User login
Sparse Hair on the Scalp
The Diagnosis: Monilethrix
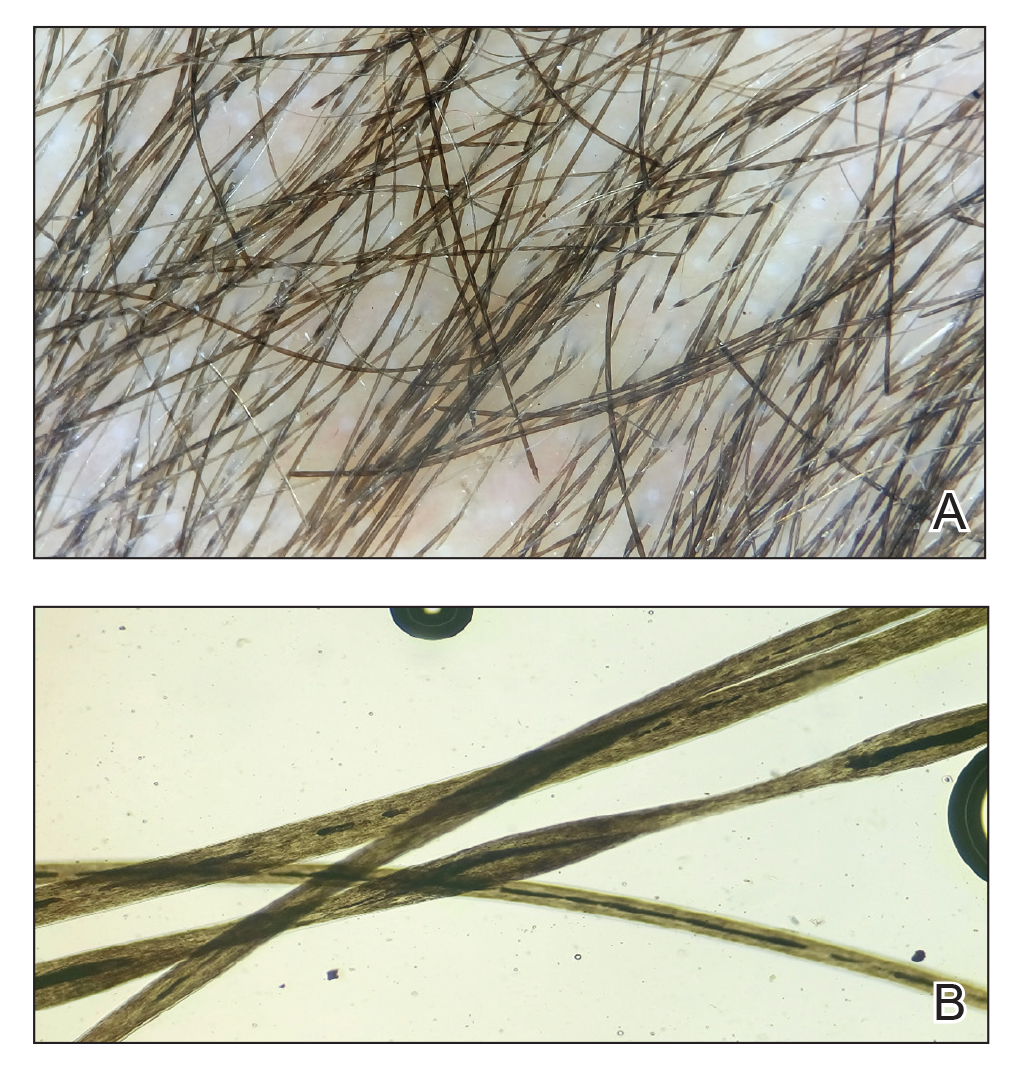
Trichoscopy showed a beaded appearance of the hair shafts (Figure, A). Light microscopy demonstrated normal medullated nodes of hair coupled with internodal, thin, nonmedullated hair at regular intervals (Figure, B). Clinical and trichoscopic findings led to a diagnosis of monilethrix.
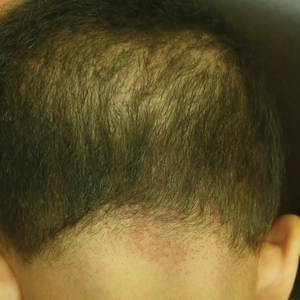
Monilethrix is a genetic hair disorder characterized by regular periodic thinning of the hair shafts, giving the strands a beaded appearance. The hair tends to break at these constricted parts, resulting in short hairs. Nodosities represent the normal hair shaft, whereas the constricted points are the site of the defect. The hair tends to be normal at birth and then becomes short, fragile, and brittle within months, leading to hypotrichosis, particularly on the occipital scalp.1 Monilethrix also may involve the eyebrows and eyelashes in addition to scalp hair. Follicular hyperkeratotic papules with perifollicular erythema frequently are noted on the occipital area. Monilethrix can be inherited in an autosomal-dominant fashion with mutations involving KRT81, KRT83, and KRT86, which code for the type II hair keratins Hb1, Hb3, and Hb6, respectively. The autosomal-recessive form is caused by mutations in the DSG4 gene, coding for the desmoglein 4 protein.2 Trichoscopy or light microscopy is essential to establish a diagnosis of monilethrix. Trichoscopy is an easy and rapid tool that is utilized to illustrate the beaded appearance of the hair shafts.3 Light microscopy shows the distinctive nodes that are medullated, with a normal hair diameter alternating with the internodes, or constrictions, that are nonmedullated and represent the sites of fracture.1 Monilethrix can improve by puberty. There is no definitive treatment; however, some patients show considerable improvement on minoxidil.4 Treatment with minoxidil was initiated in this patient; however, she was lost to follow-up.
Genetic hair disorders are rare and can be an isolated phenomenon or part of concurrent genetic syndromes. Therefore, thorough clinical examination of other ectodermal structures such as the nails and teeth is crucial as well as obtaining a detailed family history and review of systems to exclude other syndromes.2 Hypotrichosis simplex is characterized by hair loss exclusively on the scalp, sparing other ectodermal structures and with no systemic abnormalities. Ectodermal dysplasia is a heterogeneous group of disorders affecting not only the hair but also the teeth, nails, and sweat glands.2 Pili torti is another rare genetic hair disorder that is characterized by twisting of the hair fiber on its own axis. It presents clinically as sparse, depigmented, lusterless hair that is easily broken. Light microscopy demonstrates twists of hair at irregular intervals. Pili annulati is characterized by bright and dark bands when viewed with reflected light. Unlike monilethrix, there is no fragility, and the hair can grow long.5
- Mirmirani P, Huang KP, Price VH. A practical, algorithmic approach to diagnosing hair shaft disorders. Int J Dermatol. 2011;50:1-12.
- Ahmed A, Almohanna H, Griggs J, et al. Genetic hair disorders: a review. Dermatol Ther. 2019;9:421-448.
- Liu C-I, Hsu C-H. Rapid diagnosis of monilethrix using dermoscopy. Br J Dermatol. 2008;159:741-743.
- Rossi A, Iorio A, Fortuna MC, et al. Monilethrix treated with minoxidil. Int J Immunopathol Pharmacol. 2011;24:239-242.
- Singh G, Miteva M. Prognosis and management of congenital hair shaft disorders with fragility—part I. Pediatr Dermatol. 2016;33:473-480.
The Diagnosis: Monilethrix
Trichoscopy showed a beaded appearance of the hair shafts (Figure, A). Light microscopy demonstrated normal medullated nodes of hair coupled with internodal, thin, nonmedullated hair at regular intervals (Figure, B). Clinical and trichoscopic findings led to a diagnosis of monilethrix.

Monilethrix is a genetic hair disorder characterized by regular periodic thinning of the hair shafts, giving the strands a beaded appearance. The hair tends to break at these constricted parts, resulting in short hairs. Nodosities represent the normal hair shaft, whereas the constricted points are the site of the defect. The hair tends to be normal at birth and then becomes short, fragile, and brittle within months, leading to hypotrichosis, particularly on the occipital scalp.1 Monilethrix also may involve the eyebrows and eyelashes in addition to scalp hair. Follicular hyperkeratotic papules with perifollicular erythema frequently are noted on the occipital area. Monilethrix can be inherited in an autosomal-dominant fashion with mutations involving KRT81, KRT83, and KRT86, which code for the type II hair keratins Hb1, Hb3, and Hb6, respectively. The autosomal-recessive form is caused by mutations in the DSG4 gene, coding for the desmoglein 4 protein.2 Trichoscopy or light microscopy is essential to establish a diagnosis of monilethrix. Trichoscopy is an easy and rapid tool that is utilized to illustrate the beaded appearance of the hair shafts.3 Light microscopy shows the distinctive nodes that are medullated, with a normal hair diameter alternating with the internodes, or constrictions, that are nonmedullated and represent the sites of fracture.1 Monilethrix can improve by puberty. There is no definitive treatment; however, some patients show considerable improvement on minoxidil.4 Treatment with minoxidil was initiated in this patient; however, she was lost to follow-up.
Genetic hair disorders are rare and can be an isolated phenomenon or part of concurrent genetic syndromes. Therefore, thorough clinical examination of other ectodermal structures such as the nails and teeth is crucial as well as obtaining a detailed family history and review of systems to exclude other syndromes.2 Hypotrichosis simplex is characterized by hair loss exclusively on the scalp, sparing other ectodermal structures and with no systemic abnormalities. Ectodermal dysplasia is a heterogeneous group of disorders affecting not only the hair but also the teeth, nails, and sweat glands.2 Pili torti is another rare genetic hair disorder that is characterized by twisting of the hair fiber on its own axis. It presents clinically as sparse, depigmented, lusterless hair that is easily broken. Light microscopy demonstrates twists of hair at irregular intervals. Pili annulati is characterized by bright and dark bands when viewed with reflected light. Unlike monilethrix, there is no fragility, and the hair can grow long.5
The Diagnosis: Monilethrix
Trichoscopy showed a beaded appearance of the hair shafts (Figure, A). Light microscopy demonstrated normal medullated nodes of hair coupled with internodal, thin, nonmedullated hair at regular intervals (Figure, B). Clinical and trichoscopic findings led to a diagnosis of monilethrix.

Monilethrix is a genetic hair disorder characterized by regular periodic thinning of the hair shafts, giving the strands a beaded appearance. The hair tends to break at these constricted parts, resulting in short hairs. Nodosities represent the normal hair shaft, whereas the constricted points are the site of the defect. The hair tends to be normal at birth and then becomes short, fragile, and brittle within months, leading to hypotrichosis, particularly on the occipital scalp.1 Monilethrix also may involve the eyebrows and eyelashes in addition to scalp hair. Follicular hyperkeratotic papules with perifollicular erythema frequently are noted on the occipital area. Monilethrix can be inherited in an autosomal-dominant fashion with mutations involving KRT81, KRT83, and KRT86, which code for the type II hair keratins Hb1, Hb3, and Hb6, respectively. The autosomal-recessive form is caused by mutations in the DSG4 gene, coding for the desmoglein 4 protein.2 Trichoscopy or light microscopy is essential to establish a diagnosis of monilethrix. Trichoscopy is an easy and rapid tool that is utilized to illustrate the beaded appearance of the hair shafts.3 Light microscopy shows the distinctive nodes that are medullated, with a normal hair diameter alternating with the internodes, or constrictions, that are nonmedullated and represent the sites of fracture.1 Monilethrix can improve by puberty. There is no definitive treatment; however, some patients show considerable improvement on minoxidil.4 Treatment with minoxidil was initiated in this patient; however, she was lost to follow-up.
Genetic hair disorders are rare and can be an isolated phenomenon or part of concurrent genetic syndromes. Therefore, thorough clinical examination of other ectodermal structures such as the nails and teeth is crucial as well as obtaining a detailed family history and review of systems to exclude other syndromes.2 Hypotrichosis simplex is characterized by hair loss exclusively on the scalp, sparing other ectodermal structures and with no systemic abnormalities. Ectodermal dysplasia is a heterogeneous group of disorders affecting not only the hair but also the teeth, nails, and sweat glands.2 Pili torti is another rare genetic hair disorder that is characterized by twisting of the hair fiber on its own axis. It presents clinically as sparse, depigmented, lusterless hair that is easily broken. Light microscopy demonstrates twists of hair at irregular intervals. Pili annulati is characterized by bright and dark bands when viewed with reflected light. Unlike monilethrix, there is no fragility, and the hair can grow long.5
- Mirmirani P, Huang KP, Price VH. A practical, algorithmic approach to diagnosing hair shaft disorders. Int J Dermatol. 2011;50:1-12.
- Ahmed A, Almohanna H, Griggs J, et al. Genetic hair disorders: a review. Dermatol Ther. 2019;9:421-448.
- Liu C-I, Hsu C-H. Rapid diagnosis of monilethrix using dermoscopy. Br J Dermatol. 2008;159:741-743.
- Rossi A, Iorio A, Fortuna MC, et al. Monilethrix treated with minoxidil. Int J Immunopathol Pharmacol. 2011;24:239-242.
- Singh G, Miteva M. Prognosis and management of congenital hair shaft disorders with fragility—part I. Pediatr Dermatol. 2016;33:473-480.
- Mirmirani P, Huang KP, Price VH. A practical, algorithmic approach to diagnosing hair shaft disorders. Int J Dermatol. 2011;50:1-12.
- Ahmed A, Almohanna H, Griggs J, et al. Genetic hair disorders: a review. Dermatol Ther. 2019;9:421-448.
- Liu C-I, Hsu C-H. Rapid diagnosis of monilethrix using dermoscopy. Br J Dermatol. 2008;159:741-743.
- Rossi A, Iorio A, Fortuna MC, et al. Monilethrix treated with minoxidil. Int J Immunopathol Pharmacol. 2011;24:239-242.
- Singh G, Miteva M. Prognosis and management of congenital hair shaft disorders with fragility—part I. Pediatr Dermatol. 2016;33:473-480.
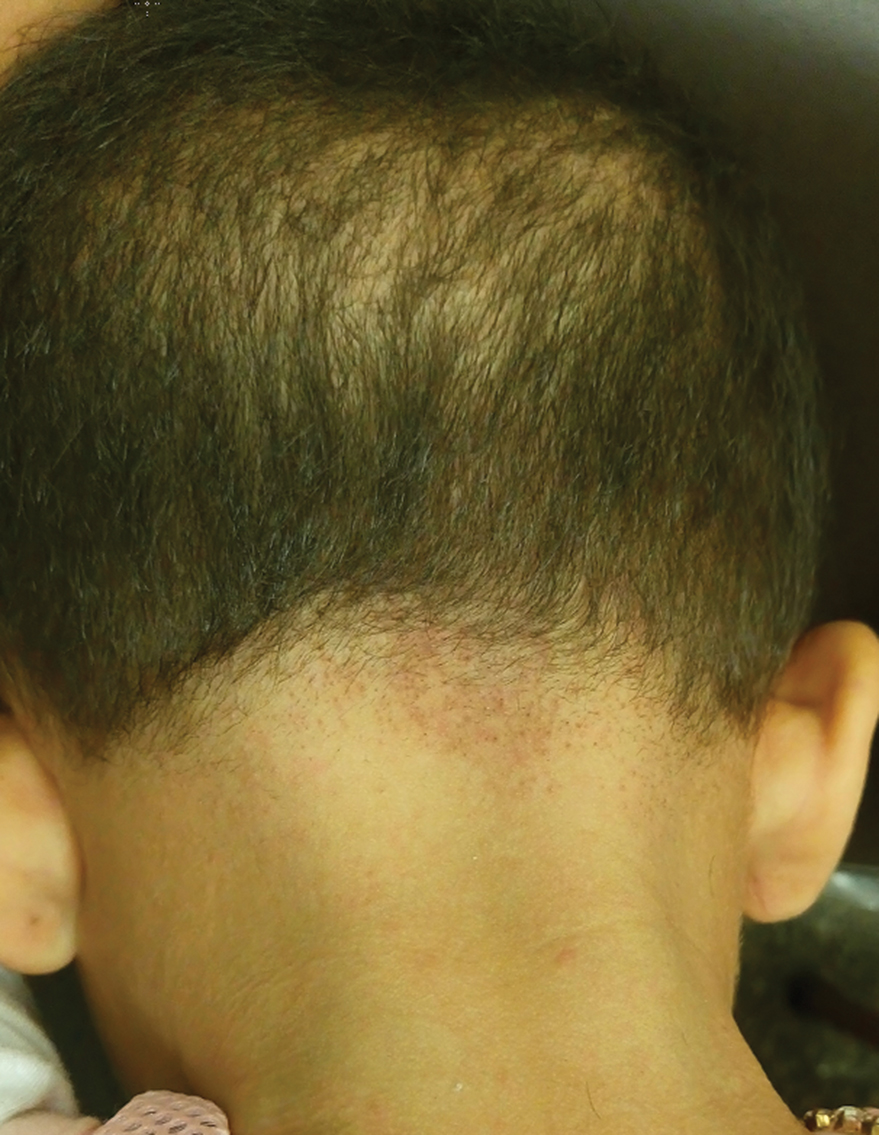
A 5-year-old girl presented to our clinic with sparse scalp hair. Her mother reported thinning of the hair and breakage that appeared shortly after birth. She also reported that the patient’s hair was dull, dry, and unable to be grown long. The patient was otherwise healthy. She was born to nonconsanguineous parents, and her family history was unremarkable. Physical examination revealed dry, brittle, and short hairs. The hair was sparser on the occipital area of the scalp, and multiple keratotic papules were noted in this area. No abnormalities were detected on the teeth or nails, and a review of systems was unremarkable. Trichoscopy and light microscopy were performed.
