User login
Cardiovascular disease remains leading cause of type 2 diabetes mortality
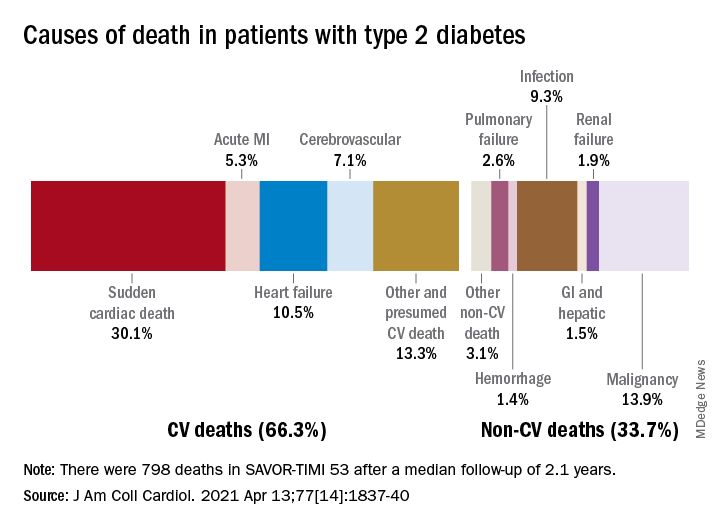
Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA okays new indication for alirocumab in homozygous FH
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
Use of complimentary and alternative medicine common in diabetes patients
An updated worldwide estimate of complementary and alternative medicine (CAM) use among individuals with diabetes found widespread use, though it varied greatly by region and is sometimes hard to define.
The report is the first literature review of the subject since 2007. The researchers looked at CAM use by region, as well as by patient categories such as those with advanced diabetes and by length of time since diagnosis. The most commonly reported CAMs in use were herbal medicine, acupuncture, homeopathy, and spiritual healing.
Only about one-third of patients disclosed their CAM use to their physician or health care provider. “We suggest that health care professionals should carefully anticipate the likelihood of their [patients’] diabetic CAM use in order to enhance treatment optimization and promote medication adherence, as well as to provide a fully informed consultation,” said first author Abdulaziz S. Alzahrani, a PhD student at the University of Birmingham (England). The study was published March 8, 2021, in the European Journal of Clinical Pharmacology.
Patients also have a responsibility, said Gregory Rhee, PhD, assistant professor of public health sciences at the University of Connecticut, Farmington. He was the lead author of a 2018 survey of CAM use in adults aged 65 years and older with diabetes in the United States using data from the 2012 National Health Interview Survey, and found that 25% had used CAM in some form in the prior year. “They need to be more up front, more proactive talking about CAM use with their doctors, and the second part is the physician. They also should be better educated in terms of CAM use. Traditionally, the physician in Western societies have pretty much ignored CAM use. But they are getting aware of CAM use and also we know that people are coming from multiple cultural backgrounds. The physicians and other health care providers should be better informed about CAM, and they should be better educated about it to provide patients better practice,” said Dr. Rhee.
He also distinguished between approaches like yoga or Tai Chi, which are physically oriented and not particularly controversial, and herbal medicines or dietary supplements. “Those can be controversial because we do not have strong scientific evidence to support those modalities for effectiveness on diabetes management,” Dr. Rhee added.
Mr. Alzahrani and colleagues conducted a meta-analysis of 38 studies, which included data from 25 countries. The included studies varied in their approach. For example, 16 studies focused exclusively on herbal and nutritional supplements. The most commonly mentioned CAMs were acupuncture and mind-body therapies (each named in six studies), religious and spiritual healing (five studies), and homeopathy (four studies). Among 31 studies focusing on herbal and nutritional supplements, the most common herbs mentioned were cinnamon and fenugreek (mentioned in 18 studies), garlic (17 studies), aloe vera (14 studies), and black seed (12 studies).
Prevalence of CAM use varied widely, ranging from 17% in Jordan to 89% in India and in a separate study in Jordan. The pooled prevalence of CAM use was 51% (95% confidence interval, 43%-59%). Subgroup analyses found the highest rate of CAM use in Europe (76%) and Africa (55%), and the lowest in North America (45%).
When the researchers examined patient characteristics, they found no significant relationship between CAM use and established ethnicity groups, or between type 1 and type 2 diabetes. The prevalence ratio was lower among men (PR, 0.86; 95% CI, 0.81-0.91). PRs for CAM use were lower among those with diabetic complications (PR, 0.81; 95% CI, 0.66-0.99). Individuals with diabetes of at least 5 years’ duration were more likely to use CAM than those with shorter duration of illness (PR, 1.71; 95% CI, 1.04-1.32).
Most (78%) CAM users employed it as an addition to their treatment regimen (95% CI, 56-94%), while 21% used it as an alternative to prescribed medicine (95% CI, 12-31%). More than two-thirds (67%) of individuals did not disclose CAM use to health care professionals (95% CI, 58-76%).
Although CAM use can be a source of friction between patients and physicians, Dr. Rhee also sees it as an opportunity. Patients from diverse backgrounds may be using CAM, often as a result of different cultural backgrounds. He cited the belief in some Asian countries that the balance of Yin and Yang is key to health, which many patients believe can be addressed through CAM. “If we want to promote cultural diversity, if we really care about patient diversity, I think CAM is one of the potential sources where the doctors should know [more about] the issue,” said Dr. Rhee.
The study was funded by the University of Birmingham. Dr. Rhee and Mr. Alzahrani have no relevant financial disclosures.
An updated worldwide estimate of complementary and alternative medicine (CAM) use among individuals with diabetes found widespread use, though it varied greatly by region and is sometimes hard to define.
The report is the first literature review of the subject since 2007. The researchers looked at CAM use by region, as well as by patient categories such as those with advanced diabetes and by length of time since diagnosis. The most commonly reported CAMs in use were herbal medicine, acupuncture, homeopathy, and spiritual healing.
Only about one-third of patients disclosed their CAM use to their physician or health care provider. “We suggest that health care professionals should carefully anticipate the likelihood of their [patients’] diabetic CAM use in order to enhance treatment optimization and promote medication adherence, as well as to provide a fully informed consultation,” said first author Abdulaziz S. Alzahrani, a PhD student at the University of Birmingham (England). The study was published March 8, 2021, in the European Journal of Clinical Pharmacology.
Patients also have a responsibility, said Gregory Rhee, PhD, assistant professor of public health sciences at the University of Connecticut, Farmington. He was the lead author of a 2018 survey of CAM use in adults aged 65 years and older with diabetes in the United States using data from the 2012 National Health Interview Survey, and found that 25% had used CAM in some form in the prior year. “They need to be more up front, more proactive talking about CAM use with their doctors, and the second part is the physician. They also should be better educated in terms of CAM use. Traditionally, the physician in Western societies have pretty much ignored CAM use. But they are getting aware of CAM use and also we know that people are coming from multiple cultural backgrounds. The physicians and other health care providers should be better informed about CAM, and they should be better educated about it to provide patients better practice,” said Dr. Rhee.
He also distinguished between approaches like yoga or Tai Chi, which are physically oriented and not particularly controversial, and herbal medicines or dietary supplements. “Those can be controversial because we do not have strong scientific evidence to support those modalities for effectiveness on diabetes management,” Dr. Rhee added.
Mr. Alzahrani and colleagues conducted a meta-analysis of 38 studies, which included data from 25 countries. The included studies varied in their approach. For example, 16 studies focused exclusively on herbal and nutritional supplements. The most commonly mentioned CAMs were acupuncture and mind-body therapies (each named in six studies), religious and spiritual healing (five studies), and homeopathy (four studies). Among 31 studies focusing on herbal and nutritional supplements, the most common herbs mentioned were cinnamon and fenugreek (mentioned in 18 studies), garlic (17 studies), aloe vera (14 studies), and black seed (12 studies).
Prevalence of CAM use varied widely, ranging from 17% in Jordan to 89% in India and in a separate study in Jordan. The pooled prevalence of CAM use was 51% (95% confidence interval, 43%-59%). Subgroup analyses found the highest rate of CAM use in Europe (76%) and Africa (55%), and the lowest in North America (45%).
When the researchers examined patient characteristics, they found no significant relationship between CAM use and established ethnicity groups, or between type 1 and type 2 diabetes. The prevalence ratio was lower among men (PR, 0.86; 95% CI, 0.81-0.91). PRs for CAM use were lower among those with diabetic complications (PR, 0.81; 95% CI, 0.66-0.99). Individuals with diabetes of at least 5 years’ duration were more likely to use CAM than those with shorter duration of illness (PR, 1.71; 95% CI, 1.04-1.32).
Most (78%) CAM users employed it as an addition to their treatment regimen (95% CI, 56-94%), while 21% used it as an alternative to prescribed medicine (95% CI, 12-31%). More than two-thirds (67%) of individuals did not disclose CAM use to health care professionals (95% CI, 58-76%).
Although CAM use can be a source of friction between patients and physicians, Dr. Rhee also sees it as an opportunity. Patients from diverse backgrounds may be using CAM, often as a result of different cultural backgrounds. He cited the belief in some Asian countries that the balance of Yin and Yang is key to health, which many patients believe can be addressed through CAM. “If we want to promote cultural diversity, if we really care about patient diversity, I think CAM is one of the potential sources where the doctors should know [more about] the issue,” said Dr. Rhee.
