User login
Osteoporosis management: Use a goal-oriented, individualized approach
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10
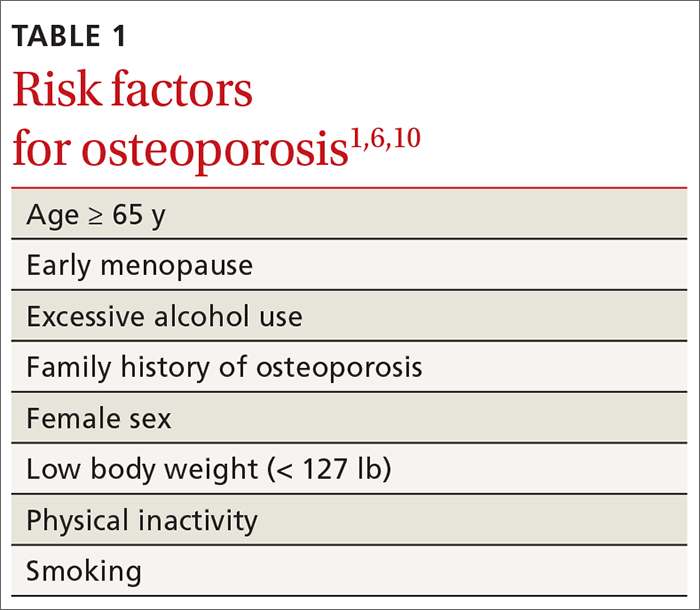
Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
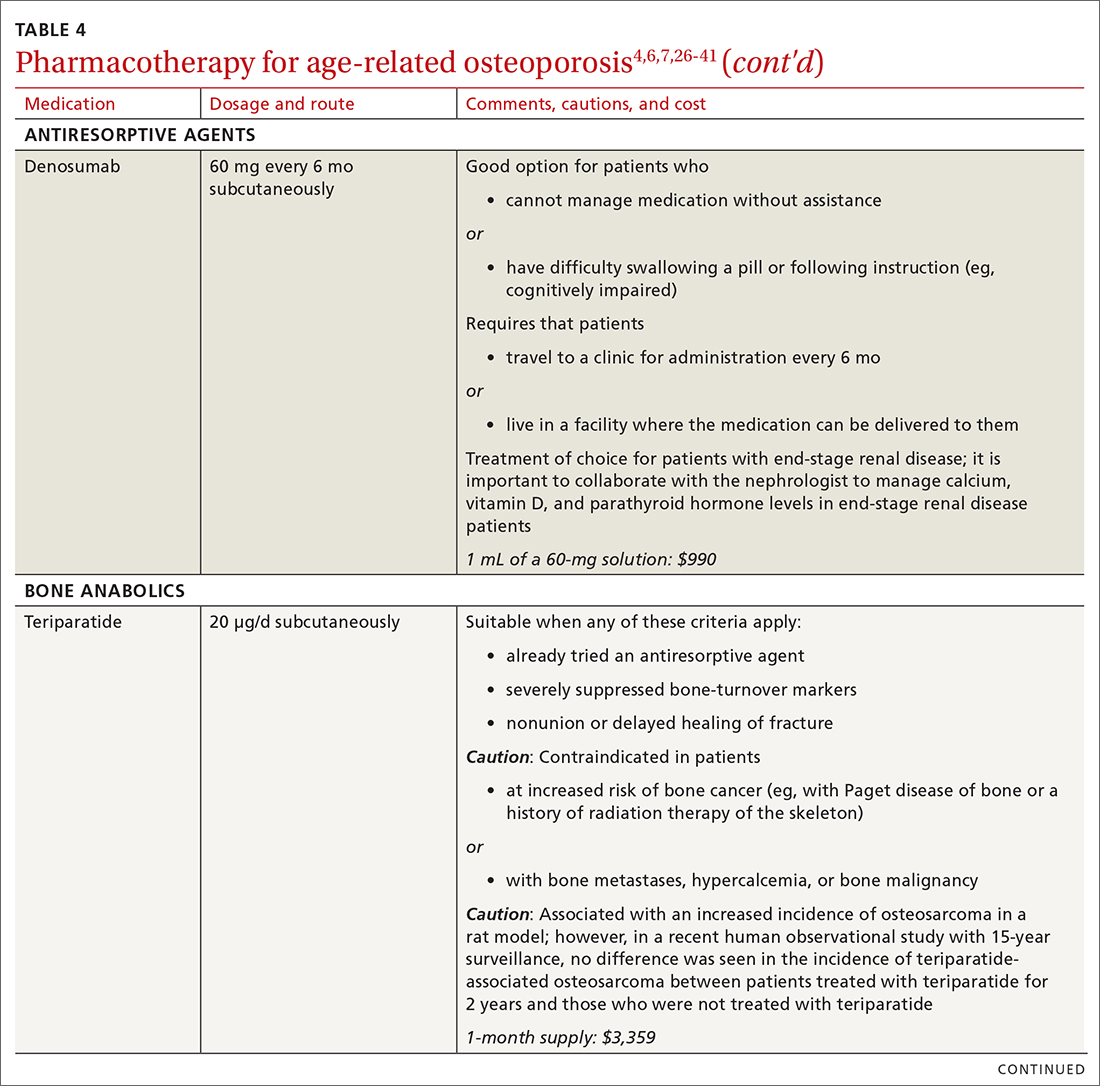
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)

❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
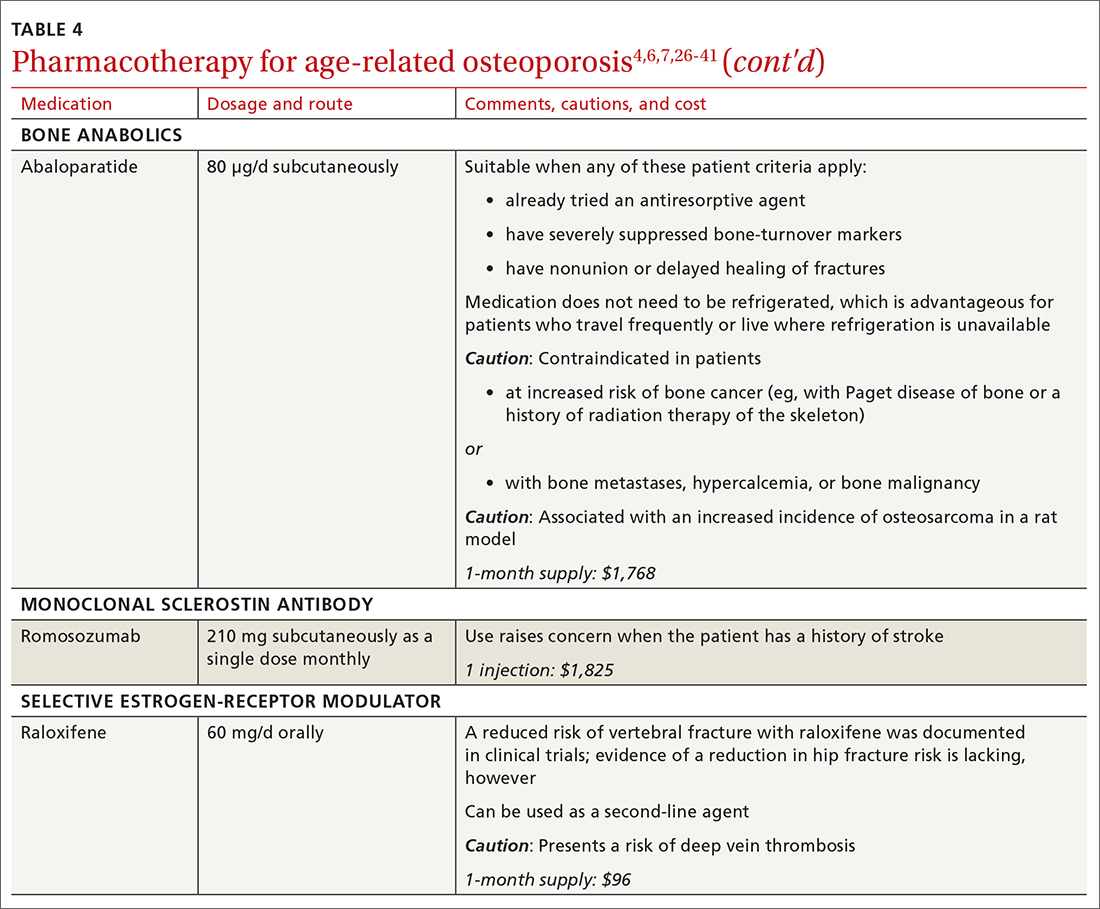
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
PRACTICE RECOMMENDATIONS
❯ Consider screening for osteoporosis, using bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA), in all postmenopausal women ≥ 65 years and in women < 65 years at high risk of osteoporosis.
❯ Consider screening in men ≥ 70 years and in younger men at high risk of fracture.
❯ Use the trabecular bone score with DXA BMD to screen patients at high risk of fracture who have a normal BMD—eg, patients with type 2 diabetes or ankylosing spondylitis.
❯ Offer individualized pharmacotherapy to older patients with a diagnosis of osteoporosis and to those at high risk of fracture.
Watchdog group demands removal of FDA leaders after aducanumab approval
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
The aducanumab revolution
The approval was hailed by advocacy groups and some practitioners as a victory for patients and families, as the drug – the first anti-Alzheimer’s agent to reach the market in 18 years – is a potentially disease-modifying therapy, which acts to clear amyloid plaques from the brain.
But several prominent Alzheimer’s researchers lambasted the agency’s decision, citing unclear evidence of benefit, trials that did not meet their primary endpoints, and reliance on a post hoc analysis of a high-dose subgroup of patients in a halted trial to argue that aducanumab (Aduhelm, Biogen, and Eisai), slowed cognitive and functional decline by 22% on one measure. In November 2020, 10 of 11 members of an independent FDA advisory committee voted against aducanumab’s approval, citing holes in the data and concerns about the quality of the evidence. After the agency went on to approve anyway, three members of that committee resigned in protest.
