User login
‘New first-line standard of care’ in cervical cancer
That declaration was made by Raza Mirza, MD, chief oncologist at Copenhagen University Hospital in Denmark, who was invited to discuss the pros and cons of the KEYNOTE-826 trial at the European Society for Medical Oncology (ESMO) Congress 2021.
The trial showed that adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard chemotherapy — with or without bevacizumab — resulted in about a one third reduction in the risk for both disease progression and death compared with chemotherapy alone.
The benefit of adding pembrolizumab was seen both in the overall study population and in patients with higher levels of programmed death ligand-1 (PD-L1), but not in those with biomarker-negative tumors, reported investigator Nicoletta Colombo, MD, PhD, from the University of Milan-Bicocca, Italy.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus platinum-based chemotherapy with or without bevacizumab may be a new first-line standard of care,” she said in a late-breaking oral abstract presentation. The study was also simultaneously published online in The New England Journal of Medicine.
Since 2014, the standard of care for treating patients with recurrent, persistent, or metastatic cervical cancer has been chemotherapy with a platinum compound, paclitaxel, plus bevacizumab, based on the results of the GOG 240 study.
Immunotherapy with PD-1 inhibitors have shown efficacy as monotherapy in second- or later-line therapy for women with cervical cancer, but until now no data about the addition of these agents to chemotherapy were available, Dr. Colombo noted.
Dr. Mirza noted that there is sound rationale for using checkpoint inhibitors targeted against PD-1 in patients with cervical cancer, because PD-L1 has been shown to be a consistent biomarker for infection of the cervix with human papillomavirus (HPV), which is responsible for more than 90% of cervical cancers.
“PD-L1 is significantly upregulated in cervical cancer and detectable by immunohistochemistry,” he said. “PD-L1 expression reduces the immune response since it is able to bind to PD-1 on T-cell lymphocytes, thereby inhibiting their function. These findings suggest that targeting the PD-1/PD-L1 pathway may be therapeutically effective and should be considered in the treatment of cervical cancer.”
KEYNOTE-826 details
This was a double-blind trial conducted in 617 patients stratified by metastatic disease status at diagnosis; PD-L1 combined positive score (CPS) either < 1, 1 to < 10, or ≥ 10. They were randomized in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus platinum-based chemotherapy, with bevacizumab added at the investigator’s discretion.
The dual primary endpoints of progression-free survival (PFS) and overall survival (OS) were each tested sequentially in patients with a PD-L1 CPS ≥ 1 in both the intention-to-treat (ITT) or “all-comers” population, and in patients with a PD-L1 CPS ≥ 10.
Patient characteristics were generally well balanced between the treatment groups, except for a slightly higher proportion of patients with squamous cell histology in the pembrolizumab versus the placebo group (76.3% vs 68.3%).
PFS and OS results
The addition of pembrolizumab was associated with improved PFS across most protocol-specified subgroups, Dr. Colombo and colleagues noted.
After a median follow-up of 22 months, the 12-month PFS rate in the biomarker-selected population (all patients with a PD-L1 CPS ≥ 1) was 45.5% for patients in the pembrolizumab group versus 34.1% in the placebo group. This translated into a hazard ratio (HR) for progression on pembrolizumab of 0.62 (P < .001).
The respective PFS rates in the ITT population were 44.7% and 33.5%, with an HR for progression of 0.65 (P < .001) with the checkpoint inhibitor.
In patients with PD-L1 CPS ≥ 10, the respective rates of PFS and the HR were 44.6%, 33.5%, and 0.58 (P < .001).
OS rates were also significantly improved, he noted.
The 12-month and 24-month OS rates in all patients with PD-L1 CPS ≥ 1 were 75.3% and 53%, respectively, for patients assigned to pembrolizumab versus 63.1% and 41.7% in patients assigned to placebo, translating to an HR for death with pembrolizumab in this group of 0.64 (P < .001).
In the all-comers (ITT) population, respective 12- and 24-month OS rates were 74.8% and 50.4% with pembrolizumab versus 63.6% and 40.4% with placebo. This difference translated into an HR for death with anti-PD-1 of 0.67 (P < .001).
Among patients with the higher PD-L1 levels (≥ CPS 10), the respective OS rates were 75.7% and 54.4% with pembrolizumab versus 61.5% and 44.6% with placebo (HR 0.61, P < .001).
Dr. Mirza emphasized that “we did not see any efficacy of pembrolizumab in the biomarker-negative population,” with an HR for PFS of 0.94 and HR for OS of 1.0 in this subgroup.
The most common grade ≥ 3 adverse events were anemia, which occurred in 30.3% of patients assigned to pembrolizumab compared with 26.9% in the placebo group, and neutropenias, which occurred in 12.4% and 9.7% of patients, respectively. One patient in the pembrolizumab group died from an immune-related event, encephalitis.