The study was funded by the University of Birmingham. Dr. Rhee and Mr. Alzahrani have no relevant financial disclosures.
An updated worldwide estimate of complementary and alternative medicine (CAM) use among individuals with diabetes found widespread use, though it varied greatly by region and is sometimes hard to define.
The report is the first literature review of the subject since 2007. The researchers looked at CAM use by region, as well as by patient categories such as those with advanced diabetes and by length of time since diagnosis. The most commonly reported CAMs in use were herbal medicine, acupuncture, homeopathy, and spiritual healing.
Only about one-third of patients disclosed their CAM use to their physician or health care provider. “We suggest that health care professionals should carefully anticipate the likelihood of their [patients’] diabetic CAM use in order to enhance treatment optimization and promote medication adherence, as well as to provide a fully informed consultation,” said first author Abdulaziz S. Alzahrani, a PhD student at the University of Birmingham (England). The study was published March 8, 2021, in the European Journal of Clinical Pharmacology.
Patients also have a responsibility, said Gregory Rhee, PhD, assistant professor of public health sciences at the University of Connecticut, Farmington. He was the lead author of a 2018 survey of CAM use in adults aged 65 years and older with diabetes in the United States using data from the 2012 National Health Interview Survey, and found that 25% had used CAM in some form in the prior year. “They need to be more up front, more proactive talking about CAM use with their doctors, and the second part is the physician. They also should be better educated in terms of CAM use. Traditionally, the physician in Western societies have pretty much ignored CAM use. But they are getting aware of CAM use and also we know that people are coming from multiple cultural backgrounds. The physicians and other health care providers should be better informed about CAM, and they should be better educated about it to provide patients better practice,” said Dr. Rhee.
He also distinguished between approaches like yoga or Tai Chi, which are physically oriented and not particularly controversial, and herbal medicines or dietary supplements. “Those can be controversial because we do not have strong scientific evidence to support those modalities for effectiveness on diabetes management,” Dr. Rhee added.
Mr. Alzahrani and colleagues conducted a meta-analysis of 38 studies, which included data from 25 countries. The included studies varied in their approach. For example, 16 studies focused exclusively on herbal and nutritional supplements. The most commonly mentioned CAMs were acupuncture and mind-body therapies (each named in six studies), religious and spiritual healing (five studies), and homeopathy (four studies). Among 31 studies focusing on herbal and nutritional supplements, the most common herbs mentioned were cinnamon and fenugreek (mentioned in 18 studies), garlic (17 studies), aloe vera (14 studies), and black seed (12 studies).
Prevalence of CAM use varied widely, ranging from 17% in Jordan to 89% in India and in a separate study in Jordan. The pooled prevalence of CAM use was 51% (95% confidence interval, 43%-59%). Subgroup analyses found the highest rate of CAM use in Europe (76%) and Africa (55%), and the lowest in North America (45%).
When the researchers examined patient characteristics, they found no significant relationship between CAM use and established ethnicity groups, or between type 1 and type 2 diabetes. The prevalence ratio was lower among men (PR, 0.86; 95% CI, 0.81-0.91). PRs for CAM use were lower among those with diabetic complications (PR, 0.81; 95% CI, 0.66-0.99). Individuals with diabetes of at least 5 years’ duration were more likely to use CAM than those with shorter duration of illness (PR, 1.71; 95% CI, 1.04-1.32).
Most (78%) CAM users employed it as an addition to their treatment regimen (95% CI, 56-94%), while 21% used it as an alternative to prescribed medicine (95% CI, 12-31%). More than two-thirds (67%) of individuals did not disclose CAM use to health care professionals (95% CI, 58-76%).
Although CAM use can be a source of friction between patients and physicians, Dr. Rhee also sees it as an opportunity. Patients from diverse backgrounds may be using CAM, often as a result of different cultural backgrounds. He cited the belief in some Asian countries that the balance of Yin and Yang is key to health, which many patients believe can be addressed through CAM. “If we want to promote cultural diversity, if we really care about patient diversity, I think CAM is one of the potential sources where the doctors should know [more about] the issue,” said Dr. Rhee.
The study was funded by the University of Birmingham. Dr. Rhee and Mr. Alzahrani have no relevant financial disclosures.
FROM THE EUROPEAN JOURNAL OF CLINICAL PHARMACOLOGY
Dapagliflozin may cut risk of HF hospitalization in patients with type 2 diabetes
Background: Dapagliflozin is a selective inhibitor of sodium-glucose transporter 2 (SGLT2) in the kidney; the drug blocks glucose reabsorption in the proximal tubule. It is taken once daily by mouth. An initial study sponsored by AstraZeneca was published January 2019 in the New England Journal of Medicine – “Dapagliflozin and cardiovascular outcomes in type 2 diabetes.” Until recently there was not an FDA-approved indication for the drug.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: 882 clinical sites in 33 countries.
Synopsis: The study randomized approximately 17,000 patients to receive either dapagliflozin or placebo in addition to any other diabetes treatments prescribed by their physician. This study demonstrated its primary safety outcome, which was that patients on dapagliflozin did not have any more major adverse cardiac events (MACE), compared with placebo. There were two primary efficacy outcomes. First, there was no change in MACE with dapagliflozin, compared with placebo. Second, and pertinent to this drug’s approval, was that dapagliflozin reduced risk of hospitalization for heart failure (HF) from 5.8% to 4.9%, compared to placebo; this includes both HF with both preserved and reduced ejection fractions.
Bottom line: Dapagliflozin now has an FDA-approved indication to reduce hospitalizations for HF in patients with type 2 diabetes. Based on this study, the number needed to treat with dapagliflozin is 111 patients to prevent one hospitalization for HF.
Citation: Farxiga approved in the US to reduce the risk of hospitalization for heart failure in patients with type-2 diabetes. AstraZeneca Press Release, 2019 Oct 21.
Dr. Como is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: Dapagliflozin is a selective inhibitor of sodium-glucose transporter 2 (SGLT2) in the kidney; the drug blocks glucose reabsorption in the proximal tubule. It is taken once daily by mouth. An initial study sponsored by AstraZeneca was published January 2019 in the New England Journal of Medicine – “Dapagliflozin and cardiovascular outcomes in type 2 diabetes.” Until recently there was not an FDA-approved indication for the drug.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: 882 clinical sites in 33 countries.
Synopsis: The study randomized approximately 17,000 patients to receive either dapagliflozin or placebo in addition to any other diabetes treatments prescribed by their physician. This study demonstrated its primary safety outcome, which was that patients on dapagliflozin did not have any more major adverse cardiac events (MACE), compared with placebo. There were two primary efficacy outcomes. First, there was no change in MACE with dapagliflozin, compared with placebo. Second, and pertinent to this drug’s approval, was that dapagliflozin reduced risk of hospitalization for heart failure (HF) from 5.8% to 4.9%, compared to placebo; this includes both HF with both preserved and reduced ejection fractions.
Bottom line: Dapagliflozin now has an FDA-approved indication to reduce hospitalizations for HF in patients with type 2 diabetes. Based on this study, the number needed to treat with dapagliflozin is 111 patients to prevent one hospitalization for HF.
Citation: Farxiga approved in the US to reduce the risk of hospitalization for heart failure in patients with type-2 diabetes. AstraZeneca Press Release, 2019 Oct 21.
Dr. Como is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: Dapagliflozin is a selective inhibitor of sodium-glucose transporter 2 (SGLT2) in the kidney; the drug blocks glucose reabsorption in the proximal tubule. It is taken once daily by mouth. An initial study sponsored by AstraZeneca was published January 2019 in the New England Journal of Medicine – “Dapagliflozin and cardiovascular outcomes in type 2 diabetes.” Until recently there was not an FDA-approved indication for the drug.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: 882 clinical sites in 33 countries.
Synopsis: The study randomized approximately 17,000 patients to receive either dapagliflozin or placebo in addition to any other diabetes treatments prescribed by their physician. This study demonstrated its primary safety outcome, which was that patients on dapagliflozin did not have any more major adverse cardiac events (MACE), compared with placebo. There were two primary efficacy outcomes. First, there was no change in MACE with dapagliflozin, compared with placebo. Second, and pertinent to this drug’s approval, was that dapagliflozin reduced risk of hospitalization for heart failure (HF) from 5.8% to 4.9%, compared to placebo; this includes both HF with both preserved and reduced ejection fractions.
Bottom line: Dapagliflozin now has an FDA-approved indication to reduce hospitalizations for HF in patients with type 2 diabetes. Based on this study, the number needed to treat with dapagliflozin is 111 patients to prevent one hospitalization for HF.
Citation: Farxiga approved in the US to reduce the risk of hospitalization for heart failure in patients with type-2 diabetes. AstraZeneca Press Release, 2019 Oct 21.
Dr. Como is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Artifactual hypoglycemia: When there’s a problem in the tube
If you are looking for zebras you might consider adrenal insufficiency, which could cause both hyperkalemia and hypoglycemia, but this would make no sense in someone asymptomatic.
This pattern is one I have seen commonly when I am on call, and I am contacted about abnormal labs. The lab reported no hemolysis seen, but this is the typical pattern seen with hemolytic specimens and/or specimens that have been held a long time before they are analyzed.
