hoc secondary analysis of the Sleep SAAF (Strong African American Families) study, a randomized clinical trial of 234 participants comparing a responsive parenting (RP) intervention with a safety control group over the first 16 weeks post partum. The original analysis studied the effects of the intervention on rapid weight gain.
In the original analysis, the authors write that “From birth to 2, the prevalence of high weight for length (above the 95th percentile) is 25% higher among African American children compared to White children. From age 2 to 19, the rate of obesity is more than 50% higher among African American children compared to White children. Similar disparities persist into adulthood: rates of obesity are approximately 25% higher among African American adults compared to White adults.”
The differences in early rapid weight gain may be driving the disparities, the authors write.
Elements of the intervention
The intervention in the current analysis included materials delivered at the 3- and 8-week home visits focused on soothing and crying, feeding, and interactive play in the babies’ first months. Families were recruited from Augusta University Medical Center in Augusta, Ga., and had home visits at 1, 3, 8, and 16 weeks post partum.
Mothers got a packet of handouts and facilitators walked through the information with them. The measures involved hands-on activities, discussion, and videos, all tailored for Black families, the authors state.
Mothers were taught about responding appropriately at night when their baby cries, including giving the baby a couple of minutes to fall back to sleep independently and by using calming messages, such as shushing or white noise, before picking the baby up.
Babies learn to fall asleep on their own
They also learned to put infants to bed early (ideally by 8 p.m.) so the babies would be calm but awake and could learn to fall asleep on their own.
The control group’s guidance was matched for intensity and session length but focused on sleep and home safety, such as reducing the risk of sudden infant death syndrome (SIDS), keeping the baby’s sleep area close to, but away from, the mother’s bed, and preventing shaken baby syndrome.
In both groups, the 3-week visit session lasted about 90-120 minutes and the 8-week visit lasted about 45-60 minutes.
Longer sleep with the intervention
A total of 212 Black mothers, average age 22.7, were randomized – 108 to the RP group and 104 to the control group. Answers on questionnaires were analyzed and at 16 weeks post partum, infants in the RP group (relative to controls) had:
- Longer reported nighttime sleep (mean difference, 40 minutes [95% confidence interval, 3-77]).
- Longer total sleep duration (mean difference, 73 minutes [95% CI, 14-131]).
- Fewer nighttime wakings (mean difference, −0.4 wakings [95% CI, −0.6 to −0.1]).
- Greater likelihood of meeting guidelines of at least 12 hours of sleep per day (risk ratio, 1.4 [95% CI, 1.1 to 1.8]) than controls.
Findings were published in JAMA Network Open.
Additionally, mothers in the RP group more frequently reported they engaged in practices such as letting babies have a few minutes to fall back to sleep on their own (RR, 1.6 [95% CI, 1.0-2.6]) and being less likely to feed their infant just before the baby’s bedtime (RR, 0.5 [95% CI, 0.3-0.8]).
In an accompanying invited commentary, Sarah M. Honaker, PhD, department of pediatrics, Indiana University, Indianapolis, and Alicia Chung, EdD, Center for Early Childhood Health and Development at New York University, write that though the added average sleep duration is one of the most significant findings, there is a possibility of desirability bias because it was reported by the mothers after specific guidance by the facilitators.
“Nonetheless,” the editorialists write, “even if the true effect were half as small, this additional sleep duration could yield notable benefits in infant development if the effect persisted over time. The difference in night wakings between the intervention and control groups (1.8 vs 1.5 per night) at 16 weeks postpartum was statistically significant, though it is unclear whether this difference is clinically meaningful to families.”
They note that it is unclear from the study how the intervention was culturally adapted and how the adaptation might have affected outcomes.
Sleep intervention trials have focused on White families
The editorialists write that much is known about the benefits of behavioral sleep intervention in controlled trials and general population settings, and no adverse effects on infant attachment or cortisol levels have been linked to the interventions.