Despite his enthusiasm for the regimen, Dr. Mirza tempered it by pointing out that there was an imbalance in the sample sizes regarding histology, and a potential bias introduced by the failure to stratify by tumor histology.
He noted that in other studies checkpoint inhibitors have had only modest activity against adenocarcinomas, which were more frequent in the placebo group in KEYNOTE-826, resulting in a potential positive bias in favor of pembrolizumab.
KEYNOTE-826 is funded by MSD. Dr. Colombo has disclosed consultant, research, and promotional speaking activities for multiple companies. Dr. Mirza has disclosed personal financial interests with Merck and other companies.
A version of this article was first published on Medscape.com.
That declaration was made by Raza Mirza, MD, chief oncologist at Copenhagen University Hospital in Denmark, who was invited to discuss the pros and cons of the KEYNOTE-826 trial at the European Society for Medical Oncology (ESMO) Congress 2021.
The trial showed that adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard chemotherapy — with or without bevacizumab — resulted in about a one third reduction in the risk for both disease progression and death compared with chemotherapy alone.
The benefit of adding pembrolizumab was seen both in the overall study population and in patients with higher levels of programmed death ligand-1 (PD-L1), but not in those with biomarker-negative tumors, reported investigator Nicoletta Colombo, MD, PhD, from the University of Milan-Bicocca, Italy.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus platinum-based chemotherapy with or without bevacizumab may be a new first-line standard of care,” she said in a late-breaking oral abstract presentation. The study was also simultaneously published online in The New England Journal of Medicine.
Since 2014, the standard of care for treating patients with recurrent, persistent, or metastatic cervical cancer has been chemotherapy with a platinum compound, paclitaxel, plus bevacizumab, based on the results of the GOG 240 study.
Immunotherapy with PD-1 inhibitors have shown efficacy as monotherapy in second- or later-line therapy for women with cervical cancer, but until now no data about the addition of these agents to chemotherapy were available, Dr. Colombo noted.
Dr. Mirza noted that there is sound rationale for using checkpoint inhibitors targeted against PD-1 in patients with cervical cancer, because PD-L1 has been shown to be a consistent biomarker for infection of the cervix with human papillomavirus (HPV), which is responsible for more than 90% of cervical cancers.
“PD-L1 is significantly upregulated in cervical cancer and detectable by immunohistochemistry,” he said. “PD-L1 expression reduces the immune response since it is able to bind to PD-1 on T-cell lymphocytes, thereby inhibiting their function. These findings suggest that targeting the PD-1/PD-L1 pathway may be therapeutically effective and should be considered in the treatment of cervical cancer.”
KEYNOTE-826 details
This was a double-blind trial conducted in 617 patients stratified by metastatic disease status at diagnosis; PD-L1 combined positive score (CPS) either < 1, 1 to < 10, or ≥ 10. They were randomized in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus platinum-based chemotherapy, with bevacizumab added at the investigator’s discretion.
The dual primary endpoints of progression-free survival (PFS) and overall survival (OS) were each tested sequentially in patients with a PD-L1 CPS ≥ 1 in both the intention-to-treat (ITT) or “all-comers” population, and in patients with a PD-L1 CPS ≥ 10.
Patient characteristics were generally well balanced between the treatment groups, except for a slightly higher proportion of patients with squamous cell histology in the pembrolizumab versus the placebo group (76.3% vs 68.3%).
PFS and OS results
The addition of pembrolizumab was associated with improved PFS across most protocol-specified subgroups, Dr. Colombo and colleagues noted.
After a median follow-up of 22 months, the 12-month PFS rate in the biomarker-selected population (all patients with a PD-L1 CPS ≥ 1) was 45.5% for patients in the pembrolizumab group versus 34.1% in the placebo group. This translated into a hazard ratio (HR) for progression on pembrolizumab of 0.62 (P < .001).
The respective PFS rates in the ITT population were 44.7% and 33.5%, with an HR for progression of 0.65 (P < .001) with the checkpoint inhibitor.
In patients with PD-L1 CPS ≥ 10, the respective rates of PFS and the HR were 44.6%, 33.5%, and 0.58 (P < .001).
OS rates were also significantly improved, he noted.
The 12-month and 24-month OS rates in all patients with PD-L1 CPS ≥ 1 were 75.3% and 53%, respectively, for patients assigned to pembrolizumab versus 63.1% and 41.7% in patients assigned to placebo, translating to an HR for death with pembrolizumab in this group of 0.64 (P < .001).
In the all-comers (ITT) population, respective 12- and 24-month OS rates were 74.8% and 50.4% with pembrolizumab versus 63.6% and 40.4% with placebo. This difference translated into an HR for death with anti-PD-1 of 0.67 (P < .001).
Among patients with the higher PD-L1 levels (≥ CPS 10), the respective OS rates were 75.7% and 54.4% with pembrolizumab versus 61.5% and 44.6% with placebo (HR 0.61, P < .001).
