User login
Cervical cancer mortality stagnates despite screening
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
FROM ADVANCING NIH RESEARCH ON THE HEALTH OF WOMEN
Convenience, not outcomes may drive robot-assisted surgeries
“The problem in minimally invasive surgery, especially in cancer surgery, is that the concept has been flip-flopped,” said Hooman Noorchashm, MD, PhD, a retired cardiothoracic surgeon turned patient advocate. “The main purpose of surgery should be removal of diseased tissue or repair of damaged tissue with adequate safety. The size of the incision on that triage scheme is secondary.”
In 2013, Dr. Noorchashm’s wife, Amy Reed, MD, an anesthesiologist, had a hysterectomy for treatment of severe uterine fibroids. The surgery was performed with a laparoscopic power morcellator, which led to the dissemination of cells from a previously undetected abdominal lesion. She was later diagnosed with stage 4 leiomyosarcoma and died in May 2017.
Dr. Noorchashm said the problem with robotic surgery isn’t the technology itself or how it’s used, but why it’s used in the first place. “Not only was there an extreme level of laxity with respect to the malignant potential of fibroids, but also that the size of the incision supersedes the safety of the procedure.”
The ultimate goal of oncologic surgery is to achieve an en bloc resection with clean surgical margins and removal of the tumor intact, Dr. Noorchashm said. The only scientific way of showing the benefits or therapeutic equivalence of new technology is through noninferiority comparison trials.
Robotic surgery inching toward $14 billion in revenue by 2028
Although robotic surgical technology has been in use since the 1990s, the technology is still considered to be its infancy. The first Food and Drug Administration–approved robotics platform, the da Vinci Surgical System (Intuitive Surgical) was approved by the FDA in 2000. And, now, with its patent expiring in 2022, competitors will be developing and launching new products for abdominal and colorectal surgery, partial knee replacements, cardiovascular procedures, head and neck surgery, and spinal procedures.
Robotic surgery is a rapidly expanding area with new product launches announced daily. In August 2021, the market research firm Grand View Research, reported the surgical robot marketplace is projected to reach $14 billion by 2028, up from $3.6 billion this year.
“This new era of robotic-assisted surgery attracts both surgeons and patients. Robotic surgery has reshaped our surgeries over the last 2 decades, and robots are now used in almost in every surgical field. Still, as surgeons, we continue to look – with great interest – to new robotic companies that may be able to provide better robots in a more cost-effective manner,” wrote urologists Ahmad Almujalhem and Koon Ho Rha in a review published in the journal BJUI Compass.
However, the authors wrote that, although the market is competitive, cost remains an issue, as are competing interests. In addition, many companies are creating replicas of existing technologies instead of focusing on new designs and new technology. “Although the da Vinci system propelled many robots to market, there has been no significant improvement in the console,” they added.
The technology is attractive to both surgeons and patients. “Surgeons are attracted to newer technologies, better vision, and easier learning curves. Patients are also attracted to robotic surgery, as this technology is considered state of the art and is associated with reduced pain and scar size,” the authors wrote.
Outcomes depend on many variables
In terms of outcomes, the literature is mixed. It largely depends on a number of variables from the site of surgery, the type of cancer, technology used, and the surgeon’s skill.
Jung Mogg Kim, MD, PhD, a microbiologist with Hanyang University, Seoul, South Korea, published a systemic review and meta-analysis of 27 clinical reports in PLoS ONE assessing clinical outcomes. They found that robot-assisted laparoscopic surgery did not result in statistically superior outcomes, compared with conventional laparoscopic surgery, except for lower estimated blood loss with robots. Operative time and total complications rates were “significantly more favorable” with conventional laparoscopic procedures.
Thomas E. Ahlering, MD, a robotic prostatectomy specialist at the University of California, Irvine, explained that the success or failure of robot-assisted surgery can be highly dependent on the body site and tumor type.
“The oncologic outcome, as long as the surgeon is up to speed, is not going to be better, but the goal is to be as good,” he said in an interview.
In most cases, Dr. Ahlering said, the goal of surgery is to remove a viable tumor with clean margins while leaving the organ intact. But in prostate surgery, the goal is to remove the entire organ while trying to preserve urinary continence and sexual function.
“One of the biggest benefits of the robot is that we’re able to use it in a laparoscopic environment meaning that we need a pneumoperitoneum [which] dramatically decreases bleeding. In prostate cancer, the area is so highly vascular that bleeding is a major issue,” he said.
The same benefits of reduced bleeding, improved visualization, and precision are also seen with robotic-assisted surgery for renal cancer, he noted.
He also emphasized that positive surgical margins, while less desirable than complete elimination of malignant cells, is not nearly as dire in prostate cancer as it is in surgery for other malignancies, such as soft-tissue sarcomas.
“The majority of cases are never going to recur, and if they do recur they essentially never lead to metastatic disease to bone, much less to prostate cancer–related death. The only thing they can do is slightly increase the PSA [prostate-specific antigen] recurrence,” he said.
Assuming that outcomes are comparable between an open procedure, conventional laparoscopic procedure, or robot-assisted approach, surgeons “will almost all go for the robot. It’s easier on the surgeon and it’s easier on the system,” Dr. Ahlering said.
In skilled hands for select patients, the use of a carefully researched and well-designed surgical assistive device can result in outcomes that are comparable with those seen in open surgical procedures, with robot-assisted surgery offering the possibility of less perioperative bleeding, lower postoperative morbidity, and faster recovery times.
“In our program we have been using robots to perform robotic radical prostatectomy and nephron-sparing surgery – partial nephrectomy and we’re also using them to perform intracorporeal bowel reconstruction and robotic radical cystectomy,” said Ashutosh Tewari, MD, of the Icahn School of Medicine at Mount Sinai, New York.
Robot-assisted surgery can be used “anywhere where you have to be selective, anywhere where you have to be reconstructive, anywhere where [assisted] vision can help, anywhere where the lack of bleeding will be of help to patients, and anywhere where a smaller incision can achieve the same goals,” Dr. Tewari said in an interview. Dr. Tewari’s Mount Sinai colleagues reported at the 2021 American Urological Association annual meeting, robotic-assisted salvage radical and partial nephrectomies were found to be safe and feasible procedures in patients with metachronous kidney tumors. For patients with early invasive cancer (stage pT1), oncologic outcomes with robotic-assisted partial nephrectomy were similar to those of patients who underwent radical surgery. The authors concluded that salvage robotic-assisted partial nephrectomy “can be considered in this group of patients due to the risk of future recurrences and need to preserve renal function.”
The National Comprehensive Cancer Network guideline for prostate cancer, updated in September 2021, states that “laparoscopic and robot-assisted radical prostatectomy are commonly used and are considered comparable to conventional approaches in experienced hands.”
In 2018, researchers in a multinational comparison trial reported that patients with cervical cancer who were randomly assigned to minimally invasive robot-assisted radical hysterectomy had significantly lower rates of both disease-free survival and overall survival than women randomized to open abdominal radical hysterectomy. The study results were published in the New England Journal of Medicine.
The use of robotically assisted surgical (RAS) devices could possibly create a “shielding layer” between the surgical team and patient reducing the risk of infection, according to Ajmal Zemmar, MD, PhD, FMH, a neurosurgeon with the University of Louisville (Ky.) Dr. Zemmar and colleagues recently published a perspective in Nature Machine Intelligence on trends in the use of surgical robots.
“In the operating theatre, robots can place intravascular lines, intubate the patient and manage the airway. The integration of a robot as a shielding layer, physically separating the health care worker and patient, is a powerful tool to combat the omnipresent fear of pathogen contamination and maintain surgical volumes,” Dr. Zemmar and colleagues wrote.
Surgical vs. clinical outcomes
In July 2021, this news organization reported that clinical trials of RAS for nipple-sparing mastectomy procedures were looking primarily at cosmetic or surgical outcomes and were not collecting cancer outcomes and if they were, it was secondary to cosmetic or surgical outcomes.
The FDA followed up by issuing a safety communication in August warning patients and providers that neither the safety nor efficacy of RAS for use in mastectomy procedures or treatment of breast cancer have been established.
“In addition, the FDA is aware of allegations that clinical studies are being conducted using RAS devices to perform mastectomies for the prevention or treatment of cancer without the FDA oversight required for such significant risk studies,” the communication stated.
Dr. Tewari disclosed relationships with various companies. Dr. Noorchashm had no relevant disclosures. Dr. Ahlering disclosed past funding or other considerations from Intuitive Robotics.
“The problem in minimally invasive surgery, especially in cancer surgery, is that the concept has been flip-flopped,” said Hooman Noorchashm, MD, PhD, a retired cardiothoracic surgeon turned patient advocate. “The main purpose of surgery should be removal of diseased tissue or repair of damaged tissue with adequate safety. The size of the incision on that triage scheme is secondary.”
In 2013, Dr. Noorchashm’s wife, Amy Reed, MD, an anesthesiologist, had a hysterectomy for treatment of severe uterine fibroids. The surgery was performed with a laparoscopic power morcellator, which led to the dissemination of cells from a previously undetected abdominal lesion. She was later diagnosed with stage 4 leiomyosarcoma and died in May 2017.
Dr. Noorchashm said the problem with robotic surgery isn’t the technology itself or how it’s used, but why it’s used in the first place. “Not only was there an extreme level of laxity with respect to the malignant potential of fibroids, but also that the size of the incision supersedes the safety of the procedure.”
The ultimate goal of oncologic surgery is to achieve an en bloc resection with clean surgical margins and removal of the tumor intact, Dr. Noorchashm said. The only scientific way of showing the benefits or therapeutic equivalence of new technology is through noninferiority comparison trials.
Robotic surgery inching toward $14 billion in revenue by 2028
Although robotic surgical technology has been in use since the 1990s, the technology is still considered to be its infancy. The first Food and Drug Administration–approved robotics platform, the da Vinci Surgical System (Intuitive Surgical) was approved by the FDA in 2000. And, now, with its patent expiring in 2022, competitors will be developing and launching new products for abdominal and colorectal surgery, partial knee replacements, cardiovascular procedures, head and neck surgery, and spinal procedures.
Robotic surgery is a rapidly expanding area with new product launches announced daily. In August 2021, the market research firm Grand View Research, reported the surgical robot marketplace is projected to reach $14 billion by 2028, up from $3.6 billion this year.
“This new era of robotic-assisted surgery attracts both surgeons and patients. Robotic surgery has reshaped our surgeries over the last 2 decades, and robots are now used in almost in every surgical field. Still, as surgeons, we continue to look – with great interest – to new robotic companies that may be able to provide better robots in a more cost-effective manner,” wrote urologists Ahmad Almujalhem and Koon Ho Rha in a review published in the journal BJUI Compass.
However, the authors wrote that, although the market is competitive, cost remains an issue, as are competing interests. In addition, many companies are creating replicas of existing technologies instead of focusing on new designs and new technology. “Although the da Vinci system propelled many robots to market, there has been no significant improvement in the console,” they added.
