User login
CDC notes sharp declines in breast and cervical cancer screening
The new data come from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), a program that provides cancer screening services to women with low income and inadequate health insurance.
The data show that the total number of screenings funded by the NBCCEDP declined by 87% for breast cancer screening and by 84% for cervical cancer screening in April 2020 in comparison with the previous 5-year averages for that month.
The declines in breast cancer screening varied from 84% among Hispanic women to 98% among American Indian/Alaskan Native women. The declines in cervical cancer screening varied from 82% among Black women to 92% among Asian Pacific Islander women.
In April 2020, breast cancer screening declined by 86% in metro areas, 88% in urban areas, and 89% in rural areas in comparison with respective 5-year averages. For cervical cancer screenings, the corresponding declines were 85%, 77%, and 82%.
The findings are consistent with those from studies conducted in insured populations, note the authors, led by the Amy DeGroff, PhD, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
“Prolonged delays in screening related to the COVID-19 pandemic may lead to delayed diagnoses, poor health consequences, and an increase in cancer disparities among women already experiencing health inequities,” the CDC states in a press release.
Women from racial and ethnic minority groups already face a disproportionate burden of cervical and breast cancers in the United States: Black women and Hispanic women have the highest rates of cervical cancer incidence (8.3 and 8.9 per 100,000 women, respectively, vs. 7.3 per 100,000 among White women) and the highest rates of cervical cancer deaths. Black women have the highest rate of breast cancer death (26.9 per 100,000 women, vs. 19.4 per 100,000 among White women), the study authors explain.
Although the volume of screening began to recover in May 2020 – test volumes for breast and cervical cancer were 39% and 40% below the 5-year average by June 2020 – breast cancer screening in rural areas remained 52% below the 5-year average, they report.
The findings were published online June 30 in Preventive Medicine.
“This study highlights a decline in cancer screening among women of racial and ethnic minority groups with low incomes when their access to medical services decreased at the beginning of the pandemic,” Dr. DeGroff comments in the CDC press release.
The findings “reinforce the need to safely maintain routine health care services during the pandemic, especially when the health care environment meets COVID-19 safety guidelines,” she adds.
The investigators used NBCCEDP administrative and program data reported to the CDC by awardees – organizations that receive funding to implement the NBCCEDP – to assess the impact of COVID-19 on the number of breast and cervical cancer screening tests administered through the program and the effects of COVID-19 on the availability of screening services and NBCCEDP awardees’ capacity to support partner clinics.
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the review period of January-June 2015-2020.
Despite COVID-related challenges, “a large number of awardees reported flexibility and creative efforts to reach women and support clinics’ resumption of clinical care, including screening, during the COVID-19 pandemic,” the authors write.
“[The] CDC encourages health care professionals to help minimize delays in testing by continuing routine cancer screening for women having symptoms or at high risk for breast or cervical cancer,” Dr. DeGroff commented. “The Early Detection Program can help women overcome barriers to health equity by educating them about the importance of routine screening, addressing their concerns about COVID-19 transmission, and helping them to safely access screening through interventions like patient navigation.”
Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically, they note.
A version of this article first appeared on Medscape.com.
The new data come from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), a program that provides cancer screening services to women with low income and inadequate health insurance.
The data show that the total number of screenings funded by the NBCCEDP declined by 87% for breast cancer screening and by 84% for cervical cancer screening in April 2020 in comparison with the previous 5-year averages for that month.
The declines in breast cancer screening varied from 84% among Hispanic women to 98% among American Indian/Alaskan Native women. The declines in cervical cancer screening varied from 82% among Black women to 92% among Asian Pacific Islander women.
In April 2020, breast cancer screening declined by 86% in metro areas, 88% in urban areas, and 89% in rural areas in comparison with respective 5-year averages. For cervical cancer screenings, the corresponding declines were 85%, 77%, and 82%.
The findings are consistent with those from studies conducted in insured populations, note the authors, led by the Amy DeGroff, PhD, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
“Prolonged delays in screening related to the COVID-19 pandemic may lead to delayed diagnoses, poor health consequences, and an increase in cancer disparities among women already experiencing health inequities,” the CDC states in a press release.
Women from racial and ethnic minority groups already face a disproportionate burden of cervical and breast cancers in the United States: Black women and Hispanic women have the highest rates of cervical cancer incidence (8.3 and 8.9 per 100,000 women, respectively, vs. 7.3 per 100,000 among White women) and the highest rates of cervical cancer deaths. Black women have the highest rate of breast cancer death (26.9 per 100,000 women, vs. 19.4 per 100,000 among White women), the study authors explain.
Although the volume of screening began to recover in May 2020 – test volumes for breast and cervical cancer were 39% and 40% below the 5-year average by June 2020 – breast cancer screening in rural areas remained 52% below the 5-year average, they report.
The findings were published online June 30 in Preventive Medicine.
“This study highlights a decline in cancer screening among women of racial and ethnic minority groups with low incomes when their access to medical services decreased at the beginning of the pandemic,” Dr. DeGroff comments in the CDC press release.
The findings “reinforce the need to safely maintain routine health care services during the pandemic, especially when the health care environment meets COVID-19 safety guidelines,” she adds.
The investigators used NBCCEDP administrative and program data reported to the CDC by awardees – organizations that receive funding to implement the NBCCEDP – to assess the impact of COVID-19 on the number of breast and cervical cancer screening tests administered through the program and the effects of COVID-19 on the availability of screening services and NBCCEDP awardees’ capacity to support partner clinics.
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the review period of January-June 2015-2020.
Despite COVID-related challenges, “a large number of awardees reported flexibility and creative efforts to reach women and support clinics’ resumption of clinical care, including screening, during the COVID-19 pandemic,” the authors write.
“[The] CDC encourages health care professionals to help minimize delays in testing by continuing routine cancer screening for women having symptoms or at high risk for breast or cervical cancer,” Dr. DeGroff commented. “The Early Detection Program can help women overcome barriers to health equity by educating them about the importance of routine screening, addressing their concerns about COVID-19 transmission, and helping them to safely access screening through interventions like patient navigation.”
Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically, they note.
A version of this article first appeared on Medscape.com.
The new data come from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), a program that provides cancer screening services to women with low income and inadequate health insurance.
The data show that the total number of screenings funded by the NBCCEDP declined by 87% for breast cancer screening and by 84% for cervical cancer screening in April 2020 in comparison with the previous 5-year averages for that month.
The declines in breast cancer screening varied from 84% among Hispanic women to 98% among American Indian/Alaskan Native women. The declines in cervical cancer screening varied from 82% among Black women to 92% among Asian Pacific Islander women.
In April 2020, breast cancer screening declined by 86% in metro areas, 88% in urban areas, and 89% in rural areas in comparison with respective 5-year averages. For cervical cancer screenings, the corresponding declines were 85%, 77%, and 82%.