However, they add, “Unfortunately, this substantial progress in our understanding of infant BSI [behavioral sleep intervention] comes with a caveat, in that most previous studies have been performed with White families from mid-to-high socioeconomic backgrounds.”
Dr. Honaker and Dr. Chung write, “[I]t is important to note that much work remains to examine the acceptability, feasibility, and efficacy of infant BSI in other groups that have been historically marginalized.”
Dr. Lavner and colleagues point out that before their study, there had been little emphasis on interventions to encourage better sleep in general for Black infants, “as most early sleep interventions for this population have focused on SIDS prevention.”
“To our knowledge, Sleep SAAF is the first study to show any benefits of [an] RP intervention on sleep and sleep practices among Black infants and their families,” they write.
The researchers note that a limitation of the study is that the study sample was limited to Black first-time mothers recruited from a single medical center in Georgia.
The study by Dr. Lavner et al. was funded by the National Institutes of Health, a Harrington Faculty Fellowship from the University of Texas, and an award from the Penn State Clinical and Translational Sciences Institute supported by the National Center for Advancing Translational Sciences. Editorialist Dr. Honaker reported receiving grants from Nationwide Children’s Hospital (parent grant, Centers for Disease Control and Prevention) to evaluate the acceptability of infant behavioral sleep intervention in Black families.
An intervention tailored for Black first-time mothers helped increase their infants’ sleep time, researchers have found, a notable result as many studies have shown Black infants get less sleep on average than White infants.
Less sleep has historically put Black children at higher risk for negative outcomes including obesity and poorer social-emotional functioning and cognitive development. These disparities persist into adulthood, the researchers note, as previous studies have shown.
Justin A. Lavner, PhD, with the department of psychology at the University of Georgia in Athens, led this post hoc secondary analysis of the Sleep SAAF (Strong African American Families) study, a randomized clinical trial of 234 participants comparing a responsive parenting (RP) intervention with a safety control group over the first 16 weeks post partum. The original analysis studied the effects of the intervention on rapid weight gain.
In the original analysis, the authors write that “From birth to 2, the prevalence of high weight for length (above the 95th percentile) is 25% higher among African American children compared to White children. From age 2 to 19, the rate of obesity is more than 50% higher among African American children compared to White children. Similar disparities persist into adulthood: rates of obesity are approximately 25% higher among African American adults compared to White adults.”
The differences in early rapid weight gain may be driving the disparities, the authors write.
Elements of the intervention
The intervention in the current analysis included materials delivered at the 3- and 8-week home visits focused on soothing and crying, feeding, and interactive play in the babies’ first months. Families were recruited from Augusta University Medical Center in Augusta, Ga., and had home visits at 1, 3, 8, and 16 weeks post partum.
Mothers got a packet of handouts and facilitators walked through the information with them. The measures involved hands-on activities, discussion, and videos, all tailored for Black families, the authors state.
Mothers were taught about responding appropriately at night when their baby cries, including giving the baby a couple of minutes to fall back to sleep independently and by using calming messages, such as shushing or white noise, before picking the baby up.
Babies learn to fall asleep on their own
They also learned to put infants to bed early (ideally by 8 p.m.) so the babies would be calm but awake and could learn to fall asleep on their own.
The control group’s guidance was matched for intensity and session length but focused on sleep and home safety, such as reducing the risk of sudden infant death syndrome (SIDS), keeping the baby’s sleep area close to, but away from, the mother’s bed, and preventing shaken baby syndrome.
In both groups, the 3-week visit session lasted about 90-120 minutes and the 8-week visit lasted about 45-60 minutes.
Longer sleep with the intervention
A total of 212 Black mothers, average age 22.7, were randomized – 108 to the RP group and 104 to the control group. Answers on questionnaires were analyzed and at 16 weeks post partum, infants in the RP group (relative to controls) had:
- Longer reported nighttime sleep (mean difference, 40 minutes [95% confidence interval, 3-77]).
