User login
Less is more: Nanotechnology enhances antifungal’s efficacy
The use of nanotechnology significantly reduced the amount of efinaconazole needed to effectively treat nail fungus in a study that pitted nitric oxide–releasing nanoparticles combined with the antifungal against reference strains of Trichophyton rubrum.
Efinaconazole has demonstrated effectiveness as a topical treatment for T. rubrum, but treatment can be expensive, with a single 4-mL bottle costing $691 at a major chain pharmacy, wrote Caroline B. Costa-Orlandi, PhD, of Universidade Estadual Paulista, Sao Paulo, Brazil, and her colleagues.
In a study published in the Journal of Drugs in Dermatology, an international research team evaluated topical efinaconazole and topical terbinafine, each combined with previously characterized, nitric oxide–releasing nanoparticles (NO-np) in a checkerboard design, to attack two reference strains of T. rubrum, ATCC MYA-4438 and ATCC 28189. NO-np was combined with 10% efinaconazole or with terbinafine.
The combination of NO-np and efinaconazole reduced the minimum inhibitory concentration (MIC) of efinaconazole by 16 times compared with treatment alone against ATCC MYA-4438; by 4 times when combined against ATCC 28189. With NO-np plus terbinafine, MICs against ATCC 28189 and ATCC MYA-4438 were reduced by four- and twofold, respectively, when compared with terbinafine alone. These data follow recently published findings in a study cited by the authors that demonstrated that NO-np is superior to topical terbinafine 1% cream in clearing infection in a mouse model of deep dermal dermatophytosis, suggesting that the combination may be even more effective (Nanomedicine. 2017 Oct;13[7]:2267-70).
“What we found was that we could impart the same antifungal activity at the highest concentrations tested of either alone by combining them at a fraction of these concentrations,” corresponding author Adam Friedman, MD, professor of dermatology, George Washington University, Washington, said in a press release issued by the university. The impact of this combination, “which we visualized using electron microscopy as compared to either product alone, highlighted their synergistic damaging effects at concentrations that would be completely safe to human cells,” he added.
Other benefits of NO-np include low cost, safety, ease of use, reduced likelihood for the development of antimicrobial resistance, and proven efficacy against other dermatophyte infections, the researchers noted.
The findings support the potential value of further research to evaluate nanoparticles combined with topical antifungals in a clinical setting, they said.
Dr. Costa-Orlandi had no financial conflicts to disclose. Authors Adam Friedman, MD, and Joel Friedman, MD, are coinventors of the nitric oxide–releasing nanoparticles used in the study. Dr. Adam Friedman is on the advisory board of Dermatology News.
SOURCE: Costa-Orlandi C et al. J Drugs Dermatol. 2018;17(7):717-20.
The use of nanotechnology significantly reduced the amount of efinaconazole needed to effectively treat nail fungus in a study that pitted nitric oxide–releasing nanoparticles combined with the antifungal against reference strains of Trichophyton rubrum.
Efinaconazole has demonstrated effectiveness as a topical treatment for T. rubrum, but treatment can be expensive, with a single 4-mL bottle costing $691 at a major chain pharmacy, wrote Caroline B. Costa-Orlandi, PhD, of Universidade Estadual Paulista, Sao Paulo, Brazil, and her colleagues.
In a study published in the Journal of Drugs in Dermatology, an international research team evaluated topical efinaconazole and topical terbinafine, each combined with previously characterized, nitric oxide–releasing nanoparticles (NO-np) in a checkerboard design, to attack two reference strains of T. rubrum, ATCC MYA-4438 and ATCC 28189. NO-np was combined with 10% efinaconazole or with terbinafine.
The combination of NO-np and efinaconazole reduced the minimum inhibitory concentration (MIC) of efinaconazole by 16 times compared with treatment alone against ATCC MYA-4438; by 4 times when combined against ATCC 28189. With NO-np plus terbinafine, MICs against ATCC 28189 and ATCC MYA-4438 were reduced by four- and twofold, respectively, when compared with terbinafine alone. These data follow recently published findings in a study cited by the authors that demonstrated that NO-np is superior to topical terbinafine 1% cream in clearing infection in a mouse model of deep dermal dermatophytosis, suggesting that the combination may be even more effective (Nanomedicine. 2017 Oct;13[7]:2267-70).
“What we found was that we could impart the same antifungal activity at the highest concentrations tested of either alone by combining them at a fraction of these concentrations,” corresponding author Adam Friedman, MD, professor of dermatology, George Washington University, Washington, said in a press release issued by the university. The impact of this combination, “which we visualized using electron microscopy as compared to either product alone, highlighted their synergistic damaging effects at concentrations that would be completely safe to human cells,” he added.
Other benefits of NO-np include low cost, safety, ease of use, reduced likelihood for the development of antimicrobial resistance, and proven efficacy against other dermatophyte infections, the researchers noted.
The findings support the potential value of further research to evaluate nanoparticles combined with topical antifungals in a clinical setting, they said.
Dr. Costa-Orlandi had no financial conflicts to disclose. Authors Adam Friedman, MD, and Joel Friedman, MD, are coinventors of the nitric oxide–releasing nanoparticles used in the study. Dr. Adam Friedman is on the advisory board of Dermatology News.
SOURCE: Costa-Orlandi C et al. J Drugs Dermatol. 2018;17(7):717-20.
The use of nanotechnology significantly reduced the amount of efinaconazole needed to effectively treat nail fungus in a study that pitted nitric oxide–releasing nanoparticles combined with the antifungal against reference strains of Trichophyton rubrum.
Efinaconazole has demonstrated effectiveness as a topical treatment for T. rubrum, but treatment can be expensive, with a single 4-mL bottle costing $691 at a major chain pharmacy, wrote Caroline B. Costa-Orlandi, PhD, of Universidade Estadual Paulista, Sao Paulo, Brazil, and her colleagues.
In a study published in the Journal of Drugs in Dermatology, an international research team evaluated topical efinaconazole and topical terbinafine, each combined with previously characterized, nitric oxide–releasing nanoparticles (NO-np) in a checkerboard design, to attack two reference strains of T. rubrum, ATCC MYA-4438 and ATCC 28189. NO-np was combined with 10% efinaconazole or with terbinafine.
The combination of NO-np and efinaconazole reduced the minimum inhibitory concentration (MIC) of efinaconazole by 16 times compared with treatment alone against ATCC MYA-4438; by 4 times when combined against ATCC 28189. With NO-np plus terbinafine, MICs against ATCC 28189 and ATCC MYA-4438 were reduced by four- and twofold, respectively, when compared with terbinafine alone. These data follow recently published findings in a study cited by the authors that demonstrated that NO-np is superior to topical terbinafine 1% cream in clearing infection in a mouse model of deep dermal dermatophytosis, suggesting that the combination may be even more effective (Nanomedicine. 2017 Oct;13[7]:2267-70).
“What we found was that we could impart the same antifungal activity at the highest concentrations tested of either alone by combining them at a fraction of these concentrations,” corresponding author Adam Friedman, MD, professor of dermatology, George Washington University, Washington, said in a press release issued by the university. The impact of this combination, “which we visualized using electron microscopy as compared to either product alone, highlighted their synergistic damaging effects at concentrations that would be completely safe to human cells,” he added.
Other benefits of NO-np include low cost, safety, ease of use, reduced likelihood for the development of antimicrobial resistance, and proven efficacy against other dermatophyte infections, the researchers noted.
The findings support the potential value of further research to evaluate nanoparticles combined with topical antifungals in a clinical setting, they said.
Dr. Costa-Orlandi had no financial conflicts to disclose. Authors Adam Friedman, MD, and Joel Friedman, MD, are coinventors of the nitric oxide–releasing nanoparticles used in the study. Dr. Adam Friedman is on the advisory board of Dermatology News.
SOURCE: Costa-Orlandi C et al. J Drugs Dermatol. 2018;17(7):717-20.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: Adding nanoparticles to antifungal medication improved the drug’s effectiveness and reduced the amount needed.
