User login
Abnormal uterine bleeding: When can you forgo biopsy?
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
EXPERT ANALYSIS FROM ACOG 2019
Bilateral salpingectomy gains favor for sterilization
NASHVILLE, TENN. –
“[It is] probably the newest thing on the block ... this is becoming super widespread,” Eve Espey, MD, said of the procedure during a contraceptive update at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Although evidence directly supporting bilateral salpingectomy for sterilization is lacking, there are good reasons to consider it, she said.
For example, the procedure is likely more effective than tubal ligation with no increased risk for complications, and is probably more likely to cut ovarian cancer risk than is tubal ligation, explained Dr. Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque.
“So we don’t actually have good [randomized controlled trials] on effectiveness for [bilateral] salpingectomy, but it is most like a partial salpingectomy, which is highly effective, so there is reason to believe that it might be more effective,” she added. The downsides are that the procedure may take longer, it may impair ovarian blood supply, and long-term population-level data on outcomes are lacking.
ACOG said in a 2015 committee opinion that when counseling women, bilateral salpingectomy can be discussed and considered “a method that provides effective contraception,” but also stressed the need for randomized controlled trials to support any related reduction in ovarian cancer risk. That opinion (#620) was replaced in April 2019 by Committee Opinion #774, which addresses opportunistic salpingectomy for epithelial ovarian cancer prevention, and which states that “the risks and benefits of salpingectomy should be discussed with patients who desire permanent sterilization.”
“[The Society of Gynecologic Oncology] is much, much more emphatic,” Dr. Espey said, citing a 2013 Clinical Practice Statement calling for discussion and consideration of risk-reducing salpingectomy in lieu of tubal ligation for women at average risk of ovarian cancer (after childbearing).
Dr. Espey also noted that during a recent grand rounds on sterilization, about 90% of participants said they were doing bilateral salpingectomy in the setting of vaginal delivery. “So I think we’re going to see this coming not just with C-section, but also with vaginal delivery.”
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. –
“[It is] probably the newest thing on the block ... this is becoming super widespread,” Eve Espey, MD, said of the procedure during a contraceptive update at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Although evidence directly supporting bilateral salpingectomy for sterilization is lacking, there are good reasons to consider it, she said.
For example, the procedure is likely more effective than tubal ligation with no increased risk for complications, and is probably more likely to cut ovarian cancer risk than is tubal ligation, explained Dr. Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque.
“So we don’t actually have good [randomized controlled trials] on effectiveness for [bilateral] salpingectomy, but it is most like a partial salpingectomy, which is highly effective, so there is reason to believe that it might be more effective,” she added. The downsides are that the procedure may take longer, it may impair ovarian blood supply, and long-term population-level data on outcomes are lacking.
ACOG said in a 2015 committee opinion that when counseling women, bilateral salpingectomy can be discussed and considered “a method that provides effective contraception,” but also stressed the need for randomized controlled trials to support any related reduction in ovarian cancer risk. That opinion (#620) was replaced in April 2019 by Committee Opinion #774, which addresses opportunistic salpingectomy for epithelial ovarian cancer prevention, and which states that “the risks and benefits of salpingectomy should be discussed with patients who desire permanent sterilization.”
“[The Society of Gynecologic Oncology] is much, much more emphatic,” Dr. Espey said, citing a 2013 Clinical Practice Statement calling for discussion and consideration of risk-reducing salpingectomy in lieu of tubal ligation for women at average risk of ovarian cancer (after childbearing).
Dr. Espey also noted that during a recent grand rounds on sterilization, about 90% of participants said they were doing bilateral salpingectomy in the setting of vaginal delivery. “So I think we’re going to see this coming not just with C-section, but also with vaginal delivery.”
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. –
“[It is] probably the newest thing on the block ... this is becoming super widespread,” Eve Espey, MD, said of the procedure during a contraceptive update at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Although evidence directly supporting bilateral salpingectomy for sterilization is lacking, there are good reasons to consider it, she said.
For example, the procedure is likely more effective than tubal ligation with no increased risk for complications, and is probably more likely to cut ovarian cancer risk than is tubal ligation, explained Dr. Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque.
“So we don’t actually have good [randomized controlled trials] on effectiveness for [bilateral] salpingectomy, but it is most like a partial salpingectomy, which is highly effective, so there is reason to believe that it might be more effective,” she added. The downsides are that the procedure may take longer, it may impair ovarian blood supply, and long-term population-level data on outcomes are lacking.
ACOG said in a 2015 committee opinion that when counseling women, bilateral salpingectomy can be discussed and considered “a method that provides effective contraception,” but also stressed the need for randomized controlled trials to support any related reduction in ovarian cancer risk. That opinion (#620) was replaced in April 2019 by Committee Opinion #774, which addresses opportunistic salpingectomy for epithelial ovarian cancer prevention, and which states that “the risks and benefits of salpingectomy should be discussed with patients who desire permanent sterilization.”
“[The Society of Gynecologic Oncology] is much, much more emphatic,” Dr. Espey said, citing a 2013 Clinical Practice Statement calling for discussion and consideration of risk-reducing salpingectomy in lieu of tubal ligation for women at average risk of ovarian cancer (after childbearing).
Dr. Espey also noted that during a recent grand rounds on sterilization, about 90% of participants said they were doing bilateral salpingectomy in the setting of vaginal delivery. “So I think we’re going to see this coming not just with C-section, but also with vaginal delivery.”
Dr. Espey reported having no relevant financial disclosures.
EXPERT COMMENTARY FROM ACOG 2019
Appropriateness of performing in-office uterine aspiration
In their article, "Uterine aspiration: From OR to office" (February 2019), Lauren Thaxton, MD, MBA, and Bri Tristan, MD, made the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard surgical management of early pregnancy failure. Their reasons included an equivalent safety profile, reduced costs, and patient-centered characteristics.
OBG Management posed this query to readers in a website poll: "Should the standard location for uterine apiration be in the office?" See how readers responded, below.
Poll results
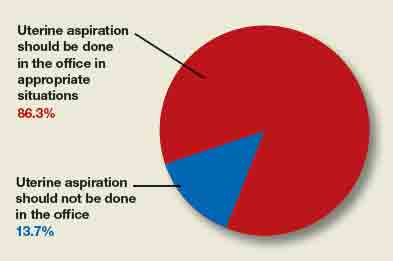
A total of 73 readers cast their vote:
- 86.3% (63 readers) said yes, in appropriate clinical situations
- 13.7% (10 readers) said no
Reader comments
"Yes, in appropriate clinical situations."
-Yardlie Toussaint-Foster, DO, Downingtown, Pennsylvania
"I have been doing it this way (in the office) for years, up to 11 to 12 weeks without complication."
-John Lane, MD, Raleigh, North Carolina
In their article, "Uterine aspiration: From OR to office" (February 2019), Lauren Thaxton, MD, MBA, and Bri Tristan, MD, made the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard surgical management of early pregnancy failure. Their reasons included an equivalent safety profile, reduced costs, and patient-centered characteristics.
OBG Management posed this query to readers in a website poll: "Should the standard location for uterine apiration be in the office?" See how readers responded, below.
Poll results
A total of 73 readers cast their vote:
- 86.3% (63 readers) said yes, in appropriate clinical situations
- 13.7% (10 readers) said no
Reader comments
"Yes, in appropriate clinical situations."
-Yardlie Toussaint-Foster, DO, Downingtown, Pennsylvania
"I have been doing it this way (in the office) for years, up to 11 to 12 weeks without complication."
-John Lane, MD, Raleigh, North Carolina

In their article, "Uterine aspiration: From OR to office" (February 2019), Lauren Thaxton, MD, MBA, and Bri Tristan, MD, made the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard surgical management of early pregnancy failure. Their reasons included an equivalent safety profile, reduced costs, and patient-centered characteristics.
OBG Management posed this query to readers in a website poll: "Should the standard location for uterine apiration be in the office?" See how readers responded, below.
Poll results
A total of 73 readers cast their vote:
- 86.3% (63 readers) said yes, in appropriate clinical situations
- 13.7% (10 readers) said no
Reader comments
"Yes, in appropriate clinical situations."
-Yardlie Toussaint-Foster, DO, Downingtown, Pennsylvania
"I have been doing it this way (in the office) for years, up to 11 to 12 weeks without complication."
-John Lane, MD, Raleigh, North Carolina

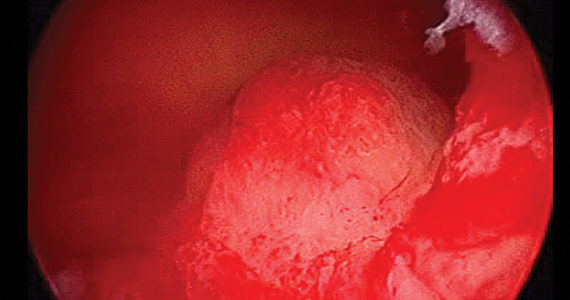
Consider cutaneous endometriosis in women with umbilical lesions
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
The Affordable Care Act, closing in on a decade
The Affordable Care Act (ACA) was enacted on March 23, 2010. Controversies, complaints, and detractors have and continue to abound. But the ACA’s landmark women’s health gains are unmistakable. Contraceptive coverage, maternity coverage, Medicaid coverage of low-income women, coverage for individuals with preexisting conditions, and gender-neutral premiums are now a part of the fabric of our society. For most.