The technology is attractive to both surgeons and patients. “Surgeons are attracted to newer technologies, better vision, and easier learning curves. Patients are also attracted to robotic surgery, as this technology is considered state of the art and is associated with reduced pain and scar size,” the authors wrote.
Outcomes depend on many variables
In terms of outcomes, the literature is mixed. It largely depends on a number of variables from the site of surgery, the type of cancer, technology used, and the surgeon’s skill.
Jung Mogg Kim, MD, PhD, a microbiologist with Hanyang University, Seoul, South Korea, published a systemic review and meta-analysis of 27 clinical reports in PLoS ONE assessing clinical outcomes. They found that robot-assisted laparoscopic surgery did not result in statistically superior outcomes, compared with conventional laparoscopic surgery, except for lower estimated blood loss with robots. Operative time and total complications rates were “significantly more favorable” with conventional laparoscopic procedures.
Thomas E. Ahlering, MD, a robotic prostatectomy specialist at the University of California, Irvine, explained that the success or failure of robot-assisted surgery can be highly dependent on the body site and tumor type.
“The oncologic outcome, as long as the surgeon is up to speed, is not going to be better, but the goal is to be as good,” he said in an interview.
In most cases, Dr. Ahlering said, the goal of surgery is to remove a viable tumor with clean margins while leaving the organ intact. But in prostate surgery, the goal is to remove the entire organ while trying to preserve urinary continence and sexual function.
“One of the biggest benefits of the robot is that we’re able to use it in a laparoscopic environment meaning that we need a pneumoperitoneum [which] dramatically decreases bleeding. In prostate cancer, the area is so highly vascular that bleeding is a major issue,” he said.
The same benefits of reduced bleeding, improved visualization, and precision are also seen with robotic-assisted surgery for renal cancer, he noted.
He also emphasized that positive surgical margins, while less desirable than complete elimination of malignant cells, is not nearly as dire in prostate cancer as it is in surgery for other malignancies, such as soft-tissue sarcomas.
“The majority of cases are never going to recur, and if they do recur they essentially never lead to metastatic disease to bone, much less to prostate cancer–related death. The only thing they can do is slightly increase the PSA [prostate-specific antigen] recurrence,” he said.
Assuming that outcomes are comparable between an open procedure, conventional laparoscopic procedure, or robot-assisted approach, surgeons “will almost all go for the robot. It’s easier on the surgeon and it’s easier on the system,” Dr. Ahlering said.
In skilled hands for select patients, the use of a carefully researched and well-designed surgical assistive device can result in outcomes that are comparable with those seen in open surgical procedures, with robot-assisted surgery offering the possibility of less perioperative bleeding, lower postoperative morbidity, and faster recovery times.
“In our program we have been using robots to perform robotic radical prostatectomy and nephron-sparing surgery – partial nephrectomy and we’re also using them to perform intracorporeal bowel reconstruction and robotic radical cystectomy,” said Ashutosh Tewari, MD, of the Icahn School of Medicine at Mount Sinai, New York.
Robot-assisted surgery can be used “anywhere where you have to be selective, anywhere where you have to be reconstructive, anywhere where [assisted] vision can help, anywhere where the lack of bleeding will be of help to patients, and anywhere where a smaller incision can achieve the same goals,” Dr. Tewari said in an interview. Dr. Tewari’s Mount Sinai colleagues reported at the 2021 American Urological Association annual meeting, robotic-assisted salvage radical and partial nephrectomies were found to be safe and feasible procedures in patients with metachronous kidney tumors. For patients with early invasive cancer (stage pT1), oncologic outcomes with robotic-assisted partial nephrectomy were similar to those of patients who underwent radical surgery. The authors concluded that salvage robotic-assisted partial nephrectomy “can be considered in this group of patients due to the risk of future recurrences and need to preserve renal function.”
The National Comprehensive Cancer Network guideline for prostate cancer, updated in September 2021, states that “laparoscopic and robot-assisted radical prostatectomy are commonly used and are considered comparable to conventional approaches in experienced hands.”
In 2018, researchers in a multinational comparison trial reported that patients with cervical cancer who were randomly assigned to minimally invasive robot-assisted radical hysterectomy had significantly lower rates of both disease-free survival and overall survival than women randomized to open abdominal radical hysterectomy. The study results were published in the New England Journal of Medicine.
The use of robotically assisted surgical (RAS) devices could possibly create a “shielding layer” between the surgical team and patient reducing the risk of infection, according to Ajmal Zemmar, MD, PhD, FMH, a neurosurgeon with the University of Louisville (Ky.) Dr. Zemmar and colleagues recently published a perspective in Nature Machine Intelligence on trends in the use of surgical robots.
“In the operating theatre, robots can place intravascular lines, intubate the patient and manage the airway. The integration of a robot as a shielding layer, physically separating the health care worker and patient, is a powerful tool to combat the omnipresent fear of pathogen contamination and maintain surgical volumes,” Dr. Zemmar and colleagues wrote.
Surgical vs. clinical outcomes
In July 2021, this news organization reported that clinical trials of RAS for nipple-sparing mastectomy procedures were looking primarily at cosmetic or surgical outcomes and were not collecting cancer outcomes and if they were, it was secondary to cosmetic or surgical outcomes.
The FDA followed up by issuing a safety communication in August warning patients and providers that neither the safety nor efficacy of RAS for use in mastectomy procedures or treatment of breast cancer have been established.
“In addition, the FDA is aware of allegations that clinical studies are being conducted using RAS devices to perform mastectomies for the prevention or treatment of cancer without the FDA oversight required for such significant risk studies,” the communication stated.
Dr. Tewari disclosed relationships with various companies. Dr. Noorchashm had no relevant disclosures. Dr. Ahlering disclosed past funding or other considerations from Intuitive Robotics.
“The problem in minimally invasive surgery, especially in cancer surgery, is that the concept has been flip-flopped,” said Hooman Noorchashm, MD, PhD, a retired cardiothoracic surgeon turned patient advocate. “The main purpose of surgery should be removal of diseased tissue or repair of damaged tissue with adequate safety. The size of the incision on that triage scheme is secondary.”
In 2013, Dr. Noorchashm’s wife, Amy Reed, MD, an anesthesiologist, had a hysterectomy for treatment of severe uterine fibroids. The surgery was performed with a laparoscopic power morcellator, which led to the dissemination of cells from a previously undetected abdominal lesion. She was later diagnosed with stage 4 leiomyosarcoma and died in May 2017.
Dr. Noorchashm said the problem with robotic surgery isn’t the technology itself or how it’s used, but why it’s used in the first place. “Not only was there an extreme level of laxity with respect to the malignant potential of fibroids, but also that the size of the incision supersedes the safety of the procedure.”
The ultimate goal of oncologic surgery is to achieve an en bloc resection with clean surgical margins and removal of the tumor intact, Dr. Noorchashm said. The only scientific way of showing the benefits or therapeutic equivalence of new technology is through noninferiority comparison trials.
Robotic surgery inching toward $14 billion in revenue by 2028
Although robotic surgical technology has been in use since the 1990s, the technology is still considered to be its infancy. The first Food and Drug Administration–approved robotics platform, the da Vinci Surgical System (Intuitive Surgical) was approved by the FDA in 2000. And, now, with its patent expiring in 2022, competitors will be developing and launching new products for abdominal and colorectal surgery, partial knee replacements, cardiovascular procedures, head and neck surgery, and spinal procedures.
Robotic surgery is a rapidly expanding area with new product launches announced daily. In August 2021, the market research firm Grand View Research, reported the surgical robot marketplace is projected to reach $14 billion by 2028, up from $3.6 billion this year.
“This new era of robotic-assisted surgery attracts both surgeons and patients. Robotic surgery has reshaped our surgeries over the last 2 decades, and robots are now used in almost in every surgical field. Still, as surgeons, we continue to look – with great interest – to new robotic companies that may be able to provide better robots in a more cost-effective manner,” wrote urologists Ahmad Almujalhem and Koon Ho Rha in a review published in the journal BJUI Compass.
However, the authors wrote that, although the market is competitive, cost remains an issue, as are competing interests. In addition, many companies are creating replicas of existing technologies instead of focusing on new designs and new technology. “Although the da Vinci system propelled many robots to market, there has been no significant improvement in the console,” they added.
The technology is attractive to both surgeons and patients. “Surgeons are attracted to newer technologies, better vision, and easier learning curves. Patients are also attracted to robotic surgery, as this technology is considered state of the art and is associated with reduced pain and scar size,” the authors wrote.
Outcomes depend on many variables
In terms of outcomes, the literature is mixed. It largely depends on a number of variables from the site of surgery, the type of cancer, technology used, and the surgeon’s skill.
Jung Mogg Kim, MD, PhD, a microbiologist with Hanyang University, Seoul, South Korea, published a systemic review and meta-analysis of 27 clinical reports in PLoS ONE assessing clinical outcomes. They found that robot-assisted laparoscopic surgery did not result in statistically superior outcomes, compared with conventional laparoscopic surgery, except for lower estimated blood loss with robots. Operative time and total complications rates were “significantly more favorable” with conventional laparoscopic procedures.
Thomas E. Ahlering, MD, a robotic prostatectomy specialist at the University of California, Irvine, explained that the success or failure of robot-assisted surgery can be highly dependent on the body site and tumor type.
“The oncologic outcome, as long as the surgeon is up to speed, is not going to be better, but the goal is to be as good,” he said in an interview.
In most cases, Dr. Ahlering said, the goal of surgery is to remove a viable tumor with clean margins while leaving the organ intact. But in prostate surgery, the goal is to remove the entire organ while trying to preserve urinary continence and sexual function.
“One of the biggest benefits of the robot is that we’re able to use it in a laparoscopic environment meaning that we need a pneumoperitoneum [which] dramatically decreases bleeding. In prostate cancer, the area is so highly vascular that bleeding is a major issue,” he said.
The same benefits of reduced bleeding, improved visualization, and precision are also seen with robotic-assisted surgery for renal cancer, he noted.
He also emphasized that positive surgical margins, while less desirable than complete elimination of malignant cells, is not nearly as dire in prostate cancer as it is in surgery for other malignancies, such as soft-tissue sarcomas.
“The majority of cases are never going to recur, and if they do recur they essentially never lead to metastatic disease to bone, much less to prostate cancer–related death. The only thing they can do is slightly increase the PSA [prostate-specific antigen] recurrence,” he said.
Assuming that outcomes are comparable between an open procedure, conventional laparoscopic procedure, or robot-assisted approach, surgeons “will almost all go for the robot. It’s easier on the surgeon and it’s easier on the system,” Dr. Ahlering said.
In skilled hands for select patients, the use of a carefully researched and well-designed surgical assistive device can result in outcomes that are comparable with those seen in open surgical procedures, with robot-assisted surgery offering the possibility of less perioperative bleeding, lower postoperative morbidity, and faster recovery times.