Major finding: Efinaconazole combined with nitric oxide–releasing nanoparticles reduced the antifungal’s minimum inhibitory concentration 16-fold, compared with the antifungal alone against T. rubrum reference strains.
Study details: The data come from an in vitro analysis of nanoparticle-enhanced efinaconazole or terbinafine against T. rubrum.
Disclosures: Dr. Costa-Orlandi had no financial conflicts to disclose. Coauthors Dr. Adam Friedman and Dr. Joel Friedman are coinventors of the nitric oxide–releasing nanoparticles used in the study.
Source: Costa-Orlandi C et al. J Drugs Dermatol. 2018;17(7):717-20.
Fish pedicures
A letter published in JAMA Dermatology describes an otherwise healthy woman in her 20s who experienced nail abnormalities some months after having a fish pedicure. Onychomadesis, or transverse splitting of the nail plate, occurs when the nail matrix has arrested in producing the nail plate. It can be thought of as more severe form of Beau’s lines, in which the nail itself actually breaks and separates from the proximal nail plate and eventually sheds.
Fish pedicures have a long-standing history in Mediterranean and Middle Eastern cultures for aiding such skin conditions as psoriasis and helping to remove scaly skin. The Garra rufa fish are nonmigratory freshwater fish native to the Persian Gulf and Eastern Mediterranean. Suction allows them to attach to rocks and eat plankton. These “doctor fish,” as they are nicknamed, when placed in a warm bath of 25°C to 30°C, will also eat human skin when starved of their natural food source. As the JAMA Dermatology letter mentions, this was demonstrated in a study in Kangal, Turkey, where Garra rufa fish were used to improve psoriasis by feeding on psoriasis plaques but not normal skin. After 3 weeks of therapy with Garra rufa in 67 patients, there was a 72% reduction in the Psoriasis Area and Severity Index (PASI) score from baseline (Evid Based Complement Alternat Med. 2006 Dec;3[4]:483-8).
Popular in the United States and Europe about a decade ago, fish pedicures have now been banned in 10 U.S. states and in some parts of Europe. While the trend in the United States has waned, fish pedicures have recently become more popular in vacation destinations, such as the Caribbean. The inherent concern of fish pedicures is risk of infection as the same fish are used successively and cannot be adequately sanitized between people.
Two cases of staphylococcus infections and one of Mycobacterium marinum have been reported after fish pedicures. Whether these infections were caused by the fish or the water source, however, remains to be determined. If the fish were transmitting infections, it seems that more infections would likely have been reported, considering the widespread popularity in the past. I, like Antonella Tosti, MD, who commented in a CNN report on the JAMA Dermatology case, also doubt that the fish pedicure alone caused onychomadesis in this woman. In order for onychomadesis to occur, there would have had to have been significant trauma to all 10 nails at the matrix. Would the fish been able to have caused the same amount of trauma to all 10 nails in one setting? While it is possible, I believe a more likely explanation would be an alternate endogenous or exogenous source.
Traditional medicine has been used to enhance beauty and cure ailments for thousands of years before the advent of modern medicine as demonstrated by the Kangal study. Before discounting fish pedicures completely, perhaps some thought should also be given to how this practice affects wildlife and the fish. The CNN report refers to a 2011 investigation by the U.K.’s Fish Health Inspectorate, which “found a bacterial outbreak among thousands of these fish, which had been transported from Indonesia to the United Kingdom pedicure spas. Fish were found with bulging eyes, many hemorrhaging around the gills and mouth. The culprit was found to be a streptococcal bacteria, a strain that is associated with fish like tilapia, according to David Verner-Jeffreys, a senior microbiologist at the Centre for Environment, Fisheries and Aquaculture Science in the U.K.”
Whether or not these fish would pose any risk to humans is unknown, but certainly, this practice adversely affects the welfare of the fish and their environment. The overharvesting of these fish has led the Turkish government to introduce legal protections for the country’s Garra rufa in an attempt to combat overfishing and exploitation.
Perhaps fish pedicures solely for aesthetic reasons should not be practiced because of the potential infection risk – as well as the harm (to both humans and fish) and overharvesting of the fish. If used properly, these fish, however, could be an aid in treating certain skin pathologies.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A letter published in JAMA Dermatology describes an otherwise healthy woman in her 20s who experienced nail abnormalities some months after having a fish pedicure. Onychomadesis, or transverse splitting of the nail plate, occurs when the nail matrix has arrested in producing the nail plate. It can be thought of as more severe form of Beau’s lines, in which the nail itself actually breaks and separates from the proximal nail plate and eventually sheds.
Fish pedicures have a long-standing history in Mediterranean and Middle Eastern cultures for aiding such skin conditions as psoriasis and helping to remove scaly skin. The Garra rufa fish are nonmigratory freshwater fish native to the Persian Gulf and Eastern Mediterranean. Suction allows them to attach to rocks and eat plankton. These “doctor fish,” as they are nicknamed, when placed in a warm bath of 25°C to 30°C, will also eat human skin when starved of their natural food source. As the JAMA Dermatology letter mentions, this was demonstrated in a study in Kangal, Turkey, where Garra rufa fish were used to improve psoriasis by feeding on psoriasis plaques but not normal skin. After 3 weeks of therapy with Garra rufa in 67 patients, there was a 72% reduction in the Psoriasis Area and Severity Index (PASI) score from baseline (Evid Based Complement Alternat Med. 2006 Dec;3[4]:483-8).
Popular in the United States and Europe about a decade ago, fish pedicures have now been banned in 10 U.S. states and in some parts of Europe. While the trend in the United States has waned, fish pedicures have recently become more popular in vacation destinations, such as the Caribbean. The inherent concern of fish pedicures is risk of infection as the same fish are used successively and cannot be adequately sanitized between people.
Two cases of staphylococcus infections and one of Mycobacterium marinum have been reported after fish pedicures. Whether these infections were caused by the fish or the water source, however, remains to be determined. If the fish were transmitting infections, it seems that more infections would likely have been reported, considering the widespread popularity in the past. I, like Antonella Tosti, MD, who commented in a CNN report on the JAMA Dermatology case, also doubt that the fish pedicure alone caused onychomadesis in this woman. In order for onychomadesis to occur, there would have had to have been significant trauma to all 10 nails at the matrix. Would the fish been able to have caused the same amount of trauma to all 10 nails in one setting? While it is possible, I believe a more likely explanation would be an alternate endogenous or exogenous source.
Traditional medicine has been used to enhance beauty and cure ailments for thousands of years before the advent of modern medicine as demonstrated by the Kangal study. Before discounting fish pedicures completely, perhaps some thought should also be given to how this practice affects wildlife and the fish. The CNN report refers to a 2011 investigation by the U.K.’s Fish Health Inspectorate, which “found a bacterial outbreak among thousands of these fish, which had been transported from Indonesia to the United Kingdom pedicure spas. Fish were found with bulging eyes, many hemorrhaging around the gills and mouth. The culprit was found to be a streptococcal bacteria, a strain that is associated with fish like tilapia, according to David Verner-Jeffreys, a senior microbiologist at the Centre for Environment, Fisheries and Aquaculture Science in the U.K.”
Whether or not these fish would pose any risk to humans is unknown, but certainly, this practice adversely affects the welfare of the fish and their environment. The overharvesting of these fish has led the Turkish government to introduce legal protections for the country’s Garra rufa in an attempt to combat overfishing and exploitation.
Perhaps fish pedicures solely for aesthetic reasons should not be practiced because of the potential infection risk – as well as the harm (to both humans and fish) and overharvesting of the fish. If used properly, these fish, however, could be an aid in treating certain skin pathologies.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A letter published in JAMA Dermatology describes an otherwise healthy woman in her 20s who experienced nail abnormalities some months after having a fish pedicure. Onychomadesis, or transverse splitting of the nail plate, occurs when the nail matrix has arrested in producing the nail plate. It can be thought of as more severe form of Beau’s lines, in which the nail itself actually breaks and separates from the proximal nail plate and eventually sheds.