The FDA decision on aducanumab was made using the agency’s accelerated approval pathway, which allows for the use of a surrogate endpoint – in this case imaging that showed amyloid clearance from the brain – to predict clinical benefit. But amyloid clearance, which a number of experimental antiamyloid antibodies have been shown capable of, has not been definitively linked to clinical benefit. Aducanumab, which is delivered by monthly intravenous infusion, will be marketed pending results from a phase 4 clinical trial, which the manufacturer has nearly a decade to complete. The drug’s price was announced at $56,000 per year, underscoring concern over its modest-at-best benefits.
Clinicians prescribing aducanumab must obtain magnetic resonance imaging at baseline and repeatedly during the course of treatment to detect brain edema and microhemorrhages, which occurred in a third of high-dose patients in clinical trials. Beyond this, there are few restrictions. The FDA label allows for its use in any patient deemed to have Alzheimer’s disease, without stipulations as to disease stage or evidence of brain amyloid. Payers, of course, are likely to restrict use to certain patient groups, and to require evidence of amyloid positivity. The FDA offered no guidance on when treatment should be ceased, leaving payers to make that call as well. Whatever aducanumab’s value and role turns out to be, the first-in-class treatment for Alzheimer’s disease is likely to have a major impact on how patients are assessed and treated in the coming years, and embolden manufactures of similar agents to seek FDA approval.
This news organization reached out to researchers, advocates, and specialists in the community to learn how they see this change playing out.
Fielding broad interest
Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association, which was a strong proponent of aducanumab’s approval, acknowledged in an interview that the months to come are likely to be confusing for practitioners and families alike as the drug makes its way into community practices.
“We understand that off the bat millions of Americans will not have access to this tomorrow, but over time that will build. And the physician community, the specialists most likely to be prescribing this, over the next few years will even expand further,” Dr. Carrillo said.
For now, those specialists are mostly just struggling to respond responsibly to a deluge of inquiries from patients and their families.
“I’ve gotten like 20 calls in the just the past 2 days,” said neurologist Philip R. Delio, MD, who practices in Santa Barbara, Calif. “This is a longstanding issue that physicians have with patients’ access to information. Patients are getting information about a drug which isn’t available yet. They don’t know that it’s not ready to be sold. They don’t necessarily realize that a biopharma company won’t go into production until the FDA approves the drug.”
Many patients, Dr. Delio said, are aware of the controversy surrounding aducanumab and eager to hear their neurologist’s opinion. “I have tried to let them know that I want to see the trial data and to better understand the FDA’s rationale in approving it. I always caution patients that the devil will be in the details.”
While aducanumab’s label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all but meaningless. Neurologist Douglas Scharre, MD, of the Ohio State University Wexner Medical Center, and a site investigator on a trial of aducanumab, said that he and his colleagues at the university’s memory center have tried to anticipate who might be deemed eligible by triaging calls.
Dr. Scharre and colleagues have been working under the assumption that payers will support aducanumab only for patients like those who seemed to benefit in the trials – people with mild cognitive impairment (MCI) or in the earliest stages of dementia with evidence of brain amyloid.
“I don’t want to fill up our new patient slots with people who are not even appropriate for this drug,” Dr. Scharre said. “We have a call center, and we have a few triage questions. After that a nurse practitioner collects some more data, and there’s a review process. Only then do we decide whether that person could be a candidate. If we deem that they are, we will want them in and to order an amyloid PET” – a type of brain scan that is seldom used outside research settings and not reimbursed by Medicare.
Dr. Scharre predicts that regardless of payer limitations, “there will be people hounding for the drug who are not appropriate for the drug. There will be very wealthy people who will want to pay for tests and get it no matter what.” Another concern, he said, was that having poorly selected patients on the drug could make definitive trial results even more elusive.
“The label the way it’s written is not going to help the drug in phase 4 trials,” he said. “It’s good to have real-world patient data, but if you have all these people in your cohort who are too early or too late, you won’t have good results.”
The challenge of delivery
Intravenous infusions are new to Alzheimer’s disease and pose all sorts of logistical hurdles. The Alzheimer’s Association’s Dr. Carrillo described the situation as “manageable,” noting that infusions are standard of care for many diseases, and that neurologists now have more than 15 years’ experience with them for multiple sclerosis.
Still, most clinicians treating Alzheimer’s disease in the community – neurologists, geriatricians, psychiatrists, and primary care physicians – do not have infusion centers in their practices. Virtually none have experience with or access to PET-amyloid, or with screening for amyloid-related imaging abnormalities–edema (ARIA-e) on MRI, as required by the FDA.
“I contacted the hospital infusion center we use and said I could end up sending five or six patients a week, can you handle this? They only have so many chairs,” Dr. Delio said. “I am one neurologist in a local community, and I might have 50 candidates for this drug. That’s a lot for them.” Patients with cognitive impairment are also difficult to infuse and may need to be treated at home, he noted.
“MRIs are easy enough to do,” Dr. Delio said. “But do we know what ARIA-e looks like on imaging? You’d have to talk to the radiologists – this is another element of uncertainty. Do we even know what we’re looking for with these scans? Will we recognize this?”
Neurologist Jeffrey L. Cummings, MD, ScD, of the University of Nevada, Las Vegas, a vocal proponent of aducanumab and lead author of a May 2021 paper defending the evidence for it, acknowledged that the field was unprepared for a wide-scale adoption of infusions in dementia treatment, pointing to a Rand Corporation study from 2017 that warned that screening, diagnosis, and availability of infusion chairs would have to be drastically scaled up to meet demand.
“There are few clinicians who know how to identify MCI, too few imaging centers, too few radiologists who know how to identify ARIA-e on MRI, so all of these things will be required to be put into place. The label doesn’t specify any of this, but good clinical practice will require that, and getting this up and running will take 18 to 24 months,” Dr. Cummings said.
Neurologist David S. Knopman, MD, of the Mayo Clinic in Rochester, Minn., a leading critic of the evidence for aducanumab who recently resigned his position on the independent committee that advises the FDA on neurology drugs, said that for large research institutions like his that have served as trial sites, the transition to offering PET-amyloid, MRI, and infusions in clinical practice will be easier.
“We have all this because this is what we do every day. And we have a very extensive understanding of MCI and mild dementia staging,” Dr. Knopman said. “But the amount of infrastructure that is implied by this, and all the extra steps it would take, would be a real challenge for people in general neurology practice.”
In addition to routine use of PET-amyloid and MRI screening for ARIA-e, Dr. Knopman said, clinicians will have to provide genetic screening and counseling before administering aducanumab, as clinical trials showed that treated patients have a higher risk of developing ARIA-e if they have APOE4, a risk variant for Alzheimer’s disease. “And that has real implications for the families and the children of patients,” he said.
Uncertainty over costs
Aducanumab’s true costs, to patients and to taxpayers, remain unknown. The $56,000 per year currently cited by its manufacturer “doesn’t count the PET scans and MRIs,” Dr. Knopman noted. “We’re probably pushing $100,00 a year for the first year of treatment.”
Most of that expense will likely be borne by Medicare, he said, and if not, “that will exacerbate existing health care disparities. People who can pay out of pocket are a pretty limited group.”
Dr. Scharre agreed that the costs of treatment were concerning, and that “at least you should be able to narrow it down and hopefully just use health care dollars for people who might stand to benefit,” he said – namely patients in an earlier stage of disease.
The Alzheimer’s Association’s Dr. Carrillo declined to address the high price of aducanumab or its implications, saying only that the association is “very invested in all aspects of access including covering costs associated with the drug and the rest of treatment.”
Access also means “infrastructure, access to physicians to diagnose, access to diagnostics,” Dr. Carrillo said.
Dr. Cummings said aducanumab’s price would likely come down through negotiations with the Centers for Medicare & Medicaid Services, copayments, and bulk purchases.
The FDA has offered no guidance on how long treatment with aducanumab should last, or what should prompt withdrawal of treatment, meaning that patients could, in theory, stay on it to the end of their lives – raising costs further.
Critics have also noted that a built-in financial incentive under Medicare Part B, which covers infusion drugs, could result in overprescription of aducanumab. Under Medicare Part B, prescribing physicians are reimbursed 6% of a drug’s average sales price.
Geriatricians wary
On social media and in the lay press, geriatricians have been among the most outspoken opponents of the FDA decision and the Alzheimer’s Association’s advocacy of aducanumab.
Eric Widera, MD, a geriatrician at the University of California, San Francisco, said that the specialty might be less likely than others to embrace aducanumab. “I think part of the reasons geriatricians don’t make a lot of money is they have strong commitment to their values,” Dr. Widera said.
The American Geriatrics Society opposed the drug’s approval, citing concerns about evidence, side effects, and cost. “Additional considerations are the unintended consequences of overstressing Medicare’s limited financial reserves, and of challenging health care systems … to divert precious resources to an expensive treatment of uncertain value,” the society’s president, Peter Hollmann, MD, and chief executive officer, Nancy E. Lundebjerg, wrote in a June 2 letter to the FDA.
Dr. Widera said the approval was likely to undermine confidence in the FDA and in the Alzheimer’s Association, which receives significant funding from drug manufacturers, including Biogen and Eisai. “There’s a lot of reasons that the Geriatrics Society could have done what the Alzheimer’s Association did, and yet they came out against it, which I applaud.”
Dr. Widera pointed to a study showing that dementia patients were less likely to be on an antidementia drug if they were treated by a geriatrician, compared with a psychiatrist or a neurologist. But whether the specialty will prove as cautious with aducanumab remains to be seen. Some geriatricians will be tempted to open lucrative infusion centers, he predicted.
What is especially worrisome, Dr. Widera said, is that aducanumab’s label offers no guidance as to when to withdraw treatment. “We’ll probably see something similar to what happened with the cholinesterase inhibitors” – the class of marginally effective antidementia drugs that includes donepezil (Aricept, Pfizer) and rivastigmine (Exelon, Novartis). “No one thinks about deprescribing them. People are prescribed them even in their last months of life. There is no reason to think these infusions won’t be continued for a very long time, well beyond how long people were dosed in the trials.”
“Taking care of someone with dementia is hard enough,” Dr. Widera added. “We can’t even get normal support in the home for someone with dementia. But we are more than happy to throw money to Biogen for a drug they have not yet showed benefit for. Hopefully in 5 years we’ll have a drug that actually works,” Dr. Widera said. “After 5 years of giving this to people at $50,000 a year.”