Many physicians and patients—many lawmakers, too—do not remember the serious problems people had with their insurance companies before the ACA. Maternity coverage was usually a free-standing rider to an insurance policy, making it very expensive. Insurance plans did not have to, and often did not, cover contraceptives, and none did without copays or deductibles. Women were routinely denied coverage if they had ever had a cesarean delivery, had once been the victim of domestic violence, or had any one of many common conditions, like diabetes. The many exclusionary conditions are so common, in fact, that one study estimated that around 52 million adults in the United States (27% of those younger than age 65 years) have preexisting conditions that would potentially make them uninsurable without the ACA’s protections.1
Before the ACA, it also was common for women with insurance policies to find their coverage rescinded, often with no explanation, even though they paid their premiums every month. And women with serious medical conditions often saw their coverage ended midway through their course of treatment. That placed their ObGyns in a terrible situation, too.
The insurance industry as a whole was running rough-shod over its customers, and making a lot of money by creatively and routinely denying coverage and payment for care. People were often insured, but not covered. The ACA halted many of these practices, and required insurers to meet high medical loss ratios, guaranteeing that 80% of the premiums’ for individual and small market insurers (and 85% for large insurers) are returned to patients in care payments or even in checks. In fact, nearly $4 billion in premiums have been rebated to insured individuals over the last 7 years under the ACA.2
The commitment of the American College of Obstetricians and Gynecologists (ACOG) to women’s health and to our members’ ability to provide the best care has centered on preserving the critical gains of the ACA for women, improving them when we can, and making sure politicians don’t turn back the clock on women’s health. We have been busy.
In this article, we will look at what has happened to these landmark gains and promises of improved women’s health, specifically preexisting condition protections and contraceptive coverage, under a new Administration. What happens when good health care policy and political enmity collide?

Preexisting coverage protections
The 1996 Health Insurance Portability and Accountability Act (HIPAA) defines a preexisting condition exclusionas a “limitation or exclusion of benefits relating to a condition based on the fact that the condition was present before the date of enrollment for the coverage, whether or not any medical advice, diagnosis, care, or treatment was recommended or received before that date.” HIPPA prohibited employer-sponsored health plans from discriminating against individuals through denying them coverage or charging them more based on their or their family members’ health problems. The ACA expanded protections to prohibit the insurance practice of denying coverage altogether to an individual with a preexisting condition.3
Continue to: Under Congress...
Under Congress
Republicans held the majority in both chambers of the 115th Congress (2017–2018), and hoped to use their majority status to get an ACA repeal bill to the Republican President’s desk for speedy enactment. It was not easy, and they were not successful. Four major bills—the American Health Care Act, the Better Care Reconciliation Act, the Health Care Freedom Act, and the Graham-Cassidy Amendment—never made it over the finish line, with some not even making it to a vote. The Health Care Freedom Act was voted down in the Senate 51-49 when Senator John McCain came back from brain surgery to cast his famous thumbs-down vote.4 These bills all would have repealed or hobbled guaranteed issue, community rating, and essential health benefits of the ACA. Of all the legislative attempts to undermine the ACA, only the 2017 Tax Cuts and Jobs Act (TCJA) was signed into law, repealing the ACA individual mandate.
Handling by the courts
The TCJA gave ACA opponents their opening in court. Twenty Republican state attorneys general and governors brought suit in February 2018 (Texas v Azar), arguing that because the ACA relies on the mandate, and the mandate has been repealed, the rest of the ACA also should be struck down. A federal district judge agreed, on December 15, 2018, declaring the entire ACA unconstitutional.5
That decision has been limited in its practical effect so far, and maybe it was not altogether unexpected. What was unexpected was that the US Department of Justice (DOJ) refused to defend a federal law, in this case, the ACA. In June 2018, the DOJ declined to defend the individual mandate, as well as guaranteed issue, community rating, the ban on preexisting condition exclusions, and discrimination based on health status in the ACA. The DOJ at that time, however, did not agree with the plaintiffs that without the mandate the entire ACA should be struck down. It said, “There is no reason why the ACA’s particular expansion of Medicaid hinges on the individual mandate.” Later, after the December 15 ruling, the DOJ changed its position and agreed with the judge, in a two-sentence letter to the court, that the ACA should be stricken altogether—shortly after which 3 career DOJ attorneys resigned.6
A legal expert observed: “The DOJ’s decision not to defend the ACA breaks with the Department’s long-standing bipartisan commitment to defend federal laws if reasonable arguments can be made in their defense. Decisions not to defend federal law are exceedingly rare. It seems even rarer to change the government’s position mid-appeal in such a high-profile lawsuit that risks disrupting the entire health care system and health insurance coverage for millions of Americans.”7
Regulatory tactics
What a policy maker cannot do by law, he or she can try to accomplish by regulation. The Administration is using 3 regulatory routes to undercut the ACA preexisting coverage protections and market stability.
Route 1: Short-Term Limited Duration (STLD) plans. These plans were created in the ACA to provide bridge coverage for up to 3 months for individuals in between health insurance plans. These plans do not have to comply with ACA patient protections, can deny coverage for preexisting conditions, and do not cover maternity care. In 2018, the Administration moved to allow these plans to be marketed broadly and renewed for up to 3 years. Because these plans provide less coverage and often come with high deductibles, they can be marketed with lower premiums, skimming off healthier younger people who do not expect to need much care, as well as lower-income families. This destabilizes the market and leaves people insured but not covered, exactly the situation before the ACA. Seven public health and medical groups sued to challenge the Administration’s STLD regulation; the lawsuit is presently pending.
Continue to: Route 2: Association Health Plans (AHPs)...
Route 2: Association Health Plans (AHPs). The Administration also has allowed the sale of AHPs, marketed to small employers and self-employed individuals. These plans also do not have to comply with ACA consumer protections. They often do not cover maternity care or other essential benefits, and can charge women higher premiums for the same insurance. This regulation, too, resulted in litigation and a federal judge enjoined the rule, but the case is now on appeal.
Route 3: ACA Section 1332 waivers. These waivers were created in the ACA to encourage state innovation to increase access to health coverage, under certain guardrails: states must ensure coverage is at least as comprehensive as the Essential Health Benefits; cost sharing protections must be at least as affordable as under the ACA; the plan must cover at least a comparable number of its residents; and the plan must not increase the federal deficit.
The Adminstration has come under fire for approving 1332 waiver plans that do not meet these guardrails, and allow insurers to exclude coverage for individuals with preexisting conditions, as well as skirt other important ACA patient protections. In response, Seema Verma, Administrator of the Centers for Medicare & Medicaid Services, promised as recently as April 23, that the Administration will not allow any weakening of the ACA preexisting coverage guarantee.8 So far, however, we do not know what action this means, and not surprisingly, House Democrats, now in the majority, are waiting to see those assurances come true. Consistent polling shows that a large majority of Americans, across political parties, think preexisting coverage protections are very important.9
Already, the House passed HR986, to repeal the Administration’s changes to the 1332 waiver rules. The bill won only 4 Republican votes in the House and now waits a Senate vote.
The House is ready to vote on HR1010, which returns the STLD rules to the original ACA version. The Congressional Budget Office has determined that this bill will reduce the federal deficit by $8.9 billion over 10 years, in part by reestablishing a large risk pool. Lower ACA premiums would mean lower federal subsidies and small federal outlays.
Contraceptive coverage
Since 2012, the ACA has required non-grandfathered individual and group health plans to cover, with no copays or deductibles, women’s preventive services, as determined by the Health Resources and Services Administration (HRSA). HRSA asked the National Academy of Medicine (the Institute of Medicine [IOM] at the time) to develop these coverage guidelines based on clinical and scientific relevance. The IOM relied heavily on ACOG’s testimony and women’s health guidelines. The guidelines are updated every 5 years, based on extensive review by the Women’s Preventive Services Initiative, led by ACOG. By law and regulation, covered services include:
- well-woman visits
- contraceptive methods and counseling, including all methods approved for women by the FDA
- breast and cervical cancer screening
- counseling for sexually transmitted infections
- counseling and screening for HIV
- screening for gestational diabetes
- breastfeeding support, supplies, and counseling
- screening and counseling for interpersonal and domestic violence.
Continue to: The previous administration offered a narrow exemption...
The previous administration offered a narrow exemption—an accommodation—for churches, religious orders, and integrated auxiliaries (organizations with financial support primarily from churches). That accommodation was expanded in the Supreme Court’s decision in Hobby Lobby, for closely held for-profit organizations that had religious objections to covering some or all contraceptives. Under the accommodation, the entity’s insurer or third-party administrator was responsible for providing contraceptive services to the entity’s plan participants and beneficiaries.