Red hair in women linked to elevated CRP levels in Nurses’ Health Study
Red-haired women were significantly more likely than were women with nonred hair to have elevated levels of C-reactive protein that may increase risk for cardiovascular conditions, according to data from nearly 9,000 women participating in the Nurses’ Health Study.
“Positive associations between red hair and cardiovascular disease and cancer in women, but not men, have been reported,” wrote Rebecca I. Hartman, MD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
In a study published in the Journal of Investigative Dermatology, they reviewed data from the Nurses’ Health Study, a 1976 cohort study of 121,700 women registered nurses in the United States. They analyzed blood specimens from 8,994 women that were collected between 1989 and 1990. Participants’ natural hair color was determined by asking them their natural hair color at age 21 years, with choices of red, blonde, light brown, dark brown, or black. Overall, dark brown/black hair was the most common color (45%) and 390 of the women (4.3%) had red hair.
The average CRP levels were significantly higher for women with red hair (3.7 mg/L), compared with those with blonde (3.3 mg/L), light brown (3.0 mg/mL), or dark brown/black (3.2 mg/L).
Using the CRP levels for red-haired women as a reference, women with blond, light brown, and dark brown/black hair averaged significantly lower CRP levels than those of red-haired women in an age-adjusted model (–15.2%, –18/1%, and –14.2%, respectively) and in a multivariate analysis (–12.7%, –14.1%, and –10.9%, respectively).
Non-red-haired women had significantly lower odds of high CRP levels compared with red-haired women, with odds ratios of 0.62, 0.60, and 0.67 for women with blonde, light brown, and dark brown/black hair, respectively, in multivariate analysis, the researchers found.
The study was limited by several factors including the use of self-reports for hair color and the relative homogeneity of the Nurses’ Health Study, which has a population of mostly white, female health professionals, the researchers noted.
However, the findings of significantly increased CRP levels “could potentially explain a prior report of increased risks of cardiovascular disease and cancer in red-haired women,” they said. “Although, we observed similar associations in the NHS between red hair and cardiovascular disease and cancer, they were not statistically significant,” they added.
Additional studies are needed to validate and examine the clinical significance of the results, they concluded.
“Elevated CRP levels, a marker of inflammation, have been associated with increased risk for several diseases, including colon cancer and heart disease,” lead author Dr. Hartman said in an interview. “Another study suggested red-haired women have elevated risks of cardiovascular disease and cancer. We wanted to see if different levels of inflammation in red-haired women could possibly explain these findings.”
She said she was not surprised by the findings, “as they were in line with our hypothesis.” In addition, “animal studies suggest that the gene most responsible for red hair, MC1R, may be linked to inflammation,” she said.
While red-haired women were found to have higher CRP levels in the study, “the underlying mechanism and clinical significance remain unknown,” and more research is needed, Dr. Hartman emphasized. “First, our findings need to be validated in women and also examined in men. If our findings are validated, future studies should examine the mechanism of CRP elevation in red-haired women, and whether these women have elevated risks of colon cancer and heart disease,” she said.
“If red-haired women do have increased levels of inflammation, and as a result have elevated risks of colon cancer and heart disease, then future interventions can focus on enhanced screening and possibly chemoprevention in this population,” she added.
The study was supported by the National Institutes of Health. Lead author Dr. Hartman was supported by an American Skin Association Research Grant.
SOURCE: Hartman RI et al. J Invest Dermatol. 2020 Oct 12. doi: 10.1016/j.jid.2020.09.015.
Red-haired women were significantly more likely than were women with nonred hair to have elevated levels of C-reactive protein that may increase risk for cardiovascular conditions, according to data from nearly 9,000 women participating in the Nurses’ Health Study.
“Positive associations between red hair and cardiovascular disease and cancer in women, but not men, have been reported,” wrote Rebecca I. Hartman, MD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
In a study published in the Journal of Investigative Dermatology, they reviewed data from the Nurses’ Health Study, a 1976 cohort study of 121,700 women registered nurses in the United States. They analyzed blood specimens from 8,994 women that were collected between 1989 and 1990. Participants’ natural hair color was determined by asking them their natural hair color at age 21 years, with choices of red, blonde, light brown, dark brown, or black. Overall, dark brown/black hair was the most common color (45%) and 390 of the women (4.3%) had red hair.
The average CRP levels were significantly higher for women with red hair (3.7 mg/L), compared with those with blonde (3.3 mg/L), light brown (3.0 mg/mL), or dark brown/black (3.2 mg/L).
Using the CRP levels for red-haired women as a reference, women with blond, light brown, and dark brown/black hair averaged significantly lower CRP levels than those of red-haired women in an age-adjusted model (–15.2%, –18/1%, and –14.2%, respectively) and in a multivariate analysis (–12.7%, –14.1%, and –10.9%, respectively).
Non-red-haired women had significantly lower odds of high CRP levels compared with red-haired women, with odds ratios of 0.62, 0.60, and 0.67 for women with blonde, light brown, and dark brown/black hair, respectively, in multivariate analysis, the researchers found.
The study was limited by several factors including the use of self-reports for hair color and the relative homogeneity of the Nurses’ Health Study, which has a population of mostly white, female health professionals, the researchers noted.
However, the findings of significantly increased CRP levels “could potentially explain a prior report of increased risks of cardiovascular disease and cancer in red-haired women,” they said. “Although, we observed similar associations in the NHS between red hair and cardiovascular disease and cancer, they were not statistically significant,” they added.
Additional studies are needed to validate and examine the clinical significance of the results, they concluded.
“Elevated CRP levels, a marker of inflammation, have been associated with increased risk for several diseases, including colon cancer and heart disease,” lead author Dr. Hartman said in an interview. “Another study suggested red-haired women have elevated risks of cardiovascular disease and cancer. We wanted to see if different levels of inflammation in red-haired women could possibly explain these findings.”
She said she was not surprised by the findings, “as they were in line with our hypothesis.” In addition, “animal studies suggest that the gene most responsible for red hair, MC1R, may be linked to inflammation,” she said.
While red-haired women were found to have higher CRP levels in the study, “the underlying mechanism and clinical significance remain unknown,” and more research is needed, Dr. Hartman emphasized. “First, our findings need to be validated in women and also examined in men. If our findings are validated, future studies should examine the mechanism of CRP elevation in red-haired women, and whether these women have elevated risks of colon cancer and heart disease,” she said.
“If red-haired women do have increased levels of inflammation, and as a result have elevated risks of colon cancer and heart disease, then future interventions can focus on enhanced screening and possibly chemoprevention in this population,” she added.
The study was supported by the National Institutes of Health. Lead author Dr. Hartman was supported by an American Skin Association Research Grant.
SOURCE: Hartman RI et al. J Invest Dermatol. 2020 Oct 12. doi: 10.1016/j.jid.2020.09.015.
Red-haired women were significantly more likely than were women with nonred hair to have elevated levels of C-reactive protein that may increase risk for cardiovascular conditions, according to data from nearly 9,000 women participating in the Nurses’ Health Study.
“Positive associations between red hair and cardiovascular disease and cancer in women, but not men, have been reported,” wrote Rebecca I. Hartman, MD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
In a study published in the Journal of Investigative Dermatology, they reviewed data from the Nurses’ Health Study, a 1976 cohort study of 121,700 women registered nurses in the United States. They analyzed blood specimens from 8,994 women that were collected between 1989 and 1990. Participants’ natural hair color was determined by asking them their natural hair color at age 21 years, with choices of red, blonde, light brown, dark brown, or black. Overall, dark brown/black hair was the most common color (45%) and 390 of the women (4.3%) had red hair.
The average CRP levels were significantly higher for women with red hair (3.7 mg/L), compared with those with blonde (3.3 mg/L), light brown (3.0 mg/mL), or dark brown/black (3.2 mg/L).
Using the CRP levels for red-haired women as a reference, women with blond, light brown, and dark brown/black hair averaged significantly lower CRP levels than those of red-haired women in an age-adjusted model (–15.2%, –18/1%, and –14.2%, respectively) and in a multivariate analysis (–12.7%, –14.1%, and –10.9%, respectively).
Non-red-haired women had significantly lower odds of high CRP levels compared with red-haired women, with odds ratios of 0.62, 0.60, and 0.67 for women with blonde, light brown, and dark brown/black hair, respectively, in multivariate analysis, the researchers found.
The study was limited by several factors including the use of self-reports for hair color and the relative homogeneity of the Nurses’ Health Study, which has a population of mostly white, female health professionals, the researchers noted.
However, the findings of significantly increased CRP levels “could potentially explain a prior report of increased risks of cardiovascular disease and cancer in red-haired women,” they said. “Although, we observed similar associations in the NHS between red hair and cardiovascular disease and cancer, they were not statistically significant,” they added.
Additional studies are needed to validate and examine the clinical significance of the results, they concluded.
“Elevated CRP levels, a marker of inflammation, have been associated with increased risk for several diseases, including colon cancer and heart disease,” lead author Dr. Hartman said in an interview. “Another study suggested red-haired women have elevated risks of cardiovascular disease and cancer. We wanted to see if different levels of inflammation in red-haired women could possibly explain these findings.”
She said she was not surprised by the findings, “as they were in line with our hypothesis.” In addition, “animal studies suggest that the gene most responsible for red hair, MC1R, may be linked to inflammation,” she said.
While red-haired women were found to have higher CRP levels in the study, “the underlying mechanism and clinical significance remain unknown,” and more research is needed, Dr. Hartman emphasized. “First, our findings need to be validated in women and also examined in men. If our findings are validated, future studies should examine the mechanism of CRP elevation in red-haired women, and whether these women have elevated risks of colon cancer and heart disease,” she said.
“If red-haired women do have increased levels of inflammation, and as a result have elevated risks of colon cancer and heart disease, then future interventions can focus on enhanced screening and possibly chemoprevention in this population,” she added.
The study was supported by the National Institutes of Health. Lead author Dr. Hartman was supported by an American Skin Association Research Grant.
SOURCE: Hartman RI et al. J Invest Dermatol. 2020 Oct 12. doi: 10.1016/j.jid.2020.09.015.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Paraneoplastic Pemphigus With Cicatricial Nail Involvement
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
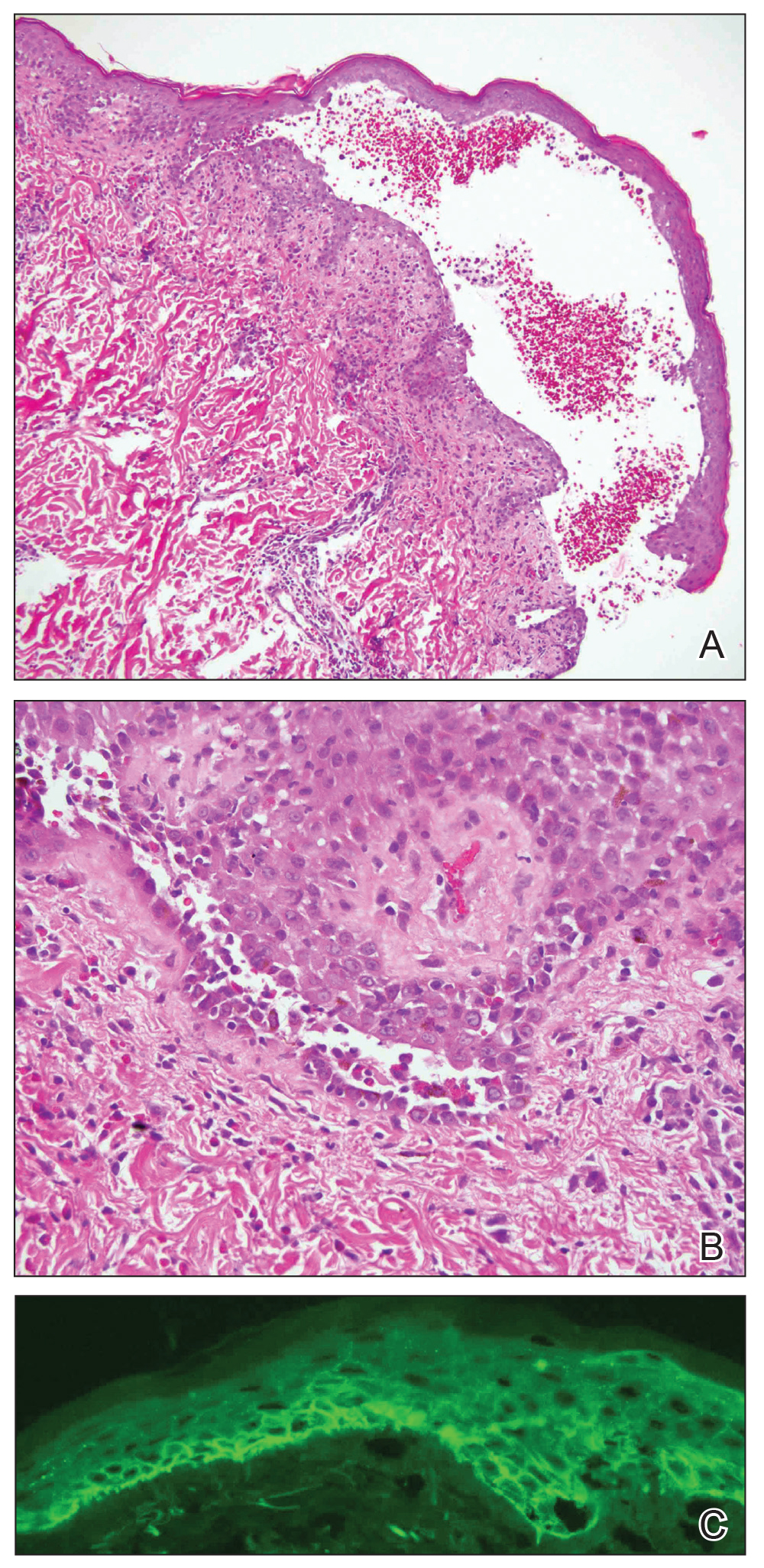
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.