- Longer total sleep duration (mean difference, 73 minutes [95% CI, 14-131]).
- Fewer nighttime wakings (mean difference, −0.4 wakings [95% CI, −0.6 to −0.1]).
- Greater likelihood of meeting guidelines of at least 12 hours of sleep per day (risk ratio, 1.4 [95% CI, 1.1 to 1.8]) than controls.
Findings were published in JAMA Network Open.
Additionally, mothers in the RP group more frequently reported they engaged in practices such as letting babies have a few minutes to fall back to sleep on their own (RR, 1.6 [95% CI, 1.0-2.6]) and being less likely to feed their infant just before the baby’s bedtime (RR, 0.5 [95% CI, 0.3-0.8]).
In an accompanying invited commentary, Sarah M. Honaker, PhD, department of pediatrics, Indiana University, Indianapolis, and Alicia Chung, EdD, Center for Early Childhood Health and Development at New York University, write that though the added average sleep duration is one of the most significant findings, there is a possibility of desirability bias because it was reported by the mothers after specific guidance by the facilitators.
“Nonetheless,” the editorialists write, “even if the true effect were half as small, this additional sleep duration could yield notable benefits in infant development if the effect persisted over time. The difference in night wakings between the intervention and control groups (1.8 vs 1.5 per night) at 16 weeks postpartum was statistically significant, though it is unclear whether this difference is clinically meaningful to families.”
They note that it is unclear from the study how the intervention was culturally adapted and how the adaptation might have affected outcomes.
Sleep intervention trials have focused on White families
The editorialists write that much is known about the benefits of behavioral sleep intervention in controlled trials and general population settings, and no adverse effects on infant attachment or cortisol levels have been linked to the interventions.
However, they add, “Unfortunately, this substantial progress in our understanding of infant BSI [behavioral sleep intervention] comes with a caveat, in that most previous studies have been performed with White families from mid-to-high socioeconomic backgrounds.”
Dr. Honaker and Dr. Chung write, “[I]t is important to note that much work remains to examine the acceptability, feasibility, and efficacy of infant BSI in other groups that have been historically marginalized.”
Dr. Lavner and colleagues point out that before their study, there had been little emphasis on interventions to encourage better sleep in general for Black infants, “as most early sleep interventions for this population have focused on SIDS prevention.”
“To our knowledge, Sleep SAAF is the first study to show any benefits of [an] RP intervention on sleep and sleep practices among Black infants and their families,” they write.
The researchers note that a limitation of the study is that the study sample was limited to Black first-time mothers recruited from a single medical center in Georgia.
The study by Dr. Lavner et al. was funded by the National Institutes of Health, a Harrington Faculty Fellowship from the University of Texas, and an award from the Penn State Clinical and Translational Sciences Institute supported by the National Center for Advancing Translational Sciences. Editorialist Dr. Honaker reported receiving grants from Nationwide Children’s Hospital (parent grant, Centers for Disease Control and Prevention) to evaluate the acceptability of infant behavioral sleep intervention in Black families.
An intervention tailored for Black first-time mothers helped increase their infants’ sleep time, researchers have found, a notable result as many studies have shown Black infants get less sleep on average than White infants.
Less sleep has historically put Black children at higher risk for negative outcomes including obesity and poorer social-emotional functioning and cognitive development. These disparities persist into adulthood, the researchers note, as previous studies have shown.
Justin A. Lavner, PhD, with the department of psychology at the University of Georgia in Athens, led this post hoc secondary analysis of the Sleep SAAF (Strong African American Families) study, a randomized clinical trial of 234 participants comparing a responsive parenting (RP) intervention with a safety control group over the first 16 weeks post partum. The original analysis studied the effects of the intervention on rapid weight gain.