Dr. Mirza emphasized that “we did not see any efficacy of pembrolizumab in the biomarker-negative population,” with an HR for PFS of 0.94 and HR for OS of 1.0 in this subgroup.
The most common grade ≥ 3 adverse events were anemia, which occurred in 30.3% of patients assigned to pembrolizumab compared with 26.9% in the placebo group, and neutropenias, which occurred in 12.4% and 9.7% of patients, respectively. One patient in the pembrolizumab group died from an immune-related event, encephalitis.
Despite his enthusiasm for the regimen, Dr. Mirza tempered it by pointing out that there was an imbalance in the sample sizes regarding histology, and a potential bias introduced by the failure to stratify by tumor histology.
He noted that in other studies checkpoint inhibitors have had only modest activity against adenocarcinomas, which were more frequent in the placebo group in KEYNOTE-826, resulting in a potential positive bias in favor of pembrolizumab.
KEYNOTE-826 is funded by MSD. Dr. Colombo has disclosed consultant, research, and promotional speaking activities for multiple companies. Dr. Mirza has disclosed personal financial interests with Merck and other companies.
A version of this article was first published on Medscape.com.
That declaration was made by Raza Mirza, MD, chief oncologist at Copenhagen University Hospital in Denmark, who was invited to discuss the pros and cons of the KEYNOTE-826 trial at the European Society for Medical Oncology (ESMO) Congress 2021.
The trial showed that adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard chemotherapy — with or without bevacizumab — resulted in about a one third reduction in the risk for both disease progression and death compared with chemotherapy alone.
The benefit of adding pembrolizumab was seen both in the overall study population and in patients with higher levels of programmed death ligand-1 (PD-L1), but not in those with biomarker-negative tumors, reported investigator Nicoletta Colombo, MD, PhD, from the University of Milan-Bicocca, Italy.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus platinum-based chemotherapy with or without bevacizumab may be a new first-line standard of care,” she said in a late-breaking oral abstract presentation. The study was also simultaneously published online in The New England Journal of Medicine.
Since 2014, the standard of care for treating patients with recurrent, persistent, or metastatic cervical cancer has been chemotherapy with a platinum compound, paclitaxel, plus bevacizumab, based on the results of the GOG 240 study.
Immunotherapy with PD-1 inhibitors have shown efficacy as monotherapy in second- or later-line therapy for women with cervical cancer, but until now no data about the addition of these agents to chemotherapy were available, Dr. Colombo noted.
Dr. Mirza noted that there is sound rationale for using checkpoint inhibitors targeted against PD-1 in patients with cervical cancer, because PD-L1 has been shown to be a consistent biomarker for infection of the cervix with human papillomavirus (HPV), which is responsible for more than 90% of cervical cancers.
“PD-L1 is significantly upregulated in cervical cancer and detectable by immunohistochemistry,” he said. “PD-L1 expression reduces the immune response since it is able to bind to PD-1 on T-cell lymphocytes, thereby inhibiting their function. These findings suggest that targeting the PD-1/PD-L1 pathway may be therapeutically effective and should be considered in the treatment of cervical cancer.”
KEYNOTE-826 details
This was a double-blind trial conducted in 617 patients stratified by metastatic disease status at diagnosis; PD-L1 combined positive score (CPS) either < 1, 1 to < 10, or ≥ 10. They were randomized in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus platinum-based chemotherapy, with bevacizumab added at the investigator’s discretion.
The dual primary endpoints of progression-free survival (PFS) and overall survival (OS) were each tested sequentially in patients with a PD-L1 CPS ≥ 1 in both the intention-to-treat (ITT) or “all-comers” population, and in patients with a PD-L1 CPS ≥ 10.
Patient characteristics were generally well balanced between the treatment groups, except for a slightly higher proportion of patients with squamous cell histology in the pembrolizumab versus the placebo group (76.3% vs 68.3%).
PFS and OS results
The addition of pembrolizumab was associated with improved PFS across most protocol-specified subgroups, Dr. Colombo and colleagues noted.
After a median follow-up of 22 months, the 12-month PFS rate in the biomarker-selected population (all patients with a PD-L1 CPS ≥ 1) was 45.5% for patients in the pembrolizumab group versus 34.1% in the placebo group. This translated into a hazard ratio (HR) for progression on pembrolizumab of 0.62 (P < .001).
The respective PFS rates in the ITT population were 44.7% and 33.5%, with an HR for progression of 0.65 (P < .001) with the checkpoint inhibitor.
In patients with PD-L1 CPS ≥ 10, the respective rates of PFS and the HR were 44.6%, 33.5%, and 0.58 (P < .001).
OS rates were also significantly improved, he noted.
The 12-month and 24-month OS rates in all patients with PD-L1 CPS ≥ 1 were 75.3% and 53%, respectively, for patients assigned to pembrolizumab versus 63.1% and 41.7% in patients assigned to placebo, translating to an HR for death with pembrolizumab in this group of 0.64 (P < .001).