The findings are consistent with those from studies conducted in insured populations, note the authors, led by the Amy DeGroff, PhD, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
“Prolonged delays in screening related to the COVID-19 pandemic may lead to delayed diagnoses, poor health consequences, and an increase in cancer disparities among women already experiencing health inequities,” the CDC states in a press release.
Women from racial and ethnic minority groups already face a disproportionate burden of cervical and breast cancers in the United States: Black women and Hispanic women have the highest rates of cervical cancer incidence (8.3 and 8.9 per 100,000 women, respectively, vs. 7.3 per 100,000 among White women) and the highest rates of cervical cancer deaths. Black women have the highest rate of breast cancer death (26.9 per 100,000 women, vs. 19.4 per 100,000 among White women), the study authors explain.
Although the volume of screening began to recover in May 2020 – test volumes for breast and cervical cancer were 39% and 40% below the 5-year average by June 2020 – breast cancer screening in rural areas remained 52% below the 5-year average, they report.
The findings were published online June 30 in Preventive Medicine.
“This study highlights a decline in cancer screening among women of racial and ethnic minority groups with low incomes when their access to medical services decreased at the beginning of the pandemic,” Dr. DeGroff comments in the CDC press release.
The findings “reinforce the need to safely maintain routine health care services during the pandemic, especially when the health care environment meets COVID-19 safety guidelines,” she adds.
The investigators used NBCCEDP administrative and program data reported to the CDC by awardees – organizations that receive funding to implement the NBCCEDP – to assess the impact of COVID-19 on the number of breast and cervical cancer screening tests administered through the program and the effects of COVID-19 on the availability of screening services and NBCCEDP awardees’ capacity to support partner clinics.
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the review period of January-June 2015-2020.
Despite COVID-related challenges, “a large number of awardees reported flexibility and creative efforts to reach women and support clinics’ resumption of clinical care, including screening, during the COVID-19 pandemic,” the authors write.
“[The] CDC encourages health care professionals to help minimize delays in testing by continuing routine cancer screening for women having symptoms or at high risk for breast or cervical cancer,” Dr. DeGroff commented. “The Early Detection Program can help women overcome barriers to health equity by educating them about the importance of routine screening, addressing their concerns about COVID-19 transmission, and helping them to safely access screening through interventions like patient navigation.”
Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically, they note.
A version of this article first appeared on Medscape.com.
Racial and economic disparities persist in endometrial cancer care
Women who were Black, Latina, American Indian, or Alaska Native were significantly less likely than White women to receive guidelines-adherent treatment for endometrial cancer, based on data from more than 80,000 women.
The incidence of uterine cancer has increased across all ethnicities in recent decades, and adherence to the National Comprehensive Cancer Network treatment guidelines has been associated with improved survival, wrote Victoria A. Rodriguez, MSW, MPH, of the University of California, Irvine, and colleagues. “To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines.”
In a retrospective study published in Obstetrics & Gynecology, the researchers used data from the SEER (Surveillance, Epidemiology, and End Results) database between Jan. 1, 2006, and Dec. 31, 2015. The study population included 83,883 women aged 18 years and older who were diagnosed with their first or only endometrial carcinoma. The primary dependent variable was adherence to the NCCN guidelines for the initial course of treatment, which included a combination of therapies based on cancer subtype and the extent of the disease, the researchers said.
The researchers combined the guidelines and the corresponding data from the SEER database to create “a binary variable representing adherence to [NCCN] guidelines (1 = adherent treatment, 0 = nonadherent treatment).”
Approximately 60% of the total patient population received guidelines-adherent treatment. In a multivariate analysis, Black women, Latina women, and American Indian or Alaska Native women were significantly less likely than White women to receive such treatment (odds ratios, 0.88, 0.92, and 0.82, respectively), controlling for factors including neighborhood socioeconomic status, age, and stage at diagnosis, year of diagnosis, histology, and disease grade. Asian women and Native Hawaiian/Pacific Islander women were significantly more likely to received guidelines-adherent treatment, compared with White women (OR, 1.14 and 1.19, respectively).
The researchers also found a significant gradient in guidelines-adherent treatment based on neighborhood socioeconomic status. Relative to the highest neighborhood socioeconomic status group, women in the lower groups had significantly lower odds of receiving guidelines-adherent treatment, with ORs of 0.89, 0.84, 0.80, and 0.73, respectively, for the high-middle neighborhood socioeconomic group, the middle group, the low-middle group, and the lowest group (P < .001 for all).
“Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer,” the researchers noted.
The study findings were limited by several factors in including the retrospective design and potential for unmeasured confounding variables not included in SEER, such as hospital and physician characteristics, the researchers said. Also, the SEER data set was limited to only the first course of treatment, and did not include information on patient comorbidities that might affect treatment.
“Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial-ethnic groups and socioeconomic status groups, with special attention to low-income women of color,” the researchers emphasized. More research on the impact of comorbidities on a patient’s ability to receive guidelines-based care should be used to inform whether comorbidities should be part of the NCCN guidelines.
However, the results were strengthened by the large sample size and diverse population, so the findings are generalizable to the overall U.S. population, the researchers said.
“Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial-ethnic or socioeconomic backgrounds,” they concluded.
Pursue optimal treatment to curb mortality
Even more concerning than the increase in the incidence of endometrial cancer in the United States is the increase in mortality from this disease, said Emma C. Rossi, MD, of the University of North Carolina at Chapel Hill, in an interview.
“Therefore, it is critical that we identify factors which might be contributing to the increasing lethality of this cancer,” she emphasized. “One such potential factor is race, as it has been observed that Black race is associated with an increased risk of death from endometrial cancer. Historically, this was attributed to the more aggressive subtypes of endometrial cancer (such as serous) which have a higher incidence among Black women. However, more recently, population-based studies have identified that this worse prognosis is independent of histologic cell type,” which suggests that something in our health care delivery is contributing to these worse outcomes.
“The present study helps to confirm these concerning associations, shedding some light on contributory factors, in this case, modifiable (adherence to recommended guidelines) and less modifiable (neighborhood socioeconomic environments) [ones],” Dr. Rossi noted. “The guidelines that are established by the NCCN are chosen after they have been shown to be associated with improved outcomes (including either survival or quality of life), and therefore lack of adherence to these outcomes may suggest inferior quality care is being delivered.”
Studies such as this are helpful in exposing the problem of treatment disparity to help identify sources of problems to develop solutions, she added.
The results should inspire clinicians “to feel agency in changing these outcomes, albeit by tackling very difficult social, political, and health system shortfalls,” she said.
Identify barriers to care
Barriers to greater adherence to guidelines-based care include varying definitions of such care, Dr. Rossi said.
“This is particularly true for surgical management of endometrial cancer, which remains controversial with respect to lymph node assessment. Lack of surgical staging with lymph node assessment was considered noncompliant care for this study; however, lymphadenectomy has not specifically, in and of itself, been associated with improved outcomes, and therefore some surgeons argue against performing it routinely,” she explained.
