User login
How to proceed when it comes to vitamin D
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
What should the family physician do?
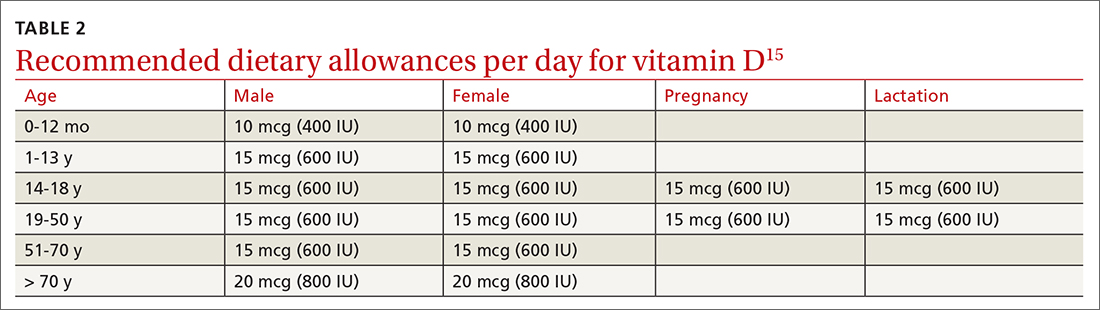
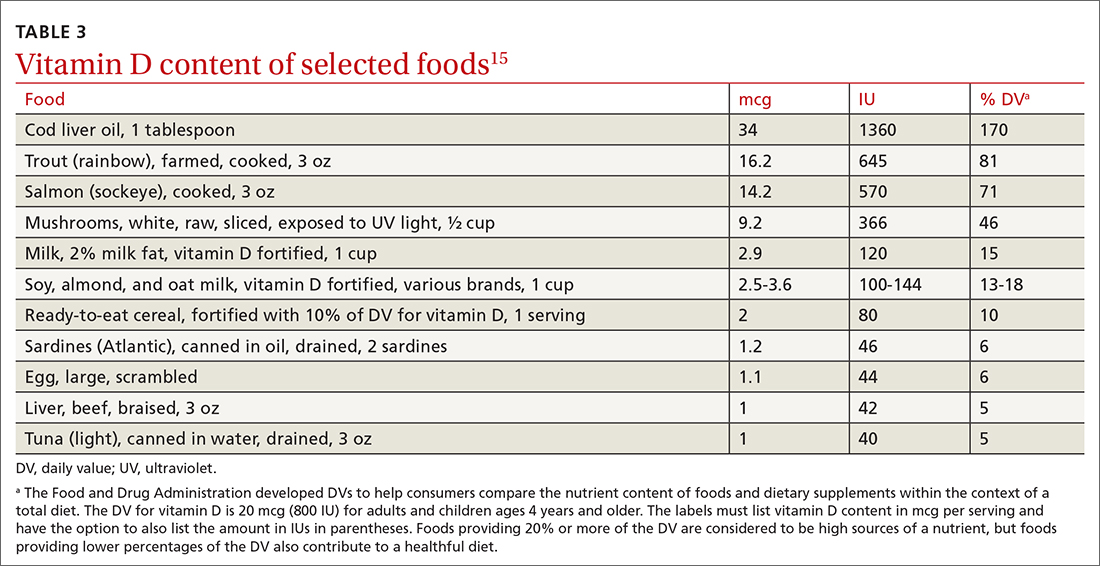
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
Zero benefit of aducanumab for Alzheimer’s disease, expert panel rules
adding to growing opposition from medical experts to the Food and Drug Administration’s approval of this controversial drug.
The Institute for Clinical and Economic Review asked one of its expert panels, the California Technology Assessment Forum, to consider the available data about aducanumab and requested that members vote on whether there was sufficient evidence of a net benefit of aducanumab plus supportive care versus supportive care alone. All 15 panelists voted no.
Several panelists, including ICER President Steven D. Pearson, MD, talked about their personal experience with family members who have the disease.
There was universal agreement among the panelists that there is an urgent need for effective medications to treat the disease. However, the panel of clinicians and researchers also agreed that the evidence to date does not show that the drug helps patients with this debilitating disease.
Panelist Sei Lee, MD, a geriatrician at the University of California, San Francisco, said he lost his mother to AD 6 years ago. In addition to his clinical work, Dr. Lee has conducted research focused on improving the targeting of preventive AD interventions for older adults to maximize benefits and minimize harms.
Dr. Lee said he frequently felt completely overwhelmed by the challenges of his mother’s disease.
“I absolutely hear everyone who is saying we need an effective therapy for this,” Dr. Lee said.
Dr. Lee added that, as an experienced researcher who has weighed the aducanumab data, he saw no clear proof of a benefit that would outweigh the drug’s documented side effects in the two phase 3 trials of the drug. Those side effects include temporary brain swelling. Dr. Lee suggested that Biogen do more to address concerns about this side effect, saying it should not be ignored.
“There’s clearly substantial uncertainty” about aducanumab, Dr. Lee said. “If I had to guess, I think the data is stronger for net harm than it is for that benefit.”
Questions persist about the data Biogen used in support of aducanumab after announcing that the drug had failed in a dual-track phase 3 program.
In March 2019, it was announced that two phase 3 clinical trials, EMERGE and ENGAGE, were scrapped because of disappointing results. The trials were intended to show that aducanumab could slow progression of cognitive and functional impairment, as measured by changes in scores on the Clinical Dementia Rating–Sum of Boxes (CDR-SB).
However, in October 2019, there was an about-face – Biogen announced that, in one of the studies, there were positive findings for a subset of patients who received a higher dose of aducanumab.
No treatment benefit was observed in either the high- or low-dose arms at week 78 in the ENGAGE trial. In the EMERGE trial, however, there was a statistically significant difference in change from baseline in CDR-SB score in the high-dose arm (difference vs. placebo, –0.39; 95% confidence interval, –0.69 to –0.09) but not in the low-dose arm, ICER noted in a draft report.
Still, there are questions about whether this difference would translate into clinical benefit. “Although statistically significant, the change in CDR-SB score in the high-dose group was less than the 1- to 2-point change that has been suggested as a minimal clinically important difference,” ICER staff wrote.
More push back
Other influential organizations also remain skeptical.
On July 14, the Cleveland Clinic announced it would not use aducanumab at this time, following a staff review of the evidence. Physicians from the clinic could prescribe it to appropriate patients, who would receive their infusions at external facilities, a spokeswoman for the clinic told this news organization. The Cleveland Clinic said it will reevaluate this position as additional data become available.
In addition, as reported by the New York Times, the Mount Sinai Health System in New York also decided not to administer the drug.
The drug received accelerated approval from the FDA. That approval was conditional upon Biogen’s conducting further research by 2030 that demonstrates that the drug has clinical benefit.
On July 14, the executive committee of the American Neurological Association issued a statement asking for a speedier timeline.
The FDA should ensure that Biogen completes the required confirmatory study “as soon as possible, preferably within 3 years, to confirm or not whether clinical efficacy is observed,” the ANA executive committee wrote in the letter.
The ANA executive committee also criticized the FDA’s decision to allow Biogen to begin sales of the drug. In light of the clinical evidence available at this time, aducanumab “should not have been approved” in the first place, the ANA executive committee stated.
drawing from the discussion at the meeting and the panel’s votes. The work of the Boston-based group is used by private insurers to inform medication coverage decisions.