In the original analysis, the authors write that “From birth to 2, the prevalence of high weight for length (above the 95th percentile) is 25% higher among African American children compared to White children. From age 2 to 19, the rate of obesity is more than 50% higher among African American children compared to White children. Similar disparities persist into adulthood: rates of obesity are approximately 25% higher among African American adults compared to White adults.”
The differences in early rapid weight gain may be driving the disparities, the authors write.
Elements of the intervention
The intervention in the current analysis included materials delivered at the 3- and 8-week home visits focused on soothing and crying, feeding, and interactive play in the babies’ first months. Families were recruited from Augusta University Medical Center in Augusta, Ga., and had home visits at 1, 3, 8, and 16 weeks post partum.
Mothers got a packet of handouts and facilitators walked through the information with them. The measures involved hands-on activities, discussion, and videos, all tailored for Black families, the authors state.
Mothers were taught about responding appropriately at night when their baby cries, including giving the baby a couple of minutes to fall back to sleep independently and by using calming messages, such as shushing or white noise, before picking the baby up.
Babies learn to fall asleep on their own
They also learned to put infants to bed early (ideally by 8 p.m.) so the babies would be calm but awake and could learn to fall asleep on their own.
The control group’s guidance was matched for intensity and session length but focused on sleep and home safety, such as reducing the risk of sudden infant death syndrome (SIDS), keeping the baby’s sleep area close to, but away from, the mother’s bed, and preventing shaken baby syndrome.
In both groups, the 3-week visit session lasted about 90-120 minutes and the 8-week visit lasted about 45-60 minutes.
Longer sleep with the intervention
A total of 212 Black mothers, average age 22.7, were randomized – 108 to the RP group and 104 to the control group. Answers on questionnaires were analyzed and at 16 weeks post partum, infants in the RP group (relative to controls) had:
- Longer reported nighttime sleep (mean difference, 40 minutes [95% confidence interval, 3-77]).
- Longer total sleep duration (mean difference, 73 minutes [95% CI, 14-131]).
- Fewer nighttime wakings (mean difference, −0.4 wakings [95% CI, −0.6 to −0.1]).
- Greater likelihood of meeting guidelines of at least 12 hours of sleep per day (risk ratio, 1.4 [95% CI, 1.1 to 1.8]) than controls.
Findings were published in JAMA Network Open.
Additionally, mothers in the RP group more frequently reported they engaged in practices such as letting babies have a few minutes to fall back to sleep on their own (RR, 1.6 [95% CI, 1.0-2.6]) and being less likely to feed their infant just before the baby’s bedtime (RR, 0.5 [95% CI, 0.3-0.8]).
In an accompanying invited commentary, Sarah M. Honaker, PhD, department of pediatrics, Indiana University, Indianapolis, and Alicia Chung, EdD, Center for Early Childhood Health and Development at New York University, write that though the added average sleep duration is one of the most significant findings, there is a possibility of desirability bias because it was reported by the mothers after specific guidance by the facilitators.
“Nonetheless,” the editorialists write, “even if the true effect were half as small, this additional sleep duration could yield notable benefits in infant development if the effect persisted over time. The difference in night wakings between the intervention and control groups (1.8 vs 1.5 per night) at 16 weeks postpartum was statistically significant, though it is unclear whether this difference is clinically meaningful to families.”
They note that it is unclear from the study how the intervention was culturally adapted and how the adaptation might have affected outcomes.
Sleep intervention trials have focused on White families
The editorialists write that much is known about the benefits of behavioral sleep intervention in controlled trials and general population settings, and no adverse effects on infant attachment or cortisol levels have been linked to the interventions.
However, they add, “Unfortunately, this substantial progress in our understanding of infant BSI [behavioral sleep intervention] comes with a caveat, in that most previous studies have been performed with White families from mid-to-high socioeconomic backgrounds.”
Dr. Honaker and Dr. Chung write, “[I]t is important to note that much work remains to examine the acceptability, feasibility, and efficacy of infant BSI in other groups that have been historically marginalized.”