“In our program we have been using robots to perform robotic radical prostatectomy and nephron-sparing surgery – partial nephrectomy and we’re also using them to perform intracorporeal bowel reconstruction and robotic radical cystectomy,” said Ashutosh Tewari, MD, of the Icahn School of Medicine at Mount Sinai, New York.
Robot-assisted surgery can be used “anywhere where you have to be selective, anywhere where you have to be reconstructive, anywhere where [assisted] vision can help, anywhere where the lack of bleeding will be of help to patients, and anywhere where a smaller incision can achieve the same goals,” Dr. Tewari said in an interview. Dr. Tewari’s Mount Sinai colleagues reported at the 2021 American Urological Association annual meeting, robotic-assisted salvage radical and partial nephrectomies were found to be safe and feasible procedures in patients with metachronous kidney tumors. For patients with early invasive cancer (stage pT1), oncologic outcomes with robotic-assisted partial nephrectomy were similar to those of patients who underwent radical surgery. The authors concluded that salvage robotic-assisted partial nephrectomy “can be considered in this group of patients due to the risk of future recurrences and need to preserve renal function.”
The National Comprehensive Cancer Network guideline for prostate cancer, updated in September 2021, states that “laparoscopic and robot-assisted radical prostatectomy are commonly used and are considered comparable to conventional approaches in experienced hands.”
In 2018, researchers in a multinational comparison trial reported that patients with cervical cancer who were randomly assigned to minimally invasive robot-assisted radical hysterectomy had significantly lower rates of both disease-free survival and overall survival than women randomized to open abdominal radical hysterectomy. The study results were published in the New England Journal of Medicine.
The use of robotically assisted surgical (RAS) devices could possibly create a “shielding layer” between the surgical team and patient reducing the risk of infection, according to Ajmal Zemmar, MD, PhD, FMH, a neurosurgeon with the University of Louisville (Ky.) Dr. Zemmar and colleagues recently published a perspective in Nature Machine Intelligence on trends in the use of surgical robots.
“In the operating theatre, robots can place intravascular lines, intubate the patient and manage the airway. The integration of a robot as a shielding layer, physically separating the health care worker and patient, is a powerful tool to combat the omnipresent fear of pathogen contamination and maintain surgical volumes,” Dr. Zemmar and colleagues wrote.
Surgical vs. clinical outcomes
In July 2021, this news organization reported that clinical trials of RAS for nipple-sparing mastectomy procedures were looking primarily at cosmetic or surgical outcomes and were not collecting cancer outcomes and if they were, it was secondary to cosmetic or surgical outcomes.
The FDA followed up by issuing a safety communication in August warning patients and providers that neither the safety nor efficacy of RAS for use in mastectomy procedures or treatment of breast cancer have been established.
“In addition, the FDA is aware of allegations that clinical studies are being conducted using RAS devices to perform mastectomies for the prevention or treatment of cancer without the FDA oversight required for such significant risk studies,” the communication stated.
Dr. Tewari disclosed relationships with various companies. Dr. Noorchashm had no relevant disclosures. Dr. Ahlering disclosed past funding or other considerations from Intuitive Robotics.
Early mortality falls in advanced ovarian cancer with neoadjuvant chemo
FROM JAMA ONCOLOGY
Cancer centers with a high use of neoadjuvant chemotherapy in patients with advanced-stage epithelial ovarian cancer show similar improvements in median overall survival and larger declines in short-term mortality than in centers with low use of this treatment. This is according to a study published in JAMA Oncology, suggesting that neoadjuvant chemotherapy may be a suitable first-line treatment approach for many patients with advanced-stage ovarian cancer.
“There is considerable variation in practice. Some centers administer neoadjuvant chemotherapy to 75% of patients with advanced ovarian cancers, others use the approach very infrequently,” said Alexander Melamed, MD, MPH, of Columbia University, New York.
“I hope that those clinicians who have been worried about the negative impacts of too frequent administration of neoadjuvant chemotherapy may be reassured by this study and may come to use this good treatment more often.”
Research has shown that, compared with primary cytoreductive surgery, the use of neoadjuvant chemotherapy has similar long-term survival and improved perioperative outcomes in patients with ovarian cancer. While the use of neoadjuvant chemotherapy has increased, many experts continue to recommend upfront surgery as the preferred treatment for these patients.
“In part, these recommendations are based on flawed interpretations of real-world data. Specifically, many observational studies have concluded that upfront surgery results in better survival than neoadjuvant chemotherapy, based on study designs that ignored the fact that patients who receive neoadjuvant chemotherapy in the real word are sicker and have more extensive cancer than those who receive upfront surgery,” Dr. Melamed said.
In this difference-in-differences comparative effectiveness analysis, researchers asked if the difference in adoption of neoadjuvant chemotherapy by U.S. cancer centers for advanced-stage epithelial ovarian cancer was associated with differences in median overall survival and 1-year all-cause mortality.
“By assessing how this divergence in practice impacted patient outcomes we were able to infer how frequent use of neoadjuvant impacts survival in ovarian cancer patients. This study design allowed us to sidestep the problem of selection bias that has plagued many other observational studies in this space,” Dr. Melamed explained.
This observational study included 39,299 women with stage IIIC and IV epithelial ovarian cancer, diagnosed between 2004 and 2015 who were followed to the end of 2018, and treated at one of 664 cancer programs. Patients treated in programs that increased neoadjuvant chemotherapy administration had greater improvements in 1-year mortality (difference-in-differences, −2.1%; 95% confidence interval, −3.7 to −0.5) and equivalent gains in median overall survival (difference-in-differences, 0.9 months; 95% CI, −1.9 to 3.7 months), compared with those treated in programs that used the treatment infrequently.
“For a long time, experts have suggested that the apparent discordance between randomized controlled trials and real-world studies that compare neoadjuvant chemotherapy to upfront surgery for ovarian cancer might mean that the randomized trials are not applicable to real-world practice. What is significant about our findings, is that, when more appropriate study methods are used to analyze the real-world data, the apparent contradiction between real-world and randomized studies is resolved.
“We found that, just as one would guess based on the findings of randomized trials, patients treated in the centers that increased the use of neoadjuvant chemotherapy did not have any decrement in long-term survival, but that short-term mortality did improve more in these centers than in centers that administered neoadjuvant chemotherapy rarely,” she said.
Dr. Melamed said that the findings should “spur a reappraisal” of what clinicians consider the default treatment for women with stage IIIC and IV ovarian cancer.
Taken together with randomized controlled trials, “the evidence may be at a point where it is now time to consider neoadjuvant chemotherapy as the default approach to patients with bulky carcinomatosis, and that primary surgery may be a reasonable alternative for a select group of healthy, young patients with low-volume metastasis.
“Other factors like the route of adjuvant chemotherapy may also need to be considered. However, I believe the belief that aggressive primary debulking is beneficial for most women with advanced ovarian cancer is outdated,” Dr. Melamed said.
No relevant conflicts of interest were reported for this research.
FROM JAMA ONCOLOGY
Cancer centers with a high use of neoadjuvant chemotherapy in patients with advanced-stage epithelial ovarian cancer show similar improvements in median overall survival and larger declines in short-term mortality than in centers with low use of this treatment. This is according to a study published in JAMA Oncology, suggesting that neoadjuvant chemotherapy may be a suitable first-line treatment approach for many patients with advanced-stage ovarian cancer.
“There is considerable variation in practice. Some centers administer neoadjuvant chemotherapy to 75% of patients with advanced ovarian cancers, others use the approach very infrequently,” said Alexander Melamed, MD, MPH, of Columbia University, New York.
“I hope that those clinicians who have been worried about the negative impacts of too frequent administration of neoadjuvant chemotherapy may be reassured by this study and may come to use this good treatment more often.”
Research has shown that, compared with primary cytoreductive surgery, the use of neoadjuvant chemotherapy has similar long-term survival and improved perioperative outcomes in patients with ovarian cancer. While the use of neoadjuvant chemotherapy has increased, many experts continue to recommend upfront surgery as the preferred treatment for these patients.
“In part, these recommendations are based on flawed interpretations of real-world data. Specifically, many observational studies have concluded that upfront surgery results in better survival than neoadjuvant chemotherapy, based on study designs that ignored the fact that patients who receive neoadjuvant chemotherapy in the real word are sicker and have more extensive cancer than those who receive upfront surgery,” Dr. Melamed said.
In this difference-in-differences comparative effectiveness analysis, researchers asked if the difference in adoption of neoadjuvant chemotherapy by U.S. cancer centers for advanced-stage epithelial ovarian cancer was associated with differences in median overall survival and 1-year all-cause mortality.
“By assessing how this divergence in practice impacted patient outcomes we were able to infer how frequent use of neoadjuvant impacts survival in ovarian cancer patients. This study design allowed us to sidestep the problem of selection bias that has plagued many other observational studies in this space,” Dr. Melamed explained.
This observational study included 39,299 women with stage IIIC and IV epithelial ovarian cancer, diagnosed between 2004 and 2015 who were followed to the end of 2018, and treated at one of 664 cancer programs. Patients treated in programs that increased neoadjuvant chemotherapy administration had greater improvements in 1-year mortality (difference-in-differences, −2.1%; 95% confidence interval, −3.7 to −0.5) and equivalent gains in median overall survival (difference-in-differences, 0.9 months; 95% CI, −1.9 to 3.7 months), compared with those treated in programs that used the treatment infrequently.
“For a long time, experts have suggested that the apparent discordance between randomized controlled trials and real-world studies that compare neoadjuvant chemotherapy to upfront surgery for ovarian cancer might mean that the randomized trials are not applicable to real-world practice. What is significant about our findings, is that, when more appropriate study methods are used to analyze the real-world data, the apparent contradiction between real-world and randomized studies is resolved.
“We found that, just as one would guess based on the findings of randomized trials, patients treated in the centers that increased the use of neoadjuvant chemotherapy did not have any decrement in long-term survival, but that short-term mortality did improve more in these centers than in centers that administered neoadjuvant chemotherapy rarely,” she said.
Dr. Melamed said that the findings should “spur a reappraisal” of what clinicians consider the default treatment for women with stage IIIC and IV ovarian cancer.
Taken together with randomized controlled trials, “the evidence may be at a point where it is now time to consider neoadjuvant chemotherapy as the default approach to patients with bulky carcinomatosis, and that primary surgery may be a reasonable alternative for a select group of healthy, young patients with low-volume metastasis.
“Other factors like the route of adjuvant chemotherapy may also need to be considered. However, I believe the belief that aggressive primary debulking is beneficial for most women with advanced ovarian cancer is outdated,” Dr. Melamed said.
No relevant conflicts of interest were reported for this research.
FROM JAMA ONCOLOGY
Cancer centers with a high use of neoadjuvant chemotherapy in patients with advanced-stage epithelial ovarian cancer show similar improvements in median overall survival and larger declines in short-term mortality than in centers with low use of this treatment. This is according to a study published in JAMA Oncology, suggesting that neoadjuvant chemotherapy may be a suitable first-line treatment approach for many patients with advanced-stage ovarian cancer.