A fractured research community
Ever since October 2019, when Biogen and Eisai announced that despite two trials halted for futility, they would go ahead and seek FDA approval for aducanumab, the Alzheimer’s research community has been bitterly divided over the drug and the FDA’s accelerated approval process.
Top researchers published critical editorials in journals, with some eventually taking their case to major newspapers as well. The Alzheimer’s Association’s position on the drug has clashed with that of many researchers whose work it supports.
“The Alzheimer’s community has been wonderfully collegial – we all have a common purpose,” Dr. Cummings said. “Now we have people taking extreme positions and I’m hoping this will not result in a permanent fracturing of the community.”
Chief among the critics’ concerns is that the FDA decision ratified the use of antiamyloid therapies based on biomarker evidence, opening the door for makers of similar drugs – those still under development or even those whose development has been halted – to seek approval on weak evidence of clinical benefit.
Whether the approval will chill research into drugs targeting pathways other than amyloid is uncertain.
Dr. Cummings said he felt that while the aducanumab decision would spur other manufacturers of antiamyloid drugs to seek accelerated approval, other classes of Alzheimer’s therapies in development also stand to get a boost. Many Alzheimer’s experts believe that a combination of drugs targeting different elements of the disease pathway – not just amyloid – will be needed in the long run.
Dr. Scharre said that the buzz over aducanumab’s approval will have at least one concrete benefit: people getting into doctors’ offices sooner.
“The people who come into our memory centers represent only a fraction of people walking around with MCI – there are people out there who may have heard that it’s normal aging; they have decreased insight; there’s denial, there’s embarrassment – there’s hundreds of reasons people avoid getting seen,” he said.
“Perhaps they come in and learn that they don’t have any degenerative process but their thyroid is out of whack, or there’s something else causing cognitive impairment. And if they do have a degenerative process, they’ll have time to start [aducanumab], and hopefully get to see a reduction in the decline.”
Dr. Knopman was a site investigator for the Biogen aducanumab trials and has consulted for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences. A former member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, he was recused from the Nov. 6, 2020, meeting that voted against aducanumab. Dr. Cummings has consulted for Biogen, Eisai, and other manufacturers. Dr. Scharre reports financial relationships with Biogen, Brain Test, Acadia, and Vascular Scientific. Dr. Widera has no disclosures. Dr. Delio is a speaker for Gore Medical, Allergan, and Biohaven Pharmaceuticals.
The approval was hailed by advocacy groups and some practitioners as a victory for patients and families, as the drug – the first anti-Alzheimer’s agent to reach the market in 18 years – is a potentially disease-modifying therapy, which acts to clear amyloid plaques from the brain.
But several prominent Alzheimer’s researchers lambasted the agency’s decision, citing unclear evidence of benefit, trials that did not meet their primary endpoints, and reliance on a post hoc analysis of a high-dose subgroup of patients in a halted trial to argue that aducanumab (Aduhelm, Biogen, and Eisai), slowed cognitive and functional decline by 22% on one measure. In November 2020, 10 of 11 members of an independent FDA advisory committee voted against aducanumab’s approval, citing holes in the data and concerns about the quality of the evidence. After the agency went on to approve anyway, three members of that committee resigned in protest.
The FDA decision on aducanumab was made using the agency’s accelerated approval pathway, which allows for the use of a surrogate endpoint – in this case imaging that showed amyloid clearance from the brain – to predict clinical benefit. But amyloid clearance, which a number of experimental antiamyloid antibodies have been shown capable of, has not been definitively linked to clinical benefit. Aducanumab, which is delivered by monthly intravenous infusion, will be marketed pending results from a phase 4 clinical trial, which the manufacturer has nearly a decade to complete. The drug’s price was announced at $56,000 per year, underscoring concern over its modest-at-best benefits.
Clinicians prescribing aducanumab must obtain magnetic resonance imaging at baseline and repeatedly during the course of treatment to detect brain edema and microhemorrhages, which occurred in a third of high-dose patients in clinical trials. Beyond this, there are few restrictions. The FDA label allows for its use in any patient deemed to have Alzheimer’s disease, without stipulations as to disease stage or evidence of brain amyloid. Payers, of course, are likely to restrict use to certain patient groups, and to require evidence of amyloid positivity. The FDA offered no guidance on when treatment should be ceased, leaving payers to make that call as well. Whatever aducanumab’s value and role turns out to be, the first-in-class treatment for Alzheimer’s disease is likely to have a major impact on how patients are assessed and treated in the coming years, and embolden manufactures of similar agents to seek FDA approval.
This news organization reached out to researchers, advocates, and specialists in the community to learn how they see this change playing out.
Fielding broad interest
Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association, which was a strong proponent of aducanumab’s approval, acknowledged in an interview that the months to come are likely to be confusing for practitioners and families alike as the drug makes its way into community practices.
“We understand that off the bat millions of Americans will not have access to this tomorrow, but over time that will build. And the physician community, the specialists most likely to be prescribing this, over the next few years will even expand further,” Dr. Carrillo said.
For now, those specialists are mostly just struggling to respond responsibly to a deluge of inquiries from patients and their families.
“I’ve gotten like 20 calls in the just the past 2 days,” said neurologist Philip R. Delio, MD, who practices in Santa Barbara, Calif. “This is a longstanding issue that physicians have with patients’ access to information. Patients are getting information about a drug which isn’t available yet. They don’t know that it’s not ready to be sold. They don’t necessarily realize that a biopharma company won’t go into production until the FDA approves the drug.”
Many patients, Dr. Delio said, are aware of the controversy surrounding aducanumab and eager to hear their neurologist’s opinion. “I have tried to let them know that I want to see the trial data and to better understand the FDA’s rationale in approving it. I always caution patients that the devil will be in the details.”
While aducanumab’s label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all but meaningless. Neurologist Douglas Scharre, MD, of the Ohio State University Wexner Medical Center, and a site investigator on a trial of aducanumab, said that he and his colleagues at the university’s memory center have tried to anticipate who might be deemed eligible by triaging calls.
Dr. Scharre and colleagues have been working under the assumption that payers will support aducanumab only for patients like those who seemed to benefit in the trials – people with mild cognitive impairment (MCI) or in the earliest stages of dementia with evidence of brain amyloid.
“I don’t want to fill up our new patient slots with people who are not even appropriate for this drug,” Dr. Scharre said. “We have a call center, and we have a few triage questions. After that a nurse practitioner collects some more data, and there’s a review process. Only then do we decide whether that person could be a candidate. If we deem that they are, we will want them in and to order an amyloid PET” – a type of brain scan that is seldom used outside research settings and not reimbursed by Medicare.
Dr. Scharre predicts that regardless of payer limitations, “there will be people hounding for the drug who are not appropriate for the drug. There will be very wealthy people who will want to pay for tests and get it no matter what.” Another concern, he said, was that having poorly selected patients on the drug could make definitive trial results even more elusive.
“The label the way it’s written is not going to help the drug in phase 4 trials,” he said. “It’s good to have real-world patient data, but if you have all these people in your cohort who are too early or too late, you won’t have good results.”
The challenge of delivery
Intravenous infusions are new to Alzheimer’s disease and pose all sorts of logistical hurdles. The Alzheimer’s Association’s Dr. Carrillo described the situation as “manageable,” noting that infusions are standard of care for many diseases, and that neurologists now have more than 15 years’ experience with them for multiple sclerosis.
Still, most clinicians treating Alzheimer’s disease in the community – neurologists, geriatricians, psychiatrists, and primary care physicians – do not have infusion centers in their practices. Virtually none have experience with or access to PET-amyloid, or with screening for amyloid-related imaging abnormalities–edema (ARIA-e) on MRI, as required by the FDA.
“I contacted the hospital infusion center we use and said I could end up sending five or six patients a week, can you handle this? They only have so many chairs,” Dr. Delio said. “I am one neurologist in a local community, and I might have 50 candidates for this drug. That’s a lot for them.” Patients with cognitive impairment are also difficult to infuse and may need to be treated at home, he noted.
“MRIs are easy enough to do,” Dr. Delio said. “But do we know what ARIA-e looks like on imaging? You’d have to talk to the radiologists – this is another element of uncertainty. Do we even know what we’re looking for with these scans? Will we recognize this?”
Neurologist Jeffrey L. Cummings, MD, ScD, of the University of Nevada, Las Vegas, a vocal proponent of aducanumab and lead author of a May 2021 paper defending the evidence for it, acknowledged that the field was unprepared for a wide-scale adoption of infusions in dementia treatment, pointing to a Rand Corporation study from 2017 that warned that screening, diagnosis, and availability of infusion chairs would have to be drastically scaled up to meet demand.
“There are few clinicians who know how to identify MCI, too few imaging centers, too few radiologists who know how to identify ARIA-e on MRI, so all of these things will be required to be put into place. The label doesn’t specify any of this, but good clinical practice will require that, and getting this up and running will take 18 to 24 months,” Dr. Cummings said.
Neurologist David S. Knopman, MD, of the Mayo Clinic in Rochester, Minn., a leading critic of the evidence for aducanumab who recently resigned his position on the independent committee that advises the FDA on neurology drugs, said that for large research institutions like his that have served as trial sites, the transition to offering PET-amyloid, MRI, and infusions in clinical practice will be easier.
“We have all this because this is what we do every day. And we have a very extensive understanding of MCI and mild dementia staging,” Dr. Knopman said. “But the amount of infrastructure that is implied by this, and all the extra steps it would take, would be a real challenge for people in general neurology practice.”
In addition to routine use of PET-amyloid and MRI screening for ARIA-e, Dr. Knopman said, clinicians will have to provide genetic screening and counseling before administering aducanumab, as clinical trials showed that treated patients have a higher risk of developing ARIA-e if they have APOE4, a risk variant for Alzheimer’s disease. “And that has real implications for the families and the children of patients,” he said.
Uncertainty over costs
Aducanumab’s true costs, to patients and to taxpayers, remain unknown. The $56,000 per year currently cited by its manufacturer “doesn’t count the PET scans and MRIs,” Dr. Knopman noted. “We’re probably pushing $100,00 a year for the first year of treatment.”