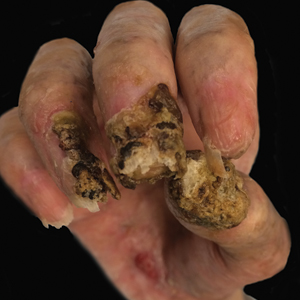
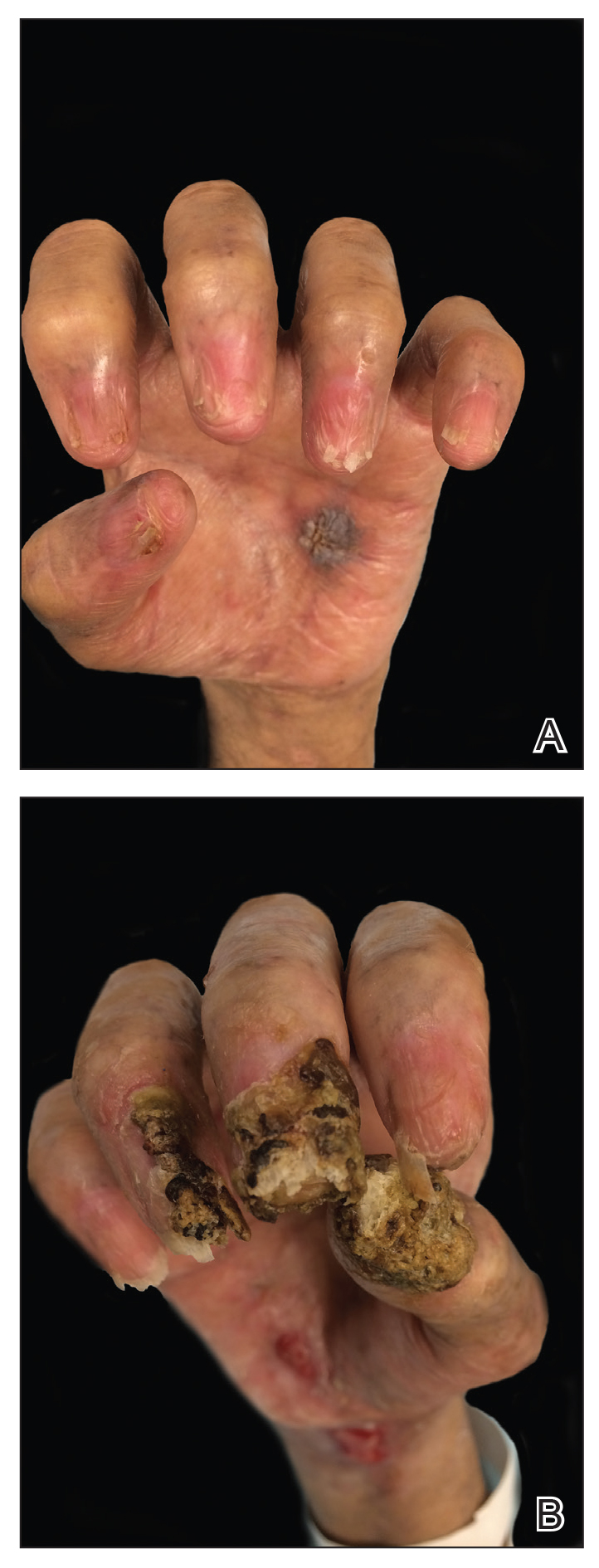
Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.
Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.

Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.

Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.

Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.

Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
Practice Points
- Paraneoplastic pemphigus (PNP) is a rare blistering skin eruption commonly associated with an underlying malignancy.
- Paraneoplastic pemphigus generally presents with erosive stomatitis with involvement of the vermillion lip but also can involve the skin and nails.
- Nail involvement can lead to scarring of the nails and can mimic lichen planus, bullous pemphigoid, or epidermolysis bullosa of the nails. These nail changes likely are due to the pronounced lichenoid interphase dermatitis seen in PNP.
New treatment options show promise for centrifugal cicatricial alopecia
according to a review of current strategies for this challenging disease, delivered at the virtual Skin of Color Update 2020.
Two case reports of favorable results with topical metformin were published earlier this year. A subsequent case in which metformin provided a major improvement in quality of life has provided further encouragement, according to Crystal Aguh, MD, director of the ethnic skin program at Johns Hopkins University, Baltimore.
In the subsequent case, there was complete scalp coverage, allowing the patient to no longer use a wig, which is “tough to achieve in patients with advanced disease,” Dr. Aguh said.
In the two published cases, 10% metformin compounded in Lipoderm (PCCA) produced notable hair growth within 6 months. Dr. Aguh said that the case studies were prompted by experimental evidence associating metformin with an antifibrotic effect.
This finding is potentially important. Most current treatments for CCCA are based on anti-inflammatory activity, according to Dr. Aguh, but fibrosis is strongly implicated in the pathogenesis of CCCA. Of several lines of evidence, one is the association between CCCA and other fibrosing conditions.
For example, women with CCCA “are several times more likely to have uterine fibroids than women without CCCA,” said Dr. Aguh, citing a study that she published in 2018. She suggested that there is an urgent need for new treatment options because of the “often-disappointing” responses to current standard therapies.
In the CCCA cases treated so far, topical metformin has been well tolerated, which is attributed to the low level of systemic absorption. No nausea or other gastrointestinal side effects common to oral metformin have been so far observed in Dr. Aguh’s cases.
“Some patients experience scalp dryness or irritation,” she said, but added that a light coating of emollient typically relieves this complaint.
Despite the promising results, topical metformin “is not a silver bullet,” Dr. Aguh cautioned. She estimated that only 10%-15% of patients respond, but this treatment can be considered “as an adjunctive option to avoid another round of intralesional steroids.”
Platelet-rich plasma (PRP) is another option that has demonstrated promise in a published case report for which Dr. Aguh served as a coauthor. In this series of two patients, only one had CCCA. The other had lichen planopilaris, but both patients experienced hair regrowth after failing standard therapies.
When treating alopecia with PRP, Dr. Aguh typically offers three or four sessions spaced 4 weeks apart. She does not start other treatments at the same time, but she does not discontinue topical treatments that patients are already taking, including topical minoxidil.
Again, like topical metformin, PRP is reasonably considered in patients who have failed standard therapies, according to Dr. Aguh. She cautioned that responses are not permanent. Patients who respond typically require retreatment a year or more later, but good responses have been seen after retreatment.
Appropriate hair care can help. Dr. Aguh recounted a case in which a patient with presumed CCCA was referred after failing intralesional triamcinolone injections. Ultimately, the patient was diagnosed with acquired trichorrhexis nodosa, but the large clinical improvements gained from better hair care practices, including avoidance of chemical relaxants and thermal styling, are relevant to CCCA, as well as other conditions resulting in hair loss.
In a book written by Dr. Aguh, titled “90 Days to Beautiful Hair,” strategies for better hair care practices include advice to reduce tension on hair follicles.
The role of increased traction is an issue in CCCA, agreed Amy McMichael, MD, chair of the department of dermatology, Wake Forest University, Winston-Salem, N.C. Although she provided data at the meeting suggesting that CCCA is a fibrosing disease linked to genetic susceptibility, she said there is also a “strong association” between the severity of CCCA and extensions, hair weaving, and other tension-associated hairstyles.
While there is an urgent need to develop therapies that address the underlying pathophysiology of CCCA, she concurred that patients with this or other conditions associated with hair loss, such as seborrheic dermatitis or frontal fibrosing alopecia, should not ignore appropriate hair care.
Dr. Aguh has financial relationships with LEO Pharma and UCB Pharma. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Nutrafol, Johnson & Johnson, and Aclaris.
according to a review of current strategies for this challenging disease, delivered at the virtual Skin of Color Update 2020.
Two case reports of favorable results with topical metformin were published earlier this year. A subsequent case in which metformin provided a major improvement in quality of life has provided further encouragement, according to Crystal Aguh, MD, director of the ethnic skin program at Johns Hopkins University, Baltimore.
In the subsequent case, there was complete scalp coverage, allowing the patient to no longer use a wig, which is “tough to achieve in patients with advanced disease,” Dr. Aguh said.
In the two published cases, 10% metformin compounded in Lipoderm (PCCA) produced notable hair growth within 6 months. Dr. Aguh said that the case studies were prompted by experimental evidence associating metformin with an antifibrotic effect.
This finding is potentially important. Most current treatments for CCCA are based on anti-inflammatory activity, according to Dr. Aguh, but fibrosis is strongly implicated in the pathogenesis of CCCA. Of several lines of evidence, one is the association between CCCA and other fibrosing conditions.
For example, women with CCCA “are several times more likely to have uterine fibroids than women without CCCA,” said Dr. Aguh, citing a study that she published in 2018. She suggested that there is an urgent need for new treatment options because of the “often-disappointing” responses to current standard therapies.
In the CCCA cases treated so far, topical metformin has been well tolerated, which is attributed to the low level of systemic absorption. No nausea or other gastrointestinal side effects common to oral metformin have been so far observed in Dr. Aguh’s cases.
“Some patients experience scalp dryness or irritation,” she said, but added that a light coating of emollient typically relieves this complaint.
Despite the promising results, topical metformin “is not a silver bullet,” Dr. Aguh cautioned. She estimated that only 10%-15% of patients respond, but this treatment can be considered “as an adjunctive option to avoid another round of intralesional steroids.”
Platelet-rich plasma (PRP) is another option that has demonstrated promise in a published case report for which Dr. Aguh served as a coauthor. In this series of two patients, only one had CCCA. The other had lichen planopilaris, but both patients experienced hair regrowth after failing standard therapies.
When treating alopecia with PRP, Dr. Aguh typically offers three or four sessions spaced 4 weeks apart. She does not start other treatments at the same time, but she does not discontinue topical treatments that patients are already taking, including topical minoxidil.
Again, like topical metformin, PRP is reasonably considered in patients who have failed standard therapies, according to Dr. Aguh. She cautioned that responses are not permanent. Patients who respond typically require retreatment a year or more later, but good responses have been seen after retreatment.
Appropriate hair care can help. Dr. Aguh recounted a case in which a patient with presumed CCCA was referred after failing intralesional triamcinolone injections. Ultimately, the patient was diagnosed with acquired trichorrhexis nodosa, but the large clinical improvements gained from better hair care practices, including avoidance of chemical relaxants and thermal styling, are relevant to CCCA, as well as other conditions resulting in hair loss.
In a book written by Dr. Aguh, titled “90 Days to Beautiful Hair,” strategies for better hair care practices include advice to reduce tension on hair follicles.
The role of increased traction is an issue in CCCA, agreed Amy McMichael, MD, chair of the department of dermatology, Wake Forest University, Winston-Salem, N.C. Although she provided data at the meeting suggesting that CCCA is a fibrosing disease linked to genetic susceptibility, she said there is also a “strong association” between the severity of CCCA and extensions, hair weaving, and other tension-associated hairstyles.
While there is an urgent need to develop therapies that address the underlying pathophysiology of CCCA, she concurred that patients with this or other conditions associated with hair loss, such as seborrheic dermatitis or frontal fibrosing alopecia, should not ignore appropriate hair care.
Dr. Aguh has financial relationships with LEO Pharma and UCB Pharma. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Nutrafol, Johnson & Johnson, and Aclaris.
according to a review of current strategies for this challenging disease, delivered at the virtual Skin of Color Update 2020.
Two case reports of favorable results with topical metformin were published earlier this year. A subsequent case in which metformin provided a major improvement in quality of life has provided further encouragement, according to Crystal Aguh, MD, director of the ethnic skin program at Johns Hopkins University, Baltimore.
In the subsequent case, there was complete scalp coverage, allowing the patient to no longer use a wig, which is “tough to achieve in patients with advanced disease,” Dr. Aguh said.
In the two published cases, 10% metformin compounded in Lipoderm (PCCA) produced notable hair growth within 6 months. Dr. Aguh said that the case studies were prompted by experimental evidence associating metformin with an antifibrotic effect.
This finding is potentially important. Most current treatments for CCCA are based on anti-inflammatory activity, according to Dr. Aguh, but fibrosis is strongly implicated in the pathogenesis of CCCA. Of several lines of evidence, one is the association between CCCA and other fibrosing conditions.
For example, women with CCCA “are several times more likely to have uterine fibroids than women without CCCA,” said Dr. Aguh, citing a study that she published in 2018. She suggested that there is an urgent need for new treatment options because of the “often-disappointing” responses to current standard therapies.
In the CCCA cases treated so far, topical metformin has been well tolerated, which is attributed to the low level of systemic absorption. No nausea or other gastrointestinal side effects common to oral metformin have been so far observed in Dr. Aguh’s cases.
“Some patients experience scalp dryness or irritation,” she said, but added that a light coating of emollient typically relieves this complaint.
Despite the promising results, topical metformin “is not a silver bullet,” Dr. Aguh cautioned. She estimated that only 10%-15% of patients respond, but this treatment can be considered “as an adjunctive option to avoid another round of intralesional steroids.”
Platelet-rich plasma (PRP) is another option that has demonstrated promise in a published case report for which Dr. Aguh served as a coauthor. In this series of two patients, only one had CCCA. The other had lichen planopilaris, but both patients experienced hair regrowth after failing standard therapies.
When treating alopecia with PRP, Dr. Aguh typically offers three or four sessions spaced 4 weeks apart. She does not start other treatments at the same time, but she does not discontinue topical treatments that patients are already taking, including topical minoxidil.
Again, like topical metformin, PRP is reasonably considered in patients who have failed standard therapies, according to Dr. Aguh. She cautioned that responses are not permanent. Patients who respond typically require retreatment a year or more later, but good responses have been seen after retreatment.
Appropriate hair care can help. Dr. Aguh recounted a case in which a patient with presumed CCCA was referred after failing intralesional triamcinolone injections. Ultimately, the patient was diagnosed with acquired trichorrhexis nodosa, but the large clinical improvements gained from better hair care practices, including avoidance of chemical relaxants and thermal styling, are relevant to CCCA, as well as other conditions resulting in hair loss.
In a book written by Dr. Aguh, titled “90 Days to Beautiful Hair,” strategies for better hair care practices include advice to reduce tension on hair follicles.
The role of increased traction is an issue in CCCA, agreed Amy McMichael, MD, chair of the department of dermatology, Wake Forest University, Winston-Salem, N.C. Although she provided data at the meeting suggesting that CCCA is a fibrosing disease linked to genetic susceptibility, she said there is also a “strong association” between the severity of CCCA and extensions, hair weaving, and other tension-associated hairstyles.
While there is an urgent need to develop therapies that address the underlying pathophysiology of CCCA, she concurred that patients with this or other conditions associated with hair loss, such as seborrheic dermatitis or frontal fibrosing alopecia, should not ignore appropriate hair care.
Dr. Aguh has financial relationships with LEO Pharma and UCB Pharma. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Nutrafol, Johnson & Johnson, and Aclaris.
FROM SOC 2020
Congenital Defect of the Toenail
The Diagnosis: Onychodystrophy Secondary to Polydactyly
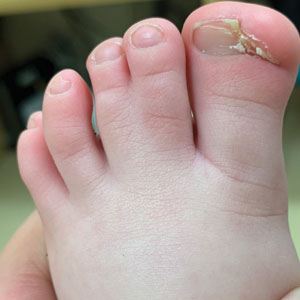
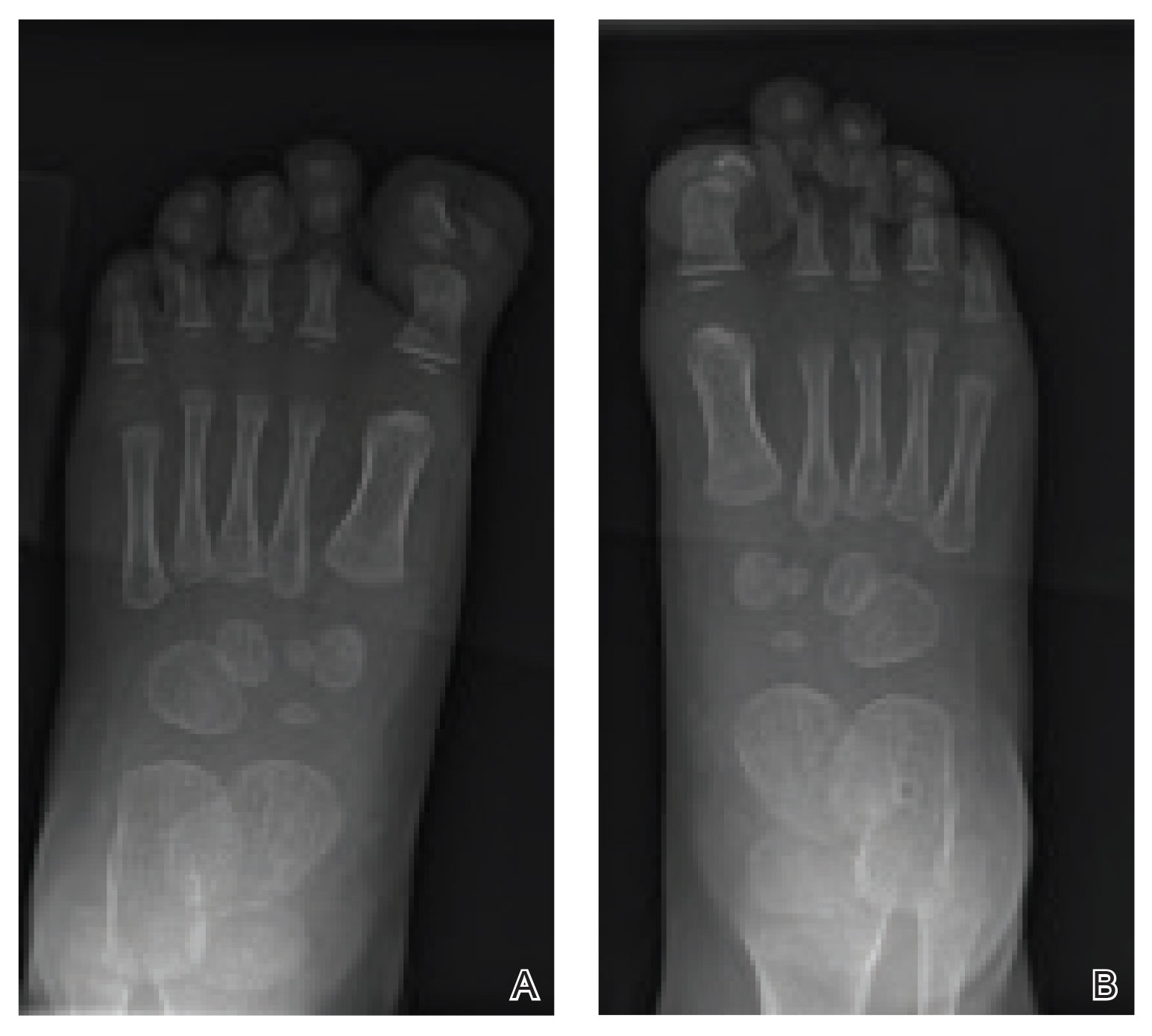
Radiographs of the feet demonstrated an accessory distal phalanx of the left great toe with a similar smaller accessory distal phalanx on the right great toe (Figure). The patient was referred to orthopedic surgery, and surgical intervention was recommended for only the left great toe given recurrent skin inflammation and nail complications. An excision of the left great toe polydactyly was performed. The patient healed well without complications.