“Lack of access to sophisticated surgical tools and advanced surgical techniques may account for nonguidelines-based care in the patients with early-stage endometrial cancer; however, there are likely other differences in the ability to deliver guideline-concordant care (such as chemotherapy and radiation therapy) for advanced-stage cancers,” Dr. Rossi said. “Patient and provider positive attitudes toward adjuvant therapy, access to transportation, supportive home environments, paid sick leave, well-controlled or minimal comorbidities are all factors which promote the administration of complex adjuvant therapies such as chemotherapy and radiation. In low-resource neighborhoods and minority communities, barriers to these factors may be contributing to nonguidelines-concordant care.”
Dr. Rossi emphasized the need to “dive deeper into these data at individual health-system and provider levels.” For example, research is needed to compare the practice patterns and models of high-performing clinical practices with lower-performing practices in terms of factors such as tumor boards, journal review, peer review, dashboards, and metrics. By doing so, “we can ensure that we are understanding where and why variations in care are occurring,” Dr. Rossi said.
The study was supported in part by the Faculty Mentor Program Fellowship from the University of California, Irvine, graduate division. Ms. Rodriguez was supported in part by a grant from the National Cancer Institute. The researchers had no financial conflicts to disclose. Dr. Rossi had no financial conflicts to disclose.
Women who were Black, Latina, American Indian, or Alaska Native were significantly less likely than White women to receive guidelines-adherent treatment for endometrial cancer, based on data from more than 80,000 women.
The incidence of uterine cancer has increased across all ethnicities in recent decades, and adherence to the National Comprehensive Cancer Network treatment guidelines has been associated with improved survival, wrote Victoria A. Rodriguez, MSW, MPH, of the University of California, Irvine, and colleagues. “To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines.”
In a retrospective study published in Obstetrics & Gynecology, the researchers used data from the SEER (Surveillance, Epidemiology, and End Results) database between Jan. 1, 2006, and Dec. 31, 2015. The study population included 83,883 women aged 18 years and older who were diagnosed with their first or only endometrial carcinoma. The primary dependent variable was adherence to the NCCN guidelines for the initial course of treatment, which included a combination of therapies based on cancer subtype and the extent of the disease, the researchers said.
The researchers combined the guidelines and the corresponding data from the SEER database to create “a binary variable representing adherence to [NCCN] guidelines (1 = adherent treatment, 0 = nonadherent treatment).”
Approximately 60% of the total patient population received guidelines-adherent treatment. In a multivariate analysis, Black women, Latina women, and American Indian or Alaska Native women were significantly less likely than White women to receive such treatment (odds ratios, 0.88, 0.92, and 0.82, respectively), controlling for factors including neighborhood socioeconomic status, age, and stage at diagnosis, year of diagnosis, histology, and disease grade. Asian women and Native Hawaiian/Pacific Islander women were significantly more likely to received guidelines-adherent treatment, compared with White women (OR, 1.14 and 1.19, respectively).
The researchers also found a significant gradient in guidelines-adherent treatment based on neighborhood socioeconomic status. Relative to the highest neighborhood socioeconomic status group, women in the lower groups had significantly lower odds of receiving guidelines-adherent treatment, with ORs of 0.89, 0.84, 0.80, and 0.73, respectively, for the high-middle neighborhood socioeconomic group, the middle group, the low-middle group, and the lowest group (P < .001 for all).
“Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer,” the researchers noted.
The study findings were limited by several factors in including the retrospective design and potential for unmeasured confounding variables not included in SEER, such as hospital and physician characteristics, the researchers said. Also, the SEER data set was limited to only the first course of treatment, and did not include information on patient comorbidities that might affect treatment.
“Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial-ethnic groups and socioeconomic status groups, with special attention to low-income women of color,” the researchers emphasized. More research on the impact of comorbidities on a patient’s ability to receive guidelines-based care should be used to inform whether comorbidities should be part of the NCCN guidelines.
However, the results were strengthened by the large sample size and diverse population, so the findings are generalizable to the overall U.S. population, the researchers said.
“Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial-ethnic or socioeconomic backgrounds,” they concluded.
Pursue optimal treatment to curb mortality
Even more concerning than the increase in the incidence of endometrial cancer in the United States is the increase in mortality from this disease, said Emma C. Rossi, MD, of the University of North Carolina at Chapel Hill, in an interview.
“Therefore, it is critical that we identify factors which might be contributing to the increasing lethality of this cancer,” she emphasized. “One such potential factor is race, as it has been observed that Black race is associated with an increased risk of death from endometrial cancer. Historically, this was attributed to the more aggressive subtypes of endometrial cancer (such as serous) which have a higher incidence among Black women. However, more recently, population-based studies have identified that this worse prognosis is independent of histologic cell type,” which suggests that something in our health care delivery is contributing to these worse outcomes.
“The present study helps to confirm these concerning associations, shedding some light on contributory factors, in this case, modifiable (adherence to recommended guidelines) and less modifiable (neighborhood socioeconomic environments) [ones],” Dr. Rossi noted. “The guidelines that are established by the NCCN are chosen after they have been shown to be associated with improved outcomes (including either survival or quality of life), and therefore lack of adherence to these outcomes may suggest inferior quality care is being delivered.”
Studies such as this are helpful in exposing the problem of treatment disparity to help identify sources of problems to develop solutions, she added.
The results should inspire clinicians “to feel agency in changing these outcomes, albeit by tackling very difficult social, political, and health system shortfalls,” she said.
Identify barriers to care
Barriers to greater adherence to guidelines-based care include varying definitions of such care, Dr. Rossi said.
“This is particularly true for surgical management of endometrial cancer, which remains controversial with respect to lymph node assessment. Lack of surgical staging with lymph node assessment was considered noncompliant care for this study; however, lymphadenectomy has not specifically, in and of itself, been associated with improved outcomes, and therefore some surgeons argue against performing it routinely,” she explained.
“Lack of access to sophisticated surgical tools and advanced surgical techniques may account for nonguidelines-based care in the patients with early-stage endometrial cancer; however, there are likely other differences in the ability to deliver guideline-concordant care (such as chemotherapy and radiation therapy) for advanced-stage cancers,” Dr. Rossi said. “Patient and provider positive attitudes toward adjuvant therapy, access to transportation, supportive home environments, paid sick leave, well-controlled or minimal comorbidities are all factors which promote the administration of complex adjuvant therapies such as chemotherapy and radiation. In low-resource neighborhoods and minority communities, barriers to these factors may be contributing to nonguidelines-concordant care.”
Dr. Rossi emphasized the need to “dive deeper into these data at individual health-system and provider levels.” For example, research is needed to compare the practice patterns and models of high-performing clinical practices with lower-performing practices in terms of factors such as tumor boards, journal review, peer review, dashboards, and metrics. By doing so, “we can ensure that we are understanding where and why variations in care are occurring,” Dr. Rossi said.
The study was supported in part by the Faculty Mentor Program Fellowship from the University of California, Irvine, graduate division. Ms. Rodriguez was supported in part by a grant from the National Cancer Institute. The researchers had no financial conflicts to disclose. Dr. Rossi had no financial conflicts to disclose.
