User login
Antidepressants for pediatric patients
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
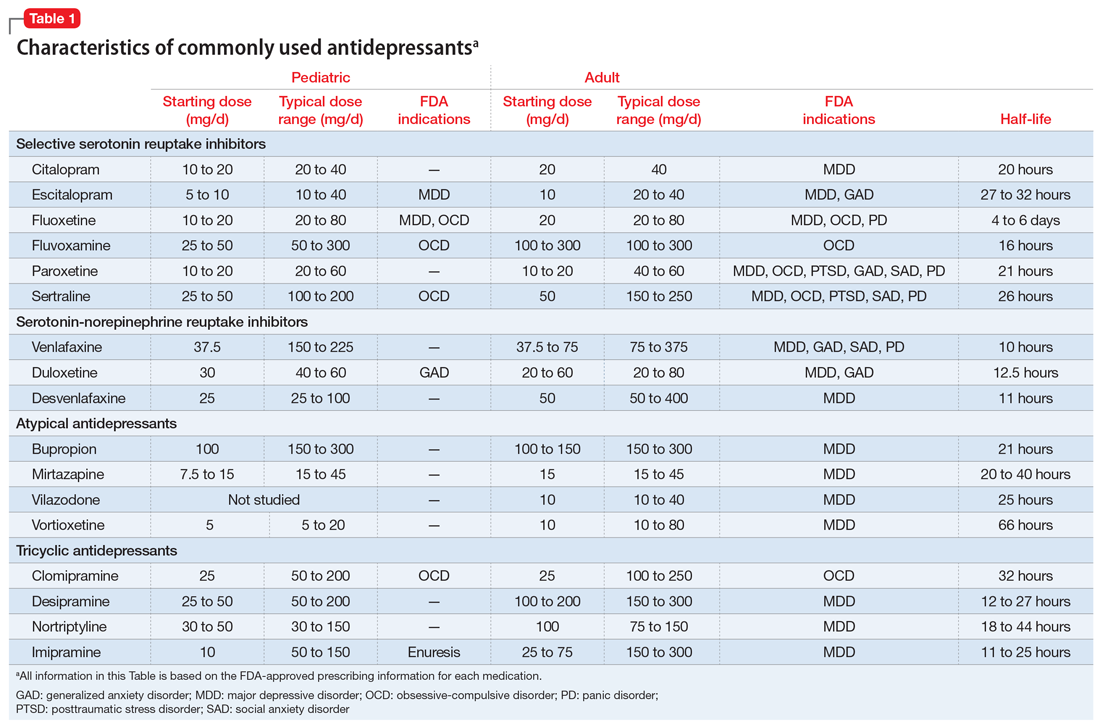
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.
There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).
Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.
In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.
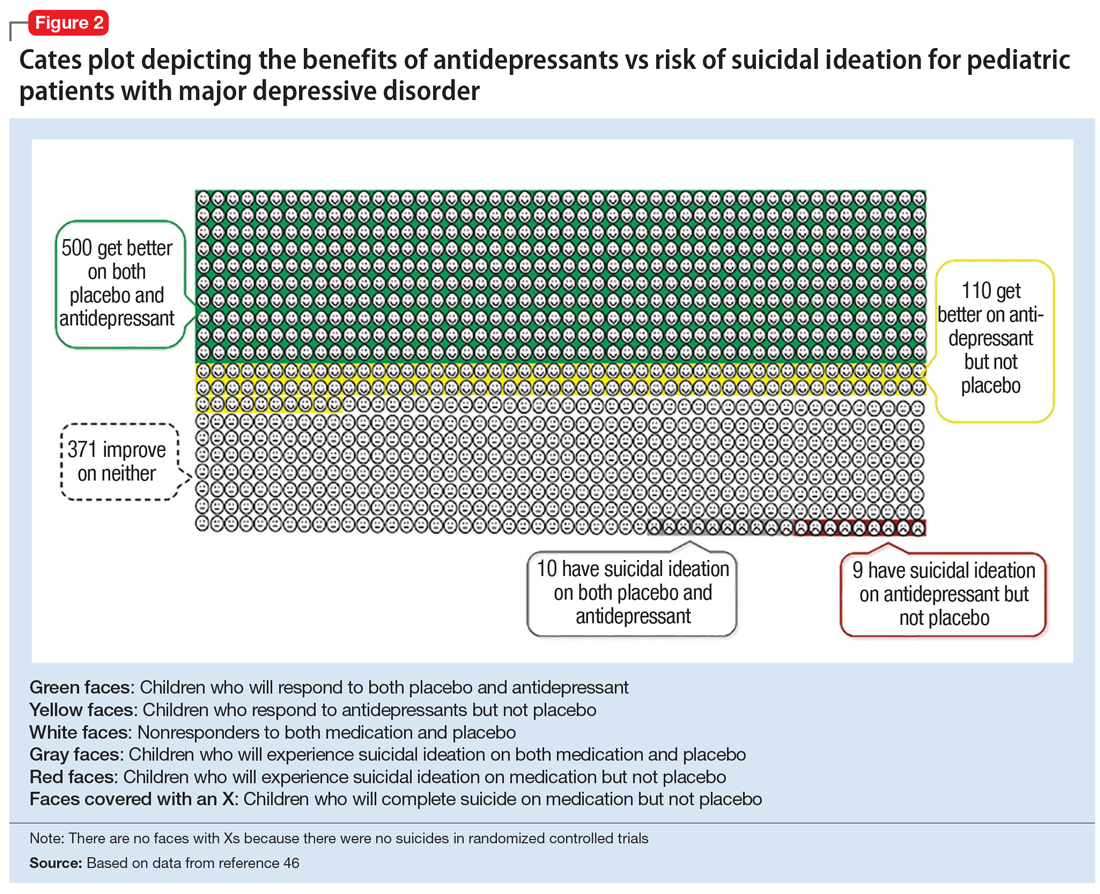
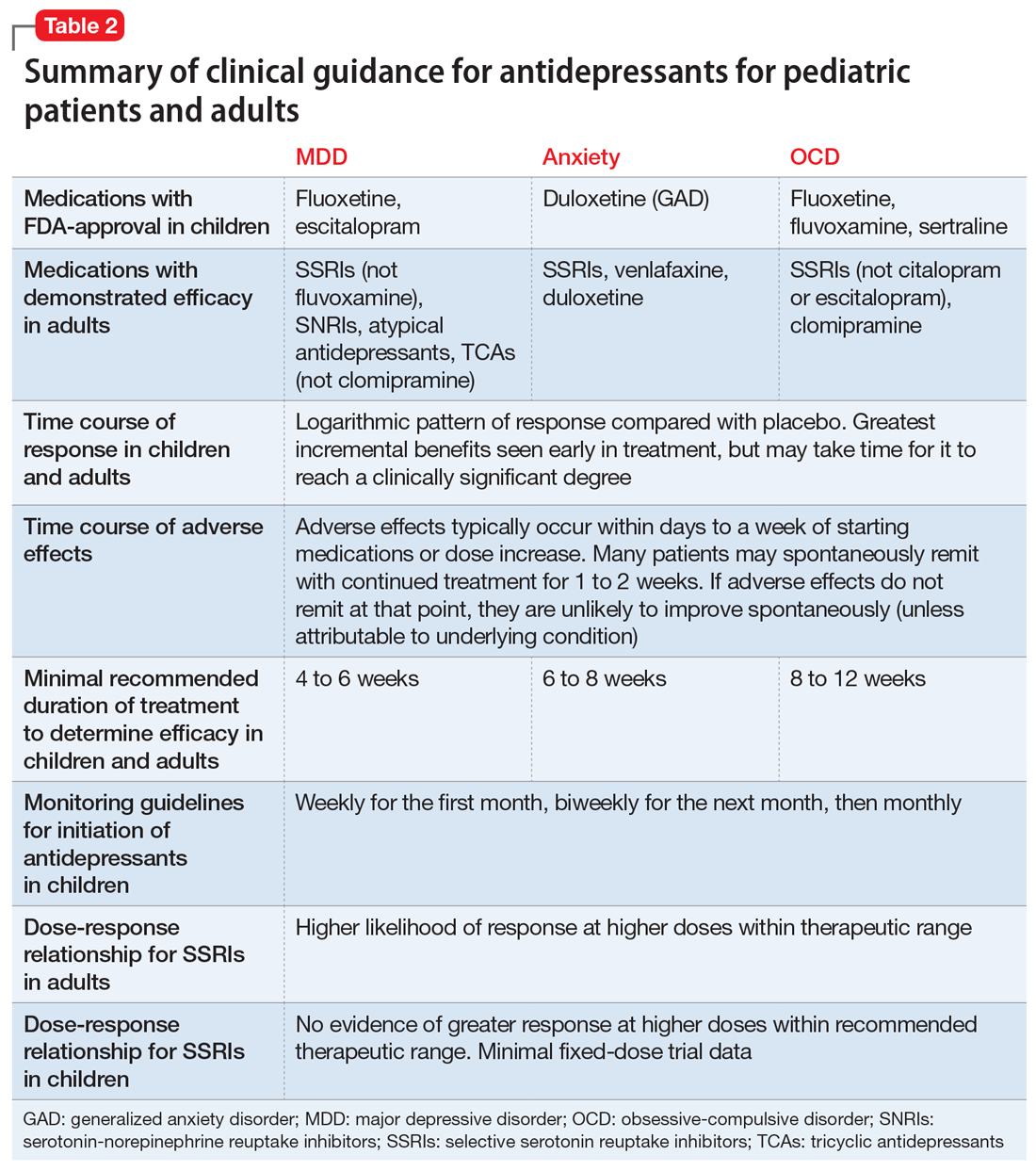
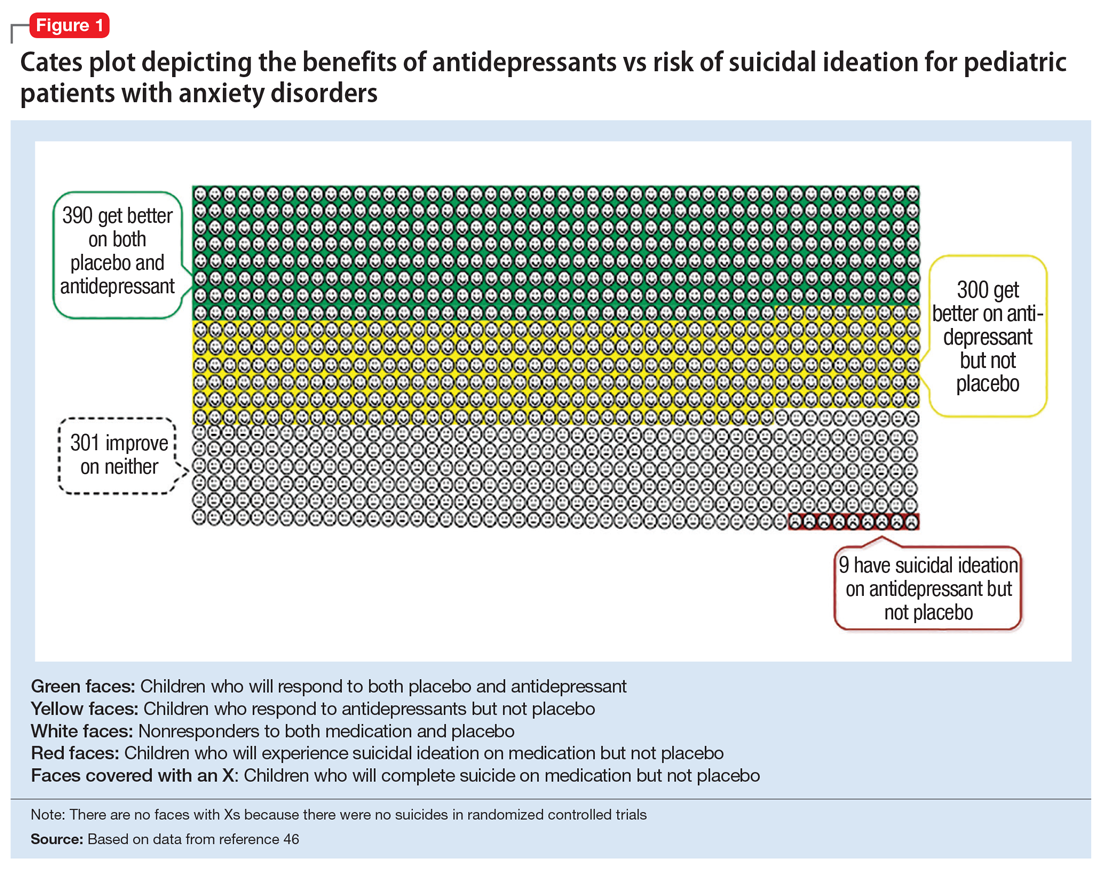
Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.
Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.

There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).

Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.

In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.

Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.

Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.

There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).

Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.

In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.

Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.

Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Combo therapy outcomes for West syndrome prove no better than monotherapy
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM IEC 2019
FDA approves istradefylline for Parkinson’s disease
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
Prices, out-of-pocket costs for MS drugs rose despite competition
according to an analysis published in JAMA Neurology. The increased prices raise concern “because they demonstrate that the approval of new therapies did not ameliorate and could have even contributed to high inflation rates observed for incumbent drugs,” wrote the authors.
Four self-administered disease-modifying therapies (DMTs) for multiple sclerosis (MS) were available before 2009, and seven new branded DMTs were introduced after that year. Previous research indicated that the prices of DMTs for MS increased at higher rates than the prices of drugs for other disorders. How these price increases affected pharmaceutical spending during the past decade is uncertain, however.
A review of Medicare claims data
Alvaro San-Juan-Rodriguez, PharmD, a fellow in pharmacoeconomics, outcomes, and pharmacoanalytics research at the University of Pittsburgh, and colleagues examined claims data from 2006 to 2016 from a 5% random sample of Medicare beneficiaries. Information for a mean of 2.8 million Medicare beneficiaries per year was available. The researchers extracted all prescription claims for self-administered DMTs for MS (that is, glatiramer acetate, interferon beta-1a, interferon beta-1b, fingolimod, teriflunomide, dimethyl fumarate, and peginterferon beta-1a).
Dr. San-Juan-Rodriguez and associates chose three main outcomes. The first was the annual cost of treatment with each medication, which was based on Medicare Part D prescription claims gross costs and Food and Drug Administration–approved recommended dosing. The second was the market share of each medication, which the researchers defined as the proportion of pharmaceutical spending accounted for by each drug. The third was pharmaceutical spending per 1,000 Medicare beneficiaries for all drugs. The investigators also examined the relative contributions of Medicare Part D Plans’ payments, patients’ out-of-pocket costs, and other payments toward pharmaceutical spending.
Prices defied market expectations
The annual costs of treatment with self-administered DMTs for MS increased more than 300%. The mean annual cost was $18,660 in 2006 and $75,847 in 2016, and the mean annual rate of price increase was 12.8%. “Prices of most self-administered DMTs for MS increased in parallel, defying standard market expectations,” the investigators wrote.
Branded formulations of glatiramer acetate maintained the largest market share throughout the study period, ranging between 32.2% and 48.4%. However, the market share of platform therapies – glatiramer acetate, interferon beta-1a, and interferon beta-1b – decreased significantly from 2006 to 2016. Market shares for brand-name glatiramers declined from 36.7% to 32.2%, for intramuscular interferon beta-1a (30 mcg) from 32.3% to 14.2%, for interferon beta-1b from 18.7% to 4.5%, and for interferon beta-1a (8.8, 22, or 44 mcg) from 12.2% to 8.3%. The market shares of newer therapies, however, increased to 7.9% for fingolimod, 9.0% for teriflunomide, and 19.2% for dimethyl fumarate.
Pharmaceutical spending per 1,000 beneficiaries increased by a factor of 10.2 throughout the study period (from $7,794 to $79,411). Patients’ out-of-pocket spending per 1,000 beneficiaries increased by a factor of 7.2 (from $372 to $2,673). Furthermore, the relative contribution of federal payments toward pharmaceutical spending increased from 68.5% to 73.8%.
“Large increases in drug prices have not been specific to MS drugs,” said Dr. San-Juan-Rodriguez in an interview. “We previously described similar trends in other specialty medications used to treat severe disease states, such as tumor necrosis factor inhibitors [TNFi] for the treatment of rheumatoid arthritis. Yet these increases took place at a slower pace. For instance, list prices of TNFi increased at an average annual rate of 9.9% in the same time period, 2006-2016.
“It is important to acknowledge that rising list prices of drugs may partially reflect competition for rebates,” he added. “Yet the specific reasons behind the faster growth of prices of MS drugs, compared with the prices of drugs used in other disease states, remain uncertain.”
Neurologists should bear in mind that, although generic drugs are substantially cheaper than branded drugs, generic specialty medications do not always reduce costs for Medicare Part D beneficiaries. “On the contrary, due to incentive misalignments created by the Medicare Part D benefit design, beneficiaries using generic drugs such as Glatopa ... may pay more than those using the branded drug,” Dr. San-Juan-Rodriguez said.
What are neurologists’ responsibilities?
Although the original annual price of interferon beta-1b ($10,920) was stunning, physicians now recall it with nostalgia, wrote Daniel M. Hartung, PharmD, associate professor of biostatistics and epidemiology, and Dennis Bourdette, MD, professor of neurology, both at Oregon Health and Science University, Portland, in an accompanying editorial. “The prices for DMTs for MS have risen dramatically over the last 15 years, far outpacing inflation, and now have a mean price of more than $86,000 per year.”
Neurologists should be concerned about these rising prices, Dr. Hartung and Dr. Bourdette wrote. They should feel responsibility toward the health care system that pays for these medications, and toward patients who pay out of their own pockets. “Neurologists should be seeking to minimize the financial adverse effects of these therapies as much as they try to minimize physical adverse effects.”
One way for neurologists to address increasing prices is to urge state and federal lawmakers to pass legislation to curb them, they wrote. Neurologists also should reexamine their relationships with pharmaceutical and biotechnology companies. “Remaining silent should not be an option. ... Neurologists should not allow the unfettered increases in price for these drugs to hurt the health care system or patients.”
The Myers Family Foundation and the National Heart, Lung, and Blood Institute funded the research. Several authors are employees of health insurance companies such as the UPMC Health Plan Insurance Services Division and Humana. One author received personal fees from Pfizer that were unrelated to this study.
SOURCEs: San-Juan-Rodriguez A et al. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2711; Hartung DM and Bourdette D. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2445.
according to an analysis published in JAMA Neurology. The increased prices raise concern “because they demonstrate that the approval of new therapies did not ameliorate and could have even contributed to high inflation rates observed for incumbent drugs,” wrote the authors.
Four self-administered disease-modifying therapies (DMTs) for multiple sclerosis (MS) were available before 2009, and seven new branded DMTs were introduced after that year. Previous research indicated that the prices of DMTs for MS increased at higher rates than the prices of drugs for other disorders. How these price increases affected pharmaceutical spending during the past decade is uncertain, however.
A review of Medicare claims data
Alvaro San-Juan-Rodriguez, PharmD, a fellow in pharmacoeconomics, outcomes, and pharmacoanalytics research at the University of Pittsburgh, and colleagues examined claims data from 2006 to 2016 from a 5% random sample of Medicare beneficiaries. Information for a mean of 2.8 million Medicare beneficiaries per year was available. The researchers extracted all prescription claims for self-administered DMTs for MS (that is, glatiramer acetate, interferon beta-1a, interferon beta-1b, fingolimod, teriflunomide, dimethyl fumarate, and peginterferon beta-1a).
Dr. San-Juan-Rodriguez and associates chose three main outcomes. The first was the annual cost of treatment with each medication, which was based on Medicare Part D prescription claims gross costs and Food and Drug Administration–approved recommended dosing. The second was the market share of each medication, which the researchers defined as the proportion of pharmaceutical spending accounted for by each drug. The third was pharmaceutical spending per 1,000 Medicare beneficiaries for all drugs. The investigators also examined the relative contributions of Medicare Part D Plans’ payments, patients’ out-of-pocket costs, and other payments toward pharmaceutical spending.
Prices defied market expectations
The annual costs of treatment with self-administered DMTs for MS increased more than 300%. The mean annual cost was $18,660 in 2006 and $75,847 in 2016, and the mean annual rate of price increase was 12.8%. “Prices of most self-administered DMTs for MS increased in parallel, defying standard market expectations,” the investigators wrote.
Branded formulations of glatiramer acetate maintained the largest market share throughout the study period, ranging between 32.2% and 48.4%. However, the market share of platform therapies – glatiramer acetate, interferon beta-1a, and interferon beta-1b – decreased significantly from 2006 to 2016. Market shares for brand-name glatiramers declined from 36.7% to 32.2%, for intramuscular interferon beta-1a (30 mcg) from 32.3% to 14.2%, for interferon beta-1b from 18.7% to 4.5%, and for interferon beta-1a (8.8, 22, or 44 mcg) from 12.2% to 8.3%. The market shares of newer therapies, however, increased to 7.9% for fingolimod, 9.0% for teriflunomide, and 19.2% for dimethyl fumarate.
Pharmaceutical spending per 1,000 beneficiaries increased by a factor of 10.2 throughout the study period (from $7,794 to $79,411). Patients’ out-of-pocket spending per 1,000 beneficiaries increased by a factor of 7.2 (from $372 to $2,673). Furthermore, the relative contribution of federal payments toward pharmaceutical spending increased from 68.5% to 73.8%.
“Large increases in drug prices have not been specific to MS drugs,” said Dr. San-Juan-Rodriguez in an interview. “We previously described similar trends in other specialty medications used to treat severe disease states, such as tumor necrosis factor inhibitors [TNFi] for the treatment of rheumatoid arthritis. Yet these increases took place at a slower pace. For instance, list prices of TNFi increased at an average annual rate of 9.9% in the same time period, 2006-2016.
“It is important to acknowledge that rising list prices of drugs may partially reflect competition for rebates,” he added. “Yet the specific reasons behind the faster growth of prices of MS drugs, compared with the prices of drugs used in other disease states, remain uncertain.”
Neurologists should bear in mind that, although generic drugs are substantially cheaper than branded drugs, generic specialty medications do not always reduce costs for Medicare Part D beneficiaries. “On the contrary, due to incentive misalignments created by the Medicare Part D benefit design, beneficiaries using generic drugs such as Glatopa ... may pay more than those using the branded drug,” Dr. San-Juan-Rodriguez said.
What are neurologists’ responsibilities?
Although the original annual price of interferon beta-1b ($10,920) was stunning, physicians now recall it with nostalgia, wrote Daniel M. Hartung, PharmD, associate professor of biostatistics and epidemiology, and Dennis Bourdette, MD, professor of neurology, both at Oregon Health and Science University, Portland, in an accompanying editorial. “The prices for DMTs for MS have risen dramatically over the last 15 years, far outpacing inflation, and now have a mean price of more than $86,000 per year.”