Lippi and colleagues reported on the clinically significant increase in potassium in samples that visually appeared not to be hemolyzed.1 Hemolyzed specimens can also drop glucose values, but not as profoundly as raising potassium values. When left unprocessed, glycolysis occurs in the white blood cells of a blood sample and may consume 5%-7% of the sample’s glucose content per hour.2
Khaled and colleagues looked at the drop in glucose levels in samples over time based on what anticoagulants were used.3 They found that, at 3 hours, glucose measurements were decreased by 28.4 mg/dL when sodium citrate is used, 58 mg/dL when EDTA was used, 15.4 mg/dL when fluoride oxalate was used, and 60.2 mg/dL when no anticoagulant is used.
Low blood sugars caused by elevated WBCs in blood samples has been well described.4 It has been described with moderate and very high WBC counts, as well as with the leukocytosis seen with polycythemia vera.5 The term “leukocyte larceny” has been used to describe high WBC counts that can not only utilize glucose, but also oxygen.
Saccheti and colleagues described a patient with a WBC greater than 500,000 who had repeatedly low oxygen levels on blood gases, that did not correlate with the normal oxygen saturations measured by pulse oximetry.6 This same issue has been seen in patients with extreme thrombocytosis.7Pearl: When labs don’t make sense clinically, always look at the possibility that there may be a problem in the tube and not in the person. Especially think of this when blood samples may have been held for a long time before they are run, such as with visiting nurse visits and blood draws at shelters and nursing homes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lippi G et al. Clin Chem Lab Med. 2006;44(3):311-6.
2. Mikesh LM and Bruns DE. Clin Chem. 2008 May;54(5):930-2.
3. Khaled S et al. Al-Mukhtar Journal of Sciences. 2018;33(2):100-6.
4. Goodenow TJ and Malarkey WB. JAMA. 1977;237(18):1961-2.
5. R Arem et al. Arch Intern Med. 1982 Nov;142(12):2199-201.
6. Sacchetti A et al. J Emerg Med. 1990;8:567–569.
7. A Mehta et al. Eur Respir J. 2008 Feb;31(2):469-72.
If you are looking for zebras you might consider adrenal insufficiency, which could cause both hyperkalemia and hypoglycemia, but this would make no sense in someone asymptomatic.
This pattern is one I have seen commonly when I am on call, and I am contacted about abnormal labs. The lab reported no hemolysis seen, but this is the typical pattern seen with hemolytic specimens and/or specimens that have been held a long time before they are analyzed.
Lippi and colleagues reported on the clinically significant increase in potassium in samples that visually appeared not to be hemolyzed.1 Hemolyzed specimens can also drop glucose values, but not as profoundly as raising potassium values. When left unprocessed, glycolysis occurs in the white blood cells of a blood sample and may consume 5%-7% of the sample’s glucose content per hour.2
Khaled and colleagues looked at the drop in glucose levels in samples over time based on what anticoagulants were used.3 They found that, at 3 hours, glucose measurements were decreased by 28.4 mg/dL when sodium citrate is used, 58 mg/dL when EDTA was used, 15.4 mg/dL when fluoride oxalate was used, and 60.2 mg/dL when no anticoagulant is used.
Low blood sugars caused by elevated WBCs in blood samples has been well described.4 It has been described with moderate and very high WBC counts, as well as with the leukocytosis seen with polycythemia vera.5 The term “leukocyte larceny” has been used to describe high WBC counts that can not only utilize glucose, but also oxygen.
Saccheti and colleagues described a patient with a WBC greater than 500,000 who had repeatedly low oxygen levels on blood gases, that did not correlate with the normal oxygen saturations measured by pulse oximetry.6 This same issue has been seen in patients with extreme thrombocytosis.7Pearl: When labs don’t make sense clinically, always look at the possibility that there may be a problem in the tube and not in the person. Especially think of this when blood samples may have been held for a long time before they are run, such as with visiting nurse visits and blood draws at shelters and nursing homes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lippi G et al. Clin Chem Lab Med. 2006;44(3):311-6.
2. Mikesh LM and Bruns DE. Clin Chem. 2008 May;54(5):930-2.
3. Khaled S et al. Al-Mukhtar Journal of Sciences. 2018;33(2):100-6.
4. Goodenow TJ and Malarkey WB. JAMA. 1977;237(18):1961-2.
5. R Arem et al. Arch Intern Med. 1982 Nov;142(12):2199-201.
6. Sacchetti A et al. J Emerg Med. 1990;8:567–569.
7. A Mehta et al. Eur Respir J. 2008 Feb;31(2):469-72.
If you are looking for zebras you might consider adrenal insufficiency, which could cause both hyperkalemia and hypoglycemia, but this would make no sense in someone asymptomatic.
This pattern is one I have seen commonly when I am on call, and I am contacted about abnormal labs. The lab reported no hemolysis seen, but this is the typical pattern seen with hemolytic specimens and/or specimens that have been held a long time before they are analyzed.
Lippi and colleagues reported on the clinically significant increase in potassium in samples that visually appeared not to be hemolyzed.1 Hemolyzed specimens can also drop glucose values, but not as profoundly as raising potassium values. When left unprocessed, glycolysis occurs in the white blood cells of a blood sample and may consume 5%-7% of the sample’s glucose content per hour.2
Khaled and colleagues looked at the drop in glucose levels in samples over time based on what anticoagulants were used.3 They found that, at 3 hours, glucose measurements were decreased by 28.4 mg/dL when sodium citrate is used, 58 mg/dL when EDTA was used, 15.4 mg/dL when fluoride oxalate was used, and 60.2 mg/dL when no anticoagulant is used.
Low blood sugars caused by elevated WBCs in blood samples has been well described.4 It has been described with moderate and very high WBC counts, as well as with the leukocytosis seen with polycythemia vera.5 The term “leukocyte larceny” has been used to describe high WBC counts that can not only utilize glucose, but also oxygen.
Saccheti and colleagues described a patient with a WBC greater than 500,000 who had repeatedly low oxygen levels on blood gases, that did not correlate with the normal oxygen saturations measured by pulse oximetry.6 This same issue has been seen in patients with extreme thrombocytosis.7Pearl: When labs don’t make sense clinically, always look at the possibility that there may be a problem in the tube and not in the person. Especially think of this when blood samples may have been held for a long time before they are run, such as with visiting nurse visits and blood draws at shelters and nursing homes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lippi G et al. Clin Chem Lab Med. 2006;44(3):311-6.
2. Mikesh LM and Bruns DE. Clin Chem. 2008 May;54(5):930-2.
3. Khaled S et al. Al-Mukhtar Journal of Sciences. 2018;33(2):100-6.
4. Goodenow TJ and Malarkey WB. JAMA. 1977;237(18):1961-2.
5. R Arem et al. Arch Intern Med. 1982 Nov;142(12):2199-201.
6. Sacchetti A et al. J Emerg Med. 1990;8:567–569.
7. A Mehta et al. Eur Respir J. 2008 Feb;31(2):469-72.
COVID-19 can cause atypical thyroid inflammation
Individuals who experience inflammation of the thyroid gland during acute COVID-19 illness may still have subacute thyroiditis months later, even if thyroid function has normalized, new research suggests.
Furthermore, the thyroiditis seems to be different from thyroid inflammation caused by other viruses, said Ilaria Muller, MD, PhD, when presenting her findings March 21 at the virtual ENDO 2021 meeting.
“SARS-CoV-2 seems to have multifactorial action on thyroid function,” said Dr. Muller, of the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy.
In July 2020, Dr. Muller and colleagues described patients hospitalized at their institution with severe COVID-19, 15% of whom had thyrotoxicosis due to atypical subacute thyroiditis, compared with just 1% of a comparison group hospitalized in the same subintensive care units during the spring of 2019, as reported by this news organization.
The “atypical” thyroiditis that occurred in the patients with COVID-19 was not associated with neck pain and affected more men than women. Moreover, it was associated with low TSH and free-triiodothyronine (T3) levels, and normal or elevated levels of free-thyroxine (T4), which is a very different presentation to classic nonthyroidal illness syndrome (NTIS) usually seen in critically ill patients, she explained.
Although transient T4 elevations can occur in acute illness, that phenomenon is not associated with low TSH. This newly described scenario appears to be a combination of thyrotoxicosis and NTIS, Dr. Muller and colleagues had speculated last summer.
Follow patients with COVID-19 and thyroid dysfunction for a year
Now, in an assessment of 51 patients 3 months after hospitalization for moderate-to-severe COVID-19 reported by Dr. Muller at ENDO 2021, both inflammatory markers and thyroid function had normalized, yet on imaging, a third of patients still exhibited focal hypoechoic areas suggestive of thyroiditis.
Of those, two-thirds had reduced uptake on thyroid scintigraphy, but few had antithyroid autoantibodies.
“The thyroid dysfunction induced by COVID-19 seems not mediated by autoimmunity. It is important to continue to follow these patients since they might develop thyroid dysfunction during the following months,” Dr. Muller emphasized.
Asked to comment, session moderator Robert W. Lash, MD, the Endocrine Society’s chief professional & clinical affairs officer, told this news organization: “When you’re ICU-level sick, it’s not unusual to have weird thyroid tests. Some viruses cause thyroid problems as well ... What makes this different is that while a lot of thyroid inflammation is caused by antibodies, this was not.”
“It looks like this was [SARS-CoV-2] causing damage to the thyroid gland, which is interesting,” he noted, adding that the thyroid gland expresses high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which allow SARS-CoV-2 to infect human cells.
“This is probably part of that same story,” Dr. Lash said.
