User login
Osteoporosis management: Use a goal-oriented, individualized approach
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10
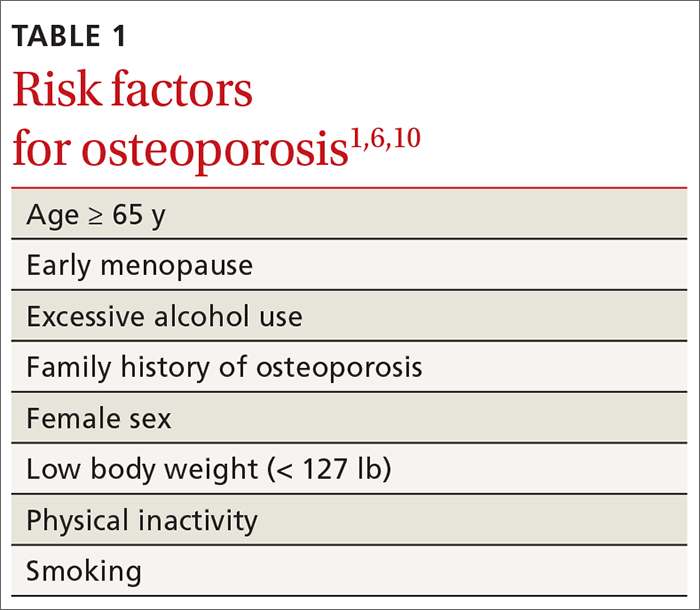
Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
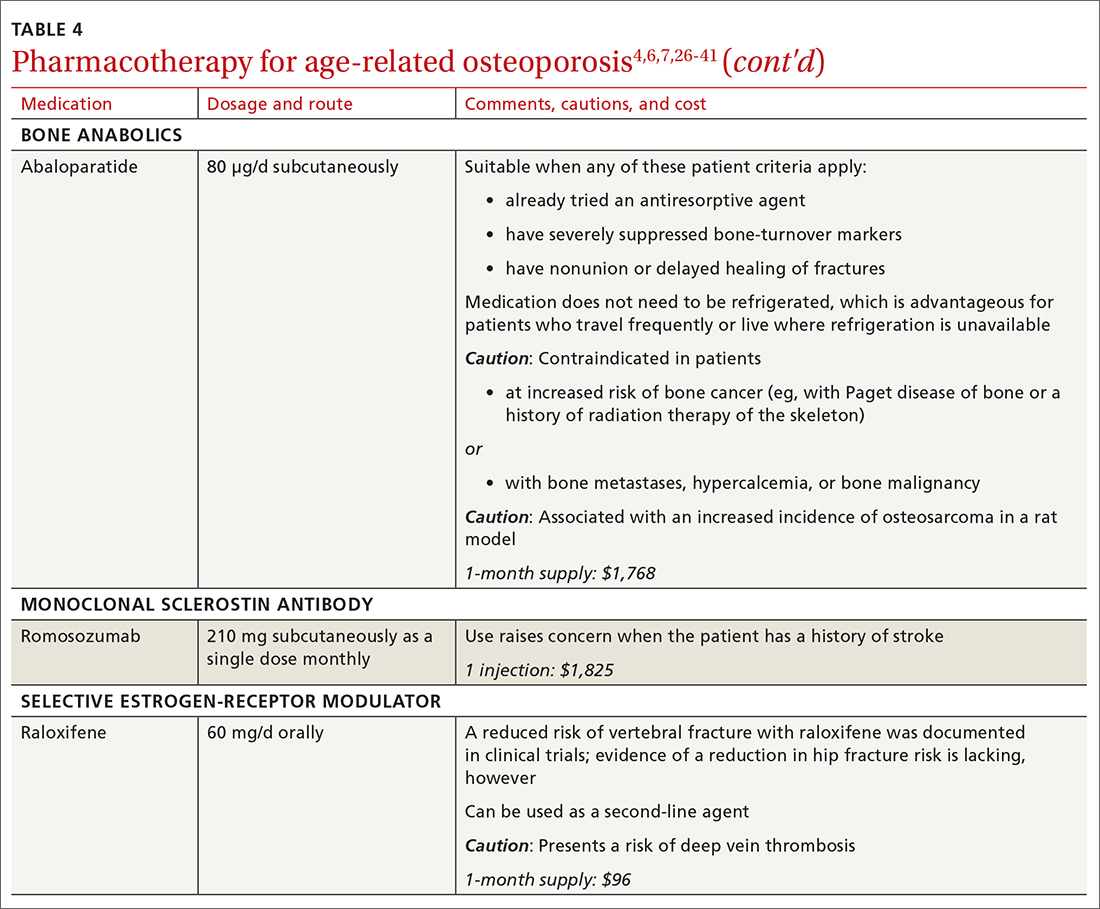
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)
❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
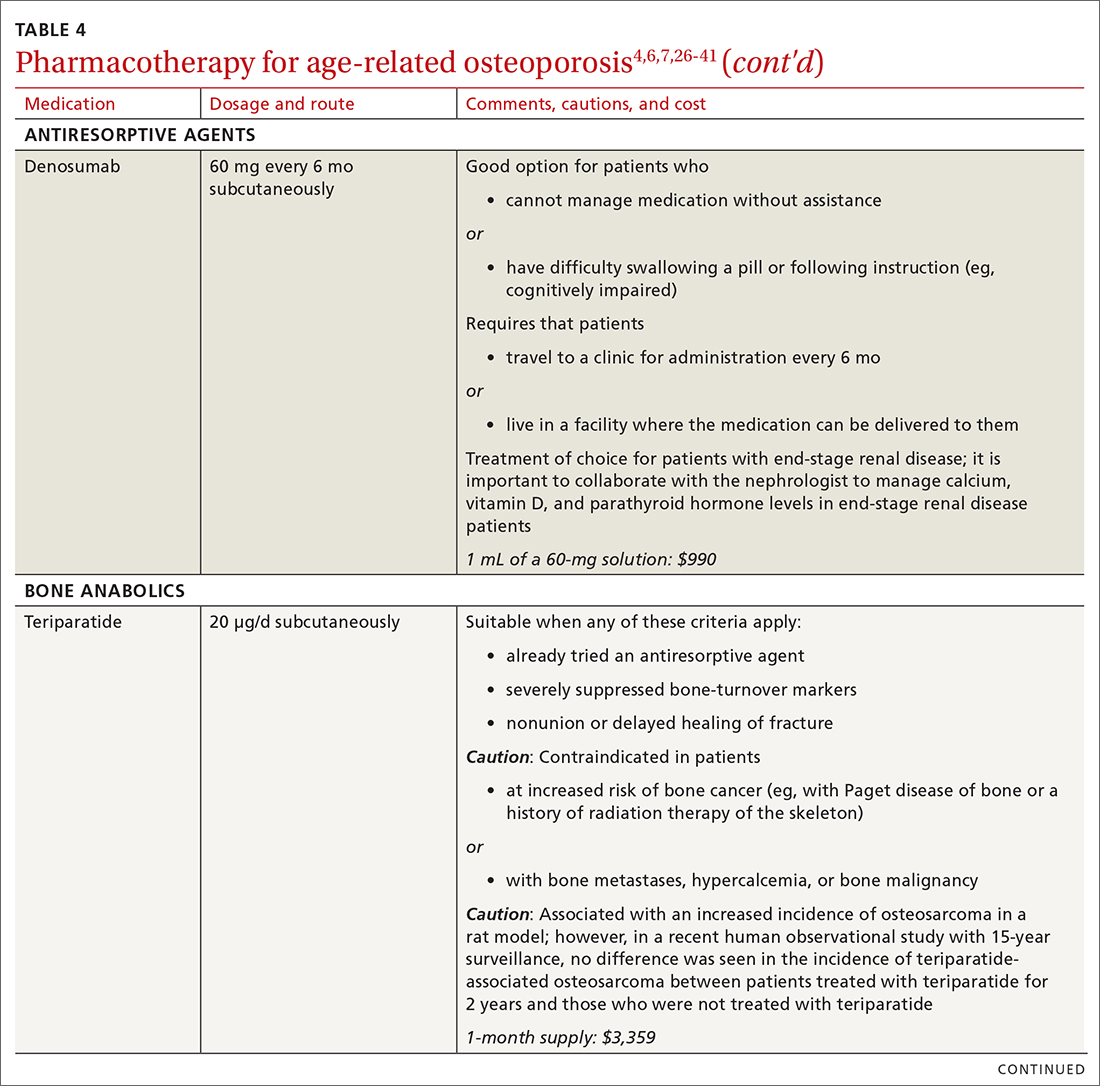
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
PRACTICE RECOMMENDATIONS
❯ Consider screening for osteoporosis, using bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA), in all postmenopausal women ≥ 65 years and in women < 65 years at high risk of osteoporosis.
❯ Consider screening in men ≥ 70 years and in younger men at high risk of fracture.
❯ Use the trabecular bone score with DXA BMD to screen patients at high risk of fracture who have a normal BMD—eg, patients with type 2 diabetes or ankylosing spondylitis.
❯ Offer individualized pharmacotherapy to older patients with a diagnosis of osteoporosis and to those at high risk of fracture.
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
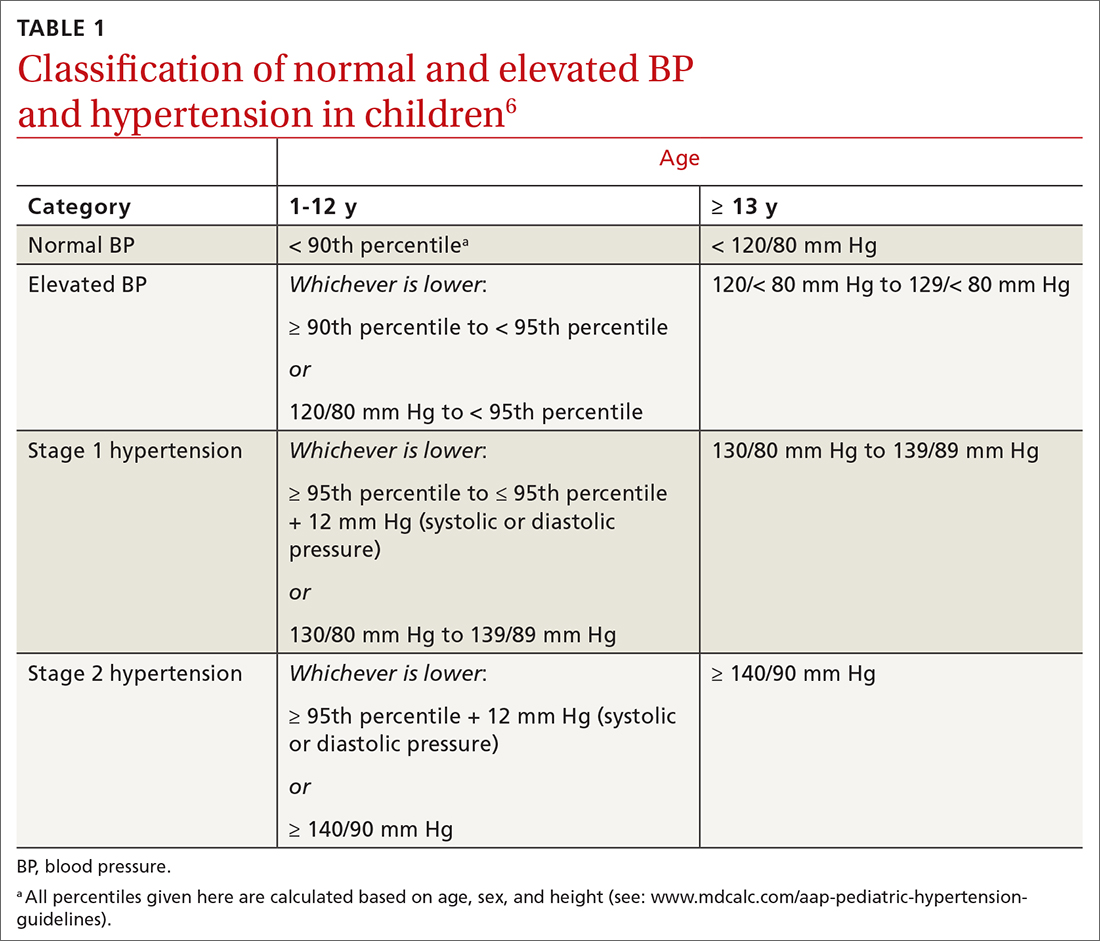
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
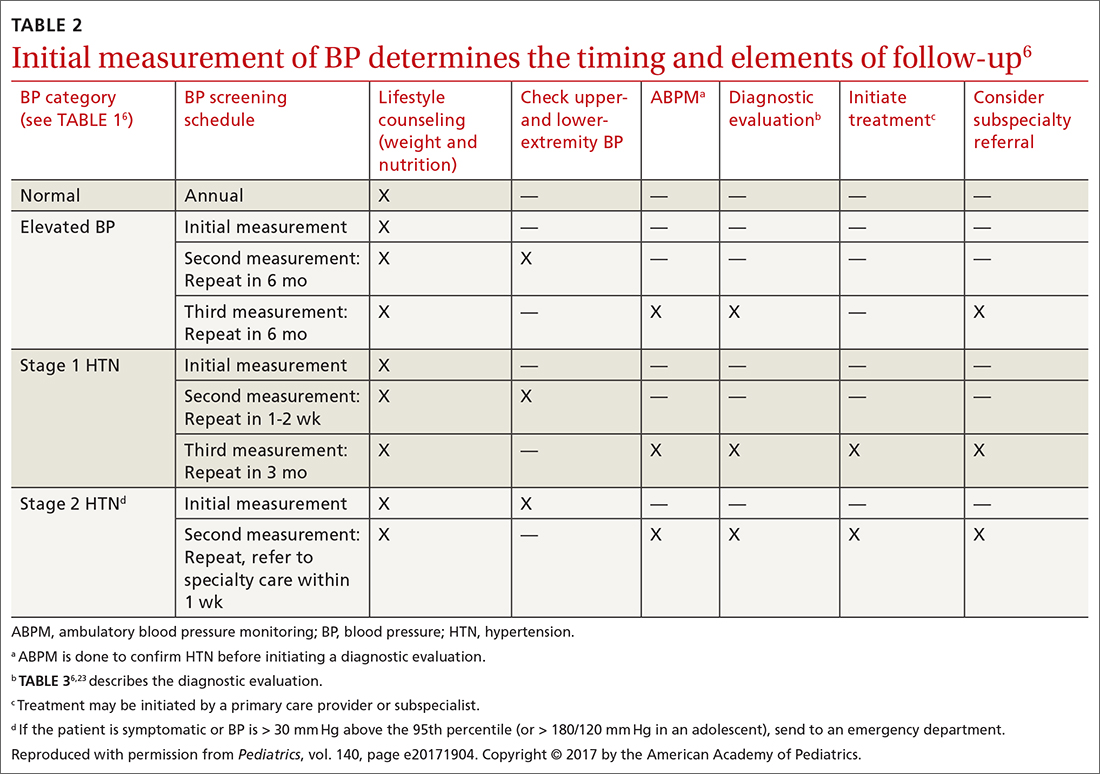
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
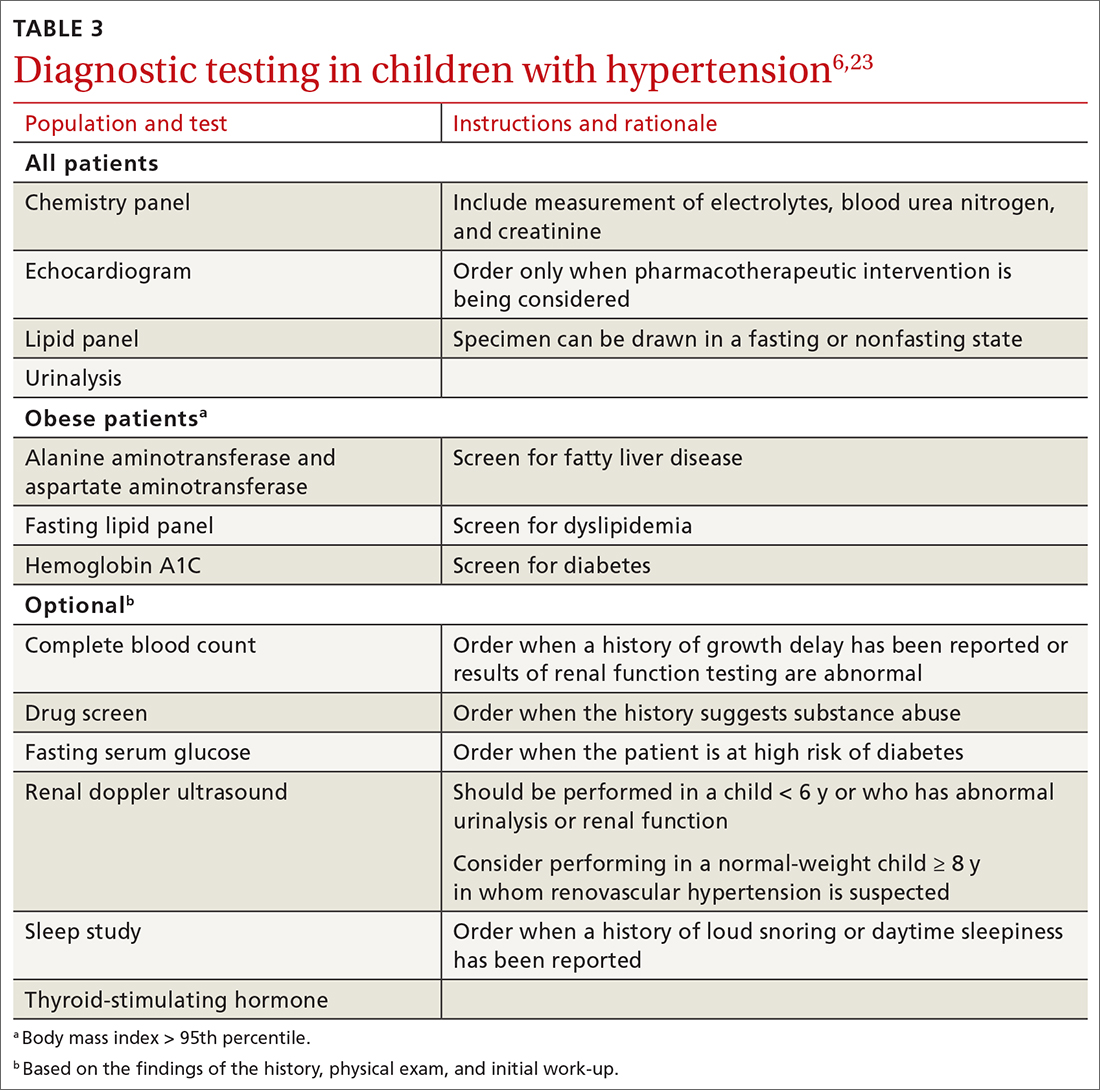
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
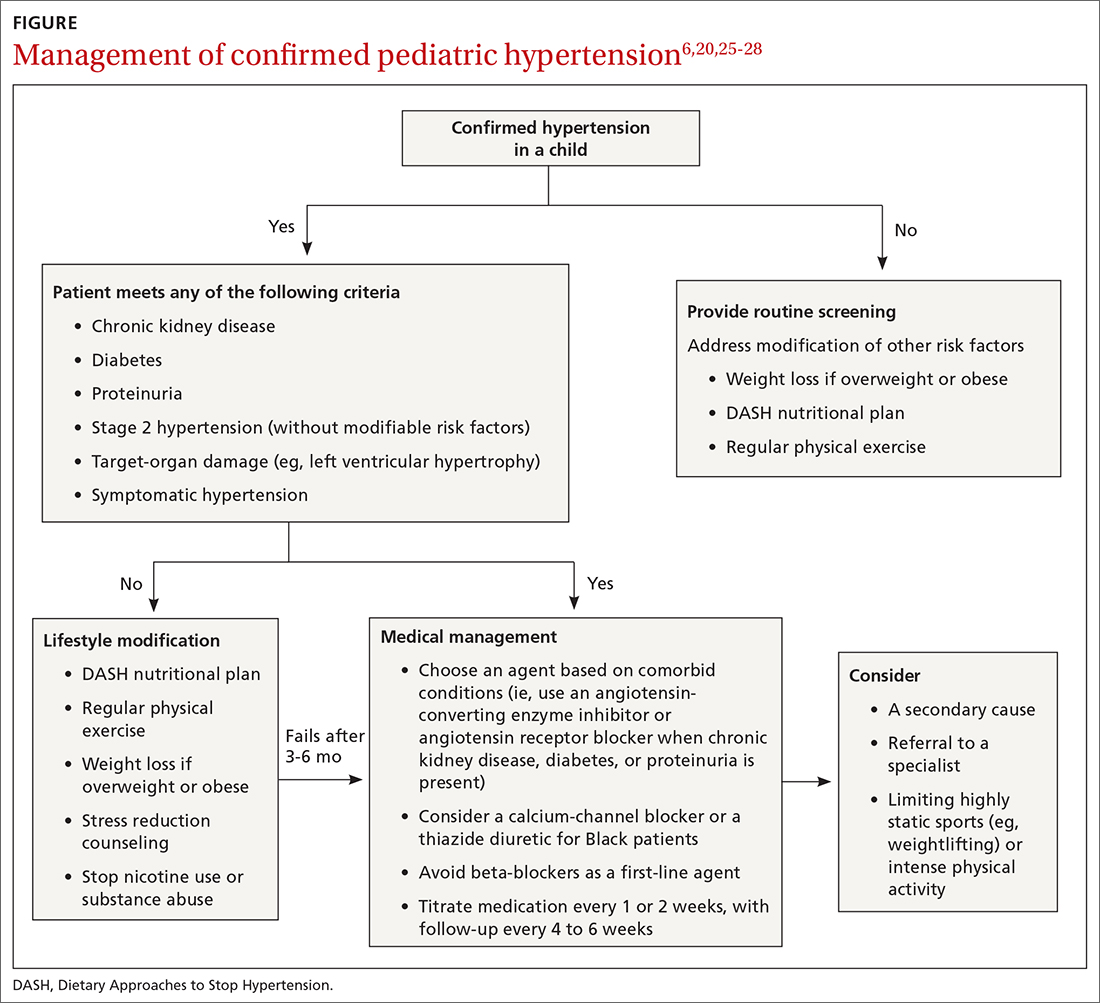
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Numerous large nodules on scalp
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
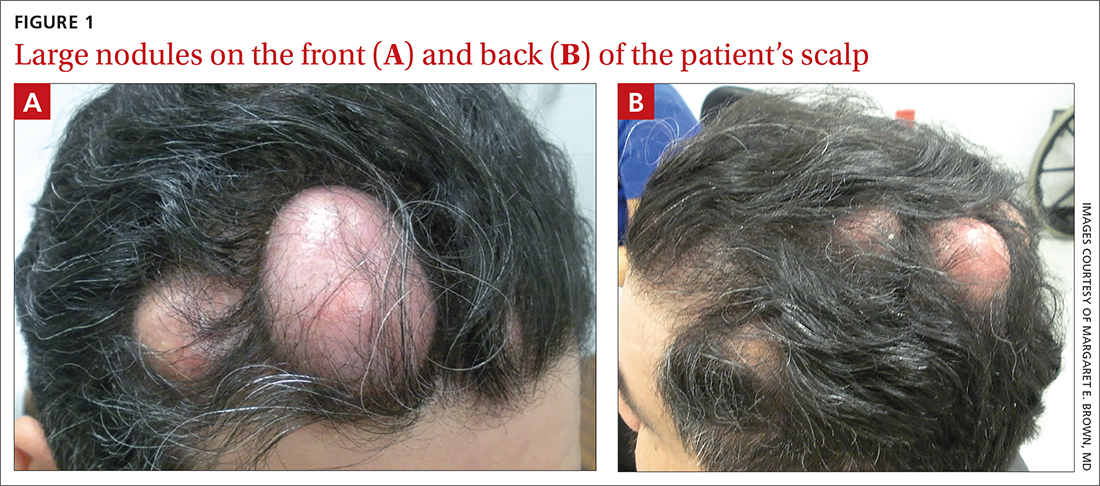
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
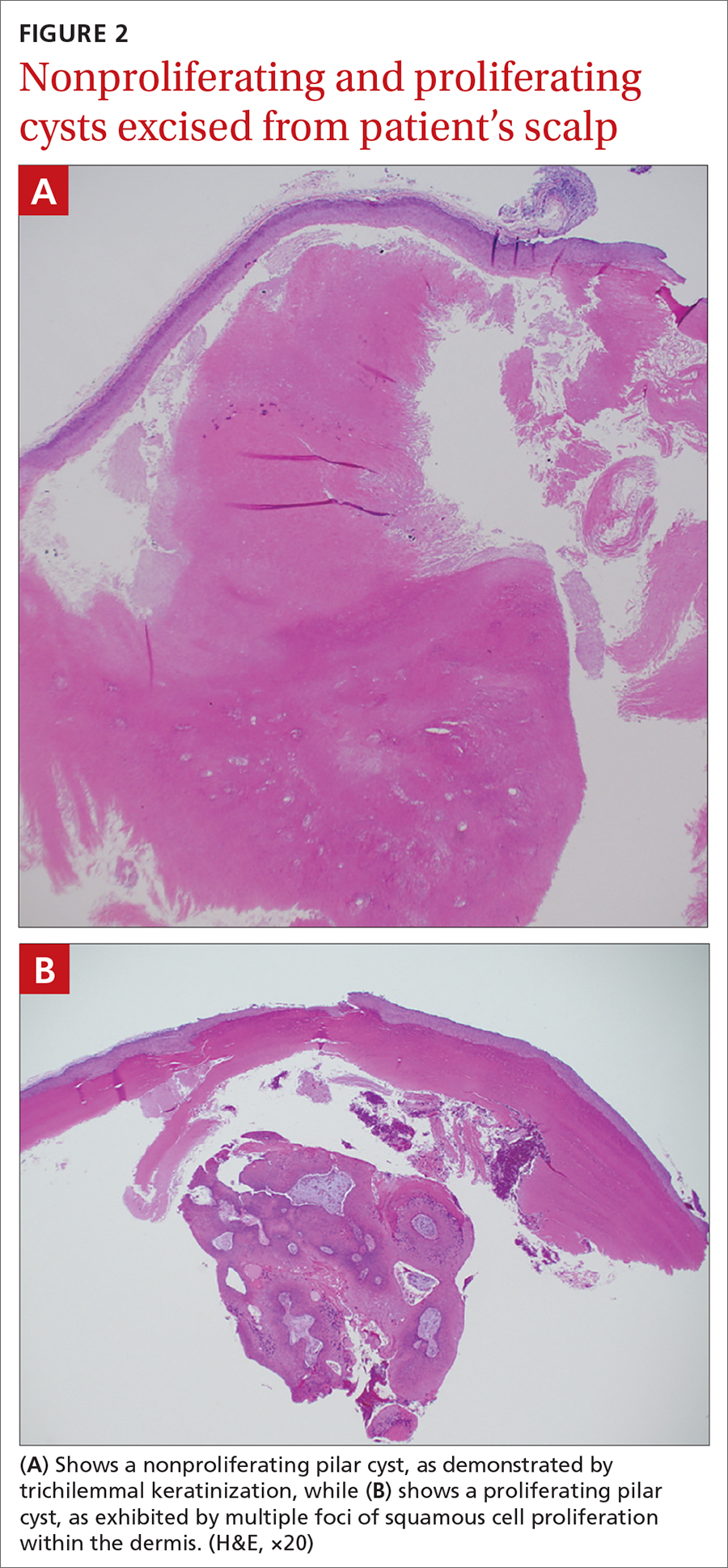
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.

Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.

With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.

Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.