Most of that expense will likely be borne by Medicare, he said, and if not, “that will exacerbate existing health care disparities. People who can pay out of pocket are a pretty limited group.”
Dr. Scharre agreed that the costs of treatment were concerning, and that “at least you should be able to narrow it down and hopefully just use health care dollars for people who might stand to benefit,” he said – namely patients in an earlier stage of disease.
The Alzheimer’s Association’s Dr. Carrillo declined to address the high price of aducanumab or its implications, saying only that the association is “very invested in all aspects of access including covering costs associated with the drug and the rest of treatment.”
Access also means “infrastructure, access to physicians to diagnose, access to diagnostics,” Dr. Carrillo said.
Dr. Cummings said aducanumab’s price would likely come down through negotiations with the Centers for Medicare & Medicaid Services, copayments, and bulk purchases.
The FDA has offered no guidance on how long treatment with aducanumab should last, or what should prompt withdrawal of treatment, meaning that patients could, in theory, stay on it to the end of their lives – raising costs further.
Critics have also noted that a built-in financial incentive under Medicare Part B, which covers infusion drugs, could result in overprescription of aducanumab. Under Medicare Part B, prescribing physicians are reimbursed 6% of a drug’s average sales price.
Geriatricians wary
On social media and in the lay press, geriatricians have been among the most outspoken opponents of the FDA decision and the Alzheimer’s Association’s advocacy of aducanumab.
Eric Widera, MD, a geriatrician at the University of California, San Francisco, said that the specialty might be less likely than others to embrace aducanumab. “I think part of the reasons geriatricians don’t make a lot of money is they have strong commitment to their values,” Dr. Widera said.
The American Geriatrics Society opposed the drug’s approval, citing concerns about evidence, side effects, and cost. “Additional considerations are the unintended consequences of overstressing Medicare’s limited financial reserves, and of challenging health care systems … to divert precious resources to an expensive treatment of uncertain value,” the society’s president, Peter Hollmann, MD, and chief executive officer, Nancy E. Lundebjerg, wrote in a June 2 letter to the FDA.
Dr. Widera said the approval was likely to undermine confidence in the FDA and in the Alzheimer’s Association, which receives significant funding from drug manufacturers, including Biogen and Eisai. “There’s a lot of reasons that the Geriatrics Society could have done what the Alzheimer’s Association did, and yet they came out against it, which I applaud.”
Dr. Widera pointed to a study showing that dementia patients were less likely to be on an antidementia drug if they were treated by a geriatrician, compared with a psychiatrist or a neurologist. But whether the specialty will prove as cautious with aducanumab remains to be seen. Some geriatricians will be tempted to open lucrative infusion centers, he predicted.
What is especially worrisome, Dr. Widera said, is that aducanumab’s label offers no guidance as to when to withdraw treatment. “We’ll probably see something similar to what happened with the cholinesterase inhibitors” – the class of marginally effective antidementia drugs that includes donepezil (Aricept, Pfizer) and rivastigmine (Exelon, Novartis). “No one thinks about deprescribing them. People are prescribed them even in their last months of life. There is no reason to think these infusions won’t be continued for a very long time, well beyond how long people were dosed in the trials.”
“Taking care of someone with dementia is hard enough,” Dr. Widera added. “We can’t even get normal support in the home for someone with dementia. But we are more than happy to throw money to Biogen for a drug they have not yet showed benefit for. Hopefully in 5 years we’ll have a drug that actually works,” Dr. Widera said. “After 5 years of giving this to people at $50,000 a year.”
A fractured research community
Ever since October 2019, when Biogen and Eisai announced that despite two trials halted for futility, they would go ahead and seek FDA approval for aducanumab, the Alzheimer’s research community has been bitterly divided over the drug and the FDA’s accelerated approval process.
Top researchers published critical editorials in journals, with some eventually taking their case to major newspapers as well. The Alzheimer’s Association’s position on the drug has clashed with that of many researchers whose work it supports.
“The Alzheimer’s community has been wonderfully collegial – we all have a common purpose,” Dr. Cummings said. “Now we have people taking extreme positions and I’m hoping this will not result in a permanent fracturing of the community.”
Chief among the critics’ concerns is that the FDA decision ratified the use of antiamyloid therapies based on biomarker evidence, opening the door for makers of similar drugs – those still under development or even those whose development has been halted – to seek approval on weak evidence of clinical benefit.
Whether the approval will chill research into drugs targeting pathways other than amyloid is uncertain.
Dr. Cummings said he felt that while the aducanumab decision would spur other manufacturers of antiamyloid drugs to seek accelerated approval, other classes of Alzheimer’s therapies in development also stand to get a boost. Many Alzheimer’s experts believe that a combination of drugs targeting different elements of the disease pathway – not just amyloid – will be needed in the long run.
Dr. Scharre said that the buzz over aducanumab’s approval will have at least one concrete benefit: people getting into doctors’ offices sooner.
“The people who come into our memory centers represent only a fraction of people walking around with MCI – there are people out there who may have heard that it’s normal aging; they have decreased insight; there’s denial, there’s embarrassment – there’s hundreds of reasons people avoid getting seen,” he said.
“Perhaps they come in and learn that they don’t have any degenerative process but their thyroid is out of whack, or there’s something else causing cognitive impairment. And if they do have a degenerative process, they’ll have time to start [aducanumab], and hopefully get to see a reduction in the decline.”
Dr. Knopman was a site investigator for the Biogen aducanumab trials and has consulted for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences. A former member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, he was recused from the Nov. 6, 2020, meeting that voted against aducanumab. Dr. Cummings has consulted for Biogen, Eisai, and other manufacturers. Dr. Scharre reports financial relationships with Biogen, Brain Test, Acadia, and Vascular Scientific. Dr. Widera has no disclosures. Dr. Delio is a speaker for Gore Medical, Allergan, and Biohaven Pharmaceuticals.
The approval was hailed by advocacy groups and some practitioners as a victory for patients and families, as the drug – the first anti-Alzheimer’s agent to reach the market in 18 years – is a potentially disease-modifying therapy, which acts to clear amyloid plaques from the brain.
But several prominent Alzheimer’s researchers lambasted the agency’s decision, citing unclear evidence of benefit, trials that did not meet their primary endpoints, and reliance on a post hoc analysis of a high-dose subgroup of patients in a halted trial to argue that aducanumab (Aduhelm, Biogen, and Eisai), slowed cognitive and functional decline by 22% on one measure. In November 2020, 10 of 11 members of an independent FDA advisory committee voted against aducanumab’s approval, citing holes in the data and concerns about the quality of the evidence. After the agency went on to approve anyway, three members of that committee resigned in protest.
The FDA decision on aducanumab was made using the agency’s accelerated approval pathway, which allows for the use of a surrogate endpoint – in this case imaging that showed amyloid clearance from the brain – to predict clinical benefit. But amyloid clearance, which a number of experimental antiamyloid antibodies have been shown capable of, has not been definitively linked to clinical benefit. Aducanumab, which is delivered by monthly intravenous infusion, will be marketed pending results from a phase 4 clinical trial, which the manufacturer has nearly a decade to complete. The drug’s price was announced at $56,000 per year, underscoring concern over its modest-at-best benefits.
Clinicians prescribing aducanumab must obtain magnetic resonance imaging at baseline and repeatedly during the course of treatment to detect brain edema and microhemorrhages, which occurred in a third of high-dose patients in clinical trials. Beyond this, there are few restrictions. The FDA label allows for its use in any patient deemed to have Alzheimer’s disease, without stipulations as to disease stage or evidence of brain amyloid. Payers, of course, are likely to restrict use to certain patient groups, and to require evidence of amyloid positivity. The FDA offered no guidance on when treatment should be ceased, leaving payers to make that call as well. Whatever aducanumab’s value and role turns out to be, the first-in-class treatment for Alzheimer’s disease is likely to have a major impact on how patients are assessed and treated in the coming years, and embolden manufactures of similar agents to seek FDA approval.
This news organization reached out to researchers, advocates, and specialists in the community to learn how they see this change playing out.
Fielding broad interest
Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association, which was a strong proponent of aducanumab’s approval, acknowledged in an interview that the months to come are likely to be confusing for practitioners and families alike as the drug makes its way into community practices.
“We understand that off the bat millions of Americans will not have access to this tomorrow, but over time that will build. And the physician community, the specialists most likely to be prescribing this, over the next few years will even expand further,” Dr. Carrillo said.
For now, those specialists are mostly just struggling to respond responsibly to a deluge of inquiries from patients and their families.
“I’ve gotten like 20 calls in the just the past 2 days,” said neurologist Philip R. Delio, MD, who practices in Santa Barbara, Calif. “This is a longstanding issue that physicians have with patients’ access to information. Patients are getting information about a drug which isn’t available yet. They don’t know that it’s not ready to be sold. They don’t necessarily realize that a biopharma company won’t go into production until the FDA approves the drug.”
Many patients, Dr. Delio said, are aware of the controversy surrounding aducanumab and eager to hear their neurologist’s opinion. “I have tried to let them know that I want to see the trial data and to better understand the FDA’s rationale in approving it. I always caution patients that the devil will be in the details.”
While aducanumab’s label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all but meaningless. Neurologist Douglas Scharre, MD, of the Ohio State University Wexner Medical Center, and a site investigator on a trial of aducanumab, said that he and his colleagues at the university’s memory center have tried to anticipate who might be deemed eligible by triaging calls.
Dr. Scharre and colleagues have been working under the assumption that payers will support aducanumab only for patients like those who seemed to benefit in the trials – people with mild cognitive impairment (MCI) or in the earliest stages of dementia with evidence of brain amyloid.
“I don’t want to fill up our new patient slots with people who are not even appropriate for this drug,” Dr. Scharre said. “We have a call center, and we have a few triage questions. After that a nurse practitioner collects some more data, and there’s a review process. Only then do we decide whether that person could be a candidate. If we deem that they are, we will want them in and to order an amyloid PET” – a type of brain scan that is seldom used outside research settings and not reimbursed by Medicare.