In October 2017, the Trump administration acted to greatly expand the ability of any employer, college or university, individual, or insurer to opt out of the ACA’s contraceptive coverage requirement. You will read more about this later.
ACOG’s business case for contraception
Early in the Trump Administration, the White House released a statement saying, “Ensuring affordable, accessible, and quality healthcare is critical to improving women’s health and ensuring that it fits their priorities at any stage of life.”10 ACOG could not agree more, and we encouraged the President to accomplish this important goal by protecting the landmark women’s health gains of the ACA. Our call to the President and the US Congress was: “Don’t turn back the clock on women’s health.”
We made a business case for continued contraceptive coverage:
Contraception reduces unintended pregnancies and saves federal dollars.
- Approximately 45% of US pregnancies are unintended.11
- No-copay coverage of contraception has contributed to a dramatic decline in the unintended pregnancy rate in the United States, now at a 30-year low.12
- When cost is not a barrier, women choose more effective forms of contraception, such as intrauterine devices and implants.13
- Unintended pregnancies cost approximately $12.5 billion in government expenditures in 2008.14
- Private health plans spend as much as $4.6 billion annually in costs related to unintended pregnancies.15
Contraception means healthier women and healthier families.
- Under the ACA, the uninsured rate among women ages 18 to 64 almost halved, decreasing from 19.3% to 10.8%.16
- More than 55 million women gained access to preventive services, including contraception, without a copay or a deductible.16
- Women with unintended pregnancies are more likely to delay prenatal care. Infants are at greater risk of birth defects, low birth weight, and poor mental and physical functioning in early childhood.17
Increased access to contraception helps families and improves economic security.
- Women saved $1.4 billion in out-of-pocket costs for contraception in 1 year.18
- Before the ACA, women were spending between 30% and 44% of their total out-of-pocket health costs just on birth control.19
- The ability to plan a pregnancy increases engagement of women in the workforce and improves economic stability for women and their families.20
Administration expands religious exemptions to contraception coverage
Still, on October 6, 2017, the Trump Administration moved to curtail women’s access to and coverage of contraception with the Religious Exemptions and Accommodations for Coverage of Certain Preventive Services under the Affordable Care Act and Moral Exemptions and Accommodations for Coverage of Certain Preventive Services Under the Affordable Care Act. In November 2018, the Administration published a revised rule, to take effect in January 2019.21 The rule immediately was taken to court by more than a dozen states and, 1 month later, was subject to an injunction by the US Court of Appeals for the Ninth Circuit, blocking the rules from going into effect in those states.
Continue to: The rule vastly expands the Obama Administration’s religious accommodation...
The rule vastly expands the Obama Administration’s religious accommodation to include “nonprofit organizations, small businesses, and individuals that have nonreligious moral convictions opposing services covered by the contraceptive mandate.” The covered entities include21:
- churches, integrated auxiliaries, and religious orders with religious objections
- nonprofit organizations with religious or moral objections
- for-profit entities that are not publicly traded, with religious or moral objections
- for-profit entities that are publicly traded, with religious objections
- other nongovernmental employers with religious objections
- nongovernmental institutions of higher education with religious or moral objections
- individuals with religious or moral objections, with employer sponsored or individual market coverage, where the plan sponsor and/or issuer (as applicable) are willing to offer them a plan omitting contraceptive coverage to which they object
- issuers with religious or moral objections, to the extent they provide coverage to a plan sponsor or individual that is also exempt.
The Administration says women losing coverage can get contraceptives through Title X clinics or other government programs. Of course, many women losing coverage are employed, and earn above the low income (100% of the federal poverty level) eligibility requirement for Title X assistance. To address that, the Administration, through its proposed Title X regulations, broadens the definition of “low income” in that program to include women who lose their contraceptive coverage through the employer-base health insurance plan. This move further limits the ability of the Title X program to adequately care for already-qualified individuals.
The Administration’s rule also relied on major inaccuracies, which ACOG corrected.22 First, ACOG pointed out that, in fact, FDA-approved contraceptive methods are not abortifacients, countering the Administration’s contention that contraception is an abortifacient, and that contraceptives cause abortions or miscarriages. Every FDA-approved contraceptive acts before implantation, does not interfere with a pregnancy, and is not effective after a fertilized egg has implanted successfully in the uterus.23 No credible research supports the false statement that birth control causes miscarriages.24
Second, ACOG offered data proving that increased access to contraception is not associated with increased unsafe sexual behavior or increased sexual activity.25,26 The facts are that:
- The percentage of teens who are having sex has declined significantly, by 14% for female and 22% for male teenagers, over the past 25 years.27
- More women are using contraception the first time they have sex. Young women who do not use birth control at first sexual intercourse are twice as likely to become teen mothers.28
- Increased access to and use of contraception has contributed to a dramatic decline in rates of adolescent pregnancy.29
- School-based health centers that provide access to contraceptives are proven to increase use of contraceptives by already sexually active students, not to increase onset of sexual activity.30,31
Third, ACOG made clear the benefits to women’s health from contraception. ACOG asserted: As with any medication, certain types of contraception may be contraindicated for patients with certain medical conditions, including high blood pressure, lupus, or a history of breast cancer.32,33 For these and many other reasons, access to the full range of FDA-approved contraception, with no cost sharing or other barriers, is critical to women’s health. Regarding VTE, the risk among oral contraceptive users is very low. In fact, it is much lower than the risk of VTE during pregnancy or in the immediate postpartum period.34
Continue to: Regarding breast cancer: there is no proven increased risk...
Regarding breast cancer: there is no proven increased risk of breast cancer among contraceptive users, particularly among those younger than age 40. For women older than 40, health care providers must consider both the risks of becoming pregnant at advanced reproductive age and the risks of continuing contraception use until menopause.35
ACOG has 2 clear messages for politicians
ACOG has remained steadfast in its opposition to the Administration’s proposals to block access to contraception. ACOG expressed its strong opposition to political interference in medical care, saying “Every woman, regardless of her insurer, employer, state of residence, or income, should have affordable, seamless access to the right form of contraception for her, free from interference from her employer or politicians.”22
ACOG’s voice has been joined by 5 other major medical associations—American Academy of Family Physicians, American Academy of Pediatrics, American Psychiatric Association, American Academy of Pediatrics, and American Osteopathic Association—together representing more than 560,000 physicians and medical students, in urging the Administration to immediately withdraw its proposals. This broad coalition unequivocally stated36:
Contraception is an integral part of preventive care and a medical necessity for women during approximately 30 years of their lives. Access to no-copay contraception leads to healthier women and families. Changes to our healthcare system come with very high stakes – impacting tens of millions of our patients. Access to contraception allows women to achieve, lead and reach their full potentials, becoming key drivers of our Nation’s economic success. These rules would create a new standard whereby employers can deny their employees coverage, based on their own moral objections. This interferes in the personal health care decisions of our patients, and inappropriately inserts a patient’s employer into the physician-patient relationship. In addition, these rules open the door to moral exemptions for other essential health care, including vaccinations.
These are challenging days for women’s health policy and legislation federally, and in many states. ACOG has two clear messages for politicians: Don’t turn back the clock on women’s health, and stay out of our exam rooms.
- Claxton G, Cox C, Damico A, et al. Pre-existing conditions and medical underwriting in the individual insurance market prior to the ACA. Kaiser Family Foundation website. Published December 12, 2016. Accessed June 25, 2019.
- Norris L. Billions in ACA rebates show 80/20 rule’s impact. HealthInsurance.org website. Published May 10, 2019. Accessed June 25, 2019.
- Patient Protection and Affordable Care Act: Preexisting condition exclusions, lifetime and annual limits, rescissions, and patient protections. Regulations.gov website. Accessed June 25, 2019.
- Jost T. The Senate’s Health Care Freedom Act. Health Affairs website. Updated July 28, 2017. Accessed June 25, 2019.
- Texas v Azar decision. American Medical Association website. Accessed June 25, 2019.
- Keith K. DOJ, plaintiffs file in Texas v United States. Health Affairs website. Published May 2 2019. Accessed June 25, 2019.
- John & Rusty Report. Trump Administration asks court to strike down entire ACA. March 26, 2019. https://jrreport.wordandbrown.com/2019/03/26/trump-administration-asks-court-to-strike-down-entire-aca/. Accessed June 29, 2019.
- Speech: Remarks by Administrator Seema Verma at the CMS National Forum on State Relief and Empowerment Waivers. Centers for Medicare & Medicaid website. Published April 23, 2019. Accessed June 25, 2019.
- Poll: The ACA’s pre-existing condition protections remain popular with the public, including republicans, as legal challenge looms this week. Kaiser Family Foundation website. Published September 5, 2018. Accessed June 25, 2019.