“There is considerable variation in practice. Some centers administer neoadjuvant chemotherapy to 75% of patients with advanced ovarian cancers, others use the approach very infrequently,” said Alexander Melamed, MD, MPH, of Columbia University, New York.
“I hope that those clinicians who have been worried about the negative impacts of too frequent administration of neoadjuvant chemotherapy may be reassured by this study and may come to use this good treatment more often.”
Research has shown that, compared with primary cytoreductive surgery, the use of neoadjuvant chemotherapy has similar long-term survival and improved perioperative outcomes in patients with ovarian cancer. While the use of neoadjuvant chemotherapy has increased, many experts continue to recommend upfront surgery as the preferred treatment for these patients.
“In part, these recommendations are based on flawed interpretations of real-world data. Specifically, many observational studies have concluded that upfront surgery results in better survival than neoadjuvant chemotherapy, based on study designs that ignored the fact that patients who receive neoadjuvant chemotherapy in the real word are sicker and have more extensive cancer than those who receive upfront surgery,” Dr. Melamed said.
In this difference-in-differences comparative effectiveness analysis, researchers asked if the difference in adoption of neoadjuvant chemotherapy by U.S. cancer centers for advanced-stage epithelial ovarian cancer was associated with differences in median overall survival and 1-year all-cause mortality.
“By assessing how this divergence in practice impacted patient outcomes we were able to infer how frequent use of neoadjuvant impacts survival in ovarian cancer patients. This study design allowed us to sidestep the problem of selection bias that has plagued many other observational studies in this space,” Dr. Melamed explained.
This observational study included 39,299 women with stage IIIC and IV epithelial ovarian cancer, diagnosed between 2004 and 2015 who were followed to the end of 2018, and treated at one of 664 cancer programs. Patients treated in programs that increased neoadjuvant chemotherapy administration had greater improvements in 1-year mortality (difference-in-differences, −2.1%; 95% confidence interval, −3.7 to −0.5) and equivalent gains in median overall survival (difference-in-differences, 0.9 months; 95% CI, −1.9 to 3.7 months), compared with those treated in programs that used the treatment infrequently.
“For a long time, experts have suggested that the apparent discordance between randomized controlled trials and real-world studies that compare neoadjuvant chemotherapy to upfront surgery for ovarian cancer might mean that the randomized trials are not applicable to real-world practice. What is significant about our findings, is that, when more appropriate study methods are used to analyze the real-world data, the apparent contradiction between real-world and randomized studies is resolved.
“We found that, just as one would guess based on the findings of randomized trials, patients treated in the centers that increased the use of neoadjuvant chemotherapy did not have any decrement in long-term survival, but that short-term mortality did improve more in these centers than in centers that administered neoadjuvant chemotherapy rarely,” she said.
Dr. Melamed said that the findings should “spur a reappraisal” of what clinicians consider the default treatment for women with stage IIIC and IV ovarian cancer.
Taken together with randomized controlled trials, “the evidence may be at a point where it is now time to consider neoadjuvant chemotherapy as the default approach to patients with bulky carcinomatosis, and that primary surgery may be a reasonable alternative for a select group of healthy, young patients with low-volume metastasis.
“Other factors like the route of adjuvant chemotherapy may also need to be considered. However, I believe the belief that aggressive primary debulking is beneficial for most women with advanced ovarian cancer is outdated,” Dr. Melamed said.
No relevant conflicts of interest were reported for this research.
True or false: Breast density increases breast cancer risk
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).
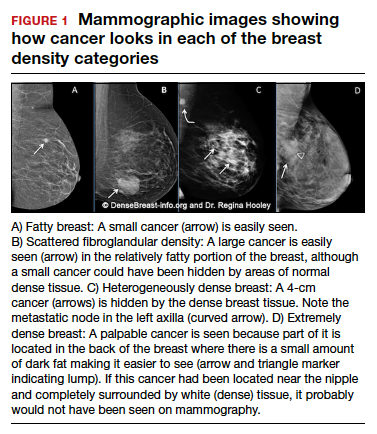
Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8
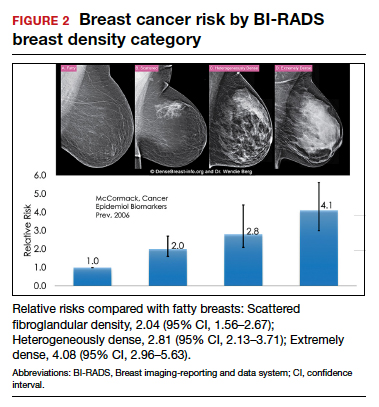
Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Quiz developed in collaboration with 
Unexpected thrombocytosis could flag occult cancer
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
Many patients, doctors unaware of advancements in cancer care
This is the main finding from two studies presented at the 2021 European Society for Medical Oncology Congress.
The survey of patients found that most don’t understand how immunotherapy works, and the survey of doctors found that many working outside of the cancer field are using information on survival that is wildly out of date.
When a patient is first told they have cancer, counseling is usually done by a surgeon or general medical doctor and not an oncologist, said Conleth Murphy, MD, of Bon Secours Hospital Cork, Ireland, and coauthor of the second study.
Noncancer doctors often grossly underestimate patients’ chances of survival, Dr. Murphy’s study found. This suggests that doctors who practice outside of cancer care may be working with the same information they learned in medical school, he said.
“These patients must be spared the traumatic effects of being handed a death sentence that no longer reflects the current reality,” Dr. Murphy said.
After receiving a diagnosis of cancer, “patients often immediately have pressing questions about what it means for their future,” he noted. A common question is: “How long do I have left?”
Nononcologists should refrain from answering patients’ questions with numbers, Dr. Murphy said.
Family doctors are likely to be influenced by the experience they have had with specific cancer patients in their practice, said Cyril Bonin, MD, a general practitioner in Usson-du-Poitou, France, who has 900 patients in his practice.
He sees about 10 patients with a new diagnosis of cancer each year. In addition, about 50 of his patients are in active treatment for cancer or have finished treatment and are considered cancer survivors.
“It is not entirely realistic for us to expect practitioners who deal with hundreds of different diseases to keep up with every facet of a rapidly changing oncology landscape,” said Marco Donia, MD, an expert in immunotherapy from the University of Copenhagen.
That landscape has changed dramatically in recent years, particularly since immunotherapy was added to the arsenal. Immunotherapy is a way to fine-tune your immune system to fight cancer.
For example, in the past, patients with metastatic melanoma would have an average survival of about 1 year. But now, some patients who have responded to immunotherapy are still alive 10 years later.
Findings from the patient survey
It is important that patients stay well informed because immunotherapy is a “complex treatment that is too often mistaken for a miracle cure,” said Paris Kosmidis, MD, the co-author of the patient survey.
“The more patients know about it, the better the communication with their medical team and thus the better their outcomes are likely to be,” said Dr. Kosmidis, who is co-founder and chief medical officer of CareAcross, an online service that provides personalized education for cancer patients
The survey was of 5,589 patients with cancer who were recruited from CareAcross clients from the United Kingdom, France, Italy, Spain, and Germany.
The survey asked them about how immunotherapy works, what it costs, and its side effects.
Almost half responded “not sure/do not know,” but about a third correctly answered that immunotherapy “activates the immune system to kill cancer cells.”
Similarly, more than half thought that immunotherapy started working right away, while only 20% correctly answered that it takes several weeks to become effective.
“This is important because patients need to start their therapy with realistic expectations, for example to avoid disappointment when their symptoms take some time to disappear,” Dr. Kosmidis said.
A small group of 24 patients with lung cancer who had been treated with immunotherapy got many correct answers, but they overestimated the intensity of side effects, compared with other therapies.
“Well-informed patients who know what to expect can do 90% of the job of preventing side effects from becoming severe by having them treated early,” said Dr. Donia, of the University of Copenhagen.
Most cancer patients were also unaware of the cost of immunotherapy, which can exceed $100,000 a year, Dr. Kosmidis said.
Results of the doctor survey
The other survey presented at the meeting looked at how much doctors know about survival for 12 of the most common cancers.
Dr. Murphy and colleagues asked 301 noncancer doctors and 46 cancer specialists to estimate the percentage of patients who could be expected to live for 5 years after diagnosis (a measure known as the 5-year survival rate).
Answers from the two groups were compared and graded according to cancer survival statistics from the National Cancer Registry of Ireland.
Both groups of doctors had a hard time estimating the survival of common cancers.
Nononcologists accurately predicted 5-year survival for just two of the cancer types, while the cancer specialists got it right for four cancer types.
However, the noncancer doctors had a more pessimistic outlook on cancer survival generally and severely underestimated the chances of survival in specific cancers, particularly stage IV breast cancer. The survival for this cancer has “evolved considerably over time and now reaches 40% in Ireland,” Dr. Murphy pointed out.
“These results are in line with what we had expected because most physicians’ knowledge of oncology dates back to whatever education they received during their years of training, so their perceptions of cancer prognosis are likely to lag behind the major survival gains achieved in the recent past,” Dr. Murphy said.
A version of this article first appeared on Medscape.com.
This is the main finding from two studies presented at the 2021 European Society for Medical Oncology Congress.
The survey of patients found that most don’t understand how immunotherapy works, and the survey of doctors found that many working outside of the cancer field are using information on survival that is wildly out of date.
When a patient is first told they have cancer, counseling is usually done by a surgeon or general medical doctor and not an oncologist, said Conleth Murphy, MD, of Bon Secours Hospital Cork, Ireland, and coauthor of the second study.
Noncancer doctors often grossly underestimate patients’ chances of survival, Dr. Murphy’s study found. This suggests that doctors who practice outside of cancer care may be working with the same information they learned in medical school, he said.
“These patients must be spared the traumatic effects of being handed a death sentence that no longer reflects the current reality,” Dr. Murphy said.
After receiving a diagnosis of cancer, “patients often immediately have pressing questions about what it means for their future,” he noted. A common question is: “How long do I have left?”
Nononcologists should refrain from answering patients’ questions with numbers, Dr. Murphy said.
Family doctors are likely to be influenced by the experience they have had with specific cancer patients in their practice, said Cyril Bonin, MD, a general practitioner in Usson-du-Poitou, France, who has 900 patients in his practice.
He sees about 10 patients with a new diagnosis of cancer each year. In addition, about 50 of his patients are in active treatment for cancer or have finished treatment and are considered cancer survivors.
“It is not entirely realistic for us to expect practitioners who deal with hundreds of different diseases to keep up with every facet of a rapidly changing oncology landscape,” said Marco Donia, MD, an expert in immunotherapy from the University of Copenhagen.
That landscape has changed dramatically in recent years, particularly since immunotherapy was added to the arsenal. Immunotherapy is a way to fine-tune your immune system to fight cancer.