Many clinically heterogeneous phenotypes exist for polydactyly and syndactyly, which both are common entities with incidences of 1 in 700 to 1000 births and 1 in 2000 to 3000 births, respectively.1 Both polydactyly and syndactyly can be an isolated variant in newborns or present with multiple concurrent malformations as part of a genetic syndrome, with more than 300 syndromic anomalies described. The genetic basis of these conditions is equally diverse, with homeobox genes, hedgehog pathways, fibroblast growth factors, and boneand cartilage-derived morphogenetic proteins implicated in their development.1
The differential diagnosis for our patient included congenital malalignment of the great toenails, nail-patella syndrome, onychodystrophy secondary to polydactyly, and congenital hypertrophy of the lateral nail fold. Given the strong family history, polydactyly was suspected.
Congenital malalignment of the great toenails results in lateral deviation of the nail plates.2 It is an underdiagnosed condition with different etiologies hypothesized, such as genetic factors with possible autosomal-dominant transmission and extrinsic factors.3 One proposed mechanism of pathogenesis is desynchronization during growth of the nail and distal phalanx of the hallux, leading to larger nail plates that grow laterally.4 Typical features associated with this disease are nail discoloration, nail plate thickening, and transversal grooves or ridges, none of which were seen in our patient.2
Children with nail-patella syndrome have dysplastic nails and associated bony abnormalities, such as absent patellae.5 This syndrome results from an autosomaldominant mutation in the LIM homeobox transcription factor 1-beta gene, LMX1B, which is responsible for dorsal-ventral patterning of the limb, as well as patterning of the nails, patellae and long bones, and even the kidney tubule.6 As such, patients with nail-patella syndrome have associated renal abnormalities. The findings in our patient were limited to the feet, making an underlying syndrome unlikely to be the cause.
First described in 1968 by Meadow,7 fetal hydantoin syndrome is a well-documented sequela in women taking phenytoin throughout pregnancy. Multiple malformations are possible, including cardiac defects, cleft lip/palate, digit and nail hypoplasia, abnormal facial features, mental disability, and growth abnormalities.8 The teratogenicity behind phenytoin results from reactive oxygen species that alter embryonic DNA, proteins, and lipids.9 The mother of this child was not on any seizure prophylaxis, eliminating it from the differential.
Congenital hypertrophy of the lateral nail fold is a defect of the soft tissue of the hallux leading to hypertrophy of the nail fold, commonly presenting with inflammation and pain10 possibly due to dyssynchronous growth between the soft tissue and nail plate.11 With this defect, a lip covering the nail plate is common, which was not seen in our patient.
As demonstrated in our patient, family history can help guide the diagnosis. Seven of 9 nonsyndromic forms of syndactyly are inherited in an autosomal-dominant fashion and range from mild presentations, as in our patient, to more severe deformations with underlying bone fusion and functional impairment.12 Polydactyly also often is expressed in an autosomal-dominant pattern, with up to 30% of patients having a positive family history. Polydactyly traditionally is classified by the location of the supernumerary digit as preaxial (radial), central, or postaxial (ulnar), and many further morphologic variations exist within these groups. Overall, preaxial polydactyly is relatively rare and represents 15% of polydactylies, with central and postaxial comprising the other 6% and 79%, respectively.13 Delineation of the underlying anatomy may reveal ray duplications (digit and corresponding metacarpal or metatarsal bone), metatarsal variants, and duplicated phalanges that may be hypoplastic or deformed. Patients may report difficulty finding comfortable footwear, cosmetic concerns, and nail-related complications. Although not always required, surgical intervention may provide definitive treatment but can leave residual deformities in the surrounding altered anatomy; thus, orthopedic or plastic surgery consultations are critical in appropriately counseling patients.
- Ahmed H, Akbari H, Emanmi A, et al. Genetic overview of syndactyly and polydactyly. Plast Reconstr Surg Glob Open. 2017;5:e1549.
- Catalfo P, Musumeci ML, Lacarrubba F, et al. Congenital malalignment of the great toenails: a review. Skin Appendage Disord. 2018;4:230-235.
- Kus S, Tahmaz E, Gurunluoglu R, et al. Congenital malalignment of the great toenails in dizygotic twins. Pediatr Dermatol. 2005;22:434-435.
- Chaniotakis I, Bonitsis N, Stergiopoulou C, et al. Dizygotic twins with congenital malalignment of the great toenails: reappraisal of the pathogenesis. J Am Acad Dermatol. 2007;57:711-715.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Dreyer SD, Zhou G, Baldini A, et al, Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47-50.
- Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2:1296.
- Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6.
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995;48:112-120.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Martinet C, Pascal M, Civatte J, et al. Lateral nail-pad of the big toe in infants. apropos of 2 cases. Ann Dermatol Venereol. 1984;111:731-732.
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824.
- Belthur MV, Linton JL, Barnes DA. The spectrum of preaxial polydactyly of the foot. J Pediatr Orthop. 2011;31:435-447.
The Diagnosis: Onychodystrophy Secondary to Polydactyly
Radiographs of the feet demonstrated an accessory distal phalanx of the left great toe with a similar smaller accessory distal phalanx on the right great toe (Figure). The patient was referred to orthopedic surgery, and surgical intervention was recommended for only the left great toe given recurrent skin inflammation and nail complications. An excision of the left great toe polydactyly was performed. The patient healed well without complications.

Many clinically heterogeneous phenotypes exist for polydactyly and syndactyly, which both are common entities with incidences of 1 in 700 to 1000 births and 1 in 2000 to 3000 births, respectively.1 Both polydactyly and syndactyly can be an isolated variant in newborns or present with multiple concurrent malformations as part of a genetic syndrome, with more than 300 syndromic anomalies described. The genetic basis of these conditions is equally diverse, with homeobox genes, hedgehog pathways, fibroblast growth factors, and boneand cartilage-derived morphogenetic proteins implicated in their development.1
The differential diagnosis for our patient included congenital malalignment of the great toenails, nail-patella syndrome, onychodystrophy secondary to polydactyly, and congenital hypertrophy of the lateral nail fold. Given the strong family history, polydactyly was suspected.
Congenital malalignment of the great toenails results in lateral deviation of the nail plates.2 It is an underdiagnosed condition with different etiologies hypothesized, such as genetic factors with possible autosomal-dominant transmission and extrinsic factors.3 One proposed mechanism of pathogenesis is desynchronization during growth of the nail and distal phalanx of the hallux, leading to larger nail plates that grow laterally.4 Typical features associated with this disease are nail discoloration, nail plate thickening, and transversal grooves or ridges, none of which were seen in our patient.2
Children with nail-patella syndrome have dysplastic nails and associated bony abnormalities, such as absent patellae.5 This syndrome results from an autosomaldominant mutation in the LIM homeobox transcription factor 1-beta gene, LMX1B, which is responsible for dorsal-ventral patterning of the limb, as well as patterning of the nails, patellae and long bones, and even the kidney tubule.6 As such, patients with nail-patella syndrome have associated renal abnormalities. The findings in our patient were limited to the feet, making an underlying syndrome unlikely to be the cause.
First described in 1968 by Meadow,7 fetal hydantoin syndrome is a well-documented sequela in women taking phenytoin throughout pregnancy. Multiple malformations are possible, including cardiac defects, cleft lip/palate, digit and nail hypoplasia, abnormal facial features, mental disability, and growth abnormalities.8 The teratogenicity behind phenytoin results from reactive oxygen species that alter embryonic DNA, proteins, and lipids.9 The mother of this child was not on any seizure prophylaxis, eliminating it from the differential.
Congenital hypertrophy of the lateral nail fold is a defect of the soft tissue of the hallux leading to hypertrophy of the nail fold, commonly presenting with inflammation and pain10 possibly due to dyssynchronous growth between the soft tissue and nail plate.11 With this defect, a lip covering the nail plate is common, which was not seen in our patient.
As demonstrated in our patient, family history can help guide the diagnosis. Seven of 9 nonsyndromic forms of syndactyly are inherited in an autosomal-dominant fashion and range from mild presentations, as in our patient, to more severe deformations with underlying bone fusion and functional impairment.12 Polydactyly also often is expressed in an autosomal-dominant pattern, with up to 30% of patients having a positive family history. Polydactyly traditionally is classified by the location of the supernumerary digit as preaxial (radial), central, or postaxial (ulnar), and many further morphologic variations exist within these groups. Overall, preaxial polydactyly is relatively rare and represents 15% of polydactylies, with central and postaxial comprising the other 6% and 79%, respectively.13 Delineation of the underlying anatomy may reveal ray duplications (digit and corresponding metacarpal or metatarsal bone), metatarsal variants, and duplicated phalanges that may be hypoplastic or deformed. Patients may report difficulty finding comfortable footwear, cosmetic concerns, and nail-related complications. Although not always required, surgical intervention may provide definitive treatment but can leave residual deformities in the surrounding altered anatomy; thus, orthopedic or plastic surgery consultations are critical in appropriately counseling patients.
The Diagnosis: Onychodystrophy Secondary to Polydactyly
Radiographs of the feet demonstrated an accessory distal phalanx of the left great toe with a similar smaller accessory distal phalanx on the right great toe (Figure). The patient was referred to orthopedic surgery, and surgical intervention was recommended for only the left great toe given recurrent skin inflammation and nail complications. An excision of the left great toe polydactyly was performed. The patient healed well without complications.

Many clinically heterogeneous phenotypes exist for polydactyly and syndactyly, which both are common entities with incidences of 1 in 700 to 1000 births and 1 in 2000 to 3000 births, respectively.1 Both polydactyly and syndactyly can be an isolated variant in newborns or present with multiple concurrent malformations as part of a genetic syndrome, with more than 300 syndromic anomalies described. The genetic basis of these conditions is equally diverse, with homeobox genes, hedgehog pathways, fibroblast growth factors, and boneand cartilage-derived morphogenetic proteins implicated in their development.1
The differential diagnosis for our patient included congenital malalignment of the great toenails, nail-patella syndrome, onychodystrophy secondary to polydactyly, and congenital hypertrophy of the lateral nail fold. Given the strong family history, polydactyly was suspected.
Congenital malalignment of the great toenails results in lateral deviation of the nail plates.2 It is an underdiagnosed condition with different etiologies hypothesized, such as genetic factors with possible autosomal-dominant transmission and extrinsic factors.3 One proposed mechanism of pathogenesis is desynchronization during growth of the nail and distal phalanx of the hallux, leading to larger nail plates that grow laterally.4 Typical features associated with this disease are nail discoloration, nail plate thickening, and transversal grooves or ridges, none of which were seen in our patient.2
Children with nail-patella syndrome have dysplastic nails and associated bony abnormalities, such as absent patellae.5 This syndrome results from an autosomaldominant mutation in the LIM homeobox transcription factor 1-beta gene, LMX1B, which is responsible for dorsal-ventral patterning of the limb, as well as patterning of the nails, patellae and long bones, and even the kidney tubule.6 As such, patients with nail-patella syndrome have associated renal abnormalities. The findings in our patient were limited to the feet, making an underlying syndrome unlikely to be the cause.
First described in 1968 by Meadow,7 fetal hydantoin syndrome is a well-documented sequela in women taking phenytoin throughout pregnancy. Multiple malformations are possible, including cardiac defects, cleft lip/palate, digit and nail hypoplasia, abnormal facial features, mental disability, and growth abnormalities.8 The teratogenicity behind phenytoin results from reactive oxygen species that alter embryonic DNA, proteins, and lipids.9 The mother of this child was not on any seizure prophylaxis, eliminating it from the differential.
Congenital hypertrophy of the lateral nail fold is a defect of the soft tissue of the hallux leading to hypertrophy of the nail fold, commonly presenting with inflammation and pain10 possibly due to dyssynchronous growth between the soft tissue and nail plate.11 With this defect, a lip covering the nail plate is common, which was not seen in our patient.
As demonstrated in our patient, family history can help guide the diagnosis. Seven of 9 nonsyndromic forms of syndactyly are inherited in an autosomal-dominant fashion and range from mild presentations, as in our patient, to more severe deformations with underlying bone fusion and functional impairment.12 Polydactyly also often is expressed in an autosomal-dominant pattern, with up to 30% of patients having a positive family history. Polydactyly traditionally is classified by the location of the supernumerary digit as preaxial (radial), central, or postaxial (ulnar), and many further morphologic variations exist within these groups. Overall, preaxial polydactyly is relatively rare and represents 15% of polydactylies, with central and postaxial comprising the other 6% and 79%, respectively.13 Delineation of the underlying anatomy may reveal ray duplications (digit and corresponding metacarpal or metatarsal bone), metatarsal variants, and duplicated phalanges that may be hypoplastic or deformed. Patients may report difficulty finding comfortable footwear, cosmetic concerns, and nail-related complications. Although not always required, surgical intervention may provide definitive treatment but can leave residual deformities in the surrounding altered anatomy; thus, orthopedic or plastic surgery consultations are critical in appropriately counseling patients.
- Ahmed H, Akbari H, Emanmi A, et al. Genetic overview of syndactyly and polydactyly. Plast Reconstr Surg Glob Open. 2017;5:e1549.
- Catalfo P, Musumeci ML, Lacarrubba F, et al. Congenital malalignment of the great toenails: a review. Skin Appendage Disord. 2018;4:230-235.
- Kus S, Tahmaz E, Gurunluoglu R, et al. Congenital malalignment of the great toenails in dizygotic twins. Pediatr Dermatol. 2005;22:434-435.
- Chaniotakis I, Bonitsis N, Stergiopoulou C, et al. Dizygotic twins with congenital malalignment of the great toenails: reappraisal of the pathogenesis. J Am Acad Dermatol. 2007;57:711-715.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Dreyer SD, Zhou G, Baldini A, et al, Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47-50.
- Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2:1296.
- Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6.
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995;48:112-120.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Martinet C, Pascal M, Civatte J, et al. Lateral nail-pad of the big toe in infants. apropos of 2 cases. Ann Dermatol Venereol. 1984;111:731-732.
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824.
- Belthur MV, Linton JL, Barnes DA. The spectrum of preaxial polydactyly of the foot. J Pediatr Orthop. 2011;31:435-447.