With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
New-onset hirsutism
A 74-year-old woman presented to the dermatology clinic for follow-up 3 months after the surgical excision of a basal cell carcinoma on her left jawline. During this postop period, the patient developed new-onset hirsutism. She appeared to be in otherwise good health.
Family and personal medical history were unremarkable. Her medication regimen included aspirin 81 mg/d and a daily multivitamin. The patient was postmenopausal and had a body mass index of 28 and a history of acid reflux and osteoarthritis.
Physical examination of the patient’s scalp showed male-pattern alopecia (FIGURE 1A). She also had coarse terminal hairs on her forearms and back, as well as on her chin (FIGURE 1B).
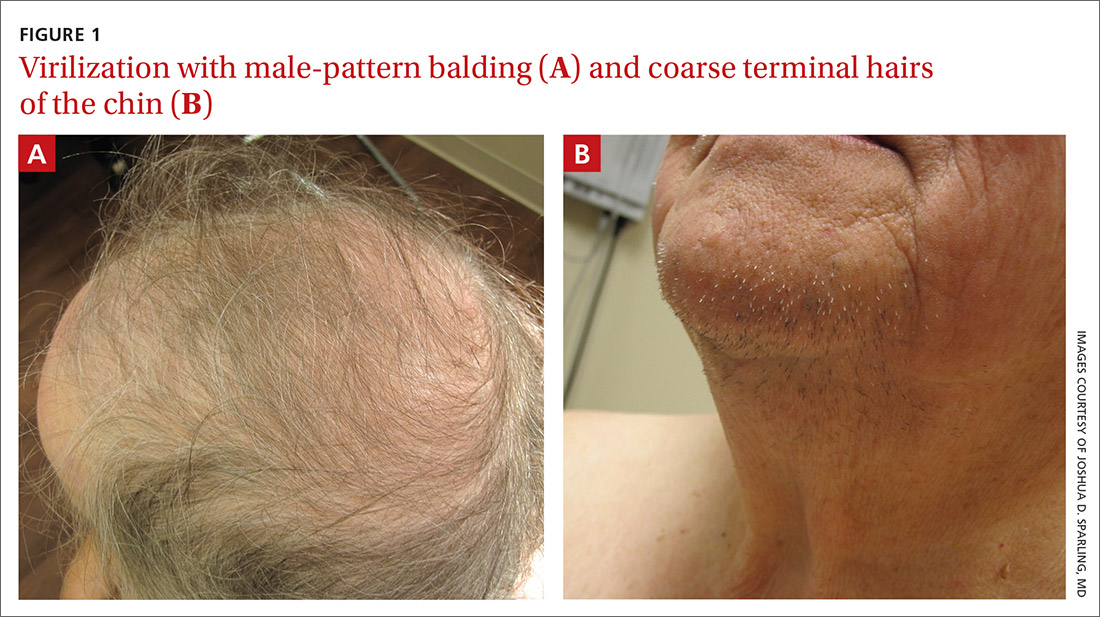
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Androgen-secreting ovarian tumor
Based on the distribution of terminal hairs and marked change over 3 months, as well as the male-pattern alopecia, a diagnosis of androgen excess was suspected. Laboratory work-up, including thyroid-stimulating hormone, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, prolactin, complete blood count, and complete metabolic panel, was within normal limits. Pelvic ultrasound of the ovaries and abdominal computed tomography (CT) of the adrenal glands were also normal.
Further testing showed an elevated testosterone level of 464 ng/dL (reference range: 2-45 ng/dL) and an elevated free testosterone level of 66.8 ng/dL (reference range: 0.2-3.7 ng/dL). These levels pointed to an androgen-secreting ovarian tumor; the androgen excess was likely the cause of her hirsutism.
Hirsutism or hypertrichosis?
Hirsutism, a common disorder affecting up to 8% of women, is defined by excess terminal hairs that appear in a male pattern in women due to production of excess androgens.1 This should be distinguished from hypertrichosis, which is generalized excessive hair growth not caused by androgen excess.
Testosterone and DHEAS—produced in the ovaries and adrenal glands, respectively—contribute to the development of hirsutism.1 Hirsutism is more often associated with adrenal or ovarian tumors in postmenopausal patients.2 Generalized hypertrichosis can be associated with porphyria cutanea tarda, severe anorexia nervosa, and rarely, malignancies; it also can be secondary to certain agents, such as cyclosporin, phenytoin, and minoxidil.
While hirsutism is associated with hyperandrogenemia, its degree correlates poorly with serum levels. Notably, about half of women with hirsutism have been found to have normal levels of circulating androgens.1 Severe signs of hyperandrogenemia include rapid onset of symptoms, signs of virilization, and a palpable abdominal or pelvic mass.3
Continue to: Is the patient pre- or postmenopausal?
Is the patient pre- or postmenopausal? Polycystic ovary syndrome (PCOS) accounts for up to three-fourths of premenopausal hirsutism.3 The likelihood of hirsutism is actually decreased in postmenopausal women because estrogen levels can drop abruptly after menopause. That said, conditions linked to hirsutism in postmenopausal women include adrenal hyperplasia, thyroid dysfunction, Cushing syndrome, and least frequently, androgen-secreting tumors (seen in this patient). (Hirsutism can also be idiopathic or iatrogenic [medications].)
Methods for detection
Research suggests that when a female patient is given a diagnosis of hirsutism, it’s important to explore possible underlying ovarian and/or adrenal tumors and adult-onset adrenal hyperplasia.1 The following tests and procedure can be helpful:
Serum testosterone and DHEAS. Levels of total testosterone > 200 ng/dL and/or DHEAS > 700 ng/dL are strongly indicative of androgen-secreting tumors.1
Imaging—including ultrasound, CT, or magnetic resonance imaging—can be used for evaluation of the adrenal glands and ovaries. However, imaging is often unable to identify these small tumors.4
Selective venous catheterization can be useful in the localization and lateralization of an androgen-secreting tumor, although a nondiagnostic result with this technique is not uncommon.4
Continue to: Dynamic hormonal testing
Dynamic hormonal testing may assist in determining the pathology of disease but not laterality.2 For example, testing for gonadotropin-releasing hormone agonists can be helpful because the constant administration of such agonists can lead to ovarian suppression without affecting adrenal androgen secretion.5
Testing with oral dexamethasone may induce adrenal hormonal depression of androgens and subsequent estradiol through aromatase conversion, which can help rule out an ovarian source.6 Exogenous administration of follicle-stimulating hormone or luteinizing hormone can further differentiate the source from ovarian theca or granulosa cell production.4
Treatment varies
The specific etiology of a patient’s hirsutism dictates the most appropriate treatment. For example, medication-induced hirsutism often requires discontinuation of the offending agent, whereas PCOS would necessitate appropriate nonpharmacologic and pharmacologic interventions.
For our patient, the elevated testosterone and free testosterone levels with normal DHEAS strongly suggested the presence of an androgen-secreting ovarian tumor. These findings led to a referral for bilateral salpingo-oophorectomy. The surgical gross appearance of the patient’s ovaries was unremarkable, but gross dissection and pathology of the ovaries (which were not postoperatively identified to determine laterality) showed one was larger (2.7 × 1.5 × 0.8 cm vs 3.2 × 1.4 × 1.2 cm).
The larger ovary contained an area of brown induration measuring 2.3 × 1.1 × 1.1 cm. This area corresponded to abundant eosinophilic cytoplasm with nuclear, rich, round-cell proliferation, consistent with the diagnosis of a benign ovarian Leydig cell tumor (FIGURE 2). Thus, the bilateral salpingo-oophorectomy was both diagnostic and therapeutic.
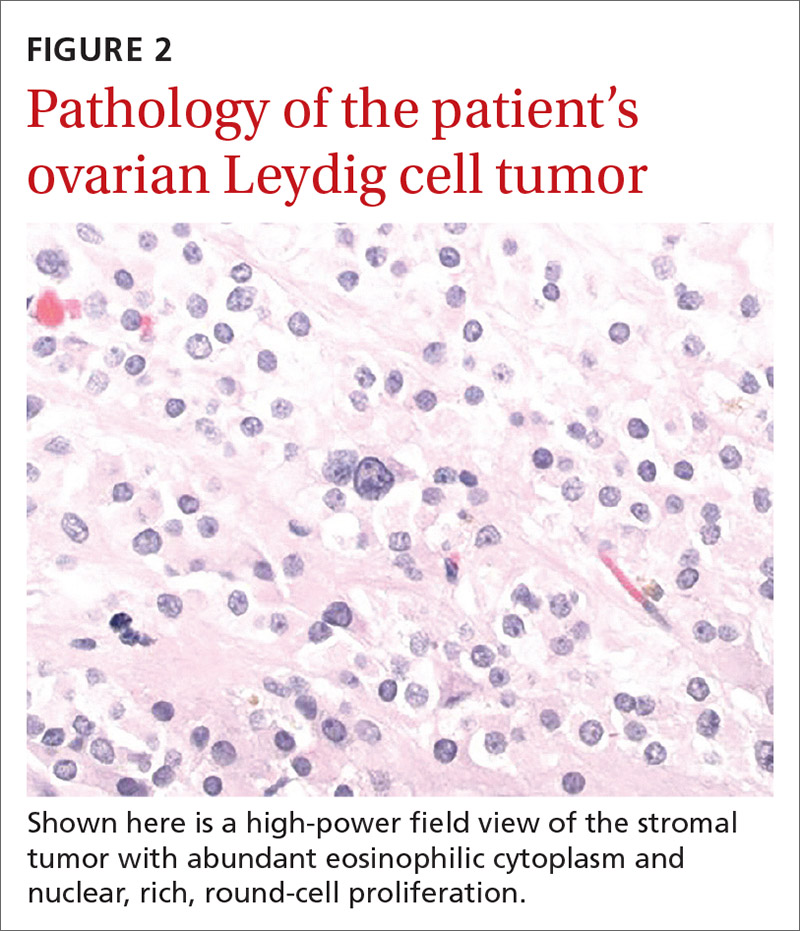
Six weeks after the surgery, blood work showed normalization of testosterone and free testosterone levels. The patient’s hirsutism completely resolved over the course of the next several months.
1. Hunter M, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67:2565-2572.
2. Alpañés M, González-Casbas JM, Sánchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588.
3. Bode D, Seehusen DA, Baird D. Hirsutism in women. Am Fam Physician. 2012;85:373-380.
4. Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neopolasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12-15.
5. Gandrapu B, Sundar P, Phillips B. Hyperandrogenism in a postmenaupsal woman secondary to testosterone secreting ovarian stromal tumor with acoustic schwannoma. Case Rep Endocrinol. 2018;2018:8154513.
6. Curran DR, Moore C, Huber T. What is the best approach to the evaluation of hirsutism? J Fam Pract. 2005;54:458-473.
A 74-year-old woman presented to the dermatology clinic for follow-up 3 months after the surgical excision of a basal cell carcinoma on her left jawline. During this postop period, the patient developed new-onset hirsutism. She appeared to be in otherwise good health.
Family and personal medical history were unremarkable. Her medication regimen included aspirin 81 mg/d and a daily multivitamin. The patient was postmenopausal and had a body mass index of 28 and a history of acid reflux and osteoarthritis.
Physical examination of the patient’s scalp showed male-pattern alopecia (FIGURE 1A). She also had coarse terminal hairs on her forearms and back, as well as on her chin (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Androgen-secreting ovarian tumor
Based on the distribution of terminal hairs and marked change over 3 months, as well as the male-pattern alopecia, a diagnosis of androgen excess was suspected. Laboratory work-up, including thyroid-stimulating hormone, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, prolactin, complete blood count, and complete metabolic panel, was within normal limits. Pelvic ultrasound of the ovaries and abdominal computed tomography (CT) of the adrenal glands were also normal.
Further testing showed an elevated testosterone level of 464 ng/dL (reference range: 2-45 ng/dL) and an elevated free testosterone level of 66.8 ng/dL (reference range: 0.2-3.7 ng/dL). These levels pointed to an androgen-secreting ovarian tumor; the androgen excess was likely the cause of her hirsutism.
Hirsutism or hypertrichosis?
Hirsutism, a common disorder affecting up to 8% of women, is defined by excess terminal hairs that appear in a male pattern in women due to production of excess androgens.1 This should be distinguished from hypertrichosis, which is generalized excessive hair growth not caused by androgen excess.
Testosterone and DHEAS—produced in the ovaries and adrenal glands, respectively—contribute to the development of hirsutism.1 Hirsutism is more often associated with adrenal or ovarian tumors in postmenopausal patients.2 Generalized hypertrichosis can be associated with porphyria cutanea tarda, severe anorexia nervosa, and rarely, malignancies; it also can be secondary to certain agents, such as cyclosporin, phenytoin, and minoxidil.
While hirsutism is associated with hyperandrogenemia, its degree correlates poorly with serum levels. Notably, about half of women with hirsutism have been found to have normal levels of circulating androgens.1 Severe signs of hyperandrogenemia include rapid onset of symptoms, signs of virilization, and a palpable abdominal or pelvic mass.3
Continue to: Is the patient pre- or postmenopausal?
Is the patient pre- or postmenopausal? Polycystic ovary syndrome (PCOS) accounts for up to three-fourths of premenopausal hirsutism.3 The likelihood of hirsutism is actually decreased in postmenopausal women because estrogen levels can drop abruptly after menopause. That said, conditions linked to hirsutism in postmenopausal women include adrenal hyperplasia, thyroid dysfunction, Cushing syndrome, and least frequently, androgen-secreting tumors (seen in this patient). (Hirsutism can also be idiopathic or iatrogenic [medications].)
Methods for detection
Research suggests that when a female patient is given a diagnosis of hirsutism, it’s important to explore possible underlying ovarian and/or adrenal tumors and adult-onset adrenal hyperplasia.1 The following tests and procedure can be helpful:
Serum testosterone and DHEAS. Levels of total testosterone > 200 ng/dL and/or DHEAS > 700 ng/dL are strongly indicative of androgen-secreting tumors.1
Imaging—including ultrasound, CT, or magnetic resonance imaging—can be used for evaluation of the adrenal glands and ovaries. However, imaging is often unable to identify these small tumors.4
Selective venous catheterization can be useful in the localization and lateralization of an androgen-secreting tumor, although a nondiagnostic result with this technique is not uncommon.4
Continue to: Dynamic hormonal testing
Dynamic hormonal testing may assist in determining the pathology of disease but not laterality.2 For example, testing for gonadotropin-releasing hormone agonists can be helpful because the constant administration of such agonists can lead to ovarian suppression without affecting adrenal androgen secretion.5
Testing with oral dexamethasone may induce adrenal hormonal depression of androgens and subsequent estradiol through aromatase conversion, which can help rule out an ovarian source.6 Exogenous administration of follicle-stimulating hormone or luteinizing hormone can further differentiate the source from ovarian theca or granulosa cell production.4
Treatment varies
The specific etiology of a patient’s hirsutism dictates the most appropriate treatment. For example, medication-induced hirsutism often requires discontinuation of the offending agent, whereas PCOS would necessitate appropriate nonpharmacologic and pharmacologic interventions.
For our patient, the elevated testosterone and free testosterone levels with normal DHEAS strongly suggested the presence of an androgen-secreting ovarian tumor. These findings led to a referral for bilateral salpingo-oophorectomy. The surgical gross appearance of the patient’s ovaries was unremarkable, but gross dissection and pathology of the ovaries (which were not postoperatively identified to determine laterality) showed one was larger (2.7 × 1.5 × 0.8 cm vs 3.2 × 1.4 × 1.2 cm).
The larger ovary contained an area of brown induration measuring 2.3 × 1.1 × 1.1 cm. This area corresponded to abundant eosinophilic cytoplasm with nuclear, rich, round-cell proliferation, consistent with the diagnosis of a benign ovarian Leydig cell tumor (FIGURE 2). Thus, the bilateral salpingo-oophorectomy was both diagnostic and therapeutic.

Six weeks after the surgery, blood work showed normalization of testosterone and free testosterone levels. The patient’s hirsutism completely resolved over the course of the next several months.
A 74-year-old woman presented to the dermatology clinic for follow-up 3 months after the surgical excision of a basal cell carcinoma on her left jawline. During this postop period, the patient developed new-onset hirsutism. She appeared to be in otherwise good health.
Family and personal medical history were unremarkable. Her medication regimen included aspirin 81 mg/d and a daily multivitamin. The patient was postmenopausal and had a body mass index of 28 and a history of acid reflux and osteoarthritis.
Physical examination of the patient’s scalp showed male-pattern alopecia (FIGURE 1A). She also had coarse terminal hairs on her forearms and back, as well as on her chin (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Androgen-secreting ovarian tumor
Based on the distribution of terminal hairs and marked change over 3 months, as well as the male-pattern alopecia, a diagnosis of androgen excess was suspected. Laboratory work-up, including thyroid-stimulating hormone, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, prolactin, complete blood count, and complete metabolic panel, was within normal limits. Pelvic ultrasound of the ovaries and abdominal computed tomography (CT) of the adrenal glands were also normal.
Further testing showed an elevated testosterone level of 464 ng/dL (reference range: 2-45 ng/dL) and an elevated free testosterone level of 66.8 ng/dL (reference range: 0.2-3.7 ng/dL). These levels pointed to an androgen-secreting ovarian tumor; the androgen excess was likely the cause of her hirsutism.
Hirsutism or hypertrichosis?
Hirsutism, a common disorder affecting up to 8% of women, is defined by excess terminal hairs that appear in a male pattern in women due to production of excess androgens.1 This should be distinguished from hypertrichosis, which is generalized excessive hair growth not caused by androgen excess.
Testosterone and DHEAS—produced in the ovaries and adrenal glands, respectively—contribute to the development of hirsutism.1 Hirsutism is more often associated with adrenal or ovarian tumors in postmenopausal patients.2 Generalized hypertrichosis can be associated with porphyria cutanea tarda, severe anorexia nervosa, and rarely, malignancies; it also can be secondary to certain agents, such as cyclosporin, phenytoin, and minoxidil.
While hirsutism is associated with hyperandrogenemia, its degree correlates poorly with serum levels. Notably, about half of women with hirsutism have been found to have normal levels of circulating androgens.1 Severe signs of hyperandrogenemia include rapid onset of symptoms, signs of virilization, and a palpable abdominal or pelvic mass.3
Continue to: Is the patient pre- or postmenopausal?
Is the patient pre- or postmenopausal? Polycystic ovary syndrome (PCOS) accounts for up to three-fourths of premenopausal hirsutism.3 The likelihood of hirsutism is actually decreased in postmenopausal women because estrogen levels can drop abruptly after menopause. That said, conditions linked to hirsutism in postmenopausal women include adrenal hyperplasia, thyroid dysfunction, Cushing syndrome, and least frequently, androgen-secreting tumors (seen in this patient). (Hirsutism can also be idiopathic or iatrogenic [medications].)
Methods for detection
Research suggests that when a female patient is given a diagnosis of hirsutism, it’s important to explore possible underlying ovarian and/or adrenal tumors and adult-onset adrenal hyperplasia.1 The following tests and procedure can be helpful:
Serum testosterone and DHEAS. Levels of total testosterone > 200 ng/dL and/or DHEAS > 700 ng/dL are strongly indicative of androgen-secreting tumors.1
Imaging—including ultrasound, CT, or magnetic resonance imaging—can be used for evaluation of the adrenal glands and ovaries. However, imaging is often unable to identify these small tumors.4
Selective venous catheterization can be useful in the localization and lateralization of an androgen-secreting tumor, although a nondiagnostic result with this technique is not uncommon.4
Continue to: Dynamic hormonal testing
Dynamic hormonal testing may assist in determining the pathology of disease but not laterality.2 For example, testing for gonadotropin-releasing hormone agonists can be helpful because the constant administration of such agonists can lead to ovarian suppression without affecting adrenal androgen secretion.5
Testing with oral dexamethasone may induce adrenal hormonal depression of androgens and subsequent estradiol through aromatase conversion, which can help rule out an ovarian source.6 Exogenous administration of follicle-stimulating hormone or luteinizing hormone can further differentiate the source from ovarian theca or granulosa cell production.4
Treatment varies
The specific etiology of a patient’s hirsutism dictates the most appropriate treatment. For example, medication-induced hirsutism often requires discontinuation of the offending agent, whereas PCOS would necessitate appropriate nonpharmacologic and pharmacologic interventions.
For our patient, the elevated testosterone and free testosterone levels with normal DHEAS strongly suggested the presence of an androgen-secreting ovarian tumor. These findings led to a referral for bilateral salpingo-oophorectomy. The surgical gross appearance of the patient’s ovaries was unremarkable, but gross dissection and pathology of the ovaries (which were not postoperatively identified to determine laterality) showed one was larger (2.7 × 1.5 × 0.8 cm vs 3.2 × 1.4 × 1.2 cm).
The larger ovary contained an area of brown induration measuring 2.3 × 1.1 × 1.1 cm. This area corresponded to abundant eosinophilic cytoplasm with nuclear, rich, round-cell proliferation, consistent with the diagnosis of a benign ovarian Leydig cell tumor (FIGURE 2). Thus, the bilateral salpingo-oophorectomy was both diagnostic and therapeutic.