For patients who had thyroid abnormalities during acute COVID-19 illness or develop symptoms that might be thyroid-related afterward, he advises: “You should keep an eye on thyroid tests. It just raises your awareness ... You might check their thyroid tests every 6 months for a year.”
Signs of focal thyroiditis despite normalized thyroid function
The 51 patients (33 men and 18 women) hospitalized with moderate-to-severe COVID-19 had no history of thyroid disease and had not been taking thyroid medications, amiodarone, or steroids before baseline TSH was measured.
From baseline to 3 months, TSH rose from 1.2 to 1.6 mIU/L, while serum concentrations of T4, T3, C-reactive protein, and full blood counts had all normalized (all P < 0.01 vs. baseline).
Thyroid ultrasound at 3 months in 49 patients showed signs of focal thyroiditis in 16 (33%).
Among 14 patients of those who further underwent thyroid 99mTc or I123 uptake scans, four (29%) were normal, eight (57%) had focally reduced uptake, and two (14%) had diffusely reduced uptake.
Of the 16 patients with focal thyroiditis, only three were positive for autoantibodies to thyroglobulin (TgAb) or thyroid peroxidase (TPOAb). All were negative for autoantibodies to the TSH receptor.
“Importantly, of the two with diffusely reduced uptake, only one was positive for TPOAb or TgAb,” Dr. Muller noted, adding, “SARS-CoV-2 disease seems to trigger some dysfunction which very likely has complex and multifactorial mechanisms.”
In response to a question about a possible role for biopsies and thyroid cytology, Dr. Muller replied: “That’s definitely the key ... So far we’re just making guesses, so the key will be cytological or histological studies to see what is really going on in the thyroid.”
“What we know is that [unlike] classical thyroiditis that has been described after viral diseases including SARS-CoV-2, these patients have a different scenario ... Probably something is going on within the thyroid with a different mechanism, so surely cytology and histology studies are what we need,” she concluded.
The study was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and by a COVID-19 research grant from the European Society of Endocrinology. Dr. Muller and Dr. Lash have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals who experience inflammation of the thyroid gland during acute COVID-19 illness may still have subacute thyroiditis months later, even if thyroid function has normalized, new research suggests.
Furthermore, the thyroiditis seems to be different from thyroid inflammation caused by other viruses, said Ilaria Muller, MD, PhD, when presenting her findings March 21 at the virtual ENDO 2021 meeting.
“SARS-CoV-2 seems to have multifactorial action on thyroid function,” said Dr. Muller, of the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy.
In July 2020, Dr. Muller and colleagues described patients hospitalized at their institution with severe COVID-19, 15% of whom had thyrotoxicosis due to atypical subacute thyroiditis, compared with just 1% of a comparison group hospitalized in the same subintensive care units during the spring of 2019, as reported by this news organization.
The “atypical” thyroiditis that occurred in the patients with COVID-19 was not associated with neck pain and affected more men than women. Moreover, it was associated with low TSH and free-triiodothyronine (T3) levels, and normal or elevated levels of free-thyroxine (T4), which is a very different presentation to classic nonthyroidal illness syndrome (NTIS) usually seen in critically ill patients, she explained.
Although transient T4 elevations can occur in acute illness, that phenomenon is not associated with low TSH. This newly described scenario appears to be a combination of thyrotoxicosis and NTIS, Dr. Muller and colleagues had speculated last summer.
Follow patients with COVID-19 and thyroid dysfunction for a year
Now, in an assessment of 51 patients 3 months after hospitalization for moderate-to-severe COVID-19 reported by Dr. Muller at ENDO 2021, both inflammatory markers and thyroid function had normalized, yet on imaging, a third of patients still exhibited focal hypoechoic areas suggestive of thyroiditis.
Of those, two-thirds had reduced uptake on thyroid scintigraphy, but few had antithyroid autoantibodies.
“The thyroid dysfunction induced by COVID-19 seems not mediated by autoimmunity. It is important to continue to follow these patients since they might develop thyroid dysfunction during the following months,” Dr. Muller emphasized.
Asked to comment, session moderator Robert W. Lash, MD, the Endocrine Society’s chief professional & clinical affairs officer, told this news organization: “When you’re ICU-level sick, it’s not unusual to have weird thyroid tests. Some viruses cause thyroid problems as well ... What makes this different is that while a lot of thyroid inflammation is caused by antibodies, this was not.”
“It looks like this was [SARS-CoV-2] causing damage to the thyroid gland, which is interesting,” he noted, adding that the thyroid gland expresses high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which allow SARS-CoV-2 to infect human cells.
“This is probably part of that same story,” Dr. Lash said.
For patients who had thyroid abnormalities during acute COVID-19 illness or develop symptoms that might be thyroid-related afterward, he advises: “You should keep an eye on thyroid tests. It just raises your awareness ... You might check their thyroid tests every 6 months for a year.”
Signs of focal thyroiditis despite normalized thyroid function
The 51 patients (33 men and 18 women) hospitalized with moderate-to-severe COVID-19 had no history of thyroid disease and had not been taking thyroid medications, amiodarone, or steroids before baseline TSH was measured.
From baseline to 3 months, TSH rose from 1.2 to 1.6 mIU/L, while serum concentrations of T4, T3, C-reactive protein, and full blood counts had all normalized (all P < 0.01 vs. baseline).
Thyroid ultrasound at 3 months in 49 patients showed signs of focal thyroiditis in 16 (33%).
Among 14 patients of those who further underwent thyroid 99mTc or I123 uptake scans, four (29%) were normal, eight (57%) had focally reduced uptake, and two (14%) had diffusely reduced uptake.
Of the 16 patients with focal thyroiditis, only three were positive for autoantibodies to thyroglobulin (TgAb) or thyroid peroxidase (TPOAb). All were negative for autoantibodies to the TSH receptor.
“Importantly, of the two with diffusely reduced uptake, only one was positive for TPOAb or TgAb,” Dr. Muller noted, adding, “SARS-CoV-2 disease seems to trigger some dysfunction which very likely has complex and multifactorial mechanisms.”
In response to a question about a possible role for biopsies and thyroid cytology, Dr. Muller replied: “That’s definitely the key ... So far we’re just making guesses, so the key will be cytological or histological studies to see what is really going on in the thyroid.”
“What we know is that [unlike] classical thyroiditis that has been described after viral diseases including SARS-CoV-2, these patients have a different scenario ... Probably something is going on within the thyroid with a different mechanism, so surely cytology and histology studies are what we need,” she concluded.
The study was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and by a COVID-19 research grant from the European Society of Endocrinology. Dr. Muller and Dr. Lash have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals who experience inflammation of the thyroid gland during acute COVID-19 illness may still have subacute thyroiditis months later, even if thyroid function has normalized, new research suggests.
Furthermore, the thyroiditis seems to be different from thyroid inflammation caused by other viruses, said Ilaria Muller, MD, PhD, when presenting her findings March 21 at the virtual ENDO 2021 meeting.
“SARS-CoV-2 seems to have multifactorial action on thyroid function,” said Dr. Muller, of the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy.
In July 2020, Dr. Muller and colleagues described patients hospitalized at their institution with severe COVID-19, 15% of whom had thyrotoxicosis due to atypical subacute thyroiditis, compared with just 1% of a comparison group hospitalized in the same subintensive care units during the spring of 2019, as reported by this news organization.
The “atypical” thyroiditis that occurred in the patients with COVID-19 was not associated with neck pain and affected more men than women. Moreover, it was associated with low TSH and free-triiodothyronine (T3) levels, and normal or elevated levels of free-thyroxine (T4), which is a very different presentation to classic nonthyroidal illness syndrome (NTIS) usually seen in critically ill patients, she explained.
Although transient T4 elevations can occur in acute illness, that phenomenon is not associated with low TSH. This newly described scenario appears to be a combination of thyrotoxicosis and NTIS, Dr. Muller and colleagues had speculated last summer.
Follow patients with COVID-19 and thyroid dysfunction for a year
Now, in an assessment of 51 patients 3 months after hospitalization for moderate-to-severe COVID-19 reported by Dr. Muller at ENDO 2021, both inflammatory markers and thyroid function had normalized, yet on imaging, a third of patients still exhibited focal hypoechoic areas suggestive of thyroiditis.
Of those, two-thirds had reduced uptake on thyroid scintigraphy, but few had antithyroid autoantibodies.
“The thyroid dysfunction induced by COVID-19 seems not mediated by autoimmunity. It is important to continue to follow these patients since they might develop thyroid dysfunction during the following months,” Dr. Muller emphasized.
Asked to comment, session moderator Robert W. Lash, MD, the Endocrine Society’s chief professional & clinical affairs officer, told this news organization: “When you’re ICU-level sick, it’s not unusual to have weird thyroid tests. Some viruses cause thyroid problems as well ... What makes this different is that while a lot of thyroid inflammation is caused by antibodies, this was not.”
“It looks like this was [SARS-CoV-2] causing damage to the thyroid gland, which is interesting,” he noted, adding that the thyroid gland expresses high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which allow SARS-CoV-2 to infect human cells.
“This is probably part of that same story,” Dr. Lash said.
For patients who had thyroid abnormalities during acute COVID-19 illness or develop symptoms that might be thyroid-related afterward, he advises: “You should keep an eye on thyroid tests. It just raises your awareness ... You might check their thyroid tests every 6 months for a year.”
Signs of focal thyroiditis despite normalized thyroid function
The 51 patients (33 men and 18 women) hospitalized with moderate-to-severe COVID-19 had no history of thyroid disease and had not been taking thyroid medications, amiodarone, or steroids before baseline TSH was measured.