- Statement from President Donald J. Trump on Women’s Health Week. White House website. Issued May 14, 2017. Accessed June 26, 2019.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374:843-852.
- Insurance coverage of contraception. Guttmacher Institute website. Published August 2018. Accessed June 26, 2019.
- Carlin CS, Fertig AR, Dowd BE. Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs. 2016;35:1608-1615.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250–255.
- Canestaro W, et al. Implications of employer coverage of contraception: cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception. 2017;95:77-89.
- Simmons A, et al. The Affordable Care Act: Promoting better health for women. Office of the Assistant Secretary for Planning and Evaluation Issue Brief, Department of Health and Human Services. June 14, 2016. Accessed June 25, 2019.
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34:1204-1211. Accessed June 25, 2019.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34(7).
- Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013.
- Department of Health and Human Services. Fact sheet: Final rules on religious and moral exemptions and accommodation for coverage of certain preventive services under the Affordable Care Act. November 7, 2018. Accessed June 26, 2019.
- American College of Obstetricians and Gynecologists. Facts are important: Correcting the record on the Administration’s contraceptive coverage roll back rule. October 2017. Accessed June 26, 2019.
- Brief for Physicians for Reproductive Health, American College of Obstetricians and Gynecologists et al. as Amici Curiae Supporting Respondents, Sebelius v. Hobby Lobby, 573 U.S. XXX. 2014. (No. 13-354).
- Early pregnancy loss. FAQ No. 90. American College of Obstetricians and Gynecologists. August 2015.
- Kirby D. Emerging answers 2007: Research findings on programs to reduce teen pregnancy and sexually transmitted diseases. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009.
- Meyer JL, Gold MA, Haggerty CL. Advance provision of emergency contraception among adolescent and young adult women: a systematic review of literature. J Pediatr Adolesc Gynecol. 2011;24:2-9.
- Martinez GM and Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15–19 in the United States. NCHS Data Brief, 2015, No. 209. Hyattsville, MD: National Center for Health Statistics; 2015.
- Martinez GM, Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15-19 in the United States. NCHS Data Brief. July 2015. Accessed June 26, 2019.
- Lindberg L, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59:577-583.
- Minguez M, Santelli JS, Gibson E, et al. Reproductive health impact of a school health center. J Adolesc Health. 2015;56:338-344.
- Knopf JA, Finnie RK, Peng Y, et al. Community Preventive Services Task Force. School-based health centers to advance health equity: a Community Guide systematic review. Am J Preventive Med. 2016;51:114-126.
- Progestin-only hormonal birth control: pill and injection. FAQ No. 86. American College of Obstetricians and Gynecologists. July 2014.
- Combined hormonal birth control: pill, patch, and ring. FAQ No. 185. American College of Obstetricians and Gynecologists. July 2014.
- Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Committee Opinion No. 540. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:1239-1242.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1–66.
- Letter to President Donald J. Trump. October 6, 2017. https://www.aafp.org/dam/AAFP/documents/advocacy/coverage/aca/LT-Group6-President-ContraceptionIFRs-100617.pdf. Accessed June 26, 2019.
The Affordable Care Act (ACA) was enacted on March 23, 2010. Controversies, complaints, and detractors have and continue to abound. But the ACA’s landmark women’s health gains are unmistakable. Contraceptive coverage, maternity coverage, Medicaid coverage of low-income women, coverage for individuals with preexisting conditions, and gender-neutral premiums are now a part of the fabric of our society. For most.
Many physicians and patients—many lawmakers, too—do not remember the serious problems people had with their insurance companies before the ACA. Maternity coverage was usually a free-standing rider to an insurance policy, making it very expensive. Insurance plans did not have to, and often did not, cover contraceptives, and none did without copays or deductibles. Women were routinely denied coverage if they had ever had a cesarean delivery, had once been the victim of domestic violence, or had any one of many common conditions, like diabetes. The many exclusionary conditions are so common, in fact, that one study estimated that around 52 million adults in the United States (27% of those younger than age 65 years) have preexisting conditions that would potentially make them uninsurable without the ACA’s protections.1
Before the ACA, it also was common for women with insurance policies to find their coverage rescinded, often with no explanation, even though they paid their premiums every month. And women with serious medical conditions often saw their coverage ended midway through their course of treatment. That placed their ObGyns in a terrible situation, too.
The insurance industry as a whole was running rough-shod over its customers, and making a lot of money by creatively and routinely denying coverage and payment for care. People were often insured, but not covered. The ACA halted many of these practices, and required insurers to meet high medical loss ratios, guaranteeing that 80% of the premiums’ for individual and small market insurers (and 85% for large insurers) are returned to patients in care payments or even in checks. In fact, nearly $4 billion in premiums have been rebated to insured individuals over the last 7 years under the ACA.2
The commitment of the American College of Obstetricians and Gynecologists (ACOG) to women’s health and to our members’ ability to provide the best care has centered on preserving the critical gains of the ACA for women, improving them when we can, and making sure politicians don’t turn back the clock on women’s health. We have been busy.
In this article, we will look at what has happened to these landmark gains and promises of improved women’s health, specifically preexisting condition protections and contraceptive coverage, under a new Administration. What happens when good health care policy and political enmity collide?

Preexisting coverage protections
The 1996 Health Insurance Portability and Accountability Act (HIPAA) defines a preexisting condition exclusionas a “limitation or exclusion of benefits relating to a condition based on the fact that the condition was present before the date of enrollment for the coverage, whether or not any medical advice, diagnosis, care, or treatment was recommended or received before that date.” HIPPA prohibited employer-sponsored health plans from discriminating against individuals through denying them coverage or charging them more based on their or their family members’ health problems. The ACA expanded protections to prohibit the insurance practice of denying coverage altogether to an individual with a preexisting condition.3
Continue to: Under Congress...
Under Congress
Republicans held the majority in both chambers of the 115th Congress (2017–2018), and hoped to use their majority status to get an ACA repeal bill to the Republican President’s desk for speedy enactment. It was not easy, and they were not successful. Four major bills—the American Health Care Act, the Better Care Reconciliation Act, the Health Care Freedom Act, and the Graham-Cassidy Amendment—never made it over the finish line, with some not even making it to a vote. The Health Care Freedom Act was voted down in the Senate 51-49 when Senator John McCain came back from brain surgery to cast his famous thumbs-down vote.4 These bills all would have repealed or hobbled guaranteed issue, community rating, and essential health benefits of the ACA. Of all the legislative attempts to undermine the ACA, only the 2017 Tax Cuts and Jobs Act (TCJA) was signed into law, repealing the ACA individual mandate.
Handling by the courts
The TCJA gave ACA opponents their opening in court. Twenty Republican state attorneys general and governors brought suit in February 2018 (Texas v Azar), arguing that because the ACA relies on the mandate, and the mandate has been repealed, the rest of the ACA also should be struck down. A federal district judge agreed, on December 15, 2018, declaring the entire ACA unconstitutional.5
That decision has been limited in its practical effect so far, and maybe it was not altogether unexpected. What was unexpected was that the US Department of Justice (DOJ) refused to defend a federal law, in this case, the ACA. In June 2018, the DOJ declined to defend the individual mandate, as well as guaranteed issue, community rating, the ban on preexisting condition exclusions, and discrimination based on health status in the ACA. The DOJ at that time, however, did not agree with the plaintiffs that without the mandate the entire ACA should be struck down. It said, “There is no reason why the ACA’s particular expansion of Medicaid hinges on the individual mandate.” Later, after the December 15 ruling, the DOJ changed its position and agreed with the judge, in a two-sentence letter to the court, that the ACA should be stricken altogether—shortly after which 3 career DOJ attorneys resigned.6
A legal expert observed: “The DOJ’s decision not to defend the ACA breaks with the Department’s long-standing bipartisan commitment to defend federal laws if reasonable arguments can be made in their defense. Decisions not to defend federal law are exceedingly rare. It seems even rarer to change the government’s position mid-appeal in such a high-profile lawsuit that risks disrupting the entire health care system and health insurance coverage for millions of Americans.”7
Regulatory tactics
What a policy maker cannot do by law, he or she can try to accomplish by regulation. The Administration is using 3 regulatory routes to undercut the ACA preexisting coverage protections and market stability.
Route 1: Short-Term Limited Duration (STLD) plans. These plans were created in the ACA to provide bridge coverage for up to 3 months for individuals in between health insurance plans. These plans do not have to comply with ACA patient protections, can deny coverage for preexisting conditions, and do not cover maternity care. In 2018, the Administration moved to allow these plans to be marketed broadly and renewed for up to 3 years. Because these plans provide less coverage and often come with high deductibles, they can be marketed with lower premiums, skimming off healthier younger people who do not expect to need much care, as well as lower-income families. This destabilizes the market and leaves people insured but not covered, exactly the situation before the ACA. Seven public health and medical groups sued to challenge the Administration’s STLD regulation; the lawsuit is presently pending.
Continue to: Route 2: Association Health Plans (AHPs)...