Six weeks after the surgery, blood work showed normalization of testosterone and free testosterone levels. The patient’s hirsutism completely resolved over the course of the next several months.
1. Hunter M, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67:2565-2572.
2. Alpañés M, González-Casbas JM, Sánchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588.
3. Bode D, Seehusen DA, Baird D. Hirsutism in women. Am Fam Physician. 2012;85:373-380.
4. Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neopolasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12-15.
5. Gandrapu B, Sundar P, Phillips B. Hyperandrogenism in a postmenaupsal woman secondary to testosterone secreting ovarian stromal tumor with acoustic schwannoma. Case Rep Endocrinol. 2018;2018:8154513.
6. Curran DR, Moore C, Huber T. What is the best approach to the evaluation of hirsutism? J Fam Pract. 2005;54:458-473.
1. Hunter M, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67:2565-2572.
2. Alpañés M, González-Casbas JM, Sánchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588.
3. Bode D, Seehusen DA, Baird D. Hirsutism in women. Am Fam Physician. 2012;85:373-380.
4. Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neopolasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12-15.
5. Gandrapu B, Sundar P, Phillips B. Hyperandrogenism in a postmenaupsal woman secondary to testosterone secreting ovarian stromal tumor with acoustic schwannoma. Case Rep Endocrinol. 2018;2018:8154513.
6. Curran DR, Moore C, Huber T. What is the best approach to the evaluation of hirsutism? J Fam Pract. 2005;54:458-473.
Theory of Planned Behavior Provides A Theoretical Explanation For Enhanced Behavior Change With Genetic-Based Lifestyle Interventions
Study Overview
Objective. To determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the Theory of Planned Behavior (TPB), a widely accepted theory used to help predict human lifestyle-related behaviors.
Design. Pragmatic, cluster, randomized controlled trial.
Settings and participants. This study took place at the East Elgin Family Health Team, a primary care clinic in Aylmer, Ontario, Canada. Recruitment occurred between April 2017 and September 2018, with staggered intervention cohorts occurring from May 2017 to September 2019. Participants enrolled in a weight management program at the clinic were invited to participate in the study if they met the following inclusion criteria: body mass index (BMI) ≥25 kg/m2, >18 years of age, English-speaking, willing to undergo genetic testing, having access to a computer with internet at least 1 day per week, and not seeing another health care provider for weight loss advice outside of the study. Exclusion criteria included pregnancy and lactation. All participants provided written informed consent.
Interventions. At baseline, weight management program cohorts (average cohort size was 14 participants) were randomized (1:1) to receive either the standard population-based intervention (Group Lifestyle Balance, or GLB) or a modified GLB intervention, which included the provision of lifestyle genomics (LGx) information and advice (GLB+LGx). Both interventions aimed to assist participants with weight management and healthy lifestyle change, with particular focus on nutrition and physical activity (PA). Interventions were 12 months long, consisting of 23 group-based sessions and 3 one-on-one sessions with a registered dietitian after 3, 6, and 12 months (all sessions were face-to-face). To improve intervention adherence, participants were given reminder calls for their one-on-one appointments and for the start of their program. A sample size was calculated based on the primary outcome indicating that a total of 74 participants were needed (n = 37 per group) for this trial. By September 2019, this sample size was exceeded with 10 randomized groups (n = 140).
The 5 randomized standard GLB groups followed the established GLB program curriculum comprising population-based information and advice while focusing on following a calorie-controlled, moderate-fat (25% of calories) nutrition plan with at least 150 minutes of weekly moderate-intensity PA. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting outlining population-based targets, including acceptable macronutrient distribution ranges for protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
The 5 randomized modified GLB+LGx groups followed a modified GLB program curriculum in which participants were given genetic-based information and advice, which differed from the advice given to the standard GLB group, while focusing on following a calorie-controlled nutrition plan. The nutrition and PA targets were personalized based on their individual genetic variation. For example, participants with the AA variant of FTO (rs9939609) were advised to engage in at least 30 to 60 minutes of PA daily 6 days per week, with muscle-strengthening activities at least 2 days per week, rather than receiving the standard population-based advice to aim for 150 minutes weekly of PA with at least 2 days per week of muscle-strengthening activity. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting, which outlined genetic-based information and advice related to protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
Measures and analysis. Change in the TPB components (attitudes, subjective norms and perceived behavioral control) were measured via a TPB questionnaire at 5 time points: baseline (2-week run-in period), immediately after the first group session (where participants received a summary report of either population-based or genetic-based recommendations depending on group assignment), and after 3-, 6- and 12-month follow-ups. Attitudes, subjective norms, and perceived behavioral control were measured on a Likert scale from 1 through 7. Self-reported measures of actual behavioral control (including annual household income, perceptions about events arising in one’s day-to-day life that suddenly take up one’s free time, perceptions about the frequency of feeling ill or tired, and highest achieved level of education) were collected via survey questions and assessed on a Likert scale of 1 through 7. Stage of change was also measured, based on the Transtheoretical Model, using a Likert scale of 1 through 6.
Linear mixed models were used to conduct within- and between-group analyses using SPSS version 26.0, while controlling for measures of actual behavioral control. All analyses were intention-to-treat by originally assigned groups, with mean value imputation conducted for missing data. A Bonferroni correction for multiple testing was used. For all statistical analyses, the level of significance was set at P < 0.05 and trending towards significance at P = 0.05–0.06.
Main results. Participants consisted of primarily middle-age, middle-income, Caucasian females. Baseline attitudes towards the effectiveness of nutrition and PA for weight management were generally positive, and participants perceived that undergoing genetic testing would assist with weight management. Participants had overall neutral subjective norms related to friends and family consuming a healthy diet and engaging in PA, but perceived that their friends, family, and health care team (HCT) believed it was important for them to achieve their nutrition and PA recommendations. Participants overall also perceived that their HCT believed genetic testing could assist with weight management. Baseline measures of perceived behavioral control were overall neutral, with baseline stage of change between “motivation” and “action” (short-term; <3 months).
In within-group analyses, significant improvements (P < 0.05) in attitudes towards the effectiveness of nutrition and PA recommendations for weight management, subjective norms related to both friends and family consuming a healthy diet, and perceived behavioral control in changing PA/dietary intake and managing weight tended to be short-term in the GLB group and long-term for the GLB+LGx group. In all cases of between-group differences for changes in TPB components, the GLB group exhibited reductions in scores, whereas the GLB+LGx group exhibited increases or improvements. Between-group differences (short-term and long-term) in several measures of subjective norms were observed. For example, after 3 months, significant between-group differences were observed in changes in perception that friends believed LGx would help with weight management (P = 0.024). After 12 months, between-group differences trending towards significance were also observed in changes in perception that family members believed genetic testing would help with weight management (P = 0.05). Significant between-group differences and differences trending towards significance were also observed at 12 months for changes in perception that family believed it was important for the participant to achieve the PA recommendations (P = 0.049) and nutrition recommendations (P = 0.05). Between-group differences trending towards significance were also observed at 3 months in attitudes towards the effectiveness of LGx for weight management (P = 0.06). There were no significant between-group differences observed in changes in perceived behavioral control.
Conclusion. Results from this study support the hypothesis that the TPB can help provide a theoretical explanation for why genetically tailored lifestyle information and advice can lead to improvements in lifestyle behavior change.
Commentary
Because health behaviors are critical in areas such as prevention, treatment, and rehabilitation, it is important to describe and understand what drives these behaviors.1 Theories are important tools in this effort as they aim to explain and predict health behavior and are used in the design and evaluation of interventions.1 The TPB is one of the most widely accepted behavior change theories and posits that attitudes, subjective norms (or social pressures and behaviors), and perceived behavioral control are significant predictors of an individual’s intention to engage in behaviors.2 TPB has been highlighted in the literature as a validated theory for predicting nutrition and PA intentions and resulting behaviors.3,4
Motivating lifestyle behavior change in clinical practice can be challenging, but some studies have demonstrated how providing genetic information and advice (or lifestyle genomics) can help motivate changes in nutrition and PA among patients.5-7 Because this has yet to be explained using the TPB, this study is an important contribution to the literature as it aimed to determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the TPB. Briefly, results from within-group analyses in this study demonstrated that the provision of genetically tailored lifestyle information and advice (via the GLB+LGx intervention) tended to impact antecedents of behavior change, more so over the long-term, while population-based advice (via the standard GLB intervention) tended to impact antecedents of behavior change over the short-term (eg, attitudes towards dietary fat intake, perceptions that friends and family consume a healthy diet, and perceptions about the impact of genetic-based advice for weight management). In addition, between-group differences in subjective norms observed at 12 months suggested that social pressures and norms may be influencing long-term changes in lifestyle habits.
While key strengths of this study include its pragmatic cluster randomized controlled trial design, 12-month intervention duration, and intent-to-treat analyses, there are some study limitations, which are acknowledged by the authors. Generalizability is limited to the demographic characteristics of the study population (ie, middle-aged, middle-income, Caucasian females enrolled in a lifestyle change weight management program). Thus, replication of the study is needed in more diverse study populations and with health-related outcomes beyond weight management. In addition, as the authors indicate, future research should ensure the inclusion of theory-based questionnaires in genetic-based intervention studies assessing lifestyle behavior change to elucidate theory-based mechanisms of change.
Applications for Clinical Practice
Population-based research has consistently indicated that nutrition interventions typically impact short-term dietary changes. Confronting the challenge of long-term adherence to nutrition and PA recommendations requires an understanding of factors impacting long-term motivation and behavior change. With increased attention on and research into genetically tailored lifestyle advice (or lifestyle genomics), it is important for clinical practitioners to be familiar with the evidence supporting these approaches. In addition, this research highlights the need to consider individual factors (attitudes, subjective norms, and perceived behavioral control) that may predict successful change in lifestyle habits when providing nutrition and PA recommendations, whether population-based or genetically tailored.
—Katrina F. Mateo, PhD, MPH
1. Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Appl Psychol. 2008;57:698-716.
2. Ajzen I. The Theory of planned behaviour: reactions and reflections. Psychol Health. 2011;26:1113-1127.
3. McDermott MS, Oliver M, Simnadis T, et al. The Theory of Planned Behaviour and dietary patterns: A systematic review and meta-analysis. Prev Med (Baltim). 2015;81:150-156.
4. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: A meta-analysis. Health Psychol Rev. 2011;5:97-144.
5. Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, et al A. An intervention study of individual, APOE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161-174.
6. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. DeAngelis MM, ed. PLoS One. 2014;9(11):e112665.
7. Egglestone C, Morris A, O’Brien A. Effect of direct‐to‐consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565-575.
Study Overview
Objective. To determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the Theory of Planned Behavior (TPB), a widely accepted theory used to help predict human lifestyle-related behaviors.
Design. Pragmatic, cluster, randomized controlled trial.
Settings and participants. This study took place at the East Elgin Family Health Team, a primary care clinic in Aylmer, Ontario, Canada. Recruitment occurred between April 2017 and September 2018, with staggered intervention cohorts occurring from May 2017 to September 2019. Participants enrolled in a weight management program at the clinic were invited to participate in the study if they met the following inclusion criteria: body mass index (BMI) ≥25 kg/m2, >18 years of age, English-speaking, willing to undergo genetic testing, having access to a computer with internet at least 1 day per week, and not seeing another health care provider for weight loss advice outside of the study. Exclusion criteria included pregnancy and lactation. All participants provided written informed consent.
Interventions. At baseline, weight management program cohorts (average cohort size was 14 participants) were randomized (1:1) to receive either the standard population-based intervention (Group Lifestyle Balance, or GLB) or a modified GLB intervention, which included the provision of lifestyle genomics (LGx) information and advice (GLB+LGx). Both interventions aimed to assist participants with weight management and healthy lifestyle change, with particular focus on nutrition and physical activity (PA). Interventions were 12 months long, consisting of 23 group-based sessions and 3 one-on-one sessions with a registered dietitian after 3, 6, and 12 months (all sessions were face-to-face). To improve intervention adherence, participants were given reminder calls for their one-on-one appointments and for the start of their program. A sample size was calculated based on the primary outcome indicating that a total of 74 participants were needed (n = 37 per group) for this trial. By September 2019, this sample size was exceeded with 10 randomized groups (n = 140).
The 5 randomized standard GLB groups followed the established GLB program curriculum comprising population-based information and advice while focusing on following a calorie-controlled, moderate-fat (25% of calories) nutrition plan with at least 150 minutes of weekly moderate-intensity PA. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting outlining population-based targets, including acceptable macronutrient distribution ranges for protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
The 5 randomized modified GLB+LGx groups followed a modified GLB program curriculum in which participants were given genetic-based information and advice, which differed from the advice given to the standard GLB group, while focusing on following a calorie-controlled nutrition plan. The nutrition and PA targets were personalized based on their individual genetic variation. For example, participants with the AA variant of FTO (rs9939609) were advised to engage in at least 30 to 60 minutes of PA daily 6 days per week, with muscle-strengthening activities at least 2 days per week, rather than receiving the standard population-based advice to aim for 150 minutes weekly of PA with at least 2 days per week of muscle-strengthening activity. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting, which outlined genetic-based information and advice related to protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
Measures and analysis. Change in the TPB components (attitudes, subjective norms and perceived behavioral control) were measured via a TPB questionnaire at 5 time points: baseline (2-week run-in period), immediately after the first group session (where participants received a summary report of either population-based or genetic-based recommendations depending on group assignment), and after 3-, 6- and 12-month follow-ups. Attitudes, subjective norms, and perceived behavioral control were measured on a Likert scale from 1 through 7. Self-reported measures of actual behavioral control (including annual household income, perceptions about events arising in one’s day-to-day life that suddenly take up one’s free time, perceptions about the frequency of feeling ill or tired, and highest achieved level of education) were collected via survey questions and assessed on a Likert scale of 1 through 7. Stage of change was also measured, based on the Transtheoretical Model, using a Likert scale of 1 through 6.
Linear mixed models were used to conduct within- and between-group analyses using SPSS version 26.0, while controlling for measures of actual behavioral control. All analyses were intention-to-treat by originally assigned groups, with mean value imputation conducted for missing data. A Bonferroni correction for multiple testing was used. For all statistical analyses, the level of significance was set at P < 0.05 and trending towards significance at P = 0.05–0.06.
Main results. Participants consisted of primarily middle-age, middle-income, Caucasian females. Baseline attitudes towards the effectiveness of nutrition and PA for weight management were generally positive, and participants perceived that undergoing genetic testing would assist with weight management. Participants had overall neutral subjective norms related to friends and family consuming a healthy diet and engaging in PA, but perceived that their friends, family, and health care team (HCT) believed it was important for them to achieve their nutrition and PA recommendations. Participants overall also perceived that their HCT believed genetic testing could assist with weight management. Baseline measures of perceived behavioral control were overall neutral, with baseline stage of change between “motivation” and “action” (short-term; <3 months).
In within-group analyses, significant improvements (P < 0.05) in attitudes towards the effectiveness of nutrition and PA recommendations for weight management, subjective norms related to both friends and family consuming a healthy diet, and perceived behavioral control in changing PA/dietary intake and managing weight tended to be short-term in the GLB group and long-term for the GLB+LGx group. In all cases of between-group differences for changes in TPB components, the GLB group exhibited reductions in scores, whereas the GLB+LGx group exhibited increases or improvements. Between-group differences (short-term and long-term) in several measures of subjective norms were observed. For example, after 3 months, significant between-group differences were observed in changes in perception that friends believed LGx would help with weight management (P = 0.024). After 12 months, between-group differences trending towards significance were also observed in changes in perception that family members believed genetic testing would help with weight management (P = 0.05). Significant between-group differences and differences trending towards significance were also observed at 12 months for changes in perception that family believed it was important for the participant to achieve the PA recommendations (P = 0.049) and nutrition recommendations (P = 0.05). Between-group differences trending towards significance were also observed at 3 months in attitudes towards the effectiveness of LGx for weight management (P = 0.06). There were no significant between-group differences observed in changes in perceived behavioral control.
Conclusion. Results from this study support the hypothesis that the TPB can help provide a theoretical explanation for why genetically tailored lifestyle information and advice can lead to improvements in lifestyle behavior change.
Commentary
Because health behaviors are critical in areas such as prevention, treatment, and rehabilitation, it is important to describe and understand what drives these behaviors.1 Theories are important tools in this effort as they aim to explain and predict health behavior and are used in the design and evaluation of interventions.1 The TPB is one of the most widely accepted behavior change theories and posits that attitudes, subjective norms (or social pressures and behaviors), and perceived behavioral control are significant predictors of an individual’s intention to engage in behaviors.2 TPB has been highlighted in the literature as a validated theory for predicting nutrition and PA intentions and resulting behaviors.3,4
Motivating lifestyle behavior change in clinical practice can be challenging, but some studies have demonstrated how providing genetic information and advice (or lifestyle genomics) can help motivate changes in nutrition and PA among patients.5-7 Because this has yet to be explained using the TPB, this study is an important contribution to the literature as it aimed to determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the TPB. Briefly, results from within-group analyses in this study demonstrated that the provision of genetically tailored lifestyle information and advice (via the GLB+LGx intervention) tended to impact antecedents of behavior change, more so over the long-term, while population-based advice (via the standard GLB intervention) tended to impact antecedents of behavior change over the short-term (eg, attitudes towards dietary fat intake, perceptions that friends and family consume a healthy diet, and perceptions about the impact of genetic-based advice for weight management). In addition, between-group differences in subjective norms observed at 12 months suggested that social pressures and norms may be influencing long-term changes in lifestyle habits.
While key strengths of this study include its pragmatic cluster randomized controlled trial design, 12-month intervention duration, and intent-to-treat analyses, there are some study limitations, which are acknowledged by the authors. Generalizability is limited to the demographic characteristics of the study population (ie, middle-aged, middle-income, Caucasian females enrolled in a lifestyle change weight management program). Thus, replication of the study is needed in more diverse study populations and with health-related outcomes beyond weight management. In addition, as the authors indicate, future research should ensure the inclusion of theory-based questionnaires in genetic-based intervention studies assessing lifestyle behavior change to elucidate theory-based mechanisms of change.
Applications for Clinical Practice
Population-based research has consistently indicated that nutrition interventions typically impact short-term dietary changes. Confronting the challenge of long-term adherence to nutrition and PA recommendations requires an understanding of factors impacting long-term motivation and behavior change. With increased attention on and research into genetically tailored lifestyle advice (or lifestyle genomics), it is important for clinical practitioners to be familiar with the evidence supporting these approaches. In addition, this research highlights the need to consider individual factors (attitudes, subjective norms, and perceived behavioral control) that may predict successful change in lifestyle habits when providing nutrition and PA recommendations, whether population-based or genetically tailored.
—Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the Theory of Planned Behavior (TPB), a widely accepted theory used to help predict human lifestyle-related behaviors.
Design. Pragmatic, cluster, randomized controlled trial.
Settings and participants. This study took place at the East Elgin Family Health Team, a primary care clinic in Aylmer, Ontario, Canada. Recruitment occurred between April 2017 and September 2018, with staggered intervention cohorts occurring from May 2017 to September 2019. Participants enrolled in a weight management program at the clinic were invited to participate in the study if they met the following inclusion criteria: body mass index (BMI) ≥25 kg/m2, >18 years of age, English-speaking, willing to undergo genetic testing, having access to a computer with internet at least 1 day per week, and not seeing another health care provider for weight loss advice outside of the study. Exclusion criteria included pregnancy and lactation. All participants provided written informed consent.
Interventions. At baseline, weight management program cohorts (average cohort size was 14 participants) were randomized (1:1) to receive either the standard population-based intervention (Group Lifestyle Balance, or GLB) or a modified GLB intervention, which included the provision of lifestyle genomics (LGx) information and advice (GLB+LGx). Both interventions aimed to assist participants with weight management and healthy lifestyle change, with particular focus on nutrition and physical activity (PA). Interventions were 12 months long, consisting of 23 group-based sessions and 3 one-on-one sessions with a registered dietitian after 3, 6, and 12 months (all sessions were face-to-face). To improve intervention adherence, participants were given reminder calls for their one-on-one appointments and for the start of their program. A sample size was calculated based on the primary outcome indicating that a total of 74 participants were needed (n = 37 per group) for this trial. By September 2019, this sample size was exceeded with 10 randomized groups (n = 140).
The 5 randomized standard GLB groups followed the established GLB program curriculum comprising population-based information and advice while focusing on following a calorie-controlled, moderate-fat (25% of calories) nutrition plan with at least 150 minutes of weekly moderate-intensity PA. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting outlining population-based targets, including acceptable macronutrient distribution ranges for protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
The 5 randomized modified GLB+LGx groups followed a modified GLB program curriculum in which participants were given genetic-based information and advice, which differed from the advice given to the standard GLB group, while focusing on following a calorie-controlled nutrition plan. The nutrition and PA targets were personalized based on their individual genetic variation. For example, participants with the AA variant of FTO (rs9939609) were advised to engage in at least 30 to 60 minutes of PA daily 6 days per week, with muscle-strengthening activities at least 2 days per week, rather than receiving the standard population-based advice to aim for 150 minutes weekly of PA with at least 2 days per week of muscle-strengthening activity. Participants were also provided with a 1-page summary report of their nutrition and PA guidelines at the first group meeting, which outlined genetic-based information and advice related to protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, sodium, calories, snacking, and PA.
Measures and analysis. Change in the TPB components (attitudes, subjective norms and perceived behavioral control) were measured via a TPB questionnaire at 5 time points: baseline (2-week run-in period), immediately after the first group session (where participants received a summary report of either population-based or genetic-based recommendations depending on group assignment), and after 3-, 6- and 12-month follow-ups. Attitudes, subjective norms, and perceived behavioral control were measured on a Likert scale from 1 through 7. Self-reported measures of actual behavioral control (including annual household income, perceptions about events arising in one’s day-to-day life that suddenly take up one’s free time, perceptions about the frequency of feeling ill or tired, and highest achieved level of education) were collected via survey questions and assessed on a Likert scale of 1 through 7. Stage of change was also measured, based on the Transtheoretical Model, using a Likert scale of 1 through 6.
Linear mixed models were used to conduct within- and between-group analyses using SPSS version 26.0, while controlling for measures of actual behavioral control. All analyses were intention-to-treat by originally assigned groups, with mean value imputation conducted for missing data. A Bonferroni correction for multiple testing was used. For all statistical analyses, the level of significance was set at P < 0.05 and trending towards significance at P = 0.05–0.06.
Main results. Participants consisted of primarily middle-age, middle-income, Caucasian females. Baseline attitudes towards the effectiveness of nutrition and PA for weight management were generally positive, and participants perceived that undergoing genetic testing would assist with weight management. Participants had overall neutral subjective norms related to friends and family consuming a healthy diet and engaging in PA, but perceived that their friends, family, and health care team (HCT) believed it was important for them to achieve their nutrition and PA recommendations. Participants overall also perceived that their HCT believed genetic testing could assist with weight management. Baseline measures of perceived behavioral control were overall neutral, with baseline stage of change between “motivation” and “action” (short-term; <3 months).
In within-group analyses, significant improvements (P < 0.05) in attitudes towards the effectiveness of nutrition and PA recommendations for weight management, subjective norms related to both friends and family consuming a healthy diet, and perceived behavioral control in changing PA/dietary intake and managing weight tended to be short-term in the GLB group and long-term for the GLB+LGx group. In all cases of between-group differences for changes in TPB components, the GLB group exhibited reductions in scores, whereas the GLB+LGx group exhibited increases or improvements. Between-group differences (short-term and long-term) in several measures of subjective norms were observed. For example, after 3 months, significant between-group differences were observed in changes in perception that friends believed LGx would help with weight management (P = 0.024). After 12 months, between-group differences trending towards significance were also observed in changes in perception that family members believed genetic testing would help with weight management (P = 0.05). Significant between-group differences and differences trending towards significance were also observed at 12 months for changes in perception that family believed it was important for the participant to achieve the PA recommendations (P = 0.049) and nutrition recommendations (P = 0.05). Between-group differences trending towards significance were also observed at 3 months in attitudes towards the effectiveness of LGx for weight management (P = 0.06). There were no significant between-group differences observed in changes in perceived behavioral control.
Conclusion. Results from this study support the hypothesis that the TPB can help provide a theoretical explanation for why genetically tailored lifestyle information and advice can lead to improvements in lifestyle behavior change.
Commentary
Because health behaviors are critical in areas such as prevention, treatment, and rehabilitation, it is important to describe and understand what drives these behaviors.1 Theories are important tools in this effort as they aim to explain and predict health behavior and are used in the design and evaluation of interventions.1 The TPB is one of the most widely accepted behavior change theories and posits that attitudes, subjective norms (or social pressures and behaviors), and perceived behavioral control are significant predictors of an individual’s intention to engage in behaviors.2 TPB has been highlighted in the literature as a validated theory for predicting nutrition and PA intentions and resulting behaviors.3,4
Motivating lifestyle behavior change in clinical practice can be challenging, but some studies have demonstrated how providing genetic information and advice (or lifestyle genomics) can help motivate changes in nutrition and PA among patients.5-7 Because this has yet to be explained using the TPB, this study is an important contribution to the literature as it aimed to determine the impact of providing genetically tailored and population-based lifestyle advice for weight management on key constructs of the TPB. Briefly, results from within-group analyses in this study demonstrated that the provision of genetically tailored lifestyle information and advice (via the GLB+LGx intervention) tended to impact antecedents of behavior change, more so over the long-term, while population-based advice (via the standard GLB intervention) tended to impact antecedents of behavior change over the short-term (eg, attitudes towards dietary fat intake, perceptions that friends and family consume a healthy diet, and perceptions about the impact of genetic-based advice for weight management). In addition, between-group differences in subjective norms observed at 12 months suggested that social pressures and norms may be influencing long-term changes in lifestyle habits.
While key strengths of this study include its pragmatic cluster randomized controlled trial design, 12-month intervention duration, and intent-to-treat analyses, there are some study limitations, which are acknowledged by the authors. Generalizability is limited to the demographic characteristics of the study population (ie, middle-aged, middle-income, Caucasian females enrolled in a lifestyle change weight management program). Thus, replication of the study is needed in more diverse study populations and with health-related outcomes beyond weight management. In addition, as the authors indicate, future research should ensure the inclusion of theory-based questionnaires in genetic-based intervention studies assessing lifestyle behavior change to elucidate theory-based mechanisms of change.
Applications for Clinical Practice
Population-based research has consistently indicated that nutrition interventions typically impact short-term dietary changes. Confronting the challenge of long-term adherence to nutrition and PA recommendations requires an understanding of factors impacting long-term motivation and behavior change. With increased attention on and research into genetically tailored lifestyle advice (or lifestyle genomics), it is important for clinical practitioners to be familiar with the evidence supporting these approaches. In addition, this research highlights the need to consider individual factors (attitudes, subjective norms, and perceived behavioral control) that may predict successful change in lifestyle habits when providing nutrition and PA recommendations, whether population-based or genetically tailored.
—Katrina F. Mateo, PhD, MPH
1. Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Appl Psychol. 2008;57:698-716.
2. Ajzen I. The Theory of planned behaviour: reactions and reflections. Psychol Health. 2011;26:1113-1127.
3. McDermott MS, Oliver M, Simnadis T, et al. The Theory of Planned Behaviour and dietary patterns: A systematic review and meta-analysis. Prev Med (Baltim). 2015;81:150-156.
4. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: A meta-analysis. Health Psychol Rev. 2011;5:97-144.
5. Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, et al A. An intervention study of individual, APOE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161-174.
6. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. DeAngelis MM, ed. PLoS One. 2014;9(11):e112665.
7. Egglestone C, Morris A, O’Brien A. Effect of direct‐to‐consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565-575.
1. Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Appl Psychol. 2008;57:698-716.
2. Ajzen I. The Theory of planned behaviour: reactions and reflections. Psychol Health. 2011;26:1113-1127.
3. McDermott MS, Oliver M, Simnadis T, et al. The Theory of Planned Behaviour and dietary patterns: A systematic review and meta-analysis. Prev Med (Baltim). 2015;81:150-156.
4. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: A meta-analysis. Health Psychol Rev. 2011;5:97-144.
5. Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, et al A. An intervention study of individual, APOE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161-174.
6. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. DeAngelis MM, ed. PLoS One. 2014;9(11):e112665.
7. Egglestone C, Morris A, O’Brien A. Effect of direct‐to‐consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565-575.
Breaking the cycle of medication overuse headache
Medication overuse headache (MOH), a secondary headache diagnosis, is a prevalent phenomenon that complicates headache diagnosis and treatment, increases the cost of care, and reduces quality of life. Effective abortive medication is essential for the headache sufferer; when an abortive is used too frequently, however, headache frequency increases—potentially beginning a cycle in which the patient then takes more medication to abort the headache. Over time, the patient suffers from an ever-increasing number of headaches, takes even more abortive medication, and so on. In the presence of MOH, there is a reduction in pain response to preventive and abortive treatments; when medication overuse is eliminated, pain response improves.1
Although MOH is well recognized among headache specialists, the condition is often overlooked in primary care. Since headache is a top complaint in primary care, however, and prevention is a major goal in family medicine, the opportunity for you to recognize, treat, and prevent MOH is significant. In fact, a randomized controlled trial showed that brief patient education about headache care and MOH provided by a primary care physician can lead to a significant reduction in headache frequency among patients with MOH.2
This article reviews the recognition and diagnosis of MOH, based on historical features and current criteria; addresses risk factors for abortive medication overuse and how to withdraw an offending agent; and explores the value of bridging and preventive therapies to reduce the overall frequency of headache.

What defines MOH?
Typically, MOH is a chronification of a primary headache disorder. However, in patients with a history of migraine who are undergoing treatment for another chronic pain condition with an opioid or other analgesic, MOH can be induced.3 An increase in the frequency of headache raises the specter of a concomitant increase in the level of disability4; psychiatric comorbidity5; and more headache days, with time lost from school and work.
The Migraine Disability Assessment (MIDAS) questionnaire, a validated instrument that helps the provider (1) measure the impact that headache has on a patient’s life and (2) follow treatment progress, also provides information to employers and insurance companies on treatment coverage and the need for work modification. The MIDAS score is 3 times higher in patients with MOH than in patients with episodic migraine.6,7
The annual associated cost per person of MOH has been estimated at $4000, resulting in billions of dollars in associated costs8; most of these costs are related to absenteeism and disability. After detoxification for MOH, annual outpatient medication costs are reduced by approximately 24%.9
Efforts to solve a common problem create another
Headache affects nearly 50% of the general population worldwide,10 accounting for about 4% of primary care visits11 and approximately 20% of outpatient neurology consultations.12 Although inpatient stays for headache are approximately half the duration of the overall average hospital stay, headache accounts for 3% of admissions.13 According to the Global Burden of Disease study, tension-type headache, migraine, and MOH are the 3 most common headache disorders.10 Headache is the second leading cause of disability among people 15 to 49 years of age.10
Continue to: The prevalence of MOH...
The prevalence of MOH in the general population is 2%.7,14,15 A population-based study showed that the rate of progression from episodic headache (< 15 d/mo) to chronic headache (≥ 15 d/mo) in the general population is 2.5% per year16; however, progression to chronic headache is 14% per year in patients with medication overuse. One-third of the general population with chronic migraine overuses symptomatic medication; in US headache clinics, roughly one-half of patients with chronic headache overuse acute medication.6
Definitions and diagnosis
MOH is a secondary headache diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3) (TABLE 1),17 which lists diagnostic criteria for recognized headache disorders.
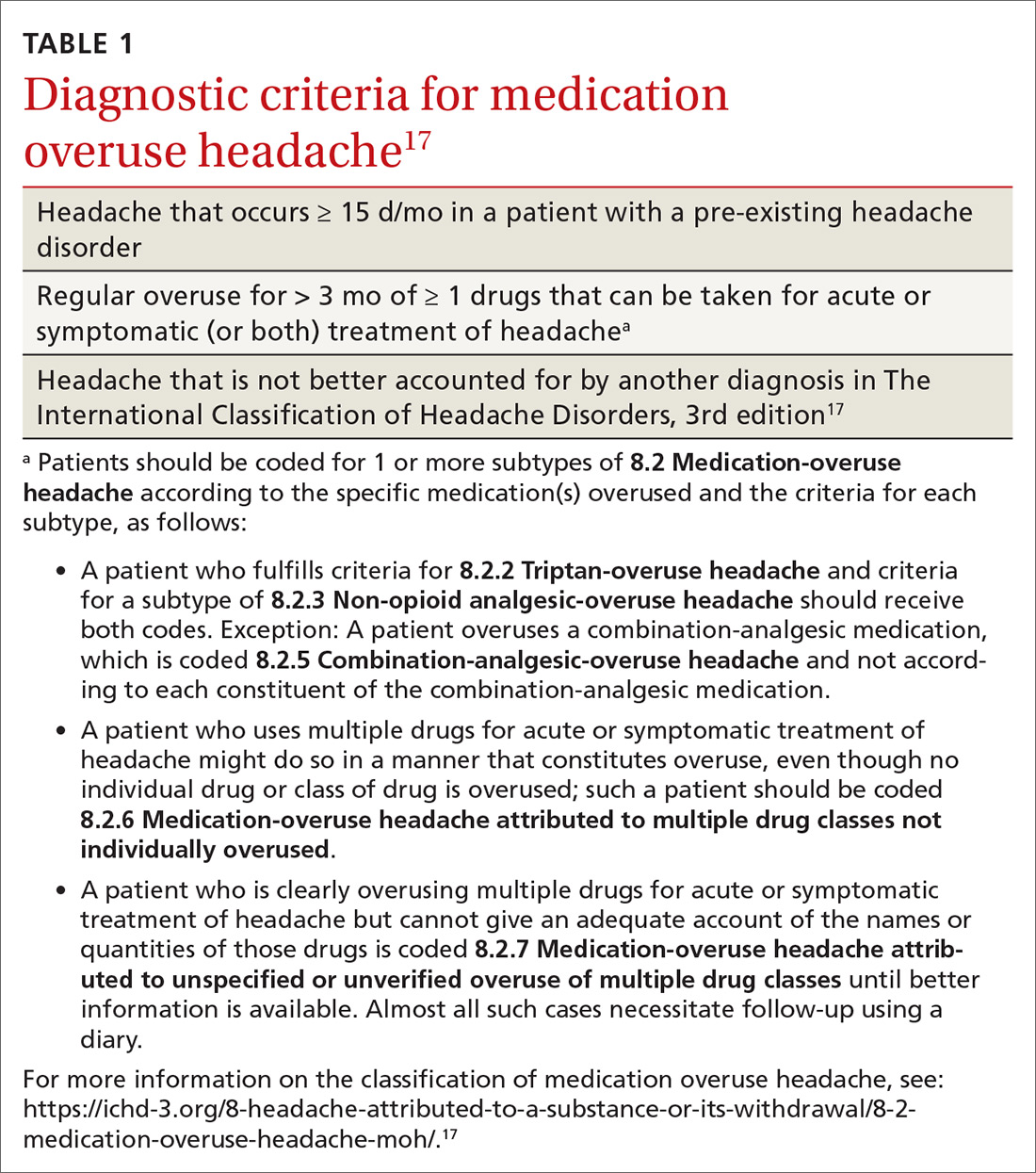
Terminology. MOH has also been called rebound headache, drug-induced headache, and transformed migraine, but these terms are outdated and are not formal diagnoses. Patients sometimes refer to substance-withdrawal headaches (not discussed in this article) as rebound headaches, so clarity is important when discussing headache with patients: namely, that MOH is an exacerbation of an existing headache condition caused by overuse of abortive headache medications, including analgesics, combination analgesics, triptans, barbiturates, and opioids.
MOH was recognized in the early 1950s and fully differentiated as a diagnosis in 2005 in the second edition of the ICHD. The disorder is subcategorized by offending abortive agent (TABLE 217) because the frequency of analgesic use required to develop MOH differs by agent.
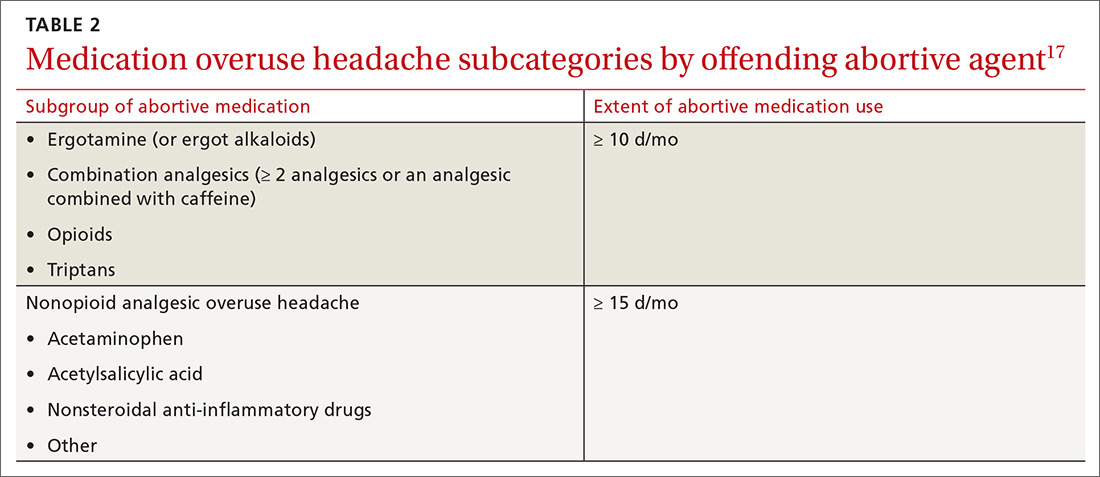
Risk factors for MOH and chronification of a primary headache disorder. There are several risk factors for developing MOH, and others that contribute to increasing headache frequency in general (TABLE 35,14,18-23). Some risk factors are common to each. All are important to address because some are modifiable.
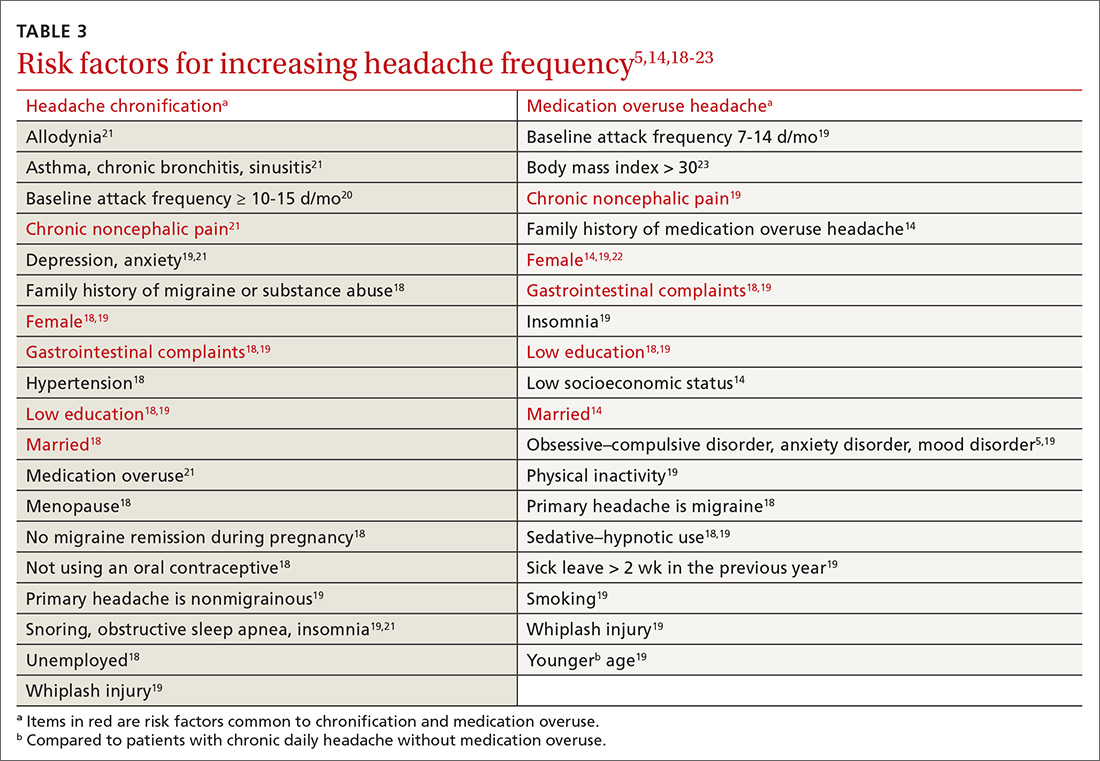
Continue to: Pathophysiology
Pathophysiology. The pathophysiology and psychology behind MOH are largely unknown. Physiologic changes in pain processing and functional imaging changes have been demonstrated in patients with MOH, both of which are reversible upon withdrawal of medication.23 Genetic factors and changes in hormone and neurotransmitter levels are found in MOH patients; this is not the case in patients who have an episodic headache pattern only.24
Presentation. Diagnostic criteria for MOH do not include clinical characteristics. Typically, the phenotype of MOH in a given patient is similar to the underlying primary headache25—although this principle can be complicated to tease out because these medications can suppress some symptoms. Diagnosis of a primary headache disorder should be documented along with the diagnosis of MOH.
Medication overuse can exist without MOH: Not every patient who frequently uses an abortive medication develops MOH.
Treatment is multifaceted—and can become complex
Mainstays of treatment of MOH are education about the disorder and detoxification from the overused agent, although specific treatments can differ depending on the agent involved, the frequency and duration of its use, and a patient’s behavioral patterns and psychiatric comorbidities. Often, a daily medication to prevent headache is considered upon, or after, withdrawal of the offending agent. The timing of introducing a preventive might impact its effectiveness. Some refractory cases require more intensive therapy, including hospitalization at a specialized tertiary center.
But before we look at detoxification from an overused agent, it’s important to review one of the best strategies of all in combatting MOH.
Continue to: First and best strategy
First and best strategy: Avoid onset of MOH
Select an appropriate abortive to reduce the risk of MOH. With regard to specific acute headache medications, some nuances other than type of headache should be considered. Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as abortive therapy by the American Headache Society for their efficacy, favorable adverse effect profile, and low cost. NSAIDs are protective against development of MOH if a patient’s baseline headache frequency is < 10/mo; at a frequency of 10 to 14 d/mo, however, the risk of MOH increases when using an NSAID.6 A similar effect has been seen with triptans.16 Longer-acting NSAIDs, such as nabumetone and naproxen, have been proposed as less likely to cause MOH, and are even used as bridging therapy sometimes (as long as neither of these was the overused medication).26
The time it takes to develop MOH is shortest with triptans, followed by ergots, then analgesics.27
Prospective cohort studies6,16 have shown that barbiturates and opioids are more likely to induce MOH; for that reason, agents in these analgesic classes are almost universally avoided unless no other medically acceptable options exist. Using barbiturate-containing compounds or opioids > 4 d/mo exponentially increases the likelihood of MOH.
Promising preclinical data demonstrate that the gepant, or small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist, class of medications used as abortive therapy does not induce medication overuse cutaneous allodynia.28
Provide education. Primary prevention of MOH involves (1) increasing patients’ awareness of how to take medications appropriately and (2) restricting intake of over-the-counter abortive medications. Often, the expert recommendation is to limit abortives to approximately 2 d/wk because more frequent use places patients at risk of further increased use and subsequent MOH.
Continue to: A randomized controlled trial in Norway...
A randomized controlled trial in Norway compared outcomes in 2 groups of patients with MOH: One group was given advice on the disorder by a physician; the other group was not provided with advice. In the “business-as-usual” group, there was no significant improvement; however, when general practitioners provided simple advice (lasting roughly 9 minutes) about reducing abortive medication use to a safe level and cautioned patients that they would be “feeling worse before feeling better,” headache days were reduced by approximately 8 per month and medication days, by 16 per month.2
A subsequent, long-term follow-up study29 of patients from the Norway trial2 who had been given advice and education showed a relapse rate (ie, into overuse of headache medication) of only 8% and sustained reduction of headache days and medication use at 16 months.
Offer support and other nondrug interventions. A recent review of 3 studies23 recommended that extra support for patients from a headache nurse, close follow-up, keeping an electronic diary that provides feedback, and undertaking a short course of psychotherapy can reduce medication overuse and prevent relapse.
If MOH develops, initiate withdrawal, introduce a preventive
Withdraw overused medication. Most current evidence suggests that withdrawal of the offending agent is the most effective factor in reducing headache days and improving quality of life. A randomized controlled trial compared the effects of (1) complete and immediate withdrawal of an abortive medication with (2) reducing its use (ie, limiting intake to 2 d/wk), on headache frequency, disability, and quality of life.30 There was a reduction of headache days in both groups; however, reduction was much greater at 2 months in the complete withdrawal group than in the restricted intake group (respectively, a 41% and a 26% reduction in headache days per month). This effect was sustained at 6 and 12 months in both groups. The study confirmed the results of earlier research2,15: Abrupt withdrawal leads to reversion to an episodic pattern at 2 to 6 months in approximately 40% to 60% of patients.
More studies are needed to determine the most appropriate treatment course for MOH; however, complete withdrawal of the causative drug is the most important intervention.
Continue to: Consider withdrawal plus preventive treatment
Consider withdrawal plus preventive treatment. Use of sodium valproate, in addition to medication overuse detoxification, led to a significant reduction in headache days and improvement in quality of life at 12 weeks but no difference after 24 weeks, compared with detoxification alone in a randomized, double-blind, placebo-controlled study.31
A study of 61 patients showed a larger reduction (by 7.2 d/mo) in headache frequency with any preventive medication in addition to medication withdrawal, compared to withdrawal alone (by 4.1 d/mo) after 3 months; however, the relative benefit was gone at 6 months.32
A study of 98 patients compared immediate and delayed initiation of preventive medication upon withdrawal of overused abortive medication.33 Response was defined as a > 50% reduction in headache frequency and was similar in both groups; results showed a 28% response with immediate initiation of a preventive; a 23% response with delayed (ie, 2 months after withdrawal) initiation; and a 48% response in both groups at 12 months.
Collectively, these studies suggest that adding a preventive medication at the time of withdrawal has the potential to reduce headache frequency more quickly than withdrawal alone. However, after 3 to 6 months, the outcome of reduced headache frequency is the same whether or not a preventive medication is used—as long as the offending agent has been withdrawn.
Do preventives work without withdrawing overused medication? Patients with MOH often show little or no improvement with addition of a preventive medication only; their response to a preventive improves after withdrawal of the overused medication. Patients without previous headache improvement after addition of a preventive, who also did not improve 2 months after withdrawal, then demonstrated an overall reduction in headache by 26% when a preventive was reintroduced after withdrawal.2
Continue to: The research evidence for preventives
The research evidence for preventives. Medications for headache prevention have not been extensively evaluated specifically for treating MOH. Here is what’s known:
- Flunarizine, amitriptyline, and beta-blockers usually are ineffective for MOH.24
- Results for topiramate are mixed: A small, double-blind, placebo-controlled chronic migraine study in Europe showed that, in a subgroup of patients with MOH, topiramate led to a small but significant reduction (3.5 d/mo) in headache frequency, compared to placebo.27 A similar study done in the United States did not show a significant difference between the active-treatment and placebo groups.34
- Findings regarding onabotulinumtoxinA are intriguing: In a posthoc analysis of onabotulinumtoxinA to treat chronic migraine, patients with MOH who did not undergo detoxification had an 8 d/mo greater reduction in headache, compared to placebo.35 However, when compared to placebo in conjunction with detoxification, onabotulinumtoxinA demonstrated no benefit.36
- Newer CGRP antagonist and CGRP receptor antagonist monoclonal antibodies are successful preventive medications that have demonstrated a reduction in acute medication use days per month and headache days per month37; these compounds have not been compared to withdrawal alone.
Reducing the severity and duration of withdrawal symptoms
Withdrawal from overused abortive headache medications can lead to worsening headache, nausea, vomiting, hypotension, tachycardia, sleep disturbances, restlessness, anxiety, and nervousness. Symptoms usually last 2 to 10 days but can persist for as long as 4 weeks; duration of withdrawal symptoms varies with the medication that is being overused. In patients who have used a triptan, for example, mean duration of withdrawal is 4.1 days; ergotamine, 6.7 days; and NSAIDs, 9.5 days.23 Tapered withdrawal is sometimes recommended with opioids and barbiturates to reduce withdrawal symptoms. It is unclear whether starting a preventive medication during withdrawal assists in reducing withdrawal symptoms.38
Bridging therapy to reduce symptoms of withdrawal is often provided despite debatable utility. Available evidence does not favor one agent or method but suggests some strategies that could be helpful:
- A prednisone taper has a potential role during the first 6 days of withdrawal by reducing rebound headache and withdrawal symptoms39; however, oral prednisolone has been shown to have no benefit.40
- Alone, IV methylprednisolone seems not to be of benefit; however, in a retrospective study of 94 patients, IV methylprednisolone plus diazepam for 5 days led to a significant reduction in headache frequency and drug consumption that was sustained after 3 months.41
- Celecoxib was compared to prednisone over a 20-day course: a celecoxib dosage of 400 mg/d for the first 5 days, tapered by 100 mg every 5 days, and an oral prednisone dosage of 75 mg/d for the first 5 days, then tapered every 5 days. Patients taking celecoxib had lower headache intensity but there was no difference in headache frequency and acute medication intake between the groups.42
Other strategies. Using antiemetics and NSAIDs to reduce withdrawal symptoms is widely practiced, but no placebo-controlled trials have been conducted to support this strategy.
Patients in withdrawal might be more likely to benefit from inpatient care if they have a severe comorbidity, such as opioid or barbiturate use; failure to respond to, tolerate, or adhere to treatment; or relapse after withdrawal.38
Continue to: Cognitive behavioral therapy...
Cognitive behavioral therapy, exercise, a headache diary, and biofeedback should be considered in every patient’s treatment strategy because a multidisciplinary approach increases adherence and leads to improvement in headache frequency and a decrease in disability and medication use.43
Predictors of Tx success
A prospective cohort study determined that the rate of MOH relapse is 31% at 6 months, 41% at 1 year, and 45% at 4 years, with the highest risk of relapse during the first year.44 Looking at the correlation between type of medication overused and relapse rate, the research indicates that
- triptans have the lowest risk of relapse,44
- simple analgesics have a higher risk of relapse than triptans,22,44 and
- opioids have the highest risk of relapse.22
Where the data don’t agree. Data on combination analgesics and on ergots are conflicting.22 In addition, data on whether the primary type of headache predicts relapse rate conflict; however, migraine might predict a better outcome than tension-type headache.22
To recap and expand: Management pearls
The major goals of headache management generally are to rule out secondary headache, reach a correct diagnosis, reduce overall headache frequency, and provide effective abortive medication. A large component of reducing headache frequency is addressing and treating medication overuse.
Seek to understand the nature of the patient’s headache disorder. Components of the history are key in identifying the underlying headache diagnosis and ruling out other, more concerning secondary headache diagnoses. The ICHD-3 is an excellent resource for treating headache disorders because the classification lists specific diagnostic criteria for all recognized headache diagnoses.
Continue to: Medication withdrawal...
Medication withdrawal—with or without preventive medication—should reduce the frequency of MOH in 2 or 3 months. If headache does not become less frequent, however, the headache diagnosis might need to be reconsidered. Minimizing the use of abortive medication is generally recommended, but reduction or withdrawal of these medications does not guarantee that patients will revert to an episodic pattern of headache.
Treating withdrawal symptoms is a reasonable approach in some patients, but evidence does not support routinely providing bridging therapy.
Apply preventives carefully. Abortive medication withdrawal should generally be completed before initiating preventive medication; however, over the short term, starting preventive therapy while withdrawing the overused medication could assist in reducing headache frequency rapidly. This strategy can put patients at risk of medication adverse effects and using the medications longer than necessary, yet might be reasonable in certain patients, given their comorbidities, risk of relapse, and physician and patient preference. A preventive medication for an individual patient should generally be chosen in line with recommendations of the American Academy of Neurology45 and on the basis of the history and comorbidities.
Provide education, which is essential to lowering barriers to success. Patients with MOH must be counseled to understand that (1) a headache treatment that is supposed to be making them feel better is, in fact, making them feel worse and (2) they will get worse before they get better. Many patients are afraid to be without medication to use as needed. It is helpful to educate them on the different types of treatments (abortive, preventive); how MOH interferes with headache prophylaxis and medication efficacy; how MOH alters brain function (ie, aforementioned physiologic changes in pain processing and functional imaging changes23); and that such change is reversible when medication is withdrawn.
ACKNOWLEDGEMENT
The author thanks Jeffrey Curtis, MD, MPH, for his support and editing assistance with the manuscript.
CORRESPONDENCE
Allison Crain, MD, 2927 N 7th Avenue, Phoenix, AZ 85013; [email protected].
1. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
2. Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 2015;86:505-512.
3. Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179-190.
4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301-315.
5. Chu H-T, Liang C-S, Lee J-T, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. 2018;58:407-415.
6. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
7. Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
8. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703-711.
9. Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665-672.
10. . Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954-976.
11. Kernick D, Stapley S, Goadsby PJ, et al. What happens to new-onset headache presenting to primary care? A case–cohort study using electronic primary care records. Cephalalgia. 2008;28:1188-1195.
12. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
13. Munoz-Ceron J, Marin-Careaga V, L, et al. Headache at the emergency room: etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PloS One. 2019;14:e0208728.
14. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010;9:391-401.
15. Tassorelli C, Jensen R, Allena M, et al; the . A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014;34:645-655.
16. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
18. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65-72.
19. Hagen K, Linde M, Steiner TJ, et al. Risk factors for medication-overuse headache: an 11-year follow-up study. The Nord-Trøndelag Health Studies. Pain. 2012;153:56-61.
20. Katsarava Z, Schneewiess S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788-790.
21. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. 2019;93:e2224-e2236.
22. Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139:405-414.
23. Ferraro S, Grazzi L, Mandelli M, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. 2012;13:255-262.
24. Diener H-C, Holle D, Solbach K, et al. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12:575-583.
25. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011-1014.
26. Mauskop A, ed. Migraine and Headache. 2nd ed. Oxford University Press; 2013.
27. Diener H-C, Bussone G, Van Oene JC, et al; . Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
28. Navratilova E, Behravesh S, Oyarzo J, et al. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache Cephalalgia. 2020;40:892-902.
29. Kristoffersen ES, Straand J, Russell MB, et al. Lasting improvement of medication-overuse headache after brief intervention—a long-term follow-up in primary care. Eur J Neurol. 2017;24:883-891.
30. Carlsen LN, Munksgaard SB, Jensen RH, et al. Complete detoxification is the most effective treatment of medication-overuse headache: a randomized controlled open-label trial. Cephalalgia. 2018;38:225-236.
31. Sarchielli P, Messina P, Cupini LM, et al; SAMOHA Study Group. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1289-1297.
32. Hagen K, Stovner LJ. A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol Scand Suppl. 2011;124(suppl 191):38-43.
33. Munksgaard SB, Bendtsen L, Jensen RH. Detoxification of medication-overuse headache by a multidisciplinary treatment programme is highly effective: a comparison of two consecutive treatment methods in an open-label design. Cephalalgia. 2012;32:834-844.
34. Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153-1162.
35. Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48-56.
36. Sandrini G, Perrotta A, Tassorelli C, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427-433.
37. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(suppl 1):99-105.
38. Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention and treatment of medication overuse headache. Lancet Neurol. 2019;18:891-902.
39. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000;20:107-113.
40. Bøe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: a randomized, double-blind study. Neurology. 2007;69:26-31.
41. Paolucci M, Altamura C, Brunelli N, et al. Methylprednisolone plus diazepam i.v. as bridge therapy for medication overuse headache. Neurol Sci. 2017;38:2025-2029.
42. Taghdiri F, Togha M, Razeghi Jahromi S, et al. Celecoxib vs prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind clinical trial. Headache. 2015;55:128-135.
43. Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795-816.
44. Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005;25:12-15.
45. Silberstein SD, Holland S, Freitag F, et al; . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78:1137-1145.
Medication overuse headache (MOH), a secondary headache diagnosis, is a prevalent phenomenon that complicates headache diagnosis and treatment, increases the cost of care, and reduces quality of life. Effective abortive medication is essential for the headache sufferer; when an abortive is used too frequently, however, headache frequency increases—potentially beginning a cycle in which the patient then takes more medication to abort the headache. Over time, the patient suffers from an ever-increasing number of headaches, takes even more abortive medication, and so on. In the presence of MOH, there is a reduction in pain response to preventive and abortive treatments; when medication overuse is eliminated, pain response improves.1
Although MOH is well recognized among headache specialists, the condition is often overlooked in primary care. Since headache is a top complaint in primary care, however, and prevention is a major goal in family medicine, the opportunity for you to recognize, treat, and prevent MOH is significant. In fact, a randomized controlled trial showed that brief patient education about headache care and MOH provided by a primary care physician can lead to a significant reduction in headache frequency among patients with MOH.2
This article reviews the recognition and diagnosis of MOH, based on historical features and current criteria; addresses risk factors for abortive medication overuse and how to withdraw an offending agent; and explores the value of bridging and preventive therapies to reduce the overall frequency of headache.