Neurologists should be concerned about these rising prices, Dr. Hartung and Dr. Bourdette wrote. They should feel responsibility toward the health care system that pays for these medications, and toward patients who pay out of their own pockets. “Neurologists should be seeking to minimize the financial adverse effects of these therapies as much as they try to minimize physical adverse effects.”
One way for neurologists to address increasing prices is to urge state and federal lawmakers to pass legislation to curb them, they wrote. Neurologists also should reexamine their relationships with pharmaceutical and biotechnology companies. “Remaining silent should not be an option. ... Neurologists should not allow the unfettered increases in price for these drugs to hurt the health care system or patients.”
The Myers Family Foundation and the National Heart, Lung, and Blood Institute funded the research. Several authors are employees of health insurance companies such as the UPMC Health Plan Insurance Services Division and Humana. One author received personal fees from Pfizer that were unrelated to this study.
SOURCEs: San-Juan-Rodriguez A et al. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2711; Hartung DM and Bourdette D. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2445.
according to an analysis published in JAMA Neurology. The increased prices raise concern “because they demonstrate that the approval of new therapies did not ameliorate and could have even contributed to high inflation rates observed for incumbent drugs,” wrote the authors.
Four self-administered disease-modifying therapies (DMTs) for multiple sclerosis (MS) were available before 2009, and seven new branded DMTs were introduced after that year. Previous research indicated that the prices of DMTs for MS increased at higher rates than the prices of drugs for other disorders. How these price increases affected pharmaceutical spending during the past decade is uncertain, however.
A review of Medicare claims data
Alvaro San-Juan-Rodriguez, PharmD, a fellow in pharmacoeconomics, outcomes, and pharmacoanalytics research at the University of Pittsburgh, and colleagues examined claims data from 2006 to 2016 from a 5% random sample of Medicare beneficiaries. Information for a mean of 2.8 million Medicare beneficiaries per year was available. The researchers extracted all prescription claims for self-administered DMTs for MS (that is, glatiramer acetate, interferon beta-1a, interferon beta-1b, fingolimod, teriflunomide, dimethyl fumarate, and peginterferon beta-1a).
Dr. San-Juan-Rodriguez and associates chose three main outcomes. The first was the annual cost of treatment with each medication, which was based on Medicare Part D prescription claims gross costs and Food and Drug Administration–approved recommended dosing. The second was the market share of each medication, which the researchers defined as the proportion of pharmaceutical spending accounted for by each drug. The third was pharmaceutical spending per 1,000 Medicare beneficiaries for all drugs. The investigators also examined the relative contributions of Medicare Part D Plans’ payments, patients’ out-of-pocket costs, and other payments toward pharmaceutical spending.
Prices defied market expectations
The annual costs of treatment with self-administered DMTs for MS increased more than 300%. The mean annual cost was $18,660 in 2006 and $75,847 in 2016, and the mean annual rate of price increase was 12.8%. “Prices of most self-administered DMTs for MS increased in parallel, defying standard market expectations,” the investigators wrote.
Branded formulations of glatiramer acetate maintained the largest market share throughout the study period, ranging between 32.2% and 48.4%. However, the market share of platform therapies – glatiramer acetate, interferon beta-1a, and interferon beta-1b – decreased significantly from 2006 to 2016. Market shares for brand-name glatiramers declined from 36.7% to 32.2%, for intramuscular interferon beta-1a (30 mcg) from 32.3% to 14.2%, for interferon beta-1b from 18.7% to 4.5%, and for interferon beta-1a (8.8, 22, or 44 mcg) from 12.2% to 8.3%. The market shares of newer therapies, however, increased to 7.9% for fingolimod, 9.0% for teriflunomide, and 19.2% for dimethyl fumarate.
Pharmaceutical spending per 1,000 beneficiaries increased by a factor of 10.2 throughout the study period (from $7,794 to $79,411). Patients’ out-of-pocket spending per 1,000 beneficiaries increased by a factor of 7.2 (from $372 to $2,673). Furthermore, the relative contribution of federal payments toward pharmaceutical spending increased from 68.5% to 73.8%.
“Large increases in drug prices have not been specific to MS drugs,” said Dr. San-Juan-Rodriguez in an interview. “We previously described similar trends in other specialty medications used to treat severe disease states, such as tumor necrosis factor inhibitors [TNFi] for the treatment of rheumatoid arthritis. Yet these increases took place at a slower pace. For instance, list prices of TNFi increased at an average annual rate of 9.9% in the same time period, 2006-2016.
“It is important to acknowledge that rising list prices of drugs may partially reflect competition for rebates,” he added. “Yet the specific reasons behind the faster growth of prices of MS drugs, compared with the prices of drugs used in other disease states, remain uncertain.”
Neurologists should bear in mind that, although generic drugs are substantially cheaper than branded drugs, generic specialty medications do not always reduce costs for Medicare Part D beneficiaries. “On the contrary, due to incentive misalignments created by the Medicare Part D benefit design, beneficiaries using generic drugs such as Glatopa ... may pay more than those using the branded drug,” Dr. San-Juan-Rodriguez said.
What are neurologists’ responsibilities?
Although the original annual price of interferon beta-1b ($10,920) was stunning, physicians now recall it with nostalgia, wrote Daniel M. Hartung, PharmD, associate professor of biostatistics and epidemiology, and Dennis Bourdette, MD, professor of neurology, both at Oregon Health and Science University, Portland, in an accompanying editorial. “The prices for DMTs for MS have risen dramatically over the last 15 years, far outpacing inflation, and now have a mean price of more than $86,000 per year.”
Neurologists should be concerned about these rising prices, Dr. Hartung and Dr. Bourdette wrote. They should feel responsibility toward the health care system that pays for these medications, and toward patients who pay out of their own pockets. “Neurologists should be seeking to minimize the financial adverse effects of these therapies as much as they try to minimize physical adverse effects.”
One way for neurologists to address increasing prices is to urge state and federal lawmakers to pass legislation to curb them, they wrote. Neurologists also should reexamine their relationships with pharmaceutical and biotechnology companies. “Remaining silent should not be an option. ... Neurologists should not allow the unfettered increases in price for these drugs to hurt the health care system or patients.”
The Myers Family Foundation and the National Heart, Lung, and Blood Institute funded the research. Several authors are employees of health insurance companies such as the UPMC Health Plan Insurance Services Division and Humana. One author received personal fees from Pfizer that were unrelated to this study.
SOURCEs: San-Juan-Rodriguez A et al. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2711; Hartung DM and Bourdette D. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2445.
FROM JAMA NEUROLOGY
Calquence earns breakthrough designation for CLL monotherapy
The Bruton tyrosine kinase inhibitor is already approved for the treatment of adults with mantle cell lymphoma who have received at least one prior therapy, and multiple trials are underway to evaluate the drug’s use in a variety of B-cell malignancies, according to the drug’s sponsor, AstraZeneca.
The current designation was based on preliminary results from two phase 3 trials – ELEVATE-TN and ASCEND. In the three-arm ELEVATE-TN trial, researchers evaluated acalabrutinib alone or in combination with obinutuzumab versus chlorambucil plus obinutuzumab in previously untreated patients with CLL. In the two-arm ASCEND trial, previously treated patients with CLL were randomized to receive acalabrutinib monotherapy or the physician’s choice of either rituximab plus idelalisib or rituximab plus bendamustine.
Interim analyses of the two trials showed that acalabrutinib alone, or in combination, significantly improved progression-free survival without raising safety concerns.
Breakthrough therapy designation allows for an expedited review by the FDA for treatments aimed at treating serious conditions where there is preliminary clinical evidence showing a substantial improvement over an available therapy or a clinically significant endpoint.
The Bruton tyrosine kinase inhibitor is already approved for the treatment of adults with mantle cell lymphoma who have received at least one prior therapy, and multiple trials are underway to evaluate the drug’s use in a variety of B-cell malignancies, according to the drug’s sponsor, AstraZeneca.
The current designation was based on preliminary results from two phase 3 trials – ELEVATE-TN and ASCEND. In the three-arm ELEVATE-TN trial, researchers evaluated acalabrutinib alone or in combination with obinutuzumab versus chlorambucil plus obinutuzumab in previously untreated patients with CLL. In the two-arm ASCEND trial, previously treated patients with CLL were randomized to receive acalabrutinib monotherapy or the physician’s choice of either rituximab plus idelalisib or rituximab plus bendamustine.
Interim analyses of the two trials showed that acalabrutinib alone, or in combination, significantly improved progression-free survival without raising safety concerns.
Breakthrough therapy designation allows for an expedited review by the FDA for treatments aimed at treating serious conditions where there is preliminary clinical evidence showing a substantial improvement over an available therapy or a clinically significant endpoint.
The Bruton tyrosine kinase inhibitor is already approved for the treatment of adults with mantle cell lymphoma who have received at least one prior therapy, and multiple trials are underway to evaluate the drug’s use in a variety of B-cell malignancies, according to the drug’s sponsor, AstraZeneca.
The current designation was based on preliminary results from two phase 3 trials – ELEVATE-TN and ASCEND. In the three-arm ELEVATE-TN trial, researchers evaluated acalabrutinib alone or in combination with obinutuzumab versus chlorambucil plus obinutuzumab in previously untreated patients with CLL. In the two-arm ASCEND trial, previously treated patients with CLL were randomized to receive acalabrutinib monotherapy or the physician’s choice of either rituximab plus idelalisib or rituximab plus bendamustine.
Interim analyses of the two trials showed that acalabrutinib alone, or in combination, significantly improved progression-free survival without raising safety concerns.
Breakthrough therapy designation allows for an expedited review by the FDA for treatments aimed at treating serious conditions where there is preliminary clinical evidence showing a substantial improvement over an available therapy or a clinically significant endpoint.
High-dose vitamin D for bone health may do more harm than good
In fact, rather than a hypothesized increase in volumetric bone mineral density (BMD) with doses well above the recommended dietary allowance, a negative dose-response relationship was observed, Lauren A. Burt, PhD, of the McCaig Institute for Bone and Joint Health at the University of Calgary (Alta.) and colleagues found.
The total volumetric radial BMD was significantly lower in 101 and 97 study participants randomized to receive daily vitamin D3 doses of 10,000 IU or 4,000 IU for 3 years, respectively (–7.5 and –3.9 mg of calcium hydroxyapatite [HA] per cm3), compared with 105 participants randomized to a reference group that received 400 IU (mean percent changes, –3.5%, –2.4%, and –1.2%, respectively). Total volumetric tibial BMD was also significantly lower in the 10,000 IU arm, compared with the reference arm (–4.1 mg HA per cm3; mean percent change –1.7% vs. –0.4%), the investigators reported Aug. 27 in JAMA.
There also were no significant differences seen between the three groups for the coprimary endpoint of bone strength at either the radius or tibia.
Participants in the double-blind trial were community-dwelling healthy men and women aged 55-70 years (mean age, 62.2 years) without osteoporosis and with baseline levels of 25-hydroxyvitamin D (25[OH]D) of 30-125 nmol/L. They were enrolled from a single center between August 2013 and December 2017 and treated with daily oral vitamin D3 drops at the assigned dosage for 3 years and with calcium supplementation if dietary calcium intake was less than 1,200 mg daily.
Mean supplementation adherence was 99% among the 303 participants who completed the trial (out of 311 enrolled), and adherence was similar across the groups.
Baseline 25(OH)D levels in the 400 IU group were 76.3 nmol/L at baseline, 76.7 nmol/L at 3 months, and 77.4 nmol/L at 3 years. The corresponding measures for the 4,000 IU group were 81.3, 115.3, and 132.2 nmol/L, and for the 10,000 IU group, they were 78.4, 188.0, and 144.4, the investigators said, noting that significant group-by-time interactions were noted for volumetric BMD.