In the all-comers (ITT) population, respective 12- and 24-month OS rates were 74.8% and 50.4% with pembrolizumab versus 63.6% and 40.4% with placebo. This difference translated into an HR for death with anti-PD-1 of 0.67 (P < .001).
Among patients with the higher PD-L1 levels (≥ CPS 10), the respective OS rates were 75.7% and 54.4% with pembrolizumab versus 61.5% and 44.6% with placebo (HR 0.61, P < .001).
Dr. Mirza emphasized that “we did not see any efficacy of pembrolizumab in the biomarker-negative population,” with an HR for PFS of 0.94 and HR for OS of 1.0 in this subgroup.
The most common grade ≥ 3 adverse events were anemia, which occurred in 30.3% of patients assigned to pembrolizumab compared with 26.9% in the placebo group, and neutropenias, which occurred in 12.4% and 9.7% of patients, respectively. One patient in the pembrolizumab group died from an immune-related event, encephalitis.
Despite his enthusiasm for the regimen, Dr. Mirza tempered it by pointing out that there was an imbalance in the sample sizes regarding histology, and a potential bias introduced by the failure to stratify by tumor histology.
He noted that in other studies checkpoint inhibitors have had only modest activity against adenocarcinomas, which were more frequent in the placebo group in KEYNOTE-826, resulting in a potential positive bias in favor of pembrolizumab.
KEYNOTE-826 is funded by MSD. Dr. Colombo has disclosed consultant, research, and promotional speaking activities for multiple companies. Dr. Mirza has disclosed personal financial interests with Merck and other companies.
A version of this article was first published on Medscape.com.
Prevalence of high-risk HPV types dwindled since vaccine approval
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multidisciplinary approach to gyn care: A single center’s experience
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
One in three cancer articles on social media has wrong info
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Advice on biopsies, workups, and referrals
Over the next 2 months we will dedicate this column to some general tips and pearls from the perspective of a gynecologic oncologist to guide general obstetrician gynecologists in the workup and management of preinvasive or invasive gynecologic diseases. The goal of these recommendations is to minimize misdiagnosis or delayed diagnosis and avoid unnecessary or untimely referrals.
Perform biopsy, not Pap smears, on visible cervical and vaginal lesions
The purpose of the Pap smear is to screen asymptomatic patients for cervical dysplasia or microscopic invasive disease. Cytology is an unreliable diagnostic tool for visible, symptomatic lesions in large part because of sampling errors, and the lack of architectural information in cytologic versus histopathologic specimens. Invasive lesions can be mischaracterized as preinvasive on a Pap smear. This can result in delayed diagnosis and unnecessary diagnostic procedures. For example, if a visible, abnormal-appearing, cervical lesion is seen during a routine visit and a Pap smear is performed (rather than a biopsy of the mass), the patient may receive an incorrect preliminary diagnosis of “high-grade dysplasia, carcinoma in situ” as it can be difficult to distinguish invasive carcinoma from carcinoma in situ on cytology. If the patient and provider do not understand the limitations of Pap smears in diagnosing invasive cancers, they may be falsely reassured and possibly delay or abstain from follow-up for an excisional procedure. If she does return for the loop electrosurgical excision procedure (LEEP), there might still be unnecessary delays in making referrals and definitive treatment while waiting for results. Radical hysterectomy may not promptly follow because, if performed within 6 weeks of an excisional procedure, it is associated with a significantly higher risk for perioperative complication, and therefore, if the excisional procedure was unnecessary to begin with, there may be additional time lost that need not be.1
Some clinicians avoid biopsy of visible lesions because they are concerned about bleeding complications that might arise in the office. Straightforward strategies to control bleeding are readily available in most gynecology offices, especially those already equipped for procedures such as LEEP and colposcopy. Prior to performing the biopsy, clinicians should ensure that they have supplies such as gauze sponges and ring forceps or packing forceps, silver nitrate, and ferric subsulfate solution (“Monsel’s solution”) close at hand. In the vast majority of cases, direct pressure for 5 minutes with gauze sponges and ferric subsulfate is highly effective at resolving most bleeding from a cervical or vaginal biopsy site. If this does not bring hemostasis, cautery devices or suture can be employed. If all else fails, be prepared to place vaginal packing (always with the insertion of a urinary Foley catheter to prevent urinary retention). In my experience, this is rarely needed.
Wherever possible, visible cervical or vaginal (or vulvar, see below) lesions should be biopsied for histopathology, sampling representative areas of the most concerning portion, in order to minimize misdiagnosis and expedite referral and definitive treatment. For necrotic-appearing lesions I recommend taking multiple samples of the tumor, as necrotic, nonviable tissue can prevent accurate diagnosis of a cancer. In general, Pap smears should be reserved as screening tests for asymptomatic women without visible pathology.