What defines MOH?
Typically, MOH is a chronification of a primary headache disorder. However, in patients with a history of migraine who are undergoing treatment for another chronic pain condition with an opioid or other analgesic, MOH can be induced.3 An increase in the frequency of headache raises the specter of a concomitant increase in the level of disability4; psychiatric comorbidity5; and more headache days, with time lost from school and work.
The Migraine Disability Assessment (MIDAS) questionnaire, a validated instrument that helps the provider (1) measure the impact that headache has on a patient’s life and (2) follow treatment progress, also provides information to employers and insurance companies on treatment coverage and the need for work modification. The MIDAS score is 3 times higher in patients with MOH than in patients with episodic migraine.6,7
The annual associated cost per person of MOH has been estimated at $4000, resulting in billions of dollars in associated costs8; most of these costs are related to absenteeism and disability. After detoxification for MOH, annual outpatient medication costs are reduced by approximately 24%.9
Efforts to solve a common problem create another
Headache affects nearly 50% of the general population worldwide,10 accounting for about 4% of primary care visits11 and approximately 20% of outpatient neurology consultations.12 Although inpatient stays for headache are approximately half the duration of the overall average hospital stay, headache accounts for 3% of admissions.13 According to the Global Burden of Disease study, tension-type headache, migraine, and MOH are the 3 most common headache disorders.10 Headache is the second leading cause of disability among people 15 to 49 years of age.10
Continue to: The prevalence of MOH...
The prevalence of MOH in the general population is 2%.7,14,15 A population-based study showed that the rate of progression from episodic headache (< 15 d/mo) to chronic headache (≥ 15 d/mo) in the general population is 2.5% per year16; however, progression to chronic headache is 14% per year in patients with medication overuse. One-third of the general population with chronic migraine overuses symptomatic medication; in US headache clinics, roughly one-half of patients with chronic headache overuse acute medication.6
Definitions and diagnosis
MOH is a secondary headache diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3) (TABLE 1),17 which lists diagnostic criteria for recognized headache disorders.

Terminology. MOH has also been called rebound headache, drug-induced headache, and transformed migraine, but these terms are outdated and are not formal diagnoses. Patients sometimes refer to substance-withdrawal headaches (not discussed in this article) as rebound headaches, so clarity is important when discussing headache with patients: namely, that MOH is an exacerbation of an existing headache condition caused by overuse of abortive headache medications, including analgesics, combination analgesics, triptans, barbiturates, and opioids.
MOH was recognized in the early 1950s and fully differentiated as a diagnosis in 2005 in the second edition of the ICHD. The disorder is subcategorized by offending abortive agent (TABLE 217) because the frequency of analgesic use required to develop MOH differs by agent.

Risk factors for MOH and chronification of a primary headache disorder. There are several risk factors for developing MOH, and others that contribute to increasing headache frequency in general (TABLE 35,14,18-23). Some risk factors are common to each. All are important to address because some are modifiable.

Continue to: Pathophysiology
Pathophysiology. The pathophysiology and psychology behind MOH are largely unknown. Physiologic changes in pain processing and functional imaging changes have been demonstrated in patients with MOH, both of which are reversible upon withdrawal of medication.23 Genetic factors and changes in hormone and neurotransmitter levels are found in MOH patients; this is not the case in patients who have an episodic headache pattern only.24
Presentation. Diagnostic criteria for MOH do not include clinical characteristics. Typically, the phenotype of MOH in a given patient is similar to the underlying primary headache25—although this principle can be complicated to tease out because these medications can suppress some symptoms. Diagnosis of a primary headache disorder should be documented along with the diagnosis of MOH.
Medication overuse can exist without MOH: Not every patient who frequently uses an abortive medication develops MOH.
Treatment is multifaceted—and can become complex
Mainstays of treatment of MOH are education about the disorder and detoxification from the overused agent, although specific treatments can differ depending on the agent involved, the frequency and duration of its use, and a patient’s behavioral patterns and psychiatric comorbidities. Often, a daily medication to prevent headache is considered upon, or after, withdrawal of the offending agent. The timing of introducing a preventive might impact its effectiveness. Some refractory cases require more intensive therapy, including hospitalization at a specialized tertiary center.
But before we look at detoxification from an overused agent, it’s important to review one of the best strategies of all in combatting MOH.
Continue to: First and best strategy
First and best strategy: Avoid onset of MOH
Select an appropriate abortive to reduce the risk of MOH. With regard to specific acute headache medications, some nuances other than type of headache should be considered. Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as abortive therapy by the American Headache Society for their efficacy, favorable adverse effect profile, and low cost. NSAIDs are protective against development of MOH if a patient’s baseline headache frequency is < 10/mo; at a frequency of 10 to 14 d/mo, however, the risk of MOH increases when using an NSAID.6 A similar effect has been seen with triptans.16 Longer-acting NSAIDs, such as nabumetone and naproxen, have been proposed as less likely to cause MOH, and are even used as bridging therapy sometimes (as long as neither of these was the overused medication).26
The time it takes to develop MOH is shortest with triptans, followed by ergots, then analgesics.27
Prospective cohort studies6,16 have shown that barbiturates and opioids are more likely to induce MOH; for that reason, agents in these analgesic classes are almost universally avoided unless no other medically acceptable options exist. Using barbiturate-containing compounds or opioids > 4 d/mo exponentially increases the likelihood of MOH.
Promising preclinical data demonstrate that the gepant, or small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist, class of medications used as abortive therapy does not induce medication overuse cutaneous allodynia.28
Provide education. Primary prevention of MOH involves (1) increasing patients’ awareness of how to take medications appropriately and (2) restricting intake of over-the-counter abortive medications. Often, the expert recommendation is to limit abortives to approximately 2 d/wk because more frequent use places patients at risk of further increased use and subsequent MOH.
Continue to: A randomized controlled trial in Norway...
A randomized controlled trial in Norway compared outcomes in 2 groups of patients with MOH: One group was given advice on the disorder by a physician; the other group was not provided with advice. In the “business-as-usual” group, there was no significant improvement; however, when general practitioners provided simple advice (lasting roughly 9 minutes) about reducing abortive medication use to a safe level and cautioned patients that they would be “feeling worse before feeling better,” headache days were reduced by approximately 8 per month and medication days, by 16 per month.2
A subsequent, long-term follow-up study29 of patients from the Norway trial2 who had been given advice and education showed a relapse rate (ie, into overuse of headache medication) of only 8% and sustained reduction of headache days and medication use at 16 months.
Offer support and other nondrug interventions. A recent review of 3 studies23 recommended that extra support for patients from a headache nurse, close follow-up, keeping an electronic diary that provides feedback, and undertaking a short course of psychotherapy can reduce medication overuse and prevent relapse.
If MOH develops, initiate withdrawal, introduce a preventive
Withdraw overused medication. Most current evidence suggests that withdrawal of the offending agent is the most effective factor in reducing headache days and improving quality of life. A randomized controlled trial compared the effects of (1) complete and immediate withdrawal of an abortive medication with (2) reducing its use (ie, limiting intake to 2 d/wk), on headache frequency, disability, and quality of life.30 There was a reduction of headache days in both groups; however, reduction was much greater at 2 months in the complete withdrawal group than in the restricted intake group (respectively, a 41% and a 26% reduction in headache days per month). This effect was sustained at 6 and 12 months in both groups. The study confirmed the results of earlier research2,15: Abrupt withdrawal leads to reversion to an episodic pattern at 2 to 6 months in approximately 40% to 60% of patients.
More studies are needed to determine the most appropriate treatment course for MOH; however, complete withdrawal of the causative drug is the most important intervention.
Continue to: Consider withdrawal plus preventive treatment
Consider withdrawal plus preventive treatment. Use of sodium valproate, in addition to medication overuse detoxification, led to a significant reduction in headache days and improvement in quality of life at 12 weeks but no difference after 24 weeks, compared with detoxification alone in a randomized, double-blind, placebo-controlled study.31
A study of 61 patients showed a larger reduction (by 7.2 d/mo) in headache frequency with any preventive medication in addition to medication withdrawal, compared to withdrawal alone (by 4.1 d/mo) after 3 months; however, the relative benefit was gone at 6 months.32
A study of 98 patients compared immediate and delayed initiation of preventive medication upon withdrawal of overused abortive medication.33 Response was defined as a > 50% reduction in headache frequency and was similar in both groups; results showed a 28% response with immediate initiation of a preventive; a 23% response with delayed (ie, 2 months after withdrawal) initiation; and a 48% response in both groups at 12 months.
Collectively, these studies suggest that adding a preventive medication at the time of withdrawal has the potential to reduce headache frequency more quickly than withdrawal alone. However, after 3 to 6 months, the outcome of reduced headache frequency is the same whether or not a preventive medication is used—as long as the offending agent has been withdrawn.
Do preventives work without withdrawing overused medication? Patients with MOH often show little or no improvement with addition of a preventive medication only; their response to a preventive improves after withdrawal of the overused medication. Patients without previous headache improvement after addition of a preventive, who also did not improve 2 months after withdrawal, then demonstrated an overall reduction in headache by 26% when a preventive was reintroduced after withdrawal.2
Continue to: The research evidence for preventives
The research evidence for preventives. Medications for headache prevention have not been extensively evaluated specifically for treating MOH. Here is what’s known:
- Flunarizine, amitriptyline, and beta-blockers usually are ineffective for MOH.24
- Results for topiramate are mixed: A small, double-blind, placebo-controlled chronic migraine study in Europe showed that, in a subgroup of patients with MOH, topiramate led to a small but significant reduction (3.5 d/mo) in headache frequency, compared to placebo.27 A similar study done in the United States did not show a significant difference between the active-treatment and placebo groups.34
- Findings regarding onabotulinumtoxinA are intriguing: In a posthoc analysis of onabotulinumtoxinA to treat chronic migraine, patients with MOH who did not undergo detoxification had an 8 d/mo greater reduction in headache, compared to placebo.35 However, when compared to placebo in conjunction with detoxification, onabotulinumtoxinA demonstrated no benefit.36
- Newer CGRP antagonist and CGRP receptor antagonist monoclonal antibodies are successful preventive medications that have demonstrated a reduction in acute medication use days per month and headache days per month37; these compounds have not been compared to withdrawal alone.
Reducing the severity and duration of withdrawal symptoms
Withdrawal from overused abortive headache medications can lead to worsening headache, nausea, vomiting, hypotension, tachycardia, sleep disturbances, restlessness, anxiety, and nervousness. Symptoms usually last 2 to 10 days but can persist for as long as 4 weeks; duration of withdrawal symptoms varies with the medication that is being overused. In patients who have used a triptan, for example, mean duration of withdrawal is 4.1 days; ergotamine, 6.7 days; and NSAIDs, 9.5 days.23 Tapered withdrawal is sometimes recommended with opioids and barbiturates to reduce withdrawal symptoms. It is unclear whether starting a preventive medication during withdrawal assists in reducing withdrawal symptoms.38
Bridging therapy to reduce symptoms of withdrawal is often provided despite debatable utility. Available evidence does not favor one agent or method but suggests some strategies that could be helpful:
- A prednisone taper has a potential role during the first 6 days of withdrawal by reducing rebound headache and withdrawal symptoms39; however, oral prednisolone has been shown to have no benefit.40
- Alone, IV methylprednisolone seems not to be of benefit; however, in a retrospective study of 94 patients, IV methylprednisolone plus diazepam for 5 days led to a significant reduction in headache frequency and drug consumption that was sustained after 3 months.41
- Celecoxib was compared to prednisone over a 20-day course: a celecoxib dosage of 400 mg/d for the first 5 days, tapered by 100 mg every 5 days, and an oral prednisone dosage of 75 mg/d for the first 5 days, then tapered every 5 days. Patients taking celecoxib had lower headache intensity but there was no difference in headache frequency and acute medication intake between the groups.42
Other strategies. Using antiemetics and NSAIDs to reduce withdrawal symptoms is widely practiced, but no placebo-controlled trials have been conducted to support this strategy.
Patients in withdrawal might be more likely to benefit from inpatient care if they have a severe comorbidity, such as opioid or barbiturate use; failure to respond to, tolerate, or adhere to treatment; or relapse after withdrawal.38
Continue to: Cognitive behavioral therapy...
Cognitive behavioral therapy, exercise, a headache diary, and biofeedback should be considered in every patient’s treatment strategy because a multidisciplinary approach increases adherence and leads to improvement in headache frequency and a decrease in disability and medication use.43
Predictors of Tx success
A prospective cohort study determined that the rate of MOH relapse is 31% at 6 months, 41% at 1 year, and 45% at 4 years, with the highest risk of relapse during the first year.44 Looking at the correlation between type of medication overused and relapse rate, the research indicates that
- triptans have the lowest risk of relapse,44
- simple analgesics have a higher risk of relapse than triptans,22,44 and
- opioids have the highest risk of relapse.22
Where the data don’t agree. Data on combination analgesics and on ergots are conflicting.22 In addition, data on whether the primary type of headache predicts relapse rate conflict; however, migraine might predict a better outcome than tension-type headache.22
To recap and expand: Management pearls
The major goals of headache management generally are to rule out secondary headache, reach a correct diagnosis, reduce overall headache frequency, and provide effective abortive medication. A large component of reducing headache frequency is addressing and treating medication overuse.
Seek to understand the nature of the patient’s headache disorder. Components of the history are key in identifying the underlying headache diagnosis and ruling out other, more concerning secondary headache diagnoses. The ICHD-3 is an excellent resource for treating headache disorders because the classification lists specific diagnostic criteria for all recognized headache diagnoses.
Continue to: Medication withdrawal...
Medication withdrawal—with or without preventive medication—should reduce the frequency of MOH in 2 or 3 months. If headache does not become less frequent, however, the headache diagnosis might need to be reconsidered. Minimizing the use of abortive medication is generally recommended, but reduction or withdrawal of these medications does not guarantee that patients will revert to an episodic pattern of headache.
Treating withdrawal symptoms is a reasonable approach in some patients, but evidence does not support routinely providing bridging therapy.
Apply preventives carefully. Abortive medication withdrawal should generally be completed before initiating preventive medication; however, over the short term, starting preventive therapy while withdrawing the overused medication could assist in reducing headache frequency rapidly. This strategy can put patients at risk of medication adverse effects and using the medications longer than necessary, yet might be reasonable in certain patients, given their comorbidities, risk of relapse, and physician and patient preference. A preventive medication for an individual patient should generally be chosen in line with recommendations of the American Academy of Neurology45 and on the basis of the history and comorbidities.
Provide education, which is essential to lowering barriers to success. Patients with MOH must be counseled to understand that (1) a headache treatment that is supposed to be making them feel better is, in fact, making them feel worse and (2) they will get worse before they get better. Many patients are afraid to be without medication to use as needed. It is helpful to educate them on the different types of treatments (abortive, preventive); how MOH interferes with headache prophylaxis and medication efficacy; how MOH alters brain function (ie, aforementioned physiologic changes in pain processing and functional imaging changes23); and that such change is reversible when medication is withdrawn.
ACKNOWLEDGEMENT
The author thanks Jeffrey Curtis, MD, MPH, for his support and editing assistance with the manuscript.
CORRESPONDENCE
Allison Crain, MD, 2927 N 7th Avenue, Phoenix, AZ 85013; [email protected].
Medication overuse headache (MOH), a secondary headache diagnosis, is a prevalent phenomenon that complicates headache diagnosis and treatment, increases the cost of care, and reduces quality of life. Effective abortive medication is essential for the headache sufferer; when an abortive is used too frequently, however, headache frequency increases—potentially beginning a cycle in which the patient then takes more medication to abort the headache. Over time, the patient suffers from an ever-increasing number of headaches, takes even more abortive medication, and so on. In the presence of MOH, there is a reduction in pain response to preventive and abortive treatments; when medication overuse is eliminated, pain response improves.1
Although MOH is well recognized among headache specialists, the condition is often overlooked in primary care. Since headache is a top complaint in primary care, however, and prevention is a major goal in family medicine, the opportunity for you to recognize, treat, and prevent MOH is significant. In fact, a randomized controlled trial showed that brief patient education about headache care and MOH provided by a primary care physician can lead to a significant reduction in headache frequency among patients with MOH.2
This article reviews the recognition and diagnosis of MOH, based on historical features and current criteria; addresses risk factors for abortive medication overuse and how to withdraw an offending agent; and explores the value of bridging and preventive therapies to reduce the overall frequency of headache.

What defines MOH?
Typically, MOH is a chronification of a primary headache disorder. However, in patients with a history of migraine who are undergoing treatment for another chronic pain condition with an opioid or other analgesic, MOH can be induced.3 An increase in the frequency of headache raises the specter of a concomitant increase in the level of disability4; psychiatric comorbidity5; and more headache days, with time lost from school and work.
The Migraine Disability Assessment (MIDAS) questionnaire, a validated instrument that helps the provider (1) measure the impact that headache has on a patient’s life and (2) follow treatment progress, also provides information to employers and insurance companies on treatment coverage and the need for work modification. The MIDAS score is 3 times higher in patients with MOH than in patients with episodic migraine.6,7
The annual associated cost per person of MOH has been estimated at $4000, resulting in billions of dollars in associated costs8; most of these costs are related to absenteeism and disability. After detoxification for MOH, annual outpatient medication costs are reduced by approximately 24%.9
Efforts to solve a common problem create another
Headache affects nearly 50% of the general population worldwide,10 accounting for about 4% of primary care visits11 and approximately 20% of outpatient neurology consultations.12 Although inpatient stays for headache are approximately half the duration of the overall average hospital stay, headache accounts for 3% of admissions.13 According to the Global Burden of Disease study, tension-type headache, migraine, and MOH are the 3 most common headache disorders.10 Headache is the second leading cause of disability among people 15 to 49 years of age.10
Continue to: The prevalence of MOH...
The prevalence of MOH in the general population is 2%.7,14,15 A population-based study showed that the rate of progression from episodic headache (< 15 d/mo) to chronic headache (≥ 15 d/mo) in the general population is 2.5% per year16; however, progression to chronic headache is 14% per year in patients with medication overuse. One-third of the general population with chronic migraine overuses symptomatic medication; in US headache clinics, roughly one-half of patients with chronic headache overuse acute medication.6
Definitions and diagnosis
MOH is a secondary headache diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3) (TABLE 1),17 which lists diagnostic criteria for recognized headache disorders.

Terminology. MOH has also been called rebound headache, drug-induced headache, and transformed migraine, but these terms are outdated and are not formal diagnoses. Patients sometimes refer to substance-withdrawal headaches (not discussed in this article) as rebound headaches, so clarity is important when discussing headache with patients: namely, that MOH is an exacerbation of an existing headache condition caused by overuse of abortive headache medications, including analgesics, combination analgesics, triptans, barbiturates, and opioids.
MOH was recognized in the early 1950s and fully differentiated as a diagnosis in 2005 in the second edition of the ICHD. The disorder is subcategorized by offending abortive agent (TABLE 217) because the frequency of analgesic use required to develop MOH differs by agent.

Risk factors for MOH and chronification of a primary headache disorder. There are several risk factors for developing MOH, and others that contribute to increasing headache frequency in general (TABLE 35,14,18-23). Some risk factors are common to each. All are important to address because some are modifiable.