Dr. Lavner and colleagues point out that before their study, there had been little emphasis on interventions to encourage better sleep in general for Black infants, “as most early sleep interventions for this population have focused on SIDS prevention.”
“To our knowledge, Sleep SAAF is the first study to show any benefits of [an] RP intervention on sleep and sleep practices among Black infants and their families,” they write.
The researchers note that a limitation of the study is that the study sample was limited to Black first-time mothers recruited from a single medical center in Georgia.
The study by Dr. Lavner et al. was funded by the National Institutes of Health, a Harrington Faculty Fellowship from the University of Texas, and an award from the Penn State Clinical and Translational Sciences Institute supported by the National Center for Advancing Translational Sciences. Editorialist Dr. Honaker reported receiving grants from Nationwide Children’s Hospital (parent grant, Centers for Disease Control and Prevention) to evaluate the acceptability of infant behavioral sleep intervention in Black families.
FROM JAMA NETWORK OPEN
Disparities in statin use persist in high-risk Americans
Disparities in statin use in minority populations persist regardless of insurance status and 10-year atherosclerotic cardiovascular disease risk.
Those are among the findings of a study that sampled a national population database and has provided robust data and granular details on those disparities.
The researchers reported in JAMA Cardiology that the overall prevalence of statin use was 25.5%, and that it varied significantly between defined ethnic groups: 20% for Blacks, 15.4% for Hispanics, and 27.9% for Whites (P < .001). Statin use rates by Asian participants, at 25.5%, didn’t differ significantly from use by Whites.
“We know that there are racial and ethnic disparities in the use of guideline-indicated statins after having established heart disease, but it was unknown if these disparities existed in the use of guideline-indicated statins for prevention of heart disease in those who just have risk factors,” lead author Joshua Jacobs, PharmD, a clinical pharmacist of cardiovascular medicine at University of Utah Intermountain Healthcare, said in written comments. “Additionally, race is included in the guideline-recommended risk factor calculation in an effort to reduce these disparities.”
Dr. Jacobs and colleagues evaluated statins for use in primary prevention, building upon previous single-center or diabetes-only cohort studies. What makes their study different from previous studies evaluating disparities in statin use is its use of temporal trends or current 10-year predicted ASCVD risk categorization, he said.
Using data from the National Health and Nutrition Examination Survey (NHANES), the researchers performed a serial, cross-sectional analysis of 3,417 participants that they said represented 39.4 million U.S. adults after applying sampling weights for age, gender, and race and ethnicity. In the weighted sample, 62.2% were men. In terms of self-reported race and ethnicity, 4.2% were of Asian descent, 12.7% were Black, 10.1% were Hispanic, and 73% were White.
Study participants completed a standardized questionnaire given by trained interviewers and also went to mobile examination centers where physical, anthropomorphic, and laboratory measurements, including height, weight, LDL cholesterol, and fasting blood glucose were collected. Pill bottle review also verified participants’ self-reported medication use.
The study noted that for primary prevention of atherosclerotic cardiovascular disease (ASCVD), the 2018 American College of Cardiology/American Heart Association Guideline recommends statins for, among other patient factors, elevated 10-year predicted ASCVD risk. The study divided ASCVD risk strata into three groups – 5% to less than 7.5%, 7.5% to less than 20%, and more than 20% – based on the 2018 ACC/AHA guideline and used pooled cohort equation to calculate 10-year ASCVD risk, which the guideline endorses.
Gaps persist despite ASCVD risk
The analysis found no statistically significant difference within each ASCVD risk strata between the White and Asian groups. But although statin use increased proportionately across each higher risk group, the gap widened noticeably in the highest risk group (more than 20% 10-year risk) between Whites, used as the reference at 37.6%, and Blacks (23.8%; prevalence ratio, .90; 95% confidence interval, .82-.98) and Hispanics (23.9%; PR, .90; 95% CI, .81-.99).