Bone strength decreased over time, but group-by-time interactions for that measure were not statistically significant, they said.
A total of 44 serious adverse events occurred in 38 participants (12.2%), and one death from presumed myocardial infarction occurred in the 400 IU group. Of eight prespecified adverse events, only hypercalcemia and hypercalciuria had significant dose-response effects; all episodes of hypercalcemia were mild and had resolved at follow-up, and the two hypercalcemia events, which occurred in one participant in the 10,000 IU group, were also transient. No significant difference in fall rates was seen in the three groups, they noted.
Vitamin D is considered beneficial for preventing and treating osteoporosis, and data support supplementation in individuals with 25(OH)D levels less than 30 nmol/L, but recent meta-analyses did not find a major treatment benefit for osteoporosis or for preventing falls and fractures, the investigators said.
Further, while most supplementation recommendations call for 400-2,000 IU daily, with a tolerable upper intake level of 4,000-10,000 IU, 3% of U.S. adults in 2013-2014 reported intake of at least 4,000 IU per day, but few studies have assessed the effects of doses at or above the upper intake level for 12 months or longer, they noted, adding that this study was “motivated by the prevalence of high-dose vitamin D supplementation among healthy adults.”
“It was hypothesized that a higher dose of vitamin D has a positive effect on high-resolution peripheral quantitative CT measures of volumetric density and strength, perhaps via suppression of parathyroid hormone (PTH)–mediated bone turnover,” they wrote.
However, based on the significantly lower radial BMD seen with both 4,000 and 10,000 IU, compared with 400 IU; the lower tibial BMD with 10,000 IU, compared with 400 IU; and the lack of a difference in bone strength at the radius and tibia, the findings do not support a benefit of high-dose vitamin D supplementation for bone health, they said, noting that additional study is needed to determine whether such doses are harmful.
“Because these results are in the opposite direction of the research hypothesis, this evidence of high-dose vitamin D having a negative effect on bone should be regarded as hypothesis generating, requiring confirmation with further research,” they concluded.
SOURCE: Burt L et al. JAMA. 2019 Aug 27;322(8):736-45.
In fact, rather than a hypothesized increase in volumetric bone mineral density (BMD) with doses well above the recommended dietary allowance, a negative dose-response relationship was observed, Lauren A. Burt, PhD, of the McCaig Institute for Bone and Joint Health at the University of Calgary (Alta.) and colleagues found.
The total volumetric radial BMD was significantly lower in 101 and 97 study participants randomized to receive daily vitamin D3 doses of 10,000 IU or 4,000 IU for 3 years, respectively (–7.5 and –3.9 mg of calcium hydroxyapatite [HA] per cm3), compared with 105 participants randomized to a reference group that received 400 IU (mean percent changes, –3.5%, –2.4%, and –1.2%, respectively). Total volumetric tibial BMD was also significantly lower in the 10,000 IU arm, compared with the reference arm (–4.1 mg HA per cm3; mean percent change –1.7% vs. –0.4%), the investigators reported Aug. 27 in JAMA.
There also were no significant differences seen between the three groups for the coprimary endpoint of bone strength at either the radius or tibia.
Participants in the double-blind trial were community-dwelling healthy men and women aged 55-70 years (mean age, 62.2 years) without osteoporosis and with baseline levels of 25-hydroxyvitamin D (25[OH]D) of 30-125 nmol/L. They were enrolled from a single center between August 2013 and December 2017 and treated with daily oral vitamin D3 drops at the assigned dosage for 3 years and with calcium supplementation if dietary calcium intake was less than 1,200 mg daily.
Mean supplementation adherence was 99% among the 303 participants who completed the trial (out of 311 enrolled), and adherence was similar across the groups.
Baseline 25(OH)D levels in the 400 IU group were 76.3 nmol/L at baseline, 76.7 nmol/L at 3 months, and 77.4 nmol/L at 3 years. The corresponding measures for the 4,000 IU group were 81.3, 115.3, and 132.2 nmol/L, and for the 10,000 IU group, they were 78.4, 188.0, and 144.4, the investigators said, noting that significant group-by-time interactions were noted for volumetric BMD.
Bone strength decreased over time, but group-by-time interactions for that measure were not statistically significant, they said.
A total of 44 serious adverse events occurred in 38 participants (12.2%), and one death from presumed myocardial infarction occurred in the 400 IU group. Of eight prespecified adverse events, only hypercalcemia and hypercalciuria had significant dose-response effects; all episodes of hypercalcemia were mild and had resolved at follow-up, and the two hypercalcemia events, which occurred in one participant in the 10,000 IU group, were also transient. No significant difference in fall rates was seen in the three groups, they noted.
Vitamin D is considered beneficial for preventing and treating osteoporosis, and data support supplementation in individuals with 25(OH)D levels less than 30 nmol/L, but recent meta-analyses did not find a major treatment benefit for osteoporosis or for preventing falls and fractures, the investigators said.
Further, while most supplementation recommendations call for 400-2,000 IU daily, with a tolerable upper intake level of 4,000-10,000 IU, 3% of U.S. adults in 2013-2014 reported intake of at least 4,000 IU per day, but few studies have assessed the effects of doses at or above the upper intake level for 12 months or longer, they noted, adding that this study was “motivated by the prevalence of high-dose vitamin D supplementation among healthy adults.”
“It was hypothesized that a higher dose of vitamin D has a positive effect on high-resolution peripheral quantitative CT measures of volumetric density and strength, perhaps via suppression of parathyroid hormone (PTH)–mediated bone turnover,” they wrote.
However, based on the significantly lower radial BMD seen with both 4,000 and 10,000 IU, compared with 400 IU; the lower tibial BMD with 10,000 IU, compared with 400 IU; and the lack of a difference in bone strength at the radius and tibia, the findings do not support a benefit of high-dose vitamin D supplementation for bone health, they said, noting that additional study is needed to determine whether such doses are harmful.
“Because these results are in the opposite direction of the research hypothesis, this evidence of high-dose vitamin D having a negative effect on bone should be regarded as hypothesis generating, requiring confirmation with further research,” they concluded.
SOURCE: Burt L et al. JAMA. 2019 Aug 27;322(8):736-45.
In fact, rather than a hypothesized increase in volumetric bone mineral density (BMD) with doses well above the recommended dietary allowance, a negative dose-response relationship was observed, Lauren A. Burt, PhD, of the McCaig Institute for Bone and Joint Health at the University of Calgary (Alta.) and colleagues found.
The total volumetric radial BMD was significantly lower in 101 and 97 study participants randomized to receive daily vitamin D3 doses of 10,000 IU or 4,000 IU for 3 years, respectively (–7.5 and –3.9 mg of calcium hydroxyapatite [HA] per cm3), compared with 105 participants randomized to a reference group that received 400 IU (mean percent changes, –3.5%, –2.4%, and –1.2%, respectively). Total volumetric tibial BMD was also significantly lower in the 10,000 IU arm, compared with the reference arm (–4.1 mg HA per cm3; mean percent change –1.7% vs. –0.4%), the investigators reported Aug. 27 in JAMA.
There also were no significant differences seen between the three groups for the coprimary endpoint of bone strength at either the radius or tibia.
Participants in the double-blind trial were community-dwelling healthy men and women aged 55-70 years (mean age, 62.2 years) without osteoporosis and with baseline levels of 25-hydroxyvitamin D (25[OH]D) of 30-125 nmol/L. They were enrolled from a single center between August 2013 and December 2017 and treated with daily oral vitamin D3 drops at the assigned dosage for 3 years and with calcium supplementation if dietary calcium intake was less than 1,200 mg daily.
Mean supplementation adherence was 99% among the 303 participants who completed the trial (out of 311 enrolled), and adherence was similar across the groups.
Baseline 25(OH)D levels in the 400 IU group were 76.3 nmol/L at baseline, 76.7 nmol/L at 3 months, and 77.4 nmol/L at 3 years. The corresponding measures for the 4,000 IU group were 81.3, 115.3, and 132.2 nmol/L, and for the 10,000 IU group, they were 78.4, 188.0, and 144.4, the investigators said, noting that significant group-by-time interactions were noted for volumetric BMD.
Bone strength decreased over time, but group-by-time interactions for that measure were not statistically significant, they said.
A total of 44 serious adverse events occurred in 38 participants (12.2%), and one death from presumed myocardial infarction occurred in the 400 IU group. Of eight prespecified adverse events, only hypercalcemia and hypercalciuria had significant dose-response effects; all episodes of hypercalcemia were mild and had resolved at follow-up, and the two hypercalcemia events, which occurred in one participant in the 10,000 IU group, were also transient. No significant difference in fall rates was seen in the three groups, they noted.
Vitamin D is considered beneficial for preventing and treating osteoporosis, and data support supplementation in individuals with 25(OH)D levels less than 30 nmol/L, but recent meta-analyses did not find a major treatment benefit for osteoporosis or for preventing falls and fractures, the investigators said.
Further, while most supplementation recommendations call for 400-2,000 IU daily, with a tolerable upper intake level of 4,000-10,000 IU, 3% of U.S. adults in 2013-2014 reported intake of at least 4,000 IU per day, but few studies have assessed the effects of doses at or above the upper intake level for 12 months or longer, they noted, adding that this study was “motivated by the prevalence of high-dose vitamin D supplementation among healthy adults.”
“It was hypothesized that a higher dose of vitamin D has a positive effect on high-resolution peripheral quantitative CT measures of volumetric density and strength, perhaps via suppression of parathyroid hormone (PTH)–mediated bone turnover,” they wrote.
However, based on the significantly lower radial BMD seen with both 4,000 and 10,000 IU, compared with 400 IU; the lower tibial BMD with 10,000 IU, compared with 400 IU; and the lack of a difference in bone strength at the radius and tibia, the findings do not support a benefit of high-dose vitamin D supplementation for bone health, they said, noting that additional study is needed to determine whether such doses are harmful.
“Because these results are in the opposite direction of the research hypothesis, this evidence of high-dose vitamin D having a negative effect on bone should be regarded as hypothesis generating, requiring confirmation with further research,” they concluded.
SOURCE: Burt L et al. JAMA. 2019 Aug 27;322(8):736-45.
FROM JAMA
FDA approves Taltz for treatment of ankylosing spondylitis
(AS), according to a press release from Eli Lilly.
AS is the third indication for ixekizumab, along with moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and active psoriatic arthritis in adults.
Approval of the humanized interleukin-17A antagonist was based on results from a pair of randomized, double-blind, placebo-controlled, phase 3 studies involving 657 adult patients with active AS: the COAST-V trial in those naive to biologic disease-modifying antirheumatic drugs (bDMARDs) and the COAST-W trial in those who were intolerant or had inadequate response to tumor necrosis factor (TNF) inhibitors. The primary endpoint in both trials was achievement of 40% improvement in Assessment of Spondyloarthritis International Society criteria (ASAS40) at 16 weeks, compared with placebo.
In COAST-V, 48% of patients who received ixekizumab achieved ASAS40, compared with 18% of controls (P less than .0001). In COAST-W, 25% of patients who received ixekizumab achieved ASAS40 versus 13% of controls (P less than .05). The adverse events reported during both trials were consistent with the safety profile in patients who receive ixekizumab for the treatment of plaque psoriasis, including injection-site reactions, upper respiratory tract infections, nausea, and tinea infections.
