User login
Morcellation use in gynecologic surgery: Current clinical recommendations and cautions
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
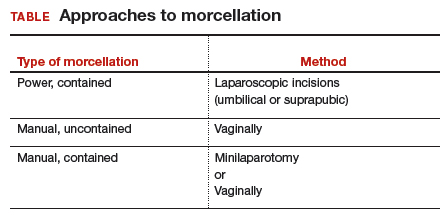
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7
Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Hormone therapy and cognition: What is best for the midlife brain?
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
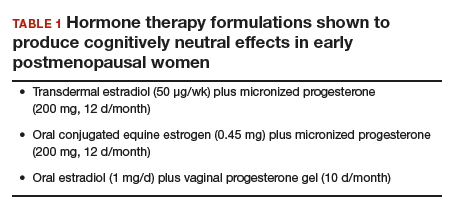
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).
What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
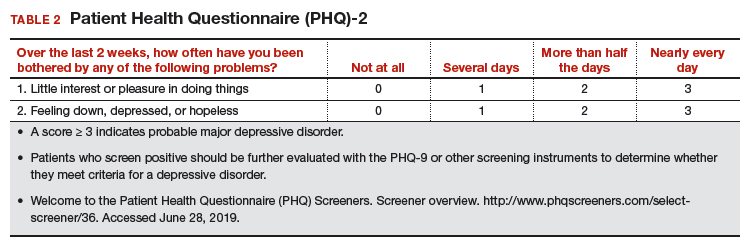
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).
Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).

What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).

Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).

What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).

Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
Office hysteroscopic evaluation of postmenopausal bleeding
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
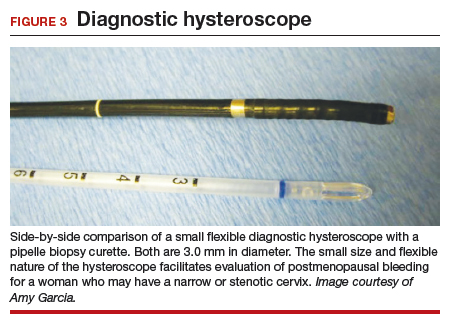
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
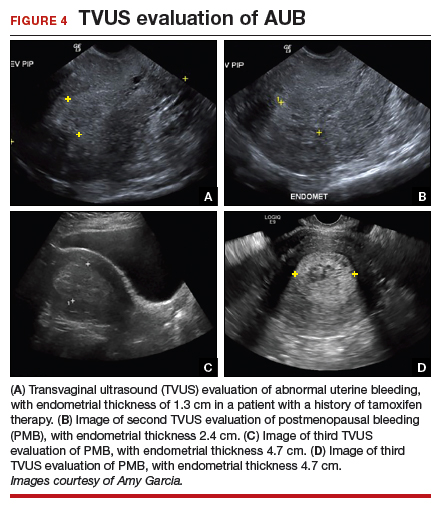
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

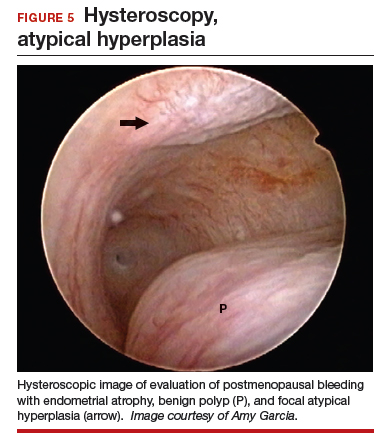
This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