For example, in the past, patients with metastatic melanoma would have an average survival of about 1 year. But now, some patients who have responded to immunotherapy are still alive 10 years later.
Findings from the patient survey
It is important that patients stay well informed because immunotherapy is a “complex treatment that is too often mistaken for a miracle cure,” said Paris Kosmidis, MD, the co-author of the patient survey.
“The more patients know about it, the better the communication with their medical team and thus the better their outcomes are likely to be,” said Dr. Kosmidis, who is co-founder and chief medical officer of CareAcross, an online service that provides personalized education for cancer patients
The survey was of 5,589 patients with cancer who were recruited from CareAcross clients from the United Kingdom, France, Italy, Spain, and Germany.
The survey asked them about how immunotherapy works, what it costs, and its side effects.
Almost half responded “not sure/do not know,” but about a third correctly answered that immunotherapy “activates the immune system to kill cancer cells.”
Similarly, more than half thought that immunotherapy started working right away, while only 20% correctly answered that it takes several weeks to become effective.
“This is important because patients need to start their therapy with realistic expectations, for example to avoid disappointment when their symptoms take some time to disappear,” Dr. Kosmidis said.
A small group of 24 patients with lung cancer who had been treated with immunotherapy got many correct answers, but they overestimated the intensity of side effects, compared with other therapies.
“Well-informed patients who know what to expect can do 90% of the job of preventing side effects from becoming severe by having them treated early,” said Dr. Donia, of the University of Copenhagen.
Most cancer patients were also unaware of the cost of immunotherapy, which can exceed $100,000 a year, Dr. Kosmidis said.
Results of the doctor survey
The other survey presented at the meeting looked at how much doctors know about survival for 12 of the most common cancers.
Dr. Murphy and colleagues asked 301 noncancer doctors and 46 cancer specialists to estimate the percentage of patients who could be expected to live for 5 years after diagnosis (a measure known as the 5-year survival rate).
Answers from the two groups were compared and graded according to cancer survival statistics from the National Cancer Registry of Ireland.
Both groups of doctors had a hard time estimating the survival of common cancers.
Nononcologists accurately predicted 5-year survival for just two of the cancer types, while the cancer specialists got it right for four cancer types.
However, the noncancer doctors had a more pessimistic outlook on cancer survival generally and severely underestimated the chances of survival in specific cancers, particularly stage IV breast cancer. The survival for this cancer has “evolved considerably over time and now reaches 40% in Ireland,” Dr. Murphy pointed out.
“These results are in line with what we had expected because most physicians’ knowledge of oncology dates back to whatever education they received during their years of training, so their perceptions of cancer prognosis are likely to lag behind the major survival gains achieved in the recent past,” Dr. Murphy said.
A version of this article first appeared on Medscape.com.
This is the main finding from two studies presented at the 2021 European Society for Medical Oncology Congress.
The survey of patients found that most don’t understand how immunotherapy works, and the survey of doctors found that many working outside of the cancer field are using information on survival that is wildly out of date.
When a patient is first told they have cancer, counseling is usually done by a surgeon or general medical doctor and not an oncologist, said Conleth Murphy, MD, of Bon Secours Hospital Cork, Ireland, and coauthor of the second study.
Noncancer doctors often grossly underestimate patients’ chances of survival, Dr. Murphy’s study found. This suggests that doctors who practice outside of cancer care may be working with the same information they learned in medical school, he said.
“These patients must be spared the traumatic effects of being handed a death sentence that no longer reflects the current reality,” Dr. Murphy said.
After receiving a diagnosis of cancer, “patients often immediately have pressing questions about what it means for their future,” he noted. A common question is: “How long do I have left?”
Nononcologists should refrain from answering patients’ questions with numbers, Dr. Murphy said.
Family doctors are likely to be influenced by the experience they have had with specific cancer patients in their practice, said Cyril Bonin, MD, a general practitioner in Usson-du-Poitou, France, who has 900 patients in his practice.
He sees about 10 patients with a new diagnosis of cancer each year. In addition, about 50 of his patients are in active treatment for cancer or have finished treatment and are considered cancer survivors.
“It is not entirely realistic for us to expect practitioners who deal with hundreds of different diseases to keep up with every facet of a rapidly changing oncology landscape,” said Marco Donia, MD, an expert in immunotherapy from the University of Copenhagen.
That landscape has changed dramatically in recent years, particularly since immunotherapy was added to the arsenal. Immunotherapy is a way to fine-tune your immune system to fight cancer.
For example, in the past, patients with metastatic melanoma would have an average survival of about 1 year. But now, some patients who have responded to immunotherapy are still alive 10 years later.
Findings from the patient survey
It is important that patients stay well informed because immunotherapy is a “complex treatment that is too often mistaken for a miracle cure,” said Paris Kosmidis, MD, the co-author of the patient survey.
“The more patients know about it, the better the communication with their medical team and thus the better their outcomes are likely to be,” said Dr. Kosmidis, who is co-founder and chief medical officer of CareAcross, an online service that provides personalized education for cancer patients
The survey was of 5,589 patients with cancer who were recruited from CareAcross clients from the United Kingdom, France, Italy, Spain, and Germany.
The survey asked them about how immunotherapy works, what it costs, and its side effects.
Almost half responded “not sure/do not know,” but about a third correctly answered that immunotherapy “activates the immune system to kill cancer cells.”
Similarly, more than half thought that immunotherapy started working right away, while only 20% correctly answered that it takes several weeks to become effective.
“This is important because patients need to start their therapy with realistic expectations, for example to avoid disappointment when their symptoms take some time to disappear,” Dr. Kosmidis said.
A small group of 24 patients with lung cancer who had been treated with immunotherapy got many correct answers, but they overestimated the intensity of side effects, compared with other therapies.
“Well-informed patients who know what to expect can do 90% of the job of preventing side effects from becoming severe by having them treated early,” said Dr. Donia, of the University of Copenhagen.
Most cancer patients were also unaware of the cost of immunotherapy, which can exceed $100,000 a year, Dr. Kosmidis said.
Results of the doctor survey
The other survey presented at the meeting looked at how much doctors know about survival for 12 of the most common cancers.
Dr. Murphy and colleagues asked 301 noncancer doctors and 46 cancer specialists to estimate the percentage of patients who could be expected to live for 5 years after diagnosis (a measure known as the 5-year survival rate).
Answers from the two groups were compared and graded according to cancer survival statistics from the National Cancer Registry of Ireland.
Both groups of doctors had a hard time estimating the survival of common cancers.
Nononcologists accurately predicted 5-year survival for just two of the cancer types, while the cancer specialists got it right for four cancer types.
However, the noncancer doctors had a more pessimistic outlook on cancer survival generally and severely underestimated the chances of survival in specific cancers, particularly stage IV breast cancer. The survival for this cancer has “evolved considerably over time and now reaches 40% in Ireland,” Dr. Murphy pointed out.
“These results are in line with what we had expected because most physicians’ knowledge of oncology dates back to whatever education they received during their years of training, so their perceptions of cancer prognosis are likely to lag behind the major survival gains achieved in the recent past,” Dr. Murphy said.
A version of this article first appeared on Medscape.com.
FDA approval for tisotumab vedotin in advanced cervical cancer
There is currently no standard option for these patients. The mainstay of therapy in this setting is monotherapy with chemotherapy, but the benefit-risk profiles are poor, and overall response rates (ORRs) are less than 15%.
In the clinical trial that led to the accelerated approval, tisotumab vedotin-tftv yielded an ORR of 24%, which an expert not connected with the trial said was “impressive.”
“Tivdak’s approval as a monotherapy in the U.S. is an important milestone for women with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy, as they are in need of a new treatment option and we look forward to making it available to them,” Jan van de Winkel, PhD, chief executive officer of Genmab, said in a statement.
Tisotumab vedotin is an antibody–drug conjugate: A human monoclonal antibody directed against tissue factor, which is highly expressed on many solid tumors, is attached to the microtubule-disrupting agent monomethyl auristatin E.
Details of clinical trial data
The accelerated approval was based on the results of the innovaTV 204, an open-label, multicenter, single-arm clinical trial, which was published online on April 9 in The Lancet Oncology, as reported at the time.
The trial included 101 women with recurrent or metastatic squamous cell, adenocarcinoma, or adenosquamous cervical cancer whose disease had progressed with or after doublet chemotherapy with bevacizumab (if eligible by local standards) and who had received two or fewer previous systemic regimens for recurrent or metastatic disease.
All patients received tisotumab vendotin intravenously at a dose of 2.0 mg/kg (up to a maximum of 200 mg) once every 3 weeks until disease progression or unacceptable toxicity.
The confirmed ORR was 24% and included seven (7%) complete responses and 17 (17%) partial responses.
The disease control rate was 72%, and the median duration of response was 8.3 months. The median progression-free survival was 4.2 months; the 6-month progression-free survival rate was 30%.
Median overall survival (OS) was 12.1 months. OS rates were 79% at 6 months and 51% at 12 months.
Overall, the safety profile with tisotumab vedotin was manageable, the trialists reported. The most common treatment-related adverse events were alopecia (38%), epistaxis (30%), nausea (27%), conjunctivitis (26%), fatigue (26%), and dry eye (23%). Adverse events of grade 3 or higher were reported by 28% of patients and included neutropenia (3%), fatigue (2%), ulcerative keratitis (2%), and peripheral neuropathies (2%). One patient died as a result of septic shock that was considered by the investigators to be related to therapy.
The new product labeling includes a boxed warning for ocular toxicity. It notes that tisotumab vedotin “caused changes in the corneal epithelium and conjunctiva resulting in changes in vision, including severe vision loss, and corneal ulceration.” It recommends that clinicians conduct an ophthalmic exam at baseline, prior to each dose, and as clinically indicated and that patients adhere to premedication and required eye care before, during, and after infusion.
Confirmatory trial underway
Continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials.
The confirmatory trial for tisotumab vedotin is already underway: The global phase 3 innovaTV 301 trial began in January 2021. It will compare tisotumab vendotin to chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed) for patients with recurrent or metastatic cervical cancer who have received one or two prior lines of systemic therapy.
A version of this article first appeared on Medscape.com.
There is currently no standard option for these patients. The mainstay of therapy in this setting is monotherapy with chemotherapy, but the benefit-risk profiles are poor, and overall response rates (ORRs) are less than 15%.
In the clinical trial that led to the accelerated approval, tisotumab vedotin-tftv yielded an ORR of 24%, which an expert not connected with the trial said was “impressive.”
“Tivdak’s approval as a monotherapy in the U.S. is an important milestone for women with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy, as they are in need of a new treatment option and we look forward to making it available to them,” Jan van de Winkel, PhD, chief executive officer of Genmab, said in a statement.
Tisotumab vedotin is an antibody–drug conjugate: A human monoclonal antibody directed against tissue factor, which is highly expressed on many solid tumors, is attached to the microtubule-disrupting agent monomethyl auristatin E.