Lawmakers have taken an interest in aducanumab. On July 12, two top Democrats in the U.S. House of Representatives released a letter that they had sent to Biogen as part of their investigation into how the FDA handled the aducanumab approval and Biogen’s pricing for the drug.
In the letter, House Energy and Commerce Chairman Frank Pallone Jr. (D-N.J.) and Oversight and Reform Chairwoman Carolyn B. Maloney (D-N.Y.) wrote that they had “significant questions about the drug’s clinical benefit, and the steep $56,000 annual price tag.”
At the ICER meeting on July 15, Dr. Lee said patients with AD and their caregivers would benefit more from increased spending on supportive services, such as home health care.
“There’s so many things we could do” with money that Biogen may get for aducanumab, Dr. Lee said. “To spend it on a medication that is more likely to do more harm than help seems really ill advised.”
A version of this article first appeared on Medscape.com.
adding to growing opposition from medical experts to the Food and Drug Administration’s approval of this controversial drug.
The Institute for Clinical and Economic Review asked one of its expert panels, the California Technology Assessment Forum, to consider the available data about aducanumab and requested that members vote on whether there was sufficient evidence of a net benefit of aducanumab plus supportive care versus supportive care alone. All 15 panelists voted no.
Several panelists, including ICER President Steven D. Pearson, MD, talked about their personal experience with family members who have the disease.
There was universal agreement among the panelists that there is an urgent need for effective medications to treat the disease. However, the panel of clinicians and researchers also agreed that the evidence to date does not show that the drug helps patients with this debilitating disease.
Panelist Sei Lee, MD, a geriatrician at the University of California, San Francisco, said he lost his mother to AD 6 years ago. In addition to his clinical work, Dr. Lee has conducted research focused on improving the targeting of preventive AD interventions for older adults to maximize benefits and minimize harms.
Dr. Lee said he frequently felt completely overwhelmed by the challenges of his mother’s disease.
“I absolutely hear everyone who is saying we need an effective therapy for this,” Dr. Lee said.
Dr. Lee added that, as an experienced researcher who has weighed the aducanumab data, he saw no clear proof of a benefit that would outweigh the drug’s documented side effects in the two phase 3 trials of the drug. Those side effects include temporary brain swelling. Dr. Lee suggested that Biogen do more to address concerns about this side effect, saying it should not be ignored.
“There’s clearly substantial uncertainty” about aducanumab, Dr. Lee said. “If I had to guess, I think the data is stronger for net harm than it is for that benefit.”
Questions persist about the data Biogen used in support of aducanumab after announcing that the drug had failed in a dual-track phase 3 program.
In March 2019, it was announced that two phase 3 clinical trials, EMERGE and ENGAGE, were scrapped because of disappointing results. The trials were intended to show that aducanumab could slow progression of cognitive and functional impairment, as measured by changes in scores on the Clinical Dementia Rating–Sum of Boxes (CDR-SB).
However, in October 2019, there was an about-face – Biogen announced that, in one of the studies, there were positive findings for a subset of patients who received a higher dose of aducanumab.
No treatment benefit was observed in either the high- or low-dose arms at week 78 in the ENGAGE trial. In the EMERGE trial, however, there was a statistically significant difference in change from baseline in CDR-SB score in the high-dose arm (difference vs. placebo, –0.39; 95% confidence interval, –0.69 to –0.09) but not in the low-dose arm, ICER noted in a draft report.
Still, there are questions about whether this difference would translate into clinical benefit. “Although statistically significant, the change in CDR-SB score in the high-dose group was less than the 1- to 2-point change that has been suggested as a minimal clinically important difference,” ICER staff wrote.
More push back
Other influential organizations also remain skeptical.
On July 14, the Cleveland Clinic announced it would not use aducanumab at this time, following a staff review of the evidence. Physicians from the clinic could prescribe it to appropriate patients, who would receive their infusions at external facilities, a spokeswoman for the clinic told this news organization. The Cleveland Clinic said it will reevaluate this position as additional data become available.
In addition, as reported by the New York Times, the Mount Sinai Health System in New York also decided not to administer the drug.
The drug received accelerated approval from the FDA. That approval was conditional upon Biogen’s conducting further research by 2030 that demonstrates that the drug has clinical benefit.
On July 14, the executive committee of the American Neurological Association issued a statement asking for a speedier timeline.
The FDA should ensure that Biogen completes the required confirmatory study “as soon as possible, preferably within 3 years, to confirm or not whether clinical efficacy is observed,” the ANA executive committee wrote in the letter.
The ANA executive committee also criticized the FDA’s decision to allow Biogen to begin sales of the drug. In light of the clinical evidence available at this time, aducanumab “should not have been approved” in the first place, the ANA executive committee stated.
drawing from the discussion at the meeting and the panel’s votes. The work of the Boston-based group is used by private insurers to inform medication coverage decisions.
Lawmakers have taken an interest in aducanumab. On July 12, two top Democrats in the U.S. House of Representatives released a letter that they had sent to Biogen as part of their investigation into how the FDA handled the aducanumab approval and Biogen’s pricing for the drug.
In the letter, House Energy and Commerce Chairman Frank Pallone Jr. (D-N.J.) and Oversight and Reform Chairwoman Carolyn B. Maloney (D-N.Y.) wrote that they had “significant questions about the drug’s clinical benefit, and the steep $56,000 annual price tag.”
At the ICER meeting on July 15, Dr. Lee said patients with AD and their caregivers would benefit more from increased spending on supportive services, such as home health care.
“There’s so many things we could do” with money that Biogen may get for aducanumab, Dr. Lee said. “To spend it on a medication that is more likely to do more harm than help seems really ill advised.”
A version of this article first appeared on Medscape.com.
adding to growing opposition from medical experts to the Food and Drug Administration’s approval of this controversial drug.
The Institute for Clinical and Economic Review asked one of its expert panels, the California Technology Assessment Forum, to consider the available data about aducanumab and requested that members vote on whether there was sufficient evidence of a net benefit of aducanumab plus supportive care versus supportive care alone. All 15 panelists voted no.
Several panelists, including ICER President Steven D. Pearson, MD, talked about their personal experience with family members who have the disease.
There was universal agreement among the panelists that there is an urgent need for effective medications to treat the disease. However, the panel of clinicians and researchers also agreed that the evidence to date does not show that the drug helps patients with this debilitating disease.
Panelist Sei Lee, MD, a geriatrician at the University of California, San Francisco, said he lost his mother to AD 6 years ago. In addition to his clinical work, Dr. Lee has conducted research focused on improving the targeting of preventive AD interventions for older adults to maximize benefits and minimize harms.
Dr. Lee said he frequently felt completely overwhelmed by the challenges of his mother’s disease.
“I absolutely hear everyone who is saying we need an effective therapy for this,” Dr. Lee said.
Dr. Lee added that, as an experienced researcher who has weighed the aducanumab data, he saw no clear proof of a benefit that would outweigh the drug’s documented side effects in the two phase 3 trials of the drug. Those side effects include temporary brain swelling. Dr. Lee suggested that Biogen do more to address concerns about this side effect, saying it should not be ignored.
“There’s clearly substantial uncertainty” about aducanumab, Dr. Lee said. “If I had to guess, I think the data is stronger for net harm than it is for that benefit.”
Questions persist about the data Biogen used in support of aducanumab after announcing that the drug had failed in a dual-track phase 3 program.