“Results from the phase 3 clinical trial program in ankylosing spondylitis show that Taltz helped reduce pain and inflammation and improve function in patients who had never been treated with a bDMARD as well as those who previously failed TNF inhibitors. This approval is an important milestone for patients and physicians who are looking for a much-needed alternative to address symptoms of AS,” said Philip Mease, MD, of Providence St. Joseph Health and the University of Washington, both in Seattle.
(AS), according to a press release from Eli Lilly.
AS is the third indication for ixekizumab, along with moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and active psoriatic arthritis in adults.
Approval of the humanized interleukin-17A antagonist was based on results from a pair of randomized, double-blind, placebo-controlled, phase 3 studies involving 657 adult patients with active AS: the COAST-V trial in those naive to biologic disease-modifying antirheumatic drugs (bDMARDs) and the COAST-W trial in those who were intolerant or had inadequate response to tumor necrosis factor (TNF) inhibitors. The primary endpoint in both trials was achievement of 40% improvement in Assessment of Spondyloarthritis International Society criteria (ASAS40) at 16 weeks, compared with placebo.
In COAST-V, 48% of patients who received ixekizumab achieved ASAS40, compared with 18% of controls (P less than .0001). In COAST-W, 25% of patients who received ixekizumab achieved ASAS40 versus 13% of controls (P less than .05). The adverse events reported during both trials were consistent with the safety profile in patients who receive ixekizumab for the treatment of plaque psoriasis, including injection-site reactions, upper respiratory tract infections, nausea, and tinea infections.
“Results from the phase 3 clinical trial program in ankylosing spondylitis show that Taltz helped reduce pain and inflammation and improve function in patients who had never been treated with a bDMARD as well as those who previously failed TNF inhibitors. This approval is an important milestone for patients and physicians who are looking for a much-needed alternative to address symptoms of AS,” said Philip Mease, MD, of Providence St. Joseph Health and the University of Washington, both in Seattle.
(AS), according to a press release from Eli Lilly.
AS is the third indication for ixekizumab, along with moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and active psoriatic arthritis in adults.
Approval of the humanized interleukin-17A antagonist was based on results from a pair of randomized, double-blind, placebo-controlled, phase 3 studies involving 657 adult patients with active AS: the COAST-V trial in those naive to biologic disease-modifying antirheumatic drugs (bDMARDs) and the COAST-W trial in those who were intolerant or had inadequate response to tumor necrosis factor (TNF) inhibitors. The primary endpoint in both trials was achievement of 40% improvement in Assessment of Spondyloarthritis International Society criteria (ASAS40) at 16 weeks, compared with placebo.
In COAST-V, 48% of patients who received ixekizumab achieved ASAS40, compared with 18% of controls (P less than .0001). In COAST-W, 25% of patients who received ixekizumab achieved ASAS40 versus 13% of controls (P less than .05). The adverse events reported during both trials were consistent with the safety profile in patients who receive ixekizumab for the treatment of plaque psoriasis, including injection-site reactions, upper respiratory tract infections, nausea, and tinea infections.
“Results from the phase 3 clinical trial program in ankylosing spondylitis show that Taltz helped reduce pain and inflammation and improve function in patients who had never been treated with a bDMARD as well as those who previously failed TNF inhibitors. This approval is an important milestone for patients and physicians who are looking for a much-needed alternative to address symptoms of AS,” said Philip Mease, MD, of Providence St. Joseph Health and the University of Washington, both in Seattle.
Intranasal midazolam as first line for status epilepticus
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
REPORTING FROM IEC 2019
‘Pot’ is still hot for Dravet, Lennox-Gastaut
BANGKOK – Interim results of long-term, open-label extension trials of add-on prescription cannabidiol in patients with Dravet syndrome or Lennox-Gastaut syndrome show sustained, clinically meaningful seizure reductions with no new safety concerns, Anup D. Patel, MD, reported at the International Epilepsy Congress.
“Overall, this is a very promising and sustainable result that we were happy to see,” said Dr. Patel, chief of child neurology at Nationwide Children’s Hospital in Columbus, Ohio.
Epidiolex is the brand name for the plant-derived, highly purified cannabidiol (CBD) in an oil-based oral solution at 100 mg/mL. Dr. Patel has been involved in the medication’s development program since the earliest open-label compassionate use study, which was followed by rigorous phase 3, double-blind, placebo-controlled randomized trials, eventually leading to Food and Drug Administration marketing approval for the treatment of Dravet syndrome and Lennox-Gastaut syndrome in patients 2 years of age or older.
“On June 25th, 2018, history was made: for the first time in United States history, a plant-based derivative of marijuana was approved for use as a medication, and it was also the first FDA-approved treatment for Dravet syndrome,” Dr. Patel noted at the congress sponsored by the International League Against Epilepsy.
A total of 96% of the 289 children with Dravet syndrome who completed the 14-week, double-blind, controlled randomized trials enrolled in the open-label, long-term extension study, during which they were on a median of three concurrent antiepileptic drugs along with a mean modal dose of CBD at 22 mg/kg/day. Although the target maintenance dose of CBD was 20 mg/kg/day, as advised in the product labeling, physicians could reduce or increase the dose up to 30 mg/kg/day.
“In the initial compassionate-use study, our site could go up to 50 mg/kg/day,” according to Dr. Patel. “We have plenty of data showing efficacy and continued safety beyond the FDA-recommended dose.”
In the open-label extension study, the median reduction from baseline in monthly seizure frequency assessed in 12-week intervals up to a maximum of week 72 was 44%-57% for convulsive seizures and 49%-67% for total seizures. More than 80% of patients and/or caregivers reported improvement in the patient’s overall condition as assessed on the Subject/Caregiver Global Impression of Change scale.
The pattern of adverse events associated with CBD has been consistent across all of the studies. The most common side effects are diarrhea in about one-third of patients, sleepiness in one-quarter, and decreased appetite in about one-quarter. Seven percent of patients discontinued the long-term extension trial because of adverse events.
Seventy percent of patients remained in the long-term extension study at 1 year.
Twenty-six patients developed liver transaminase levels greater than three times the upper limit of normal, and of note, 23 of the 26 were on concomitant valproic acid. None met criteria for severe drug-induced liver injury, and all recovered either spontaneously or after a reduction in the dose of CBD or valproic acid. But this association between CBD, valproic acid, and increased risk of mild liver injury has been a consistent finding across the clinical trials program.
“This is a very important clinical pearl to take away,” commented Dr. Patel, who is also a pediatric neurologist at Ohio State University.
The interim results of the long-term, open-label extension study of add-on CBD in patients with Lennox-Gastaut syndrome are similar to the Dravet syndrome study. Overall, 99% of the 368 patients with Lennox-Gastaut syndrome who completed the 14-week, double-blind, randomized trials signed up for the open-label extension. During a median follow-up of 61 weeks, the median percent reduction in seizure frequency as assessed in serial 12-week windows was 48%-70% for drop seizures and 48%-63% for total seizures. Twenty-four percent of patients withdrew from the study. Eighty-eight percent of patients or caregivers reported an improvement in overall condition when assessed at weeks 24 and 48. Forty-seven patients developed elevated transaminase levels – typically within the first 2 months on CBD – and 35 of them were on concomitant valproic acid.
More on drug-drug interactions
Elsewhere at IEC 2019, Gilmour Morrison of GW Pharmaceuticals, the Cambridge, England, company that markets Epidiolex, presented the findings of a series of drug-drug interaction studies involving coadministration of their CBD with clobazam (Sympazan and Onfi), valproate, stiripentol (Diacomit), or midazolam (Versed) in adult epilepsy patients and healthy volunteers. The researchers reported a bidirectional drug-drug interaction between Epidiolex and clobazam resulting in increased levels of the active metabolites of both drugs. The mechanism is believed to involve inhibition of cytochrome P450 2C19. However, there were no interactions with midazolam or valproate, and the slight bump in stiripentol levels when given with CBD didn’t reach the level of a clinically meaningful drug-drug interaction, according to the investigators.
On the horizon, Canadian researchers are investigating the possibility that since both the tetrahydrocannabinol (THC) and CBD components of marijuana have been shown to have anticonvulsant effects, adding a bit of THC to CBD will result in even better seizure control than with pure CBD in patients with Dravet syndrome. Investigators at Toronto’s Hospital for Sick Children have conducted a prospective, open-label study of a product containing CBD and THC in a 50:1 ratio as add-on therapy in 20 children with Dravet syndrome. The dose was 2-16 mg/kg/day of CBD and 0.04-0.32 mg/kg/day of THC. The cannabis plant extract used in the study was produced by Tilray, a Canadian pharmaceutical company.
Nineteen of the 20 patients completed the 20-week study. The sole noncompleter died of SUDEP (sudden unexpected death in epilepsy) deemed treatment unrelated. Patients experienced a median 71% reduction in motor seizures, compared with baseline. Sixty-three percent of patients had at least a 50% reduction in seizure frequency. Elevated liver transaminases occurred in patients on concomitant valproic acid, as did platelet abnormalities, which have not been seen in the Epidiolex studies, noted Dr. Patel, who was not involved in the Canadian study (Ann Clin Transl Neurol. 2018 Aug 1;5[9]:1077-88).
Dr. Patel reported serving as a consultant to Greenwich Biosciences, a U.S. offshoot of GW Pharmaceuticals. He receives research grants from that company as well as from the National Institutes of Health and the Pediatric Epilepsy Research Foundation.
BANGKOK – Interim results of long-term, open-label extension trials of add-on prescription cannabidiol in patients with Dravet syndrome or Lennox-Gastaut syndrome show sustained, clinically meaningful seizure reductions with no new safety concerns, Anup D. Patel, MD, reported at the International Epilepsy Congress.
“Overall, this is a very promising and sustainable result that we were happy to see,” said Dr. Patel, chief of child neurology at Nationwide Children’s Hospital in Columbus, Ohio.
Epidiolex is the brand name for the plant-derived, highly purified cannabidiol (CBD) in an oil-based oral solution at 100 mg/mL. Dr. Patel has been involved in the medication’s development program since the earliest open-label compassionate use study, which was followed by rigorous phase 3, double-blind, placebo-controlled randomized trials, eventually leading to Food and Drug Administration marketing approval for the treatment of Dravet syndrome and Lennox-Gastaut syndrome in patients 2 years of age or older.
“On June 25th, 2018, history was made: for the first time in United States history, a plant-based derivative of marijuana was approved for use as a medication, and it was also the first FDA-approved treatment for Dravet syndrome,” Dr. Patel noted at the congress sponsored by the International League Against Epilepsy.
A total of 96% of the 289 children with Dravet syndrome who completed the 14-week, double-blind, controlled randomized trials enrolled in the open-label, long-term extension study, during which they were on a median of three concurrent antiepileptic drugs along with a mean modal dose of CBD at 22 mg/kg/day. Although the target maintenance dose of CBD was 20 mg/kg/day, as advised in the product labeling, physicians could reduce or increase the dose up to 30 mg/kg/day.
“In the initial compassionate-use study, our site could go up to 50 mg/kg/day,” according to Dr. Patel. “We have plenty of data showing efficacy and continued safety beyond the FDA-recommended dose.”
In the open-label extension study, the median reduction from baseline in monthly seizure frequency assessed in 12-week intervals up to a maximum of week 72 was 44%-57% for convulsive seizures and 49%-67% for total seizures. More than 80% of patients and/or caregivers reported improvement in the patient’s overall condition as assessed on the Subject/Caregiver Global Impression of Change scale.
The pattern of adverse events associated with CBD has been consistent across all of the studies. The most common side effects are diarrhea in about one-third of patients, sleepiness in one-quarter, and decreased appetite in about one-quarter. Seven percent of patients discontinued the long-term extension trial because of adverse events.