Route 2: Association Health Plans (AHPs). The Administration also has allowed the sale of AHPs, marketed to small employers and self-employed individuals. These plans also do not have to comply with ACA consumer protections. They often do not cover maternity care or other essential benefits, and can charge women higher premiums for the same insurance. This regulation, too, resulted in litigation and a federal judge enjoined the rule, but the case is now on appeal.
Route 3: ACA Section 1332 waivers. These waivers were created in the ACA to encourage state innovation to increase access to health coverage, under certain guardrails: states must ensure coverage is at least as comprehensive as the Essential Health Benefits; cost sharing protections must be at least as affordable as under the ACA; the plan must cover at least a comparable number of its residents; and the plan must not increase the federal deficit.
The Adminstration has come under fire for approving 1332 waiver plans that do not meet these guardrails, and allow insurers to exclude coverage for individuals with preexisting conditions, as well as skirt other important ACA patient protections. In response, Seema Verma, Administrator of the Centers for Medicare & Medicaid Services, promised as recently as April 23, that the Administration will not allow any weakening of the ACA preexisting coverage guarantee.8 So far, however, we do not know what action this means, and not surprisingly, House Democrats, now in the majority, are waiting to see those assurances come true. Consistent polling shows that a large majority of Americans, across political parties, think preexisting coverage protections are very important.9
Already, the House passed HR986, to repeal the Administration’s changes to the 1332 waiver rules. The bill won only 4 Republican votes in the House and now waits a Senate vote.
The House is ready to vote on HR1010, which returns the STLD rules to the original ACA version. The Congressional Budget Office has determined that this bill will reduce the federal deficit by $8.9 billion over 10 years, in part by reestablishing a large risk pool. Lower ACA premiums would mean lower federal subsidies and small federal outlays.
Contraceptive coverage
Since 2012, the ACA has required non-grandfathered individual and group health plans to cover, with no copays or deductibles, women’s preventive services, as determined by the Health Resources and Services Administration (HRSA). HRSA asked the National Academy of Medicine (the Institute of Medicine [IOM] at the time) to develop these coverage guidelines based on clinical and scientific relevance. The IOM relied heavily on ACOG’s testimony and women’s health guidelines. The guidelines are updated every 5 years, based on extensive review by the Women’s Preventive Services Initiative, led by ACOG. By law and regulation, covered services include:
- well-woman visits
- contraceptive methods and counseling, including all methods approved for women by the FDA
- breast and cervical cancer screening
- counseling for sexually transmitted infections
- counseling and screening for HIV
- screening for gestational diabetes
- breastfeeding support, supplies, and counseling
- screening and counseling for interpersonal and domestic violence.
Continue to: The previous administration offered a narrow exemption...
The previous administration offered a narrow exemption—an accommodation—for churches, religious orders, and integrated auxiliaries (organizations with financial support primarily from churches). That accommodation was expanded in the Supreme Court’s decision in Hobby Lobby, for closely held for-profit organizations that had religious objections to covering some or all contraceptives. Under the accommodation, the entity’s insurer or third-party administrator was responsible for providing contraceptive services to the entity’s plan participants and beneficiaries.
In October 2017, the Trump administration acted to greatly expand the ability of any employer, college or university, individual, or insurer to opt out of the ACA’s contraceptive coverage requirement. You will read more about this later.
ACOG’s business case for contraception
Early in the Trump Administration, the White House released a statement saying, “Ensuring affordable, accessible, and quality healthcare is critical to improving women’s health and ensuring that it fits their priorities at any stage of life.”10 ACOG could not agree more, and we encouraged the President to accomplish this important goal by protecting the landmark women’s health gains of the ACA. Our call to the President and the US Congress was: “Don’t turn back the clock on women’s health.”
We made a business case for continued contraceptive coverage:
Contraception reduces unintended pregnancies and saves federal dollars.
- Approximately 45% of US pregnancies are unintended.11
- No-copay coverage of contraception has contributed to a dramatic decline in the unintended pregnancy rate in the United States, now at a 30-year low.12
- When cost is not a barrier, women choose more effective forms of contraception, such as intrauterine devices and implants.13
- Unintended pregnancies cost approximately $12.5 billion in government expenditures in 2008.14
- Private health plans spend as much as $4.6 billion annually in costs related to unintended pregnancies.15
Contraception means healthier women and healthier families.
- Under the ACA, the uninsured rate among women ages 18 to 64 almost halved, decreasing from 19.3% to 10.8%.16
- More than 55 million women gained access to preventive services, including contraception, without a copay or a deductible.16
- Women with unintended pregnancies are more likely to delay prenatal care. Infants are at greater risk of birth defects, low birth weight, and poor mental and physical functioning in early childhood.17
Increased access to contraception helps families and improves economic security.
- Women saved $1.4 billion in out-of-pocket costs for contraception in 1 year.18
- Before the ACA, women were spending between 30% and 44% of their total out-of-pocket health costs just on birth control.19
- The ability to plan a pregnancy increases engagement of women in the workforce and improves economic stability for women and their families.20
Administration expands religious exemptions to contraception coverage
Still, on October 6, 2017, the Trump Administration moved to curtail women’s access to and coverage of contraception with the Religious Exemptions and Accommodations for Coverage of Certain Preventive Services under the Affordable Care Act and Moral Exemptions and Accommodations for Coverage of Certain Preventive Services Under the Affordable Care Act. In November 2018, the Administration published a revised rule, to take effect in January 2019.21 The rule immediately was taken to court by more than a dozen states and, 1 month later, was subject to an injunction by the US Court of Appeals for the Ninth Circuit, blocking the rules from going into effect in those states.
Continue to: The rule vastly expands the Obama Administration’s religious accommodation...
The rule vastly expands the Obama Administration’s religious accommodation to include “nonprofit organizations, small businesses, and individuals that have nonreligious moral convictions opposing services covered by the contraceptive mandate.” The covered entities include21:
- churches, integrated auxiliaries, and religious orders with religious objections
- nonprofit organizations with religious or moral objections
- for-profit entities that are not publicly traded, with religious or moral objections
- for-profit entities that are publicly traded, with religious objections
- other nongovernmental employers with religious objections
- nongovernmental institutions of higher education with religious or moral objections
- individuals with religious or moral objections, with employer sponsored or individual market coverage, where the plan sponsor and/or issuer (as applicable) are willing to offer them a plan omitting contraceptive coverage to which they object
- issuers with religious or moral objections, to the extent they provide coverage to a plan sponsor or individual that is also exempt.
The Administration says women losing coverage can get contraceptives through Title X clinics or other government programs. Of course, many women losing coverage are employed, and earn above the low income (100% of the federal poverty level) eligibility requirement for Title X assistance. To address that, the Administration, through its proposed Title X regulations, broadens the definition of “low income” in that program to include women who lose their contraceptive coverage through the employer-base health insurance plan. This move further limits the ability of the Title X program to adequately care for already-qualified individuals.
The Administration’s rule also relied on major inaccuracies, which ACOG corrected.22 First, ACOG pointed out that, in fact, FDA-approved contraceptive methods are not abortifacients, countering the Administration’s contention that contraception is an abortifacient, and that contraceptives cause abortions or miscarriages. Every FDA-approved contraceptive acts before implantation, does not interfere with a pregnancy, and is not effective after a fertilized egg has implanted successfully in the uterus.23 No credible research supports the false statement that birth control causes miscarriages.24
Second, ACOG offered data proving that increased access to contraception is not associated with increased unsafe sexual behavior or increased sexual activity.25,26 The facts are that:
- The percentage of teens who are having sex has declined significantly, by 14% for female and 22% for male teenagers, over the past 25 years.27
- More women are using contraception the first time they have sex. Young women who do not use birth control at first sexual intercourse are twice as likely to become teen mothers.28
- Increased access to and use of contraception has contributed to a dramatic decline in rates of adolescent pregnancy.29
- School-based health centers that provide access to contraceptives are proven to increase use of contraceptives by already sexually active students, not to increase onset of sexual activity.30,31
Third, ACOG made clear the benefits to women’s health from contraception. ACOG asserted: As with any medication, certain types of contraception may be contraindicated for patients with certain medical conditions, including high blood pressure, lupus, or a history of breast cancer.32,33 For these and many other reasons, access to the full range of FDA-approved contraception, with no cost sharing or other barriers, is critical to women’s health. Regarding VTE, the risk among oral contraceptive users is very low. In fact, it is much lower than the risk of VTE during pregnancy or in the immediate postpartum period.34
Continue to: Regarding breast cancer: there is no proven increased risk...