Women who were Black, Latina, American Indian, or Alaska Native were significantly less likely than White women to receive guidelines-adherent treatment for endometrial cancer, based on data from more than 80,000 women.
The incidence of uterine cancer has increased across all ethnicities in recent decades, and adherence to the National Comprehensive Cancer Network treatment guidelines has been associated with improved survival, wrote Victoria A. Rodriguez, MSW, MPH, of the University of California, Irvine, and colleagues. “To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines.”
In a retrospective study published in Obstetrics & Gynecology, the researchers used data from the SEER (Surveillance, Epidemiology, and End Results) database between Jan. 1, 2006, and Dec. 31, 2015. The study population included 83,883 women aged 18 years and older who were diagnosed with their first or only endometrial carcinoma. The primary dependent variable was adherence to the NCCN guidelines for the initial course of treatment, which included a combination of therapies based on cancer subtype and the extent of the disease, the researchers said.
The researchers combined the guidelines and the corresponding data from the SEER database to create “a binary variable representing adherence to [NCCN] guidelines (1 = adherent treatment, 0 = nonadherent treatment).”
Approximately 60% of the total patient population received guidelines-adherent treatment. In a multivariate analysis, Black women, Latina women, and American Indian or Alaska Native women were significantly less likely than White women to receive such treatment (odds ratios, 0.88, 0.92, and 0.82, respectively), controlling for factors including neighborhood socioeconomic status, age, and stage at diagnosis, year of diagnosis, histology, and disease grade. Asian women and Native Hawaiian/Pacific Islander women were significantly more likely to received guidelines-adherent treatment, compared with White women (OR, 1.14 and 1.19, respectively).
The researchers also found a significant gradient in guidelines-adherent treatment based on neighborhood socioeconomic status. Relative to the highest neighborhood socioeconomic status group, women in the lower groups had significantly lower odds of receiving guidelines-adherent treatment, with ORs of 0.89, 0.84, 0.80, and 0.73, respectively, for the high-middle neighborhood socioeconomic group, the middle group, the low-middle group, and the lowest group (P < .001 for all).
“Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer,” the researchers noted.
The study findings were limited by several factors in including the retrospective design and potential for unmeasured confounding variables not included in SEER, such as hospital and physician characteristics, the researchers said. Also, the SEER data set was limited to only the first course of treatment, and did not include information on patient comorbidities that might affect treatment.
“Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial-ethnic groups and socioeconomic status groups, with special attention to low-income women of color,” the researchers emphasized. More research on the impact of comorbidities on a patient’s ability to receive guidelines-based care should be used to inform whether comorbidities should be part of the NCCN guidelines.
However, the results were strengthened by the large sample size and diverse population, so the findings are generalizable to the overall U.S. population, the researchers said.
“Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial-ethnic or socioeconomic backgrounds,” they concluded.
Pursue optimal treatment to curb mortality
Even more concerning than the increase in the incidence of endometrial cancer in the United States is the increase in mortality from this disease, said Emma C. Rossi, MD, of the University of North Carolina at Chapel Hill, in an interview.
“Therefore, it is critical that we identify factors which might be contributing to the increasing lethality of this cancer,” she emphasized. “One such potential factor is race, as it has been observed that Black race is associated with an increased risk of death from endometrial cancer. Historically, this was attributed to the more aggressive subtypes of endometrial cancer (such as serous) which have a higher incidence among Black women. However, more recently, population-based studies have identified that this worse prognosis is independent of histologic cell type,” which suggests that something in our health care delivery is contributing to these worse outcomes.
“The present study helps to confirm these concerning associations, shedding some light on contributory factors, in this case, modifiable (adherence to recommended guidelines) and less modifiable (neighborhood socioeconomic environments) [ones],” Dr. Rossi noted. “The guidelines that are established by the NCCN are chosen after they have been shown to be associated with improved outcomes (including either survival or quality of life), and therefore lack of adherence to these outcomes may suggest inferior quality care is being delivered.”
Studies such as this are helpful in exposing the problem of treatment disparity to help identify sources of problems to develop solutions, she added.
The results should inspire clinicians “to feel agency in changing these outcomes, albeit by tackling very difficult social, political, and health system shortfalls,” she said.
Identify barriers to care
Barriers to greater adherence to guidelines-based care include varying definitions of such care, Dr. Rossi said.
“This is particularly true for surgical management of endometrial cancer, which remains controversial with respect to lymph node assessment. Lack of surgical staging with lymph node assessment was considered noncompliant care for this study; however, lymphadenectomy has not specifically, in and of itself, been associated with improved outcomes, and therefore some surgeons argue against performing it routinely,” she explained.
“Lack of access to sophisticated surgical tools and advanced surgical techniques may account for nonguidelines-based care in the patients with early-stage endometrial cancer; however, there are likely other differences in the ability to deliver guideline-concordant care (such as chemotherapy and radiation therapy) for advanced-stage cancers,” Dr. Rossi said. “Patient and provider positive attitudes toward adjuvant therapy, access to transportation, supportive home environments, paid sick leave, well-controlled or minimal comorbidities are all factors which promote the administration of complex adjuvant therapies such as chemotherapy and radiation. In low-resource neighborhoods and minority communities, barriers to these factors may be contributing to nonguidelines-concordant care.”
Dr. Rossi emphasized the need to “dive deeper into these data at individual health-system and provider levels.” For example, research is needed to compare the practice patterns and models of high-performing clinical practices with lower-performing practices in terms of factors such as tumor boards, journal review, peer review, dashboards, and metrics. By doing so, “we can ensure that we are understanding where and why variations in care are occurring,” Dr. Rossi said.