Fish pedicures have a long-standing history in Mediterranean and Middle Eastern cultures for aiding such skin conditions as psoriasis and helping to remove scaly skin. The Garra rufa fish are nonmigratory freshwater fish native to the Persian Gulf and Eastern Mediterranean. Suction allows them to attach to rocks and eat plankton. These “doctor fish,” as they are nicknamed, when placed in a warm bath of 25°C to 30°C, will also eat human skin when starved of their natural food source. As the JAMA Dermatology letter mentions, this was demonstrated in a study in Kangal, Turkey, where Garra rufa fish were used to improve psoriasis by feeding on psoriasis plaques but not normal skin. After 3 weeks of therapy with Garra rufa in 67 patients, there was a 72% reduction in the Psoriasis Area and Severity Index (PASI) score from baseline (Evid Based Complement Alternat Med. 2006 Dec;3[4]:483-8).
Popular in the United States and Europe about a decade ago, fish pedicures have now been banned in 10 U.S. states and in some parts of Europe. While the trend in the United States has waned, fish pedicures have recently become more popular in vacation destinations, such as the Caribbean. The inherent concern of fish pedicures is risk of infection as the same fish are used successively and cannot be adequately sanitized between people.
Two cases of staphylococcus infections and one of Mycobacterium marinum have been reported after fish pedicures. Whether these infections were caused by the fish or the water source, however, remains to be determined. If the fish were transmitting infections, it seems that more infections would likely have been reported, considering the widespread popularity in the past. I, like Antonella Tosti, MD, who commented in a CNN report on the JAMA Dermatology case, also doubt that the fish pedicure alone caused onychomadesis in this woman. In order for onychomadesis to occur, there would have had to have been significant trauma to all 10 nails at the matrix. Would the fish been able to have caused the same amount of trauma to all 10 nails in one setting? While it is possible, I believe a more likely explanation would be an alternate endogenous or exogenous source.