Regarding breast cancer: there is no proven increased risk of breast cancer among contraceptive users, particularly among those younger than age 40. For women older than 40, health care providers must consider both the risks of becoming pregnant at advanced reproductive age and the risks of continuing contraception use until menopause.35
ACOG has 2 clear messages for politicians
ACOG has remained steadfast in its opposition to the Administration’s proposals to block access to contraception. ACOG expressed its strong opposition to political interference in medical care, saying “Every woman, regardless of her insurer, employer, state of residence, or income, should have affordable, seamless access to the right form of contraception for her, free from interference from her employer or politicians.”22
ACOG’s voice has been joined by 5 other major medical associations—American Academy of Family Physicians, American Academy of Pediatrics, American Psychiatric Association, American Academy of Pediatrics, and American Osteopathic Association—together representing more than 560,000 physicians and medical students, in urging the Administration to immediately withdraw its proposals. This broad coalition unequivocally stated36:
Contraception is an integral part of preventive care and a medical necessity for women during approximately 30 years of their lives. Access to no-copay contraception leads to healthier women and families. Changes to our healthcare system come with very high stakes – impacting tens of millions of our patients. Access to contraception allows women to achieve, lead and reach their full potentials, becoming key drivers of our Nation’s economic success. These rules would create a new standard whereby employers can deny their employees coverage, based on their own moral objections. This interferes in the personal health care decisions of our patients, and inappropriately inserts a patient’s employer into the physician-patient relationship. In addition, these rules open the door to moral exemptions for other essential health care, including vaccinations.
These are challenging days for women’s health policy and legislation federally, and in many states. ACOG has two clear messages for politicians: Don’t turn back the clock on women’s health, and stay out of our exam rooms.
The Affordable Care Act (ACA) was enacted on March 23, 2010. Controversies, complaints, and detractors have and continue to abound. But the ACA’s landmark women’s health gains are unmistakable. Contraceptive coverage, maternity coverage, Medicaid coverage of low-income women, coverage for individuals with preexisting conditions, and gender-neutral premiums are now a part of the fabric of our society. For most.
Many physicians and patients—many lawmakers, too—do not remember the serious problems people had with their insurance companies before the ACA. Maternity coverage was usually a free-standing rider to an insurance policy, making it very expensive. Insurance plans did not have to, and often did not, cover contraceptives, and none did without copays or deductibles. Women were routinely denied coverage if they had ever had a cesarean delivery, had once been the victim of domestic violence, or had any one of many common conditions, like diabetes. The many exclusionary conditions are so common, in fact, that one study estimated that around 52 million adults in the United States (27% of those younger than age 65 years) have preexisting conditions that would potentially make them uninsurable without the ACA’s protections.1
Before the ACA, it also was common for women with insurance policies to find their coverage rescinded, often with no explanation, even though they paid their premiums every month. And women with serious medical conditions often saw their coverage ended midway through their course of treatment. That placed their ObGyns in a terrible situation, too.
The insurance industry as a whole was running rough-shod over its customers, and making a lot of money by creatively and routinely denying coverage and payment for care. People were often insured, but not covered. The ACA halted many of these practices, and required insurers to meet high medical loss ratios, guaranteeing that 80% of the premiums’ for individual and small market insurers (and 85% for large insurers) are returned to patients in care payments or even in checks. In fact, nearly $4 billion in premiums have been rebated to insured individuals over the last 7 years under the ACA.2
The commitment of the American College of Obstetricians and Gynecologists (ACOG) to women’s health and to our members’ ability to provide the best care has centered on preserving the critical gains of the ACA for women, improving them when we can, and making sure politicians don’t turn back the clock on women’s health. We have been busy.
In this article, we will look at what has happened to these landmark gains and promises of improved women’s health, specifically preexisting condition protections and contraceptive coverage, under a new Administration. What happens when good health care policy and political enmity collide?

Preexisting coverage protections
The 1996 Health Insurance Portability and Accountability Act (HIPAA) defines a preexisting condition exclusionas a “limitation or exclusion of benefits relating to a condition based on the fact that the condition was present before the date of enrollment for the coverage, whether or not any medical advice, diagnosis, care, or treatment was recommended or received before that date.” HIPPA prohibited employer-sponsored health plans from discriminating against individuals through denying them coverage or charging them more based on their or their family members’ health problems. The ACA expanded protections to prohibit the insurance practice of denying coverage altogether to an individual with a preexisting condition.3
Continue to: Under Congress...
Under Congress
Republicans held the majority in both chambers of the 115th Congress (2017–2018), and hoped to use their majority status to get an ACA repeal bill to the Republican President’s desk for speedy enactment. It was not easy, and they were not successful. Four major bills—the American Health Care Act, the Better Care Reconciliation Act, the Health Care Freedom Act, and the Graham-Cassidy Amendment—never made it over the finish line, with some not even making it to a vote. The Health Care Freedom Act was voted down in the Senate 51-49 when Senator John McCain came back from brain surgery to cast his famous thumbs-down vote.4 These bills all would have repealed or hobbled guaranteed issue, community rating, and essential health benefits of the ACA. Of all the legislative attempts to undermine the ACA, only the 2017 Tax Cuts and Jobs Act (TCJA) was signed into law, repealing the ACA individual mandate.
Handling by the courts
The TCJA gave ACA opponents their opening in court. Twenty Republican state attorneys general and governors brought suit in February 2018 (Texas v Azar), arguing that because the ACA relies on the mandate, and the mandate has been repealed, the rest of the ACA also should be struck down. A federal district judge agreed, on December 15, 2018, declaring the entire ACA unconstitutional.5
That decision has been limited in its practical effect so far, and maybe it was not altogether unexpected. What was unexpected was that the US Department of Justice (DOJ) refused to defend a federal law, in this case, the ACA. In June 2018, the DOJ declined to defend the individual mandate, as well as guaranteed issue, community rating, the ban on preexisting condition exclusions, and discrimination based on health status in the ACA. The DOJ at that time, however, did not agree with the plaintiffs that without the mandate the entire ACA should be struck down. It said, “There is no reason why the ACA’s particular expansion of Medicaid hinges on the individual mandate.” Later, after the December 15 ruling, the DOJ changed its position and agreed with the judge, in a two-sentence letter to the court, that the ACA should be stricken altogether—shortly after which 3 career DOJ attorneys resigned.6
A legal expert observed: “The DOJ’s decision not to defend the ACA breaks with the Department’s long-standing bipartisan commitment to defend federal laws if reasonable arguments can be made in their defense. Decisions not to defend federal law are exceedingly rare. It seems even rarer to change the government’s position mid-appeal in such a high-profile lawsuit that risks disrupting the entire health care system and health insurance coverage for millions of Americans.”7
Regulatory tactics
What a policy maker cannot do by law, he or she can try to accomplish by regulation. The Administration is using 3 regulatory routes to undercut the ACA preexisting coverage protections and market stability.
Route 1: Short-Term Limited Duration (STLD) plans. These plans were created in the ACA to provide bridge coverage for up to 3 months for individuals in between health insurance plans. These plans do not have to comply with ACA patient protections, can deny coverage for preexisting conditions, and do not cover maternity care. In 2018, the Administration moved to allow these plans to be marketed broadly and renewed for up to 3 years. Because these plans provide less coverage and often come with high deductibles, they can be marketed with lower premiums, skimming off healthier younger people who do not expect to need much care, as well as lower-income families. This destabilizes the market and leaves people insured but not covered, exactly the situation before the ACA. Seven public health and medical groups sued to challenge the Administration’s STLD regulation; the lawsuit is presently pending.
Continue to: Route 2: Association Health Plans (AHPs)...
Route 2: Association Health Plans (AHPs). The Administration also has allowed the sale of AHPs, marketed to small employers and self-employed individuals. These plans also do not have to comply with ACA consumer protections. They often do not cover maternity care or other essential benefits, and can charge women higher premiums for the same insurance. This regulation, too, resulted in litigation and a federal judge enjoined the rule, but the case is now on appeal.
Route 3: ACA Section 1332 waivers. These waivers were created in the ACA to encourage state innovation to increase access to health coverage, under certain guardrails: states must ensure coverage is at least as comprehensive as the Essential Health Benefits; cost sharing protections must be at least as affordable as under the ACA; the plan must cover at least a comparable number of its residents; and the plan must not increase the federal deficit.
The Adminstration has come under fire for approving 1332 waiver plans that do not meet these guardrails, and allow insurers to exclude coverage for individuals with preexisting conditions, as well as skirt other important ACA patient protections. In response, Seema Verma, Administrator of the Centers for Medicare & Medicaid Services, promised as recently as April 23, that the Administration will not allow any weakening of the ACA preexisting coverage guarantee.8 So far, however, we do not know what action this means, and not surprisingly, House Democrats, now in the majority, are waiting to see those assurances come true. Consistent polling shows that a large majority of Americans, across political parties, think preexisting coverage protections are very important.9
Already, the House passed HR986, to repeal the Administration’s changes to the 1332 waiver rules. The bill won only 4 Republican votes in the House and now waits a Senate vote.
The House is ready to vote on HR1010, which returns the STLD rules to the original ACA version. The Congressional Budget Office has determined that this bill will reduce the federal deficit by $8.9 billion over 10 years, in part by reestablishing a large risk pool. Lower ACA premiums would mean lower federal subsidies and small federal outlays.