Details of clinical trial data
The accelerated approval was based on the results of the innovaTV 204, an open-label, multicenter, single-arm clinical trial, which was published online on April 9 in The Lancet Oncology, as reported at the time.
The trial included 101 women with recurrent or metastatic squamous cell, adenocarcinoma, or adenosquamous cervical cancer whose disease had progressed with or after doublet chemotherapy with bevacizumab (if eligible by local standards) and who had received two or fewer previous systemic regimens for recurrent or metastatic disease.
All patients received tisotumab vendotin intravenously at a dose of 2.0 mg/kg (up to a maximum of 200 mg) once every 3 weeks until disease progression or unacceptable toxicity.
The confirmed ORR was 24% and included seven (7%) complete responses and 17 (17%) partial responses.
The disease control rate was 72%, and the median duration of response was 8.3 months. The median progression-free survival was 4.2 months; the 6-month progression-free survival rate was 30%.
Median overall survival (OS) was 12.1 months. OS rates were 79% at 6 months and 51% at 12 months.
Overall, the safety profile with tisotumab vedotin was manageable, the trialists reported. The most common treatment-related adverse events were alopecia (38%), epistaxis (30%), nausea (27%), conjunctivitis (26%), fatigue (26%), and dry eye (23%). Adverse events of grade 3 or higher were reported by 28% of patients and included neutropenia (3%), fatigue (2%), ulcerative keratitis (2%), and peripheral neuropathies (2%). One patient died as a result of septic shock that was considered by the investigators to be related to therapy.
The new product labeling includes a boxed warning for ocular toxicity. It notes that tisotumab vedotin “caused changes in the corneal epithelium and conjunctiva resulting in changes in vision, including severe vision loss, and corneal ulceration.” It recommends that clinicians conduct an ophthalmic exam at baseline, prior to each dose, and as clinically indicated and that patients adhere to premedication and required eye care before, during, and after infusion.
Confirmatory trial underway
Continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials.
The confirmatory trial for tisotumab vedotin is already underway: The global phase 3 innovaTV 301 trial began in January 2021. It will compare tisotumab vendotin to chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed) for patients with recurrent or metastatic cervical cancer who have received one or two prior lines of systemic therapy.
A version of this article first appeared on Medscape.com.
There is currently no standard option for these patients. The mainstay of therapy in this setting is monotherapy with chemotherapy, but the benefit-risk profiles are poor, and overall response rates (ORRs) are less than 15%.
In the clinical trial that led to the accelerated approval, tisotumab vedotin-tftv yielded an ORR of 24%, which an expert not connected with the trial said was “impressive.”
“Tivdak’s approval as a monotherapy in the U.S. is an important milestone for women with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy, as they are in need of a new treatment option and we look forward to making it available to them,” Jan van de Winkel, PhD, chief executive officer of Genmab, said in a statement.
Tisotumab vedotin is an antibody–drug conjugate: A human monoclonal antibody directed against tissue factor, which is highly expressed on many solid tumors, is attached to the microtubule-disrupting agent monomethyl auristatin E.
Details of clinical trial data
The accelerated approval was based on the results of the innovaTV 204, an open-label, multicenter, single-arm clinical trial, which was published online on April 9 in The Lancet Oncology, as reported at the time.
The trial included 101 women with recurrent or metastatic squamous cell, adenocarcinoma, or adenosquamous cervical cancer whose disease had progressed with or after doublet chemotherapy with bevacizumab (if eligible by local standards) and who had received two or fewer previous systemic regimens for recurrent or metastatic disease.
All patients received tisotumab vendotin intravenously at a dose of 2.0 mg/kg (up to a maximum of 200 mg) once every 3 weeks until disease progression or unacceptable toxicity.
The confirmed ORR was 24% and included seven (7%) complete responses and 17 (17%) partial responses.
The disease control rate was 72%, and the median duration of response was 8.3 months. The median progression-free survival was 4.2 months; the 6-month progression-free survival rate was 30%.
Median overall survival (OS) was 12.1 months. OS rates were 79% at 6 months and 51% at 12 months.
Overall, the safety profile with tisotumab vedotin was manageable, the trialists reported. The most common treatment-related adverse events were alopecia (38%), epistaxis (30%), nausea (27%), conjunctivitis (26%), fatigue (26%), and dry eye (23%). Adverse events of grade 3 or higher were reported by 28% of patients and included neutropenia (3%), fatigue (2%), ulcerative keratitis (2%), and peripheral neuropathies (2%). One patient died as a result of septic shock that was considered by the investigators to be related to therapy.
The new product labeling includes a boxed warning for ocular toxicity. It notes that tisotumab vedotin “caused changes in the corneal epithelium and conjunctiva resulting in changes in vision, including severe vision loss, and corneal ulceration.” It recommends that clinicians conduct an ophthalmic exam at baseline, prior to each dose, and as clinically indicated and that patients adhere to premedication and required eye care before, during, and after infusion.
Confirmatory trial underway
Continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials.
The confirmatory trial for tisotumab vedotin is already underway: The global phase 3 innovaTV 301 trial began in January 2021. It will compare tisotumab vendotin to chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed) for patients with recurrent or metastatic cervical cancer who have received one or two prior lines of systemic therapy.
A version of this article first appeared on Medscape.com.
Management of advanced endometrial cancer
Endometrial cancer is most commonly diagnosed at an early stage. Unfortunately, there is a trend toward the diagnosis of more advanced disease, for which cure is rare, and this is an important contributing factor toward the overall increasing mortality trend for endometrial cancer.
Histology is a major risk factor for advanced disease. For example, serous carcinoma, which accounts for approximately only 10% of all endometrial cancer diagnoses, comprises 25% of cases of advanced cases. Similarly, carcinosarcoma, a cell type known to be particularly aggressive, is relatively overrepresented among cases of advanced disease.
Advanced endometrial cancer includes cases of stage III (involvement of lymph nodes, ovaries, and vagina) and stage IV disease (with direct extension into pelvic viscera and distant metastases). In most cases of stage III disease, extrauterine metastases are microscopic and are detected only at the time of surgical staging. Bulky nodal disease within the pelvic and para-aortic nodal basins is less common but associated with worse prognosis than for patients with microscopic nodal metastases. Stage IV disease usually presents with peritoneal spread of disease including carcinomatosis, omental disease, and involvement of the small and large intestine.
Once advanced, endometrial cancer requires more than surgery alone, relying heavily on adjuvant therapies to achieve responses, particularly systemic therapy with platinum and taxane chemotherapy. In some cases, molecularly targeted therapy (such as trastuzumab for serous carcinomas that demonstrate overexpression of HER2) has been shown to be superior in efficacy.1 Surgery may involve either radical nodal dissections to the infrarenal aortic basin, and/or peritoneal debulking procedures similar to that required for ovarian cancer. Perhaps because of patterns of disease distribution so similar to ovarian cancer, historically, sequencing of therapy focused on radical primary debulking surgery (PDS) followed by chemotherapy.
In 2000, a retrospective series from Johns Hopkins University documented the outcomes of 65 patients with advanced endometrial cancer who had undergone primary debulking surgery followed by chemotherapy.2 They noted that survival was directly associated with degree of cytoreduction, with the best outcomes seen for those patients whose surgery resulted in no gross residual disease. Following these data, PDS with complete resection of all disease became the goal of primary therapy.
However, unlike ovarian cancer (which shares a similar disease distribution with advanced endometrial cancer) patients with endometrial cancer are more obese, older, and typically have more comorbidities. Therefore, radical primary debulking surgeries may be associated with poor patient perioperative outcomes, and feasibility of complete cytoreduction, particularly in very obese patients, can be limited. For this reason, neoadjuvant chemotherapy (NACT) has been explored as an option. The potential virtue of NACT is that it allows for tumor deposits to decrease in size, or be eliminated, prior to surgery, resulting in a less morbid procedure for the patient.
Observed outcomes for NACT relative to PDS are mixed. When small series have compared the two for the treatment of advanced serous endometrial cancer, NACT was associated with decreased perioperative morbidity, with similar overall survival observed.3,4
However, in larger series exploring patients within the National Cancer Database (a collection of over 1,500 hospitals accredited by the Commission on Cancer) outcomes appear different for the two approaches.5,6 While PDS was initially associated with worse survival, at approximately 5-6 months from diagnosis, this changed and survival was observed to be consistently superior for this group. These data suggest that patients undergoing primary surgical cytoreduction may experience an early mortality risk, possibly secondary to the impact of surgery, but that if they are to survive beyond this point, they experience better outcomes. While the researchers attempted to control for risk factors of poor outcomes that might have systematically differed between the two groups, this specific database is limited in its ability to account for all fundamental differences between them. Only approximately 15% of women with advanced endometrial cancer were offered NACT during those time periods. This observation alone suggests that this likely represents a group specially selected for their poor candidacy for upfront debulking surgery, and inherently increased risk for death from all causes.
The question remains, is NACT appropriate for all patients or just those who are considered poor surgical candidates? Could all patients benefit from the decreased morbidity associated with surgery after NACT without compromising survival? Randomized controlled trials are necessary to answer this question as they are the only way to ensure that risk factors for poor outcomes (such as histology, disease distribution, medical comorbidities) are equally distributed among both groups.
In the meantime, gynecologic oncologists should take a cautious approach to decision making regarding sequencing of surgery and chemotherapy in the setting of a new diagnosis of advanced endometrial cancer. Arguably more important than surgical interventions, access to molecularly targeted systemic therapy is likely to bring the best outcomes for advanced endometrial cancer. Carboplatin and paclitaxel are the current gold standard of care for frontline systemic therapy; however, response rates with this regimen are less favorable for endometrial cancer than for ovarian cancer. Work is being done to test novel therapies against actionable targets to use as alternatives or as adjuncts to traditional chemotherapy regimens. In doing so, clinicians are learning to distinguish endometrial cancers by more than simply their histologic features, but also by their molecular profiles.
Advanced endometrial cancer is a serious disease with high lethality. Future research should focus on ways to ensure toxicities of therapy, including surgery, are minimized while improving upon existing poor clinical outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no financial disclosures.
References
1. Fader AN et al. J Clin Oncol 2018;36(20):2044-51.
2. Bristow RE et al. Gynecol Oncol 2000;78(2):85-91.
3. Bogani G et al. Tumori 2019;105(1):92-97.
4. Wilkinson-Ryan I et al. Int J Gynecol Cancer. 2015;25(1):63-8.
5. Tobias CJ et al. JAMA Netw Open 2020;3(12):e2028612.
6. Chambers LM et al. Gynecol Oncol 2021;160(2):405-12.
Endometrial cancer is most commonly diagnosed at an early stage. Unfortunately, there is a trend toward the diagnosis of more advanced disease, for which cure is rare, and this is an important contributing factor toward the overall increasing mortality trend for endometrial cancer.