The study was supported in part by the Faculty Mentor Program Fellowship from the University of California, Irvine, graduate division. Ms. Rodriguez was supported in part by a grant from the National Cancer Institute. The researchers had no financial conflicts to disclose. Dr. Rossi had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
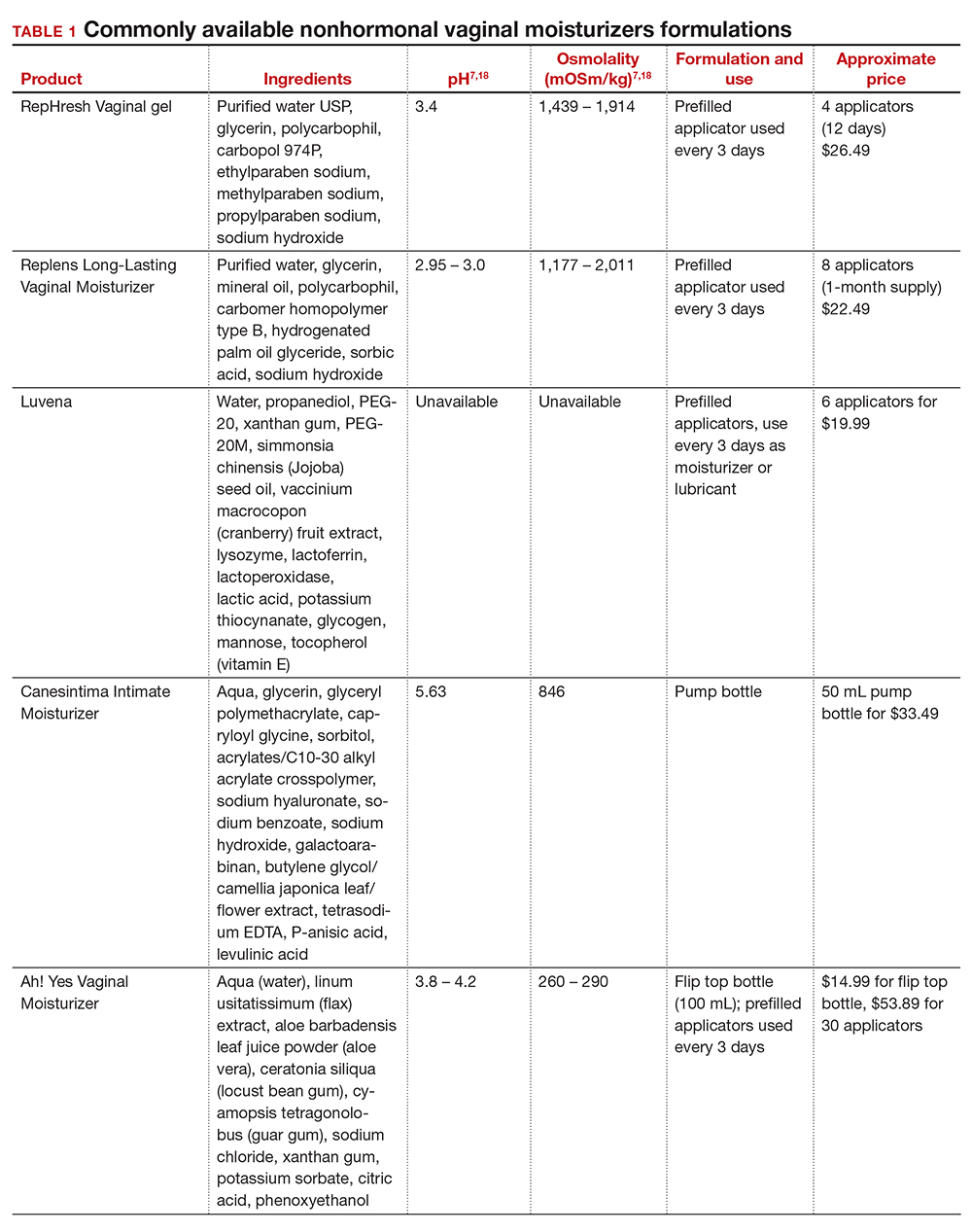
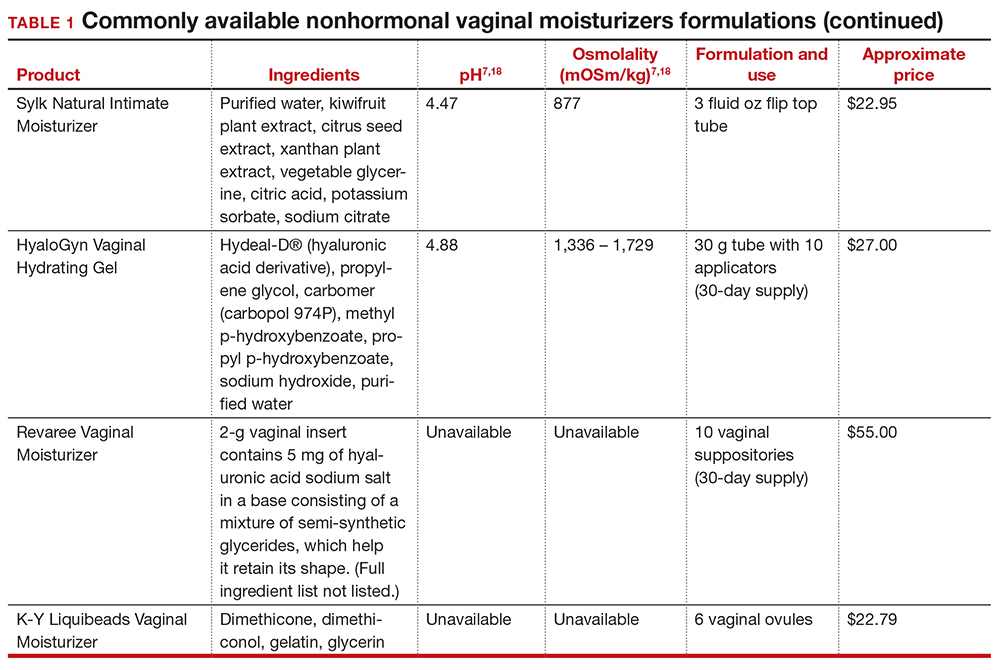
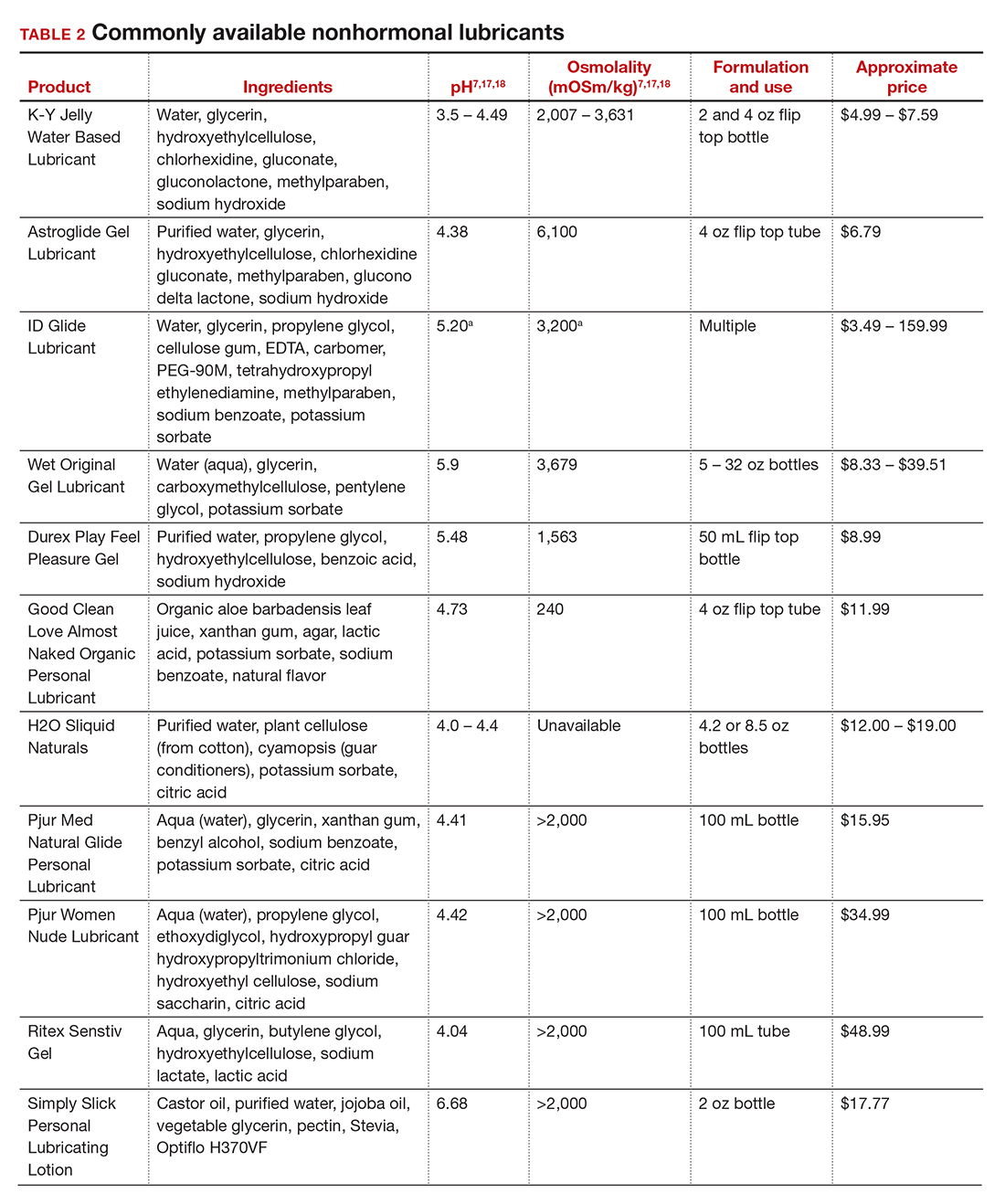
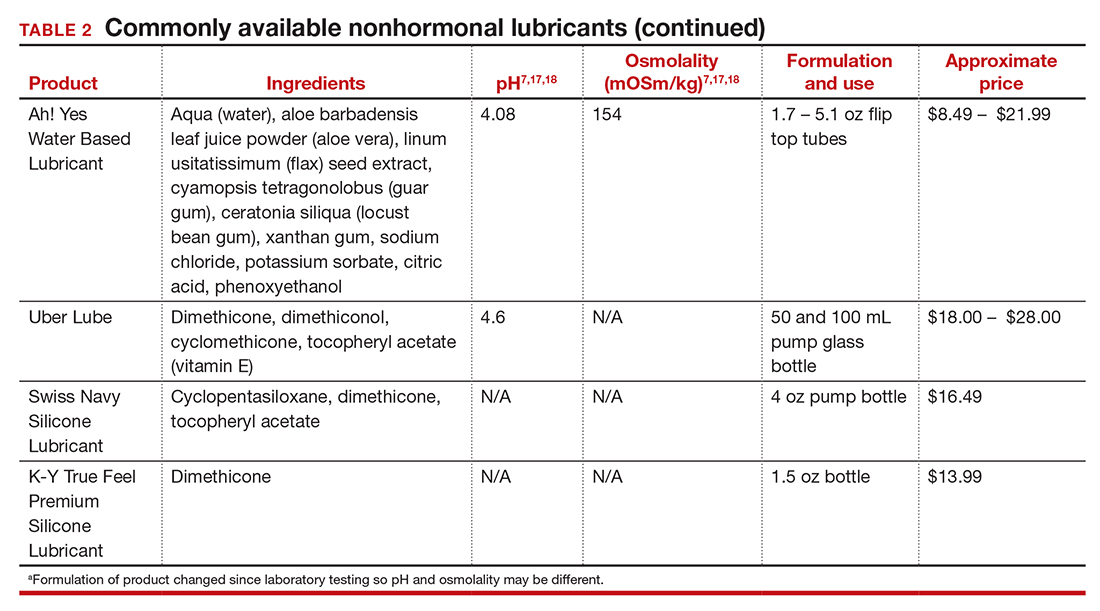
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Decision making regarding LEEP versus cone biopsy for excision of cervical dysplasia
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 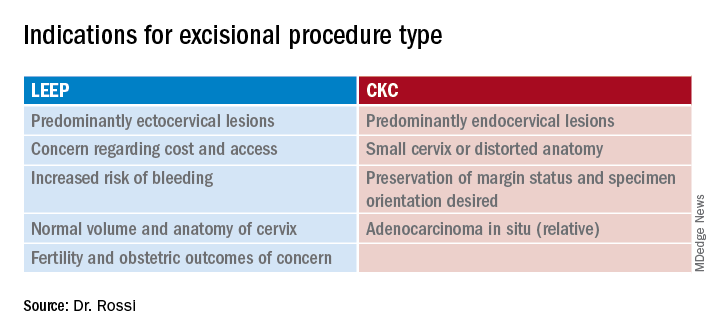
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Cervical cancer rates fall, but other HPV cancers increase
Cervical cancer incidence in the United States decreased by about 1% per year from 2001 to 2017, but at the same time there was an increase in the incidence of other human papillomavirus (HPV)–related cancers, a new study reveals.
Over the same period, there was an overall 1.3% annual increase in oropharyngeal, anal, rectal, and vulvar cancers in women, and a 2.3% annual increase in these cancers in men.
HPV is associated with more than 90% of cervical cancers and between 60% and 75% of oropharyngeal, vulvar, vaginal, and penile cancer in the United States, the researchers noted.