- Ahmed H, Akbari H, Emanmi A, et al. Genetic overview of syndactyly and polydactyly. Plast Reconstr Surg Glob Open. 2017;5:e1549.
- Catalfo P, Musumeci ML, Lacarrubba F, et al. Congenital malalignment of the great toenails: a review. Skin Appendage Disord. 2018;4:230-235.
- Kus S, Tahmaz E, Gurunluoglu R, et al. Congenital malalignment of the great toenails in dizygotic twins. Pediatr Dermatol. 2005;22:434-435.
- Chaniotakis I, Bonitsis N, Stergiopoulou C, et al. Dizygotic twins with congenital malalignment of the great toenails: reappraisal of the pathogenesis. J Am Acad Dermatol. 2007;57:711-715.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Dreyer SD, Zhou G, Baldini A, et al, Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47-50.
- Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2:1296.
- Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6.
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995;48:112-120.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Martinet C, Pascal M, Civatte J, et al. Lateral nail-pad of the big toe in infants. apropos of 2 cases. Ann Dermatol Venereol. 1984;111:731-732.
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824.
- Belthur MV, Linton JL, Barnes DA. The spectrum of preaxial polydactyly of the foot. J Pediatr Orthop. 2011;31:435-447.
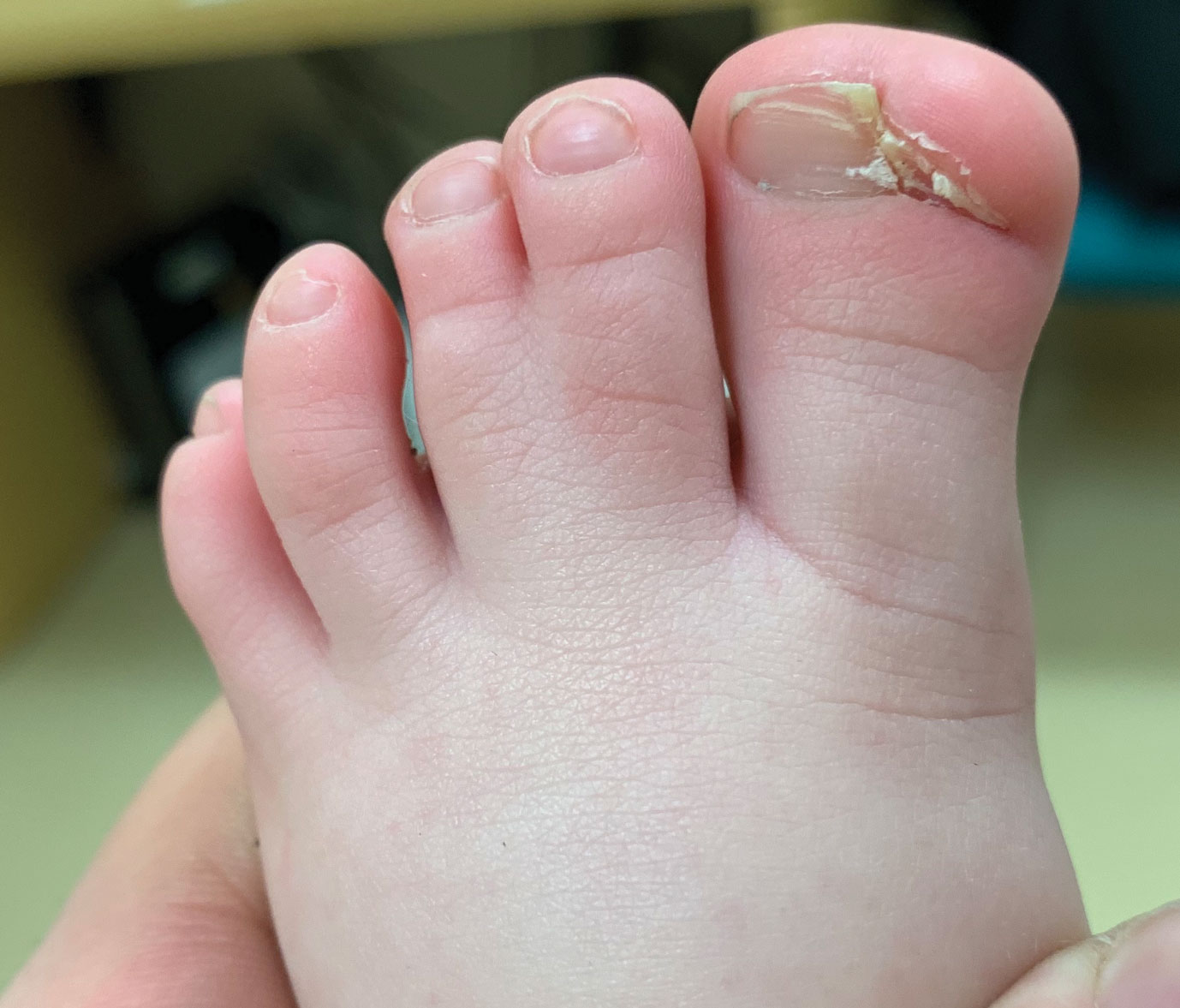
An 18-month-old girl presented for evaluation of nail dystrophy. The patient’s parents stated that the left great toenail had been dystrophic since birth, leading to skin irritation and “snagging” of the toenail on socks and footwear. Additional history revealed that the patient also had webbed toes, and there was a paternal family history of polydactyly and syndactyly. Physical examination revealed webbing of the second and third toes to the distal interphalangeal joints on both feet, marked nail plate dystrophy on the left big toe, and an irregularly shaped nail plate on the right big toe. The patient had no similar findings on the hands.
Persistent hair loss after radiation improved with minoxidil
The first study to systematically address the problem of persistent hair loss in patients who undergo radiation to the scalp for central nervous system or head and neck tumors has found that treatment with topical minoxidil leads to improvement in hair loss.
The study was published online on Aug. 5 in JAMA Dermatology.
Minoxidil has been used for many years to treat age-associated baldness in men, noted the authors. It was used off label in this study to treat radiation-associated persistent hair loss; 82% of patients showed at least some improvement.
For patients who do not improve with minoxidil, hair transplant and scalp reconstruction with plastic surgery were other options, the authors comment.
“Almost in all instances, there is something that can be done to improve persistent hair loss after radiation and give patients a sense of control,” senior author Mario E. Lacouture, MD, said in an interview. He is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York City.
About 60% of people with CNS tumors and 30% with head and neck cancer receive radiation to the head, and 75%-100% of these patients experience acute hair loss. For many, hair grows back in 2-3 months. However, for about 60%, hair loss persists for 6 or more months after completion of radiotherapy, the authors note.
In past work, Dr. Lacouture and colleagues found that persistent hair loss in cancer survivors is associated with depression, anxiety, and psychosocial distress.
“There are therapies and procedures that may mitigate persistent radiation therapy hair loss that can bring back psychosocial well-being to many of these patients,” Dr. Lacouture said. “These approaches are likely underutilized because patients are not being referred to specialists in hair or scalp reconstruction.”
Specialists can be found through the International Society for Hair Restoration Surgery and the American Academy of Dermatology, he added.
Study details
The retrospective cohort study included 71 children and adults who developed persistent hair loss after radiotherapy for primary CNS tumors (90%, n = 64) or head and neck sarcoma (10%, n = 7). The median age of the patients was 27 years (range, 4-75 years); 72% (n = 51) were female and 82% (n = 58) were White.
These patients had been treated at Memorial Sloan Kettering Cancer Center in New York City or St. Jude Children’s Hospital in Memphis from January 2011 to January 2019.
The team analyzed standardized clinical photographs of the scalp using the Common Terminology Criteria for Adverse Events version 5.0. Grade 1 alopecia was defined as hair loss of less than 50% of normal that does not require camouflage with hair pieces, scarves, or similar items. Grade 2 alopecia was defined as hair loss greater than 50% of normal that requires camouflage and is associated with negative psychosocial effects.
Over half of patients (56%, 40/70) had grade 1 hair loss. Clinical images were available for 54 patients; for most of these patients, hair loss was attributable to radiation alone (74%, n = 40). Evaluation of clinical imaging showed three variants of hair loss: localized (54%, 29/54), diffuse (24%, 13/54), and mixed pattern (22%, 12/54). Data on dermatologic imaging of the scalp (trichoscopy) were available for 28 patients; the main finding was white patches (57%, 16/28).
The median scalp radiation dose was 39.6 Gy (range, 15.1-50.0 Gy). The researchers estimate that a dose of 36.1 Gy (95% CI, 33.7-39.6 Gy) was sufficient to induce grade 2 hair loss in 50% of patients.
Severity of hair loss appeared to increase with radiation dose. For every 1-unit increase in radiation dose, the odds of grade 2 hair loss increased by 15% (odds ratio, 1.15; 95% CI, 1.04-1.28; P < .001). Proton irradiation was associated with even higher odds of severe hair loss (OR, 5.7; 95% CI, 1.05-30.8; P < .001). Results remained significant when analyses were controlled for sex, age at time of radiotherapy, and concurrent chemotherapy.
The majority of evaluable patients who were treated with topical minoxidil (5%) twice daily showed a response (82%, 28/34) during a median follow-up of 61 weeks (interquartile range, 21-105 weeks).
Among 25 of these patients for whom clinical images were available, 16% (4/25) showed complete response. Two patients improved with hair transplant, and one showed complete response with plastic surgery reconstruction of the hair.
The study had several limitations, including its retrospective design and a lack of complete data for certain variables, such as standardized clinical photographs, trichoscopy images, and radiotherapy treatment plans.
On the basis of these results, the authors are seeking funding for a prospective study of the use of minoxidil for persistent radiation-induced alopecia.
The study was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center. One or more authors has relationships with pharmaceutical companies, as listed in the original article.
This article first appeared on Medscape.com.
The first study to systematically address the problem of persistent hair loss in patients who undergo radiation to the scalp for central nervous system or head and neck tumors has found that treatment with topical minoxidil leads to improvement in hair loss.
The study was published online on Aug. 5 in JAMA Dermatology.
Minoxidil has been used for many years to treat age-associated baldness in men, noted the authors. It was used off label in this study to treat radiation-associated persistent hair loss; 82% of patients showed at least some improvement.
For patients who do not improve with minoxidil, hair transplant and scalp reconstruction with plastic surgery were other options, the authors comment.
“Almost in all instances, there is something that can be done to improve persistent hair loss after radiation and give patients a sense of control,” senior author Mario E. Lacouture, MD, said in an interview. He is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York City.
About 60% of people with CNS tumors and 30% with head and neck cancer receive radiation to the head, and 75%-100% of these patients experience acute hair loss. For many, hair grows back in 2-3 months. However, for about 60%, hair loss persists for 6 or more months after completion of radiotherapy, the authors note.
In past work, Dr. Lacouture and colleagues found that persistent hair loss in cancer survivors is associated with depression, anxiety, and psychosocial distress.
“There are therapies and procedures that may mitigate persistent radiation therapy hair loss that can bring back psychosocial well-being to many of these patients,” Dr. Lacouture said. “These approaches are likely underutilized because patients are not being referred to specialists in hair or scalp reconstruction.”
Specialists can be found through the International Society for Hair Restoration Surgery and the American Academy of Dermatology, he added.
Study details
The retrospective cohort study included 71 children and adults who developed persistent hair loss after radiotherapy for primary CNS tumors (90%, n = 64) or head and neck sarcoma (10%, n = 7). The median age of the patients was 27 years (range, 4-75 years); 72% (n = 51) were female and 82% (n = 58) were White.
These patients had been treated at Memorial Sloan Kettering Cancer Center in New York City or St. Jude Children’s Hospital in Memphis from January 2011 to January 2019.
The team analyzed standardized clinical photographs of the scalp using the Common Terminology Criteria for Adverse Events version 5.0. Grade 1 alopecia was defined as hair loss of less than 50% of normal that does not require camouflage with hair pieces, scarves, or similar items. Grade 2 alopecia was defined as hair loss greater than 50% of normal that requires camouflage and is associated with negative psychosocial effects.
Over half of patients (56%, 40/70) had grade 1 hair loss. Clinical images were available for 54 patients; for most of these patients, hair loss was attributable to radiation alone (74%, n = 40). Evaluation of clinical imaging showed three variants of hair loss: localized (54%, 29/54), diffuse (24%, 13/54), and mixed pattern (22%, 12/54). Data on dermatologic imaging of the scalp (trichoscopy) were available for 28 patients; the main finding was white patches (57%, 16/28).
The median scalp radiation dose was 39.6 Gy (range, 15.1-50.0 Gy). The researchers estimate that a dose of 36.1 Gy (95% CI, 33.7-39.6 Gy) was sufficient to induce grade 2 hair loss in 50% of patients.
Severity of hair loss appeared to increase with radiation dose. For every 1-unit increase in radiation dose, the odds of grade 2 hair loss increased by 15% (odds ratio, 1.15; 95% CI, 1.04-1.28; P < .001). Proton irradiation was associated with even higher odds of severe hair loss (OR, 5.7; 95% CI, 1.05-30.8; P < .001). Results remained significant when analyses were controlled for sex, age at time of radiotherapy, and concurrent chemotherapy.
The majority of evaluable patients who were treated with topical minoxidil (5%) twice daily showed a response (82%, 28/34) during a median follow-up of 61 weeks (interquartile range, 21-105 weeks).
Among 25 of these patients for whom clinical images were available, 16% (4/25) showed complete response. Two patients improved with hair transplant, and one showed complete response with plastic surgery reconstruction of the hair.
The study had several limitations, including its retrospective design and a lack of complete data for certain variables, such as standardized clinical photographs, trichoscopy images, and radiotherapy treatment plans.
On the basis of these results, the authors are seeking funding for a prospective study of the use of minoxidil for persistent radiation-induced alopecia.
The study was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center. One or more authors has relationships with pharmaceutical companies, as listed in the original article.
This article first appeared on Medscape.com.
The first study to systematically address the problem of persistent hair loss in patients who undergo radiation to the scalp for central nervous system or head and neck tumors has found that treatment with topical minoxidil leads to improvement in hair loss.
The study was published online on Aug. 5 in JAMA Dermatology.
Minoxidil has been used for many years to treat age-associated baldness in men, noted the authors. It was used off label in this study to treat radiation-associated persistent hair loss; 82% of patients showed at least some improvement.
For patients who do not improve with minoxidil, hair transplant and scalp reconstruction with plastic surgery were other options, the authors comment.
“Almost in all instances, there is something that can be done to improve persistent hair loss after radiation and give patients a sense of control,” senior author Mario E. Lacouture, MD, said in an interview. He is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York City.
About 60% of people with CNS tumors and 30% with head and neck cancer receive radiation to the head, and 75%-100% of these patients experience acute hair loss. For many, hair grows back in 2-3 months. However, for about 60%, hair loss persists for 6 or more months after completion of radiotherapy, the authors note.
In past work, Dr. Lacouture and colleagues found that persistent hair loss in cancer survivors is associated with depression, anxiety, and psychosocial distress.
“There are therapies and procedures that may mitigate persistent radiation therapy hair loss that can bring back psychosocial well-being to many of these patients,” Dr. Lacouture said. “These approaches are likely underutilized because patients are not being referred to specialists in hair or scalp reconstruction.”
Specialists can be found through the International Society for Hair Restoration Surgery and the American Academy of Dermatology, he added.
Study details
The retrospective cohort study included 71 children and adults who developed persistent hair loss after radiotherapy for primary CNS tumors (90%, n = 64) or head and neck sarcoma (10%, n = 7). The median age of the patients was 27 years (range, 4-75 years); 72% (n = 51) were female and 82% (n = 58) were White.
These patients had been treated at Memorial Sloan Kettering Cancer Center in New York City or St. Jude Children’s Hospital in Memphis from January 2011 to January 2019.
The team analyzed standardized clinical photographs of the scalp using the Common Terminology Criteria for Adverse Events version 5.0. Grade 1 alopecia was defined as hair loss of less than 50% of normal that does not require camouflage with hair pieces, scarves, or similar items. Grade 2 alopecia was defined as hair loss greater than 50% of normal that requires camouflage and is associated with negative psychosocial effects.