Seventy percent of patients remained in the long-term extension study at 1 year.
Twenty-six patients developed liver transaminase levels greater than three times the upper limit of normal, and of note, 23 of the 26 were on concomitant valproic acid. None met criteria for severe drug-induced liver injury, and all recovered either spontaneously or after a reduction in the dose of CBD or valproic acid. But this association between CBD, valproic acid, and increased risk of mild liver injury has been a consistent finding across the clinical trials program.
“This is a very important clinical pearl to take away,” commented Dr. Patel, who is also a pediatric neurologist at Ohio State University.
The interim results of the long-term, open-label extension study of add-on CBD in patients with Lennox-Gastaut syndrome are similar to the Dravet syndrome study. Overall, 99% of the 368 patients with Lennox-Gastaut syndrome who completed the 14-week, double-blind, randomized trials signed up for the open-label extension. During a median follow-up of 61 weeks, the median percent reduction in seizure frequency as assessed in serial 12-week windows was 48%-70% for drop seizures and 48%-63% for total seizures. Twenty-four percent of patients withdrew from the study. Eighty-eight percent of patients or caregivers reported an improvement in overall condition when assessed at weeks 24 and 48. Forty-seven patients developed elevated transaminase levels – typically within the first 2 months on CBD – and 35 of them were on concomitant valproic acid.
More on drug-drug interactions
Elsewhere at IEC 2019, Gilmour Morrison of GW Pharmaceuticals, the Cambridge, England, company that markets Epidiolex, presented the findings of a series of drug-drug interaction studies involving coadministration of their CBD with clobazam (Sympazan and Onfi), valproate, stiripentol (Diacomit), or midazolam (Versed) in adult epilepsy patients and healthy volunteers. The researchers reported a bidirectional drug-drug interaction between Epidiolex and clobazam resulting in increased levels of the active metabolites of both drugs. The mechanism is believed to involve inhibition of cytochrome P450 2C19. However, there were no interactions with midazolam or valproate, and the slight bump in stiripentol levels when given with CBD didn’t reach the level of a clinically meaningful drug-drug interaction, according to the investigators.
On the horizon, Canadian researchers are investigating the possibility that since both the tetrahydrocannabinol (THC) and CBD components of marijuana have been shown to have anticonvulsant effects, adding a bit of THC to CBD will result in even better seizure control than with pure CBD in patients with Dravet syndrome. Investigators at Toronto’s Hospital for Sick Children have conducted a prospective, open-label study of a product containing CBD and THC in a 50:1 ratio as add-on therapy in 20 children with Dravet syndrome. The dose was 2-16 mg/kg/day of CBD and 0.04-0.32 mg/kg/day of THC. The cannabis plant extract used in the study was produced by Tilray, a Canadian pharmaceutical company.
Nineteen of the 20 patients completed the 20-week study. The sole noncompleter died of SUDEP (sudden unexpected death in epilepsy) deemed treatment unrelated. Patients experienced a median 71% reduction in motor seizures, compared with baseline. Sixty-three percent of patients had at least a 50% reduction in seizure frequency. Elevated liver transaminases occurred in patients on concomitant valproic acid, as did platelet abnormalities, which have not been seen in the Epidiolex studies, noted Dr. Patel, who was not involved in the Canadian study (Ann Clin Transl Neurol. 2018 Aug 1;5[9]:1077-88).
Dr. Patel reported serving as a consultant to Greenwich Biosciences, a U.S. offshoot of GW Pharmaceuticals. He receives research grants from that company as well as from the National Institutes of Health and the Pediatric Epilepsy Research Foundation.
BANGKOK – Interim results of long-term, open-label extension trials of add-on prescription cannabidiol in patients with Dravet syndrome or Lennox-Gastaut syndrome show sustained, clinically meaningful seizure reductions with no new safety concerns, Anup D. Patel, MD, reported at the International Epilepsy Congress.
“Overall, this is a very promising and sustainable result that we were happy to see,” said Dr. Patel, chief of child neurology at Nationwide Children’s Hospital in Columbus, Ohio.
Epidiolex is the brand name for the plant-derived, highly purified cannabidiol (CBD) in an oil-based oral solution at 100 mg/mL. Dr. Patel has been involved in the medication’s development program since the earliest open-label compassionate use study, which was followed by rigorous phase 3, double-blind, placebo-controlled randomized trials, eventually leading to Food and Drug Administration marketing approval for the treatment of Dravet syndrome and Lennox-Gastaut syndrome in patients 2 years of age or older.
“On June 25th, 2018, history was made: for the first time in United States history, a plant-based derivative of marijuana was approved for use as a medication, and it was also the first FDA-approved treatment for Dravet syndrome,” Dr. Patel noted at the congress sponsored by the International League Against Epilepsy.
A total of 96% of the 289 children with Dravet syndrome who completed the 14-week, double-blind, controlled randomized trials enrolled in the open-label, long-term extension study, during which they were on a median of three concurrent antiepileptic drugs along with a mean modal dose of CBD at 22 mg/kg/day. Although the target maintenance dose of CBD was 20 mg/kg/day, as advised in the product labeling, physicians could reduce or increase the dose up to 30 mg/kg/day.
“In the initial compassionate-use study, our site could go up to 50 mg/kg/day,” according to Dr. Patel. “We have plenty of data showing efficacy and continued safety beyond the FDA-recommended dose.”
In the open-label extension study, the median reduction from baseline in monthly seizure frequency assessed in 12-week intervals up to a maximum of week 72 was 44%-57% for convulsive seizures and 49%-67% for total seizures. More than 80% of patients and/or caregivers reported improvement in the patient’s overall condition as assessed on the Subject/Caregiver Global Impression of Change scale.
The pattern of adverse events associated with CBD has been consistent across all of the studies. The most common side effects are diarrhea in about one-third of patients, sleepiness in one-quarter, and decreased appetite in about one-quarter. Seven percent of patients discontinued the long-term extension trial because of adverse events.
Seventy percent of patients remained in the long-term extension study at 1 year.
Twenty-six patients developed liver transaminase levels greater than three times the upper limit of normal, and of note, 23 of the 26 were on concomitant valproic acid. None met criteria for severe drug-induced liver injury, and all recovered either spontaneously or after a reduction in the dose of CBD or valproic acid. But this association between CBD, valproic acid, and increased risk of mild liver injury has been a consistent finding across the clinical trials program.
“This is a very important clinical pearl to take away,” commented Dr. Patel, who is also a pediatric neurologist at Ohio State University.
The interim results of the long-term, open-label extension study of add-on CBD in patients with Lennox-Gastaut syndrome are similar to the Dravet syndrome study. Overall, 99% of the 368 patients with Lennox-Gastaut syndrome who completed the 14-week, double-blind, randomized trials signed up for the open-label extension. During a median follow-up of 61 weeks, the median percent reduction in seizure frequency as assessed in serial 12-week windows was 48%-70% for drop seizures and 48%-63% for total seizures. Twenty-four percent of patients withdrew from the study. Eighty-eight percent of patients or caregivers reported an improvement in overall condition when assessed at weeks 24 and 48. Forty-seven patients developed elevated transaminase levels – typically within the first 2 months on CBD – and 35 of them were on concomitant valproic acid.
More on drug-drug interactions
Elsewhere at IEC 2019, Gilmour Morrison of GW Pharmaceuticals, the Cambridge, England, company that markets Epidiolex, presented the findings of a series of drug-drug interaction studies involving coadministration of their CBD with clobazam (Sympazan and Onfi), valproate, stiripentol (Diacomit), or midazolam (Versed) in adult epilepsy patients and healthy volunteers. The researchers reported a bidirectional drug-drug interaction between Epidiolex and clobazam resulting in increased levels of the active metabolites of both drugs. The mechanism is believed to involve inhibition of cytochrome P450 2C19. However, there were no interactions with midazolam or valproate, and the slight bump in stiripentol levels when given with CBD didn’t reach the level of a clinically meaningful drug-drug interaction, according to the investigators.
On the horizon, Canadian researchers are investigating the possibility that since both the tetrahydrocannabinol (THC) and CBD components of marijuana have been shown to have anticonvulsant effects, adding a bit of THC to CBD will result in even better seizure control than with pure CBD in patients with Dravet syndrome. Investigators at Toronto’s Hospital for Sick Children have conducted a prospective, open-label study of a product containing CBD and THC in a 50:1 ratio as add-on therapy in 20 children with Dravet syndrome. The dose was 2-16 mg/kg/day of CBD and 0.04-0.32 mg/kg/day of THC. The cannabis plant extract used in the study was produced by Tilray, a Canadian pharmaceutical company.
Nineteen of the 20 patients completed the 20-week study. The sole noncompleter died of SUDEP (sudden unexpected death in epilepsy) deemed treatment unrelated. Patients experienced a median 71% reduction in motor seizures, compared with baseline. Sixty-three percent of patients had at least a 50% reduction in seizure frequency. Elevated liver transaminases occurred in patients on concomitant valproic acid, as did platelet abnormalities, which have not been seen in the Epidiolex studies, noted Dr. Patel, who was not involved in the Canadian study (Ann Clin Transl Neurol. 2018 Aug 1;5[9]:1077-88).
Dr. Patel reported serving as a consultant to Greenwich Biosciences, a U.S. offshoot of GW Pharmaceuticals. He receives research grants from that company as well as from the National Institutes of Health and the Pediatric Epilepsy Research Foundation.
REPORTING FROM IEC 2019
ASCO VTE guideline update: DOACs now an option for prevention, treatment
The direct oral anticoagulants (DOACs) apixaban and rivaroxaban are now among the options for thromboprophylaxis in high-risk cancer outpatients with low risk for bleeding and drug interactions, according to a practice guideline update from the American Society of Clinical Oncology.
Rivaroxaban also has been added as an option for initial anticoagulation for venous thromboembolism (VTE), and both rivaroxaban and edoxaban are now options for long-term anticoagulation, Nigel S. Key, MB ChB, and colleagues wrote in the updated guideline on the prophylaxis and treatment of VTE – including deep vein thrombosis (DVT) and pulmonary embolism (PE) – in cancer patients (J Clin Oncol. 2019 Aug 5. doi: 10.1200/JCO.19.19.01461).
The addition of DOACs as options for VTE prophylaxis and treatment represents the most notable change to the guideline.
“Oral anticoagulants that target thrombin (direct thrombin inhibitor, dabigatran) or activated factor X (antifactor Xa inhibitors, rivaroxaban, apixaban, and edoxaban) are now approved for treatment of DVT or PE as well as for DVT prophylaxis following orthopedic surgery and for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation,” the guideline panel wrote.
A systematic review of PubMed and the Cochrane Library for randomized controlled trials (RCTs) and meta-analyses of RCTs published from Aug. 1, 2014, through Dec. 4, 2018, identified 35 publications on VTE prophylaxis and treatment, including 2 RCTs of DOACs for prophylaxis and 2 others of DOAC treatment, as well as 8 publications on VTE risk assessment. A multidisciplinary expert panel appointed by ASCO and cochaired by Dr. Key of the University of North Carolina, Chapel Hill, used this evidence to develop the updated guideline.
The work was guided by “the ‘signals’ approach that is designed to identify only new, potentially practice-changing data – signals – that might translate into revised practice recommendations,” the authors explained.
DOAC-related updates
VTE prophylaxis. Based in part on findings from the recently published AVERT trial of apixaban in patients initiating a new course of chemotherapy and from the CASSINI trial of rivaroxaban in patients with solid tumors or lymphoma starting systemic antineoplastic therapy, the panel added both agents as thromboprophylactic options that can be offered to high-risk cancer outpatients with no significant risk factors for bleeding or drug interactions (N Engl J Med. 2019;380:711-19; N Engl J Med. 2019;380:720-8).