Don’t treat or refer low-grade dysplasia, even if persistent
Increasingly we are understanding that low-grade dysplasia of the lower genital tract (CIN I, VAIN I, VIN I) is less a precursor for cancer, and more a phenomenon of benign HPV-associated changes.2 This HPV change may be chronically persistent, may require years of observation and serial Pap smears, and may be a general nuisance for the patient. However, current guidelines do not recommend intervention for low-grade dysplasia of the lower genital tract.2 Interventions to resect these lesions can result in morbidity, including perineal pain, vaginal scarring, and cervical stenosis or insufficiency. Given the extremely low risk for progression to cancer, these morbidities do not outweigh any small potential benefit.
When I am conferring with patients who have chronic low-grade dysplasia I spend a great deal of time exploring their understanding of the diagnosis and its pathophysiology, their fears, and their expectation regarding “success” of treatment. I spend the time educating them that this is a sequela of chronic viral infection that will not be eradicated with local surgical excisions, that their cancer risk and need for surveillance would persist even if surgical intervention were offered, and that the side effects of treatment would outweigh any benefit from the small risk of cancer or high-grade dysplasia.
In summary, the treatment of choice for persistent low-grade dysplasia of the lower genital tract is comprehensive patient education, not surgical resection or referral to gynecologic oncology.
Repeat sampling if there’s a discordance between imaging and biopsy results
Delay in cancer diagnosis is one of the greatest concerns for front-line gynecology providers. One of the more modifiable strategies to avoid missed or delayed diagnosis is to ensure that there is concordance between clinical findings and testing results. Otherwise said: The results and findings should make sense in aggregate. An example was cited above in which a visible cervical mass demonstrated CIN III on cytologic testing. Another common example is a biopsy result of “scant benign endometrium” in a patient with postmenopausal bleeding and thickened endometrial stripe on ultrasound. In both of these cases there is clear discordance between physical findings and the results of pathology sampling. A pathology report, in all of its black and white certitude, seems like the most reliable source of information. However, always trust your clinical judgment. If the clinical picture is suggesting something far worse than these limited, often random or blind samplings, I recommend repeated or more extensive sampling (for example, dilation and curettage). At the very least, schedule close follow-up with repeated sampling if the symptom or finding persists. The emphasis here is on scheduled follow-up, rather than “p.r.n.,” because a patient who was given a “normal” pathology result to explain her abnormal symptoms may not volunteer that those symptoms are persistent as she may feel that anything sinister was already ruled out. Make certain that you explain the potential for misdiagnosis as the reason for why you would like to see her back shortly to ensure the issue has resolved.
Biopsy vulvar lesions, minimize empiric treatment
Vulvar cancer is notoriously associated with delayed diagnosis. Unfortunately, it is commonplace for gynecologic oncologists to see women who have vulvar cancers that have been empirically treated, sometimes for months or years, with steroids or other topical agents. If a lesion on the vulva is characteristically benign in appearance (such as condyloma or lichen sclerosis), it may be reasonable to start empiric treatment. However, all patients who are treated without biopsy should be rescheduled for a planned follow-up appointment in 2-3 months. If the lesion/area remains unchanged, or worse, the lesion should be biopsied before proceeding with a change in therapy or continued therapy. Once again, don’t rely on patients to return for evaluation if the lesion doesn’t improve. Many patients assume that our first empiric diagnosis is “gospel,” and therefore may not return if the treatment doesn’t work. Meanwhile, providers may assume that patients will know that there is uncertainty in our interpretation and that they will know to report if the initial treatment didn’t work. These assumptions are the recipe for delayed diagnosis. If there is too great a burden on the patient to schedule a return visit because of social or financial reasons then the patient should have a biopsy prior to initiation of treatment. As a rule, empiric treatment is not a good strategy for patients without good access to follow-up.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Sullivan S. et al Gynecol Oncol. 2017 Feb;144(2):294-8.
2. Perkins R .et al J Low Genit Tract Dis. 2020 Apr;24(2):102-31.
Over the next 2 months we will dedicate this column to some general tips and pearls from the perspective of a gynecologic oncologist to guide general obstetrician gynecologists in the workup and management of preinvasive or invasive gynecologic diseases. The goal of these recommendations is to minimize misdiagnosis or delayed diagnosis and avoid unnecessary or untimely referrals.