From baseline to 3 months, TSH rose from 1.2 to 1.6 mIU/L, while serum concentrations of T4, T3, C-reactive protein, and full blood counts had all normalized (all P < 0.01 vs. baseline).
Thyroid ultrasound at 3 months in 49 patients showed signs of focal thyroiditis in 16 (33%).
Among 14 patients of those who further underwent thyroid 99mTc or I123 uptake scans, four (29%) were normal, eight (57%) had focally reduced uptake, and two (14%) had diffusely reduced uptake.
Of the 16 patients with focal thyroiditis, only three were positive for autoantibodies to thyroglobulin (TgAb) or thyroid peroxidase (TPOAb). All were negative for autoantibodies to the TSH receptor.
“Importantly, of the two with diffusely reduced uptake, only one was positive for TPOAb or TgAb,” Dr. Muller noted, adding, “SARS-CoV-2 disease seems to trigger some dysfunction which very likely has complex and multifactorial mechanisms.”
In response to a question about a possible role for biopsies and thyroid cytology, Dr. Muller replied: “That’s definitely the key ... So far we’re just making guesses, so the key will be cytological or histological studies to see what is really going on in the thyroid.”
“What we know is that [unlike] classical thyroiditis that has been described after viral diseases including SARS-CoV-2, these patients have a different scenario ... Probably something is going on within the thyroid with a different mechanism, so surely cytology and histology studies are what we need,” she concluded.
The study was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and by a COVID-19 research grant from the European Society of Endocrinology. Dr. Muller and Dr. Lash have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Combo thyroid hormones as good as levothyroxine for hypothyroidism
Patients with hypothyroidism treated with the three most common pharmacologic strategies of levothyroxine (LT4) alone, LT4 in combination with triiodothyronine (T3), or desiccated thyroid extract showed no differences in thyroid symptoms or secondary outcomes in a double-blind, randomized study.
“There are now proven good treatment options for the more than 1 in 10 patients with hypothyroidism who continue to experience symptoms of fatigue, mental fogginess, weight gain, and other symptoms despite taking levothyroxine,” first author Thanh Duc Hoang, DO, an endocrinologist at the Walter Reed National Military Medical Center, in Bethesda, Md., said in a press statement.
The findings were presented at the annual meeting of the Endocrine Society.
Commenting on the study, Alan P. Farwell, MD, said these new results are a valuable contribution to the understanding of treatment effects. “I think this is an interesting and important study and further studies are needed to clarify the optimal way to treat hypothyroidism,” said Dr. Farwell, who is director of endocrine clinics at Boston University.
Importantly, “the findings are different than studies where the patients are aware of what medication they are receiving,” he stressed in an interview, underscoring the importance of the double-blind design of the trial.
But Anne Cappola, MD, of the University of Pennsylvania, Philadelphia, pointed out that “the study was small and unlikely to have the statistical power to detect differences that could have been clinically important.”
Nevertheless, she too agreed that the double-blind study design is key: “My experience with patients is [the effects] are affected by patients’ perceptions about their thyroid medication. That is why studies designed so that patients do not know which treatment they are receiving are so important in this area.”
Randomized, double-blind comparison
Prior to the widespread availability of the current gold standard hypothyroidism treatment of LT4, the condition was typically treated with desiccated (animal) thyroid extract. And with many patients continuing to have a preference for this therapeutic approach, it is still commonly used.
Additionally, some patients treated with LT4 alone report greater improvements in symptoms with the addition of T3 – despite studies showing no benefits from the two together – leading to many clinicians commonly trying the combination approach.
To compare the efficacy of the three approaches in a prospective, double-blind, cross-over fashion, 75 patients received three therapeutic approaches each for 3 months: desiccated thyroid extract, an LT4/T3 combination, or LT4 alone.
After each 3-month treatment, patients completed a 36-point thyroid symptom questionnaire.
There was no significant differences in symptom relief, the primary outcome, between the three treatments (P = .32).
Overall, 45% of patients indicated they preferred desiccated thyroid as their first choice of treatment, 32% preferred LT4/T3 as their first choice, and 23% preferred LT4 alone.
For the secondary endpoints of weight, general health, depression (assessed using the Beck Depression Inventory), memory (Wechsler Memory Scale), lipids, and thyroid function, again, there were no significant differences between groups in any of the measures.
When switched to desiccated thyroid, many felt ‘much better’
A further exploratory analysis revealed that those who experienced symptoms while taking LT4 alone reported greater alleviation of symptoms with the other two treatments.
“As a whole group, there was no significant difference between the three treatment arms,” Dr. Hoang explained in an interview.
“However, with the subgroup analysis based on the scores of symptom questionnaires, we found that symptomatic patients on LT4 improved while being treated with LT4/T3 or desiccated thyroid,” he said.
Reports of improvements in switching to desiccated thyroid were notable, Dr. Hoang added. “Many patients when switched from LT4 to desiccated thyroid extract said they felt much better, [with] more energy, less mental fogginess, a better outlook, less flair of lupus symptoms, easier to lose weight, etc.”
The study also showed more patients with Hashimoto’s disease preferred desiccated thyroid extract and LT4/T3, compared with LT4 alone, however, the differences were not significant.
Treatment adjustments a helpful first step
Dr. Farwell noted that his approach when patients are still reporting symptoms despite LT4 treatment is to first try tweaking the dose.
“In my own practice, I prefer to adjust LT4 dosing first, and on occasion add T3, with a goal of getting both hormone levels in the upper half of the normal range,” he said. “I find that to be a better approach than desiccated thyroid extract. T3 should be taken twice a day due to its half-life.”
The approach is generally successful, he added. “Even those that come in asking for desiccated thyroid extract whom I am able to convince to try LT4/T3 end up being happy with their treatment in the end.
“The key is that you need to spend time discussing the options with patients and come to a consensus as to the therapy that will best resolve their symptoms and that they are most comfortable with,” he concluded.
In response to mounting evidence of different hypothyroidism treatment responses according to various subgroups of patients, experts recently called for the initiation of more thorough clinical trials on the issue of combination therapy, as recently reported by this news organization.
Dr. Hoang reported being a speaker for Acella Pharmaceuticals. Dr. Farwell and Dr. Cappola reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with hypothyroidism treated with the three most common pharmacologic strategies of levothyroxine (LT4) alone, LT4 in combination with triiodothyronine (T3), or desiccated thyroid extract showed no differences in thyroid symptoms or secondary outcomes in a double-blind, randomized study.
“There are now proven good treatment options for the more than 1 in 10 patients with hypothyroidism who continue to experience symptoms of fatigue, mental fogginess, weight gain, and other symptoms despite taking levothyroxine,” first author Thanh Duc Hoang, DO, an endocrinologist at the Walter Reed National Military Medical Center, in Bethesda, Md., said in a press statement.
The findings were presented at the annual meeting of the Endocrine Society.
Commenting on the study, Alan P. Farwell, MD, said these new results are a valuable contribution to the understanding of treatment effects. “I think this is an interesting and important study and further studies are needed to clarify the optimal way to treat hypothyroidism,” said Dr. Farwell, who is director of endocrine clinics at Boston University.
Importantly, “the findings are different than studies where the patients are aware of what medication they are receiving,” he stressed in an interview, underscoring the importance of the double-blind design of the trial.
But Anne Cappola, MD, of the University of Pennsylvania, Philadelphia, pointed out that “the study was small and unlikely to have the statistical power to detect differences that could have been clinically important.”
Nevertheless, she too agreed that the double-blind study design is key: “My experience with patients is [the effects] are affected by patients’ perceptions about their thyroid medication. That is why studies designed so that patients do not know which treatment they are receiving are so important in this area.”
Randomized, double-blind comparison
Prior to the widespread availability of the current gold standard hypothyroidism treatment of LT4, the condition was typically treated with desiccated (animal) thyroid extract. And with many patients continuing to have a preference for this therapeutic approach, it is still commonly used.
Additionally, some patients treated with LT4 alone report greater improvements in symptoms with the addition of T3 – despite studies showing no benefits from the two together – leading to many clinicians commonly trying the combination approach.
To compare the efficacy of the three approaches in a prospective, double-blind, cross-over fashion, 75 patients received three therapeutic approaches each for 3 months: desiccated thyroid extract, an LT4/T3 combination, or LT4 alone.
After each 3-month treatment, patients completed a 36-point thyroid symptom questionnaire.
There was no significant differences in symptom relief, the primary outcome, between the three treatments (P = .32).
Overall, 45% of patients indicated they preferred desiccated thyroid as their first choice of treatment, 32% preferred LT4/T3 as their first choice, and 23% preferred LT4 alone.
For the secondary endpoints of weight, general health, depression (assessed using the Beck Depression Inventory), memory (Wechsler Memory Scale), lipids, and thyroid function, again, there were no significant differences between groups in any of the measures.
When switched to desiccated thyroid, many felt ‘much better’
A further exploratory analysis revealed that those who experienced symptoms while taking LT4 alone reported greater alleviation of symptoms with the other two treatments.
“As a whole group, there was no significant difference between the three treatment arms,” Dr. Hoang explained in an interview.
“However, with the subgroup analysis based on the scores of symptom questionnaires, we found that symptomatic patients on LT4 improved while being treated with LT4/T3 or desiccated thyroid,” he said.