Histology is a major risk factor for advanced disease. For example, serous carcinoma, which accounts for approximately only 10% of all endometrial cancer diagnoses, comprises 25% of cases of advanced cases. Similarly, carcinosarcoma, a cell type known to be particularly aggressive, is relatively overrepresented among cases of advanced disease.
Advanced endometrial cancer includes cases of stage III (involvement of lymph nodes, ovaries, and vagina) and stage IV disease (with direct extension into pelvic viscera and distant metastases). In most cases of stage III disease, extrauterine metastases are microscopic and are detected only at the time of surgical staging. Bulky nodal disease within the pelvic and para-aortic nodal basins is less common but associated with worse prognosis than for patients with microscopic nodal metastases. Stage IV disease usually presents with peritoneal spread of disease including carcinomatosis, omental disease, and involvement of the small and large intestine.
Once advanced, endometrial cancer requires more than surgery alone, relying heavily on adjuvant therapies to achieve responses, particularly systemic therapy with platinum and taxane chemotherapy. In some cases, molecularly targeted therapy (such as trastuzumab for serous carcinomas that demonstrate overexpression of HER2) has been shown to be superior in efficacy.1 Surgery may involve either radical nodal dissections to the infrarenal aortic basin, and/or peritoneal debulking procedures similar to that required for ovarian cancer. Perhaps because of patterns of disease distribution so similar to ovarian cancer, historically, sequencing of therapy focused on radical primary debulking surgery (PDS) followed by chemotherapy.
In 2000, a retrospective series from Johns Hopkins University documented the outcomes of 65 patients with advanced endometrial cancer who had undergone primary debulking surgery followed by chemotherapy.2 They noted that survival was directly associated with degree of cytoreduction, with the best outcomes seen for those patients whose surgery resulted in no gross residual disease. Following these data, PDS with complete resection of all disease became the goal of primary therapy.
However, unlike ovarian cancer (which shares a similar disease distribution with advanced endometrial cancer) patients with endometrial cancer are more obese, older, and typically have more comorbidities. Therefore, radical primary debulking surgeries may be associated with poor patient perioperative outcomes, and feasibility of complete cytoreduction, particularly in very obese patients, can be limited. For this reason, neoadjuvant chemotherapy (NACT) has been explored as an option. The potential virtue of NACT is that it allows for tumor deposits to decrease in size, or be eliminated, prior to surgery, resulting in a less morbid procedure for the patient.
Observed outcomes for NACT relative to PDS are mixed. When small series have compared the two for the treatment of advanced serous endometrial cancer, NACT was associated with decreased perioperative morbidity, with similar overall survival observed.3,4
However, in larger series exploring patients within the National Cancer Database (a collection of over 1,500 hospitals accredited by the Commission on Cancer) outcomes appear different for the two approaches.5,6 While PDS was initially associated with worse survival, at approximately 5-6 months from diagnosis, this changed and survival was observed to be consistently superior for this group. These data suggest that patients undergoing primary surgical cytoreduction may experience an early mortality risk, possibly secondary to the impact of surgery, but that if they are to survive beyond this point, they experience better outcomes. While the researchers attempted to control for risk factors of poor outcomes that might have systematically differed between the two groups, this specific database is limited in its ability to account for all fundamental differences between them. Only approximately 15% of women with advanced endometrial cancer were offered NACT during those time periods. This observation alone suggests that this likely represents a group specially selected for their poor candidacy for upfront debulking surgery, and inherently increased risk for death from all causes.
The question remains, is NACT appropriate for all patients or just those who are considered poor surgical candidates? Could all patients benefit from the decreased morbidity associated with surgery after NACT without compromising survival? Randomized controlled trials are necessary to answer this question as they are the only way to ensure that risk factors for poor outcomes (such as histology, disease distribution, medical comorbidities) are equally distributed among both groups.
In the meantime, gynecologic oncologists should take a cautious approach to decision making regarding sequencing of surgery and chemotherapy in the setting of a new diagnosis of advanced endometrial cancer. Arguably more important than surgical interventions, access to molecularly targeted systemic therapy is likely to bring the best outcomes for advanced endometrial cancer. Carboplatin and paclitaxel are the current gold standard of care for frontline systemic therapy; however, response rates with this regimen are less favorable for endometrial cancer than for ovarian cancer. Work is being done to test novel therapies against actionable targets to use as alternatives or as adjuncts to traditional chemotherapy regimens. In doing so, clinicians are learning to distinguish endometrial cancers by more than simply their histologic features, but also by their molecular profiles.
Advanced endometrial cancer is a serious disease with high lethality. Future research should focus on ways to ensure toxicities of therapy, including surgery, are minimized while improving upon existing poor clinical outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no financial disclosures.
References
1. Fader AN et al. J Clin Oncol 2018;36(20):2044-51.
2. Bristow RE et al. Gynecol Oncol 2000;78(2):85-91.
3. Bogani G et al. Tumori 2019;105(1):92-97.
4. Wilkinson-Ryan I et al. Int J Gynecol Cancer. 2015;25(1):63-8.
5. Tobias CJ et al. JAMA Netw Open 2020;3(12):e2028612.
6. Chambers LM et al. Gynecol Oncol 2021;160(2):405-12.
Endometrial cancer is most commonly diagnosed at an early stage. Unfortunately, there is a trend toward the diagnosis of more advanced disease, for which cure is rare, and this is an important contributing factor toward the overall increasing mortality trend for endometrial cancer.
Histology is a major risk factor for advanced disease. For example, serous carcinoma, which accounts for approximately only 10% of all endometrial cancer diagnoses, comprises 25% of cases of advanced cases. Similarly, carcinosarcoma, a cell type known to be particularly aggressive, is relatively overrepresented among cases of advanced disease.
Advanced endometrial cancer includes cases of stage III (involvement of lymph nodes, ovaries, and vagina) and stage IV disease (with direct extension into pelvic viscera and distant metastases). In most cases of stage III disease, extrauterine metastases are microscopic and are detected only at the time of surgical staging. Bulky nodal disease within the pelvic and para-aortic nodal basins is less common but associated with worse prognosis than for patients with microscopic nodal metastases. Stage IV disease usually presents with peritoneal spread of disease including carcinomatosis, omental disease, and involvement of the small and large intestine.
Once advanced, endometrial cancer requires more than surgery alone, relying heavily on adjuvant therapies to achieve responses, particularly systemic therapy with platinum and taxane chemotherapy. In some cases, molecularly targeted therapy (such as trastuzumab for serous carcinomas that demonstrate overexpression of HER2) has been shown to be superior in efficacy.1 Surgery may involve either radical nodal dissections to the infrarenal aortic basin, and/or peritoneal debulking procedures similar to that required for ovarian cancer. Perhaps because of patterns of disease distribution so similar to ovarian cancer, historically, sequencing of therapy focused on radical primary debulking surgery (PDS) followed by chemotherapy.
In 2000, a retrospective series from Johns Hopkins University documented the outcomes of 65 patients with advanced endometrial cancer who had undergone primary debulking surgery followed by chemotherapy.2 They noted that survival was directly associated with degree of cytoreduction, with the best outcomes seen for those patients whose surgery resulted in no gross residual disease. Following these data, PDS with complete resection of all disease became the goal of primary therapy.
However, unlike ovarian cancer (which shares a similar disease distribution with advanced endometrial cancer) patients with endometrial cancer are more obese, older, and typically have more comorbidities. Therefore, radical primary debulking surgeries may be associated with poor patient perioperative outcomes, and feasibility of complete cytoreduction, particularly in very obese patients, can be limited. For this reason, neoadjuvant chemotherapy (NACT) has been explored as an option. The potential virtue of NACT is that it allows for tumor deposits to decrease in size, or be eliminated, prior to surgery, resulting in a less morbid procedure for the patient.
Observed outcomes for NACT relative to PDS are mixed. When small series have compared the two for the treatment of advanced serous endometrial cancer, NACT was associated with decreased perioperative morbidity, with similar overall survival observed.3,4
However, in larger series exploring patients within the National Cancer Database (a collection of over 1,500 hospitals accredited by the Commission on Cancer) outcomes appear different for the two approaches.5,6 While PDS was initially associated with worse survival, at approximately 5-6 months from diagnosis, this changed and survival was observed to be consistently superior for this group. These data suggest that patients undergoing primary surgical cytoreduction may experience an early mortality risk, possibly secondary to the impact of surgery, but that if they are to survive beyond this point, they experience better outcomes. While the researchers attempted to control for risk factors of poor outcomes that might have systematically differed between the two groups, this specific database is limited in its ability to account for all fundamental differences between them. Only approximately 15% of women with advanced endometrial cancer were offered NACT during those time periods. This observation alone suggests that this likely represents a group specially selected for their poor candidacy for upfront debulking surgery, and inherently increased risk for death from all causes.
The question remains, is NACT appropriate for all patients or just those who are considered poor surgical candidates? Could all patients benefit from the decreased morbidity associated with surgery after NACT without compromising survival? Randomized controlled trials are necessary to answer this question as they are the only way to ensure that risk factors for poor outcomes (such as histology, disease distribution, medical comorbidities) are equally distributed among both groups.
In the meantime, gynecologic oncologists should take a cautious approach to decision making regarding sequencing of surgery and chemotherapy in the setting of a new diagnosis of advanced endometrial cancer. Arguably more important than surgical interventions, access to molecularly targeted systemic therapy is likely to bring the best outcomes for advanced endometrial cancer. Carboplatin and paclitaxel are the current gold standard of care for frontline systemic therapy; however, response rates with this regimen are less favorable for endometrial cancer than for ovarian cancer. Work is being done to test novel therapies against actionable targets to use as alternatives or as adjuncts to traditional chemotherapy regimens. In doing so, clinicians are learning to distinguish endometrial cancers by more than simply their histologic features, but also by their molecular profiles.
Advanced endometrial cancer is a serious disease with high lethality. Future research should focus on ways to ensure toxicities of therapy, including surgery, are minimized while improving upon existing poor clinical outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no financial disclosures.
References
1. Fader AN et al. J Clin Oncol 2018;36(20):2044-51.
2. Bristow RE et al. Gynecol Oncol 2000;78(2):85-91.
3. Bogani G et al. Tumori 2019;105(1):92-97.
4. Wilkinson-Ryan I et al. Int J Gynecol Cancer. 2015;25(1):63-8.
5. Tobias CJ et al. JAMA Netw Open 2020;3(12):e2028612.
6. Chambers LM et al. Gynecol Oncol 2021;160(2):405-12.
Most community-based oncologists skip biomarker testing
A recent survey shows that fewer than half of community oncologists use biomarker testing to guide patient discussions about treatment, which compares with 73% of academic clinicians.
The findings, reported at the 2020 World Conference on Lung Cancer, which was rescheduled for January 2021, highlight the potential for unequal application of the latest advances in cancer genomics and targeted therapies throughout the health care system, which could worsen existing disparities in underserved populations, according to Leigh Boehmer, PharmD, medical director for the Association of Community Cancer Centers, Rockville, Md.