Continue to: Pathophysiology
Pathophysiology. The pathophysiology and psychology behind MOH are largely unknown. Physiologic changes in pain processing and functional imaging changes have been demonstrated in patients with MOH, both of which are reversible upon withdrawal of medication.23 Genetic factors and changes in hormone and neurotransmitter levels are found in MOH patients; this is not the case in patients who have an episodic headache pattern only.24
Presentation. Diagnostic criteria for MOH do not include clinical characteristics. Typically, the phenotype of MOH in a given patient is similar to the underlying primary headache25—although this principle can be complicated to tease out because these medications can suppress some symptoms. Diagnosis of a primary headache disorder should be documented along with the diagnosis of MOH.
Medication overuse can exist without MOH: Not every patient who frequently uses an abortive medication develops MOH.
Treatment is multifaceted—and can become complex
Mainstays of treatment of MOH are education about the disorder and detoxification from the overused agent, although specific treatments can differ depending on the agent involved, the frequency and duration of its use, and a patient’s behavioral patterns and psychiatric comorbidities. Often, a daily medication to prevent headache is considered upon, or after, withdrawal of the offending agent. The timing of introducing a preventive might impact its effectiveness. Some refractory cases require more intensive therapy, including hospitalization at a specialized tertiary center.
But before we look at detoxification from an overused agent, it’s important to review one of the best strategies of all in combatting MOH.
Continue to: First and best strategy
First and best strategy: Avoid onset of MOH
Select an appropriate abortive to reduce the risk of MOH. With regard to specific acute headache medications, some nuances other than type of headache should be considered. Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as abortive therapy by the American Headache Society for their efficacy, favorable adverse effect profile, and low cost. NSAIDs are protective against development of MOH if a patient’s baseline headache frequency is < 10/mo; at a frequency of 10 to 14 d/mo, however, the risk of MOH increases when using an NSAID.6 A similar effect has been seen with triptans.16 Longer-acting NSAIDs, such as nabumetone and naproxen, have been proposed as less likely to cause MOH, and are even used as bridging therapy sometimes (as long as neither of these was the overused medication).26
The time it takes to develop MOH is shortest with triptans, followed by ergots, then analgesics.27
Prospective cohort studies6,16 have shown that barbiturates and opioids are more likely to induce MOH; for that reason, agents in these analgesic classes are almost universally avoided unless no other medically acceptable options exist. Using barbiturate-containing compounds or opioids > 4 d/mo exponentially increases the likelihood of MOH.
Promising preclinical data demonstrate that the gepant, or small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist, class of medications used as abortive therapy does not induce medication overuse cutaneous allodynia.28
Provide education. Primary prevention of MOH involves (1) increasing patients’ awareness of how to take medications appropriately and (2) restricting intake of over-the-counter abortive medications. Often, the expert recommendation is to limit abortives to approximately 2 d/wk because more frequent use places patients at risk of further increased use and subsequent MOH.
Continue to: A randomized controlled trial in Norway...
A randomized controlled trial in Norway compared outcomes in 2 groups of patients with MOH: One group was given advice on the disorder by a physician; the other group was not provided with advice. In the “business-as-usual” group, there was no significant improvement; however, when general practitioners provided simple advice (lasting roughly 9 minutes) about reducing abortive medication use to a safe level and cautioned patients that they would be “feeling worse before feeling better,” headache days were reduced by approximately 8 per month and medication days, by 16 per month.2
A subsequent, long-term follow-up study29 of patients from the Norway trial2 who had been given advice and education showed a relapse rate (ie, into overuse of headache medication) of only 8% and sustained reduction of headache days and medication use at 16 months.
Offer support and other nondrug interventions. A recent review of 3 studies23 recommended that extra support for patients from a headache nurse, close follow-up, keeping an electronic diary that provides feedback, and undertaking a short course of psychotherapy can reduce medication overuse and prevent relapse.
If MOH develops, initiate withdrawal, introduce a preventive
Withdraw overused medication. Most current evidence suggests that withdrawal of the offending agent is the most effective factor in reducing headache days and improving quality of life. A randomized controlled trial compared the effects of (1) complete and immediate withdrawal of an abortive medication with (2) reducing its use (ie, limiting intake to 2 d/wk), on headache frequency, disability, and quality of life.30 There was a reduction of headache days in both groups; however, reduction was much greater at 2 months in the complete withdrawal group than in the restricted intake group (respectively, a 41% and a 26% reduction in headache days per month). This effect was sustained at 6 and 12 months in both groups. The study confirmed the results of earlier research2,15: Abrupt withdrawal leads to reversion to an episodic pattern at 2 to 6 months in approximately 40% to 60% of patients.
More studies are needed to determine the most appropriate treatment course for MOH; however, complete withdrawal of the causative drug is the most important intervention.
Continue to: Consider withdrawal plus preventive treatment
Consider withdrawal plus preventive treatment. Use of sodium valproate, in addition to medication overuse detoxification, led to a significant reduction in headache days and improvement in quality of life at 12 weeks but no difference after 24 weeks, compared with detoxification alone in a randomized, double-blind, placebo-controlled study.31
A study of 61 patients showed a larger reduction (by 7.2 d/mo) in headache frequency with any preventive medication in addition to medication withdrawal, compared to withdrawal alone (by 4.1 d/mo) after 3 months; however, the relative benefit was gone at 6 months.32
A study of 98 patients compared immediate and delayed initiation of preventive medication upon withdrawal of overused abortive medication.33 Response was defined as a > 50% reduction in headache frequency and was similar in both groups; results showed a 28% response with immediate initiation of a preventive; a 23% response with delayed (ie, 2 months after withdrawal) initiation; and a 48% response in both groups at 12 months.
Collectively, these studies suggest that adding a preventive medication at the time of withdrawal has the potential to reduce headache frequency more quickly than withdrawal alone. However, after 3 to 6 months, the outcome of reduced headache frequency is the same whether or not a preventive medication is used—as long as the offending agent has been withdrawn.
Do preventives work without withdrawing overused medication? Patients with MOH often show little or no improvement with addition of a preventive medication only; their response to a preventive improves after withdrawal of the overused medication. Patients without previous headache improvement after addition of a preventive, who also did not improve 2 months after withdrawal, then demonstrated an overall reduction in headache by 26% when a preventive was reintroduced after withdrawal.2
Continue to: The research evidence for preventives
The research evidence for preventives. Medications for headache prevention have not been extensively evaluated specifically for treating MOH. Here is what’s known:
- Flunarizine, amitriptyline, and beta-blockers usually are ineffective for MOH.24
- Results for topiramate are mixed: A small, double-blind, placebo-controlled chronic migraine study in Europe showed that, in a subgroup of patients with MOH, topiramate led to a small but significant reduction (3.5 d/mo) in headache frequency, compared to placebo.27 A similar study done in the United States did not show a significant difference between the active-treatment and placebo groups.34
- Findings regarding onabotulinumtoxinA are intriguing: In a posthoc analysis of onabotulinumtoxinA to treat chronic migraine, patients with MOH who did not undergo detoxification had an 8 d/mo greater reduction in headache, compared to placebo.35 However, when compared to placebo in conjunction with detoxification, onabotulinumtoxinA demonstrated no benefit.36
- Newer CGRP antagonist and CGRP receptor antagonist monoclonal antibodies are successful preventive medications that have demonstrated a reduction in acute medication use days per month and headache days per month37; these compounds have not been compared to withdrawal alone.
Reducing the severity and duration of withdrawal symptoms
Withdrawal from overused abortive headache medications can lead to worsening headache, nausea, vomiting, hypotension, tachycardia, sleep disturbances, restlessness, anxiety, and nervousness. Symptoms usually last 2 to 10 days but can persist for as long as 4 weeks; duration of withdrawal symptoms varies with the medication that is being overused. In patients who have used a triptan, for example, mean duration of withdrawal is 4.1 days; ergotamine, 6.7 days; and NSAIDs, 9.5 days.23 Tapered withdrawal is sometimes recommended with opioids and barbiturates to reduce withdrawal symptoms. It is unclear whether starting a preventive medication during withdrawal assists in reducing withdrawal symptoms.38
Bridging therapy to reduce symptoms of withdrawal is often provided despite debatable utility. Available evidence does not favor one agent or method but suggests some strategies that could be helpful:
- A prednisone taper has a potential role during the first 6 days of withdrawal by reducing rebound headache and withdrawal symptoms39; however, oral prednisolone has been shown to have no benefit.40
- Alone, IV methylprednisolone seems not to be of benefit; however, in a retrospective study of 94 patients, IV methylprednisolone plus diazepam for 5 days led to a significant reduction in headache frequency and drug consumption that was sustained after 3 months.41
- Celecoxib was compared to prednisone over a 20-day course: a celecoxib dosage of 400 mg/d for the first 5 days, tapered by 100 mg every 5 days, and an oral prednisone dosage of 75 mg/d for the first 5 days, then tapered every 5 days. Patients taking celecoxib had lower headache intensity but there was no difference in headache frequency and acute medication intake between the groups.42
Other strategies. Using antiemetics and NSAIDs to reduce withdrawal symptoms is widely practiced, but no placebo-controlled trials have been conducted to support this strategy.
Patients in withdrawal might be more likely to benefit from inpatient care if they have a severe comorbidity, such as opioid or barbiturate use; failure to respond to, tolerate, or adhere to treatment; or relapse after withdrawal.38
Continue to: Cognitive behavioral therapy...
Cognitive behavioral therapy, exercise, a headache diary, and biofeedback should be considered in every patient’s treatment strategy because a multidisciplinary approach increases adherence and leads to improvement in headache frequency and a decrease in disability and medication use.43
Predictors of Tx success
A prospective cohort study determined that the rate of MOH relapse is 31% at 6 months, 41% at 1 year, and 45% at 4 years, with the highest risk of relapse during the first year.44 Looking at the correlation between type of medication overused and relapse rate, the research indicates that
- triptans have the lowest risk of relapse,44
- simple analgesics have a higher risk of relapse than triptans,22,44 and
- opioids have the highest risk of relapse.22
Where the data don’t agree. Data on combination analgesics and on ergots are conflicting.22 In addition, data on whether the primary type of headache predicts relapse rate conflict; however, migraine might predict a better outcome than tension-type headache.22
To recap and expand: Management pearls
The major goals of headache management generally are to rule out secondary headache, reach a correct diagnosis, reduce overall headache frequency, and provide effective abortive medication. A large component of reducing headache frequency is addressing and treating medication overuse.
Seek to understand the nature of the patient’s headache disorder. Components of the history are key in identifying the underlying headache diagnosis and ruling out other, more concerning secondary headache diagnoses. The ICHD-3 is an excellent resource for treating headache disorders because the classification lists specific diagnostic criteria for all recognized headache diagnoses.
Continue to: Medication withdrawal...
Medication withdrawal—with or without preventive medication—should reduce the frequency of MOH in 2 or 3 months. If headache does not become less frequent, however, the headache diagnosis might need to be reconsidered. Minimizing the use of abortive medication is generally recommended, but reduction or withdrawal of these medications does not guarantee that patients will revert to an episodic pattern of headache.
Treating withdrawal symptoms is a reasonable approach in some patients, but evidence does not support routinely providing bridging therapy.
Apply preventives carefully. Abortive medication withdrawal should generally be completed before initiating preventive medication; however, over the short term, starting preventive therapy while withdrawing the overused medication could assist in reducing headache frequency rapidly. This strategy can put patients at risk of medication adverse effects and using the medications longer than necessary, yet might be reasonable in certain patients, given their comorbidities, risk of relapse, and physician and patient preference. A preventive medication for an individual patient should generally be chosen in line with recommendations of the American Academy of Neurology45 and on the basis of the history and comorbidities.
Provide education, which is essential to lowering barriers to success. Patients with MOH must be counseled to understand that (1) a headache treatment that is supposed to be making them feel better is, in fact, making them feel worse and (2) they will get worse before they get better. Many patients are afraid to be without medication to use as needed. It is helpful to educate them on the different types of treatments (abortive, preventive); how MOH interferes with headache prophylaxis and medication efficacy; how MOH alters brain function (ie, aforementioned physiologic changes in pain processing and functional imaging changes23); and that such change is reversible when medication is withdrawn.
ACKNOWLEDGEMENT
The author thanks Jeffrey Curtis, MD, MPH, for his support and editing assistance with the manuscript.
CORRESPONDENCE
Allison Crain, MD, 2927 N 7th Avenue, Phoenix, AZ 85013; [email protected].
1. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
2. Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 2015;86:505-512.
3. Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179-190.
4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301-315.
5. Chu H-T, Liang C-S, Lee J-T, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. 2018;58:407-415.
6. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
7. Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
8. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703-711.
9. Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665-672.
10. . Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954-976.
11. Kernick D, Stapley S, Goadsby PJ, et al. What happens to new-onset headache presenting to primary care? A case–cohort study using electronic primary care records. Cephalalgia. 2008;28:1188-1195.
12. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
13. Munoz-Ceron J, Marin-Careaga V, L, et al. Headache at the emergency room: etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PloS One. 2019;14:e0208728.
14. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010;9:391-401.
15. Tassorelli C, Jensen R, Allena M, et al; the . A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014;34:645-655.
16. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
18. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65-72.
19. Hagen K, Linde M, Steiner TJ, et al. Risk factors for medication-overuse headache: an 11-year follow-up study. The Nord-Trøndelag Health Studies. Pain. 2012;153:56-61.
20. Katsarava Z, Schneewiess S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788-790.
21. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. 2019;93:e2224-e2236.
22. Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139:405-414.
23. Ferraro S, Grazzi L, Mandelli M, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. 2012;13:255-262.
24. Diener H-C, Holle D, Solbach K, et al. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12:575-583.
25. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011-1014.
26. Mauskop A, ed. Migraine and Headache. 2nd ed. Oxford University Press; 2013.
27. Diener H-C, Bussone G, Van Oene JC, et al; . Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
28. Navratilova E, Behravesh S, Oyarzo J, et al. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache Cephalalgia. 2020;40:892-902.
29. Kristoffersen ES, Straand J, Russell MB, et al. Lasting improvement of medication-overuse headache after brief intervention—a long-term follow-up in primary care. Eur J Neurol. 2017;24:883-891.
30. Carlsen LN, Munksgaard SB, Jensen RH, et al. Complete detoxification is the most effective treatment of medication-overuse headache: a randomized controlled open-label trial. Cephalalgia. 2018;38:225-236.
31. Sarchielli P, Messina P, Cupini LM, et al; SAMOHA Study Group. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1289-1297.
32. Hagen K, Stovner LJ. A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol Scand Suppl. 2011;124(suppl 191):38-43.
33. Munksgaard SB, Bendtsen L, Jensen RH. Detoxification of medication-overuse headache by a multidisciplinary treatment programme is highly effective: a comparison of two consecutive treatment methods in an open-label design. Cephalalgia. 2012;32:834-844.
34. Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153-1162.
35. Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48-56.
36. Sandrini G, Perrotta A, Tassorelli C, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427-433.
37. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(suppl 1):99-105.
38. Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention and treatment of medication overuse headache. Lancet Neurol. 2019;18:891-902.
39. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000;20:107-113.
40. Bøe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: a randomized, double-blind study. Neurology. 2007;69:26-31.
41. Paolucci M, Altamura C, Brunelli N, et al. Methylprednisolone plus diazepam i.v. as bridge therapy for medication overuse headache. Neurol Sci. 2017;38:2025-2029.
42. Taghdiri F, Togha M, Razeghi Jahromi S, et al. Celecoxib vs prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind clinical trial. Headache. 2015;55:128-135.
43. Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795-816.
44. Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005;25:12-15.
45. Silberstein SD, Holland S, Freitag F, et al; . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78:1137-1145.
1. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
2. Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 2015;86:505-512.
3. Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179-190.
4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301-315.
5. Chu H-T, Liang C-S, Lee J-T, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. 2018;58:407-415.
6. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
7. Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
8. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703-711.
9. Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665-672.
10. . Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954-976.
11. Kernick D, Stapley S, Goadsby PJ, et al. What happens to new-onset headache presenting to primary care? A case–cohort study using electronic primary care records. Cephalalgia. 2008;28:1188-1195.
12. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
13. Munoz-Ceron J, Marin-Careaga V, L, et al. Headache at the emergency room: etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PloS One. 2019;14:e0208728.
14. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010;9:391-401.
15. Tassorelli C, Jensen R, Allena M, et al; the . A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014;34:645-655.
16. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
18. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65-72.
19. Hagen K, Linde M, Steiner TJ, et al. Risk factors for medication-overuse headache: an 11-year follow-up study. The Nord-Trøndelag Health Studies. Pain. 2012;153:56-61.
20. Katsarava Z, Schneewiess S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788-790.
21. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. 2019;93:e2224-e2236.
22. Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139:405-414.
23. Ferraro S, Grazzi L, Mandelli M, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. 2012;13:255-262.
24. Diener H-C, Holle D, Solbach K, et al. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12:575-583.
25. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011-1014.
26. Mauskop A, ed. Migraine and Headache. 2nd ed. Oxford University Press; 2013.
27. Diener H-C, Bussone G, Van Oene JC, et al; . Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
28. Navratilova E, Behravesh S, Oyarzo J, et al. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache Cephalalgia. 2020;40:892-902.
29. Kristoffersen ES, Straand J, Russell MB, et al. Lasting improvement of medication-overuse headache after brief intervention—a long-term follow-up in primary care. Eur J Neurol. 2017;24:883-891.
30. Carlsen LN, Munksgaard SB, Jensen RH, et al. Complete detoxification is the most effective treatment of medication-overuse headache: a randomized controlled open-label trial. Cephalalgia. 2018;38:225-236.
31. Sarchielli P, Messina P, Cupini LM, et al; SAMOHA Study Group. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1289-1297.
32. Hagen K, Stovner LJ. A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol Scand Suppl. 2011;124(suppl 191):38-43.
33. Munksgaard SB, Bendtsen L, Jensen RH. Detoxification of medication-overuse headache by a multidisciplinary treatment programme is highly effective: a comparison of two consecutive treatment methods in an open-label design. Cephalalgia. 2012;32:834-844.
34. Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153-1162.
35. Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48-56.
36. Sandrini G, Perrotta A, Tassorelli C, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427-433.
37. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(suppl 1):99-105.
38. Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention and treatment of medication overuse headache. Lancet Neurol. 2019;18:891-902.
39. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000;20:107-113.
40. Bøe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: a randomized, double-blind study. Neurology. 2007;69:26-31.
41. Paolucci M, Altamura C, Brunelli N, et al. Methylprednisolone plus diazepam i.v. as bridge therapy for medication overuse headache. Neurol Sci. 2017;38:2025-2029.
42. Taghdiri F, Togha M, Razeghi Jahromi S, et al. Celecoxib vs prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind clinical trial. Headache. 2015;55:128-135.
43. Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795-816.
44. Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005;25:12-15.
45. Silberstein SD, Holland S, Freitag F, et al; . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78:1137-1145.
PRACTICE RECOMMENDATIONS
› Avoid prescribing barbiturates or opioids for a headache disorder. A
› Limit use of a headache-abortive medication to twice a week when starting a patient on the drug. C
› Consider providing bridging therapy during detoxification of the overused medication. C
› Do not provide a preventive medication without withdrawing the overused agent. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to refine your approach to peripheral arterial disease
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1
Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.
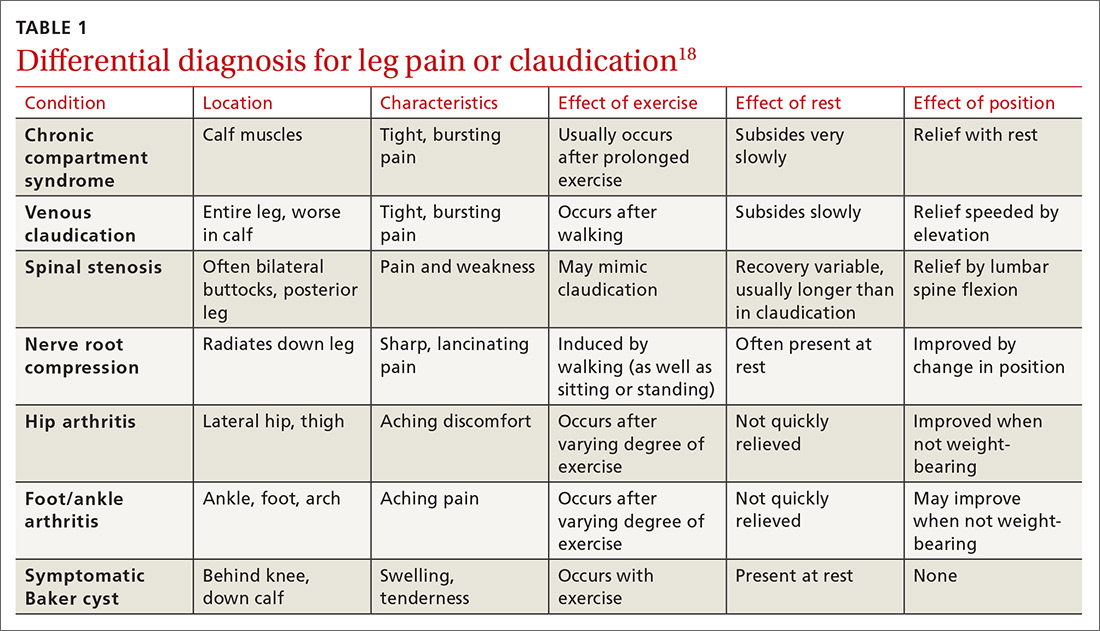
Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.
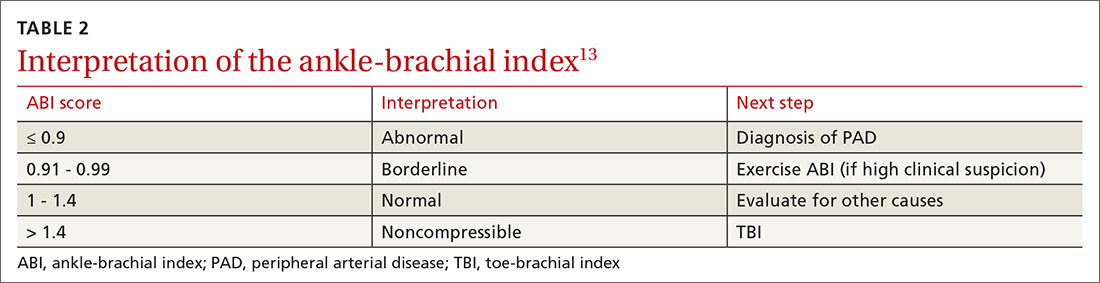
An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.
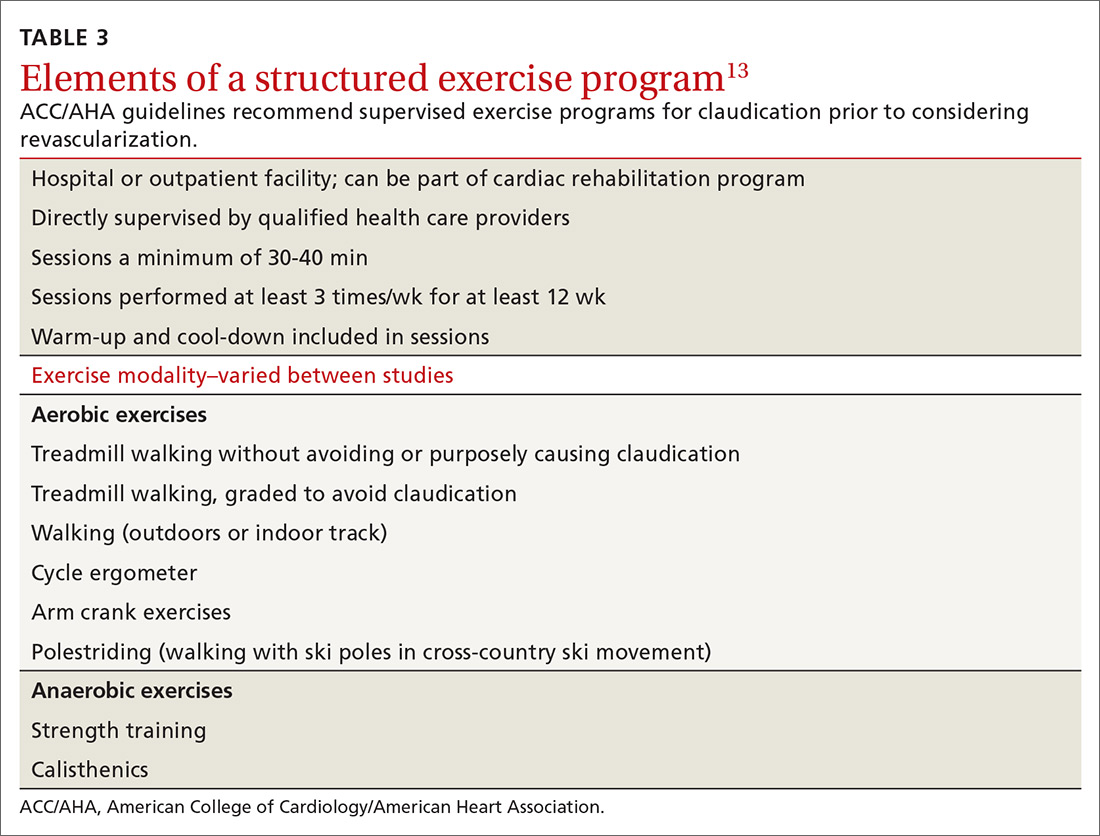
Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.
Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1

Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.

Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.

An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.

Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.

Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1

Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.

Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.

An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.

Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.

Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
PRACTICE RECOMMENDATIONS
❯ Use the ankle-brachial index for diagnosis in patients with history/physical exam findings suggestive of peripheral arterial disease (PAD). A
❯ Strongly encourage smoking cessation in patients with PAD as doing so reduces 5-year mortality and amputation rates. B
❯ Use structured exercise programs for patients with intermittent claudication prior to consideration of revascularization; doing so offers similar benefit and lower risks. A
❯ Recommend revascularization for patients who have limb ischemia or lifestyle-limiting claudication despite medical and exercise therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Pediatric cholestatic liver disease: Successful transition of care
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
KEY POINTS
- The causes of cholestasis in children are different from those in adults, with genetic inherited causes more common in childhood.
- Cholestasis in children can be caused by biliary tract obstruction such as in biliary atresia or defects in forming and excreting bile acids and other components of bile.
- With the growing number of people with childhood-onset liver disease surviving into adulthood, it is important for internists to be aware of unique problems and challenges in continuing management of this population.
- In addition to medical comorbidities, these patients may also have impaired psychosocial functioning and quality of life.
Laboratory tests in rheumatology: A rational approach
Laboratory tests are often ordered inappropriately for patients in whom a rheumatologic illness is suspected; this occurs in both primary and secondary care.1 Some tests are available both singly and as part of a battery of tests screening healthy people without symptoms.
The problem: negative test results are by no means always reassuring, and false-positive results raise the risks of unnecessary anxiety for patients and clinicians, needless referrals, and potential morbidity due to further unnecessary testing and exposure to wrong treatments.2 Clinicians should be aware of the pitfalls of these tests in order to choose them wisely and interpret the results correctly.
This article provides practical guidance on requesting and interpreting some common tests in rheumatology, with the aid of case vignettes.
RHEUMATOID FACTOR AND ANTICITRULLINATED PEPTIDE ANTIBODY
A 41-year-old woman, previously in good health, presents to her primary care practitioner with a 6-week history of pain and swelling in her hands and early morning stiffness lasting about 2 hours. She denies having any extraarticular symptoms. Physical examination reveals synovitis across her right metacarpophalangeal joints, proximal interphalangeal joint of the left middle finger, and left wrist. The primary care physician is concerned that her symptoms might be due to rheumatoid arthritis.
Would testing for rheumatoid factor and anticitrullinated peptide antibody be useful in this patient?
Rheumatoid factor is an antibody (immunoglobulin M, IgG, or IgA) targeted against the Fc fragment of IgG.3 It was so named because it was originally detected in patients with rheumatoid arthritis, but it is neither sensitive nor specific for this condition. A meta-analysis of more than 5,000 patients with rheumatoid arthritis reported that rheumatoid factor testing had a sensitivity of 69% and specificity of 85%.4
Anticitrullinated peptide antibody, on the other hand, is much more specific for rheumatoid arthritis (95%), as it is seldom seen in other conditions, but its sensitivity is similar to that of rheumatoid factor (68%).4–6 A positive result would thus lend strength to the diagnosis of rheumatoid arthritis, but a negative result would not exclude it.
Approach to early arthritis
When faced with a patient with early arthritis, some key questions to ask include7,8:
Is this an inflammatory or a mechanical problem? Inflammatory arthritis is suggested by joint swelling that is not due to trauma or bony hypertrophy, early morning stiffness lasting longer than 30 minutes, and elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein). Involvement of the small joints of the hands and feet may be suggested by pain on compression of the metacarpophalangeal and metatarsophalangeal joints, respectively.
Is there a definite identifiable underlying cause for the inflammatory arthritis? The pattern of development of joint symptoms or the presence of extraarticular symptoms may suggest an underlying problem such as gout, psoriatic arthritis, systemic lupus erythematosus, or sarcoidosis.
If the arthritis is undifferentiated (ie, there is no definite identifiable cause), is it likely to remit or persist? This is perhaps the most important question to ask in order to prognosticate. Patients with risk factors for persistent disease, ie, for development of rheumatoid arthritis, should be referred to a rheumatologist early for timely institution of disease-modifying antirheumatic drug therapy.9 Multiple studies have shown that patients in whom this therapy is started early have much better clinical, functional, and radiologic outcomes than those in whom it is delayed.10–12
The revised American College of Rheumatology and European League Against Rheumatism criteria13 include the following factors as predictors of persistence:
- Number of involved joints (with greater weight given to involvement of small joints)
- Duration of symptoms 6 weeks or longer
- Elevated acute-phase response (erythrocyte sedimentation rate or C-reactive protein level)
- A positive serologic test (either rheumatoid factor or anticitrullinated peptide antibody).
If both rheumatoid factor and anticitrullinated peptide antibody are positive in a patient with early undifferentiated arthritis, the risk of progression to rheumatoid arthritis is almost 100%, thus underscoring the importance of testing for these antibodies.5,6 Referral to a rheumatologist should, however, not be delayed in patients with negative test results (more than one-third of patients with rheumatoid arthritis may be negative for both), and should be considered in those with inflammatory joint symptoms persisting longer than 6 weeks, especially with involvement of the small joints (sparing the distal interphalangeals) and elevated acute-phase response.
Rheumatoid factor in healthy people without symptoms
In some countries, testing for rheumatoid factor is offered as part of a battery of screening tests in healthy people who have no symptoms, a practice that should be strongly discouraged.
Multiple studies, both prospective and retrospective, have demonstrated that both rheumatoid factor and anticitrullinated peptide antibody may be present several years before the clinical diagnosis of rheumatoid arthritis.6,14–16 But the risk of developing rheumatoid arthritis for asymptomatic individuals who are rheumatoid factor-positive depends on the rheumatoid factor titer, positive family history of rheumatoid arthritis in first-degree relatives, and copresence of anticitrullinated peptide antibody. The absolute risk, nevertheless, is still very small. In some, there might be an alternative explanation such as undiagnosed Sjögren syndrome or hepatitis C.
In any event, no strategy is currently available that is proven to prevent the development of rheumatoid arthritis, and there is no role for disease-modifying therapy during the preclinical phase.16
Back to our patient
Blood testing in our patient reveals normal complete blood cell counts, aminotransferase levels, and serum creatinine concentration; findings on urinalysis are normal. Her erythrocyte sedimentation rate is 56 mm/hour (reference range 0–15), and her C-reactive protein level is 26 mg/dL (normal < 3). Testing is negative for rheumatoid factor and anticitrullinated peptide antibody.
Although her rheumatoid factor and anticitrullinated peptide antibody tests are negative, she is referred to a rheumatologist because she has predictors of persistent disease, ie, symptom duration of 6 weeks, involvement of the small joints of the hands, and elevated erythrocyte sedimentation rate and C-reactive protein. The rheumatologist checks her parvovirus serology, which is negative.
The patient is given parenteral depot corticosteroid therapy, to which she responds briefly. Because her symptoms persist and continue to worsen, methotrexate treatment is started after an additional 6 weeks.
ANTINUCLEAR ANTIBODY
A 37-year-old woman presents to her primary care physician with the complaint of tiredness. She has a family history of systemic lupus erythematosus in her sister and maternal aunt. She is understandably worried about lupus because of the family history and is asking to be tested for it.
Would testing for antinuclear antibody be reasonable?
Antinuclear antibody is not a single antibody but rather a family of autoantibodies that are directed against nuclear constituents such as single- or double-stranded deoxyribonucleic acid (dsDNA), histones, centromeres, proteins complexed with ribonucleic acid (RNA), and enzymes such as topoisomerase.17,18
Protein antigens complexed with RNA and some enzymes in the nucleus are also known as extractable nuclear antigens (ENAs). They include Ro, La, Sm, Jo-1, RNP, and ScL-70 and are named after the patient in whom they were first discovered (Robert, Lavine, Smith, and John), the antigen that is targeted (ribonucleoprotein or RNP), and the disease with which they are associated (anti-ScL-70 or antitopoisomerase in diffuse cutaneous scleroderma).
Antinuclear antibody testing is commonly requested to exclude connective tissue diseases such as lupus, but the clinician needs to be aware of the following points:
Antinuclear antibody may be encountered in conditions other than lupus
These include19:
- Other autoimmune diseases such as rheumatoid arthritis, primary Sjögren syndrome, systemic sclerosis, autoimmune thyroid disease, and myasthenia gravis
- Infection with organisms that share the epitope with self-antigens (molecular mimicry)
- Cancers
- Drugs such as hydralazine, procainamide, and minocycline.
Antinuclear antibody might also be produced by the healthy immune system from time to time to clear the nuclear debris that is extruded from aging cells.
A study in healthy individuals20 reported a prevalence of positive antinuclear antibody of 32% at a titer of 1/40, 15% at a titer of 1/80, 7% at a titer of 1/160, and 3% at a titer of 1/320. Importantly, a positive result was more common among family members of patients with autoimmune connective tissue diseases.21 Hence, a positive antinuclear antibody result does not always mean lupus.
Antinuclear antibody testing is highly sensitive for lupus
With current laboratory methods, antinuclear antibody testing has a sensitivity close to 100%. Hence, a negative result virtually rules out lupus.
Two methods are commonly used to test for antinuclear antibody: indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA).22 While human epithelial (Hep2) cells are used as the source of antigen in immunofluorescence, purified nuclear antigens coated on multiple-well plates are used in ELISA.
Although ELISA is simpler to perform, immunofluorescence has a slightly better sensitivity (because the Hep2 cells express a wide range of antigens) and is still considered the gold standard. As expected, the higher sensitivity occurs at the cost of reduced specificity (about 60%), so antinuclear antibody will also be detected in all the other conditions listed above.23
To improve the specificity of antinuclear antibody testing, laboratories report titers (the highest dilution of the test serum that tested positive); a cutoff of greater than 1/80 is generally considered significant.
Do not order antinuclear antibody testing indiscriminately
To sum up, the antinuclear antibody test should be requested only in patients with involvement of multiple organ systems. Although a negative result would make it extremely unlikely that the clinical presentation is due to lupus, a positive result is insufficient on its own to make a diagnosis of lupus.
Diagnosing lupus is straightforward when patients present with a specific manifestation such as inflammatory arthritis, photosensitive skin rash, hemolytic anemia, thrombocytopenia, or nephritis, or with specific antibodies such as those against dsDNA or Sm. Patients who present with nonspecific symptoms such as arthralgia or tiredness with a positive antinuclear antibody and negative anti-dsDNA and anti-Sm may present difficulties even for the specialist.25–27
Back to our patient
Our patient denies arthralgia. She has no extraarticular symptoms such as skin rashes, oral ulcers, sicca symptoms, muscle weakness, Raynaud phenomenon, pleuritic chest pain, or breathlessness. Findings on physical examination and urinalysis are unremarkable.
Her primary care physician decides to check her complete blood cell count, erythrocyte sedimentation rate, and thyroid-stimulating hormone level. Although she is reassured that her tiredness is not due to lupus, she insists on getting an antinuclear antibody test.
Her complete blood cell counts are normal. Her erythrocyte sedimentation rate is 6 mm/hour. However, her thyroid-stimulating hormone level is elevated, and subsequent testing shows low free thyroxine and positive thyroid peroxidase antibodies. The antinuclear antibody is positive in a titer of 1/80 and negative for anti-dsDNA and anti-ENA.
We explain to her that the positive antinuclear antibody is most likely related to her autoimmune thyroid disease. She is referred to an endocrinologist.
ANTIPHOSPHOLIPID ANTIBODIES
A 24-year-old woman presents to the emergency department with acute unprovoked deep vein thrombosis in her right leg, confirmed by ultrasonography. She has no history of previous thrombosis, and the relevant family history is unremarkable. She has never been pregnant. Her platelet count is 84 × 109/L (reference range 150–400), and her baseline activated partial thromboplastin time is prolonged at 62 seconds (reference range 23.0–32.4). The rest of her blood counts and her prothrombin time, liver enzyme levels, and serum creatinine level are normal.
Should this patient be tested for antiphospholipid antibodies?
Antiphospholipid antibodies are important because of their association with thrombotic risk (both venous and arterial) and pregnancy morbidity. The name is a misnomer, as these antibodies are targeted against some proteins that are bound to phospholipids and not only to the phospholipids themselves.
According to the modified Sapporo criteria for the classification of antiphospholipid syndrome,28 antiphospholipid antibodies should remain persistently positive on at least 2 separate occasions at least 12 weeks apart for the result to be considered significant because some infections and drugs may be associated with the transient presence of antiphospholipid antibodies.
Screening for antiphospholipid antibodies should include testing for IgM and IgG anticardiolipin antibodies, lupus anticoagulant, and IgM and IgG beta-2 glycoprotein I antibodies.29,30
Anticardiolipin antibodies
Anticardiolipin (aCL) antibodies may be targeted either against beta-2 glycoprotein I (beta-2GPI) that is bound to cardiolipin (a phospholipid) or against cardiolipin alone; the former is more specific. Antibodies directed against cardiolipin alone are usually transient and are associated with infections and drugs. The result is considered significant only when anticardiolipin antibodies are present in a medium to high titer (> 40 IgG phospholipid units or IgM phospholipid units, or > 99th percentile).
Lupus anticoagulant
The antibody with “lupus anticoagulant activity” is targeted against prothrombin plus phospholipid or beta-2GPI plus phospholipid. The test for it is a functional assay involving 3 steps:
Demonstrating the prolongation of a phospholipid-dependent coagulation assay like the activated partial thromboplastin time (aPTT). (This may explain the prolongation of aPTT in the patient described in the vignette.) Although the presence of lupus anticoagulant is associated with thrombosis, it is called an “anticoagulant” because of this in vitro prolongation of phospholipid-dependent coagulation assays.
Mixing study. The phospholipid-dependent coagulation assay could be prolonged because of either the deficiency of a coagulation factor or the presence of the antiphospholipid antibodies. This can be differentiated by mixing the patient’s plasma with normal plasma (which will have all the clotting factors) in a 1:1 ratio. If the coagulation assay remains prolonged after the addition of normal plasma, clotting factor deficiency can be excluded.
Addition of a phospholipid. If the prolongation of the coagulation assay is due to the presence of an antiphospholipid antibody, addition of extra phospholipid will correct this.
Beta-2 glycoprotein I antibody (anti-beta-2GPI)
The beta-2GPI that is not bound to the cardiolipin can be detected by separately testing for beta-2GPI (the anticardiolipin test only detects the beta-2GPI that is bound to the cardiolipin). The result is considered significant if beta-2GPI is present in a medium to high titer (> 99th percentile).
Studies have shown that antiphospholipid antibodies may be present in 1% to 5% of apparently healthy people in the general population.31 These are usually low-titer anticardiolipin or anti-beta-GPI IgM antibodies that are not associated with thrombosis or adverse pregnancy outcomes. Hence, the term antiphospholipid syndrome should be reserved for those who have had at least 1 episode of thrombosis or pregnancy morbidity and persistent antiphospholipid antibodies, and not those who have asymptomatic or transient antiphospholipid antibodies.
Triple positivity (positive anticardiolipin, lupus anticoagulant, and anti-beta-2GPI) seems to be associated with the highest risk of thrombosis, with a 10-year cumulative incidence of 37.1% (95% confidence interval [CI] 19.9–54.3) for a first thrombotic event,32 and 44.2% (95% CI 38.6–49.8) for recurrent thrombosis.33
The association with thrombosis is stronger for lupus anticoagulant than with the other 2 antibodies, with different studies34 finding an odds ratio ranging from 5 to 16. A positive lupus anticoagulant test with or without a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a high-risk profile, while a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a moderate-risk profile. A low titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a low-risk profile that may not be associated with thrombosis.35
Antiphospholipid syndrome is important to recognize because of the need for long-term anticoagulation to prevent recurrence.36 It may be primary, when it occurs on its own, or secondary, when it occurs in association with another autoimmune disease such as lupus.
Venous events in antiphospholipid syndrome most commonly manifest as lower-limb deep vein thrombosis or pulmonary embolism, while arterial events most commonly manifest as stroke or transient ischemic attack.37 Obstetric manifestations may include not only miscarriage and stillbirth, but also preterm delivery, intrauterine growth retardation, and preeclampsia, all occurring due to placental insufficiency.
The frequency of antiphospholipid antibodies has been estimated as 13.5% in patients with stroke, 11% with myocardial infarction, 9.5% with deep vein thrombosis, and 6% for those with pregnancy morbidity.38
Some noncriteria manifestations have also been recognized in antiphospholipid syndrome, such as thrombocytopenia, cardiac vegetations (Libman-Sachs endocarditis), livedo reticularis, and nephropathy.
Back to our patient
Our patient’s anticardiolipin IgG test is negative, while her lupus anticoagulant and beta-2GPI IgG are positive. She has no clinical or laboratory features suggesting lupus.
She is started on warfarin. After 3 months, the warfarin is interrupted for several days, and she is retested for all 3 antiphospholipid antibodies. Her beta-2GPI I IgG and lupus anticoagulant tests are again positive. Because of the persistent antiphospholipid antibody positivity and clinical history of deep vein thrombosis, her condition is diagnosed as primary antiphospholipid syndrome. She is advised to continue anticoagulant therapy indefinitely.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY
A 34-year-old man who is an injecting drug user presents with a 2-week history of fever, malaise, and generalized arthralgia. There are no localizing symptoms of infection. Notable findings on examination include a temperature of 38.0°C (100.4°F), needle track marks in his arms, nonblanching vasculitic rash in his legs, and a systolic murmur over the precordium.
His white blood cell count is 15.3 × 109/L (reference range 3.7–11.0), and his C-reactive protein level is 234 mg/dL (normal < 3). Otherwise, results of blood cell counts, liver enzyme tests, renal function tests, urinalysis, and chest radiography are normal.
Two sets of blood cultures are drawn. Transthoracic echocardiography and the antineutrophil cytoplasmic antibody (ANCA) test are requested, as are screening tests for human immunodeficiency virus, hepatitis B, and hepatitis C.
Was the ANCA test indicated in this patient?
ANCAs are autoantibodies against antigens located in the cytoplasmic granules of neutrophils and monocytes. They are associated with small-vessel vasculitides such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and isolated pauciimmune crescentic glomerulonephritis, all collectively known as ANCA-associated vasculitis (AAV).39
Laboratory methods to detect ANCA include indirect immunofluorescence and antigen-specific enzyme immunoassays. Indirect immunofluorescence only tells us whether or not an antibody that is targeting a cytoplasmic antigen is present. Based on the indirect immunofluorescent pattern, ANCA can be classified as follows:
- Perinuclear or p-ANCA (if the targeted antigen is located just around the nucleus and extends into it)
- Cytoplasmic or c-ANCA (if the targeted antigen is located farther away from the nucleus)
- Atypical ANCA (if the indirect immunofluorescent pattern does not fit with either p-ANCA or c-ANCA).
Indirect immunofluorescence does not give information about the exact antigen that is targeted; this can only be obtained by performing 1 of the antigen-specific immunoassays. The target antigen for c-ANCA is usually proteinase-3 (PR3), while that for p-ANCA could be myeloperoxidase (MPO), cathepsin, lysozyme, lactoferrin, or bactericidal permeability inhibitor. Anti-PR3 is highly specific for GPA, while anti-MPO is usually associated with MPA and EGPA. Less commonly, anti-PR3 may be seen in patients with MPA and anti-MPO in those with GPA. Hence, there is an increasing trend toward classifying ANCA-associated vasculitis into PR3-associated or MPO-associated vasculitis rather than as GPA, MPA, EGPA, or renal-limited vasculitis.40
Several audits have shown that the ANCA test is widely misused and requested indiscriminately to rule out vasculitis. This results in a lower positive predictive value, possible harm to patients due to increased false-positive rates, and increased burden on the laboratory.41–43 At least 2 separate groups have demonstrated that a gating policy that refuses ANCA testing in patients without clinical evidence of systemic vasculitis can reduce the number of inappropriate requests, improve the diagnostic yield, and make it more clinically relevant and cost-effective.44,45
The clinician should bear in mind that:
Current guidelines recommend using one of the antigen-specific assays for PR3 and MPO as the primary screening method.48 Until recently, indirect immunofluorescence was used to screen for ANCA-associated vasculitis, and positive results were confirmed by ELISA to detect ANCAs specific for PR3 and MPO,49 but this is no longer recommended because of recent evidence suggesting a large variability between the different indirect immunofluorescent methods and improved diagnostic performance of the antigen-specific assays.
In a large multicenter study by Damoiseaux et al, the specificity with the different antigen-specific immunoassays was 98% to 99% for PR3-ANCA and 96% to 99% for MPO-ANCA.50
ANCA-associated vasculitis should not be considered excluded if the PR3 and MPO-ANCA are negative. In the Damoiseaux study, about 11% to 15% of patients with GPA and 8% to 24% of patients with MPA tested negative for both PR3 and MPO-ANCA.50
If the ANCA result is negative and clinical suspicion for ANCA-associated vasculitis is high, the clinician may wish to consider requesting another immunoassay method or indirect immunofluorescence. Results of indirect immunofluorescent testing results may be positive in those with a negative immunoassay, and vice versa.
Thus, the ANCA result should always be interpreted in the context of the whole clinical picture.51 Biopsy should still be considered the gold standard for the diagnosis of ANCA-associated vasculitis. The ANCA titer can help to improve clinical interpretation, because the likelihood of ANCA-associated vasculitis increases with higher levels of PR3 and MPO-ANCA.52
Back to our patient
Our patient’s blood cultures grow methicillin-sensitive Staphylococcus aureus in both sets after 48 hours. Transthoracic echocardiography reveals vegetations around the tricuspid valve, with no evidence of valvular regurgitation. The diagnosis is right-sided infective endocarditis. He is started on appropriate antibiotics.
Tests for human immunodeficiency virus, hepatitis B, and hepatitis C are negative. The ANCA test is positive for MPO-ANCA at 28 IU/mL (normal < 10).
The positive ANCA is thought to be related to the infective endocarditis. His vasculitis is most likely secondary to infective endocarditis and not ANCA-associated vasculitis. The ANCA test need not have been requested in the first place.
HUMAN LEUKOCYTE ANTIGEN-B27
A 22-year-old man presents to his primary care physician with a 4-month history of gradually worsening low back pain associated with early morning stiffness lasting more than 2 hours. He has no peripheral joint symptoms.
In the last 2 years, he has had 2 separate episodes of uveitis. There is a family history of ankylosing spondylitis in his father. Examination reveals global restriction of lumbar movements but is otherwise unremarkable. Magnetic resonance imaging (MRI) of the lumbar spine and sacroiliac joints is normal.
Should this patient be tested for human leukocyte antigen-B27 (HLA-B27)?
The major histocompatibility complex (MHC) is a gene complex that is present in all animals. It encodes proteins that help with immunologic tolerance. HLA simply refers to the human version of the MHC.53 The HLA gene complex, located on chromosome 6, is categorized into class I, class II, and class III. HLA-B is one of the 3 class I genes. Thus, a positive HLA-B27 result simply means that the particular gene is present in that person.
HLA-B27 is strongly associated with ankylosing spondylitis, also known as axial spondyloarthropathy.54 Other genes also contribute to the pathogenesis of ankylosing spondylitis, but HLA-B27 is present in more than 90% of patients with this disease and is by far considered the most important. The association is not as strong for peripheral spondyloarthropathy, with studies reporting a frequency of up to 75% for reactive arthritis and inflammatory bowel disease-associated arthritis, and up to 50% for psoriatic arthritis and uveitis.55
About 9% of healthy, asymptomatic individuals may have HLA-B27, so the mere presence of this gene is not evidence of disease.56 There may be up to a 20-fold increased risk of ankylosing spondylitis among those who are HLA-B27-positive.57
Some HLA genes have many different alleles, each of which is given a number (explaining the number 27 that follows the B). Closely related alleles that differ from one another by only a few amino-acid substitutions are then categorized together, thus accounting for more than 100 subtypes of HLA-B27 (designated from HLA-B*2701 to HLA-B*27106). These subtypes vary in frequency among different racial groups, and the population prevalence of ankylosing spondylitis parallels the frequency of HLA-B27.58 The most common subtype seen in white people and American Indians is B*2705. HLA-B27 is rare in blacks, explaining the rarity of ankylosing spondylitis in this population. Further examples include HLA-B*2704, which is seen in Asians, and HLA-B*2702, seen in Mediterranean populations. Not all subtypes of HLA-B27 are associated with disease, and some, like HLA-B*2706, may also be protective.
When should the clinician consider testing for HLA-B27?
Peripheral spondyloarthropathy may present with arthritis, enthesitis (eg, heel pain due to inflammation at the site of insertion of the Achilles tendon or plantar fascia), or dactylitis (“sausage” swelling of the whole finger or toe due to extension of inflammation beyond the margins of the joint). Other clues may include psoriasis, inflammatory bowel disease, history of preceding gastrointestinal or genitourinary infection, family history of similar conditions, and history of recurrent uveitis.
For the initial assessment of patients who have inflammatory back pain, plain radiography of the sacroiliac joints is considered the gold standard.59 If plain radiography does not show evidence of sacroiliitis, MRI of the sacroiliac joints should be considered. While plain radiography can reveal only structural changes such as sclerosis, erosions, and ankylosis, MRI is useful to evaluate for early inflammatory changes such as bone marrow edema. Imaging the lumbar spine is not necessary, as the sacroiliac joints are almost invariably involved in axial spondyloarthropathy, and lesions seldom occur in the lumbar spine in isolation.60
The diagnosis of ankylosing spondylitis previously relied on confirmatory imaging features, but based on the new International Society classification criteria,61–63 which can be applied to patients with more than 3 months of back pain and age of onset of symptoms before age 45, patients can be classified as having 1 of the following:
- Radiographic axial spondyloarthropathy, if they have evidence of sacroiliitis on imaging plus 1 other feature of spondyloarthropathy
- Nonradiographic axial spondyloarthropathy, if they have a positive HLA-B27 plus 2 other features of spondyloarthropathy (Table 7).
These new criteria have a sensitivity of 82.9% and specificity of 84.4%.62,63 The disease burden of radiographic and nonradiographic axial spondyloarthropathy has been shown to be similar, suggesting that they are part of the same disease spectrum. Thus, the HLA-B27 test is useful to make a diagnosis of axial spondyloarthropathy even in the absence of imaging features and could be requested in patients with 2 or more features of spondyloarthropathy. In the absence of imaging features and a negative HLA-B27 result, however, the patient cannot be classified as having axial spondyloarthropathy.
Back to our patient
The absence of radiographic evidence would not exclude axial spondyloarthropathy in our patient. The HLA-B27 test is requested because of the inflammatory back pain and the presence of 2 spondyloarthropathy features (uveitis and the family history) and is reported to be positive. His disease is classified as nonradiographic axial spondyloarthropathy.
He is started on regular naproxen and is referred to a physiotherapist. After 1 month, he reports significant symptomatic improvement. He asks if he can be retested for HLA-B27 to see if it has become negative. We tell him that there is no point in repeating it, as it is a gene and will not disappear.
SUMMARY: CONSIDER THE CLINICAL PICTURE
When approaching a patient suspected of having a rheumatologic disease, a clinician should first consider the clinical presentation and the intended purpose of each test. The tests, in general, might serve several purposes. They might help to:
Increase the likelihood of the diagnosis in question. For example, a positive rheumatoid factor or anticitrullinated peptide antibody can help diagnose rheumatoid arthritis in a patient with early polyarthritis, a positive HLA-B27 can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging, and a positive ANCA can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
Reduce the likelihood of the diagnosis in question. For example, a negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pains.
Monitor the condition. For example DNA antibodies can be used to monitor the activity of lupus.
Plan the treatment strategy. For example, one might consider lifelong anticoagulation if antiphospholipid antibodies are persistently positive in a patient with thrombosis.
Prognosticate. For example, positive rheumatoid factor and anticitrullinated peptide antibody increase the risk of erosive rheumatoid arthritis.
If the test was requested in the absence of a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test and not attach a diagnostic label prematurely. None of the tests can confirm or exclude a condition, so the results should always be interpreted in the context of the whole clinical picture.
- American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 2002; 47(4):429–433. doi:10.1002/art.10381
- Rang M. The Ulysses syndrome. Can Med Assoc J 1972; 106(2):122–123. pmid:5058884
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35(6):727–734. doi:10.1155/2013/726598
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146(11):797–808. pmid:17548411
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038. doi:10.4061/2011/815038
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48(10):2741–2749. doi:10.1002/art.11223
- Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004; 97(9):421–424. doi:10.1258/jrsm.97.9.421
- Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61(4):290–297. pmid:11874828
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76(6):948–959. doi:10.1136/annrheumdis-2016-210602
- Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year follow up of a prospective double blind placebo controlled study. J Rheumatol 1995; 22(12):2208–2213. pmid:8835550
- van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124(8):699–707. pmid:8633829
- Andreson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9):2569–2581. doi:10.1002/art.27584
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50(2):380–386. doi:10.1002/art.20018
- del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988; 31(10):1239–1244. pmid:3178905
- Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010; 36(2):213–241. doi:10.1016/j.rdc.2010.02.001
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000; 124(1):71–81. doi:10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2
- Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br J Hosp Med (Lond) 2007; 68(10):538–541. doi:10.12968/hmed.2007.68.10.27324
- Illei GG, Klippel JH. Why is the ANA result positive? Bull Rheum Dis 1999; 48(1):1–4. pmid:10028188
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40(9):1601–1611. doi:10.1002/art.1780400909
- Langkilde H, Voss A, Heegaard N, Laustrup H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017; 26(7):723–728. doi:10.1177/0961203316676378
- Rondeel JM. Immunofluorescence versus ELISA for the detection of antinuclear antigens. Expert Rev Mol Diagn 2002; 2(3):226–232. doi:10.1586/14737159.2.3.226
- Solomon DH, Kavanaugh AJ, Schur PH; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002; 47(4):434–444. doi:10.1002/art.10561
- Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996; 156(13):1421–1425. pmid:8678710
- Maddison PJ. Is it SLE? Best Pract Res Clin Rheumatol 2002; 16(2):167–180. doi:10.1053/berh.2001.0219
- Price E, Walker E. Diagnostic vertigo: the journey to diagnosis in systemic lupus erythematosus. Health (London) 2014; 18(3):223–239. doi:10.1177/1363459313488008
- Blumenthal DE. Tired, aching, ANA-positive: does your patient have lupus or fibromyalgia? Cleve Clin J Med 2002; 69(2):143–146, 151–152. pmid:11990644
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157(1):47–58. doi:10.1111/j.1365-2141.2012.09037.x
- Giannakopoulos B, Passam F, Iannou Y, Krillis SA. How we diagnose the antiphospholipid syndrome. Blood 2009; 113(5):985–994. doi:10.1182/blood-2007-12-129627
- Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010; 9(5):A299–A304. doi:10.1016/j.autrev.2009.11.013
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118(17):4714–4718. doi:10.1182/blood-2011-03-340232
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8(2):237–242. doi:10.1111/j.1538-7836.2009.03674.x
- Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101(5):1827–1832. doi:10.1182/blood-2002-02-0441
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378(21):2010–2021. doi:10.1056/NEJMra1705454
- Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122(5):817–824. doi:10.1182/blood-2013-04-496257
- Cervera R. Lessons from the “Euro-Phospholipid” project. Autoimmun Rev 2008; 7(3):174–178. doi:10.1016/j.autrev.2007.11.011
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013; 65(11):1869–1873. doi:10.1002/acr.22066
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol 2010; 160(2):143–160. doi:10.1111/j.1365-2249.2009.04078.x
- Cornec D, Cornec-Le-Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016; 12(10):570–579. doi:10.1038/nrrheum.2016.123
- Edgar JD, McMillan SA, Bruce IN, Conlan SK. An audit of ANCA in routine clinical practice. Postgrad Med J 1995; 71(840):605–612. pmid:8545289
- McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic testing in a routine clinical setting. QJM 2001; 94(11):615–621. pmid:11704691
- Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch Intern Med 2002; 162(13):1509–1514. pmid:12090888
- Sinclair D, Saas M, Stevens JM. The effect of a symptom related “gated policy” on ANCA requests in routine clinical practice. J Clin Pathol 2004; 57(2):131–134. pmid:14747434
- Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol 2010; 63(8):678–680. doi:10.1136/jcp.2009.072504
- Savige J, Gills D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999; 111(4):507–513. pmid:10191771
- Robinson PC, Steele RH. Appropriateness of antineutrophil cytoplasmic antibody testing in a tertiary hospital. J Clin Pathol 2009; 62(8):743–745. doi:10.1136/jcp.2009.064485
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017; 13(11):683–692. doi:10.1038/nrrheum.2017.140
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53(3):743–753. doi:10.1046/j.1523-1755.1998.00807.x
- Damoiseaux J, Csemok E, Rasmussen N, et al. Detection of antineutrophil antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen specific immunoassays. Ann Rheum Dis 2017; 76(4):647–653. doi:10.1136/annrheumdis-2016-209507
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J 2006; 82(970):483–488. doi:10.1136/pgmj.2005.042648
- Vermeersch P, Blockmans D, Bossuyt X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin Chem 2009; 55(10):1886–1888. doi:10.1373/clinchem.2009.130583
- Bowness P. HLA-B27. Annu Rev Immunol 2015; 33:29–48. doi:10.1146/annurev-immunol-032414-112110
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
- Khan MA. Thoughts concerning the early diagnosis of ankylosing spondylitis and related diseases. Clin Exp Rheumatol 2002; 20(6 suppl 28):S6–S10. pmid:12463439
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41(1):58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984; 27(3):241–249. pmid:6608352
- Sheehan NJ. HLA-B27: what’s new? Rheumatology (Oxford) 2010; 49(4):621–631. doi:10.1093/rheumatology/kep450
- Baraliakos X, Maksymmowych WP. Imaging in the diagnosis and management of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2016; 30(4):608–623. doi:10.1016/j.berh.2016.09.011