Low-molecular-weight heparin (LMWH) also remains an option in such patients; consideration of therapy should involve discussion with the patient about relative benefits and harms, drug costs, and “the uncertainty surrounding duration of prophylaxis in this setting,” they wrote.
Anticoagulation for VTE. Options for initial anticoagulation include LMWH, unfractionated heparin (UFH), fondaparinux, and now rivaroxaban, with the latter added based on findings from two RCTs – the SELECT-D trial and the Hokusai VTE-Cancer study – and multiple meta-analyses (J Clin Oncol. 2018;36:2017-23; N Engl J Med. 2018;378:615-24).
Long-term anticoagulation can involve treatment with LMWH, edoxaban, or rivaroxaban for at least 6 months, all of which have improved efficacy versus vitamin K agonists (VKAs), the panel noted. However, VKAs may be used if LMWH and DOACs are not accessible.
Importantly, the literature indicates an increased risk of major bleeding with DOACs, particularly in patients with gastrointestinal malignancies and potentially in those with genitourinary malignancies. “Caution with DOACs is also warranted in other settings with high risk for mucosal bleeding,” the panel wrote.
Additional updates
CNS metastases. The anticoagulation recommendations were also updated to include patients with metastatic central nervous system malignancies (those with primary CNS malignancies were included previously). Both those with primary and metastatic CNS malignancy should be offered anticoagulation for established VTE as described for patients with other types of cancer. However, the panel stressed that “uncertainties remain about choice of agents and selection of patients most likely to benefit.”
“Patients with intracranial tumors are at increased risk for thrombotic complications and intracranial hemorrhage (ICH), but the presence of a stable or active primary intracranial malignancy or brain metastases is not an absolute contraindication to anticoagulation,” they wrote.
Limited evidence suggests that therapeutic anticoagulation does not increase ICH risk in patients with brain metastases, but it may increase risk in those with primary brain tumors, the panel added.
Additionally, preliminary data from a retrospective cohort of patients with metastatic brain disease and venous thrombosis suggest that DOACs may be associated with a lower risk of ICH than is LMWH in this population.
Long-term postoperative LMWH. Extended prophylaxis with LMWH for up to 4 weeks is recommended after major open or laparoscopic abdominal or pelvic surgery in cancer patients with high-risk features, such as restricted mobility, obesity, history of VTE, or with additional risk factors. Lower-risk surgical settings require case-by-case decision making about appropriate thromboprophylaxis duration, according to the update.
A 2014 RCT looking at thromboprophylaxis duration in 225 patients undergoing laparoscopic surgery for colorectal cancer prompted the addition of laparoscopic surgery to this recommendation. In that study, VTE occurred by 4 weeks in nearly 10% of patients receiving 1 week of prophylaxis and in no patients in the 4-week arm. Major bleeding occurred in one versus zero patients in the thromboprophylaxis arms, respectively (Ann Surg. April 2014;259[4]:665-9).
Reaffirmed recommendations
Based on the latest available data, the panel reaffirmed that most hospitalized patients with cancer and an acute medical condition require thromboprophylaxis for the duration of their hospitalization and that thromboprophylaxis should not be routinely recommended for all outpatients with cancer.
The panel also reaffirmed the need for thromboprophylaxis starting preoperatively and continuing for at least 7-10 days in patients undergoing major cancer surgery, the need for periodic assessment of VTE risk in cancer patients, and the importance of patient education about the signs and symptoms of VTE.
Perspective and future directions
In an interview, David H. Henry, MD, said he was pleased to see ASCO incorporate the latest DOAC data into the VTE guideline.
The AVERT and CASSINI studies, in particular, highlight the value of using the Khorana Risk Score, which considers cancer type, blood counts, and body mass index to predict the risk of thrombosis in cancer patients and to guide decisions regarding prophylaxis, said Dr. Henry, vice chair of the department of medicine and clinical professor of medicine at Penn Medicine’s Abramson Cancer Center, Philadelphia.
The DOACs also represent “a nice new development in the treatment setting,” he said, adding that it’s been long known – since the 2003 CLOT trial – that cancer patients with VTE had much lower recurrence rates with LMWH versus warfarin (Coumadin).
“Now fast forward to the modern era ... and DOACs now appear to be a good idea,” he said.
Dr. Henry also addressed the recommendation for expanded postoperative LMWH use.
“That I found interesting; I’m not sure what took them so long,” he said, explaining that National Comprehensive Cancer Network and European Society of Medical Oncology recommendations have long stated that, for patients with abdominal cancers who undergo abdominopelvic surgery, DVT prophylaxis should continue for 4 weeks.
Dr. Henry said that a survey at his center showed that those recommendations were “very poorly followed,” with surgeons giving 4 weeks of prophylaxis in just 5% of cases.
“The good news from our survey was that not many people had a VTE, despite not many people following the recommendations, but I must say I think our surgeons are catching on,” he said.
Overall, the updated guideline highlights the importance of considering the “cancer variable” when it comes to VTE prevention and treatment.
“We’ve known forever that when we diagnose a DVT or PE in the outpatient setting – and this is independent of cancer – that you should treat it. Add the cancer variable and we now know that we should worry and try to prevent the VTE in certain high-risk patients, and there are some drugs to do it with,” he said, adding that “you should worry about the person you’ve just provoked [with surgery] as well.”
An important question not addressed in the guideline update is the indefinite use of DOACs in cancer patients with ongoing risk, he said.
“When we see DVT or PE, we usually treat for 3 months – that’s the industry standard – and at the end of 3 months ... you do a time out and you say to yourself, ‘Was this person provoked?’ ” he said.
For example, if they took a long flight or if pregnancy was a factor, treatment can usually be safely stopped. However, in a cancer patient who still has cancer, the provocation continues, and the patient may require indefinite treatment.
Questions that remain involve defining “indefinite” and include whether (and which of) these drugs can be used indefinitely in such patients, Dr. Henry said.
Dr. Key reported receiving honoraria from Novo Nordisk, research funding to his institution from Baxter Biosciences, Grifols, and Pfizer, and serving as a consultant or advisor for Genentech, Roche, Uniqure, Seattle Genetics, and Shire Human Genetic Therapies. Numerous disclosures were also reported by other expert panel members.
The direct oral anticoagulants (DOACs) apixaban and rivaroxaban are now among the options for thromboprophylaxis in high-risk cancer outpatients with low risk for bleeding and drug interactions, according to a practice guideline update from the American Society of Clinical Oncology.
Rivaroxaban also has been added as an option for initial anticoagulation for venous thromboembolism (VTE), and both rivaroxaban and edoxaban are now options for long-term anticoagulation, Nigel S. Key, MB ChB, and colleagues wrote in the updated guideline on the prophylaxis and treatment of VTE – including deep vein thrombosis (DVT) and pulmonary embolism (PE) – in cancer patients (J Clin Oncol. 2019 Aug 5. doi: 10.1200/JCO.19.19.01461).
The addition of DOACs as options for VTE prophylaxis and treatment represents the most notable change to the guideline.
“Oral anticoagulants that target thrombin (direct thrombin inhibitor, dabigatran) or activated factor X (antifactor Xa inhibitors, rivaroxaban, apixaban, and edoxaban) are now approved for treatment of DVT or PE as well as for DVT prophylaxis following orthopedic surgery and for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation,” the guideline panel wrote.
A systematic review of PubMed and the Cochrane Library for randomized controlled trials (RCTs) and meta-analyses of RCTs published from Aug. 1, 2014, through Dec. 4, 2018, identified 35 publications on VTE prophylaxis and treatment, including 2 RCTs of DOACs for prophylaxis and 2 others of DOAC treatment, as well as 8 publications on VTE risk assessment. A multidisciplinary expert panel appointed by ASCO and cochaired by Dr. Key of the University of North Carolina, Chapel Hill, used this evidence to develop the updated guideline.
The work was guided by “the ‘signals’ approach that is designed to identify only new, potentially practice-changing data – signals – that might translate into revised practice recommendations,” the authors explained.
DOAC-related updates
VTE prophylaxis. Based in part on findings from the recently published AVERT trial of apixaban in patients initiating a new course of chemotherapy and from the CASSINI trial of rivaroxaban in patients with solid tumors or lymphoma starting systemic antineoplastic therapy, the panel added both agents as thromboprophylactic options that can be offered to high-risk cancer outpatients with no significant risk factors for bleeding or drug interactions (N Engl J Med. 2019;380:711-19; N Engl J Med. 2019;380:720-8).
Low-molecular-weight heparin (LMWH) also remains an option in such patients; consideration of therapy should involve discussion with the patient about relative benefits and harms, drug costs, and “the uncertainty surrounding duration of prophylaxis in this setting,” they wrote.
Anticoagulation for VTE. Options for initial anticoagulation include LMWH, unfractionated heparin (UFH), fondaparinux, and now rivaroxaban, with the latter added based on findings from two RCTs – the SELECT-D trial and the Hokusai VTE-Cancer study – and multiple meta-analyses (J Clin Oncol. 2018;36:2017-23; N Engl J Med. 2018;378:615-24).
Long-term anticoagulation can involve treatment with LMWH, edoxaban, or rivaroxaban for at least 6 months, all of which have improved efficacy versus vitamin K agonists (VKAs), the panel noted. However, VKAs may be used if LMWH and DOACs are not accessible.
Importantly, the literature indicates an increased risk of major bleeding with DOACs, particularly in patients with gastrointestinal malignancies and potentially in those with genitourinary malignancies. “Caution with DOACs is also warranted in other settings with high risk for mucosal bleeding,” the panel wrote.
Additional updates
CNS metastases. The anticoagulation recommendations were also updated to include patients with metastatic central nervous system malignancies (those with primary CNS malignancies were included previously). Both those with primary and metastatic CNS malignancy should be offered anticoagulation for established VTE as described for patients with other types of cancer. However, the panel stressed that “uncertainties remain about choice of agents and selection of patients most likely to benefit.”
“Patients with intracranial tumors are at increased risk for thrombotic complications and intracranial hemorrhage (ICH), but the presence of a stable or active primary intracranial malignancy or brain metastases is not an absolute contraindication to anticoagulation,” they wrote.
Limited evidence suggests that therapeutic anticoagulation does not increase ICH risk in patients with brain metastases, but it may increase risk in those with primary brain tumors, the panel added.
Additionally, preliminary data from a retrospective cohort of patients with metastatic brain disease and venous thrombosis suggest that DOACs may be associated with a lower risk of ICH than is LMWH in this population.
Long-term postoperative LMWH. Extended prophylaxis with LMWH for up to 4 weeks is recommended after major open or laparoscopic abdominal or pelvic surgery in cancer patients with high-risk features, such as restricted mobility, obesity, history of VTE, or with additional risk factors. Lower-risk surgical settings require case-by-case decision making about appropriate thromboprophylaxis duration, according to the update.
A 2014 RCT looking at thromboprophylaxis duration in 225 patients undergoing laparoscopic surgery for colorectal cancer prompted the addition of laparoscopic surgery to this recommendation. In that study, VTE occurred by 4 weeks in nearly 10% of patients receiving 1 week of prophylaxis and in no patients in the 4-week arm. Major bleeding occurred in one versus zero patients in the thromboprophylaxis arms, respectively (Ann Surg. April 2014;259[4]:665-9).