The survey – a mixed-methods approach for assessing practice patterns, attitudes, barriers, and resource needs related to biomarker testing among clinicians – was developed by the ACCC in partnership with the LUNGevity Foundation and administered to clinicians caring for patients with non–small cell lung cancer who are uninsured or covered by Medicaid.
Of 99 respondents, more than 85% were physicians and 68% worked in a community setting. Only 40% indicated they were very familiar or extremely familiar with 2018 Molecular Testing Guidelines for Lung Cancer from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Clinicians were most confident about selecting appropriate tests to use, interpreting test results, and prognosticating based on test results, with 77%, 74%, and 74%, respectively, saying they are very confident or extremely confident in those areas. They were less confident about determining when to order testing and in coordinating care across the multidisciplinary team, with 59% and 64%, respectively, saying they were very confident or extremely confident in those areas, Dr. Boehmer reported at the conference.
The shortcomings with respect to communication across teams were echoed in two focus groups convened to further validate the survey results, he noted.
As for the reasons why clinicians ordered biomarker testing, 88% and 82% of community and academic clinicians, respectively, said they did so to help make targeted treatment decisions.
“Only 48% of community clinicians indicated that they use biomarker testing to guide patient discussions, compared to 73% of academic clinicians,” he said. “That finding was considered statistically significant.”
With respect to decision-making about biomarker testing, 41% said they prefer to share the responsibility with patients, whereas 52% said they prefer to make the final decision.
“Shedding further light on this situation, focus group participants expressed that patients lacked comprehension and interest about what testing entails and what testing means for their treatment options,” Dr. Boehmer noted.
In order to make more informed decisions about biomarker testing, respondents said they need more information on financial resources for patient assistance (26%) and education around both published guidelines and practical implications of the clinical data (21%).
When asked about patients’ information needs, 23% said their patients need psychosocial support, 22% said they need financial assistance, and 9% said their patients have no additional resource needs.
However, only 27% said they provide patients with resources related to psychosocial support services, and only 44% share financial assistance information, he said.
Further, the fact that 9% said their patients need no additional resources represents “a disconnect” from the findings of the survey and focus groups, he added.
“We believe that this study identifies key areas of ongoing clinician need related to biomarker testing, including things like increased guideline familiarity, practical applications of guideline-concordant testing, and … how to optimally coordinate multidisciplinary care delivery,” Dr. Boehmer said. “Professional organizations … in partnership with patient advocacy organizations or groups should focus on developing those patient education materials … and tools for improving patient-clinician discussions about biomarker testing.”
The ACCC will be working with the LUNGevity Foundation and the Center for Business Models in Healthcare to develop an intervention to ensure that such discussions are “easily integrated into the care process for every patient,” he noted.
Such efforts are important for ensuring that clinicians are informed about the value of biomarker testing and about guidelines for testing so that patients receive the best possible care, said invited discussant Joshua Sabari, MD, of New York University Langone Health’s Perlmutter Cancer Center.
“I know that, in clinic, when meeting a new patient with non–small cell lung cancer, it’s critical to understand the driver alteration, not only for prognosis, but also for goals-of-care discussion, as well as potential treatment option,” Dr. Sabari said.
Dr. Boehmer reported consulting for Pfizer. Dr. Sabari reported consulting and advisory board membership for multiple pharmaceutical companies.
A recent survey shows that fewer than half of community oncologists use biomarker testing to guide patient discussions about treatment, which compares with 73% of academic clinicians.
The findings, reported at the 2020 World Conference on Lung Cancer, which was rescheduled for January 2021, highlight the potential for unequal application of the latest advances in cancer genomics and targeted therapies throughout the health care system, which could worsen existing disparities in underserved populations, according to Leigh Boehmer, PharmD, medical director for the Association of Community Cancer Centers, Rockville, Md.
The survey – a mixed-methods approach for assessing practice patterns, attitudes, barriers, and resource needs related to biomarker testing among clinicians – was developed by the ACCC in partnership with the LUNGevity Foundation and administered to clinicians caring for patients with non–small cell lung cancer who are uninsured or covered by Medicaid.
Of 99 respondents, more than 85% were physicians and 68% worked in a community setting. Only 40% indicated they were very familiar or extremely familiar with 2018 Molecular Testing Guidelines for Lung Cancer from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Clinicians were most confident about selecting appropriate tests to use, interpreting test results, and prognosticating based on test results, with 77%, 74%, and 74%, respectively, saying they are very confident or extremely confident in those areas. They were less confident about determining when to order testing and in coordinating care across the multidisciplinary team, with 59% and 64%, respectively, saying they were very confident or extremely confident in those areas, Dr. Boehmer reported at the conference.
The shortcomings with respect to communication across teams were echoed in two focus groups convened to further validate the survey results, he noted.
As for the reasons why clinicians ordered biomarker testing, 88% and 82% of community and academic clinicians, respectively, said they did so to help make targeted treatment decisions.
“Only 48% of community clinicians indicated that they use biomarker testing to guide patient discussions, compared to 73% of academic clinicians,” he said. “That finding was considered statistically significant.”
With respect to decision-making about biomarker testing, 41% said they prefer to share the responsibility with patients, whereas 52% said they prefer to make the final decision.
“Shedding further light on this situation, focus group participants expressed that patients lacked comprehension and interest about what testing entails and what testing means for their treatment options,” Dr. Boehmer noted.
In order to make more informed decisions about biomarker testing, respondents said they need more information on financial resources for patient assistance (26%) and education around both published guidelines and practical implications of the clinical data (21%).
When asked about patients’ information needs, 23% said their patients need psychosocial support, 22% said they need financial assistance, and 9% said their patients have no additional resource needs.
However, only 27% said they provide patients with resources related to psychosocial support services, and only 44% share financial assistance information, he said.
Further, the fact that 9% said their patients need no additional resources represents “a disconnect” from the findings of the survey and focus groups, he added.
“We believe that this study identifies key areas of ongoing clinician need related to biomarker testing, including things like increased guideline familiarity, practical applications of guideline-concordant testing, and … how to optimally coordinate multidisciplinary care delivery,” Dr. Boehmer said. “Professional organizations … in partnership with patient advocacy organizations or groups should focus on developing those patient education materials … and tools for improving patient-clinician discussions about biomarker testing.”
The ACCC will be working with the LUNGevity Foundation and the Center for Business Models in Healthcare to develop an intervention to ensure that such discussions are “easily integrated into the care process for every patient,” he noted.
Such efforts are important for ensuring that clinicians are informed about the value of biomarker testing and about guidelines for testing so that patients receive the best possible care, said invited discussant Joshua Sabari, MD, of New York University Langone Health’s Perlmutter Cancer Center.
“I know that, in clinic, when meeting a new patient with non–small cell lung cancer, it’s critical to understand the driver alteration, not only for prognosis, but also for goals-of-care discussion, as well as potential treatment option,” Dr. Sabari said.
Dr. Boehmer reported consulting for Pfizer. Dr. Sabari reported consulting and advisory board membership for multiple pharmaceutical companies.
A recent survey shows that fewer than half of community oncologists use biomarker testing to guide patient discussions about treatment, which compares with 73% of academic clinicians.
The findings, reported at the 2020 World Conference on Lung Cancer, which was rescheduled for January 2021, highlight the potential for unequal application of the latest advances in cancer genomics and targeted therapies throughout the health care system, which could worsen existing disparities in underserved populations, according to Leigh Boehmer, PharmD, medical director for the Association of Community Cancer Centers, Rockville, Md.
The survey – a mixed-methods approach for assessing practice patterns, attitudes, barriers, and resource needs related to biomarker testing among clinicians – was developed by the ACCC in partnership with the LUNGevity Foundation and administered to clinicians caring for patients with non–small cell lung cancer who are uninsured or covered by Medicaid.
Of 99 respondents, more than 85% were physicians and 68% worked in a community setting. Only 40% indicated they were very familiar or extremely familiar with 2018 Molecular Testing Guidelines for Lung Cancer from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Clinicians were most confident about selecting appropriate tests to use, interpreting test results, and prognosticating based on test results, with 77%, 74%, and 74%, respectively, saying they are very confident or extremely confident in those areas. They were less confident about determining when to order testing and in coordinating care across the multidisciplinary team, with 59% and 64%, respectively, saying they were very confident or extremely confident in those areas, Dr. Boehmer reported at the conference.
The shortcomings with respect to communication across teams were echoed in two focus groups convened to further validate the survey results, he noted.
As for the reasons why clinicians ordered biomarker testing, 88% and 82% of community and academic clinicians, respectively, said they did so to help make targeted treatment decisions.
“Only 48% of community clinicians indicated that they use biomarker testing to guide patient discussions, compared to 73% of academic clinicians,” he said. “That finding was considered statistically significant.”
With respect to decision-making about biomarker testing, 41% said they prefer to share the responsibility with patients, whereas 52% said they prefer to make the final decision.
“Shedding further light on this situation, focus group participants expressed that patients lacked comprehension and interest about what testing entails and what testing means for their treatment options,” Dr. Boehmer noted.
In order to make more informed decisions about biomarker testing, respondents said they need more information on financial resources for patient assistance (26%) and education around both published guidelines and practical implications of the clinical data (21%).
When asked about patients’ information needs, 23% said their patients need psychosocial support, 22% said they need financial assistance, and 9% said their patients have no additional resource needs.
However, only 27% said they provide patients with resources related to psychosocial support services, and only 44% share financial assistance information, he said.
Further, the fact that 9% said their patients need no additional resources represents “a disconnect” from the findings of the survey and focus groups, he added.
“We believe that this study identifies key areas of ongoing clinician need related to biomarker testing, including things like increased guideline familiarity, practical applications of guideline-concordant testing, and … how to optimally coordinate multidisciplinary care delivery,” Dr. Boehmer said. “Professional organizations … in partnership with patient advocacy organizations or groups should focus on developing those patient education materials … and tools for improving patient-clinician discussions about biomarker testing.”
The ACCC will be working with the LUNGevity Foundation and the Center for Business Models in Healthcare to develop an intervention to ensure that such discussions are “easily integrated into the care process for every patient,” he noted.
Such efforts are important for ensuring that clinicians are informed about the value of biomarker testing and about guidelines for testing so that patients receive the best possible care, said invited discussant Joshua Sabari, MD, of New York University Langone Health’s Perlmutter Cancer Center.
“I know that, in clinic, when meeting a new patient with non–small cell lung cancer, it’s critical to understand the driver alteration, not only for prognosis, but also for goals-of-care discussion, as well as potential treatment option,” Dr. Sabari said.
Dr. Boehmer reported consulting for Pfizer. Dr. Sabari reported consulting and advisory board membership for multiple pharmaceutical companies.
REPORTING FROM WCLC 2020
Immunotherapy for cancer patients with poor PS needs a rethink
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.