Perform biopsy, not Pap smears, on visible cervical and vaginal lesions
The purpose of the Pap smear is to screen asymptomatic patients for cervical dysplasia or microscopic invasive disease. Cytology is an unreliable diagnostic tool for visible, symptomatic lesions in large part because of sampling errors, and the lack of architectural information in cytologic versus histopathologic specimens. Invasive lesions can be mischaracterized as preinvasive on a Pap smear. This can result in delayed diagnosis and unnecessary diagnostic procedures. For example, if a visible, abnormal-appearing, cervical lesion is seen during a routine visit and a Pap smear is performed (rather than a biopsy of the mass), the patient may receive an incorrect preliminary diagnosis of “high-grade dysplasia, carcinoma in situ” as it can be difficult to distinguish invasive carcinoma from carcinoma in situ on cytology. If the patient and provider do not understand the limitations of Pap smears in diagnosing invasive cancers, they may be falsely reassured and possibly delay or abstain from follow-up for an excisional procedure. If she does return for the loop electrosurgical excision procedure (LEEP), there might still be unnecessary delays in making referrals and definitive treatment while waiting for results. Radical hysterectomy may not promptly follow because, if performed within 6 weeks of an excisional procedure, it is associated with a significantly higher risk for perioperative complication, and therefore, if the excisional procedure was unnecessary to begin with, there may be additional time lost that need not be.1
Some clinicians avoid biopsy of visible lesions because they are concerned about bleeding complications that might arise in the office. Straightforward strategies to control bleeding are readily available in most gynecology offices, especially those already equipped for procedures such as LEEP and colposcopy. Prior to performing the biopsy, clinicians should ensure that they have supplies such as gauze sponges and ring forceps or packing forceps, silver nitrate, and ferric subsulfate solution (“Monsel’s solution”) close at hand. In the vast majority of cases, direct pressure for 5 minutes with gauze sponges and ferric subsulfate is highly effective at resolving most bleeding from a cervical or vaginal biopsy site. If this does not bring hemostasis, cautery devices or suture can be employed. If all else fails, be prepared to place vaginal packing (always with the insertion of a urinary Foley catheter to prevent urinary retention). In my experience, this is rarely needed.
Wherever possible, visible cervical or vaginal (or vulvar, see below) lesions should be biopsied for histopathology, sampling representative areas of the most concerning portion, in order to minimize misdiagnosis and expedite referral and definitive treatment. For necrotic-appearing lesions I recommend taking multiple samples of the tumor, as necrotic, nonviable tissue can prevent accurate diagnosis of a cancer. In general, Pap smears should be reserved as screening tests for asymptomatic women without visible pathology.
Don’t treat or refer low-grade dysplasia, even if persistent
Increasingly we are understanding that low-grade dysplasia of the lower genital tract (CIN I, VAIN I, VIN I) is less a precursor for cancer, and more a phenomenon of benign HPV-associated changes.2 This HPV change may be chronically persistent, may require years of observation and serial Pap smears, and may be a general nuisance for the patient. However, current guidelines do not recommend intervention for low-grade dysplasia of the lower genital tract.2 Interventions to resect these lesions can result in morbidity, including perineal pain, vaginal scarring, and cervical stenosis or insufficiency. Given the extremely low risk for progression to cancer, these morbidities do not outweigh any small potential benefit.
When I am conferring with patients who have chronic low-grade dysplasia I spend a great deal of time exploring their understanding of the diagnosis and its pathophysiology, their fears, and their expectation regarding “success” of treatment. I spend the time educating them that this is a sequela of chronic viral infection that will not be eradicated with local surgical excisions, that their cancer risk and need for surveillance would persist even if surgical intervention were offered, and that the side effects of treatment would outweigh any benefit from the small risk of cancer or high-grade dysplasia.
In summary, the treatment of choice for persistent low-grade dysplasia of the lower genital tract is comprehensive patient education, not surgical resection or referral to gynecologic oncology.
Repeat sampling if there’s a discordance between imaging and biopsy results
Delay in cancer diagnosis is one of the greatest concerns for front-line gynecology providers. One of the more modifiable strategies to avoid missed or delayed diagnosis is to ensure that there is concordance between clinical findings and testing results. Otherwise said: The results and findings should make sense in aggregate. An example was cited above in which a visible cervical mass demonstrated CIN III on cytologic testing. Another common example is a biopsy result of “scant benign endometrium” in a patient with postmenopausal bleeding and thickened endometrial stripe on ultrasound. In both of these cases there is clear discordance between physical findings and the results of pathology sampling. A pathology report, in all of its black and white certitude, seems like the most reliable source of information. However, always trust your clinical judgment. If the clinical picture is suggesting something far worse than these limited, often random or blind samplings, I recommend repeated or more extensive sampling (for example, dilation and curettage). At the very least, schedule close follow-up with repeated sampling if the symptom or finding persists. The emphasis here is on scheduled follow-up, rather than “p.r.n.,” because a patient who was given a “normal” pathology result to explain her abnormal symptoms may not volunteer that those symptoms are persistent as she may feel that anything sinister was already ruled out. Make certain that you explain the potential for misdiagnosis as the reason for why you would like to see her back shortly to ensure the issue has resolved.