Contraceptive coverage
Since 2012, the ACA has required non-grandfathered individual and group health plans to cover, with no copays or deductibles, women’s preventive services, as determined by the Health Resources and Services Administration (HRSA). HRSA asked the National Academy of Medicine (the Institute of Medicine [IOM] at the time) to develop these coverage guidelines based on clinical and scientific relevance. The IOM relied heavily on ACOG’s testimony and women’s health guidelines. The guidelines are updated every 5 years, based on extensive review by the Women’s Preventive Services Initiative, led by ACOG. By law and regulation, covered services include:
- well-woman visits
- contraceptive methods and counseling, including all methods approved for women by the FDA
- breast and cervical cancer screening
- counseling for sexually transmitted infections
- counseling and screening for HIV
- screening for gestational diabetes
- breastfeeding support, supplies, and counseling
- screening and counseling for interpersonal and domestic violence.
Continue to: The previous administration offered a narrow exemption...
The previous administration offered a narrow exemption—an accommodation—for churches, religious orders, and integrated auxiliaries (organizations with financial support primarily from churches). That accommodation was expanded in the Supreme Court’s decision in Hobby Lobby, for closely held for-profit organizations that had religious objections to covering some or all contraceptives. Under the accommodation, the entity’s insurer or third-party administrator was responsible for providing contraceptive services to the entity’s plan participants and beneficiaries.
In October 2017, the Trump administration acted to greatly expand the ability of any employer, college or university, individual, or insurer to opt out of the ACA’s contraceptive coverage requirement. You will read more about this later.
ACOG’s business case for contraception
Early in the Trump Administration, the White House released a statement saying, “Ensuring affordable, accessible, and quality healthcare is critical to improving women’s health and ensuring that it fits their priorities at any stage of life.”10 ACOG could not agree more, and we encouraged the President to accomplish this important goal by protecting the landmark women’s health gains of the ACA. Our call to the President and the US Congress was: “Don’t turn back the clock on women’s health.”
We made a business case for continued contraceptive coverage:
Contraception reduces unintended pregnancies and saves federal dollars.
- Approximately 45% of US pregnancies are unintended.11
- No-copay coverage of contraception has contributed to a dramatic decline in the unintended pregnancy rate in the United States, now at a 30-year low.12
- When cost is not a barrier, women choose more effective forms of contraception, such as intrauterine devices and implants.13
- Unintended pregnancies cost approximately $12.5 billion in government expenditures in 2008.14
- Private health plans spend as much as $4.6 billion annually in costs related to unintended pregnancies.15
Contraception means healthier women and healthier families.
- Under the ACA, the uninsured rate among women ages 18 to 64 almost halved, decreasing from 19.3% to 10.8%.16
- More than 55 million women gained access to preventive services, including contraception, without a copay or a deductible.16
- Women with unintended pregnancies are more likely to delay prenatal care. Infants are at greater risk of birth defects, low birth weight, and poor mental and physical functioning in early childhood.17
Increased access to contraception helps families and improves economic security.
- Women saved $1.4 billion in out-of-pocket costs for contraception in 1 year.18
- Before the ACA, women were spending between 30% and 44% of their total out-of-pocket health costs just on birth control.19
- The ability to plan a pregnancy increases engagement of women in the workforce and improves economic stability for women and their families.20
Administration expands religious exemptions to contraception coverage
Still, on October 6, 2017, the Trump Administration moved to curtail women’s access to and coverage of contraception with the Religious Exemptions and Accommodations for Coverage of Certain Preventive Services under the Affordable Care Act and Moral Exemptions and Accommodations for Coverage of Certain Preventive Services Under the Affordable Care Act. In November 2018, the Administration published a revised rule, to take effect in January 2019.21 The rule immediately was taken to court by more than a dozen states and, 1 month later, was subject to an injunction by the US Court of Appeals for the Ninth Circuit, blocking the rules from going into effect in those states.
Continue to: The rule vastly expands the Obama Administration’s religious accommodation...
The rule vastly expands the Obama Administration’s religious accommodation to include “nonprofit organizations, small businesses, and individuals that have nonreligious moral convictions opposing services covered by the contraceptive mandate.” The covered entities include21:
- churches, integrated auxiliaries, and religious orders with religious objections
- nonprofit organizations with religious or moral objections
- for-profit entities that are not publicly traded, with religious or moral objections
- for-profit entities that are publicly traded, with religious objections
- other nongovernmental employers with religious objections
- nongovernmental institutions of higher education with religious or moral objections
- individuals with religious or moral objections, with employer sponsored or individual market coverage, where the plan sponsor and/or issuer (as applicable) are willing to offer them a plan omitting contraceptive coverage to which they object
- issuers with religious or moral objections, to the extent they provide coverage to a plan sponsor or individual that is also exempt.
The Administration says women losing coverage can get contraceptives through Title X clinics or other government programs. Of course, many women losing coverage are employed, and earn above the low income (100% of the federal poverty level) eligibility requirement for Title X assistance. To address that, the Administration, through its proposed Title X regulations, broadens the definition of “low income” in that program to include women who lose their contraceptive coverage through the employer-base health insurance plan. This move further limits the ability of the Title X program to adequately care for already-qualified individuals.
The Administration’s rule also relied on major inaccuracies, which ACOG corrected.22 First, ACOG pointed out that, in fact, FDA-approved contraceptive methods are not abortifacients, countering the Administration’s contention that contraception is an abortifacient, and that contraceptives cause abortions or miscarriages. Every FDA-approved contraceptive acts before implantation, does not interfere with a pregnancy, and is not effective after a fertilized egg has implanted successfully in the uterus.23 No credible research supports the false statement that birth control causes miscarriages.24
Second, ACOG offered data proving that increased access to contraception is not associated with increased unsafe sexual behavior or increased sexual activity.25,26 The facts are that:
- The percentage of teens who are having sex has declined significantly, by 14% for female and 22% for male teenagers, over the past 25 years.27
- More women are using contraception the first time they have sex. Young women who do not use birth control at first sexual intercourse are twice as likely to become teen mothers.28
- Increased access to and use of contraception has contributed to a dramatic decline in rates of adolescent pregnancy.29
- School-based health centers that provide access to contraceptives are proven to increase use of contraceptives by already sexually active students, not to increase onset of sexual activity.30,31
Third, ACOG made clear the benefits to women’s health from contraception. ACOG asserted: As with any medication, certain types of contraception may be contraindicated for patients with certain medical conditions, including high blood pressure, lupus, or a history of breast cancer.32,33 For these and many other reasons, access to the full range of FDA-approved contraception, with no cost sharing or other barriers, is critical to women’s health. Regarding VTE, the risk among oral contraceptive users is very low. In fact, it is much lower than the risk of VTE during pregnancy or in the immediate postpartum period.34
Continue to: Regarding breast cancer: there is no proven increased risk...
Regarding breast cancer: there is no proven increased risk of breast cancer among contraceptive users, particularly among those younger than age 40. For women older than 40, health care providers must consider both the risks of becoming pregnant at advanced reproductive age and the risks of continuing contraception use until menopause.35
ACOG has 2 clear messages for politicians
ACOG has remained steadfast in its opposition to the Administration’s proposals to block access to contraception. ACOG expressed its strong opposition to political interference in medical care, saying “Every woman, regardless of her insurer, employer, state of residence, or income, should have affordable, seamless access to the right form of contraception for her, free from interference from her employer or politicians.”22
ACOG’s voice has been joined by 5 other major medical associations—American Academy of Family Physicians, American Academy of Pediatrics, American Psychiatric Association, American Academy of Pediatrics, and American Osteopathic Association—together representing more than 560,000 physicians and medical students, in urging the Administration to immediately withdraw its proposals. This broad coalition unequivocally stated36:
Contraception is an integral part of preventive care and a medical necessity for women during approximately 30 years of their lives. Access to no-copay contraception leads to healthier women and families. Changes to our healthcare system come with very high stakes – impacting tens of millions of our patients. Access to contraception allows women to achieve, lead and reach their full potentials, becoming key drivers of our Nation’s economic success. These rules would create a new standard whereby employers can deny their employees coverage, based on their own moral objections. This interferes in the personal health care decisions of our patients, and inappropriately inserts a patient’s employer into the physician-patient relationship. In addition, these rules open the door to moral exemptions for other essential health care, including vaccinations.
These are challenging days for women’s health policy and legislation federally, and in many states. ACOG has two clear messages for politicians: Don’t turn back the clock on women’s health, and stay out of our exam rooms.
- Claxton G, Cox C, Damico A, et al. Pre-existing conditions and medical underwriting in the individual insurance market prior to the ACA. Kaiser Family Foundation website. Published December 12, 2016. Accessed June 25, 2019.
- Norris L. Billions in ACA rebates show 80/20 rule’s impact. HealthInsurance.org website. Published May 10, 2019. Accessed June 25, 2019.