Dr. Scharre predicts that regardless of payer limitations, “there will be people hounding for the drug who are not appropriate for the drug. There will be very wealthy people who will want to pay for tests and get it no matter what.” Another concern, he said, was that having poorly selected patients on the drug could make definitive trial results even more elusive.
“The label the way it’s written is not going to help the drug in phase 4 trials,” he said. “It’s good to have real-world patient data, but if you have all these people in your cohort who are too early or too late, you won’t have good results.”
The challenge of delivery
Intravenous infusions are new to Alzheimer’s disease and pose all sorts of logistical hurdles. The Alzheimer’s Association’s Dr. Carrillo described the situation as “manageable,” noting that infusions are standard of care for many diseases, and that neurologists now have more than 15 years’ experience with them for multiple sclerosis.
Still, most clinicians treating Alzheimer’s disease in the community – neurologists, geriatricians, psychiatrists, and primary care physicians – do not have infusion centers in their practices. Virtually none have experience with or access to PET-amyloid, or with screening for amyloid-related imaging abnormalities–edema (ARIA-e) on MRI, as required by the FDA.
“I contacted the hospital infusion center we use and said I could end up sending five or six patients a week, can you handle this? They only have so many chairs,” Dr. Delio said. “I am one neurologist in a local community, and I might have 50 candidates for this drug. That’s a lot for them.” Patients with cognitive impairment are also difficult to infuse and may need to be treated at home, he noted.
“MRIs are easy enough to do,” Dr. Delio said. “But do we know what ARIA-e looks like on imaging? You’d have to talk to the radiologists – this is another element of uncertainty. Do we even know what we’re looking for with these scans? Will we recognize this?”
Neurologist Jeffrey L. Cummings, MD, ScD, of the University of Nevada, Las Vegas, a vocal proponent of aducanumab and lead author of a May 2021 paper defending the evidence for it, acknowledged that the field was unprepared for a wide-scale adoption of infusions in dementia treatment, pointing to a Rand Corporation study from 2017 that warned that screening, diagnosis, and availability of infusion chairs would have to be drastically scaled up to meet demand.
“There are few clinicians who know how to identify MCI, too few imaging centers, too few radiologists who know how to identify ARIA-e on MRI, so all of these things will be required to be put into place. The label doesn’t specify any of this, but good clinical practice will require that, and getting this up and running will take 18 to 24 months,” Dr. Cummings said.
Neurologist David S. Knopman, MD, of the Mayo Clinic in Rochester, Minn., a leading critic of the evidence for aducanumab who recently resigned his position on the independent committee that advises the FDA on neurology drugs, said that for large research institutions like his that have served as trial sites, the transition to offering PET-amyloid, MRI, and infusions in clinical practice will be easier.
“We have all this because this is what we do every day. And we have a very extensive understanding of MCI and mild dementia staging,” Dr. Knopman said. “But the amount of infrastructure that is implied by this, and all the extra steps it would take, would be a real challenge for people in general neurology practice.”
In addition to routine use of PET-amyloid and MRI screening for ARIA-e, Dr. Knopman said, clinicians will have to provide genetic screening and counseling before administering aducanumab, as clinical trials showed that treated patients have a higher risk of developing ARIA-e if they have APOE4, a risk variant for Alzheimer’s disease. “And that has real implications for the families and the children of patients,” he said.
Uncertainty over costs
Aducanumab’s true costs, to patients and to taxpayers, remain unknown. The $56,000 per year currently cited by its manufacturer “doesn’t count the PET scans and MRIs,” Dr. Knopman noted. “We’re probably pushing $100,00 a year for the first year of treatment.”
Most of that expense will likely be borne by Medicare, he said, and if not, “that will exacerbate existing health care disparities. People who can pay out of pocket are a pretty limited group.”
Dr. Scharre agreed that the costs of treatment were concerning, and that “at least you should be able to narrow it down and hopefully just use health care dollars for people who might stand to benefit,” he said – namely patients in an earlier stage of disease.
The Alzheimer’s Association’s Dr. Carrillo declined to address the high price of aducanumab or its implications, saying only that the association is “very invested in all aspects of access including covering costs associated with the drug and the rest of treatment.”
Access also means “infrastructure, access to physicians to diagnose, access to diagnostics,” Dr. Carrillo said.
Dr. Cummings said aducanumab’s price would likely come down through negotiations with the Centers for Medicare & Medicaid Services, copayments, and bulk purchases.
The FDA has offered no guidance on how long treatment with aducanumab should last, or what should prompt withdrawal of treatment, meaning that patients could, in theory, stay on it to the end of their lives – raising costs further.
Critics have also noted that a built-in financial incentive under Medicare Part B, which covers infusion drugs, could result in overprescription of aducanumab. Under Medicare Part B, prescribing physicians are reimbursed 6% of a drug’s average sales price.
Geriatricians wary
On social media and in the lay press, geriatricians have been among the most outspoken opponents of the FDA decision and the Alzheimer’s Association’s advocacy of aducanumab.
Eric Widera, MD, a geriatrician at the University of California, San Francisco, said that the specialty might be less likely than others to embrace aducanumab. “I think part of the reasons geriatricians don’t make a lot of money is they have strong commitment to their values,” Dr. Widera said.
The American Geriatrics Society opposed the drug’s approval, citing concerns about evidence, side effects, and cost. “Additional considerations are the unintended consequences of overstressing Medicare’s limited financial reserves, and of challenging health care systems … to divert precious resources to an expensive treatment of uncertain value,” the society’s president, Peter Hollmann, MD, and chief executive officer, Nancy E. Lundebjerg, wrote in a June 2 letter to the FDA.
Dr. Widera said the approval was likely to undermine confidence in the FDA and in the Alzheimer’s Association, which receives significant funding from drug manufacturers, including Biogen and Eisai. “There’s a lot of reasons that the Geriatrics Society could have done what the Alzheimer’s Association did, and yet they came out against it, which I applaud.”
Dr. Widera pointed to a study showing that dementia patients were less likely to be on an antidementia drug if they were treated by a geriatrician, compared with a psychiatrist or a neurologist. But whether the specialty will prove as cautious with aducanumab remains to be seen. Some geriatricians will be tempted to open lucrative infusion centers, he predicted.
What is especially worrisome, Dr. Widera said, is that aducanumab’s label offers no guidance as to when to withdraw treatment. “We’ll probably see something similar to what happened with the cholinesterase inhibitors” – the class of marginally effective antidementia drugs that includes donepezil (Aricept, Pfizer) and rivastigmine (Exelon, Novartis). “No one thinks about deprescribing them. People are prescribed them even in their last months of life. There is no reason to think these infusions won’t be continued for a very long time, well beyond how long people were dosed in the trials.”
“Taking care of someone with dementia is hard enough,” Dr. Widera added. “We can’t even get normal support in the home for someone with dementia. But we are more than happy to throw money to Biogen for a drug they have not yet showed benefit for. Hopefully in 5 years we’ll have a drug that actually works,” Dr. Widera said. “After 5 years of giving this to people at $50,000 a year.”
A fractured research community
Ever since October 2019, when Biogen and Eisai announced that despite two trials halted for futility, they would go ahead and seek FDA approval for aducanumab, the Alzheimer’s research community has been bitterly divided over the drug and the FDA’s accelerated approval process.
Top researchers published critical editorials in journals, with some eventually taking their case to major newspapers as well. The Alzheimer’s Association’s position on the drug has clashed with that of many researchers whose work it supports.
“The Alzheimer’s community has been wonderfully collegial – we all have a common purpose,” Dr. Cummings said. “Now we have people taking extreme positions and I’m hoping this will not result in a permanent fracturing of the community.”
Chief among the critics’ concerns is that the FDA decision ratified the use of antiamyloid therapies based on biomarker evidence, opening the door for makers of similar drugs – those still under development or even those whose development has been halted – to seek approval on weak evidence of clinical benefit.
Whether the approval will chill research into drugs targeting pathways other than amyloid is uncertain.
Dr. Cummings said he felt that while the aducanumab decision would spur other manufacturers of antiamyloid drugs to seek accelerated approval, other classes of Alzheimer’s therapies in development also stand to get a boost. Many Alzheimer’s experts believe that a combination of drugs targeting different elements of the disease pathway – not just amyloid – will be needed in the long run.
Dr. Scharre said that the buzz over aducanumab’s approval will have at least one concrete benefit: people getting into doctors’ offices sooner.
“The people who come into our memory centers represent only a fraction of people walking around with MCI – there are people out there who may have heard that it’s normal aging; they have decreased insight; there’s denial, there’s embarrassment – there’s hundreds of reasons people avoid getting seen,” he said.
“Perhaps they come in and learn that they don’t have any degenerative process but their thyroid is out of whack, or there’s something else causing cognitive impairment. And if they do have a degenerative process, they’ll have time to start [aducanumab], and hopefully get to see a reduction in the decline.”
Dr. Knopman was a site investigator for the Biogen aducanumab trials and has consulted for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences. A former member of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, he was recused from the Nov. 6, 2020, meeting that voted against aducanumab. Dr. Cummings has consulted for Biogen, Eisai, and other manufacturers. Dr. Scharre reports financial relationships with Biogen, Brain Test, Acadia, and Vascular Scientific. Dr. Widera has no disclosures. Dr. Delio is a speaker for Gore Medical, Allergan, and Biohaven Pharmaceuticals.
Choosing the right R-CHOP dosage for elderly patients with DLBCL
Physicians often face the choice of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone + rituximab), according to Edward J. Bataillard of the Imperial College Healthcare National Health Service Trust, London, and colleagues.
To address this issue, the researchers conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. They found that greater than 80 years of age is an important cutoff for treating patients with a reduced R-CHOP dosage, according to their results, published in Blood Advances (2021;5[9]:2426-37).
Cutoff at 80 years of age
Their final review comprised 13 studies including 5,188 patients. Overall, the lower DI (intended or relative) was associated with inferior survival in seven of nine studies reporting crude survival analyses. In addition, most studies and those larger studies of higher quality showed poorer outcomes associated with reduced R-CHOP DI.
However, in subgroups of patients aged 80 years or more, survival was not consistently affected by the use of lower dosage R-CHOP, according to the researchers.
“We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged < 80 years, but the literature to date does not support full-dose intensity in those 80 years [or older],” they stated.