Biopsy vulvar lesions, minimize empiric treatment
Vulvar cancer is notoriously associated with delayed diagnosis. Unfortunately, it is commonplace for gynecologic oncologists to see women who have vulvar cancers that have been empirically treated, sometimes for months or years, with steroids or other topical agents. If a lesion on the vulva is characteristically benign in appearance (such as condyloma or lichen sclerosis), it may be reasonable to start empiric treatment. However, all patients who are treated without biopsy should be rescheduled for a planned follow-up appointment in 2-3 months. If the lesion/area remains unchanged, or worse, the lesion should be biopsied before proceeding with a change in therapy or continued therapy. Once again, don’t rely on patients to return for evaluation if the lesion doesn’t improve. Many patients assume that our first empiric diagnosis is “gospel,” and therefore may not return if the treatment doesn’t work. Meanwhile, providers may assume that patients will know that there is uncertainty in our interpretation and that they will know to report if the initial treatment didn’t work. These assumptions are the recipe for delayed diagnosis. If there is too great a burden on the patient to schedule a return visit because of social or financial reasons then the patient should have a biopsy prior to initiation of treatment. As a rule, empiric treatment is not a good strategy for patients without good access to follow-up.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Sullivan S. et al Gynecol Oncol. 2017 Feb;144(2):294-8.
2. Perkins R .et al J Low Genit Tract Dis. 2020 Apr;24(2):102-31.
Over the next 2 months we will dedicate this column to some general tips and pearls from the perspective of a gynecologic oncologist to guide general obstetrician gynecologists in the workup and management of preinvasive or invasive gynecologic diseases. The goal of these recommendations is to minimize misdiagnosis or delayed diagnosis and avoid unnecessary or untimely referrals.
Perform biopsy, not Pap smears, on visible cervical and vaginal lesions
The purpose of the Pap smear is to screen asymptomatic patients for cervical dysplasia or microscopic invasive disease. Cytology is an unreliable diagnostic tool for visible, symptomatic lesions in large part because of sampling errors, and the lack of architectural information in cytologic versus histopathologic specimens. Invasive lesions can be mischaracterized as preinvasive on a Pap smear. This can result in delayed diagnosis and unnecessary diagnostic procedures. For example, if a visible, abnormal-appearing, cervical lesion is seen during a routine visit and a Pap smear is performed (rather than a biopsy of the mass), the patient may receive an incorrect preliminary diagnosis of “high-grade dysplasia, carcinoma in situ” as it can be difficult to distinguish invasive carcinoma from carcinoma in situ on cytology. If the patient and provider do not understand the limitations of Pap smears in diagnosing invasive cancers, they may be falsely reassured and possibly delay or abstain from follow-up for an excisional procedure. If she does return for the loop electrosurgical excision procedure (LEEP), there might still be unnecessary delays in making referrals and definitive treatment while waiting for results. Radical hysterectomy may not promptly follow because, if performed within 6 weeks of an excisional procedure, it is associated with a significantly higher risk for perioperative complication, and therefore, if the excisional procedure was unnecessary to begin with, there may be additional time lost that need not be.1
Some clinicians avoid biopsy of visible lesions because they are concerned about bleeding complications that might arise in the office. Straightforward strategies to control bleeding are readily available in most gynecology offices, especially those already equipped for procedures such as LEEP and colposcopy. Prior to performing the biopsy, clinicians should ensure that they have supplies such as gauze sponges and ring forceps or packing forceps, silver nitrate, and ferric subsulfate solution (“Monsel’s solution”) close at hand. In the vast majority of cases, direct pressure for 5 minutes with gauze sponges and ferric subsulfate is highly effective at resolving most bleeding from a cervical or vaginal biopsy site. If this does not bring hemostasis, cautery devices or suture can be employed. If all else fails, be prepared to place vaginal packing (always with the insertion of a urinary Foley catheter to prevent urinary retention). In my experience, this is rarely needed.
Wherever possible, visible cervical or vaginal (or vulvar, see below) lesions should be biopsied for histopathology, sampling representative areas of the most concerning portion, in order to minimize misdiagnosis and expedite referral and definitive treatment. For necrotic-appearing lesions I recommend taking multiple samples of the tumor, as necrotic, nonviable tissue can prevent accurate diagnosis of a cancer. In general, Pap smears should be reserved as screening tests for asymptomatic women without visible pathology.
Don’t treat or refer low-grade dysplasia, even if persistent
Increasingly we are understanding that low-grade dysplasia of the lower genital tract (CIN I, VAIN I, VIN I) is less a precursor for cancer, and more a phenomenon of benign HPV-associated changes.2 This HPV change may be chronically persistent, may require years of observation and serial Pap smears, and may be a general nuisance for the patient. However, current guidelines do not recommend intervention for low-grade dysplasia of the lower genital tract.2 Interventions to resect these lesions can result in morbidity, including perineal pain, vaginal scarring, and cervical stenosis or insufficiency. Given the extremely low risk for progression to cancer, these morbidities do not outweigh any small potential benefit.