Oropharyngeal cancer incidence increased by 2.3% overall, with a 2.7% increase in men and a 0.77% increase in women. The incidence of this cancer was nearly fivefold greater in men at 8.89 per 100,000 population versus 1.68 per 100,000 population for women, the study found.
In addition, among women over age 50 years, anal and rectal cancer incidence increased by 3.5% per year; at the same time, cervical cancer incidence decreased 1.5% per year.
The increase in the incidence of oropharyngeal cancer and in anal and rectal cancers is expected to continue, the authors said.
The data showing these new trends come from an analysis of 657,317 individuals obtained from the U.S. Cancer Statistics program, conducted by Cheng-I Liao, MD, of Kaohsiung (Taiwan) Veterans Hospital and colleagues.
The study was highlighted at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented June 6.
These incidence trends may reflect the availability of clear guidelines for screening and vaccination for the prevention of HPV-related cervical cancer – and the dearth of guidelines and standardized screening and vaccination for the other HPV-related cancers, the authors said.
The team also found cervical cancer accounted for 52% of all HPV-related cancers during the study period. The decrease in the incidence of cervical cancer over time was greater among women aged 20-24 (4.6% per year), compared with those aged 25-29 years (1.6%) and 30-34 years (1.1%),
Dr. Liao speculated that this age-based difference suggests a potential effect of HPV vaccination, greater vaccine acceptance among younger women, and clear guidelines for screening and vaccination.