However, the researchers concluded that: “In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.”
Two of the authors reported being previously employed by Roche. A third served as a consultant and adviser and received honoraria from Roche and other pharmaceutical companies. Several authors reported disclosures related to multiple other pharmaceutical companies.
Physicians often face the choice of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone + rituximab), according to Edward J. Bataillard of the Imperial College Healthcare National Health Service Trust, London, and colleagues.
To address this issue, the researchers conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. They found that greater than 80 years of age is an important cutoff for treating patients with a reduced R-CHOP dosage, according to their results, published in Blood Advances (2021;5[9]:2426-37).
Cutoff at 80 years of age
Their final review comprised 13 studies including 5,188 patients. Overall, the lower DI (intended or relative) was associated with inferior survival in seven of nine studies reporting crude survival analyses. In addition, most studies and those larger studies of higher quality showed poorer outcomes associated with reduced R-CHOP DI.
However, in subgroups of patients aged 80 years or more, survival was not consistently affected by the use of lower dosage R-CHOP, according to the researchers.
“We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged < 80 years, but the literature to date does not support full-dose intensity in those 80 years [or older],” they stated.
However, the researchers concluded that: “In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.”
Two of the authors reported being previously employed by Roche. A third served as a consultant and adviser and received honoraria from Roche and other pharmaceutical companies. Several authors reported disclosures related to multiple other pharmaceutical companies.
Physicians often face the choice of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone + rituximab), according to Edward J. Bataillard of the Imperial College Healthcare National Health Service Trust, London, and colleagues.
To address this issue, the researchers conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. They found that greater than 80 years of age is an important cutoff for treating patients with a reduced R-CHOP dosage, according to their results, published in Blood Advances (2021;5[9]:2426-37).
Cutoff at 80 years of age
Their final review comprised 13 studies including 5,188 patients. Overall, the lower DI (intended or relative) was associated with inferior survival in seven of nine studies reporting crude survival analyses. In addition, most studies and those larger studies of higher quality showed poorer outcomes associated with reduced R-CHOP DI.
However, in subgroups of patients aged 80 years or more, survival was not consistently affected by the use of lower dosage R-CHOP, according to the researchers.
“We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged < 80 years, but the literature to date does not support full-dose intensity in those 80 years [or older],” they stated.
However, the researchers concluded that: “In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.”
Two of the authors reported being previously employed by Roche. A third served as a consultant and adviser and received honoraria from Roche and other pharmaceutical companies. Several authors reported disclosures related to multiple other pharmaceutical companies.
FROM BLOOD ADVANCES
FDA approves controversial Alzheimer’s drug aducanumab (Aduhelm)
In November, the Peripheral and Central Nervous System Drugs Advisory Committee voted eight to one against approving the drug because, based on clinical trial results, evidence of efficacy was not strong enough. Two other members said they were uncertain on the issue of efficacy.
In a company release Michel Vounatsos, Biogen’s Chief Executive Officer, said, “this historic moment is the culmination of more than a decade of groundbreaking research in the complex field of Alzheimer’s disease. We believe this first-in-class medicine will transform the treatment of people living with Alzheimer’s disease and spark continuous innovation in the years to come.
Rocky road
The road to approval has been extremely rocky for aducanumab, an anti-amyloid-beta human monoclonal antibody, previously known as BIIB037.
As reported by this news organization, two phase 3 trials evaluating the drug were initially scrapped in March 2019 because of interim futility analysis. At the time, Biogen released a statement saying that aducanumab was unlikely to meet primary endpoints in the ENGAGE and EMERGE randomized controlled trials.
However, in an about-face 7 months later, Biogen and Eisai announced that a new analysis showed the drug met its primary endpoint of reduction in clinical decline, including cognition and function, in the EMERGE trial.
Although ENGAGE still didn’t meet its primary endpoint, data from its new analysis “supported” the EMERGE findings, the drug companies said at the time.
However, 1 year later, a majority of the members of the FDA’s advisory panel were against the drug’s approval. Details of that decision were published online March 30 in the Journal of the American Medical Association.
As reported by this news organization, a Viewpoint written by three of the committee members notes that results from the drug’s only large positive clinical trial fell short.
“There is no persuasive evidence to support approval of aducanumab at this time,” they write.
Groups such as Public Citizen’s Health Research Group not only agree with the Viewpoint’s authors, they also criticized the FDA for its collaboration with the drug’s manufacturers on briefing documents and more.
On April 1, Health Research Group members sent a letter to the U.S. Secretary of Health and Human Services requesting the temporary suspension of the FDA’s neuroscience chief, Bill Dunn, MD, because of his role in supervising the collaboration.
Alzheimer association weighs in
The Alzheimer’s Association has been a proponent of the drug throughout its development.
Ahead of today’s news, the organization noted in a statement that a decision to approve “would be historic” because it would make aducanumab “the first drug to slow Alzheimer’s disease” and would mark the beginning of a new future for AD treatments.
“The Alzheimer’s Association urgently supports FDA approval of the treatment based on clinical trial results that showed a 22% reduction in cognitive and function decline — something that could make a meaningful difference” for patients with AD, it said.
Kristen Clifford, chief program officer for the Alzheimer’s Association, said in an interview at the time that approval would be considered a “victory” for patients with AD and for the field overall.
“For individuals who would potentially be eligible for the treatment, this drug could mean more quality time. Slowing decline, particularly in early diagnosis, could add weeks or months or maybe even years of active life,” Clifford said.
“If approved, this would really be a landmark moment. And it could provide hope for those living with Alzheimer’s and their families,” she added.
Clifford noted that approval of this type of drug would also underscore the importance of early detection for AD. “This treatment would encourage earlier diagnosis of the disease,” she said.
In a new statement released just after approval for aducanumab was announced, the organization said that today’s news is a win-win for all patients with AD and their families.
A version of this article first appeared on Medscape.com.
In November, the Peripheral and Central Nervous System Drugs Advisory Committee voted eight to one against approving the drug because, based on clinical trial results, evidence of efficacy was not strong enough. Two other members said they were uncertain on the issue of efficacy.
In a company release Michel Vounatsos, Biogen’s Chief Executive Officer, said, “this historic moment is the culmination of more than a decade of groundbreaking research in the complex field of Alzheimer’s disease. We believe this first-in-class medicine will transform the treatment of people living with Alzheimer’s disease and spark continuous innovation in the years to come.
Rocky road
The road to approval has been extremely rocky for aducanumab, an anti-amyloid-beta human monoclonal antibody, previously known as BIIB037.
As reported by this news organization, two phase 3 trials evaluating the drug were initially scrapped in March 2019 because of interim futility analysis. At the time, Biogen released a statement saying that aducanumab was unlikely to meet primary endpoints in the ENGAGE and EMERGE randomized controlled trials.
However, in an about-face 7 months later, Biogen and Eisai announced that a new analysis showed the drug met its primary endpoint of reduction in clinical decline, including cognition and function, in the EMERGE trial.
Although ENGAGE still didn’t meet its primary endpoint, data from its new analysis “supported” the EMERGE findings, the drug companies said at the time.
However, 1 year later, a majority of the members of the FDA’s advisory panel were against the drug’s approval. Details of that decision were published online March 30 in the Journal of the American Medical Association.
As reported by this news organization, a Viewpoint written by three of the committee members notes that results from the drug’s only large positive clinical trial fell short.
“There is no persuasive evidence to support approval of aducanumab at this time,” they write.
Groups such as Public Citizen’s Health Research Group not only agree with the Viewpoint’s authors, they also criticized the FDA for its collaboration with the drug’s manufacturers on briefing documents and more.
On April 1, Health Research Group members sent a letter to the U.S. Secretary of Health and Human Services requesting the temporary suspension of the FDA’s neuroscience chief, Bill Dunn, MD, because of his role in supervising the collaboration.
Alzheimer association weighs in
The Alzheimer’s Association has been a proponent of the drug throughout its development.
Ahead of today’s news, the organization noted in a statement that a decision to approve “would be historic” because it would make aducanumab “the first drug to slow Alzheimer’s disease” and would mark the beginning of a new future for AD treatments.
“The Alzheimer’s Association urgently supports FDA approval of the treatment based on clinical trial results that showed a 22% reduction in cognitive and function decline — something that could make a meaningful difference” for patients with AD, it said.
Kristen Clifford, chief program officer for the Alzheimer’s Association, said in an interview at the time that approval would be considered a “victory” for patients with AD and for the field overall.
“For individuals who would potentially be eligible for the treatment, this drug could mean more quality time. Slowing decline, particularly in early diagnosis, could add weeks or months or maybe even years of active life,” Clifford said.
“If approved, this would really be a landmark moment. And it could provide hope for those living with Alzheimer’s and their families,” she added.
Clifford noted that approval of this type of drug would also underscore the importance of early detection for AD. “This treatment would encourage earlier diagnosis of the disease,” she said.
In a new statement released just after approval for aducanumab was announced, the organization said that today’s news is a win-win for all patients with AD and their families.
A version of this article first appeared on Medscape.com.
In November, the Peripheral and Central Nervous System Drugs Advisory Committee voted eight to one against approving the drug because, based on clinical trial results, evidence of efficacy was not strong enough. Two other members said they were uncertain on the issue of efficacy.
In a company release Michel Vounatsos, Biogen’s Chief Executive Officer, said, “this historic moment is the culmination of more than a decade of groundbreaking research in the complex field of Alzheimer’s disease. We believe this first-in-class medicine will transform the treatment of people living with Alzheimer’s disease and spark continuous innovation in the years to come.
Rocky road
The road to approval has been extremely rocky for aducanumab, an anti-amyloid-beta human monoclonal antibody, previously known as BIIB037.
As reported by this news organization, two phase 3 trials evaluating the drug were initially scrapped in March 2019 because of interim futility analysis. At the time, Biogen released a statement saying that aducanumab was unlikely to meet primary endpoints in the ENGAGE and EMERGE randomized controlled trials.
However, in an about-face 7 months later, Biogen and Eisai announced that a new analysis showed the drug met its primary endpoint of reduction in clinical decline, including cognition and function, in the EMERGE trial.