Traditional medicine has been used to enhance beauty and cure ailments for thousands of years before the advent of modern medicine as demonstrated by the Kangal study. Before discounting fish pedicures completely, perhaps some thought should also be given to how this practice affects wildlife and the fish. The CNN report refers to a 2011 investigation by the U.K.’s Fish Health Inspectorate, which “found a bacterial outbreak among thousands of these fish, which had been transported from Indonesia to the United Kingdom pedicure spas. Fish were found with bulging eyes, many hemorrhaging around the gills and mouth. The culprit was found to be a streptococcal bacteria, a strain that is associated with fish like tilapia, according to David Verner-Jeffreys, a senior microbiologist at the Centre for Environment, Fisheries and Aquaculture Science in the U.K.”
Whether or not these fish would pose any risk to humans is unknown, but certainly, this practice adversely affects the welfare of the fish and their environment. The overharvesting of these fish has led the Turkish government to introduce legal protections for the country’s Garra rufa in an attempt to combat overfishing and exploitation.
Perhaps fish pedicures solely for aesthetic reasons should not be practiced because of the potential infection risk – as well as the harm (to both humans and fish) and overharvesting of the fish. If used properly, these fish, however, could be an aid in treating certain skin pathologies.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Scalp Psoriasis With Increased Hair Density
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
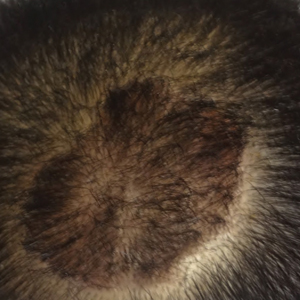
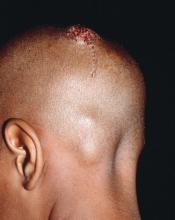
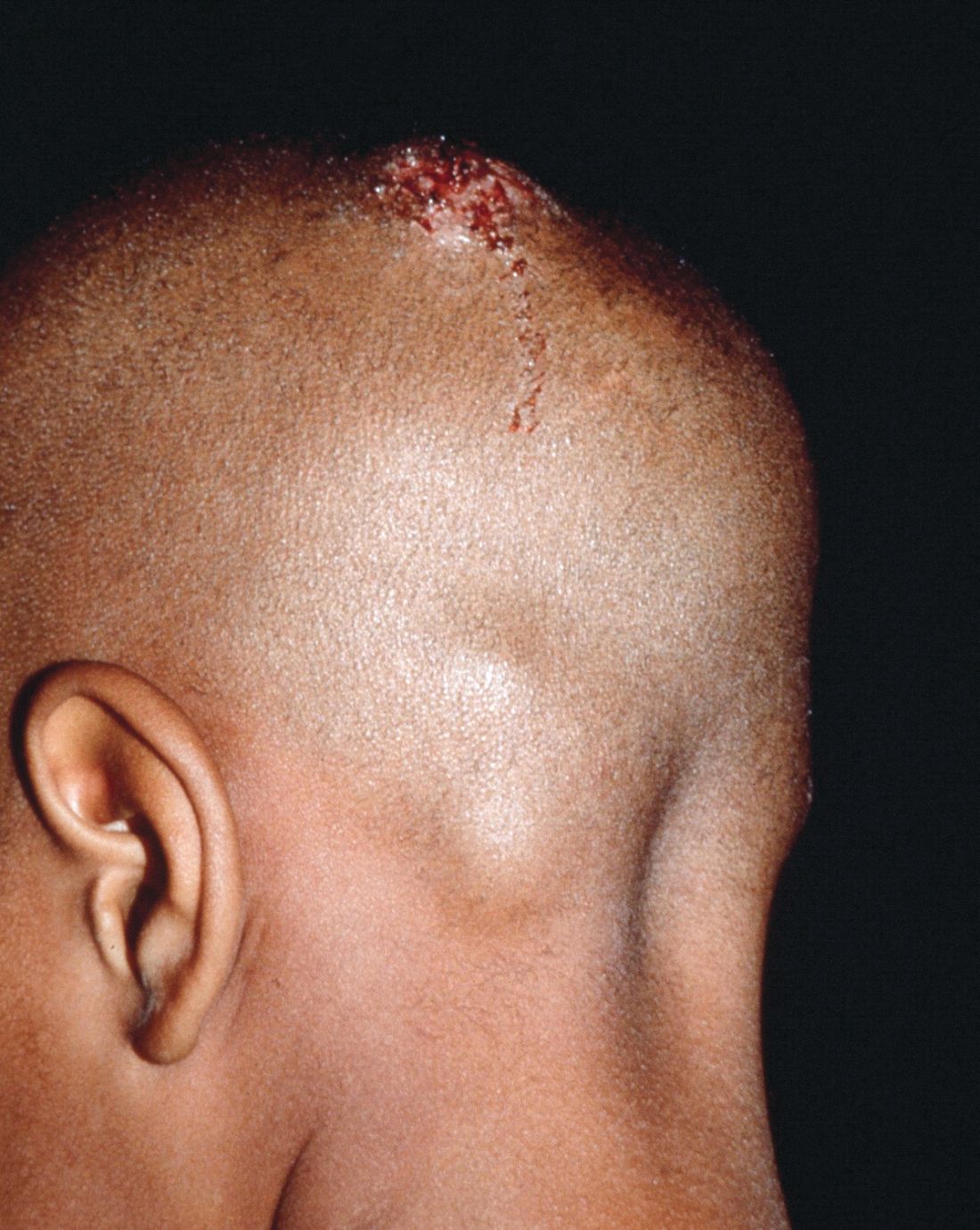
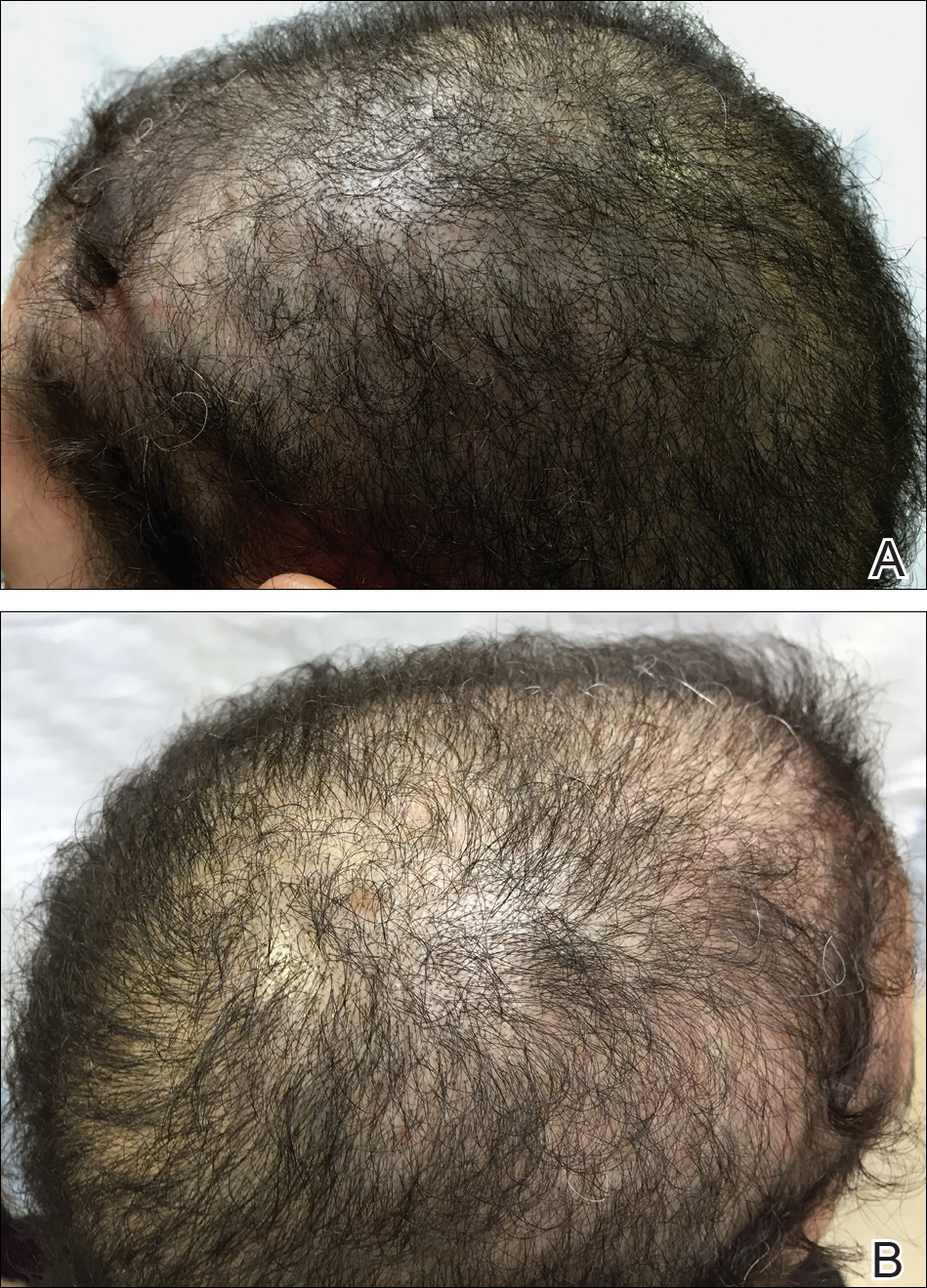
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Practice Points
- Scalp psoriasis may present with hair loss or increased hair density.
- Psoriasis with increased hair density may make topical medications more difficult to apply.
Nonscarring Alopecia Associated With Vitamin D Deficiency
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
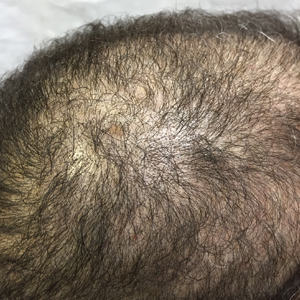
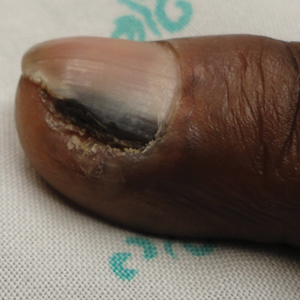
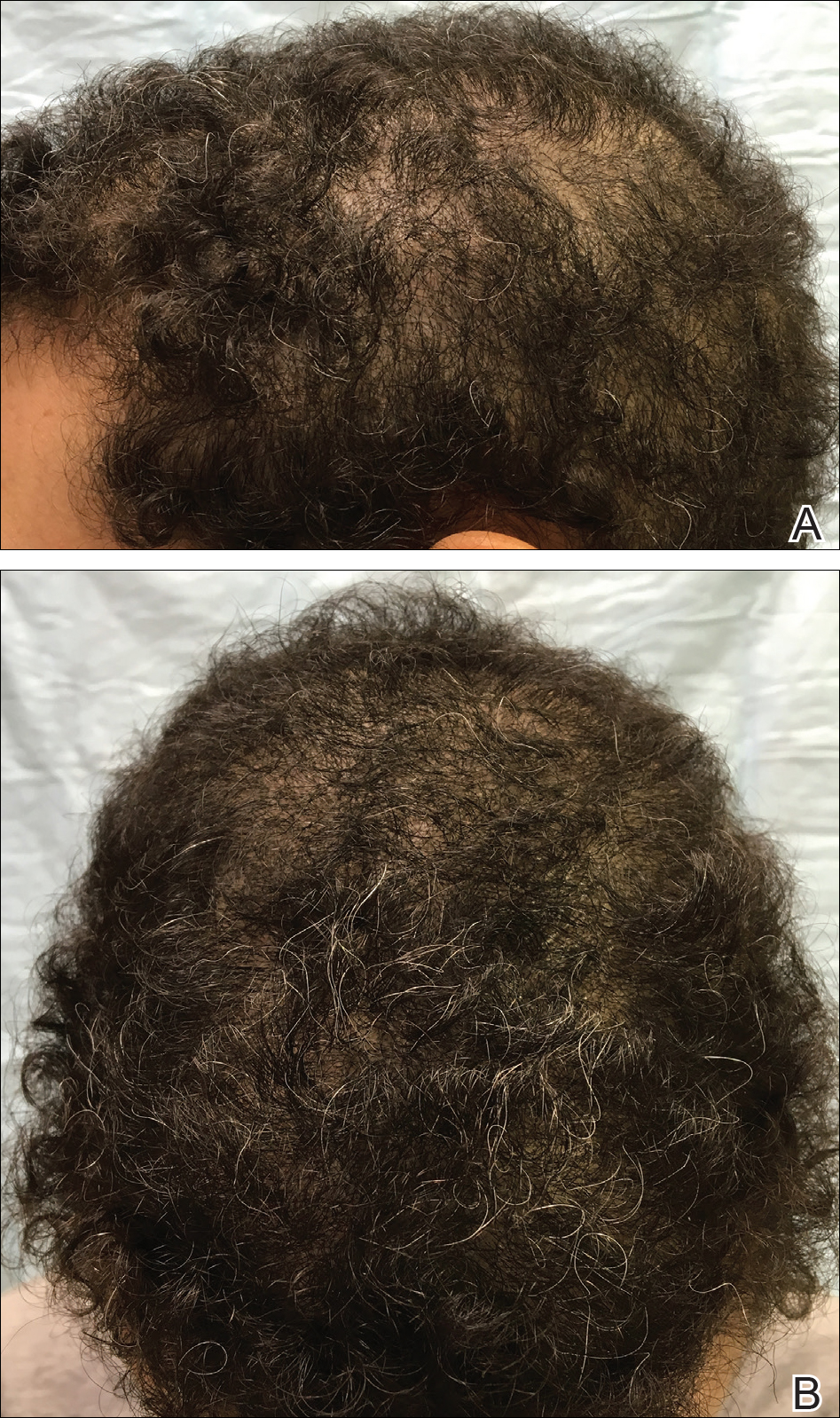
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.
On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.
Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Practice Points
- The evaluation of vitamin D levels is important in the management of nonscarring alopecia.
- Vitamin D deficiency can present as nonscarring alopecia not associated with alopecia areata, androgenetic alopecia, or telogen effluvium.
Relapsing scabies? Nails may hold a clue
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Pediatric relapse estimated as high as 66%.