However, an expert approached for comment was not so sure. It is likely too soon to give HPV vaccination too much credit for lower cervical cancer rates, said Jennifer Young Pierce, MD, MPH, a gynecologic oncologist at the Mitchell Cancer Institute, University of South Alabama, Mobile.
The continued rise in HPV-related cancers other than cervical cancers supports the point that screening – rather than vaccination – accounts for much of the decline observed in cervical cancer incidence, Dr. Pierce said in an interview.
Vaccination in men lags behind that of women, and there is a lack of good screening methods for head and neck cancers, she explained.
“When we have both vaccination and screening in these other cancers at high rates, we’re going to see significant declines in those cancers also,” she said.
“I’m very excited by the data but I do not believe it is related to vaccination as a method of prevention,” said Dr. Pierce, a professor of interdisciplinary clinical oncology who has been involved in numerous HPV vaccine–related studies and initiatives to improve vaccine uptake since its approval in 2006.
HPV vaccination
The HPV vaccine was first approved for preventing HPV-related cervical cancer in 2006 with an indication for girls and women aged 9-26 years. The vaccine indication was expanded in 2011 to include boys aged 11-12 years and is now approved for those up to age 45 years.
However, neither standardized screening nor HPV vaccination is currently recommended for any HPV-related cancer other than cervical cancer, Dr. Liao said.
Vaccination during much of the current study time frame (2001-2017) didn’t apply to most of the people who got cancer, Dr. Pierce explained in an interview, noting that the vaccinated individuals “still aren’t old enough to be part of the group we’re talking about.”
Rather, the increased use of HPV screening along with Pap testing for cervical cancer was becoming much more widespread at the time and was likely picking up more precancerous lesions – and thereby helping to decrease cervical cancer incidence in women in their 40s, 50s, 60s, and 70s, she said.
Dr. Pierce does, however, credit the vaccine movement for improving awareness of HPV risk.
“It has done a great job of educating the population about the dangers of these cancers ... and that there’s more we can do to prevent them,” she said.
Like Dr. Liao, she stressed the need for research focused on finding more effective screening modalities and on vaccine efficacy.
Also commenting on the study, ASCO president Lori J. Pierce, MD, a radiation oncologist, professor, and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, said the findings underscore the need for ongoing exploration of potential strategies such as HPV screening for high-risk populations.
“We can pick out higher risk populations so it would make sense to do a screen,” she said.
“Clearly, this study shows that we still have a great deal of work to do in order to reverse the increasing incidence rates of other HPV-related cancers,” she added in a press statement.
In an interview prior to the press conference, Dr. Pierce said in an interview that the findings are important because the outcome “opens all of our eyes into the trends of HPV-related cancers in the United States.
“This is something that hasn’t been studied well over time,” she added, noting that, where guidelines do exist for HPV-related cancers other than cervical cancer, they are inconsistent.
Further, it is possible that the vaccine will “cover a significant portion of the etiologic viruses that cause these cancers,” thereby helping to prevent the other HPV-related cancers.
For that reason, additional research and strategies for overcoming vaccine hesitancy, increasing overall vaccination rates, and for developing consistent guidelines are needed.
“I think there needs to be further resources and research to address the lack of screening for these other HPV-related cancers and we need to have consistent vaccination guidelines, because these cancers are preventable,” she said
Dr. Liao and Dr. Pierce disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer incidence in the United States decreased by about 1% per year from 2001 to 2017, but at the same time there was an increase in the incidence of other human papillomavirus (HPV)–related cancers, a new study reveals.
Over the same period, there was an overall 1.3% annual increase in oropharyngeal, anal, rectal, and vulvar cancers in women, and a 2.3% annual increase in these cancers in men.
HPV is associated with more than 90% of cervical cancers and between 60% and 75% of oropharyngeal, vulvar, vaginal, and penile cancer in the United States, the researchers noted.
Oropharyngeal cancer incidence increased by 2.3% overall, with a 2.7% increase in men and a 0.77% increase in women. The incidence of this cancer was nearly fivefold greater in men at 8.89 per 100,000 population versus 1.68 per 100,000 population for women, the study found.
In addition, among women over age 50 years, anal and rectal cancer incidence increased by 3.5% per year; at the same time, cervical cancer incidence decreased 1.5% per year.
The increase in the incidence of oropharyngeal cancer and in anal and rectal cancers is expected to continue, the authors said.
The data showing these new trends come from an analysis of 657,317 individuals obtained from the U.S. Cancer Statistics program, conducted by Cheng-I Liao, MD, of Kaohsiung (Taiwan) Veterans Hospital and colleagues.
The study was highlighted at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented June 6.
These incidence trends may reflect the availability of clear guidelines for screening and vaccination for the prevention of HPV-related cervical cancer – and the dearth of guidelines and standardized screening and vaccination for the other HPV-related cancers, the authors said.
The team also found cervical cancer accounted for 52% of all HPV-related cancers during the study period. The decrease in the incidence of cervical cancer over time was greater among women aged 20-24 (4.6% per year), compared with those aged 25-29 years (1.6%) and 30-34 years (1.1%),
Dr. Liao speculated that this age-based difference suggests a potential effect of HPV vaccination, greater vaccine acceptance among younger women, and clear guidelines for screening and vaccination.
However, an expert approached for comment was not so sure. It is likely too soon to give HPV vaccination too much credit for lower cervical cancer rates, said Jennifer Young Pierce, MD, MPH, a gynecologic oncologist at the Mitchell Cancer Institute, University of South Alabama, Mobile.
The continued rise in HPV-related cancers other than cervical cancers supports the point that screening – rather than vaccination – accounts for much of the decline observed in cervical cancer incidence, Dr. Pierce said in an interview.
Vaccination in men lags behind that of women, and there is a lack of good screening methods for head and neck cancers, she explained.
“When we have both vaccination and screening in these other cancers at high rates, we’re going to see significant declines in those cancers also,” she said.
“I’m very excited by the data but I do not believe it is related to vaccination as a method of prevention,” said Dr. Pierce, a professor of interdisciplinary clinical oncology who has been involved in numerous HPV vaccine–related studies and initiatives to improve vaccine uptake since its approval in 2006.
HPV vaccination
The HPV vaccine was first approved for preventing HPV-related cervical cancer in 2006 with an indication for girls and women aged 9-26 years. The vaccine indication was expanded in 2011 to include boys aged 11-12 years and is now approved for those up to age 45 years.
However, neither standardized screening nor HPV vaccination is currently recommended for any HPV-related cancer other than cervical cancer, Dr. Liao said.
Vaccination during much of the current study time frame (2001-2017) didn’t apply to most of the people who got cancer, Dr. Pierce explained in an interview, noting that the vaccinated individuals “still aren’t old enough to be part of the group we’re talking about.”
Rather, the increased use of HPV screening along with Pap testing for cervical cancer was becoming much more widespread at the time and was likely picking up more precancerous lesions – and thereby helping to decrease cervical cancer incidence in women in their 40s, 50s, 60s, and 70s, she said.