Reports of improvements in switching to desiccated thyroid were notable, Dr. Hoang added. “Many patients when switched from LT4 to desiccated thyroid extract said they felt much better, [with] more energy, less mental fogginess, a better outlook, less flair of lupus symptoms, easier to lose weight, etc.”
The study also showed more patients with Hashimoto’s disease preferred desiccated thyroid extract and LT4/T3, compared with LT4 alone, however, the differences were not significant.
Treatment adjustments a helpful first step
Dr. Farwell noted that his approach when patients are still reporting symptoms despite LT4 treatment is to first try tweaking the dose.
“In my own practice, I prefer to adjust LT4 dosing first, and on occasion add T3, with a goal of getting both hormone levels in the upper half of the normal range,” he said. “I find that to be a better approach than desiccated thyroid extract. T3 should be taken twice a day due to its half-life.”
The approach is generally successful, he added. “Even those that come in asking for desiccated thyroid extract whom I am able to convince to try LT4/T3 end up being happy with their treatment in the end.
“The key is that you need to spend time discussing the options with patients and come to a consensus as to the therapy that will best resolve their symptoms and that they are most comfortable with,” he concluded.
In response to mounting evidence of different hypothyroidism treatment responses according to various subgroups of patients, experts recently called for the initiation of more thorough clinical trials on the issue of combination therapy, as recently reported by this news organization.
Dr. Hoang reported being a speaker for Acella Pharmaceuticals. Dr. Farwell and Dr. Cappola reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with hypothyroidism treated with the three most common pharmacologic strategies of levothyroxine (LT4) alone, LT4 in combination with triiodothyronine (T3), or desiccated thyroid extract showed no differences in thyroid symptoms or secondary outcomes in a double-blind, randomized study.
“There are now proven good treatment options for the more than 1 in 10 patients with hypothyroidism who continue to experience symptoms of fatigue, mental fogginess, weight gain, and other symptoms despite taking levothyroxine,” first author Thanh Duc Hoang, DO, an endocrinologist at the Walter Reed National Military Medical Center, in Bethesda, Md., said in a press statement.
The findings were presented at the annual meeting of the Endocrine Society.
Commenting on the study, Alan P. Farwell, MD, said these new results are a valuable contribution to the understanding of treatment effects. “I think this is an interesting and important study and further studies are needed to clarify the optimal way to treat hypothyroidism,” said Dr. Farwell, who is director of endocrine clinics at Boston University.
Importantly, “the findings are different than studies where the patients are aware of what medication they are receiving,” he stressed in an interview, underscoring the importance of the double-blind design of the trial.
But Anne Cappola, MD, of the University of Pennsylvania, Philadelphia, pointed out that “the study was small and unlikely to have the statistical power to detect differences that could have been clinically important.”
Nevertheless, she too agreed that the double-blind study design is key: “My experience with patients is [the effects] are affected by patients’ perceptions about their thyroid medication. That is why studies designed so that patients do not know which treatment they are receiving are so important in this area.”
Randomized, double-blind comparison
Prior to the widespread availability of the current gold standard hypothyroidism treatment of LT4, the condition was typically treated with desiccated (animal) thyroid extract. And with many patients continuing to have a preference for this therapeutic approach, it is still commonly used.
Additionally, some patients treated with LT4 alone report greater improvements in symptoms with the addition of T3 – despite studies showing no benefits from the two together – leading to many clinicians commonly trying the combination approach.
To compare the efficacy of the three approaches in a prospective, double-blind, cross-over fashion, 75 patients received three therapeutic approaches each for 3 months: desiccated thyroid extract, an LT4/T3 combination, or LT4 alone.
After each 3-month treatment, patients completed a 36-point thyroid symptom questionnaire.
There was no significant differences in symptom relief, the primary outcome, between the three treatments (P = .32).
Overall, 45% of patients indicated they preferred desiccated thyroid as their first choice of treatment, 32% preferred LT4/T3 as their first choice, and 23% preferred LT4 alone.
For the secondary endpoints of weight, general health, depression (assessed using the Beck Depression Inventory), memory (Wechsler Memory Scale), lipids, and thyroid function, again, there were no significant differences between groups in any of the measures.
When switched to desiccated thyroid, many felt ‘much better’
A further exploratory analysis revealed that those who experienced symptoms while taking LT4 alone reported greater alleviation of symptoms with the other two treatments.
“As a whole group, there was no significant difference between the three treatment arms,” Dr. Hoang explained in an interview.
“However, with the subgroup analysis based on the scores of symptom questionnaires, we found that symptomatic patients on LT4 improved while being treated with LT4/T3 or desiccated thyroid,” he said.
Reports of improvements in switching to desiccated thyroid were notable, Dr. Hoang added. “Many patients when switched from LT4 to desiccated thyroid extract said they felt much better, [with] more energy, less mental fogginess, a better outlook, less flair of lupus symptoms, easier to lose weight, etc.”
The study also showed more patients with Hashimoto’s disease preferred desiccated thyroid extract and LT4/T3, compared with LT4 alone, however, the differences were not significant.
Treatment adjustments a helpful first step
Dr. Farwell noted that his approach when patients are still reporting symptoms despite LT4 treatment is to first try tweaking the dose.
“In my own practice, I prefer to adjust LT4 dosing first, and on occasion add T3, with a goal of getting both hormone levels in the upper half of the normal range,” he said. “I find that to be a better approach than desiccated thyroid extract. T3 should be taken twice a day due to its half-life.”
The approach is generally successful, he added. “Even those that come in asking for desiccated thyroid extract whom I am able to convince to try LT4/T3 end up being happy with their treatment in the end.
“The key is that you need to spend time discussing the options with patients and come to a consensus as to the therapy that will best resolve their symptoms and that they are most comfortable with,” he concluded.
In response to mounting evidence of different hypothyroidism treatment responses according to various subgroups of patients, experts recently called for the initiation of more thorough clinical trials on the issue of combination therapy, as recently reported by this news organization.
Dr. Hoang reported being a speaker for Acella Pharmaceuticals. Dr. Farwell and Dr. Cappola reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metyrapone for Cushing’s syndrome: Safe, effective in first test
Metyrapone, an inhibitor of endogenous adrenal corticosteroid synthesis currently used in U.S. practice to test adrenocorticotropic hormone (ACTH) function, was safe and effective for treating endogenous Cushing’s syndrome in a multicenter, open-label, single-arm study of 50 patients, the first prospective test of metyrapone (Metopirone) as a therapeutic agent.
Treatment with metyrapone for 12 weeks normalized mean levels of urinary free cortisol (UFC) in 23 of the 49 patients (47%) in the efficacy analysis, and cut pretreatment mean UFC levels by at least 50% in another 16 patients (33%). Treatment also improved clinical signs of hypercortisolism, associated comorbidities, and quality of life, and was well tolerated, Lynnette K. Nieman, MD, said at the annual meeting of the Endocrine Society.
“This prospective study confirms that metyrapone is effective, has a rapid onset of action, and is a safe medical treatment for endogenous Cushing’s syndrome,” declared Dr. Nieman, chief of the endocrinology consultation service of the National Institutes of Health Clinical Center in Bethesda, Md.
The study included a 24-week extension phase of continued metyrapone treatment in patients whose mean UFC level fell to less than two times the upper limit of normal (ULN), but Dr. Nieman did not report results from this extension.
Confirmation of off-label and European experience
“This was the first prospective study of metyrapone, albeit a small study and with only short-term data presented. It confirms what we have known from its off label use in the U.S. and retrospective studies in the U.K. and Europe: Metyrapone normalizes mean UFC in approximately half of patients. Probably with more aggressive up titration efficacy will have been even higher, but of course with the trade-off of adrenal insufficiency,” said Maria Fleseriu, MD, professor and director of the pituitary center at Oregon Health & Science University in Portland, who was not involved with the study.
“Longer-term data from this prospective study is clearly needed to evaluate for possible loss of response, as well as adverse events related to precursors accumulation. We also need data on tumor size with long-term use in patients with Cushing’s disease, “Dr. Fleseriu added in an interview.
“Metyrapone, an 11-hydroxylase inhibitor, is not [Food and Drug Administration–approved for therapy] and thus it will be hard for it to become a first-line medical therapy,” she continued. “Furthermore, multiple times a day administration is not ideal for most patients; however if metyrapone is readily available and cheaper than other drugs, its use might increase over time. Hirsutism in women (though not all women develop this) and hypertension could be issues with long-term use,” she cautioned.
“We have used metyrapone off label for many years. It has a rapid onset of action, and we also have experience using it in combination therapy with ketoconazole, especially in patients with severe Cushing’s, although ketoconazole is not [FDA] approved for Cushing’s syndrome, and all combination therapies are off-label, too,” Dr. Fleseriu noted.
Metyrapone is approved by the European Medicines Agency for treating Cushing’s syndrome based “on observational, retrospective studies published over more than 50 years,” according to Dr. Nieman. The drug has FDA approval only for diagnostic purposes.
The PROMPT (Effects of Metyrapone in Patients With Endogenous Cushing’s Syndrome) study enrolled patients in eight European countries who were newly diagnosed with endogenous Cushing’s syndrome of any etiology. The study excluded patients with an advanced adrenal carcinoma, as well as patients with recurrent or persistent Cushing’s disease following transsphenoidal surgery. Patients also needed three 24-hour measures of UFC that were at least 50% above the ULN (165 nmol/24 hours).