Major finding: Nail scabies found in great toenail, not fingernails.
Study details: Observational multicenter prospective study of 47 pediatric patients with common scabies.
Disclosures: The authors had no relevant financial disclosures.
Source: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
Early diagnosis, treatment key to prevent permanent baldness in tinea capitis
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The psychosocial impact and overall lower quality of health associated with tinea capitis is significant.
Major finding: Wood’s light should not be the only method of organism identification.
Study details: A systematic literature review of 4,190 studies.
Disclosures: The researchers had no relevant financial disclosures.
Source: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
Ropivacaine called top anesthesia for nail surgery
CHICAGO – Ropivacaine has a fast onset of action, longer duration than either lidocaine or bupivacaine, and it’s the only one of the three that’s inherently vasoconstrictive. For Brienne Cressey, MD, those features make ropivacaine the local anesthetic of choice in performing nail surgery.
“Local anesthesia is really key for nail surgery. If you don’t have good anesthesia it’s not a good experience for either the surgeon or the patient,” she observed at the annual meeting of the American College of Mohs Surgery.
Lidocaine has a fast onset – less than 1 minute – but a problematic short duration of 30-120 minutes. Bupivacaine has the disadvantage of a slow onset of up to 5 minutes, albeit with a longer duration of anesthesia at 2-4 hours. Ropivacaine has a fast onset, plus a duration of up to 8 hours. And unlike lidocaine and bupivacaine, which are vasodilatory, ropivacaine is vasoconstrictive.
“With lidocaine, you get a lot of blood right after you take off your tourniquet. With ropivacaine, you get really nice reperfusion, but it’s not too much. You take off the tourniquet, check to see you’ve got reperfusion, then you add a little ropivacaine – about 0.5 mL – on either side of the base of the distal phalanx. It stops the bleeding immediately and you can easily put on a pressure dressing. It’s a nice way to get the patient over the hump of those first hours of pain and lets them drive home in comfort,” explained Dr. Cressey, a dermatologist working in a group practice at Dermatology Professionals in East Greenwich, R.I.
Ropivacaine is less cardiotoxic than bupivacaine. And ropivacaine offers an additional advantage: Its pH is such that no buffering is necessary. “Ropivacaine doesn’t require any compounding. You can just use it at 1% straight out of the bottle. That’s what we do in our office, and we’ve had very good experience with it,” according to the dermatologist.
Achieving smooth sailing with local anesthesia
Dr. Cressey delivers ropivacaine slowly through a 30-gauge needle, which makes for a smaller, less painful puncture. She utilizes a topical cold spray, and places a vibrating machine as a distractant proximal to where she is injecting. She keeps the anesthetic at room temperature or warms it to body temperature in a water bath as another means of reducing the pain of injection.
The distal digital block
This is a cross between a traditional proximal digital block and a wing block. It works well for the second, third, and fourth digits, which are mostly volar dominant. The block bathes the volar nerve branch in anesthesia at the midline of the finger or toe.
Dr. Cressey begins by injecting ropivacaine proximal and lateral to the junction of the proximal nail fold and lateral nail fold. After creating a dermal wheal, she directs her needle perpendicularly downward toward the finger or toe pad, injecting 1-4 mL of anesthesia, depending upon digit size. Visible blanching will progress digitally. If resistance is encountered, it suggests the needle has penetrated a ligament or other fibrous tissue. Simply withdraw the needle and continue injecting.
“What’s nice about the distal digital block is you get an immediate effect, and there’s good hemostasis during the procedure as well,” she said.
Dr. Cressey reported no financial conflicts regarding her presentation.
CHICAGO – Ropivacaine has a fast onset of action, longer duration than either lidocaine or bupivacaine, and it’s the only one of the three that’s inherently vasoconstrictive. For Brienne Cressey, MD, those features make ropivacaine the local anesthetic of choice in performing nail surgery.
“Local anesthesia is really key for nail surgery. If you don’t have good anesthesia it’s not a good experience for either the surgeon or the patient,” she observed at the annual meeting of the American College of Mohs Surgery.
Lidocaine has a fast onset – less than 1 minute – but a problematic short duration of 30-120 minutes. Bupivacaine has the disadvantage of a slow onset of up to 5 minutes, albeit with a longer duration of anesthesia at 2-4 hours. Ropivacaine has a fast onset, plus a duration of up to 8 hours. And unlike lidocaine and bupivacaine, which are vasodilatory, ropivacaine is vasoconstrictive.
“With lidocaine, you get a lot of blood right after you take off your tourniquet. With ropivacaine, you get really nice reperfusion, but it’s not too much. You take off the tourniquet, check to see you’ve got reperfusion, then you add a little ropivacaine – about 0.5 mL – on either side of the base of the distal phalanx. It stops the bleeding immediately and you can easily put on a pressure dressing. It’s a nice way to get the patient over the hump of those first hours of pain and lets them drive home in comfort,” explained Dr. Cressey, a dermatologist working in a group practice at Dermatology Professionals in East Greenwich, R.I.
Ropivacaine is less cardiotoxic than bupivacaine. And ropivacaine offers an additional advantage: Its pH is such that no buffering is necessary. “Ropivacaine doesn’t require any compounding. You can just use it at 1% straight out of the bottle. That’s what we do in our office, and we’ve had very good experience with it,” according to the dermatologist.
Achieving smooth sailing with local anesthesia
Dr. Cressey delivers ropivacaine slowly through a 30-gauge needle, which makes for a smaller, less painful puncture. She utilizes a topical cold spray, and places a vibrating machine as a distractant proximal to where she is injecting. She keeps the anesthetic at room temperature or warms it to body temperature in a water bath as another means of reducing the pain of injection.
The distal digital block
This is a cross between a traditional proximal digital block and a wing block. It works well for the second, third, and fourth digits, which are mostly volar dominant. The block bathes the volar nerve branch in anesthesia at the midline of the finger or toe.
Dr. Cressey begins by injecting ropivacaine proximal and lateral to the junction of the proximal nail fold and lateral nail fold. After creating a dermal wheal, she directs her needle perpendicularly downward toward the finger or toe pad, injecting 1-4 mL of anesthesia, depending upon digit size. Visible blanching will progress digitally. If resistance is encountered, it suggests the needle has penetrated a ligament or other fibrous tissue. Simply withdraw the needle and continue injecting.
“What’s nice about the distal digital block is you get an immediate effect, and there’s good hemostasis during the procedure as well,” she said.
Dr. Cressey reported no financial conflicts regarding her presentation.
CHICAGO – Ropivacaine has a fast onset of action, longer duration than either lidocaine or bupivacaine, and it’s the only one of the three that’s inherently vasoconstrictive. For Brienne Cressey, MD, those features make ropivacaine the local anesthetic of choice in performing nail surgery.
“Local anesthesia is really key for nail surgery. If you don’t have good anesthesia it’s not a good experience for either the surgeon or the patient,” she observed at the annual meeting of the American College of Mohs Surgery.
Lidocaine has a fast onset – less than 1 minute – but a problematic short duration of 30-120 minutes. Bupivacaine has the disadvantage of a slow onset of up to 5 minutes, albeit with a longer duration of anesthesia at 2-4 hours. Ropivacaine has a fast onset, plus a duration of up to 8 hours. And unlike lidocaine and bupivacaine, which are vasodilatory, ropivacaine is vasoconstrictive.
“With lidocaine, you get a lot of blood right after you take off your tourniquet. With ropivacaine, you get really nice reperfusion, but it’s not too much. You take off the tourniquet, check to see you’ve got reperfusion, then you add a little ropivacaine – about 0.5 mL – on either side of the base of the distal phalanx. It stops the bleeding immediately and you can easily put on a pressure dressing. It’s a nice way to get the patient over the hump of those first hours of pain and lets them drive home in comfort,” explained Dr. Cressey, a dermatologist working in a group practice at Dermatology Professionals in East Greenwich, R.I.