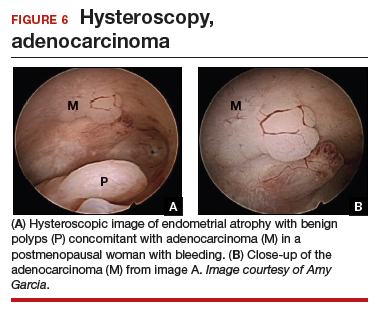
This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Why do so many women aged 65 years and older die of cervical cancer?
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1
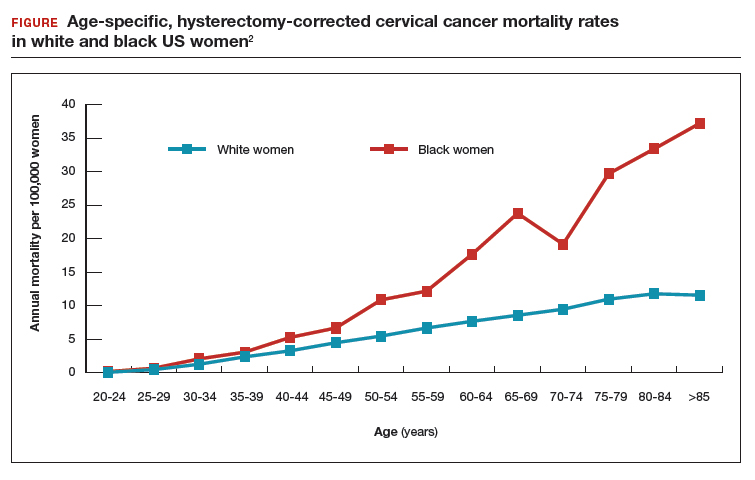
A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Diagnosis, treatment, and prevention of ovarian remnant syndrome
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
The ovarian remnant syndrome
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
Tips to improve immunization rates in your office
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
Universal adolescent education on healthy relationships needed
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
FROM OBSTETRICS & GYNECOLOGY
Gynecologic surgeries linked with persistent opioid use
– showing that persistent opioid use can follow such surgeries.
For a study published in Obstetrics & Gynecology, Jason D. Wright, MD, of Columbia University, New York, and colleagues looked at insurance claims data from 729,625 opioid-naive women, median age 44 years, who had undergone a myomectomy; a minimally invasive, vaginal, or abdominal hysterectomy; an open or laparoscopic oophorectomy; endometrial ablation; tubal ligation; or dilation and curettage. The vast majority of subjects, 93%, had commercial health insurance, with the rest enrolled in Medicaid. Women undergoing multiple surgical procedures, with serious comorbidities, or who underwent another surgery within 6 months of the initial one, were excluded from the analysis.
Dr. Wright and colleagues found that 60% of patients in the cohort received an initial opioid prescription in the perioperative period. Additional opioids were then prescribed to 6.8% (P less than .001) of those women between 90 and 180 days after surgery. The rate of additional prescriptions varied by year across the study period, from 2009 to 2016, and declined to 6% by the final year of the study. The rate of further opioid prescriptions varied according to procedure: 4.8% for myomectomy, 6.6% for minimally invasive hysterectomy, 6.7% for abdominal hysterectomy, 6.3% for endometrial ablation, 7% for tubal ligation, and 7.2% for dilation and curettage (P less than .001).
Factors significantly increasing likelihood of a new prescription included younger age and a history of depression, anxiety, or a substance abuse disorder. Also, a higher total dose of opioids initially prescribed, and a greater number of days supplied, were associated with increased risk for an additional prescription.
“These data demonstrate that the rate of new persistent opioid use after common gynecologic procedures is substantial,” Dr. Wright and colleagues wrote in their analysis, noting that prior studies across a wide range of surgeries have shown rates of new persistent opioid use to be between 3% and 8%. “Careful risk assessment of patients preoperatively may be useful to mitigate opioid misuse in high risk populations,” the investigators wrote. “Women with underlying psychosocial disorders, medical comorbidities, or a history of substance use disorder are at particular risk for persistent opioid use and should be prescribed opioids with extra caution.”
Dr. Wright and colleagues’ study “provides powerful data that should cause gynecological surgeons to pause when writing an opioid prescription,” David M. Jaspan, DO, chairman of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Is an opioid the best first line medication for this patient? Would an NSAID work better? Is multimodal medication an option? What are the patient characteristics that may be associated with persistent use?”
Dr. Wright and colleagues noted among the study’s limitations the fact that actual opioid use could not be measured, nor could use of nonopioid painkillers.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Alfred I. Neugut disclosed relationships with various pharmaceutical firms. Dr. Dawn L. Hershman received a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. The remaining coauthors had no relevant financial disclosures.
SOURCE: Wright JD et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003358.
– showing that persistent opioid use can follow such surgeries.
For a study published in Obstetrics & Gynecology, Jason D. Wright, MD, of Columbia University, New York, and colleagues looked at insurance claims data from 729,625 opioid-naive women, median age 44 years, who had undergone a myomectomy; a minimally invasive, vaginal, or abdominal hysterectomy; an open or laparoscopic oophorectomy; endometrial ablation; tubal ligation; or dilation and curettage. The vast majority of subjects, 93%, had commercial health insurance, with the rest enrolled in Medicaid. Women undergoing multiple surgical procedures, with serious comorbidities, or who underwent another surgery within 6 months of the initial one, were excluded from the analysis.
Dr. Wright and colleagues found that 60% of patients in the cohort received an initial opioid prescription in the perioperative period. Additional opioids were then prescribed to 6.8% (P less than .001) of those women between 90 and 180 days after surgery. The rate of additional prescriptions varied by year across the study period, from 2009 to 2016, and declined to 6% by the final year of the study. The rate of further opioid prescriptions varied according to procedure: 4.8% for myomectomy, 6.6% for minimally invasive hysterectomy, 6.7% for abdominal hysterectomy, 6.3% for endometrial ablation, 7% for tubal ligation, and 7.2% for dilation and curettage (P less than .001).