In March 2019, it was announced that two phase 3 clinical trials, EMERGE and ENGAGE, were scrapped because of disappointing results. The trials were intended to show that aducanumab could slow progression of cognitive and functional impairment, as measured by changes in scores on the Clinical Dementia Rating–Sum of Boxes (CDR-SB).
However, in October 2019, there was an about-face – Biogen announced that, in one of the studies, there were positive findings for a subset of patients who received a higher dose of aducanumab.
No treatment benefit was observed in either the high- or low-dose arms at week 78 in the ENGAGE trial. In the EMERGE trial, however, there was a statistically significant difference in change from baseline in CDR-SB score in the high-dose arm (difference vs. placebo, –0.39; 95% confidence interval, –0.69 to –0.09) but not in the low-dose arm, ICER noted in a draft report.
Still, there are questions about whether this difference would translate into clinical benefit. “Although statistically significant, the change in CDR-SB score in the high-dose group was less than the 1- to 2-point change that has been suggested as a minimal clinically important difference,” ICER staff wrote.
More push back
Other influential organizations also remain skeptical.
On July 14, the Cleveland Clinic announced it would not use aducanumab at this time, following a staff review of the evidence. Physicians from the clinic could prescribe it to appropriate patients, who would receive their infusions at external facilities, a spokeswoman for the clinic told this news organization. The Cleveland Clinic said it will reevaluate this position as additional data become available.
In addition, as reported by the New York Times, the Mount Sinai Health System in New York also decided not to administer the drug.
The drug received accelerated approval from the FDA. That approval was conditional upon Biogen’s conducting further research by 2030 that demonstrates that the drug has clinical benefit.
On July 14, the executive committee of the American Neurological Association issued a statement asking for a speedier timeline.
The FDA should ensure that Biogen completes the required confirmatory study “as soon as possible, preferably within 3 years, to confirm or not whether clinical efficacy is observed,” the ANA executive committee wrote in the letter.
The ANA executive committee also criticized the FDA’s decision to allow Biogen to begin sales of the drug. In light of the clinical evidence available at this time, aducanumab “should not have been approved” in the first place, the ANA executive committee stated.
drawing from the discussion at the meeting and the panel’s votes. The work of the Boston-based group is used by private insurers to inform medication coverage decisions.
Lawmakers have taken an interest in aducanumab. On July 12, two top Democrats in the U.S. House of Representatives released a letter that they had sent to Biogen as part of their investigation into how the FDA handled the aducanumab approval and Biogen’s pricing for the drug.
In the letter, House Energy and Commerce Chairman Frank Pallone Jr. (D-N.J.) and Oversight and Reform Chairwoman Carolyn B. Maloney (D-N.Y.) wrote that they had “significant questions about the drug’s clinical benefit, and the steep $56,000 annual price tag.”
At the ICER meeting on July 15, Dr. Lee said patients with AD and their caregivers would benefit more from increased spending on supportive services, such as home health care.
“There’s so many things we could do” with money that Biogen may get for aducanumab, Dr. Lee said. “To spend it on a medication that is more likely to do more harm than help seems really ill advised.”
A version of this article first appeared on Medscape.com.
Contentious Alzheimer’s drug likely to get national coverage plan, CMS says
On July 12, a process that will take until next year to complete.
The Centers for Medicare & Medicaid Services said it will accept public comments about how Medicare should cover aducanumab through Aug. 11. The agency intends to post a draft decision memo on its coverage approach by Jan. 12, 2022, and then finalize this policy by April 12. Coverage decisions about aducanumab now are being made at the local level by Medicare’s administrative contractors, CMS said in a press release.
The announcement followed separate public calls for such a review by America’s Health Insurance Plans (AHIP) and the Alzheimer’s Association.
On June 30, AHIP submitted a formal request to the CMS. In it, AHIP requests that CMS take “swift action” on a national coverage determination for aducanumab. In the request, the organization specifically urged CMS to use a policy known as coverage with evidence development (CED) for Aduhelm.
This CED approach would allow access for patients considered most likely to benefit from the drug while Biogen continues research needed to definitively show its clinical benefit, said AHIP chief executive Matt Eyles.
In June, the Food and Drug Administration approved aducanumab based on data suggesting the drug might slow AD progression using the surrogate marker of a reduction in amyloid plaque.
The FDA’s accelerated approval letter set a 2030 deadline for Biogen to produce evidence from a phase 3 clinical trial definitively proving the drug’s efficacy.
Hefty price tag
Even if Biogen meets the FDA’s deadline, patients with AD, their families, clinicians, and insurers likely will wrestle for years with questions about whether to use this costly drug without clear evidence of benefit. The drug is estimated to cost $56,000 per year.
In addition, patients taking the drug will be required to undergo MRI scans to monitor for brain swelling or bleeding, complications that were experienced by those participating in previous studies of the drug, Mr. Eyles noted in his letter to CMS, which AHIP provided to this news organization.
About 80% of those eligible for aducanumab in the United States are enrolled in Medicare, write James D. Chambers, PhD, MPharm, Tufts University, Boston, and coauthors in a June article in the journal Health Affairs. Like AHIP, these authors also recommended CMS consider the CED path for the drug.
CMS has used the CED approach since 2003 to evaluate interventions such as amyloid PET for clinical evaluation of AD to implantable cardioverter defibrillators.
Applying CED to aducanumab “would provide the medical community, patients, caregivers, and payers with additional information long before the FDA’s required postapproval studies are completed,” Dr. Chambers and coauthors wrote. “It would also ensure that data on every patient treated would add to the knowledge base about how aducanumab impacts patient outcomes such as cognition, function, and quality of life.”
In the AHIP request to CMS, Mr. Eyles also noted that an independent review organization, the Institute for Clinical and Economic Review, said the evidence from studies done to date on aducanumab is “insufficient” to show a net health benefit for patients with mild cognitive impairment because of AD or mild AD.
At the ICER meeting, which will take place July 15, one of ICER’s expert panels, the California Technology Assessment Forum, said it will further consider all of the available scientific data on aducanumab and vote on a series of questions about its efficacy and value.
ICER’s reports have clout because insurers use its recommendations to help determine how to cover drugs and medical treatments. Among the questions ICER has posted online ahead of the meeting is one about the relative effects of aducanumab plus supportive care versus supportive care alone.
‘Dark irony’
Even as the medical community waits for Biogen to present clear evidence of a benefit for aducanumab, clinics specializing in AD may get a financial boost, said Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, and codirector of Penn’s Memory Center.
Some clinicians see the arrival of the drug as a “win” for the field despite lingering concerns about its approval, said Dr. Karlawish at a panel discussion held July 12 by the nonprofit Hastings Center, a bioethics research institute. Dr. Karlawish is a fellow at Hastings.
In May, Dr. Karlawish published an article in STAT titled “If the FDA approves Biogen’s Alzheimer’s treatment, I won’t prescribe it.” Dr. Karlawish told this news organization that he was a site investigator for Biogen studies of aducanumab and has worked on studies sponsored by Lilly and Eisai.
During the discussion July 12, Dr. Karlawish said he had altered his view and now might be a “reluctant prescriber.” This shift is because of his commitment “to preserve, protect and defend their autonomy” of patients with AD.
He also noted the drug could draw more money into the field to help care for patients with AD by providing increased access to diagnostics. Additionally, funds provided to clinics for administering aducanumab will aid specialty memory centers, “which have been basically impoverished since their creation,” Dr. Karlawish said.