Reaffirmed recommendations
Based on the latest available data, the panel reaffirmed that most hospitalized patients with cancer and an acute medical condition require thromboprophylaxis for the duration of their hospitalization and that thromboprophylaxis should not be routinely recommended for all outpatients with cancer.
The panel also reaffirmed the need for thromboprophylaxis starting preoperatively and continuing for at least 7-10 days in patients undergoing major cancer surgery, the need for periodic assessment of VTE risk in cancer patients, and the importance of patient education about the signs and symptoms of VTE.
Perspective and future directions
In an interview, David H. Henry, MD, said he was pleased to see ASCO incorporate the latest DOAC data into the VTE guideline.
The AVERT and CASSINI studies, in particular, highlight the value of using the Khorana Risk Score, which considers cancer type, blood counts, and body mass index to predict the risk of thrombosis in cancer patients and to guide decisions regarding prophylaxis, said Dr. Henry, vice chair of the department of medicine and clinical professor of medicine at Penn Medicine’s Abramson Cancer Center, Philadelphia.
The DOACs also represent “a nice new development in the treatment setting,” he said, adding that it’s been long known – since the 2003 CLOT trial – that cancer patients with VTE had much lower recurrence rates with LMWH versus warfarin (Coumadin).
“Now fast forward to the modern era ... and DOACs now appear to be a good idea,” he said.
Dr. Henry also addressed the recommendation for expanded postoperative LMWH use.
“That I found interesting; I’m not sure what took them so long,” he said, explaining that National Comprehensive Cancer Network and European Society of Medical Oncology recommendations have long stated that, for patients with abdominal cancers who undergo abdominopelvic surgery, DVT prophylaxis should continue for 4 weeks.
Dr. Henry said that a survey at his center showed that those recommendations were “very poorly followed,” with surgeons giving 4 weeks of prophylaxis in just 5% of cases.
“The good news from our survey was that not many people had a VTE, despite not many people following the recommendations, but I must say I think our surgeons are catching on,” he said.
Overall, the updated guideline highlights the importance of considering the “cancer variable” when it comes to VTE prevention and treatment.
“We’ve known forever that when we diagnose a DVT or PE in the outpatient setting – and this is independent of cancer – that you should treat it. Add the cancer variable and we now know that we should worry and try to prevent the VTE in certain high-risk patients, and there are some drugs to do it with,” he said, adding that “you should worry about the person you’ve just provoked [with surgery] as well.”
An important question not addressed in the guideline update is the indefinite use of DOACs in cancer patients with ongoing risk, he said.
“When we see DVT or PE, we usually treat for 3 months – that’s the industry standard – and at the end of 3 months ... you do a time out and you say to yourself, ‘Was this person provoked?’ ” he said.
For example, if they took a long flight or if pregnancy was a factor, treatment can usually be safely stopped. However, in a cancer patient who still has cancer, the provocation continues, and the patient may require indefinite treatment.
Questions that remain involve defining “indefinite” and include whether (and which of) these drugs can be used indefinitely in such patients, Dr. Henry said.
Dr. Key reported receiving honoraria from Novo Nordisk, research funding to his institution from Baxter Biosciences, Grifols, and Pfizer, and serving as a consultant or advisor for Genentech, Roche, Uniqure, Seattle Genetics, and Shire Human Genetic Therapies. Numerous disclosures were also reported by other expert panel members.
The direct oral anticoagulants (DOACs) apixaban and rivaroxaban are now among the options for thromboprophylaxis in high-risk cancer outpatients with low risk for bleeding and drug interactions, according to a practice guideline update from the American Society of Clinical Oncology.
Rivaroxaban also has been added as an option for initial anticoagulation for venous thromboembolism (VTE), and both rivaroxaban and edoxaban are now options for long-term anticoagulation, Nigel S. Key, MB ChB, and colleagues wrote in the updated guideline on the prophylaxis and treatment of VTE – including deep vein thrombosis (DVT) and pulmonary embolism (PE) – in cancer patients (J Clin Oncol. 2019 Aug 5. doi: 10.1200/JCO.19.19.01461).
The addition of DOACs as options for VTE prophylaxis and treatment represents the most notable change to the guideline.
“Oral anticoagulants that target thrombin (direct thrombin inhibitor, dabigatran) or activated factor X (antifactor Xa inhibitors, rivaroxaban, apixaban, and edoxaban) are now approved for treatment of DVT or PE as well as for DVT prophylaxis following orthopedic surgery and for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation,” the guideline panel wrote.
A systematic review of PubMed and the Cochrane Library for randomized controlled trials (RCTs) and meta-analyses of RCTs published from Aug. 1, 2014, through Dec. 4, 2018, identified 35 publications on VTE prophylaxis and treatment, including 2 RCTs of DOACs for prophylaxis and 2 others of DOAC treatment, as well as 8 publications on VTE risk assessment. A multidisciplinary expert panel appointed by ASCO and cochaired by Dr. Key of the University of North Carolina, Chapel Hill, used this evidence to develop the updated guideline.
The work was guided by “the ‘signals’ approach that is designed to identify only new, potentially practice-changing data – signals – that might translate into revised practice recommendations,” the authors explained.
DOAC-related updates
VTE prophylaxis. Based in part on findings from the recently published AVERT trial of apixaban in patients initiating a new course of chemotherapy and from the CASSINI trial of rivaroxaban in patients with solid tumors or lymphoma starting systemic antineoplastic therapy, the panel added both agents as thromboprophylactic options that can be offered to high-risk cancer outpatients with no significant risk factors for bleeding or drug interactions (N Engl J Med. 2019;380:711-19; N Engl J Med. 2019;380:720-8).
Low-molecular-weight heparin (LMWH) also remains an option in such patients; consideration of therapy should involve discussion with the patient about relative benefits and harms, drug costs, and “the uncertainty surrounding duration of prophylaxis in this setting,” they wrote.
Anticoagulation for VTE. Options for initial anticoagulation include LMWH, unfractionated heparin (UFH), fondaparinux, and now rivaroxaban, with the latter added based on findings from two RCTs – the SELECT-D trial and the Hokusai VTE-Cancer study – and multiple meta-analyses (J Clin Oncol. 2018;36:2017-23; N Engl J Med. 2018;378:615-24).
Long-term anticoagulation can involve treatment with LMWH, edoxaban, or rivaroxaban for at least 6 months, all of which have improved efficacy versus vitamin K agonists (VKAs), the panel noted. However, VKAs may be used if LMWH and DOACs are not accessible.
Importantly, the literature indicates an increased risk of major bleeding with DOACs, particularly in patients with gastrointestinal malignancies and potentially in those with genitourinary malignancies. “Caution with DOACs is also warranted in other settings with high risk for mucosal bleeding,” the panel wrote.
Additional updates
CNS metastases. The anticoagulation recommendations were also updated to include patients with metastatic central nervous system malignancies (those with primary CNS malignancies were included previously). Both those with primary and metastatic CNS malignancy should be offered anticoagulation for established VTE as described for patients with other types of cancer. However, the panel stressed that “uncertainties remain about choice of agents and selection of patients most likely to benefit.”
“Patients with intracranial tumors are at increased risk for thrombotic complications and intracranial hemorrhage (ICH), but the presence of a stable or active primary intracranial malignancy or brain metastases is not an absolute contraindication to anticoagulation,” they wrote.
Limited evidence suggests that therapeutic anticoagulation does not increase ICH risk in patients with brain metastases, but it may increase risk in those with primary brain tumors, the panel added.
Additionally, preliminary data from a retrospective cohort of patients with metastatic brain disease and venous thrombosis suggest that DOACs may be associated with a lower risk of ICH than is LMWH in this population.
Long-term postoperative LMWH. Extended prophylaxis with LMWH for up to 4 weeks is recommended after major open or laparoscopic abdominal or pelvic surgery in cancer patients with high-risk features, such as restricted mobility, obesity, history of VTE, or with additional risk factors. Lower-risk surgical settings require case-by-case decision making about appropriate thromboprophylaxis duration, according to the update.
A 2014 RCT looking at thromboprophylaxis duration in 225 patients undergoing laparoscopic surgery for colorectal cancer prompted the addition of laparoscopic surgery to this recommendation. In that study, VTE occurred by 4 weeks in nearly 10% of patients receiving 1 week of prophylaxis and in no patients in the 4-week arm. Major bleeding occurred in one versus zero patients in the thromboprophylaxis arms, respectively (Ann Surg. April 2014;259[4]:665-9).
Reaffirmed recommendations
Based on the latest available data, the panel reaffirmed that most hospitalized patients with cancer and an acute medical condition require thromboprophylaxis for the duration of their hospitalization and that thromboprophylaxis should not be routinely recommended for all outpatients with cancer.
The panel also reaffirmed the need for thromboprophylaxis starting preoperatively and continuing for at least 7-10 days in patients undergoing major cancer surgery, the need for periodic assessment of VTE risk in cancer patients, and the importance of patient education about the signs and symptoms of VTE.
Perspective and future directions
In an interview, David H. Henry, MD, said he was pleased to see ASCO incorporate the latest DOAC data into the VTE guideline.
The AVERT and CASSINI studies, in particular, highlight the value of using the Khorana Risk Score, which considers cancer type, blood counts, and body mass index to predict the risk of thrombosis in cancer patients and to guide decisions regarding prophylaxis, said Dr. Henry, vice chair of the department of medicine and clinical professor of medicine at Penn Medicine’s Abramson Cancer Center, Philadelphia.
The DOACs also represent “a nice new development in the treatment setting,” he said, adding that it’s been long known – since the 2003 CLOT trial – that cancer patients with VTE had much lower recurrence rates with LMWH versus warfarin (Coumadin).
“Now fast forward to the modern era ... and DOACs now appear to be a good idea,” he said.
Dr. Henry also addressed the recommendation for expanded postoperative LMWH use.
“That I found interesting; I’m not sure what took them so long,” he said, explaining that National Comprehensive Cancer Network and European Society of Medical Oncology recommendations have long stated that, for patients with abdominal cancers who undergo abdominopelvic surgery, DVT prophylaxis should continue for 4 weeks.
Dr. Henry said that a survey at his center showed that those recommendations were “very poorly followed,” with surgeons giving 4 weeks of prophylaxis in just 5% of cases.
“The good news from our survey was that not many people had a VTE, despite not many people following the recommendations, but I must say I think our surgeons are catching on,” he said.
Overall, the updated guideline highlights the importance of considering the “cancer variable” when it comes to VTE prevention and treatment.
“We’ve known forever that when we diagnose a DVT or PE in the outpatient setting – and this is independent of cancer – that you should treat it. Add the cancer variable and we now know that we should worry and try to prevent the VTE in certain high-risk patients, and there are some drugs to do it with,” he said, adding that “you should worry about the person you’ve just provoked [with surgery] as well.”
An important question not addressed in the guideline update is the indefinite use of DOACs in cancer patients with ongoing risk, he said.
“When we see DVT or PE, we usually treat for 3 months – that’s the industry standard – and at the end of 3 months ... you do a time out and you say to yourself, ‘Was this person provoked?’ ” he said.
For example, if they took a long flight or if pregnancy was a factor, treatment can usually be safely stopped. However, in a cancer patient who still has cancer, the provocation continues, and the patient may require indefinite treatment.
Questions that remain involve defining “indefinite” and include whether (and which of) these drugs can be used indefinitely in such patients, Dr. Henry said.
Dr. Key reported receiving honoraria from Novo Nordisk, research funding to his institution from Baxter Biosciences, Grifols, and Pfizer, and serving as a consultant or advisor for Genentech, Roche, Uniqure, Seattle Genetics, and Shire Human Genetic Therapies. Numerous disclosures were also reported by other expert panel members.