The average age of the patients was 46 years; 69% were women, 90% had Cushing’s disease, and 8% had ectopic ACTH secretion. The average time from initial symptom onset was 4 years. Sixty-one percent had a history of pituitary surgery, 69% were hypertensive, 43% had diabetes or glucose intolerance, and 41% had osteoporosis. The median mean UFC at entry was 570 nmol/24 hours, which is 3.5 times the ULN, and ranged from 291 to 8,476 nmol/24 hours.
Patients began on a metyrapone dosage of 750 mg/day unless their mean UFC exceeded 5 times the ULN, in which case the dosage doubled to 1,500 mg/day. During the 12-week period, clinicians up- or down-titrated the dosage to ideally achieve a UFC less than the ULN while maintaining serum cortisol levels of 7-12 mcg/dL to preclude adrenal insufficiency effects. The median dosage at the end of 12 weeks was 1,500 mg/day, and ranged from 250 to 5,500 mg/day. One of the 50 patients dropped out because of an unrelated acute medical condition, and two patients underwent pituitary surgery despite a response to metyrapone and were included in the efficacy analysis.
After the first week on treatment, patients had a median 49% cut from their baseline UFC level, and after 12 weeks this rose to a median 74% cut from baseline. The study’s primary endpoint was normalization of UFC after 12 weeks, which occurred in 47% of patients, while 22% had a normal level in a late-night salivary cortisol measurement.
Two-thirds of patients had an improvement or resolution of their signs and symptoms, on average quality of life scores improved, median systolic and diastolic blood pressures decreased by 4-5 mm Hg, and average A1c levels were stable, but the mean cholesterol level decreased significantly, and testosterone levels rose significantly in women.
Proper dose titration makes a difference
Adverse events occurred in 26 of the 50 patients (52%) who received any treatment; 1 patient had a serious adverse event, 7 patients required a dosage adjustment because of adverse events, and 6 patients stopped treatment. The most common adverse events were gastrointestinal – nausea in 24% and decreased appetite in 18% – as well as mild symptoms consistent with adrenal insufficiency such as fatigue and headache. Six patients (12%) were identified with reversible adrenal insufficiency, and no patients complained of worsening acne or hirsutism.
“I think the adverse events are a function of [less than optimal] dose titration and variability in UFC levels,” said Dr. Nieman.
This test of metyrapone’s efficacy comes a year after the FDA approved osilodrostat (Isturisa) for treating Cushing’s disease (but not Cushing’s syndrome). Like metyrapone, osilodrostat controls cortisol overproduction by blocking the enzyme 11-beta-hydroxylase and preventing cortisol synthesis, and osilodrostat was the first agent with these properties to receive an FDA label for therapy.
Osilodrostat “is the only adrenal steroidogenesis inhibitor assessed in randomized controlled long-term trials – over 200 patients with Cushing’s disease – and it has been shown to be highly effective at maintaining normal urinary free cortisol in large majority of patients with Cushing’s disease, as well as Cushing’s syndrome in a study in Japan. Adrenal insufficiency was high [with osilodrostat], especially with the high dose in the trial with forced uptitration. In my clinical practice I have noticed less adrenal insufficiency, but I use much slower drug titration,” said Dr. Fleseriu.
“I think these drugs [metyrapone and osilodrostat] are relatively equivalent,” Dr. Nieman said during discussion of her report. “One nonmedical judgment will be cost,” she added. “Everyone is looking forward to what the pricing structure will be for the new drugs.”
Dr. Fleseriu noted that “for most patients, surgery is first-line therapy, and rarely medication is an alternative first option, especially for Cushing’s disease. However, medical therapy is essential in the management of patients with Cushing’s syndrome when curative surgery fails, surgery is not feasible, when a patient is awaiting radiation’s effect, and for recurrent cases of Cushing’s syndrome.”
PROMPT was sponsored by HRA Pharma, the company that markets metyrapone. Dr. Nieman had no disclosures, but several of her associates on the study are HRA employees. Dr. Fleseriu has been a consultant to Novartis, Recordati, Sparrow, and Strongbridge.
Metyrapone, an inhibitor of endogenous adrenal corticosteroid synthesis currently used in U.S. practice to test adrenocorticotropic hormone (ACTH) function, was safe and effective for treating endogenous Cushing’s syndrome in a multicenter, open-label, single-arm study of 50 patients, the first prospective test of metyrapone (Metopirone) as a therapeutic agent.
Treatment with metyrapone for 12 weeks normalized mean levels of urinary free cortisol (UFC) in 23 of the 49 patients (47%) in the efficacy analysis, and cut pretreatment mean UFC levels by at least 50% in another 16 patients (33%). Treatment also improved clinical signs of hypercortisolism, associated comorbidities, and quality of life, and was well tolerated, Lynnette K. Nieman, MD, said at the annual meeting of the Endocrine Society.
“This prospective study confirms that metyrapone is effective, has a rapid onset of action, and is a safe medical treatment for endogenous Cushing’s syndrome,” declared Dr. Nieman, chief of the endocrinology consultation service of the National Institutes of Health Clinical Center in Bethesda, Md.
The study included a 24-week extension phase of continued metyrapone treatment in patients whose mean UFC level fell to less than two times the upper limit of normal (ULN), but Dr. Nieman did not report results from this extension.
Confirmation of off-label and European experience
“This was the first prospective study of metyrapone, albeit a small study and with only short-term data presented. It confirms what we have known from its off label use in the U.S. and retrospective studies in the U.K. and Europe: Metyrapone normalizes mean UFC in approximately half of patients. Probably with more aggressive up titration efficacy will have been even higher, but of course with the trade-off of adrenal insufficiency,” said Maria Fleseriu, MD, professor and director of the pituitary center at Oregon Health & Science University in Portland, who was not involved with the study.
“Longer-term data from this prospective study is clearly needed to evaluate for possible loss of response, as well as adverse events related to precursors accumulation. We also need data on tumor size with long-term use in patients with Cushing’s disease, “Dr. Fleseriu added in an interview.
“Metyrapone, an 11-hydroxylase inhibitor, is not [Food and Drug Administration–approved for therapy] and thus it will be hard for it to become a first-line medical therapy,” she continued. “Furthermore, multiple times a day administration is not ideal for most patients; however if metyrapone is readily available and cheaper than other drugs, its use might increase over time. Hirsutism in women (though not all women develop this) and hypertension could be issues with long-term use,” she cautioned.
“We have used metyrapone off label for many years. It has a rapid onset of action, and we also have experience using it in combination therapy with ketoconazole, especially in patients with severe Cushing’s, although ketoconazole is not [FDA] approved for Cushing’s syndrome, and all combination therapies are off-label, too,” Dr. Fleseriu noted.
Metyrapone is approved by the European Medicines Agency for treating Cushing’s syndrome based “on observational, retrospective studies published over more than 50 years,” according to Dr. Nieman. The drug has FDA approval only for diagnostic purposes.
The PROMPT (Effects of Metyrapone in Patients With Endogenous Cushing’s Syndrome) study enrolled patients in eight European countries who were newly diagnosed with endogenous Cushing’s syndrome of any etiology. The study excluded patients with an advanced adrenal carcinoma, as well as patients with recurrent or persistent Cushing’s disease following transsphenoidal surgery. Patients also needed three 24-hour measures of UFC that were at least 50% above the ULN (165 nmol/24 hours).
The average age of the patients was 46 years; 69% were women, 90% had Cushing’s disease, and 8% had ectopic ACTH secretion. The average time from initial symptom onset was 4 years. Sixty-one percent had a history of pituitary surgery, 69% were hypertensive, 43% had diabetes or glucose intolerance, and 41% had osteoporosis. The median mean UFC at entry was 570 nmol/24 hours, which is 3.5 times the ULN, and ranged from 291 to 8,476 nmol/24 hours.
Patients began on a metyrapone dosage of 750 mg/day unless their mean UFC exceeded 5 times the ULN, in which case the dosage doubled to 1,500 mg/day. During the 12-week period, clinicians up- or down-titrated the dosage to ideally achieve a UFC less than the ULN while maintaining serum cortisol levels of 7-12 mcg/dL to preclude adrenal insufficiency effects. The median dosage at the end of 12 weeks was 1,500 mg/day, and ranged from 250 to 5,500 mg/day. One of the 50 patients dropped out because of an unrelated acute medical condition, and two patients underwent pituitary surgery despite a response to metyrapone and were included in the efficacy analysis.
After the first week on treatment, patients had a median 49% cut from their baseline UFC level, and after 12 weeks this rose to a median 74% cut from baseline. The study’s primary endpoint was normalization of UFC after 12 weeks, which occurred in 47% of patients, while 22% had a normal level in a late-night salivary cortisol measurement.
Two-thirds of patients had an improvement or resolution of their signs and symptoms, on average quality of life scores improved, median systolic and diastolic blood pressures decreased by 4-5 mm Hg, and average A1c levels were stable, but the mean cholesterol level decreased significantly, and testosterone levels rose significantly in women.
Proper dose titration makes a difference
Adverse events occurred in 26 of the 50 patients (52%) who received any treatment; 1 patient had a serious adverse event, 7 patients required a dosage adjustment because of adverse events, and 6 patients stopped treatment. The most common adverse events were gastrointestinal – nausea in 24% and decreased appetite in 18% – as well as mild symptoms consistent with adrenal insufficiency such as fatigue and headache. Six patients (12%) were identified with reversible adrenal insufficiency, and no patients complained of worsening acne or hirsutism.