“There is a dark irony that it takes a questionably beneficial drug to bring in the revenue to finally get memory centers up and functioning,” Dr. Karlawish said, adding that there needs to be “a larger conversation about how a big, vast, and problematic disease is being treated.”
Aducanumab’s approval shows that diseases in the U.S. are not fully considered as diseases until they have “a business model, and much of that business model relies on the pharmaceutical industry,” he noted.
Dr. Woodcock’s ‘personal commitment’
In early July, the FDA took two highly publicized steps to address criticism of its handling of the aducanumab approval. It revised the drug’s label to limit its use to patients with mild cognitive impairment likely related to AD or those in the mild stages of the disease.
In addition, Janet Woodcock, MD, the FDA’s acting commissioner, took to Twitter and posted a letter she sent to the Office of the Inspector General that called for a federal investigation into the drug’s approval that would examine agency staff interactions with Biogen.
AHIP spokesperson Kristine Grow said July 12 that her organization is still seeking a national Medicare coverage decision, but that the label revision was a “step in the right direction.”
“Patients with Alzheimer’s disease, and their families and caregivers, deserve safe, effective treatments. We applaud the FDA for this label adjustment, which brings indicated patients a bit closer to those included in clinical trials,” Ms. Grow said in an interview.
“At the same time, we remain concerned about the limited clinical evidence demonstrating efficacy and the serious safety risks that aducanumab poses for patients. We look forward to additional information from the FDA and other regulators, including CMS’ coverage guidance for patients who are Medicare eligible,” she added.
The controversy surrounding the approval of aducanumab is drawing more attention to the lack of a confirmed FDA commissioner. But in her letter to OIG, Dr. Woodcock wrote as if she intends to remain at the helm of the agency for at least a while longer. She wrote in her letter that OIG has her “personal commitment” that the FDA will fully cooperate if the investigative unit decides to undertake a review.
Dr. Woodcock also urged that a review be conducted as soon as possible, noting “should such a review result in actionable items, you also have my commitment to addressing these issues.”
A former FDA adviser who resigned over the agency’s handling of aducanumab said July 12 there needs to be a broader investigation of the FDA’s actions.
Attending the Hastings Center event was Aaron S. Kesselheim, MD, JD, MPH, of Harvard Medical School, Boston, one of three former members of an FDA advisory committee who resigned over the agency’s handling of aducanumab. Dr. Kesselheim said in an interview that he has no financial relationships to disclose in connection with this discussion.
“I would suggest that instead all aspects of this approval process should be investigated,” Dr. Kesselheim said, including the relationship between FDA and Biogen.
Dr. Karlawish said he was also concerned that Dr. Woodcock’s request for an investigation was “very narrow,” and noted members of Congress have said they are examining the FDA’s handling of this drug.
In a July 9 joint statement, House Committee on Energy and Commerce Chairman Frank Pallone Jr (D-N.J.), and House Committee on Oversight and Reform Chairwoman Carolyn B. Maloney (D-N.Y.) said they were “pleased” by Dr. Woodcock’s announcement, but they will keep digging into ongoing questions about the drug. In their view, the OIG review of FDA staff interactions with Biogen officials would complement their committees’ “robust investigation of this matter.”
“We continue to have concerns about the approval process for Aduhelm, how Biogen set its price, and the implications for seniors, providers, and taxpayers,” Mr. Pallone and Ms. Maloney added.
A version of this article first appeared on Medscape.com.
On July 12, a process that will take until next year to complete.
The Centers for Medicare & Medicaid Services said it will accept public comments about how Medicare should cover aducanumab through Aug. 11. The agency intends to post a draft decision memo on its coverage approach by Jan. 12, 2022, and then finalize this policy by April 12. Coverage decisions about aducanumab now are being made at the local level by Medicare’s administrative contractors, CMS said in a press release.
The announcement followed separate public calls for such a review by America’s Health Insurance Plans (AHIP) and the Alzheimer’s Association.
On June 30, AHIP submitted a formal request to the CMS. In it, AHIP requests that CMS take “swift action” on a national coverage determination for aducanumab. In the request, the organization specifically urged CMS to use a policy known as coverage with evidence development (CED) for Aduhelm.
This CED approach would allow access for patients considered most likely to benefit from the drug while Biogen continues research needed to definitively show its clinical benefit, said AHIP chief executive Matt Eyles.
In June, the Food and Drug Administration approved aducanumab based on data suggesting the drug might slow AD progression using the surrogate marker of a reduction in amyloid plaque.
The FDA’s accelerated approval letter set a 2030 deadline for Biogen to produce evidence from a phase 3 clinical trial definitively proving the drug’s efficacy.
Hefty price tag
Even if Biogen meets the FDA’s deadline, patients with AD, their families, clinicians, and insurers likely will wrestle for years with questions about whether to use this costly drug without clear evidence of benefit. The drug is estimated to cost $56,000 per year.
In addition, patients taking the drug will be required to undergo MRI scans to monitor for brain swelling or bleeding, complications that were experienced by those participating in previous studies of the drug, Mr. Eyles noted in his letter to CMS, which AHIP provided to this news organization.
About 80% of those eligible for aducanumab in the United States are enrolled in Medicare, write James D. Chambers, PhD, MPharm, Tufts University, Boston, and coauthors in a June article in the journal Health Affairs. Like AHIP, these authors also recommended CMS consider the CED path for the drug.
CMS has used the CED approach since 2003 to evaluate interventions such as amyloid PET for clinical evaluation of AD to implantable cardioverter defibrillators.
Applying CED to aducanumab “would provide the medical community, patients, caregivers, and payers with additional information long before the FDA’s required postapproval studies are completed,” Dr. Chambers and coauthors wrote. “It would also ensure that data on every patient treated would add to the knowledge base about how aducanumab impacts patient outcomes such as cognition, function, and quality of life.”
In the AHIP request to CMS, Mr. Eyles also noted that an independent review organization, the Institute for Clinical and Economic Review, said the evidence from studies done to date on aducanumab is “insufficient” to show a net health benefit for patients with mild cognitive impairment because of AD or mild AD.
At the ICER meeting, which will take place July 15, one of ICER’s expert panels, the California Technology Assessment Forum, said it will further consider all of the available scientific data on aducanumab and vote on a series of questions about its efficacy and value.
ICER’s reports have clout because insurers use its recommendations to help determine how to cover drugs and medical treatments. Among the questions ICER has posted online ahead of the meeting is one about the relative effects of aducanumab plus supportive care versus supportive care alone.
‘Dark irony’
Even as the medical community waits for Biogen to present clear evidence of a benefit for aducanumab, clinics specializing in AD may get a financial boost, said Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, and codirector of Penn’s Memory Center.
Some clinicians see the arrival of the drug as a “win” for the field despite lingering concerns about its approval, said Dr. Karlawish at a panel discussion held July 12 by the nonprofit Hastings Center, a bioethics research institute. Dr. Karlawish is a fellow at Hastings.
In May, Dr. Karlawish published an article in STAT titled “If the FDA approves Biogen’s Alzheimer’s treatment, I won’t prescribe it.” Dr. Karlawish told this news organization that he was a site investigator for Biogen studies of aducanumab and has worked on studies sponsored by Lilly and Eisai.