Over half of patients (56%, 40/70) had grade 1 hair loss. Clinical images were available for 54 patients; for most of these patients, hair loss was attributable to radiation alone (74%, n = 40). Evaluation of clinical imaging showed three variants of hair loss: localized (54%, 29/54), diffuse (24%, 13/54), and mixed pattern (22%, 12/54). Data on dermatologic imaging of the scalp (trichoscopy) were available for 28 patients; the main finding was white patches (57%, 16/28).
The median scalp radiation dose was 39.6 Gy (range, 15.1-50.0 Gy). The researchers estimate that a dose of 36.1 Gy (95% CI, 33.7-39.6 Gy) was sufficient to induce grade 2 hair loss in 50% of patients.
Severity of hair loss appeared to increase with radiation dose. For every 1-unit increase in radiation dose, the odds of grade 2 hair loss increased by 15% (odds ratio, 1.15; 95% CI, 1.04-1.28; P < .001). Proton irradiation was associated with even higher odds of severe hair loss (OR, 5.7; 95% CI, 1.05-30.8; P < .001). Results remained significant when analyses were controlled for sex, age at time of radiotherapy, and concurrent chemotherapy.
The majority of evaluable patients who were treated with topical minoxidil (5%) twice daily showed a response (82%, 28/34) during a median follow-up of 61 weeks (interquartile range, 21-105 weeks).
Among 25 of these patients for whom clinical images were available, 16% (4/25) showed complete response. Two patients improved with hair transplant, and one showed complete response with plastic surgery reconstruction of the hair.
The study had several limitations, including its retrospective design and a lack of complete data for certain variables, such as standardized clinical photographs, trichoscopy images, and radiotherapy treatment plans.
On the basis of these results, the authors are seeking funding for a prospective study of the use of minoxidil for persistent radiation-induced alopecia.
The study was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center. One or more authors has relationships with pharmaceutical companies, as listed in the original article.
This article first appeared on Medscape.com.
Assessment of Nail Content in the American Academy of Dermatology Patient Education Website
To the Editor:
Patients with skin, hair, or nail concerns often utilize online resources to self-diagnose or learn more about physician-diagnosed conditions. The American Academy of Dermatology (AAD) website offers the public access to informational pages categorized by disease or treatment (https://www.aad.org/public). We sought to evaluate the nail content by searching the Patients and Public section of the AAD website to qualitatively and quantitatively describe mentions of nail conditions. Psoriasis, psoriatic arthritis, atopic dermatitis, and ringworm content also were analyzed and compared to nail content. The analysis was performed on September 7, 2019.
Of the 73 topics listed in the Diseases and Treatments section of the site, 17 (23%) specifically mentioned nail symptoms or pathology (Table). Three additional topics—atopic dermatitis, cellulitis, and neurodermatitis—recommended keeping nails short to prevent injury from scratching. There was 1 mention of obtaining fungal cultures, 2 of nail scraping microscopy, 2 of nail clippings, and 2 of nail-related cancers. There were no mentions of nail biopsies. The total number of unique clinical images across all sections was 300, with 12 of nails. The video library contained 84 videos, of which 6 focused on nail health.
Our study demonstrated that nail content is underrepresented in the public education section of the AAD website. If patients are unable to find nail disease material on the AAD website, they may seek alternative sources that are unreliable. Prior studies have shown that patient Internet resources for subungual melanoma and onychomycosis often are inadequate in quality and readability.1,2
Representative photographs and key information on common nail diseases could be added to improve patient education. The atopic dermatitis section should include text on related nail changes with accompanying images. We also recommend including paronychia information and images as either a separate topic or in the cellulitis section. The contact dermatitis section mentions nail cosmetics as causative factors, but an image of roller-coaster onycholysis may be more helpful.3 Although the alopecia areata section mentions nail changes, this information should be added to the general hair loss section of the site, as many patients may initially seek out the latter category. Herpes simplex may affect nails, and an image showing these changes would be instructive. In addition, pyogenic granulomas and paronychia occur with isotretinoin use.4
Many of the included images were not representative of common clinical findings. The nail lichen planus image showed pitting instead of more typical findings of nail plate atrophy and pterygium. The nail melanoma image showed thickened yellow toenails and the fifth toenail with a thin gray-brown band instead of an isolated wide black band. The nail fungus section included images of superficial onychomycosis and severe onychodystrophy instead of showing more common changes such as distal onycholysis with subungual hyperkeratosis, which is typical of the most common subtype, distal lateral subungual onychomycosis.5 Onychomycosis was referenced again in the ringworm section with 1 image repeated from the nail fungus section and another image that appeared to be a subungual hematoma.
The AAD website offers important patient education resources; however, nail content is underrepresented on this platform. Dermatologists are experts on nail disease, and increased efforts are needed to educate the public about frequently encountered nail signs and symptoms that could signify a serious underlying condition.
After our original search and analysis, new nail topics, images, and videos have been added; therefore, there has been a positive trend toward new nail content being added to site, which will greatly benefit patients.
- Kang R, Lipner S. Assessment of internet sources on subungual melanoma [published online August 30, 2018]. Melanoma Res. doi:10.1097/CMR.0000000000000508.
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Rieder EA, Tosti A. Cosmetically induced disorders of the nail with update on contemporary nail manicures. J Clin Aesthet Dermatol. 2016;9:39-44.
- Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, et al. Uncommon side effects of isotretinoin therapy: paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
To the Editor:
Patients with skin, hair, or nail concerns often utilize online resources to self-diagnose or learn more about physician-diagnosed conditions. The American Academy of Dermatology (AAD) website offers the public access to informational pages categorized by disease or treatment (https://www.aad.org/public). We sought to evaluate the nail content by searching the Patients and Public section of the AAD website to qualitatively and quantitatively describe mentions of nail conditions. Psoriasis, psoriatic arthritis, atopic dermatitis, and ringworm content also were analyzed and compared to nail content. The analysis was performed on September 7, 2019.
Of the 73 topics listed in the Diseases and Treatments section of the site, 17 (23%) specifically mentioned nail symptoms or pathology (Table). Three additional topics—atopic dermatitis, cellulitis, and neurodermatitis—recommended keeping nails short to prevent injury from scratching. There was 1 mention of obtaining fungal cultures, 2 of nail scraping microscopy, 2 of nail clippings, and 2 of nail-related cancers. There were no mentions of nail biopsies. The total number of unique clinical images across all sections was 300, with 12 of nails. The video library contained 84 videos, of which 6 focused on nail health.

Our study demonstrated that nail content is underrepresented in the public education section of the AAD website. If patients are unable to find nail disease material on the AAD website, they may seek alternative sources that are unreliable. Prior studies have shown that patient Internet resources for subungual melanoma and onychomycosis often are inadequate in quality and readability.1,2
Representative photographs and key information on common nail diseases could be added to improve patient education. The atopic dermatitis section should include text on related nail changes with accompanying images. We also recommend including paronychia information and images as either a separate topic or in the cellulitis section. The contact dermatitis section mentions nail cosmetics as causative factors, but an image of roller-coaster onycholysis may be more helpful.3 Although the alopecia areata section mentions nail changes, this information should be added to the general hair loss section of the site, as many patients may initially seek out the latter category. Herpes simplex may affect nails, and an image showing these changes would be instructive. In addition, pyogenic granulomas and paronychia occur with isotretinoin use.4
Many of the included images were not representative of common clinical findings. The nail lichen planus image showed pitting instead of more typical findings of nail plate atrophy and pterygium. The nail melanoma image showed thickened yellow toenails and the fifth toenail with a thin gray-brown band instead of an isolated wide black band. The nail fungus section included images of superficial onychomycosis and severe onychodystrophy instead of showing more common changes such as distal onycholysis with subungual hyperkeratosis, which is typical of the most common subtype, distal lateral subungual onychomycosis.5 Onychomycosis was referenced again in the ringworm section with 1 image repeated from the nail fungus section and another image that appeared to be a subungual hematoma.
The AAD website offers important patient education resources; however, nail content is underrepresented on this platform. Dermatologists are experts on nail disease, and increased efforts are needed to educate the public about frequently encountered nail signs and symptoms that could signify a serious underlying condition.
After our original search and analysis, new nail topics, images, and videos have been added; therefore, there has been a positive trend toward new nail content being added to site, which will greatly benefit patients.
To the Editor:
Patients with skin, hair, or nail concerns often utilize online resources to self-diagnose or learn more about physician-diagnosed conditions. The American Academy of Dermatology (AAD) website offers the public access to informational pages categorized by disease or treatment (https://www.aad.org/public). We sought to evaluate the nail content by searching the Patients and Public section of the AAD website to qualitatively and quantitatively describe mentions of nail conditions. Psoriasis, psoriatic arthritis, atopic dermatitis, and ringworm content also were analyzed and compared to nail content. The analysis was performed on September 7, 2019.
Of the 73 topics listed in the Diseases and Treatments section of the site, 17 (23%) specifically mentioned nail symptoms or pathology (Table). Three additional topics—atopic dermatitis, cellulitis, and neurodermatitis—recommended keeping nails short to prevent injury from scratching. There was 1 mention of obtaining fungal cultures, 2 of nail scraping microscopy, 2 of nail clippings, and 2 of nail-related cancers. There were no mentions of nail biopsies. The total number of unique clinical images across all sections was 300, with 12 of nails. The video library contained 84 videos, of which 6 focused on nail health.

Our study demonstrated that nail content is underrepresented in the public education section of the AAD website. If patients are unable to find nail disease material on the AAD website, they may seek alternative sources that are unreliable. Prior studies have shown that patient Internet resources for subungual melanoma and onychomycosis often are inadequate in quality and readability.1,2
Representative photographs and key information on common nail diseases could be added to improve patient education. The atopic dermatitis section should include text on related nail changes with accompanying images. We also recommend including paronychia information and images as either a separate topic or in the cellulitis section. The contact dermatitis section mentions nail cosmetics as causative factors, but an image of roller-coaster onycholysis may be more helpful.3 Although the alopecia areata section mentions nail changes, this information should be added to the general hair loss section of the site, as many patients may initially seek out the latter category. Herpes simplex may affect nails, and an image showing these changes would be instructive. In addition, pyogenic granulomas and paronychia occur with isotretinoin use.4
Many of the included images were not representative of common clinical findings. The nail lichen planus image showed pitting instead of more typical findings of nail plate atrophy and pterygium. The nail melanoma image showed thickened yellow toenails and the fifth toenail with a thin gray-brown band instead of an isolated wide black band. The nail fungus section included images of superficial onychomycosis and severe onychodystrophy instead of showing more common changes such as distal onycholysis with subungual hyperkeratosis, which is typical of the most common subtype, distal lateral subungual onychomycosis.5 Onychomycosis was referenced again in the ringworm section with 1 image repeated from the nail fungus section and another image that appeared to be a subungual hematoma.
The AAD website offers important patient education resources; however, nail content is underrepresented on this platform. Dermatologists are experts on nail disease, and increased efforts are needed to educate the public about frequently encountered nail signs and symptoms that could signify a serious underlying condition.
After our original search and analysis, new nail topics, images, and videos have been added; therefore, there has been a positive trend toward new nail content being added to site, which will greatly benefit patients.
- Kang R, Lipner S. Assessment of internet sources on subungual melanoma [published online August 30, 2018]. Melanoma Res. doi:10.1097/CMR.0000000000000508.
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Rieder EA, Tosti A. Cosmetically induced disorders of the nail with update on contemporary nail manicures. J Clin Aesthet Dermatol. 2016;9:39-44.
- Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, et al. Uncommon side effects of isotretinoin therapy: paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kang R, Lipner S. Assessment of internet sources on subungual melanoma [published online August 30, 2018]. Melanoma Res. doi:10.1097/CMR.0000000000000508.
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Rieder EA, Tosti A. Cosmetically induced disorders of the nail with update on contemporary nail manicures. J Clin Aesthet Dermatol. 2016;9:39-44.
- Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, et al. Uncommon side effects of isotretinoin therapy: paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
Practice Points
- Patients often utilize online resources to research skin, hair, and nail conditions.
- Nail signs and symptoms may represent a serious underlying condition, and nail content is underrepresented on the American Academy of Dermatology (AAD) Patients and Public section of the website.
- There is a need for more information on nail conditions on the AAD website, offering patients a more comprehensive online dermatology resource. Subsequently, there has been a positive trend toward new nail content being added to the site.
For suspected hair disorders, consider trichoscopy before biopsy
In the clinical experience of Bianca Maria Piraccini, MD,
Dermoscopic imaging, also known as trichoscopy, “avoids invasive procedures and provides immediate results,” Dr. Piraccini, of the University of Bologna’s division of dermatology in the department of experimental, diagnostic, and specialty medicine, said during the virtual annual meeting of the Society for Pediatric Dermatology. “It is helpful for diagnosing all sorts of alopecia, starting with those that appear at birth, such as aplasia cutis congenita to those that appear in adolescence, such as androgenetic alopecia.”
Dr. Piraccini noted that lanugo hair is produced at 16-20 weeks’ gestation and is shed in utero and replaced by thicker hair at 32-36 weeks. “The speed of transition from vellus to intermediate and terminal hair varies from child to child,” she said. “The scalp at birth presents with thin, intermediate, or thick hair.”
In a dermoscopic evaluation of hair in 45 neonates during their first 30 days of life, Dr. Piraccini and colleagues found that 70% had low density hair while the remaining 30% had high density hair (Br J Dermatol 2013; 169:896-900). Two neonates presented a frontal-temporal pattern of hair loss. Trichoscopy revealed that nine neonates, all in the poor hair density group, had a particular hair shaft dermoscopic feature, characterized by the presence of widespread thin hair.
In some children, she continued, hair in the occipital area does not enter the telogen phase until after birth. These hairs remain on the scalp for 8-12 weeks and then fall out, resulting in neonatal occipital alopecia, which is the most common form of transient neonatal hair loss. Neonatal occipital alopecia is characterized by a band-like shape or oval alopecic patch with a sharp lower margin, but it often goes unnoticed by parents.
“It occurs with higher prevalence in infants born to mothers younger than age 34, in those with a non-cesarean birth, and in those with a gestational age greater than 37 weeks,” Dr. Piraccini said. “There are different degrees of severity. On trichoscopy, the condition appears as thin regrowing hair. The outcome is totally benign, with normal hair growth within the first year of life.”
Any aspect of alopecia in the occipital area in young children may be a sign of hair shaft disorders, which are characterized by increased hair fragility. “Trichoscopy is diagnostic,” she said. “When applied to the hair you see monilethrix, a rare inherited disorder characterized by sparse, brittle hair that often breaks before reaching a few inches in length. As the child grows, the hair gradually acquires the characteristics it will have in adulthood. “It may remain thin and with a short anagen phase for several years, but acute shedding is rare,” she said.
When an older child presents with increased hair shedding, the first exam to perform is the pull test. If it results in painless traction of several anagen hair without sheaths and with ragged cuticles, think about loose anagen hair syndrome. This condition affects females more than males, usually occurs between the ages of 2 and 5, and is characterized by a defective anchoring of the hair shaft to the hair follicle. The three clinical types of loose anagen hair syndrome are short, rough sparse hair; increased shedding; and areas of alopecia. The syndrome “tends to be inherited but spontaneously improves with aging,” Dr. Piraccini said.
Alopecia areata, another common pediatric hair disorder, occurs in 20% of patients younger than 16 years of age and 9% of those with Down syndrome, and is associated with a family history of the condition. Young age at onset is a negative prognostic factor. “On trichoscopy, common features of alopecia areata are yellow dots, black dots, exclamation mark hairs, and broken hair,” she said. “Trichoscopy can also help you distinguish acute from chronic alopecia areata. The risk of relapse is common, and psychological support is mandatory, because it is very stressful for children.”
Another form of patchy alopecia, trichotillomania, occurs mainly in school-aged children and appears as irregular patches of alopecia with hairs broken at different lengths. “The pull test is negative because all telogen hairs have been pulled out by the patient,” Dr. Piraccini said. “Parents often do not accept the diagnosis as they do not see the child touching his or her hair. It has a good prognosis.”
Trichoscopic signs of trichotillomania include black dots, hair broken at different length, flame hair, clots of hair, and tulip hair. Treatment typically consists of psychological counseling and N-acetylcysteine 600-2,400 g/day.
Dr. Piraccini reported having no relevant financial disclosures.