Ropivacaine is less cardiotoxic than bupivacaine. And ropivacaine offers an additional advantage: Its pH is such that no buffering is necessary. “Ropivacaine doesn’t require any compounding. You can just use it at 1% straight out of the bottle. That’s what we do in our office, and we’ve had very good experience with it,” according to the dermatologist.
Achieving smooth sailing with local anesthesia
Dr. Cressey delivers ropivacaine slowly through a 30-gauge needle, which makes for a smaller, less painful puncture. She utilizes a topical cold spray, and places a vibrating machine as a distractant proximal to where she is injecting. She keeps the anesthetic at room temperature or warms it to body temperature in a water bath as another means of reducing the pain of injection.
The distal digital block
This is a cross between a traditional proximal digital block and a wing block. It works well for the second, third, and fourth digits, which are mostly volar dominant. The block bathes the volar nerve branch in anesthesia at the midline of the finger or toe.
Dr. Cressey begins by injecting ropivacaine proximal and lateral to the junction of the proximal nail fold and lateral nail fold. After creating a dermal wheal, she directs her needle perpendicularly downward toward the finger or toe pad, injecting 1-4 mL of anesthesia, depending upon digit size. Visible blanching will progress digitally. If resistance is encountered, it suggests the needle has penetrated a ligament or other fibrous tissue. Simply withdraw the needle and continue injecting.
“What’s nice about the distal digital block is you get an immediate effect, and there’s good hemostasis during the procedure as well,” she said.
Dr. Cressey reported no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
Do black women pay a price for hair care regimens?
A new analysis of 18 hair products used by black women finds that they contain 45 endocrine-disrupting or asthma-associated chemicals, a finding that could help explain why this population suffers from higher rates of chemical exposure and hormone-related health conditions.
“We found multiples of our targeted chemicals in all of our products,” said study lead author Jessica S. Helm, PhD, of the Silent Spring Institute, Newton, Mass., in an interview. “We’re concerned about the additive effect of multiple products being used together.”
According to the study, previous research has found that, compared with white women, U.S. black women have higher urinary levels of chemicals like phthalates and parabens. Black women also have higher rates of asthma and hormone-related health conditions like uterine fibroids and infertility, Dr. Helms said.
The researchers launched their study to better understand the possible role of hair care products in raising chemical levels in black women, Dr. Helm said.
The researchers tested 18 types of hair care products shown by a 2004-2005 survey to be popular among black women: hot oil treatments, anti-frizz products and polishes, leave-in conditioners, root stimulators, hair lotions, and relaxers. Researchers had purchased the products in 2008.
The researchers detected 45 of 66 target chemicals in the samples, including some that are banned in the European Union or regulated in California based on health concerns, according to Dr. Helms.
Most of the products contained parabens and phthalates (both 78%), UV filters (72%), and cyclosiloxanes (67%).
All products contained at least 1 of 19 targeted fragrances, while “hair lotions, root stimulators, and hair relaxers contained multiple fragrance chemicals per product, with an average of five to eight targeted fragrance chemicals detected per product versus an average of two in the anti-frizz products.”
How do the findings compare with previous research? “They’re roughly consistent with what’s been found before, but potentially on the higher end,” Dr. Helms said. “For some of these chemicals, there’s not a lot of data from the past.”
Most of the chemicals aren’t listed on product labels, Dr. Helm said. “It’s possible that some of the ingredients were unintentionally added as part of manufacturing or other processes.”
Dr. Helm urged physicians to consider the connections between hair care products and chemical exposure. “Maybe there’s an opportunity to use fewer products,” she said.
Dr. Helm acknowledged that it is difficult to find hair care products that don’t include fragrance. She recommends the use of products made from plants or organic ingredients, and she pointed to a Silver Spring Institute–affiliated app called DetoxMe that offers suggestions about reducing chemical exposure from consumer products.
The study was funded by the Rose Foundation, the Goldman Fund, and Hurricane Voices Breast Cancer Foundation. The authors report no relevant disclosures.
SOURCE: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
A new analysis of 18 hair products used by black women finds that they contain 45 endocrine-disrupting or asthma-associated chemicals, a finding that could help explain why this population suffers from higher rates of chemical exposure and hormone-related health conditions.
“We found multiples of our targeted chemicals in all of our products,” said study lead author Jessica S. Helm, PhD, of the Silent Spring Institute, Newton, Mass., in an interview. “We’re concerned about the additive effect of multiple products being used together.”
According to the study, previous research has found that, compared with white women, U.S. black women have higher urinary levels of chemicals like phthalates and parabens. Black women also have higher rates of asthma and hormone-related health conditions like uterine fibroids and infertility, Dr. Helms said.
The researchers launched their study to better understand the possible role of hair care products in raising chemical levels in black women, Dr. Helm said.
The researchers tested 18 types of hair care products shown by a 2004-2005 survey to be popular among black women: hot oil treatments, anti-frizz products and polishes, leave-in conditioners, root stimulators, hair lotions, and relaxers. Researchers had purchased the products in 2008.
The researchers detected 45 of 66 target chemicals in the samples, including some that are banned in the European Union or regulated in California based on health concerns, according to Dr. Helms.
Most of the products contained parabens and phthalates (both 78%), UV filters (72%), and cyclosiloxanes (67%).
All products contained at least 1 of 19 targeted fragrances, while “hair lotions, root stimulators, and hair relaxers contained multiple fragrance chemicals per product, with an average of five to eight targeted fragrance chemicals detected per product versus an average of two in the anti-frizz products.”
How do the findings compare with previous research? “They’re roughly consistent with what’s been found before, but potentially on the higher end,” Dr. Helms said. “For some of these chemicals, there’s not a lot of data from the past.”
Most of the chemicals aren’t listed on product labels, Dr. Helm said. “It’s possible that some of the ingredients were unintentionally added as part of manufacturing or other processes.”
Dr. Helm urged physicians to consider the connections between hair care products and chemical exposure. “Maybe there’s an opportunity to use fewer products,” she said.
Dr. Helm acknowledged that it is difficult to find hair care products that don’t include fragrance. She recommends the use of products made from plants or organic ingredients, and she pointed to a Silver Spring Institute–affiliated app called DetoxMe that offers suggestions about reducing chemical exposure from consumer products.
The study was funded by the Rose Foundation, the Goldman Fund, and Hurricane Voices Breast Cancer Foundation. The authors report no relevant disclosures.
SOURCE: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
A new analysis of 18 hair products used by black women finds that they contain 45 endocrine-disrupting or asthma-associated chemicals, a finding that could help explain why this population suffers from higher rates of chemical exposure and hormone-related health conditions.
“We found multiples of our targeted chemicals in all of our products,” said study lead author Jessica S. Helm, PhD, of the Silent Spring Institute, Newton, Mass., in an interview. “We’re concerned about the additive effect of multiple products being used together.”
According to the study, previous research has found that, compared with white women, U.S. black women have higher urinary levels of chemicals like phthalates and parabens. Black women also have higher rates of asthma and hormone-related health conditions like uterine fibroids and infertility, Dr. Helms said.
The researchers launched their study to better understand the possible role of hair care products in raising chemical levels in black women, Dr. Helm said.
The researchers tested 18 types of hair care products shown by a 2004-2005 survey to be popular among black women: hot oil treatments, anti-frizz products and polishes, leave-in conditioners, root stimulators, hair lotions, and relaxers. Researchers had purchased the products in 2008.
The researchers detected 45 of 66 target chemicals in the samples, including some that are banned in the European Union or regulated in California based on health concerns, according to Dr. Helms.
Most of the products contained parabens and phthalates (both 78%), UV filters (72%), and cyclosiloxanes (67%).
All products contained at least 1 of 19 targeted fragrances, while “hair lotions, root stimulators, and hair relaxers contained multiple fragrance chemicals per product, with an average of five to eight targeted fragrance chemicals detected per product versus an average of two in the anti-frizz products.”
How do the findings compare with previous research? “They’re roughly consistent with what’s been found before, but potentially on the higher end,” Dr. Helms said. “For some of these chemicals, there’s not a lot of data from the past.”
Most of the chemicals aren’t listed on product labels, Dr. Helm said. “It’s possible that some of the ingredients were unintentionally added as part of manufacturing or other processes.”