“I think the adverse events are a function of [less than optimal] dose titration and variability in UFC levels,” said Dr. Nieman.
This test of metyrapone’s efficacy comes a year after the FDA approved osilodrostat (Isturisa) for treating Cushing’s disease (but not Cushing’s syndrome). Like metyrapone, osilodrostat controls cortisol overproduction by blocking the enzyme 11-beta-hydroxylase and preventing cortisol synthesis, and osilodrostat was the first agent with these properties to receive an FDA label for therapy.
Osilodrostat “is the only adrenal steroidogenesis inhibitor assessed in randomized controlled long-term trials – over 200 patients with Cushing’s disease – and it has been shown to be highly effective at maintaining normal urinary free cortisol in large majority of patients with Cushing’s disease, as well as Cushing’s syndrome in a study in Japan. Adrenal insufficiency was high [with osilodrostat], especially with the high dose in the trial with forced uptitration. In my clinical practice I have noticed less adrenal insufficiency, but I use much slower drug titration,” said Dr. Fleseriu.
“I think these drugs [metyrapone and osilodrostat] are relatively equivalent,” Dr. Nieman said during discussion of her report. “One nonmedical judgment will be cost,” she added. “Everyone is looking forward to what the pricing structure will be for the new drugs.”
Dr. Fleseriu noted that “for most patients, surgery is first-line therapy, and rarely medication is an alternative first option, especially for Cushing’s disease. However, medical therapy is essential in the management of patients with Cushing’s syndrome when curative surgery fails, surgery is not feasible, when a patient is awaiting radiation’s effect, and for recurrent cases of Cushing’s syndrome.”
PROMPT was sponsored by HRA Pharma, the company that markets metyrapone. Dr. Nieman had no disclosures, but several of her associates on the study are HRA employees. Dr. Fleseriu has been a consultant to Novartis, Recordati, Sparrow, and Strongbridge.
Metyrapone, an inhibitor of endogenous adrenal corticosteroid synthesis currently used in U.S. practice to test adrenocorticotropic hormone (ACTH) function, was safe and effective for treating endogenous Cushing’s syndrome in a multicenter, open-label, single-arm study of 50 patients, the first prospective test of metyrapone (Metopirone) as a therapeutic agent.
Treatment with metyrapone for 12 weeks normalized mean levels of urinary free cortisol (UFC) in 23 of the 49 patients (47%) in the efficacy analysis, and cut pretreatment mean UFC levels by at least 50% in another 16 patients (33%). Treatment also improved clinical signs of hypercortisolism, associated comorbidities, and quality of life, and was well tolerated, Lynnette K. Nieman, MD, said at the annual meeting of the Endocrine Society.
“This prospective study confirms that metyrapone is effective, has a rapid onset of action, and is a safe medical treatment for endogenous Cushing’s syndrome,” declared Dr. Nieman, chief of the endocrinology consultation service of the National Institutes of Health Clinical Center in Bethesda, Md.
The study included a 24-week extension phase of continued metyrapone treatment in patients whose mean UFC level fell to less than two times the upper limit of normal (ULN), but Dr. Nieman did not report results from this extension.
Confirmation of off-label and European experience
“This was the first prospective study of metyrapone, albeit a small study and with only short-term data presented. It confirms what we have known from its off label use in the U.S. and retrospective studies in the U.K. and Europe: Metyrapone normalizes mean UFC in approximately half of patients. Probably with more aggressive up titration efficacy will have been even higher, but of course with the trade-off of adrenal insufficiency,” said Maria Fleseriu, MD, professor and director of the pituitary center at Oregon Health & Science University in Portland, who was not involved with the study.
“Longer-term data from this prospective study is clearly needed to evaluate for possible loss of response, as well as adverse events related to precursors accumulation. We also need data on tumor size with long-term use in patients with Cushing’s disease, “Dr. Fleseriu added in an interview.
“Metyrapone, an 11-hydroxylase inhibitor, is not [Food and Drug Administration–approved for therapy] and thus it will be hard for it to become a first-line medical therapy,” she continued. “Furthermore, multiple times a day administration is not ideal for most patients; however if metyrapone is readily available and cheaper than other drugs, its use might increase over time. Hirsutism in women (though not all women develop this) and hypertension could be issues with long-term use,” she cautioned.
“We have used metyrapone off label for many years. It has a rapid onset of action, and we also have experience using it in combination therapy with ketoconazole, especially in patients with severe Cushing’s, although ketoconazole is not [FDA] approved for Cushing’s syndrome, and all combination therapies are off-label, too,” Dr. Fleseriu noted.
Metyrapone is approved by the European Medicines Agency for treating Cushing’s syndrome based “on observational, retrospective studies published over more than 50 years,” according to Dr. Nieman. The drug has FDA approval only for diagnostic purposes.
The PROMPT (Effects of Metyrapone in Patients With Endogenous Cushing’s Syndrome) study enrolled patients in eight European countries who were newly diagnosed with endogenous Cushing’s syndrome of any etiology. The study excluded patients with an advanced adrenal carcinoma, as well as patients with recurrent or persistent Cushing’s disease following transsphenoidal surgery. Patients also needed three 24-hour measures of UFC that were at least 50% above the ULN (165 nmol/24 hours).
The average age of the patients was 46 years; 69% were women, 90% had Cushing’s disease, and 8% had ectopic ACTH secretion. The average time from initial symptom onset was 4 years. Sixty-one percent had a history of pituitary surgery, 69% were hypertensive, 43% had diabetes or glucose intolerance, and 41% had osteoporosis. The median mean UFC at entry was 570 nmol/24 hours, which is 3.5 times the ULN, and ranged from 291 to 8,476 nmol/24 hours.
Patients began on a metyrapone dosage of 750 mg/day unless their mean UFC exceeded 5 times the ULN, in which case the dosage doubled to 1,500 mg/day. During the 12-week period, clinicians up- or down-titrated the dosage to ideally achieve a UFC less than the ULN while maintaining serum cortisol levels of 7-12 mcg/dL to preclude adrenal insufficiency effects. The median dosage at the end of 12 weeks was 1,500 mg/day, and ranged from 250 to 5,500 mg/day. One of the 50 patients dropped out because of an unrelated acute medical condition, and two patients underwent pituitary surgery despite a response to metyrapone and were included in the efficacy analysis.
After the first week on treatment, patients had a median 49% cut from their baseline UFC level, and after 12 weeks this rose to a median 74% cut from baseline. The study’s primary endpoint was normalization of UFC after 12 weeks, which occurred in 47% of patients, while 22% had a normal level in a late-night salivary cortisol measurement.
Two-thirds of patients had an improvement or resolution of their signs and symptoms, on average quality of life scores improved, median systolic and diastolic blood pressures decreased by 4-5 mm Hg, and average A1c levels were stable, but the mean cholesterol level decreased significantly, and testosterone levels rose significantly in women.
Proper dose titration makes a difference
Adverse events occurred in 26 of the 50 patients (52%) who received any treatment; 1 patient had a serious adverse event, 7 patients required a dosage adjustment because of adverse events, and 6 patients stopped treatment. The most common adverse events were gastrointestinal – nausea in 24% and decreased appetite in 18% – as well as mild symptoms consistent with adrenal insufficiency such as fatigue and headache. Six patients (12%) were identified with reversible adrenal insufficiency, and no patients complained of worsening acne or hirsutism.
“I think the adverse events are a function of [less than optimal] dose titration and variability in UFC levels,” said Dr. Nieman.
This test of metyrapone’s efficacy comes a year after the FDA approved osilodrostat (Isturisa) for treating Cushing’s disease (but not Cushing’s syndrome). Like metyrapone, osilodrostat controls cortisol overproduction by blocking the enzyme 11-beta-hydroxylase and preventing cortisol synthesis, and osilodrostat was the first agent with these properties to receive an FDA label for therapy.
Osilodrostat “is the only adrenal steroidogenesis inhibitor assessed in randomized controlled long-term trials – over 200 patients with Cushing’s disease – and it has been shown to be highly effective at maintaining normal urinary free cortisol in large majority of patients with Cushing’s disease, as well as Cushing’s syndrome in a study in Japan. Adrenal insufficiency was high [with osilodrostat], especially with the high dose in the trial with forced uptitration. In my clinical practice I have noticed less adrenal insufficiency, but I use much slower drug titration,” said Dr. Fleseriu.
“I think these drugs [metyrapone and osilodrostat] are relatively equivalent,” Dr. Nieman said during discussion of her report. “One nonmedical judgment will be cost,” she added. “Everyone is looking forward to what the pricing structure will be for the new drugs.”
Dr. Fleseriu noted that “for most patients, surgery is first-line therapy, and rarely medication is an alternative first option, especially for Cushing’s disease. However, medical therapy is essential in the management of patients with Cushing’s syndrome when curative surgery fails, surgery is not feasible, when a patient is awaiting radiation’s effect, and for recurrent cases of Cushing’s syndrome.”
PROMPT was sponsored by HRA Pharma, the company that markets metyrapone. Dr. Nieman had no disclosures, but several of her associates on the study are HRA employees. Dr. Fleseriu has been a consultant to Novartis, Recordati, Sparrow, and Strongbridge.
FROM ENDO 2021
PCOS equivalent in men: No ovaries required
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
FROM ENDO 2021
Women with PCOS at increased risk for COVID-19
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.