During the discussion July 12, Dr. Karlawish said he had altered his view and now might be a “reluctant prescriber.” This shift is because of his commitment “to preserve, protect and defend their autonomy” of patients with AD.
He also noted the drug could draw more money into the field to help care for patients with AD by providing increased access to diagnostics. Additionally, funds provided to clinics for administering aducanumab will aid specialty memory centers, “which have been basically impoverished since their creation,” Dr. Karlawish said.
“There is a dark irony that it takes a questionably beneficial drug to bring in the revenue to finally get memory centers up and functioning,” Dr. Karlawish said, adding that there needs to be “a larger conversation about how a big, vast, and problematic disease is being treated.”
Aducanumab’s approval shows that diseases in the U.S. are not fully considered as diseases until they have “a business model, and much of that business model relies on the pharmaceutical industry,” he noted.
Dr. Woodcock’s ‘personal commitment’
In early July, the FDA took two highly publicized steps to address criticism of its handling of the aducanumab approval. It revised the drug’s label to limit its use to patients with mild cognitive impairment likely related to AD or those in the mild stages of the disease.
In addition, Janet Woodcock, MD, the FDA’s acting commissioner, took to Twitter and posted a letter she sent to the Office of the Inspector General that called for a federal investigation into the drug’s approval that would examine agency staff interactions with Biogen.
AHIP spokesperson Kristine Grow said July 12 that her organization is still seeking a national Medicare coverage decision, but that the label revision was a “step in the right direction.”
“Patients with Alzheimer’s disease, and their families and caregivers, deserve safe, effective treatments. We applaud the FDA for this label adjustment, which brings indicated patients a bit closer to those included in clinical trials,” Ms. Grow said in an interview.
“At the same time, we remain concerned about the limited clinical evidence demonstrating efficacy and the serious safety risks that aducanumab poses for patients. We look forward to additional information from the FDA and other regulators, including CMS’ coverage guidance for patients who are Medicare eligible,” she added.
The controversy surrounding the approval of aducanumab is drawing more attention to the lack of a confirmed FDA commissioner. But in her letter to OIG, Dr. Woodcock wrote as if she intends to remain at the helm of the agency for at least a while longer. She wrote in her letter that OIG has her “personal commitment” that the FDA will fully cooperate if the investigative unit decides to undertake a review.
Dr. Woodcock also urged that a review be conducted as soon as possible, noting “should such a review result in actionable items, you also have my commitment to addressing these issues.”
A former FDA adviser who resigned over the agency’s handling of aducanumab said July 12 there needs to be a broader investigation of the FDA’s actions.
Attending the Hastings Center event was Aaron S. Kesselheim, MD, JD, MPH, of Harvard Medical School, Boston, one of three former members of an FDA advisory committee who resigned over the agency’s handling of aducanumab. Dr. Kesselheim said in an interview that he has no financial relationships to disclose in connection with this discussion.
“I would suggest that instead all aspects of this approval process should be investigated,” Dr. Kesselheim said, including the relationship between FDA and Biogen.
Dr. Karlawish said he was also concerned that Dr. Woodcock’s request for an investigation was “very narrow,” and noted members of Congress have said they are examining the FDA’s handling of this drug.
In a July 9 joint statement, House Committee on Energy and Commerce Chairman Frank Pallone Jr (D-N.J.), and House Committee on Oversight and Reform Chairwoman Carolyn B. Maloney (D-N.Y.) said they were “pleased” by Dr. Woodcock’s announcement, but they will keep digging into ongoing questions about the drug. In their view, the OIG review of FDA staff interactions with Biogen officials would complement their committees’ “robust investigation of this matter.”
“We continue to have concerns about the approval process for Aduhelm, how Biogen set its price, and the implications for seniors, providers, and taxpayers,” Mr. Pallone and Ms. Maloney added.
A version of this article first appeared on Medscape.com.
On July 12, a process that will take until next year to complete.
The Centers for Medicare & Medicaid Services said it will accept public comments about how Medicare should cover aducanumab through Aug. 11. The agency intends to post a draft decision memo on its coverage approach by Jan. 12, 2022, and then finalize this policy by April 12. Coverage decisions about aducanumab now are being made at the local level by Medicare’s administrative contractors, CMS said in a press release.
The announcement followed separate public calls for such a review by America’s Health Insurance Plans (AHIP) and the Alzheimer’s Association.
On June 30, AHIP submitted a formal request to the CMS. In it, AHIP requests that CMS take “swift action” on a national coverage determination for aducanumab. In the request, the organization specifically urged CMS to use a policy known as coverage with evidence development (CED) for Aduhelm.
This CED approach would allow access for patients considered most likely to benefit from the drug while Biogen continues research needed to definitively show its clinical benefit, said AHIP chief executive Matt Eyles.
In June, the Food and Drug Administration approved aducanumab based on data suggesting the drug might slow AD progression using the surrogate marker of a reduction in amyloid plaque.
The FDA’s accelerated approval letter set a 2030 deadline for Biogen to produce evidence from a phase 3 clinical trial definitively proving the drug’s efficacy.
Hefty price tag
Even if Biogen meets the FDA’s deadline, patients with AD, their families, clinicians, and insurers likely will wrestle for years with questions about whether to use this costly drug without clear evidence of benefit. The drug is estimated to cost $56,000 per year.
In addition, patients taking the drug will be required to undergo MRI scans to monitor for brain swelling or bleeding, complications that were experienced by those participating in previous studies of the drug, Mr. Eyles noted in his letter to CMS, which AHIP provided to this news organization.
About 80% of those eligible for aducanumab in the United States are enrolled in Medicare, write James D. Chambers, PhD, MPharm, Tufts University, Boston, and coauthors in a June article in the journal Health Affairs. Like AHIP, these authors also recommended CMS consider the CED path for the drug.
CMS has used the CED approach since 2003 to evaluate interventions such as amyloid PET for clinical evaluation of AD to implantable cardioverter defibrillators.
Applying CED to aducanumab “would provide the medical community, patients, caregivers, and payers with additional information long before the FDA’s required postapproval studies are completed,” Dr. Chambers and coauthors wrote. “It would also ensure that data on every patient treated would add to the knowledge base about how aducanumab impacts patient outcomes such as cognition, function, and quality of life.”
In the AHIP request to CMS, Mr. Eyles also noted that an independent review organization, the Institute for Clinical and Economic Review, said the evidence from studies done to date on aducanumab is “insufficient” to show a net health benefit for patients with mild cognitive impairment because of AD or mild AD.
At the ICER meeting, which will take place July 15, one of ICER’s expert panels, the California Technology Assessment Forum, said it will further consider all of the available scientific data on aducanumab and vote on a series of questions about its efficacy and value.
ICER’s reports have clout because insurers use its recommendations to help determine how to cover drugs and medical treatments. Among the questions ICER has posted online ahead of the meeting is one about the relative effects of aducanumab plus supportive care versus supportive care alone.
‘Dark irony’
Even as the medical community waits for Biogen to present clear evidence of a benefit for aducanumab, clinics specializing in AD may get a financial boost, said Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, and codirector of Penn’s Memory Center.
Some clinicians see the arrival of the drug as a “win” for the field despite lingering concerns about its approval, said Dr. Karlawish at a panel discussion held July 12 by the nonprofit Hastings Center, a bioethics research institute. Dr. Karlawish is a fellow at Hastings.