- Mandl P, Navarro-Compan V, Terslev L, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74(7):1327–1339. doi:10.1136/annrheumdis-2014-206971
- McAllister K, Goodson N, Warburton I, Rogers G. Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 2017; 356:j839. doi:10.1136/bmj.j839
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74(8):1483–1487. doi:10.1136/annrheumdis-2014-207151
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68(6):777–783. doi:10.1136/ard.2009.108233
Laboratory tests are often ordered inappropriately for patients in whom a rheumatologic illness is suspected; this occurs in both primary and secondary care.1 Some tests are available both singly and as part of a battery of tests screening healthy people without symptoms.
The problem: negative test results are by no means always reassuring, and false-positive results raise the risks of unnecessary anxiety for patients and clinicians, needless referrals, and potential morbidity due to further unnecessary testing and exposure to wrong treatments.2 Clinicians should be aware of the pitfalls of these tests in order to choose them wisely and interpret the results correctly.
This article provides practical guidance on requesting and interpreting some common tests in rheumatology, with the aid of case vignettes.
RHEUMATOID FACTOR AND ANTICITRULLINATED PEPTIDE ANTIBODY
A 41-year-old woman, previously in good health, presents to her primary care practitioner with a 6-week history of pain and swelling in her hands and early morning stiffness lasting about 2 hours. She denies having any extraarticular symptoms. Physical examination reveals synovitis across her right metacarpophalangeal joints, proximal interphalangeal joint of the left middle finger, and left wrist. The primary care physician is concerned that her symptoms might be due to rheumatoid arthritis.
Would testing for rheumatoid factor and anticitrullinated peptide antibody be useful in this patient?
Rheumatoid factor is an antibody (immunoglobulin M, IgG, or IgA) targeted against the Fc fragment of IgG.3 It was so named because it was originally detected in patients with rheumatoid arthritis, but it is neither sensitive nor specific for this condition. A meta-analysis of more than 5,000 patients with rheumatoid arthritis reported that rheumatoid factor testing had a sensitivity of 69% and specificity of 85%.4
Anticitrullinated peptide antibody, on the other hand, is much more specific for rheumatoid arthritis (95%), as it is seldom seen in other conditions, but its sensitivity is similar to that of rheumatoid factor (68%).4–6 A positive result would thus lend strength to the diagnosis of rheumatoid arthritis, but a negative result would not exclude it.
Approach to early arthritis
When faced with a patient with early arthritis, some key questions to ask include7,8:
Is this an inflammatory or a mechanical problem? Inflammatory arthritis is suggested by joint swelling that is not due to trauma or bony hypertrophy, early morning stiffness lasting longer than 30 minutes, and elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein). Involvement of the small joints of the hands and feet may be suggested by pain on compression of the metacarpophalangeal and metatarsophalangeal joints, respectively.
Is there a definite identifiable underlying cause for the inflammatory arthritis? The pattern of development of joint symptoms or the presence of extraarticular symptoms may suggest an underlying problem such as gout, psoriatic arthritis, systemic lupus erythematosus, or sarcoidosis.
If the arthritis is undifferentiated (ie, there is no definite identifiable cause), is it likely to remit or persist? This is perhaps the most important question to ask in order to prognosticate. Patients with risk factors for persistent disease, ie, for development of rheumatoid arthritis, should be referred to a rheumatologist early for timely institution of disease-modifying antirheumatic drug therapy.9 Multiple studies have shown that patients in whom this therapy is started early have much better clinical, functional, and radiologic outcomes than those in whom it is delayed.10–12
The revised American College of Rheumatology and European League Against Rheumatism criteria13 include the following factors as predictors of persistence:
- Number of involved joints (with greater weight given to involvement of small joints)
- Duration of symptoms 6 weeks or longer
- Elevated acute-phase response (erythrocyte sedimentation rate or C-reactive protein level)
- A positive serologic test (either rheumatoid factor or anticitrullinated peptide antibody).
If both rheumatoid factor and anticitrullinated peptide antibody are positive in a patient with early undifferentiated arthritis, the risk of progression to rheumatoid arthritis is almost 100%, thus underscoring the importance of testing for these antibodies.5,6 Referral to a rheumatologist should, however, not be delayed in patients with negative test results (more than one-third of patients with rheumatoid arthritis may be negative for both), and should be considered in those with inflammatory joint symptoms persisting longer than 6 weeks, especially with involvement of the small joints (sparing the distal interphalangeals) and elevated acute-phase response.
Rheumatoid factor in healthy people without symptoms
In some countries, testing for rheumatoid factor is offered as part of a battery of screening tests in healthy people who have no symptoms, a practice that should be strongly discouraged.
Multiple studies, both prospective and retrospective, have demonstrated that both rheumatoid factor and anticitrullinated peptide antibody may be present several years before the clinical diagnosis of rheumatoid arthritis.6,14–16 But the risk of developing rheumatoid arthritis for asymptomatic individuals who are rheumatoid factor-positive depends on the rheumatoid factor titer, positive family history of rheumatoid arthritis in first-degree relatives, and copresence of anticitrullinated peptide antibody. The absolute risk, nevertheless, is still very small. In some, there might be an alternative explanation such as undiagnosed Sjögren syndrome or hepatitis C.
In any event, no strategy is currently available that is proven to prevent the development of rheumatoid arthritis, and there is no role for disease-modifying therapy during the preclinical phase.16
Back to our patient
Blood testing in our patient reveals normal complete blood cell counts, aminotransferase levels, and serum creatinine concentration; findings on urinalysis are normal. Her erythrocyte sedimentation rate is 56 mm/hour (reference range 0–15), and her C-reactive protein level is 26 mg/dL (normal < 3). Testing is negative for rheumatoid factor and anticitrullinated peptide antibody.
Although her rheumatoid factor and anticitrullinated peptide antibody tests are negative, she is referred to a rheumatologist because she has predictors of persistent disease, ie, symptom duration of 6 weeks, involvement of the small joints of the hands, and elevated erythrocyte sedimentation rate and C-reactive protein. The rheumatologist checks her parvovirus serology, which is negative.
The patient is given parenteral depot corticosteroid therapy, to which she responds briefly. Because her symptoms persist and continue to worsen, methotrexate treatment is started after an additional 6 weeks.
ANTINUCLEAR ANTIBODY
A 37-year-old woman presents to her primary care physician with the complaint of tiredness. She has a family history of systemic lupus erythematosus in her sister and maternal aunt. She is understandably worried about lupus because of the family history and is asking to be tested for it.
Would testing for antinuclear antibody be reasonable?
Antinuclear antibody is not a single antibody but rather a family of autoantibodies that are directed against nuclear constituents such as single- or double-stranded deoxyribonucleic acid (dsDNA), histones, centromeres, proteins complexed with ribonucleic acid (RNA), and enzymes such as topoisomerase.17,18
Protein antigens complexed with RNA and some enzymes in the nucleus are also known as extractable nuclear antigens (ENAs). They include Ro, La, Sm, Jo-1, RNP, and ScL-70 and are named after the patient in whom they were first discovered (Robert, Lavine, Smith, and John), the antigen that is targeted (ribonucleoprotein or RNP), and the disease with which they are associated (anti-ScL-70 or antitopoisomerase in diffuse cutaneous scleroderma).
Antinuclear antibody testing is commonly requested to exclude connective tissue diseases such as lupus, but the clinician needs to be aware of the following points:
Antinuclear antibody may be encountered in conditions other than lupus
These include19:
- Other autoimmune diseases such as rheumatoid arthritis, primary Sjögren syndrome, systemic sclerosis, autoimmune thyroid disease, and myasthenia gravis
- Infection with organisms that share the epitope with self-antigens (molecular mimicry)
- Cancers
- Drugs such as hydralazine, procainamide, and minocycline.
Antinuclear antibody might also be produced by the healthy immune system from time to time to clear the nuclear debris that is extruded from aging cells.
A study in healthy individuals20 reported a prevalence of positive antinuclear antibody of 32% at a titer of 1/40, 15% at a titer of 1/80, 7% at a titer of 1/160, and 3% at a titer of 1/320. Importantly, a positive result was more common among family members of patients with autoimmune connective tissue diseases.21 Hence, a positive antinuclear antibody result does not always mean lupus.
Antinuclear antibody testing is highly sensitive for lupus
With current laboratory methods, antinuclear antibody testing has a sensitivity close to 100%. Hence, a negative result virtually rules out lupus.
Two methods are commonly used to test for antinuclear antibody: indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA).22 While human epithelial (Hep2) cells are used as the source of antigen in immunofluorescence, purified nuclear antigens coated on multiple-well plates are used in ELISA.
Although ELISA is simpler to perform, immunofluorescence has a slightly better sensitivity (because the Hep2 cells express a wide range of antigens) and is still considered the gold standard. As expected, the higher sensitivity occurs at the cost of reduced specificity (about 60%), so antinuclear antibody will also be detected in all the other conditions listed above.23
To improve the specificity of antinuclear antibody testing, laboratories report titers (the highest dilution of the test serum that tested positive); a cutoff of greater than 1/80 is generally considered significant.
Do not order antinuclear antibody testing indiscriminately
To sum up, the antinuclear antibody test should be requested only in patients with involvement of multiple organ systems. Although a negative result would make it extremely unlikely that the clinical presentation is due to lupus, a positive result is insufficient on its own to make a diagnosis of lupus.
Diagnosing lupus is straightforward when patients present with a specific manifestation such as inflammatory arthritis, photosensitive skin rash, hemolytic anemia, thrombocytopenia, or nephritis, or with specific antibodies such as those against dsDNA or Sm. Patients who present with nonspecific symptoms such as arthralgia or tiredness with a positive antinuclear antibody and negative anti-dsDNA and anti-Sm may present difficulties even for the specialist.25–27
Back to our patient
Our patient denies arthralgia. She has no extraarticular symptoms such as skin rashes, oral ulcers, sicca symptoms, muscle weakness, Raynaud phenomenon, pleuritic chest pain, or breathlessness. Findings on physical examination and urinalysis are unremarkable.
Her primary care physician decides to check her complete blood cell count, erythrocyte sedimentation rate, and thyroid-stimulating hormone level. Although she is reassured that her tiredness is not due to lupus, she insists on getting an antinuclear antibody test.
Her complete blood cell counts are normal. Her erythrocyte sedimentation rate is 6 mm/hour. However, her thyroid-stimulating hormone level is elevated, and subsequent testing shows low free thyroxine and positive thyroid peroxidase antibodies. The antinuclear antibody is positive in a titer of 1/80 and negative for anti-dsDNA and anti-ENA.
We explain to her that the positive antinuclear antibody is most likely related to her autoimmune thyroid disease. She is referred to an endocrinologist.
ANTIPHOSPHOLIPID ANTIBODIES
A 24-year-old woman presents to the emergency department with acute unprovoked deep vein thrombosis in her right leg, confirmed by ultrasonography. She has no history of previous thrombosis, and the relevant family history is unremarkable. She has never been pregnant. Her platelet count is 84 × 109/L (reference range 150–400), and her baseline activated partial thromboplastin time is prolonged at 62 seconds (reference range 23.0–32.4). The rest of her blood counts and her prothrombin time, liver enzyme levels, and serum creatinine level are normal.
Should this patient be tested for antiphospholipid antibodies?
Antiphospholipid antibodies are important because of their association with thrombotic risk (both venous and arterial) and pregnancy morbidity. The name is a misnomer, as these antibodies are targeted against some proteins that are bound to phospholipids and not only to the phospholipids themselves.
According to the modified Sapporo criteria for the classification of antiphospholipid syndrome,28 antiphospholipid antibodies should remain persistently positive on at least 2 separate occasions at least 12 weeks apart for the result to be considered significant because some infections and drugs may be associated with the transient presence of antiphospholipid antibodies.
Screening for antiphospholipid antibodies should include testing for IgM and IgG anticardiolipin antibodies, lupus anticoagulant, and IgM and IgG beta-2 glycoprotein I antibodies.29,30
Anticardiolipin antibodies
Anticardiolipin (aCL) antibodies may be targeted either against beta-2 glycoprotein I (beta-2GPI) that is bound to cardiolipin (a phospholipid) or against cardiolipin alone; the former is more specific. Antibodies directed against cardiolipin alone are usually transient and are associated with infections and drugs. The result is considered significant only when anticardiolipin antibodies are present in a medium to high titer (> 40 IgG phospholipid units or IgM phospholipid units, or > 99th percentile).
Lupus anticoagulant
The antibody with “lupus anticoagulant activity” is targeted against prothrombin plus phospholipid or beta-2GPI plus phospholipid. The test for it is a functional assay involving 3 steps:
Demonstrating the prolongation of a phospholipid-dependent coagulation assay like the activated partial thromboplastin time (aPTT). (This may explain the prolongation of aPTT in the patient described in the vignette.) Although the presence of lupus anticoagulant is associated with thrombosis, it is called an “anticoagulant” because of this in vitro prolongation of phospholipid-dependent coagulation assays.
Mixing study. The phospholipid-dependent coagulation assay could be prolonged because of either the deficiency of a coagulation factor or the presence of the antiphospholipid antibodies. This can be differentiated by mixing the patient’s plasma with normal plasma (which will have all the clotting factors) in a 1:1 ratio. If the coagulation assay remains prolonged after the addition of normal plasma, clotting factor deficiency can be excluded.
Addition of a phospholipid. If the prolongation of the coagulation assay is due to the presence of an antiphospholipid antibody, addition of extra phospholipid will correct this.
Beta-2 glycoprotein I antibody (anti-beta-2GPI)
The beta-2GPI that is not bound to the cardiolipin can be detected by separately testing for beta-2GPI (the anticardiolipin test only detects the beta-2GPI that is bound to the cardiolipin). The result is considered significant if beta-2GPI is present in a medium to high titer (> 99th percentile).
Studies have shown that antiphospholipid antibodies may be present in 1% to 5% of apparently healthy people in the general population.31 These are usually low-titer anticardiolipin or anti-beta-GPI IgM antibodies that are not associated with thrombosis or adverse pregnancy outcomes. Hence, the term antiphospholipid syndrome should be reserved for those who have had at least 1 episode of thrombosis or pregnancy morbidity and persistent antiphospholipid antibodies, and not those who have asymptomatic or transient antiphospholipid antibodies.
Triple positivity (positive anticardiolipin, lupus anticoagulant, and anti-beta-2GPI) seems to be associated with the highest risk of thrombosis, with a 10-year cumulative incidence of 37.1% (95% confidence interval [CI] 19.9–54.3) for a first thrombotic event,32 and 44.2% (95% CI 38.6–49.8) for recurrent thrombosis.33
The association with thrombosis is stronger for lupus anticoagulant than with the other 2 antibodies, with different studies34 finding an odds ratio ranging from 5 to 16. A positive lupus anticoagulant test with or without a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a high-risk profile, while a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a moderate-risk profile. A low titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a low-risk profile that may not be associated with thrombosis.35
Antiphospholipid syndrome is important to recognize because of the need for long-term anticoagulation to prevent recurrence.36 It may be primary, when it occurs on its own, or secondary, when it occurs in association with another autoimmune disease such as lupus.
Venous events in antiphospholipid syndrome most commonly manifest as lower-limb deep vein thrombosis or pulmonary embolism, while arterial events most commonly manifest as stroke or transient ischemic attack.37 Obstetric manifestations may include not only miscarriage and stillbirth, but also preterm delivery, intrauterine growth retardation, and preeclampsia, all occurring due to placental insufficiency.
The frequency of antiphospholipid antibodies has been estimated as 13.5% in patients with stroke, 11% with myocardial infarction, 9.5% with deep vein thrombosis, and 6% for those with pregnancy morbidity.38
Some noncriteria manifestations have also been recognized in antiphospholipid syndrome, such as thrombocytopenia, cardiac vegetations (Libman-Sachs endocarditis), livedo reticularis, and nephropathy.
Back to our patient
Our patient’s anticardiolipin IgG test is negative, while her lupus anticoagulant and beta-2GPI IgG are positive. She has no clinical or laboratory features suggesting lupus.
She is started on warfarin. After 3 months, the warfarin is interrupted for several days, and she is retested for all 3 antiphospholipid antibodies. Her beta-2GPI I IgG and lupus anticoagulant tests are again positive. Because of the persistent antiphospholipid antibody positivity and clinical history of deep vein thrombosis, her condition is diagnosed as primary antiphospholipid syndrome. She is advised to continue anticoagulant therapy indefinitely.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY
A 34-year-old man who is an injecting drug user presents with a 2-week history of fever, malaise, and generalized arthralgia. There are no localizing symptoms of infection. Notable findings on examination include a temperature of 38.0°C (100.4°F), needle track marks in his arms, nonblanching vasculitic rash in his legs, and a systolic murmur over the precordium.
His white blood cell count is 15.3 × 109/L (reference range 3.7–11.0), and his C-reactive protein level is 234 mg/dL (normal < 3). Otherwise, results of blood cell counts, liver enzyme tests, renal function tests, urinalysis, and chest radiography are normal.
Two sets of blood cultures are drawn. Transthoracic echocardiography and the antineutrophil cytoplasmic antibody (ANCA) test are requested, as are screening tests for human immunodeficiency virus, hepatitis B, and hepatitis C.
Was the ANCA test indicated in this patient?
ANCAs are autoantibodies against antigens located in the cytoplasmic granules of neutrophils and monocytes. They are associated with small-vessel vasculitides such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and isolated pauciimmune crescentic glomerulonephritis, all collectively known as ANCA-associated vasculitis (AAV).39
Laboratory methods to detect ANCA include indirect immunofluorescence and antigen-specific enzyme immunoassays. Indirect immunofluorescence only tells us whether or not an antibody that is targeting a cytoplasmic antigen is present. Based on the indirect immunofluorescent pattern, ANCA can be classified as follows:
- Perinuclear or p-ANCA (if the targeted antigen is located just around the nucleus and extends into it)
- Cytoplasmic or c-ANCA (if the targeted antigen is located farther away from the nucleus)
- Atypical ANCA (if the indirect immunofluorescent pattern does not fit with either p-ANCA or c-ANCA).
Indirect immunofluorescence does not give information about the exact antigen that is targeted; this can only be obtained by performing 1 of the antigen-specific immunoassays. The target antigen for c-ANCA is usually proteinase-3 (PR3), while that for p-ANCA could be myeloperoxidase (MPO), cathepsin, lysozyme, lactoferrin, or bactericidal permeability inhibitor. Anti-PR3 is highly specific for GPA, while anti-MPO is usually associated with MPA and EGPA. Less commonly, anti-PR3 may be seen in patients with MPA and anti-MPO in those with GPA. Hence, there is an increasing trend toward classifying ANCA-associated vasculitis into PR3-associated or MPO-associated vasculitis rather than as GPA, MPA, EGPA, or renal-limited vasculitis.40
Several audits have shown that the ANCA test is widely misused and requested indiscriminately to rule out vasculitis. This results in a lower positive predictive value, possible harm to patients due to increased false-positive rates, and increased burden on the laboratory.41–43 At least 2 separate groups have demonstrated that a gating policy that refuses ANCA testing in patients without clinical evidence of systemic vasculitis can reduce the number of inappropriate requests, improve the diagnostic yield, and make it more clinically relevant and cost-effective.44,45
The clinician should bear in mind that:
Current guidelines recommend using one of the antigen-specific assays for PR3 and MPO as the primary screening method.48 Until recently, indirect immunofluorescence was used to screen for ANCA-associated vasculitis, and positive results were confirmed by ELISA to detect ANCAs specific for PR3 and MPO,49 but this is no longer recommended because of recent evidence suggesting a large variability between the different indirect immunofluorescent methods and improved diagnostic performance of the antigen-specific assays.
In a large multicenter study by Damoiseaux et al, the specificity with the different antigen-specific immunoassays was 98% to 99% for PR3-ANCA and 96% to 99% for MPO-ANCA.50
ANCA-associated vasculitis should not be considered excluded if the PR3 and MPO-ANCA are negative. In the Damoiseaux study, about 11% to 15% of patients with GPA and 8% to 24% of patients with MPA tested negative for both PR3 and MPO-ANCA.50
If the ANCA result is negative and clinical suspicion for ANCA-associated vasculitis is high, the clinician may wish to consider requesting another immunoassay method or indirect immunofluorescence. Results of indirect immunofluorescent testing results may be positive in those with a negative immunoassay, and vice versa.
Thus, the ANCA result should always be interpreted in the context of the whole clinical picture.51 Biopsy should still be considered the gold standard for the diagnosis of ANCA-associated vasculitis. The ANCA titer can help to improve clinical interpretation, because the likelihood of ANCA-associated vasculitis increases with higher levels of PR3 and MPO-ANCA.52
Back to our patient
Our patient’s blood cultures grow methicillin-sensitive Staphylococcus aureus in both sets after 48 hours. Transthoracic echocardiography reveals vegetations around the tricuspid valve, with no evidence of valvular regurgitation. The diagnosis is right-sided infective endocarditis. He is started on appropriate antibiotics.
Tests for human immunodeficiency virus, hepatitis B, and hepatitis C are negative. The ANCA test is positive for MPO-ANCA at 28 IU/mL (normal < 10).
The positive ANCA is thought to be related to the infective endocarditis. His vasculitis is most likely secondary to infective endocarditis and not ANCA-associated vasculitis. The ANCA test need not have been requested in the first place.
HUMAN LEUKOCYTE ANTIGEN-B27
A 22-year-old man presents to his primary care physician with a 4-month history of gradually worsening low back pain associated with early morning stiffness lasting more than 2 hours. He has no peripheral joint symptoms.
In the last 2 years, he has had 2 separate episodes of uveitis. There is a family history of ankylosing spondylitis in his father. Examination reveals global restriction of lumbar movements but is otherwise unremarkable. Magnetic resonance imaging (MRI) of the lumbar spine and sacroiliac joints is normal.
Should this patient be tested for human leukocyte antigen-B27 (HLA-B27)?
The major histocompatibility complex (MHC) is a gene complex that is present in all animals. It encodes proteins that help with immunologic tolerance. HLA simply refers to the human version of the MHC.53 The HLA gene complex, located on chromosome 6, is categorized into class I, class II, and class III. HLA-B is one of the 3 class I genes. Thus, a positive HLA-B27 result simply means that the particular gene is present in that person.
HLA-B27 is strongly associated with ankylosing spondylitis, also known as axial spondyloarthropathy.54 Other genes also contribute to the pathogenesis of ankylosing spondylitis, but HLA-B27 is present in more than 90% of patients with this disease and is by far considered the most important. The association is not as strong for peripheral spondyloarthropathy, with studies reporting a frequency of up to 75% for reactive arthritis and inflammatory bowel disease-associated arthritis, and up to 50% for psoriatic arthritis and uveitis.55
About 9% of healthy, asymptomatic individuals may have HLA-B27, so the mere presence of this gene is not evidence of disease.56 There may be up to a 20-fold increased risk of ankylosing spondylitis among those who are HLA-B27-positive.57
Some HLA genes have many different alleles, each of which is given a number (explaining the number 27 that follows the B). Closely related alleles that differ from one another by only a few amino-acid substitutions are then categorized together, thus accounting for more than 100 subtypes of HLA-B27 (designated from HLA-B*2701 to HLA-B*27106). These subtypes vary in frequency among different racial groups, and the population prevalence of ankylosing spondylitis parallels the frequency of HLA-B27.58 The most common subtype seen in white people and American Indians is B*2705. HLA-B27 is rare in blacks, explaining the rarity of ankylosing spondylitis in this population. Further examples include HLA-B*2704, which is seen in Asians, and HLA-B*2702, seen in Mediterranean populations. Not all subtypes of HLA-B27 are associated with disease, and some, like HLA-B*2706, may also be protective.
When should the clinician consider testing for HLA-B27?
Peripheral spondyloarthropathy may present with arthritis, enthesitis (eg, heel pain due to inflammation at the site of insertion of the Achilles tendon or plantar fascia), or dactylitis (“sausage” swelling of the whole finger or toe due to extension of inflammation beyond the margins of the joint). Other clues may include psoriasis, inflammatory bowel disease, history of preceding gastrointestinal or genitourinary infection, family history of similar conditions, and history of recurrent uveitis.
For the initial assessment of patients who have inflammatory back pain, plain radiography of the sacroiliac joints is considered the gold standard.59 If plain radiography does not show evidence of sacroiliitis, MRI of the sacroiliac joints should be considered. While plain radiography can reveal only structural changes such as sclerosis, erosions, and ankylosis, MRI is useful to evaluate for early inflammatory changes such as bone marrow edema. Imaging the lumbar spine is not necessary, as the sacroiliac joints are almost invariably involved in axial spondyloarthropathy, and lesions seldom occur in the lumbar spine in isolation.60
The diagnosis of ankylosing spondylitis previously relied on confirmatory imaging features, but based on the new International Society classification criteria,61–63 which can be applied to patients with more than 3 months of back pain and age of onset of symptoms before age 45, patients can be classified as having 1 of the following:
- Radiographic axial spondyloarthropathy, if they have evidence of sacroiliitis on imaging plus 1 other feature of spondyloarthropathy
- Nonradiographic axial spondyloarthropathy, if they have a positive HLA-B27 plus 2 other features of spondyloarthropathy (Table 7).
These new criteria have a sensitivity of 82.9% and specificity of 84.4%.62,63 The disease burden of radiographic and nonradiographic axial spondyloarthropathy has been shown to be similar, suggesting that they are part of the same disease spectrum. Thus, the HLA-B27 test is useful to make a diagnosis of axial spondyloarthropathy even in the absence of imaging features and could be requested in patients with 2 or more features of spondyloarthropathy. In the absence of imaging features and a negative HLA-B27 result, however, the patient cannot be classified as having axial spondyloarthropathy.
Back to our patient
The absence of radiographic evidence would not exclude axial spondyloarthropathy in our patient. The HLA-B27 test is requested because of the inflammatory back pain and the presence of 2 spondyloarthropathy features (uveitis and the family history) and is reported to be positive. His disease is classified as nonradiographic axial spondyloarthropathy.
He is started on regular naproxen and is referred to a physiotherapist. After 1 month, he reports significant symptomatic improvement. He asks if he can be retested for HLA-B27 to see if it has become negative. We tell him that there is no point in repeating it, as it is a gene and will not disappear.
SUMMARY: CONSIDER THE CLINICAL PICTURE
When approaching a patient suspected of having a rheumatologic disease, a clinician should first consider the clinical presentation and the intended purpose of each test. The tests, in general, might serve several purposes. They might help to:
Increase the likelihood of the diagnosis in question. For example, a positive rheumatoid factor or anticitrullinated peptide antibody can help diagnose rheumatoid arthritis in a patient with early polyarthritis, a positive HLA-B27 can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging, and a positive ANCA can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
Reduce the likelihood of the diagnosis in question. For example, a negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pains.
Monitor the condition. For example DNA antibodies can be used to monitor the activity of lupus.
Plan the treatment strategy. For example, one might consider lifelong anticoagulation if antiphospholipid antibodies are persistently positive in a patient with thrombosis.
Prognosticate. For example, positive rheumatoid factor and anticitrullinated peptide antibody increase the risk of erosive rheumatoid arthritis.
If the test was requested in the absence of a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test and not attach a diagnostic label prematurely. None of the tests can confirm or exclude a condition, so the results should always be interpreted in the context of the whole clinical picture.
Laboratory tests are often ordered inappropriately for patients in whom a rheumatologic illness is suspected; this occurs in both primary and secondary care.1 Some tests are available both singly and as part of a battery of tests screening healthy people without symptoms.
The problem: negative test results are by no means always reassuring, and false-positive results raise the risks of unnecessary anxiety for patients and clinicians, needless referrals, and potential morbidity due to further unnecessary testing and exposure to wrong treatments.2 Clinicians should be aware of the pitfalls of these tests in order to choose them wisely and interpret the results correctly.
This article provides practical guidance on requesting and interpreting some common tests in rheumatology, with the aid of case vignettes.
RHEUMATOID FACTOR AND ANTICITRULLINATED PEPTIDE ANTIBODY
A 41-year-old woman, previously in good health, presents to her primary care practitioner with a 6-week history of pain and swelling in her hands and early morning stiffness lasting about 2 hours. She denies having any extraarticular symptoms. Physical examination reveals synovitis across her right metacarpophalangeal joints, proximal interphalangeal joint of the left middle finger, and left wrist. The primary care physician is concerned that her symptoms might be due to rheumatoid arthritis.
Would testing for rheumatoid factor and anticitrullinated peptide antibody be useful in this patient?
Rheumatoid factor is an antibody (immunoglobulin M, IgG, or IgA) targeted against the Fc fragment of IgG.3 It was so named because it was originally detected in patients with rheumatoid arthritis, but it is neither sensitive nor specific for this condition. A meta-analysis of more than 5,000 patients with rheumatoid arthritis reported that rheumatoid factor testing had a sensitivity of 69% and specificity of 85%.4
Anticitrullinated peptide antibody, on the other hand, is much more specific for rheumatoid arthritis (95%), as it is seldom seen in other conditions, but its sensitivity is similar to that of rheumatoid factor (68%).4–6 A positive result would thus lend strength to the diagnosis of rheumatoid arthritis, but a negative result would not exclude it.
Approach to early arthritis
When faced with a patient with early arthritis, some key questions to ask include7,8:
Is this an inflammatory or a mechanical problem? Inflammatory arthritis is suggested by joint swelling that is not due to trauma or bony hypertrophy, early morning stiffness lasting longer than 30 minutes, and elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein). Involvement of the small joints of the hands and feet may be suggested by pain on compression of the metacarpophalangeal and metatarsophalangeal joints, respectively.
Is there a definite identifiable underlying cause for the inflammatory arthritis? The pattern of development of joint symptoms or the presence of extraarticular symptoms may suggest an underlying problem such as gout, psoriatic arthritis, systemic lupus erythematosus, or sarcoidosis.
If the arthritis is undifferentiated (ie, there is no definite identifiable cause), is it likely to remit or persist? This is perhaps the most important question to ask in order to prognosticate. Patients with risk factors for persistent disease, ie, for development of rheumatoid arthritis, should be referred to a rheumatologist early for timely institution of disease-modifying antirheumatic drug therapy.9 Multiple studies have shown that patients in whom this therapy is started early have much better clinical, functional, and radiologic outcomes than those in whom it is delayed.10–12
The revised American College of Rheumatology and European League Against Rheumatism criteria13 include the following factors as predictors of persistence:
- Number of involved joints (with greater weight given to involvement of small joints)
- Duration of symptoms 6 weeks or longer
- Elevated acute-phase response (erythrocyte sedimentation rate or C-reactive protein level)
- A positive serologic test (either rheumatoid factor or anticitrullinated peptide antibody).
If both rheumatoid factor and anticitrullinated peptide antibody are positive in a patient with early undifferentiated arthritis, the risk of progression to rheumatoid arthritis is almost 100%, thus underscoring the importance of testing for these antibodies.5,6 Referral to a rheumatologist should, however, not be delayed in patients with negative test results (more than one-third of patients with rheumatoid arthritis may be negative for both), and should be considered in those with inflammatory joint symptoms persisting longer than 6 weeks, especially with involvement of the small joints (sparing the distal interphalangeals) and elevated acute-phase response.
Rheumatoid factor in healthy people without symptoms
In some countries, testing for rheumatoid factor is offered as part of a battery of screening tests in healthy people who have no symptoms, a practice that should be strongly discouraged.
Multiple studies, both prospective and retrospective, have demonstrated that both rheumatoid factor and anticitrullinated peptide antibody may be present several years before the clinical diagnosis of rheumatoid arthritis.6,14–16 But the risk of developing rheumatoid arthritis for asymptomatic individuals who are rheumatoid factor-positive depends on the rheumatoid factor titer, positive family history of rheumatoid arthritis in first-degree relatives, and copresence of anticitrullinated peptide antibody. The absolute risk, nevertheless, is still very small. In some, there might be an alternative explanation such as undiagnosed Sjögren syndrome or hepatitis C.
In any event, no strategy is currently available that is proven to prevent the development of rheumatoid arthritis, and there is no role for disease-modifying therapy during the preclinical phase.16
Back to our patient
Blood testing in our patient reveals normal complete blood cell counts, aminotransferase levels, and serum creatinine concentration; findings on urinalysis are normal. Her erythrocyte sedimentation rate is 56 mm/hour (reference range 0–15), and her C-reactive protein level is 26 mg/dL (normal < 3). Testing is negative for rheumatoid factor and anticitrullinated peptide antibody.
Although her rheumatoid factor and anticitrullinated peptide antibody tests are negative, she is referred to a rheumatologist because she has predictors of persistent disease, ie, symptom duration of 6 weeks, involvement of the small joints of the hands, and elevated erythrocyte sedimentation rate and C-reactive protein. The rheumatologist checks her parvovirus serology, which is negative.
The patient is given parenteral depot corticosteroid therapy, to which she responds briefly. Because her symptoms persist and continue to worsen, methotrexate treatment is started after an additional 6 weeks.
ANTINUCLEAR ANTIBODY
A 37-year-old woman presents to her primary care physician with the complaint of tiredness. She has a family history of systemic lupus erythematosus in her sister and maternal aunt. She is understandably worried about lupus because of the family history and is asking to be tested for it.
Would testing for antinuclear antibody be reasonable?
Antinuclear antibody is not a single antibody but rather a family of autoantibodies that are directed against nuclear constituents such as single- or double-stranded deoxyribonucleic acid (dsDNA), histones, centromeres, proteins complexed with ribonucleic acid (RNA), and enzymes such as topoisomerase.17,18