- Patient Protection and Affordable Care Act: Preexisting condition exclusions, lifetime and annual limits, rescissions, and patient protections. Regulations.gov website. Accessed June 25, 2019.
- Jost T. The Senate’s Health Care Freedom Act. Health Affairs website. Updated July 28, 2017. Accessed June 25, 2019.
- Texas v Azar decision. American Medical Association website. Accessed June 25, 2019.
- Keith K. DOJ, plaintiffs file in Texas v United States. Health Affairs website. Published May 2 2019. Accessed June 25, 2019.
- John & Rusty Report. Trump Administration asks court to strike down entire ACA. March 26, 2019. https://jrreport.wordandbrown.com/2019/03/26/trump-administration-asks-court-to-strike-down-entire-aca/. Accessed June 29, 2019.
- Speech: Remarks by Administrator Seema Verma at the CMS National Forum on State Relief and Empowerment Waivers. Centers for Medicare & Medicaid website. Published April 23, 2019. Accessed June 25, 2019.
- Poll: The ACA’s pre-existing condition protections remain popular with the public, including republicans, as legal challenge looms this week. Kaiser Family Foundation website. Published September 5, 2018. Accessed June 25, 2019.
- Statement from President Donald J. Trump on Women’s Health Week. White House website. Issued May 14, 2017. Accessed June 26, 2019.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374:843-852.
- Insurance coverage of contraception. Guttmacher Institute website. Published August 2018. Accessed June 26, 2019.
- Carlin CS, Fertig AR, Dowd BE. Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs. 2016;35:1608-1615.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250–255.
- Canestaro W, et al. Implications of employer coverage of contraception: cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception. 2017;95:77-89.
- Simmons A, et al. The Affordable Care Act: Promoting better health for women. Office of the Assistant Secretary for Planning and Evaluation Issue Brief, Department of Health and Human Services. June 14, 2016. Accessed June 25, 2019.
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34:1204-1211. Accessed June 25, 2019.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34(7).
- Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013.
- Department of Health and Human Services. Fact sheet: Final rules on religious and moral exemptions and accommodation for coverage of certain preventive services under the Affordable Care Act. November 7, 2018. Accessed June 26, 2019.
- American College of Obstetricians and Gynecologists. Facts are important: Correcting the record on the Administration’s contraceptive coverage roll back rule. October 2017. Accessed June 26, 2019.
- Brief for Physicians for Reproductive Health, American College of Obstetricians and Gynecologists et al. as Amici Curiae Supporting Respondents, Sebelius v. Hobby Lobby, 573 U.S. XXX. 2014. (No. 13-354).
- Early pregnancy loss. FAQ No. 90. American College of Obstetricians and Gynecologists. August 2015.
- Kirby D. Emerging answers 2007: Research findings on programs to reduce teen pregnancy and sexually transmitted diseases. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009.
- Meyer JL, Gold MA, Haggerty CL. Advance provision of emergency contraception among adolescent and young adult women: a systematic review of literature. J Pediatr Adolesc Gynecol. 2011;24:2-9.
- Martinez GM and Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15–19 in the United States. NCHS Data Brief, 2015, No. 209. Hyattsville, MD: National Center for Health Statistics; 2015.
- Martinez GM, Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15-19 in the United States. NCHS Data Brief. July 2015. Accessed June 26, 2019.
- Lindberg L, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59:577-583.
- Minguez M, Santelli JS, Gibson E, et al. Reproductive health impact of a school health center. J Adolesc Health. 2015;56:338-344.
- Knopf JA, Finnie RK, Peng Y, et al. Community Preventive Services Task Force. School-based health centers to advance health equity: a Community Guide systematic review. Am J Preventive Med. 2016;51:114-126.
- Progestin-only hormonal birth control: pill and injection. FAQ No. 86. American College of Obstetricians and Gynecologists. July 2014.
- Combined hormonal birth control: pill, patch, and ring. FAQ No. 185. American College of Obstetricians and Gynecologists. July 2014.
- Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Committee Opinion No. 540. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:1239-1242.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1–66.
- Letter to President Donald J. Trump. October 6, 2017. https://www.aafp.org/dam/AAFP/documents/advocacy/coverage/aca/LT-Group6-President-ContraceptionIFRs-100617.pdf. Accessed June 26, 2019.
- Claxton G, Cox C, Damico A, et al. Pre-existing conditions and medical underwriting in the individual insurance market prior to the ACA. Kaiser Family Foundation website. Published December 12, 2016. Accessed June 25, 2019.
- Norris L. Billions in ACA rebates show 80/20 rule’s impact. HealthInsurance.org website. Published May 10, 2019. Accessed June 25, 2019.
- Patient Protection and Affordable Care Act: Preexisting condition exclusions, lifetime and annual limits, rescissions, and patient protections. Regulations.gov website. Accessed June 25, 2019.
- Jost T. The Senate’s Health Care Freedom Act. Health Affairs website. Updated July 28, 2017. Accessed June 25, 2019.
- Texas v Azar decision. American Medical Association website. Accessed June 25, 2019.
- Keith K. DOJ, plaintiffs file in Texas v United States. Health Affairs website. Published May 2 2019. Accessed June 25, 2019.
- John & Rusty Report. Trump Administration asks court to strike down entire ACA. March 26, 2019. https://jrreport.wordandbrown.com/2019/03/26/trump-administration-asks-court-to-strike-down-entire-aca/. Accessed June 29, 2019.
- Speech: Remarks by Administrator Seema Verma at the CMS National Forum on State Relief and Empowerment Waivers. Centers for Medicare & Medicaid website. Published April 23, 2019. Accessed June 25, 2019.
- Poll: The ACA’s pre-existing condition protections remain popular with the public, including republicans, as legal challenge looms this week. Kaiser Family Foundation website. Published September 5, 2018. Accessed June 25, 2019.
- Statement from President Donald J. Trump on Women’s Health Week. White House website. Issued May 14, 2017. Accessed June 26, 2019.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374:843-852.
- Insurance coverage of contraception. Guttmacher Institute website. Published August 2018. Accessed June 26, 2019.
- Carlin CS, Fertig AR, Dowd BE. Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs. 2016;35:1608-1615.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250–255.
- Canestaro W, et al. Implications of employer coverage of contraception: cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception. 2017;95:77-89.
- Simmons A, et al. The Affordable Care Act: Promoting better health for women. Office of the Assistant Secretary for Planning and Evaluation Issue Brief, Department of Health and Human Services. June 14, 2016. Accessed June 25, 2019.
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34:1204-1211. Accessed June 25, 2019.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34(7).
- Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013.
- Department of Health and Human Services. Fact sheet: Final rules on religious and moral exemptions and accommodation for coverage of certain preventive services under the Affordable Care Act. November 7, 2018. Accessed June 26, 2019.
- American College of Obstetricians and Gynecologists. Facts are important: Correcting the record on the Administration’s contraceptive coverage roll back rule. October 2017. Accessed June 26, 2019.
- Brief for Physicians for Reproductive Health, American College of Obstetricians and Gynecologists et al. as Amici Curiae Supporting Respondents, Sebelius v. Hobby Lobby, 573 U.S. XXX. 2014. (No. 13-354).
- Early pregnancy loss. FAQ No. 90. American College of Obstetricians and Gynecologists. August 2015.
- Kirby D. Emerging answers 2007: Research findings on programs to reduce teen pregnancy and sexually transmitted diseases. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009.
- Meyer JL, Gold MA, Haggerty CL. Advance provision of emergency contraception among adolescent and young adult women: a systematic review of literature. J Pediatr Adolesc Gynecol. 2011;24:2-9.
- Martinez GM and Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15–19 in the United States. NCHS Data Brief, 2015, No. 209. Hyattsville, MD: National Center for Health Statistics; 2015.
- Martinez GM, Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15-19 in the United States. NCHS Data Brief. July 2015. Accessed June 26, 2019.
- Lindberg L, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59:577-583.
- Minguez M, Santelli JS, Gibson E, et al. Reproductive health impact of a school health center. J Adolesc Health. 2015;56:338-344.
- Knopf JA, Finnie RK, Peng Y, et al. Community Preventive Services Task Force. School-based health centers to advance health equity: a Community Guide systematic review. Am J Preventive Med. 2016;51:114-126.
- Progestin-only hormonal birth control: pill and injection. FAQ No. 86. American College of Obstetricians and Gynecologists. July 2014.
- Combined hormonal birth control: pill, patch, and ring. FAQ No. 185. American College of Obstetricians and Gynecologists. July 2014.
- Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Committee Opinion No. 540. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:1239-1242.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1–66.
- Letter to President Donald J. Trump. October 6, 2017. https://www.aafp.org/dam/AAFP/documents/advocacy/coverage/aca/LT-Group6-President-ContraceptionIFRs-100617.pdf. Accessed June 26, 2019.
The mesh mess, enmeshed in controversy
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
Opioid prescriptions: 2019 snapshot


Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2019 Update on abnormal uterine bleeding
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.