In May, Dr. Karlawish published an article in STAT titled “If the FDA approves Biogen’s Alzheimer’s treatment, I won’t prescribe it.” Dr. Karlawish told this news organization that he was a site investigator for Biogen studies of aducanumab and has worked on studies sponsored by Lilly and Eisai.
During the discussion July 12, Dr. Karlawish said he had altered his view and now might be a “reluctant prescriber.” This shift is because of his commitment “to preserve, protect and defend their autonomy” of patients with AD.
He also noted the drug could draw more money into the field to help care for patients with AD by providing increased access to diagnostics. Additionally, funds provided to clinics for administering aducanumab will aid specialty memory centers, “which have been basically impoverished since their creation,” Dr. Karlawish said.
“There is a dark irony that it takes a questionably beneficial drug to bring in the revenue to finally get memory centers up and functioning,” Dr. Karlawish said, adding that there needs to be “a larger conversation about how a big, vast, and problematic disease is being treated.”
Aducanumab’s approval shows that diseases in the U.S. are not fully considered as diseases until they have “a business model, and much of that business model relies on the pharmaceutical industry,” he noted.
Dr. Woodcock’s ‘personal commitment’
In early July, the FDA took two highly publicized steps to address criticism of its handling of the aducanumab approval. It revised the drug’s label to limit its use to patients with mild cognitive impairment likely related to AD or those in the mild stages of the disease.
In addition, Janet Woodcock, MD, the FDA’s acting commissioner, took to Twitter and posted a letter she sent to the Office of the Inspector General that called for a federal investigation into the drug’s approval that would examine agency staff interactions with Biogen.
AHIP spokesperson Kristine Grow said July 12 that her organization is still seeking a national Medicare coverage decision, but that the label revision was a “step in the right direction.”
“Patients with Alzheimer’s disease, and their families and caregivers, deserve safe, effective treatments. We applaud the FDA for this label adjustment, which brings indicated patients a bit closer to those included in clinical trials,” Ms. Grow said in an interview.
“At the same time, we remain concerned about the limited clinical evidence demonstrating efficacy and the serious safety risks that aducanumab poses for patients. We look forward to additional information from the FDA and other regulators, including CMS’ coverage guidance for patients who are Medicare eligible,” she added.
The controversy surrounding the approval of aducanumab is drawing more attention to the lack of a confirmed FDA commissioner. But in her letter to OIG, Dr. Woodcock wrote as if she intends to remain at the helm of the agency for at least a while longer. She wrote in her letter that OIG has her “personal commitment” that the FDA will fully cooperate if the investigative unit decides to undertake a review.
Dr. Woodcock also urged that a review be conducted as soon as possible, noting “should such a review result in actionable items, you also have my commitment to addressing these issues.”
A former FDA adviser who resigned over the agency’s handling of aducanumab said July 12 there needs to be a broader investigation of the FDA’s actions.
Attending the Hastings Center event was Aaron S. Kesselheim, MD, JD, MPH, of Harvard Medical School, Boston, one of three former members of an FDA advisory committee who resigned over the agency’s handling of aducanumab. Dr. Kesselheim said in an interview that he has no financial relationships to disclose in connection with this discussion.
“I would suggest that instead all aspects of this approval process should be investigated,” Dr. Kesselheim said, including the relationship between FDA and Biogen.
Dr. Karlawish said he was also concerned that Dr. Woodcock’s request for an investigation was “very narrow,” and noted members of Congress have said they are examining the FDA’s handling of this drug.
In a July 9 joint statement, House Committee on Energy and Commerce Chairman Frank Pallone Jr (D-N.J.), and House Committee on Oversight and Reform Chairwoman Carolyn B. Maloney (D-N.Y.) said they were “pleased” by Dr. Woodcock’s announcement, but they will keep digging into ongoing questions about the drug. In their view, the OIG review of FDA staff interactions with Biogen officials would complement their committees’ “robust investigation of this matter.”
“We continue to have concerns about the approval process for Aduhelm, how Biogen set its price, and the implications for seniors, providers, and taxpayers,” Mr. Pallone and Ms. Maloney added.
A version of this article first appeared on Medscape.com.
Dance training ‘drastically’ reduces Parkinson’s progression, eases symptoms
Over 3 years, weekly participation in dance training classes “drastically” reduced the expected decline in motor function and significantly improved speech, tremors, balance, and stiffness, the researchers reported.
Dance training also appeared to have benefits regarding cognition, hallucinations, depression, and anxiety.
“These findings strongly suggest the benefits of dance for people with PD as a supplement to a normal treatment regimen,” the investigators noted.
Although the mechanism of benefit is unclear, dance training may help “train neural network nodes that helps either strengthen networks damaged or builds neural road maps that pass the damage,” study investigator Joseph DeSouza, PhD, principal investigator and associate professor, department of psychology, York University, Toronto, said in an interview.
The study was published online July 7, 2021, in Brain Sciences.
Multiple benefits
PD is a neurodegenerative disease associated with progression of motor dysfunction within the first 5 years of diagnosis. The annual rate of motor decline, as determined with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), is between 5.2 and 8.9 points.
Prior studies that assessed various styles of dance by patients with PD showed beneficial effects regarding gait speed, balance, locomotion, and aspects of quality of life.
To investigate further, DeSouza and coauthor Karolina Bearss, a PhD candidate at York University, followed 16 patients with mild to moderate PD who participated in a weekly dance class at Canada’s National Ballet School and Trinity St. Paul’s church.
Dance for Parkinson’s Disease, which is an established dance curriculum, involves aerobic and anaerobic movements. The protocol begins with a seated warm-up, followed by barre work, and ends with moving across the floor. All participants learn choreography for an upcoming performance.
In the study, 16 patients with PD who did not participant in the dance classes served as control patients.
Over 3 years, the daily rate of motor decline, as indicated by scores on part III of the MDS-UPDRS, was zero among the dancers (slope = 0.000146), indicating no motor impairment, whereas among the nondancers, the motor decline during follow-up was as expected (P < .01), the researchers reported.
In modeling the data, the researchers determined that after completing 1,000 days of dance training, dancers will have a motor score of 19.07, compared with a score of 28.27 for nondancers.
“Our data further showed that training in dance will slow the rate of PD motor impairment progression, as measured by the UPDRS III, by close to 3 points annually in comparison to our PD subjects who did not train,” the researchers reported.
Dance training also had a beneficial effect on motor or nonmotor aspects of daily living and on motor complications, for which there was no significant decline among the PD dancers.
“For those with Parkinson’s disease, even when it’s mild, motor impairment can impact their daily functioning – how they feel about themselves. Many of these motor symptoms lead to isolation because once they get extreme, these people don’t want to go out,” Dr. DeSouza said in a news release.
“These motor symptoms lead to further psychological issues, depression, social isolation and eventually the symptoms do get worse over time. Our study shows that training with dance and music can slow this down and improve their daily living and daily function,” he added.
‘Great potential’
Reached for comment, Demian Kogutek, PhD, director of music therapy, University of Evansville (Indiana), said that these preliminary findings from a longitudinal study are “promising.”
“I believe that dance therapy has a great potential for PD. The longitudinal aspect of this study undoubtedly adds to the current literature. Although it is a standardized assessment, it is somewhat subjective,” Dr. Kogutek said in an interview.
Going forward, Dr. Kogutek said he’d like to see other objective outcomes measured, such as objective assessments of balance, gait, hand strength, and dexterity.