Dr. Helm urged physicians to consider the connections between hair care products and chemical exposure. “Maybe there’s an opportunity to use fewer products,” she said.
Dr. Helm acknowledged that it is difficult to find hair care products that don’t include fragrance. She recommends the use of products made from plants or organic ingredients, and she pointed to a Silver Spring Institute–affiliated app called DetoxMe that offers suggestions about reducing chemical exposure from consumer products.
The study was funded by the Rose Foundation, the Goldman Fund, and Hurricane Voices Breast Cancer Foundation. The authors report no relevant disclosures.
SOURCE: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
Key clinical point: Endocrine-disrupting and asthma-associated chemicals are commonly found in hair care products used by black women.
Major finding: Of the 66 target chemicals, 45 were found in the 18 products tested.
Study details: Analysis of 18 hair care products purchased in 2008.
Disclosures: The study was funded by the Goldman Fund, Hurricane Voices Breast Cancer Foundation, and the Rose Foundation. The authors report no relevant disclosures.
Source: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
Pigmented Squamous Cell Carcinoma Presenting as Longitudinal Melanonychia in a Transplant Recipient
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).
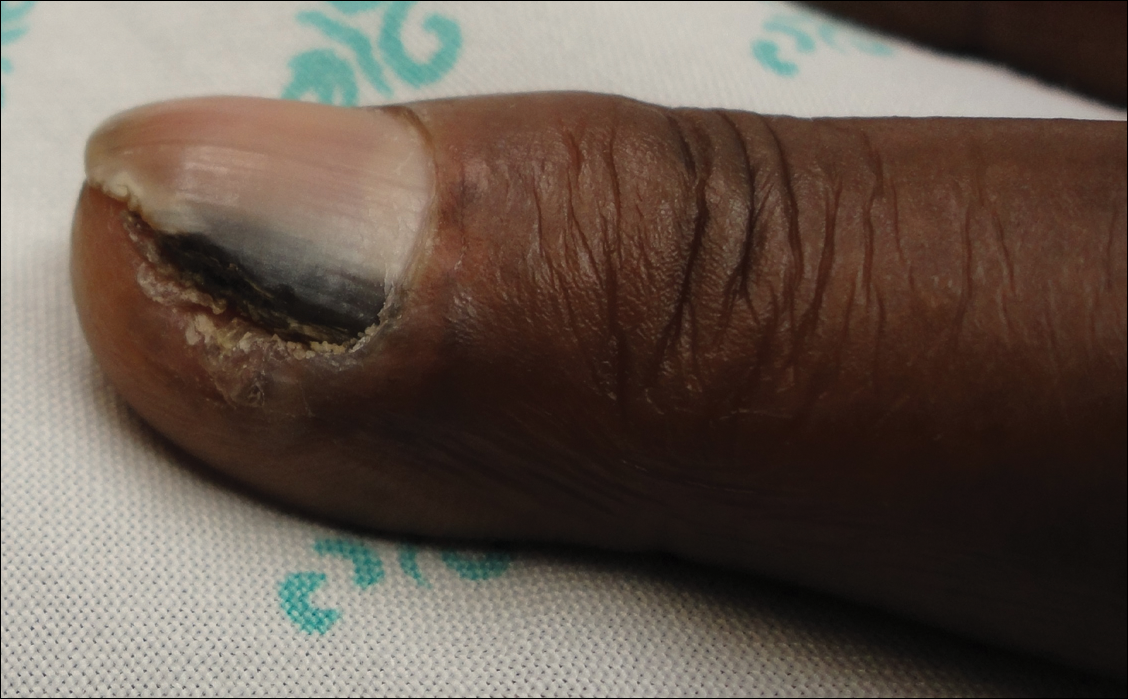
Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).
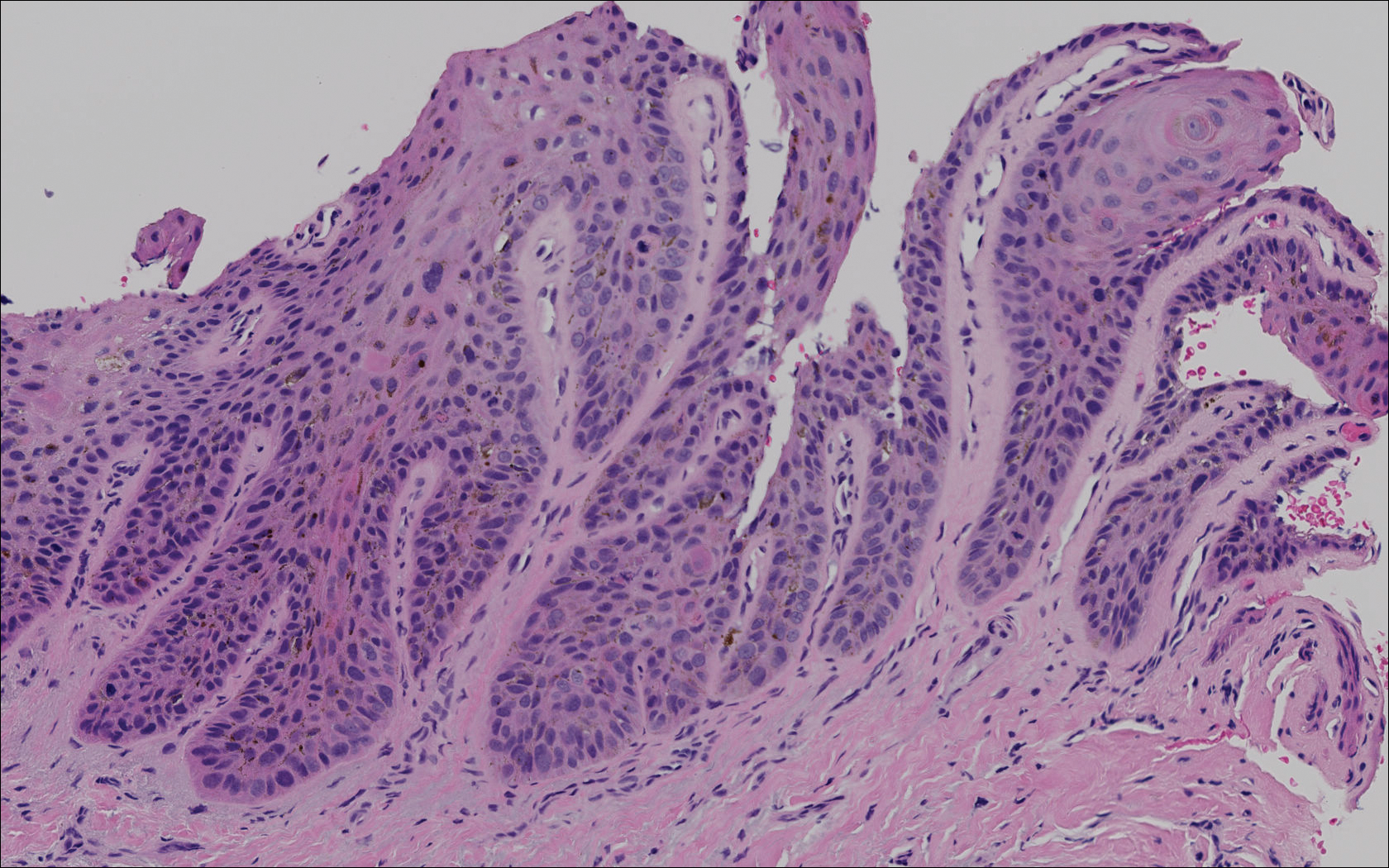
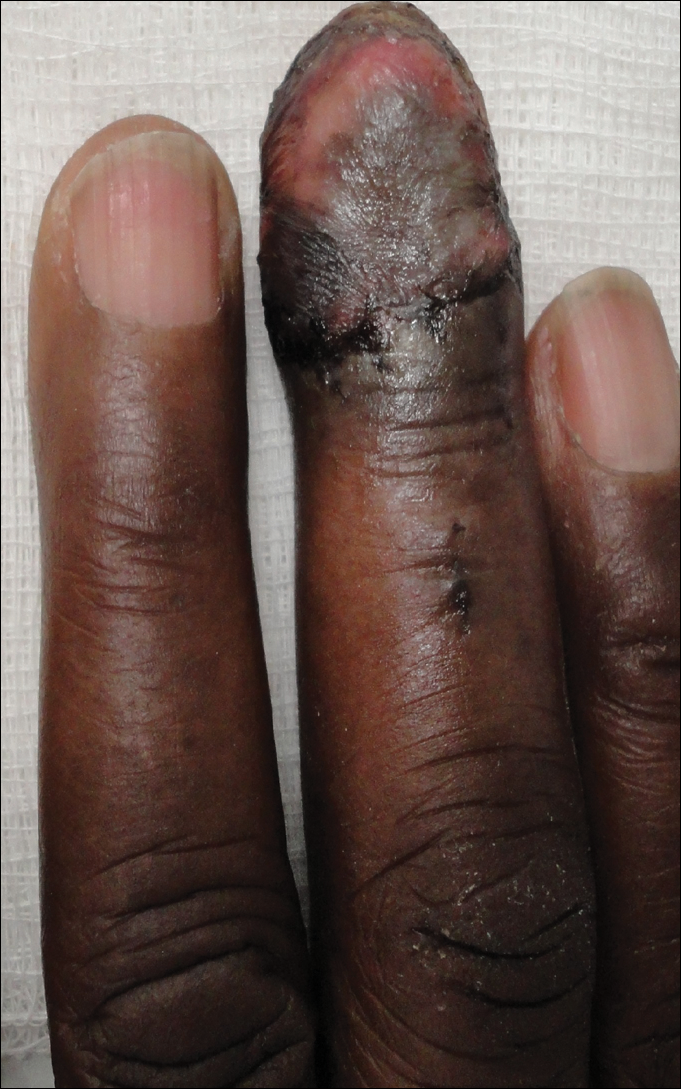
Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).

Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).


Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).

Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).


Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
Practice Points
- Risk factors for the development of pigmented squamous cell carcinoma (pSCC) include older age, male sex, and use of immunosuppressant medications.
- Subungual pSCC can present as longitudinal melanonychia and should be considered in the differential diagnosis for melanonychia in patients with skin of color or those who are immunosuppressed.
Picosecond 755-nm laser found effective for neck rejuvenation
DALLAS –
“It’s important to note that response was variable, but many patients were satisfied with the treatment,” Hana Jeon, MD said at the annual conference of the American Society for Laser Medicine and Surgery, Inc. “Further studies are needed to identify the clinical characteristics of neck laxity that would most benefit from this treatment.”
The researchers enrolled 25 patients with an average age of 58 years. The laser treatment settings were a 6-mm spot side-delivered at a fluence of 0.71 J/cm2 in a pulse width of 750 picoseconds. The patients were treated five times on the neck every 2-4 weeks, and follow-up visits were scheduled for 1 month and 3 months after the last treatment. Digital photos were taken at each visit. Formal assessment tools included patient and physician satisfaction scores and the Global Aesthetic Improvement Scale. In all, 21 women and 3 men completed the study. The majority (72%) had Fitzpatrick skin type II, while 16% had type III, 8% had type I, and 4% had type IV. An average of 5,042 pulses were delivered during each treatment session. The majority of patients (84%) required no anesthesia, while the rest used topical numbing medicine from 30 minutes to an hour prior to the procedure.
Dr. Jeon reported that the average pain score during the procedure was 4.7 on a 10-point scale. Forced-air cooling was used for comfort, and on average, mild redness following the treatment lasted less than 1 day (a mean of 0.6 days, with a range of 0-5 days). Mild pain also lasted less than 1 day (a mean of 0.1 days, with a range of 0-2 days). No swelling, crusting, bruising, bleeding, infection, blistering, scarring, burn, or dyspigmentation occurred.
On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved,” 17% and 18% of cases as “much improved,” and 4% and 9% of cases as “extremely improved.”
At 3 months, 35% of patients said they would be “somewhat likely” to recommend the procedure, and 30% said they would be “extremely likely” to recommend it.
Dr. Jeon reported having no financial disclosures.
DALLAS –
“It’s important to note that response was variable, but many patients were satisfied with the treatment,” Hana Jeon, MD said at the annual conference of the American Society for Laser Medicine and Surgery, Inc. “Further studies are needed to identify the clinical characteristics of neck laxity that would most benefit from this treatment.”
The researchers enrolled 25 patients with an average age of 58 years. The laser treatment settings were a 6-mm spot side-delivered at a fluence of 0.71 J/cm2 in a pulse width of 750 picoseconds. The patients were treated five times on the neck every 2-4 weeks, and follow-up visits were scheduled for 1 month and 3 months after the last treatment. Digital photos were taken at each visit. Formal assessment tools included patient and physician satisfaction scores and the Global Aesthetic Improvement Scale. In all, 21 women and 3 men completed the study. The majority (72%) had Fitzpatrick skin type II, while 16% had type III, 8% had type I, and 4% had type IV. An average of 5,042 pulses were delivered during each treatment session. The majority of patients (84%) required no anesthesia, while the rest used topical numbing medicine from 30 minutes to an hour prior to the procedure.
Dr. Jeon reported that the average pain score during the procedure was 4.7 on a 10-point scale. Forced-air cooling was used for comfort, and on average, mild redness following the treatment lasted less than 1 day (a mean of 0.6 days, with a range of 0-5 days). Mild pain also lasted less than 1 day (a mean of 0.1 days, with a range of 0-2 days). No swelling, crusting, bruising, bleeding, infection, blistering, scarring, burn, or dyspigmentation occurred.
On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved,” 17% and 18% of cases as “much improved,” and 4% and 9% of cases as “extremely improved.”
At 3 months, 35% of patients said they would be “somewhat likely” to recommend the procedure, and 30% said they would be “extremely likely” to recommend it.
Dr. Jeon reported having no financial disclosures.
DALLAS –
“It’s important to note that response was variable, but many patients were satisfied with the treatment,” Hana Jeon, MD said at the annual conference of the American Society for Laser Medicine and Surgery, Inc. “Further studies are needed to identify the clinical characteristics of neck laxity that would most benefit from this treatment.”
The researchers enrolled 25 patients with an average age of 58 years. The laser treatment settings were a 6-mm spot side-delivered at a fluence of 0.71 J/cm2 in a pulse width of 750 picoseconds. The patients were treated five times on the neck every 2-4 weeks, and follow-up visits were scheduled for 1 month and 3 months after the last treatment. Digital photos were taken at each visit. Formal assessment tools included patient and physician satisfaction scores and the Global Aesthetic Improvement Scale. In all, 21 women and 3 men completed the study. The majority (72%) had Fitzpatrick skin type II, while 16% had type III, 8% had type I, and 4% had type IV. An average of 5,042 pulses were delivered during each treatment session. The majority of patients (84%) required no anesthesia, while the rest used topical numbing medicine from 30 minutes to an hour prior to the procedure.
Dr. Jeon reported that the average pain score during the procedure was 4.7 on a 10-point scale. Forced-air cooling was used for comfort, and on average, mild redness following the treatment lasted less than 1 day (a mean of 0.6 days, with a range of 0-5 days). Mild pain also lasted less than 1 day (a mean of 0.1 days, with a range of 0-2 days). No swelling, crusting, bruising, bleeding, infection, blistering, scarring, burn, or dyspigmentation occurred.
On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved,” 17% and 18% of cases as “much improved,” and 4% and 9% of cases as “extremely improved.”
At 3 months, 35% of patients said they would be “somewhat likely” to recommend the procedure, and 30% said they would be “extremely likely” to recommend it.
Dr. Jeon reported having no financial disclosures.
REPORTING FROM ASLMS 2018
Key clinical point: Response to using a picosecond 755-nm laser with focus lens array for neck rejuvenation was variable.
Major finding: On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved.”
Study details: A single-center study of 25 patients treated for neck laxity.
Disclosures: Dr. Jeon reported having no financial disclosures.