When I am conferring with patients who have chronic low-grade dysplasia I spend a great deal of time exploring their understanding of the diagnosis and its pathophysiology, their fears, and their expectation regarding “success” of treatment. I spend the time educating them that this is a sequela of chronic viral infection that will not be eradicated with local surgical excisions, that their cancer risk and need for surveillance would persist even if surgical intervention were offered, and that the side effects of treatment would outweigh any benefit from the small risk of cancer or high-grade dysplasia.
In summary, the treatment of choice for persistent low-grade dysplasia of the lower genital tract is comprehensive patient education, not surgical resection or referral to gynecologic oncology.
Repeat sampling if there’s a discordance between imaging and biopsy results
Delay in cancer diagnosis is one of the greatest concerns for front-line gynecology providers. One of the more modifiable strategies to avoid missed or delayed diagnosis is to ensure that there is concordance between clinical findings and testing results. Otherwise said: The results and findings should make sense in aggregate. An example was cited above in which a visible cervical mass demonstrated CIN III on cytologic testing. Another common example is a biopsy result of “scant benign endometrium” in a patient with postmenopausal bleeding and thickened endometrial stripe on ultrasound. In both of these cases there is clear discordance between physical findings and the results of pathology sampling. A pathology report, in all of its black and white certitude, seems like the most reliable source of information. However, always trust your clinical judgment. If the clinical picture is suggesting something far worse than these limited, often random or blind samplings, I recommend repeated or more extensive sampling (for example, dilation and curettage). At the very least, schedule close follow-up with repeated sampling if the symptom or finding persists. The emphasis here is on scheduled follow-up, rather than “p.r.n.,” because a patient who was given a “normal” pathology result to explain her abnormal symptoms may not volunteer that those symptoms are persistent as she may feel that anything sinister was already ruled out. Make certain that you explain the potential for misdiagnosis as the reason for why you would like to see her back shortly to ensure the issue has resolved.
Biopsy vulvar lesions, minimize empiric treatment
Vulvar cancer is notoriously associated with delayed diagnosis. Unfortunately, it is commonplace for gynecologic oncologists to see women who have vulvar cancers that have been empirically treated, sometimes for months or years, with steroids or other topical agents. If a lesion on the vulva is characteristically benign in appearance (such as condyloma or lichen sclerosis), it may be reasonable to start empiric treatment. However, all patients who are treated without biopsy should be rescheduled for a planned follow-up appointment in 2-3 months. If the lesion/area remains unchanged, or worse, the lesion should be biopsied before proceeding with a change in therapy or continued therapy. Once again, don’t rely on patients to return for evaluation if the lesion doesn’t improve. Many patients assume that our first empiric diagnosis is “gospel,” and therefore may not return if the treatment doesn’t work. Meanwhile, providers may assume that patients will know that there is uncertainty in our interpretation and that they will know to report if the initial treatment didn’t work. These assumptions are the recipe for delayed diagnosis. If there is too great a burden on the patient to schedule a return visit because of social or financial reasons then the patient should have a biopsy prior to initiation of treatment. As a rule, empiric treatment is not a good strategy for patients without good access to follow-up.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Sullivan S. et al Gynecol Oncol. 2017 Feb;144(2):294-8.
2. Perkins R .et al J Low Genit Tract Dis. 2020 Apr;24(2):102-31.
Hematologic cancer increases risk of delivery complications
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
Cancer mortality continues to drop in females as breast cancer reversal looms
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.
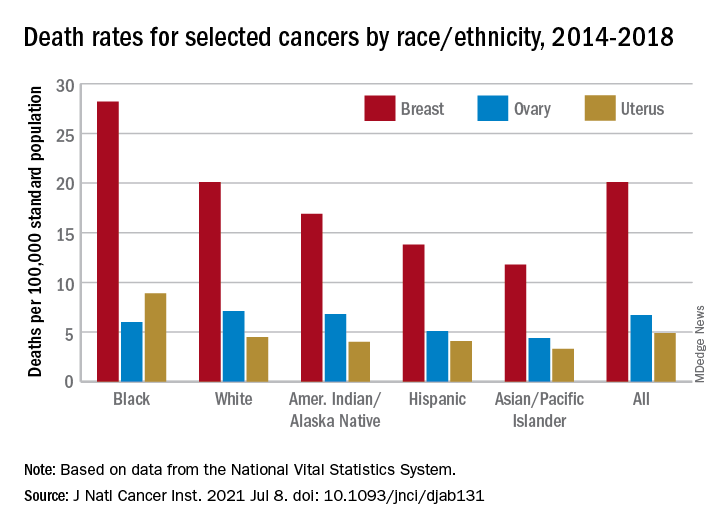
The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.

The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.

The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Placental allograft, cytology processor, cell-free RNA testing, and male infertility
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Focus on cancer risk
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.