In the clinical experience of Bianca Maria Piraccini, MD,
Dermoscopic imaging, also known as trichoscopy, “avoids invasive procedures and provides immediate results,” Dr. Piraccini, of the University of Bologna’s division of dermatology in the department of experimental, diagnostic, and specialty medicine, said during the virtual annual meeting of the Society for Pediatric Dermatology. “It is helpful for diagnosing all sorts of alopecia, starting with those that appear at birth, such as aplasia cutis congenita to those that appear in adolescence, such as androgenetic alopecia.”
Dr. Piraccini noted that lanugo hair is produced at 16-20 weeks’ gestation and is shed in utero and replaced by thicker hair at 32-36 weeks. “The speed of transition from vellus to intermediate and terminal hair varies from child to child,” she said. “The scalp at birth presents with thin, intermediate, or thick hair.”
In a dermoscopic evaluation of hair in 45 neonates during their first 30 days of life, Dr. Piraccini and colleagues found that 70% had low density hair while the remaining 30% had high density hair (Br J Dermatol 2013; 169:896-900). Two neonates presented a frontal-temporal pattern of hair loss. Trichoscopy revealed that nine neonates, all in the poor hair density group, had a particular hair shaft dermoscopic feature, characterized by the presence of widespread thin hair.
In some children, she continued, hair in the occipital area does not enter the telogen phase until after birth. These hairs remain on the scalp for 8-12 weeks and then fall out, resulting in neonatal occipital alopecia, which is the most common form of transient neonatal hair loss. Neonatal occipital alopecia is characterized by a band-like shape or oval alopecic patch with a sharp lower margin, but it often goes unnoticed by parents.
“It occurs with higher prevalence in infants born to mothers younger than age 34, in those with a non-cesarean birth, and in those with a gestational age greater than 37 weeks,” Dr. Piraccini said. “There are different degrees of severity. On trichoscopy, the condition appears as thin regrowing hair. The outcome is totally benign, with normal hair growth within the first year of life.”
Any aspect of alopecia in the occipital area in young children may be a sign of hair shaft disorders, which are characterized by increased hair fragility. “Trichoscopy is diagnostic,” she said. “When applied to the hair you see monilethrix, a rare inherited disorder characterized by sparse, brittle hair that often breaks before reaching a few inches in length. As the child grows, the hair gradually acquires the characteristics it will have in adulthood. “It may remain thin and with a short anagen phase for several years, but acute shedding is rare,” she said.
When an older child presents with increased hair shedding, the first exam to perform is the pull test. If it results in painless traction of several anagen hair without sheaths and with ragged cuticles, think about loose anagen hair syndrome. This condition affects females more than males, usually occurs between the ages of 2 and 5, and is characterized by a defective anchoring of the hair shaft to the hair follicle. The three clinical types of loose anagen hair syndrome are short, rough sparse hair; increased shedding; and areas of alopecia. The syndrome “tends to be inherited but spontaneously improves with aging,” Dr. Piraccini said.
Alopecia areata, another common pediatric hair disorder, occurs in 20% of patients younger than 16 years of age and 9% of those with Down syndrome, and is associated with a family history of the condition. Young age at onset is a negative prognostic factor. “On trichoscopy, common features of alopecia areata are yellow dots, black dots, exclamation mark hairs, and broken hair,” she said. “Trichoscopy can also help you distinguish acute from chronic alopecia areata. The risk of relapse is common, and psychological support is mandatory, because it is very stressful for children.”
Another form of patchy alopecia, trichotillomania, occurs mainly in school-aged children and appears as irregular patches of alopecia with hairs broken at different lengths. “The pull test is negative because all telogen hairs have been pulled out by the patient,” Dr. Piraccini said. “Parents often do not accept the diagnosis as they do not see the child touching his or her hair. It has a good prognosis.”
Trichoscopic signs of trichotillomania include black dots, hair broken at different length, flame hair, clots of hair, and tulip hair. Treatment typically consists of psychological counseling and N-acetylcysteine 600-2,400 g/day.
Dr. Piraccini reported having no relevant financial disclosures.
In the clinical experience of Bianca Maria Piraccini, MD,
Dermoscopic imaging, also known as trichoscopy, “avoids invasive procedures and provides immediate results,” Dr. Piraccini, of the University of Bologna’s division of dermatology in the department of experimental, diagnostic, and specialty medicine, said during the virtual annual meeting of the Society for Pediatric Dermatology. “It is helpful for diagnosing all sorts of alopecia, starting with those that appear at birth, such as aplasia cutis congenita to those that appear in adolescence, such as androgenetic alopecia.”
Dr. Piraccini noted that lanugo hair is produced at 16-20 weeks’ gestation and is shed in utero and replaced by thicker hair at 32-36 weeks. “The speed of transition from vellus to intermediate and terminal hair varies from child to child,” she said. “The scalp at birth presents with thin, intermediate, or thick hair.”
In a dermoscopic evaluation of hair in 45 neonates during their first 30 days of life, Dr. Piraccini and colleagues found that 70% had low density hair while the remaining 30% had high density hair (Br J Dermatol 2013; 169:896-900). Two neonates presented a frontal-temporal pattern of hair loss. Trichoscopy revealed that nine neonates, all in the poor hair density group, had a particular hair shaft dermoscopic feature, characterized by the presence of widespread thin hair.
In some children, she continued, hair in the occipital area does not enter the telogen phase until after birth. These hairs remain on the scalp for 8-12 weeks and then fall out, resulting in neonatal occipital alopecia, which is the most common form of transient neonatal hair loss. Neonatal occipital alopecia is characterized by a band-like shape or oval alopecic patch with a sharp lower margin, but it often goes unnoticed by parents.
“It occurs with higher prevalence in infants born to mothers younger than age 34, in those with a non-cesarean birth, and in those with a gestational age greater than 37 weeks,” Dr. Piraccini said. “There are different degrees of severity. On trichoscopy, the condition appears as thin regrowing hair. The outcome is totally benign, with normal hair growth within the first year of life.”
Any aspect of alopecia in the occipital area in young children may be a sign of hair shaft disorders, which are characterized by increased hair fragility. “Trichoscopy is diagnostic,” she said. “When applied to the hair you see monilethrix, a rare inherited disorder characterized by sparse, brittle hair that often breaks before reaching a few inches in length. As the child grows, the hair gradually acquires the characteristics it will have in adulthood. “It may remain thin and with a short anagen phase for several years, but acute shedding is rare,” she said.
When an older child presents with increased hair shedding, the first exam to perform is the pull test. If it results in painless traction of several anagen hair without sheaths and with ragged cuticles, think about loose anagen hair syndrome. This condition affects females more than males, usually occurs between the ages of 2 and 5, and is characterized by a defective anchoring of the hair shaft to the hair follicle. The three clinical types of loose anagen hair syndrome are short, rough sparse hair; increased shedding; and areas of alopecia. The syndrome “tends to be inherited but spontaneously improves with aging,” Dr. Piraccini said.
Alopecia areata, another common pediatric hair disorder, occurs in 20% of patients younger than 16 years of age and 9% of those with Down syndrome, and is associated with a family history of the condition. Young age at onset is a negative prognostic factor. “On trichoscopy, common features of alopecia areata are yellow dots, black dots, exclamation mark hairs, and broken hair,” she said. “Trichoscopy can also help you distinguish acute from chronic alopecia areata. The risk of relapse is common, and psychological support is mandatory, because it is very stressful for children.”
Another form of patchy alopecia, trichotillomania, occurs mainly in school-aged children and appears as irregular patches of alopecia with hairs broken at different lengths. “The pull test is negative because all telogen hairs have been pulled out by the patient,” Dr. Piraccini said. “Parents often do not accept the diagnosis as they do not see the child touching his or her hair. It has a good prognosis.”
Trichoscopic signs of trichotillomania include black dots, hair broken at different length, flame hair, clots of hair, and tulip hair. Treatment typically consists of psychological counseling and N-acetylcysteine 600-2,400 g/day.
Dr. Piraccini reported having no relevant financial disclosures.
FROM SPD 2020
Brilliant Green Staining of the Fingernails
Case Report
A 92-year-old Eastern European woman presented to our nail clinic with a history of onychodystrophy and arthralgia of the digits of several months’ duration. Her dermatologic history was notable for irritant hand dermatitis. A prior nail plate clipping with histopathologic examination was negative for fungal elements. Physical examination revealed onychorrhexis of all fingernails as well as onycholysis and subungual hyperkeratosis of the right fourth fingernail. Blue-green staining was incidentally noted on the right second and third fingernails and nail folds (Figure 1). Contact dermoscopy using ultrasound gel revealed translucent areas with sparse pigment, though denser areas had a fine branching pattern (Figure 2). When questioned, the patient reported use of “zelyonka,” a brilliant green solution, to self-treat the nails. Histopathology on repeat nail clippings showed parakeratosis and serum, which was most consistent with her known history of irritant hand dermatitis. Radiographs of the hands revealed osteoarthritis that was most prominent at the distal interphalangeal joints.
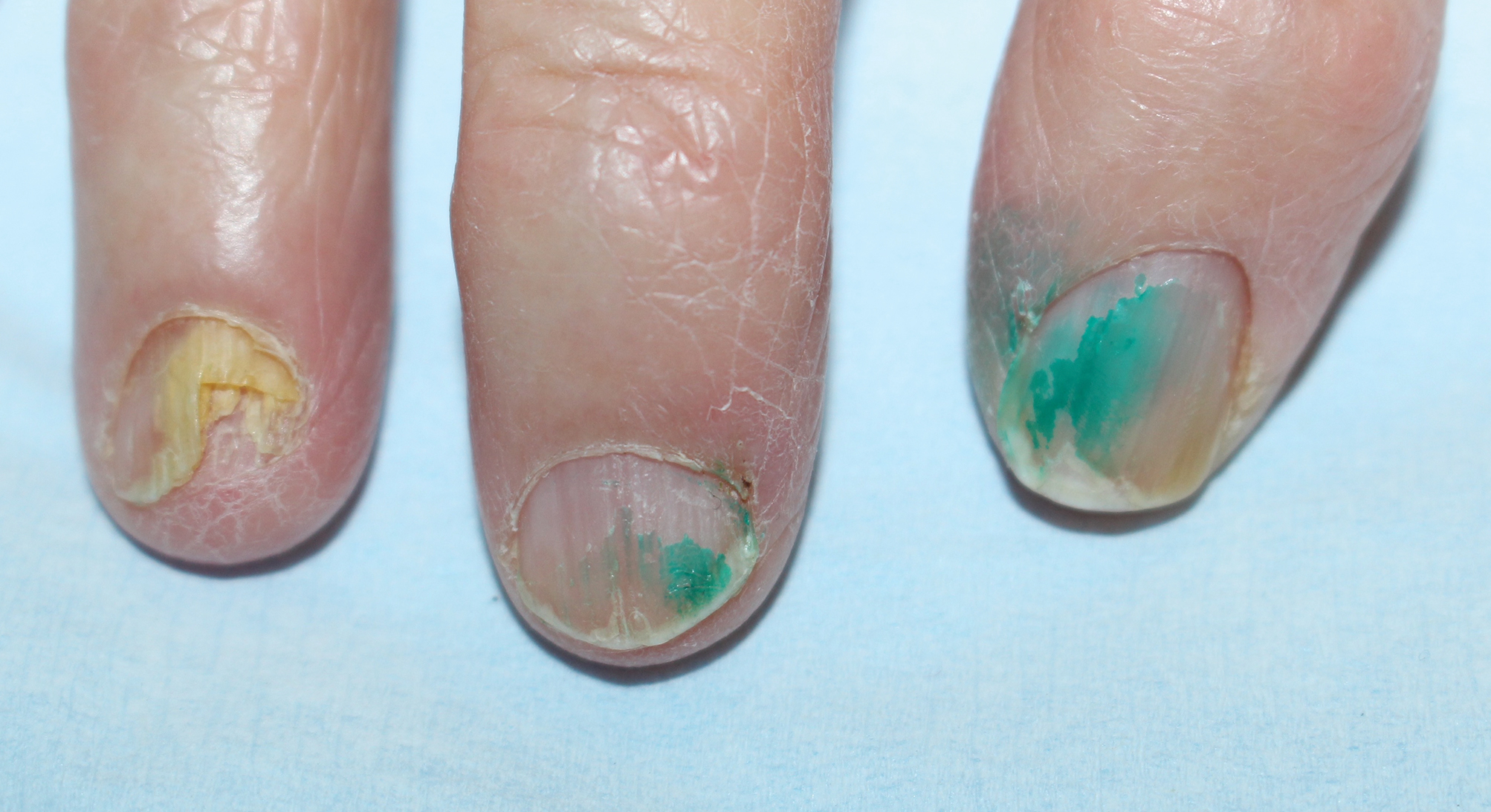
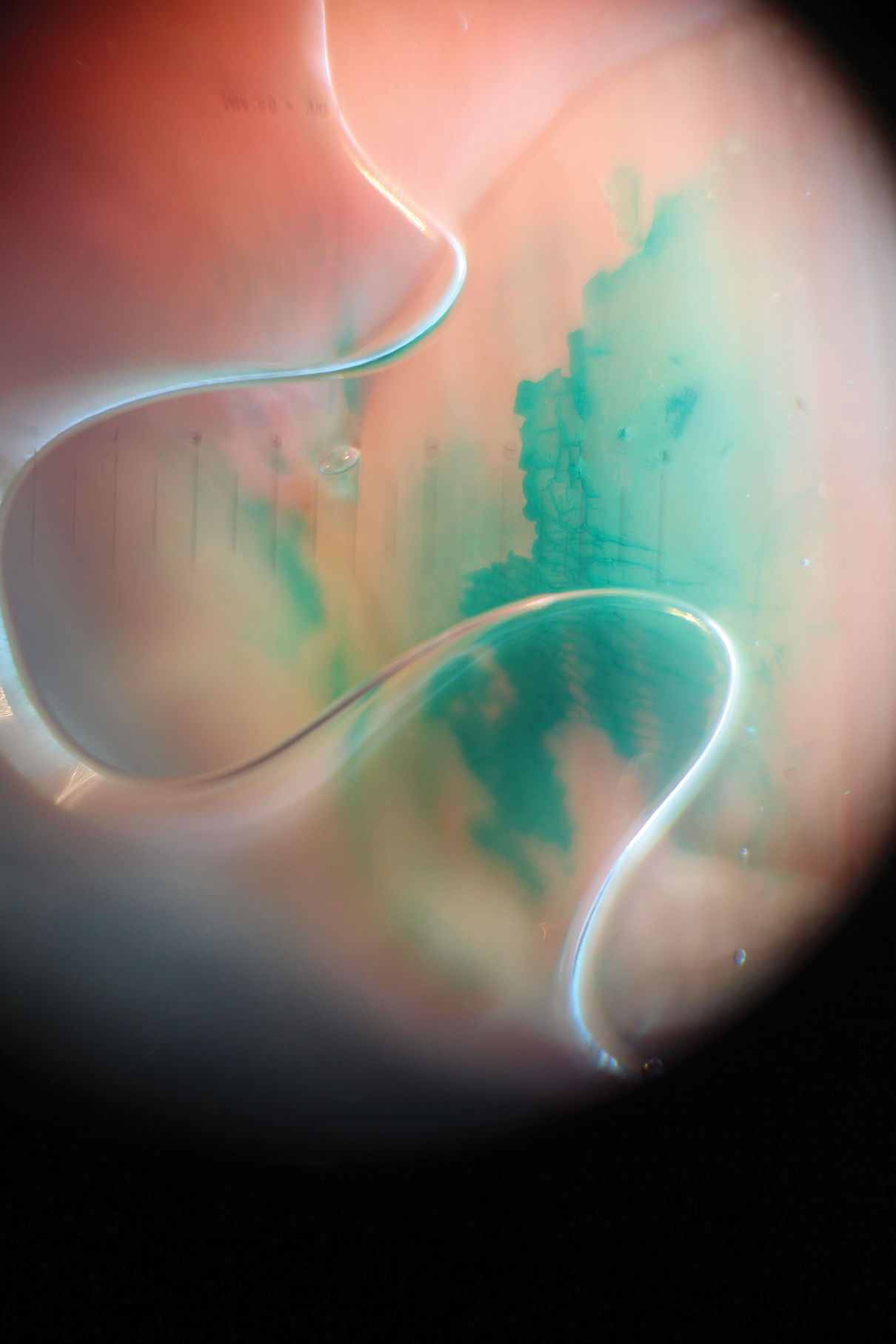
Comment
Brilliant green is a triphenylmethane dye commonly used in Eastern Europe and other regions for the treatment of superficial skin infections and onychomycosis.1 Its use as an antiseptic and wound healing agent has been investigated in the scientific literature since at least the early 20th century.2 Brilliant green typically is applied in a 0.1% to 2% ethanol solution.1 The dye has bactericidal activity against gram-positive organisms, particularly staphylococci and streptococci.2,3 It has been used for the treatment of fungal skin and nail infections since at least the early 20th century, with anecdotal success.4 Although there have been no studies investigating use of brilliant green alone for the treatment of onychomycosis, it is sometimes used in combination with conventional oral agents for this purpose.5 Because of its availability, safety, ease of use, and low cost, brilliant green has been promoted as an antiseptic in resource-poor settings.3 The revival of brilliant green and other antiseptic dyes in these settings has been suggested as an alternative to oral antibiotic agents, to which resistance is rising, and as a potential cancer therapy.6,7 Although brilliant green’s mechanism of action in treating skin infections is unclear, it has been shown to form covalent adducts with thioredoxin reductase 2, a protein conserved from bacteria to humans with an essential function for cellular activity.7
Early case studies suggested that brilliant green was beneficial in treating wounds2; however, this indication is controversial. In a guinea pig study, brilliant green was shown to inhibit wound healing and the formation of granulation tissue.8 It also should be noted that when used topically, brilliant green may cause skin sensitization, necrotic skin reactions, and permanent staining of clothing. It has no known anti-inflammatory properties and also may cause skin irritation.8 Brilliant green may cause blindness if it comes in contact with the eyes.1
Brilliant green has other potential dermatologic indications. For example, a combination of brilliant green and gentian violet, a related dye, has demonstrated efficacy in the treatment of cutaneous hemangiomas in mouse models by blocking expression of angiopoietin-2.7
Dermatologists should be familiar with brilliant green and its common uses as well as adverse effects. Brilliant green is commercially available for a low cost ($5 to $20) in specialty pharmacies or online (eg, Amazon). It is sold alone or in combination with gentian violet and proflavine hemisulfate, and a prescription is not required. Due to its low cost and accessibility, patients may use brilliant green to self-treat dermatologic conditions. Green nails due to staining with brilliant green dye must be distinguished from other etiologies causing green nail discoloration, such as infection with Pseudomonas aeruginosa or Aspergillus, bullous disorders, jaundice, “old” hematomas, nail polish, and other exogenous pigments.