Although ENGAGE still didn’t meet its primary endpoint, data from its new analysis “supported” the EMERGE findings, the drug companies said at the time.
However, 1 year later, a majority of the members of the FDA’s advisory panel were against the drug’s approval. Details of that decision were published online March 30 in the Journal of the American Medical Association.
As reported by this news organization, a Viewpoint written by three of the committee members notes that results from the drug’s only large positive clinical trial fell short.
“There is no persuasive evidence to support approval of aducanumab at this time,” they write.
Groups such as Public Citizen’s Health Research Group not only agree with the Viewpoint’s authors, they also criticized the FDA for its collaboration with the drug’s manufacturers on briefing documents and more.
On April 1, Health Research Group members sent a letter to the U.S. Secretary of Health and Human Services requesting the temporary suspension of the FDA’s neuroscience chief, Bill Dunn, MD, because of his role in supervising the collaboration.
Alzheimer association weighs in
The Alzheimer’s Association has been a proponent of the drug throughout its development.
Ahead of today’s news, the organization noted in a statement that a decision to approve “would be historic” because it would make aducanumab “the first drug to slow Alzheimer’s disease” and would mark the beginning of a new future for AD treatments.
“The Alzheimer’s Association urgently supports FDA approval of the treatment based on clinical trial results that showed a 22% reduction in cognitive and function decline — something that could make a meaningful difference” for patients with AD, it said.
Kristen Clifford, chief program officer for the Alzheimer’s Association, said in an interview at the time that approval would be considered a “victory” for patients with AD and for the field overall.
“For individuals who would potentially be eligible for the treatment, this drug could mean more quality time. Slowing decline, particularly in early diagnosis, could add weeks or months or maybe even years of active life,” Clifford said.
“If approved, this would really be a landmark moment. And it could provide hope for those living with Alzheimer’s and their families,” she added.
Clifford noted that approval of this type of drug would also underscore the importance of early detection for AD. “This treatment would encourage earlier diagnosis of the disease,” she said.
In a new statement released just after approval for aducanumab was announced, the organization said that today’s news is a win-win for all patients with AD and their families.
A version of this article first appeared on Medscape.com.
Differences in Palliative Care Delivery Among Adults With Cancer and With Terminal Noncancer Illness in Their Last Year of Life
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
Fall prevention advice for patients with Parkinson’s
A 75-year-old man with Parkinson’s disease has had three falls over the past 4 weeks. He has been compliant with his Parkinson’s treatment. Which of the following options would most help decrease his fall risk?
A. Vitamin D supplementation
B. Vitamin B12 supplementation
C. Calcium supplementation
D. Tai chi
There has been recent evidence that vitamin D supplementation is not helpful in preventing falls in most community-dwelling older adults. Bolland and colleagues performed a meta-analysis of 81 randomized, controlled trials and found that vitamin D supplementation does not prevent fractures or falls.1 They found no difference or benefit in high-dose versus low-dose vitamin D supplementation.
The U.S. Preventive Services Task Force recommends against vitamin D supplementation for the purpose of preventing falls in community-dwelling adults over the age of 65.2 The same USPSTF report recommends exercise intervention, as having the strongest evidence for fall prevention in community-dwelling adults age 65 or older who are at risk for falls.
The benefits of tai chi
Tai chi with it’s emphasis on balance, strength training as well as stress reduction is an excellent option for older adults.
Lui and colleagues performed a meta-analyses of five randomized, controlled trials (355 patients) of tai chi in patients with Parkinson disease.3 Tai chi significantly decreased fall rates (odds ratio, 0.47; 95% confidence interval, 0.30-0.74; P = .001) and significantly improved balance and functional mobility (P < .001) in people with Parkinson disease, compared with no training.
Tai chi can also help prevent falls in a more general population of elderly patients. Lomas-Vega and colleagues performed a meta-analysis of 10 high-quality studies that met inclusion criteria evaluating tai chi for fall prevention.4 Fall risk was reduced over short-term follow-up (incident rate ratio, 0.57; 95% CI, 0.46-0.70) and a small protective effect was seen over long-term follow-up (IRR, 0.87; 95% CI, 0.77-0.98).
Pearl: Consider tai chi in your elderly patients with fall risk to increase their balance and reduce risks of falls.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Bolland MJ et al. Lancet Diabetes Endocrinol. 2018;6(11):847.
2. U.S. Preventive Services Task Force. JAMA. 2018;319(16):1696.
3. Liu HH et al. Parkinsons Dis. 2019 Feb 21;2019:9626934
4. Lomas-Vega R et al. J Am Geriatr Soc. 2017;65(9):2037.
A 75-year-old man with Parkinson’s disease has had three falls over the past 4 weeks. He has been compliant with his Parkinson’s treatment. Which of the following options would most help decrease his fall risk?
A. Vitamin D supplementation
B. Vitamin B12 supplementation
C. Calcium supplementation
D. Tai chi
There has been recent evidence that vitamin D supplementation is not helpful in preventing falls in most community-dwelling older adults. Bolland and colleagues performed a meta-analysis of 81 randomized, controlled trials and found that vitamin D supplementation does not prevent fractures or falls.1 They found no difference or benefit in high-dose versus low-dose vitamin D supplementation.
The U.S. Preventive Services Task Force recommends against vitamin D supplementation for the purpose of preventing falls in community-dwelling adults over the age of 65.2 The same USPSTF report recommends exercise intervention, as having the strongest evidence for fall prevention in community-dwelling adults age 65 or older who are at risk for falls.
The benefits of tai chi
Tai chi with it’s emphasis on balance, strength training as well as stress reduction is an excellent option for older adults.
Lui and colleagues performed a meta-analyses of five randomized, controlled trials (355 patients) of tai chi in patients with Parkinson disease.3 Tai chi significantly decreased fall rates (odds ratio, 0.47; 95% confidence interval, 0.30-0.74; P = .001) and significantly improved balance and functional mobility (P < .001) in people with Parkinson disease, compared with no training.
Tai chi can also help prevent falls in a more general population of elderly patients. Lomas-Vega and colleagues performed a meta-analysis of 10 high-quality studies that met inclusion criteria evaluating tai chi for fall prevention.4 Fall risk was reduced over short-term follow-up (incident rate ratio, 0.57; 95% CI, 0.46-0.70) and a small protective effect was seen over long-term follow-up (IRR, 0.87; 95% CI, 0.77-0.98).
Pearl: Consider tai chi in your elderly patients with fall risk to increase their balance and reduce risks of falls.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Bolland MJ et al. Lancet Diabetes Endocrinol. 2018;6(11):847.
2. U.S. Preventive Services Task Force. JAMA. 2018;319(16):1696.
3. Liu HH et al. Parkinsons Dis. 2019 Feb 21;2019:9626934
4. Lomas-Vega R et al. J Am Geriatr Soc. 2017;65(9):2037.
A 75-year-old man with Parkinson’s disease has had three falls over the past 4 weeks. He has been compliant with his Parkinson’s treatment. Which of the following options would most help decrease his fall risk?
A. Vitamin D supplementation
B. Vitamin B12 supplementation
C. Calcium supplementation
D. Tai chi
There has been recent evidence that vitamin D supplementation is not helpful in preventing falls in most community-dwelling older adults. Bolland and colleagues performed a meta-analysis of 81 randomized, controlled trials and found that vitamin D supplementation does not prevent fractures or falls.1 They found no difference or benefit in high-dose versus low-dose vitamin D supplementation.
The U.S. Preventive Services Task Force recommends against vitamin D supplementation for the purpose of preventing falls in community-dwelling adults over the age of 65.2 The same USPSTF report recommends exercise intervention, as having the strongest evidence for fall prevention in community-dwelling adults age 65 or older who are at risk for falls.
The benefits of tai chi
Tai chi with it’s emphasis on balance, strength training as well as stress reduction is an excellent option for older adults.
Lui and colleagues performed a meta-analyses of five randomized, controlled trials (355 patients) of tai chi in patients with Parkinson disease.3 Tai chi significantly decreased fall rates (odds ratio, 0.47; 95% confidence interval, 0.30-0.74; P = .001) and significantly improved balance and functional mobility (P < .001) in people with Parkinson disease, compared with no training.
Tai chi can also help prevent falls in a more general population of elderly patients. Lomas-Vega and colleagues performed a meta-analysis of 10 high-quality studies that met inclusion criteria evaluating tai chi for fall prevention.4 Fall risk was reduced over short-term follow-up (incident rate ratio, 0.57; 95% CI, 0.46-0.70) and a small protective effect was seen over long-term follow-up (IRR, 0.87; 95% CI, 0.77-0.98).
Pearl: Consider tai chi in your elderly patients with fall risk to increase their balance and reduce risks of falls.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Bolland MJ et al. Lancet Diabetes Endocrinol. 2018;6(11):847.
2. U.S. Preventive Services Task Force. JAMA. 2018;319(16):1696.
3. Liu HH et al. Parkinsons Dis. 2019 Feb 21;2019:9626934
4. Lomas-Vega R et al. J Am Geriatr Soc. 2017;65(9):2037.
Novel rehab program fights frailty, boosts capacity in advanced HF
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
Use of Comprehensive Geriatric Assessment in Oncology Patients to Guide Treatment Decisions and Predict Chemotherapy Toxicity
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
When to refer patients with new memory loss
Initial questions should zero in on what the patient is forgetting, said Megan Richie, MD, a neurohospitalist at the University of California, San Francisco, who spoke to a virtual audience at the American College of Physicians (ACP) annual Internal Medicine meeting.
Is the patient forgetting to buy things in a store, having trouble recalling events, forgetting important dates? How often do these incidents occur?
These questions “will help get at how pervasive and how likely the memory loss is affecting their lives, versus a subjective complaint that doesn’t have much impact on the day-to-day function,” she said.
It’s also important to ask whether other neurocognitive symptoms accompany the memory loss, Dr. Richie noted.
Does the patient search for words, struggle with attention, or have problems with executive function? Does the patient have psychiatric symptoms, such as hallucinations or delusions, or other neurologic complaints, including weakness, numbness, vision change, or movement disorders?
“When you know how many neurocognitive symptoms they have, think about how [those symptoms] are affecting their safety and functional status. How are they on their activities of daily living?” Dr. Richie suggests.