Also weighing in on the results, Karen Lee, PhD, president and CEO of Parkinson Canada, said her organization is “encouraged by these preliminary findings as exercise and healthy activities are important for people with Parkinson’s. This study is part of a growing body of research that explores the link between the impact of activities and both motor and nonmotor symptoms of Parkinson’s.
“This research adds to growing evidence about the importance of exercise as part of the management of Parkinson’s, and we encourage people living with Parkinson’s to incorporate exercise as part of their approach to managing their health,” Dr. Lee said in an interview.
Funding for the project is provided in part by a National Science and Engineering Research Council Discovery Grant and by donations from the Irpinia Club of Toronto and others. Dr. Dr. DeSouza, Ms. Bearss, Dr. Kogutek, and Dr. Lee disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 3 years, weekly participation in dance training classes “drastically” reduced the expected decline in motor function and significantly improved speech, tremors, balance, and stiffness, the researchers reported.
Dance training also appeared to have benefits regarding cognition, hallucinations, depression, and anxiety.
“These findings strongly suggest the benefits of dance for people with PD as a supplement to a normal treatment regimen,” the investigators noted.
Although the mechanism of benefit is unclear, dance training may help “train neural network nodes that helps either strengthen networks damaged or builds neural road maps that pass the damage,” study investigator Joseph DeSouza, PhD, principal investigator and associate professor, department of psychology, York University, Toronto, said in an interview.
The study was published online July 7, 2021, in Brain Sciences.
Multiple benefits
PD is a neurodegenerative disease associated with progression of motor dysfunction within the first 5 years of diagnosis. The annual rate of motor decline, as determined with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), is between 5.2 and 8.9 points.
Prior studies that assessed various styles of dance by patients with PD showed beneficial effects regarding gait speed, balance, locomotion, and aspects of quality of life.
To investigate further, DeSouza and coauthor Karolina Bearss, a PhD candidate at York University, followed 16 patients with mild to moderate PD who participated in a weekly dance class at Canada’s National Ballet School and Trinity St. Paul’s church.
Dance for Parkinson’s Disease, which is an established dance curriculum, involves aerobic and anaerobic movements. The protocol begins with a seated warm-up, followed by barre work, and ends with moving across the floor. All participants learn choreography for an upcoming performance.
In the study, 16 patients with PD who did not participant in the dance classes served as control patients.
Over 3 years, the daily rate of motor decline, as indicated by scores on part III of the MDS-UPDRS, was zero among the dancers (slope = 0.000146), indicating no motor impairment, whereas among the nondancers, the motor decline during follow-up was as expected (P < .01), the researchers reported.
In modeling the data, the researchers determined that after completing 1,000 days of dance training, dancers will have a motor score of 19.07, compared with a score of 28.27 for nondancers.
“Our data further showed that training in dance will slow the rate of PD motor impairment progression, as measured by the UPDRS III, by close to 3 points annually in comparison to our PD subjects who did not train,” the researchers reported.
Dance training also had a beneficial effect on motor or nonmotor aspects of daily living and on motor complications, for which there was no significant decline among the PD dancers.
“For those with Parkinson’s disease, even when it’s mild, motor impairment can impact their daily functioning – how they feel about themselves. Many of these motor symptoms lead to isolation because once they get extreme, these people don’t want to go out,” Dr. DeSouza said in a news release.
“These motor symptoms lead to further psychological issues, depression, social isolation and eventually the symptoms do get worse over time. Our study shows that training with dance and music can slow this down and improve their daily living and daily function,” he added.
‘Great potential’
Reached for comment, Demian Kogutek, PhD, director of music therapy, University of Evansville (Indiana), said that these preliminary findings from a longitudinal study are “promising.”
“I believe that dance therapy has a great potential for PD. The longitudinal aspect of this study undoubtedly adds to the current literature. Although it is a standardized assessment, it is somewhat subjective,” Dr. Kogutek said in an interview.
Going forward, Dr. Kogutek said he’d like to see other objective outcomes measured, such as objective assessments of balance, gait, hand strength, and dexterity.
Also weighing in on the results, Karen Lee, PhD, president and CEO of Parkinson Canada, said her organization is “encouraged by these preliminary findings as exercise and healthy activities are important for people with Parkinson’s. This study is part of a growing body of research that explores the link between the impact of activities and both motor and nonmotor symptoms of Parkinson’s.
“This research adds to growing evidence about the importance of exercise as part of the management of Parkinson’s, and we encourage people living with Parkinson’s to incorporate exercise as part of their approach to managing their health,” Dr. Lee said in an interview.
Funding for the project is provided in part by a National Science and Engineering Research Council Discovery Grant and by donations from the Irpinia Club of Toronto and others. Dr. Dr. DeSouza, Ms. Bearss, Dr. Kogutek, and Dr. Lee disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 3 years, weekly participation in dance training classes “drastically” reduced the expected decline in motor function and significantly improved speech, tremors, balance, and stiffness, the researchers reported.
Dance training also appeared to have benefits regarding cognition, hallucinations, depression, and anxiety.
“These findings strongly suggest the benefits of dance for people with PD as a supplement to a normal treatment regimen,” the investigators noted.
Although the mechanism of benefit is unclear, dance training may help “train neural network nodes that helps either strengthen networks damaged or builds neural road maps that pass the damage,” study investigator Joseph DeSouza, PhD, principal investigator and associate professor, department of psychology, York University, Toronto, said in an interview.
The study was published online July 7, 2021, in Brain Sciences.
Multiple benefits
PD is a neurodegenerative disease associated with progression of motor dysfunction within the first 5 years of diagnosis. The annual rate of motor decline, as determined with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), is between 5.2 and 8.9 points.
Prior studies that assessed various styles of dance by patients with PD showed beneficial effects regarding gait speed, balance, locomotion, and aspects of quality of life.
To investigate further, DeSouza and coauthor Karolina Bearss, a PhD candidate at York University, followed 16 patients with mild to moderate PD who participated in a weekly dance class at Canada’s National Ballet School and Trinity St. Paul’s church.
Dance for Parkinson’s Disease, which is an established dance curriculum, involves aerobic and anaerobic movements. The protocol begins with a seated warm-up, followed by barre work, and ends with moving across the floor. All participants learn choreography for an upcoming performance.
In the study, 16 patients with PD who did not participant in the dance classes served as control patients.
Over 3 years, the daily rate of motor decline, as indicated by scores on part III of the MDS-UPDRS, was zero among the dancers (slope = 0.000146), indicating no motor impairment, whereas among the nondancers, the motor decline during follow-up was as expected (P < .01), the researchers reported.
In modeling the data, the researchers determined that after completing 1,000 days of dance training, dancers will have a motor score of 19.07, compared with a score of 28.27 for nondancers.
“Our data further showed that training in dance will slow the rate of PD motor impairment progression, as measured by the UPDRS III, by close to 3 points annually in comparison to our PD subjects who did not train,” the researchers reported.
Dance training also had a beneficial effect on motor or nonmotor aspects of daily living and on motor complications, for which there was no significant decline among the PD dancers.
“For those with Parkinson’s disease, even when it’s mild, motor impairment can impact their daily functioning – how they feel about themselves. Many of these motor symptoms lead to isolation because once they get extreme, these people don’t want to go out,” Dr. DeSouza said in a news release.
“These motor symptoms lead to further psychological issues, depression, social isolation and eventually the symptoms do get worse over time. Our study shows that training with dance and music can slow this down and improve their daily living and daily function,” he added.