- Balabanova M, Popova L, Tchipeva R. Dyes in dermatology. Clin Dermatol. 2003;21:2-6.
- Browning CH, Gulbransen R, Kennaway EL, et al. Flavine and brilliant green, powerful antiseptics with low toxicity to the tissues: their use in the treatment of infected wounds. Br Med J. 1917;1:73-78.
- Bakker P, Doorne H, Gooskens V, et al. Activity of gentian violet and brilliant green against some microorganisms associated with skin infections. Int J Dermatol. 1992;31:210-213.
- Montgomery RM, Casper EA. Cutaneous manifestations of the fungi causing dermatophytosis and onychomycosis and their treatment. J Am Med Assoc. 1945;128:77-83.
- Tchernev G, Cardoso JC, Ali MM, et al. Primary onychomycosis with granulomatous Tinea faciei. Braz J Infect Dis. 2010;14:546-547.
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29:69-73.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22:775-80.
- Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol. 1986;115:41-44
Case Report
A 92-year-old Eastern European woman presented to our nail clinic with a history of onychodystrophy and arthralgia of the digits of several months’ duration. Her dermatologic history was notable for irritant hand dermatitis. A prior nail plate clipping with histopathologic examination was negative for fungal elements. Physical examination revealed onychorrhexis of all fingernails as well as onycholysis and subungual hyperkeratosis of the right fourth fingernail. Blue-green staining was incidentally noted on the right second and third fingernails and nail folds (Figure 1). Contact dermoscopy using ultrasound gel revealed translucent areas with sparse pigment, though denser areas had a fine branching pattern (Figure 2). When questioned, the patient reported use of “zelyonka,” a brilliant green solution, to self-treat the nails. Histopathology on repeat nail clippings showed parakeratosis and serum, which was most consistent with her known history of irritant hand dermatitis. Radiographs of the hands revealed osteoarthritis that was most prominent at the distal interphalangeal joints.


Comment
Brilliant green is a triphenylmethane dye commonly used in Eastern Europe and other regions for the treatment of superficial skin infections and onychomycosis.1 Its use as an antiseptic and wound healing agent has been investigated in the scientific literature since at least the early 20th century.2 Brilliant green typically is applied in a 0.1% to 2% ethanol solution.1 The dye has bactericidal activity against gram-positive organisms, particularly staphylococci and streptococci.2,3 It has been used for the treatment of fungal skin and nail infections since at least the early 20th century, with anecdotal success.4 Although there have been no studies investigating use of brilliant green alone for the treatment of onychomycosis, it is sometimes used in combination with conventional oral agents for this purpose.5 Because of its availability, safety, ease of use, and low cost, brilliant green has been promoted as an antiseptic in resource-poor settings.3 The revival of brilliant green and other antiseptic dyes in these settings has been suggested as an alternative to oral antibiotic agents, to which resistance is rising, and as a potential cancer therapy.6,7 Although brilliant green’s mechanism of action in treating skin infections is unclear, it has been shown to form covalent adducts with thioredoxin reductase 2, a protein conserved from bacteria to humans with an essential function for cellular activity.7
Early case studies suggested that brilliant green was beneficial in treating wounds2; however, this indication is controversial. In a guinea pig study, brilliant green was shown to inhibit wound healing and the formation of granulation tissue.8 It also should be noted that when used topically, brilliant green may cause skin sensitization, necrotic skin reactions, and permanent staining of clothing. It has no known anti-inflammatory properties and also may cause skin irritation.8 Brilliant green may cause blindness if it comes in contact with the eyes.1
Brilliant green has other potential dermatologic indications. For example, a combination of brilliant green and gentian violet, a related dye, has demonstrated efficacy in the treatment of cutaneous hemangiomas in mouse models by blocking expression of angiopoietin-2.7
Dermatologists should be familiar with brilliant green and its common uses as well as adverse effects. Brilliant green is commercially available for a low cost ($5 to $20) in specialty pharmacies or online (eg, Amazon). It is sold alone or in combination with gentian violet and proflavine hemisulfate, and a prescription is not required. Due to its low cost and accessibility, patients may use brilliant green to self-treat dermatologic conditions. Green nails due to staining with brilliant green dye must be distinguished from other etiologies causing green nail discoloration, such as infection with Pseudomonas aeruginosa or Aspergillus, bullous disorders, jaundice, “old” hematomas, nail polish, and other exogenous pigments.
Case Report
A 92-year-old Eastern European woman presented to our nail clinic with a history of onychodystrophy and arthralgia of the digits of several months’ duration. Her dermatologic history was notable for irritant hand dermatitis. A prior nail plate clipping with histopathologic examination was negative for fungal elements. Physical examination revealed onychorrhexis of all fingernails as well as onycholysis and subungual hyperkeratosis of the right fourth fingernail. Blue-green staining was incidentally noted on the right second and third fingernails and nail folds (Figure 1). Contact dermoscopy using ultrasound gel revealed translucent areas with sparse pigment, though denser areas had a fine branching pattern (Figure 2). When questioned, the patient reported use of “zelyonka,” a brilliant green solution, to self-treat the nails. Histopathology on repeat nail clippings showed parakeratosis and serum, which was most consistent with her known history of irritant hand dermatitis. Radiographs of the hands revealed osteoarthritis that was most prominent at the distal interphalangeal joints.


Comment
Brilliant green is a triphenylmethane dye commonly used in Eastern Europe and other regions for the treatment of superficial skin infections and onychomycosis.1 Its use as an antiseptic and wound healing agent has been investigated in the scientific literature since at least the early 20th century.2 Brilliant green typically is applied in a 0.1% to 2% ethanol solution.1 The dye has bactericidal activity against gram-positive organisms, particularly staphylococci and streptococci.2,3 It has been used for the treatment of fungal skin and nail infections since at least the early 20th century, with anecdotal success.4 Although there have been no studies investigating use of brilliant green alone for the treatment of onychomycosis, it is sometimes used in combination with conventional oral agents for this purpose.5 Because of its availability, safety, ease of use, and low cost, brilliant green has been promoted as an antiseptic in resource-poor settings.3 The revival of brilliant green and other antiseptic dyes in these settings has been suggested as an alternative to oral antibiotic agents, to which resistance is rising, and as a potential cancer therapy.6,7 Although brilliant green’s mechanism of action in treating skin infections is unclear, it has been shown to form covalent adducts with thioredoxin reductase 2, a protein conserved from bacteria to humans with an essential function for cellular activity.7
Early case studies suggested that brilliant green was beneficial in treating wounds2; however, this indication is controversial. In a guinea pig study, brilliant green was shown to inhibit wound healing and the formation of granulation tissue.8 It also should be noted that when used topically, brilliant green may cause skin sensitization, necrotic skin reactions, and permanent staining of clothing. It has no known anti-inflammatory properties and also may cause skin irritation.8 Brilliant green may cause blindness if it comes in contact with the eyes.1
Brilliant green has other potential dermatologic indications. For example, a combination of brilliant green and gentian violet, a related dye, has demonstrated efficacy in the treatment of cutaneous hemangiomas in mouse models by blocking expression of angiopoietin-2.7
Dermatologists should be familiar with brilliant green and its common uses as well as adverse effects. Brilliant green is commercially available for a low cost ($5 to $20) in specialty pharmacies or online (eg, Amazon). It is sold alone or in combination with gentian violet and proflavine hemisulfate, and a prescription is not required. Due to its low cost and accessibility, patients may use brilliant green to self-treat dermatologic conditions. Green nails due to staining with brilliant green dye must be distinguished from other etiologies causing green nail discoloration, such as infection with Pseudomonas aeruginosa or Aspergillus, bullous disorders, jaundice, “old” hematomas, nail polish, and other exogenous pigments.
- Balabanova M, Popova L, Tchipeva R. Dyes in dermatology. Clin Dermatol. 2003;21:2-6.
- Browning CH, Gulbransen R, Kennaway EL, et al. Flavine and brilliant green, powerful antiseptics with low toxicity to the tissues: their use in the treatment of infected wounds. Br Med J. 1917;1:73-78.
- Bakker P, Doorne H, Gooskens V, et al. Activity of gentian violet and brilliant green against some microorganisms associated with skin infections. Int J Dermatol. 1992;31:210-213.
- Montgomery RM, Casper EA. Cutaneous manifestations of the fungi causing dermatophytosis and onychomycosis and their treatment. J Am Med Assoc. 1945;128:77-83.
- Tchernev G, Cardoso JC, Ali MM, et al. Primary onychomycosis with granulomatous Tinea faciei. Braz J Infect Dis. 2010;14:546-547.
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29:69-73.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22:775-80.
- Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol. 1986;115:41-44
- Balabanova M, Popova L, Tchipeva R. Dyes in dermatology. Clin Dermatol. 2003;21:2-6.
- Browning CH, Gulbransen R, Kennaway EL, et al. Flavine and brilliant green, powerful antiseptics with low toxicity to the tissues: their use in the treatment of infected wounds. Br Med J. 1917;1:73-78.
- Bakker P, Doorne H, Gooskens V, et al. Activity of gentian violet and brilliant green against some microorganisms associated with skin infections. Int J Dermatol. 1992;31:210-213.
- Montgomery RM, Casper EA. Cutaneous manifestations of the fungi causing dermatophytosis and onychomycosis and their treatment. J Am Med Assoc. 1945;128:77-83.
- Tchernev G, Cardoso JC, Ali MM, et al. Primary onychomycosis with granulomatous Tinea faciei. Braz J Infect Dis. 2010;14:546-547.
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29:69-73.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22:775-80.
- Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol. 1986;115:41-44
Practice Points
- Chloronychia, or green nail syndrome, is due to Pseudomonas aeruginosaPalatino LT Std infection and is a common etiology of green nail discoloration. Green nail discoloration also may be secondary to use of the antiseptic dye brilliant green.
- Brilliant green is bactericidal but has no known antifungal or anti-inflammatory activity; it should be considered in the differential diagnosis of green nail discoloration and also may cause blindness with eye contact.
Utilization of a Stress Ball to Diminish Anxiety During Nail Surgery
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.
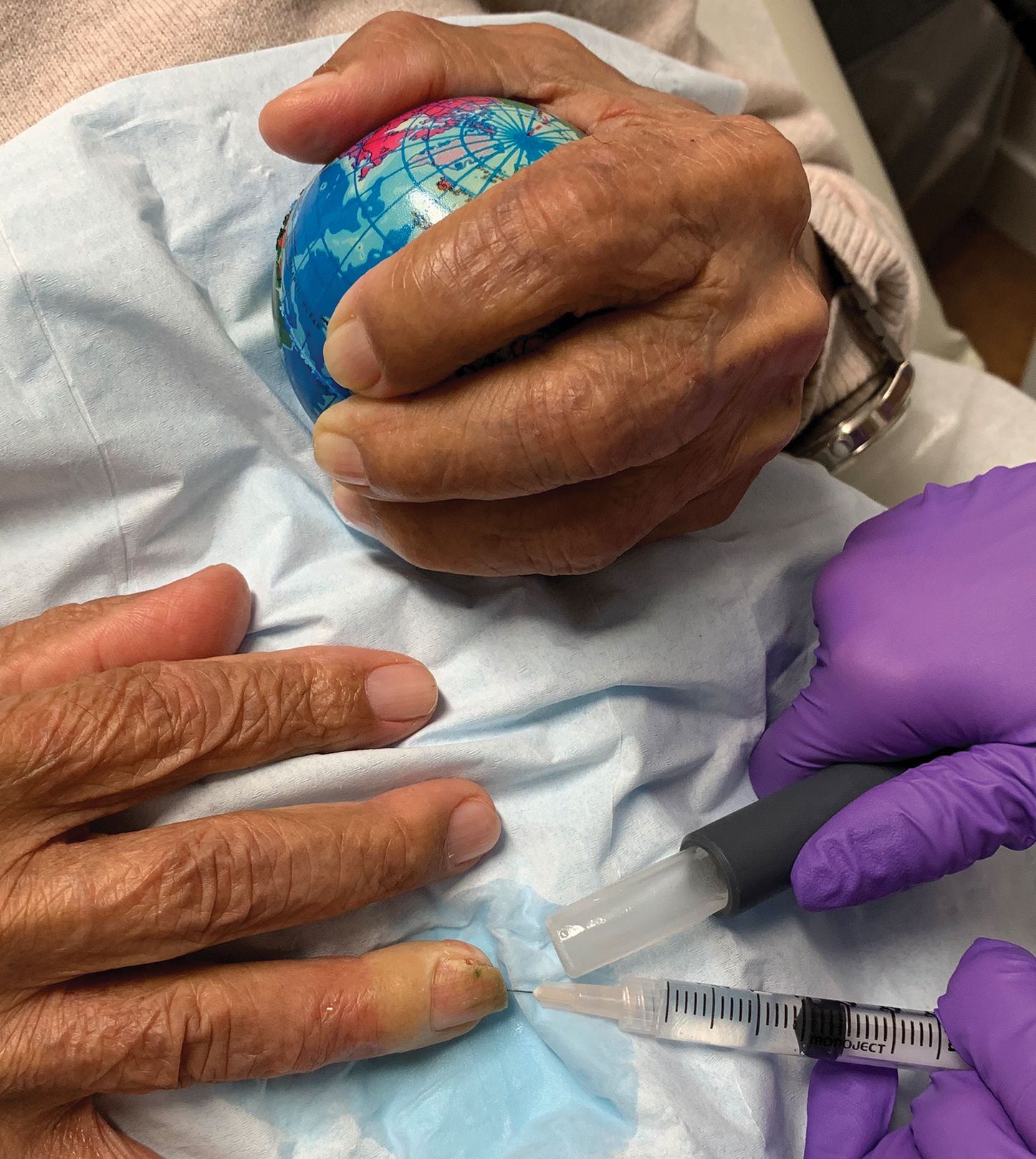
Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.

Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.

Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.