Also ask whether the patient is taking medications and whether they drive a vehicle. If they do drive, do they get lost?
“These are all going to help you determine the acuity of the workup,” she said.
After a thorough history, cognitive screening is the next consideration.
Cognitive screening can be performed in minutes
One of the tests Dr. Richie recommends is the Mini-Cog. It takes 3 minutes to administer and has been formally recommended by the Alzheimer’s Association because it can be completed in the time frame of a Medicare wellness visit, she said.
It entails a three-word recall and clock-drawing test.
Dr. Richie said it’s important to eliminate some key causes first: “Certainly if the patient has signs and symptoms of depression, pseudodementia is a very real and treatable disease you do not want to miss and should consider in these patients,” she pointed out.
Systemic medical conditions can also lead to memory loss.
If there’s an acute component to the complaint, a new infection or medication withdrawal or a side effect could be driving it, so that’s key in questioning.
Dr. Richie explained that the American Academy of Neurology recommends a very limited workup.
“It’s really just to check their thyroid, their vitamin B12 levels, and then a one-time picture of their brain, which can be either MRI or a CT, to look for structural problems or vascular dementia or hydrocephalus, etc.”
“You do not routinely need spinal fluid testing or an EEG,” she emphasized.
Signs that a neurologist should be involved include a rapid decline, signs of potential seizures, or that the patient doesn’t seem safe in their condition.
Neuropsychological testing is helpful, but it takes nearly 3 hours and may not be a good choice for restless or aggressive patients, Dr. Richie said.
Such testing is often not available, and if it is, insurance coverage is often a barrier because many plans don’t cover it.
Patients often ask about drugs and supplements they see advertised to help with memory loss. Medications are not helpful for mild cognitive impairment, although there is evidence that some are beneficial for patients with dementia, Dr. Richie said.
Celine Goetz, MD, assistant professor of internal medicine at Rush University Medical Center, Chicago, Illinois, told this news organization that it’s easy to relate to the fear that patients and families feel when cognitive impairment begins to emerge.
“[Dr.] Richie’s talk was right on point for internists like myself who see many patients with memory complaints, cognitive impairment, and dementia. I think we’re all terrified of losing our memory and the social and functional impairment that comes with that,” she said.
Although cognitive impairment and dementia aren’t curable or reversible, Dr. Goetz noted, internists can help patients optimize management of conditions such as diabetes and heart disease, which can affect cognitive function.
Dr. Richie pointed out that some interventions lack evidence for the treatment of mild cognitive impairment, but Dr. Goetz emphasized that resources are plentiful and can be effective in combination.
“Engaging social workers, pharmacists, nutritionists, physical and occupational therapists, and, on the inpatient side, delirium protocols, chaplains, and music therapists make a huge difference in patient care,” she said.
Dr. Richie and Dr. Goetz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initial questions should zero in on what the patient is forgetting, said Megan Richie, MD, a neurohospitalist at the University of California, San Francisco, who spoke to a virtual audience at the American College of Physicians (ACP) annual Internal Medicine meeting.
Is the patient forgetting to buy things in a store, having trouble recalling events, forgetting important dates? How often do these incidents occur?
These questions “will help get at how pervasive and how likely the memory loss is affecting their lives, versus a subjective complaint that doesn’t have much impact on the day-to-day function,” she said.
It’s also important to ask whether other neurocognitive symptoms accompany the memory loss, Dr. Richie noted.
Does the patient search for words, struggle with attention, or have problems with executive function? Does the patient have psychiatric symptoms, such as hallucinations or delusions, or other neurologic complaints, including weakness, numbness, vision change, or movement disorders?
“When you know how many neurocognitive symptoms they have, think about how [those symptoms] are affecting their safety and functional status. How are they on their activities of daily living?” Dr. Richie suggests.
Also ask whether the patient is taking medications and whether they drive a vehicle. If they do drive, do they get lost?
“These are all going to help you determine the acuity of the workup,” she said.
After a thorough history, cognitive screening is the next consideration.
Cognitive screening can be performed in minutes
One of the tests Dr. Richie recommends is the Mini-Cog. It takes 3 minutes to administer and has been formally recommended by the Alzheimer’s Association because it can be completed in the time frame of a Medicare wellness visit, she said.
It entails a three-word recall and clock-drawing test.
Dr. Richie said it’s important to eliminate some key causes first: “Certainly if the patient has signs and symptoms of depression, pseudodementia is a very real and treatable disease you do not want to miss and should consider in these patients,” she pointed out.
Systemic medical conditions can also lead to memory loss.
If there’s an acute component to the complaint, a new infection or medication withdrawal or a side effect could be driving it, so that’s key in questioning.
Dr. Richie explained that the American Academy of Neurology recommends a very limited workup.
“It’s really just to check their thyroid, their vitamin B12 levels, and then a one-time picture of their brain, which can be either MRI or a CT, to look for structural problems or vascular dementia or hydrocephalus, etc.”
“You do not routinely need spinal fluid testing or an EEG,” she emphasized.
Signs that a neurologist should be involved include a rapid decline, signs of potential seizures, or that the patient doesn’t seem safe in their condition.
Neuropsychological testing is helpful, but it takes nearly 3 hours and may not be a good choice for restless or aggressive patients, Dr. Richie said.
Such testing is often not available, and if it is, insurance coverage is often a barrier because many plans don’t cover it.
Patients often ask about drugs and supplements they see advertised to help with memory loss. Medications are not helpful for mild cognitive impairment, although there is evidence that some are beneficial for patients with dementia, Dr. Richie said.
Celine Goetz, MD, assistant professor of internal medicine at Rush University Medical Center, Chicago, Illinois, told this news organization that it’s easy to relate to the fear that patients and families feel when cognitive impairment begins to emerge.
“[Dr.] Richie’s talk was right on point for internists like myself who see many patients with memory complaints, cognitive impairment, and dementia. I think we’re all terrified of losing our memory and the social and functional impairment that comes with that,” she said.
Although cognitive impairment and dementia aren’t curable or reversible, Dr. Goetz noted, internists can help patients optimize management of conditions such as diabetes and heart disease, which can affect cognitive function.
Dr. Richie pointed out that some interventions lack evidence for the treatment of mild cognitive impairment, but Dr. Goetz emphasized that resources are plentiful and can be effective in combination.
“Engaging social workers, pharmacists, nutritionists, physical and occupational therapists, and, on the inpatient side, delirium protocols, chaplains, and music therapists make a huge difference in patient care,” she said.
Dr. Richie and Dr. Goetz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initial questions should zero in on what the patient is forgetting, said Megan Richie, MD, a neurohospitalist at the University of California, San Francisco, who spoke to a virtual audience at the American College of Physicians (ACP) annual Internal Medicine meeting.
Is the patient forgetting to buy things in a store, having trouble recalling events, forgetting important dates? How often do these incidents occur?
These questions “will help get at how pervasive and how likely the memory loss is affecting their lives, versus a subjective complaint that doesn’t have much impact on the day-to-day function,” she said.
It’s also important to ask whether other neurocognitive symptoms accompany the memory loss, Dr. Richie noted.
Does the patient search for words, struggle with attention, or have problems with executive function? Does the patient have psychiatric symptoms, such as hallucinations or delusions, or other neurologic complaints, including weakness, numbness, vision change, or movement disorders?
“When you know how many neurocognitive symptoms they have, think about how [those symptoms] are affecting their safety and functional status. How are they on their activities of daily living?” Dr. Richie suggests.
Also ask whether the patient is taking medications and whether they drive a vehicle. If they do drive, do they get lost?
“These are all going to help you determine the acuity of the workup,” she said.
After a thorough history, cognitive screening is the next consideration.
Cognitive screening can be performed in minutes
One of the tests Dr. Richie recommends is the Mini-Cog. It takes 3 minutes to administer and has been formally recommended by the Alzheimer’s Association because it can be completed in the time frame of a Medicare wellness visit, she said.
It entails a three-word recall and clock-drawing test.
Dr. Richie said it’s important to eliminate some key causes first: “Certainly if the patient has signs and symptoms of depression, pseudodementia is a very real and treatable disease you do not want to miss and should consider in these patients,” she pointed out.
Systemic medical conditions can also lead to memory loss.
If there’s an acute component to the complaint, a new infection or medication withdrawal or a side effect could be driving it, so that’s key in questioning.
Dr. Richie explained that the American Academy of Neurology recommends a very limited workup.
“It’s really just to check their thyroid, their vitamin B12 levels, and then a one-time picture of their brain, which can be either MRI or a CT, to look for structural problems or vascular dementia or hydrocephalus, etc.”
“You do not routinely need spinal fluid testing or an EEG,” she emphasized.
Signs that a neurologist should be involved include a rapid decline, signs of potential seizures, or that the patient doesn’t seem safe in their condition.
Neuropsychological testing is helpful, but it takes nearly 3 hours and may not be a good choice for restless or aggressive patients, Dr. Richie said.
Such testing is often not available, and if it is, insurance coverage is often a barrier because many plans don’t cover it.
Patients often ask about drugs and supplements they see advertised to help with memory loss. Medications are not helpful for mild cognitive impairment, although there is evidence that some are beneficial for patients with dementia, Dr. Richie said.
Celine Goetz, MD, assistant professor of internal medicine at Rush University Medical Center, Chicago, Illinois, told this news organization that it’s easy to relate to the fear that patients and families feel when cognitive impairment begins to emerge.
“[Dr.] Richie’s talk was right on point for internists like myself who see many patients with memory complaints, cognitive impairment, and dementia. I think we’re all terrified of losing our memory and the social and functional impairment that comes with that,” she said.
Although cognitive impairment and dementia aren’t curable or reversible, Dr. Goetz noted, internists can help patients optimize management of conditions such as diabetes and heart disease, which can affect cognitive function.
Dr. Richie pointed out that some interventions lack evidence for the treatment of mild cognitive impairment, but Dr. Goetz emphasized that resources are plentiful and can be effective in combination.
“Engaging social workers, pharmacists, nutritionists, physical and occupational therapists, and, on the inpatient side, delirium protocols, chaplains, and music therapists make a huge difference in patient care,” she said.
Dr. Richie and Dr. Goetz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2021