Protein antigens complexed with RNA and some enzymes in the nucleus are also known as extractable nuclear antigens (ENAs). They include Ro, La, Sm, Jo-1, RNP, and ScL-70 and are named after the patient in whom they were first discovered (Robert, Lavine, Smith, and John), the antigen that is targeted (ribonucleoprotein or RNP), and the disease with which they are associated (anti-ScL-70 or antitopoisomerase in diffuse cutaneous scleroderma).
Antinuclear antibody testing is commonly requested to exclude connective tissue diseases such as lupus, but the clinician needs to be aware of the following points:
Antinuclear antibody may be encountered in conditions other than lupus
These include19:
- Other autoimmune diseases such as rheumatoid arthritis, primary Sjögren syndrome, systemic sclerosis, autoimmune thyroid disease, and myasthenia gravis
- Infection with organisms that share the epitope with self-antigens (molecular mimicry)
- Cancers
- Drugs such as hydralazine, procainamide, and minocycline.
Antinuclear antibody might also be produced by the healthy immune system from time to time to clear the nuclear debris that is extruded from aging cells.
A study in healthy individuals20 reported a prevalence of positive antinuclear antibody of 32% at a titer of 1/40, 15% at a titer of 1/80, 7% at a titer of 1/160, and 3% at a titer of 1/320. Importantly, a positive result was more common among family members of patients with autoimmune connective tissue diseases.21 Hence, a positive antinuclear antibody result does not always mean lupus.
Antinuclear antibody testing is highly sensitive for lupus
With current laboratory methods, antinuclear antibody testing has a sensitivity close to 100%. Hence, a negative result virtually rules out lupus.
Two methods are commonly used to test for antinuclear antibody: indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA).22 While human epithelial (Hep2) cells are used as the source of antigen in immunofluorescence, purified nuclear antigens coated on multiple-well plates are used in ELISA.
Although ELISA is simpler to perform, immunofluorescence has a slightly better sensitivity (because the Hep2 cells express a wide range of antigens) and is still considered the gold standard. As expected, the higher sensitivity occurs at the cost of reduced specificity (about 60%), so antinuclear antibody will also be detected in all the other conditions listed above.23
To improve the specificity of antinuclear antibody testing, laboratories report titers (the highest dilution of the test serum that tested positive); a cutoff of greater than 1/80 is generally considered significant.
Do not order antinuclear antibody testing indiscriminately
To sum up, the antinuclear antibody test should be requested only in patients with involvement of multiple organ systems. Although a negative result would make it extremely unlikely that the clinical presentation is due to lupus, a positive result is insufficient on its own to make a diagnosis of lupus.
Diagnosing lupus is straightforward when patients present with a specific manifestation such as inflammatory arthritis, photosensitive skin rash, hemolytic anemia, thrombocytopenia, or nephritis, or with specific antibodies such as those against dsDNA or Sm. Patients who present with nonspecific symptoms such as arthralgia or tiredness with a positive antinuclear antibody and negative anti-dsDNA and anti-Sm may present difficulties even for the specialist.25–27
Back to our patient
Our patient denies arthralgia. She has no extraarticular symptoms such as skin rashes, oral ulcers, sicca symptoms, muscle weakness, Raynaud phenomenon, pleuritic chest pain, or breathlessness. Findings on physical examination and urinalysis are unremarkable.
Her primary care physician decides to check her complete blood cell count, erythrocyte sedimentation rate, and thyroid-stimulating hormone level. Although she is reassured that her tiredness is not due to lupus, she insists on getting an antinuclear antibody test.
Her complete blood cell counts are normal. Her erythrocyte sedimentation rate is 6 mm/hour. However, her thyroid-stimulating hormone level is elevated, and subsequent testing shows low free thyroxine and positive thyroid peroxidase antibodies. The antinuclear antibody is positive in a titer of 1/80 and negative for anti-dsDNA and anti-ENA.
We explain to her that the positive antinuclear antibody is most likely related to her autoimmune thyroid disease. She is referred to an endocrinologist.
ANTIPHOSPHOLIPID ANTIBODIES
A 24-year-old woman presents to the emergency department with acute unprovoked deep vein thrombosis in her right leg, confirmed by ultrasonography. She has no history of previous thrombosis, and the relevant family history is unremarkable. She has never been pregnant. Her platelet count is 84 × 109/L (reference range 150–400), and her baseline activated partial thromboplastin time is prolonged at 62 seconds (reference range 23.0–32.4). The rest of her blood counts and her prothrombin time, liver enzyme levels, and serum creatinine level are normal.
Should this patient be tested for antiphospholipid antibodies?
Antiphospholipid antibodies are important because of their association with thrombotic risk (both venous and arterial) and pregnancy morbidity. The name is a misnomer, as these antibodies are targeted against some proteins that are bound to phospholipids and not only to the phospholipids themselves.
According to the modified Sapporo criteria for the classification of antiphospholipid syndrome,28 antiphospholipid antibodies should remain persistently positive on at least 2 separate occasions at least 12 weeks apart for the result to be considered significant because some infections and drugs may be associated with the transient presence of antiphospholipid antibodies.
Screening for antiphospholipid antibodies should include testing for IgM and IgG anticardiolipin antibodies, lupus anticoagulant, and IgM and IgG beta-2 glycoprotein I antibodies.29,30
Anticardiolipin antibodies
Anticardiolipin (aCL) antibodies may be targeted either against beta-2 glycoprotein I (beta-2GPI) that is bound to cardiolipin (a phospholipid) or against cardiolipin alone; the former is more specific. Antibodies directed against cardiolipin alone are usually transient and are associated with infections and drugs. The result is considered significant only when anticardiolipin antibodies are present in a medium to high titer (> 40 IgG phospholipid units or IgM phospholipid units, or > 99th percentile).
Lupus anticoagulant
The antibody with “lupus anticoagulant activity” is targeted against prothrombin plus phospholipid or beta-2GPI plus phospholipid. The test for it is a functional assay involving 3 steps:
Demonstrating the prolongation of a phospholipid-dependent coagulation assay like the activated partial thromboplastin time (aPTT). (This may explain the prolongation of aPTT in the patient described in the vignette.) Although the presence of lupus anticoagulant is associated with thrombosis, it is called an “anticoagulant” because of this in vitro prolongation of phospholipid-dependent coagulation assays.
Mixing study. The phospholipid-dependent coagulation assay could be prolonged because of either the deficiency of a coagulation factor or the presence of the antiphospholipid antibodies. This can be differentiated by mixing the patient’s plasma with normal plasma (which will have all the clotting factors) in a 1:1 ratio. If the coagulation assay remains prolonged after the addition of normal plasma, clotting factor deficiency can be excluded.
Addition of a phospholipid. If the prolongation of the coagulation assay is due to the presence of an antiphospholipid antibody, addition of extra phospholipid will correct this.
Beta-2 glycoprotein I antibody (anti-beta-2GPI)
The beta-2GPI that is not bound to the cardiolipin can be detected by separately testing for beta-2GPI (the anticardiolipin test only detects the beta-2GPI that is bound to the cardiolipin). The result is considered significant if beta-2GPI is present in a medium to high titer (> 99th percentile).
Studies have shown that antiphospholipid antibodies may be present in 1% to 5% of apparently healthy people in the general population.31 These are usually low-titer anticardiolipin or anti-beta-GPI IgM antibodies that are not associated with thrombosis or adverse pregnancy outcomes. Hence, the term antiphospholipid syndrome should be reserved for those who have had at least 1 episode of thrombosis or pregnancy morbidity and persistent antiphospholipid antibodies, and not those who have asymptomatic or transient antiphospholipid antibodies.
Triple positivity (positive anticardiolipin, lupus anticoagulant, and anti-beta-2GPI) seems to be associated with the highest risk of thrombosis, with a 10-year cumulative incidence of 37.1% (95% confidence interval [CI] 19.9–54.3) for a first thrombotic event,32 and 44.2% (95% CI 38.6–49.8) for recurrent thrombosis.33
The association with thrombosis is stronger for lupus anticoagulant than with the other 2 antibodies, with different studies34 finding an odds ratio ranging from 5 to 16. A positive lupus anticoagulant test with or without a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a high-risk profile, while a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a moderate-risk profile. A low titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a low-risk profile that may not be associated with thrombosis.35
Antiphospholipid syndrome is important to recognize because of the need for long-term anticoagulation to prevent recurrence.36 It may be primary, when it occurs on its own, or secondary, when it occurs in association with another autoimmune disease such as lupus.
Venous events in antiphospholipid syndrome most commonly manifest as lower-limb deep vein thrombosis or pulmonary embolism, while arterial events most commonly manifest as stroke or transient ischemic attack.37 Obstetric manifestations may include not only miscarriage and stillbirth, but also preterm delivery, intrauterine growth retardation, and preeclampsia, all occurring due to placental insufficiency.
The frequency of antiphospholipid antibodies has been estimated as 13.5% in patients with stroke, 11% with myocardial infarction, 9.5% with deep vein thrombosis, and 6% for those with pregnancy morbidity.38
Some noncriteria manifestations have also been recognized in antiphospholipid syndrome, such as thrombocytopenia, cardiac vegetations (Libman-Sachs endocarditis), livedo reticularis, and nephropathy.
Back to our patient
Our patient’s anticardiolipin IgG test is negative, while her lupus anticoagulant and beta-2GPI IgG are positive. She has no clinical or laboratory features suggesting lupus.
She is started on warfarin. After 3 months, the warfarin is interrupted for several days, and she is retested for all 3 antiphospholipid antibodies. Her beta-2GPI I IgG and lupus anticoagulant tests are again positive. Because of the persistent antiphospholipid antibody positivity and clinical history of deep vein thrombosis, her condition is diagnosed as primary antiphospholipid syndrome. She is advised to continue anticoagulant therapy indefinitely.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY
A 34-year-old man who is an injecting drug user presents with a 2-week history of fever, malaise, and generalized arthralgia. There are no localizing symptoms of infection. Notable findings on examination include a temperature of 38.0°C (100.4°F), needle track marks in his arms, nonblanching vasculitic rash in his legs, and a systolic murmur over the precordium.
His white blood cell count is 15.3 × 109/L (reference range 3.7–11.0), and his C-reactive protein level is 234 mg/dL (normal < 3). Otherwise, results of blood cell counts, liver enzyme tests, renal function tests, urinalysis, and chest radiography are normal.
Two sets of blood cultures are drawn. Transthoracic echocardiography and the antineutrophil cytoplasmic antibody (ANCA) test are requested, as are screening tests for human immunodeficiency virus, hepatitis B, and hepatitis C.
Was the ANCA test indicated in this patient?
ANCAs are autoantibodies against antigens located in the cytoplasmic granules of neutrophils and monocytes. They are associated with small-vessel vasculitides such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and isolated pauciimmune crescentic glomerulonephritis, all collectively known as ANCA-associated vasculitis (AAV).39
Laboratory methods to detect ANCA include indirect immunofluorescence and antigen-specific enzyme immunoassays. Indirect immunofluorescence only tells us whether or not an antibody that is targeting a cytoplasmic antigen is present. Based on the indirect immunofluorescent pattern, ANCA can be classified as follows:
- Perinuclear or p-ANCA (if the targeted antigen is located just around the nucleus and extends into it)
- Cytoplasmic or c-ANCA (if the targeted antigen is located farther away from the nucleus)
- Atypical ANCA (if the indirect immunofluorescent pattern does not fit with either p-ANCA or c-ANCA).
Indirect immunofluorescence does not give information about the exact antigen that is targeted; this can only be obtained by performing 1 of the antigen-specific immunoassays. The target antigen for c-ANCA is usually proteinase-3 (PR3), while that for p-ANCA could be myeloperoxidase (MPO), cathepsin, lysozyme, lactoferrin, or bactericidal permeability inhibitor. Anti-PR3 is highly specific for GPA, while anti-MPO is usually associated with MPA and EGPA. Less commonly, anti-PR3 may be seen in patients with MPA and anti-MPO in those with GPA. Hence, there is an increasing trend toward classifying ANCA-associated vasculitis into PR3-associated or MPO-associated vasculitis rather than as GPA, MPA, EGPA, or renal-limited vasculitis.40
Several audits have shown that the ANCA test is widely misused and requested indiscriminately to rule out vasculitis. This results in a lower positive predictive value, possible harm to patients due to increased false-positive rates, and increased burden on the laboratory.41–43 At least 2 separate groups have demonstrated that a gating policy that refuses ANCA testing in patients without clinical evidence of systemic vasculitis can reduce the number of inappropriate requests, improve the diagnostic yield, and make it more clinically relevant and cost-effective.44,45
The clinician should bear in mind that:
Current guidelines recommend using one of the antigen-specific assays for PR3 and MPO as the primary screening method.48 Until recently, indirect immunofluorescence was used to screen for ANCA-associated vasculitis, and positive results were confirmed by ELISA to detect ANCAs specific for PR3 and MPO,49 but this is no longer recommended because of recent evidence suggesting a large variability between the different indirect immunofluorescent methods and improved diagnostic performance of the antigen-specific assays.
In a large multicenter study by Damoiseaux et al, the specificity with the different antigen-specific immunoassays was 98% to 99% for PR3-ANCA and 96% to 99% for MPO-ANCA.50
ANCA-associated vasculitis should not be considered excluded if the PR3 and MPO-ANCA are negative. In the Damoiseaux study, about 11% to 15% of patients with GPA and 8% to 24% of patients with MPA tested negative for both PR3 and MPO-ANCA.50
If the ANCA result is negative and clinical suspicion for ANCA-associated vasculitis is high, the clinician may wish to consider requesting another immunoassay method or indirect immunofluorescence. Results of indirect immunofluorescent testing results may be positive in those with a negative immunoassay, and vice versa.
Thus, the ANCA result should always be interpreted in the context of the whole clinical picture.51 Biopsy should still be considered the gold standard for the diagnosis of ANCA-associated vasculitis. The ANCA titer can help to improve clinical interpretation, because the likelihood of ANCA-associated vasculitis increases with higher levels of PR3 and MPO-ANCA.52
Back to our patient
Our patient’s blood cultures grow methicillin-sensitive Staphylococcus aureus in both sets after 48 hours. Transthoracic echocardiography reveals vegetations around the tricuspid valve, with no evidence of valvular regurgitation. The diagnosis is right-sided infective endocarditis. He is started on appropriate antibiotics.
Tests for human immunodeficiency virus, hepatitis B, and hepatitis C are negative. The ANCA test is positive for MPO-ANCA at 28 IU/mL (normal < 10).
The positive ANCA is thought to be related to the infective endocarditis. His vasculitis is most likely secondary to infective endocarditis and not ANCA-associated vasculitis. The ANCA test need not have been requested in the first place.
HUMAN LEUKOCYTE ANTIGEN-B27
A 22-year-old man presents to his primary care physician with a 4-month history of gradually worsening low back pain associated with early morning stiffness lasting more than 2 hours. He has no peripheral joint symptoms.
In the last 2 years, he has had 2 separate episodes of uveitis. There is a family history of ankylosing spondylitis in his father. Examination reveals global restriction of lumbar movements but is otherwise unremarkable. Magnetic resonance imaging (MRI) of the lumbar spine and sacroiliac joints is normal.
Should this patient be tested for human leukocyte antigen-B27 (HLA-B27)?
The major histocompatibility complex (MHC) is a gene complex that is present in all animals. It encodes proteins that help with immunologic tolerance. HLA simply refers to the human version of the MHC.53 The HLA gene complex, located on chromosome 6, is categorized into class I, class II, and class III. HLA-B is one of the 3 class I genes. Thus, a positive HLA-B27 result simply means that the particular gene is present in that person.
HLA-B27 is strongly associated with ankylosing spondylitis, also known as axial spondyloarthropathy.54 Other genes also contribute to the pathogenesis of ankylosing spondylitis, but HLA-B27 is present in more than 90% of patients with this disease and is by far considered the most important. The association is not as strong for peripheral spondyloarthropathy, with studies reporting a frequency of up to 75% for reactive arthritis and inflammatory bowel disease-associated arthritis, and up to 50% for psoriatic arthritis and uveitis.55
About 9% of healthy, asymptomatic individuals may have HLA-B27, so the mere presence of this gene is not evidence of disease.56 There may be up to a 20-fold increased risk of ankylosing spondylitis among those who are HLA-B27-positive.57
Some HLA genes have many different alleles, each of which is given a number (explaining the number 27 that follows the B). Closely related alleles that differ from one another by only a few amino-acid substitutions are then categorized together, thus accounting for more than 100 subtypes of HLA-B27 (designated from HLA-B*2701 to HLA-B*27106). These subtypes vary in frequency among different racial groups, and the population prevalence of ankylosing spondylitis parallels the frequency of HLA-B27.58 The most common subtype seen in white people and American Indians is B*2705. HLA-B27 is rare in blacks, explaining the rarity of ankylosing spondylitis in this population. Further examples include HLA-B*2704, which is seen in Asians, and HLA-B*2702, seen in Mediterranean populations. Not all subtypes of HLA-B27 are associated with disease, and some, like HLA-B*2706, may also be protective.
When should the clinician consider testing for HLA-B27?
Peripheral spondyloarthropathy may present with arthritis, enthesitis (eg, heel pain due to inflammation at the site of insertion of the Achilles tendon or plantar fascia), or dactylitis (“sausage” swelling of the whole finger or toe due to extension of inflammation beyond the margins of the joint). Other clues may include psoriasis, inflammatory bowel disease, history of preceding gastrointestinal or genitourinary infection, family history of similar conditions, and history of recurrent uveitis.
For the initial assessment of patients who have inflammatory back pain, plain radiography of the sacroiliac joints is considered the gold standard.59 If plain radiography does not show evidence of sacroiliitis, MRI of the sacroiliac joints should be considered. While plain radiography can reveal only structural changes such as sclerosis, erosions, and ankylosis, MRI is useful to evaluate for early inflammatory changes such as bone marrow edema. Imaging the lumbar spine is not necessary, as the sacroiliac joints are almost invariably involved in axial spondyloarthropathy, and lesions seldom occur in the lumbar spine in isolation.60
The diagnosis of ankylosing spondylitis previously relied on confirmatory imaging features, but based on the new International Society classification criteria,61–63 which can be applied to patients with more than 3 months of back pain and age of onset of symptoms before age 45, patients can be classified as having 1 of the following:
- Radiographic axial spondyloarthropathy, if they have evidence of sacroiliitis on imaging plus 1 other feature of spondyloarthropathy
- Nonradiographic axial spondyloarthropathy, if they have a positive HLA-B27 plus 2 other features of spondyloarthropathy (Table 7).
These new criteria have a sensitivity of 82.9% and specificity of 84.4%.62,63 The disease burden of radiographic and nonradiographic axial spondyloarthropathy has been shown to be similar, suggesting that they are part of the same disease spectrum. Thus, the HLA-B27 test is useful to make a diagnosis of axial spondyloarthropathy even in the absence of imaging features and could be requested in patients with 2 or more features of spondyloarthropathy. In the absence of imaging features and a negative HLA-B27 result, however, the patient cannot be classified as having axial spondyloarthropathy.
Back to our patient
The absence of radiographic evidence would not exclude axial spondyloarthropathy in our patient. The HLA-B27 test is requested because of the inflammatory back pain and the presence of 2 spondyloarthropathy features (uveitis and the family history) and is reported to be positive. His disease is classified as nonradiographic axial spondyloarthropathy.
He is started on regular naproxen and is referred to a physiotherapist. After 1 month, he reports significant symptomatic improvement. He asks if he can be retested for HLA-B27 to see if it has become negative. We tell him that there is no point in repeating it, as it is a gene and will not disappear.
SUMMARY: CONSIDER THE CLINICAL PICTURE
When approaching a patient suspected of having a rheumatologic disease, a clinician should first consider the clinical presentation and the intended purpose of each test. The tests, in general, might serve several purposes. They might help to:
Increase the likelihood of the diagnosis in question. For example, a positive rheumatoid factor or anticitrullinated peptide antibody can help diagnose rheumatoid arthritis in a patient with early polyarthritis, a positive HLA-B27 can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging, and a positive ANCA can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
Reduce the likelihood of the diagnosis in question. For example, a negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pains.
Monitor the condition. For example DNA antibodies can be used to monitor the activity of lupus.
Plan the treatment strategy. For example, one might consider lifelong anticoagulation if antiphospholipid antibodies are persistently positive in a patient with thrombosis.
Prognosticate. For example, positive rheumatoid factor and anticitrullinated peptide antibody increase the risk of erosive rheumatoid arthritis.
If the test was requested in the absence of a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test and not attach a diagnostic label prematurely. None of the tests can confirm or exclude a condition, so the results should always be interpreted in the context of the whole clinical picture.
- American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 2002; 47(4):429–433. doi:10.1002/art.10381
- Rang M. The Ulysses syndrome. Can Med Assoc J 1972; 106(2):122–123. pmid:5058884
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35(6):727–734. doi:10.1155/2013/726598
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146(11):797–808. pmid:17548411
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038. doi:10.4061/2011/815038
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48(10):2741–2749. doi:10.1002/art.11223
- Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004; 97(9):421–424. doi:10.1258/jrsm.97.9.421
- Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61(4):290–297. pmid:11874828
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76(6):948–959. doi:10.1136/annrheumdis-2016-210602
- Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year follow up of a prospective double blind placebo controlled study. J Rheumatol 1995; 22(12):2208–2213. pmid:8835550
- van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124(8):699–707. pmid:8633829
- Andreson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9):2569–2581. doi:10.1002/art.27584
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50(2):380–386. doi:10.1002/art.20018
- del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988; 31(10):1239–1244. pmid:3178905
- Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010; 36(2):213–241. doi:10.1016/j.rdc.2010.02.001
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000; 124(1):71–81. doi:10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2
- Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br J Hosp Med (Lond) 2007; 68(10):538–541. doi:10.12968/hmed.2007.68.10.27324
- Illei GG, Klippel JH. Why is the ANA result positive? Bull Rheum Dis 1999; 48(1):1–4. pmid:10028188
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40(9):1601–1611. doi:10.1002/art.1780400909
- Langkilde H, Voss A, Heegaard N, Laustrup H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017; 26(7):723–728. doi:10.1177/0961203316676378
- Rondeel JM. Immunofluorescence versus ELISA for the detection of antinuclear antigens. Expert Rev Mol Diagn 2002; 2(3):226–232. doi:10.1586/14737159.2.3.226
- Solomon DH, Kavanaugh AJ, Schur PH; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002; 47(4):434–444. doi:10.1002/art.10561
- Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996; 156(13):1421–1425. pmid:8678710
- Maddison PJ. Is it SLE? Best Pract Res Clin Rheumatol 2002; 16(2):167–180. doi:10.1053/berh.2001.0219
- Price E, Walker E. Diagnostic vertigo: the journey to diagnosis in systemic lupus erythematosus. Health (London) 2014; 18(3):223–239. doi:10.1177/1363459313488008
- Blumenthal DE. Tired, aching, ANA-positive: does your patient have lupus or fibromyalgia? Cleve Clin J Med 2002; 69(2):143–146, 151–152. pmid:11990644
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157(1):47–58. doi:10.1111/j.1365-2141.2012.09037.x
- Giannakopoulos B, Passam F, Iannou Y, Krillis SA. How we diagnose the antiphospholipid syndrome. Blood 2009; 113(5):985–994. doi:10.1182/blood-2007-12-129627
- Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010; 9(5):A299–A304. doi:10.1016/j.autrev.2009.11.013
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118(17):4714–4718. doi:10.1182/blood-2011-03-340232
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8(2):237–242. doi:10.1111/j.1538-7836.2009.03674.x
- Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101(5):1827–1832. doi:10.1182/blood-2002-02-0441
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378(21):2010–2021. doi:10.1056/NEJMra1705454
- Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122(5):817–824. doi:10.1182/blood-2013-04-496257
- Cervera R. Lessons from the “Euro-Phospholipid” project. Autoimmun Rev 2008; 7(3):174–178. doi:10.1016/j.autrev.2007.11.011
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013; 65(11):1869–1873. doi:10.1002/acr.22066
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol 2010; 160(2):143–160. doi:10.1111/j.1365-2249.2009.04078.x
- Cornec D, Cornec-Le-Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016; 12(10):570–579. doi:10.1038/nrrheum.2016.123
- Edgar JD, McMillan SA, Bruce IN, Conlan SK. An audit of ANCA in routine clinical practice. Postgrad Med J 1995; 71(840):605–612. pmid:8545289
- McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic testing in a routine clinical setting. QJM 2001; 94(11):615–621. pmid:11704691
- Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch Intern Med 2002; 162(13):1509–1514. pmid:12090888
- Sinclair D, Saas M, Stevens JM. The effect of a symptom related “gated policy” on ANCA requests in routine clinical practice. J Clin Pathol 2004; 57(2):131–134. pmid:14747434
- Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol 2010; 63(8):678–680. doi:10.1136/jcp.2009.072504
- Savige J, Gills D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999; 111(4):507–513. pmid:10191771
- Robinson PC, Steele RH. Appropriateness of antineutrophil cytoplasmic antibody testing in a tertiary hospital. J Clin Pathol 2009; 62(8):743–745. doi:10.1136/jcp.2009.064485
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017; 13(11):683–692. doi:10.1038/nrrheum.2017.140
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53(3):743–753. doi:10.1046/j.1523-1755.1998.00807.x
- Damoiseaux J, Csemok E, Rasmussen N, et al. Detection of antineutrophil antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen specific immunoassays. Ann Rheum Dis 2017; 76(4):647–653. doi:10.1136/annrheumdis-2016-209507
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J 2006; 82(970):483–488. doi:10.1136/pgmj.2005.042648
- Vermeersch P, Blockmans D, Bossuyt X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin Chem 2009; 55(10):1886–1888. doi:10.1373/clinchem.2009.130583
- Bowness P. HLA-B27. Annu Rev Immunol 2015; 33:29–48. doi:10.1146/annurev-immunol-032414-112110
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
- Khan MA. Thoughts concerning the early diagnosis of ankylosing spondylitis and related diseases. Clin Exp Rheumatol 2002; 20(6 suppl 28):S6–S10. pmid:12463439
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41(1):58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984; 27(3):241–249. pmid:6608352
- Sheehan NJ. HLA-B27: what’s new? Rheumatology (Oxford) 2010; 49(4):621–631. doi:10.1093/rheumatology/kep450
- Baraliakos X, Maksymmowych WP. Imaging in the diagnosis and management of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2016; 30(4):608–623. doi:10.1016/j.berh.2016.09.011
- Mandl P, Navarro-Compan V, Terslev L, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74(7):1327–1339. doi:10.1136/annrheumdis-2014-206971
- McAllister K, Goodson N, Warburton I, Rogers G. Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 2017; 356:j839. doi:10.1136/bmj.j839
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74(8):1483–1487. doi:10.1136/annrheumdis-2014-207151
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68(6):777–783. doi:10.1136/ard.2009.108233
- American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 2002; 47(4):429–433. doi:10.1002/art.10381
- Rang M. The Ulysses syndrome. Can Med Assoc J 1972; 106(2):122–123. pmid:5058884
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35(6):727–734. doi:10.1155/2013/726598
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146(11):797–808. pmid:17548411
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038. doi:10.4061/2011/815038
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48(10):2741–2749. doi:10.1002/art.11223
- Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004; 97(9):421–424. doi:10.1258/jrsm.97.9.421
- Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61(4):290–297. pmid:11874828
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76(6):948–959. doi:10.1136/annrheumdis-2016-210602
- Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year follow up of a prospective double blind placebo controlled study. J Rheumatol 1995; 22(12):2208–2213. pmid:8835550
- van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124(8):699–707. pmid:8633829
- Andreson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9):2569–2581. doi:10.1002/art.27584
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50(2):380–386. doi:10.1002/art.20018
- del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988; 31(10):1239–1244. pmid:3178905
- Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010; 36(2):213–241. doi:10.1016/j.rdc.2010.02.001
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000; 124(1):71–81. doi:10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2
- Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br J Hosp Med (Lond) 2007; 68(10):538–541. doi:10.12968/hmed.2007.68.10.27324
- Illei GG, Klippel JH. Why is the ANA result positive? Bull Rheum Dis 1999; 48(1):1–4. pmid:10028188
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40(9):1601–1611. doi:10.1002/art.1780400909
- Langkilde H, Voss A, Heegaard N, Laustrup H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017; 26(7):723–728. doi:10.1177/0961203316676378
- Rondeel JM. Immunofluorescence versus ELISA for the detection of antinuclear antigens. Expert Rev Mol Diagn 2002; 2(3):226–232. doi:10.1586/14737159.2.3.226
- Solomon DH, Kavanaugh AJ, Schur PH; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002; 47(4):434–444. doi:10.1002/art.10561
- Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996; 156(13):1421–1425. pmid:8678710
- Maddison PJ. Is it SLE? Best Pract Res Clin Rheumatol 2002; 16(2):167–180. doi:10.1053/berh.2001.0219
- Price E, Walker E. Diagnostic vertigo: the journey to diagnosis in systemic lupus erythematosus. Health (London) 2014; 18(3):223–239. doi:10.1177/1363459313488008
- Blumenthal DE. Tired, aching, ANA-positive: does your patient have lupus or fibromyalgia? Cleve Clin J Med 2002; 69(2):143–146, 151–152. pmid:11990644
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157(1):47–58. doi:10.1111/j.1365-2141.2012.09037.x
- Giannakopoulos B, Passam F, Iannou Y, Krillis SA. How we diagnose the antiphospholipid syndrome. Blood 2009; 113(5):985–994. doi:10.1182/blood-2007-12-129627
- Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010; 9(5):A299–A304. doi:10.1016/j.autrev.2009.11.013
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118(17):4714–4718. doi:10.1182/blood-2011-03-340232
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8(2):237–242. doi:10.1111/j.1538-7836.2009.03674.x
- Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101(5):1827–1832. doi:10.1182/blood-2002-02-0441
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378(21):2010–2021. doi:10.1056/NEJMra1705454
- Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122(5):817–824. doi:10.1182/blood-2013-04-496257
- Cervera R. Lessons from the “Euro-Phospholipid” project. Autoimmun Rev 2008; 7(3):174–178. doi:10.1016/j.autrev.2007.11.011
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013; 65(11):1869–1873. doi:10.1002/acr.22066
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol 2010; 160(2):143–160. doi:10.1111/j.1365-2249.2009.04078.x
- Cornec D, Cornec-Le-Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016; 12(10):570–579. doi:10.1038/nrrheum.2016.123
- Edgar JD, McMillan SA, Bruce IN, Conlan SK. An audit of ANCA in routine clinical practice. Postgrad Med J 1995; 71(840):605–612. pmid:8545289
- McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic testing in a routine clinical setting. QJM 2001; 94(11):615–621. pmid:11704691
- Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch Intern Med 2002; 162(13):1509–1514. pmid:12090888
- Sinclair D, Saas M, Stevens JM. The effect of a symptom related “gated policy” on ANCA requests in routine clinical practice. J Clin Pathol 2004; 57(2):131–134. pmid:14747434
- Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol 2010; 63(8):678–680. doi:10.1136/jcp.2009.072504
- Savige J, Gills D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999; 111(4):507–513. pmid:10191771
- Robinson PC, Steele RH. Appropriateness of antineutrophil cytoplasmic antibody testing in a tertiary hospital. J Clin Pathol 2009; 62(8):743–745. doi:10.1136/jcp.2009.064485
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017; 13(11):683–692. doi:10.1038/nrrheum.2017.140
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53(3):743–753. doi:10.1046/j.1523-1755.1998.00807.x
- Damoiseaux J, Csemok E, Rasmussen N, et al. Detection of antineutrophil antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen specific immunoassays. Ann Rheum Dis 2017; 76(4):647–653. doi:10.1136/annrheumdis-2016-209507
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J 2006; 82(970):483–488. doi:10.1136/pgmj.2005.042648
- Vermeersch P, Blockmans D, Bossuyt X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin Chem 2009; 55(10):1886–1888. doi:10.1373/clinchem.2009.130583
- Bowness P. HLA-B27. Annu Rev Immunol 2015; 33:29–48. doi:10.1146/annurev-immunol-032414-112110
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
- Khan MA. Thoughts concerning the early diagnosis of ankylosing spondylitis and related diseases. Clin Exp Rheumatol 2002; 20(6 suppl 28):S6–S10. pmid:12463439
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41(1):58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984; 27(3):241–249. pmid:6608352
- Sheehan NJ. HLA-B27: what’s new? Rheumatology (Oxford) 2010; 49(4):621–631. doi:10.1093/rheumatology/kep450
- Baraliakos X, Maksymmowych WP. Imaging in the diagnosis and management of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2016; 30(4):608–623. doi:10.1016/j.berh.2016.09.011
- Mandl P, Navarro-Compan V, Terslev L, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74(7):1327–1339. doi:10.1136/annrheumdis-2014-206971
- McAllister K, Goodson N, Warburton I, Rogers G. Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 2017; 356:j839. doi:10.1136/bmj.j839
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74(8):1483–1487. doi:10.1136/annrheumdis-2014-207151
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68(6):777–783. doi:10.1136/ard.2009.108233
KEY POINTS
- If a test was requested without a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test; immunologic tests have limited specificity.
- A positive rheumatoid factor or anticitrullinated peptide antibody test can help diagnose rheumatoid arthritis in a patient with early polyarthritis.
- A positive HLA-B27 test can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging.
- Positive antinuclear cytoplasmic antibody (ANCA) can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
- A negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pain.
Bicuspid aortic valve: Basics and beyond
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
KEY POINTS
- Associated aortopathies such as aortic root dilation, aneurysm, dissection, and coarctation may initially be asymptomatic.
- Regular surveillance with transthoracic echocardiography (TTE) is required.
- Transesophageal echocardiography should be performed if TTE does not clearly show the aorta and aortic root. Magnetic resonance imaging or computed tomographic angiography may also be needed to measure the aortic root and ascending thoracic aorta.
- If initial imaging is normal and there is no aortic dilation, repeat imaging should be done every 5 to 10 years. If any abnormality is found, annual surveillance is needed.
- Women with a bicuspid aortic valve who are contemplating pregnancy should undergo echocardiography first, and some may need to undergo surgery.