The study also evaluated a number of social determinants of health factors. Health insurance and access to routine health care were significantly associated with greater statin use in Black, Hispanic, and White participants; marital status and food insecurity were not. However, even when variables such as education, household income, and health insurance were applied, statin use was still significantly higher in Whites than in Blacks and Hispanics. For those with health insurance, statin use was 28.6% (95% CI, 25-32), 21.1% (95% CI, 17.3-25.4) and 19.9% (95% CI, 15.9-24.5), respectively.
The study noted that the pooled cohort equation-guided approach to statins for primary prevention, which the 2018 ACC/AHA guideline endorsed, should promote greater use of statins among Black patients. “Equitable use of statin therapy for prevention of heart disease is needed for Black and Hispanic adults,” Dr. Jacobs said. “Improvements in access to care, such as having a routine primary care clinician and health insurance, may decrease these health disparities.”
A goal of the study was to identify if disparities in statin use held up across different risk groups, senior author Ambarish Pandey, MD, said in an interview. Use of the NHANES data makes this study unique among analyses of statin use disparities, he said.
“A lot of the work that has been done previously has focused on secondary prevention among patients who have atherosclerotic cardiovascular disease or have focused on single-center or hospital-based cohorts and have not really focused on a national representative cohort like NHANES,” said Dr. Pandey, of the UT Southwestern Medical Center, Dallas.
The next step is to do community-based participatory research focusing on different implementation strategies to increase the uptake of preventive statin use among Black and Hispanic communities, Dr. Jacobs said.
Dr. Jacobs has no relevant relationships to disclose. Dr. Pandey disclosed relationships with Gilead Sciences, Applied Therapeutics, Myovista, Tricog Health, Eli Lilly, Cytokinetics, Rivus, Roche Diagnostics, Pieces Technologies, Palomarin, Emmi Solutions, and Axon.
Disparities in statin use in minority populations persist regardless of insurance status and 10-year atherosclerotic cardiovascular disease risk.
Those are among the findings of a study that sampled a national population database and has provided robust data and granular details on those disparities.
The researchers reported in JAMA Cardiology that the overall prevalence of statin use was 25.5%, and that it varied significantly between defined ethnic groups: 20% for Blacks, 15.4% for Hispanics, and 27.9% for Whites (P < .001). Statin use rates by Asian participants, at 25.5%, didn’t differ significantly from use by Whites.
“We know that there are racial and ethnic disparities in the use of guideline-indicated statins after having established heart disease, but it was unknown if these disparities existed in the use of guideline-indicated statins for prevention of heart disease in those who just have risk factors,” lead author Joshua Jacobs, PharmD, a clinical pharmacist of cardiovascular medicine at University of Utah Intermountain Healthcare, said in written comments. “Additionally, race is included in the guideline-recommended risk factor calculation in an effort to reduce these disparities.”
Dr. Jacobs and colleagues evaluated statins for use in primary prevention, building upon previous single-center or diabetes-only cohort studies. What makes their study different from previous studies evaluating disparities in statin use is its use of temporal trends or current 10-year predicted ASCVD risk categorization, he said.
Using data from the National Health and Nutrition Examination Survey (NHANES), the researchers performed a serial, cross-sectional analysis of 3,417 participants that they said represented 39.4 million U.S. adults after applying sampling weights for age, gender, and race and ethnicity. In the weighted sample, 62.2% were men. In terms of self-reported race and ethnicity, 4.2% were of Asian descent, 12.7% were Black, 10.1% were Hispanic, and 73% were White.
Study participants completed a standardized questionnaire given by trained interviewers and also went to mobile examination centers where physical, anthropomorphic, and laboratory measurements, including height, weight, LDL cholesterol, and fasting blood glucose were collected. Pill bottle review also verified participants’ self-reported medication use.