Dr. Pierce does, however, credit the vaccine movement for improving awareness of HPV risk.
“It has done a great job of educating the population about the dangers of these cancers ... and that there’s more we can do to prevent them,” she said.
Like Dr. Liao, she stressed the need for research focused on finding more effective screening modalities and on vaccine efficacy.
Also commenting on the study, ASCO president Lori J. Pierce, MD, a radiation oncologist, professor, and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, said the findings underscore the need for ongoing exploration of potential strategies such as HPV screening for high-risk populations.
“We can pick out higher risk populations so it would make sense to do a screen,” she said.
“Clearly, this study shows that we still have a great deal of work to do in order to reverse the increasing incidence rates of other HPV-related cancers,” she added in a press statement.
In an interview prior to the press conference, Dr. Pierce said in an interview that the findings are important because the outcome “opens all of our eyes into the trends of HPV-related cancers in the United States.
“This is something that hasn’t been studied well over time,” she added, noting that, where guidelines do exist for HPV-related cancers other than cervical cancer, they are inconsistent.
Further, it is possible that the vaccine will “cover a significant portion of the etiologic viruses that cause these cancers,” thereby helping to prevent the other HPV-related cancers.
For that reason, additional research and strategies for overcoming vaccine hesitancy, increasing overall vaccination rates, and for developing consistent guidelines are needed.
“I think there needs to be further resources and research to address the lack of screening for these other HPV-related cancers and we need to have consistent vaccination guidelines, because these cancers are preventable,” she said
Dr. Liao and Dr. Pierce disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer incidence in the United States decreased by about 1% per year from 2001 to 2017, but at the same time there was an increase in the incidence of other human papillomavirus (HPV)–related cancers, a new study reveals.
Over the same period, there was an overall 1.3% annual increase in oropharyngeal, anal, rectal, and vulvar cancers in women, and a 2.3% annual increase in these cancers in men.
HPV is associated with more than 90% of cervical cancers and between 60% and 75% of oropharyngeal, vulvar, vaginal, and penile cancer in the United States, the researchers noted.
Oropharyngeal cancer incidence increased by 2.3% overall, with a 2.7% increase in men and a 0.77% increase in women. The incidence of this cancer was nearly fivefold greater in men at 8.89 per 100,000 population versus 1.68 per 100,000 population for women, the study found.
In addition, among women over age 50 years, anal and rectal cancer incidence increased by 3.5% per year; at the same time, cervical cancer incidence decreased 1.5% per year.
The increase in the incidence of oropharyngeal cancer and in anal and rectal cancers is expected to continue, the authors said.
The data showing these new trends come from an analysis of 657,317 individuals obtained from the U.S. Cancer Statistics program, conducted by Cheng-I Liao, MD, of Kaohsiung (Taiwan) Veterans Hospital and colleagues.
The study was highlighted at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented June 6.
These incidence trends may reflect the availability of clear guidelines for screening and vaccination for the prevention of HPV-related cervical cancer – and the dearth of guidelines and standardized screening and vaccination for the other HPV-related cancers, the authors said.
The team also found cervical cancer accounted for 52% of all HPV-related cancers during the study period. The decrease in the incidence of cervical cancer over time was greater among women aged 20-24 (4.6% per year), compared with those aged 25-29 years (1.6%) and 30-34 years (1.1%),
Dr. Liao speculated that this age-based difference suggests a potential effect of HPV vaccination, greater vaccine acceptance among younger women, and clear guidelines for screening and vaccination.
However, an expert approached for comment was not so sure. It is likely too soon to give HPV vaccination too much credit for lower cervical cancer rates, said Jennifer Young Pierce, MD, MPH, a gynecologic oncologist at the Mitchell Cancer Institute, University of South Alabama, Mobile.
The continued rise in HPV-related cancers other than cervical cancers supports the point that screening – rather than vaccination – accounts for much of the decline observed in cervical cancer incidence, Dr. Pierce said in an interview.
Vaccination in men lags behind that of women, and there is a lack of good screening methods for head and neck cancers, she explained.
“When we have both vaccination and screening in these other cancers at high rates, we’re going to see significant declines in those cancers also,” she said.
“I’m very excited by the data but I do not believe it is related to vaccination as a method of prevention,” said Dr. Pierce, a professor of interdisciplinary clinical oncology who has been involved in numerous HPV vaccine–related studies and initiatives to improve vaccine uptake since its approval in 2006.
HPV vaccination
The HPV vaccine was first approved for preventing HPV-related cervical cancer in 2006 with an indication for girls and women aged 9-26 years. The vaccine indication was expanded in 2011 to include boys aged 11-12 years and is now approved for those up to age 45 years.
However, neither standardized screening nor HPV vaccination is currently recommended for any HPV-related cancer other than cervical cancer, Dr. Liao said.
Vaccination during much of the current study time frame (2001-2017) didn’t apply to most of the people who got cancer, Dr. Pierce explained in an interview, noting that the vaccinated individuals “still aren’t old enough to be part of the group we’re talking about.”
Rather, the increased use of HPV screening along with Pap testing for cervical cancer was becoming much more widespread at the time and was likely picking up more precancerous lesions – and thereby helping to decrease cervical cancer incidence in women in their 40s, 50s, 60s, and 70s, she said.
Dr. Pierce does, however, credit the vaccine movement for improving awareness of HPV risk.
“It has done a great job of educating the population about the dangers of these cancers ... and that there’s more we can do to prevent them,” she said.
Like Dr. Liao, she stressed the need for research focused on finding more effective screening modalities and on vaccine efficacy.
Also commenting on the study, ASCO president Lori J. Pierce, MD, a radiation oncologist, professor, and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, said the findings underscore the need for ongoing exploration of potential strategies such as HPV screening for high-risk populations.
“We can pick out higher risk populations so it would make sense to do a screen,” she said.
“Clearly, this study shows that we still have a great deal of work to do in order to reverse the increasing incidence rates of other HPV-related cancers,” she added in a press statement.
In an interview prior to the press conference, Dr. Pierce said in an interview that the findings are important because the outcome “opens all of our eyes into the trends of HPV-related cancers in the United States.
“This is something that hasn’t been studied well over time,” she added, noting that, where guidelines do exist for HPV-related cancers other than cervical cancer, they are inconsistent.
Further, it is possible that the vaccine will “cover a significant portion of the etiologic viruses that cause these cancers,” thereby helping to prevent the other HPV-related cancers.
For that reason, additional research and strategies for overcoming vaccine hesitancy, increasing overall vaccination rates, and for developing consistent guidelines are needed.
“I think there needs to be further resources and research to address the lack of screening for these other HPV-related cancers and we need to have consistent vaccination guidelines, because these cancers are preventable,” she said
Dr. Liao and Dr. Pierce disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
The power and promise of social media in oncology
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM COSMO 2021
For cervical cancer screening, any strategy is acceptable
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
Success in LGBTQ+ medicine requires awareness of risk
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.