‘Great potential’
Reached for comment, Demian Kogutek, PhD, director of music therapy, University of Evansville (Indiana), said that these preliminary findings from a longitudinal study are “promising.”
“I believe that dance therapy has a great potential for PD. The longitudinal aspect of this study undoubtedly adds to the current literature. Although it is a standardized assessment, it is somewhat subjective,” Dr. Kogutek said in an interview.
Going forward, Dr. Kogutek said he’d like to see other objective outcomes measured, such as objective assessments of balance, gait, hand strength, and dexterity.
Also weighing in on the results, Karen Lee, PhD, president and CEO of Parkinson Canada, said her organization is “encouraged by these preliminary findings as exercise and healthy activities are important for people with Parkinson’s. This study is part of a growing body of research that explores the link between the impact of activities and both motor and nonmotor symptoms of Parkinson’s.
“This research adds to growing evidence about the importance of exercise as part of the management of Parkinson’s, and we encourage people living with Parkinson’s to incorporate exercise as part of their approach to managing their health,” Dr. Lee said in an interview.
Funding for the project is provided in part by a National Science and Engineering Research Council Discovery Grant and by donations from the Irpinia Club of Toronto and others. Dr. Dr. DeSouza, Ms. Bearss, Dr. Kogutek, and Dr. Lee disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart failure med undertreatment because of older age common, flouts evidence
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
Chronic stress and genetics can raise the risk of Alzheimer’s disease
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
FROM BIOLOGICAL REVIEWS
Hearing loss tied to decline in physical functioning
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA fast-tracks lecanemab for Alzheimer’s disease
Lecanemab (formerly BAN2401) is a humanized monoclonal antibody that selectively binds to large, soluble aggregated Abeta protofibrils. The antibody was developed following the discovery of a mutation in amyloid precursor protein that leads to a form of Alzheimer’s disease that is marked by particularly high levels of Abeta protofibrils.
“As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease,” Eisai and Biogen said in a joint news release.
The breakthrough therapy designation for lecanemab is based on results of a randomized, double-blind, phase 2b proof-of-concept study published April 17 in Alzheimer’s Research & Therapy.
The study enrolled 856 patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease with confirmed presence of amyloid pathology.
At the highest doses, treatment with lecanemab led to a reduction in brain amyloid accompanied by a consistent reduction of clinical decline across several clinical and biomarker endpoints.
Phase 3 testing underway
In March, Eisai and Biogen completed enrollment in a phase 3 study designed to confirm the safety and efficacy of lecanemab in patients with symptomatic early Alzheimer’s disease.
The CLARITY AD study includes 1,795 patients with early Alzheimer’s disease, and initial results are expected by the end of September 2022. The core study will compare lecanemab against placebo on the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months. The study will also evaluate the long-term safety and tolerability of lecanemab in the extension phase and whether the long-term effects of lecanemab, as measured by the CDR-SB at the end of the core study, are maintained over time.
Additionally, the phase 3 AHEAD 3-45 clinical study is currently exploring lecanemab in adults with preclinical Alzheimer’s disease (clinically normal but with intermediate or elevated brain amyloid).
On June 7, the FDA – amid significant controversy – approved aducanumab (Aduhelm), the first anti-amyloid agent for the treatment Alzheimer’s disease, disregarding the recommendation by its own advisory panel not to approve the drug. Three members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee subsequently resigned in protest following the agency’s approval of aducanumab.
In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
A version of this article first appeared on Medscape.com.
Lecanemab (formerly BAN2401) is a humanized monoclonal antibody that selectively binds to large, soluble aggregated Abeta protofibrils. The antibody was developed following the discovery of a mutation in amyloid precursor protein that leads to a form of Alzheimer’s disease that is marked by particularly high levels of Abeta protofibrils.
“As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease,” Eisai and Biogen said in a joint news release.
The breakthrough therapy designation for lecanemab is based on results of a randomized, double-blind, phase 2b proof-of-concept study published April 17 in Alzheimer’s Research & Therapy.
The study enrolled 856 patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease with confirmed presence of amyloid pathology.
At the highest doses, treatment with lecanemab led to a reduction in brain amyloid accompanied by a consistent reduction of clinical decline across several clinical and biomarker endpoints.
Phase 3 testing underway
In March, Eisai and Biogen completed enrollment in a phase 3 study designed to confirm the safety and efficacy of lecanemab in patients with symptomatic early Alzheimer’s disease.
The CLARITY AD study includes 1,795 patients with early Alzheimer’s disease, and initial results are expected by the end of September 2022. The core study will compare lecanemab against placebo on the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months. The study will also evaluate the long-term safety and tolerability of lecanemab in the extension phase and whether the long-term effects of lecanemab, as measured by the CDR-SB at the end of the core study, are maintained over time.
Additionally, the phase 3 AHEAD 3-45 clinical study is currently exploring lecanemab in adults with preclinical Alzheimer’s disease (clinically normal but with intermediate or elevated brain amyloid).
On June 7, the FDA – amid significant controversy – approved aducanumab (Aduhelm), the first anti-amyloid agent for the treatment Alzheimer’s disease, disregarding the recommendation by its own advisory panel not to approve the drug. Three members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee subsequently resigned in protest following the agency’s approval of aducanumab.
In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
A version of this article first appeared on Medscape.com.
Lecanemab (formerly BAN2401) is a humanized monoclonal antibody that selectively binds to large, soluble aggregated Abeta protofibrils. The antibody was developed following the discovery of a mutation in amyloid precursor protein that leads to a form of Alzheimer’s disease that is marked by particularly high levels of Abeta protofibrils.
“As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease,” Eisai and Biogen said in a joint news release.
The breakthrough therapy designation for lecanemab is based on results of a randomized, double-blind, phase 2b proof-of-concept study published April 17 in Alzheimer’s Research & Therapy.
The study enrolled 856 patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease with confirmed presence of amyloid pathology.
At the highest doses, treatment with lecanemab led to a reduction in brain amyloid accompanied by a consistent reduction of clinical decline across several clinical and biomarker endpoints.
Phase 3 testing underway
In March, Eisai and Biogen completed enrollment in a phase 3 study designed to confirm the safety and efficacy of lecanemab in patients with symptomatic early Alzheimer’s disease.
The CLARITY AD study includes 1,795 patients with early Alzheimer’s disease, and initial results are expected by the end of September 2022. The core study will compare lecanemab against placebo on the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months. The study will also evaluate the long-term safety and tolerability of lecanemab in the extension phase and whether the long-term effects of lecanemab, as measured by the CDR-SB at the end of the core study, are maintained over time.
Additionally, the phase 3 AHEAD 3-45 clinical study is currently exploring lecanemab in adults with preclinical Alzheimer’s disease (clinically normal but with intermediate or elevated brain amyloid).
On June 7, the FDA – amid significant controversy – approved aducanumab (Aduhelm), the first anti-amyloid agent for the treatment Alzheimer’s disease, disregarding the recommendation by its own advisory panel not to approve the drug. Three members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee subsequently resigned in protest following the agency’s approval of aducanumab.
In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
A version of this article first appeared on Medscape.com.
Stopping statins linked to death, CV events in elderly
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Memory benefit seen with antihypertensives crossing blood-brain barrier
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
FROM HYPERTENSION