The study noted that for primary prevention of atherosclerotic cardiovascular disease (ASCVD), the 2018 American College of Cardiology/American Heart Association Guideline recommends statins for, among other patient factors, elevated 10-year predicted ASCVD risk. The study divided ASCVD risk strata into three groups – 5% to less than 7.5%, 7.5% to less than 20%, and more than 20% – based on the 2018 ACC/AHA guideline and used pooled cohort equation to calculate 10-year ASCVD risk, which the guideline endorses.
Gaps persist despite ASCVD risk
The analysis found no statistically significant difference within each ASCVD risk strata between the White and Asian groups. But although statin use increased proportionately across each higher risk group, the gap widened noticeably in the highest risk group (more than 20% 10-year risk) between Whites, used as the reference at 37.6%, and Blacks (23.8%; prevalence ratio, .90; 95% confidence interval, .82-.98) and Hispanics (23.9%; PR, .90; 95% CI, .81-.99).
The study also evaluated a number of social determinants of health factors. Health insurance and access to routine health care were significantly associated with greater statin use in Black, Hispanic, and White participants; marital status and food insecurity were not. However, even when variables such as education, household income, and health insurance were applied, statin use was still significantly higher in Whites than in Blacks and Hispanics. For those with health insurance, statin use was 28.6% (95% CI, 25-32), 21.1% (95% CI, 17.3-25.4) and 19.9% (95% CI, 15.9-24.5), respectively.
The study noted that the pooled cohort equation-guided approach to statins for primary prevention, which the 2018 ACC/AHA guideline endorsed, should promote greater use of statins among Black patients. “Equitable use of statin therapy for prevention of heart disease is needed for Black and Hispanic adults,” Dr. Jacobs said. “Improvements in access to care, such as having a routine primary care clinician and health insurance, may decrease these health disparities.”
A goal of the study was to identify if disparities in statin use held up across different risk groups, senior author Ambarish Pandey, MD, said in an interview. Use of the NHANES data makes this study unique among analyses of statin use disparities, he said.
“A lot of the work that has been done previously has focused on secondary prevention among patients who have atherosclerotic cardiovascular disease or have focused on single-center or hospital-based cohorts and have not really focused on a national representative cohort like NHANES,” said Dr. Pandey, of the UT Southwestern Medical Center, Dallas.
The next step is to do community-based participatory research focusing on different implementation strategies to increase the uptake of preventive statin use among Black and Hispanic communities, Dr. Jacobs said.
Dr. Jacobs has no relevant relationships to disclose. Dr. Pandey disclosed relationships with Gilead Sciences, Applied Therapeutics, Myovista, Tricog Health, Eli Lilly, Cytokinetics, Rivus, Roche Diagnostics, Pieces Technologies, Palomarin, Emmi Solutions, and Axon.
Disparities in statin use in minority populations persist regardless of insurance status and 10-year atherosclerotic cardiovascular disease risk.
Those are among the findings of a study that sampled a national population database and has provided robust data and granular details on those disparities.
The researchers reported in JAMA Cardiology that the overall prevalence of statin use was 25.5%, and that it varied significantly between defined ethnic groups: 20% for Blacks, 15.4% for Hispanics, and 27.9% for Whites (P < .001). Statin use rates by Asian participants, at 25.5%, didn’t differ significantly from use by Whites.
“We know that there are racial and ethnic disparities in the use of guideline-indicated statins after having established heart disease, but it was unknown if these disparities existed in the use of guideline-indicated statins for prevention of heart disease in those who just have risk factors,” lead author Joshua Jacobs, PharmD, a clinical pharmacist of cardiovascular medicine at University of Utah Intermountain Healthcare, said in written comments. “Additionally, race is included in the guideline-recommended risk factor calculation in an effort to reduce these disparities.”