Factors significantly increasing likelihood of a new prescription included younger age and a history of depression, anxiety, or a substance abuse disorder. Also, a higher total dose of opioids initially prescribed, and a greater number of days supplied, were associated with increased risk for an additional prescription.
“These data demonstrate that the rate of new persistent opioid use after common gynecologic procedures is substantial,” Dr. Wright and colleagues wrote in their analysis, noting that prior studies across a wide range of surgeries have shown rates of new persistent opioid use to be between 3% and 8%. “Careful risk assessment of patients preoperatively may be useful to mitigate opioid misuse in high risk populations,” the investigators wrote. “Women with underlying psychosocial disorders, medical comorbidities, or a history of substance use disorder are at particular risk for persistent opioid use and should be prescribed opioids with extra caution.”
Dr. Wright and colleagues’ study “provides powerful data that should cause gynecological surgeons to pause when writing an opioid prescription,” David M. Jaspan, DO, chairman of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Is an opioid the best first line medication for this patient? Would an NSAID work better? Is multimodal medication an option? What are the patient characteristics that may be associated with persistent use?”
Dr. Wright and colleagues noted among the study’s limitations the fact that actual opioid use could not be measured, nor could use of nonopioid painkillers.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Alfred I. Neugut disclosed relationships with various pharmaceutical firms. Dr. Dawn L. Hershman received a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. The remaining coauthors had no relevant financial disclosures.
SOURCE: Wright JD et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003358.
– showing that persistent opioid use can follow such surgeries.
For a study published in Obstetrics & Gynecology, Jason D. Wright, MD, of Columbia University, New York, and colleagues looked at insurance claims data from 729,625 opioid-naive women, median age 44 years, who had undergone a myomectomy; a minimally invasive, vaginal, or abdominal hysterectomy; an open or laparoscopic oophorectomy; endometrial ablation; tubal ligation; or dilation and curettage. The vast majority of subjects, 93%, had commercial health insurance, with the rest enrolled in Medicaid. Women undergoing multiple surgical procedures, with serious comorbidities, or who underwent another surgery within 6 months of the initial one, were excluded from the analysis.
Dr. Wright and colleagues found that 60% of patients in the cohort received an initial opioid prescription in the perioperative period. Additional opioids were then prescribed to 6.8% (P less than .001) of those women between 90 and 180 days after surgery. The rate of additional prescriptions varied by year across the study period, from 2009 to 2016, and declined to 6% by the final year of the study. The rate of further opioid prescriptions varied according to procedure: 4.8% for myomectomy, 6.6% for minimally invasive hysterectomy, 6.7% for abdominal hysterectomy, 6.3% for endometrial ablation, 7% for tubal ligation, and 7.2% for dilation and curettage (P less than .001).
Factors significantly increasing likelihood of a new prescription included younger age and a history of depression, anxiety, or a substance abuse disorder. Also, a higher total dose of opioids initially prescribed, and a greater number of days supplied, were associated with increased risk for an additional prescription.
“These data demonstrate that the rate of new persistent opioid use after common gynecologic procedures is substantial,” Dr. Wright and colleagues wrote in their analysis, noting that prior studies across a wide range of surgeries have shown rates of new persistent opioid use to be between 3% and 8%. “Careful risk assessment of patients preoperatively may be useful to mitigate opioid misuse in high risk populations,” the investigators wrote. “Women with underlying psychosocial disorders, medical comorbidities, or a history of substance use disorder are at particular risk for persistent opioid use and should be prescribed opioids with extra caution.”
Dr. Wright and colleagues’ study “provides powerful data that should cause gynecological surgeons to pause when writing an opioid prescription,” David M. Jaspan, DO, chairman of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Is an opioid the best first line medication for this patient? Would an NSAID work better? Is multimodal medication an option? What are the patient characteristics that may be associated with persistent use?”
Dr. Wright and colleagues noted among the study’s limitations the fact that actual opioid use could not be measured, nor could use of nonopioid painkillers.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Alfred I. Neugut disclosed relationships with various pharmaceutical firms. Dr. Dawn L. Hershman received a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. The remaining coauthors had no relevant financial disclosures.
SOURCE: Wright JD et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003358.
FROM OBSTETRICS & GYNECOLOGY
Expert advice for immediate postpartum LARC insertion
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.
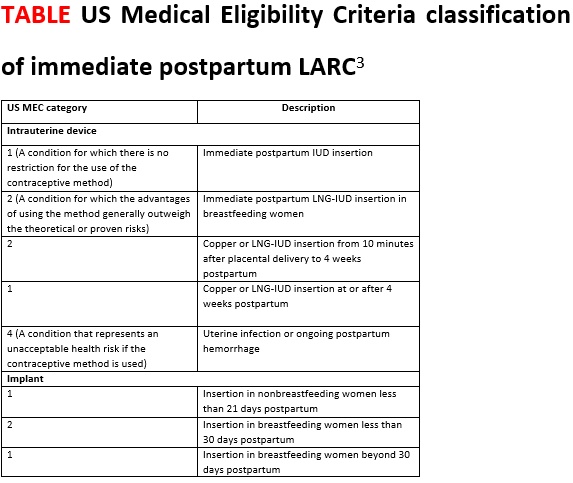
OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.

OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.

OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.