Dr. Jacobs and colleagues evaluated statins for use in primary prevention, building upon previous single-center or diabetes-only cohort studies. What makes their study different from previous studies evaluating disparities in statin use is its use of temporal trends or current 10-year predicted ASCVD risk categorization, he said.
Using data from the National Health and Nutrition Examination Survey (NHANES), the researchers performed a serial, cross-sectional analysis of 3,417 participants that they said represented 39.4 million U.S. adults after applying sampling weights for age, gender, and race and ethnicity. In the weighted sample, 62.2% were men. In terms of self-reported race and ethnicity, 4.2% were of Asian descent, 12.7% were Black, 10.1% were Hispanic, and 73% were White.
Study participants completed a standardized questionnaire given by trained interviewers and also went to mobile examination centers where physical, anthropomorphic, and laboratory measurements, including height, weight, LDL cholesterol, and fasting blood glucose were collected. Pill bottle review also verified participants’ self-reported medication use.
The study noted that for primary prevention of atherosclerotic cardiovascular disease (ASCVD), the 2018 American College of Cardiology/American Heart Association Guideline recommends statins for, among other patient factors, elevated 10-year predicted ASCVD risk. The study divided ASCVD risk strata into three groups – 5% to less than 7.5%, 7.5% to less than 20%, and more than 20% – based on the 2018 ACC/AHA guideline and used pooled cohort equation to calculate 10-year ASCVD risk, which the guideline endorses.
Gaps persist despite ASCVD risk
The analysis found no statistically significant difference within each ASCVD risk strata between the White and Asian groups. But although statin use increased proportionately across each higher risk group, the gap widened noticeably in the highest risk group (more than 20% 10-year risk) between Whites, used as the reference at 37.6%, and Blacks (23.8%; prevalence ratio, .90; 95% confidence interval, .82-.98) and Hispanics (23.9%; PR, .90; 95% CI, .81-.99).
The study also evaluated a number of social determinants of health factors. Health insurance and access to routine health care were significantly associated with greater statin use in Black, Hispanic, and White participants; marital status and food insecurity were not. However, even when variables such as education, household income, and health insurance were applied, statin use was still significantly higher in Whites than in Blacks and Hispanics. For those with health insurance, statin use was 28.6% (95% CI, 25-32), 21.1% (95% CI, 17.3-25.4) and 19.9% (95% CI, 15.9-24.5), respectively.
The study noted that the pooled cohort equation-guided approach to statins for primary prevention, which the 2018 ACC/AHA guideline endorsed, should promote greater use of statins among Black patients. “Equitable use of statin therapy for prevention of heart disease is needed for Black and Hispanic adults,” Dr. Jacobs said. “Improvements in access to care, such as having a routine primary care clinician and health insurance, may decrease these health disparities.”
A goal of the study was to identify if disparities in statin use held up across different risk groups, senior author Ambarish Pandey, MD, said in an interview. Use of the NHANES data makes this study unique among analyses of statin use disparities, he said.
“A lot of the work that has been done previously has focused on secondary prevention among patients who have atherosclerotic cardiovascular disease or have focused on single-center or hospital-based cohorts and have not really focused on a national representative cohort like NHANES,” said Dr. Pandey, of the UT Southwestern Medical Center, Dallas.
The next step is to do community-based participatory research focusing on different implementation strategies to increase the uptake of preventive statin use among Black and Hispanic communities, Dr. Jacobs said.
Dr. Jacobs has no relevant relationships to disclose. Dr. Pandey disclosed relationships with Gilead Sciences, Applied Therapeutics, Myovista, Tricog Health, Eli Lilly, Cytokinetics, Rivus, Roche Diagnostics, Pieces Technologies, Palomarin, Emmi Solutions, and Axon.
FROM JAMA CARDIOLOGY
Pilot study evaluates sensitive skin burden in persons of color
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
AT AAD 2023
Mediterranean diet linked to 24% reduction in CVD risk in women
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Racial morphing: A conundrum in cosmetic dermatology
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR